13 |
Ancillary Neurodiagnostic Procedures—Lumbar Puncture and Neuroimaging |
We have instruments of precision in increasing numbers with which we and our hospital assistants at untold expense make tests and take observations, the vast majority of which are but supplementary to, and as nothing compared with, the careful study of the patient by a keen observer using his eyes and ears and fingers and a few simple aids.
I. ARRAY OF NEURODIAGNOSTIC PROCEDURES
After completing the history and neurologic examination (NE) and proposing a tentative diagnosis, the examiner (Ex) has to decide whether further studies are required. Table 13-1 reviews the array of standard diagnostic tests. The goal is to choose the one or two safest, least invasive, and most economical procedures that will best confirm or refute the tentative diagnosis. Do not order every conceivable test to cover every diagnostic possibility. Go for the jugular. If you fail initially to secure the diagnosis, select successive tests in a logical order. In this section we will discuss the clinical use of lumbar puncture (LP) and neuroimaging studies.
TABLE 13-1 • Ancillary Neurodiagnostic Procedures
A. Neuroradiologic imaging 1. Plain films 2. CT (enhanced and unenhanced) 3. MRI (enhanced and unenhanced) 4. Angiography:MRA, direct injection, CTA, Doppler sonography 5. Cranial ultrasonography 6. Radionuclide scanning: PET, SPECT B. Electroneurodiagnosis 1. Electroencephalography (EEG) 2. Electromyography (EMG) 3. Nerve conduction velocity (NCV) 4. Evoked responses: visual, auditory, and somatosensory 5. Audiogram C. Punctures 1. Subarachnoid (LP or cisternal) for CSF 2. Subdural for blood, pus, or fluid 3. Ventricular for blood or CSF 4. Stereotaxic for lesion biopsy D. Blood biochemistry 1. Complete metabolic profile 2. Toxicology screen 3. Quantitative amino acids and organic acids 4. Serum enzymes 5. Long-chain fatty acids, cholesterol, cholestanol 6. Serum ceruloplasmin 7. B1, B12 and folic acid 8. Lysosomal enzymes 9. Collagen vascular screen 10. Disease-specific tests for inborn errors of metabolism 11. Hypercoagulable screen 12. Protein fractionation E. Neuro-ophthalmology 1. Visual acuity 2. Visual fields, central and peripheral 3. Ophthalmoscopy direct and indirect, slit-lamp inspection of media and fundus 4. Visual evoked response (VER) 5. Electroretinogram (ERG) 6. Electronystagmography 7. Optical coherence tomography (OCT) F. Urinalysis 1. Routine including specific gravity 2. Screen for inborn errors of metabolism 3. Toxicology screen 4. Catecholamines G. Neuropsychological tests 1. Developmental 2. IQ 3. Achievement 4. Neuropsychological battery 5. Personality profile H. Biopsy 1. Muscle 2. Nerve 3. Skin 4. Brain I. Microbiologic tests 1. Serologic 2. Culture of microorganisms 3. Immunologic screen, T and B cells, blood titers, γ-globulin 4. Polymerase chain reaction J. Genetic tests 1. Karyotype 2. Genotype |
ABBREVIATIONS: CSF = cerebrospinal fluid; CT = computed tomography; CTA = Cranial computed tomography angiography; LP = lumbar puncture; MRA = magnetic resonance angiography; MRI = magnetic resonance imaging; PET = positron emission tomography; SPECT = single-photon emission computed tomography. |
II. THE CEREBROSPINAL FLUID EXAMINATION
A. Location, origin, and circulation of the cerebrospinal fluid
1. Examination of the cerebrospinal fluid (CSF) dates to Heinrich Iraneous Quincke who introduced spinal puncture in 1891 for the treatment of hydrocephalus.
2. Location of the CSF: The CSF occupies the ventricles and subarachnoid spaces. The total volume of CSF is about 150 mL. The subarachnoid space separates the arachnoid membrane from the pia mater. In addition to the CSF, the subarachnoid space contains the blood vessels that enter or leave the central nervous system (CNS).
3. Formation of the CSF
a. The CSF is formed by:
i. The choroid plexuses of the lateral, III, and IV ventricles.
ii. Water produced by oxidative metabolism.
iii. An ultrafiltrate through the blood–brain barrier of the cerebral capillaries (Fishman, 1992; May et al, 1990).
b. The rate of production is 0.3 to 0.4 mL/min, or about 500 mL/day.
4. Circulation of the CSF
a. The CSF exits the ventricles by flowing out of the foramina into the IV ventricle, the Lateral foramina of Luschka and the Median foramen of Magendie. The ependyma lining the ventricles absorb some CSF (Rando and Fishman, 1992). Learn Fig. 13-1.
b. After exiting from the foramina of the IV ventricle, the CSF enters the subarachnoid space. It may then percolate downward around the spinal cord or upward over the cerebral hemispheres to the superior sagittal sinus.
i. Pacchionian granulations that extend from the subarachnoid space into the lumen of the sinus allow absorption of the CSF into the venous blood (Fig 13-2).
ii. At the spinal level, drainage takes place at the root sleeves, where the pia and arachnoid fuse with the connective tissue of the spinal nerves.
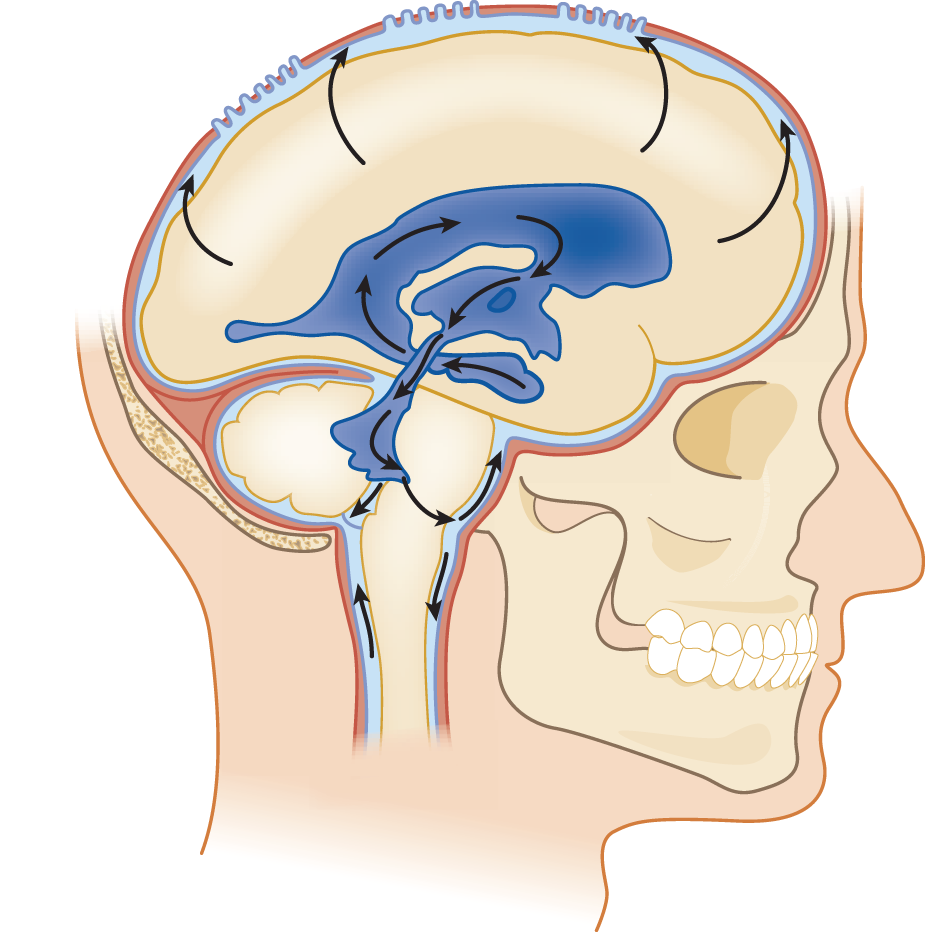
FIGURE 13-1. Lateral view of the ventricular system and CSF circulation. Beginning in the temporal horn, trace a drop of CSF through the III ventricle, aqueduct, out the IV ventricle, into the subarachnoid space, and up over the convexity of the hemisphere to the Pacchionian granulations along the superior sagittal sinus. CSF = cerebrospinal fluid.
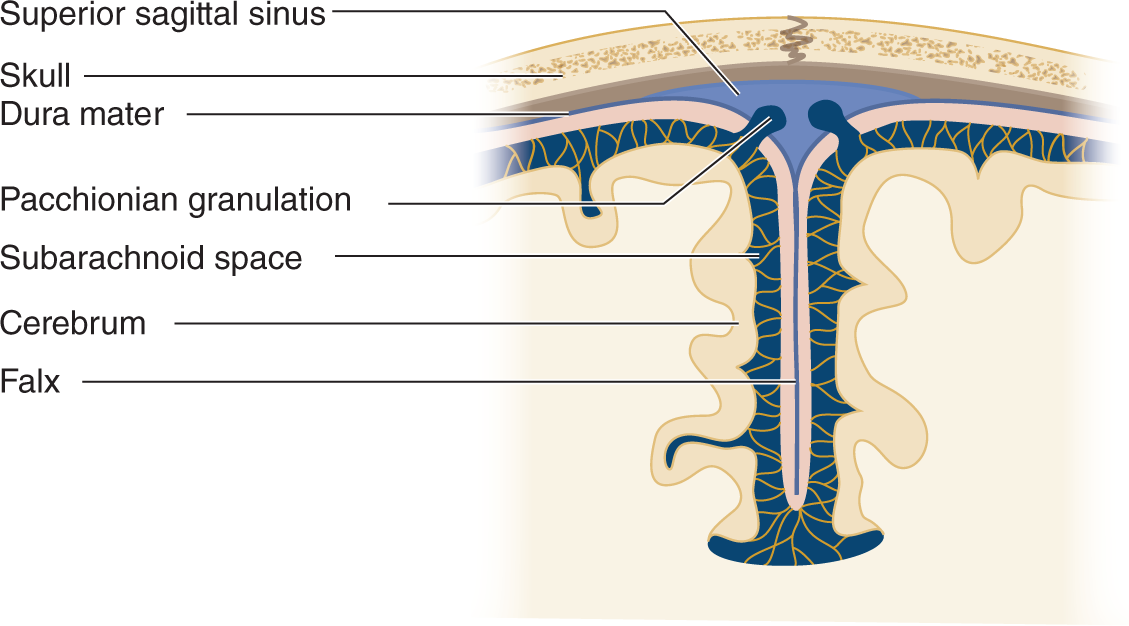
FIGURE 13-2. Coronal section through the cerebral falx to show the Pacchionian granulations projecting directly into the superior sagittal sinus. The CSF passes through the granulations into the venous blood of the sinus. CSF = cerebrospinal fluid.
5. Trace a drop of CSF from the temporal horn of the lateral ventricle to its absorption into the blood.
_________
_________
B. Functions of the cerebrospinal fluid
1. The CSF in the subarachnoid space provides a flotation layer around the brain and spinal cord that cushions them from trauma.
2. The CSF permits circulation of metabolites and electrolytes, neurotransmitters, peptide hormones, antibodies, leukocytes, and various other normal and abnormal cells.
3. The CSF aids in regulating the pH and electrolyte balance of the extracellular space of the CNS.
C. Composition of the cerebrospinal fluid
The CSF is a sparkling clear salt solution containing a few white blood cells (WBCs), proteins, sugar, traces of enzymes, neurohumors, neurotransmitters, and other metabolites (Fishman, 1992). The cell count, sugar, and protein levels change with age (see the top rows of Table 13-2 and Fig. 13-3) (Video 13-1).
TABLE 13-2 • Typical Cerebrospinal Fluid Profiles in Normal Individuals and in Various Diseases

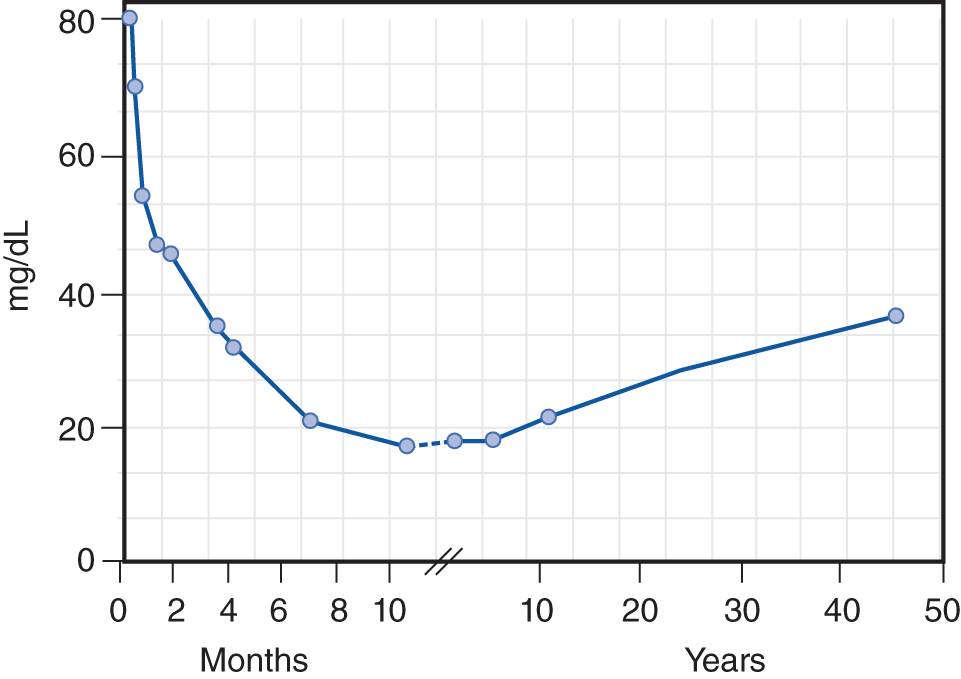
FIGURE 13-3. Age-related changes in the average total protein content of the cerebrospinal fluid. (Reproduced with permission from Widell S. On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo-encephalitis. Acta Paediatr. 1958 November;47(6):711–713.)

Video 13-1. Patient with HIV and aseptic meningitis attributed to immune reconstitution inflammatory syndrome (IRIS).
D. Normal pressure of the cerebrospinal fluid
Measure the pressure of the CSF by attaching a manometer to a needle inserted into the subarachnoid space (Fig. 13-4A). The normal CSF pressure depends on the patient’s (Pt’s) age (Table 13-2): 10 to 100 mm of water for young infants, 80 to 180 mm of water for normal mature individuals, and up to 250 mm of water for grossly obese individuals, perhaps because of increased intra-abdominal pressure (Whiteley et al, 2006). Diseases may increase or decrease intracranial pressure. Increased intracranial pressure is by far the most common problem.
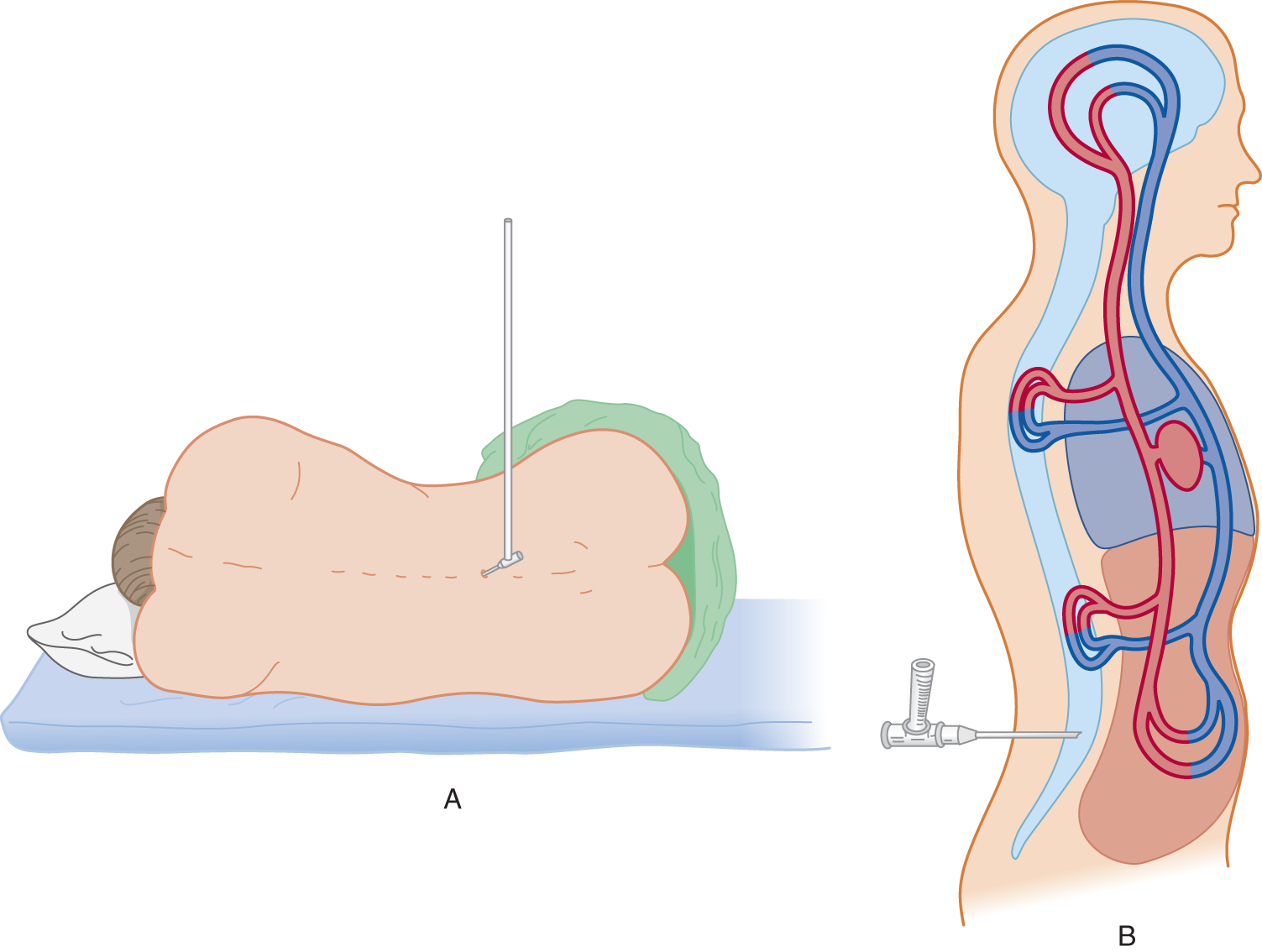
FIGURE 13-4. (A) Patient in left lateral position with legs and back flexed. A manometer is attached to a needle inserted into the subarachnoid space. (B) Top view of patient diagrammatically representing the continuity of vessels inside and outside the craniovertebral cavity.
E. Increased intracranial pressure
1. Symptoms of increased intracranial pressure: Symptoms consist of headaches, nausea and vomiting, dizziness, transient blurring of vision or blindness (transient obscurations of vision), and mild obtundation. Mothers of children who have shunts to treat increased pressure often can tell by overall changes in the child’s behavior that the shunt is blocked or malfunctioning.
2. Signs of increased intracranial pressure
a. Increased pressure in infants causes a bulging fontanel, split sutures, and an increasing occipitofrontal circumference that moves successively upward on the percentile lines of an occipitofrontal circumference chart (Fig. 1-23).
b. In older Pts, papilledema occurs and often a VI nerve palsy develops as a consequence of compression of the nerve during its long intracranial course.
3. Causes of increased intracranial pressure
a. Expanding lesions within the craniovertebral space, such as hematomas, neoplasms, abscesses, and brain edema. Rarely, increased production of CSF may cause increased pressure.
b. Prolonged status epilepticus or hypoxia, which causes brain edema.
c. Metabolic encephalopathies: Hepatic, uremic, Reye syndrome, idiopathic intracranial hypertension (pseudotumor cerebri), and endocrinopathies.
d. CNS infections: Meningitis and encephalitis may incite extreme edema and increased pressure.
e. Obstructive lesions that impede the flow of CSF from the ventricles to the subarachnoid space and through the Pacchionian granulations. The most common sites of blockage are
i. The interventricular foramen of Monro, usually by a neoplasm.
ii. The cerebral aqueduct, usually by congenital atresia or stenosis, inflammatory adhesions, or neoplastic compression.
iii. The IV ventricle and its outlets, usually by posterior fossa neoplasms, inflammatory adhesions or failure of outlets to perforate, as in the Dandy–Walker malformation.
iv. The subarachnoid space, usually by adhesions after meningitis or subarachnoid hemorrhage.
v. The Pacchionian granulations, because of clogging of the granulations (and root sleeves) by blood or extremely high CSF protein.
vi. The intracranial venous sinuses, because of thrombosis (Video 13-2 CVT).

Video 13-2. Patient with advanced bilateral papilledema due to superior sagittal sinus venous thrombosis.
4. Increased intracranial pressure and Pascal law
a. Physically, the CSF is essentially water and the CNS is about 80% water. Students who have handled only the stiff, formalin-fixed brain of the cadaver do not appreciate the supple softness of the living brain. Its compliancy resembles a foam sponge pillow or a balloon (the pia-arachnoid) filled with molasses. Therefore, a physicist, in studying intracranial hydrodynamics, might represent the CNS and the CSF as a single homogeneous fluid. To duplicate biologic conditions, the combined CNS–CSF model includes the vascular space within the craniovertebral space, as shown in Fig. 13-4B.
b. The CNS–CSF in the craniovertebral space is itself incompressible, like any fluid. According to Pascal law, pressure exerted on a fluid in a closed container is transmitted equally in all directions. The pressure transmission is independent of the size or shape of the container. Therefore, pressure in the lumbar CSF reflects the intracranial pressure, although biological factors alter the simple expression of Pascal law.
c. As intracranial pressure increases, it displaces CSF and the intravascular blood (Fig. 12-9). If these compensations fail, any further increase in pressure causes the brain to herniate down through the foramen magnum, the only place it can go, the only way out of the brain case (Chapter 12).
F. Low cerebrospinal fluid (CSF) volume (pressure) syndrome
1. Symptoms and signs: The Pt experiences orthostatic headache, vertigo, tinnitus, nausea, and vomiting, especially when rising from a reclining to a vertical position, and may faint. The symptoms could occur because of loss of flotation, allowing the cerebrum to sag onto the brainstem. A variety of connective tissue disorders have been associated with the development of this syndrome (Schievink, 2006).
2. Causes of low CSF pressure
a. Medical procedures that pierce the meninges and establish a CSF leak include lumbar puncture and neurosurgical operations.
b. Basal skull fractures that create fistulae in the nose or middle ear, causing CSF rhinorrhea or otorrhea (Video 13-3).
c. Severe dehydration.
d. Leakage along nerve roots (Rando and Fishman, 1992).
e. Idiopathic aliquorrhea (Rando and Fishman, 1992).

Video 13-3. Cranial fossa fracture in a patient with bilateral raccoon’s eyes and a right Battle sign describing CSF otorrhea. The patient also exhibits asterixis of the upper extremities. CT also demonstrates pneumocephalus.
G. Indications for lumbar puncture (spinal puncture or spinal tap)
1. To identify infections of the CSF. Repeated lumbar punctures serve to monitor the effect of treatment.
2. To identify subarachnoid bleeding.
3. To identify neoplastic invasion or seeding of the subarachnoid space by gliomas, carcinomas, or leukemias and lymphomas.
4. To measure and fractionate CSF proteins in suspected immunologic diseases, especially multiple sclerosis, and for the differential diagnosis of some neuropathies, such as Guillain–Barré syndrome (albuminocytologic dissociation).
5. To measure pH, electrolytes, enzymes, neurotransmitters, and trace constituents in the diagnosis of the genetic/metabolic encephalopathies (Hyland and Arnold, 1999).
6. To introduce chemotherapeutic or antibacterial agents or anesthetics.
7. To introduce contrast agents for myelography or radionuclides for study of CSF flow dynamics and to locate leaks.
8. To measure for increased CSF in patients with possible pseudotumor cerebri (idiopathic intracranial hypertension). In such cases, imaging studies have excluded a mass lesion that would cause herniation. An LP is also required to document low CSF pressure in low-pressure syndromes.
H. Contraindications to a lumbar puncture
1. Infection of the lumbar skin or deeper tissues through which the needle must pass.
2. Coagulopathies: Spinal subarachnoid hemorrhage or spinal subdural hematomas may follow an LP in these Pts (Masdeu et al, 1979). Diseases, such as hemophilia and thrombocytopenia, or anticoagulant therapy represent relative rather than absolute contraindications.
3. Cervical cord lesions: Removal of CSF from the lumbar region may cause the cord to shift against the lesion, resulting in quadriplegia, apnea, and death.
4. Increased intracranial pressure from a suspected or known intracranial mass lesion.
a. If the history, NE, or ophthalmoscopic examination suggest a mass lesion or increased intracranial pressure, an LP generally should not be done. The presence of distinct venous pulsations virtually excludes increased intracranial pressure. Nearly 90% of normal persons show pulsations if both eyes are carefully examined (Levin, 1978). The absence of venous pulsations does not establish increased pressure because some normal Pts do not show such pulsations.
b. The most common mass lesions that cause brain herniation include neoplasms, hematomas, abscesses, cerebral edema, and massive cerebral or cerebellar hemispheric infarction or hemorrhage. Of 22 Pts with brain abscess, five showed evidence of herniation within 2 hours of an LP (Samson and Clark, 1973). Do not perform LPs in Pts with brain abscess. The diagnosis depends on radiographic imaging findings.
c. Posterior fossa lesions, which may cause transforaminal herniation, also pose a great threat. Signs of impending transforaminal herniation include a stiff neck, hiccups, irregular breathing, apnea, hypotension, and quadriparesis (Hartmann et al, 1994). Note also that posterior fossa masses may cause upward herniation out of the posterior fossa, thereby compressing the midbrain. If the clinical findings raise any question of a mass lesion that may cause transtentorial or transforaminal or upward herniation, always order head computed tomography (CT) or, better, brain magnetic resonance imaging (MRI) before doing an LP. The radiographic examination may clinch the diagnosis and render the LP useless and potentially harmful (Hasbun et al, 2001).
d. Sometimes clinical exigencies will call for an LP even in the presence of increased pressure, to identify or exclude a specifically treatable disorder. Conditions with possible increased pressure that may require an LP include suspected meningitis or encephalitis and idiopathic intracranial hypertension. Then the Ex has to weigh the indications against the contraindications (Fishman, 1992). In these cases, consider pretreating the Pt with mannitol and hyperventilation to reduce intracranial pressure before the LP.
e. In the management of increased pressure from acute head injuries and other emergencies and coma, neurosurgeons implant a pressure transducer within the skull for continuous monitoring of the results of treatment.
I. Complications of a lumbar puncture
1. Transtentorial or transforaminal herniation of the brain, causing death. Typically, this occurs within 2 hours of the LP.
2. Back pain at the puncture site.
3. Bleeding: Epidural, subdural, or subarachnoid; a so-called bloody tap (Masdeu et al, 1979). Spinal epidural hematoma is a rare and devastating condition which may be due to trauma, surgery, epidural catheterization, coagulation disorders, anticoagulation therapy, arteriovenous malformations, cavernous malformations, and Paget disease. Thrombocytopenia or other bleeding diathesis or systemic heparin or warfarin administration increase the risk of puncture-induced spinal canal hemorrhage (epidural, subdural or subarachnoid). Spinal epidural hematoma or spinal hematoma after neuraxial anesthesia is a rare complication with anticoagulation therapy (Wysowski et al, 1998). A spinal epidural hematoma may produce progressive radicular pain and sphincter disturbances, and represents a neurological emergency requiring urgent investigation and treatment. Most neurologic sequelae seem to relate to inadvertent insertion of the needle at too high a level (Hamandi et al, 2002). Thus, strict application of guidelines for performing spinal procedures in anticoagulated patient is recommended (Horlocker et al, 2003)
4. Headache and post-LP low CSF volume (pressure) syndrome: See Section F. Most Pts have some backache. Up to 32% (wide range of reported frequency) have headache after an LP (Armon and Evans, 2005). The use of atraumatic needles has reduced the incidence of post LP headache to about 3% (Lavi et al, 2006). Low CSF pressure from CSF leakage presumably causes post-LP headache.
a. The headache begins a day or two after the LP, is often throbbing, mainly occurs in the upright position, is worsened by coughing or straining, and improves when the Pt reclines. The Pt may have a stiff neck, nausea and vomiting (Kuntz et al, 1992).
b. Most post-LP headaches resolve spontaneously by approximately 10 to 14 days. Bed rest (none, 2-8 hours, or up to 48 hours), forcing fluids, or intravenous caffeine are of questionable benefit in helping to prevent post-LP headaches (Roos, 2003a).
c. Refractory headaches respond to an epidural injection of 15 to 20 mL of autologous blood at the puncture site (Sencakova et al, 2001), presumably because it seals the puncture wound (Boonmak and Boonmak, 2010).
5. Double vision, which usually results from unilateral or bilateral cranial nerve VI palsies is usually self-limited.
6. Iatrogenic infection: very rare.
7. Implantation of epidermoid tumors in the lumbosacral canal: a very rare complication (Park et al, 2003).
8. In summary, the three most dangerous circumstances for an LP are increased intracranial pressure, a mass lesion capable of producing herniation, and a high cervical cord lesion.
J. Preparation for a lumbar puncture
1. Always get an MRI or CT scan whenever the Pt has the new onset of overt neurologic signs and symptoms, an altered consciousness, or seizures (Hasbun et al, 2001).
2. Always get an MRI if the Pt might have a compressive spinal cord lesion.
3. Ensure that the radiographs are competently read, particularly with respect to ventricular size, presence of absence of sulci and cisterns, and the absence of a posterior fossa lesion. MRI is usually superior to CT, except for emergency screening for large mass lesions, edema, or recent bleeding.
4. Review the history and medical record for a bleeding tendency. The platelet count should exceed 50,000 and the international normalized ratio (INR) should be less than 1.2. While preprocedural administration of aspirin therapy has not been associated with a high risk of bleeding with LP or other spinal anesthetic interventions, data on clopidogrel are lacking (Horlocker et al, 1995; Doherty and Forbes, 2014).
5. Decide whether to send a simultaneous blood sample for comparison with the CSF with respect to chemistry and rising antibody titers.
6. Psychological preparation of the Pt for an LP: Everyone dreads needles, particularly a stab in the back. Every layperson, it seems, knows of someone who had one of “those taps” and afterward had a permanent backache or never walked again. Of course, that conclusion has reversed cause and effect: the LP was done because of the disability, but litigious Pts today blame the doctor, not the disease. The Ex must ensure that the indications are valid and that the Pt understands the reasons for the procedure. Because an LP counts as surgery, have the Pt sign a consent form listing complications such as backache, headache, infection, and bleeding.
K. Technique for lumbar puncture
1. Positioning of the Pt for an LP
a. The Pt assumes the lateral recumbent position with the head, spine, and extremities flexed (the fetal position; Fig. 13-4A). A pillow under the head keeps it aligned with the spine. In the trunk-flexed position, the distance between the dorsal processes and lamina of adjacent vertebrae  increases/
increases/ decreases. (
decreases. ( increases) (Spinal flexion thus increases the target area for the needle. Review Fig. 12-24 if you erred.)
increases) (Spinal flexion thus increases the target area for the needle. Review Fig. 12-24 if you erred.)
b. Cooperative and not acutely ill Pts may sit with the spine flexed. However, in this position, the measurement of the intraspinal pressure is unreliable. If the pressure reading is important (eg, in a patient with possible pseudotumor cerebri), the lateral recumbent position is preferred.
2. Needle insertion and manometry for measuring CSF pressure
a. Clean the lower lumbar area with Betadine or 70% alcohol and apply a sterile drape.
b. Select the vertebral interspace, between L3 and L4, between L4 and L5, or between L5 and S1, by palpation or by lining up the vertebral interspace with the top of the iliac crests, which is at the interspace between L3 and L4. Insertion of the needle rostral to that level may damage the distal tip of the spinal cord, which usually ends at L2 (Fishman, 1992).
c. Depending on the Pt, anesthetize the insertion point with 1% lidocaine.
d. Insert a 20- to 22-gauge needle, with its stylet in place, into the interspace between the dorsal processes of the vertebrae at the selected level. Angle the needle slightly cephalad to parallel the slant of the dorsal spines of the vertebrae (Fig. 12-24). Insert the needle until a slight “pop” is felt as the needle pierces the dura and enters the subarachnoid space. The larger the bore of the needle, the easier it is to reach the subarachnoid space, but the greater the leakage of fluid after withdrawal of the needle.
i. Insert the needle with the bevel of the needle turned parallel to the long axis of the spine (Roos, 2003b). The bevel then is thought to more cleanly separate rather than transect the longitudinal fibers of the dura, thus reducing post-LP leakage of CSF. A problem with the theory is that dural fibers run in a variety of directions, not just longitudinally.
ii. To reduce post-LP leakage and post-LP headaches, Whitacre or Sprotte dull-tipped needles have been advocated to replace the standard sharp-tipped Quincke needle (Strupp et al, 2001). These needles have a blunt tip alleged to separate rather than cut the dural fibers. The needles drain CSF through an oval opening on the lateral aspect of the shaft, just proximal to the blunt tip, rather than through the tip itself (Evans et al, 2000).
iii. Before insertion of a blunt-tipped needle, the Ex uses a sharp-tipped “introducer” needle to cut a path about two-thirds of the way in.
e. After feeling the initial pop, the Ex withdraws the stylet. A drop of CSF should appear at the hub of the three-way stopcock on the LP needle. Attach a manometer and allow the fluid level to stabilize in the manometer. Record the opening pressure. At the end of the procedure, record the closing pressure.
f. If the history or ophthalmoscopic examination raises a concern about increased pressure, proceed this way. Insert a needle through the skin but stop it just short of the subarachnoid space. Remove the stylet and attach a manometer. With the manometer in place and with a fingertip blocking the open bore at the top of the manometer, advance the needle into the subarachnoid space and allow the fluid to fill the manometer gradually. Otherwise, the Ex may insert the needle with its regular stylet in place, withdraw it, and quickly attach a three-way stopcock and manometer.
g. For premature and young infants, use a standard butterfly needle (Greensher et al, 1971). The Ex should limit the depth of any needle puncture to 2.5 cm in these infants.
3. Fluctuations in the meniscus in the manometer
a. The meniscus of CSF in the manometer will fluctuate around some mean value, for example, 120 mm of water (Fig. 13-5).
b. Causes for fluctuations: As shown in Fig. 13-4B, the intracranial and intraspinal veins communicate directly with the extracranial and extravertebral veins. Because these communications lack valves, the extracranial veins transmit any change in their pressure directly into the craniovertebral space, in particular the changes in intrathoracic and intra-abdominal pressure (Whiteley et al, 2006). When a person inhales air, the CNS–CSF pressure  increases/
increases/ decreases/
decreases/ does not change. (
does not change. ( decreases) (The intrathoracic pressure decreases with inspiration. Inspiration sucks blood from the CNS veins and the CNS-CSF pressure drops. Breathing causes rhythmic excursions of the manometer meniscus at the rate of 16 per minute, as shown in Fig. 13-5.)
decreases) (The intrathoracic pressure decreases with inspiration. Inspiration sucks blood from the CNS veins and the CNS-CSF pressure drops. Breathing causes rhythmic excursions of the manometer meniscus at the rate of 16 per minute, as shown in Fig. 13-5.)
c. Contraction of the abdominal muscles would  increase/
increase/ decrease the intracranial pressure. Explain.
decrease the intracranial pressure. Explain.
_________
_________ increase) (The intervertebral veins reflect the increased intra-abdominal pressure backward into the craniovertebral space, thus reducing the outflow of blood from the CNS. Because the arterial input continues, the CNS–CSF pressure increases.)
increase) (The intervertebral veins reflect the increased intra-abdominal pressure backward into the craniovertebral space, thus reducing the outflow of blood from the CNS. Because the arterial input continues, the CNS–CSF pressure increases.)
d. The pulse causes smaller excursions of the meniscus (not shown in Fig. 13-5 because of their small amplitude). Because the arterial walls absorb much of the arterial pressure, the CNS–CSF pressure most closely reflects the capillary and venous pressures.
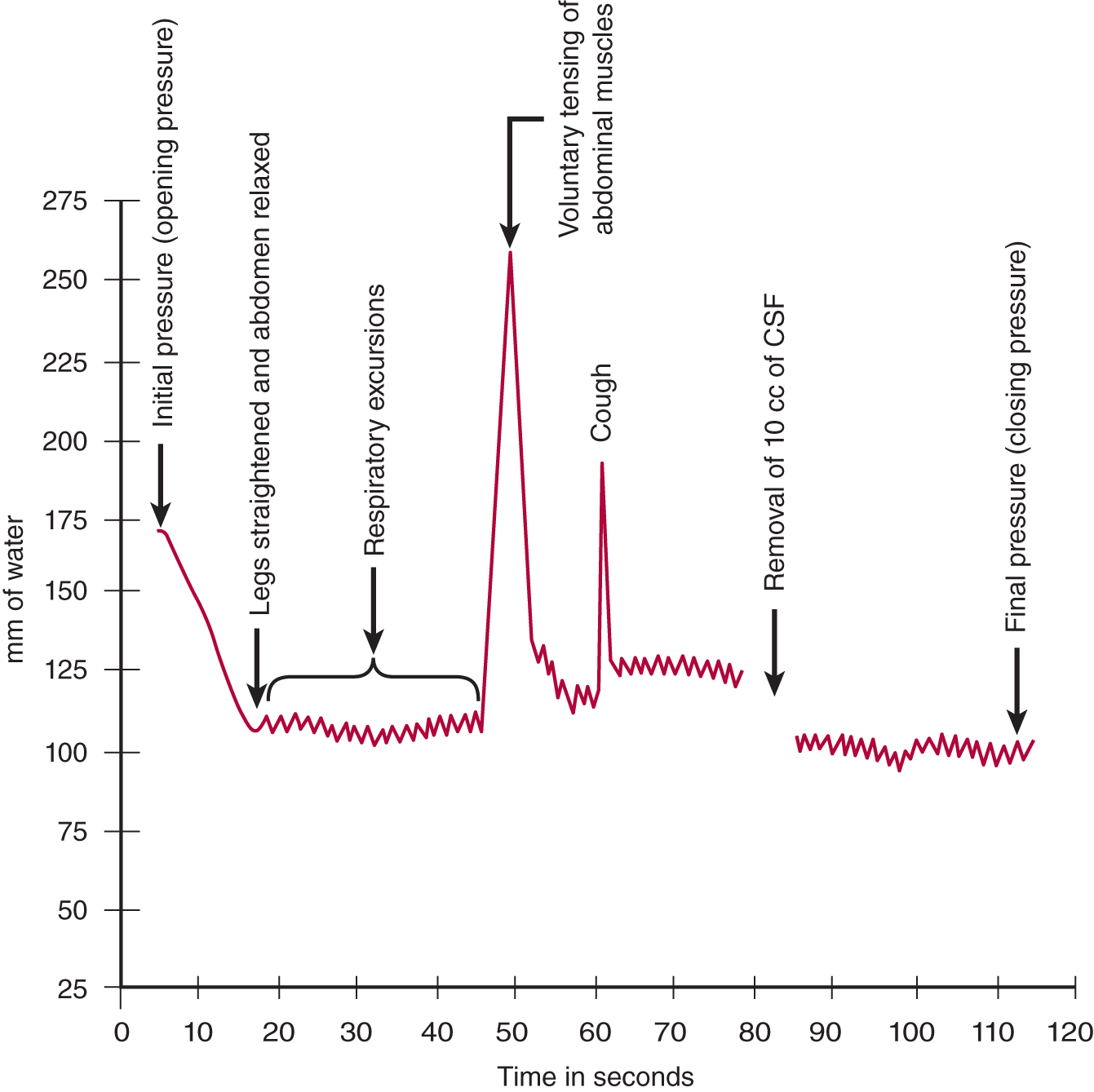
FIGURE 13-5. Graph of cerebrospinal fluid pressure, as measured by manometry. Starting at the left, read through the legends just above the arrows.
L. Clinical evaluation of increased pressure as registered by manometry
1. Assume that the opening pressure in the manometer is 240 mm of water, which is  normal/
normal/ low/
low/ high. (
high. ( high)
high)
2. Two explanations exist:
a. The intrinsic CNS–CSF pressure is too high.
b. The pressure reflects factors extrinsic to the craniovertebral space.
3. An LP always makes the Pt anxious and causes increased tension in the skeletal muscles. What, then, is a simple extrinsic cause for increased CNS-CSF pressure? _________
4. Encourage the Pt to relax the flexed position and to take a few deep breaths. In the Pt with the opening pressure of 240 mm of water, these maneuvers dropped the pressure to 210 mm of water. This value is  normal/
normal/ low/
low/ high. (
high. ( high)
high)
5. The flexed posture has caused the Pt to flex the head on the chest, and it may have bent somewhat to the side while resting on the pillow. Because flexion or turning of the head may compress the jugular veins, the Ex straightened the Pt’s head and readjusted it on the pillow. These maneuvers failed to drop the pressure below 200 mm of water, indicating that the Pt had slightly increased intrinsic intracranial pressure.
6. If an expanding intracranial lesion raises the CNS–CSF pressure, visualize what might happen after removal of CSF from the lumbar region (Fig. 13-6).
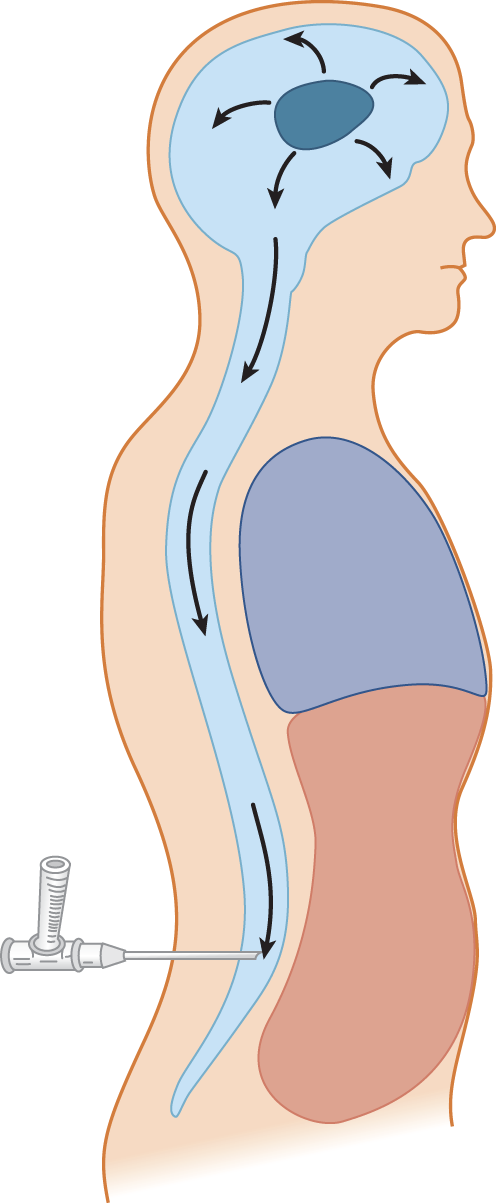
FIGURE 13-6. Depiction of an expanding intracranial lesion (oval black mass) causing increased intracranial pressure. The pressure exerts itself equally in all directions. Escape of cerebrospinal fluid through a lumbar needle allows the intracranial contents to flow (herniate) toward the region of lowered pressure.
7. Increased intracranial pressure may have caused impending herniation of the brain. The system may be delicately balanced, with the uncus and parahippocampal gyrus poised ready to plunge over the edge of the tentorium, or the cerebellar tonsils may be ready to herniate through the foramen magnum. These two potentially fatal herniations are called _________
8. Withdrawal of fluid from the lumbar region will allow the brain to flow down toward the point of low pressure, according to Pascal law. The potential herniation is converted to actual herniation. The Pt will _________
9. If, after careful measurement, the Ex concludes that the Pt truly has intrinsic increased intracranial pressure, what is the next step?  Withdraw fluid rapidly./
Withdraw fluid rapidly./ Withdraw fluid cautiously./
Withdraw fluid cautiously./ Withdraw the needle immediately! (
Withdraw the needle immediately! ( Withdraw the needle immediately!) (The small amount of fluid in the manometer suffices for a cell count or to see blood in the CSF, which is often the most important information needed.)
Withdraw the needle immediately!) (The small amount of fluid in the manometer suffices for a cell count or to see blood in the CSF, which is often the most important information needed.)
10. Describe the safe maneuvers used to exclude extrinsic factors as the cause for high spinal fluid pressure._________
M. Cessation of cerebrospinal fluid flow
1. Frequently, the flow of fluid starts but stops. Very rarely, transforaminal herniation, like a plug in a drain, blocks the transmission of intracranial pressure after a small amount of CSF has drained out. In this case, the Pt shows dramatic signs of apnea and quadriplegia. Most commonly, the cause is far more benign: Something has blocked the needle (Table 13-3).
TABLE 13-3 • Common Causes for Cessation of Cerebrospinal Fluid Flow and Their Remedies
Cause |
Remedy |
Blood clot in the needle lumen |
Replace stylet to ream out the needle |
Nerve root has fallen over the bevel of the needle |
Rotate the shaft of the needle |
Displacement of the tip of the needle from the subarachnoid space or incomplete penetration |
If you think the needle tip is too deep, withdraw it slightly; if too shallow, insert it farther |
2. After trying the maneuvers in Table 13-3, reattach the manometer to measure the pressure again. If the system is open from the subarachnoid space through the needle to the manometer, the manometer meniscus should show excursions at the rate of 16 per minute, caused by _________
3. The CSF may also stop flowing through the needle if a lesion occludes the vertebral canal.
4. What not to do if CSF stops flowing through the needle: Exasperation may tempt the Ex to try to suck CSF out with a syringe. If at this point you do not understand the dangers, this text has totally failed.
N. Collection and appearance of the cerebrospinal fluid
1. After measuring the opening pressure, collect 10 to 15 mL of CSF by allowing several milliliters to drip into each of three or four tubes. The normal fluid appears sparkling clear in all tubes. Inspect all tubes for cloudiness, redness (erythrochromia), and yellowness (xanthochromia). The attending physician, not the laboratory technician, bears the responsibility for proper inspection of CSF after collection.
2. To end the LP, measure the closing pressure and replace the stylet before withdrawing the needle. Replacing the stylet is thought to keep arachnoid strands from being sucked through the dural puncture site, which might prolong CSF leakage.
O. Cloudiness of the cerebrospinal fluid
1. Cloudiness usually means an increased number of WBCs in the CSF. Rarely, numerous bacteria cause cloudiness. Normally, the CSF contains five or fewer WBCs per mm3. More than 300 polymorphonuclear WBCs/mm3 or more than 400 to 500/mm3 of lymphocytes or monocytes will cause detectable cloudiness, if the Ex inspects the CSF properly. Obvious cloudiness occurs with counts of 600 to 800 WBCs/mm3.
2. To detect minimal cloudiness with counts in the range of several hundred WBCs per cubic millimeter, do the following:
a. Obtain an exact duplicate of the tube used to collect the CSF and add the same amount of water as is in the CSF tube. The duplicate tube must have the same translucency, color, and refractive index as the CSF tube.
b. Hold the duplicate and CSF tubes side-by-side against a white sheet of paper, against a dark background, and then against a light source. Use daylight, if at all possible. Compare the CSF with the water by looking through the sides of the tubes, as in using a colorimeter. The direct, side-by-side comparison enables the Ex to detect very subtle cloudiness or color changes.
c. In bright sunlight, even mild CSF pleocytosis with WBCs or red blood cells (RBCs) can cause the Tyndall effect, a snowy iridescence.
d. Students may suppose that this is all unnecessary because the CSF goes to the laboratory for the “official” examination and cell count anyway, so why bother? Well, the careful physician inspects the CSF personally and does the cell count on the spot, before the cells have undergone the autolysis that commences in an hour, and to gain the information quickly and reliably. In the second place, personal inspection checks the accuracy of the laboratory, a check that unfortunately is necessary. If the laboratory reports 20 WBCs/mm3 after the Ex has seen a cloudy fluid, the laboratory must be wrong. If the Pt has meningitis, you must avoid an error on this critical point.
P. Bloody taps, erythrochromia, and xanthochromia
1. RBCs get into the CSF at one of two times in relation to the tap:
a. From preexisting bleeding caused by a ruptured blood vessel or other CNS lesion.
b. From inadvertent bleeding caused by the needle puncture, a “traumatic” or “bloody tap.”
2. Frank bleeding causes a red CSF, called erythrochromia. It takes an RBC count of 100 to 300/mL to cause erythrochromia. Normally the RBC count in the CSF is _______. (zero)
3. In contrast to erythrochromia, a yellowish CSF is called xanthochromia.
4. Differentiation of preexisting bleeding from a bloody tap: A traumatic tap is generally inconsequential, whereas preexisting bleeding may foretell a life-threatening lesion. Three tests distinguish the two sources of RBCs, the four-tube test, centrifugation for xanthochromia, and cytologic demonstration of erythrophagocytosis by monocytes.
a. The four-tube test: Compare the redness of the successive tubes. Blood that enters the CSF before the tap will have mixed freely with the CSF. All tubes display the same color and will have the same cell count. Blood from a traumatic tap tends to clear in successive tubes.
b. Centrifugation for xanthochromia: If the tubes are uniform in color, centrifuge one to deposit the RBCs on the bottom and compare the supernatant fluid with a control tube, as described above. If the CSF is completely colorless, the RBCs entered the CSF recently, either less than 2 to 4 hours ago or from the tap itself. Discoloration of the supernatant fluid means that the RBCs entered more than 2 to 4 hours before and have undergone lysis. Free hemoglobin in solution causes the xanthochromia. If the bleeding occurred days before the tap, degradation products of hemoglobin, methemoglobin, and bilirubin cause the color. Because RBCs undergo crenation in the CSF, crenation does not signify preexisting blood.
c. Erythrophagocytosis: Cytologic examination proves preexisting bleeding by demonstrating phagocytosed RBCs in macrophages, but it takes many hours to days to develop.
5. Correction of the WBC for blood contamination: Subtract 1 WBC/mm3 for each 700 RBCs/mm3. The remainder approximates the true CSF WBC count.
Q. Cerebrospinal fluid xanthochromia
1. Xanthochromia means a yellowish discoloration of the CSF detected by controlled comparison of the CSF sample with water. The most common causes are free hemoglobin, oxyhemoglobin, methemoglobin, bilirubin, and high protein (usually >200 mg/dL). Less likely causes of xanthochromia include jaundice secondary to liver disease or hemolytic disease in the newborn, diet high in carotenes, and rifampin therapy (Fishman, 1992). Distinguish the possibilities this way:
a. Xanthochromia from very high protein will clot quickly when the CSF stands in the tube (Froin syndrome). With protein values above 150 mg/mL, CSF may look slightly yellow because of albumin-bound bilirubin that does not come from blood. Or if bleeding causes more than 150,000 RBCs/mL, the amount of serum may tinge the CSF.
b. Dip a hemoglobin test tape into the CSF to identify hemoglobin. An Ictotest reagent tablet identifies bilirubin. Neither reagent reacts if only protein is present.
2. The final arbiter is spectrophotometry, which can distinguish the successive degradation products of red cell lysis: oxyhemoglobin, methemoglobin, and bilirubin. Spectroscopy can be positive even with xanthochromia too faint for detection by the unaided eye.
R. Laboratory examination of the cerebrospinal fluid
1. The routine tests include cell count and cell identification, sugar, total protein, protein fractionation, and the Venereal Disease Research Laboratory (VDRL) test for syphilis. Chloride determination has little value with the routine specimen.
2. Successful exploitation of the CSF depends on careful planning. Molecular biology advances so rapidly that the Ex almost has to consult the literature for the best tests to apply to each new CSF sample. For example, the CSF level of hypocretin in narcolepsy (Mignot et al, 2002), the measurement of CSF total tau (T-tau), phosphorylated tau (P-tau) and the 42 amino acid form of β-amyloid (Aβ42) in Alzheimer disease (Andreasen et al, 2001; Riemenschneider et al, 2002; Blennow, 2004; Anoop et al, 2010), the identification of ferritin indicating activation of phagocytosis by microglia as an index of intracranial bleeding (Thompson, 1995), the identification of beta-2-transferrin protein to detect a CSF fistula in rhinorrhea, the increase in prolactin (Aydln et al, 2002) in CSF from seizures, the reduced levels of homovanillic acid, biopterin, and neopterin in dopa-responsive dystonia (Nygaard, 1993), and the levels of CSF iron, ferritin and transferrin in restless legs syndrome (Mizuno et al, 2005) now qualify as potential diagnostic tests. The vast array of possibilities places even more value on the clinical findings to guide the selection of tests for the particular Pt. For example, in patients with rapidly progressive dementias, for whom the diagnosis remains uncertain, the 14-3-3 protein assay in the CSF is highly sensitive and specific for the diagnosis of various forms of Creutzfeldt-Jakob disease (neurocognitive disorder due to prion disease). However, false positive results occur with a large range of central nervous system (CNS) inflammatory, vascular, metabolic and neoplastic disorders associated with rapid neuronal destruction (Muayqil et al, 2012) (Fig. 13-7).

FIGURE 13-7. Dendrogram showing methods for examining the cerebrospinal fluid (reference). CSF = cerebrospinal fluid; ELISA = enzyme-linked immunosorbent assay; IgG = immunoglobulin G; PCR = polymerase chain reaction; RBC = red blood cell; TB = tuberculosis; VDRL = Venereal Disease Research Laboratory test; WBC = white blood cell (Anoop et al, 2010; Blennow and Hampel, 2003; Leib et al, 1999).
3. Glucose content of CSF
a. The CSF glucose normally is about 66% of the blood glucose or higher in preterm or term infants. The CSF glucose concentration lags some hours behind changes in the blood glucose. In general, the Ex should determine the blood glucose at the time of the LP for comparison. A 50-mL bolus of 50% glucose IV will equilibrate with the CSF in 30 minutes to several hours. When feasible, do the LP in the morning to allow for overnight equilibration of blood and CSF levels.
b. High CSF glucose reflects high blood values.
c. Low CSF glucose implies diffuse meningeal disease, usually because of increased WBCs or neoplastic seeding. The usual causes include:
i. Acute bacterial meningitis, tuberculous meningitis, and fungal meningitis.
ii. Herpes simplex encephalitis (but not typically with most viral infections).
iii. Neoplastic seeding of the CSF with lymphoma or acute leukemias or carcinomatosis of the meninges.
iv. Systemic causes of hypoglycemia, including prolonged insulin shock.
v. Sometimes with subarachnoid hemorrhage.
vi. Idiopathic aglycorrachia (Fraser, 1991).
4. Identification of abnormal proteins in the CSF
a. The total protein is measured and the proteins are fractionated (Thompson, 1995). Abnormal immunoglobulins can be detected even without elevation of the total protein (Swaiman, 1999).
b. The normal amount of protein varies with the Pt’s age (Fig. 13-3). The total protein is lower in the ventricular and cisternal CSF than in the lumbar CSF.
c. Even for bloody CSF, the Ex can estimate the total CSF protein by subtracting 1 mg/dL of protein for each 1000 RBCs/mm3.
d. A striking albuminocytologic dissociation occurs in Guillain-Barré syndrome (GBS). The protein increases to several 100 mg/dL without corresponding pleocytosis, but often the increase in protein or the peak does not occur for days to weeks after the onset of the illness. The CSF below a block in the spinal canal also shows a strong albuminocytologic dissociation.
e. CSF findings in multiple sclerosis and other immunopathies: The CSF may display a mild pleocytosis. Cytologic examination reveals plasma cells and immunologically altered lymphocytes. The CSF contains increased γ-globulin, mainly immunoglobulin G (IgG), and an increased CSF IgG index. IgG is manufactured intrathecally but may enter through a disrupted blood–brain barrier or by bleeding into the CSF. Agarose electrophoresis demonstrates oligoclonal gammopathy as discrete bands, whereas normal CSF immunoglobulins migrate as a diffuse band. Isoelectric focusing shows heavy and light chains of immunoglobulins. Myelin basic protein is frequently increased in all demyelinating diseases. The clinical findings, CSF profile, and MRI findings together certify the diagnosis of multiple sclerosis (Swaimann, 1999).
5. Cytologic analysis and staining
a. Normal cell content of the CSF: The normal total CSF WBC counts are fewer than 20 WBCs/mm3 for preterm infants to neonates, fewer than 15 WBCs/mm3 at 4 to 8 weeks, and generally no more than 5 WBCs/mm3 in older infants, children, and adults. Newborns have about 60% polymorphonuclear cells, but in older individuals lymphocytes make up 75% of the total and monocytes 25% make up the rest. Normally CSF contains no polymorphonuclear leukocytes, eosinophiles, plasma cells, and RBCs, but it may rarely contain ependymal cells.
b. The most common causes of CSF pleocytosis are meningitis, encephalitis, and neoplastic seeding. Convulsions alone can cause a slight pleocytosis that may then cause confusion with encephalitis (Edwards et al, 1983). If the cell count exceeds 5 WBCs/mm3, the cells should be smeared on a slide and stained for organisms. To estimate the total WBC count to compensate for a bloody tap, subtract 1 WBC/mm3 for each 700 RBCs. Also compare the WBC count in the first and last of the four tubes collected. Decreasing amounts of blood should cause a decreased cell count in the last tube, if the blood comes from the tap. Preexisting blood is evenly mixed.
c. Staining: The cells in the CSF are concentrated by centrifugation or by sedimentation methods. Depending on the diseases suspected clinically, the cell stains include Gram, Papanicolaou, Romanowsky, and Wright Giemsa stains, the Diff-Quik methods, or India ink for fungi (Bigner, 1992).
d. Identification of neoplastic cells in the CSF: Neoplastic cells in the CSF arise from seeding by intrinsic gliomas, blood dyscrasias, or metastatic carcinoma. An immunohistochemistry panel aids in differentiating the various types of neoplastic cells (Bigner, 1992). In acute encephalitis, the activated lymphocytes require differentiation from neoplastic cells. The neoplastic cells of lymphomas and acute leukemia commonly invade the CSF, but chronic leukemias uncommonly do so. Immunophenotyping helps distinguish lymphomas, which are usually monoclonal B cells.
i. The most common carcinomas that seed the subarachnoid space arise from the breast, lung, and skin (melanoma).
ii. The common gliomas that seed the CSF are medulloblastoma, pineoblastoma, retinoblastoma, and neuroblastoma. The glial cells stain with antibodies to glial fibrillary acid protein.
S. Cerebrospinal profiles in acute bacterial meningitis and viral meningoencephalitis
1. CSF in acute bacterial meningitis: Polymorphonuclear pleocytosis with more than 100 to 500 WBCs/mm3, increased pressure, decreased glucose, and increased protein (Table 13-2). Brief pretreatment with antibiotics does not reduce the cell count but does reduce the demonstration of the organism by stain or culture.
2. CSF in viral meningitis: Lymphocytic pleocytosis, normal glucose, slightly to moderately increased protein, and increased pressure (Table 13-2). The most common causes are enteroviruses, herpes viruses, and human immunodeficiency virus (HIV; Johnson, 1998; Roos, 1997).
T. Identification of infectious agents in the cerebrospinal fluid
1. Molecular biology has greatly improved the diagnosis of meningitis and encephalitis. Previously, the diagnosis depended mainly on the Gram stain, India ink, culture, and antibody titers. These remain as staples, but the newer techniques prove particularly valuable when pretreatment with antibiotics prevent demonstration of microorganisms by stain or culture. Many serologic tests depend on matching serum and CSF titers and estimating the rate of production of antibodies and their passage through the blood–brain barrier (Roos, 1997). Newer techniques include:
a. The polymerase chain reaction (PCR) for nucleic acids of most common viruses and bacteria (Video 13-4).
b. Improved test batteries for virus-specific antibodies.
c. Counterimmune electrophoreses and latex particle agglutination tests for antigens of common bacterial meningitides such as Haemophilus influenzae type B, Neisseria meningitidis, Streptococcus pneumoniae and other streptococci, and Escherichia coli (screening panel for acute meningitides).
d. The limulus amebocyte lysate assay for Gram-negative endotoxins and Gram-negative meningitis.

Video 13-4. Young patient with viral meningitis due to Epstein-Barr Virus (EBV).
2. Herpes simplex: Identified by PCR (Kennedy and Chaudhuri, 2002). The detection of this virus, with its predilection for the temporal lobe, is extremely important because of the potential for treatment with acyclovir.
3. Enteroviruses: Identified by viral culture or reverse transcriptase PCR.
4. Arthropod borne viruses: The diagnosis depends on a fourfold or greater increase in virus-specific IgG between acute and convalescent sera or by identification of virus-specific IgM in CSF or serum.
5. HIV: Identified by PCR. HIV is recoverable from the CSF in all stages of infection, even before any clinical signs of acquired immunodeficiency syndrome and in the absence of virus recovery from the blood. The neurologic complications span the gamut: dementia, aseptic meningitis, myelopathy, polyneuropathy, secondary opportunistic meningitides, and vasculitis (Harrison and McArthur, 1995; Sharer, 1992).
6. Epstein-Barr (infectious mononucleosis): Quantitative PCR level for the virus (Weinberg et al, 2002) (Video 13-2).
7. Syphilis: Identified by a positive Venereal Disease Research Laboratory test which is highly specific but only 30% to 70% sensitive for neurosyphilis (Castro et al, 2008). The CSF fluorescent antibody absorption test (FTA-ABS) is more sensitive but not specific as false positives are common (Marra et al, 1995).
8. Cryptococcus: The most frequent fungal infection of the CSF is detected by India ink staining, culture, and latex agglutination tests.
9. Borrelia burgdorferi (Lyme disease): Detected by the Western blot test.
10. Tuberculous meningitis: Detected by staining, culture, PCR, and nucleic acid amplification (Pai et al, 2003).
U. Summary of the cerebrospinal fluid examination
1. Review Table 13-2 for the CSF changes in various disorders, but do not memorize the information.
2. Describe the differences in the pressure, cell count, and protein values during maturation from preterm infants to adults.
_________
_________
BIBLIOGRAPHY · The Cerebrospinal Fluid Examination
Andreasen N, Minthon L, Davidsson P, et al. Evaluation of CSF-tau and CSFA 42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001;58:373–379.
Anoop A, Singh PK, Jacob RS, Maji SK. CSF biomarkers for Alzheimer’s disease diagnosis. International Journal of Alzheimer’s Disease. 2010;1–12.
Armon C, Evans RW. Addendum to assessment: prevention of post-lumbar puncture headaches: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65(4)2:510–512.
Aydln GB, Köse G, De ğerliyurt A, et al. Prolactin levels in cerebrospinal fluid of patients with infantile spasms. Pediatr Neurol. 2002;27:267–270.
Bigner SH. Cerebrospinal fluid (CSF) cytology: current status and diagnostic applications. J Neuropathol Exp Neurol. 1992;51:235–245.
Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. The Lancet Neurology. 2003;2:605–613.
Blennow K. Cerebrospinal Fluid Protein Biomarkers for Alzheimer’s Disease. J Am Soc Exp NeuroTherapeutics. 2004;1:213–225.
Boonmak P, Boonmak S. Epidural blood patching for preventing and treating post-dural puncture headache. Cochrane Database Syst Rev. 2010;(i)Cd001791.
Castro R. Prieto ES, da Luz Martins Pereira F. Nontreponemal tests in the diagnosis of neurosyphilis: an evaluation of the Venereal Disease Research Laboratory (VDRL) and the Rapid Plasma Reagin (RPR) tests. J Clin Lab Anal. 2008;22(4):257–261.
Doherty CM, Forbes RB. Diagnostic lumbar puncture. Ulster Med J. 2014;83(2):93–102.
Edwards R, Schmidley GW, Simon RP. How often does a CSF pleocytosis follow generalized convulsions? Ann Neurol. 1983;13:460–461.
Evans RW, Armon C, Frohman EM, et al. Assessment: prevention of post-lumbar puncture headaches. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2000;55:909–914.
Fishman RA. Cerebrospinal Fluid in Diseases of the Nervous System. Philadelphia, PA: WB Saunders; 1992.
Fraser JL. Persistent lumbar aglycorrachia of unknown cause. Neurology. 1991;41:1323–1324.
Greensher J, Mofenson HC, Borofsky LG, et al. Lumbar puncture in the neonate: a simplified technique. J Pediatr. 1971;78:1034–1035.
Hamandi K, Mottershead J, Lewis T, et al. Irreversible damage to the spinal cord following spinal anesthesia. Neurology. 2002;59:624–626.
Harrison, MJ, McArthur JC, eds. AIDS and Neurology. New York, NY: Churchill Livingstone; 1995.
Hartmann A, Stingele R, Schnitzer, M. General treatment strategies for elevated intracerebral pressure, in Hanley DF, Einhaupl KM, Bleck TP, et al, eds. Neural Critical Care. Berlin: Springer-Verlag; 1994.
Hasbun R, Abrahams J, Jekel J, et al. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345:1727–1733.
Horlocker TT, Wedel DJ, Schroeder DR, et al. Preoperative antiplatelet therapy does not increase the risk of spinal hematoma associated with regional anesthesia. Anesth Analg. 1995;80(2):303–309.
Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003;28(3):172–197.
Hyland K, Arnold LA. Value of lumbar puncture in the diagnosis of genetic metabolic encephalopathies. J Child Neurol. 1999;14(Suppl 1):S9–S15.
Johnson RT. Viral Infections of the Nervous System. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 1998.
Kennedy PDE, Chaudhuri A. Herpes simplex encephalitis. J Neurol Neurosurg Psychiatry. 2002;73:237–238.
Kuntz KM, Kokmen E, Stevens JC, et al. Post lumbar puncture headache: experience in 501 consecutive procedures. Neurology. 1992;42:1884–1887.
Lavi R, Yamitsky D, Yernitzky D, et al. Standard vs atraumatic whitacre needle for diagnostic lumbar puncture; a randomized trial. Neurology. 2006;67(8):1492–1494.
Leib SL, Boscacci R, Gratzl O, et al. Predictive value of cerebrospinal fluid (CSF) lactate level versus CSF/blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clinic Infect Dis. 1999;29(1):69–74.
Levin BE. The clinical significance of spontaneous pulsations of the retinal vein. Arch Neurol. 1978;35:37–40.
Marra CM, Critchlow CW, Hook EW, et al. Cerebrospinal fluid treponemal antibodies in untreated early syphilis. Arch Neurol. 1995;52(1):68–72.
Masdeu JC, Breuer AC, Schoene WC. Spinal subarachnoid hematomas: clue to a source of bleeding in traumatic lumbar puncture. Neurology. 1979;29:872–876.
May C, Kaye JR, Atack MB, et al. Cerebrospinal fluid production is reduced in healthy aging. Neurology. 1990;40:500–502.
Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–1562.
Mizuno S, Mihara T, Miyaoka T, et al. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14(1):43–47.
Muayqil T, Gronseth G, Camicioli R. Evidence-based guideline: diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2012;79(14):1499–1506.
Nygaard TG. Dopa-responsive dystonia. Delineation of the clinical syndrome and clues to pathogenesis. Adv Neurol. 1993;60:577–585.
Pai M, Flores LL, Pai N, et al. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2003;3(10):633–643.
Park J, Chung C, Kim H. Iatrogenic spinal epidermoid tumor. A complication of spinal puncture in an adult. Clin Neurol Neurosurg. 2003;105:281–285.
Quincke H. Über hydrocephalus. Verhandlungen des Congresses für innere Medizin. Vol 10. Wiesbaden: JF Bergman, 1891, 321.
Rando TA, Fishman RA. Spontaneous intracranial hypotension: report of two cases and review of the literature. Neurology. 1992;42:481–487.
Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44(31):590–591.
Riemenschneider M, Lautenschlager N, Wagenpfeil S, et al. Cerebrospinal Tau and b-amyloid 43 proteins identify Alzheimer disease in subjects with mild cognitive impairment. Arch Neurol. 2002;59:1729–1734.
Roos KL, ed. Central Nervous System Infectious Diseases and Therapy. New York, NY: Marcel Dekker; 1997.
Roos KL. Lumbar puncture. Semin Neurol. 2003a;23(1):105–114.
Roos KL. Cerebrospinal fluid. In Joynt RJ, Griggs RC, eds. Baker’s Clinical Neurology. Baltimore, MD: Lippincott, Williams & Wilkins; 2003b.
Samson DS, Clark K. A current review of brain abscess. Am J Med. 1973;54:201–210.
Sencakova D, Mokri B, McClelland RL. The efficacy of epidural blood patch in spontaneous CSF leaks. Neurology. 2001;57:1921–1923.
Sharer LR. Pathology of HIV-1 infection of the central nervous system. J Neuropathol Exp Neurol. 1992;51:3–11.
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286–2296.
Strupp M, Schueler O, Straube A, et al. “Atraumatic” Sprotte needle reduces the incidence of post-lumbar puncture headaches. Neurology. 2001;57:2310–2312.
Swaiman KF. Spinal fluid examination. In: Swaiman KF, Ashwal S, eds. Pediatric Neurology. 3rd ed. St. Louis, MO: CV Mosby; 1999, Chap. 10, 115–121.
Thompson EJ. Cerebrospinal fluid. J Neurol Neurosurg Psychiatry. 1995;59:349–357.
Van Der Meulen J. Cerebrospinal fluid xanthochromia: an objective index. Neurology. 1966;16:170–178.
Weinberg A, Shaobing L, Palmer M, et al. Quantitative CSF PCR in Ebstein-Barr virus infection of the central nervous system. Ann Neurol. 2002;52:543–548.
Whiteley W, Al-Shahi R, Wardlow CP, et al. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67:1690–1691.
Wysowski DK, Talarico L, Bacsanyi J, Bostein P. Spinal and epidural hematoma and low-molecular-weight heparin. N Engl J Med. 1998;338:1774–1775.
III. NEURORADIOLOGY
A. Plain skull and spine radiographs
1. Structures visualized by plain films
a. Plain skull films show bone, teeth, and air-filled cavities including the nasopharynx. The films show the contours of bone, its thickness, density, vascular markings, foramina, orbits, and the state of the sutures. Plain films show calcifications, normal, as in the pineal body, and in calcified lesions. Plain films do not show the ventricles and subarachnoid spaces, brain parenchyma or its lesions (unless calcified), or normal blood vessels.
b. Bony lesions shown include hyperostoses, erosions, fractures, and synostosis.
c. Plain spine films show vertebral contours, malformations, fractures, and the intervertebral disc spaces.
2. Indications for plain skull or vertebral radiographs
a. In most instances, you should bypass plain films in favor of CT or MRI, which visualize CNS parenchyma and its lesions, the CSF spaces, and vessels, thus providing far more information.
b. Plain skull films quickly and inexpensively screen for infection in the sinuses and mastoids by showing mucosal thickening, clouding, and fluid levels. Skull films are also useful in the evaluation of shunt integrity in previously shunted patients with hydrocephalus. Order lateral, Waters, and Towne views to visualize all of the sinuses and the mastoid air cells. Sinus-dedicated CT is currently the best method of sinus visualization but it is far more expensive. Plain films serve little purpose if MRI or CT is required, because these procedures also show sinus disease.
c. Vertebral radiographs aid in screening for malformation syndromes involving bone, for spondylosis, and for cervical fractures and dislocations in unconscious Pts with acute head injuries.
d. Surveys of the skull, ribs, and long bones by plain radiographs identify previous fractures in battered infants.
3. Risk of plain radiographs: Minimal radiation.
B. Computerized axial tomography
1. Structures visualized: CT serves many of the previously listed indications for plain radiographs, but, in addition to showing bone and air filled cavities, it shows brain parenchyma and many parenchymal lesions, calcifications, and the CSF spaces. These structures appear as black or shades of gray, depending on their ability to absorb radiation (Table 13-4 and Figs. 13-8A to 13-8C). Intravenous injection of iodinated contrast material demonstrates the larger brain vessels.
TABLE 13-4 • Gray Scale for Structures and Fluids in Radiographs
Normal gray matter |
Normal white matter |
CSF spaces |
Bone |
Air-filled cavities |
Usual lesions |
|
Plain films |
Not seen |
Not seen |
Not seen |
White |
Black |
Seen if calcified |
CT |
Light gray |
Intermediate gray |
Black |
White |
Black |
Bright |
T1-MRI |
Intermediate gray |
Light gray |
Black |
— |
Black |
Dark (fat bright) |
T2-MRI |
Light gray |
Very dark |
Bright |
— |
Black |
Bright |
FLAIR |
Gray |
Medium gray |
Black |
— |
Black |
Bright |
DWI |
Light gray |
Medium gray |
Dark |
— |
— |
Bright |
ABBREVIATIONS: CSF = cerebrospinal fluid; CT = computed tomography; DWI = diffusion-weighted imaging; FLAIR = fluid attenuating inversion recovery; T1-MRI = T1-weighted magnetic resonance imaging; T2-MRI = T2-weighted magnetic resonance imaging. |
||||||
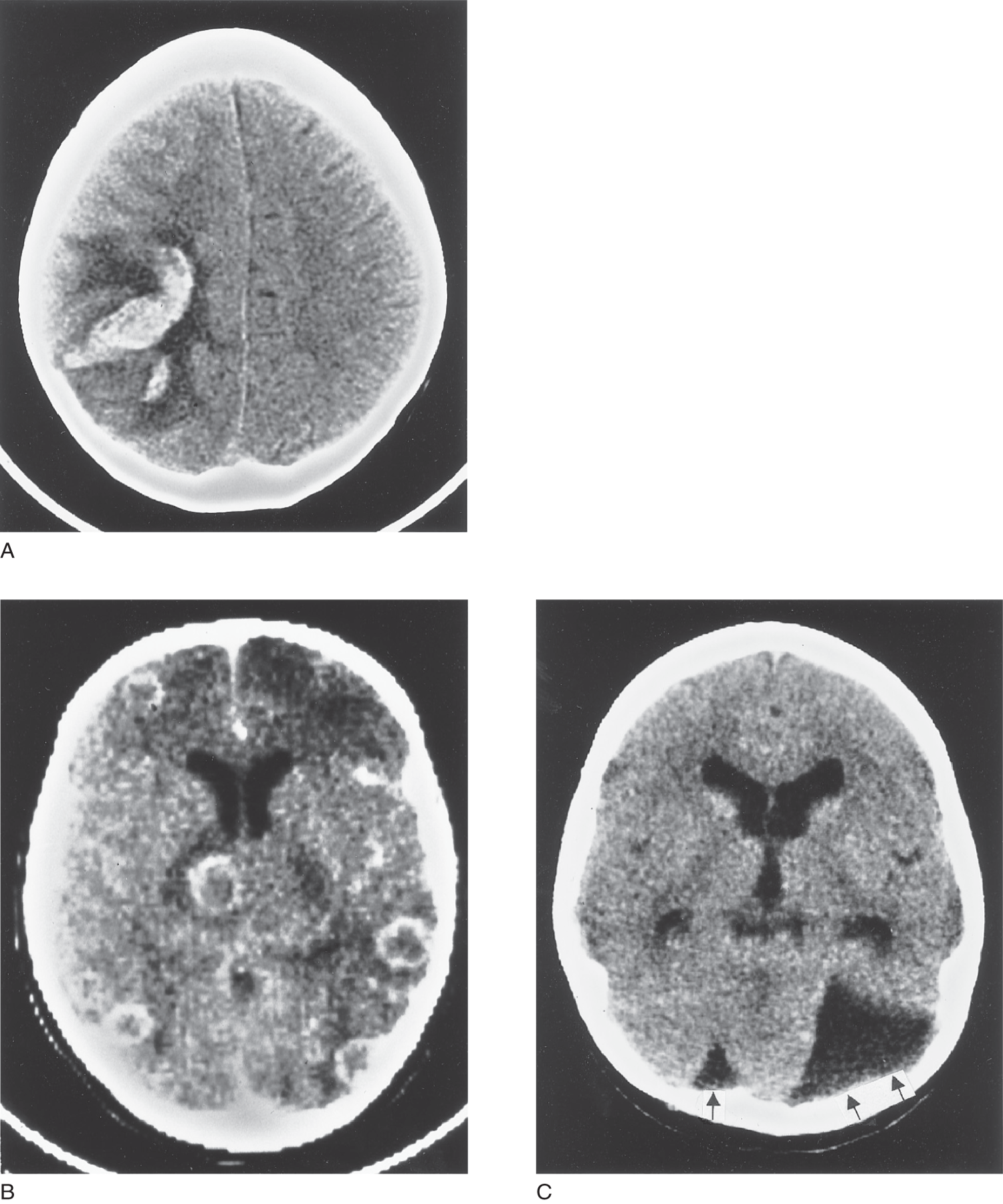
FIGURE 13-8. (A) CT scan, horizontal section at a high level of the cerebrum, above the ventricles, shows a recent hemorrhage in the left parieto-occipital region. Edema causes the dark zone around the hemorrhage. (B) CT scan, horizontal section of the cerebrum, shows contrast-enhanced white rings of vascularity around multiple abscess cavities. (C) CT scan, horizontal section of the brain, shows congenital arachnoid cyst of the posterior fossa (two arrows). It has displaced the cerebellum and caused slight ventricular enlargement from obstructive hydrocephalus. The single arrow (to the reader’s left) points to the displaced cisterna magna cerebelli. CSF = cerebrospinal fluid; CT = computed tomography.
2. Indications for CT
a. For most Pts requiring an imaging procedure, order an MRI. Substitute CT for MRI if the Pt has magnetic metal in the body, certain electronic devices (eg, pacemaker) (Greenspan and Montesanno, 1993).
b. Substitute CT for MRI when time is critical, as in the diagnosis of acute intracranial bleeding from a head injury or when you have to identify or exclude quickly a lesion requiring surgical intervention. CT often shows intracranial bleeding better than MRI.
c. CT suffices to follow the changes in ventricular size after shunt operations for hydrocephalus.
d. CT shows normal intracranial calcification and calcified lesions (Fig. 13-9).
e. CT can visualize the skull and sutures in three dimensions, enabling surgeons to better plan the correction of craniofacial malformations and some facial injuries.
f. CT can be obtained more conveniently than MRI for imaging of deeply comatose intensive care Pts or determination of brain death.
g. Angiography with CT: Iodinated contrast material injected into a peripheral vein will display blood vessels and enhance many lesions that have abnormal blood vessels or blood–brain barrier disruption (Fig. 13-8B). CT angiography provides a three-dimensional view of the vessels of the brain and neck (Mazziotta, 2000).
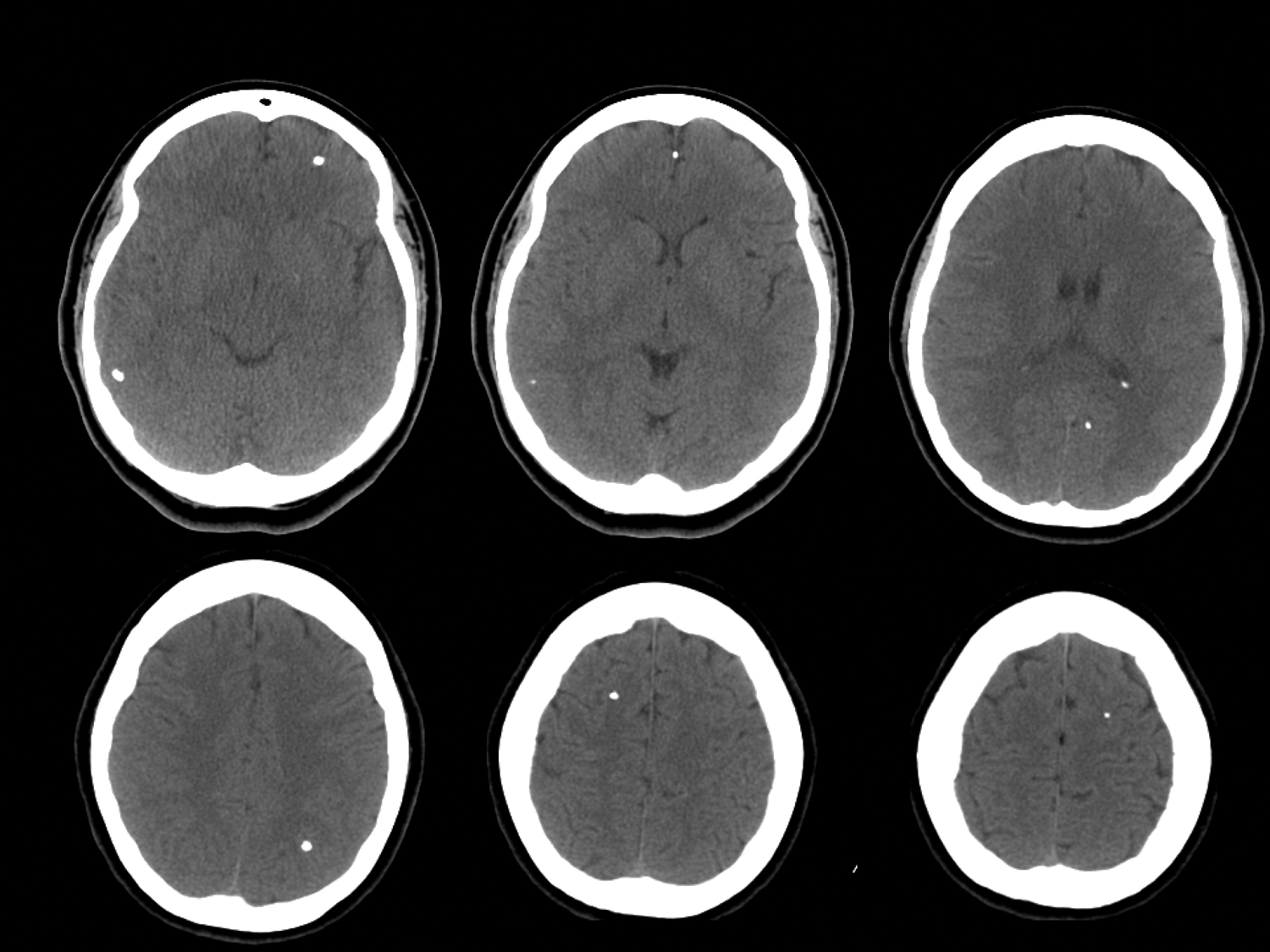
FIGURE 13-9. Axial noncontrast head computed tomography (CT) shows multiple calcifications in a patient with neurocysticercosis. (Used with permission from Jordan Rosenblum, MD.)
3. Comparison of CT with MRI
a. CT is cheaper and more widely available. Its wider availability, shorter scanning time, and lower cost make it suitable as a substitute for MRI in some cases. It shows calcifications, whereas MRI does not.
b. CT poorly visualizes lesions of the posterior fossa or base of the skull because the dense petrous bone degrades the image.
4. Risk of CT: Some uncooperative Pts and infants require sedation. Some Pts may have allergic reactions to injected contrast material containing iodine. The fluid load from IV contrast may be of danger to a patient with renal impairment.
C. Magnetic resonance imaging of the head and spine
1. The superiority of MRI to CT: MRI exploits the paramagnetic signals from the protons and does not expose the Pt to radiation. Because of its vastly superior specificity and sensitivity, MRI is the procedure of choice for imaging the brain and spinal cord (Gilman, 1998; Greenberg, 1999; Modic et al, 1993; Osborn, 1994), with the few exceptions listed above. Radiographs show the normal anatomy and lesions of the CNS as black or on a gray scale (Figs. 13-10 and 13-11A to 13-11E and Table 13-4).

FIGURE 13-10. (A) Coronal section, T2-weighted image, at the level of the genu of the internal capsule. The upper arrows point to the genu of the internal capsule at the foramen of Monro. The two foramina of Monro lead from the lateral ventricles into the III ventricle. The lower arrows show the Y of the internal carotid artery (shown as a black flow void) as it bifurcates into the middle and anterior cerebral arteries. (B) Horizontal section, T2-weighted image, at the level of the genu of the internal capsule. The arrows point to the genu of the internal capsule at the foramen of Monro.

FIGURE 13-11. Montage of lesions demonstrated by MRI. The left side of the patient’s brain is to the reader’s right. All horizontal scans are mounted with the frontal lobes up. (A) MRI, horizontal T2-weighted section of the brain, shows a subacute infarct (arrow) in the left thalamus. (B) MRI, horizontal T2-weighted section of the cerebrum, shows multiple areas of periventricular demyelination (arrows) in multiple sclerosis. (C) MRI, horizontal T1-weighted section of brain, gadolinium enhanced, shows an astrocytoma with cystic cavities (arrows). The tumor is bright, and the fluid-filled cysts and CSF are black in T1-weighted images, in contrast to bright areas in T2-weighted images. (D) MRI, horizontal T1-weighted section of the brain, shows bilateral subdural hematomas (arrows) and atrophic cerebrum with enlarged ventricles and subarachnoid spaces. The patient, a battered child, had received multiple head injuries. (E) MRI, sagittal T1-weighted section of the brain. The medial wall of the hemisphere shows severe atrophy of the frontal lobe gyri, with extreme widening of the sulci. Notice the normal size of the gyri and sulci in the parts of the cerebrum posterior to the white marker. The patient has a form of dementia called Pick disease. MRI = magnetic resonance imaging.
2. The number and variety of imaging procedures, far from replacing the history and NE, places an even greater premium on the clinical findings to enable the Ex to select the appropriate procedures that are safe, decisive, and as economical as possible. The more focused the clinical information, the better the clinician can “go for the jugular” in selecting the one or two best tests.
3. Types of MRI procedures: Table 13-5 (Atlas, 1995; Krings et al, 2001; Osborn, 1994; Orrison, 2000).
TABLE 13-5 • MRI Techniques for Brain Imaging and Their Application
Procedure |
Principle |
Application |
Standard T1 and T2 MRI |
Exploits paramagnetic properties of protons, most of which are in extracellular space |
Shows normal anatomy of parenchyma and CSF spaces and most lesions; vessels appear as flow voids or bright with injection of contrast material |
Diffusion-weighted imaging (DWI) |
Measures changes in diffusion; diffusion means thermally driven Brownian movement; decreased diffusion shows bright signal |
Detects fluid shifts caused by membrane dysfunction (cytotoxic edema); in early stroke, it shows bright areas within minutes; conventional MRI takes hours; shows response to thrombolytic therapy; delineates neoplasms, abscesses, trauma and demyelination; some tumors usually dark |
Diffusion tensor imaging (DTI) |
Maps anisotropic and isotropic diffusion |
Identifies some white matter lesions missed on standard MRI and further characterizes others; can trace CNS tracts, normal or degenerating |
Perfusion weighted imaging (PWI) |
Injected gadolinium discloses regional differences in blood flow by paramagnetic changes in neighboring molecules; areas of low blood flow appear black |
Identifies sites of abnormal blood flow; DWI shows the core infarct, and perfusion MR defines the sites of potentially reversible damage; used to localize and grade brain tumors and in measuring response to treatment |
Functional MRI (fMRI) = BOLD = Blood Oxygen Level Dependent |
Shows regional blood flow in relation to changes in deoxyhemoglobin level |
Shows sites of increased or decreased blood flow dependent on functional activity or lesions; used in presurgical mapping of sensorimotor cortex and localization of speech, memory, and other mental and sensorimotor functions; EEG can be time locked to trigger fMRI during epileptogenic discharges to localize the site |
Fluid attentuation inversion recovery (FLAIR) |
Images resemble T2 films but the signal from CSF is suppressed, making it dark rather than bright |
Especially shows lesions adjacent to CSF spaces, such as periventricular leukomalacia and other parenchymal lesions; shows mesial temporal sclerosis in temporal lobe epilepsy |
ABBREVIATIONS: CSF = cerebrospinal fluid; EEG = electroencephogram; MRI = magnetic resonance imaging. |
||
a. “Standard” MRI
i. T1-weighted imaging
ii. T2-weighted imaging
b. Diffusion-weighted imaging
c. Perfusion-weighted imaging
d. MRA
e. Functional MRI (fMRI).
f. Magnetic resonance spectroscopy (MRS).
4. Indications for MRI
a. History or NE that raises the suspicion of an extra-axial or parenchymal lesion of the CNS or surrounding tissues (Yock, 2002).
b. Suspected cerebrovascular disease: infarction (Fig. 13-11A) (Videos 13-5 and 13-6), hemorrhage, aneurysms, and arteriovenous malformations.
i. MRA demonstrates the intracranial and neck vessels, in lieu of invasive angiography. Various imaging techniques taken together, as listed in C 3 a to 3 f, define the necrotic core of an infarct that cannot recover, the penumbra of ischemic but recoverable tissue, the age of the lesion, and the time that blood has been in the tissue (Baldoli et al, 2002; Choi et al, 2000; Davis et al, 2003; Schlaug et al, 1999).
ii. MRA and other angiographic techniques aid in determining the site of occlusion of the vessels and provide insight as to the mechanism of the infarct (Osborn, 1994; Fig. 13-12).
iii. Autopsy examination (Kelly et al, 2001) and agreement between MRI techniques confirm the accuracy of the MRI procedures in cerebrovascular disease (Lansberg et al, 2000; Neumann-Hafelin et al, 2000).
c. Suspected demyelinating disease of adults or children (Fig. 13-11B).
d. Neoplasia: primary or metastatic (Fig. 13-11C) (Video 13-7 VHL).
e. Craniospinal trauma (Fig. 13-11D).
f. Dementia (Fig. 13-11E).
g. Acute infections: meningitis, encephalitis, and abscesses (Fig. 13-8B).
h. Investigation of the developmental level of the brain (Thatcher et al, 1996), neuropsychiatric disorders in adults and children (Garreau, 1998), and developmental retardation as related to microcephaly, macrocephaly, hydrocephaly, cerebral palsy, anoxic encephalopathy, and malformations (Barkovich, 2000).
i. Investigation of the cause of seizures and the origin of epileptogenic activity.
j. Acute or new onset headaches, in particular “thunderclap” headaches that may signify subarachnoid hemorrhage (CT is also useful in detecting acute subarachnoid hemorrhage).
k. MRI can estimate the age of intracerebral hemorrhage by changes in paramagnetic properties of the decomposition products from oxyhemoglobin through deoxyhemoglobin, methemoglobin, transferrin, and hemosiderin (Osborn, 1994).

Video 13-5. Right crural predominant hemiparesis due to left anterior cerebral artery (ACA) territory infarction. MRA shows left A3CA occlusion.

Video 13-6. Patient with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) misdiagnosed as multiple sclerosis.

Video 13-7. Patient with Von Hippel Lindau and spinal cord (conus medullaris region) hemangioblastoma.
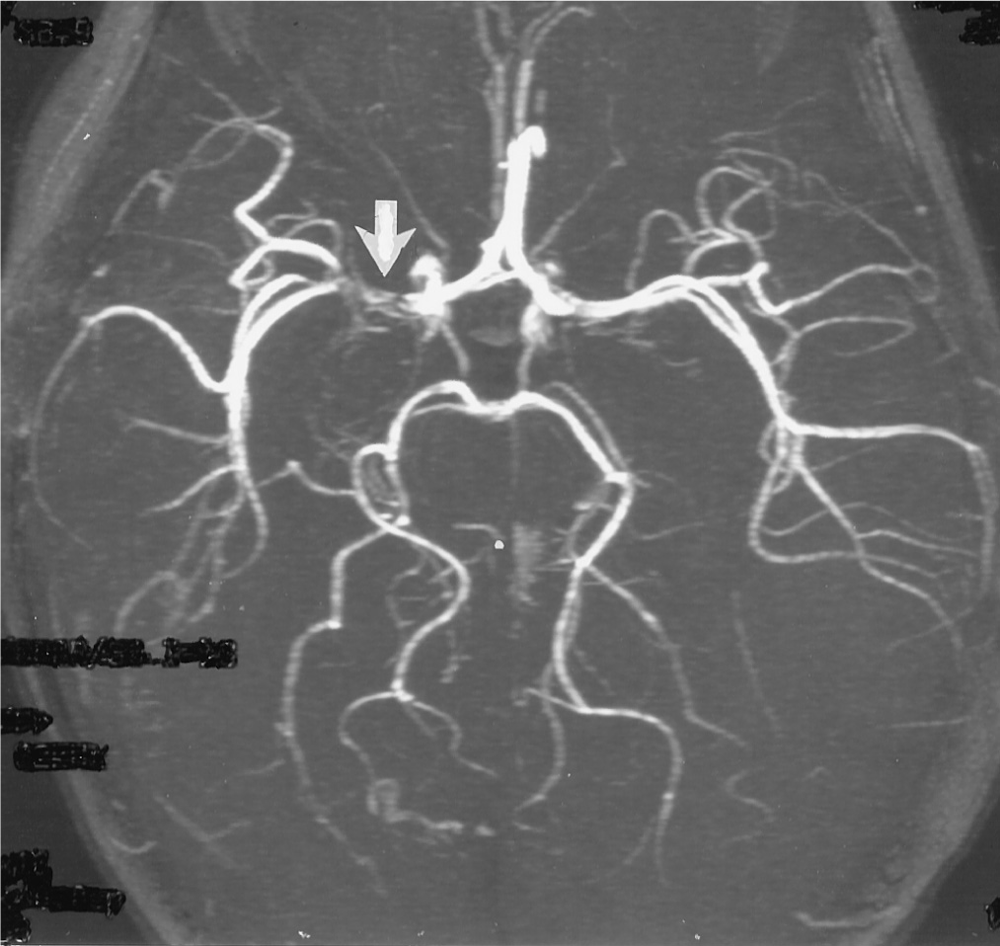
FIGURE 13-12. Magnetic resonance angiogram. The arrow shows the site of embolic occlusion of the right middle cerebral artery.
5. Enhancement with gadolinium: Gadolinium is a magnetic metallic element of the rare earth group (atomic weight = 157.25). The IV injection of gadolinium shows the blood vessels and sites of increased blood–brain barrier permeability, as in infarcts, neoplasms, and demyelinating lesions (Fig. 13-11C) or contusions. If you are selecting the right Pts for MRI, those with reasonably strong clinical evidence of a neurologic lesion, gadolinium enhancement is generally indicated (Elster, 1993). Even if normal, a gadolinium scan provides valuable evidence against highly aggressive or destructive lesions.
6. Risks of MRI: MRI per se causes no known adverse biological effects, but the strong magnetic field precludes MRI for Pts with certain paramagnetic implants and certain electronic devices. Some Pts get claustrophobia when inserted into the MRI tube. Uncooperative Pts and infants require sedation or anesthesia to prevent movement during an MRI scan. Avoid gadolinium in pregnant women and Pts with a history of allergic reactions. Also always check renal function before giving gadolinium as rarely this agent has been linked with occurrence of nephrogenic systemic fibrosis (NSF) also known as nephrogenic fibrosing dermopathy (NFD) (Marckmann et al, 2006).
D. Computed tomography and magnetic resonance myelography
Visualization of the spinal cord and vertebral lesions by CT or MRI has reduced the need for direct injection of contrast material into the subarachnoid space via an LP (Atlas, 1995; Greenspan and Montesonno, 1993; Modic et al, 1993; Pui and Husen, 2000). Myelography is still needed when MR is contraindicated, when searching for certain CSF leaks, or when artifacts from spinal fusion devices preclude adequate visualization of the spinal cord.
E. Diffusion-weighted imaging (diffusion-weighted magnetic resonance imaging)
This technique shows cytotoxic edema as bright areas. In acute infarction it visualizes the lesion sooner than with conventional MRI or CT, and it supplements standard MRI in many other disorders that cause anatomic lesions. Perfusion-weighted imaging adds additional information about blood flow and blood–brain barrier disruption (Tables 13-4 and 13-5). Diffusion tensor imaging shows normal and degenerating CNS tracts (Ciccarelli et al, 2001; Werring et al, 2000).
F. Magnetic resonance spectroscopy
1. Standard MRI displays anatomy by using the signals from water protons. MRS displays metabolites by using signals from nonwater protons or phosphorus. (Proton MRS = PMRS = 1H-MRS; phosphorous MRS = 31P-MRS.) By using voxels of 1 to 2 cm2, proton MRS displays spectral peaks for the metabolites involved in the mitochondrial electron-transport chain, oxidative phosphorylation, Krebs cycle, aerobic glycolysis, and anaerobic metabolism (Fig. 13-13).
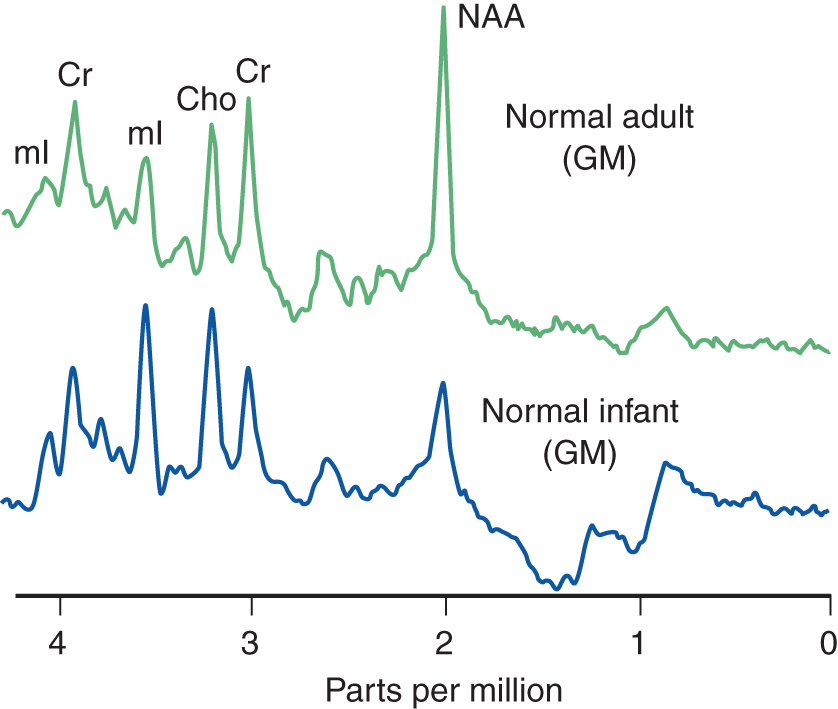
FIGURE 13-13. Magnetic resonance spectroscopy of the gray matter. Notice the higher NAA peak in the adult GM, indicating further neuronal development. Cho = choline; Cr = creatine; GM = gray matter; mI = myo-inositol; NAA = N-acetyl aspartate. (Adapted from Danielsen ER, Ross B. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. New York, NY: Marcel Dekker; 1999.)
2. The major resonances charted are from lactate, N-acetyl aspartate, glutamine, creatine, choline, and myo-inositol (Fig. 13-13).
a. Lactate is an end product of glycolysis. It does not show a distinct peak in normal persons but increases in anaerobic metabolism and marks sites of tissue damage. Lactate peaks:
i. In the acutely ischemic or necrotic tissue, when oxidative metabolism fails.
ii. In tissue infiltrated by inflammatory cells. The lactate in ischemic tissue may come from the infiltrating macrophages.
iii. In mitochondrial encephalopathies.
iv. In fluid-filled cysts.
b. N-acetylaspartate (NAA) is a marker of neuronal density and viability. NAA is an amino acid of unknown function located almost exclusively in mature perikarya and their processes, including axons. Its presence reflects neuronal density in the gray matter and axonal integrity in the white matter. Its decrease reflects neuronal destruction, as in degenerative diseases, infarction, and glial tumors. NAA appears as early as 16 weeks in the fetal cortex, increases rapidly, and reaches the adult level by around 16 years.
c. Creatine is distributed fairly uniformly and serves as an internal standard for comparison of other metabolites. It occurs alone or as phosphocreatine. It increases in some lesions, such as tumors. Neuronal loss causes a decrease in the NAA-to-creatine ratio because the creatine is considered the basis for comparison of spectral peaks.
d. Choline (Cho) levels reflect free choline and phosphoryl and glucophosphoryl choline of the phospholipids of cell membranes. Membrane disruption, as in acute demyelination, releases phospholipids and increases choline levels. Brain tumors have a high Cho level because of increased cellular density.
e. Myo-inositol serves as a marker of astrocytes, where it is found almost exclusively.
3. Clinical application of MRS (Danielsen and Ross, 1999; Rudkin and Arnold, 1999).
a. Cerebrovascular disease: Decreases of NAA and increases of lactate in the ischemic zone allows estimation of the distribution and severity of neuronal damage.
b. Epilepsy: Localizes epileptogenic foci by elevated lactate during seizure discharge and by decreased NAA levels in the focus.
c. Multiple sclerosis: Decreased NAA shows axonal damage in plaques and in adjacent tissue in chronic multiple sclerosis. Increased Cho indicates demyelination in plaques.
d. HIV: NAA decreases and Cho increases before clinical signs and changes on standard MRI.
e. Brain tumors: Aids in differentiating histologic type of tumor and in guiding stereotaxic biopsy.
f. Neurodegenerative diseases: Spectroscopy shows neuronal loss in numerous degenerative diseases including amyotrophic lateral sclerosis, parkinsonism, and Parkinson plus syndromes, Huntington chorea, and various metabolic disorders.
g. Inborn errors of metabolism: Currently, MRS lacks the sensitivity to detect the primary defect in most cases but can document the neuronal and axonal loss, demyelination, and lactate level. For example, MRS may be diagnostic in Canavan disease, a childhood autosomal recessive leukodystrophy caused by mutations in the gene for human aspartoacylase, demonstrating a characteristic elevated NAA peak (Cakmarkci et al, 2010). Future technical improvements undoubtedly will make MRS more valuable in these diseases.
BIBLIOGRAPHY · Computed Tomography, Magnetic Resonance Imaging, and Magnetic Resonance Spectroscopy
Atlas SW. Magnetic Resonance Imaging of the Brain and Spine. 2nd ed. New York, NY: Raven; 1995.
Baldoli C, Righini A, Parrazini C. Demonstration of acute ischemic lesions in the fetal brain by diffusion magnetic resonance imaging. Ann Neurol. 2002;52:243–246.
Barkovich JA. Pediatric Neuroimaging. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000.
Cakmarkci H, Pekcevik Y, Yis U, et al. Diagnostic value of proton MR spectroscopy and diffusion-weighted MR imaging in childhood inherited neurometabolic brain diseases and review of the literature. Eur J Radiol. 2010;74(3):161–171.
Choi SH, Na DL, Chung CS, et al. Diffusion-weighted MRI in vascular dementia. Neurology. 2000;54:83–89.
Ciccarelli O, Werring DJ, Wheeler-Kingshott CAM, et al. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology. 2001;56:926–934.
Danielsen ER, Ross B. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. New York, NY: Marcel Dekker; 1999.
Davis S, Fisher M, Warach S, eds. Magnetic Resonance Imaging in Stroke. New York, NY: Cambridge Univ. Press; 2003.
Elster AD. Is gadolinium required for routine cranial MR imaging? An update. MRI Decisions. 1993, September/October.
Garreau B, ed. Neuroimaging in Child Neuropsychiatric Disorders. Heidelberg: Springer-Verlag; 1998.
Gilman S. Imaging of the brain. N Engl J Med. 1998;338:812–820, 889–896.
Greenberg JO, ed. Neuroimaging. 2nd ed. New York, NY: McGraw-Hill; 1999.
Greenspan A, Montesanno P. Imaging of the Spine in Clinical Practice. St Louis, MO: CV Mosby; 1993.
Kelly PJ, Hedley-Whyte ET, Primavera J, et al. Diffusion MRI in ischemic stroke compared to pathologically verified infarction. Neurology. 2001;56:914–920.
Krings T, Schreckenberger M, Rohde V, et al. Metabolic and electrophysiological validation of functional MRI. J Neurol Neurosurg Psychiatry. 2001;71:762–771.
Lansberg MG, Norbash AM, Marks MP, et al. Advantages of adding diffusion-weighted magnetic resonance imaging to conventional magnetic resonance imaging for evaluating acute stroke. Arch Neurol. 2000;57:1311–1316.
Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359–2362 (Medline).
Mazziotta JC. Imaging: window on the brain. Arch Neurol. 2000;57:1413–1421.
Modic MT, Masaryk TJ, Ross JS. Magnetic Resonance Imaging of the Spine. St Louis, MO: CV Mosby; 1993.
Neumann-Hafelin T, Moseley ME, Albers GW. New magnetic resonance imaging methods for cerebrovascular disease: emerging clinical applications. Ann Neurol. 2000;47:559–570.
Orrison W, ed. Neuroimaging. Philadelphia, PA: WB Saunders; 2000.
Osborn AG. Diagnostic Neuroradiology. St Louis, MO: CV Mosby; 1994.
Pui MH, Husen YA. Value of magnetic resonance myelography in the diagnosis of disc herniation and spinal stenosis. Australas Radiol. 2000;44(3):281–284.
Rudkin TM, Arnold DL. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol. 1999;56:919–926.
Schlaug G, Benfield A, Baird AE, et al. The ischemic penumbra operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–1537.
Thatcher RW, Lyon GR, Rumsey J, et al. Developmental Neuroimaging. San Diego, CA: Academic Press; 1996.
Werring, DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry. 2000;69:269–272.
Yock DH. Magnetic Resonance Imaging of CNS Diseases. A Teaching File. 2nd ed. St Louis, MO: CV Mosby; 2002.
G. Introduction to dynamic techniques for localization of functions and lesions in the brain
1. Functional localization means detecting sites of brain function involved in the performance of specified mental, motor, and sensory processes. Examples of localized mental process include word generation and reactions to anxiety or pain. Localized motor actions include finger tapping or tongue movement. Rather than mapping the brain as a mosaic of isolated areas, the newer functional methods demonstrate distributed systems or linked areas, including the cerebellum. These methods extend the localization doctrines derived from classic clinicopathologic correlations at autopsy and from static radiographic films (Frank and Pavlakis, 2001). Functional techniques fall into two large groups: radiographic and electromagnetic.
2. The functional radiographic techniques demonstrate focal differences in metabolism and cerebral blood flow that depend on neuronal activity (Orrison, 2000). Radiographic techniques for recording focal variations in blood flow include fMRI, positron emission tomography (PET), and single-photon emission computed tomography (SPECT). MRS will evolve to include more and more functional applications.
3. The functional electromagnetic techniques depend on the fact that masses of neurons produce recordable electrical potentials and recordable differences in magnetic field. Electroencephalography (EEG) noninvasively records electrical potentials from masses of neurons from scalp electrodes. Electrocorticography records from electrodes placed directly on the cortex or electrodes can also be inserted into the depths of the brain. Various sensory stimuli applied to receptors or sensory pathways evoke recordable electrical potentials from farther along the pathway or at the cortical receptive areas (Section IV). Magnetoencephalography involves recording magnetic fields produced by the electrical potentials from masses of active neurons.
4. Most of the functional techniques for studying brain lesions and localization of functions are also applied to the analysis of normal and abnormal development of the brain (Thatcher et al, 1996). The supplement to the December 2002 issue of the Journal of Cell Biology, pp. 1–248, thoroughly reviews all aspects of molecular imaging.
H. Functional magnetic resonance imaging
When active neurons at a site increase the local metabolic rate and blood flow, the increased blood flow more than meets O2 demand, paradoxically causing the deoxy-hemoglobin level to fall. Deoxyhemoglobin reduces the magnetic signal from neighboring water molecules (Buxton, 2002; Pritchard and Cummings, 1997). Functional MRI exploits this difference as a measure of increased flow and increased neuronal activity. The focal increases in cortical blood flow occur within seconds when the brain engages in specified mental, motor, or sensory processes. More commonly used as a research tool, fMRI is beginning to have clinical application (Table 13-5).
I. Radionuclide scanning
1. Radionuclide scans use injected or inhaled radioactive substances detected by external scanning. The substances are administered by breathing, IV injection, or injection into the subarachnoid space, as for detecting a CSF fistula.
2. The two methods most widely used are SPECT and PET (Diksic and Rega, 1991; Gjedde et al, 2000).
a. SPECT typically uses technetium, a gamma emitter. It is relatively cheap and widely available.
b. PET uses a positron-emitting isotope of C, O, or F, typically fluorodeoxyglucose. Fluorodeoxyglucose is metabolized only to the first step, phosphorylation. Because it does not proceed down the metabolic path, it accumulates in the tissue for measurement. PET scanning is more expensive than SPECT, but the cost has been decreasing due to greater availability of the isotope. PET scanning now plays an important part in the clinical management of many cancers (Fig. 13-14). PET scanning is now a routine clinical procedure for many oncology patients.
FIGURE 13-14. F-18 FDG PET images (top) demonstrate a focus of hypermetabolic activity near the vertex of the skull (arrow) just to the left of the midline. Fusion imaging with a recent MRI study (bottom) is useful to more precisely localize the focus. The finding is consistent with recurrent glioblastoma in this patient. (Courtesy of Robert H. Wagner, MD.)
3. Radionuclide scans show blood flow through the various regions of the brain. When using fluorodeoxyglucose as the radioactive signal, PET shows sites of glucose use. By combining other chemicals such as neurotransmitters with radioactive isotopes, a wide range of metabolic activity can be investigated.
4. DaTscan (Ioflupane I 123 injection, also known as phenyltropane) is a radiopharmaceutical indicated for striatal dopamine transporter visualization using SPECT brain imaging, and only FDA approved to distinguish potential Parkinson disease from essential tremor.
5. Clinical applications
a. Studying the dynamics of glucose metabolism or other metabolites.
b. Studying blood flow, whether hyperperfusion or hypoperfusion.
c. Determining the absence of cerebral blood flow in the diagnosis of brain death (SPECT). First and foremost, the clinician needs to determine irreversible cessation of all functions of the cerebrum and brainstem. In brain death, blood flow superior to the circle of Willis circulation is completely absent. However, with its reported false positive and false negative rates, cerebral perfusion scintigraphy is seldom performed (Costa et al, 1999).
d. Studying the dynamics of CSF flow (Fig. 13-15) and detecting fistulae (SPECT), or slow-flow CSF leak in cases of low CSF volume headaches (spontaneous intracranial hypotension) (Rondo and Fishman, 1992; Mokri et al, 2002).
e. Localizing an active epileptogenic focus before surgical removal (SPECT and PET).
f. Localizing brain activity during specified mental tasks, sensory stimuli, or motor actions (PET).
g. Analysis of neuropsychiatric disorders of adults and children (O’Tuama et al, 1999).
h. PET with the use of amyloid tracers is potentially useful as a noninvasive method to determine regional cerebral patterns of amyloid plaques and neurofibrillary tangles, but amyloid PET is not a substitute for a careful history and thorough examination, as there is a high prevalence of amyloid positivity in non-Alzheimer dementias and normal older individuals (Small GW et al, 2006; Ikonomovic et al, 2008; Koivunen et al, 2011; Johnson et al, 2013).
i. DaTscan may be a potential addition to the clinician armamentarium in the evaluation of parkinsonism, but is often overutilized, and should not replace the clinical examination (de la Fuente-Fernandez, 2012) (Fig. 13-16).
FIGURE 13-15. Cisternography. The 4-hour images (figure 1) demonstrate early visualization of the lateral ventricles. Delayed images at 24 hours (figure 2) and at 48 hours demonstrate persistence of activity in the ventricles consistent with the diagnosis of normal pressure hydrocephalus. (Courtesy of Robert H. Wagner, MD.)
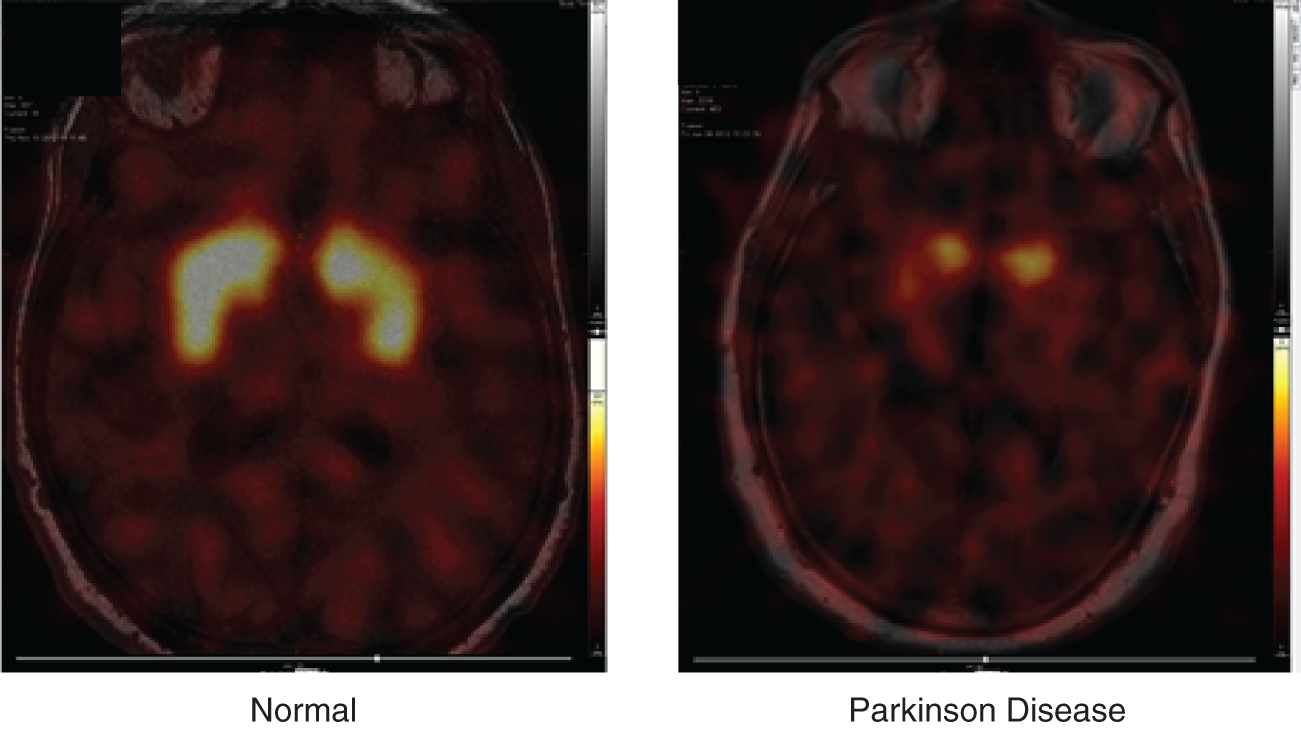
Figure 13-16. DaTscan. Normal subject and patient with Parkinson disease. (Courtesy of Robert H. Wagner, MD.)
BIBLIOGRAPHY · Functional Imaging and Radionuclide Scanning
Buxton RB. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques. New York, NY: Cambridge Univ. Press; 2002.
Costa DC, Spilowsky L, Ell PJ. Nuclear medicine in neurology and psychiatry. The Lancet. 1999; 354(9184):1107–1111.
de la Fuente-Fernandez R. Role of DaTSCAN and clinical diagnosis in Parkinson disease. Neurology. 2012;78(10):696–701.
Diksic M, Rega RC. Radiopharmaceuticals and Brain Pathophysiology Studied with PET and SPECT. Boca Raton, FL: CRC Press; 1991.
Frank Y, Pavlakis SG. Brain imaging in neurobehavioral disorders. Pediatr Neurol. 2001;25:278–287.
Gjedde A, Hansen SB, Knudsen GM, et al, eds. Physiological Imaging of the Brain with PET. San Diego, CA: Academic Press; 2000.
Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(6):1630–1645.
Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. J Nucl Med. 2013; 54(3):476–490.
Koivunen J, Scheinin N, Virta JR, et al. Amyloid PET imaging in patients with mild cognitive impairment. A 2-year follow-up study. Neurology. 2011;76(2):1085–1090.
Mazziotta JC, Toga AW, Frackowiak RSJ. Brain Mapping: The Disorders. New York, NY: Cambridge Univ. Press; 2000.
Mokri B, Maher CO, Sencakova D. Spontaneous CSF leaks: underlying disorder of connective tissue. Neurology. 2002;58(5);814–816.
O’Tuama LA, Dickstein DP, Neeper R, et al. Functional brain imaging in neuropsychiatric disorders of childhood. J Child Neurol. 1999;14:207–221.
Pritchard JW, Cummings JL. The insistent call from functional MRI. Neurology. 1997;48:797–800.
Rondo TA, Fishman RA. Spontaneous intracranial hypotension. Report of two cases and review of the literature. Neurology. 1992;42(3):481–487.
Small GW, Kepe V, Ercoli LM, et al. PET of brain amyloid and Tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–2663.
Thatcher RW, Lyon GR, Rumsey J, et al. Developmental Neuroimaging. San Diego, CA: Academic Press; 1996.
J. Angiography of the central nervous system: arteriography and venography
1. Definition: Angiography means the radiographic visualization of the course, distribution, and caliber of blood vessels (Huber, 1982; Newton and Potts, 1974; Osborn, 1999). The process differs from methods for detecting regional differences in blood flow by functional imaging or Doppler ultrasound.
2. Methods for demonstrating CNS vessels
a. CT shows vessels, albeit crudely, after IV injection of iodinated compounds. 3D reconstructions from CT demonstrates the vessels in three dimensions.
b. Standard MRI films show blood vessels as flow voids (Fig. 13-11A). An IV injection of gadolinium increases the visibility of the vessels.
c. MRA shows the vessels satisfactorily, but not as well as with injection of contrast agents by catheterization (Fig. 13-12). Improvements in MRA have reduced the need for, and may ultimately eliminate, direct injection angiography.
d. Direct injection angiography of iodinated contrast material into an intra-arterial catheter demonstrates the arteries and veins best of all the methods.
e. Doppler ultrasound studies demonstrate blood flow in the neck and some intracranial vessels but do not produce angiograms.
3. Indications for invasive angiography (as a supplement to MRA)
a. Demonstration of degrees of narrowing of vessels in the neck and intracranial arteries (eg, CNS vasculitis).
b. Investigation of subarachnoid hemorrhage and demonstration of aneurysms and arteriovenous malformations of the brain and spinal cord (Djindjian, 1970).
c. Preoperative planning of surgical procedures that may involve or displace vessels.
4. Risk of invasive angiography: Arterial thrombosis, embolization, dissection, and allergic reactions to the contrast medium. Because the kidneys excrete the contrast material, renal failure may preclude contrast angiography (this is a relative contraindication as limited angiography may still be possible), but MRA without contrast is still safe.
BIBLIOGRAPHY · Angiography
Djindjian R. Angiography of the Spinal Cord. Baltimore, MD: University Park Press; 1970.
Huber P. Krayenbuhl/Yasargil Cerebral Angiography. 2nd ed. Stuttgart: Georg Thieme Verlag; 1982.
Newton TH, Potts DG, eds. Radiology of the Skull and Brain. Vols. 1-3. St. Louis, MO: CV Mosby; 1974.
Osborn AG. Diagnostic Cerebral Angiography. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999.
 Learning Objectives for Chapter 13
Learning Objectives for Chapter 13