
CHEMICAL REACTIONS & ENERGETICS
CHEMICAL REACTIONS & ENERGETICS
GLOSSARY
acid A substance that produces H+ ions in solution. Acids neutralize bases, producing water as a product.
base A substance that produces OH- ions in solution. Bases neutralize acids, producing water as a product.
catalyst A substance that increases the rate of a reaction without being consumed by the reaction.
chemical energy The energy that can be obtained from a chemical reaction when the reactants have greater potential energy than the products.
chemical reaction A process in which the atoms in one or more substances (the reactants) rearrange to form different substances (the products).
electrolysis The use of electrical current to drive a chemical reaction that would not happen spontaneously.
electrolyte A substance that produces an electrically conductive solution when dissolved in water.
entropy A thermodynamic quantity related to the amount of energy dispersed into a substance at a given temperature.
enzyme A protein that acts as a biological catalyst to increase the rate of a biochemical reaction.
exothermic A reaction that emits energy into the surroundings.
filtration A process of separation in which a solid is separated from a liquid using a filtration device such as a funnel and filter paper.
greenhouse gas An atmospheric gas that is transparent to visible light, but absorbs infrared light. These atmosphere gases act like glass in a greenhouse, allowing light to enter but preventing heat energy from escaping. The three most important greenhouse gases in Earth’s atmosphere are water vapour, carbon dioxide and methane.
hydrocarbon An organic compound containing only carbon and hydrogen.
Kelvin scale An absolute scale used for measuring temperature. On the Kelvin scale, water freezes at 273 K and boils at 373 K. The lowest possible temperature (at which point molecular motion stops) is zero on the Kelvin scale.
kinetic energy The energy associated with the motion of an object or particle.
neutralization A chemical reaction between an acid and a base that typically produces water and a salt.
oxide An oxygen-containing compound.
potential energy The energy associated with the position (within a field) or composition of an object.
precipitation A reaction between two solutions in which a solid (or precipitate) forms.
reactant Any one of the substances that undergoes a chemical reaction. In a reaction, reactants react to form products.
thermal energy The energy associated with the random thermal motion of atoms and molecules.
thermodynamics The study of energy and its conversions from one form to another.
transition-metal oxides Compounds containing a transition metal and oxygen.
voltaic cell A chemical cell that employs a spontaneous chemical reaction to produce electrical current.
CHEMICAL EQUATIONS
the 30-second chemistry
Chemists must move seamlessly between three related worlds: the macroscopic world that exists in the lab within beakers, flasks and test tubes; the atomic and molecular world, which we can’t see but are constantly trying to imagine and understand; and the symbolic world, which is how we represent the atomic and molecular world on paper. A chemical equation is a way to symbolically represent changes that occur in the atomic and molecular world. These changes are called chemical reactions and they often (although not always) result in significant changes in the macroscopic world. For example, the burning of natural gas is a chemical reaction. In this reaction methane gas combines with oxygen to form carbon dioxide and water. In the macroscopic world, we see the reaction as a blue flame on our stove top. In the molecular world, methane molecules combine with oxygen molecules and transform into carbon dioxide molecules and water molecules. In the symbolic world, we represent the reaction with the following chemical equation:
CH4 + 2 O2 → CO2 + 2 H2O.
Chemical equations must be balanced: they must contain the same number of each type of atom on either side of the equation. Why? Because in a chemical reaction, matter is conserved. Atoms can’t just vanish or form out of nothing.
3-SECOND NUCLEUS
A chemical equation is a way to precisely represent a chemical reaction, a change in which the atoms that compose one or more substances rearrange to form one or more different substances.
3-MINUTE VALENCE
Chemical reactions occur all around us all the time. For example, our cars are powered by chemical reactions; cooking is a chemical reaction; and our bodies maintain a myriad of reactions that allow us to think, move, eat and reproduce. Chemical equations not only represent the identities of the reactants and products in a chemical reaction; they also give us quantitative relationships between the amounts that react.
RELATED TOPICS
See also
COMBUSTION REACTIONS & ENERGY SOURCES
3-SECOND BIOGRAPHIES
ROBERT BOYLE
1627–91
Anglo-Irish chemist who formulated some of the earliest ideas about chemical reactions
ANTOINE LAVOISIER
1743–94
French chemist who contributed significantly to our understanding of chemical reactions, especially combustion
30-SECOND TEXT
Nivaldo Tro
We witness chemical reactions, such as the burning of natural gas, every day.

COMBUSTION REACTIONS & ENERGY SOURCES
the 30-second chemistry
The first chemical reaction that early humans used was burning or combustion. In a combustion reaction, a substance combines with oxygen and most commonly produces carbon dioxide, water and other oxide products. Combustion reactions are useful because they emit heat as they occur – they are exothermic. Certain molecules, especially hydrocarbons, have an inherently high potential energy that can be released through combustion. For this reason, our society’s fuels are largely based on hydrocarbons. Natural gas is primarily methane (CH4). Liquefied petroleum (LP) gas is a mixture of propane (C3H8) and butane (C4H10). Petrol is a mixture of hydrocarbons, such as octane (C8H18), containing five or more carbon atoms. Coal is also a major part of our energy equation: it is mostly carbon and combines with oxygen to form carbon dioxide. Together these fuels are known as fossil fuels because they originated from ancient plant and animal life. However, the combustion of fossil fuels is not without problems. The most vexing problem is probably the emission of carbon dioxide, a greenhouse gas that is affecting Earth’s climate. Since the Industrial Revolution, atmospheric carbon dioxide levels have risen by about 38 per cent, and average global temperatures have risen by about 0.8°C (1.4°F).
3-SECOND NUCLEUS
Our society’s energy comes largely from the combustion of fossil fuels, which produce large amounts of energy when burned.
3-MINUTE VALENCE
Energy is critical to a society’s standard of living. In general, as the standard of living rises, so does energy consumption. Our current energy sources are dwindling, however, and creating environmental problems. The shift to alternative energy sources – such as solar power or wind power – has been slow, but steady. The main challenges of the alternative sources are their cost and their inherent intermittence.
RELATED TOPICS
See also
3-SECOND BIOGRAPHY
ANTOINE LAVOISIER
1743–94
French chemist who contributed significantly to our understanding of chemical reactions, especially combustion
30-SECOND TEXT
Nivaldo Tro
Combustion or burning is common in energy generation and industry.

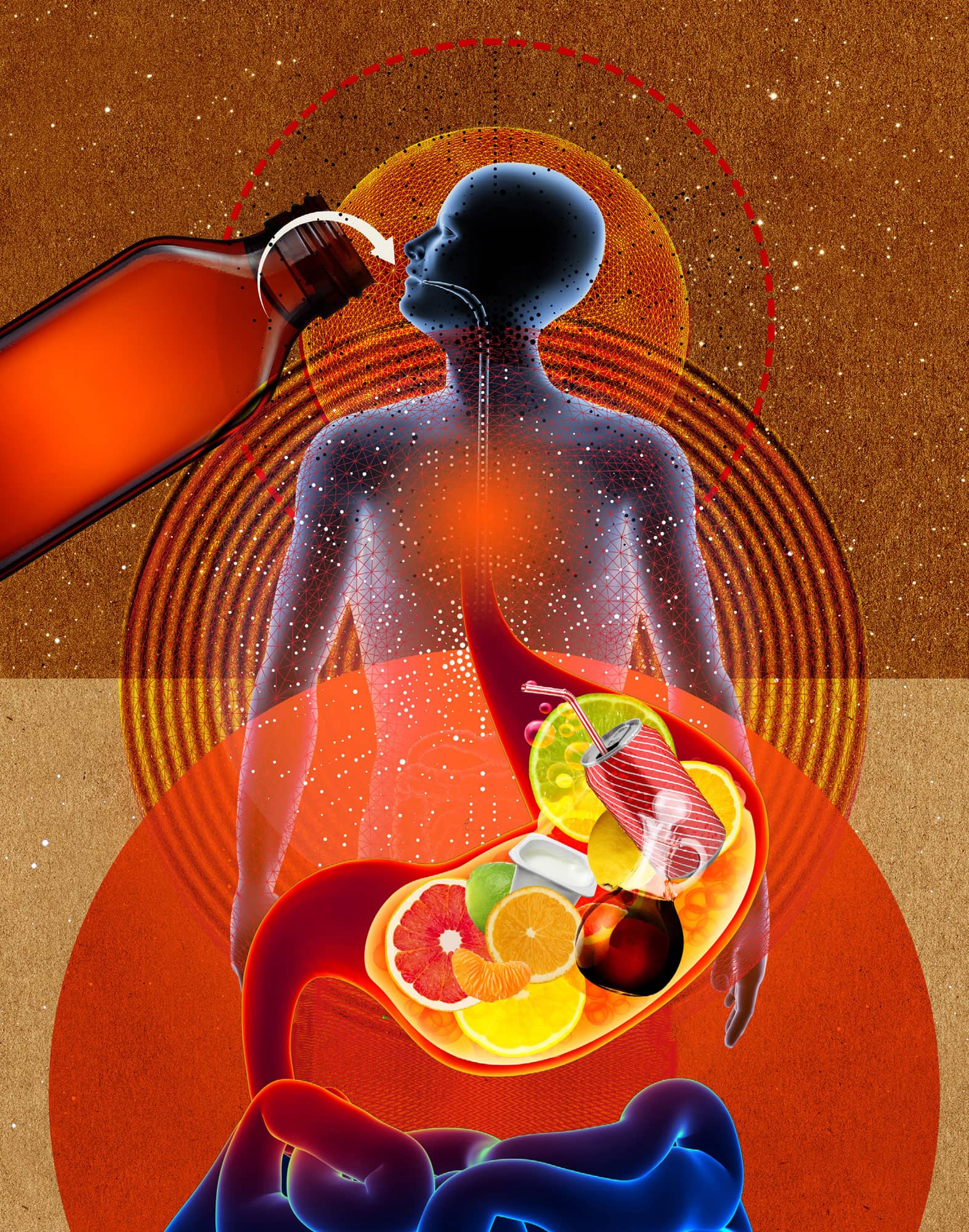
NEUTRALIZING: ACIDS & BASES
the 30-second chemistry
Most of us experience acids by taste – they are sour. Citrus fruits, vinegar, carbonated beverages, yoghurt and sour sweets all owe their tangy deliciousness to the acids they contain. Chemists prefer not to taste their work and often define acids as chemicals that produce the hydrogen ion (H+) when dissolved in water. If acids are a chemical yin, then bases are the yang. Bases tend to taste bitter and produce hydroxide (OH–) in water, the chemical opposite of H+. When an acid and base are mixed, the hydrogen ion combines with the hydroxide ion to form HOH (water):
H+ + OH– → H2O.
This type of chemical reaction is called neutralization. If exactly equal amounts of H+ and OH– are mixed, the resulting solution will contain neither (because all of the H+ and OH– ions reacted to form water), and it won’t be acidic or basic. Our stomachs use hydrochloric acid (HCl) to help digest our food. If we eat too much, especially acidic or fatty foods, our stomachs can produce too much HCl, causing us to feel uncomfortable (sometimes called ‘sour stomach’). To neutralize the excess acid, we can take an antacid. Antacids are bases like aluminium hydroxide (Al(OH)3), magnesium hydroxide (Mg(OH)2) and calcium carbonate (CaCO3) that neutralize the excess stomach acid.
3-SECOND NUCLEUS
Acids produced H+ in water while bases produce OH–. An acid and a base react to produce water, effectively neutralizing each other.
3-MINUTE VALENCE
The pH scale is used to measure the acidity or basicity of a solution. The lower the pH value, the higher the concentration of H+, and the more acidic the solution. The higher the pH value, the higher the concentration of OH–, and the more basic the solution. Pure water is neutral and has a pH of 7. Stomach acid is pH 1.6, tomato juice 4.2, sea water 8.2 and milk of magnesia (Mg(OH)2) 10.4.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
SVANTE ARRHENIUS
1859–1927
Swedish winner of the Nobel Prize for Chemistry (1903), who first suggested the acid/base definitions given here
JOHANNES BRØNSTED & MARTIN LOWRY
1879–1947 & 1874–1936
Danish and British chemists who defined acids and bases on how they react
30-SECOND TEXT
Jeff C. Bryan
Antacids contain bases that neutralize stomach acid, the cause of heartburn.
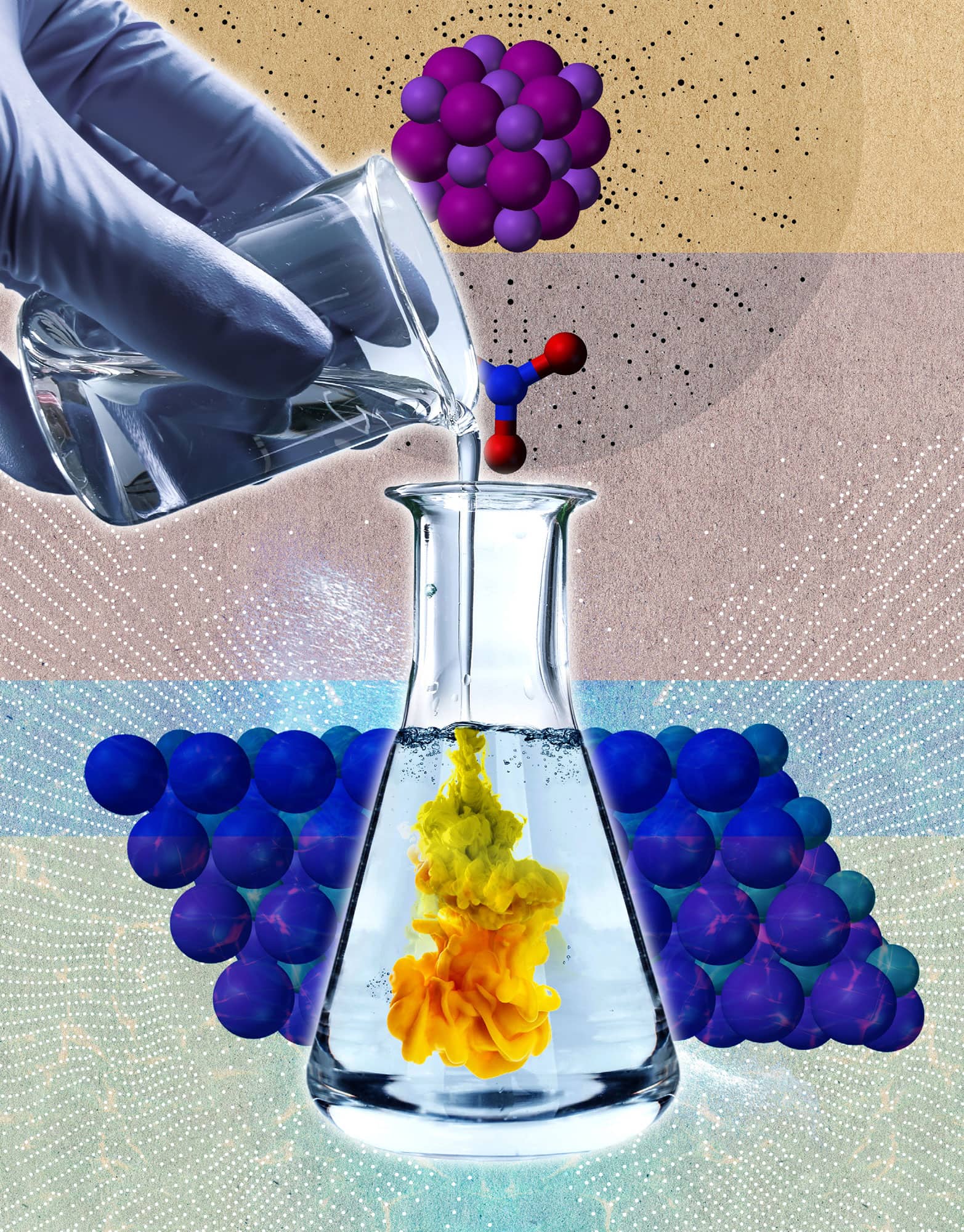
CREATING SOLIDS: PRECIPITATION REACTIONS
the 30-second chemistry
Two friends are out for a night on the town, both looking for love. One is drawn to an incredibly attractive stranger. Once together, the friend and the stranger never separate, because the attraction is so strong. They drop out of sight, losing touch with their old friends. This scenario is similar to a particular type of chemical reaction called a precipitation reaction. When two or more ions are mixed in water, they are initially attracted to nearby water molecules. However, as they move around (due to thermal energy), the ions encounter each other and are drawn together. If the attraction is strong enough, the two ions will stick together and fall out of solution (form a solid). If the attraction isn’t very strong, the ions don’t get together and just stay in solution. As examples, when silver (Ag+) and chloride (Cl–) are mixed they stick together and form a solid precipitate. However, sodium (Na+) is much less attractive to chloride, so they don’t form a precipitate. The ‘bathtub ring’ that you sometimes see if you bathe in hard water is due to a precipitation reaction between the ions in hard water and the ions in soap.
3-SECOND NUCLEUS
A precipitation reaction occurs when two ions are so strongly attracted to each other that they form a solid.
3-MINUTE VALENCE
Precipitation reactions are particularly useful when something needs to be removed from a solution. For example, water treatment plants can use precipitation reactions to remove undesirable contaminants from our water. If a chemical reaction takes place in a solution, and the product is insoluble in that solution, then it falls out of solution as a solid and can be isolated by filtration.
RELATED TOPICS
See also
3-SECOND BIOGRAPHY
LINUS PAULING
1901–94
American winner of the 1954 Nobel Prize in Chemistry, who developed our understanding of how atoms and molecules are attracted to each other. He was also a peace activist and won the 1962 Nobel Peace Prize
30-SECOND TEXT
Jeff C. Bryan
In a precipitation, a solid forms when two liquid solutions are mixed.
USING CHEMISTRY TO GENERATE ELECTRICITY
the 30-second chemistry
Chemical reactions in which electrons migrate from one chemical substance to another are called oxidation-reduction reactions. These types of reactions can be used to generate electricity by arranging the chemical substances so that the substance gaining electrons (being reduced and called the oxidizing agent) is not in physical contact with the substance losing electrons (being oxidized and called the reducing agent). The electrons are then forced to travel through an external circuit to get from the reducing agent to the oxidizing agent. This arrangement is called a voltaic cell. Self-contained voltaic cells – either by themselves or connected in series – function as batteries. They produce electricity. The lead storage battery used to start a car engine is comprised of six voltaic cells containing lead and lead oxide immersed in a solution of sulfuric acid (battery acid). Dry cell batteries, such as those used in torches, use zinc and magnesium dioxide. Button batteries (used in calculators or watches) also use zinc, but they have mercuric oxide or silver oxide substituted as the oxidizing agent. Lithium ion batteries use lithium between planes of graphite as the reducing agent and a lithium metal oxide as the oxidizing agent.
3-SECOND NUCLEUS
Loss of electrons is oxidation, while gain of electrons is reduction; reactions that involve transfer of electrons between reactants are oxidation-reduction or redox reactions.
3-MINUTE VALENCE
Corrosion results from oxidation of metals exposed to oxidizing agents in the environment. When the metal is iron, the process is rusting. Rust is hydrated forms of iron(III) oxide generated when iron is exposed to moisture and oxygen. The rate of rusting depends on the acidity of the environment and the presence of electrolytes to help carry electric current. Having a metal that is more easily oxidized (a sacrificial electrode) in contact with iron can retard rusting.
RELATED TOPIC
See also
3-SECOND BIOGRAPHIES
MICHAEL FARADAY
1791–1867
English scientist who developed the system of oxidation numbers and coined many terms associated with electrochemistry
WALTHER HERMANN NERNST
1864–1941
German chemist who developed the equation for the relationship between concentration and voltage
30-SECOND TEXT
John B. Vincent
Batteries use chemical reactions that involve the transfer of electrons to produce electricity.

REACTION RATES & CHEMICAL KINETICS
the 30-second chemistry
Chemical reactions occur at a variety of speeds or rates. Chemical explosions occur rapidly, with the creation of large volumes of hot gas. Many chemicals are stable, however; they react so slowly that they can be placed in a bottle. Fortunately, the rates (or speeds) at which chemical reactions occur can be controlled – and studying these processes is the field of chemical kinetics. One way to increase the rate of a reaction is by increasing the temperature. For this reason, the reactions that cook food happen faster the higher the temperature. Increasing the concentration of the reactant substances or surface area also increases the rates of chemical reactions. You can hold an iron nail in your hand, but if it is ground to a fine powder (greatly increasing its surface area) the iron can burst into flames in air. The concentration of many chemicals in your body must be carefully regulated for you to remain healthy. Your body accomplishes this by regulating the rates of a wide range of chemical reactions. The rates of slow reactions are accelerated by proteins called enzymes; these biological molecules are catalysts (substances that change the rate of a chemical reaction without being consumed by the reaction).
3-SECOND NUCLEUS
The rate of a chemical reaction is the speed at which the reaction occurs and depends on reactant concentration, temperature and whether or not a catalyst is present.
3-MINUTE VALENCE
Although nitrogen and oxygen gas are stable, at the temperature of a car engine they react to produce nitric oxide gas (NO), an air pollutant that is a cause of acid rain and smog. NO is removed from a car’s exhaust gas by a catalytic converter. In the converter, the exhaust passes over a honeycomb-like structure of alumina impregnated with solid transition-metal oxides that catalyse the conversion of NO back to O2 and N2.
RELATED TOPICS
See also
USING CHEMISTRY TO GENERATE ELECTRICITY
3-SECOND BIOGRAPHIES
JACOBUS HENRICUS VAN ’T HOFF
1852–1911
Dutch chemist who won the first Nobel Prize in Chemistry in part for determining graphical methods to establish that reaction rates depend on the concentrations of the reactants
HENRY TAUBE
1915–2005
American chemist who won the 1983 Nobel Prize in Chemistry for relating rates of chemical reactions to electronic structure
30-SECOND TEXT
John B. Vincent
Controlling how fast a chemical reaction occurs allows us to reduce pollution and create new molecules.

ENERGY & THE FIRST LAW OF THERMODYNAMICS
the 30-second chemistry
The universe has a quantity we call energy. Objects can possess it and can transfer it to other objects, but it can neither be created nor destroyed. The total amount of energy that exists is constant. This principle is known as the first law of thermodynamics. (A more nuanced treatment includes mass/energy as the constant, but we simplify a bit here.) We formally define energy as the capacity that an object has to exert a force on another object across a distance. For example, a moving car has energy because it has the capacity to strike another object and exert a force on it over a distance. Energy can come in many different forms. The moving car has kinetic energy, the energy associated with its motion. All substances above zero kelvin have thermal energy, a type of kinetic energy associated with the random, temperature-dependent motion of the particles that compose the substance. The higher the temperature, the greater the thermal energy. The book you are holding contains potential energy, the energy associated with an object’s position. Chemical substances have chemical energy, a type of potential energy associated with the positions of all of their electrons and protons. Energy can be transferred or exchanged but, according to the first law, it can never be created or destroyed.
3-SECOND NUCLEUS
Energy can be transferred or exchanged but, according to the first law of thermodynamics, it can never be created or destroyed.
3-MINUTE VALENCE
The first law of thermodynamics implies that energy cannot be made out of nothing. Any human attempt to create energy out of thin air has failed. The law has not, of course, prevented humans from trying. But as far as we know, the spontaneous creation of energy is impossible. In other words, when it comes to energy, you can’t win – you can’t get something for nothing.
RELATED TOPICS
See also
ENTROPY & THE SECOND LAW OF THERMODYNAMICS
ENTROPY & THE THIRD LAW OF THERMODYNAMICS
ENTROPY & SPONTANEOUS PROCESSES
3-SECOND BIOGRAPHY
RUDOLF CLAUSIUS
1822–88
German physicist who formulated one of the earliest versions of the first law of thermodynamics
30-SECOND TEXT
Nivaldo Tro
A steam engine is powered by the energy transferred from burning fuel.

ENTROPY & THE SECOND LAW OF THERMODYNAMICS
the 30-second chemistry
We have seen that in energy transactions, you can’t win – you can’t get energy out of nothing. But it gets worse – you can’t even break even. In our universe, energy always spreads out or randomizes itself as much as possible. The second law of thermodynamics describes this pervasive tendency: in any spontaneous process, a quantity called entropy (which you can think of as a measure of energy randomization or energy dispersion) always increases. You have no doubt experienced the second law every time you hold a warm drink. The thermal energy in the drink disperses itself into the surroundings – the drink spontaneously cools down (and the air surrounding the drink warms up a bit). Imagine a universe in which the hot drink gets hotter (and the surroundings slightly cooler) as energy transfers from the surroundings into the drink! Not possible according to the second law. The second law implies that, in any energy transaction, some energy must be dispersed if the transaction is to occur at all. In other words, nature always takes a heat tax. For example, recharging a battery will always take more energy than the amount of energy you can use as you discharge the battery. Such is the second law – when it comes to energy, you can’t break even.
3-SECOND NUCLEUS
For all spontaneous processes entropy increases.
3-MINUTE VALENCE
The second law of thermodynamics implies that a perpetual motion machine – one that keeps on moving forever without the need for energy input – is impossible. With each cycle of the machine’s motion, some energy must be dispersed in order for the motion to occur at all. As a result, the energy of the machine must necessarily decrease over time, and it must eventually stop moving.
RELATED TOPICS
See also
ENERGY & THE FIRST LAW OF THERMODYNAMICS
ENTROPY & THE THIRD LAW OF THERMODYNAMICS
ENTROPY & SPONTANEOUS PROCESSES
3-SECOND BIOGRAPHIES
NICOLAS LÉONARD SADI CARNOT
1796–1832
French physicist instrumental in the development of thermodynamics
RUDOLF CLAUSIUS
1822–88
German physicist who was instrumental in formulating the second law of thermodynamics
30-SECOND TEXT
Nivaldo Tro
A perpetual motion machine cannot exist according to the second law of thermodynamics.

ENTROPY & THE THIRD LAW OF THERMODYNAMICS
the 30-second chemistry
According to the first law of thermodynamics, you can’t win – you can’t get something for nothing. According to the second law, you can’t break even – every energy transaction necessarily results in a loss to the surroundings. According to the third law of thermodynamics, you can’t get out of the game. In the case of thermodynamics ‘getting out of the game’ means getting to the lowest possible temperature, zero kelvin or absolute zero. Absolute zero is the temperature at which atomic and molecular motion essentially stops. The third law of thermodynamics states that the entropy of a perfect crystal is zero at zero kelvin. This law has two implications. The first one is that entropy, unlike other thermodynamic quantities, can be measured on an absolute scale. All perfect crystals have zero entropy at zero kelvin. As the temperature rises, energy is dispersed into the crystal and its temperature and entropy increases. The second implication is that absolute zero can never be reached in a finite number of steps. It would take an infinite number of cooling steps to arrive at the absolute zero of temperature, so it can never be achieved.
3-SECOND NUCLEUS
The absolute entropy of a perfect crystal at zero kelvin is zero.
3-MINUTE VALENCE
Entropy is a measure of the energy dispersed into a system per unit temperature. For this reason, dispersing the same amount of energy into a system at a colder temperature produces greater entropy than dispersing that energy into a warmer system. As we will see in the next entry, this is the reason that ice melts above its melting point but not below it.
RELATED TOPICS
See also
ENERGY & THE FIRST LAW OF THERMODYNAMICS
ENTROPY & THE SECOND LAW OF THERMODYNAMICS
ENTROPY & SPONTANEOUS PROCESSES
3-SECOND BIOGRAPHY
WALTHER HERMANN NERNST
1864–1941
German chemist who formulated the third law of thermodynamics and received the 1920 Nobel Prize in Chemistry for his work
30-SECOND TEXT
Nivaldo Tro
The third law of thermodynamics, as formulated by Walther Nernst, implies that the absolute zero of temperature cannot be reached.

ENTROPY & SPONTANEOUS PROCESSES
the 30-second chemistry
The criteria for determining whether any process will happen is simple: will the process result in an increase in entropy? Consider the freezing of water. Water freezes spontaneously below 0°C (32°F). Why? When water freezes, the water molecules become more organized, and the energy they contain becomes less randomized - their entropy decreases. How then is this process ever spontaneous? Because when water freezes it gives off heat (energy is dispersed) and the resulting entropy increase in the surroundings is temperature-dependent. We can understand this with a simple analogy. If you give a poor person £1,000, you significantly increase his or her net worth. But if you give a rich person the same £1,000, the impact is negligible. Similarly, if you disperse a given amount of energy into cold surroundings, you significantly increase its entropy, but if you disperse the same amount of energy into warm surroundings, the increase is less. When the freezing of water occurs below 0°C, the heat emitted into the surroundings is enough to increase the entropy of the surroundings so much that it more than compensates for the decrease in entropy of the water molecules themselves, resulting in an overall increase in entropy of the universe and therefore a spontaneous process.
3-SECOND NUCLEUS
A process is spontaneous if it increases the entropy (energy dispersion) of the universe.
3-MINUTE VALENCE
Processes that result in a decrease in entropy are not impossible, they just don’t happen spontaneously. Iron spontaneously reacts with oxygen to form iron oxide (rust). This process causes an increase in the entropy of the universe. However, iron oxide can be turned back into iron. In fact, the manufacture of iron metal from iron oxide depends on it.
RELATED TOPICS
See also
ENERGY & THE FIRST LAW OF THERMODYNAMICS
ENTROPY & THE SECOND LAW OF THERMODYNAMICS
ENTROPY & THE THIRD LAW OF THERMODYNAMICS
3-SECOND BIOGRAPHIES
JOSIAH WILLARD GIBBS
1839–1903
American physicist who developed the main criteria for the spontaneity of a process
LUDWIG EDUARD BOLTZMANN
1844–1906
Austrian physicist who developed a statistical description of the second law of thermodynamics
30-SECOND TEXT
Nivaldo Tro
When ice melts at a temperature above its melting point, the entropy of the universe increases.
