Post-Fermentation Treatments and Related Topics
Post-fermentation treatments may begin almost immediately after fermentation. These may involve any necessary adjustments to the wine’s physicochemical composition, as well as procedures such as sur lies maturation. Subsequent modifications involve various forms of clarification and fining, and chemical and biologic stabilization (including oxidation control). Both before and after bottling, further spontaneous chemical changes occur (aging). These affect the wine’s visual, gustatory, and olfactory attributes, with potential beneficial or detrimental consequences. These changes may occur in either inert or wood containers. Because of the significance of maturing premium wines in barrels before bottling, the taxonomy, distribution, and structural and chemical attributes of oak are noted, as well as barrel construction, conditioning, and care, as well as their alternatives. The origin and chemical and physical attributes of cork are explored, as well as stopper production, insertion, faults, and sorting, plus closure alternatives. The properties and production of various storage/transport containers (notably glass) are subsequently described. A discussion of wine spoilage follows including: cork-related problems; the various forms of microbial spoilage; sulfur and other off-odor development and control; and accidental contamination. An examination of winery waste water and treatment completes the chapter.
Keywords
wine; post-fermentation; sur lies; stabilization; clarification; fining; wine aging; oak; barrels; cork; wine spoilage; off-odors; winery waste water treatment
All wines undergo a period of adjustment (maturation) before bottling. Maturation involves the deposition and/or removal of particulate and colloidal material. In addition, the wine undergoes a range of physical, chemical, and biological changes that usually maintain or improve its sensory qualities. Many of these changes occur spontaneously, but may be promoted by the winemaker to speed their occurrence. Although undue intervention can disrupt the wine’s inherent attributes, shunning any intervention can be equally deleterious. What is important is that rational, data-based action takes precedence, not philosophical (or marketing) dictates.
Wine Adjustments
Adjustments attempt to correct deficiencies found in the grapes and/or sensory imbalances that developed during fermentation. In certain jurisdictions, certain types of adjustments to acidity and sweetness are permitted only before fermentation. This is regrettable, because it is impossible to predict the course of fermentation precisely. Judicious adjustment after vinification can improve the wine so that its stylistic, geographic, and varietal attributes can be fully expressed. Regrettably, once enacted, laws seem to develop a life of their own, independent of need or logic.
Acidity and pH Adjustment
Theoretically, acidity and pH adjustment can be conducted at almost any stage during vinification. Nevertheless, postfermentative correction is probably optimal. During fermentation, deacidification often occurs spontaneously, due to acid precipitation or yeast and bacterial metabolism. In addition, some strains of Saccharomyces cerevisiae synthesize significant amounts of malic acid during fermentation (Farris et al., 1989). Thus, the truest assessment of wine acidity and any need for adjustment is possible only at the end of fermentation. However, if the juice is above pH 3.4, prefermentative lowering of the pH is advisable. It favorably influences fermentation and avoids large adjustments following fermentation, especially with white wines.
Typically, red wines have higher pH values than white wines. This partially results from red wines being more frequently produced in warmer regions. Therefore, they tend to have lower malic acid contents at harvest. In addition, more potassium is extracted during the extended maceration red wines receive. Consequently, more tartaric acid exists in a salt form. These have a greater tendency to crystallize and precipitate than non-salt forms.
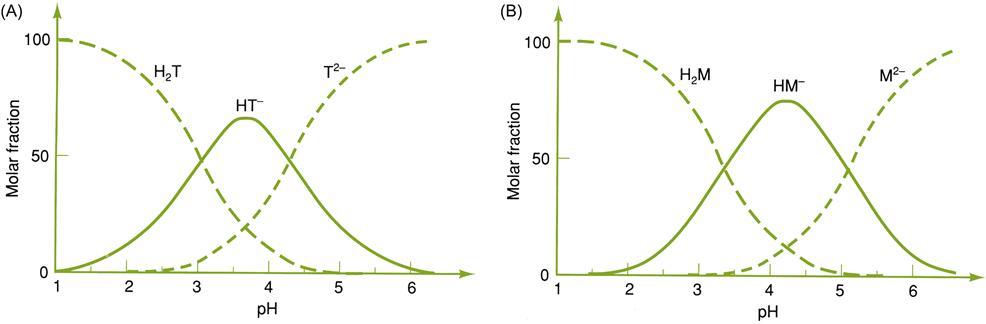
Precise recommendations for optimal acidity are impossible. They reflect stylistic, regional, and traditional preferences. More fundamentally, acidity and pH are complexly interrelated. The major fixed acids in grapes (tartaric and malic) occur in a dynamic equilibrium of ionized and nonionized states (Fig. 8.1). These include undissociated (nonionized) acids, half-ionized states (with one ionized carboxyl group), fully ionized states (with both carboxyl groups ionized), half-salts (with one carboxyl group associated with a cation), full salts (with both carboxyl groups bound to cations), or as double salts with other acid molecules and cations. The proportion of these interconvertible states depends largely on the pH, concentration of the respective acids, and potassium ions. Because of the complexity of the equilibria, and how they are affected by wine colloids, precise prediction of the consequences of changing any one of these factors on acidity is nigh impossible. Nevertheless, a range between 0.55 and 0.85% total acidity is generally considered appropriate. Red wines are customarily preferred at the lower end of the range, whereas white wines are preferred at the upper end.

Another important aspect of acidity is pH. It represents the proportion of H+ to OH− ions in an aqueous solution – the higher the proportion of H+ ions, the lower the pH; conversely, the higher the proportion of OH− ions, the higher the pH. Wines vary considerably in pH, with values below 3.1 being perceived as sour, and those above 3.7 being considered flat. White wines are commonly preferred at the lower end of the pH range, whereas red wines are frequently favored in the midrange.
Relatively low pH values in wine are preferred for many reasons: they give wines their fresh taste; improve microbial stability; reduce browning; diminish the need for SO2; and enhance the production and stability of fruit esters. The concentrations of monoterpenes may also be affected (Rapp et al., 1985). For example, the concentration of geraniol, citronellol, and nerol may rise at low pH values, whereas those of linalool, α-terpineol, and hotrienol decline. In red wines, color intensity and hue are enhanced at lower pH values.
Because of the importance of pH, the method of acidity-correction is influenced considerably by how it affects pH. Because tartaric acid is more highly ionized than malic acid, within the usual range of wine pH values, adjusting the concentration of tartaric acid has a greater effect on pH than an equivalent change in the concentration of malic acid (Fig. 8.2). Thus, adjusting the concentration of tartaric acid affects pH more than total acidity. In contrast, adjusting malic acid content affects total acidity more than pH. Which is preferable depends on the rationale for adjustment. Prefermentative adjustments are discussed in Chapter 7.
Deacidification
Wine may be deacidified by either physicochemical or biological means. Physicochemical deacidification involves either acid precipitation or column ion-exchange. Biological deacidification usually involves malolactic fermentation (see Chapter 7).
The advantages of eliminating excess acidity extend beyond simply removing an excessively sour taste. One of the side-benefits relates to shifts in wine fragrance. The effect on monoterpene concentration has already been noted. In addition, pH can modify the equilibrium between glycosides and esters, and their aglycones and constituent acids and alcohols, respectively.
Precipitation
Precipitation primarily entails the neutralization of tartaric acid; malic acid is less involved due to the higher solubility of its salts. Neutralization occurs when cations (positively charged ions) of a salt exchange with hydrogen ion(s) of an acid. Salt formation can reduce acid solubility, inducing crystallization and precipitation. The removal of the precipitated salt during racking, filtration, or centrifugation makes the reaction irreversible.
To induce the neutralization and precipitation of tartaric acid, finely ground calcium carbonate may be added to the wine. The reaction produces a calcium double salt of tartaric acid.
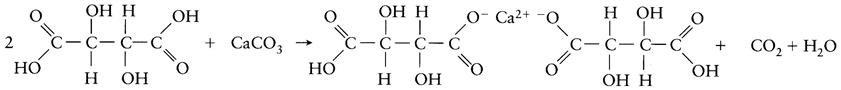
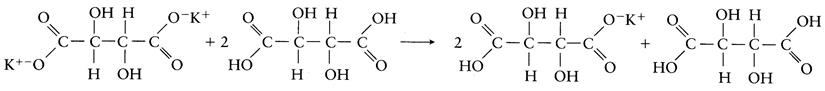
Neutralization can also result from the formation of insoluble half salts. Deacidification with potassium tartrate by this method is illustrated below.
Of available methods, deacidification with calcium carbonate is probably the most common. Of alternate procedures, the use of potassium tartate is more expensive and potassium carbonate prohibited in several countries. Although widely used, calcium carbonate has a number of disadvantages. Its primary drawback is the slow rate at which calcium tartrate precipitates. In addition, formation of calcium malate may generate a salty taste if the wine has not already undergone malolactic fermentation. Furthermore, if tartrate removal is excessive, the resulting increase in pH may leave the wine with a flat taste, and increase susceptibility to microbial spoilage.
Some of the disadvantages of calcium carbonate addition may be avoided using double-salt deacidification. The name refers to the belief that the technique functioned primarily via the formation of an insoluble double calcium salt between malic and tartaric acids. It now appears that very little of the hypothesized double salt actually forms. Nonetheless, the procedure does both speed the precipitation of calcium tartrate and facilitate the partial precipitation of calcium malate (Cole and Boulton, 1989).
The major difference between the single- and double-salt procedures is that the latter involves the addition of calcium carbonate to only a small proportion (~10%) of the wine to be deacidified. Sufficient calcium carbonate is added to raise the pH to above 5.1. This assures adequate dissociation of both malic and tartaric acids (see Fig. 8.2). It also induces the rapid formation and precipitation of the salts. A patented modification of the double-salt procedure (Acidex) incorporates 1% calcium malate–tartrate with the calcium carbonate. The double salt possibly acts as seed crystals, promoting rapid crystallization.
In double-salt procedures, the remainder of the wine is slowly blended back into the treated portion, with vigorous stirring. Subsequent crystal removal occurs by filtration, centrifugation, or settling. Stabilization may take 3 months, during which residual salts precipitate before bottling.
Although precipitation works well with wines of medium to high total acidity (6–9 g/mL), and medium to low pH (<3.5), it can result in an excessive pH rise in wines showing both high acidity (>9 g/mL) and high pH (>3.5). This situation is most common in cool climatic regions, where malic acid constitutes the major acid and the potassium content is high. In this situation, column ion exchange may be used (Bonorden et al., 1986), or tartaric acid may be added to the wine before the addition of calcium carbonate in double-salt deacidification (Nagel et al., 1988). Precipitation following neutralization removes the excess potassium with acid salts, and the added tartaric acid lowers the pH to an acceptable value.
Because protective colloids can significantly affect the precipitation of acid salts, it is important to conduct deacidification trials on small samples of the wine. This will establish the amount of calcium carbonate or Acidex required for the desired degree of deacidification.
Ion-Exchange Column
Ion exchange involves passing the wine through a resin-containing column. During passage, ions in the wine exchange with those in the column. The types of ions replaced can be adjusted by modifying the type of resin and ions present.
For deacidification, the column is packed with an anion-exchange resin. Tartrate ions are commonly exchanged with hydroxyl ions (OH−), thus removing tartrate from the wine. The hydroxyl ions released from the resin associate with hydrogen ions, forming water. Alternatively, malate may be removed by exchange with a tartrate-charged resin. The excess tartaric acid may be subsequently removed by neutralization and precipitation. The major limiting factor in ion-exchange use, other than legal restrictions and cost, is its tendency to remove flavorants and color from the wine, reducing wine quality.
Biological Deacidification
Biological deacidification, via malolactic fermentation, is possibly the most common means of acidity correction. Because malolactic fermentation can occur before, during, and after alcoholic fermentation, it is discussed in Chapter 7. Alternatively, use of malic-acid-degrading strains of S. cerevisiae can achieve partial deacidification.
Acidification
If wines are too low in acidity, or possess an undesirably high pH, tartaric acid may be added. As an acidulent, tartaric acid has several distinct advantages. These include its fresh crisp taste, high microbial stability, and a dissociation constant (Ka) that allows it to markedly reduce pH. The main disadvantage of tartaric acid addition is its cost, especially when added to wines high in potassium content. Crystal formation results in most of the tartaric acid being lost due to precipitation. The addition of citric acid avoids these problems and can assist in preventing ferric casse, via its chelating action. Nevertheless, the ease with which citric acid is metabolized by many microbes means that it is microbially unstable. Alternatively, ion exchange may be used to lower pH by exchanging H+ for the Ca2+ or K+ of tartrate and malate salts.
Sweetening
In the past, stable naturally sweet wines were rare. Most of the sweet wines of antiquity probably contained boiled-down must or honey – crystalline sugar becoming common in the second half of the nineteenth century. Stabilization by adding distilled alcohol, as in port and madeira, is a comparatively recent innovation. The stable, naturally sweet table wines of the past few centuries seem to have been produced from highly botrytized grapes (see Chapter 9). In contrast, present-day technology can produce a wide range of sweet wines, without recourse to botrytization, baking, or fortification.
Wines may be sweetened with sucrose, for example, sparkling wines. However, most still wines with a sweet character obtain this attribute from the addition of partially fermented or unfermented grape juice, termed sweet reserve (süssreserve). The base wine is typically fermented dry and sweetened just before bottling. To avoid microbial spoilage, both the wine and sweet reserve are sterilized by filtration or pasteurization; the blend being bottled under aseptic conditions, employing sterile bottles and corks. Regardless, peace of mind demands that sulfur dioxide and sorbic acid be added to protect against in-bottle yeast and bacterial spoilage.
Various techniques are used in preparing and preserving sweet reserve. One procedure involves separating a small portion of the juice as the sweet reserve. Thus, it possesses the same varietal, vintage, and geographic origin as the wine it sweetens. If the sweet reserve is partially fermented, yeast activity is terminated prematurely by chilling, filtration, and centrifugation, or by trapping the carbon dioxide released during fermentation. If the sweet reserve is stored as unfermented juice, microbial activity is inhibited/restricted by cooling to−2°C after clarification, pasteurizing, applying CO2 pressure, or sulfiting to above 100 ppm of free SO2. In the last instance, desulfiting is achieved by flash heating or sparging with nitrogen gas before use. Complete desulfiting is not required (or possible). What is left can be calculated into what is required in the finished wine at bottling. If desired, juice can also be concentrated by reverse osmosis or cryoextraction. Heat and vacuum concentration are additional possibilities, but are likely to result in greater flavor modification and fragrance loss.
Dealcoholization
In the past few decades, an increasing market for low-alcohol and dealcoholized wines has developed. Conversely, delayed harvesting to increase flavor content has resulted in wines with high alcohol content. Although a small portion of this latter tendency may be due to global warming, most of it appears to be intentional (Alston et al., 2011) – despite the wines often being criticized as unbalanced and ‘hot.’ Interestingly, Meillon et al. (2010) found unmodified control wines were preferred to their partially (1.5 and 3%) dealcoholized versions. The alcohol contents of the control Chardonnay, Sauvignon blanc, Merlot, and Syrah wines were 14.2, 13.6, 13.4, and 12.7, respectively. Only less experienced consumers tended to prefer the partially dealcoholized wines. Reconstituting the most dealcoholized versions to their original alcohol content did not fully compensate for aspects lost during reverse osmosis dealcoholization.
Previously, dealcoholization involved heat-induced alcohol evaporation. Although successful, it generated detectable baked or cooked odors, and drove off important flavorants. Correspondingly, it was appropriate only for the production of inexpensive, low-alcohol wines, for an uncritical niche market. With the advent of vacuum distillation, the temperature required could be reduced, avoiding heat-generated flavor distortion. Despite this, it still had the problem that many important volatiles escaped with the alcohol. Although some of these could be retrieved and added back to the wine, the final product still lacked its original character.
Alternative procedures include strip-column distillation, dialysis, pervaporation, spinning cone column, and stripping with carbon dioxide (see Wollan, 2010b). Strip-column distillation can lower ethanol contents down to 0.9 g ethanol/liter (Duerr and Cuénat, 1988; Ireton, 1990). Dialysis can have similar effects, with apparently little loss in flavor (Wucherpfennig et al., 1986). Pervaporation selectively removes ethanol, permitting the rest of the permeate to be added back to the wine (Takács et al., 2007). The spinning cone column (Rieger, 1994) and volatilization with carbon dioxide (Antonelli et al., 1996; Scott and Cooke, 1995) both appear to have the advantages of speed and minimal flavor disruption. Nevertheless, the most widely used dealcoholization techniques appear to be vacuum distillation and reverse osmosis, despite their potential for removal of fruity aromatics and accentuating unpleasant odors (Fischer and Berger, 1996). Until recently, these procedures required expensive installations and extensive technical skill. Thus, their application was limited to very large wineries, where the cost–benefit return could justify their purchase. Currently, though, specialized firms offer the service on a contract basis. In addition, affordable bench top units are becoming available, which are appropriate for boutique wineries desiring slight alcohol reduction.
When overly alcoholic wines are treated to bring their alcohol contents down to traditional values, measurable losses in aromatic have generally been relatively minor. Because alcohol content influences wine flavor and balance, alcohol adjustment has the potential to provide the winemaker with an opportunity (albeit for a price) to tweak a wine’s sensory characteristics.
As noted in Chapter 7, another solution has been described by Kontoudakis et al. (2011). A portion of the crop is picked unripe, fermented, and treated with bentonite and charcoal to produce a flavorless, low-alcohol acidic wine. Its subsequent addition to wine made from mature grapes decreases the blended wine’s alcohol content while compensating for reduced acidity. The resultant wine possesses a more traditional alcohol content, while the acidity enhances color intensity and freshness, apparently without negatively affecting flagrance.
Simpler still is harvesting earlier, at a °Brix that will achieve a lower alcohol content. Recent studies in Australia have shown that consumers preferred Cabernet Sauvignon and Shiraz wines in the 13.5% alcohol range (Anonymous, 2011). This was achieved with grapes picked earlier than is currently standard.
Where the addition of water is permissible to produce low-alcohol beverages, dilution is clearly the simplest and least expensive dealcoholization technique. Flavor enhancement, as with wine coolers, can offset flavor dilution.
Flavor Enhancement
Many grape flavorants, notably terpenes, norisoprenoids and volatile phenols, are bound in nonvolatile glycosidic complexes. Consequently, releasing this potential has drawn considerable attention. Glycosidic bonds may be broken by either acidic or enzymic hydrolysis. Because acid-induced hydrolysis is slow, heating the wine to increase the reaction rate has been investigated (Leino et al., 1993). Although successful, it tends to increase the production of methyl disulfide, accentuate terpene oxidation, and promote the hydrolysis of fruit esters. These features diminish the floral character of the wine, but enhance oaky, honey, and smoky aspects – attributes typically associated with bottle-aged wine.
Because of the flavor distortion associated with heat-accentuated acidic hydrolysis, enzymic hydrolysis has received most of the attention. Of these, β-glycosidase preparations have been extensively studied. Because flavorants are often bound to a variety of sugars, not just glucose, preparations with some α-arabinosidase, α-rhanmosidase, β-xylanosidase, and β-apiosidase activities are preferred. Commercial enzyme preparations are usually derived from filamentous fungi. Their enzymes are relatively insensitive to the acidic conditions typical of wine, unlike those produced by grapes or yeasts.
When employed, the enzymes are added at the end of fermentation – with glucose in the juice/must acting as an inhibitor. In addition, enzymic action can be quickly terminated, as desired, by adding bentonite; it absorbs and removes the enzymes by precipitation. More efficient (but expensive) regulation can be achieved with immobilization of the enzymes in a column through which the wine is passed (Caldini et al., 1994).
As yet, commercial sources of enzymes capable of releasing varietally significant thiols from their cysteinylated and glutathionylated complexes are unavailable.
Sur lies Maturation
Sur lies (on the lees) maturation is an old procedure enjoying considerable renewed interest and application (Dubourdieu et al., 2000). It has been used traditionally with Burgundian and some Loire white wines for decades, if not centuries. It is now employed fairly extensively worldwide, and occasionally with red wines. In red wines, it is credited with diminished astringency and an enhanced perception of sweetness (Marchal et al., 2011). Nonetheless, it is also associated with reduced color intensity (Rodríguez et al., 2005).
Sur lies maturation involves leaving the wine in contact with the lees at the end of fermentation. The duration can range from 3 to 6 months. This usually takes place in the same barrel in which fermentation occurred. The large surface area/volume ratio of small cooperage, and periodic stirring (bâttonage), favors the diffusion of nutrients, mannoproteins, and flavorants from yeasts into the wine (Fig. 8.3).

 ) tank aged on light lees; (
) tank aged on light lees; ( ) barrel aged on light lees; (
) barrel aged on light lees; ( ) barrel aged on fermentation lees; (
) barrel aged on fermentation lees; ( ) barrel aged on fermentation lees periodically resuspended. (From Llaubères et al., 1987. Copyright 1987, American Chemical Society, reproduced by permission.)
) barrel aged on fermentation lees periodically resuspended. (From Llaubères et al., 1987. Copyright 1987, American Chemical Society, reproduced by permission.)The effects associated with sur lies maturation occur to some degree in all wines, except when they are racked early and frequently. Nonetheless, they are accentuated with the extended lees contact associated with sur lies maturation.
During maturation, dead and dying yeast cells begin to autolyse. This involves a series of stages. The first two stages, identified by Charpentier and Feuillat (1993), relate to cell-membrane integrity and hydrolytic enzyme activation. The last three stages involve cytoplasmic disintegration, degradation, and increased cell-wall porosity. Although occurring slowly under cool storage conditions, and within the pH range of wine, autolysis releases many cellular constituents (Charpentier, 2000). Lees autolysis also favors the degradation of grape-derived arabinans and arabinogalactan-proteins. These tend to characterize red wines more than white wines, due to the former’s prolonged contact with grape skins (Doco et al., 2003).
The release of volatile yeast metabolites, such as ethyl octanoate and ethyl decanoate, can add a fruity element to the wine, whereas enzymatic reduction diminishes the sensory impact of carbonyl compounds, such as diacetyl. They can also activate the release of aromatic compounds from their precursors (Loscos et al., 2009). Lees may also reduce the sensory defect generated by 4-ethylphenols (Chassagne et al., 2005; Loscos et al., 2009), and the synthesis of sotolon (possessing a curry-like odor) during bottle aging (Lavigne et al., 2008).
Susceptibility to oxidative browning may decrease, due to the release of various amino acids (increasing the supply of oxidizable substrates). Although yeast hydrolytic enzymes can liberate glycosidically bound flavorants (Zoecklein et al., 1997), they may also degrade other grape aromatics. However, the most significant influence of sur lies maturation appears to result from the liberation of mannoprotein cell-wall constituents.
Mannoproteins have a wide range of effects (Caridi, 2006). These glycoproteins appear to have a protective effect on the monomeric anthocyanin content (Palomero et al., 2007). They also soften the taste of red wines, by complexing with and precipitating grape- or oak-derived tannins (Vidal, S. et al., 2004). Mannoproteins also diminish the likelihood of phenolic pinking in white wines (Dubourdieu, 1995). The latter feature is linked with the release of a hydrolytic breakdown product of yeast invertase (Dubourdieu and Moine, 1998a). In addition, mannoproteins favor the early completion of malolactic fermentation (Guilloux-Benatier et al., 1995), and minimize haze production from heat-unstable proteins (Dupin et al., 2000a). Improved protein stability is especially valuable in reducing the need for bentonite fining. To this end, Gonzalez-Ramos et al. (2009) have developed yeast strains with increased mannoprotein release; it apparently can reduce the need for bentonite fining by some 20–40%.
Mannoproteins also promote tartrate stability (Dubourdieu and Moine, 1998b). This clearly benefits wines consumed early, but the long-term benefits of tartrate stability are less clear. For example, breakdown of the soluble complexes may eventually lead to in- bottle tartrate deposition, as well as protein-haze formation. At least, delayed tartrate crystallization is of less concern, because the wine is most likely to have been consumed before the process commences. Aficionados usually know what the crystals are, and are not concerned.
In addition, mannoproteins reduce adsorption of aromatic compounds, such as fruit esters by oak cooperage (Ramirez-Ramirez et al., 2004). They can also favor the volatilization of some compounds, but bind others (Lubbers et al., 1994; Chalier et al., 2007). Particularly important in this regard may be a reduction in the volatility of thiols such as ethanethiol and methanethiol.
To avoid the development of a low redox potential in the lees, and the consequential production of reduced-sulfur off-odors, the wine is periodically stirred (bâttonage). This may occur on a weekly to monthly basis. It also favors the oxidization of hydrogen sulfide. However, in large cooperage, hydrostatic pressure exerted on the lees appears to promote the production of hydrogen sulfide and several reduced organic sulfur compounds (Lavigne, 1995). Anaerobic conditions in the lees also favor their production. Thus, sur lies maturation takes place almost exclusively in small cooperage. Under these conditions, lees appear to metabolize or absorb mercaptans; they escape or are oxidized during racking.
Nevertheless, sur lies maturation has been noted to increase susceptibility to the development of a sunstruck odor (goût de lumière) (La Follette et al., 1993). In addition, the release of significant amounts of glucose during autolysis (Guilloux-Benatier et al., 2001) increases the risks of microbial spoilage. Thus, it is particularly crucial that the cooperage be properly cleaned and sanitized to avoid contamination with yeasts such as Brettanomyces. When conducted slowly, bâttonage-induced oxygen exposure appears to avoid significant oxygen accumulation in the wine (Castellari et al., 2004). Thus, activation of acetic acid bacteria is minimized, when combined with cool storage temperatures. This can avoid the need for adding sulfur dioxide. This could retard or prevent malolactic fermentation that may be desired to occur simultaneously with sur lies maturation.
A distinctive form of sur lies maturation, though not so called, occurs during sparkling wine production. During the long, yeast-contact period, following the second, in-bottle fermentation, autolysis donates the toasty bouquet that characterizes fine sparkling wines. Part of this may be associated with the release of thiols, such as phenylmethanethiol and ethyl 3-sulfanylpropionate (Tominaga et al., 2003). The release of mannoproteins also stabilizes dissolved carbon dioxide, promoting the formation of long-lasting chains of bubbles. In addition, mannoproteins slow the release of aromatic compounds from the wine (Dufour and Bayonove, 1999), as well as bind potentially undesirable thiols (Tominaga et al., 2003). Another special example of a sur lies maturation-type process involves the solera aging of fino sherries.
Color Adjustment
The bevy of studies on micro-oxygenation, in relation to red wine production (see below), is one expression of the interest in color adjustment. The slow or periodic addition of oxygen favors the polymerization of anthocyanins with tannins (proanthocyanidins). Boulton et al. (1996) suggest that red wines can benefit from up to 60 mL O2 per liter (~10 periodic saturations), but show obvious deterioration with more than 25 saturations. Nonetheless, micro-oxygenation must be carefully monitored to avoid risks of activating dormant acetic acid bacteria, aggravating potential spoilage by Brettanomyces, and inducing the precipitation (loss) of polymeric pigments.
Another technique relates to the effect of temperature on enhancing polymerization. Correspondingly, Somers and Pocock (1990) recommended heating the wine during maturation as an alternative to aeration, to encourage early color stabilization.
In other instances, with varieties that either possess low tannin contents or tannins that are not easily extracted, adding enologic tannins may improve color depth and stability. Currently, the results of such investigations have been ambivalent, being encouraging with some cultivars, but not with others.
More frequently, though, color adjustment after fermentation refers to its reduction, especially with white wines. All wines can be partially or completely decolored by ultrafiltration. Depending on the permeability characteristics of the membrane, ultrafiltration retains macromolecules above a specific size. With membranes of lower cut-off values (~500 Da), ultrafiltration can also remove phenolic pinking. The use of filters, with even lower cut-off values, can produce blush or white wines from red or rosé wines. The major factor limiting the more widespread use of ultrafiltration is its potential for removing important flavorants along with macromolecules.
Adding PVPP (polyvinylpolypyrrolidone) is another procedure used to remove brown or pink pigments (Lamuela-Raventós et al., 2001). By binding tannins into large macromolecular complexes, PVPP facilitates their removal by filtration or centrifugation. Several white wines, such as Sauvignon blanc, have a tendency to turn pinkish within days of oxygen exposure (Simpson, 1977), especially those assiduously protected from oxidation during and after crushing. Considerable oxygen may be required for development of full pink expression (Singleton et al., 1979). Pinking is thought to occur when flavan-3,4-diols (leucoanthocyanins) slowly dehydrate to flavenes under reducing conditions. These can quickly oxidize to their corresponding colored flavylium-forms on exposure to oxygen. The use of moderate levels of sulfur dioxide helps to limit pinking (Simpson, 1977). Anecdotally, erythorbic acid, a diastereoisomer of ascorbic acid, has been reported to be more effective than ascorbic acid in limiting pinking in bottled white wine (Clark et al., 2010).
Other means of color removal involve the addition of casein or special preparations of activated carbon. With activated carbon, the simultaneous removal of aromatic compounds and the occasional donation of off-odors have limited its use. The addition of yeast hulls has also been studied as a means of removing brownish pigments from white wines (Razmkhab et al., 2002).
Blending
Blending is a standard feature in winemaking. It can vary from the selective combination of free- and press-run juice or wine; to wine made from different lots of grapes or juice derived from a single or several adjacent vineyards, growing the same or different cultivars; to wine made from grapes grown in different regions or countries. When blending wine, it is important to establish the physicochemical and sensory attributes of each to better predict the best potential blends.
Of blended wines, those made by combining wines from different varieties is probably the most well known to consumers. In Bordeaux, both red and white wines are rarely made from a single variety. For red wines, more than five are permitted, and for whites, up to three are authorized. In Chianti, up to five varieties may be included, and in Châteauneuf-du-Pape, 13 different cultivars may be involved. Even more may be blended in the production of porto.
In the past, varietal combination frequently occurred at harvest. Cultivars were often dispersed throughout the vineyard and harvested simultaneously, without separation as to variety. Although this is rare today, it may still occur in some regions. Where varieties are fermented together (cofermentation), the musts are typically combined after crushing. Usually, only the juice or must from either white or red cultivars is blended, though sometimes this is blended from white and red cultivars. Examples of the latter are Chianti and some Côte Rôtie wines. The addition of white juice to red must has often been explained as a means of ‘softening’ the wine. While possibly valid, it may be more valuable in color enhancement (Gigliotti et al., 1985). If the red wine is low in copigment factors, for which the white variety has an abundant supply, the addition of some juice from the white variety can enhance coloration. The addition of white juice could also assist in the formation of pigment polymers, which are important in long-term color stability. Where this is valuable, but dilution with the juice undesired, only the skins may be employed (depending on the phenolic components needed). It is more common, though, to ferment the must of individual cultivars separately, followed by blending of their wines. This has the advantage of permitting selective blending, based on the attributes of the specific wines. Separating grapes into lots at harvest also permits the distinctive qualities of the fruit from different sites or maturity grades to be realized. These can be blended later, if desired.
The skill and experience of the blender are especially important in the production of fortified and sparkling wines. Without blending, the creation and maintenance of house-styles would be impossible. The production of proprietary table wines also is largely dependent on the judicious combination of diverse wines. Consistency of character typically is more important than the vintage, variety, or vineyard origin. The skill of the blender is often amazing, given the number of wines potentially involved. It may also help that most consumers are ill-adept at remembering subtle sensory differences.
Blending also is used in the production of many premium table wines. In this case, however, the wines typically come from the same geographic region, and are often from a single vineyard (or holding) and vintage. Limitations on blending are usually precisely articulated in Appellation Control laws – the more renowned the region, the more restrictive the legislation.
There is little to guide blenders other than past experience. Blending to achieve specific attributes (relative to taste or some legal requirements) is usually comparatively easy. Simple calculations based on the relative composition, and the proportion of the wines blended, may approximate the desired result. However, many aspects are so complex and nonlinear that major discrepancies between physicochemical prediction and sensory perception are common. Evident examples relate to acidity, color, and flavor. To date, few studies have directly investigated the scientific basis of blending. Those that have have focused on methods predicting color, based on pigmentation of the base wines. Color is particularly significant due to its strong influence on quality perception, at least to critics, connoisseurs and trained tasters.
Blending diagrams may be developed using colorimeter readings. The diagrams are founded on the reflectance of the wines in the red, green, and blue portions of the visible spectrum. From these data, the lots required to achieve a desired color can be estimated. Pérez-Magariño and González-San José (2002) have proposed simple absorbance measurements for wineries without the equipment or software necessary to make the complex measurements normally involved.
Because of the limitations of individual blenders, and the nonlinear manner in which many sensory attributes combine, computer-aided systems have been proposed to facilitate blending (Datta and Nakai, 1992; Ferrier and Block, 2001). If nothing else, they may assist in helping us understand the origins of how various attributes interact.
Despite our lack of understanding into the subtleties of blending, on a practical level it is clear that some of the major advantages associated with blending are improved flavor, balance, and complexity. These properties were even known to the ancient Greeks; for example, Theophrastus (371–287 B.C.) notes in his Concerning Odours (ΠEPI OΣMΩN 11.2):
… for instance, if wine of Heraclea be mixed with wine of Erythrae, since the latter contributes its mildness and the former its fragrance; for the effect is that they simultaneously destroy one another’s inferior qualities through the mildness of the one and the fragrance of the other.
Loeb edition
In contrast, the Roman author, Columella (De Re Rustica 3.21.6–10) was opposed to blending, at least before vinification. He considered the qualities of the better wine to be worsened by the inferior one, and that the blended wine would not age well. However, subsequent comments indicate that part of this negative opinion is based on problems associated with mixed planting (e.g., the inability to prune or grow cultivar vines relative to their unique requirements).
In a classic study by Singleton and Ough (1962), similar pairs of wines, ranked comparably, but recognizably distinct, were reassessed along with their 50:50 blend. In no case was the blended wine ranked more poorly than the lower ranked of the base wines. More significantly, about 20% of the blends were ranked higher than either of the component wines (Fig. 8.4). Because the relationship between perceived intensity and flavorant concentration is nonlinear, blending does not necessarily diminish the desirable sensory characteristics of the individual wines. The reverse is more common.

 ) compared with their 50:50 blend (×), indicating the value of a complex flavor derived from blending. (Based on data from Singleton and Ough, 1962, from Singleton, 1990, reproduced by permission.)
) compared with their 50:50 blend (×), indicating the value of a complex flavor derived from blending. (Based on data from Singleton and Ough, 1962, from Singleton, 1990, reproduced by permission.)Although the origin of the improved sensory quality of blended wines is unknown, it may relate to the increased flavor subtlety and complexity. This has been used to commercial advantage when wines of intense flavor have been blended with large volumes of neutral wine. The blend still tends to express the characteristics of the fragrant component. In addition, the negative perception of some off-odors often diminishes with dilution, as they approach their threshold of detection (see Chapter 11).
When blending should take place depends largely on the type and style of wine involved. In sherry production, for example, fractional blending occurs periodically throughout maturation. With sparkling wines, blending (development of the cuvée) occurs in the spring following harvest. At this point, the unique features of the base wines are apparent. Blending of red table wines also typically occurs in the spring following fermentation.
Important in the deliberation is not only the proportional amount of each wine, but also whether wine derived from later pressings should be included. Wines from poor vintages customarily benefit from the addition of extra press wine than wines from better vintages. Later press fractions contain a higher proportion of pigment and tannins than the free-run or first pressing. The addition of pressings can also provide extra body and color to white wines. After blending, the wine is often aged for several weeks, months, or years before bottling. This is intended to ‘marry’ their flavors, as well as allow a new equilibrium to develop relative to acidity, their salts, protein, and pigment stabilization.
Wines may not be blended for a number of reasons. Wines produced from grapes of especially high quality are usually kept and bottled separately, to retain their distinctive attributes. For wines produced from famous vineyards, blending with wine from other sites, regardless of quality, would prohibit the owner from using the site name. This would significantly reduce the market value of the wine. With famous names, origin can be more important to sales than inherent quality.
Stabilization and Clarification
Stabilization and clarification involve procedures designed to produce a brilliantly clear wine with no flavor faults. Because the procedures can themselves create problems, it is essential that they be used judiciously, and only to the degree necessary.
Stabilization
Tartrate and Other Crystalline Salts
Tartrate stabilization is one of the facets of wine technology most influenced by consumer perception. The presence of even a few tartrate crystals can too easily be misinterpreted by neophytes. As a consequence, considerable effort is expended to avoid the formation of crystalline deposits in bottled wine (they have too often been misinterpreted as glass slivers). Stabilization is normally achieved by enhancing crystallization, followed by removal. In wines likely to be consumed shortly after bottling, a simpler delaying of crystallization can be used.
Potassium Bitartrate Instability
Juice is typically supersaturated with potassium bitartrate at crushing. As the alcohol content rises during fermentation, the solubility of the bitartrate decreases. This induces the slow precipitation of potassium bitartrate (cream of tartar). Given sufficient time, the salt crystals precipitate spontaneously. In northern regions, low cellar temperatures may induce adequately rapid precipitation. This is seldom satisfactory in warmer areas. Early bottling aggravates the problem. Where spontaneous precipitation is inadequate, refrigeration typically achieves rapid and satisfactory bitartrate stability. For a comparison of two stabilization techniques, from an environment impact perspective, see Bories et al. (2011).
Because the rate of bitartrate crystallization is directly dependent on the degree of supersaturation, wines that are only mildly unstable may be insufficiently stabilized by cold treatment. In addition, protective colloids may retard crystallization (Lubbers et al., 1993). Typically, protective colloids have been viewed negatively, as their precipitation after bottling releases tartrates that could subsequently crystallize. Although this may be true for most protective colloids, some mannoproteins appear sufficiently stable to donate adequate tartrate stability in bottled wine (Dubourdieu and Moine, 1998b). Different types of mannoproteins are released early during fermentation, and especially later during maturation as yeast cells in the lees autolyse. Alternatively, the addition of yeast cell-wall enzymic digest (yeast hulls) can promote tartrate stability, without cold or other stabilization treatments.
Potassium bitartrate exists in a dynamic equilibrium between ionized and salt states, as well as being associated with protective colloids. Under supersaturated conditions, salt crystals begin to form, eventually reaching a critical mass that provokes precipitation. Crystallization continues until an equilibrium develops. If sufficient crystallization and removal occur before bottling, bitartrate stability is achieved. Because chilling decreases solubility (provoking crystallization), bitartrate stability is of particular concern where bottled wines are likely to experience cold temperatures for any significant period during transport or storage.

In red wines, crystallization during maturation is often associated with yeast cells. They may constitute about 20% of crystal weight (Vernhet et al., 1999b). This compares with about 2% in white wines (Vernhet et al., 1999a). Potassium hydrogen tartrate crystals may also associate with other materials, for example, small amounts of phenolic compounds (notably anthocyanins and tannins in red wines), and polysaccharides, such as rhamnogalacturonans and mannoproteins.
Thus, although chilling tends to establish bitartrate stability, charged particles can interfere with crystal initiation and growth. For example, positively charged bitartrate crystals are attracted to negatively charged colloids, blocking growth. The charge on the crystals is created by the tendency for more potassium than bitartrate ions to associate with crystals early in growth (Rodriguez-Clemente and Correa-Gorospe, 1988). Crystal growth may also be delayed by the binding of bitartrate ions to positively charged proteins. This reduces the amount of free bitartrate and, thereby, the rate of crystallization. Because both bitartrate and potassium ions may bind with tannins, crystallization tends to be delayed more in red than in white wines. The binding of potassium with sulfites is another source of delayed bitartrate stabilization.
For cold stabilization, table wines are routinely chilled to near the wine’s freezing point. Five days is usually sufficient at−5.5°C, but 2 weeks may be necessary at−3.9°C. Fortified wines are customarily chilled to between−7.2 and−9.4°C, depending on their alcoholic strength. The stabilization temperature can be estimated using the empirical formula established by Perin (1977):
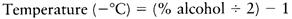
Adding potassium bitartrate crystals is often used to stimulate crystal growth. Crystal initiation (nucleation) is the principal obstacle to crystal growth. Another technique employing the same principle involves filters incorporating seed crystals. The chilled wine is agitated and then passed through the filter. Crystal growth is encouraged by the dense concentration of seed nuclei in the filter. The filter acts as a support medium for the crystal nuclei.
At the end of chilling, the wine is either filtered or centrifuged to remove the crystals. Crystal removal must be performed before the wine warms to ambient temperatures to prevent resolubilization.
Because of the expense of refrigeration, various procedures have been developed to determine the need for cold stabilization. Regrettably, none appears to be fully adequate. Potassium conductivity, although valuable, is too complex for regular use in most wineries. Thus, empirical freeze tests are the most common. For details on the various tests, the reader is directed to Goswell (1981) and Zoecklein et al. (1995). Despite the expense of cold stabilization, a recent comparison with several alternative techniques actually found the former to be the most cost-effective (Low et al., 2008).
Reverse osmosis is an alternative stabilization technique. By removing water, the concentration of bitartrate is increased, favoring crystallization and precipitation. After crystal removal, the water is added back.
Electrodialysis is another membrane technique occasionally used in bitartrate stabilization (Soares et al., 2009; Wollan, 2010a). Electrically charged membranes selectively prevent the passage of ions of the opposite charge. Passing wine between oppositely charged membranes can remove both anions and cations. In practice, though, potassium ions are more rapidly removed than tartrate ions. This not only limits crystallization, which requires both ions, but tends to lower the pH. Reducing the potassium content by about 10% seems to be effective in achieving adequate bitartrate stability. The procedure has the benefit of avoiding the simultaneous precipitation of polysaccharides and polyphenols, associated with cooling the wine to near freezing. It also reduces the energy costs associated with cooling, and wine loss with the crystals that form.
Another technique particularly useful for wines with high potassium contents is ion exchange. Passing the wine through a column packed with sodium-containing resin exchanges sodium for potassium. Sodium bitartrate is more soluble than its potassium equivalent, and is therefore much less likely to precipitate. Although effective, ion exchange is not the method of choice. Not only is it prohibited in certain jurisdictions, for example the EU, but it also increases the wine’s sodium content. The high potassium/low sodium content of wine is one of its positive health benefits.
If the wine is expected to be consumed shortly after bottling, treatment with metatartaric acid is an inexpensive alternative. Metatartaric acid is produced by the formation of ester bonds between the hydroxyl and acid groups of tartaric acid. The polymer is generated during prolonged heating of tartaric acid at 170°C. When added to wine, metatartaric acid restricts potassium bitartrate crystallization. It also interferes with the growth of calcium tartrate crystals. Because metatartaric acid slowly hydrolyzes back to tartaric acid, its effect is only temporary. At storage temperatures between 12 and 18°C it may be effective for about 1 year. Because hydrolysis is temperature-dependent, the stabilizing action of metatartaric acid quickly disappears above 20°C. If this treatment is used, metatartaric acid is added just before bottling. Other agents that can retard if not totally prevent crystallization include gum arabic and carboxymethylcellulose. They do not appear to modify the wines sensory attributes, possess no health risks, and remain active significantly longer than metatartaric acid (Bosso et al., 2010; Gerbaud et al., 2010).
Calcium Tartrate Instability
Instability caused by calcium tartrate is more difficult to control than its potassium counterpart. Fortunately, it is less common. Calcium-induced problems usually arise from the excessive use of calcium carbonate in deacidification, but can also arise from using cement fermentors, filter pads, and fining agents.
Several organic acids significantly influence calcium tartrate crystallization (McKinnon et al., 1995). For example, malolactic fermentation removes a major inhibitor of crystallization (malic acid) and, thus, promotes earlier calcium tartrate stability. However, for wines consumed shortly after bottling, such as champagnes, retarding crystallization may be preferable. Thus, malic acid may be added to sparkling wine with the dosage. Because grapes, used in making the base wine for sparkling wines, are pressed whole, they contain little polygalacturonic acid (a pectin breakdown product). Polygalacturonic acid is a potent retardant of calcium tartrate crystallization.
Calcium tartrate stabilization is more complex because precipitation is not effectively activated by chilling. Despite crystal growth and precipitation occurring optimally between 5 and 10°C, it can still take months for spontaneous stability to develop. Seeding with calcium tartrate crystals, while deacidifying with calcium carbonate, greatly enhances precipitation (Fig. 8.5). Because the formation of crystal nuclei requires more free energy than crystal growth, seeding circumvents the major limiting factor in stability development. A racemic mixture of calcium tartrate seed nuclei, containing both L and D isomers, is preferred. The racemic mixture is about one-eighth as soluble as the naturally occurring L-tartrate salt. This may result from the more favorable (stable) packing of both isomers within crystals (Brock et al., 1991). The slow conversion of the L form to the D form is a major factor provoking crystallization in bottled wine. Because clarification removes ‘seed’ crystals that promote crystallization, wine filtration should be delayed until calcium tartrate stability is no longer considered a hazard. Protective colloids such as soluble proteins and tannins can restrict crystal nucleation, but they do not inhibit crystal growth (Postel, 1983).

If protective colloids are a problem, agar may be added to the wine. Agar, an algal polysaccharide, tends to neutralize the charges on protective colloids. This eliminates their protective property, allowing colloid-tartrate complexes to dissociate. This favors both colloid precipitation and calcium tartrate crystallization.
Alternatively, calcium content may be directly reduced through ion exchange. Because of the efficiency of ion removal, typically only part of the wine needs to be treated. This proportion is then mixed back into the main volume. Treating only a small portion of the wine minimizes the flavor loss often associated with ion exchange.
Other treatments that show promise are the addition of stable colloids, such as pectic and alginic acids. They restrict crystallization and keep calcium tartrate in solution (Wucherpfennig et al., 1984).
Other Calcium Salt Instabilities
Occasionally, crystals of calcium oxalate form in wine. The development occurs late, commonly after bottling. The redox potential of most young wines stabilizes the complex formed between oxalic acid and metal ions, such as iron. However, as the redox potential rises during aging, ferrous oxalate changes into the unstable ferric form. After dissociation, oxalic acid may bond with calcium, forming calcium oxalate crystals.
Oxalic acid is commonly derived from grape must, but small amounts may originate from iron-induced structural changes in tartaric acid. Oxalic acid can be removed by blue fining early in maturation (Amerine et al., 1980), but avoiding the development of high calcium levels in the wine is preferable.
Other potentially troublesome sources of crystallization are saccharic and mucic acids. Both are produced by the pathogen Botrytis cinerea. They may form insoluble calcium salts. The addition of calcium carbonate for bitartrate stability often induces their crystallization, precipitation, and separation before bottling.
Protein Stabilization
Protein-induced haze is another significant concern for winemakers, potentially causing economic loss. Protein instability is primarily a concern with white wine, but occasionally can affect rosé wines. It is seldom a problem with red wines, presumably due to the precipitation of the causal proteins with tannins, and removal before bottling.
Haze (i.e., turbidity/clouding) results from the clumping of dissolved proteins into light-dispersing, colloidal particles. Heat exposure during transport accelerates colloid formation (Dufrechou et al., 2010), but it can develop at standard temperatures. This results as soluble proteins denature in the presence of polyphenolics, metals, and/or sulfates (Pocock et al., 2007). Denaturation appears to involve protein unfolding, followed by interaction with other constituents to form colloidal-sized aggregates. Nonetheless, a complete understanding of the causes of haze development remains elusive (Batista et al., 2009), possibly due to variation in secondary factors involved in different wines.
The majority of proteins suspended in wine have an isoelectric point (pI) above the pH range of wine. The isoelectric point is the pH at which a protein is electrically neutral. Consequently, most soluble proteins in wine possess a net positive charge, generated by the ionization of amino groups. This charge slows clumping, while Brownian movement and hydration (coating with water) delay settling. In contrast, denaturation favors coalescing, producing clouding.
The proteins primarily involved in haze production have only recently been identified. The situation was confusing because protein instability was poorly correlated with protein content. The principal proteins involved are pathogenesis-related (PR) protein (Waters et al., 1996b,c; Dambrouck et al., 2003), including thaumatin-like proteins and chitinases. Other proteins may also be present in the precipitate, for example β-(1-3)-glucanase and a ripening-related protein (grip22) precursor (Esteruelas et al., 2009a). In reconstruction experiments, chitinase was the most active protein provoking haze formation (Gazzola et al., 2012).
Pathogenesis-related proteins often constitute the majority of the small soluble proteins found in pressed juice and wine. Their occurrence typically ranges from 50 to 100 mg/liter, but can vary from 200 to 250 mg/liter in Muscat of Alexandria and Sauvignon blanc, to 62 mg/liter and 31 mg/liter for Pinot noir and Shiraz, respectively (Pocock et al., 2000). Mechanical harvesting, associated with prolonged transport to (or storage at) the winery, can activate increased production. The result can be a doubling of the amount of bentonite needed for stabilization (from 0.5 to 1.0 g/liter) (Pocock et al., 1998).
As the name pathogenesis-related proteins suggests, their principal role in grapes is to protect maturing fruit from infection. Nonetheless, their production in post-véraison healthy fruit suggests a possible additional developmental role (Tattersall et al., 2001). Despite being the principal causal agents of protein instability in wine, it is their acid stability, resistant to proteolytic action, and their minimal bonding with tannins that create the problem. Their stability permits them to survive through fermentation and maturation, denaturing slowly during prolonged in-bottle aging. It is this slow denaturation that produces the instability that can be so costly.
The proteins involved in protein instability are unrelated to those involved in haze production in beer (Siebert et al., 1996). In beer, proteins possessing a high proline content combine selectively with polyphenolics possessing two- or three-vincinal (adjacent) OH groups (Siebert and Lynn, 1998). Such complexes in wine would precipitate long before bottling.
Additional soluble proteins in wine, such as yeast mannoproteins and grape arabinogalactan–protein complexes may aggravate heat-induced protein haze, even though specific members in both groups can reduce protein-induced haze (Pellerin et al., 1994). Particularly interesting is the action of mannoproteins in reducing the size of haze particles to the threshold of human detection (Waters et al., 1993; Dupin et al., 2000b). Because the active glycoprotein fractions do not appear to be associated with the haze particles, they presumably have their action on secondary factors involved in aggregation (Dupin et al., 2000a). Although commercial mannoprotein preparations are available that limit protein-haze development, use of yeast strains releasing increased amounts of mannoproteins is an alternative solution (Dupin et al., 2000a,b).
A number of procedures have been developed to achieve protein stability. The most common involves the addition of bentonite (Fig. 8.6). It is typically added after fermentation as a fining agent. However, data from Pocock et al. (2011) suggest that two-stage addition (with and after fermentation) is more efficient, both in terms of total amount required and effectiveness.

Because of the abundance of cations associated with bentonite, extensive exchange of ions can occur with ionized protein amino groups. By weakening their association with water, the cations favor protein coalescence and precipitation. Flocculation and precipitation are further enhanced by adsorption onto the negatively charged plates of bentonite. Because of the variable amounts and ionizing potential of the different proteins involved, it is not surprising that the dynamics of their precipitation can vary considerably (Fig. 8.7).

Sodium bentonite is preferred because it separates more readily into individual silicate plates. This generates the largest surface area of any clay and, therefore, the greatest potential for cation exchange and protein adsorption. Regrettably, bentonite generates considerable sediment and associated wine loss. Although much of the wine lost can be recovered by vacuum rotary drum filtration, the oxidation potential associated with the process can reduce its quality. Despite these limitations, bentonite’s additional clarification properties (see below) still make it the preferred fining agent for protein stabilization.
Because haze-inducing proteins can be desorbed from bentonite by increasing the pH, there is interest in developing a continuous flow stabilization system. Bentonite immobilization would not only permit its regeneration, but also dramatically reduce the amount of wine lost with currently practiced batch fining. An alternate procedure being investigated is in-line dosing, and bentonite removal with centrifugation (Muhlack et al., 2006).
Other fining agents, such as tannins, are occasionally used in lieu of bentonite. However, the addition of tannins is often ill-advised. They can leave an off-odor and generate an astringent mouth-feel. Kieselsol, a colloidal suspension of silicon dioxide, has also occasionally been used to remove proteins. Another alternative procedure showing promise is zirconium dioxide (Marangon et al., 2011a). It is supplied enclosed in a metal cage to facilitate its removal at the end of treatment. The addition of carrageenan and pectin before fermentation is another alternative showing promise (Marangon et al., 2012). Ultrafiltration has been investigated as another alternative to bentonite or other types of fining (Hsu et al., 1987). It has the advantage of minimizing wine loss, and the need for a final polishing centrifugation or filtration.
Traditionally, protein stability and treatment need have been estimated empirically by treating wine samples to 80°C for 8 h. However, studies by Pocock and Waters (2006) suggest that 2 h may be fully adequate. In a comparison of various protein stabilization tests, Esteruelas et al. (2009b) recommend exposing samples to 90°C for 1 h in a water bath, followed by cooling to 4°C for 6 h in a refrigerator. Cooling is usually required for the rapid development of haze in treated samples. Its development also depends on the ionic strength and sulfite content of the wine, with thaumatin-like proteins and cutinases responding differently (Marangon et al., 2011b). Haze is either measured subjectively by eye, or objectively with a nephelometer or some other optical density devise. If demonstrated to be unstable, samples are treated and retested to determine whether further treatment is still required.
Polysaccharide Removal and Stability
Pectinaceous and other mucilaginous polysaccharides can cause difficulty with filtration, as well as provoke haze development. Polysaccharides can also act as protective colloids, binding with other suspended materials, slowing or preventing precipitation. For example, negatively charged pectins collect around positively charged grape solids. In addition, multiple hydrogen bonds formed between water and pectins help these complexes remain in suspension. During fermentation, alcohol production tends to disrupt hydration, provoking pectin precipitation.
Where concentration of pectins is high, the must is usually treated with pectinases. These contain a mixture of enzymes, including a pectin lyase. They split the pectin polymer into simpler, noncolloidal, galacturonic acid subunits. In so doing, positively charged areas of the pectin are exposed and can bind to the negatively charged surfaces of other colloids. As these complexes increase in mass, they tend to precipitate, becoming easier to remove during fining.
Other grape-derived polysaccharides, such as arabinans and galactans, have little effect on haze formation or filtration. Nonetheless, their degradation can be beneficial in producing denser lees. This reduces wine loss during racking. Like grape-derived polysaccharides, yeast-derived mannans have no detrimental effects relative to haze production or filtration.
The most vexing group of polysaccharides is the β-glucans. They are produced in, and easily extracted from, botrytized grapes. They can cause serious filtration problems, even at low concentrations (Fig. 8.8). This is especially serious in highly alcoholic wines, in which aggregation is enhanced. A Kieselsol–gelatin mixture is apparently effective in removing the mucilaginous polymers. Alternatively, the wine may be treated with a formulation of β-glucanases (Villettaz et al., 1984). The enzymes hydrolyze the polymers, eliminating both their protective colloidal property and filter-plugging action. To minimize extraction, noble-rotted grapes are manually harvested and slowly pressed without prior crushing.
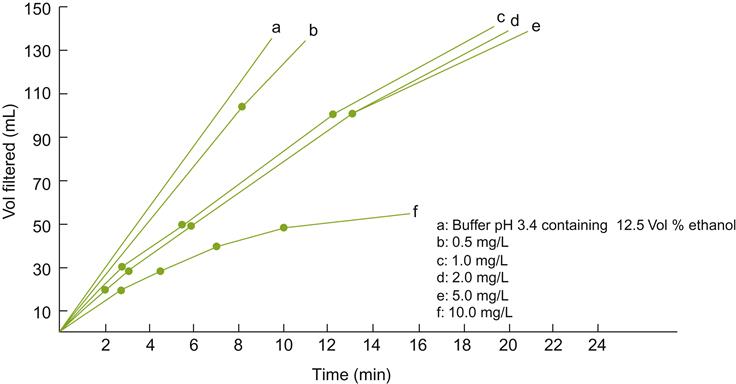
Tannin Removal and Oxidative Casse (Haziness)
Tannins may be both directly and indirectly involved in haze (casse) formation. After exposure to oxygen, catechins and other phenolics oxidize and may polymerize into brown, light-diffracting colloids, potentially causing oxidative casse. Shortly after crushing, as grape polyphenol oxidases are inactivated, oxidative reactions become slow and nonenzymatic. Depending on the timing and degree of oxidation, tannin oxidation can result in a loss of color intensity, a shift in hue, and an enhancement in long-term color stability. The addition of sulfur dioxide limits oxidation through its antioxidant and antienzymatic properties. However, wine from moldy fruit, contaminated with fungal polyphenol oxidases (laccases), is particularly susceptible to oxidative casse. Because laccases are poorly inactivated by sulfur dioxide, at permissible concentrations, pasteurization may be the only convenient means of protecting moldy juice from oxidative casse. Healthy grapes rarely develop oxidative casse. Because casse usually develops early during maturation and precipitates before bottling, it is seldom involved in in-bottle clouding.
Chilling wine to achieve bitartrate stability may provoke formation of a protein–tannin haze. Filtration before the wine warms removes these protein–tannin complexes before their dissociation, preventing their reformation post-bottling.
Removal of excess proanthocyanins and tannins is normally achieved by adding fining agents such as gelatin, egg albumin, isinglass, or casein. Because most are complex collections of proteins, with different amino acid compositions, or their denatured products, each has somewhat different effects, as do individual commercial versions (e.g., Maury et al., 2001; Cosme et al., 2009). Thus, it is only possible to provide general tendencies here.
These agents take effect through their positively charged sites associating with negatively charged sites on flavonoids. The interaction produces large protein– phenolic complexes. Their formation is a function of the balance between the potential binding sites of both the tannins and proteins. Excess in either tends to diminish binding. Once formed, the complexes may be removed by filtration or centrifugation, if early bottling is desired. Otherwise, adequate spontaneous sedimentation normally occurs during maturation. The removal of excess tannins reduces a major source of astringency, generates a smoother mouth-feel, reduces the likelihood of oxidative casse, and limits the accumulation of sediment following bottling.
In white wines, the addition of PVPP is a particularly effective means of removing flavonoids and their dimers. Ultrafiltration may also be used to remove excess proanthocyanidins and other polyphenolic compounds in white wines. Ultrafiltration is seldom used with red wines, due the simultaneous removal of important flavorants and anthocyanins.
Additional but infrequent sources of phenolic instability include oak chips or shavings, used to give an oaked character (Pocock et al., 1984), and the accidental incorporation of excessive amounts of leaf material in the grape crush (Somers and Ziemelis, 1985). Both can generate in-bottle precipitation, especially if the wine is bottled early. These problems can be avoided by permitting sufficient time for spontaneous precipitation. The instability associated with oak-chip use results from the overextraction of ellagic acid. The phenolic deposit produced consists of a fine precipitate of off-white to fawn-colored ellagic acid crystals. A flavonol haze in white wine, usually associated with excessive leaf material in the crush, is produced by the formation of fine, yellow, quercetin crystals (Somers and Ziemelis, 1985). Its occurrence, if suspected, can be reduced with PVPP (Laborde et al., 2006). An excessive use of sulfur dioxide has also been associated with cases of phenolic haze in red wines.
Many premium-quality red wines develop a tannin-based sediment during prolonged in-bottle aging. This potential source of haziness is typically not viewed as a fault. It develops only if the sediment is resuspended by turbulence generated by injudicious bottle handling or wine pouring. Individuals who customarily consume aged wines know its origin. They often consider sediment a quality indicator (incorrectly assuming that fining is inherently prejudicial to wine quality).
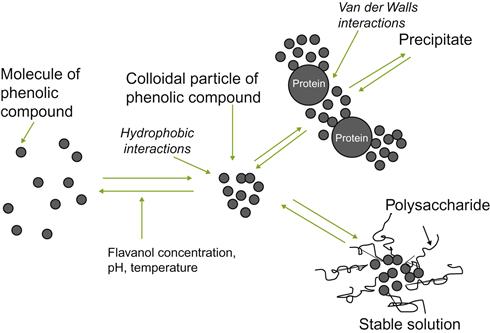
Some of the interactions in wine stabilization are illustrated in Fig. 8.9.
Metal Casse Stabilization
Several metals can form insoluble salts and induce additional forms of haziness. Although occurring much less frequently than in the past (largely due to the replacement of iron, copper, bronze, and brass fitting with stainless steel), metal casse occasionally still causes problems.
The most important metallic ions involved in casse formation are iron (Fe3+ and Fe2+) and copper (Cu2+ and Cu+). They may be derived from grapes, soil contaminants, fungicidal residues, or winery equipment. Most metallic ions so derived are lost during fermentation, by coprecipitation with yeast cells. Troublesome concentrations of metal contaminants usually are associated with pickup after vinification. Corroded stainless steel, improperly soldered joints, unprotected copper or bronze piping, and tap fixtures are the prime sources of any current contamination. Additional sources may be fining and decoloring agents, such as gelatin, isinglass, activated carbon, and cement cooperage.
Ferric (Iron) Casse
Two forms of ferric (iron) casse are known – white and blue (Fig. 8.10). Although uncommon, white casse occasionally develops in white wine. It occurs when soluble ferrous phosphate (FePO4) is oxidized to insoluble ferric phosphate (Fe3(PO4)2). The white haziness may be due solely to ferric phosphate, or to a complex between it and soluble proteins. In red wines, the oxidation of ferrous ions (Fe2+) to the ferric state (Fe3+) can result in the formation of a blue casse. Ferric ions form insoluble particles with anthocyanins and tannins. The oxidation of ferrous to ferric ions usually occurs when the wine is exposed to air. In an unstable wine, sufficient oxygen may be absorbed during bottling to induce clouding.
The development of ferric casse is dependent both on the wine’s metallic content and on its redox potential. Its occurrence is also affected by pH, temperature, and the concentration of certain acids. White casse forms only below pH 3.6 and is generally suppressed at cool temperatures. In contrast, blue casse is accentuated at cold temperatures. The frequency of white casse increases sharply as the iron concentration rises above 15–20 mg/liter. Recommended maximum amounts for iron in wine are in the range of≤4 mg/liter. Critical iron concentrations for the formation of blue casse have been more difficult to estimate. Its occurrence is markedly affected by the wine’s phosphate content and traces of copper (1 mg/liter). In addition, citric acid can chelate ferric and ferrous ions, reducing their effective (free) concentration in wine. Correspondingly, the addition of citric acid (~120 mg/liter) has been suggested as a means of limiting ferric casse development where it has been a problem (Amerine and Joslyn, 1970).
Wines may be directly stabilized against ferric casse by iron removal. For example, the addition of phytates, such as calcium phytate, selectively removes iron ions (Trela, 2010). EDTA (ethylenediaminetetraacetic acid), pectinic acid, and alginic acid can also be used to remove iron and copper ions. Removal with ferrocyanide is probably the most efficient method, as it precipitates most metal ions, including iron, copper, lead, zinc, and magnesium. The process is known as blue fining. Because of cyanide’s toxicity, blue fining is prohibited in many countries, and is strictly regulated where permitted. Filtration removes the insoluble metal–ferrocyanide complexes.
Ferric casse may also be controlled by the addition of agents that limit the flocculation of insoluble ferric complexes. Gum arabic acts in this manner. It functions as a protective colloid, restricting haze formation. Because gum arabic limits the clarification of colloidal material, it can only be safely applied after the wine has undergone all other stabilization procedures.
Copper Casse
Whereas iron casse develops on exposure to oxygen, copper casse forms solely in its absence. It develops only after bottling and is associated with a decrease in redox potential. Light exposure speeds the reduction of copper, critical in casse development. Sulfur dioxide is important, if not essential, for its development. In a series of incompletely understood reactions, involving the generation of hydrogen sulfide, cupric and cuprous sulfides form. The sulfides produce a fine, reddish-brown deposit, or they flocculate with proteins to form a reddish haze. Copper casse is particularly a problem with white wines, but can also cause haziness in rosé wines. Wines with copper contents greater than 0.5 mg/liter are particularly susceptible to copper casse development (Langhans and Schlotter, 1985). Values for copper in wine are generally recommended to be less than 0.2 mg/liter.
Masque
Occasionally, a deposit termed masque, forms on the inner surfaces of sparkling wine bottles. It results from the deposition of material formed by the interaction of albumin (used as a fining agent) and fatty acids (Maujean et al., 1978). Riddling and disgorging, used to eliminate yeast sediment, do not remove masque. Masque is a problem only with traditionally produced (méthode champenoise) sparkling wines, in which the wine is sold in the same bottle as that used for the second fermentation.
Lacquer-Like Bottle Deposits
In the 1990s, there was an increase in the worldwide incidence of a lacquer-like deposit in bottles of red wine. It appeared especially in higher-priced wines. The deposit developed within a few years, and could cover the entire inner surfaces of the bottle. It is not associated with a reduction in wine quality, but impeded sales due to the turbid perception.
The thin, film-like layer resulted from the deposition of a complex of tannins, anthocyanins and proteins (Waters et al., 1996a). The protein component was unexpected because the high tannin content of red wines has usually been thought to induce the protein precipitation before bottling. Although the mechanism of deposit formation is unknown, several factors can reduce its occurrence. These include the use of bentonite (>50 g/liter) and cold stabilization (−4°C for 5 days, followed by centrifugation at−4°C to remove insoluble material).
Microbial Stabilization
Microbial stability is not necessarily synonymous with microbial sterility. At bottling, wines may contain upwards of 103–104 viable cells/mL (Renouf et al., 2008). Under most situations, they provoke no stability or sensory problems. They remain metabolically inactive under in-bottle conditions.
The simplest procedure for conferring limited microbial stability is racking. Racking removes cells that have fallen out of the wine by flocculation, or have coprecipitated with tannins and proteins. The sediment includes both viable and nonviable microorganisms. The latter slowly undergo autolysis and release nutrients that could favor subsequent microbial growth. Microbial stability also relates to the wine’s basic chemistry, which is improved with minimal residual sugar (≤0.5 g/liter) and malic acid (≤30 mg/liter) contents, and low pH (≤3.3). Cool temperatures help maintain microbial viability, but retard or prevent metabolic action.
For long-term microbial stability, notably with sweet wines, the addition of antimicrobial compounds and sterilization may be required. The antimicrobial agent most frequently used is sulfur dioxide. It may be added at various times during wine production, but almost always after fermentation. Concentrations of 0.8–1.5 mg/liter (molecular) sulfur dioxide inhibit the growth of most yeasts and bacteria. Nevertheless, the precise amounts will depend on the temperature, pH, ethanol content, nutrient availability, concentration of sulfur-binding compounds, the microbial population, and the species and strains involved. For example, spoilage yeasts, such as Saccharomycodes ludwigii, Zygosaccharomyces bailii, and Brettanomyces spp., often require>3 ppm molecular SO2 for limiting the initiation of contamination (Thomas and Davenport, 1985). Current data indicate that standard levels of sulfur dioxide are inadequate to control acetic acid bacteria in wine (see Romano and Suzzi, 1993); thus, the importance of cool storage during maturation. The modern trend of lowering the concentration of sulfur dioxide can actually favor the growth of resistant strains (by eliminating competition with susceptible strains and species), accentuating future problems.
The free sulfur dioxide content should be determined about 24 h after addition – when an equilibrium has developed between the various free and bound forms of sulfur dioxide. The proportional molecular SO2 content can be estimated from the free sulfur dioxide content and the wine’s pH. Only molecular SO2 appears to be taken up by cells and, therefore, acts antimicrobially. Table 8.1 illustrates the amount of free sulfur dioxide required to have approximately 0.8 mg/liter available within the normal range of wine pH values. Figure 8.11 illustrates the relative toxicity of sulfur dioxide to several wine microbes, and the marked influence of pH on toxicity.
Table 8.1
Amount of free sulfur dioxide required to obtain a concentration of 0.8 ppm molecular SO2 as a function of pH
| pH | Free SO2 |
| 2.8 | 9.7 |
| 2.9 | 11 |
| 3.0 | 13 |
| 3.1 | 16 |
| 3.2 | 21 |
| 3.3 | 26 |
| 3.4 | 32 |
| 3.5 | 40 |
| 3.6 | 50 |
| 3.7 | 63 |
| 3.8 | 79 |
| 3.9 | 99 |
| 4.0 | 125 |
Source: Data from Smith (1982).

Although generally less effective than sulfur dioxide, sorbic acid (200 mg/L) is actively fungistatic against numerous yeasts. This natural fatty acid, usually supplied as a potassium salt, is food safe and frequently used in the nutrition industry. It is often added to enhance yeast control in sweet wines. Its effectiveness is largely limited to lower pH conditions, as typical of wine, where most of the compound exists in its toxic, undissociated state. Regrettably, it is relatively ineffective against Zygosaccharomyces or Brettanomyces (Rankine and Pilone, 1973), as well as bacteria. Thus, sulfur dioxide needs to be used to complement sorbate’s action. Because sorbate binds with SO2, as well as sugars, these factors must be taken into account in determining the appropriate amount of sulfur dioxide required. In addition, sulfur dioxide is required to protect against the potential conversion of sorbic acid to sorbyl alcohol by lactic acid bacteria. Esterification of sorbyl alcohol with ethanol generates 2-ethoxyhexa-3,4-diene, a compound with an intense geranium-like odor.
Benzoic acid and sodium benzoate were once employed as yeast inhibitors, but their general ineffectiveness and taste modification have eliminated their use.
Dimethyl dicarbonate (DMDC) may be used to sterilize wine (Costa et al., 2008), usually as a substitute for, or to reduce the addition of, sulfur dioxide. DMDC rapidly decomposes to carbon dioxide and methanol, neither leaving a residue nor modifying the wine’s sensory attributes (Calisto, 1990). Its action is little affected by pH. Unfortunately, its action against Saccharomyces cerevisiae and Oenococcus oeni, its poor solubility, and its corrosive action limit its more general application. In the absence of sulfur dioxide or DMDC, bottled wines can only be securely stabilized against microbial growth by physical means, namely, pasteurization and sterile filtration.
Pasteurization is the older of the two techniques. It has the advantage of promoting protein and copper casse stabilization, by denaturing and precipitating proteins. Although pasteurization may generate increased amounts of protective colloids, cause slight decolorization, and modify wine fragrance, it appears not to influence phenolic polymerization (Somers and Evans, 1986).
Wine pasteurization usually occurs for shorter periods or at lower temperatures than typical for products such as milk. This is possibly due to wine’s low pH and ethanol content, both of which markedly depresses the thermal resistance of yeasts and bacteria. Barillère et al. (1983) indicate that approximately 3 min at 60°C should be sufficient for a wine at 11% ethanol. Flash pasteurization at 80°C usually requires only a few seconds. Sulfur dioxide reduces still further the need for heating. High temperatures markedly increase the proportion of free SO2 in wine. Although pasteurization kills most microbes, it does not inactivate the endospores of Bacillus species. On rare occasions, these bacteria may induce wine spoilage.
Partially because of the complexities of establishing the most appropriate time and temperature conditions for pasteurization, membrane filters have replaced pasteurization in most situations. Filters also result in few physical or chemical disruptions to the sensory characteristics of wine. Membrane filters with a pore size of 0.45 μm or less are standard, although Renouf et al. (2008) recommend filters with pore sizes≤0.3 μm.
Wine sterilization also requires the simultaneous application of measures to avoid recontamination. This involves sterilizing all parts of the bottling line, as well as the use of sterile bottles and closures. Sulfur dioxide is commonly added before wines are pasteurized or sterile-filtered to confer protection against oxidation.
Oxidation Control/Regulation During Maturation (Micro-Oxygenation)
In most situations, exposure to air during maturation is avoided. Nevertheless, despite a winemaker’s best intentions, oxygen uptake does occur. Cellar activities such as tartrate stabilization, filtration, clarification, refrigeration, and bottle filling all potentially expose wine to air and oxygen uptake (Vidal et al., 2001; Castellari et al., 2004; Valade et al., 2006). With due precaution, oxygen uptake is usually no more than a few mg/L. Additional periodic oxygen uptake is associated with racking and barrel topping, as well as with slow, continuous diffusion through joints and barrel ends. The amount of oxygen absorbed during racking varies considerably. For example, measurements taken by Valade et al. (2006) varied, on average, from about 0.25–3 mg O2/liter, based on bottom vs. top filling of the cooperage. During in-barrel maturation most of the oxygen gains access where the head and bilge staves meet (Moutounet et al., 1998). Whether oxygen can permeate directly through barrel staves in significant amounts is a contentious issue, with little solid evidence either way.
Regardless of origin, limited oxygen uptake – either periodically or slowly over a protracted period – apparently results in no measurable oxygen buildup. The small amounts of oxygen inadvertently absorbed are consumed fairly rapidly, especially with red wines. Quinones are probably the principal oxidation by-product. Their subsequent polymerization and restructuring regenerate phenol groups capable of consuming additional oxygen (see Fig. 6.16). These reactions also favor color stabilization in red wines (by encouraging early anthocyanin–proanthocyanidin polymerization). In addition, flavonoid (tannin–tannin) complexes reduce the bitterness and astringency of tannins, donating a smoother mouth-feel. Furthermore, acetaldehyde, produced in the oxidation of ethanol with peroxide (a by-product of phenol oxidation), can combine with other wine constituents, notably sulfur dioxide, anthocyanins, and tannins. This limits acetaldehyde accumulation, avoiding its significant contribution to the development of an oxidized attribute in table wines.
During maturation, oxidation is limited by restricting air access, as well as by the addition of SO2. However, new knowledge related to the benefits of limited oxygen ingress for red wine maturation is changing long-held views. Although red wines may benefit from up to 60 mL O2 per liter (10 saturations), they show obvious deterioration with more than 25 saturations (Boulton et al., 1996). Correspondingly, there has been considerable interest in regulating oxygen uptake. This is termed micro-oxygenation (Dykes and Kilmartin, 2007). It has been of particular interest to producers who desire the perceived benefits of minimal oxygen uptake, but wish to avoid the oak flavors associated with maturation in oak cooperage. Micro-oxidation may also be used in the presence of oak chips or slats to achieve the attributes of maturation in barrels, without the expense and upkeep of the latter. The presence of oak chips or slats does not appear to alter the dynamics of oxygen uptake using micro- oxidation procedures (Laurie et al., 2008).
The relative value of micro-oxygenation appears to depend on the cultivar. It seems most applicable to varieties with ample catechin and proanthocyanin content, to form stable complexes with anthocyanins. Occasionally, though, the term has been used to refer to small amounts of oxygen supplied during fermentation and before in-barrel maturation (Sánchez-Iglesias et al., 2009). Micro-oxygenation has also been reported to reduce the incidence of reduced off-odors in the wine.
Micro-oxygenation may involve the use of high- density polyethylene cooperage (Flecknoe-Brown, 2005). These can be designed to have a relatively precise oxygen diffusion rate. Oxygen uptake directly through oak cooperage is thought to be negligible. As noted, whatever absorption occurs most likely occurs through the ends of the staves (Moutounet et al., 1998), or directly via the bung hole (if not tightly bunged). The rates most often quoted are estimates and could vary considerably, due to differences in barrel construction, wood porosity, wood thickness, and repeat use.
Because of complexities in determining actual rates of oxidation, as well as the levels of sulfur dioxide, hydrogen peroxide, and important aromatics during in-barrel maturation, studies are ongoing on using spectrophotometric techniques to automatically assay all their levels. Examples are the PreSens oxygen sensor (or bungs) fitted with fiber optics, associated with absorption and fluorescence spectroscopy (Plates 8.1 and 8.15).
Alternative means of micro-oxygenation involve silicone tube diffusers; silicone being about a thousand times more oxygen-permeable than other plastics. These are especially applicable for use in large cooperage. Oxygen ingress can be controlled by adjusting the length, thickness, and oxygen pressure in the tubing. Instruments, such as Microdue® or Parsec®, can also supply oxygen at various rates, via microporous diffusers. Castellari et al. (2004) consider that about 5 mL O2/liter/month (at 15–20°C) is roughly equivalent to barrel uptake, and lower than the rate of oxygen consumption by red wine. Typical rates for micro-oxidation are in the range of 5–10 mL O2/liter/month. Treatment may last for a few weeks to several months, depending on the cultivar, wine pH, and style desired (see Dykes and Kilmartin, 2007). The amounts of dissolved oxygen can vary considerably, depending largely on the rate of micro-oxygenation and temperature. The latter markedly affects oxygen solubility. Absorption values approximate about 10 times that of wine without micro-oxygenation (Laurie et al., 2008). An example of wine-dissolved oxygen content during micro-oxidation is given in Fig. 8.12.
 Median value; Box, 25–75 percentiles. (From Castellari et al., 2004, reproduced by permission.)
Median value; Box, 25–75 percentiles. (From Castellari et al., 2004, reproduced by permission.)Although often viewed as beneficial, frequent sampling is advised to reduce the likelihood of sudden sensory deterioration. An alternative to direct injection of oxygen involves electrochemical oxidation (Fell et al., 2007). Whether these new procedures adequately mimic the limited oxidation in barrels is a moot point (du Toit et al., 2006). What they clearly do offer is better regulation.
Despite the positive effect of limited oxygen ingress for red wines, it can favor the development of several types of hazes, notably ferric and oxidative casse. Oxygen uptake also reduces the concentration of free sulfur dioxide, resulting in its disassociation with acetaldehyde, and the redevelopment of color in bleached pigments. The combined effects of reduced sulfur dioxide content and the ingress of oxygen could also favor the reactivation of dormant spoilage microbes in wine. For example, micro-oxygenation may increase the incidence of Brettanomyces, and the presence of a barnyardy/medicinal attribute (du Toit et al., 2006). Although micro-oxygenation is unlikely to lead to vinegarization, the activation of acetic acid bacteria could give wine a sharp vinegary aspect. Thus, it is important that maturation occur at cool (12–16°C) temperatures, to limit the activity of spoilage yeasts and acetic acid bacteria (Vivas et al., 1995). Reduction in antimicrobial action of SO2 also means that sulfite application and timing need to be adjusted, but not to the degree that it inhibits the desired formation of color-stabilizing, flavonol-anthocyanin adducts (Tao et al., 2007). Nonetheless, with adequate winery hygiene and the use of inert cooperage, this is less of a concern.
Notably with white wine, sulfur dioxide is added for its joint antimicrobial and antioxidative properties after alcoholic (or malolactic) fermentation. Sulfur dioxide addition and concentration significantly influence oxidative browning (Fig. 8.13) and limit the participation of quinones in further oxidative reactions (by directly binding with quinones), as well as bleaching brown pigments. The addition of sulfur dioxide and air exclusion protect 3-mercaptohexanol and other important thiols from oxidative degradation (Blanchard et al., 2004; Nikolantonaki et al., 2010). Glutathione may also be added to aid in protecting against oxidative browning. In a comparative study of fining agents, casein, potassium caseinate, and isinglass were found to be especially effective at removing low-molecular-weight flavonoids, and thus reducing browning potential (Cosme et al., 2008).
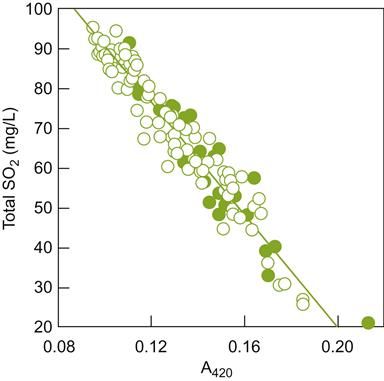
Fining
Fining is commonly used to accelerate the precipitation of suspended material. By binding to or adsorbing particulate matter, fining agents generate aggregates of sufficient mass to precipitate quickly. Removal can also be further accelerated by centrifugation or filtration. In addition to facilitating clarification, fining can help stabilize wines against haze formation (by precipitating the compounds involved in haze production), eliminate certain off-odors, and remove excessive amounts of bitter and astringent phenolics. Its effects on pesticide removal are often pesticide-specific, as well as significantly affected by pesticide solubility (Ruediger et al., 2004). Although fining may not completely remove undesired compounds, it facilitates negating their sensory impact.
Because fining is an aid to (not a replacement for) spontaneous stabilization, it should be used only to the extent necessary, and after clarification. It is important to avoid sensory disruption by minimizing unnecessary changes to the wine’s chemical and physical balance. Figure 8.14 illustrates the potential of several fining agents to produce flavor modification through the removal of aromatic compounds. Fining should also be conducted as quickly as possible to avoid unintended oxygen uptake.

Description of the various tests designed to determine the need for fining are beyond the scope of this book, but can be obtained from references such as Zoecklein et al. (1995). Regrettably, such trials often do not adequately reproduce conditions found under winery conditions. For example, mixing and settling are likely to be much more uniform and complete in a lab sample than how they occur in winery practice. Although laboratory trials may not be perfect, they yield predictive data not obtainable otherwise.
Activated Carbon (Charcoal)
Activated carbon is purified, powdered charcoal. It is treated physically or chemically to generate microfissures that vastly increase its adsorptive surface area. The large surface area (between 500 and 1500 m2/g) and electrical charge effectively adsorb a wide range of polar compounds, notably phenols and their derivatives. Activated carbon is used primarily to decolorize wine or remove off-odors. Different preparations are available for specific applications.
Decolorizing carbons are often employed to selectively remove flavonoid monomers and dimers. Larger polymers poorly penetrate the micropores of activated carbon (Singleton, 1967). Deodorizing carbons are valuable in removing mercaptan off-odors, but may also remove desirable flavor compounds. Activated carbon may also give the treated wine an atypical odor. Furthermore, activated carbon has an oxidizing property. Although this can be valuable, trials using small wine samples are vital to avoiding undesirable, unexpected effects. Typical doses vary from 2.5 to 50 g/hL.
Albumin
Albumin is the principal protein in egg white and is a classic fining agent. To improve its fining action, the egg whites are whisked with a small amount of salt and water. Currently, the active ingredient in egg white, albumin, is supplied in the form of a pure, dried powder. As albumin has a net positive change, it is added primarily to remove excessive bitter/astringent tannins from red wines. The peptide linkages of albumin form hydrogen bonds with hydroxyl groups on tannins. The opposing charges on the molecules favor the formation of large protein–tannin aggregates. These precipitate, or can be removed by, filtration.
One of the disadvantages of albumin is its relative solubility. If used to excess, it may donate a detectable meringue-like attribute to the treated wine. In addition, it may cause problems for those sensitive to egg products. It activates the release of histamine from mast cells possessing albumin-receptive IgE antibodies. The physiologic affects depend on the location of the mast cells; for example, in bronchioles contraction of smooth muscle provokes constriction.
Bentonite
Bentonite is a type of montmorillonite clay frequently used in clarifying juice and wines (both white and red). It is useful in removing unstable colloidal material from red wine, especially from grapes damaged by rough handling before or during destemming. Bentonite is also effective in removing heat-unstable proteins. Their positively charged sites associate with negative charged regions on the surfaces of bentonite plates. The resulting protein–clay complex flocculates and settles out. Because these effects partially depend on the wine’s pH, fining is delayed until after any intended blending is complete. Otherwise, a rise in pH of the blended product could reduce protein solubility and increase the potential for subsequent haze formation. Bentonite’s ability to remove proteins also depends on the presence of other cations in the wine and ethanol content (Blade and Boulton, 1988). Bentonite has little anion-exchange capacity. Thus, it is relatively ineffective in removing either neutral or negatively charged proteins. Thankfully, the latter have few, if any, significant sensory influences.
Through ion exchange, cations such as copper, potassium and zinc also tend to be removed. Thus, bentonite can be employed to prevent as well as treat copper casse. Depending on its intended use, bentonite’s tendency to induce partial decoloration and remove nutrients, such as amino acids, is either an additional benefit or a disadvantage. Bentonite is also used to promote yeast flocculation during the second, in-bottle fermentation of sparkling wines. In this instance, though, its addition must be used judiciously, to avoid removing important mannoproteins. The latter contribute significantly to the effervescent qualities of sparkling wines (Vanrell et al., 2007).
Together with other fining agents, such as tannins and casein, bentonite speeds the settling of particulate matter. It can also correct for the addition of excessive amounts of proteinaceous fining agents, by inducing their precipitation. Because bentonite settles out relatively quickly, and is easily filtered, it is one of the few fining agents that does not itself potentially create a stability or clarification problem. Bentonite also has, in comparison with other fining agents, a minimal effect on the sensory properties of the treated wine (Fig. 8.15).

The major drawbacks to bentonite use are color loss in red wines and a tendency to produce voluminous sediment. The latter can cause considerable wine loss during racking. Correspondingly, small-scale laboratory tests are usually conducted in advance to estimate the minimum quantity required to achieve the desired results. Details on such tests are given in Weiss et al. (2001). Such tests are prudent as different commercial bentonites can vary significantly in their effects on protein and aroma removal (Fig. 8.15). Nonetheless, Lambri et al. (2010) found little significant direct effect on aromatic constituents, most removal being limited to indirect effects due to flavorants binding to the proteins precipitated.
The bentonite often preferred in the United States is Wyoming bentonite. Its crystalline structure is based on multiple overlaying sheets, each consisting of a sandwich of two layers of tetrahedral silicon oxide and a central plate of octahedral aluminum hydroxide. Because its predominant cation is monovalent sodium, the particles swell readily in water and disperse into separate sheets of alumina-silicate. The crystalline sheets are about 1 nm thick and 500 nm wide, with each being about 100 Å apart (1 Å=10−10 m) when fully swollen in water. Their separation provides an immense contact area over which cation exchange, adsorption, and hydrogen bonding can occur (Gougeon et al., 2002). When fully expanded (after about 2–3 days in warm water), sodium bentonite has a surface area of about 700–800 m2/g. Swelling the bentonite before its addition to wine significantly improves its efficacy (Marchal et al., 2002).
Calcium bentonites are less commonly used. They tend to clump on swelling and thus provide less surface area for fining. Their sheets are separated by only about 10 Å after swelling. Nevertheless, they do have the advantage of producing a denser sediment, resulting is less wine loss during removal. Calcium bentonite also has the advantage of liberating fewer sodium ions, but has the disadvantage of being less efficient at removing proteins, nonetheless, this latter feature can occasionally be of value when used to promote yeast flocculation in sparkling wine production (avoiding undue removal of mannoproteins). Calcium bentonites may be ‘activated’ by exposure of a wet slurry to sodium carbonate at 80°C. This results in an exchange of sodium for calcium in the crystalline structure of the clay, donating properties similar to those of sodium bentonite.
Examples of the variability among the physicochemical attributes of several bentonite preparations can be found in Marchal et al. (1995). Part of their variation, besides their predominant cation (Na+ vs. Ca2+), may arise from other mineral constituents. These may include small amounts of quartz, chalcedony, feldspars, calcite, dolomite, analcime, and pyrite. In addition, some of the aluminum in the octahedral position may be replaced by ions, such as Mg2+, Fe2+, and Fe3+.
Most commercial bentonite preparations are modified before use. They may be treated with sulfuric acid or alkali, followed by adjustment of their hydrogen, sodium, or calcium contents. The treatment both removes undesirable mineral contaminants and adjusts their fining attributes.
Kieselsol
Kieselsol is an aqueous suspension of silicon dioxide. Because it is available in both positively and negatively charged forms, Kieselsol can be formulated to selectively adsorb and remove either positively and negatively charged colloidal materials. It is commonly used to remove bitter polyphenolic compounds from white wine. Combined with gelatin, it is effective in clarifying wines containing mucilaginous protective colloids, such as those found in botrytized wines. Kieselsol tends to produce a less voluminous sediment than bentonite, removes little color from red wines, and has no tendency to add taste. It is also useful where rapid fining is important due to the production schedule.
Casein
Casein is the major milk protein. In association with sodium or potassium ions, it forms a readily soluble caseinate. In wine, it dissociates and insoluble caseinate is released. This settles rapidly, and as it does, the casein fibrils adsorb and remove negatively charged particles. It is more effective in removing simpler flavonoids than more polymerized polymers. Casein finds its primary use as a decolorant in white wines. Its deodorizing potential can be either a blessing (off-odor removal) or a detriment (removing desirable aromatics). Because it is so insoluble at low pH values, it must be well mixed with water before addition, as well as after. This property also results in little residue, avoiding overfining. Nonetheless, minute but detectable levels of residues may remain, leading to the (unestablished) possibility of allergic reactions for those people particularly sensitive to milk proteins.
Gelatin
Gelatin is a soluble albumin-like protein derived from the prolonged boiling or enzymatic treatment of animal tissues (typically bones, skin, and tendons). The former process leads to larger protein complexes, whereas the latter yields smaller versions. Prolonged heating or enzymatic treatment results in the gelatin losing some of its gelling properties, but results in a more effective fining agent. Depending on the formulation, the modified proteins can range from about 15,000 Da up to 380,000 Da. The lower-molecular-weight fractions appear more selective in removing more polymerized tannins than the heavier fractions (Maury et al., 2001).
Gelatin formulations with more of a net positive charge are used primarily to remove excessive astringency, caused by condensed tannins. Gelatine addition has little effect on hydrolyzable tannins or simple flavonoid phenolics. Gelatin is usually added early in maturation. This avoids color loss that would be more pronounced if it were added later (due to the continuing polymerization of anthocyanins with tannins). When gelatin is added to white wine, there is a risk of leaving a gelatin-derived haze. This may be avoided by the simultaneous addition of flavorless tannins, Kieselsol, or other protein-binding agents. These materials favor the formation of a fine meshwork of gelatin fibers that removes tannins and other negatively charged particles. Excessive fining with gelatin can result in undesirable color loss in red wines.
Although the risks were minimal, wines fined with beef-derived gelatin in Europe were feared as a possible source of contamination with prions of Bovine Spongiform Encephalopathy (BSE, or mad-cow disease as it is commonly referred to). The internal bondings of this infectious protein are so remarkable that the rendering process used in producing gelatin does not inactivate the infectious agent. In the United States, most gelatin is derived from pig skins, a source free of BSE prions. Although the actual risk of gelatin use to human health is unknown, the possibility of any risk has prompted the study of substitutes made from plant proteins. Recent studies include Iturmendi et al. (2010) and Tschiersch et al. (2010). However, because of potential residues, if wheat gluten is used, due to potential allergic reactions mention of its use may become a label requirement (Simonato et al., 2011).
Gum Arabic
Gum arabic is a complex gel, consisting primarily of high-molecular-weight, hydroxyproline-rich, arabinogalactan-protein complexes. It is a natural gum, derived principally from the sap that exudes from cuts or holes in the bark of two species of Acacia, a tree native to West Africa. Its use in wine is limited, but it has been used successfully to treat ferric casse and stabilize the color of red wine. It can also be used to smooth out aggressive astringency and adjust a wine’s flavor profile (Obradovic and Hancock, 2010).
Isinglass
Isinglass is a gelatin derived from collagen-like proteins extracted from fish air bladders, notably sturgeon. Similar to most other proteinaceous fining agents, isinglass is primarily used to remove tannins. In addition, it has little tendency to add or remove aromatics. Because it is less subject to overfining, isinglass requires less added tannin than gelatin to function in fining white wine. Unfortunately, it produces a voluminous sediment that can plug filters.
Polyvinylpolypyrrolidone
Polyvinylpolypyrrolidone (PVPP) is a resinous polymer that acts similarly to proteinaceous fining agents. It is particularly useful in the selective removal of flavans and mono- and dimeric phenolics. As such, PVPP has particular value in diminishing undesirable bitterness. For this reason, it is usually added relatively early in maturation. It is also efficient in preventing oxidative browning and removing its brown by-products from white wines. It functions well at cool temperatures and precipitates spontaneously.
Some grades of PVPP can be isolated from the sediment, purified, and reused. Alternatively, PVPP may be bonded to a silica support, over or through which the wine is passed. Regrettably, PVPP, along with charcoal and casein, effectively removes resveratrol (Castellari et al., 1998), one of the wine components frequently credited with some of the health benefits associated with moderate wine consumption.
A different formulation of related resins – a copolymer of vinylimidazole and vinylpyrrolidone (PVI-PVP) – is used to remove excess metal ions from wine. It effectively removes copper, iron, lead, cadmium, and aluminum.
Tannin
The tannins used in fining are usually extracted from pulverized insect galls that develop on oak leaves. They are commonly combined with gelatin. The tannin–gelatin mixture forms a delicate meshwork that sweeps colloidal proteins out of wine. Tannins in the mesh join with soluble proteins to form both weak and strong chemical bonds. The weak bonds involve nonionized tannin carboxyl and hydroxyl groups, and hydrogen bonds in protein peptide linkages. Strong bonds involve covalent links between tannin quinone groups and protein amino and sulfur groups. The latter form stable links between soluble proteins and the tannin–gelatin meshwork.
Copper Sulfate
Copper sulfate (or occasionally silver salts) may be used to remove or prevent the accumulation of sulfur off-odors. Such treatment may become more frequent due to the increased adoption of screw cap (ROTE) closures. Screw caps are generally much more effective at excluding oxygen than cork or other closures, causing some to ascribe an apparent increase in reduced sulfur off-odors to their adoption. This potential, if real, may be countered by a variety of options (see the section on ‘Sulfur Off-odors’ below), including copper fining.
Copper fining involves adding up to 0.5 mg/liter of copper sulfate, at least 1 month before bottling. The copper reacts with hydrogen sulfide and various thiols (notably mercaptans). The odorless copper complexes precipitate readily, and are eliminated during racking or filtration. Residual levels of copper should not exceed 0.2 mg/liter (the usual legal limit). If disulfides are also a problem, sulfur dioxide and ascorbic acid (usually 50 mg/liter) may also be added. Sulfite generated from sulfur dioxide binds and eventually splits disulfides, generating thiols that can react with the copper (Bobet et al., 1990). Regrettably, the splitting of disulfides is slow. The ascorbic acid appears to act as an antioxidant, preventing the reoxidation of thiols to disulfides.
Clarification
In contrast to fining, clarification involves only physical means to remove suspended particulate matter. As such, various steps occur both before and after fining. With the juice from white grapes, however, fermentation is usually preceded by an initial clarification to improve flavor development. After fermentation, the first racking initiates clarification. It removes material that sediments spontaneously, thereby helping to prevent microbial spoilage. Subsequently, additional rackings and centrifugation or filtration remove finer material.
Racking
Until the twentieth century, racking and fining were the principal clarification methods available. Racking consisted primarily of manually decanting wine from storage vessel to storage vessel. Currently, it can still be as simple, but has evolved in large wineries to highly sophisticated, automated, tank-to-tank transfer systems. In all cases, decanting attempts to achieve minimal lees resuspension. It stops when unavoidable turbulence begins to noticeably cloud the wine. The residue is often filtered to retrieve wine otherwise lost with the lees. Racking is generally more effective in clarifying wine matured in small cooperage than in large tanks. This is due to the distance (time taken) for sedimentation, plus the development of highly reductive conditions in the deep lees layer at the bottom.
The first racking is conventionally done several weeks after alcoholic fermentation. If malolactic fermentation is desired, and has not already come to completion, racking is delayed. Racking may also be delayed to permit prolonged lees contact, such as in sur lies maturation.
By the first racking, most of the yeast, bacteria, and grape-cell fragments have settled out. Subsequent rackings remove most of the residual microbial population, along with precipitated tannins, pigments, and crystalline material. Later rackings separate the wine from sediment generated as a consequence of fining.
If sufficient time is available, racking and fining can produce stable, crystal clear wines. However, the trend to early bottling, a few weeks or months after fermentation, provides insufficient time for racking and spontaneous precipitation to generate adequate clarification. Consequently, centrifugation and filtration are often employed to achieve the necessary clarity and stability.
In addition to aiding clarification, racking plays several additional valuable roles in wine maturation. By removing microbial cells and other sources of nutrients, racking enhances microbial stability. The transfer process also disrupts stratification that may develop within the wine. This is particularly important in large storage tanks, in which stratification can lead to variations in redox potential and rates of maturation throughout the wine. Racking also removes the primary sources of reduced-sulfur taints, notably hydrogen sulfide and mercaptans. These may form under the low-redox conditions that develop in thick layers of lees. These benefits accrue not only from the separation of the wine from the lees, but also due to partial volatilization and oxidation of existant reduced sulfur compounds.
Additional benefits connected with racking- associated, incidental aeration involve the improved color stability of red wines. The value of such incidental aeration in white wine maturation is more controversial. Slight aeration is required with sur lies matured white wines, but is avoided otherwise. Where desired, oxygen exposure can be minimized with automatic pumping systems, using carbon dioxide or nitrogen as a blanketing gas. Because nitrogen is less dense than air (1.25 g/L vs 1.275 g/L at 0°C) (in contrast to CO2–1.98 g/L), its use in excluding air is largely limited to sealed cooperage.
The turbulence generated during pumping and filling helps liberate carbon dioxide that is present in a supersaturated state following fermentation. The escape is essential for wine to lose its slight petillance before bottling. If considered necessary, sulfur dioxide addition is usually timed to coincide with racking.
The number of rackings recommended varies considerably from region to region, depending on empirically established norms. Cooperage size is also a determining factor – the larger the cooperage, the more frequent the racking. This is necessary to avoid the development of a thick sediment layer conducive to off-odor production.
The method of racking depends largely on cooperage size, and the economics of manual versus mechanical transfer. Manual draining by gravity, or with a simple hand pump, is adequate where volumes tend to be small and labor costs low. Hand pumps for transferring wine from one barrel to another have been used at least since the late 1400s, when an illustration of this activity was produced in Nuremberg. For most large wineries, however, manual racking would be prohibitively expensive. Mechanical pumping (besides gravity feed) is the only reasonable option. Also, if aeration and sulfiting are deemed desirable, they can be controlled more precisely through mechanical rather than manual racking.
Centrifugation
Centrifugation employs rotation at high speed to expedite settling. It is equivalent to spontaneous sedimentation, but occurs within minutes, rather than months. It often replaces multiple rackings when early bottling is desired. Centrifugation is also useful when the wine is heavily laden with particulate matter. Highly turbid musts and wine are prone to off-odor development if they are permitted to clarify spontaneously. Centrifugation is much more efficient in removing large amounts of particulates than plate filters. Centrifugation also avoids potential health problems (dust and worker allergy) associated with the use and disposal of diatomaceous earth and other filter aids.
Blanketing the wine with an inert gas has minimized a former liability of centrifugation – oxidation. Automation, combined with continuous centrifugation, has improved the efficiency and economy of the process to such an extent that centrifugation is often the preferred clarification technique.
Filtration
When filtration is mentioned, it is usually in association with matured wine being prepared for bottling. However, it can also relate to reducing turbidity before wine maturation, and, thereby, the frequency and need for racking. This variation in procedure can influence the accumulation of volatile compounds during maturation. For example, the content of furanilic aldehydes and vanillin were higher in filtered wine, but were lower in γ-butryolactone and eugenol concentrations (Moreno and Azpilicueta, 2006). Changes in the development of esters in red wine were also noted (Moreno et al., 2007).
Filtration involves the physical retention of material on or within a fibrous or porous support. Depending on the pore size, filtration removes coarse particles with diameters larger than 100 μm down to molecules and ions with diameters less than 10−3 μm. However, the greater the retentive property, the greater the likelihood of plugging. As a consequence, filtration typically is preceded by preliminary clarification, using racking, fining, or centrifugation. This is especially important when employing membrane sterilization or ultrafiltration.
With the development of new filters and support systems, filtration now tends to be subdivided into four categories. Conventional filtration employs depth-type fibrous filters. These can remove particles down to about 1 μm in diameter. Other filtration techniques involve membranes containing crevices, channels, or pores. Depending on the size range of the perforations, the sieving action is termed microfiltration, ultrafiltration, reverse osmosis, or dialysis. Microfiltration and ultrafiltration usually are differentiated on the basis of their nominal (approximate minimal) pore size, 1.0−0.1 μm and 0.2−0.05 μm, respectively. Microfiltration is used primarily to remove fine particles and in sterilization. Ultrafiltration is employed to remove macromolecules and colloidal material. Reverse osmosis and dialysis are used to remove or concentrate low-molecular-weight molecules or ions. For example, they may be used to adjust alcohol content by direct extraction (or removing sugar before fermentation), as well as eliminate excessive amounts of acetic acid (Fig. 8.16) or off-odors (Ugarte et al., 2005). Dialysis involves the same principle (diffusion) as reverse osmosis, but does not use pressure to reverse the direction of flow. Electrodialysis uses the development of an electrical differential across the membrane to influence the flow of charged particles.

Filtration primarily acts by blocking the passage of material larger than the minimum pore size of the filter (Fig. 8.17). However, because material smaller than the smallest perforations may be retained by a filter, other principles may be involved. Surface adsorption by electrical attraction can be more important than physical blockage at the lower limit of filtration. Adsorption is generally important with depth filters, but less significant with membrane filters, where retention occurs principally only at the surface. Capillary forces may facilitate movement through filters. With depth filters, microbial growth in the filter can result in ‘grow through.’ Thus, it is essential that depth filters be frequently cleaned and sterilized, or replaced. Other complicating factors involve the potential for molecules to become hydrated (coated by a layer of water), or be enveloped by or bond to other molecules. Thus, measurement of the particle in terms of its molecular weight (Da) may considerably underestimate its diameter in terms of filter passage. The particle’s propensity to be deformed upon passage or inherent deviation from oval shape can become significant factors in permeability, especially under pressure. Thus, the rule of thumb of 500 Da equating to roughly 1 nm is exactly that, an approximation that may be considerably off from reality.
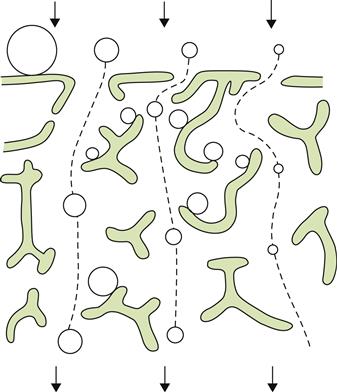
Filtration has often been avoided by premium winemakers, supposedly because of flavor loss. Although there is little objective data confirming this view, Ribeiro-Corréa et al. (1996) indicate that the contents of several flavorants are reduced. However, the sensory impact of these influences was not statistically detectable when evaluated by a sensory panel. In another study, the concentration of esters in filtered wines was lower after maturation in oak than in equivalent unfiltered wines (Fig. 8.18). The esters most affected were isoamyl acetate, ethyl butyrate, and ethyl hexanoate. Whether such changes were sensorially detectable was not investigated.
Depth Filters
Depth filters are composed of randomly overlapping fibers of relatively inert material. They may be purchased preformed (filter pads) or produced during the filtration process (filter beds). Most filters are cellulose-based, but may consist of glass fibers (see Fig. 8.22A).
Filter pads come in a broad range of porosities, permitting differential flow rates and selective particle removal. Tight filters remove smaller particles, but retain most of the material on the filter surface. Consequently, they tend to plug quickly. In contrast, loose filters retain most of the material within the tortuous channels of the pad, plug less quickly, but remove only larger particles. Tight filters are commonly used just before bottling for a final ‘polishing’ filtration.
Filter beds develop as a filter aid, suspended in the wine, is progressively deposited on an internal framework during filtration. Filter beds may be employed prior to polishing, sterilization, or ultrafiltration. The filter aid most commonly used has been diatomaceous earth. This consists of the silicaceous cell wall remains of countless generations of diatoms – microscopic, unicellular algae (Fig. 8.19). Depending on the filtration rate, and the particle size to be removed, different formulations of diatomaceous earths are available. Diatomaceous earth is added to the wine during filtration at about 1–1.5 g/liter. Loose cellulose fibers, treated to have a positive charge, may be added to facilitate the adsorption of colloidal materials. Perlite, the pulverized amorphous remains of heat-treated volcanic glass, has a very fine structure. It is occasionally used instead of diatomaceous earth.

Plates possessing a screen of cloth, plastic, or stainless steel are covered with a precoat of filter aid. These are inserted into the framework of a filter press (Fig. 8.20, Plate 8.2). The filter aid is continuously added and mixed with the wine being filtered. Pressure forces the wine through the filter bed. Porous metal or plastic sheets support the filter and provide channels through which the filtered wine escapes. During filtration, the depth of the filter bed grows. The continual addition of filter aid is essential for maintaining a high flow rate at low pressures. Without additional filter aid, the bed would soon plug. Use of higher pressures tends to compact the filter material, aggravating plugging. Choosing the correct grade of filter aid is essential. Particle size affects both flow and plugging rates and, consequently, filtration efficiency. After operation, filtration is temporarily halted to allow removal of the accumulated filter aid and retained material.

Because extensive exposure to the sharp, silicaceous nature of diatomaceous earth can cause eye and respiratory tract irritation (Cook et al., 2005), it is now considered a workplace hazard. Thus, its disposal has become complicated. Correspondingly, there is a shift to other filtering materials or procedures.
Filter beds are usually associated with plate-and-frame, recessed-plate, or leaf press construction. Plate-and-frame presses consist of alternating precoated plates and frames that provide space for cake development. Recessed-plate presses are similar, but each plate serves both plate and frame functions. However, filter beds can be constructed quite differently. The rotary vacuum drum is a prime example (Fig. 8.21). It consists of a large, perforated, cloth-covered, hollow drum. The drum is precoated with 5–10 cm of filter aid, usually diatomaceous earth. Part of the drum is immersed in the wine being filtered. The filter aid is added and kept uniformly dispersed as the wine is drawn through the filter bed into the drum. Shaving the accumulated filter aid and particulate matter off the drum occurs automatically as it rotates. Rotary vacuum drums work particularly well with wines that are highly charged with particulate matter or mucilaginous colloids. Other than high purchase and operation costs, the major drawback of rotary vacuum filtration has been the potential for wine oxidation. Aeration is difficult to limit because part of the drum is raised out of the wine during rotation. Nevertheless, a blanketing atmosphere, devoid of oxygen, could significantly limit the oxidation problem.
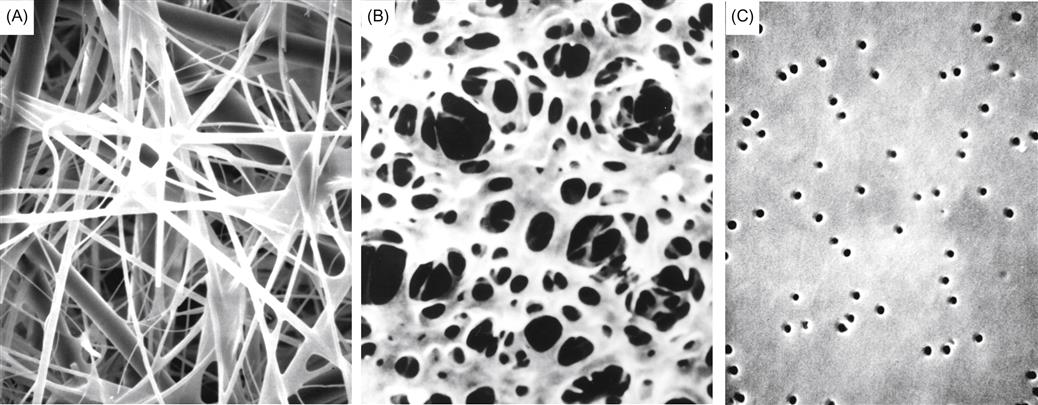
Because filter aids and pads can occasionally be a source of metal and calcium contamination, the material is commonly treated with a tartaric acid wash. In addition, the first sample of wine filtered is often kept aside, at least until its freedom from metal contamination, or earthy, paper-like odors has been assessed.
Membrane Filters
Membrane filters are constructed out of a wide range of synthetic materials, including cellulose acetate, cellulose nitrate (collodion), polyamide (nylon), polycarbonate, polypropylene, and polytetrafluoroethylene (Teflon) (Fig. 8.22B). With the exception of polycarbonate filters, most form a complex network of fine, interconnected channels. Polycarbonate (Nuclepore) filters contain cylindrical pores of uniform diameter that pass directly through the filter (Fig. 8.22C). Because polycarbonate filters have a small pore-surface area, they are seldom used for regular wine filtration. In comparison, most other membrane filters contain 50–85% filtering surface. Thus, they have an improved flow rate for the same cut-off point (rated pore size). A relatively new inorganic membrane filter, Anapore, possesses both uniform capillary pores and higher flow rate. Another introduction is a sintered, stainless-steel membrane that provides both an inert and extremely robust membrane system, combined with high flow rates.
Because of their small pore-diameter, membrane filters tend to have a slower flow rate than depth filters. They are also more likely to plug, because much, if not all, of the retention occurs at the surface. To circumvent rapid plugging, filter holders may direct the flow parallel to, rather than straight through, the filter (Fig. 8.23). This favors particles being swept along by the flow, significantly retarding plugging of the membrane. The parallel system is termed tangential or cross-flow filtration. The conventional, perpendicular flow is termed dead-end filtration. Membrane polarity is particularly important to the adherence of polyphenolics to, and in, the pores of the membrane (Vernhet and Moutounet, 2002). Premature fouling of the membrane, and the resulting reduced permeate flow, is one of the primary factors limiting the more extensive use of tangential membrane filters. Short, periodic back-flushing, back-pulsing, ultrasound, or an external electric field are among techniques that can greatly increase the functional life of filters, and minimize flux variation. Recent studies on the sources of plugging (Boissier et al., 2008) are permitting the life of filters to be extended, as well as improving permeate flow rate.
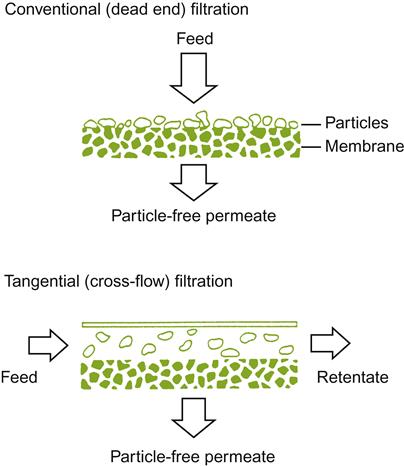
Cross-flow filtration has several distinct advantages over dead-end versions. It is automated, and can both effectively and quickly reduce turbidity to a desirable range (<2 NTU, nephelometric turbidity units), as well as sterilize wine. Thus, it can simultaneously eliminate several separate filtration steps, as well as reduce water consumption and effluent production. Further details on use can be found in El Rayess et al. (2011).
Advanced forms of tangential filtration often employ cylinders of complex internal structure. The external portion, exposed to the wine, contains pores that become progressively smaller toward the center. The central region contains pores of constant diameter, to assure the retention of particles or molecules above a certain size or molecular weight. Cartridge filters contain some of the nonplugging features of depth filters and offer the particle-size retention characteristics of conventional membrane filters. These polypropylene filters are resistant to most chemical reagents. This allows them to be cleansed and used repeatedly. Such developments may reduce, if not eliminate, the need to conduct filterability tests prior to sterile filtration. Filterability tests are discussed in Peleg et al. (1979) and de la Garza and Boulton (1984).
Microfiltration is extensively used to sterilize wines and avoids the flavor modification occasionally associated with pasteurization. The cut-off pore size for sterile filtration has traditionally been 0.45 μm, but is ideally between 0.3 and 0.2 μm, due to variation in the actual minimum pore size and to avoid ‘grow through.’
Ultrafiltration (Fig. 8.24) has been used to a limited extent in protein stabilization. Although effective, it removes most colloidal material. Thus, ultrafiltration can remove important pigments, tannins, and mannoproteins (Fig. 8.25). Its use with white wines appears not to produce unacceptable flavor loss (Flores et al., 1991).


Aging
The tendency to improve, or at least change, during aging is one of wine’s most beguiling properties. Regrettably, most wines improve only for a few years before showing irreversible loss in sensory pleasure. In contrast, red wines produced from varieties such as Cabernet Sauvignon, Shiraz, Tempranillo, Nebbiolo, and Pinot noir, may continue to improve in flavor and subtlety for years to decades. White wines produced from varieties such as Riesling, Chardonnay, Sauvignon blanc, and Viura also show excellent aging potential.
Quality loss is commonly explained as a dissipation of its young, fresh, fruity bouquet (Fig. 8.26), along with any aroma donated by the grape variety. Wines noted for their aging potential typically show similar aromatic losses, but these are replaced by what is termed an aged bouquet. These changes are considered desirable when the aged bouquet, subtle modifications in flavor, and development of a smoother texture (in red wines) more than compensate for the wine’s fading varietal and fruity character.
Recognition of the loss of the young bouquet, and its replacement in better wines by a more delicate and complex fragrance and flavor, must have been noted early on, explaining the wondrous salutation bestowed on aged wines by numerous Roman authors. Precise written descriptions in terms now clear are, however, uncommon; one of the earliest being those recorded by Count Haraszthy on his visit to Kloster Erbach, Germany in 1861 (Haraszthy, 1862). Nonetheless, the fact that wines were being ‘dissected’ in sensory detail is noted by Eiximenis (1384).
Only since the early 1980s have sufficiently precise analytical tools become available to begin unraveling the chemical mysteries of wine aging. However, as aging has been studied in only a few varietal wines, caution must be exercised in generalizing from these findings. More is known about why most wines decline in sensory interest than why some retain or improve in character for decades.
Knowledge of how wines age, and how this process might be influenced, is important to all involved or interested in wine. At the very least, sensory deterioration adversely affects the shelf-life and the financial return to the producer. On the other hand, the prestige associated with long aging potential adds greatly to the desirability and appeal of premium wines. It also permits consumers to actively participate in the aging process, through the conditions and duration of storage they provide/permit. Because the factors affecting aging are poorly understood, a mystique has built up around vineyards and varieties associated with wines that are perceived or thought to improve with age.
Aging is occasionally considered to possess two phases. The first, called maturation, refers to changes that occur between alcoholic fermentation and bottling. Although maturation frequently lasts from 6 to 24 months, it may continue for decades. During maturation, the wine may undergo malolactic fermentation, be stored in oak cooperage, be racked, and treated to one or more filtration and/or clarification procedures. During in-barrel maturation, racking, and clarification, wines may absorb about 40 mL O2/year. Although insufficient to give the wine a noticeably oxidized character (at least in the short term), it is frequently viewed as beneficial to the color stability of red wines. Only in some fortified wines is obvious oxidation an important and critical component of maturation.
The second phase of aging commences with bottling. Because this stage occurs largely in the absence of oxygen, it has been called reductive aging. This contrasts with oxidative and biological aging, terms used to describe the maturation of some fortified wines and sherries, respectively.
Effects of Aging
Age-related chemical changes have long been recognized. Initially, these modifications are viewed favorably. They result in the dissipation of the yeasty aspect and spritzy character of newly fermented wines, loss of turbidity, improvements in microbial stability, and improved color stability in red wines. Subsequently, and usually post-bottling, there is a progressive loss in the wine’s fresh fruity bouquet. If this is associated with the development of an appreciated aged bouquet and smoother mouth-feel, the consequences of aging are highly desirable. To encourage these latter processes, most wine connoisseurs store their wine in cool cellars for years to decades. Regrettably, most wines do not age particularly well. Most white wines are recommended to be consumed within a few years of production. Most red wines improve or retain their flavor for little more than 5–10 years. In reality, though, these views reflect professional opinion. It is often thought that most consumers prefer the fresh fruity character of young wines versus the more general, subtle aspects of an aged bouquet. However, this may simply reflect their disinterest in storing wine for extended periods, or their acceptance of (or insensitivity or indifference to) the rough astringency of many young red wines.
Nonenzymatic oxidative reactions produce significant sensory changes during aging. This involves the transfer of an electron (or hydrogen atom) from the oxidized compound to oxygen, or another acceptor. In bottled wine, reactions involving molecular oxygen are limited to what little diffuses into the bottle via the cork, or between the cork and the neck. Temperature, pH, and the phenolic content significantly affect a wine’s oxidative potential. Other oxidative reactions (not directly involving molecular oxygen) occur during wine aging. Their influence on wine fragrance and taste are little known. The presence of iron and copper ions are the best-known wine oxidative catalysts (Danielewicz, 2011). Because the redox potential of wine changes post-bottling, the relative importance and types of oxidative/reductive reactions occurring undoubtedly vary over time.
As with other aspects of wine chemistry, determining the sensory significance of these changes is more difficult than detecting them. To establish significance, it is necessary to show that the changes detectably impact sensory perception. Because most chemicals occur at concentrations below their sensory threshold, most modifications are unlikely to affect either wine flavor or the development of an aged bouquet.
Appearance
One of the more obvious consequences of wine aging relates to color change. Initially, red wines may deepen in color, but their intensity slowly fades as the tint takes on a ruby and then a brickish hue. These shifts result from the disruption of self-association and copigment anthocyanin complexes (typical of young wines), the progressive formation of new pigments (e.g., pyranoanthocyanins, catechinpyrylium, and xanthylium pigments), and the polymerization of proanthocyanidins and anthocyanins into tannin–tannin and anthocyanin–tannin complexes. These alterations were initially predicted from a decline in optical density and a shift in the absorption spectrum (Fig. 8.27). They have subsequently been confirmed by various methods (Peng et al., 2002; Remy et al., 2000), including radioactive isotope analysis (Zimman and Waterhouse, 2004). The degree of polymerization is typically measured with a diluted sample placed in a cuvette and its optical density measured at 520 and 420 nm (Somers and Evans, 1977). High 520/420 nm ratios indicate a bright-red color, whereas low values indicate a shift to brickish hues. In contrast, the transition from almost colorless to yellow, gold, and finally brownish shades in white wines has usually been assessed by measuring the optical density at 420 nm. Skouroumounis et al. (2003) have developed a spectrophotometric technique applicable to unopened wine. This offers the possibility that samples showing premature browning may be detected, and removed, before reaching the consumer.

