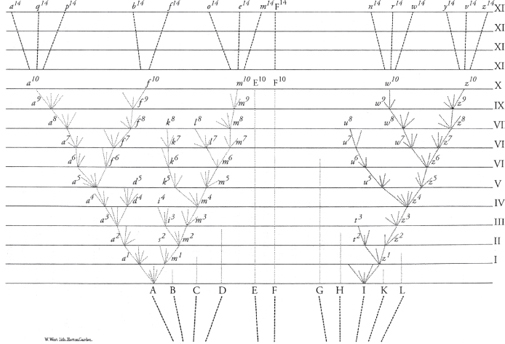
Fig. 14-0: Darwin first introduced the concept of “the Tree of Life” with this figure in The Origin of Species, published in 1859. He wrote: “From the first growth of the tree, many a limb and branch has decayed and dropped off; and these lost branches of various sizes may represent those whole orders, families, and genera which have now no living representatives, and which are known to us only from having been found in a fossil state.… As buds give rise by growth to fresh buds, and these, if vigorous, branch out and overtop on all sides many a feebler branch, so by generation I believe it has been with the great Tree of Life, which fills with its dead and broken branches the crust of the earth, and covers the surface with its ever branching and beautiful ramifications.”
All of life has remarkable unity in terms of its cellular structure, metabolism, and chemical pathways. As we look around us, however, we can only marvel at life’s diversity. Early naturalists categorized the diversity of life by a classification system, ranging from kingdom at the broadest scale to species for unique organisms. Originally plants and animals were the two kingdoms, but the deepening revelation of the microbial world led to five or six kingdoms within three “domains” of life—bacteria, archaea, and eukarya. Both plants and animals are made up of the complex eukaryotic cells, and are in one domain. The single-celled prokaryotes have much of the genetic diversity of the planet and are so different from one another that they make up two domains, bacteria and archaea. How could such diversity arise from a common origin?
The links between past and present are preserved as the fossils of the geological record. The progressive changes in life through Earth’s history were revealed through the careful study of layered sedimentary rocks, revealing a biological history where generations of organisms went extinct and new species appeared. Current organisms can be traced back to common ancestors in the geological record. The geological timescale is divided largely on the basis of sudden changes in the life assemblage. For this reason there are far more divisions after the first fossils with hard body parts appeared 543 million years ago, separating the Phanerozoic from the Precambrian. The Phanerozoic is divided into three eras, bounded by mass extinction events. Charles Darwin investigated current life and the fossil record. He observed vast differences in life between different continents and smaller differences between neighboring islands. He proposed the theory of evolution—that life would progressively diversify through time by the process of natural selection, where competition and environmental change would stress populations and lead to more successful reproduction of individuals with certain characteristics. Small changes over long periods of time could lead to the diversity of life. Darwin’s intuitive theory was vindicated by the discovery of DNA that provided a mechanism for inherited characteristics and for steady small changes in DNA sequences. Current DNA sequences also provide a living record of ancient change. Organisms with similar DNA have a recent common ancestor, while those with very different DNA diverged from each other in the ancient past.
Evolution of macroscopic life is too slow to be seen on human timescales, because new species appear by very gradual modification of existing ones. For bacteria that can undergo multiple generations in a day, evolution is fast enough that it can be observed in the laboratory. Concrete experience of evolution can be seen for macroscopic life by understanding that evolution requires both species origination and species extinction. The geological record shows that barring mass extinctions, the background extinction rate is about 0.00001% per year, which leads to 99% species reduction in 40–50 million years. During geological time, however, the number of species has greatly increased, showing that species generation has outpaced species extinction. While species origination is gradual, species extinction can be abrupt. Human domination of the planet has vastly increased the extinction rate, by a factor of 10,000, and consequently the extinction half of evolution is very evident to us. If current human-induced extinction rates continue, much of the current diversity of macroscopic life will be gone in a few hundred years.
In the previous chapter we emphasized the unity of life when viewed on the cellular level and considered how the formation of a first protocell, the universal common ancestor to all of life today, might have come into being. These cells would have been so small that they would be visible only under a microscope, and life might not have been obvious to the untrained and unaided eye examining Earth’s surface.
What a contrast to life today! Life is everywhere, and its diversity can be awe inspiring: giant sequoias, oysters, cobras, honey bees, human beings, and mold growing on old food—everywhere we look, even in deserts and glaciated regions, life can be found. Clearly, habitability has increased markedly over Earth’s history. Our habitable planet is not just one where life was able to begin, but where life has flourished to its current omnipresent state. The building of a habitable planet includes the initial conditions that have been discussed thus far, and the evolution of the planet from the first primitive life more than three billion years ago to the complex and diverse ecosystems that we can observe today. Our task then is to describe this change and to explore the mechanisms by which it has come about.
How can we adequately characterize life’s diversity? At the same time as we see diversity, we also see great commonalities among different types of life. Mammals share many characteristics, as do many flowering plants. We intuitively perceive a scale of similarity, with some organisms very similar to one another, others slightly similar, and others vastly different. Such obvious observations have long led people to try to classify life systematically. Naturalists in the eighteenth century went to great lengths to try to describe, categorize, and make sense of the diversity of life that they could see (they were only vaguely aware of the vast microbial world). Carolus Linnaeus, a Swedish naturalist, was one of the most influential and created a hierarchical classification of plants and animals whose essential form has survived to the present day. The term Homo sapiens, for example (“man wise”), is an example of his binomial nomenclature for the naming of different species.
Linnaeus linked species by their common characteristics into groups, in ways that we can readily understand. Human beings, chimpanzees, and gorillas share many characteristics, as do wolves, dogs, and coyotes or bobcats, leopards, and tigers. A dog is more similar to a coyote than to a gorilla or a leopard. And all of these have common characteristics not shared by fish and lizards, let alone ants, flowering plants, and seaweed. Very careful consideration of these issues allows a classification of macroscopic life, where some characteristics (like plant and animal) are very broad and common to huge groups and others (such as having a single hoof and walking on four legs) are shared by few, down to the species level where the collection of characteristics is unique. Early naturalists had a passion for a careful classification of all of Earth’s life that they could see. They were willing to undertake long arduous voyages, stopping at ports plagued with incurable diseases, in order to explore, collect, systematize, and try to understand life’s diversity, and to preserve the specimens in newly established museums. The diversity of life and its organization into distinct groups based on shared characteristics was a great and exciting process of discovery of the life of the planet. These early systematists organized life into a hierarchical structure in order of decreasing generality and numbers of organisms—kingdom, phylum, class, order, family, genus, species (Fig. 14-1). Since they were little aware of the microbial world, the two kingdoms were plants and animals.
The development of the microscope permitted the discovery and investigation of microbes, and the discovery of the cellular structure of all of life. In the mid-nineteenth century a new kingdom, protista, was suggested for the microbial world. Once the prokaryotes and eukaryotes were clearly differentiated, it became clear that both plants and animals had a kinship in that they were both made up of eukaryotic cells. Ultimately, in the late twentieth century DNA analysis revealed that prokaryotes have fundamentally different genetic lineages, and they were divided into bacteria and archaea, also called eubacteria and archaebacteria. One is left then with three domains of life (eukaryotes, bacteria, and archaea), which can be divided into five or six kingdoms—two that are prokaryotic (eubacteria and archaebacteria), two that are the unicellular or simple multicellular eukaryotes (protists and fungi), and two for the diversified eukaryotic organisms (plants and animal) (Fig. 14-1).
DNA provides a rigorous and quantitative measure of diversity. A small but rapidly growing number of organisms have had their DNA sequenced, and the results can be compared to the visual and qualitative classification carried out by the early naturalists. The macroscopic changes we can see correspond to changes in the detailed molecular sequences of the DNA molecules that direct the appearance and metabolism of all of life. Human beings individually have 99.9% of their DNA in common, and the human species has 96% DNA in common with chimpanzees, 90% with mice, and 80% with the distant mammal, the platypus. Mammals and plants have only 22% of their genes in common. The correspondence between visual differences and DNA variation validates the comparison of living organisms with extinct organisms, for which visible features are preserved as fossils but DNA is no longer present. The discovery of the microbial world, where visual differences are few, adds a new dimension to this picture, which we will discuss further later in this chapter.
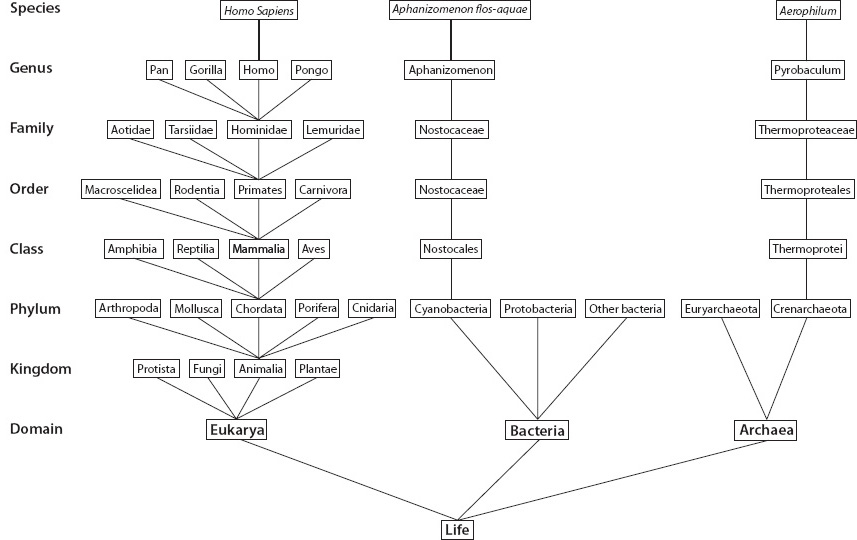
Fig. 14-1: Early classification scheme for currently existing life as a nested hierarchy, with domain and kingdom as the broadest classes and species as the most specific. We, for example are Homo sapiens: Kingdom: Animalia; Phylum: Chordata; Class: Mammalia; Order: Primates; Family: Hominidae; Genus: Homo; Species: Sapiens.
During the same time period that early biologists were identifying and classifying the diversity of modern life, geologists were working out the stratigraphic record, where they would try to order different rock layers from oldest to youngest. This also became practical and important to predict where coal seams might be found to fuel the burgeoning industrial revolution. Since radioactive dating did not exist in the eighteenth and nineteenth centuries, it was not possible to assign exact ages to particular rock formations. Instead, geologists had to rely on the relative ages of sedimentary rocks. The simple principles of relative age were deduced by the Danish geologist Nicolas Steno in the seventeenth century. He noted that sediments were generally formed by particles falling from or precipitating from water, and that individual sediment layers could be traced for long distances. Furthermore, the layer on top conformed in its shape to the layer beneath, showing that it was deposited after the deeper layer was already in place. The law of superposition codifies this principle: for a series of flat-lying strata, the ages become progressively more recent upward in the stratigraphic section. Of course, in detail there are exceptions to this principle. Sediments can be deposited on slopes, and can be thrust on top of one another by faults, but the principle is fundamentally sound. Undeformed sequences abound in the geological record (Fig. 14-2). The law of superposition can also be used to unravel complex sequences of deformed and faulted rocks.
Fig. 14-2: Two examples of horizontal strata, laid down one on top of the other following the law of superposition—in undeformed sequences, the younger rock is found on top of the older one. Top panel: sedimentary rocks from the Grand Canyon; bottom panel: basaltic lava flow from the Columbia River basalts in Oregon. (Courtesy of U.S. Geological Survey)
This procedure worked well for limited areas where the layers could be continually traced, but how could rocks from different places be compared to one another? The rocks themselves were rarely distinctive—one shale, sandstone, or limestone can look very much like another. Fossils provided the Rosetta Stone (Fig. 14-3). The thousands of animal and plant species identified in fossils could be carefully catalogued into fossil assemblages, the groups of animals that lived together in a particular time period. Fossil assemblages were found to vary in the same stratigraphic order on every continent. Even though the exact sequence of rock types might differ from one region to another, the changes in fossil assemblage were always regular. While no one location contained a complete stratigraphic record, the fossil assemblages permitted the partial columns to be cross-linked into a single whole.
The oldest sedimentary rocks did not contain fossils, and this nonfossiliferous time was initially all lumped together as Precambrian (Fig. 14-4). Well-preserved fossils with hard body parts appeared suddenly in the stratigraphic record, and these first layers were called the Cambrian period, for which a characteristic fossil is the trilobite (seen in Fig. 13-7, Chapter 13). Trilobites vary in detail as one ascends through the stratigraphic column, and then they disappear, never to be found in younger rocks. Much later, the first fishes appeared. Trees were even younger. In this way, a global stratigraphic column, ordered in relative time series with the oldest rocks at the bottom and the youngest at the top, was able to be constructed (Fig. 14-4).
In the absence of precise dates, either in our personal lives or in ancient history, we refer to time periods with names, such as “teenager,” the Bronze Age, or the Middle Ages, brackets of time defined by beginning and ending events. Geologists used the same convention for their relative timescale, applying names to periods with characteristic fossil assemblages and creating a boundary where the fossil assemblage showed a distinct change, usually associated with the sudden disappearance of abundant species. The names were applied to a hierarchical system, eons, eras, periods, epochs, and—the finest time division—stages. (Only specialists have a detailed knowledge of the epochs and stages.) The appearance of animals with hard body parts was a defining event in Earth’s history, and it was used to divide the Phanerozoic eon from the great expanse of earlier time initially called the Precambrian. Within the Phanerozoic, there were occasional disappearances of large numbers of species, now referred to as mass extinctions. The largest extinctions were used to define the eras. The extinction between the Permian and Triassic led to the demise of some 80% of existing genera and separates the Paleozoic and Mesozoic eras. The disappearance of the dinosaurs (and about half of all other species) at the end of the Cretaceous is defined as the boundary between the Mesozoic and Cenozoic eras. The large numbers of extinctions, deforestation, and environmental change associated with the rise of human civilization probably merits another era boundary, as the whole surface of Earth is transformed by our activities.

Fig. 14-3: Fossil assemblages differ in major ways for different periods of Earth history. On top is an artistic rendition of an Ordovician ecosystem (500–425 Ma) where invertebrates dominate, and there were no vertebrates or plants. (Figure © C. Langmuir) On the bottom is an assemblage from the Permian, with advanced plants and dominant reptiles. (Image © by Karen Carr (www.karencarr.com)) Rocks from these two periods collect completely distinct fossil assemblages that allow rocks on different continents to be place in stratigraphic order.
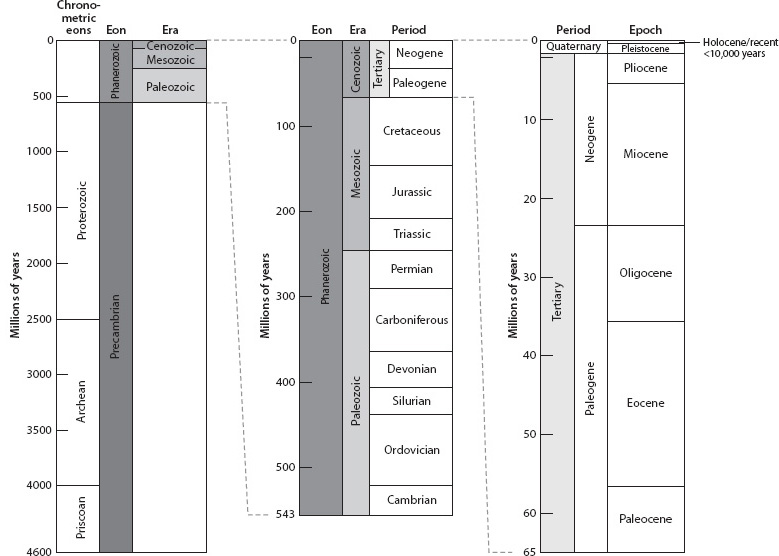
Fig. 14-4: Geological timescale originally constructed from stratigraphic relations and the fossil record, and confirmed and quantified by radiometric dating. The timescale on the left is all of Earth history and shows that the Phanerozoic era where fossils exist occupies only 10% of Earth’s history. The two columns to the right expand short intervals of columns to their left. (Courtesy of U.S. Geological Survey)
For early geologists this record of temporal change was relative. Radiometric dating, as discussed in Chapter 4, allowed the hypothesis of a biologically constructed stratigraphic timescale to be tested. While sediments were made up of diverse particles weathered from rocks of different ages, igneous rocks found in the same intervals provided discrete dates. These dates verified the validity of the stratigraphic column and also assigned exact times to the geological periods (Fig. 14-4). The Phanerozoic/Precambrian boundary was found to occur at 542 Ma—only 12% of Earth’s history!
Radiometric dating was gradually able to reveal more about the Precambrian, where the fossil record did not permit accurate cross-calibration of sections from different regions. The quantitative timescale has allowed the Precambrian to be divided into intervals that correspond with the eon-era-period framework. The expanse of time between the formation of Earth and the oldest known rocks is called the Hadean. (The younger bound of the Hadean is subject to change as older rocks are found.) The oldest rocks, currently the Acasta Gneiss in Canada dated at 4.0 Ga, define the beginning of the Archean eon, which extends to 2500 Ma. The next youngest eon is called the Proterozoic and spans 2500 Ma to the start of the Cambrian at 542 Ma. Because the Archean and Proterozoic encompass such vastness of time, they are each divided into three somewhat arbitrarily defined eras, and the Proterozoic also has well-defined periods. The youngest rocks of the Proterozoic eon are the Neoproterozoic era, from 1000 Ma to 542 Ma, a time of great importance. During this time there were multiple “snowball Earth” episodes in the aptly named Cryogenian period, and the early development of multicellular life in the youngest period of the Neoproterozoic, the Ediacaran, which immediately underlies the Cambrian period of the Phanerozoic eon. While the names initially seem somewhat arcane, they become like old friends with frequent usage. This overall naming of Earth time provides us with the vocabulary we will use to discuss events in Earth’s history.
Early nineteenth-century scientists developed two macroscopic avenues to explore the diversity of life—existing organisms, showing diversity today, and the fossil record, showing diversity through time. How could these be related? How does life in the fossil record compare to the newly discovered systematics of living organisms, and has life changed through time? The remarkable discovery was that most fossils had no living counterparts. There are no living trilobites or dinosaurs, and even large mammals present in the most recent fossil record, such as mastodons and saber-toothed tigers, are extinct today. Then the task became to systematize the changes in the fossil record, using techniques similar to those which had been used for living organisms. Since most classification of organisms is based on morphological differences (e.g., how many legs does the organism have, does a beetle have five or six segments to its antennae, and so on), and fossils also reveal morphology, it is possible to relate current life to the fossil record. This comparison reveals that much of the current diversity of characteristics of similar species can be related to a common ancestor in the geological record. Working backwards, this common ancestor can be related morphologically to common ancestors of other species, finding a mutual common ancestor even deeper in geological time. These relationships give rise to the concept of the Tree of Life (Fig. 14-5), where the current diversification of species are the growing ends of the individual twigs of the tree and the ultimate source of life would be at the base of the trunk. Note that the Tree of Life shows changes through time, not to be confused with the treelike classification of modern life (Fig. 14-1), which deals only with currently living species.
The great leap in understanding the history and diversity of life was made in the mid-nineteenth century. At this time there was convincing evidence of a vast expanse of geological time and a developing understanding of the details of life’s diversity as a whole. Charles Darwin in his travels saw that physical separation of continents like Australia led to large differences in living species, and in the Galapagos Islands he observed that small changes emerged for populations from neighboring islands that were barely isolated (Fig. 14-6). The accumulating body of information and recognition of the vast expanse of time suggested by the geological record gave rise to the grand synthesis of Darwin’s theory of evolution. Darwin proposed that life would progressively diversify through time by the process of natural selection, where competition and environmental change would stress populations and lead to more successful reproduction of individuals with certain characteristics. Small changes over long periods of time could progressively lead to the diversity of life. This could be seen by differences that corresponded with the physical separation of living organisms, and also through the progressive change observed in the fossil record. In this way both the diversity today and the change through time could be related to a common process.
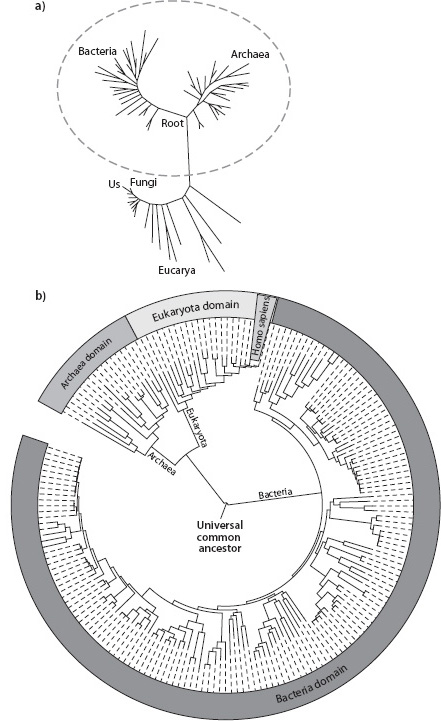
Fig. 14-5: Two modern representations of the Tree of Life. (a) Tree of Life (adapted from Pace, Science 276 [1997]:734–40); (b) Alternative Tree of Life based on genome sequencing. The center is the root of the Tree of Life and corresponds to the universal common ancestor of life. The different shadow areas represent the three domains of life: dark gray represents bacteria, gray represents archaea, and light gray represents eukaryota (protista, fungi, animalia, and plantae). Note the presence of Homo sapiens (humans) second from the rightmost edge of the light gray segment, and that most of the genetic diversity of life rests with the single-celled organisms (modified after iTOL: Interactive Tree Of Life; http://itol.embl.de/itol.cgi).
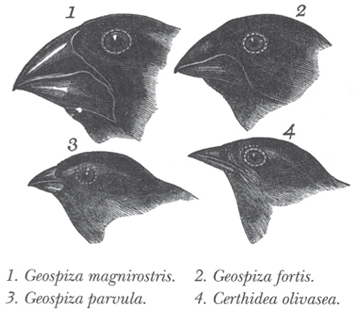
Fig. 14-6: Darwin’s original illustration of the slight differences in finches found on different islands of the Galapagos archipelago off the coast of Ecuador. Darwin noted that the finches had only slight differences from one another and were also similar to related species in South America, even though the climate was very different. He wrote: “There is nothing in the conditions of life, in the geological nature of the islands.… in fact there is a considerable dissimilarity in all these respects. On the other hand, there is a considerable degree of resemblance in the volcanic nature of the soil, in climate, height, and size of the islands, between the Galapagos and Cape de Verde Archipelagos: but what an entire and absolute difference in their inhabitants! The inhabitants of the Cape de Verde Islands are related to those of Africa, like those of the Galapagos to America.”
Darwin’s ideas were controversial in the eighteenth century and remain difficult to understand and accept for many nonbiologists today. One of the difficulties has been that evolution operates over millions to billions of years, and our lifetimes are more than ten thousand times shorter than that, so we do not see new species emerging in clear ways within our human experience. Instead, life seems relatively unchanging—humans have always been here, oak trees are oak trees, dogs are dogs. The theory of evolution when it was developed was based on differences in life in different locations, on historical inference and reasoning, rather than direct observation of change and experiment. Perhaps we can accept the likelihood of small changes. But how can these lead to the large diversity of living organisms? Could a whale and a tree really have a common ancestor?
The same genre of issue was faced with geological time, where Earth seems relatively unchanging within human experience, and it is difficult to fathom the consequences of hundreds of millions of years of change. A similar disconnect made it difficult to comprehend plate tectonics. We are now forced to accept as fact the geological timescale, based on radioactive dating. And plate tectonics is a proven fact from the accurate measurements of plate movements today that correspond precisely with the inference from magnetic anomalies. Is there similar conclusive evidence for evolution?
A stunning contribution to the understanding and development of the ideas of evolution was the discovery of DNA as the genetic material that controls the specificity of species and provides the mechanisms of inheritance. Since the turn of the century, as the genomes of various species become precisely described, we understand in ever greater detail the origins of differences within and among species. Mutations are no longer the hypothetical qualitative concept they were in Darwin’s era; they reflect precise changes in the base sequence of DNA strands. Mutations can come about in several ways. Replication efficiency is not 100%, and sections of DNA can be snipped, pasted, or duplicated, providing inevitable mechanisms of progressive biological change. Some changes will lead to fatality, some to a competitive advantage. The advantageous changes survive. DNA provides the mechanism for evolution and the inevitable transfer of changed characteristics from one generation to the next (Fig. 14-7).
There is also one domain where evolution can be observed experimentally on laboratory timescales, and that is in the microbial world. The number of generations and DNA replications that occur along with the selective pressure of environmental change is the control on the rate of evolution. For human beings with a generational change of roughly 30 years, a thousand generations is 30,000 years, well beyond our historical record or even perspective. To see evolution in action requires organisms with lifetimes short enough that thousands of generations can occur on human timescales, and environmental change can be manipulated. The simplest bacteria can divide every hour or so, leading to the possibility of twenty or more generations in a day. A single bacterium, call him Adam, could in a week go through one hundred generations, giving rise to a population of billions or more, depending on the ratio of reproduction rate to the lifetime of an individual bacterium and the availability of nutrients. A crisis such as lack of nutrients, change of physical conditions, or introduction of a toxic disease could lead to precipitous decline in population with only a few survivors with the special mutations that allowed them to survive, from which again a genetically distinct population could arise. If this species of bacteria were writing their history, it would be filled with grand events of population rise and decline occurring over hundreds of generations—an apocalyptic history in a test tube on a timescale less than a human summer vacation. In some laboratories these kinds of experiments have been carried out over a decade or more, leading to substantial genetic diversity.
Through these detailed experiments and observations, evolution has been observed in the laboratory. Biologists have been able to start with a single cell and watch it evolve through tens of thousands of generations, developing diverse capabilities and behaviors in response to environmental change, and then mapping these changes in the DNA of the evolved organism. Gene transfer of various types and the development of dependent symbiotic behavior have also been able to be observed experimentally. Thus, while changes in large organisms with long lifetimes are poorly accessible to us by direct experience, use of microorganisms with very short lifetimes reveals the reality of evolution by genetic change.
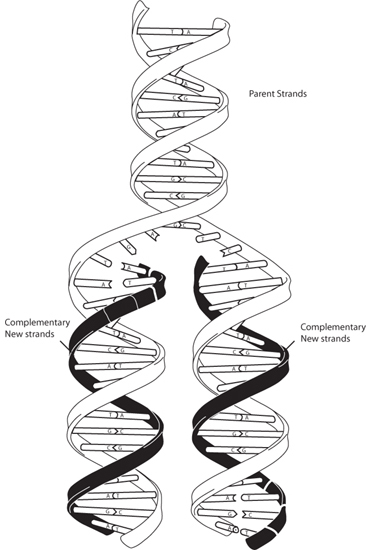
Fig. 14-7: Schematic illustration of DNA replication that permits inherited characteristics and mutations to be passed on from one generation to the next. (Illustration by artist Darryl Leja, courtesy of National Human Genome Research Institute)
On the macroscopic scale, changes in complex species on the scale of centuries are evident to us upon reflection. The diversity and specialization of dogs and domestic animals and plants, and the common use of breeding to develop particular characteristics, are evident. Some of these changes approach species differentiation—breeding between St. Bernards and Chihuahuas is virtually impossible physically and would likely lead to the death of the mother if she were the Chihuahua. While less familiar to most city dwellers, the changes in crops through human intervention are also great. And the appearance of new diseases, as well as the development of new strains that are resistant to antibiotics, is evolution of great importance to the future health of human beings.
Knowing that progressive changes in DNA sequences are inevitable, and are observed in laboratory experiments with living organisms, evolution can be understood as an inexorable statistical process. If two populations of a species are isolated on two different islands, or isolated into two different ecosystems by environmental parameters, each one will gradually change in different directions. As time passes, these changes become progressively greater, until the point where the genetic similarity is too disparate for interbreeding. The extent of the genetic difference will increase with time, providing a kind of clock that dates the time since the two new species were genetically identical. This process, repeated over and over again over millions of years, leads to groups that had a recent common ancestor, and hence have similar DNA, and groups that only have a very ancient common ancestor, and greater differences in their DNA. It explains why species on nearby islands are subtly different, and why animals in Australia, such as the kangaroo, are very different from those found in North America. This process explains beautifully the fact from the fossil record that organisms in Africa and South America were the same at the time the two continents were joined together and then progressively changed to the distinct flora and fauna that are present on these continents today.
The quantitative character of species identification through DNA allows the Tree of Life to be constructed using quantitative methods rather than inferences from physical characteristics. The degree of similarity of the DNA quantifies the differences among species and allows estimates of the time since there was a common ancestor. In this way the present genetic diversity also contains information about past history. How many genetic changes would be necessary to make the genome of organism A identical to that of organism B? If genetic changes happen at a more or less regular rate, that makes possible a genetic clock providing an estimate of the time when two different branches on the tree of life divided (Fig. 14-5).
This concept shows that no two living species are descended from each other. A common misconception about evolution is illustrated by the statement, for example, that “man is descended from the apes.” We often interpret this statement to mean that humans were descended from gorillas or chimpanzees, which is incorrect. Instead, humans and chimpanzees are cousins descended from a common ancestor. The two species now have about 96% of their DNA in common, fully quantified thanks to the sequencing of the human and chimpanzee genomes. Using the mutation clock, the differences require about 6 million years. The identity of the common ancestor can then be related to specific species in the fossil record, linking current diversity, established fossil history, and quantitative DNA measurements. The places in the DNA where we differ from chimps will eventually reveal details of the mutation paths that have led to the diversification of the two species from their common ancestor.
DNA quantification has also permitted the detailed investigation of the microbial world, where visual differences are not so evident as for macroscopic, multicellular life. While human beings have more than 3 billion base pairs, a typical prokaryote contains on the order of 1 million base pairs. As more and more bacterial species were sequenced, the remarkable discovery was that these unicellular organisms have such variable genes that they contain most of the genetic diversity of the planet. Carl Woese pioneered the new view of life’s diversity based on DNA, and the eukarya that represent all the life we can see are only a small fraction of the total genetic diversity that has developed over Earth’s history (see Fig. 14-5b).
Examining the bacterial DNA relationships in detail has also revealed another mechanism for evolution besides gradual mutations. Bacteria can sometimes transfer a gene from one organism to another. This type of change in DNA is referred to as horizontal gene transfer because it occurs laterally between different branches of the tree of life, rather than linearly along a single branch of the tree. Viruses are another mechanism of gene transfer other than mutation, since some viruses inject their DNA into a cell where it is incorporated in the host cell DNA. Horizontal gene transfer is abundantly observed in microbial life, to such an extent that many biologists think the strict linear tree-of-life concept no longer is relevant to the microbial domain and that the deep roots of the tree have many exchanges and interconnections, allowing one branch to exchange genetically with another. Microbial life is a vast storehouse of genetic information that can be transferred among organisms as they adapt to competitive pressures and diverse environments.
SIDEBAR
EVOLUTION OF LANGUAGE
The evolution of language is a useful parallel to consider on a shorter timescale the effects of small changes and interactions leading to diversity over time. While we are scarcely aware of language changes in our lifetime, the contrasts between modern English and Shakespearian English are clear to anyone who reads a Shakespeare play, and these changes have occurred in less than four hundred years. The differences between modern English and Chaucer, and Chaucer and Beowulf, are even greater, occurring over a thousand years. The differentiation of European languages from Latin has occurred in only two thousand years. The common Indo-European root that has led to languages as diverse as Hindi, Farsi, Russian, and Gaelic was about 5000 BC. Some of the changes occur through slow progressive change in a single isolated language. Some occur when there is an invasion or development of communication between two different language groups, and a new language that makes use of ingredients from both groups evolves. Longer times and greater isolation lead to greater differences. Greater communication leads to more sharing and commonality. The global language exchange that is now taking place is leading to the extinction of many minor languages. Languages also contain a history of where they came from, when they diverged from ancestral tongues, and how they were combined with other languages. And language is passed quite faithfully, but not quite perfectly, from one generation to the next. Language diversity demonstrates clearly how small changes and combinations that occur progressively through time lead to large differences, such that two populations no longer are able to communicate with one another.
The analogies with biological evolution are evident—small changes over time lead to separate languages where communication is no longer possible. Change is fostered by time and separation. New languages do not suddenly appear but emerge from small changes over time. Current languages have ancestral roots that can be traced to their common parent, and a “tree of language” can be constructed with many similarities to the tree of life. In mathematical terms, language differentiation has many similarities to biological evolution. DNA is the “language” of the cell. While biological changes happen more slowly than spoken language, there is much in common in the principles and outcome.
The magic of DNA from the perspective of the theory of evolution is that it provides a mechanism for inheritance, a simple and quantitative basis for understanding of gradual evolutionary change, and the possibility of rigorously quantifying diversity. It also contains history—a record of how the current diversity has evolved through time. DNA thus provides a very precise and quantitative mechanism for evolution. Scientists in the lab today can manipulate DNA strands to change the characteristics of organisms and produce new evolutionary mechanisms—human-mediated gene transfer.
Another perspective on evolution is to realize that there must always be two complementary processes occurring—the extinction of old species as well as the gradual development of new ones. The total number of species on the planet will depend on the balance between those going extinct and those coming into being. The overall trend of numbers of species through time that emerges from the fossil record is shown in Figure 14-8. The geological record shows that there is a background extinction rate, punctuated by short periods of mass extinctions. During the largest of the mass extinctions, at the boundary that defines the transition from the Paleozoic to the Mesozoic eras, some 70–90% of species went extinct in only a few million years. After this precipitous decline, diversity and the number of species increased during the Mesozoic, only to undergo another sudden decline at the boundary between the last period in the Mesozoic, the Cretaceous, and the first period of the Cenozoic era, the Tertiary. Since that mass extinction, the number of species again climbed substantially until recently, when the latest mass extinction precipitated by humans began to influence the planet.
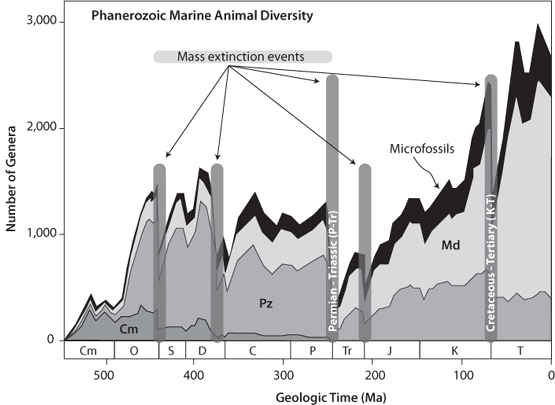
Fig. 14-8: Quantification of the number of distinct genera through geological time. Note that the number of genera has increased substantially over the Phanerozoic, but this overall increase has been punctuated by periodic abrupt extinctions, called mass extinctions. The five largest mass extinctions are indicted by the gray bars. The distinctively Cambrian fauna went permanently extinct at the Permo-Triassic boundary that separates the Phanerozoic and Mesozoic eras. (Modified after Sepkoski, Bulletins of American Paleontology 363 (2002). See also strata.geology.wisc.edu/jack)
Studies of the fossil record suggest that the background extinction rate is about 0.00001% per year. That number can be viewed analogously to a decay constant for radioactive decay—the rate per year at which species disappear is analogous to the proportion of atoms that will decay in a given year. In this terminology, the decay constant for species extinction is 10–7, which corresponds to a mean life of species of 10 million years. Using this number as a “decay constant” leads to a half-life for all species of 6.9 million years. With this extinction rate, 99% of all species go extinct in 43 million years.
The data shown in Figure 14-8, however, show that the number of species has not decreased with time. No complex organisms were even present long before the Cambrian, and there is an overall increase in numbers of species through the geological record. If species only went extinct, diversity and numbers of species would inevitably decline, so species creation has to have been occurring at a faster pace than species extinction. Using the background extinction rate, this suggests almost complete species turnover in the time periods of 43 million years where 99% of extant species go extinct—and this is less than 1% of Earth’s history. Biological change has a very dynamic pace on Earth timescales. Such change is fortunate because it allows life to adapt to inevitable environmental change, provided that change is not too abrupt.
On our human timescale, however, emergence of new species appears to be a very slow process. For every million species, only sixteen new species would emerge each century. And this emergence is not like some new species popping up and appearing out of nowhere—it would be a gradual evolutionary, mutation-by-mutation change where two species that used to be approximately the same have now become just different enough to be identified as separate. Emergence of new species is thus subtle, gradual, and almost invisible to us on human timescales.
The extinction half of evolution, however, is easier to see, because extinctions can be abrupt. Extinction on human timescales is obvious to all of us, as the rapid changes in habitat imposed by the exponential increase in human population and associated civilization lead to large numbers of extinctions. Biologists who are specialists in this area estimate current extinction rates of about 0.1% per year for macroscopic life, or a decay constant of 10–3, ten thousand times the background level. Human beings have thus accelerated extinction rates by ten thousand times, allowing this aspect of the evolutionary process to become obvious to us. If emergence of new species had been similarly accelerated, then more than 20% of Earth species would be new in the past two centuries, and this aspect of evolution would also be obvious. Extinctions are subject to rapid rate changes, while species emergence is limited by the inexorably slow molecular process of DNA mutations. This accounts for the observation that species emergence is not obvious to us, while the extinction half of evolution is so much in evidence.
The evidence for evolution is thus firmly based in the geological record of life, understood in molecular detail through DNA, obviously necessary from observations of extinction, and subject to laboratory verification. Like the Big Bang and plate tectonics, it ranks a 10 on our theory scale and is one of the firm foundations of scientific understanding of the world we inhabit.
From the perspectives of current diversity of life, the fossil record, the theory of evolution, and the modern evidence from DNA studies, we can understand the present complexity and diversity of life as a progressive evolutionary process whose molecular basis can be understood and verified. Going backward through time we find common ancestors for all current species, which themselves have common ancestors that occurred early on Earth. The unity of all of life points to a common ancestry. The details of the evolutionary process show the mechanisms by which diversity has emerged within this unity.
It is difficult to encompass the intuitive genius of Darwin’s concept. He intuited that characteristics were inherited, that they could undergo small changes progressively through time, and that the selection of favorable (i.e., successful in terms of surviving and reproducing) changes would lead to gradual changes over time, as revealed in the fossil record and both the diversity and commonality of life as we know it. These ideas had no known mechanism when Darwin proposed them. DNA provided the precise description of the mechanism of transfer of inherited characteristics from one generation to the next, and the means of mutation and gradual change through small mutations in the specific DNA sequences. DNA thus provided a link between rigorous, detailed modern biochemistry and the overall understanding of life and its evolution proposed by Darwin. This synthesis is one of the great moments of the history of science, where two independent approaches converged to provide an understanding that united biochemistry, biology, paleontology, and the history of Earth.
The two halves of the evolutionary process are the emergence of new species and the extinction of existing ones. Both must occur for ancient species to no longer exist, and the total number of species on Earth to have increased during the Phanerozoic. Emergence is so gradual as to be almost imperceptible for macroscopic life on human timescales, though it is readily apparent in the microbial realm. Extinctions are abrupt, and the massive environmental change caused by human domination of all ecosystems has increased the extinction rate by a factor of 10,000, making the extinction half of the evolutionary process all too evident.
Lynn Margulis and Michael F. Dolan. 2002. Early Life; Evolution on the Pre-Cambrian Earth. Sudbury, MA: Jones & Bartlett Learning.
Andrew H. Knoll. 2003. Life on a Young Planet: The First Three Billion Years of Evolution on Earth. Princeton, NJ: Princeton University Press.
Richard Dawkins. 2004. The Ancestor’s Tale: A Pilgrimage to the Dawn of Evolution. Boston: Houghton Miffllin Harcourt.
Charles Darwin and E. O. Wilson. 2005. Darwin’s Four Great Books (Voyage of the Beagle, The Origin of Species, The Descent of Man, The Expression of Emotions in Man and Animals). New York: W. W. Norton & Co.