1 |
Examination of the Face and Head |
Disease is of antiquity and nothing about it changes. It is we who change as we learn to recognize what was formerly imperceptible.
I. INTRODUCTION TO THE NEUROLOGICAL EXAMINATION
A. Symptoms and signs of neurologic disease
Diseases that affect the nervous system manifest by cognitive, motor, or sensory symptoms and signs and by abnormal body contours (Fig. 1-1).

FIGURE 1-1. Dendrogram summarizing neurologic symptoms and signs.
B. Steps in the neurologic examination
1. The neurologic examination (NE) consists of a series of simple, standardized steps. Each step focuses on a specific, readable endpoint. Most steps test a known neuroanatomic circuit. As endpoints, the examiner (Ex) selects:
a. Simple behaviors, such as a pupil constricting to light or a finger flexing.
b. Complex behaviors, such as walking, speaking, or writing.
c. Specific body contours, such as head size or shape.
2. The Ex compares the result of each step with a “standard person” of same age, sex, and culture and judges each result as normal, borderline, or abnormal.
3. The steps use four types of operations: inspections, questions, requests, and maneuvers.
a. Inspections disclose the patient’s (Pt’s) bodily contours and spontaneous and elicited behaviors (Video 1-1).
b. Questions determine the Pt’s mental status and sensory perceptions.
c. Requests or commands test the Pt’s volitional responses.
d. Maneuvers impose stimuli to elicit sensations and reflexes.

Video 1-1. Left hemiatrophy and hyperreflexia in a patient with Moya Moya, ischemic stroke during infancy and poststroke seizures.
C. The NE as standardized assessment of designated behaviors
1. For the NE, we may define behavior as any detectable change produced by neural activation of an effector. The neural activation may arise voluntarily or reflexly.
2. Because only two types of effectors exist, namely glands and muscles, humans can produce behaviors by only two actions: by secreting something and by adjusting the length of their muscle fibers.
a. By secreting we produce sweat, tears, saliva, mucus, hormones and digestive juices, and semen.
b. By adjusting the length of muscle fibers we can:
i. Operate our skeletal levers to move ourselves and objects around.
ii. Open and close or vibrate our apertures: vocal cords, eyelids, mouth, and other sphincters.
iii. Move gases and liquids through our tubes (air, blood, secretions, food, feces, gametes, and urine).
c. All human behavior consists of secreting substances or changing the length of muscles’ fibers. Whatever the behavior, it originates from nerve impulses traveling through neural circuits.
d. The definition of behavior excludes thinking per se because the Ex cannot directly observe it.
D. Corollaries of the definition of behavior
1. All behaviors and all thoughts depend on neuroanatomic circuits.
2. Any behavior conveys some information about the integrity of some neuroanatomic circuit. For example,
a. Normal movements mean that the peripheral nerves are intact; that their endings are secreting adequate quantities of acetylcholine to the skeletal muscles; that the pyramidal and cerebellar pathways are intact; and that the substantia nigra is secreting adequate quantities of dopamine to the striatum.
b. Normal movement means that a large amount of neural circuitry and a large volume of neural tissue are not the site of a lesion.
3. The concept of behavior and brain function as circuitry applies not only to the rational, conscious Pt but also to newborn infants, those in coma or a persistent vegetative state, and the diagnosis of brain death.
a. If the brain is alive, not depressed by medication, toxins, or metabolic imbalance, and has the right temperature, the Ex can prove it is alive by using the NE to elicit some behavior.
b. Conversely, the total absence of any behavior dependent on brain circuits proves that the brain is dead, if the requirements in a, above, are fulfilled.
II. LOOKING AT EYES
You can see an awful lot just by looking.
If restricted to one part of the examination, choose inspection, the most efficient method of physical diagnosis. Inspection begins the moment you approach your Pt. Immediately you might notice pinpoint pupils and numerous needle scars over the antecubital veins. In two glances you suspect a drug addict. This is the diagnostic power of inspection. But wait a minute. Eyedrops to treat glaucoma may have constricted the pupils, and repeated blood transfusions may have scarred the antecubital veins. The diagnostic value of signs emerges only after integration with a complete history and physical examination. No single diagnostic technique is sufficient.
After a lifetime of looking, you may consider yourself a keen observer. To test how well you have observed something, try to draw it. What you have seen well, you can draw well. Complete the requested drawings faithfully. They are tremendous tools.
Before starting this section, please get a hand mirror and a transparent millimeter ruler. Come on now; be fair. Give the tactics a chance.
A. Relation of the eyelid margins to the iris
1. An opening, the palpebral fissure, separates the upper and lower eyelid margins. See the vertical arrow in Fig. 1-2A.
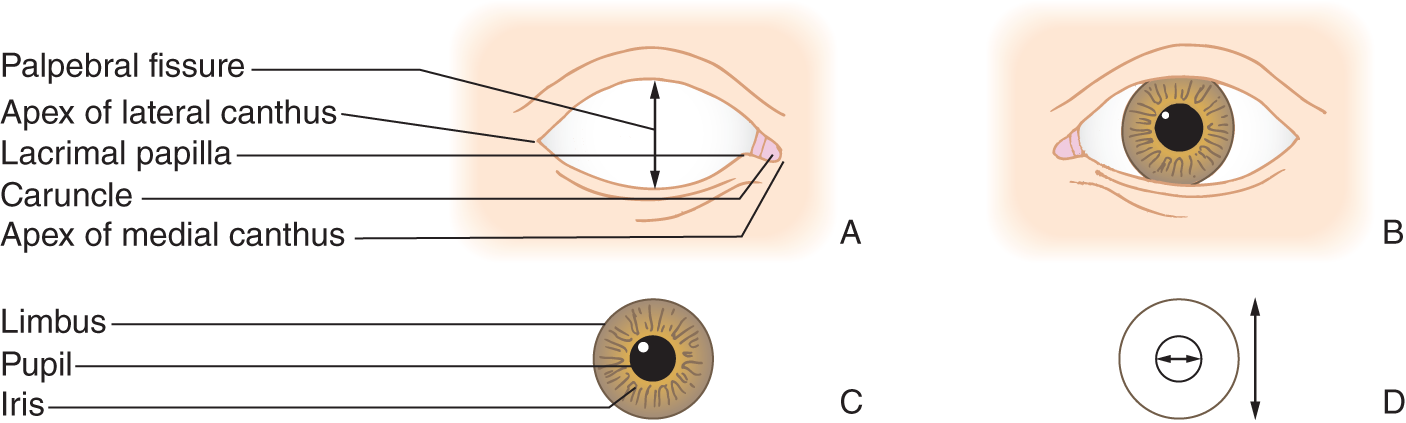
FIGURE 1-2. (A–D) Nomenclature of the external eye.
2. Observe your eyes in a hand mirror; in the space on the left side of this page, draw the exact contours of the margins of your upper and lower eyelids. Especially notice the configuration at the apices of the medial and lateral canthi (medial and lateral angles) of the palpebral fissure.
3. Compare your drawing with the one shown in Fig. 1-2A. Then look again into the mirror and identify the parts of your eye, as listed in Fig. 1-2A.
4. In Fig. 1-2A and in your mirror notice the caruncle, the tiny mound of meaty tissue that occupies the apex of the medial canthus.
5. In the mirror study the iris, the colored disc, surrounded by the white sclera (Fig. 1-2C). Is it uniform in color?
6. Next, in your mirror identify your limbus and pupil.
a. The external circumference of the iris, at its junction with the white of the eye, forms the limbus.
b. The internal circumference of the iris forms the pupil, the opening that admits light into the eye (horizontal arrow in Fig. 1-2D).
c. The cornea, a transparent disc, covers the iris and pupil. The limbus also marks the external circumference of the cornea.
d. Two types of abnormal corneal rings occur near the limbus. A golden-brown or brownish green corneal ring, the Kayser-Fleischer ring, formed by the deposition of copper in the Descemet membrane considered to be pathognomonic of Wilson hepatolenticular degeneration (may also be seen in other conditions such as aceruloplasminemia, primary biliary cirrhosis, Hardikar syndrome [Nydegger et al, 2008], and hypercupremia). A grayish-white ring, the arcus senilis or arcus cornealis, is more prevalent with increasing age and is more frequently observed in men. A unilateral corneal arcus may be a diagnostic sign of carotid artery stenosis (absent on the side of the stenotic artery).
7. In your mirror study the relation of the upper and lower lid margins to the limbus and iris as you look straight ahead. Contrast where the margins of the upper and lower lids cross the iris. _________
8. Set aside your mirror; from memory, draw the iris in the left eye of Fig. 1-3, showing the exact relation of the lid margins to the iris. (Check against Fig. 1-2B. If you erred, redraw the iris in the right eye shown in Fig. 1-3.)
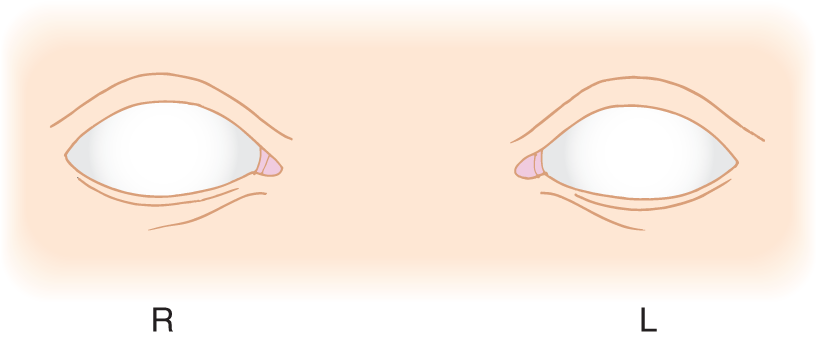
FIGURE 1-3. Blank for drawing the relation of the limbus, iris, and pupil to the lid margins when the patient looks straight ahead. L = left; R = right.
9. From memory, label the structures shown in Fig. 1-3 with the terms from Fig. 1-2, and check your results against Fig. 1-2.
10. Relation of limbus to canthi and caruncles when the eyes deviate.
a. Look in your mirror and study another person to learn the relation of the limbus to the canthi and caruncles when the eyes turn as far as possible to the right or left. Remove eye glasses.
b. With the eyes turned to one side as far as possible, how much scleral white shows between the limbus and the apex of the lateral canthus of the abducted eye? _________
c. Although the lateral arc of the limbus reaches the apex of the lateral canthus, the medial arc cannot reach the apex of the medial canthus because the caruncle occupies it (Fig. 1-2A). Instead, the medial arc of the limbus reaches to, or nearly to, the lateral margin of the caruncle. Thus, with the eyes to one side, the limbus of the abducted eye reaches to, or nearly to, the _________
11. In Fig. 1-4, draw the relation of the limbus, iris, and pupils to the lids when the Pt looks all of the way to the left.
FIGURE 1-4. Blank for drawing the relation of the limbus, iris, and pupil to the lid margins when the patient looks to the left as far as possible.
12. A line drawn through the apex of the medial and lateral canthi of one eye defines the angle of the palpebral fissure (Fig. 1-5).
FIGURE 1-5. Left eye, showing angulations of the palpebral fissure.
B. Anatomic variations of the medial canthus
1. With the eyes at rest, the iris normally is nearly centered between the medial and lateral angles of the eyelids. See Fig. 1-6A.
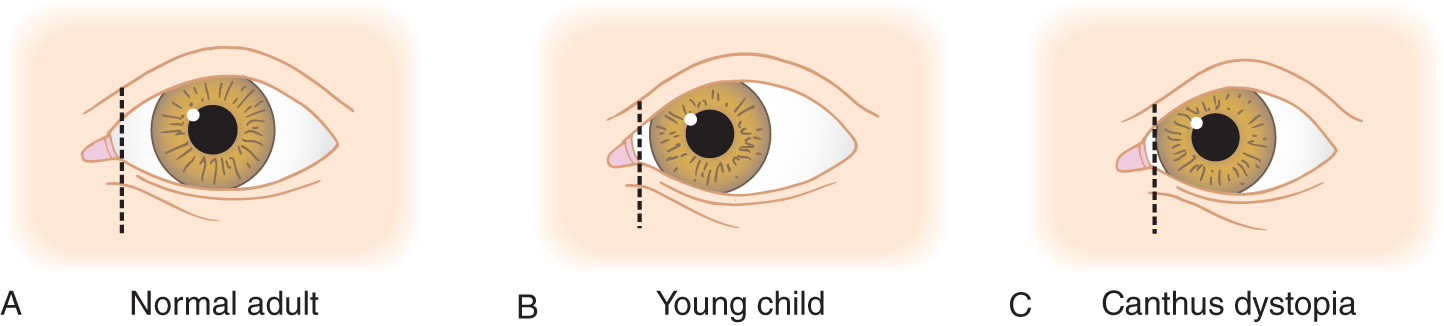
FIGURE 1-6. Left eye, showing variations in the relation of the medial canthus and lacrimal papilla (vertical line) to the corneal limbus.
2. Look at an infant or young child and note that the medial canthus covers more of the conjunctiva than in adults. When the medial canthus is displaced laterally relative to the limbus, as in many young children, their eyes appear to deviate inward, although the eyes are perfectly straight, as shown in Fig. 1-6B.
3. Lateral displacement of the medial canthus, which moves the lacrimal punctum out toward the limbus, is termed canthus dystopia (dys = bad; topos = place; hence, badly placed canthus).
4. Sometimes a skinfold covers the medial canthus. Because the fold is on the canthus, it is called an epicanthal fold. In spaces A, B, and C in Figs. 1-7A to 1-7C, write down your diagnosis: epicanthal fold, normal, or canthus dystopia.
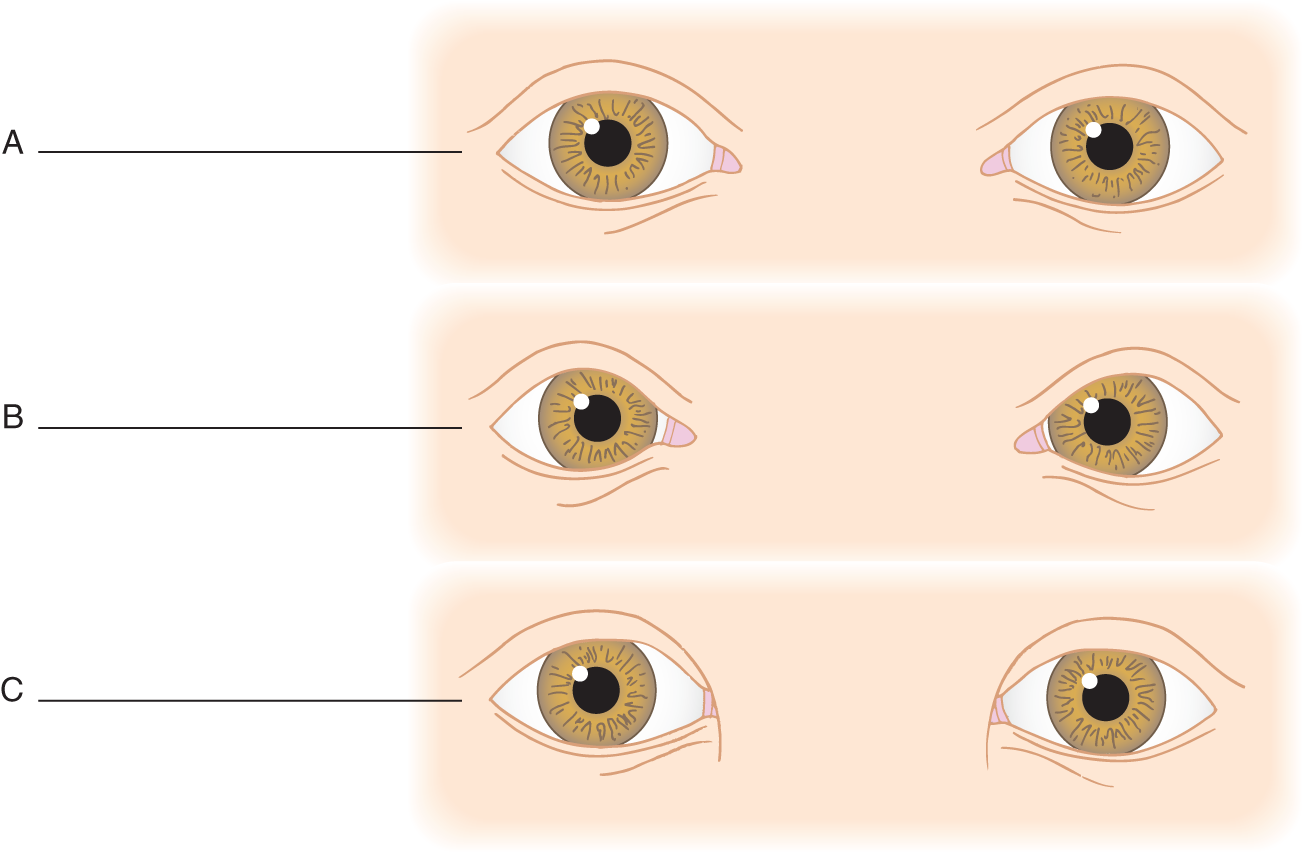
FIGURE 1-7. Write your diagnosis in blanks (A), (B), and (C).
C. Intercanthal and interorbital distances
1. In Figs. 1-7A and 1-7B, measure the distance between the apices of the medial canthi in the normal eyes and those with canthus dystopia. The intercanthal distance of normal eyes is _________
2. The measurement of 1.9 cm is the normal distance for a newborn. Measure your own intercanthal distance by holding up your ruler while looking in a mirror. It will be about 3 cm. See Jones (1997) for graphs of facial measurements.
3. Canthus dystopia or epicanthal folds cause the illusion of an increased distance between the eyes. The bone that forms the medial walls of the orbits sets the actual distance. This distance, the actual interorbital distance, can be measured only from skull radiographs, computed tomography (CT), or magnetic resonance imaging (MRI).
a. If the medial orbital walls, and consequently the eyes, are set too far apart, the Pt has orbital hypertelorism (hyper = excessive; tele = far, as in telephone).
b. If the medial orbital walls and consequently the eyes are set too close together, the Pt has orbital hypo_________
4. What canthal or lid anomalies could produce the illusion of hypertelorism, even with a short interorbital distance? _________
5. What procedure would you order to decide whether a Pt has an abnormal interorbital distance? _________
6. If the interorbital distance is too large, the Pt has _________
7. Hypotelorism or hypertelorism increases the likelihood that the Pt has an abnormal brain (DeMyer, 1967, 1975, 1977).
D. Pupillary size
1. While holding up your millimeter ruler as you look in the mirror, measure and record the size of one pupil: ________ mm. Then measure it with the scale shown in Fig. 12-30 by holding it next to the Pt’s eye.
2. Observe whether both pupils are exactly round and have the same diameter.
a. Most people have exactly round, equal pupils, or isocoria (iso = equal; cor = pupil). The core is the center of something. The prefix a- or an- negates the term that follows. Thus, any congenital or acquired difference in pupillary size is called _________
b. An abnormally small pupil is called cormiosis or simply miosis.
c. Study the width of your iris and its concentricity with the pupils. An eccentric pupil is called corectopia (core = center; ectopia = out of place). A form of corectopia with dorsomedially displaced pupils can occur in unconscious Pts (Kinnier Wilson pupil).
3. Pupils undergo an age-related change in size. The pupils of the newborn infant are small. By adolescence, the pupils are their largest; the “wide-eyed,” innocent look of the adolescent is a look once favored by women who used drops of atropine, or bella donna (= beautiful lady), to give them enlarged, pupils. By old age, the pupils have become small, giving a flinty countenance.
E. Height of the palpebral fissure
1. Although the pupils normally are exactly equal, the height of the right and left palpebral fissures may differ slightly in normal persons because of slight drooping of an eyelid. Pathologic drooping of the upper lid is called ptosis. Look in your mirror to see whether one of your lids droops more than the other.
2. Hold your mirror straight in front of your eyes and then move your mirror up and down but follow it only with your eyes while observing the surface area of your upper lid. In which direction do you see most of the surface area of the upper lid?  Up/
Up/ Straight ahead/
Straight ahead/ Down (
Down ( Down)
Down)
3. Notice in your mirror that, as the eyeballs rotate up and down, automatic adjustments keep the height of the palpebral fissure and the relation of the lid margins to the iris nearly the same. Such automatic adjustments are called associated movements. What would happen to vision if the eyelid margins did not adjust when the eyeballs rotated up or down? _________
4. Hyperthyroid Pts often show a sign called lid lag. As the eyeballs rotate down, the upper lid does not drop. You will see scleral white between the upper lid and the upper arc of the limbus.
5. Protrusion of an eyeball, called exophthalmos or proptosis, widens the palpebral fissure. A sunken eyeball, called enophthalmos, results in a narrow palpebral fissure.
6. Two conditions that reduce the height of the palpebral fissure are drooping of an eyelid, called _________
7. Microphthalmia means a pathologically small eyeball, and macrophthalmia means a pathologically large eyeball. Correspondingly, the eyeball may have a microcornea or a macrocornea.
8. Write the correct diagnosis in Figs. 1-8A to 1-8F. Always compare the two eyes systematically: pupils, iris, and lids.
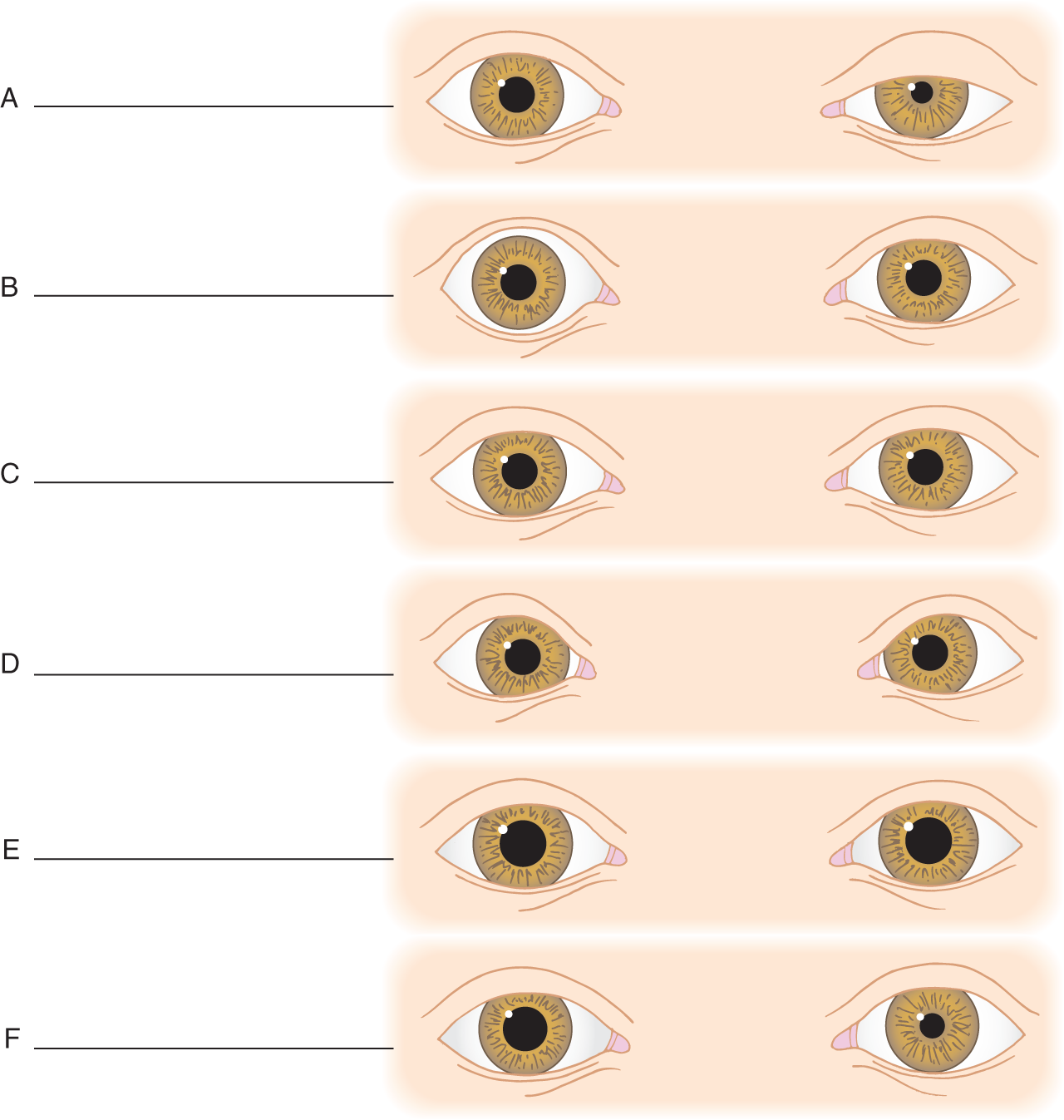
FIGURE 1-8. Write your diagnosis in blanks (A) to (F). (A. Ptosis and cormiosis on L (anisocoria)) (B. Exophthalmia on R) (C. Normal) (D. Canthus dystopia) (E. Macrocornea and pupillodilation (corectasia)) (F. Miosis on L (anisocoria))
9. The foregoing ocular anomalies frequently occur in facial malformation syndromes. Measurement of the intercanthal, interpupillary, and interorbital distances aids in their diagnosis (Jones, 1997).
10. While inspecting the eyelids, note the rate of blinking. Infrequent blinking accompanies a number of conditions from hyperthyroidism to parkinsonism. The absence of blinking causes the eyes to have a “reptilian stare.”
III. INSPECTION OF THE FACE, EARS, HAIR, AND SKIN
The human features and countenance, although composed of but some ten parts or little more, are so fashioned that among so many thousands of men there are no two in existence who cannot be distinguished from one another.
A. Inspection of the nose, mouth, chin, and ears
From abnormalities in the face alone, the perceptive Ex can diagnose literally hundreds of disorders, ranging from infectious diseases, such as leprosy, to endocrinopathies, mental, and neurologic disorders. After inspecting the entire face, start at the eyes and look systematically at the forehead, nose, mouth, chin, and ears.
1. Nose: Consider the bridge, the nostrils, and the relation of the nose to other facial proportions.
2. Mouth: Consider the vermillion border of the lips, the philtrum (the groove between the upper lip and the columella of the nose), median labial tubercle of the upper lip, and the line formed by lip closure. Do the lips make a horizontal closure line? Are the lips closed when the Pt’s face is at rest? Does the mouth hang open, forming an inverted-U upper lip? Does the Pt have microstomia or macrostomia?
3. Chin: Look for a small chin, micrognathia, or a large protuberant chin, macrognathia, as in acromegaly.
4. Ears: Check for contour, shape, and asymmetry. Learn to draw a normal ear and label its parts as shown in Fig. 1-9.
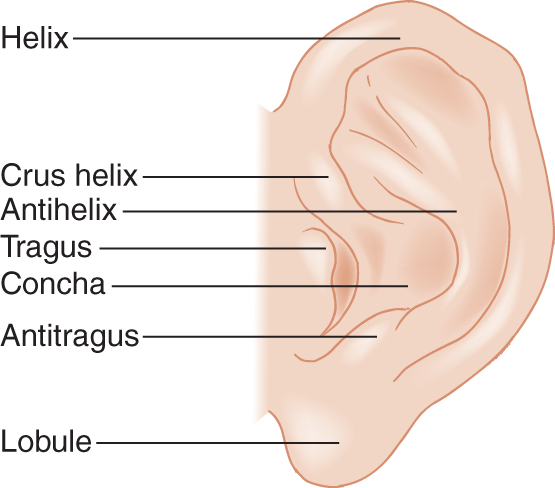
FIGURE 1-9. Anatomy of the normal ear, lateral view. Compare your own ear with that in the drawing and identify the parts.
B. Inspection of the hair of the scalp, eyebrows, and beard
1. Notice the border of the hairline. How does it relate to the forehead and to the nape of the neck? Is the hairline too high or too low? What is the texture of the scalp hair? Is the color nonuniform (poliosis). Is the hair absent in spots (alopecia)?
2. Observe whether the eyebrows are full, scanty, absent, or joined in the midline. The absence of the lateral part of the eyebrows is common in hypothyroidism. Midline union of the eyebrows (synophrys) occurs in some malformation syndromes.
3. Inspect the hair of the beard and face for its distribution and texture. Ask a male Pt how often he has to shave. Many disorders affect the distribution and texture of the hair: infections, congenital malformations, endocrine, and intersex syndromes. Use the Tanner Sexual Maturity Scale to describe secondary sexual characteristics.
C. Inspection of the skin of the face and general body surface
Various neurocutaneous stigmata are virtually pathognomonic of the underlying disease or correlate with neurologic deficits (Gomez, 1987; Hurko and Provost, 1999; Karabiber et al, 2002):
1. Multiple flat brown spots (café-au-lait spots): von Recklinghausen disease or neurofibromatosis (NF-1).
2. Irregular linear blotches of brown pigmentation of the infant’s skin: incontinentia pigmenti.
3. Ash leaf-shaped white spots (naevus anemicus) and facial angiofibromas on the butterfly area and chin: tuberous sclerosis complex. Viewing the Pt in a dark room with a Wood’s lamp (ultraviolet light of 360 nm) enhances discovery of the white spots.
4. Facial hemangiomas (Nevus flammeus) in the V1 distribution of the trigeminal nerve: Sturge-Weber syndrome (Bodensteiner and Roach, 1999).
5. Hypopigmented whorls: Hypomelanosis of Ito.
6. Hemangiomatous hypertrophy of one limb (most commonly the leg): Klippel-Trenaunay-Weber syndrome.
7. Individuals with alcoholic use disorder often have flushed skin when acutely intoxicated. Chronic alcohol use disorder may account for spider nevi over the upper thorax, neck, and head; palmar erythema; multiple bruises; cigarette burns on the fingers; jaundice; gynecomastia; scaly, beriberi-like skin if malnourished; glossitis; and sparse chest, pubic, and axillary hair.
D. How to look at a face to diagnose malformation syndromes
1. If the Pt’s facial gestalt or some individual feature seems odd, the Pt may have a malformation syndrome, and it may affect the face and brain or other internal organs. The face of such a Pt often predicts with some certainty the Pt’s brain anomaly and intellectual potential, as in Down syndrome (Video 1-2) and holoprosencephaly. Thus, in many instances, the face predicts the defective brain (DeMyer, 1975). Even if you cannot recite all of the possible facial malformations, by knowing the limits of normal, you can recognize the abnormal. The face is so important to humans that we have a special area of the cerebrum, the inferomedial temporo-occipital region, wired for facial recognition. (See prosopagnosia in Chapter 11.)

Video 1-2. 47-year-old man with Down syndrome, had posterior wiring and fusion between the inferior posterior margin of the occipital bone and the posterior arch of C3 in 1989. Prior to surgery, he was reading at the 2nd grade level. Down syndrome occurs in 1 of every 691 live births in the United States. Approximately 40-80% of individuals with Down syndrome develop Alzheimer disease-like dementia by the fifth to sixth decade of life.
2. An understanding of facial embryology greatly expedites the diagnosis of malformation syndromes or other disorders affecting the face. The face derives from two morphogenetic sectors, the frontonasal process sector and the branchial arch sector.
a. Figures 1-10 and 1-11 show these two sectors.
b. Notice in Figs. 1-10 and 1-11 that the embryonic frontonasal process (=frontal prominence) produces the forehead, upper eyelids, nose, and medial third of the upper lip.
c. Notice that the branchial arch sectors produce:
i. The ears (and the malleus, incus, and stapes of the inner ear).
ii. The maxillary and mandibular processes and lower eyelids, jaw, and the lateral thirds of the upper lip and the entire lower lip and chin.
d. Notice that the frontonasal process produces the medial third of the upper lip and that the branchial arches produce the lateral thirds of the upper lip.
e. Notice the fusion sites between the medial third and the two lateral thirds of the upper lip. Failure of this fusion produces the common lateral cleft lip (harelip), whereas failure of development of the medial third of the lip produces a median cleft lip.
f. Defects of fusion between the processes of the branchial arches result in branchial cleft cysts in the neck.

FIGURE 1-10. Embryogenesis of the face. Note the frontal prominence (frontonasal process) that forms the forehead and nose and the maxillary and mandibular processes that form the branchial arches.
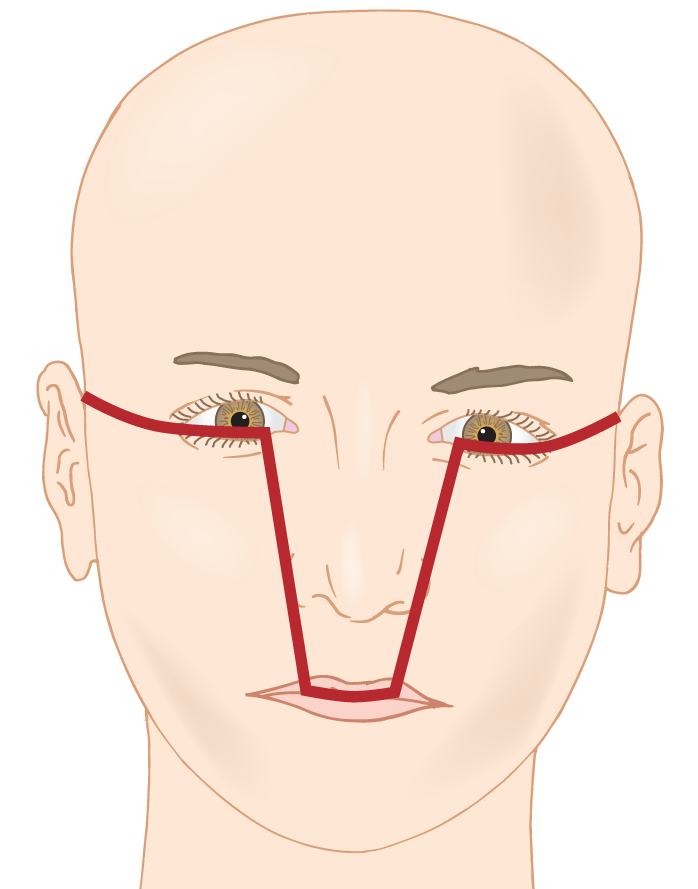
FIGURE 1-11. The heavy line divides the frontonasal sector of the face and the branchial arch sectors.
3. A second method of localization of facial malformations involves dividing the face into three transverse sectors that cut across the morphogenetic sectors (Fig. 1-12).
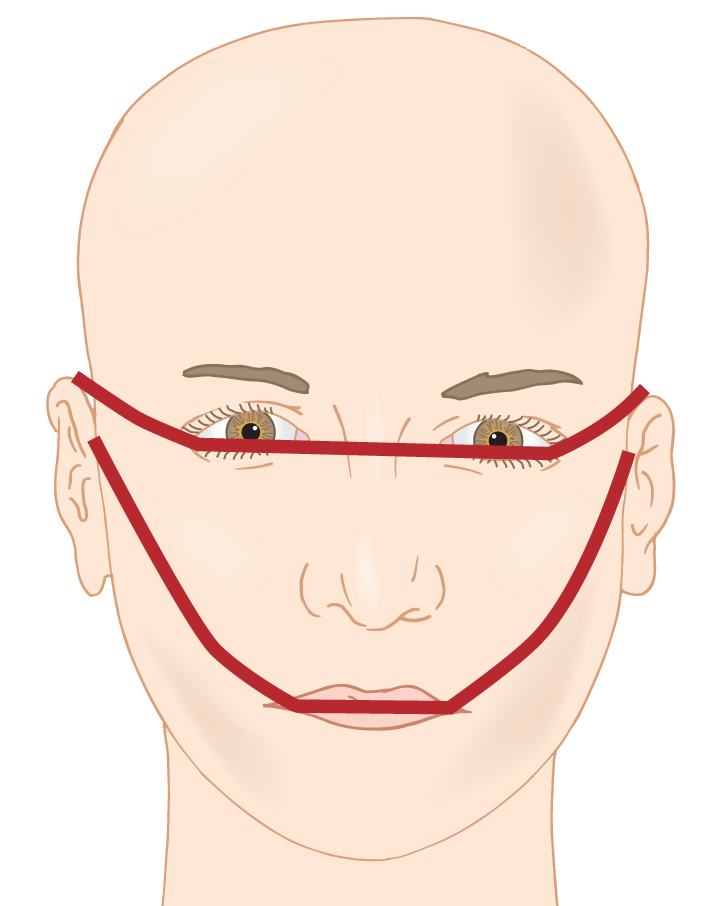
FIGURE 1-12. The lines divide the face transversely for descriptive purposes into upper, middle, and lower parts. Malformations may affect one, two, or all three parts.
4. Malformations may affect the frontonasal process only, resulting in orbital hypo- or hypertelorism or nasal malformations (DeMyer, 1967, 1975; Guion-Almeida et al, 1996); malformations may affect only the branchial arch section, resulting in lateral clefts of the lip and anomalies of the outer and middle ear and jaw; or malformations may affect derivatives of the frontonasal process and the branchial arch sector, resulting in a panfacial malformation syndrome that affects the entire face.
5. Procedure for facial analysis
a. First observe the Pt’s facial gestalt.
b. Then systematically divide the face into parts for individual inspection. Start at the hair and forehead and work down to the chin.
c. Imagine the face as a pair of eyes. Are they too close together or too far apart? Are the pupils unequal? Simply ask and answer such questions as you inspect the Pt’s forehead, nose, mouth, chin, and ears.
6. Decide whether the Pt has a panfacial anomaly, such as Down syndrome, or whether the malformation localizes mainly or exclusively to a sector of the face, based on its morphogenesis.
7. Finally, having identified the anomalies, consult a syndrome compendium to make the diagnosis (Bysse, 1990; Canepa et al, 2001; Gorlin et al, 2001; Jones, 1997; Winter and Baraitser, 2001).
E. A test panel for facial diagnosis
To test your acumen in facial diagnosis, study each Pt in Figs. 1-13A to 1-13L from the hairline down and record any abnormalities. Then check your findings in frame F. Grade yourself on the “honor system.”


FIGURE 1-13. Montage of facial and skin abnormalities detected by inspection. See (F) after you have described the Pts yourself. Figures 1-13E and 1-13F show the same Pt.
F. Descriptions of patients in montage of Fig. 1-13
1. Figure 1-13A shows an infant with rounded face, slight obliquity of the palpebral fissures, open mouth, inverted-U-shaped upper lip, and short upper extremities. The frog-legged position of the lower extremities reflects hypotonia: Down syndrome, trisomy 21.
2. Figure 1-13B shows an infant with high forehead (abnormal upper sector as shown in Fig. 1-12), large head, anti obliquity of the palpebral fissures, and orbital hypertelorism. Radiographic examination disclosed agenesis of the corpus callosum. The infant had mild intellectual developmental disorder.
3. Figure 1-13C shows a boy with “widow’s peak” hairline (midline V-shaped extension of hair pointing downward), hypertelorism plus canthus dystopia plus epicanthal folds, flat nasal bridge with slight median groove, and broad philtrum: median cleft face syndrome (DeMyer, 1967, 1975), also called frontonasal dysplasia. Such patients generally are mentally normal.
4. Figure 1-13D shows a newborn infant with microcephaly, orbital hypotelorism, flat nasal bridge, and median cleft of the upper lip. The apparent ptosis is caused by some lid edema and the fact that the infant has its eyes turned somewhat downward. The facial malformation is restricted to the frontonasal process sector (Figs. 1-10 and 1-11). This face is pathognomonic for holoprosencephaly, a brain malformation of the type shown in Fig. 1-24. Such Pts have severe intellectual disability (DeMyer, 1975).
5. Figure 1-13E shows an infant girl showing normal hairline and forehead. On the left side notice the small palpebral fissure, hypoplastic zygoma and mandible, downward displaced ear, and slight macrostomia: unilateral first branchial arch syndrome (affecting the mandible and maxilla, from the first branchial arch). The frontonasal process sector of the face is normal. Notice the symmetrical corneal light reflections, indicating intact ocular muscles, which derive from somites, not from branchial arches. A small pigmented nevus is present on the right side of the forehead.
6. Figure 1-13F shows the lateral view of patient shown in Fig. 1-13E. Notice the malformed tragus and extra tag of tissue and the hypoplastic left mandible (hypoplasia of the first branchial arch). The rest of the ear, from the second branchial arch, is well-formed.
7. Figure 1-13G shows a boy with midline eyebrows (synophrys), small midfacial segment (the middle of the three horizontal sectors of the face; Fig. 1-12), little or no philtrum, mild micrognathia, and intellectual developmental disorder: Cornelia de Lange syndrome. The patient also had a lumbar hair patch and hypoplasia of the radius and thumb, typical of this syndrome. The apparent ptosis was caused by squinting from the bright light used for photography.
8. Figure 1-13H shows a boy with megalocephaly and high forehead, coarse facial features, midline eyebrows, slight hypertelorism, concave nasal bridge, very broad philtrum with thick lips, and an open mouth (from thickened tongue): Hurler syndrome, mucopolysaccharidosis. The megalocephaly is secondary to metabolic megalencephaly (DeMyer, 1999).
9. Figure 1-13I shows a young woman with very slight ptosis on the right, bilateral temporal and masseter muscle atrophy (hollowing of the temples and posterior cheeks giving a so-called “hatchet face” appearance), expressionless face, and sagging jaw with inverted-U-shaped upper lip: myotonic dystrophy. Males have frontal baldness.
10. Figure 10-13J shows a young man with white forelock, slight synophrys, slight hypertelorism, and heterochromia of the iris. Notice especially the medial part of the iris of the left eye. He was mildly deaf. All of these features are diagnostic of Waardenburg syndrome, type II. Type I patients have dystopia canthorum. He also has incidental acne.
11. Figure 1-13K shows a boy with port wine stain, affecting the maxillary division of the trigeminal nerve (see Fig. 10-2) and, to a lesser extent, the ophthalmic division: Sturge-Weber syndrome, sometimes referred as encephalotrigeminal angiomatosis (Bodensteiner and Roach, 1999). The patient had epileptic seizures due to meningeal and cortical involvement by the angiomatosis.
12. Figure 1-13L shows the back of boy with multiple neurofibromas and café-au-lait spots (arrows): neurofibromatosis, type I. This disorder, like Sturge-Weber syndrome, belongs to the neurocutaneous syndromes, a group of congenital diseases that affect the brain and skin.
BIBLIOGRAPHY · Inspection of the Eyes, Face, Hair, and Skin
Norms for Ocular and Facial Measurements
Farkas LG. Anthropometry of the Head and Face in Medicine. Amsterdam: Elsevier/North Holland; 1981.
Jones KL. Smith’s Recognizable Patterns of Human Malformation. 5th ed. Philadelphia, PA: W.B. Saunders; 1997.
Nydegger A, Van Dyck M, Fisher RA, et al. Hardiker syndrome: long term outcome of a rare genetic disorder. Am J Med Genet A. 2008;146A(19):2468–2472.
Craniofacial Anomalies
Bodensteiner J, Roach ES, eds. Sturge-Weber Syndrome. Mt. Freedom: Sturge-Weber Foundation; 1999.
Bysse ML, ed. Birth Defects Encyclopedia. Cambridge, MA: Blackwell Scientific Publications;1990.
Canepa G, Maroteaux P, Pietrogrande V, eds. Dysmorphic Syndromes and Constitutional Diseases of the Skeleton. Padova: Piccin Nuova Libraria; 2001.
DeMyer W. The median cleft face syndrome: differential diagnosis of cranium bifidum, hypertelorism, and median cleft nose, lip, and palate. Neurology. 1967;17:961–971.
DeMyer W. Median facial malformations and their implications for brain malformations. Birth Defects. 1975;11:155–181.
DeMyer W. Orbital hypertelorism. In: Vinken PJ, Bruyn GW, eds. Handbook of Clinical Neurology. Vol. 30. Amsterdam: North-Holland Publishing Company; 1977, Chap. 9, 235–255.
Gomez M, ed. Neurocutaneous Diseases. A Practical Approach. Boston, MA: Butterworths; 1987.
Gorlin RJ, Cohen MM, Hennekam RCM, eds. Syndromes of the Head and Neck. 4th ed. New York: Oxford University Press; 2001.
Guion-Almeida ML, Richieri-Costa A, Saavedra D, et al. Frontonasal dysplasia: analysis of 21 cases and literature review. Int J Oral Maxillofac Surg. 1996;25:91–97.
Hurko O, Provost TT. Neurology and the skin. J Neurol Neurosurg Psychiatry. 1999;66:417–430.
Jones KL. Smith’s Recognizable Patterns of Human Malformation. 5th ed. Philadelphia, PA: W.B. Saunders; 1997.
Karabiber H, Sasmaz S, Turanh G, et al. Prevalence of hypopigmented maculae and caféau-lait spots in idiopathic epileptic and healthy children. J Child Neurol. 2002;17:57–59.
Stricher M, Van Der Meulen J, Raphael B, Mazzola R, eds. Craniofacial Malformations. Edinburgh: Churchill Livingstone; 1990.
Winter EM, Baraitser M. London Dysmorphology Database and Photolibrary. 3rd ed. Oxford: Oxford Electronic Publishing; 2001.
IV. PALPATION OF THE HEAD, AUSCULTATION, AND THE NEUROVASCULAR EXAMINATION
A. Palpation of the head
1. The laying on of hands, an ancient habit of healers, serves at once as a source of information to the physician and of comfort to the Pt. After inspection, grasp the Pt’s head between your fingertips (or your own head in lieu of a Pt) and, using fairly firm pressure, search for soft spots, lumps, depressions, and areas of tenderness. Palpate your own frontal and parietal eminences. Which region is the widest, the frontal or parietal? By feeling along the midline and then out laterally, locate the depression, the site of the coronal suture, between the frontal and parietal eminences. Feel in the midline posteriorly, where the nape of the neck meets the skull, for the skull bump called the external occipital protuberance.
2. Section VI describes the palpation of the sutures and fontanels of the infant calvaria.
B. The neurovascular examination
Patients suspected of cerebrovascular disease require a thorough neurovascular examination involving palpation and auscultation of the head and arteries and blood pressure measurements (Roach et al, 2010). Check for asymmetry of the right- and left-side arteries.
1. Palpation of arteries
a. Temporal arteries: Lay your index finger lightly, just in front of your tragus, and follow the pulsating temporal artery as far distally as possible. Note any prominence, nodularity, or tenderness of the artery.
b. Subclavian arteries: Lightly place your fingertips 2 to 3 cm across the proximal part of the clavicle, just lateral to the origin of the sternocleidomastoid muscle. Try this in thin-skinned, lightly muscled individuals to learn how to locate the artery (Video 1-3).
c. Remaining arteries: Palpate the radial, femoral, and dorsalis pedis arteries. If you suspect occlusive disease of the carotid artery, avoid palpating it.

Video 1-3. Left upper extremity claudication in a patient with advanced left subclavian artery stenosis associated with Takayasu’s arteritis.
2. Auscultation of the head and neck for bruits
a. A bruit is but an indication of nonlaminar (turbulent) flow and can have many causes. Aneurysms, arteriovenous malformations or fistulae, occlusive vascular disease, and hyperdynamic circulatory states (hemodialysis, fever, anemia, hyperthyroidism) may cause bruits over the carotid arteries or head (Sandock et al, 1982; Roach et al, 2010). Normal infants and children to age 5 years often have benign bruits over the head. A continuous venous hum is generally innocuous. Sometimes normal adults have benign bruits over the carotid arteries, but a strong, localized bruit over the carotid bifurcation increases the likelihood of a stroke but not necessarily in the arterial territory corresponding to the bruit (Howard et al, 1989). Particularly, if the Pt has hypertension, diabetes, or coronary artery disease. Cervical bruits may also be due to flow augmentation because of contralateral carotid artery stenosis, external carotid artery pathology, arterial kinks, sounds transmitted from a more proximal source, turbulent flow due to anemia, hemodialysis, or hyperthyroidism.
b. Pathologic bruits become audible over the carotids after approximately 50% luminal diameter stenosis. The loudness may increase as the stenosis increases. At approximately 90% stenosis, the most ominous bruit appears, which has a soft, high-pitched sound that continues throughout systole into diastole. At 95% or greater stenosis, the bruit disappears.
c. To survey for bruits of the neck vessels, gently place a rubber-edged stethoscope bell at various sites along the carotid and vertebral arteries (Fig. 1-14A) and over the supraclavicular fossa for the subclavian artery. If you suspect occlusive arterial disease, avoid compressing the carotid artery. Carotid bruits ordinarily are heard along the anterior edge of the sternocleidomastoid muscle, and vertebral artery bruits are heard along the posterior edge. Listen carefully at the carotid bifurcation, below the angle of the mandible.
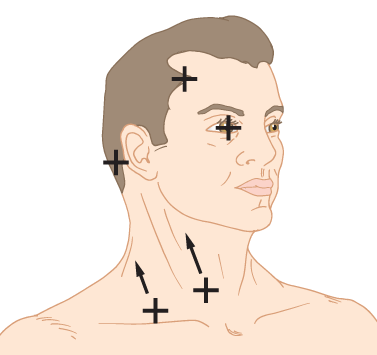
d. Then, place the bell over the mastoid processes, frontal and parietal regions, and over each eye after the Pt gently closes and then relaxes the eyelid. The bell will keep the lid closed, protecting the cornea. Place the bell of the stethoscope over your own eye and listen to the sound as you tense and relax your eyelids.
e. In Fig. 1-14B, draw lines showing where to place the stethoscope bell to listen for carotid or vertebral artery bruits and then mark X’s at the other sites to place the bell to listen for a cranial bruit.
f. Using your stethoscope, listen to what is the silence over your own head. Can you hear heart or breath sounds when you auscultate your carotid arteries? _________
g. When you place the bell over the eye, after the Pt has closed the eye, what do you have to ask the Pt to do to eliminate muscle noise? _________
h. In summary, you will hear intracranial bruits best with the bell of the stethoscope over the eyes, carotid artery bruits over the bifurcation, and subclavian bruits over the supraclavicular fossa.
i. Further investigation of a bruit may require duplex ultrasonography, magnetic resonance angiography (MRA), computed tomography angiography (CTA), or catheter cerebral angiography.
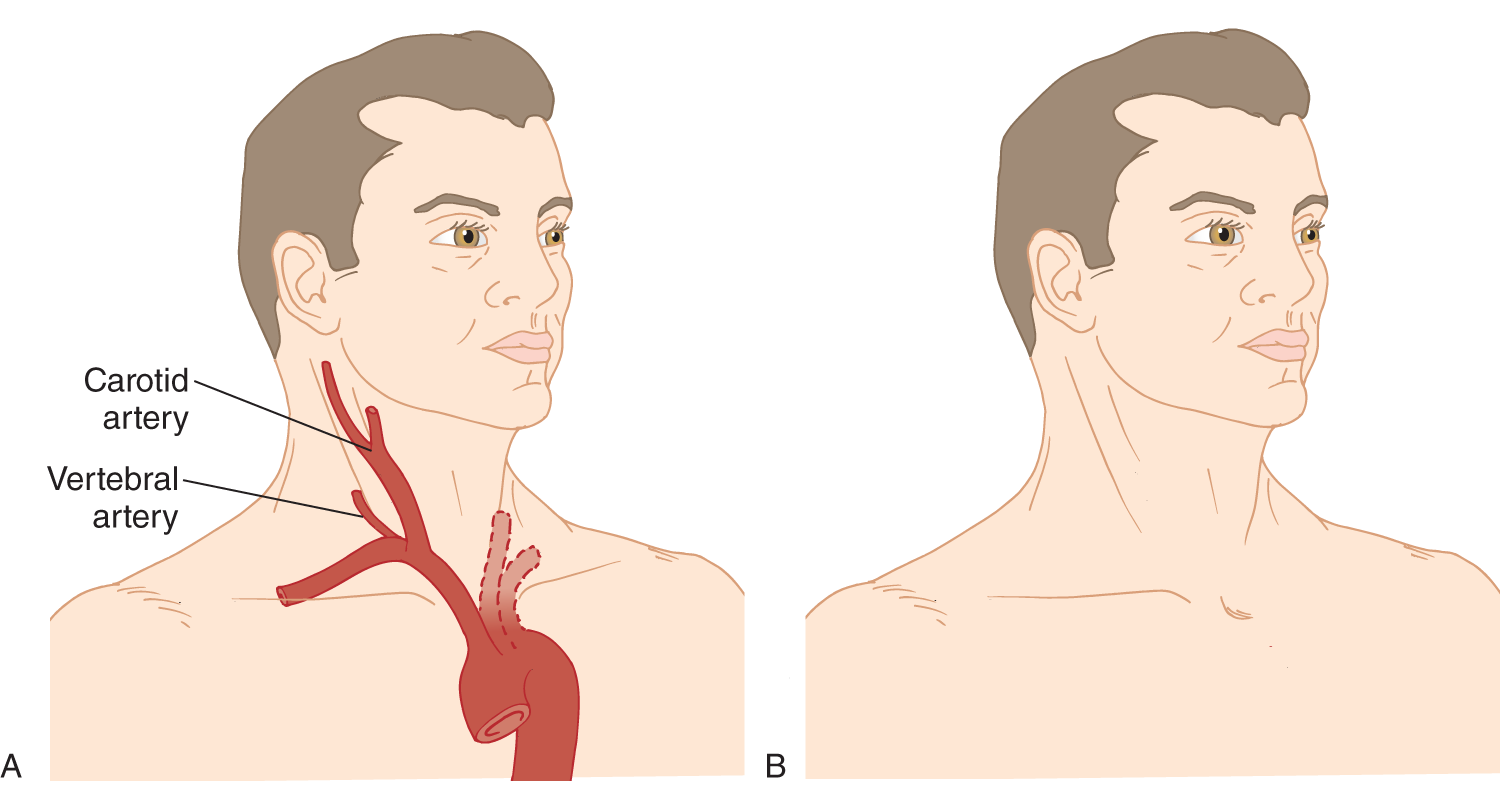
FIGURE 1-14. Auscultation of the neck vessels for bruits. (A) Phantom view of carotid and vertebral arteries in the neck. (B) Blank to mark sites of auscultation for bruits.
3. Blood pressure measurement in patients suspected of cerebrovascular disease
a. Measure the blood pressure in both arms. A consistent difference of more than 10 to 20 mm Hg between the two arms suggests proximal occlusive disease, usually of one of the subclavian arteries. See Chapter 12 for blood pressure measurement in suspected orthostatic hypotension.
b. Measure the blood pressure of the femoral arteries and palpate the pulse. Hypertension in the arms and a reduced pulse and blood pressure in the femoral artery suggest coarctation of the aorta.
BIBLIOGRAPHY · Palpation of the Head, Auscultation, and the Neurovascular Examination
Howard VJ, Howard G, Harpold GJ, et al. Correlation of carotid bruits and carotid atherosclerosis detected by B-mode real-time ultrasonography. Stroke. 1989;20:1331–1335.
Roach ES, Bettermann K, Biller J. Toole’s Cerebrovascular Disorders. 6th ed. Cambridge: Cambridge Press; 2010.
Sandock BA, Whisnant JP, Furlan AJ, Mickell JL. Carotid artery bruits: prevalence survey and differential diagnosis. Mayo Clin Proc. 1982;57:227–230.
C. Percussion
1. Percussion or compression over the frontal and maxillary sinuses and mastoid processes may disclose tenderness when these cavities are infected, but the percussion noted from the skull itself has little diagnostic value.
2. To test for the pain of sinusitis, have the sitting Pt lean forward with the trunk on the legs and head down and then have the Pt perform a Valsalva maneuver. The increased venous pressure acting on the already swollen mucosa increases sinus pain, which will then have a pounding character corresponding to the pulse.
3. Try transillumination of the frontal and maxillary sinuses.
4. To further check for sinusitis and mastoiditis, obtain plain skull films, CT, or MRI to look for mucosal thickening, an air-fluid level, or opacification.
V. TRANSILLUMINATION OF THE INFANT’S HEAD
A. Purpose
Transillumination of an infant’s head may disclose excessive amounts of extra and intracranial fluid and may indicate the need for CT or MRI of the brain (Fig. 1-15).

FIGURE 1-15. Localized transillumination of the parietal region. A parietal craniotomy was done to relieve combined intracerebral and subdural hematoma caused by a head injury. Clear fluid then accumulated between the dura and cerebrum (subdural hygroma) and gradually bulged through the surgical defect in the parietal bone. Observe the scalp vessels coursing over the bulge.
B. Technique
Take the infant into a completely dark closet and allow several minutes for your eyes to adapt to the darkness. Place an ordinary flashlight fitted with a rubber adaptor against the infant’s head and move it over the entire calvarium, including the posterior fossa (Rabe, 1967; Sjögren and Engsner, 1972).
C. Results
Normally, a small halo of light, smaller than 1 cm in diameter, appears around the adaptor margin. Four conditions may enlarge the halo or even cause the entire head to light up.
1. Edema of the scalp, caused by birth injury or infiltration of intravenous fluid (Fig. 1-16A).
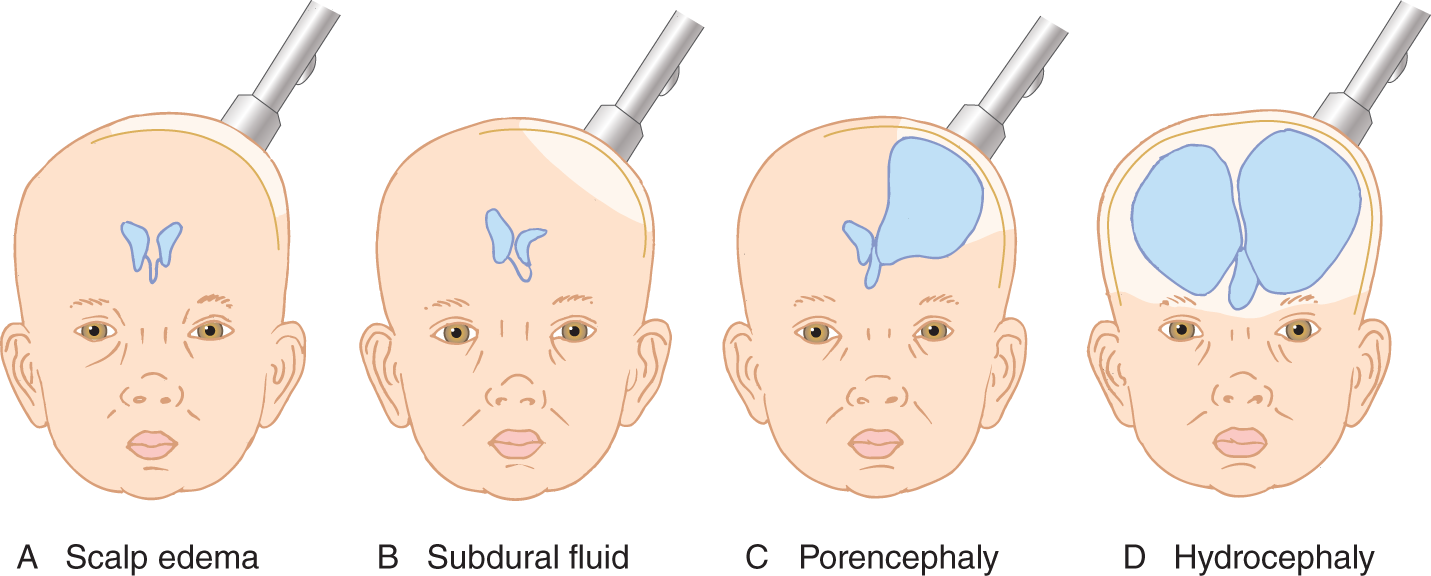
FIGURE 1-16. Head transillumination with lesions at different sites.
2. Increased fluid in the subdural space (subdural hygroma) or in the subarachnoid space (Fig. 1-16B).
3. Increased fluid within the brain because of thinning by hydrocephalic stretching, destruction, atrophy, or hypoplasia (Figs. 1-16C and 1-16D).
4. Premature infants with huge fontanels and very thin cranial bones.
D. Systematic analysis of the causes for cranial transillumination
First consider whether the fluid that transilluminates is outside or inside the calvarium. If outside, the fluid must be in the scalp or subgaleal space.
1. Detection of scalp edema by the pitting test: Bruising and infiltration of fluid given through a scalp vein most commonly cause scalp edema. The pitting test discloses it: press the ball of your finger firmly against the scalp in the area of transillumination. Continue pressing until your fingertip directly feels the bone. After removal of your finger, a pit remains.
2. Detection of subgaleal fluid: Subgaleal fluid elevates the scalp from the calvarium. The bone can be felt by ballottement. Finger pressure readily discloses the distance between the scalp and skull, but the scalp does not pit (unless it is also edematous).
3. Location of fluid inside the skull
a. Fluid inside the skull occupies the subdural or subarachnoid spaces or is intracerebral. If intracerebral, the fluid occupies the ventricles or a cyst (Figs. 1-16C and 1-16D).
b. Acutely after a head injury, epidural blood is too opaque to transilluminate. Exact localization of intracranial fluid often requires imaging (Chapter 13). Sometimes subdural fluid is diagnosed by inserting a needle through the lateral angle of the anterior fontanel or a burr hole, if the fontanels have closed.
4. To prove that you can systematically analyze transillumination, place your hand on top of your head and recite the possible extracranial and intracranial locations of the fluid. Then complete Table 1-1.
TABLE 1-1 • Tests for Locating Excess Fluid When the Head Transilluminates
Location of fluid |
Test procedure to verify |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
_________ |
(Scalp; fingertip pressure for pitting edema) (Subgaleal space; fingertip ballottement for elevation of scalp) (Subdural space; radiographic imaging or taps) (Within the brain; radiographic imaging)
a. In the absence of the cerebellum or in the presence of a large cyst, the posterior fossa (inferior occipital region) transilluminates. Thus, for a thorough examination, move the flashlight over the entire cranium.
b. Transillumination has little value in Pts older than 18 months. The thicker, well-ossified skull will not transmit light. Transillumination in an older Pt would usually mean that the fluid was located _________
5. By adding transillumination to the NE, which consists of inspection, palpation, auscultation, and occipitofrontal circumference (OFC) measurement, you will appreciate Harvey Cushing’s (1869–1939) remark that we have increasing numbers of laboratory tests, “… the vast majority of which are but supplementary to, and as nothing compared with, the careful study of the Pt by a keen observer using his eyes and ears and fingers and a few simple aids.”
BIBLIOGRAPHY · Transillumination of the Infant’s Head
Rabe E. Skull transillumination in infants. Gen Pract. 1967;36:78–88.
Sjögren I, Engsner G. Transillumination of the skull in infants and children. Acta Paediatr Scand. 1972;61:426–428.
VI. THE BONES AND SUTURES OF THE CRANIUM
A. Origin of the skull bones
The cranial bones arise in cephalic mesoderm by two different histogenetic sequences. The endochondral bone of the cranial base develops from preformed cartilage. The membranous bone of the cranial vault and facial skeleton develops directly from osteoid.
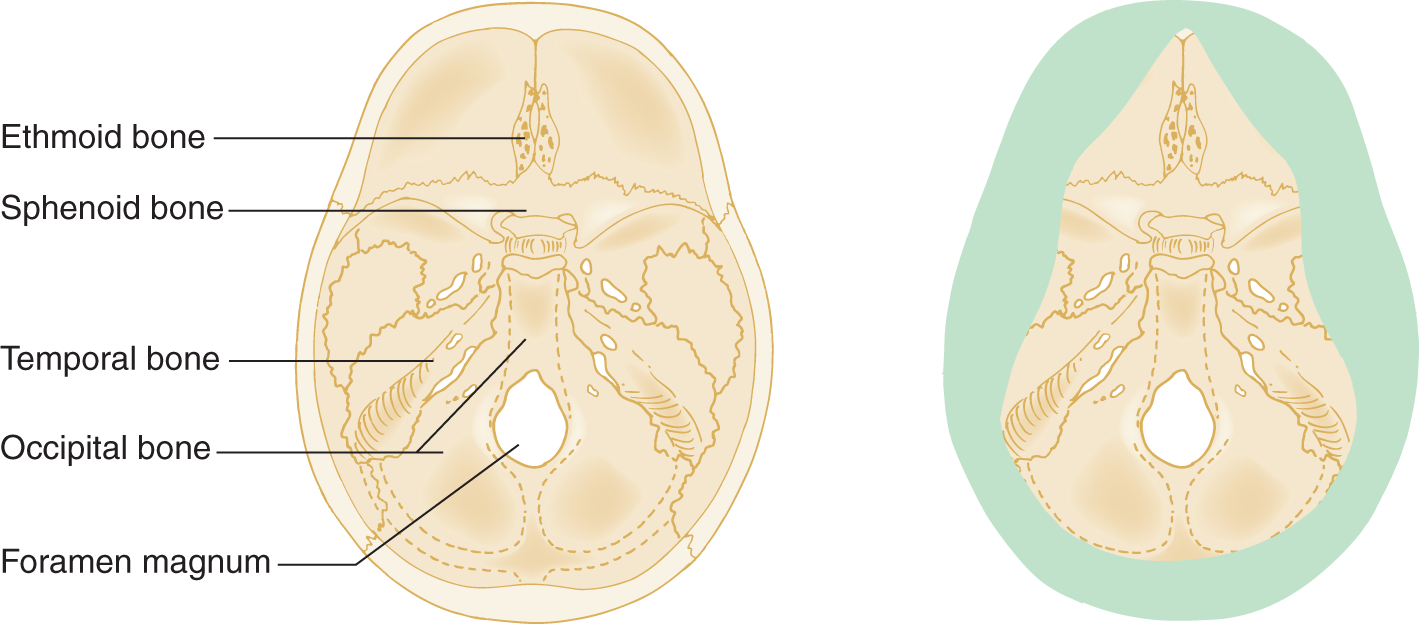
FIGURE 1-17. Interior view of skull base on the left. The right side shows only the endochondral bones.
B. Functional arthrology of the cranium
1. When a cavity separates the two bones at a joint, the joint is a diarthrosis. Connective tissue forms a capsule around the joint space and its cavity. If connective tissue also occupies the space between the bones, leaving no joint cavity, the joint is a synarthrosis.
2. The temporomandibular joints and joints of the ear ossicles are diarthroses. All other skull joints are synarthroses.
3. During morphogenesis, cartilaginous connective tissue united the joints of the endochondral bones at the cranial base. Such synarthroses are called synchondroses. Similarly, fibrous connective tissue united the membranous bones of the cranial vault and of the face to each other and to endochondral bones. Those synarthroses where the large, flat bones of the cranium contact each other are sutures.
4. Wherever membranous bones contact each other or endochondral bones, the joints are called _________
5. In fetuses, the fibrous connective tissue at the sutures is broad and somewhat pliant. Where the fibrous connective tissue forms large, nonossified membranes between the margins of some skull bones, the sites are called fontanels. Learn the sutures, fontanels, and bones in Fig. 1-18.
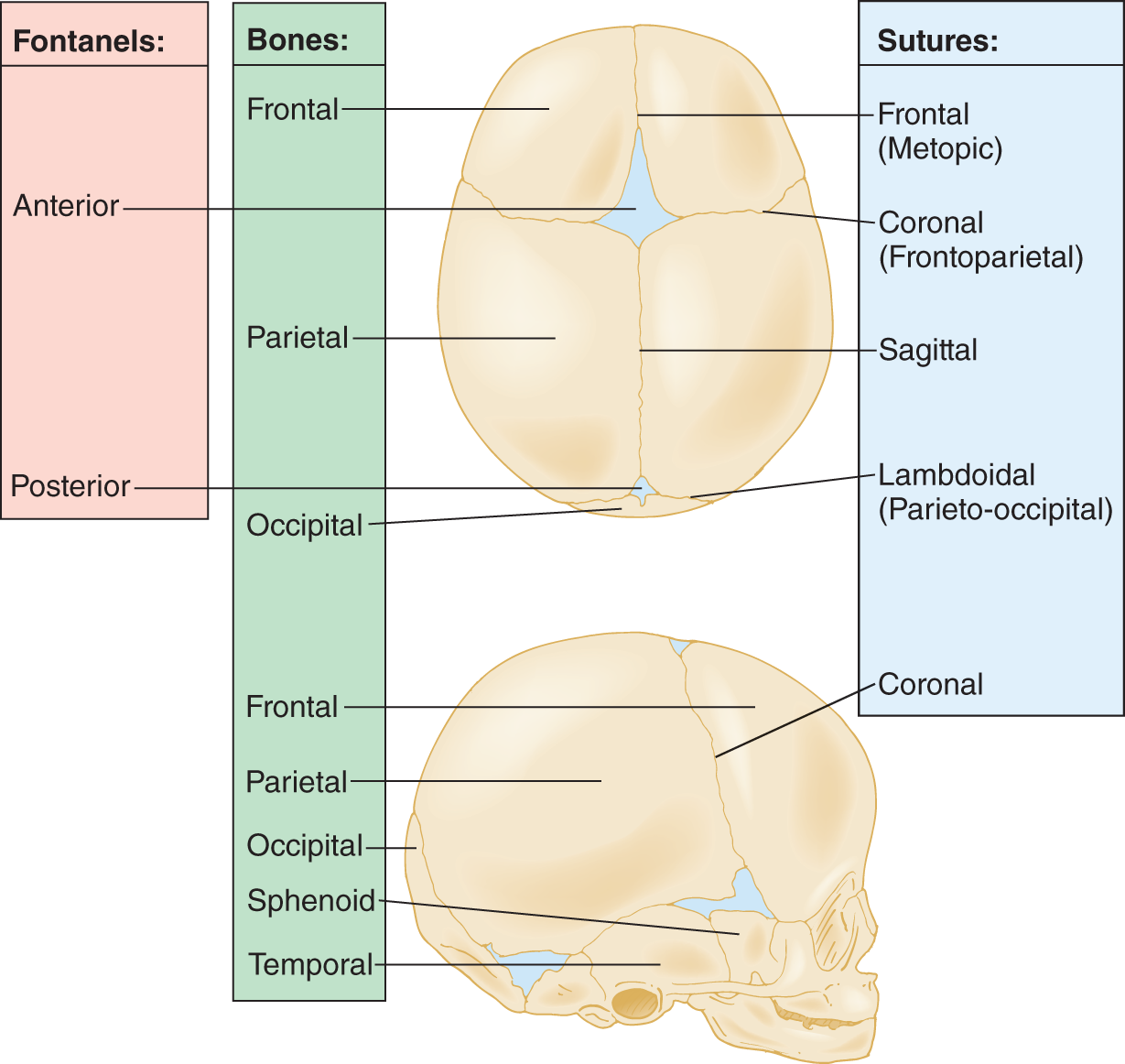
FIGURE 1-18. Fontanels, bones, and sutures of the infant cranium. Top: superior view of cranium. Bottom: lateral view of cranium.
6. The bones that border the largest fontanel, the anterior, are the _________
C. Pliancy of the synarthroses in the infant’s cranium
1. A fontanel is a sheet of fibrous connective tissue that bridges the gaps at the intersections of the sutures in the neonatal skull. Later the fontanels ossify. The sheet of connective tissue at the fontanels allows an up and down, diaphragm-like action, whereas the narrower connective tissue strip at the sutures permits only a limited, hinging action. Thus, the infant skull is most pliant at the  sutures/
sutures/ fontanels. (
fontanels. ( fontanels)
fontanels)
2. The cartilage uniting the synchondroses of the cranial base is less pliant than fibrous connective tissue. Rank the three skull synarthroses, the synchondroses, fontanels, and sutures, in order from most to least pliant: _________
3. During morphogenesis and birth, the cranium serves the contradictory functions of plasticity and rigidity. The plasticity of the cranium permits expansion and deformation, whereas the rigidity of the bones protects the brain during passage through the birth canal. The suture margins may overlap one another, thus damaging the brain by compression or by rupturing veins and venous sinuses.
4. The relative rigidity of the synchondroses prevents buckling of the skull base against the brainstem during birth, which would imperil the passenger even more than deformation of the calvarium.
D. Response of the anterior fontanel to changes in intracranial pressure
1. To the palpating finger, the anterior fontanel is pliant, as every mother knows because she calls it the “soft spot.” Physicians often mistakenly describe the normal anterior fontanel as “flat.” The normal anterior fontanelle in fact will be slightly concave, if you support the infant upright when it is not struggling or crying, because gravity causes the brain to sag away from the calvarium. As you run your finger across the anterior fontanel from one side to the other, the finger will dip down a little as it crosses the fontanel. In addition, a beam of light that crosses the fontanel tangentially will cast a shadow on the concavity of the normal fontanel. The fontanel will also pulsate.
2. If the infant is held upside down, gravity distends the intracranial veins with blood and forces the brain against the roof of the skull. The anterior fontanel, the soft spot, flattens or bulges slightly. When the infant is held upright, the opposite shift in intracranial contents occurs, and the fontanel assumes its normal  concave/
concave/ flat/
flat/ convex contour. (
convex contour. ( concave)
concave)
3. When crying, the infant expires against a partly closed glottis. Contraction of the expiratory muscles increases intrathoracic pressure. The venous system transmits the pressure intracranially. As the intracranial pressure increases, the anterior fontanel  becomes sunken/
becomes sunken/ bulges/
bulges/ does not change. (
does not change. ( bulges)
bulges)
4. Dehydration reduces blood and tissue volume, thereby reducing intracranial pressure. The anterior fontanel  sinks more/
sinks more/ bulges more/
bulges more/ does not change. (
does not change. ( sinks more)
sinks more)
5. Write a statement relating the contour of the anterior fontanel to the intracranial pressure. _________
6. Record the size of the fontanel in the infant’s medical record.
E. Mechanism of normal enlargement of the cranium
1. During the gentle expansion of the fetal and infant brain, bone progressively deposits along the suture margins, keeping them from separating. The most important growth occurs along the sagittal, coronal, and lambdoidal sutures. Study Fig. 1-19.
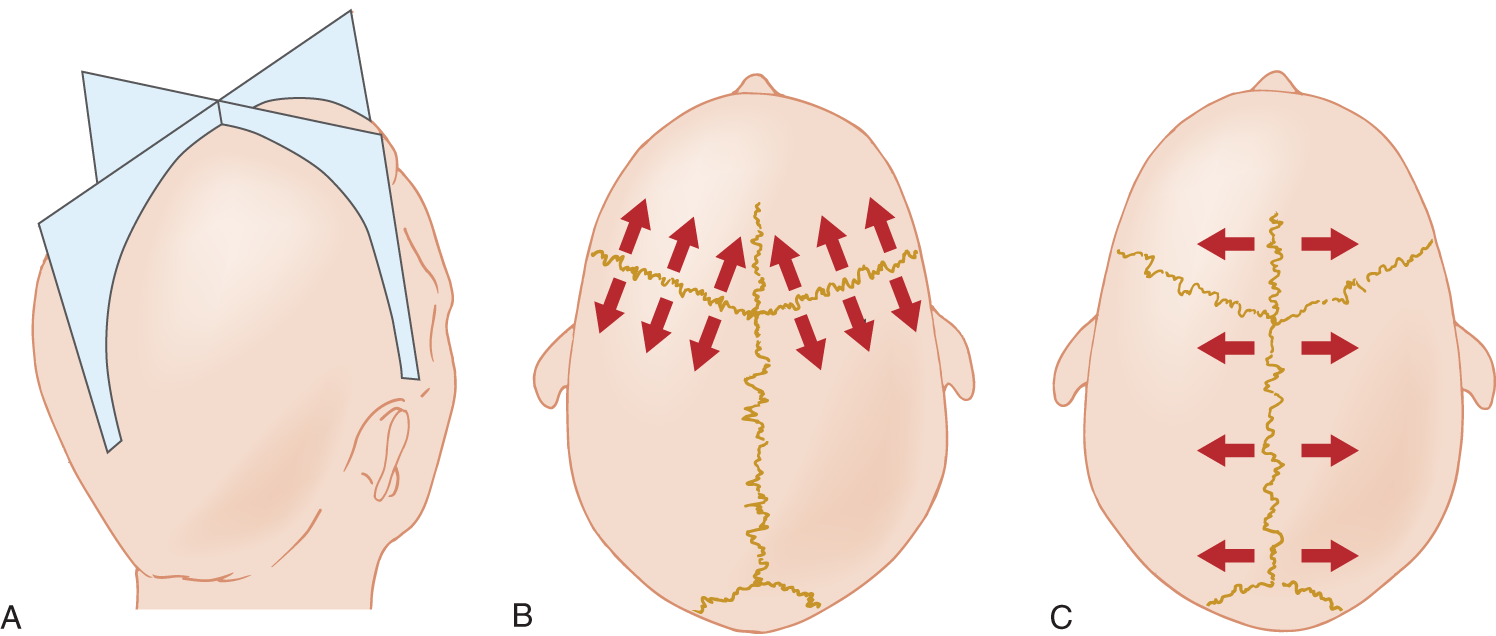
FIGURE 1-19. The sagittal and coronal sutures and the direction of head enlargement from their growth zones. (A) Schematic projection of the planes of the sagittal and coronal sutures. (B) Growth along the coronal suture increases the anteroposterior diameter of the head. (C) Growth along the sagittal suture increases the lateral diameter of the head.
2. Bone deposition along the coronal suture increases the head diameter that is perpendicular to it, ie, the  anteroposterior/
anteroposterior/ lateral diameter. (
lateral diameter. ( anteroposterior)
anteroposterior)
3. Bone deposition along the sagittal suture increases the head diameter that is perpendicular to it, ie, the _________
4. Bone deposition along the parieto-occipital (lambdoidal) suture would increase the _________
5. As a general rule, we can state that growth along a suture margin increases the head diameter that is _________
6. Study the head shapes of your classmates. Some will have unusually short and wide heads, others will have long and thin heads. Both groups will have heads with approximately the same circumference. Therefore, a head that is relatively small in one diameter will, in compensation, enlarge in the opposite diameter. Anthropologists designate short-headedness as brachycephaly (brachy = short) and long-headedness as dolichocephaly (dolicho = long). Which head type would have the greatest lateral diameter? _________
7. In brachycephaly, growth from the _________
8. In dolichocephaly, growth from the _________
F. Closure of the sutures and fontanels
1. With maturation, the cranium becomes increasingly rigid because the synchondroses, sutures, and fontanels progressively unite and finally ossify completely. Before the synarthroses ossify, we say they are “open.” Afterward, they are “closed.” The time of closure of the various sutures by ossification varies considerably (Table 1-2).
TABLE 1-2 • Time of Ossification of Some Cranial Synarthroses
Synarthrosis |
Time ossification completed |
Frontal (metopic) suture |
2 y |
Coronal suture |
30 y |
Basilar synchondroses |
2-20 y |
Anterior fontanel |
1.5 y (range, 3-27 mo) |
Posterior fontanel |
Birth to 2 mo |
2. The anterior fontanel usually closes by 18 months (normal range, 4-27 months). If increased intracranial pressure occurs before closure, the anterior fontanel reflects it first by bulging. Suppose increased intracranial pressure began after that time. Which synarthrosis would yield next to the pressure, the  synchondroses of the cranial base or
synchondroses of the cranial base or  the sutures of the cranial vault? (
the sutures of the cranial vault? ( sutures of the cranial vault)
sutures of the cranial vault)
3. Palpation will disclose wide separation, or “splitting,” of the sutures. Otherwise, skull radiographs or CT are essential. If the sutures split, what happens to the head size? _________
4. Although suture closure by ossification continues into adulthood, functional closure by dense connective tissue occurs much earlier. By 10 to 12 years of age, the sutures have adhered so firmly, although not ossified, that they no longer yield to increased intracranial pressure. If a 16-year-old girl has a big head and split sutures, as shown by a skull radiograph, what is the minimum time she could have had increased intracranial pressure? _________
5. List three physical signs of pathologically increased intracranial pressure in a young infant._________
VII. MISSHAPEN HEADS: CRANIOSYNOSTOSES, DEFORMATIONAL (POSITIONAL) MOLDING, AND CONGENITAL DEFORMITIES
A. The craniosynostoses
1. In the craniosynostoses, one or more sutures and fontanels do not form or, if formed, ossify and close prematurely, resulting in a misshapen head. The excessive bone growth along the suture lines causes palpable ridges (Video 1-4).

Video 1-4. Pfeiffer syndrome. The patient had broad, short thumbs and big toes and had prior surgical correction of craniosynostosi.
2. If the major sutures close in the fetus or young infant during the brain’s growth phase, how would it affect head size and brain size? _________
3. In the absence of sutures and fontanels, what would happen to the intracranial pressure? _________
4. To treat craniosynostoses, surgeons create artificial sutures by removal of strips of bone from the calvarium. The Ex must recognize craniosynostosis in young infants because early treatment produces the best cosmetic results and prevents subsequent complications: increased intracranial pressure, brain damage from restricted brain expansion, and blindness from optic nerve compression (Shillito and Matson, 1968).
5. Diagnostic terms for abnormal head shapes caused by craniosynostosis:
a. The terms dolichocephaly and brachycephaly describe normal variations in the length or width of the skull. The sutures are basically normal. Clinicians use special terms to differentiate the abnormal head shapes caused by craniosynostosis from normal variations. (The terms here follow Ford’s usage [1973], but you will find many synonyms and some discrepancies across investigators.)
b. The pathologic condition of short-headedness from craniosynostosis is acrobrachycephaly (Fig. 1-20A). The closed suture is the _________
c. In acrobrachycephaly, the head becomes too wide because it expands perpendicular to the less affected suture, the _________
d. The pathologic condition of long-headedness from craniosynostosis is called scaphocephaly (scapho = skiff or boat). The head resembles an inverted boat (Fig. 1-20B). The closed suture is the _________
e. In scaphocephaly, the head becomes long because it expands perpendicular to the less affected suture, the _________
f. The pathologic head shape shown in Fig. 1-20C is called oxycephaly (oxy = keen or sharp). The lateral and anteroposterior diameters are reduced. What does this head shape imply about the cranial sutures? _________
g. Complete Table 1-3.
h. Would acrobrachycephaly, scaphocephaly, or oxycephaly most likely cause microcephaly and increased intracranial pressure? Explain. _________
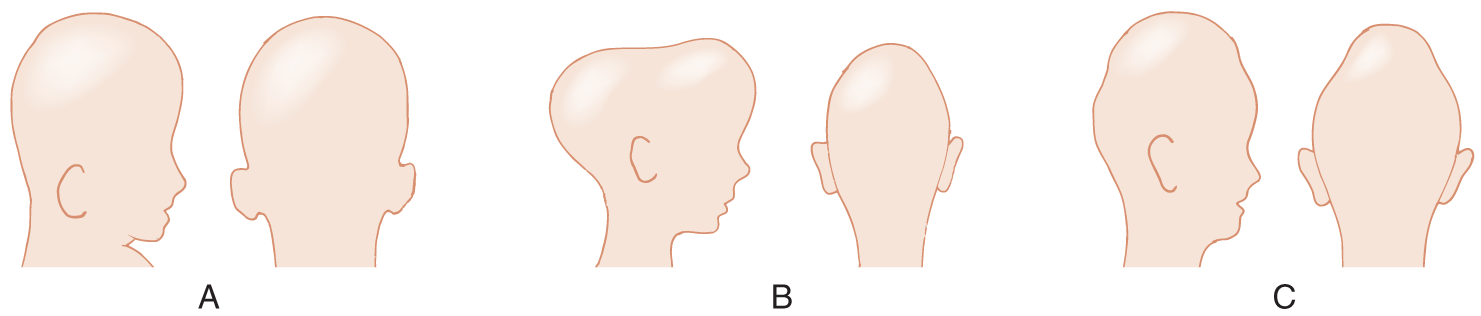
FIGURE 1-20. Silhouettes of abnormal head caused by craniosynostosis. (A) Acrobrachycephaly, (B) scaphocephaly, and (C) oxycephaly.
TABLE 1-3 • Comparison of Terms for Head Shapes
Skull shape |
Term for extreme variation of normal skull |
Pathologic term for craniosynostosis |
Skull too short and wide |
||
Skull too long |
||
Skull too short and narrow |
None |
(Brachycephaly; acrobrachycephaly Dolichocephaly; scaphocephaly Oxycephaly)
6. The increased intracranial pressure in craniosynostosis causes the weakest part of the calvarium to yield. Because it cannot expand at the sutures or fontanels, the thin orbital plates bulge downward, causing the eyes to protrude. The general term for eyeball protrusion is _________
7. Clinical detection of craniosynostosis
a. Clinical recognition of craniosynostosis requires inspection of the infant’s head shape and palpation along the sites of the major sutures. The finger will detect the absence of the sutures and fontanels and replacement by a bony ridge along the suture lines.
b. Skull radiographs or CT confirms the bony overgrowth and the absence of the sutures (CT directly shows bone, whereas MRI does not).
8. A 2-month-old baby with a short head would raise the suspicion of a deficiency of growth from the _________
9. How would you determine whether the child has brachycephaly, a normal variant, or craniosynostosis of the coronal suture, ie, acrobrachycephaly? _________
10. Complete Table 1-4 to fix the diagnostic terms for head shapes firmly in mind.
TABLE 1-4 • Terms for Abnormal Head Shapes
_________ |
Too short a skull because of a lack of coronal suture |
_________ |
Too long a skull because of a lack of sagittal suture |
_________ |
Too long a skull without craniosynostosis |
_________ |
Short, narrow skull because of craniosynostosis |
_________ |
Short skull without craniosynostosis |
(Acrobrachycephaly) (Scaphocephaly) (Dolichocephaly) (Oxycephaly) (Brachycephaly)
11. Craniosynostosis of various types occurs in numerous syndromes, many with congenital malformations of the brain and extremities. See Ashwal (1999) and Cohen (1986).
BIBLIOGRAPHY · Craniosynostosis
Ashwal S. Congenital structural defects. In: Swaiman KE, Ashwal S, eds. Pediatric Neurology. Principles & Practice. 3rd ed. St. Louis, MO: Mosby; 1999, Chap. 17, 234–300.
Cohen MM, ed. Craniosynostosis: Diagnosis, Evaluation, and Management. New York: Raven Press; 1986.
Ford F. Diseases of the Nervous System in Infancy, Childhood and Adolescence. 6th ed. Springfield, IL: Charles C. Thomas; 1973.
Shillito J Jr, Matson D. Craniosynostosis: a review of 519 surgical patients. Pediatrics. 1968;41:829–853.
B. The asymmetrical head: plagiocephaly and deformational molding of the cranium
1. Intrinsic diseases such as craniosynostosis, rickets, and syphilis alter head shape, as does extrinsic pressure. Striking examples of molding of the cranium and the feet by extrinsic pressure occurred in certain societies that sought to beautify girls by tightly bandaging the head and feet during infancy.
2. If an infant has an abnormal position in utero or if a neurologic deficit, such as hemiplegia or hemianopia, causes it to recline on one part of its head after birth, the head deforms to become flat on the “down” side.
3. If the young infant reclines too much on its back, the occiput becomes symmetrically flattened (DeMyer, 2002). The prolonged sleeping position of infants on their backs to avoid sudden infant death syndrome has caused a distinct increase in deformational or positional occipital flattening (Huang et al, 1996; Marshall et al, 1997; Mulliken, 1999). The brachycephaly is usually mild, more or less symmetrical, and self-correcting as the infant learns to sit up and spends less time on its back. The long periods that premature infants spend on the sides of their heads causes a deformational long-headedness, a dolichocephaly, that also tends to self-correction.
4. If the infant reclines on one parieto-occipital region, it flattens, and the head becomes skewed or asymmetrical. When the head molding, prenatally or postnatally, produces head asymmetry, the condition is called plagiocephaly. Extracranial lesions that may accompany plagiocephaly are torticollis and vertebral malformations. Sometimes no cause for skull asymmetry can be identified.
5. Although usually bilateral, craniosynostosis may obliterate the sutures only on one side of the head. The affected hemicranium is small, causing an asymmetrical calvarium and face. Some investigators prefer to limit the term plagiocephaly to the deformity caused by craniosynostosis.
6. Plagiocephaly affects the whole base of the skull and often causes a tilt of the base. With deformational plagiocephaly, the unilaterally flattened occiput displaces or rotates the ipsilateral ear and cheek forward (Fig. 1-21A).

FIGURE 1-21. Top view of plagiocephaly. (A) The two left drawings show simple deformational or positional molding of the head and its effect on the prominence of the occiput and cheek. The sutures are open on both sides. (B) The two right drawings show the effect of unilateral craniosynostosis in restricting head growth on the right side. Notice the difference in the forward positioning of the forehead and cheeks between (A) and (B). (Reproduced with permission from Marshall D, Fenner GC, Wolfe A, et al. Abnormal head shape in infants. Int Pediatr. 1997;12:172-177.)
7. With the much rarer craniosynostotic plagiocephaly, which may occur from unilateral lambdoidal synostosis, the restricted hemicranium fails to expand, and the ear and cheek ipsilateral to the flattening are displaced backward relative to the normal forward growth on the opposite side (Fig. 1-21B).
8. Use the “two-forefinger” test to determine the degree of rotation of the skull base: look down on top of the infant’s head and insert your forefingers lightly into the external end of each ear canal. Normally, the fingers should align exactly. In plagiocephaly, the degree of offset of the fingers reflects the degree of rotation of the calvarial base.
9. Because torticollis may accompany the plagiocephaly, palpate the sterno-cleidomastoid muscle for a hematoma or fibrotic tightening.
10. Plagiocephaly from lambdoidal synostosis may warrant surgical correction, whereas the much commoner deformational plagiocephaly usually responds to positioning and application of a molding helmet (Marshall et al, 1997; Mullikan et al, 1999).
11. In any event, an abnormal size or shape of the skull raises the question of an abnormal brain (Miller and Clarren, 2000), often requiring further investigation by MRI or CT. Computed tomography can depict the skull and sutures in three dimensions.
C. Misshapen heads in neonates, other than plagiocephaly
1. The most common conditions are molding of the head during birth and consequent scalp edema, frank caput succedaneum, subgaleal hematoma, and cephalohematoma, all from birth trauma, and meningocele or meningoencephalocele, which are malformations. In the conditions caused by birth trauma, the scalp has closed in the midline.
2. Meningoceles or meningoencephaloceles are saclike protrusions in the midline and may occur from the occipital region to the forehead. They resemble a “topknot.” Meningoceles generally transilluminate, whereas meningoencephaloceles generally do not.
3. In scalp edema or in the frankly hemorrhagic scalp edema called caput succedaneum, the infant has a scalp swelling that alters the head contour smoothly, usually symmetrically, and at the vertex (Volpe, 2001). Scalp edema, whether from injury or infiltration of intravenous fluid, transilluminates readily, but the greater the hemorrhage, the more opaque the lesion.
4. In subgaleal hemorrhage, the blood occupies a space between the external periosteum and the galea aponeurotica of the scalp. It does not transilluminate. It may cause significant blood loss.
5. In cephalohematoma, blood dissects the external periosteum away from one of the large, flat calvarial bones, usually the parietal. The lesion thus causes a localized bulge, limited by suture lines. The adherence of the periosteum at the sutures arrests the spread of the lesion to adjacent bones. A unilateral parietal hematoma is limited medially by the sagittal suture, frontally by the coronal suture, and posteriorly by the parieto-occipital (lambdoidal) suture. The underlying bone sometimes has a fracture.
6. By palpation, scalp edema pits, subgaleal hematoma is fluctuant or allows ballottement, and a cephalohematoma is very firm. None of these extracranial lesions pulsate, whereas meningoceles or any other lesion connected to the intracranial space do. Final diagnosis of the lesion and its consequences for the brain may require imaging by ultrasound, CT, or MRI.
BIBLIOGRAPHY · Misshapen Heads
DeMyer W. Small, large or abnormally shaped heads. In: Maria BL, ed. Current Management in Child Neurology. 2nd ed. Hamilton, BC: Decker Inc.; 2002, Chap. 49, 299–304.
Huang M, Gruss J, Mouradian WE, et al. The differential diagnosis of posterior plagiocephaly: true lambdoid synostosis versus positional molding. Plast Reconstr Surg. 1996;98:765–774.
Marshall, D, Fenner GC, Wolfe A, et al. Abnormal head shape in infants. Int Pediatr. 1997;12:172–177.
Miller RI, Clarren SK. Long-term developmental outcomes in patients with deformational plagiocephaly. Pediatrics. 2000;105:e26.
Mulliken JB, Woude DL, Vander DL, et al. Analysis of posterior plagiocephaly: deformational versus synostotic. Plast Reconstr Surg. 1999;103:371–380.
Volpe JJ. Neurology of the Newborn. 4th ed. Philadelphia, PA: W.B. Saunders; 2001.
VIII. THE SIZE OF THE HEAD AND BRAIN
A. Determinants of head size
The head size of any given individual depends on the factors listed in Table 1-5. Learn this table.
TABLE 1-5 • Determinants of Occipitofrontal Circumference of Infants and Children
Age Genetic background Sex Family somatotype Volume of intracranial contents Brain size Amount of fluid in the ventricles and meningeal spaces Volume of blood in the intracranial vessels Space-occupying lesions Ability of calvarium to expand (open or closed sutures) Thickness of the calvarial bones, scalp, and hair |
B. Measuring the head size: the occipitofrontal circumference (OFC)
1. The two most important measurements of the newborn infant are its birth weight in relation to gestational age and its OFC (Raymond and Holmes, 1994; Roche et al, 1987; Sher and Brown, 1975; Sheth et al, 1995; Volpe 2001). Record every infant’s OFC at every examination (DeMyer, 1999, 2002). Place a tape measure around the maximum OFC, from the external occipital protuberance (inion) to the glabella. Figures 1-22A to 1-22D show the normal values, which differ by age and sex.

FIGURE 1-22. Growth curve for the occipitofrontal circumference. The areas shown for males and females include 2 standard deviations above and below the mean. Ninety-five percent of normal children fall within these limits.
2. Mnemonic for the infant’s OFC: The average full-term newborn weighs approximately 3500 g. The birth OFC is approximately 35 cm. The OFC increases, on average, about 1 cm per month, making the average OFC at 1 year about 47 to 48 cm.
3. An OFC beyond +2 standard deviations from the mean always raises suspicion of an abnormal brain, particularly with normal body weight, chest circumference, length, and body proportions. Measure the OFCs of parents and siblings to make allowance for normal variations of somatotype (DeMyer, 1999; Weaver and Christian, 1980).
4. Too large a head is called macrocephaly (megalocephaly), and one that is too small is called microcephaly (Opitz and Holt, 1990). An infant’s head may be too large or too small at birth, or the head may become abnormal in size later on. To best recognize an abnormal trend in head size as the infant develops, plot successive OFC measurements on a chart (Fig. 1-23). In Fig. 1-23, trend line A represents an abnormally enlarging head, and trend line B represents a lagging head size.
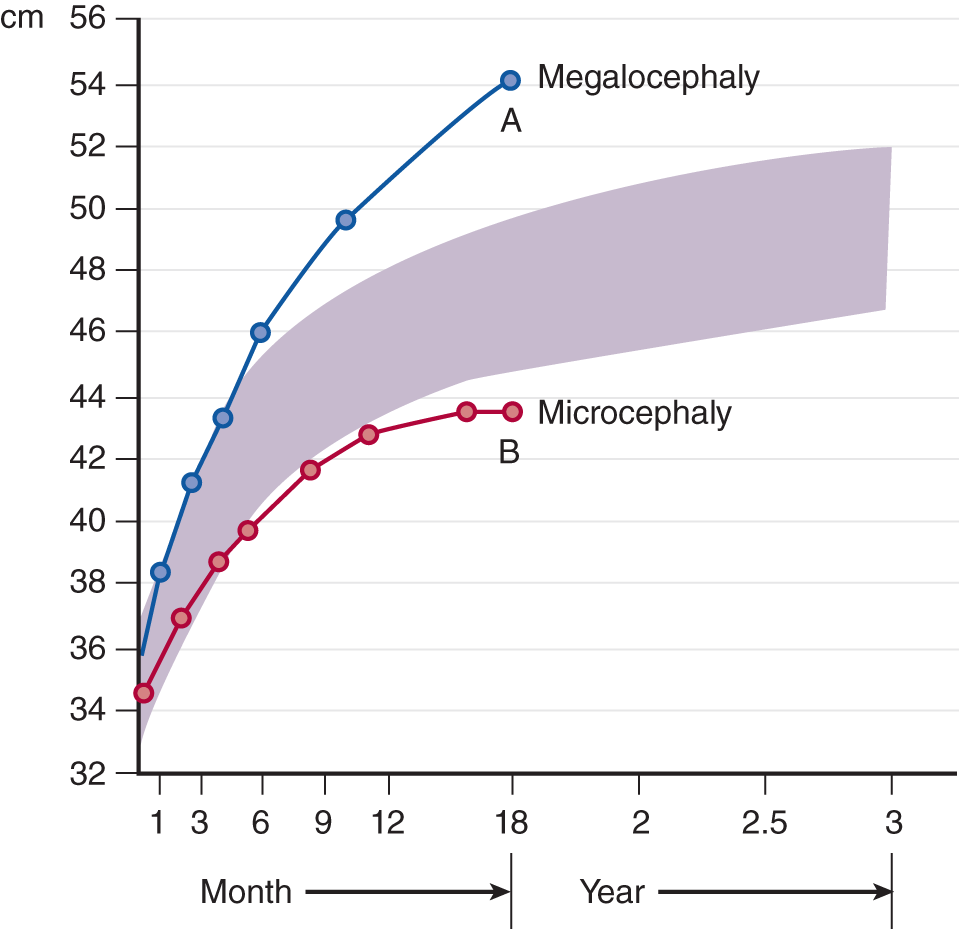
FIGURE 1-23. Successive plots of abnormal head sizes. The shaded area represents ±2SD from the mean.
BIBLIOGRAPHY · Measuring the Head Size
DeMyer W. Microcephaly, micrencephaly, megalocephaly and megalencephaly. In: Swaiman K, Ashwal S, eds. Pediatric Neurology. 3rd ed. St. Louis, MO: Mosby; 1999, Chap. 18, 301–311.
DeMyer W. Small, large and abnormally shaped heads. In: Maria BL, ed. Current Management in Child Neurology. 2nd ed. Hamilton, BC: Decker Inc.; 2002, Chap. 49, 299–304.
Opitz JM, Holt MC. Microcephaly: general considerations and aids to nosology. J Craniofac Genet Dev Biol. 1990;10:175–204.
Raymond GV, Holmes LB. Head circumference standards in neonates. J Child Neurol. 1994;9:63–66.
Roche AF, Mukherjee D, Guo S, et al. Head circumference reference data: birth to 18 years. Pediatrics. 1987;79:706–712.
Sher PK, Brown SB. A longitudinal study of head growth in pre-term infants. II. Differentiation between “catch-up” head growth and early infantile hydrocephalus. Dev Med Child Neurol. 1975;17:711–718.
Sheth RD, Mullett MD, Bodensteiner JB, et al. Longitudinal head growth in developmentally normal preterm infants. Arch Pediatr Adolesc Med. 1995;149:1358–1361.
Volpe JJ. Neurology of the Newborn. 4th ed. Philadelphia, PA: W.B. Saunders; 2001.
Weaver D, Christian J. Familial variation of head size and adjustment for parental head circumference. J Pediatr. 1980;96:990–994.
C. Relation of head size to brain size
1. The technical term for the head is cephalon and that for the brain is encephalon. If the brain or encephalon is too small and weighs too little, the condition is called micrencephaly. If the brain is too large and weighs too much, it is called _________
2. Of necessity, a Pt with microcephaly has  megalencephaly/
megalencephaly/ micrencephaly/
micrencephaly/ a normal brain size. (
a normal brain size. ( micrencephaly)
micrencephaly)
3. Of necessity, a Pt with megalencephaly must have megalocephaly, but a Pt with megalocephaly does not necessarily have megalencephaly. Megalocephaly is simply a statistical description of head size, not a diagnosis or a synonym for megalencephaly. In megalocephaly, the brain may be normal in weight, too large, or too small. In fact, Pts with little or no cerebrum (hydranencephaly) may have microcephaly, normocephaly, or megalocephaly. Thus, the encephalon may be much smaller than suggested by the OFC. When the brain fails to fill the cranium, fluid occupies the extra space. Study Fig. 1-24.
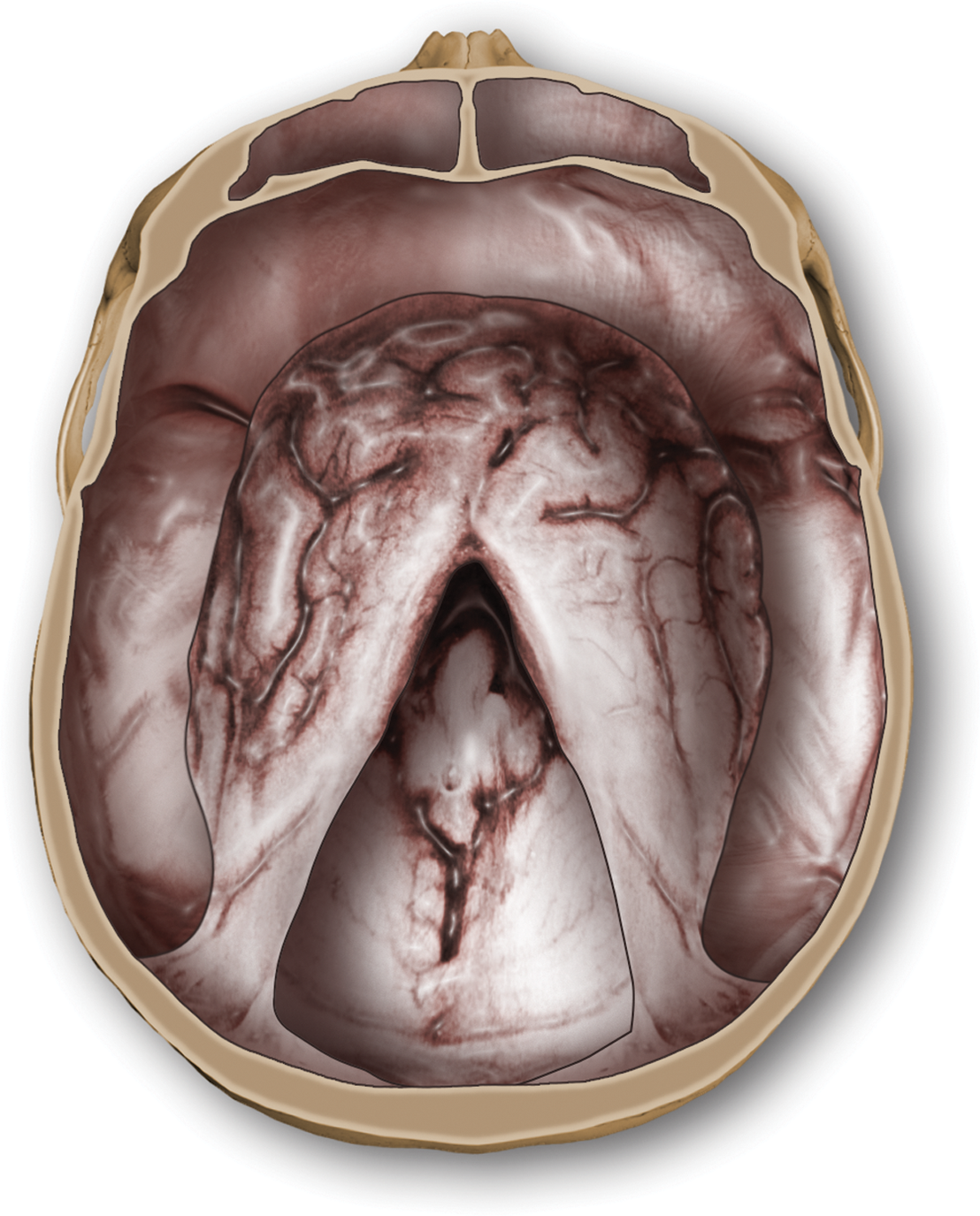
FIGURE 1-24. Postmortem photograph of a micrencephalon in a microcephalic skull. When this patient died at the age of 4 months, the occipitofrontal circumference was only 32 cm (normal, 41 cm). Despite the tiny head, the disproportionately small brain failed to fill the intracranial space.
4. Select the correct statement about head size and brain size:
a. The OFC and brain weight have a strict, linear correlation in normal and pathologic conditions.
b. Even though the OFC and brain weight generally correlate, the OFC predicts the maximum weight the brain may have, but not the minimum.
c. Even though the OFC and brain weight generally correlate, the OFC predicts the minimum weight the brain may have, but not the maximum.
d. None of these answers apply. ( b)
b)
5. The relation of head size to intelligence is complex. As the OFC approaches and exceeds ±2 standard deviations from the mean, the likelihood of an abnormal brain increases. Some evidence suggests that a head size slightly larger than average correlates with increased intelligence and protects against dementia (Tisserand et al, 2002), but children with neurofibromatosis, muscular dystrophy, and autism have heads larger than average and yet have below-average IQs.
D. Head size, brain size, and hydrocephalus
1. Whenever the volume of cerebrospinal fluid (CSF) is significantly increased, the condition is called hydrocephaly (=hydrocephalus), irrespective of brain size or head size, irrespective of the location of the CSF, whether within the ventricles or subarachnoid space, and irrespective of the intracranial pressure. Thus, the one condition necessary to justify the term hydrocephaly is an excessive volume of _________
2. The Pt shown in Fig. 1-24 happened to have microcephaly and micrencephaly. The disproportionately small brain left a large fluid-filled space. Thus, this Pt had hydrocephaly, microcephaly, and micrencephaly.
3. Hydrocephaly has a second meaning, a triad of increased volume of CSF, increased intracranial pressure, and usually increased head size. If the excessive CSF is under pressure in the ventricles, the cerebral wall thins, the ventricles dilate, and the head size increases, if the sutures have not closed (Fig. 1-25).

FIGURE 1-25. Coronal sections of the brain. (A) Normal brain showing configuration of the anterior horns of the lateral ventricles. (B) Normal anterior horns as shown by radiography (in this case, pneumoencephalography). (C) Dilated anterior horns in a patient with obstructive hydrocephalus. (D) Radiograph of dilated anterior horns in a patient with obstructive hydrocephalus.
4. With an undersized brain, as from atrophy, hypoplasia, or destructive lesions, the excessive CSF may merely fill in the unoccupied space (nature abhors a vacuum). In this condition, called hydrocephalus ex vacuo, the intracranial pressure is normal. Does the term hydrocephaly apply correctly?  Yes/
Yes/ No. Explain. _________
No. Explain. _________ Yes. The first definition, the purely quantitative one, applies even though the Pt does not have increased intracranial pressure)
Yes. The first definition, the purely quantitative one, applies even though the Pt does not have increased intracranial pressure)
5. Thus far, the terms micro-, megalo-, and hydro- have been used quantitatively to mean too much or too little of something. Thus, they are descriptive terms, like gigantism or dwarfism, not diagnoses. An abnormal head size usually requires radiographic visualization of the size and shape of the brain and its ventricles and subarachnoid spaces. Visualization techniques include ultrasound in fetuses and very young infants and CT or MRI (Chapter 13).
E. Causes of megalocephaly
1. One of five conditions usually causes megalocephaly. Be able to recite these. See DeMyer (1999) for a full list of differential diagnoses for megalocephaly (Fig. 1-26).
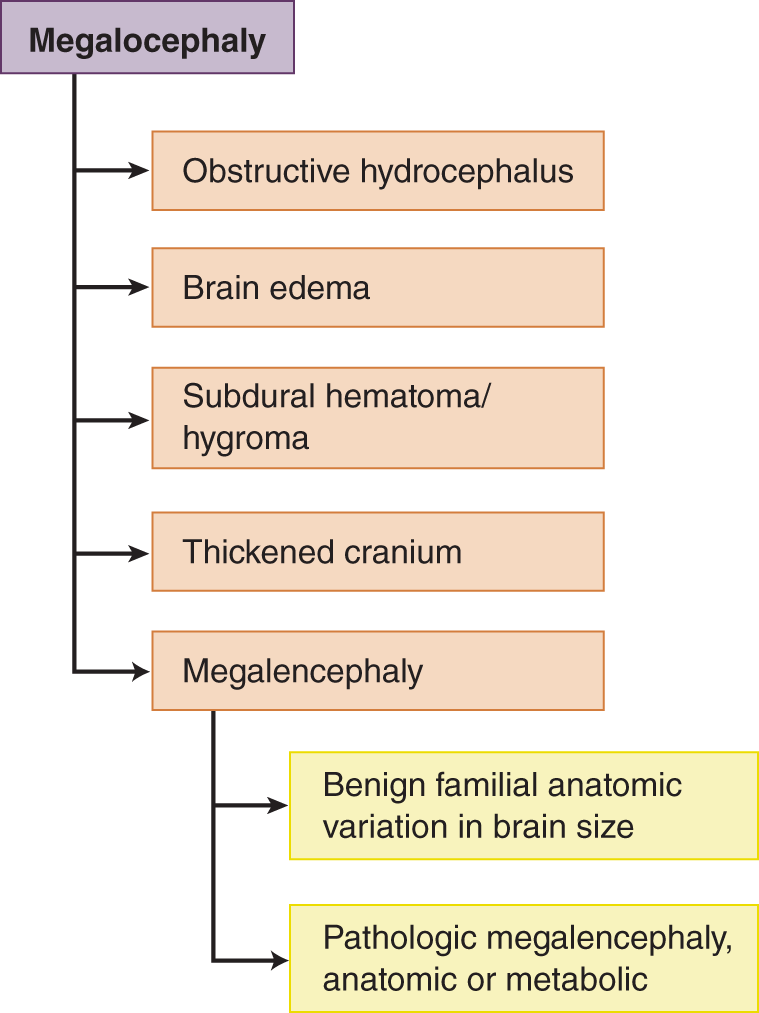
FIGURE 1-26. The five most common causes for megalocephaly.
2. An unfortunate, all too common cause of a bulging fontanel and an enlarged head is a battered or shaken infant who has a subdural hematoma and brain edema and who most likely has had seizures. The caretakers provide an implausible history: “He rolled off of the couch” or “He crawled out of the crib”—remarkable feats for a 3-month-old infant. The NE shows an obtunded infant with multiple bruises involving the scalp, ear, or remainder of the body, a bulging fontanel, split sutures, retinal hemorrhages, and often fractures of the skull, ribs, or long bones. The lesions are of various ages, indicating repeated assaults. The shaken rather than the battered baby may lack the skin signs and multiple fractures but has the brain lesions (Kempe et al, 1962).
F. Review of steps in the NE of a patient with megalocephaly or microcephaly (DeMyer, 1999)
1. In infants, palpate the fontanelle and sutures and attempt to transilluminate the cranium.
2. Compare the somatotype and OFC of the Pt with those of siblings and parents. Does the Pt match or differ from the family pattern?
3. Obtain MRIs if the history or examination discloses slow development. Note that MRI is almost always superior to CT in diagnosing intracranial lesions except in a few acute emergencies when intracranial bleeding is suspected. Use CT to show the cranial bones, the sutures, and calcified intracranial lesions (Alper et al, 1999; Barkovich, 2000).
BIBLIOGRAPHY · Abnormalities of Head Size and Brain Size
Alper G, Ekinci G, Yilmaz Y, et al. Magnetic resonance imaging characteristics of benign macrocephaly in children. J Child Neurol. 1999;14:678–682.
Barkovich AJ. Pediatric Neuroimaging. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000.
DeMyer W. Microcephaly, micrencephaly, megalocephaly and megalencephaly. In: Swaiman K, Ashwal S, eds. Pediatric Neurology. 3rd ed. St. Louis, MO: Mosby; 1999, Chap. 18, 301–311.
DeMyer W. Small, large or abnormally shaped heads. In: Maria BL, ed. Current Management in Child Neurology. 2nd ed. Hamilton, BC: Decker; 2002, Chap. 49, 299–304.
Kempe CH, Silverman FN, Steele BF, et al. The battered-child syndrome. JAMA. 1962;181(1): 17–24.
Tisserand DJ, Bosma H, Van Boxtel MPJ, et al. Head size and cognitive ability in non-demented older adults are related. Neurology. 2002;56:969–971.
IX. THE GENERAL RULES FOR THE NEUROLOGIC EXAMINATION
Having learned some of the steps of the NE, you can now appreciate the general, operational, analytic, and attitudinal rules that determine success or failure.
A. Operational rules for the neurologic examination
1. Follow the prescribed technique for each step of the NE.
2. Systematize the NE: The actual order is less important than the fact that you follow an order:
a. Anatomic order: Work from top to bottom (rostrocaudal order).
b. Functional order: Test for mental, motor, and sensory dysfunctions in packages; the Standard NE (see Introduction) follows this rule.
3. Quantify the NE: Measure the measurable and grade or scale the rest. Numbers comprise the language of science.
a. Record pupillary size, OFC, limb circumference, weight, etc, when relevant to the clinical problem.
b. Grade muscular strength from 0 to 5, muscle stretch reflexes from 0 to 4+, or other functions as minimally, mildly, moderately, or severely abnormal.
c. Match or titrate the Pt’s functions against your own whenever possible: visual acuity, extent of visual fields, sensory thresholds, and strength (biceps pull against biceps pull).
d. To decide whether a body part deviates from normal, compare it against three standards:
i. Compare each part with the established norm for a person of like age and sex.
ii. Compare each part with its mate on the opposite side.
iii. Compare the Pt with the family members. Does the Pt match or deviate from the genetic traits of the family?
4. Do a minimum screening NE on every Pt (Chapter 15): You must examine everything pertinent to the clinical problem, but you cannot and need not do every conceivable test on every Pt. You can extend or reduce the Standard NE to adapt to the clinical problem. The Pt with acute pain radiating down a leg requires a considerably different NE from that for the unconscious Pt. The Pt’s age, mental status, the symptoms that require investigation, and the diseases suspected require intelligent adaptation of the Standard NE.
a. Gather in the gestalt of the Pt: Appraise the total impact of the Pt’s personality, facial appearance, and somatotype. Then inspect the Pt part by part.
b. Inspect every square centimeter of skin and mucous membrane.
c. Look into every opening.
d. Feel every part.
e. Listen over the chest, abdomen, head, and blood vessels.
f. Smell every odor.
B. Analytic rules for the NE
1. Record all endpoint results objectively: Record what actually occurred during each operation of the NE, not an interpretation.
Interpretation: The Pt withdrew his leg from pain.
Observation: Pinprick elicited leg flexion.
Notice how the second statement records the operation performed (pinprick) and the behavioral result (leg flexion). A brain-dead Pt with an intact spinal cord or a paraplegic might reflexly withdraw the leg but could not have “felt pain.”
2. Think circuitry: After recording the findings objectively, interpret their pathophysiologic implications and convert to technical terminology, eg, the observed weakness and hyperreflexia on one side becomes hemiplegia from pyramidal tract interruption. The interpretation locates the lesion in the circuits of the nervous system. Thus far, Chapter 1 has not introduced circuitry, but subsequent chapters will discuss it extensively.
a. As corollaries of the definition of behavior on p. 1, we can state that:
i. All behavior depends on neuroanatomic circuitry.
ii. Every behavior, spontaneous or induced, that the Pt can or cannot produce conveys valuable information as to the integrity of the underlying circuits.
b. By “thinking through” the circuits responsible for each end action tested, the Ex plugs into and can determine which circuits of the nervous system are intact or impaired, eg:
i. If the pupil constricts to light, the circuit from the retina to the midbrain and back to the pupilloconstrictor muscle is intact. If the pupil does not react, a lesion, anatomic or pharmacologic, blocks the circuit at some point.
ii. If the large toe flexes in response to plantar stimulation, the pyramidal tract and the afferent and efferent nerve fibers of the foot are intact; if the toe extends (Babinski sign), the pyramidal tract is interrupted (Video 1-5). Thus, blockage of certain circuits may alter the effector actions in characteristic ways.

Video 1-5. Triple flexor response (Babinski sign). Application of a stimulus to the lateral aspect of the left foot provoked hip flexion, knee flexion, dorsiflexion of the foot, and extension of the large toe with extension and abduction of the other toes.
3. Localize the lesion: Postulate an anatomic site where the lesion has interrupted a nerve, pathway, or circuit.
a. Because the clinical signs of lesions at different levels of a circuit vary, the NE discloses which level the lesion has interrupted.
b. For a weak or paralyzed Pt, the NE localizes the lesion to the pyramidal tract, ventral motoneuron, nerve root, plexus, peripheral nerve, neuromyal junction, or the muscle itself.
c. For a blind Pt, the NE localizes the lesion to the retina, optic nerve, chiasm or optic tract, the geniculocalcarine tract, or the calcarine (visual) cortex itself.
d. By knowing the anatomic conjunctions of the various neural circuits, the Ex can localize the lesion to a specific anatomic site. A VI cranial nerve palsy and a contralateral hemiplegia localize the lesion to the basis pontis, near the pontomedullary junction, at the conjunction of the VI cranial nerve and the pyramidal tract.
4. Propose an etiologic diagnosis: For example, craniosynostosis is the cause of the Pt’s abnormal head shape. By integrating the history, anatomic localization, pathophysiology, and the probability of various diseases, the Ex achieves a presumptive etiologic diagnosis, which in turn determines any required laboratory workup to reach a final etiologic diagnosis. The final etiologic diagnosis sets the prognosis and therapy.
5. This catechism summarizes the analytic process applied to every Pt:
a. Is there a lesion?
b. Where is the lesion?
c. What is the lesion or disease present?
d. How do I confirm the diagnosis?
e. What is the therapeutic and preventive management?
C. Attitudinal and ethical rules for success in the NE
First and foremost, the success of the NE depends on the Ex’s mindset and ability to react nonjudgmentally.
1. React professionally: Accept every Pt with equal humility and grace. Accept all of the Pt’s behavior objectively as clinical phenomena, as the activation of glands and muscles by nerve impulses shuttling through neuroanatomic circuits. You cannot complete a competent, analytical evaluation while plagued with emotions of attraction or repulsion or while making moral judgments about the Pt’s lifestyle and worthiness.
2. Communicate with the Pt during the examination
a. Proper communication ensures full Pt cooperation and confidence. Reassure the Pt that a particular test will not hurt or prepare the Pt if the test causes discomfort.
b. Remember that everyone has doubts about how they will measure up under scrutiny and whether the physician will discover something dreadful. During the NE, you may want to comment briefly on your findings: “Your blood pressure is OK.” “Your eyes check out normal.” Patients will appreciate the communication, but avoid extensive discussions. Wait until the end of the examination for that.
i. For the anxious but neurologically normal Pt, the recital of normal findings will provide some assurance.
ii. For the neurologically impaired Pt, the recital of abnormal findings assures the Pt of your care and concern. When you summarize your conclusions at the end of the examination, the Pt will understand the evidence for your conclusions and will more readily accept the correct management.
3. Expect the abnormal: Expect that every observation, question, request, or maneuver will disclose an abnormality. The trained mind discovers what it is trained to discover. Look for abnormalities, anomalies, asymmetries, malalignments, and dysfunctions. The expectation of normality inevitably results in loss of vigilance and a sloppy or incomplete examination (Fowkes, 1986). If you expect that the eardrums or rectum will be normal, you will not examine them carefully, if at all.
4. Enjoy each NE
a. Convert parts of the NE into a friendly contest or game.
i. Visual fields: Ask the Pt, “Let’s find out whether you can see out as far to the side as I can.”
ii. Strength testing: “Do not let me win.”
iii. Sensory testing: “Let’s see how light a touch you can feel.”
b. Such challenges elicit the Pt’s interest and best performance and maintain your own interest. Then both you and the Pt will enjoy the NE. If you do something 20 times a day for the rest of your career, you may as well learn how to enjoy it.
c. The NE gives you the privilege of entering and sorting through the Pt’s neural circuits to discover the way lesions alter cognitive, motor, and sensory functions. If you remain curious, inquisitive, and take pride in your knowledge, technique, and competence, the whole process of the NE and its derivative conclusions becomes immensely gratifying or downright fun. Viewed this way, each NE becomes an opportunity for enjoyment. That is what we want this text to offer you: the skill to do a competent, enjoyable NE.
BIBLIOGRAPHY
Fowkes FGR. Diagnostic vigilance. Lancet. 1986;1:493–494.
D. Review of the initial examination of the head and face
To ensure mastery you need to review and rehearse these general principles and the techniques of this chapter. Although you may prefer to call it practicing, behavioral psychologists would say that they want you to emit the terminal behavior of the learning sequence. By terminal behavior, the psychologist means that you should produce those recitations and performances that provide evidence to you and anyone watching that you have learned what you should have. First, review the Learning Objectives for Section VIII, p. 48. Then, with a partner, practice the “terminal behavior” outlined in Section IV, A to E, of the Standard NE (see Introduction).
 Learning Objectives for Chapter 1
Learning Objectives for Chapter 1