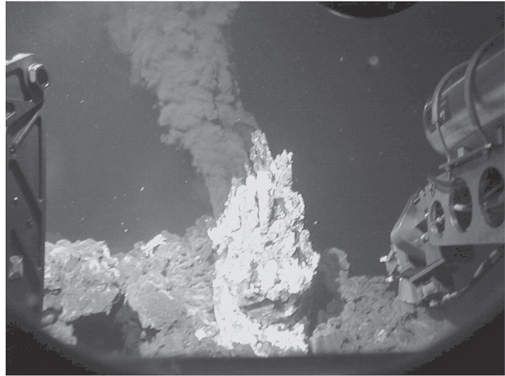
Fig. 12-0: Pictures of black smoking chimneys on the East Pacific Rise. The mineral-rich fluids are clear at depth in the crust and precipitate sulfides and oxides upon encountering seawater, creating the “smoke,” which is not carbon! Exit temperatures of the fluids are as high as 400°C. See color plate 23. (Photograph courtesy of K. H. Rubin)
The physical description of plate tectonics is only the beginning of an understanding of its importance for Earth. The chemical circulation that takes place during that process, the plate tectonic geochemical cycle, is central to Earth’s habitability. It is an important control on the steady-state composition of seawater, produces the oceanic and continental crust, transfers the elements necessary for life to Earth’s surface, and lowers the viscosity of the mantle to permit active convection.
The cycle begins at ocean ridges where magma is transferred from the mantle to create the ocean crust. At the spreading center magma at 1,200°C is in close proximity to the ocean at a few degrees C. Seawater circulating through cracks in the crust becomes strongly heated, leading to intense hydrothermal activity that manifests as fields of black smokers, high-temperature chimneys that can reach heights of ten-story buildings and belch fluids at temperatures as hot as 400°C. These reactive fluids modify the igneous rocks of the ocean crust to create new minerals that contain H2O and CO2 as solid constituents. These minerals persist as the plate ages, and the bulk composition of the solid crust, beginning with essentially no H2O, ends up with an H2O content of about 2% and substantial CO2. As seawater modifies the compositions of the rocks, the composition of the ocean is changed by the exchange with the rocks. The fluids coming out of hydrothermal vents have a very different composition from seawater itself, balancing the input of river water in ways that maintain the steady-state composition of the ocean.
As the plate journeys across the seafloor, diverse sediments accumulate, leading to a complex sandwich of materials rich in water and other elements that were not initially present at the ocean ridge. As the plate subducts, these materials undergo processes of mineralogical change called metamorphism that release the mineral-bound H2O and CO2. The released water lowers the melting temperature of the slab and the mantle wedge overlying the slab, producing wet magmas that rise to become the hydrous, explosive magmas that build volcanoes at convergent margins. The hydrous magmas of the convergent margins differentiate upon cooling to compositions that are rich in silica and low in Mg and Fe, creating low-density materials that are added to the continents and permit the continental crust to float above sea level. The material remaining in the slab is recycled back into the mantle, where it contributes to the creation of a mantle of heterogeneous volatile and trace element composition. The recycling of CO2 at convergent margins leads to long-term climate stability on Earth. Recycling of H2O maintains the steady state volume of the oceans and lowers the viscosity of the mantle, enhancing convection. Further transfers to the surface come from the volcanism from mantle plumes that likely originate at the core/mantle boundary. The solid Earth geochemical cycle then relates the mantle, ocean ridges, oceans, volcanism at convergent margins, creation of the continental crust, mantle convection, and long-term climate stability. The cycles of the solid Earth and their interactions with surface reservoirs are essential for the long-term habitability of the planet.
The basic structure of plate tectonics is plate formation by mantle melting at ocean ridges, movement of the plates across surface, and their return to the mantle at subduction zones. Through this process, plate tectonics recycles material from the interior to the surface and back again in a grand cycle of the solid Earth. This process, however, is only the physical framework of a comprehensive system of exchanges, circulations and feedbacks among Earth’s inner and outer layers, including mantle, crust, ocean, atmosphere, life, and even the core. This total process, which we will call the plate tectonic geochemical cycle (Fig. 12-1), is not simply the physical recirculation of rock through the plates. It is an essential aspect of planetary habitability that maintains climate stability during Earth’s history, keeps seawater volume and composition near steady state, and permits the formation and persistence of the continental crust where life on land has had the chance to flourish. The cycle also provides ecosystems in the sunless deep sea, which as we shall learn may be important for the origin and maintenance of early life. Our aim in this chapter is to understand the overall chemical exchanges involved with plate tectonics, and the processes that contribute to the steady state characteristics far from equilibrium that characterize Earth’s reservoirs. This aim requires an exploration in some detail of the processes of plate creation and destruction at spreading centers and convergent margins. In this chapter we explore this cycle from the creation of the plate at ocean ridges with the attendant interactions with the ocean, the transport of the plate across the seafloor, and the complex processing that occurs as the plate is returned to the mantle at convergent margins, where arc volcanism builds continents and releases volatiles to the atmosphere. These processes relate mantle convection and plate tectonics to the ocean and atmosphere.
In early presentations of plate tectonics, ocean ridges were drawn as a line on a map with transform offsets. Investigation of the ridge reveals it instead to be a dynamic system extending from mantle to microbe, linking mantle, crust, ocean, and life. The chemical fluxes associated with ocean ridges create new environments for life, permit volcanism thousands of kilometers away at convergent margins when the crust is recycled, and contribute to the steady-state chemical composition of seawater. All of these have significance for global habitability. The suitability of Earth for advanced life on continents depends on the hidden operation of the ocean ridge system, hidden from view and virtually unknown even to specialists only decades ago.
Simple volumetric considerations show that most of Earth’s volcanism occurs at ocean ridges. The amount of new magma delivered from the mantle to ocean ridges can be estimated from the product of crustal thickness (6 km) times mean spreading rate (about 5 cm/yr) times the ridge length of about 70,000 km. This amount is about 21 km3 per year. Calculations for convergent margin volcanoes yield estimates of about 2–3 km3/yr, only a tenth as much. Estimates for intraplate volcanism yield numbers similar to those of convergent margins. Hence, ocean ridge volcanoes are responsible for 80% of Earth’s volcanic output.
Fig. 12-1: Cartoon of the plate tectonic geochemical cycle discussed in this chapter. Mantle upwelling creates the crust at ocean ridges and drives hydrothermal circulation. The hydrothermal activity modifies seawater chemistry and creates hydrous minerals in the crust. Sediments accumulate as the crust ages. The crust may also be influenced by hot spots. The complex package of materials is subducted. Metamorphism of the crust releases water and causes melting and formation of island arcs and ultimately continental crust. The slab returns to the deep mantle and creates mantle heterogeneity, later sampled by volcanism. See color plate 19.
Careful mapping of the seafloor in the last decades of the twentieth century gradually revealed that ocean ridge volcanoes are not the symmetrical cones that come to mind with the word “volcano” but are long linear features where magma rises to fill the crack created by the separation of the plates. The ridge is also “segmented” by transform faults that offset the volcanic crack, and therefore ridge volcanoes are not referred to by names of volcanoes but by names of segments, often associated with their bounding transform faults.
The detailed morphology of ocean ridge volcanoes depends on the spreading rate. At the fast spreading rates (>10 cm/yr) of the East Pacific Rise (Fig. 12-2), volcanoes are very long and narrow and do not rise very high off the seafloor. While a volcano like Mount Fuji might be a circular feature some 20 km across that rises 3,000 m above its surroundings, East Pacific Rise volcanoes can be 100 km long, 2–3 km wide, with relief of only a few hundred meters. Their shape can be understood from the fact that at fast spreading rates the hot mantle rises so close to the surface that the magma oozes up all along the opening crack of the spreading center, making a long linear feature—what Jeff Fox has described as “the wound that never heals.” At a spreading rate of 10 cm/yr, the crust moves like a geological race car, 100 km per million years. Since the volcanoes are only a few kilometers wide, old lavas are rapidly spread away, so there is not the opportunity to build up a high undersea cone.
At slow-spreading ridges (< 4 cm/yr), the spreading center is still a long crack, but the hot mantle upwells slowly enough that the hottest temperatures are kept at bay. The lithosphere thickens rapidly close to the spreading axis and toward the transform faults where the ridge abuts older lithosphere. Shallow magma chambers are either ephemeral or absent. The lithosphere forms a kind of cold tent that causes deeper faulting and also causes mantle-derived magmas to be focused along the ridge axis (see Fig. 12-3). The deep faults lead to a prominent rift valley, and the focused magmatism leads to large depth changes along the ridge—shallower toward the centers of segments and deepening toward the transform faults.
Fig. 12-2: Bathymetric map of the East Pacific Rise off the coast of South America from south of the Siqueiros transform to north of the Clipperton transform. Notice that near 9°N there is also an overlapping spreading center, which is a ridge offset smaller than a transform fault where two ridge volcanoes overshoot and overlap. In order to help visualize the topography, the bathymetric maps (bathymetry is the word used for undersea topography) of the seafloor are usually presented with different shades corresponding to different depths. Of course, these shades are not the colors of the actual seafloor but are simply a visual aid to see shapes. The long linear features between offsets are the characteristic form of ocean ridge volcanoes at fast spreading rates (>8cm/yr). The vertical scale of the figure is ~200 km. The total depth change across-axis is a fewer hundreds of meters. See color plate 21. (Image courtesy of Stacey Tighe, University of Rhode Island)
Ridge volcanoes also differ from those on land in that all of them are covered by a 2–3 km blanket of ocean. This makes the ridge an interface between cold seawater near 0°C and mantle-derived magma at 1,200°C. The eruptions and the faults created by spreading create pathways for fluids to pass through the crust and come in very close contact with molten magma and very hot rock. The interaction leads to vigorous hydrothermal systems with chemical exchanges that modify both seawater composition and the composition of the ocean crust.
Fig. 12-3: Bathymetric map of a portion of the northern Mid-Atlantic ridge, looking north. The region, near 36°N, is known as the FAMOUS (French American Undersea Study) area. The segment is about 55 km in length, bounded by transform offsets on the north and south. Notice that the spreading axis is located within a rift valley with steep walls. The magnitude of bathymetric variations is much greater than the fast-spreading ridge, with the rift valley being 1,000 m deeper than the walls of the rift mountains that bound it. The gray scale is very different from Fig. 12-2, since the amount of relief is about five times greater. See color plate 22. (Figure courtesy of Javier Escartin, Institut de Physique du Globe, Paris, using data from Cannat et al., Earth Planet. Sci. Lett. 173 (2001):257–69, and Escartin et al., J. Geophys. Res. 106 (2001):21,719–35)
The vigor of ridge hydrothermal systems caught most marine geologists by surprise. Exploring the northern portions of the East Pacific Rise using a submersible, spires were discovered with billowing clouds of black “smoke” coming out the top (see frontispiece). Arriving at the smoking “chimneys,” the manipulator arm stuck a temperature sensor into the fluid flow, and the sensor went off scale and then appeared to fail. Upon return to the surface it was found that the temperature sensor had melted! When the temperatures of black smoker vents were finally able to be measured, the temperatures were found to be as hot as 400°C—far hotter than the 100°C of boiling water at Earth’s surface.
How could such high temperatures be possible? The key is the influence of pressure on boiling points. If we camp at high altitudes, the pressure is lower and therefore water boils at a lower temperature and cooking takes longer. Just the opposite occurs when the pressure is higher—the boiling point goes up. This is apparent from the pressure-temperature phase diagram for H2O seen in Figure 4-3. The weight of water causes the pressure to increase greatly with depth so water can be heated to much higher temperatures before boiling. The molten magma rising from the mantle has a temperature of 1,200°C and heats seawater all the way to its boiling point. Therefore, rather than being a cold blanket that suppresses hydrothermal activity, the high pressures of the ocean allow far higher temperatures and more vigorous activity than is possible on land.
The hot, saline water is chemically very reactive. Magma coming from the mantle contains almost no water, and there is disequilibrium between the rock and hot seawater. The subsequent chemical reactions cause both rock and water to change composition. Rocks that originally consisted of the minerals pyroxene, plagioclase, and olivine react to form hydrous minerals such as chlorite, amphibole, and epidote, discussed further below. The water changes its composition at the same time, losing virtually all of its Mg, some Na, and dissolving Fe, Mn, Cu, Zn, Pb, and other metals as its oxidized sulfate transforms to reduced sulfide. At high temperatures the density of this mineral-laden solution decreases, and it becomes buoyant and rises turbulently to the surface. As it rises it deposits sulfide minerals along veins in the crust and then flows out of surficial vents at high velocities. Here it contacts deep seawater with a temperature of only a few degrees C. The rapid cooling of the reduced and acidic hydrothermal fluid causes the metals dissolved in the fluid to precipitate, creating the dramatic “black smoke” and leading to the construction of chimneys that rise from the seafloor.
While the study of ocean ridge hydrothermal systems is still in its infancy, certain generalizations are emerging about their type and distribution. The ocean ridge hydrothermal systems relate to magmatism and faulting. At fast spreading rates, a shallow magma chamber 2–3 km below the surface exists in the ocean crust. Above the magma chamber are shallow faults that serve as pathways for fluid motion. Seawater penetrates the cracks, is heated by the underlying magmatic heat, and buoyancy drives it forcefully upward to the surface. The hydrothermal system is an active convective system driven by a shallow layer with very large thermal contrast at its base. The convection is by way of flow of water through cracks and porous rock, rather than convection of the rock itself. The Rayleigh number for the fluid is very high, because the viscosity of water is low and the temperature contrast large. Since the spreading rate is fast, magma supply is large and frequent eruptions reset the hydrothermal system. Hydrothermal activity manifests as a rapidly changing assembly of small groups of “black smokers” distributed quite extensively along the ridge segment (Fig. 12-4, right panel).
At slow-spreading ridges, the magma supply is smaller, lithosphere thicker, and faulting deeper (see Fig. 12-4, left panel). Shallow magma chambers, when they exist, are intermittent. Locations of faults and access to the heat source that they provide are important controls on the locations of hydrothermal systems. These tectonic contrasts influence the hydrothermal system and create a variety of hydrothermal environments. Where water penetrates more deeply along faults, heat is extracted from large volumes of hot rock rather than the boundary layer of a magma chamber. This leads to large systems that may be separated from the zone of active volcanism and persist for relatively long periods of time. In periods when magma approaches the surface to form a shallow magma system, usually at the center of a ridge segment, the systems that develop are more similar to fast-spreading ridges. Slow-spreading ridges can also host a very different form of hydrothermal system, at lower temperatures, that arises when hot mantle peridotite is raised by faults to be close to the surface, and is altered by its interaction with seawater. This type of system can release reduced species of gases such as CH4 and H2 that we shall see may be important for the origin of life.
The influence of hydrothermal systems does not stop at the seafloor. The rising plumes from the hydrothermal vents contain reactive particles that rise above the ocean ridge until they reach a level of neutral buoyancy in the water column, similar to the behavior of plumes coming from utility smokestacks. These plumes spread far and wide in the deep sea. This became evident from study of the distribution of the isotope 3He. 3He is present in gases released from ocean ridge basalts and incorporated in the hydrothermal plumes but is steadily lost from seawater as it diffuses into the atmosphere and eventually out to space. Because 3He is created only during nucleosynthesis, and not as a result of radioactive decay, and all old helium has escaped from the top of the atmosphere, there is no 3He naturally in the oceans. That allows 3He to be a reliable tracer of the amount and extent of hydrothermal fluids in the oceans. The trace of the plumes can be mapped by looking at the distribution of 3He dissolved in the water. Figure 12-5 shows that the 3He plume from the East Pacific Rise extends far across the Pacific Ocean.
Fig. 12-4: Cartoons illustrating hydrothermal flow at fast- and slow-spreading ridges. At intermediate and fast spreading ridges (bottom panel), a shallow magma chamber is common, and hydrothermal circulation is near the center of the ridge above the chamber, supported by shallow faults. (Figure courtesy of D. Kelly and J. Delaney) At slow-spreading ridges (top panel), such as the TAG area near 20°N on the Mid-Atlantic Ridge, magma reservoirs are often absent and hydrothermal circulation is often associated with deep faults slightly off axis.
Fig. 12-5: Map of the distribution of 3He in waters of the Pacific Ocean. The extensive plume with significant 3He concentrations shows the extent of hydrothermal fluid distribution from the East Pacific Rise spanning the Pacific basin. (Figure modified from J. Lupton and H. Craig, Science, 1981 v. 214, no. 4516, 13–18)
The most surprising aspect of the deep-sea vent systems was the discovery of lush and diverse ecosystems of animals surrounding the hydrothermal vents. Abundant life was thought to require sunlight, and most of the deep sea was very limited in biological productivity, relying on scavenging of organic materials falling form the surface. Some early photographs showed pictures of what looked like clams and mussels present more than 2 km beneath the surface. Clams and mussels are ordinarily found in the tidal environment! While many of the animals bore superficial resemblance to those we are familiar with, most were unique new species. Some of the animals were dissimilar from any species found on land. Most colorful were the tall white tubeworms with bright red tops that could rapidly extend and retract from their white stalks (Fig. 12-6). The density of life in the immediate vicinity of the vents is staggering. Living in an environment considered to be the most barren part of Earth, isolated from the life-giving sun, the deep-sea vent communities can have densities of life that are locally as rich as tropical rainforests, though of course far smaller in extent.
The base of the food chain for these communities is sulfur-oxidizing bacteria. The sulfide in the hydrothermal fluids is out of equilibrium with oxidized seawater, and this coupled with the high temperatures provides the conditions for the sulfur-oxidizing bacteria to flourish. These bacteria form the base of the food web of the vent ecosystems, and they have developed symbiotic relationships with many of the animals that frequent seafloor near the vents. Some of the bacteria have been found to thrive at temperatures higher than 100°C, something that was previously considered impossible. The ecosystems are robust and well adapted to an environment that on land would be toxic and uninhabitable. Organisms on land look to the sun as the ultimate source of their nourishment and well-being. If intelligent life at vents evolved, the sun would be a minor and distant inference, and their gods of nurturing and destruction would be the active volcanism that controls all aspects of their life cycles. The simple fact of spreading plates creates a linked system from mantle to microbe, where life is sustained by the vertical movement of energy and mass from the mantle to the exterior.
The revolutionary aspect of discovery of life at hydrothermal vents is that the base of the food chain is supported by volcanic energy from the planetary interior, rather than energy from the sun. That this was possible greatly expanded views of the potential habitats for life on other planets. Even distant from a star, or in the complete absence of starlight, life is possible.
Fig. 12-6: Picture of a vent community around a hydrothermal vent in the same region of the East Pacific Rise as Fig. 12-2. Some of the animals bear superficial resemblance to animals found in tidal zones. Others, such as the long thin “tubeworms” have a unique appearance. All have a metabolism very different from surface organisms, depending on symbiotic relationships with sulfur-oxidizing bacteria supported by the vent fluids. See also color plate 24. (Photograph courtesy of Cindy Lee Van Dover)
While ocean ridges are clearly central to plate tectonics, and support their own unique biosphere, are they also central to habitability? Yes, chemical processes during formation, transport and recycling of the ocean crust are integral aspects of a habitable Earth. Four aspects of their formation and recirculation are particularly important, none of them apparent from consideration of the tectonic aspects of plate movements alone:
(1) Geochemical processes at ridges sustain the chemical composition of the oceans;
(2) ridges store and transport water and other elements to the subduction zone that permits volcanism and continental growth to occur there;
(3) ridges play an important role in the water and carbon cycles that provide long-term climate stability for Earth (discussed in Chapter 9);
(4) ridges may have played an important role in the origin of life on Earth, with implications for the viability of life elsewhere in the galaxy (discussed in Chapter 13).
From the pre-plate tectonic perspective, the ocean mass balance appears to be rather straightforward: the ocean receives inputs from river water and wind-driven dust and creates outputs from evaporation of salts in isolated seas and the deposit of sediments to the seafloor. The evaporated water that rises to form rain is very pure. It first collects some elements by reacting with gases and dust in the atmosphere. Then, when it rains and runs over the rocks of the continents, it weathers the rocks and dissolves some of their mineral constituents, so that upon its return to the ocean it has a much higher mineral content. Table 12-1 compares the compositions of seawater, rainwater, river water, and hydrothermal fluids. While river water is far less mineral rich than seawater, it contains far higher quantities of various elements than does pure rainwater. And seawater has high concentrations of many elements, but far less than a saturated solution such as the Great Salt Lake.
Table 12-1
Compositions of Earth’s water (concentrations in parts per million)
*R. Chester, Marine Geochemistry (Oxford: Blackwell Science, 2000); and H. Elderfield and A. Schultz, Annu Rev. Earth Planet Sci. 24 (1996):191–224.
**Elderfield and Schultz (1996); hydrothermal fluids have a considerable range in composition. Na and Cl in the hydrothermal fluid are similar to seawater.
In this simple version of events, pure water is removed from the oceans by evaporation, and more mineral-rich river water is added to the oceans in an equal amount. Therefore the water cycle causes more and more chemicals to be added to the ocean, and the mineral content of seawater should steadily increase with time. The ratios of elements would be the same in river water and seawater. It would be a bit like having a tub full of water that you distill to take a shower while standing in the tub. The distilled water you shower with is always clean. At first the tub water is clean, but the dirt and soap would steadily accumulate in it as you took shower after shower, and ultimately the tub water would be so rich in soap and salt that it would become saturated.
The ocean, however, is not overly mineral rich and is not saturated with salt and other minerals. Mineral-saturated water, such as the Great Salt Lake in Utah, has much higher element concentrations than seawater. In fact, there is so little Na in seawater that the entire budget of Na would be added by rivers in only 47 million years. (This was actually one of the early methods that some geologists used to calculate Earth’s age.) Somehow seawater is maintained at steady state far below saturation. Furthermore, ratios of many elements in seawater are vastly different from the river water that supplies the oceans (see Table 12-1). This requires active sinks that are removing elements as fast as they are added, creating a kinetic balance, or steady-state disequilibrium, that has been maintained over limited ranges over Earth’s history. The steady-state composition of seawater below saturation requires a balance between sources and sinks.
The water cycle also does not fractionate radiogenic isotopic ratios. If oceans received all their inputs from the continents, then the radiogenic elements in seawater should have the same radiogenic isotopic ratios as the continental crust that is weathered by the rivers. The most abundant of such elements in seawater is our old friend 87Sr/86Sr. The average 87Sr/86Sr ratio of continental crust is >0.712, while the seawater ratio is much lower, near 0.709. The Sr isotope evidence shows that continents cannot be the only source of material added to the oceans. Seawater requires some other process contributing to both sources and sinks!
One sink is life in the ocean. Organisms with siliceous shells remove Si and those with carbonate shells remove Ca, leading to low Si/K and Ca/K in the remaining water. But then why does seawater have low Mg/K as well? And life does not fractionate radiogenic isotope ratios and cannot explain the Sr isotope data.
The mystery process that balances seawater composition is the ocean ridge hydrothermal circulation. The disequilibrium encountered by seawater as it passes through hot rock causes some new minerals to form, some elements to be removed from seawater, and others to be added. The modified solution, which is the hydrothermal vent fluid, then encounters the ocean. Some elements are added to the ocean, some immediately precipitate in the chimneys, and some create reactive particles that scavenge other elements from the water column and remove them to the sediments. The water circulation through the crust removes a substantial proportion of the riverine input of Na, and it quantitatively removes the Mg from the seawater that circulates (note the zero concentration of Mg in the hydrothermal fluid), contributing to the low Mg/Na of seawater (~0.1) compared to river water (~0.6). And the 87Sr/86Sr composition of the ocean crust and of vent fluids is about 0.703. Mixing of Sr in this fluid with continentally derived Sr (0.712) leads to the intermediate Sr isotope composition of seawater (0.709).
While each individual hydrothermal vent is small, the total hydrothermal system including the thousands of vents along the ocean ridge is large. High temperature flow completely processes the volume of the ocean in tens of millions of years. Lower temperature flow off axis is far more extensive, and processes the ocean volume in a few hundred thousand years. Both of these fluxes are small, however, compared to river water, which supplies one ocean volume every 30,000–40,000 years. Since rivers are a much greater volume, how can the hydrothermal flux be so important? The answer lies in the very high concentrations of some elements in high-temperature vent fluids, as is apparent in Table 12-1. Some elements have concentrations a thousand or more times higher than river water and for many elements hydrothermal fluxes are as large or larger than the global river flux.
Two elements that are very concentrated in vent fluids are Fe and Mn, and yet these elements have essentially zero concentration in seawater. How is this possible? As we shall discuss at length in Chapter 17, Fe and Mn are quite soluble in water when they have a 2+ valence, and they are readily dissolved in hot, acidic, reduced hydrothermal fluids. When these elements encounter alkaline and oxidized seawater, they are oxidized to their 3+ form, and immediately precipitate in the hydrothermal plumes above the vent chimneys. These precipitated particles have very reactive surfaces and scavenge many other metals from the water before falling to the seafloor as sediment. The hydrothermal fluids are a large source that then produces a large sink. The sink includes other metals in the oceans, because the plumes from these chimneys spread out over large expanses of the ocean, and the particles encounter large volumes of seawater. The plumes have an ocean processing time of 4,000–8,000 years and are an important means of interaction between hydrothermal systems and the larger ocean.
It is one of the remarkable aspects of plate tectonics that geochemical consequences of volcanism include a pivotal partnership in the maintenance of seawater composition that is a central aspect of Earth’s habitability.
The mantle rising to melt beneath the ocean ridge has very low water content, and the lavas making up the crust at ocean ridges (mid-ocean ridge basalts, referred to as MORB) are made up of minerals like plagioclase, olivine, and pyroxene that do not contain any water at all. Any other volatiles that were in the mantle were also removed from the mantle during melting, and from the magmas by degassing as they solidify. The new plate when it forms is essentially anhydrous and volatile free.
Hydrothermal interactions with seawater change the composition of the plate. Interaction with seawater at both high and low temperatures “alters” the rocks, transforming the original dry minerals to hydrous minerals such as amphibole and layer silicates. These minerals, akin to mica, are not damp to the touch but contain water as an essential part of their mineral structure. For example, in the amphibole mineral formula—Ca2(Mg,Fe)5Si8O22(OH)2—and chlorite mineral formula—(Mg,Al,Fe)12 (Si,Al)8O20(OH)16—the OH group reflects the structural water that is part of the solid mineral. Formation of these minerals changes the rock from shiny black basalt to a metamorphic rock, called greenschist or amphibolite, depending on the mineralogy, that contains several percent water locked in its minerals (Table 12-2). As the crust ages, the interactions with water continues, albeit at lower temperatures, and there is some hydration of the mantle beneath the crust, where olivine and orthopyroxene are modified to serpentine Mg3(Si2O5)(OH)4.
As the crust traverses the ocean basin on its way to the subduction zone, a rain of sediments gradually accumulates leading to typically 500–1000 m of sediment that form a part of the subducting plate. The sediments come from several sources—from weathering of continents both from rivers and from wind, from accumulated particles originally from hydrothermal vents that settle through the water column, and from accumulation of dead organisms. Most of the minerals in these sediments have even higher proportions of water than the altered ocean crust. CO2 is also added to the plate locked in solid form in carbonate minerals, both through deposition of carbonate sediments and precipitation of carbonates in veins in the ocean crust and mantle. An average composition of subducting sediment, GLOSS (global subducting sediment), is included in Table 12-2.
Table 12-2
Compositions of mantle, altered mantle, oceanic crust, altered oceanic crust, and global subducting sediment
aChemical compositions of average primitive mantle (W. McDonough and S. Sun (Chemical Geology 120 (1995) 223–253).
bAverage altered serpentinite (harzburgite aver. (OM94) K. Hanghoj et al., J. Petrol. 51 (2010) 201–227).
cAverage ocean crust (Gale, Langmuir, and Dalton, in press).
dAltered ocean crust (SUPER, K. Kelley and T. Plank, Geochem. Geophys. Geosys. 4(6) (2003) 8910).
eGLOSS (Global Subducting Sediment composition (T. Plank and C. Langmuir, Chemical Geology 145 (1998) 325–394).
Note the very high volatile contents of altered materials and sediment.
Fig. 12-7: Illustration of the change in plate composition leading to the volatile-rich package of materials that enters the subduction zone.
Volatiles are not the only materials added to the plate through alteration and sediment accumulation. Many elements, such as U, Rb, Ba, K, and B, are taken up by the hydrous minerals (see Table 12-2). Elements such as Pb, Cu, and Zn are accumulated in the sulfides formed by deposition from hydrothermal fluids. The reactions with oxidized seawater also convert the oxidation state of the crust so that much of the Fe2+ is converted to Fe3+.
This entire package of material—altered mantle, altered basalt, and sediments—then moves down into the mantle at subduction zones (see Fig. 12-7). The descending plate is very different from the water- and CO2-poor magma that came out at the spreading center tens of millions of years before, because of all the interactions with the surface reservoirs. Interactions with seawater modified the crustal composition, adding volatiles and other elements. Continental erosion, biogenic accumulation, and deposition of particles from hydrothermal vents created a rich and diverse sedimentary package above the crust. Movement of the plates transports this diverse assemblage to the subduction zone, creating flux from the surface to the mantle. As we shall see in the following section, this flux is what permits volcanism at convergent margins, creates a heterogeneous mantle, and leads to the formation of the continental crust.
The kinematic description of plate tectonics associated the downward moving plate with the seismically defined Benioff zones. Standing above the Benioff zones with striking regularity are linear chains of conical volcanoes, such as the Pacific “Ring of Fire.” When they are built on the seafloor, their great heights barely rise above sea level. When built on the high plains of central Mexico or the Altiplano of the Andes, where the thick crust results in a base level of 2,000–3,000 m, the volcanoes rise to 5,500 m or more, forming some of the highest peaks in the world. The existence of most of these volcanoes was well known hundreds of years before the advent of plate tectonics. Plate tectonics showed their ubiquitous relationship to subduction zones. More careful examination of the detailed locations of earthquakes revealed a remarkable regularity with respect to the down-going plate—when the positions of the volcanoes were compared to the seismicity below, most were found to occur about 110 km above the Benioff zone. Subduction and volcanism are systematically related (Fig. 12-8).
There is a predicament when trying to understand why subduction leads to volcanism. At ocean ridges, depressurization of hot material causes melting. At subduction zones, in contrast, the cold plate is descending and produces a predominantly downward flow of the mantle in the mantle wedge, as was illustrated in Figure 11-9. Downwelling of cold material is the opposite of the hot upwelling at ocean ridges and makes it more and more difficult for the mantle to melt. At subduction zones, why isn’t melting suppressed?
The key to melting at convergent margins lies with water. Several lines of evidence show that convergent margin magmas, unlike those at ocean ridges, are water rich.
Fig. 12-8: Top: Map of the Juan de Fuca Plate and Cascades volcanoes showing their linear alignment and relationship to the down-going plate. Bottom: Aerial photograph of the Aleutian volcanic front. Mt. Kanaga is in the foreground. Moffett in the middle distance and Great Sitkin further away. (Photo courtesy of Chris Nye, Alaska Division of Geological and Geophysical Surveys and Alaska Volcano Observatory)
Fig. 12-9: Temperature vs. water concentration diagram illustrating how water affects melting temperature for mantle peridotite and subducted basalt and sediment. T2 is the melting point of dry peridotite. The freezing point depression effect depends on how much water can be dissolved in the magma, which is pressure sensitive. Low-pressure magmas cannot contain significant water, and therefore water has no effect on melting. At higher pressures, magmas can dissolve 20% or more of water, leading to a very strong depression of melting temperatures, as illustrated at T1, a wet melting temperature.
• Analyses of small inclusions of magma trapped in crystals that preserve initial volatile contents contain 5% or more of water (as well as high CO2).
• The volcanoes are often explosive, and dissolved water converting to gas is a major cause of such explosive eruptions.
• Differentiation under hydrous conditions leads to higher silica magmas (andesites, rhyolite, granites), which would account for the preponderance of such magmas at convergent margin volcanoes.
High water contents are the key to how melting can occur at convergent margins (Fig. 12-9). Melting experiments of mantle peridotite show that water is an extremely effective agent of freezing point depression (see Chapter 7). The extent of freezing point depression is proportional to the amount of water that can be dissolved in the liquid magma. If too much water is added, it simply creates a fluid or gas and does not further lower the melting temperature. Because water vapor is so compressible, the maximum water content (the “solubility” of water in magma) increases greatly with pressure. At one atmosphere, almost no water can stay dissolved in magma; but at pressures of 10–30 kb in the mantle wedge (30–90 km below the surface) as much as 20% water can enter the magma, lowering the melting temperature by hundreds of degrees. The vast differences in water content cause melting at divergent and convergent margins to occur by different mechanisms. Whereas melting at ridges is by decompression of hot mantle, melting at convergent margins is caused by flux melting from lowered melting temperatures due to the freezing point depression caused by the addition of water.
For flux melting to occur, water must somehow be transported to the mantle beneath convergent margin volcanoes. The obvious source is the volatile-laden crust of the down-going plate. The water-rich minerals that formed near the surface are not stable at high pressures and temperatures. They undergo mineral transformations that lead to new structures and minerals with lower water content that release the excess water as a fluid. This overall process is called metamorphism. Metamorphism is solid-state transformation of rocks in response to changes in temperature and pressure. With increasing pressure and temperature, metamorphic reactions usually involve the progressive dehydration and decarbonation of rocks and the release of H2O and CO2 to the surroundings. During subduction, hydrous phases such as amphibole and chlorite that formed at the ocean ridge are transformed by a series of reactions to the anhydrous assemblage of pyroxene and garnet, making the dense rock eclogite, which helps to drag the slab down into the mantle (see Fig. 11-11). Carbonate minerals also become unstable, and high pressures and temperatures cause carbonates and silicates to react and release the CO2 as a gas. For example,
CaCO3 + MgSiO3 + SiO2 = CaMgSi2O6 + CO2
As we learned in Chapter 9, this decarbonation reaction is one of the vital pathways for preservation of Earth’s equable climate on long timescales.
The melting temperature of the basalts and sediments of the ocean crust in the presence of water is far lower than the melting temperature of mantle peridotite (~800°C rather than the ~1,500°C solidus of anhydrous mantle peridotite at a depth of 100 km). Because of these low melting temperatures in the presence of water, portions of the slab may also melt during subduction. The slab temperature becomes hottest where it is in contact with the mantle wedge. Since sediments are the uppermost slab layer, the sediments are most likely to melt. Geochemical evidence suggests such melting commonly occurs in the sediment. Melting may also occur in the ocean crust, depending on the detailed thermal environment of subduction. For example, very slow subduction rates give the slab much more time to heat up as it descends, making slab melting more likely. In the ancient Earth, when the mantle temperature in the wedge was considerably higher, slab melting was inevitable. Melts of the slab would have high concentrations of H2O and CO2 and would be another mechanism of volatile transport.
The fluids and melts from the slab that form at the relatively low temperatures at the top of the slab are much less dense than the mantle and will rise into the mantle wedge. The mantle wedge at this depth has an inverted temperature gradient—temperatures are much higher in the “core” of the wedge than they are in the older slab (see Fig. 11-9). Although the mantle immediately adjacent to the cold slab is too cold to melt, once the water rises far enough it enters the region of hotter mantle, where the added water lowers the melting temperature sufficiently to cause mantle melting to occur (Fig. 12-9). Whereas human beings usually associate melting with increased temperatures, and the mantle beneath ridges melts by decreasing pressures, melts at convergent margins are formed by a third mechanism—flux melting, where melting temperature is lowered by addition of another chemical component. We make use of the same principle to make roads safe for driving in the winter. Adding salt to our roads lowers the melting temperature of the ice even when the temperature is below the freezing point of pure water. At convergent margins, the migration of CO2 and H2O from the slab lowers the melting temperature of the mantle to produce the volatile-rich magmas that rise to the surface to form the explosive arc volcanoes.
The high concentrations of water also explain some of the major differences between the eruptive behavior of divergent and convergent margins. Ocean ridge basalts do not erupt explosively, and most flows are small and move slowly across the seafloor. In contrast, continental volcanoes are renowned for their explosive behavior. The tops of many of these volcanoes consist of large craters that are the remnants of the explosive removal of the volcano summits. Such eruptions became well known when Mount St. Helens erupted in May 1980 (Fig. 12-10). The solubility of water in magmas explains much of this contrast in behavior. The high water content of convergent margin magmas can all remain dissolved in the magma as long as the pressure is high. But as the pressure drops, the solubility decreases and some of the water escapes as a vapor. This vapor creates bubbles in the magma, which can lead to an extreme buildup of pressure, and the volcano filled with magma is a bit like an out-of-control pressure cooker or an overgassed bottle of champagne. If an earthquake makes a crack in the surface of the volcano, or the pressure builds up enough that the rocks near the surface of the volcano cannot support the stress any longer, the volcano explodes catastrophically. It is owing to their high water content, ultimately derived from the subducted slab, that convergent margin volcanoes are explosive while ocean ridge volcanoes are not. This differential solubility of volatiles is also what leads to the flux of gas from convergent margin volcanoes that is essential to the composition of the atmosphere and climate stability.
Fig. 12-10: Mount St. Helens before it erupted in May 1980 (top) and after it erupted (bottom). (Courtesy of U.S. Geological Survey)
Water is not the only element transferred from the down-going slab. Many elements are strongly enriched in sediments and altered ocean crust relative to the mantle. Some elements are soluble in hot water-rich solutions at high pressure, and these elements should be efficiently extracted from the slab. Other elements are carried by slab melts and are effectively transferred. And sediments are enriched in many elements by a factor of one hundred or more compared to the mantle, so any sediment contribution leads to high abundances of certain elements.
Is it possible to prove that elements subducted at the trench are recycled through the mantle to come out at convergent margin volcanoes? Fortunately, there is a magic bullet in the geochemical arsenal that provides such proof. The interaction of cosmic rays with Earth’s atmosphere produces a number of radioactive isotopes, the most familiar one being 14C. Another cosmogenic radionuclide with a much longer half-life of 1.6 million years is 10Be. The 10Be formed in the atmosphere falls to the surface leading to small but measurable amounts in the sediments that fall to the seafloor to create the sediment layer of the ocean crust. The topmost oceanic sediments have the most 10Be, and the concentration gradually decreases with depth as the 10Be decays away. In sediments that are older than 10–15 Ma all of the cosmogenic nuclide has decayed away to its daughter product 10B. This decay occurs no matter where the 10Be resides. In the column of sediments being subducted, the 10Be exists only in the youngest sediments of the top few meters (Fig. 12-11). There is no 10Be in the mantle, and none in ocean ridge basalts. Nonetheless, many recently erupted convergent margin magmas contain some 10Be! The presence of 10Be in freshly erupted arc volcanics requires that Be from the uppermost sediments is carried down the trench, off the slab, into the wedge, and out the volcano in a few million years. This is conclusive evidence that elements from subducted sediments contribute to arc magmas. An evaluation of many other elements shows good correlations between element ratios in the sediments of the down-going slab and elements in the volcanic rocks of the overlying arc, confirming that subducted sediments are recycled to contribute to convergent margin volcanism.
Fig. 12-11: 10Be content of deep-sea sediments. Because of the 1.6 Ma half-life of 10Be, it gradually decays away in older sediments as younger ones accumulate on top of them. (Figure courtesy of Terry Plank)
More subtle evidence that we will not delve into here shows that elements from ocean crust are also recycled. The flux of elements from the various layers of the slab then makes the chemical compositions of arc volcanics vastly different from those of ocean ridge basalts. Elements that are enriched in sediments or are easily transported in metamorphic fluids or partial melts of the slab are greatly increased in abundance in the sources of convergent margin magmas. Whereas the volcanics at ocean ridges have very low abundances of magmaphile elements, those at convergent margins have large enrichments (10–100 times of many magmaphile elements, e.g., K, Rb, Cs, Ba, Th, U, and Pb). Over Earth’s history, the fluxes from the slab have lead to progressive concentration of magmaphile and fluid-loving elements toward the surface, including Na, K, and P, three elements that are essential for living organisms.
High water contents also lead to higher SiO2 contents of magmas, and to the precipitation of minerals that cause magmas to evolve toward higher SiO2 as they cool. Basalts at ocean ridges all have relatively low SiO2, near 50%, while convergent margin magmas commonly have 55% SiO2 or more. The higher silica magmas also have low density. Convergent margin magmatism then leads to light crust, filled with magmaphile elements, that is not able to be subducted and becomes a permanent resident of the surface, riding buoyantly on top of plates. Continental crust formation can then be viewed conceptually as arising from the solid Earth geochemical cycle: first, basaltic crust forms at an ocean ridge; then, it is modified and hydrated by interaction with the surface; when subducted, it serves as the agent for delivery of the necessary elements to form the continental crust, where most of advanced life on Earth now resides.
Water clearly is the central element in this process. Recycling of water to the mantle is necessary to form continental crust. And at the same time, for the longevity of the oceans, the water that is subducted must be returned to the surface. Were the water in altered ocean crust and sediment simply returned to the mantle, the oceans would gradually empty over just a few hundred million years as water was returned to the interior. Since water is essential for life, there would be no planetary habitability.
After the plate has been processed at convergent margins, it continues its journey into the mantle. Also, not all of the material transferred to the mantle wedge makes it out to subduction zone volcanoes, so some of the material exiting the slab remains in the mantle. The end products of subduction are over time gradually mixed back in to the mantle and play a pivotal role in mantle evolution.
Fig. 12-12: Left: Illustration of the “marble cake” concept where the mantle is a folded mixture of diverse layers. Right: An outcrop of mantle rock that is layered with diverse veins. Some of these veins may reflect recycled crust. (Photography courtesy of Peter Kelemen)
One of the most important consequences of this recycling stems from the role that H2O plays in mantle viscosity. While most of the water is returned to the surface, a small fraction of recycled water is able to be included in nominally anhydrous mantle minerals—those that have no “OH” in their formula but nonetheless can include tens of parts per million of water in their mineral structure. Mantle minerals thus become “moistened” by their contact with fluids from the slab, and the slightly hydrated minerals are much weaker than perfectly dry minerals. The small amount of water causes the viscosity of the mantle to decrease by 1–2 orders of magnitude. The change in viscosity has a significant effect on the Rayleigh number, and hence the vigor of mantle convection. Some scientists think that Venus has no plate tectonics because its mantle is dry and therefore stiff. The presence of an ocean on Earth, coupled with the plate tectonic geochemical cycle, may be what permits steady, active convection and exchange between Earth’s interior and the surface.
The process of plate recirculation also influences the major element composition of the mantle and how it is distributed. The recycled plate differs from what melted at ocean ridges by having separated crust from residual mantle and being processed at convergent margins. The 6-km-thick crust in particular has mineralogy, density, and physical properties different from mantle peridotite. While convection is “vigorous” from a planetary point of view, it is still a rather slow stirring and folding that is taking place in the solid state. The recycled material is not efficiently stirred and homogenized into the mantle but instead will be slowly deformed and thinned, in a fashion described by Claude Allègre and Don Turcotte as a “marble cake.” This process leads to extended veins of diverse thicknesses distributed through the mantle (Fig. 12-12).
There is also the possibility that some subducted plates, or portions of them, are sufficiently dense that they fall through the mantle and accumulate at the core/mantle boundary. If this boundary is also the location where mantle plumes form, the crust would preferentially contribute to the plumes. These plumes then rise to the surface, generating magmas and releasing volatiles. Some of the outpourings are so intense that they significantly impact climate and life (see Chapter 17). Plumes relate subducting crust and the heat output from the core to surface reservoirs.
Since the recycled crust also has a low melting temperature relative to the surrounding mantle, it also melts more easily during mantle convection, so that low-degree melts of the subducted plate, highly enriched in magmaphile elements, are a possible consequence of plate recirculation. All these diverse processes lead to mantle heterogeneity on various scales—variations in mantle composition that are created and preserved through plate recycling. Such heterogeneity then leads to a diverse set of basalt compositions appearing at the ocean ridge and at ocean islands hundreds of millions to billions of years later. The complement of continental crust formation and maintenance of seawater composition is the formation of the heterogeneous mantle through plate recirculation.
The plate tectonic geochemical cycle relates mantle, crust, ocean and atmosphere. Magma emplacement at ocean ridges produces a large temperature contrast between crust and the deep ocean that drives vast hydrothermal systems at the ridge axis. These systems provide important sources and sinks for seawater, greatly influencing the chemical composition of the ocean. At the same time, the crustal mineralogy is transformed to contain volatile-rich minerals such as chlorite and carbonate. During passage of the plate to the subduction zone, low-temperature hydrothermal circulation and sediment deposition continue to influence the chemical composition. As the plate descends into the mantle at the convergent margin, the volatile-rich minerals undergo metamorphism and release their H2O and CO2 as well as many other magmaphile elements, such as Na, P, K, and Pb. The water depresses the melting temperature of the slab, possibly causing melting, particularly of the upper sedimentary layer. Fluids and hydrous melts are light and percolate upward into the hot mantle wedge. There they lower the melting temperature of the mantle and cause the formation of water-rich magmas, which ascend to the surface to form arc volcanoes. The high water contents make these volcanoes very explosive, as the water degasses during eruption.
Geochemical data show the importance of the subducted materials from the oceanic plate for the creation of convergent margins. The mystery of convergent margin volcanism—how do volcanoes form in a cold, downwelling environment—can be understood as a simple consequence of volcanism at mid-ocean ridges, with the attendant hydrothermal interactions adding volatiles and other elements to the crust which are then transported by plate tectonics to subduction zones. The water exploding out of convergent margin volcanoes originated through the hydrothermal systems circulating two miles beneath the surface at ocean ridges.
High water also contributes to lower-density magma compositions higher in SiO2, and the low-density crust is stabilized at the surface and is not subject to subduction. Since convergent margins are the locations where continents are being formed, the origin and continued existence of the continental landmasses depends on the functioning and recirculation of the oceanic plates hidden beneath the sea. The distinctive compositions of continental rocks owe much to the transport of elements in fluids off the slab, and the recycling of sediments through erosion, deposition in the deep sea, and subduction into the mantle. Continents then become a natural outgrowth of oceanic volcanism and plate tectonics taking place beneath an ocean. As we saw in Chapter 9, the ocean itself is able to persist because of the climate feedbacks involving interactions of the atmosphere with the sun coupled with the CO2 recycling controlled by subduction. The persistence of plate tectonics and the convective vigor of Earth’s mantle may also be maintained by the plate tectonic geochemical cycle, as recycled water lowers mantle viscosity by 1 to 2 orders of magnitude. This also contributes to the viability of mantle plumes rising through the entire mantle to the surface to contribute to surface reservoirs. Atmosphere, ocean, ocean crust, mantle, core, continents, and plate tectonics form a linked system that sustains the conditions of our habitable planet.
Special Issue on InterRidge 2007. Oceanography 20, no. 1.
J. D. Morris and J. G. Ryan. 2003. “Subduction Zone Processes and Implications for Changing Composition of the Upper and Lower Mantle.” In H. W. Carlson, ed., The Mantle and Core, vol. 2 of Treatise on Geochemistry. Oxford: Elsevier Science. Pp. 451–70.