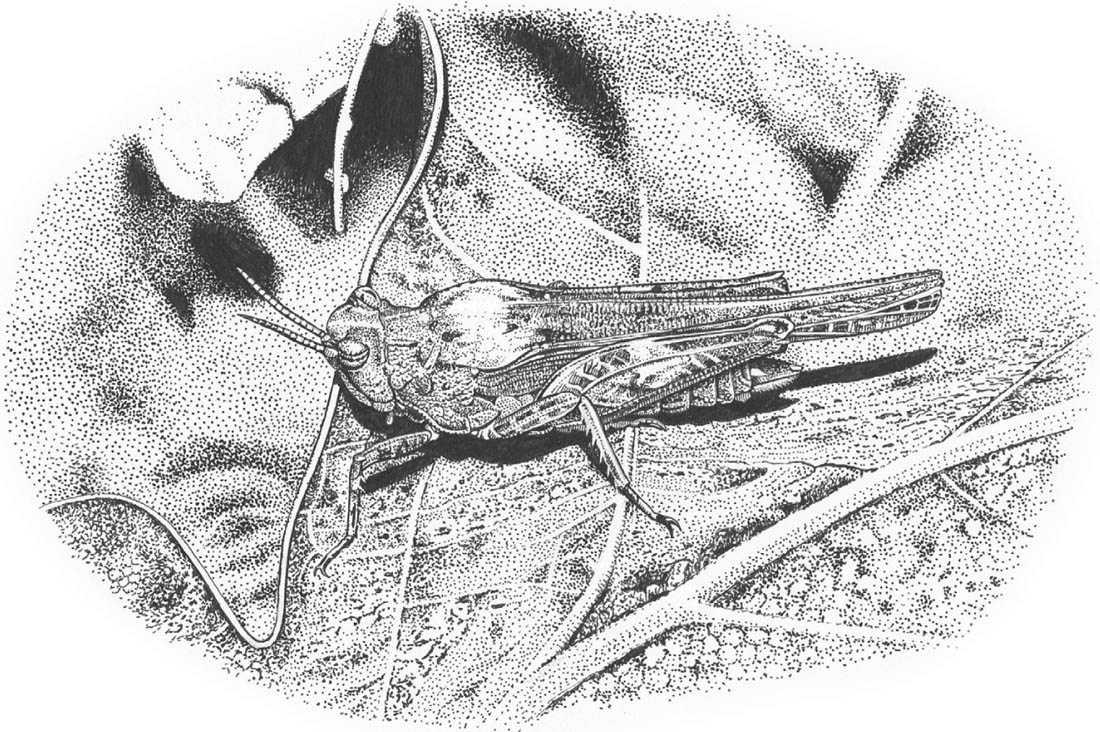
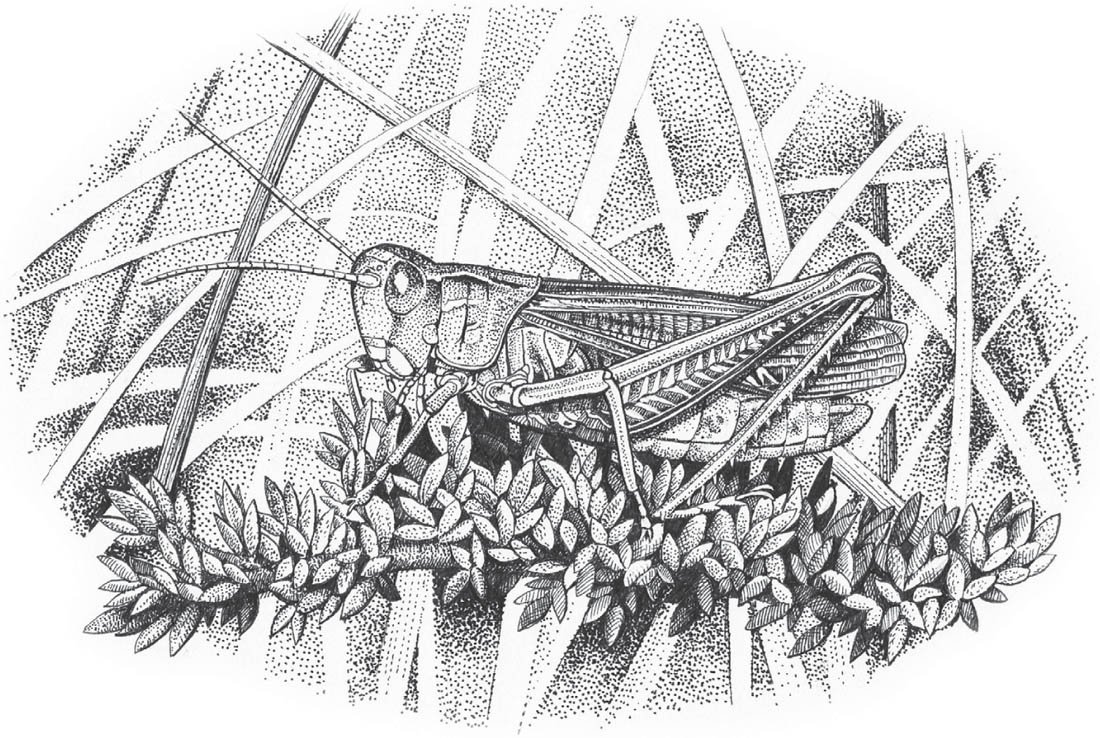
Slender groundhopper (© Tim Bernhard)
The British species, part 2: Caelifera
Groundhoppers and grasshoppers
GROUNDHOPPERS (TETRIGIDAE)
These are sometimes referred to as ‘pygmy grasshoppers’ or ‘grouse locusts’. They are believed to have changed relatively little from the evolutionary ancestors of today’s Orthoptera, and their preference for relatively simple plants such as soil algae and mosses as food sources can be understood as a legacy of their evolutionary ancestry (Blackith, 1987; Paranjape et al., 1987).
There are only three British species of this fascinating group. They superficially resemble tiny grasshoppers, but on closer inspection one can see significant structural differences. The most obvious is that the pronotum section of the thorax projects back over the abdomen, tapering towards or beyond its tip. The tarsi of the fore and mid legs have only two segments (three in grasshoppers). The male genitalia and the female ovipositor are structured very differently from those of grasshoppers.

Slender groundhopper (© Tim Bernhard)
CEPERO’S GROUNDHOPPER
Tetrix ceperoi (Bolivar, 1887)
MEASUREMENTS: Male: 9.5–10.5 mm in length; female: 11–13 mm in length.

FIGS A–C. Male; dorsal view; female
Description
Like the other groundhoppers, T. ceperoi is small and inconspicuous. The pronotum has a fine median ridge running along it and tapers back, usually reaching 2.5 mm to 4 mm beyond the tip of the abdomen. The fan-shaped hind wings are kept folded under the pronotum, and usually terminate just beyond the tip of the pronotum. The fore wings are vestigial and take the form of small pads at the side of the insect’s body, just below the pronotum. The mid femora have wavy edges dorsally and ventrally, and the anterior edge of the vertex (front of the head) is approximately continuous with the front of the eyes.
Variation
Although they are generally quite cryptically coloured, there is considerable individual variation in both the ground colour and the patterning on the dorsal surface of the pronotum. Combining ground colour and pattern variation, Paul (1988) distinguished 10 varieties, while Hochkirch et al. (2007) distinguished 20 different colour forms, which they were able to reduce to a simpler 6-fold classification: light brown, dark brown, black, mottled green, red-brown and grey. Elsewhere, Hochkirch et al. (2008a) distinguished nine different pronotal patterns.
The colour forms are classified according to a mix of basic colour and pattern into nine categories, although some defied classification in such a simplified way:
These variant colour patterns coexist in many populations, but differ in their relative frequency. Previous discussions in the literature have usually proposed explanations of the persistence of this diversity in terms of assortative mating, differential selective pressures and various genetic mechanisms on the assumption that colour patterns are strictly under genetic control (Nabours, 1929; Paul, 1988; Caesar et al., 2007). A recent study by Hochkirch et al. (2008a) showed that some aspects of adult coloration are influenced by substrate colour during individual development, but their results are contested by Karlsson et al. (2009) (see Chapter 3 for more details).

FIGS D–K. Brown with pale median dorsal stripe; green with pale dorsal stripe; grey-brown; mottled green with pale shoulders; mottled green; red-brown mottled; red-brown mottled with green; rust brown.
Similar species
T. ceperoi could be confused with either of our other two groundhoppers.
T. undulata is larger bodied (more ‘stocky’ in appearance), and has a relatively much shorter pronotum, which reaches back approximately to the tip of the abdomen (significantly further in T. ceperoi). In addition, T. undulata has a more prominent raised keel down the middle of the dorsal surface of the pronotum.
The slender groundhopper, T. subulata, is very similar to T. ceperoi and careful examination is needed to separate them.
There are five main features.
In T. ceperoi the width of the vertex is approximately 1.3 to 1.5 times the width of the eye. In T. subulata it is relatively wider, 1.5 to 1.8 or more times the width of the eye. These differences are, of course, difficult to determine with living insects in the field – but with experience the general impression of T. ceperoi is that its eyes are more prominent than those of T. subulata, when viewed from above.
In T. ceperoi the vertex does not project significantly in front of the eyes (giving the impression of a smoothly curved front edge when viewed from above). In T. subulata the vertex projects a small distance in front of the eyes. In profile, the front of the face is more vertical in T. subulata but curves back towards the top in T. ceperoi. One way of determining this is by observing (from the side) the angle formed by the meeting of the front of the face and the vertex. This is usually slightly obtuse in T. ceperoi, and acute in T. subulata.
In all, it is probably advisable to check several characters – and, even better, several specimens – for confident identification. (See the Key, Figs K12–16.)
Life cycle
The main period for mating and egg-laying in Britain is May and June. Eggs are laid in clusters held together by a sticky secretion, in damp ground or among low vegetation. They hatch in 3 to 4 weeks and the resulting nymphs pass through five (male) or six (female) instars, usually reaching adulthood by autumn. The winter is spent as either a late instar nymph or an immature adult. They may be active on warm days from early spring onwards, and reach full maturity by May.
Habitat
Despite its very restricted distribution in Britain, T. ceperoi occurs in a wide variety of habitats. These include the landward side of dune systems, on muddy or stony edges of ponds or lakes, on seepages from sea cliffs, on bare peat at the edge of streams and ponds, in wet sand pits and on muddy edges of drainage ditches. What all these habitats have in common is patches of damp or wet bare ground, interspersed with vegetation cover. The presence of soil algae, mosses or other small, delicate plants is also necessary. Most known sites in Britain are close to the coast.
Behaviour
Both sexes are inactive in dull or cool weather. In warm, sunny spells they graze on young shoots of mosses, on algae, or on decaying plant material. As they do so, their palps are continually in action, probing and ‘testing’ the substrate on which they feed. Females are relatively static, usually either feeding or occasionally moving around over mud, pebbles or larger plants. The males are more active, presumably in search of potential mates, and spend less of their time feeding. Hochkirch et al. (2007) showed that males spent more time than females did on bare ground and mosses, with greater intensity of incident sunlight. Females spent more time among leaf litter and higher plants than males did. There was a related difference in the frequency of two colour forms: black males were much more common than black females, and mottled-green females much more common than males with that background colour. Black males would be better camouflaged on a muddy background, and females on their less exposed, more vegetated preferred microhabitat. Tentative evidence that males might also be more exposed to predation was the greater incidence of missing legs in the males. In the absence of stridulation, males find mates by visual searching, and it is supposed that the clear view they get by occupying bare ground enhances their chances of success, the resulting behaviour representing a ‘trade-off between natural and sexual selection.
Males of T. ceperoi have a distinctive ‘courtship’ display, referred to by Hochkirch et al. (2006) as ‘pronotal bobbing’. This looks like a fast, deep ‘bow’ in the direction of the female (see DVD). The male simultaneously raises his hind legs and dips down his head, so raising the rest of his body at a steep angle, then returns to his usual stance and moves rapidly toward the female. In the analysis carried out by Hochkirch et al. the pronotal bobbing took on average only 0.8 of a second. The male next mounts the female and, if successful, holds on to her pronotum while curving his abdomen down between the rear portion of her pronotum and the nearside femur. Contact is then made by moving the open valves of his genitalia up to engage with the underside of the tip of the female’s abdomen. Mating usually lasts only a few seconds and the male then moves on. However, two males often compete to mate with a single female, both attempting to mount her at once. Females are polyandrous and are often prepared to mate with rival males in quick succession. However, they also reject some males by not opening the space between the hind femur and pronotum, and sometimes by vibrating vigorously until a mounted but unwanted male is shaken off (see DVD).
Where T. ceperoi occurs together with T. subulata mating attempts and actual mating take place between the two species. Gröning et al. (2007) studied ‘reproductive interference’ between the two species both in the laboratory and in the field. In both situations males of both species appeared not to discriminate between the females of the two species. However, possibly because of the distinctive visual courtship signal of T. ceperoi (‘pronotal bobbing’), females of that species rejected males of T. subulata more often than they rejected males of their own species (see Chapter 6).
Presumably their cryptic coloration, and tendency to rest on colour-matching substrates, is their main defence against predators. However, they can jump out of the way when disturbed (especially in warm weather), and they can augment their leaps with flight. They can also swim by powerful strokes of their hind legs if their escape attempts land them in open water, although there is no evidence of their entering the water independently of provocation.
Enemies
See under slender groundhopper (T. subulata).
Distribution
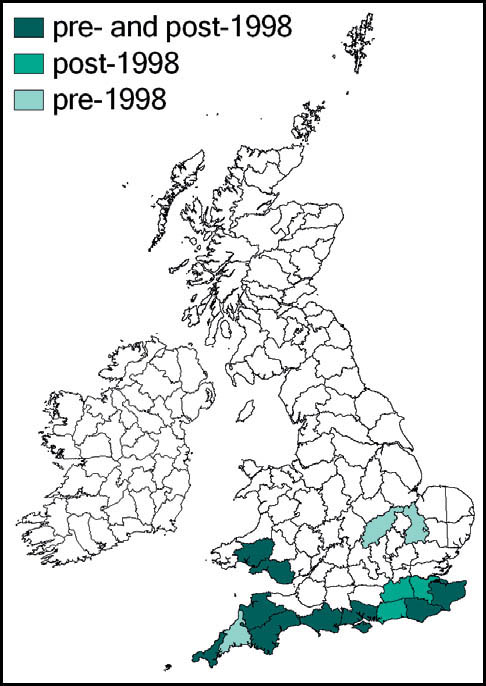
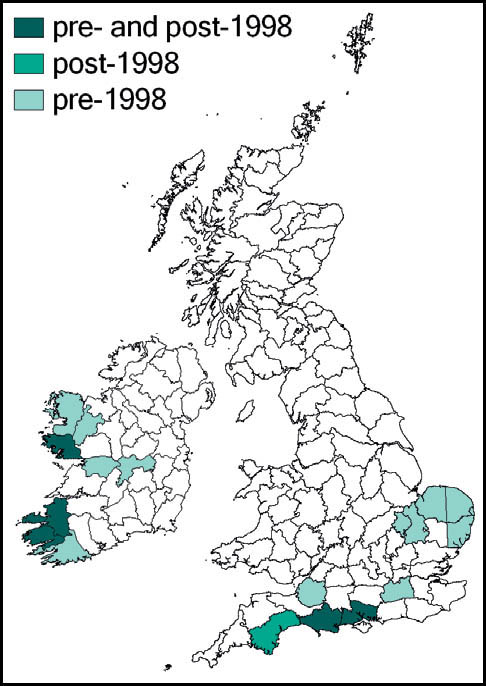
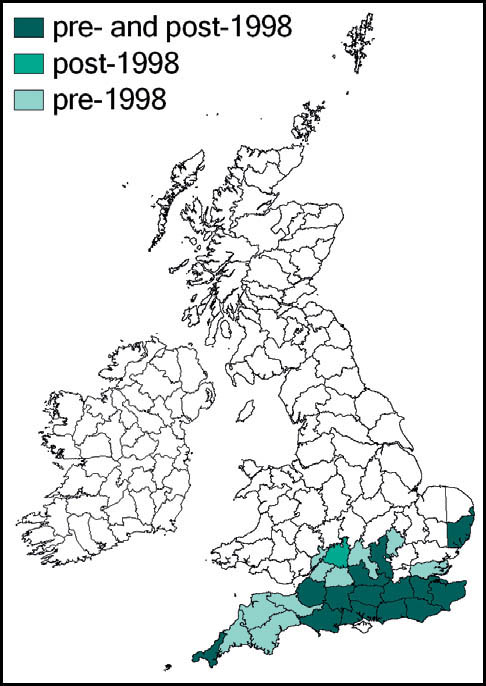
This species is quite widespread in western and southern Europe and North Africa. In Britain it is at the northern edge of its range. Here it is regarded as a localised and scarce species, although it can be abundant in suitable sites. Its known localities are mainly coastal and in the southern counties of England and Wales. It is present in coastal districts of Kent, Sussex, Hampshire and the Isle of Wight, Dorset, Devon and Cornwall in England and Carmarthenshire and Glamorgan in south Wales. There are also inland records from Surrey and Hampshire (New Forest), and isolated records from Northamptonshire and Cambridgeshire. It is not known from Ireland.
Status and conservation
Cepero’s groundhopper was first distinguished from the slender groundhopper, Tetrix subulata, among British populations, and so recognised as a British species, by B. P. Uvarov (1940). The scarcity of T. ceperoi in Britain is probably the result of a combination of its rather specialised habitat preferences and its geographical range. It does not appear to be threatened, and, indeed, could well be under-recorded given its close similarity to T. subulata. The discovery of the species near Peterborough, many kilometres outside its previously known range, is of interest. It could be that this population is the result of an incidental introduction, but if it is not, then the possibility arises that the species may have been overlooked elsewhere outside its previously known range.
References: Brown, 1950; Caesar et al., 2007; Evans & Edmondson, 2007; Gröning et al., 2007; Hochkirch et al., 2006, 2007, 2008a; Karlsson et al., 2009; Nebours, 1929; Paul, 1988; Uvarov, 1940.
SLENDER GROUNDHOPPER
Tetrix subulata (Linnaeus, 1758)
MEASUREMENTS: Male: 9–12.5 mm in length; Female: 11–15 mm in length.

FIGS A–C. Male; dorsal view; female

FIG D. F. bifasciata
Description
This species, as its English name implies, is long and narrow in overall shape. The pronotum tapers back over the body and terminates several millimetres (3–4.5) beyond the tip of the abdomen. The fan-shaped wings, when not in use, are folded below and along the underside of the pronotum, and usually terminate just beyond its tip. The vertex is relatively broad and projects a small distance in front of the eyes. The edges of the mid femur, when viewed from the side, are straight or gently curved, not wavy, as in Cepero’s groundhopper.
Variation
There is a form of the slender groundhopper (f. bifasciata) in which the pronotum and wings are shorter than they are in the typical form, terminating only just beyond the tip of the genitalia at the rear end of the abdomen. This form varies in frequency among populations (apparently one northern locality has mostly this form (see Widgery, 1998)), and there are intermediate forms.
As in other groundhoppers, there are many different colour patterns, and usually several in any population. Some common variants are illustrated.

FIGS E–M. Dark brown with ochre patches; pale median stripe; pale shoulders; mottled form; white spot; rust-brown with dark sides; fawn with dark brown sides; dark with white flashes; dark grey unmarked.
Another colour form (not illustrated) was unmarked white, and found on exposed chalk in a disused chalk quarry (T. Tamblin, pers. obs.).
Similar species
The typical form is very similar to Cepero’s groundhopper, and close examination with a hand lens is necessary for confirmation. (For details, see under that species.)
Form bifasciata could be confused with the common groundhopper (T. undulata), but the latter is a more ‘stocky’ insect, with a strongly raised median keel on the pronotum.
Life cycle
In early spring, slender groundhoppers become fully active a little later than their close relative, the common groundhopper. Initially, much of their time is spent basking or feeding. Courtship and mating take place from early April to June in sunny weather. The eggs are approximately 3 mm in length and are loosely sausage-shaped with a horn-like projection at one end. They are laid in small holes, usually in damp soil, in clutches of 10 to 20, stuck together with an adhesive secretion. They hatch in 3 to 4 weeks. As in other Orthoptera, the initial phase is a worm-like larva, which quickly sheds its outer coating to reveal the first instar nymph. Development takes place through the spring and summer months, males passing through five, females six, nymphal stages. The previous generation of adults dies out during the summer, so that later in the year only various nymphal stages are present. The winter is spent as a final instar nymph, or as an immature adult. Full sexual maturity is reached early in the following spring.
Habitat
The slender groundhopper is usually found in damp or marshy habitats such as river edges, pond margins, wet meadows, ditches and damp hollows in heathland. However, it can also be found well away from open water, in damp woodland rides, or cart tracks. Areas of bare ground, open to sunlight, but also damp, with simple plants such as soil algae, mosses and fungi are essential. The preference for damp conditions may be associated with the relatively specialised diet, but also may reflect water demands for the eggs (Hochkirch et al., 2000). It may be that patches of bare ground are specifically required for courtship and mating, as, in many of the habitats, patches of open ground become shaded out by vigorous growth of grasses, sedges and rushes by late spring or early summer. At this time, especially, the groundhoppers climb up to sun themselves on exposed vegetation or other perches (see under ‘Behaviour’).

FIG N. Nymph.
Behaviour
Although apparently dormant during the coldest months, the groundhoppers emerge to bask in sheltered spots during sunny weather from early March (in southern England). They are present in greater numbers by the beginning of April, and by the middle of April, courtship and mating are under way.
The groundhoppers are active mainly in warm and sunny weather and later in the season, when exposed patches of bare ground are often shaded out by denser vegetation, they climb onto the higher leaves and stems of grasses or sedges to bask. They can also be seen on the reflective surfaces of containers and other litter. In one former willow plantation by a small river they could be found resting or feeding on the raised platforms provided by the mossy and part-rotted stumps of the trees (pers. obs.).
They graze on soil algae and fresh, tender shoots of mosses, but will also consume detritus, and females sometimes eat young, tender shoots of grasses or other flowering plants. The somewhat different diets of males and females might be explained in terms of the nutritional needs of the latter for egg production (Hochkirch et al., 2000). Feeding is generally accompanied by frequent antennation of the substrate, and probing with the palps (see DVD).
The females are relatively inactive, and spend most of their time resting or feeding on the ground or among detritus, while the males spend much of their time actively searching for potential mates, especially in the afternoon. Initial location of a female is probably visual, but, in close proximity, males frequently point their antennae directly towards the female and ‘twirl’ them in a way that suggests that scent may be involved. Commonly, but not always, the male faces the female and performs a very brief courtship signal. Hochkirch et al. (2006) distinguish two such signals which they term ‘frontal swinging’ and ‘lateral swinging’. Both these signals are of very brief duration (around one second), and involve raising the body of the insect slightly by stretching the fore and mid legs and swinging forward, or stretching the legs on one side to produce a sideways motion. The amplitude of the signal is very small and it is easily missed by the human observer. However, on film it is readily detectable (see DVD).
The signal may be repeated several times as the male approaches the female. As the approach is usually from the front, he commonly mounts frontally, and climbs onto her pronotum, facing backwards. He then turns around, and, gripping her pronotum with fore and mid legs, curves his abdomen down between the side of the female’s pronotum and the femur of that side. Females appear to vary in their receptiveness, and sometimes males attempt to force their way between these structures. Once this position has been achieved, the male searches with the tip of his abdomen for the genitalia of the female and mating takes place. This is usually very brief – a few seconds only – and is usually accompanied by antennal contact, the male curving his antennae down, the female raising hers. The male disconnects his genitalia from those of the female, dismounts and walks away. Frequently the female continues to feed throughout the operation.
However, this ‘textbook’ sequence is often varied. Competition between males for mates is quite intense, and it commonly happens that the mating attempts of one male are interrupted by the arrival of another suitor. The latter will often attempt to pull off the first, but, if that fails, he will simply clamber over it and attempt to mate from the other side of the female. Sometimes it appears that the second male successfully mates with the female as soon as the first completes copulation and dismounts. Often, if a male fails to elicit the appropriate response from a female he will resume the initial posture, facing backwards while standing on the pronotum of the female. This brings the tip of his abdomen over the antennae of the female. He then reverses to the standard position for copulation and is often successful. Another probable consequence of the intensity of male competition is that males often dash at and mount a female directly and without any observable courtship signal.
In addition to the two ‘swing’ signals described above, both males and females signal to one another by rapidly raising and then lowering one hind leg (or sometimes both). This ‘leg flick’ signal is most often used when males encounter one another, and is followed by one or both moving away.

FIG O. Mating pair.
Another pattern of movement that appears to have a communicative role is a rapid vibration of the whole body that looks like a brief fit of rage or frustration. It could be that this is less a visual signal than a form of vibratory communication that is sensed either by direct physical contact or via the substrate (Benediktov, 2005). However, what appears to the human eye as the same ‘vibration’ signal is used in a range of different contexts. Most frequently it is used when two or more males encounter one another. In a head-on encounter, one or both may vibrate and one moves off. Sometimes one male climbs onto another, which then vibrates until the first dismounts. This is sometimes followed by a fit of vibration from the first. In fact, males frequently make physical contact with one another, sometimes climbing onto or over one another, and often with mutual antennation or vibratory signals. Hochkirch et al. (2006) interpret this as indicating that males will attempt to mate with more or less anything of roughly the right size. Occasionally, indeed, these encounters do look like mating attempts, but more often they have the appearance of mildly antagonistic or neutral contacts. The locations where individuals congregate and perform courtship and mating appear to be strongly physically delimited within the wider habitat. It could be that these contacts function in some way to maintain the cohesion of localised aggregations, which could be important for mate location in a species without sound communication that lives in habitats offering little long-distance visibility.
Where T. ceperoi and T. subulata occur together (as they do in some British localities, such as Rye Harbour), males of both species mis-direct courtship and mating attempts to females of the ‘wrong’ species (see under T. ceperoi).
In laboratory tests of reproductive interference between T. subulata and T. undulata (Hochkirch et al., 2008a), males of both species showed a strong preference for females of T. undulata, while females of each species were equally receptive to males of either. This may be because of the close similarity of the courtship signals of the males. However, in the field, where the two species intermingle, males of T. undulata seem to court or mate with females of their own species and those of T. subulata in roughly equal proportions. Females of both species accept or resist mating attempts from males of either species without any obvious discrimination. In one extraordinary filmed sequence, a male T. undulata mounts a female T. subulata, but is pulled off by a rival male T. subulata, which then walks over the male T. undulata (eliciting a fit of vibration from the latter), to successfully mate with the female T. subulata. She then wanders off and re-encounters the original male T. undulata, who promptly mounts and successfully mates with her! (See Chapter 6 for more details, and the DVD.)
Enemies
There appear to be no literature reports of predation on groundhoppers, but it is possible that they are taken by water-side birds, amphibians or, like many other orthopterans, by spiders.
Loss of one of the back legs is common in most populations, and it is sometimes assumed that this is evidence of predation (autotomy is used by orthopterans as a means of escape from predators) but it may also be a result of imperfect moulting of nymphal cuticle during development. Cryptic coloration and the apparent tendency to select matching substrates must be an important defence against visual predators. However, when basking, and sometimes when feeding, they rest on contrasting substrates, presumably reflecting a trade-off between those priorities and predator avoidance. Their most common response to disturbance is to jump – often landing on a colour-matching surface. Especially in warm weather, they may respond by flying some four or five metres, and they can change direction to some extent while in flight. Like the other two groundhopper species, they are able to swim, using powerful kicks of the back legs. This is presumably an aid in escaping from terrestrial predators.
Distribution
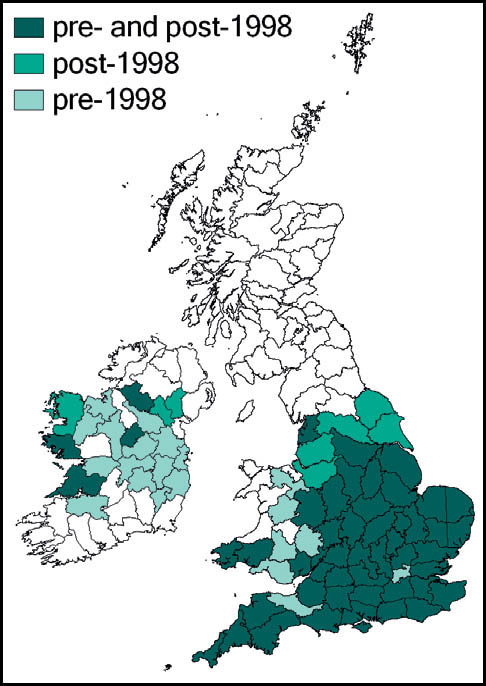
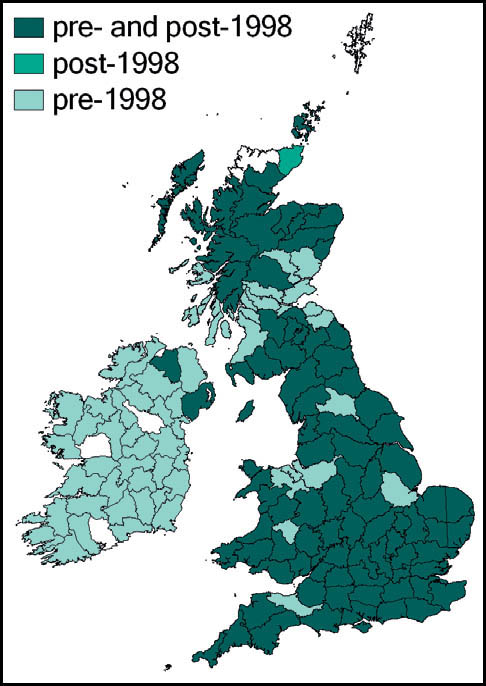
This species has a very wide distribution throughout the temperate regions of the northern hemisphere, including most of Europe, temperate Asia, north Africa and north America. In Britain it is common and widespread in south-western, central-southern and south-eastern England and East Anglia. Kevan (1961) gives north Lincolnshire as its most northerly outpost, but it has since been recorded from Lancashire and Yorkshire (Drax power station, at Selby in 1998), and subsequently in a number of other localities in Yorkshire (Sutton, 2005a). There is also a scattering of records from southern and some eastern counties of Wales, and across Ireland.
Status and conservation
It seems likely that many of the slender groundhopper’s localities are rather temporary and vulnerable to shading out of the required bare patches by vegetative succession, and to the drying out of ponds and damp hollows. Extensive drainage of wetlands for agriculture must also have greatly affected its populations. However, it appears to be a relatively effective coloniser of newly created habitats and is regarded as a ‘pioneer’ species. Careful searching of any apparently suitable habitat within its climatic range generally reveals its presence. In view of this, it seems unlikely that this species is significantly threatened in Britain.
References: Hochkirch et al., 2000, 2006, 2008a; Gröning et al., 2007; Kevan, 1961; Sutton, 2005a.
COMMON GROUNDHOPPER
Tetrix undulata (Sowerby, 1806)
MEASUREMENTS: Male: 8–9 mm in length; female: 9–11 mm in length.

FIGS A–C. Male; male dorsal view; female.
Description
The common groundhopper is a more compact, robust insect than its two close relatives. This appearance is partly the result of the much shorter rear extension of the pronotum, the tip of which reaches back only so far as, or a little beyond, the rear end of the abdomen and genitalia. The reduced hind wings are largely concealed under the pronotum with just a narrow section of the leading edge visible below it, and to the rear of the small ridged pads that are the reduced vestiges of the fore wings. The longitudinal ridge on the dorsal surface of the pronotum forms a prominent keel in this species and has a convex outline when viewed from the side, giving a slightly ‘hump-backed’ appearance to the insect. The curvature is even in the male, but more abrupt towards the front of the pronotum in the female.
There is an uncommon fully-winged form (f. macroptera Haij) listed from two Scottish sites by Kevan (1952), and, more recently in 1991, also from Scotland (Haes & Harding, 1997).
As in the other groundhoppers, there is a great diversity of colour forms and many of these are similar in all three species – possibly reflecting their overlapping habitat preferences. (See Chapter 3, Fig 72.)
Similar species
The adults of this species can easily be distinguished from those of the other two British groundhopper species by the much shorter pronotum, and also by the raised central keel along the pronotum. However, confusion with the short-winged form bifasciata of the slender groundhopper is possible. The prominent keel in the common groundhopper is the most obvious distinguishing feature here. Early instar nymphs of all three species are very difficult to distinguish, but in the final nymphal instar the hind wing pads are clearly visible in the other two species, but not in T. undulata.

FIG D. Nymph.
Life cycle
Although they are reputed to be active in warm days through the year, common groundhoppers are rarely seen before the middle of March in southern Britain. However, by the end of that month they are very active in good weather. Courtship and mating begins at that time, and continues for much longer into the summer than is the case for the other two groundhopper species. The eggs, which are similar in form to those of the other groundhoppers (see under slender groundhopper), are laid into the ground or among low vegetation. They hatch in 3 to 4 weeks. Subsequent development is as in the other groundhoppers – an initial vermiform larva gives way to a series of five (in the male) or six (in the female) nymphal instars – but as the breeding season is more extended in this species, both adults and nymphs of various stages can be found together through the year. Overwintering nymphs complete their development the following spring, and so become fully adult later in the season than those that overwintered as adults.
Habitat
As noted by many observers, this species can be found in a much wider range of habitats than the other two British species of groundhopper. However, it does have markedly overlapping habitat preferences, so that the damp pond margins, stream edges and hollows where the slender groundhopper occurs frequently harbour this species too. It also inhabits a range of other biotopes: along path edges and in clearings in open woodland – especially among mosses and leaf litter, and on heathland among gorse and heathers. A key requirement seems to be patches of bare ground open to the sun, and plants such as algae and mosses that form its main diet. Even in apparently suitable habitat the population is often aggregated in small local patches.
Behaviour
Common groundhoppers are inactive in cool or overcast weather, and on warm, sunny days divide their time between basking, feeding and reproductive activity. Females are most often seen on the ground, feeding on various small plants, while males are generally more active. When not feeding or basking, they run in a rather jerky fashion on haphazard routes around open patches, or through leaf litter. This is presumably a mate-searching strategy, as when a female is approached the male generally stops, facing the female. Several brief and inconspicuous ‘swinging’ courtship signals may be given before the male moves forward, often making antennal contact with the female and mounting. Mounting is often from the front, so the male is initially standing on the pronotum of the female, facing backwards. He quickly turns around and probes with his abdomen to open a space between the hind femur and pronotum of the female. If the female is receptive, she allows this and the male then rubs his abdomen against that of the female and reaches down to make contact with her genitalia as she curves her abdomen in his direction. Mating usually lasts only a few seconds, and the male moves off, usually with the tip of his abdomen drooping, and the genital plates still parted.
However, there are many deviations from this pattern. Often males appear to approach directly, and mount and attempt to mate either without any obvious preliminaries, or with antennal contact only. In some examples, apparent female resistance is overcome by a mounted male reversing his position on the pronotum of the female, then reverting back to the normal mating position. One filmed sequence shows a female lacking one hind leg. A male who mounts her appears confused, dismounts, re-mounts and dismounts a further two times, despite her opening the space between her pronotum and the remaining hind femur. The sequence is completed by her walking over him, leaving him in a fit of vibration!
During their mate-searching activity, the males frequently encounter one another. When they do so, a variety of signals ensues. Commonly, two males exchange ‘leg flick’ signals with their hind legs. This usually results in one of them departing. On other occasions one or both of two adjacent males will perform a rapid up-down vibration, followed by the departure of one of them. On still other occasions, the initial ‘leg flick’ signal is ignored by another approaching male who may simply climb onto the first. This usually elicits strong vibration from the underling until the other dismounts, and is often followed by a subsequent fit of vibration on the part of the ‘abused’ male. As in T. subulata, many of these interactions between males do not appear to involve mating attempts, and may possibly have some function either in male-to-male competition, or in maintaining the cohesion of the local population.

FIG E. Male slender groundhopper attempts to mate with female common groundhopper.
These interactions between males are often replicated when males of T. undulata encounter male T. subulata. Where the two species mingle (as is very frequent in the damper parts of the habitat of T. undulata), courtship, mating attempts and mating between the two species are common (Hochkirch at al., 2006, 2008a; see under T. subulata; and see also Chapter 6 for more detail, and the DVD).
Enemies
As in the other groundhopper species, the cryptic coloration, and the associated behavioural disposition to settle on colour-matching surfaces, suggests selection by visual predation. Although camouflage appears to be their best defence, they are effective jumpers and, like their relatives, good swimmers.
Distribution
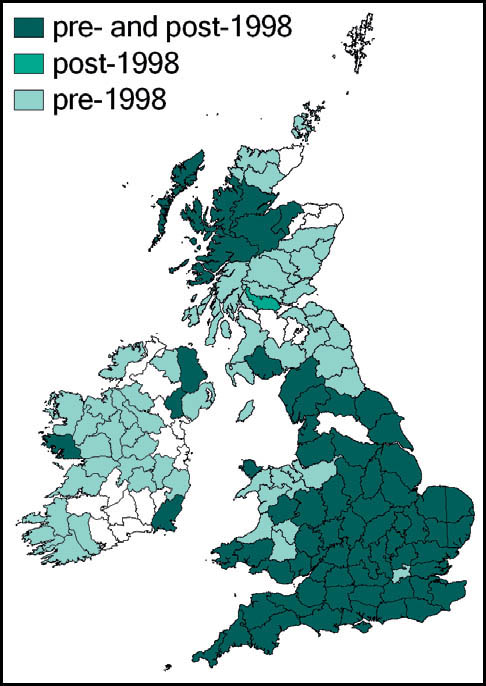
This species is widespread throughout most of Europe, except for southern Spain and northern Scandinavia.
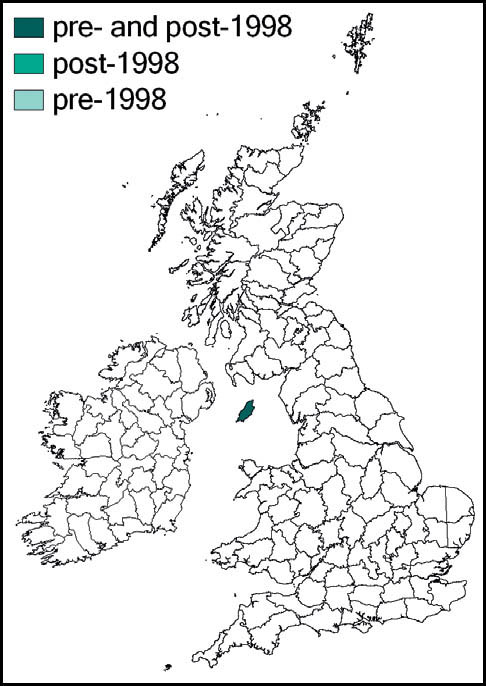
T. undulata is widespread throughout Britain, and has even been recorded in the Outer Hebrides (Barra and, more recently, South Uist (Sutton, 2007e)) and Orkney. In the Scottish highlands there are strong populations in ancient pine and birch woods (Marshall & Haes, 1988). In Monmouth it is recorded at altitudes up to 300m (Sutton, 2008a).
It also appears to be widespread in Ireland. Although there are gaps in the available distribution maps, this probably results from under-recording of this cryptic insect.
Status and conservation
Although T. undulata is a widespread and often abundant species, it has quite exacting habitat requirements and is vulnerable to vegetation succession as well as to habitat loss to agriculture and ‘development’. Unlike its sister species, T. subulata, it cannot fly and is likely to be relatively poor at dispersal to new sites. It is unclear whether the rare occurrence of the macropterous form is related to dispersal ability.
References: Haes & Harding, 1997; Hodgson, 1963; Hochkirch et al., 2006, 2008a; Kevan, 1952; Marshall & Haes, 1988; Sutton, 2007e, 2008a.
GRASSHOPPERS (ACRIDIDAE)
There are eleven British species in this large family. They are familiar, often abundant, inhabitants of grassland and heath. The pronotum is saddle-shaped and usually has a median keel along the dorsal surface, and a keel on each side-edge. There is also a groove, or sulcus, that cuts across the dorsal surface of the pronotum at approximately the middle. The shape of the side-edge keels and the position of the transverse sulcus are often crucial characteristics for identification.
The head bears a pair of relatively short and thickened antennae (sometimes broadened at the tip). The mouthparts are adapted for chewing vegetable matter – usually grass-blades. The female has a short, inconspicuous ovipositor, composed of two pairs of valves, while the male has an up-turned subgenital plate at the rear end of its abdomen, so the different shape of the tip of the abdomen is a useful way of distinguishing the sexes.
Stripe-winged grasshopper (© Tim Bernhard)
THE LARGE MARSH GRASSHOPPER
Stethophyma grossum (Linnaeus, 1758)
MEASUREMENTS: Male: 22–9 mm in length; female: 29–36 mm in length.

FIGS A–E. Male; male dorsal view; female; female dorsal view; dark female.
Description
This is the largest of the British grasshoppers – and perhaps the most beautifully patterned. The wings are fully developed, although varying in length to some degree – extending almost to, or a few millimetres beyond, the tip of the abdomen. The leading edge of the fore wing bulges outwards towards the base. The side keels of the pronotum are gently incurved, and diverge laterally on the rear portion of the pronotum. The cerci are relatively long in the male, and the valves of the female ovipositor are long and distinctively shaped. The aperture of the hearing organ is wide, and clearly visible on each side at the anterior end of the abdomen.
In this species the colour patterns are quite distinctive, although variable. In both sexes the hind legs are ringed with black – usually around the ‘knees’, and often with other contrasting black patches on the femora or tibiae. The femora are bright red ventrally. The colour pattern of the males is usually more contrasting than that of the females. The dorsal surface of the head and pronotum is usually olive-green (sometimes brownish), shading to yellow laterally, and on the fore and mid legs and hind tibiae. The side keels of the pronota are outlined in yellow, and there is a black band along each side of the head behind the eye. The abdomen is green, shading to yellow ventrally, with variable black markings on each segment.
The females are usually olive green, shading to greenish yellow on the sides and underside of the abdomen, with the legs frequently green, rather than contrasting yellow, as in the males. In both sexes the fore wings are usually tinted brown, usually with a yellow stripe along the leading edge. There is often black on the paranota as well as on the sides of the head.
In some females the olive-green shades to greyish brown, and there is an uncommon purple form.
Similar species
This species is quite unlike any other British species. Its large size and colour pattern on the hind femora are unique.
Life cycle
The adults emerge rather late in the season, usually from the last week in July onwards. They continue through August and September, into October. The eggs are laid in batches of up to 14 at the base of grass tufts, enclosed in an elongated pod. They enter a resting-stage, and do not hatch until May or early June the following year. The nymphs pass through five instars before emerging as adults later in the summer.
Habitat
Earlier in the last century, this species inhabited a wider range of wet habitats than is the case now in Britain, including rough, wet meadows, marshes and moorland, as well as wet heaths. Further south, in Europe, it still does. According to Lucas (1920) it was found among rank grasses by the river Bure in Norfolk in 1892, and it formerly occurred in the Norfolk broads, the fens of Cambridgeshire, and the lower Thames marshes. In the west of Ireland it is still found by rivers and lakes, in Normandy it is found in ‘fairly dry’ riparian grassland (Sutton, 2007b), and in the Swiss Alps it occurs on rocky banks of mountain streams (pers. obs.).
Its current strongholds in England are acidic quaking bogs, often associated with purple moor-grass (Molinia caerulea), bog myrtle (Myrica gale), cross-leaved heath (Erica tetralix), Sphagnum mosses, broad-leaved cotton grass (Eriophorum latifolium) and white-beaked sedge (Rhynchospora alba). It is usually found in the wettest parts of such habitats, which it shares with few other orthopterans, although it is often found in the same habitat as the bog bush-cricket (Metrioptera brachyptera), especially in the New Forest and Dorset heaths.
Behaviour
For such a large and spectacular insect, the large marsh grasshopper is surprisingly difficult to spot. On warm days the males produce their unique ‘ticking’ stridulation by flicking one hind leg (sometimes both, but out of synchronisation) against a wing tip. The sound is repeated in bursts of four to ten at a rate of two to three per second. Usually the male is static while stridulating, but continues to move through the vegetation before delivering its next burst. However, sometimes a male delivers several bursts from the same perch, in alternation with a nearby competitor (see DVD). The ‘song’ is frequently produced from a perch as much as 20 or 30 cm above the ground, from the stem of a rush or small shrub. The male is very alert while stridulating, and any sudden movement from the observer sends it running backwards and down into the undergrowth. More active disturbance, especially in warm weather, results in the grasshopper taking to the wing, in a swift, direct flight path, often of 10 metres or more. Lucas’s (1920) description of its flight and associated escape behaviour could hardly be bettered:

FIG F. Male on song perch.
One that flew near me had its long legs stretched out behind it, like those of a heron on the wing. When stalked it sometimes rises once or twice, but if thoroughly disturbed hides amongst the rank bog vegetation, with which its colours so harmonise that it is seldom again found… (Lucas, 1920: 228)
The females are usually less active, and are frequently seen lower down among low-growing plants such as Sphagnum moss. Both sexes are reluctant to fly in cool weather, and rely on their camouflage and ability to disappear into deep cover if alarmed.
They feed on blades and stems of grasses, rushes and sedges, sometimes biting through stems of rushes to access the more nutritious seed heads.
Enemies
Lucas (1920) reports having seen a male of this species being carried off by the hornet robber-fly, Asilus crabroniformis. Its cryptic coloration and the alertness of the males while stridulating suggest adaptation to visual or auditory predation – presumably from birds.
Distribution
This species is widespread (but not common, owing to its distinctive habitat requirements) throughout Europe north of the Alps, and northwards in Scandinavia to approximately 68 degrees latitude. It occurs in wet grassland and seepages in the Alps up to 2,400 metres. It is localised in northern Spain and north Italy. In the east, its range extends through eastern Europe and the former Soviet Union to Siberia.
This species has not been recorded in England north of a line from the Bristol Channel to the Wash, and even within its range it has always been restricted by its habitat requirements. It has not been recorded from Scotland or Wales, but is present in the west and south of Ireland.
It was regarded by Burr (1936) as extinct in the Cambridgeshire fens as a result of drainage, and has also been lost from its Norfolk and Thames-side localities (Marshall, 1974).
A population discovered in the Somerset Levels in 1942 was adversely affected by peat cutting and agricultural intensification. Since the 1980s there were records only from Shapwick Heath (1989) and Westhay Moor (1995), until a single specimen was reported in 2006. However, subsequent searches of the locality have proved unsuccessful (Sutton, 2007d).
It was deliberately introduced to Thursley Common, Surrey, in 1967 (Marshall & Haes, 1988) but appears to have become extinct both there, and also in what may have been a natural habitat in Surrey, where it was found in 1982. Subsequent searches of this locality have not been successful (Baldock, 1999; Sutton, 2003c).
Currently, the species appears to be confined to sphagnum-dominated mires in heathland in east Dorset and the New Forest, Hampshire. However, where suitable habitat exists in these areas, it maintains substantial populations.
Status and conservation
Haes (in Shirt 1987) rated it ‘vulnerable’, and considered the main threats to be drainage and shading of its habitat by afforestation.
As the species survives in a wider range of habitats in mainland Europe, it might be speculated that, with predicted warming of the UK climate, it might adopt a wider range here, and still be retained even if its current habitats in Dorset and Hampshire become too dry for it. Sutton (2007b) is sceptical about this possibility, however, as the Norfolk, Cambridgeshire, Surrey and Somerset populations were lost as habitat dried out.
However, reported extensions of its range in the Netherlands give rise to some cautious optimism (Sutton, 2008b), and the projected restoration of former East Anglian fens might allow for its reintroduction to that part of its range.
In the west of Ireland, it is threatened by peat extraction.
References: Baldock, 1999; Burr, 1936; Harz, 1975; Kleukers & Krekels, 2004; Lucas, 1920; Marshall, 1974; Marshall & Haes, 1988; Ragge, 1965; Shirt, 1987; Sutton, 2003c, 2007b, 2007d, 2008b.
THE STRIPE-WINGED GRASSHOPPER
Stenobothrus lineatus (Panzer, 1796)
MEASUREMENTS: Male: 15–19 mm in length; female: 17–22mm in length.
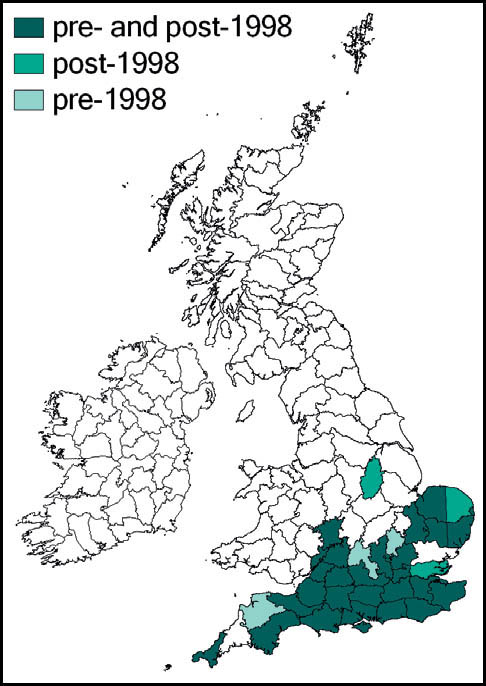

FIG A–D. Male; male dorsal view; face; female.
Description
This handsome, medium-sized grasshopper is best identified by the unique pattern formed by the veins in the fore wings. The median area of each wing is enlarged, and the cross veins form an almost parallel series, giving a ladder-like impression. Both pairs of wings are fully developed; they reach to just beyond the tip of the abdomen in males, but are often significantly shorter in females. There is no bulge on the leading edge of the fore wing. The side keels of the pronotum are moderately inflexed or incurved, and diverge toward the rear edge of the pronotum. The males have from 300 to 450 minute stridulatory pegs on the inner surface of each hind femur, and a rather elongated subgenital plate that tapers to a point. In the female, the ovipositor valves (which usually protrude slightly from the rear end of the abdomen) have a small tooth (see the Key, Fig K21b).
There is considerable variation in colour pattern, but what is probably the most common form is green on the head, pronotum and rear edges of the wings (forming a tapering green dorsal ‘roof’ over the abdomen when the insect is at rest). The green shades paler down the sides of the pronotum and head, and may be considerably paler on the face, with palps sometimes white. The eyes vary from fawn to darker brown. The side keels of the pronotum are outlined in whitish to cream or pink, edged with black externally in the anterior part of the pronotum, and internally on the posterior part. The pale lines are continued forward onto the head and eventually meet at the front of the head. The wings often appear dark brown to black, but are paler in freshly emerged specimens. There is usually a white stripe close to the leading edge of the fore wing, and a white spot, or ‘stigma’ towards the outer tip. However, one or both of these may be absent. The upper (anterior) surface of the hind femora is commonly green, but may be fawn, brown, pinkish, or even purple.
A minority colour form has the green on the top of the head and pronotum and the rear margins of the fore wings replaced by brown or grey-brown, with a pale median longitudinal stripe on the top of the head and pronotum. In this form, the sides of the head and pronotum are green, but the anterior edge of the hind femora is generally brown. There is also a rare form in which the head, pronotum and hind legs are pink (B. Pinchen, pers. corr.)
In newly emerged individuals, the abdomen is pale grey, with dark brown patches on the sides of most segments. However, with sexual maturity, the hind three to four segments and genital plate become bright red in the male. The colour often extends further up the abdomen, and also suffuses the hind tibiae and posterior edge of the hind femora. Similar, but less spectacular colour changes also occur in the females, with orange coloration on several of the abdominal segments.

FIGS E–F. Two-colour forms.
Similar species
The wing venation is the best diagnostic feature to use, but this is not always obvious in the field. The male ‘parsons nose’ subgenital plate, and the toothed ovipositor valves in the female are also reliable characters, but still less easy to use in the field.
The most closely similar species, and one that often occurs together with the striped-winged, is the common green grasshopper (Omocestus viridulus). Many colour forms of this have brown or grey paranota, but in the stripe-winged grasshopper, these are always green, even in the ‘brown’ form. However, the fully green form of the common green grasshopper can look very similar indeed to the stripe-winged. Useful clues are:
These indications are not entirely reliable, however, and for full confirmation anatomical characters should be used. In addition to the wing venation and shape of the male subgenital plate, these include the shape of the valves of the ovipositor in females: elongated and untoothed in the common green grasshopper.
Other species that could be confused with the stripe-winged grasshopper include the following:
Life cycle
The eggs are laid during the summer above the surface of the soil, among the bases of tufts of grasses. The pods contain up to eight eggs, and are oval in shape, with plant fragments attached to the outer coating. The eggs remain dormant through the winter, and hatch in April or May the following year. The resulting nymphs pass through four instars prior to reaching adulthood by June or July. In favourable seasons and localities they may reach adulthood as early as the first week in June, becoming fully sexually mature a little later. The adults continue to be active through July, August and September, with a few surviving until October.

FIG G. Final instar nymph.
Habitat
The stripe-winged grasshopper favours rough, uncultivated and well-drained grasslands and heaths. It is often found, along with the rufous grasshopper (Gomphocerippus rufus), on south-facing, rabbit-grazed downland slopes. Elsewhere it is also found on the drier parts of heather heaths and on sandy, acid soils, as on the Suffolk heaths, and the Brecks of East Anglia. In the latter habitat it favours areas with bare ground and sparse vegetation, consisting of mosses, lichens and fine grasses, adjacent to areas of longer grass and scrub and often in association with rabbit activity.
Behaviour
Both sexes are well camouflaged in their habitat, the females, especially, being rather inactive and staying close to the ground. Both males and females retreat into deeper cover when disturbed, but also can fly effectively. If pursued, they frequently fly towards their tormenter, a very effective surprise tactic. The males are more active than the females, often wandering about and pausing every few seconds to produce a burst of stridulation. Because of the densely packed stridulatory pegs on the hind femora, the sound can be produced by a rather slow movement of the legs against the wings. Usually both legs are moved, usually out of synchronisation, and the resulting sound is a very distinctive ‘wheezing’ that lasts for some 10 to 20 seconds. Sometimes only one leg is used, and individuals that have lost a hind leg can still stridulate. The song is rather quiet and unobtrusive, and one frequently sees the slowly moving hind legs of a male before hearing the song. In the presence of a female, the character of the sound changes to a ‘courtship’ song.
In a prolonged film sequence of the courtship of this species, the male faced the female, a few centimetres away, and produced a quiet stridulation by rapid, low amplitude vibrations of the hind femora against the wings. This was continued for many minutes until the female moved off. She was soon re-found by the male who then continued with his low-amplitude stridulation (see DVD for clips of this). However, Ragge (1965) describes a more elaborate courtship pattern, consisting of an alternation between an extended version of the normal song, and a more subdued series of ‘ticks’ produced by more rapid leg movements. This continues for some time, culminating in a series of louder sounds produced by quick movements of the hind legs, followed by the male attempting to mate with the female. Ragge notes that the females, too, sometimes stridulate, but it is unclear what part this plays in the mating system of the species.
Distribution
The stripe-winged grasshopper is distributed throughout much of Europe, from Spain in the west, through to Siberia and Northern Mongolia in the east. Further south in Europe it is limited to moderate altitudes in mountains, for example, from 1,400 to 2,500 metres in the Alps.
In Britain, it is mainly confined to southern counties and East Anglia, with very few records from Cornwall and Devon. It occurs on the chalk downland of Dorset and the south of the Isle of Wight, and is common and widespread on south-facing slopes of the North and South Downs. Further north, it occurs, but is more localised, on the Mendips, the Cotswolds and the Chiltern hills, and (rarely) as far north as Nottinghamshire. It also occurs on heaths in Hampshire, Surrey and West Sussex, as well as in Suffolk and Norfolk. In the Brecks it is widespread, but thinly distributed on heather heath and dry grassland along rides and clearings in pine forest.
There are some indications that it is spreading into new localities, and possibly also expanding its range (Sutton, 2003c). Richmond reports new records that indicate that the species is spreading from its strongholds in the Brecks (Sutton, 2007d, 2008b, 2011), and it was discovered at a site in Epping Forest (the first Essex record) in 2010 (Wilde, 2009; Sutton, 2010a).
Status and conservation
It seems probable that a combination of agricultural intensification, afforestation and the decline of grazing by rabbits and livestock has rendered this species much more localised than in the past. However, as many of the remaining localities are managed by organisations with a conservation brief, it seems unlikely that this species is seriously endangered. In the Brecks, the open rides and large areas of clear fell associated with current management by the Forestry Commission appear to be very favourable to this species. The recent indications of range expansion could be a response to climate change, and close monitoring of suitable habitat away from its current range would be of interest.
References: Harz, 1975; Kleukers & Krekels, 2004; Lucas, 1920; Marshall & Haes, 1988; Ragge, 1965; Sutton, 2003c, 2007d, 2008b, 2010a, 2011; Wilde, 2009.
THE LESSER MOTTLED GRASSHOPPER
Stenobothrus stigmaticus (Rambur, 1839)
MEASUREMENTS: Male: 10 to 12 mm in length; Female: 12 to 15 mm in length.

FIGS A–D. Male; male dorsal view; female; female dorsal view.
Description
The lesser mottled grasshopper is very small, with gently incurved side-keels to the pronotum. Both pairs of wings are fully developed, but are usually quite short. In the male they reach back almost to the tip of the abdomen, but in females they are relatively shorter – often leaving two or three abdominal segments exposed. The wings are also narrow, without the expanded median zone characteristic of the stripe-winged grasshopper. The male antennae are slightly thickened towards the tip. The ovipositor valves are toothed.
In the most frequent colour pattern, the head, pronotum, side plates of the thorax to the rear of the paranota, and the anterior edges of the hind femora are all bright green. The side keels of the pronotum are outlined in cream to pale brown, with black edging externally on the anterior section of the pronotum, and, somewhat widened as wedges, internally on the rear section of the pronotum.
The pale lines are carried forward onto the head and join at the front of the head. The ground colour of the abdomen in freshly emerged specimens is grey. The fore and mid legs, as well as the outer surfaces of the hind femora, are also grey. The fore wings are fawn to cream in colour, often with a white stripe close to the leading edge, and one or two white stigmata in the outer half of each fore wing. There are often dark brown or black spots along the middle area of the fore wing, and black patches at the sides of the first few abdominal segments. There is a pale brownish rectangular patch at the front of the paranota, and the lower part of the face is creamy white to pale brown.
As the adults become sexually mature, they develop a red tint to the posterior segments of the abdomen, which sometimes extends to almost the whole of the abdomen as well as the legs and wings. This is particularly pronounced in the males.
Two minority colour forms have been noticed. In one of these, the green on the dorsal surface of the head, pronotum and edges of the femora is replaced by pale brown. In another form, all the green is replaced by brown. When this form is suffused with red, the effect is very striking.
Similar species
In general appearance this species is quite similar to its close relative, the stripe-winged grasshopper, although this does not occur in the Isle of Man. The lesser mottled grasshopper lacks the expanded central area to the fore wings, and the associated pattern in the wing venation, that characterise its relative. The subgenital plate in the male is less pronounced in the lesser mottled, and the grey or brownish patch at the front of the paranota in the lesser mottled grasshopper is distinctive, but not evident in all colour forms. The green form of the lesser mottled has fawn-brown rear edges to the fore wings (arched over the back at rest), while the green form of the stripe-winged always has green in this area.
Because of its small size, the lesser mottled grasshopper could be confused with the mottled grasshopper (M. maculatus), which does occur on the Isle of Man. However, that species has much more acutely inflexed side keels to the pronotum, and a noticeably larger head relative to the pronotum. The male antennae of the mottled grasshopper are clubbed, whereas those of the lesser mottled are only gradually thickened towards the tip.
The common green grasshopper (O. viridulus) is also quite similar, but lacks the grey or brownish patches on the paranota, has relatively longer wings, does not develop reddish tints with sexual maturity, and is considerably larger. Although O. viridulus does occur on the Isle of Man it has not been recorded in the habitats occupied by the lesser mottled.
The common field grasshopper (C. brunneus) does have an uncommon colour form that resembles the brown form of the lesser mottled grasshopper, but the field grasshopper has more strongly inflexed side keels and relatively longer wings, and is generally a much larger insect.
Life cycle
The eggs are laid during the summer, and enter a dormant stage over winter. Embryonic development has a high temperature threshold and so the eggs hatch late in the spring. Subsequent development is favoured by warm and sunny conditions that enable rapid ‘catch-up’ nymphal development (van Wingerden et al., 1991; Cherrill & Selman, 2007). There are usually four nymphal instars, but (as in C. brunneus) females sometimes develop through five instars. The adults are present from late July until September.
Habitat
On the European mainland, the subspecies (ssp. faberi) to which the British population is assigned is reported to occur on Calluna heaths, dry meadows and steppe grassland, as well as inland dunes (Harz, 1975) and sheep pasture (Bellmann, 1988). In mainland Europe there is no special association with the coast. On the Isle of Man it exists in discrete colonies on patches of grass and heathers (Calluna vulgaris) and bell-heather (Erica cinerea), in the vicinity of rocky coastal outcrops. It occurs among shorter grasses in company with the mottled grasshopper (M. maculatus), but will tolerate tussocky grassland, where it occurs together with the common field grasshopper (C. brunneus). However, it does not occur in areas of taller grasses, or where heathers and other shrubs have more than 50 per cent ground cover (Cherrill, 1994; Cherrill & Selman, 2007). The habitats favoured by this species have in common that they are warm and dry, with low-growing vegetation on nutrient-poor soils. In most localities moderate grazing by livestock, rabbits or deer seems to be required. Small populations were found close to the rough on the golf course that occupies the landward part of the Langness peninsula, on the Isle of Man (Cherrill & Selman, 2007; see also Chapter 9 for more detail.)
Behaviour
The Manx population seems to live in discrete colonies, with numerous males sharing favoured spots (e.g. a platform of flattened grass stems, or the flat top of a bank). In cool weather they remain inactive, but with an increase in temperature the males run actively through and over vegetation in their ‘patch’. This seems to be a mate-location strategy, and when a female is encountered, males begin a courtship display. Ragge (1965) gives a detailed description of this. The process begins with a burst of soft stridulation lasting from four to six seconds in which one hind leg is moved more vigorously than the other. That leg is then moved quickly downwards to make a louder sound. After a pause of three to seven seconds there is a brief period of noiseless movement of the hind legs, followed by another burst of stridulation, which is like the first, only with the other leg moved more vigorously. The whole cycle is repeated several times, usually culminating with a mating attempt on the part of the male.
Most usually, the males are rejected, the female either jumping away, or moving off more slowly, in which case she is usually followed by the male, who then resumes courtship.
Frequently, two or more males compete with one another in courting a female, in which case antagonistic signals between the males disrupt the courtship routine described by Ragge, and brief bursts of stridulation in this context appear to have more to do with male-to-male communication than courtship (see DVD).
The calling song of the male is a rather subdued ‘chirp’ lasting from two to four seconds, starting soft and becoming louder. It is repeated at irregular intervals, but only in warm, sunny weather.
Distribution
Internationally, three subspecies are recognised, but the subspecies faberi, which includes the Isle of Man population, is widespread in central and western Europe, from lower altitudes in the Alps northwards, with north-west Germany marking the northern limit of its range on the European mainland. Eastwards, it occurs through eastern Europe to the western parts of the former Soviet Union.
In Britain it is known only from the southern tip (the Langness peninsula) of the Isle of Man, where it was first recognised in 1962 (Ragge, 1963). This is its northernmost outpost.
Status and conservation
The species is reputedly in decline in mainland Europe, where it is threatened by cessation of grazing on traditional pastures, with subsequent loss of its short-grass habitat through natural succession, and by agricultural intensification and ‘improvement’ of grassland (Bellmann & Luquet, 2009; van Wingerden & Dimmers, 1993).
Given the unique British distribution of this species, there has been some speculation that it might have been an accidental introduction. However, there is no obvious route by which this might have occurred. Also, Manx specimens are smaller than the ones from the continental mainland (the latter given as 11–13 mm (males) and 14–18 mm (females) in length), and this suggests that they may have been isolated for a long time. For both reasons, Ragge (1965) and Burton (1963) tentatively concluded that S. stigmaticus is probably a native species.
A species whose British distribution is as highly localised as that of the lesser mottled grasshopper must be considered vulnerable, despite its inclusion in the Isle of Man Wildlife Act of 1990, Section 5, and the inclusion of its habitat in a designated ASSI. An application to extend the existing golf course into the part of the peninsula occupied by the grasshopper was rejected at a public enquiry in 1990. However, the habitats continue to be privately owned, and there remains a question about appropriate management for the species. Grazing by sheep and cattle ceased around 1985, and it seems that parts of the habitat became unsuitable for S. stigmaticus as a result of the development of a tall, cool grass sward and encroachment by scrub (Cherrill & Selman, 2007). Resumption of grazing in one part of the peninsula in 2003 had resulted in recolonisation by the time the site was surveyed in 2006. Although rabbit grazing on habitat close to the rocky shore line may be adequate to maintain the habitat, the security of the species in its only known British locality will depend on appropriate management over the whole of the site. In the longer term, it is possible that some parts of the habitat will be threatened by sea-level rise.
References: Bellmann, 1988; Bellmann & Luquet, 2009; Burton, 1963; Cherrill, 1994; Cherrill & Selman, 2007; Evans & Edmondson, 2007; Harz, 1975; Marshall, 1974; Marshall & Haes, 1988; Ragge, 1963, 1965; van Wingerden et al., 1991; van Wingerden & Dimmers, 1993.
THE WOODLAND GRASSHOPPER
Omocestus rufipes (Zetterstedt, 1821)
MEASUREMENTS: Male: 13 to 17.5 mm in length; female: 18 to 22 mm in length.

FIGS A–G. Male; female dorsal; female; male face; female face; male; dark male dorsal.
Description
This is a medium-sized grasshopper, with both pairs of wings fully developed, reaching back to or beyond the tip of the abdomen in both sexes. There is no bulge on the leading edge of the fore wings. The transverse groove (sulcus) on the pronotum is anterior to the midpoint, and the side keels are strongly incurved. The ovipositor valves are short, and without teeth. The male has 90 to 130 stridulatory pegs on each hind femur.
In both sexes the side keels of the pronotum are finely outlined in white, but this may be obscured in very dark males (Fig G). When fully mature, males may be mainly black, with brown on the dorsal surface of the head, pronotum, rear-margins of the fore wings (folded over the abdomen when at rest), and the distal part of the hind femora. In these specimens, the final segments of the abdomen, the tibiae and sometimes part of the femora are bright red. The black of the face contrasts sharply with the chalk white of the palps.
The females are more variable in colour, but have two main colour forms: green and brown. In the green form, the top of the head, the pronotum and the rear edge of the fore wings are a rather dull green, sometimes shading paler medially. The white outlining of the side keels of the pronotum is clear, and bordered on the inner edge by black wedge-shaped marks on the rear section of the pronotum. The sides of the head, the pronotum and the hind femora vary from greyish through to chestnut brown. The palps, as in the male, are white, although they contrast less strongly with the face. The abdomen is greyish, with an orange-red flush on the hind segments in mature females, and black patches on the sides of the anterior segments. There are dark blackish blotches along the middle of the fore wings, usually with a white stigma towards the tip.
In the ‘brown’ form of the females, greyish or olive brown replaces the green dorsally, and the black wedge-shaped marks on the dorsal surface of the pronotum are larger, and continue to the rear margin of the pronotum.

FIGS E–F. Female with grey sides; brown female.
Similar species
The fully mature male is unmistakable, given its extensively black and red coloration, and the white palps are decisive in identification.
Either form of the female could be confused with one or other colour form of the common field grasshopper (Chorthippus brunneus). That species has more sharply inflexed side keels to the pronotum, and a small bulge on the leading edge of the fore wing which is absent in the woodland grasshopper.
The green form of the female could be confused with the following.
The ‘brown’ form of the female can be confused with the heath grasshopper (Chorthippus vagans). This rare species is sometimes found close to woodland grasshopper habitat on heathland, and is very similar in appearance to the brown form of the female of the woodland grasshopper. A useful (but not entirely reliable) field character is that the anterior (upper, when at rest) edges of the hind femora of the heath grasshopper are usually pale brown and quite distinctly marked with dark brown blotches. The hind femora of the brown form of the woodland grasshopper are usually more uniformly coloured or mottled. More reliably, the transverse sulcus on the dorsal surface of the pronotum in the heath grasshopper cuts across the pronotum at the midpoint, or slightly posterior to it (so that the front section of the pronotum is equal to or slightly longer than the rear section), whereas in the common green grasshopper, the sulcus is further forward. Finally, the fore wings of the heath grasshopper have a small bulge on the leading edge, which is absent in the woodland grasshopper.
Life cycle
The eggs are laid from June to September in batches of five or six, and enclosed in a pod with a convex lid. The pods are deposited just below the surface of the soil, and the outer wall includes soil particles. The eggs hatch in April or May, depending on weather conditions, and the resulting nymphs pass through four instars to reach adulthood in June or early July. As the adults emerge early in this species, they are among the first to die off in late summer and early autumn. In southern France and Switzerland the species completes two full life cycles in the year, with adults present from April to June, and again from August to November (Bellmann & Luquet, 2009).
Habitat
In southern England the woodland grasshopper – as its name suggests – is to be found in open woodland, woodland edges, rides and clearings. For the first few years after clear felling or coppicing, numbers can increase very quickly. However, they decline again with woodland succession, and the species often seems to disappear altogether from a site. It appears to favour ancient deciduous woodland, but can also be found in conifer plantations.
An alternative habitat is moist or dry heathland, where it occurs in grassy hollows, often with heathers, but usually close to patches of gorse or other scrub, or stands of trees. This is characteristic of its habitat in the New Forest, Hampshire. In Cornwall it occurs on heathy areas and sea cliffs, away from woodland (Haes & Harding, 1997).
Further south in Europe, it is reported to live in a much wider range of habitats, including dry pine forests, dunes, and rocky slopes (Kleukers & Krekels, 2004; Bellmann & Luquet, 2009).
Behaviour
The calling song of the male is usually delivered from a perch above the ground – on low scrub or a tall grass stem. The hind legs move rapidly over the fore wings, almost synchronously (although in anticipation of a burst of song the male usually raises one leg). The stridulation begins noiselessly and slowly builds in intensity (although never becoming loud!) and stops suddenly. It may last from five to twenty seconds, and consists of a continuously repeated rapid series of ‘ticks’. The resulting sound resembles that of a stick being drawn very rapidly across a set of railings (see DVD).
The courtship song consists of repetitions of the calling song, followed by a modified and quieter version of it ‘in which the hind legs are vibrated in a rather ragged manner’. One or more quick downstrokes of the hind femora then follow and lead to the male’s mating attempt (Ragge, 1965, 1986).
Virgin females apparently also stridulate, but it is not clear what role this has in their mating system.
Both sexes are inactive in overcast weather, and spend much of their time basking during sunny spells.
Distribution
The woodland grasshopper is widely distributed through Europe, especially in the south. It occurs in Spain and Portugal, through France and central Europe to Rumania and Bulgaria, and on to Turkey, Kazakhstan and southern Siberia. Its European distribution extends northwards into Norway and Finland.
In Britain it is confined to the southern counties, with some coastal records further north, in East Anglia. It has a scattered presence in coastal districts of Cornwall and in south Devon, one known locality in Somerset, and numerous sites in Dorset. However, its strongholds are in Hampshire (especially the New Forest) and in wooded areas of Surrey and Sussex, including the North and South Downs. It is common in parts of east Kent, but there is only one past record for Essex (from J. H. Flint, 1974, cited in Wake, 1997). However, after considerable unsuccessful searching of the area, Wake concluded that the record must be considered doubtful. Subsequent searches of coastal heathland in Suffolk, where the species were recorded in the 1990s by Mike Edwards (pers. corr.) proved unsuccessful, until the species was rediscovered by T. Gardiner in 2011 (pers. corr.). However, the tendency of this species to undergo wild fluctuations in population in response to heath and woodland management could well explain the failure to re-find it in these localities.
North of the river Thames, the woodland grasshopper is either absent or very localised. However, there are recent reports from north Gloucestershire – the northernmost known localities of the species in England – that indicate range expansion (Sutton, 2003c, 2010b).
Status and conservation
This species is abundant in its favoured localities, but is vulnerable to shading out of its woodland habitats. It may have benefited from modern forestry methods in the Weald of Kent and in east Kent (Marshall & Haes, 1988). As it is at the northern limit of its European range in southern England, its distribution is very localised in the northern fringes of its distribution in this country. However, with climate change it is possible that the reports of its range expansion in Gloucestershire herald a more general northerly spread. Suitable habitats at and beyond its range boundary should be carefully monitored.
References: Bellmann & Luquet, 2009; Haes & Harding, 1997; Harz, 1975; Kleukers & Krekels, 2004; Marshall & Haes, 1988; Ragge, 1965, 1986; Sutton, 2003c, 2010b; Wake, 1997.
THE COMMON GREEN GRASSHOPPER
Omocestus viridulus (Linnaeus, 1758)
MEASUREMENTS: Male 15 to 19mm in length; female 17 to 22 mm in length.

FIGS A–D. Green male; green female; brown male dorsal view; greysided female.
Description
This medium-sized grasshopper has fully developed wings, reaching back almost to the tip of the abdomen in females, and usually to a few millimetres beyond in males. There is no bulge on the leading edge of the fore wings. The side keels of the pronotum are strongly incurved, and the transverse sulcus is anterior to the midpoint of the dorsal surface of the pronotum. The ovipositor valves in the female are elongated and untoothed. The male has from 100 to 140 stridulatory pegs on each femur.
There is considerable variation in colour patterns, the males having ‘green’ or ‘brown’ forms.
The green form has a green head, pronotum (including paranota), hind femora, and rear edge of the fore wings (above when at rest). The side keels of the pronotum have a whitish outline, with black edging externally on the front section, and narrow wedge-shaped black markings internally on the rear section. The lower part of the face, and the antennae, palps and abdomen may be green, grey or brownish. The eyes are brown, and there are dark brown to black markings on the abdominal segments. The main central area of the wings is brown, shading to black towards the tip, often with a white stigma.
The ‘brown’ form of the male is more variable, possibly as a result of colour changes occurring with age. This form has brown replacing the green on the dorsal surface of the head and pronotum, and the rear edge of the fore wings. However, the side of the head and face, the paranota and the hind femora vary in colour from olive-green through grey to pale brown, often with black mottling. The sides of the abdomen often have larger areas of black on each segment in this form, and the black wedge-shaped markings on the dorsal surface of the pronotum are wider than in the green form, giving a rather striped appearance when viewed from above.
The females are green on the dorsal surface of the head, the pronotum and the rear edge of the fore wings (sometimes the green on the fore wings is more extensive, reaching the leading edge of the wing basally). There is sometimes a pale median line along the dorsal surface of the head and pronotum. The side keels of the pronotum are outlined in whitish, and they are edged with black, as in the male, but the black edging is less extensive, especially on the rear section of the pronotum. There is often a pale whitish or fawn median line along the head and the dorsal surface of the pronotum. The sides and front of the head and the paranota may be of the same green as the dorsal surfaces, or may be greyish or fawn-brown, sometimes with darker mottling. The ground colour of the abdomen may be greyish or very pale green, with black lateral markings on the first four or five segments. The fore wings often have a white stripe close to the leading edge, and a faintly marked white stigma.

FIGS E–F. Green female with brown side; brown male.
There is also a rare form with purple coloration on the sides of the head, on the paranota, and sometimes on the legs and the basal areas of the fore wings.
Similar species
Life cycle
The egg pods are deposited during the summer at the bases of tufts of grass. They are broadly elliptical and contain up to ten eggs. These hatch the following spring, in April or early May in most years, and the resulting nymphs pass through four instars, emerging as adults from the end of May onwards. In the south the stridulation of the male can usually be heard from the first week in June onwards (as early as 19 May in the exceptionally warm spring of 2011 (pers. obs.)), later in the north and at higher altitudes. They are at their most abundant from mid-June through to early September, adults becoming scarcer into early October.
Habitat
This species is said to favour moist, grassy sites, including ditch banks, stream valleys, and damp meadows. Its habitats include parkland, open areas and ride edges in woodland, and, at higher altitudes, unimproved pastures and moorland up to 1,000 metres in Britain. It is regarded as an indicator species of unimproved pasture (Marshall & Haes, 1988). It prefers relatively tall, cool and moist grasslands, frequently with purple moor grass (Molinea caerulea) or Yorkshire fog (Holcus lanatus). It is regarded as physiologically adapted to cooler climates as it is relatively effective in raising its temperature above the ambient, and poor at cooling when ambient temperatures are high (Willott, 1997).
However, it also occurs in drier but tall grassland, and in woodland rides on well-drained soils in the East Anglian Brecks, as well as on heather heaths.
Behaviour
On sunny days the males stridulate from perches 50 cm or more above the ground on a tall grass stem or sprig of heather or gorse. The song is produced in quite extended bursts of 10 to 30 seconds or more. The movement of the hind legs against the wing veins is fast, and the whole insect may rock with the exertion involved (see DVD). The sound produced is a continuous series of rapidly repeated ‘ticks’, that sound very much like the song of the woodland grasshopper, only louder. The burst of song begins inaudibly and gradually gains in volume until it reaches a maximum about halfway through. Usually the insect moves to another perch after each burst of song, but it sometimes produces one or more subsequent bursts after a pause of 45 seconds or more. In open grassland, numerous males may be singing at the same time, spaced out evenly across the habitat. It is unclear whether this is chorusing behaviour or simply an incidental consequence of adjacent males alternating their songs with one another (see Chapter 5).
In the presence of a female, the male faces her and commences a prolonged and repetitive courtship performance. This consists of one or more bursts of a sharp clicking sound produced by striking the tip of the fore wings with the extended hind tibiae (a similar action to the stridulation of the large marsh grasshopper (S. grossum). Each burst of ‘kick stridulation’ consists of four to twelve kicks, and is followed directly by a prolonged but much quieter version of the normal stridulation. This is produced by low amplitude vibrations of the hind femora against the fore wings, with one hind leg working harder than its partner (either hind leg may play this role in successive repetitions). This phase of the courtship may last from 20 seconds to more than 90 seconds, after which the male remains motionless (except for twitching movements of the abdomen) for a few seconds before embarking on a new series of kick stridulations which, again, terminate in a prolonged low-intensity phase of stridulation. After many repetitions the male may move forwards, kick stridulating as he does so, to attempt mating (see clips on DVD).
According to Ragge (1965) a receptive female may respond by producing a short burst of quieter stridulation.
When stridulating, the males are very alert. Their favoured response to mild disturbance is to run backwards down a grass stem into the cover of deep vegetation. More vigorous disturbance will result in their taking to the wing, in low flights of 10 metres or more, after which they are usually extremely difficult to relocate.
Distribution
This species is widespread throughout almost all of Europe, including Scandinavia, to approximately 68 degrees north, from northern Spain in the west, and through central and eastern Europe to Siberia and Mongolia in the east. In southern Europe it is found at higher altitudes, up to 2,500 metres or more in the Alps, and as far south as central Italy.
It is present throughout almost the whole of the British Isles wherever its habitat persists. In the north it is present on the Hebrides, and the Orkneys (Sutton, 2003a), although it is absent from parts of mainland Scotland, including the northwest Highlands. It is widespread through Wales and reported to be the most widespread grasshopper in Ireland. In England it is particularly common in the northern and western uplands, the North and South Downs, Chilterns, Cotswolds and the downs of Wiltshire and Dorset. It is absent from parts of East Anglia and the south-east of England, such as parts of north Kent, East Essex and Suffolk.
Status and conservation
Although common and widespread in most parts of Britain, it has acquired conservation significance in those areas where it is scarce. Sutton (2003b and 2003c) reports its decline in parts of mainland Europe, the New Forest and also in Somerset and Devon and elsewhere in southern England. It is plausible that these declines in the southern parts of its range might be explained by warmer and drier conditions associated with climate change. Decline in the New Forest may have also occurred as part of an overall decline in orthopterans in the forest after fencing and gridding in the early 1960s led to overgrazing (Tubbs, 1986; Pinchen, pers. corr.). Significantly, it was reported to be thriving in Bushy Park, London (a humid habitat) and on Salisbury Plain (cool and damp).
In Essex its strongholds are in the west of the county, in Hatfield and Epping Forests. Elsewhere it is very local on remnants of old pasture, heaths and commons. It has not been recorded in the east of the county, which has a warmer, drier climate and predominantly sandy, well-drained soils. A decline of 50 per cent in its remaining sites in the county led to its inclusion in the county’s Biodiversity Action Plan (Gardiner & Harvey, 2004; Thompson & Maclean, 1999). Gardiner & Gardiner (2009) provide an instructive account of its local extinction at a small area of relict heathland common in the ancient Writtle Forest, in mid-Essex. Formerly the species was ‘widespread but local’ on the common (Wake, 1997), but surveys from 2000 onwards have failed to encounter it. The lack of management of the site since the 1950s has led to encroachment by bracken, gorse scrub, and birch and oak woodland. These now occupy some 80 per cent of the site.
In some upland habitats (the Malvern Hills and the Cotswolds) a combination of lack of grazing at lower altitudes with intensive sheep grazing on hilltops limits the species to a narrow band of habitat between the two (Gardiner, 2011a).
Current evidence suggests that in most habitats some grazing is required to prevent shading out of the habitat, but that if this is so intense as to produce a short sward this, too, eliminates the grasshopper. Gardiner (2010b) notes that in Epping Forest low-intensity grazing (1–2 cows per 10 hectares) by English Longhorn cattle seems to be beneficial.
References: Gardiner, 2010b, 2011a; Gardiner & Gardiner, 2009; Gardiner & Harvey, 2004; Harz, 1975; Kleukers & Krekels, 2004; Marshall & Haes, 1988; Ragge, 1965; Sutton, 2003b, 2003c; Thompson & Maclean, 1999; Wake, 1997; Willott, 1997.
THE COMMON FIELD GRASSHOPPER
Chorthippus brunneus (Thunberg, 1815)
MEASUREMENTS: Male 15–19 mm in length; Female 19–25 mm in length.
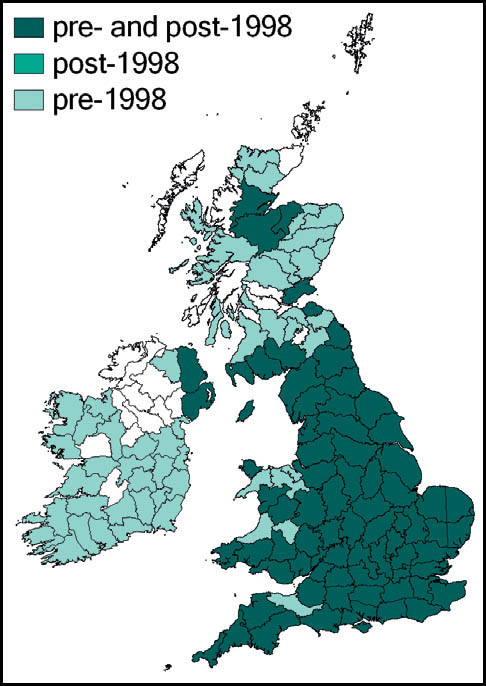

FIGS A–C. Male; dorsal view; female.
Description
This medium-sized grasshopper has strongly inflexed side keels to the pronotum, and the transverse sulcus on the pronotum is anterior to the midpoint. The wings are long, usually clearly reaching back beyond the hind knees and tip of the abdomen. Both sexes have a ‘bulge’ on the leading edge of each fore wing, located close to the opening of the hearing organ at the side of the abdomen when the wings are folded. The underside of the thorax is densely hairy. Males are generally noticeably smaller than females, with relatively longer antennae. They have 50 to 90 stridulatory pegs on each hind femur.
The colour patterns are extremely varied in both males and females. Ragge (1965) distinguishes the following 12 categories (with considerable detailed variation within each!).

FIGS D–G. Striped female; mottled male; semi-mottled female; green male.

FIGS H–N. Green brown sides female; green lilac sides female; purple female; purple brown sides female; buff female; black male; black pale sides.
The mix and proportions of these colour forms varies greatly from one local population to another. However, in general it can be said that forms 1, 2, 3, 5 and 10 are relatively common, whereas the others are less so and may not occur at all in some populations.
Sexually mature males develop bright orange-red tints on the dorsal surface of the final four or five abdominal segments, and females, too, develop orange coloration on the rear abdominal segments, although this is not as striking as in the males. The underside of the abdomen is yellow or pale greenish yellow.
Some colour forms have a white stripe along the leading edge of the fore wings, and may also have a whitish stigma.
Similar species
The most closely similar British species is the very rare heath grasshopper (Chorthippus vagans). That species is so rare that in most localities confusion with the common field can be ruled out by location alone. However, where the heath grasshopper does occur (or might yet be discovered!) it is important to be able to separate the two species. The heath grasshopper is generally smaller than the common field, and less variable in its colour patterns. The most reliable character (not one easily used in the field, but quite clearly recognisable in sharp photographs) is the position of the transverse sulcus on the dorsal surface of the pronotum. This cuts across in front of the midpoint in C. brunneus, but at or behind the midpoint in C. vagans (i.e. the rear section of the pronotum is distinctly longer than the front section in C. brunneus, equal or shorter in C. vagans). In C. vagans the wings are usually relatively shorter, rarely reaching beyond the hind knees, and the underside of the thorax is only sparsely hairy (densely so in C. brunneus). The width of the head is narrow relative to the distance from the tip of the head to hind margin of the pronotum in C. vagans, slightly wider in C. brunneus (giving C. vagans, especially in the males, a ‘slimmer’, more elongated appearance when viewed from above). A very useful field character that seems to be reliable is that the black wedge-shaped markings on the rear portion of the dorsal surface of the pronotum reach back to the rear margin of the pronotum in C. vagans, but stop short of the rear margin in C. brunneus (when present at all). C. vagans has alternating pale and dark brown areas on the upper edges of the hind femora – not a common feature in C. brunneus.
The various green forms of C. brunneus could be mistaken for green forms of stripe-winged, common green or lesser mottled grasshoppers. Often the resemblance in colour pattern can be remarkably close. However, the sharply inflexed side keels to the pronotum, the long wings and the bulge on the leading edge of the fore wings serve to distinguish it readily from these other species.
The very localised rufous grasshopper (Gomphocerippus rufus) also has similar colour forms to some of those exhibited by the common field, and also has rather similarly inflexed pronotal side keels. However, G. rufus has relatively shorter wings, and noticeably club-tipped antennae.
Life cycle
The eggs are laid in the soil, at a depth of about one centimetre, with up to 14 forming two or three rows in a large pod, equipped with a lid (see Chapter 3, Fig 61). They hatch from late April through to June the following year. Males pass through four nymphal instars before emerging as adults from the beginning of July onwards. Some females go through five nymphal stages, while others complete development in only four. Warmer conditions during early nymphal development and with abundant food increase the likelihood of females adding the extra nymphal stage. The switch between the four- or five-stage developmental pathways is the moult at the end of the second instar. In female nymphs destined to complete their development in four instars, this moult leads to the normal penultimate (third) stage of development, in which the wing stubs are inverted and point back with their leading edge along the midline of the dorsal surface of the abdomen. However, in female nymphs destined to develop through five nymphal instars, the moult gives rise to a larger nymph that retains the main features of the second instar. This then goes on to pass through two further instars (fourth and fifth) that are broadly similar in stage of development of the genitalia and wings to stages 3 and 4 of the ‘standard’ four-stage developmental process. Normally the adult females that emerge from the five-stage process are larger than the others and capable of laying larger egg clutches (see Chapter 3, Fig 65, for images of nymphal stages).
Recent research has shown that there is a seasonal dimension to this variation in life-history strategies. It seems that eggs laid early in the summer are smaller than those that are laid later. The earlier-laid eggs develop at various rates, but all enter diapause at a certain stage of embryonic development, and overwinter at that stage. They hatch more or less synchronously early the following year. These early hatchlings are smaller than the offspring of eggs laid later the previous year, but given favourable early summer climatic conditions, the females are more likely to go on to develop through five nymphal instars. Conversely, eggs laid later in the summer tend to be larger, and do not reach the diapause stage by the onset of winter. As a consequence they avoid diapause, and go on to develop the following year with later, and more divergent hatching dates. These later hatchlings are larger than the earlier ones and the females of these are more likely to complete their development in just four nymphal stages.
This difference of developmental strategy can be explained in terms of a trade-off between, on the one hand, better survival chances of the offspring that develop earlier in the year, and on average greater fecundity of the larger females produced, and, on the other hand, the greater female ‘parental investment’ in the later-laid eggs, given the shorter period and less favourable conditions for development and reproduction that they are likely to encounter as they emerge later the following summer (Collins, 2001, 2006; Cherrill, 2002, 2004, 2005; Cherrill & Begon, 1989a, 1991; Cherrill & Haswell, 2006; see also Chapter 3 for more detail).
There is evidence that this seasonal difference in life-history strategies is also played out geographically, with larger, five-instar females being less frequent in more northerly localities (Telfer & Hassall, 1999).
Possibly owing to these complexities in its life history, adults of this species can be found from as early as the end of May (Pinchen, pers. corr.) through June, July, August and September, less commonly in October, and into early November in the south. An exceptionally late observation of a singing male was by J. Paul on 11 December 2004 (Sutton, 2005a).
Habitat
This species flourishes in a wide range of habitats – often ones that might appear hostile to the survival of any species. These include: roadside verges; sand dunes; dry rocky slopes; sandy or gravelly banks; the edges of car parks; recently disturbed land, such as eroding coastal cliffs, quarries or building sites; dry pastures; and open areas in woodland. However, these share common features: at least some sparse grasses, patches of bare ground with loose soil or fine gravel, and exposure to sunshine. Compared with other species, it is effective at raising its body temperature above the ambient, as well as avoiding overheating. For this it does require habitats open to sunshine, but it appears not to need tall, cooler grass tussocks for shelter from high temperatures (Willott, 1997). Although tolerant of a wide range of habitats, it is rarely found in moist or lush grassland. It is also absent from intensively managed and ‘improved’ farm grasslands (Gardiner, 2009a).
Behaviour
Both sexes spend much of their time on the ground or in low vegetation, frequently sunning themselves on exposed substrates such as rocks, items of litter, or concrete structures. Males are particularly active in warm conditions, and spend much of their time chasing females – often three or four in hot pursuit of a single female. Sometimes the males deliver a solitary calling song. This consists of a series of 6 to 10 short chirps (each chirp lasting half a second), with one or two seconds between chirps. The whole performance is repeated at regular intervals. However, the males are highly gregarious, and a still, silent male will be provoked into chirping by the approach of another male. Two males together will chirp alternately and the chirp rate becomes faster, producing an even more complex interaction when other males gather. Typically this happens as males assemble around a solitary female as she basks or feeds on grass blades. With rather little warning (sometimes by the male antennating the female, or by his producing a sharp noise by a quick downstroke of the hind legs) one of the males will make a mating attempt. If the female is not receptive she may kick him away with her hind legs, or she may hop away, in which case the males will usually follow and gather round her, chirping as before (see DVD).
Females, too, are able to stridulate, but their chirp is quieter than that of the male. Receptive females stridulate in response to the male stridulation.
There are reports of swarming behaviour, with numbers flying up to several hundred metres (Haes, 1976; Marshall & Haes, 1988). This is presumably a significant aid to colonisation of new sites.
Enemies
Eggs are attacked by parasitic wasps of the genus Scelio. Richards and Waloff (1954) reported 19 to 28 per cent of pods affected. A study of predation on this species and the mottled grasshopper (M. maculatus) in north-west England (Cherrill & Begon, 1989b) revealed very high rates of predation by wolf spiders of the genus Pardosa (Lycosidae) on early instars. Both nymphs and adults are taken by the solitary wasp Tachysphex pompilformis (Richards & Waloff, 1954; N. Owens, pers. corr.).
The adults are frequent victims of large web-spinning spiders, such as the garden spider (Araneus diadematus) and wasp spider (Argiope bruennichi). Also, C. brunneus is among a small number of common species that form a significant part of the diet of declining farmland birds, such as cirl bunting (Emberiza cirlus), skylark (Alauda arvensis) and grey partridge (Perdix perdix) (Brickle et al., 2000; Robinson et al., 2001).
Distribution
This very widespread species occurs throughout Europe, eastwards through the middle-east to northern China, and southwards into north Africa.
In England the common field grasshopper is very common and widespread south of Yorkshire, but is more localised further north, becoming primarily a coastal species into Scotland. In Wales it is also widespread, but mainly in the lowland areas. It is present on the Isle of Man, the Scilly Isles and the Inner Hebrides.
Status and conservation
Although both common and widespread, this species does have definite habitat requirements. A survey of roadside verges in Essex, for example, found this species to be much more specialist in its habitat requirements than its relative, the common meadow grasshopper (C. parallelus) (pers. obs.). Dry, sparsely vegetated habitats with well drained nutrient poor substrates that are exposed to the sun – often on south-facing banks – are characteristic. Many of its habitats are provided as unintended consequences of human activity, and it could be locally threatened by excessive tidy-mindedness in the management of open spaces and marginal patches of land in urban and suburban areas. Fertilisation, frequent mowing, intensive grazing, and prolonged neglect leading to scrubbing over of habitat, are all threats to the survival of local populations. However, as it flies readily and well, it is likely to be effective at dispersal to new sites.
References: Brickle et al., 2000; Cherrill, 2002, 2004, 2005; Cherrill & Begon, 1989a, 1989b, 1991; Cherrill & Haswell, 2006; Collins, 2001, 2006; Gardiner, 2009a; Haes, 1976; Harz, 1975; Marshall & Haes, 1988; Ragge, 1965; Richards & Waloff, 1954; Robinson et al., 2001; Sutton, 2005a; Telfer & Hassall, 1999; Willott, 1997.
THE HEATH GRASSHOPPER
Chorthippus vagans (Eversmann, 1848)
MEASUREMENTS: Male 13 to 16mm in length; females 16 to 21 mm in length.
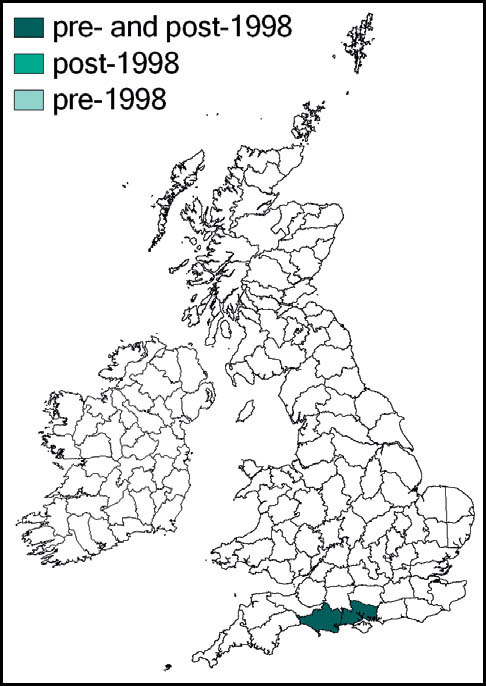

FIGS A–D. Male; male dorsal view; female; female dorsal view.

FIG E. Grey form.
Description
This rare species has strongly inflexed side keels to the pronotum, fully developed wings which, however, are usually relatively short: reaching back to the hind knees, or just short of them. There is a slight bulge on the leading edge of each fore wing, close to the opening of the hearing organ on the abdomen when the wings are folded.
The transverse sulcus on the dorsal surface of the pronotum cuts across at the midpoint, or slightly posterior to it, so that the front section of the pronotum is equal to or longer than the rear section. The underside of the thorax is only sparsely hairy.
This species shows relatively little variation in colour pattern. The dorsal surface of the head and pronotum varies from olive-brown to grey, with the rear portion of the fore wings (uppermost when the wings are folded) usually of the same colour. The side keels on the pronotum are outlined in white or pale brown and edged externally dark brown to black, and internally on the rear portion by black wedges of varying width, but always reaching to the rear margin of the pronotum (in British specimens). In one common form, the sides of the head and thorax are plain grey, often with some shading to brown dorsally. In others there is irregular patterning of orange and chestnut-brown. The anterior segments of the abdomen have black patches at the sides, and in sexually mature specimens of both sexes, the rear segments of the abdomen are coloured orange-red, especially on the dorsal surface. This also tints the hind tibiae and femora. In most specimens there are dark blotches on a paler ground colour on the hind femora, especially on the anterior (upper) edge.
Similar species
There are two species that might be confused with the heath grasshopper.

FIGS F–G. Dorsal surface of the pronotum of C. vagans; dorsal surface of the pronotum of C. brunneus.
Life cycle
The egg pods contain batches of approximately six to eight eggs, and they are deposited 0.5 to 1.5 cm below the surface of the soil. The eggs hatch during May or June the following year. The resulting nymphs pass through four instars and emerge as adults from early July and through August. They are at their peak in August, but can also be found in September, although in smaller numbers.
Habitat
In Britain this species occurs exclusively on dry heathland, often on the south-facing slopes of ridges or small hills, and associated with heathers as well as low-growing gorse scrub. Sparsely vegetated patches with bare, sandy ground appear also to be important.
Elsewhere in Europe it occurs in a wider range of habitats, including forest/dune transitional zones, arid grasslands, rocky slopes, quarries and railway embankments (Bellmann, 1988; Kleukers & Krekels, 2004). A German study found it positively associated with bare ground, and negatively with tree cover and leaf litter (Hochkirch et al., 2008b) in a transitional zone between forest and inland dunes. The eggs are resistant to desiccation (Ingrisch, 1983), and the species seems adapted to hot, dry conditions. However, Hochkirch et al. found that nearby areas of taller vegetation and higher moisture content were used for shelter at high ambient temperatures.
Behaviour
They are active only during warm, sunny days. When weather conditions are favourable, and especially around the middle of the day, the males move around in vegetation and over bare ground, occasionally stopping to stridulate. The song is rather subdued, and consists of extended bursts of five seconds or more. It resembles the song of the meadow grasshopper, but is softer, and of longer duration. The song is repeated at irregular intervals. Interacting males exchange shorter, more frequent bursts of stridulation.
There is no distinct courtship song, but in the presence of a female the male makes a more abrupt sound by single down strokes of the hind legs.
Unusually for a British grasshopper, it feeds on plants such as heather and gorse as well as grasses (see DVD). Its preferred method of escape is to retreat into dense vegetation, except on hot days when it flies more readily. Hochkirch et al. (2008b) measured the dispersal ability of the species, showing that a proportion of their study population were able to disperse to an adjacent colony – the maximum distance moved by a single male being 180 metres.
History
The species was first thought to occur in Britain when a specimen labelled ‘New Forest’ was discovered in the collection of the Natural History Museum, London, by B. P. Uvarov (1922). Subsequently W. R. Frazer identified two specimens he had collected at Wareham, Dorset in 1934 (Frazer, 1944). Another collection, made at Studland, Dorset, in 1933, and subsequently passed to the Natural History Museum, was also found to contain this species. In 1947 others were found on Sopley Common, Hampshire. Subsequently Ragge was able to confirm and study these populations, reporting on the then-known status of the species (Ragge, 1954, 1965; Marshall, 1974).
Distribution
This species occurs from Denmark in the north, through Europe to Calabria in the south, and southern Spain in the west. To the east in occurs in Romania, Bulgaria, Slovenia, and the European part of the former Soviet Union as far as Kazakhstan.
In southern Britain it is at the northern edge of its climatic range, and confined to heathland in Dorset and Hampshire. In occurs locally on the heaths to the south and west of Poole Harbour, in a few remaining heathland sites in the Bournemouth area, and further east on the western fringe of the New Forest.
Status and conservation
This species is declining over much of its European range and regarded as vulnerable or endangered in a number of European countries (e.g. Germany, Switzerland, Belgium, the Netherlands and Luxemburg). Factors responsible for the decline include agricultural intensification, urbanisation, and conversion of some of its habitats for recreational use.
A study by Hochkirch et al. (2008b) on an inland dune in north-west Germany confirmed their view of it as a species of the transitional zone between forest and open dunes. A population on the open dune tended to seek out cooler, less dry microhabitats with taller grasses, whereas those in a recently cleared but previously forested area tended to occupy areas with greater irradiation and less leaf litter. Their conclusion was that the main threat to the species was the elimination of transitional zones between forest and dry, low-nutrient open habitats such as dunes (presumably also sandy heathland and other sparsely vegetated habitats). Sharp boundaries between these habitat types, as well as shading, either by succession or plantations, were seen as major threats. On their study site, thinning of forest trees and removal of leaf litter to increase both insolation and bare ground was followed by a large increase in population.
Marshall & Haes (1988) describe the species as vulnerable in Britain. Even at its favoured localities, numbers of this species fluctuate greatly from year to year, presumably in relation to weather. The main threats to it here have been loss and fragmentation of its heathland habitats, especially in the Bournmouth area, where heathland has been lost to agriculture and urban development. Afforestation of open heathland, as well as shading out by succession to scrub and secondary woodland is also a threat where heathland is not under conservation management.
Sutton (2003c) expressed concern about the threat of heathland fires, especially in hot, dry summers, although it was reported to have survived a recent fire on Upton Heath, Dorset. More research on the ecology of the few remaining UK populations and appropriate management regimes is required.
References: Bellmann, 1988; Frazer, 1944; Harz, 1975; Hochkirch et al., 2008b; Ingrisch, 1983; Kleukers & Krekels, 2004; Marshall, 1974; Marshall & Haes, 1988; Ragge, 1954; Ragge, 1965; Sutton, 2003c; Uvarov, 1922.
THE MEADOW GRASSHOPPER
Chorthippus parallelus (Zetterstedt, 1821)
MEASUREMENTS: Male 10 to 17mm in length; female 16 to 23 mm in length.
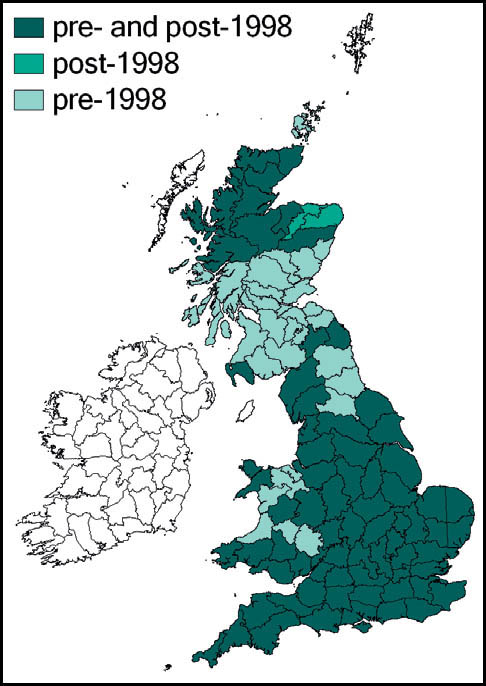

FIGS A–C. Male; male dorsal view; female.
Description
This very common species is usually recognisable because of its very much reduced wings. This is particularly true of the females whose wings generally do not reach back beyond the rear margin of the second or third abdominal segment. The wings of the male are better developed, but still do not reach back as far as the hind ‘knees’. There is a small bulge near the base of the leading edge of each fore wing. This is easier to make out in the male, but is also present in the female. The side keels of the pronotum are slightly incurved (not inflexed). The males are considerably smaller than the females, and have noticeably longer antennae.

FIGS D–I. Green female; green brown legs; green brown sides; dorsal stripe female; brown female; purple female (© J. Dobson).
There is an uncommon form (f. explicatus (Sélys)) with fully developed wings, and, to make matters more complicated, there are also intermediate forms (see Chapter 3, Figs 68 and 70d).
This species, like its relative, the common field grasshopper, displays immense variability in its colour forms. The permutation of patterns includes various distributions of green, pink, purple, brown, rust-brown, grey, yellow and black.
Ragge (1965) offers a useful simplifying classification that fits each of these forms into six categories.
The eyes are usually chestnut to dark brown, and the side keels of the pronotum are often (but not always) outlined in whitish. Sometimes there is also a narrow black edging to the side keels, and occasionally this is extended internally to form wedge-shaped marks on the dorsal surface of the pronotum like those of the common field grasshopper. Either sex may also have a white stripe (usually indistinct) along the leading edge of each fore wing, and/or a white stigma towards the tip of the wing.
The abdomen often has a median dorsal stripe of a different colour (noticeable in females and nymphs), and there are black markings of variable extent on the sides of the abdominal segments.
The males almost always have black or very dark brown hind ‘knees’, which contrast with the rest of the visible parts of the femora.
Similar species
The very short wings of the females distinguish them from all other British species.
In both sexes, the slightly incurved, not inflexed, side keels of the pronotum distinguish this species from all others except the large and lesser marsh grasshopper (Stethophyma grossum and Chorthippus albomarginatus). This species is unlikely to be mistaken for the large marsh grasshopper on grounds of size and wing length. However, the lesser marsh grasshopper has parallel or sometimes slightly incurved side keels to the pronotum, and males of C. parallelus with relatively well-developed wings can be difficult to distinguish with confidence in the field from males of C. albomarginatus. Usually the wings of male C. albomarginatus reach almost to, or slightly beyond, the hind ‘knees’, while in normal forms of C. parallelus they fall well short of the hind ‘knees’. Males of C. parallelus have a definite basal bulge on the leading edge of the fore wing, whereas this is absent (or vestigial) in male C. albomarginatus (but present in females). Males of C. parallelus also have a distinctive posture when at rest, with head and pronotum slightly upward sloping, as if they are ‘sitting up’. Males of C. albomarginatus tend to align their bodies more closely with the vegetation they are resting on – ‘sitting tight’. In sunny weather, of course, the songs of the two species are a very reliable diagnostic character.
Finally, a useful, if not entirely reliable, guide to field identification is the black-brown colour of the ‘knees’ of male C. parallelus – usually the same colour, or paler than the rest of the visible parts of the femora in C. albomarginatus. If in doubt, it is best to use a combination of the above characters.
The long-winged form of C. parallelus is often very long-winged – i.e., its wings are considerably longer than those of C. albomarginatus. For intermediate forms it is advisable to use other characters for identification.
Life cycle
Clutches of up to 10 eggs are enclosed in a pear-shaped pod, deposited just below the surface of the soil. The winter is passed in this stage, and the nymphs emerge early the following spring – usually from April onwards. The males pass through four nymphal stages, and the females either four or five, with adults usually emerging from the beginning of June onwards in the south (see Chapter 3, Fig 64). In the unusually warm spring of 2011 males were heard singing on Wanstead Flats, London, on 18 May (pers. obs.). They are abundant from July through to the end of September, with numbers declining in October.
Habitat
The meadow grasshopper can be found on most types of grassland, including quite small strips on roadside verges and central reservations, along the edges of arable fields as well as in flood meadows, rough pastures, open woodland and rides, both dry and damp heathland, coastal and inland marshes, downs, and moorland up to 1,800 metres in the north of its range. It can be particularly abundant on lush, damp unimproved grassland. However, it is one of the few species that persists, albeit in reduced numbers, on farm grasslands. It favours grassland with intermediate sward heights of 10 to 20 cm. Although it is positively associated with fine grasses, such as Agrostis species, coarser grasses, such as Lolium perenne, are selected for food (Gardiner & Hill, 2004b), implying that sward structure is of greater significance than food source in shaping its habitat preferences.
Behaviour
The males sing from a perch, often 10 to 20 cm above the ground on a grass stem. The sound is a rather rough ‘churring’, delivered in distinct bursts (echemes) each of which lasts from one to three seconds, beginning quietly and increasing in volume. It consists of 10 to 15 pulses, each produced by a single up-down motion of the hind femora. The song is repeated, usually at irregular intervals of some fifteen to twenty seconds (longer at low temperatures), and usually from the same perch. Where populations are relatively dense, ‘duets’ may be established between adjacent singing males, each alternating with the chirps of the other. Such alternations can go on for several minutes, and the intervals between chirps become more regular than when a male sings solo (see DVD).
There is no distinct courtship song, but in the presence of a female the male faces her, with antennae pointing towards her, and delivers a quieter version of the calling song. This may persist for many minutes before the male eventually moves towards the female and attempts to mate. Usually (as with most species!) the female moves away, only to be followed by the male, with a repetition of the courtship sequence. According to Ragge (1965) receptive females make silent stridulatory movements with their hind legs, and males may make a sound with single down strokes of the hind femora prior to making a mating attempt. Females are polyandrous, probably mating once every six or seven days. Copulation lasts about 15 minutes (Reinhardt et al., 2007).
Females are relatively inactive, and usually rest or feed low down among grasses, or seek oviposition sites in patches of bare ground or among surface litter (see DVD).
A behavioural study carried out by Gardiner on a population in a grazed hay meadow showed directional patterns of dispersal. Adults, in particular, moved uphill from the short turf of the introduction site towards an area of longer grass some three metres away. It seems probable that the more favourable habitat was detected visually (Gardiner & Hill, 2004a; Gardiner, 2009a).
Enemies
The eggs are parasitised by wasps of the genus Scelio, with as many as 19 to 28 per cent of pods affected in the study by Richards & Waloff (1954). Adults and nymphs are taken by the wasp Tachysphex pompiliformis. The adults are among the most frequent prey of large, web-spinning spiders, including the garden spider (Araneus diadematus) and wasp spider (Argiope bruennichi).
Like C. brunneus, they form an important component of the diet of several declining farmland bird species, including skylark (Alauda arvensis), grey partridge (Perdix perdix) and cirl bunting (Emberiza cirlus).
Distribution
The meadow grasshopper is distributed throughout Europe, including Scandinavia, up to the Arctic circle, and eastwards to Turkey, Mongolia and Siberia. In southern Europe it may be found up to altitudes of 2,200 metres.
It is abundant and widespread in southern England, East Anglia, the Midlands and most of Wales. It persists more locally northwards to the north of Scotland, and has been recorded from the Orkneys and Inner Hebrides. However, it appears to be absent from the Isle of Man and from Ireland.
Form f. explicatus was reported to be particularly frequent in 1941 (Kevan, 1953).
Status and conservation
C. parallelus is possibly our most common and widespread species, surviving in most types of grassland apart from the most intensively grazed, cut or fertilised. However, its conservation is still important in those parts of southern and central England where the spread of intensive agriculture and silage production have greatly reduced the availability of habitat for this species. There is a further conservation significance in the importance of this as one of a small number of still common grasshopper species in the diet of several declining farmland birds. Studies by Tim Gardiner and co-workers have established the value of a range of locations – remaining patches of unimproved grassland; set-aside strips; various stewardship prescriptions; varied cutting and grazing regimes; hedgerows; and footpaths – for the survival of this species in the context of predominantly agricultural landscapes (Gardiner, 2007b, 2009a; Gardiner & Haines, 2008; Gardiner & Hill, 2005a, 2005b; Gardiner et al., 2002, 2008; and for a detailed discussion of this literature, see Chapter 9).
References: Gardiner, 2007b, 2009a; Gardiner & Haines, 2008, Gardiner & Hill, 2004a, 2004b, 2005a, 2005b; Gardiner et al., 2002, 2008; Harz, 1975; Kevan, 1953; Marshall & Haes, 1988; Ragge, 1965; Reinhardt et al., 2007.
THE LESSER MARSH GRASSHOPPER
Chorthippus albomarginatus (De Geer, 1773)
MEASUREMENTS: Male 14 to 16.5 mm in length; female 17 to 21 mm in length.
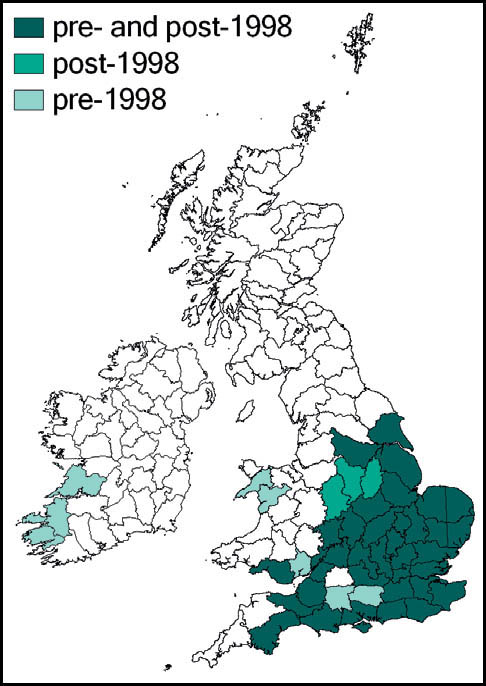

FIGS A–C. Male; dorsal view; female.

FIGS D–H. Brown male; green female; green brown sides male; dorsal stripe female; dorsal stripe (purple) female.
Description
This medium-sized grasshopper has fully developed, but rather short wings that reach back almost to, or just beyond, the hind ‘knees’. There is a definite bulge towards the base of the leading edge of each fore wing in the female. This is either absent or vestigial in the male. The side keels of the pronotum are either straight and parallel to one another, or very slightly incurved. In some females the abdomen projects well beyond the hind knees and wing tips, giving the appearance of a short-winged species. Females are significantly larger than the males, and the latter have noticeably longer antennae. The males have 90 to 140 stridulatory pegs on each hind femur.
Although this species is not as variable in colour pattern as the common field and meadow grasshoppers, there is still a good deal of variation. Again, Ragge (1965) offers a useful classification.
Within each of Ragge’s categories there is considerable variation in ground colour. For example, the ‘brown’ form may be grey, brown or straw-coloured. The various green forms generally have predominantly green fore wings, too. Both green and brown forms tend to have well-marked white stripes close to the leading edge of the fore wings in the females, less commonly in the males. The side keels of the pronotum are usually outlined in white, and often have a narrow black edging externally. The hind femora are usually unmarked, lacking dark bands or spots.
Similar species
The parallel side keels on the pronotum and the fully developed wings should serve to distinguish this species from all other British grasshoppers. However, the pronotal side keels are slightly incurved in some individuals, and confusion with males of C. parallelus is quite possible. The black/dark-brown hind ‘knees’ in C. parallelus males are a useful field character, but for other, more reliable, features, see under that species.
Life cycle
The eggs are laid in batches of three to ten, enclosed in an irregularly-shaped pod deposited at the base of grass stems. They hatch in May the following year, and the nymphs pass through four instars, emerging as adults from the beginning of July onwards. The adults are most frequent from late July through to the end of September (see Chapter 3, Fig 63 for images of the life history stages).
Habitat
This species was formerly strongly associated with moist grasslands, especially coastal flood defences, grazing marshes, dune systems, and damp meadows in river flood plains. However, in recent decades it has been recorded frequently from dry habitats, including track sides on farmland, dry hillside pastures, roadside verges and dry grass heathland, such as the East Anglian Brecks. This may indicate a change in its habitat requirements, but earlier commentators also noted its ability to occupy dry grassland sites (e.g. Payne, 1957). In its coastal localities it is associated with grassland dominated by salt-marsh grasses (Puccinellia spp.), as well as among marram grass (Ammophila arenaria) on dune systems, but a study of its Essex habitats found it commonly on lightly grazed and marginal areas of farm grassland, including agricultural set-aside, where it was associated with finer-leaved grasses such as creeping bent (Agrostis stolonifera) and rough meadow grass (Poa trivialis) (Gardiner et al., 2002) with sward heights between 10 and 20 cm.
Behaviour
The males are active among the grasses in search of females, or, on warm, sunny days, singing from a perch on a grass stem. The song is a brief, unobtrusive ‘burr’ lasting about half a second, and repeated three or four times, with a pause of approximately one second between bursts. The insect usually moves to another perch before repeating its song.
In the vicinity of a female, the male produces a quite distinct courtship song. The male moves close to the female, facing her and spreading his long antennae wide in her direction. The courtship song begins as a prolonged and continuous stridulation, in which the hind legs are held low down, almost parallel with the line of the body, and vibrated through a very narrow arc. This phase is hardly, if at all, audible to humans. Eventually, the male shifts to a more vigorous stridulation, with the hind legs raised almost to the vertical. At this stage the insect’s body begins to rock, and the stridulation is audible. From this point on there is a variable alternation between low-and raised-leg stridulation, eventually culminating in a mating attempt. This is (as is usual among grasshoppers!) generally rejected by the female moving off, or kicking the unfortunate male away (see clips from several courtship sequences on the DVD). The male then resumes the chase, takes up position and continues to court. According to Ragge (1965) a receptive female responds to the male courtship with a brief vibration of her hind femora, and mating ensues. In fact, courtship sequences are more often than not interrupted by other searching males, as groups of as many as ten individuals of both sexes tend to congregate. In these clusters there is frequent disturbance, with males making repeated direct mating attempts and being repulsed by females, while the males flick up their hind femora in response to one another.
Occasionally males perform the courtship sequence in the absence of a female (see DVD). Presumably, the calling song of the male functions to bring these groups together, rather than to locate or attract a receptive female.
Enemies
Along with C. parallelus and C. brunneus, this species forms a valuable component of the diet of declining farmland birds. Adults are frequent victims of large, web-spinning spiders, including Argiope bruennichi and Araneus quadratus.
Distribution
This species is widespread in mainland Europe from Spain in the west through to the former Soviet Union and Central Asia in the east. It occurs as far north as 64 degrees latitude in Scandinavia, and southwards to Italy and southern France. It occurs at altitudes up to 1,500 metres in the south. In the Netherlands it is common in the north and west, and in Belgium it is predominantly coastal (Kleukers & Krekels, 2004).
In Britain, the situation is confused by uncertainty about the validity of some earlier records, but it seems clear that the species has been extending its geographical range in recent years, possibly as a result of becoming more generalist in its habitat requirements (Wake, 1997; Evans & Edmondson, 2007). It is now widespread in southern, eastern and central counties of England, reaching north to Derbyshire (Sutton, 2003a) and south Yorkshire. Westwards it occurs in south Wales and is abundant around the Severn estuary. Further south and west it is much more localised in Dorset and Devon. In Ireland it appears to be confined to a small number of sites in the west.
Expansion of range from the south into greater London was reported by Sutton (2003c), and continued expansion north and west along a front from the Severn estuary to the Humber estuary was noted in 2004. New localities in Norfolk were discovered by Richmond and reported by Sutton (2007c). Range expansion seems to have been accompanied by readiness to occupy drier grassland habitats, including roadside verges. An intriguing report of one captured flying at 240 metres above the ground suggests a possible mode of dispersal (Sutton 2004b).
Status and conservation
The lesser marsh grasshopper is widespread and often abundant in southern and central Britain, and is possibly favoured by agricultural set-aside. With the cessation of set-aside, the fate of inland populations on farmland will be dependent on agri-environmental stewardship treatments that take account of its habitat requirements. Similar considerations apply to its habitats on flood defences, roadside verges, churchyards, cart tracks and other patches of unimproved and extensively managed grassland. It is adversely affected by hay-cutting, both suffering direct mortality, and becoming exposed to predation in short turf (see references to studies by Gardiner and colleagues under C. parallelus; see also Chapter 9).
References: Evans & Edmondson, 2007; Gardiner et al., 2002; Harz, 1975; Kleukers & Krekels, 2004; Mashall & Haes, 1988; Payne, 1957; Ragge, 1965; Sutton, 2003a, 2003c, 2004b, 2007c; Wake, 1997.
THE RUFOUS GRASSHOPPER
Gomphocerippus rufus (Linnaeus, 1758)
MEASUREMENTS: Male: 14 to 18 mm. in length; female: 16 to 21 mm. in length.
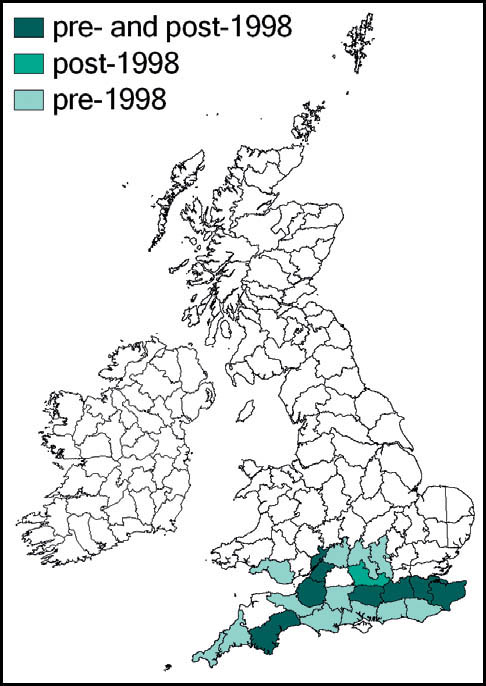

FIGS A–C. Male; dorsal view; female.
Description
The rufous grasshopper is one of only two British species with clubbed antennae. The antennae of the male are very long, and terminate with a pear-shaped club which tapers to a point at the tip. The female’s antennae are shorter, and also thickend towards the tip but not as prominently as in the male. The side keels of the pronotum are strongly inflexed. The wings are fully developed, and in the male reach back to the hind ‘knees’ or a little beyond. In the female, the wings are relatively shorter, falling short of the tip of the abdomen and hind knees, so that the final two or three abdominal segments are exposed. There is a slight bulge basally on the leading edge of each fore wing in both sexes.
The eyes are usually dark red-brown, the antennal clubs black, with white tips, and, in sexually mature individuals, there is some reddish coloration on the rear segments of the abdomen (more vivid in the males) and on the hind tibiae and ventral edge of the hind femora. The face and palps of the male are pale greenish or fawn, and the palps, especially, are whitish.
The colour patterns of the wings and body are very variable, but Ragge (1965) organised the diversity into three main categories.

FIGS D–E. purple; wedge; plain
Within these broad categories there is considerable variation of ground colour and patterning. The ground colour can vary from pale grey, through olive green and orange-brown, to a darker brown with black mottling. In some forms, especially the purple and brown varieties, the wings may be orange-brown with black spotting. In some forms the coloration of head and pronotum is plain, but in others there is darker mottling. In one striking form of the ‘wedge’ variety there is a dorsal cream stripe on the head, pronotum and rear margin of the fore wings.
In some localities, as well as the fully purple form distinguished by Ragge, there are forms in which the sides of the head and pronotum are purple, with various other colours dorsally.
Similar species
This is a distinctive species, unlikely to be confused with any other.
Some forms might be mistaken for the common field grasshopper (Chorthippus brunneus) on the basis of their inflexed pronotal side keels and comparable colour forms. However, the relatively longer wings and the lack of clubs to the antennae of that species are diagnostic.
The mottled grasshopper (Myrmeleotettix maculatus) also has clubbed antennae, but that species is very much smaller, and the side keels to the pronotum are much more acutely inflexed than those of G. rufus.
Life cycle
The egg pod is deposited just below the surface of the soil. It is roughly cylindrical with an apical lid, and contains up to 10 eggs. The eggs hatch rather late the following spring, from mid to late May, and the resulting nymphs pass through four instars before reaching adulthood in late July or August. Final instar nymphs can be seen as late as early September (Lucas, 1920; pers. obs.).
The antennal club is clearly visible in both sexes by the fourth nymphal instar.
Depending on weather conditions the adults persist through October, and into November. They have even been seen as late as the first week in December, and are reputed to take shelter under fallen leaves in cold weather (Marshall & Haes, 1988).
Habitat
This species is almost always found on calcareous grassland, especially south-facing slopes of chalk downland, although it is also reported from chalk sea cliffs and sand dunes as well as the edges of woodland. Where it occurs, the population is centred among patches of low scrub, notably dogwood or bramble among tall grasses. Male stridulation, courtship and mating, as well as basking, all seem to take place on the stems or broad leaves of woody plants in such patches. However, as a sun-loving species, it depends on regular scrub management and is vulnerable to being shaded out. Bare patches of ground are also required for oviposition.
Further south in Europe it is also associated with wooded areas, being found in sunny clearings and along woodland rides as well as in dry grasslands.
Behaviour
This species is rather inactive except on warm, sunny days. Both sexes are frequently to be seen on a perch – usually in low, open scrub, basking in the sun. Like most basking orthopterans, they sit sideways to the sun, with the hind leg that faces the sun lowered, so that the side of the abdomen is exposed. The males sing from perches 10 to 20 cm or so above ground level. The typical song is a rather subdued trill, lasting some five seconds and repeated at irregular intervals. Males spend much of their time moving through vegetation in search of females.
Courtship and mating usually takes place in patches of low scrub, especially dogwood, with both male and female using the leaves as platforms.
In the presence of a female, the male performs the most extraordinary courtship song and dance of any British grasshopper. This consists of a series of distinct phases which are usually performed in a set sequence, although this is capable of modification under experimental manipulation (Riede, 1986; see also Chapter 6 for a detailed description; Ragge & Reynolds for an analysis, together with oscillograms; and the DVD for film clips illustrating the various phases of the courtship).
The female is largely motionless through the performance, and her only obvious signs of interest appear to be:
Occasionally males run through the courtship routine in the absence of a female – as though practicing! (see the DVD.)
Enemies
Both sexes tend to remain in the loose cover of stands of dogwood or other low scrub, and either climb down into deeper cover or fly when disturbed. Males delivering the calling song are alert, but when engaged in the courtship performance they seem impervious to disturbance, and continue even when buffeted by rough winds. Some colour forms seem to match their substrates very closely, notably the purple, or partly purple forms, which are surprisingly well camouflaged against the early autumnal tints of dogwood leaves.
This, like other small and medium-sized grasshoppers, is vulnerable to predation by spiders, notably the wasp spider (Argiope bruennichi).
Distribution
This species occurs in mainland Europe from southern parts of Scandinavia, south-west through to France, especially the north, and eastwards through eastern Europe, the former Soviet Union, Siberia and Manchuria. In Italy it occurs in the Alps and Apennines, especially in forested zones. It is known from only one area in the Netherlands, but is more widespread in Belgium, especially in the south east.
In Britain this species is confined to the southern counties of England and a small area of south Wales. It is common on the North Downs in Surrey through to east Kent, but is less widespread on the South Downs. It has also been reported on calcareous grassland in Hampshire, Dorset and Wiltshire, on the Mendips (where it is rare) and in the Cotswolds, and on the south Devon coast. An isolated population is recorded from north Cornwall, where it occurs on calcareous dunes (Marshall & Haes, 1988).
The striking purple form is reported from Branscombe (Sutton, 2004a) and is also quite frequent on the North Downs (Baldock, 1999; pers. obs.)
Status and conservation
As this species is close to the northern limit of its geographical range in southern England, it is dependent on warm spring and summer weather, and so is highly vulnerable to prolonged poor weather, or successive poor summers, after which its numbers can be drastically reduced (Marshall & Haes, 1988). Many of its favoured habitats, such as on the North Downs and parts of the South Downs, are managed for conservation, but this is not necessarily favourable for this species. Many of the charismatic downland insects, notably butterflies such as the silver-spotted skipper (Hesperia comma) and Adonis blue (Polyommatus bellargus), require the hot microclimates produced by short turf. The rufous grasshopper, in contrast, favours areas of tall, tussocky grasses with low scrub. Haes and Harding (1997) suggest that this species benefited from the outbreak of myxomatosis in the 1950s, which reduced rabbit numbers and allowed coarser grasses to flourish. Despite the seeming incompatibility between management prescriptions for key downland butterflies and the rufous grasshopper, the management regime on the North Downs near Dorking seems favourable for both.
References: Baldock, 1999; Bellman & Luquet, 2009; Haes & Harding, 1997; Harz, 1975; Kleukers & Krekels, 2004; Lucas, 1920; Marshall & Haes, 1988; Ragge, 1965; Ragge & Reynolds, 1998; Riede, 1986; Sutton, 2004a.
THE MOTTLED GRASSHOPPER
Myrmeleotettix maculatus (Thunberg, 1815)
MEASUREMENTS: Male: 12–15 mm in length; female: 13–19 mm in length.
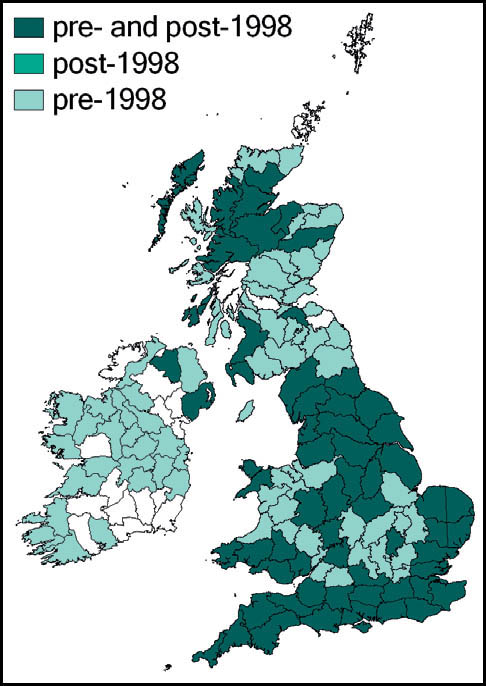

FIGS A–D. male; dorsal view; face and antennae (male); female
Description
The wings are fully developed in both sexes, reaching back to the hind ‘knees’ or just beyond in males and many females. In some females, however, the wings are slightly shorter in relation to the body, and the final one or two segments of the abdomen may be exposed. There is no bulge on the leading edge of the fore wings in either sex. The side keels of the pronotum are very strongly inflexed. The antennae of the male are very long, clubbed and turned outwards towards the tips. The female antennae are significantly shorter, and thickened towards the tips, rather than clearly clubbed.
There is a bewildering range of colour patterns, many individuals having irregular patterns of various colours that not only blend with the substrate, but also break up the outline of the insect’s body. Constant features seem to be the pale coloured abdomen, with dark patches on the first three or four segments, and a diffuse reddish tint to the final few segments of the abdomen, and parts of the hind legs in sexually mature males.
Ragge (1965) offers a classification that reduces the colour variation to twelve broad categories.
Forms 1 to 6 and 11 and 12 have no areas of green on the head or pronotum. (See Chapter 3, Fig 71 for images of a selection of these colour forms.)
There are some intermediate forms, especially between 1 and 2, and 3 and 4, where the side keels to the pronotum are faintly, but not clearly outlined pale, and vague outlines of the wedge-shaped markings on the dorsal surface of the pronotum are just visible.
As in the groundhoppers, the balance between different colour forms seems to vary with the character of the substrate, leading to speculation as to the causal mechanisms involved both in maintaining the diversity of colour forms and in modifying the proportions between them in local populations. An increase in the proportion of dark or black forms after heath fires (‘fire melanism’) is often noticed. For example, Baldock reported a population of this species as virtually all of the black form after a heath fire in Surrey (Sutton, 2003c). N. Owens made a quantitative study of the proportions of green and non-green individuals one year after a heath fire in Norfolk, comparing the populations on the burnt area with those on other substrates nearby. More than 75 per cent of those on the burnt area were ‘non-green’, compared with only 34 per cent non-green on a sandy cliff (Owens, 2010). There seem to be three possible explanations: high rates of selection by predators on non-matching insects over a single season; a phenotypic response to substrate colour during development; or selective movement into colour-matching areas by the different colour morphs. Initial results in 2011 suggest that the ratios of the colour morphs of early instars at different locations are very similar to those of the previous year (Owens, pers. corr.). This evidence tells against the explanation in terms of a phenotypic response to the substrate, but leaves open the other two possibilities. Selective movement across different parts of the habitat should not be ruled out in view of Gardiner’s observations on the movements of C. parallelus (see under that species).
Similar species
This very small species is often recognisable by size alone.
On closer inspection, the clubbed or apically thickened antennae, as well as the rather more strongly inflexed side keels to the pronotum serve to distinguish this species from small specimens of the common field grasshopper (Chorthippus brunneus), which often occurs in the same habitats as the mottled. Also, the common field has a bulge on the leading edge of each fore wing, absent in the mottled grasshopper.
The only other British species with clubbed antennae is the rufous grasshopper (Gomphocerippus rufus), which is noticeably larger, is unlikely to be found in the same habitat as the mottled, and does have a basal bulge on the leading edge of the fore wing.
Confusion is also possible between females of the mottled grasshopper and the heath grasshopper (Chorthippus vagans) in the latter’s very few localities. However, the heath grasshopper lacks the apical thickening of the antennae, has less markedly inflexed side-keels to the pronotum, and has a bulge on the leading edge of each fore wing (absent in M. maculatus).
Life cycle
The females deposit the egg-pods just below the surface of the soil on bare patches or among mosses or other low-growing plants. Each egg pod contains up to six eggs. These usually hatch in April or May the following year, and final instar nymphs are present as early as the second week in May, but also as late as mid-July. Adults begin to appear from late May in the south, and males can usually be heard as early as the first week in June. The adults are most abundant through July, but numbers begin to decline in the latter half of August, and continue to do so until early October.

FIGS E–H. Life-history stages: 2nd instar nymph; 3rd instar nymph; 4th instar nymph, green form; 4th instar nymph, semi-mottled form.
Habitat
This is a species of dry, exposed habitats, with short vegetation, areas of bare ground, and maximum exposure to the sun. Among its favoured haunts are: south-facing hillsides on sand, chalk or limestone, especially where intensively grazed by rabbits; sparsely vegetated acidic grass heathlands; sandy heather heaths; coastal cliffs; and dune systems. In uplands of western Britain it is found commonly on moorland hillsides. Possibly because of its small size it is relatively poor at raising its body temperature above the ambient, and this may explain its strong association with habitats exposed to the sun with short turf and bare ground (Willott, 1997). Another consequence is that it is less liable to overheating than are species with better thermoregulatory ability, and so does not require adjacent stands of cooler longer grasses for shelter. However, its feeding preference is for coarser, broad-leaved grasses that are relatively scarce on the preferred habitat.
In many localities it is associated with rabbit grazing and with mosses typical of dry heathy habitats, such as Polytrichum species.
Behaviour
Males are especially active on sunny days, sometimes singing from a fixed location on the ground, or on mosses or other very low vegetation, but more often running rapidly on what appear to be random routes, criss-crossing a patch of open ground a square metre or so in area. As they do so, they stop occasionally to emit a short burst of song, then resume their ‘patrol’. The song consists of a series of 10 to 30 soft ‘burrs’ that are repeated approximately every half-second, becoming louder towards the end. Females tend to be much more sedentary, spending much of their time feeding on grass blades, basking, or egg-laying. Once a male approaches a female he stops, points both antennae forward, then, after a short interval, approaches more closely, commencing his elaborate courtship sequence as he does so. Often this attracts the attention of other males and as many as three or four may assemble and collectively chase the female – normally without success.
The complexity of the male courtship performance almost matches that of the rufous grasshopper. (A detailed description of this is given in Chapter 6, and clips can be seen on the DVD; see also the analyses and oscillograms given in Ragge & Reynolds (1998).)
If there is no response from the female, or if, as frequently happens, she hops away a short distance, the whole process is repeated. Occasionally, during the male courtship sequence the female may make a few desultory stridulatory movements with her hind legs. However, it seems that the male is impervious to incoming sensory information in the course of the courtship performance, as, if the female leaves part way through, he continues to the end and then lunges at the empty space that was occupied by the desired female at the beginning of the sequence (see DVD).
Males often ‘flick’ a hind leg up and down when they encounter others during their perambulations. This has the appearance of an antagonistic signal, but may simply be a gender advertisement. This speculation is supported by an extraordinary event-sequence on film. A male was observed to perform a persistent courtship dance to what was visibly another male, but which remained still. The courting male eventually attempted to mate, jumping on to the back of the other male and then spinning around and leaving. On investigation, it was discovered that the motionless male was dead and in the grip of a spider. It seems likely that in this species at least, gender is indicated by behavioural signals, rather than by mere physical appearance.
Enemies
As the mottled grasshopper’s usual habitats are relatively open and bare, they are presumably vulnerable to attack from birds and other vertebrate visual predators. Their exceptionally variable colour patterns and tendency to rest against matching substrates is likely to be their main defence against such predators. However, they also fly readily in warm weather when disturbed.
Adults and nymphs are subject to considerable predation from spiders. Early-instar nymphs were found to be the main prey of wolf spiders of the genus Pardosa (Lycosidae) in a study of a population of this species and C. brunneus at a coastal locality in north-west England (Cherrill & Begon, 1989b). Both adults and nymphs are taken by the wasp Tachysphex pompiliformis (N. Owens, pers. corr.)
Distribution
This species is widely distributed throughout Europe as far as approximately 63 degrees north in Scandinavia, and eastwards to Turkey and Siberia. In southern Europe it can be found in mountains up to 2,000 metres or more.
In Britain it occurs almost throughout, wherever there is suitable habitat. In mainland Scotland it seems to be absent or very local in the west, but is widespread in the east and north. It is present on both the Inner and Outer Hebrides, and was recorded for the first time in Lewis in 2006 (Sutton, 2006b).
It is widespread throughout Wales, and has a scattered distribution in Ireland, being more frequent in the west. It is one of the few species that is present and common on the Isle of Man.
Status and conservation
This species seems to be able to maintain populations on relatively small and isolated relict patches of heathland, but is very vulnerable to succession to more vigorous grasses and scrub. Nutrient enrichment for agricultural purposes or cessation of grazing are therefore a threat to it. In many of its habitats active management is needed to maintain its key requirements of short turf and bare earth. Irregular disturbance by vehicles (as on Ministry of Defence land; see Gardiner & Benton, 2009) as well as rabbit-grazing disrupt natural succession and expose areas of bare ground.
In many lowland areas its marginal habitats are liable to be seen as ripe for building ‘development’, and many local extinctions must have resulted from this.
Although mineral extraction, road construction and other industrial activities often produce apparently suitable habitats for this species, its ability to colonise new sites seems to be very limited (Wake, 1997; Marshall & Haes, 1988). Although it is one of our most widespread species geographically, it is very localised in some parts of its range. In Essex, for example, Wake reported only five populations in the whole county, and county recorders T. Gardiner and P. Harvey included it in their Red Data list for the county (Gardiner & Harvey, 2004). In order to increase the number of Essex populations, Gardiner attempted translocation of the species from its strongest Essex population to nearby coastal habitat recently planted with marram grass as a flood prevention measure. Initial evidence suggests that the effort has been successful (Gardiner, 2010e)
References: Cherrill & Begon, 1989b; Gardiner, 2010e; Gardiner & Benton, 200g; Gardiner & Harvey, 2004; Harz, 1975; Marshall & Haes, 1988; Owens, 2010; Ragge, 1965; Ragge & Reynolds, 1998; Sutton, 2003c, 2006b; Wake, 1997; Willott, 1997.