The metabolic intermediates needed for cell growth and maintenance are generally synthesized from components of the glycolytic and PPP pathways, and the TCA (tricarboxylic acid) cycle (see Fig. 7.20). However, during vinification, most of the TCA-cycle enzymes in the mitochondrion are inactive. Isozymic versions of most of these enzymes (located in the cytoplasm) take over the function of generating the necessary metabolic intermediates used in the biosynthesis of some amino acids and nucleotides (see Fig. 7.20). Full operation of the TCA cycle would produce an excess of NADH, disrupting the redox balance (respiratory oxidative phosphorylation is inoperative during fermentation). This is avoided because NADH, produced during the oxidation of citrate to succinate (the ‘right-hand’ side of the TCA cycle), can be oxidized back to NAD+ by reducing oxaloacetate to succinate (the ‘left-hand’ side of the TCA cycle). The result is redox balance. In addition, NADH generated in glycolysis may be oxidized in the reduction of oxaloacetate to succinate, rather than in the reduction of acetaldehyde to ethanol. In both scenarios, excess succinate is generated. This probably explains why succinate is one of the major by-products of yeast fermentation. In yeasts, the PPP functions primarily in the generation of particular amino acids and pentose sugars involved in the synthesis of nucleotides.
The replacement of TCA-cycle intermediates lost to biosynthesis probably comes from pyruvate. Pyruvate may be directly channeled through acetate, carboxylated to oxaloacetate, or indirectly routed via the glyoxylate pathway. The last pathway, if active, is probably functional only near the end of fermentation. Glucose suppresses the glyoxylate pathway. The involvement of biotin in the carboxylation of pyruvate to oxaloacetate probably accounts for its primary requirement by yeast cells.
The accumulation of another major by-product of fermentation, glycerol, also has its origin in the need to maintain a favorable redox balance, as well as its function as an osmoticum. In some strains, glycerol accumulation appears to enhance the synthesis of another osmoticum, trehalose (Li et al., 2010). The importance of glycerol synthesis to redox balance is suggested by the inability of mutants, defective in glycerol synthesis, to grow under anaerobic conditions (Nissen et al., 2000). In addition, Roustan and Sablayrolles (2002) present evidence suggesting that glycerol synthesis, at least during the stationary phase, is associated with redox balance by eliminating excess reducing power. The reduction of dihydroxyacetone phosphate to glycerol 3-phosphate can oxidize the NADH generated in the oxidation of glyceraldehyde 3-phosphate in glycolysis (Fig. 7.23). However, the coupling of these two reactions does not generate ATP, and is therefore an energy-neutral form of glucose fermentation. This is in contrast to the net production of two ATP molecules, and release of two CO2 molecules, during the fermentation of glucose to ethanol. The separate functions of redox balance and osmotolerance appear to be regulated by different isozymes of glycerol-3-phosphate dehydrogenase, GPD1 and GPD2 (Ansell et al., 1997).
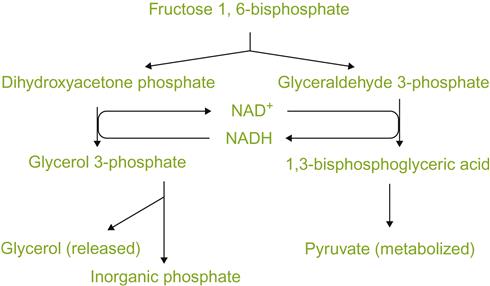
The presence of sulfur dioxide increases the production of glycerol. This probably comes from an indirect effect, requiring an alternative route to regenerate NAD+. When sulfur dioxide binds with acetaldehyde, its reduction to ethanol is inhibited, blocking the usual means by which alcoholic fermentation regenerates NAD+.
Throughout fermentation, yeast cells adjust physiologically to the changing conditions in the juice/must to produce adequate levels of ATP, maintain favorable redox and ionic balances, and synthesize necessary metabolic intermediates. Consequently, the concentration of yeast by-products in the cytoplasm and juice changes continuously throughout fermentation (see Figs. 7.21 and 7.22). Because several by-products are aromatic, for example acetic acid, acetoin (primarily by conversion from diacetyl), and succinic acid, their presence can affect bouquet development. The accumulation of acetyl CoA (as a result of limited activity of Krebs Cycle enzymes) may explain the accumulation and release of acetate esters during fermentation. The alcoholysis of acetyl CoA (and other acyl SCoA complexes) during esterification would release CoA for other metabolic functions. In addition, the formation of other aromatics, notably higher alcohols, reflects the relative availability of amino acids and other nitrogen sources in the juice. Adequate availability permits amino acids to be used as an energy source, or generates organic acids, fatty acids, and reduced-sulfur compounds.
Although all strains of S. cerevisiae possess the same set of enzymes, their catalytic activities may vary due to allelic differences. In addition, slight differences in regulation, or gene copy number, mean that any two strains are unlikely to respond identically under the same conditions. This variability undoubtedly accounts for many of the subtle, and not so subtle, differences between fermentations conducted by different strains. For example, overexpression of cytoplasmic malate dehydrogenase increases not only the accumulation of malic acid, but also fumaric and citric acids (Pines et al., 1997). In addition, overexpression of glycerol 3-phosphate dehydrogenase not only results in a marked increase in glycerol production, but also augments the accumulation of acetaldehyde, pyruvate, acetate, 2,3-butanediol, succinate, and especially acetoin (Michnick et al., 1997). This is the mechanism by which glycerol production may be enhanced by heat shocking reactivated yeast before inoculation (Berovic and Herga, 2007). The shift in metabolism toward glycerol synthesis is of enologic interest as it could result in ‘normal’ alcohol contents in wines produced from grapes having high °Brix values. This is becoming more common, as delaying harvest to achieve maximum flavor development can lead to table wines possessing up to 15% ethanol.
Influence on Grape Constituents
Yeasts have their major effect on the sugar content of the juice or must. If fermentation goes to completion, only minute amounts of fermentable sugars remain (preferably≤1 g/liter). Small amounts of nonfermentable sugars, such as arabinose, rhamnose, and xylose, also remain (∼0.2 g/liter). These small quantities have no sensory significance and leave the wine tasting ‘dry.’
Yeasts may increase pH by metabolizing malic acid to lactic acid. However, the proportion converted is highly variable, differing among strains by 3–45% (Rankine, 1966). Some strains of S. paradoxus can also degrade malic acid up to 40% (Orlic et al., 2007). Some strains of Saccharomyces cerevisiae can also synthesize malic acid, maximally at 25 °C (Farris et al., 1989). However, this is a more common property of S. bayanus var. uvarum.
Schizosaccharomyces pombe can completely decarboxylate malic acid to lactic acid (Benito et al., 2013). It also has the potential to limit urea and alcohol accumulation. The former reduces the potential for ethyl carbamate production, and the latter the high alcohol contents associated with overmaturation of grapes to achieve higher flavor potential. Nonetheless, this yeast has been little used because its sensory impact has generally, but not consistently, been viewed as negative. The chemical reasons for this are unclear. Delaying its inoculation until after S. cerevisiae has been active for several days, or has completed fermentation, apparently reduces its potential negative impact of (Carre et al., 1983). Immobilization appears to be an alternative and effective approach that does not spoil the wine’s character (Silva et al., 2003). It also permits greater control over the degree of deacidification.
During fermentation, the release of alcohols and other organics helps dissolve compounds from seeds and skins. Quantitatively, the most significant chemicals extracted are anthocyanins and various flavonoid phenolics, notably tannins. The latter are partially dependent on the solubilizing action of ethanol. Anthocyanin extraction often reaches a maximum within 3–5 days, when the alcohol content has reached about 5–7% (Somers and Pocock, 1986). As the alcohol concentration continues to rise, color intensity may begin to fall. This can result from the coprecipitation of anthocyanins with grape and yeast cells, to which they may bind. Nevertheless, the primary reason for color loss appears to be disruption of weak anthocyanin complexes present in the juice. Freed anthocyanins may convert into uncolored states. Although extraction of tannins occurs more slowly, their content often reaches higher values than anthocyanins. Tannin extraction from stems (rachis), if present, may reach a plateau after about 7 days. Seed tannins are the slowest to be liberated; their accumulation may still be active after several weeks (Siegrist, 1985).
Ethanol also aids the solubilization of certain aromatic compounds from grape cells. Unfortunately, little is known about the dynamics of the process. Conversely, ethanol decreases the solubility of other grape constituents, notably pectins and other carbohydrate polymers. The pectin content may fall by upward of 70% during fermentation.
As noted, the metabolic action of yeasts produces many important wine volatiles, notably higher alcohols, fatty acids, and esters. Yeast metabolism may also degrade some grape aromatics, notably aldehydes. This potentially could limit the expression of the herbaceous odor generated by C6 aldehydes and alcohols produced as a consequence of oxidation during the grape crush. Yeasts can also influence wine flavor by decarboxylating hydroxycinnamic acids to their equivalent vinylphenols. More significantly, fermentation may play a major role in the liberation of varietal aromatics, notably those bound in complexes with glycosides (Williams et al., 1996) or cysteine (Tominaga et al., 1998). Because yeast strains differ significantly in these attributes (Howell et al., 2004), strain choice can either enhance or diminish varietal expression.
Indirect effects of yeast action include wine color modification (Eglinton et al., 2004; Medina et al., 2005). This may involve pigment loss, by adherence to grape cell remnants and yeast mannoproteins, or color stabilization by the release of carbonyls, such as pyruvic acid and acetaldehyde, and the generation of vinylphenols. Because yeast strains also vary in these characteristics, strain selection can influence color depth and stability in red wines (Bartowsky et al., 2004b; Morata et al., 2006).
Yeasts
Classification and Life Cycle
Yeasts are a diverse group of fungi characterized by possessing a unicellular growth habit. Cell division may involve either budding (extrusion of a daughter cell from the mother cell) (Plate 7.6) or fission (division of the mother cell into one or more cells by localized ingrowths). Occasionally, yeasts may form short chains. Yeasts are also distinguished by possessing a single nucleus – in contrast to the frequently variable number of nuclei found in filamentous fungi. In addition, the composition of their cell walls is unique. The major fibrous cell-wall component of most fungi, chitin, occurs only as a minor component in yeast cell walls. It is localized to the bud scar, the site at which daughter cells are produced. The major constituent of yeast cells consists of chains of glucose molecules, specifically bonded together to form β-1,3-D-glucans. Also present are mannoproteins, complex polymers of the sugar, mannose, and particular proteins. These are covalently linked to either β-1,3-D-glucans or smaller amounts of glucose molecules bonded together into β-1,6-D-glucan chains.
Although characterized by a distinctive set of properties, yeasts are not a single evolutionarily related group. A yeast-like growth habit has evolved independently in at least three major fungal taxa – the Zygomycota, Ascomycota, and Basidiomycota. Only yeast members of the Ascomycota (and related imperfect forms) are significant in wine production. Most imperfect yeasts, i.e., those that have lost the ability to undergo sexual reproduction, are derived from ascomycete yeasts. Under appropriate conditions, the cells of most ascomycete yeasts differentiate into asci – the structures in which haploid spores are produced through meiosis and cytoplasmic division.
In Saccharomyces cerevisiae and related species, four haploid spores are produced as a consequence of meiosis (Fig. 7.24). After rupture of the ascal wall (originally the mother cell wall), spores typically germinate to produce haploid vegetative cells. Those of opposite mating type usually fuse shortly after germination to reestablish the diploid state. Fusion may occur even before rupture of the ascal wall. Although individual cells only have the capacity to grow and bud about eight times before dying, newly budded cells have the same capacity as the mother cell – to produce (bud) eight new cells. Cellular death apparently results from disruption caused by the accumulation of circular copies of rDNA in the nucleus (Sinclair and Guarente, 1997). Under appropriate conditions, diploid cells can differentiate into asci and ascospores. However, wine strains rarely express this potential, at least in must or wine. Ascal development is suppressed by high concentrations of glucose, ethanol, and CO2.
If sporulation is desired, as in breeding experiments, nutrient starvation, the addition of sodium acetate, or both can induce ascospore production in S. cerevisiae. Bicarbonate accumulation in the growth medium also acts as a meiosis-promoting factor (Ohkuni et al., 1998).
Until the late 1970s, yeast classification was, by necessity, based on physiological properties and the few morphological traits readily observable under the light microscope. These have now been supplemented with nucleotide sequence analysis and DNA–DNA hybridization. These procedures will hopefully open a new period of more stable classification, based on evolutionary relationships.
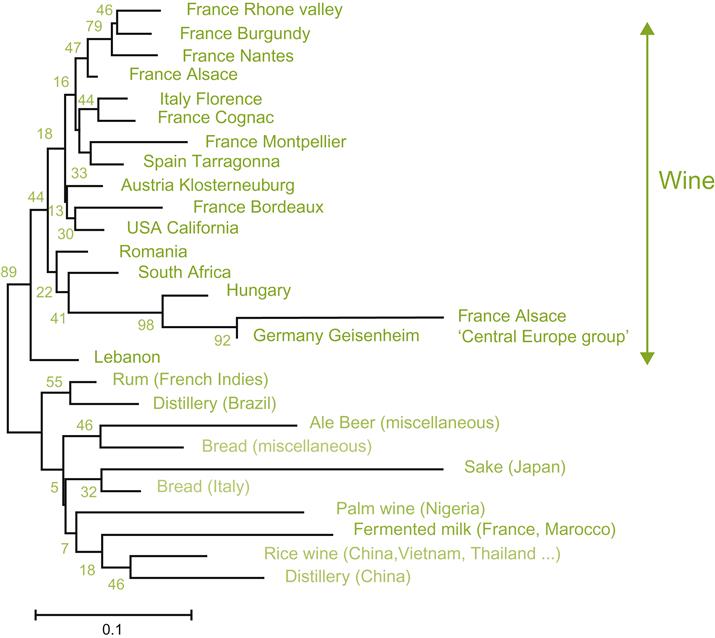
In most recent taxonomic treatments (e.g., Kurtzman et al., 2011), many named species of Saccharomyces have been reduced to synonymy. This does not refute the differences formerly used to distinguish these ‘species,’ but rather indicates that they are either minor genetic variants or genetically unstable. Most of these former species are viewed as physiological races of recognized species, or occasionally members of different genera. For example, S. fermentati and S. rosei are considered to be strains of Torulaspora rosei. Species such as S. bayanus, S. uvarum, and S. pastorianus (S. carlsbergensis) are variously viewed as subspecies of S. cerevisiae (Nguyen and Gaillardin, 2005), as distinct varieties of a separate species (S. bayanus var. bayanus or S. bayanus var. uvarum), or species (S. pastorianus) by Kurtzman et al. (2011). Figure 7.25 illustrates a modern interpretation of the relatedness among Saccharomyces and related genera, species, and strains.

Saccharomyces cerevisiae and related species (Saccharomyces sensu stricto) are apparently evolved from an ancient chromosome doubling (autopolyploidy) (Wolfe and Shields, 1997; Wong et al., 2002). This was followed by inactivation or loss of most of the duplicate chromosomes (diploidization). Although this is estimated to have occurred millions of years ago, hybridization and polyploidy still occur within the genus (de Barros Lopes et al., 2002; Naumova et al., 2005). For example, S. pastorianus is thought to be an alloploid hybrid between S. cerevisiae and S. bayanus, or another subspecies. A list of accepted names and synonyms for some of the more commonly found yeasts on grapes or in wine is given in Appendix 7.1. Differences between some of the physiological races of S. cerevisiae (formerly given species status) are noted in Appendix 7.2.
Yeast Identification
Identification procedures, based on mitochondrial DNA, PCR, and other molecular technologies, now permit the rapid (hours vs. days) identification of species, and even strains from small samples (Cocolin et al., 2000; Martorell et al., 2005). Without these procedures it would be impossible to know what strains were actually conducting fermentation, or to detect the dynamics of strain fluctuation during fermentation. Thus, not only have these techniques been a boon to research, but they also have spawned renewed interest in endemic strains as potential sources of regional or site-specific wine individuality.
The speed and precision of molecular techniques are replacing traditional identification procedures, based on physiological properties and production of the sexual phase. Molecular techniques are also particularly useful in the early detection of potential spoilage organisms. They are also being applied to assessing the dynamics of physiological changes during fermentation. Regrettably, these procedures are costly, technically demanding, and currently only available in research centers, commercial laboratories, or the largest of wineries.
Alternative techniques for the differentiation between fermentative and spoilage yeasts involve free fatty acid analysis, gas chromatography, or pulsed-field electrophoresis. However, these are equally unavailable to the vast majority of wineries. Computer-based analysis of data via synoptic keys, in contrast to structured dichotomous keys (Payne, 1998), would facilitate identification using standard culture techniques. In their absence, specialized culturing procedures, such as those given by Cavazza et al. (1992), or the standard methods noted in Kurtzman et al. (2011) are effective, but not speedy.
Standard procedures require that the organism be isolated as a single cell and grown on laboratory media. This is typically achieved by dilution from the source, containing upward of a billion cells/mL. Typically, selective media are required for the isolation of different species. It is essential that each species or strain be isolated and cultured individually. Identification is based on the capacity to grow with or without particular nutrients. Depending on how the isolation is done, data on the number of viable cells of each species and strain may be obtained. However, just because an organism is isolated does not necessarily mean that it was growing or physiologically active in the juice, must, or wine. Conversely, inability to culture an organism does not necessarily mean it was not present in a viable state. Yeast cells may survive for extended periods in a dormant (unculturable) state (Cocolin and Mills, 2003).
Because of the time, equipment, and experience required for the effective use of even traditional identification techniques, they are not discussed here. In all but large wineries, yeast identification is typically contracted out to commercial laboratories.
Yeast Evolution and Grape Flora
Saccharomyces cerevisiae is undoubtedly the most important of all yeast species. In various forms, it functions as the wine yeast, brewer’s yeast, distiller’s yeast, and baker’s yeast. Laboratory strains are extensively used in industry and in fundamental studies on genetics, biochemistry, and molecular biology. For all its importance, the natural habitat of S. cerevisiae is only now becoming clear. Current evidence indicates that its indigenous niche is the sap and bark of oak trees, and possibly adjacent soil. In these sites, it co-inhabits with a sibling species, S. paradoxus. The latter is often viewed as the progenitor of S. cerevisiae. This view is supported by their extensive genetic and physiological similarities, equivalent natural habitats, and ability to ferment wines to dryness (Redžepović et al., 2002). Other Saccharomyces species also inhabit bark, notably oak bark, for example, S. kudriavzevii and S. uvarum (Sampaio and Gonçalves, 2008).
Wild strains of Saccharomyces cerevisiae and S. paradoxus differ primarily by being reproductively isolated (i.e., having an inability to mate under natural conditions); possessing genomic sequence divergence; and showing different thermal growth profiles. They also vary in that wild strains of S. cerevisiae demonstrate limited genetic distinction across continents, vs. the partial reproductive isolation between dispersed populations of S. paradoxus (Sniegowski et al., 2002; Kuehne et al., 2007). Wild strains of S. cerevisiae are distinguishable from wine strains by the absence of chromosomal polymorphisms, by being prototrophic, and by being sporulation-proficient. In contrast, wine strains exhibit significant DNA sequence diversity, and chromosome length polymorphisms, often possess auxotrophic or lethal mutants, express considerable heterozygosity, and show variable spore viability (see Mortimer, 2000). Currently, there is no consensus as to the evolutionary relationship between wild and domesticated strains of S. cerevisiae. Nevertheless, it appears that feral S. cerevisiae gave rise to two domesticated lines, one that developed into wine yeasts (and subsequently beer and bread yeasts). The other line gave rise to saké yeasts (Fay and Benavides, 2005). The greatest diversity was found in yeasts derived from fermented products in Africa, lending support to an ‘out-of-Africa’ origin of domesticated yeast (Fay and Benavides, 2005). Did humans unsuspectedly take S. cerevisiae along with them when our ancestors migrated out of Africa? Relative to wine yeasts, specifically, present data fits a Near Eastern center of origin (Fig. 7.26).
Although Saccharomyces cerevisiae has occasionally been isolated from fruit flies (Drosophila spp.), and may be transmitted by bees and wasps, the importance of insect dispersal is unresolved (Wolf and Benda, 1965; Phaff, 1986). S. cerevisiae is usually absent or rare on healthy grapes. Even in long-established vineyards, the isolation of S. cerevisiae (in small numbers) occurs only near the end of ripening. Mortimer and Polsinelli (1999) estimate about one healthy berry per thousand carries wine yeasts. However, on surface-damaged fruit, the frequency may rise to one in four (1×105 to 1×106 yeast cells/berry). In Croatia, Redžepović et al. (2002) found that S. paradoxus was more frequently found on grapes than S. cerevisiae. A comparison of the attributes contributed to wine by S. paradoxus vs. S. cerevisiae is provided by Majdak et al. (2002) and Orlić et al. (2010).
Strains of the related species, Saccharomyces bayanus, can also conduct effective alcoholic fermentations. Nevertheless, they are less encountered, and their use typically associated with special winemaking situations. For example, S. bayanus var. bayanus has properties especially well adapted to the production of sparkling wines and fino sherries. S. bayanus is also well adapted to fermenting white wines from relatively neutral flavored grapes grown in warm climates. It tends to produce little volatile acidity, augments the malic acid, succinic acid, and glycerol contents, and generates more aromatic alcohols and ethyl esters (Castellari et al., 1994; Antonelli et al., 1999). For cool fermentations (below 15 °C), the cryotolerant S. bayanus var. uvarum is of particular value. It is often involved in the production of tokaji, amarone, and sauternes wines. In some regions, such as Alsace, it has been reported to be the predominant fermentative yeast (Demuyter et al., 2004). The origin of these two species (often considered subspecies of S. cerevisiae) is unknown, as are their natural habitats. Wild strains have occasionally been isolated from the caddis fly, some mushroom species, and hornbeam tree exudate (Carpinus).
General attribute tendencies of the different species, subspecies, and strains of wine yeast have been investigated, but precise prediction of their actions under specific conditions is impossible. Individual experimentation over several years is the only way to determine their effects under in-house conditions.
Although wine yeasts rarely occur in significant numbers on the fruit, leaves, or stems of grapevines, other yeast species are frequently found. These include species of Hanseniospora (Kloeckera), Candida, Pichia, Hansenula, Metschnikowia, Sporobolomyces, Cryptococcus, Rhodotorula, and Aureobasidium. Their population numbers change during fruit ripening (Renouf et al., 2005), increasing markedly during the last few weeks of maturation. Endemic yeasts may be found on the fruit pedicel, but occur more frequently on the callused terminal ends, the receptacle. They are most frequently isolated from around stomata on the fruit, or next to cracks in the cuticle. In the last site, they routinely form small colonies (Belin, 1972). They presumably grow on nutrients seeping out of openings in the fruit, receptacle, and pedicel. Yeasts do not grow on the plates of wax that cover much of the berry surface, the matte-like bloom. In fact, yeasts cease to grow where they come in contact with the waxy cuticular plates.
Commonly, the cells are dormant or only slowly reproducing. This unquestionably results from the dry state of the cuticle. Microbes require at least a thin coating of water to be metabolically active. This occurs only during rainy spells, or when fog or dew condenses on grape surfaces. In contrast, damaged fruit surfaces, where juice may escape, may be slightly hygroscopic, providing a more favorable, albeit highly osmotic, site for yeast (and bacterial) growth.
As noted, grapes do not appear to have been the natural (ancestral) habitat for S. cerevisiae (or its progenitor). Nonetheless, winery equipment, and the winery itself, act as a significant inoculum source for spontaneous fermentations (Ciani et al., 2004; Santamaría et al., 2005). This is suggested by the repeat isolation of one or a few dominant strain(s) from particular wineries. Outside the winery, strain spread seems limited, and occurs only over short distances (Valero et al., 2005). A possible exception occurs where pomace is spread as a vineyard soil conditioner. In addition, regional strains isolated from vineyards often differ (Khan et al., 2000). Thus, this lends credence to the view that endemic yeast populations could contribute to a site’s regional character.
The most frequently occurring yeast species on mature grapes is Kloeckera apiculata. In warm regions, the perfect state of Kloeckera (Hanseniaspora) tends to replace the asexual form. Filamentous fungi, such as Aspergillus, Botrytis, Penicillium, Plasmopara, and Uncinula, are rarely isolated, except from diseased or damaged fruit. Similarly, acetic acid bacteria are usually found in significant numbers only on diseased or damaged fruit.
Under most conditions, especially where must is inoculated with a particular strain of S. cerevisiae, the indigenous yeast flora has often been viewed to be of little significance in winemaking. The acidic, highly osmotic conditions of grape juice were thought to retard the growth of most yeasts, fungi, and bacteria. Also, the rapid development of anaerobic conditions and the accumulation of ethanol compromise the growth of competitive species. Nevertheless, in musts low in sugar content, the activity of yeasts less tolerant of high sugar and ethanol contents may be important (Ciani and Picciotti, 1995). Diseased grapes also have significantly modified and enhanced populations of epiphytic yeasts. These can markedly affect the outcome of fermentation, even with inoculation.
Even on healthy grapes, other yeasts may occur at concentrations approaching those typically used in inoculated fermentations (Fig. 7.27). Molecular evidence indicates that they may continue to persist in a viable, but unculturable state, throughout fermentation (Cocolin and Mills, 2003). At present, it is not established how commonly, or for how long, these species remain metabolically active (Millet and Lonvaud-Funel, 2000). Thus, their significance to vinification remains uncertain, and possibly underestimated. However, in some instances, they maintain or increase their numbers during fermentation (Mora and Mulet, 1991; Fig. 7.27). This appears to be more common when fermentation temperatures are low (Heard and Fleet, 1988), or yeast inoculation is delayed (Petering et al., 1993). Only in diseased or damaged grapes is the microbial grape flora clearly known to play an important role in vinification.
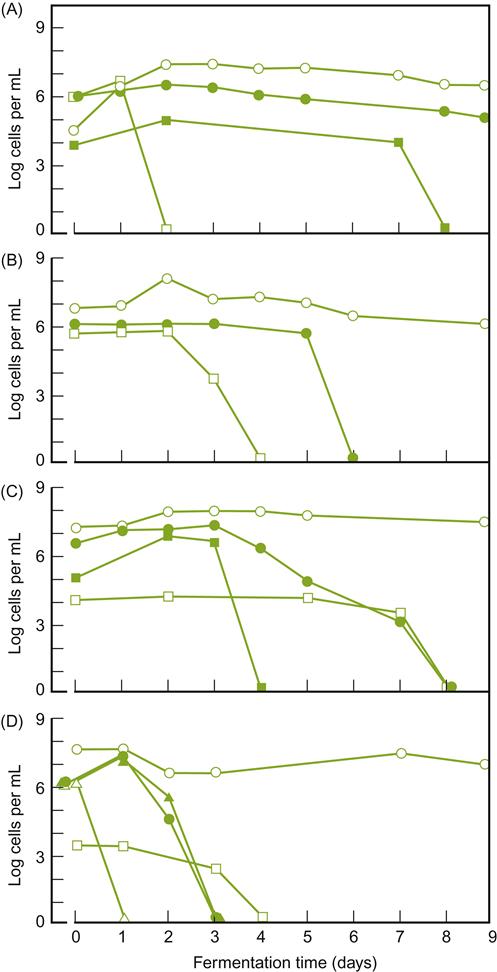
 , Saccharomyces cerevisiae;
, Saccharomyces cerevisiae;  , Kloeckera apiculata;
, Kloeckera apiculata;  , Candida stellata; ■, C. pulcherrima;
, Candida stellata; ■, C. pulcherrima;  , C. colliculosa;
, C. colliculosa;  , Hansenula anomala. The initial population of S. cerevisiae comes predominantly from the inoculation conducted in the fermentations. (From Heard and Fleet, 1985, reproduced by permission.)
, Hansenula anomala. The initial population of S. cerevisiae comes predominantly from the inoculation conducted in the fermentations. (From Heard and Fleet, 1985, reproduced by permission.)In addition to the epiphytic yeast inoculum, juice and must may become inoculated from winery equipment (notably crushers, presses, and sumps) and the winery environment. This is especially true in old wineries, in which the equipment and buildings are thinly covered with wine yeasts. In addition, Hansenula anomala and Pichia membranaefaciens commonly occur in wineries, with Brettanomyces and Aureobasidium pullulans often being isolated from the walls of moist cellars. The hygienic operation of present-day wineries greatly limits juice and must inoculation, or contamination from the winery and its equipment. In such situations, the vineyard is presumably the main source of S. cerevisiae in spontaneous fermentations (Török et al., 1996).
Succession During Fermentation
In spontaneous fermentations, there is a rapid and early succession of yeast species. Initially, Kloeckera apiculata and Candida stellata may occur in the range of 103–106 cells/mL. This number increases rapidly if the grapes or must are left at warm temperatures. Although these endemic yeasts may grow at the beginning of fermentation, most strains soon pass into decline. The culturable population usually decreases, becoming a minor component of the yeast population. This has frequently been interpreted as a result of the increasing concentration of ethanol produced by Saccharomyces, and/or the addition of sulfur dioxide. Recent studies indicate that other interstrain and interspecies effects and influences may also play a role. Examples may involve the selective action of acetaldehyde (Cheraiti et al., 2005), and relative abilities to translocate glucose into the cell (Nissen et al., 2004).
During the initial stages of fermentation, the activities of non-Saccharomyces yeasts contribute to the production of compounds such as acetic acid, glycerol, and various esters (Ciani and Maccarelli, 1998; Romano et al., 2003). This can be sufficient to significantly influence the wine’s aroma (Eglinton et al., 2000; Soden et al., 2000). For example, some strains of Kloeckera apiculata can potentially produce up to 25 times the amounts of acetic acid typically produced by S. cerevisiae. K. apiculata, along with other members of the grape epiphytic flora, may also produce above-threshold amounts of 2-aminoacetophenone. This compound has been associated with the naphthalene-like odor characteristic of untypical aging (UTA) (Sponholz and Hühn, 1996). In addition, K. apiculata may inhibit some S. cerevisiae strains from completing fermentation (Velázquez et al., 1991), possibly due its production of acetic acid, octanoic and decanoic acids, or ‘killer’ factors. In addition, the indigenous grape flora may enhance amino acid availability by their proteolytic activities (Dizy and Bisson, 2000). At cool fermentation temperatures (10 °C), yeasts such as K. apiculata may remain active (or at least viable) throughout alcoholic fermentation (Heard and Fleet, 1988; Erten, 2002).
Occasionally, strains of Candida stellata persist in fermenting juice (Fleet et al., 1984). Their ability to produce high concentrations of glycerol could play a role in enhancing a wine’s smooth mouth-feel. Jolly et al. (2003) report that the perceived quality of Chenin blanc wine was increased when Candida pulcherrima was combined with Saccharomyces cerevisiae. Torulaspora delbrucecki also has the potential to positively influence the sensory properties of wine, due to its low production of acetic acid and synthesis of succinic acid. Although Kloeckera apiculata and C. stellata typically ferment only up to approximately 4 and 10% alcohol, respectively, they are able to survive much higher alcohol concentrations (Gao and Fleet, 1988).
Of equal or possibly greater significance is the property of some Hanseniaspora, Debaryomyces, and Dekkera spp. to produce glycosidases (Villena et al., 2007). Their action in releasing terpenoids and other volatiles could positively affect the development of the varietal character of a wine. The role of their other enzymatic activities on flavor development is largely unknown, except for the negative influence of Dekkera spp. on the decarboxylation of hydroxycinnamic acids in generating 4-ethylphenol and 4-ethylguaiacol.
Nonetheless, most members of the grape flora are either slow growing or typically suppressed by the low pH, high initial osmolarity of the juice/must, augmenting ethanol content of the ferment, oxygen deficiency, or sulfur dioxide. Their relative inability to incorporate glucose under vinous conditions may also play a significant role in their early demise (Nissen et al., 2004). Consequently, most species of Candida, Pichia, Cryptococcus, and Rhodotorula probably do not contribute significantly to fermentation. Their populations seldom rise above 104 cells/mL, and the species usually disappear quickly from the ferment. However, if warm conditions prevail, and active fermentation is delayed, these yeasts may initiate severe spoilage. Under such conditions, Pichia guilliermondii has the potential to produce sufficient 4-ethylphenol to generate odors variously described as burnt beans, band-aid, wet dog, horse sweat, and barnyard (Barata et al., 2006).
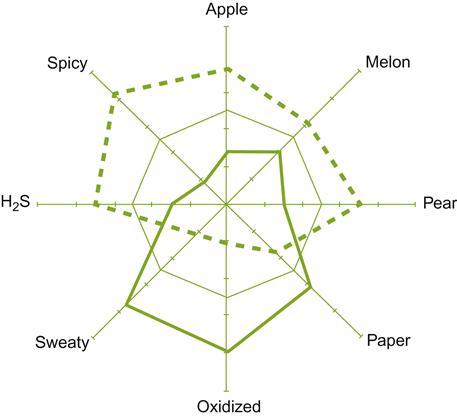
Because of their potential to affect wine quality (Egli et al., 1998), there is increasing interest among winemakers in using endemic yeasts to give their wines distinctiveness (Fig. 7.28). Nevertheless, as with any technique, it needs to be used with discretion and tempered with experience. For example, cool fermentation without sulfur dioxide runs the risk that non-Saccharomyces yeast may dominate fermentation, generating sufficient acetic acid and fusel alcohols to mask grape varietal aromas. In addition, other yeasts can lead to stuck fermentation, especially under stressful vinifications (Ciani et al., 2006).
The few bacteria able to grow in juice or must are usually inhibited by Saccharomyces cerevisiae, with the exception of lactic acid bacteria. Thus, S. cerevisiae (or occasionally other Saccharomyces spp.) find few if any organisms capable of competing with them in grape must. Not surprisingly, S. cerevisiae tends to dominate and complete fermentation, even when its initial presence in must is rare (less than 1/5000 colonies), as in many spontaneous fermentations (Holloway et al., 1990). Subsequently, however, other yeasts may multiply in association with lactic acid bacteria during or following malolactic fermentation, notably Pichia spp. (Fleet et al., 1984).
Several investigations indicate that local populations of S. cerevisiae are heterogeneous, consisting of many strains, differing considerably even among regional wineries (see Mortimer, 2000). Although their numbers are usually low, they may occasionally reach 104–105 cells/mL, possibly derived from winery equipment, or as residents on damaged grapes. There may also be shifts in their dominance from year to year (Polsinelli et al., 1996) and from site to site (Guillamón et al., 1996). Inoculated strains typically dominate fermentation, but other strains may remain active for several days before being suppressed (Querol et al., 1992). Thus, indigenous S. cerevisiae strains may play a significant sensory role even in induced fermentations. Shifts in population numbers may also occur throughout fermentation when must is inoculated with several strains (Schütz and Gafner, 1993a; Sipiczki et al., 2004). Because of the low incidence of sexual recombination, infrequent ascospore production, and poor ascospore viability, it is suspected that mitotic recombination and mutation are the primary sources of this variability (Puig et al., 2000). However, strain selection in an existing heterogenous population could be at work as well.
Must Inoculation
During inoculation, sufficient Saccharomyces cerevisiae is added to reach a population of about 105–106 cells/mL. With active dry yeasts, this is equivalent to about 0.1–0.2 g/liter of juice/must. Active dry yeast often contains about 20–30×109 cells/g. In its production, the culture is treated so that the cells possess adequate amounts of protein, ergosterol, unsaturated fatty acids, and reserve materials for several divisions. Principal among the latter is trehalose, important in providing resistance to both drying and rehydration.
Inoculation is particularly common in the production of white wine, due to its lower nutrient status and yeast population (short maceration followed by clarification), and cool fermentation temperature. The different processes involved in red wine production mean inoculation is less necessary (i.e., fermentation with the pomace, warmer fermentation temperatures, possibility of some aeration during pumping over). Nonetheless, inoculation still has significant benefits in assuring a clean, predictable fermentation. With stuck fermentations, inoculation is essential.
Before addition, the inoculum is placed in water or dilute juice. Rehydration is recommended to occur at about 37 °C for 20 min (details depending on the strain and supplier). Supplementation of the rehydration medium with small amounts of sterols (often in the form of yeast cell fragments) (Soubeyrand et al., 2005) increases both yeast viability and improves their fermentative ability. A short interval at 25 °C before inoculation also improves survival, during which damaged cytoplasmic and membrane structures (Beker and Rapoport, 1987) are repaired and metabolism adjusts itself to the new conditions. This is important before the cells are exposed to the full osmotic potential of grape must. Readaptation occurs progressively (Fig. 7.29) and is estimated to involve about 2000 genes (Rossignol et al., 2006). Inoculation of cool musts requires even longer and progressive adaptation. Fractional addition of further juice, before final incorporation of the inoculum into the must, avoids rapid temperature changes and promotes retention of high cell viability.
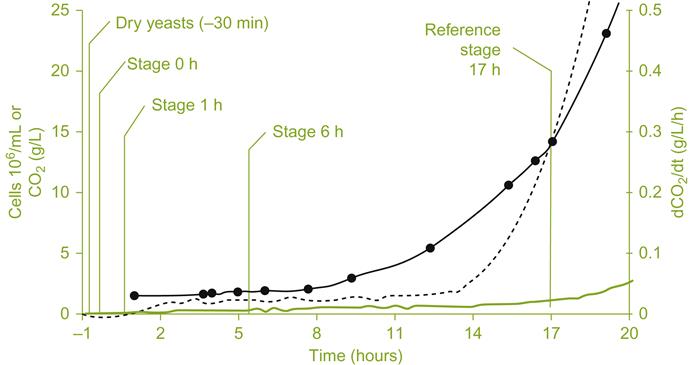
 , cumulated CO2 released. (Reprinted from Rossignol et al., 2006, with kind permission of Springer Scientific and Business Media.)
, cumulated CO2 released. (Reprinted from Rossignol et al., 2006, with kind permission of Springer Scientific and Business Media.)Commercially available strains of S. cerevisiae possess a wide range of characteristics, suitable for almost any winemaking situations. These include those that enhance the release of varietal flavorants (glycosides and/or cysteinylated/glutathionylated complexes); secrete acid proteases (potentially valuable in achieving protein stability) (Younes et al., 2011); produce an abundance of fruit esters (especially valuable in fermenting neutral flavored cultivars); are of neutral character (allowing the varietal character to be highlighted); or possess high hydroxycinnamate decarboxylase activity (enhancing the formation of stable pyranoanthocyanin pigments). Strains are also available that are notable for their production of low levels of acetic acid, hydrogen sulfide, or urea. Others may be chosen because of their relative fermentation speed; their ability to synthesize or degrade malic or lactic acid; their ability to augment the concentration of glycerol; their ability to restart stuck fermentation; or their known value in producing particular wine styles, notably carbonic maceration, late-harvest, or early- versus late-maturing reds. In addition, there are locally selected strains that are reported to produce regionally distinctive wines.
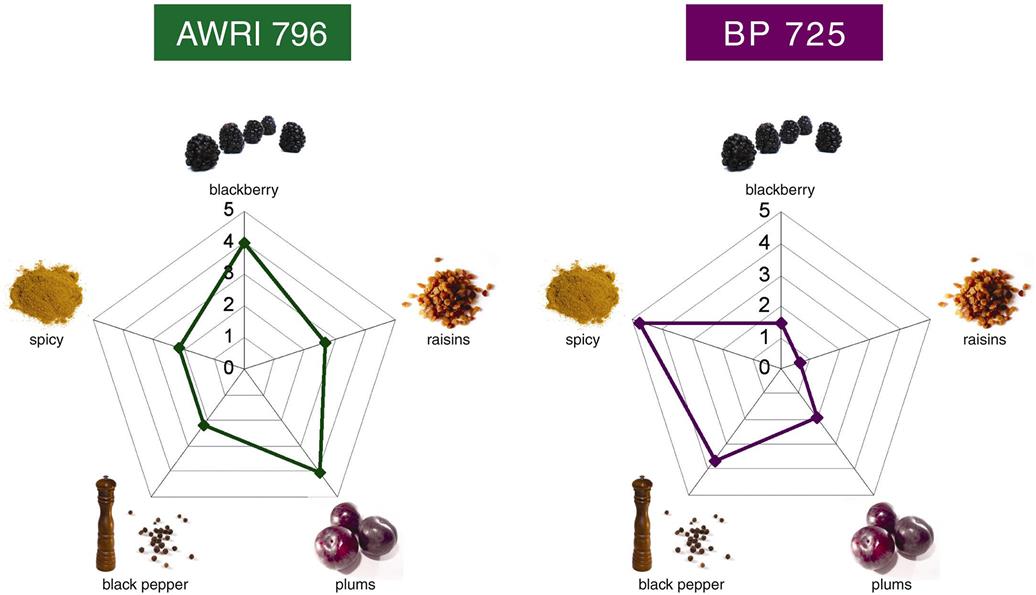
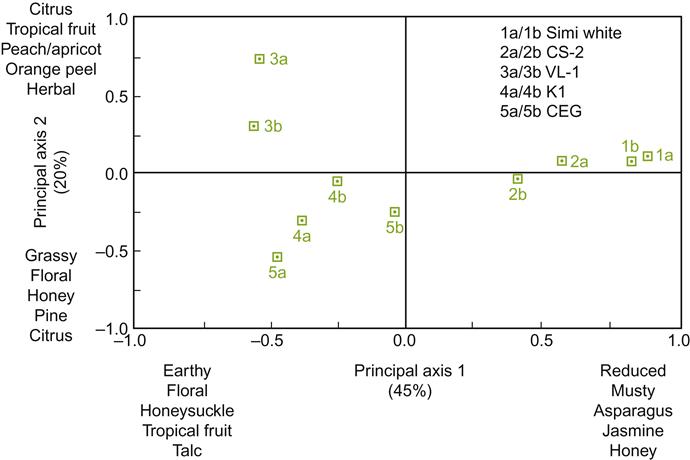
Despite all this information, or possibly because of it, the winemaker’s choice is far from simple. The main characteristics of most commercial strains are known, but most of their other properties are not, or if known they are buried in research papers not readily accessible to most winemakers. In addition, much information is anecdotal. Commercial suppliers provide data and suggestions on the various species and strains they provide, but cross comparison between suppliers is fraught with difficulty, combined with the knowledge that local conditions often are crucial to feature expression. Thus, regrettably, it is again up to the winemaker to do their own individual experimentation to determine what best suits their situations and preferences. Figures 7.30 and 7.31 give visual representations of the clear sensory differences between yeast strains.
In only a few instances is inoculation absolutely essential. With thermovinification or pasteurized juice, most of the endogenous yeast population is destroyed, requiring inoculation to achieve a rapid initiation of fermentation. In addition, inoculation is necessary to restart ‘stuck’ fermentations, and to promote fermentation of juice containing a significant number of moldy grapes. Moldy grapes generally possess various inhibitors, such as acetic acid, that slow yeast growth and metabolism. Finally, inoculation is required to assure the initiation of the second fermentation in sparkling wine production. However, the predominant reason for using a specific yeast strain or strains is to avoid the production of undesirable flavors that are occasionally associated with spontaneous fermentations.
Spontaneous vs. Induced Fermentation
There has been much discussion over the years concerning the relative merits of spontaneous versus induced fermentation. That various strains of Saccharomyces cerevisiae supply distinctive sensory attributes is indisputable (Cavazza et al., 1989; Grando et al., 1993; Walsh et al., 2006). This is particularly important for aromatically neutral cultivars. Their use may also play an increasingly important role in restricting the higher alcohol potentials of late-harvested grapes – conducted on the belief that this enhances wine flavor (Donaldson, 2008). Yeast strains differ significantly in ethanol production under identical conditions. In addition, strain choice can equally affect the varietal character of aromatically distinctive cultivars, by influencing the liberation or synthesis of specific grape flavorants (Ugliano et al., 2006). These influences not only affect the sensory attributes of young wines, but can still be detected after at least 3 years (King et al., 2011). This may be even more significant with non-Saccharomyces yeasts. They appear to have greater activity in breaking glycosidic bonds (Mendes-Ferreira et al., 2001). These effects can be further modified or enhanced by sur lies maturation. Regrettably, our knowledge of the chemical origin of their sensory effects is insufficient to permit prediction of results. Nevertheless, using established strains provides the winemaker with the greatest confidence that fermentation will initiate rapidly, go to completion cleanly (diminishing the possibility of undesirable microbial activity, disruption from killer factors, or must oxidation), and possess relatively predictable flavor and quality attributes.
In contrast, spontaneous fermentations may accentuate yearly variations in character. Capitalizing on vintage uniqueness is part of the marketing strategy of many European regions, and often considered part of the appeal of terroir distinctiveness. Spontaneous fermentations are often believed to generate greater aromatic complexity. Whether this is beneficial or not depends on the perceiver. It is commonly viewed by wine writers and other wine professionals to be of significance to the consumer. Sadly, this is probably wishful thinking (justifying research), and may apply only to those influenced by the comments of wine critics. The vast majority of consumers seem oblivious or indifferent to such subtleties, with their purchasing habits little influenced by factors actually affecting the wine’s sensory attributes.
Although spontaneous fermentation can influence a wine’s sensory character (Hernández-Orte et al., 2008; Varela et al., 2009), its effects can be inconsistent and relatively unpredictable. It also has a greater risk of leading to the development of off-odors and other undesirable sensory properties. For example, the activity of Kloeckera and Hanseniaspora can increase the incidence of non-protein haze formation (Dizy and Bisson, 2000). Spontaneous fermentations associated with oxidative yeasts are also frequently associated with higher concentrations of volatile acidity and ethyl acetate (Salvadores et al., 1993). They also tend to possess noticeable lag periods (most likely due to the low S. cerevisiae inoculum) and thus are more susceptible to disruption by killer factors (see below), or suppression by other yeast species.
Those who favor spontaneous fermentation believe that the endemic grape flora supplies a desired subtle or regional character (Mateo et al., 1991), supposedly missing with induced fermentations. Large-scale wineries, where brand-name continuity is essential, must avoid such risks of inconsistency. Because even induced fermentations are not pure-culture fermentations, they already run enough hazards with the indigenous microbial flora. Juice and must always contain sizable populations of epiphytic yeasts and bacteria, unless pasteurized or treated to thermovinification. In either case, it is preferable to do yearly blind tasting with competitor wines to ascertain, in an objective manner, whether beliefs are supported by results. An outline of such techniques is provided in Chapter 11 and in greater detail in Jackson (2009).
An alternative to either spontaneous or standard induced fermentation is inoculation with a mix of local and commercial yeast strains (Moreno et al., 1991). The combination appears to diminish individual differences, producing a more uniform and distinctive character. This may also involve the joint inoculation of Saccharomyces cerevisiae with species such as Candida stellata (Soden et al., 2000), Debaryomyces vanriji (Garcia et al., 2002), or Pichia kluyveri (Anfang et al., 2009). In the latter instance, the liberation of the aromatically significant thiol, 3-mercaptohexyl acetate, from Sauvignon blanc was enhanced. The activity and survival of members of a mixture is highly dependent on a variety of factors, notably species and temperature (Fig. 7.32). Aeration of the must also favors prolonged activity of non-Saccharomyces yeasts (Hansen et al., 2001). Because joint inoculation may result in partial suppression of one or more strains or species, sequential inoculation of non-Saccharomyces species, followed by later inoculation with Saccharomyces cerevisiae, is an intriguing option (Languet et al., 2005; Raynal et al., 2011). This generates, under semi-controlled conditions, a sequence similar to spontaneous fermentation.
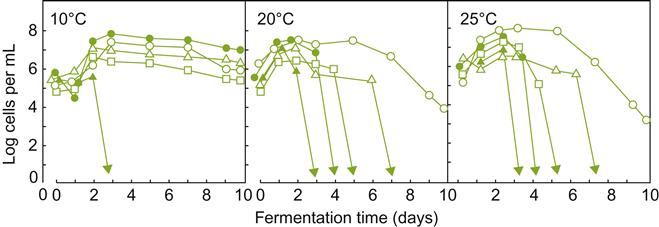
 , Saccharomyces cerevisiae;
, Saccharomyces cerevisiae;  , Kloeckera apiculata;
, Kloeckera apiculata;  , Candida stellata;
, Candida stellata;  , Candida krusei;
, Candida krusei;  , Hansenula anomala. (From Fleet et al., 1989, reproduced by permission.)
, Hansenula anomala. (From Fleet et al., 1989, reproduced by permission.)As noted earlier, on-site experimentation is the only sure means of determining the value of any practice. The metabolic products released by any strain, species, or their combination depends largely on the specific fermentation conditions (Thorngate, 1998; Regodón Mateos et al., 2006).
Another choice for winemakers searching to add a distinctive aspect to their wine is to use cryotolerant yeasts, notably S. bayanus var. uvarum. It is characterized not only by its ability to ferment at cold temperatures (Fig. 7.33), but also by its potential to direct the development of desirable sensory characteristics. For example, cryotolerant yeasts generally produce higher concentrations of glycerol, succinic acid, 2-phenethyl alcohol, and isoamyl and isobutyl alcohols; synthesize malic acid; and produce less acetic acid than many mesophilic S. cerevisiae strains (Castellari et al., 1994; Massoutier et al., 1998).
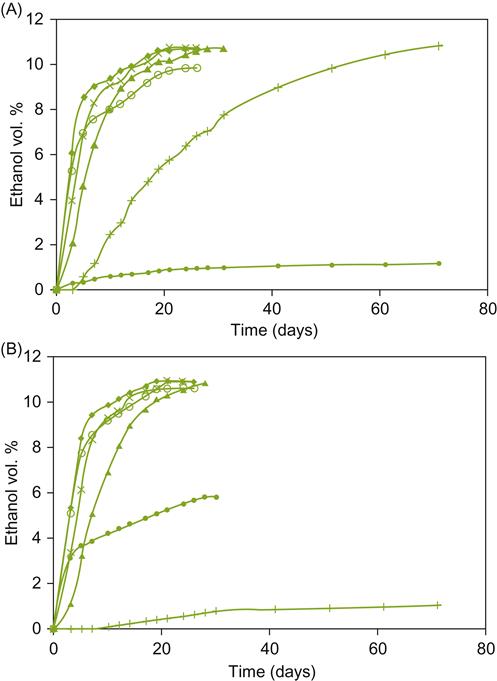
 , 12 °C;×, 18 °C; ♦, 24 °C;
, 12 °C;×, 18 °C; ♦, 24 °C;  , 30 °C;
, 30 °C;  , 36 °C) for (A) cryotolerant and (B) non-cryotolerant strains. (From Castellari et al., 1995, reproduced by permission.)
, 36 °C) for (A) cryotolerant and (B) non-cryotolerant strains. (From Castellari et al., 1995, reproduced by permission.)Although different strains, species, and their combination can affect the sensory attributes of wines, until recently the question of whether these differences affected consumer preference had not been studied. Under the conditions of an experiment in a relatively recent study, the answer was affirmative (King et al., 2010). Whether consumer impression is sufficient to noticeably affect subsequent purchase and for how long are quite different questions for which there are currently no answers.
Yeast Breeding
Genetically, Saccharomyces cerevisiae is the best understood eucaryote, having been used for decades as a favorite laboratory organism. It is easily cultured and has a comparatively small genome (~13000 kb), possessing relatively little repetitive DNA and few introns. The complete genome of a laboratory strain was deciphered in 1997, indicating that it possessed about 5800 protein-coding genes, located on 16 chromosomes. Furthermore, its protenome (full set of proteins) may be defined in the near future. In addition, recent developments in DNA microarray technology may make it easier to understand the intricacies and interconnections of its metabolic pathways (see Cavalieri et al., 2000). These advances should permit a shift from the traditional cross-and-select approach to breeding to one based on the selective elimination, modification, or introduction of specific genes.
Although S. cerevisiae is admirably suited to its role as the predominant fermenter of grape must, and there exists a wide diversity of strains possessing distinctive and useful properties, improved expression or new properties would be useful. Such modifications can vary from subtle variations in aromatic synthesis (Fig. 7.34) to the incorporation of properties such as malolactic fermentation.
Genetic Modification
In contrast to industrial fermentations, winemaking has made little use of genetically engineered microbes (Cebollero et al., 2007). This is partially explained by the complex chemical origin of wine quality, which makes delineating specific improvements difficult. In addition, other factors such as grape variety, fruit maturity, and fermentation temperature are generally, but not necessarily, considered to be of greater importance. In addition, more is known about the negative influences of certain yeast properties than about their positive sensory attributes. Finally, public suspicion of genetic engineering is regrettably, but undoubtedly, a prime reason for its minimal use.
In genetic improvement, features controlled by one or a few genes are the most easily influenced. For example, inactivating the gene that encodes sulfite reductase limits the conversion of sulfite to H2S. Improvement in other attributes, such as flocculation, has been more difficult. Flocculation is regulated by several genes, epistatic (modifier) genes, and possibly cytoplasmic genetic factors (Teunissen and Steensma, 1995). The major locus encodes for cell-surface proteins, notably lectins and lectin receptors. These are not constitutively present on the cell surface, but appear later in colony growth. This may result from the proteins being selectively deposited where budding has occurred, and at the tips of buds (Bony et al., 1998). In addition, the flocculation mechanism employed by different yeast strains can differ, as is the case with top- versus bottom-fermenting brewing yeasts (Dengis and Rouxhet, 1997).
Most important enologic properties are likely to be under multigenic control (Marullo et al., 2004). Alcohol tolerance and the ability to ferment steadily and cleanly at low temperatures are undoubtedly multigenic; most individual genes possessing only slight or synergistic effects. Genetic improvement is possible, but probably will take considerable effort and time to achieve. Chances of improvement are significantly enhanced if the biochemical basis of such factors is understood. This knowledge could pinpoint the genes most likely worth modifying.
Even more convoluted problems arise from our inability to predict the consequences of changing the direction of metabolic pathways (Prior et al., 2000). Modifying the regulation of one pathway can have important and unforeseen consequences on another (Guerzoni et al., 1985; de Barros Lopes et al., 2003). Unanticipated metabolic disruptions are less likely when the compound concerned is the end by-product of a metabolic pathway. Thus, terpene synthesis has been incorporated, by transfer of farnesyl diphosphate synthetase from a laboratory strain into a wine strain, without apparent undesirable side-effects (Javelot et al., 1991). Use of the modified yeast gives the wine a Muscat-like attribute.
Many techniques are available to the researcher interested in improving wine yeasts. The most direct approach involves simple selection, and often is initially effective. This method is much facilitated if a selective culture medium can be devised to permit only cells containing the desired trait to multiply. Otherwise, cells must be laboriously isolated and individually studied for the presence of the desired trait.
Alternatively, adaptive evolution has had a long and successful history in developing industrial microbes. Surprisingly, it has been little employed in wine yeast breeding. Its applicability has, however, been illustrated by McBryde et al. (2006). It avoids problems associated with the reticence of many winemakers and consumers to accept wine produced using generically engineered strains. However, for certain changes, more direct measures of modifying the genetic makeup are required. This can involve procedures such as hybridization, backcrossing, and mutagenesis, or newer techniques such as somatic fusion and genetic engineering.
Interspecific hybridization has apparently occurred naturally in the past within Saccharomyces sensu stricto species (de Barros Lopes et al., 2002), as well as currently (Cubillos et al., 2009). This may be facilitated by passage of the spores through the gut of Drosophila, a known vector of S. cerevisiae (Reuter et al., 2007). Nonetheless, wine yeast breeding has proven difficult. One of the principal complications results from the early (intra-ascal) fusion of spores of opposite mating-type. This precludes designed mating between haploid cells of different strains. Further complicating matters is the tendency of haploid cells to switch mating type shortly after ascospore germination, followed by nuclear fusion to form diploid cells. This eliminates any possibility of their designed mating.
Problems with crossing can occasionally be avoided by tetrad analysis – the early physical isolation and separation of ascospores. Physically placing spores from desired strains next to one another often results in fusion and successful crossing. Designed mating can be made easier if the strains crossed are first made heterothallic (Bakalinsky and Snow, 1990b). Most wine strains are homothallic (self-fertile), with haploid nuclei fusing within the ascus or shortly thereafter, obviating the possibility of designed crosses. Introduction of a recessive allele of the HO gene prevents switching of the mating-type gene, and correspondingly increases the probability of successful designed crosses. An alternate technique has been described by Ramírez et al. (1998). Regrettably, techniques that work successfully with well-characterized, haploid, laboratory strains do not necessarily work equivalently with industrial strains, such as wine yeasts.
Breeding wine strains is also exacerbated by the low frequency of sexual reproduction and spore germination (Bakalinsky and Snow, 1990a). Many strains undergo meiosis infrequently, a clear precondition for sexual reproduction. Of the haploid spores produced, infertility is frequent, probably due to the high incidence of aneuploidy (unequal numbers of similar chromosomes) (Bakalinsky and Snow, 1990a). Wine strains may also show chromosomal polymorphism, associated with rearrangement. It has been suggested that aneuploidy and other chromosomal abnormalities, due to their frequent occurrence, may possess unsuspected selective value under winemaking conditions.
Even in successful matings, the typical diploid state of wine yeasts can mask the presence of potentially desirable recessive alleles. In haploid organisms, the phenotype of recessive genes is expressed – no corresponding dominant (usually functional) allele being present. Although a complication in crossing experiments, diploidy permits ‘genome renewal’ by permitting the selective elimination of growth-retarding alleles. These slowly accumulate due to mutation (Ramírez et al., 1999).
If only a single genetic trait needs to be incorporated into an existing strain, backcross breeding is the preferred method. Figure 7.35 illustrates a situation in which a dominant flocculant gene is transferred from a donor strain to a valuable nonflocculant strain. Hybridized cells, containing the flocculant gene, are repeatedly backcrossed to the recipient strain. Combined with strong selection for flocculation, backcrossing rapidly eliminates undesired donor genes, unintentionally incorporated in the original cross.
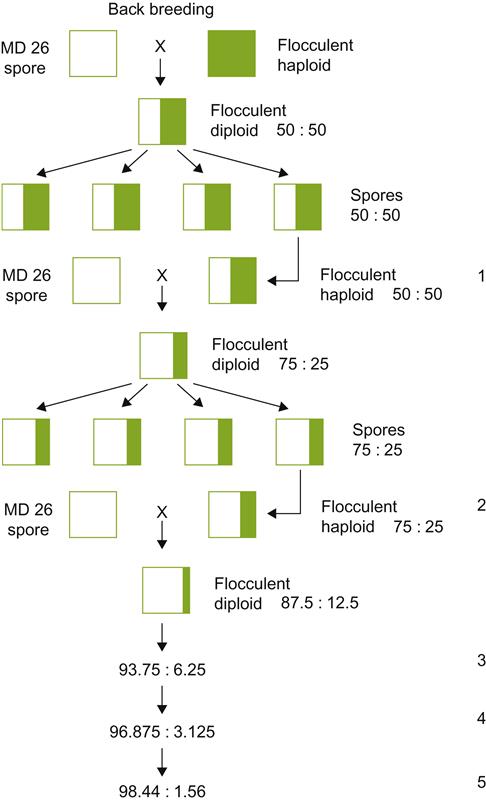
Where the genetic nature of the desired trait is unknown, or is under complex genetic control, crossing potentially suitable strains requires growing out tens of thousands of progeny. These must be individually assessed for their respective characteristics. Because this is so arduous, time-consuming, and expensive, breeders try to incorporate selective techniques that permit only the growth of desirable progeny.
In situations where standard procedures are not applicable (existing strains do not or are not known to possess the desired trait), ‘unconventional’ techniques must be sought. For example, somatic fusion may permit the incorporation of traits from yeast species with which traditional mating is impossible. Somatic fusion requires enzymatic, cell-wall dissolution, and subsequent mixing of the protoplasts. This usually occurs in the presence of polyethylene glycol, or some other agent that facilitates protoplast fusion. Fusion permits the possibility of gene exchange by combining the genes of both species in a single cytoplasm.
A serious limitation and disadvantage with somatic fusion is the frequent instability of the association. Fused cells often revert to one of the original species. The incorporation of foreign genes can also interfere with expression of existing traits.
Less disruptive is the incorporation of one or only a few genes from a donor organism. The procedure is called transformation, or more popularly, genetic engineering. It has the distinct advantage that the donor and recipient need not be closely related, as they need to be for crossing or somatic fusion to work. In transformation, yeast protoplasts may be bathed in a solution containing DNA from the donor organism. The DNA segments also require appropriate initiator and termination sequences for proper function when incorporated. Upon uptake, the gene needs to be transported into the nucleus and inserted into a chromosome. Without this, it is potentially unstable and may be lost in subsequent generations. Alternatively, the gene may be spliced into a plasmid before uptake. Plasmids are circular, cytoplasmic DNA segments that partially control their own replication. Thus, even without chromosomal insertion, the gene may be replicated, along with the plasmid in the cytoplasm. Although most eucaryotic cells do not possess plasmids, wine yeasts frequently carry copies of a 2-μm plasmid. Despite their presence, the plasmid is nonessential to the host cell.
Using transformation, the malolactic gene from Lactococcus lactis has been transferred to S. cerevisiae, along with the malate permease transport gene from Schizosaccharomyces pombe (Bony et al., 1997). The possession of several copies of the malolactic enzyme facilitates the almost complete conversion of malic acid to lactic acid within 4 days. Recently, a commercial strain (ML01) of this type has been released, based on a construct of genes from S. pombe and Oenococcus oeni (Husnik et al., 2007). The strain conducts simultaneous alcoholic and malolactic fermentations. This permits rapid deacidification, without the development of the buttery and other flavors usually associated with bacterial malolactic fermentation. Wine stabilization procedures can commence immediately after alcoholic fermentation, without waiting (sometimes for months) for bacterial malolactic fermentation to come to completion.
Other genes of enologic interest that have been incorporated into yeasts are β-(1,4)-endoglucanase (to increase flavor by hydrolyzing glucosidically bound aromatics) and alcohol acetyltransferase (to increase fruity flavors). Both features might benefit wines produced from aromatically neutral varieties. Yeasts have also been transformed with polysaccarase genes so that they can degrade glucans and xylans (Louw et al., 2006). They can increase the proportion of free-run wine and enhance the color intensity and stability of red wines.
Other examples involve combining the flocculation gene (FLO1) with a late-fermentation promotor (HSP30). Exposing the yeast to heat-shock can induce flocculation ‘on demand.’ In another case, a homozygous strain, recessive for one of the aldehyde dehydrogenase genes (ALD6), was transformed with a high-copy 2-μm plasmid, containing the glycerol 3-phosphate dehydrogenase gene (GPD2). This diverted more sugar to glycerol production, without an overproduction of acetic acid (Eglinton et al., 2002). This has the potential benefits of reducing ethanol production (producing more balanced wines from fully mature grapes) and increasing the fermentation rate (by enhancing osmotolerance) (Remize et al., 1999), as well as enhancing the smooth mouth-feel of the wine.
However, with characteristics based on many unlinked genes, it is a moot point whether genetic engineering has any selective advantage. In addition, genetic engineering is still complex and expensive. Part of this involves the long and complicated set of tests required before getting approval from the US Food and Drug Administration, or equivalent regulatory agencies. There is also considerable controversy about the safety of releasing genetically modified organisms into the environment. Although caution is always prudent, imprudent caution impedes progress. When reflecting on the incredible modification that has occurred historically during crop domestication (Zohary and Hopf, 2000), notably with corn (Mangelsdorf, 1974), the modifications involved in genetic engineering seem almost infinitesimal.
A requirement for all useful strains, no matter how they are derived, is genetic stability. Although a property of most traits, genetic stability is not a universal characteristic (Ambrona et al., 2005). For instance, flocculant strains often lose their ability to form large clumps of cells, settling out as a powdery sediment. Genetic instability may arise due to aneuploidy, mutation, or epigenetic modification. The latter is a previously unsuspected but major factor in gene regulation. Being often influenced by environmental conditions or transposon movement, prediction seems impossible.
A final caveat relates to the difference between conditions in the laboratory and micro-vinification, and commercial production conditions. Not only do vinification procedures vary from winery to winery, but the indigenous yeasts and bacterial flora of the grapes can significantly influence the attributes expressed by an inoculated strain. These differences frequently modulate the characteristics of the added strain, to such a degree that its intended effects may be largely negated or unpredictably qualified. Occasionally, indigenous yeasts displace inoculated strains. As always, experience under actual winery conditions for several years is essential to have relative confidence in the actual value of ‘new and improved’ strains.
Environmental Factors Affecting Fermentation
Carbon and Energy Sources
The major carbon and energy sources for fermentation are glucose and fructose. Other nutrients may be used, but they are present either in small amounts (amino acids), are poorly incorporated into the cell (glycerol), or can be utilized (respired) only in the presence of oxygen (acetic acid and ethanol). Sucrose can be fermented, but it is seldom present in significant amounts in grapes. It may be added, however, during processes such as chaptalization and amelioration, or as a nutrient for the second fermentation in the production of sparkling wine. For its metabolism, sucrose must be enzymatically split into its component monosaccharides, glucose and fructose. This occurs under the action of one of several invertases. Hydrolysis usually occurs external to the cell membrane by an invertase located between the cell wall and plasma membrane (periplasm). Most other sugars cannot be fermented by Saccharomyces cerevisiae as they lack the requisite enzymes and/or transport proteins. Significantly, though, other grape sugars can be utilized as an energy source by several spoilage microorganisms.
A variety of transport mechanisms moves glucose across the plasma membrane (Kruckeberg, 1996). Saccharomyces cerevisiae shows an extraordinary duplication and diversification of its HXT (hexose transport) gene family. Each of the approximately 18 variants possesses distinct regulatory and transport-kinetic properties. Some are low-affinity systems that typically work at high substrate concentrations. For example, the Hxt1 carrier is expressed only at the beginning of fermentation (Perez et al., 2005), Hxt2 (an intermediate carrier) is active only during the lag phase, whereas Hxt3 is functional throughout fermentation (but maximally at the onset of the stationary phase). Low-affinity systems appear to function by facilitated diffusion (without direct expenditure of metabolic energy). Thus, they are most effective at high sugar concentrations. In contrast, high-affinity systems are energy-requiring, and are particularly valuable at low glucose concentrations. They tend to be activated by a membrane protein, Snf3p, when sugar levels fall. Despite this, the major high-affinity systems of wine yeasts (Hxt6 and Hxt7) become active when the yeast colony enters the stationary phase – when considerable amounts of sugars still remain in the must. Although these transport proteins are named because of their transport capabilities, deletion mutants suggest that they have additional, regulatory roles that are not fully understood.
Glucose concentration not only differentially affects the activation of sugar transport mechanisms, but also regulates expression of enzymes in the TCA and glyoxylate pathways. Nevertheless, continued expression of selected chromosomal (vs. mitochondrial) genes results in some TCA enzymes being found in the cytoplasm even at high sugar concentrations. They are required for biosynthetic reactions essential for growth.
At maturity, the sugar concentration of most wine grapes ranges between 20 and 25%. At this concentration, the osmotic influence of sugar can delay the onset of fermentation. The resulting partial plasmolysis of yeast cells may be one of the causes of any lag period before active fermentation (Nishino et al., 1985). In addition, cell viability may be reduced; cell division retarded; and sensitivity to alcohol toxicity enhanced. At sugar concentrations above 25–30%, the likelihood of fermentation terminating prematurely increases considerably. Strains of Saccharomyces cerevisiae differ greatly in their sensitivity to sugar concentration.
The nature of the remarkable tolerance of wine yeasts to the plasmolytic action of sugar is unclear, but appears to be related to increased synthesis, or reduced permeability, of the cell membrane to glycerol (see Brewster et al., 1993). These responses to increased environmental osmolarity permit glycerol to equilibrate the osmotic potential of the cytoplasm to that of the surrounding medium. The accumulation of trehalose may also be involved.
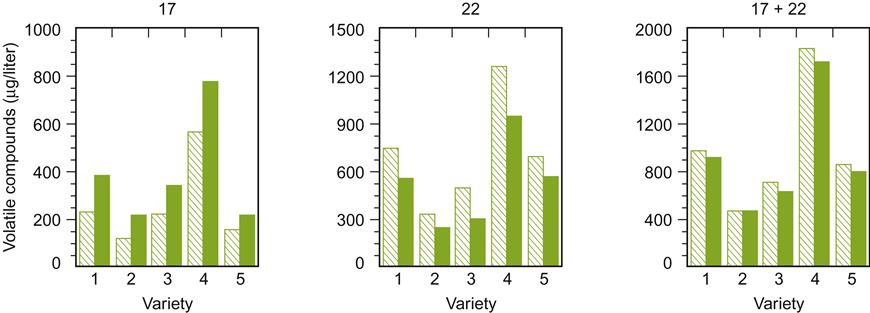
Sugar content affects the synthesis of several important aromatic compounds. High sugar concentrations increase the production of acetic acid (Fig. 7.36) and its esters. However, as indicated in Fig. 7.37, the effect of total soluble solids on esterification is not solely due to sugar content. For example, the synthesis of isoamyl acetate and 2-phenethyl acetate during fermentation decreases with increasing maturity (associated with a decreased synthesis of their corresponding fatty acids), but increases in juice from immature grapes, augmented with sugar to achieve the same °Brix. The importance of precursors in the must have been directly confirmed by Dennis et al. (2012). They found that the concentrations of C6 alcohols and aldehydes directly influence the synthesis of acetates such as benzyl, hexyl, and octyl acetates during fermentation.
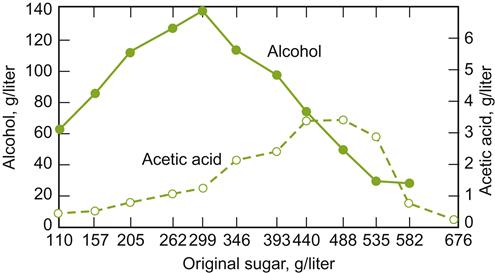
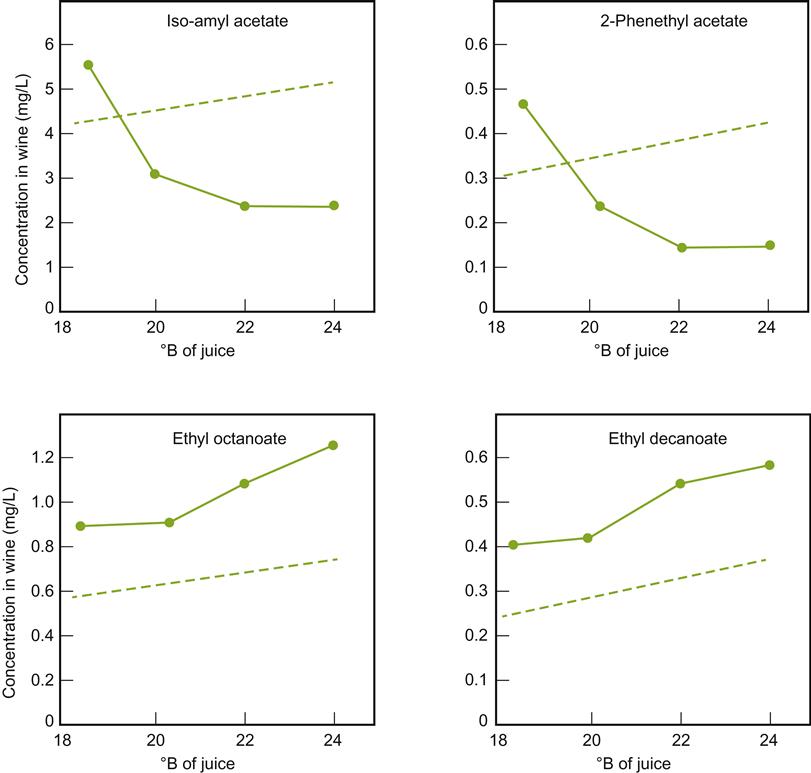
Over a wide range of sugar concentrations, ethanol production is directly related to sugar content. However, above 30%, ethanol production per gram sugar begins to decline (Fig. 7.36).
Alcohols
All alcohols are toxic to varying degrees. Because Saccharomyces cerevisiae shows considerable insensitivity to ethanol toxicity, much effort has been spent attempting to understand the nature of this tolerance, and why it breaks down at high concentrations. Several factors appear to be associated with ethanol tolerance. These include activation of glycerol and trehalose synthesis (Hallsworth, 1998; Lucero et al., 2000); accumulation of Hsp104 (a general stress-related protein) and Hsp12 (Sales et al., 2000); and modification of the plasma membrane, such as activating membrane ATPase, substitution of ergosterol for lanosterol, increasing the portion of phosphatidyl inositol vs. phosphatidyl choline, and augmenting the incorporation of palmitic acid (Aguilera et al., 2006). These membrane changes decrease permeability (Mizoguchi and Hara, 1998), minimizing the loss of nutrients and cofactors from the cell, notably magnesium and calcium. That these factors may disrupt cellular redox potential is suggested by the growth stimulation provided by acetaldehyde (increasing the availability of NAD+) (Vriesekoop et al., 2007). Vacuolar membrane function is also crucial for the retention of toxic substances stored in vacuoles (Kitamoto, 1989). In addition to disrupting membrane function, ethanol can inactivate some enzymes by inducing structural modifications.
Although alcohol buildup eventually inhibits fermentation, it begins disrupting yeast metabolism at much lower concentrations. For example, suppression of sugar uptake can begin at about 2% ethanol (Dittrich, 1977). This property is partially dependent on the fermentation temperature, and is reflected in yeast growth potential (Casey and Ingledew, 1986), possibly through it effect on membrane fluidity. Disruption of ammonium transport and inhibition of general amino acid permeases also occurs as alcohol content increases. These influences can be partially offset by enrichment with unsaturated fatty acids (e.g., adding yeast hulls). Although higher (fusel) alcohols are more inhibitory than ethanol, their much lower concentration substantially limits their toxic influence.
Although most strains of S. cerevisiae can ferment up to 13–15% ethanol, there is wide variation in this ability. Cessation of growth routinely occurs at concentrations below those that inhibit fermentation. It is generally believed that this results from disruption of the semifluid nature of the cell membrane, thus lowering water activity (Hallsworth, 1998). This destroys the ability of the cell to control cytoplasmic function, leading to nutrient loss and disruption of the electrochemical gradient across the membrane. The latter is vital for nutrient transport (see Cartwright et al., 1989). Lowered water activity also disrupts hydrogen bonding, essential to enzyme function. High sugar contents enhance ethanol toxicity.
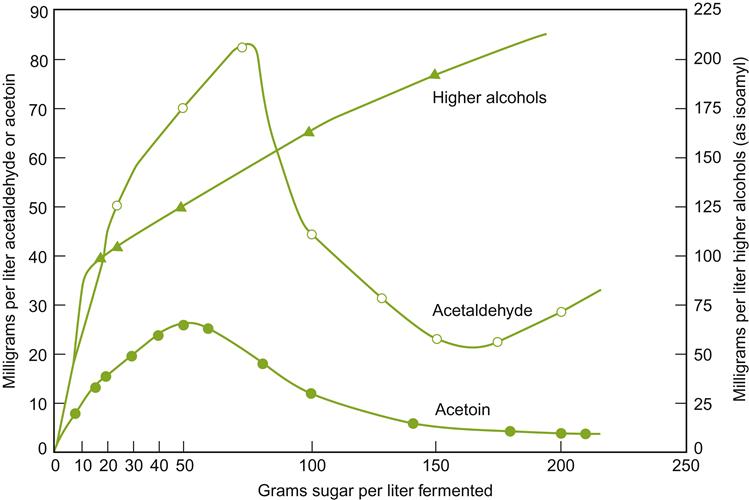
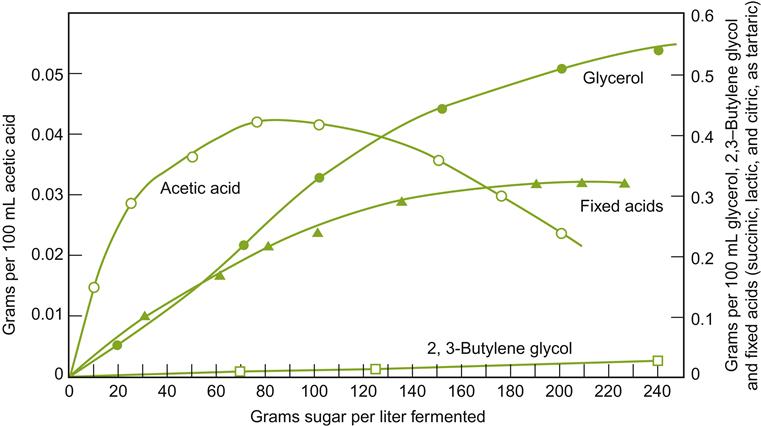
Ethanol is occasionally added to fermenting must or wine, usually in the form of distilled wine spirits (unmatured brandy), to arrest or prevent yeast and other microbial activity. This property is used selectively in sherry, port, and madeira production. In port, the brandy is added early during fermentation to retain about half the sugar content of the must. This leaves the wine with the aromatic attributes typical of early fermentation. For example, young port is likely to be higher in acetic acid, acetaldehyde, and acetoin content, but lower in glycerol, fixed acids, and higher alcohols (see Figs. 7.21 and 7.22) than had it fermented to dryness. In sherry production, wine spirits are added at the end of fermentation to inhibit the growth of acetic acid bacteria (>15% ethanol) and flor yeasts (>18% ethanol) during solera aging.
The accumulation of alcohol during fermentation has an important dissolving action on phenolic compounds. This effect is most pronounced at the beginning of fermentation. Given sufficient maceration time, though, phenolic compounds dissolve in water (Hernández-Jiménez et al., 2012). Most of the distinctive taste of red wines depends on the facilitated extraction of flavanols by ethanol during fermentation. Ethanol aids but is less critical to the extraction of anthocyanins. Phenolic extraction can be further enhanced in the presence of sulfur dioxide (Oszmianski et al., 1988).
Nitrogenous Compounds
Next to sugars, nitrogenous compounds are quantitatively the most important yeast nutrients. Under most circumstances, juice and must contain sufficient nitrogen for fermentation. Nitrogen contents can, however, vary considerably – values reported for juice in California can range from 60 to 2400 mg/liter. In addition, some cultivars have a tendency to experience nitrogen deficiency more frequently than others, for example, Chardonnay and Colombard. This is especially true when the juice has been given undue prefermentative centrifugation or filtration.
Nitrogen is required not only for the synthesis of structural, transport, and enzymatic proteins (essential for growth and metabolism), but is also an essential constituent in information molecules (nucleic acids) and in electron transport compounds. The importance of the ready availability of nitrogen for synthesis is illustrated by the rapid turnover in sugar transport proteins. These essential molecules have a half-life of about 6 h. Production of reduced sulfur odors can also commence within 30 min of the onset of nitrogen starvation.
Most of the nitrogen in grape must is in the form of free amino acids, notably proline and arginine. The proline content can be disregarded as it is poorly incorporated under anaerobic conditions. Thus, most of the assimilable nitrogen available to yeasts (YAN) consists of arginine, in addition to any ammonium ions present. Because arginine is largely localized in the skins, the duration of maceration and the method of pressing (with or without prior crushing) can significantly influence its extraction. With vineyard fertilization, the content of other available amino acids, notably glutamine, is increased.
Uptake is highly correlated with the dynamics of yeast growth (Fig. 7.38), being initially stored before use. Optimum levels suggested by Henschke and Jiranek (1993) are in the range of 400–500 mg/liter, specific values depending on the demands of the yeast species and strain, as well as fermentation conditions, such as temperature and must sugar content. Significantly higher concentrations can be a disadvantage. They promote unnecessary cell multiplication and, correspondingly, reduce the conversion of sugar to alcohol. In contrast, low values or amino acid imbalance can enhance the release of higher alcohols; the accumulation of reduced sulfur off-odors; and an increased likelihood of sluggish or stuck fermentation.
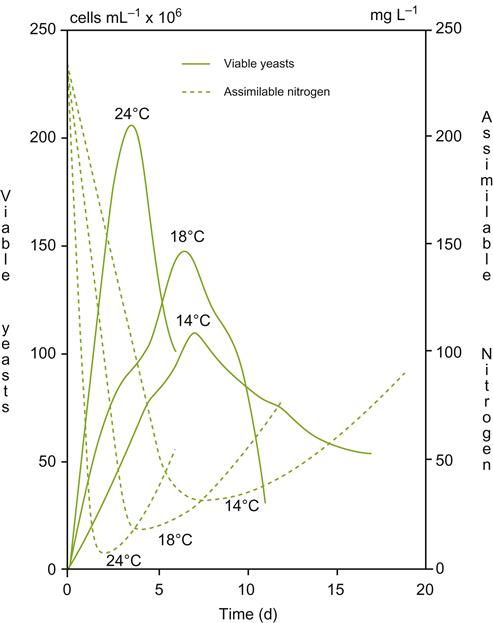
When nitrogen is required, it is usually supplied as DAP. Ammonia is the least energy-demanding form of nitrogen available to yeasts. Amino acid uptake is associated with simultaneous hydrogen uptake. This eventually requires the expenditure of ATP for the expulsion of H+, to avoid lowering the pH of the cytoplasm. As ethanol content rises, its disruption of the cell membrane results in the spontaneous diffusion of H+ into the cytoplasm, and uptake of amino acids declines or ceases.
If added, DAP is usually supplied halfway through fermentation, near the onset of the stationary phase (Bely et al., 1990). As such, it does not interfere with the early synthesis of sugar transport proteins (Bely et al., 1994). Earlier addition also reduces yeast uptake of amino acids early during fermentation, when alcohol accumulation is still low. This has the potential to influence flavor development, due to the involvement of amino acids in higher alcohol and, thereby, ester formation and other sensory attributes (Fig. 7.39). Late addition is ill-advised as residual amounts can favor microbial spoilage.
In situations where nitrogen deficiency is severe, significant amounts of hydrogen sulfide are released as a consequence of restricted amino acid synthesis and degradation. This is most marked if nitrogen becomes limiting during the exponential phase. It is countered by the availability of ammonia (or most amino acids, with the notable exception of cysteine and proline). The breakdown of cysteine has the potential to increase the release of H2S and mercaptans. Under such conditions, earlier addition of DAP is advisable (Jiranek et al., 1995). Avoiding nitrogen deficiency also minimizes arginine degradation, and the subsequent release of urea. This in turn reduces the likelihood of ethyl carbamate generation, especially in wine exposed to heating. Ethyl carbamate is a suspected carcinogen.
Although a valuable fermentation aid, addition of DAP is best minimized or avoided if possible. Excessive nitrogen can predispose some strains to high hydrogen sulfide production, as much as low nitrogen.
Development of rapid methods to estimate assimilable nitrogen (Dukes and Butzke, 1998), including infrared spectroscopy (FTIR), permits wineries to better assess actual need (Gardner et al., 2002). In addition, fermentation temperature, juice sugar content, yeast strain, and effects on flavor development need to be considered when estimating the ‘ideal’ nitrogen supply (O’Kennedy and Reid, 2008). Nitrogen requirements have aspects relating both to the avoidance of problems, as well as quality development issues. Because of the multiplicity of factors affecting the beneficial/detrimental aspects of nitrogen supplementation, it is often recommended that when conditions change (such as yeast strain or other viniviticultural practices) that microvinification be conducted to assess results prior to conducting full-scale fermentation.
Several conditions can reduce must nitrogen content. Nitrogen deficiency in the vineyard and must clarification can limit or diminish, respectively, the assimilable nitrogen content. If sufficiently marked, inadequate nitrogen levels slow fermentation and are one potential cause of ‘stuck’ fermentations. This may result from the partially irreversible inactivation of sugar transport by ammonia starvation (Lagunas, 1986). The half-life of the main glucose transport system is approximately 12 h, with complete inactivation occurring within approximately 50 h (Schulze et al., 1996). This results from the cessation of protein synthesis and enzyme degradation. The lack of ammonia can also negate the allosteric activation of crucial glycolytic enzymes, such as phosphofructokinase and pyruvic kinase. This in turn further inhibits glucose uptake (Bely et al., 1994).
Juice nitrogen content may be decreased by 33–80% due to infection by Botrytis cinerea (Rapp and Reuther, 1971). Nitrogen deficiency is also a frequent problem relative to the second fermentation in sparkling wine production and results both from consumption during the initial fermentation and clarification of the cuvée wines. It is countered by the addition of ammonium salts, notably DAP.
Nitrogen demand and incorporation occurs most rapidly during fermentation’s exponential growth phase. This correlates with the period when cell growth and division are most active. Subsequently, there is a slow release of nitrogen-containing compounds back into the fermenting juice/must (Fig. 7.40).
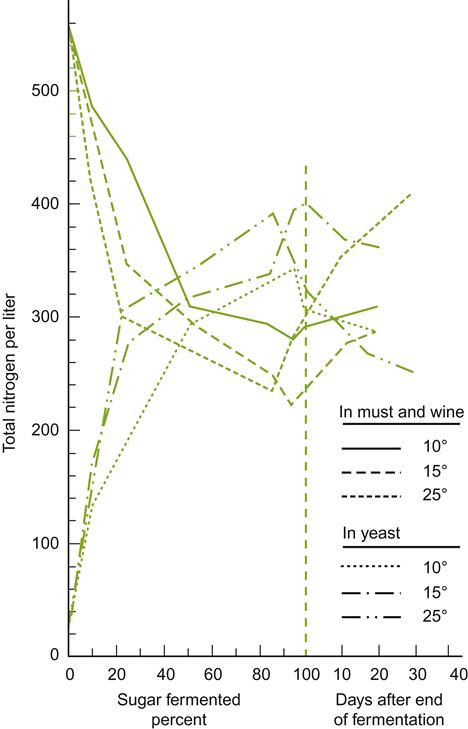
Of inorganic nitrogen sources, ammonia is incorporated preferentially. Wine yeasts tend to overexpress ammonia (MEP2) transport genes (Cavalieri et al., 2000). The oxidized state of ammonia permits its direct incorporation into organic compounds. Although ammonia is potentially capable of repressing the uptake of amino acids, its normal concentration in grape juice is insufficient to exert this effect. Saccharomyces cerevisiae has several amino acid transport systems (Cartwright et al., 1989). One is nonspecific and directs the uptake of all amino acids, with the exception of proline. The other systems are more selective, transporting only particular groups of amino acids. These properties probably explain why certain amino acids are preferentially incorporated (phenylalanine, leucine, isoleucine, and tryptophan), whereas others are more slowly assimilated (alanine, arginine) (Ough et al., 1991).
The principal amino acids available in grape must are proline and arginine. Because proline metabolism requires molecular oxygen for its metabolism (Tomenchok and Brandriss, 1987), arginine is the primary amino acid nitrogen source. Unfortunately, arginine may be degraded to ornithine and urea. If the ammonia derived from the breakdown of urea is not incorporated by cell metabolism, urea may accumulate and be secreted from the cell. This, as noted, could participate in the generation of ethyl carbamate (Ough et al., 1990). Although proline is not metabolized under fermentative conditions, its presence may be important in stabilizing the yeast-cell membrane.
Amines and peptides may also be incorporated as nitrogen sources, but not protein nitrogen. Wine yeasts are incapable of either transporting proteins into the cell, or enzymatically degrading them to amino acids outside the plasma membrane.
Yeast cells generally synthesize their own amino acid and nucleotide requirements from inorganic nitrogen and sugar. Consequently, most yeast strains do not need these metabolites in the medium. Nevertheless, they can and are assimilated from the medium when available. Assimilation avoids the diversion of metabolic intermediates and energy to their biosynthesis.
Nitrogen content can influence the synthesis and release of aromatic compounds during fermentation. Noticeable in this regard is the reduction in fusel alcohol content in the presence of ammonia or urea. The effect can be reversed by the presence and assimilation of certain amino acids. These opposing effects are the consequence of fusel alcohol use in amino acid biosynthesis, and their release during amino acid deamination, respectively. Under nitrogen starvation, there is a dramatic increase in the production of glycerol and trehalose (Schulze et al., 1996).
During and especially after fermentation, nitrogen and other constituents are slowly released into the wine. This is associated with the autolysis of dead and dying yeast cells (see Fig. 7.40). Because this may activate undesirable microbial activity, the first racking typically occurs shortly after fermentation. When malolactic fermentation is desired, however, racking is delayed until the bacterial conversion of malic to lactic acid is complete. Racking is also delayed if sur lies contact is desired. When the liberation of assimilable nitrogen into the wine is desired, small cooperage is preferred as it provides better contact between the wine and the lees (larger surface area/volume ratio).
Large-molecular-weight nitrogen-containing compounds are also released during yeast autolysis at the end of fermentation. This is particularly notable in relation to mannoproteins during sur lies maturation. The amount released often reaches 150–200 mg/liter, but actual amounts depend on the yeast strain, agitation, duration of contact, and the ambient temperature.
Lipids
Lipids are the basic constituents of cell membranes (phospholipids and sterols), function in energy storage (oils), act as pigments (carotenoids), and are components in proteins (lipoproteins) and carbohydrates (glycolipids).
Yeasts synthesize their own lipid requirements when grown aerobically, but are unable to produce long-chain, unsaturated fatty acids and sterols under anaerobic conditions. This is less significant in red wine production, where limited supplies of precursors are derived from the pomace during fermentation. The anaerobic limitation of lipid synthesis can, however, cause sluggish fermentation in highly clarified white juice. Clarification can remove more than 90% of the fatty acid content. This is particularly marked with unsaturated fatty acids, notably oleic, linoleic, and linolenic acids (Bertrand and Miele, 1984). Sterols are probably removed as well. Nevertheless, yeasts typically possess sufficient reserves of these vital nutrients to initiate fermentation and complete several cell divisions (typically four to five when a yeast inoculum is used). However, in spontaneous fermentation, up to 16 or more cell divisions may be involved in reaching the typical stationary population. Thus, a deficit in sterol and unsaturated fatty acid content can aggravate ethanol-induced reduction in glucose uptake, resulting in stuck fermentation.
Wine yeasts are sensitive to mid-chain (C8 and C10) saturated fatty acids, such as octanoic and decanoic acid. As by-products of yeast metabolism, they accumulate during fermentation. Because they increase membrane fluidity, proton influx increases, acidifying the cytoplasm. Cytoplasmic acidification favors the ethanol-induced leakage of nutrients, such as amino acids (Sá Correia et al., 1989), and can inhibit both high- and low-affinity sugar-transport systems (Zamora et al., 1996). Because toxicity increases with a decrease in pH, injury is likely associated with the undissociated form of these fatty acids. Toxic effects are limited or reversed by the addition of ergosterol and long-chain unsaturated fatty acids (Fig. 7.41), or the addition of various absorptive substances, such as activated charcoal, bentonite, silica gel, or yeast hulls (consisting primarily of cell-wall remnants following controlled autolysis). By removing octanoic and decanoic acids (via absorption), their potential for membrane disruption is reduced. In addition, yeast hulls may act as a source of required sterols and unsaturated fatty acids (see Munoz and Ingledew, 1990).
Another possible solution to problems associated with low sterol and unsaturated fatty acid content is the use of yeasts with high sterol contents. Sterol synthesis is commonly suppressed in the presence of glucose, but some strains do not show this effect. Growing yeast under highly aerobic conditions, as in active dry yeast production, generates cells elevated in unsaturated fatty acids. They may contain up to three times the sterol content of cells grown semiaerobically (Tyagi, 1984). Musts inoculated with strains possessing enhanced sterol contents frequently ferment more sugar than strains possessing low sterol contents.
Phenols
The phenolic content of must can have various effects on the course of fermentation. In red grapes, anthocyanins stimulate fermentation, whereas proanthocyanidins in white grapes can be slightly inhibitory (Cantarelli, 1989). Phenolic compounds are also a determining factor in the activation of film formation, important in fino sherry production (Cantarelli, 1989). Certain phenolics, notably the esters of gallic acid, are toxic, whereas others, such as chlorogenic and isochlorogenic acids, stimulate fermentation. The principal situation in which phenols suppress yeast metabolism is during the second fermentation of sparkling wine production. This is one of the primary reasons why few red sparkling wines are produced using standard procedures.
In addition to affecting fermentation, phenols may also be modified by yeast action – the most sensory significant example being the decarboxylation of ferulic and p-coumaric acids to aromatic vinyl phenols (4-vinyl guaiacol and 4-vinyl phenol, respectively) (Chatonnet et al., 1989). Other phenolic constituents in the must may influence this conversion.
Sulfur Dioxide
Sulfur dioxide is one of the most common wine additives. However, sulfur dioxide is also a by-product of yeast amino acid metabolism. In most instances, yeast-derived sulfur dioxide is rapidly bound to organic compounds in yeast cells or the fermenting must. Thus, its antimicrobial potential is unlikely to play a significant role in the success in S. cerevisiae in out-competing other microbes during fermentation. Interestingly, sulfur dioxide addition favors not only the growth of strains resistant to sulfur dioxide, but also appears to select strains that produce greater amounts of sulfur dioxide.
The differential action of sulfur dioxide against the grape epiphytic flora can be used to selectively control the action of indigenous yeasts. As our understanding of their role increases in wine fragrance development (Henick-Kling et al., 1998), their modulation versus inhibition is being viewed as an important tool in adjusting wine character.
At the concentrations typically used (less than 50 ppm total with healthy grapes), sulfur dioxide is not known to affect the fermentation rate. However, it can slow the onset of fermentation. The presence of 15–20 ppm can reduce the viability of a yeast inoculum from 106 to 104 cells/mL or less (Lehmann, 1987). Although sulfur dioxide limits the growth of indigenous yeasts and bacteria, this may be unnecessary as inoculated yeasts rapidly come to dominate fermentation, even in the absence of sulfur dioxide (Petering et al., 1993; Henick-Kling et al., 1998).
Yeast resistance to sulfur dioxide is correlated with several factors. For example, the SSU1R gene that controls sulfite efflux is overexpressed in wine yeasts (Hauser et al., 2001). This apparently results from its translocation to a position where it is under the control of the ECM34 promotor (Pérez-Ortín et al., 2002). In addition, it appears to be influenced by the presence of various nutrients, for example adenine which increases resistance and methionine which decreases it (Aranda et al., 2006).
In addition to its antimicrobial activities, sulfur dioxide can significantly influence yeast metabolism. Sulfur dioxide readily binds with several carbonyl compounds, notably acetaldehyde, pyruvic acid, and α-ketoglutaric acid. Binding increases their biosynthesis and potential release into the fermenting must. Thus, their concentration in finished wine often correlates with the concentration of sulfur dioxide added to the must. Sulfur dioxide also favors glycerol synthesis, whereas it tends to limit acetic acid production. Fixed acidity generally does not change, partially because sulfur dioxide suppresses the metabolism of both lactic and acetic acid bacteria.
As sulfur dioxide binds to carbonyl compounds, it inadvertently increases the amount of sulfur dioxide needed to suppress the action of spoilage microbes. Bound sulfur dioxide is much less antimicrobial than molecular SO2.
Sulfur dioxide favors the extraction of phenolic compounds, including anthocyanins. However, it can also reversibly decolorize anthocyanins. In addition, anthocyanins bound to sulfur dioxide are unable to polymerize with proanthocyanidins. This can adversely affect long-term color stability.
Although sulfur dioxide is the best available wine antimicrobial, it does not control certain spoilage yeasts. Many strains of Saccharomycodes ludwigii, Zygosaccharomyces bailii, and Brettanomyces spp. are particularly tolerant to sulfur dioxide (Hammond and Carr, 1976, see Fig. 8.11).
Elemental sulfur, potentially present as a residue from its use as a fungicide, can be assimilated and used in the synthesis of sulfur-containing amino acids and coenzymes. It may also be oxidized to sulfate and sulfur dioxide, or reduced to hydrogen sulfide. The reduction of sulfur to hydrogen sulfide may be a means, albeit aromatically unpleasant, of maintaining a favorable redox balance in yeast cells under anaerobic conditions.
Oxygen and Aeration
The process of fermentation itself requires no oxygen. Even in the presence of oxygen, S. cerevisiae preferentially ferments. Nevertheless, trace amounts of oxygen can indirectly favor fermentation by permitting the biosynthesis of sterols and long-chain unsaturated fatty acids. The production and proper functioning of the yeast cell membrane require sterols (primarily ergosterol), as well as C16 and C18 fatty acids. An alternative source comes from the juice and compounds extracted from the pomace during maceration. Molecular oxygen is also required in the synthesis of the vitamin nicotinic acid. In addition, anaerobic conditions favor the accumulation of toxic (C8 and C10) fatty carboxylic acids (Alexandre and Charpentier, 1995). They collect because they are not acylated in the synthesis of required long-chain fatty acids.
Juice oxidation during stemming and crushing is usually sufficient to permit adequate yeast growth during vinification. The amount of oxygen absorbed depends on the duration of skin contact. Because phenolic extraction increases with extended skin contact, the capacity of the juice to consume oxygen rises correspondingly. In removing free oxygen, conditions shift from oxidative to reductive. Aeration beyond that which occurs coincidentally during stemming and crushing (hyperoxygenation) is variously viewed as being beneficial (Cheynier et al., 1991) or detrimental (Dubourdieu and Lavigne, 1990). These different views may result from strain response to oxygen; the degree and timing of oxygen uptake; and increased synthesis of higher alcohols and esters (Valero et al., 2002). Nevertheless, hyperoxygenation does favor cell growth and promotes fermentation. A similar process, conducted post-fermentation during sur lies maturation, is termed bâttonage. It diminishes the procedure’s reductive influence (Fornairon-Bonnefond et al., 2003).
The initial browning associated with crushing is acceptable because the colored compounds that form are primarily lost during fermentation (attachment to yeast cells), or precipitated during subsequent clarification. It also gives white wine a degree of resistance against in-bottle oxidative browning, by eliminating readily oxidizable phenols early in vinification.
During red wine fermentation, oxygen may be absorbed during pumping over. The resulting incorporation of about 10 mg O2/liter often speeds the process of fermentation. This is more marked when aeration occurs at the end of the exponential phase (Sablayrolles and Barre, 1986). The yeast population increases and average cell viability is enhanced. Aeration also increases the production of acetaldehyde, thus favoring color stability by assisting the early formation of anthocyanin–tannin copolymers.
During the fermentation of white wines, winemakers assiduously avoid oxygen exposure. It increases a tendency to synthesize fusel alcohols and acetaldehyde. Increased levels of volatile acidity (acetic acid) may also result from the activation of acetic acid bacteria. In addition, semiaerobic conditions can depress the synthesis of esters (Nykänen, 1986). However, the absence of oxygen can enhance the likelihood of hydrogen sulfide accumulation. Depending on the timing of aeration, oxygen uptake not only oxidizes H2S, but can also enhance its synthesis (Houtman and du Plessis, 1981). To offset H2S accumulation, short aeration at the beginning or a few days after the commencement of fermentation (Bertrand and Torres-Alegre, 1984) has been recommended. The timing and extent of aeration can also influence urea accumulation and, therefore, the potential for ethyl carbamate production (Henschke and Ough, 1991). In situations where stuck fermentation has been a problem, the addition of nitrogen (DAP) and slight aeration (1 mg/L) at the onset of the stationary phase has been suggested (Julien et al., 2000). The cuvée blend in sparkling wine production also benefits from aeration prior to the second fermentation. Because the relative benefits of oxygen uptake vary with the yeast strain and cultivar, tests of its value need on-site verification.
Following fermentation, limited slow oxygen uptake (∼40 mg O2/liter) is considered to benefit the maturation of red wines. It favors color stability and reduces the typical reduction in color associated with malolactic fermentation (Pérez-Magariño et al., 2007). In contrast, most white wines are painstakingly protected from air exposure following fermentation. The exception to this practice occurs when wines are given extended contact with the lees (sur lies maturation). In this situation, limited oxygen uptake is desired and achieved by periodically stirring the lees (bâttonage). This limits the accumulation of an excessively reduced environment in the lees (and the associated development of reduced-sulfur off-odors).
Oxygen absorption is influenced by many factors, including clarification, skin contact, phenol concentration, sulfur dioxide content, the presence of polyphenol oxidases, sugar concentration, temperature, pumping over, rate of fermentation, and, of course, protection from air before, during, and after fermentation.
Carbon Dioxide and Pressure
During fermentation, large volumes of carbon dioxide are generated – about 260 mL/g glucose. This equates to over 50 times the volume of the juice fermented. The escape of carbon dioxide is estimated to remove about 20% of the heat generated during fermentation. Some of the heat loss is associated with water evaporation.
Various volatile compounds are also carried off with the carbon dioxide. Ethanol loss is estimated to be about 1–1.5% of that produced (Williams and Boulton, 1983), but varies with sugar use and temperature. Higher alcohols and monoterpenes are lost to about the same degree (∼1%). In contrast, significant dissipation of both ethyl and acetate esters can occur (Fig. 7.42). Depending on the variety, and especially the fermentation temperature, up to 25% of these aromatically important compounds may be lost (Miller et al., 1987). On average, more acetate esters volatilize than ethyl esters. This degree of escape could noticeably reduce the fruity character of a wine. Trapping these compounds for readdition to the wine is an interesting concept (Muller et al., 1993; Bach, 2001; Schäfer and Crespo, 2007).
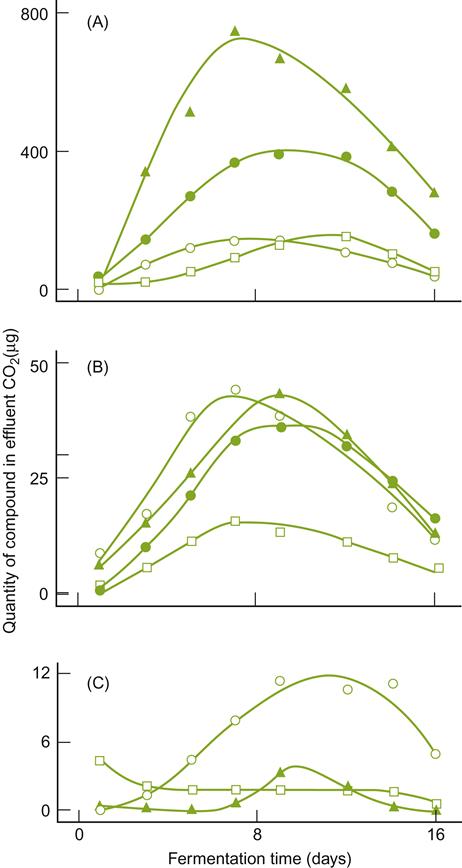
 , Isoamyl acetate;
, Isoamyl acetate;  , ethyl n-hexanoate;
, ethyl n-hexanoate;  , ethyl n-octanoate;
, ethyl n-octanoate;  isoamyl alcohol; (B)
isoamyl alcohol; (B)  , Isobutyl acetate;
, Isobutyl acetate;  , hexyl acetate;
, hexyl acetate;  , ethyl n-butanoate;
, ethyl n-butanoate;  , isobutanol; (C)
, isobutanol; (C)  , Ethyl n-decanoate;
, Ethyl n-decanoate;  , 1-hexanol;
, 1-hexanol;  , 2-phenylethanol. (From Miller et al., 1987, reproduced by permission.)
, 2-phenylethanol. (From Miller et al., 1987, reproduced by permission.)Volatilization from fermenting juice is a function of concentration; the rates of synthesis and degradation, where relevant; weak associations with matrix constituents and their comparative solubilities in carbon dioxide and the increasingly alcoholic juice. Dissipation is further affected by fermentor size and shape. For example, small fermentors possess higher surface area/volume ratios and lower liquid pressures than larger fermentors. This favors volatility. In addition, although the reduction of vapor pressure at low temperatures tends to limit volatilization, the slower release of carbon dioxide could partially offset this factor by favoring incorporation and loss with CO2.
The generation of carbon dioxide produces strong convection currents within the ferment. These help equilibrate the nutrient and temperature status throughout the juice. However, the presence of a floating or submerged cap disrupts equilibration in red musts (Fig. 7.46).
In vats, and in most tank fermentors, the carbon dioxide produced during fermentation is allowed to escape into the surrounding air. When the gas is trapped, pressure in the tank rapidly rises (Fig. 7.43A). At pressures above 700 kPa (∼7 atm), yeast growth ceases, although pressure-related effects have been reported at pressures as low as 30 kPa. Low pH and high alcohol content increase yeast sensitivity to CO2 pressure (Kunkee and Ough, 1966). This has its most significant influence during sparkling wine production. Even pressures of 20 kPa, which are easily found in young cuvée wines, can have a minor but noticeable effect in slowing fermentation and cell division (Fig. 7.43B). Pressures upward of 600 kPa are typically reached by the end of the second in-bottle fermentation. Nevertheless, the fermentative ability of yeasts may not be completely inhibited until about 3000 kPa. In addition, carbon dioxide accumulation may affect metabolism by influencing the balance between carboxylation and decarboxylation reactions (Table 7.1). The effect of pressure on the synthesis of aromatic compounds during vinification appears not to have been investigated.
Table 7.1
Possible effects of carbon dioxide on key enzymes of S. cerevisiae
| Reaction | Comment |
 |
Reduced production of ethanol |
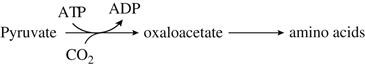 |
Stimulation, less available pyruvate for ethanol production |
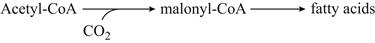 |
Stimulation, less available pyruvate for ethanol production |
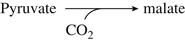 |
Stimulation, less available pyruvate but malate enzyme level is not high |
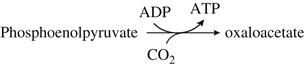 |
Stimulation, less available pyruvate but enzyme is repressed by glucose |
 |
Reduced production of biosynthetic precursors, thus cell yield will decrease; will reduce rate of production of ethanol |
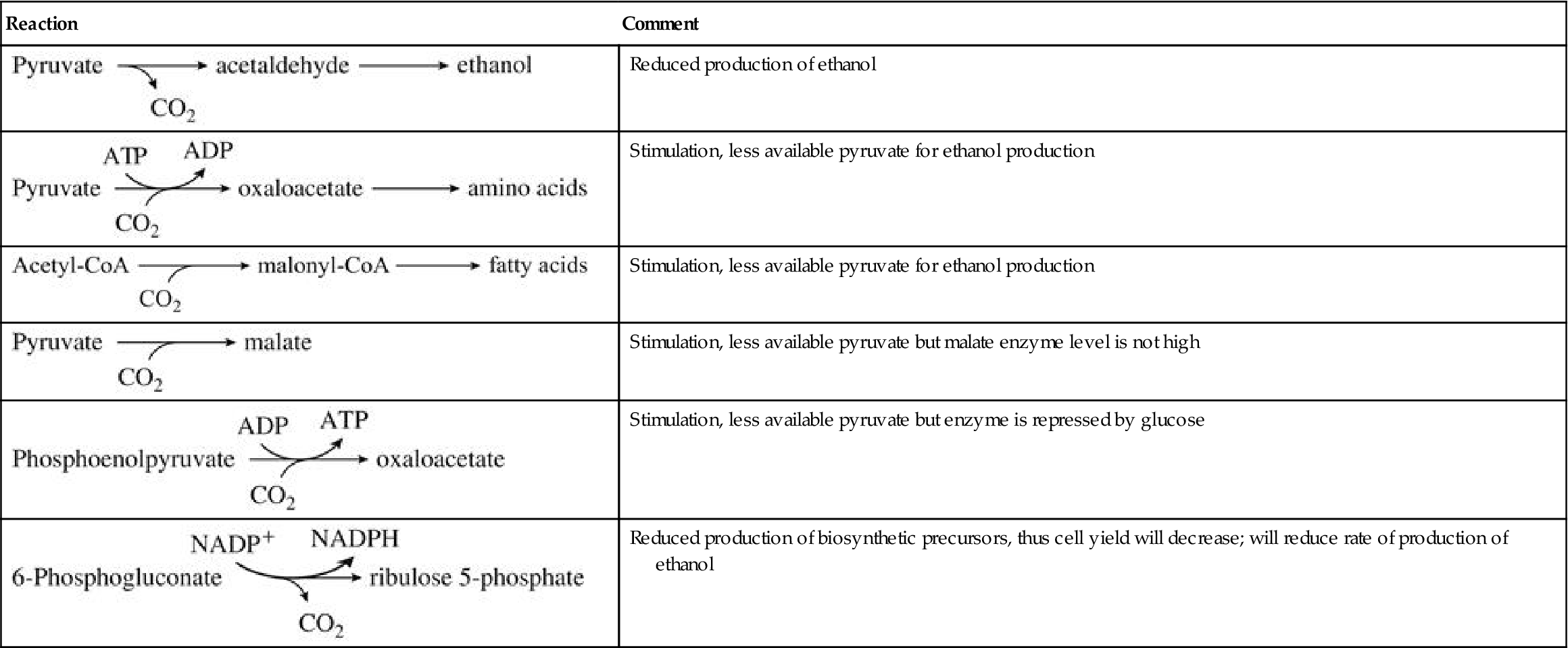
From Jones et al., 1981, reproduced by permission.
Some of the consequences of high pressure on cell growth and metabolism may accrue from a decrease in water viscosity (Bett and Cappi, 1965). This could disrupt the intramolecular hydrogen bonding vital to protein structure and function. In addition, critical changes appear to involve damage to cellular organelles (Iwahashi et al., 2003). Synthesis of heat-shock proteins (such as Hsp104) and trehalose can limit protein denaturation (Hottiger et al., 1994) and stabilize membrane fluidity (Iwahashi et al., 1995).
The pressure created by trapping the carbon dioxide produced during fermentation has occasionally been used to encourage a more constant rate of fermentation. It also has been used to induce the premature termination of fermentation, leaving the wine with a sweet finish. However, care must be used with the latter. Spoilage yeasts, such as Torulopsis and Kloeckera, are less sensitive to high pressures than S. cerevisiae. Their production of acetic acid could generate a vinegary taint. Caution also needs to be taken because lactic acid bacteria (Lactobacillus) are little affected by the pressures that affect wine yeasts (Dittrich, 1977).
pH
The pH range normally found in juice and must has little effect on the rate of fermentation, or on the synthesis and release of aromatic compounds. Only at abnormally low pH values (<3.0) is fermentation impeded. However, low pH may assist in the uptake of some amino acids. It supplies protons used in activating transport across the cell membrane (Cartwright et al., 1989).
The most important effects of pH on fermentation are indirect, such as noted previously in discussing the antibiotic action of sulfur dioxide. Low pH also prevents many potentially competitive organisms from growing in must or wine. In addition, pH affects the stability of some fermentation by-products. The best-known example relates to the hydrolysis of ethyl and acetate esters, in which breakdown occurs more rapidly at low pH values. pH also has a marked effect on the coloration of anthocyanins and the relative oxidizability of phenolic compounds.
Vitamins
Vitamins play a crucial role in the regulation of yeast metabolism, functioning as coenzymes and enzyme precursors (Table 7.2). Although vitamins are not metabolized as energy sources, their concentrations decrease markedly during fermentation (see Amerine and Joslyn, 1970). Yeast requirements typically are satisfied by either biosynthesis or assimilation from the juice. Certain conditions can, however, significantly reduce their concentration or availability. Fatty acids produced during fermentation can inhibit thiamine uptake; oversulfiting (or long-term storage of grape juice at high sulfur dioxide concentrations) degrades thiamine; and grape infection (notably by Botrytis cinerea) or contamination of stored juice by fungi and wild yeasts can lower the vitamin content. Under such conditions, a vitamin supplement may improve fermentation or be required to reinitiate stuck fermentation.
Table 7.2
The role of vitamins in yeast metabolism
| Vitamin | Active form | Metabolic role | Optimum conc. (mg/liter) |
| Biotin | Biotin | All carboxylation and decarboxylation reactions | 0.005–0.5 |
| Pantothenate | Coenzyme A | Keto acid oxidation reactions; fatty acid, amino acid, carbohydrate, and choline metabolism | 0.2–2.0 |
| Thiamine (B1) | Thiamine-pyrophosphate | Fermentative decarboxylation of pyruvate; oxo acid oxidation and decarboxylation | 0.1–1.0 |
| Pyridoxine | Pyridoxal phosphate | Amino acid metabolism; deamination, decarboxylation, and racemization reactions | 0.1–1.0 |
| p-Aminobenzoic acid and folic acid | Tetrahydro-folate | Transamination; ergosterol synthesis; transfer of one-carbon units | 0.5–5.0 |
| Niacin (nicotinic acid) | NAD+, NADP+ | Dehydrogenation reactions | 0.1—1.0 |
| Riboflavin (B2) | FMN, FAD | Dehydrogenation reactions and some amino acid oxidations | 0.2–0.25 |
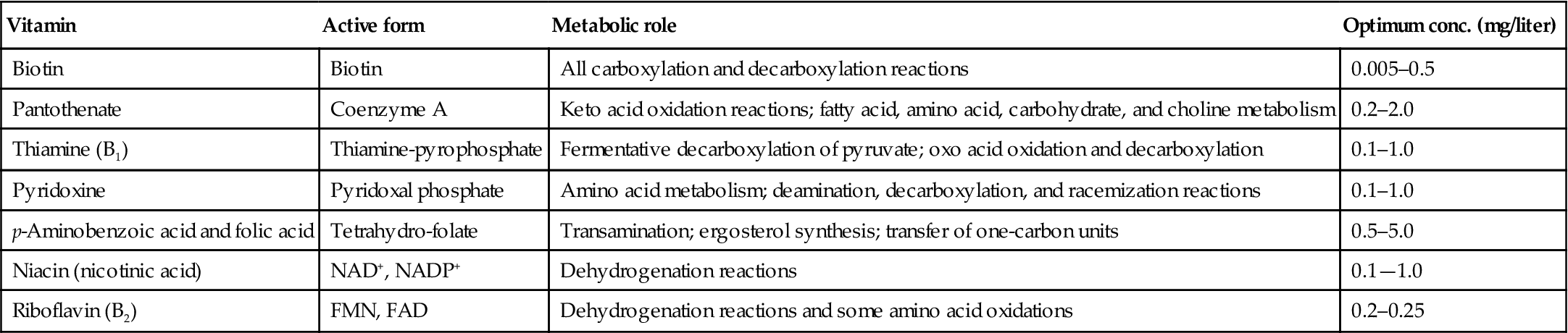
From Jones et al., 1981, reproduced by permission.
Adequate concentrations of thiamine reduce the synthesis of carbonyl compounds that bind to sulfur dioxide, thereby diminishing the amount of SO2 needed to control spoilage organisms. In addition to limiting carbonyl synthesis, thiamine also reduces the concentration and relative proportions of higher alcohols produced during fermentation. Although seldom a problem, deficiencies in pyridoxine and pantothenic acid can disrupt yeast metabolism, resulting in increased hydrogen sulfide synthesis.
Occasionally, prophylactic vitamin addition is recommended in situations where sluggish or stuck fermentations are a frequent and recurring problem. Yeast extract, where permitted, can be a good source of missing vitamins, as well as other nutrients. Although this is of value where deficiencies are known, such supplements can increase volatile acidity of some wines (Eglinton et al., 1993).
Inorganic Elements
Inorganic elements are often essential components in the active (catalytic) sites of enzymes. They also play active roles in regulating cellular metabolism and maintaining cytoplasmic pH and ionic balance (Table 7.3). For example, magnesium is involved in the catalytic action of several key glycolytic enzymes and stabilizing membrane structure. As such, magnesium helps adapt yeast cells to rapidly increasing alcohol concentrations (Dasari et al., 1990) and limits cell damage (Walker, 1998). Because calcium tends to restrict magnesium uptake, it is important not to inadvertently augment calcium levels (Birch et al., 2003), such as by the addition of calcium carbonate to neutralize excessive acidity.
Table 7.3
Major inorganic elements required for yeast growth and metabolism
| Ion | Role | Concentration (μM) |
| K+ | Enhances tolerance to toxic ions; involved in control of intercellular pH; K+ excretion is used to counterbalance uptake of essential ions, e.g., Zn2+, Co2+; K+ stabilizes optimum pH for fermentation | 20×103 |
| Mg2+ | Levels regulated by divalent cation transport system; Mg2+ seems to buffer cell against adverse environmental effects and is involved in activating sugar uptake | 5×103 |
| Ca2+ | Actively taken up by cells during growth and incorporated into cell wall proteins; Ca2+ buffers cells against adverse environments; Ca2+ counteracts Mg2+ inhibition and stimulates effect of suboptimal concentrations of Mg2+ | 1.5×103 |
| Zn2+ | Essential for glycolysis and for synthesis of some vitamins; uptake is reduced below pH 5, and two K+ ions are excreted for each Zn2+ taken up | 50 |
| Mn2+ | Implicated in regulating the effects of Zn2+; Mn2+ stimulates synthesis of proteins | 15 |
| Fe2+, Fe3+ | Present in active site of many yeast proteins | 10 |
| Na+ | Passively diffuses into cells; stimulates uptake of some sugars | 0.25 |
| Cl− | Acts as counterion to movement of some positive ions | 0.1 |
| Mo2+, Co2+, B2+ | Stimulates growth at low concentrations | 0.5 |
From Jones et al., 1981, reproduced by permission.
Although inorganic elements are normally assumed to be in adequate supply, there is difficulty in accurately assessing yeast requirements and the available ion concentrations in grape juice and must. Not only do organic compounds, such as amino acids, sequester elements, thereby reducing their effective concentration, but ions can antagonize each other’s uptake. Occasionally, as in the case of potentially toxic aluminum ions, this may be beneficial.
The abundance of potassium ions probably makes K+ the most significant metallic cation in juice and must. High potassium contents can interfere with the efficient uptake of amino acids, such as glycine. Under anaerobic conditions, potassium excretion may be necessary to maintain an acceptable ionic balance, due to the simultaneous incorporation of protons (H+) with glycine (Cartwright et al., 1989). At low pH values, abnormally high potassium contents may result in sluggish or stuck fermentation. This probably results from the joint uptake of potassium with glucose, and the subsequent decrease in pH associated with the excretion of hydrogen ions to maintain cytoplasmic ionic equilibrium (Kudo et al., 1998). A high potassium concentration can also generate tartrate instability, which is associated with high juice and wine pH. High pH can also lead to microbial instability, increasing the tendency of white wines to brown, and inducing color instability in red wines.
Temperature
Temperature is one of the most influential factors affecting fermentation. Not only does it directly and indirectly influence multiple aspects of yeast metabolism, but it is also one of the features over which the winemaker has the greatest control.
At the upper and lower limits, temperature can cause cell death. However, inhibitory effects can be experienced well within these extremes. Relative tolerance to high temperatures appears to depend, at least partially, on production of a particular heat-shock protein – Hsp104. This limits or reverses the aggregation of essential cellular proteins (Parsel et al., 1994). The disruptive influences of high temperatures are increased under growth-limiting conditions, such as the presence of ethanol and C8 to C10 carboxylic acids. In contrast, low temperatures tend to diminish the toxic effects of ethanol. This may be partially a consequence of the higher proportion of unsaturated fatty acid residues in the plasma membrane (Rose, 1989). This property may help to explain the higher maximum viable cell count at the end of fermentations conducted at cooler temperatures (Ough, 1966a).
Yeast growth rate is particularly sensitive to the fermentation temperature during the exponential growth phase. For example, cell division was found to occur every 12 h at 10 °C, every 5 h at 20 °C, and every 3 h at 30 °C (Ough, 1966a). Charoenchai et al. (1998) provide comparable data on the relative growth rates of a wide range of wine yeasts. Michelet et al. (2004) report that with the commercial yeast strains they tested, all strains completed fermentation without problems at either 12 or 20 °C, the differences being a slower completion at the cooler temperature, and variation in their aromatic by-products.
At temperatures above 20 °C, yeasts experience a rapid decline in viability at the end of fermentation. At cooler temperatures, cell growth is retarded but viability enhanced. Cool temperatures also prolong any fermentative lag phase. For this reason, winemakers may warm white juice to 20 °C before adding the yeast inoculum. Once fermentation has commenced, the juice may be cooled to the preferred temperature.
The temperature at which active dry yeast is rehydrated is critical, especially if cool fermentation temperatures are intended (Llauradó et al., 2005). It is important that rehydration should occur at the recommended temperature, with slow acclimation to the intended fermentation temperature. This minimizes the likelihood of stuck or sluggish fermentations. Growth and fermentation ability may also be assisted by the addition of ergosterol, inactive dry yeasts, or other constituents (Pozo-Bayón et al., 2009). Alternatively, or in addition, cryogenic yeast strains or species may be chosen when cool fermentation temperatures are selected.
In addition to affecting growth and survival, temperature has many subtle, and not so subtle, effects on yeast metabolism. One of the most marked, as noted, is its influence on fermentation rate. A quick onset and completion of fermentation have advantages. For example, this limits juice oxidation and the growth of epiphytic yeasts or those derived from the winery environment. Thus, the preferred fermentation temperature may have little to do with the optimum for ethanol production or yeast growth. Because yeast strains differ in their temperature response, the optimum temperature for vinification can vary widely. Equally and potentially more significant is the temperature’s modulation of enzymatic pathways and their effects on aromatic production, both beneficial and detrimental.
The preference in most wine-producing regions is to conduct white wine fermentation within a range of 10–20 °C. General consensus considers the wine’s quality to be higher at the cooler end of the range (e.g., du Plessis, 1983). Nonetheless, some European regions still prefer fermentation temperatures within the range of 20–25 °C. Most New World winemakers favor cooler temperatures because they yield fresher, more fruity wines. These properties in a white wine are highly valued throughout much of the world. Freshness and fruitiness in young white wines is partially due to the increased synthesis and retention of fruit esters, such as isoamyl, isobutyl, and hexyl acetates (Fig. 7.44A). Fatty acid ethyl esters may also contribute to a fruity aspect; for example caproate and caprylate ethyl esters possess apple-like aspects. Many of these ethyl esters are also produced more effectively at 15 °C. In contrast, 2-phenethyl acetate achieves its highest concentration at 20 °C (Fig. 7.44B). Production of ethanol and higher alcohols may also be enhanced at cool temperatures. Cooler fermentation temperatures also reduce the release of yeast colloids, facilitating clarification. On the negative side, cool temperatures slow fermentation, and may augment the likelihood of sluggish fermentation.