Evolution of Sex Chromosomes
Doris Bachtrog
OUTLINE
1. Origin of sex chromosomes
2. Y (W)-chromosome degeneration
3. Dosage compensation of the X
4. Gene content evolution of sex chromosomes
5. Diversity of sex determination
Sex is universal among most groups of eukaryotes, yet a remarkable diversity of sex-determining (SD) mechanisms exist. The evolution of separate sexes has been accompanied by the acquisition of sex chromosomes many times across fungi, plants, and animals. Despite independent origins, sex chromosomes of many organisms share common features, reflecting similar evolutionary forces acting on them. Sex chromosomes are of particular interest to biologists for different reasons. First, sex chromosomes determine the gender of many species; thus they contain the gene ultimately responsible for sex determination. Second, Y or W chromosomes often lack recombination and undergo chromosome-wide degeneration. Finally, sex chromosomes show sex-biased transmission (that is, they spend different amounts of time in males and females), and they occur in different copy number in the two sexes (for example, in mammals, the X is diploid in females, but haploid in males). These features drive many unusual patterns of genome evolution, and sex chromosomes uniquely contribute to many evolutionary processes, such as speciation and evolutionary conflict.
GLOSSARY
Dosage Compensation. A process that balances expression of sex-linked and autosomal genes in the heterogametic sex.
Environmental Sex Determination (ESD). The process by which sex differentiation is determined by external environmental factors (e.g., temperature or pH) during offspring development.
Female Heterogamety. A sex chromosome system in which males have two identical sex chromosomes (two Z chromosomes) and females have two different sex chromosomes (a Z and a W chromosome).
Genotypic Sex Determination (GSD). The process by which sex differentiation is determined primarily by genetic factors, most commonly on the sex chromosomes.
Haplodiploidy. A sex-determination system in which sex is determined by ploidy level. Males are haploid and develop from unfertilized eggs, whereas females are diploid and develop from fertilized eggs. Females typically have control over fertilization.
Hemizygosity. A state in which only one copy of a gene is functioning in an otherwise-diploid organism (for example, the X chromosome in an XY male).
Heterogametic Sex. The sex with a pair of different sex chromosomes (e.g., male XY in mammals; female ZW in birds). The heterogametic sex produces two different types of gametes, one with one type of sex chromosome and one with the other.
Homogametic Sex. The sex with a pair of identical sex chromosomes (e.g., female XX in mammals; male ZZ in birds), therefore producing only gametes with one type of sex chromosome.
Male Heterogamety. A sex chromosome system in which females have two identical sex chromosomes (two X chromosomes) and males have two different sex chromosomes (an X and a Y chromosome).
Sex Determination. Any of various mechanisms in which the sex of an individual is determined.
Sex-Biased Expression. Expression of genes that show different absolute expression levels in males and females.
Sexually Antagonistic Selection. Selection that differs in direction between males and females; that is, an allele is favored in one sex and unfavored in the other.
Male versus Female Heterogamety
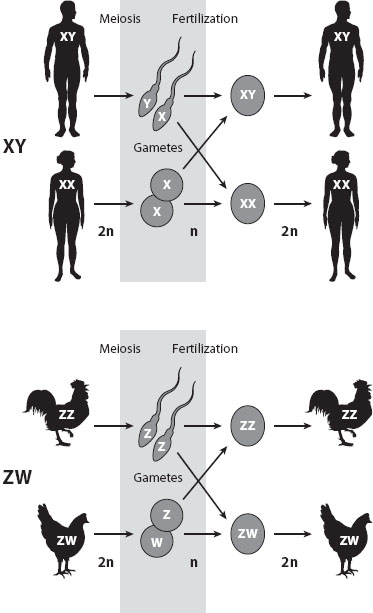
In many taxa, including some plants and many animal species, sex is determined by a pair of sex chromosomes. The most familiar sex chromosome system is that of humans, in which females have two identical sex chromosomes, called X chromosomes (i.e., a female is XX), and males have two different chromosomes (an X and a Y chromosome). In some species, including birds, snakes, and butterflies, this pattern is reversed: females carry two different sex chromosomes (termed the Z and W chromosomes), and males carry two identical sex chromosomes (ZZ). The sex with the identical pair of sex chromosomes (the homogametic sex) produces gametes with only one type of sex chromosome, while the sex with different sex chromosomes (the heterogametic sex) produces gametes of two different types (figure 1).

Figure 1. Male versus female heterogamety. In some species, including mammals and Drosophila, males have an X and a Y chromosome, while females have two X chromosomes. Here, females produce gametes with one type of sex chromosome (an X), and males produce gametes bearing either an X or a Y chromosome. In female-heterogametic species, such as birds or butterflies, males are the homogametic sex (producing Z-carrying gametes only), while females are heterogametic (producing Z- and W-carrying gametes).
Independent Origins of Sex Chromosomes
Sex chromosomes are phylogenetically widespread and originated many times independently in different organisms, including plants and animals. For example, the XY sex chromosome system shared by all mammals originated about 150 million years ago and is not homologous to the ZW sex chromosome system of birds. Among other vertebrates, ZW sex chromosomes evolved independently in snakes, and both ZW and XY systems have evolved multiple times in other reptiles, amphibians, and fish. Sex chromosomes are also widespread in invertebrates, which contain both male- and female-heterogametic systems. Many Diptera, including the fruit fly Drosophila, are XY, while Lepidoptera (moths and butterflies) are ZW. In addition, sex chromosomes arose multiple times in plant species. Examples of independently evolved sex chromosomes include those of papaya (XY), cannabis (XY), wild strawberry (ZW), and white campion (XY). Despite independent origins, sex chromosomes share many similar characteristics. Notably, the chromosome present only in the heterogametic sex (the Y or W chromosome) is often gene poor and has accumulated repetitive DNA. In contrast, the X and Z chromosomes superficially resemble autosomes, apart from their difference in copy number between males and females; however, X chromosomes have often evolved special regulatory mechanisms to compensate for this gene dose difference (see section 3 below). The similar appearance of sex chromosomes in different organisms suggests that similar selective pressures have acted to shape the evolution of sex chromosomes in different taxa. But where do sex chromosomes come from, and what evolutionary processes drive their evolution?
Sex Chromosomes Originate from Autosomes
Sex chromosomes arise from autosomes. The first step in the evolution of sex chromosomes is the acquisition of a sex-determining function on a proto-sex chromosome (genetic sex determination). Genetic sex determination can arise in a species that has no separate sexes (i.e., hermaphrodites, in which individuals carry both female and male reproductive functions), or in a species with separate sexes but where sex is determined by environmental cues (for example, temperature, such as in many turtles and crocodiles). If sex chromosomes arise in a hermaphroditic species, as is the case for most plants and many animals, a likely path for the evolution of separate sexes and sex chromosomes is that one proto-sex chromosome acquires a male-sterility mutation and the other proto-sex chromosome acquires a female-sterility mutation. Depending on the dominance relationship of these mutations, this would generate a proto-X/proto-Y or proto-Z/proto-W chromosome and the population would transition through a stage in which both hermaphrodites and females, or hermaphrodites and males, are present. There is then strong selection to restrict recombination between the male-sterility and female-sterility mutations on the different chromosomes, since a recombination event could place both mutations on the same chromosome and would generate a sterile individual. In a species with environmental sex determination, a dominant male-determining mutation on a former autosome would create a proto-Y chromosome, while a dominant female-determining mutation would result in the origination of a proto-W chromosome (figure 2).
Figure 2. The origin of sex chromosomes (shown is the XY system). Sex chromosomes arise from a pair of initially identical autosomes with the same sets of homologous genes (white boxes). The first step in the evolution of heteromorphic sex chromosomes is the acquisition of a sex-determining gene (in black) on one of the autosomes. The accumulation of sexually antagonistic mutations close to the sex-determining region (shaded boxes) selects for a repression of recombination between nascent sex chromosomes (darker-shaded area along the chromosome). The nonrecombining Y chromosome loses most of its original genes (gray boxes indicate pseudogenes) and degenerates. This results in a pair of heteromorphic sex chromosomes. Different scenarios are possible. If the initial mutation is a female-determining mutation, a ZW system could evolve. In the case where sex determination arises in a hermaphroditic species, evolving separate sexes (and sex chromosomes) would actually require mutations at two loci (a male and a female sterility mutation).
The SRY gene in mammals is such a dominant master sex-determining locus and is found on the Y chromosome; it initiates the differentiation of the developing embryo into a male. Thus, acquisition of the SRY gene on an autosome likely triggered the formation of a proto-Y chromosome in mammals, and its former homologue that lacked the SRY gene became a proto-X chromosome. On autosomes, recombination homogenizes the gene content between the homologous paternal and maternal chromosomes and shuffles segregating mutations across different chromosomal backgrounds. To allow nascent sex chromosomes to evolve independently, it is necessary that recombination between the proto-Y and proto-X chromosomes becomes suppressed, allowing each chromosome to accumulate independent mutations; the X and the Y can then diverge from each other in sequence and function. But why should recombination become restricted on a pair of proto-sex chromosomes beyond the sex-determining region?
Sexual Antagonism Drives Recombination Suppression
The driving force for the evolution of restricted recombination between proto-sex chromosomes is generally thought to result from sexually antagonistic alleles accumulating close to the sex-determining region. Sexual antagonism refers to a situation in which genes cause opposing fitness effects in the two sexes. Males and females in many species differ in their morphology, behavior, and physiology; however, in the absence of sex chromosomes, they share identical sets of genes. It is possible that a large number of genes or mutations may have opposing fitness benefits in the two sexes; specific mutations can be good for one sex, but bad for the other. For example, in guppies, females prefer males with bright, colorful ornaments; despite increased predation risk, such males have a mating advantage over less brightly colored ones. Less colorful females, however, maximize their fitness by avoiding predation. Thus, a mutation that causes a brightly colored spot is selected for in males, but selected against in females. Such a sexually antagonistic mutation can become established in the population only if the benefit to males outweighs its harmful effects in females; however, if this mutation arises in close proximity to the male-determining region, it will find itself more often in males, the sex in which it is beneficial, and it can become established more easily in the population. Thus, sexually antagonistic mutations are expected to accumulate close to sex-determining genes. Indeed, several color genes in guppies are closely linked to a male-determining gene. Sporadic recombination events between the male-beneficial mutations and the male-determining region would transfer these color genes onto the X chromosome, and they would be expressed in females. Thus, there is selection to eliminate recombination between the sexually antagonistic alleles and the sex-determining region, to ensure that such genes are restricted to the favored sex. Recurrent accumulation of sexually antagonistic mutations on the proto-sex chromosomes can select for the repression of recombination over most or all of the length of the proto-sex chromosomes (figure 2). A consequence of the restriction of recombination between the nascent sex chromosomes is that the heterogametic sex chromosome (the Y or W) is completely sheltered from recombination, while the other sex chromosome (X or Z) can still recombine in the homogametic sex. The lack of recombination on the Y or W chromosome results in its degeneration.
2. Y (W)-CHROMOSOME DEGENERATION
A Lack of Recombination Causes Y Degeneration
The most dominant characteristic of heteromorphic sex chromosomes is the lack of functional genes on the Y or W chromosome. Y (or W) chromosomes degenerate because of their lack of recombination. Autosomes and the X in female mammals (or Z in male birds) always exist in two copies, a paternal and a maternal one, and undergo meiotic recombination. As explained below, this enables selection to efficiently purge deleterious mutations, and allows the X or Z to maintain its original gene content. Y (or W) chromosomes, in contrast, completely lack meiotic recombination for most of their length. The efficacy of natural selection is reduced on a nonrecombining chromosome, and is the basis for the degeneration of the Y (or W) chromosome (figure 3). Natural populations are subject to recurrent mutations. Some of these mutations increase the fitness of their carrier, for example, by increasing survivorship, or increasing fertility (beneficial mutations); most mutations, however, are detrimental and reduce the function of a well-adapted gene (deleterious mutations). The general role of natural selection is to incorporate beneficial mutations (i.e., adaptation) and remove deleterious ones (purifying selection; see chapter V.1). On a recombining chromosome, natural selection can act on individual mutations by reshuffling mutations and putting them on different genomic backgrounds. In contrast, in the absence of recombination, new gene combinations cannot be generated and selection must act on the entire chromosome. That is, different selected mutations on a nonrecombining chromosome can interfere with each other, thereby reducing the efficacy of natural selection. This reduction can lead either to an accumulation of deleterious mutations, by a process known as Muller’s ratchet or by genetic hitchhiking with beneficial mutations, or to reduced rates of adaptive evolution (the ruby in the rubbish model; see figure 3 for a description of these processes). Under the Muller’s ratchet and genetic hitchhiking model, Y-linked genes continuously decrease in fitness relative to their X homologues, as a result of the accumulation of deleterious mutations. The ruby in the rubbish model, in contrast, states that reduced fitness of Y-linked genes relative to the X instead results from a lower rate of incorporation of beneficial mutations. Under both scenarios, dysfunctional Y-linked alleles will eventually become silenced and lost from the degenerating Y, and in the long run, only a few genes remain on old Y chromosomes, if any.
Figure 3. Models of Y degeneration. Y/W chromosomes degenerate since they accumulate deleterious mutations at ancestral genes. Two main processes have been proposed to explain this accumulation: Muller’s ratchet, the irreversible accumulation of deleterious mutations (gray circles) in a finite population, and genetic hitchhiking of deleterious mutations together with beneficial alleles (white circles). Y or W chromosomes may also undergo less adaptive evolution, as a result of linkage of beneficial alleles with deleterious mutations (the ruby in the rubbish model). (A) Muller’s ratchet. Mutation-free chromosomes can be lost in finite populations as a result of stochastic effects. Recombination allows the re-creation of mutation-free chromosomes, whereas this loss is irreversible on a nonrecombining Y chromosome. (B) Genetic hitchhiking. Newly arising beneficial mutations might occur on a chromosome that also contains deleterious mutations. Recombination enables the beneficial allele to disassociate from the deleterious mutation, while the fixation of the beneficial mutation on a nonrecombining Y chromosome will drag along the deleterious mutation. (C) Ruby in the rubbish. Beneficial mutations of weak effect linked to more strongly deleterious mutations will be eliminated by purifying selection on a nonrecombining Y chromosome, since such chromosomes will have no net fitness advantage.
Old Y Chromosomes
The degeneration of Y (or W)-linked functional genes is associated with an accumulation of repetitive DNA, such as transposable elements (TEs) or satellite DNA (see chapter V.2). In parts of the genome that recombine, such insertions can normally be efficiently purged. On the Y (W), however, repetitive DNA can accumulate as a result of the reduced efficacy of natural selection. In addition, as more and more genes degenerate, the chance that a new TE inserts into a functional gene decreases, and TEs can start to accumulate neutrally. Thus, the size of an evolving Y chromosome can increase dramatically because of the accumulation of repetitive DNA; however, as the gene density becomes lower and lower, large deletions can occur, and the Y or W chromosome can shrink in size and carry fewer and fewer genes (figure 2). Eventually, the Y (W) may carry only the sex determining gene and a few other genes beneficial to the heterogametic sex. Ultimately, a species might evolve an alternative sex determination signal, for example, the ratio of X to autosomes can determine sex (as is the case in Drosophila). In such a situation, the Y chromosome can be lost entirely, and a species becomes XX (females) and X0 (i.e., only one X chromosome and no Y in males, as has happened in Caenorhabditis elegans; see section 5 below).
3. DOSAGE COMPENSATION OF THE X
Gene Dose Deficiency in Heterogametic Sex
The amount of gene product correlates with the number of gene copies for a gene. Y degeneration creates the problem of reduced gene dose in males; genes that degenerate from the Y are expressed at a lower level in males. In many gene networks, however, the dose of genes is important, and gene dose imbalances may have negative fitness consequences. Thus, many organisms have evolved compensatory mechanisms to counterbalance this gene dose deficiency in the heterogametic sex, and different species have found different strategies to achieve dosage compensation (i.e., the balancing of gene product of sex-linked genes in the heterogametic sex). The primary selective pressure driving the evolution of dosage compensation is to balance expression levels between autosomal and sex-linked genes in the heterogametic sex, which has too little gene product for genes that have been lost from the Y chromosome. A by-product of the acquisition of dosage compensation is that expression levels for X-linked genes become similar between the sexes; that is, dosage compensation equalizes expression levels of X-linked genes between the sexes. Note that this is a consequence of selection for dosage compensation in males, and not the primary selective pressure driving it. Different organisms with independently evolved Y chromosomes have found vastly different evolutionary solutions to achieve dosage compensation.
Different Paths to Dosage Compensation
The most direct way to compensate for a deficiency in X chromosomal gene product is to upregulate X-linked genes specifically in the heterogametic sex. This requires a compensatory mechanism that is male-specific and exclusively recognizes and targets X-linked genes. Drosophila has evolved dosage compensation along this path, using a male-specific ribonucleoprotein complex (a complex that consists of RNA and proteins) that specifically targets the X chromosome and results in transcriptional upregulation of the X (figure 4). This mechanism restores the balance between X and autosomal gene product in males, without changing expression levels of the X in females, and it results in similar levels of X-linked gene product in both sexes. A more indirect path to evolve dosage compensation is followed in mammals and C. elegans. Here, it is believed that the initial upregulation of X-linked genes was not sex limited. Instead, selection in males to upregulate the X also resulted in increased X expression in females. Thus, while a general upregulation of X-linked genes restores the gene dose problem in males, females now produce too much gene product from their two X chromosomes relative to their autosomal expression; this in turn creates selective pressure in females to evolve a compensatory mechanism to reduce elevated expression levels of the X. In mammals, this is achieved by females completely inactivating one of their two X chromosomes using noncoding RNAs. C. elegans XX hermaphrodites, on the other hand, approximately halve expression from each of their two active X chromosomes. Thus, dosage compensation in these organisms is achieved through a two-step process: upregulation of the X in both sexes, followed by downregulation or inactivation of the X in females. Again, this results in balanced X-autosome expression in males (and females), and similar levels of X-linked expression in both sexes.
Figure 4. The evolution of dosage compensation. Y degeneration results in reduced gene dose of X-linked genes, selecting for an upregulation of X-linked genes in males. This upregulation may be male specific (as in Drosophila). Alternatively, this initial upregulation may not be sex specific, thus resulting in selective pressure to downregulate the X in females. Mammals completely inactivate one of their X chromosomes, whereas C. elegans halve expression from both their X chromosomes in hermaphrodites.
Lack of Dosage Compensation in Some Systems
Not all taxa appear to have evolved dosage compensation. This could be a consequence of recently evolved sex chromosomes that have not yet had time to have acquired dosage compensation (as in some fish species), or because the X chromosome contains only few genes, none of which is dosage sensitive. However, recent empirical data show that birds, butterflies, and schistostomes (a trematode worm) all lack dosage compensation; these taxa are all considered to have “old” and large sex chromosomes. A common feature of these three groups is that they all have female heterogametic sex determination. W chromosomes closely resemble Y chromosomes; that is, they are degenerate with few active genes. Thus, in ZW systems, females have reduced expression of Z-linked genes, and dosage compensation should function to upregulate Z genes in females, to compensate for their degenerate W chromosome. At present, it is unclear whether the lack of dosage compensation in ZW species is a general feature of these systems, and what the evolutionary explanation for this might be.
4. GENE CONTENT EVOLUTION OF SEX CHROMOSOMES
Differential Accumulation of Sexually Antagonistic Mutations
As discussed above, the driving force in the evolution of restricted recombination between the proto-sex chromosomes is sexually antagonistic selection. Males and females share the same genome, but they often differ in their morphology, behavior, and physiology. This implies that many genes may have different optimal functions in males and females. Sex-biased transmission and hemizygosity shape patterns of gene content evolution of sex chromosomes. In particular, Y chromosomes are limited to males and W chromosomes to females, whereas X chromosomes are transmitted more often through females (females have two X chromosomes, and males, only one; thus, an X chromosome is found two-thirds of the time in a female, and only one-third of the time in a male) and Z chromosomes show male-biased transmission. In general, if a chromosome spends more time in one sex, it should be better adapted to the specific needs of that sex. Autosomes spend equal amounts of time in males and females and are thus exposed to selection equally in the two sexes, while sex-biased transmission of the sex chromosomes implies that they might accumulate sexually antagonistic genes. In addition, X and Z chromosomes are hemizygous in the heterogametic sex, greatly influencing the fixation probability of recessive mutations, especially for mutations favoring the heterogametic sex. Together, these peculiarities uniquely affect the evolutionary dynamics of sex chromosomes, making them a hot spot for the accumulation of sexually antagonistic mutations.
Gene Content of Y Chromosome
Y chromosomes are male limited, and thus selected only in males, whereas W chromosomes are under selection only in females; the Y should therefore accumulate male-beneficial and the W female-beneficial genes. Indeed, most of the genes on the Y chromosome of Drosophila and mammals, which are the best-studied sex chromosome systems to date, have testis-specific expression. Also, Drosophila males that lack a Y chromosome (which are X0) are viable but sterile. Thus, the Drosophila Y contains no genes necessary for viability, but harbors genes required for male fertility. Extensive sequence analysis has revealed a peculiar structure of genes present on the human Y chromosome. In particular, most testis-specific genes on the human Y are contained within ampliconic and palindromic structures (i.e., present in multiple copies in opposite orientations). The gene copies in these palindromes frequently undergo Y-to-Y gene conversion (a recombination-related process), which can be an efficient way to prevent degeneration of Y-linked gene functions. Much less is known about the gene content of W chromosomes, but similar ampliconic gene families have been detected for the chicken W chromosome. This suggests that gene amplification and intrachromosomal gene conversion may be an important way to retard degeneration and preserve gene function on a nonrecombining chromosome.
Sex-Biased Transmission versus Hemizygosity on X and Z
Expectations for gene content evolution are straightforward for Y and W chromosomes, but the situation is more complex for the X and Z, since sex-biased transmission and hemizygosity may work in opposite directions. On one hand, the X shows female-biased transmission, which favors the accumulation of female-beneficial genes on the X, since X-linked genes are more often under selection in females than in males. Conversely, Z chromosomes are more often transmitted through males and male-beneficial genes should accumulate; however, hemizygosity of the X and Z chromosomes may favor an accumulation of mutations beneficial to the hemizygous sex (i.e., male-beneficial mutations on the X, and female-beneficial mutations on the Z), depending on their dominance coefficient. Many beneficial mutations, including those with sexually antagonistic fitness effects, may be recessive. Selection is more effective in incorporating recessive mutations on sex chromosomes. In particular, hemizygosity of the X in males implies that recessive, male-beneficial mutations can become incorporated more easily on the X relative to autosomes, while hemizygosity of the Z in females favors the accumulation of recessive female-beneficial alleles. Thus, X chromosomes may simultaneously accumulate dominant female-beneficial mutations (due to sex-biased transition) and recessive male-beneficial mutations (due to hemizygosity of the X in males), while dominant male-beneficial and recessive female-beneficial mutations may accumulate on Z chromosomes.
Gene Content of X and Z Chromosomes
Empirical patterns of gene content evolution are complex on X or Z chromosomes, as expected given contrasting selective forces. In particular, genome-wide expression studies have shown that genes with sex-biased expression (that is, genes expressed at different levels in males and females) show a nonrandom distribution on sex chromosomes. Different expression levels of genes in the two sexes could be a consequence of the resolution of sexual antagonism. By reducing the expression level of a gene in the sex that suffers a selective disadvantage from the phenotype encoded by that gene, fitness of that sex would increase; thus, genes with sex-biased expression may represent genes that are (or were in the past) under sexually antagonistic selection. Genes with male-biased expression are depleted from the X chromosomes of Drosophila, while female-biased genes are more common on the X, relative to autosomes. In mammals, female-biased genes expressed in ovaries and placenta are also overrepresented on the X. This suggests that the X chromosome in these species has become “feminized.” Such a pattern is consistent with sexually antagonistic mutations being at least partially dominant, and with female-beneficial genes accumulating on the X, which is more often transmitted through females.
Male-biased genes expressed during early spermatogenesis also appear to be in excess on the X of mammals, which suggests that recessive, male-beneficial genes also accumulate on the X. Studies of the genomic distribution of sex-biased genes in ZW systems found similar patterns. There is a deficit of female-biased genes on the Z chromosome of chickens, and testis-specific genes appear enriched on the Z of Bombyx. Again, this is consistent with an accumulation of dominant, male-beneficial mutations and a removal of female-beneficial mutations on the Z chromosome.
Recent research, however, has suggested that the biased distribution of male or female genes on the X also depends on the evolutionary age of the gene, and it is unclear how good sex-biased expression really is as a proxy for identifying past sexual conflict. Thus, while genes with different functions and expression patterns clearly show biased distributions on X chromosomes, the underlying causes for this are not yet clear. It is likely that a complex suite of evolutionary forces, such as the transcriptional inactivation of the X chromosome during spermatogenesis in some species, affects the distribution of sex-biased genes in the genome.
5. DIVERSITY OF SEX DETERMINATION
Mechanisms of Sex Determination
Sex is a universal feature of eukaryotic organisms (see chapter IV.4), and sex determination is a vital biological process: imprecise sex determination leads to the production of faulty, intersexual individuals, and consequently to reproductive impairment. Sex determination must therefore be subject to strong selective pressures; nevertheless, sex-determination mechanisms can undergo rapid evolutionary change, and tremendous variation in sex-determination mechanisms exists, not only within major phylogenetic groups, but also occasionally even within species. Although the number and range of modes of sex determination is large, they can be broadly divided into two major categories: genetic sex determination (GSD) and environmental sex determination (ESD). Sex can be determined based on the genetic makeup of the fertilized zygote (GSD), or sexual development can be under the control of environmental cues (ESD); note, however, that this simple dichotomy is somewhat misleading, and sex determination is always controlled by genes. With ESD, an initial external agent is used to stimulate either male or female development, but the pathway for sexual differentiation of course involves genes. In addition, in some species both mechanisms exist simultaneously, and GSD systems can sometimes be overridden by environmental cues, such as bacterial infections. The principal exception to this evolutionary instability of sex-determining mechanisms is the case of systems with highly evolved, heteromorphic sex chromosomes, since transitions involving a degenerate Y or a dosage-compensated X are difficult. In general, however, the evolution of sex-determination mechanisms presents a conundrum: the trait itself is under strong selective control, yet it can undergo frequent and rapid evolutionary change. The reasons for this diversity of mechanisms and the forces driving transitions between different systems are unclear.
Environmental Sex Determination
With ESD, the primary signal to trigger development into either a male or a female is given by environmental cues. Environmentally derived signals utilized for sex determination include temperature of egg incubation during a critical period, which is employed by several reptiles such as crocodiles and turtles, as well as by some invertebrates, including the gall midge Heteropeza, and the fungus gnat Sciara. Other environmental clues used for sex determination include photoperiod, as utilized by some amphipods that develop into males early during the season, and into females later. ESD systems also include species that practice sex change: in some snail species such as slipper limpets, young mobile adults are males and later change into sessile females. In some fish species, sex change is induced by social organization. Anemone fish, for example, form social units with a size-based dominance hierarchy composed of a breeding pair and several nonbreeders. If the female of a group—which is the largest individual—dies, the male grows and changes sex to become the breeding female, while the largest nonbreeder grows and becomes the breeding male. In the echiuran worm Bonellia viridis, the vast majority of sexually undifferentiated larvae metamorphose into dwarf males that live inside the female when exposed to females, but differentiate into females when developing in the absence of other females. In some arthropods, sex is determined by infection with certain bacteria. Wolbachia, for example, infects a high proportion of insect species and can result in feminization of infected males. In this case, an environmental stimulus (Wolbachia infection) overrides a GSD system.
Genetic Sex Determination: Sex Chromosomes
The mechanisms of GSD can vary enormously, with clearly distinguishable sex chromosomes being only one possibility. As mentioned above, chromosomal sex determination includes systems in which males are the heterogametic sex (XX/XY sex chromosomes), such as found in mammals, many insects and invertebrates, and several plant species. Alternatively, females can be the heterogametic sex (ZW/ZW), and this system is found in birds, some reptiles and amphibians, some insects, and invertebrates. In some species, the Y chromosome has been entirely lost; here females have two X chromosomes (XX) and males, only a single X (X0), as in a number of insects, including some crickets, grasshoppers, and cockroaches, as well as in C. elegans. The mechanism by which sex chromosomes trigger sex determination functions either through a dominant male- or female-determining gene on the Y or W chromosome, or through the ratio of sex chromosomes to autosomes (the X:A ratio). Mammals have a dominant masculinizing gene on the Y (the SRY gene), whereas Drosophila and X0 species use the X:A ratio for sex determination. Some species contain multiple sex chromosomes instead of a single pair of sex chromosomes; for example, some invertebrates, fish, and mammals contain two X chromosomes (males are X1X2Y and females are X1X1X2X2). A very peculiar sex chromosome configuration is found in platypuses, which have ten sex chromosomes. Males have five X and five Y chromosomes (X1X2X3X4X5Y1 Y2Y3Y4Y5), while females have ten X chromosomes (X1X1X2X2X3X3X4X4X5X5). In other species, sex is determined by a single locus (genic sex determination), but they have no visually distinguishable sex chromosomes (e.g., phorid flies, several fish species).
Genetic Sex Determination: Other Systems
Another familiar mode of sex determination is haplodiploidy (1N-2N). Here, unfertilized eggs develop into haploid individuals that are males, and fertilized eggs develop into diploid females. This mechanism is utilized in hymenoptera (bees, ants, and wasps) and some mites and beetles. Other, rare mechanisms of genetic sex determination include paternal genome elimination, X chromosome elimination, and monogeny. In some species of scale insects, for example, both sexes develop from fertilized eggs and all embryos are initially diploid. However, during early development of the male offspring, the paternal half of the genome is either deactivated through heterochromatinization or completely eliminated (paternal genome elimination; i.e., males are either functionally or actually haploid). In other systems, such as pea aphids, only the X chromosome is eliminated to form X0 males (while females are XX), but the autosomes remain diploid in males (X chromosome elimination). Gall midges (Diptera) reproduce by a mechanism in which all offspring of each individual female are either exclusively male or exclusively female, and is a result of a single maternal effect autosomal gene (monogeny).
Thus, despite the antiquity of the two sexes, a vast diversity of sex-determination mechanisms exist, and these mechanisms evolve rapidly in some lineages. The evolutionary forces that drive rapid change in sex-determination pathways remain largely a mystery but are an area of active research.
FURTHER READING
Bachtrog, D. 2006. A dynamic view of sex chromosome evolution. Current Opinion in Genetics and Development 16(6): 578–585. Focuses on the dynamics of processes shaping sex chromosomes over evolutionary time.
Bachtrog, D., M. Kirkpatrick, J. E. Mank, S. F. McDaniel, J. C. Pires, W. Rice, and N. Valenzuela. 2011. Are all sex chromosomes created equal? Trends in Genetics 27(9): 350–357. Contrasts various sex chromosome systems, including female and male heterogametic systems, and sex chromosomes in haploid organisms.
Charlesworth, B., and D. Charlesworth. 2000. The degeneration of Y chromosomes. Philosophical Transactions of the Royal Society B 355(1403): 1563–1572. A comprehensive overview of the evolutionary processes driving Y degeneration.
Charlesworth, D., and J. E. Mank. 2010. The birds and the bees and the flowers and the trees: Lessons from genetic mapping of sex determination in plants and animals. Genetics 186(1): 9–31. An overview of the diversity of sex chromosome systems.
Lahn, B. T., N. M. Pearson, and K. Jegalian. 2001. The human Y chromosome, in the light of evolution. Nature Reviews Genetics 2(3): 207–216. An overview of the evolution and gene content of the human Y chromosome.
Marshall Graves, J. A. 2008. Weird animal genomes and the evolution of vertebrate sex and sex chromosomes. Annual Review of Genetics 42: 565–586. Describes the diversity of sex chromosome systems among vertebrates.
Ming, R., A. Bendahmane, and S. S. Renner. 2011. Sex chromosomes in land plants. Annual Review of Plant Biology 62: 485–514. An overview of sex chromosomes in land plants.
Vicoso, B., and D. Bachtrog. 2009. Progress and prospects toward our understanding of the evolution of dosage compensation. Chromosome Research 7(5): 585–602. An overview of current understanding of the process of dosage compensation and its evolution in model species.
Vicoso, B., and B. Charlesworth. 2006. Evolution on the X chromosome: Unusual patterns and processes. Nature Reviews Genetics 7(8): 645–653. A comprehensive overview of the evolutionary processes operating on X chromosomes.