(A) Aphids
INSECTS AND MITES THAT SUCK FLUIDS FROM LEAVES AND NEEDLES
INSECTS IN TWO ORDERS (Hemiptera, Thysanoptera), along with several families of mites, feed by sucking fluids from plants. Plant injuries produced differ completely from those produced by insects with chewing mouthparts, and many produce distinct symptoms related to how the mouthparts function and what site of the plant they feed. Excreted waste materials can also be useful for diagnosis.
Insects in the order Hemiptera have “piercing-sucking” mouthparts in the form of long stylets that penetrate the plant; within the stylet bundle is a channel for introduction of saliva and a food canal for removing the fluids on which the insect feeds. Many of the most common insects in this order (e.g., aphids, soft scales, mealybugs, psyllids, many leafhoppers and treehoppers) use the mouthparts to reach the phloem of the plant, extracting the sugar-rich sap. Usually, individual insects with this habit produce little cell injury, but when high numbers are sustained, plants may show wilting, stunted growth, and color changes. Leaf curling can also be produced by some insects, notably aphids, which feed on emerging leaves; however, phloem feeding insects also normally excrete sugary honeydew as a waste product, which can be very useful for diagnosis. Furthermore, surfaces on which honeydew lands and persists support growth of sooty mold fungi. Waste sugars may also be converted into wax that may cover the body or be expelled in the form of small pellets or threads.
Other insects (e.g., some leafhoppers, lace bugs, thrips) and spider mites remove contents from interior parenchyma cells of the leaves. Often this results in light spotting, known as stippling. Excreted waste associated with this feeding is often in the form of small dark droplets, known as tar spots.
More extensive damage is done by insects that feed in a manner known as “lacerate and flush” where large numbers of cells are killed during feeding events. This can produce highly visible spots of dead tissue, and when feeding of this type occurs on emerging growth, leaves become distorted. Plant bugs, along with many stink bugs and leaffooted bugs, are common insects that feed in this manner.
Mouthparts of thrips and spider mites are quite small and function differently from those of hemipterans. Both groups puncture surface cells in the course of feeding, which results in scarring wounds from thrips and small stippling wounds from spider mites. Because of their small size, eriophyid mites are limited to feeding on only the outer epidermal cells, producing leaf bronzing when in high populations.

(B) leafhoppers

(C) squash bugs are representative insects in the order Hemiptera that feed on fluids from plants using piercing-sucking mouthparts. A, B DAVID SHETLAR. C WHITNEY CRANSHAW

D. Sticky honeydew is the waste fluid excreted by insects, such as soft scales, that suck fluids from the phloem. WHITNEY CRANSHAW

E. Wax may be excreted or used to cover the body of many insects, such as woolly aphids, that suck fluids from the phloem of plants. WHITNEY CRANSHAW

F. Insects that suck fluids from the xylem of plants, such as spittlebugs, produce watery excretions. JEFF HAHN, UNIVERSITY OF MINNESOTA

G. True bugs, such as plant bugs, which use their mouthparts in a “lacerate and flush” manner often cause areas of localized cell death at feeding sites. DAVID SHETLAR

H. Thrips have mouthparts that penetrate and remove the fluid from plant cells, producing silvery scarring injuries. WHITNEY CRANSHAW

I. The old exoskeletons of spider mites, as well as the insects that feed with sucking mouthparts, remain after molting and can often be helpful signs to diagnose their presence. WHITNEY CRANSHAW

J. Leaf bronzing is a common response to injuries produced by spider mites and eriophyid mites, which damage surface cells. WHITNEY CRANSHAW
Whiteflies (Aleyrodidae family) are primarily tropical or subtropical insects. Several species survive year-round in southern areas that rarely freeze, but in cooler areas survival over winter is limited to plants grown indoors or in protected sites. Adults are small insects (ca.  inch), typically covered with powdery whitish wax. Immature stages of whiteflies are scalelike, feeding on sap from plants and rarely moving after the first stage. Whiteflies pass through a unique final development stage that does not feed, sometimes termed a “pupa,” from which the adults emerge.
inch), typically covered with powdery whitish wax. Immature stages of whiteflies are scalelike, feeding on sap from plants and rarely moving after the first stage. Whiteflies pass through a unique final development stage that does not feed, sometimes termed a “pupa,” from which the adults emerge.
GREENHOUSE WHITEFLY (Trialeurodes vaporariorum)1
HOSTS Greenhouse whitefly has a wide host range and is known to develop on more than 250 ornamental and vegetable plants. Poinsettia, hibiscus, nicotiana, aster, calendula, cucumber, lantana, tomato, grape, ageratum, bean, and begonia are among the commonly infested plants.
DAMAGE Greenhouse whitefly sucks sap from the plant, primarily from the phloem. Heavy infestations cause decline of plant vigor. Stunting, yellowing of foliage, and premature leaf drop are among the symptoms of injury. Infestations are also associated with production of honeydew excreted by the whitefly during feeding.
DISTRIBUTION Greenhouse whitefly is found worldwide and the most common species associated with greenhouses and houseplants. Greenhouse whitefly cannot survive winter outdoors in areas with freezing winter temperatures but is commonly annually introduced into gardens on infested transplants.
APPEARANCE All the immature stages of greenhouse whitefly are inconspicuous and easily overlooked. They are usually pale in color, almost translucent, and superficially resemble certain scale nymphs. Late-stage nymphs and the nonfeeding pupae may have numerous thin waxy threads along the sides of the body, giving them a somewhat spiny appearance. The spininess of the immature stages is variable and affected by population density and the hairiness of the leaf surface. Adults have nearly pure white wings because of a white waxy coating on them; the wings are held rooflike over the abdomen.
LIFE HISTORY AND HABITS Eggs are attached to leaf undersurfaces and are a creamy yellow color before darkening after 24 hours. Females prefer to attach eggs to the youngest leaves, often in a semicircular pattern. Egg hatch typically occurs within 5–7 days, and the newly hatched nymphs move a short distance before flattening themselves against the leaf to feed. All remaining immature stages of greenhouse whitefly are immobile. There are three nymphal stages that feed on the plant, spaced at 2- to 4-day intervals, followed by a nonfeeding “pupal” stage lasting almost a week. Under highly favorable conditions, a generation of greenhouse whitefly can take as little as 3–4 weeks to complete. Each female is capable of laying 400 eggs over a period of up to 2 months, although usually far fewer eggs are produced.
OTHER WHITEFLIES
SWEETPOTATO WHITEFLY (Bemisia tabaci)1 was known to occur in the southern U.S. for more than a century but was not a serious pest. In the mid-1980s, however, a new strain of this insect, known as biotype B, became established. This strain rapidly spread across the southern U.S. and developed into a far more serious pest because of its wider host range, ability to vector plant viruses, and resistance to several categories of insecticides. Furthermore, it appears to have completely replaced the former strain (biotype A) previously present in North America.

B. Greenhouse whitefly eggs and young nymphs. JIM KALISCH, UNIVERSITY OF NEBRASKA

C. Mixed life stages (hatched eggs, nymphs) of greenhouse whitefly. WHITNEY CRANSHAW

D. Last nymph (“pupa”) of greenhouse whitefly. DAVID SHETLAR

E. Adult and nymphs of greenhouse whitefly. JIM KALISCH, UNIVERSITY OF NEBRASKA

F. Sweetpotato whitefly. DAVID SHETLAR

G. Mixed life stages of sweetpotato whitefly. DAVID SHETLAR

H. Newly emerged adult of sweetpotato whitefly. DAVID SHETLAR
Sweetpotato whitefly biotype B has an extremely wide host range, including poinsettia, hibiscus, chrysanthemum, tomato, pepper, squash, cucumber, cotton, bean, eggplant, cabbage, watermelon, broccoli, potato, and peanut. As with other whiteflies, adults and nymphs remove sap while feeding, causing reduction of plant vigor during heavy infestations. However, introduced saliva has some toxic effects, producing disorders such as silvering of squash foliage, yellowing of lettuce, and whitening of roots and stems of carrot and broccoli. Affected tomato may also show irregular ripening. These symptoms have also led to the name “silverleaf whitefly.” Sweetpotato whitefly can also transmit a few plant viruses, including those associated with tomato yellow leaf curl, tomato mottle, and bean golden mosaic.
Sweetpotato whitefly biotype B is a common insect in greenhouses. It is generally similar in appearance to greenhouse whitefly but has a slightly more yellowish body, and the adults tend to wrap their wings around their abdomens when at rest. The last instar (pupa) is the most distinguishing form, being teardrop-shaped, lacking waxy spines, and being more flattened than that of greenhouse whitefly.
More recently an additional biotype, biotype Q, has been detected in greenhouse plantings in many North American sites. However, only recently have outdoor reproducing populations been observed, in southern Florida. The primary difference between the Q and B biotypes is in susceptibility to certain insecticides used in whitefly management.
BANDEDWINGED WHITEFLY (Trialeurodes abutilonea)1 is a close relative of greenhouse whitefly but rarely a pest. Adult bandedwinged whiteflies can be differentiated by the presence of two smoky gray, zigzag bands on the wings; pupae are distinguished by a dark band down the middle of the body. Poinsettia, geranium, hibiscus, and petunia are the most common hosts in greenhouses. In southern states this species may breed outdoors on various weed hosts as well as on cotton and some ornamentals.
MULBERRY WHITEFLY (Tetraleurodes mori)1 is found throughout most of the U.S. although rarely as a pest. Common hosts include mahonia, hackberry, mountain laurel, sweetgum, maple, dogwood, sycamore, citrus, and mulberry. The last-stage nymph (“pupa”) has an unusual and conspicuous appearance, being shiny black with a white fringe. Multiple overlapping generations are produced during the growing season, and the pupa is the overwintering stage that can withstand freezing temperatures. A species of similar appearance is AZALEA WHITEFLY (Pealius azalaeae),1 an introduced species now found throughout the southeastern U.S. wherever azalea is grown. Rhododendrons may host RHODODENDRON WHITEFLY (Dialeurodes chittendeni).1
ASH WHITEFLY (Siphonius phillyreae)1 is an introduced insect discovered in southern California in the late 1980s that has since spread to many states. Hosts include ash, mulberry, crabapple, flowering pear, serviceberry, western redbud, crape myrtle, tuliptree, lilac, pyracantha, and privet. Severe infestations, with associated problems of honeydew production and sooty mold, occurred shortly after its introduction. Subsequent establishment of the parasitic wasp Encarsia inaron has since had a great effect on suppressing populations.
A. Silverleaf symptom associated with sweetpotato whitefly, strain B. ALTON N. SPARKS JR., UNIVERSITY OF GEORGIA, BUGWOOD.ORG

B. Irregular ripening of tomato fruit associated with sweetpotato whitefly, strain B. JOHN CAPINERA, UNIVERSITY OF FLORIDA

C. Bandedwinged whitefly. RONALD SMITH, AUBURN UNIVERSITY, BUGWOOD.ORG

D. Nymphs of bandedwinged whitefly. TOM MURRAY

E. Mulberry whitefly. WHITNEY CRANSHAW

F. Nymphs of rhododendron whitefly. BRUCE WATT, UNIVERSITY OF MAINE, BUGWOOD.ORG

G. Ash whitefly adult. ROBIN ROSETTA, OREGON STATE UNIVERSITY

H. Ash whitefly, last-instar nymphs (“pupae”). ROBIN ROSETTA, OREGON STATE UNIVERSITY
CITRUS BLACKFLY (Aleurocanthus woglumi)1 is an important whitefly associated with citrus in southern Florida. As it feeds it produces considerable amounts of honeydew, further promoting unattractive sooty molds on leaves and developing fruit. As in mulberry whitefly, the last-stage nymph is shiny black with a white wax fringe. Adults are slate blue, considerably darker than most common whiteflies. CITRUS WHITEFLY (Dialeurodes citri)1 is found throughout the southern U.S. It can be an important pest of citrus but develops on a wide range of hosts including ivy, gardenia, lilac, and privet. Development is continuous, with generations being completed in about 2 or 3 months during the growing season. Winter in the northern areas of the range is spent as late-stage nymphs on leaves. WOOLLY WHITEFLY (Aleurothrixus floccosus)1 also develops on citrus, as well as on Eugenia. It is found in areas of southern California and Florida. Woolly whitefly became a serious pest following its accidental introduction around 1909 until several parasitic wasps were established that since have provided excellent biological control. As the name suggests, the body of the nymph is covered in long, loose waxy threads.
GIANT WHITEFLY (Aleurodicus dugesii)1 is a non-native species of spreading distribution that is substantially larger than most whiteflies. Giant whiteflies tend to reproduce on the same leaf on which they developed, causing clustering of colonies, and waxy filaments dislodged from the body may spread across the leaf surface. Furthermore, while laying eggs they make distinct waxy spirals on the underside of leaves. Giant whitefly has a very wide host range of ornamental plants, including begonia, hibiscus, mulberry, and giant bird-of-paradise. Banana, some citrus, and many vegetables are other hosts. More than 60 species of plants serve as hosts of the RUGOSE SPIRALING WHITEFLY (A. rugioperculatus), with gumbo-limbo, coconut palm, and Calophyllum among the plants that are most heavily infested in southern Florida. The species, as well as other “spiraling whiteflies” (Aleurodicus spp.) lay their eggs in a distinctive spiral pattern, mixed with wax, on the underside of leaves. In addition to causing plant stresses that produce foliage yellowing and leaf drop, rugose spiraling whitefly also excretes large amounts of honeydew.
IRIS WHITEFLY (Aleyrodes spiraeoides)1 is reportedly common in California and sometimes injurious to iris and gladiolus. It has a wide host range including many vegetables, strawberry, and cotton. Adults have a slightly darkened dot on each wing. Eggs are laid in distinct circles, and fine powdery wax is produced about the eggs and nymphs on the leaf. BRASSICA WHITEFLY or “cabbage whitefly” (Aleyrodes proletella) is a European species originally discovered on the East Coast but which has since spread extensively across the northern U.S. and into many areas of Canada. This whitefly can infest most cabbage family plants and has been a problem mostly on kale. Sowthistle (Sonchus) is a noncrop plant that also commonly supports this insect.
Other whiteflies have recently become established in south Florida. FICUS WHITEFLY (Singhiella simplex)1 has developed into a potentially serious pest of Ficus spp., particularly weeping fig (F. benjamana). BONDAR’S NESTING WHITEFLY (Paraleyrodes bondari)1 has also been primarily associated with ficus, although it has a potentially wider range of host plants.
1 Hemiptera: Aleyrodidae
A. Citrus blackfly. FLORIDA DIVISION OF PLANT INDUSTRY, BUGWOOD.ORG

B. Citrus blackfly eggs. FLORIDA DIVISION OF PLANT INDUSTRY, BUGWOOD.ORG

C. Citrus blackfly nymphs. CHAZZ HESSELEIN, ALABAMA COOPERATIVE EXTENSION SERVICE, BUGWOOD.ORG

D. Citrus whitefly. LYLE J. BUSS, UNIVERSITY OF FLORIDA, BUGWOOD.ORG

E. Citrus whitefly nymphs. FLORIDA DIVISION OF PLANT INDUSTRY, BUGWOOD.ORG

F. Woolly whitefly. CHAZZ HESSELEIN, ALABAMA COOPERATIVE EXTENSION SERVICE, BUGWOOD.ORG

G. Woolly whitefly nymphs. FLORIDA DIVISION OF PLANT INDUSTRY, BUGWOOD.ORG

H. Giant whitefly. DAVID CAPPAERT

I. A spiraling whitefly (Aleurodicus species). DAVID SHETLAR.

J. Brassica whitefly. ROBIN ROSETTA, OREGON STATE UNIVERSITY

K. Brassica whitefly nymphs. ROBIN ROSETTA, OREGON STATE UNIVERSITY

L. Iris whitefly. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM
More than 1,300 species of aphids (Aphididae family) occur in North America, including scores of species that are associated with and may be damaging to yard and garden plants. All are small, rarely exceeding inch, but they reproduce prolifically and frequently become extremely abundant. Normally wingless adult forms are produced, but aphids can produce both winged and wingless adults. Production of winged forms increases when conditions exist that favor leaving the plant, such as overcrowding and decline of plant quality. Winged forms are also produced at certain times of year, often in response to changes in day length, prompting them to move to new host plants.
Aphids feed by sucking sap from the phloem of plants and can cause several kinds of injuries. In high numbers they can cause a decline in plant vigor, leaf yellowing, and wilting. Aphids also excrete large amounts of honeydew, which may become a significant nuisance, attracting nuisance flies and wasps and supporting growth of associated sooty molds. Aphids feeding on new growth of some plants may cause leaf curling or discoloration. In addition, some aphids can transmit viruses that produce plant disease.
Normal aphid reproduction is asexual. Females produce an egg, without mating, but the egg is retained internally, hatches, and the nymph emerges as a live birth. Furthermore, the daughter aphid at birth has already begun to mature her own eggs—literally born pregnant. The ability of aphids to reproduce asexually (parthenogenesis) and hatch eggs internally to give live birth (ovoviviparity) helps aphids to build large populations rapidly and requires only a single foundress to colonize a new plant.
In most areas of North America, however, conditions can occur that are adverse to aphid reproduction, notably freezing temperatures that kill host plants and are lethal to aphids. Adapting to this condition, most aphid species have shifted their habit so that at the end of the growing season they lay eggs externally that can survive between seasons. The production of eggs is preceded by a generation in which special winged forms of both males and females are produced (sexuales). These meet and mate on the winter host plant, producing oviparae, special forms that lay eggs that survive winter. An aphid life cycle that involves production of males during one generation, followed by the production of an egg, is called a holocyclic life cycle.
When conditions are continuously favorable for reproduction, the production of sexual forms and overwintering eggs is dispensed with, and asexual reproduction occurs year-round. Some insects, such as cabbage aphid, normally reproduce year-round on outdoor host plants and never produce an egg, having an anholocylic life cycle. Also, some aphids may shift to an anholocyclic life cycle in indoor conditions. For example, GREEN PEACH APHID (Myzus persicae) and POTATO APHID (Macrosiphum euphorbiae) may reproduce indoors year-round strictly through parthenogenesis but produce overwintering eggs on outdoor plants.
Many species of aphids compound the complexity of their life cycles by alternating host plants during the growing season. In this type of life cycle, termed two-host or heteroecious holocycly, eggs are produced on a winter host, and spring populations arising after egg hatch occur on this host. They then disperse in late spring to an alternate, summer host. About half the aphids in North America have this habit of alternating between host plants in winter and summer. Those that produce overwintering eggs but do not alternate hosts in winter and summer are said to have an autoecious holocyclic life cycle.

B. Norway maple aphids and associated honeydew. WHITNEY CRANSHAW

C. Cottonwood aphid giving birth. WHITNEY CRANSHAW

D. Honeydew and cast skins of green peach aphid. WHITNEY CRANSHAW

E. Sooty mold on sidewalk under linden tree infested by linden aphid. WHITNEY CRANSHAW

F. Mating pair of aphids on sycamore. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

G. Egg producing forms (oviparae) and eggs of a Ceruraphis species of aphid. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

H. Overwintering egg of the mealy plum aphid. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM
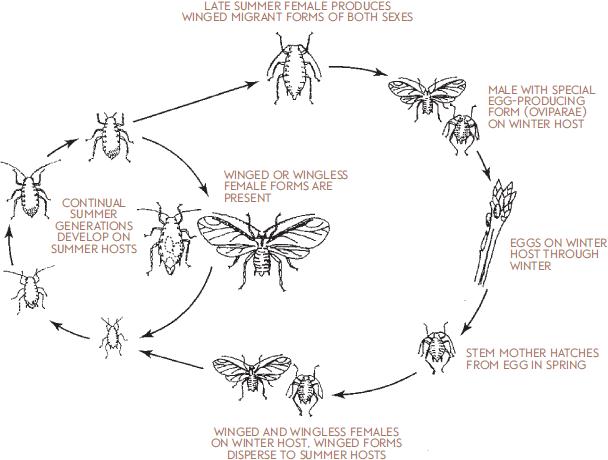
I. Life cycle of an aphid that has host alternation (heteroecious, holocyclic).
GREEN PEACH APHID (Myzus persicae)1
HOSTS Winter (primary) hosts include peach, apricot, and rarely certain cherries and plums. Summer hosts include more than 200 species of herbaceous plants, many of them vegetables and ornamentals. Green peach aphid is also one of the most common damaging aphids of greenhouse crops.
DAMAGE On winter hosts such as peach and apricot, spring generations can produce serious leaf curling. A wide variety of vegetables, herbs, and other herbaceous plants are colonized during the growing season. Green peach aphid is also adapted to developing on many plants grown indoors and is one of the most important greenhouse aphids.
Leaf curling symptoms produced by green peach aphid are restricted to certain species of Prunus in spring. However, in high population green peach aphid can cause stunting, wilting, and premature leaf drop and produce abundant amounts of excreted honeydew. Green peach aphid is also an efficient vector of many plant viruses including potato virus Y, cucumber mosaic, plum pox, and bean common mosaic. Strains of green peach aphid that are highly resistant to many insecticides have become common in many areas.
DISTRIBUTION Green peach aphid occurs throughout North America, both outdoors and as a common greenhouse pest.
APPEARANCE Nymphs and wingless adults are usually straw colored, sometimes pale green. Nymphs that will ultimately transform to winged forms may be pale orange or red. Winged females have a black head and dark patch on the abdomen.
LIFE HISTORY AND HABITS Outdoors in cold climate areas, green peach aphid alternates between a primary winter host and various secondary summer hosts. On the primary host, winter is spent as eggs laid near buds of peach or apricot. Eggs hatch following bud break to produce wingless females (first generation). Upon reaching adulthood they give live birth, producing two or three generations on the winter host. In the last spring generation, winged forms are produced that abandon the winter host and migrate to herbaceous summer host plants.
On summer hosts, individual aphids may become mature within 2 weeks after birth. Under optimal conditions females may produce two or more young per day for about 2–3 weeks. Most adults are wingless, but some winged forms occur, particularly when colonies become crowded. A dozen or more generations may be produced during the growing season. During late summer and early fall, new winged forms (fall migrant males and females) are produced that migrate to the Prunus winter hosts. After mating, the overwintering eggs are laid.
In greenhouses and areas where warm temperatures occur throughout the year, the forms of green peach aphid associated with the Prunus winter hosts do not occur. Instead reproduction is continuous from females that give live birth without mating. Adults may be winged or wingless.
1 Hemiptera: Aphididae

B. Green peach aphid colony, showing range of color. WHITNEY CRANSHAW

C. Overwintering egg of the green peach aphid. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

D. Green peach aphid hatched from overwintered egg (fundatrice) and nymphs. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

E. Leaf curling produced by green peach aphid. WHITNEY CRANSHAW

F. Black cherry aphids. WHITNEY CRANSHAW

G. Currant aphids. WHITNEY CRANSHAW

H. Spirea aphids. WHITNEY CRANSHAW

I. Black bean aphid on euonymus. DAVID SHETLAR

J. Symptoms produced by snowball viburnum aphid. WHITNEY CRANSHAW

K. Symptoms produced by leafcurl plum aphid. WHITNEY CRANSHAW

L. Rosy apple aphid colony. WHITNEY CRANSHAW
SOME COMMON APHIDS IN NORTH AMERICA THAT ALTERNATE BETWEEN PRIMARY (WINTER) AND SECONDARY (SUMMER) HOST PLANTS1
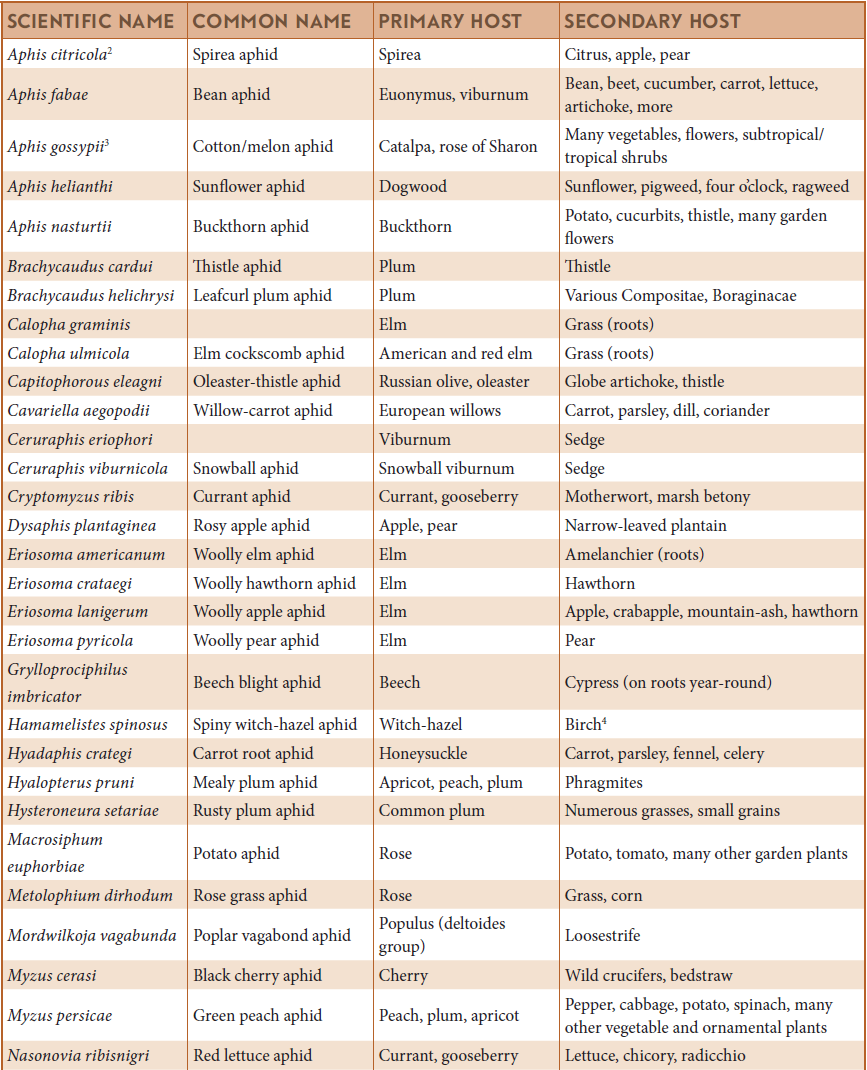
1 All species on this list commonly produce overwintering eggs and have a heteroecious holocyclic life cycle.
2 May be known as green citrus aphid when associated with citrus.
3 In much of the southern half of U.S. and in greenhouses, reproduces continuously in normal asexual manner without use of alternate primary hosts.
4 Alternates between two woody plants where it can overwinter as an egg (on witch-hazel) or hibernating female (on birch).

A. Thistle aphid colony. WHITNEY CRANSHAW

B. Willow-carrot aphids on dill. WHITNEY CRANSHAW

C. Mealy plum aphid colony. WHITNEY CRANSHAW

D. Waterlily aphids. WHITNEY CRANSHAW

E. Bird cherry-oat aphids. JIM KALISCH, UNIVERSITY OF NEBRASKA
COTTON/MELON APHID (Aphis gossypii)1
HOSTS A wide range that includes hundreds of plants. Among garden plants, cotton/melon aphid is particularly damaging to melon, cucumber, squash, and related vine crops. However, it is also commonly found on plants such as pepper, eggplant, spinach, asparagus, okra, hibiscus, crape myrtle, bougainvillea, pittosporum, and many tropical and subtropical shrubs. Cotton/melon aphid is also one of the most common aphid pests of greenhouse crops.
DAMAGE Sap feeding can cause wilting, yellowing, and in extreme cases, death of older leaves. Infestation of new growth results in leaf curling. In some vegetable production areas, cotton/melon aphid is often most important because of its ability to readily transmit many viruses, including cucumber mosaic virus, watermelon mosaic virus 2, and zucchini yellow mosaic virus. Many populations of cotton/melon aphid have developed high levels of resistance to insecticides, further complicating management.
DISTRIBUTION Cotton/melon aphid is cosmopolitan and can occur throughout North America but is particularly damaging in the southern and southwestern U.S.
APPEARANCE Wingless females are about  inch and usually a mottled light green. They can vary, however, from pale yellow to dark green or almost blackish. A light coating of wax gives them a dull appearance. Winged adults have a black head and thorax, yellowish-green abdomen, and light gray wings.
inch and usually a mottled light green. They can vary, however, from pale yellow to dark green or almost blackish. A light coating of wax gives them a dull appearance. Winged adults have a black head and thorax, yellowish-green abdomen, and light gray wings.
LIFE HISTORY AND HABITS In the southern U.S. and in greenhouses, reproduction occurs continually as long as temperatures allow. Unlike most aphids, cotton/melon aphid thrives under warm conditions. Optimal temperatures are in the range of 70–80° F, and the life cycle may be completed in as little as 1 week.
In northern areas, cotton/melon aphid may produce an overwintering egg that is laid on catalpa or rose of Sharon. When these eggs hatch in spring, there are usually two generations on the winter host before the species moves to vegetables, flowers, and other herbaceous plants. In the south and in greenhouses, eggs are not produced and continual generations occur year-round.
CABBAGE APHID (Brevicoryne brassicae)1
HOSTS Cabbage aphid feeds solely on crucifers and can be damaging to broccoli, cabbage, Brussels sprout, cauliflower, canola, and other plants in the mustard family (Brassicaceae).
DAMAGE Large numbers of cabbage aphids removing sap retard plant growth. Infestation of new growth distorts heads and produces severe leaf curl. The presence of cabbage aphid as a contaminant can be very important, particularly with Brussels sprout. Flowers and seedpods may produce poorly.
DISTRIBUTION Cabbage aphid is present throughout North America.
APPEARANCE Mature females are grayish green with a dark head and dark cornicles. A double row of dark bars is present on the back, but the entire body is covered with fine powdery wax. Winged forms have a single row of dark dorsal bars and dark wing veins. Cabbage aphids are about 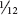 inch long. After a molt, the powdery wax covering is absent, allowing markings to be visible. Wax is again produced shortly after the aphids resume feeding.
inch long. After a molt, the powdery wax covering is absent, allowing markings to be visible. Wax is again produced shortly after the aphids resume feeding.
LIFE HISTORY AND HABITS In southern areas, reproduction can be continual, with adult females giving live birth as long as temperatures permit. There are three nymphal stages followed by the adult form, and the complete life cycle can be completed in less than 2 weeks under optimal conditions. The great majority of adults are wingless unless host plants greatly deteriorate. They may live for about a month, during which time they can produce more than 80 young. Winged forms are weak fliers but readily infest nearby plantings. Relatively few young (ca. 6–10) are produced by winged females. In northern areas where winter conditions prevent continual reproduction, winter is spent as an egg, laid on a mustard-family plant.
A. Cotton-melon aphids. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

B. Cotton-melon aphids on primrose. JIM KALISCH, UNIVERSITY OF NEBRASKA

C. Pea aphids. WHITNEY CRANSHAW

D. Crapemyrtle aphids. DAVID SHETLAR

E. Oleander aphids. DAVID CAPPAERT, BUGWOOD.ORG

F. Spotted hawthorn aphids. DAVID SHETLAR

G. Oak aphids. DAVID SHETLAR

H. Asparagus aphids. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

I. Norway maple aphids. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

J. Overwintering eggs of Norway maple aphid. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

K. Aphid eggs on pine needles. JIM KALISCH, UNIVERSITY OF NEBRASKA

L. Colony of cabbage aphids. WHITNEY CRANSHAW

M. Cabbage aphids on Brussels sprouts. WHITNEY CRANSHAW
APHIDS IN NORTH AMERICA THAT DO NOT COMMONLY ALTERNATE BETWEEN PRIMARY (WINTER) AND SECONDARY (SUMMER) HOST PLANTS
SCIENTIFIC NAME |
COMMON NAME |
HOST |
SPECIES THAT COMMONLY PRODUCE OVERWINTERING EGGS (HOLOCYCLIC LIFE CYCLE) |
||
Acyrthosiphon pisum |
Pea aphid |
Pea, alfalfa, clover, other legumes |
Acyrthosiphum lactucae |
Lettuce aphid |
Lettuce |
Aphis ceanothi |
Ceanothus |
|
Aphis hederae |
Ivy aphid |
English ivy |
Aphis middletoni |
Corn root aphid |
Aster, cornflower |
Aphis pomi |
Apple aphid |
Apple, pear, quince, hawthorn |
Aphis nerii |
Milkweed aphid |
Oleander, milkweeds, vinca |
Aphis sedi |
Sedum |
|
Aulacorthum solani |
Foxglove aphid |
Numerous hosts including foxglove, lettuce, potato, clover, bulbs |
Brachycorynella asparagi |
Asparagus aphid |
Asparagus |
Brevicoryne brassicae |
Cabbage aphid |
Brussels sprout, cabbage, other crucifers |
Callaphis betulaecolens |
Common birch aphid |
Birch |
Callaphis juglandis |
Dusky-winged walnut aphid |
Persian walnut |
Callipterinella callipterus |
European white birch |
|
Chaitophorus populicola |
Poplar leaf aphid |
Poplar, cottonwood |
Chaitophorus populifolii |
Poplar, cottonwood |
|
Chaitophorus viminalis |
Small black and green willow aphid |
Willow |
Chromaphis juglandicola |
Walnut aphid |
Persian walnut |
Cinara coloradensis |
Black polished spruce aphid |
Spruce |
Cinara curvipes |
Bowlegged fir aphid |
Fir, Engelmann spruce |
Cinara fornacula |
Green spruce aphid |
Spruce |
Cinara laricis |
Larch aphid |
Larch |
Cinara sabiniae |
Rocky Mountain juniper aphid |
Juniper |
Cinara strobi |
White pine aphid |
Eastern white pine |
Drepanosiphum platanoides |
Sycamore aphid |
Sycamore |
Eucallipterus tiliae |
Linden aphid |
Linden |
Hyadaphis tartaricae |
Honeysuckle witches’-broom aphid |
Tartarian honeysuckle |
Macrosiphoniella sanborni |
Chrysanthemum aphid |
Chrysanthemum |
Macrosiphum rosae |
Rose aphid |
Rose |
Melanocallis caryaefoliae |
Black pecan aphid |
Hickory, pecan |
Monellia caryella |
Blackmargined aphid |
Hickory |
Monelliopsis pecanis |
Yellow pecan aphid |
Pecan |
Myzocallis alhambra |
Dusky-winged oak aphid |
Oak |
Myzocallis coryli |
Filbert aphid |
Filbert, hazelnut |
Norway maple aphid |
Norway maple |
|
Periphyllus negundinis |
Boxelder aphid |
Boxelder maple |
Phyllaphis fagi |
Woolly beech aphid |
Beech |
Phorodon cannabis |
Cannabis aphid |
Cannabis |
Meliarhizophagus fraxinifolii |
Leafcurl ash aphid |
Ash |
Pterocomma smithiae |
Black willow aphid |
Willow, poplar |
Sarucallis kahawaluokalani |
Crapemyrtle aphid |
Crape myrtle |
Shivaphis celti |
Asian woolly hackberry aphid |
Hackberry |
Shizaphis graminum |
Greenbug |
Bluegrass, corn, small grains |
Sitobion avenae |
English grain aphid |
Corn, small grains |
Tinocallis saltans |
Siberian elm |
|
Tinocallis ulmifolii |
Elm leaf aphid |
Elm, particularly American elm |
Uroleucon ambrosiae |
Brown ambrosia aphid |
Aster family plants including lettuce, echinacea, rudbeckia, goldenrod |
Uroleucon pseudambrosiae |
Lettuce, sowthistle, endive |
|
Uroleucon rudbeckiae |
Goldenglow aphid |
Goldenglow, larkspur, delphinium |
Utamphorphora crataegi |
Fourspotted hawthorn aphid |
Hawthorn |
SPECIES THAT RARELY OR NEVER PRODUCE OVERWINTERING EGGS (ANHOLOCYCLIC LIFE CYCLE) |
||
Aphis craccivora |
Cowpea aphid |
Legumes (cowpea, kidney bean, lima bean), asparagus, lettuce, carrot |
Aulacorthum circumflexum |
Crescentmarked lily aphid |
Columbine, aster, lily, vinca, violet, cyclamen |
Chaetosiphon fragaefolii |
Strawberry aphid |
Strawberry |
Dysaphis tulipae |
Tulip bulb aphid |
Tulip, iris, gladiolus |
Hyadaphis foeniculi |
Fennel aphid |
Carrot, parsley, fennel, dill, celery |
Forda formicaria |
Grass (roots) |
|
Lipaphis pseudobrassicae |
Turnip aphid |
Many crucifers |
Longistigma caryae1 |
Giant bark aphid |
Oak, beech, nut trees |
Myzus ascalonicus |
Shallot aphid |
Numerous, including alliums, dandelion, tulip, lettuce, strawberry |
Rhopalosiphum maidis |
Corn leaf aphid |
Corn, small grains |
Rhopalosiphum rufiabdomalis |
Rice root aphid |
Primarily roots of grains. Common on many plants in hydroponic production |
Toxoptera aurantii |
Black citrus aphid |
Camellia, citrus, crape myrtle, ficus |
Toxoptera citricidus |
Brown citrus aphid |
Citrus, ficus, camellia, gardenia |
Tuberolachnus salignis |
Giant willow aphid |
Willow |
1 Overwintering eggs may be produced in northern areas of range.
Some aphids cover their body with long waxy threads, an effective deterrent to many natural enemies. Most of these “woolly” aphids are in the subfamily Pemphaginae1 and generally share life cycle similarities, including an affinity for developing on the roots or stems of secondary (summer) hosts. Differences occur in the habits of the sexual form and the production of only a single overwintering egg. Leaf curling is often produced by forms that colonize foliage.
Several Eriosoma1 species are associated with elm as a winter host, with most alternating with other species as summer hosts where they feed on stems or roots. (Note: These species are also treated on pages 368 and 526.) WOOLLY PEAR APHID (E. pyricola), most common in the Northwest, alternates between elm foliage in spring and the twigs of pear. Summer generations of the WOOLLY HAWTHORN APHID (E. crataegi) occur on branches and stems of hawthorn; rosette leaf distortions are produced on elm. WOOLLY ELM APHID (E. americanum) develops on the roots of Amelanchier in the summer after moving from American elm, on which it tightly rolls leaves in the spring. Perhaps best known is the WOOLLY APPLE APHID (E. lanigerum), which can build up dense colonies on suckers, branches, and, in warmer areas, roots of apple and crabapple. In much, if not most, of North America the woolly apple aphid does not have an alternate generation on elm. WOOLLY ELM BARK APHID (E. rileyi) is solely associated with elm, colonizing branches, and does not have a leaf curling generation.
LEAFCURL ASH APHID (Meliarhizophagus fraxinifolii)1 develops on the expanding new growth of ash, particularly green ash, creating tightly rolled and thickened leaves sometimes referred to as “pseudo-galls.” Some associated distortion and twisting of twigs may also occur. The aphids are yellow-green with a brown head but are covered with white, waxy threads. Often they are found in curled ash leaves in dense mixtures of old cast skins and droplets of wax-coated honeydew. Winter is spent on the roots of ash. Prociphilus americanus1 produces injuries similar to leafcurl ash aphid in eastern North America during late spring but migrates to fir (Abies spp.), where summer generations occur on the roots. Prociphilus caryae alternates between serviceberry (Amelanchier), where it curls leaves, and the roots of white pine. WOOLLY ALDER APHID (P. tessellatus) develops on the branches of alder in summer; overwintering eggs are laid on silver maple and the subsequent spring generations may curl leaves of this host.

Woolly alder aphid on maple. LACY L. HYCHE, AUBURN UNIVERSITY, BUGWOOD.ORG
Two common woolly aphids develop on beech. Colonies of BEECH BLIGHT APHID (Grylloprociphilus imbricator)1 develop on the foliage of beech in spring, later expanding to petioles and twigs. WOOLLY BEECH APHID (Phyllaphis fagi)2 is a European species now widely distributed in North America. All stages occur on beech foliage, and the species is frequently abundant.
ASIAN WOOLLY HACKBERRY APHID (Shivaphis celti)2 is a recently introduced species that has spread throughout much of the southern U.S. and is also found in California. It produces conspicuous colonies on hackberry leaves, with pale bluish wax surrounding the developing insects.
Woolly aphids associated with conifers, known as adelgids, colonize needles, twigs, and trunks. Most that are associated with needles are in the genus Pineus.3
1 Hemiptera: Aphididae (Pemphiginae)
2 Hemiptera: Aphididae (Drepanosiphinae)
3 Hemiptera: Adelgidae
A. Leafcurl produced by woolly elm aphid. STEVEN KATOVICH, USDA FOREST SERVICE, BUGWOOD.ORG

B. Colony of woolly elm aphids. WHITNEY CRANSHAW

C. Woolly apple aphid colony on crabapple stem. WHITNEY CRANSHAW

D. Damage produced by leafcurl ash aphid. WHITNEY CRANSHAW

E. Colony of leafcurl ash aphid. WHITNEY CRANSHAW

F. Woolly beech aphid. DAVID SHETLAR

G. Asian woolly hackberry aphid. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

H. Winged adult Asian woolly hackberry aphid. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

I. A Pineus species adelgid on pine needle. WHITNEY CRANSHAW
MEALYBUGS ASSOCIATED PRIMARILY WITH FOLIAGE
Note: Mealybugs associated primarily with twigs are treated on page 372. Species that develop primarily on roots are described on page 528.
CITRUS MEALYBUG (Planoccoccus citri)1
HOSTS Many plants grown in greenhouses are susceptible, as are several common plants used in interiorscapes such as ficus and philodendron. Coleus, fuchsia, gardenia, apple, stone fruits (Prunus spp.), and rose are common hosts. Citrus mealybug is an important pest of citrus orchards, particularly grapefruit, where it tends to settle on the underside of fruit.
DAMAGE Citrus mealybug damages plants in several ways. Feeding can weaken the plant by removing sap, and the saliva introduced into plants causes vascular blockage, resulting in distortion to new growth and leaf drop. The wax-covered bodies of the mealybugs and the honeydew they excrete degrade plant appearance.
DISTRIBUTION Widespread in greenhouses and on indoor plants in the U.S. in extreme southern areas not normally susceptible to hard winter freezing.
APPEARANCE Generally oval and marked by having 17–18 pairs of short wax filaments along the side of the body and no tail filaments. An indistinct purplish stripe can often be observed along the back.
LIFE HISTORY AND HABITS Eggs are produced by the adult females in a large egg sac (ovisac) which may contain up to 600 eggs. The eggs hatch after 7–10 days, and the young yellowish nymphs, known as crawlers, move about the plant seeking favorable sites for feeding. Terminal growth, cracks, and crotches are common areas where the crawlers settle. During development, females undergo two additional molts before becoming full grown. They maintain their legs throughout life but usually move little in later stages of development. However, they may move off plants and in greenhouses have been observed to lay eggs on benches, containers, and other nonplant surfaces. Off the plant, citrus mealybug may survive more than 2 weeks.
The rarely observed males undergo an additional (fourth) development stage that is nonfeeding and occurs in a small cocoon of loose wax. Adult males are much smaller than the females, have wings, and do not feed. Females can lay eggs in the absence of males.
A generation of citrus mealybug can be completed in about 1 month under optimal conditions indoors. Two to three generations are produced annually outdoors in Florida.
LONGTAILED MEALYBUG (Pseudococcus longispinus)1
HOSTS A wide variety of plants including begonia, citrus, dracaena, gardenia, ivy, impatiens, philodendron, tomato, coleus, poinsettia, fig, fuchsia, ferns, begonia, pyracantha, holly, yew, and rhododendron.
DAMAGE Longtailed mealybug feeds on the sap and in moderate numbers can induce leaf abscission. It also produces nuisance amounts of honeydew.
DISTRIBUTION Occurs outdoors in the southern states, as far north as Maryland. This mealybug is one of the most common and widely distributed insects associated with greenhouse and interiorscape plants.
APPEARANCE Oval, about ⅛ inch long, and covered with powdery wax. Longtailed mealybug lacks distinctive striping along the back but has numerous waxy filaments extending along the sides of the body. The presence of long threadlike tails, one pair of which may exceed the length of the body, is a distinguishing feature.

B. Citrus mealybug infesting fruit. JIM KALISCH, UNIVERSITY OF NEBRASKA

C. Citrus mealybug producing egg sac. WHITNEY CRANSHAW

D. Male of the citrus mealybug. WHITNEY CRANSHAW

E. Citrus mealybug crawlers and young nymphs. WHITNEY CRANSHAW

F. Longtailed mealybug. DAVID SHETLAR

G. Longtailed mealybugs with egg sacs. WHITNEY CRANSHAW
LIFE HISTORY AND HABITS Longtailed mealybug produces eggs that hatch almost immediately once outside the mother, essentially in the form of live birth. The young may remain under the mother for a few days and are released into a loose, snowy white mass of wax produced by the female that is conspicuous but much less developed than the ovisacs of some other species. The young feed on leaves and small branches, but females migrate to more protected sites when rearing young.
Under optimal conditions, development may be completed in about 45 days, after which there is a fairly extended period during which eggs mature. Females produce young over a period of a month, but some 90% are typically laid in the first 10 days. Outdoors, typically two or three generations are completed annually. Males are produced and are required for successful reproduction.
OTHER MEALYBUGS OBSERVED ON FOLIAGE
COMSTOCK MEALYBUG (Pseudococcus comstocki) is found primarily in the northeastern quarter of the U.S., parts of southern Canada, California, and Washington. Historically it has been a pest primarily of pear and other fruits such as apple and peach. Its host range is broad, however, and includes (but is not limited to) privet, mulberry, maple, hibiscus, catalpa, buckeye, pine, and yew. Young nymphs usually feed on leaves, whereas older stages tend to aggregate on twigs, often around nodes and scars. Two generations are annually produced. OBSCURE MEALYBUG (P. viburni) is an important pest of ornamentals with a host range of more than 50 plant genera. It can occur on the upper roots as well as the aboveground portion of plants. CITROPHILUS MEALYBUG (P. calceolariae) is associated primarily with citrus, but it does occur on other fruit crops, including grape.
MADEIRA MEALYBUG (Phenacoccus madeirensis)1 and MEXICAN MEALYBUG (P. gossypii) are two closely related species that are virtually identical in shape and form, with 3 rows of short, waxy tufts along the back and short terminal filaments. Eggs are laid in a large elongate egg sac that may be twice the length of the female. They are subtropical species with wide host range that can be found outdoors in the southern U.S. and are common greenhouse/interior plant pests throughout North America. Mexican mealybug is sometimes found on the upper roots as well as on leaves, stems, and flowers. Madeira mealybugs often overwhelm, even kill, hosts, while Mexican mealybugs are less damaging to hosts.
PINK HIBISCUS MEALYBUG (Maconellicoccus hirsutus)1 has attracted considerable attention and concern since its discovery in south Florida in 2002. Despite its name, it has a wide host range, including a variety of fruits and ornamentals and even certain vegetables. Adults are pinkish but covered with white wax. The eggs turn pinkish shortly after they are produced. As many as 15 generations can be produced annually. It has become a serious pest throughout south Florida and has been detected in southern Texas and California.
COCONUT MEALYBUG (Nipaecoccus nipae)1 is found primarily on palms but sometimes feeds on other plants including sago palm, avocado, bird-of-paradise, dracaena, and orchids. Females are generally oval in form but have thick pyramids of wax on the back and 10-12 pairs of wax filaments along the sides. The related GOLDEN MEALYBUG (N. aurilanatus) infests twigs of pines and araucarias in California. It has a dark purple body marked with a dorsal yellow stripe.
Two related species are associated primarily with herbaceous, often annual, plants. SOLENOPSIS MEALYBUG (Phenacoccus solenopsis) feeds on a wide range of plants in the mallow and nightshade families, including hibiscus, cotton, and tomato. Adult females have an oval body thinly covered with wax with green stripes running parallel to a ridge in the center. Females produce a large ovisac. The SOLANUM MEALYBUG (P. solani) feeds on a variety of plants in the lily, aster, mallow, and nightshade families, often concentrated at the base of plants. It is covered with whitish wax, possesses only short waxy filaments along the sides, and does not have filament “tails.” It does not produce an ovisac.
A. Comstock mealybug. CLEMSON UNIVERSITY-USDA COOPERATIVE EXTENSION SLIDE SERIES, BUGWOOD.ORG

B. Obscure mealybug. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

C. Citrophilus mealybug. USDA AGRICULTURAL RESEARCH SERVICE, BUGWOOD.ORG

D. Madeira mealybug. ANNE W. GIDEON, BUGWOOD.ORG

E. Striped mealybug. JIM KALISCH, UNIVERSITY OF NEBRASKA

F. Mexican mealybug, mixed life stages including male. DAVID SHETLAR

G. Solenopsis mealybug. USDA AGRICULTURAL RESEARCH SERVICE, BUGWOOD.ORG
STRIPED MEALYBUG (Ferrisia virgata)1 is an introduced species found primarily in the eastern states. It has a gray body covered with white wax, except where two stripes on the abdomen distinctly mark this species. A pair of filaments on the tip of the abdomen, about half the length of the body, is also present. Dogwood, hawthorn, azalea, holly, magnolia, apple, and mulberry are among the hosts. Two generations are produced, with winter spent as a nymph. GILL’S MEALYBUG (Ferrisia gilli) is a species recently described that is present in California pistachio production. It is also found on several deciduous ornamentals, grapes, persimmons, and Prunus.
MISCANTHUS MEALYBUG (Miscanthiococcus miscanthi)1 develops at the base of the leaf sheaths of Miscanthus, producing dwarfing and poor growth. Roots are also commonly colonized. Three generations are produced per year in the central U.S., with winter spent as a fertilized adult female.
RHODESGRASS MEALYBUG (Antonia graminis)1 is a turfgrass-infesting species presently established in many areas of the southern U.S. Rhodesgrass is the primary host plant, but other warm season grasses may also be infested. Colonies are often concentrated at the base of the plants. Adults are characterized by a very long slender thread at the end of the abdomen that protrudes from the massed mealybugs. Large colonies may excrete considerable amounts of honeydew that is attractive to ants and wasps. A closely related species is NOXIOUS BAMBOO MEALYBUG (A. pretiosa),1 which develops at the nodes of Bambusca and Phyllostachys species of bamboo.
Two species of mealybugs are associated with buffalograss, Tridiscus sporoboli (BUFFALOGRASS MEALYBUG) and a Trionymus species. These are small mealybugs that are easily overlooked and can usually be found inside or near leaf sheaths. Symptoms during heavy infestations are similar to those produced by drought stress, and plants often have a reddish-purple discoloration.
1 Hemiptera: Pseudococcidae
COCHINEAL SCALES
Cochineal scales (Dactylopius spp.)1 develop on prickly pear and sometimes other cacti. They produce large, conspicuous cottony masses of wax that cover the mature female and eggs. First-stage nymphs (crawlers) feed for about 3 weeks before settling, after which they remain immobile. Males develop in a small cocoon during their later stages. Multiple overlapping generations are produced in warmer areas of the southwestern states and Florida, where D. opuntiae is present. Two generations are normal for D. confusus in the Rocky Mountain States.
Cochineal scales have long been prized as a source of natural red dye, a color due to high levels of carminic acid in their blood. The species D. coccus is the cochineal scale usually used commercially for this purpose and is less covered with wax than D. confusus. Dactylopius coccus has been introduced into the U.S. and is now present in most southwestern states.
1 Hemiptera: Dactylopiidae

B. An Antonia species of mealybug on bamboo. DAVID SHETLAR

C. Rhodesgrass mealybug. DAVID SHETLAR

D. Mealybugs on buffalograss. JIM KALISCH, UNIVERSITY OF NEBRASKA

E. Cochineal scales on prickly pear cactus. WHITNEY CRANSHAW

F. Cochineal scales with crawlers. WHITNEY CRANSHAW

G. Dark red blood (carmine) of cochineal scale. WHITNEY CRANSHAW
SOFT SCALES ASSOCIATED PRIMARILY WITH FOLIAGE
Soft scales suck fluids from the phloem of plants and most excrete conspicuous amounts of honeydew. Unlike the armored scales, nymphal stages of soft scales retain some mobility during development and they migrate between foliage and twigs. Most soft scales ultimately settle on twigs in the adult stage and are discussed in chapter 4, but a few are most commonly observed on leaves.
BROWN SOFT SCALE (Coccus hesperidum)1
HOSTS Ficus, citrus, Schefflera, and English ivy are among the most common hosts; however, brown soft scale has an extremely wide range of potential hosts.
DAMAGE Heavy infestations of twigs and leaves can cause wilting, premature leaf drop, and dieback. A more commonly observed problem is the abundant amount of honeydew produced by this insect, which is often later associated with sooty molds.
DISTRIBUTION Soft brown scale is found throughout North America and the most common scale insect associated with indoor-grown houseplants. Cold winter temperatures restrict it as a year-round species on outdoor plants to southern areas where freezing temperatures are rare.
APPEARANCE Full-grown female scales (males are rare) are light brown, oval, and generally flattened in shape. Younger scales are yellowish brown with splotchy dark areas along the middle and sides.
LIFE HISTORY AND HABITS Eggs hatch over an extended period, underneath the bodies of the females. After crawling about the plant, the nymphs settle, begin to feed, and molt a couple of times. Upon reaching adulthood, the female exoskeleton hardens into a shell-like cover. Late-stage nymphs and adults remain immobile for the rest of their lives. Generations are produced continually and overlap. A single generation is typically completed in 2–3 months.
OTHER SOFT SCALES OBSERVED ON FOLIAGE
CITRICOLA SCALE (Coccus pseudomagnoliarum) is similar in appearance to brown soft scale but gets slightly larger and is grayish. Citrus, elm, hackberry, pomegranate, and walnut are common hosts in California. One generation is produced annually. GREEN SCALE (C. viridis) is elongate oval in shape and a distinctive light green color when alive. It can cover foliage and small stems. It is established in the southern half of Florida but can survive on greenhouse plants and houseplants. It commonly infests citrus, gardenia, Schefflera, Cuban laurel, and several other woody houseplants.
COTTONY MAPLE LEAF SCALE (Pulvinaria acericola)1 develops on maple, dogwood, and holly in the eastern U.S. and southern Canada. Its life history differs a bit from that of cottony maple scale (page 382) in that mature females migrate back to leaves in spring, where they subsequently produce cottony egg sacs. COTTONY CAMELLIA SCALE (P. floccifera), sometimes known as “cottony taxus scale,” has a similar life history. It produces a long, narrow egg sac, and eggs hatch over an extended period of about 6 weeks. Holly, camellia, jasmine, and yew are among the common hosts.

B. Brown soft scales and associated honeydew. WHITNEY CRANSHAW

C. Honeydew produced by brown soft scale collecting on lower leaf. WHITNEY CRANSHAW

D. Green scale. DAVID SHETLAR

E. Citricola scale. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

F. Sooty mold growing on honeydew excreted by green scale. DAVID SHETLAR

G. Cottony maple leaf scale. DAVID SHETLAR

H. Cottony maple leaf scale stage on twigs. DAVID SHETLAR
PYRIFORM SCALE (Protopulvinaria pyriformis)1 is a fairly large, irregularly pear-shaped scale that settles on the underside of the leaves of many tropical and semitropical ornamental plants. Adults are light reddish brown with a darker margin. Females lay the eggs within a flattened ovisac that surrounds the body.
TURFGRASS SCALE (Lecanopsis formicarum)1 is a potential pest of Kentucky bluegrass, red fescue, and some other cool-season grasses. It is most visible in late spring and early summer when females produce a cottony egg sac at the base of stems and leaves. Turfgrass scale is a European species found in eastern Canada and upper New York. Early-stage nymphs (crawlers) are reddish, later stages more yellow.
TESSELLATED SCALE (Eucalymnatus tessellatus)1 is a tropical species that can occur in greenhouses and has established outdoors in parts of Florida, where it is associated mostly with palms, mango, and crepe-jasmine.
FLORIDA WAX SCALE (Ceroplastes floridensis)1 can be common on citrus and hollies in the southeastern states. Stages that settle on leaves often line up along main veins. INDIAN WAX SCALE (C. ceriferus) develops on many plants but is particularly common on holly and can produce large amounts of honeydew. These species, along with other wax scales (Ceroplastes spp.), are also often found on twigs.

Florida wax scale, flipped to expose eggs. CHAZZ HESSELEIN, ALABAMA COOPERATIVE EXTENSION SERVICE, BUGWOOD.ORG

Indian wax scale. DAVID SHETLAR
CROTON SCALE (Phalacrococcus howertoni) is a recently (2008) established scale in southern Florida that has shown great damage potential. Croton, several figs, guava, gumbo-limbo, and mango are among its hosts.

Sooty mold associated with Indian wax scale infestation of holly. DAVID SHETLAR
1 Hemiptera: Coccidae

B. Turfgrass scale. DAVID SHETLAR

C. Tesselated scale. DAVID SHETLAR

D. Croton scale. DOUG CALDWELL, UNIVERSITY OF FLORIDA

E. Florida wax scale. CHAZZ HESSELEIN, ALABAMA COOPERATIVE EXTENSION SERVICE, BUGWOOD.ORG

F. Indian wax scale. DAVID SHETLAR
ARMORED SCALES OBSERVED PRIMARILY ON FOLIAGE
Armored scales suck fluids from the mesophyll cells of plants and, unlike soft scales and mealybugs, do not excrete honeydew. Armored scales also differ by losing their legs after the first molt and are only active during the first-instar “crawler” stage. Most armored scales occur on twigs and branches and are discussed in chapters 4 and 5, but a few are most commonly observed on needles or leaves of evergreen deciduous trees and shrubs.
PINE NEEDLE SCALE (Chionaspis pinifoliae)1
HOSTS A wide variety of conifers, particularly certain pines and spruce. Douglas-fir and hemlock are infrequent hosts.
DAMAGE Pine needle scale feeds on needles, often producing some localized discoloration around the feeding site. In high numbers, it can produce premature needle shed and some dieback of branches.
DISTRIBUTION Generally distributed throughout North America but is most common in the northern half of the U.S. and southern Canada.
APPEARANCE Adult females are almost pure white, elongated, and armored. They are slender and slightly yellow at the front end, widening at the rear. Males, when present, have a smaller, narrower scale cover. Crawler stages are light purple.
LIFE HISTORY AND HABITS The life history varies in different parts of North America. In most areas, the primary overwintering stage is eggs, underneath the mother scale. In some areas, particularly with mild winters, some females may survive and produce eggs throughout winter and into early spring. Eggs usually hatch in mid-spring, often coincident with the peak bloom of common lilac. Crawlers settle within a few days of hatch and subsequently remain in place for the rest of their lives.
In much of the eastern U.S. a second generation occurs, with eggs hatching in early summer and the adults maturing in early fall. (Small, partial third generations have sometimes been observed.) Single-generation strains typically predominate in the west. Also, males are common in eastern areas but largely absent in western populations.
OTHER ARMORED SCALES OBSERVED ON FOLIAGE
PINELEAF SCALE (Chionaspis heterophyllae)1 is closely related to pine needle scale, nearly identical in form, and associated with pine in eastern North America. BLACK PINELEAF SCALE (Nuculaspis californica)1 can be found on the needles of pines, firs, and Douglas-fir, occasionally producing outbreaks in pines under droughty conditions. Non-native pines, such as Scotch and Austrian pine, are particularly susceptible. One generation is produced per season in the north; two in southern states.

B. Pine needle scale with eggs. DAVID SHETLAR

C. Infestation of pine needle scale. DAVID SHETLAR

D. Pine needle scale, adult female exposed from under cover. DAVID SHETLAR
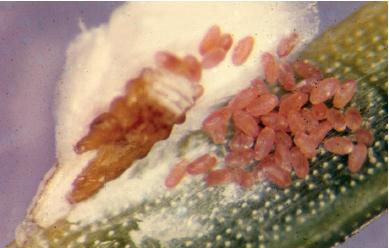
E. Pine needle scale exposed while producing eggs. WHITNEY CRANSHAW

F. Pine needle scale crawlers. DAVID SHETLAR

G. Pineleaf scale. J. A. DAVIDSON, UNIVERSITY OF MARYLAND, BUGWOOD. ORG

H. Pine needle scale nymphs. WHITNEY CRANSHAW

I. Recently settled nymphs of black pineleaf scale. DONALD OWEN, CALIFORNIA DEPARTMENT OF FORESTRY AND FIRE PROTECTION, BUGWOOD.ORG

J. Black pineleaf scale. WHITNEY CRANSHAW
JUNIPER SCALE (Carulaspis juniperi)1 has females that are round but commonly distorted by the rough surface of juniper leaves. The tiny males are elongate drop-shaped. They are bright white with yellow exuviae (central on the female and on one end on the male), but can become gray over time. Heavy infestations can completely cover juniper foliage, causing severe dieback, even whole plant death.
HEMLOCK SCALE (Abgrallaspis ithacae)1 females form small, circular to oval-shaped, gray shells on the needles of hemlock, pine, fir, and Douglas-fir. It is most common in the native range of eastern hemlock. It produces two generations per year with second-stage nymphs overwintering. ELONGATE HEMLOCK SCALE (Fiorinia externa)1 can often be found alongside the hemlock scale, but the cover of F. externa is parallel-sided and three times the length of the hemlock scale. This is a non-native species that caused severe dieback of hemlock until parasitic wasps and a lady beetle were introduced that effectively achieved biological control. TEA SCALE (Fiorinia theae) is an imported pest that commonly attacks holly and camellias. The female covers are brown to black and spear-shaped, while males appear as clusters of tiny white exclamation marks. Heavy infestations cause yellowing of leaves and early drop.
OLEANDER SCALE (Aspidiotus nerii)1 feeds on plants in more than 100 families, but oleander is a particularly common host in the U.S. Mature female scales are circular and range from white to light brown in color. Males, if they occur, are only about half the size of females, but many parthenogenetic strains (absent males) are known. Increasing evidence suggests that A. nerii is a species complex, with differences in host range and reproduction. CRYPTOMERIA SCALE (A. cryptomeriae) is a common insect on true firs (Abies spp.) and hemlock in the eastern states.
FLORIDA RED SCALE (Chrysomphalus aonidum)1 is a common pest of evergreen holly, especially in southern states. It can also infest many other plants, especially citrus, where it can cause leaf drop and early fruit drop. The scale is fairly small, round, and dark reddish brown in color.
EUONYMUS SCALE (Unaspis euonymi)1 is a common species on evergreen shrub types of euonymus and can occur on a few other shrubs. Females are brown and somewhat oystershell-shaped and males are fuzzy white and elongated. Females develop primarily on twigs, but some females and the more conspicuous white-colored males occur on leaves.
SWEETGUM SCALE (Diaspidiotus liquidambaris)1 is usually first noticed when yellow, red, or dark green spots appear on sweetgum leaves. This scale has a leaf-feeding phase in which first instars settle on the undersurface of leaves. Their feeding activity causes the leaf to swell around the scale, which then develops in a pitlike cavity. Upon maturing, the second-stage scale moves back to the stems to finish development. This scale has two generations per year with unmated females and males overwintering on stems and branches.

Sweetgum scales on sycamore leaf. DAVID SHETLAR

B. Hemlock scale. PENNSYLVANIA DEPARTMENT OF CONSERVATION AND NATURAL RESOURCES-FORESTRY, BUGWOOD.ORG

C. Elongate hemlock scale. ERIC R. DAY, VIRGINIA POLYTECHNIC INSTITUTE AND STATE UNIVERSITY, BUGWOOD.ORG

D. Oleander scale. CHARLES OLSEN, USDA APHIS PPQ, BUGWOOD.ORG

E. Florida red scale. LORRAINE GRANEY, BARTLETT TREE CARE, BUGWOOD.ORG

F. Tea scale. CHAZZ HESSELEIN, ALABAMA COOPERATIVE EXTENSION SERVICE, BUGWOOD.ORG

G. Cryptomeria scale. LORRAINE GRANEY, BARTLETT TREE CARE, BUGWOOD.ORG

H. Euonymus scale. DAVID SHETLAR

I. Sweetgum scale close-up. DAVID SHETLAR

J. Euonymus scale leaf injury symptoms. DAVID SHETLAR
CACTUS SCALE (Diaspis echinocacti) occurs on a wide variety of cacti and is the most common armored scale on these plants. Under suitable temperature conditions, a generation can be completed within a month, with adult females laying eggs over a 1–2-month period. BOISDUVAL SCALE (Diaspis boisduvalii) is a general feeder of many tropical plants but primarily a pest of orchids, yucca, and palms.

Cactus scale. DAVID SHETLAR
There are several Lepidosaphes species associated primarily with foliage. PURPLE SCALE (L. beckii) is very similar in appearance to oystershell scale but associated with citrus in parts of California and Florida. Three generations per year commonly occur in Florida. CAMELLIA SCALE (L. camelliae), widely distributed in the eastern half of the U.S., is associated with camellia, holly (particularly Burford holly), privet, Cleyera, Ternstroemia, and Rhaphiolepsis. As many as 4–5 generations are reported in Georgia. WINGED EUONYMUS SCALE (L. yanangicola) develops damaging populations on winged euonymus in some mid-Atlantic and midwestern states. It is commonly mistaken as small oystershell scales. MASKELL SCALE (L. pallida) develops on Cryptomeria and Taxus. It is part of a species complex that includes PINE OYSTERSHELL SCALE (L. pini) on Pinus thunbergii and P. densiflora, and UMBRELLA PINE SCALE (L. sciadopitysi) on Sciadopitys. Two generations are commonly produced by Maskell scale, with crawlers appearing in June and August.
FERN SCALE (Pinnaspis aspidistrae) is pear- to oystershell-shaped and usually light brown in color. This is a tropical species that can occur on ferns grown inside. The scales settle on leaves, stems, and bark.
Aulacaspis yasumatsui,1 known variously as the “Asian cycad scale,” “cycad aulacaspis scale,” or “sago scale,” is an insect first discovered in the U.S. in 1997 that has proved devastating to king and queen sago cycads. Large populations build up quickly on foliage, with generation times of about 1 month, and heavily infested plants can be killed. Control is complicated by the occurrence of many individual scales on belowground parts of the plant. Originally found in Florida, it is now also known from Texas. Natural enemies of this insect were collected from areas where it is native (Southeast Asia), and efforts to establish them appear to have successfully improved biological control of this insect.

Asian cycad scale. DAVID SHETLAR
PINYON NEEDLE SCALE (Matsucoccus acalyptus)2 develops on pinyon in the southwestern U.S., occasionally producing outbreaks that cause foliage yellowing and premature needle drop. The most conspicuous stage of this insect are the small, black, second-instar nymphs, known as the “bean stage,” attached to needles through winter into early spring. Later stages migrate to trunks in spring and lay eggs. A life cycle that also involves alternation between leaves and stems occurs with SYCAMORE SCALE (Stomacoccus platani).1 Sycamore scale is present in the southwestern U.S. and can produce 3–5 generations in a year.
1 Hemiptera: Diaspididae
2 Hemiptera: Margarodidae
A. Maskells scale. LORRAINE GRANEY, BARTLETT TREE CARE, BUGWOOD.ORG

B. Pine oystershell scale. LORRAINE GRANEY, BARTLETT TREE CARE, BUGWOOD.ORG

C. Camellia scale. ERIC R. DAY, VIRGINIA POLYTECHNIC INSTITUTE AND STATE UNIVERSITY, BUGWOOD.ORG

D. Fern scale. J. A. DAVIDSON, UNIVERSITY OF MARYLAND, BUGWOOD.ORG

E. Bermudagrass scale. DAVID SHETLAR

F. Heavy infestation of Asian cycad scale. DOUG CALDWELL, UNIVERSITY OF FLORIDA

G. Pinyon needle scale. WHITNEY CRANSHAW

H. Sycamore scale crawler. LORRAINE GRANEY, BARTLETT TREE CARE, BUGWOOD.ORG
Psyllids are small insects, about 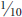 –
–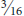 inch in length when full grown. Adults somewhat resemble miniature cicadas and are often referred to as jumping plant lice because of their ability to jump. Nymphs are flattened and often scalelike in appearance, found attached usually to the underside of leaves or within leaf pockets or folds. Taxonomically, psyllids are considered a superfamily (Psylloidea) comprising three families: Psyllidae, Triozidae, and Calophyidae.
inch in length when full grown. Adults somewhat resemble miniature cicadas and are often referred to as jumping plant lice because of their ability to jump. Nymphs are flattened and often scalelike in appearance, found attached usually to the underside of leaves or within leaf pockets or folds. Taxonomically, psyllids are considered a superfamily (Psylloidea) comprising three families: Psyllidae, Triozidae, and Calophyidae.
Psyllids tend to be quite specific in their hosts, usually restricting their feeding to a single genus or family of plants, and some types of plants (e.g., willows, sumac) support many psyllid species. The overwhelming majority of the 300-plus North American species develop on dicots.
Many psyllid species cause little, if any, plant damage and have habits that rarely attract attention. Others can cause significant plant injury, not only from removal of plant fluids during feeding, but also through effects induced by their introduced saliva. Distortions of leaf growth and production of pits or swellings on leaves are among the symptoms caused by some psyllids. A few have saliva that is systemically toxic to the plant and can produce a wide range of disorders, and some psyllids are important as vectors of phytoplasmas that can cause serious plant diseases.
Many psyllids excrete materials as they feed that are quite distinctive and can be useful in diagnosis. Many excrete honeydew, which may crystalize over and cover the developing nymphs, but others excrete distinctive waxy threads or wax-covered pellets. Note: Many psyllids produce leaf galls in the form of pits or lumps on leaves. These are discussed later in this chapter on page 326.
POTATO/TOMATO PSYLLID (Bactericera cockerelli)1
HOSTS Many solanaceous plants can host potato/tomato psyllid, including eggplant, pepper, tomatillo, Lycium spp., and some other nightshade family plants. Only tomato and potato appear to sustain significant injury as a result of feeding by the insect.
DAMAGE Saliva injected during feeding causes various disruptions of plant growth, collectively described as PSYLLID YELLOWS. Slowed plant growth, leaf curling, and color changes are common results. Effects on potato tubers include reduced size, premature sprouting, and rough skin. Tomatoes damaged by this species produce small fruits that are soft and of poor quality.
In some areas, particularly in Texas, southern California, and much of Mexico, the potato psyllid is also important as a vector of the phytoplasma, Candidatus Liberibacter solanacearum. This can produce a disease known as ZEBRA CHIP that causes dark streaking in potato tubers, particularly after they are cooked. This pathogen can also damage tomato and pepper.
DISTRIBUTION Texas, southern California, and areas of northern Mexico are most commonly infested during cool-season months. Dispersal through the High Plains and Rocky Mountain regions occurs during late spring and early summer.
APPEARANCE Mature adults are generally dark gray or black with distinct white bands and markings. These markings take a few days to develop, so newly emerged adults are pale-colored. Nymphs are flattened and have a series of minute waxy projections surrounding the body. Young nymphs are pale brown or tan. Older nymphs become increasingly greenish and develop noticeable wing pads. Eggs are minute but laid on a characteristic small stalk.
LIFE HISTORY AND HABITS Potato/tomato psyllid is a migratory insect wintering in the extreme southwestern U.S. and Mexico. It annually migrates northward in late spring, when temperatures begin to get hot in the overwintering breeding areas. Females lay small yellow-orange eggs in small groups, usually on the underside of leaves. The nymphs are flattened and somewhat scalelike. Nymphs rarely move and tend to concentrate on the underside of leaves in more shaded areas of the plant.

B. Tomato/potato psyllid nymphs. WHITNEY CRANSHAW

C. Tomato/potato psyllid adult laying eggs. WHITNEY CRANSHAW

D. Mixed stages of tomato/potato psyllid adult on tomato leaf. WHITNEY CRANSHAW

E. “Psyllid sugar” excrement produced by tomato/potato psyllid adult. WHITNEY CRANSHAW

F. “Psyllid yellows” foliar color change of potato produced by tomato/potato psyllid feeding. WHITNEY CRANSHAW

G. Premature tuber sprouting associated with tomato/potato psyllid injury. WHITNEY CRANSHAW

H. Dull fruit color symptomatic of tomato injury by tomato/potato psyllid. WHITNEY CRANSHAW
After 3–4 weeks the adults emerge and repeat the cycle. During a season, 3–4 generations may be completed in a region. A reverse migration to southern areas is assumed to occur in early fall. Potato/tomato psyllid may breed continuously in greenhouses and has developed as a pest of greenhouse tomatoes.
PEAR PSYLLA (Cacopsylla pyricola)2

Eggs of pear psylla on twigs. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY
HOSTS Restricted to fruit-producing pear. Species of ornamental pear do not appear to be susceptible to this insect.
DAMAGE Pear psylla feeds on the leaves of pear trees, excreting large amounts of honeydew that cover leaves and fruit and allow growth of sooty molds. High numbers of psyllids on trees can reduce plant vigor, cause necrotic spotting of leaves, and even induce a condition known as PSYLLA SHOCK, resulting in leaf drop and suppressed growth from which it may take a plant several years to recover. In some areas pear psylla is also important in the spread of the phytoplasma (Candidatus Phytoplasma pyri) that produces PEAR DECLINE DISEASE.
DISTRIBUTION Of European origin, pear psylla can be found wherever pear is grown. It is particularly damaging in the Pacific States and in the Northeast.
APPEARANCE Adults are dark reddish brown with clear wings. Young nymphs are pale yellowish but develop dark markings and wing pads as they get older. Nymphs are often covered with droplets of honeydew.
LIFE HISTORY AND HABITS Pear psylla adults overwinter in protected areas (under bark, plant debris on soil, or other cover) in the vicinity of previously infested trees. It becomes active in late winter or early spring and moves to pear trees, laying yellow-orange eggs as pear buds begin to swell. The emerging nymphs then move to feed on the tender new growth.
As they feed, young pear psylla nymphs become covered with the honeydew droplets they excrete. During the final nymphal stage, conspicuous wing pads develop and the nymphs leave the honeydew droplet, subsequently molting to the adult stage. Later generations lay eggs on the new leaves, often concentrating on sucker sprouts late in the season. Two to three generations are normally produced in a season. At the end of the year, dark-colored winter adult forms move to shelter.
OTHER PSYLLIDS
Perhaps the most important Cacopsylla species, aside from pear psylla, is BOXWOOD PSYLLID (C. buxi).1 Common in the eastern and northwestern states, this species can produce conspicuous leaf-cupping distortion to American boxwood. Nymphs cover themselves with long threads of wax as they feed on the newly developing leaves in spring. One generation occurs per year, with adults laying overwintering eggs around bud scales in early summer. Adults occasionally settle on skin of humans when infested plants are disturbed; they can produce a bite that may be felt as a small prick but is not painful.
Approximately 60 Cacopsylla species occur in North America, about half of which occur on willow. Among the others that may occasionally be noticed by gardeners include APPLE SUCKER PSYLLID (C. mali), which feeds primarily on sprouts of fruit trees; BOXELDER PSYLLID (C. negundinis), which may produce modest leaf distortion and noticeable honeydew; and SUMAC PSYLLID (C. triozimima), which can be abundant on Rhus. Cacopsylla tobirae is a non-native species associated with Japanese pittosporum (Pittosporum tobira). Feeding can induce a tight curling of the new growth and they excrete honeydew and produce conspicuous threads of wax. This psyllid has recently been found in California and its occurrence reported in North Carolina.

B. Pear psylla nymphs in honeydew droplets. WHITNEY CRANSHAW

C. Upper leaf symptom produced by pear psylla feeding. WHITNEY CRANSHAW

D. Boxwood psyllid nymphs and associated wax thread excrement. JOHN CAPINERA, UNIVERSITY OF FLORIDA

E. Boxwood psyllid adult. DAVID SHETLAR

F. Boxelder psyllids. WHITNEY CRANSHAW

G. Leaf cupping produced in response to boxwood psyllid feeding. WHITNEY CRANSHAW

H. Sumac psyllid adults. WHITNEY CRANSHAW
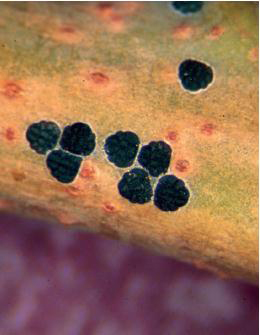
I. Overwintering nymphs of sumac psyllid. WHITNEY CRANSHAW
ASIAN CITRUS PSYLLID, Diaphorina citri,2 is an insect recently introduced into North America that has high potential to devastate the citrus industry. The insects feed on the stems and young leaves, and the feeding injuries result in some cupping distortion of new growth and dieback of new shoots. The importance of Asian citrus psyllid can be enormously increased, however, by its ability to vector the pathogen that produces the huanglongbing (HLB) disease, also known as citrus greening. Since its first detection in southeast Florida in 1998, Asian citrus psyllid has spread to infest all of Florida and some areas of all states where citrus is grown.
The pathogen that produces citrus greening is a type of bacterium (phytoplasma), Candidatus Liberibacter asiaticus. It is introduced into plants during feeding by Asian citrus psyllids that previously fed on an infected tree. Once introduced, the bacteria grow in the phloem of the trees and produces a progressive, irreversible disease that is usually fatal in about 5 years. Early symptoms include a blotchiness of leaves, followed by increasing foliage yellowing. Fruit production declines and individual fruits decline in quality, become misshapen, smaller, and show incomplete ripening. Citrus greening is now found throughout the citrus-producing areas of Florida and was more recently discovered in Los Angeles. The disease is also present in Mexico and progressing northward with the movement of infected psyllids.
The Asian citrus psyllid is relatively large (ca. ⅙ inch), mottled brown, and perhaps most recognizable by the way the adult insects position themselves on leaves, tipped at a 45° angle. Eggs are laid on the tips of shoots and the nymphs feed on young shoots and leaves. As many as 8–10 generations can be produced, although development slows during winter, as nymphs can develop only on newly produced growth.
Several non-native psyllids associated with eucalyptus have been accidentally introduced and become established in parts of North America, particularly California. REDGUM LERP PSYLLID (Glycaspis brimblecomei)2 spread quickly after it became established in California in the late 1990s and was later discovered to be established in Florida. In addition to causing feeding injuries and nuisance problems with excreted honeydew, it forms a conspicuous, conical wax cap (lerp) over the nymphs. Eucalyptus globulus is most commonly infested. BLUEGUM PSYLLID (Ctenarytaina eucalptyi)2 is also a recent introduction and has developed into an important pest of Eucalyptus pulverulenta grown to provide foliage for flower arrangements. Blastopsylla occidentalis1 occurs on eucalyptus in Florida. LEMONGUM PSYLLID (Cryptoneossa triangula)2 and SPOTTED GUM PSYLLID (Eucalyptolyma maidenii)2 are minor pests on lemon-scented gum (Eucalyptus citriodora) and spotted gum (Eucalyptus maculata) in California.
ACACIA PSYLLID (Acizzia uncatoides)2 is a common species in California, associated with acacia and silk tree, sometimes occurring in nuisance or even damaging numbers. The nymphs of RUDBECKIA LEAFSPOT PSYLLID (Bactericera antennata)2 produce a purplish blotch on the leaves of black-eyed Susan and other Rudbeckia species in the Midwest. More recently it has also been reported to feed on hibiscus. Adults have pink to purple bodies with clear wings, and adults seem not to cause noticeable damage.
COTTONY ASH PSYLLID (Psyllopsis discrepans)2 can cause severe leaf distortion of black and Manchurian ash. It is a European species that appears to have spread widely across Canada since its North American detection in Nova Scotia and is presently most damaging in the Plains provinces and Dakotas. A related species, P. fraxinicola, has recently become established in Oregon, where it develops on European and narrow-leaved ash.
1 Hemiptera: Triozidae
2 Hemiptera: Psyllidae
3 Hemiptera: Calophyidae
A. Adult citrus psyllid. DAVID HALL, USDA AGRICULTURAL RESEARCH SERVICE, BUGWOOD.ORG

B. Life stages of the Asian citrus psyllid. DAVID HALL, USDA AGRICULTURAL RESEARCH SERVICE, BUGWOOD.ORG

C. Asian citrus psyllids infesting terminal growth. JEFFREY W. LOTZ, FLORIDA DEPARTMENT OF AGRICULTURE AND CONSUMER SERVICES, BUGWOOD.ORG

D. Leaf yellowing and irregular fruit ripening associated with Asian citrus psyllid. JEFFREY W. LOTZ, FLORIDA DEPARTMENT OF AGRICULTURE AND CONSUMER SERVICES, BUGWOOD.ORG

E. Bluegum psyllid colony. ROBIN ROSETTA, OREGON STATE UNIVERSITY

F. Redgum lerp psyllid. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

G. Acacia psyllids. DAVID SHETLAR

H. Leaf spotting produced by rudbeckia psyllid. DAVID SHETLAR

I. Rudbeckia psyllid adults. DAVID SHETLAR

J. Rudbeckia psyllid nymph. DAVID SHETLAR
Leafhoppers1 are small insects, typically about ⅛–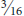 inch in length, elongate and somewhat wedge-shaped, and tapering at the end. Eggs are inserted as small groups in plant tissues, usually in leaf veins or small twigs. The nymphs are active insects, usually moving readily when disturbed, often with a side-to-side (crablike) walk. Adults are winged and fly readily, leaping from plants with their enlarged hind legs.
inch in length, elongate and somewhat wedge-shaped, and tapering at the end. Eggs are inserted as small groups in plant tissues, usually in leaf veins or small twigs. The nymphs are active insects, usually moving readily when disturbed, often with a side-to-side (crablike) walk. Adults are winged and fly readily, leaping from plants with their enlarged hind legs.
More than 2,500 species of leafhoppers are found in North America, and some may be associated with almost any plant except those with thick, waxy foliage. All suck plant fluids with stylet mouthparts from leaves and, less commonly, succulent stems. Most species feed on the phloem of plants, removing only modest amounts of sap and excreting some honeydew. Other leafhoppers restrict their feeding to the parenchyma cells of the leaf mesophyll layer, producing white flecking wounds. These insects often excrete dark spots in the manner characteristic of other mesophyll-feeding insects, such as lace bugs and some thrips. Some members of the genus Empoasca can injure the vascular system of plants, producing a condition known as hopperburn. One group of leafhoppers known as the “sharpshooters” feed on the xylem fluids; they tend to be more elongated than other leafhoppers, with an elongated head. They get their name from the habit of some species of flicking small droplets during excretion, which may be detectable as fine spray.
Some leafhoppers are important because of their ability to transmit plant pathogens that cause plant diseases. These include viruses (curly top), phytoplasmas (aster yellows, ash yellows, elm phloem necrosis), and the xylem-limited bacteria (bacterial leaf scorch, Pierce’s disease of grape).
POTATO LEAFHOPPER (Empoasca fabae)1
HOSTS A wide variety of plants, particularly legumes such as bean and alfalfa. Potato, raspberry, wisteria, and several trees, including maple, birch, and apple, are common hosts.
DAMAGE Destruction of cells during feeding and injection of saliva toxic to plants disrupt the phloem and sap flow of plants. Photosynthesis is reduced and foliage discoloration is common, typically a yellowing. Symptoms are usually first evident as leaf tip wilting that progresses backward as tissues die, resulting in a condition known as hopperburn. Severe leaf injury and even premature plant death are common on potato; more subtle leaf discoloration and curling are more characteristic on bean. On trees, reductions in shoot elongation result in stunting and close-spaced leaves
DISTRIBUTION Potato leafhopper is restricted to eastern North America, only infrequently being found as far west as Wyoming and Colorado.
APPEARANCE Pale green, about ⅛ inch, elongate, and gradually tapering to the hind end. Some pale spotting behind the head may be observed on close observation. The nymphs are lighter colored and highly active. They are found on leaf undersides and readily move sideways when disturbed.
LIFE HISTORY AND HABITS Winter is spent in areas around the Gulf Coast, where potato leafhoppers feed in alfalfa and various weeds. A northward migration occurs annually, and often suddenly, in May or early June. Alfalfa and bean are often early season hosts, with potato increasingly favored later in the summer. Potato leafhopper may breed year-round in southern parts of its range but dies out in the north during winter.
Females insert eggs into veins on the underside of the leaf. The young nymphs are pale green and gradually darken with age, molting four times over the course of 2–3 weeks before reaching the adult form. Three to five generations per year are typically produced in the midwestern states. This pest can suddenly appear in trees and shrubs following the harvesting of alfalfa, a favored host plant.

B. Sharpshooter leafhopper excreting watery droplet. WHITNEY CRANSHAW

C. Leaf spotting (stippling) produced by leafhopper that feeds on mesophyll. WHITNEY CRANSHAW

D. Potato leafhopper adult. JIM KALISCH, UNIVERSITY OF NEBRASKA

E. Potato leafhopper on wisteria. DAVID SHETLAR

F. Potato leafhopper nymph. DAVID SHETLAR

G. Leaf symptom associated with hopperburn injury by potato leafhopper. JIM KALISCH, UNIVERSITY OF NEBRASKA

H. Potato field killed prematurely by potato leafhopper. TED RADCLIFFE, UNIVERSITY OF MINNESOTA

I. Hopperburn injury to maple. DAVID SHETLAR
Several other less commonly damaging species of Empoasca leafhoppers, sometimes described as garden leafhoppers, occur throughout North America. All are similar in general appearance—small green leafhoppers—to potato leafhopper and can be separated only by experts. Hopperburn-type symptoms caused by E. recurvata have been observed on winter squash in Colorado. SOUTHERN GARDEN LEAFHOPPER (E. solana) apparently may also produce mild hopperburn symptoms on potato, bean, and lettuce in southern states.
Other Empoasca species are not phloem feeders, however, and feed primarily on the mesophyll, producing white flecking (stippling) injuries to foliage. WESTERN POTATO LEAFHOPPER (E. abrupta) and INTERMOUNTAIN LEAFHOPPER (E. filamenta) are two western species that apparently produce white flecking injuries to potato but do not induce hopperburn. White flecking of foliage is produced on apple, hawthorn, pear, and some stone fruits by APPLE LEAFHOPPER (E. maligna).
ROSE LEAFHOPPER (Edwardsiana rosae)1
HOSTS Rosa species and Rubus species are overwintering hosts. Dogwood, oak, elm, hawthorn, apple, poplar, maple, and oak are among the summer hosts.
DAMAGE Rose leafhoppers feed on the sap of mesophyll and produce white flecking wounds (stippling) on foliage. Damage occurs early in the season, after which time the insects disperse to summer hosts. Eggs are inserted into canes, and occasionally these wounds serve as entry courts for pathogens.
DISTRIBUTION Throughout North America in association with rose and bramble hosts.
APPEARANCE Rose leafhopper is pale yellow to creamy white in both the adult and nymphal stages. Early-stage nymphs have reddish eyes, but those of older nymphs and adults are white. Dark spotting on the nymphs can distinguish them from white apple leafhopper. One behavioral feature of the pale yellow nymphs is that, unlike most leafhoppers, they cannot move sideways.
LIFE HISTORY AND HABITS Eggs survive winter inserted into stems of rose canes, and pimple-like swellings develop where eggs are laid. At egg hatch in early spring, nymphs move to foliage and feed on the leaf underside. All nymphal stages can usually be completed in 2–3 weeks. Individuals may remain on a single leaf the entire time, but rose leafhopper nymphs are quite active and readily move forward (but not sideways) when disturbed. The winged adults usually disperse to alternate summer host plants, returning to rose in late summer for overwintering egg-laying. Eggs during the summer generations are laid into large veins and petioles of leaves. In addition to the first generation on rose, two additional generations are produced during the summer. In the Pacific Northwest, peak numbers of nymphs are usually observed in early June, late July/early August, and mid-September.

B. Hopperburn injury to pumpkin. WHITNEY CRANSHAW

C. Western potato leafhopper. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

D. Southern garden leafhoppers. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

E. Stippling injury produced by western potato leafhopper. WHITNEY CRANSHAW

F. Rose leafhopper adult. DAVID SHETLAR

G. Rose leafhopper nymph. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

H. Leaf symptoms produced by rose leafhopper feeding. WHITNEY CRANSHAW
OTHER MESOPHYLL-FEEDING LEAFHOPPERS
Small white flecking wounds in leaves (stippling) are a characteristic symptom produced by leafhoppers that feed on the parenchyma cells of the mesophyll, removing cell contents. Edwardsiana commisuralis1 is a common western species that produces injuries similar to rose leafhopper on dogwood and alder. E. hippocastani occurs on elm and E. australis on mountain-ash.
WHITE APPLE LEAFHOPPER (Typhlocyba pomaria) is physically almost identical to rose leafhopper. It produces wounds around feeding sites that appear as whitish flecking (stippling) on leaves of apple, cherry, peach, prune, and hawthorn. Winter is spent as eggs inserted under the bark of twigs and small branches of these hosts. Nymphs are uniformly colored translucent white, occasionally yellow. Two generations typically are produced, but they overlap considerably and adults may be present from late May through October. White apple leafhopper is a major pest of apple orchards in much of North America, damaging plants by removing chlorophyll from leaves as it feeds and by excreting small dark droplets that may spot fruit.
Erythroneura leafhoppers also produce white flecking wounds. Nymphs are cream-colored or slightly yellow and have some spotting. WESTERN GRAPE LEAFHOPPER (E. vulnerata)1 is an important pest of grapes grown in the western states. Winter is spent in the adult stage under sheltering debris in the vicinity of previously infested plants. The adults emerge when spring temperatures reach the mid-60s and fly to the vines to feed shortly after the new growth emerges. After several weeks, the females begin egg-laying, inserting the egg just underneath the leaf surface; this appears as a small bubble when closely examined. Eggs hatch in 1-2 weeks, and the nymphs feed on the mesophyll of cells on the lower leaf surface. They become full grown in approximately 3 weeks. Depending on location, three to five generations may be produced annually and may overlap and be present on plants continuously, as long as foliage remains. In fall, the adults disperse to cover for wintering.
Related species have similar habits. Grape also hosts VARIEGATED LEAFHOPPER (Erythroneura variabilis) in southern California and EASTERN GRAPE LEAFHOPPER (E. comes) in eastern states. VIRGINIA CREEPER (ZICZAC) LEAFHOPPER (E. ziczac) is common in the High Plains and Rocky Mountain region where it develops on grape, Virginia creeper, elm, and Boston ivy. THREEBANDED LEAFHOPPER (E. tricincta) may occur on grape, Virginia creeper, and apple in the east. E. gleditsia feeds on black locust, Aesculus species, and hawthorn, and E. lawsoniana feeds on sycamore.
MAPLE LEAFHOPPER (Alebra albostriella)1 produces stippling injuries on leaves of several trees and shrubs, including various maples, American elm, basswood, oak, beech, hickory, hawthorn, and sumac. It produces one generation per year, with peak populations observed in late spring in the East and in midsummer in central California. It overwinters as eggs inserted into twigs.
Feeding by Paraphlepsius strobi1 produces purplish spotting on beets and lambsquarter.

B. White apple leafhopper nymphs. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

C. Injury produced by white apple leafhopper. WHITNEY CRANSHAW

D. Variegated leafhopper, adult. HAROLD LARSEN, COLORADO STATE UNIVERSITY

E. Variegated leafhopper, nymphs. HAROLD LARSEN, COLORADO STATE UNIVERSITY

F. Western grape leafhopper. TOM MURRAY

G. Zic-zac leafhoppers. HAROLD LARSEN, COLORADO STATE UNIVERSITY

H. Leafhopper damage to grape. WHITNEY CRANSHAW

I. Zic-zac leafhopper damage to Virginia creeper. WHITNEY CRANSHAW

J. Injury produced by maple leafhopper. WHITNEY CRANSHAW

K. Leaf spot injury to beet produced by Paraphlepsius strobi. WHITNEY CRANSHAW
ASTER (OR SIXSPOTTED) LEAFHOPPER (Macrosteles quadrilineatus)1
HOSTS An extremely wide range of plants, including small grains and turfgrasses, many vegetables, and flower crops.
DAMAGE Direct feeding effects are insignificant, producing minor spotting at most; however, aster leafhopper can transmit the phytoplasma (Candidatus Phytoplasma asteris) that produces the eastern strain of aster yellows disease. Aster yellows is damaging to head lettuce, carrot, celery, cosmos, marigold, aster, and many other garden plants.
DISTRIBUTION Aster leafhopper can be found throughout most of North America but is most common and important in the Midwest, northeastern U.S., and eastern Canada.
APPEARANCE Overall color of adults is usually grayish green. The presence of three pairs of dark markings on the head is the most distinguishing feature of this insect in separating it from other common leafhoppers.
LIFE HISTORY AND HABITS Aster leafhopper survives poorly in areas of harsh winter temperatures but may survive as eggs as far north as New England and Manitoba if conditions are suitable. The primary wintering areas are along the Gulf of Mexico and the southern Great Plains. Winged adults are highly migratory, and large annual flights allow them to colonize a wide area each growing season, often moving on favorable winds in late May and early June. Spring grains are important hosts used after migrations, and populations may increase rapidly.
Eggs are laid in leaves and stems of plants and hatch in about a week. Nymphs pass through four instars during 4–6 weeks. Adults can live for months. Two or four generations are typically produced during the growing season.
Leafhoppers that feed on plants infected with aster yellows acquire the causal phytoplasma but cannot transmit the pathogen to new plants until the phytoplasma has circulated in the leafhopper and moved to its salivary glands, a period of between 10 days to 3 weeks. Typically, somewhere between 1 and 5% of all leafhoppers carry the aster yellows phytoplasma in the Midwest during the growing season.
Several other leafhoppers are involved in transmitting the phytoplasma strains that produce aster yellows. In the far west and southeastern U.S., Scaphytopius irroratus1 is important in transmitting the western strain of this pathogen. Scaphytopius acutus is a vector involved in transmission in the Northeast. Other vectors include Ceratagallia abrupta,1 MOUNTAIN LEAFHOPPER (Colladonus montanus),1 and Fieberiella florii.1 The latter two species are also vectors of the phytoplasma strains that produce WESTERN X-DISEASE in stone fruits.
MISCELLANEOUS LEAFHOPPERS THAT FEED ON PHLOEM
BEET LEAFHOPPER, Circulifer (= Neoaliturus) tenellus,1 is the vector of the virus that causes beet curly top. This produces disease in several plants, notably pepper, tomato, beet, and bean. Damage by beet leafhopper feeding in the absence of the virus is insignificant. Beet leafhopper is originally of European origin but has become widely established in the western U.S., particularly the southwest.
HONEYLOCUST LEAFHOPPER (Macropsis fumipennis)1 is common on honeylocust and many other shade trees in much of North America. Feeding produces a slight flecking of the foliage, and the insect is a minor honeydew producer. The bright green nymphs are commonly confused with those of honeylocust plant bug, and early in the season both are present on foliage. At least two generations of honeylocust leafhopper are produced annually. Macropsis ocellata is common on willow in the northeastern quadrant states, and M. graminea occurs on Populus species in the northern U.S. and Canada. PLUM LEAFHOPPER (M. trimaculata) develops on stone fruits and is known to vector peach yellow leaf curl virus.

B. Hairy roots and witches’-broom symptomatic of aster yellows infection of carrot. WHITNEY CRANSHAW

C. Aster yellows symptoms in statice. WHITNEY CRANSHAW

D. Changes in flower development (phyllody) symptomatic of aster yellow infection. WHITNEY CRANSHAW

E. Fieberiella florii. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

F. Colladanus montanus. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

G. Beet leafhopper adult. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

H. Newly hatched beet leafhopper nymph and egg. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

I. Beet leafhopper nymph. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

J. Honeylocust leafhopper adult. WHITNEY CRANSHAW

K. Honeylocust leafhopper nymph. WHITNEY
WHITEBANDED ELM LEAFHOPPER (Scaphoideus luteolus)1 is important as the vector of the phytoplasma that produces elm phloem necrosis disease. The generally brown-colored nymphs are distinctive with a broad white band across the abdomen. One generation per year is produced, with winter spent as eggs inserted into twigs.
Leafhoppers in the subfamily Cicadellinae are known as “sharpshooters.” These leafhoppers are generally distinguishable by having an unusually enlarged head area, often pointed. This area contains the muscles that allow them to pull out fluids from the xylem and they, along with some spittlebugs, psyllids, and treehoppers, are among the few insects that can feed on xylem fluids. The origin of the name “sharpshooter” is questionable but often proposed to be related to the insects’ ability to forcefully flick droplets of excreted watery fluids away from their bodies.
Sharpshooters include some of the most brightly colored of the leafhoppers. Graphocephala coccinea,1 known variously as the “candy-striped leafhopper” or RED-BANDED LEAFHOPPER, is a gaudily colored species with alternating bands of magenta and green or blue. It can be common on many garden flowers and caneberries but causes little injury. Egg scars on leaves of woody plant hosts may appear as small blisters on shrubs on which nymphs develop (e.g., rhododendron, laurel, azalea). The related BLUE-GREEN SHARPSHOOTER, Hordnia (=Graphocephala) atropunctata,1 is more commonly associated with vines and woody plants. It is not damaging to plants on which it feeds, but it has some importance in coastal areas of California because of its ability to transmit the bacterium (Xylella fastidiosa) that produces PIERCE’S DISEASE OF GRAPE. More than a dozen other Graphocephala/Hordnia species occur in North America, many also brightly colored and patterned.
Pierce’s disease is the most important pest problem of the California grape industry. The bacterium grows in the xylem of the plant and produces a progressive decline of vines, ultimately killing the plants. Of greatest importance as a vector is the GLASSY-WINGED SHARPSHOOTER (Homalodisca vitripennis),1 a species that is highly mobile and can develop on many common host plants, wild and cultivated. Citrus is a particularly important host, but other common plants on which it breeds in California include bird of paradise, eucalyptus, euonymus, crape myrtle, pittosporum, sunflower, hibiscus, xylosma, and cottonwood. Several other sharpshooters can transmit X. fastidiosa but are much less important as vectors of the Pierce’s disease pathogen in California. These include the GREEN SHARPSHOOTER (Draeculacephala minerva)1 and the REDHEADED SHARPSHOOTER (Carneocephala fulgida),1 both of which breed on grasses.
Xylella fastidiosa produces diseases in several other plants. In California, important diseases produced include OLEANDER LEAF SCORCH, ALMOND LEAF SCORCH, and MULBERRY LEAF SCORCH. In the eastern U.S. diseases produced by X. fastidiosa include PHONY PEACH DISEASE, PLUM LEAF SCALD, and BACTERIAL LEAF SCORCH, which affects many common shade trees, including American elm, maple, oak, sycamore, sweetgum, and hackberry. The pathogen is also common on many other plants, including turfgrasses, but does not produce disease in many hosts. Vectors of X. fastidiosa in the eastern U.S. include several sharpshooter species, including Homalodisca insolita, Graphocephala spp., Draeculacephala spp., Oncometopia spp., and, in areas where it has spread, the glassy-winged sharpshooter.
Many leafhoppers are associated with lawns and turfgrasses. None appear to cause much more than minor spotting and are not seriously damaging except during seedling establishment. Common species include LAWN LEAFHOPPER (Deltocephalus hospes),1 GRAY LAWN LEAFHOPPER (Exitianus exitiosus),1 LESSER LAWN LEAFHOPPER (Graminella sonora),1 PAINTED LEAFHOPPER (Endria inimica),1 and CLOVER LEAFHOPPER (Ceratagallia sanguinolenta).1 Several species in the genera Deltoacephalus, Draeculcephala, Forcipata, Cuerna, Latalus, Polyamia, Dikraneura, and Balclutha may also be found on turf and ornamental grasses.
1 Hemiptera: Cicadellidae

B. Glassy-winged sharpshooter and eggs. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

C. Blue-green sharpshooters. DAVID SHETLAR

D. Glassy-winged sharpshooter and adult and nymphs. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

E. Redheaded sharpshooter and adult and nymphs. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

F. An Endria species of leafhopper. TOM MURRAY

G. Clover leafhopper. TOM MURRAY

H. A Deltocephala species of leafhopper. TOM MURRAY

I. A Draeculacephala species of leafhopper. DAVID SHETLAR
LEAFFOOTED BUGS ASSOCIATED WITH FOLIAGE
Leaffooted bugs (Coreidae) are moderate to large-sized insects, with a prominently projecting head possessing piercing-sucking mouthparts. Many have a pronounced flattening of the hind legs, from which the family name is derived. Most leaffooted bugs feed primarily on seeds and are discussed on page 602.
SQUASH BUG (Anasa tristus)1
HOSTS Winter types of squash, including pumpkin. Rarely, summer squash and melon are damaged.
DAMAGE Adults and nymphs suck sap from the stems and leaves, causing localized injuries that kill and collapse tissues. Initial symptoms are small yellow flecks on foliage that later turn brown. Later, foliage often wilts and dies beyond damaged areas. Feeding may occur on fruit, causing wounds that are readily colonized by rotting organisms. The wilting associated with squash bug is sometimes termed Anasa wilt of cucurbits.
More recently squash bugs have been found associated with a bacterium, Serratia marcescens, that can produce cucurbit yellow vine disease. The bacteria survive winter within squash bugs and are introduced into plants when the insects feed. Yellow vine disease often produces bright yellowing of foliage, as well as more general symptoms (stunting, wilting) that can be produced by fungal root rots and bacterial wilt, making diagnosis complicated. Yellow vine disease has been found over a wide area, encompassing much of the eastern half of the U.S.
DISTRIBUTION Squash bug is generally distributed throughout the U.S., excluding the Northwest, and southern Canada and extending south into Central America. This species is most common and damaging in southern areas.
APPEARANCE Adults are grayish brown and about 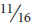 inch in length. They lay distinctive shiny coppery red masses of eggs, and the newly hatched nymphs are pale green. Older nymphs are gray and develop wing pads that become increasingly prominent.
inch in length. They lay distinctive shiny coppery red masses of eggs, and the newly hatched nymphs are pale green. Older nymphs are gray and develop wing pads that become increasingly prominent.
LIFE HISTORY AND HABITS Squash bugs spend winter in the adult stage in protected sites around previously infested plantings. They become active and first appear and feed in June, shortly after plant emergence. At this time they mate, and females lay masses of eggs on leaf undersides and occasionally stems. After hatching, the nymphs feed together in groups, usually on the shaded undersides of the plant.
Only one generation per year is produced in the northern range of squash bug, but 2 or even 3 generations may be present in more southerly areas. Adults that develop late in the season do not lay eggs and leave the field for overwintering shelter.
RELATED SPECIES
HORNED SQUASH BUG (Anasa armigera) occurs in the southeastern U.S. as far west as Texas and occasionally damages plants in a manner similar to squash bug. Life histories are apparently also similar, but the two species differ markedly in appearance. Horned squash bug nymphs are white until the fifth instar, later turning variegated brown and yellow. Adults have a broad thorax with sharp angles.
OPUNTIA BUG (Chelinidea vittiger)1 develops on the pads of opuntia cacti. Overwintered adults move to opuntia in late spring and lay eggs in small masses. Nymphs feed on the plants and in high numbers may cause wilting. Two generations are produced annually. On cholla cacti the leaffootted bug Narnia snowi1 is often common.
1 Hemiptera: Coreidae

B. Mating pair of squash bugs. WHITNEY CRANSHAW

C. Squash bug egg masses. WHITNEY CRANSHAW

D. Nymphs and associated injury by squash bugs. WHITNEY CRANSHAW

E. Squash bugs nymphs. WHITNEY CRANSHAW

F. Wilting of pumpkin due to squash bug feeding injury. WHITNEY CRANSHAW

G. Symptoms of cucurbit yellow vine disease. JOE JASINSKI, OHIO STATE UNIVERSITY EXTENSION, BUGWOOD.ORG

H. Horned squash bug. JOHN CAPINERA, UNIVERSITY OF FLORIDA

I. Opuntia bug adults. WHITNEY CRANSHAW

J. Opuntia bug nymphs. WHITNEY CRANSHAW
The plant bugs (Miridae) are a large family of moderate-sized (ca. ¼ inch) insects. The great majority feed on plant sap, but a few are predators of other insects or omnivores. When feeding on plants, plant bugs use their mouthparts in a fairly destructive manner, known as lacerate-flush, which involves physically breaking many cells and flushing the feeding wound with digestive enzymes. This produces localized areas of dead cells. Feeding on developing leaves, buds, and flowers is common among plant bugs, resulting in secondary symptoms such as distortions and abortion. Plant bugs damaging to fruit and flowers are covered on page 592.
FOURLINED PLANT BUG (Poecilocapsus lineatus)1
HOSTS Fourlined plant bug will feed on a very wide range of plants. Damage is most commonly found on perennials (especially those in the mint family or composites), but they commonly attack shrubs, including azalea, dogwood, forsythia, viburnum, and weigelia.
DAMAGE Discrete white or dark spots are originally produced at feeding sites. These damaged areas often become watery as cells die and collapse. Damage is concentrated on the younger tissues on upper areas of plants, and injuries to new growth may cause wilting or growth distortion.
DISTRIBUTION Northeastern quadrant of the U.S. and adjacent areas of southern Canada.
APPEARANCE Adults are yellow, sometimes green, with 4 dark stripes and have an overall length of ¼ to ⅓ inch. Immature forms are bright red-orange with black dots on the thorax. The characteristic striping becomes apparent in later stages.
LIFE HISTORY AND HABITS Winter is spent as eggs, laid in small clusters in slits of shoots. Nymphs hatch in mid- to late spring and develop in about a month. Adults usually appear in July and may be present for several weeks, mating and laying eggs. One generation is produced annually.
GARDEN FLEAHOPPER (Halticus bractatus)1
HOSTS A wide range of plants, with leaves of legumes preferred but also including garden vegetables such as pumpkin, squash, tomato, potato, beet, and pepper. This species is also found on many weeds.
DAMAGE Pale yellow spotting typically develops at feeding sites of garden leafhopper. Feeding can cause stunting and may kill seedlings. Dark fecal spots are produced that further detract from the appearance of infested plants.
DISTRIBUTION Eastern U.S., particularly southern areas.
APPEARANCE Adults are shiny black with yellowish legs and antennae. They are among the smallest of the plant bugs (ca.  inch) with females having rounded bodies and males being slender. The hind legs are greatly enlarged and allow adults to hop when disturbed, similar to flea beetles, for which they are often mistaken. Both short-winged and long-winged females may be present. Males always have long wings. The nymphs are pale green and darken with age.
inch) with females having rounded bodies and males being slender. The hind legs are greatly enlarged and allow adults to hop when disturbed, similar to flea beetles, for which they are often mistaken. Both short-winged and long-winged females may be present. Males always have long wings. The nymphs are pale green and darken with age.
LIFE HISTORY AND HABITS Garden fleahopper may winter as adults in extreme southern parts of its range but normally survives as eggs inserted into vegetation. Eggs hatch around April, and the nymphs develop over the course of a month. Numerous generations are produced (5 reported in Virginia) that overlap considerably by midseason.

B. Nymph of fourlined plant bug and associated injury. DAVID SHETLAR

C. Water soaked wounds produced by fourlined plant bug feeding on cucumber. WHITNEY CRANSHAW

D. Scabby callous growth in response to leaf-feeding injuries by fourlined plant bug. WHITNEY CRANSHAW

E. Fourlined plant bugs and associated injury to daisy. DAVID SHETLAR

F. Garden fleahopper, adult females. JIM KALISCH, UNIVERSITY OF NEBRASKA

G. Garden fleahopper, adult males. JIM KALISCH, UNIVERSITY OF NEBRASKA

H. Garden fleahopper, adults and nymphs. JIM KALISCH, UNIVERSITY OF NEBRASKA

I. Garden fleahopper injury symptoms on squash leaf. JIM KALISCH, UNIVERSITY OF NEBRASKA
HONEYLOCUST PLANT BUG (Blepharidopterus chlorionis)1 is a serious pest of honeylocust in much of the northern U.S. Nymphs feed on developing buds and leaves in May and June and often kill them. Older leaves may survive but show discoloration and deformation because of localized necrosis around feeding points. Heavy infestations may greatly retard foliage development in spring and have been associated with twig and branch dieback. One generation is produced annually, with overwintering eggs inserted into twigs in June and early July.
ASH PLANT BUG (Tropidosteptes amoenus)1 produces whitish flecking wounds on leaves of ash (particularly green ash) in much of the U.S. and southern Canada. Emerging leaves that are damaged show distortion. Dark tarry spots of excrement are present around feeding sites on the leaf underside. Adults are light brown, about ⅜ inch long, with heart-shaped markings on the plate behind the head (scutellum). The nymphs are more oval, shiny yellowish or reddish brown, and lack wings. Overwintering eggs are laid under loose bark, and nymphs usually hatch in late April and May. Most injury occurs when the nymphs approach maturity in late May. A second generation occurs in July and August and adults produced in this latter generation produce the overwintering eggs.
In eastern North America, Tropidosteptes brooksi may also cause spotting of ash leaves. This insect is greenish, and its life history is presumed similar to that of ash plant bug. WESTERN ASH PLANT BUG (T. pacificus) is the common species in the Pacific States. Adults are brown and the nymphs green with black spots. Tropidosteptes illitus is a yellow and brown or black species found in western states.
Plant bugs in the genus Plagiognathus1 cause leaf spotting, with damaged areas often dropping out. Close to 90 species occur in North America, and they have been collected on plants in more than 3 dozen families, but, with few exceptions, little is known about the plants on which they develop. Plagiognathus delicatus may co-occur with honeylocust plant bug on honeylocust. Plagiognathus punctatipes can be a common species associated with black walnut. SYCAMORE PLANT BUG (P. albatus) develops on leaves of sycamore and planetree. Spotting injuries it produces may look like anthracnose, a common fungal disease on these plants.
YUCCA PLANT BUG (Halticotoma valida)1 feeds on yucca foliage, producing white mottling marked with dark tarry spots of waste. It is found over the natural range of yucca and occurs in ornamental plantings as far north as Ohio and southern Pennsylvania. This is a small plant bug, about 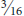 inch, with black wings and orange-red legs, that has 3–5 generations per year in the Atlantic States. PHLOX PLANT BUG (Lopidea davisi)1 feeds on leaves, buds, and flowers of phlox. White or yellow flecking injuries are typical on leaves. Flowers and seeds are also damaged. It is a colorful species with orange immature stages (nymphs); the winged adults have both black and orange coloration. Similar injuries can be produced on hollyhock by the HOLLYHOCK PLANT BUG (Brooksetta althaeae),1 a dark green plant bug.
inch, with black wings and orange-red legs, that has 3–5 generations per year in the Atlantic States. PHLOX PLANT BUG (Lopidea davisi)1 feeds on leaves, buds, and flowers of phlox. White or yellow flecking injuries are typical on leaves. Flowers and seeds are also damaged. It is a colorful species with orange immature stages (nymphs); the winged adults have both black and orange coloration. Similar injuries can be produced on hollyhock by the HOLLYHOCK PLANT BUG (Brooksetta althaeae),1 a dark green plant bug.
Leaves of royal palm in Florida now often show injuries associated with feeding by Xlastodoris luteolus,2 known as the “royal palm bug.” Leaf spotting on the underside of the leaf is a typical symptom, but in high populations large areas of leaves may show dark streaking.
ASH-GRAY LEAF BUGS3 (Parapiesma spp.) are inconspicuous insects that rarely attract the attention of a gardener but they can be common on pigweed as well as grain-producing amaranth and many related weeds common in gardens, including lambsquarters. They are gray or brown with reticulated wings held flat over the body, giving a resemblance to lace bugs. Feeding on foliage may produce minor spotting and distortion of young leaves, but seed feeding on Amaranthus has been shown to reduce yield.
1 Hemiptera: Miridae
2 Hemiptera: Thaumastocoridae
3 Hemiptera: Piesmatidae

B. Honeylocust plant bug adult. WHITNEY CRANSHAW

C. Honeylocust plant bug nymphs massed on twig. JIM KALISCH, UNIVERSITY OF NEBRASKA

D. Ash plant bug nymphs and injury. DAVID SHETLAR

E. Ash plant bug adult. DAVID SHETLAR

F. Walnut plant bug adult. WHITNEY CRANSHAW

G. Sycamore plant bug nymph. DAVID SHETLAR

H. Sycamore plant bug injury. DAVID SHETLAR

I. Royal palm bugs and associated injury. DOUG CALDWELL, UNIVERSITY OF FLORIDA

J. Royal palm bugs. LYLE J. BUSS, UNIVERSITY OF FLORIDA

K. Yucca plant bugs. DAVID SHETLAR

L. Ash-gray leaf bugs. DAVID SHETLAR
HAIRY CHINCH BUG (Blissus leucopterus hirtus)1
HOSTS Most turfgrass species, but Kentucky bluegrass and bentgrasses are particularly damaged; perennial ryegrass and fine fescues can be damaged if they lack endophytic fungi.
DAMAGE Chinch bug feeding apparently interferes with the ability of plants to transport water. Damage resembles that caused by drought and tends to occur in patches, with killed turfgrass appearing strawlike and failing to respond to watering.
DISTRIBUTION Hairy chinch bug is a common species throughout the Midwest, northeastern U.S., and southeastern Canada.
APPEARANCE Adults are about ⅙ inch, gray-black, and covered with fine hairs. Wings are white with a dark spot in the middle. Forms of chinch bugs with short wings are common. Young nymphs have a bright orange abdomen that darkens to blue-black as they develop.
LIFE HISTORY AND HABITS Hairy chinch bug winters in the adult stage, at the base of grass stems among the thatch. It becomes active when daytime temperatures begin to regularly reach 70° F. From Pennsylvania west to Nebraska two generations per year are produced. Into southern Canada, one generation occurs. The bugs usually begin laying eggs from mid-April through May. Egg-laying begins later but occurs over a longer period in northern areas. The eggs are laid in leaf sheaths or thatch, with peak egg-laying in the New Jersey area approximately when white clover is in peak bloom.
The nymphs feed for about 4–6 weeks and may occur in large masses in midsummer. The second generation usually peaks in late August and early September. Damage from the second generation rarely occurs when the turf has entered summer dormancy. The fungus Beauveria bassiana and the predatory big-eyed bugs (Geocoris spp.) are particularly important in biological control of hairy chinch bug.
RELATED AND SIMILAR SPECIES
SOUTHERN CHINCH BUG (Blissus insularis) is an important pest of turf from South Carolina to Oklahoma and south. It is very similar in appearance to hairy chinch bug, but their ranges do not overlap. Several warm-season grasses are hosts, but southern chinch bug is most damaging to St. Augustinegrass. Three or four generations are typically produced annually, with peak damage by summer generations occurring during hot, dry weather.
WESTERN CHINCH BUG (B. occiduus) is a species that has been reported damaging to buffalograss in the Central Plains, where it is also known as the “buffalograss chinch bug.” Overwintering populations consist primarily of short-winged forms. A mixture of long-winged and short-winged (brachypterus) forms occurs during the summer. The general life history appears to be similar to that of hairy chinch bug. COMMON CHINCH BUG (B. leucopterus leucopterus) is generally distributed throughout the central U.S. It can be a serious pest of small grains, sorghum, and corn in the Midwest but rarely causes significant injury to turfgrass.

B. Lawn area showing chinch bug injury. DAVID SHETLAR

C. Hairy chinch bug nymphs exposed around the base of grass plants. DAVID SHETLAR

D. Adult and nymph of the southern chinch bug. DAVID SHETLAR

E. Western chinch bug adults, showing short- and longer-winged forms. JIM KALISCH, UNIVERSITY OF NEBRASKA

F. False chinch bug adults. JIM KALISCH, UNIVERSITY OF NEBRASKA

G. False chinch bugs, mixed life stages. WHITNEY CRANSHAW

H. Damage to lettuce by false chinch bugs. WHITNEY CRANSHAW

I. False chinch bugs massed on drying seed pods. WHITNEY CRANSHAW
FALSE CHINCH BUGS (Nysius spp.)2 are about ⅙ inch, winged, and slightly more elongate in form than “true” chinch bugs (Blissus spp.). General coloration is mottled gray, with the thorax and head somewhat darker. Nymphs are gray-brown and have some reddish or orange markings on the abdomen. False chinch bugs are generally distributed throughout most of western North America but are particularly common in the High Plains and Intermountain West.
False chinch bugs suck the sap from plants during feeding. The adults also commonly aggregate and occur in large numbers on individual plants, causing plants to wilt and die rapidly. Outbreaks are sporadic but can destroy plantings, particularly early in the year. Later in the season, aggregations tend to be greatest on developing seed heads. False chinch bugs are sometimes a nuisance pest of homes and buildings during hot summers when they may migrate into buildings. Most garden plants can be damaged during outbreaks; crucifers and beet family plants are favored. Wild hosts include many weeds such as tansy mustard, kochia, Russian thistle, and sagebrush.
False chinch bugs spend the winter as nymphs or adults under protective debris near winter annual mustards they use for hosts. They become active in early spring and move to developing mustards to feed. Adults lay eggs in loose soil or soil cracks around plants and eggs hatch in about 4 days. Under summer conditions, the wingless, gray nymphs feed for about 3 weeks and then reach the adult stage. Adults live for several weeks, fly readily, and can disperse over wide areas. About three generations are usually produced, with peak numbers often appearing in July and early August.
1 Hemiptera: Blissidae
2 Hemiptera: Lygaeidae
STINK BUGS THAT FEED PRIMARILY ON LEAVES
Stink bugs (Pentatomidae family) are moderately large insects, about ½ inch long, with a characteristic, broad shield form. Most species produce a disagreeable odor when handled. Most are rather uniform green or brown, but some species have bold patterning. Nymphs are generally similar to adults in appearance but tend to be more rounded in form and often have distinct patterning on the abdomen. Stink bug eggs are laid in masses and are a unique form, barrel-shaped and often with distinct spines around the top.
Stink bugs feed with mouthparts designed to suck sap but also cause localized injury around the feeding site. On foliage this often results in a somewhat cloudy area surrounding a central feeding puncture. Feeding is often concentrated on younger foliage, resulting in leaf distortions and dieback.
Plant-feeding stink bugs are damaging primarily to the fruiting parts of plants. Young fruit may abort from stink bug damage. Seeds of legumes are sometimes killed or shrunken following pod feeding. Fruit that has limited injury may continue to grow, but indented areas develop at the feeding site, producing an injury known as catfacing, discussed in more detail on page 594. Many stink bugs, such as TWOSPOTTED STINK BUG, FLORIDA PREDATORY STINK BUG, and SPINED SOLDIER BUG, are beneficial predators of other insects (page 628). A few others, such as ROUGH STINK BUGS (page 595), may feed on both plants and insects.
HARLEQUIN BUG (Murgantia histrionica)1 is a gaudily colored insect that feeds primarily on various mustard family (Brassicaceae) plants, with cabbage and mustards grown for greens among those plants most seriously damaged. However, harlequin bug will occasionally feed on a fairly wide variety, and some plants, notably cleome (spider plant), are also highly favored. Areas around the feeding site typically turn cloudy, and when the insect feeds on young tissue, growth develops in a distorted manner and may turn brown and die. It is widely distributed throughout the southern U.S.
All stages of this insect are brightly patterned with red, white, and black. Adults have the typical broad form of a stink bug, about ⅜ inch in length. Nymphs have a more rounded shape with similar coloration. Harlequin bugs normally survive winters only in the adult stage, hidden in protected sites such as crop debris, and do not undergo true dormancy. The adults emerge from winter shelter in mid-spring and typically feed first on wild mustard weed hosts. By June or early July they may be found in gardens, feeding on cabbage, radish, and other crucifers. Females then lay masses of eggs that resemble rows of small black-and-white banded barrels on the leaves of these plants. The immature nymphs usually develop in the same plants where eggs were laid and feed for about 2 months before becoming full grown. If warm weather persists, 3 and sometimes 4 generations are produced annually.
Another brightly colored stink bug that feeds on plants of the cabbage family is the PAINTED BUG (Bagrada hilaris),1 also known as the “bagrada bug.” It is much smaller (¼ inch) than the harlequin bug but can cause similar damage to the growing leaves of many crop plants. Wild mustards are important early season hosts and in high populations non-brassica crops may be injured. The present distribution of this insect, native to Africa, now includes areas within most of the southwestern states.
The KUDZU BUG (Megacopta cribraria)2 is a recently introduced species to North America, first detected (2009) in Georgia. Since then it has steadily spread in the southeastern U.S. and is presently known from parts of the Carolinas, Georgia, and Alabama. It feeds primarily on the leaves and stems of various legumes, including kudzu, kidney bean, soybean, and lima bean. In high numbers they can cause significant crop losses, but this insect also attracts attention and concern as it has a habit of massing on the sides of buildings in autumn.
1 Hemiptera: Pentatomidae
2 Hemiptera: Plataspidae

B. Harlequin bug egg mass. WHITNEY CRANSHAW

C. Harlequin bug egg mass just after hatch. WHITNEY CRANSHAW

D. Harlequin bug nymphs. WHITNEY CRANSHAW

E. Plant injury produced by harlequin bugs. WHITNEY CRANSHAW

F. Harlequin bug adults showing range in coloration. WHITNEY CRANSHAW

G. Kudzu bug. RUSS OTTENS, UNIVERSITY OF GEORGIA, BUGWOOD.ORG

H. Massed kudzu bugs. RUSS OTTENS, UNIVERSITY OF GEORGIA, BUGWOOD.ORG

I. Painted bugs. SURENDRA K. DARA, UNIVERSITY OF CALIFORNIA COOPERATIVE EXTENSION SAN LUIS OBISPO COUNTY, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROJECT
The lace bugs (Tingidae family) are relatively small insects (ca. ⅕ inch) with delicately sculptured wings held flat over the back. They feed on the underside of leaves, producing irregular white or yellow spotting that is evident on the upper leaf surface. Droplets of a varnishlike excrement are also deposited around feeding sites, assisting in diagnosis. More than 150 lace bug species occur in North America, with many of the most damaging species occurring in the eastern coastal states. Members of the genus Stephanitis1 are associated with broadleaf evergreens, and the injuries they produce are particularly conspicuous on these plants since they retain their foliage. These lace bug species winter as eggs inserted into or cemented to leaves with a covering of crusty dark excrement. Eggs hatch in late spring, and there may be 2 generations.
AZALEA LACE BUG (S. pyrioides) is the most damaging lace bug associated with landscape plants, producing severe injury to azalea foliage. It is particularly damaging in the southeastern and Mid-Atlantic states but occurs over a broad area in the eastern U.S. where azalea is grown and recently became established in the Pacific Northwest, where it has shown high potential to cause injury. On rhododendron and mountain laurel along the East Coast, a similar type of leaf injury is produced by the RHODODENDRON LACE BUG (S. rhododendri). ANDROMEDA LACE BUG (S. takeyai) develops on Japanese andromeda, with leucothoe, styrax, and willow as incidental hosts.
Corythuca1 is the largest and most diverse genus of lace bugs, and most of the 50-odd species in this genus develop on woody plants. All winter as adults under protective cover in the vicinity of previously infested plants. Eggs are laid in small groups inserted into leaf veins. Development typically takes about a month, and two generations are produced annually by most species.
HAWTHORN LACE BUG (C. cydoniae) is probably the most commonly encountered species in this group of lace bugs. It occurs throughout most of the U.S. and southern Canada and is associated with various roseaceous plants, particularly hawthorn, cotoneaster, amelanchier, quince, and pyracantha. Some differences in susceptibility to injury among different species and cultivars have been identified. Other commonly encountered lace bugs in this group include OAK LACE BUG (C. arcuata) on various oaks; BIRCH LACE BUG (C. pallipes) on birch, maple, and mountain-ash; ANGULATE TINGID (C. angulata) on ceanothus; SYCAMORE LACE BUG (C. ciliata) on sycamore; HACKBERRY LACE BUG (C. celtidis) on hackberry; ELM LACE BUG (C. ulmi) on elm; WALNUT LACE BUG (C. juglandis) on walnut, butternut, and basswood; and CHERRY LACE BUG (C. pruni) on cherry, chokecherry, and raspberry.
A few species of lace bugs are also found on herbaceous plants. CHRYSANTHEMUM LACE BUG (Corythuca marmorata) occurs on goldenrod, aster, and chrysanthemum. Nymphs and adults can be found on upper leaf surfaces. DISTINCT LACE BUG (C. distincta) is most commonly found feeding on various thistles (Cirsium) and can be common on Canada thistle.
EGGPLANT LACE BUG (Gargaphia solani)1 is a minor pest of eggplant in parts of the central and southern U.S. This species has the unusual habit of adult guarding of eggs and young nymphs. BASSWOOD LACE BUG (G. tilia) is common on linden and basswood in the midwestern states.
LANTANA LACE BUG (Teleonemia scrupulosa)1 is native to parts of Florida and Texas, occurring naturally southward into northern Chile. It develops on the underside of leaves of lantana and sage, producing the characteristic spotting typical of other lace bugs. Since lantana has become a noxious weed in some areas of the world where the plant was introduced, lantana lace bug has been introduced to assist in its control.
1 Hemiptera: Tingidae

B. Egg mass of the rhododendron lace bug. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

C. Adult of the rhododendron lace bug. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

D. Rhododendron lace bug nymph. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

E. Azalea lace bug damage. WHITNEY CRANSHAW

F. Adults and nymphs of azalea lace bug. DAVID SHETLAR

G. Sycamore lace bugs, mixed stages. DAVID SHETLAR

H. Walnut lace bugs, mixed stages. DAVID SHETLAR

I. Injury symptoms on upper leaf surface from cherry lace bug. WHITNEY CRANSHAW

J. Injury symptoms on lower leaf surface from cherry lace bug. WHITNEY CRANSHAW

K. Foliage bronzing produced by hawthorn lace bug. DAVID SHETLAR

L. Chrysanthemum lace bug. JIM KALISCH, UNIVERSITY OF NEBRASKA

M. Eggplant lace bug. WHITNEY CRANSHAW

N. Damage produced by lantana lace bug. CHAZZ HESSELEIN, ALABAMA COOPERATIVE EXTENSION SERVICE, BUGWOOD.ORG

O. Lantana lace bug. CHAZZ HESSELEIN, ALABAMA COOPERATIVE EXTENSION SERVICE, BUGWOOD.ORG
Thrips are minute insects, rarely more than 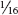 inch, of elongate form. The order name, Thysanoptera, means “fringe wing,” a reference to the fringe of long hairs on both pairs of narrow wings. Thrips also possess unusual mouthparts, with a single mandible that functions as a spike to puncture the leaf surface and a pair of finer stylets (maxillae) that penetrate interior cells. Thrips then suck the released fluids. Plant injuries appear typically as silvery scars marked with small varnishlike fecal droppings. Feeding by some thrips cause leaves to fold, roll, or curl. Despite their small size, thrips that land on human skin may produce a bite that can be felt as a very mild pinprick. Thrips that develop primarily in flowers, such as the “flower thrips” group (Frankliniella spp.), are discussed more thoroughly on page 590. Predaceous thrips are discussed on page 628.
inch, of elongate form. The order name, Thysanoptera, means “fringe wing,” a reference to the fringe of long hairs on both pairs of narrow wings. Thrips also possess unusual mouthparts, with a single mandible that functions as a spike to puncture the leaf surface and a pair of finer stylets (maxillae) that penetrate interior cells. Thrips then suck the released fluids. Plant injuries appear typically as silvery scars marked with small varnishlike fecal droppings. Feeding by some thrips cause leaves to fold, roll, or curl. Despite their small size, thrips that land on human skin may produce a bite that can be felt as a very mild pinprick. Thrips that develop primarily in flowers, such as the “flower thrips” group (Frankliniella spp.), are discussed more thoroughly on page 590. Predaceous thrips are discussed on page 628.
The biology of thrips is unusual as well. Eggs of most species are inserted into plant tissue. Two active feeding stages (larvae) subsequently occur. These are followed by two inactive and nonfeeding stages (prepupa and pupa) which usually develop in soil.
Note: The common name for insects of this order, “thrips,” is both singular and plural. Use of the word “thrip” in any context is incorrect.
ONION THRIPS (Thrips tabaci)1
HOSTS An extremely wide range, with onion, cabbage, and bean among the most commonly damaged plants. Onion thrips is often the most common thrips found on leaves of vegetables and flowers.
DAMAGE During feeding, onion thrips puncture the leaf surface and remove cell sap. Damaged areas appear as light flecking wounds and silvery scars, often with dark fecal spots. Newly expanding leaves may curl when damaged, and some varieties of cabbage react to injuries by producing wartlike growths. Onion thrips may vector viruses in the Tospovirus group, such as tomato spotted wilt virus, impatiens necrotic spot virus, and iris yellow spot virus.
DISTRIBUTION Throughout North America, common.
APPEARANCE Adults are usually yellowish or yellow-brown, tending to be darker with cooler weather. Larvae are creamy yellow.
LIFE HISTORY AND HABITS Onion thrips overwinters in the adult stage throughout its range in protected sites and old plant materials. It may also be introduced into a field on infested transplants and is common on onion sets. Eggs are inserted into leaves and stems. They hatch in about one week and pass through two wingless feeding stages as larvae on the plant for about two weeks. These are followed by two nonfeeding stages (“prepupa,” “pupa”) that occur in the soil or in crevices on the plant. The winged adult stage commonly disperses throughout an area and may fly long distances aided by winds. Several generations occur annually, and all life stages may be present by early spring.
OTHER THRIPS ASSOCIATED WITH FOLIAGE
GLADIOLUS THRIPS (Thrips simplex) feeds on lily, iris, and gladiolus, although damage is restricted to the last. Feeding at the base of emerging leaves produces silvery scarring, that later often turns brown. Infestation of flowers produces serious scarring and often distortion. Heavy infestations can prevent flower production. Gladiolus thrips can also damage the corms in storage, causing them to become sticky and dark from plant sap at wounds. Infested corms produce poorly when replanted and are the primary source of new infestations, as gladiolus thrips fail to survive outdoors in most areas. Daylily is occasionally infested by DAYLILY THRIPS (T. hemerocallis).
A. Adult onion thrips. ALTON N. SPARKS JR., UNIVERSITY OF GEORGIA, BUGWOOD.ORG

B. Leaf injury to onion produced by onion thrips. WHITNEY CRANSHAW

C. Onion thrips nymphs and injury to base of onion leaf. WHITNEY CRANSHAW

D. Onion thrips and injury to cabbage. WHITNEY CRANSHAW

E. Scabby spotting produced in response to onion thrips injury to cabbage. GERALD HOLMES, CALIFORNIA POLYTECHNIC STATE UNIVERSITY AT SAN LUIS OBISPO, BUGWOOD.ORG

F. Gladiolus thrips. JIM KALISCH, UNIVERSITY OF NEBRASKA

G. Leaf injury produced by gladiolus thrips. WHITNEY CRANSHAW
MELON THRIPS (Thrips palmi) is a tropical species that became established in southern Florida in the early 1990s. High populations develop on leaves, which become yellow or white and prematurely die. Melon, squash, tomato, pepper, and potato have been particularly damaged. INTRODUCED BASSWOOD THRIPS (T. calcaratus) is broadly distributed in the upper midwestern and northeastern U.S. It feeds on several hardwood trees, but American basswood is particularly susceptible and the only plant seriously damaged. Introduced basswood thrips has a single late-spring generation that appears on plants around bud break. A small amount of feeding at this time may cause basswood buds to die and drop. Eggs are laid in newly expanding leaves, and feeding may cause defoliation.
PEAR THRIPS (Taeniothrips inconsequens)1 is an introduced species now found throughout the northeastern quadrant of North America and in regions of the Pacific Northwest. It develops on several fruit trees and is of some concern to orchardists; however, it has been most damaging to sugar maple, causing leaf distortion as new foliage emerges and premature defoliation during outbreaks.
PRIVET THRIPS (Dendrothrips ornatus)1 produces foliage flecking primarily on California and regal privet but it can also attack lilac and ash. Adults are generally brown with thin light bands on each of the abdominal segments. Multiple generations are produced beginning in late spring. TOYON THRIPS (Rhyncothrips ilex)1 develops on the new growth of Christmas berry, causing twisting and curling of foliage during spring. Adults are black and nymphs orangish. Only one generation is produced annually. IRIS THRIPS (Iridothrips iridis)1 can be very damaging to Japanese iris, as feeding injuries scar leaves. Nymphs are white and can thus be distinguished from nymphs of other thrips associated with iris.
GREENHOUSE THRIPS (Heliothrips haemorrhoidalis)1 develops on the foliage of a wide variety of plants, including avocado, viburnum, dogwood, azalea, grape, palms, orchids, philodendron, maples, magnolia, dahlia, ferns, and hibiscus. It is a common outdoor pest in southern California and Florida but restricted to indoor plants in areas of temperate climate. Greenhouse thrips feed primarily on foliage, first on the lower surface and often later moving to the upper surface of shaded leaves. Damage typically appears as discolored areas between the lateral veins. Leaf distortion and dimpling of fruit can also occur. Small droplets of reddish fluid, later turning black, are excreted. Adult greenhouse thrips are fairly small (ca.  inch) and dark black with a silver sheen. Larvae are pale yellowish with red eyes.
inch) and dark black with a silver sheen. Larvae are pale yellowish with red eyes.
BANDED GREENHOUSE THRIPS (Hercinothrips femoralis)1 may also scar foliage of indoor-grown plants, including African violet, chrysanthemum, fig, gardenia, jasmine, tomato, dieffenbachia, hoya, philodendron, rubber tree, and schefflera. Adults are generally brown with white patches on the forewing.
BEAN THRIPS (Caliothrips fasciatus)1 feed primarily on the underside of leaves, typically concentrating feeding at points in the leaf surface. This results in conspicuous white spotting along with which are dark fecal droplets. Legumes are the most common hosts, but they can be found on a considerably wider range of plants, including some grasses, and are a common insect contaminant of navel oranges.
More than a dozen Anaphothrips species occur in North America, all of which develop on grasses. Anaphothrips obscurus1 is common on many grasses, including turfgrasses and small grains, and has been associated with early-season turf injury during periods in the Rocky Mountain region. These thrips are occasionally associated with biting human skin when turfgrasses enter periods of dormancy.
A. Melon thrips. FLORIDA DIVISION OF PLANT INDUSTRY, FLORIDA DEPARTMENT OF AGRICULTURE AND CONSUMER SERVICES, BUGWOOD.ORG

B. Nymphs of pear thrips. DAVID SHETLAR

C. Pear thrips adult. PENNSYLVANIA DEPARTMENT OF CONSERVATION AND NATURAL RESOURCES-FORESTRY, BUGWOOD.ORG

D. Leaf injury symptoms produced by pear thrips. DAVID SHETLAR

E. Privet thrips adults. DAVID SHETLAR

F. Privet thrips nymphs. WHITNEY CRANSHAW

G. Privet thrips leaf injury. WHITNEY CRANSHAW

H. Nymph of a grass thrips, Anaphothrips species. KEN GRAY COLLECTION, OREGON STATE

I. Greenhouse thrips. WHITNEY CRANSHAW

J. Injury symptoms produced by bean thrips. WHITNEY CRANSHAWUNIVERSITY
CHILLI THRIPS (Scirtothrips dorsalis)1 is a relatively recent introduction (Florida in 1991 and Texas in 2005) that is becoming a major pest of many plants, including field and vegetable crops, ornamental trees, shrubs, and flowers. The thrips are small and generally light colored, though the adults have black hairs on their wings. The typical thrips life cycle is followed, though all stages tend to stay on the plant. This pest causes severe scarring of leaves, stems, and fruits and is a capable vector of several viruses that produce plant disease.
Thrips in the genus Frankliniella are generally known as “flower thrips” as they often develop in flowers and may damage developing fruit and seedpods (page 590); however, they also feed on foliage, sometimes producing serious leaf scarring and distortion of new growth. Important species include WESTERN FLOWER THRIPS (F. occidental), FLOWER THRIPS (F. tritici), and TOBACCO THRIPS (F. fusca). Many are also important as vectors of tospoviruses that cause plant disease, including tomato spotted wilt and impatiens necrotic spot.
REDBANDED THRIPS (Selenothrips rubrocinctus)1 is a tropical species that occurs in parts of Florida. Mango, avocado, and sweetgum are among the most common hosts and may be disfigured by the leaf scarring and conspicuous dark fecal spotting produced by the thrips.
CUBAN LAUREL THRIPS (Gynaikothrips ficorum)2 is most commonly associated with Ficus nitida and F. microcarpa, producing spotting and thickened leaf folds of new growth. They are fairly large thrips (ca. ⅛ inch) and adults are jet black. Adults move to the emerging leaves and induce a leaf fold, within which they lay a large group of eggs and where the pale yellow nymphs develop. A closely related species, the WEEPING FIG THRIPS (G. uzeli), is also present in the southeastern states and, more recently, in southern California, where it is damaging primarily to weeping fig (F. benjamina).

Ring spot symptoms on fruit produced by tomato spotted wilt. WHITNEY CRANSHAW

Western flower thrips injury to bean leaf. WHITNEY CRANSHAW
1 Thysanoptera: Thripidae
2 Thysanoptera: Phlaeothripidae