Fig. 7-0: Photograph of a pallasite meteorite. Dark areas are olivine crystals. Lighter areas are metal. (Courtesy of Harvard Museum of Natural History)
After the planets and moons formed from planetesimals, they underwent profound internal changes that created their primary internal structure. The grand scheme of planetary differentiation can be summarized as a progressive stratification of the planet, where dense materials sink to the interior and light materials rise to the surface. Earth, for example, has become layered with an Fe-metal core, a silicate mantle, a solid crust that differs between ocean and continent, an ocean, and an atmosphere. Core separation is a consequence of the immiscibility of metallic and silicate liquids and the much higher density of metal, leading to a metallic core underlying silicate mantle. At the very high temperatures of Earth’s interior, the solid silicate mantle convects, bringing hot, deep material toward the surface. This ascent causes the crust to form by melting of Earth’s mantle at shallow depths, where melting points are lower. The melt is lighter than the mantle that surrounds it and rises buoyantly to the surface. Melts of the mantle form the rocks rich in Mg and Fe (mafic rocks) of the ocean crust. Further igneous processing leads to the creation of the rocks rich in feldspar and quartz (felsic rocks) of the continents. Both oceanic and continental crusts are lighter than the underlying mantle and float on top of it. The lower density and greater thickness of the continental crust cause the continents to float at higher levels than the ocean crust. The outermost layers of liquid ocean and gaseous atmosphere likely formed largely by degassing of the mantle, but may also have been influenced by the continuing influx of volatile-rich objects from space. Evidence from short-lived radionuclides shows that core and atmosphere formation happened in the earliest few tens of millions of years of Earth’s history. The crust that we see today formed much later. The ocean floors are geologically young (<160 Ma) because they are continually being created and destroyed. The continents preserve a longer record, but there are only tiny remnants as old as 4,000 Ma, leaving Earth’s earliest history with no direct record, apart from the important clues from meteorites. Achondritic meteorites that are older than 4,000 Ma suggest that the overall process of immiscibility, melting, and degassing to produce distinct compositional layers stratified by density is a common planetary process.
The net effect of interior stratification is to distribute the elements according to their chemical tendencies. Siderophile (metal-loving) elements end up in the core. Most lithophile (rock-loving) elements end up in the mantle. A small group of lithophile elements concentrate into magmas (magmaphile elements) and are concentrated at the surface. Magmaphile elements include the volatiles (H2O, CO2, N2) and P, Na, K, Cl—an assemblage that is focused to the surface and will then provide the environments and molecules for the establishment of a stable climate and the origin and evolution of life.
Segregation of planets from the solar nebula explains features such as the bulk density, the low relative abundances of many volatile elements, and the preponderance of the big four planet-forming elements in terrestrial planets. Planets today, however, are not homogenous mixtures of matter. They have structure and have been differentiated into layers with distinct compositions. This is readily apparent from the meteorites, many of which provide us with samples from the interiors of disrupted parent bodies circling around the solar system. Some of these nonchondritic meteorites are Fe-rich metals, some are mixtures of metal and rocks, and others are volcanic rocks that reflect partial melting of planetary interiors. Processes of melting and separation of metal and silicate within planetary bodies clearly operated in early solar system history and therefore must have affected Earth and the moon as well. The meteorites provide clues as to what may have happened within Earth.
Since Earth has not been disrupted into little fragments, we have no direct access to its interior. The deepest drill holes only penetrate 10 km or so, a trivial distance compared to Earth’s 6371 km radius. Therefore, while we can determine the compositions of the liquid ocean and gaseous atmosphere by direct measurement, and we have rocks from the exposed surfaces of the crust, we must rely on other lines of evidence to determine Earth’s structure and its total composition.
As we learned in Chapter 5, the first line of evidence comes from the estimate of Earth’s density. To determine density (mass/volume), we need to know the volume and mass of Earth. Volume is readily measured. To determine mass requires the application of Newton’s Laws. One method based on the moon’s orbit was discussed in Chapter 5. An alternative is possible from surface measurements. A mass, m, near Earth’s surface falls toward the surface with an acceleration commonly referred to as g. The gravitational force driving this acceleration is then :

This force is also related to Newton’s third law that gives the gravitational force between two masses:

R is the radius of the earth, Me the mass of the earth, and G the universal constant of gravitation. Since the two forces are equal,

R and g are easily measured, but G requires a much more difficult measurement of the gravitational attraction between two objects of known mass. Through painstaking experiment, Lord Cavendish determined a value for G in 1798. He then calculated the density of the earth to be 5.45 grams per cubic centimeter, close to the value of 5.25 gm/cm3 determined by more accurate modern methods. Common rocks at Earth’s surface have a density of about 2.7 gm/cm3 (water is 1.0 gm/cm3), so there must be very dense materials in Earth’s interior to make the average so high. What is this dense, interior material, and where is it located?
This question can be approached from the ellipsoidal “shape” of the Earth. Since Earth spins around an axis, the equator is spinning at high speeds of 1,668 km/hour, while the North and South Poles are stationary. The centrifugal force caused by the great velocities at the equator causes the equatorial regions to bulge relative to the poles, making Earth slightly ellipsoidal in shape. The size of the equatorial bulge depends on how the mass is distributed within Earth. If the mass is concentrated toward the interior, the bulge is less. One can understand this by imagining (or actually trying) to swing a weight around in a circle over one’s head. If the weight is on the end of a long string making one revolution per second, there is substantial pull on the arms. If at the same revolutions per second the weight is close to the body, there is much less pull (and of course the weight is moving at a much slower velocity). This property has the name moment of inertia. Earth has a moment of inertia that is about 20% less than if it were a sphere of uniform density. If the mass were uniformly distributed, Earth would have a larger equatorial bulge. Therefore, dense material must be concentrated toward Earth’s center.
The mean density and moment of inertia can be combined to infer Earth’s general density distribution. The result is that Earth must have a core with a density of about 11 gm/cm3 that makes up roughly half Earth’s radius. Few elements that we know of have such high densities at Earth’s surface. Iron, with a density at one atmosphere of 5.6 gm/cm3, is too light. Only very heavy metals such as gold and silver, with densities of 10–12 gm/cm3 seemed to fulfill the requirements. Could it be that Earth has a core of solid gold?! This conundrum was resolved once it was realized that under the high pressures of Earth’s interior solids are compressible, and their density at depth in Earth is greater than their surface density, just as we saw for the densities of the outer planets in Chapter 5. The effect of pressure on density leads to a gradual increase of density with depth for all of Earth’s layers.
Much more clarity about internal earth structure came about in the early twentieth century from the field of seismology, the study of earthquakes. The shock of an earthquake causes Earth to ring like a bell and creates waves with so much energy that they traverse around and through the entire globe. It was discovered that these waves could be recorded with very precise pendulums (now called seismometers), which produced seismograms that recorded the detailed pattern of the waves (Fig. 7-1).
Fig. 7-1: (a) Illustration of the difference between compressional waves, where matter displaces in the direction of motion, and shear waves, where matter moves perpendicular to the direction of motion; (b) seismogram record showing the arrival of P and S waves produced by an earthquake. P waves have higher velocity and arrive first. Horizontal axis is time. (Courtesy of U.S. Geological Survey)
The timing of the arrival of the waves recorded at different places around the globe gives information about the velocity of the waves through the planet. This velocity is dependent on the physical properties of the material through which the wave travels, including the density. With the seismic velocity data, the density structure of Earth was able to be determined (Fig. 7-2). This structure shows large regions where the density increases gradually with depth and some depths where abrupt jumps in density show major changes in chemical composition. The biggest jump, from ~6 to 10 gm/cm3 was used to define the core/mantle boundary.
Fig. 7-2: Density profile through Earth determined by the velocity of seismic waves. Density increases progressively in each layer largely due to compression. Abrupt changes in density occur where the material composition changes abruptly. Some of the small changes in the upper mantle are due to changes in mineralogy for the same peridotite composition.
As the complex patterns of recorded waves became better understood, seismologists realized from the seismograms that earthquakes create three major types of waves: compressional waves, where the material is moving forward and back in the direction the wave is moving; shear waves, where the material is moving perpendicular to the direction of motion; and surface waves, which pass around Earth’s surface rather than through its interior (see Fig. 7-1). Surprisingly, shear waves abruptly disappear from what is seen by seismometers that are located a little more than halfway around Earth from the location of the earthquake! The region where no shear waves appear became called the shadow zone (Fig. 7-3).
Fig. 7-3: Paths of earthquakes through Earth for an earthquake located at the North Pole. The waves bend depending on the changes in the density of the material through which they pass. The solid lines show the paths of the waves. The regions where no direct waves arrive are generally referred to as shadow zones.
The shadow zone can be understood from the recognition that shear waves are unable to propagate through a liquid. Compressional and shear waves both propagate through solids, at slightly different velocities. In liquids, however, shear waves die out because fluids do not sustain shearing—you can bend a stick or a metal rod, but not a fluid because it does not have the strength to sustain the shearing force. Whales and dolphins can communicate at very long distances by sound waves, which are compressional, but the back and forth movement of their huge tails dissipates rapidly. The shadow zone showed shear waves disappear in a portion of Earth’s interior, and that portion must then be liquid. The very systematic spatial distribution of the shadow zone permitted precise mapping of the interior liquid layer, which begins exactly at the abrupt change in density that defines the core/mantle boundary (Fig. 7-3).
The definition of layers of distinct density combined with information about whether the layer was solid or liquid provided the basic description of Earth’s interior stratification. At the surface is the crust, with a thickness of about 35 km beneath continents, and about 6 km in the ocean basins. The base of the crust is defined by a change in seismic velocity called the Mohorovicic discontinuity (the “Moho”), where the density abruptly increases from about 2.7–3.3 gm/cm3. Beneath the crust is the solid mantle, which extends all the way to a depth of 2,900 km, where the Gutenberg discontinuity in velocity defines the core/mantle boundary. Below that depth lies the 2,100 km of the outer, liquid core. The base of the liquid core is defined by the Lehman discontinuity, where the density jumps again to the >1,000 km solid inner core.
The next step was to determine the chemical composition of these layers. This step required knowledge of the density and seismic velocities of materials under the appropriate temperatures and pressures (Table 7-1). A long period of careful experimentation provided the densities and seismic velocities of a wide variety of minerals that could be used to calibrate the seismic results. Earth’s core consists of Fe and Ni with a small percentage of some lighter elements (whose identity is still in dispute) that could produce slightly lower seismic velocities than pure Fe-Ni. Earth’s crust is amenable to direct inspection, and its composition corresponds to the observed seismic velocities. The continental crust, with a density of about 2.7 gm/cm3 is made up primarily of the minerals quartz and feldspar—the principal constituents of granite, with a small amount of Fe-Mg–bearing minerals such as pyroxenes and amphiboles. The oceanic crust has no quartz, about 50% feldspar, and a much higher proportion of mafic minerals. Its mean density is about 3.0 gm/cm3.
Table 7-1
Common rocks of the crust and mantle
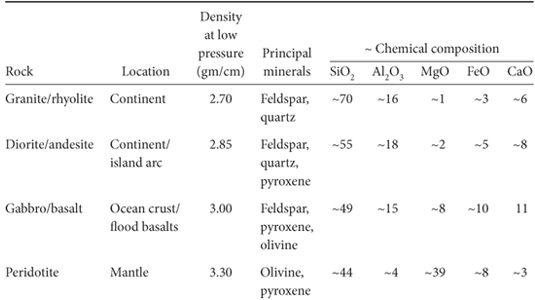
Earth’s mantle proved the most difficult material to identify with certainty. Because the seismic data were insufficient to fully constrain the mantle composition, observations of rare mantle rocks exposed at the surface, experimentation and geochemical reasoning provided necessary constraints. The upper mantle is now well constrained to be predominantly made up of the rock type peridotite, consisting of the mafic minerals olivine and pyroxenes, with a density of about 3.33 gm/cm3. The evidence is as follows:
(1) The understanding of nucleosynthesis, meteorite compositions, and the density constraints given above show that Earth must consist dominantly of the big four inner planet–building nuclides—Fe, Mg, O, and Si. Although much Fe is in the core, substantial Fe is left for the rest of the earth, so the mantle must consist of some combination of MgO, SiO2 and FeO. To be consistent with meteoritic ratios for these elements, the mantle would have to consist, at low pressure, of olivine and pyroxene.
2) The places where mantle has been thrust up to the surface along faults reveal the rock peridotite, consisting of about 55% olivine, 35% pyroxene, and 5–10% of phases that contain CaO and Al2O3.
(3) Some rare rocks called kimberlites erupt explosively from Earth’s interior and contain rock fragments captured at various depths below the crust. Some fragments contain diamonds (kimberlites are the source of all natural diamonds). Because diamonds only form at pressures far below the crust, the kimberlites must come from and sample the mantle. The rock fragments from these depths, called ultramafic nodules are dominantly peridotite.
(4) At ocean ridges, where the crust is very thin, the volcanic rocks must form by partial melting of the mantle. The compositions of these rocks require that peridotite be the material that is melted.
All this information points to a mantle composition of peridotite. Experiments on olivines and pyroxenes have shown that these minerals change structure as pressures increase at greater depth, and this explains why the density curves for the upper mantle in Figure 7-2 are not perfectly smooth. When there is a conversion to a more dense mineral structure, the seismic velocity rises.
For the core, an obvious question that emerges is how there could be a layer of molten metal (the outer core) surrounded by solids above and below it, producing a kind of internal liquid metal ocean. At the core/mantle boundary, one possibility is that the temperature increases substantially. Alternatively, at the pressures that exist at a depth of 2,900 km, metallic iron may melt at a lower temperature than magnesium silicate, permitting the metal and silicate at the same temperature to be liquid and solid respectively. The primary cause of the outer core being liquid is the lower melting temperature of Fe metal compared to lower mantle rock, but as it turns out the temperature also jumps at the core/mantle boundary (Fig. 7-4).
The deeper solid/liquid boundary between the inner and outer core has another explanation. The melting point of all rocks and metals increases substantially with pressure, because melting involves expansion and breaking of chemical bonds, and higher pressures make this more difficult to accomplish. The pressure is sufficiently high in the inner core that despite its higher temperature the Fe metal once again becomes solid (Fig. 7-4). The liquid/solid boundary between inner and outer core results from the effects of pressure on melting temperature.
The diverse evidence and lines of reasoning then leads to a clear definition of Earth’s inner structure (Fig. 7-5). This structure is supported by all geophysical data and is as close to an established fact as possible in the absence of actually being able to directly penetrate Earth’s deep interior. It ranks a 9 on our theory scale. While the major structural elements have this certainty, the details of deep Earth structure in the core and lowermost mantle, such as their exact composition and mineralogy, remain to be fully elucidated.
The various elements of the periodic table are not evenly distributed among the four major Earth layers of core, mantle crust, atmosphere/ocean. To understand where the various elements reside, we need to consider their affinities for different types of materials and states of matter.
Fig. 7-4: Temperature profile through Earth. The figure also illustrates that the state of matter of Earth’s interior depends on the different melting points of rock and metal and how they vary with pressure. The inner core can be solid and the outer core liquid even though the temperature of the inner core is higher than the temperature of the outer core, because of the increase in melting temperature with depth. The outer core is liquid while the mantle above it is solid because the temperature of melting of Fe is lower than that of silicate at great depths in Earth, and there is a large jump in temperature at the core/mantle boundary. (Data from Lay et al. Nat. Geosci 1 (2008):25–32; Madon, Mantle, in Encyclopedia of Earth System Science, vol. 3 (San Diego: Academic Press, 1992), 85–99; Alfé et al., Mineralogical Magazine 67 (2003):113–23; Duffy, Philosophical Transactions of the Royal Society of London A 366 (2008):4273–93; and Fiquet et al., Science 329 (2010):1516–18)
Fig. 7-5: Illustration of Earth’s major layers and how they are distributed with depth.
It is convenient to divide the elements into four major groups (Fig. 7-6). The atmophile elements are those which are very volatile and tend to occur as gas or liquid molecules under the conditions on Earth. (Phile is a suffix that means “has an affinity for.” A francophile is someone who loves France.) Atmophiles include the noble gases (such as helium, neon, and argon), water, carbon dioxide, and nitrogen. These elements have very low density and are overwhelmingly concentrated in the ocean and atmosphere.
The lithophile elements are those which prefer to be in silicate rocks. These include silicon, magnesium, oxygen, calcium, aluminum, titanium, etc. These elements are overwhelmingly in Earth’s mantle and crust.
The siderophile elements are those which prefer the metallic state. These are the metals we are all familiar with—nickel, gold, silver, copper, iron, platinum and so on.
The chalcophile elements are sulfur loving and occur in sulfur-bearing minerals. They include lead, copper, zinc, platinum, and arsenic. There is substantial overlap between chalcophile and siderophile elements. Iron is unique because it lies in all three groups. Whether it is metal or silicate depends on the oxygen content. It also forms the most common sulfide, pyrite, otherwise known as “fool’s gold.”
In addition, there is a subset of lithophile elements that need special consideration—those that are concentrated into silicate liquid. Because molten rock is called magma, these elements can be called magmaphile. Magmaphile elements are so large that they do not fit readily into silicate minerals and are strongly concentrated into the liquid phase when a rock melts, since the liquid has more flexible “sites” that are able to accommodate the larger elements. Magmaphile elements and molecules tend to become concentrated in Earth’s crust, which, we will see shortly, is created by partial melting of Earth’s interior. Many of them then end up in the ocean or atmosphere. The magmaphile elements are generally those lithophile elements toward the bottom of the periodic table, where the high atomic number generally creates large ionic sizes (e.g., Rb, Cs, Ba, Sr, La, Pb, Th, and U), as well as the atmophile elements and molecules such as CO2 and H2O. Some siderophile and chalcophile elements (e.g., W, Sb) are also magmaphile.
Fig. 7-6: Periodic table with element affinities based on Goldschmidt’s classification. Elements are grouped according to their preferred host phases into lithophile (silicate loving), siderophile (iron loving), chalcophile (sulfur loving), and atmophile (gas loving). Magmaphile elements, which preferentially enter the silicate liquid when both solid and liquid are present, are indicated by diagonal lines. Elements in italics are short-lived radionuclides.
These affinities predict where most of Earth’s elements reside. For example, although nickel is as abundant as calcium and aluminum in meteorites, most of Earth’s nickel is in the core because of its siderophile tendencies. Gold, silver, platinum, and tungsten (W) were also depleted from the mantle by core formation, making these already rare elements even more precious. As we shall see in a later chapter, because Earth’s iridium resides largely in the core, it has been possible to show that a large iridium-rich asteroid hit Earth 66 million years ago. Lithophile elements reside largely in the mantle, with the exception of the subgroup of magmaphile elements such as K and the volatiles that are largely in the crust, ocean, and atmosphere.
These considerations allow us to estimate a bulk composition of Earth and where the various elements reside (Fig. 7-7). As an example, consider the abundance of an element such as Fe. The mass of Earth’s core is 1.87 × 1027 gm. That of the mantle is 4.02 × 1027 gm. Together the masses of Earth’s thin outer crust, ocean, and atmosphere come to only 0.029 × 1027 gm. The iron content of the crust can be measured directly, but there is so little crust that it is a negligible contribution to the total. The iron content of mantle material can be estimated about 8% by weight. The iron content of the core is estimated from density and seismic velocity calibrated with experimental data to be 85% by weight. From these various results the iron content of the planet can be estimated:
Mass of iron in core |
1.6 × 1027 gm |
Mass of iron in mantle |
0.26 × 1027 gm |
Mass of iron in crust |
0.002 × 1027 gm |
Total mass of iron |
1.86 × 1027 gm |
Total mass of Earth |
6.0 × 1027 gm |
This yields an iron content for the bulk earth of 31.9 %. The abundances of most other elements can also be estimated by a similar approach that makes use of the geochemical behavior of the different elements (Table 7-2).
Also, we can now reconsider the hypothesis that Earth formed from meteoritic materials. If that hypothesis were true, then we would expect the silicate earth for which we can make direct measurements (Earth excluding the core) to have chondritic proportions of refractory lithophile elements and strong depletions in the siderophile and chalcophile elements that would have been segregated into the core. In general this corresponds with the observations. However, precise estimates of bulk silicate earth compositions are models that make use of the assumption that Earth is chondritic. Within the uncertainties in the data, Earth could deviate slightly from chondritic proportions. At a level of high precision, much remains to be understood about Earth’s exact composition.
Fig. 7-7: Distribution of the elements among Earth’s layers. The vertical axis is a list of 63 elements. The horizontal axis shows the proportion of the element in each layer normalized to 1, considering the relative mass of each layer: the mass of Earth: 5.997; mass of the crust: 0.0223; mass of the mantle: 4.043; mass of the core: 1.932. Chalcophile and siderophile elements have large proportions in the core; lithophile elements are in Earth’s silicate layers, with the magmaphile elements concentrated in the continental crust. (Data from W. F. McDonough, Chemical Geology 120 (1995):223–253)
Table 7-2
Composition of the bulk Earth*
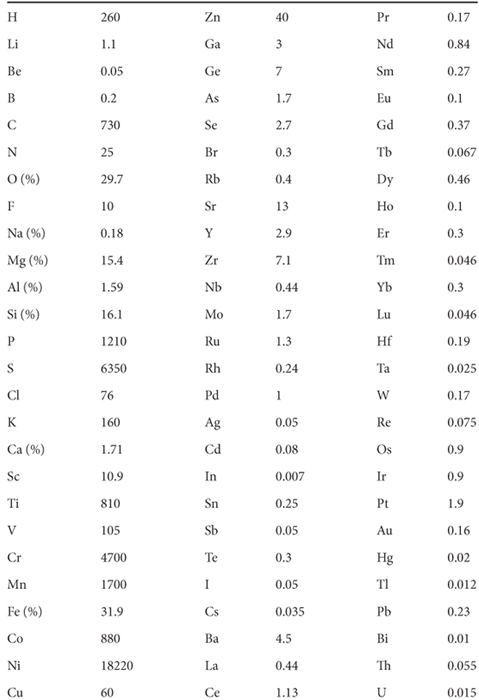
* Data from W. F. McDonough, Chemical Geology 120 (1995): 223–253. Concentrations in ppm unless otherwise noted.
The combination of moment of inertia, seismology, geological observations, experimentation, geochemistry, and cosmochemistry thus provides substantial knowledge of the compositions of Earth’s layers. This knowledge in its broad aspects is universally accepted because it accounts for a large variety of observations, from seismic velocities to siderophile element abundances. The composition of crust and upper mantle within a small range of error bounds rank an 8–9 on our certainty scale because there are so many lines of evidence, including direct measurement and experimentation. We also know with high certainty that the lower mantle is silicate and the core is Fe-Ni metal because seismic data and bulk earth properties constrain their composition. The precise compositions of lower mantle and core rank only a 7–8 on the scale because there are no direct chemical measurements, and the very high pressures limit the experimental constraints.
Armed with knowledge of the existence, physical properties, and overall composition of Earth’s layers, we now face the question of how these layers came about. As we learned in Chapter 5, some meteorites appear to be the remnants of small planetary objects that were broken apart by subsequent impacts. These various meteorites show that planetary bodies other than Earth ended up with discrete layers of metal, silicate, and volcanic material. Venus and Mars, like Earth, also have greater mean densities than their silicate crusts and must have large amounts of metal in their interiors, have evidence of crust formation by volcanism, and an atmosphere. Taking this body of evidence, it appears that the process of planetary differentiation to core, mantle, crust and volatile-rich surface is a general phenomenon—how does it happen?
A critical question is whether the layers were created as the planet formed, with a metal core coming together first, followed by the other layers, or whether metal and silicate were initially all mixed together and later segregated into the layers we see today. The first model can be called heterogeneous accretion because the materials that were initially added to the Earth would have been very different from one another and varied with time. The metal material would first gather to form the core, then the silicate material added on top of the core, and finally the gas and water to form the ocean and atmosphere sprinkled onto the surface. The second model is called homogenous accretion because the materials accreted to Earth were initially homogeneous and the separation of layers would happen after accretion, with the core sinking into Earth’s center and the volatiles outgassing to form ocean and atmosphere. As for the crustal layer, we see that layer being created and recycled today, so heterogeneous accretion is not an option. It is, however, a potential model for the origin of core, mantle, and volatile-rich exterior.
Since planets accrete from solid objects in the solar nebula, heterogeneous accretion of the core would require a period when all solid objects in the early inner solar system were metal, and all the silicates remained in the gas. That would require that metals were more refractory (= less volatile) than the lithophile elements, so metal would be the first material to precipitate from the nebular gas, followed at lower temperatures by materials containing Si, Al, Ca, Ti, etc. Experiments, theoretical calculations, and observations of carbonaceous chondrites, however, all suggest that calcium and aluminum-rich silicates were the first materials to solidify (Table 7-3). Dating of meteorites also shows that the iron meteorites, thought to be remnants of cores of small solar system objects, formed slightly after the oldest materials in chondrites. Therefore, there is no evidence for a period in solar system history where metal was available to accrete in the absence of silicate. The metal cores of planetary bodies must have formed by separation of metal from rock after accretion.
Furthermore, astronomers studying interstellar clouds where star formation is occurring have found evidence for both metal and silicate in the clouds surrounding the star. This observation provides evidence for the simultaneous presence of both materials during solar system formation. In its pure form, therefore, the heterogeneous accretion scenario is not well supported.
Table 7-3
Condensation sequence
| Temperature (K) | Element condensing | Form of condensate |
3695 |
W (Tungsten) |
Tungsten oxide – WO3; wolframite – FeWO4/MnWO4 |
1760–1500 |
Al, Ti, Ca |
Aluminium oxides: Al2O3, CaO, MgAl2O4 |
1400 |
Fe, Ni |
Nickel-iron grains |
1300 |
Si |
Silicates: olivine (Mg, Fe)2SiO4, feldspars (Na, K) AlSi3O8 |
450–300 |
C |
Carbonaceous compounds and hydrous minerals |
<300 |
Ices |
Ice particles: water, H2O; ammonia, NH3; methane, CH4; argon-neon ices |
Since heterogeneous accretion is not viable for formation of core and mantle, core and mantle must have separated from one another after accretion. Is this physically reasonable? Two characteristics of metal and silicate account for this separation:
(1) The high density of metal relative to silicate means the metal would sink through the silicate under the force of gravity;
(2) this density separation is facilitated because metal and silicate are immiscible.
Immiscibility is a concept familiar to us from “kitchen science.” Water is denser than alcohol, but addition of water to alcohol does not lead to the creation of a layer of pure water at the bottom of the glass. The two liquids are miscible; they mix to form a single homogeneous material. In contrast, oil and vinegar are immiscible; they remain separate phases when mixed together. Even after being vigorously stirred, the less dense oil floats to the top to form a pure layer, with the denser vinegar (which is largely water) underneath. Immiscibility and density differences lead to discrete layers with a sharp boundary between them. Metal and silicate are like oil and vinegar, they are immiscible. The combination of the large density difference and immiscibility makes downward separation of metal from silicate inevitable and irreversible.
When did the core form and how long did core formation actually take? One possibility would be that melting and vigorous convection at the time of accretion would cause core formation to happen instantaneously with accretion. Alternatively, core formation could be progressive and even be happening in small amounts today.
Both geophysical arguments and geochemistry have a bearing on this question. Consideration of heat sources in the early Earth makes it hard to avoid the conclusion that substantial melting must have occurred during its formation. Heat sources in the early Earth include the heat released by impacts, heat created by the now extinct radionuclides, and heat from long-lived radionuclides. In addition, the process of core separation generated a great deal of heat as the metal “fell” to Earth’s center. Calculations involving these heat sources show that so much heat was available at this point in Earth’s history it is highly likely that substantial portions of Earth melted. If the moon formed from impact of a Mars-size protoplanet (discussed in Chapter 8), the impact alone would have generated enough heat to melt Earth’s interior.
Good evidence also exists from studies of lunar rocks that the moon melted early in its history, as we will see in Chapter 8. Because of both heat sources and heat sinks, it is likely that if the moon melted, Earth melted also. The moon has smaller sources of heat than Earth. The smaller gravitational field causes heat generated by impacts to be less. The moon is volatile depleted, so the major heat producer 40K was lower in abundance. The moon has no significant core, so heat generation by core formation would not contribute. Therefore, much more heat must have been available to melt Earth than to melt the moon.
Heat loss would also be more efficient on the moon because it is a smaller object. This can be understood experientially by considering the different time it takes a drop of hot water to cool compared to a cup of coffee. Small objects cool much more rapidly than large objects because heat contained in the entire volume is lost through the surface. The larger the ratio of surface area to volume, the more rapidly is heat lost. Since the moon has fewer sources of heat and more rapid dissipation of heat than Earth, if the moon melted, it is likely that Earth did also. Melting of substantial portions of Earth in the first few tens of millions of years of its history would have led to efficient segregation of core from mantle.
Quantitative estimates of the time to segregate the core come from our geochemical clocks—the radiogenic isotopes. In this case, the key is an extinct lithophile radioactive nuclide, 182Hf, with a half-life of 9 million years, that decays to siderophile 182W. 182Hf accreted by Earth decayed completely to 182W in the first 100 million years of Earth’s history. If the core formed 100 million years or more after Earth accreted, then all the 182Hf would have decayed away prior to core formation, and the tungsten isotopes of late-forming core would be the same as the mantle and bulk earth. Furthermore, the isotope ratios would also be the same as other solar system materials, such as chondrites, that never experienced metal-silicate separation (Fig. 7-8). On the other hand, if the core formed very quickly, then most of the tungsten would segregate into the core before 182Hf had decayed away, and the mantle left behind would have had a very high Hf/W ratio while some radiogenic 182Hf was still present. As the remaining 182Hf decayed, the small amount of tungsten remaining in the mantle would become enriched in the daughter isotope, 182W. Then the silicate Earth we can measure would have an excess of 182W relative to undifferentiated objects such as chondrites. If Earth’s rocks and chondrites have the same W isotope ratios, then core formation occurred >100 Ma after Earth accreted. If the W isotope ratios differ, core formation happened earlier. The earlier that core formation occurred, the more extreme would be the W isotope differences. Because Hf and W are rare elements, the measurements are technically difficult, and only in 2002 were definitive data obtained by several different laboratories. These measurements show enrichment of 182W in the silicate Earth compared to chondrites. The magnitude of the anomaly suggests core formation took place in the first 30 Ma of Earth’s history. Since the W isotope data show definitively that the core formed after Earth accreted, the data provide further evidence for homogeneous rather than heterogeneous accretion.
The various lines of evidence, then, suggest a dominantly homogeneous accretion of Earth, followed by widespread melting and rapid segregation of core from mantle by immiscibility and density separation in the first few tens of millions of years of Earth’s history.
Fig. 7-8: Illustration of the effect of core formation on tungsten isotope evolution. T0 is the time when Earth accreted. If the core formed >100 million years after T0, all the radioactive 182Hf would have decayed to 182W, and core, mantle, and carbonaceous chondrites would all have the same tungsten isotope ratio. Because the silicate Earth has a different W isotope composition than chondrites, with enrichment in 182W, the core must have formed before the completion of Hf isotope decay. (Data from Quing-zhu Yin et al. Nature 418 (2002): 949–52 and Schoenberg et al., Geochim. Cosmochim. Acta 66 (2002):3151)
This time frame fits well with evidence from other meteorites and isotope systems. These studies have shown that smaller planetary objects broken apart to form achondritic meteorites formed and differentiated into layers of metallic core, silicate mantle, and volcanic crust in as little as 5.0 million years. The heat source for such rapid differentiation is thought be 26Al, which with its 0.7 million year half-life decays away fully in less than 7.0 Ma. Meteorites thought to be derived from Mars, which is about one-eighth the mass of Earth, give a timescale for formation of a Martian core of about 15.0 Ma. It appears that small objects formed and differentiated rapidly, while larger objects such as Mars and Earth took slightly longer.
From the perspective of the age of the solar system (4,565 million years), planetary formation and differentiation, powered by impacts and extinct radionuclides, occurred within the first 1% of solar system history. This is roughly equivalent in terms of percentages to the gestation periods of most mammals, when their primary physical structure becomes well defined.
The crust is the uppermost part of the solid Earth above the seismically defined Mohorovicic discontinuity. The ocean and continental crust are distinct in their seismic properties and chemical composition. The ocean crust is made up largely of the mafic igneous rock basalt and its plutonic equivalent, gabbro. The continental crust is roughly granitic in composition (see Table 7-1), with a layer of sediments at the uppermost levels.
The origin of the crust is understood well in general terms, though many details remain to be elucidated. The major process that forms the crust is partial melting of Earth’s interior. The magma produced is buoyant and either rises to the surface to erupt as volcanic lava or remains at slightly greater depths, where it cools more slowly and creates the coarsely crystalline plutons—gabbros in the ocean crust and granites in the continental crust. These processes can be observed today, and ancient rocks are sufficiently similar to modern ones to suggest that they formed by the same general process.
Processes of melting and crystallization near Earth’s surface are subject to experimental investigation and well-constrained modeling, and geochemists over the last century have come to understand well the essential aspects of melting. Our normal human intuition is that melting is quite simple. If you heat something up, when it reaches its melting point, it melts. For example, H2O has a single melting point of 0°C. Two factors contribute to more complex melting behavior for rocks: melting points depend on pressure (e.g., Figs. 4-3 and 7-4), and melting of complex mixtures like rocks melt over a range of temperatures, rather than a single temperature.
Unlike the constant pressure world we inhabit, Earth is subject to huge pressure variations owing to the vast amounts of rock that press down on the interior. For all substances for which the volume of the solid is less than the volume of the liquid (all rocks have this property), pressure and temperature have the opposite effect on melting of rocks. Increased pressure and decreased temperature enhance the stability of the solid. The high pressures of Earth’s interior are the reason the mantle can be solid, even though the mantle is at higher temperatures than the melting temperature of mantle rock at the surface.
Since rocks are collections of minerals, and hence an assemblage of phases—not a single phase like water or ice—their melting behavior is different from pure compounds. For example, pure salt turns to liquid at a temperature of 350°C, and ice at 0°C, but an equal mixture of ice and salt melts at –20°C. We make use of these properties frequently. Small amounts of salt easily melt in water (we say “dissolve”) at room temperature. In winter, adding salt to roads turns the ice to saltwater, even at temperatures below freezing. This general principle where adding one substance to another lowers the melting temperatures is called freezing point depression and can be represented graphically in a “phase diagram” as illustrated in the sidebar.
Careful experimental work also reveals that mixtures of minerals melt over a range of temperatures, not a single temperature. This range is bounded by the inception of melting, called the solidus, and the completion of melting, at the liquidus. Below the solidus, the mixture is solid. Between solidus and liquidus, the system is only partially melted. Above the liquidus, the mixture is entirely liquid. Because melting occurs over a range of temperatures, partial melting can occur, permitting segregation and separation of liquid from solid. We now arrive at the critical aspect for separation of Earth’s crustal layers: the composition of the partial melt is different from the bulk composition. Partial melting to a different composition coupled with the buoyancy of the liquid relative to the solid permits segregation of the melt to form crustal layers that differ from the mantle that melted to produce them. These properties of melting mixtures can be viewed graphically and quantitatively using temperature-composition diagrams, called phase diagrams (see sidebar).
SIDEBAR
MELTING OF ROCKS
Our intuition about melting is based primarily on ice, which melts at a single temperature of 0°C. When there is more than one mineral present, however, melting takes place over a range of temperatures. Rocks are made up of many minerals, and therefore the details of the melting are quite complex. The important principles can be illustrated using mixtures of two minerals. In the first example, there are two distinct minerals that do not dissolve in one another in the solid state. In the liquid, however, all molecules mix together to form a single solution (analogous to salty water for ice/salt mixtures). In the second example, the molecules mix and form solutions in both liquid and liquid states (such as alloys), so there is only one solid phase present, but the solid can have varying proportions of its constituent molecules. Rocks are made up of both kinds of minerals—those of uniform composition and those which form solid solutions.
Equilibrium Melting in the Diopside-Anorthite Binary Eutectic Phase Diagram
The binary eutectic phase diagram (Fig. 7-9) illustrates melting where there are two distinct solid phases that do not form a solid solution, and a liquid phase where all the molecules are fully miscible. The horizontal axis represents the proportion of each mineral in the mixture. Each pure mineral plots at one end of the horizontal axis, and melts at a single temperature. All other compositions along the axis are mixtures of the two minerals. Temperature is plotted along the vertical axis, increasing upward.
Below the solidus, all mixtures are solid. Above the liquidus, all mixtures are a single liquid phase. What interests us is the transition between the two. To illustrate the transition, we explore the melting of one arbitrarily chosen mixture, which we call a bulk composition, indicated by a vertical line on the diagram. The vertical line in Figure 7-9 has a composition of 52% anorthite (An) and 48% diopside (Di). We could arbitrarily have chosen any bulk composition. Whatever the choice, when the bulk composition is either entirely solid or entirely liquid, it has exactly the proportions that it started with.
For systems of this type, there is a single minimum melting temperature for all mixtures, called the eutectic (E on the diagram), which then defines the solidus. As we heat our chosen bulk composition from an entirely solid state, the solid mixture encounters the solidus at point S1. Here the mixture will begin to melt. The first liquid, L1, necessarily appears at the “eutectic point,” E, since it is the minimum melting temperature for all mixtures. Because this liquid is richer in Di than the bulk composition, as the liquid forms the solid composition becomes relatively depleted in Di, causing its composition to move along the horizontal axis directly away from S1 to the anorthite axis. As long as both Di and An are present, the liquid is stuck at L1 (= E) and the temperature remains constant. When the solid composition reaches the An axis, the last crystal of Di has melted out, and the system consists of 80% liquid of composition E and 20% An. Then the remaining anorthite begins to melt out as the temperature increases. Melting the anorthite causes the liquid to become more An rich, and it moves along the line from L1 toward L2. As the temperature increases, the solid composition (pure anorthite) increases in temperature to S2 as the liquid composition moves to L2. When the liquid composition arrives at the bulk composition, the system is entirely liquid, as the last crystal of An dissolves, at S2. Note that the liquid compositions (L1 to L2) always differ from the bulk composition until the system is entirely liquid. Melting occurs over a range of temperature. Over this temperature range, partial melting occurs.
Fig. 7-9: Phase diagram of diopside-anorthite, illustrating melting of two phases that mix in the liquid state but remain separate in the solid state. The point labeled E is the minimum melting temperature, called the eutectic. BC stands for an arbitrarily selected bulk composition. Pressure is one atmosphere.
Fig. 7-10: Phase diagram illustrating the principles of solid solution, using the olivine solid solution. Olivine is the most abundant mineral in Earth’s mantle. BC stands for an arbitrarily selected bulk composition. Pressure is one atmosphere.
Forsterite-Fayalite Solid Solution Phase Diagram
When there is solution in both solid and liquid, there is a single solid phase, but it can be of variable composition. The most common such solid solution in Earth’s upper mantle is the mineral olivine, which is a solution between pure forsterite (Mg2SiO4) and pure fayalite (Fe2SiO4). Figure 7-10 is the olivine phase diagram. During melting, all solid compositions lie along the solidus and all liquid compositions lie along the liquidus. Co-existing solid and liquid always have the same temperature. Again we choose an arbitrary bulk composition, BC, indicated by the vertical line with arrow. At temperatures below the solidus, only solid olivine is present. As the temperature rises, the bulk composition arrives at the solidus and the first liquid appears at S1, with composition L1. As the temperature continues to rise, the solid follows the path S1–S2 and the liquid follows the path L1–L2. When the liquid arrives at the bulk composition, the system is entirely molten, and with further heating the liquid rises above the liquidus to the liquid field. Notice that in this system as well, partial melting occurs over a range of temperatures, and during melting the liquid composition always differs from the bulk composition.
The phase diagrams illustrated in the sidebar are for experimental data determined at one atmosphere pressure. Since pressure variations are very significant for Earth, we need to consider carefully the effects of pressure. Higher pressures cause the denser solid phases to become stabilized relative to the lighter liquids, and therefore it takes higher temperatures to cause melting to occur. For Earth, melting temperatures generally increase by 5°–10°C for every thousand bars (one kilobar, or 0.1 Gigapascals [GPa]) of pressure (equivalent to about 3 km of depth). At 120 km below the surface, the pressure is about 40 kbar (4 GPa), and the melting temperature of rocks is about 400°C higher than at the surface! A rock that is entirely solid at this depth could be well above its solidus at the low pressures of the surface, so if mantle material rises, it can melt because of the decrease in melting temperature with decreasing pressure. This process is called pressure release melting.
Pressure release occurs on Earth whenever mantle material rises. As we will learn in Chapter 11, the mantle convects slowly, and beneath ocean ridges and ocean islands the mantle ascends from depth toward the surface. As a given parcel of mantle rises, there is increasingly less weight of rock above it, and the pressure decreases. Ultimately the pressure decreases enough that the mantle crosses the solidus (the temperature at which melting begins) and starts to melt (Fig. 7-11). As it ascends, the mantle is farther and farther above its solidus and the extent of partial melting increases. The proportion of melt produced depends on the extent of rise above the depth where the solidus is crossed. This explanation of mantle melting contradicts our experience of melting by the application of heat and the raising of temperature, since we live in a constant pressure environment. Instead, the mantle cools down as it melts! Melting occurs by pressure decrease rather than temperature increase.
Now we come to the separation of chemically distinct crustal layers. Partial melts of the mantle have a different composition from the mantle itself. Instead of the 45% SiO2 and 40% MgO of mantle peridotite, the partial melts have about 50% SiO2 and about 15% MgO. Such liquids, which become basalts, have a density that is 10% less than the mantle and rise readily from the molten zone toward the surface. Partial melting of the mantle to produce basalts is the mechanism that forms the ocean crust, discussed at length in Chapter 12.
Fig. 7-11: Illustration of how the mantle melts on Earth by depressurization. Melting of mantle rock (peridotite) depends on temperature and pressure. The onset of melting is called the solidus; the temperature above which melting is complete is called the liquidus. Between the two is a partially molten region, as indicated by the contours of percent melting. The path for melting during mantle ascent changes after crossing of the solidus because melting takes energy, which lowers the temperature of the ascending mantle. (Solidus line after Hirschmann, Geochem. Geophys. Geosyst. 1(2000), paper no. 2000GC000070; liquidus temperature after Katz et al., Geochem. Geophys. Geosyst. 4 (2003), no. 9)
Continental crust formation follows many of the same principles, but there are multiple steps of partial melting. Multiple steps are necessary because the granite rocks and high Si and K contents of the continents cannot be produced by melting of the mantle. Instead, the continents represent the final product of sequential melting and cooling of magmas. When the mantle melts, basalts are formed. When these basalts melt in turn, granites form. Granites are also the end point of many other likely processes. When granites melt, granites are formed. When the mafic lower crust is melted, less dense granitic magmas are formed and rise to the upper crust. When granites or basalts are eroded to form sediments and these sediments melt, granites are formed. Therefore granites represent the logical endpoint of a sequence of many different melting and cooling events. The low densities of granitic rocks then ensure that they remain at the surface, floating on top of the mantle, well above the levels of the ocean crust.
Since continents are the ultimate endpoint of multiple melting processes, they have very effectively concentrated the magmaphile elements that prefer liquid to solid. Trace elements such as Th, U, Ba, Rb, K, and La have a substantial fraction of their total Earth budget in the continental crust. Perhaps as much as 70% of Earth’s Rb, for example, has been concentrated in the outermost layer, effectively distilled to the surface by magmatic processes.
While this general conception of continent formation is sound, the specific physical processes involved in the initial creation of continental crust and the timing of when it happened are not well understood. If basalts come to the surface and then melt to produce granites, and if both granitic partial melt and mafic solid residue were to remain in the crust, the total composition of the crust would not change, it would simply be divided into two different layers. This occurs in part—the lower crust is more mafic than the upper crust—but the total crust is too high in SiO2 and low in FeO and MgO to be a mantle melt. Furthermore, materials being added today to the continents by volcanic and plutonic rocks do not have the same composition as average continent—they are too mafic and do not have appropriate ratios of important trace elements. What we determine by experiments to be a mantle melt is dominantly basalt, what we observe being added to the continent is dominantly basalt, so how did this material become transformed to the granitic masses that form much of the continents we live on?
Three general models have been proposed to account for this puzzle. The first suggestion is that processes have changed though Earth’s history, and in the ancient Earth the mantle was hotter and melted recycled basalt materials at depth to give rise to the continents. The residue remained in the mantle. The second idea is that continents form by a multistage process, where first a basaltic layer is formed, then the mafic residue that melted to produce granite “delaminates” and falls back into the mantle (Fig. 7-12). A third intriguing possibility is that weathering is as important a process as igneous activity in controlling continental crust composition. Today we know that weathering of mafic minerals that make up basalt is a much more rapid process than weathering of the felsic minerals that make up granite. Weathering, therefore, might selectively remove the more mafic elements and deliver them to the ocean, leaving the felsic continent behind.
Table 7-4
Compositions of crust and mantle
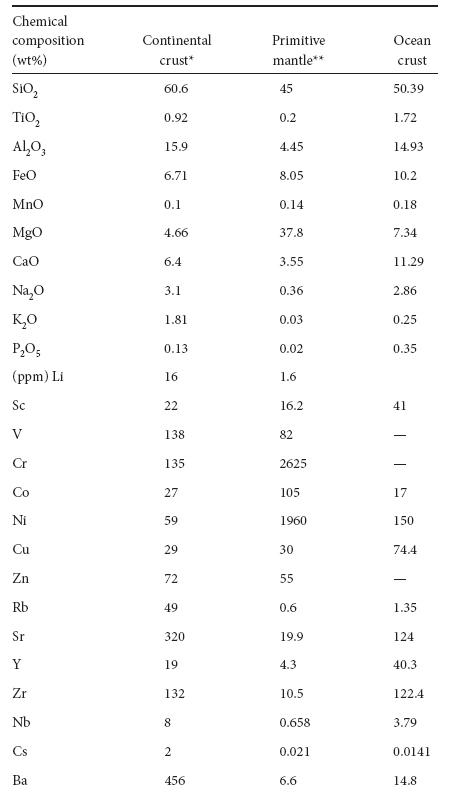
* Continental crust composition from Rudnick and Gas Composition of the Continental Crust Treatise on Geochemistry #3, 1–64.
** Primitive mantle from Sun and McDonough Chemical and isotope systematics of ocean basalts: implications for mantle composition and process. Geol. Soc. London Special Pas 42 (1989):313–345.
However they formed, granitic continents are an ancient feature of Earth. The oldest preserved continental rocks are very similar in composition to average continent today, suggesting that continent formation has been a repeatable process throughout Earth’s history. In fact, the very oldest rocks are granites and sediments that were formed by weathering of granites. Both types of rocks are not “juvenile”—they require an extended history. This tells us that crustal, granite-forming processes must have been active in earliest Earth’s history, even before the rock record.
The scenario given above for the solid Earth also relates closely to the origin of Earth’s atmosphere and ocean layers. The important volatiles H2O and CO2 were able to accrete in significant amounts because they can reside in the solid state in some of the minerals that make up rocks. Even minerals that have no volatile element in their chemical formula can contain small amounts of volatiles. Other minerals, such as amphibole or mica, contain substantial amounts of water. Limestone (CaCO3) is by far the largest reservoir of CO2 in Earth’s crust. When these minerals break down and release gas, either by being heated or melted, the volatiles will rise to the surface, providing carbon, hydrogen, and nitrogen, the elements most essential for life. Degassing is an important process of atmosphere creation. When did the atmosphere form in Earth’s history?
Once again radiogenic isotopes come to our aid with this question, making use of another exotic element, xenon (Xe). The noble gases such as xenon have an important place in the discussion of the atmosphere, because they are nonreactive and always remain in the gaseous state. Since they do not react with other elements to form minerals, a significant proportion of the noble gas budget of the Earth resides in the atmosphere. One of the xenon isotopes, 129Xe, is the product of the short-lived radioactive isotope 129I, with a half-life of 16 million years. While xenon is atmophile, iodine (I) is lithophile. Therefore just as Hf and W provide evidence on the separation of the core and mantle, I and Xe provide evidence for the separation of mantle and atmosphere.
Fig. 7-12: Illustration of the role continental delamination may play in formation of the continents. Continental collision or arc magmatism thickens the crust and could lead to sufficient crustal thickening that melting takes place at depth. The granitic melts rise to the surface. The high temperature residue is sufficiently dense that it sinks into the mantle, leaving behind a high SiO2 continental crust. Alternatively, magmas could crystallize dense minerals rich in Fe and Mg at depth in the crust, and these accumulated crystals could delaminate.
In 1983 Claude Allègre and coworkers determined the xenon isotope composition of volcanic rocks from ocean ridges and showed that they have an excess of 129Xe compared to the atmosphere. Because ocean ridge basalts are derived by partial melting of the mantle, the inference is that the upper mantle has the 129Xe anomaly. If the separation of mantle and atmosphere occurred 100 Ma or more after Earth accreted, then all the 129I would have decayed away and all of Earth’s reservoirs would have the same xenon isotopic composition. If on the other hand the atmosphere separated from the mantle very early in Earth’s history, removing much of the xenon, then the remaining iodine would keep on producing 129Xe. Since there was little xenon left in the mantle, an excess of 129Xe relative to other xenon isotopes would result. The constraints from 129I also suggest a period of about 30 Ma, similar to the timescale inferred from Hf-W for core/mantle differentiation. This evidence provides a consistent story where formation of the major layers of core, mantle, and atmosphere occurred over the first few tens of millions of years of Earth’s history. The atmosphere and ocean would then be the result of homogenous accretion and mantle degassing.
For the atmosphere, however, the devil is in the details. Other noble gas isotope ratios are not readily explained either by degassing from Earth’s interior or late addition of gases. Impacts of comets seem inevitable from models of solar system formation, but some of the measurements of isotope ratios of modern cometary material are not the same as those of the atmosphere and ocean. Giant impacts (see Chapter 8) or vigorous solar wind early in solar system history might have stripped the original atmosphere, so perhaps multiple atmospheres formed during the early Earth. A complex history that includes heterogeneous accretion of materials with different volatile budgets seems necessary to explain Earth’s inner and outer volatile abundances. These remaining puzzles make the atmosphere the least understood aspect of formation of Earth’s layers.
A family of different processes has led to the gradual differentiation of the Earth into layers sorted by density whose extent and composition are well constrained, with error bars and questions increasing with increasing depth. The innermost layer is a dense solid core of iron, nickel, and a small proportion of lighter elements. The liquid outer core is also metallic. Much lower in density is the mantle, consisting largely of solid solutions of ferro-magnesian silicates. Melting of the mantle leads to outgassing to create ocean and atmosphere and eruption of silicate magmas to form the crust. The denser, basaltic ocean crust is a result of mantle melting and resides at deeper levels than lighter, granitic continental crust, which must be derived by a sequence of melting processes.
Short-lived radioisotopes in the Hf-W and I-Xe systems both indicate that core and atmosphere formed early in Earth’s history, and of course these reservoirs remain separated today. In contrast, crust formation and destruction are ongoing processes. Ocean crust formation can be observed today and is a direct result of mantle melting, but all of ocean crust is young, <150 Ma. Continental crust has a vast range of ages. Even the oldest continental rocks show evidence of a long previous history, and the earliest crustal differentiation events are hidden from view. Various theories are still being actively considered for the exact mechanisms by which continental crust has formed.
The diverse processes that have created Earth’s layers have also led to a striking separation of elements. The siderophile elements reside largely in the core, and the large ion lithophile elements have been concentrated into mantle and crust. The magmaphile elements have been effectively concentrated into the crust, and the special classes of volatile magmaphile elements have been outgassed to form the least dense layers—ocean and atmosphere. This interior stratification sets the framework for life, which must rely on the molecules concentrated toward the surface. In particular, CO2, H2O, and N, which are so central to all living molecules, are placed at the surface by planetary differentiation, as are the magmaphile elements central to life—K, Na, Cl, and P. As we shall see in Chapter 9, CO2 and H2O and their interactions with the crust are also key players in establishing the long-lived climate stability upon which the origin and evolution of life depends.