Inbreeding
Deborah Charlesworth
OUTLINE
1. Inbreeding
2. Measuring the degree of inbreeding
3. Measuring inbreeding coefficients and rates of self-fertilization and other inbreeding
4. Long- and short-term consequences of inbreeding
5. Consequences of inbreeding for molecular evolution and genome evolution
6. Inbreeding depression, heterosis, and purging
Inbreeding (mating between individuals with recent common ancestors) led to populations of organisms being more homozygous than predicted by the familiar Hardy-Weinberg formula for random-mating populations. Inbreeding is one form of nonrandom mating, and it can occur in populations where numbers of potential mates are limited because of small population size or restricted dispersal, or in species or populations with preferential mating with related individuals, either natural (e.g., in naturally self-fertilizing organisms such as many hermaphrodite plants and animals) or enforced (e.g., in sib-mated “inbred lines” of mice, or when inbred strains or breeds are created by crop breeders or animal breeders). Inbreeding has many consequences: the action of natural selection and the amount of genetic variation in inbreeding populations both differ from the situations in outcrossers. Because so many situations of interest to plant and animal breeders involve inbreeding, and because several “model species” important in modern biology are inbreeders, it is interesting to understand these differences. This chapter also outlines the concepts of inbreeding depression and hybrid vigor.
GLOSSARY
Allele. The “type” of a gene, for example, whether the allele is wild type or a mutant.
Diploid. An organism, or stage in the life cycle of an organism, in which individuals carry alleles from a maternal and a paternal parent (as opposed to haploid individuals or stages, with only one allele of each locus).
Genotype Frequencies. The proportions of the different homozygous and heterozygous genotypes at a gene with more than one allele present in a population or sample of individuals. These frequencies depend on the allele frequencies and on the mating system.
Hermaphroditic Organisms. Organisms with both male and female functions that can potentially mate in isolation (as opposed to having separate male and female individuals, which can reproduce only by mating with another individual). Some plants have separate male and female flowers on the same individual; as far as the mating system is concerned, such “monoecious” plants can often be treated as hermaphrodites.
Inbreeding Depression. The lower survival or fertility of progeny of inbred matings compared with progeny produced by outcrossing.
Self-fertilization (Selfing). Mating of a hermaphroditic individual (or a monoecious plant) with its own gametes, or mating between individuals that are genetically the same individual. The most extreme form of selfing occurs in haploid plants, when self-mating produces progeny that are homozygous at all loci. Selfing in other hermaphrodites increases the proportion of homozygous loci, but more slowly.
Sib-mating. Mating between full siblings.
1. INBREEDING
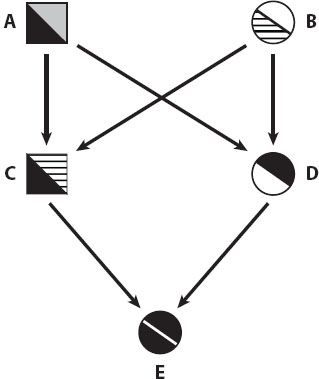
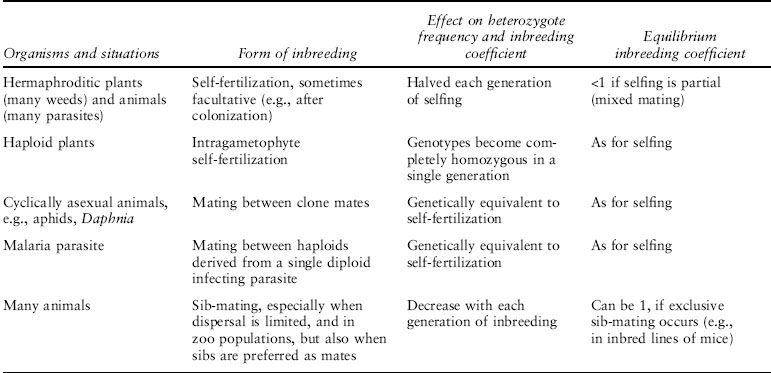
Inbreeding is the occurrence of mating between individuals with common ancestors (figure 1). This can happen in various ways (table 1), and an important division is between two general types of situations, as follows:
• inbreeding caused by lack of opportunities to encounter and mate with unrelated individuals, for example, when population size is restricted, including populations that have experienced a recent bottleneck
• inbreeding caused by patterns of mating with related individuals, for example, through self-fertilization in hermaphroditic plants or animals (often called selfing), or in populations in which individuals regularly or frequently mate with their siblings (e.g., when a preference for sibs as mating partners exists, as in some human cultures)

Figure 1. Identity by descent in an inbreeding pedigree (a full sib-mating). Males and females are symbolized by squares and circles, respectively, and the individuals are labeled with letters, and their different alleles are shown with different shadings. Individual E, produced by a full sib-mating, inherits the black allele from her grandfather (individual A) via both her father (C) and her mother (D).
Table 1. Some biological situations in which inbreeding occurs
This chapter deals mostly with the second type of inbreeding, but inbreeding cannot always be neatly categorized into these two types. For example, the situation with animals in which the progeny of females do not disperse and must mate with their siblings, as occurs in social spiders, cannot be categorized as one type or the other. More generally, given that dispersal in real organisms never leads to fully random mating throughout the entire species (i.e., mating with no relationship to the origins of the individuals), some inbreeding must often occur in most species, even if at a very low frequency. Despite its prominence in textbooks, random mating and the familiar Hardy-Weinberg genotype frequencies are not the reality for most species (see chapter I.3). Even populations of large, mobile animals such as humans show evidence of some subdivision, clearly indicating that, at least until recent times, matings have been mostly between individuals from close localities.
Many hermaphroditic animals and plants (including monoecious plants with separate male and female flowers on each individual) have self-incompatibility systems preventing inbreeding populations (see chapter IV.8). On the other hand, sib-mating may occur in many randomly mating animals and plants, because dispersal is generally insufficient to guarantee that all potential mates are unrelated, and many organisms have no means of recognizing sibs or avoiding them as mates (this is an example of the first kind of inbreeding listed above).
2. MEASURING THE DEGREE OF INBREEDING
One important measure of inbreeding is the proportion of progeny in a generation or cohort that are generated by the inbreeding. For instance, for hermaphroditic plant populations, we can estimate the selfing rate. Clearly, such selfing rates implicitly assume that the inbreeding occurs according to a regular system (with the same rate for all individuals in the population, remaining the same every generation). It is in principle possible to estimate selfing rates for individuals and determine whether they are genetically controlled and/or related to particular morphologies (such as larger flowers) or individual characteristics (e.g., the occasional self-compatible individuals in self-incompatible plant species), though this is rarely done. However, it is clear that inbreeding is often temporally variable or context dependent. For example, the selfing rate in a plant or animal population may be higher in years or locations where the density of members of the species is lower, or it can be higher when mating partners are unavailable to snails or other animals than when they are present (e.g., Escobar et al., 2011).
More generally, inbreeding is measured in terms of inbreeding coefficients, which express the probability that both the copies of a gene in a diploid individual were inherited from a copy in a common ancestor—the probability that they are “identical by descent.” The increase in the inbreeding coefficient under a regular system of inbreeding can be predicted over the generations under a given mating system. For instance, Mendel’s rules of inheritance show that for progeny produced by selfing, this probability is 0.5, while an outcrossed mating produces progeny whose inbreeding coefficient is zero (for the offspring of sib-mating, it is 0.25). Over the generations, a mixture of selfing with some outcrossing therefore leads to an inbreeding coefficient that is less than 1 (figure 2 shows this for several selfing rates), whereas slower inbreeding can lead to an inbreeding coefficient of 1 if no outcrossing occurs (e.g., complete sib-mating in figure 2). Pedigrees of much greater complexity than these simple examples include information about the possible lines of descent, and they can also be used to predict the inbreeding coefficient of any member of the pedigree, relative to that of the earliest ancestors in the pedigree (Falconer and Mackay 1996).
Figure 2. Approach to equilibrium inbreeding coefficients (f) in populations under regular self-fertilization at different self-fertilization rates (denoted by S), and under regular full sib-mating. At equilibrium under the mixed mating model, the expected f value is S/(2 – S).
Inbreeding coefficients are a very general and useful measure, because they can be applied to any form of inbreeding and compared between different populations or experiments. For inbreeding of the first category above, the inbreeding coefficient can be predicted by modeling the situation in the population of interest, and calculating the time since common ancestry of alleles in any generation (Falconer and Mackay 1996). Methods to do this often use the concept of “inbreeding effective population size,” which takes common ancestry into account (see chapter IV.1).
3. MEASURING INBREEDING COEFFICIENTS AND RATES OF SELF-FERTILIZATION AND OTHER INBREEDING
Several reviews have recently been published of procedures for estimating inbreeding rates in natural populations (e.g., Jarne and David 2008). One procedure for estimating inbreeding coefficients is based on the probabilities of identity by descent of the alleles in individuals, which determines the genotype frequencies. This is easily understood for an autosomal locus with two variants, A1 and A2, with frequencies p and q. For one of the two alleles of an individual taken from the population, the probability that it is A1 is simply the A1 frequency in the population, p, and the chance that the allele is identical by descent (IBD) with the individual’s other allele is its inbreeding coefficient, f; ignoring mutation, this allele must also be A1 because it is identical by descent. The probability that the other allele is non-IBD is 1 – f, in which case the probability that the individual’s genotype is A1A1 is p2, neglecting mutation. Therefore, the total probability that the individual’s genotype is A1A1 is equal to
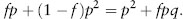
Similarly, the frequency of A2A2 is q2 + fpq; by subtraction from 1, the frequency of A1A2 is 2(1 – f)pq. Therefore, heterozygosity is reduced by inbreeding in proportion to f.
Genetic markers allow one to measure the genotype and allele frequencies in a population of interest, giving an estimate of the f value in these equations, and to test whether the frequencies of homozygotes and heterozygotes correspond with random mating (f = 0), or whether there is a deficiency of heterozygotes, indicating inbreeding (at least if selection against heterozygotes can be discounted; such selection is implausible, as a factor affecting many loci, and in practice, estimates of mating system usually employ genetic markers such as microsatellites or allozymes that are assumed to be selectively neutral). If so, and if the population’s biology suggests that a particular regular system of inbreeding is likely, for example, selfing, the rate of inbreeding can be estimated by assuming that the population has reached equilibrium under that system, and finding the rate that yields the observed genotype frequencies.
Note that inbreeding estimates may not correspond to the values predicted from known pedigrees, because genes may become homozygous more slowly than expected, because of linkage to loci where homozygosity lowers fitness; this is explained in more detail below.
Alternatively, selfing rates can be estimated using family arrays. It may even be possible to estimate the number of generations of inbreeding in the ancestry of individuals. With this kind of approach, individual outcrossing rates can sometimes be estimated using paternity assignment employing suitably informative markers, and it may then be possible to associate selfing rates with characteristics such as, in a plant, different anther-stigma distances in the flowers of different individuals. Both these approaches indicate that, although most populations are found to be either largely outcrossing or largely selfing, intermediate mating systems also exist in both plants and animals (Escobar et al. 2011). Some very important model species in modern biology are among the high inbreeders, notably the plant Arabidopsis thaliana and the nematode Caenorhabditis elegans.
4. LONG- AND SHORT-TERM CONSEQUENCES OF INBREEDING
Effects on Genotype and Allele Frequencies and on Genetic Variation
Inbreeding increases the frequencies of homozygotes. If inbreeding occurs at a high rate in a population, as in highly selfing species, frequencies of heterozygotes quickly decrease. If a population becomes highly inbred, its individual members can become homozygous at all or most loci. A very important consequence of homozygosity is inbreeding depression, which will be discussed below.
Overall, inbreeding decreases genetic variation within populations and increases isolation between populations, for the following reasons. A longer-term consequence of homozygosity is that the genetic drift process affects homozygous genotypes, rather than individual alleles. Given enough time, populations lose genetic variants through genetic drift, and the difference just mentioned makes this happens faster under inbreeding than in an outcrossing population. Because genetic drift acts independently in different populations, and inbreeding increases isolation between populations by decreasing gene flow, inbreeding leads to increased population differentiation. This can be enhanced by extinction and recolonization processes.
The Effect of Inbreeding on Genetic Recombination, and Differences between Inbreeding and Asexual Reproduction
If a population becomes highly inbred, so that its individual members are homozygous at all or most loci, as in highly selfing species, recombinant genotypes will rarely be formed, even if meiosis and sexual reproduction occur normally, with fertilization of eggs by sperm. This effect occurs simply because double heterozygotes for any pair of loci are rare (rare heterozygotes that are formed when individuals outcross will, of course, yield recombinant progeny), and crossing-over produces recombinant progeny only if the parent is heterozygous at both loci. At the extreme of complete self-fertilization for multiple generations, all individuals will be homozygotes, and reproduction yields progeny whose genotypes are the same as those of their mother, as also occurs with many kinds of asexual reproduction, but the cause is very different. Indeed, asexual species (see chapter IV.4) are often highly heterozygous (such species are often formed by hybridization events).
5. CONSEQUENCES OF INBREEDING FOR MOLECULAR EVOLUTION AND GENOME EVOLUTION
Long-Term Consequences: Hitchhiking
The lowered recombination rate in highly inbreeding populations means that genetic “hitchhiking” events affect larger genome regions than would be affected in an outcrosser. For example, the spread of an advantageous mutation will reduce diversity across a region of genome, and the region will be large in a selfing species. This may make it difficult to detect natural selection in selfers through the low diversity regions caused by selective sweeps.
These hitchhiking effects add to the effect of genetic drift explained above, and can greatly reduce diversity in highly inbreeding populations versus comparable outcrossers (with similar values of the main determinants of diversity, the number of individuals, and the mutation rate). This lower diversity occurs because selective sweeps in selfing species lead to low diversity across large regions of genome, and also because selective elimination of deleterious mutations will cause greater lowering of diversity than under outcrossing. These processes act within populations, so different populations may remain genetically different, and species-wide diversity may not be low. However, in subdivided populations species-wide diversity will generally also be reduced. The net effect on the distribution of diversity within and between populations is generally to greatly reduce within-population diversity in highly inbreeding species, and thus increase the proportion of total diversity that is found between populations.
Haldane’s Sieve
Another very interesting consequence of inbreeding is that advantageous mutations will be more likely to spread, even if recessive, than in more outcrossing populations. In a diploid population, advantageous mutations first appear in heterozygotes, and in outcrossing species, homozygotes will remain rare until a high allele frequency is reached. Therefore the selection in heterozygotes determines whether mutations can spread (i.e., the product hs, where s is the selection coefficient and h is the dominance coefficient). Recessive mutations (low h values) therefore have a low chance of fixation. In partially self-fertilizing species, however, less dominant mutations can spread more readily, because inbreeding produces homozygotes even for rare mutations. In contrast, under outcrossing, advantageous mutations are most likely to spread if they are dominant. It is sometimes suggested that inbreeding is advantageous, because it allows the population to take advantage of both dominant and recessive mutations; however, this is a population-level advantage, and (as further discussed below) unlikely to outweigh the strong immediate disadvantages to individuals of inbreeding caused by inbreeding depression.
Chromosome Evolution
Another consequence of high homozygosity produced by inbreeding is that the disadvantages to a new chromosome arrangement are reduced, because these disadvantages often arise through recombination (in translocation heterozygotes) or mis-segregation in heterozygotes. Chromosome rearrangements are indeed observed to be commoner in inbreeding than in outcrossing plants.
Sex Allocation
Hermaphroditic inbreeding populations are predicted to allocate reproductive resources more toward female than male functions. In hermaphroditic selfing organisms, this is indeed found, as detected by lower pollen-ovule ratios in plants, and lower amounts of testis tissue in animals (Petersen and Fischer 1996). In species with separate sexes, inbreeding populations may have female-biased sex ratios.
6. INBREEDING DEPRESSION, HETEROSIS, AND PURGING
Inbreeding Depression and Heterosis
The survival and fertility of offspring of related individuals is usually reduced. Such inbreeding depression effects are well documented in many organisms, including higher frequencies in inbred (consanguineous) families than in outcrosses of major abnormalities such as chlorophyll-deficient albino seedlings in plants and developmental defects in fish, or genetic diseases, as in humans (Bittles 2003). The survival and fertility of individuals in experimentally produced inbred lines is frequently so low that many such lines go extinct. Hybrids made by intercrossing surviving lines often have higher quality than their inbred parents, frequently exceeding the best parent values for several characters. This increased performance of F1 hybrids is called heterosis, or hybrid vigor.
Models and Empirical Evidence
Inbreeding depression is caused by increased homozygosity of individuals, and its occurrence implies that genetic variation in fitness traits exists within the population. What kind of variation is involved, and why is it present in natural populations? Two distinct kinds of variation can contribute:
• alleles at loci with heterozygote advantage (overdominance)
• partially recessive detrimental mutations present in populations at low frequencies due to mutation-selection balance
However, it seems unlikely that hybrid vigor will often be caused by overdominant alleles present in two inbred lines that are intercrossed. This would require that by chance, one of the lines has become homozygous for one allele at the locus, and the other line for the other. In a given cross, heterosis is manifested in many different characteristics, and it becomes implausible that many genes with overdominant alleles will undergo such lucky chances (most inbred lines will become homozygous for the allele whose homozygotes have the highest fitness).
In contrast, the mutation-selection balance hypothesis can readily explain the occurrence of hybrid vigor. Heterosis in an intercross between two populations or genetically uniform strains depends on the existence of genetic differences between them. Although detrimental mutations are expected to be individually rare within large populations, minor-effect mutations can reach high frequencies by genetic drift in small populations, or even become fixed. In different populations, different mutations will reach high frequencies; thus, when two such populations are intercrossed, there is a high chance that the parent genotypes involved will each be homozygous for a different set of deleterious mutations, and so the progeny will be heterozygotes. If the mutations are wholly or partially recessive, even with almost intermediate dominance, the deleterious mutation effects will be wholly or partially masked, leading to heterosis if enough such loci contribute.
The mutation-selection balance hypothesis can also readily explain the occurrence of inbreeding depression. While, as just explained, heterosis will be due largely to mutations with small detrimental effects that can reach high frequencies in populations, rare mutations with moderately large effects predominate in inbreeding depression. For inbreeding within a population to lower fitness, homozygotes for mutations must have much lower fitness than heterozygotes; in other words, the small number of mutations that become homozygous in a given individual must be quite recessive. There is good evidence supporting the involvement of rare recessive alleles (Charlesworth and Willis 2009).
This hypothesis can account quantitatively, without invoking overdominant loci, for the magnitude of inbreeding depression observed in several species where the relevant spontaneous deleterious mutation rates and their effects can be estimated (Charlesworth and Willis 2009). There is also little direct evidence for overdominance from estimates of the magnitude of additive versus dominance genetic variance, which could potentially distinguish whether mutational load or heterozygote advantage contributes most to genetic variation in fitness (overdominance would yield high dominance variance). With few exceptions, characters related to fitness give results consistent with the mutational model, and the exceptions do not suggest a major role of heterozygote advantage (Charlesworth and Willis 2009).
The most obvious approach to determining the genetic basis of the variants causing inbreeding depression or heterosis would seem to be genetically mapping them; however, this is not currently practicable. One problem is that the low resolution of QTL mapping cannot tell us whether a genetic factor that is detected is a single gene versus several genes being involved. Thus, an apparent genetic factor showing heterosis could prove to be a pseudo-overdominant situation, in which deleterious mutations at two distinct loci complement one another in heterozygotes. Indeed, fine mapping of particular cases has separated some apparently overdominant factors into situations with deleterious recessive alleles in repulsion. Another difficulty is that, if inbreeding depression is indeed caused by rare mutations, different families should carry different QTLs, so that mapping on one family does not help discover genes important in other families.
The existence of inbreeding depression is probably an important reason why outcrossing has evolved and is often maintained by natural selection. Yet many organisms inbreed by self-fertilization, or exhibit some tendency to prefer matings with relatives. The evolution of inbreeding is discussed in chapter IV.8.
Purging and Failure to Purge Deleterious Mutation
The deleterious mutation hypothesis predicts that inbreeding will expose the mutations to selection in homozygotes, and that inbreeding should thus reduce the load of such mutations in the population; this is called purging. Theoretical models of mutations show that major purging effects occur mainly when the mutations greatly lower fitness, and are minor for slightly detrimental mutations. In addition, large purging effects occur only in certain selective situations, and purging is less effective in organisms with large chromosome numbers and high recombination rates.
There is much evidence that deleterious mutations exist in organisms and can fail to be purged during inbreeding. For example, in Drosophila, multiple generations of inbreeding lead to lower homozygote frequencies than predicted by the inbreeding coefficient expected from the pedigree, indicating that natural selection against homozygotes for some loci or genome regions is preventing homozygosity. Even in organisms with very high levels of inbreeding in nature, such as Caenorhabditis elegans, some genome regions resist becoming homozygous.
A question that has interested many biologists working on mating systems is whether inbreeding leads to lower levels of adaptation than outbreeding, and therefore to a greater chance of extinction. Recall that high levels of inbreeding cause effects similar to those in small populations, and that genetic drift of weakly deleterious mutations is therefore of greater importance than for an outbreeder (see section on heterosis above). Fertility and survival of individuals in inbreeding populations might therefore be low because of high frequencies of such deleterious mutations. On the other hand, the removal of recessive and largely recessive mutations due to exposure to selection in homozygotes (see explanation of purging above) acts in the opposite direction. We have already seen that purging does not completely remove such mutations. Empirical studies are starting to uncover evidence that selfing plants may indeed show maladaptation or genetic degradation. The lineage leading to the highly selfing plant A. thaliana has been found to have accumulated more substitutions in its coding sequences (i.e., the DNA encoding proteins) at sites that change the amino acid, compared with a related outcrossing lineage (Slotte et al. 2010).
It is less clear whether these effects are strong enough to reduce the long-term survival of inbreeding species. Inbreeders have long been claimed to be “evolutionary dead ends,” and it is now becoming possible to use phylogenetic trees based on DNA sequences to infer when changes from outbreeding to inbreeding, and the reverse, have occurred in suitable taxa. These studies have shown that inbreeding probably evolves often, but rarely persists for long evolutionary time, and that there are few large taxa of highly inbreeding organisms (Takebayashi and Morrell 2001).
This, however, does not imply that the failure of inbreeders to generate large taxa of descendant species is because their loss of genetic diversity leads to a lower ability to adapt to changing environments. As explained above, the lower genetic diversity under inbreeding is a long-term consequence, involving genetic drift. It is unlikely to cause a mating system shift to evolve, or to influence such shifts. The short-term effect of homozygosity in causing inbreeding depression is likely to be a much more important cause of failure of inbreeding lineages to evolve, or to persist for long if they do evolve. Self-fertilization is certainly often strongly selectively favored in the short term, through its well-known “transmission advantage,” as well as “reproductive assurance” when density is low so that mating opportunities are scarce (or because pollinators are scarce for an animal-pollinated plant), and also when locally adapted genotypes are selected to avoid receiving gametes from different environments. With its many advantages, inbreeding is not a great puzzle—the bigger puzzle is why so many organisms outcross (see chapter IV.8). Possibly, inbreeding often arises in local populations of a subdivided species, but severe inbreeding depression selects for outcrossing with conspecifics from other populations (Schoen and Busch 2008). There is certainly no sign that selfing plants are unable to adapt to diverse environments, as witnessed by many studies of local adaptation in A. thaliana.
FURTHER READING
Bittles, A. 2003. Consanguineous marriage and childhood health. Developmental Medicine and Child Neurology 45: 571–576.
Charlesworth, D., and J. H. Willis. 2009. The genetics of inbreeding depression. Nature Reviews Genetics 10: 783–796.
Escobar, J. S., J. R. Auld, A. C. Correa, et al. 2011. Patterns of mating-system evolution in hermaphroditic animals: Correlations among selfing rate, inbreeding depression, and the timing of reproduction. Evolution 65: 1233–1253.
Falconer, D. S, and T.F.C. Mackay. 1996. Introduction to Quantitative Genetics. 4th ed. Harlow, Essex: Longman.
Jarne, P., and P. David. 2008. Quantifying inbreeding in natural populations of hermaphroditic organisms. Heredity 100: 431–439.
Petersen, C. W., and E. A. Fischer. 1996. Intraspecific variation in sex allocation in a simultaneous hermaphrodite: The effect of individual size. Evolution 50: 636–645.
Schoen, D. J., and J. W. Busch. 2008. On the evolution of self-fertilization in a metapopulation. International Journal of Plant Sciences 169: 119–127.
Slotte, T., J. Foxe, K. Hazzouri, and S. I. Wright. 2010. Genome-wide evidence for efficient positive and purifying selection in Capsella grandiflora, a plant species with a large effective population size. Molecular Biology and Evolution 27: 1813–1821.
Takebayashi, N., and P. P. Morrell. 2001. Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. American Journal of Botany 88: 1143–1150.