
Fig. 15-0: Forest fire, which is an uncontrolled release of the energy stored in Earth’s planetary fuel cell, as reduced organic carbon molecules react with oxygen. This image is from the Biscuit fire, which was Oregon’s largest forest fire of the last century, consuming almost 500,000 acres. (Photo © Lou Angelo Digital on Flickr, with permission)
Biological evolution has been closely coupled to planetary evolution throughout Earth’s history. The origin of life required reducing conditions and the earliest Earth provided them, with a surface devoid of free oxygen. Modern life requires oxidizing conditions and free O2 for its metabolism. Between the ancient and modern Earth, during the Archean and Proterozoic, a planetary transformation progressively oxidized the atmosphere, ocean, and crust.
This transformation was the result of life. To make its constituent organic molecules, life requires hydrogen and “reducing power,” electrons that can be added to the oxidized carbon in CO2, where C has a 4+ valence, to make the reduced carbon of organic matter, CH2O, where carbon has a neutral valence. This change requires a source of hydrogen and a source of electrons to reduce the carbon. Early life was limited by sources of hydrogen and reducing power, but at some point in the Archean life developed photosynthesis to take advantage of the ubiquitous water molecule as a source of both hydrogen and electrons. Photosynthesis converts the energy of stellar nuclear fusion to an electron flow that stores energy in the chemical bonds of organic matter.
The complement of the reduced compounds of organic matter is the production of oxidizing power. Every molecule of CO2 converted to reduced carbon and stored in the Earth creates an equivalent molecule of the highly reactive O2. The oxidizing power released by life reacted with other planetary materials and gradually oxidized the ocean, soils, and atmosphere. The O2 waste product of photosynthesis was initially toxic to organisms, but the formation of oxidized and reduced reservoirs also created a large potential energy source, and life evolved to take advantage of it. The development of aerobic respiration provided eighteen times as much energy per glucose molecule, empowering new biological potential. After a long process of progressive planetary oxidation, O2 was able to reach high enough concentrations in the atmosphere for complex multicellular life to evolve, and for an ozone shield that protects from the harmful effects of ionizing radiation, permitting abundant life on land.
The progressive oxidation of the surface has transformed Earth from a homogeneous oxidation state in the interior and exterior to one where the planet has become a kind of giant fuel cell, with a reduced interior and largely oxidized exterior, whose combination produces energy. In this sense, life has energized the planet, using solar energy through photosynthesis to separate electrons and create reduced and oxidized reservoirs whose reaction powers life and planet. The development of chemical mechanisms that transitioned from a reduced early Earth, where no oxygen was produced, to a modern Earth, where high levels of oxygen are essential for multicellular life, was a linked biological and planetary evolution. This process is the gradual transformation of planet and life, a story that closely links Earth, life, and the sun.
Life at the beginning was a planetary process. In Chapter 13 we found that life’s origin was not an isolated biological event but depended on the existence of an ocean, a stable climate, an appropriate atmosphere, volcanism, and mineral surfaces—all planetary phenomena. Today, terrestrial life and Earth are also inextricably interdependent. Life depends on water, soil, air, and climate, which are planetary, and those domains are also influenced by life. The oxygen in the air and ocean that makes animal life possible is biologically produced, as is the organic matter that makes soils fertile for plants. Climate stability depends on the carbon cycle, which links life and climate to volcanism and the rock cycle, and life also enhances weathering through breaking down minerals. Biological and geological processes are linked through the cycles of most elements through the various earth reservoirs. Life at the beginning was a planetary process; life today is a planetary process.
There are nonetheless vast differences between the early Earth and Earth today for both life and planet. The early Earth was a barren landscape populated by unicelled organisms even simpler than the most primitive prokaryotic cells, and O2 was toxic for these early organisms. The atmosphere had a very different chemical composition, much higher in CO2 and lacking in oxygen. Today multicellular organisms dominate every nook and cranny of the planetary surface, and the atmosphere has 21% oxygen, sustained by plants and essential for modern animals.
There is thus a long journey from early barely colonized, anoxygenic Earth to modern fully inhabited, oxidized Earth—the story of planetary evolution. To understand the gradual development of the habitable planet we experience today—exquisitely attuned with the survival of the species that abound on the surface—we need to unearth the planetary history that transformed ancient past to familiar present, which is our aim in the next three chapters. A central aspect of this history is how life and the planetary surface coevolved to generate reduced and oxidized reservoirs whose interactions provide the energy for modern life.
Life’s metabolism produces organic molecules and processes energy. The energy for life can be viewed as a kind of slow electric current involving electron transport. Carbon is an essential medium for this transfer of electrons. With valence states between +4 and –4, carbon contains the maximum potential of any element in the periodic table for electron transfer. The source of carbon for most life is CO2 from volcanic outgassing, where carbon has a 4+ valence. Organic molecules in contrast are made up of carbon in more reduced states and have carbon-hydrogen bonds. The generic formula for organic matter is CH2O, where carbon has been reduced from a 4+ valence in CO2 to a neutral valence—e.g., a common product of organic synthesis is glucose, C6H12O6 or 6(CH2O). Carbon can become even more reduced, as in the compound CH4, where carbon has a 4– valence and has eight more electrons than carbon in CO2. To reduce the carbon and form organic molecules life requires a source of electrons and of hydrogen. Schematically the overall production of organic matter on Earth can then be summarized as:
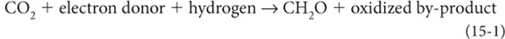
The electron donor element, called the reductant, becomes oxidized by transferring electrons to carbon.
Reaction (1) is the formation of organic molecules, and such formation requires energy, which must come from the sun, Earth, or some disequilibrium that has energetic potential. Reaction (1) can also run in reverse, releasing energy. Plants use energy from the sun to run Reaction (1) in the forward direction; animals then consume the CH2O to make energy for metabolism by running Reaction (1) in reverse. Both involve electron transport—the slow electrical current. All of life involves such transport.
Chemical reactions that involve electron transport are called oxidation/reduction reactions, and to maintain charge balance every molecule that is reduced by electron addition must be balanced by another molecule that is oxidized by electron removal. This means that life, forming reduced molecules, requires partners that can be oxidized. This is the chemical coupling between life and planet, as most of the elements that can become oxidized are molecules such as Fe and S (as well as O2–) that we associate with rocks and the solid Earth rather than life itself. Life and Earth process energy in an energy partnership.
The separation of reduced and oxidized compounds then creates an energy potential that is released when the compounds come into contact. The amount of energy produced depends on the number of electrons that are transported. Maximum electron transport occurs when highly reduced molecules encounter highly oxidized ones. An example of such release, Reaction (1) running in reverse, is when we heat our homes with natural gas. Natural gas is methane (CH4), where carbon is in its most reduced –4 valence. When we burn it, it reacts with the highly oxidized O2 molecule:
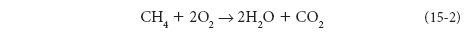
Fig. 15-1: Schematic illustration of a fuel cell, where the oxidation/reduction reaction of H2 plus ½ O2 to make water is used to generate an electrical current.
Each oxygen accepts two electrons to change from neutral valence to 2–, so eight electrons are transferred from the carbon, releasing large amounts of energy. Heating homes involves flames and release of heat. Another way the energy potential can be released is directly into an electrical current, as takes place in a fuel cell (Fig. 15-1). The fuel cell analogy can apply to all reactions between reduced and oxidized compounds that produce controlled electron flow. All of modern animal life depends on such controlled reactions between highly reduced molecules (food) and O2.
The starting point for Earth’s history is based on the ages of the meteorites and moon near 4.55 billion years ago. Apart from the tiny zircons discussed in Chapter 9, there is no terrestrial rock record for what happened between that time and tiny continental terrains that contain the oldest reliably dated rocks—the Acasta Gneiss in Canada, with an age of 4.03 billion years. The Hadean is an apt term for this portion of Earth’s history, since Earth would have been a kind of fiery hell from our current perspective, with much more abundant volcanism and frequent meteorite impacts. For this time period much of the scientific interpretation is inevitably guesswork, because there is little direct evidence of life and Earth’s surface conditions apart from what can be gleaned from zircons and planetary science.
We are slightly better off for the Archean era, covering the time span from 4 Ga to 2.5 Ga. “Slightly” is the appropriate word, because the volume of Archean rocks preserved today is only a few percent of current continental crust. The few rocks that have survived have been substantially modified during their long history in the crust, leaving a record that is difficult to interpret. To put the early Earth problem in perspective, the Hadean and Archean are when the first continents formed, plate tectonics may have begun to operate, and life first appeared. This two-billion-year time span is four times longer than the entire record of animal life in the Phanerozoic. With no preserved rocks for the first 550 million years of Earth’s history, and few metamorphosed rocks for the next billion years, the challenge to understand earliest Earth’s history is daunting. Nonetheless, the evidence that does exist is sufficient to show that the early Earth was a very different place from Earth today.
In particular, all the evidence suggests the early Earth was lacking in free O2. The present atmosphere composition with 21% oxygen is a striking disequilibrium state, because O2 is such a highly reactive molecule. Left to itself, oxygen cannot persist at equilibrium as a separate gaseous molecule as long as there are reduced compounds with which it can react. Oxygen reacts with metals, carbon, sulfur, and other atoms to form oxides. Some reactions, such as the weathering of rocks, are relatively slow by human standards, others are rapid enough to create fire or even violent explosions. Only the continuous production of oxygen by plants allows the oxic present conditions of Earth’s atmosphere to persist. Absent such production, the oxygen in the atmosphere would react away, ridding Earth’s surface of organic matter in a few hundred years, and then gradually disappearing over a few hundred thousand years as the remaining O2 reacted with rocks and reduced gases coming from Earth’s interior. How can we tell if the early Earth might also have had free O2?
There are no samples of ancient atmospheres remaining, but there is an abundance of other evidence showing that free O2 was lacking from Earth’s origin through much of the Archean. Because O2 cannot be measured directly, the evidence comes from other elements with multiple oxidation states that would have reacted with O2 if it had been present.
Many atoms can have multiple valence states and combine with varying amounts of oxygen. As more and more oxygen is consumed, the valence state of the metal progressively increases. For Fe and O, two of the major planet-forming elements, some of the possible molecules are Fe, FeO, Fe3O4, and Fe2O3. Notice that in this series the oxygen proportion of the molecule increases as the valence state of Fe increases from neutral to 2+ to 3+. For this reason, we call the higher valence states the oxidized forms of the atom. Rusting of iron and the red color of many soils are visible examples of iron becoming oxidized by reaction with oxygen.
Sulfur is another abundant element with multiple oxidation states under terrestrial conditions. Sulfide minerals such as troilite (FeS), the form of sulfide found in meteorites, has S with a –2 valence. Pyrite (FeS2), has S with a –1 valence. These minerals can be oxidized to sulfates (e.g., FeSO4 or CaSO4) where S has a +6 valence. Many other elements have multiple oxidation states and provide additional clues for the geological detective (Fig. 15-2). Elements with different oxidation states form different minerals, and the presence or absence of these minerals indicates the oxidation state of the surface at the time the minerals formed. When free O2 is available, only fully oxidized minerals are stable. The mineralogy of rocks thus reveals the oxidation state of Earth’s reservoirs.
The first rocks of interest are the meteorites that combined to form the early Earth. Chondritic meteorites contain Fe metal, silicate minerals with FeO, and the most reduced form of sulfur, FeS. There is no excess oxygen available, so the Fe and S are in reduced states. The volatile-bearing meteorites, carbonaceous chondrites, contain iron metal and reduced carbon compounds, materials that cannot exist in the presence of O2. We saw in Chapter 7 that early planetary differentiation involved reaction between Fe metal and silicates. Free oxygen is also lacking in the solar nebula, where there is always an excess of oxygen-hungry elements, such as H, C, and Fe, to combine with oxygen to form oxides. This evidence shows that materials that formed Earth were highly reduced. Furthermore, the moon, which formed from similar materials near the same time, is reduced even today. There is no ferric iron on the moon, and lunar basalts appear to have been in equilibrium with Fe metal. Since the moon is a “planetary fossil” that did not undergo co-evolution with life, it provides further evidence for a reduced state for the early Earth before life began its transformative process.
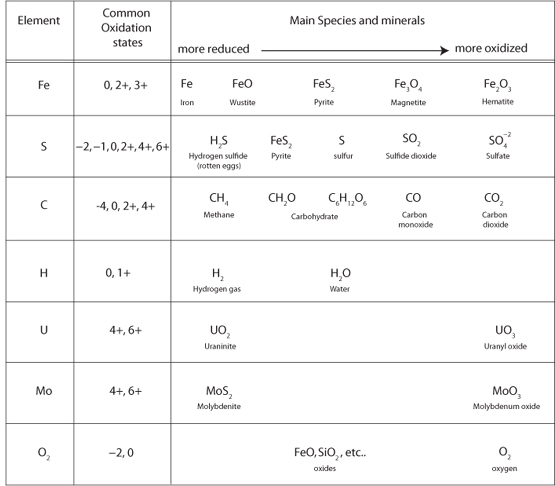
Fig. 15-2: Illustration of the various oxidation states that important minerals can have during Earth processes. Reduced forms are on the left, and more oxidized forms on the right. The early Earth had all elements but carbon in their reduced forms. During Earth’s history, life has taken oxidized carbon from CO2 to make reduced organic carbon, and this electron flow has been balanced by oxidation of all other species. Because different oxidation states have different solubilities in water and form different minerals, preserved minerals in ancient rocks record the oxidation state at the time they formed.
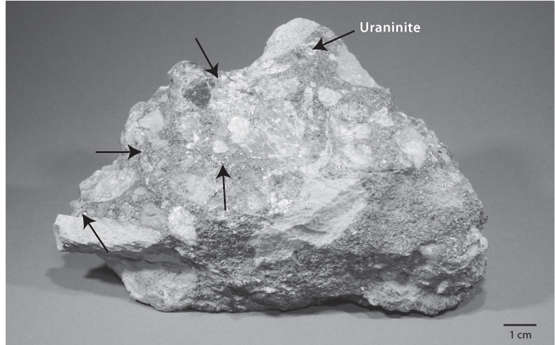
Fig. 15-3: Ancient river gravel containing grains of uraninite, indicated by arrows, where U is in the +4 valence state. The gravel was subsequently buried and turned into a hard rock that was recently unearthed by erosion. Uraninite can persist and be included in river gravels only under reducing atmospheric conditions, showing the Archean atmosphere had no oxygen. (Courtesy of Harvard Museum of Natural History, Dick Holland Collection)
We can move forward in time by looking at some of the oldest Earth rocks. To infer past conditions of the atmosphere, it is key that the minerals formed at the surface and were not buried deep in the crust, where even today oxygen does not penetrate efficiently. Sedimentary rocks form at the surface, and river sediments in particular are inevitably in contact with the atmosphere. Some ancient river sediments, a type of gravel called a placer deposit (Fig. 15-3), survive from the Archean. The mineralogy of these sediments can be used to test for the presence of oxygen in the atmosphere.
The two critical minerals in the Archean placer deposits are reduced sulfides and a uranium-bearing mineral called uraninite (UO2). Uranium can have multiple oxidation states, of which the two found in minerals are U 4+ and U6+. U6+ is relatively soluble in water and does not form stable minerals on Earth in the presence of oxygen. Uranium in its reduced 4+ form, however, is insoluble in water and precipitates to form uraninite. When uraninite is exposed to the surface today, the U4+ is oxidized to U6+, the uraninite mineral rapidly disappears, and U6+ is carried away by the water. Uraninite persists only in reducing environments lacking in oxygen, and does not exist in modern river sediments. The placer deposits formed by Archean rivers, however, formed ancient rocks that were buried and shielded from later atmospheres. When these rocks are freshly brought to the surface, they contain uraninite, showing that when these gravels were formed, no oxygen was available at the surface to oxidize the uraninite. Similar arguments apply to pyrite. While pyrite is found in modern environments, it occurs only in reduced rocks, such as sediments rich in organic matter. Pyrite does not survive for long in contact with the atmosphere, and no river gravels today contain the mineral. The presence of sedimentary pyrite in ancient rocks exposed to the atmosphere also indicates reducing conditions with no atmospheric oxygen.
Another source of indirect evidence about the oxidation state of the early Earth comes from consideration of the origin of life. Life is made up of organic molecules that have reduced forms of carbon, and organic molecules react with oxygen and are converted to their oxidized forms where organic molecules are not possible. For life to get started, organic molecules must exist stably in the environment, and this is not possible with an oxidizing atmosphere, so reducing conditions are probably essential for the origin of life. Once the first cell formed, it would also survive by making use of molecular building blocks in its environment. The organic molecules required by early organisms would be destroyed by oxygen. Both life’s origin and its early persistence seem to require anaerobic—oxygen-free—conditions.
This evidence and reasoning about a reduced early Earth are also consistent with our understanding of the source of oxygen in the atmosphere today. Oxygen-producing photosynthetic bacteria were absent in earliest Earth’s history, there were no plants, and there was a large mass of reduced compounds such as FeO, FeS2, and probably CH4 and H2 ready to attach to any trace amounts of oxygen that might have been produced—e.g., by reactions in the upper atmosphere.
All these lines of evidence—meteorites, early planetary differentiation with metal present, the oxidation state of the moon, the mineralogy of ancient sediments, the origin of life, and where O2 comes from today—lead to a convincing conclusion of a reduced early Earth. The early atmosphere was so reducing that O2 contents are estimated to be less than 10–10 of the present atmospheric level (PAL) of O2.
In contrast to the early Earth, the habitability of Earth today and the existence of multicellular organisms depend on the presence of the high levels of O2 in the atmosphere. Without O2, little of the life currently on the surface would survive, and there would be no ozone shield protecting surface life from high-energy ultraviolet radiation coming from the sun. Clearly the oxygen content of the atmosphere has changed through Earth’s history, and this change is closely associated with life. Why did life start to produce O2? As we will see in the next section, O2 production is a manifestation of the gradual development of biological technology to harvest and make use of the energy coming from the sun. This development took place through a series of “energy revolutions” that enabled life to have progressively increasing access to energy over Earth’s history.
As we saw from Reaction (1) above, formation of organic molecules requires input of energy and creates an oxidized atom. Organic molecules need to be formed not only to make living matter but also as a source of food that can be “burned” to release energy for metabolic processes. The energy currency in the cell is adenosine triphosphate (ATP), which is used to power cellular reactions. As ATP, the molecule is charged with energy; conversion of ATP to ADP (adenosine diphosphate) provides energy for the cell, and then conversion back to ATP recharges the molecule with energetic potential. The breakdown of carbohydrates such as glucose provides electron transport and chemical pathways that allow production of ATP from ADP. Organisms “burn” the sugar, using the special ATP-ADP mechanism to capture the energy so it can be used for metabolic processes.
Ultimately, formation of ATP requires an external energy source. Some organisms make use of external energy sources such as sunlight or chemicals that are out of equilibrium with their surroundings to produce ATP as well as glucose, which is later burned for cellular operations. Organisms that produce their own organic molecules by making use of an external energy source are called autotrophs. Autotrophs make use of available energy to create their own food. Other organisms, called heterotrophs, absorb (e.g., eat) organic molecules made by other organisms and make use of them for the energy for cellular processes. Plants are autotrophs because they make their organic molecules by harvesting sunlight. Animals are heterotrophs—we eat for essential molecules and for our energy source. There are also autotrophic and heterotrophic bacteria.
It is not clear whether earliest life was autotrophic or heterotrophic. One idea is that earliest life may have been heterotrophic, eating the organic chemicals that were made abiotically in the early Earth. Such life would be limited by the availability of nonbiogenic “food” provided by the planetary environment. In this case there would be distinct advantages for organisms that were able to make their own food using energy directly, rather than relying on their surroundings to provide them with food. Alternatively, the earliest organisms may have developed means to convert sunlight or other external energy to make their own food. In either case, the development of autotrophy, allowing life to make its own food through access to planetary and solar energy, was such a major step in the evolution of life that we can call it the first energy revolution.
Chemoautotrophs made use of chemical energy, e.g., in hydrothermal vents. Methanogens, for example, derive energy to make organic matter from the reaction
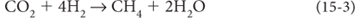
oxidizing hydrogen from neutral to +1, and releasing methane in the process.
Photoautotrophs were able to harvest light energy from the sun to gain electrons from reductant molecules such as H2 and H2S, which also served as a source of hydrogen. These organisms made glucose from reactions such as:
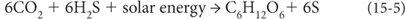
In the first reaction, the H2 is the source of electrons and hydrogen as it is oxidized from neutral to +1. In the second, H2S is the source of hydrogen, and electrons are released by the oxidation of sulfur from –2 to neutral valence. Notice that no oxygen is produced in these reactions, and the reactions require the reduced molecules H2 and H2S and can take place only in reducing environments. The photosynthetic processes for these anaerobic bacteria are of two types—photosystem 1 and photosystem 2 (PS1 and PS2)—with the numbers simply indicating the order in which they were discovered.
The glucose that is formed can then be processed to produce ATP for other cellular reactions, for example, by fermentation:
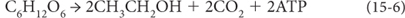
Glucose → 2 alcohol (ethanol) + 2 carbon dioxide + 2 ATP
Another method is glycolysis, which has a more complex reaction that also produces two ATP for each glucose molecule. Both of these metabolic pathways are anaerobic methods of ATP production. The waste products are not fully oxidized, and cells that use these mechanisms need to have means to dispose of the waste. Aerobic metabolism, discussed below, is able to further process these “waste products,” and through the citric acid cycle—or the Krebs cycle—makes use of the energy available in their chemical bonds to generate much more ATP.
These mechanisms permitted bacteria to make their own food and process it to gain ATP. When they died or leaked molecules into the environment, they provided resources for heterotrophic bacteria, leading to the development of early ecosystems and the first simple food webs. These mechanisms still exist in cellular metabolisms today. Prokaryotic cells that thrive in anaerobic environments use photosynthetic mechanisms that are most closely related to early life.
The disadvantages of PS1 and PS2 used in Reactions (15-4) and (15-5) are that they rely on availability of H2S and H2 as a source of electrons and hydrogen, and these molecules are always at low concentrations in seawater. This limits both the amount of organic matter that can be formed and the extent of the biosphere. Earliest life then likely required a reducing environment to gain a planetary foothold, but it was limited by the availability of the reducing power and the H-containing molecules that it needed to form organic matter. Given these limitations, it is unlikely that early life was very abundant.
Within the liquid environment, however, was an almost infinite reservoir of hydrogen and electrons in the H2O molecules. One of the great evolutionary innovations occurred when organisms developed the chemical machinery to break down the H2O molecule as a combined source of reducing power and hydrogen. This innovation required the combination and modification of PS1 and PS2 acting together, leading to the oxygenic photosynthesis that is at the base of most food chains even to the present day:
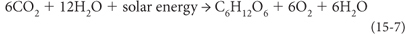
The electrons to reduce the C come from converting the O2– in the H2O molecule to neutrally charged O2, so Reaction (15-7) requires breaking the strong H-O bonds of water. PS2 was modified to be able to break the H-O bonds, and then PS1 was able to complete the process. Oxygen is the reductant, supplying electrons to reduce the carbon as oxygen changes its valence from –2 to neutral, and each atom of organic carbon produced leads to a by-product of one molecule of O2. Cyanobacteria are the diverse descendants of early organisms that developed this capability.
The development of oxygenic photosynthesis was a second energy revolution that allowed much greater access to the energy of the sun, enabling it to be captured and stored in the form of reduced organic molecules. The new mechanism of oxygenic photosynthesis was no longer limited by a source of hydrogen and electrons—there was an almost infinite source of hydrogen and reducing power once the very strong bonds holding the water molecule together could be broken, and there was a vast energy supply in the form of sunlight. Of course, while oxygenic photosynthesis eliminated the problem of hydrogen availability, there were still limits to growth, owing to the limited supply of other critical nutrients for life such as phosphorous and nitrogen. Furthermore, early prokaryotic organisms would have had to rely on anaerobic metabolism of glucose to generate their energy. For every molecule of glucose they were able to obtain, they could still get only two ATP units of energy for cellular processes.
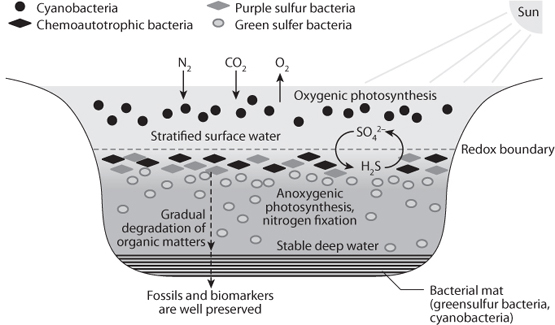
Fig. 15-4: Illustration of conditions in the Black Sea, one of the few places in the ocean where oxygen is absent just below the surface. Oxygenic photosynthesis by aerobic cyanobacteria takes place in the top layer. Oxidation of sinking organic matter uses up all the oxygen in the water below, preventing cyanobacteria from surviving. In their place, anaerobic bacteria (purple and green sulfur bacteria) make use of the variable oxidation states of sulfur to generate their own thriving ecosystem. The anaerobic conditions of the early Earth would have been fully occupied by similar anaerobic bacteria, which today are relegated to minor niches of the biosphere that have avoided oxidation.
All of these forms of photosynthesis survive today. For example, in the Black Sea cyanobacteria occupy the shallow water where they carry out oxygenic photosynthesis. As they die and fall through the water column, the oxygen they produced is consumed in the breakdown of the organic matter, and the deeper water is anaerobic. There, purple and green bacteria that make up colorful layers in anaerobic environments are carrying out photosynthesis using PS1 and PS2 separately (Fig. 15-4).
As can be seen from Reactions (4) and (5) above, anaerobic operation of PS1 and PS2 created benign waste products like H2O. Oxygenic photosynthesis, however, created a difficult pollution problem. While today we think of O2 as a beneficent aspect of photosynthesis, this was not the case for early organisms. O2 breaks down organic molecules and would have been a devastating poison for early life. Even for modern life oxygen is a potent poison, as reflected in our popular consumption of “antioxidants” to prevent deterioration of our cells. Absent the evolution of molecular protection against this poison, the waste problem would have limited the spread of oxygenic photosynthesis.
Within this crisis, however, there was also latent opportunity that contained the potential for a third energy revolution. Oxygen is a highly reactive molecule that spontaneously reacts with all kinds of metals and reduced organic matter, and such reactions release abundant energy. Where energy is available, some evolutionary adaptation comes along to take advantage of it. This adaptation was a major boon for life’s energy, because while anaerobic breakdown of glucose by Reaction (15-5) produces only two molecules of ATP, aerobic (using oxygen) breakdown of glucose is able to produce thirty-six molecules of ATP!
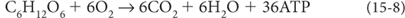
Glucose → 6 carbon dioxide + 6 water + 36 ATP
This ability to respire oxygen in a controlled way required major evolutionary adaptation and was the third energy revolution for Earth’s life, because it increased by a factor of 18 the energy that could be gained from the glucose molecule (see Fig. 15-5). Every breath you are taking as you read this chapter delivers the oxygen and releases the CO2 from Reaction (8), and every thought you are having is making use of the ATP released in the process.
The potential energy of Reaction (15-8) could not be fully exploited, however. To take advantage of aerobic respiration requires that there be plenty of O2 available. And because O2 is so highly reactive, it is gobbled up by reduced species, which were in abundance in the early Earth in the form of CH4, H2S, FeO, and FeS2. Raising the oxygen level of the atmosphere to allow life to fully make use of oxygen for energy production would require producing enough oxygen to overcome the reducing power of the planetary surface. The planetary exterior needed to become oxidized before the full potential of the third energy revolution would be able to be realized.
So we see that evolution of life is closely coupled to evolution of the oxidation state of Earth’s surface. The atmosphere and ocean start with essentially no O2, and the abundance of life is kept at low levels by the limited supply of reductants that enable conversion of CO2 to organic carbon. Then life evolves oxygenic photosynthesis to break the water molecule and have an essentially infinite supply of hydrogen and of reductants in the O2– of the water molecule. The O2 produced by the growth in organic matter is at first a pollutant and poison, but life evolves to make use of it as a much more efficient energy source. Once sufficient O2 is available, life even comes to depend on high levels of O2 in the environment. Ultimately, breathing and inner circulation systems evolve to transport oxygen efficiently to multicelled organisms, allowing oxygen-hungry organs like the brain to evolve. Evolution thus leads to and is influenced by a progressively oxidized surface. The entire process leads to an increasing ability to harness energy from the sun and send it through the biosphere via electron flow.
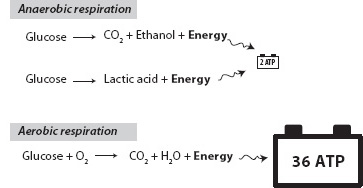
Fig. 15-5: Illustration of the different energy production that can be obtained by anaerobic and aerobic metabolism of glucose to form the energy currency of life, adenosine triphosphate (ATP). Anaerobic respiration of glucose produces only two ATP per glucose molecule, indicated by the small battery. Aerobic respiration fully metabolizes the glucose to produce 36 ATP, indicated by the large battery. The greater energy production is an energy revolution for life.
A fuel cell is a device that converts the chemical potential of oxidized and reduced molecules into an electrical current. A fuel cell is like a battery, except that it relies on replenishment of reduced and oxidized molecules to maintain its energy potential. The fuel cell becomes charged by the separation of positive and negative electrical potential enabling electron flow.
From a planetary perspective, over billions of years of earth evolution, the electrical current generated by life has been used to “charge” a planetary fuel cell, using solar energy to convert CO2 and H2O to reduced carbon and oxidized species. The solid Earth also plays a role. The interior reservoir of the Earth is so vast that its overall oxidation state is little affected by organic processes at the surface. Earth’s interior then provides a sustained reduced reservoir for the fuel cell. Life adds to the reduced reservoir by burial of organic carbon, and every atom of carbon sequestered by burial releases a molecule of O2 to oxidize the surface. Surface oxidation is a long process, because the abundant reservoirs of reduced sulfur and iron provide a large sink that absorbs the oxygen. Only when this reservoir has become sufficiently saturated with oxygen is an oxygenic atmosphere able to be developed. All of this occurs while life consists only of single-cell organisms, a process taking 2 billion years or more.
Once sufficient free O2 is available at the surface, multicellular life is able to evolve by making use of the planetary fuel cell to generate high energy during aerobic respiration, when reduced organic molecules are reacted with O2 to produce energy. For example, at hydrothermal vents, reduced species from the mantle encounter oxidized seawater, supplying the energy potential for the microbes that lie at the base of deep-sea food chains. Life evolved to take advantage of the energy flow, leading at hydrothermal vents to the spectacular abundance of life in the absence of sunlight. Energy can be released in the absence of sunlight by the flow between the two “poles” of Earth’s fuel cell, reduced reservoirs of organic carbon or Earth’s interior encountering the oxidized pole of the planetary surface (Fig. 15-6).
Nonbiological processes are also influenced by contact between the reduced interior and the oxidized surface. When new reduced rock is exposed to the surface, it reacts and becomes oxidized, enhancing weathering and contributing to geochemical cycles. When oxidized ocean crust is subducted into the interior, it adds ferric (Fe3+) iron and water to the mantle wedge above the slab, oxidizing the local region of the mantle and influencing the compositions of magmas, and their cooling path, to produce high SiO2 magmas and oxidized gases characteristic of the continental crust.)
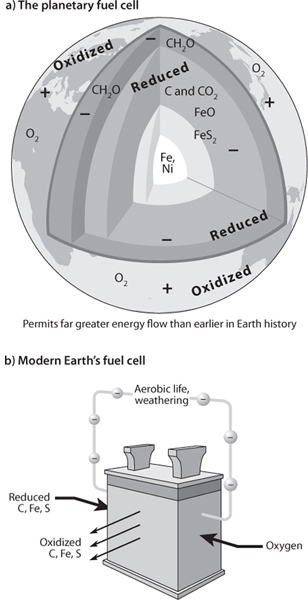
Fig. 15-6: Top panel: This illustrates the state of the modern Earth with oxidized and reduced reservoirs that when combined release energy. The reduced reservoirs are organic carbon and Earth’s interior. The oxidized reservoirs are the oxidized surface rocks and the O2 in the atmosphere. Bottom panel: The fuel cell concept of Figure 15-1 with the Earth’s chemical reservoirs as the reduced and oxidized species that power Earth processes.
The fuel cell can also be released in catastrophic and uncontrolled energy loss to heat. This occurs, for example, when the organic carbon buried in Earth’s crust is burned by contact with the atmosphere. As we shall see in Chapter 17, extreme climate change and important punctuation points in biological evolution often result from catastrophic connections between Earth’s reduced and oxidized reservoirs. And all of modern civilization depends on our access to and exploitation of Earth’s fuel cell through extraction and burning of fossil fuels.
Earth’s history began with both interior and exterior at a similar reduced oxidation state. This reduced state lasted more than 1 billion years and was an essential condition for the origin of life, during which reduced molecules must be stable in the surface environment. Early life discovered how to make its own food by making use of chemical energy from Earth and light energy from the sun. These autotrophs were nonetheless limited by sources of reductants. The discovery of oxygenic photosynthesis, sometime during the Archean period, vastly increased life’s access to solar energy by obtaining hydrogen and electrons from the ubiquitous water molecule. Life would then have been limited only by other critical reagents such as N and P. The O2 by-product of oxygenic photosynthesis was initially a poisonous pollutant, but it also contained the possibility of much more cellular energy through aerobic respiration of glucose, increasing energy available for life by a factor of 18. The buildup of oxygen in the atmosphere would have been limited, however, by all the reduced species resident at the surface and continually added from Earth’s reduced interior. Only after life had produced enough oxygen to saturate the surface reservoirs could the O2 content of the atmosphere rise to modern levels, permitting the development of multi-cellular life in the late Proterozoic and Phanerozoic. The progressive oxidation of the surface by production of organic matter through Earth’s history has created vast oxidized and reduced reservoirs that serve as the poles of a planetary fuel cell. Reactions between these reservoirs provide the essential energy for modern life and planetary processes.