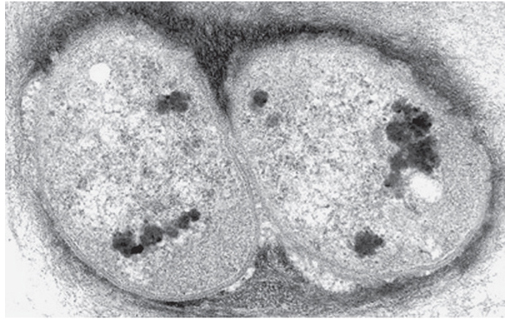
Fig. 13-0: TEM image of a complete division of a bacteria cell. Note the cell membrane delimiting their cytoplasm from the extracellular matrix. Magnification 20,000×. (Image reprinted by permission from the author, M. Halit Umar, and the copyright-holder, © Microscopy UK or their contributors, Copyright 2000)
How life began is the least understood aspect of the development of our habitable planet. There is evidence for primitive life between 3.5 and 3.0 Ga, but a good fossil record exists only for the most recent 10% of Earth’s history, since the beginning of the Cambrian period, 543 million years ago. Earth’s earliest history, when life likely began, has no direct record at all. Understanding life’s origin then requires careful detective work to infer a process hidden in the deepest planetary past.
The most important evidence comes from life itself. Life depends on the alpha-particle nuclide carbon, which can build three-dimensional molecules of immense variety and size and has many oxidation states that facilitate the transfer of electrons that are essential for life’s energetic processes. The other critical elements that make up more than 98% of living matter are H, O, N, and S. Phosphorous plays a critical role but is low in actual concentration. All of these elements are made in abundance by nucleosynthesis. With a few notable exceptions such as hydrogen and the heavy elements that make up rocks, the chemical composition of life is very similar to the sun, showing the strong influence of the laws of nucelosynthesis on life’s development.
A single origin for life is evident from the commonalities among all living organisms. All life is cellular, and the earliest evidence for life is unicellular organisms that have similarities to the most primitive organisms living today. All cells today have the same sets of molecules as essential building blocks—carbohydrates, lipids, amino acids, and nucleic acids. The amino acids have a particular chirality—i.e., they are “left-handed.” All cells also have the same chemical machines, the most central of which are the pathways from DNA to RNA to proteins that govern cellular operations, the role of DNA in inheritance, and the processes that store and release energy through adenosine triphosphate (ATP).
The unity of life and the gradual changes in life through time point to a first common ancestor, the primitive cell from which all subsequent life evolved. The origin of life can then be viewed as a series of steps leading to this first cell. These steps involve (1) formation of the molecular building blocks in the correct state of matter; (2) construction of complex molecules from simpler components; (3) development of an outer membrane to contain the cell contents; (4) a chiral selection process; and (5) self-replicating chemical cycles. There is clear evidence for the first three of these, and examples of emerging possibilities for the remaining steps.
Life is often viewed as going “against nature” because life involves increasing order and decreasing entropy, which appear to violate thermodynamics. Life also has many “chicken and egg” paradoxes. The increasing order is possible because life is a nested system, transforming energy from sun and Earth. Life facilitates this energy transformation and leads to faster production of entropy in the larger system than would happen absent life. The “chicken-egg” relationship is inevitable when seen as a progressive evolutionary sequence of chemical cycles that become dependent on one another. Such processes have the great advantage of being self-sustaining.
The origin and evolution of life is a solar system process, deriving energy from Earth and sun and fully dependent on planetary cycles. The origin of life cannot be solved absent understanding of the planetary conditions that made it possible. If life is viewed as an efficient and natural planetary process, then it is likely to occur widely throughout the universe.
Earth today is fully inhabited. On the microscopic scale, millions of species, most of them unidentified, occupy every ecological niche, even apparently hostile ones such as saline brines in oil fields, toxic waste dumps, or cracks deep in Earth’s crust. Life is so successful that each milliliter of seawater contains more than 10 million micro-organisms, and every square centimeter of our skin is home to a zoo of millions of minute cells, thriving off our waste products. Where did all this life come from, and how did it start? Is life a planetary accident or part of normal planetary functioning? Is life a passive passenger on Earth’s surface or an integral part of the planetary system? Have the inhabitants influenced and modified the planet’s habitability? The next chapters address these issues that are central to our understanding of Earth as an inhabited planet.
Life is a chemical phenomenon based on molecules that transfer material and energy in complex cycles within and among organisms and in exchange with the environment. Like Earth itself, life is a system and shares the characteristics of natural systems outlined in Chapter 1. Life is distinguished from other natural systems, however, by being capable of and undergoing Darwinian evolution. Life is also is based on a fundamentally different chemical structure than the rock and metal that make up the solid planet. Both life and rock, however, have the commonality that they depend upon the chemical behavior of a single element that occupies a position in the middle of the Periodic Table, with a 4+ valence that makes bonds in three dimensions and can build three-dimensional building blocks. For rocks, the 4+ element is Si, and the fundamental building block is the silica tetrahedron discussed in Chapter 4. For life, the 4+ element is carbon, and the structures are the organic molecules on which all life is based. As alpha particle nuclides, both C and Si are very abundant in the universe. What about other elements that are also centrally located in the periodic table? Beneath C and Si in the periodic table is the element germanium (Ge), which is also a 4+ element and can also form a complex class of three-dimensional molecules, called the germanates. Germanium, however, has a mass of 72, higher than 56Fe, and very little Ge is made in stars. Even though it is a refractory element, the terrestrial abundance is only 1 part per million—250,000 times less than Si. The stellar processes of nucleosynthesis relegate Ge to trivial rather than planetary importance.
Carbon has five major advantages compared to silicon that permit more complex three-dimensional structures, more diverse chemical reactions, and easier chemical transport:
(1) Under normal planetary conditions carbon can bond to itself as well as to other elements—carbon-carbon bonds are at the backbone of many organic molecules.
(2) Organic molecules can bend and fold, creating large, complex three-dimensional structures like proteins and DNA that are central to life processes, whereas silicate minerals are relatively rigid and inflexible.
(3) Carbon forms various common molecules that can be solid (e.g., bone, limestone, and wood), liquid (e.g., alcohol, gasoline, and acetone), and gas (e.g., carbon dioxide and methane [natural gas]) at the same temperature and pressure. This allows transport and exchange of carbon among solid, liquid, and gaseous reservoirs.
(4) Carbon can form molecules, some of which are soluble in water (e.g., sugar, alcohol) and some insoluble (e.g., wood and oil), permitting the coexistence of and exchange between solid and liquid.
(5) Carbon can have multiple valence states (e.g., 4+ in CO2, neutral in C, and 4– in CH4) that enable electron transfer reactions permitting energy flow and storage.
The greater flexibility of carbon bonds leads to a far greater assemblage of molecules than is possible with silicates, and millions of different organic molecules are known (Fig. 13-1), compared to thousands of silicate minerals. In contrast to silicates, organic molecules are almost infinite in their capacity for variation and modification. Their ability to be present as different states of matter and in one form to be stable in the presence of fluid and in another to be transported by the fluid permits chemical cycles. The electron transfer processes allows collection of energy from the environment (e.g., by eating or by photosynthesis) and its storage and transfer, permitting energy systems within an organism and in ecosystems. While the solid earth transfers mass and energy on geological timescales of thousands to millions of years, and relies mostly on large changes in temperature and pressure to implement changes in state and transfer of energy, organic compounds can perform similar functions over the diversity of timescales that are pertinent to life—shorter than microseconds in terms of energy transfer within a cell and as long as years to decades for the storage of food and transfer of matter and energy through ecosystems.
Fig. 13-1: Ball-and-stick models of a simple and a complex organic molecule. On the left is glucose C6H12O6, consisting of twenty-four atoms with a molecular weight of 180. The gray balls that have four bonds are the carbon atoms. On the right is hemoglobin (C738H1166N812O203S2Fe)4, with a molecular weight of about 67,000. Each tiny dot is one of the atoms. The large gray balls are the four Fe atoms in the hemoglobin. Hemoglobin is a protein made up of 574 amino acids of twenty different types. (University of Arizona)
One of the largest questions about life in the universe and early life on Earth is whether other forms of life very different from our own (e.g., not based on carbon) might be possible. While one cannot definitively rule out such a possibility, from the perspectives just given the chance of a life system based on an element other than carbon seems remote indeed. Silicate-based life in particular would have huge disadvantages compared to carbon-based life systems. Instead, it appears from our limited sample that there is a straightforward consequence of the abundances of the elements produced by nucleosynthesis and the nature of the properties of elements in the Periodic Table. Silicates and metals with a central role for Si and Fe form the three-dimensional structure of rocky planets. Organic molecules with a central role for C form the structure of life. Both of them are produced in abundance in the universe and depend on the unique characteristics and three-dimensional capabilities inherited from the fundamental atomic structure revealed in the Periodic Table.
In Chapter 6 we were able to understand the abundances of the different elements in the entire Earth by comparing them to nongaseous elements in the sun and in chondrites, and we noted the overall correspondence between the chondritic meteorites and the solid Earth, adjusted for the relative volatility of the different elements. This led to an understanding of the dominance of Fe, Mg, Si, and O, which make up more than 90% of the planet.
As the solid planetary system is dominated by only four elements, organic life also consists largely of a small number of elements. H, O, and C make up 98% of the human body in terms of numbers of atoms, and 93% by weight. The next three most abundant elements are N, S, and P, and 99% of organic molecules are made up of just these six elements. It is clear that life has a fundamentally different chemical composition than rock and metal, and we are not simply made up of representative fragments of Earth.
If we view the chemical composition of life from a cosmic perspective, however, the dominance of H, C, O, N, and S is not surprising. For the solid Earth we could understand the dominance of the elements Fe, Mg, Si, and O by considering the amounts made during nucleosynthesis and the loss of volatile elements during planetary formation (see Table 5-5). We can similarly understand the chemical composition of life, recognizing that life relies on volatile elements and largely excludes the refractory lithophile elements that go predominantly into rocks and the siderophile elements that go into the core. Let’s revisit the first twenty-eight elements of the Periodic Table from this perspective (refer to Table 5-5). Hydrogen is the most abundant element in the universe, and also very important for life. Helium and the other noble gases are not chemically reactive and therefore do not participate significantly in low-temperature chemical systems such as life. Li, Be, and B are only produced in tiny quantities during the Big Bang. The next two most abundant elements produced during nucleosynthesis are the alpha-particle nuclides 12C and 16O, and 14N is the even nuclide that occurs between them on the Periodic Table. All of these are produced in abundance by nucleosynthesis and are central to life. F and Na are odd, and Ne is a noble gas. Mg, Al, and Si are made in abundance, but are refractory lithophiles that go into rocks. Phosphorous appears at first to be more of a puzzle. It is next to Si and below N on the Periodic Table, and substantial amounts are formed during nucleosynthesis, but much less than the alpha-particle nuclides and nitrogen that are otherwise dominant. This paradox is partially resolved when we note that while phosphorous is a common constituent of vital organic molecules, it is a minor constituent in terms of number of atoms. For example, the molecule of adenosine triphosphate that lies at the heart of energy transfer in life contains three P atoms and forty-four C, H, and N atoms. The role of phosphorous is important; its overall abundance is small. Sulfur, the next most abundant element in life, is also an alpha-particle nuclide. While central to life, it is actually low in relative abundance, which may be understood by its combination with Fe and incorporation into the core. All of the elements heavier than S are either odd, noble gases, or lithophile and/or siderophile.
Figure 13-2 shows graphically that for all elements but noble gases and the rock forming elements Fe, Mg, Si, and Al, there is an approximate correspondence between solar abundances and human abundances. The chemical composition of life makes some sense from a cosmic perspective. From a chemical perspective, life is the complement of the solid Earth. The solid Earth is representative of the sun and solar system minus most of the volatile elements; life is representative of the sun and solar system minus the rock- and metal-forming elements that make up the solid Earth.
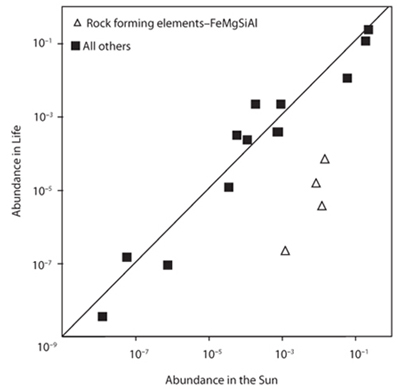
Fig. 13-2: Comparison of relative abundances of Li, Be, B, C, N, O, F, Na, Ca, Al, Si, P, S, and Fe in living tissue and in the sun. Values are normalized to H/1000. Note that with exception of the strongly lithophile Si, Al, Mg, and siderophile Fe, there is broad similarity between life and sun in relative abundances.
Of course, just as minor elements such as H2O and CO2 play an important role in planetary systems, minor elements such as Fe, Ca, and Zn play a very important role in living systems. Bones and skeletons require Ca. Hemoglobin has Fe as its central and all-important molecule. About one-half of all enzymes have a metal atom as an important constituent. Life is fully planetary in its chemical composition.
For most of us, the impression of the life that surrounds us is one of great diversity. Mold growing on old food, giant sequoias, oysters, cobras, cockroaches, and human beings seem very dissimilar from one another. At the same time, we see great commonalities among different types of life—mammals share many characteristics, as do many flowering plants.
While we tend to see the differences among living organisms and can marvel at life’s diversity, examination of life at the microscopic and molecular levels provides a very different perspective, showing that all of life shares essential characteristics. It is this fact that allows the question of the origin of life to be reduced to the origin of the simplest single-celled organism that has the essential characteristics shared by all of life today. What are those characteristics?
All of life is made up of cells with similar attributes. Whether an organism is a single-celled bacteria or complex assemblage of tens of trillions of cells of some 210 different types that make up a person, life is cellular.1 Figure 13-3 illustrates this simple fact, where a unicelled fungus is compared to a cell from the human body. Examine any organism under a microscope, and all of them are made up of cells with an exterior membrane to provide a boundary with the external world, and across which selective transport takes place, and an interior where similar molecules and similar geochemical reactions and cycles carry out metabolism and replication. Animal and plant cells have important differences—plant cells also have an external cell wall and cellulose is a most important molecule—but the commonalities are much greater than the differences.
Fig. 13-3: Comparison of two eukaryotic cells. The top sketch is the fungus cell; the bottom cell is a human cell. Notice that broad similarity in appearance and structure, with an external cell membrane, a nucleus, organelles, and protoplasm.
The second half of the twentieth century saw the rise of biochemistry, where life could be investigated on the molecular level. Close examination of life revealed still more stunning similarities among all organisms. On the atomic level, this similarity is expressed in terms of the small number of elements that make up all of life. And in turn these elements combine to form a small number of building blocks, such as H2O, CH3, NH3, CO2, PO4, and so on that combine to form the enormous diversity of larger organic molecules.
These larger molecules, while showing huge variety in detail, nonetheless can be classified into four groups of macromolecules, common to all cells, that fulfill the basic functions of the cellular machine. These four classes are carbohydrates, amino acids, lipids, and nucleic acids.
Carbohydrates are the fuel source for cellular operations. Carbohydrates are hydrated carbon atoms—carbon atoms that combine with whole numbers of H2O molecules. In terms of the chemical reaction that summarizes the creation of organic carbon through oxygenic photosynthesis,
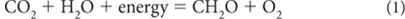
Note that the valence state of the carbon changes from +4 in CO2 to +0 in CH2O, as the valence state of two oxygens changes from –2 to 0. This results from a transfer of electrons to the carbon atoms. Such a transfer of electrons is at the heart of most organic reactions. Carbon is special in this respect because it can lose or gain up to four electrons and have a completed electron shell.
A simple carbohydrate such as the sugar glucose (Fig. 13-1) has a simple formula, C6H12O6, and structure. Fructose has the same formula with the atoms arranged in a different structure. Combining fructose and glucose together makes sucrose. There are also very large and complex carbohydrates, such as the starches or cellulose, which have formulas consisting of a hundred atoms or more.
Oxidation of carbohydrates releases energy that can be used by the cell through reactions that can be simplified as, for example:
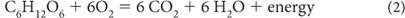
The organic carbon is converted to CO2 as electron transfer goes in the opposite direction from reaction (1), and energy is produced. When we burn wood in our fireplaces, we are facilitating this electron transfer and producing heat in the process. Our bodies “burn” carbohydrates in a more controlled fashion to produce the energy needed for our cellular metabolism.
Lipids have much less oxygen than carbohydrates, and the carbon is in a more reduced form. There is a higher potential energy content, because of the larger electron transfer that occurs. Lipids are a very efficient way to store high energy per molecule, and our bodies convert carbohydrates to fat in order to store the extra food energy in compact form. Lipids are the fats found in animals and the oils found in plants, and they also have other functions, such as creating the basic structure of cell membranes.
Amino acids are the twenty-two molecules that are the building blocks of proteins. Amino acids have a particular chemical structure consisting of a central carbon whose four bonds are connected to an “amino” group (NH2), a carboxyl group (COOH), a hydrogen atom, and a side chain that is called an R group (Fig. 13-4). The first three are the same in all amino acids. The identity of the molecule that makes up the side chain is what distinguishes one amino acid from another. Their chemical formula can be written as H2NCHRCOOH. The R group molecules may be hydrophobic—i.e., they do not want to coexist with water—or hydrophilic—they do want to be next to water molecules (this latter class of amino acids is called the polar class). A third type of amino acid, called the charged class, has an R group that contains a positive or negative charge. Within these classes, the amino acids can also be of different sizes and shapes. The largest (and rarest) amino acid found in terrestrial life, for example, tryptophan, has a side chain consisting of eighteen atoms. The smallest, glycine, H2NCH2COOH, has only one additional hydrogen atom as its “R group.” Many more amino acids can be made in the laboratory than exist as the protein building molecules of terrestrial life. Amino acids are also commonly found in carbonaceous chondrites, showing an important organic component to molecule creation in interstellar space.
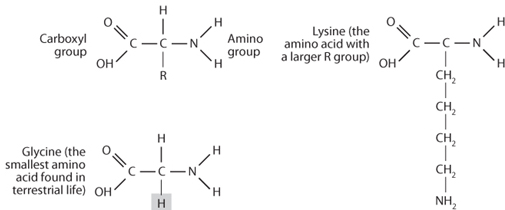
Fig. 13-4: This diagram shows the general structure of amino acids. The amino group and acid group are common to all, the identity of the R-group changes from one amino acid to another, as illustrated with the simple R-group for glycine and the larger R-group for lysine.
Fig. 13-5: Illustration of the peptide bond reaction that permits amino acids to join together to form proteins. Note that the reaction involves dehydration, the removal of a water molecule as the amino and acid groups of the two different amino acids become bonded together. Proteins commonly consist of hundreds of amino acids joined together through peptide bonds.
An important aspect of amino acids is that the carboxyl and amino groups can join together to form a peptide bond (Fig. 13-5). This universal capacity of amino acids to bond together is what makes the construction of huge protein molecules possible. The proteins form more than 10,000 different molecules that include the basic structure of the organism as well as enzymes, hormones, etc. They are involved in oxygen transport, muscle contractions, and countless other metabolic activities. If proteins are thought of as “words,” the twenty-two amino acids found in living organisms are the protein alphabet, and their combination is able to create the immense diversity of proteins found in living organisms. Very complex molecules can be built by amino acids. For example, hemoglobin is a protein with a chemical formula of C2952H4664N812O832 S8Fe4, consisting of more than five hundred amino acids surrounding four Fe atoms (Fig. 13-1).
Fig. 13-6: Illustration of the chirality of amino acid molecules. Both left- and right-handed forms are made in most natural processes, but life uses only left-handed amino acids. This requires an early selection process that was able to distinguish between the two. Notice how the “shape” of a protein where these two forms were joined together with a peptide bond would be very different depending on the chirality. (Image courtesy of NASA)
Each type of amino acid can also occur in left-handed and right-handed forms—i.e., they can be mirror images of each other (Fig. 13-6). This chirality is very important for how the amino acids can fit together. For example, it is very difficult for human beings to shake hands in the conventional way if one person uses a left hand and the other a right hand. And it is not possible to stack left and right hands on top of one another so that the shapes match. Molecules with different chirality can also have very different effects on the body. Thalidomide, for example, in left-handed form, was an effective antidepressant that was used for pregnant women in the 1950s. The right-handed form, produced in small amounts during the manufacturing, produced birth defects in babies.
Many amino acids can be made in the laboratory—some seventy are known, and all of them occur in right-handed and left-handed forms. The remarkable feature of life on Earth is that all organisms make use of only twenty-two left-handed amino acids.
Nucleic acids carry out information, communication, and memory functions within the cell. Like the amino acids, nucleic acids also have commonality of structure, with a sugar backbone, a phosphate group, and five basic building blocks consisting of two classes of molecules: the purines, adenine and guanine, and pyrimidines, thymine, cytosine, and uracil. Deoxyribonucleic acid, DNA, makes use of the adenine, guanine, cytosine, and thymine. Ribonucleic acid, RNA, substitutes uracil for thymine. The important characteristic of the nucleic acids is that they make complementary chains. This permits the molecules to replicate and communicate. RNA can match up with DNA to carry the information necessary to make proteins to other parts of the cell. DNA can split and replicate to pass almost identical information and instruction from one generation to the next.
The four classes of molecules can be looked at from an organizational standpoint, where each class of molecules provides a different set of functions: energy source, energy storage, structure, and instruction and communications. Carbohydrates are the immediate energy source, and oxidation of the carbohydrate back to CO2 plus H2O provides the basic fuel for cellular operations. Lipids allow excess energy to be stored efficiently for possible future use. Fats build up when an excess of carbohydrate accumulates in the organism, and then can be released during times of food shortage. Lipids have other important functions (such as cholesterol in the blood) and are also important constituents of cell membranes. Amino acids combine to form the remarkably diverse proteins and make up the physical structures of life. They also act as important enzymes, the catalysts that enable efficient cellular functioning. Nucleic acids provide the instruction kit for cellular operations and the means of communication both within the cell and from one generation to the next. All of life—all plants, animals, and single-celled organisms—use the same molecules and same basic structural organization. From these perspectives, life is a unity.
In addition to the commonalities of basic cellular appearance and the same restricted groups of molecules present in cells, a limited number of chemical machines are fundamental to the operation of all cells.
Perhaps the most fundamental machine is the relationship among nucleic acids and proteins, whereby the instruction kit contained in DNA is put into operation in the cell. The DNA carries code that specifies which amino acid will be added to a protein. The RNA reads the code and then carries it to the protein where the appropriate amino acid can be placed. DNA to RNA to protein is the “central dogma” of cellular operations. Each one of the amino acids is coded within DNA by a series of three distinct bases, called a codon. Since there are four bases, the total number of codon instructions is 43, or 64, which code for the twenty-two amino acids, as well as “start” and “stop” commands, which are essential because a given strand of DNA may code for many proteins, so the RNA needs to know when the job is complete. Since the number of possible commands is larger than the number of amino acids, there is some redundancy, with different codons able to specify the same amino acid. This mechanism of protein synthesis, information storage, and transfer of genetic information from one generation to the next operates in all cells.
Each cell also has a fundamental energy driver, which is an electrical charge set up across the cell membrane. This electrical potential acts as a microbattery that causes electron flow, which is necessary for the basic chemical reactions of cellular function.
The chemical cycles that mediate energy conversion in cells are also remarkably similar. The currency of cellular energy, discussed at length in Chapter 15, is the conversion between adenosine diphosphate (ADP) and adenosine triphosphate (ATP) involving the addition or removal of a phosphate molecule. In most cells, a fundamental mechanism for this conversion is the citric acid cycle, a complex series of chemical steps that converts between ADP and ATP and can run in both directions, depending on whether energy is being used or created.
These shared characteristics show the great commonality of all of life on Earth. All of life uses the same chemical building blocks, down to the detail of having a limited number of amino acids with the same chirality, and consists of cells that operate using the same fundamental machinery for protein building, transfer of information from one generation to the next, and energy production, storage, and use. The discoveries of the late twentieth century have revealed the remarkable unity of life from the cellular to the atomic realm.
Our view of the history of life on Earth is informed by detailed study of living organisms, and by fossils—the remains of once-living organisms preserved in sedimentary rocks (Fig. 13-7). The fossil record reveals a remarkable diversity of organisms, most of which have no living examples today. What is largely unappreciated to the nonspecialist is that the visible fossil record begins only 543 million years ago, at the boundary between the Precambrian and Cambrian periods. In fact, it is the appearance of mineralized skeletons that produce visible, macroscopic fossils that defines this boundary. While 543 million years is a long time by human standards, it is only 12% of Earth’s history. If we visited Earth a billion years ago—less than 25% of Earth’s history—the planet would be unrecognizable to us: no grasses, trees, or shrubs, no plants; no mammals, no fish, worms, or insects. There would be nothing for us to eat and very little to see aside from barren landscape. From this perspective, time travel has its disadvantages.
There is abundant evidence, however, for the presence of earlier life that did not have the hard parts that could be preserved as fossils. While plants and animals were absent, the most abundant form of life was thriving and omnipresent. That life is the millions of species of unicellular organisms, the building blocks for the more complex multicellular organisms that exploded onto the scene in the Cambrian.
Unicellular life can be divided into two major groups, called prokaryotic and eukaryotic (fig. 13-8). While both groups share the common characteristics of life discussed above, the two types of cells are very different from one another. Prokaryotic cells are usually small, less than 1 micron (one-thousandth of a millimeter) in diameter. They have a minute quantity of DNA, have no cell nucleus, and can divide and double population in twenty minutes. The inner structure of these cells is not differentiated. They are essentially a membrane sack with the basic ingredients necessary for cellular metabolism and reproduction. In these primitive organisms, many of the important aspects of photosynthesis or respiration take place in the cell membrane.
Prokaryotic life continues in unbelievable abundance on, around, and within us. While our eukaryotic cells make up the parts of our anatomy that we recognize, there are ten times as many prokaryotic as eukaryotic cells within a human being. Prokaryotes surround us and inhabit us by the billions, too small for us to see. Each square centimeter of our skin is home to a million of these organisms. Our armpits have ten times more. More of them reside on the surface of each human body than there are people on Earth. Each cubic centimeter of seawater contains 10 million of them. Each cubic centimeter of soil is a thriving metropolis of 100 million. In their diversity, population, and flexibility they beat out plants and animals hands down. They are an invisible world that is fundamental to most geochemical cycles and are the invisible backbone that makes life sustainable. Their effects are everywhere—from the health of soil, to the photosynthetic capacity of the oceans, to the proper operation of our digestive system, to mold on old food and many diseases.
Fig. 13-7: Trilobite fossil from the Cambrian period from Schlotheim, Czech Bohemia. Prior to the Cambrian, before 543 million years, no fossils with hard body parts have been found. (Photograph courtesy of Museum of Comparative Zoology, Harvard University)
Fig. 13-8: Schematic illustrations of prokaryotic and eukaryotic cells. While the images are the same size, the actual sizes of the cells are very different. The small symbol to the left of the eukaryotic cell gives an indication of relative size. Prokaryotic cells are usually small, generally less than 1 micron in length, while eukaryotic cells are commonly 10 microns. This makes a difference of a factor of a thousand in terms of volume, and provides credence to the idea that eukaryotes evolved from incorporation of or symbiotic relationships among prokaryotes.
Eukaryotic cells are complex factories compared to their prokaryotic cousins. They are far larger (1–10 microns in diameter, so a thousand times larger in volume) and have a complex inner structure, with DNA contained in a cell nucleus and the interior populated by a series of molecular machines called organelles that undertake functions such as respiration (in mitochondria) and photosynthesis (in chloroplasts). In contrast, prokaryote cell interiors are much less differentiated. Eukaryotes have a thousand times more DNA than prokaryotes, and replicate in about twenty-four hours.
The organelles that carry out important functions within eukaryotic cells have their own DNA and a strong kinship with some prokaryotic cells. Lynn Margulis pioneered the idea, now largely accepted, that organelles in eukaryotic cells developed by evolving symbioses among diverse prokaryotic cells, which eventually fully merged into discrete individuals that preserved the essential functions of their ancestors. Eukaryotes may be evolved products of earlier prokaryotic communities. That said, there are still major debates on how the original eukaryotes arose.
The multi-cellular life that appeared about 600 million years ago is made up of groups of eukaryotic cells, which themselves had become specialized to fulfill specific functions. In our bodies, for example, kidney cells, liver cells, nerve cells, blood cells, muscle cells, and so on are all eukaryotic cells that have become adapted to their own specialized and coordinated function. The overall development of life can then be viewed in simplistic terms as an early stage of primitive prokaryotic cells, which combine and are transformed to form the larger and more complex eukaryotes, which in turn combine and transform to form the multicellular, macroscopic life that emerges in the Cambrian and now forms the visible life we see all around us.
These overall trends in the history of life need a timescale, including the time of appearance of the first organism in the geological record. While individual microorganisms are too small to see without a good microscope, large communities of microorganisms do make visible communities. Particularly important microbiological communities for the geological record are those that create rocks, called stromatolites. Stromatolites are growth structures preserved in carbonate sediments and are most often created by photosynthetic microbiological communities that live in shallow seas. The metabolism of some bacteria causes precipitation of calcium carbonate between the cells. As the carbonate precipitates, the living bacteria propagate upward toward the sun and then precipitate another thin layer of carbonate. Over thousands of years, this creates a characteristic rock structure. Sometimes under special circumstances these structures even preserve the cellular remains of the microorganisms that caused them to be deposited, though in most cases the progressive cementation by carbonate tends to destroy these remains.
Stromatolites may be present in some of the oldest rocks, become common through billions of years of early Earth’s history, and are preserved in rare environments on Earth today. Therefore it is possible to examine present-day examples and try to relate their sedimentary structures to those that can be observed in the distant past. Figure 13-9a shows modern examples of stromatolites from the classic locality of Shark Bay, Australia, and sedimentary structures similar to stromatolites that are preserved today and in some of the earliest rocks of the geological record from 3.5 billion years ago (Fig. 13-9b). The stromatolite evidence is considered by some to be evidence for thriving bacterial life at 3.5 Ga, about the same age as the oldest rocks.
Further evidence for earliest life comes from the stable isotopes of carbon. Life significantly prefers the light 12C isotope over the heavier 13C isotope, by 2.5%. Using the stable isotope nomenclature discussed in Chapter 9, the 2.5% preference means that carbon compounds made by life have δ13C (a normalized measure of the 13C/12C ratio) about 25 per mil lighter (more negative) than inorganic compounds such as CaCO3. Some carbon compounds separated from ancient rocks with an age of 3.5 Ga have just this signature of “light” carbon.
Another line of evidence comes from biomarkers, complex organic molecules that do not break down easily and would be the result only of life. Roger Summons and colleagues found evidence for biomarkers made during photosynthesis in rocks 2.7 billion years old. On the basis of this evidence, there was confidence that Earth was inhabited by 3. 5 Ga. It even appeared that photosynthesis had evolved as early as 2.7 Ga, substantially before the rise of oxygen in the atmosphere near 2.4 Ga (discussed at length in Chapters 15 and 16).
However, all of this evidence for very ancient life and its capabilities has become subject to doubt. Structures similar to ancient stromatolites have been shown by John Grotzinger to be able to be formed by inorganic processes. Evidence from carbon compounds also has the problem that the rocks in question have existed in the crust for billions of years of Earth’s history, and during all of this period, life has been present. All the water circulating through the cracks and porosity of Earth’s crust contains microorganisms, and it would be exceedingly difficult to keep a rock isolated from these effects over billions of years. Furthermore, petroleum compounds, made from living matter and therefore containing “light” carbon, are formed at depth in the crust and migrate here and there, providing further sources of contamination. For these reasons, evidence from carbon compounds cannot be regarded as definitive. Indeed, careful recent work has shown that the biomarker evidence in the 2.7 Ga rocks is from young rather than ancient compounds, taking away the main line of evidence for a very ancient date for the beginning of photosynthesis.
Fig. 13-9: Top: Modern stromatolites from Shark Bay Australia (photograph courtesy of Paul Hoffman and Francis Macdonald, Harvard University). Bottom: Right panel shows an example of stromatolite structure found in the 3.45 Ga Warawoona formation from Australia. A variety of evidence suggests these stromatolites were formed in association with microbial mats. Scale bar: 15 cm (photograph courtesy of Andrew Knoll, Harvard University, based on Allwood et al., Proc. Natl. Acad. Sci. 106 [2009], no. 24: 9548–55).
Definitive evidence for earliest life then needs a combination of evidence and reasoning—reliable visual evidence, carbon isotopes, how pristine the rocks are, what geological environment they come from, etc. Textural evidence that lacks carbon isotopes, microfossils and biomarkers comes from 3.45 Ga stromatolites (Fig. 13-9b). The most definitive evidence (as of 2010) comes from 3.2 billion-year-old rocks (Fig. 13-10a), where structures such as cell membranes are still preserved from ancient microorganisms. Many geoscientists also consider that visual and carbon isotope evidence from 3.5 Ga rocks indicates life existed at that time. Photosynthesizing cyanobacteria are found in rocks as old as 2.0 Ga, and must have occurred earlier than that to account for the first rise of O2 in the atmosphere. If the 3.45 Ga stromatolites included photosynthetic bacteria, photosynthesis may have had a much earlier start. Beautiful visual evidence for eukaryotes occurs in rocks at 1.5 Ga (Fig. 13-10b), and there is substantial evidence for them at 2.0 Ga.
The progression from prokaryote to eukaryote to multicelled organisms is crudely correlative with the maximum size of organism. Jonathan Payne and others have looked at the maximum size of organisms from diverse lines of evidence to produce a plot of maximum size through time, which shows well the progression of life over time (Fig. 13-11). All of this evidence indicates that the earliest life we can recognize was similar to the prokaryotic life-forms whose ancestors still thrive today. From the perspective of the geobiological detective, this is great good fortune, because study of prokaryotic cells and the communities they form today gives clues of what to look for in the rock record that would indicate when primitive ancestral prokaryotes first appeared. The question of the origin of life can then be reduced to the origin of the simplest life we know—a cell that has the necessary characteristics to be the common ancestor from whom all subsequent life has evolved.
Fig. 13-10: Images of early micro-fossils. Images were produced with a transmitted light microscope, a backscattered environmental SEM, and a TEM. The images on the left, (a)–(d), are features interpreted as indicating cellular life in rocks as old as 3.2 Ga (Javaux et al., Nature 463[2010]:18). Images on the right are of the eukaryotic Shuiyousphaeridium macroreticulatum from the Ruyang Group, northern China, showing definitive evidence for eukayotic cells at about 1.5 Ga. Image size of (e) is about 300 microns across, and for (f) showing a feature of the cell, about 40 microns across (Javaux et al., Geobiology 2 [2004], no. 3: 121–32).
Fig. 13-11: Plot of maximum body size through time of organisms from rocks (and the present day.) Triangles are prokaryotes, circles are eukaryotes, squares are animals, and diamonds are vascular plants. (Modified from Payne, et al., Photosynth. Res. (2010) DOI 10.1007/s11120-010-9593-1)
The background given in the previous section provides a framework for an understanding of life’s origin. Life evolved in early Earth’s history, most likely before 3.5 Ga where direct evidence is lacking. All of life today has a startling unity of chemical composition that reflects the solar system and a great specificity of process that shows the relationship among all living organisms. Evolved life can be understood as a progressive development from the simplest prokaryotic organism, which we can examine today and evidence for which we see in the rock record. The origin of life then becomes the question of how the early planetary environment gave rise to the most primitive common ancestor of all of life in a fairly short period of time early in planetary history. What are the steps that would be necessary for this simplest life form to develop?
Fifty years ago this question was not readily accessible to scientific investigation, and while there are still great challenges, that is no longer the case. The increasing understanding of the molecular machinery of cells makes the chemical description of life’s operations ever more precise, and the developing understanding of early planetary environments leads to specific laboratory experiments where the potential development of this machinery can be investigated. The origin of life then stops being a vague puzzlement—how did this mystery of life begin?—and can be broken down into a precise series of more specific questions. Given the increasing knowledge of the conditions of early planets, how can the various components of life arrive naturally in the absence of living organisms? How can the basic organic building blocks form? How can these molecules join together into the larger polymers that are needed? How can the basic structure of a cell membrane be formed? How can steady state chemical cycles come into being?
This change from imponderable big questions to focused, specific questions is characteristic of the progression of science. Throughout this book we have encountered questions that not so long ago were imponderable mysteries, now revealed to be subject to quantitative understanding. How the universe began—now revealed from evidence of the Big Bang. Where the elements came from—revealed in the understanding of stellar interiors. How old Earth is, and how it has stayed hot enough to be geologically active for so long—revealed by the discoveries of radioactivity and convection. How characteristics such as family resemblance are passed from one generation to another—revealed in the structure of DNA. Why continents fit like a puzzle across the Atlantic—revealed in the precise operation of plate tectonics. “How did life begin?” is another of these great questions, and one for which a satisfying understanding is not yet determined. What we will see in the remainder of this chapter, however, is that from the ability to pose increasingly precise questions, the architecture of an understanding of life’s origin is rapidly developing. By “architecture” we mean the overall structure of a solution, with a much more satisfactory understanding likely to emerge in the coming decades of this century. This framework comes from two directions:
(1) a more thorough understanding of how life works through the continuing revolution in chemical biology;
(2) a more thorough investigation of present and past planetary environments that might provide the raw materials and flow of energy that would make early life possible.
Our understanding of the history of life shows us that the most primitive living organism we know, and that found most deeply in the fossil record, is the prokaryotic cell, which contains the fundamental chemical machinery that is the basis of all complex life. The fossil record reveals the progressive diversification and growing complexity of life, the theory of evolution provides the framework or understanding for this development (see Chapter 14), and the understanding of DNA provides the detailed chemical mechanism by which it takes place. Fossils, evolution, and chemical biology all conjoin to relate the most primitive cell to the present diversity.
The situation is not quite so simple, however, because there is no known organism that can be placed at the base of the Tree of Life, from which all other organisms can be descended. All of present life is evolved, and the earliest record of life’s evolution remains hidden. Prokaryotes today are not the representatives of early life, but rather their very distant descendants that have undergone billions of years of evolution. Nonetheless, life points in the direction of a common ancestor, whose characteristics we can infer. This unknown organism can be referred to as the universal common ancestor (UCA). The UCA would have the principal characteristics shared by all of life:
All cells are made up of the same limited set of elemental building blocks—H2O, C, N, P, and so on that are in the appropriate state of matter.
All cells have a cell membrane that isolates the organism from its surroundings and across which chemical exchange can take place.
All cells operate with the same set of organic chemicals to carry out the basic mechanisms of life—carbohydrates, amino acids, nucleic acids, and lipids.
All cells work with amino acids that are left handed, and nucleic acids that are right handed, i.e. they have a definite “chirality” that is not random.
All cells have a cellular organization that manages chemical cycles within the cell, permitting a steady-state existence in the face of environmental change.
All cells have the means to replicate and pass information from one generation to the next.
We now turn to an exploration of how each of these steps may have developed.
Hydrogen, carbon, oxygen, and nitrogen make up >98% of the mass of cells. As noted previously, the basic building blocks for life are among the most abundant elements produced by nucleosynthesis and are not in short supply throughout the galaxy. A greater challenge than the presence of particular elements is that the elements need to be in the correct chemical form and state of matter. Most scientists currently believe, for example, that carbon needs initially to be in a reduced chemical state, with some C combined with H rather than O, because it is much easier to build organic molecules from carbon in reduced (CH4) form than in oxidized (CO2) form.
An even more important requirement is liquid water. All living cells are composed dominantly of water. By weight, cells are about 70% water. Water has unusual chemical properties that give it an important role for life as well as for climate stability. It is a polar substance, which means that many substances can dissolve in it. We will see below that the polar property is also essential for forming the first cellular containers. It has high heat of fusion, heat of vaporization, and heat capacity, which allow it to remain in a liquid state over wide changes in conditions. And its solid form is lighter than the liquid form, which enhances convection and vertical circulation of bodies of water. These characteristics make water an indispensable medium for life. Molecules dissolve in it and are transported by it, and it provides a persistent and stable environment in which the various reactions necessary for life to begin could take place. Water is clearly in great abundance on Earth’s surface today. Early Earth’s history, filled with meteorite bombardments and even the massive bombardment that is believed to have led to the formation of the moon, would have caused surface temperatures that would boil water, and therefore liquid water is not likely in earliest Earth’s history. As we saw in Chapter 9, however, there is evidence from the oxygen-isotope compositions of zircons for a liquid ocean as early as 4.4 Ga. Liquid water appears not to be an impediment to an origin of life far earlier than the first evidence for it in the geological record.
With the necessary elements and molecules in place, the next step is to form the organic molecules that are the basis of living processes. The word organic is applied to these molecules because it was initially believed that they could be constructed only by living organisms and not by purely physical mechanisms. We now know that there are many physical processes that create organic molecules—there are so many, in fact, that it is difficult to know which of them may have been most important. In Chapter 3 we provided the evidence for organic molecules in the galactic interstellar medium and remarked that carbonaceous chondrites that arrive on Earth today contain organic molecules, including amino acids. Studies of comets have also revealed organic molecules. One possibility, then, is that Earth does not need to make the organic precursors to life, because they were delivered from the very reduced environment of the solar nebula during planetary formation. We cannot be sure to what extent they would not be destroyed by impact, nor can we be assured that they were present in sufficient quantities. As it turns out, Earth itself also has diverse means to make simple organic molecules.
One of the most important experiments in this direction was carried out by Stanley Miller in 1952, when he was a student in the laboratory of Nobel prize–winning chemist Harold Urey. Miller designed an apparatus (Fig. 13-12) in which electrical discharges were applied to a mixture of water vapor, CH4, H2, and NH3 that underwent cycles of evaporation and precipitation. This experiment produced a wealth of amino acids and other organic molecules. Subsequent experiments under varying conditions have been able to produce all the necessary amino acids, sugars, the bases essential for nucleotides and adenosine triphosphate (ATP) through reactions taking place in conditions appropriate to those which might have existed in certain environments on the early Earth.
Fig. 13-12: Schematic of the Miller-Urey apparatus where it was shown that atmospheric processes were capable of producing a large variety of organic molecules, including amino acids.
Another environment that is promising is deep-sea hydrothermal vents. The vents have favorable attributes for chemical reactions—ubiquitous seawater, warm temperatures and large temperature gradients, gaseous compounds including H2, other reduced molecules, disequilibrium conditions, metals that are often useful catalysts to organic reactions, abundant and diverse mineral surfaces, and mixing of fluids of diverse chemical compositions, all of which lead to many chemical reactions. These conditions are often difficult to replicate in the laboratory, since the reactions are often not at equilibrium and occur at high temperatures, high pressures, and with large temperature gradients. There has been substantial effort, however, to calculate from thermodynamics the diverse organic chemicals that could be produced under a variety of conditions. These calculations have shown that diverse synthesis of organic molecules can be generated by fluid mixing of hot, moderately reduced submarine hydrothermal solutions with seawater.
The various experiments, observations, and thermodynamic calculations have shown that amino acids, lipids, carbohydrates, and the building blocks for nucleic acids can be formed in a variety of potential planetary environments. Abiotic synthesis of many of the essential molecular ingredients of life has been demonstrated, proving the feasibility to form the fundamental building blocks of the complex organic molecules needed for life.
The molecules involved in life today are mostly more complex than the simpler organic building blocks generated in experiments. Some of the smaller building blocks need to join together to make more complex monomers. The nucleotides that make up RNA and DNA, for example, require joining of the nucleobases with the sugar backbone and phosphate groups. The monomers then need to come together in long chains called polymers, groups of the simpler molecules joined together like links on a chain. Amino acids combine to form the remarkable diversity of proteins and enzymes, following very specific rules. Nucleotides need to join in long chains to make up the nucleic acids of RNA and DNA. Simple sugars combine to form complex carbohydrates, and simple fats combine to make the large group of lipids, which in turn combine to make membranes. The essential next step to life is to form the more complex monomers and to join them together in polymers.
A plethora of possible processes to form polymers have been proposed and are under active investigation, but the problems are not straightforward ones. Forming nucleotides from the base, phosphate, and sugar components is not a problem for which there is yet a clear solution. Polymers do not form automatically after the monomers exist. There needs to be a high concentration of the monomers, so they need to be concentrated in some manner. Many of the reactions involve the loss of water, and this is difficult when dissolved in seawater. Furthermore, the polymers of amino acids have a left-handed chirality, and those that make DNA and RNA are right handed. Not only do polymers need to form; there needs to be a selective process that distinguishes right from left. None of these challenges are definitively resolved.
Most of the processes that generate the organic building blocks of more complex molecules create them in dilute concentrations, and further chemical reactions among these molecules require that they become concentrated. For example, if an amino acid is made in the atmosphere by a Miller-Urey process and then is carried into the ocean by rainwater, its concentration in the ocean is minuscule. Furthermore, many molecules have short lifetimes, because they are progressively modified by other chemical reactions or by heat or cold. Therefore, it is necessary not only to make the necessary molecular building blocks but also to concentrate them, and the available time is limited before they break down.
Since water is the common solvent needed for all life-forming processes, freezing and evaporation of water are two possible mechanisms of concentration. Amino acids can become strongly concentrated by evaporating the water from a dilute solution of them. The bond that combines amino acids, called the peptide bond, is produced by dehydration, and therefore the same process that concentrates the amino acids could also facilitate their combination. For example, tide pools, which may have been more abundant on the early Earth because of the much larger tides that would have resulted from the moon being much closer to Earth than it is today, could undergo repeated replenishment and evaporation. These concentration processes also lower the water content of the system, making dehydration reactions more possible. For example, letting amino acid–laden water evaporate on hot rock can lead to the formation of the peptide bond and the formation of amino acid polymers.
Concentrating the organic ingredients is not alone sufficient to create the characteristic chemistry of living organisms, because life as we know it today is highly selective in “handedness” of the molecules. All of the amino acids but one (glycine) can occur in both right-handed and left-handed forms, and the natural planetary processes that are able to make amino acids produce approximately equal amounts of the left-handed and right-handed varieties. But only the left-handed form appears in living organisms. Chirality is essential because an important property of proteins is their physical shape, and this depends on how they angle and bend as the many amino acids that make them up are combined together. The chiral uniformity of amino acids is central to the operation of life as we know it.
Chirality is also important for RNA and DNA, because the sugar ribose can occur in both left-handed and right-handed forms. Only the right-handed form is found in nature. This selectivity is what permits the formation of the right-handed double helix, always turned in the same direction, and symmetrically opposite to a left-handed helix. Selective chirality is an essential aspect of terrestrial organisms.
The origin of selective chirality is not yet clearly understood. Some experiments have shown that incorporation of left-handed components can terminate the growth of a right-handed helix. In this case, only chirally uniform helices would grow, leading to their ultimate success. For amino acids, the origin of the chirality is even less clear. In living cells, the chirality is controlled by the enzymes that are involved in protein synthesis—they are chiral and therefore preserve the uniform handedness of protein synthesis. The initiation of such chiral selectivity thus seems to require a chiral template that would selectively bond only with one of the two forms of amino acids, even though both were present in the initial chemical soup.
One speculation is that mineral surfaces may pose a possible solution to both the concentration and chirality questions, and also aid in the formation of much larger molecules from the simpler organic building blocks. Experiments have shown that minerals can form single layers of molecules on many mineral surfaces. Clays are of particular interest because their layered structure and fine grain size provide many regular surfaces with properties that are useful for the arrangement of molecular layers on the surfaces of the minerals. These surfaces provide a mechanism for concentration of molecules from water, for interactions between the surface-bound molecules and other molecules in the water, and for formation of polymers as one monomer after another is absorbed onto mineral sites that have the same configuration. Clays are also of fine-enough grain size that they are often suspended in the water, enabling greater scope for interaction and transport. An intriguing possibility is that mineral surfaces may also contribute to monochirality. Some mineral surfaces are chiral, and layers absorbed onto them could also have a single chirality. Therefore, a mineral surface could be a site of molecular concentration, select for particular molecules, provide an environment for the formation of polymers, and accept only molecules with the same chirality.
One could even speculate that there might have been left- and right-handed forms of early life. They would not be able to interact effectively with each other—organic reactions taking place in one form would likely be inert or lethal to the other. Having both survive would not be stable, and therefore inevitably one died out and the other persisted.
All cells are contained within a cell membrane that isolates the chemical contents from the external environment and allows selective transport of matter across the membrane to maintain stability, import nutrients, and export waste. Creation of a suitable container is essential for life as we know it.
The characteristics of the membrane container have much to do with the particular chemical properties of water. Water has two small, positively charged hydrogen atoms on one side of the molecule and a large, negatively charged oxygen on the other end. This makes water a polar molecule with positively and negatively charged ends, and these ends align like little magnets to create an ordered array in the liquid. The polar properties of water have a large influence on what other molecules can be dissolved in it—polar molecules generally dissolve easily in water, while nonpolar molecules, like fats and oils, do not. There are a class of molecules that have one end that is polar, and hence hydrophilic, and another end that is nonpolar, and hydrophobic. The hydrophilic end likes to be dissolved in water, while the hydrophobic end wants to avoid the water. Detergents have such properties, which explains their tendency to form bubbles.
Fig. 13-13: Illustration of how early cell containers may have formed. Fatty acid molecules with hydrophobic and hydrophilic ends form bilayers (a) so that only the hydrophilic ends are in contact with water. These then bend into spheres, called liposomes (b) that completely isolate the hydrophobic ends of the layer. Modern cell membranes have this sort of structure. Image (c) shows the experimental formation of such structures, and how they can combine and incorporate other liposomes. They are also able to split and divide.
Cell membranes are made up of fatty acids. Once formed, the fatty acids are very stable and are not easily destroyed, and their longevity gives them ready availability for prebiotic processes. These acids have hydrophilic and hydrophobic ends. When placed in water, the fatty acids prefer to have their hydrophilic ends in contact with the water and their hydrophobic ends isolated. This leads to the formation of bilayers, with hydrophilic ends on the outside and hydrophobic ends on the inside (Fig. 13-13). An even more stable configuration is to wrap the bilayers into a sphere, with the inner and outer surfaces consisting of the hydrophilic ends of the molecules, which completely isolates the hydrophobic ends from water. These little spherules, called liposomes, are very similar to essential features of the cell membrane, though modern membranes have evolved much intricate cellular machinery to facilitate transport between the cell interior and the external environment.
These various considerations suggest a simple process for the formation of early fatty acid containers that could incorporate other organic components in the initial steps leading to cellular precursors.
At this point we reach the largest gap in our understanding of life’s origin. Thus far we have discussed the plenitude of the necessary elements for life, the diverse environments for making the simple organic building blocks, the potential for concentration of monomers and the formation of polymers, possible mechanisms for selection for one chiral form, and the arising of a cellular membrane. All of these are essential steps for life—but life is a self-sustaining and self-replicating process that is well beyond any of these steps. None of the steps discussed thus far lead us to the universal common ancestor from which all life evolved. There remains a large gap between the view looking backward in time from the present toward the universal ancestor that no longer exists and forward in time through early planetary processes that can lead to the essential building blocks of life. The unresolved gap contains the essential steps where all the necessary ingredients work together to form self-replicating chemical systems (Fig. 13-14).
A number of aspects make this gap in our understanding exceedingly difficult to fill. The first is that we are looking at a vast expanse of time, possibly as much as a billion years, with no good historical record. A lot can happen in a billion years—for example, in about half that time life has evolved from single celled organisms to the complex ecosystems that we see around us today. The recognition of the vast expanse of time that is available is difficult to encompass. Within this time period, hosts of processes could have taken place, and events with minuscule statistical probability may become inevitable. If, for example, there were a one in a million chance of some event, but there were millions of opportunities, the event moves from unlikely to inevitable. It would be as if one could purchase a lottery ticket every week for a million weeks—eventually one would become a winner. The long timescales create a difficult problem for laboratory experiments that must take place on the timescale of weeks to months.
Fig. 13-14: Illustration of the bottleneck in our understanding of the origin of life. (Modified from Jonathan I. Lunine, Earth: Evolution of a Habitable World (Cambridge: Cambridge University Press, 1999))
Life’s development must also have involved many sequential steps, taking place in environments for which we have few constraints. Laboratory experiments normally try to explore and constrain single environments—experiments with clay surfaces, or with hot water interacting with sulphides, or reactions in atmospheres subject to ultraviolet radiation—and emerging life probably involved exchanges and reactions depending on the diversity of environments offered by the Earth and the cycles among them. Chemicals made in one environment would be transported and react with others formed in an entirely different environment. And there could have been profound catastrophic events that were pivotal, such as impacting comets and meteorites, massive volcanism, and abrupt climate change. We know that such events have profoundly influenced life in the last billion years, and their frequency would have been much greater in early Earth’s history.
It is evident that modern life as we see it is too complicated and interconnected to relate it simply to the basic ingredients discussed above. There must be a long series of intermediate steps that connect the fundamental building blocks of life to a fully functioning and self-replicating system. One of the intricacies of all of modern life is the relationship among DNA, RNA, and protein. DNA carries the cellular memory and instructions; RNA reads the instructions and enables the building of the proteins and enzymes. The proteins and enzymes are needed in turn to enable the communication between the DNA and RNA. Therefore, we have a classic “chicken and egg” problem—DNA is needed to code for protein formation, and proteins are needed to enable DNA to give the instructions. How could such a system evolve?
It seems almost inevitable that certain steps that were essential to the life-forming process disappeared as life moved on to the next step. We can consider this in an overly simplified and schematic outline. Imagine there are different environments where a series of chemical reactions take place ending up with the production of molecules A and B. These environments are specific to a certain period of Earth’s history and no longer exist. Then A and B react to form molecule C, C converts back to A and B, and they become part of a stable cycle involving production and release of energy, sustained by sunlight or a hydrothermal vent, for example. From present observation, A and B are needed to make C, and C is needed to make A and B. This chemical cycle has, again, a “chicken and egg” character. The cycle survives because it is a cycle, not a one-way reaction that goes to completion. This cycle can then interact with other cycles to create far more complex relationships, which will also have precursors that disappear from view. Now imagine the scientist who comes along at the end trying to figure out how it all began, with no direct knowledge of the environments that gave rise to the precursors to the current system. “Chicken and egg” paradoxes arise from a temporal series of reactions and relationships that become progressively linked and evolve with time.
The DNA-RNA-protein cycle is a complex cycle involving innumerable steps with highly specialized enzymes to make it efficient. It surely resulted from a complex history of thousands of small changes. This recognition has led to the idea of a simpler form of replication and protein formation that might have preceded the fully developed system that survives today in living organisms.
One idea is that the DNA-RNA-protein connection was preceded by an “RNA world” where DNA did not yet play a part. RNA has the advantages that it carries information in its nucleotides, like DNA (only one of the four nucleotides of the DNA and RNA differ from one another); it can serve as the facilitator of protein construction; and it is more amenable to prebiotic synthesis. What gave additional support to the idea of an RNA world was the discovery that one form of RNA could also serve as an enzyme, called a ribozyme. Therefore, RNA alone has the potential to fulfill the major necessary functions for a primitive cell—memory, replication, protein synthesis, and enzymatic activity. This led to the idea of the “RNA world,” which may have preceded a more evolved world in which DNA took over the function of inherited memory from one generation to the next. DNA is more stable than RNA and is a better storage device for cellular memory. In the long run, a DNA-based system will have an evolutionary advantage, while an RNA-world may be a necessary but no longer existing precursor.
The various steps that we have discussed show the progress and existing problems with developing a verifiable understanding of life’s origin. Important new developments in this field appear frequently in the scientific literature. One of the difficulties is the diversity of environments on Earth. If life is a planetary process, then it is not a test tube process. For example, some have proposed that ancient hydrothermal vents are favorably locations for the origin of life. Some of the most primitive bacteria are thermophilic (liking heat), consistent with a vent location. And hydrothermal vents are a source of concentrated energy and chemical gradient, shielded in large part from the ultraviolet radiation and destructive impacts that would characterize near-surface reservoirs. Such environments are very difficult to replicate in a laboratory—most modern vent organisms cannot be cultivated in the lab. The natural scientific method is to strictly control variables and experimental parameters, where it may be fluctuating and diverse conditions that are important for aspects of life’s origin. It is perhaps even more likely that it is the interactions among diverse planetary environments that are necessary. With this diversity and the hundreds of millions of years of time available, the human laboratory challenges are daunting.
There is a larger question, however, which is whether there is a fundamental tendency toward life on planetary systems or whether life on Earth is a rare and highly unusual occurrence, requiring a whole series of statistically unlikely steps. For this larger question, two aspects of life can seem particularly perplexing. One is the argument that life violates the laws of thermodynamics. In thermodynamics you always lose—there is an inevitable tendency toward increasing disorder, or entropy, and there is an inevitable energy loss in every process, so that you never get as much energy out as you put in. Life appears to violate these principles because life is the appearance of order from disorder, and with evolution one could argue that the extent of order has increased with time. And life also creates energy—plants take raw ingredients from the air and soil and through photosynthesis turn them into more energetic compounds whose consumption provides the basis for animal life. Separation of carbon from oxygen creates an energy potential that can be used up the food chain or burned and used to power a modern civilization. So how can we understand the creation of order and energy within a universe that is bound by the laws of thermodynamics?
The question of energy is solved by the fact that order and higher energy compounds are created in nested systems, where the smaller system is making use of the energy from the larger system. There is enormous energy flow coming into the atmosphere from the sun, and coming from Earth’s interior. Life on Earth’s surface lives off these external energy sources. Within the solar system as a whole, energy is flowing downhill, and most of the energy is lost. Plants make use of solar energy, but the efficiency is not complete, and less energy is produced by photosynthesis than is received from the incoming photons. Therefore, life makes use with inevitable inefficiency of the dissipating energy of the universe. Life is possible only because it is a small part of a larger system. We owe our existence entirely to ongoing energy flow from the Universe,
The question of arising order is perhaps more intriguing, because it seems to violate the law of increasing entropy. Life makes sense from a thermodynamic point of view, however, if the scale is again adjusted so that there is maximum entropy production in the larger system, i.e., that the rate of change in entropy is maximized. Processes are more successful if they are able to generate entropy with greater efficiency. This principle can be easily appreciated from simple physical systems. If a pan has two holes in it, one larger than the other, most of the water flows out of the larger hole. If there are two water wheels, one with less friction than the other, the lubricated wheel will spin faster, process more water, and generate more energy per unit time. Whether liquids convect or not depends on which process more efficiently dissipates the available energy. Rocks take the steepest path if they fall down a slope. Processes that make use of the available energy with most efficiency garner that energy, and “win” relative to less efficient processes.
Life can also be viewed in this context. For example, a piece of wood is out of equilibrium with the atmosphere—the organic compounds in the wood are thermodynamically unstable in the presence of oxygen. Dry wood in a sterile environment, however, decays very slowly. We build long-lived houses from it. If the wood is put in the ground with moisture and bacteria, the bugs use the available energy and cause the wood to rot much more rapidly. Termites are even more efficient entropy producers than bacteria. Similarly, bacteria are essential for efficient operation of a compost heap. Worms in compost heaps are even better. Our human bodies are very efficient in this respect. We ingest plant and animal matter and in a few hours convert it to lower energy forms, while decay of the same matter sitting on the kitchen counter or even in a compost heap takes weeks or months.
What about plants? If you imagine green-colored ground with the same reflection characteristics as a leaf, and no photosynthesis, and you leave it in the sun for a brief period of time, it will warm up, and then the heat will gradually dissipate if it is taken out of the sunlight. A leaf undergoing photosynthesis stays cool because it is immediately converting the sunlight to chemical energy. The leaf causes greater entropy production compared to inert matter.
Any process that can take a potential energy and make the reaction run more efficiently to completion is favored, i.e., it succeeds relative to less efficient processes. Viewed from this perspective, life is a process that maximizes processing of energy. Even evolution might be viewed from this context, where evolutionary change is a series of steps of progressively greater rates of entropy production. Far from struggling against entropy production, life maximizes it! And this explains its great success. Life occupies every ecological niche, makes use of a host of energy sources, and appears in abundance out of nowhere once water, energy and nutrients become available. If we view life as an inevitable outcome of maximizing energy use, then this characteristic of life seems natural. Our own success as a species makes sense in this context—by use of tools and fuels we are able to harness the available energy from our environment far more efficiently than any other organism.
If one views life as a process that leads to more efficient dissipation of energy, then the origin of life no longer seems a statistical improbability but rather a natural outcome of the energetics of the universe. From the perspective of entropy, rather than defying the fundamental thermodynamic law of increasing entropy, life ruthlessly obeys it. The creation of order entails creation of greater disorder in the larger system, so the combination of energy flow and order creation maximizes entropy production. The series of steps leading to life’s origin would then be driven by their success at maximizing energy use and the rate of production of entropy. Enzymes, the efficient drivers of the chemical reactions of life, succeed because they allow the available energy to be processed more efficiently. Symbioses occur because the interactions allow each organism to process energy more efficiently. And systems that are able to grow and replicate and make use of cycles to replenish the necessary raw materials will be more successful than systems that use up the energy and material without cycles and reproducibility. From this perspective, while many of the detailed mechanisms of life’s formation remain to be elucidated, the driving force for planetary life is a fundamental feature of the Universe.
The chemical composition of life roughly corresponds with the solar composition, and the basic chemical ingredients for life are present in abundance. Central to life is the element carbon and the water molecule. Carbon has unique attributes among all the elements of the periodic table, making it ideally suited for life. Water also has exceptional chemical properties that give it a central role. Both water and carbon molecules were present in earliest Earth’s history, providing suitable environments for life’s origin.
All of life is remarkably coherent in terms of its fundamental molecules, molecular machinery, and cellular structure suggesting a common origin. Earliest life, likely beginning before 3.5 Ga, was prokaryotic cells, and evolution of life since that time can logically be related to them. The task for discovery of the origin of life is to find a candidate for a universal common ancestor that could give rise to the simplest prokaryote, from which all subsequent life could have evolved over billions of years. A possible framework of steps leading to life’s origin can be laid out, and many of the steps can be approximated in laboratory settings. Because there must also be many unknown steps that depended on the details of the planetary environment prior to the oldest rock record, a true biochemical replication of an origin of life remains problematic. The most difficult step is the passage from polymers contained in a “cellular” container to a self-replicating organism that can evolve by natural selection.
By the creation of order and increased efficiency of energy dissipation, life enhances the creation of entropy in the larger system. In this sense, life is a natural response to fundamental thermodynamic laws. Given a suitable planetary environment, life can then be viewed with some justification as a natural consequence of planetary evolution.
Andrew A. Knoll. 2004. Life on a Young Planet: The First Three Billion Years of Evolution on Earth. Princeton, NJ: Princeton University Press.
Jonathan I. Lunine. 2005. Astrobiology: A Multidisciplinary Approach. Boston: Pearson Addison-Wesley.
J. William Schopf, ed. 2002. Life’s Origin: The Beginnings of Biological Evolution. Berkeley: University of California Press.
Alonzo Ricardi and Jack W. Szostak. 2009. The origin of life on Earth. Scientific American 301(3):54–61.
1 Viruses are a notable exception to this observation, but they do not live independently of cellular life on which they depend for replication.