
Fig. 20-0: Images of Brazilian rain forest showing destruction between 2000 and 2009. Note the 40 km scale bar in the lower left-hand corner. The area shown is about the size of the state of Wyoming or the country of Poland. (Images from NASA Earth Observatory (earth observatory.nasa.gov/Features/WorldofChange/deforestation.php))
The rise of human civilization is a transformative event in planetary history. For the first time a single species dominates the entire surface, sits at the top of all terrestrial and oceanic food chains, and has taken over much of the biosphere for its own purposes. We are also influencing the physical environment by changing the composition of the atmosphere and ocean, modifying the water cycle, eliminating soils, and constructing huge communities on a scale never before seen. To an external observer there is also a change in the capabilities of the planet as a whole. Human beings permit access to planet scale sensing from space, land, and sea; we are capable of conscious direction of the planetary systems; and we could communicate with other planetary civilizations, should they exist. We have also vastly increased the rate of and capacity for planetary change through our access to energy, global communication, technology, and our developing ability to direct evolution through modification of DNA. Such changes are monumental in the context of all of Earth’s history, and they certainly warrant the name of a new geological epoch—as per the suggestion of Paul Crutzen’s “Anthropocene.” The vastness of planetary change and capability, however, is more equivalent to the great events of the past that mark boundaries between geological eras or even eons, such as the origin of life, the rise of oxygen, the origin of multicellular life, or the Permo-Triassic extinction. We have entered a potential Anthropozoic era, an era of planetary consciousness and directed evolution where a single species controls the fate of the planet. Eras of the past have lasted hundreds of millions of years—will the Anthropozoic have such longevity? Can we usher in a new era with wisdom and conscience, or will we be a failed and abortive attempt at further planetary evolution?
One practical challenge is to manage planetary resources so that we do not run out of essential materials—and food. Even more important, human activities are now so vast that they impact planetary systems. Energy use has increased greenhouse gases in the atmosphere by 40% for CO2 and 100% for CH4, greatly exceeding natural variations, and causing the warming of the atmosphere, the melting of the Arctic ice cap, and an inexorable rise in sea level. Part of the anthropogenic CO2 is absorbed by the oceans, making the oceans more acidic. The effects on the biosphere are even more marked. At sea, the more acid ocean contributes to the decline of coral reefs and impacts the entire oceanic ecosystem. On land, 25% of terrestrial plant and animal productivity now relates to human food production. This has caused massive destruction of habitat for species other than ourselves. Rain forests, the greatest remaining reservoir of biodiversity, are being cut down at the rate of 40,000 km2 per year, an area larger than the state of Massachusetts (see frontispiece). The diversity of species that can exist depends on contiguous land area; as wild areas diminish, extinctions inevitably follow. Human population has thus far been the driver for loss of biodiversity. Climate change would lead to even greater losses. Loss of biodiversity is a reduction in the genetic potential of the planet and its ability to respond to planetary change.
We have as yet shown little willingness to take responsibility for our effects on the planet or respond to planetary change. In our current economic model, both energy use and population growth cause negative environmental impact, and few are willing to limit either. The planet is free of charge—environmental costs are rarely meaningfully included in economic models, and including them would require societal readjustments, which are inevitably resisted. Solutions are largely a matter of personal and political choice, because effective steps are possible at a small fraction of what is spent on being able to destroy each other’s existence with weapons. While a dependence on fossil fuels is inevitable for coming decades, removal of CO2 from the atmosphere (carbon capture and sequestration) is an emerging capability that could be applied on a global scale. If it were, it could buy enough time for solar, wind, and nuclear power to grow to fill our energy needs. Ultimately fusion power is likely to become economic, providing a source of energy that is inexhaustible from our current perspective. This alone would not avoid planetary crisis, however. A revaluation of the planet that supports us will be necessary to avoid further planetary deterioration. Humanity is the result of billions of years of planetary evolution, and it has flourished from the bounty provided by Earth. Are we entitled, or appreciative? Only a revolution in human attitudes from “planetary user” to “planetary conservator” would permit effective planetary management.
The discussion in the previous chapter presents Earth’s resources as if Earth were a gift box containing treasure that needed to be managed. Instead, Earth is a dynamic system whose health is determined by a myriad of biogeochemical cycles involving atmosphere, hydrosphere, biosphere, and the solid Earth. If our use of resources did not influence this system then the problems we face would be a matter of intelligent resource management. But if we are a planetary force on a global scale that changes these cycles, then we need also to consider the overall health of the system and how that is influenced by human activities. The evidence summarized below shows that we have rapidly become a dominant influence on the planetary surface, unlike any other single species in the history of Earth. We have changed climate, modified the chemistry of the oceans, taken control of much of the biosphere, and are extinguishing other species at rates equivalent to a mass extinction.
Because of our influence, we live in a time of profound and rapid planetary change. Human actions have modified climate and the oceans and may end in global catastrophe, not only for other species but also for our own. At the same time, human civilization provides radical new capabilities that could have a beneficent planetary influence, if humans became able to view themselves and act as an integral and responsible part of a planetary system. As human beings we face choices that will forever affect our planet’s future. Will we choose planetary decline or planetary husbandry? We cannot avoid these choices, because our impacts are drastic and global in scale. For better or worse, we are now at the helm—the fate of the planet is in our hands.
Human activity influences all the reservoirs of Earth’s surface: atmosphere, ocean, soils, and biosphere.
We learned in Chapter 13 that the stability of Earth’s climate has been exquisitely tuned by the greenhouse effect to maintain Earth’s surface temperature in a narrow range, despite the increase in solar luminosity over Earth’s history. This range has encompassed relatively warm periods, with no glaciers at the poles, which last existed more than 30 million years ago, as well as colder periods when Milankovitch cycles caused oscillations between ice ages and interglacials, as well as more permanent glaciations. While the range in states has been large, changes have been gradual relative to life spans of organisms. The change from a greenhouse state with no ice ages to an icehouse state with periodic ice ages took several million years. Even the “rapid” change from glacial to interglacial is a 10,000-year process, 50 to 100 centuries, encompassing 200 generations even of long-lived species such as humans. Life is forced to adapt to these changes, but the short lifetimes of all species permit gradual movement and modification so that ecosystems have the time to migrate and adjust over thousands to millions of years.
Are human-induced changes significant in this planetary context? To address this question we can examine the detailed record of climate change over the last 700,000 years, preserved in the contents of CO2 in the air trapped in bubbles in polar ice. CO2 has oscillated between minimum values near 190 parts per million at glacial maxima to values of about 270 ppm during the brief interglacials, as temperature also varies (Fig. 20-1). CO2 varies in lockstep with climate change. Prior to the industrial revolution, the atmosphere’s CO2 content was consistent with interglacial values at a steady 280 ppm. As industrial emissions began, CO2 began to increase. Over the last 50 years for which a very accurate record is available, the atmosphere’s CO2 content has risen from 315 to more than 390 ppm, an average rate of 1.2 ppm per year. More recent CO2 emissions are so large that the rate has increased to 2 ppm per year. The current atmospheric CO2 content greatly exceeds any values over the last 700,000-year ice-core record and likely exceeds any values for the last several million years. These are large changes on a global scale. But wait—atmospheric CO2 in the Cretaceous period was likely close to 2,000 ppm, higher even than the most dire predictions for the next century. Of course, at this time Earth was in a “hothouse” state, with no ice sheets and shallow seas that covered much of the continents. But it is natural change, so the total range of CO2 variations over tens of millions of years is large. How do we know that the recent CO2 rise is due to human impact, and not simply natural variations?
Fig. 20-1: Methane, temperature, and CO2 from the Dome C ice core showing that CO2, temperature, and methane have varied in lockstep over the last 700,000 years. During the brief interglacials, all parameters are high, and during glacials all are low. (European Project for Ice Coring in Antarctica (EPICA), project members, 2006)
There are two independent approaches that confirm the human cause of the atmospheric change. The first is simply to add up the amount of CO2 we have pumped into the atmosphere. The number is so vast that CO2 in the atmosphere has actually increased less than would be expected from the amount of carbon we have burned. Instead, about 45% of the emitted CO2 must have been absorbed by the ocean and biosphere, and only the remaining 55% has accumulated in the atmosphere. A second approach turns to the sources of natural variations in atmospheric CO2. For the CO2 budget, the elephants in the room are the ocean and solid Earth. The ocean contains fifty times as much CO2 as the atmosphere, so small variations in the ocean budget could be important. The solid Earth contains even vaster quantities of CO2, so couldn’t solid Earth emissions lead to the rise?
The latter question is addressed by measurements of volcanic emissions of CO2. Earth’s volcanic CO2 emissions are 0.2 gigatons (Gt) per year. The passage from glacial to interglacial is associated with a temporary increase to as much as 0.5 Gt per year. In comparison, human emissions in 2008 were 30.0 Gt, 150 times the natural background. Solid Earth emissions are trivial in comparison to recent human emissions and produce change only on long timescales.
The ocean as a source of increased CO2 is also ruled out. First, the ocean must have been a net absorber of CO2 to account for the fate of all the human emissions. This conclusion can also be tested by measurements of atmospheric O2 concentrations and the change in carbon isotopes.
Carbon released from the oceans is already in molecules of CO2, and release of CO2 from the oceans has no effect on atmospheric oxygen. When we burn carbon, however, the carbon combines with atmospheric oxygen, reducing atmospheric concentrations. If fossil fuels are the cause of increasing CO2 in the atmosphere, the atmospheric O2 concentrations should decrease in lockstep with CO2. One difficulty is to make sufficiently precise measurements of O2, which would vary by only a few parts per million (2 ppm of annual CO2 rise would only decrease O2 from 22.9% to 22.8998%). Charles David Keeling mastered such measurements to provide a record of variations in O2, seen in Figure 20-2. The steady decline of atmospheric O2 corresponds with carbon burning.
Carbon isotopes are another independent test. Organic carbon in fossil fuels is isotopically light. Burning adds this light carbon to the atmosphere. Indeed, the carbon isotope composition of atmospheric CO2 has been steadily decreasing, showing the massive input of burned organic carbon (Fig. 20-2). It is a simple fact that atmospheric CO2 is increasing because of human emissions. It merits a 10 on our theory scale.
One of the intriguing aspects of the glacial record is that coming out of glacial periods, temperatures begin to rise before CO2 does. Doesn’t this prove that CO2 does not cause warming? In the nonscientific community, this is often interpreted to mean that “atmospheric temperature causes CO2 to change, and therefore CO2 is a consequence and not a cause of warming.” This misunderstanding results because CO2 is a positive feedback on warming during deglaciations, whereas it is driving warming today. The increased solar luminosity in the Northern Hemisphere driven by Earth’s orbital variations causes a slight temperature rise. This rise causes some CO2 to be released from the oceans, which causes further warming, which causes CO2 to rise more—a positive feedback. Melting of the ice sheets then causes increased volcanism that releases further CO2, augmenting warming. Deglacial warming is the result of multiple feedbacks, with CO2 playing a pivotal role. The warming effect of greenhouse gases is a fundamental fact of physics, not a matter of “belief” or a political issue.
Another important greenhouse gas is methane. Like CO2, methane has oscillated regularly with ice age cycles, from 350–400 parts per billion during glacial maxima, spiking to brief peaks near 650 ppb during interglacials, followed by a rapid decline to intermediate values in the subsequent few thousand years (Fig. 20-3). In the last 150 years methane concentrations in the atmosphere have more than doubled, to 1,750 ppb. Human sources of methane to the atmosphere are dominantly from livestock, landfills, and natural gas exploitation, and these exceed natural sources, leading to the atmospheric rise. While the ~1 ppm rise in methane appears small, the effect on atmospheric warming is substantial because methane is twenty times more powerful as a greenhouse gas than CO2.
Fig. 20-2: Proof that the increase in CO2 in the atmosphere is due to human emissions from burning organic carbon. (a) Burning carbon uses up oxygen to produce CO2, so carbon burning should lead to a decline in O2. The decline in O2 is actually greater than would be expected from the atmospheric increase in CO2, showing that much of the CO2 from burning is taken up by the oceans and biosphere. This observation is also consistent with simple mass balance constraints—almost twice as much CO2 has been produced by fossil fuel burning as has accumulated in the atmosphere. The two O2 curves are data from two different locations. (b) Organic carbon in fossil fuels is preferentially depleted in the heavy carbon isotope 12C leading to low values of δ13C. Adding this isotopically light carbon to the atmosphere decreases the δ13 of atmospheric CO2, as is observed. Note that the y-axis scale is inverted. (©2007 IPCC AR-4 WG1, chap. 2, fig. 2.3)
Fig. 20-3: Variations in methane over the last 250,000 years from the Antarctica ice cores, and the near tripling of CH4 during the last century. (Data from Loulergue et al., Nature 453 (2008):383–86, and Etheridge et al., J. Geophys. Res. 103 (1998):15,979–93)
Other greenhouse gases are also of emerging importance. NO2 has increased markedly and has a significant warming influence. Chlorofluorocarbons (CFCs), the chemical used for refrigerants and other industrial applications in the twentieth century, are also greenhouse gases. CFCs also lead to destruction of Earth’s protective ozone shield. The effects of CFCs on ozone were established scientifically in the 1970s, but international action took place only after the discovery of the “ozone hole” over Antarctica in 1985, leading to a 1987 international agreement (the Montreal Protocol) that called for an elimination of CFC emissions. The worldwide agreement, the clear implications for human health, and the expiration of patents on CFCs that reduced their profitability, led to support from the chemical industry, and CFC emissions have been largely eliminated. The cessation has led to a decline in ozone-depleting gases of 10% from their peak in 1995. The ozone hole has not yet recovered, because of the residence time of CFCs in the atmosphere, and is not expected to recover until late in the twenty-first century. The replacement gases for CFCs (notably hydrofluorcarbons, or HFCs) do not destroy the ozone but are nonetheless potent greenhouse gases. Their use is increasing rapidly, particularly in developing countries, and projections suggest they may have an atmospheric influence equivalent to 100 ppm CO2 by midcentury.
To put these human-induced changes in context, we can recall that an atmospheric greenhouse has been essential for Earth’s climate stability over billions of years and that absent a greenhouse, Earth would freeze. Stability has been maintained over the last several million years by 180–280 ppm CO2 and <650 ppb methane in the atmosphere. On the thousands-of-years timescale, CO2 and methane have been important parts of the climate oscillations that have taken us in and out of ice ages. We also know that when Earth has been in a “hothouse” state when CO2 concentrations in the atmosphere were elevated, and sea level was much higher because there were no ice sheets. Earth’s history shows us that greenhouse gases matter. They permit and maintain planetary habitability and have participated in changes from ice age to interglacial and icehouse to hothouse. From this perspective, are human impacts significant? We are now in the process of certainly doubling, and potentially quadrupling, the CO2 content of the atmosphere. We have already more than doubled the methane contents and are adding other potent greenhouse gases for which the atmospheric concentration has been zero since Earth’s formation. Human impacts are of larger magnitude than the changes between ice ages and interglacials, and they are approaching the magnitude of hothouse vs. icehouse.
It is not only the magnitude of the atmospheric changes, it is also the rate of change, which is perhaps more important for ecosystem effects. Earth’s reservoirs are large and adjust slowly to change. Higher CO2 in the atmosphere will be absorbed by the oceans over a thousand years or so. Over tens of thousands of years higher CO2 leads to enhanced weathering, which reduces CO2. Organisms adapt over thousands of years and evolve over millions of years to changing environments. Earth systems are adapted to slow change. Change over decades is exceedingly rapid, and does not permit these gradual accommodations. Recent changes in CO2 are 1.8 ppm per year. For comparison, during the glacial terminations CO2 changes by 100.0 ppm in 5,000 years, or 0.02 ppm per year. Recent changes are ninety times faster! In both magnitude of change and rate of change, humans are profoundly influencing the planetary atmosphere and Earth’s thermostat, the climate system.
Fig. 20-4: The radiative forcing of the three most important greenhouse gases compared to the changes from the last glacial maximum 20,000 years ago through the present. Light gray bars show the range of natural variations over the last 800,000 years. Significant but gradual changes occurred during the deglaciation, followed by stable values during most of the Holocene. Abrupt changes occur in the Anthropocene. Human-induced forcing of climate of more than 2 watts/m2 is of the same order as the change from last glacial maximum to the interglacial, but it is occurring over 100 years rather than 10,000 years. Further increase of CO2 to 500–1,000 ppm will lead to much greater forcing. (©2007 IPCC AR-4 WG1, chap. 6, fig. 6.4)
How large will these changes be? From fundamental physics the warming effect on the atmosphere can be rigorously calculated, as shown in Figure 20-4. Human beings are causing the atmosphere to exert a major warming effect on Earth. Note also that the rate of change of these effects in Figure 20-4d.
Fig. 20-5: Annual anomalies of global land-surface air temperature (°C), 1850–2005, relative to the 1961–90 mean. (Modified after ©2007 IPCC AR-4 WG1, chap. 3, fig. 3.1)
The atmospheric changes have in recent decades created measurable increases in the mean temperature of the atmosphere. While global temperature increased from 1900 to 1940, there was little change between 1940 and 1980. The longer record (Fig. 20-5) that is now available shows a marked temperature rise over the last century and also that the rate of rise is increasing, from about 0.035°C per decade between 1850 and 1950, to 0.067°C per decade between 1950 and 1980, to 0.177°C per decade for the last thirty years. The last decade of temperatures is the warmest on record. Temperature has increased about 0.8°C (1.6°F) since 1880; 2009 was the second warmest year on record.
Of course, the increase in global temperature does not preclude an unusually cold day or year in local regions. Temperature increase is also not evenly distributed around the world. Figure 20-6 shows the map of how warming was distributed in the 2000–2009 decade. Warming has been much more extensive over continents than oceans and is particularly marked at high northern latitudes. In part, this reflects the greater heat capacity of the ocean and its longer equilibration time.
Fig. 20-6: This map illustrates how much warmer temperatures were during the decade 2000–2009, compared to average temperatures recorded between 1951 and 1980 (a common reference period for climate studies). Note that the most extreme warming, shown in dark gray, was in the Arctic. White areas over Africa and parts of the Southern Ocean are places where temperatures were not recorded. A small region near Antarctica has shown slight cooling. See color plate 27. (NASA images by Robert Simmon, based on data from the Goddard Institute for Space Studies)
The increase in measured temperature is consistent with a wealth of other studies, including the first date of spring blooming or planting, the length of the growing season, the northern extent of various wildlife, etc. Another direct measure of temperature trends comes from measurements of ice and snow cover. Figure 20-7 summarizes the evidence that, contemporaneously with the temperature increase since 1980, the extent of sea ice, frozen ground, glaciers, and snow cover have all decreased substantially in the Northern Hemisphere. This effect is particularly noteworthy in the Arctic (Fig. 20-8), owing to the concentration of warming at high northern latitudes.
Fig. 20-7: Summary of observed variations in ice, snow, and frozen ground for the period 1993–2003 when global temperatures have been increasing. (Modified from ©2007 IPCC AR-4 WG1, chap. 4, fig. 4.23)
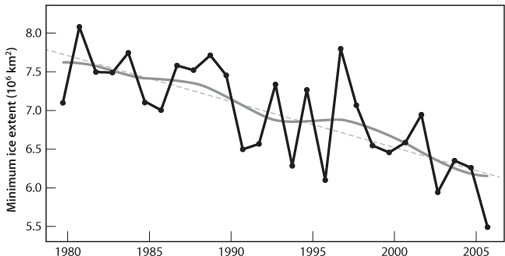
Fig. 20-8: Change in the minimum amount of ice in the Arctic over the last twenty-five years. As global temperature increases, the effect is largest at high northern latitudes. This has caused much greater melting of the Arctic ice through the summer months. The figure shows that the minimum ice extent has decreased by 30% over this time interval. (©2007 IPCC AR-4 WG1, chap. 4, fig. 4.8)
When it is dissolved in water, CO2 forms carbonic acid, increasing the acidity (lowering the pH) of the water. This experiment, which can be performed simply on a bench top, is being performed on a grand scale by fossil fuel burning. The higher CO2 in the atmosphere is being partially absorbed by the ocean, lowering its pH. The pH of the surface ocean has been lowered by about 0.1 pH unit, corresponding to a 30% increase in hydrogen ion concentration (Fig. 20-9). This effect is not a consequence of temperature change but is a simple and direct result of increased CO2 in the atmosphere. As CO2 continues to increase, the surface oceans where life resides will become increasingly acidic.
The pH of the oceans has a major effect on shell-producing organisms, because the CaCO3 that makes up the shells is less stable in more acidic water. (When geologists want to determine whether a rock is a carbonate, they put a drop of acid on the rock and see if it “fizzes” as the CaCO3 breaks down and CO2 gas is released.) Such effects will become important if the ocean’s acidity continues to increase. Even the small changes that have occurred thus far cause a decrease in the carbonate ion concentration in the ocean that inhibits growth, already contributing to the global decline in coral reefs (Fig. 20-10). The effects for some other calcifying organisms are less clear. The response to increased CO2 is likely to differ from one organism to another and may not be detrimental for all organisms. For some organisms, higher CO2 could potentially lead to increased shell thickness, for example, provided adequate other nutrients were available Therefore the situation for the ocean is akin to that of the atmosphere. Increased CO2 in the atmosphere from fundamental physics leads to warming, but there are other feedbacks that need to be considered for precise calculation of the effects. Similarly, adding CO2 to the atmosphere from burning of fossil fuels leads to a more acid ocean. What the system-wide effects of such changes are, and what other feedbacks may kick in, will require extensive study. And as with the atmosphere, it is the rate of change as well as the magnitude of change that is the striking human influence.
Note that the effects of ocean acidification occur independently of “global warming” and do not relate to change in atmospheric temperature. Lower oceanic pH is an inevitable consequence of increased atmospheric CO2. Warming of the atmosphere will also lead to warming of the surface ocean, with unknown effects on the oceanic biosphere. Both temperature and acidity are significant parameters for ocean ecosystems. Here the rate of emissions is very important relative to natural cycles, because little compensation through dissolution of CaCO3 has time to occur.
Fig. 20-9: Time series of (a) atmospheric CO2 at Mauna Loa (in parts per million volume, ppmv), surface ocean pH and pCO2 in the subtropical North Pacific Ocean. Left-hand scale is for the atmospheric CO2 data, right-hand scale for the ocean pH. (b) Further change in pH of the surface ocean that will occur for different CO2 levels in the atmosphere. Note that pH is the logarithm of hydrogen ion, so one pH unit is a factor of ten more acidic. (Doney et al., Annu. Rev. Mar. Sci. 1 (2009):169–92. Reprinted, with permission, from the Annual Review of Marine Science, Volume 1 © 2009 by Annual Reviews)
Fig. 20-10: Photos of coral Oculina patagonica after being maintained for twelve months in (a) normal seawater (pH = 8.2) and (b) acidified seawater (pH = 7.4). The higher acidity dissolves the protective carbonate around the living coral. (From Fine and Tchernov, Science 315 (2007):1811. Reprinted with permission from AAAS.
Source: Doney et al. (2009; see Fig. 20-09))
Every animal is driven by a search for food, and Homo sapiens is no exception. The trail of human migrations is marked by extinctions, likely associated with hunting. As the ancient Polynesians colonized the Pacific islands, as many as 75% of the native bird species went extinct, likely because they had never seen predators and were an easy source of food. About 45,000 years ago, glacially lowered sea level allowed Aborigines to reach Australia. Their arrival corresponds to, and likely caused, a great reduction in native vegetation (as recorded in the carbon isotope ratios in radiocarbon-dated fossil egg shells) and to the extinction of several species, such as the Tasmanian tiger and many large marsupials. When humans first arrived in North America across the Bering Strait, they found wildlife more diverse even than in Africa—saber-toothed tigers (Fig. 20-11), mammoths, long-horned bison, camels, giant wolves, antelopes, tapirs, and a multitude of large birds. In North and South America, 70–80% of the large mammals are now extinct. Similar extinctions occurred with the first arrival of humans in Madagascar and New Zealand, as well as Australia (Fig. 20-12).

Fig. 20-11: Some of the great mammals that were common before Homo sapiens entered their environment.
Fig. 20-12: Decline of large mammals in the last 20,000 years likely owing to the presence of Homo sapiens on new continents. Rapid extinctions occurred immediately following our arrival. North America used to have a diversity of large mammals even greater than modern Africa. (Modified from E. O. Wilson, ed. Biodiversity (Washington, D.C.: National Academy of Sciences, 1988))
The advent of agriculture and domestication of animals led to even greater environmental impact. What grew in our soils and roamed in our grasslands was to an ever-greater extent determined by us. Genetic engineering started at a very early stage by selection of crops and animals for human needs. To keep livestock in and predators out, fences were constructed; to compensate for fluctuations in nature’s supply of water, dams and irrigation systems were put in place, all in the name of increased access to food. Estimates are that human activities are now responsible for one-quarter of terrestrial biomass production.
Today the reach of human appetites is not restricted to their local environment but has become global. Scientific data published in 2003 determined that large fish—such as tuna, swordfish, marlin, and the large bottom-dwelling fish such as cod, halibut, skate, and flounder—have declined throughout the oceans by 90% compared to 1950 levels. The 1950 levels were likely already diminished relative to preindustrial levels. The decreasing fish supply occurred owing to massive growth in the ocean harvest from factory fishing ships. Stocks are now so low that fish harvest is in decline.
Extinctions have spread to the less visible animals through loss of habitat. Humans have taken land for agriculture, converting an ecosystem with an assemblage of diverse species to large expanses of a single grain, where any other plant is a “weed.” Deforestation has been a human footprint. Vast expanses of current landscapes, such as the Scottish highlands and much of Europe, were once rich forests. Land is also converted for animal husbandry, clearing existing plant species and eliminating habitat for wildlife. Global mapping now shows that some 50% of global grasslands, tropical dry forests, and broadleaf forests have been converted to human uses.
The greatest repositories of global biodiversity are the tropical rain forests, where many of the current species are not even known. Modern technology permits very efficient deforestation. Rain forests are being cleared at the rate of 40,000 km2 per year, ten times the area of the state of Rhode Island—annually (see frontspiece and Fig. 20-13). Some 70% of the Indonesian rain forest is now gone. Rain forest soils are so poor that remedial forestation is problematic.
The destruction of habitat and species is not restricted to land. In some regions coral reefs, which support enormous biodiversity in their environments, have decreased by 90% from levels since the 1970s. Phosphate pollution in lakes and rivers has also created massive “dead zones,” where algae thrive but animals cannot.
Fig. 20-13: Modern rates of destruction of rain forests. (a) Annual rates of destruction of the Amazon rain forest, which accounts for more than half of global loss. Total global loss per year is 40,000 km2, an area 200 km on a side. For visualization, (b) shows an area of the northeastern United States equivalent to what is being deforested annually in rain forests. (All figures derived from official National Institute for Amazonian Research (INPA) figures)
While extinction of large species is apparent to us, smaller species extinctions are relatively invisible and are more difficult to quantify. General effects can be inferred from laws of the effects of habitat destruction on the abundance of species have been determined by careful studies of specific regions. E. O. Wilson was able to use islands to investigate and understand the importance of habitat size on species diversity. Figure 20-14 shows how the number of species of reptiles and amphibians depends on the contiguous land area available. A reduction in area of 90% leads to a 50% species reduction. As humans take over habitat, even without direct killing, they diminish habitat, causing extinctions and a reduction in global biodiversity. The diminishment is not only at the species extinction level, but also at the important level of variation within a species. Genetic diversity within a species leads to greater survivability and a richer gene pool.
Fig. 20-14: Study of ocean islands shows that the number of species that an island can support decreases progressively with diminishing island size. The same principles apply to “biodiversity islands” on continents. To preserve species, large contiguous areas are necessary, not just isolated pockets. Also, as climate change forces life to change latitudes, the lack of contiguous space leads to extinctions. (Adapted from Biogeography by E. O. Wilson)
In most ecosystems, species compete and evolve into a diverse mix of organisms that are in an ever-moving balance. Introduction of new species without predators, or against which the indigenous species have no defense, can lead to exponential population growth of the introduced species and extinctions and loss of diversity of local species. Humans have transported nonnative species (often called invasive species or exotic species) to new continents. Early humans were themselves invasive and also introduced species such as goats, rats, and cats that became a major factor in local ecosystems. In the modern era, the advent of international transport and communication provides easy transport of species from one continent to another. This is apparent with the zebra mussel in North America that eliminates native mussels and now dominates many lake and river ecosystems in the United States and Canada; the African bee in the Americas; the tree snake that has wiped out the bird population on the Pacific island of Guam; the rabbit, sheep, and goat in Australia; bark beetles that ravage forests; and hundreds of plant species.
The combination of factors attributed to humans—overharvesting, habitat takeover, habitat and biological destruction by pollution, introduction of exotic species—all have greatly affected the total biodiversity on Earth. These effects to date have not been caused by climate change but by the growth of human population and our needs for land and food. Climate change is a new factor that will be added on top of these existing effects. It may have even more widespread repercussions for the planet’s organisms, because the rate of climate change induced by human activities is so much greater than the natural rate to which ecosystems ordinarily respond.
In a sense, biodiversity is the end consequence of planetary change. As we induce climate change, modify the oceans, destroy habitat, lose soils, and overharvest species for food and sport, we eliminate much of the genetic diversity accumulated by Earth over billions of years. Like the accumulation of oil and coal over hundreds of millions of years, biodiversity is also an accumulating resource, with more species and greater genetic variation through geological time. Genetic diversity is the tool chest of life, and the greater the diversity, the more life is able to adapt to changing conditions, and the greater the opportunity for genetic resiliency. Destruction of biodiversity is the diminishment of the gene pool. By destroying biodiversity, we reduce the evolutionary potential of the planetary biosphere.
All human beings want to prosper, and that prosperity is generally measured by economic output, of which a common measure is gross domestic product (GDP). The GDP of nations correlates closely with their energy use (Fig. 20-15a), and since the overwhelming proportion of energy comes from fossil fuels, energy use also correlates closely with CO2 emissions (Fig. 20-15b). Nations and individuals wish to be prosperous; prosperity requires energy; energy production releases CO2. This fundamental conflict between increasing prosperity and reducing CO2 emissions makes a very difficult problem.
Compounding the difficulties are the differences among nations. North America and Europe are responsible for the CO2 problem, having released 70% of integrated CO2 emissions between 1800 and 2000. These nations also continue to dominate emissions on a per capita basis (Fig. 20-16a). CO2 emitted per person in the United States is five times higher than the global average, ten times higher than Africa and Asia (minus China) and more than twice as high as Europe. There are also differences in economic efficiency from the point of view of CO2 emitted per dollar of GDP (Fig. 20-16b). To obtain their GDP, the former Soviet Union and Middle East, both resource-rich regions, emit the most CO2 per dollar of GDP, followed by the United States, China, Canada, and Australia. The rest of the world is far more CO2 efficient. The economies of Europe and Japan are 1.6 times more energy efficient than the United States. Despite having about 5% of the world’s population, the United States has emitted the most CO2 over the last two centuries, has the largest present-day per capita emissions of populous nations, and is the least energy-efficient large economy of the developed world.
Against this present situation and history, the growth in global CO2 emissions now comes from the developing world. Asia (less Japan) accounts for more than 70% of the growth in CO2 emissions from 2001 to 2010. China overtook the United States in terms of total CO2 emissions in 2006, and China constructs one new coal-fired utility plant per week, with a useable life of more than thirty years. Automobile sales in China are growing at a rapid rate. Because of its large population, poor energy efficiency, reliance on coal, and rapidly growing GDP, growth of CO2 emissions from China is inevitable and will increasingly dominate global CO2 budgets. India also aspires to such rapid growth.
Fig. 20-15: Graph of total primary energy (tons of oil equivalent per capita) vs. (a) gross national product (GDP) per capita and (b) tons of CO2 emissions per capita. (Data from International Energy Agency, Key World Energy Statistics, 2009)
Fig. 20-16: (a) CO2 emissions vs. region and (b) CO2/GDP (PPP) versus region. Units are kg CO2 per dollar. (Data from International Energy Agency, Key World Energy Statistics, 2009)
These facts create a contentious political problem. The world is competitive, and the countries that use the most energy are the most powerful. No country is willing to reduce its economic prosperity or its global power—no politician could be elected on such a platform. The West created the mess, and one could argue that those responsible for a mess should clean it up. The West also still uses a disproportionate share of energy on a per capita basis. But the growth in global CO2 comes from the developing world. They are not responsible for the current problem, and their citizens do not get their “fair share” of the economic prosperity provided by energy. Why should they have to cut emissions?
This backdrop was encountered in the Kyoto conference on global warming, where there were attempts at a global plan to reduce CO2 emissions. Europe agreed to reduce emissions, the developing world was exempted, and the United States refused to sign. An unanticipated consequence of the protocol was to export CO2 emissions from the developed world to the developing world. For example, the United Kingdom reduced its own emissions by 5% but its consumption-based emissions increased by 17%! The net result is that global CO2 emissions continued to rise by 29% between 2000 and 2008. Growth of CO2 emissions by China dwarfs any economizing measures taken by the rest of the world. A subsequent conference in Copenhagen in 2009 led to no new agreements, despite a more willing U.S. administration. The politics have made the problem simply intractable, and CO2 emissions will continue to increase. What then is likely to happen?
As we saw in the previous chapter, the limited quantities of oil make it likely, if not inevitable, that petroleum use will decline over coming decades. There is enough coal, however, to fuel the entire world for another century or two. Further, unlike the reserves of oil that are concentrated in the volatile Middle East, those of coal reside in the United States, Russia, and China. Coal is currently five times cheaper than oil as an energy source for electricity, and as long as economics does not include environmental costs, its use will continue to increase.
There is, of course, much talk about the surge of renewable energy—principally solar and wind. Solar and wind energy today make up less than 1% of energy production in the United States. Both of them are capital-intensive industries, with substantial installation costs. The electrical grid also would need to be redone in order to make good use of their energy. Fossil fuels have the great advantage that they can be easily transported and burned on demand anytime, anywhere. In contrast, the most economical regions for sun and wind power are often far from where electricity is needed. They are also currently not as cheap as fossil fuels. Even with huge growth in renewable energy, it is unlikely to make up more than 10% of energy needs for a very long time.
For all these reasons, our atmosphere is in for further large increases in CO2 content. Despite optimistic hypothetical scenarios of cutting CO2 emissions, there is currently no prospect that emissions will decrease on a global basis. This is apparent by comparing actual experience with the various “scenarios” constructed by the International Panel on Climate Change (IPCC). The group made predictions on the basis of the “middle-of-the-road scenario,” which would include substantial global efforts to reduce the growth in emissions. What has happened in practice is that global emissions have exceeded the most pessimistic “business as usual” scenario. There is no evidence that our political systems are capable of meaningful reduction in global CO2 emissions. The timescale of CO2 rise may be quite short. In 2008 CO2 rose by 2.4 parts per million to 390 ppm, the largest annual increase on record. If emissions grow at 2.5% per year as they have for the last decade, 560 ppm would be reached by 2050, and CO2 rise would continue after that unless emissions suddenly went to zero! To keep the atmosphere below 560 ppm, CO2 emissions would need to be drastically reduced to almost zero by midcentury.
As CO2 rises, the acidity of the ocean will continue to increase, with unknown effects on oceanic ecosystems. As population expands and economies grow, biodiversity will continue to decrease. Coral reefs and rain forests may largely disappear.
As concentrations of greenhouse gases rise, so also will Earth’s temperature (Fig. 20-17). Estimates of the magnitude of this change require models, and the models are imperfect. The climate system is a complex beast! The models are becoming increasingly complex, can reproduce current global warming, and account for short-term cooling effects caused by large volcanic eruptions (Fig. 20-20). The models suggest that at twice the preindustrial content (560 ppm CO2) mean global warming would be about 3°–5°C (i.e., 6°F). This would give New York City the climate of Atlanta, Georgia. Keeping CO2 to these levels would require major efforts over the next decades to cut CO2 emissions. Absent such efforts, a second doubling (i.e., from 560 to 1,020 ppm) would lead to an additional 3.5°C warming. New York would have the climate of Florida.
Fig. 20-17: Temperature projections, global map. Multimodel of annual mean surface warming (surface air temperature change, °C) for the A1B model where significant steps are taken to slow the growth in CO2 emissions and have them decline after 2050. Projections are shown for three time periods, 2011–2030 (left), 2046–65 (middle), and 2080–99 (right). Anomalies are relative to the average of the period 1980–99. See also color plate 28. (©2007 IPCC AR-4 WG1, chap. 10, fig. 10.8)
Not only will our planet become warmer, but the distribution of precipitation will change. The same computer simulations that provide estimates of the magnitude of the coming warming also suggest that as Earth warms, rainfall will be ever more strongly focused on the tropics. If the models are correct, Earth’s dry lands will become even more parched (Fig. 20-18).
The paleoclimatic record is consistent with these predictions. The last glacial period provides us with an excellent cold analog. On this colder Earth, the dry lands were much less dry and the tropics less wet than now. The evidence comes from several sources, but the most convincing and easiest to understand is the size of lakes that have no outlet to the sea. The water they receive from the precipitation in their catchments basins is lost entirely by evaporation from the lake’s surface. Such closed basin lakes exist only in the dry regions of our planet. This makes them excellent recorders of aridity. If rainfall decreases, they shrink in size. If it increases, they grow in size.
Fig. 20-18: Predicted changes in precipitation. (a) Multimodel mean changes in precipitation. Changes are predicted annual means for the period 2080–99 relative to 1980–99 (©2007 IPCC AR-4 WG1, chap. 10, fig. 10.12). (b) The mean predictions for latitude zones, expressed as the change in precipitation minus evaporation (P-E). Note the predicted drying of midlatitudes (Held and Soden, J. Climate 19 (2006): 5686).
Two of these lakes are well known. One is our own Great Salt Lake in the state of Utah. The other is the Dead Sea, which occupies the rift zone separating Israel and Jordan. Both of these lakes were far larger during the last glacial period, as were lakes in New Mexico and Nevada, in northwestern China, and in the Patagonian drylands of Argentina. We know this because the ages of paleoshore lines, which stand out like bathtub rings, have been established using the radiocarbon method.
At the same time that the lakes in Earth’s mid-latitude dry zones were several times larger than now, equatorial lakes, such as Africa’s Lake Victoria, were dry. We know this because cores penetrating the lake sediments terminate in a soil horizon. Further, the radiocarbon ages of organic matter from the lake sediments deposited on this soil reveal that the lake came back into existence at the onset of the Bolling Allerod warm period, which marked the end of the last glacial period. The record from cold times lends support both to the predictions based on computer simulations that increased CO2 will warm the planet and also that it will focus its precipitation more strongly in the tropics.
Not only will the warming and the shift in precipitation patterns impact our lives, but these changes will lead to mayhem for our planet’s wildlife. As the CO2-induced warming intensifies, one species after another will be squeezed out of its current habitat and opportunists will move in as replacements. There will no longer be what might be called a steady-state grouping of plants, animals, and insects. Rather, all these residents will be in a state of flux. And of course species adapted to polar conditions (e.g., polar bears) may survive only in artificially cooled “zoos.”
As is already happening, Earth’s glaciers will continue to shrink. In Peru, where these masses of mountaintop ice serve as reservoirs providing meltwater during dry seasons and droughts, this disappearance may lead to severe water shortages. While the melting of the world’s mountain glaciers will lead to a modest rise in sea level, that of the Greenland and Antarctic ice caps surely will lead to very serious changes. If melted, Greenland ice would raise sea level by about 6 m, causing most of southern Florida to disappear beneath the sea. The West Antarctic ice sheet, considered vulnerable to warming, would create another 6 m of rise. Most projections place the timescale required for Greenland’s meltdown at several centuries. But the recent pronounced speedup of its main outlet glaciers and the observation of ever more widespread summer melt pools call for a reevaluation of this timescale. Photos of water accumulated in melt pools cascading into two abysses thought to extend to the base of the ice dramatically demonstrate that these outlet glaciers have a self-lubricating mechanism that might greatly increase their rate of flow into the sea. There is simply inadequate experience and understanding of the melting of ice sheets to fully evaluate the appropriate timescale.
For all these consequences, the present is the key to the future. Given the long timescale involved in changing our economy and infrastructure, there is little time to be wasted.
Our future prospects also need to take into account the overall condition of Earth during the last several thousand years, which has been a golden age of stability. Based on historical data, we cannot assume such stability will continue.
Figure 20-19 compares global temperatures for the Holocene to comparable periods during interglacials of the last 430,000 years. The length and stability of the current warm period have been the longest and most stable, although the interglacial 400,000 years ago comes close. There have also not been regional droughts of many decades but these are common in the historical record.
This stability is in part a consequence of the fact that the last significant volcanic eruption occurred in the early 1800s, and no truly large volcanic eruption has occurred during the Holocene. This last statement may seem perplexing, since most of us are familiar with the spectacular volcanic eruptions of Mount St. Helens in 1980 and Mount Pinatubo in 1991. Mount St. Helens erupted 2–3 km3 of material; Pinatubo was significantly larger at 10 km3 and also ejected 20 million tons of SO2 into the atmosphere. SO2 in the stratosphere leads to cooling, and Pinatubo caused about 0.5°C of global cooling between 1991 and 1993 (Fig. 20-20). This cooling also provided a useful calibration of climate models, which were able to predict the cooling effects very precisely. A much earlier basalt eruption from Laki volcano in Iceland (1783–1785) ejected close to 15 km3 of basaltic lava and likely large amounts of SO2. At this time the eastern United States recorded winter temperatures almost 5.0°C below average. Iceland lost most of its livestock and one quarter of its population to famine. In 2010 a much smaller Icelandic eruption led to major disruption of European air traffic. It is difficult to fathom what would be the consequences of a Laki eruption were it to occur today.
Fig. 20-19: Comparison of global temperatures for the Holocene compared to comparable periods during interglacials of the last 430,000 years. Note the long and stable temperatures during the Holocene that have supported the rise of human civilization and that are very unusual in recent Earth history. Each of the horizontal timescales encompasses 40,000 years. (Data from the Vostok ice core)
Fig. 20-20: (a) Photograph of the eruption of Mt. Pinatubo in 1991. The eruption injected 20 million tons of SO2 into the upper atmosphere, causing a temporary cooling of global climate. Much larger eruptions in the past (and future) would have had an even more dramatic effect. (Courtesy of U.S. Geological Survey.) (b) Solid line shows the observed changes in land surface air temperature caused by the Pinatubo eruption. This provided a test for climate models. Light gray shows the model results, which compare well with the observations. (Figure modified from Hansen et al. (1996), A Pinatubo climate modeling investigation, in The Mount Pinatubo Eruption, NATO AbI Series, vol. 1, 42, pp. 233–272, Springer Verlag)
In the context of the historical record of volcanism, however, even Pinatubo and Laki are babies. The Tambora eruption in Indonesia in 1815 was 160 km3 of ejecta and led to the “year without a summer” in North America and Europe. Heavy snow and frost gripped New England in June, and most crops were lost in North America, where animals were slaughtered for food. Europe experienced the worst famine of the ninetenth century, and riots, arson, and looting took place in many European cities.
Even Tambora is a modest eruption relative to the massive eruptions of “supervolcanoes.” Some 74,000 years ago, Toba in Sumatra erupted 2,800 km3 of ejecta, almost twenty times the volume of Tambora, leaving a crater (now a lake) that is 100 km long and 30 km wide. Although Tambora is in Indonesia, so much material was erupted that ash layers up to 6 m thick were deposited in India. The record of this eruption is preserved globally, including a marked signal in the Greenland ice core. The amount of SO2 erupted was likely >100 times larger than Mount Pinatubo. Model simulations of the Toba eruption suggest global cooling of 12°C (20°F) for six years, and destruction of much of the vegetation on the globe, including all the broadleaf trees. The Toba eruption has been called upon to explain a “bottleneck” in human population near that time, when human population is believed to have declined to as few as 10,000 individuals. While volcanic eruptions such as Tambora and Toba occur infrequently, eruptions of that magnitude will happen again periodically. They are the normal volcanic functioning of the solid Earth system.
These examples illustrate the fact that short-term climate change and natural disasters on a global scale are an inevitable aspect of Earth’s surface. The smooth and optimal climate conditions of the last two hundred years are exceptional. Our perspective of long-term global stability is an accident of the benign conditions that have prevailed since 1850.
Most of us are also not aware of the delicate balance between food production and supply that characterizes our global stability. Most arable land is cultivated, and there is a percent or two difference between food production and consumption. One billion people are malnourished. There are a couple of months of food reserves globally. We accept that modern civilization is able to feed itself, thanks to the takeover of all easily arable land, extensive use of fertilizer, and the green revolution. What we do not realize is that this has all been possible thanks to a remarkably benign period of planetary history. Between about 1550 and 1850 Earth experienced the “little ice age,” where the Thames froze in Britain, skating occurred on Amsterdam canals, and George Washington’s troops experienced the deprivations of Valley Forge. The exit from the “little ice age” led to exceptionally recent benign climate conditions for agriculture. So in the growth of human population between 1850 and 2010 there have been no major volcanic eruptions, and climate has been remarkably stable. None of the major short-term oscillations that are so apparent in the ice-core record have occurred. Assuming continued stability and no planetary crisis, all should be well, but we live on the edge of famine. Ultimately, planetary change will occur. Instability is a natural planetary condition. Given this reality, is it prudent to live at the edge of our means and cause perturbations to the planetary systems for which we do not understand the consequences?
In the absence of a change in human behavior, many of the negative planetary consequences discussed above will increase greatly in the twenty-first century, because the root causes continue to increase. Our influence scales either directly with population (e.g., food, land use), or exponentially with population owing to the combination of population and economic growth (e.g., energy consumption and associated CO2 emissions). Controlling population growth is not generally on the table as an acceptable environmental solution. Economic growth is based on free resources and increased energy production, for which the only viable option for decades has been fossil fuels, and this remains the global economic model. As long as biodiversity, soils, and fossil fuels are not recognized as rare and precious planetary resources, they will continue to decline until their scarcity causes crisis. At that point they can never recover on timescales pertinent to us. And if greenhouse gases accumulate sufficiently and climate change ceases to be gradual, nothing can be done because of the long residence time of CO2 in the atmosphere.
The future need not be so bleak. We have the capacity to change our behavior and manage the planet for the mutual benefit of all species. We simply eliminate population growth by having one to two children per family globally, become largely vegetarian in order to make more efficient use of land and food, make a concerted global effort toward sustainable energy sources, travel in small vehicles and trains, live in only the space we need, wear sweaters in winter and sweat a little in summer, adopt sustainable farming practices, reduce our waste of resources, remove and sequester CO2 from the atmosphere, and start to promote forests and grasslands instead of destroying them. We can carefully monitor the other life that shares and supports the planet and preserve its capacity for life as well as our own. All of these are within our power of technology and choice. Would these changes drastically reduce our quality of life? Actually, many of them would improve it. Some of these changes would save money. Others could take place only by adding environmental costs to use of resources.
A principal factor that prevents action is that our economic model has no simple way to account for environmental costs. Agriculture does not take into account the costs of soil depletion. Fossil fuel burning does not take into account modification of the atmosphere. People do not pay for the CO2 they emit. Habitat destruction does not take into account the destruction of species. Fishermen do not pay for the fish they take from the sea; lumber, oil, coal, and mining companies do not pay for their resources, aside from the cost to buy the land. Economic costs are only those of extraction and delivery—Earth is free. It is as if there were a bank in town, filled with gold that has accumulated over vast periods of time, and without asking where it came from or even any appreciation, we were able to walk into the bank, perhaps pay a small entrance fee, and take all the gold we want and use it. Our unquestioned attitude is that Earth’s resources are free—the only major costs should be those to extract and ship them. The driving force is “what would make me more money now,” irrespective of the long-term consequences for myself or others. Our economic model does not take into account environmental costs and consequences or planetary history.
This economic model of industrial civilization is justifiable when environmental impacts are small enough that they do not have planetary significance and when resources—the Earth bank—are believed to be infinite. If environmental impacts are large enough that they need to be taken into account, we have no free market model for doing so. If resources are finite and environmental impacts detrimental, cost should reflect those facts as well as the cost of extraction.
An additional problem is that we do not know well the cost that would be involved. E. O. Wilson has estimated that $50 billion would be sufficient to save the remaining biodiversity hot spots that contain the majority of threatened species, but this estimate does not include the costs of mitigating climate change that would modify the habitat. Costs to conserve energy can be profitable over time frames of decades; $100 billion dollars per year would make a vast difference in CO2 emissions. Sustainable farming practices can be economically favorable over the long term. To put these costs in context, these expenses are small relative to the amount we spend globally on the military. Fifteen percent of global expenses on war and its instruments would protect the planet. If the United States were to fund its defense budget with a gas tax, gasoline would cost $8 per gallon, yet even ten cents per gallon is viewed as unacceptable for costs of damage to the planet. So the money is there, the technology can be developed. We have the practical capacity for planetary management. It is a matter of choice. Do we choose to protect the planet and conserve its treasure and thereby also prevent wars over resources, or do we continue on our present course and spend more on military capability and war in order to access the resources?
There is a positive solution where strategic, energy, and climate interests converge. Reducing energy consumption and converting to wind and solar power would reduce the necessity to access oil reserves, lessen the need for costly military interventions, and stem a huge export of national currencies to other nations. Climate, the economy, and national security all benefit.
The lack of action is one of political and personal choice, not one of technological capability. The willingness to act could occur if we were to realize at the level of actions rather than concepts that we are an integral part of the planetary system, not just a user of it. If this choice of planetary conservation were made, what could be done?
The area of human influence that has received the most attention is what to do about energy and CO2 emissions. To reduce CO2 emissions we can:
• increase our productivity per unit of energy (e.g., energy conservation);
• reduce our dependence on fossil fuels for energy and adopt energy sources that do not release CO2;
• remove CO2 from the atmosphere by carbon capture and sequestration.
All of these in concert could limit the CO2 buildup in the atmosphere with its consequences for global warming, sea-level rise, ocean acidification, and biosphere impacts.
One of the easiest means to reduce energy consumption would be to be more efficient. The United States and China in particular are 160% less energy efficient than Europe and Japan per dollar of GDP. Vast changes in this respect would be possible, and would occur quite naturally if the cost of energy were to rise. Such actions would only limit the rise in CO2 emissions, however, and the ultimate goal must be to have them drop to near zero. This is a much longer-term task, requiring conversion of our entire energy infrastructure. Eighty-five percent of our energy is currently derived from the burning of coal, petroleum, and natural gas. Most electricity globally is produced by coal, which is about one-fifth the cost of oil per unit of energy. To move away from fossil fuels would involve a switch to some combination of other energy sources (nuclear, wind, hydro, solar, geothermal, vegetation …). Many of these have their own associated problems. A spread in nuclear power globally could lead to nuclear accidents, nuclear proliferation, and an enhanced terrorist threat, and energy from vegetation competes with crops, raising food prices and limiting food availability. Energy conversion is a multifaceted and complex problem.
Two major routes exist by which energy from the sun might be harnessed. One involves photovoltaic cells and the other biofuels. The photovoltaic route is presently stalled due to high costs. Further, such an energy source would have to be coupled with an energy storage system that would provide power during nights and periods of heavy cloud cover. While an ideal solution because the energy from the sun is so vast and is everywhere, large-scale use of photovoltaic energy awaits substantial technological advances or a large carbon tax that makes it competitive.
The other form of solar energy is biofuels, which is already being actively pursued. Biofuels are a closed loop for CO2. The plants remove CO2 from the atmosphere converting it to organic carbon, which is then burned to return the CO2 to the atmosphere with no net CO2 addition. The rub is that production of biofuels takes energy to fertilize the soil, grow and harvest the plants, and produce and transport the fuel. In the United States, biofuel production likely uses more energy than is produced, or at best uses 90% of the energy. At the same time, corn for biofuels competes with food, raising food prices. It is a great boon for agribusiness, because almost all the increase in grain production in the United States has been for ethanol. U.S. biofuels do not contribute meaningfully to CO2 reduction. In Brazil gasohol made from sugar cane is widely used, and because of the tropical climate and long growing season, such production is energy efficient and the ethanol is cheaper. (Because Brazilian ethanol is cheaper, the United States put major restrictions on its import.) While in principle more land could be devoted to biofuels, that choice would also lead to greatly increased habitat destruction, accompanied by inevitable loss of biodiversity and competition between food and energy that would lead to higher prices for both. The sensible future of biofuels may be to provide liquid energy for transportation once petroleum reserves begin to seriously diminish.
Wind is currently economically competitive, and installation of wind turbines is growing rapidly. Wind has a fundamental limitation in that were it to take over a major segment of the energy supply, it would sap 10–20% of the energy carried by surface winds. The consequent climate change might rival that from a large CO2 buildup. Wind, like solar, is also a highly distributed energy source, and is not easily stored and cannot be called up on demand. On cloudy and windless days there would be no energy available! Using these sources for more than 10% or so of the energy supply would require a reworking of the national electricity grid. Wind and solar are part of the long-term solution, but the time frame is long and the challenges great.
As things now stand, no combination of these alternative energy sources can make a major contribution. Solar electricity remains far too expensive. Hydro- and geothermal powers are already near their capacity and are too limited. Both wind and solar in large amounts are inconsistent with our current electrical grid.
Nuclear power has supplied significant amounts of electricity in Japan, Switzerland, and particularly France. After a history of safe operation for decades, the 2011 nuclear accident in Japan showed the potential dangers of nuclear power and the widespread consequences that such an accident can have. Even with this accident, however, the long-term environmental costs, amount of radiation released, and industrial safety record are all better for nuclear power than for coal! Construction of new nuclear power plants, however, is such a long and slow process that it could make only a modest increment to our energy needs over the next few decades. The need for enhanced regulation and safety after the accident stemming from the Japan earthquake can only make the process slower. Safety issues may even rule out an important role for nuclear power. Germany, for example, plans to abandon all nuclear power by 2022.
If costs of energy reflected atmospheric change, all these options would become cheaper than fossil fuels, leading to rapid growth in many of these industries. The timescale, however, is long. The pot at the end of the rainbow would be power from nuclear fusion, which has vast potential and is environmentally clean. Advocates claim that fusion power by midcentury may begin to provide portions of our energy needs. If fossil fuel energy became more expensive, therefore, there are long-term prospects of low CO2 emissions. With conservation, renewable energy, and nuclear energy and fusion, our energy needs could be fulfilled by non-fossil fuels by midcentury. What would be required to make this happen, however, would be coordinated and concerted action now and a revised economic model for energy costs. Even with that optimistic scenario, there is clearly a need for a stopgap over the next decades to moderate CO2 buildup.
Fortunately, there is a route by which an unacceptable CO2 rise can be avoided. It involves the capture of CO2 coupled with long-term storage. The obvious target would be to scrub CO2 from the stack effluent of coalfired electrical power plants. However, as this would likely be prohibitively expensive, a more economical alternative has been identified. Coal gasification runs steam over coal to produce CO and H2 (i.e., coal + H2O → H2 + CO) instead of burning the coal in atmospheric oxygen. The CO is then oxidized to CO2, and the H2 is fed to an electricity-generating fuel cell (i.e., a flow-through battery). Such plants can be far more cheaply modified for CO2 capture. This route also yields to more efficient use of the chemical energy contained in coal. The top few thousand sites of CO2 emissions, mostly utility plants, account for more than 30% of total emission. Concentrated CO2 capture at these locations would make a major contribution.
No matter how it’s accomplished, capture of the CO2 produced in electrical power plants is only part of the answer. CO2 also has to be removed from small sources. Today roughly two-thirds of the fossil fuels are burned in small units (automobiles, homes, etc.). For each tankful of gasoline (~50 kg) burned in an ordinary automobile, about 150 kg of CO2 are produced (that’s about 1 pound per mile!). Two routes might be taken to abate CO2 emissions by the transportation fleet, which today accounts for one-third of fossil fuel consumption. One would be to power vehicles with rechargeable batteries or with hydrogen fuel cells. In either case, the energy would ultimately come from electrical power plants. Nice idea, but no cigar. No battery has yet been developed that can power a vehicle for more than short runs, and electrical vehicles still use substantial gasoline. Nor has any feasible means been found to store enough H2 aboard a vehicle to allow it to operate for many days. So, barring a major breakthrough, this is not the answer.
Klaus Lackner, a scientist at Columbia University, has suggested that removal of CO2 from the atmosphere may be doable at a modest cost. His case is based on an analogy to wind-generated electricity. In order to supply the energy required to support the average American, a rotor intercepting 100 square meters of brisk wind is required. By contrast, Lackner shows that in order to remove from the atmosphere the CO2 produced, if this energy were instead obtained by burning fossil fuels, one would have to capture the CO2 from only 1 square meter of the same wind stream. Capture would either be into a CO2 absorbing liquid such as Ca(OH)2 or on a plastic that was loaded with chemical receptors able to capture and release CO2. The advantages of this form of carbon capture are that it can be done anywhere, and it provides a means to capture CO2 emitted by small sources such as cars and homes. Once captured, the CO2 would have to be recovered and the absorber recycled. An advantage of air extraction is that it could be done anywhere on the planet rather than close to the place where the energy is produced, as is the case with electrical power plants.
Fig. 20-21: Illustration of the different modes of CO2 storage after capture, as discussed in the text.
All these mechanisms lead to the capturing of CO2. The problem then is what to do with it. Several options exist for storing the CO2 once it’s captured (Fig. 20-21).
Currently only about one-sixth of the ocean’s capacity for CO2 uptake is being utilized. The reason is that subsurface waters are replaced by waters that have been in contact with the atmosphere only very slowly. The deeper the water, the slower its replacement. As the deeper parts of the ocean will not take up their share for hundreds of years, the idea is to short-circuit the delivery by pumping liquid CO2 directly into the deep sea. Although liquid CO2 is less dense than surface ocean water, it is more compressible. At a depth of 3,500 m, the densities of seawater and liquid CO2 become equal. Below this depth liquid CO2 is denser than seawater. Hence, if injected below a depth of 3,500 meters, liquid CO2 would sink to the seafloor. Further, it would not remain a liquid, for under the cold and high-pressure conditions that prevail in the deep sea, the CO2 would combine with H2O to form a solid whose formula is 6H2O × CO2. Chemists refer to this solid as a clathrate. As the clathrate is denser than either liquid CO2 or seawater, it would pile up on the bottom. Of course, over time the clathrate would dissolve and the CO2 would be dispersed throughout the deep sea, where it would react with the resident carbonate ions to form bicarbonate ions. In this way, delivery of CO2 to the deep sea could be greatly speeded.
Antarctica’s ice cap is underlaid by hundreds of lakes. They form because Earth’s internal heat, diffusing up from beneath, warms and in some places melts the basal ice. The idea would be to pipe liquid CO2 down through the ice into these lakes. Upon arrival, the CO2 would react with the lake water and form a clathrate, which would sink to the lake bottom. As it would be prohibitively expensive to pipe liquid CO2 to Antarctica, were this option to be implemented, it would have to be coupled with CO2 extraction from the air over the ice cap. As the atmosphere mixes extremely rapidly, CO2 removal could be carried out anywhere on the planet. Just as the air over regions like the New York metropolitan area does not experience a significant buildup in CO2, neither would the air over Antarctica experience a significant depletion.
The pores in the deep strata of sedimentary basins are invariably filled with very salty waters known as brines. As the brines have been trapped in these reservoirs for millions of years, another option is to pump liquid CO2 into these salty waters. Unlike the deep ocean and the lakes beneath Antarctica, these brines are too warm for CO2 clathrates to be stable. Hence, the CO2 would remain in liquid form. This is fortunate, because were clathrates to form, they would clog the sediment pores and prevent the liquid CO2 from spreading out into the aquifer. Statoil, a Norwegian energy company, is already doing this. They recover methane from a reservoir beneath the North Sea. The 15% CO2 this gas contains must be separated before the methane can be burned. Normally, this separated CO2 would be released to the atmosphere. But as Norway has an emission tax of $50 per ton of CO2, Statoil decided it would be cheaper to liquefy the separated CO2 and pump it back down into a water-filled stratum. This is now being done routinely. A tiny beginning!
As pressure increases with depth, CO2 turns from a gas to a liquid and its density becomes greater than seawater. Kurt House and Dan Schrag have proposed that this CO2 could be injected into sediments below the sea floor and remain there stably with no impact on the deep ocean biosphere and no tendency to escape. This solution would be particularly appropriate for power plants adjacent to a continental shelf, which has sediments at the appropriate depth. Storage in deep-sea sediments is also promising for coastal locations.
With somewhat more effort, it is possible to permanently immobilize CO2. This option involves reacting CO2 with MgO to form a tough and resistant magnesium carbonate mineral. One option would be to obtain MgO by grinding up and dissolving ultrabasic rock whose dominant mineral is olivine with a chemical formula, Mg2SiO4. Hence, the reaction would be:
Mg2SiO4 + 2CO2 → 2MgCO3 + SiO2
Although nearly all of Earth’s ultrabasic rock resides in its mantle and hence is unavailable to us, surface outcrops do exist in many places. So, large electrical power plants and air extraction facilities would be constructed at the sites of these ultrabasic rock outcrops.
Nature also makes large quantities of carbonate-rich veins in mantle rocks that are exposed at the surface. An intriguing new possibility being proposed by Peter Kelemen is that this reaction might be able to be self-sustaining if the rocks were cracked and injected with CO2-rich fluids.
None of these storage options is without engineering challenges or environmental impacts. Concern has already been raised about the possible impacts of deep-sea storage on organisms inhabiting the ocean deeps. Greenpeace has already taken a strong stand against this option. In order to implement Antarctic disposal, it would be necessary to modify an existing treaty that bans mining on the continent of Antarctica. Before permitting large quantities of liquid CO2 to be injected into saline aquifers beneath their homes, people would want to be assured that this activity would not trigger damaging earthquakes or lead to catastrophic releases of CO2. Finally, even the conversion of CO2 to MgCO3 is not free of environmental problems. Large mines or injection operations with a substantial infrastructure would need to be constructed.
An additional alternative to CO2 sequestration is to adopt an approach of “geo-engineering” that would attempt to modify the atmosphere to reduce solar radiation reaching the surface. Models tell us that doubling CO2 is equivalent to dialing up the sun by about 2%. If so, then to counter a doubling, we would have to reflect away 2% of the sunlight reaching the top of the atmosphere. While several ways to do this have been suggested, one stands out as the cheapest and least environmentally intrusive. It involves injecting SO2 gas into the stratosphere. As revealed by nature’s experiments (i.e., the eruption of El Chichón in 1984 and of Mount Pinatubo in 1991, the SO2 is rapidly converted to tiny sulfuric acid (H2SO4) aerosols. These aerosols backscatter about 10% of the sunlight that encounters them. Thus, to reflect away 2% of the sun’s rays, they would have to intercept 20% of the incoming rays. This would require a standing inventory of 32 million tons of SO2. As the residence time of the aerosols in the stratosphere is only one to two years, they would have to be replaced regularly. This, of course, has the advantage that if the side effects proved unacceptable, the injections could be terminated and the aerosols would soon be gone. It, of course, has the disadvantage that if for some reason the injections were stopped due to war or political upheaval, the warmth would return. Furthermore, such engineering does nothing to halt the acidification of the oceans or the destruction of biodiversity, and there might be unforeseen consequences as well.
Clearly, any solution to the CO2 problem will have its own set of environmental concerns. As this is unavoidable, the goal would be that the environmental damage stemming from the solution be far smaller than that stemming from the CO2 itself.
Regardless of the route taken, the solution to the CO2 problem will be a huge enterprise. Its magnitude is most easily appreciated by considering the amount of liquid CO2 that would have to be disposed of, if fossil fuels were to continue to dominate the energy market. At today’s rate of usage, about 24 km3 of liquid CO2 would be produced each year. If in 2060, there are 10 billion people, and if by then poverty has been largely eliminated, the amount would rise to perhaps 64 km3 each year of liquid CO2 and some 2,500 km3 of CO2 would have been stored, more than the combined volumes of Lakes Erie and Ontario!
Clearly, the capture and storage of CO2 would raise the cost of fossil fuel energy, but the increases are modest. It is estimated that the cost increase will be on the order of 25±10%. In total, estimates are that controlling CO2 would reduce global GDP by growth by only 0.1–.15% annually to 2050. This is a very small number, particularly when compared to other governmental costs such as defense or health.
Despite the gravity of the problem and the reasonableness of the cost to take effective action, there is remarkably stiff resistance to any significant steps in the service of planetary conservation. If we are to prevent an objectionable CO2 rise, we face an uphill battle. The technology required to do the job must be created pretty much from scratch, a plan for payment must be developed, 180 nations must be brought on board, a skeptical public must be convinced.… These tasks will take decades, and implementing the technology and infrastructure decades more. At the moment, there is no indication that the flow of CO2 into the atmosphere will be brought to a halt.
The steps outlined above are possible solutions to the modification of the atmosphere and the acidification of the oceans. They do not address the living environment of soils and biosphere. Atmospheric change is apparent from measurements of gases and in recent decades from increasing temperature and sea level rise. Both of these can be felt and imagined as directly influencing human well-being. Destruction of soils and the biosphere, however, has no immediate impacts and is essentially invisible to the majority of human population that has little contact with land or nature. Soils could be considered as important as the atmosphere to our long-term well-being, but the coverage of soil deterioration in the media is minuscule to absent, as compared to climate change, which appears in most news media on a daily basis. Biosphere destruction is the concern of a small group of conservationists but is peripheral to most of us. For neither soils nor biosphere are there equivalent organizations to the IPCC to bring global attention and scientific expertise to the issues. Both of these problems are as important as climate change in terms of their long-term impact. Both have structural parallels to climate change—the modern economic model does not take into account the long-term costs, which leads to inevitable deterioration. Solving the energy and atmospheric problems could give the impression of a clean slate for further population and economic growth based on human consumption without consideration of consequences. So the problem we face is not simply the technical challenge of climate change; it is the economic model and human attitude toward the planet on which we live.
Another important aspect is the prevalence of food shortages and poverty in the developing world. For the billion people who are the most destitute poor, the main consideration is to have food, shelter, and sufficient fuel to survive. There is no choice of reducing carbon emissions or not cutting down forests for food or fuel. Faced with poverty or famine, none of us is capable of broader questions of planetary health. The poor are also in areas where the population is increasing most rapidly and where biodiversity is most threatened. If we are to solve the planetary crisis, we must also solve the human crisis. The challenge we face is not only our attitude toward our planet, but also our attitude toward our fellow human beings.
The scope and rate of change induced by human civilization is planet altering. This has led Paul Crutzen to suggest that we now live in a new geological epoch, the Anthropocene, and that the Holocene epoch ended as human civilization began. Epoch boundaries are generally minor planetary events, and the change wrought by humans is not minor. Human civilization has led to the first global community of a single species, destruction of billions of years of accumulation of resources, a change in atmospheric composition, a fourth planetary energy revolution, and a mass extinction. Furthermore, our technology now permits directed evolution, a sensing awareness of planetary systems from land and space, and potential communication with other planets in the galaxy. The creation of intelligent life and civilization has produced a fundamentally different planetary system than existed ten thousand years ago. One could argue that the potential for planetary change is almost as great as that caused by origin of life or the rise of oxygen.
The only other boundaries in Earth’s history that have encompassed such vast changes are era boundaries. The second rise of O2 and development of multicellular life were one such boundary. The Permo-Triassic extinction was arguably not as significant, because while it involved a massive change in life, it did not involve an energy revoluton, a change in the character of evolution, or fundamental change in life’s capacity for planetary change. It appears, therefore, that we have not simply changed epochs from Holocene to Anthropocene; one could argue that we have changed eras and eons, from Cenozoic and Phanerozoic to Anthropozoic. There is, of course, one aspect of eons that remains a great unknown. Eon and era boundaries of the past have separated spans of time of hundreds of millions of years. We are a few hundred years into the human era. Will we survive? Will we have the wisdom and conscience to realize our potential place in planetary history? There is the potential in human civilization for Earth to pass from “habitable planet” to “inhabited planet,” i.e., one that carries intelligence and consciousness on a global scale, for the benefit and further development of the planet and all its life. Or the Anthropozoic era could be an abortive and failed mutation, as the intelligent species destroys itself and its environment. Should we fail and another form of intelligent life comes along in a few tens of millions of years, they would find a planet devoid of most of its treasure chest. A second effort at planetary civilization would be correspondingly more difficult.
Even in small numbers, human beings exerted a dramatic influence on the planet and its biosphere. Evidence for early interventions comes from extinctions of large animals on the heels of our migration into hitherto “virgin” lands. With the advent of favorable climate conditions and our access to energy, we became able to modify Earth for our needs and to have incomparable advantages compared to other species. Our growth in population and use of resources has enabled all the wonders of modern civilization and also created the potential for environmental catastrophe. Human beings are influencing the atmosphere, ocean, soils, and biosphere on planetary scales. Our success has been possible because of the long evolution of our planet toward increasing habitability and a relatively long period of planetary stability in terms of climate and volcanism. Our recent actions have made the planet more habitable for ourselves in the short term and far less habitable for all other species that we do not protect and harvest for our food. In the long term, our modifications of the physical environment and destruction of other species and entire ecosystems may lead to planetary crises that affect our own preservation. This situation creates a unique problem in the history of life. We have won the battle of biological evolution. We have won it to such an extent that we are able to destroy other life, use up irreplaceable planetary resources, and unconsciously modify the planetary environment. Because of our impacts, planetary evolution now depends on our behavior. The health and future direction of Earth will depend on our actions.
E. O. Wilson. The Diversity of Life. 1999. New York: W. W. Norton & Co.
Fourth Assessment Report (AR4) of the Intergovernmental Panel on Climate Change (IPCC). http://www.ipcc.ch/ipccreports/ar4-wg1.htm.
Comprehensive Assessment of Water Management in Agriculture. 2007. Water for Food, Water for Life: A Comprehensive Assessment of Water Management in Agriculture. London: Earthscan, and Colombo: International Water Management Institute. http://www.iwmi.cgiar.org/assessment.
Peter Gleick et al. 2006. The World’s Water 2006–2007. The Biennial Report on Freshwater Resources. Washington, DC: Island Press.
ISRIC—World Soil Information. http://www.isric.org.