Specific and Distinctive Wine Styles
Although wine has been made for millennia, most current styles are of comparative recent origin, some having no ancient equivalent. Sweet wines were produced in the past, but largely by techniques little used today. Modern versions include those based on juice concentrated biologically (botrytized wines) or by physical processes (freezing, desiccation, or heating), or sweetened by the addition of a süssreserve. Unique red table wines examined include those involving drying (appassimento and occasionally botrytized), refermentation (governo), and carbonic maceration (e.g., Beaujolais). Production of the various types of sparkling wines is reviewed, principally those employing the traditional (méthode champagnoise) process. Also discussed is an account of the physico-chemistry of effervescence and mousse development. Subsequently, details of the production and attributes of the major stylistic forms of fortified wines are provided, notably sherries, ports, madeiras, and vermouths. This is followed by an exploration of the types, production, and characteristics of brandies.
Keywords
Sweet wines; botrytized wines; icewines; vin santo; appassimento; amarone; governo process; carbonic maceration; champagnes; effervescence; fortified wines; sherry; port; madeira; vermouth; brandies
Wine has been produced for millennia, but many modern styles have no ancient equivalent. Wine styles often reflect the unique climatic and politico-socio-economic environment under which they arose. For example, botrytized wines emerged in regions favoring the selective development of noble-rot; sparkling wine evolved under atypically cold conditions unsuitable for standard red wine production in Champagne; and port arose out of expanded trade between Portugal and England, due to conflicts and trade restrictions with France. Some of these wine styles, such as those noted, have spread throughout the world. Others have remained local specialties, if not idiosyncrasies. This chapter covers some of the more important, interesting, and unique wine styles, and their main variants.
Sweet Table Wines
Sweet table wines encompass a wide diversity of styles, possessing little in common other than sweetness. They may be white, rosé, or red; still or sparkling; and may range from aromatically simple to complexly fragrant. The most famous are those made from noble-rotted (Botrytis-infected) grapes.
Botrytized Wines
Wines made from grapes partially infected by Botrytis cinerea have probably been made for centuries. The fungus is ubiquitous. Normally, it induces a destructive bunch rot (see Chapter 4). If significant numbers of infected grapes are included in the crush, the wine made from them is unpalatable. The wine has a brownish color, shows high volatile acidity, is difficult to clarify, and possesses multiple, unpleasant, moldy odors. Nevertheless, under very specific, unique, climatic conditions, infection is restricted, generating what is termed ‘noble’ rot. These grapes beget some of the most seraphic white wines that have ever been produced. This is despite my having studied Botrytis much of my life.
When noble-rotted grapes were first intentionally used for wine production is unknown. Historical evidence favors the Tokaj region of Hungary, in the mid-sixteenth century (ca. 1560). There is documentary evidence that wine, made specifically from botrytized (aszú) grapes, was of sufficient worth to be mentioned by name in a will, dated 1571 (Fig. 9.1). The commercial renown of this special Tokaji wine was already well established by the early 1600 s (Zimányi, 1987). Documentary evidence, denoting the production of botrytized wine at Schloss Johannisberg, Germany in 1775, and possibly earlier elsewhere, is alluded to in Johnson (1989). When the deliberate production of botrytized wines began in France is uncertain, but production appears to have been well established in Sauternes between 1830 and 1850. Isolated production of botrytized wines occurs throughout Europe, wherever conditions are favorable for noble rot development. The idea of using Botrytis-infected grapes for wine production has been slow to catch on in the New World, but botrytized wines are now produced to a limited degree in Australia, Canada, New Zealand, South Africa, and the United States.

Infection
Early in the season, most infections develop from spores produced on overwintered fungal tissue, either in tissue remains or resting structures called sclerotia (Fig. 4.55). Infections begin on aborted and senescing flower parts, notably the stamens and petals. Infected flower parts, entrapped within the developing fruit cluster, may initiate infection later on in the season. Although early fruit infections usually cease as the hyphae penetrate the young berry, the hyphal cells remains viable. Reactivation of these latent infections is particularly important under dry autumnal conditions. As the fruit reaches maturity, resistance to fungal growth declines. This most likely involves the reduction in acidity, as the grapes ripen, and a decline in antifungal phenolic content. Under moist conditions, though, new infections are thought to be more important than latent infections.
Disease susceptibility, and its direction (bunch vs. noble rot), depend on several factors. Skin toughness and open fruit clusters reduce disease incidence, whereas rain, protracted moist periods, and shallow rooting increase susceptibility. The latter encourages rapid water uptake and berry splitting, both favoring bunch-rot development. In contrast, noble rot develops late in the season, under a protracted sequence of fluctuation moist/dry conditions. This typically involves cool, still nights, in vineyards adjacent to warm water. Fog development during the evening and/or early morning favors fungal growth. If this is sequentially followed by dry, sunny days, berries begin to dehydrate, slowing and severely restricting fungal growth. Repeated cycles of activation/repression result in the progressive concentration as well as modification of berry content.
Depending on the temperature and humidity, spores (conidia) are produced on specialized, branched hyphae (conidiophores) that erupt through the berry skin. The shape of the spore clusters (Fig. 9.2) so resembles grape clusters that the botanical name for the fungus is derived from the Greek word meaning ‘grape cluster’ – βοτσυς. Because the microclimate of the fruit cluster markedly affects fungal development, various stages of healthy, noble-, and bunch-rotted grapes may frequently occur within the same cluster (Plate 9.1).

During infection, several hydrolytic enzymes are released by Botrytis. Particularly destructive are the pectolytic enzymes. They degrade the components that hold plant cells together. The enzymes also incite disruption, resulting in the collapse and death of adjacent tissue. With loss of physiological control, the fruit can easily begin to dehydrate under dry conditions. Disruption of the vascular connections between the pedicel and the fruit, as ripening advances, means that moisture lost via evaporation is not replaced from the vine. Additional water may be lost by evaporation through conidiophores, which can act as wicks.
The resultant dehydration is a crucial factor in determining disease development. Drying retards fungal growth and appears to modify its metabolism. This may result from the combined influences of the increasing osmolarity and acidity of the juice. Juice concentration is also a feature crucial in the development of the wine’s sensory properties. Finally, water loss limits secondary invasion by saprophytic bacteria and fungi. Invasion by fungi, including Penicillium, Aspergillus, and Mucor, probably generates most of the moldy off-odors and tastes associated with bunch rot. For example, P. frequentans produces plastic and moldy off-odors, via the synthesis of styrene (Jouret et al., 1972) and 1-octen-3-ol (Kaminiski et al., 1974), respectively. Saprophytic fungi are commonly present during the development of Botrytis bunch rot, but almost completely absent during noble-rotting. In addition, the phenolic flavors, commonly associated with wines made from botrytized red grapes, is undetectable in botrytized white wines. This may result from pressing being conducted without prior crushing, and the lower phenolic content of white grapes. Despite this, noble rot is associated with a color change in the grape skins. White grapes take on a pink to purplish coloration (Plate 9.1). This presumably results from pigment formation as leucoanthocyanins (flavan-3,4-ols) oxidize.
One of the typical chemical indicators of Botrytis infection is the presence of gluconic acid. Although B. cinerea produces gluconic acid, the acetic acid bacterium, Gluconobacter oxydans, is even more active in this regard. Because they frequently invade grapes infected by Botrytis, they are probably responsible for most of the gluconic acid found in diseased grapes (Sponholz and Dittrich, 1985). The bacteria also produce acetic acid and ethyl acetate. Thus, their action may be partially responsible for the elevated concentrations of these compounds in some botrytized wines. The elevated sugar content of the juice is also known to accentuate the production of volatile acidity and ethyl acetate. Inoculation with Torulaspora delbrueckii has been suggested to reduce the likelihood of this potential fault in botrytized wines (Renault et al., 2009).
Chemical Changes Induced in Association with Noble Rotting
Berry dehydration, combined with the metabolic action of Botrytis, are the principal causes of the sensorial influences of noble rotting. Some of the effects of drying simply have concentrating effects, such as the increase in the citric acid content (Table 9.1). With other compounds, fungal metabolism is sufficiently active to result in a decrease, despite the concentrating effect of water loss. This is particularly noticeable with tartaric acid and ammonia (Table 9.1). The selective metabolism of tartaric acid, versus malic acid, is crucial in avoiding a marked decline in pH. In addition, the enhanced acidity, associated with the relative increase in malic acid content, counteracts the cloying effect of the wine’s high, residual sugar content.
Table 9.1
Comparison of juice from healthy and Botrytis-infected grapes
| Component | Sauvignon berries | Sémillon berries | ||
| Healthy | Infected | Healthy | Infected | |
| Fresh weight/100 berries (g) | 225 | 112 | 202 | 98 |
| Sugar content (g/liter) | 281 | 326 | 247 | 317 |
| Acidity (g/liter) | 5.4 | 5.5 | 6.0 | 5.5 |
| Tartaric acid (g/liter) | 5.2 | 1.9 | 5.3 | 2.5 |
| Malic acid (g/liter) | 4.9 | 7.4 | 5.4 | 7.8 |
| Citric acid (g/liter) | 0.3 | 0.5 | 0.26 | 0.34 |
| Gluconic acid (g/liter) | 0 | 1.2 | 0 | 2.1 |
| Ammonia (mg/liter) | 49 | 7 | 165 | 25 |
| pH | 3.4 | 3.5 | 3.3 | 3.6 |
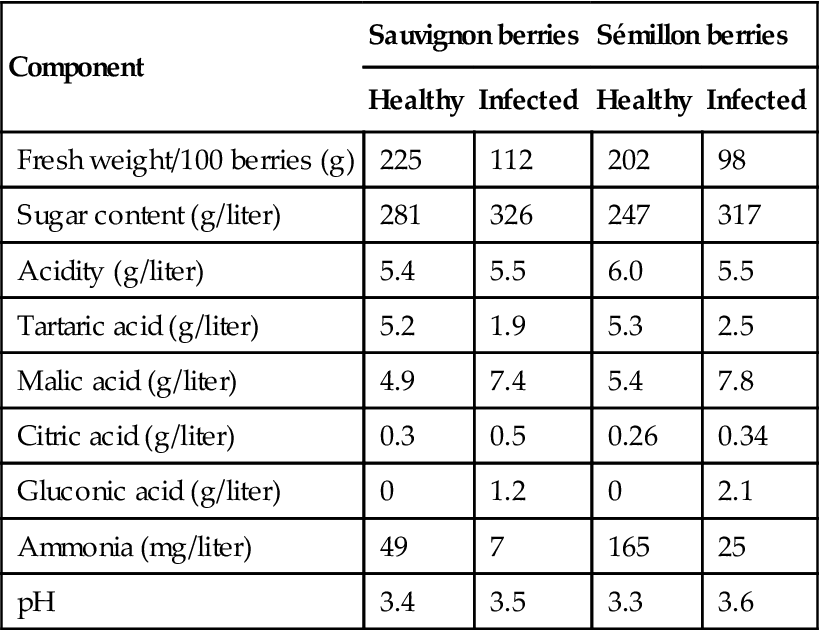
Source: After Charpentié (1954) from Ribéreau-Gayon et al., 1980, reproduced by permission.
One of the most notable changes during noble rotting is the increase in sugar content. This occurs in spite of the metabolism of up to 35–45% of the original grape sugars. With Brix levels reaching up to 60 or above, there is a corresponding decline in osmotic potential. This may explain the inability of B. cinerea to metabolize a higher proportion of sugars, and other metabolic disturbances during infection (Sudraud, 1981). Occasionally, selective glucose metabolism is reflected in an atypically high fructose/glucose ratio. The breakdown of pectins and grape polysaccharides also results in the accumulation of sugars, such as arabinose, galactose, mannose, rhamnose, xylose, and the sugar acid, galacturonic acid.
Production of glycerol during infection and fermentation, combined with the concentrating effect of berry dehydration, can result in values reaching or exceeding 30 g/L. At such levels, it could augment the smooth mouth-feel of the wine. This potential may be enhanced by the simultaneous synthesis and concentration of other polyols, such as arabitol, erythritol, myo-inositol, sorbitol, xylitol, and, in particular, mannitol (Magyar, 2011).
A distinctive feature of noble rotting is a general loss in varietal aroma. This is particularly noticeable with Muscat cultivars. Aroma impairment is explained largely by the degradation of terpenes that give these varieties their distinctive fragrance. Examples are the metabolism of linalool, geraniol, and nerol to less volatile compounds, such as β-pinene, α-terpineol, and various furan and pyran oxides (Bock et al., 1986, 1988). These may be involved in the phenolic and iodine-like odors reported in some botrytized wines (Boidron, 1978). Botrytis also produces esterases that can degrade fruit esters, that give many young white wines their fruity character (Dubourdieu et al., 1983). The significance of these effects depends on their relative importance to wine fragrance. Muscat varieties often lose more character than they gain from Botrytis, whereas Riesling and Sémillon generally gain more aromatic complexity than they lose in varietal distinctiveness. Another potential flavorant, seemingly destroyed by B. cinerea, is 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) (Sponholz and Hühn, 1996). At subthreshold levels, it contributes to an aged bouquet, but above about 20 μg/L, it can donate a kerosene-like odor.
An exception to the typical loss of varietal character applies to some thiols, notably those generated in cultivars such as Sauvignon blanc. In this instance, the concentrations of S-3-(hexanol-1-ol)-L-cysteine (Cys-3MH) in grapes, and 3-mercaptohexanol (3MH) in wines, increases in association with botrytization (Thibon et al., 2009). Additional thiols, derived from precursors formed during infection, are liberated as a consequence of fermentation (Thibon et al., 2010). Some of those present in young sauternes, possessing roasted or citrus resemblances, are undetectable after several years (Bailly et al., 2009).
In addition to the frequent reduction in varietal distinctiveness, Botrytis provides its own special attributes. One of the more significant flavorants appears to be sotolon. In combination with other aromatics, found or produced in botrytized wines, sotolon was considered to contribute to the honey-like fragrance of many botrytized wines (Masuda et al., 1984). Nonetheless, no correlation between the degree of botrytization and sotolon content has been detected (Sponholz and Hühn, 1993). Infected grapes also contain the mushroom alcohol, 1-octen-3-ol. Its relevance to the fragrance of botrytized wines appears in doubt, though, as it can be converted by Saccharomyces cerevisiae to a less aromatic ketone, 1-octen-3-one (Darriet et al., 2002). Among additional compounds found are terpene derivatives. More than 20 terpenes have been isolated from infected grapes (Bock et al., 1985). Additional compounds, typical of botrytized sauternes, have been identified by Sarrazin et al. (2007). Some of these include homofuraneol, theaspirane, γ-decalactone and abhexon (Bailly et al., 2009). During aging theaspirane accumulates. It appears to be associated with an apricot odor, a characteristic attribute of botrytized wines.
Infection by Botrytis cinerea not only affects the wine’s taste and fragrance, but it also restricts harvesting options. By disrupting skin integrity, rupture is facilitated. Correspondingly, harvesting is done manually. Surprisingly, infection retards separation of the berry from the pedicel (Fregoni et al., 1986).
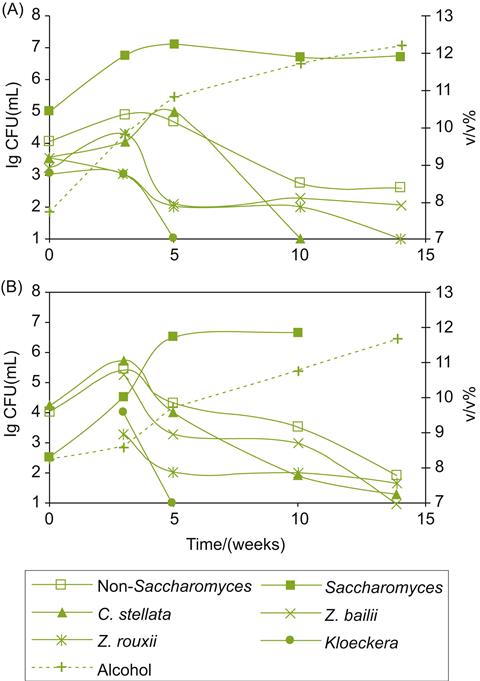
Infection by Botrytis affects the activity of other microorganisms on and in grapes and juice before, during (Fig. 9.3), and after fermentation. In the vineyard, infection facilitates secondary invasion by acetic acid bacteria and several saprophytic fungi. Infection also affects the epiphytic yeast flora, both increasing their numbers and modifying species composition. For example, Candida stellata, Torulaspora delbrueckii, and Saccharomyces bayanus may dominate the yeast flora of infected grapes. In tokaji, Candida pulcherrima may be the dominant yeast on botrytized grapes. This shifts to C. stellata after collection, transport to, and storage at the winery (Bene and Magyar, 2004; Fig. 9.4). The dependence of T. delbrueckii on oxygen may partially explain its early demise during fermentation (Mauricio et al., 1991), whereas the high population of Candida is probably associated fructose metabolism (Mills et al., 2002). In addition, molecular techniques have detected the presence of viable, but nonculturable, populations of Candida and Hanseniaspora at the end of fermentation (Mills et al., 2002). Saccharomyces uvarum appears to be particularly able to withstand the high sugar concentration, and low nitrogen, sterol and thiamine conditions, as well as the cool temperatures typically associated with botrytized wine fermentation.
Because Botrytis cinerea significantly lowers juice thiamine content, small amounts of the vitamin (0.5 mg/L) are frequently added before fermentation (Dittrich et al., 1975; Dubourdieu, 1999). This limits the decrease in keto-acid decarboxylation, associated with low thiamine levels, and the undesirable, marked increase in SO2-binding carbonyl compounds.
There is considerable variation in the influence of different strains of Botrytis cinerea on yeast growth. Suppression may result from the release of toxic fatty acids by fungal esterases. In addition, some Botrytis strains produce compounds that can either stimulate (Minárik et al., 1986) or suppress (Dubourdieu, 1981) yeast metabolism.
Little is known about the specific effects of noble rotting on malolactic fermentation. The presence of high residual sugar and glycerol contents presumably could favor its development. However, the addition of up to 200 or 250 mg/L SO2, to retain a noticeable residual sugar content free from undesirable microbial activity during maturation and after bottling, precludes malolactic fermentation. Although the addition of sulfur dioxide rapidly reduces the culturable yeast population, a variable portion enters a viable, but nonculturable state (Divol and Lonvaud-Funel, 2005). Depending on conditions, these cells may reinitiate growth, causing spoilage.
Laccases are one of the most significant enzymes produced by B. cinerea. Their pathologic function is uncertain, but it is suspected that they inactivate antifungal grape phenolics, such as pterostilbene and resveratrol (Pezet et al., 1991). Different laccases are induced in grape juice, and in the presence of gallic and p-coumaric acids. In addition, pectin may augment laccase synthesis in the presence of phenolic compounds (Marbach et al., 1985). In wine, laccases oxidize a wide range of phenolics, including p-, o-, and some m-diphenols, diquinones, anthocyanins, tannins, and a few other compounds, such as ascorbic acid. This may partially explain the comparatively high phenolic content of botrytized wines, and the atypical absence of hydroxycinnamates. In addition, the oxidation of 2-S-glutathionylcaftaric acid may contribute to much of the golden coloration of botrytized white wine (see Macheix et al., 1991).
Unlike grape polyphenol oxidase, laccase is particularly active at wine pH values, as well as in the presence of typical levels of sulfur dioxide used in other wines. Concentrations of about 50 mg/L SO2 at pH 3.4 are required to inhibit the action of laccase in wine (about 125 mg/L added to the must) (Kovać, 1979). Another difference between grape and fungal phenol oxidases is the minimal effect their oxidized by-products have on laccase activity (Dubernet, 1974). In contrast, hydrogen sulfide can completely inhibit laccase activity at contents as low as 1–2.5 mg/L (added as a sulfide salt) (see Macheix et al., 1991).
The activity and stability of laccase in must and wine have serious consequences for red wines. Because laccase rapidly and irreversibly oxidizes anthocyanins, even low levels of infection can result in considerable browning and loss of red coloration. In contrast, the gold color produced from oxidized phenolics in white grapes is considered a positive quality feature. Color shifts depend largely on the grape variety, as well as the degree and nature of infection.
In addition to laccase, B. cinerea synthesizes other oxidases, for example, glucose, amine and glycerol oxidases, catalase, and peroxidase. Of these, only glucose oxidase is of significance in must or wine, due to its stability, activity at low pH values, and relative insensitivity to sulfur dioxide (Fig. 9.5). These properties make it likely that it is one of the most significant oxidases in botrytized grapes and juice (Vivas et al., 2010). By oxidizing glucose to gluconic acid, it releases hydrogen peroxide. It subsequently oxidizes other compounds, for example, tartaric acid, ethanol, and glycerol, respectively to glyoxylic acid, acetaldehyde, and glyceraldehyde. These, in their turn, can interact with catechins and proanthocyanins, generating yellowish pigments.
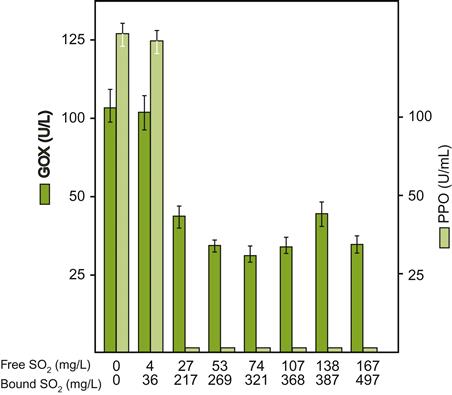
During infection, Botrytis synthesizes a series of high-molecular-weight polysaccharides. These form two distinct subgroups. One group consists primarily of polymers of mannose and galactose, with small amounts glucose and rhamnose. They vary in mass between 20,000 and 50,000 Da, and induce increased production of acetic acid and glycerol during fermentation (Dubourdieu, 1981). The other group consists of branched, β-1,6 glucan chains. These polymers can range from 100,000 to 1,000,000 Da. They have little, if any, effect on yeast metabolism, but form strand-like lineocolloids in the presence of alcohol. As little as 2–3 mg/L can seriously retard filtration (Wucherpfennig, 1985). Consequently, they can cause serious plugging problems during clarification. If assessed to be a problem, they can be degraded by the addition of β-glucanases. To minimize their release, botrytized grapes are usually harvested manually and pressed whole. This is effective because of their localization just underneath the skin, in association with the fungal cells that produce them.
Botrytis may also induce a form of calcium salt instability. The fungus produces an enzyme that oxidizes galacturonic acid (a breakdown product of pectin) to mucic (galactaric) acid. Mucic acid slowly binds with calcium, forming an insoluble salt. This may produce the sediment that occasionally forms in bottles of botrytized wines.
In addition to the direct and indirect effects of Botrytis infection, changes result from the action of secondary invaders – notably the synthesis of gluconic acid by acetic acid bacteria. Often used as an indicator of Botrytis infection, gluconic acid has no known sensory significance. The sweetness of its intramolecular cyclic esters (γ- and δ-gluconolactone) is apparently too slight to be perceptible. However, these lactones, combined with two other bacterial by-products, notably 5-oxofructone and dihydroxyacetone, constitute the principal SO2-binding compounds in botrytized must and wine (Barbe et al., 2002). High concentrations of hydroxypropanedial (a triose reductone) may even further reduce free sulfur dioxide concentrations and, thereby, its antioxidative and antimicrobial effects. It is also another chemical indicator of Botrytis (and other fungal) infections (Guillou et al., 1997).
As typical of fine wines, they cannot be produced inexpensively. Dehydration results in a marked loss in juice volume; there are considerable risks in leaving grapes on the vine to overmature; and fermentation and clarification can be difficult. Nevertheless, their production is one of the crowning achievements of winemaking.
Types of Botrytized Wines
As with any style, having had independent origins in different locations and times, there is considerable variation in the cultivars used, viticultural techniques practiced, and production procedures employed. Thus, it should not be surprising that botrytized wines can be remarkably diverse. Nonetheless, there is still a noticeable similarity, imposed by the action of Botrytis cinerea, and the effects of juice concentration. They are all characterized by high residual sugar, acid, and glycerol content, and the presence of a distinctive fragrance. This is typically characterized as resembling apricot, peach, pear, and honey. Despite residual sugar contents often being above 200 g/L, the acid content is usually adequate to avoid the wine being either cloying or syrupy.
Tokaji Aszú
Documentary evidence strongly supports tokaji having been the first, intentionally produced, botrytized wine (Fig. 9.1). The grapes are grown and the wine produced in a relatively small, semi-mountainous, region of northeastern Hungary (Tokaj Hegyalja). The traditional cultivars are Furmint (70%), and Hárslevelű (25%), although in recent times, Muscat lunel, and Zéta have been cultivated, along with the return of an ancient local cultivar, Kövérszőlő. The region is sheltered by the Zemplén hills, permitting a late harvest, associated with humidity coming from the Tisza and Bodrog rivers.
Its most famous version, tokaji eszencia, is derived from juice that spontaneously seeps out of highly botrytized (aszú) berries. These have historically been placed in small wicker tubs. About 1–1.5 liters of eszencia (juice that seeps out on its own) is obtained from 30 liters of aszú berries. After several weeks, the collected eszencia is transferred to small wooden barrels for fermentation and maturation (~10 °C). Because of the very high sugar content (occasionally more than 50%), fermentation occurs slowly, seldom reaching 5% alcohol before termination. After fermentation ceases, the bungs may be left slightly ajar. Oxygen uptake was thought to be restricted by the growth of a common cellar mold, Racodium cellare, growing on the wine’s surface (Sullivan, 1981). Despite its common development on humid cellar walls (Plate 9.2), the fungus is not known to grow on the wine’s surface. Any velum cover is more likely of yeast origin. Velum development is no longer favored, except for certain dry szamorodni styles (Atkin, 2001), to which it donates a slight sherry-like attribute. The juice used in its production comes from both healthy and aszú clusters (extracted by pressing). Fermentation employs standard white wine production techniques. After a variable period, the barrels of tokaji eszencia are bunged tight. Up to 20 years in-barrel maturation may ensue before bottling. Although occasionally bottled and sold by itself, the eszencia is more commonly used for blending with aszú style wines (Eperjesi, 2010).
Aszú style wines are made from mixing young or fermenting white wine, made from healthy grapes, with various proportions of shriveled aszú paste, or occasionally berries. The paste is produced by gently crushing the berries. The aszú proportion may come from grapes before or after the eszencia (free-run) has drained away. Legislation detailing the latter process was passed in 1655 (Asvany, 1987).
The various designations of tokaji auzú wines were originally based on the amount (number of puttony) of aszú paste added to the traditional (Gönci) barrel, before being topped up with wine made from healthy grapes. The Gönci had a capacity of 136 liters, and a puttony contained about 20–22 kg of paste. Regulations for each category are now based on minimum sugar content (60, 90, 120, and 150 g/L for 3, 4, 5 and 6 puttony aszú wines, respectively). For example, a 5 puttonyos aszú would be derived from 100 (5×20) kg aszú paste combined with about 100 kg juice or wine (Eperjesi, 2010). For continuity, the old puttonyos designations have been retained on the label. The mixture is macerated for 24–48 h, during which material is extracted from the paste. This is followed by gentle pressing and clarification by natural settling. Subsequently, the juice is transferred to small barrels (holding ~136 liters) for a continuation of, or re-fermentation. Because of cool cellar temperatures, and the presence of alcohol (if wine is used), fermentation typically proceeds slowly. Traditionally, the barrels were left partially empty, and the bung left loose for 1–3 months. This produced an oxidized attribute not found in other botrytized wines. This is attested to by the concentrations of acetaldehyde, acetals, and acetoin in the wine (see Schreier, 1979). This practice has ceased, except as noted above for certain dry szamorodni styles. The wines are only lightly sulfited. Thus, residual sulfur dioxide contents range in the level of 20 to 30 mg/L (Magyar, 2011). Fermentation may take several weeks to months to finish. Alternately, the mixture may be placed in larger cooperage. If fermentation needs to be terminated prematurely, to retain a predetermined residual sugar content, this is more commonly achieved by filtration than by sulfiting. Because of these potential variations in cellar procedures, wines from different producers often show marked individuality.
The alcohol content of the finished wine often tends to be in the 10 to 13% range. During the Communist era, a distillate made from tokaji wine was often added to prematurely terminate fermentation, to achieve an even sweeter finish, or to replace volume lost by evaporation during maturation. This may explain the alcohol contents noted for the sweeter versions listed in Table 9.2. The principal yeast involved in fermentation is Saccharomyces bayanus. Candida stellata and C. zemplinina may also be active (Sipiczki 2003).
Table 9.2
Chemical composition of Tokaji wines
| Quality grade | Total Extract (g/liter) | Extract residue (g/liter) | Sugar content (g/liter) | Ethanol content (%, v/v) |
| Two puttonyos | 55 | 25 | 30 | 14 |
| Three puttonyos | 90 | 30 | 60 | 14 |
| Four puttonyos | 125 | 35 | 90 | 13 |
| Five puttonyos | 160 | 40 | 120 | 12 |
| Six puttonyos | 195 | 45 | 150 | 12 |
| Eszencia | 300 | 50 | 250 | 10 |
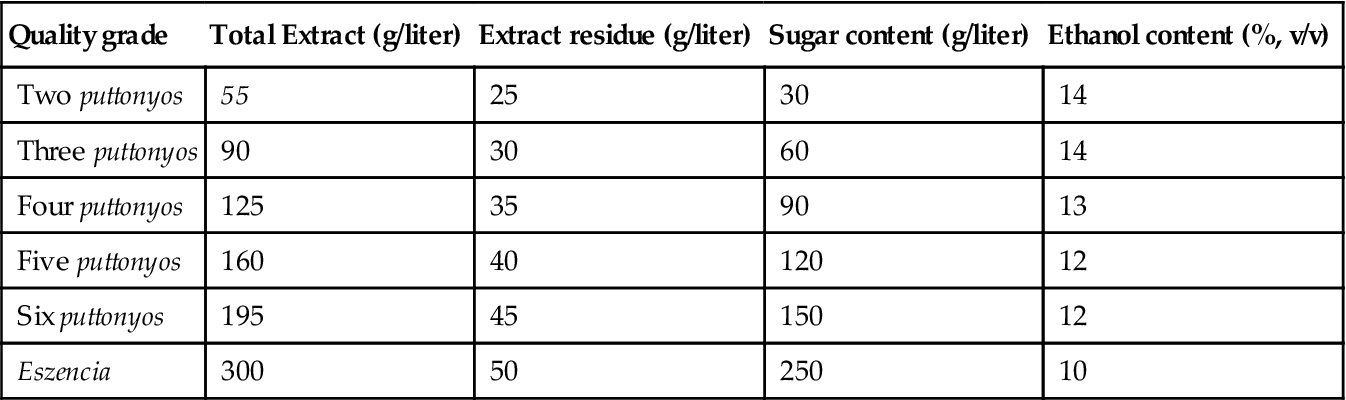
Source: After Farkaš (1988), reproduced by permission.
German Botrytized Wines
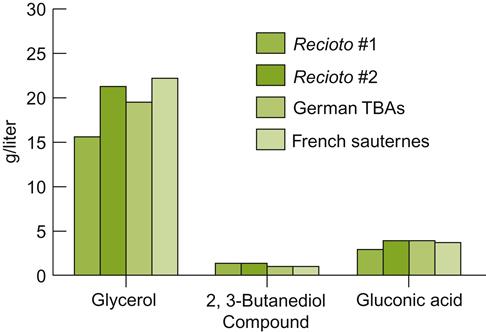
German botrytized wines come in a variety of categories. Their basic characteristics are indicated by their Prädikat designation: auslese, beerenauslese and trockenbeerenauslese. Auslesen wines are derived from specially selected clusters (or parts thereof) of late-harvested fruit. Beerenauslesen (BA) and trockenbeerenauslesen (TBA) wines are derived, respectively, from individual berries, or dried berries, selected from clusters of late-harvested fruit. Auslesen is typically botrytized, but not necessarily (Anonymous, 1979). In contrast, BA and TBA versions require Botrytis-induced concentration. Each category is also characterized by an increasingly high minimum grape sugar content (see Table 10.1). The chemical and sensory attributes of TBA wines are very similar to those of a 6 puttony tokaji aszú.
Riesling is the preferred cultivar for producing botrytized wines. Nonetheless, other cultivars are occasionally used. These may include Gewürztraminer, Ruländer, Scheurebe, Silvaner, and Huxelrebe. Similar wines are produced in Austria, but frequently from Pinot blanc, Pinot gris, Welsch Riesling, Neuburger, and other cultivars. The most renowned region for their production is Ruster, where vines are grown adjacent to Lake Neusiedl. The most propitious locations in Germany are along the Rhine and Mosel rivers, notably the Rheingau and central Mosel-Saar-Ruwer regions, respectively.
BA and TBA juices typically contain more sugar than is converted to alcohol during fermentation. The wines are consequently sweet and low in alcoholic strength, commonly 6–8% (Table 9.3). Auslesen wines may be fermented dry or may be processed to retain residual sweetness, depending on the preferences of the winemaker.
Table 9.3
Composition of some 1971 Beerenauslesen and Trockenbeerenauslesen wines
| Wine typea | Total extract (g/liter) | Sugar content (g/liter) | Alcohol content (%, w/v) | Total acidity (g/liter) | Glycerol content (g/liter) | Acetaldehyde content (mg/liter) | Tannin content (mg/liter) | pH |
| BA | 163 | 74 | 7.9 | 8.7 | 12.0 | 73 | 250 | 3.2 |
| BA | 152 | 103 | 6.3 | 9.4 | 13.6 | 62 | 390 | 3.2 |
| BA | 119 | 78 | 7.9 | 7.9 | 10.9 | 139 | 390 | 3.0 |
| TBA | 299 | 224 | 5.3 | 11.4 | 13.0 | 56 | 291 | 3.6 |
| TBA | 303 | 194 | 6.4 | 10.5 | 40.0 | 163 | 446 | 3.5 |
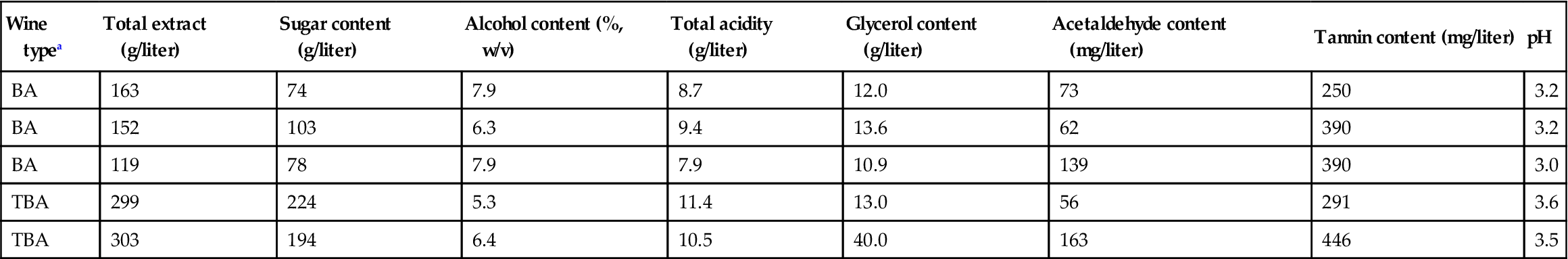
aBA, beerenauslese; TBA, trockenbeerenauslese.
Source: Data from Watanabe and Shimazu (1976).
The other main Prädikat wine categories, namely, Kabinett and Spätlese, may be derived from botrytized grapes, but seldom are. Their sweetness is usually derived from the addition of süssreserve – unfermented or partially fermented juice kept aside for sweetening. Süssreserve is added just before bottling to a dry wine, produced from the majority of the harvest. Various techniques have been used to restrict microbial growth in the süssreserve. These include high doses of sulfur dioxide, storage at temperatures near or below freezing, and hyperbaric CO2.
French Botrytized Wines
In France, the best-known botrytized wines are sauternes. The confluence of two rivers, the Garonne and a tributary, the Ciron, tends to generate misty conditions that favor noble rot development at harvest time. Over a period of several weeks, noble-rotted grapes may be selectively harvested from clusters in the vineyard. This technique is also used in Tokaj to maximize the collection of the largest number of aszú grapes. Because of the cost of multiple harvesting, most producers may harvest only several times, selecting the more botrytized clusters or portions for sauternes production. This is similar to the procedure followed in Germany, except that there, different styles are produced depending on the proportion of healthy grapes included and the degree of botrytization. Uninfected grapes are separately used in the production of dry white bordeaux.
When noble-rotted grapes are not individually harvested, the whole or partial clusters are pressed without stemming. This facilitates juice escape, while leaving most of the glucan polymers in the pomace. The juice may be left to soak in some of the pomace overnight, to augment flavor extraction. Because of the high viscosity of the juice, a slow, gentle, repeat, pressing is required to extract the juice. As with carbonic maceration (see below), the second and subsequent pressings tend to have the highest quality. Because of the high pressures required to extract the juice, the must or grapes may be pretreated to cryoextraction (cooling to below freezing). This ruptures cell membranes, facilitating juice release (Dubourdieu, 1999).
Typically, only one sweet style is produced for sauternes production, in contrast to the gradation of botrytized styles in Germany, Austria, and Tokaj. The hierarchical system of wine classification is based on historical prestige of the various Sauternes producer/vineyards.
A major stylistic difference between French and most German botrytized wines is the alcohol level attained. French styles commonly exceed 11–13% alcohol, whereas German versions seldom exceed 10%. Additional differences arise from whether the wines are matured in oak (up to 2 years for sauternes, or more for tokaji, but little to none in Germany), and the preferred fermentation temperatures. For example, in Sauternes, temperatures in the range of 20–24 °C are typical (Ribéreau-Gayon et al., 2006), and occasionally reach 28 °C (Donèche, 1993). In contrast, cool cellar temperatures are preferred and typical in Germany and Tokaj. The other main sensory differences come from the varieties used in their production. For example, the use of Sauvignon blanc and Sémillon explains the presence of a wide range of thiols in sauternes (Bailly et al., 2006), and their general absence in German or Hungarian botrytized wines.
Occasionally, sauternes is spoilt by yeast reactivation (Divol et al., 2006). These are strains that presumably went temporarily into a nonculturable state. They possess characteristics similar to flor yeasts, being highly resistant to sulfite, acetaldehyde, ethanol, and high concentrations of sugar. The addition of sufficient sulfur dioxide usually prevents its occurrence. The application of ethidium monoazide and quantitative PCR may permit the differentiation between viable (nonculturable) and nonliving yeasts (Shi et al., 2012).
Sweet botrytized wines are produced, in a more or less similar manner, in other French regions. The main locations are Alsace and the Loire Valley. In the Loire, Chenin blanc is the preferred cultivar, whereas in Alsace, Gewürztraminer is the principal cultivar used. It produces wines termed Sélection de Grain Nobles. Alternative cultivars used in Alsace include Riesling, Pinot gris, and Muscat.
Desirable Varietal Attributes
As noted, many grape varieties are used in the production of botrytized wines. Their appropriateness depends on several factors. Essentially all are white cultivars, thus avoiding the brown coloration produced by anthocyanin oxidation. Most varieties mature late, thus, the time of ripening coincides with weather conditions suitable for noble rot development. Late maturity also retains endogenous systems of resistance up until the early fall, reducing the likelihood of early bunch rot. The cultivars are also relatively thick-skinned. Because of the tissue softening induced by Botrytis, harvesting infected varieties with soft skins would be very difficult. Thick-skinned varieties are also less susceptible to splitting and bunch rot.
Induced Botrytization
The production of botrytized wines is both a risky and an expensive procedure. Leaving mature grapes on the vine increases the likelihood of bird damage, bunch rot, and other fruit losses. These dangers may be partially diminished by successive harvesting, but labor costs limit its use to only the most expensive estate-bottled wines. In Germany, most of the crop is usually harvested to produce a nonbotrytized wine. Only a variable portion is left on the vine for noble rot development or eiswein production.
Where climatic conditions are unfavorable for noble-rot development, harvested grapes have occasionally been exposed to conditions that favor its development (Nelson and Amerine, 1957). The fruit is sprayed with a solution of B. cinerea spores. They are subsequently placed on trays and held at about 90–100% humidity for 24–36 h at 20–25 °C. These conditions encourage spore germination and fruit penetration. Subsequently, cool dry air is passed over the fruit to induce partial dehydration and restrict fungal development. After 10–14 days, infection has developed sufficiently that the fruit can be pressed and the juice fermented. Induced botrytization has been used successfully, but only on a limited scale in California and Australia.
Inoculation of juice with spores or mycelia of B. cinerea, followed by aeration, apparently induces many of the desirable sensory changes that occur in vineyard infections (Watanabe and Shimazu, 1976). It appears not to have been used commercially. In addition, whether the artificial nature of its production might limit consumer acceptance is unknown. It does not have the ‘romance’ of the vagaries of nature.
Nonbotrytized Sweet White Wine
Sweet, nonbotrytized, white wines are produced in most, if not all wine-producing regions. Most have evolved slowly into their present-day forms. On the other hand, several modern versions have developed quickly, in response to perceived consumer preferences. Coolers are a prime example.
In most modern versions, some form of sweet reserve is used. This may even have ancient precursors. The ancient Romans occasionally placed grape clusters in cold water (e.g., a well) until late winter or early spring. The juice extracted (semper mustum) was added to sweeten dry wine.
Alternatively, sugars may be retained by prematurely terminating fermentation. This can be achieved by filtering out the yeasts; by allowing CO2 pressure to rise in reinforced fermentors; or from the addition of sulfur dioxide. In the latter, at least 50 mg/L free sulfite in required (Donèche, 1993). Because of the high concentration of SO2-binding carbonyls in juice, and that accumulate during fermentation, achieving this level of free sulfur dioxide may require the addition of sulfite at 200–300 mg/L (Divol et al., 2006). Further additions are required during aging to prevent the reinitiation of fermentation. Consequently, up to 400 mg/L total sulfur dioxide is permitted in European sweet wines. A supplementary benefit of this sulfite level is its limiting of the oxidizing action of laccase. Despite this, there is a desire to reduce the value. One possibility showing promise is the selective removal of SO2-binding carbonyls, notably acetaldehyde, pyruvic acid, 2-oxoglutaric acid, and 5-oxofructose. This has been achieved with phenylsulfonylhydrazine, attached to a porous polymer support (Blasi et al., 2008). It apparently does not cause noticeable wine quality deterioration.
Because the residual sugar content makes the wine microbially unstable, stringent measures must be taken to avoid microbial spoilage. Sterile filtration of the wine into sterile bottles, sealed with sterile corks, is common. Sterile bottling has supplanted the previous standard use of high sulfite additions at bottling.
Drying
Drying is probably the oldest and simplest procedure used in producing sweet wines. This traditionally involved placing grape clusters on mats or trays in the sun, or shade, to dehydrate. Alternatively, grapes grown in hot sunny climates were left on the vine to dehydrate. Because of the high sugar contents obtained, the wines often reached alcohol contents in the range of fortified wines, without the addition of fortifying spirits. A modern alternative, hot-air drying, can speed the process, avoiding potential browning due to sunburn, or fungal attack if drying is too slow or delayed. Drying rate largely depends on berry size and skin toughness, cuticle and wax thickness.
After several weeks or months, partially dehydrated grapes are crushed, and the concentrated juice fermented. Variations on these procedures are common throughout southern Europe, leading to a wide variety of wines, under an equally extensive proliferation of names. These may carry specific stylistic names, such as vin santo or vino tostado, or generic terms, such as recioto, passito, passiti1, appended to the name of the cultivar used, and/or its provenance. Examples are Recioto di Soave, Greco di Bianco Passito, or Passito di Pantelleria. In Friuli, sweet wine made from Picolit may be made either from grapes dried on mats or left on the vine to raisin. The latter are often affected with noble rot and, thus, possess a character combining passito and botrytized attributes. Drying grapes on mats are also used in the production of fortified sweet wines, notably Marsala, Malága, and Sherry.
Drying not only concentrates grape constituents, but also modifies grape metabolism and chemistry. Many of the changes, such as increases in abscisic acid (Costantini et al., 2006) and proline content, presumably reflect only a response to water stress. They are not known to have any sensory significance. However, activation of lipoxygenases can increase the concentration of hexanal, hex-1-enol, and hex-2-enal, whereas alcohol dehydrogenase activation promotes the synthesis of ethanol and acetaldehyde. Increased respiration and ethylene production provoke a loss in volatiles and changes in polyphenol content. An example of the latter is the synthesis of type A and type B vistins (Marquez et al., 2012). The requisite pyruvic acid and acetaldehyde for these cycloaddition pigments apparently results from the activation of anaerobic metabolism in the grapes. Additional metabolic alterations may increase the concentration of higher alcohols (Bellincontro et al., 2004). Franco et al. (2004) found that sun-drying was associated with enhanced concentrations of isobutanol, benzyl alcohol, 2-phenylethanol, 5-methylfurfural, γ-butyrolactone and γ-hexalactone. Modification in terpene content also reflects the drying method (Eberle et al., 2007).
Variations in style often depend on the grape variety or varieties used, and the treatments applied before, during, or after fermentation. For example, Moscato grapes in Sicily may be cured in a solution of saltwater and volcanic ash (at least in the past), before being crushed and fermented.
Vin santo is one of the more internationally available sweet wines derived from partially dehydrated grapes (reaching °Brix of 30 and above). It is produced in several northern Italian regions, but is particularly a specialty of Tuscany. An aromatic style is also produced on the Greek island of Santorini. The grape cultivars used in different regions are usually distinct, including both aromatic and nonaromatic varieties, such as Malvasia Bianca and Trebbiano, respectively. Occasionally, red cultivars are employed, such as Sangiovese and Canaiolo. They are used to make a slightly rosé version (occhio di pernice – eye of the partridge). Varieties possessing thicker skins and open clusters are more suitable, due to their greater resistance to infection during drying. Sulfite salts may be applied to limit mold growth.
In Italy, grapes used to produce vin santo are partially dehydrated on trays, arranged in naturally ventilated rooms (fruttaio) for up to 4 months. The degree of juice concentration depends on the preferences of the producer. The juice must reach a legal minimum of 26% sugar, but can almost double that. During drying, there may be a progressive increase in the presence of Metschnikowia pulcherrima (Balloni et al., 1989), or apiculate yeasts such as Hanseniaspora uvarum and Kloeckera apiculata. These alternations in yeast flora appear to enhance the aromatic complexity of the wine (Domizio et al., 2007). Of particular importance, due to the high osmolarity of the juice, may be the activity of osmotolerant species, such as Candida zemplinina and Zygosaccharomyces rouxii. These may facilitate the subsequent dominance of Saccharomyces yeasts under warmer (16–18 °C) conditions.
After any decayed fruit is removed, the grapes are pressed, and the juice allowed to clarify for several days, before fermentation. Fermentation typically occurs in small barrels (caraelli) of 50 to 200 L capacity (Stella, 1981; Domizio and Lencioni, 2011). These may be coopered from chestnut, cherry, or oak, preferably untoasted. Maturation in partially (80% to 90%) filled barrels can vary from 2 to 6 years, often occurring in attics (vinsantaia). Here the wine is exposed to the annual cycle of temperature extremes. Several rackings are typical during maturation.
The finished wine can vary from light to dark golden, to orange, or amber. Its sensory attributes are characterized, as typical of wines permitted oxidative aging, of raisin, nuts, and hay, with elements of honey, caramel, and cream. Alcohol content can vary from 14% to 17% (or up to 21% in dry styles). They can also range from dry (secco) to sweet (dolce).
Heating
Another ancient technique entails concentrating the juice or semisweet wine by heating or boiling. A classic version is vino catto, produced in the Marche and Abruzzo regions of Italy. It involves heating Trebbiano, Passerina, or Moscato musts in copper vessels over a fire to 80–95 °C, until reduced by 30 to 70%. The treatment results in a loss in varietal character, but generates a caramelized or baked odor, dark melanoidin pigments, and raises the sugar content up to about 55%. After cooling to 35 °C, fresh must is added to contribute some varietal attributes. The mixture is fermented in large cooperage at about 25 °C for 6 weeks. Fermentation involves osmotolerant strains of Saccharomyces cerevisiae, Candida apicola, C. zemplinina, and Zygosaccharomyces bailii (Tofalo et al., 2009). Aging occurs in barrel. Depending on production procedures, the alcohol content can vary from 8 to 16%, and residual sugar levels range from 83 to 350 g/L (Di Mattia et al., 2007).
Cooked must is also used in the production of marsala in Sicily, and added for color to oloroso sherries in Spain and some madeiras. Another use of heat in wine production is employed in the maturation of madeira.
Icewine
Juice concentration can also be achieved as grapes freeze and most of their water content turns to ice. If the grapes are harvested and pressed frozen, the juice can be used to make icewine (eiswein). Eiswein is reported to have been first produced in Germany in the late 1700s. More recently, the process has spread, and is now a standard technique used in cool viticultural regions, for example Canada and China.
For icewine production, grapes are left on the vine until winter temperatures fall to or below−7 °C to−8 °C (Plate 9.3). During the prolonged overmaturation period, grape chemistry changes, partially due to dehydration. In some cultivars, this has been correlated with thickening of the skin (Rolle et al., 2010). This could be of value in protecting the fruit from splitting in rainy spells; the cellular disruption induced by partial freezing, before temperatures reaching permissible values for harvesting; and may limit invasion by saprophytic or parasitic microbes. Those cultivars typically used in icewine production inherently have relatively thick skins, for example, Vidal and Riesling. Other desired cultivar traits are late maturity, fruit retention at maturity, high acidity, and winter hardiness.
At−7 °C to−8 °C, most of the water in the fruit forms ice crystals, forcing out dissolved substances into the remaining liquid. Harvesting and pressing (under freezing temperatures – Plates 9.4, 9.5 and 9.6) minimize thawing as the concentrated juice slowly escapes. The ice remains in the press with the seeds and skins. Pressing can take upward of 12 h. The presence of stalks facilitates juice escape by producing drainage channels. Rice hulls may also be added, to further assist in juice flow (Plate 7.2). Juice yield is estimated to be about 15% to 20% of what it would have been from the same grapes at regular harvest time. The sugar concentration is typically so high (often above 35°Brix) that fermentation occurs very slowly and stops prematurely, leaving the wine with a high residual sugar content. Incomplete fermentation probably results from the stresses of the high sugar content, combined with the accumulation of ethanol, acetic acid, and toxic C8 and C10 carboxylic acids. One of the adaptations yeasts make to these unfavorable conditions involves aldehyde dehydrogenase (ALD3) (Pigeau and Inglis, 2005). The enzyme converts acetaldehyde to acetic acid. Acetic acid accumulates partially due to a downregulation of enzymes converting acetic acid to acetyl CoA, and its use in fatty acid synthesis. The energy so derived appears to be used to augment glycerol synthesis (Fig. 9.6). Glycerol helps provide osmotolerance by limiting water loss from the cytoplasm.
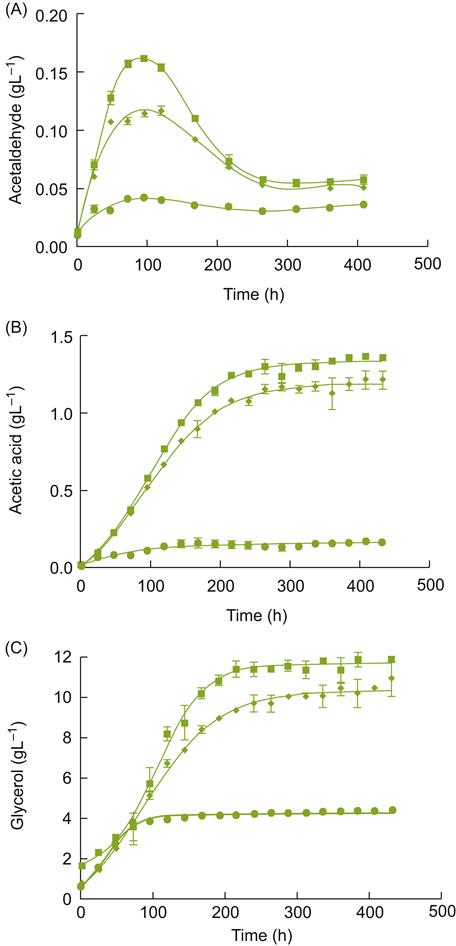
 ); chaptalized diluted icewine juice, 35.6°Brix (♦); and diluted icewine juice, 21.3°Brix (
); chaptalized diluted icewine juice, 35.6°Brix (♦); and diluted icewine juice, 21.3°Brix ( ) were inoculated with the commercial yeast K1-V1116. Acetaldehyde (A), acetic acid (B) and glycerol (C) were measured daily throughout the course of the fermentations. Values represent the average±standard deviation of the mean of duplicate fermentations. (From Pigeau and Inglis, 2007, reproduced by permission.)
) were inoculated with the commercial yeast K1-V1116. Acetaldehyde (A), acetic acid (B) and glycerol (C) were measured daily throughout the course of the fermentations. Values represent the average±standard deviation of the mean of duplicate fermentations. (From Pigeau and Inglis, 2007, reproduced by permission.)Because of the difficult fermentation conditions, yeasts are often acclimated before being added to the must (Kontkanen et al., 2004). However, this practice is not universal. Kontkanen et al. (2005) have shown that acclimation and nutrient supplementation can significantly modify the flavor characteristics of the wine. Thus, these procedures may be a means by which the wine can be given a distinctive character, varying from raisiny, buttery and spicy, to peach/terpene-like, to honey and orange, or pineapple/alcoholic. Choice of yeast strain has also been found to significantly affect the accumulation of acetic acid, glycerol, reduced-sulfur odors, and color (Erasmus et al., 2004). Inoculation with about 0.5 g/L active dry yeast is normally required to achieve the preferred 10% alcohol content.
Prolonged overripening may result in a loss of aromatic compounds, but concentration appears to more than compensate for the loss (Bowen and Reynolds, 2012). Cold weather conditions result in an absolute loss in acidity (potassium tartrate crystallization), but juice concentration again assures sufficient acidity to balance the high residual sugar content in the wine (frequently>12.5%). The golden color probably results from the joint effects of juice concentration, caftaric acid oxidation, and the release of catechins on freezing. This counters the reduction in flavonoid and non-flavonoid phenolics associated with overmaturation and partial dehydration at cool temperatures (Kilmartin et al., 2007; Mencarelli et al., 2010).
Although eiswein has been made for at least two centuries in Germany, the worldwide popularity of icewines is comparatively recent. This appears to be correlated with its production in southern Ontario and neighboring portions of the United States. The climatic conditions in these regions seem particularly favorable to fine icewine production. Despite its newfound fame, there is a surprising lack of technical information on the biochemistry involved. Thus, little is known about the changes that occur during overripening, pressing, and fermentation, or the microbiology of its production.
One of the major problems connected with icewine production involves protecting the fruit from birds and other predators, awaiting the first occurrence of an adequately hard freeze. Additional problems include molding during rainy spells, and the less than ideal conditions for manual harvesting and pressing (Plate 9.7). From 20 to 60% of the fruit may be lost before sufficient freezing occurs. Difficulties in achieving adequate fermentation are another frequent complication.
In regions where climatic conditions do not permit natural icewine production, cryoextraction can provide its technological equivalent. This has the advantage of permitting the degree of juice concentration to be selected in advance (Chauvet et al., 1986). It also avoids the risks of leaving the fruit on the vine for months, and the difficulties of harvesting and crushing grapes during frigid winter weather. Reverse osmosis has also been used to produce concentrated juices for making icewine-like wines. These techniques, however, seem to lack the flavor changes that develop during long vineyard overripening. They also lack the mystique, so often important in consumer appeal.
Addition of Juice Concentrate (Sweet Reserve)
The addition of unfermented grape juice (sweet reserve, süssreserve) to dry wine is a widespread technique, first perfected in Germany. It has the advantages of retaining the varietal distinctiveness of the juice, as well as producing no supplemental flavors. If the juice is derived from the same grapes used in making the wine, varietal, vintage, and appellation of origin are not compromised. It may also augment varietal distinctiveness, which is occasionally lost during fermentation. Furthermore, the procedure is technologically simpler and more easily controlled than most other sweetening processes.
If storage is short, refrigeration at or just below 0 °C is adequate, requiring no sulfiting. However, prolonged storage at low temperatures requires the addition of sulfur dioxide (100 mg/L), or cross-flow microfiltration to restrict yeast growth. Several spoilage yeasts are known to grow at near 0 °C. Another option is to treat the juice with heavy sulfiting (~1000 mg/L). Prior to use, the sulfur dioxide is reduced to about 100 mg/L by flash-heating with steam, followed by rapid cooling; heating with nitrogen sparging; or use of a spinning cone apparatus.
An alternative procedure noted above, and typical of many botrytized wines, involves terminating fermentation prematurely. Although successful in retaining residual sugars, it also affects the wine’s chemical composition, retaining the attributes of the fermentation stage at which it ceased (see Figs. 7.21 and 7.22).
Red Wine Styles
Recioto-Style Wines
In contrast to white wines, few red wines are produced with a sweet finish. In this regard, Italy is probably the major producer. The wines are often produced with moderate petillance, for example, Lambrusco from Emilia Romagna, or fortified, as with the Vin Doux Naturels from southern France. The latter is characterized by oxidative and Maillard fragrances (Schneider et al., 1998; Cutzach et al., 1999).
Even fewer red wines are produced from dried (passito) grapes. The process is ancient, being noted by Cassiodorus (A.D. c.485–c.585) in his book Acinatico. The following describes the process being used in the Veneto:
… in the autumn grapes are chosen in the domestic bowers, hung up by the bottom tip, then conserved in jars and in ordinary repositories. They hardened during time, do not liquefy, useless humors are exuded, and the grapes become sweet. This goes on until December, until winter begins, and wine becomes new when in all the wine cellar it is already old.
The recioto wines of Veneto (Valpolicella) and Lombardy are particularly unique in that a portion of the grapes frequently develop noble rot during the drying phase. It appears to develop from grapes possessing quiescent Botrytis cinerea infections, presumably established early in berry development. Usseglio-Tomasset et al. (1980) appear to have been the first to have noticed, or at least published on this occurrence.
Recioto Valpolicella is made from a variable blending of musts from Corvina, Corvinone, Rondinella and Molinara. Nebbiolo or Groppello are used in Lombardy. In Valpolicella, sweet (amabile), sparkling (spumante) and dry (amarone) versions may be produced from the partially dried (passito) grapes. Of these, the most famous and internationally well known is the dry, amarone style. Another example of a red wine, using at least some partially dried grapes, is traditionally made chianti wines, involving what is termed the governo process.
Drying affects grape constituents, notably the total phenol as well as caftaric and coutaric acid contents. The specific influences depend on the temperature at which dehydration occurs, and the degree of dehydration (Mencarelli et al., 2010). Stilbene and flavanol contents may also be enhanced, notably with moderate drying (10 to 20%) at 20 °C. During fermentation, the wines develop a distinctive fragrance and flavor, presumably derived from the processes occurring in the fruit before fermentation. In amarone, the fragrance often contains elements that resemble the sharp phenolic odor of tulip and daffodil flowers. This may arise from phenolic oxidation by laccases (Boubals, 1982). However, this distinctive attribute is limited if the grapes are unaffected by Botrytis. The relative occurrence of B. cinerea probably depends on conditions during flowering. These significantly affect the incidence of nascent grape infections. For the consumer, it is regrettable that the action of Botrytis is not specified, as it is it that donates the most distinctive (desirable) attributes. Otherwise, the result is only slightly different from overmaturation on the vine, and generation of an overly alcoholic wine.
Recioto wines typically have a higher than usual alcohol content, but should have a smoother, more harmonious taste than the majority of full-bodied red wines. Because of the unique botrytized fragrance, recioto wines supply winemakers with an additional means of producing wines with a distinctive character.
Production of Amarone
Healthy, fully mature clusters, or their most mature portions, are placed in a single layer on trays designed to ease air flow around the fruit. The trays are stacked in rows several meters high, in well-ventilated storage areas (fruttaio) (Fig. 9.7; Plate 9.8). Natural ventilation may be augmented with fans to keep the relative humidity below 90%. The grapes are left under cool ambient temperatures to partially dry for several months. The fruit is usually turned every few weeks to promote uniform dehydration. Cool temperatures (3–12 °C), and humidity levels below 90% are crucial to restricting microbial spoilage. Some producers are now experimenting with sophisticated means for controlling humidity and temperature (see Paronetto and Dellaglio, 2011). It is hoped that, by selecting to regulate the drying process (appassimento), it will not reduce the action of Botrytis cinerea.

During the 3–4 month storage period, the physical and chemical characteristics of the grapes undergo major changes These relate partially to the activation of genes associated with dehydration stress (Zamboni et al., 2008). The most obvious effect is the 25 to 40% drop in moisture content. Other critically important changes appear to accrue from the action of B. cinerea. As noted, fungal growth likely originates from latent fruit infections acquired in the spring following berry inception. Under the dry, cool, storage conditions, the fungus reinitiates a slow development. Grape coloration may change from bluish purple to pale red (with less dehydration) (Plate 9.9), or remain typical (with more marked dessication). Surface fungal sporulation, so characteristic of botrytized grapes in the vineyard, is seldom observed in fruttaio.
The percentage of fruit showing infection usually increases in relation to the duration of storage, the actual percentage depending on the vintage and variety (Fig. 9.8). Corvina seems the most susceptible, Rondinella the most resistant, and Molinara moderately resistant (Usseglio-Tomasset et al., 1980). The proportion of each cultivar used can vary considerably from producer to producer, but roughly in the following proportions: Corvina (and Corvinone) (40–70%), Rondinella (20–40%), and Molinara (5–25%).
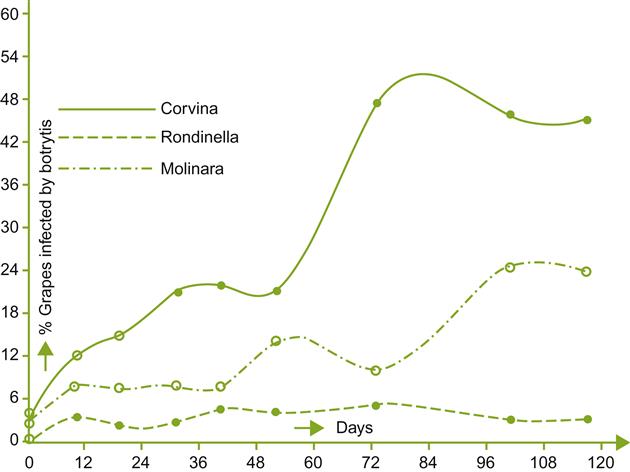
As infection progresses, the grapes become flaccid, and the skin loses its strength. The visual and mechanical manifestations of infection are reflected in even more pronounced chemical alterations (Table 9.4). These changes can show the distinctive effects of noble rotting. Although fungal metabolism reduces the absolute sugar content, water loss results in a marked relative increase in sugar concentration. The °Brix can rise from 25° to over 40° in heavily noble-rotted grapes. Due to the selective metabolism of glucose, the relative proportion of fructose rises. The grapes can also show a marked (10- to 20-fold) increase in gluconic acid and glycerol concentration (Fig. 9.9). Total acidity declines marginally, if at all. Despite dehydration, the tartaric acid concentration remains relatively constant (B. cinerea metabolizes tartaric acid), whereas that of malic acid declines. These data may indicate that selective acid metabolism by B. cinerea during storage differs from that occurring in the vineyard. Alternatively, the atypically low malic acid content may reflect the metabolic action of grapes during prolonged storage.
Table 9.4
Changes in must composition during the drying of grapes for recioto wines
| Duration of drying (days) | |||||
| 0 | 19 | 40 | 73 | 101 | |
| Sugar (g/liter) | 181 | 188 | 193 | 204 | 230 |
| Glucose (g/liter) | 92 | 90 | 91 | 95 | 103 |
| Fructose (g/liter) | 89 | 98 | 102 | 109 | 128 |
| Total extract (g/liter) | 205 | 216 | 228 | 232 | 261 |
| Total acidity (g/liter) | 6.9 | 7.5 | 6.4 | 6.5 | 6.6 |
| pH | 3.1 | 3.0 | 3.1 | 3.1 | 3.2 |
| Volatile acidity (mg/liter) | 50 | 90 | 90 | 100 | 120 |
| Tartaric acid (g/liter) | 7.3 | 7.4 | 7.7 | 5.9 | 7.1 |
| Malic acid (g/liter) | 5.7 | 5.2 | 3.9 | 4.4 | 3.8 |
| Glycerol (g/liter) | 0.2 | 0.7 | 0.8 | 2.9 | 5.1 |
| Gluconic acid (g/liter) | 0.2 | 0.7 | 1.1 | 1.0 | 1.7 |
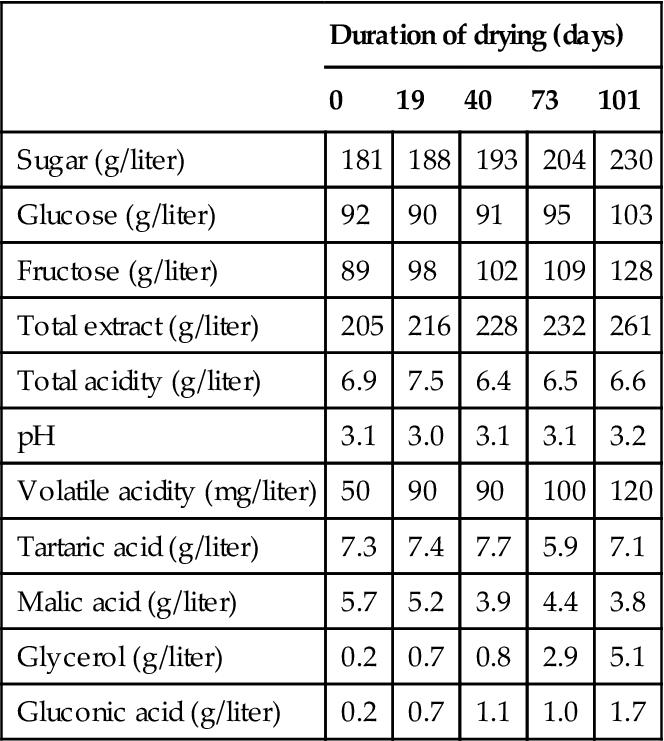
Source: Data from Usseglio-Tomasset et al. (1980).
Browning and red color loss are noticeable, but are less marked than might be expected, considering the presence of B. cinerea. Reduced synthesis, or the relative inactivity, of laccase seems supported by the resveratrol content of amarone, being up to 10 times higher than in regular (nonbotrytized) Valpolicella (Brenna et al., 2005). This may result from suppression by the high grape sugar content (Donèche, 1991), skin tannins (Marquette et al., 2003), poorly understood factors (Guerzoni et al., 1979), or the resistance of some varieties to infection. In visibly infected botrytized grapes, the must concentration of laccase was about five times higher than that in healthy must (Tosi et al., 2012). Still, this seems considerably lower than that found for botrytized white grapes (Grassin and Dubourdieu, 1989), albeit measured with a different assay technique.
Following storage, the grapes are stemmed, crushed, and allowed to ferment under the action of indigenous yeasts. When a noticeable residual sweetness is desired, the must is kept cool (≤12 °C) throughout alcoholic fermentation. For the dry amarone style, the must may be warmed to, or allowed to rise to, about 20 °C during fermentation. Despite fermentation commencing at 4–6 °C, the alcohol content and temperature can rise to 14–16% and 16–18 °C, respectively, within 3 to 4 weeks (Usseglio-Tomasset et al., 1980).
Fermentation temperature influences the relative presence of different endemic yeasts. Studies have associated various Saccharomyces species with spontaneous amarone fermentations, notably S. uvarum and S. cerevisiae (Usseglio-Tomasset et al., 1980; Dellaglio et al., 2003; Tosi et al., 2009). Each affects the aromatic chemistry of the wine differently. S. uvarum may predominate under cool temperatures, finally giving way to S. cerevisiae as the ferment warms. Fermentation can vary from 20 to 40 days.
Alcoholic fermentation may recommence when ambient temperatures rise in the spring. This may be either prevented by yeast removal (filtration for the amabile style) or encouraged (for production of the spumante style).
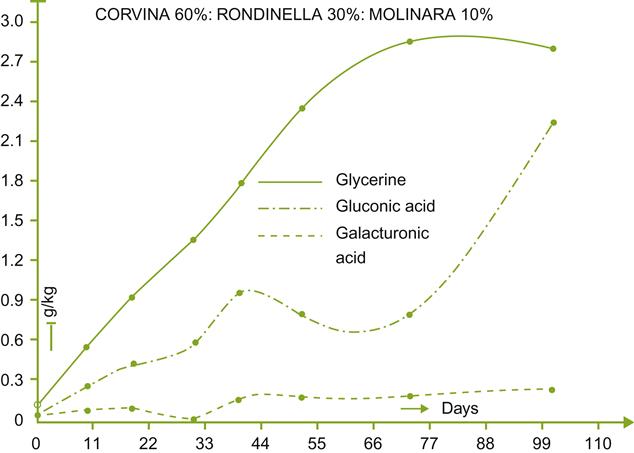
Figure 9.10 shows that the concentrations of glycerol, 2,3-butanediol, and gluconic acid are roughly comparable to those expected of highly botrytized wines. The high levels of both glycerol and alcohol contribute to the smooth texture of the wine. The smooth sensation is undoubtedly aided by the limited extraction of tannins during the cool fermentation phase. Cool temperatures also encourage the production and retention of fragrant esters. In a comparison of amarone wines, derived from healthy and noble-rotted grapes, the presence of 1-octen-3-ol, typically produced by fungi, was surprisingly not increased (Tosi et al., 2012). In addition, there were significant increases in the concentration of phenylacetaldehyde, furaneol, and γ-nonalactone, but a decrease in sherry lactones (diastereoisomers of 5-hydroxy-4-hexanolide). Unexpectedly, there were negligible changes in terpene content.
Because of the cool storage conditions, and wine’s high alcohol content, it is usually necessary to inoculate to induce malolactic fermentation. Alternately, simultaneous inoculation with an acclimated culture of Oenococcus oeni and yeast achieves earlier malic acid reduction (Zapparoli et al., 2009). Spontaneous malolactic fermentation is more likely to occur in the spring and summer in sweet recioto versions. Malolactic fermentation may be the source of the carbon dioxide that occasionally gives the amabile style a slight effervescence.
Amarone wines commonly are aged in oak for 3 years before bottling. This is considered to improve the wine’s fragrance and harmony. For example, tentative studies have shown that benzenoid derivatives (e.g., benzaldehyde, phenyl acetaldehyde, syringaldehyde and vanillin) show significant increases, while most sulfide compounds decrease (Fedrizzi et al., 2011). This is in contrast to similar wine not exposed to the appassimento process. Old cooperage is preferred, to avoid giving the wine a marked oaky character.
Governo Process
Another uniquely Italian process, partially involving grapes undergoing appassimento is the governo process. About 3 to 10% of the grape harvest is kept aside. During a 2-month storage period, these grapes undergo slow partial drying, and associated changes to their chemistry and flora. The population of apiculate yeasts, such as Kloeckera apiculata declines markedly, whereas the proportion of Saccharomyces cerevisiae increases (Messini et al., 1990).
After storage, the grapes are crushed and allowed to commence fermentation. At this point, the fermenting must is added to wine previously made from the main portion of the crop. The cellar may be heated to facilitate the slow refermentation of the mixture. The second yeast fermentation, induced primarily by S. cerevisiae, donates a light frizzante that enhances the early drinkability of the wine. The process also appears to delay the onset, if not the eventual occurrence, of malolactic fermentation.
Few studies of this traditional process have been conducted. This is regrettable, not only for this, but also for other traditional but regional procedures. Knowledge and experience, developed over centuries, if not, millennia, are at risk of being lost, under the relentless advance of market dictates and economics.
Carbonic Maceration Wines
In its simplest form, a procedure resembling carbonic maceration may be as old as winemaking itself. The involvement of incidental berry autofermentation in winemaking was probably widespread until the introduction of efficient mechanical crushers in the nineteenth century. Mechanical crushers permitted complete, rather than partial fruit crushing – the result of treading underfoot or other procedures. Regrettably, for historians, there is little direct recorded evidence of its involvement. Nonetheless, from descriptions of how wines were made at estates, such as Château Lafite in the 1800s, berry autofermentation must have been common (Henderson, 1824; Cocks, 1846). In addition, grape pressing, often a week or more after harvesting, appears to have been extensive in Champagne (Guyot, 1861). Depending on how they were stored, autofermentation is a distinct possibility. The procedure’s beneficial effect was considered common knowledge, such that Louis Pasteur (1876) could say ‘… tout le monde sait …’. Currently, only in a few regions, notably Beaujolais, has this process remained an integral, essential, and dominant feature of wine production. Documentary evidence of its extensive use is noted in Chauvet (1971).
The classic beaujolais procedure differs from carbonic maceration in not flushing the grapes with carbon dioxide in the fermentor; uses a shorter autofermentation period (5 to 6 days); involves some pumping over; and is associated with about 20% berry rupture during fermentor loading. Little sensory difference between the two procedures was noted (Chauvet, 1971). The use of a carbonic maceration-like process is reported to have been used in Rioja, Spain, at least as early as 1947 (Amerine and Ough, 1968). Its use is probably much older.
Grapes are well known to metabolize malic acid, especially during ripening under warm conditions. Although carbonic maceration favors malic acid decarboxylation, the process is not specifically used for de-acidification. Its primary intent has been to produce early maturing wines. This certainly would have been a desirable feature during the so-called Dark Ages. In addition, the presence of a unique and distinctly fruity aroma, combined with creative marketing, generated the Beaujolais Nouveau est ici frenzy of the 1980 and 1990s. The craze still gushes, but more tranquilly today. Nevertheless, their image as light, quaffable wines has led to many critics to unjustly malign all wines produced using carbonic maceration or its variants.
Because of the technique’s largely regional use, the study of grape berry fermentation has garnered little attention outside southern France and parts of Italy. With the popularity of nouveau-type wines, interest in the process expanded. The Institut National de la Recherche Agronomique in Montfavet, France has been the primary center for investigation. Interestingly, the study of autofermentation evolved not out of an interest in nouveau-style wines, but as a means of extending berry storage. Grapes were placed under a blanket of carbon dioxide, similar to that used commercially for apple storage. Although unsuccessful in its intended purpose, submerging grapes in an atmosphere of carbon dioxide resulted in Flanzy proposing a new vinification procedure (Flanzy, 1935). The process came to be called carbonic maceration. Incidentally, it shed light on the ancient, and now traditional, process used in producing beaujolais, and to a limited extent, other European wines. The coiners of the term are opposed to extending its use to these ancient procedures (personal communication). Nonetheless, much of the world has adopted carbonic maceration to describe all winemaking procedures involving the extensive use of grape berry autofermentation. This practice is applied here.
Figure 9.11 compares carbonic maceration-based vinifications with traditional vinification. Carbonic maceration differs fundamentally from standard procedures in that the berries undergo self-fermentation, before being crushed and the onset of standard alcoholic and malolactic fermentations. For maximal benefit, it is essential that the fruit be harvested with minimal breakage.
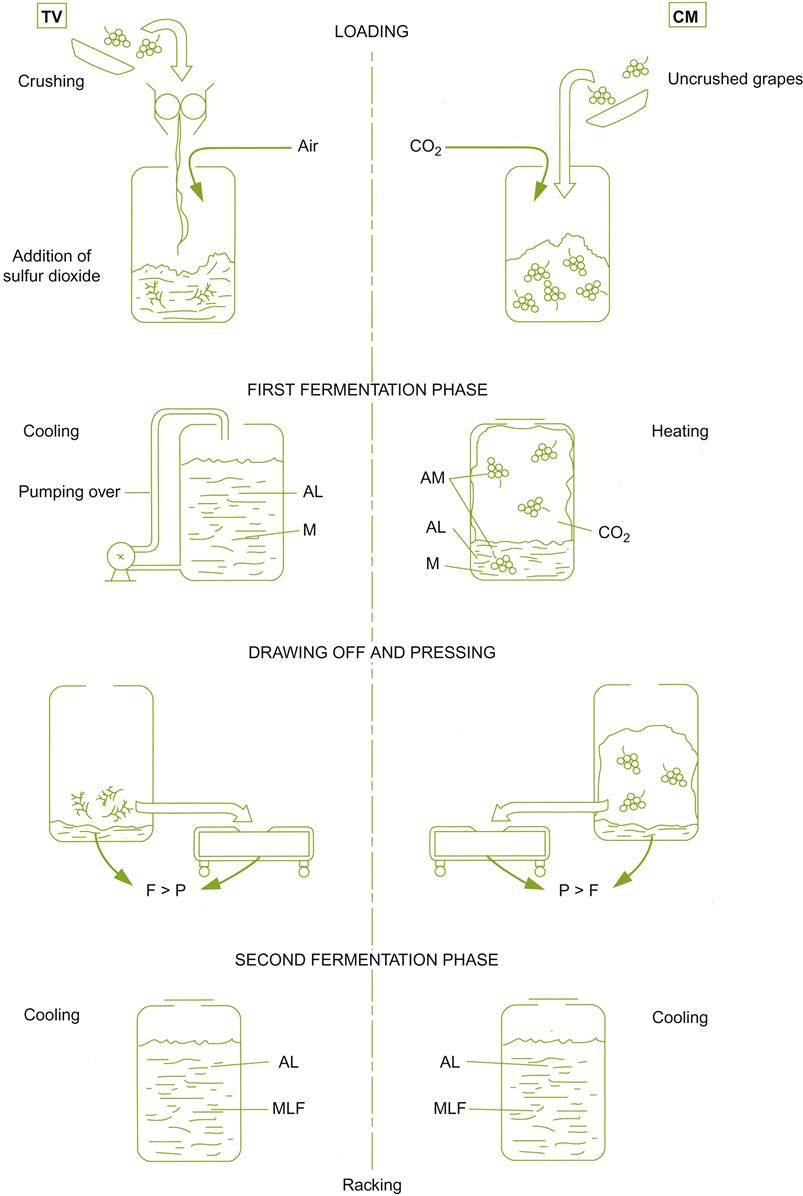
Typically, beaujolais-style carbonic maceration involves the presence of a small amount of must. It is released when some of the fruit ruptures when being loaded into the fermentor. Thus, berry fermentation typically occurs simultaneously with limited alcoholic (yeast) fermentation.
If fruit is not dumped into the fermentor, anaerobic maceration occurs (at least initially) in the absence of free juice – pure carbonic maceration. This occurs when grapes, still in their harvest containers, are placed in sealed chambers (Fig. 9.12), or left in the containers in which they were transported to the winery, and then wrapped in plastic film (e.g., polyvinylidene chloride). Pigment extraction during this phase is usually poor. Thus, the juice is left to ferment in contact with the seeds and skins after crushing, until the desired color has been achieved. If, however, deep coloration is not critical, sufficient berries may collapse and rupture during carbonic maceration to achieve adequate pigmentation.
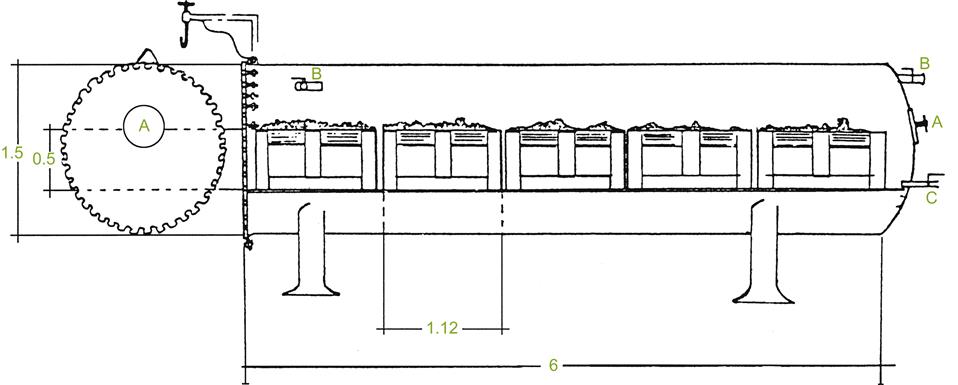
Typically, the whole harvest undergoes initial carbonic maceration. However, in some regions, only part of the crop may undergo carbonic maceration. For example, carbonic maceration may be used to partially mask the intense aroma of cultivars, such as Concord (Vitis labrusca) (Fuleki, 1974), or several French-American hybrids (Garino-Canina, 1948). With Bordeaux cultivars, such as Cabernet, more than 85% of the fruit must undergo carbonic maceration for the varietal aroma to be suppressed (Martinière, 1981). Thus, modifying the uncrushed proportion of the crop has the potential to adjust the relative contribution of carbonic maceration vs. varietal character. The intensity of the carbonic maceration aroma may also be modified by influencing its duration and temperature. Alternatively, carbonic maceration wine may be blended with must vinified by standard procedures.
At the end of carbonic maceration, the grapes are pressed and the juice allowed to ferment to dryness by yeast action. Malolactic fermentation typically occurs shortly after the termination of alcoholic fermentation. After completing malolactic fermentation, the wine typically receives a light dosing with sulfur dioxide (20–50 mg/L). This helps prevent further microbial action. Racking commonly occurs at the same time. Racking may be delayed for several weeks, however, to derive flavor attributes donated by yeast autolysis.
Although carbonic maceration is used most extensively in the production of light, fruity, red wines, it can yield wines capable of long aging. The procedure has also been used to a limited extent in producing rosé and white wines.
Advantages and Disadvantages
Carbonic maceration donates a unique, fruity aroma. This feature has been variously described as possessing kirsch, cherry, or raspberry aspects. Additional descriptors are noted in Table 9.5.
Table 9.5
Sample descriptors used to describe certain carbonic maceration wines
| Full carbonic maceration | Semicarbonic maceration (beaujolais) | |
| Visual appearance | Ruby red | Ruby red |
| Fragrance | Kirsch | Hyacinth |
| Coffee | Coffee | |
| English candy | English candy | |
| Vanilla | Vanilla | |
| Grilled almonds | Cherry | |
| Russian leather | Banana | |
| Resin | Raspberry | |
| Quality | Fine (predominantly vegetal and lactic) | Rich (predominantly winy and phenolic) |
| Taste | Subtle | Rough |
| Buttery | Tannic |
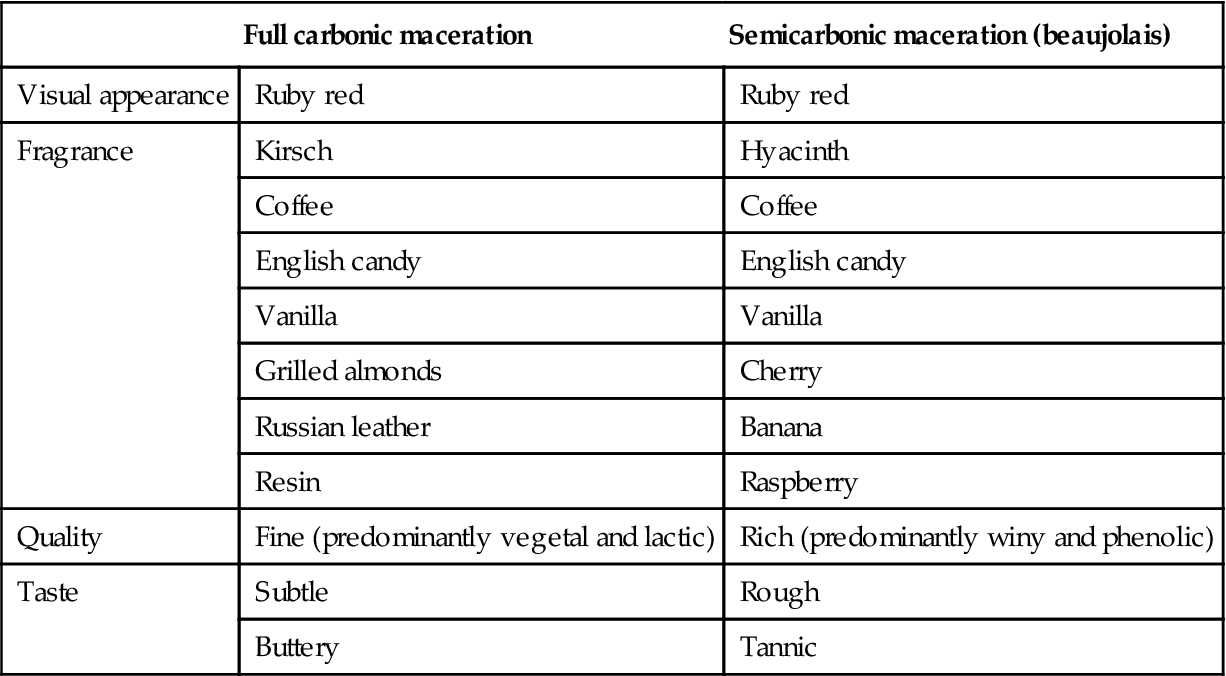
Source: After Flanzy et al. (1987). © INRA, Paris, reproduced by permission.
For relatively neutral varieties, such as Aramon, Carignan, and Gamay, carbonic maceration provides an appealing fruitiness that standard vinification does not yield. With cultivars, such as Cabernet Sauvignon, Merlot, and Concord, carbonic maceration may, to varying degrees, mask the varietal aroma. This may be desirable or not, depending on the appeal of the aroma. With other cultivars, such as Syrah and Maréchal Foch, carbonic maceration has been reported to enhance the complexity of the varietal fragrance. The observed reduction in the herbaceous character of several French-American hybrids may result from the curtailed production of hexan- and hexen-ols (Salinas et al., 1998). Carbonic maceration has even been reported to enhance the varietal aroma detected in some white wines (Bénard et al., 1971).
Because standard vinification extracts more tannins than carbonic maceration (Pellegrini et al., 2000), the process may be preferable when used with highly tannic grapes. The potential for deacidification might also partially justify its use with acidic grapes.
Carbonic maceration wines seldom demonstrate a yeasty bouquet after completing vinification. The wine, which also has a smoother taste, can thus be enjoyed sooner. This has financial benefits, because the wines can be bottled and sold within a few weeks of production. Thus, capital is not tied up for years in cellar stock.
Regrettably, the carbonic maceration aroma does not improve on aging, and fades relatively quickly. Unless a varietal aroma or pleasing aged bouquet replaces the fading carbonic maceration fragrance, the wine commonly has a shelf-life of little more than 6 to 12 months. Carbonic maceration does not, in itself, limit shelf-life. The aging potential depends primarily on grape quality and the use of press-run fractions. Consequently, some carbonic maceration wines show long aging potential, notably those from northern Beaujolais, the Rhône Valley, and Rioja. Extended fermentative maceration after carbonic maceration favors the extraction of sufficient aromatics, anthocyanin, and tannins to give the wine aging potential.
In the past, the comparative simplicity of carbonic maceration supplemented the benefits of early drinkability. It required neither destemming nor grape treading. Whole grape clusters could simply be loaded into wide shallow vats. Crushing and pressing were easier, because the grapes became weak and flaccid, a consequence of carbonic maceration.
Some of these advantages are still relevant today, for example, early drinkability and easier pressing of pulpy grapes. However, other aspects of carbonic maceration are incompatible with present-day harvesting and winemaking. In most situations, mechanical harvesting is preferred, due to its cost effectiveness. However, for carbonic maceration, it might rupture more berries than normally thought desirable. In addition, independent containers are required for the carbonic maceration and alcoholic fermentation phases. Most fermentors are not designed for loading and evacuating whole grape clusters. Loading could also risk excessive fruit rupture.
Care must be taken at all stages, from harvesting to loading, to minimize fruit rupture. In Beaujolais, broad, shallow (∼2.5 m) vats are preferred. They facilitate fruit loading and, thereby, reduce berry rupture. Vats permit the ready displacement of air with carbon dioxide at the beginning of maceration. This occurs slowly, over the course of several days, if there is no initial flushing with CO2. Subsequently, the vat opening is covered to restrict air access, while permitting carbon dioxide generated by berry autofermentation to escape.
In Beaujolais, the juice that slowly accumulates, as an increasing proportion of the fruit ruptures, is periodically pumped over the fruit. Although frequently practiced, pumping over is not recommended for strict carbonic maceration. The procedure can increase oxidation and bacterial action, leading to undesirably high concentrations of ethyl acetate (≥150 mg/L) (Descout, 1986). By temporarily removing the buoyant action of the juice, pumping over also induces further fruit rupture. The ethanol produced by yeast action, in the liberated juice, also favors breakage, by weakening and killing skin cells. Rupture curtails the generation of the metabolic consequences of carbonic maceration.
One of the more serious drawbacks of carbonic maceration is the high demand it places on fermentor capacity. Because the fruit is neither stemmed nor crushed, they displace considerably more volume than would the must derived from the same amount of fruit. Furthermore, the initial grape-cell fermentation significantly prolongs the fermentation period (Fig. 9.13). Although malolactic fermentation typically commences shortly after yeast fermentation, this does not offset the need for increased fermentor capacity at this critical time of the year.
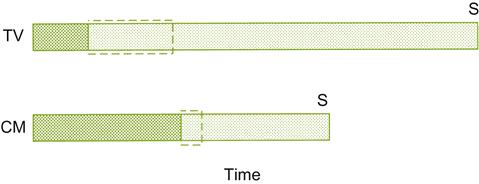
The problem of fermentor capacity can be sidestepped with the use of specially designed storage containers (Fig. 9.12), or by adequately wrapping the fruit in plastic (Rankine et al., 1985). This is easiest when the grapes are left in the same containers as those in which they were harvested. Both significantly reduce the berry rupture that inevitably occurs during vat loading.
Phase I – Carbonic Maceration
Whole-Grape (Auto-)Fermentation
In the absence of oxygen, grape cells switch from respiratory to fermentative metabolism. This shift is more rapid if air is immediately flushed out with carbon dioxide. Because carbon dioxide is more dense than air, it displaces air around the fruit. Carbon dioxide is customarily preferred to nitrogen, due to the former’s uniquely desirable properties. It is readily dissolved by cytoplasm, where it induces ion leakage (Yurgalevitch and Janes, 1988). It also shifts the equilibria of cellular decarboxylation reactions (Isenberg, 1978). In addition, carbon dioxide may accelerate the breakdown of pectins, by inducing the synthesis of grape pectinases.
Biochemically, grape-cell alcoholic fermentation is similar to that found in yeasts and most other cells. The primary end product is ethanol, with smaller accumulations of glycerol, acetaldehyde, acetic acid, and succinic acid.
The generation of ethanol during autofermentation is very limited, rarely rising above 2%. This results from the early inactivation of alcohol dehydrogenase in grape cells. However, ethanol accumulation is, by itself, insufficient to fully explain enzyme inactivation (Molina et al., 1986). Instead, enzyme activity probably ceases as an indirect result of ethanol-induced membrane disruption and cell death (Romieu et al., 1989). The resultant release of organic acids, stored in cell, would inhibit alcohol dehydrogenase activity by lowering cytoplasmic pH.
During grape-cell fermentation, malic acid is metabolized to other acids (primarily oxaloacetic, pyruvic, and succinic acids), as well as ethanol. Depending on the grape variety, and fermentation temperature (Flanzy et al., 1987), upward of 15–60% of the malic acid content is metabolized during carbonic maceration. Significant decarboxylation to lactic acid does not occur. The other major grape acids (tartaric and citric) are occasionally metabolized. Their metabolism appears to depend predominantly on the grape variety.
Associated with grape-cell fermentation is a modified operation of the shikimic acid pathway (Fig. 9.14). Shikimic acid accumulates, along with volatile by- products, such as ethyl cinnamate, benzaldehyde, vinylbenzene, and salicylic acid. The last is not itself volatile, but can react with ethanol to form an aromatic ethyl ester. Higher concentrations of ethyl decanoate, eugenol, methyl and ethyl vanillates, ethyl and vinyl guaiacols, and ethyl and vinyl phenols develop during carbonic maceration than during standard vinification (Ducruet, 1984). The high ethyl cinnamate and ethyl decanoate levels may be sufficiently distinctive to serve as indicators of carbonic maceration.
The precise chemical nature of the characteristic fragrance of carbonic maceration wines remains unclear. However, some elements have been tentatively ascribed to ethyl cinnamate and benzaldehyde. These may generate some of the strawberry–raspberry (Versini and Tomasi, 1983), and cherry–kirsch (Ducruet, 1984) fragrances that distinguish carbonic maceration wines. Isoamyl acetate and higher alcohols also appear to be significant contributors to aromatic distinctiveness (Fondville-Bagnol, 1996). Both free and bound terpenoid contents may also show marked increases from Muscat, at least at high (32 °C) maceration temperatures (Bitteur et al., 1996). In contrast, low hexyl acetate and hexanol contents are typical. Without prefermentative crushing, there is less chance for fatty acid oxidation, and the generation of vegetative odors.
One of the distinctive consequences of carbonic maceration is a reduction in the amount of free ammonia and a rise in the concentration of amino acids (Flanzy et al., 1987). Some of the amino acids undoubtedly arise from the enzymatic breakdown of proteins. Others may be biosynthesized from glycolytic or tricarboxylic acid (TCA) cycle intermediates and ammonia. Although the total concentration of amino acids rises, the content of specific amino acids varies independently. For example, the concentrations of aspartic and glutamic acids decline, due to their metabolism during carbonic maceration (Nicol et al., 1988).
The release of organic nitrogen during maceration probably helps to explain the rapid onset and completion of both alcoholic and malolactic fermentation. Whether the high amino acid content plays any role in the development of the characteristic carbonic maceration fragrance is unknown.
During carbonic maceration, pectins break down in the fruit. Consequently, the attachment of cells to one another weakens, and the pulp loses its solid texture. If the carbon dioxide produced inside the intact fruit escapes, the berries become flaccid. Otherwise, the CO2 pressure maintains berry shape, but not strength.
At the beginning of carbonic maceration, the fruit absorbs carbon dioxide from the surrounding environment. The amount dissolved depends on the temperature, varying from approximately 60% of berry volume at 15 °C to 15% at 40 °C (Chambroy and Flanzy, 1984). As berries become saturated, carbon dioxide liberated during fermentation begins to be released. Production rate is slower and more steady at cooler (25 °C) than warmer (35 °C) temperatures, not reaching a peak for 8 and 4 days, respectively (Chambroy and Flanzy, 1984). Carbon dioxide production ceases when the cells die, due to alcohol toxicity, or when the energy supply from fermentation is insufficient to sustain cellular integrity. The termination of gas release has occasionally been used as an indicator of the end of autofermentation, and when to crush the grapes for the onset of full alcoholic fermentation.
As grape cells die, the metabolic regulation of movement across cellular membranes ceases. This enables the release of various substances from the cells, notably phenolic compounds. The extraction of phenols by the juice is complex and often highly specific. The major controlling factors are the temperature and duration of carbonic maceration, as well as the amount of fermenting juice around the fruit.
Anthocyanins are more rapidly and extensively dissolved than tannins. Because high temperatures speed color stability, by favoring anthocyanin–tannin poly-merization, winemakers prefer short maceration at temperatures above 30 °C. Tannin extraction appears to be primarily from the skins, with little coming from the seeds.
Nonflavonoid phenolics are both extracted and structurally modified during maceration. Chlorogenic acid dissolves, and the tartrate esters of p-coumaric and caffeic acids hydrolyze rapidly. As a result, small quantities of free p-coumaric and caffeic acids accumulate.
Submersion of the fruit in fermenting juice, common at the base of the vat, markedly increases anthocyanin and tannin extraction (Fig. 9.15). This presumably results from the solvent action of the alcohol that accumulates in the fermenting must. As alcohol diffuses into intact grapes, ethanol dissolves phenols in the fruit. Thus, pigment and tannin extraction is not limited at this stage just to berries that break open at the bottom of the vat.
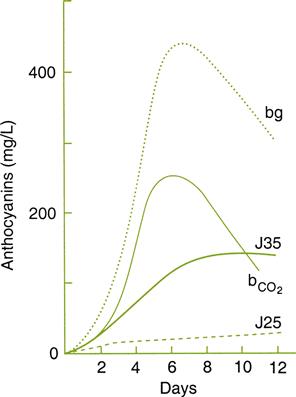
Of the factors influencing grape-cell fermentation, temperature is probably the most significant and easily controlled. The initial phase of carbonic maceration is considered to be optimal at between 30 and 32 °C. This shortens its duration, promotes grape autofermentation, and favors pigment and tannin extraction. To encourage the rapid onset of grape-cell fermentation, the fruit is often picked late in the afternoon on warm sunny days. Alternatively, the fruit may be heated to the desired temperature. Despite this, the preferred (traditional) initial temperature in Beaujolais is reported to be between 18 and 22 °C (Descout, 1983). Heating is uncommon.
Fermentation of Released Juice
In Beaujolais, the fruit is fermented in shallow vats. The breakage and amount of juice released depends on the maturity and health of the fruit, grape variety, tank depth, and the mechanism of loading. To minimize berry rupture, the tanks are commonly no more than 2.5 m deep. Nonetheless, 10–20% of the grapes may break during loading. This level increases during maceration, as the berries weaken and the cumulative mass of the fruit ruptures those at the bottom (Fig. 9.16). Pumping over augments fruit collapse and the amount of free-run liberated. The proportion of juice released by fruit rupture varies widely, but, by the end of maceration, can reach 35–55%.
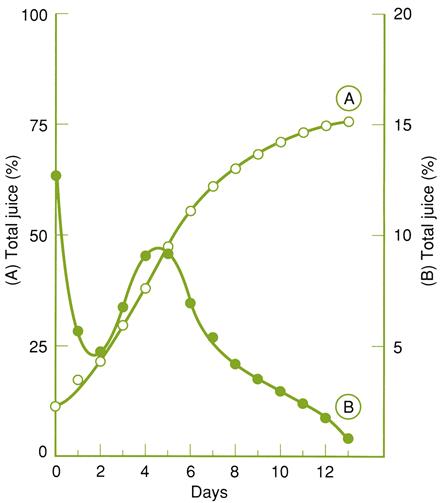
If the juice is low in acidity, tartaric acid may be added to the free-run. Addition of sulfur dioxide at this time usually brings the level of SO2 up to 20–50 mg/L. Sulfiting is limited to avoid either the production of hydrogen sulfide or delay the onset of malolactic fermentation. Early completion of malolactic fermentation is essential for the production of primeur or nouveau wines. Because of early bottling, microbial stability needs to be established within a few weeks of vinification.
Occasionally, chaptalization is conducted before pressing. Normally, though, when the °Brix value is too low, sugar is added after pressing, when alcoholic fermentation is at its apex (Descout, 1983).
Because juice inoculation occurs spontaneously upon berry rupture, some yeast fermentation occurs concurrently with carbonic maceration. This has a marked effect on the course and duration of grape-cell fermentation. Yeast fermentation has its most marked effect on fruit submerged in the fermenting juice. Even in the absence of released juice, however, the population of yeasts and bacteria on the grapes increases. The absence of oxygen is not a factor as their metabolism is largely fermentative.
Few studies of the yeast flora during carbonic maceration have been conducted. S. cerevisiae appears to be the dominant species, although Schizosaccharomyces pombe may constitute up to 25% of the yeast population (Barre, 1969). The yeast population reaches approximately 8–12×107 cells by the time of pressing. Fungicides on the fruit can modify this value significantly, especially because of the small juice volume initially released. Correspondingly, the juice may be inoculated with an active yeast culture to offset potential yeast suppression by fungicide residues.
Thermotolerant yeast strains are required if optimal temperatures for carbonic maceration are used. Nevertheless, it is still important to prevent excessive heat buildup during fermentation. Temperatures above 35 °C can induce yeast death and leave the must open to spoilage yeasts and bacteria.
Yeast inoculation tends to reduce the accumulation of ethyl acetate (Descout, 1986). The origin of this compound is not precisely known. Because its increase is not directly correlated with the simultaneous buildup of acetic acid, it presumably is synthesized directly by grapes or the indigenous flora. Pumping over and periodic chaptalization (frequent but small additions of sugar) have the regrettable consequence of encouraging ethyl acetate accumulation.
Yeast activity has a considerable influence on the course of carbonic maceration. If flushing with CO2 is not used to displace air from the fermentor, yeast action supplements that generated by grape-cell fermentation. If the fruit is cool and not heated artificially, yeast metabolism also generates most of the heat that warms the fruit during carbonic maceration. Yeasts quickly convert released sugars to ethanol, carbon dioxide and heat, in contrast to the limited fermentation of whole grapes.
Alcohol vapors generated during carbonic maceration are partially absorbed by the fruit. Not surprisingly, more ethanol diffuses into the fruit submerged in fermenting juice (Fig. 9.17). By acting as a sink for alcohol, intact berries aid yeast fermentation by slowing the accumulation of ethanol in the juice. Malic acid released from broken berries tends to diffuse inward, permitting its continued metabolism by living grape cells. If lactic acid bacteria are active in the juice, the flow of malic acid may reverse. Sugars slowly diffuse out of intact berries, adding to those released by progressive fruit rupture. These sugars provide a continuing nutrient supply for yeast metabolism. By the end of carbonic maceration, the sugar content of intact fruit has usually fallen to about 50 to 70 g/L (Descout, 1986). Throughout carbonic maceration, nutrients and liquid released as berries rupture help minimize the accumulation of toxic octanoic and decanoic acids.
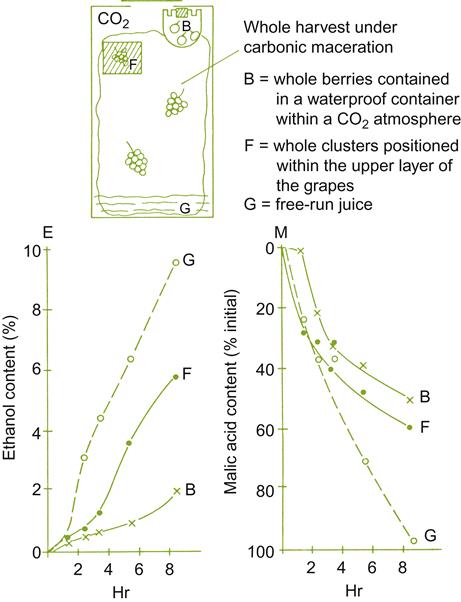
Although alcohol accumulation eventually inhibits grape-cell fermentation, it appears to activate the production of aromatic compounds that characterize carbonic-maceration wines (Tesnière et al., 1991).
As noted, winemakers occasionally use the end of carbon dioxide release as an indicator of the termination of carbonic maceration. Another clue is the drop in juice specific gravity to 1.02 or below. Alternatively, carbonic maceration may be terminated when juice color or flavor has reached a desirable value.
Maceration typically lasts 6–8 days, but can last up to 2 weeks. Long maceration is more common when there is no simultaneous yeast fermentation. Extended contact between the juice and fruit often leads to the development of a bitter character. This presumably results from extraction of phenolic compounds from the stems.
Phase II – Alcoholic Fermentation
Once the decision has been taken to terminate carbonic maceration, the free-run is allowed to escape and the remaining intact grapes and pomace pressed to extract the juice. If the free-run shows no signs of active malolactic fermentation, it is common to combine all the juice fractions for alcoholic fermentation. However, if the free-run juice is undergoing malolactic fermentation, the press-run juice is usually fermented separately. There is a concern that the higher content of fermentable sugars in the press-run may spur acetic acid production by the bacteria.
The free- and press-run fractions may also be fermented separately to permit blending based on their respective qualities, and the intentions of the winemaker. Free-run juice, in contrast to traditionally vinified wines, is viewed of lower quality than the press-run fractions. Free-run juice produces wine that is less alcoholic (by 1–2%) than the press-run juice. Free-run wine is also lighter in color, more herbaceous, bitter tasting, and higher in acetaldehyde and 2,3-butanediol content. The press-run fraction generates wine that is more aromatic, alcoholic, and colored. It also contains most of the esters, fusel alcohols, and aromatic compounds that give carbonic maceration wines their distinctive fragrance. Although the total phenolic content in both fractions is nearly identical, the specific composition differs. Tannins in the press-run are softer tasting and less bitter than are those in the free-run.
For the lighter, primeur-style, typical of beaujolais nouveau, a higher proportion of free-run wine is used in the blend. When a wine of longer aging potential is desired, the blend contains mostly press-run wine.
For the second phase of vinification, a temperature of between 18 and 20 °C is generally preferred. This is believed to retain the distinctive fragrance donated by carbonic maceration. If the initial phase has taken place or reached temperatures considerably above 18 to 20 °C, cooling is required. Some cooling occurs spontaneously, when carbonic maceration comes to completion and during pressing. Nevertheless, additional cooling is often required. Even at 18 to 20 °C, fermentation is tumultuous and customarily complete within 48 h.
In addition to consuming fermentable sugars, yeasts modify the concentration of volatile phenols. Malolactic fermentation further alters phenolic composition. These effects are more pronounced in carbonic maceration wines than in traditionally produced wines. Both 4-vinylguaiacol and 4-vinylphenol content increases, whereas 4-ethylphenol decreases during alcoholic fermentation (Etiévant et al., 1989). Whether the volatile phenols are a function of carbonic maceration, the presence of Brettanomyces, or contaminant bacteria in the samples, as suspected by Tesnière and Flanzy (2011), remains unresolved. Total volatile phenols increase during malolactic fermentation.
Malolactic fermentation typically begins immediately after the completion of alcoholic fermentation, if not before. This is favored by the limited use of sulfur dioxide, storage at warm temperatures, reduced wine acidity, and the ready availability of nitrogenous and other nutrients. If malolactic fermentation is slow to commence, the wine is commonly inoculated. This usually involves the addition of wine that has just undergone successful malolactic fermentation. Natural inoculation appears to induce quicker fermentation than commercially available cultures.
Aging
Most carbonic maceration wines are produced for rapid consumption, with only a smaller proportion vinified for extended aging. A few of the changes that can occur during aging are shown in Fig. 9.18. They are similar to those occurring in traditionally vinified wines, but occur more quickly and are more pronounced. Subjectively, it is known that the fruity aroma induced by carbonic maceration soon fades from nouveau-style beaujolais. It is often replaced, after several years, with attributes reminiscent of sewage.
Maturation in oak is uncommon. However, short exposure to oak can add complexity to the wine. Winemakers differ considerably in their opinions concerning whether oak benefits or detracts from the fruity character of the wine. Because of early bottling (for nouveau versions), maturation in oak is an option only for styles based primarily on press-run wine. These typically can age well for several years.
Use with Rosé and White Wines
Although carbonic maceration is predominantly used for the production of red wines, rosé and white wines are occasionally vinified using the technique. In the production of rosé wines, grapes are kept from being submerged in free-run juice. This limits pigment and tannin extraction, and maximizes the development of a fruity aroma. If pigment extraction is insufficient, the grapes are crushed and the second, alcoholic phase of fermentation conducted briefly in contact with the seeds and skins. Once sufficient color has been obtained, the fermenting must is pressed to separate the juice from the pomace.
Similarly, white grapes, treated by carbonic maceration, are kept isolated from fermenting juice. The duration of the process for white wines is commonly shorter than for either red or rosé versions, often being little more than 48 h. The precise duration and temperature chosen depend on winemaker preferences and how maceration affects the varietal aroma. Carbonic maceration can either suppress or enhance varietal character (Bénard et al., 1971).
For the second phase of vinification, juice fermentation is conducted without skin contact. If it is low in pH, the wine may be acidified on pressing. Alternatively, the wine may be cooled and sulfited to prevent deacidification by malolactic fermentation.
An alternative to the typical short carbonic maceration for white grapes at warm temperatures is maceration at 5 °C for approximately 3 days (Montedoro et al., 1974). The procedure favors ester synthesis and retention, as well as reducing phenolic extraction. Centrifugation may also be used to reduce the phenol content and diminish color intensity.
Occasionally, the harvest is divided into lots – one treated according to standard procedures, the other treated to carbonic maceration. The fractions may be blended together to provide a wine of enriched fragrance and improved acid balance.
Sparkling Wines
Sparkling wine owes much of its initial development to technical advancements unrelated to production of the wine itself. These involved the, almost providential, simultaneous, reintroduction of cork closures and improvements in glass manufacture. The availability of strong glass bottles, able to withstand the high pressures that develop in sparkling wine, was an absolute. These became available when a switch from wood- to coal-fired furnaces permitted higher kiln temperatures. This occurred in England during the reign of King James I (1603–1625). England’s forest trees were better employed in its desire to establish naval prowess. Production of strong glass was further improved with the addition of lead oxide to the mixture. This is credited to George Ravenscroft in 1675 (Charleston and Angus-Butterworth, 1957). Similarly, a closure able to withstand the carbon dioxide pressures generated by the second, in-bottle fermentation was essential. Cork began its use as a bottle closure in the latter part of the 1500s. These developments coincided with an atypically long spell of cold weather in Europe, termed the Little Ice Age (Le Roy Ladurie, 1971). This may have triggered the evolution of sparkling wine. The string of poor vintages reduced the quality of the red wines traditionally being produced in the Champagne region. One of the principal goals of Dom Perignon was to prefect the production of a superior white wine from Pinot noir, and avoid the development of a sparkle. In his intent to prevent the development of a fizzy wine, Dom Perignon was undone. What is now called ‘consumer demand’ took over when the social dilettantes in Paris fell in love with the bubbles, and la joie de vie that it came to epitomize.
Wine from Champagne, like elsewhere back then, was exported in barrel. In arrival in England, it was bottled and closed with cork (Simon, 1971). The wine typically became spritzy in the spring, due to either reactivated alcoholic fermentation on residual sugars, or to malolactic fermentation. This novel ‘sparkling’ wine had become sufficiently renowned by 1676 to be noted by George Etherege in The Man of Mode (Act IV, scene 1). The effervescence effect of adding sugar was also sufficiently known and noteworthy to have been the subject of a communique by Christopher Merret to the Royal Society, December 17, 1662. It was entitled Some Observations Concerning the Ordering of Wines. In addition, by 1675, the procedure was described in a book On the Art and Mystery of Vintners and Wine Coopers (Simon, 1971). Establishing the correct amount of sugar to add, to avoid frequent and unpredictable bottle explosion, had to await developments in chemistry more than century later. The appropriate formula (4 g sugar/100 kPa CO2/liter) was established by François (1837). Its precise application itself required being able to accurately assess the residual sugar content in the base wine. This became possible when Ernst Abbe invented the first refractometer in the late 1800s. Although not ideal for measuring the sugar content of wine, its measurements could be adjusted to rapidly approximate the wine’s sugar content. Additional developments involved the introduction of machines to insert the cork in 1827, supply the dosage and attach agrafes in 1844, and apply the wire mesh to the finishing cork in 1846 (Loubère, 1978).
By the 1720s, the popularity of bubbly champagne was well established, both in England and at the French court. A flying cork is clearly illustrated in a painting by Jean François de Troy, entitled The Oyster Lunch (circa 1720). Nonetheless, there is no documentary evidence that Dom Perignon (1638–1715), cellar master in the Benedictine Abbey, Hautvillers, in Champagne, had any direct role in its development. His role relates to practices he encouraged in the vineyard, during pressing, and with the art of blending wines from separate vineyards. These aspects are ascribed to Dom Perignon in an anonymous work, entitled Mémoire sur la Manière de cultiver la Vigne et de faire le Vin. The book, published in 1718, is thought to have been penned by Jean Godinot, Canon of Riems. Dom Perignon discouraged the use of white grapes, preferring Pinot noir, presumably due to a desire to produce red wine, and the propensity of white wine to referment in the spring. The preference for red wine could, alone, be interpreted as indicating he was opposed to bubbly wine.
Even with strong bottles and cork closures, the early inability to judge accurately if, and how much, sugar to add to the base wine led to extensive, explosive, bottle loss. Cellar workers wore head masks for protection. In addition, discovering how to efficiently remove the accumulated yeast sediment occurred only in the early 1800s. This is ascribed to Mme Clicquote, and her cellar master Antoine de Müller. Thus, sparkling champagne, as we know it, was long in developing. The same is true, relative to its transformation from its early, very sweet renderings to its current dry version.
The finesse that is now the hallmark of champagne was slow to be championed. It became associated with Parisian nightlife in its original, sweet version. As such, it was considered vulgar by the cognoscenti. The taste for dry champagne came later, and developed as an English predilection, apparently beginning in the 1850s. This penchant quickly spread, becoming the accepted style among the social elite. The transition in preference from sweet to dry is reflected in the evolution of champagne terminology: doux being very sweet, demi-sec being sweet, sec still detectably sweet, extra sec approaching dryness, and only brut actually perceptibly dry. Only the nature style has no sugar added to the wine after disgorging (yeast removal). Subsequently, the methode champagnoise, as it came to be called, spread throughout most of the winemaking world. The twentieth century saw additional improvements, designed primarily to minimize production costs.
A classification of sparkling wines is given in Table 1.2, with the three major processes compared in Fig. 9.19. Although sparkling wines are usually classified by production method, this is of little practical value to consumers. Wines produced by these techniques are frequently distinguishable only by close and careful sensory evaluation. More obvious sensory differences develop from the color and aroma of the base wines, the degree of carbon dioxide supersaturation, the duration of lees contact, and the sweetness given the finished wine. Here, sparkling wines are discussed traditionally, that is, relative to their method of production. Figure 9.20 outlines the traditional (champenoise) method described below.
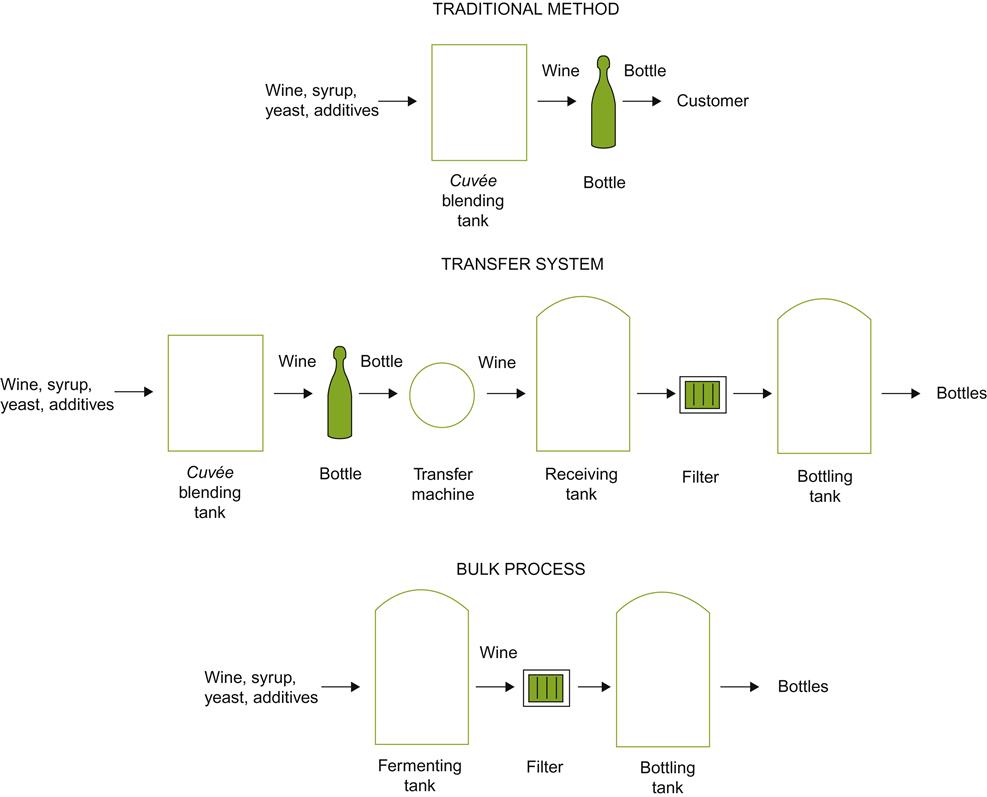
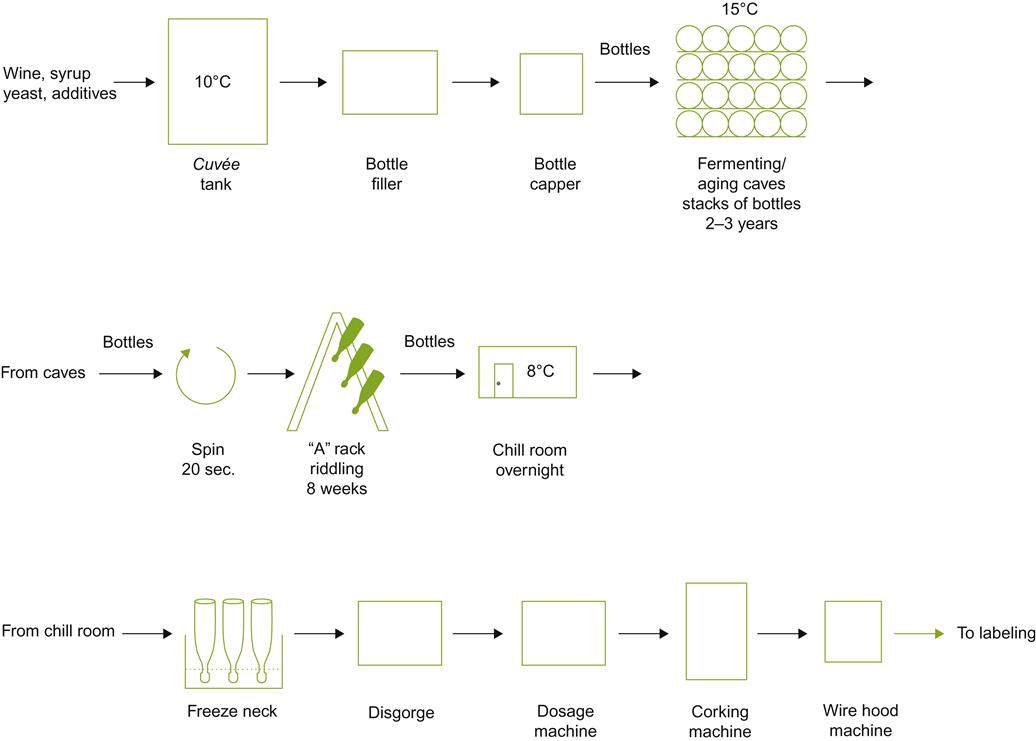
Standard Process
Grape Cultivars Employed
Although white or red grapes are vinified to produce base wine(s), most sparkling wines are white. Thus, when red grapes are used, particular attention must be taken during harvest and pressing to severely limit pigment extraction.
In Champagne, three grape varieties are used – one white (Chardonnay), and two red (Pinot noir and Meunier). Although the varieties may be used separately, most champagnes are derived from a blend of all three. Each variety is deemed to contribute unique qualities to the blend – Chardonnay providing finesse and elegance, Pinot noir donating body, and Meunier giving fruitiness and roundness. However, published evidence for such claims is absent, and what little has been written on the topic does not appear to support these views (de la Presa-Owens et al., 1998). The study showed an enhancement in flavor intensity but a reduction in varietal attributes.
Traditional wisdom also suggests that each variety matures at a different rate – Chardonnay wines the slowest, and Meunier wines the quickest. Thus, Meunier features prominently in nonvintage blends, aged about 1 year in-bottle before disgorging (separation from the yeast). Conversely, Chardonnay is commonly an important component in vintage blends, matured for at least 3 years before disgorging. The varieties are also considered to differ in their tendency to effervesce and develop a cordon – a ring of bubbles around the edge of a glass. Pinot noir has the greatest tendency to generate a stable effervescence, whereas Chardonnay the least (Marchal et al., 2001). Surface foam stability is an independent property, unrelated to the wine’s effervesce attributes (a function of bubble nucleation and size). Foam stability relates to surfactants that collect around the bubble. Flavor retention in the mouth is also reported to be a varietal characteristic (Penning-Rowsell, 1979), but again without documentary support.
In other areas of France, regional cultivars, such as Chenin blanc in the Loire Valley, are often the dominant or only varieties employed. Outside France, either indigenous or imported cultivars are used. In Spain, the native varieties Parellada, Xarel-lo, and Viura are employed. Each variety is considered to contribute a different and important characteristic to the blend – Parellada providing fragrance and softness, Viura donating finesse and elegance, and Xarel-lo imparting strength and a golden color.
Harvesting
Where financial returns permit, harvesting of both white and red grapes occurs manually. Manual harvesting permits both pre- and post-harvest selection to exclude infected grapes. This is especially critical where red grapes are used. Laccase and glucose oxidase, released by Botrytis cinerea, can cause serious oxidative browning. It can also negatively affect foam stability (Marchal et al., 2006). Manual picking minimizes fruit rupture, the release of juice, subsequent oxidation of the juice, and pigment and tannin extraction. However, because of the slowness of manual harvesting, fruit may not be picked at optimal quality. The inability to harvest quickly can occasionally lead to considerable quality loss under inclement weather conditions.
Harvesting occurs earlier than is usual for table wine production. This yields grapes higher in total acidity and of lower pH. Acidity and pH are important features regulating the freshness desired in sparkling wines. Early picking also assures lower °Brix, yielding wines of reduced alcohol content. An alcohol content of between 9 and 10.5% is preferred for base wines. In addition, slightly immature fruit have less varietal aroma, appropriate for the production of most sparkling wines. Finally, the grapes are more likely to be healthy. It is not surprising that Champagne, the most northerly wine-producing region in France, became associated with the production of sparkling wine. Grapes from the region yield relatively poorly colored, acidic juice, relatively low in sugar content and varietal character, what was called vin gris. These are all features now considered desirable for the production of dry sparkling wines, accentuating subtlety and finesse. If the sugar content is too low, it can be adjusted by chaptalization.
Harvesting preferentially occurs during cooler parts of the day and/or under cloudy conditions. This has the advantage of avoiding the expense of cooling the juice before inoculation and fermentation. It also retards oxidation of the juice during pressing and any premature initiation of fermentation.
Pressing
Grapes are pressed whole, without prior stemming or crushing. This gently releases the juice, minimizing pigment extraction, grape solids release, polyphenol oxidase liberation, and potassium solubilization. The juice so liberated is called the cuvée. It has little oxidation potential. Thus, little sulfur dioxide is required, or bentonite to promote clarification. Whole-grape pressing also limits the extraction of varietal aroma compounds localized in the skins. They could mask the subtle, aged, fermentation bouquet so desired in champagne and equivalent, dry, sparkling wines. Pressing whole grapes is also considered to promote early malolactic fermentation, and favor the onset of the second, in-bottle, yeast fermentation.
Pressing whole grapes takes considerably longer than conventional pressing. Using the large-diameter, vertical presses historically preferred in Champagne, it could take upwards of 2 h for the release of the cuvée. Although the slow liberation exposed the juice to oxygen, any pigment formation tended to precipitate during settling or fermentation. This provided protection against subsequent, in-bottle, oxidative browning.
In pressing whole clusters, the stems provide channels for the juice to escape, minimizing the pressures required. Large shallow presses also provide an expanded surface area for juice release, further minimizing the pressures needed. Nevertheless, pneumatic horizontal presses have essentially replaced traditional presses for all but illustrative purposes. Pneumatic presses generally work well with unstemmed, uncrushed grapes. They also have the advantages of taking up less space, being easier to load and unload, and permitting more efficient pomace crumbling between successive pressings. Whether automated versions of traditional presses can sustain their diminished use is a moot point, even in Champagne.
Approximately 62.5 liters of juice/100 kg grapes is permitted in champagne production. The initial and largest fraction (~80%) is officially termed the cuvée (Fig. 9.21). When juice flow slows to a trickle, pressure is released, the grapes mixed (retrousse), and pressure reapplied. This may occur several times during the liberation of the 2500 liters permitted from 4000 kg grapes (the amount added to a traditional vertical press). Subsequent fractions are called the taille. For their release, pressures exceeding 100 kPa are required. The première taille refers to the first 500-liter fraction. Its lower acidity and higher tannin content usually preclude its use in champagne production. The fraction also possesses more fruit flavors. Nevertheless, the première taille may be useful in particular proprietary blends. The 2emetaille is not used in champagne production. For details on the chemical composition of these various fractions, see Valade and Blanck (1989).
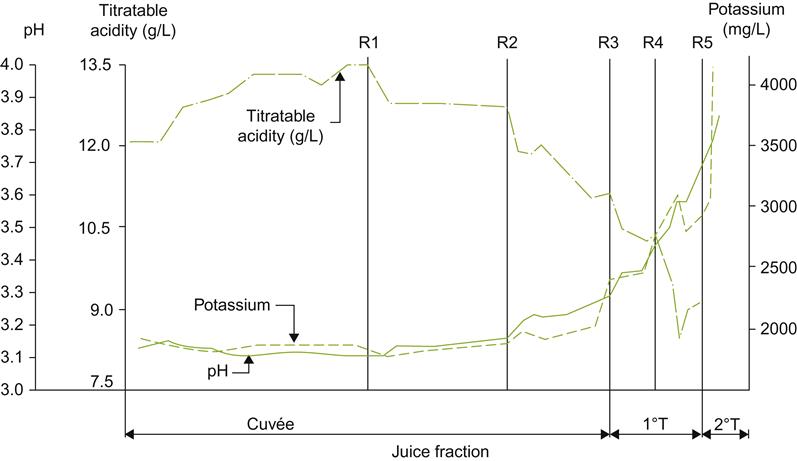
In much of the New World, quality and pricing constraints, not legislation, are the major factors influencing juice yield. Increasing the volume pressed from the grapes, beyond a particular point, compromises quality. This results as the increased flavor extraction and phenolic content begins to detract from the wine’s subtle bouquet. Tannins are particularly undesirable. They can impede the second, in-bottle fermentation, and augment the wine’s tendency to gush on agitation.
Sulfur dioxide is added to the juice as it comes from the press, 40–60 mg/L being typical in Champagne. Depending on the quality of the vintage (the maturity and proportion of diseased fruit), sugar, acid, bentonite, charcoal, and pectinase may be added. In cold climatic regions, sugar addition may be required as the °Brix value may be insufficient, whereas in warm climates, tartaric or citric acid supplementation may be necessary to compensate for deficiency in total acidity. Bentonite, activated carbon, pectinase, and additional sulfur dioxide may be added to remove pigments, degrade glucans, or inactivate enzymes released from diseased fruit. Hyperoxidation has been studied as a substitute for activated carbon in decoloration and astringent phenol removal from must, principally in the taille of Pinot noir and Meunier (Blanck, 1990).
Juice not already cool at harvest is routinely chilled down to approximately 10 °C, and left to clarify by settling for 12–24 h before fermentation. Alternatively, the must may be clarified more quickly with centrifugation or filtration.
As noted, grapes pressed whole liberate fewer solids than those pressed after crushing (0.5 vs. 2–4%) (see Randall, 1987). Where grapes are crushed before pressing, the extra solids are normally removed prior to fermentation via bentonite-facilitated settling, centrifugation, or filtration. The use of peristaltic pumps in transporting the juice to temporary storage tanks minimizes particulate generation following crushing and pressing.
Even under optimal conditions, the juice obtained from red grapes may contain a slight pinkish tinge. The anthocyanins involved usually coprecipitate with yeasts during fermentation, or later during fining. Anthocyanase addition, or other forms of decolorization, are customarily unnecessary with juice from healthy grapes.
Primary Fermentation
Juice fermentation follows procedures typical for most white wines. Fermentation occurs primarily in large-volume, stainless steel tanks, but special lots may still be barrel fermented. Fermentation usually occurs at approximately 15–18 °C. Lower temperatures are reported to give a grassy odor, whereas higher temperatures yield wines lacking in finesse (Moulin, 1987). Bentonite or casein, if not added earlier, may be added to aid fermentation and remove excess polyphenolics. Occasionally, a mixture of bentonite, potassium caseinate and microcrystalline cellulose may be substituted (Puig-Deu et al., 1999). Inoculation with selected yeast strains is almost universal. It helps avoid the production of perceptible amounts of sulfur dioxide, acetaldehyde, acetic acid, or other undesired volatiles potentially synthesized by indigenous yeasts.
If the juice is too low in pH (≤3.0), malolactic deacidification is commonly encouraged. This permits a greater proportion of the wine to be left dry (brut). In addition, some producers believe that malolactic fermentation donates a desirable, subtle bouquet. As the bacterial sediment produced is difficult to remove by riddling, it is important that malolactic fermentation be complete before the second, in-bottle fermentation. Malolactic fermentation is encouraged by minimal SO2 addition and maturation of the wine at or above 18 °C. Producers may also inoculate the wine with a particular strain of Oenococcus oeni to encourage its rapid onset. If producers wish to avoid malolactic deacidification (to enhance aging potential), the wine is sterile-filtered.
Finally, wines are clarified and cold-stabilized by cultivar, site, and vintage. Maturation may last for several months to years. Aging typically occurs in stainless steel, but occasionally occurs in large or small oak cooperage. Certain producers are reported to mature some of the base wines on lees under light CO2 pressure (100–150 kPa) in 1.5-liter bottles (Randall, 1987).
After maturation, the wines are ready for preparation of the cuvée. The second application of the term refers to the blend of base wines that will be used in the production of the sparkling wine.
Preparation of the Cuvée
The blending of wines derived from different sites, varieties, and vintages is one of the hallmarks of sparkling wine production. Because single wines seldom possess all the features producers desire, samples from different base wines are combined to obtain a small number of primary blends, upon which the formula for the cuvée is developed. The selection process is based solely on sensory evaluation. In addition to improving the quality of the sparkling wine, blending helps abate yearly variations in quality and supply. This is essential in producing the consistency required for proprietary brands.
Because blending can disrupt tartrate equilibrium, the cuvée is typically cold-stabilized to reestablish stability. The cuvée is loose-filtered cold, to remove any tartrate crystals that form. Tight filtration is less desirable as it may remove proteins and polysaccharides important in the formation of a fine and stable mousse.
Tirage
Tirage involves adding a concentrated (50–65%) sucrose solution, plus other nutrients or adjuvants, to the cuvée. The mixture is added just before yeast inoculation. The tirage may be made up in water or the cuvée itself. When wine is not the solvent, citric acid may be added at 1–1.5% to activate the hydrolysis of sucrose to glucose and fructose.
Sufficient tirage is added to supply about 24 g sucrose/liter. During fermentation, this produces a pressure considered appropriate for most sparkling wines, about 600 kPa (6 atm). Because the pressure exerted by carbon dioxide varies with the temperature and other factors, the concentration of CO2 is occasionally expressed in terms of mass. For most 750-mL bottles, this equates to 9 g CO2 (5 liters of compressed gas). This sucrose/CO2 conversion ratio results from the poor growth conditions. These permit essentially only catabolic (CO2 generating) metabolism.
If the cuvée blend contains residual fermentable sugars, this is subtracted from the amount added with the tirage. Approximately 4.2 g sugar is required for the generation of 2 g carbon dioxide. During the second, in-bottle fermentation, the alcohol content generally rises by about 1%.
Thiamine and nitrogen (DAP – diammonium hydrogen phosphate) are typically added in the tirage to supply 0.5 and 100 mg/L, respectively. Thiamine appears to counteract the alcohol-induced inhibition of sugar uptake by yeast cells (Bidan et al., 1986). Nitrogen addition is unnecessary if the concentration of assimilable nitrogen in the cuvée is above 15 mg/L. When insufficient, supplementation helps suppress the production of hydrogen sulfide. Occasionally, trace amounts of copper salts (≤0.5 mg/L) are added to further reduce hydrogen sulfide accumulation (Berti, 1981). Some producers incorporate bentonite, casein, gelatin, or isinglass to aid yeast flocculation at the end of fermentation. Evidence suggesting a negative effect of most fining agents on effervescence indicates that their use may be ill-advised (Maujean et al., 1990), or at least minimized. Data from Dambrouck et al. (2005) suggest that the detrimental effects of bentonite on protein removal (and foam stability) are largely counteracted by the simultaneous addition of casein. There is circumstantial evidence that removal of invertase, a glycoprotein that constitutes 10–20% of wine proteins, may be important to foam stability.
If the base wines have not undergone malolactic fermentation, the cuvée may be sterile-filtered. Providing a sulfur dioxide content of greater than 10 mg/L free SO2 is effective in inhibiting lactic acid bacteria, but less preferable. It can further complicate initiation of the second, in-bottle fermentation.
Yeasts and Culture Acclimation
The second fermentation involves inoculation of the cuvée with a special yeast strain. Because of the special and exacting conditions that prevail during this fermentation, yeasts must be capable of commencing fermentation at alcohol contents between 8 and 12%, at temperatures around 10 °C, at pH values as low as 2.8, and with free sulfur dioxide contents up to 25 mg/L. The suppressive influences of pH and sulfur dioxide are illustrated in Figs. 9.22 and 9.23. If cold tolerance is of particular concern, strains of S. uvarum may be used. Their suitability may be compromised, however, by their tendency to produce increased amounts of isoamyl and isobutyl alcohol, and quadrupled levels of 2-phenyl alcohol (Massoutier et al., 1998). In addition, S. uvarum does not flocculate well. This has recently been overcome by interspecific hybridization, between S. uvarum and a flocculent strain of S. cerevisiae (Coloretti et al., 2006).
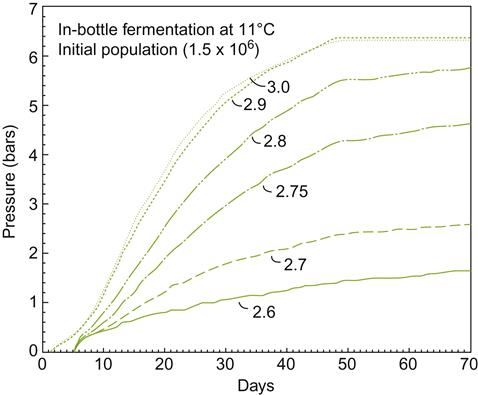
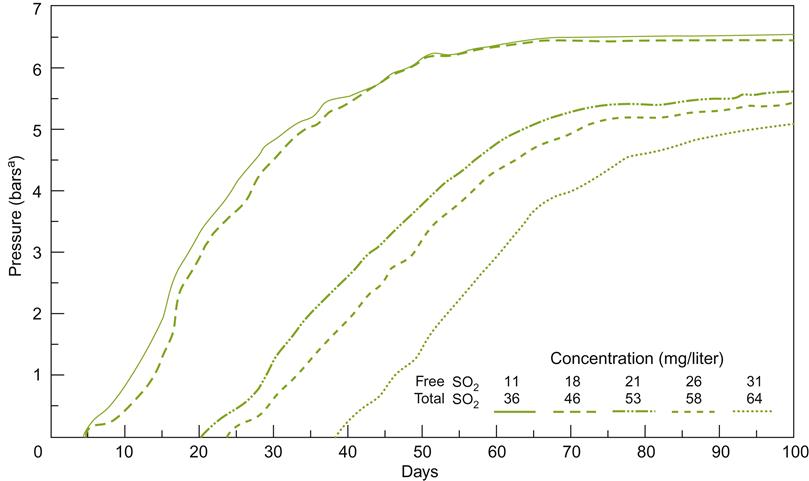
Flocculation, generating a coarse sediment, is essential for efficient lees removal during riddling. Developments in the use of encapsulated yeast may avoid both the need for flocculation and the expense of the riddling–disgorging process, but have yet to become standard practice.
The yeast strain must also have low tendencies to produce hydrogen sulfide, sulfur dioxide, acetaldehyde, acetic acid, and ethyl acetate. The presence of an active proteolytic ability after fermentation is also desirable. It promotes amino acid and oligopeptide release during yeast autolysis.
Because of the unfavorable fermentation conditions, the yeast inoculum is acclimated before addition. Otherwise, most of the yeast cells die, resulting in a prolonged latency before fermentation commences. Acclimation usually starts with inoculation of a glucose solution at about 20 to 25 °C. The culture is aerated to ensure adequate production of unsaturated fatty acids and sterols (required primarily in this instance for proper membrane function). Once the yeasts are actively growing, the culture may be added to enough cuvée to produce a 60:40 mix. Over the next few days, cuvée wine is added to reach a 80–90% cuvée mixture. Simultaneously, the culture is slowly cooled to adapt the cells to the desired fermentation temperature (Juroszek et al., 1987).
The cuvée is inoculated with the acclimated culture to reach a concentration of approximately 3–4×106 cells/mL (often equating to 2–5% the cuvée volume). Higher inoculation levels are thought to increase the likelihood of hydrogen sulfide production, whereas lower levels increase the risk of failed or incomplete fermentation.
Second Fermentation
Once the cuvée has been mixed with the tirage and yeast inoculum, the wine is bottled. In the past, bottles were sealed with a cork stopper, held by a reusable metal clamp, called an agrafe. Although some producers are again adopting the technique (Valade et al., 2011), cork stoppers have largely been replaced with an especially designed crown cap. The cap possesses a polyethylene bidule. It is a hollow, indented plug (typically 17 mm by 14 mm) that separates the wine from the cap. This helps retain the lees that collect during riddling, and facilitates a cleaner disgorging. Crown caps are also less expensive, less oxygen permeable, and more easily removed by automated machines than agrafe-attached corks during disgorging. Nonetheless, even crown caps can vary in oxygen and carbon dioxide permeability (Valade et al., 2011). Lower permeability characteristics can increase the accumulation of dimethyl sulfide (Vasserot et al., 2001a). Despite this potential, champagne has not been characterized by the undesirable sensory consequences of excessive dimethyl sulfide accumulation.
Occasionally 375-mL, 1500-mL, and larger volume bottles are used, but the 750-mL bottle is standard. Unless a brand-distinctive shape or color is used, the bottle typically has pronounced sloping shoulders and a greenish tint. The glass is thicker (4.5–8 mm) than for table wines. This helps them withstand the high pressures that develop during the second fermentation (Volovik, 1968). Special care is also taken during annealing, to minimize stress retention in the glass. Such stress could lead to bottle explosion.
Filled bottles may be stacked on their sides in large, freestanding piles (Fig. 9.24), in cases, or in specially designed containers ready for mechanical riddling. The wine is kept at a relatively stable temperature, preferably between 10 and 15 °C, at least for the second fermentation. Cooler temperatures may result in premature termination of fermentation, whereas warmer temperatures may result in both a rapid rise in alcohol content and a drop in redox potential. The latter is likely to increase hydrogen sulfide production (Markides, 1987). A stable temperature also helps maintain yeast viability under difficult fermentation conditions.

At 11 °C, a common fermentation temperature in Champagne, the second fermentation often takes about 50 days (Fig. 9.25). Temperatures up to 15 °C may also be used. During the early stages of fermentation, the yeast population goes through three to four cell divisions, reaching a final concentration of approximately 1–1.5×107 cells/mL. The rate of fermentation depends primarily on the temperature, initial CO2 content, pH, and sulfur dioxide content.
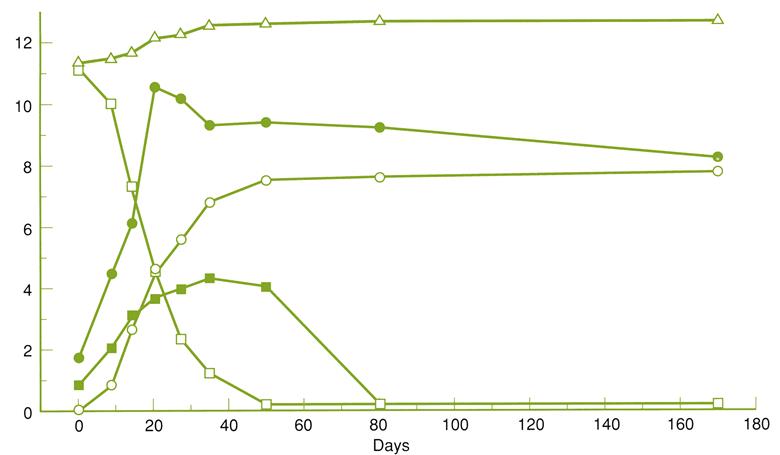
 , total yeasts (×106);
, total yeasts (×106);  , viable yeasts (×106);
, viable yeasts (×106);  sugars/2 (g/liter);
sugars/2 (g/liter);  , pressure (bars);
, pressure (bars);  , ethanol (%). (After Bidan et al., 1986, reproduced by permission.)
, ethanol (%). (After Bidan et al., 1986, reproduced by permission.)After fermentation, the bottles may be transferred to a new site for maturation, typically at 10 °C. The dead and dying yeast remain in contact with the wine for a minimum of 9 months, but may continue for 3 or more years, depending on the wine attributes desired. For effervescence production and foam stability, a maturation period of about 18 months seems optimal. These properties appear principally correlated with the accumulation of polysaccharides (Andrés-Lacueva et al., 1997). Further aging seems to result in polysaccharide hydrolysis.
During in-bottle maturation, the number of viable cells drops rapidly. After approximately 80 days, the viable yeast population drops to below 106 cells/mL. By disgorgement, normally 9 months to 1 year after tirage, few if any viable (culturable) cells remain. Even within 6 weeks, cells show atypical, large, expanded vesicles. By 3 months, the cells become plasmolyzed and most typical membrane-bound organelles have disappeared (Piton et al., 1988). This is associated with equally marked changes in membrane lipid content. Changes in cell wall structure also occur, notably the disappearance of the innermost layer. The rapid decline in viability contrasts greatly with the slow decline following the primary fermentation.
These structural changes are associated with major metabolic perturbations. As the wine becomes depleted in fermentable sugars, the cells begin to metabolize internal energy reserves, notably glycogen. As nutrient conditions deteriorate further, cells start to show autophagy (Cebollero and Gonzalez, 2006) and die. One of the first indicators of degeneration is the leakage of cellular nutrients, due to disrupted membrane function. As cells die, autolysis commences, despite unfavorable low pH and temperature conditions. Autolysis involves the release and activation of cellular hydrolytic enzymes. These degrade structural cell components. Along with cell wall mucopolysaccharides, several potentially significant flavor enhancers (nucleotides and glutamate) are released. Deletion of the BCY1 gene from yeast strains appears to speed autolysis (Tabera et al., 2006). Up to 50% of the yeast biomass may be liberated during autolysis (Leroy et al., 1990).
Yeast strain, grape variety, storage conditions, and duration of lees contact all influence the release of nitrogenous compounds from autolysing yeast cells. Yeast strains differ not only in the amount, but also in the specific amino acids released. A temperature of 10 °C has generally been considered optimal. Higher temperatures increase the rate of nitrogen release, and change the attributes of the compounds liberated. Temperature also influences the rate and types of aromatic compounds freed.
The release of amino acids and oligopeptides has frequently been associated with the development of a toasty bouquet. This may arise from the generation of thiols, such as benzenemethanethiol, 2-furanmethanethiol, and ethyl 3-mercaptopropionate (Tominaga et al., 2003). Amino acids can also be precursors for other significant aromatics, such as sotolon (from threonine), ethoxy-5-butyrolactone (from glutamic acid), benzaldehyde (from phenylalanine), and vitispirane (from methionine) (see Bidan et al., 1986). However, changes in their concentration seem not readily correlated with the content of the pertinent amino acids. The concentration of some, such as 1,2-dihydro-1,1,6-trimethylnaphthalene (TDN) and vitispirane increase during lees contact (Francioli et al., 2003). Nucleotides, with potential flavor influences, also occur in champagne (Charpentier et al., 2005). Individually, their concentrations do not appear to accumulate sufficiently to affect the wine’s sensory attributes. This does exclude the possibility that they may act in conjunction.
Besides the distinctive toasty profile, typically associated with sparkling wines aged on lees for several years, other prominent aromatic aspects include those resembling lemon and ripe fruit (Priser et al., 1996). Minor attributes may include floral, caramel, vanilla, buttery, vegetal, yeasty, and animal aspects. Figure 9.26 presents additional sensory differences associated with lees contact.
Changes in the concentrations and types of fatty acids and lipids have also been noted during lees contact. The level of fatty acids may increase initially, but subsequently decline. Polar lipids decrease in concentration, whereas neutral lipids increase. Such changes may continue for at least 11 years (Troton et al., 1989). The triacylglycerol accumulated may act as an important precursor for aromatic compounds during aging.
Modification in the concentration of esters has been reported during in-bottle maturation. Similar to still wines, most fruity/floral aspects, commonly derived from acetate and ethyl esters of fatty acids, decline (Francioli et al., 2003). In contrast, those formed from the major organic acids increase (Silva et al., 1987). Although contact with lees typically lasts no more than 3 years, an example of changes in the volatile character with extended contact is shown in Fig. 9.27. Extended lees contact, when provided, is usually designated on the label by the expression ‘late disgorged.’ Additional details are provided in Riu-Aumatell et al. (2006). Modification associated with post-disgorgement aging is discussed later in this section.