2. Predators can enhance species coexistence
3. Predators can sometimes hamper prey species coexistence
4. The impact of predator and prey behavior on community organization
5. Predators can initiate trophic cascades
6. The diverse effects of predator diversity
Acts of predation are among the most dramatic events one can see in nature, but the impact of predation on ecological communities goes well beyond the effect of direct mortality on the prey species itself. Because species are embedded in complex food webs, predation on one species can lead to chains of indirect interactions affecting many other species. Predation can sometimes enhance diversity, for instance, if it is differentially inflicted on dominant or abundant competitors. This can free up space or resources, thus permitting inferior or scarce competitors that are better able to withstand predation to persist. However, indiscriminate predation can instead shift the relative competitive rankings of species without enhancing coexistence. Prey species that are highly productive can sometimes sustain predators at levels where less productive prey species are vulnerable to exclusion. There are many complexities in predator-prey interactions that have implications for community organization, including behavioral games between predators and prey and interactions among predators themselves, altering their net effects on their prey. A deeper understanding of all these dimensions of predator-prey interactions is essential for developing wiser policies of conservation and resource management in our rapidly changing world.
apparent competition. An indirect interaction between prey species where a given prey species experiences more intense predation because of the presence of the alternative prey as a result of changes in either predator abundance or predator behavior.
community. The assemblage of species that are found together at one place at one time that can potentially interact.
community module. A small number of species involved in a clearly defined pattern of interactions, such as two consumers competing for a shared resource, or two prey species interacting indirectly via their impacts on a shared predator.
community organization. A term that broadly encompasses the number of species found in a community, their relative abundances, and their pattern of interconnections via competition, exploitation, and mutualism.
indirect interactions. When there are three or more species, a given pair of species may influence each other via changing the abundance, activity, or traits of other species (one to many).
keystone predator. A predator that strongly interacts with its prey and facilitates their coexistence.
natural enemy. A species that utilizes another species (the “victim”) as a resource and harms that other species in so doing. Natural enemies include “true” predators, parasitoids, pathogens, and herbivores.
predator. A natural enemy that kills its victim in order to utilize resources contained in that victim.
switching. A behavioral response by predators to relative prey abundance such that common prey are disproportionately attacked.
trophic cascade. A chain reaction in a community across trophic levels in which predation on one species relaxes consumption by that species of its own resource population.
Few things in the world thrill the nature lover as much as the sight of a predator in action or repose—a lion lazing in the sun, a killer whale gamboling in the waves, a diamondback rattler coiled menacingly under a desert shrub, a spider spinning a silken coffin around its quivering moth prey. Predators provide intellectual thrills too, for some of the most dramatic and intellectually rich stories in ecology involve elucidating the impact of predators on communities. Before recounting some of these tales, which I will use to illustrate principles governing how predators influence communities, it is useful to clarify some terms and to provide a sense of the overall complexity of this issue.
Community organization denotes the number of species that co-occur and their relative abundances, along with those processes that control these structural features, including in particular interspecific interactions such as predation, competition, and facilitation. Predators as sources of mortality directly reduce prey and, if sufficiently severe, can cause extinction. Via such direct impacts, predation can profoundly influence community organization. Yet direct mortality (albeit dramatic and compelling) is just one of the many causal pathways by which predators govern community organization. Individual prey can respond adaptively to avoid predation, for instance by changing habitats, feeding rates, or even morphological traits. These responses may reduce predation, but at the cost of hampering resource acquisition, competitive ability, dispersal, or stress tolerance. Because predators and their prey are almost always embedded in complex communities where they interact with other species, the direct impact of predators on prey numbers, behaviors, and traits can set in motion many chains of indirect interactions across the community (see chapter III.5). Beyond the direct and indirect effects of predators on the average abundance of species, predators also provide feedback effects in communities, which sometimes stabilize interactions but, in other settings, can be dramatically destabilizing. Most communities have multiple predator species, and interactions among the predators can strongly influence the role of predation in community organization. Many species are both predator and prey. A top predator has no obvious predator consuming it (although top predators do have parasites and pathogens).
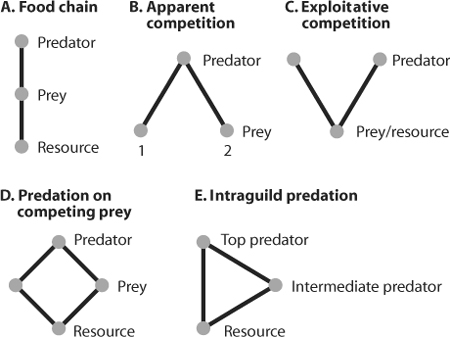
Understanding these ramifying impacts of predation presents an immense challenge. One avenue that has proven useful in analyzing the role of predation in communities is to examine community modules, small sets of species (or well-defined functional groups) interacting in configurations such as those shown in figure 1. In this figure, each species shown is assumed to be dynamically responsive to the other species connected to it by a feeding relationship; usually more species are present than the few shown here, but for some purposes these additional species can be ignored, either because they have negligible effects or because their action can be subsumed in some manner. Theoretical and experimental analyses of modules allow one to think clearly about important processes without getting bogged down in a proliferation of details.
Figure 1. Community modules. These are simple subwebs drawn out of more complex community webs. In intraguild predation (E), top predators and intermediate predators utilize the same resources, and the top predator feeds as well on the intermediate predator.
One theme that has received considerable attention is the role of predators in governing coexistence within guilds of their prey, where a guild is a set of species that utilize resources in similar ways and thus potentially compete (i.e., the prey are engaged in exploitative competition, comparable to figure 1C but one trophic level down). If predators are inefficient consumers or swamped by a surfeit of prey, there may be little effect of predation on prey species richness. But in other circumstances, predators can be essential in permitting species to coexist.
A celebrated experiment by Robert Paine in the intertidal of eastern Washington exemplifies the power of experiments to reveal the key role of predators in communities and highlights several important features of predation in a community context. A thick band of mussels (Mytilus californianus) and several species of barnacles dominate the rocky midintertidal. The lower intertidal has lower biomass and much higher species diversity, including immature individuals of these species as well as other space occupiers such as macroscopic benthic algae and a sponge, which in turn sustain browsers such as chitons and limpets. The top predator in the system is the starfish Pisaster ochraceus. Paine’s experimental protocol was elegantly simple: he systematically removed Pisaster from a strip of the lower intertidal, with appropriate controls nearby. Within a few years, diversity on the rock surface was collapsing into a mussel monoculture. Mussels are superior competitors for space, crowding out algae and barnacles and indirectly forcing abandonment by browsing invertebrates.
This experiment crisply shows that predation can sustain components of diversity in a community, and do so dramatically. Note I said “components” of diversity. Mussel beds support hundreds of small invertebrate and plant species, living on and among the shells, a dependent community that might disappear when the beds are demolished by the starfish. It is unclear without further study if Pisaster enriches the entire community or just the guild of species that directly compete with mussels for space (and of course any species that largely depend on them). Pisaster in the lower rocky intertidal is the canonical keystone species, a species with such a large impact on the community that, in its absence, the community radically changes in species composition. We can draw out some key lessons of this study that pertain to many systems.
First, for predators to enhance diversity, it must hold that in their absence there is strong competition leading to exclusion. In the intertidal, space is contended for by species differing in their ability to colonize and monopolize space, and the mussel is clearly the dominant competitor. This broadly fits the exploitative competition module (figure 1C). Models of exploitative competition for a single resource predict that in the absence of mitigating factors (e.g., temporal or spatial heterogeneity), a single species should persist at the lowest level of the shared resource (here, empty space), excluding all others.
Second, the impact of Pisaster was particularly strong on a dominant competitor–the mussel. Predator selectivity helps determine whether or not predators enhance, or instead reduce, prey diversity. For selectivity to promote prey coexistence, there should be a negative trade-off in prey traits across species, so that those prey species superior in competition are more vulnerable to the predator. However, predator selectivity alone does not permit competitive coexistence. Imagine that the numbers of Pisaster are determined at a broader spatial scale than Paine’s study site and that their attack behavior is fixed, independent of prey numbers. Pisaster predation might then act, to a reasonable approximation, as a fixed, density-independent mortality factor, but with different magnitudes on different prey. Figure 2 shows a simple graphic model for exploitative competition with added predation. The solid lines denote the birthrate of each competitor on the resource. The dotted line shows background mortality experienced by both prey species, when the predator is absent. Species 1 wins in competition because it persists at a lower resource level (its so-called R*; note that 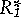 <
< 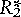 ). When the predator is present, it selectively feeds on prey species 1, and so increases the level of mortality experienced by that species to the level shown by the dashed line. The resource levels now required by species 1 to sustain itself in the face of predation (increased to
). When the predator is present, it selectively feeds on prey species 1, and so increases the level of mortality experienced by that species to the level shown by the dashed line. The resource levels now required by species 1 to sustain itself in the face of predation (increased to 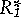 ′) exceed those needed by species 2, so species 2 will win. Density-independent selective predation clearly is important because it determines which species dominates. But on its own, it will not maintain prey diversity.
′) exceed those needed by species 2, so species 2 will win. Density-independent selective predation clearly is important because it determines which species dominates. But on its own, it will not maintain prey diversity.
Figure 2. A graphic model of reversal of dominance in exploitative competition as a result of predation. The curved solid lines show how each consumer’s birthrate increases with resource R in the absence of predation. The dotted line is background mortality experienced equally by both species. Species 1 has a lower maximal birthrate than does species 2 at high resource levels but can reproduce more effectively when resources are scarce. If species 1 is present at equilibrium, it sustains the resource at level 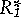 , and species 2 becomes extinct at this low resource level (because it needs at least
, and species 2 becomes extinct at this low resource level (because it needs at least 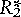 to persist). The dashed line includes the additional mortality suffered by species 1 when a selective predator is present. Consumerspecies 1 now requires a much higher resource level than
to persist). The dashed line includes the additional mortality suffered by species 1 when a selective predator is present. Consumerspecies 1 now requires a much higher resource level than 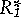 to persist (indicated by
to persist (indicated by 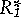 ′), and this level is higher than
′), and this level is higher than 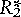 , so now species 2 can exclude species 1–predation has reversed competitive dominance. (In this example, the same outcome holds even if the predator is an indiscriminate generalist, because prey 2 could still persist at a somewhat lower level of the shared resource, and so will win in competition.)
, so now species 2 can exclude species 1–predation has reversed competitive dominance. (In this example, the same outcome holds even if the predator is an indiscriminate generalist, because prey 2 could still persist at a somewhat lower level of the shared resource, and so will win in competition.)
Now imagine that the predator is a generalist imposing mortality uniformly on both prey species. Prey numbers will decline, and resource levels will rise. In the example shown, such uniform predation shifts dominance from species 1 to species 2, but again without coexistence. In general, in competition for a single resource, predation that acts as a simple source of additional mortality on either or both species does not permit coexistence (although it may determine the winner).
This is not what is implied by Paine’s study: reduction in mussel abundance leads to a large increase in diversity rather than a simple replacement of one competitor by another. For a diversifiying effect of predation, one of several mechanisms must come into play. In general, for predation to enhance coexistence, interspecific density dependence must be weakened relative to intraspecific density dependence.
A wide array of mechanisms can permit predators to facilitate coexistence, operating over different temporal and spatial scales. Space precludes a full treatment of these, so I will mention just a few highlights. The predator itself can show density-dependent and discriminatory feedback responses to its prey, in effect providing independent modes of regulation controlling prey numbers in addition to resource competition, and so stabilize competitive coexistence. Sometimes, a lowered overall abundance of a prey guild caused by predation may permit coexistence mechanisms to operate that are simply less effective at higher overall abundance. For instance, the starfish does not uniformly reduce mussel numbers but instead clears out patches. These patches can be colonized by species that are rapid dispersers but poor competitors. Alternatively, if refuges are present and in limited supply, prey may compete for refuges to avoid predation. If different prey species use different refuges (e.g., because of body size differences), the presence of predators leads to competition for enemy-free space. This may provide an axis for niche differentiation that does not exist in the absence of predation.
If a dominant competitor is attacked by a specialist predator, the added density-dependent feedback needed to facilitate coexistence can come from a strong numerical response of this specialist predator. The predator keeps the dominant below its carrying capacity, thus freeing resources for other species; when the dominant declines, so too (with a lag) does the predator, and the dominant can then rebound from low numbers. Specialization by predators on competitive dominants requires a trade-off between competitive ability and vulnerability to predation. Trade-offs emerge from morphological, behavioral, life history, or phenological constraints. For instance, turtles are protected from predators by a thick shell, but this reduces their ability to move rapidly over the landscape in search of food. Understanding trade-offs is fundamental to tying the details of organismal biology to the dynamics of predator-prey interactions. With a multiplicity of prey species, each with its own specialist predator, in principle an entire species-rich ensemble could be supported despite strong potential competition among the prey. Many parasitoids are relatively specialized and effective at limiting their insect hosts, so this speciose group of natural enemies may help explain the hyperdiversity of herbivorous insects. But such tight specialization is much less common in terrestrial and aquatic food webs with vertebrate predators and their prey.
In many circumstances, predation makes coexistence more difficult. This can happen in several ways. For instance, if prey respond behaviorally to predators by crowding into a limited supply of refuges, they may compete for those refuges directly or may compete more intensely for food resources inside refuges. If prey on their own do not strongly compete, predation can reduce species richness. There are many examples. For instance, experimental studies have examined the impact of Anolis lizards on web-building spider communities on Staniel Cay in the Bahamas. The lizards sharply reduced total spider abundance and species richness. The latter effect arose because spiders that were rare in the controls were differentially absent in the lizard sites; spiders that were relatively more common were less strongly affected. The lizards preferentially feed on species that are rare even in the absence of lizard predation, so added mortality from lizards pushes these spider species to the point of local extinction.
Predators have particularly devastating impacts on prey communities when the prey species have not had an evolutionary history equipping them with appropriate defenses. A tragic example comes from the island of Guam. During the last 40 years, Guam’s native forest bird species have plummeted in abundance, and several species are now believed extinct or nearly so. The culprit is an introduced predatory snake, the brown tree snake Boiga irregularis, which was apparently transported to Guam in the mid-twentieth century. Boiga, a major predator on bird nests in its native range, has become very abundant on Guam, with declines in bird numbers paralleling increases in its range and abundance on the island. One reason the brown tree snake has so thoroughly decimated the native avifauna of Guam is that its numbers soared and remained high even after its bird prey largely disappeared. This reflects two features of Guam. First, Boiga has neither competitors nor predators that could keep its own numbers in check. It is the top predator in the community. Second, and crucially, Boiga is a generalist and also consumes small lizards such as skinks and geckos. These lizards are abundant on Guam. Because of their high reproductive potential, they withstand predation more readily than do species with lower reproductive potential (many island birds have notably low clutch sizes). These alternative prey species sustain a high snake density; the snake can then overexploit bird populations to the point of extinction without endangering its own persistence.
This kind of negative indirect interaction between prey is called apparent competition (figure 1B) because in many respects its outcome resembles the impact of direct or exploitative competition. If one could do a radical experiment and remove alternative prey species from Guam, the working hypothesis would be that snake numbers would be held in check, weakening predation pressure on the native birds. In general, the intensity of predation on any particular prey species indirectly depends on alternative prey in the diet of the shared predator. By supporting a predator, some prey species may indirectly limit the abundance of a dominant competitor, thereby facilitating their own persistence. Models of keystone predation (figure 1D) show that coexistence can occur if one prey species is superior at competing for a shared resource, and the other prey species is superior at withstanding—and sustaining—the shared predator, i.e., at apparent competition. But this mechanism of coexistence depends on a “Goldilocks effect.” If prey productivity is low, few predators will occur, and so what mainly matters is competition among prey. If productivity is high, many predators will be sustained, and the ability to withstand predation looms large. Coexistence involving a balance between exploitative and apparent competition is most likely at intermediate productivity.
Predators and prey are not automatons but can respond behaviorally to shifts in each others’ abundance and behavior, with important consequences for community organization and dynamics that are still being elucidated by ecologists.
A basic feature of predation is that the capacity of individual predators to consume prey is limited: the rate of predation saturates as prey density becomes large. Over short time spans, increases in one prey species can benefit another, simply because the predator’s capacity to capture and process prey becomes saturated. This indirect mutualism between prey species may explain a number of phenomena such as mixed-species aggregations of ungulates or forest birds. But saturation on its own does not explain the maintenance of prey diversity because basically it just permits prey to escape predation and grow to numbers where they are likely to be limited by food or other resources.
Flexible predatory behaviors can lead to frequency-dependent predation that both keeps prey numbers low and helps maintain diversity. If rare species are relatively ignored by predators, they may enjoy a kind of protection at low numbers. For instance, predatory fish in coral reefs concentrate on cardinalfish when they are abundant, allowing recruits of many other fish species to escape unharmed. Such switching behaviors can permit stable coexistence of prey on a single resource. This has been long known as a theoretical possibility, but there are surprisingly few (if any) rigorously documented examples. In the coral reef example, there does not appear to be direct competition between the cardinalfish and beneficiary species such as butterfly fish. Many examples of indirect mutualism between prey involve predators shifting their attention between habitats with different prey species, relaxing predation when prey numbers in a habitat are temporarily low. In such cases, the spatial segregation of the prey that allows predator switching also means the prey may not be strongly competing in the first place. Moreover, because additional prey species should often boost predator numbers, positive interactions among prey species via predator saturation or switching may be outweighed by longer-term numerical responses leading to apparent competition. Wolves, for instance, are well known for flexible hunting behaviors, including switching. But having moose as an abundant and productive alternative prey for wolves has been implicated in the decline and local extirpation of woodland caribou in parts of Canada, because wolf numbers are substantially boosted by the availability of moose.
Beyond the issue of modulating species coexistence, flexible predator behaviors can strongly affect system stability. Theoretical studies suggest that mobile predators in heterogeneous landscapes that respond adaptively to changes in local prey numbers can have strong stabilizing effects. This is a compelling idea and surely helps explain the persistence of some complex ecological communities. However, flexible predator behaviors, including switching, can also at times destabilize systems. The North Pacific has seen a sequential collapse of marine mammals—first seals, then sea lions, and finally sea otters—over the last few decades. One might at first suspect that this has been caused by global change, such as shifts in climate, but recent evidence implicates switching by a top predator—the killer whale. Historically, killer whales focused their predation on the great whales, such as sperm whales, but whale numbers were strongly reduced by an upsurge of whaling after World War II, particularly by the Japanese whaling fleet. This sharp reduction in their primary food source led killer whales to start feeding on seals, and then when seal numbers were depleted, on smaller-bodied sea lions. When these in turn had been sufficiently cleaned out, the killer whales started feeding on the even smaller-bodied sea otters. Calculations suggest switching behavior by a relatively small number of killer whales suffice to explain the observed collapse of sea otter populations in the Aleutian Islands. Thus, flexible predator switching behavior has amplified an initial disturbance caused by humans, with reverberating impacts across an enormous oceanic ecosystem.
Behavioral responses by prey to predators are also important. Prey individuals faced with predators often reduce their foraging. There is increasing evidence that these nonlethal effects of predation can be quantitatively large, at times even more important than the direct lethal effects of predation on prey communities. Analyses of such trait-mediated indirect interactions between species is a very active area in community ecology (see chapter III.5).
If predators reduce the abundance or shift the behavior of their prey, this can have strong second-order effects on the resources consumed by those prey. This chain of indirect interactions is called a trophic cascade. There are an increasing number of examples of dramatic trophic cascades in both terrestrial and aquatic systems.
In North America, European colonists eliminated wolves over wide areas, but in recent years the wolf has returned as a result of growing sympathy by the public for such predators. Wolves prey on large ungulates such as elk. Researchers have compared areas of Banff National Park where wolves colonized to areas avoided because of human activities. Elk were more numerous by an order of magnitude in low-wolf areas, with higher survival and calf recruitment. This led to substantially lower aspen recruitment and willow production because of intense browsing.
This recent study is a microcosm of a widespread shift in trophic interactions that plausibly occurred over huge landscapes during the settlement of the American West. Large carnivores are highly vulnerable to direct elimination by humans and indirect impacts via habitat fragmentation. In the Great Plains, settlers, as they spread across the prairie in the late nineteenth century, substituted cattle for native migratory bison and systematically exterminated top predators such as wolves and grizzly bears. In protected areas such as Wind Cave National Park in southwestern South Dakota, in the twentieth century, there was an upsurge in the abundance of wild ungulates such as deer and elk, freed from predation by both wild and human predators. Analyses of cottonwood and bur oak stands reveal essentially zero recruitment for more than a century because of high levels of browsing. (Inside exclosures, there has been substantial recruitment.) Thus, a trophic cascade initiated by human decimation of top predators may have had profound consequences for Great Plains ecosystems.
Simple models of trophic cascades consider unbranched food chains (figure 1A) with one species at each trophic level. These models show that effective predators can free the basal species of control by the intermediate prey species. One worrisome effect of human impacts on the Earth’s ecosystems is that top predators are particularly vulnerable to anthropogenic disturbance, leading to disrupted control of herbivore numbers. For instance, an accidental introduction of the pathogen canine parvovirus onto Isle Royale around 1980 caused a dramatic crash in wolves, which in turn permitted an upsurge of moose numbers and more intense browsing.
The topic of trophic cascades has been the focus of an intense and continuing debate among ecologists (see also chapters III.5 and III.6). Many feel that simple models matching the food chain of figure 1A leave out critical complicating factors. The strength of trophic cascades can vary with many factors, such as the overall complexity of the food web and the magnitude of direct plant defenses against herbivory. If a long coevolutionary struggle between plants and herbivores has created an armory of defenses, such as toxins, structural defenses, and low-quality tissues, herbivores may be more a dynamic annoyance than a prime driver, even in the absence of predators. Moreover, increases in herbivory can drive shifts in plant community composition so that the final community is dominated by species that the herbivores cannot readily eat. Shortterm manipulative experiments may not capture such compositional changes (which may require colonization from external species pools).
John Terborgh has argued that few natural plant communities are immune to the strong effect of herbivores unchecked by predation. The huge artificial Lake Guri in Venezuela isolated hundreds of islands of varying sizes. On smaller islands, predators such as jaguars were absent, and folivorous howler monkeys increased to high numbers. Likewise, anteaters and armadillos disappeared, and the colonies of their prey, leafcutting ants, burgeoned. These hyperabundant herbivores turned to plants not normally part of their diets and devastated their preferred forage plants, with dramatic effects on tree recruitment. In effect, the absence of the original top predator means that the herbivores can grow until limited by their own resources. This sets up the opportunity for apparent competition between different plant species in the herbivore’s diet, and plants with low tolerance of herbivory are vulnerable to extinction. The removal of the top predator then results in plant extinctions, two trophic levels down.
Turning this process around, we can return to the original theme of this chapter—identifying ways predation can facilitate prey species coexistence—and link this issue to both trophic cascades and apparent competition. If prey species themselves depend on living resources and are left unchecked, they can overexploit some of those resources to the point of extinction, driven by apparent competition (in this case, via the numerical response of the prey to its own resources). This can preclude niche partitioning among the prey. Conversely, a reduction in prey abundance or activity caused by predation can permit a wider range of resource species to persist, opening up avenues for potential niche partitioning and weakening interspecific competition relative to intraspecific competition. In effect, trophic cascades can mediate coexistence via niche partitioning at intermediate trophic levels, which is permitted because the top predator indirectly relaxes apparent competition at the basal trophic level.
Relatively few systems have just a single top predator, matching the simple modules of figure 1. Predator diversity has a wide variety of impacts on community organization and functioning, many of which have been poorly explored either theoretically or empirically.
Experimental studies in kelp forests have shown that increasing predator diversity strengthens the trophic cascade when both predator and herbivore trophic levels are diverse. The reason is that herbivores respond behaviorally to predation by reducing foraging rates, and different herbivores respond to different predators. With more predator species, fewer avenues of escape are open to the prey. Predator diversity thus has a synergistic effect on limitation of herbivore numbers, indirectly boosting kelp biomass. Conversely, in a study of invertebrate predation on planthoppers feeding in salt marshes, increased predator diversity dampened the trophic cascade. The reason is that this system included intraguild predation (figure 1E), a kind of omnivory where some predators eat other predators as well as a shared resource. For instance, spiders eat ladybugs, and both prey on planthoppers. Models of intraguild predation suggest that in the absence of factors such as alternative prey, for the predators to coexist, the top predator has to be less efficient at utilizing the shared resource. A mixture of predators will thus reduce the total impact of predation on the basal species and weaken trophic cascades. However, these models have yet to incorporate complexities such as trait-mediated interactions, spatial heterogeneity, and other realistic factors, and so this conclusion should be viewed as a tentative hypothesis
In this essay, I have just scratched the surface of the rich topic of predation and community organization. There are many important issues, such as the role of life history variation and population structure, the implications of spatial dynamics such as metapopulation processes, and the relationship between predation and the classic complexity-stability debate, which deserve much further scrutiny. The examples sketched above show that it is vital to understand the impact of predators on community organization, not just in terms of basic science but to inform conservation and management policies. It is difficult to understand the origin and maintenance of the diversity of life without paying attention to the profound impact of predation. It is even more difficult to imagine that we can preserve the biota with which we share the planet without paying due attention to the compelling drama of predation as a key driver in ecological systems.
Chase, J. M., P. A. Abrams, J. P. Grover, S. Diehl, P. Chesson, R. D. Holt, S. A. Richards, R. M. Nisbet, and T. J. Case. 2002. The interaction between predation and competition: A review and synthesis. Ecology Letters 5: 302–315.
Holt, R. D. 1997. Community modules. In A. C. Gange and V. K. Brown, eds., Multitrophic Interactions in Terrestrial Ecosystems, 36th Symposium of the British Ecological Society. Oxford: Blackwell Science, 333–349.
Holt, R. D., and J. H. Lawton. 1994. The ecological consequences of shared natural enemies. Annual Review of Ecology and Systematics 25: 495–520.
Levin, S. A. 1970. Community equilibria and stability, and an extension of the competitive exclusion principle. American Naturalist 104: 413–423.
Rosenheim, J. A. 2007. Special feature—intraguild predation. Seven papers on this topic. Ecology 88: 2679–2728.
Paine, R. T. 1966. Food web complexity and community stability. American Naturalist 100: 65–75.
Ray, J. C., K. H. Redford, R. S. Steneck, and J. Berger, eds. 2005. Large Carnivores and the Conservation of Biodiversity. Washington, DC: Island Press.
Ripple, W. J., and R. L. Beschta. 2007. Hardwood tree decline following large carnivore loss on the Great Plains, USA. Frontiers in Ecology and the Environment 5: 241–246.
Savidge, J. A. 1987. Extinction of an island forest avifauna by an introduced snake. Ecology 68: 660–668.
Spiller, D. A., and T. W. Schoener. 1998. Lizards reduce spider species richness by excluding rare species. Ecology 79: 503–516.
Springer, A. M., J. A. Estes, G. B. van Vliet, T. M. Williams, D. F. Doak, E. M. Danner, K. A. Forney, and B. Pfister. 2003. Sequential megafaunal collapse in the North Pacific Ocean: An ongoing legacy of industrial whaling? Proceedings of the National Academy of Sciences, U.S.A. 100: 12223–12228.
Terborgh, J., K. Feeley, M. Silman, P. Nunez, and B. Balukjian. 2006. Vegetation dynamics of predator-free landbridge islands. Journal of Ecology 94: 253–263.