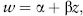
Responses to Selection: Natural Populations
Joel G. Kingsolver and David W. Pfennig
OUTLINE
1. Measuring selection in natural populations
2. Strength and patterns of phenotypic selection
3. Microevolution in natural populations
4. Local adaptation and population divergence
5. Limits to selection and evolutionary responses
GLOSSARY
Allopatry. A geographical distribution in which populations (or species) occur in different locations or habitats (contrast with sympatry).
Divergent Selection. The situation in which selection acts in contrasting directions in two populations.
Local Adaptation. The evolution of features in separate populations that render the members of each such population better able to survive and reproduce in its particular habitat.
Microevolution. Generally refers to inherited change in the characteristics of a group of organisms across generations that occurs within populations and species. Often contrasted with macroevolution, generally regarded as large-scale evolutionary change, ranging from the origin of species and major new features (e.g., novel traits or even new body plans) to long-term evolutionary trends.
Natural Selection. Variation in reproductive success that is correlated with variation in phenotypic traits among individuals. Natural selection can produce evolutionary change when such trait variation is inherited.
Phenotypic Selection. A form of selection that occurs when individuals with particular phenotypes survive and produce offspring at higher rates than do individuals with other phenotypes within a population.
Selection Gradient. A measure of the strength of selection acting on quantitative traits. For selection on a single trait, it is equal to the slope of the best-fit regression line in a scatterplot showing relative fitness as a function of phenotype. For selection acting on multiple traits, it is equal to the slope of the partial regression in a scatterplot showing relative fitness as a function of all phenotypic traits.
Sexual Selection. A form of natural selection that arises from variation in fitness resulting from either within-sex competition for reproduction or between-sex choice of mates.
Sympatry. A geographical distribution in which populations (or species) occur in the same location and habitat (contrast with allopatry).
1. MEASURING SELECTION IN NATURAL POPULATIONS
In On the Origin of Species, Darwin proposed a new mechanism that drives evolution and generates adaptation: natural selection (see chapter III.1). Yet despite the centrality of natural selection to his theory, Darwin never actually attempted to measure selection in nature. Furthermore, in the century following the publication of The Origin, selection was generally regarded as too weak, and evolutionary change too slow, to be observed directly in natural populations.
Research in the past four decades has demonstrated that selection and evolution in natural populations can be faster and more dynamic than Darwin and other early evolutionary biologists thought possible. Selection has now been detected in hundreds of populations in nature; moreover, numerous examples of rapid evolutionary change—microevolution on a timescale of 1 to 100 years—have also been reported.
What have we learned from these studies of selection and evolution in natural populations? Here we focus on phenotypic selection, because natural selection acts on the phenotypes of individual organisms. We describe how the strength of selection can be quantified and the patterns of phenotypic selection observed in nature. We explore the conditions that have promoted rapid evolution in nature and how such evolution can lead to local adaptation. Finally, we consider some of the limits to selection that can slow or alter evolutionary change.
Phenotypic selection occurs when individuals with particular phenotypes survive and produce offspring at rates higher than those of individuals with other phenotypes within a population. Phenotypic selection requires phenotypic variation, whereby individuals differ in some characteristics, and differential reproduction, whereby some individuals have more surviving offspring than others because of their distinctive characteristics. Thus, phenotypic selection results from differences in relative fitness (i.e., relative to the mean fitness or reproductive success) of individuals with different phenotypes.
To determine whether selection is acting on some trait in a population, we must first estimate the fitness associated with various trait values (see chapter III.5). Typically, this is done by measuring trait values for a sample of individuals of similar age (e.g., hatchlings), marking each individual, then following them over time to determine their survival and reproductive success. Ideally, these data can be used to determine the relationship between the total fitness and trait values of each individual; in practice, however, most investigators measure only individual components of fitness: survival, mating success, or fecundity.
Once we estimate the fitness associated with different trait values, we then plot fitness against trait value and fit a regression line (i.e., the best-fit line) through the data points. From the slope and shape of this regression line, we can determine the strength and mode of selection acting on the focal trait. When this fitness function is monotonic (always increasing or always decreasing, indicating directional selection; see chapter III.4), the fitness (w) of the trait (z) can be estimated by the simple linear regression equation:
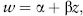
where α is the y-intercept of the fitness function and β is the fitness function’s slope. In this case, β measures the strength of directional selection (figure 1A). By contrast, when the fitness function has curvature (indicating stabilizing and disruptive selection; see figures 1B and 1C), quadratic regression is required to estimate the strength of selection. Here, fitness is estimated by:
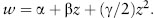
Here γ measures the amount of curvature in the fitness function—that is, the strength of quadratic selection. When β = 0 and γ is significantly negative (i.e., when fitness is highest at an intermediate phenotypic value), we conclude that stabilizing selection is acting on the trait of interest (figure 1B). By contrast, when β = 0 and γ is significantly positive (i.e., when the fitness function contains an intermediate performance minimum), we conclude that disruptive selection is acting (figure 1C).
Figure 1. Three different modes of selection can act on a quantitative trait: (A) directional selection, in which extreme phenotypes on one end of the phenotype distribution have the highest fitness and those on the other end have the lowest; (B) stabilizing selection, in which intermediate phenotypes have the highest fitness and extreme phenotypes on either end have the lowest; and (C) disruptive selection, in which extreme phenotypes on both ends of the phenotype distribution have the highest fitness and intermediate phenotypes have the lowest. The graph on the top row shows the distribution of phenotypes in a hypothetical population before selection, the graphs in the middle row show fitness associated with different phenotypes during each of three different modes of selection, and the graphs in the bottom row show the distribution of phenotypes following selection (in each panel, the dashed line shows the distribution of phenotypes before selection).
The coefficients β and γ (called the directional selection gradient and quadratic selection gradient, respectively) provide useful measures of the strength of phenotypic selection in a population. To allow comparisons among different types of traits and organisms, we can standardize selection gradients by the amount of variation in the trait (e.g., by the standard deviation) to obtain standardized measures of selection, βs and γs.
2. STRENGTH AND PATTERNS OF PHENOTYPIC SELECTION
Scores of studies have quantified phenotypic selection in natural populations using this approach over the past three decades; thousands of estimates of the strength of phenotypic selection are now available, especially for terrestrial plants, birds, lizards, frogs, and insects. Several general patterns emerge from these studies. First, there is abundant evidence for directional selection on morphological and life history traits in many study systems. The strength of such selection varies widely among species and traits, but it is often sufficiently strong to generate rapid evolution change (assuming that genetic variation is available; see below). Moreover, the magnitude of directional selection is not always consistent over time; indeed, in any given population, directional selection can vary in magnitude and even direction over time (i.e., reversals in the direction of selection are sometimes detected). Another common pattern associated with directional selection is that it often acts on body size in diverse taxa. Indeed, in most natural populations studied, directional selection favors increasing body size; that is, larger size tends to be associated with higher survival, fecundity, and mating success.
Studies have also found that the strength of directional selection depends on the component of fitness (e.g., survival, fecundity, mating success) involved. For example, directional selection through mating success and fecundity is generally greater, and more consistent in direction over time, than directional selection through survival. This basic pattern holds both among and within studies, as well as for different types of traits and organisms. These results suggest that sexual selection (selection due to differences in mating success) is frequently stronger than viability selection (selection due to differences in survival). In this sense, the struggle for existence may be less intense than the struggle for mates.
If populations of organisms are well adapted to current environments, then we would expect stabilizing selection (figure 1B) to be common; however, field studies of phenotypic selection in natural populations provide little evidence for significant stabilizing selection in most study systems. In particular, current estimates of quadratic selection gradients suggest that stabilizing selection is no more common than disruptive selection (figure 1C).
This surprising result can be explained in several ways. First, there may be trade-offs among various components of fitness, such that (for example) a trait value that increases survival may also decrease mating success or fecundity (see chapter III.12). Consequently, net selection on the trait may be less than directional selection via each fitness component. Second, phenotypic and genetic correlations between traits may cause indirect, correlated selection (see chapter III.4); as a result, direct selection on a trait may be balanced by opposing indirect selection on a correlated trait. Third, directional selection on a trait may alternate in direction in time or space, reducing the cumulative effects of selection. However, for most traits in most populations, none of these hypotheses is strongly supported by the available data. It is likely that experimental manipulations of traits or environments will be needed to reliably detect stabilizing selection in the field.
3. MICROEVOLUTION IN NATURAL POPULATIONS
If directional selection is common in natural populations, does this lead to detectable microevolutionary changes? For convenience, we can arbitrarily define rapid, contemporary evolution as detectable changes in the mean phenotype of a population in a few human generations—for example, within the 150 years or so since Darwin’s On the Origin of Species. Rapid microevolution has now been reported for both morphological and life history traits in numerous field populations of microbes, plants, and animals. The rates of evolutionary change vary widely, with many slower rates and a long tail of rapid rates.
What ecological conditions promote rapid microevolution? A common theme is colonization of new geographic regions and environments, leading to newly adapted populations. Drosophila subobscura provides an elegant example of rapid, repeatable microevolution following colonization. In its native range from northern Africa to Scandinavia, D. subobscura exhibits a strong geographic cline in wing and body size. During the late 1970s, D. subobscura was introduced independently into both South (by 1978) and North (by 1982) America, and rapidly expanded its geographic range on each continent. Studies in the mid-1980s showed that when reared under the same temperature conditions, there was no significant geographic differentiation in size among populations within either North or South America. However, by 2000, population divergence had generated size clines in both North and South America that paralleled the European cline. Substantial evolutionary increases in size at higher latitude populations in North and South America were particularly important in these clines. Rapid evolutionary changes in size and age of reproductive maturity have also been detected in newly introduced populations of salmon, and other animal and plant species.
Colonizing species can also generate selection and microevolution in native species. For example, the soapberry bug (Jadera haematoloma) is a plant-feeding insect native to the southeastern United States. It uses its long beak to feed on the fruit capsules of its host plants. Prior to 1925, soapberry bug populations in Florida fed exclusively on fruits of the native balloon vine. Starting in 1926, flat-podded golden rain tree, an Asian relative of balloon vine with flatter and narrower fruit capsules, was introduced and widely used by gardeners in Florida as an ornamental. By the 1960s, many soapberry bug populations had evolved a shorter beak, enabling them to feed more effectively on flat-podded golden rain tree. Comparable rates of evolutionary change in native species in response to colonizing competitors or predators have also been reported.
A second major cause of rapid evolution is recent environmental change due to human activities. Local adaptation of populations in response to herbicides, heavy metals, insecticides, and soil pH has been detected in many plants and insects; resistance to antibiotics and other antimicrobial agents has occurred in numerous human pathogens; and evolutionary change in response to recent global climate change has been detected. For example, evolutionary changes in the timing of flowering in annual plants, and in the seasonal cues that control active development in mosquitoes, have been detected in association with the recent elevation in mean environmental temperatures. The frequency and rates of microevolution in nature may increase as human activities increasingly alter climate and other major components of our physical, chemical, and biological environments.
4. LOCAL ADAPTATION AND POPULATION DIVERGENCE
Selection does not always act in a similar way on all populations of any given species; indeed, the mode (figure 1), magnitude, and even direction of selection might differ in different populations. Regarding the latter, when directional selection acts in opposing directions in different populations, it is referred to as divergent selection (figures 2A and 2B). Divergent selection is important, for two main reasons. First, divergent selection can promote local adaptation, in which different populations evolve different adaptive trait values or even different adaptive traits altogether (see chapter IV.3). Second, divergent selection may ultimately favor the evolution of barriers to genetic exchange between populations and thereby lead to speciation (see chapter VI.4). Here, we briefly review the causes of divergent selection before discussing its role in local adaptation and speciation.
Figure 2. Selection is divergent when it acts in contrasting directions in different populations. (A) For example, an ancestral population of fish that splits into two separate populations that come to occupy two different environments–fast-moving water and slow-moving water–might experience divergent selection on body shape if (B) different body shapes are favored in different water-flow regimes. Moreover, such divergent selection might even promote the evolution of reproductive isolation and, possibly, speciation. Specifically, (B) if offspring produced by matings between populations perform poorly in both environments, postzygotic isolating mechanisms might prevent gene flow between populations on secondary contact; (C) additionally, if individuals prefer mates with a similar body shape, prezygotic isolating mechanisms might also prevent gene flow.
There are three main agents of divergent selection. First, divergent selection can arise because of differences between populations in their abiotic environments. For instance, populations might diverge from one another as a result of experiencing different soil chemistries, climates, or resources. As an example, populations of plants that grow on serpentine soils must adapt to extreme soil conditions, including the absence of essential nutrients and the presence of heavy metals. Such populations may diverge from conspecific populations residing on nonserpentine soil to such a degree that they eventually become separate species (indeed, serpentine soils are characterized by high levels of endemism). Generally, differences in the abiotic environment can be potent agents of divergent selection.
Second, divergent selection can arise when populations differ in their interactions with other species, especially predators, parasites, or competitors (see chapter III.5 and III.15). For example, when conspecific populations differ in their exposure to a heterospecific competitor––such that some populations co-occur with the heterospecific and others do not––those populations in sympatry with the heterospecific will experience a different selective environment from that experienced by conspecifics in allopatry. Consequently, populations might diverge from one another in traits associated with resource use, reproduction, or both. In particular, the sympatric population might experience selection for traits that minimize resource competition and/or costly reproductive interactions with the heterospecific (e.g., mismatings or signal interference), and thereby undergo a form of divergent trait evolution known as character displacement.
The classic example of character displacement comes from finches from the Galápagos Islands. As Lack first observed, different species of finches typically differ in beak morphology more in areas where they are sympatric than where they are allopatric. Such divergence likely reflects selection to minimize competition between species (beak size and shape correlates with the types of seeds on which each species feeds, so differing in beak size reduces overlap in diet and thereby competition for food). More generally, character displacement has been detected in numerous species, and it has been shown to affect traits involved in both resource use and reproduction.
Third, divergent selection can arise when the direction or strength of sexual selection differs between different populations. For instance, populations might diverge in display traits, sensory systems, and/or mate preferences owing to differences in either abiotic or biotic environments. As an example, two species of threespine sticklebacks (of the Gasterosteus aculeatus complex) have undergone character displacement in certain small lakes in southwestern Canada, which has resulted in one species expressing a distinctive “benthic” ecomorph and the other a distinctive “limnetic” ecomorph. The benthic ecomorph inhabits the heavily vegetated littoral zone of lakes, whereas the limnetic ecomorph occupies open water. As a result of occupying different habitats, the light environments experienced by these two ecotypes also differ. In the littoral zone, where the benthic ecomorph forages and mates, red coloration is more difficult to detect. By contrast, in open water, where the limnetic ecomorph forages and mates, red coloration is more discernible. Interestingly, benthic females are less sensitive to variation in red than are limnetic females, and, unlike limnetic females, benthic females do not tend to prefer redder males. Male red coloration, in turn, is “tuned” to female perception of red color: males are redder in populations where females are actually sensitive to, and thus prefer, redder males. There are a number of other examples in which display traits, sensory systems, and/or mate preferences have diverged between populations experiencing different selective regimes.
As the examples above illustrate, an important consequence of divergent selection is that it can promote local adaptation. But how frequently does divergent selection lead to local adaptation, and what are the patterns it produces? A standard means for assessing local adaptation is reciprocal transplant experiments, in which samples of individuals (and genotypes) from different populations are reared together in a “common garden” at each site. These experiments can determine whether genotypes from the native or “home” population have higher relative fitness at their native site than genotypes from populations at other (“away”) sites: that is, whether local genotypes are locally adapted to their own sites.
A recent review (Hereford 2009) of more than 70 reciprocal transplant studies in field populations indicates an overall frequency of local adaptation of 71 percent and an average fitness advantage of native genotypes at their native site of 45 percent. The magnitude of local adaptation was greater when there were larger environmental differences among sites, as we would expect if these environmental differences create the divergent selection that caused the local adaptation. Interestingly, trade-offs in relative fitness across sites were weak, suggesting that strong local adaptation to one site does not necessarily produce low fitness at other sites. Overall, these results suggest that local adaptation in natural populations is widespread. The phenotypic basis for local adaptation is not always known, but may frequently result from population differences in many traits.
Local adaptation is not the only significant consequence of divergent selection, however. As noted above, divergent selection can even promote speciation. Indeed, differences that arise between populations owing to divergent selection can reduce or prevent gene flow when populations come into secondary contact, thereby reproductively isolating them. This route to speciation, dubbed ecological speciation, occurs when barriers to gene exchange between populations evolve as a consequence of ecologically based divergent selection (see chapter VI.4).
To illustrate how divergent selection can promote speciation, imagine that different populations of a fish invade two different environments: one containing only slow-moving water and one containing only fast-moving water (figure 2A). These two different populations would likely experience divergent selection pressures (figure 2B) and might therefore evolve different body shapes. Consequently, hybrids produced by any matings that might occur between such populations (if they were ever to come into secondary contact) would likely be disfavored because of ecologically based divergent selection; in this case, because these hybrids would likely have an intermediate body shape, which would perform poorly in either parental environment (see figure 2B). Such reduced fitness of between-population hybrids might act as a postzygotic isolating mechanism preventing gene flow between ancestral and derived populations. Additionally, if individuals prefer mates with a similar body shape (figure 2C), prezygotic isolating mechanisms might also prevent gene flow between these populations, thereby potentially completing the speciation process. Generally, ecological speciation becomes more likely if a strong association exists between the gene(s) conferring local adaptation and the gene(s) conferring reproductive isolation.
As support for ecological speciation, numerous studies have found that divergent selection has promoted differences between populations in traits such as body shape, size, or coloration that influence mate preferences. The ecological speciation model is also supported by laboratory experimental evolution studies, comparative studies, and by instances of parallel speciation, in which reproductive isolation has evolved independently and repeatedly between populations adapting to contrasting environments.
Finally, broadly defined, divergent selection includes the special case of disruptive selection (figure 1C). As noted above, disruptive selection occurs when two or more modal phenotypes in a population have higher fitness than the intermediate phenotypes between them. Recent empirical studies suggest that disruptive selection might be more widespread than formerly presumed; indeed, several studies of natural populations have documented disruptive selection; two examples are shown in figure 3. Disruptive selection might play a general and important role in maintaining diversity within species, especially when such selection leads to the evolution of a mating polymorphism or a resource polymorphism (as in the two examples depicted in figure 3).
Figure 3. Two examples of disruptive selection in the wild. (A) The adult population of Darwin’s finches (Geospiza fortis) at El Garrapatero (Santa Cruz Island, Galápagos archipelago) consists of a small-beaked morph and a large-beaked morph. The data come from a study by Podos and colleagues. (B) This population experiences disruptive selection, in which individuals with small and large beaks have higher fitness than those with intermediate-sized beaks. Selection on beak morphology is depicted as a cubic spline (heavy line) with 95% confidence intervals (lighter lines). These data are from work by Hendry and colleagues. (C) Similarly, disruptive selection disfavors individuals with intermediate trophic phenotypes in Mexican spadefoot toad tadpoles (Spea multiplicata). This graph shows the probability of survival for individuals expressing different ecomorphs—omnivores, carnivores, and intermediates—based on a mark-recapture experiment in a natural pond in Arizona. Individuals expressing an intermediate phenotype had lowest survival (numbers above each bar show sample sizes), demonstrating that this population experiences disruptive selection. (D) Disruptive selection can also be visualized by a cubic-spline estimate of body size (a fitness proxy) on a composite shape variable of trophic morphology (morphological index). The cubic spline (solid line) is bracketed by 95% confidence intervals (dashed lines). As in panel (B), the presence of an intermediate fitness minimum suggests that disruptive selection acts on trophic morphology. The data in panels (C) and (D) are from work by Martin and Pfennig.
5. LIMITS TO SELECTION AND EVOLUTIONARY RESPONSES
In this article, we have emphasized that directional selection and local adaptation are common in nature. We also described how colonization events and anthropogenic environmental changes have generated microevolutionary changes in many populations during the past century, but directional selection does not inevitably lead to evolutionary change and adaptation. First, evolution also requires appropriate genetic variation in the traits under selection (see chapter III.4). Although genetic variation has been documented for numerous phenotypic traits for many natural populations, lack of genetic variation can still limit the response of some populations to environmental change. For example, tropical species of Drosophila with narrow geographical distributions have little genetic variability in resistance to cold and to desiccation. This lack of genetic variation limits their potential to adapt to cooler or drier environmental conditions beyond their current geographic ranges––and their capacity for evolutionary responses to future climate changes. Limits to genetic variance and other factors might constrain evolutionary responses to selection in a variety of ways (see chapter III.8).
Additionally, environmental change not only causes selection but can also alter patterns of phenotypic and genetic variation in important ways. Long-term population studies with mammals and birds illustrate this point. Yellow-bellied marmot populations in the Rocky Mountains in the western United States hibernate as adults during the long, snowy winter months. Adult mass at the start of hibernation has increased markedly over the past 35 years, largely as a result of three factors related to weather: earlier seasonal emergence from hibernation, earlier weaning of young, and a longer growing season. The longer growing season and larger adult size at hibernation have also reduced adult mortality, causing large increases in population density in recent years. Selection and adaptive evolution have likely played little direct role in these phenotypic changes.
Environmental change can also affect both selection and genetic variation, however, in ways that alter the rate of microevolution. Higher spring temperatures in the Netherlands during the past 35 years have generated directional selection on the timing of breeding in populations of the great tit, a common bird in northern Europe. Additionally, increasing spring temperature has increased the heritability of breeding time in the population, by altering patterns of phenotypic and genetic variation. The increase in both selection and heritability has accelerated the rate of microevolution of breeding time in this population.
These examples illustrate how ecology and evolution are intimately interconnected in the responses of populations to environmental change. The interactions among genetic variation (see chapter III.4), phenotypic plasticity (chapter III.10), life history (chapter III.11), and selection are essential for a full understanding of the evolutionary responses and adaptation of natural populations in a changing world.
FURTHER READING
Boughman, J. W. 2001. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411: 944–948. The paper presents an empirical example in which divergent sexual selection has apparently favored the evolution of reproductive isolation in natural populations.
Hereford, J. 2009. A quantitative survey of local adaptation and fitness trade-offs. American Naturalist 173: 579–588. This paper presents a meta-analysis of field experimental studies, and it documents that local adaptation is widespread in natural populations.
Hoffmann, A. A., and C. M. Sgrò. 2011. Climate change and evolutionary adaptation. Nature 470: 479–485. This paper illustrates how lack of genetic variation in key phenotypic traits can limit the adaptive responses of some tropical species to climate change.
Kingsolver, J. G., and S. E. Diamond. 2011. Phenotypic selection in natural populations: What limits directional selection? American Naturalist 177: 346–357. This synthetic review explores an important paradox: the abundant evidence for directional selection, and the limited evidence for stabilizing selection.
Kingsolver, J. G., and D. W. Pfennig. 2004. Individual-level selection as a cause of Cope’s rule of phyletic size increase. Evolution 58: 1608–1612. In this paper, the authors present the results of a meta-analysis that suggests that selection favors larger body size in many species.
Nosil, P., and H. D. Rundle. 2009. Ecological speciation: Natural selection and the formation of new species. In S. A. Levin, ed., The Princeton Guide to Ecology. Princeton, NJ: Princeton University Press, 134–142. This book chapter presents a concise overview of the role of ecological factors, especially biotic interactions, in speciation.
Reznick, D. N., and C. K. Ghalambor. 2001. The population ecology of contemporary adaptations: What do empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112–113: 183–198. This review documents how anthropogenic environmental change, species invasions, and other ecological factors contribute to rapid adaptive evolution in contemporary populations.
Siepielski, A. M., J. D. DiBattista, J. A. Evans, and S. M. Carlson. 2011. Differences in the temporal dynamics of phenotypic selection among fitness components in the wild. Proceedings of the Royal Society B 278: 1572–1580. This synthetic review explores the evidence for temporal variation in the strength and direction of selection in natural populations.