
FIG 92. The alpine bush-cricket, male. (© G. Carron)
The patterns of interaction through which reproduction is managed in each species are the outcome of the evolutionary history of their lineage, and bear the legacy of the responses of past generations to a great range of different sorts of selective pressure. These include climatic and other environmental conditions, together with predation and parasitism and other factors that affect survival chances. But these factors combine with and often modify the various ways in which the behaviour and condition of individuals of one sex influence the reproductive success rates of the other. While it is recognised that sexual reproduction necessarily involves cooperative interactions between males and females, it is also true that males and females frequently have asymmetrical and even conflicting reproductive interests. Since neither sex has complete control over the reproductive process, the mating and reproductive sequence of each species can be understood as embodying a (possibly still evolving) co-evolutionary compromise between male and female reproductive interests, modified and constrained in various ways by natural selection (refer to Chapter 4 for more on these ideas).
As we saw in Chapter 4, the sequence of interactions that make up the reproductive system of sexually reproducing species can be analysed as a series of eight distinct (but sometimes overlapping) phases, each the outcome of a distinct set of selective pressures (Alexander, et al. 1997): rapprochement, courtship, mating, insemination, post-copulatory behaviour, fertilisation (in Orthoptera, coinciding with egg-laying), care of offspring, and maintenance of long-term pair bonds.
The first of these phases – rapprochement – was the main topic of the previous chapter. However, in gregarious species, such as the greenhouse camel-cricket (Diestrammena (Tachycines) asynamorus) no distinct phase of pair formation is required. In many species of grasshoppers, populations are often so dense that active searching for females on the part of males is an advantageous alternative to the calling song, and in the case of groundhoppers it is the only available strategy.
The second, courtship, phase is initiated when a male and female are at close quarters, and at least one – usually the male – is showing some sign of sexual interest in the other. In some species there is a distinct courtship song, but this is often combined (as becomes possible when the pair are physically close) with other channels of communication – visual, chemical and tactile. The courtship phase is considered to function as a way of inducing in the female a motivational change so that she accepts the male’s eventual mating attempt. Because even prolonged courtship often ends in rejection, and/or disruption and usurpation by rival males, it must also provide the basis for females to exercise choice (Robinson & Hall, 2002). Courtship songs and performances, therefore, should be understood as at least in part the outcome of sexual selection acting on ancestral populations. However, these and other pressures are likely to have worked to some extent in contradictory directions. As well as fixing the attention of the target female and influencing her physiological state, the male’s courtship should avoid attracting the attention of rival males or predators. This may help to explain why courtship songs, in those species that have them, are generally delivered at much lower intensity than calling songs. There is no benefit to be gained from the energetic costs and risks associated with projecting their advertisement over long distances once there is a target female close by, and presumably there is an advantage to be gained from a discreet assignation that is not noticed by other males in the vicinity.
In a few species there is no courtship phase, and males forcibly mate with females. This reproductive strategy, ‘coercive copulation’, is an extreme expression of conflicting male and female reproductive interests. It is adopted in some grasshopper species and, among the Tettigoniidae, in the Alpine bush-cricket Anonconotus alpinus (Vahed, 2002; Vahed & Carron, 2008).

FIG 92. The alpine bush-cricket, male. (© G. Carron)
In Orthoptera, as in most sexually reproducing species, there is usually a time lapse between copulation, insemination and actual fertilisation of the eggs. This lapse may allow females to choose which male’s sperm will fertilise her eggs and also reflects her greater interest in the timing and placing of the fertilised eggs. As most of the female’s strategies for controlling which male’s sperm fertilise her eggs are internal, physiological processes, they are termed ‘cryptic female choice’ (Eberhard, 1991, 1997. See also Chapter 4). In many species, males have evolved strategies in response to this that commonly prolong copulation or, by various means, limit the probability that the female will mate with another male prior to laying eggs fertilised by him.
Phases three to six in the mating sequence (copulation, insemination, fertilisation and post-copulatory behaviour) often overlap with one another, and have provided many opportunities for conflicting reproductive interests between males and females to have evolved into a great variety of strategies and counter-strategies in the mating systems of different subgroups among the Orthoptera. Perhaps the most intriguing and challenging of these elements in orthopteran mating systems are the nuptial gifts, which are especially prevalent among bush-crickets. The often ingenious research effort that has focused on the exploration and interpretation of this phenomenon has addressed some of the most fundamental theoretical questions concerning sexual selection, conflict of reproductive interest, and the evolution of sexual difference itself.
The final two phases of the sequence of reproductive behaviour as listed by Alexander et al., (1997) are rarely, if ever, encountered among orthopterans. The penultimate phase, cooperation between the sexes in care for the offspring, is found only in monogamous species, in which the male has equal interest in the welfare of the jointly produced offspring. A few groups of beetles and cockroaches exhibit joint care of the young, but there is as yet little or no evidence of this in any group of orthopterans. However, in some deep-burrowing species of crickets (Anurogryllus and Gymnogryllus species, for example), ground weta (Hemiandrus) and some mole-crickets (Gryllotalpidae), females take care of eggs and young nymphs. There is some evidence, too, that females of some of these species take over the underground chambers of males, and so may indirectly benefit from resources provided by them (Alexander & Otte, 1967; Walker, 1983b).
The many different groups of orthopterans have evolved strikingly divergent reproductive patterns, in which the various strategies mentioned above have been exemplified, and some of the sequential phases either omitted or collapsed into one another. This diversity has been a popular topic for researchers because it offers great opportunities to test the expectations derived from general evolutionary theory, as well as theoretical frameworks with a more specific focus on insects. What follows is a selection of case studies drawn from the vast literature on orthopteran reproductive behaviour. Some of these are readily understood in terms of theoretical expectations, while others remain puzzling and demand further research effort. The focus of this chapter will be on reproductive interactions following the successful completion of the rapprochement phase.
CAELIFERA
Examples of mating systems will be drawn from two major subgroups of the Caelifera: groundhoppers (Tetrigidae) and grasshoppers (Acrididae). The groundhoppers are of interest because their courtship and mating are conducted without the use of sound communication, but rely on visual (and probably chemical) signals. Another focus of research interest has been the incomplete development of signals to prevent mating between different species.
Grasshoppers exhibit a great variety of mating systems. Of particular interest are the sometimes highly elaborate courtship performances, including both song and dance, and the great persistence of male courtship in the face of repeated female rejection. In a small number of species there is evidence of male competition for, and defence of, territories holding resources attractive to females (‘resource defence polygyny’) – a pattern more frequently observed among the Ensifera.
In the Caelifera, mating involves the male mounting the female and curving his abdomen down and round so as to engage the female’s genitalia with his own. The spermatophore is inserted directly into the genital opening of the female.
Groundhoppers (family Tetrigidae)
The groundhoppers superficially resemble very small grasshoppers. However, there are significant structural and behavioural differences (see the Key and Chapters 1 and 8). They have no stridulatory or hearing organs, although they do make use of vibratory communication, presumably mediated by the substrate. This group of orthopterans has attracted rather little research attention until quite recently, but during the last decade Axel Hochkirch, Julia Gröning and their associates and others including Anders Forsman, and Sofia Caesar have produced a series of remarkable studies on three species of European groundhopper that also occur in Britain – the slender (Tetrix subulata), Cepero’s (T. ceperoi) and common (T. undulata). Several of their studies explore distinctive features of the reproductive behaviour of these species, and pose interesting questions about the lack of fully developed behavioural mechanisms preventing courtship and mating across the species divide (notably Hochkirch et al., 2006; Gröning et al., 2007; Hochkirch et al., 2008).

FIG 93. Habitat (mid Essex) shared by both common and slender groundhoppers.
Courtship, mating attempts and actual mating between males and females of different species are referred to as ‘reproductive interference’, and its persistence in this group of insects is puzzling from a theoretical point of view. Actual hybridisation has not been recorded, and apparently on genetic grounds is considered unlikely, so there are presumably post-mating barriers to fertilisation or embryonic development. As there are costs associated with interspecific reproductive activity – in terms of wasted time and energy, and unnecessary risk of predation – one would expect that better perceptual cues and communicative behaviours would have evolved to secure pre-mating reproductive isolation. Alternatively, one might expect that species lacking reliable means of discriminating between potential mates of their own and other species would either have evolved in discrete geographical zones, or, if overlapping in their ranges, would at least have diverged in their habitat preferences so that de facto encounters between individuals of the different species would be unlikely.
In fact, Hochkirch and his colleagues sometimes consider reproductive interference as a possible explanation for the rarity of shared habitat between pairs of groundhopper species, but in other discussions the problem is posed the other way round: how can we explain the fact that the species do coexist, despite failure to develop isolating mechanisms in their reproductive behaviour? T. ceperoi is a rare species in Britain, so the pattern of its coexistence with the other members of the genus is difficult to ascertain. However, careful searching of numerous sites where the slender groundhopper (T. subulata) occurs in my part of south-east England suggests that it commonly coexists in mixed communities with the common groundhopper (T. undulata). However, the common groundhopper does have a much wider ecological tolerance and is often found on dry heath and open grassland, by paths and so on, whereas the slender groundhopper does seem to be confined to damp or wet habitats.
All three species occur in rather dense aggregations, usually in open habitats with patches of bare ground or stones and plants such as algae, mosses and liverworts. Males are more active than females and move around their habitat, apparently in search of potential mates. They frequently interact with each other, sometimes signalling visually by raising one or both of the hind legs (‘leg flick’). If this occurs, both males usually go their separate ways. However, males frequently mount other males, which usually provokes a sudden (and quite amusing!) vibratory response like a temper tantrum from the one mounted. This, again, usually leads to mutual separation. The vibratory signal is sometimes used in addition to the leg-flick signal between approaching males, and also by a male after dismounting from another male.
On approaching a female a male will usually stop, and, facing her, extend his antennae forwards, twirling them as he does so (see DVD). This strongly suggests some form of chemical communication, although there appears to be no research evidence to support this.

FIG 94. A male slender groundhopper detects a female.
The next phase consists of a series of one or more bodily movements that appear to have a courtship function. The male of Cepero’s groundhopper performs an antic referred to by Hochkirch et al. (2006) as ‘pronotal bobbing’. He simultaneously dips his head and raises his hind legs, giving the impression of a very brief (0.8 of a second) and deep ‘bow’. He then approaches the female directly and attempts to mate. In both the slender and common groundhoppers, there are also visual displays. However, these are less marked than those of Cepero’s, and may be of two sorts. Sometimes a male will raise his body by stretching fore and mid legs, and swing his body forwards (‘frontal swing’), or he may swing his body sideways by stretching his legs on one side. Both slender and common groundhoppers use these signals, so that there is no obvious visual differentiation between their courtship signalling.

FIG 95. A male slender groundhopper performing a ‘frontal swing’ signal.
Mounting and copulation in all three species are very similar. Males clamber onto the back of the female, often back-to-front and with a good deal of physical re-orientation. When appropriately perched on top of the pronotum of the female, facing the same way, and grasping her with his legs, the male, with genital valves open, probes between the near-side of the female’s pronotum and her hind femur on that side. If she is receptive she allows this space to open up, and the male curves his abdomen down, twisting the tip sideways to make contact with the genitalia of the female from below. The process frequently involves antennal contact, the male lowering his towards the female, while she raises hers. Usually mating is very brief – often no more than a few seconds – but in cool or wet weather a pair may remain in copula for several hours.

FIG 96. A male slender groundhopper begins to mount a female, and now mounted, back-to-front.
The male quickly dismounts and continues searching, frequently re-mating soon afterwards. Similarly, females may re-mate with a series of males in rapid succession. However, females also regularly reject males in various ways, including simply walking away, or walking over them. More characteristically, however, rejection takes the more passive form of refusal to open sufficient space for the male to push his abdomen between her hind femur and pronotum. Sometimes females will vibrate their bodies violently to shake off particularly persistent and unwanted males. Occasionally, however, a female approaches a male and appears to invite mating by performing the ‘leg flick’ signal and waving her abdomen at the male. (The DVD shows examples of most of these behaviours).

FIG 97. A mating pair of slender goundhoppers; a mating pair of common groundhoppers, with surplus male.
Gröning et al. (2007) carried out studies of heterospecific mating attempts between T. ceperoi and T. subulata in both laboratory and field conditions. Their results suggested that males of both species did not discriminate between females of their own and those of the other species. Indeed, they even sometimes attempted to mate with other males. However, females did discriminate between con- and heterospecific males, rejecting the latter more frequently. The authors suggest that the differences between the visual displays of the males of the two species might enable females to identify the ‘right’ species. Gröning et al. consider that coexistence between the two species despite lack of complete reproductive isolation is possible because of differences in local population density between them. Frequencies of mating attempts by males were correlated with the number of encounters, irrespective of the species encountered, so if they encountered mainly females of their own species this would reduce the frequency of counter-adaptive mating effort being spent on the ‘wrong’ species.
Where the common (T. undulata) and slender (T. subulata) groundhoppers coexist, heterospecific courtship, mating attempts and actual mating are common. In laboratory studies by Hochkirch et al. (2008), females of each species were equally receptive to males of the other. Possibly, they argue, this may be to do with the similarity of the visual displays of the males of the two species. However, males of both species showed a preference for females of T. undulata. In fact, the preference of male T. subulata for females of T. undulata was so marked that in a mixed population experiment there were no conspecific T. subulata matings. The possible interpretation here is that the more bulky appearance of T. undulata females is preferred by male T. subulata in line with the common view that male insects tend to prefer larger females as they are likely to be more fecund.
However, filming at a site in Essex, where both species coexist, revealed frequent heterospecific courtship and mating between these two species and in both directions. In two of five filmed examples of male T. subulata attempting to mate with female T. undulata the male was successful. In three out of six examples of male T. undulata attempting to mate with female T. subulata, three were apparently successful. However, there was some evidence of conflict surrounding these mating attempts. Females of T. subulata sometimes resisted mating attempts by T. undulata males, in one instance by an episode of vibration. In two cases males of T. subulata attempted to disrupt mating between male T. undulata and female T. subulata, one of them by attempting to drag its rival off the female by grabbing its genital valves in its mandibles (see DVD). However, it is not clear whether these examples of competition between males or conflict between males and females are more common in relation to heterospecific mating attempts than they are in conspecific encounters. In both species, males frequently attempt to disrupt mating pairs of their own species. One common male tactic is to cling onto the opposite side of a female to the one already occupied by a rival male, and probe with his abdomen between his side of the female’s abdomen and her near-side femur. This tactic is frequently successful, as the second male may be allowed to mate as soon as the first has departed.
Hochkirch et al. (2008) consider that these species may coexist despite incomplete behavioural reproductive isolation, because although their distributions and habitat preferences overlap, they are not identical. If the two species predominantly occupy different niches within the same habitat, reproductive interference will be minimised by the relative rarity of heterospecific encounters. However, while it is true that T. undulata can occupy a wider range of habitats than can T. subulata, populations of the two species interact freely where they overlap. My own observations in southern Britain suggest that this is a common situation. One possible explanation might be that where population densities are high and males are persistent, the costs to females of resisting male harassment may be more than the costs of tolerating them, even when mating cannot lead to fertilisation. Although the minimal courtship displays described by Hochkirch et al. (2008) and Gröning et al. (2007) are often used as preludes to mating, males also proceed directly to attempted mountings and sometimes succeed in mating with no apparent courtship. This may be a measure of the intensity of male competition for mating opportunities.

FIG 98. Heterospecific mating: male slender groundhopper with female common groundhopper: (a) male attempts to mount but is blocked by the raised left femur of the female, but the male clings on (b), then dismounts (c), and tries again (d), this time succeeding (e). (f) Rear view of mating attempt of slender with common groundhopper.
Apparently the spermatophore transferred by male groundhoppers is relatively large (Farrow as cited by Marshall & Haes, 1988: 43) and consists mainly of protein. If, indeed, this constitutes a form of ‘nuptial gift’, it is surprising that male reproductive behaviour is so promiscuous, and that the refractory period between successive matings is virtually non-existent. Compared with most grasshoppers, among which it is quite rare to observe a female accepting a courting male under natural conditions, males of groundhoppers mate frequently, and females are also highly polyandrous. The relatively high success rate of males in persuading females to mate, which seems to characterise the mating systems of all three species, poses the question whether females may have evolved mechanisms of cryptic choice over which male’s sperm fertilise her eggs. Equally, there is no obvious male counter-strategy to prolong copulation or limit female promiscuity as one might expect in a mating system involving significant male parental investment. Caesar & Forsman (2009) conducted a laboratory study of the effects of polyandry on offspring viability in T. subulata. Surprisingly the survival of the offspring of multiply-mated females was lower than those of monogamous females. However, these results differed considerably between nymphs reared in shaded versus sunny conditions, implying that any benefits that might accrue from multiple matings could depend on the environmental conditions to which offspring are exposed.
The instances of males mounting other males, and associated interactions, seem to be open to interpretation. Gröning et al. (2007) and Hochkirch et al. (2008) describe these as copulation trials and see this apparently homosexual behaviour as an indication of the promiscuous mating motivation of the males. Sometimes these homosexual mountings do seem similar to mating attempts, but often they appear to have the character either of antagonistic encounters, or merely contact behaviour. An alternative interpretation of the rather high frequency of physical contacts and non-sexual mounting between individuals in these species may be that they are mechanisms involved in maintaining mating aggregations. This may be important in species that lack acoustic or other means of communication over long distances.
Grasshoppers (family Acrididae)
Courtship among grasshoppers varies greatly from species to species. Even among the three common British species of the genus Chorthippus there is a great deal of variation not only between species, but also from population to population in response to such factors as climate and density. In the nominate subspecies of the common meadow grasshopper, Chorthippus parallelus, there appears to be no distinct courtship song, but instead the male continues to produce the repeated buzz of the calling song when at close quarters to the female (see DVD). According to Ragge (1965) a receptive female makes soundless stridulatory movements in response, and males may sometimes make a sudden loud sound by a down-stroke of the hind legs immediately prior to mating. A close relative, the common field grasshopper, Chorthippus brunneus, also has no distinct courtship song. However, in this species, receptive females also stridulate, in response to other females as well as to males. Mating occurs as the outcome of exchanges of brief chirps between a male and a responding female.

FIG 99. Courtship and mating in the lesser marsh grasshopper: (a) phase 1 courtship, legs held low; (b) phase 2 courtship, legs held higher; (c) successful mating.
A third common British species in this genus, the lesser marsh grasshopper, Chorthippus albomarginatus, has a courtship song that is quite different from the calling song. Once a male encounters a female at close quarters he faces her and begins a prolonged sequence of stridulation that is repeated many times. Ragge divides this into four phases – a modified chirp, produced by the vibrating hind legs held at a low angle, almost parallel to the insect’s body, and ending with them held at a more acute angle. The second phase is a more subdued sound produced with the hind legs retained at this higher angle. The third phase consists of a variable number of continuous repetitions of phase one. After a short pause (phase four) the whole sequence is repeated (see clips on the DVD). Throughout the sequence the sounds produced are much more subdued than the buzzing chirps of the calling song, but even so they are frequently interrupted by the arrival of competing males.
In all three species the courtship sequence may be repeated many times over before a male attempts to mate. A male that attempts to mount an unreceptive female may receive a sharp kick from one or both of the hind legs of the female, or, less decisively, will have his mating attempt obstructed by the female raising her hind femur on the relevant side. At any point in the courtship, a female may either jump clear, or simply move off and settle again nearby. In the latter eventuality, the male will often follow and resume his courtship.
These species frequently occur in quite dense populations, and it is relatively rare for males to be allowed to continue courtship without interruption by one or more rival males. Males that encounter one another in the course of searching behaviour will usually exchange a leg flick signal with one or both hind legs and move on their separate ways. However, in some species, notably Chorthippus brunneus (and, in other genera, Omocestus stigmaticus and Myrmeleotettix maculatus) small clusters of two to four or five males may engage in collective chases of an unwilling female, often signalling to one another with hind leg flicks or brief bursts of stridulation as they do so. In C. albomarginatus, groups of up to ten individuals of both sexes gather together, with repeated direct mating attempts by assembled males, and leg-flick signals exchanged between them (see the species account and DVD clips).

FIG 100. Two males of the lesser marsh grasshopper compete for the same female: one courts while the female blocks an attempted mounting by the other; the rejected male departs, while the other attempts to mount from the rear.
Some species exhibit much more complex courtship routines (see Berger, 2008; Ostrowski et al., 2009), and these include two species that occur in Britain – the rufous grasshopper, Gomphocerippus rufus and the mottled grasshopper, Myrmeleotettix maculatus. Males of the mottled grasshopper have a rather quiet calling song, which is often uttered at intervals as males run around patches of open habitat in search of females. As a female is encountered (either visually or by chemical cues) the male stops a short distance away, and begins his elaborate courtship routine. This is characterised by Ragge and Reynolds (1998: 366) as one of the most complex so far known among the western European grasshoppers. The sequence consists of a series of three phases, the first two of which may be repeated many times over before a male makes a mating attempt. If he moves on from phase two to three, there is usually a mating attempt as the culmination of phase three. However, if this is unsuccessful, the male will usually return to phase one and continue the courtship.

FIG 101. A mating pair of mottled grasshoppers, with the female still courted by another male (and a common groundhopper looks on!). (© N. Owens)
Phase one takes the form of a prolonged (20 or more echemes) quiet stridulation, with the hind femora held relatively high, and describing a narrow arc as the sound is produced. The antennae are raised gradually with each stroke of the hind legs until they are raised vertically above the head, but spread out laterally in a wide ‘v’ shape. In the course of this phase, the insect sways both sideways and vertically by regular alteration of the angles of the various leg segments. These movements become more intense until phase one is terminated by a sudden swinging forward and back of the hind legs, sweeping back of the antennae and lifting of the face. Phase two continues with a new pattern of stridulation, in which the hind legs are vibrated alternately at low and higher angles in the production of each echeme. The antennae are kept raised during this phase, and the process of increasingly intense rocking, or swaying movements of the body is repeated as in phase one. This phase, too, is terminated by the sudden, jerky, throwing back of the antennae, facial movement and hind-leg swing. These phases may be repeated many times, but the routine culminates in a third phase in which the repetition rate of echemes is increased, giving a low-intensity continuous sound, while the rocking movements of the body are accompanied by a repeated sideways swivelling of the head. As this phase reaches a peak of intensity, the male lunges at the female.
Almost always this approach is rejected by the female. She may leap decisively away or, more usually, hop or walk a few centimetres away, and remain motionless. Invariably the male will follow and recommence the courtship routine. Sometimes the female, too, may issue a brief burst of stridulation, but this does not seem to indicate immediate readiness to mate. This ‘cat-and-mouse’ game can continue seemingly interminably – for 30 minutes or more – before the male gives up or succeeds in mating. Ragge and Reynolds note that the sequence in this species is not as stereotyped as in some others, with some variation in the passage between phases. Interestingly, the transition from phase two to three is variable, often being preceded by numerous repetitions of the first two phases. However, there appears to be a stage reached in the sequence when phase three follows ‘automatically’ from a final repetition of phase two. This is evidenced in a sequence (see DVD) in which a female leaps away from a courting male at the end of phase one, but he continues through phases two and three and finally lunges at the now absent female. He then appears to search the immediate vicinity as if confused by her absence. This seems to suggest that visual inputs after a certain point in the routine are not processed, or, at least, do not issue in appropriate motor responses to modify or terminate the routine.
The courtship of the rufous grasshopper (Gomphocerippus rufus) is, if anything, still more striking. It too can be conveniently analysed as comprising three phases. First, on encountering a female, the male faces her with antennae held low, and palps wide apart. He produces a low-intensity stridulation with the hind femora describing a narrow arc, and held low (i.e. almost parallel to the line of the body), and waves his antennae up and down, independently of one another. The next phase, which may or may not involve continued low-intensity stridulation, consists of side-to-side head-wagging, with the palps swinging conspicuously along with the head movements. This pattern is accelerated until phase three takes over. This is initiated by a sudden swinging back and then forward of the antennae, now spread wide, while the head-wagging is greatly speeded up and accompanied by an intense lateral shaking of the palps. This continues for approximately one second, after which the insect jerks back a few millimetres and repeats the exercise two or three times. These actions are accompanied by rapid vibration of the hind legs. Phase three usually culminates in the male lunging at the female, frequently hitting her sharply with his head. He may repeat this two or three times, unless the female departs (see DVD).
As with the mottled grasshopper, the first two phases may be repeated many times before a male moves into the final phase of the courtship and then, in the (usual!) event of the mating attempt failing, the whole performance is repeated. In one courtship sequence observed, the rebound from the violence of the male’s lunge at the female was sufficient to throw him off the dogwood leaf on which both were perched. This male laboriously negotiated the complex shrub structure to climb back and continue the courtship. As with the mottled grasshopper, a female frequently departs during a performance, leaving the male to complete ‘solo’ and lunge at the space she had previously occupied. Again, the courtship behaviour of the rufous grasshopper resembles that of the mottled in that females show little sign of interest, if any, in the male performance, sometimes leaping well away or, alternatively, making less decisive signs of rejection. In the case of rufous grasshoppers, courted females frequently turn away from their suitors, in which eventuality males usually manoeuvre so as to present themselves frontally to the female (presumably to ensure that she has a good view). Sometimes male attempts to mount are blocked by the simple expedient of the female raising the relevant hind femur. Like males of the mottled grasshopper, male rufous grasshoppers also continue at least to the end of the current phase of the courtship routine, and often repeat it even if the female jumps away. An experimental study by Riede (1986) designed to investigate the control mechanisms of the courtship performance in this species showed that in fact courting males can process visual inputs during most parts of the routine. However, detailed visual cues are involved in the initial male orientation to the female prior to courtship, and thereafter even replacement by a dummy does not disrupt the courtship. Partially blinded males continue to repeat phases of the courtship performance many times over in the absence of the female.
Another species with elaborate courtship behaviour is the north American band-winged grasshopper Trimerotropis maritima. As noted in Chapter 5, despite the vivid flight displays of the males, pairs are generally formed by male searching behaviour, using visual or chemical cues. According to the detailed study of the mating system of this species (Steinberg & Willey, 1983), males respond to encounters with females either with a direct mating attempt, or by embarking on a courtship routine. This begins with a signal they refer to as ‘fluttering’ (small-amplitude movements of the hind femora) followed by bouts of courtship chirping, interspersed with periods of silence in which the male continues to face the female. Signals used by the female include ‘fluttering’, as well as what Steinberg and Willey refer to as ‘femur raising’ and ‘waving off’. The former, as the name suggests, involves raising the hind femur (or femora) to 90 degrees or more, with the angle between tibia and femur open or closed. ‘Waving off’ consists in either a waving or a kicking movement of the raised hind femur. These female responses appear to indicate unreceptiveness on the part of the female, as a courting male never mounted after presentation of all three signals by a female, and usually did not mount after the femur-raising or waving-off responses. However, males did not abandon courtship after these signals from the females, and Steinberg and Willey suggest that they may even have the function of further stimulating the male’s courtship activity. In seven out of eight cases of courtship leading to copulation, femur-raising and/or waving-off responses had occurred in earlier bouts. The number of bouts involved in the instances of courtship observed varied greatly, but in some cases males showed great persistence, in one case continuing a courtship for over 27 minutes. Courtships were terminated by males mounting females, but these often did not result in copulation, as females either did not open their genitalia, or revealed spermatophore mating plugs from previous matings.
This mating system has several interesting features. First, the ability of males to identify the sex of static females suggests a possible role for chemical communication in this mating system. Second, the role of various female signals in response to male courtship cuts against common assumptions about the passive character of female involvement in mating systems. Steinberg and Willey consider that courtship in this species may have several functions – enabling sex and species identification on the part of females, changing the motivational state of the females to overcome their resistance to mating, and displaying the ‘fitness’ of the male. It might not be easy to discriminate between the last two of these supposed functions, but possibly the persistence of male suitors despite repeated rejection signals is one way in which females may assess the fitness of individual males. Finally, a feature that defies easy explanation is that males frequently court nymphs. There is clearly no chance of copulation, and the visual appearance of the nymphs is quite different from that of adult females. Courted nymphs femur-raise and wave off males, and the males do not generally attempt to mount them.
The elaborate courtship performances that appear to be required by the females in each of these species are presumably the result of a long history of sexual selection acting on the males. This suggests an ‘operational sex ratio’ strongly biased against males. One possible explanation for this could be that the females mate only once, or very few times, so males get very few mating opportunities. Extremely elaborate and prolonged courtship could be explained either as a means whereby females assess the ‘quality’ of males by their vigour and persistence, or as the outcome of a ‘Fisherian’ process of sexual selection, through which female preferences for elaborate courtship routines co-evolve with the inherited ability of males to produce them (see Chapter 4). Either way, the extreme persistence of the males in the face of repeated apparent rejection by courted females suggests that persistence itself has been rewarded, with persistent males eventually mating successfully. Bull (1979) reported that only 10 per cent of 1,610 courtship routines initiated by males of M. maculatus actually reached the final phase, and mating attempts even then ‘rarely succeeded’. The high energetic and other costs to the males of these prolonged performances do seem to imply a powerful role for sexual selection in defiance of survival-related selective pressures. In the virtual absence in the world of grasshoppers of vivid colours and eye-catching patterns, perhaps these elaborate and impressive courtship routines are the closest orthopteran parallel to the peacock or the bird of paradise?
The intensity and direction of sexual selection depends, among other things, on the relative frequency with which males and females mate, the common view being that it is usually in the reproductive interest of males to mate more frequently; females less frequently (See Chapter 4). However, very little is known about mating frequencies of grasshoppers under natural conditions. Reinhardt et al. (2007) carried out a field study to establish the mating frequency of females of C. parallelus. The population chosen was dense, with frequent encounters between males and females, but females were estimated to re-mate only every 6 to 7 days, compared with the greater frequency of once every 2.6 days when kept under confined conditions in captivity. This is interpreted as an effect of greater female control over courtship and mating under natural conditions. Nevertheless, it seems that females in this and many other grasshopper species are generally multiply mated, despite mating less frequently under natural conditions. It has been independently shown that multiple mating is not usually necessary to fertilise the complement of the female’s eggs, so a question arises as to the potential benefits to females of multiple mating (in those species in which it occurs). One hypothesis is that sperm deteriorates in some way while it is stored in the spermatheca, with female re-mating explained in terms of the benefits of replenishing the store with fresh sperm. Reinhardt et al. (1999) compared singly with multiply-mated females, with respect to numbers of eggs per egg pod, fertilisation rates, hatching rate and sex ratios, and found no negative effect of single mating or age of stored sperm on any of these measures of fitness.
However, Reinhardt et al. did find that multiply-mated females produced heavier offspring. A possible explanation for this is that females derive some nutrients from the protein-rich spermatophore delivered by the male. Amino acids derived from spermatophores have been shown to be directly involved in the formation of the eggs in some grasshopper species, and there is associated evidence of increased fecundity of multiply-mated females as well as greater longevity (e.g. Butlin et al., 1987; Pardo et al., 1994, 1995; Belovsky et al., 1996). In C. parallelus, both singly and multiply-mated females were supplied with abundant food in the study by Reinhardt et al., suggesting that general shortage of nutrients was not the cause of willingness to re-mate. However, it could be that the male spermatophore delivers some specialised nutrients important for egg formation. In the north American migratory grasshopper Melanoplus sanguinipes, laboratory trials by Belovsky et al. (1996) indicated a female preference for males that fed more effectively. These males provided greater spermatophore mass. This result is consistent with female choice related to paternal investment, with poorer male foragers resorting to coercive copulation. However, in this study males that had recently mated and so produced smaller spermatophores were still preferred by the females.
The evidence of nuptial feeding and paternal investment in the offspring in grasshoppers is still rather limited, but, as we shall see, these aspects of bush-cricket mating systems have been intensively researched and discussed.
The transfer of nutrients along with the sperm presumably involves some additional cost to males, which, in any case, have a reproductive interest in increasing the probability that their sperm will fertilise the eggs of the female with which they have mated. This is a possible source of conflict of reproductive interest between the two sexes, and we might expect male strategies to have evolved that induce the female to lay eggs soon after mating, and/or to restrict her opportunities to re-mate with another male before laying her eggs. In C. parallelus (and probably most other grasshoppers), sperm from successive matings are not stored separately in the spermatheca. Clearly, the eggs laid by a female who has mated only once will all be fertilised by that male. However, assuming that not all the stored sperm from that mating are used up prior to a second mating, some of the first male’s sperm are likely to fertilise at least some of the eggs laid after the second mating. On the theoretical assumption of free mixing of the sperm in the spermatheca, and no ‘passive’ loss of sperm (e.g. from leakage or absorption), the first male would have roughly half the paternity of eggs laid after the second mating.
In a study of C. parallelus and its relative C. biguttulus (Reinhardt, 2000) the proportion of eggs fertilised by a second male showed considerable variation among individual females of each species. In some females, the proportion of eggs fertilised by the second male was initially high, but declined over time (and successive egg pods). In others, the percentage of eggs fertilised by the second male remained high and constant at 80 to 90 per cent. In both species, significant passive loss of sperm was noted, contributing to the reduction in the first male’s paternity, to an extent dependent on the time elapsing between first and second matings. The ‘last male sperm precedence’ shown in those cases where the second male continued to fertilise most of the eggs laid subsequently is consistent with a displacement model in which the sperm left over from the first mating are displaced by the second. Studies of other grasshoppers and locusts have indicated a range of strategies employed by females to control the process of fertilisation after mating, including ejection or dissolution of the spermatophore, and re-positioning of the sperm from different matings in relation to the oviduct (cited in Reinhardt, 2000), but so far the results of experimental studies are inconclusive.
There appears to be little research on male strategies to enhance paternity in grasshoppers, but it has been established that in some species chemical stimulants delivered by the male by way of the spermatophore are responsible for accelerated formation of eggs, stimulation of oviposition or increases in the numbers of eggs laid. Increased rates of oviposition in females of Melanoplus sanguinipes and the locust, Schistocera gregaria, were produced by implantation of male accessory gland secretions (Friedel & Gillott, 1976, 1977), and similar results were obtained for the migratory locust, Locusta migratoria (Lange & Loughton 1985). In other species (e.g. Ailopus thalassinus), volatile chemical secretions – pheromones – emitted from the male abdomen and detected by the female antennae can induce increased egg production independently of either visual or tactile contact with the male (Siddiqi & Kahn, 1981; Whitman, 1982; Schmidt & Osman, 1988; see also Whitman, 1990).
Among male grasshoppers, the most common type of mating system is that sometimes described as ‘scramble competition’. However, a genus of gomphocerine grasshoppers, Ligurotettix, is very unusual in that the two recognised species, L. coquilletti and L. planum, exemplify the ‘resource-defence polygyny’ form of mating system (more frequent among the Ensifera). Aspects of acoustic communication in these species were introduced in Chapter 5, and here we consider other aspects of their mating systems, as studied by Greenfield, Shelly and others. Both species occur in desert ecosystems in southwestern USA. Their distinctive mating systems have probably evolved in the context of highly specialised food sources that are particularly important for females, giving selective advantages to males that can establish and maintain territories on those (relatively scarce) shrubs favoured by females. L coquilletti is associated with creosote bushes (Larrea tridentata) while L. planum is confined to bushes of tarbrush (Flourensia cernua). The nutritional value of individual shrubs varies greatly, and there are toxins in the less nutritious plants that inhibit growth. Both males and females in both species tend to congregate on the more nutritious shrubs, and males call, court and mate with females on a shrub that they may occupy persistently – for up to 21 days in the case of L. coquilletti males. A female approaches a shrub in response to the calling song of a territorial male, but once on the shrub, does not approach further. The male occupant uses visual cues to spot her, approaches, and proceeds to court her, often continuing to stridulate but also producing soundless hind leg movements. This usually leads to the male mounting the female, but if she is not receptive, he dismounts. One in three or four mountings leads to mating, which lasts from ten to fifteen minutes, depending on the species.
Males compete for territory on the more nutritious shrubs by a series of escalating behaviours. An intruding male is approached by the incumbent, and if the former does not retreat, a bout of competitive calling ensues, as a result of which the male who sustains a higher signal rate often succeeds in causing his rival to retreat or become silent. If he does not do so, then direct fighting ensues, involving kicking, grappling and biting. However, retreating to less favoured shrubs has disadvantages: defeated males may still remain on the same bush as silent satellites, and ‘co-dominant’ males might coexist, despite conflict, on the same bush. In L. planum, rather different ecological conditions have favoured a greater tendency for males to depart and move from shrub to shrub. Males on nutritious shrubs mate, on average, every five to ten days. Male encounter rates with females, and mating success, are higher for those congregating on nutritious bushes than for solitary males on less favoured perches.
This is clearly a system in which strong sexual selection is at work, but it is not clear whether females are attracted to calling males on the basis of their ‘quality’, or whether they, and possibly the males themselves, are independently attracted to the more nutritious shrubs because of the resources they offer. Ingenious experiments carried out by Shelly and Greenfield (1991) favour the interpretation that females of L. coquilletti were attracted to and settled on the more nutritious Larrea shrubs independently of the intensity and number of calling males, although they did not approach shrubs that lacked a calling male. However, some observational evidence suggested that females left shrubs when some occupant males left. Greenfield (1997) suggests that, once present on a shrub with one or more rival males, females would be well placed to evaluate male ‘quality’ on the basis of a wide range of characteristics, not just according to calling intensity or signal rate.
Evidence on whether males were attracted to the calling of other males was also rather equivocal, leaving open the question of whether males tended to congregate on and defend territories on nutritious shrubs solely on the basis of assessment of the quality of the shrubs, or whether they were also using the calls of incumbent males as a short cut to identification of shrubs offering favourable resources and/or more frequent mating opportunities. Given that males who maintained territories among congregations on the most nutritious shrubs were the ones with the highest mating rates, it seems likely that sexual selection would favour the male attributes that lead to successful holding of advantageous territories. In the case of L. coquilletti, prime among these attributes was the date at which they became adult, with early maturation linked to finding and maintaining favourable shrubs. Early occupants usually won subsequent conflict with potential usurpers. However, mating success does not necessarily imply successful fertilisation – and nothing is known about the ability of females of these species to evaluate the fecundity of rival males.
ENSIFERA
This diverse grouping includes the crickets, mole-crickets and their relatives (Grylloidea), bush-crickets (Tettigoniidae), camel-crickets (Rhaphidophoridae), New Zealand weta (Anostostomatidae), ambidextrous crickets (Haglidae), and others (see Chapter 1).
In the Ensifera, copulation is usually achieved by the male’s courtship performance stimulating the female to mount him, although in some species that live among tall grasses or rushes, contact is achieved by mutual orientation from opposite sides of a plant stem. The active cooperation of the female is needed for successful mating in most species, and this is commonly associated with distinct courtship interactions. Generally, the Grylloidea have courtship songs, while the Tettigonioidea tend to rely more on chemical and tactile interactions, especially involving mutual antennation and palpation of body surfaces. However, more recent research has emphasised the multiplicity of the channels of communication involved in courtship, mating and post-copulatory behaviour in all subgroups of the Ensifera.
The sperm are contained in a spermatophore, which is attached to the genital opening in the abdomen of the female during copulation. Some time is taken for the sperm to pass from the spermatophore into the spermatheca of the female, where, in some groups, sperm from each mating are stored separately (Vahed, 2003a). In bush-crickets, camel-crickets and some gryllid crickets, the spermatophore includes not just an ampulla containing the sperm and seminal fluid, but also a large gelatinous spermatophylax. Much of the fascination of bush-cricket mating systems is associated with rival hypotheses about the role of the spermatophylax in relation to conflicts of reproductive interest between males and females. In most true crickets there is no spermatophylax, and the conflict of interest between males and females is played out in rather different ways, with various sorts of male coercion – including mate-guarding – often playing a greater role.
Stenopelmatoidea
Tree weta (Hemideina species)
Only relatively recently have the extraordinary mating systems represented among the Stenopelmatoidea been subject to close study, and their evolutionary relationships and classification remain controversial. Among the best-studied are the mating systems of the New Zealand tree weta of the genus Hemideina (family Anostostomatidae).
Two species, H. femorata and H. crassidens, have similar, well-studied mating systems. Both species are closely associated with ‘galleries’ – holes in trees of several species that are initially made by wood-boring beetles or moths, and subsequently widened or deepened by occupying tree weta. Occupancy of galleries is very variable. Many apparently suitable holes are not occupied, while others may contain solitary males or females, male/female pairs, or single males with ‘harems’ of up to as many as 13 females. Males compete aggressively for control over galleries containing females, and a case has been made for their mating system to be included, like that of the Ligurotettix grasshoppers described above, in the category ‘resource-defence polygyny’. This is because the galleries are important means of shelter from a wide range of predators, and so are sought by females. Control over access to a large gallery would therefore increase the chances of a male encountering and mating with one or more females.
Adult males of both species have strikingly enlarged heads and mandibles, and these are used in conflicts between males over possession of galleries. Aggressive responses are graded, from a minimal palpation and antennation on the part of an intruding male who then departs, through kicking of the intruder by the occupant with his spined hind legs, and aggressive stridulation, to grappling, biting and lunging with gaping mandibles. The larger, better-equipped males more often succeed in these conflicts, and larger males tend to be the ones occupying the larger galleries with harems. It is supposed, therefore, that these males have greater reproductive success, and the large size and large heads and mandibles are secondary sexual characteristics that have evolved by sexual selection (Field & Sandlant, 1983; Field & Jarman, 2001).
There is no distinct courtship song, but a male tree weta approaches a female and at close quarters uses antennal contact and touching with the mandibular and maxillary palps prior to gripping the hind tibia of the female, and dragging her out of the gallery if necessary. He then curves his abdomen to probe various parts of the female’s body. The response of the female involves various levels of resistance, such as reorienting her body, moving away, kicking the male or attempting to re-enter the gallery. However, these signs of apparent female rejection do not usually deter the male, who continues to ‘court’, with variable eventual success rates (23 per cent successful in H. femorata; 64 per cent in crassidens are reported – see Field & Jarman, 2001). Mating behaviour in the male is triggered by non-volatile chemicals present on the cuticle of the female, but it is unclear whether there are specific organs of chemical sense in the male, as distinct from widely distributed sensilla. Copulation is usually achieved by the male standing behind the female, grasping her with one or more pairs of legs, and curving his abdomen-tip under hers. However, the ‘female on top’ pattern that is more typical among the Ensifera has also been observed. The dorsal surface of the ninth and tenth segments of the male has a structure known as the ‘gin trap’, which ensures genital connection and transfer of the spermatophore. Copulation in Hemideina is brief (lasting 1.5 minutes, according to Field & Jarman). However, complete transfer of sperm may take as long as 5 hours (Kelly, 2008), and, in this group, males lack the spermatophylax or other nuptial gift that might serve to distract the female and prevent her from consuming the spermatophore before its contents have been fully transferred.
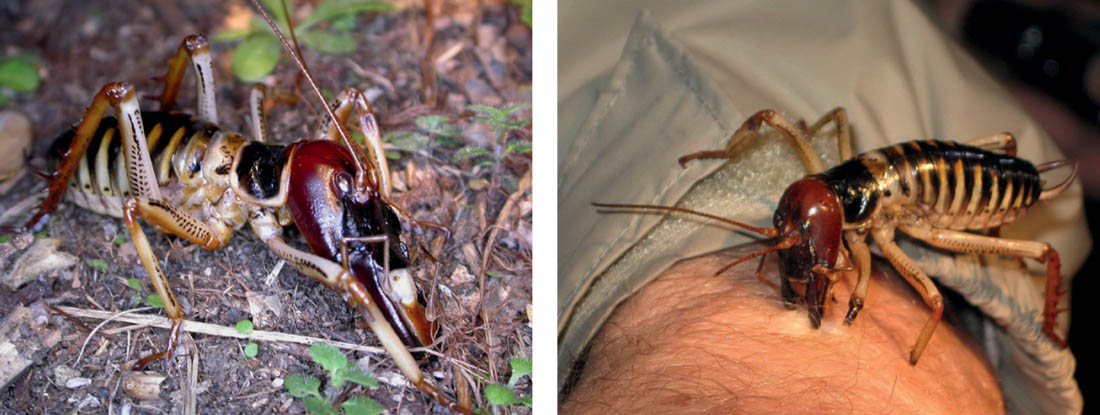
FIG 102. A tree weta, Hemideina crassidens: a male, showing enlarged head and mandibles; a male making use of its mandibles! (both photos © D. Gwynne)
One possible interpretation of the male occupancy of galleries is that it enables them to guard females with which they have mated, ensuring both that the full complement of sperm is transferred to the female, and also that she has no opportunity to mate with other males prior to ovipositing. Evidence that supports this interpretation is given by Jarman (1982) who observed male guarding behaviour for an average of 36.5 minutes after copulation in H. crassidens. However, males also sometimes react to female attempts to leave during the guarding period with what appears to be aggressive behaviour, similar to that shown in male-to-male encounters. This usually results in the departure of the female and appears to contradict the reproductive interest of the male.
As previously demonstrated in an ecological study by Field and Sandlant (2001), many apparently suitable holes are not used, suggesting that galleries per se are not a limiting resource. Studies by Kelly (2006, 2008) suggest that females prefer otherwise unoccupied holes to ones already occupied either by other females, or by a male (or both). He explains this in terms of the hypothetical costs to females of cohabitation with males (or with other females whose presence is likely to attract males). These costs have to do with male harassment which, as we have seen, can involve high levels of male aggressive behaviour. This often damages the females and renders them less mobile. Also, according to Kelly’s account, when matings occur at the entrance to galleries, males throw females off the tree after mating. This, along with other evidence on male aggression towards mates, tells against the interpretation of the gallery system simply as one adopted by males to monopolise mating opportunities with one or more females. The picture is further complicated by evidence that males are not in general more sedentary than females. Males do persist in a gallery for longer, the more females they have with them, but they and the females frequently depart without apparent usurpation by a competing male. Kelly interprets his findings as fitting the mating system of H. crassidens more with ‘male dominance polygynandry’ than with the usual model of resource-defence polygyny (Emlen & Oring, 1977). In male dominance polygynandry, both males and females mate multiply, and males compete for and mate with groups of females as they become receptive. On this interpretation, the males defend galleries only by virtue of the presence of females, and remain in charge of galleries in order to mate with the females, subsequently moving on to search for further mating opportunities.
The puzzling tendency of males to aggressively evict females after mating, throwing them off the tree onto substrate below, was confirmed in both field and experimental studies by Kelly (2008). One possible explanation for this is prompted by the novel observation that when females are confronted by a subsequent mating attempt, they consume the spermatophore from their previous mating. As other males are much less likely to encounter females that have been evicted in this way (because of the low population density), the eviction may serve the same function as mate-guarding. This leaves open the question of why a female consumes the spermatophore from a previous mating when she encounters a subsequent mating attempt. Kelly suggests that this may be because of the likelihood that a subsequent mating attempt will be made by a rival male who has successfully displaced her previous mate (Kelly, 2006). Spermatophore consumption, on this interpretation, would be an expression of female preference for a dominant male. The frequency with which females mate, their longevity (approximately one year as adults), and evidence from examination of wild-caught females that they store sperm, taken together suggest that the habit of spermatophore consumption imposes no risk that they will acquire too little sperm to fertilise their eggs.
It seems that the complexity of observed behaviour so far defies consistent interpretation in terms of prevailing theory. Even more demanding is the discovery that in H. crassidens, some males become sexually mature at an early developmental stage – in their eighth or ninth instar – rather than when fully adult (tenth instar). These males are smaller, with much smaller head and mandibles relative to total body size. They tend to occupy smaller holes than the larger males, and call from the entrances to their galleries. They do not compete aggressively with larger males, but attempt to mate with females as the latter move about outside galleries. Their mating success is supposed to be lower than that of the larger males, but ninth instar males are sometimes able to hold galleries with females. It may be that sexual selection has given rise to two alternative male mating strategies.
Finally, H. femorata provides an interesting example of potential ‘run-away’ sexual selection in favour of large heads and mandibles being off-set by natural selection. In the population studied by Field and Sandlant (2001), H. femorata galleries were almost exclusively made in the holes of a single species of wood-boring beetle, which, on emergence from the tree, leaves a hole of 10–13 mm in diameter. This diameter sets an outer limit to the head-size of H. femorata males, for if they were unable to enter these abandoned holes they would have no protection from predators and would be unable to establish harems. In fact, although large-headed males are dominant and probably have greater mating success, smaller males can occupy smaller galleries and avoid aggressive usurpations from larger males that are too big to enter their galleries.

FIG 103. A female ground weta with her eggs; and with newly hatched nymphs in an artificial chamber. (© D. Gwynne)
Ground weta (Hemiandrus species)
Unlike most weta, males of this group offer ‘nuptial gifts’ to females. These are delivered to a separate receptor-organ on the abdomen of the female. The ground weta are also unusual among the Orthoptera in that females take care of both eggs and early instar nymphs (Gwynne, 2004).
Hagloidea: Ambidextrous crickets (genus Cyphoderris)
The ambidextrous crickets belonging to the genus Cyphoderris (family Prophalangopsidae) are relics of an ancient grouping that may have been ancestral to the bush-crickets. Only three species that occur in north-western USA have been thoroughly studied, but even they remain somewhat mysterious. The calling and mating of C. strepitans, which inhabits sage bush meadows in mountainous regions of Colorado and Wyoming, takes place early in the Spring, just after snow melt. Males suffer the cost of having to sing at very low ambient temperatures, and it may be that this feature of the mating system has evolved as avoidance of temporal overlap between overt calling activity and peak activity of predators such as voles and owls (Sakaluk & Eggert, 2009). Males sing from perches at various heights, but often from the tops of sage bushes, from late afternoon into the night. They are equipped with powerful mandibles, and can show aggression to one another if crowded artificially, but under natural conditions they sing from evenly spaced-out perches. It seems that male song is involved in the spacing out of the males, but is also a means of attracting sexually receptive females. On locating a potential mate, the female mounts him. The male then ceases calling and spreads out his tegmina to expose the soft fleshy hind wings below. The female feeds on these, while the male attempts to connect his abdomen-tip with her genitalia, using a ‘gin-trap’ structure on his final abdominal segment. Females are able to prevent copulation, and extended periods of mounted females feeding from the males’ wings without copulation are reported. However, receptive females copulate with the male, whereupon he makes pumping movements with his abdomen and a spermatophore, including a large spermatophylax, is attached externally to the female’s genital opening. Copulation may last from one to five minutes, varying by species and in relation to the mating history of the individuals involved. Mating is terminated by the male, but females sometimes continue to feed on his wings and the haemolymph that flows from the exposed tissue. There is a considerable delay before the females consume the spermatophylax. Males begin to call again directly after mating, but re-mating in the same night has not been reported, and females appear not to mate again for some time, if at all.

FIG 104. Cyphoderris strepitans: (a) a singing male; (b) a pair mating. (both photos © D. Funk)
This mating system appears to be unique in the provision by the male of a double nuptial gift. The high cost to the male of his role in the mating system has been assessed by changes in weight. Calling itself demands a high level of energy, and weight loss as a result of calling for just one hour was reported to be 1.9 per cent of the males’ body weight (Dodson et al., 1998). Copulation, including the transfer of the nuptial gifts, was shown to be still more costly, with weight loss measured at approximately 10 per cent of body weight. The most plausible interpretation of the male nuptial gift in this case appears to be that it is a form of ‘mating effort’ on the part of the male, a result of sexual selection by females who benefit either in the form of nutritional input for themselves, or in the form of greater resources devoted to egg-laying (or both). If females do benefit from the transfer of the dual nuptial gift, then it is to be expected that they will prefer males that have more to offer. One obvious indication of this might be the relative mating success of ‘virgin’ males, with fully intact hind wings, and non-virgins, who will have less – or less that is palatable – to offer. An experimental study conducted by Morris et al. (1989) on a population of Cyphoderris strepitans confirmed the expectation that virgin males would be preferred. It was not clear how this discrimination occurred. It might have simply been a consequence of the depressed calling of just-mated males, who might have to be preoccupied with feeding or healing their wounds. Alternatively, females might detect wing damage in males, either by direct contact or by the small change in the character of the song caused by the reduced size of the hind wings.
Subsequent experimental work by Sakaluk and colleagues (Eggert & Sakaluk, 1994; Sakaluk et al., 2004) demonstrated that a distinct reduction in male mating success after their first mating was imposed by the loss of haemolymph, independently of the costs of restoration of the spermatophore. It was also shown that removal of the hind wings had no effect on the attractiveness of the male song, or of the willingness of females to mount males. However, males that were able to provide haemolymph feeding were more likely to succeed in transferring their spermatophore to the female. As males tend to withdraw from the female once the spermatophore is attached, it is supposed that the function of the haemolymph nuptial gift is to sustain copulation for long enough for transfer of the spermatophore to take place.
Tettigonioidea: the bush-crickets (Tettigoniidae)
Bush-crickets generally do not have a courtship song distinct from their calling song, although, in many species, courtship does include a subdued version of the calling song. Once a pair is formed – either by female phonotaxis towards a male calling song, or by mutual orientation through exchange of calls (sometimes aided by vibratory communication) – courtship is usually initiated with extended ‘fencing’ with the long, filamentous antennae. It is likely that chemical communication is also involved, either in the form of contact stimulants or volatile pheromones, although this has been fully demonstrated in only a few species. Where both male and female are receptive, they orient themselves in such a way to enable genital contact and ‘locking’ to take place. In some species (e.g. Conocephalus discolor – see DVD) this involves male and female passing each other in opposite directions on a plant stem, with the male curving his abdomen round in an effort to engage the female’s genitalia as he passes. Even where both individuals appear to be receptive, this seems to be a difficult feat, as numerous repetitions are often required, and failure is frequent. In other species, the male has to persuade the female to mount him and curve her abdomen round to contact his genitalia. Often the mounting behaviour of the female appears to be stimulated by chemical secretions on the dorsal surface of the male abdomen, and a female can be observed to rapidly palpate or ‘nibble’ the male abdomen as she mounts (Vahed, 1998. See this in P. griseoaptera on the DVD). In most species, genital locking is achieved by projections on the inner side of the male abdominal cerci fitting into pits on both sides of the female abdomen close to the genital plate. The eventual mating position varies – with the male curled up behind the female, facing in the opposite direction to her, and often clinging onto her ovipositor, or with the female mounted on the male (a more common pattern among the Gryllidae).

FIG 105. Courtship in Roesel’s bush-cricket: a male both sings and communicates with the female with his antennae; and at close quarters continues to ‘fence’ with his antennae.

FIG 106. Male cerci of Roesel’s bush-cricket. Note the sharp inner-directed points.
Although the pattern of courtship described above is very widespread among the bush-crickets, there is one genus in which the males of all species so far studied dispense with courtship entirely (Vahed, 2002; Vahed & Carron, 2008). In the genus Anonconotus, males stalk females silently, gradually closing in on them. After briefly contacting the target female with his antennae, the male usually leaps onto her back, curves his abdomen round and attempts to grip her abdomen with his sharp-pointed, pincer-like cerci. While holding the female in position he then re-positions himself in the standard bush-cricket copulatory posture – curved around behind the female, holding on to her ovipositor. Females frequently resist this form of ‘coercive copulation’ by jumping away when males attempt to mount them, kicking or attempting to shake them off, or even biting them. However, in A. alpinus, the male usually places his hind tarsi on the hind femora or tibiae of the female, apparently locking her hind legs into a posture that would prevent her jumping away or kicking back. Although not stridulating prior to mounting the female, males of these species do utter a ‘disturbance’ chirp while mounting, and also at intervals when provoked by movements of the female. In these species, males and females mate frequently, and sperm transferred per mating are relatively few in number. It may be that this unusual mating system has evolved under conditions of high population densities in open montane habitats where males encounter frequent mating opportunities.

FIG 107. Female of Poecilimon jonicus (Macedonia) mounted on the male.
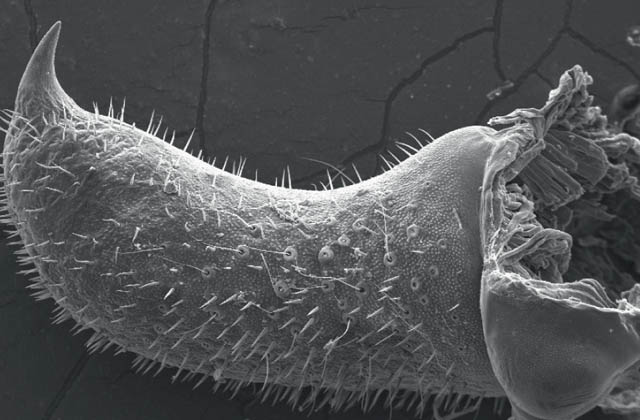
FIG 108. Scanning electron micrograph of a sharply pointed cercus of the alpine bush-cricket. (© K. Vahed)
In these and almost all other bush-crickets, once genital contact has been secured, the male attaches a spermatophore to the female’s genital opening. This consists of an ampulla which contains the sperm and seminal fluid, together with a large, gelatinous mass (the spermatophylax) which is attached to the abdomen of the female, covering the ampulla. Sperm, together with seminal fluid, begin to flow into the female’s spermatheca. One of the most dramatic early descriptions of this aspect of bush-cricket mating is provided by the great French naturalist, J. Henri Fabre. After observing the courtship of a pair of captive Decticus albifrons over several days, he finally witnesses their mating:
The two ventral extremities curve into a hook, seek each other, meet; and soon from the male’s convulsive loins there is seen to issue, in painful labour, something monstrous and unheard-of, as though the creature were expelling its entrails in a lump.
It is an opalescent bag, similar in size and colour to a mistletoe-berry, a bag with four pockets marked off by faint grooves, two larger ones above, and two smaller ones below … The strange concern remains hanging fom the lower end of the sabre of the future mother, who solemnly retires with the extraordinary wallet … (Fabre, 1917: 153)
But Fabre’s shock at the sight of the spermatophylax is compounded by his observation of what happens next:
At intervals she draws herself up on her shanks, curls into a ring and seizes her opalescent load in her mandibles, nibbling it calmly and squeezing it … The huge, sticky mass is not let go for a moment, but is munched, ground and kneaded by the insect’s mandibles and at last gulped down whole. (Fabre, 1917:154)
Initially Fabre saw this ‘horrible banquet’ as an aberration, but soon found similar patterns in other species – the ‘green grasshopper’ (Tettigonia viridissima), Ephippiger vitium (= provincialis?), the Alpine bush-cricket (Anonconotus alpinus), and the sickle-bearing bush-cricket (Phaneroptera falcata). Fabre’s vivid descriptions of these events also anticipate the most widely proposed explanations of the role of the male’s nuptial gift and its consumption by the female: as a ‘fertilising capsule’ the spermatophore is responsible for the transfer of sperm to the female, but is also ‘possibly a powerful stimulant’, as well as providing a ‘source of life for the ovules’.
Studies carried out in the first decades of the 20th century in Germany by Gerhardt, and by the Russian, Boldyrev (cited in Gwynne, 2001: 123ff), yielded the hypothesis that the spermatophylax had evolved in various ensiferan groups primarily as a means deployed by males to ensure full transfer of sperm and seminal fluid from the ampulla to the female’s genital tract. Boldyrev complemented his ‘ejaculate-protection’ hypothesis by observations on prolongation of copulation and post-copulatory guarding in some species that served the same function in preventing the female from prematurely consuming the ampulla and its contents. This hypothesis assumes that the ancestral condition that led to the evolution of the nuptial gift was one in which the sperm capsule was not completely enclosed in the female genital cavity, while multiply-mated females would have benefited from consuming all or part of the nutritious capsule containing the sperm. Sexual selection would have led to the development of the spermatophylax as a counter-adaptation on the part of males.

FIG 110. Female bush-crickets with spermatophylax attached: (a) Tylopsis lilifolia, green form; (b) Tylopsis lilifolia, brown form; (c) Roesel’s bush-cricket (© J. Dobson); (d) long-winged conehead, female consuming the spermatophylax.
The ejaculate-protection hypothesis has subsequently been extended by evidence that chemical substances transferred to the female either induce a period of reduced readiness for further mating (‘refractory period’) or stimulate her to lay eggs at an increased rate (or both). In many orthopterans, for example, males produce an enzyme (prostaglandin synthase) which is transferred to females in the ejaculate, and subsequently converts a fatty acid into prostaglandin. This compound is known to stimulate female reproduction, and so favours male reproductive interests (Arnqvist & Rowe, 2005). The combination of nuptial feeding together with hormonal modification of female reproductive behaviour can be understood as male strategies to enhance the likelihood of both insemination and fertilisation of the female’s eggs. The other major hypothesis, hinted at by Fabre, is that the spermatophylax contains nutrients that enhance the fecundity of the female and/or increase the survival chances of the offspring of the pair. On this ‘paternal-investment’ hypothesis, the reproductive interests of both parents are served by the spermatophylax – possibly to a degree that the usual direction of sexual competition is reversed, and females compete with one another for the chance of sex with a good meal on offer.
Unfortunately it is not easy to design decisive tests for these rival hypotheses, and it may be that in some cases the spermatophylax serves both functions. Also, as Gwynne (2001) is careful to point out, explaining how and why the spermatophylax evolved is not the same thing as explaining how it functions now in the mating systems of existing species. In some lineages it could well have acquired new functions. In fact, nuptial gifts of various sorts are widespread among invertebrates, and broad comparisons are useful both in generating and in considering the plausibility of different explanatory possibilities (see Simmons & Parker, 1989; Vahed, 1998; Gwynne, 2008).
Comparative studies across a wide diversity of bush-cricket taxa have tended to confirm expectations based on the ejaculate-protection hypothesis. Wedell (1993, 1994) compared bush-crickets of 19 genera and discovered correlations between spermatophylax size, ampulla size and the length of the female refractory period. Using a different method of comparison, Vahed & Gilbert (1996) analysed the relationship between ampulla mass and spermatophylax mass in 43 European species, and added sperm number for 31 of these in which it was known. Their study, which controlled for overall male body size and for phylogenetic relationships, showed close correlation across species in the relationship between spermatophylax mass, ampulla mass and sperm number. On the assumption that spermatophylax mass is a reasonable indicator of the time a female takes to consume it, this is what might be expected if its function is to ensure complete transfer of the sperm, so both of these studies seem to support the evolutionary hypothesis of ejaculate protection. However, as Vahed and Gilbert note, these results do not rule out the paternal-investment hypothesis.
Other aspects of mating systems are highly relevant here. One important variable is the extent to which females are polyandrous, as this affects both the likelihood that a given male’s paternal investment will be ‘wasted’ on the offspring of another male, and the risk that time and energy devoted to production of the spermatophylax might reduce his chances of mating with other females. Also relevant is information about the contents of both ampulla and spermatophylax. If the spermatophylax may be large but of little nutritional value, then this tends to support the ejaculate-protection hypothesis. However, it is not decisive: there may be small quantities of nutrients that have specialised importance for the fecundity of the female. Equally, highly nutritive spermatophylaxes may be required to keep the female distracted, or to enable her to delay seeking another nutritive gift from a subsequent male, quite independently of whether the nutrient content of the spermatophylax is involved directly in the formation of the eggs.
Alongside comparative studies aimed at investigating the rival evolutionary hypotheses, there are several detailed studies of the function of the spermatophylax in the mating systems of a number of particular species. These suggest that, although it may have evolved primarily as a male strategy to protect his paternity, and still has this function in many species, the function of the spermatophylax may have changed in a few lineages over evolutionary time. A study of the wartbiter (Decticus verrucivorus) by Wedell and Arak (1989) established that the average spermatophore was just large enough to distract the female while the sperm were transferred, to induce a refractory period of five days and to increase her rate of egg-laying. Smaller spermatophores would have resulted in incomplete insemination. Increasing the size of the male’s gift had no detectable effect on the number or size of eggs produced by the female. Moreover, females of this species tend to re-mate before the nutritional content from the male’s spermatophylax can be transferred to their eggs. Finally, the male wartbiter also has a high re-mating rate, and low protein content in his spermatophylax, indicating that his gift is relatively low-cost. All of this is consistent with the ejaculate-protection hypothesis for the wartbiter. Similar support for the ejaculate-protection hypothesis was provided by studies of two species of the genus Leptophyes. In the case of L. laticauda, neither food shortage in the female nor deprivation of the spermatophylax made any difference to the number or weight of eggs laid, despite the relatively large gift in this species (approximately one third of the male weight) (Vahed & Gilbert, 1997). In the speckled bush-cricket, Leptophyes punctatissima, multiply-mated females did lay more eggs than singly mated ones. However, artificial removal of the spermatophore from some of the females made no difference to the number or weight of the eggs laid (Vahed, 2003b).

FIG 111. A female wartbiter, laying her eggs in soil.

FIG 112. Female Leptophyes laticauda, consuming the large spermatophylax. (© K. Vahed)
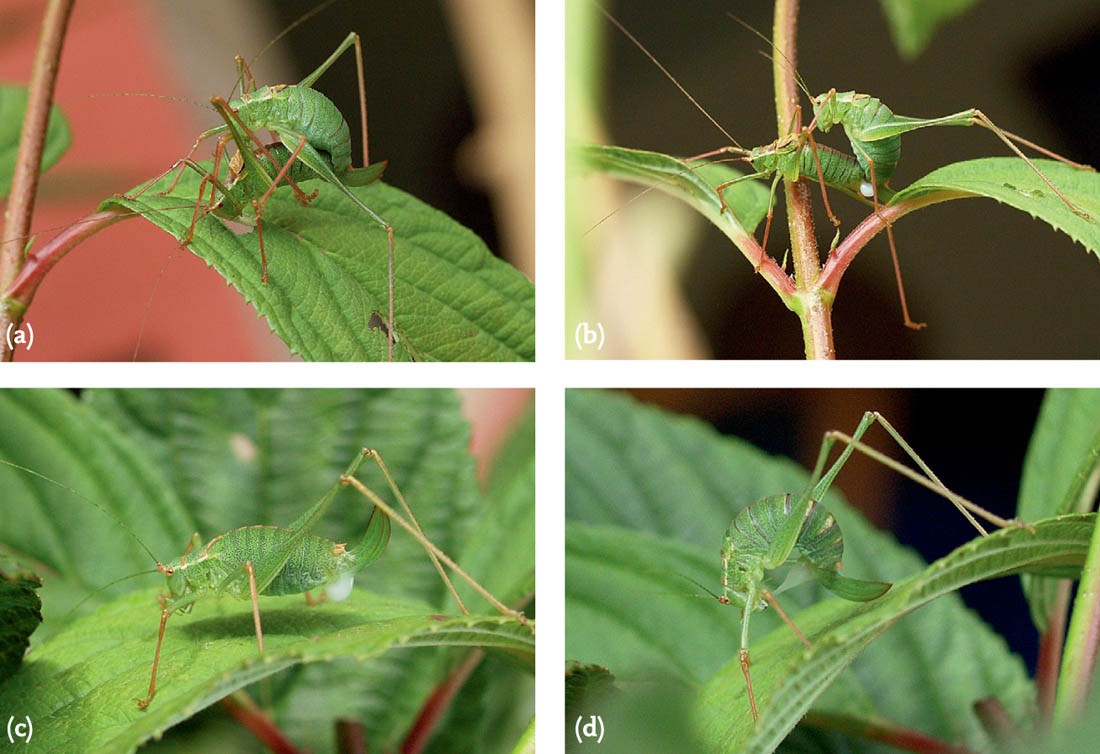
FIG 113. Mating sequence of the speckled bush-cricket: (a) a mating pair; (b) transfer of the spermatophylax; (c) female with attached spermatophylax; (d) female bites and pulls the spermatophylax. (© M. Hall)
The Australian listroscelidine bush-cricket, Requena verticalis, provides a marked contrast. In this species, the time taken by the female to consume the spermatophylax is very variable, but always as long as, or, usually, much longer than, is required for transfer of the ejaculate. In addition, gift size has been shown to significantly enhance egg size, over-wintering survival rates of eggs and growth rates of resulting nymphs. There is evidence that nutrients derived from the male are incorporated into the eggs, that females do not re-mate before the eggs from a prior mating have matured, and that females give sperm precedence to the first male they mate with (Bowen et al., 1984; Gwynne, 1984a, 1988a, 1988b, 1990, 2001; Gwynne & Snedden, 1995; Simmons & Gwynne, 1991). These features of the mating system of this species strongly favour the paternal-investment hypothesis.
On the basis of a comparative study of 19 bush-cricket species from 14 genera, Wedell (1994) proposed that variations in the spermatophylax offering represented two distinct male reproductive strategies. In many species, a spermatophylax of low nutritional value serves to distract the female for just long enough to allow for (on average) complete transfer of the ejaculate. This is true of the wartbiter and other species that have been studied in sufficient detail, except for Requena verticalis. The latter species, on Wedell’s account, belongs to a cluster of species in which the spermatophylax is relatively large and nutritious and has measurable impact on female fecundity (measured in Wedell’s study as mass of eggs laid). Subsequent work has provided evidence in support of some element of paternal investment in several bush-cricket species, such as the European phaneropterine Poecilimon veluchianus (Reinhold, 1999) and the Australian pollen bush-cricket (Zaprochilinae) Kawanaphila nartee (e.g. Simmons & Gwynne, 1991; Simmons, 1994). In these species, it seems likely that the spermatophylax serves both to ensure paternity and to contribute to female fecundity and offspring survival.
Indirect comparative support for the ejaculate-protection hypothesis comes from the mating behaviour of some species of bush-cricket that have little or no spermatophylax. In at least some of these species, such as the southern oak bush-cricket (Meconema meridionale), males appear to be able to prolong copulation by grasping the female with their cerci while insemination takes place (Vahed, 1996). In one of the species of Anonconotus, A. baracunensis, the male copulates coercively, the spermatophylax is very small, and mating is very prolonged, possibly to give time for full insemination to take place. Evidence that it is the male that prolongs copulation is provided by the interesting example of inter-specific matings of A. baracunensis males with females of A. pusillus, a species in which copulation is much more brief. The duration of these interspecific matings was just as great as that of normal A. baracunensis matings (Vahed & Carron, 2008), implying male control over the duration of copulation in this species.
It seems that prolonged copulation, with duration under male control, may have evolved as an alternative male strategy to the provision of a substantial spermatophylax. A comparative study of pairs of closely related species drawn from a range of tettigoniid groups suggests that male genital structures may have evolved to secure such extended copulations. The inwardly directed spines on the cerci of Anonconotus males are used to grip – and often pierce – the cuticle of the sides of the female abdomen (see Fig 108), while some meconematines and phasmodines have extended linear cerci that powerfully encircle the female abdomen. In other cases (e.g some copiphorines), differently angled stiff points on the male cerci must also limit the ability of the female to break free. In each of these cases, the duration of copulation is very much extended compared with related taxa that lack these structural features (Vahed, in prep.)

FIG 114. Cerci of male southern oak bush-cricket, which are used to constrain the female and prolong copulation.

FIG 115. Male large conehead. (© B. Thomas)
In some other species that produce a small spermatophylax, it seems that the females do consume it, but that they delay feeding on the ampulla. The large conehead (Ruspolia nitidula) is one such species (Vahed, cited in Gwynne, 2001) and the unwillingness of the female to consume the ampulla may be explained by the finding that she is likely to mate only once. In singly-mated, or ‘monandrous’, species, the reproductive interest of the female requires that the ampulla remains in place until all the sperm have been transferred.
The number of times a female mates in her lifetime will significantly affect the balance of reproductive interests between the sexes. In monandrous species, females will tend to be ‘choosy’ in selecting a mate, and will seek to achieve full insemination of their eggs from one mating. In monandrous mating systems, where a male can be sure that his investment will benefit offspring he has fathered, selective pressures may favour the evolution of male parental investment. Arnqvist and Rowe (2005) therefore suggest that we should expect nutritional content of nuptial gifts to increase, as against substances that manipulate female reproductive rate, to the extent that mating systems approach monandry.
Assuming that males are polygynous, it is also to be expected that there would be a high degree of competition among males, and strong sexual selection acting on them. But what of species (probably the majority) in which females mate more than once? Recent research has focused on the degree of polyandry among bush-cricket females, and its relationship to male reproductive strategy. As we have seen, there is evidence that substances passed to the female in the ejaculate (and possibly in the spermatophylax) induce a refractory period during which the female is unreceptive to further male courtship. This refractory period is thought to be dose-dependent – that is, the greater the amount of seminal fluid transferred, the longer the refractory period (Simmons & Gwynne, 1991; Simmons, 1995; Vahed, 2006). This is generally understood to be an evolved tactic on the part of the male to increase the chances that his sperm fertilise the eggs laid by the female prior to her re-mating with another male. However, it also seems likely that induced refractory periods will affect the life-time number of matings of the females. A comparative study of 18 bush-cricket species conducted by Vahed (2006) revealed a significant negative correlation between the mass of the ampulla (relative to the body mass of the male) and the degree of polyandry of the females, by species. In general, the larger the ampulla – so, presumably, the greater the volume of ejaculate – the smaller the average number of female lifetime matings. This suggests (although not conclusively) that male manipulation of female receptivity reduces the degree of polyandry, possibly against the reproductive interests of the female.
However, it remains uncertain what the optimal number of matings for females might be. In the examples of coercive copulation exhibited by Anonconotus species, the lifetime mating frequency is estimated to be far higher than is usual for bush-crickets – between 18 and 34 for female A. baracunensis, compared with 2 to 4 in Metrioptera roeselii, or 1 to 7 in Tettigonia viridissima (based on numbers of spermatodoses in the spermathecae of field-caught individuals; Vahed, 2006). This suggests that the Anonconotus coercive mating system imposes higher than optimum mating frequency on the females. However, female resistance is often successful, which may provide some support for the alternative interpretation that the coercive behaviour of males is actually sexually selected by females.
A subsequent comparative study (Vahed et al., 2011) has confirmed the expectation from theoretical models that the mass of male testes increases with the degree of polyandry. This might be expected on the basis of a greater frequency of male mating opportunities in species in which females mate more frequently. However, this study found no significant association between testis mass and sperm number per mating, and revealed a negative relationship between testis mass and ampulla mass. These findings are open to interpretation, but it may be that the high cost to males of large ejaculates (which are correlated with large spermatophylaces) also reduces their own re-mating rate. If so, smaller testes may still allow recovery of sufficient sperm in time for a repeated mating. As the non-sperm contents of the ejaculate are produced by other glands, there may also be increased investment in production of other contents of the ejaculate relative to sperm. Confirmation of the cost to males has been provided by another study by Vahed. Comparison across 23 species of bush-crickets showed that larger ejaculates and larger nuptial gifts were positively correlated not only with longer refractory periods in females but also with the duration of a male refractory period after each mating (Vahed, 2007; see also Vahed & Parker, 2012). This is virtually absent in species, such as Anonconotus species, that transfer a small or even no spermatophylax (Vahed, 2002; Vahed & Carron, 2008).

FIG 116. Platycleis affinis, male. A species with exceptionally large testes mass relative to body weight (© Vahed 2011); P. affinis, male, with dissected testes. (© K. Vahed)
Another remarkable and puzzling feature of the mating system of at least some bush-crickets may also be connected to the provision of the nuptial gift. In most species of Orthoptera (as with other sexually reproducing species) males compete with one another for mating opportunities with females, while females either passively or actively choose successful male competitors, or ones with preferred traits. In some species of bush-crickets, at least some of the time, there is sex-role reversal. That is, females compete with one another to mate with males, and males choose from the available females. This is evidenced in the unusual sight of males rejecting the mating attempts of females (see the example of this in the dark bush-cricket (Pholidoptera griseoaptera) included in the DVD). The most fully studied species in which sex-role reversal has been observed is the Australian pollen bush-cricket Kawanaphila nartee (Simmons, 1990, 1994; Simmons & Bailey, 1990; Shelly, 1993; Gwynne et al., 1998; Gwynne & Bailey, 1999). In populations of this species that occur in food-poor habitats, females approaching a calling male are observed to jostle one another, grappling with front legs and kicking. Even when a female has succeeded in mounting a male she could be displaced by usurping females. Even despite a male’s attempts to remove a female that has made genital contact, she may continue to cling on, although without receiving a spermatophylax gift. By contrast, the sex-role behaviour of populations of the same species in food-rich habitats reverts to the norm, with males competing for females.
Reversal of sex roles has been reported in several other species, such as the European white-faced decticus (Decticus albifrons), the American mormon bush-cricket (Anabrus simplex), Australian Metaballus litus and Requena verticalis, and European species of Ephippiger and Uromenus (Hartley, 1993; Gwynne, 2001; Ritchie et al., 1998). In some of these species, role reversal is observed in some sites and not in others and this has provided an opportunity to use experimental techniques to explore the conditions under which reversal takes place, and to test wider theoretical ideas about sexual selection and the evolution of sex differences.
Studies by Gwynne, Bailey, Shelly, Simmons and others of role reversal in the pollen bush-cricket K. nartee, the garden bush-cricket Requena verticalis, and the mormon bush-cricket Anabrus simplex, have yielded important insights into these topics. In these species, food-stress leads to relatively longer refractory periods for males between matings, presumably because of resource limitations on the ability to produce the spermatophylax. But food-stress increases the readiness of females to re-mate, presumably in response to their requirement for the nutritious content of the nuptial gift. So, combining these two effects, mating frequency in females is increased, but male mating frequency is reduced under conditions of food shortage. The consequence is female competition for mating opportunities and increased male choosiness. This might be explained in terms of the shift in balance of operational sex ratios – that is, the numerical proportions of sexually receptive females to receptive males. However, role reversal under these conditions may also be explained in terms of increased male choosiness in response to increased differences among the females in terms of their quality as potential mates. If females have to compete for food, and some are more successful than others, then the larger, presumably higher-quality (in the sense of more fecund) females are expected to be preferred by the males. This was, in fact, confirmed in the experimental studies. That female size is a cue for male choice, rather than an effect of greater mating success (and therefore more nutritional intake from consumption of male gifts), was demonstrated by measuring the pronotum length of the females, which does not alter after the final moult (Gwynne, 1984b, 1993).

FIG 117. A male Kawanaphila nartee transfers his spermatophylax to his mate. (© D. Gwynne)

FIG 118. Anabrus simplex, female, with a spermatophylax (© D. Gwynne); female Ephippiger species (Sardinia)
At least two of the most thoroughly studied role-reversal species have nutrient-rich spermatophylaxes and show paternal investment. In these species it might be expected that males would exercise choice over potential female recipients of a valuable and costly gift, and also that poorly fed females would compete for it. However, this is less clear in the case of species such as Uromenus stali or Ephippiger ephippiger, which have very large spermatophylaces that appear to be low in protein. It could be, as Gwynne suggests, that these apparently nutrient-poor gifts contain specialised nutrients that are important to the females. A study by Ritchie et al. (1998) suggests that this might be the case in E. ephippiger. On high-quality diets, females rejected males more frequently than males rejected females, but on low-quality diets the reverse occurred. In addition, on high-quality diets all conflict was among males, while on low-quality diet all conflict was among females. It seems hard to explain the persistence of this behavioural response to food shortage on the part of females, other than on the assumption that they gain some nutritional benefit from the male spermatophore.
In general, as the production of the spermatophylax is generally very costly to the male, and leads to a male refractory period of several days in some species, it seems likely that, even short of full sex-role reversal, males could be expected to exercise choice of female partners where these vary in fecundity (generally taken to be correlated with size).
Most comparative and species-focused studies of gift-giving species have found evidence of various sorts of male manipulation of female behaviour associated with the practice. These include distraction of the female for long enough for full insemination to take place, induction of a refractory period, or increased rate of egg-laying. However, in some species there is good evidence that the spermatophylax provides nutritional benefits that enhance female fecundity and the survival chances of the offspring, supporting the paternal investment interpretation of gift-giving in these species.
In those species, apparently the majority, where the spermatophylax appears to function primarily as a sperm-protection device, enhancing the reproductive interests of the male, there is good evidence of asymmetrical interests between the two sexes. What is less clear is whether a stronger thesis of sexual conflict is supported. Is female reproductive potential depressed, for example, by reduced opportunities to mate with other males? If the female derives no nutritional or other benefit, but only costs, as a result of the male manipulation of her behaviour, it might be expected that she would have evolved counter-strategies, such as disposing speedily of the gift without taking the time to consume it all.
Gwynne addresses this question in a review of nuptial gift-giving across a wide range of arthropod mating systems (Gwynne, 2008). He argues that most studies suggest that females obtain net direct benefits from nuptial gifts. These may be in the form of nutrients, or transfer of immune functions or other chemical defences. Indirect evidence is provided by increased mating frequency of females of some species when food from other sources is limited and by competition between females for mating opportunities in several bush-cricket species that exhibit sex-role reversal. The prolonged refractory periods in females following consumption of a nuptial gift do not necessarily imply a cost to females. An alternative explanation is that the nutritional contents of nuptial gifts take time to process. Complex interactions between food availability, nutrient content of the spermatophylax, operational sex ratios and other variables make it difficult to specify the optimum mating rate for females.
Gwynne (2001) considers the implications for sexual difference in bush-crickets of the possibility that role reversal has been a longstanding evolutionary trait in some species. If this were the case, we might expect sexual selection to have produced inverted sexual dimorphisms – that is, exaggerated traits in females that have developed as a result of successive generations of expression of male preferences. Although detailed studies are awaited, Gwynne lists: sex differences in size; sharper hearing in females, and associated differences in ear anatomy between males and females; modified genital holding adaptations in females of Kawanaphila species; exceptionally complex stridulatory organs on the tegmina of females in some species; and, finally, weapon-like facial horns in females of a few species. One of the few empirical studies of sexual selection on females in a role-reversing species is Robson and Gwynne (2010). In aggregations of mormon crickets (Anabrus simplex) where food is limited, females aggressively compete with one another and scramble for opportunities to obtain male nuptial gifts. Robson and Gwynne predicted that females equipped with larger head width, longer mandibles, and other traits that would be advantageous in competition for mates, would have greater reproductive success. The results were generally inconsistent with this expectation – females with smaller heads and shorter mandibles being favoured. Possible explanations of these puzzling results include trade-offs in male preferences between sexually selected ornamentation and fecundity, with males giving priority to the latter. This might provide a general explanation for the lack of exaggerated secondary sexual characteristics in females to compare with the weaponry and striking visual displays sometimes developed by males.
Grylloidea: Crickets
The family Grylloidea includes several subgroupings, with rather different characteristic mating systems. These include, among others, the ‘ground crickets’ (Nemobiidae), scaly crickets (Mogoplistidae), tree crickets (Oecanthidae) and field crickets and their allies (Gryllidae). Research into mating systems has tended to focus on the Gryllidae, and several Gryllus species, such as G. bimaculatus, in particular.
In the gryllids, pair formation and courtship commonly make use of acoustic communication, as in the bush-crickets and grasshoppers. However, in some species, notably those that live in dense aggregations, calling and courtship songs have been secondarily lost. In common with some grasshoppers, but unlike most bush-crickets, many crickets have distinct calling and courtship songs. There is also a high level of mutual aggression among males of many species, and this is often associated with a distinct aggressive stridulation. Females (as well as males in some species) are attracted to the conspecific calling song, while at close quarters male courtship frequently involves a distinct, usually more subdued, stridulation together with bodily movements, pheromonal communication and antennation. Male crickets have to induce females to mount them, and mutual alignment often involves close cooperation; courtship routines appear to play a decisive role in this.
Recent work on cricket mating systems has recognised the role of multiple sensory cues in courtship, mating and post-copulatory behaviour (Rence & Loher, 1977). An influential study by Tregenza and Wedell (1997) on Gryllus bimaculatus demonstrated a role for hydrocarbons on the cuticle of females in stimulating courtship singing in males. Some of the males courted dead females, but none did so after the compounds on their cuticles had been removed. Replacement of the compounds on the body-surfaces of the females led to renewed male courtship. Chemical analysis suggested that relative concentrations of different compounds were the cue for sex recognition, rather than any single ‘sex pheromone’. Subsequent work, using similar techniques to those pioneered by Tregenza and Wedell, have shown that chemical cues are critically involved in the mating systems of other crickets – notably Teleogryllus oceanicus (Balakrishnan & Pollock, 1997; Murakami & Itoh, 2003; Thomas & Simmons, 2008, 2009) and the southern US decorated, or house cricket, Gryllodes sigillatus. In the latter species, experimental removal as well as chemical ablation of the antennae in males reduced the probability of production of the courtship song, or delayed it. Females deprived of their antennae or of their chemical sensory powers were less likely to mount males in response to the courtship song, and, if they did, responded more slowly than females with fully functional antennae (Ryan & Sakaluk, 2009). Removal and replacement of cuticular hydrocarbons on dead females had the same effects as in G. bimaculatus, while replacement of removed cuticular compounds with others removed from males produced only one male courtship (out of 120!).
However, this study showed that the antennae communicate tactile as well as chemical information, and this is particularly important in guiding the female in achieving the necessary alignment with the male for successful copulation. Visual perception of behavioural responses is also clearly involved, as, although some 25 per cent of males courted dead females with cuticular compounds intact, this is clearly far less than would court living ones. In some species the ability of males to produce the courtship song is also decisive. Crankshaw (1979) showed that artificially silenced males of the house cricket Acheta domesticus were unable to elicit mounting by females, despite being able to produce all other elements of the courtship behaviour (see also Hardy & Shaw, 1983).
Copulation requires the female to climb onto the back of the male (or he backs under her) to make genital contact. Once in the copulatory position the male attaches a spermatophore (already formed in the case of crickets) to the external genitalia of the female. Sperm and seminal fluid then pass into the female genital duct over a period of a few minutes to an hour, depending on species. In most gryllids, the spermatophore lacks the additional spermatophylax typical of the bush-cricket. In most species, females mate more than once, and this, combined with the absence of the distraction provided by the spermatophylax, puts males at the risk of failing to fertilise the eggs of their mates – either because the female consumes the spermatophore before complete transfer of the ejaculate, or because she re-mates with another male before egg-laying. Post-copulatory mate-guarding is a distinctive male reproductive strategy, widespread among the Gryllidae, that may be an evolved response to this risk. Various interpretations of this trait have been advanced and subjected to ingenious observational and experimental tests. Finally, in a few cricket species, notably the short-tailed crickets (Anurogryllus), the females tend their eggs and nourish the resulting nymphs. These are among the very small number of orthopterans that include in their reproductive behaviour any element of the final phases listed by Alexander et al. (1997) – care of offspring following oviposition (see Chapter 4).

FIG 119. male wood cricket – glands in the fore wings are ‘licked’ by the female during copulation; and Allonemobius fasciatus, pair mating, with the female feeding from the tibial spur of the male (© D. Funk).
Rather different mating systems have been evolved in other groups of crickets. These include provision by males of a range of nuptial gifts other than the spermatophylax of the bush-crickets. Among the ground crickets, Nemobiidae, females of several species feed from male tibial spurs (e.g. Allonemobius species, see Fedorka & Mousseau, 2002), while those of the British wood cricket (Nemobius sylvestris) receive double spermatophores and also feed from glands in the upper surfaces of the male fore wings, which may provide chemical cues rather than offer nutrition (Prokop & Maxwell, 2008. See species account for more detail). In the tree cricket genera Oecanthus and Neoxabea, males secrete a fluid into a hollow in the dorsal surface of the thorax, and females feed from this prior to, during and after extended copulation. There is evidence that nuptial gifts in at least some species play a part in mate choice (e.g. Brown, 1999; Bussière et al., 2005a, 2005b).
Research on several species – Acheta domesticus, Gryllus integer, G. bimaculatus, G. pensylavaticus, G. veletis, Aneurogryllus arboreus, Teleogryllus commodus, Gryllodes sigillatus, and others – has discovered a variety of male traits that are apparently preferred by females: large size, age, symmetry and low parasite load. Males that have previously won competitive interactions with others are also more likely to win conflicts with other males and more likely to experience mating success. Some of these traits, presumed to be indicative of male quality, might be cued by features of song, such as intensity, duration, or other elements of courtship behaviour. (See review in Zuk & Simmons, 1997, and more recent work, e.g. Bailey, 2008; Bentsen et al., 2006; Champagnon & Castillo, 2008; Holzer et al., 2003; Hunt et al., 2005; Simmons et al., 2001; see also Chapter 5.) In the Polynesian field cricket, Teleogryllus oceanicus, aggressive interactions between males are common, and include antennal contact, kicking, loud chirping, lunging and biting (Burk, 1983). Fights produce a dominance hierarchy among males, and success in any particular encounter is a function of two factors: previous fighting success and burrow occupancy. Males defending a burrow were found to have an 84 per cent success rate in fights with intruding males. Dominant males also had a higher rate of mating success, too – but not as a direct result of female choice. Dominant males were more likely to produce the courtship song, and less likely to have their courtship disrupted by intruding males. Even without the courtship song, males were able to elicit responses from females to the early stages of courtship, but only males that produced the courtship song were allowed to mate. However, the recent loss of the ability to produce song on the part of most males in one population of this species has led to significant changes in both male and female reproductive behaviour (Zuk et al., 2006; see also Chapter 5 for more detail).
A recent experimental study of aggressiveness in four species of north American Gryllus species found marked differences among species (Jang et al., 2008). The research involved standardised encounters between males in a confined space, and aggressive responses were scaled, from antennation, through delivering an aggressive stridulation or flaring the mandibles, to grappling and pushing with heads or mandibles engaged. Two species (G. fultoni and G. vernalis) never reached the ‘grapple’ stage, while in the other two species (G. rubens and G. pennsylvanicus) most agonistic encounters escalated to that stage. The authors argue that the higher levels of aggression probably represent the ancestral condition, with subsequent reduction in aggressive tendencies in two species. One plausible explanation is that G. rubens occupies and defends burrows, and G. pennsylvanicus also shows evidence of territoriality. Aggressiveness in these species may be related to their predisposition to defend resources valued for obtaining mates, or for shelter from predators. By contrast, the other two species do not use burrows or occupy other sorts of territory.
Viewed in the light of this study, the British population of the field cricket G. campestris presents some puzzles. Males often sing from a platform at the entrance to a burrow, and this, together with reports of their aggressive tendencies (e.g White, [1789] 1937: 244, and Darwin, 1874, Ragge, 1965), has led to the supposition that they are strongly territorial. This might, for example, align their mating system to the ‘resource defence polygyny’ model, in which males fight to control resources attractive to females. However, a study of one remaining British colony revealed high rates of mobility from one burrow to another of both males and females (Edwards et al., 1996). In undisturbed colonies, the fierce fighting between males reported by Gilbert White and others is not in evidence, and may have been an effect of attempts to introduce them into a different habitat, or keep males together in confinement. It is also possible that aggressive interactions are functions of population density or some other parameter. Although the study of American field crickets by Jang et al., (2008) seems to have been designed to reveal innate differences in aggressiveness, the standardised conditions under which the test males were placed may have produced misleading results. Experience with G. campestris suggests that aggressive interactions are highly conditional on circumstances.
It seems likely that in G. campestris, the burrows function as shelters from predators in the open habitats that are characteristic of field crickets, but they also seem to play a key part in the post-copulatory behaviour. Courtship and mating usually take place close to the burrow entrance, with the male alternating between calling song and the less musical ‘ticking’ courtship stridulation. Depending on the receptivity of the female, the courtship can be very brief or apparently non-existent, or very prolonged. In prolonged courtship bouts the male faces the female with tegmina raised prominently, stridulating and ‘fencing’ with his antennae. Periodically he turns to present his rear end to her and backs towards her as if inviting her to mount. If she does not respond the whole sequence is repeated. However, a receptive female may arrive at the male’s song platform and immediately mount him. During copulation the male makes vigorous side-to-side motions. When the pair separate, the small white spermatophore can be seen attached to the underside of the female’s abdomen (see the sequences included in the DVD).

FIG 120. Burrow of the field cricket Gryllus campestris; courtship in the field cricket.
Courtship and mating in Teleogryllus oceanicus is still more complex and precarious. The male initially crouches down, strokes the female with its antennae and jerks slowly back and forth. If she stays, he turns 180 degrees to face away from her and walks forward. She follows, touching his abdomen or cerci. The male then flattens himself against the substrate and spreads out his rear wings, enabling her to walk onto his back while he moves backwards. Only if she is positioned directly on top of him, and only if he sings during the courtship process will copulation ensue. If the angle is wrong, they may try again. It seems that a high degree of mutual cooperation is required. Attempted matings are frequently abandoned either because of obvious rejection by one or other partner, interruption by a rival male, or, apparently, by mere failure to get it right (Burk, 1983)!
In the field cricket (G. campestris) the female usually remains for some time after mating in, or close to the entrance of, the male’s burrow. This is generally regarded as an example of ‘mate-guarding’, a common feature of gryllid mating systems. In Gryllus bimaculatus, Teleogryllus commodus and other species, mate-guarding is described as involving antennation, and various aggressive responses on the part of the male to female movement. Three main (but not necessarily mutually exclusive) hypotheses have been advanced that interpret mate-guarding as a predominantly male reproductive strategy:

FIG 121. Teleogryllus oceanicus, male. (© D. Rentz)
The possible roles of mate-guarding should be understood in terms of the reproductive biology and behaviour of this group of crickets. Females of most species are polyandrous, and there is an extensive research literature on the benefits, if any, that females derive from multiple matings, and what difference it makes whether these are with the same male or several (reviewed by Simmons, 2005; see also the discussion of multiple mating in bush-crickets). A distinction is made between direct benefits (such as the life-time fecundity or fertility, or longevity of the female) and indirect benefits (fitness of the offspring). An experimental design pioneered by Tregenza and Wedell (1998) has been used in numerous studies to measure both direct and indirect effects of multiple mating of females with varying numbers of males. Multiple matings have generally been shown to increase the daily rate of egg production, life-time egg production, and the proportion of eggs that are fertile. Multiple matings, then, generally confer direct benefits on female gryllid crickets, either because of materials derived from the male during mating, or because the risks of incomplete fertilisation of the eggs, or deterioration of stored sperm are avoided. However, studies of a number of cricket species using Tregenza and Wedell’s protocol have yielded very different results concerning indirect benefits. The most frequently used measures of offspring fitness have been the proportion of fertile eggs that hatch (a measure of embryonic survival), survival rate of early instar nymphs, or hatchling size. On these measures, polyandry, compared with multiple mating with the same male, resulted in increased offspring fitness in some species, but not in others. For example, indirect benefits from polyandry were reported for the southern field cricket Gryllus bimaculatus in the original study by Tregenza and Wedell (1998), but not for the decorated cricket, Gryllodes sigillatus in a study by Ivy and Sakaluk (2005). The positive finding of indirect benefits from polyandry in G. bimaculatus is consistent with experimental evidence that females of this species prefer novel partners over previous partners when they mate more than once (Bateman, 1998). Where there is evidence of increased offspring fitness associated with polyandry, it remains unclear how far this is a result of female cryptic choice in favour of preferred males (‘good genes’), differences in compatibility between the genetic endowments of different males, or non-genetic maternal influences. An experimental study of the effects of polyandry in an Australian black field cricket, Teleogryllus commodus, reported by Jennions et al. (2007) yielded no evidence of either increased hatching of fertilised eggs or offspring survival. This is consistent with their finding that the identity of male partners in this species had no effect on offspring survival.
Studies of another gryllid, the US vocal field cricket (Gryllus vocalis), presented females with many more opportunities to mate with different males than in other studies of polyandry (Gershman, 2007, 2010). Virgin females were presented with different males each day for 5, 10 and 15 days. Females that were mated 10 times laid more eggs, and laid proportionally more fertile eggs, than those that were mated only 5 times. However, there was no significant increase in either effect from 10 to 15 matings. Comparison of the offspring hatching rates of females mated many times to a single male with that of females mated to numerous different males showed no benefits from polyandry. These studies suggest that females continue to obtain direct benefits from large numbers of matings, possibly in part because of replenishment of their sperm stores with fresher and more viable sperm. As in T. commodus, there may be no indirect benefit from polyandry, or, if there is a benefit, it is acquired with relatively smaller numbers of male partners than the 5 to 15 used in these studies.
In gryllid females, the sperm from successive matings is mixed in the spermatheca, and there appears to be no internal selection in the fertilisation of the eggs. In general, males whose spermatophore remains attached for longer, or who manage more matings with a given female than their rivals, are likely to have paternity over the larger proportion of offspring. In view of this, it might be expected that males will have been sexually selected to prolong spermatophore attachment, secure multiple matings with the same female, and resist the female’s preference for mating with rivals. Females’ reproductive interests may differ depending on whether they have mated previously. Virgin females may benefit from full sperm transfer at their first mating as an insurance policy in case they have no further matings. So, for a female’s first mating there may be no conflict of reproductive interests between male and female. However, for subsequent matings, females may have an interest in mating with a rival male and/or prematurely detaching and consuming the spermatophore before full ejaculate transfer, either to obtain direct benefits or possibly as a method of exercising choice between different mates.
Experimental work involving various combinations of captive males and females of several species of gryllid crickets has produced rather different results, suggesting that mate-guarding has evolved to play different parts in the mating systems of diverse cricket species. It also seems probable that there is flexibility in behaviour within species, depending on contingencies such as population density. At least some of the diversity in the function of mate-guarding can be understood in terms of other aspects of the biology of the species concerned. Experimental studies of mate-guarding in several species have been consistent with the ejaculate-protection hypothesis. Hockham and Vahed (1997) studied a captive-reared population of the South African field cricket Teleogryllus natalensis. In this species (as in most gryllid crickets) there is no spermatophylax. In this study, presence or absence of a guarding male made no significant difference to the success of rival males in dislodging the spermatophore, the presence of a rival male did not affect the duration of spermatophore attachment, and males with continuous access to females ceased guarding long before the females were ready to re-mate. These results are interpreted as counting against both ‘spermatophore-renewal’ and ‘rival-exclusion’ hypotheses, while the ejaculate-protection hypothesis was supported by the prolongation of spermatophore attachment where females were guarded, and a significant positive correlation between guarding and spermatophore attachment durations. In addition, there were behavioural observations of the males responding aggressively to female movements toward consumption of the spermatophore. Studies have supported the ejaculate-protection hypothesis in some other species too, including Gryllus campestris and Teleogryllus commodus. Species comparisons give indirect support to the ejaculate-protection hypothesis, as in many (but not all) species that have evolved a spermatophylax, mate-guarding has been lost. An alternative male strategy, as in some bush-crickets, is prolongation of copulation for as long as it takes for full insemination to be completed.
However, the other two hypotheses gain support from studies of two more crickets – the Asian and southern-US decorated cricket, Gryllodes sigillatus, and the southern field cricket, Gryllus bimaculatus. The decorated cricket is unusual in this group, as the male does transfer a spermatophylax, and this is large enough to distract the female while sperm transfer takes place. The interval before the male is ready to re-mate is prolonged in this species. This may be because the production of the spermatophylax is costly to the male, as indicated by a reduction in the male’s immune response associated with spermatophore production (Kerr et al. 2010), although in this species the spermatophylax contains amino acids that stimulate a feeding response in females independently of nutritional value (Warwick et al., 2009).
Despite the transfer of a spermatophylax, mate-guarding has been retained in this species. The ejaculate-protection hypothesis is not supported, as females do not remove the spermatophore more quickly if they are left unguarded. Also, the refractory period before the male is ready to re-mate is much longer than the duration of post-copulatory guarding, seeming to rule out the spermatophore-renewal hypothesis. However, spermatophore attachment is prolonged by guarding in the presence of rival males, supporting the rival-exclusion hypothesis in this species, the female of which is described as ‘promiscuous’ (Frankino & Sakaluk, 1994; Sakaluk, 1984).

FIG 122. A male G. sigillatus extruding the spermatophylax during copulation; a female consuming the spermatophylax following copulation. (both photos © D. Funk)
The southern field cricket (G. bimaculatus) has been subjected to particularly intensive study, although there is some inconsistency in the findings of different studies. Simmons (1986) found that there was a correspondence between mean duration of guarding and the time taken for sperm to pass into the spermatheca of the female in this species – a finding that is at least consistent with the ejaculate-protection hypothesis. Simmons also detected two divergent patterns of female post-copulatory behaviour. Some females departed within 15 minutes, while others stayed with the male for the full guarding period of between one and two hours. Matings that occurred away from the burrow were not followed by guarding. Where females did not stay for the full guarding period, the spermatophore was removed and consumed more quickly than it was among those that were guarded. Simmons’s studies also found that females were more inclined to leave the male the more closely related they were (Simmons, 1989), and also that females laid more eggs when allowed to choose their mates than when they were allocated a mate (Simmons, 1987). Also, the size of the male had a positive effect, although relatively small, on egg-laying (see also Parker & Vahed, 2010).
Some studies have shown female preference for larger males in this species, as evidenced by a tendency to reject small males or retain their spermatophores for a shorter time when they have prior mating experience with larger males (Bateman et al., 2001). However, a study by Wynn and Vahed (2004) detected no evidence of female preference for heavier males. Their study also discounted the ejaculate-protection hypothesis, as un-guarded females showed no inclination to premature detachment of the spermatophore, and in fact retained the spermatophore for longer in the absence of a guarding male. This was because of the frequency with which males dislodged the spermatophore in subsequent mating attempts (or females removed it in advance of repeat mating attempts by males). However, males did guard females for long enough to resume their ability to mate and transfer a spermatophore, a result consistent with the spermatophore-renewal hypothesis. Results of trials in which females were presented with rival males also supported the rival-exclusion hypothesis, as unguarded females re-mated with rivals more quickly than did ones whose original mate was allowed to continue guarding. Females showed a significant preference for the rival mate for their second copulation.
Wynne and Vahed’s study is of particular interest in revealing a difference in the behaviour of virgin females compared with previously mated ones (also noted by Bateman et al., 2001). As expected, virgin females tended to retain the spermatophore from the first mating for a longer period than that of subsequent copulations. Interestingly, this difference was consistent through each of the test-situations, indicating that females had a high degree of control over the duration of spermatophore attachment. This poses the further question how far mate-guarding is an example of males overriding the reproductive interests of females, and how far it might be understood as a form of cryptic female choice: if females are able to evade their guards, but do not do so, it may be that this is an expression of female mate choice – although there appears to be little clear evidence as to what male traits are preferred. In the case of the British field cricket, G. campestris, cohabitation between males and females after copulation appears to be consensual, as females may be observed to leave the male’s burrow and explore the surrounding area only to return to his side (pers. obs.; see also DVD).
In most field crickets the females use their long, spear-shaped ovipositors to lay their eggs under the surface of the ground, or in crevices. However, in the short-tailed crickets (Anurogryllus species) the female lacks the elongated ovipositor of her relatives, and this is indicative of a quite different feature of the reproductive behaviour of this group, and probably other genera of the subfamily Brachytrupinae, such as Gymnogryllus (Alexander et al., 1997). Anurogryllus arboreus (formerly A. muticus) is a widespread and often abundant species in the Americas, sometimes considered a serious pest. The calling and courtship behaviour of this species have been intensively studied, but what is of interest here is the exceptional role of maternal care of eggs and early-stage nymphs. Strangely, it seems that since the pioneering study by West and Alexander (1963) this aspect of reproductive behaviour has received only passing mention, with many publications concerned exclusively with pest control methods. Although most acoustic signalling and some courtship and mating take place in the open (Walker, 1983b), most of the lives of these insects is spent in underground chambers and tunnels. Burrows may be dug by both males and females, and usually consist of one or more short entrance tunnels leading to a shallow open chamber. Passing down from this is a longer ‘retreat shaft’, with one or more lower chambers at a depth of up to 50 cm, with offshoots where faeces, waste food and other debris are deposited (Weaver & Sommers, 1969). Mating is confined to a relatively brief season in spring and may occur in the burrow of either male or female, or in the open. Walker reports examples of mating occurring in male burrows, followed by the male leaving the female in occupation. In one instance a male that attempted to return was repelled by an aggressive display of ‘gaping mandibles’ (Walker, 1983b). There is some evidence that female occupation of male burrows, with food stocks, may constitute a form of male parental investment.
FIG 123. Aspects of the behaviour of Anurogryllus muticus, from West & Alexander, 1963. (a) Female of Anurogryllus muticus (De Geer) in brood chamber with new pile of cut grass stems. Note antennal contact between juvenile and female. Filled defecation chamber is at left. Egg pile is partly covered with sand near female’s left front leg. Tunnels leading into the interior of the jar in which the female is confined begin directly on her right. The female burrowed up under chunks of apple, as shown, and plastered substrate particles being removed from the burrow alongside the apple, creating ‘push-ups’ of the sort indicated. The female’s hind leg was lost while she was juvenile.
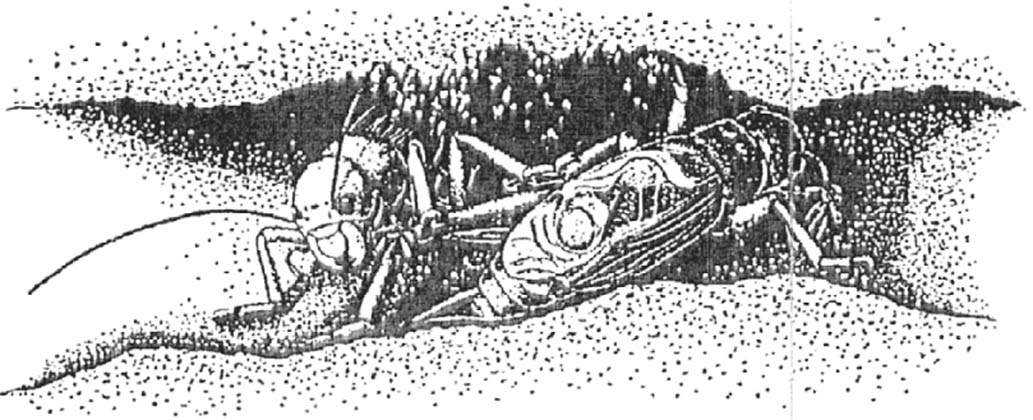
(b) Female ousting an intruding male from her burrow by kicking him after tearing off his right hind leg with her mandibles. Note the male’s lop-sided position, and the retracted labrum and spread mandibles of the female.

(c) Female responding to bright light by rearing up, approaching the glass side of the brood chamber, pressing against it, and touching it with her forelegs and maxillary palps. Temperature change may be involved Note egg pile between her front legs.
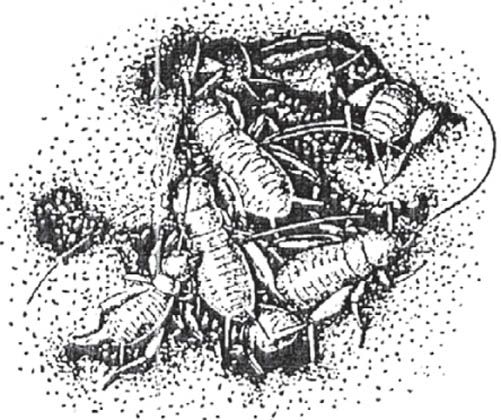
(d) Juvenile crickets (probably second and third instars) disturbed by breaking burrow above them. This is about the age at which the female dies and the juveniles begin to fend for themselves. These juveniles are more plump and soft-bodied than those of most other crickets.
Fertilised females occupy a burrow and proceed to lay a batch of eggs. Weaver and Sommers (1969) counted eggs in excavated burrows at various dates, with numbers varying between 5 and 129, but with an average of 49 per burrow across 10 burrows at the peak of the egg-laying season in May. The eggs are kept in piles by the female, and are of two different sizes. The female tends the eggs, ‘mouthing’ them, presumably to clean them of fungi, until they hatch. The resulting nymphs compete intensely for the smaller, presumably infertile, eggs and eat them. The female forages on the surface and brings food items down into the brood chamber for the nymphs. These remain with their mother through the first three instars and disperse to form their own burrows when she dies – usually towards the end of June or early July (West & Alexander, 1963; Weaver & Sommers, 1969).
The mole-crickets (Gryllotalpidae)
The mole-cricket Gryllotalpa gryllotalpa, which once occurred quite commonly in Britain, is another species in which there is continued parental care of offspring after the eggs have been laid. The mole-cricket spends most of its life underground, and although males do sometimes sing from the entrances to their burrows it seems likely that most encounters between males and females take place underground (Pinchen, pers. comm.). In the presence of a female the male performs a courtship display, continuing to sing while lowering its hind wings at the sides of its body, and swaying from side to side. If the female is receptive she climbs onto his back, as in other ensiferans, and mating is completed by the transfer of a spermatophore.
The female lays her eggs in an underground chamber that may be just below the surface or up to a foot deep. The eggs are piled into heaps in the chamber, and are said to number from 100–300 normally, although up to 640 have been recorded. The female stands guard over the eggs, and mouths them from time to time as do female Anurogryllus crickets. This is presumed to protect them from fungal attack. The eggs hatch after two to four weeks, and the early instar nymphs remain in the chamber for several weeks, after which they come to the surface but continue to be guarded by the mother (Ragge, 1965; Pinchen, 2009).
IF  THEN GO TO
THEN GO TO
* Antennae long, tapered and thread-like, as long as or longer than the body, or, if shorter, with body flattened dorsoventrally.  Ensifera: Key A
Ensifera: Key A
* Antennae relatively short and stiff, not tapered, but parallel sided or thickened towards the tip; body not flattened dorsoventrally.  Caelifera: Key B
Caelifera: Key B
Key A. Ensifera (crickets, bush-crickets and relatives)
IF  THEN IT is … or
THEN IT is … or  THEN GO TO
THEN GO TO
* Fore legs enlarged and adapted for digging (Fig. K.1); ovipositor of female inconspicuous.  mole cricket (Gryllotalpa gryllotalpa) (extremely rare, occasional imports)
mole cricket (Gryllotalpa gryllotalpa) (extremely rare, occasional imports)
* Fore legs not enlarged and adapted for digging; ovipositor of female conspicuous and sword or needle shaped.  A1
A1
A1 * Body shape roughly tubular (i.e. not flattened dorsoventrally); ovipositor of female long and straight or upturned, tapering towards the tip (sword shaped); head profile as in Fig. K.2a or b; tarsi with four segments.  A2
A2
* Body flattened dorsoventrally; ovipositor of female straight and parallel-sided, sometimes thickened at tip (spear or needle shaped); head profile as in Fig. K.2c; tarsi with three segments.  A17
A17
A2 * Fully winged (when at rest, wings reach back to or beyond the tip of the abdomen).  A3
A3
* When at rest, wings do not reach back to the tip of the abdomen (may be reduced to small flaps or completely absent).  A11
A11
A3 * Head triangular in profile (angle between the frons and vertex 50° to 60°) (Fig. K.2a).  A4
A4
* Head not triangular in profile (angle between frons and vertex approximately 70° or more, and transition between them smoothly curved) (Fig. K.2b).  A6
A6
A4 * Large (30 mm plus), uniformly green, with pale band on the front of the head and a black line on each tibia.  large conehead (Ruspolia nitidula) (very rare vagrant/early colonist in UK)
large conehead (Ruspolia nitidula) (very rare vagrant/early colonist in UK)
* Smaller (24 mm or less), brown or green, with brown stripe on dorsal surface of head and pronotum.  A5
A5
A5 * Female with up-curved ovipositor (Fig. K.3a); male with pair of central projections on final abdominal segment and cerci sharply narrowed and up-turned towards the tips (lateral view) (Fig. K.4a).  short-winged conehead long-winged form (Conocephalus dorsalis f. burri)
short-winged conehead long-winged form (Conocephalus dorsalis f. burri)
* Female with long, straight ovipositor (Fig. K.3b); rear margin of dorsal plate of final abdominal segment of male smoothly curved, and cerci taper evenly towards the tip (Fig. K.4b).  long-winged conehead (Conocephalus discolor)
long-winged conehead (Conocephalus discolor)
A6 * When at rest, wings reach back well beyond tip of the abdomen, and hind wings protrude beyond the tip of the fore wings; body green with minute black spots; female ovipositor broad, short and strongly up-curved (Fig. K.3c); male cerci up-curved, tapering to a sharp point (Fig. K.5a).  sickle-bearing bush-cricket (Phaneroptera falcata) (very rare, recent colonist of Britain)
sickle-bearing bush-cricket (Phaneroptera falcata) (very rare, recent colonist of Britain)
* Wings reach back approximately to the tip of the abdomen, or, if well beyond, fore wings as long as or longer than hind wings; body green or brown, without minute black spots; female ovipositor straight, or up-curved (Fig. K.3d, e, f, g, k); male cerci straight, slightly incurved or, if strongly incurved, not tapering to a sharp point (Fig. K.5b, c, g, i).  A7
A7
A7 * Wings do not reach as far back as the hind knees; robust, ‘plump’ build; median keel along the dorsal surface of the pronotum (Fig. K.6a); usually green, with variable black blotches; female ovipositor long and straight or slightly up-curved, minutely serrated towards the tip (Fig. K.3k).  wartbiter (Decticus verrucivorus) (very rare, in southern England only)
wartbiter (Decticus verrucivorus) (very rare, in southern England only)
* Wings usually reach as far back as hind knees or beyond; lacking median keel along the dorsum of the pronotum (Fig. K.6b); may be slender or robust in build; green or brown, but without black blotches; female ovipositor straight or up-curved, lacking serrations (K3d, e, f, g).  A8
A8
A8 * Large species (40 mm plus); body and wings mainly green, but with variable brown dorsal markings; wings reach back well beyond the tip of the abdomen; female ovipositor straight or slightly down-curved (Fig. K.3d); male cerci long and moderately incurved (Fig. K.5b).  great green bush-cricket (Tettigonia viridissima)
great green bush-cricket (Tettigonia viridissima)
* Smaller species (30mm or less); body and wings green or grey/brown; if green, then lacking brown dorsal markings; wings reach back approximately to the tip of the abdomen, or, if well beyond, then brown; female ovipositor up-curved (Fig. K.3e, f, g); male cerci long and strongly incurved, or, if short, straight or only slightly incurved (Fig. K.5c, g, i).  A9
A9
A9 * Body and wings green, with small yellow and reddish markings on the dorsum of the pronotum; wings reach back approximately to the tip of the abdomen; female ovipositor long, slightly up-curved and mainly green (Fig. K.3e); male cerci long, thin and strongly incurved (Fig. K.5c).  oak bush-cricket (Meconema thalassinum)
oak bush-cricket (Meconema thalassinum)
* Body and wings mainly grey/brown (may have some yellow/green markings on the sides of the body, but wings always brown); wings reach back to the tip of the abdomen or beyond; female ovipositor markedly up-curved and dark brown or black (Fig. K.3f, g); male cerci relatively short and straight, or slightly incurved (Fig. K.5g, i).  A10
A10
A10 * Side plates of the pronotum with clear cream/yellow border (Fig. K.7b); body and wings mainly plain brown, or with greenish tints along the sides of the abdomen; wings may reach well beyond the tip of the abdomen; female ovipositor relatively short, markedly up-curved and dark brown (Fig. K.3f); female subgenital plate with deep median cleft (Fig. K.8a); male cerci slightly incurved, and with inner projection approximately two thirds along from the base (Fig. K.5g).  Roesel’s bush-cricket, long-winged form (Metrioptera roeselii f. diluta)
Roesel’s bush-cricket, long-winged form (Metrioptera roeselii f. diluta)
* Mottled grey/brown, without clear pale borders to the side plates of the pronotum; wings reach back approximately to the tip of the abdomen; female ovipositor longer, more gently up-curved and black (Fig. K.3g); female subgenital plate with slight median cleft (Fig. K.8c); male cerci with inner projection approximately half-way along, and slightly curved outwards toward the tip (Fig. K.5i).  grey bush-cricket (Platycleis albopunctata) (mainly coastal and southern)
grey bush-cricket (Platycleis albopunctata) (mainly coastal and southern)
A11 * Wings absent; antennae extremely long (more than 2x length of the body); dorsal outline of the body markedly convex; palps, cerci and legs long relative to the body, giving a spider-like appearance; body pale brown with darker blotches.  greenhouse camel cricket (Diestrammena (Tachycines) asynamorus) (only in artificially heated buildings)
greenhouse camel cricket (Diestrammena (Tachycines) asynamorus) (only in artificially heated buildings)
* Wings present, even if vestigial; antennae long, but not more than twice the length of the body; dorsal outline of the body not markedly convex, and not spider-like in appearance; may be green or grey/brown.  A12
A12
A12 * Head triangular in profile (angle between vertex and frons 50° to 60°) (Fig. K.2a); green with brown on the dorsal surfaces of head and pronotum; female ovipositor long, narrow and up-curved (Fig. K.3a); male with pair of central projections on the final abdominal segment, and cerci abruptly narrowed and up-turned towards the tip in lateral view (Fig. K.4a).  short winged conehead (Conocephalus dorsalis)
short winged conehead (Conocephalus dorsalis)
* Head not triangular (angle between vertex and frons 70° or more, and the transition between them smoothly curved) (Fig. K.2b); may be predominantly green or brown; female ovipositor broader and up-curved or, if long, narrow and up-curved, then the insect lacks brown markings on dorsal surfaces of head and pronotum, and wings are vestigial.  A13
A13
A13 * Wings vestigial; body mainly green.  A14
A14
* Wings short (approximately one-third to two-thirds of the length of the abdomen), or, if wings vestigial, grey/brown (not green).  A15
A15
A14 * Body and legs with minute black spots; female ovipositor relatively short, broad and strongly up-curved (Fig. K.3h); usually with a brown/purple median band on the dorsal surface of abdomen, and orange/brown wing-stubs in the male; male cerci relatively short, strongly incurved and tapering to a point at the tip (Fig. K.5d).  speckled bush-cricket (Leptophyes punctatissima)
speckled bush-cricket (Leptophyes punctatissima)
* Body and legs pale green, without black spots; usually with a median yellowish line along dorsal surface of head, pronotum and abdomen, with a pair of reddish marks on the dorsal surface of the pronotum; female ovipositor long, narrow and gently up-curved (Fig. K.3i); male cerci long, incurved and not tapering to a point (Fig. K.5e).  southern oak bush-cricket (Meconema meridionale)
southern oak bush-cricket (Meconema meridionale)
A15 * Wings vestigial in female, reduced to stridulatory apparatus only in male; side flaps of the pronotum more or less evenly patterned, lacking clear pale border; male cerci straight, with small inner projection near the base (Fig. K.5f).  dark bush-cricket (Pholidoptera griseoaptera)
dark bush-cricket (Pholidoptera griseoaptera)
* Wings one-third to two-thirds of the length of the abdomen, more extensive than the stridulatory apparatus in the male; side flaps of the pronotum with clear pale border along at least one edge (Fig. K.7a, b); male cerci with prominent inner projection (Fig. K.5g, h).  A16
A16
A16 * Pale border on the side plates of the pronotum on both front and rear edges (Fig. K.7b); paired yellow spots on the sides of the thorax; female ovipositor relatively short and strongly up-curved (Fig. K.3f); female subgenital plate with deep median cleft (Fig. K.8a); male cerci with inner projection approximately two-thirds along from the base (Fig. K.5g).  Roesel’s bush-cricket (Metrioptera roeselii) (long grass)
Roesel’s bush-cricket (Metrioptera roeselii) (long grass)
* Pale border on the side plates of the pronotum on rear edge only (Fig. K.7a); lacks yellow spots on the sides of the thorax; female ovipositor longer and more gently up-curved (Fig. K.3j); female subgenital plate with shallow median cleft (Fig. K.8b); male cerci with inner projection approximately midway along (Fig. K.5h).  bog bush-cricket (Metrioptera brachyptera) (damp heath/bog)
bog bush-cricket (Metrioptera brachyptera) (damp heath/bog)
A17 * Entirely wingless; small (less than 15mm).  scaly cricket Pseudomogoplistes vicentae) (rare, under stones on shingle beaches)
scaly cricket Pseudomogoplistes vicentae) (rare, under stones on shingle beaches)
* Winged species (often wings reduced or vestigial).  A18
A18
A18 * Wings vestigial in female, reduced to stridulatory apparatus only in male; small species (less than 12mm); dark brown with pale ‘y’ marking on vertex.  wood cricket (Nemobius sylvestris) (in leaf litter, southern)
wood cricket (Nemobius sylvestris) (in leaf litter, southern)
* Fully winged, or wings at least two thirds of the length of the abdomen; larger (14mm or more).  A19
A19
A19 * Pale brown with darker markings; folded wings extend well beyond the tip of the abdomen; without yellow patches at the base of the fore wings.  house cricket (Acheta domesticus) (only in artificially warmed habitats; also similar species kept for the pet trade)
house cricket (Acheta domesticus) (only in artificially warmed habitats; also similar species kept for the pet trade)
* Body dark brown or black, with yellow patches at the base of the fore wings.  A20
A20
A20 * Wings reach back approximately to the tip of the abdomen (males), or are shorter (females).  field cricket (Gryllus campestris) (very rare, open sandy habitats)
field cricket (Gryllus campestris) (very rare, open sandy habitats)
* When folded, wings reach back well beyond the tip of the abdomen.  southern field cricket (Gryllus bimaculatus) (only occasional short-lived colonies in Britain)
southern field cricket (Gryllus bimaculatus) (only occasional short-lived colonies in Britain)
Key B. Caelifera (groundhoppers and grasshoppers)
Note 1: Some characters used in this key apply to one sex only, or are clearer in one sex than in the other. The distinction between males and females in grasshoppers and groundhoppers is less obvious than in crickets and bush-crickets, as the female ovipositor is usually less prominent. Sex differences in the appendages at the tip of the abdomen, as seen from the side, are shown in Fig. K.9.
Note 2: The colour patterns of several species are highly variable, so identification depends largely on structural features. A small bulge towards the base of the leading edge of the fore wing is present in some species, and its presence or absence is often useful for identification (but often obscured in photographs). The shape of the side keels on the dorsal surface of the pronotum is another very important character. The key distinguishes between ‘incurved’ and ‘inflexed’ side keels. The former refers to a pattern in which the keels are smoothly curved, as distinct from the latter, in which the keels are inwardly angled. In a few species, this distinction may not be clear in all specimens, and for these cases the key is designed to work whichever option is taken.
IF  THEN IT is … or
THEN IT is … or  THEN GO TO
THEN GO TO
* Pronotum projects back over the abdomen dorsally; fore and mid tarsi with only two segments (Fig. 10b).  B1
B1
* Pronotum saddle-shaped, with dorsal rear margin curved (i.e. not projecting back over the abdomen); all tarsi with three segments (Fig. K.10a).  B3
B3
B1 * Pronotum reaches back approximately as far as the hind knees, or less; median keel on dorsal surface forms a raised ridge, giving a markedly convex profile (Fig. K.11a); wings do not extend beyond the tip of the pronotum.  common groundhopper (Tetrix undulata)
common groundhopper (Tetrix undulata)
* Pronotum reaches back beyond the hind knees; median keel on dorsal surface of the pronotum less prominent, giving straight or slightly convex profile (Fig. K.11b); wings extend beyond the tip of the pronotum.  B2
B2
B2 * Mid femur with both edges smoothly curved (Fig. K.12a); vertex is at least 1.5 times as wide as the eye (Fig. K.13a), and reaches forward beyond the front margin of the eyes (view from above), (this character is less clear in males); the angle between the vertex and frons (view from the side) is 90° or less (Fig. K.14a); edges of female ovipositor valves are densely and shallowly toothed (Fig. K.15a); dorsal ridge along the edge of the hind femur is straight (Fig. K.16a).  slender groundhopper (Tetrix subulata)
slender groundhopper (Tetrix subulata)
* Mid femur with wavy edges (Fig. K.12b); vertex is less than 1.5 times as wide as the eye, and the front edge is in line with the front margin of the eyes (view from above) (Fig. K.13b) (this character less clear in the males); the angle between the vertex and frons (view from the side) is more than 90° (Fig. K.14b); edges of the female genital valves have longer, less densely packed teeth (Fig. K.15b); dorsal ridge along the edge of the hind femur bends outwards towards the hind ‘knee’ (Fig. K.16b).  Cepero’s groundhopper (Tetrix ceperoi) (rare, southern, often coastal)
Cepero’s groundhopper (Tetrix ceperoi) (rare, southern, often coastal)
NB: The groundhoppers are difficult to identify with certainty. The key is designed to work with adult specimens, not nymphs. The length of the pronotum is quite variable in both T. subulata and T. ceperoi, so this character alone should not be used to separate either from T. undulata. The distinction between T. ceperoi and T. subulata is particularly difficult. Viewing the mid femur with a hand lens is a useful guide, and the ‘kink’ in the dorsal ridge of the hind femur of T. ceperoi is also visible both with a hand lens and in a sharp photo. However, confident identification should be based on several characters. It is possible that T. ceperoi is under-recorded because of its similarity to T. subulata.
B3 * Side keels of the pronotum parallel or incurved (Fig. K.17a–g).  B4
B4
* Side keels of the pronotum inflexed (Fig. K.17h–k).  B12
B12
B4 * Large species (usually 25 mm plus); hind femur greenish with black knees and red on the rear edge; side keels of the pronotum gently incurved, diverging towards the rear edge (Fig. K.17a); pale stripe along the leading edge of the fore wing; male lacks stridulatory file on the inner surface of the hind femur.  large marsh grasshopper (Stethophyma grossum) (rare, wetlands, southern)
large marsh grasshopper (Stethophyma grossum) (rare, wetlands, southern)
* Smaller species (less than 25mm); hind femur not coloured as above; side keels of the pronotum parallel or incurved; in males a stridulatory file on the inner surface of the hind femur (Fig. K.18).  B5
B5
B5 * Side keels of the pronotum parallel or almost so, but often diverging towards the rear (Fig. K.17b); in females, the wings terminate just short of the tip of the abdomen, in males they reach the tip, or a short way beyond; often a prominent white stripe on the fore wing; there is a small bulge on the leading edge of the fore wing in females (‘costal bulge’) (Fig. K.19), inconspicuous or absent in males.  lesser marsh grasshopper (Chorthippus albomarginatus)
lesser marsh grasshopper (Chorthippus albomarginatus)
* Side keels of the pronotum incurved (Fig. K.17c–g).  B6
B6
B6 * both sexes have costal bulge (Fig. K.19).  B7
B7
* no costal bulge in either sex.  B9
B9
B7 * Side keels of the pronotum slightly incurved (Fig. K.17c).  B8
B8
* Side keels of the pronotum more strongly incurved (or inflexed) (Fig. K.17d–g, i)  B12
B12
B8 * Wings reach back significantly beyond the tip of the abdomen; distinct costal bulge in males and females; hind knees usually black (rarely so in C. albomarginatus).  common meadow grasshopper, long-winged form (Chorthippus parallelus f. explicatus)
common meadow grasshopper, long-winged form (Chorthippus parallelus f. explicatus)
* Wings greatly reduced in the female (half the length of the abdomen or less), and, in the male, falling short of the tip of the abdomen; bulge on the leading edge of the fore wing in both sexes; hind knees usually black.  common meadow grasshopper normal form (Chorthippus parallelus)
common meadow grasshopper normal form (Chorthippus parallelus)
B9 * Wings do not reach as far back as the tip of the hind knees; pronotal side keels broadly outlined pale (Fig. K.17d); with a pale ‘panel’ at the front of the side plates of the pronotum (Fig. K.20); small species (15mm or less); in mature individuals often a red suffusion on the abdomen.  lesser mottled grasshopper (Stenobothrus stigmaticus) (in UK known only from Isle of Man)
lesser mottled grasshopper (Stenobothrus stigmaticus) (in UK known only from Isle of Man)
* Wings reach back approximately to the tip of the hind knees or beyond; pale outlining of the pronotal side keels, if present, may be relatively broad or narrow (Fig. K.17e, f, g); side plates of the pronotum lack pale panel; larger species (usually more than 15mm).  B10
B10
B10 * Chalk-white palps, contrasting with face, especially in the males; mature males with extensive red on the abdomen, and black head and pronotum; females green, brown or grey dorsally, with brown, grey/black or rust-coloured sides; side keels of the pronotum are strongly incurved (Fig. K.17e); except where obscured by dark coloration (especially in males), the side keels of the pronotum are narrowly outlined in white, and bordered by two prominent black wedges; female ovipositor valves smoothly curved to point at tip (Fig. K.21a).  woodland grasshopper (Omocestus rufipes)
woodland grasshopper (Omocestus rufipes)
* Palps may be pale but not chalk-white; mature males may develop reddish suffusion on the abdomen, but head and pronotum never black; side keels of the pronotum less strongly incurved (Fig. K.17f, g); female ovipositor valves as in Fig. K.21b or c.  B11
B11
B11 * Enlarged cells in the fore wing have parallel cross veins, giving a ladder-like impression (Fig. K.22); pale outlining of the side keels of the pronotum is relatively broad (Fig. K.17f); mature specimens develop reddish tints to the tip of the abdomen (and hind tibiae in males); wings of mature specimens often black with white stripe and crescent marking; female ovipositor valves have acute dorsal projection (Fig. K.21b).  stripe-winged grasshopper (Stenobothrus lineatus)
stripe-winged grasshopper (Stenobothrus lineatus)
* Wing venation not as above; pale outlining of the pronotal side keels relatively narrow (Fig. K.17g); neither sex develops reddish tints on the abdomen; female ovipositor valves are unevenly curved, but lack a distinct dorsal projection (Fig. K.21c).  common green grasshopper (Omocestus viridulus)
common green grasshopper (Omocestus viridulus)
B12 * Antennae clubbed or distinctly thickened towards the tip.  B13
B13
* Anennae not clubbed or thickened towards the tip.  B14
B14
B13 * Pronotal side keels very strongly inflexed (Fig. K.17h); antennae clubbed in male, but only widened towards the tip in females, and not generally white-tipped (Fig. 23a, b).  mottled grasshopper (Myrmeleotettix maculatus) (usually on bare ground or low vegetation)
mottled grasshopper (Myrmeleotettix maculatus) (usually on bare ground or low vegetation)
* Pronotal side keels less markedly inflexed (Fig. K.17i); both sexes have whitetipped and clubbed antennae (Fig. K.23c, d).  rufous grasshopper (Gomphocerippus rufus) (usually in taller grasses or low scrub)
rufous grasshopper (Gomphocerippus rufus) (usually in taller grasses or low scrub)
B14 * Costal bulge present on fore wing (K19).  B15
B15
* Lacking costal bulge on fore wing.  B9
B9
B15 * The transverse sulcus on the dorsal surface of the pronotum is anterior to the mid point (K17j); wedge-shaped dark markings on the rear portion of the dorsal surface of the pronotum do not reach the rear margin (Fig. K.17j); the underside of the thorax is densely hairy.  common field grasshopper (Chorthippus brunneus) (widespread and common)
common field grasshopper (Chorthippus brunneus) (widespread and common)
* The transverse sulcus on the dorsal surface of the pronotum cuts across at the mid point or to the rear (Fig. K.17k); wedge-shaped dark markings on the rear portion of the dorsal surface of the pronotum reach back to the rear margin (Fig. K.17k); the underside of the thorax is sparsely hairy.  heath grasshopper (Chorthippus vagans) (very rare, on heaths in Dorset and Hampshire)
heath grasshopper (Chorthippus vagans) (very rare, on heaths in Dorset and Hampshire)
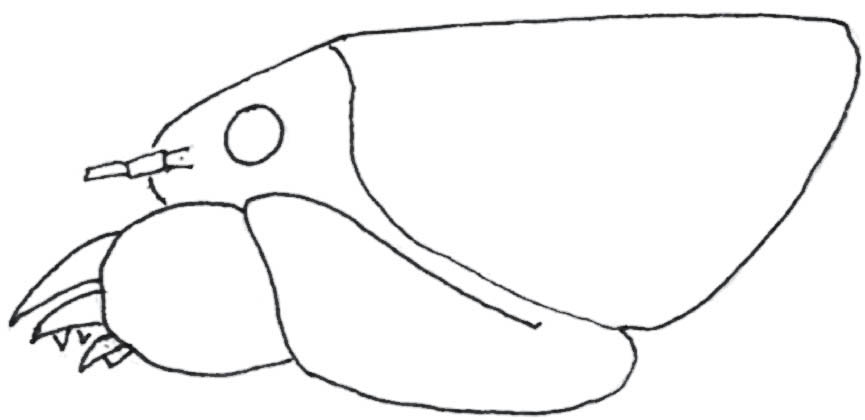
FIG. K.1 Mole cricket: fore legs modified for digging.
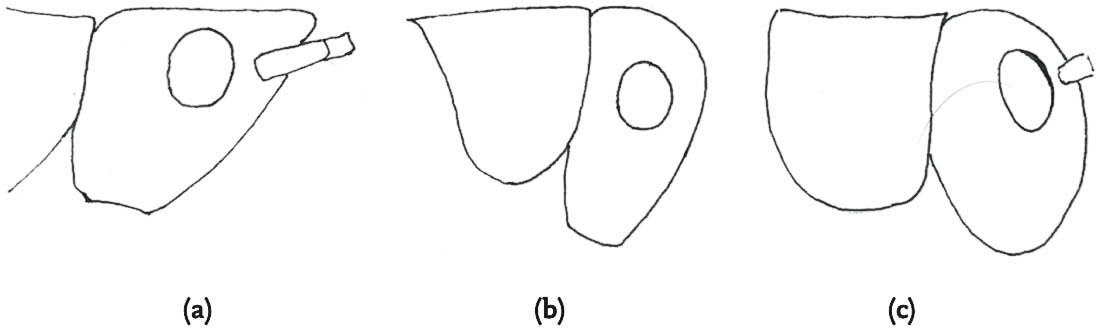
FIG. K.2 Profiles of heads: (a) conehead; (b) bush-cricket; (c) cricket.
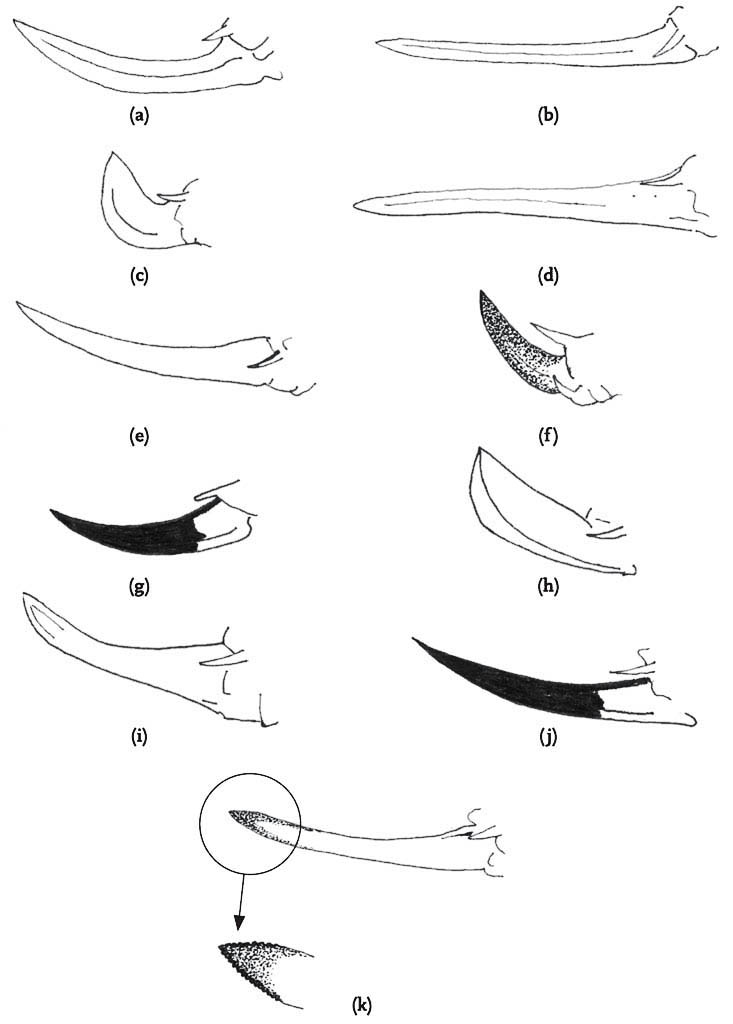
FIG. K.3 Ovipositors of female bush-crickets: (a) Conocephalus dorsalis; (b) Conocephalus discolor, (c) Phaneroptera falcata; (d) Tettigonia viridissima; (e) Meconema thalassinum; (f) Metrioptera roeselii; (g) Platycleis albopunctata; (h) Leptophyes punctatissima; (i) Meconema meridionale; (j) Metrioptera brachyptera; (k) Decticus verrucivorus.
FIG. K.4 Male cerci and tip of the abdomen (lateral view and dorsal view): (a) Conocephalus dorsalis; (b) Conocephalus discolor.
FIG. K.5 Cerci of male bush-crickets: (a) Phaneroptera falcata; (b) Tettigonia viridissima (dorsal view); (c) Meconema thalassinum (dorso-lateral view); (d) Leptophyes punctatissima (dorsal view); (e) Meconema meridionale (dorso-lateral view); (f) Pholidoptera griseoaptera (dorsal view); (g) Metrioptera roeselii; (h) Metrioptera brachyptera; (i) Platycleis albopunctata.
FIG. K.6 (a) Pronotum of Decticus verrucivorus, showing median keel on dorsal surface of the pronotum; (b) pronotum of Tettigonia viridissima: median dorsal suture, but lacking a keel.
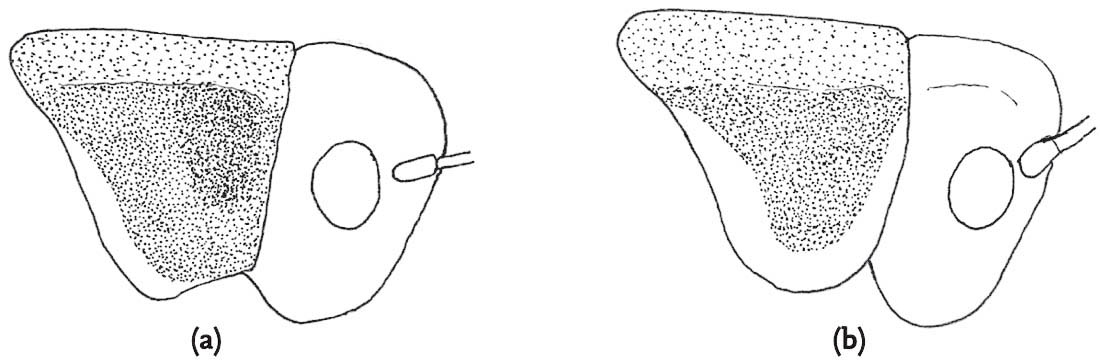
FIG. K.7 Side view of pronotum: (a) Metrioptera brachyptera; (b) Metrioptera roeselii.
FIG. K.8 Female subgenital plates of three bush-cricket species: (a) Metrioptera roeselii; (b) Metrioptera brachyptera; (c) Platycleis albopunctata.
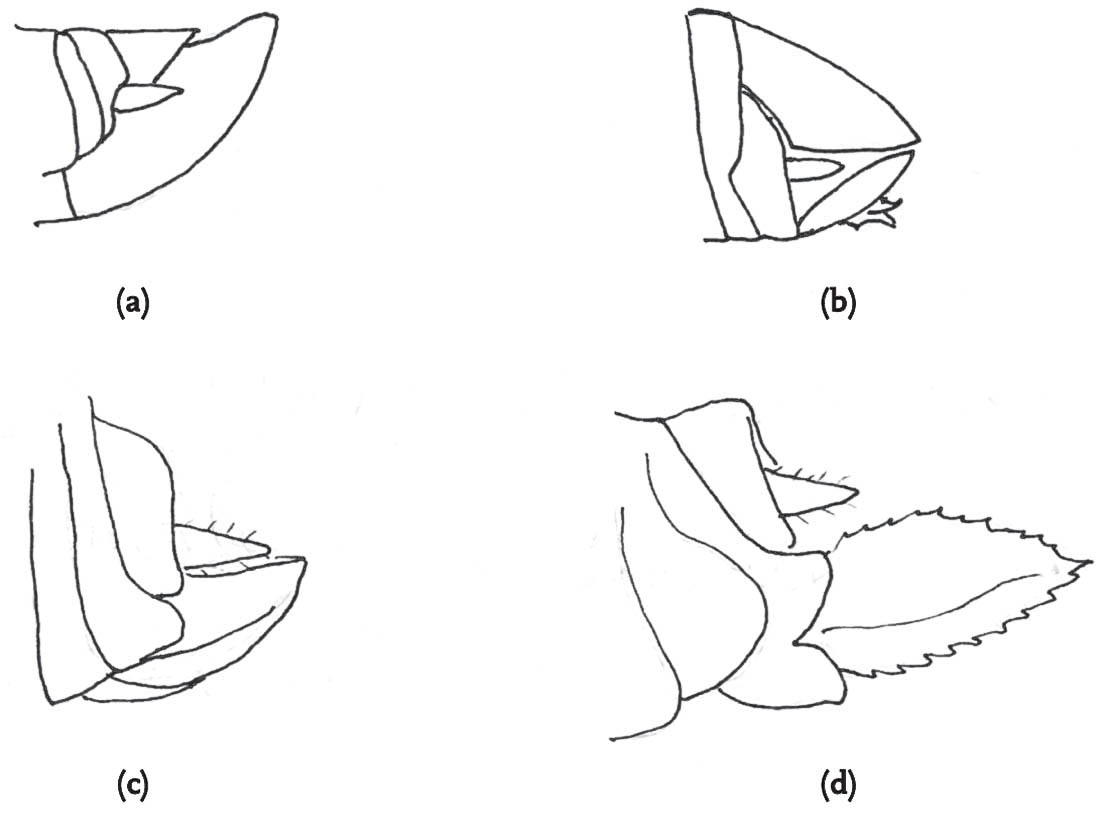
FIG. K.9 Tip of abdomen and associated structures in: (a) male grasshopper; (b) female grasshopper; (c) male groundhopper; (d) female groundhopper.
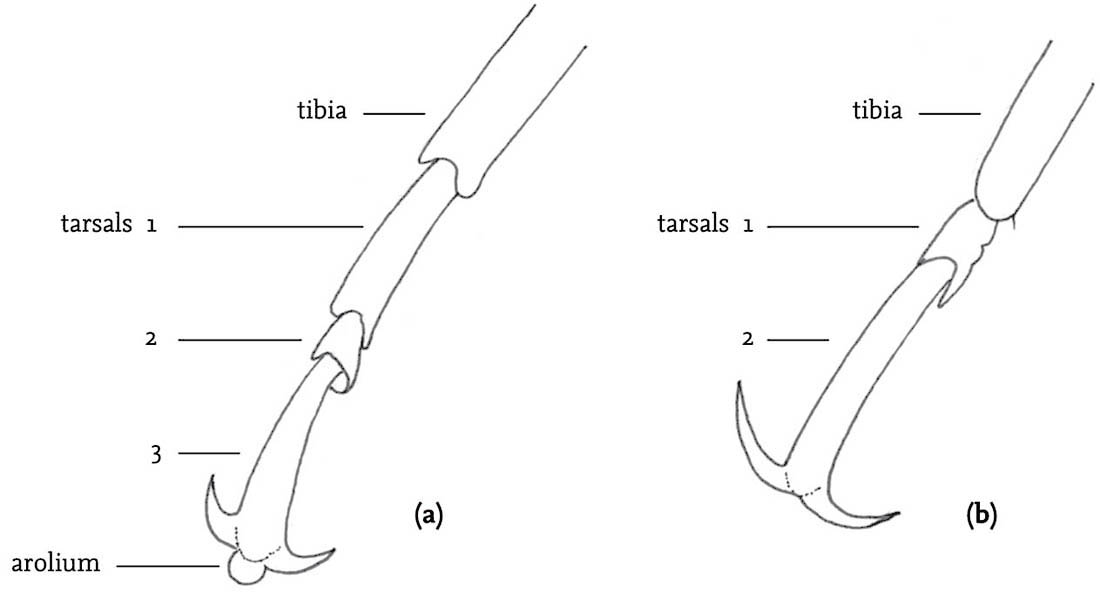
FIG. K.10 Tarsi of fore legs: (a) grasshopper (three segments, and arolium); (b) groundhopper two segments, and arolium absent).
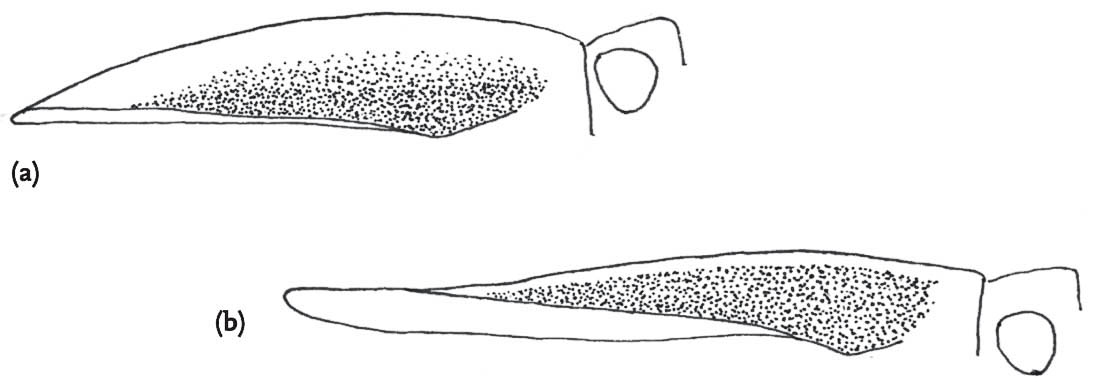
FIG. K.11 Side views of pronotum and head of groundhoppers: (a) Tetrix undulata; (b) Tetrix subulata and ceperoi.
FIG. K.12 Mid femur: (a) Tetrix subulata; (b) Tetrix ceperoi.
FIG. K.13 Dorsal view of head of (a) Tetrix subulata; and (b) Tetrix ceperoi (females).
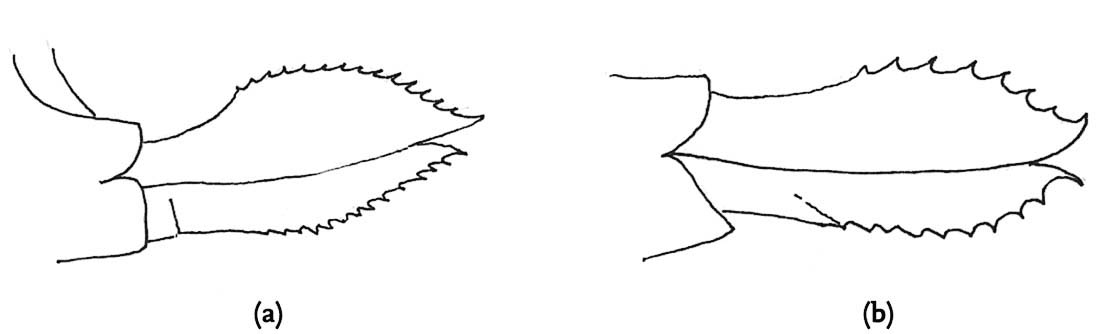
FIG. K.15 Ovipositor valves of females: (a) Tetrix subulata; (b) Tetrix ceperoi.
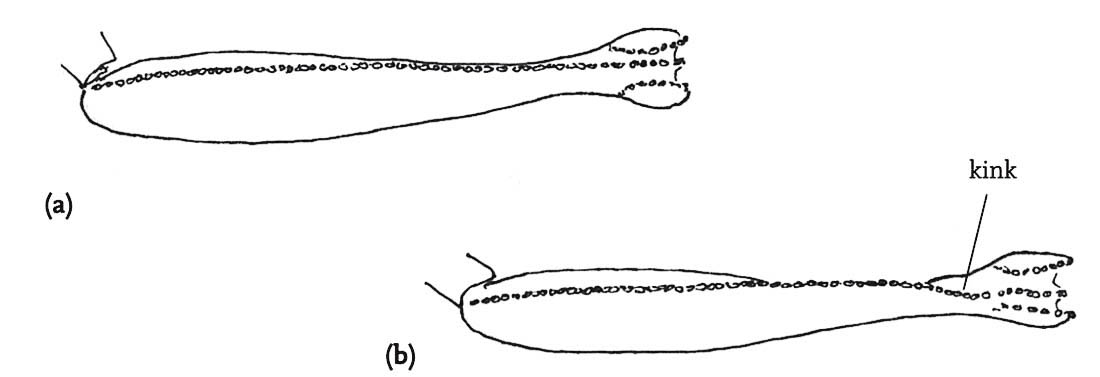
FIG. K.16 Dorsal view of hind femur: (a) Tetrix subulata; (b) Tetrix ceperoi (showing ‘kink’ in dorsal ridge).
FIG. K.17 Dorsal view of pronotum: (a) Stethophyma grossum; (b) Chorthippus albomarginatus; (c) Chorthippus parallelus; (d) Stenobothrus stigmaticus; (e) Omocestus rufipes; (f) Stenobothrus lineatus; (g) Omocestus viridulus; (h) Myrmeleotettix maculatus; (i) Gomphocerippus rufus; (j) Chorthippus brunneus; (k) Chorthippus vagans.
FIG. K.18 Inner surface of hind femur of male grasshopper (Chorthippus brunneus) showing stridulatory file.
FIG. K.19 Fore wing of Chorthippus brunneus, showing costal bulge.
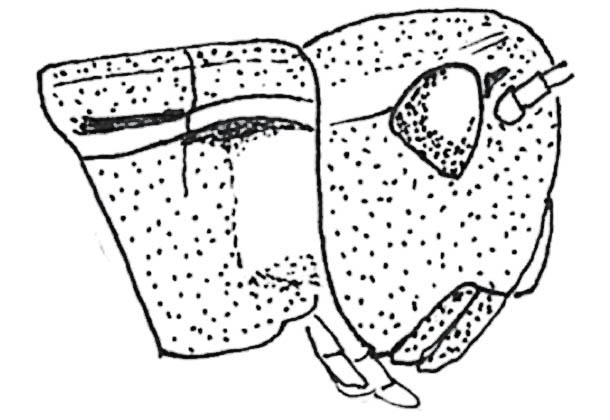
FIG. K.20 Side view of the head and pronotum of Stenobothrus stigmaticus.
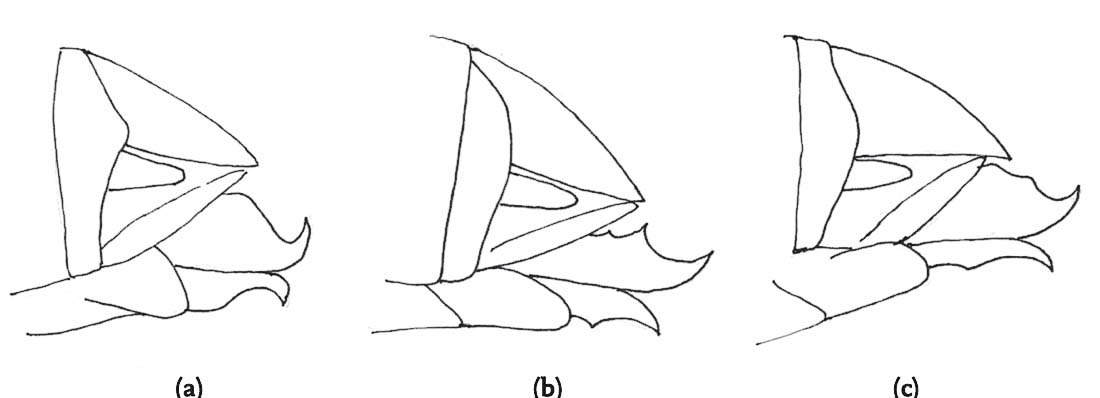
FIG. K.21 Ovipositor values of female grasshopper species: (a) Omocestus rufipes; (b) Stenobothrus lineatus; (c) Omocestus viridulus.
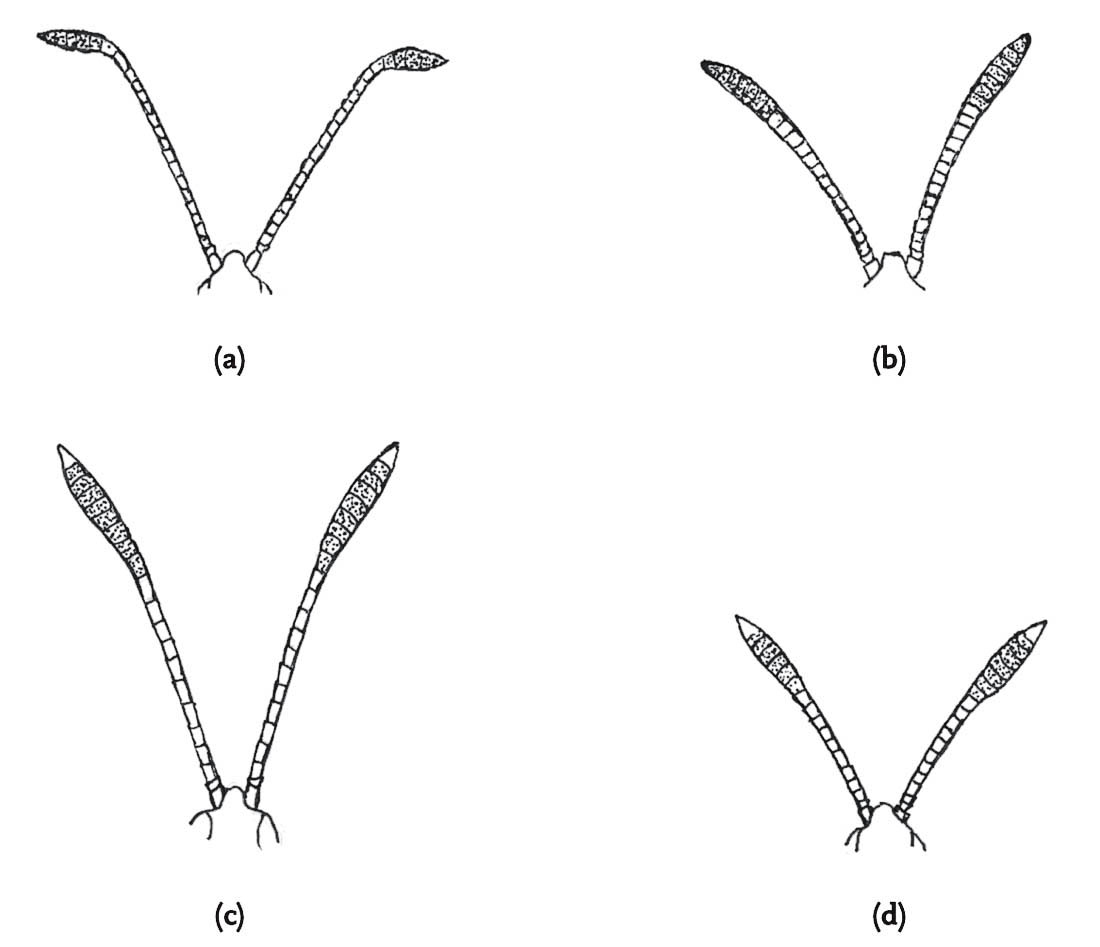
FIG. K.23 Antennae of two grasshopper species: (a) male Myrmeleotettix maculatus; (b) female Myrmeleotettix maculatus; (c) male Gomphocerippus rufus; (d) female Gomphocerippus rufus.