The Evidence for Evolution
Gregory C. Mayer
OUTLINE
1. The fossil record
2. Comparative biology
3. Biogeography
4. Evolution in action
5. Evolution as fact and theory
The evidence for evolution was first comprehensively assembled by Charles Darwin, who succeeded in convincing essentially all his scientific contemporaries of the fact of descent with modification. A signal factor in Darwin’s achievement was that he was able to weave together numerous strands of natural history—paleontology, systematics, embryology, morphology, biogeography—into a coherent framework. Since Darwin, genetics has joined this synthesis, and, in a development that might have surprised Darwin, evolution in natural populations has proven to occur sufficiently rapidly that it can be observed on human timescales. The most direct evidence of evolution comes from the fossil record, in which the dynamic changes of life over time are recorded, including many transitions between major taxa. A host of phenomena in comparative biology (e.g., systematics, morphology, embryology, genomics) and biogeography that otherwise appear inexplicable or anomalous are readily explained under the hypothesis of descent with modification. In addition, direct observation of natural and artificial populations shows the process of evolutionary change in action. Together, these sources of evidence lead to a “consilience of inductions” that makes the fact of evolution one of the most securely established generalizations in science.
GLOSSARY
Adaptation. A feature of an organism that fits it to its conditions of existence, giving rise to similarity among organisms leading the same or similar ways of life.
Homology. The correspondence, determined by their relative positions and connections, of organs in different organisms, which is indicative of affinity; the cause of this correspondence is inheritance from a common ancestor.
Oceanic Island. An island that has never been connected to a mainland and thus has received its fauna and flora over water by occasional means of transport.
Phylogenetic Tree. A representation of the history of life, with branching indicating the splitting of lineages, and the connection of the branches indicating the passage of genetic information and materials from one generation to the next.
Progression. The pattern in the fossil record in which earlier forms of life differ from later forms, with major groups first appearing in the record in a generalized form and later as more diversified members of the same group. Some of the earlier forms may become extinct, and later forms may more closely resemble modern forms. Not to be confused with progress, a different concept, according to which evolution proceeds toward some externally defined goal.
Speciation. The splitting of a lineage into two or more daughter lineages reproductively isolated and evolutionarily independent from other lineages.
Tetrapods. The group of four-limbed vertebrates comprising amphibians, reptiles, mammals, and birds; includes species that have secondarily lost their limbs, such as snakes.
Unity of Type. Similarities among organisms leading different ways of life under diverse conditions of existence, going beyond any functional need for similarity.
The evidence for evolution, it has been remarked, is not the result of some crucial experiment but something more like the contents of the American Museum of Natural History. And so it is: the evidence for evolution comes from a plethora of biological and geological subdisciplines—systematics, paleontology, stratigraphy, geochronology, biogeography, morphology, botany, zoology, embryology, genetics—many of which find their objects of study in the vast and varied collections of natural history museums.
It is the diversity of these sources of evidence, all leading to the conclusion that life on earth has undergone a long history of descent with modification, that is the great strength, and the most striking aspect, of the evidence for evolution. The varied sources of evidence are all brought into the unified explanatory scheme of evolution, forming what the philosopher William Whewell (1794–1866) called a “consilience of inductions,” each piece of evidence reinforcing the whole. Charles Darwin (1809–1882) in On the Origin of Species used precisely such a form of argumentation, marshaling the disparate facts of geology, systematics, morphology, embryology, and biogeography to support his theory of descent with modification.
Progression, Unity of Type, and Adaptation
By the time Darwin began his scientific career, it was already well established that the earth was old and that the fossil record was progressive, that is, that earlier forms of life differed from later forms, that some of the earlier forms had become extinct, and that later forms more closely resembled modern forms. It was also becoming clear that in this progression not only were older forms replaced by newer ones but later forms were in some way related to earlier ones. Thus, a major group would appear in the fossil record in a generalized form and would be succeeded by more diversified members of the same group.
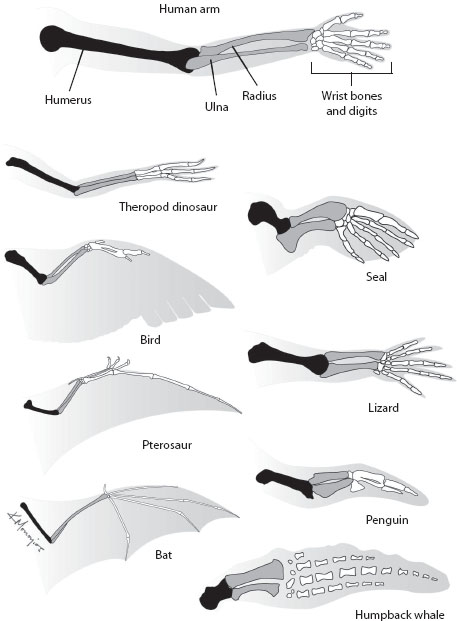
In addition to progression, two other great, but unexplained, classes of phenomena occupied biologists at this time: unity of type and adaptation. Unity of type refers to the similarities among organisms living different ways of life, similarities that extend far beyond any functional needs. The same basic skeletal plan of the forelimb—a humerus, then radius and ulna, then carpals, then metacarpals, then phalanges—occurs in all tetrapods, even though the limbs might appear quite different externally and be used for very different functions (figure 1). Such similarities extend to embryological features as well: all tetrapod embryos, for example, have four limb buds, even if the adults (e.g., whales, snakes) lack one or two sets of limbs.

Figure 1. Unity of type and adaptation, illustrated by the forelimbs of tetrapods. The pattern of one bone (humerus), two bones (radius and ulna), many bones (wrist and digits) is present in all, even though the size, shape, and relative proportions of the elements have been modified for varied ways of life, including walking, flying, grasping, and swimming. (Copyright Kalliopi Monoyios.)
Adaptation refers to those features of organisms that suit them to their conditions of existence (see chapter III.1; for a nuanced discussion of terminology, see chapter II.6), which may be shared by organisms with similar ways of life. Thus, sharks and whales share flattened tail flukes and dorsal fins, both features being of obvious functional importance for aquatic organisms. Despite these adaptive similarities, sharks and whales differ in many features, such as their respiratory, circulatory, and reproductive systems, that mark them as belonging to different major groups of organisms—fish and mammals, respectively.
Darwin provided in his theory of descent with modification a unified explanation of the geological (progression), structural (unity of type), and functional (adaptation) phenomena. Unity of type reflects inheritance of features from common ancestors, and the changes from the common ancestor are due to modification. The most important means of modification, natural selection, leads to adaptation. And when played out over geological time, descent with modification leads to progression. Once admitted, descent with modification and common ancestry would also account for the hierarchical relationships revealed by systematics and for the distributions of organisms across the surface of the globe. It was Darwin’s triumph to combine geological, morphological, embryological, systematic, and biogeographic evidence into a single explanatory theory.
This chapter considers the classes of evidence adduced by Darwin to support his theory of descent with modification, including examples from post-Darwinian disciplines such as genetics, and adds as an additional class of evidence observations of evolution in action, a class that was largely unavailable to Darwin.
1. THE FOSSIL RECORD
Progression
The earliest fossils are of simple (prokaryotic), single-celled, photosynthetic bacteria that lived in mats called stromatolites 3.5 billion years ago (see chapter II.11). One and a half to 2 billion years ago, more complex, organelle-bearing (eukaryotic) but still single-celled forms appear, and then multicellular forms. The origins of eukaryotes and multicellularity are not well documented in the record, and molecular data suggest that they may have occurred considerably earlier than the record shows (see chapter II.12). In the last part of the Precambrian, about 600 million years ago, diverse forms of marine invertebrates appear, and then in the Cambrian many more invertebrate groups arise, as well as the first vertebrates (see chapter II.15).
The earliest vertebrates, such as the recently discovered Myllokunmingia and Haikouichthys, which look remarkably like previously hypothesized generalized vertebrates, were soft-bodied jawless forms that lived about 525 million years ago. Diverse jawless fishes with mineralized hard tissues followed. The first jawed fishes appear in the Late Ordovician, about 445 million years ago. The bony fishes, or Osteichthyes, the most diverse living group of jawed fishes, are first found in the Late Silurian, about 420 million years ago. The first four-legged vertebrates (tetrapods) are the approximately 365 million-year-old amphibians Acanthostega and Ichthyostega from the Devonian (see chapter II.17). Reptiles, the first tetrapods to be independent of water (amniotes), make their appearance in the Pennsylvanian, about 315 million years ago. In the Mesozoic era, the last two major tetrapod groups arise: the first mammals are known from the Triassic/Jurassic boundary (about 200 million years ago), and the first bird is from the Late Jurassic, about 150 million years ago.
The fossil record of vertebrates, then, exemplifies the sequential origin and diversification of the major groups of organisms through geological time. Moreover, as one moves toward the present, many taxa go extinct and others dwindle in diversity, and the array of major groups comes to progressively resemble that of today.
Transitions
Darwin did not have closely spaced transitional forms that could demonstrate evolutionary continuity between major groups; he attributed their absence to the imperfections of the geological record. The record is indeed imperfect: only hard parts of organisms are readily fossilized, only certain sedimentary environments are conducive to fossilization, fossils must survive erosion and metamorphism and, finally, must be exposed on the surface and discovered. As a consequence, fewer than 1 percent of all species that ever lived are represented in the fossil record, and the record is spotty temporally, geographically, and taxonomically (see chapter II.9). For example, only about 30 localities have yielded important fossil faunas of tetrapods from the 60 million years of the Carboniferous, when the first major radiation of amphibians occurred.
Despite the imperfections, the transitional forms that Darwin hoped might be found soon started turning up. In 1861, Archaeopteryx was discovered and became the first of the proverbial “missing links” to be found. Clothed in the feathers of a bird with broad wings, it nonetheless had the long bony tail, toothed jaws, and free, clawed fingers of a reptile. T. H. Huxley (1825–1895) saw it clearly as intermediate between reptiles and birds and suggested a relationship to dinosaurs, a suggestion now well documented. Extraordinarily well-preserved fossils show not only the close skeletal resemblance of Archaeopteryx and early birds to theropod dinosaurs but also that feathers are not peculiar to birds; they were present on quite a few nonflying dinosaurs as well.
A few years later, Richard Owen (1804–1892) and E. D. Cope (1840–1897) both recognized that certain ancient reptiles (synapsids) bore an affinity to mammals. The origin of mammals from the synapsids is now known in exquisite detail (figure 2). Reptiles have several bones in the lower jaw, and one of the more posterior ones, the articular, forms the jaw joint with the quadrate bone of the skull. Mammals have a single bone in the lower jaw, the dentary (also present in reptiles), which articulates with the squamosal bone of the skull. A long series of fossils, beginning with fully reptilian forms in the Pennsylvanian, lead gradually to the mammals at the Triassic/Jurassic boundary. The dentary enlarges, becoming the largest bone of the lower jaw. The quadrate and articular become smaller. Eventually, the dentary contacts the squamosal, leading to several forms, such as Probainognathus, that have a dual jaw joint—both the old reptilian one and the new mammalian one. The transition is so gradual that it becomes a matter of convention to decide which form is the first “mammal”: Morganucodon is often so regarded, but it, too, has a dual jaw joint. In later forms the quadrate and articular detach from the jaw and become two of the three mammalian ear bones. Many other features that change during this transition—for example, the dentition becomes cusped, a secondary palate forms, the ilium becomes rod shaped—are likewise documented in the fossil record.
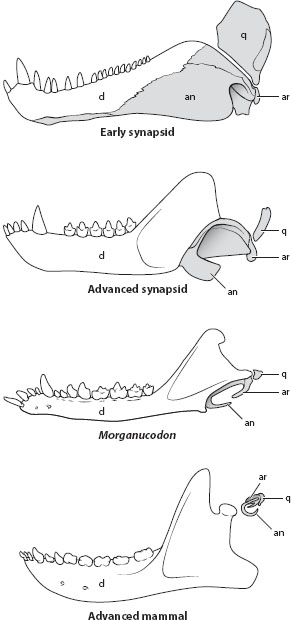
Figure 2. Homology of jaw bones of reptiles and ear bones of mammals. In early synapsids the lower jaw comprises the tooth-bearing dentary (d) and several postdentary bones, including the angular (an) and articular (ar). The latter articulates with the quadrate (q), forming the reptilian jaw joint. In advanced synapsids the latter bones are reduced, while the dentary enlarges. In the earliest mammals (Morganucodon) these bones are reduced further, and the dentary makes contact with the upper jaw, forming the mammalian jaw joint. In advanced mammals the angular, articular, and quadrate detach from the jaw entirely, becoming the tympanic, malleus, and incus of the mammalian ear. (After D. Davis 1991, K. Kardong 2012, and R. Carroll 1988.)
Another well-documented transition is that between the lobe-finned osteolepiform fishes and tetrapods (figure 3). Osteolepiforms were “typical” fish, with dorsal and anal fins, rounded heads with short snouts, and their shoulder girdles connected to their heads. But their pectoral and pelvic fins extended from the body in fleshy lobes, and within the lobe was a skeleton that, starting at the base (the end near the body), had a pattern of “one bone, two bones, many bones.” This is the same pattern as in tetrapods: the plan that exemplifies the unity of type of the tetrapod forelimb extends to osteolepiform fishes. In Panderichthys, from about 380 million years ago, the head and body are flattened, the snout is elongated, and the eyes are on top of the head; the anal and dorsal fins have been lost. In the remarkable Tiktaalik from 375 million years ago, the shoulder girdle (equivalent to our collarbone and shoulder blades) has been freed from the skull—Tiktaalik had a neck; and there is a joint within the “many bones” of the forelimb—it also had a wrist. Ten million years later we have the first actual tetrapods, Acanthostega and Ichthyostega, which have legs with toes but retain some of the gill-cover bones and the caudal fin of their fish ancestors.
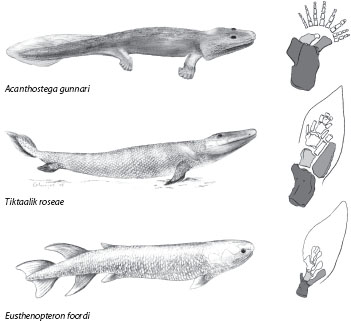
Figure 3. Transition from lobe-finned fish to tetrapods. The lobe-finned osteolepiform fish Eusthenopteron has the tetrapod-like “one bone, two bones, many bones” pattern in its fin. Tiktaalik, the “fishapod,” has lost the dorsal and anal fins, the head is free of the shoulder girdle, the snout is elongated with the eyes directed upward, and there is a wrist joint in the forelimb. Acanthostega, one of the first tetrapods, has digits but retains the caudal fin of its fish ancestors. The bones of the left forelimb shown in gray are, from darkest to lightest, the humerus, the radius, and the ulna, respectively; more distal elements are unshaded. (Copyright Kalliopi Monoyios.)
The origins of birds, mammals, and tetrapods represent fairly large changes in morphology and way of life, and the intermediate forms bridge the differences between these major taxa. The much smaller transitions that occur as one species evolves into another are also documented in the fossil record, but continuous sedimentary deposition is necessary to record such fine-scale temporal events. Such conditions are not common but occur most often in the fossil record of shelled marine planktonic protists, such as foraminifera and radiolarians, that can be recovered by extracting cores from the seabed. The shells of dead individuals rain down onto the ocean floor, forming an essentially continuous sedimentary record. In these organisms, such as the foraminiferans in the genera Globorotalia and Contusotruncana, and the radiolarian genus Eucyrtidium, such fine-scale changes can be observed, and in the latter even the split into two species from an original one has been recorded.
There are many other examples of transitional forms in the fossil record—such as ancestral whales and snakes with hindlegs—and more are being discovered every year.
2. COMPARATIVE BIOLOGY
Unity of Type
While the fossil record provides direct evidence of change of life over time, comparisons among organisms, either living or extinct, give evidence that the link between the different forms at different times is genealogical. Primary among these comparisons are the observed similarities in fundamental structure among organisms referred to as “unity of type.” These similarities, which extend from morphology to development to the genome, are homologies, that is, similarities due to inheritance from a common ancestor (see chapter II.6).
Famous homologies are the “one bone, two bones, many bones” pattern of the tetrapod limb (figure 1), and the jaw and ear bones of reptiles and mammals, both mentioned previously (figure 2). In the case of the limbs, the strong similarity of the same bone among the various tetrapods allows the homologous bones to be easily recognized: for example, humerus, radius, ulna. The similarity in plan is not accounted for by the functional requirements of the limbs but by inheritance from a common ancestor. In the jaw and ear, the homologies are traced with the aid of fossil and embryological (see the section Markers of History) evidence. The angular and articular bones of the reptilian lower jaw are homologues of the tympanic and malleus bones of the mammalian ear, while the quadrate of the reptilian upper jaw is the incus of the mammalian middle ear.
These patterns can be seen not only in the skeleton but in the genome itself at the cellular level. The human genome contains 23 pairs of chromosomes, whereas the genome of great apes has 24 pairs. The difference of one pair can readily be accounted for: human chromosome 2 is homologous to two ape chromosomes, which have become fused in the human lineage. The homology has been confirmed by the discovery of remnants of the central (centromere) and end (telomere) portions of the ape chromosomes within the human chromosome, showing that the latter arose from what were originally two chromosomes.
At perhaps the most basic level, the near universality of the genetic code is another homology that argues for the common ancestry of all life. The genetic code associates each amino acid with a codon of three nucleotides, which carries the information for the making of proteins. But the identity of the three nucleotides is arbitrary, so the code’s universality cannot be due to functional constraint but arises as a legacy of the code established in distant progenitors.
Common Ancestry
Although the common ancestry indicated by the genetic code embraces all (or nearly all) living beings, homologies do not generally have such a wide distribution. Rather, homologies characterize smaller groups, and these groups are nested within larger homology-characterized groups, which in turn are nested within yet larger such groups, and so on. This nesting of homologies results from the branching history of life, represented by the phylogenetic tree (see chapter II.1). When a lineage divides, giving rise to a new branch in the tree of life, the characteristics of the splitting lineage are passed on to its descendants. Modifications may occur in a descendant lineage, which will in turn be passed on to its, and only its, descendants. Each homology is the origin of a new feature, shared with descendants but not with collateral relatives. These nested sets of homologies, which are exactly what one would expect from a process of descent with modification, are powerful evidence for evolution.
For example, possession of a toepad, composed of laterally expanded scales at the end of the digit, characterizes lizards of the genus Anolis (figure 4). Anoles also have a hemipenis (oddly named, as this means that they have two penises, rather than half a penis, as the word might imply) as the male intromittent organ, but this organ characterizes a larger group, the squamates (lizards plus snakes), within which anoles are nested. The squamates, in turn, are nested within a yet-larger group characterized by the presence of an amniotic membrane around their embryos. The amniotes (reptiles, birds, and mammals) also have four legs, a trait shared with amphibians as well, which together make up the tetrapods. The tetrapods, also in turn, share features of the limb with certain fishes, forming again a more inclusive group. Continuing in this manner, the bony fishes, vertebrates, chordates, and deuterostomes constitute successively larger groups within which anoles are nested, and this nesting is indicative of the history of common ancestry.
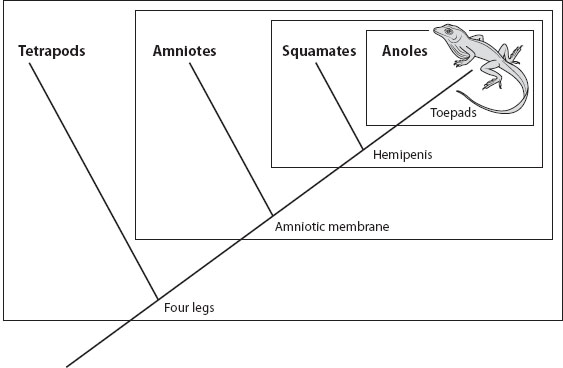
Figure 4. Phylogenetic tree of anole lizards showing nested homologies. The common ancestor of all tetrapods possessed four legs, a trait that, with modifications, was passed on to all of its descendants. One of these descendants evolved the amniotic membrane, thus nesting the amniotes (reptiles, birds, and mammals) within the tetrapods. Squamates (snakes and lizards), which evolved hemipenes, in turn are nested within amniotes. And finally, anoles are characterized by the possession of expanded toepads. Anoles thus possess toepads, hemipenes, amniotic membranes, and four legs, each trait inherited from a successively more distant ancestor, and each marking a more inclusive group. The nested boxes in the figure indicate the named clades.
Eventually all, or almost all, life can be subsumed within the nested tree. For organisms as divergent as insects and vertebrates it is hard to recognize morphological homologies, but genetic data show that homologies of gene and genome structure can be recognized in, for example, the presence of homologous sets of developmental regulatory genes (Hox genes) in both arthropods and vertebrates. While nestedness holds true for eukaryotes, there is some question whether at the base of the tree of life transfer of genetic material between prokaryotes may be so common as to obscure or efface the nested pattern (see chapter II.11).
Markers of History
Among the most striking evidences of evolution are the features of organisms that appear to reflect a constraint: organisms inherit from their ancestors a developmental system, and evolutionary modifications must take that inherited system as a starting point. Indeed, organismal features give every indication of having arisen by “tinkering” with this inherited preexisting developmental system, and make sense only as a result of descent with modification.
This constraint is evident in the similarities among the embryos of jawed vertebrates. The embryos go through a stage in which they resemble one another in the possession of pharyngeal arches, limb buds, tails, and other traits. After this stage, embryos diverge, developing into the varied forms they will become as adults. What perhaps is most striking is that structures present in the embryo are not always present in the eventual adult. Thus, humans and apes have an embryonic tail that largely disappears in the adult; only a bony vestige remains internally. Humans develop a coat of fine fur, the lanugo, which is lost just before or shortly after birth. All four limb buds develop in tetrapods, such as whales and snakes, that in the adult lack one or both pairs of limbs. In some snakes, a vestigial leg is still visible externally (figure 5). In each case, the eventual adult form develops from a shared state and subsequently passes through stages shared with smaller nested groups until finally arriving at its own specific state.

Figure 5. External hindlimb of a snake, the ball python (Python regius). Located just lateral to the anal scale, the keratinous claw is here shown slightly pushed away from the body by a probe. The claw is underlain internally by rudiments of the femur and, deeper in the body, the pelvis. (Photo by G. C. Mayer.)
Many structures show the traces of ancestry. In fetal mammals, the bones that eventually become the bones of the middle ear begin their development in positions along the jaw corresponding to those occupied by the homologous jaw bones of reptiles. The “thumb” of pandas (figure 6) is not homologous to other mammals’ inner digits, nor is it even a digit: it is a modified radial sesamoid bone, pressed into service as a makeshift digit, in an exquisite example of tinkering—that is, the modification of available structures, rather than an engineering ideal. In the Australian lungfish (Neoceratodus), there is a single dorsal lung, unlike in other lungfish (and tetrapods), in which the lungs are paired (figure 7). In all lungfish (and tetrapods), the lung attaches to the ventral part of the esophagus. In Australian lungfish, the pneumatic duct travels up alongside the right side of the esophagus to the dorsally positioned lung, seeming to trace the course of its morphological modification. Confirming this movement, the left pulmonary artery, instead of going directly to the dorsal lung, travels down the left side of the esophagus, curls under the esophagus, and follows the pneumatic duct up to the lung.
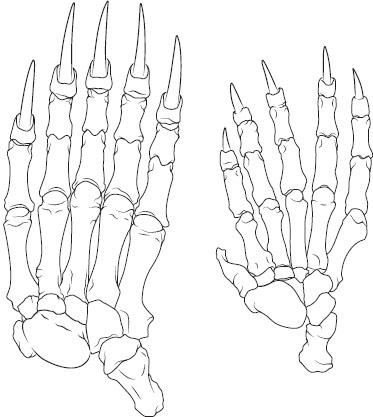
Figure 6. The right manus in the brown bear (Ursus arctos; left) and the giant panda (Ailuropoda melanoleuca; right). The panda’s thumb is not a true digit but a modified radial sesamoid bone. (After D. D. Davis 1964.)
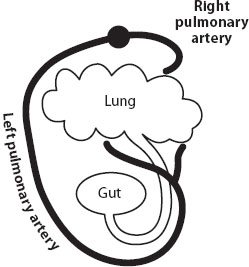
Figure 7. The lung and its arterial blood supply in the Australian lungfish (Neoceratodus). Note that the pneumatic duct swings around the gut to make the lung dorsal, and the left pulmonary artery, after branching from the dorsal aorta, follows it down and around (instead of going straight to the lung). (After E. S. Goodrich 1909.)
Such examples are not limited to morphology: pseudogenes, nonfunctional versions of genes that are functional in related species or even in other copies in the same organism, occur commonly in organism’s genomes (see chapters V.3 and V.5). For example, in most mammals, vitamin C is synthesized by a battery of enzymes. In primates, which get vitamin C in their diet, a gene for one of the necessary enzymes is present as a nonfunctional pseudogene, so that the vitamin is not synthesized. The broken gene is a relict from earlier mammals that do use it in their synthesis of the vitamin. In primates, the gene has been disabled by a mutation, but the now-inactive gene remains as a marker of primates’ forebears.
3. BIOGEOGRAPHY
Historically, the biogeographic evidence for evolution was crucial, because it was the evidence that convinced Darwin himself. Many features of the distribution of organisms that are anomalous or merely curious under a hypothesis of special creation became explicable and expected under the hypothesis of descent with modification. Under the latter hypothesis, related species should occur in geographically connected areas or in areas that could have been reached by a common ancestor of the related species. The connectedness and “reachability” of areas are determined chiefly by the distance and geographic barriers between them, and the ability of organisms to cross such barriers. Geographic conditions that either present barriers to dispersal (e.g., the ocean around islands) or facilitate it (e.g., the continuous land of continents), and organisms’ abilities to overcome or utilize these geographic barriers and bridges, are thus key determinants of the distribution of life on earth.
Islands
Bermuda is a small group of coral islands in the North Atlantic, 1000 km to the east of North America. Sitting atop a long-extinct volcanic platform and surrounded by waters of abyssal depth, islands such as Bermuda are called oceanic—they have never been connected by land to a mainland. The native non-marine vertebrate inhabitants of Bermuda are few—several land birds, migratory bats, a lizard, a terrapin—and show closest affinity to forms from the North American mainland. Some are identical or nearly so to the North American forms, whereas others are distinct endemic species found nowhere else. Several major groups common on the mainland are lacking entirely—there are, for example, no nonflying terrestrial mammals and no amphibians. Subsequent to human colonization, there have been many successful introductions of vertebrates, including land birds, reptiles, amphibians, and land mammals (figure 8). In its features, the fauna of Bermuda is typical of oceanic islands.

Figure 8. Endemic and invasive oceanic island species. Left: The endemic Bermuda skink (Eumeces [Plestiodon] longirostris) is the only extant native terrestrial reptile of Bermuda. Its nearest relatives are from the nearest mainland, North America, 1000 km to the west. Right: A Bermudian specimen of Bufo (Rhinella) marinus, native to Middle and South America, and introduced to Bermuda in 1885. It is one of several exotic amphibian and reptile species that have thrived and even become pests there, after the barrier to dispersal was overcome by human agency. (Skink photo by Richard Ground; toad photo by G. C. Mayer.)
All these characteristics are readily explained by the hypothesis of descent with modification. As a geologically oceanic island, Bermuda lacks organisms that cannot cross 1000 km of ocean (land mammals and amphibians) and is inhabited by descendants of a limited number of successful colonists, whose characteristics, such as the ability to fly, have permitted cross-oceanic dispersal. The nearest relatives of these colonists reside in the adjacent landmass of North America, because the islands are most accessible from there by “occasional means of transport” (as Darwin called them). Some of the colonists have been isolated sufficiently long to have diverged into endemic forms (e.g., the lizard), whereas others more recently arrived have not so diverged (e.g., the terrapin). The success of invasive introduced forms shows that the island fauna was, as Darwin put it, insufficiently stocked by the “creative force”: it was difficulties of dispersal, not habitat suitability, that caused the fauna to be restricted in species richness and taxonomic diversity.
Continents
On continents, the accessibility of adjacent areas leads, in general, to related forms being found throughout. Darwin noted this for the mammals of South America. The current mammals of the varied South American habitats—tropical, temperate, alpine—are all related to one another and not to mammals of similar habitats in distant places. Although organisms can sometimes disperse over large distances and across barriers (as in the case of many oceanic islands), dispersal occurs more readily between nearby areas, so that the biota of nearby areas, such as the different habitats of South America, have similar compositions. Similarly, the fossil South American mammals Darwin found were related to the ones now present. Thus, related organisms live in places they can reach by dispersal, and descend from the previous inhabitants.
This pattern is also well illustrated by the mammal fauna of Australia, a continent that became isolated from the other continents tens of millions of years ago. Most of the mammals of Australia are marsupials, a group now found elsewhere only in the Americas, where they are much less diverse. In Australia, the marsupials occupy all the major habitats and have a rich fossil record going back tens of millions of years. Having been isolated in Australia, the marsupials have prevailed and diversified into most ecological roles: herbivores (kangaroos), carnivores (thylacine), burrowers (wombats), climbers (koala), gliders (phalangers), and many more. The only native placental mammals are bats and murid rodents. Bats can fly, and rodents are able to colonize across water barriers (although not ones as wide as those that can be crossed by bats).
Geographic distributions that might seem anomalous under the hypothesis of descent with modification are explicable when the movement of the continents is taken into account. For example, Cynognathus, a terrestrial synapsid of the Triassic, about 240 million years ago, has been found in South America, Africa, and Antarctica, all now widely separated by seas unlikely to be crossed by a stout, meter-long animal like Cynoganthus. But in the Triassic all these continents were connected in a single landmass, Gondwana. Far from being a problematic case, Cynognathus shows again the importance of historical connectedness, both genealogical and geological, in the distribution of organisms.
4. EVOLUTION IN ACTION
Changes within Populations
Although fossils record descent with modification over billions of years, evolution often occurs quickly enough to be observed within one or a few human lifetimes, and such cases allow the full panoply of evolutionary mechanisms—mutation, gene flow, genetic drift, and natural selection—to be seen in action.
Darwin’s chief examples of observed evolution involved the work of animal and plant breeders in producing and elaborating the features of domesticated organisms (see chapter VIII.5). Studies of such species show the importance of selection in establishing and fixing desired characteristics. The many varieties of domestic dog, differing so much in size, shape, and behavior, have all been produced from the wolf in the last few thousands of years. Corn, one of the most highly modified and economically important organisms ever created by humans, was developed by selective breeding from a wild species of grass.
Rapid evolution has also unintentionally been caused by humans, who changed the environment in ways that prompted evolutionary responses from affected organisms (see chapters VIII.2 and VIII.3). Industrial melanism, the evolution of darker coloration in animals living in environments darkened by pollution, is widespread in a variety of insects in industrialized areas of both Europe and North America. When pollution controls have been enacted, the evolutionary change has been reversed, and the lighter-colored forms have again increased in frequency. Both the spread of melanism and its reversal have been observed in Britain in the moth Biston betularia over a period of about 150 years.
The use of pesticides and antibiotics has frequently led to the undesired evolution of resistance in the targeted organisms, including rodents, insects, bacteria, and viruses. Multiple-antibiotic-resistant strains of bacteria have become a major health problem, and one of the greatest difficulties in treating acquired immune deficiency syndrome (AIDS) has been the rapid evolution of resistance by the human immunodeficiency virus (HIV). In the evolution of HIV, high mutation rates, short generation time, large population sizes, and strong selection have all combined to make the virus adapt extremely rapidly to drugs. Drug mixtures, which attack the metabolism of the virus in different ways simultaneously, have proven more effective, as the multiple mutations required for resistance to all the drugs are less likely to occur.
Species introduced into new geographic areas, whether by humans or by natural means, are likely to find themselves in new environments and thus are more likely to undergo evolutionary changes. Such introductions have indeed produced many examples of divergence in features such as size, shape, and coloration. One example is the house sparrow (Passer domesticus), introduced into North America from Europe about 1851. By the middle of the twentieth century, the sparrow had geographically differentiated in coloration, size, shape, and physiology in a manner parallel to that of native species.
Natural populations have also been observed evolving in response to natural environmental changes (see chapter III.7). Perhaps the best-studied case is that of Darwin’s finches (Geospiza) in the Galápagos, where populations have been carefully tracked over decades. During this time span, repeated episodes of morphological evolution have occurred in response to climatically induced variations in the food supply. Further, the genetic basis of these changes has been demonstrated by observations in nature. These studies show that natural populations are not evolutionarily static but can adaptively track changes in the environment.
Speciation
Speciation, the origin of new lineages reproductively isolated and evolutionarily independent from other lineages, is a key part of evolution (see Section VI: Speciation and Macroevolution). Without it, evolution might occur within a lineage, but there would be no increases in biodiversity. Divergence of lineages is enhanced by their geographic separation (since it reduces or eliminates the homogenizing force of gene flow). Isolated populations may differentiate independently—including in characteristics affecting reproductive compatibility—to the point that they can no longer exchange genes if brought again into contact. The insensible gradation over space of populations varying from mere geographic isolates to highly differentiated populations approaching genus-level distinction provides evidence for speciation by geographic isolation, and there are many examples, such as in kingfishers of the genera Tanysiptera and Halcyon on the islands of the southwest Pacific. In most cases though, divergence takes too long to be observed within a human lifetime. Laboratory studies have shown that incipient reproductive isolation can arise in separated populations undergoing differential adaptation, and that reproductive isolation can be enhanced by selection against individuals who hybridize with the “wrong” population.
In some cases, though, speciation occurs sufficiently quickly to be observed in the wild within a human lifetime. Speciation by polyploidy (i.e., the duplication of chromosome sets) is an important mode of splitting in plants (see chapter VI.9). A well-studied case is the recent origin of the British salt marsh grass Spartina anglica, a polyploid derived from the natural crossing of the introduced American species S. alterniflora (diploid chromosome number 2n = 60) with the native S. maritima (2n = 62). Spartina alterniflora was accidentally introduced in 1829. In 1870, sterile hybrids with S. maritima were first recorded. Sterility of the hybrids, due to mismatching of the parental chromosomes, was overcome by chromosome doubling, and the fertile S. anglica (2n = 122) was first recorded in 1892. The new species has since spread along coastlines throughout Britain.
5. EVOLUTION AS FACT AND THEORY
It is sometimes noted pejoratively that evolution is a “theory.” This pejorative usage confuses a vernacular notion of “theory” as something uncertain or conjectural, with the word’s scientific usage. In science, a theory is not a mere conjecture but a connected series of propositions supported by, and explanatory of, many and varied lines of evidence. We refer to the “germ theory of disease” not because we are unsure that microbes cause disease but because the theory is a set of high-level and powerful generalizations that account for and are, in turn, supported by a huge amount of data.
Regarding evolution, it was Darwin who first convincingly assembled the many and varied lines of evidence that could be accounted for by, and provide evidence of, descent with modification. His many successors have carried on his work, and Darwin would have been both pleased and astonished by the further lines of evidence that have been brought to bear. In his own lifetime, transitional fossils began to be found, and we now have them in abundance. Darwin’s own attempts at formulating principles of inheritance failed, so he would have been gratified by the explosive growth of genetics and now genomics filling in what he did not know. Crucially, the facts of genetics could have turned out to be incompatible with Darwin’s evolutionary views but, instead, his ideas have passed this important test. He would perhaps have been most astonished by the evidence for the rapidity with which evolution can occur, including the formation of new species within a human lifetime.
As briefly reviewed here, all these lines of evidence, all leading to the same conclusion, serve to make descent with modification one of the most securely established high-level generalizations in science and allow us to speak confidently of the fact of evolution.
FURTHER READING
Carroll, R. 2009. The Rise of Amphibians: 365 Million Years of Evolution. Baltimore: Johns Hopkins University Press. Includes an account of the origins of vertebrates and reptiles, as well as of the origin of amphibians.
Coyne, J. A. 2009. Why Evolution Is True. New York: Viking Penguin. An account of the evidence for evolution for a general audience.
Dawkins, R. 2009. The Greatest Show on Earth. New York: Free Press. Another account of the evidence for evolution for a general audience.
Futuyma, D. J. 2013. Evolution. 3rd ed. Sunderland, MA: Sinauer. The leading textbook of evolutionary biology.
Grant, P. R., and Grant, B. R. 2008. How and Why Species Multiply: The Radiation of Darwin’s Finches. Princeton, NJ: Princeton University Press. An account of the detailed evolutionary studies conducted by the authors and their associates over four decades, including several episodes of closely observed evolutionary changes.
Mayr, E. 2001. What Evolution Is. New York: Basic Books. A summary of the evidence for evolution and its mechanisms by the “Darwin of the twentieth century.”
Prothero, D. R. 2007. Evolution: What the Fossils Say and Why It Matters. New York: Columbia University Press. A richly illustrated account of the fossil record emphasizing transitions between major groups.
Shubin, N. 2008. Your Inner Fish. New York: Pantheon Books. A popular account of the discovery of the fish-amphibian transitional form Tiktaalik, and of the traces of common ancestry within vertebrates (including man), and of the common ancestry of vertebrates with other animals.
Young, D. 2007. The Discovery of Evolution. 2nd ed. Cambridge: Cambridge University Press. Intended for a general audience, this superb and richly illustrated history serves as a text for evolutionary biology itself, because it introduces and explicates not just the ideas and historical figures but the evidence on which the major discoveries of evolutionary biology are based.