 , benzaldehyde;
, benzaldehyde;  , unknown;
, unknown;  , vitispirane;
, vitispirane;  , nerolidol;
, nerolidol; 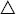 , hexyl acetate and isoamyl butyrate;
, hexyl acetate and isoamyl butyrate;  , total volatile acidity. (From Loyaux et al., 1981, reproduced by permission.)
, total volatile acidity. (From Loyaux et al., 1981, reproduced by permission.)Although the changes noted above are correlated to the second in-bottle fermentation and extended lees contact, how they arise remains unclear. Some, such as the release of glucans, amino acids, peptides, and mannoproteins are clearly associated with yeast autolysis. In contrast, the involvement of autolysis in the development of the distinctive aromatic characteristics of sparkling wines is uncertain. In addition, the potential significance of aromatic absorption by lees should not be overlooked (Gallardo-Chacón et al., 2010).
Correlations between bubble size and foam stability, and the presence of certain polysaccharides and hydrophobic proteins, have been demonstrated (Maujean et al., 1990). Colloidal protein content can double or triple within the first year.
Riddling
One of the more intricate procedures in sparkling-wine production involves removal of the yeast sediment (lees). The first step entails repeated loosening and suspending the cells in the wine. Progressing positioning of the bottle upside down moves the lees to the neck. The associated agitation also aids optimal flocculation (Stratford, 1989).
Historically, riddling (remuage) was done by hand. It took about 3 to 8 weeks, with the bottles finally being positioned neck downward in A-shaped racks (pupitres). Initially, they were arrayed to position the bottles 30° from vertical. Periodically, the sides of the pupitres were moved further apart, so that the bottles eventually came to be positioned about 10–15° from vertical. Rapid, short, vigorous bottle twisting (about one-eighth of a turn) dislodged the sediment. The bottle was then dropped back into the rack, a quarter turn (alternately to the right or left) of its original position. This action was repeated at roughly 2-day intervals.
Manual riddling has largely been replaced by automated mechanical riddling. It is less expensive, takes only about 7 to 10 days, and requires much less space (Plate 9.10). When fermentation and storage occur in the same container as riddling, bottle handling is greatly reduced. Various automated riddling systems are available. However, over zealous attempts to reduce the duration of riddling can leave the wine with a bentonite haze (Jeandet et al., 2000). The particles may also interfere with foam stability (Senée et al., 1998).
Disgorging, Dosage, and Corking
At the completion of riddling, the bottles are often left neck down for several weeks, in preparation for sediment removal (disgorging). For disgorging, the bottles are cooled to approximately 7 °C. Cooling increases the solubility of carbon dioxide, reducing the likelihood of gushing upon opening. Subsequently, the necks are immersed in an ice bath (a glycol- or CaCl2-ice solution, approximately−20 °C). This quickly freezes the sediment in the neck. Freezing commonly occurs in a trough, while the bottles are being transported through the freezing solution on way to the disgorging machine. Congealing the lees in ice, located within the bidule, facilitates ejection.
In rapid succession, the disgorging machine inverts the bottle, removes the cap, allows ejection of the frozen yeast plug, and then covers the mouth of the bottle with a sequence of devices. To minimize oxygen exposure, the headspace volume is usually flushed with carbon dioxide or an inert gas prior to cork insertion. These prevent further wine escape and adjust the wine to the desired volume (by either wine addition or removal). Adjustment is necessary because the amount of wine lost during disgorging can vary considerably. It is during this volume adjustment phase that any dosage liqueur is added.
The dosage typically consists of a concentrated sucrose solution (60–70%), dissolved in high-quality aged white wine. Preferably, the dosage wine is the same as the cuvée. Occasionally, brandy may be added to the dosage, as well as a small quantity of sulfur dioxide (15 to 20 mg/L). At this concentration, it helps restrict microbial spoilage and limit oxidation, without favoring the development of a reduced odor (Valade et al., 2006). The latter could arise from hydrogen sulfide and mercaptans, derived from cysteine residues in the wine (Tirelli et al., 2010). Without flushing the neck, disgorging may permit the uptake of about 1 mg O2 /L. Amounts, in about the same range, can be derived from oxygen dissolved in the dosage liqueur, or wine added to achieve the desired volume. An additional 3 mg O2 may slowly dissolve out of the lumen of cork cells. Typically, the oxygen is completely consumed within 6 months. Subsequently, oxygen uptake appears negligible, for at least several months (Bunner et al., 2010). A small quantity of malic acid may also be added to the dosage. This retards calcium tartrate crystallization and, thereby, instability problems. The dosage is prepared several weeks in advance, to ascertain that turbidity does not develop. The dosage volume depends on the sweetness desired, and, therefore, its sugar content.
A few sparkling wines receive no or minimal sweetening. Nature (<0.3% sugar) and Extra Brut (<0.6% sugar) wines are rare because the cuvée is seldom considered to have sufficient balance to be harmonious when bone dry. Brut wines tend to be adjusted with dosage up to 1.5% sugar. Extra-sec wines generally contain between 1.2 and 2% sugar; sec wines commonly possess between 2 and 4% sugar; demi-sec wines have between 3 and 5% sugar. Doux styles (now rare) contain more than 5% sugar. The range of sugar found in each category may vary beyond that indicated, depending on whether sugar remains following the second, in-bottle fermentation.
After volume adjustment and dosage are complete, the bottles are sealed with special corks (31 mm in diameter and 48 mm long). They are commonly composed of agglomerate cork, to which two disks of natural cork have been attached. They also possess a silicone coating on the surface in contact with the wine. Both the agglomerate portion and the silicone liner appear to limit contamination of the wine with TCA (Vasserot et al., 2001b). Although laminated corks were developed around the end of the 1800s (Sharf and Lyon, 1958), their use did not become standard until much later.
Once the cork is inserted, and just before addition of the wire hood, the upper 10 mm of the cork is compressed into its familiar, rounded shape. After the wire hood has been fastened, the bottle is agitated to disperse the dosage. The bottles are stored for 1–3 months, during which time the dosage marriages with the wine, and the cork sets in the neck. Before setting, cork extraction is particularly difficult. The rest period may occur either before or after the bottles are cleaned, in preparation for adding the capsule and label. Special glues are commonly used to retard label separation in water.
In a creative break with custom, a new closure for sparkling wine has been developed (Plate 9.11). It is in essence a resealable screw cap, but adheres to standard sparkling wine bottles, and retains most of the appearance of traditionally closed sparkling wines. Another new alternative involves a modified standard RO (Roll-on) closure. Regrettably, its use requires the adoption of bottles designed specifically for their application.
Yeast Enclosure
Incorporation of yeasts and other microbes into a stable gel matrix is increasingly being used in industrial fermentations. Investigation of its potential applicability to winemaking is comparatively recent and still tentative (Fumi et al., 1988; Martynenko and Gracheva, 2003). By injecting a yeast–gel mixture through fine needles into a fixing agent, small beads of encapsulated yeasts are generated (Fig. 9.28). Each bead contains several hundred cells. Because of the bead’s mass, simple inversion of the bottle results in rapid settling to the neck, thus eliminating the need for riddling.
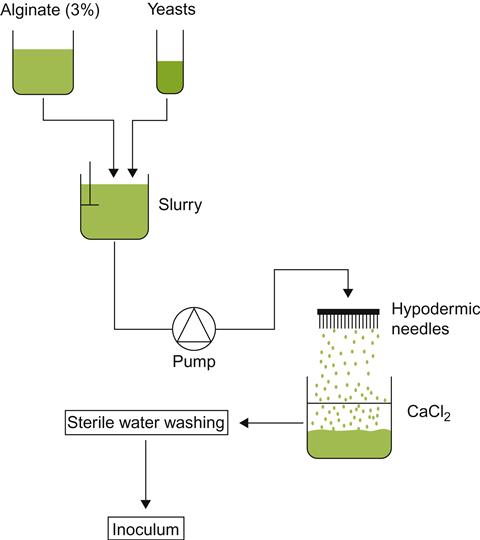
Wines produced and aged with encapsulated yeasts show only subtle chemical differences from their traditionally produced counterparts (Hilge-Rotmann and Rehm, 1990). These differences appear not to influence the sensory properties of the wine. Nonetheless, these procedures have as yet to enter mainstream sparkling wine production.
Another innovative concept involved retaining yeasts, free, within a porous cartouche inserted into the bottle – the Millispark (Jallerat, 1990). Apparently, it was abandoned due to problems with diffusion of nutrients and aromatics into and out of the enclosure.
Transfer Method
The transfer method (Fig. 9.19) was developed in the 1940s as a means of avoiding both the expense of manual riddling and the low quality of the wines then being produced by the bulk method (see below). With advancements in automated riddling, most of the advantages of the transfer method disappeared. Furthermore, advances in the bulk method have eliminated the sources of poor quality that initially plagued the process. Because the transfer system is capital-intensive, but does not have the prestige and pricing advantage of the standard method, its continued existence is in doubt. The one remaining advantage of the transfer technique consists of its avoiding bottle-to-bottle variation that can occur with the traditional method.
Preparation of the wine up to riddling is identical to that described for the standard method. Because the wines are not riddled, fining agents need not be added to aid yeast sedimentation. Typically, the bottles are stored neck down in cartons for aging. After maturation, the wine is chilled to below 0 °C before discharge. The bottles are opened by a transfer machine and the wine poured into special, pressurized receiving tanks. The wine is usually sweetened (dosage) and sulfited at this stage. Subsequently, the wine is clarified by filtration and decolored if necessary. The wine is typically sterile-filtered, just prior to bottling.
Bulk Method
Current versions are modifications of the technique initially developed by Charmat about 1907 (Charmat, 1925). The procedure (Fig. 9.19) works well with sweet sparkling wines, designed to accentuate varietal character. The best known examples are those produced from Muscat grape varieties, notably the sparkling (spumante) wines from Asti. The marked varietal character of Muscat grapes would mask the subtle bouquet generated, at considerable cost, by the standard method.
Occasionally, the wine may be aged on lees for up to 9 months, if a traditional lees-matured attribute is desired. However, because expensive pressurized tanks are tied up for months, many of the economic advantages of the system are voided.
One of the features generally thought to characterize bulk-processed sparkling wine is poorer effervescence. However, an accurate means of assessing this view has only recently become available (Maujean et al., 1988; Liger-Belair et al., 1999). Objective proof of this assertion awaits verification.
Base wine production may go to dryness or be terminated prematurely. Occasionally, fermentation is arrested at about 6% alcohol to retain sugars for the second fermentation. Termination is either by exposure to cold, followed by yeast removal, or directly by yeast removal. Yeast removal is achieved by a combination of centrifugation and filtration, or by a series of filtrations. Once the cuvée has been formulated, the wines are combined with yeast additives (ammonia and vitamins) and sugar, if necessary. The second fermentation takes place in reinforced stainless steel tanks, similar to those employed in the transfer process. If a dosage is not employed, and residual sweetness is desired, the second fermentation must be arrested early. This is most easily achieved by cooling the fermentor to about 8 °C.
If an extended contact period with yeasts is desired, for bouquet development, the lees are intermittently stirred. Left undisturbed, a thick layer of yeast cells would form, favoring the generation of reduced-sulfur taints. Mixing also helps release amino acids thought to be involved in evolution of a toasty bouquet. However, stirring also releases fat particles not easily removed by filtration. These may interfere with effervescence production (Schanderl, 1965).
At the end of fermentation, or lees contact, the wine is cold-stabilized to precipitate tartrates. Yeast removal involves centrifugation or filtration. It is imperative that these operations be conducted under isobarometric pressure conditions. Otherwise, carbon dioxide may be lost, or gained, if the pressurizing gas is carbon dioxide. Sugar and sulfur dioxide contents are adjusted just before sterile filtration and bottling.
Occasionally, still wine may be added to the sparkling wine before final filtration and bottling. This technique may be used to produce wines of reduced carbon dioxide pressure, such as Cold Duck.
Other Methods
A small amount of sparkling wine is produced by the rural or natural method. The primary fermentation is terminated early by repeated filtration to remove the yeasts. This also removes essential nutrients from the juice, notably nitrogen. Formerly, fermentation was stopped by repeatedly skimming off the cap of the fermenting juice. After fermentation has ceased, the wine is bottled, and a second in-bottle fermentation slowly converts the remaining sugars to carbon dioxide. Yeast removal usually entails manual riddling and disgorging.
Other wines have derived their sparkle from malolactic fermentation. The primary example is vinho verde from northern Portugal. The grapes are commonly harvested low in sugar, but high in acidity. They consequently produce wines low in alcohol, high in acidity. The addition of little sulfur dioxide and late racking favored the development of malolactic fermentation. Cool cellar conditions typically resulted in its occurrence in late winter or early spring. Because the wines were kept tightly bunged after fermentation, the small volume of carbon dioxide produced was trapped. The pétillant wine that resulted was consumed directly from the barrel. When maturation shifted to large tanks, much of the carbon dioxide liberated by malolactic fermentation escaped. This was especially pronounced when the wine was filtered to produce a stable, crystal-clear wine for bottling. Correspondingly, the wine is often carbonated to reintroduce its characteristic pétillance. Occasionally, vinho verdes are produced without carbonation or malolactic fermentation, when they are low in malic acid content.
In Italy, some red wines become pétillant following in-bottle malolactic fermentation. Often the same wine is produced in both still and spumante (sparkling) versions.
In the former Soviet Union, sparkling wines were commonly produced in a continuous fermentation process. Though extensively used in Russia, it has been used only sparingly outside the former communist state, for example, Portugal. Multistage, bioreactor, continuous fermentors have also been investigated in Japan (Ogbonna et al., 1989).
Carbonation
The injection of carbon dioxide under pressure is undoubtedly the least expensive method of producing a sparkling wine. It is also the least prestigious. Prestige must, by definition, be exclusive. Consequently, carbonation is used only for the least expensive effervescent wines.
Because of the comparative lack of surfactants, bubbles tend to be larger than in sparkling wines. Essentially, no mousse (semi-stable form) develops. Nonetheless, the effervescence can accentuate any faults the wine may possess. Thus, the base wine needs to be of good quality.
Although carbonated wines are generally discounted as unworthy of serious attention, carbonation has the advantage of leaving the aromatic and taste characteristics of the wine unmodified. No secondary microbial activity affects the sensory attributes of the wine. It is really a matter of preference and choice.
Production of Rosé and Red Sparkling Wines
Although red grapes may be used in the production of sparkling wines, they are normally processed to make a white wine. Only occasionally are red grapes, fermented on their skins, used to produce a rosé or light-red sparkling wine. The tannins extracted along with the pigments complicate the second fermentation and accentuate gushing. Consequently, the bulk method is preferred in their production. The base wines are almost universally encouraged to undergo malolactic fermentation prior to the second fermentation. This gives the wine a smoother mouth-feel.
Rosé sparkling wines may be produced from rosé base wines. However, rosé champagnes are typically produced by blending small amounts of red wine into a white cuvée. Anthocyanins donate most of the pinkish countenance, although some may also originate from the presence of pyranoanthocyanins (Pozo-Bayón et al., 2004).
Most rosé and red sparkling wines are finished sweet, and with low carbon dioxide pressures. They are typically either pétillant (≥7 g CO2/L) or crackling (≥9 g CO2/L). The specific carbon dioxide levels applying to each of these terms can vary considerably from jurisdiction to jurisdiction. In contrast, most white sparkling wines contain at least 12 g CO2/L.
Effervescence and Foam Characteristics
The appeal of sparkling wine, to consumer and critic alike, is often intimately intertwined with their effervescence and foam attributes. Correspondingly, the origin and factors affecting their development have come under considerable scrutiny (Senée et al., 1999; Liger-Belair et al., 2008b, 2010; Coelho et al., 2011). For effervescence, the appearance, size, and duration of bubble formation are central features, whereas the degree (height and extent), duration, and stability of the foam (mousse) are supplemental properties. These aspects are assessed subjectively in separate scoring systems devised by Obiols et al. (1998) and Gallart et al. (2004). Objectively, effervescence is assessed with highspeed video and strobe lighting (Liger-Belair, 2005), while foam parameters are assessed using a Mosalux (Maujean et al., 1990).
Carbon dioxide may exist in five states in water – microbubbles, dissolved gas, carbonic acid, carbonate ions, and bicarbonate ions. Within the normal pH range of wine, carbon dioxide exists predominantly in the form of dissolved gas, with no carbonate species (Liger-Belair, 2005).
Many factors affect the solubility of carbon dioxide and, therefore, the pressure it can exert (Lonvaud-Funel and Matsumoto, 1979). The most significant factor is temperature (Fig. 9.29), with sugar and ethanol contents being secondary. Increasing these factors decreases gas solubility and augments the pressure exerted. Once the bottle is opened, ambient atmospheric pressure puts the wine into a supersaturated state. If the wine is shaken, the gas can exert sufficient force to eject a cork at velocities of up to 50–60 km/h (Liger-Belair, 2004).
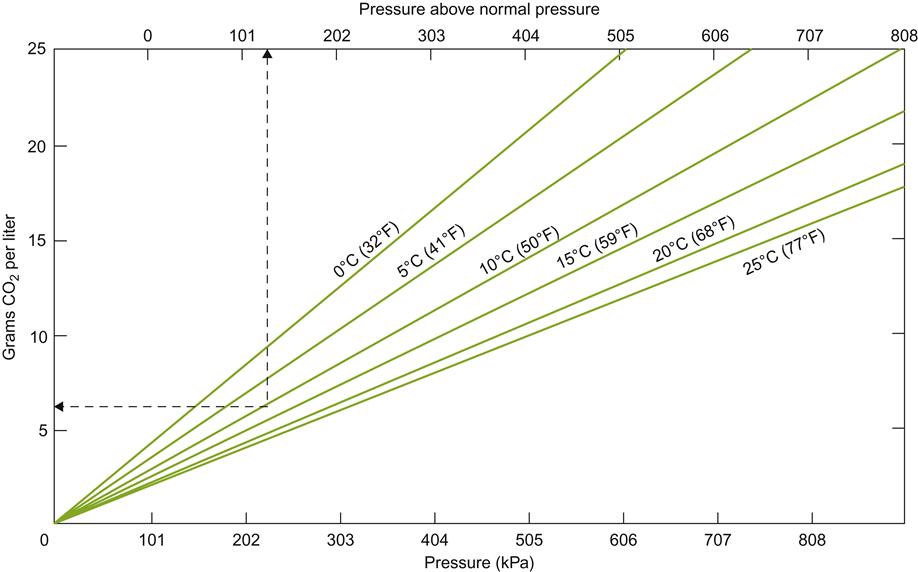
Upon opening, the headspace pressure over the wine drops from about 600 to 100 kPa (ambient atmospheric pressure). The partial pressure of carbon dioxide in air is ~0.03 kPa. This decreases carbon dioxide solubility in the wine from approximately 12 to 2 g/L, resulting in the eventual liberation of almost 5 liters of carbon dioxide gas (from a 750-mL bottle) (Jordan and Napper, 1987). Liger-Belair (2005) estimates that in a champagne flute (100 mL), full degassing would involve the release of about 10 million, 500-μm bubbles. The gas does not escape immediately, because there is insufficient free energy for bubble formation. Most of the carbon dioxide enters a metastable state, from which it is slowly liberated, if not agitated.
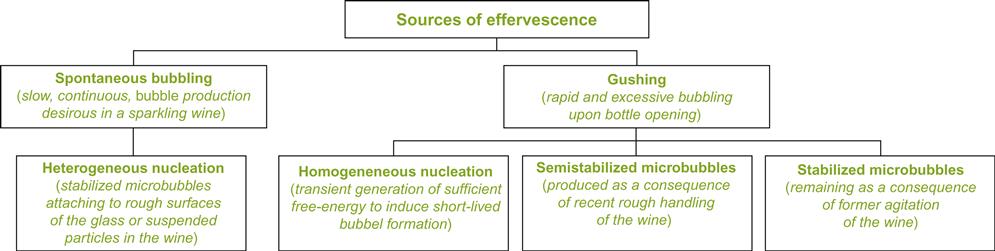
Carbon dioxide escapes from the wine either via diffusion or bubble formation (Fig. 9.30). The slowest and least significant to the sensory characteristics of sparkling wine is diffusion. Bubble nucleation may arise spontaneously, or through the action of various physical forces. Spontaneous effervescence from nucleation sites is the source for the continuous stream of bubbles so appreciated in sparkling wines. Provoked effervescence is undesirable (except during sport celebrations or ship launchings) as it enhances gushing and wine loss.
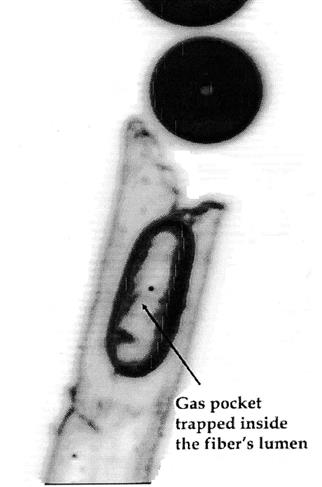
Spontaneous effervescence results from heterogeneous nucleation (Jordan and Napper, 1987). This occurs due to the formation of nucleation sites, associated with imperfections on the glass surface or extraneous suspended material. Most sites appear associated with lint fibers, adhering to the sides of the glass or floating in the wine. The latter are termed ‘fliers,’ due to their erratic or circular movements within the wine (Plate 9.12). They probably originate from glass drying or settle out of the air. They frequently possess cavities, of appropriate dimensions, to form microscopic air pockets. When wine is poured into the glass, they act as nucleation sites into which carbon dioxide can diffuse (Fig. 9.31). Nucleation sites may also develop in crevasses on tartrate salt crystals. The minimum diameter required for a nucleation site has been calculated to be about 0.25 μm (Liger-Belair et al., 2008b). Carbon dioxide, under the aegis of its supersaturated status, begins to diffuse into any entrapped air pocket. The associated formation and release of bubbles accounts for the slow liberation of almost 60% of the CO2 over a period of approximately 1 h (Fig. 9.32). As the degree of supersaturation decreases, so does the rate of bubble formation, and the size of the bubbles at the surface. The presence of even traces of detergent can inhibit this process. They coat nucleation sites, preventing carbon dioxide ingress and, correspondingly, bubble enlargement.
When the accumulating gas reaches the mouth of the nucleation site, its diameter begins to swell, developing into a nascent bubble. On reaching a critical size – from 14 to 31 μm (Liger-Belair et al., 2002), buoyancy provokes detachment (Fig. 9.31). Unknown factors can result in various bubble release scenarios, where bubbles may form in groups, or transient disruption in bubble formation occurs (Liger-Belair et al., 2006). During the ascent, carbon dioxide continues to diffuse into the bubble, increasing its diameter to upwards of 600μm. This apparently results in a dilution of surfactants coating the bubble (Liger-Belair and Jeandet, 2003). Ascent starts slowly, at about 0.2 cm/s. As the bubbles enlarge, their rate of ascent increases, reaching more than 6 cm/s as they approach the surface. Consequently, the distance between bubbles increases as they rise (Fig. 9.33). New bubbles tend to form at a rate of about 15/s, but this can vary from 1 to 30/s.
One of the sensory consequences of effervescence, other than its pleasurable appearance, is its effect on fragrance detection. The bubble chains generate convection currents and eddies (Plate 9.13). This refreshes the supply of aromatics available at the surface for volatilization. In addition, bubbles bursting at the surface propel wine microdroplets into the headspace above the wine (Plate 9.14), facilitating the escape of aromatics into the air. The effect is a selective release of volatile compounds (Liger-Belair et al., 2009). This includes decanoic acid, a compound possessing a toast-like fragrance. Although fascinating, the significance of these factors to the sensory perception of sparkling wines is questionable. Under normal tasting conditions, the wine is repeatedly swirled by the taster, generating more volatile liberation than effervescence.
In contrast to the slow, effervescent, liberation of CO2 in wine flutes, gushing can arise from a number of distinct nucleation processes (Jordan and Napper, 1987). The mechanical shock of opening or pouring provides sufficient free energy to weaken the bonds between water and carbon dioxide. Disruption of these van der Waals forces permits carbon dioxide to form nascent bubbles throughout the wine, in a process termed homogeneous nucleation. If the bubbles reach a critical size, they incorporate more CO2 than they lose. They continue to grow and begin their rapid ascent to the surface. Because the energy source for homogeneous nucleation is transient, so is the effervescence it provokes.
Another potential source of gushing comes from stabilized microbubbles. These develop from bubbles generated by agitation during handling. Most of the bubbles so formed float to the surface and break. The carbon dioxide released dissolves back into the wine, assuming the cork is still in place. However, other bubbles lose carbon dioxide to the wine, partially dissolving before reaching the surface. In the process, surfactants coating their face, produce a gas-impermeable membrane that stabilizes the bubble. After the bottle is opened, these bubbles rise to the surface. Gushing from this source takes a few seconds to develop. Semistabilized microbubbles, formed shortly after rough handling, may aggravate gushing. They can act as additional sites for bubble growth.
As noted, a membrane surrounds the bubbles as they rise. This allows them to mound on the surface, initially at the center, but also collect around the rim of the glass (Plate 9.15). This accumulation (foam) is termed the mousse. The bubbles soon burst, depending on the combined effects of the wine’s alcohol content, various surface-active ingredients, liquid drainage from between the bubbles, and the concentration of rigidifying agents, such as proteins and glycoproteins. The wine’s alcohol content (~12%) seems optimal for both foam formation and stability. When bubbles rupture, they implode on themselves. The resulting shockwave propels miniature columns of wine up from what was the submerged base of the bubble. As these columns rise, they break into a series of microscopic droplets. They can be ejected a few centimeters at several meters per second (Liger-Belair et al., 2001). Because several hundred bubbles may burst per second, the surface of the wine is spiked with these thin, cone-shaped spires (Plate 9.14). Their millisecond duration and minuscule size make them nigh invisible to the naked eye. They do, however, induce a perceptible touch sensation on the tongue and palate by stimulating nocioreceptor trigeminal nerve endings.
Because aromatic compounds (such as various alcohols, aldehydes and organic acids) may adsorb onto the bubble surface, or into the enclosed gas, they too are ejected into the headspace as the bubble ruptures. These aromatic droplets (or their remnants) can flow with air into the nose (Liger-Belair et al., 2001). This may initially enhance detection of the subtle fragrance that tends to characterize most sparkling wines. However, as time passes, the accumulation of protein and glycoprotein surfactants on the wine’s surface modifies, and eventually limits the ejection of aromatic laden droplets (Liger-Belair, 2001).
The formation of durable, continuous chains of small bubbles is an important quality attribute. The factors that regulate this property are still incompletely understood. Cool fermentation and maturation temperatures, and extended contact with the lees, are thought to favor the property. The sustained formation of fine bubble chains appears to depend on the joint effects of high-molecular-weight, and hydrophobic, low-molecular-weight mannoproteins with monoacylglycerol and glycerylethylene glycol fatty acid derivatives (Núñez et al., 2006; Coelho et al., 2011).
The formation and persistence of a cordon de mousse (Plate 9.15) is also considered an important property. It develops around the rim of the glass. In contrast to beer, the foam rapidly collapses and must be continuously replenished. Its formation and durability are largely dependent on the nature of the surfactants and the type and number of metallic ions in the wine. The surfactants, notably soluble proteins, polyphenols, and polysaccharides, decrease surface tension. Several fatty acid esters have also been found to favorably affect mousse development and stability, whereas free fatty acids negatively affect both attributes (Gallart et al., 2002). Bubble surfactants appear to collect at the wine–air interface (Péron et al., 2003), forming an amphiphilic layer composed of about 35% protein and 65% polysaccharides (Aguié-Béghin et al., 2009). Their coating of the bubbles is thought to donate a degree of stability to the mousse. The cultivar or cultivars used in preparing the base wine also affect foam attributes. For example, of several varieties assessed, Chardonnay wines had the best propensity to develop foam, but were the worst in terms of its stability (Andrés-Lacueva et al., 1997). The potential for mousse formation initially increases after the second fermentation, but may decline thereafter (Andrés-Lacueva et al., 1997). Subsequently, mousse stability may again increase.
Gravity progressively removes fluid from between the bubbles, forcing them to assume polyhedral shapes. As a result, uniformity of pressure on the sides of the bubble is lost. This forces further liquid into the angled corners of the bubbles, inducing further compaction. Thinning of the interstitial fluid layer induces fusion. Carbon dioxide in small bubbles increasingly comes under more pressure than in larger bubbles, promoting CO2 diffusion from smaller to larger bubbles. As the remaining bubbles enlarge, they become increasingly susceptible to rupture.
The presence of proteinaceous or polysaccharide surfactants tends to restrict bubble compression. Interaction between surfactants may give a degree of rigidity and elasticity to the mousse. Elasticity can absorb the energy of mechanical shocks, limiting fusion, and bubble rupture. Although formation of a mousse is desirable, it is also traditional that it be relatively evanescent. The relative absence of stabilizing surfactants limits its duration.
Aging
In contrast to the extensive research on the consequence of lees contact during in-bottle maturation, changes post-disgorgment have been little studied. This is not too surprising. Sparkling wines are rarely aged by the consumer after purchase. Nonetheless, there is a long history of a subset of connoisseurs preferring aged champagnes, either late-disgorged or post-disgorgment.
Late-disgorging (aging on the lees longer than the traditional 3 years), appears to retain the wine’s freshness longer, at least initially. However, prolonged lees contact (up to 60 months) increases the rate of oxidation. Flavor deterioration also occurs faster than that of the same wine disgorged traditionally (Stevenson, 2012).
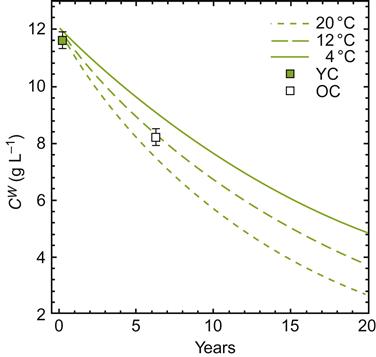
During aging, sparkling wines also slowly lose their effervescence. Only recently has this phenomenon been investigated. If the theoretical projections illustrated in Fig. 9.34 are substantiated, there should be about a 5% loss in carbon dioxide per year at 20 °C. The rate should be considerably slower at 4 °C, but, theoretically, could still result in a 60% reduction in carbon dioxide content over a 20-year period. Because champagnes may remain noticeably effervescent for about 20 years, and can still possess some fizz after 50 years, unknown factors, other than bottle volume, appear to be acting, slowing the projected loss in CO2.
 ) and old (
) and old ( ) sample. (From Liger-Belair and Villaume, 2011. Copyright 2011 American Chemical Society, reproduced by permission.)
) sample. (From Liger-Belair and Villaume, 2011. Copyright 2011 American Chemical Society, reproduced by permission.)Not only does carbon dioxide dissipate during and after disgorging, but also during the wine’s maturation on the lees. Even under a crown cap, the wine may lose 60 kPa CO2/year, depending on the seal provided (Valade et al., 2011). Oxygen uptake during this period can also sensorially affect the wine (Valade et al., 2011).
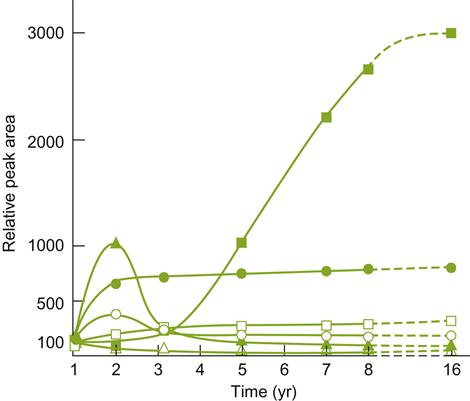
As typical for white wines, aged champagne shows a loss of floral and fruit attributes (Escudero et al., 2000). There is also the development of distinctive attributes, described as resembling roasted coffee beans, toast, and brioche (Tominaga et al., 2003). This profile has been partially associated with the presence of several thiols, for example, 2-furanmethanethiol. It is considered to donate a roasted coffee aroma to barrel-matured wines (Tominaga et al., 2000). Other thiols that accumulate to above threshold values include benzenemethanethiol and ethyl 3-mercaptopropionate. Furfural also accumulates with aging, but unlike the thiols, its increase was not associated with disgorging, as was the increase in the thiols. Additional compounds, associated with a toasty flavor, include m-cresol and decanoic acid (Escudero and Etiévant, 1999). Dihydroxyacetone has also been reported to possess a crust-like aroma.
Fortified Wines
Fortified wines are classified together because of their elevated alcohol content. This is usually derived from the addition of brandy or highly rectified spirits, at some stage in production. The marked flavor of fortified wines gives the grouping an additional unifying property. Because of this property, they are seldom consumed with meals, normally being served as aperitifs or dessert wines. Unfortunately, governments often combine them for the purposes of higher taxation.
Most fortified wines have evolved in the last two to three hundred years, primarily in southern Europe. Examples are sherry (southern Spain), port (Portugal), marsala (Sicily), madeira (Madeira) and vermouth (northern Italy). The production of some of these is discussed below.
Sherry and Sherry-Like Wines
Sherry evolved into its near-present-day form in southern Spain, possibly as late as the early 1800s. The details of its development from a young table wine, transported to England in the 1600s, are unclear (Gonzalez Gordon, 1972). The original sack imported to England, and made famous to later generations by Shakespeare, was not the sherry we know today. Sack was a table wine, coming in either red or white versions. The solera system, so associated with modern sherry, is thought to have originated in the early nineteenth century (Jeffs, 1982), and was established as standard practice by the 1850s. In its current form, sherry exists only in white to tawny versions.
In Spain, the designation sherry is used as a geographic appellation. It is restricted to wines produced in and around Jerez de la Frontera in Andalucia. Similar wines produced elsewhere in Spain, or the rest of Europe, are not permitted to use the sherry appellation. Nevertheless, similar wines may use the stylistic terms fino, amontillado, and oloroso.
Outside Europe, the designation ‘sherry’ is used generically for wines that, to varying degrees, may resemble Spanish sherries. The provenance of these products is typically appended to the term sherry. Such sherries are seldom produced by techniques similar to those employed in Jerez.
Three distinctly different techniques are used worldwide for the production of wines designated ‘sherry.’ Each is described separately. These are the traditional solera procedure (used almost exclusively in Spain), the submerged fino technique, and the baked method.
Solera System
The solera system that developed in southern Spain is a form of fractional blending (Fig. 9.35). In summary, it consists of several distinct collections of casks (termed butts), each termed a criadera. When a portion of wine is removed from the criadera containing the oldest blend, termed the solera, it is replaced with an equivalent amount of wine from the next oldest criadera. This sequence continues until wine is removed from the youngest criadera. It is in turn replaced with young wine. The wine removed from the solera is prepared for bottling. The technique is ideally suited for the production of wine that is both brand-distinctive and consistent from year-to-year.
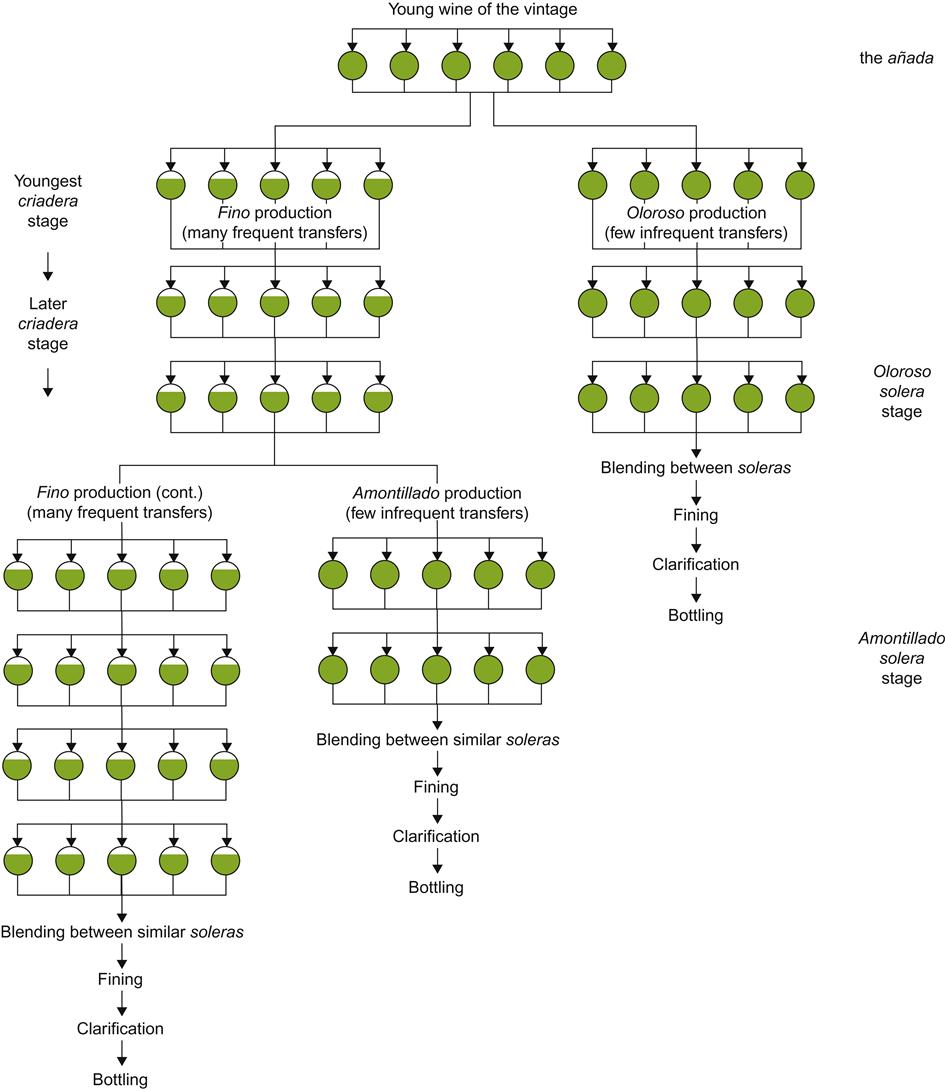
The frequency and proportion of wine transferred is adjusted to the style desired. The transfer rate and number of criaderas are particularly important. They direct the wine’s development. For example, fino sherries undergo frequent transfers and possess many criadera stages. In contrast, oloroso sherries develop best with few criaderas and involve infrequent transfers.
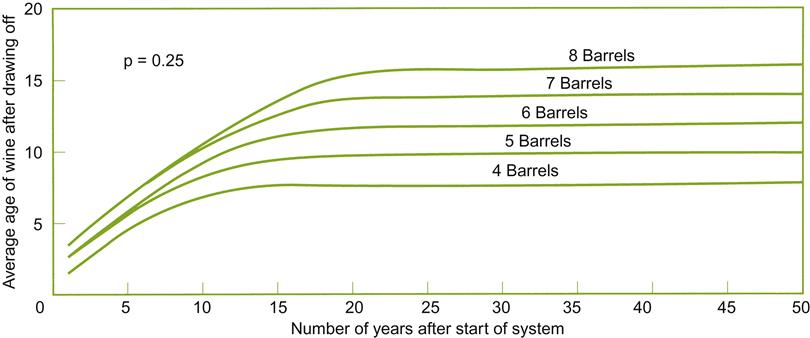
These factors also influence the average age of the sherry produced. When a solera system is initiated (a series of criaderas), the average age of the wine rises rapidly (Fig. 9.36). Subsequently, the mean age increases progressively more slowly, finally reaching what approximates a constant age. The plateau is reached more quickly when the frequency and proportion of the wine transferred is increased. Correspondingly, the number of criaderas in a solera system influences the rate and maximal age achieved. The greater the number of criaderas, the older the stable age finally achieved. Formulas for calculating the effects of these factors are discussed in Baker et al. (1952).
Spanish sherry is subdivided into three main categories – fino, amontillado, and oloroso (Fig. 9.35). They may also be classified based on where the wines are matured (e.g., Sanlúcar de Barrameda vs. Jerez de la Frontera), by their sensory characteristics (e.g., palo cortado versus raya olorosos), or on how they are sweetened (e.g., cream-type sherries).
Base Wine Production
In the past, grapes were laid out in the sun for several weeks before being stemmed and crushed. This was done to augment the sugar level of the grapes by dehydration. While still done with Pedro Ximénez and Muscat, primarily for producing sweetening wines, it is no longer applied to the principal variety, Palomino (Palomino Fino). Harvesting is done when the grapes have reached the desired maturity (≥23°Brix). Acidity level is less critical as it can be augmented with tartaric acid if insufficient. Models of maturity have been presented by Palacios et al. (1997).
Whereas the development of a sherry into a fino or oloroso once seemed arbitrary, almost mysterious, it is now largely predictable, as well as directed. Experience has shown that juice derived from grapes grown in cooler vineyards, or in cooler years, is more predisposed to becoming a fino. Vineyards containing a high proportion of chalk in the soil also tend to favor fino development. Gentle grape pressing, and the inclusion of little press-run juice, further shift evolution toward a fino. Conversely, juice derived from grapes ripened under hot conditions, grown on soils containing less chalk, pressed in hydraulic vertical presses, and incorporating press-run fractions generally promote transformation into an oloroso. Slightly higher initial phenolic contents are desired in wines designed for oloroso production (encouraging oxidation). These tendencies can also be directed by the level of fortification. Contents of 15.5 and 18% alcohol favor fino or oloroso development, respectively. The level of cask (butt) filling and maturation temperature also direct the wine’s evolution.
Production of the base wine generally follows standard procedures, except that fermentation occurs between 20 and 27 °C – higher than generally preferred elsewhere for white table wines. Pressing almost immediately follows crushing, thereby limiting tannin extraction. Spontaneous settling for several hours brings the suspended solids content down to 0.5–1%. The effect of suspended solids on the chemical composition of the wine is illustrated in Table 9.6. Increasing tannin content gives a roughness, inconsistent with accepted sherry norms. Values of≤200 mg/L total phenolic content are desired. Because the juice often has an undesirably high pH, tartaric acid is commonly added to correct this deficiency. A value lower than pH 3.45 is desired. The older procedure (called plastering) involved adding yeso, a crude form of gypsum (calcium sulfate). Plastering both lowered the pH and provided a source of sulfate. After conversion to sulfite, it had the additional advantage of inhibiting the growth of spoilage bacteria, notably Lactobacillus trichodes. Adding sulfur dioxide directly has the same effect, but avoids the addition of calcium, and other potential mineral contaminants in yeso.
Table 9.6
Concentration of several compounds after the fermentation of a must with a different concentration of solidsa,b
| M1 | M2 | M3 | |
| Volatile acidity (g/L) | 0.19 | 0.25 | 0.41 |
| Sugars (g/L) | 1.7 | 1.4 | 1.4 |
| Ethanol (% v/v) | 10.6 | 10.8 | 10.7 |
| Acetaldehyde (mg/L) | 163 | 56 | 67 |
| Ethylacetate (mg/L) | 23 | 23 | 27 |
| Methanol (mg/L) | 31 | 32 | 36 |
| N-propanol (mg/L) | 15 | 15 | 16 |
| Isobutanol (mg/L) | 27 | 25 | 31 |
| Isoamyls (mg/L) | 236 | 189 | 164 |
| Glycerol (g/L) | 7.3 | 6.5 | 6.2 |
| Citric acid (g/L) | 0.74 | 0.77 | 0.64 |
| Malic acid (g/L) | 0.07 | 0.15 | 0.24 |
| Succinic acid (g/L) | 0.52 | 0.38 | 0.89 |
| Lactic acid (g/L) | 0.11 | 0.12 | 0.13 |
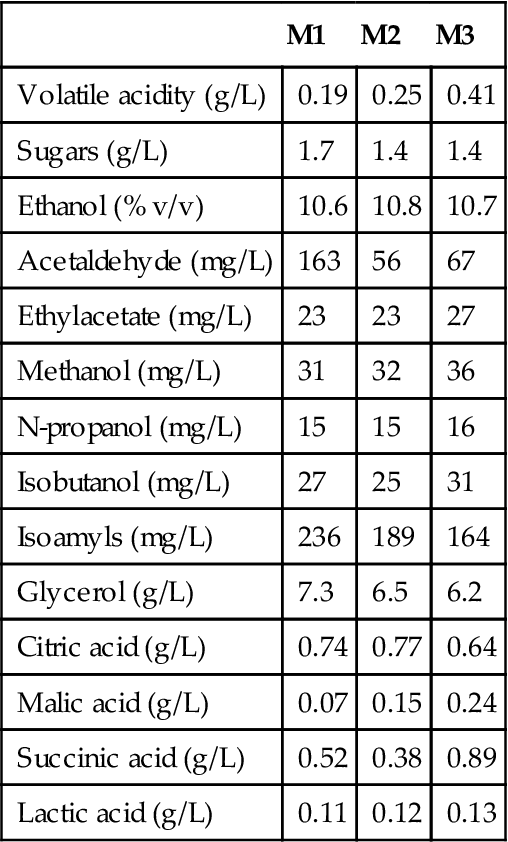
aM1, not decanted (5 g/liter solids); M2, decanted (1.1 g/liter solids); M3, decanted and filtered (0 g/liter solids).
bAnalyses correspond to the sixth day after inoculation. The experiment was done per triplicate with a coefficient of variation of<10%.
Source: From Martínez et al., 1998, reproduced by permission.
Inoculation with specific yeast strains still seems uncommon, with fermentation developing spontaneously from the indigenous grape and winery (bodega) flora. If yeast inoculation is employed, it is usually added to one-third of the must. Once fermentation has become turbulent (usually 4–5 days), an equivalent volume of must is added. When this volume is clearly fermenting, the final must portion is added. Esteve-Zarzoso et al. (2001) report that despite this acclimation, the inoculated strain is occasionally replaced by wild strains. Unlike in the past, most fermentations are now conducted in stainless steel tanks, rather than in oak barrels (butts).
To avoid interference with the sherry flavor, the base wine should have little varietal aroma. In Spain, the neutral-flavored Palomino is used for dry sherry production, whereas Pedro Ximénez or Muscat tend to be preferred for making sweet, darker sherries.
Stylistic Forms of Jerez Sherry
Finos
Fino sherries are the lightest, driest, and most subtly flavored sherries. They are also characterized by possession of a flor bouquet. This develops from the action of a yeast film (velum) that grows on the wine’s surface during solera maturation (Plate 9.16). The film-forming yeasts (flor) are typically related to those that induced the original fermentation. If flor development does not occur rapidly, an inoculum may be transferred from casks containing an active culture.
After the first racking, the base wine is fortified to bring the alcohol content up to 15–15.5%, in a process termed encabezado. This involves preparing a 50:50 blend of rectified (aromatically neutral) wine spirits (∼95% ethanol) and aged sherry, called miteado. Storage for approximately 3 days permits settling of any cloud that forms, and limits haze production in the young wine. At 15%, the alcohol favors flor development, as well as restricts the growth of acetic acid bacteria. A velum (pellicle, biofilm) forms because the elevated alcohol content promotes the production of a hydrophobic cell wall (Alexandre et al., 1999). Unlike other microbial biofilms, no protein or polysaccharide extracellular matrix forms between the cells. In contrast, direct interaction between the hydrophobic Flo11 glycoprotein, with mannobiose terminal units of oligosaccharide ligands on adjacent cells, links them together (Veelders et al., 2010). Film formation depends on activation and regulation of a particular isogenic variant, FLO11. It shows high gene expression, resulting in large numbers of Flo11 glycoproteins on the cell surface (Fidalgo et al., 2006). Expression of the gene is increased in the presence of ethanol and glucose (Ishigami et al., 2006). The hydrophobicity of these proteins results in floating vs. the more typical settling of the clumped cells. Other genes indirectly involved in velum development include BTN2, associated with cellular protein localization (Espinazo-Romeu et al., 2008), and HSP12, encoding one of the heat-shock proteins active during the stationary phase (Zara et al., 2002). Low pH and the presence of biotin (Iimura et al., 1980), pantothenate (Martínez et al., 1997c), and phenolic compounds (Cantarelli, 1989) further favor velum formation.
Yeast aggregation also entraps carbon dioxide generated by yeast metabolism, further increasing buoyancy (Martínez et al., 1997a; Zara et al., 2005). This permits the aggregated cells to float to the surface, forming the velum (flor). Thus, velum development is considered an adaptive mechanism, whereby starved yeast cells gain access to oxygen, permitting ethanol and acetaldehyde respiration (Ibeas et al., 1997a). Sulfur dioxide content is commonly adjusted to approximately 100 mg/L, to limit the growth of lactic acid bacteria. Nonetheless, small numbers of these bacteria, mostly Lactobacillus spp., have been detected during the early stages of sherry production (Moreno-Arribas and Polo, 2008). Thus, if and when malolactic fermentation occurs, it appears to take place early during maturation, and not involve the action of Oenococcus oeni.
Because maturation under a velum involves yeast metabolism, it is often referred to as biological aging. This differentiates the wine’s reductive aging (absence of oxygen under the velum) from the more traditional reductive aging of table wines, and the partially oxidative aging of most fortified wines.
The wine is matured in American oak cooperage. The butts have a capacity of about 490 liters. Typically, they have been used previously to ferment wine. Prior conditioning minimizes oak-flavor extraction that might otherwise mask the fino bouquet. Barrels are left with 10–20% ullage to provide sufficient surface, and favorable growth conditions, for flor development.
During the initial storage (añada), the development of the new wine (sobretablas) is periodically checked to assess its development. It may remain in the añada for from 1 to 2 years. Flor begins to develop and may soon cover the wine. If flor does not form as desired, even with inoculation, the wine is either used for the production of another sherry style or distilled.
During fractional blending, when wine is removed from the youngest criadera, it is replaced from an añada (Fig. 9.35). In each transfer, about one-quarter of the wine (100 liters) is removed and replenished. The transfer frequency depends on development of the wine, as determined by sensory analysis. Typically, transfers occur about twice a year, but may occur more frequently. There are generally four or five criaderas in a fino solera system. There may, however, be considerably more, especially with Manzanilla fino produced in Sanlúcar.
The butts of a criadera are arrayed in rows, in above-ground, spacious buildings called bodegas (Plate 9.17). The floors are usually bare soil, so that they can be easily moistened to maintain a relative humidity of above 60%.
Generally, butts are stacked no more than three to four high. This avoids structural damage to the cooperage. The criaderas in any particular solera series may be housed throughout the bodega. Large firms generally have numerous solera series, in various stages of development. The finished sherry may be, and usually is, a blend of wine from several separate soleras, with different initiation dates.
Each transfer involves combining the fractions from the different butts in each criadera stage, before gentle dispersion to butts in the next older criadera. This helps minimize differences among butts. The transfer process involves a series of siphons that extract the desired proportion of wine from underneath the velum. After blending, the wine is transferred to butts in the next criadera, also under its flor covering. The procedure attempts to disturb as little as possible both the surface flor and the bottom lees. The process is being automated to obviate the arduous task of manual siphoning, blending, and subsequent pouring. In the past, the process was facilitated by several ingenious devices, involving perforated tubes (rociadors) and wedge-shaped funnels. The angled spout (canoas) minimized velum disturbance.
Frequent wine transfer (about every 3 months) is critical to the development and maintenance of an active flor, refreshening the nutrient supply (Berlanga et al., 2004a). Proline is the principal nitrogen source, whereas biotin favors production of a hydrophobic cell wall. Providing a favorable surface area/volume (SA/V) ratio is also important. Leaving the butts with about 20% ullage creates a SA/V ratio of about 15 cm2/L (Fornachon, 1953). The practice provides sufficient contact with the primary carbon and energy sources, and supplies oxygen for respiration. The bung hole is left slightly ajar to allow gradual air exchange. Oxygen is required for the action of yeast mitochondrial aldehyde dehydrogenase, that converts ethanol to acetaldehyde (Millán and Ortega, 1988). Acetaldehyde accumulation often averages between 260 and 360 mg/L. Ethanol metabolism is particularly active during the first few months of velum development. As ethanol is both metabolized and escapes by volatilization, periodic wine spirit addition may be required to maintain the ethanol content at between 15 and 15.5%.
The taxonomic nature of the flor population is still contentious. This may relate as much to changing views of yeast taxonomy as to barrel-to-barrel and winery-to-winery diversity. The dominant flor-inducing yeasts have been variously identified as strains of Saccharomyces cerevisiae, S. bayanus, Torulaspora delbrueckii, or Zygosaccharomyces rouxii. Recent research suggests that unique strains of S. cerevisiae constitute the majority of flor yeasts (Ibeas et al., 1997b; Esteve-Zarzoso et al., 2004). They all appear to possess a common deletion in their 5.8 S ribosomal gene.
The proportion and genetic characteristics of the dominant yeast strains change during fermentation, and throughout solera aging. For example, the frequency of cells with functional mitochondria (capable of respiration) is highest in flor strains (Martínez et al., 1995). Whether this reflects selection of existing, or mutant strains, is unknown. Flor yeasts also show a higher expression of heat shock protein (HSP) genes, and correspondingly higher resistance to the toxicity of ethanol and acetaldehyde (Esteve-Zarzoso et al., 2001). They also have greater insensitivity to osmotic stress than fermentative strains. In addition, they overexpress SSU1, a gene that encodes for a sulfite pump that translocates sulfite out of the cell. This enhances cellular resistance to sulfur dioxide. Finally, and most distinctively, flor strains show high expression of FLO11 (alternatively called MUC1), encoding for the surface glycoprotein that is responsible for velum formation.
Flor strains often vary in their temperature preference, thickness of the velum they generate, and in their synthesis of volatile compounds, such as esters, higher alcohols, and terpenes. Whether these differences are of sensory significance is unclear (Cabrera et al., 1988). Most yeast vela are a mix of strains and species. Criddle et al. (1981) considered that mixed cultures form more uniform pellicles than pure cultures.
Flor yeasts are critical to the development of fino sherries. In the absence of fermentable sugars, yeast growth depends on a shift to respiratory metabolism. As the film grows, covering the wine, diffusion of oxygen into the wine is restricted. Thus, the redox potential of the wine increases, although the wine is seemingly exposed to air. This, plus yeast-induced inhibition of phenol oxidation, probably explain the wine’s pale color (Martínez et al., 1998; Lopez-Toledano et al., 2002). An alternative, or additional factor, appears to be the action of phenolic compounds on membrane lipids. They favor the adsorption of polyphenolics, notably those colorless intermediates in browning reactions (Márquez et al., 2009).
Flor yeasts respire ethanol, glycerol, acetic and several other organic acids, producing acetaldehyde and various aromatic by-products. Examples are 1,1-diethoxyethane, diacetyl, acetoin, 2,3-butanedione, and C4 organic acids (Cortés et al., 1999). During solera maturation, ethanol consumption remains fairly constant (in the range of 5 to 6 L per year) (Martínez et al., 1998). In contrast, glycerol consumption rapidly declines (Bravo, 1984). Its availability is not replaced. Most of the acetaldehyde generated is respired via the TCA cycle by yeast cells at the surface. It reaches its highest concentration during the añada phase, declines early in solera aging, and then slowly rises again (Martínez et al., 1997b). Limited fermentation in the lower, submerged portion of the film probably depends on residual sugars.
The accumulation of acetaldehyde (not respired during yeast metabolism) gives sherry its oxidized bouquet. Subsequent reaction of acetaldehyde with ethanol, glycerol, and other polyols generates acetals. Of these, only 1,1-diethoxyethane likely accumulates sufficiently to add a ‘green’ note (see Etiévant, 1991). Small amounts of terpenes, such as linalool, cis- and trans-nerolidol, and trans, trans-farnesol are synthesized (Fagan et al., 1981). Several lactones, notably substituted γ-butyrolactones, have been isolated from fino sherries. They are generally regarded as important in the development of a fino character (Kung et al., 1980). The lactone, sotolon, is probably important in contributing to the characteristic walnut-like fragrance of fino sherries. Sotolon has also been isolated from vin jaune, a sherry-like wine produced in the south of France (Martin et al., 1992). Sotolon forms during a slow reaction between α-ketoglutaric acid and acetaldehyde (Pham et al., 1995). Nevertheless, the typical fino fragrance appears to depend on the combined effects of several aromatics, including lactones, acetals, terpenes, and aldehydes. Examples of other chemical changes during solera maturation are provided in Fig. 9.37.
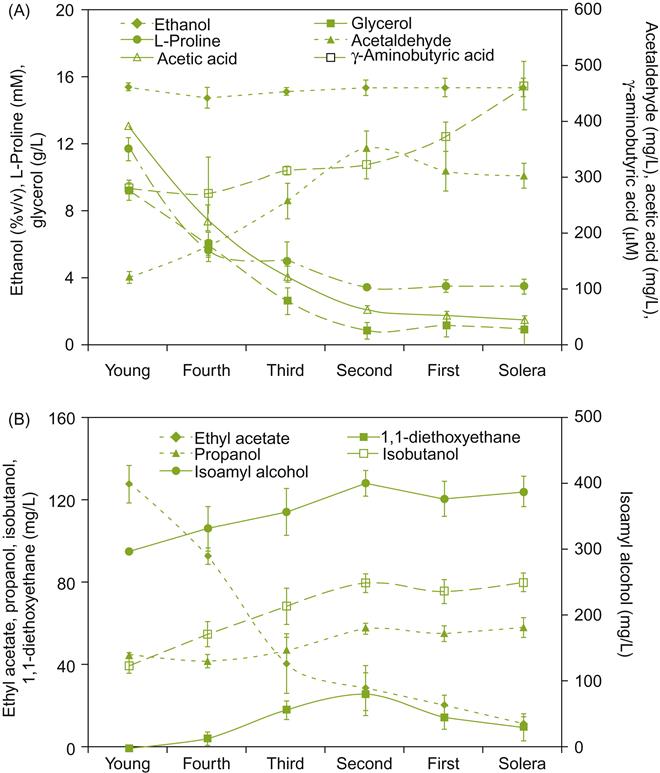
In addition to the oxidative metabolism of film yeasts, volatile compounds are lost through the sides and bung hole of the cooperage. Conversely, the evaporation of water from the butts can increase the concentration of various compounds (Martínez de la Ossa et al., 1987). This could potentially increase the alcohol content by about 0.2% v/v per year (Martínez et al., 1998). Water evaporation for barrel surfaces can be reduced by increasing the relative humidity in the bodega. This is commonly, and most simply, achieved by sprinkling water on the bodega floor.
During maturation, both amino acid and peptide content fall dramatically, especially proline (Villamiel et al., 2008). Tartaric acid, acetic acid and ethanol content also decline. In contrast, the content of higher alcohols increases (Muñoz et al., 2006).
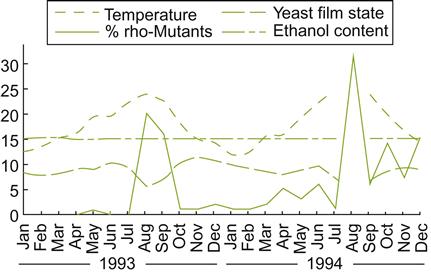
Although flor coverage is commonly complete, being about 3–6 mm thick, yeast activity is not constant. Growth is usually most active in the spring and fall, when the ambient temperatures in the bodega are between 15 and 20 °C. During the winter and summer months, unfavorable temperature conditions slow growth, and flor coverage may become patchy. This is particularly noticeable when temperatures rise above 22.5 °C (Fig. 9.38). This also correlates with an increase in the number of rho° strains (lacking mitochondria). Because these are respiratory deficient, they die and settle down to the lees.
As the velum thickens, lower sections break off and fall to the bottom. Because of yeast autolysis, sediment rarely accumulates to any degree, and the cooperage seldom needs cleaning. Nutrients released by autolysis, notably glucans, fatty acids, amino acids, peptides, and nucleotides are probably important to continued flor growth. Substances released during autolysis are also likely to be important in the development of the typical fino bouquet, similar to sparkling wines.
After maturation is complete, the wine removed from one solera is usually blended with wines from other similar soleras. Subsequently, the alcohol content is adjusted to 16.5% alcohol, or an amount considered appropriate for the export market. Increasing the alcohol content stops any further flor activity. During maturation, the malic acid level falls, which may leave the wine with insufficient acidity. If so, tartaric acid may be added. A polishing clarification and cold stabilization prepare the wine for bottling. Fino sherries are seldom blended with sweetening or color wines. They are sold as dry, pale-colored, aperitif wines.
Despite fino sherries being considered oxidized (due to the presence of acetaldehyde), newly bottled versions do not possess the oxidized character of white table wines. These attributes, or loss of fresh fino attributes, occur only after bottling. Thus, fino sherries are best consumed shortly after bottling. Although uninvestigated, loss of its initial flavor may be due to the use of short, chamfered, T-corks. These may permit significant oxygen ingress. If so, the current shift to RO closures may permit fino sherries to have a much longer shelf-life, allowing more people to have a true experience of its inherent properties. Browning and development of oxidized flavors are accelerated by exposure to light and high-temperature storage (Benítez et al., 2003, 2006).
Amontillado
Amontillado sherries begin development as a fino sherry. However, after transfer through several criaderas, the frequency of transfer is slowed, decreasing the rate of nutrient replenishment. This favors water loss, tending to increase the relative alcohol content. All these features slowly lead to the cessation of flor growth. This can be induced by fortification to reach 17–17.5% alcohol. This also protects the exposed wine from the activity of acetic acid bacteria. In addition, because an exposed wine surface is neither desired nor required, the butts are usually filled. Without flor protection, the wine shifts from reductive to a slow oxidative aging. Thus, it becomes darker in color, and develops a richer, oxidized flavor, associated with aspects derived from the earlier biological (flor) aging. Its character is dominated by the presence of ethyl esters (ethyl octanoate, ethyl butanoate and ethyl isobutanoate), eugenol, and sotolon (Moyano et al., 2010). These and other compounds donate a sensory profile described as spicy, fruity, and nutty (Zea et al., 2008). In comparison with fino soleras, there may be few to many criadera stages in the maturation of amontillado – the number depending on the flavor intended. Due to the extended period in each of the amontillado criaderas, maturation often takes up to 8 years. Most amontillado soleras are initiated intentionally, rather than, as in the past, occurring as if by accident.
When drawn from the solera, amontillado sherries may be sweetened and fortified to meet particular market demands. In Spain, the wine is usually left unmodified. After cold stabilization, and a polishing filtration, the wine is ready for bottling. Amontillados may also be used in preparing cream-type sherry blends.
Oloroso
The first step in producing an oloroso sherry involves fortifying añada wine to about 18% alcohol. This inhibits both yeast and bacterial growth, and makes oloroso maturation less sensitive to temperature fluctuation, as compared to other sherry types. Consequently, the butts are located in areas of the bodega showing the greatest temperature fluctuation. This can vary from as low as 5 °C during winter to almost 40 °C in summer. If stacked together with fino butts, the oloroso butts are on top, where climatic variation is the greatest. The butts are commonly filled to about 95% capacity, and irregular topping limits the rate and degree of oxidation. This may partially explain the minimal increase in acetaldehyde content observed during oloroso maturation. However, an additional reason may be the conversion of acetaldehyde to acetic acid, and its subsequent esterification with ethanol to ethyl acetate. This is suggested by the progressive increase in the concentration of acetic acid and ethyl acetate during oloroso maturation (Martínez de la Ossa et al., 1987).
Because of the long maturation in oak, the concentration of phenolic compounds is generally higher in amontillado and oloroso than in fino sherries (Estrella et al., 1986; Fig. 9.39). The higher phenolic content also partially arises from the original añada wine coming from press fractions in the later stages of grape pressing. In oloroso sherries, the most marked changes occur during the first few years of oxidative aging (Ortega et al., 2003). Sugar and alcohol content also rise during maturation. This probably arises from concentration due to water evaporation during the 7 to 8 years in-barrel maturation, that can last up to 14 years. Sugars may also come from the degradation of glycosides in the wood.
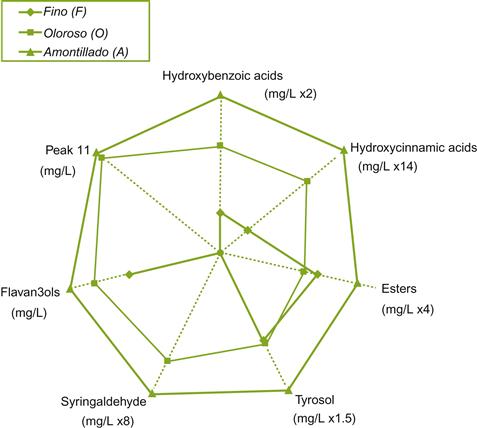
There are typically few criadera stages in an oloroso solera. Transfer rates are infrequent, often amounting to only 15% per year. Because fractional blending is limited, the wine shows considerable barrel-to-barrel variation. The cellar master establishes brand consistency through blending.
Although, like all sherries, olorosos are initially dry, they are seldom found that way on the international market. Most are available as cream sherries or their variants. Although predominantly based on olorosos, depending on their formulation, they may also contain some amontillado and fino components. They will typically also possess proprietary amounts of sweetening and color wines, and are usually brought up to about 21% alcohol. Thus, their flavor attributes are a combination of both those changes that occurred during the wine’s oxidative aging, with potentially some flor character, and the characteristics donated by the treatment given the sweetening and color wines. These aspects also explain why their flavor is so stable, even months after the bottle has been opened. After clarification and stabilization, they are ready for bottling. Palo cortado and raya sherries are special oloroso sherries. They are more subtle and rougher versions, respectively.
Distinguishing Sensory Differences
For a detailed chemical comparison of all three basic sherry types, see Zea et al. (2001). They differentiate the styles as follows. Finos were distinguished by floral and fruity notes, apparently derived from farnesol, β-citronellol and β-ionone, as well as the presence of cheesy/rancid (butanoic acid), and pungent/apple (acetaldehyde) attributes. The acetaldehyde concentration is often in the 350 to 450 mg/L range. Additional aromatics, at significantly above threshold concentration, were 1,1-diethoxyethane (fresh, fruity, green aromatic aspects) and sotolon (nut and curry notes). In contrast, oloroso profiles were characterized by ethereal and smoky aspects, for example ethyl acetate, and 4-ethylguaiacol, respectively. The latter probably has its origin as a lignin breakdown product, from the oak in which the wine was matured. Amontillados were differentiated by possessing flavor characteristics supplied by both flor (biological) and oxidative aging processes. Consequently, they had the most complex sensory attributes of the three. Most of these attributes develop early during maturation, but appear to continue accentuating, but at a slower rate.
Sweetening and Color Wines
Sweetening is typically achieved by adding one of two special sweetening wines, PX or mistela, although variations are occasionally used (Reader and Dominguez, 2003). PX is juice extracted from sun-dried berries (soleo) of Pedro Ximénez. Extended exposure to sun favors the dehydration of fructose to HMF (5-hydroxymethylfurfural), and the formation of significant accumulations of several henenols, henenals, phenolic alcohols, lactones and other furfurals (Franco et al., 2004). Because the increase in sugar content does not necessarily correlate with the development of the desired flavors, an electronic (e-) nose combined with gas chromatographic analysis has been used to facilitate rapid assessment of the optimal exposure period (Lopez de Lerma et al., 2012). To improve juice extraction, pectinases may be added to the must before pressing. This is reported to improve the product’s sensory quality (Espejo and Armada, 2010). The juice, possessing a marked raisiny flavor, is fortified to about 9% alcohol, allowed to settle, and aged for several months. After racking, the alcohol content is raised to about 18%, and the PX placed in special soleras for further maturation. At maturity, PX is dark in color, possesses about 40% sugar, and is typically used in the formulation of cream sherries.
In contrast, mistela is produced from Palomino grapes, the principal variety used in sherry production. The free-run juice and first pressing are initially fortified to about 15% alcohol, allowed to settle, racked, and fined. The product is subsequently raised to about 17–18% alcohol, and aged in casks or tanks. Mistela is not fractionally blended through a solera system. It generally contains about 16% sugar.
Alternatively, sweetening may be derived from the addition of color wine. This is normally obtained from the second pressings of Palomino grapes. Boiling brings the volume down to approximately one-fifth of its original. The froth that forms during boiling is periodically skimmed off. The product, called arrope, is a thick, dark, highly caramelized, 70% sugar solution. Addition of arrope to fermenting Palomino juice successively slows the rate of fermentation after each addition, until fermentation ceases. It often possesses an alcohol strength of about 8% and contains about 22% sugar. The wine may be raised to about 15% alcohol and be solera-aged. Alternatively, arrope may be added at the end of fermentation (one part arrope to two parts wine). The end product in either case is called vino de color.
European Sherry-Like Wines
The major source of sherry-like wines, other than Jerez, is Montilla-Moriles. It lies about 160 km northeast of Jerez. Its wines were once transported to Jerez for maturation and used in the production of Jerez sherry. This practice is no longer permitted. In Montilla-Moriles, Pedro Ximénez is the predominant cultivar. Grapes of this variety can, without solar drying, yield wines of up to 15.5 to 16% alcohol. Thus, flor tends to develop spontaneously, without fortification.
Fino sherries are produced from a combination of free-run and first press-run fractions. Oloroso sherries are produced from free-run juice plus several press fractions. Fermentation traditionally occurs in large earthenware vessels termed tinajas. These possess capacities between 6000 and 9000 liters. They resemble storage vessels (pithoi), used by the ancient Greeks and Romans. The wines are solera-aged, in a procedure analogous to that used in Jerez.
Small amounts of solera-aged sweet wine are also produced in Málaga, about 180 km east of Jerez. Most Málaga wine is produced without solera aging. Those winemakers who use fractional blending employ fermentation procedures distinct from those practiced in Jerez and Montilla. Pedro Ximénez and Moscatel grapes are harvested at about 23 to 25°Brix. They are then placed on mats and frequently turned to speed drying and overripening in the sun. This is the same as the procedure formerly common in Jerez. The grapes are covered at night to prevent the formation of dew on the surface. Both features reduce the incidence of fungal infection. The process takes 7 to 10 days, in temperatures that often reach above 40 °C. Details on chemical changes that occur during drying can be found in Ruiz et al. (2010). The most marked is an increase in acetoin content.
After a short maceration period, the grapes are pressed. Juice, fortified to 7% alcohol, may be added before fermentation. Fermentation is slow and often incomplete. The resulting wine may have an alcohol level of 15–16%, and a residual sugar content of 160–200 g/L. The wine may be further sweetened with PX and mistela. Solera aging, when employed, occurs without the interaction of flor, in a manner similar to that of an oloroso. The wine may be colored with sancocho, a product possessing a specific gravity of about 1.24, and similar to the color wine of Jerez. Because sancocho is concentrated to only one-third of the original volume, it is lighter in color and less caramelized than color wine. It is added slowly to the fermenting must to supply a distinctive arrope flavor and color. The brownish color comes from Maillard-generated pigments (melanoidins) (Rivero-Pérez et al., 2002). If aged in an oxidative solera system, the wine develops a complex flavor and an astringent aftertaste.
European sherry-like wines, produced outside Spain, include Vernaccia di Oristano and Malvasia di Bosa (Sardinia), as well as vin jaune (Jura, France). The Sardinian wines are flor-matured wines, made from fully mature grapes of the Vernaccia and Malvasia cultivars, respectively. Natural ripening on the vine commonly produces grapes with sufficient sugar content to yield wines of more than 15% alcohol. Thus, the wines often need no fortification to favor flor development.
Vin jaune is usually produced from the Savagnin cultivar, a mild-flavored strain of Traminer. The grapes are harvested late and allowed to dry for several months to develop a high sugar content. The wine is barrel-aged for slightly more than 6 years, without racking (Dos Santos et al., 2000). Flor yeast development occurs spontaneously, or following specific inoculation. Like some other sherry-like wines, its development occurs without fortification. The dry wine, often over 13% ethanol, is stored in tightly bunged barrels, after the completion of malolactic fermentation. Without fractional blending, velum completeness tends to decrease with time. Velum coverage also depends on the cellar temperature. This can vary from 5 °C in winter to 17 °C during the summer. Often a thick lees layer forms on the bottom of the barrel. Yeast autolysis is presumably important to renewed velum development as temperatures warm in the spring. As with other similar wines, the acetaldehyde content rises during aging. It can occasionally reach 600 to 700 mg/L (Pham et al., 1995). Similarly, vin jaune is characterized by the presence of sotolon, at values well above threshold. Traces of diethoxy-1-ethane also appear typical. Additional significant aromatics, recently detected, are abhexon and theaspirane-derived compounds (Collin et al., 2012).
Non-European Sherry-Like Wines
Solera-Aged Sherries
Production of solera-aged wine, similar to that practiced in Spain, is uncommon in the New World. The expense of fractional blending and the prolonged maturation undoubtedly explain this situation. Up to 10 times the volume of wine may be maturing as sold each year using fractional blending.
South African sherries are produced with a form of solera blending, but the details are quite different from those in Spain. Palomino and Chenin blanc (Steen) are the varieties normally used. The juice is inoculated with selected yeast strains, chosen for their fermentation and film-forming habits.
Wine designed to become flor-matured sherries are initially fortified to 15–15.5% alcohol. They are placed, without clarification, in 450 liter butts for 2–4 years. A 10% ullage provides surface for flor development. After the initial maturation, storage of both lees and wine occurs in casks containing about 1500 liters. There are generally two criaderas and one solera stage. Each stage has only one or two casks. Consequently, little blending occurs between transfers. Wine is generally drawn off in 450-liter lots, equivalent to the contents of añada barrels. Due to the proportionally higher evaporation of water through the wood, the alcohol content reaches a level that inhibits flor activity. The wine generated is apparently intermediate in character between a fino and an amontillado.
Wines intended for oloroso production are fortified to about 17% alcohol after fermentation. Subsequent storage occurs for about 10 years in butts without fractional blending. Sweetening mistela, derived from Palomino or Chenin blanc juice, is also fortified to 17% alcohol and matured for upward of 10 years in oak casks. Color wines are produced from arrope, blended into young sherry, and stored in butts for prolonged periods.
In Australia, flor sherries are seldom fractionally blended. After fortification, the wine may be inoculated with a film-forming yeast and matured for upward of 2 years in barrels (~275 liters), or cement tanks (~1000 liters). When the desired flor character has been reached, the wine is fortified to 18 to 19% alcohol. Further maturation occurs in oak for 1–3 years.
Submerged-Culture Sherries
A biological aging procedure, markedly different from that based on the Spanish model, has been pioneered in Australia, California, and Canada. It involves a submerged- culture technique. Respiratory growth of the flor yeasts is maintained with agitation and aeration.
The base wine is fortified to about 15% alcohol, and inoculated with an acclimated culture of flor yeast. Optimal growth conditions include a pH of about 3.2, a temperature of 15 °C, and SO2 contents close to 100 mg/L. Oxygen is provided by bubbling filtered air or oxygen through the wine. The use of porcelain sparging bulbs finely disperses the gas, improving oxygen adsorption, while minimizing the loss of aldehydes and other aromatics. The yeasts are kept suspended and highly dispersed by mechanical agitation. The process has the advantage of rapidly producing high levels of acetaldehyde (Fig. 9.40). By adjusting the duration of yeast action, slightly (~200 mg/L) to heavily (>1000 mg/L) aldehydic wines can be obtained.
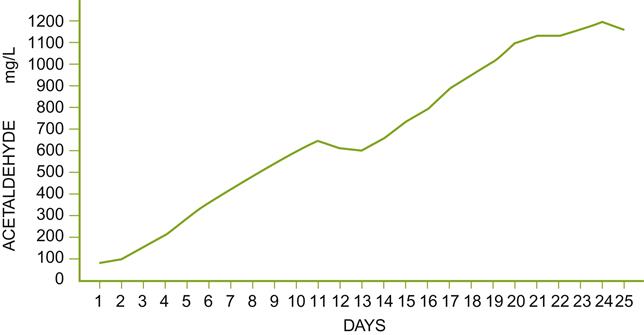
After flor treatment, fortification with relatively neutral spirits raises the alcohol content to 17 to 19%. Fortification appears to intensify the flor character. Because the wine generally lacks the complexity and finesse of solera-aged wines, it is customarily used to enhance the complexity of baked sherries (see below), rather than used independently as a beverage. The lack of finesse may result from the absence of the reductive phase that occurs under the flor growth, plus products released during yeast autolysis.
Baked Sherries
Baking has been the most common technique for producing sherries in Canada and the United States. It involves a process that more resembles the production of madeira than it does Jerez sherry. Not surprisingly, the resulting wines more resemble madeira than Jerez sherry or at least used to.
Varieties that oxidize fairly readily are preferred in the production of baked sherries. In eastern North America, the variety Niagara has routinely been used, whereas in California, varieties such as Thompson seedless, Palomino, and Tokay, have typically been used. Both white and red grape varieties may be used. Baking destroys the original color of the wine. Posson (1981) recommends juice possessing a pH no higher than 3.4 for submerged-culture sherries, whereas pH values between 3.4 and 3.6 are suggested for baked sherries.
Slow baking occurs when barrels of wine are exposed to the sun. More rapid and controlled baking is achieved in artificially heated rooms. Heating coils may also be inserted directly into wine-storage tanks. Heating is variously provided by passing steam or hot water through the coils. California winemakers are reported to prefer baking at 49 °C for 4 weeks, rather than the former 10 weeks at 60 °C (Posson, 1981).
Heating induces the formation of a wide variety of oxidative and Maillard products, including furfurals, caramelization compounds, and melanoid by-products. Baking also promotes ethanol oxidation to acetaldehyde (Kundu et al., 1979). Air or oxygen gas may be bubbled through the heated wine to accelerate oxidation.
The desired level of baking may be measured chemically by the production of 5-(hydroxymethyl)-2-furaldehyde, or colorimetrically to assess the development of brown pigments. Nevertheless, final decisions are made based on sensory analysis.
After baking, especially by rapid heating, the wine requires maturation to lose some of the resulting strong flavors and rough mouth-feel. Maturation in oak is preferred, with used barrels being employed to avoid giving the wine an oaky attribute. Aging may last for from 6 months to more than 3 years.
Baked wines are always finished sweet. The sweetness may come from fortified grape juice added to a base wine. Alternatively, premature termination of fermentation (by fortification) can retain residual sweetness in the base wine.
Porto and Port-Like Wines
The beginnings of port development, or porto as it is called in Portugal, are unclear. Fortification may have been used by the end of the 1670s. However, this appears to have been to limit spoilage during transport and improve its acceptability, not to preserve sweetness by arresting fermentation early. The latter practice seems not to have become standard until the mid-nineteenth century, but did have earlier predecessors (see Younger, 1966; Unwin, 1991).
The premature termination of fermentation, achieved with the addition of brandy spirits, is essential to modern port production. The retention of a high sugar content, and the higher (fusel) alcohols supplied with the brandy spirits, give port two of its most distinguishing features. The brandy spirits also contribute esters (e.g., ethyl hexanoate, ethyl octanoate, ethyl decanoate) and terpenes (e.g., α-terpineol, linalool). These donate a fruity, balsamic, and spicy profile (Rogerson and de Freitas, 2002). In addition, brandy spirits are rich in aldehyde content, such as acetaldehyde, propionaldehyde, isovaleraldehyde, isobutyraldehyde, benzaldehyde (Pissarra et al., 2005). These not only influence the fragrance, but also contribute to color development in the young wine. They participate in the formation of alkyl-linked anthocyanin/tannin polymers. Subsequent aging and blending differentiate the various port styles.
Porto
Port is predominantly a red wine produced in the upper Douro Valley of northern Portugal. Although originating in the upper, mountainous reaches of the Douro, the wine is typically transported downriver to Oporto for maturation and bottling. These processes occur primarily in buildings called lodges, in Vila Nova de Gaia, at the mouth of the Douro River, across from Oporto. Its closeness to the sea provides a more stable climate, appropriate for wine maturation. A small amount of white port is also produced. Plate 9.18 illustrates the range in color that may be found in port.
Most port is not vintage dated. Producers blend samples from several vintages and localities to produce brand-named wines of consistent character. After maturation for 2–3 years in large oak cooperage (Plate 9.19), the wine may be bottled and sold as Ruby port. Blending small quantities of white port into a ruby port produces most inexpensive brands of Tawny ports. However, only long aging in oak produces high-quality Tawny port. During aging, the bright-red color fades to a tawny hue, and a mild, complex, oxidized character develops. Wines of superior quality, from a single vintage, bottled between the second and third year of maturation in cask, are designated Vintage port. After long bottle-aging, Vintage port develops a distinctive and exquisitely complex fragrance. What is essentially a ruby port, but from a single vintage, and aged in large-volume cooperage for 5 to 6 years, is designated Late-Bottled Vintage (LBV) port. It matures more rapidly than Vintage port, generates no sediment after bottling, and is considerably less expensive. A few single-estate (quinta) ports are produced, usually from a single vintage. Crusted ports are a blend of wine from different vintages, matured in oak cooperage longer than a Vintage, but intended to receive and benefit from aging in bottle. They are viewed as intermediate in properties between LBV, ready to consume upon purchase, and Vintage ports, benefitting from prolonged in-bottle aging. Like Vintage port, Crusted ports will develop a significant sediment in the bottle. Vintage-character ports are often produced from finer-quality Ruby ports, coming from the Cima Corgo region of the Douro (see Fig. 10.20).
Base Wine Production
Port wine can be produced from a diverse range of grape varieties. There are 28 red and 19 white cultivars authorized in the Douro. This diversity has advantage in a region that experiences marked diversity in climate over short distances, and extremes in annual variation in humidity, rainfall, and temperature. Formerly, white and red cultivars were interdispersed in the vineyard, the grapes being harvested, crushed, and vinified together. Currently, plantings are separated, permitting separate harvesting and vinification.
Touriga Nacional, Touriga Franca, Mourisco Tinto, Tinta Barroca, Tinta Cão, Tinta Francaisco, Tinta Roriz, and Bastardo, are among the principal red cultivars. Touriga Nacional is the most highly regarded cultivar, due to its intense fragrance. This presumably comes from its high, free terpenoid content, including α-terpineol, linalool, nerol, and geraniol, as well as the norisoprenoid, β-ionone (Guedes de Pinho et al., 2007; Oliviera et al., 2006). Surprisingly, it constitutes only a small portion of the varieties cultivated. This may change due to selection for resistance to physiological disorders that can severely reduce its yield. Touriga Franca, Tinta Roriz, and Tinta Barroca are the most commonly grown varieties, at about 25%, 12% and 12%, respectively. A comparison of some of the attributes of several of these cultivars is provided in Table 9.7. They possess the stable coloration, fruity aromas, and sugar content required to produce good quality port. Esgana Cão, Malvasia, and Verdelho are among the more preferred cultivars for white port.
Table 9.7
Enologic parameters of recommended red grape cultivars
| Parameter | Probable alcohol (v/v) | pH | Total polyphenols index | Anthocyanins (mg/L) | Dry extract (g/L) | Intensity of colour |
| Bastardo | 13.0 | 3.87 | 23.94 | 98 | 28.6 | 2.78 |
| Mourisco Tinto | 11.6 | 3.61 | 16.99 | 85 | 24.1 | 1.70 |
| Tinta Amarela | 12.7 | 3.46 | 36.4 | 429 | 28.7 | 11.0 |
| Tinta Francisca | 12.6 | 3.71 | 52.7 | 451 | 28.5 | 11.1 |
| Tinta Roriz | 11.8 | 3.65 | 48.71 | 453 | 27.0 | 8.64 |
| Tinto Cão | 12.7 | 3.75 | 52.33 | 500 | 28.8 | 10.1 |
| Touriga Nacional | 13.3 | 3.69 | 71.06 | 703 | 30.8 | 17.3 |
| Touriga Franca | 13.6 | 3.78 | 76.24 | 707 | 33.0 | 18.0 |
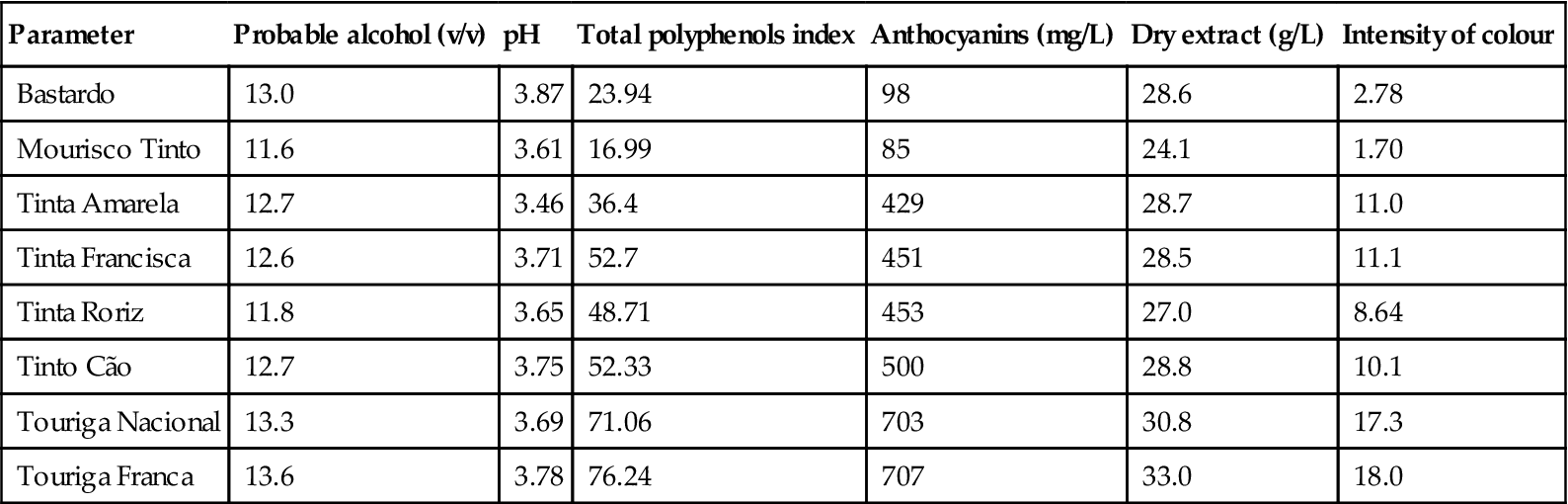
Source: From Moreira and Guedes de Pinho, 2011, adapted from Abade and Guerra, 2008, reproduced by permission.
Formerly, grapes were vinified on vineyard premises, in shallow stone vats called lagars. These granite troughs were approximately 3–6 m across and 60 cm deep. Currently, most wine is vinified by regional cooperatives, using modern crushing, pressing, and fermenting equipment. Autofermentors or other means are employed to promote early extraction of anthocyanins and tannins. Thermovinification may be used when the crop possesses more than the usual level of fungal infection. Little wine is currently produced by the old, traditional, foot-treading procedure.
As in most regions, inoculation of the must with a specific yeast strain is becoming standard practice. The primary exception is with treading, in which fermentation by the indigenous flora remains canonic.
A major problem in port production is extracting sufficient anthocyanins to provide an intensely red color before the fermenting must is fortified. Pigment extraction is largely dependent on the heat and ethanol generated during the truncated (2–3 day) fermentation period. Fermentation often occurs at temperatures between 26 and 28 °C. Because the ferment is separated from the pomace (pressed) when the sugar level falls to about 14.5°Brix, the opportunity for pigment extraction is short. Extraction is aided by extensive juice and pomace mixing during fermentation. Autofermentors achieve this automatically, in contrast with the arduous task of treading traditionally employed in lagar fermentation. Other procedures involve rotary fermentors, pneumatic plungers, and tanks possessing automatic pumping over (see Reader and Dominquez, 2003). Initially, sensorially significant differences may occur between traditional lagar and modern systems, but these differences tend to dissipate during maturation (e.g., Bakker et al., 1996). The use of deeply pigmented varieties, such as Sousão and Tinta Cão, and the addition of sulfur dioxide (100 mg/L), further help the extraction of sufficient anthocyanins.
Adding brandy spirits not only stops fermentation midstream, but it results in the wine retaining a high acetaldehyde content, present at this stage during fermentation (Fig. 7.21). Aldehydes can also come from the fortifying brandy spirits. They promote color stability, by favoring the production of anthocyanin–tannin polymers. This occurs, despite a drop in color depth (and redness), from a peak a few months after vinification. This change is initially associated with a decline in the concentration of acetaldehyde in the wine (Bakker and Timberlake, 1986). Subsequent shifts in color appear to be correlated with additional modification of anthocyanin structure, notably the formation of pyranoanthocyanins. The retention of a high residual sugar content also tends to mask the bitterness, but less so the astringency, of its tannin content.
Fortification typically occurs after the fermenting must is run off from the skins. A variable amount of press-run ferment, extracted from the wet pomace, is usually added to the free-run. Especially tannic fractions of press-run are fermented separately for distillation. The brandy spirits (aguardente) used have an alcohol content of about 77%. By the time fermentation has stopped, the must has dropped another 2°Brix. The result is a young wine possessing an alcohol content in the range of 19%, and a sugar content of between 9 and 10%. Figure 9.41 illustrates the relationship between the initial and final °Brix of a wine, fortified to 20.5% alcohol. Fortification prevents malolactic fermentation that, because of the high residual sugar content and pH, could favor the production of too much acetic acid. Tartaric acid addition is the standard method for pH adjustment.
The amount of brandy spirits required depends on the volume of the fermenting must, its alcohol content at fortification, the alcoholic strength of the brandy spirits, and the desired degree of fortification. The proportion of spirit to must can be determined from their respective alcoholic strengths (Joslyn and Amerine, 1964). The respective volumes are calculated by subtracting the alcoholic strength of the must being fortified (i.e., 8%) from the desired alcoholic strength (i.e., 18%). The corresponding must proportion is calculated by subtracting the desired alcoholic strength (i.e., 18%) from that of the fortifying spirit (i.e., 78%). In this example, 10 parts of the spirit (18−8=10) would be required per 60 parts (78−18=60) of the fermenting must to achieve the desired 18% alcohol.
The first press fraction from red port is fortified to the same level as the free-run. Each press fractions may be kept separate for independent aging, or combined immediately with the free-run. Addition of press fractions are typically required to achieve the desired concentration of anthocyanins and phenolic flavors.
Previously, white wines were fermented on the skins in a manner similar to that for red port. However, the trend is for a shortened maceration period. As with red ports, most white ports are fortified when half the original sugar content has been fermented. Semidry and dry white ports are fortified later, or when fermentation is complete.
The fortifying spirits (aguardente) are derived from distilled wine. Unlike most fortifying spirits, what is used in Portugal is not highly rectified. Consequently, it contains more higher alcohols and other flavorants than brandy sold for drinking. This feature gives Portuguese port part of its distinctive character. It is an attribute seldom present in non-Portuguese ports. The latter are customarily fortified with highly rectified (neutral) spirits, at about 96% alcohol. In addition, the wine distillate used to fortify port is, by comparison, relatively low in alcohol content (~77%).
To ensure the complete termination of fermentation, the wine is thoroughly mixed with the fortifying spirit. Storage occurs in wood or cement cooperage. The first racking usually occurs between November and March. Additional fortification brings the ethanol concentration up to 19–20.5%.
Transport to the lodges in Vila Nova de Gaia occurs the following spring. Here, the wine receives most of its maturation and blending. In contrast, most white port is matured in the upper Douro.
Maturation and Blending
Maturation occurs in large wooden (or cement) tanks, or oak casks of 600 to 650 liter capacity (pipes). The type and duration of maturation depend largely on the style intended. Racking may vary from quarterly to yearly. Slight fortification may be required after each racking. This maintains or brings the alcoholic strength to about 21%, and compensates for volume lost via evaporation from the cooperage.
The maturation of Tawny ports occurs in pipes, often left with a slight ullage. This enables development of a slightly oxidized character. In contrast, most other ports are initially aged in tanks (balseiros) of 10,000 to 100,000 liter capacity. This limits oxygen exposure and the incorporation of a significant oak-derived attribute. Ruby ports are not considered to benefit from reductive (in-bottle) aging. They are released shortly after bottling. In contrast, Vintage port receives prolonged in-bottle aging before release. They are also thought to benefit from even longer aging. They are often considered to begin reaching their optimum character after some 20 years. LBV ports may benefit from some in-bottle, reductive aging, but are usually considered near optimal shortly after bottling. Crusted ports are a blend of wines from different vintages, that are neither fined nor filtered, like Vintage port. They are matured in wood cooperage for 2 years and aged in-bottle for 3 years prior to release. The date noted on the label refers to the year in which it was bottled.
Because of the large number of producers in the Douro, blending of individual wines usually begins shortly after transfer to Oporto. As the character of each combination becomes more evident, further mixing reduces the number of blends to a more manageable figure. Blending during the first 2 years is usually confined to wine produced from a single vintage. Later, wines not used in one of the vintage- style ports may be combined fractionally with older, reserve blends. Several different reserve blends may be used in the preparation of the final blends for 2- or 3-year-old Ruby ports. Portions of these blends may become incorporated into reserve blends for subsequent years. Also involved in the preparation of the final blend may be optional amounts of sweeter or drier wines. The final blend is left to mature in oak cooperage for several months prior to fining, stabilization, and bottling.
Inexpensive Tawny ports are not necessarily older than Ruby ports, being produced from the lighter-colored Mourisco cultivar and aged at warmer temperatures. Alternatively, they may be derived from a mixture of Ruby and White ports. High quality, long-aged, Tawny ports are produced in a manner similar to Ruby port, but with extended maturation in pipes. White port is not added in the development of aged Tawny ports. With extensive maturation, bottles may indicate the minimum average age of the wine contained –10, 20, 30, or 40 plus years. Cohleita ports are Tawnies from a single vintage, matured in pipes for at least 7 years.
Most Ruby and Tawny ports are tartrate stabilized by rapid cooling, and holding the wine at−10 °C for approximately 2 weeks. The addition of Kieselsol to the cold wine before filtration helps yield a stably clear wine.
Vintage ports are not filtered before bottling. The thick sediment that forms is considered important in the development and aging potential of the wine.
Sweetening and Blending Wines
During racking, blending, and maturation, the sugar content of the port may decline. To bring the sugar content back to the desired level, special sweetening wines may be added. The main sweetening wine is called jeropiga. It is fortified to 20% alcohol when a cap begins to form on the fermenting must. Both white (branca) and reddish (loira) jeropigas are produced. Intensely red (tinta) jeropigas, produced with the addition of elderberry juice, are no longer produced or permitted. Juice concentrated under vacuum occasionally may be used for sweetening.
In addition, special wines may be used for coloration, in a process called repisa. After half the must is run off in the usual manner, the remaining must is treaded, or extensively pumped over to extract additional color.
Port-Like Wines
Many countries produce wines by techniques more or less similar to those used in Portugal. In only a few instances, though, are the wines serious international competitors to porto. Australian and South African ports are the primary alternatives.
In regions where intensely colored varieties are not grown, extracting sufficient pigmentation is a particularly serious problem. One solution is thermovinification. Various procedures have been used, including exposing the fruit to steam, plunging the fruit into boiling water, or heating the juice and pomace. Exposure to steam or boiling water is commonly used in Australia and eastern North America.
Occasionally, the must may be fermented dry before fortification. This improves pigment extraction, but can lead to excessive tannin extraction. Sweetening comes from must fortified shortly after fermentation has begun, similar to jeropiga production. Alternatively, a must concentrate may be used. If it is important that the end product resembles porto, avoiding prolonged heating during must concentration is essential. This is insignificant when baked port is produced.
Baking may take various forms, from storing wine in barrels on the top of wineries for months, to direct heating with oxygen sparging. The duration of baking is generally shorter than that used in producing baked sherries. Baking gives the wine a distinctive oxidized–caramelized bouquet.
Many cultivars are used in producing New World ports. Shiraz, Grenache, and Carignan have often been used in Australia. Hermitage (Cinsaut) and Portuguese varieties are commonly employed in South Africa. Carignan, Petite Sirah, and Zinfandel are typically used in the cooler regions of California, whereas Sousão, Rubired, and Royalty are preferred in hotter regions. Concord has customarily been used in the eastern parts of Canada and the United States.
Aromatic Character of Ports
The chemical nature of port fragrance has received comparatively little attention until recently. The common view is that the port-like bouquet comes from the combined effects of many compounds, not a single or a few unique aromatics (see Williams et al., 1983). As noted, higher alcohols derived from fortifying spirits are important in the distinctiveness of porto. Ports, given extensive wood-aging, show high concentrations of diethyl and other succinate esters. These may contribute to the basic port fragrance. Oak lactones (β-methyl-γ-octalactone isomers) and other oxygen heterocycles have also been isolated. Some of the latter are furan derivatives, such as dihydro-2-(3 H)-furanone. They may donate a sugary oxidized fragrance. However, the most distinctive in barrel-aged port appears to be sotolon (Silva Ferreira et al., 2003). It may impart a nutty aspect often detected in old Tawnies. The concentration of several norisoprenoids also increases during aging. Examples are β-ionone and β-damascenone in Vintage ports, and vitispirane, 2,2,6-trimethylcyclohexanone (TCH) and 1,1,6-trimethyl-1,2-dihydronapthalene (TDN) in Tawny ports (Silva Ferreira and Guedes de Pinho, 2004). Examples of some of these changes are noted in Fig. 9.42. Acetals, derived from glycerol and acetaldehyde, also appear to be involved in the flavor of old Tawny ports (Silva Ferreira et al., 2002). Esters of 2-phenylethanol may also contribute to the fruity, sweet fragrance of ports, as diacetyl may to its caramel aspect (Rogerson et al., 2001). Many acetals have been isolated from Tawny ports, but their participation in the oxidized character of the wine is unclear.
Madeira
Madeira wine comes from an island of the same name, some 640 km off the coast of North Africa, in the Atlantic Ocean. It is primarily characterized by its distinct baked bouquet, obtained by intentional heating (baking). Subsequent maturation occurs in wooden cooperage for several years.
Heat processing of wine has not been widely adopted in other parts of the world. Outside Madeira, it is most commonly used in North America, for the production of baked sherries and some ports. Not surprisingly, such wines resemble madeira more than Jerez sherries or porto.
Madeira wines are produced in an incredible range of styles. Some are very sweet, others almost dry. They range from versions produced from a single grape cultivar and vintage-dated, to those that are highly blended and carry only a brand name. Some are fractionally blended, using a solera-like system, others are not. Although the variations produce subtle differences in style and character, the predominant factor that distinguishes madeira from most other fortified wines is the exposure to heating, termed esteufagem.
Base Wine Production
Most madeiras are produced from white grapes. The preferred varieties are Malvasia, Sercial, Verdelho, and Bual. Several red cultivars are grown, notably Tinta Negra Mole. Terrantez, and Bastardo are still cultivated, but less so than in the past. Grapes from better sites and preferred cultivars are crushed, fermented, and stored separately to retain their distinctive attributes, at least until blending. Harvesting can begin as early as the end of August and end as late as mid-October.
Vinification procedures, except for the duration of fermentation, tend to be standard. Fermentation typically occurs in large cement fermentors, containing approximately 200–300 hL. Fermentation tends to develop spontaneously from indigenous yeasts. The duration of fermentation depends on the style intended. Very sweet madeiras, commonly called malmsey, are fortified shortly after the onset of fermentation. They are designed to retain a high residual sugar content (~120 g/L). They possess a dark color, with a rich coffee/caramel aspect. Medium sweet buals (boals) are fortified when about half the sugars have been fermented (~95 g/L residual sugars). Ideally, they should possess a dark color and express raisiny flavors. Verdelhos are often fortified at a point to retain about 70 g/L residual sugar content. They aim to possess high acidity and smoky flavors. Dry sercial styles are fermented near to 25–50 g residual sugars per liter before fortification. Fermentation to dryness may take upward of 4 weeks under cool winery conditions. Sercials have a profile characterized by lighter colors, refreshing acidity and almond flavors. Acidity and/or pH adjustment is rarely required, due to the high titratable acidity and a pH less than 3.5. This attribute helps avoid a cloying aspect to the sweetness of most styles. Cooling assures that fermentation temperatures do not rise above 26 °C. A partial chemical characterization of these styles is provided in Nogueira and Nascimento (1999). For a recent review, see Perestrelo et al. (2011).
In the past, the stylistic names were not necessarily identical to the varieties used in their production. This is no longer permitted. For much of the less expensive madeira, made primarily from Tinta Negra Mole, only stylistic terms such as dry, medium dry, medium sweet and sweet are permitted. Labels may also denote indications of age. Wines matured for at least 3, 5, 10, and 15 years can be designated as ‘superior,’ ‘reserve,’ ‘old reserve’ and ‘extra reserve,’ respectively. Designations such as ‘Rainwater’ imply a dry to medium dry, golden, high quality version, being at least 3 years of age.
Fortification involves the addition of neutral wine spirits (~96% alcohol). Sufficient spirits are added to raise the alcohol content to 14–18%. After fortification, clarification occurs (or occurred) with Spanish earth, a form of bentonite. At this point, the wine, called vinho claro, is ready for heat processing.
Heat Processing
If quantities permit, wine from different varieties are separately sealed in large capacity, coated, cement or stainless steel tanks. Smaller lots are placed in elongated wooden casks (charuto), or shorter casks (ponche) for heating. The size and type of cooperage appear to have little influence on the wine’s eventual attributes.
Formerly, heating was achieved by heating the storage room over a period of possibly 2 weeks (~5 °C per day), up to a maximum of 45–50 °C. Submerging rods, through which hot water (45–50 °C) is passed, or heating a double-jacketed stainless steel estufas, are now standard, more effective means of achieving the same goal. The wine is customarily exposed to the desired temperature for a minimum of 3 months. After ‘baking,’ the wine is slowly cooled to ambient. Cooling may be speeded by passing cold water through the same coils previously used to heat the wine. Additional heating, at a cooler temperature, may take place in wooden casks, positioned directly above heating rooms.
Alternatively, small lots of wine may be stored in butts, on the top floors of non-air-conditioned warehouses. In this location, the wine is exposed to annual cycles of heat and cold, for upwards of 8 or more years (minimum 2 years). This old technique is referred to as the canterio system. The system is preferred for the best lots of wine. Its slower, more gentle heating (generally only up to 30 to 35 °C) is less drastic, and always occurs in oak casks. Thus, it has more oak-derived attributes than typical for wines exposed to esteufagem.
Further Maturation
Fining removes most of the heavy brown sediment produced during heating. The use of charcoal achieves any additional decolorization deemed necessary. Any further maturation occurs in wooden cooperage of diverse capacities, for at least 3 months (estagio). Oak is standard, but other woods were used in the past, for example chestnut, satinwood, and mahogany. Addition of wine spirits supplies the alcohol lost during heating and brings the alcohol content back up to 18–19%.
Blending is used to achieve the desired house style(s). This can involve different lots from separate properties, reserve wines (as in port blending), as well as color and sweetening (surdo) wines (see below).
The wine cannot be released until the second year after the harvest. Small lots of wine from exceptional vintages may be matured in wood for at least 20 years. After a further 2 years in-bottle, the wine may be called Vintage madeira, and the year noted on the label. Such wines are commonly designated as garrafeira (or frasqueira) wines. In contrast, lower-quality madeiras are often matured for only 13 months before release. Much of this is reportedly used in making madeira sauce in cooking. Better-quality madeiras are matured for at least 5 years after esteufagem.
Baking, and especially extended maturation, appear to nullify many of the differences based on grape varietal origin (compare Figs. 9.43 and 9.44). In contrast, the gentler canterio process permits more of the varietal character to remain.
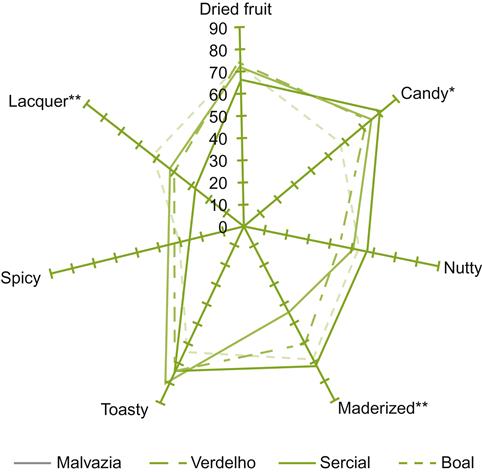
Sweetening and Blending Wines
Juice from grapes grown on the adjacent island of Porto Santo are commonly used to produce a special sweetening wine called surdo. The hotter climate of Porto Santo yields grapes with a higher sugar content than is typical for the main island. The juice is fortified shortly after it begins to ferment. Surdo is customarily heated, similar to madeira. Occasionally, however, some may be left unheated. This leaves the surdo with a fresh, fruity flavor, useful in producing certain proprietary blends. Fortified juice, without fermentation, is called abafado.
Coloring wine is produced from must heat- concentrated to about one-third of its original volume. It is dark, possessing a distinct caramelized fragrance.
Blending
Wines from different vintages and varieties are usually kept separate, at least during the first 2 years of wood maturation. Subsequently, producers begin the process of blending. Further maturation and combination eventually produce the final blend. Surdo and coloring wines are added to madeira if considered beneficial.
Chemical Nature of the Bouquet
During esteufagem, oxidative processes produce a series of aldehydes, notably acetaldehyde and acetals. However, heterocyclic acetal (cis- and trans-isomers of dioxanes and dioxolanes) production appears to be unaffected by heating (Câmara et al., 2003). Their synthesis is correlated primarily with wine age. A similar finding was found with the synthesis of sotolon (Câmara et al., 2004). As expected, heating promotes the breakdown of sugars, notably to furfurals. 5-Ethoxymethyl-2-furfural (derived from 5-hydroxymethyl-2-furfural and 2-furfural) is apparently important in the sweet aroma of madeira wines (Câmara et al., 2006). Dark pigments are derived from Maillard processes. Additional details can be found in Câmara et al. (2007) and Campo et al. (2006). These studies have been used, in combination with sensory evaluation, to investigate the optimum temperature-duration conditions for madeira development (Silva et al., 2008).
As a result of the wine’s acidity, fortification, oxidation, and heating, madeira has probably the longest aging potential of any wine. Even upon opening, it may not detectably lose its desired sensory attributes for months. The wines are considered to be characterized sensorially by aspects resembling combinations of nuts, dried fruit, toast, brown sugar, and mushrooms (Alves et al., 2005; Silva et al., 2008). Regrettably, the epithet madeirized is too often used pejoratively for all sweet, fortified, golden to brownish, white wines, in the same sense as rancio has been employed against many sweet, fortified, red wines.
Vermouth
Wine has been flavored with aromatic and bitter botanicals, probably for millennia, but certainly since ancient Greek and Roman times. However, the production of a specific, herb/spice-flavored, fortified wine, now known as vermouth, began being formulated and commercialized in Italy only in the later 1700s. The German name for one of the original ingredients, wormwood (Artemisia absinthium) – wermut, is the likely etymological origin of the term vermouth.
All vermouths have a bitter aspect, partially mollified by sweetening. In world commerce, these are generally subdivided into two broad categories: the sweeter Italian and dryer French styles. Italian vermouths are usually about 16–18% alcohol and may contain up to 4–16% sugar, for dry and sweet versions, respectively. French vermouths typically contain 18% alcohol and 4% sugar. The sweetening may come from the addition of mistelle – grape juice to which ethanol has been added to bring its alcohol content up to about 18 to 22%. As a beverage, they are taken straight, as an aperitif, or used as a base for various cocktails.
The base wine is often a neutral-flavored white wine, although in Italy, the best vermouths are (or were) produced from the aromatic Muscato bianco variety grown in Piedmont. White cultivars that may be used are Trebbiano, French Colombard, and Thompson Seedless. Red cultivars, such as Tokay and Baco Noir may also be used, depending on availability and preferences of the region. In various countries, other fruits may be fermented to produce a base product for vermouth production (Panesar et al., 2011).
Vinification follows standard procedures. For sweetening, a sugar syrup or grape juice is fortified with wine spirits or brandy. In the past, a distinct red color was derived from the addition of cochineal (an extract from the insect Dactylopius coccus). This is apparently no longer permitted – the amber color of red vermouths coming from the addition of caramel.
Upward of 50 herbs and spices may be used in flavoring vermouths. How they are produced, dried, ground (if done), and stored can significantly affect the intensity and quality of the flavors they donate. The types and quantities employed in any particular commercial brand are usually a proprietary secret. Nonetheless, the range of botanicals (barks, roots, leaves, flowers, seeds) is known. Examples of some of the more common herbs and spices that may be employed include allspice, angelica, anise, bitter almond, cinnamon, chinchona, clove, coriander, dittany of Crete, juniper, marjoram, nutmeg, orange peel, rhubarb, summer savory, and wormwood. A discussion of the plants that have been, or are being, used for vermouth flavoring can be found in Panesar et al. (2011).
For the production of Italian vermouths, extracts are prepared by soaking (macerating) the herbs and spices (7–11 g/L) in highly rectified alcohol (~85%). The botanicals may receive preliminary exposure to hot water. Occasionally, due to the requirements of individual ingredients, they are soaked separately, or in select groups. Heating may also be part of the extraction process to facilitate flavor release. If a darker tawny color is preferred, after the addition of the flavor extracts, caramel may be added. Once prepared, the formulated extract is added to the base wine. In France, extraction usually involves soaking a smaller quantity of the herb and spice mixture (4–8 g/L) directly in the base wine, following fortification. To avoid the uptake of undesired herbaceous flavors, extraction usually lasts little more than 1–2 weeks. Periodic mixing speeds extraction. Distillation may be employed to obtain selected essences, although the milder technique of supercritical fluid extraction (SFE) may soon take its place.
Because of the proprietary nature and compositional complexity of vermouths, no general discussion of the chemical nature of their flavor characteristics is possible. However, for carvacrol-type vermouths, their dominant aromatic attribute comes from the monoterpenoid phenol, carvacrol, a major flavorant in summer savory (Satureja hortenis), as well as flavorants from Crete, a type of oregano (Origanum dictamus). In addition, the presence of thujones and artemisia ketone indicates the use of Artemisia species (Tonutti and Liddle, 2010). The bitter attributes of vermouths can be variously ascribed to alkaloids (e.g., Chinchona), anthroquinones (e.g., aloes and/or rhubarb), quassinoids (Quassia bark), and secoiridoids (Gentiana).
After flavorant incorporation, the wine is aged for 4–6 months, but this can last for years for special styles. For this, maturation tends to occur in oak cooperage. During maturation, the components ‘marry.’ Finally, the vermouth is fined, cold-stabilized (at−10 °C for 10 days) and filtered. Before bottling, the wine may be sterile-filtered or pasteurized.
Brandy
Brandy is distilled wine aged in small oak cooperage. It is produced in most winemaking regions, but is best known from two versions produced in the southwest of France – cognac from Charentes and armagnac from Gascony. Similar beverages, termed eau-de-vie de vin, are produced elsewhere in France.
A distilled product, of similar nature, may be derived from the pomace remains of wine pressings, to which water, and occasionally sugar, have been added to promote fermentation. It is stored under anaerobic (ensilage) conditions, until the sugars are fermented (Da Porto, 1998). Distillation follows procedures typical of most brandies, the major difference being the extra attention required to keep the methanol content within legal limits (due to the high pectin content of the pomace). Maturation is typically shorter than for most brandies, and often does not include maturation in wood. If employed, a much wider variety of cooperage woods have been used. The product is sold under a bewildering cornucopia of vernacular names. Examples include eau-de-vie de marc (France), grappa (Italy), orujo (Spain), aguardente bagaceira (Portugal), tsipouro (Greece), and zivania (Cyprus).
Formerly, pomace was used to produce a wine (piquette) for vineyard laborers. It was often considered part of their pay. With the modern shift to mechanization, pomace became a residue, available for producing eau-de-vie in its various national guises. Otherwise, it was (is) simply a disposal by-product of wine production.
In comparison with wine, brandy and other distilled spirits have a relatively short history. Arnaud de Villeneuve (Catalonia, Spain) is reported to have distilled wine as early as 1250 (Léauté, 1990). It may also have been produced as early as 1100 in Dalerno, Italy. However, its first commercial applications appear to have been in the distillation of flower oils for perfumes and cosmetics (Forbes, 1948, p. 91), or herbal infusions combined with wine as medicine. Some of the latter evolved into modern aperitifs and liqueurs, for example Benedictine (~30 botanicals) and Chartreuse (~130 botanicals).
Distillation of alcoholic beverages in Europe began in earnest only in the 1500s, with some commercially distilled wine produced in Gascony in the mid-1400s (Bertrand, 2003b). Brandy production in the region of Charentes started in the early 1600s. It was principally produced by Dutch importers in the Netherlands, desiring to produce a more stable product for the home market. However, they soon switched to encouraging its production in Charentes and Gascony, it being more economic to transport the brandy than their base wines. Both Gascony and Charentes produced cheap, low-quality wine, ideal for distillation. The regions also had ample, readily available supplies of wood for still operation (Unwin, 1991). The involvement of the Dutch, and their name for the product, brandwijn (brunt wine), is reflected in the English term, brandy. Eventually, the adoption of refined distillation practices, extended maturation in oak and extensive blending, softened the rough edges of the raw brandy, donating the aromatic finesse that now characterizes commercial brands.
By the late 1500s, distilling wine was practiced fairly widely in Europe. Besides its use to produce an aqua vitae, it was occasionally added to wine. Gesner (1565) recommends it to prevent wine spoilage, though he considers sulfur better in this regard. Subsequently, it was suggested by Estienne and Liebavlt (1616) to improve wine flavor. However, these suggestions appear to have been for domestic use, by shippers, or tavern owners. When its addition became standard practice in the preparation of modern sherries, ports, and madeiras is unclear, but appears to have occurred considerably later.
Up until the 1500s, stills were principally used in Europe by alchemists, in their ill-fated attempts to transmute base metals into gold or other precious metals. It was also used on a limited scale to produce aqua vitae (from wine) as a medicine (Forbes, 1948). The primitive stills were themselves derived from models used, if not developed, by Greco-Egyptians in Hellenistic Alexandria (primarily used in alchemy and to concentrate aromatic plant oils). Illustrations of some of these devices, from ancient manuscripts, are reproduced in Holmyard (1956). The head of the device was called an ambix (Gr.). Eventually, the term came to be applied to the whole device. The term, alembic, for the classic type of still, came into English indirectly, via its Arabic derivative, al-anbïq.
Base Wine Production
Production of the base wine follows procedures standard for most white wines. Typically, nonaromatic white grape varieties are preferred. In Charentes, the predominant cultivar employed for cognac production is Trebbiano (termed Ugni blanc in France), whereas in Gascony, Baco 22A and Ugni blanc are the standard white cultivars grown for armagnac production. Some Folle blanche, Colombard, and a few other white cultivars are also used. Both standard varieties benefit from being relatively resistant to fungal disease. Baco 22A has the additional advantage of not requiring grafting to avoid problems associated with phylloxera. Nonetheless, its cultivation is scheduled to end, to conform with a regulation excluding French-American hybrid usage in Europe.
That these cultivars mature late has several advantages. It minimizes aroma development; limits disease development; retains high acidity (6–10 g/L); curtails ethanol production (8–11.5%, depending on the region); and facilitates mechanical harvesting (now standard). Some of these attributes, undesirable for table wine production, are ideal for brandy production. For example, high yield is not viewed negatively. It partially compensates for the conversion rate of about 5 liters of base wine to 1 liter of finished brandy.
In California, cultivars with little distinctive varietal character, such as Chenin blanc, Folle blanche, French Colombard, Palomino, and Thompson Seedless are typically used. In South Africa, Colombard and Chenin blanc are preferred (van Jaarsveld et al., 2005). Nevertheless, there is growing interest in other cultivars. These permit the production of brandies that possess a distinct regional (varietal) character. For example, Gewürztraminer donates rose and tea-like notes; Chardonnay and Riesling supply noticeable floral aromas; and Muscat provides its distinctive muscat fragrance (Versini et al., 1993). The latter is used predominantly in the production of pisco, a distinctive brandy from Chile (Bordeu et al., 2012). Short maceration periods avoid the uptake of pectinaceous material from the grapes. On enzymatic breakdown, pectins release methanol. It is concentrated during distillation.
Pressing is gentle to avoid the excessive uptake of hexanols, and to limit the uptake of a high solids content. Hexanols donate an undesirable ‘green’ aspect to brandy, and high levels of suspended solids leads to undesirable higher alcohol contents. In spontaneous fermentations, several yeasts are involved, with Saccharomyces cerevisiae initially in the minority (Versavaud et al., 1993). However, it soon dominates spontaneous fermentations. As in most modern fermentations, the tendency is to inoculate with specific strains. They can be chosen to minimize the production of undesired odors that could become concentrated in the distillate. In this regard, Saccharomyces bayanus may be selected as their flavor attributes seem particularly appropriate for brandy production. Appropriate strains are those that produce desirable flavorants, such as 2-phenylethanol, but synthesize limited amounts of acetic acid and sulfur dioxide (Riponi et al., 1997).
Neutral-flavored grape varieties are usually chosen to avoid concentrating varietal aromas that could mask traditional brandy flavors. The retention of high acidity minimizes grape infection (and associated off-odors, such as acetic acid or moldy aspects), and obviates the need for sulfur dioxide addition. Consequently, the synthesis of acetaldehyde and hydrogen sulfide is minimized. The addition of sulfur dioxide to the must is avoided, and added to wine only if considered absolutely necessary. Even here, it is held to≤2 mg/L, because of its corrosive action on stills. Fermentation typically is preferred to occur at about 22 °C. Racking off the yeast is uncommon during maturation.
Distillation
Distillation preferably occurs shortly after the completion of fermentation. If distillation is delayed, the wine is stored at a cool temperature. This helps limit the loss of fruit-smelling ethyl and acetate esters (Cantagrel, 2003), reduces the accumulation of ethyl acetate and acetals, and retards both oxidation and microbial spoilage (a risk in the absence of sulfur dioxide). Although accentuating some flavors, such as chocolate and caramel, malolactic fermentation may also donate solvent off-odors (du Plessis et al., 2004), and diminish the fruit ester content. Consequently, malolactic fermentation is generally discouraged, either by cold storage, or the addition of lysozyme. If malolactic fermentation does occur, distillation is delayed until it comes to completion.
Depending on the desires of the producer, a fraction of the lees is included with the wine for distillation. This is uncommon in armagnac production. Lees are the primary source of long-chain, fatty acid esters (Table 9.8). These high-molecular-weight esters, closely bound to the yeast cell membrane, are believed to contribute to the fruitiness of cognacs. Fatty acid esters also act as fixing agents, retaining other aromatic compounds. Lees also increase the presence of fragrant, amino acid, degradation products.
Table 9.8
Effect of lees on ester content of brandy distilled in an Alambic still (mg/liter spirit at 70% alcohol)
| Distillation | ||
| Constituents | With few lees | With lees |
| Ethyl caproate | 6.76 | 8.3 |
| Ethyl caprylate | 8.95 | 23.6 |
| Ethyl caprate | 13.8 | 63.0 |
| Ethyl laurate | 12.45 | 36.2 |
| Ethyl myristate | 5.4 | 9.8 |
| Ethyl palmitate | 9.77 | 13.2 |
| Ethyl palmitoleate | 1.44 | 1.8 |
| Ethyl stearate | 0.59 | 0.61 |
| Ethyl oleate | 1.19 | 1.22 |
| Ethyl linoleate | 7.69 | 9.52 |
| Ethyl linolenate | 1.86 | 2.58 |
| Isoamyl caprylate | 0.42 | 2.48 |
| Isoamyl caprate | 1.67 | 5.76 |
| Isoamyl laurate | 0.78 | 1.83 |
| 2-Phenylethyl caprylate | Trace | 1.20 |
| 2-Phenylethyl caprate | 0.25 | 1.55 |
| Total aromatic esters | 73.02 | 182.65 (=+150%) |
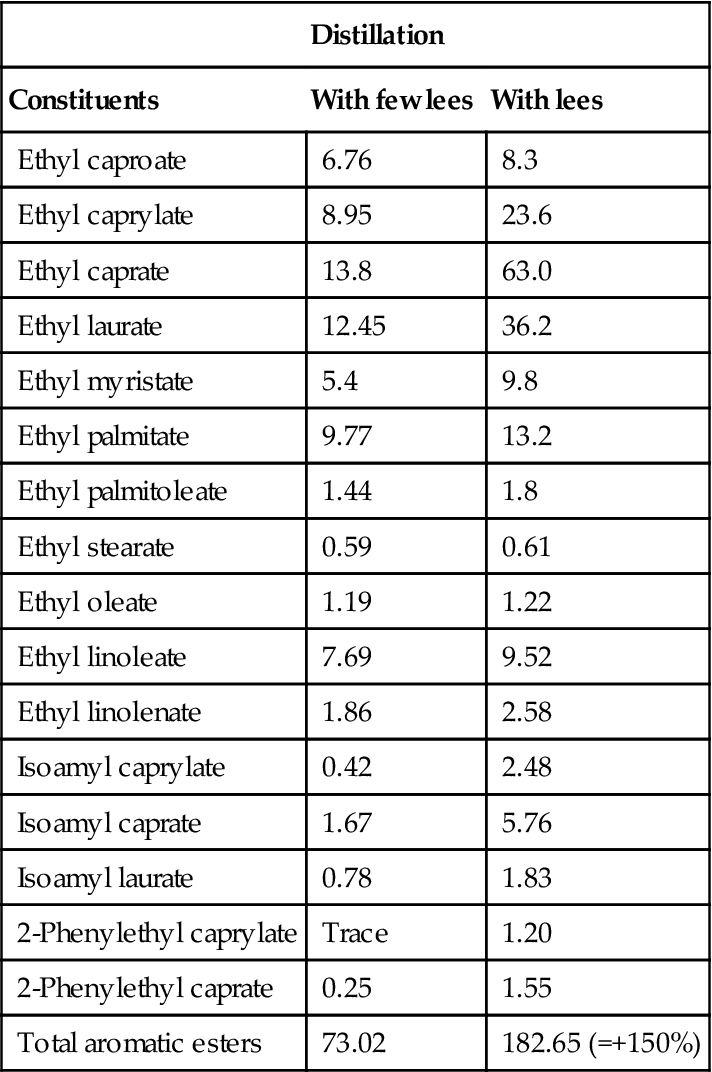
Source: From Cantagrel and Vidal 1993, reproduced by permission.
During heating, aromatic constituents vaporize at different rates and times during distillation. Volatilization depends on the individual vapor pressures of the various constituents, variations in their relative solubility in the two main solvents (water and alcohol), and the dynamics of the wine’s changing composition throughout distillation (primarily, but not exclusively the alcohol/water ratio) (Léauté, 1990). Subsequently, the compounds selectively condense as the vapors cool. Depending on how the still is operated, various distillate fractions are collected, retaining the specific composition of each fraction. The result is a crude chromatographic separation and concentration (and elimination) of wine constituents, plus the synthesis of heat-generated compounds.
In most areas, speed and economic efficiency favor the use of continuous stills. The major disadvantage of most continuous stills is limited control over separation of the various vaporized constituents. Thus, depending on how they are run, their distillates may include more wine flavorants and fusel alcohols. Batch (pot or alembic) stills permit more precise separation, and the selective inclusion (or exclusion) of particular ingredients from the distillate. Their main drawbacks are that the course of distillation is prolonged, the process is considerably less energy efficient, and requires cleaning between batches.
Continuous stills come in a variety of forms, possessing one to several columns. Here, only split- (two) column stills are described. In split stills, wine flows down a spiral tube. It coils extensively in the first (rectifying) column. During its descent, the wine is heated by hot distillate vapors ascending the column, after escaping from the second (analyzer) column. As the heated wine from the first column is pumped up and released into the top of the second (analyzer) column, it flows down and through a set of perforated plates. Superheated steam ascending in the analyzer column vaporizes the incoming, preheated wine. The vapors are directed into the rectifying column, where it is released at its bottom. As the vapors rise, they are cooled, as heat is transferred to fresh wine flowing down, through the coils in the column. Compounds with very low vapor pressures (such as acetaldehyde) are allowed to escape through the top of the column. Ethanol and most aromatics condense in various layers and are separated off near the top of the rectifying column. Compounds with high vapor pressures tend to collect near the bottom of the column. They are collected and a portion incorporated with new wine entering the still for redistillation. The retained portions constitute the nascent brandy. It is placed in barrels for the initiation of the maturation phase.
In contrast, the operation of a batch (pot) still is more complicated. Constituents are separated both by initial volatilization as well as condensation dynamics. In addition, the heating involved generates pyrolytic by-products not generated in continuous stills. Pot still use is often considered more an art than a science (Léauté, 1990). Their use in brandy production also usually involves double distillation. The alembic still, employed in the production of cognac, is typical of its type (Prulho, 1993; Fig. 9.45). The boiler is usually onion shaped, with a slightly convex base. They usually have a capacity of up to 30 hL. In the first distillation, the boiler is partially filled, and the base heated over an open flame. The wine reaches a temperature of between 90 °C and 102 °C in about 90 min. The vapors pass into the reflux cap (chapiteau) at the top of the still. The chapiteau has a volume equivalent to about 10–12% of the boiler capacity. Some of the vapors condense here, and return to the boiler for redistillation. The chapiteau is about 5–8 °C cooler than the boiler. The rest of the vapors pass through a neck to a progressively narrower tubular helix (serpentine). It descends through a water-cooled condenser. Cold water, pumped up from the bottom, cools the hot gases in the serpentine, exiting at the top. The distillate is filtered, and its temperature, alcohol and volatile content assessed, as it is collected in separate fractions at the end of the serpentine (hydrometer port). Initially, the upper part of the condenser tube passes through a preheater, where additional wine samples are heated to about 50 °C, before being added to the boiler. Boiling continues until the ethanol content of the distillate (tails) approaches 0%.
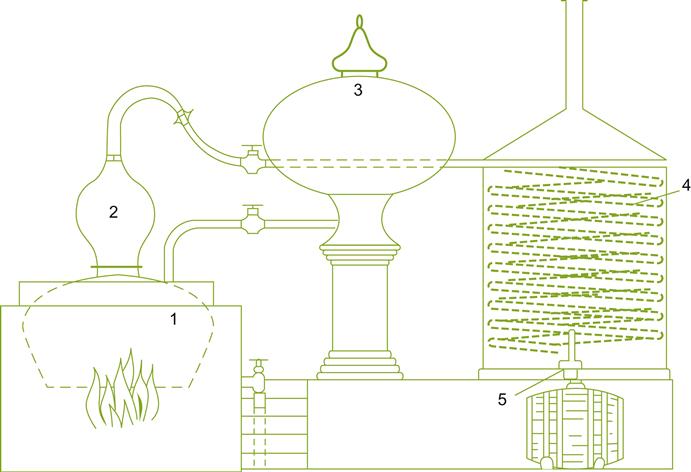
The condensate, termed the brouillis, is usually separated into portions. The first fraction that condenses (~4%) is enriched in acetaldehyde and ethyl acetate. It is termed the heads, and is often added to the main portion of the distillate for redistillation. Other highly volatile aromatics, such as ethyl caproate, ethyl caprate, and isoamyl acetate collect primarily in the early portions of the brouillis. Constituents with higher boiling points accumulate in later fractions of the brouillis. Examples are methanol and higher alcohols (1-propanol, isobutanol, methyl-2-butanol and methyl-3-butanol). Near the middle and end of distillation, compounds of lower volatility accumulate, notably ethyl lactate, diethyl succinate, acetic acid, and 2-phenylethanol (see Léauté, 1990). Terpenes tend to concentrate at the end of the brouillis, and in the tails fractions. Nonetheless, some such as geraniol and linalool may distill off early in the heads (Versini et al., 1993). Figure 9.46 provides details on the collection sequence of some chemical groups during condensation. The major portion of the brouillis is combined with similar fractions from other distillations for redistillation. Depending on the preferences of the distiller, the last portion (about the last 17%) and concentrated in furfurals, may or may not be added to wine about to be distilled, similarly to the heads fractions. The initial distillation phase often takes about 10 h.
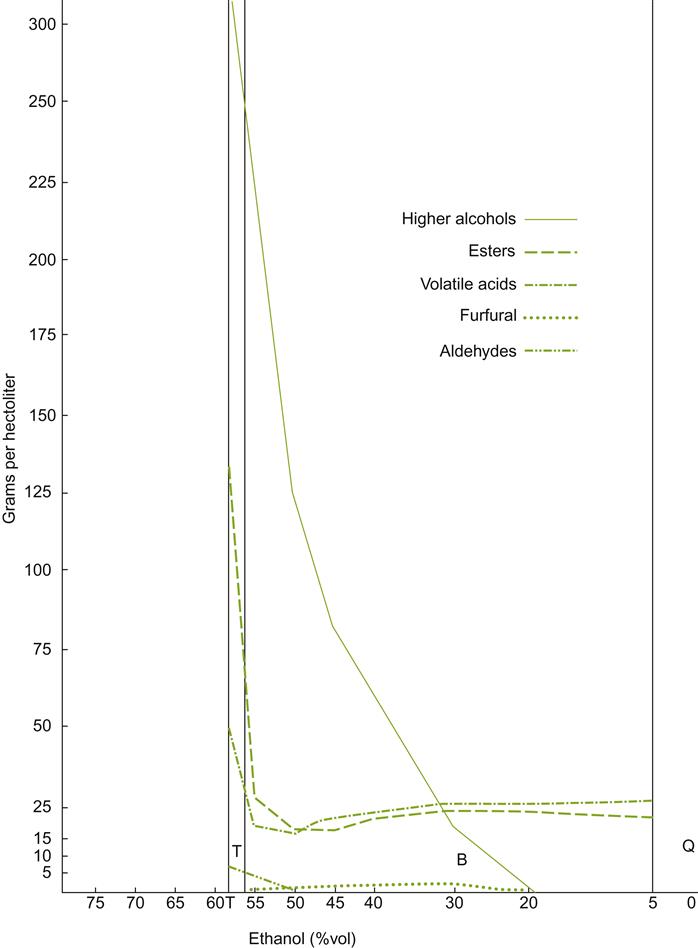
When sufficient brouillis has been collected (about 26 to 32% ethanol), it is redistilled. Alternately, brouillis may be combined with a portion of fresh base wine for redistillation. The second distillation (bonne chauffe) is similar to the first in process, except that it occurs at a slightly lower temperature. The distillate is usually separated into four fractions. After the heads has been collected (~1.5%), the next 50% of the distillate (heart or coeur) is isolated, becoming the nascent brandy. The alcohol content in this fraction is about 70% (67–72%) ethanol. The next two fractions (heart 2) (about 40%), and the tails (about 10%) are combined with the heads and added to fresh brouillis ready for redistillation. The temperature of the distillate, as it runs out of the still, is ideally about 18 °C. The second phase of distillation lasts about 14 h. Some details on the separation of compounds during the second distillation are noted in Fig. 9.47. For further details, see Cantagrel and Galy (2003).
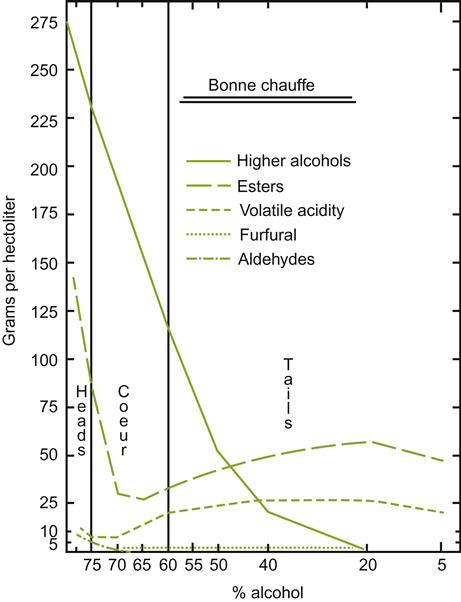
The high temperatures to which the wine is exposed (up to 800 °C at the base of the still) induce many Maillard and Strecker degradation reactions. These include reactions between sugars and amino acids, producing heterocyclics, such as furans, pyridines, and pyrazines, as well as aldehydes and acetals from the degradation of α-amino acids. In addition, heat promotes hydrolytic decomposition of nonvolatile terpene glycosides and polyols, to liberate free, volatile terpenes (Strauss and Williams, 1983), ketones (such as α- and β-ionones), and norisoprenoids (such as vitispirane and TDN).
Pot stills are typically constructed of copper. This has the advantages of being resistant to wine acids, is a good conductor of heat, and is malleable (Verre, 1993). However, sulfur dioxide can react with copper, corroding the boiler. Thus, stainless steel is occasionally substituted, except for the uppermost portions of the rectifying column. Nevertheless, copper remains the preferred construction material for distilling cognac and armagnac. Copper ions, dissolved in the wine, have the advantage of combining with fatty acids, such as caprylic, caproic and lauric acids. Their insoluble salts are subsequently filtered out at the hydrometer port. Thus, the potential of these cheesy to soapy-smelling fatty acids to distort the flavor of the distillate is reduced. Even the trace amounts of copper can oxidize compounds such as terpenes (Nguyen et al., 2009). Copper also fixes hydrogen sulfide, typically found in young wines. Where stainless steel is used, copper finings or copper sulfate may be added to the boiler.
In contrast to cognac, armagnac production employs a simple column still, possessing five to 15 distillation plates. These began to replace the older, alembic stills in the early 1900s. Although now standard, some armagnac is still made with alembic stills. The armagnacais differs from most modern, continuous stills in its use of a direct-fired boiler (Fig. 9.48). They range in capacity from 5 to 35 hL. At initiation, water is added to the boiler and column. As soon as the water starts to vaporize, wine is transferred into the preheater. Here, it is heated by exposure to the hot vapors in the condensing coils. The preheated wine (70 to 85 °C) passes over to the distilling column, where it flows down and over the column plates. Alcohol and various aromatics are continuously volatilized as hot vapors rise upward from the boiler. When the non-volatized wine remnants reach the bottom, it boils, generating vapors that rise and bubble through the descending wine. To increase contact between the rising, hot vapors and descending wine, the distillation plates are variously grooved, fitted with mushroom-shaped caps, or possess bell-shaped tunnels. The rising vapors escape into the neck. Here they pass through the preheater before reaching and descending through the condensing coils. A small condenser, usually positioned on top of the distilling column, collects the least volatile constituents. These are usually collected and sent back for redistillation. The condensate that collects at the bottom of the condenser generates a distillate possessing about 50–54% alcohol. Armagnac is significantly higher in both total and volatile acidity, as well as ester content, than cognac, but with lower C8, C10, and C12 fatty acids and ethyl esters. Depending on the distiller, some light lees may be included with the wine for distillation. Their presence supplies the principal source of fatty acids involved in the synthesis of C8, C10, and C12 ethyl esters. They provide a fruity aspect to the distillate. Fatty acids are also the source of methylketones, contributing a rancio attribute to old, oak-aged brandies.
Adjusting the degree of heating and rate of wine flow are the principal means by which the chemical makeup of the distillate is regulated. For example, increasing the flow rate lowers the temperature, increases the relative alcohol content, but reduces the concentration of flavorants, such as phenethyl alcohol, ethyl lactate, and 2,3-butanediol (Fig. 9.49), and the generation of pyrolytic by-products. Separation, and selective inclusion or exclusion of the various fractions, also significantly affects the flavorant profile of the nascent brandy.
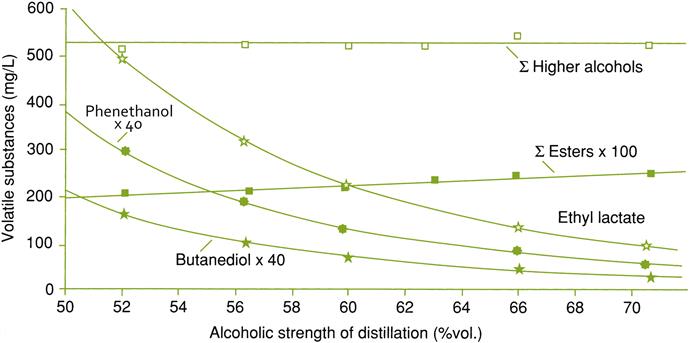
Spent wine is drawn off from the lowest level of the still. Periodically, the still needs to be shut down to clean out the sediment that accumulates in the boiler, and on the distillation plates. Thus, most armagnac stills function in a semicontinuous manner. This is termed the Verdier système. This minimizes the formation and accumulation ethyl carbamate in the brandy, in a similar manner to the cleansing of pot still after each batch.
Maturation
The second critical step in brandy production involves aging in small oak cooperage. The casks used may vary between 200- and 600-liter capacity. Those used for cognac are mostly about 350 liters, while those used for armagnac are between 400 and 420 liters. The preferred source of the oak for these brandies comes from the adjacent forests of Gascony or Limousin. The wood is more porous, facilitating volatile and gas diffusion, and contains more readily extractable tannins. The barrels are typically not filled to capacity, exposing the distillate to oxygen. Slow oxidation during maturation promotes the conversion of aldehydes to more pleasant smelling acetals. In addition, dialdehydes may be generated, as when sinapaldehyde is oxidatively split into glyoxal and syringaldehyde. Some ethanol is also slowly oxidized to acetic acid, while oxidation of some higher alcohols results in the significant accumulation of aldehydes, such as hexanal and 3-methylbutanal (Bertrand, 2003a). For other brandy appellations, the source of the oak preferred often reflects regional traditions for aging table wine. For example, Brandy de Jerez (Sherry Brandy) uses American oak.
In a study by Caldeira et al. (2002), heavy barrel toasting was considered the most favorable in terms of vanilla, woody, spicy, caramel, and smoky attributes, and the lowest in fruity, green, tails, and glue aspects. Distinct, but sensorially pleasant attributes were also found in brandies aged in chestnut cooperage. Studies are under way related to the use of wood fragments in lieu of cooperage (Caldeira et al., 2010). Initial results seem encouraging.
It is in-cooperage maturation that converts the sharp roughness of a young brandy distillate into a soft mellow beverage. Maturation typically lasts from 2 to 5 years, but may continue for 20 or more years. Maturation typically begins in new oak cooperage, but continues in used barrels, following transfer after 8 to 12 months.
During maturation, more alcohol than water is lost through the wood, resulting in a drop in the alcohol content. For example, the initial 70% ethanol content of a cognac may fall to about 60% after maturing for some 12 years. The changes in alcohol content during maturation also affect the selective extraction of oak constituents. Thus, extraction of aromatic phenolic aldehydes, such as vanillin, are favored initially, with sugar and polyol removal accentuated later on. Sugars, such as glucose, fructose, arabinose, galactose and xylose, accumulate as breakdown products of hemicellulose hydrolysis. Up to 2 g/L have apparently been recorded in brandy aged for 40 years (Montero et al., 2005).
Traditionally preferred balances of extractives appear to be optimal at alcohol contents in the range of 50–55% (Cantagrel et al., 1992). During maturation, the selective diffusion of small, more volatile compounds through the cooperage induces a slight concentration of the larger, less volatile constituents in the nascent brandy. The slow uptake of oxygen also activates oxidative reactions in the brandy (Cantagrel and Galy, 2003). These changes can be modulated by adjustments to the cellar environment (e.g., moisture level, temperature), and the attributes of the barrels chosen.
Occasionally, an infusion, made from oak shavings, may be added to provide extra extract, especially when barrel maturation is short. Addition of a sugar syrup (about 6 g/L) may be used to soften the burning sensation donated by the high alcohol content.
One of the first noticeable changes during maturation involves the extraction and oxidation of ellagitannins from the cooperage. They generate brandy’s typical golden color. Lignins degrade and are extracted much more slowly (Fig. 9.50). The flavorants extracted include oak lactones (β-methyl-γ-octalactones) and lignin breakdown products, notably vanillin, syringaldehyde, coniferaldehyde, and sinapaldehyde (Puech, 1984; Table 9.9). Ethanolysis is important in lignin degradation.
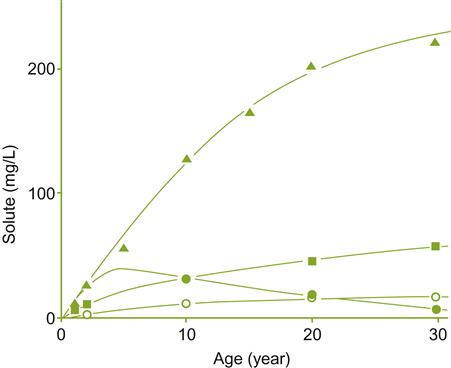
 ) ellagitannins, (
) ellagitannins, ( ) ellagic acid, (
) ellagic acid, ( ) lignin oligomers, and (
) lignin oligomers, and ( ) syringaldehyde. (From Viriot et al., 1993. Copyright 1993 American Chemical Society, reproduced by permission.)
) syringaldehyde. (From Viriot et al., 1993. Copyright 1993 American Chemical Society, reproduced by permission.)Table 9.9
Example of the effect of aging period on the presence of oak extracts in brandy
| Constituent | 0.7 years | 5 years | 13 years |
| Gallic acid | 4.6 | 9.0 | 15.3 |
| Vanillic acid | 0.3 | 1.4 | 2.8 |
| Syringic acid | 0.6 | 2.6 | 7.0 |
| 5 -HydroxymethyIfurfural | 4.2 | 4.2 | 6.3 |
| Furfural | 26.8 | 24.7 | 21.3 |
| 5-Methylfurfural | 1.5 | 1.4 | 1.6 |
| Vanillin | 0.9 | 4.4 | 8.8 |
| Syringaldehyde | 2.25 | 8.9 | 17.6 |
| Coniferaldehyde | 3.65 | 5.9 | 6.7 |
| Sinapaldehyde | 9.45 | 17.8 | 17.0 |
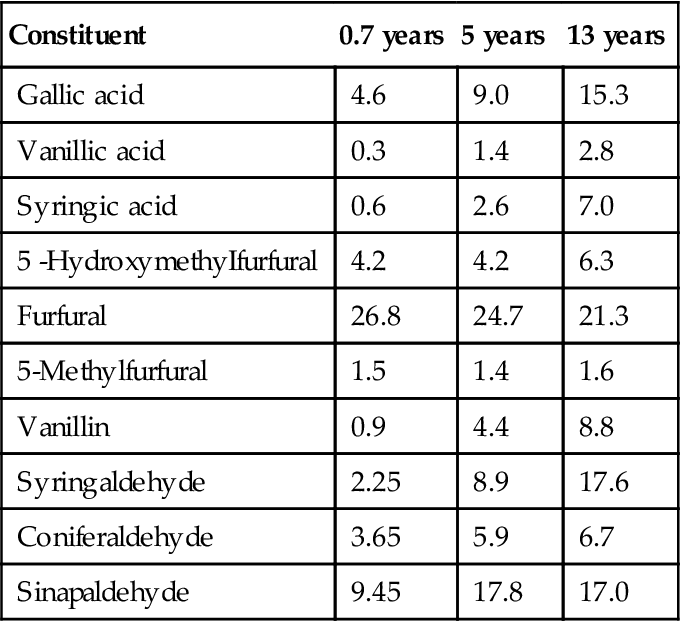
Source: From Cantagrel and Vidal, 1993, reproduced by permission.
The nature and amounts of compounds extracted are functions of the duration of in-barrel maturation, the inherent chemistry of the oak, and how these have been modified by wood seasoning and toasting during cooperage construction. In addition to adding flavor, compounds extracted from the wood reduce the volatility of undesirable esters, notably those with longer carbon chains (ethyl octanoate to ethyl hexadecanoate) (Piggott et al., 1992). The latter esters produce the undesirable sour, soapy, oily flavors detectable in a young brandy. Oak extracts (phenolics and organic acids) reduce the concentration at which ethanol forms pseudo-micelle clusters, normally above 19–20% ethanol (D’Angello et al., 1994; Nose et al., 2005). This may partially explain the less alcoholic, smoother taste sensation of aged brandies. Brandy appears to act like an alcoholic microemulsion, as well as an aqueous ethanol solution. Ethanol soluble compounds, such as higher alcohols and aldehydes, accumulate in the ethanolic micelles, reducing their volatility and headspace presence by increasing solubility (Conner et al., 1998; Escalona et al., 1999).
Although aging in oak is essential for brandy maturation, the use of new oak cooperage is kept to a minimum (often no more than 6 months to a year before transfer to used barrels). Details relative to the situation in Charentes (cognac) are described in Calvo et al. (1993). In Jerez, barrels that have been used previously to mature sherry are employed. This donates a distinctive flavor to the brandy, based on the particular sherry style matured in the barrels.
The net effect of limited contact with new oak is to avoid the uptake of excessive amounts of tannins and oak flavors. For inexpensive brandies, oak extract may be substituted for long maturation in oak. This does not, however, provide attributes donated by slow in-barrel oxidation and lignin ethanolysis. In addition, the consequences of acetate ester hydrolysis and the synthesis of fatty acid ethyl esters are less marked when maturation is foreshortened.
Shortly after aging begins, blending with older brandies commences. The barrels are usually racked yearly, and the wines from one or more series mixed before being transferred to barrels for further maturation. As with fortified wines, brand-name identification and distinctiveness are the hallmarks of the brandy industry. Nevertheless, some vintage-dated brandies, notably armagnacs, are produced. In Jerez, Spain, brandies are usually aged in the soleras y criaderas system, similar to that of sherry (Diez et al., 1985; Quiros Carrasco and Carrascal Garcia, 1993).
During maturation, the alcohol content of the blend is gradually reduced to about 40% alcohol (with the addition of distilled water), the precise level depending on the producing country. Because of clouding, exposure to refrigeration may be required for adequate clarification. Caramel may be added to enhance its yellow-gold cast. In some countries, it is also permissible to add some sweetening, which can vary from dessert wine, grape must, to honey. A final cold treatment and polishing filtration prepare the product for bottling.
The major designations of brandy are based on a combination of the minimum age of the youngest distillate in the blend and the minimum average age of the blend. These are Three Stars (2/2 years); V.O., V.S.O.P. (4/5 years); X.O., Extra, Napoleon, Vieille Réserve, Hors d’Age (5/6 years).
Quality is as difficult to define in brandy as in any other grape-derived beverage. By tradition, its characteristics have become associated with moderate levels of higher alcohols, generally in the range of 65–100 mg/L (pungency), aldehydes and acetals (sharpness), oak lactones (coconut fragrance), phenolic aldehyde derivatives from lignin degradation (vanilla and sweet fragrances), ethyl esters of C8 to C12 fatty acids (fruity/floral notes), the oxidation and transformation of fatty acids into ketones, and heat-derived furans and pyrazines (caramel and roasted notes). Excessive amounts of low volatile constituents, such as ethyl lactate and 2-phenylethanol, tend to donate an atypical heavy flavor, whereas highly volatile constituents provide sharp, irritating notes. Terpenes typically add their particular character to the brandy only when Muscat cultivars are used as the base wine. Of the main market styles, armagnac and cognac have considerably more congeners (about twice) than those of other brandies. A representative comparison is provided in Table 9.10.
Table 9.10
Compositional comparison of average values of a range of armagnacs, cognacs and brandies
| Amount (mg/L) | Armagnac | Cognac | Brandy |
| Alcohol content (% vol) | 41 | 40 | 45 |
| Acetic acid | 107 | 60 | 20 |
| 1-Butanol | 0.2 | 0.1 | 1.3 |
| 2-Butanol | 0.5 | 0.7 | 3.4 |
| Ethyl acetate | 76 | 46 | 39 |
| Furfural | 1.2 | 2.5 | 0.6 |
| Methanol | 47 | 50 | 70 |
| 2-methyl-1-propanol | 105 | 122 | 55 |
| 1-Propanol | 50 | 43 | 25 |
| Total aldehydes | 23 | 19 | 25 |
| Total esters | 109 | 73 | 55 |
| Total volatile substances | 682 | 632 | 357 |
Source: Modified from Bertrand, 2003b.
Gas chromatography–olfactometry has identified compounds central to the typical flavors of young cognacs. Important attributes associated with particular compounds are buttery (diacetyl), hay (nerolidol); grass (mainly Z-3-hexen-1-ol); pear and banana (2- and 3-methylbutyl acetates), rose (2-phenylethyl acetate), and lime tree (linalool) (Ferrari et al., 2004). In addition, the rancio attribute typical of old (15–20 year) barrel-aged brandies appears to be primarily due to the presence of methylketones, notably 2-heptanone, 2-nonanone, 2-undecanone and 2-tridecanone (Fig. 9.51). These form from the β-oxidation and decarboxylation of long-chain fatty acids. These are also important to the flavor of blue cheeses. Lignans and lactones, derived from the oxidation of oak lignins, apparently impart the balsamic aspect of the rancio attribute (Marche et al., 1975).
More recently, aroma extract dilution analysis (AEDA) has been employed to demonstrate the central significance of ethyl esters to brandy flavor (Zhao et al., 2009). Additional aromatic attributes were contributed by higher alcohols, notably 2-methyl propanol and 3-methyl butanol (fusel notes); β-damascenone, trans-β-methyl-γ-octalactone, and several fatty acid ethyl esters (fruit, sweet, and coconut); and 1,1-diethoxyethane and cis-β-methyl-γ-octalactone (cream and coconut aspects).
The sensory perception of these compounds is markedly affected by the alcohol content of the beverage. For example, as the alcohol content increases above 17%, ethanol molecules begin to cluster, reducing hydrophobic hydration. For example, the formation of ethanol-rich regions reduces the volatility of ethyl esters (Conner et al., 1998). Additional factors that influence the equilibrium between liquid and headspace concentration involve the specific concentration of individual compounds, and their interaction with the brandy matrix (Conner et al., 1993).
Despite all the clear advantages to aging in oak, there are disadvantages. Capital is tied up for long times; considerable losses of alcohol occur through the wood; and barrels, being derived from natural products and the work of man, are subject to variable quality. Thus, there is interest in shortening the aging process; reducing the variability inherent in oak cooperage; and the loss of alcohol to the air (see van Jaarsveld et al., 2009a,b,c). Although interesting, it is a moot point as to how far technological solutions can advance brandy maturation, or equivalent studies being conducted on sherry maturation. At a certain point, it is the mystique and appeal of ‘natural’ processes that attracts the connoisseur. Without the latter, will the purchaser be willing to pay the same premium?