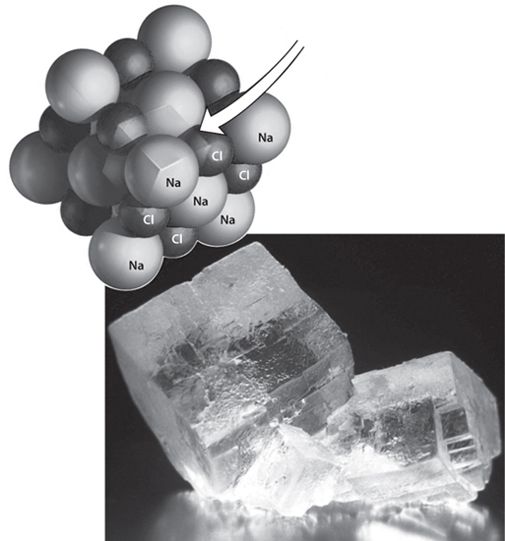
Fig. 4-0: The atomic structure and physical form of the halite mineral. The transparent box in the ball diagram shows the cubic “unit cell,” which is apparent in the cubic form of the visible mineral in the photograph. The symmetry of the mineral reflects the structure on the atomic scale. Minerals are the materials of which solid planets are made.
In stellar interiors all the important reactions involved the nuclei themselves, and one atom being transformed to another was the routine activity. But outside of stars, energies fall by orders of magnitude and a different set of laws operates. Atoms become the fundamental, unchangeable building block of matter. The number of protons in the tiny nucleus of the atom controls the number of electrons required for charge balance, and this electron cloud extends 100,000 times farther than the size of the nucleus. Interactions among atoms involve interactions among the electron clouds. The laws of electron cloud interaction control the formation of molecules in interstellar space, the formation of planets, and all the processes that subsequently take place on them. With the exception of the rare radioactive nuclides that retain the vestiges of their stellar origin, everything that happens on Earth deals with reactions among the electron clouds. For stars and atomic chemistry, the fundamental unit was the nucleus that made an isotope with a single mass, and the chart that summarized our knowledge was the Chart of the Nuclides. For planets, the fundamental control lies in the configuration of the electron clouds—elements rather than isotopes become the fundamental chemical substance. The Periodic Table, organized around electron shell structure and with all isotopes of the same element combined, presents in concise form the fundamental organization of electron clouds. Electron cloud interactions cause atoms to combine to form molecules, and almost all the chemical reactions we deal with involve interactions among molecules.
The first molecular building blocks are constructed in the vast clouds of interstellar space to form the inorganic molecules known as minerals, and also the simplest organic molecules. The minerals will become the building blocks for solid planets, and the organic molecules will contribute to larger, gaseous planets and also serve as the first building blocks for life.
Our discussions in Chapter 3 dealt with stellar processes at temperatures of millions of degrees. At these temperatures, the positively charged nuclei move so quickly that they collide and react, following the laws of nuclear physics. At this stellar level, events that are foreign to our human experience become normal. Atoms are created and destroyed; no molecules exist; there are no such things as rocks or minerals; life as we conceive of it is impossible.
Outside of this stellar realm, temperatures drop from millions of degrees to less than thousands of degrees. At these temperatures, positively charged nuclei are much less energetic and become surrounded by negatively charged electron shells. The nuclear chemistry of stellar interiors is no longer applicable, and we arrive in the realm of “normal chemistry” that we see all around us on Earth.
The basic understanding of planetary chemistry came of age in the eighteenth and nineteenth centuries, when chemists explored matter and tried to break down materials into their fundamental components. For centuries alchemists tried in vain to produce precious metals (gold and silver) from more common materials like lead and copper. They failed, but their attempts showed that other substances, such as water and air, could be separated into components with very different masses and properties. Gradually it emerged that there were fundamental building blocks of matter with specific masses and chemical affinities that could not be split into parts. The relative masses of these building blocks could be determined by seeing what mass of iron or hydrogen would react with a specific volume of oxygen, for example. In this way, the physical and chemical properties of each substance could be uniquely determined. The substances that could not be broken apart became known as elements, made up of individual, indivisible particles called atoms. “Atoms are the fundamental building block of matter that can neither be created nor destroyed” came to be a guiding principle of the new chemistry. The commonsense point that since atoms exist, they must have been created somewhere, was relegated to the class of philosophical questions that were beyond the realm of observation and scientific laws.
Some of the newly discovered elements had similar chemical behavior, and it was possible to divide them into groups based on their chemical affinity. Lithium (Li), sodium (Na), and potassium (K), for example, all could make similar salts when combined with fluorine (F), chlorine (Cl) or bromine (Br). Classes of elements with similar chemical affinities also showed quite regular increases in mass. For example, for the triad Li, Na, and K, the mass of Na (~23) is the mean of the weights of Li (~7) and K (~39). Similarly, strontium (Sr, mass 88) is the mean of calcium (Ca, 40) and barium (Ba, 136) and so on.
In 1869 the Russian chemist Dmitri Mendeleev (1843–1907) suggested the elements as a whole reflected an organized system: “The properties of the elements, such as their forms and the way they combine with other elements, are a periodic function of their atomic weight.” He constructed a table, now known as the Periodic Table, where the sixty-three elements identified at that time were arranged with those of the same affinity in the same column in order of increasing mass, and in each row the masses increasing regularly to the right. The regularity of the table was interrupted by some gaps, and Mendeleev predicted that other elements would be discovered to fill the gaps. Over the next short interval of time, many of these elements were discovered, offering convincing proof of the regularity that Mendeleev had been able to see. The Periodic Table of the Elements (Fig. 4-1) elegantly and concisely describes the most fundamental principles of chemistry. Spectra from other stars show that exactly the same elements exist everywhere in the universe, and the elements studied here on Earth reflect the same laws and processes that happen over the vast universal reaches of time and space.
The original conception of atoms was that they were indivisible particles with no internal structure. This idea gave way after the discovery of radioactivity by Henri Becquerel and Marie and Pierre Curie in the 1890s. Some of the heaviest atoms were emitting energy and were dubbed “radio-active.” This energy had an electric charge, so it could be focused in a beam. Ernest Rutherford directed such a beam at thin gold foil to see what would happen. A majority of the radiation passed straight through the foil, and some of it bounced back! This could be most simply explained if the beam was a collection of particles (called alpha particles) of positive charge that encountered something else with positive charge that would repel them. This experiment showed that atoms were not indivisible objects but had distinguishable parts. Since almost all of the alpha particles passed through the gold foil, areas of positive charge in the gold foil were extremely rare. Therefore, atoms were discovered to be mostly empty space, with a very small positively charged nucleus surrounded and balanced by an equivalent number of negatively charged electrons. Essentially, the entire volume of the atom is taken up by the surrounding electron cloud. The size of the nucleus relative to the electron cloud is about 1:100,000. If the nucleus took up the distance between the sun and Earth, the electron cloud would extend beyond the distance to the nearest star, Alpha Centauri. Or if the nucleus were the size of an apartment building, the electron cloud would be the size of the whole Earth!
Fig. 4-1: The modern rendition of Mendeleev’s Periodic Table of the Elements. Each row in the table corresponds with a particular electron shell and orbital, so the table is a kind of symbol of the electron shell structure of the atom.
Research in the early twentieth century revealed the more complex modern concept of the atom, including the discovery of neutrons as a component of the nucleus, and the concentric “electron shells” that hold gradually increasing numbers of electrons—2, 8, 18, 32, 50. Each of the outer shells was also shown to consist of multiple types of orbitals, leading to complex shell configurations that took some time to sort out in detail.
The number of electrons and the configuration of the electron cloud control all interactions between atoms. The number of electrons that surround a neutral atom are controlled by the number of protons in the nucleus; hence the number of protons determines the identity and chemical behavior of the element. As we learned in Chapter 3, while the number of protons within the nucleus of a given element is always the same, the number of neutrons may vary, giving rise to different isotopes of that element. Because the electron clouds of the different isotopes with the same number of protons are the same, the isotopes are chemically almost identical. (As we shall see in later chapters, however, the slight mass differences cause isotopes of the same element to have very slightly differing chemical behavior, which is a very useful tool to use in understanding Earth processes.) In the Periodic Table, all the isotopes of a single element are combined and averaged, which is the main reason that the atomic weights of many of the elements, particularly those with even atomic numbers, are not close to being exact integers.
The modern presentation of the Periodic Table reflects in its structure the details of the electron shell configuration for the various elements. The rows reflect the number of electron shells around the atom. The first row has two elements, after which the first electron shell is filled. The second row has eight elements, corresponding to the eight electrons of the second shell. The innermost orbitals of the third shell become filled after the addition of eight more electrons. The next two electrons are added to the fourth shell. Then, from Sc to Zn, ten electrons are successively added to the other orbitals of the third shell. Each shell can accommodate more and more electrons, and hence the outer shells become more complicated in their behavior than the inner ones.
The columns of the Periodic Table are organized around the configuration of the outermost electrons, which is where reactions with other elements take place. Elements in the first column have one electron in the outermost shell. Those in the second have two electrons. The large filled interior shows atoms where the additional electrons are filling one of the inner shells rather than the outermost shell. On the far right are the noble gases, where the outermost layer is completely filled. The Periodic Table thus not only provides useful data but is also a kind of symbol that summarizes knowledge about all the elements found in nature. It ranks a 10 on our theory scale of knowledge of the universe.
Because electron shells are energetically more stable when they are complete, atoms share, donate, and receive electrons to combine in ways that increase their stability under the conditions in which they find themselves. For this reason, with the exception of the noble gases, most elements on Earth appear in molecules that satisfy the needs of the elements to have complete electron shells. Lithium, with one electron in its outermost shell, combines very well with fluorine, which lacks one electron in its outer shell, because donation and reception lead to a “winwin” condition where both atoms achieve a more stable electron shell structure (Fig. 4-2). In the water molecule two hydrogen atoms each provide an electron to the oxygen atom, providing it with a filled shell. Such bonding between elements leads to the formation of molecules, ranging from very simple ones such as NaCl and H2O to huge organic molecules that can contain thousands of atoms.
Fig. 4-2: Ionic bonds are created when excess electrons in the outer shell of one atom are donated to fill vacant spaces in the shells of another atom, as in LiF. White circles represent electrons, dark gray circles neutrons, and light gray circles protons. Nucleii are greatly enlarged in order to be visible.
The only atoms with completely filled electron shells are the noble gases, found on the far right of the Periodic Table. Their electron shell stability means they have no tendency to react with other elements, so each atom of a noble gas is content to exist in complete isolation. Argon (Ar) in air, while rather abundant, reacts with nothing. The oxygen in air is easy to identify because oxygen reacts vigorously with many elements, as we see when iron rusts, or wood burns, or, for that matter, with every breath we take. The lack of reactivity of all the noble gas atoms is what made them so difficult to discover in the first place. Lack of reactivity is a kind of invisibility.
Atoms are even willing to lose their charge balance in order to have a filled shell structure. This leads to the creation of ions. Sodium easily loses an electron to become a positive ion, called a cation, with a single charge. Oxygen readily accepts two electrons to become a negative ion, called an anion, with two negative charges, and so on. Charged ions play a very important role in chemical reactions.
A few hundred thousand different molecules are known, but the number of different molecules that is possible is essentially infinite, and new molecules are still commonly being discovered or created in the laboratory. The most common molecules, however, are relatively simple combinations of the reactive elements (i.e., not the noble gases) that are created most abundantly in the universe. These elements (see Chapter 3) are (1) the primordial element from the Big Bang—hydrogen; (2) the alpha-particle nuclides that are not noble gases—carbon, oxygen, magnesium, silicon, sulfur, and calcium; and (3) the most stable nucleus that is the final product of nuclear fusion—iron. Nitrogen is also relatively important, especially to living organisms. Of these elements, hydrogen is the most abundant. Apart from hydrogen, the remaining six elements make up more than 98% of the reactive matter in the universe. Therefore, molecules involving these elements will be dominant, and the molecules in naturally occurring substances that are most familiar to us—rock, water, air, and life—are primarily made up of these elements.
Solid, liquid, and gas are the three states of matter most familiar to us, and elements and molecules can occur in all three of these states. At sufficiently high temperatures, no molecules can exist, and there is a fourth state of matter, plasma. Plasmas can be described as ionized gases where the elements are stripped of their electrons and there is a chaotic mixture of ionized nuclei and negative electrons. Plasma is the most common state of matter in the universe, and we observe it in our terrestrial experience through the solar aurora, the northern lights, neon lamps, and flames.
Our intuition about states of matter comes from our experience on Earth’s surface, where the pressure is uniformly low and variations in temperature cause changes in the state of matter from solid to liquid to gas. Therefore, when we think of melting or boiling or the creation of plasma, we intuitively assume it reflects an increase in temperature. This bias comes from the fact that we live in a very constant pressure environment. Even small changes in pressure, such as those we experience when under water or on high mountaintops, can have very large effects on our metabolism. But the pressure changes we experience are trivial compared to the pressure range of the overall planetary environment. Since pressure is controlled by the weight of overlying material, pressures increase rapidly with depth. Imagine the pressures generated by the weight of rock a mile thick! For this reason a planet’s pressure ranges are enormous—from essentially zero pressure in space to pressures of millions of atmospheres (megabars) in planetary interiors.
Fig. 4-3: Phase diagrams for (a) H2O and (b) CO2, showing the importance of both pressure and temperature in influencing the state of matter. Different shades of gray show the fields of solid, liquid, and gas. At constant temperature, pressure changes can convert materials from solid to liquid to gas, or vice versa. At very low pressures a direct solid-to-gas transition occurs, called sublimation. Vertical dashed lines with arrows illustrate phase changes produced at constant temperature by changing pressure.
A diagram illustrating this reality is presented in Figure 4-3, showing states of matter for two common substances—water and carbon dioxide. At a temperature of 25°C and pressure of one bar, water of course is liquid and CO2 is gas. At these pressures CO2 is never a liquid—at very cold temperatures, it is solid, and upon heating, it sublimates from solid to gas. But at higher pressures, liquid CO2 is stable—as is the case in many fire extinguishers, for example, where the CO2 is strongly compressed. Water, on the other hand, goes from solid to liquid to gas at one atmosphere, but you can see from the diagram that at very low pressures it would also sublimate, and that the melting and boiling points of water, considered by us to be very fixed quantities, vary substantially as the pressure changes.
The potential importance of pressure can also be seen from the diagrams where CO2 and H2O can change their state at constant temperature (T) as the pressure (P) changes. These effects of T and P can be qualitatively understood by recognizing that melting or boiling requires the atoms to become progressively freer of their neighbors. In solid crystals the elements are strongly bound to one another and do not move readily. In liquids the atoms or molecules are more energetic and are more loosely bound, and liquids easily deform. In gases, connections between elements or molecules are even more tenuous as the particles move randomly and chaotically, bouncing off each other. Increasing temperature increases the energy of the molecule, ultimately leading to a gaseous state. Increasing pressure tends to push the molecules closer together, making a higher density state more favorable. For almost all substances (water is the notable exception), the crystal is denser than the liquid, and therefore increased pressure makes solids more stable. Therefore, either lowering pressure or raising temperature can often have similar effects on the state of matter.
Plasmas are also sensitive to both temperature and pressure, as shown in Figure 4-4. Low temperature plasmas are possible and common in space, where the pressure is exceedingly low. Thus, for all elements, the four states of matter are traversed in various ways as pressure and temperature change.
Volatility determines whether a molecule is a solid, liquid or gas under particular conditions of temperature and pressure. Highly volatile elements have very low melting and boiling points, such as all the noble gases and N2. These substances are gaseous even at very low temperatures. Refractory elements have very high melting and boiling points. Refractory materials such as alumina (Al2O3) and magnesia (MgO) are used in the walls of blast furnaces, because they melt at temperatures higher than 2,000°C. They remain in the solid state even when other somewhat less refractory materials, such as metallic iron, melt. The refractory materials permit the molten iron to exist in a solid container.
Fig. 4-4: Illustration of the pressure temperature fields for the four states of matter. At very low pressures (low number density) or high temperatures, a fourth state of matter, plasma, becomes important. While plasmas are somewhat unusual on Earth, in the universe they are a very common state of matter. Note the logarithmic scale and the extremely high temperatures of many plasmas.
There is a large range of volatility between these extremes, and volatility can be ordered on a relative scale (see Table 4-1). Water is less volatile than carbon dioxide. Grease is less volatile than water, thereby permitting liquid grease to be stable at higher temperatures than boiling water, which gives us the difference between boiled potatoes and french fries. Iron and aluminum are still less volatile, permitting the two metals to exist in solid form even as water boils and grease melts.
Table 4-1
Physical constants of common molecules at one atmosphere pressure, ordered from most volatile to most refractory
| Compound | Melting point of solid (°C) | Boiling point of liquid (°C) |
CH4 |
–182.47 |
–161.48 |
NH3 |
–77.73 |
–33.33 |
CO2* |
–78.46 |
No liquid state |
Hg |
–38.83 |
356.62 |
H2O |
0 |
100 |
Fe |
1538 |
2861 |
SiO2 |
1713 |
2950 |
Mg2SiO4 |
1897 |
— |
Al2O3 |
2054 |
2977 |
* Sublimation point
Another important property of molecules is their density. The density of different elements varies substantially because the diameters of individual atoms vary only by a factor of 4, while the masses of the atoms vary by the number of total neutrons plus protons in the nucleus, from 1 in hydrogen atoms to 238 in uranium. As a good first approximation, the heavier an element, the greater the density of the substances the element makes. For example, solid lithium has a density of about 0.5 grams per cubic centimeter (gm/cm3), iron about 6 gm/cm3, gold 12 gm/cm3, and uranium 18 gm/cm3.
The same overall regularities apply to molecules made up of combinations of elements. Water (H2O), which has a density of 1.0 gm/cc, consists of two hydrogens with mass 1 and one oxygen with mass 16, so 18 nuclear particles in the molecule, or an average of 6 nuclear particles per atom. Forsterite (Mg2SiO4) has an average 20 particles per atom and a density of 2.8 gm/cc. Iron has 56 particles per atom and a density of 7.5 gm/cc. The proportionality between the number of nuclear particles per atom and the density is a little less than 1.0 because the size of the heavier atoms increases slightly with the increased numbers of electrons in the outermost shells. Other examples are given in Table 4-2. This regular behavior will be important for us as we try to determine the chemical compositions of distant planets for which we can determine density but as yet have no samples to measure.
Table 4-2
Densities and number of nuclear particles per molecule of common terrestrial components.
In general, molecules occupy two grand groups with rather different characteristics—organic and inorganic. Organic molecules contain carbon combined with hydrogen, and often oxygen, nitrogen, phosphorous, and traces of other elements (there are a few exceptions that have C-N bonds and no C-H bonds). The molecules are called organic because it was originally thought they could be created only by living organisms. They are all rather volatile—even the high-temperature carbon compounds we call plastics tend not to be stable above temperatures of a few hundred degrees. Most organic chemistry takes place at temperatures close to room temperature. Inorganic molecules are all those that are not carbon bearing, as well as carbon compounds with no C-H bonds (e.g., CO2, CaCO3). Inorganic molecules that occur in nature in solid form are called minerals, and virtually all solid inorganic materials (e.g., rocks) have minerals as their fundamental constituents. Some knowledge of the structure and nomenclature of both organic molecules and minerals is necessary before we consider the construction of planetary bodies and their organic components.
A mineral is defined as a naturally occurring, inorganic solid with an ordered atomic structure, distinct physical properties, and a chemical composition that can be written as a molecular formula. Well-known examples of minerals are quartz (SiO2), pyrite (FeS2), magnetite (Fe3O4), diamond (C), and muscovite mica (KAl3Si3O11[OH]2). These and all other minerals can be clearly identified by their chemical formula. Each one also has distinctive physical properties. For example, mica has a prominent cleavage that allows it to break easily along parallel planes, and quartz has no cleavage. Instead, when a quartz crystal is split apart it has what is called a conchoidal fracture. All minerals have a specific hardness, or “scratchability.” Diamond is the hardest of all minerals and will scratch any other; hence it makes the ideal jewel but easily scratches glass covers of copy machines! Graphite, another mineral with the same chemical formula as diamond but a different atomic structure, is one of the softest materials and can be scratched even by our fingernails. Other physical properties include the density, color, luster, streak (the color a mineral leaves when it is scratched on a hard surface), and whether or not the mineral is magnetic. The existence of a specific set of properties associated with each mineral often permits its identification in hand specimen without undertaking a chemical analysis.
The molecular unit cell of a crystal contains all the essential structural properties apparent in the macroscopic specimen and is what leads to beautiful, symmetric crystals. Minerals permit the microscopic molecule to have its essential characteristics viewed in macroscopic form. In a way, crystals make the invisible visible.
What does it take to make a mineral? To make a geometrically stable structure, atoms must fit together in terms of their size and charge. Atoms with many electrons are very large, whereas those with small electron clouds are very small. The atoms have to fit together so that their electron shells can interact with one another and produce a neutral molecule. For these reasons the sizes and electron shell structure of atoms determine what element combinations are possible and the geometrical form that various minerals take.
Because electrons are donated and received, it is the ionic radius of the element that controls the size and determines how atoms fit together in minerals. Size differences for ionic radii are larger than for neutrally charged atoms, because cations (positively charged ions) lose an electron and have their electron clouds pulled in by the positively charged nucleus, and anions (negatively charged ions) gain electrons, causing their electron clouds to expand. So Si4+ has a radius 0.32, while Cl– has a radius of 1.72. These characteristics mean that most of the volume of minerals is made up of the anions. Figure 4-5 shows the sizes of various ions arranged in accord with the Periodic Table. Three trends are apparent. First, the anions on the right of the table are very large. Second, ions with the same charge (columns in the Periodic Table) show an increase in size with increasing atomic number down the columns (because their electron cloud gets larger). Third, ions with the same electron shell configuration, the rows in the Periodic Table, show a decrease in size with increasing positive charge to the right. As the charge increases, there are the same number of electrons in the outer shell, but an increasing number of protons in the nucleus. Hence the positively charged nucleus exerts more of a pull on the electron shell, making the atom smaller. For the same reason, as the oxidation state of individual atoms increases (i.e., they have more net positive charges), the atoms get smaller. The up-shot is that ions of K+, Na+ and Ca2+ are larger than the ions Mg2+ and Fe2+, which are in turn larger than the ions Al3+ and Si4+. The 2– anions are larger than then 1– anions, and so on.
Fig. 4-5: Illustration of the different sizes of common elements in minerals. Note that the anions are large. Cations increase in size with increasing numbers of electron shells and decrease in size with increasing charge of the cation. Numbers in the circles represent the ionic radii of the ions. (Ionic radii from R. D. Shannon, Acta Cryst A32 (1976):751–67)
Linus Pauling, the Nobel Prize–winning chemist, noted some simple regularities in the architecture of oxide minerals. Pauling’s rules stem from the concept, now confirmed through x-ray imaging, that negatively charged oxygen ions are arranged in polyhedra around positively charged metal ions. The number of oxygen atoms in the polyhedra controls the size of interior spaces where the cation resides (Fig. 4-6). Using some simple trigonometry, Pauling calculated that a (small) Si4+ ion could hold apart four oxygen ions in a tetrahedron, but not six oxygen atoms (in an octahedron) because the interior space would be too large. Mg2+ and Fe2+ are larger than Si2+ and fit inside an octahedron. Because Si, Mg, Fe, and O are the most abundant elements that make minerals, most minerals are three-dimensional arrangements of tetrahedra and octahedra, with O– making up the polyhedral container and the cations occupying the interstices.
Fig. 4-6: Illustration of the various polyhedra made up of large anions (normally oxygen) that leave small interior spaces for cations to fill. The larger the coordination number, the larger the size of the interior cavity and the larger the cation that can be accommodated. Rc and Ra refer to the radii of the cation and anion, respectively.
In addition to size and charge constraints, in order to grow in a regular pattern the particular arrangement of the atoms must be infinitely repeatable. If two molecules combine and create a surface template where the next atoms satisfy their electron shell requirements in exactly the same way, then growth can take place indefinitely. If atoms were combined in a way that did not permit the addition of the same molecular structure, no ordered growth could occur. An extreme example of this is the noble gases, where each individual atom remains separate because no connections with other atoms are possible. Ordered growth is also energetically more stable than a disordered collection of molecules, giving ordered structures a great advantage in their development.
Infinite repeatability is the governing principle of the laws of symmetry, and all minerals are symmetrical. Symmetry permits a pattern to be infinitely repeated, as, for example, in tiling a floor or building a wall. What tile shapes can you buy that will ensure no gaps imposed by the tile shape? Why, for example, are there no pentagonal tiles, despite the beautiful form of pentagons? Such practical questions, as well as the appeal of symmetrical patterns, have made the study of symmetry a human passion for thousands of years, understood by the ancient Egyptians and extensively developed by Islamic culture. The mosaics of the Alhambra, the Islamic palace in Granada, southern Spain, built in the thirteenth century, contain almost all possible symmetries (Fig. 4-7).
The remarkable aspect of symmetry is that throughout the universe only symmetries of twofold, threefold, fourfold, and sixfold rotations are possible, as well as mirror images. The same principles for tiling a floor or building a wall apply equally to minerals, but in three dimensions. In all there are only thirty-two symmetry groups that are possible in all of nature, and every mineral belongs to one of them.
The common minerals are those made up of the most abundant elements that form solid substances, and these are the oxides of Si, Fe, Mg, Ca, and Al. The Si4+ cation needs to share four electrons to satisfy charge balance. Because of its small size, it must be tetrahedrally coordinated. Four O– anions, the largest of the common elements, fit well around Si4+ in the shape of a tetrahedron, with each silicon sharing one electron with each oxygen, leaving the oxygens with a need for one more electron to fill their outer shell. This creates the possibility for stable building blocks of silica tetrahedra that can either bond with each other or with another metal atom. Silica tetrahedra are the fundamental building blocks of most minerals, equivalent to the central role that carbon with its four bonds plays in the world of organic chemistry. Carbon atoms form the backbone for almost all the molecules of life. Silica tetrahedra form the backbone for almost all the molecules of rocks. The fourfold coordination of each of these atoms, and their ability to create structural units that can bond both with themselves and with other atoms, are what permits symmetrical three-dimensional structures to be created.
The silica tetrahedron has a number of ways it can be organized, and these create the great classes of silicate minerals (see Fig. 4-8). If the tetrahedra remain isolated from one another with no Si-O-Si bonds, then each oxygen atom bonds with another metal. This is the structure of the olivine group. Olivine, consisting of mixtures of forsterite (Mg2SiO4) and fayalite (Fe2SiO4), is the most abundant mineral in Earth’s upper mantle. The next major group has as its structural backbone individual chains of silica tetrahedra. Each tetrahedron is joined to two others. Other metals then form the connecting bonds between the chains. Single-chain silicates are called pyroxenes. A large variety of different metals and minerals can fit into these flexible structures and form part of the pyroxene class. When two chains join together, the amphibole group is formed. The large hole in the center of the double chains permits the inclusion of larger cations such as K into the amphibole structure, whereas K is effectively excluded from all pyroxenes because of its large size.
Fig. 4-7: Top: An example of tiles from the Alhambra in Spain, illustrating threefold symmetry with no mirror planes or twofold axes. Bottom: a classic quilting pattern exhibiting fourfold symmetry.
Fig. 4-8: The silica tetrahedron is the fundamental building block of the most common rock-forming minerals, the silicates. Tetrahedra can either be isolated, forming the olivine group, or combined in single chains (pyroxenes), double chains (amphiboles), sheets (micas), or three-dimensional frameworks (quartz and feldspars).
The next group occurs when silica tetrahedra form continuous two-dimensional sheets. Because the sheets of silica tetrahedra are tightly bonded within the sheet, but the bonds connecting the sheets are weaker, they have a very characteristic sheety cleavage that we readily associate with the sheet silicate group of minerals, which includes the micas. Finally, the tetrahedra can form three-dimensional framework structures. Quartz is the most common example, but the feldspar class of minerals, which is the most abundant mineral group in Earth’s crust, also has a framework structure.
An important aspect of most silicate minerals is their potential to make solid solutions. Just as there can be liquid solutions (like salty water or alcoholic drinks) that mix together to form a single substance, there can also be solid solutions if atoms have similar sizes and charges. The great geochemist Goldchmidt pointed out that if two atoms have their charge and radii within 15% of each other, extensive solution is possible. For example, Fe and Mg both have a charge of +2, and their ionic radii are 0.32 and 0.35, respectively. Because Mg2+ and Fe2+ are very close in size and have the same charge, they freely substitute for one another in minerals. As size difference increases, substitution becomes increasingly difficult, because the structure has to deform to accommodate the larger atom, and ultimately it is no longer possible to continue the symmetrical structure. For example, pyroxenes with only Mg and Fe coordinating with the chains of silica tetrahedra form more symmetrical minerals than pyroxenes that incorporate significant amounts of the larger metal calcium (Ca). The amount of Ca is limited to 50%—greater amounts cause such disruption that pyroxene symmetry is no longer possible.
There are also nonsilicate mineral groups, although these are of lesser abundance on Earth. They include the sulfides (the most common of which is pyrite, FeS2 which makes “fool’s gold”), the oxides (such as magnetite, Fe3O4), halides (such as salt NaCl) and the carbonates (CaCO3), which are the dominant constituents of limestones. Carbonates are the rocks that link the geological and organic life cycles on Earth.
To sum up, the important minerals are determined by the overall abundances of elements available, the electron shell requirements for their combinations, the relative sizes of those elements, and the symmetry of the structures they can create. These features lead to the overwhelming importance of silicate minerals in creating the solid substrate of Earth.
Organic molecules are those where carbon atoms are joined with hydrogen. Organic molecules also often have a carbon backbone and combine with other elements such as oxygen, nitrogen, phosphorous, and others. They were originally identified as those molecules which were made exclusively by living processes. But in the early nineteenth century experiments were done that showed that organic molecules could be made by normal physicochemical processes, demonstrating that the bridge between the inorganic and the organic could be made in the absence of life. Furthermore, some molecules such as methane (CH4) are made in abundance in space and by inorganic reactions involving rocks, CO2, and water. Experimentation has also shown the existence of thousands of organic molecules that do not exist in nature. Therefore, “organic” now refers to the broad class of molecules that contain carbon and hydrogen, many of which are created and used by living organisms, as well as many others not created by biological processes. This definition covers most organic molecules and suffices for our purposes, but it is not perfect, because certain carbon compounds that are definitely associated with living processes, such as urea, have C-N bonds but no C-H bonds. That said, organic molecules are the stuff of life, and inorganic molecules are generally the stuff of rocks and minerals, and the distinction is important and necessary.
Fig. 4-9: The three simplest hydrocarbons. Methane is also known as natural gas and is the most abundant organic molecule on earth. All hydrocarbons can burn to form water and carbon dioxide. For example, CH4 + 2O2 → CO2 + 2H2O.
An important aspect of organic chemistry is the inorganic solvent, water. Many organic molecules contain water and undergo transformations associated with the incorporation or release of water. Most reactions among organic molecules take place in the presence of water—the average cell is about 80% water. Therefore, while water is an inorganic molecule and ice a mineral, the world of organic molecules, at least on Earth, is very much dependent on the hydrous environment. Even in interstellar space, where liquid water does not exist, ice may play an important role. And it also appears that much of the synthesis of organic molecules in space requires an inorganic mineral substrate as a catalyst. Therefore, in general organic molecules depend very much on the inorganic molecules that either host them or give rise to their creation.
The simplest organic molecule, and one of the most abundant in the universe, is methane (CH4) (Fig. 4-9). Methane is the simplest form of hydrocarbon—organic molecules made up of a carbon backbone connected to hydrogen atoms. Many more complex hydrocarbons exist—oil and gas are largely made up of a complex assemblage of hydrocarbons. All hydrocarbons burn in the presence of oxygen, which, if the burning is complete, converts them to the inorganic molecules CO2 and H2O.
More complex organic molecules that have essential roles in biological processes can generally be divided into four groups—carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates contain carbon combined with oxygen and hydrogen in the same proportions as water. Common examples are glucose, starch, and cellulose. Lipids have much less oxygen than the carbohydrates and a much higher energy content per gram. They are very efficient storage molecules for energy and include fats in animals and oils in plants. Proteins are by far the most diverse group of organic molecule. They are long chains of amino acids. The amino acids have at one end an amine group of a nitrogen atom bonded to two hydrogens and a carbon atom, and at the other end an acid (COOH), connected to what is referred to as an R group. The identity of the R group is what varies from one amino acid to another. A huge number of amino acids are theoretically possible and can be synthesized in the laboratory. Remarkably, only twenty of them are used to build the proteins of terrestrial life. But just as twenty-six letters allows a large number of words, having twenty different amino acids permits remarkable diversity in proteins. Some 100,000 different proteins are known to occur in living organisms, but the potential number of proteins that could be constructed is vastly greater. Some protein molecules are huge collections of amino acids—hemoglobin, for example, is a structure made up of 10,000 amino acids, and there are many even larger protein molecules.
The nucleic acids are long double chains that form naturally as double helices. The backbones of the nucleic acids are alternating groups of sugar and phosphate molecules. The links between the chains consist of pairs of bases—adenine, guanine, cytosine, and thymine or uracil. The combination of the base with the sugar and phosphates is called a nucleotide. The nucleotides then combine into very long nucleic acid chains. Of course, such chains are the fundamental carriers of information—they form the genes of all life—and are the means by which all cells can replicate.
The organic molecules on Earth generally are associated with the liquid state of matter. Even mammals are largely liquid, and it is the inorganic parts of our anatomy—bones—that give us some rigidity. Because of the liquid medium, organic processes generally take place as interactions among individual molecules, and the general principles discussed above for crystals and symmetry in minerals rarely find application in the organic realm.
As the universe cooled down after the Big Bang, gaseous hydrogen and helium were the only elements of significant abundance in the universe. The first generation of stars, therefore, was made up exclusively of these elements, and there were no solid particles or planetary systems in the universe, and no organic or inorganic molecules! Only after nucleosynthesis and distribution by supernovas were the elements available to create the molecules essential for planets and life. After formation these elements rapidly become surrounded by electron shells and combine into the simplest molecules. Hydrogen as the most abundant element is an important ingredient, as in CH4. Because oxygen is made in abundance in these massive stars, there is sufficient oxygen to make oxides of all the important metals. The oxygen left over combines with hydrogen to make water. Therefore, the earliest and most abundant molecules apart from hydrogen and CH4 are oxides, such as CO and H2O, and also oxides of all the other metals in accord with their abundance created during nucleosynthesis.
The metallic oxides then combined with SiO2 to make tiny grains of silicates. The existence of grains of minerals such as olivine in interstellar clouds is now verified by astronomical observations from telescopes in orbit around Earth. These grains are so small that they would hardly qualify as dust if we observed them on earth—they are more like silicate smoke. These silicate grains would generally be surrounded by tiny mantles of ice, since H2O would be far below its melting point in the very cold environment of interstellar space. Later production of reduced species in the absence of oxygen would add molecules such as CN, CH, and HCN to the mix. Mixing in space would not necessarily lead to chemical equilibrium among all these species, because the temperature is just a bit higher than absolute zero and particle densities are lower than the most extreme vacuum that can be generated in a terrestrial laboratory. Chemical reactions that we consider normal, therefore, are rare.
The interstellar environment has two additional factors that make it very different from molecular construction on Earth. In addition to the starlight that we see in the visible part of the spectrum, stars also emit stellar winds and ultraviolet radiation. Stellar winds became known through the discovery of the solar wind in the second half of the twentieth century. Theoretical calculations by Eugene Parker in 1958 showed that the intensely hot solar corona should emit high velocity particles. The theory was confirmed by spacecraft that measured energetic particles at high velocities streaming through space from the sun. The mean composition of these particles is very similar to the mean composition of the sun. Even more energetic particles were discovered as well, showing that other stars also emit such particles, giving rise to a general cosmic radiation. Some of this radiation is far more energetic than the solar wind, coming from much more energetic objects, such as large stars and supernovas.
The atoms involved in our everyday life are not particularly energetic. Molecules of air, for example, have low energies and move as fast as bullets, though still quite a bit slower than a typical satellite. When such molecules collide, they bounce off each other and do not affect the electron shell structure of the colliding molecules. The cosmic radiation is far more energetic, with speeds up to one-tenth the speed of light. The energies are high enough that collisions can knock molecules apart or knock off electrons and create ionic species. Therefore these particles can have an important effect on what chemical species exist.
Stars also emit ultraviolet radiation. We learned in Chapter 2 about blackbody radiation, and how the radiation emitted by an object becomes increasingly short in wavelength (more energetic) as the temperature increases. Hot massive stars, therefore, emit much more energetic radiation. The total amount of radiation emitted also goes up exponentially with temperature, so stars of many solar masses emit huge amounts of ultraviolet radiation, and this radiation can have important effects on chemical species, causing them to be ionized or break apart. Massive stars, through their winds, UV radiation, and ultimate explosions as supernovae, dominate energy injection into the interstellar medium.
These sources of elements, energetic particles, and UV radiation are not isolated in space, because most stars are created in massive clouds that serve as “stellar nurseries” where thousands of new stars are born. Some of them are the massive stars emitting radiation so intense that they clear space around them. Supernovae are common in these environments, spewing newly made elements that will then be incorporated into small stars and planetary systems. The interstellar clouds (see frontispiece of Chapter 5) are the largest factories in the universe, not only for element creation but for the evolution of molecules.
Where the radiation is most intense in the cloud, complex molecules cannot form because they are continually broken apart. When the cloud becomes denser, however, the thicker dust protects the interior particles and gases from much of the radiation. In these environments, given the long time frames of interstellar space, complex reactions can take place and hundreds of different molecules are created. An important aspect of these reactions is that they differ substantially from reactions observed on Earth. On Earth, for example, virtually all organic reactions take place in the presence of water. But in space the pressures and temperatures are so low that water exists only as ice. The sluggish chemical reaction rates that one might expect there, however, are overcome by the energy supplied by the ultraviolet light from nearby stars. The rarity of interactions in the high vacuum is overcome by reactions that take place on the surfaces of the interstellar dust. Some molecules form when atoms stick to the surface of an olivine crystal and through diffusion encounter each other. Others require a combination of particles and radiation. For example, carbon dioxide can form when an ice containing carbon monoxide is irradiated with UV light in the presence of oxygen atoms.
The net result of these reactions has only been observable in the last few years, with the launching into space of telescopes that are able to explore wavelengths previously obscured by Earth’s atmosphere. These studies have revealed more than a hundred different molecules, and the list grows every year. These molecules include not only the major silicates but a host of organic molecules, including “prebiotic” molecules that would likely be those required to start life on Earth—such as water, methanol, formaldehyde, and hydrocyanic acid. Thus the interstellar clouds are not only incubators for the formation of new stars, they also make the molecular raw material that ultimately combines to form planets.
Through the study of comets, which are the relics of icy planetesimals that accreted in the outer solar nebula, we can infer that the processes that are now observed in interstellar clouds in our galaxy were also pertinent to the formation of our own solar system.. The last decade has permitted astronomical study of the compositions of comets, and many of the same molecules discovered in protoplanetary disks in distant space are found in the comets as well. While this fast-moving area of science is by no means fully developed, it is clear that all the molecules necessary for the beginning of a habitable planet are abundant and widespread throughout the universe. Processes that we can infer for the solar system at the time of its creation are beginning to be observed happening now in other parts of the galaxy (see frontispiece to Chapter 5).
Elements do not remain isolated but come together to form molecules shortly after they are formed by nucleosynthesis. The laws that govern their combination relate to their size and the detailed structure of their electron shells. Large anions form the polyhedral framework of inorganic molecules that come together to form minerals, and the size of the polyhedra controls what cations can reside in the interior. The most important structural unit for minerals is made up of the two alpha-particle nuclides Si and O, forming the silica tetrahedron. The silica tetrahedra can join in a variety of ways to produce an astonishing number of silicate minerals that we will see are the main building blocks of planets. Organic molecules are those where carbon combines with itself and with hydrogen, nitrogen, and other elements to form the basic building blocks that ultimately will come together to form primitive life. The physical properties of volatility and density will play an important role in how these molecules distribute themselves during subsequent events in the universe. Volatile compounds remain as gas even to low temperatures, and cannot accrete as solid materials. They will become the primary constituents of planetary atmospheres. Refractory materials such as silicates remain solid even at high temperatures and will become the solid materials of planets. Density will then control at what levels within planets the various molecules reside.
Huge interstellar clouds are the likely nurseries where both element formation by nucleosynthesis and the early molecules and their reactions with one another take place in an environment that is very foreign to us as residents of a benign planetary surface. Supernovae distribute new elements and create massive energy fluxes through the nebular cloud that contribute to molecular construction. Smaller concentrations of matter come together to form smaller stars such as our sun that can give rise to long-lived planetary systems necessary for life.