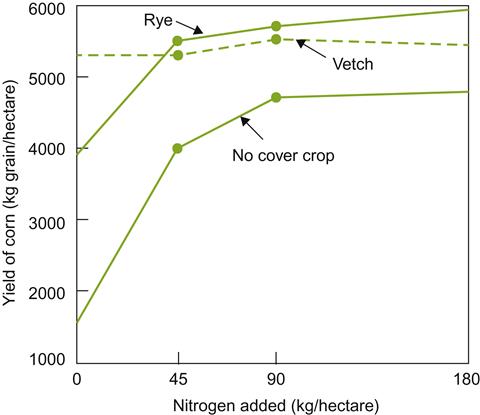
High seed costs, the need for Rhizobium inoculation, and higher water demands can limit legume feasibility. Occasionally, these disadvantages may be avoided with self-seeding legumes, such as burr clover. Cereal crops require less water and can often penetrate and loosen compacted soil. Consequently, a mix of legumes and grasses is frequently used (Winkler et al., 1974). This has the additional advantage of providing a greater range of habitats for beneficial insects, enhancing potential pest control. Nevertheless, the habitat provided must be carefully selected to avoid favoring rodent populations. Positioning nesting or resting sites for owls and hawks in or adjacent to vineyards can help control rodent pests and have the additional advantage of minimizing bird damage (Saxton et al., 2009; Saxton and Kean, 2010).
The effect of groundcover choice on the grape flavor has rarely been considered. Work by Feng et al. (2011) is beginning to shed light on this potentially significant issue. For Pinot noir, cover crops can influence the wine’s sensory attributes.
Composting
Except for green manures, most organic fertilizers are best initially composted. Composted material is less variable in chemical composition, and its nutrient content released more slowly. Compost can also have additional benefits, such as acting as a mulch (reducing water evaporation) and helping in annual weed control. Co-composting with winery wastes (stalks, seeds, and pomace), winery or vineyard wastewater, or sewage sludge helps convert these materials into a product that can be more safely added to vineyard soils. Experimentation is required to establish an effective formula for efficient and effective composting. For example, excessive water content complicates composting. Occasionally, sewage sludge is contaminated with heavy metals, obviating its use (Perret and Weissenbach, 1991).
Simple composting involves mounding the waste into windrows, frequently 1.5 m high, 2 m wide, and 4 m or more in length. These have sufficient size to insulate the interior, permitting its temperature to rise to 55–70°C. The heat, generated by microbial activity, selects thermophilic bacteria and fungi that quickly degrade most readily decomposable organic material. The combination of heat and microbial decomposition also tends to pasteurize (but not sterilize) the waste, reducing most plant pathogens and weeds to undetectable levels (Noble et al., 2009). Its effect on grape virus inactivation appears not to have been investigated. Data on other viruses suggest that exposure to temperatures near 70°C for 3 weeks is effective in eliminating most viruses. Thus, systems that do not expose all the material to adequate temperatures are probably best avoided if the compost is to be applied to vineyards.
Because composting is most efficient when microaerobic, the mounds are turned periodically. Frequency depends on the rapidity with which the internal temperature rises. Temperatures above 65–70°C inhibit the action of the most desirable thermophilic microbes. Turning not only avoids overheating and incorporates material from the exterior, but also provides adequate oxygenation for the required microbial activity. This also avoids off-odor production from anaerobic activity. To further assist oxygen penetration, bulking agents such as wood chips, corn cobs, corn stalks, or straw are often incorporated. They provide aeration channels, as well as drainage passageways for water escape. Wood chips have the advantage that they do not decompose readily and can be separated for reuse. In so doing, they can act as a natural inoculum of desirable microbes for the next compost pile. Commercial inocula are available, but are seldom necessary if some finished compost is incorporated into the new piles.
Moisture levels ideally should be between 50 and 60%. Higher levels tend to shift metabolism toward anaerobiosis and malodor production. In addition, higher moisture levels can promote continued fermentation of ethanol to acetic acid, reducing the pH. At below 40% moisture content, decomposition is retarded.
Alternative composting techniques include windrows with aeration supplied by pumping air through perforated tubes under the static piles, silo-like vertical systems, agitated beds, plug-flow systems, and rotating drums (Arvanitoyannis et al., 2006). Choice depends primarily on space availability, need for speed, and economy. Where the first two are not of primary concern, windrows are usually the preferred choice.
Where the substrate consists primarily or solely of spent pomace, the pH must be raised to near neutral (6.5–7). This is required for activity of the thermophilic microbes involved in decomposition. Raising the pH usually involves the incorporation of lime into the mix. It is estimated that such use can return up from a third to half of all the nutrients removed at harvest back to the soil. Rates of application tend to vary between 2.5 to 10 kg/hectare. If the soil is saline or sodic, it may be necessary to add a source of calcium before application. Sodium levels may be higher in the pomace than desired, especially if winery wastewater has been incorporated. This can come from sodium-based cleansing agents used in the winery. Potassium contents may also be undesirably high. If the wastewaters come from municipal sources, additional sources of potential soil contamination can be a concern.
Once bacterial mineralization brings the carbon/nitrogen ratio close to 20:1, microbial activity in the compost slows, and the temperature falls. Further composting shifts to fungal action. The latter is particularly important in the humification of much of the remaining organic material. This partially involves the condensation of lignin-derived phenolics with ammonia. The humus so derived is refractory and mineralizes only slowly in soil. Thus, it makes an excellent soil amendment. Subsequent maturation involves degradation or volatilization of potential phytotoxins, such as ethylene oxide, ammonia, and low-molecular-weight fatty acids (notably acetic, propionic, and butyric acids). After some 12–24 weeks (depending on the temperature, moisture, and nutrient content, as well as turning frequency), the compost can be safely used as an organic amendment or mulch.
Disease, Pest, and Weed Management
Changes similar to those affecting vineyard fertilization are affecting disease, pest, and weed management. At the extreme edge of this change are schemes relying totally on organic and biodynamic concepts (Jenkins, 1991; Reeve et al., 2005). Although the latter are currently in vogue, principles of sustainable viticulture, which include advances in integrated pest management (IPM), at least have a solid scientific foundation (Broome and Warner, 2008). They are based on verifiable data, not quasi-philosophic concepts related to the long-disgraced concepts, the equivalent to élan vital. ‘Natural’ pesticides, such as phosphoric acid (Foli-R-Fos®), canola oil (Synertrol®) (Magarey et al., 1993), and potassium silicate (Reynolds et al., 1996) can occasionally be as effective as standard pesticides. This is not a consistent finding, though, with higher concentrations often being required (Schilder et al., 2002). In addition, there is no guarantee that ‘organic’ control agents are any more environmentally friendly or safe for human consumption than ‘synthetic’ control agents (Bahlai et al., 2010). After all, to be effective, they must be inherently toxic. Only those compounds or adjustments that modify the ecological niche (to the detriment of the pest) or modifications of the canopy microclimate are likely to have minimal or limited environmental impact.
Little, if any, research has been conducted on the effects of pesticides (natural or man-made) on the aromatic composition of wine. One study showed an effect, but most influences were below threshold values, with the exception of ethyl acetate and isoamyl acetate (Oliva et al., 1999). Other investigations have shown that organically produced grapes or wines can be as good quality as those traditionally produced (Henick-Kling, 1995; Hill, 1988). However, the wines were not shown to be demonstrably better. That organically produced wines are ‘healthier,’ or more beneficial to the environment, is unsubstantiated. Increases in the diversity of the soil fauna and flora are not inherently better (Lotter et al., 1999; Mäder et al., 2002). If ‘natural’ was always better, then disease should not be controlled. Disease is part of the natural environment.
That organic procedures may be adopted as a concession to vocal advocacy groups, or as part of a marketing strategy to gain access to a niche market, is one thing, but claims of superiority are another. If consumers, desiring wine produced by organic or biodynamic concepts, are willing to pay for these options, fine. What is discouraging are unsubstantiated claims that conventional procedures are inherently bad, and medieval procedures automatically good. In an ideal world, consumers would be taught the realities of pest management. Regrettably, this is neither feasible nor would it be effective against a mind set that is distrustful of agricultural firms and science in general. Blind faith is often comforting, reality uncomfortable.
Minimizing environmental damage is a laudable goal, but, as noted, natural pesticides are no more guaranteed to be safer than their synthetic counterparts. For example, Stylet-Oil reduced grapevine photosynthesis and the accumulation of soluble solids in the fruit (Finger et al., 2002). The effects were volume- and frequency-dependent. In an extensive study of natural vs. synthetic pesticides, the percentage potentially carcinogenic was the same, about half (Gold et al., 1992; Ames and Gold, 1997). (Because of their low concentration in food, none was considered to constitute a significant cancer risk.) In addition, accepted pesticides used in organic viticulture may disrupt the action of natural disease control agents. For example, sulfur can increase the mortality of Anagrus spp., a biocontrol agent of leafhoppers (Martinson et al., 2001), and suppress tydeid mite (Orthotydeus lambi) populations. This mite can reduce the incidence of powdery mildew (English-Loeb et al., 1999). These comments should not be construed as an attack on so-called ‘natural’ controls. No one, at least not me, advocates using anything more toxic or disruptive than necessary. What is required is honesty and objectivity, based on verifiable data, not apocryphal statements pontificated with missionary zeal. As more data on biological control accumulates, one will be able to establish its real value, and limitations, from potential.
In this regard, natural pesticides should require the same exhaustive assessment for efficacy, safety, and residue accumulation as synthetic agents now require. Lack of data should not inherently give rise to a false sense of security. This can lead to cases, such as with pyrethroids, where increased use leads to their accumulation in stream sediments to toxic levels for bottom dwellers (Weston et al., 2004). In addition, various oils used in organic pest control can affect the taste and odor of grapes and wine (Redl and Bauer, 1990). Although health concerns about the risks of pesticide use have induced several governments to contemplate pesticide deregistration, little thought has gone into the human health risks of the potential human health risks of increased mycotoxin contamination. This could have unconscionable consequences, as have other well-intentioned but ill-advised government reactions. Nevertheless, the inability of current pesticides and herbicides to provide adequate crop protection, and the need to reduce production costs, has wisely spurred research into alternative control measures. In addition, natural toxins can direct the search for, and synthesis of, even more effective and stable synthetic products, as has been the case with human medications (Petroski and Stanley, 2009).
One of the main concepts generating better and more cost-effective pest and disease control is IPM. The term ‘management’ reflects a paradigm shift – that of limiting damage to an economically acceptable level (its economic threshold). Limiting damage to an economic or sensory insignificant level is more feasible and prudent than attempting what is often impossible – absolute control or eradication. Integrated pest management combines the expertise of specialists in diverse but cognate fields, notably plant pathology, economic entomology, plant nutrition, weed control, soil science, microclimatology, statistics, and computer science. Coordinated programs usually reduce pesticide use significantly (Broome and Warner, 2008), while achieving improved effectiveness. IPM programs are more pragmatic and data-based than organic or biodynamic approaches. IPM often includes factors such as environmental modification and assessment, biological control, better synchronization of pesticide use and rotation, and the avoidance or limitation of undesirable interactions. Timing pesticide application to coincide with vulnerable periods in a pathogen’s life cycle usually reduces the number of applications, while increasing effectiveness. It also promotes optimal pesticide selection and application, appropriate to the situation; for example, use of a simpler (less specific) protective agent when disease stress is low, and application of a curative agent (more selective and often systemic) when disease incidence is likely to be high. This approach is much less disruptive to indigenous biological control agents. Advances in disease forecasting, combined with monitoring pest or disease incidence, permit more accurate prediction of potential damage. Because most models have been developed with data collected over a fairly large area, their predictive value may be inappropriate at sites deviating significantly from the norm. Only with extensive use is it possible to adjust predictions to specific vineyards and vineyard conditions.
In addition, risk assessment (the cost/benefit ratio) is being increasingly used to regulate if, and when, application is warranted. For example, spider mites are often considered a serious grapevine pest; however, little significant effect on yield and quality may develop even with heavy infestations of the European red mite (Panonychus ulmi) (Candolfi et al., 1993). Vine capacity may be less affected than appearance might suggest, due to compensation by the root and/or shoot system to foliage damage (Hunter and Visser, 1988; Candolfi-Vasconcelos and Koblet, 1990; Fournioux, 1997). Compensation is more evident early in the season; less so later.
Timing applications has been greatly facilitated by the combination of developments in computer hardware and software, miniaturization of solar-powered weather stations (Plate 4.13) (Hill and Kassemeyer, 1997), and geographic information system (GIS) technology. Their joint use has permitted the development of expert systems that can predict the incidence of disease and pest outbreaks, similar to weather forecasts. Such systems can also provide data to adjust irrigation schedules. Computer programs are now available for major diseases in several viticultural regions.
Although the economic and ecologic benefits of IPM are obvious, its successful implementation has been far from simple. The major factor involves its considerable developmental costs. It takes years to develop an effective program, requiring the dedication of specialists in many fields. Without their various skills, forecasting consequences with the requisite accuracy would be impossible. For example, the most efficient fungicides against a particular plant pathogen may be toxic to parasites of an equivalently significant pest. As well, reduction in pesticide use may lead to secondary pest outbreaks. For example, adoption of an IPM program, largely dependent on biological control agents for the western grape leafhopper in California, coincided with an infestation of variegated leafhoppers. Even seemingly unrelated changes in viticultural practice can affect IPM efficiency. For example, herbicide use to reduce loss of soil organic matter, and eliminate sites for pest overwintering, can increase the incidence of certain fungal diseases. In addition, improved nitrogen availability can diminish inherent disease resistance.
IPM systems must also be sufficiently flexible to accommodate variability in disease/pest severity, associated with regional and annual climatic fluctuations. Finally, predicting the economic benefits of IPM programs is fraught with enigmas. In most instances, the financial losses actually ascribable to specific pest and disease agents are guesstimates at best. In some instances, reduced yield may permit the optimal ripening of the remaining fruit. Thus, calculating the cost/benefit ratio of control strategies is fraught with difficulty. Pesticide application is too often driven more by fear than need – the ‘ounce of prevention’ concept. As a result, full implementation of IPM strategies tends to be regional, and more directed toward the most economically significant disease and pest agents. Although not perfect, IPM offers a more integrated and rational approach to disease and pest management than any other process. It can also deliver both economic savings and environmental benefits.
Although IPM is preferable, individual components can be of considerable value alone. For example, research on pesticide combinations can avoid potential mutual interference, and reduce application rates and frequency (Marois et al., 1987). Improvements in nozzle and sprayer design now provide better and more uniform chemical spread, reducing runoff and drift, while achieving better pest or pathogen contact. Assuring that a greater percentage of the target receives a toxic dose can delay the development of resistant strains. In contrast, sublethal doses selectively favor the survival (and reproduction) of pathogens and pests that inherently possess partial resistance to the control agent.
The most efficient nozzles available are those that give a small negative charge to the droplets as they are released (electrostatic sprayers). Plant surfaces (positively charged) attract the droplets, facilitating their uniform deposition, and limit removal by rain. Restricted variation in droplet size (optimum between 25 and 100 μm), and reduced water volume, minimize runoff and drift (from the vaporization of very small droplets). Improved uniform coverage also has the advantage of reducing the development of pesticide resistance. Non-uniform spread facilitates resistance evolution by selectively eliminating susceptible and moderately tolerant strains, leaving only the most insensitive to propagate and multiply. Greater efficiency of application may be achieved by spraying at night, when conditions are calm and evaporation from droplets is retarded. In addition, assessment of effective leaf area can be used to fine-tune dosage application (Siegfried et al., 2007).
IPM emphasizes a diversity of control measures rather than any one technique (Basler et al., 1991). Experience has indicated that dependence on any single control measure is likely to be ultimately unsuccessful. The same is equally probable with biological or genetic control schemes, especially if used to the exclusion of other control procedures. Because most pathogenic agents multiply and can evolve exceedingly rapidly, they are disappointingly adept at developing resistance to single selective pressures. They outnumber us by multiple orders of magnitude – a pathology example of ‘For many are called, but few are chosen’ (Matthew 22:14).
Pathogen Control
Chemical Methods
Faced with increasing pesticide resistance, and the difficulty and expense of finding new control agents, limiting resistance development has become imperative (Staub, 1991). Relatively nonselective contact agents are best used when prophylactic protection against a wide range of pathogenic fungi and bacteria is required – their broad-spectrum action minimizes the likelihood of them developing tolerance. Conversely, their broad-spectrum action is also one of their disadvantages – they are likely to be toxic to biological control agents as well. Nonetheless, occasional use, as needed, can preserve the selective and curative action of systemic agents for critical situations, where their precise action is most needed. By being incorporated into the plant, and frequently translocated beyond the site of application, systemic agents can inactivate pathogens and pests within host tissues, something contact pesticides cannot do. Systemic agents also tend to be less toxic to other organisms, notably the host. As such they are likely to be less toxic, or nontoxic, to potential biocontrol agents. In addition, they can often be applied at lower dosages. Dosage rates for contact agents tend to be in the range of 1000–3000 g/ha, whereas systemic application rates are more in the 400–500 g/ha range. Disease forecasting, the prediction of disease outbreaks based on meteorological data, is particularly useful in timing their application. It reduces the need for frequent application, and increases the effectiveness of what is applied. Where disease forecasting is not available, appropriate timing of application can often be scheduled based on the phenologic (growth) stage of the vine, and the pathogen’s life cycle. The precision permitted by the Modified E–L System (see Fig. 4.2) is well adapted to the spray timing.
Resistance to nonspecific (contact) pesticides tends to be slow to nonexistent. Single mutations are unlikely to provide adequate protection against the nonselective, extensive disruption of membrane structure and enzyme function induced by contact pesticides. When the availability of many nonselective fungicides began in the late 1940s, the destructive potential of several marginally important pests was forgotten. Their potential destructiveness reappeared only when the use of selective pesticides began to replace nonselective pesticides (Mur and Branas, 1991). For example, the increased incidence of the omnivorous leafroller in California can be partially attributed to reduced use of nonselective pesticides against grape leafhoppers (Flaherty et al., 1982). This has, in the case of organic viticulture, encouraged the use of nonselective pesticides, such as oils (e.g., Stylet-Oil), plant poisons (e.g., pyrethroids), or bacterial toxins (e.g., Bt toxin).
In contrast, selective and systemic agents tend to have highly specific toxic actions. These usually involve precise molecular sites on, for example, a particular mitochondrial enzyme or specific membrane component. Regrettably, this specificity facilitates their inactivation (tolerance) by single mutations in the pathogen. Initially, resistant populations tend to be less viable, due to a partial impairment of the active site associated with the mutation. However, after prolonged selective pressure, subsequent mutations may restore biological fitness, such as overexpression of genes of an alternative metabolic pathway. Other examples of competitively neutral resistance factors could involve mutations regulating ABC (ATP-binding cassette) and MFS (major facilitators super-family) transport proteins (Del Sorbo, 2000). These have the capacity to translocate fungicides out of the cell before they can produce significant metabolic damage. At this point, terminating application of the particular pesticide has minimal effect in reducing the population of resistant strains. They have become as ecologically competitive as pesticide-sensitive strains. Thus, for long-term effectiveness, selective and systemic chemicals are best limited to use only in situations where their precise in-tissue toxicity is required (Delp, 1980; Northover, 1987).
Another approach, which tends to prolong the effective ‘life span’ of selective pesticides, involves rotational application. By alternating among pesticides that have differing modes of action, the advantage of resistance genes against any one control agent may be delayed, if not completely avoided. Furthermore, absence of sustained pesticide pressure minimizes the likelihood of enhanced ecologic fitness being selected amongst resistant strains. Unfortunately, success in retarding the accumulation of resistance using rotation is not guaranteed (Baroffio et al., 2003).
An alternative approach involves a combination of nonselective and selective pesticides. Nonselective fungicides nonspecifically reduce the size of the pest or pathogen population – thereby reducing the likelihood of resistant gene selection in those escaping a toxic exposure. This approach has been accredited with delaying the emergence of resistance genes in some pathogen populations (Delp, 1980). Surveying resistance in local populations is an important component in assessing the continuing efficacy and value of any pesticide.
Another approach to delaying the development of stable pesticide resistance is the joint application of two or more selective pesticides. Theoretically, this vastly reduces the chances of resistance development. For example, if the probability of insensitivity to any one agent were equal to 1×10−8, the combined likelihood of any organism surviving (possessing or developing simultaneous resistance) to both agents would be (1×10−8)(1×10−8)=1×10−16. If three pesticides were applied together, the likelihood of a pest developing resistance to all three would fall to 1×10−24 (effectively nil). Although feasible, the cost of such application argues against it. An alternative is the joint application of a less expensive contact with the selective agent.
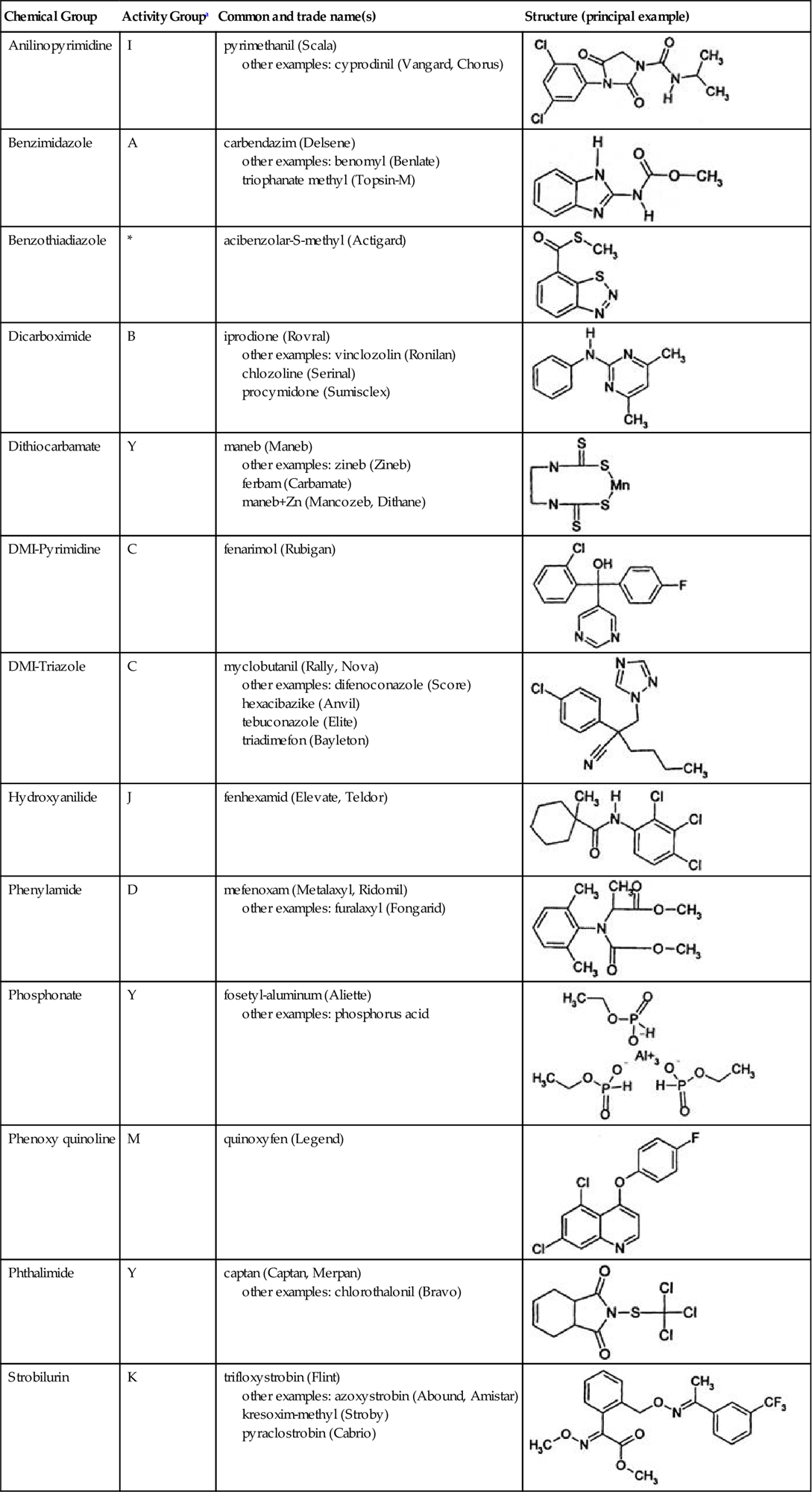
In both approaches it is important that each agent possesses unrelated toxic actions, typically associated with being in different chemical groups (Table 4.13). If the chemicals have similar modes of toxicity, the selective pressures they produce are likely to be equivalent. In this situation, resistance to any member of a group may donate resistance to other members of the group – a phenomenon termed cross-resistance.
Table 4.13
Examples of Fungicides Used in Controlling Grape Diseases, Arranged According to Chemical Family
| Chemical Group | Activity Groupa | Common and trade name(s) | Structure (principal example) |
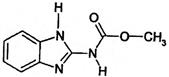
| Anilinopyrimidine | I | pyrimethanil (Scala) other examples: cyprodinil (Vangard, Chorus) |
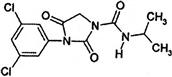 |
| Benzimidazole | A | carbendazim (Delsene) other examples: benomyl (Benlate) triophanate methyl (Topsin-M) |
 |
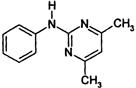
| Benzothiadiazole | * | acibenzolar-S-methyl (Actigard) | 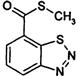 |
| Dicarboximide | B | iprodione (Rovral) other examples: vinclozolin (Ronilan) chlozoline (Serinal) procymidone (Sumisclex) |
 |
| Dithiocarbamate | Y | maneb (Maneb) other examples: zineb (Zineb) ferbam (Carbamate) maneb+Zn (Mancozeb, Dithane) |
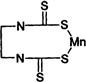 |
| DMI-Pyrimidine | C | fenarimol (Rubigan) | 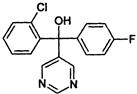 |
| DMI-Triazole | C | myclobutanil (Rally, Nova) other examples: difenoconazole (Score) hexacibazike (Anvil) tebuconazole (Elite) triadimefon (Bayleton) |
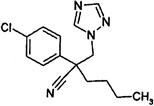 |
| Hydroxyanilide | J | fenhexamid (Elevate, Teldor) | 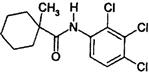 |
| Phenylamide | D | mefenoxam (Metalaxyl, Ridomil) other examples: furalaxyl (Fongarid) |
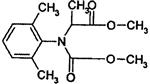 |
| Phosphonate | Y | fosetyl-aluminum (Aliette) other examples: phosphorus acid |
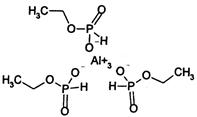 |
| Phenoxy quinoline | M | quinoxyfen (Legend) | 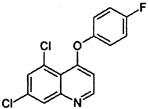 |
| Phthalimide | Y | captan (Captan, Merpan) other examples: chlorothalonil (Bravo) |
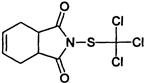 |
| Strobilurin | K | trifloxystrobin (Flint) other examples: azoxystrobin (Abound, Amistar) kresoxim-methyl (Stroby) pyraclostrobin (Cabrio) |
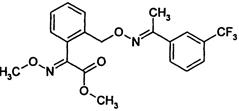 |
aGrouping of fungicides by their similar mode of action.
Another valuable means of retarding pesticide resistance is to improve application effectiveness. This frequently involves the use of better sprayers (Furness, 2002), combined with improved assessment of spray coverage (Furness 2009). Uniform crop cover is important. Sites where pesticide coverage is inadequate provide conditions favoring resistance development (selectively favoring the most resistant strains). The incorporation of adjuvants (stickers and/or spreaders) into spray mixtures can also improve the likelihood of agent effectiveness (MacGregor et al., 2003). Spreaders facilitate uniform dispersion over plant surfaces (by reducing surface tension that can disrupt distribution), whereas stickers minimize washoff. Regrettably, stickers also limit redistribution of the agent over leaf and fruit surfaces as they enlarge. Adjuvants, due to their action on cuticular wax structure, may also disrupt one of the pre-existing plant defenses against invasion. This is one of the potential problems with all soap/oil mixtures used in pest management.
Dusts, by virtue of their generally smaller mass, tend to spread more uniformly and disperse more effectively into dense canopies than droplets of a wettable agent. Conversely, dusts tend to settle and adhere more poorly to plant surfaces, with more potential for wastage due to wind drift.
Finally, application of other control measures (sanitation, environmental modification, etc.) can further delay the development of pesticide resistance. Because mutation is largely a random event, and therefore partially dependent on population size, the smaller the pest population, the less likely (i.e., more slowly) resistant mutations are to occur and be propagated.
None of these techniques will necessarily prevent the eventual development of resistance, but they can significantly delay its occurrence. The delay will depend on factors such as the widespread use of protective measures, the number of growth cycles per year, existing genetic diversity in the pest or pathogen population, the population size, and the facility with which the pest or pathogen is dispersed.
Traditionally, control agents have been either applied as liquid sprays or dusts. Both have potential problems, such as loss due to drift and washoff, dilution by rain, contamination of ground water, and damage to other organisms. Direct injection of systemically translocated compounds into the trunk would counter many of these factors. The application of metalaxyl against downy mildew by this method is as effective as spraying (Düker and Kubiak, 2009). Its disadvantage is the complexity of injecting individual vines.
Recently, a new and novel class of control agents has entered the market. They work by inducing systemic acquired resistance in the host. Their appearance is encouraging, as they take a distinctly different and ‘natural’ approach to disease and pest management. Examples are β-aminobutrytic acid, which enhances vine resistance to downy mildew (Cohen, 2002; Harm et al., 2011); acibenzolar-S-methyl, which is effective against powdery mildew (Campbell and Latorre, 2004); benzothiadiazol, which limits the development of bunch rot (Iriti et al., 2004) and downy mildew (Harm et al., 2011); and jasmonic acid against Pacific spider mites (Tetranychus pacificus) and phylloxera (Omer et al., 2000). Methyl jasmonic acid is a naturally synthesized, intracellular regulator mediating several diverse defense responses in plants. One of these involves a marked increase in the production of resveratrol and related stilbene phytoalexins (Vezzulli et al., 2007).
Partially limiting the application of some of the procedures noted above are archaic regulations, not only in the region of production, but also in importing countries (e.g., restrictions on pesticide residues). Because these regulations too often vary from year to year, and region to region, the issue is far more complicated than it needs to be and is discouraging to innovation.
Biological Control
Although synthetic and organic pesticides are likely to remain the main arsenal of agents against pests and diseases into the foreseeable future, increasing emphasis is being placed on biological control. Although some insect predators have developed pesticide resistance (Englert and Maixner, 1988), most remain sensitive to insecticides. Thus, to use indigenous insect or mite control agents, pesticide applications must be delayed, minimized, or avoided.
In addition to restricting insecticide use, it is often necessary to maintain a broad diversity of plants in the vicinity (Thomson and Hoffmann, 2009). These supply the variety of alternate hosts, food sources (pollen or nectar), and protection to support a sufficiently high population of control agents. Their population numbers often cycle considerably throughout the seasons (Bernard et al., 2006). Maintenance is also aided by providing interrow crops of flowering plants (Scarratt et al., 2007), but may still not be sufficient for meaningful control (English-Loeb et al., 2003). Thus, the release of commercially reared competitive species may also be required to effectively control pest populations. By establishing themselves on the host, competitors restrict the colonizing potential of the pest species. An example of competitive exclusion is the action of the Willamette spider mite (Eotetranychus willamettei) against the Pacific spider mite (Karban et al., 1997). Various fungi, yeasts, and bacteria can also displace fungal pathogens from their invasion sites.
Although insects are susceptible to many bacterial, fungal, and viral pathogens, few have shown promise in becoming effective biocontrol agents. Exceptions include the granulosis virus against the western grape leaf skeletonizer (Harrisina brillians) (Stern and Federici, 1990) and Beauveria bassiana (GHA strain) against western flower thrips. The major success story, though, has been Bt toxin, produced by Bacillus thuringiensis. The toxin is active against several lepidopteran pests, such as the omnivorous leafroller (Platynota stultana) and the grape leaffolder (Desmia funeralis).
Bt toxin produces ‘holes’ in the intestinal lining of insects, permitting intestinal bacterial access to the hemolymph (insect ‘blood’). The latter can induce septicemia (Broderick et al., 2006). In some regions, where Bt toxin has been extensively used, signs of resistance have appeared. This may be countered by the use of a subgroup of Bt toxins, in the Cry1Ca complex. They are toxic to lepidopteran insects currently resistant to standard Bt toxin (Avisar et al., 2009). An unsuspected benefit of its widespread use on some crops, such as corn and cotton, has been area-wide suppression of pests in varieties and crops not possessing the Bt toxin (Naranjo, 2011). Nonetheless, the development of resistance, and some expression difficulties in transgenetic varieties (De Rocher et al., 1998), may make the expense of engineering cultivars to produce Bt toxins of short-term value. The same fate might also befall genetically engineering grapevines to possess arthropod resistance with the Galanthus nivalis aggulitin (GNA) gene, or fungal resistance supplied by endochitinase genes from Trichoderma harzianum (Lorito et al., 2001).
Bacterial toxins also possess the potential for controlling fungal pathogens. Examples are syringomycin E and rhamnolipids, produced by Pseudomonas syringae and P. aeruginosa, respectively. Their combination is effective against a range of fungal pathogens (Takemoto et al., 2010).
Pheromones are another agent in the arsenal of biocontrol agents available for insect management. Pheromones are species-specific, airborne hormones produced by insects to locate mates. Thus, they can selectively attract particular insects to pesticide traps, or induce mating disorientation when applied throughout a vineyard. An alternative attractant that could selectively attract pests to traps are grape volatiles. Some of these are used by fertile females to locate oviposition sites on grapes (Tasin et al., 2005).
Another technique that has been used with some success has been the release of large numbers of artificially reared, but sterile, individuals during the mating season. This can so disrupt successful mating as to achieve effective control.
Although the initial cost of grafting to resistant rootstocks is considerable, its beneficial effects are long term. In some cases, grafting may be the only effective means of limiting pest or disease damage. This strategy was first used in the late 1800s to control the phylloxera infestation in Europe. Resistant or tolerant rootstocks are also one the principal means by which the damage of several nematodes, viruses, and soil-based toxicities is limited. Little investigated, however, is the role of grapevine rootstocks in limiting damage by leaf and fruit pathogens (Erb et al., 2009).
The biological control of fungal pathogens is less developed than for arthropod pests. This partially reflects the growth of fungal pathogens within plants, away from exposure to parasites or predators. Nevertheless, inoculation of leaf surfaces with epiphytic microbes may prevent the germination and subsequent penetration of fungal pathogens. The phylloplane flora may inhibit pathogens by competing for organic nutrients required for germination, activating systemic host defenses, inciting mycoparasitism, or provoking antibiosis. A commercially successful example involves Trichoderma harzianum (Trichodex®). It and related agents are especially effective against wood-invading fungi, such as those inducing Eutypa, Esca, and Petri diseases (Hunt, 1999). In contrast, control of Botrytis cinerea is inadequate and requires rotation with conventional fungicides. Of commercial biofungicides, the most effective against B. cinerea appears to be Greygold®. It is a mixture of the filamentous fungus Trichoderma hamatum, the yeast Rhodotorula glutinis, and the bacterium Bacillus megaterium. With powdery mildew, where the mycelium remains predominantly on the plant surface, the mycoparasite Ampelomyces quisqualis (AQ10®)has proven commercially effective (Falk et al., 1995) in some cases.
Another new and fascinating approach being investigated involves endophytes. Endophytes are microbes that grow and multiply asymptomatically within plant tissues. They are typically considered to be symbiotic and beneficial (Rodriguez and Redman, 2008), though some may be latent pathogens (see below). Their presence is detected primarily by the presence of distinctive DNA or fatty acid sequences, or by culturing. Only occasionally can they be observed microscopically within plant tissues, such as with Trichoderma. Endophytes may be localized in the xylem, as with most endophytic bacteria, or the root cortex, as with Trichoderma. Some are also found throughout the plant.
Those found in annual crops can desensitize the host to a variety of environmental stresses, such as salinity, heavy metals, drought, or heat; improve nutrient availability; or enhance disease resistance. The latter can involve activation of various systemic defense mechanisms, or synthesizing antibiotics (Barrow et al., 2008; Shoresh et al., 2010). The ability and diversity of endophytic organisms in reducing susceptibility to a wide range of environmental stresses has led some researchers to advocate their use in lieu of more expensive and controversial genetic breeding procedures (Barrow et al., 2008; Rodriguez et al., 2008). This could be expanded to include adjustment of the rhizoflora in and around roots (Pineda et al., 2010). In this regard, the presence of strains of Pseudomonas spp., carrying the biocontrol genes ph1D and hchAB, has been correlated with improved photosynthesis and grape aroma (Svercel et al., 2010). These strains were isolated from vineyards under long-term viticulture, but not young vineyard soils. This is an interesting example of long-term monoculture appearing to be associated with a beneficial effect.
Most bacterial endophytes appear to be neutral to beneficial, with the exception of latent strains of Rhizobium (Agrobacterium) vitis. Most are fastidious, xylem-inhabiting strains of Pseudomonas and Enterobacter. They are distinct from those found in the rhizosphere (Bell et al., 1995). In contrast, the endophyte Burkholderia colonizes both the rhizosphere and internal tissues (Compant et al., 2005). West et al. (2009) found that entrance occurred primarily via pruning wounds. Endophytic strains of Pseudomonas flourescens and Bacillus subtilis have been shown to reduce the size of crown gall in infected plants (Eastwell et al., 2006).
At least some fungal endophytes appear to be latent or non-damaging strains of pathogens, for example Phaeomoniella chlamydospora and Phaeoacremonium spp. (Halleen et al., 2003). In contrast, other fungal endophytes parasitize pathogens, for example Acremonium byssoides and Alternaria alternata on Plasmopara viticola (Burruano et al., 2008; Musetti et al., 2006). Mycorrhizae (specialized semiendophytic fungi that reside primarily outside the plant) can also provide resistance to some pest and disease agents.
One of the more challenging aspects in using biocontrol agents can be the special requirements for storing and application. Because spores and biological toxins often have relatively short half-lives, they need to be kept under cool, dry conditions. In addition, they are often quickly inactivated on exposure to solar UV radiation. Thus, if applied to aerial parts of the vine, they are best sprayed in the evening to extend their effective period. These limitations have retarded grower acceptance (Hofstein et al., 1996).
One of the more intriguing examples of biological control involves cross-protection. Cross-protection is a phenomenon in which virally infected cells are immune to subsequent infection by related or more virulent strains of the virus. Transforming vines with viral coat or dispersal genes can potentially have the same effect (Beachy et al., 1990; Krastanova et al., 1995).
Another component in many biological control schemes is augmentation of the soil’s organic content, either by the application of manure, compost, or a mulch (Hoitink et al., 2002). It has its greatest applicability when dealing with root pests and pathogens. The effect is thought to arise primarily by suppression of the growth and survival of the pathogenic agent, by enhancing the complexity and activity of the soil’s microflora and fauna. This applies especially to the region just around the roots – the rhizosphere. Thus, some of the benefits may also accrue from improved vine nutrition. This could involve better access to soil nutrients (directly or via mycorrhizal association), and, thereby, direct augmentation of host defenses.
Similar in concept is the application of a solution of organic nutrients (e.g., liquid manure). Augmentation of the phylloplane flora may both directly and indirectly suppress the germination or growth of leaf and fruit pathogens (Sackenheim et al., 1994).
Biological control has generally been assumed to be safer to humans and the environment than chemical control. Risk assessment concerning non-target organisms has usually supported this view, but improved techniques may limit the chance of exceptions (Barratt et al., 2010). For example, the fungal biocontrol agents Trichoderma and Gliocladium may provoke allergic respiratory problems and a range of cellular toxic effects in humans, respectively (see Brimmer and Borland, 2003). In other instances, some agents have shown action against beneficial mycorrhizal associations; may disrupt plant metabolism; or, when used prophylactically, induce energy expenditure in activating unnecessary systemic plant defenses. Like all defense mechanisms, they are a drain on plant metabolism.
Environmental Modification
Modifying the microclimate around plants has long been known to potentially minimize disease and pest incidence. By improving the light and air exposure around grapevines, canopy management can increase the toughness and thickness of the epidermis and its cuticular covering. An open canopy structure also facilitates the rapid drying of fruit and foliage surfaces. This reduces the time available for fungal penetration, and may limit or inhibit spore production. Furthermore, an exposed canopy enables more efficient application of pesticides, synthetic or organic.
Berry exposure can be enhanced by applying gibberellic acid. It promotes cluster stem elongation and separation of the fruit (Plate 3.9). Reduced berry compactness also favors production and retention of a typical epicuticular wax coating (Fig. 4.52). However, the effect of gibberellic acid on grape composition is complex, and appears to be cultivar-specific (Teszlák et al., 2005). For example, it can affect grape coloration, phenolic content, and mineral content. In addition, it can increase the formation of o-aminoacetophenone (Christoph et al., 1998; Pour Nikfardjam et al., 2005b), a compound frequently associated with the development of an ‘untypical aged flavor’ in wine. By suppressing the action of IAA oxidase, gibberellic acid can increase auxin content. It appears to favor o-aminoacetophenone synthesis (Pour Nikfardjam et al., 2005a). Less compact clusters also occur in association with minimal pruning. This probably explains part of the reduced incidence of some diseases, notably Botrytis bunch rot, with minimal pruning.
Fruit exposure may also be enhanced by basal leaf removal. The technique has been so successful in reducing dependence on fungicidal sprays that several wineries have written the practice into their contracts with growers (Stapleton et al., 1990). Basal leaf removal also eliminates most first-generation nymphs of the grape leafhopper (Erythroneura elegantula) (Stapleton et al., 1990). This can improve subsequent biological control by Anagrus epos, a parasitic wasp. It provides more time for wasp populations to increase, and spread to grapevines from overwintering sites on wild blackberries and other plants.
Balanced plant nutrition generally favors disease and pest resistance, by promoting optimal development of anatomical and physiological defenses. Nutrient excess or deficiency can have the inverse effect. For example, high nitrogen levels suppress the synthesis of a major group of grape antifungal compounds, the phytoalexins (Bavaresco and Eibach, 1987). They also favor cell elongation, associated with weaker walls, as well as promoting vigorous growth, dense foliage, and the development of high inner-canopy humidity.
With adequate irrigation, the consequences of nematode root damage are often mitigated. Adequate irrigation also avoids the serious consequences of water deficit. Conversely, excessive irrigation can favor disease development by promoting luxurious canopy development and increasing berry-cluster compactness. This is particularly important for cultivars that inherently produce compact clusters, notably Zinfandel and Chenin blanc.
Weed control usually reduces disease incidence. This may result from the removal of alternate hosts, on which pests and disease-causing agents may survive and propagate. For example, dandelions and plantain are often carriers of tomato and tobacco ringspot viruses, and Bermuda grass is a reservoir for sharpshooter leafhoppers – the primary vectors of Pierce’s disease. Conversely, cover crops are generally viewed as beneficial, supporting populations of biocontrol agents. Nonetheless, if not well chosen they can also be carriers of vine pests and disease.
Soil tillage can occasionally be beneficial in disease control. For example, the burial of Botrytis cinerea, sclerotia, and infected vine tissue promotes their degradation in the soil. In addition, the emergence of adult grape root borers (Vitacea polistiformis) is restricted by burial of the pupae (All et al., 1985).
Although environmental modification can limit the severity of some pathogens, it can enhance other problems. For example, soil acidification, used in the control of Texas root rot (Phymatotrichum omnivorum), has increased the incidence of phosphorus deficiency in Arizona vineyards (Dutt et al., 1986). The elimination of weeds may inadvertently limit the effectiveness of some forms of pest biological control (removing survival sites for pest predators and parasites). The carrier of one pest may be the reservoir of predators for another.
Genetic Control
Improved disease resistance is one of the major goals of grapevine breeding. It was first seriously investigated as an alternative to grafting in phylloxera control. Subsequently, breeding has focused primarily on developing rootstocks possessing improved drought, salt, lime, virus, and nematode resistance. Work has also progressed, but more slowly, on developing new scions, with improved pest and disease resistance. Regrettably, consumer (and especially critic) resistance to new varieties has limited acceptance. However, at the lower end of the market, new varieties often have a distinct advantage. Their production costs are reduced and their yield higher. Resistant varieties are probably most appropriate for ‘organic’ viticulture, where synthetic control agents are proscribed and varietal origin may have less marketing value.
Although valuable sources of disease resistance may exist in remnants of the original population of wild vinifera vines, most potentially useful resistance genes occur in other Vitis species. Regrettably, the EU has passed legislation against the use of interspecies crosses, as well as genetically modified crops. In so doing, they have cut themselves off from almost all sources of disease resistance. Genetic engineering has the best chance of enhancing cultivar disease resistance, without modifying enologically essential varietal traits.
It is only with rootstocks that traditional breeding has much potential to improve vine resistance to root and/or shoot diseases. This is especially the case with the recently discovered potential of roots to influence defense against foliar diseases and pests (Erb et al., 2009).
In addition to pre-existing structural and chemical defenses, plants possess two broad response-based defense mechanisms against pathogen attack. The first, often referred to as non-host resistance, appears to depend on transmembrane receptors recognizing penetration. Activating factors include constituents of most fungal cell walls (chitin), peptides typically found on bacterial flagella, and mechanical pressure (as applied during fungal penetration). Plant response activates cytological and histochemical responses that may prevent successful penetration and infection (Dry et al., 2010). Jasmonic acid and ethylene-dependent signaling pathways are though to be involved, but are not necessarily crucial to effectiveness (Glazebrook, 2005; Humphry et al., 2006).
Pathogens that overcome these primary defenses may prompt a second line of defense. This depends on specialized receptor (R) proteins. They induce a cascade of reactions involving hydrogen peroxide and nitric oxide. When rapid and intense, localized tissue death results in what is termed a hypersensitive response. If effective, it kills the invading pathogen (Tameling and Takken, 2008). As part of the cascade, a salicylic acid-dependent signaling pathway is activated (Jung et al., 2009). It primes plant defenses throughout the plant, a phenomenon termed systemic acquired resistance (Gurr and Rushton, 2005a,b). Unfortunately, excessively and unnecessary activation of the response can ‘stress’ the plant, compromising growth.
Traditionally, enhanced disease resistance has involved the resistance (R) genes noted above. They act in a dominant manner, donating immunity to the pathogen; that is, until the pathogen mutates to circumvent the resistance. Thus, the breeder and pathogen are often interlocked in a cat-and-mouse game of adding new R gene alleles, and the pathogen mutating to negate their effects. Nonetheless, R-type genes from V. rotundifolia, such as Run1 and Rvp1, appear to exhibit durability (Dry et al., 2010). Run1 donates resistance to powdery mildew. Ren1 from Vitis vinifera has a similar effect.
An alternative, and potentially longer-term approach, may involve adjustment to components of non-host resistance – the general immunity of plants to the vast majority of pathogens. One element of this phenomenon, in relation to powdery mildew, is the absence of specific MLO proteins. Grapevines possess up to 17 variants in the MLO gene family (Winterhagen et al., 2008). Homozygosity for recessive (mlo) alleles results in unsuccessful penetration by the more than 100 species of powdery mildew, except Uncinula necator (Humphry et al., 2006). Which of these variants are dominant, presumably generating susceptibility to U. necator, is under investigation (Dry et al., 2010). Inactivation of those variants might donate immunity to U. necator, equivalent to that which they possess against other powdery mildew species.
Resistance to some pathogens is also associated with the production of pathogenesis-related (PR) proteins. Examples are the chitinases, associated with haze production in wines. Enhancing their earlier activation or overexpression might improve disease resistance. This has been shown to enhance disease resistance in several transgenic crops. Enhancing baseline phytoalexin production is another potential approach.
Another option, using genetic engineering, but not involving resistance genes per se, would be the incorporation of genes coding for one of several viral genes. As noted earlier, these can induce immunity (cross-protection) against the source virus.
In most instances, complete resistance (immunity) to infection is preferred. However, even slowing the rate of disease spread (tolerance) may provide adequate protection in most years, especially where several cycles of pathogen/pest reproduction are required for the expression of severe damage. Regrettably, tolerance to one pest can occasionally favor infection by another (e.g., tolerance to Xiphinema index increases the likelihood of grapevine fanleaf virus transmission). In addition, tolerance to a systemic pathogen (by masking presence) favors the likelihood of its spread by grafting or other forms of mechanical transmission.
The increasing availability of molecular markers, such as SSRs, has begun to simplify mapping the location of resistance genes (Fischer et al., 2004; Di Gaspero et al., 2007). This potentially facilitates their isolation, amplification, and subsequent insertion via transduction. In addition, these markers can be used as indicators of the presence of resistance genes in the progeny – in a process termed marker-assisted breeding (Eibach et al., 2007). The similarity of many of these genes in other plants also aids identification of potentially useful genes in grapevines. Locating a useful allele in one cultivar or related species would theoretically permit its transfer via genetic engineering, without crossing broad generic boundaries. The latter is a frequent complaint leveled against most GM plants. The difference is termed cisgenic vs. transgenic engineering (Schouten et al., 2006). In addition, it would not disrupt existing cultivar traits. Dhekney et al. (2011) have recently isolated the VVTL-1 gene from Chardonnay, modified it to become constitutive, and reinserted it into Thompson Seedless. It expresses significantly enhanced disease resistance to several serious fungal grapevine pathogens.
Simply inserting the protein-coding component of a resistance gene is not necessarily adequate for activity. It requires the insertion of appropriate promoter, terminator, and controller regions for proper function. Thankfully, most resistance loci possess several related R genes, as well as control regions (see Dry et al., 2010). This may enhance durable resistance, especially necessary with grapevines possessing prolonged vineyard ‘life spans.’
Finally, a caveat for all such research is the possibility that improving a particular property may unexpectedly negatively impact another. Thus, as always, field trials over several years, and in diverse locations with different climates, are necessary to determine whether the benefits are both sustainable and outweigh any potential disadvantages. All this adds expense, something governments and agricultural industries seem increasingly loath to do, at a time when it is increasingly urgent to do so.
Eradication and Sanitation
In most situations, the eradication of established pathogens is impossible, due to their survival on alternative hosts, such as weeds or native plants. Eradication has greater potential for success with newly introduced, exotic pathogens. In this situation, it is normal practice to destroy all the vines in affected areas. Clearly, this induces extreme hardship on the owners involved, and requires at least financial compensation from the government. Consequently, studies are in progress to assess the feasability of reducing the impact of eradication. For above ground pathogens, removing the vine down to the crown is being investigated. The production of new shoots from long dormant buds permits the more rapid reestablishment of the vineyard. The upper parts of the vines are either burnt or buried, with a straw layer applied to aid in the decomposition of any residual infected material (Sosnowski et al., 2009).
Seed propagation is frequently used to eliminate (eradicate) host-specific systemic pathogens from a crop. However, this is inapplicable with grapevines – it would disrupt the combination of genetic traits that makes each cultivar unique (see Chapter 2). Thus, except where disease-free individuals can be found, the elimination of systemic pathogens from cultivars must involve thermotherapy, meristem culture, or a combination of both.
Thermotherapy can vary from placing young rooted shoots at 35–38°C for 2–3 months to dormant canes being given higher temperature treatment, but for shorter periods. The treatment is effective against several viruses, such as the grapevine fanleaf, tomato ringspot, and fleck viruses, as well as the leafroll agent (typically associated with closteroviruses). Hot-water immersion is also appropriate and preferable for other pathogens – the specifics depending on the pathogen and it location, i.e., external vs. internal. It can be effective in eliminating pathogens such as the bacteria Xylella fastidiosa, Xanthomonas ampelina, and Rhizobium (Agrobacterium) vitis; the phytoplasmas associated with grapevine yellows diseases (e.g., flavescence dorée); the fungus Phytophthora cinnamoni and those causing Petri disease; and root nematodes, such as Xiphinema index and Melidoyne spp. Procedures and recommended precautions for hot-water treatment are given in Hamilton (1997), Waite et al. (2001), and Waite (2005).
Because hot-water treatment stresses vine tissues, it is recommended that treated cuttings be protected from adverse weather conditions, usually in a greenhouse or equivalent, for several months prior to planting out in field nurseries. Following treatment, the cutting may be dipped in a combination of biocontrol agents to reduce the incidence of reinfection (Graham 2007). Treating vines infected by several systemic pathogens may require a combination of procedures.
The use of thermotherapy and meristem culture in the preparation of nursery stock is often of concern to growers. The treatment can modify the morphological and physiological traits of the treated vines. Expression of juvenile traits, such as spiral phyllotaxy, reduction in tendril production, more jagged and pubescent leaves, stem coloration by anthocyanins, and reduced fertility are fairly typical. These usually disappear, though, as individual vines mature or are propagated repeatedly (Mullins, 1990; Grenan, 1994).
For systemic pathogens that are not eliminated by heat treatment, but do not invade meristematic tissues, elimination is often possible by culturing meristematic sections. Direct propagation from these small fragments has often been used to eliminate viruses, such as grapevine fanleaf virus (GFLV) and grapevine leafroll-associated virus-1 (GLRaV-1); the virus-like agents of stem pitting, corky bark, and leafroll; the viroid of yellow speckle; and the bacterium Rhizobium (Agrobacterium) vitis.
Once freed of systemic pathogens, vines usually remain disease-free, if grafted to disease-free rootstock and planted in pathogen-free environments. Most serious grapevine viruses are not insect transmitted, but resistant rootstock is advisable where soil is infested with root-feeding nematodes. Alternatives are leaving the land fallow for up to 10 years, or fumigating the soil.
Sanitation and hygiene may not eliminate disease or pest problems, but they usually reduce their severity by destroying resting stages or by removing survival sites.
Quarantine
Most, if not all, wine-producing countries possess laws regulating the importation of grapevines. Some of the best examples illustrating the need for quarantine laws are those involving grapevine pathogens. Two of the major grapevine diseases in Europe (downy and powdery mildew) were imported unknowingly from North America in the nineteenth century. The phylloxera root louse was also accidentally introduced, probably on rooted cuttings. Several viral and virus-like agents are now widespread in all major wine-growing regions. They are thought to have been spread, surreptitiously, through the importation of asymptomatic, but infected, rootstocks or scions. Examples are the agents causing leafroll, corky bark, and stem pitting.
Thankfully, some other potentially devastating diseases have as yet to become widespread. For example, Pierce’s disease is still largely confined to southeastern North America and Central America. However, it is causing severe problems in parts of California, due to spread by the glassy-winged sharpshooter. Although phylloxera is present in most wine-producing regions, it has not spread throughout all parts of the countries in which it occurs. Thus, limiting grapevine movement within regions can still have a significant impact on the spread of those pathogens with limited natural means of dispersal. Disinfection of footwear and machinery is another component of limiting spread, both within and between vineyards.
Because it is difficult to detect the presence of some pests and disease-causing agents, only dormant canes are permitted entrance into most jurisdictions. This impedes the introduction of root and foliar pathogens. Nonetheless, fungal spores, insect eggs, and other minute dispersal agents may go undetected. Consequently, imported canes are typically quarantined for several years, until they are determined to be free, or freed, of known pathogens. Most pests, as well as fungal and bacterial pathogens, express their presence during the detention period. For latent viruses and viroids, detection usually requires grafting or mechanical transmission to indicator plants. Detection of systemic pathogens, through their transmission to sensitive (indicator) plants, is termed indexing. Less time-consuming analytic techniques, such as ELISA (enzyme-linked immunosorbent assay) (Clark and Adams, 1977), cDNA (Koenig et al., 1988), or PCR probes (Constable et al., 2012) can both facilitate and speed the detection of systemic pathogens. Nonetheless, because no technique is 100% perfect all the time, repeat sampling is advisable. For example, it may take up to a year after infection for some viruses to become reliably detectable. Even with these caveats, modern assessment techniques permit the earlier release of imported stock.
Unfortunately, quarantine is not only expensive, but can also irritatingly delay importer access to new material. A possible solution is the development of encapsulated somatic embryos (Das et al., 2006). Being produced under sterile conditions, and from healthy tissue, they could be shipped directly to propagation facilities in the host country. Theoretically, this should completely avoid the potential for incidental pathogen introduction along with cuttings.
Consequences of Pathogenesis for Fruit Quality
The negative influence of pests and diseases is obvious in symptoms such as blighting, distortion, shriveling, decay, and tissue destruction. More subtle effects involve vine vigor, berry size, and fruit ripening. Sequelae such as reduced root growth, poor grafting success, decreased photosynthesis, and increased incidence of bird damage on weak vines (Schroth et al., 1988) are more easily missed. In some instances, detection of infection is impeded by minimal symptoms or the absence of uninfected individuals for comparison. This was initially the case with several viral and viroid diseases.
Most pest and disease research is concerned with understanding the pathogenic state and how its effects can be minimized. However, in making practical decisions on disease control, it is important to know the effects of disease not only on vine health and yield, but also on grape and winemaking quality.
All pests and disease agents disrupt vine physiology to some degree and, therefore, potentially influence fruit yield and quality. For example, the sensory quality of wine was detectably reduced from vineyards possessing as little as 5% Esca-affected vines (Lorrain et al., 2012). However, agents that attack berries clearly have the greatest impact on fruit quality. These include three of the major fungal grapevine pathogens – Botrytis cinerea, Plasmopara viticola, and Uncinula necator. Grapevine viruses and viroids, being systemic, can both directly and indirectly affect berry characteristics. For example, leafroll-associated viruses reduce grape °Brix, increase titratable acidity, and delay ripening. Insect pests can cause fruit discoloration and malformation, as well as create lesions favoring invasion by pathogens and saprophytes as well as secondary pests.
Of fruit-infecting fungi, the effects of Botrytis cinerea have been the most extensively studied. Under special environmental conditions, infection produces a ‘noble’ rot, yielding superb wines (see Chapter 9). Typically, however, the fungus produces a bunch (ignoble) rot. Subsequent invasion by acetic acid bacteria probably explains the high levels of fixed and volatile acidity in the fruit.
Under moist conditions, secondary invaders, such as Penicillium and Aspergillus, contribute additional off-flavors, such as geosmin, 2-methylisoborneol, 1-octen-3-one, and 1-octen-3-ol (La Guerche et al., 2006). These saprophytes can also produce mycotoxins. Examples are isofumigaclavine, festuclavine, and roquefortine, produced by Penicillium spp. (Moller et al., 1997); ochratoxin A, primarily derived from Aspergillus carbonarius (O’Brien and Dietrich, 2005); fumonisins, synthesized by Aspergillus niger (Mogensen et al., 2010); and trichothecenes, generated by Trichothecium roseum (Schwenk et al., 1989). In sufficient amounts, they can be mammalian cytotoxins and carcinogens. The presence of secondary saprophytes, such as Mucor species, can disrupt the activity of lactic acid bacteria during malolactic fermentation (San Romáo and Silva Alemáo, 1986). B. cinerea produce several phytotoxins, but none known to affect humans (Krogh and Carlton, 1982). Their presence may be the source of the disrupted yeast growth noted by Blakeman (1980).
In addition to increased fixed and volatile acidity, fungi associated with bunch rots reduce nitrogen and sugar contents. This can create difficulties during fermentation. Large accumulations of β-glucans, synthesized by B. cinerea, can create clarification problems. More than a minimal level of bunch rot generally excludes their use in the making of red wines, if for no other reason than the oxidation of anthocyanins and other grape phenolics by fungal laccases. Cladosporium bunch rot also reduces the quality (color and flavor) of wines produced from affected red grapes (Briceño et al., 2009). Botrytis infection can also increase problems associated with protein haze (Girbau et al., 2004). This appears to result from the enhanced production of thaumatin-like (PR) proteins.
The effects of sour rot on wine quality has been studied by Barata et al. (2008). Sour rot is the result of microbial invasion of the fruit by a diverse set of agents, notably following feeding by insects and birds. Other than the origin of a sweet, honey-like note, generated by marked increases in ethyl phenylacetate and phenylacetic acid, and reduction in fatty acid and their esters, it is uncertain what specific feature(s) trigger consumer rejection.
Little information is available on the direct consequences of most pathogens on grape and wine quality. One exception is the higher pH and phenol contents of wine produced from fruit infected by powdery mildew. They may donate a bitterish attribute (Ough and Berg, 1979), or other flavor modifications (Fig. 4.53). Reduced anthocyanin content appears to be due to physiologic disruption during grape development (Amati et al., 1996). The flavor consequences of infection are augmented with increased skin contact, prior to or during fermentation. This may involve the conversion of several ketones to 3-octanone and (Z)-5-octen-3-one (Darriet et al., 2002). Flavor distortion has been noted in wine made from grapes with as little as 1–5% infection (Stummer et al., 2005), although not consistently. This anomaly may be related to the degree to which diseased grapes are colonized by secondary saprophytes. Yield, Brix, and anthocyanin synthesis in red grapes are reduced as a consequence of infection (Amati et al., 1996). Browning is common, but can be partially offset by the bleaching action of sulfur dioxide in white wines. Infected white grapes also possess higher concentrations of haze-producing proteins. Fermentation may also take up to twice as long to complete (Ewart et al., 1993).
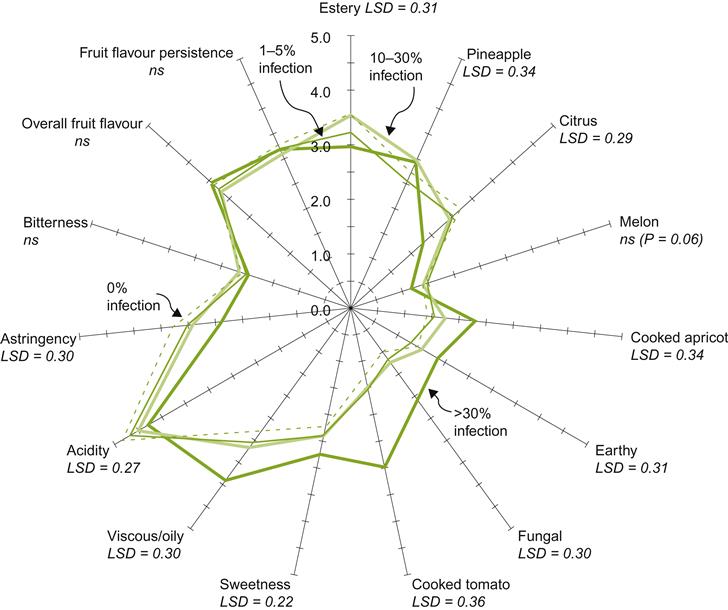
In flavescence dorée, the production of a dense, bitter pulp makes commercial wine production from affected fruit virtually impossible. Of virus and virus-like infections, leafroll has been the most investigated relative to grape and winemaking quality. Potassium transport is affected and berry titratable acidity decreased. This typically generates wines of higher pH and poorer color. Sugar accumulation in the berries is usually decreased, due to suppressed transport from the leaves. Ripening is often delayed. GLRaV-1 and rugose wood viruses shift the relative proportion of phenolics from seeds and skins, but do not appear to affect grape anthocyanin or total phenolic contents (Tomažič et al., 2003). In contrast, the level of the more stable (oxidation-resistant) anthocyanins is reduced in Nebbiolo grapes, in association with joint infection with GFV and GFkV viruses (Santini et al., 2011).
The physiological disorder, bunch-stem necrosis (dessèchement de la rafle, Stiellähme), causes grape shriveling and fruit fall around and after véraison. Wines produced from grapevines so affected often lack balance, are high in acidity, and low in ethanol, as well as several higher alcohols and esters (Ureta et al., 1982). Susceptibility to this disorder most likely has a genetic basis, as some cultivars (i.e., Silvaner and members of the Pinot family) are particularly resistant to its development.
The effects of disease on aroma have seldom been reported. Exceptions are the reduced varietal character of grapes infected by B. cinerea, or their modification by the presence of the ajinashika virus (Yamakawa and Moriya, 1983).
Although not a direct consequence of pathogenesis, the application of protective chemicals may indirectly affect wine quality. For example, the copper in Bordeaux mixture can compromise the quality of Sauvignon blanc wines and related cultivars. It can reduce the concentration of important varietal aroma compounds, such as 4-mercapto-4-methylpentan-2-one. This effect can be reduced by prolonged maceration (Hatzidimitriou et al., 1996), or avoiding the use of Bordeaux mixture (Darriet et al., 2001). Fungal and pest control agents, as well as herbicides, may also have phytotoxic effects on the vine. These can vary from direct visible damage, as caused by sulfur at high temperatures, to more subtle changes, such as reduced sugar accumulation in berries (Hatzidimitriou et al., 1996), and disruptions to photosynthesis (Saladin et al., 2003). Even more indirect influences may affect the soil flora and fauna. For example, long-term use of Bordeaux mixture has resulted in the substantial accumulation of copper in vineyards worldwide – being in the range of 130–1280 mg/kg in European vineyards (Wightwick et al., 2010). This partially results from copper’s binding with organic matter in the soil, but mainly from the formation of copper and iron oxyhydroxides; the latter bind tightly to the soil’s clay fraction. The highest concentrations tend to be found in the upper layers of the soil. An indirect effect is suppression of the soil’s microbial activity, and an indirect augmentation of its organic content (Parat et al., 2002).
Examples of Grapevine Diseases and Pests
Grapevines can be attacked by a wide diversity of biologic agents. There is insufficient space in this book to deal with all these maladies. Thus, only a few of the more important and/or representative examples of the major categories of grapevine disorders are provided. Detailed discussions of grapevine maladies for specific countries can be found in specialized works such as Pearson and Goheen (1988) and Flaherty et al. (1992) (North America), Galet (1991) and Larcher et al. (1985) (Europe), and Coombe and Dry (1992) and Nicholas et al. (1994) (Australia).
Fungal Pathogens
With few exceptions, fungal pathogens grow as long, thin, branching, microscopic filaments called hyphae, and collectively termed mycelia. Most fungi produce cell-wall ingrowths along the hyphae termed septa. The ingrowths are usually incomplete and leave a central opening through which nutrients, cytoplasm, and cell organelles may pass. Thus, fungi possess the potential to adjust the number and proportion of nuclei and various organelles within the organism as they grow. This gives fungi a degree of genetic flexibility unknown in other organisms. The filamentous growth habit also provides them with the ability to physically puncture plant-cell walls. This property, combined with their degradative powers (by secreting hydrolytic enzymes) and prodigious spore production, helps explain why fungi are the predominant disease-causing agents of plants.
Most parasitic fungi reproduce primarily by forming asexually generated spores, commonly termed conidia. They may or may not be produced in an enclosing structure. They may also produce spores generated by meiosis, usually in the spring. The latter are named relative to their taxonomic grouping (ascospores – Ascomycota, basidiospores – Basidiomycota, and oospores – Oomycota). Fungi that primarily reproduce without a sexual mode are variously termed hyphomycetes (members of the Fungi Imperfecti).
Botrytis Bunch Rot
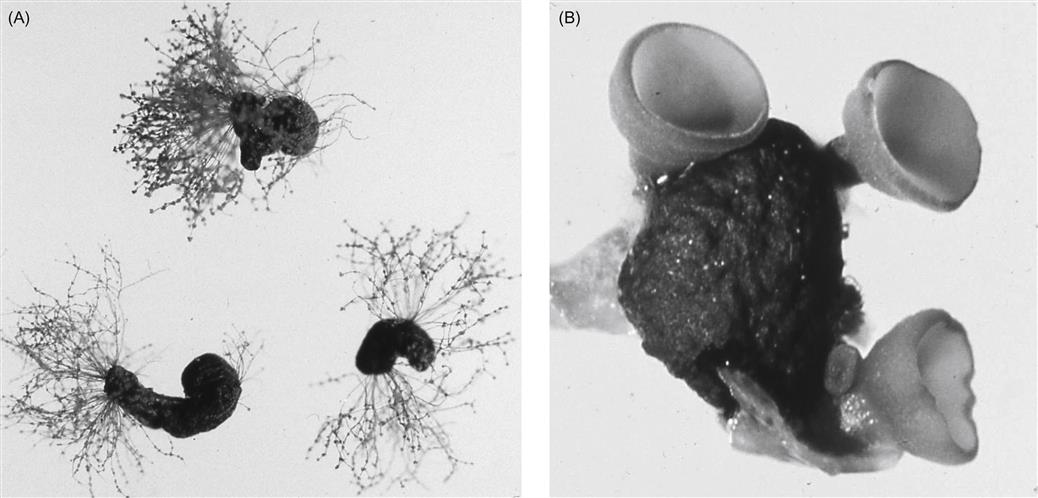
Several hyphomycetes can induce bunch rot, either alone or together. However, the principal causal agent is Botrytis cinerea. The pathogen appears to exist as two co-inhabiting subpopulations (Fournier et al., 2005), with Group II divided into two divisions based on features such as fungicide resistance, virulence, compatibility, and transposable elements. All forms infect grapevines and may occur sympatrically, but tend to possess different frequencies of fungicide resistance. Unlike many grape pathogens, B. cinerea is a necrotroph – attacking primarily damaged or senescent tissues, and provoking tissue necrosis in advance of penetration. In addition, unlike most other Botrytis species (Staats et al., 2005) B. cinerea is a nonspecialized pathogen, infecting a wide diversity of plants and tissues. As a consequence, spores infecting grapes may arise from a wide range of host species, within and around vineyards. Nonetheless, most early infections develop from spores produced on overwintered mycelia within the vineyard (Jacometti et al., 2007) (Fig. 4.54). In addition, another source of spores may arise from black, multicellular, resting structures called sclerotia (Fig. 4.55A). In both instances, these are usually conidia (asexual spores). Occasionally, ascospores may be produced from the sclerotia. They are generated in multicellular fructifications called apothecia (Fig. 4.55B).
Pathogenesis results from the combination of a wide range of toxins and enzymes. The first to be studied extensively were pectinases. They have a macerating action, by degrading the pectins that hold plant cells together. Their action is likely aided by the production of oxalic acid. By lowering the pH of intercellular fluids, it would favor the activity of pectinases. Pectinases may also initiate disruption of the cell membrane. This action, combined with the release of phytotoxic chemicals (such as secobotrytriendiol [Durán-Patrón et al., 2000], botcinolide, and botrydial), necrosis and ethylene-inducing proteins (NEP1 and NEP2) (Staats et al., 2007), as well as laccase, probably induces tissue necrosis. The potent polyphenol oxidase activity of laccase can generate a wide range of toxic quinones. The release of cutinases and lipases undoubtedly aids the penetration of plant surfaces by B. cinerea.
Initial infections usually develop on aborted and senescing flower parts. When the remnants of flowers are trapped within growing fruit clusters, they are well positioned to initiate fruit infections later in the season. Another source of fruit infection originates from latent infections that occurred in the spring. These form when hyphae invade the vessels of young green berries (Pezet and Pont, 1986). The fungus subsequently becomes inactive, until the fruit begin to ripen. At this time cells walls soften, acidity levels fall, sugar contents rise, flavonoid phenolics have become more polymerized, and the concentration of some antifungal compounds have declined (Keller et al., 2003). Such latent infections appear to be the primary source of infection, notably those during dry autumn conditions. Thus, the initial focus of control is directed at limiting early infections, with spraying coinciding with flowering, 80% cap fall, and bunch closure. Nevertheless, under protracted rainy or humid conditions destructive bunch rot can rapidly develop from de novo infections at any time during the season. Such infections are often particularly destructive. They may arise from spores produced from activated latent infections or external sources. Such infections often involve secondary invaders, notably Acetobacter, and saprophytic fungi, such as Penicillium, Aspergillus, Cladosporium, and Rhizopus, spp. The metabolism of tartaric acid and the degradation of resveratrol and other phenolic phytoalexins by B. cinerea facilitate the growth of secondary invaders. The latter can often be more damaging to fruit quality than the initial infection by B. cinerea. For example, Penicillium expansum is the likely source of the earthy smelling compound geosmin in infected grapes (La Guerche et al., 2005), whereas Acetobacter is well known to be primary source of acetic and gluconic acids.
Several insects, such as the European grape berry moth, light-brown apple moth, and fruit flies can aggravate disease incidence. They can be agents for both transporting and infecting fruit with conidia (Mondy et al., 1998b). Infections of leaves, shoots, and other vine parts occur, but are primarily important as overwintering sites.
In some regions, late-season physiological and anatomical changes have been strongly correlated with increased susceptibility to attack (Kretschmer et al., 2007). Microfissures may develop around stomata (Fig. 4.56A), and micropores form in the cuticle (Fig. 4.56B). Both provide sites facilitating fungal penetration and the release of plant nutrients that aid spore germination. The weathering of cuticular waxy plates also favors infection by aiding spore adherence. The loss of wax is most noticeable where berries press and rub against one another (see Fig. 4.52B). The cutin content can decrease by more than 60% from its preanthesis level by véraison (Comménil et al., 1997). Rapid berry enlargement, especially during heavy rains, can induce skin splitting and the release of juice, further favoring infection. Loss of polymeric procyanidins late in the season has also been correlated with relative cultivar sensitivity to Botrytis (Pezet et al., 2003).
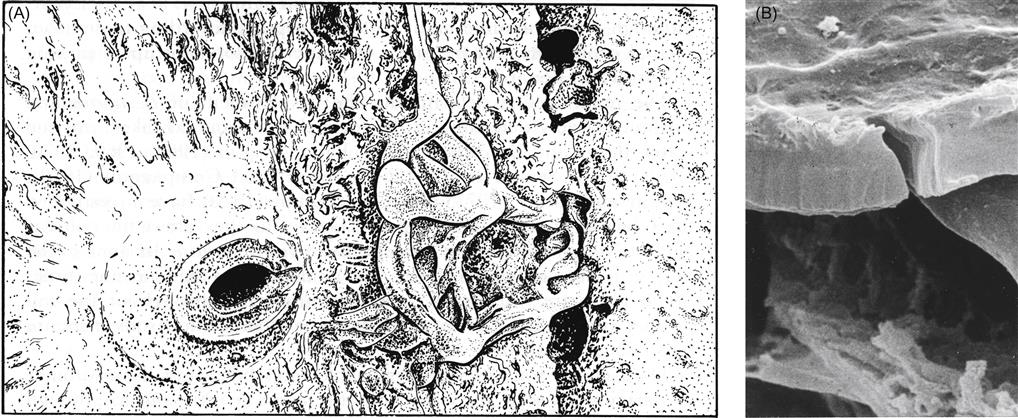
Many factors affect bunch rot susceptibility. Skin toughness and open fruit clusters reduce bunch rot incidence, whereas heavy rains, protracted periods of high humidity, and shallow vine-rooting increase susceptibility. Shallow rooting exposes the vine to waterlogging, which can favor rapid water uptake and berry splitting. Berry splitting can also result from the osmotic uptake of water through the skin under rainy conditions (Lang and Thorpe, 1989). In addition, protracted moist periods provide conditions that favor spore production, germination, and invasion.
After germination, spores produce one or more germ tubes that grow out through the spore wall. Fruit penetration occurs shortly thereafter, often through microfissures in the epidermis. Subsequent ramification initially progresses more or less parallel to the berry surface through the hypodermal tissues. Infection may fail to spread to the mesocarp (Glidewell et al., 1997) under dry conditions.
Depending on the temperature and humidity, copious numbers of spores may be produced within days. Conidia are borne on elongated, branched filaments. These erupt either directly through the epidermis or via stoma. The white to gray color of the young spores gives rise to the common name for most Botrytis diseases – gray mold. On maturity, the spores turn brown. They can be so densely packed as to give the infected tissues a felt-like appearance. Early in infection, white grapes may take on a purplish coloration. All infected fruit eventually turn brown, presumably due to the phenol oxidizing action of laccase.
Effective management often requires both sprays and environmental modification. Some fungicides remain localized on the surface and act protectively, whereas others are incorporated into plant tissues. Systemic agents possess both protective and curative properties. With the development of fungicide resistance (dicarboximides), or deregulation (benzimidazoles), there has been a shift to chemicals such as iprodione (Rovral®), cyprodinil (Vangard®), fludioxonil (Switch®), pyrimethanil (Scala®), fenhexamid (Elevate®, Teldor®), tolylfluanid (Euparen multi®), and purified paraffinic oil (Stylet oil). Their application, especially at early flowering, late flowering, and pre-bunch closure often can achieve 80–90% control. Latter application is usual required only when conditions become especially conducive to infection, especially under rainy spells just prior to harvest. Neither sulfur- nor copper-based fungicides are effective against B. cinerea. Effective fungicide application is enhanced by leaf removal around the clusters.
Benzothiadiazole, a new class of disease control agents, possesses the novel property of inducing systemic acquired resistance (Iriti et al., 2004). It has been found to activate phenol synthesis, including anthocyanin and resveratrol, the latter of which enhances disease resistance (Iriti et al., 2004).
Registration of fungicides for use is regulated by government agencies, as are dosage rates and residual levels in wine. The latter typically sets limits on the last spraying prior to harvest. Because importing countries may set even more stringent maximum permitted residual levels, growers and wineries may need to adjust application relative to the countries to which their wines are exported.
Plowing under infested plant remains, as well as applying a green manure, helps to reduce vineyard survival of the fungus. By itself, though, it is ineffective as a control measure. The use of less vigorous rootstocks, canopy management, and basal leaf removal can help generate a more open canopy and speed drying of vine surfaces. The application of gibberellic acid can also favor drying by opening tight fruit clusters. This is probably applicable only for table and raisin grapes as gibberellic acid increases the incidence of untypical aged (UTA) flavor in wine.
Because of the significance of Botrytis cinerea infection to many crops, biological control is under investigation in many parts of the world. It varies from the application of compost and manure extracts to adding suppressive mycoviral, bacterial, yeast (Pichia membranifaciens), or filamentous fungal (Pythium radiosum) agents. Rhamnolipids from Psuedomonas aeruginosa, noted earlier, may also be effective, not only as a direct inhibitor, but also as an activator of plant defense mechanisms (Varnier et al., 2009). The most commercially successful biocontrol agent for Botrytis infections appears to be Trichdex®, a formulation of Trichoderma harzianum spores (O’Neill et al., 1996). Because the action of biocontrol agents may be suppressed by fungicides, joint application is counter-indicated. An excellent review of control practices, stressing alternative control measures, can be found in Jacometti et al. (2010).
Powdery Mildew (Oidium)
The fungus that induces powdery mildew in grapevines is a member of a large group of generally host-specific, obligate, parasitic fungi. They cause related diseases on a wide range of flowering plants. The species that attacks grapevines, Uncinula (Erysyphe) necator, is one specialized to members of the Vitaceae (Halleen and Holz, 2001) (Fig. 4.57). Even low levels of infection may negatively affect grape quality. In addition, U. necator can augment susceptibility to other diseases, pests, and spoilage organisms (Gadoury et al., 2007). It is often the most destructive pathogen afflicting V. vinifera.
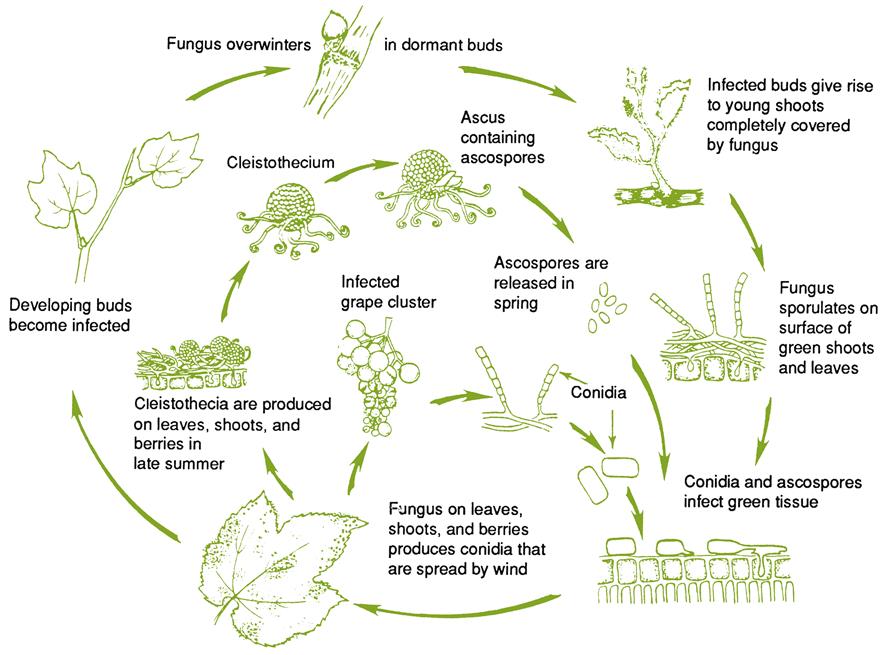
After germination, spores of Uncinula necator produce mycelium that grows over the epidermis. Periodically, specialized projections penetrate downward into epidermal cells. These generate short extensions within the cell, termed haustoria, establishing intimate contact with the cytoplasm. Nutrients extracted by the haustoria permit continued growth and sporulation of the surface mycelium. The underlying palisade cells express the greatest physiological disruption, soon becoming necrotic. This likely results from redirection of nutrients from these and adjacent cells to infected epithelial cells, resulting in their starvation. Most of the fungal mycelium remains external to the vine.
Initially, Uncinula necator was localized and endemic only to eastern and central North America. Being sympatric, indigenous Vitis species evolved partial resistance. In contrast, Vitis vinifera evolved in isolation from the pathogen. Its young tissues possess little inherent resistance to the pathogen. When it was accidentally introduced into Europe about 1840, presumably on imported vines from North America, the result was the first of a series of devastating diseases to strike European vineyards. Genome studies indicate that there were probably two separate introductions (Brewer and Milgroom, 2010). The same investigation suggests that movement of the pathogen to California, and subsequently Australia, was associated with importation of cuttings from Europe.
Unlike many pathogens, U. necator is not highly dependent on specific climatic conditions for sporulation or infection. The fungus infects over a wide range of temperatures (10–32°C) and is little affected by low humidity. It can attack any green vine tissue. Thus, vineyard conditions that favor higher tissue temperatures, such as basal leaf removal, open canopy training systems, avoidance of excessive vine vigor, and partial rootzone drying can limit disease severity. These often act synergistically (Austin et al., 2011)
Fungal overwintering often depends on dormant hyphae that survive as nascent infections on the inner scales (prophylls) of buds. Sporulation may commence within the bud and initiate infection upon budbreak. In cool climates, survival may also involve microscopic, round, reddish-black resting structures called cleistothecia (chasmothecia). Mature cleistothecia, washed from diseased tissue, may lodge in bark crevices. In this position, they are ideally situated to initiate infections in the spring (Gubler and Ypema, 1996). After rains, overwintering cleistothecia swell, rupture, and eject ascospores. These may wash or be blown onto young tissues and initiate early infection following budbreak (Pearson and Gadoury, 1987). For reasons that are still unclear, cleistothecial formation is, or was, rarely found in some countries. Thus, the principal means of survival in many locations is via dormant hyphae. If shoots are sufficiently infected early in the season, usually from mycelium overwintering on inner bud scales, they become severely stunted, forming what are called flag shoots.
In regions where cleistothecia participate in the infection cycle, it may be essential to initiate control measures earlier than when infection develops only from overwintered hyphae. Application of lime-sulfur or flowable sulfur, prior to budbreak, is often effective in limiting early cleistothecial-based infections (Gadoury et al., 1994).
Early infections can result in leaf and fruit distortion, by killing surface tissues before they expand and reach maturity. Severe infection leads to leaf and fruit drop, as well as death of shoot tips. Rapid spore (conidial) production generates the white powdery appearance (mildew) typical of infected tissue. These spores initiate further rounds of infection during the season. Fungal growth and sporulation are optimal between about 20–30°C. Above 32°C, fungal metabolism essentially stops. Later in the season, cleistothecial production can give infected tissues a distinctive red- to black-speckled appearance. Fruit is most sensitive to infection shortly after initiation, many (but not all) cultivars rapidly developing immunity thereafter (Gadoury et al., 2003). This appears to involve hyphal inability to effectively penetrate the mature epidermis. Immunity may be complete within as little as 4 weeks (Ficke et al., 2003). This may also be due to enhanced synthesis of stress and pathogenesis-related (PR) proteins, such as osmotin and traumatin-like proteins (Monteiro et al., 2003). Although growing tissue remains susceptible, older tissues become increasingly resistant, with mature leaves seldom being infected. Despite this, fruit may succumb to a diffuse, non-sporing form of infection visible only microscopically. It predisposes the fruit to insect and other pathogen attack, and the wine quality is degraded by abnormally high concentrations of volatile acidity and ethyl acetate (Gadoury et al., 2007).
Early control is not only important for fruit protection, but also minimizes foliage damage and its consequences for current and subsequent years’ growth. Although the rachis remains susceptible for a prolonged period, this does not appear to affect fruit development or augment fruit infection. Control of berry infection is most effective when applied at and shortly after flowering and fruit set, but may need to be extended under conditions favorable to the diffuse infection noted above. Where early leaf infection has historically been serious, control should commence earlier, and may need to be extended up to véraison. Early termination of treatment with sulfur is desirable to reduce its presence in crushed juice. Residual sulfur may disrupt yeast growth and augment hydrogen sulfide production during fermentation (Thomas et al., 1993).
Disease management is based primarily on sanitation and fungicidal sprays, principally sulfur and/or DMI fungicides. Because spore dispersal during the season is limited, removal of overwintering fungal tissue on leaves, stems, and fruit limits early disease onset. Delaying early onset can often postpone any potentially severe disease development until after harvest. Developing an open canopy also reduces disease incidence. It also improves the potential for uniform spray application. Wettable or sulfur dusts are commonly applied during the growing season. Although not incorporated into plant tissues, sulfur acts both as a preventative and curative agent. This results from the majority of the fungal tissue existing exterior to the plant being directly exposed to the fungicide. Unfortunately, sulfur effectiveness is temperature-dependent, being much less active below 20°C, and becoming increasingly phytotoxic above 30°C. In addition, sulfur use may promote increased spider mite populations, by suppressing predators such as the mycophagous mite Orthotydeus lambi. Demethylation-inhibiting (DMI) fungicides (e.g., Bayleton®, Rally®, and Rubigan®), or strobilurin fungicides (e.g., Abound®, Flint®, and Sovran®) are more effective, less phytotoxic, but more costly. The potential for rapid resistance to these highly specific agents requires that they be used sparingly, and never more than twice in sequence. Agents such as silicon, bicarbonates (e.g., baking soda), oils (e.g., canola oil), cinnamic aldehyde, and phosphate fertilizer may also be effective. Chitosan (a deacetylated derivative of chitin) activates chitin/chitosan receptors. These can induce a series of systemic acquired resistance factors, providing effective control even under high disease pressure conditions (Iriti et al., 2011). Most of these agents are available in commercial form. Ampelomyces quisqualis (AQ10), as well as other mycoparasitic fungi, have been used as biological control agents. Regrettably, they tend to be limited by the environmental conditions they require.
Early prevention is essential to avoid rapid spread, and before disease severity becomes evident. In addition, yield loss is often directly related to the timing of the initial infection. The development and widespread use of disease-risk models (Broome et al., 1995) have improved the timing and effectiveness of fungicide application, while reducing the amount required.
Downy Mildew (Peronospora)
Downy mildew is the second of three disease/pest introductions that devastated European vineyards in the mid- to late 1800s – the others being powdery mildew and phylloxera. All were alien pathogens to Europe, and readily attacked Vitis vinifera. Despite the partial similarity in name to powdery mildew, the causal agent Plasmopara viticola (Fig. 4.58) is unrelated to Uncinula necator.
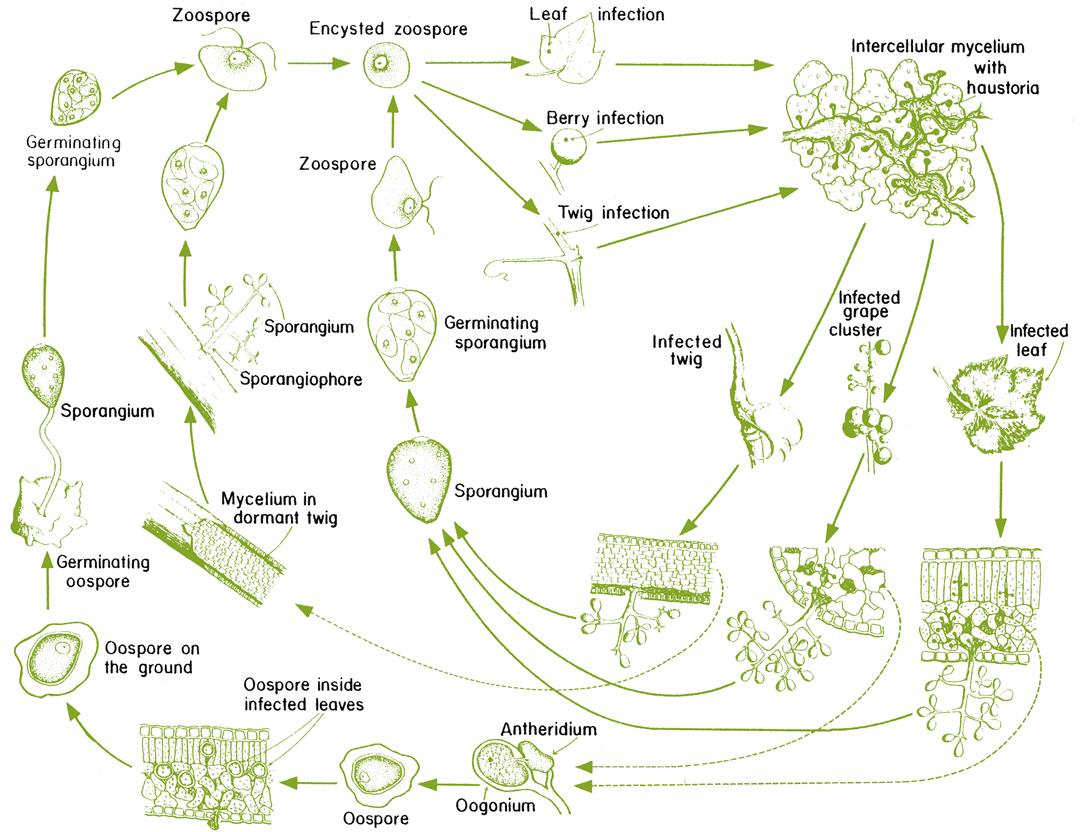
The spores, called sporangia, germinate to produce several flagellated zoospores. Zoospores possess a short motile stage, during which they swim in a thin film of water on the host tissue toward stoma. Here, they adhere to the epithelium, lose their flagella, and begin to penetrate the host. Shortly after successfully establishing itself in the host, the fungus may initiate sporangial production. This typically occurs at night. The sporangia remain viable for only a few hours after sunrise. This may explain the highly diverse and localized dispersion of P. viticola genotypes (Gobbin et al., 2005). Because infection is highly dependent on rainfall or dew, high humidity, and a restricted temperature range, downy mildew tends to produce less widespread damage than powdery mildew.
Similar to powdery mildew, downy mildew attacks all green parts of the vine, is an obligate parasite, and produces haustoria. However, Plasmopara viticola hyphae do not remain exterior to the plant. In contrast, they ramify extensively throughout host tissues. Under moist conditions, sporulation develops rapidly. On leaves, spore-bearing hyphae (sporangiophores) erupt preferentially through stomata on the lower surface. Leaf invasion is the primary source for spores inducing fruit infections. Infected shoot tips become white with spore production and show a distinct ‘S’-shaped distortion. The shoot subsequently turns brown and dies. Grapes are most vulnerable when young, but all parts of the fruit cluster remain susceptible until maturity. As with leaf infection, severe development of the disease can result in premature fruit abscission. Fruit infection has the greatest direct effect on grape quality and yield. Nonetheless, severe leaf infection can indirectly affect yield in the subsequent year, due to reduced carbohydrate generation.
During the summer, the fungus produces a resting stage within infected tissues, termed an oospore. Oospores may remain dormant for several years. When conditions favorable to germination occur, they produce a sporangium, within which spores (sporangiospores) develop. Typically, this occurs in the spring, but can occur throughout the season (Kennelly et al., 2007). In mild climates, both oospores and dormant mycelia (in infected leaves) may initiate spring infections, though in most locations infections are induced by spores derived from oospores.
Bordeaux mixture and several nonsystemic fungicides provide protection against infection, but are not curative. For this, systemic fungicides such as fosetyl aluminum and phenylamides (e.g., Metalaxyl®) are required. The uptake of these fungicides by plant tissues reduces their dilution or removal by rain. Depending on local conditions, early and effective control often limits the need for subsequent or multiple fungicide applications (Jermini et al., 2010).
No biological control measures are currently effective, although several fungi have been shown to be toxic to P. viticola. These include Alternaria alternata, Epicoccum nigrum, Acremonium byssoides, and Fusarium proliferatum. In addition, application of chitosan oligomers (a deacetylated derivative of chitin) activates resistance by enhancing phytoalexin, chitinase, and β-1,3-glucanase production in plant tissues (Aziz et al., 2006). The production of an open canopy by basal leaf removal, or a more open training system, has only a minimal effect on disease incidence. Consequently, chemicals remain the only effective treatment for this pathogen, under conditions favorable for disease development.
Black Rot of Grapes
Unlike the diseases noted thus far, black rot is of economic significance only in eastern North America and selected regions in Europe, South America, and Asia. Most indigenous Vitis spp. show considerable resistance to attack, having evolved in the presence of the pathogen for millions of years. In contrast, cultivars of V. vinifera are very susceptible. Depending on the occurrence of favorable (humid) weather conditions, and the initial inoculum, the disease can cause crop losses of up to 80%.
Three subspecies of the causal fungus Guignardia bidwellii (Phyllosticta ampelicida) are recognized. One affects only species of the Vitis subgenus; a second infects both subgenera (Vitis and Muscadinia); while the third attacks only species of the related genus Parthenocissus.
Infection can occur on new growth at any time during the growing season (Fig. 4.59), but does not develop on mature leaves or fruit following véraison. On leaves, lesions develop as small creamy circular spots. As these enlarge (up to 10 mm), they darken to a tan color, and finally turn reddish brown. The spots are surrounded by a band of dark-brown tissue. Characteristic small, black, roundish raised structures (spore-bearing pycnidia) develop in older parts of the lesion. Black, elongated lesions usually develop on petioles and the fruit stalk. These may encircle the structure, killing any tissue distal to the lesion. On young shoots, similar but larger black cankers develop. These produce pycnidia during the growing season. Lesions develop surprisingly quickly on the fruit, converting hole berries into blue-black, shriveled ‘mummies’ within a few days.
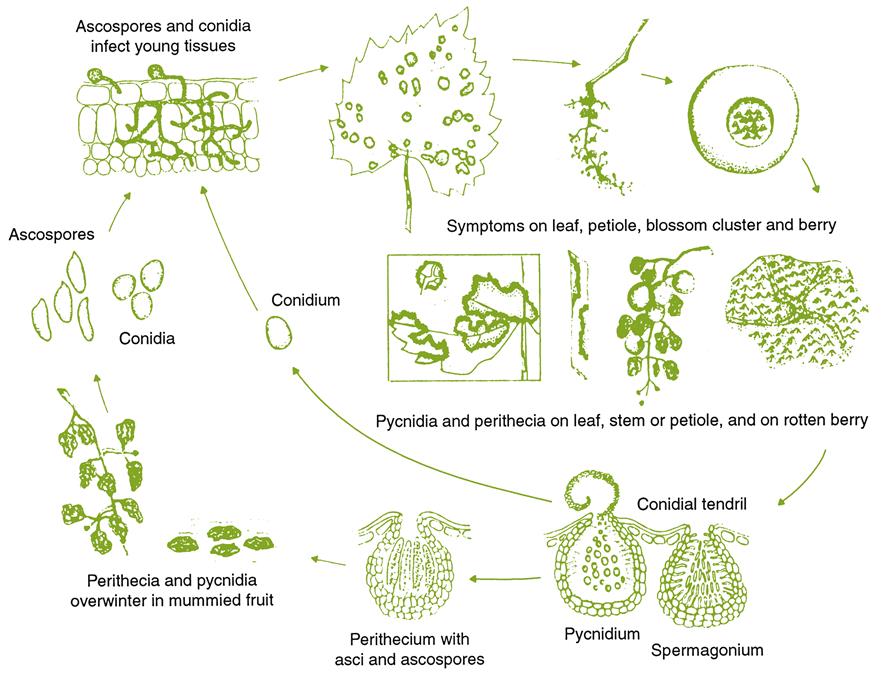
Many cycles of localized infection may occur during a single season. During the fall and winter months, structures superficially resembling pycnidia develop in mummies on the ground. These fruiting bodies, called pseudothecia, develop as a result of sexual reproduction. In the spring, the ascospores mature and are ejected into the air following even light rainfalls. They initiate a new round of infection.
On wild vines, the fungus probably survives most effectively on fruit mummies. However, in commercial vineyards, most mummies are collected and destroyed. Thus, survival is most probably associated with infected portions of canes not removed during pruning. Spore production from overwintered tissue can continue well into midsummer.
Control used to be based on the use of protective, contact fungicides, such as maneb and ferbam. However, current preference is to use systemic fungicides, such as tridimefon (Bayleton®) and myclobutanil (Eagle 40®). They have the added benefit of being potentially curative. Strobilurin fungicides are less curative, but may be valuable due to their simultaneous action against other pathogens, notably powdery and downy mildews (Hoffman and Wilcox, 2002). Sanitation (destruction of mummified fruit and infected cane wood) is particularly important in reducing the spring inoculum load. This can significantly delay and reduce the economic significance of infection. In climates where the incidence of black rot is frequent, and especially where mechanical pruning is practiced, inspection and destruction of infected cane wood should be practiced. For a discussion of integrated control measure see Hoffman et al. (2004).
Eutypa Dieback
Eutypa dieback is a serious disease, inducing a slow but insidious attack on the woody components of the vine. It often kills the vine within 10 years if unchecked. The effect on vineyard yield over time, in susceptible and more resistant cultivars, is compared in Fig. 4.60.
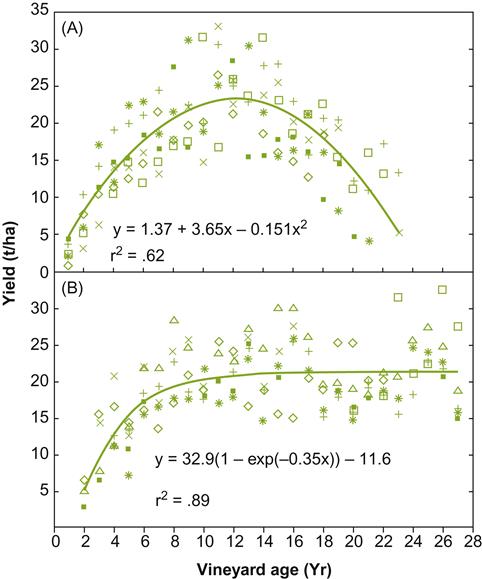
The pathogen Eutypa lata (E. armeniacae) preferentially invades wounds of the perennial shoot system (Fig. 4.61). Its most serious manifestations derive from xylem infections, principally originating from wounds produced during graft conversion or training system conversion. Wounds produced during annual pruning can also produce infection sites. Nonetheless, these infections tend to be less significant, as cane wood is less susceptible than the permanent older wood. In addition, much of cane wood is removed during pruning, before the fungus can advance into more permanent parts of the vine. However, cane wood infection may be more significant where minimal pruning is employed. Where vines are spur pruned, a double pruning technique has been suggested by Weber et al. (2007). In the procedure, nonselective trimming of the canes to 30–45 cm occurs in late fall, followed by a more traditional pruning to two-node spurs in late winter. At this time, the likelihood of infection is much reduced.
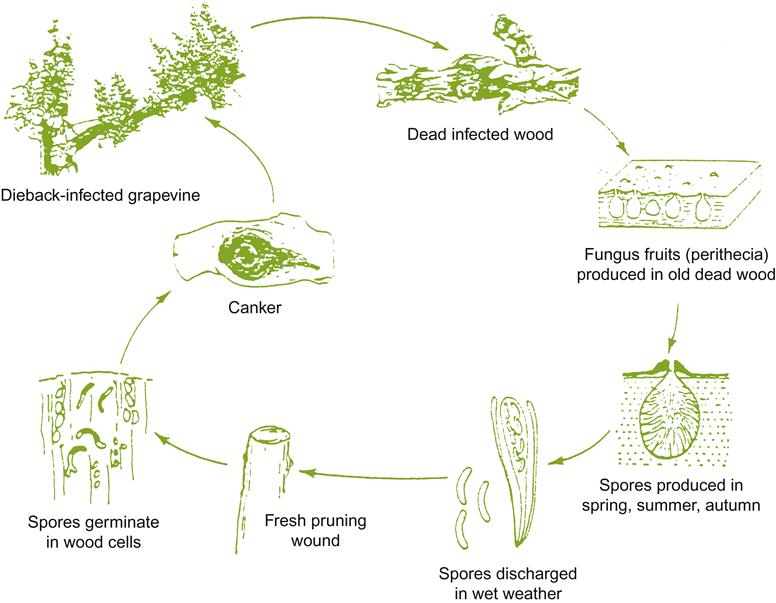
Fungal growth in the xylem is slow, with minimal invasion of the cambium and phloem. As invasion progresses, an enlarging, elongated, lens-shaped canker forms. It often remains undetected for years, due to the overlying bark. Disease symptoms often appear months or years after infection, and are typically detected when distinctive leaf and shoot symptoms develop. These are usually localized to shoots directly associated with and above the lesion. Symptoms are most apparent in the spring, when stunted shoots are most evident, compared with rapidly elongating healthy shoots. The young leaves of affected shoots are usually upturned, small, distorted, chlorotic, and possess a tattered margin. Shoot internodes are markedly dwarfed. Fruit formation on infected wood is limited and usually dehisces before maturity. These effects are caused by mycotoxins produced by the fungus. Eventually, the portion of the vine associated with the lesion dies. This feature gives rise to the expression ‘dead arm.’
The canker is usually observed only by removing the outer bark. Well-established cankers contain rows of flask-shaped perithecia (pseudothecia), structures in which ascospores are produced. Cutting tangentially along the wood, and through the canker, exposes the perithecia as round objects containing a jelly-like material (translucent when wet; white and sheet-like when dry), or mat black when empty after spore discharge. The infected wood typically possesses a V-shaped appearance and has a light-grayish to dark-brown color.
Spores in new perithecia generally mature in late winter or early spring. Subsequent release follows periods of sufficient rainfall (>1 mm), often being most marked in very early spring. Sprinkler irrigation may be an important source of water for spore discharge in dry regions.
Eutypa dieback is particularly difficult to control. Except in areas where grapevines are the major woody plant, sanitation alone is relatively ineffective in reducing disease incidence. This results from E. lata infecting many indigenous woody species common to grape-growing regions. Spores are also effectively wind dispersed over more than 100 km. Thus, a local source of infection is not necessary for the development of a serious disease outbreak. Finally, normal (surface) fungicidal spraying is ineffective in preventing spore germination within the xylem.
Management techniques include wood removal to about 5–10 cm below any area of apparent xylem staining. This is required as the fungus may occur considerably in advance of any staining. The wound is usually treated with a creamy suspension of Flusilozolr®, Carbendazim®, or 20% boric acid. The suspension must soak into the exposed wood to provide adequate protection. Destruction (preferably burning) of the infected wood is recommended. Diseased tissue can remain a source of spore production for a considerable period after removal. In addition, any pruning wounds on 2-year or older wood should be treated. Application is performed as soon as possible after pruning, especially when cuts are performed in the fall or winter months. This protects the site from infection by spores landing on the wound. Alternatively, a suspension of Trichoderma harzianum may be applied (John et al., 2008). Where possible, pruning should occur when the xylem is active and spore production low. The formation of lignin and suberin, in reaction to wounding, markedly reduces disease incidence (Munkvold and Marois, 1990). Thus, very early-autumn or late-spring pruning further minimizes the likelihood of infection.
Another disease resembling some of the symptoms of Eutypa dieback, but without foliar symptoms, is induced by several genera in the Botryosphaeriaceae (Pitt et al., 2010). They produce wedge-shaped cankers, dieback, necrotic canes, bud necrosis or whitening, graft failure, and fruit rot. These fungi have also been implicated in young vine decline, such as Petri disease (see below).
ESCA, Black Measles, Petri, and Black-Foot Diseases
These terms refer to various expressions of a disease complex caused principally, but not exclusively, by Phaeomoniella chlamydospora. Esca and Black Measles are respectively European and North American terms for its expression in mature vines (Mugnai et al., 1999). Petri disease, slow decline, and black goo are various names given to its expression in young grafted vines. Drought stress is an important factor inducing symptomatic expression, which otherwise remains largely or completely hidden. Grapevine ‘replant disease’ may partially be another expression of this disease complex, although assorted bacteria and nematodes have also been implicated.
Much confusion about the origin of these disease syndromes has been caused by the frequent association of symptoms with the presence of other fungi, and uncertainties as to their correct identification. Fungi not infrequently isolated from diseased and dying vines, or aggravating symptoms, are Phaeoacremonium aleophilum and several other hyphomycetes, as well as several white rot fungi (notably Fomitiporia punctata and F. mediterreanea). The latter are thought to be particularly important in Esca expression in Europe.
The disease has been present for centuries, but only recently has its presence become particularly apparent. One of the factors awakening people to its significance was the extensive replanting necessitated by an outbreak of phylloxera in California. This occurred with vines grafted to A×R#1, the predominant rootstock used at the time. The replanting provided conditions where the appearance of a slow vine decline was accentuated by the numbers observed. Once identified, other trunk syndromes were recognized as being alternative expressions of possibly the same disease. Another probable cause for an increase in disease expression, especially in mature vines, is the abandonment of sodium arsenate several weeks after pruning. Depending on when pruning is done, wounds may remain susceptible to infection by P. chlamydospora for weeks or months.
Esca expression is intermittent, and may reveal itself either in chronic or acute forms. Symptoms of chronic disease are characterized by a progressive foliage deterioration, whereas the acute phase results in sudden vine death. In the chronic form, leaf symptoms can begin at any time. They start in basal leaves and move apically, appearing as yellow to red patches. These develop necrotic centers as the patches coalesce (‘tiger strips’). They eventually become irregular brown zones of necrosis between the veins and margins of the leaf, leading eventually to leaf dehiscence. Fruit symptoms vary with the region and cultivar. In France and northern Italy, berries are often visually asymptomatic, but do not fill or mature properly. In southern Europe and California, affected berries may develop brown/violet patches (the black measles syndrome). Foliar and fruit symptoms may occur simultaneously or separately. Vines showing chronic symptoms one year may appear healthy and symptomless in subsequent years.
Symptoms of infection in the wood appear as pale brown discolored sections in the xylem, surrounded by a dark ring, especially in the trunk or cordon. This lesion may occur alone, or in combination with lesions produced by white rot fungi or Eutypa dieback (see Creaser et al., 2002). Frequently, infection occurs in the absence of leaf or fruit symptoms.
Expression of the disease in young vines (Petri disease) most frequently results in, or from, partial graft failure associated with infection by P. chlamydospora. However, infection by itself does not necessarily induce disease development (Edwards and Pascoe, 2003). Stress conditions, such as water deficit or overcropping before the vine is established, appear to favor disease expression. The vine may show a slow decline within the next few years. If decline occurs later, it would normally be considered an example of Esca. On investigation, the graft site usually shows lesions (streaks or dots). These may exude a thick black ooze (‘black goo’). Microscopically, xylem vessels may show tyloses that have grown in from infected parenchyma cells. The xylem exudate involves phenolic substances synthesized by the necrosing parenchyma cells. External symptoms include poor budbreak, stunted shoot growth, mild foliar chlorosis associated with necrotic edges, wilting, and dieback. This presumably results from toxins produced by the fungus or diseased tissues. These prevent full development of the callus. The resultant poor xylem connections severely restrict water and nutrient transport under hot, dry conditions. This probably explains why extensive watering and nutrient application (compost) may diminish symptoms or revive diseased plants. Although most prevalent and severe in grafted vines, own-rooted vines can also develop the disease.
Although the origin of infection in young vines is still not fully established, the prevailing view is that it originates principally from symptomless infected vines, from which the rootstock or scion wood was obtained. This is consistent with the use of hot-water treatment to eradicate the pathogen (Fourie and Halleen, 2004). Treating the wood prior to grafting with several fungicides has also been found to be beneficial (Fourie and Halleen, 2006). Subsequent vine establishment in pathogen-free potting soil, prior to planting in the field, might prevent early reinfection (when vines seem to be the most susceptible). The same may apply to black-foot disease (below). In contrast, Esca may develop from infection of pruning wounds, though infection from scion mother plants is a distinct possibility.
Another disease that develops under similar conditions is Cylindrocarpon black-foot disease. It can be another source of grapevine decline in new plantations. In this case, though, the fungus causes a root and butt rot. In cold climates, the practice of removing vines from their supports and burying them for winter protection may also produce lesions facilitating fungal entrance (Petit et al., 2011). Subterranean symptoms include few feeder roots, low root biomass, and necrotic root lesions. Vegetation is chlorotic and stunted. No effective specific control exists, although interesting results with endomycorrhizal infection have been promising (Petit and Gubler, 2005). Inoculation of roots with Glomus intraradices reduced both the number of root lesions, as well as disease severity, while increasing root dry weight.
Bacterial Pathogens
Bacteria are an ancient group of microorganisms, existing predominantly as colonies of more or less independent cells. Most possess a rigid wall, providing them with their standard spherical to rod shapes. However, one group – the phytoplasmas – possess no cell wall and are amorphous, making them almost impossible to identify under a regular light microscope.
Bacteria are typically restricted to entering plants either via natural openings (e.g., stoma and lenticels), or through wounds. Nevertheless, root pathogens produce enzymes that can degrade plant cell walls, permitting direct penetration of young roots. Alternatively, bacteria may enter the host through grafting wounds, or via insect and nematode feeding sites. Overwintering occurs as dormant cells in soil, plant parts, or various vectors. Grapevines may also act as hosts of fastidious, endophytic bacteria (Bell et al., 1995), as well as rhizosphere bacteria. Some of these are growth-promoting, such as Burkholderia (Compant et al., 2005). Their presence may also reduce disease severity.
Crown Gall
Crown gall is a disease affecting many woody plants. In grapevines, it is typically induced by a specialized species, Rhizobium vitis (Palacio-Bielsa et al., 2009). Formerly designated Agrobacterium vitis, all members of the genus Agrobacterium have recently been transferred to Rhizobium (Young et al., 2001). In contrast, R. radiobacter (formerly A. tumifaciens) provokes crown gall in a wide range of angiosperms, but rarely in grapevines. Exterior to the vine, R. vitis is essentially isolated only from the rhizosphere around young grapevine roots. It can survive for at least 2 years, possibly longer, in dead and dying roots (Burr et al., 1995).
Rhizobium vitis can invade and induce lesions in young vine roots (Fig. 4.62), possibly through its production of polygalacturonidases. These enzymes may also assist the bacterium in gaining access to the xylem. Alternatively, or in addition, wounds produced by nematode feeding, such as Meloidogyne hapla, may facilitate root penetration (Süle et al., 1995). Other potential sites of penetration probably involve wounds generated as a result of grafting or soil cultivation. Upon gaining access to the xylem, where the bacterium primarily grows, passive transport in the xylem sap can translocate it into the shoot. Such movement primarily occurs in early spring. Subsequently, the bacterial population drops precipitously in the shoot system, rising again only in the fall (Bauer et al., 1994). Thus, although the bacterium can grow systemically throughout the vine, its distribution is far from uniform, and is most frequently localized to the root system.
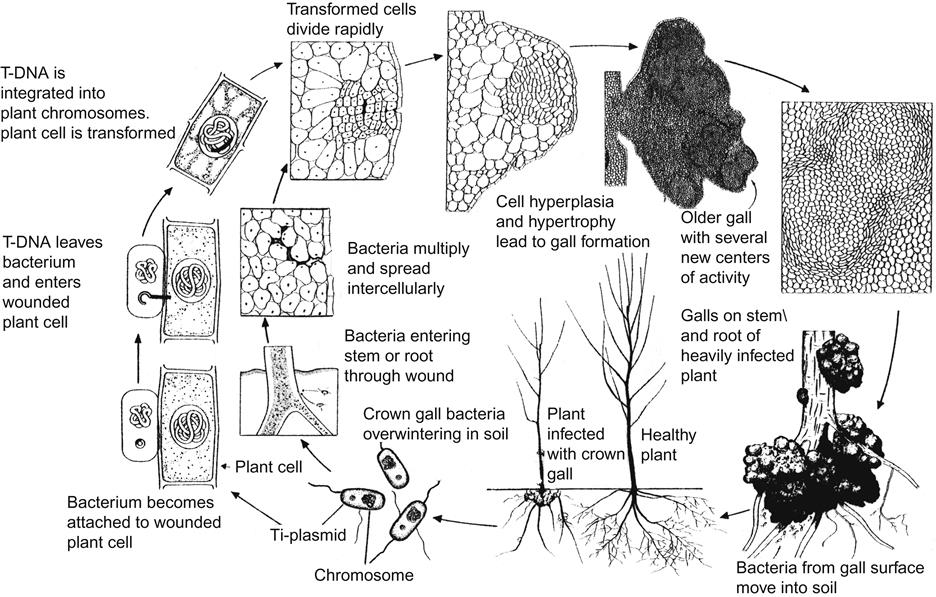
The strains of Rhizobium vitis that inhabit wild vines in North America and Europe (Burr et al., 1998) appear to be non-tumorigenic. In contrast, most strains in commercial vineyards are tumorigenic (gall forming). This divergence may have originated from the accidental dispersion of asymptomatic, tumorigenic strains on rootstocks, associated with their global distribution in phylloxera control.
Despite R. vitis primarily inhabiting the root xylem, and occasionally inciting localized root necrosis, its serious pathogenicity occurs at the trunk base. In this location, R. vitis can provoke uncoordinated cell division in the vascular tissue, inducing gall formation. Individual galls are commonly self-limiting, and subsequently rot and separate from the stem. New galls may originate next to old galls. In young vines, galls can girdle the trunk, killing the vine. In mature vines, the consequences of gall formation depend on their number, size, and distribution. Heavy galling significantly disrupts vascular flow, reducing vine vigor and fruit yield (Schroth et al., 1988). Galls are also invasion sites for other grapevine pathogens, notably Pseudomonas syringae f. syringae and Armellariella mellea.
Gall formation appears to require cellular microlesions in parenchyma cells, such as those produced by frost damage. Correspondingly, gall initiation typically occurs during the spring in cold climates. Galls develop most commonly near the transition from root to shoot (crown), hence the name ‘crown gall.’ Graft conversion (topping) can also be a significant gall activator, resulting in graft failure.
Wounding indirectly stimulates gall induction by activating parenchyma cell multiplication. This favors bacterial attachment and the transfer of a tumorigenic (Ti) plasmid from the bacterium into the dedifferentiating parenchyma cell. Upon plasmid transfer and activation, the infected cells transform into tumorous cells. This is associated with the overproduction of auxins and cytokinins, provoking an uncoordinated multiplication of callus-like tissue. It is this tissue that generates the gall. The plasmid also codes for the production of a unique group of amino compounds: the opines nopaline, octapine, or vitopine. Opines serve as specific nutrients for bacterial growth.
No fully effective control of crown gall currently exists. The best option, where possible, is to plant disease-free stock in virgin soil. In already infested soils, the best choices are either to graft to a crown-gall-resistant rootstock, or use biological control. Resistant rootstocks such as NAZ4, NAZ6, Gloire de Monpellier, C3309, or 101-14 MGT do not prevent infection, but reduce gall severity. Severity may also be reduced by prior infection with the HLB-2 strain of Rhizobium vitis (Pu and Goodman, 1993), nonvirulent (Ti plasmid-deficient) strains (Zäuner et al., 2006), or Rahnella aquatilis HX2 (Chen et al., 2007). Inoculation of disease-free rootstocks with Pseudomonas aureofaciens and P. fluorescens also appears to markedly reduce infection and disease severity (Khmel et al., 1998). Where crown gall is serious, as in northeastern North America, multiple trunking is often practiced as a precaution. It is unlikely that all trunks will succumb simultaneously, leaving one or more trunks to sustain and reestablish the vine. The disease is typically more serious on V. vinifera cultivars than on V. labrusca and French-American hybrids.
Eradicating the bacterium from scions and rootstocks used in propagation can often, but not consistently, be achieved with hot-water treatment (Burr et al., 1996). Thus, micropropagation from apical shoot tissue is used where elimination is obligated, as with international movement of cuttings. When propagating disease-free clones, the use of pasteurized soil and greenhouse equipment is essential to prevent the spread of R. vitis during bench grafting.
Pierce’s Disease
Pierce’s disease is induced by another xylem-inhabiting bacterium, Xylella fastidiosa (Hopkins and Purcell, 2002). It causes a similar disease in many tree crops. Considerable genetic diversity exists within the species (Hendson et al., 2001), but most pathotypes are ill-adapted to multiplying and provoking disease in grapevines. In addition, many annual crops, vines, and weeds may carry X. fastidiosa as asymptomatic endosymbiont (Wistrom and Purcell, 2005). These can act as reservoirs for the bacterium, from which transmission to susceptible hosts can occur via xylem-feeding insects (Redak et al., 2004). Because of host specificity, and differential cultivar sensitivity, transmission does not necessarily result in disease. The primary insect transmitters are sharpshooter leafhoppers (Cicadellidae) and related spittle bugs (Cercopidae).
Geographical distribution of the disease is limited by the presence of suitable vectors and the bacterium. Except for several isolated observations in Europe (Boubals, 1989; Berisha et al., 1998), and its recent significant occurrence in parts of California, Pierce’s disease is primarily isolated to its endemic habitats in the southeastern United States, Mexico, and Central America. The tropical to subtropical localization of Xylella-induced diseases suggests that winter temperatures are a significant factor limiting pathogen spread. In areas where the pathogen is endemic, indigenous species of Vitis are resistant or relatively tolerant to infection. Disease severity is often a reflection of the relative incidence of the pathogen (Ruel and Walker, 2006). Although its occurrence can severely restrict the commercial cultivation of V. vinifera, there is considerable variability in cultivar susceptibility. Of popular varieties, Chardonnay and Pinot noir are particularly sensitive, Cabernet Sauvignon and Sauvignon blanc less so, and Riesling and Zinfandel show moderate resistance.
Until recently, Pierce’s disease was of limited significance in California – presumably due to the absence of an effective vector. This situation has changed since the appearance of the glassy-winged sharpshooter (Homalodisca coagulata). It is an indigenous insect within the historical range of the disease in the southeastern USA and northeastern Mexico. It is capable of flourishing on the nutritionally meager solution found in the xylem.
In sensitive vines, transmission often results in rapid bacterial colonization and movement throughout the plant. Subsequently, the bacteria adhere to the interior surfaces of xylem vessels. This is accompanied by the accumulation of xanthan gums in the vessel lumina. Later, tyloses grow into affected vessels, causing further occlusion (Stevenson et al., 2004). These sequelae disrupt water flow and place the vine under potential water deficit. Symptoms are exacerbated in hot or arid climates, but are not in themselves produced by water stress (Thorne et al., 2006). Details of the mechanisms of pathogenesis can be found in Chatterjee et al. (2008).
Leaf symptoms (scorch) develop as a progressive inward browning and desiccation of the blade. In advance of the necrosing region, concentric areas of discoloration commonly develop. They are yellow in white cultivars and red-purple in red cultivars. The blade may eventually drop, leaving the petiole still attached to the shoot (Stevenson et al., 2005). This produces a symptom called ‘matchstick.’ Late in the season, ‘green islands’ may remain on canes, surrounded by brown mature bark. These islands are associated with regions where periderm differentiation is absent.
In severely affected vines, budbreak is delayed, and shoot growth is slow and stunted. The first four to six leaves are dwarfed, and the main veins are bordered by dark-green bands. Subsequent leaves generally are more typical in size and appearance.
Infected V. vinifera cultivars may survive for 1–5 years, depending on the age of the vine when infected, the variety, and local conditions. Young vines are particularly susceptible and frequently succumb within 2 years. Until the late 1990s, with the spread of the glassy-winged sharpshooter, infection in southern California developed in mid- to late season. Frequently, the bacterium did not survive the winter, and recovery was common (Hill and Purcell, 1995). This is no longer the situation with the new, more effective vector.
In warm climates, where the pathogen and vector are common, the only effective control is growing resistant or tolerant cultivars. Where the disease is established, but localized, vineyard plantings should ideally avoid areas where reservoirs of the pathogen and vector are common, notably river banks populated with vines and shrubs, such as blackberry and elderberry. Because transfer from indigenous plants is less efficient, replanting riparian environments with native plants can reduce the likelihood of transmission. Transfer is predominantly from symptomless carriers, rather than from vine to vine. Thus, planting a 7-m-wide conifer or hardwood belt around vineyards in susceptible sites has been investigated as a transmission buffer. Insecticidal control of vectors has generally been unsuccessful in halting disease spread, but can be effective in dramatically reducing local vector populations. For example, imidacloprid and thiamethoxam are particularly active against sharpshooters. Thankfully, these insecticides appear to have little effect on sharpshooter egg-parasitoids. In California, release of egg-parasitoid wasps (Gonatocerus spp.) has markedly reduced the population of glassy-winged sharpshooters. Other biological control agents of potential interest include mycoparasites, such as Hirsutella spp. and Beauveria bassiana, as well as various natural predators. In addition, competitive exclusion, associated with infection by asymptomatic strains of X. fastidiosa, may provide resistance to subsequent exposure to pathogenic strains.
Yellows Diseases
Several grapevine yellows syndromes have been identified; all induced by one or more phytoplasmas (cell-wall-less bacteria) (Lee et al., 2000). Because of their amorphous shape and small size, they are almost impossible to differentiate microscopically from cell fragments. Disease symptoms are also often insufficiently diagnostic. Thus, unambiguous identification requires the use of molecular techniques, such as PCR (polymerase chain reaction).
Although associated with infection by members of the provisional genus Candidatus (Anonymous, 2004), they are usually classified by their 16S ribosomal RNA fragments (Tran-Nguyen et al., 2008). For example, flavescence dorée is induced by a member of the elm yellows disease group (16SrV); grapevine yellows in the eastern United States and northern Italy is associated with a member of the western X disease group (16SrIII); Vergilbungskrankheit (Germany), bois noir (France), and southern European (Mediterranean) grapevine yellows appear to be associated with members of the stolbur subgroup of aster yellows (16SrIG); while Australian grapevine yellows is incited by an Australian subgroup of aster yellows (16SrIJ). Each group appears to be transmitted by different plant- or leaf-hoppers: Vergilbungskrankheit and bois noir by Hyalesthes obsoletus; Australian grapevine yellows, probably by Orosius argentatus; and flavescence dorée and eastern American forms by Scaphoideus titanus (littoralis). The vectors are not specialized grapevine pests, feeding and multiplying on a wide range of host plants. Typically, alternative so-called ‘dead-end’ hosts (such as grapevines) often express more severe symptoms than those on which the pathogen and standard host developed a form of co-existence.
In grapevines, phytoplasmas multiply exclusively in phloem cells, where they may disrupt nutrient flow and appear to cause profound growth substance disturbances. During feeding, the vector acquires the bacterium in its salivary glands. After reproducing in the insect, the bacterium accumulates in the salivary glands. This favors transmission via the fluid ejected by the proboscis just prior to feeding. Some forms of the disease are also transmissible, at a low frequency, by grafting.
Because the vector, S. titanus, is endemic to eastern North America, it was thought that the phytoplasma inducing flavescence dorée may have been introduced into Europe, along with S. titanus in the late 1940s (Maixner et al., 1993). However, this appears unlikely as the strains causing yellows diseases in France and eastern North America are genetically distinct.
Grapevine yellows diseases are frequently characterized by the following symptoms. Newly infected vines show delayed bud burst and shoot growth in the spring. Internodes are shortened and cane development may show a zigzag pattern. Leaf blades may become partially necrotic and roll downward, more or less overlapping one another, and become brittle. The foliage often turns yellow in white cultivars, and red in red cultivars. Alternatively, angular colored spots may develop on leaf blades. In sensitive cultivars, the most distinctive symptom is a drooping posture that develops in the summer, due to poor lignification of the vascular tissues. In addition, shoots do not turn brown as they mature, or develop only patchy brown areas. Shoot tips may die back during the growing season and develop black pustules. Affected shoots usually die during winter. The fruit tends to shrivel and develop a dense, fibrous, bitter pulp. Symptoms typically begin to develop in the year following infection, termed the crisis year. Symptoms may be more pronounced with joint phytoplasma/virus infections. Symptoms may disappear in subsequent years, associated with induction of responses typical of systemic acquired resistance (Landi and Romanazzi, 2011).
Two distinct expressions of flavescence dorée occur in Europe. In the Nieluccio type, the disease becomes progressively more severe each year until the vine dies. In the Baco 22A type, the vine recovers after symptoms develop in the crisis year. If they are reinfected within a few years of a previous infection recovered vines show only a localized, rather than a systemic, reaction. There is also considerable variation in cultivar sensitivity to the various forms of grapevine yellows. For example, Pinot noir is particularly susceptible to flavescence dorée, but is little affected by bois noir. Other forms of yellows diseases may or may not show remission. In European regions, where flavescence dorée and bois noir occur sympatrically, symptoms of flavescence dorée appear earlier in the season than those of bois noir (Angelini et al., 2006). A characteristic feature of bois noir that gives it its name is the frequent blackening of poorly ripened wood in the fall.
No effective disease control is known for regions where both vectors and pathogens are established. The only effective option is growing varieties that show recovery (Baco 22A expression), or that are relatively insensitive. Insecticide spraying delays but does not stop pathogen spread, although care should be taken in vine propagation, as the pathogen may be spread by grafting. Nonetheless, this recommendation is difficult to apply effectively, with graft spread being most likely during the infection year, when the source material is symptomless. Recovered vines apparently are noninfectious, and can be safely used as stock for propagation. Dormant scion wood can be cured of infection by hot-water treatment. Vector eggs are also simultaneously killed (Caudwell et al., 1997).
Viruses, Virus-Like, and Viroid Pathogens
Viruses and related pathogens are submicroscopic, noncellular, infectious agents, dependent on host cells for self-replication. Those that attack grapevines possess only RNA as their genetic material. Differentiation between viruses and viroids is based primarily on the presence or absence, respectively, of a protein coat enveloping the nucleic acid. Viroids also possess a much smaller RNA genome than the majority of plant viruses. Virus-like diseases are those possessing transmission characteristics similar to viruses, but for which no consistent association with a pathogenic agent has yet been established. Infection is usually systemic, affecting all tissues, with the occasional exception of the apical meristem, pollen, and seed.
Where apical-meristem infection is absent, viral elimination is possible via micropropagation (excision of meristematic tissue and embryogenesis in tissue culture). This is particularly important where traditional vegetative propagation techniques facilitate the perpetuation of systemic infections. Because of the systemic spread possible following grafting, the widespread adoption of grafting worldwide probably explains the global dispersion of most grapevine viruses and viroids. Although grapevine viruses and viroid infections are graft-transmissible, some are also spread by nematode, aphid, mealybug, and fungal vectors (Walter, 1991). Some can also be mechanically transmitted via pruning equipment; thus the importance of frequent surface sterilization of pruning equipment.
In addition to micropropagation, cultivars may be cured of some viral infections by heat treating dormant stem cuttings or young vines. Regrettably, the treatment appears ineffective in eliminating viroids (Duran-Vila et al., 1988).
The production and propagation of virus- and viroid-free nursery stock are ongoing projects in most wine-producing countries. This is based on the belief that cured clones grow better and generate better quality grapes than infected vines (Komar et al., 2007). Although commonly valid, clones free of all known systemic pathogens do not consistently outperform their infected counterparts (Woodham et al., 1984). Nonetheless, eliminating all systemic infections is desirable, if only because symptomless carriers can be a source of agents inducing debilitating diseases or limit grafting success in susceptible cultivars.
Detection of viral and viroid infection has historically been based on indexing. This involves inoculation of an ‘indicator’ plant that produces distinctive disease symptoms after infection. Identification with serological techniques, especially with ELISA, cDNA, or PCR probes, can confirm indexing results, and may eventually replace the long and expensive indexing process. Because disease-free vines remain pathogen-free while micropropagated in culture vessels, their use may reduce, if not eliminate, the need for quarantining and indexing imported vine cuttings.
The major viruses infecting grapevines fall into one of three main groups – nepoviruses, closteroviruses, and vitiviruses (formerly classified under trichoviruses). Nepoviruses are polyhedral (spherical), single-stranded RNA (ssRNA) viruses, with a genome divided unequally into two linear segments. They include the fanleaf, tomato ringspot, tobacco ringspot, and peach rosette mosaic viruses. All are nematode transmitted and cause several forms of grapevine decline. Closteroviruses are flexuous, filamentous, ssRNA viruses, with a single linear genome. A few are known to be occasionally transmissible by mealybugs or scale insects. They probably cause most instances of leafroll. Vitiviruses are also flexuous, filamentous, ssRNA viruses. The main examples are GVA and GVB (grapevine viruses A and B). They are most frequently associated with instances of stem pitting (Kober stem grooving) and corky bark, respectively. Both viruses can be transmitted by mealybugs. Examples of other viral groups causing or associated with grapevine diseases are trichoviruses (grapevine berry inner necrosis virus), luteoviruses (grapevine Ajinashika virus), grapevine fleck virus (possibly belonging to the tymovirus group), a foveavirus, inducing Rupestris stem pitting, and a capillovirus-like virus, occasionally associated with rugose wood. Most, if not all, grapevine viruses are graft-transmissible.
The exact causal nature of many presumably viral and viroid diseases in grapevines remains unclear. Investigation is confounded by many cultivars being symptomless carriers, the slow development of symptoms, the apparent inconsistent association with recognizable pathogen(s), and the complex etiology of symptom expression. In some instances, several viroids or viruses, or both, may be required for expression, or may modify disease expression. For example, vein-banding disease only develops when grapevines are jointly infected with both the grapevine fanleaf virus (GFLV) and a grapevine yellow speckle viroid (GYSVd-1) (Szychowski et al., 1995).
Fanleaf Degeneration
Fanleaf degeneration possesses the longest known historical record of any grapevine virus. Its symptoms are identifiable in herbarium specimens more than 200 years old. Because of its long history in Europe, and the absence of infection in free-living North American grapevines, grapevine fanleaf virus is assumed to be of European or Near Eastern origin. Its current distribution is believed to be due to grafting and the dispersion of European cultivars worldwide. Natural spread in vineyards is slow because of the limited movement in soil of its major nematode vectors, Xiphinema index and X. italiae (about 1.5 m/year). Because grapevine roots typically do not form natural grafts, vine-to-vine transfer is unlikely. Although transferable to other plants, the virus is limited to grapevines under field conditions. This may result from the limited host range of its vectors.
The impact of infection varies widely, depending on cultivar tolerance and environmental conditions. Tolerant cultivars are little affected by infection, whereas susceptible varieties show progressive decline. Nonetheless, fanleaf degeneration is generally viewed as the most damaging grapevine virus. Yield losses can reach up to 80%, with any fruit that does mature of poor quality. Infected vines have a shortened life span, increased sensitivity to environmental stress, and reduced grafting and rooting potential. Three distinctive syndromes have been recognized, based on particular strain–cultivar combinations. These are malformation, yellow mosaic, and veinbanding.
Fan-shaped leaf malformation is the most distinctive foliage expression. Chlorotic speckling and a leathery texture commonly accompany leaf distortion. Shoots may be misshapen, showing fasciation (stem flattening), a zigzag pattern at the nodes, atypically variable internode lengths, double nodes, and other aberrations. Fruit set is poor and bunches are reduced in size. The yellow mosaic (chromatic) syndrome develops as a strikingly bright-yellow mottling of the leaves, tendrils, shoots, and inflorescences in the early spring. Discoloration can vary from isolated chlorotic spots to uniform yellowing. The third expression, veinbanding, develops as a speckled yellowing on mature leaves, bordering the main veins in mid- to late-summer. In both discoloration patterns, leaf shape is normal, but fruit set is poor, with many ‘shot’ berries (‘hen and chickens’ appearance). Fanleaf degeneration also shows a characteristic intracellular development of trabeculae. These appear as strands of cell-wall material spanning the lumen of xylem vessels.
Where both vector and virus are established, planting vines grafted on rootstock resistant to the virus is usually required (vector tolerance still permits infection). Fumigation of the soil with nematicides can, to varying degrees, reduce but not eradicate nematode vector presence. Allowing the land to lie fallow can be useful in reducing nematode populations, but the strategy often requires an impractical 6–10 years. The long requisite fallow period probably results from nematode survival on undislodged roots. Roots occasionally can remain viable for up to 8 or more years following vine uprooting (McKenry and Buzo, 1996).
Leafroll
Leafroll is another debilitating, globally widespread, virus disease of grapevines. It is associated with infection by one or more, of up to 10, grapevine leafroll-associated viruses (GLRaVs) (Boscia et al., 1995). They are all members of the closterovirus group, with GLRaV-1 and GLRaV-3 being the most widespread and economically significant. Two vitiviruses (GVA and GVB) have also been associated with leafroll, although they are more commonly associated with Kober stem grooving and corky bark, respectively. Symptomatic expression varies considerably, but generally does not lead to vine degeneration. Many scion and rootstock cultivars are symptomless carriers of the infectious agent(s). As with fanleaf degeneration, the agents probably originated in Europe, or the Near East; feral North American grapevines do not show infection.
Spread of the causal agent(s) depends primarily on graft transmission. Nevertheless, insect vectors may occasionally be involved. For example, GLRaV-1, -3, -5 can be transmitted by phloem feeders, such as mealybugs or soft scale insects (Sforza et al., 2003). In the phloem, the virus induces callose accumulation. This can disrupt the translocation of organic nutrients from the leaves to other parts of the vine. Several species of mealybugs have been reported to transfer grapevine viruses A (GVA) and B (GVB). Partial vector control may be achieved with several coccinellid predators and parasitic Hymenoptera. However, their effectiveness is limited by ants feeding on mealybug honeydew. Thus, chemical stem barriers may be required to increase the efficacy of biological control (Addison, 2002).
The discovery that the mealybug Planococcus ficus can feed on grapevine roots (Walton and Pringle, 2004) is of considerable concern. Most control measures to date have been directed at aboveground feeding. This feature also means that roguing only aboveground parts of infected plants can leave viruliferous insects to survive on root remnants, infesting replacement healthy vines. This mealybug is the key pest in South Africa, the Mediterranean, Argentina, and now in some regions of California.
The disease complex derives its name from a marked, basal leaf down-rolling. It most frequently occurs late in the season. The interveinal areas of leaves may also turn pale yellow or deep red, depending on the cultivar. In contrast, the main veins remain distinctly green. Infected vines can occasionally be detected by their retention of leaves much longer than adjacent healthy vines. In addition, leaf blades may fall, leaving petioles still attached to the cane. Whole vines, as well as individual shoots and leaves, are dwarfed in comparison to healthy plants. Fruit production may be decreased by up to 40%, and the berries may show delayed ripening, reduced sugar contents, and altered pigmentation.
Control is based primarily on destruction of infected vines and replacement with disease-free stock. Disease-free nursery stock may be generated by thermotherapy or by micropropagation. Because the identity of all causal agents still remains unestablished, confirmation of elimination of the infectious agent(s) is performed by grafting to sensitive cultivars (indexing). The economic implications of the various control options are discussed by Atallah et al. (2012).
Yellow Speckle
Yellow speckle is a widespread, but relatively minor viroid disease of grapevines. Other viroids occur in grapevines, but their economic significance and relationship to recognized grapevine diseases remain unclear. Symptoms of infection by grapevine yellow speckle viroids (GYSVd1 and GYSVd2) are often temporary, and develop only under special climatic conditions. Foliar symptoms generally develop at the end of the summer, and consist of leaf spotting. When sufficiently marked, the scattered chlorotic spots may resemble the veinbanding symptom of fanleaf degeneration. Studies suggest that shoot growth is slightly curtailed and grape acidity is reduced (Wolpert et al., 1996).
Control is dependent on planting viroid-free vines. Elimination of the causal agent can be achieved by micropropagation. Thermotherapy is ineffective (Barlass and Skene, 1987).
Nematode Pathogens
Nematodes are a large group of microscopic, unsegmented roundworms that live predominantly as saprobes in soil. However, some are parasitic on plants, fungi, and animals. Those attacking grapevines are restricted to feeding on roots. They derive their nutrition from extracting the cytoplasmic fluids from root cells. This is accomplished with a spear-like stylet that punctures host cells.
Feeding may be restricted to the surface of roots or, following burrowing, may occur in the root cortex. In addition to the direct damage caused by feeding, and the resultant root disruption, nematodes may transmit viruses and facilitate infection by other pathogens. Active dispersal by nematodes in soil is both slow and limited, with most long-distance movement being through the action of wind and water, on vineyard machinery, or by the translocation of infested plants.
Reproduction occurs via egg production, with or without the interaction of males. The multi-year survival of eggs in a dormant state often markedly reduces the effectiveness of fallowing in nematode control. The degree of damage caused by nematodes (including that of the viruses they transmit) was poorly understood until the effects of applying pre-plant soil fumigants became common. Combined with fallow, fumigants can dramatically reduce nematode populations in shallow, sandy soils. They have more limited efficacy in deep or clayey soils, where fumigant penetration is restricted. Effective sterilant action requires penetration to a depth of 40–120 cm (zone of highest root density – depending on soil structure). The most effective and widely used nematicide, methyl bromide, is scheduled for deregulation. Methyl iodide appears to be an effective alternative (Ohr et al., 1996), and is not a stratosphere ozone depleter. Other potential options include sodium methyldithiocarb (Vapam®), chloropicrin+iodomethane, propargyl bromide, dimethyl disulfide, sulfuryl fluoride, and possibly sodium and potassium azide (Schneider et al., 2006; Cabrera et al., 2011). Another potential substitute is DiTera®, a selective nematicide that reportedly does not kill beneficial mycorrhizal fungi or saprophytic nematodes. It contains a formulation of the hyphomycete Myrothecium.
Application of specific yeasts (Hashem et al., 2008) and/or compost (Weckert et al., 2009) has also shown promise, and can be used in functioning vineyards. Nonetheless, effective nematode control in the future may require integrated nematicide applications, increased use of resistant/tolerant rootstock, and modified cultural practices (Zasada et al., 2010).
Generally, the most effective, long-term means of limiting nematode damage entails the use of nematode-resistant or -tolerant rootstocks. Although no current rootstock cultivar is resistant to all grapevine-attacking nematodes, some are resistant or tolerant to one or more of the important pathogenic genera. Therefore, determination of both the actual and potential nematode pests (and viruses they might transmit) can be a critical component of rootstock selection (Walker, 2009). For example, 10-17A provides broad nematode resistance, but not for fanleaf degeneration (GFLV), whereas O39-16 supplies better protection in situations where both X. index and GFLV are present. Problems in selecting the ‘best’ rootstock are illustrated in McKenry et al. (2004). Before replanting an infested vineyard, the old vines are usually cut off at just above the soil, the trunks painted with a systemic herbicide to kill the roots prior to removal, and the field left fallow for 1 year. Without resistant rootstock use, fallow periods of at least 7–10 years are required without fumigation.
Where groundcovers are used in vineyards, planting nematode-resistant crops may be an important factor. For example, Cahaba white vetch (Vicia sativa), barley, and Blando bromegrass have an advantage in sites known to harbor parasitic nematodes. Conversely, nematode-favorable hosts, such as peas, lupins, clovers, and most vetch crops, should be avoided. Mulching of underrow cover crops, such as biofumigant Brassica spp., can be of value. They can reduce the nematode population in infested vineyards (McLeod and Steel, 1999; Rahman and Somers, 2005). Brassicas produce glucosinolates that remain inactive while in the living plant. However, upon mulching, these compounds are hydrolyzed by the enzyme myrosinase, releasing volatile isothiocyanates. Immediate incorporation into the soil liberates sufficient isothiocyanates to kill nematodes. For a review on botanical nematicides see Ntalli and Caboni (2012).
The detection and identification of nematode problems usually require microscopic examination of the root system. Aboveground symptoms are insufficient diagnostic. Many nematode species can attack grapevine roots, but few induce significant damage. The most serious are the root-knot (Meloidogyne) and dagger (Xiphinema) nematodes. Other nematodes occasionally found feeding on grapevine roots include species of Pratylenchus (lesion nematodes), Tylenchulus (citrus nematodes), and Criconemella (ring nematodes).
Root-Knot Nematodes
Root-knot nematodes (Meloidogyne spp.) are sedentary endoparasites that penetrate young feeder roots. After penetration, adjacent cells are stimulated to divide and increase in size, producing gall-like swellings (Fig. 4.63). Where multiple infections occur, knot formation may give the root a chain-of-beads appearance. Reproduction commonly occurs during the spring and fall root-growth periods (de Klerk and Loubser, 1988). Each female may produce up to 1500 eggs. Second-stage juveniles have been shown to travel up to 30 cm in 3 days through sandy loam (Flaherty et al., 1992). The most important species are M. incognita, M. javanica, M. arenaria, and M. hapla.
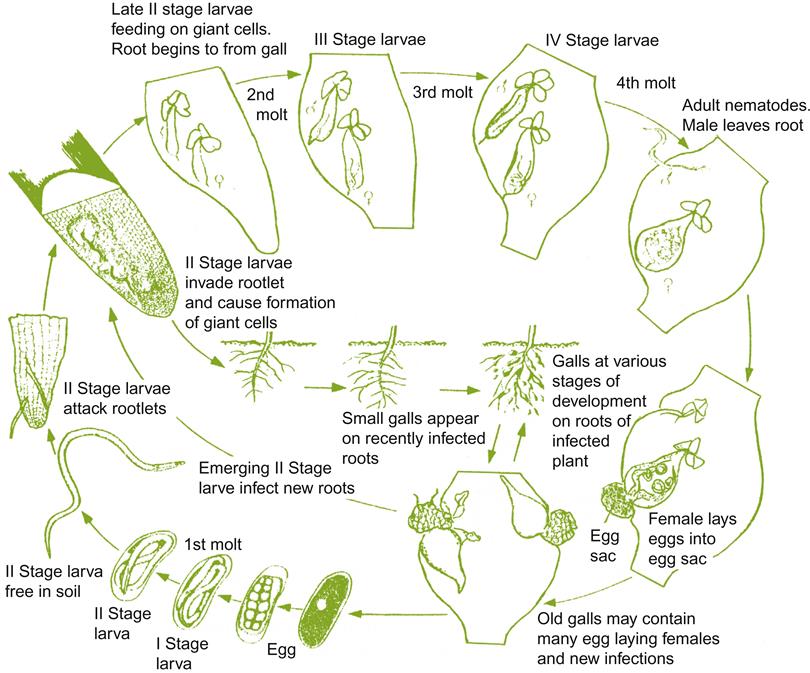
Infection results in a decline in vigor and yield, as well as increased susceptibility to water and nutrient stress. Symptomatic severity can be limited by increasing the water supply and pruning to prevent overcropping. Damage is typically most marked in young developing vines planted in highly infested, light-textured soils. Older vines with deep root systems seem to be less affected by root-knot nematodes.
Dagger Nematodes
In contrast to root-knot nematodes, dagger nematodes (Xiphinema spp.) are migratory and feed on epidermal cells near the root tip. Concentrated feeding may initiate root bending, followed by lesion darkening. Extensive attack causes root death and induces the production of tufts of lateral roots. In addition to destroying roots, X. index is a principal vector of the grapevine fanleaf virus. The combined action of both nematode and viral infections can quickly make a vineyard commercially unproductive. Other species of Xiphinema may transmit a range of other, but less serious, viruses to grapevines (see Walter, 1991).
Insect and Mite Pests
Insects and mites can cause extensive grapevine damage. Although most of these pests infest only a single part of the vine, all components are attacked by one or more species. Most control measures have been based on synthetic pesticides. However, because of problems associated with their use, greater emphasis is being placed on cultural and biological controls.
Insects and mites are both distinguished by a hard exoskeleton. This is shed several times during growth. Mites and some insects pass through several immature, adult-like stages before becoming reproductive, whereas most insects pass through several worm-like (larval) and a pupal stage before becoming a short-lived adult.
Insects are distinguished by possessing three main body parts (head, thorax, and abdomen), sensory antennae, three pairs of legs attached to the central thorax, and a segmented abdomen. In contrast, mites possess a fused cephalothorax, broadly attached to an unsegmented abdomen, lack antennae, and four pairs of legs, except in the initial, immature stage. Two pairs of legs are attached to the cephalothorax and the other pairs are located on the abdomen. Both groups reproduce primarily by egg production.
Although some insects have evolved intimate relationships with grapevines, notably phylloxera, others infest a wide range of hosts. As with fungal pathogens, infestation does not necessarily correlate with severity. Severity depends more on the specific genetic properties of the cultivar–pest interaction, macro- and microclimatic conditions, soil properties, and resistance to control agents.
Phylloxera
Of all grapevine pests, the obligate parasite phylloxera (Daktulosphaira vitifoliae) has had the greatest impact on viticulture and wine production. A relatively insignificant, endemic pest of North American Vitis species (east of the Rocky Mountains), phylloxera devastated European vineyards when it was inadvertently introduced about 1860. It was often considered to have been equally decimating on the feral population of V. vinifera. However, human destruction of its natural habitats along flood plains and riverbanks, and the introduction of powdery and downy mildews, may have been more important in the extensive demise of wild (sylvestris) populations. Investigation of the incidence of phylloxera in indigenous populations in central and southern Europe has found few signs of phylloxera infestation (Ocete et al., 2011). This is hypothesized to be due to the anoxic conditions generated by the annual flooding and addition of sediment, as well as the gravelly and/or sandy nature of its riverine habitats. There is current concern that indigenous Chinese Vitis spp., which are susceptible to phylloxera, may be endangered by the recent accidental introduction of the pest into China (Du et al., 2009).
At the height of its spread in Europe, phylloxera destroyed more than 2 million vineyard hectares and almost brought European wine production to a halt in severely affected areas. It also demonstrated the urgency for laws regulating the importation of vine material (still inadequately appreciated, or at least applied, in some countries), and provided the first clear example of the effectiveness of biological control.
Grafting sensitive V. vinifera cultivars to resistant rootstocks was so successful that the danger posed by phylloxera was partially forgotten in some parts of the world. For example, the predominant use of a single resistant rootstock cultivar (A×R#1) in California resulted in considerable hardship when a new biotype of phylloxera arose that could infest A×R#1. It necessitated replanting of some 5000 ha in Napa and Sonoma between 1988 and 1995. Biotypes, other than the two main current variants, have also been identified in California (De Benedictis et al., 1996).
The need to replant vineyards on a massive scale has apparently permitted several relatively minor vascular pathogens of older grapevines to make their presence known. Both Phaeomoniella chlamydospora (Petri disease) and Cylindrocarpon (black-foot disease) have caused significant young vine decline in replanted vineyards (Scheck et al., 1998).
The complexities of the aphid-like phylloxera life cycle are detailed in Forneck and Huber (2009), and in a simplified version in Fig. 4.64. It illustrates that several asexually reproduced generations may occur per year. Despite a winged stage that can arise from root galls long-distance dispersal without human involvement is slow. For the sexual stage to typically occur, the insect must pass sequentially through both leaf- and root-galling phases. However, many Vitis spp. are comparatively resistant to one of these galling phases (Table 4.14). Thus, absence of the leaf-galling phase (Fig. 4.65; Plate 4.14) probably explains the absence of the sexual cycle in California. This also applies to most other viticultural areas where phylloxera is not indigenous. Occasionally, grafted cultivars may develop leaf galls. These are thought to develop from nymphs that migrate from leaf galls on shoots that sporadically arise from American rootstocks (Remund and Boller, 1994).
Table 4.14
Susceptibility of Vitis Species to Root and Leaf Expressions of Phylloxera
| Host reaction | Resistant (bearing none to few galls on roots) | Tolerant (bearing many galls on roots) |
| Vitisspecies already exposed to phylloxera (eastern North America) | ||
| Resistant (bearing none to few galls on leaves) | V. rotundifolia | V. aestivalis |
| V. berlandieri | V. girdiana | |
| V. candicans | V. labrusca | |
| V. cinerea | V. lincecumii | |
| V. cordifolia | V. monticola | |
| V. rubra | ||
| Tolerant (bearing many galls on leaves) | V. riparia (V. vulpina) | None |
| V. rupestris | ||
| Vitisspecies not previously exposed to phylloxera (western North America, Asia, Europe, and Middle East) | ||
| Resistant (bearing none to few galls on leaves) | V. coignetiae | V. arizonica |
| V. californica | ||
| V. davidii | ||
| V. romanetti | ||
| V. ficifolia (=V. flexuosa) | ||
| V. vinifera (incl. V. sylvestris) | ||
| Susceptible (bearing many galls on leaves) | V. betulifolia | V. amurensis |
| V. reticulate | V. piazeskii | |
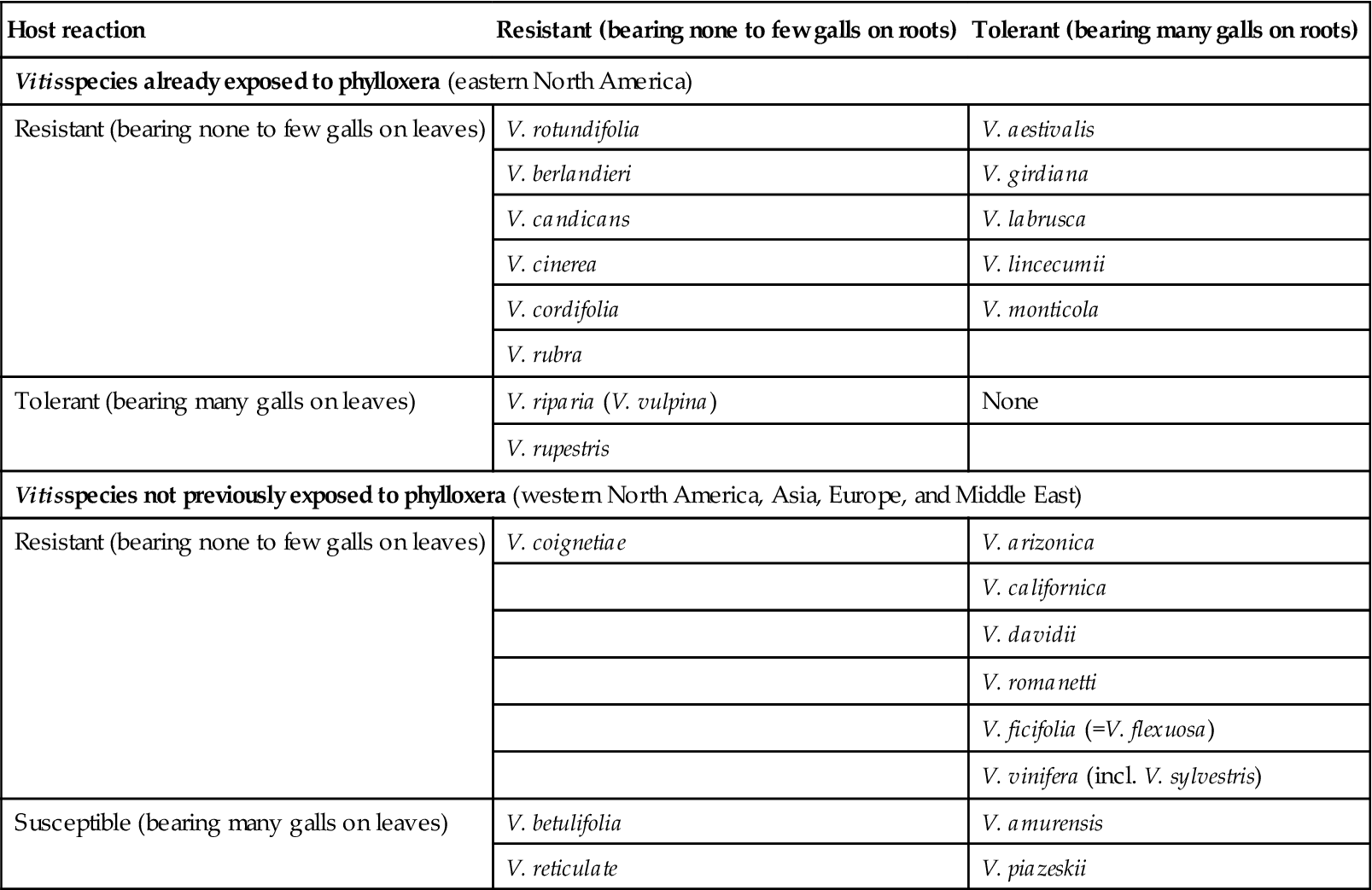
Source: From Wapshere and Helm, 1987, reproduced by permission.

Phylloxera biotypes, differentially pathogenic on grapevine cultivars, appear to be common (King and Rilling, 1991; Forneck et al., 2000). This occurs in spite of the rarity of the sexual stage in most infested areas. Differences in pathogenicity may be expressed in the ability of phylloxera biotypes to feed, stimulate gall formation, or reproduce rapidly.
Susceptible hosts respond to root feeding by increasing the nutrient supply to the damaged region, and gall formation. The latter is probably induced by the auxin IAA, one of the main constituents in phylloxera saliva (Schäller, 1968). Feeding also induces the formation of distinctive hook-shaped bends and swellings on young roots called nodosities (Plate 4.15). These soon succumb to secondary infection by fungi, such as Fusarium, Pythium, and Cephalosporium spp. (Granett et al., 1998). In contrast, more mature roots produce semispherical swellings termed tuberosities. These give the root a roughened, warty appearance. Their development is the more serious manifestation of infestation. They markedly disrupt water and nutrient flow in the root’s vascular tissues. The insect population is maintained primarily on tuberosities. Rapid nodosity decay, and the seasonal production of new feeder roots, can limit the development of dense populations of feeding insects (Williams and Granett, 1988).
Resistant rootstock cultivars show limited, if any, galling, followed by rapid healing. In Borner, for example, the roots react to feeding by localized hypersensitivity and necrosis (Dietrich et al., 2010). In contrast, tolerant rootstocks show galling. Nonetheless, vigorous root growth normally compensates for any damage, and nodosities support only limited insect populations (Boubals 1966). Adverse cultural conditions may, however, lead to progressive damage resulting in vine decline, termed phylloxera ‘comeback.’
Aboveground symptoms of root infestation are relatively indistinct, and are primarily expressed as a progressive vine decline. The first clear indications of infection usually appear as stunted growth and premature leaf yellowing. With sensitive cultivars, these effects annually become more marked, finally culminating in vine death.
Phylloxera root infestation can be confirmed only by root observation. The distinctive lemon-yellow eggs (Plate 4.16) and clusters of yellowish-green nymphs and adults on young roots are diagnostic. Tuberosities isolated from dying vines usually possess few phylloxera. Nevertheless, the presence of the tyroglyphid mite (Rhizoglyphus elongatus) appears to be an indicator of past phylloxera presence. The mite lives on the decaying cortical tissues of tuberosities.
Many environmental conditions can affect the severity of vine attack. It is well known that phylloxera infestation is much less significant in sandy soils. It has been suggested that this results from the higher silicon content, either in the soil solution or in vine roots (Ermolaev, 1990). The higher temperatures (>32°C) that may occur in sandy soils are also unfavorable to phylloxera, and limit its damage (Foott, 1987). Irrigation and fertilization can occasionally diminish the damage, whereas drought increases severity (Flaherty et al., 1982).
Quarantine is a vital component in limiting phylloxera spread to non-infested areas. Even where the pest is present, quarantine may prevent the importation and distribution of new biotypes.
In nurseries, vines can be disinfected by placing the washed root system in hot water (52–54°C) for about 5 min. Nursery soils can be disinfected by pasteurization or fumigation. If the vineyard soil is already infested, the major control measure remains grafting to resistant rootstocks. Typically, rootstocks with some V. vinifera parentage should be avoided (Granett et al., 1996). Nevertheless, 1202C and O39-16 seem exceptions to this rule, at least in California. Whether rootstocks of pure North American Vitis parentage will continue to remain resistant to phylloxera under vineyard monoculture is unknown. Reports of decline have been noted in Germany. Hopefully advances in categorizing phylloxera strains, via DNA fingerprinting, will assist in understanding the occasionally conflicting worldwide data on rootstock resistance or tolerance to phylloxera.
Although chemical agents such as aldicarb (Temik®) (Loubser et al., 1992) have shown some promise against phylloxera, control will remain dependent on quarantine and grafting for the foreseeable future.
Leafhoppers (Sharpshooters)
Specialized xylem-feeding insects, notably leafhoppers and related forms, are not only harmful pests in their own right, but also critical vectors in the transmission of several destructive pathogens. In many grape-growing regions of North America, the western and eastern grape leafhoppers (Erythroneura elegantula and E. comes, respectively) are the most important species; in southern California, the variegated leafhopper (E. variabilis) and the nonindigenous glassy-winged sharpshooter (Homalodisca coagulata) are the major species whereas the potato leafhopper (Empoasca fabae) is particularly significant in the southeastern United States. About 20 species of sharpshooters and leafhoppers are vectors of Xylella fastidiosa, the causal agent of Pierce’s disease. In Europe, Empoasca leafhoppers tend to be particularly damaging, especially E. vitis. Scaphoideus littoralis (synonym S. titanus) is the primary vector of flavescence dorée. In South Africa, Acia lineatifrons is the prominent species. Most of these insects have fairly similar life cycles and are controlled by similar techniques.
Depending on the species and prevailing conditions, the insects may go through one to three generations per year. Those species passing through several generations per year are generally the most serious, as their numbers can increase dramatically throughout the season.
Leafhoppers typically lay their eggs on the underside of leaves. After hatching, the nymphs usually pass through five molts before reaching the adult stage (Fig. 4.66). All stages feed on the cytoplasmic fluid of leaf and fruit tissue. Feeding results in the formation of white spots, which on heavily infested leaves leads to a marked loss in color. Growth of one or more hyphomycetes on escaped sap and insect honeydew can produce a sooty appearance on plant surfaces. Pronounced damage is usually caused only when infestations reach>10–15 leafhoppers/leaf. Severe infestation can lead to leaf necrosis, premature defoliation, delayed berry ripening, and reduced fruit quality. Some varieties, notably late-maturing cultivars, tend to sustain greater damage than early-maturing varieties. Such cultivars not only endure leafhopper infestation for a longer period, but may also suffer from the migration of leafhoppers from early-maturing varieties. Most leafhopper species affecting grapevines do not infest grapes exclusively. Thus, in the fall and early spring they often survive and multiply on other plants. Adult overwintering can occur under leaves, weeds, or debris, in and around vineyards.
Control measures have been increasingly based on enhancing the population of indigenous parasites and predators. In California, the wasp Anagrus epos is an effective parasite on the eggs of the western grape leafhopper (less so on the variegated leafhopper). The short life cycle permits up to 10 wasp generations per year. By July, the parasite population may reach levels sufficient to destroy 90–95% of leafhopper eggs. The numbers of A. epos can be augmented by selective habitat diversification, such as planting prune trees upwind from vineyards (Murphy et al., 1996). The windbreak produced by the trees further enhances parasite concentration. Another parasitic wasp, Aphelopsis cosemi, attacks nymphs, resulting in their sterilization. Several predatory insects, such as lacewings and ladybugs, as well as the general predatory mite Anystis agilis, attack leafhoppers. The release of commercially reared green lacewings, Chryosoperla spp., can be both effective and economically feasible if the timing is correct. Release should coincide with egg hatching, and at a population of about 15–25 leafhoppers per leaf (Daane et al., 1993). Of cultural practices, basal leaf removal is particularly useful in removing most first-generation leafhoppers. They occur most frequently on basal leaves.
Members of the genus Gonatocerus (Pilkington et al., 2005) are the principal parasitoid wasps feeding on eggs of the glassy-winged sharpshooter (Homalodisca coagulata). They can infest 10–50% of the eggs during the first generation, and up to 90–100% on the second late-summer population. Anagrus epos may also prove effective. One of the problems associated with this, or any other control measure against the glassy-winded sharpshooter, is that its major significance derives from its being a vector for Xylella fastidiosa, the agent of Pierce’s disease. As such, just a single feeding by a carrier can result in effective X. fastidiosa transmission. The feeding damage caused by the sharpshooter is itself relatively insignificant.
An additional biocontrol mechanism of potential value is the use of Alcaligenes. It is a symbiotic bacterium that limits the multiplication of X. fastidiosa in the insect vector (Bextine et al., 2004). Symbiotic control is also under investigation to control Scaphoideus titanus, the major carrier of the phytoplasma causing flavescence dorée (Marzorati et al., 2006).
Chemical control of leafhoppers has shifted from synthetic pesticides, such as organophosphates, to ‘softer,’ nicotine-based compounds (e.g., imidacloprid). When required, it has the advantage of causing minimal disruption to beneficial biocontrol agents.
Tortricid Moths
Grapevines are attacked by a wide diversity of tortricid moths. In southern regions of Europe Lobesia botrana is the major pest species, whereas in more northern regions Eupoecilia ambiguella is the most significant species. In much of eastern and central North America the important tortricid is the grape berry moth Paralobesia (Endopiza) viteana (Fig. 4.67A). In California the omnivorous leafroller Platynota stultana (Fig. 4.67B) and the orange tortrix Argyrotaenia citrana are the notable forms in warmer and cooler regions, respectively. In Australia the light-brown apple moth Epiphyas postvittana is the major tortricid of significance. It has recently been found in California, and the species is now naturalized throughout much of New Zealand.
Because of their taxonomic affinity, tortricid moths possess relatively similar life cycles. Adult females lay egg clusters on or close to flowers and grape bunches. Those less specialized to grapevines lay eggs on leaves. The eggs hatch into pale-colored larvae that feed predominantly on flowers and developing fruit or on leaves, depending on the species. Following several molts, the larvae form a web-like cocoon in which they metamorphose into pupae. After a variable period, adult moths emerge and mate, initiating the next generation. Adults are small, relatively inconspicuous brown moths. They generally possess a bell-shaped wing profile, and develop projecting, snout-like mouth parts. Depending on the species, and prevailing climatic conditions, two to four generations develop per year.
Although tortricid moths possess many properties in common, significant differences occur. For example, European berry moths form cocoons under rough bark, and in the crevices and cracks on trellis posts. American berry moths form folds in leaves in which they spin their cocoons. These eventually fall to the ground. Although tortricid moths affecting grapes in California and Australia form cocoons, the insects do not hibernate as pupae, as do other American and European berry moths. The Californian tortricid moths survive as larvae in web nests formed in mummified grape clusters, or on other vine and vineyard debris. These hibernating characteristics influence the type and success of sanitation used in reducing their overwintering success.
Second- and third-generation larvae are the most damaging, due to their increase in numbers throughout the season. In addition to direct-feeding damage, larvae produce wounds subsequently infected by bunch rot fungi, as well as spoilage yeasts and bacteria. Their action can further attract infestations by fruit flies (Drosophila spp.). Not only can tortricid larvae produce wounds, facilitating bunch rot, but several species are carriers of Botrytis. The details of transmission are most well established for the European berry moth (Lobesia botrana), but the light-brown apple moth has the same potential in Australia (Bailey et al., 1997). Adult females of L. botrana preferentially select Botrytis-infected berries on which to lay their eggs, while the larvae selectively feed on infected berries (Mondy et al., 1998b). In addition, females raised on Botrytis-infected grapes produce more eggs (Mondy et al., 1998a).
As with other insect pests, increased emphasis is being placed on control by endemic pests and parasites. The diminished use of pesticides and the establishment of habitats for sustaining populations of indigenous parasites and predators are essential to sustained biological control (Sengonca and Leisse, 1989). In addition, synthetic pesticides are being replaced by commercial preparations of Bacillus thuringiensis, or by pheromone applications to disrupt mating success. The effectiveness of pheromones in disorientating male Lobesia botrana is affected by the height of the applicators and development of the leaf canopy (Sauer and Karg, 1998). Grape leaves partially absorb pheromones, slowly releasing them into the atmosphere (Schmitz et al., 1997).
In many instances, the release of artificially reared egg parasites, such as Trichogramma minutum and T. embryophagum (Plate 4.17), has been successful in controlling several tortricid pests. Another species, Trichogramma carverae, appears to provide effective control of the light-brown apple moth (Epiphyas postvittana). Green lacewings (Crysoperla spp.) and many spiders are also active predators. Egg and pupal parasites kill the insect before damage can be done, whereas larval predators and parasitoids limit population buildup by preventing reproduction. Frequently, biological control provides adequate control of these pests. However, where necessary, for example when biological controls prove inadequate (Varela et al., 2010), the availability of several selective pesticides provides a safety net.
Where insects overwinter on the ground, row cultivation to bury hibernating pupae or larvae can be valuable. This is of particular use as it often takes several years for tortricid populations to build up to critical levels (Flaherty et al., 1992). For species not specialized to grapevines, such as Lobesia botrana, the use of grass vs. broadleaf cover crops (which the pest prefers) can diminish their incidence on vines.
Mealybugs
Mealybugs are a group of small (<5 mm) gregarious insects that are readily distinguishable due to the presence of a fringe of white filaments around their waxy covered bodies. Only at certain stages in their life cycle are wings produced. They pass through several nymph stages before reaching maturity, and frequently show several to multiple generations per year. They overwinter as eggs or small nymphs, usually under the bark. As a group, they obtain nourishment by plunging their mouth parts into the phloem and extracting sap. As a consequence they are potential virus vectors. Currently, they are known to occasionally transmit agents of grapevine leafroll virus, notably GLRaV-3. Thus, like many grapevine pests, they not only cause direct damage, by debilitating the vine when infestation is heavy, but can still be serious vectors of grapevine pathogens at low population numbers. In addition, their release of honeydew on grapes favors the growth of sooty molds. The latter can both interfere with fermentation and generate off-flavors.
Depending on the region, the most significant genera and species vary. These often include members of the genera Pseudococcus, Planococcus, Scaphoideus, Ferresia, and Maconellicoccus.
Control is hindered by the ability of many species to feed on all aerial plant parts, and occasionally the roots; their preference for tissues buried under a thick canopy; and their capacity to survive and multiply on a wide range of native plants and weeds. Nonetheless, they are susceptible to an equally wide range of predators and parasites. These include ladybugs, lacewings, midges, mites, pirate bugs, and parasitoid wasps (Daane et al., 2008). The implantation of some of these natural control agents from indigenous mealybug habitats often provides adequate control. Covering piles of stemmer/cursher detritus or whole grape press remains with plastic for at least a week has been shown to drastically reduce mealybug survival (Smith and Varela, 2008). This can limit mealybug dispersal if the remains are subsequently spread on vineyards.
Mites
Several types of mites inflict damage on grapevines. Of these, spider mites tend to be the most significant. Spider mites are most serious under dusty conditions, especially when vines are water stressed. The most significant species differ from region to region. The European red spider mite (Panonychus ulmi) and the two-spotted spider mite (Tetranychus urticae) tend to be the most important species in much of Europe, whereas the yellow vine spider mite (Eotetranychus carpini) is the primary species in Mediterranean France and Italy. In eastern North America the European red spider mite is the principal damaging species, whereas in California the Pacific spider mite (Tetranychus pacificus) is the most destructive form (Fig. 4.68). The Willamette spider mite (Eotetranychus willamettei) is found in California, but is less damaging than the Pacific spider mite. This property has been used in biological control (habitat exclusion), through the release of artificially reared Willamette spider mites in infested vineyards (Karban et al., 1997). The Willamette spider mite can also serve as a host for enhancing the predator population against the Pacific spider mite. In Chili, Oligonychus vitis is the most injurious species.