
| AMERICAN BADGER Taxidea taxus |
HB 42–72cm; T 10–15.5cm; W 4–12kg
Squat, low-slung badger with a broad, wedge-shaped head. Coat is grizzled yellow-grey, with buff underparts and dark limbs. Face has a black mask, black cheek stripes and a distinctive white blaze from the nose to the nape, sometimes extending along the spine. Distribution and Habitat SW Canada and USA (mostly west of the Mississippi river, south of Tennessee, and west of Ohio River) to C Mexico. Inhabits mainly open habitats from sea-level to 3,600m, including prairie, grassland, open woodland, scrubland and desert. Occurs on farmland but cannot tolerate intensive agriculture. Feeding Ecology Omnivorous, but strongly reliant on small burrowing mammals such as ground squirrels, marmots, prairie dogs, pocket gophers, voles, mice and lagomorphs. Other prey includes arthropods, birds, eggs and carrion, and occasionally reptiles, amphibians, fish and molluscs. Consumes grains, seeds and grass, but most vegetation is probably taken incidentally while eating prey. Does not kill livestock except for very rare kills of newborn lambs and poultry. Foraging is solitary and usually nocturnal. Very well adapted to excavate prey from burrows; uses its keen sense of smell to locate burrows and plug their entrances with soil and sod, or sometimes snow, rocks or other objects, to block escape routes. Coyotes often form hunting ‘partnerships’ with American Badgers, which is supposedly advantageous to both species, but there is little evidence that badgers benefit. Caches surplus food in burrows and by burying carcasses, usually to jackrabbit size, but 2 records from Utah involved solitary badgers burying cattle calves weighing 18–27kg, and feeding from them for 41–52 days. Scavenges carrion and occasionally from human refuse. Social and Spatial Behaviour Solitary. Adults occupy stable ranges; male ranges are usually, but not always, 2–4 times larger than female ranges. Territorial behaviour is limited; there is high range overlap, adults actively avoid each other and individuals often use the same dens at different times. Average range estimates include 2.4km2 (♀s) to 5.8km2 (♂s) in Utah, to 13km2 (♀s) to 44km2 (♂s) in Illinois, with very large ranges at the distribution’s extreme (SW Canada) of 9–87.3km2 (♀s) and 51–450km2 (♂s). Density estimates 40–500/100km2, but as low as 0.4/100km2 in SW Canada. Reproduction and Demography Seasonal. Mating late July–August; births late March–early April. Gestation 210–240 days, with delayed implantation. Litter size 1–5, averaging 2. Weaning at 6 weeks, dispersal at 3–4 months. Some females breed at 4–5 months, but most breeding follows the first winter. MORTALITY Natural mortality is mostly from starvation during prey crashes and predation; adult American Badgers are occasionally killed by bears, Puma, Grey Wolf and Coyote. LIFESPAN 14 years in the wild, 26 in captivity. Status and Threats Generally common and widespread, with an estimated global population of several hundred thousand. Ongoing declines are driven by agricultural intensification on grassland, associated declines of ground squirrels and prairie dogs, and retributive killing for badgers’ excavations, which can damage crops and equipment, and (rarely) cause injury to livestock. Endangered in British Columbia (<100 remaining) and Ontario (<200 remaining). Red List LC, population trend Decreasing.

| HONEY BADGER Mellivora capensis |
RATEL
HB 74–96cm; T 14.3–26cm; W ♀ 6.2–13.6kg, ♂ 7.7–14.5kg
Powerfully built and conspicuously bicoloured, with black sides and underparts contrasting with a silver-grey to dark grey cape. Melanistic individuals occur, mostly in African rainforest. Skin is very thick and loose, providing protection against snakebites, bee stings and predators. A formidable and tenacious animal that sometimes deters Lions and Leopards, but there are few carnivores surrounded by as many falsehoods, and they are killed by larger carnivores. Distribution and Habitat Sub-Saharan Africa, the Arabian Peninsula and C and S Asia, from SE Kazakhstan to Nepal and most of the Indian subcontinent. Occupies all kinds of wet and dry forests, woodland, grassland, alpine heath (to 4,050m), steppes, scrub, wetland, semi-desert and true desert. Occurs in agricultural areas with cover. Feeding Ecology Omnivorous and highly opportunistic. The most important prey is small mammals to the size of Springhare (2kg), and reptiles including monitors, African Rock Python and highly venomous snakes, e.g. Cape Cobra, Puff Adder (Honey Badgers are very resistant to snake neurotoxins). Occasionally kills larger vertebrates, e.g. Aardwolf, but reports of it killing large ungulates by castration are specious. Also consumes invertebrates, birds, nestlings, eggs, carrion, fruits (including berries) and seeds. Bees, and their honeycomb and honey, are readily eaten, but there is no evidence that Greater Honeyguide birds direct Honey Badgers to hives. Raids domestic beehives and poultry coops. Foraging is mainly nocturno-crepuscular, with increased diurnalism during cold winters. Forages alone, but congregates at food-rich patches. Foraging is largely by its excellent sense of smell, and most prey is excavated after following scent trails. Climbs strongly and raids nests for eggs and nestlings; raptor chicks are important seasonal prey in the Kalahari. Scavenges, including from other carnivores’ kills, and at campgrounds and dumps. Social and Spatial Behaviour Solitary. Male ranges are massive, marked by high overlap and avoidance rather than active territorial defence; each male range overlaps as many as 13 female ranges (Kalahari). Female ranges are more exclusive, but also with little evidence of territorial defence. Moves constantly while foraging, covering up to 40km/day, averaging 14km (♂s) and 8km (♀s) between rest periods. Kalahari range size 85–194km2, averaging 126km2 (♀s), and 229–776km2, averaging 541km2 (♂s). Occurs naturally at low densities, 3/100km2 (S Kalahari). Reproduction and Demography Aseasonal. Gestation 50–70 days. Litter size 1, rarely 2. Weaning at 2–3 months, but cubs are entirely dependent on their mothers to 10–12 months, gradually diminishing to independence at 16–22 months. MORTALITY Annual mortality under protection estimated at 46% (cubs, to independence) and 34% (adults, Kalahari), mainly by starvation, predation by large cats and hyaenas, and infanticide. LIFESPAN 7 years in the wild, 28 in captivity. Status and Threats Widespread, adaptable and has a broad habitat tolerance. However, it has an unusually low reproductive rate, is naturally rare and is vulnerable where directly persecuted, especially by apiarists and livestock owners; the use of poisons to target Honey Badgers is particularly grave, and has extirpated populations locally. Also killed for traditional medicinal uses and superstitious beliefs, such as claims that it excavates gravesites, and appears to be increasingly sought for bushmeat in some areas (e.g. W Africa), perhaps as other, preferred species decline. CITES Appendix III – Botswana; Red List LC, population trend Decreasing.
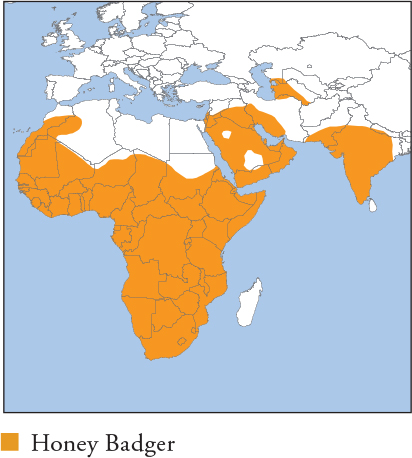
Plate 73

| SUMATRAN HOG BADGER Arctonyx hoevenii |
HB 51–71cm; T 8–18cm; W c.4–8kg
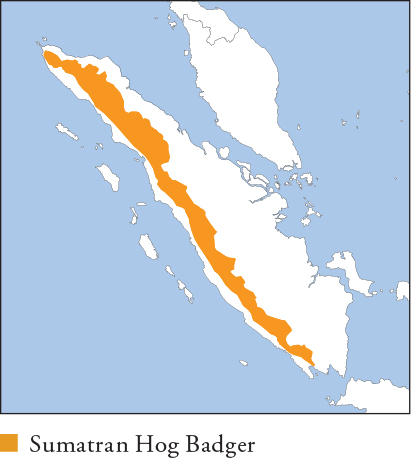
Hog Badgers were considered to be a single species, A. collaris, until 2008, when a long-overdue review of the genus recognised 3 species. Sumatran Hog Badger is the smallest and darkest species, with sparse dirty-black body fur and a creamy-white tail, throat, chin and blaze. Distribution and Habitat Endemic to Sumatra. Restricted to 700–3,780m in the forested foothills and slopes of the Barisan Mountains. Occurs in montane and mossy forests as well as subalpine meadows; camera-trap surveys indicate it is more common in higher montane forest than in lower foothill forest. Feeding Ecology Thought to have a diet consisting almost entirely of soil-living invertebrates, primarily earthworms, beetle larvae and ants. Captive animals refuse raw meat, although 1 animal was trapped using a squirrel carcass as bait. Foraging thought to be mainly by a well-developed sense of smell. Grubs in soft soil with its muzzle to locate prey, which is excavated and lapped up with its long cylindrical tongue, leaving characteristic funnel-shaped depressions. Limited observations indicate it is cathemeral. Social and Spatial Behaviour Unknown. Early accounts suggest adults form pair bonds, but most sightings and camera-trap images are of single animals. Reproduction and Demography Unknown. Females have 3 pairs of mammae, suggesting litters numbering up to 6, but there are no records. MORTALITY Unknown. Reported as aggressive when threatened, and like all hog badgers uses pungent secretions from well-developed anal scent glands to deter predation. LIFESPAN Unknown. Status and Threats Status poorly known. Camera-trap photographs and frequency of diggings suggest it is common in intact forest at 800–2,600m. Considered a local delicacy in some areas, and snared intentionally and accidentally, but impacts are unknown. Small numbers appear in markets, e.g. in Jakarta, for the novelty pet trade. Red List LC, population trend Stable.
| NORTHERN HOG BADGER Arctonyx albogularis |
CHINESE HOG BADGER
HB 54.6–70cm; T 11.4–22cm; W c.5–10kg
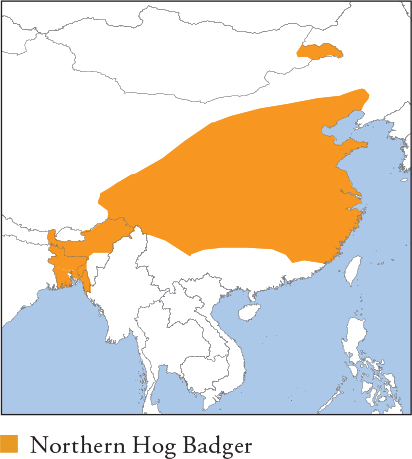
Medium-sized hog badger with a shaggy coat that is long and soft in winter. Colour is blackish with interspersed white hairs on the hindquarters, mid-back and sides, becoming near white in some individuals. More white on the face and throat than in Sumatran Hog Badger. Distribution and Habitat E to S China, extreme E Mongolia, NE India and probably sub-Himalayan Bhutan, Nepal and N Bangladesh. Considered the most generalist of the hog badgers, with a wide habitat tolerance. Occurs from sea-level to 4,300m in temperate forest, scrubland and montane meadows. Lives in agricultural habitats and close to villages. Feeding Ecology Omnivorous and opportunistic, with a high proportion of small rodents and snails in the diet, as well as herptiles, birds, earthworms, beetles, larvae, roots, acorns and leaves. Earthworms are especially important from late spring to autumn. Foraging is solitary and primarily nocturnal. Unlike other hog badgers, hibernates over winter (November–March) in its northern range (C China); it is not clear if it hibernates in milder areas of its range. Social and Spatial Behaviour Unknown. Most records are of single adults; adult pairs live amicably in captivity. Reproduction and Demography Poorly known, but thought to be seasonal (C China). Mating apparently April–May; births February–March, indicating a long period of delayed implantation (estimated at 5–9.5 months for a captive female). Litter size 1–6. Captive juveniles first eat solid food at 85 days; weaning and independence co-occur at 4 months. MORTALITY Unknown; allegedly preyed on by Dhole, Leopard, Grey Wolf and Asiatic Black Bear in C China. LIFESPAN 13.9 years in captivity. Status and Threats Status poorly known. It is the most widespread hog badger species and is common in some areas, but heavily hunted for human consumption and killed as ‘by-catch’ in snares. Severely threatened in SE China. Red List LC, population trend Decreasing.
| GREATER HOG BADGER Arctonyx collaris |
HB 65–104cm; T 19–29cm; W 7–15kg
Largest hog badger. Robust, with a massively built skull, and described as resembling a small bear in the field. It is the lightest coloured species, pale grizzled grey to yellowish grey, with more white on the face, head and neck than in Northern Hog Badger (with which it overlaps in E Bangladesh and NE India, and possibly S China and N Myanmar); some individuals have almost entirely white or creamy-white heads. Lower limbs are black, extending variably over the shoulders and neck. Distribution and Habitat Southeast Asia, from E Bangladesh and NE India through Myanmar, Thailand, Laos, Cambodia and Vietnam. It probably occurs in Yunnan, China; records from Malaysia are equivocal. Occurs at 500–1,500m, primarily in undisturbed lowland and hill forests as well as bamboo stands. Reported close to villages, but rarely in modified habitat; occurs in rubber plantations close to forests. Feeding Ecology Poorly known; thought to be omnivorous and possibly specialises partially in earthworms (which captive animals relish) like Sumatran Hog Badger, but not to the same extent. Captive animals consume meat, reptiles, fish, bread, milk and fruits, especially plantains. Cathemeral. Social and Spatial Behaviour Unknown. Assumed to be solitary. Reproduction and Demography Very poorly known. Litter size thought to be 2–3. MORTALITY Important prey of Leopard in Thailand and also killed by Tiger. LIFESPAN 7 years (minimum estimate) in captivity. Status and Threats Relatively widespread, but its large size and apparent lack of wariness of humans and dogs make it a common target of hunters. Intensively hunted in much of its range and overhunted to extinction in much of Indochina. Severely threatened in Laos, Vietnam and perhaps Myanmar, where it now occurs only patchily. Hunting intensity is lower in Thailand, where the species is considered relatively secure. Red List VU, population trend Decreasing.

Plate 74

| EUROPEAN BADGER Meles meles, ASIAN BADGER Meles leucurus, JAPANESE BADGER Meles anakuma |
NORTHWEST ASIAN BADGER (M. leucurus)
ANAGUMA (M. anakuma)
European Badger: HB 56–87cm; T 11.5–20.5cm; W 4.8–17.1kg (exceptionally >30kg during autumn hypophagia).
Asian Badger: HB 48.5–70cm; T 11–20cm; W 3.2–10.5kg.
Japanese Badger: HB 50.5–80cm; T 14–20cm; W 4.2–9kg Until recently, considered 1 species, Eurasian Badger (Meles meles), with 3 regional subspecies distributed throughout Eurasia from the UK to Japan; each subspecies is now recognised as a full species based on molecular, morphological and distributional differences. European Badger population from Turkey south of the Caucasus Mountains and Caspian Sea, to S Tajikistan, is genetically and morphologically somewhat distinct, and may be a fourth species, Southwest Asian Badger (M. canescens); this awaits wider consensus. European Badger is the largest badger species; Asian and Japanese badgers are smaller on average. European Badger is typically brindled silvery grey, with black legs and alternating black-and-white facial stripes. Albino, melanistic and erythristic European individuals occur. Coloration in Asian and Japanese badgers is variable. Some individuals resemble a slightly paler version of the European Badger with reduced facial stripes, but body fur colour varies from yellowish grey to greyish black or pure black; and varies similarly widely for facial markings, from narrow stripes to dark spectacles, or markings absent entirely. The degree of facial striping is least in Japanese Badger and the Far Eastern form of the Asian Badger (‘Amur Badger’).
Distribution and Habitat
European Badger: UK, W Europe from Iberian Peninsula to Fenno-Scandinavia, to the west bank of Volga River, and south through Turkey to Sinai Peninsula, through N Iraq and N Iran to Afghanistan–Tajikistan border. Asian Badger: from Volga River east across S Russia, C Asia, Mongolia, E China and Korean Peninsula, including Jeju Island. Japanese Badger: endemic to Japan (Honshu, Kyushu, Shikoku, Shodoshima). Asian and European badgers are sympatric in Volga River region and Uzbekistan–Tajikistan borderlands. European Badger and Japanese Badger inhabit mainly forest and associated scrub or grassland habitats, as well as farmland, fields and urban habitats. Asian Badger inhabits forest, scrubland, grassland, steppes and semi-desert with scrub cover.
Feeding Ecology
Omnivorous, feeding mainly on soil-living invertebrates and insects; wild and cultivated fruits (including berries), hard mast, grains, tubers and mushrooms; and small mammals such as mice, voles and shrews. Earthworms are a major component of the diet in many populations, especially of European and Japanese badgers. Asian Badgers generally occupy more arid habitats with harsh winters, where earthworms are less common in the diet. Hedgehogs, rabbits, small birds, herptiles and eggs are opportunistically consumed; European Rabbit is the main component of European Badger diet in Doñana NP, Spain. Exceptionally kills very young lambs and poultry (recorded for European and Asian badgers). Foraging is mainly solitary. European Badgers congregate, sometimes in large groups of >20, at food-rich patches, including artificial feeding sites. Nocturno-crepuscular, with increased diurnalism where undisturbed. In parts of the range experiencing severe winters, all species undergo partial hibernation with opportunistic foraging, but can remain underground for months in protracted winters, living entirely off fat reserves. Scavenges from carrion, bird feeders, pet bowls and human refuse.
Social and Spatial Behaviour
Sociality is flexible and complex, and linked to availability of clumped, rich food sources, especially earthworms. It is most gregarious and reaches highest densities where food patches are dense and frequent, e.g. lowland UK, living in large communal clans numbering up to 29, averaging 5–8 adults. Clan adults are mostly inter-related, with a minority of unrelated immigrants. Clans share territory, with extensive burrow systems called setts, where members gather to interact before setting off to forage alone. Clans actively repel strangers; neighbouring ranges may overlap, but core areas with main setts are defended, sometimes violently. Clans tend to be smaller for European Badger in southern and eastern parts of the range (linked to lower food availability), where mated pairs with shared ranges are typical. Japanese Badgers are mainly solitary in Japan, with females maintaining small, relatively exclusive ranges, which they share with offspring, often for extended periods (up to 14 months for female and 26 months for male offspring); adult males have larger ranges, which are flexible, approximately doubling in size during the breeding season, when they attempt to visit as many females ranges as possible. Asian Badgers are poorly known but assumed to follow broadly similar social patterns linked to food patch availability. Territory size 0.1–0.4km2 (Japan) and 0.3–1.5km2 (UK), to 4–24.4km2 (Poland). Density estimates: 0.2–0.25/km2 (Finland), 0.9/km2 (Ireland), 1.6–2.6/km2 (Poland), 4/km2 (Japan, suburbs) and 4.7–25.3/km2 (UK).
Reproduction and Demography
In clans, multiple adults of both sexes breed, and females often mate with males from neighbouring clans. Breeding occurs year-round, but mating peaks February–May (European Badger, UK) and April–August (Japanese Badger), with most births December–April. Gestation includes a very variable period of delayed implantation with a range of 90–300 days. Litter size 1–5, averaging 2–3. Weaning begins at 12 weeks, but extends to 6 months under low food availability. MORTALITY Annual mortality rates estimated at approximately 50% of cubs and 30% of adults. Natural mortality occurs mainly from starvation (especially cubs) and predation; Asian Badger is preyed upon by Amur Tiger; Russia). LIFESPAN European Badger: 14 years in the wild, 16 in captivity; poorly known for other species.
Status and Threats
Widespread, common and found in many protected areas. Main threat in Europe is roadkill, which claims 50,000 individuals in Britain and 10–15% of Danish and Dutch populations annually. Persecuted as a pest and used for illegal ‘baiting’ with terriers. Controversially culled as a carrier of bovine tuberculosis (bTB) in the UK, despite strong evidence that culls do not reduce incidence of the disease. Asian Badgers are widely hunted for bushmeat and traditional medicinal purposes; badger farms, e.g. in South Korea, are mostly stocked with wild-caught animals, the sustainability of which is dubious. About 200 Japanese Badgers are legally culled each year in Kyushu, apparently to reduce crop damage; this number rose to more than 6,000 in 2016 as a result of an ill-advised government bounty programme, which also killed many Masked Palm Civets and Raccoon Dogs. All 3 species are persecuted for damage to crops, heavily in China and Japan. European Badger: Red List LC, population trend Stable. Asian Badger: Red List LC, population trend Unknown. Japanese Badger: Red List LC, population trend Decreasing.
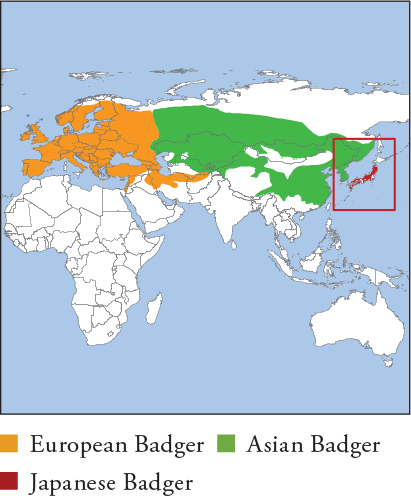
Plate 75

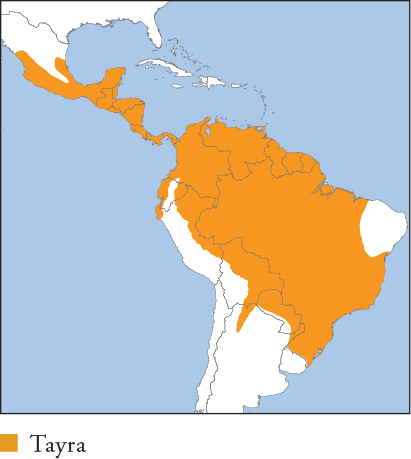
| TAYRA Eira barbara |
GREY-HEADED TAYRA, EIRA
HB 55.9–71.2cm; T 36.5–47cm; W 2.7–7kg
Largest mustelid in Latin America, excluding otters. Typically dark smoky brown to black, often with a creamy-yellow or white throat patch and a pale head. Completely pale individuals occur, including a uniformly golden form (probably leucistic) recorded from N South America to S Brazil. The Tayra belongs in a unique genus, and its closest relatives are thought to be the Fisher and Wolverine. Distribution and Habitat C Mexico, through Central America to N Argentina, Paraguay and C Uruguay. Occurs in various dry and wet forests and forest woodland, and in meadows, grassland and savannah in forested mosaics. Recorded up to 3,100m (E Andean slopes, Ecuador), generally rare above 2,000m; absent from high Andes and Brazilian caatinga. Tolerates agriculture, plantations and pasture in association with forest. Feeding Ecology Omnivorous, eating mammals to the size of agoutis, Southern Opossum, small primates (Common Marmosets, squirrel monkeys and tamarins) and neonate sloths, as well as reptiles to the size of Green Iguanas, birds, eggs, invertebrates (adults, eggs and pupae), fruits, honey and carrion. Pursues large prey, including brocket deer and large adult primates, but successful hunts are unknown; an observed attack on an adult Pale-throated Three-toed Sloth was unsuccessful. Tayras raid the nests of land-nesting reptiles for eggs, including those of various caiman species. They are recorded caching unripe fruit (plantains and sapote) in bromeliads and tree cavities for later consumption. Usually forages alone, although adult pairs and family groups are observed. Cathemeral, and equally at home on the ground or in trees. Social and Spatial Behaviour Poorly known. Assumed to be mainly solitary. Small social groups occur, usually assumed to comprise a female with large offspring, but this has not been confirmed. Limited data indicate that adult ranges overlap extensively. Range estimates 5.3–16km2 (♀s) and 24.4km2 (1 ♂) in Belize. Reproduction and Demography Thought to breed year-round. Gestation 63–70 days. Litter size 1–3. Weaning at around 2–3.5 months. Males are occasionally recorded with family groups, but apparently do not help raise juveniles. MORTALITY Unknown. LIFESPAN 18 years in captivity. Status and Threats Widespread and often common. Main threat is loss of forested habitat and agricultural intensification, leading to local endangerment, e.g. in Mexico. It is hunted in parts of its range for fur or meat, and killed as a predator of poultry, which may contribute to local declines. CITES Appendix III – Honduras; Red List LC, population trend Decreasing.
| WOLVERINE Gulo gulo |
GLUTTON, SKUNK-BEAR
HB 65–105cm; T 17–26cm; SH 36.5–43.2cm; W ♀ 6.6–14.8kg, ♂ 11.3–18.2kg
Largest terrestrial mustelid. Heavily built, with a bear-like head, short, powerful limbs and a bushy tail. Dark brown with a blond to rusty-brown fringe running from the shoulders along the sides; this extends in some individuals to cover the entire upper body in a pale cape. Forehead is often grizzled grey to blond. Cream to white markings on the chest are common; they extend to the front legs and feet in some individuals. Distribution and Habitat Circumpolar, mostly north of 50ºN from Fenno-Scandinavia through Russia, N Mongolia, N China, Canada, Alaska and W USA in the Rockies. Inhabits coniferous and deciduous forests, open rocky terrain and Arctic tundra. Strongly associated with deep snow and dead timber for denning. Actively avoids areas of human disturbance, including agricultural land, roads, skiing fields and recent logging. Feeding Ecology Relies heavily on ungulates, particularly winter-killed or predator-killed carrion, but actively hunts ungulates (mainly juveniles; capable of killing adult Moose and Reindeer in deep snow), e.g. 28% of food items in Norway consist of Reindeer killed by Wolverines. Also actively hunts small to mid-sized vertebrates such as marmots, porcupines, beavers, ground squirrels, lagomorphs, meso-carnivores (e.g. Red Foxes), small rodents and birds, especially ground-feeders such as ptarmigans and grouse. Consumes eggs, invertebrates, fruits and fungi. Known to raid trap-lines for captured fur-bearers, scavenges hunter refuse (gut-piles etc.) and sometimes kills livestock (mainly sheep lambs). Semi-domestic, free-ranging Reindeer, both killed and scavenged, make up 40–95% of prey items in N Norway and Sweden. Scavenges whale and seal carcasses in coastal Alaska. Foraging is cathermal, solitary and mostly terrestrial, although it is a strong climber and swimmer known to forage in trees and water. Hoards surplus food under rock piles, ice or snow, sometimes creating large caches, e.g. 20 Red Foxes and 100 ptarmigans in one cache (Russia). Social and Spatial Behaviour Solitary, with very large, stable ranges. Male ranges are larger and overlap multiple female ranges. Adults exclude same-sex conspecifics, but range overlap can be considerable. Covers large daily distances to 35km, driven in part by the search for carrion; summer movements tend to be larger than winter ones. Territory size estimates include 31–560km2 (♀s) and 133–1,131km2 (♂s) in N Sweden, 53–232km2 (♀s) and 488–917km2 (♂s) in NW Alaska, and 175–692km2 (♀s) and 845–2,127km2 (♂s) in Idaho. Subadults have ranges 2–3 times as large as adults in the same population, e.g. on average 400km2 (adult ♀s) and 1,175km2 (subadult ♀s), and 1,160km2 (adult ♂s) and 3,292km2 (subadult ♂s; Greater Yellowstone Ecosystem). Density poorly known, but it is naturally rare with low densities, estimated at 3.5/1,000km2 (Greater Yellowstone) to 15.3/1,000km2 (N Montana). Reproduction and Demography Seasonal. Mating May–August; births January–April. Wolverines require snow cover for denning, and reproduction is limited to areas with snow persisting to mid-May, the end of the denning period. Gestation 215–272 days (captivity), with delayed implantation. Litter size 1–5, averaging 2–3. Weaning at 7–8 weeks. Independence at around 8–10 months, dispersal at 12–13 months. Wolverines are excellent dispersers, regularly covering >200km and occasionally 400–500km. Females breed on average at 3.4 years. MORTALITY Annual adult and subadult mortality, respectively, is 26% and 43% (trapped), and 12% and 7% (not trapped). Most natural mortality is due to starvation and predation by large carnivores, including Grey Wolf, Puma; North America) and other Wolverines. LIFESPAN 13 years in the wild, 18 in captivity. Status and Threats Wide distribution, with numerous large and continuous populations, but the species occurs in very low densities, and it is sensitive to persecution and disturbance. It has declined in large areas of W USA and S Europe. Threatened by overtrapping, predator-control programmes, illegal killing for livestock depredation and habitat conversion. Given the species’ strong reliance on snow cover for reproduction, climatic warming is predicted to reduce suitable habitat and fragment populations. Red List LC (VU in Europe), population trend Decreasing.
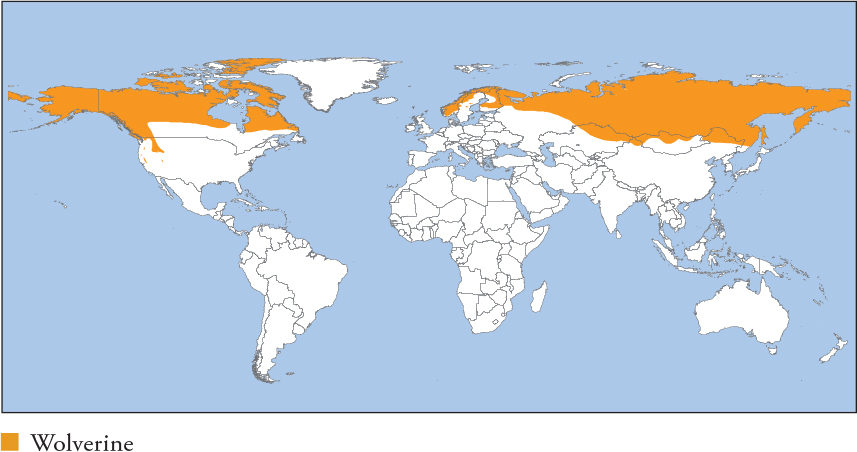
Plate 76

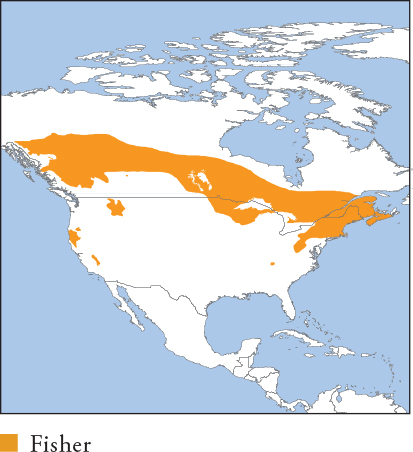
| FISHER Pekania pennanti |
HB 45–65cm; T 25.3–50cm; W ♀ 1.3–3.2kg, ♂ 3.5–5.5kg (exceptionally to 9kg)
Classified in its own genus, reflecting very early divergence (with Tayra) from true martens (Martes). Grey-brown to silver-tipped black with paler head and shoulders, sometimes with white or cream throat, chest and groin. Distribution and Habitat S Canada, extreme SW Alaska, W, Mid-west and NE USA. Strongly prefers intact forest generally <1,250m with dense canopy. Uses logged forest but avoids large open areas, clear-cuts and human disturbance. Feeding Ecology Often specialises in North American Porcupine and Snowshoe Hare, but also eats other lagomorphs, squirrels, small rodents, birds, eggs, herptiles, invertebrates, fungi and carrion. Foxes, raccoons and skunks are recorded prey, and cannibalism is documented. Rarely kills domestic poultry. Foraging is solitary, nocturno-crepuscular and mainly terrestrial, but Fishers are extremely capable climbers; they chase porcupines to the ground, where they are killed. Fishers cache small kills, and scavenge from refuse and pet bowls. Social and Spatial Behaviour Solitary and territorial. Average range size generally 2.1–29.9km2 (♀s) and 9.2–38.7km2 (♂s); largest ranges in N British Columbia, averaging 33km2 (summer, ♀s), and 122km2 (summer, ♂s). Rapidly covers large distances, up to 90km in 3 days. Density 8.6–11.2/1,000km2 (low-quality spruce forest, N British Columbia) to 14–52/100km2 (high-quality habitat, California). Reproduction and Demography Seasonal. Mating March–May; births late February–early May (following year). Gestation 236–275 days, with delayed implantation (total embryonic development ~50–55 days). Litter size 1–6, averaging 2–3. Weaning at 2–3 months, and dispersal from 7–9 months. MORTALITY In California, where trapping is illegal, mortality is mainly from predation (60–77% of known deaths) by Pumas, Bobcats, Coyotes and domestic dogs, natural disease (13–23%) and human causes. LIFESPAN 7.5 years in the wild, 10 in captivity. Status and Threats Extirpated from most of historic distribution by overtrapping for furs; now recovered or reintroduced in much of eastern and Great Lakes range; recovery is poor in the Pacific Northwest. Legally trapped in most of its range, impacting populations in low-quality habitat. Red List LC, population trend Unknown.
| SABLE Martes zibellina |
JAPANESE SABLE
HB 35–56cm; T 11.5–19cm; W ♀ 0.7–1.6kg, ♂ 0.8–1.8kg
Honey brown to very dark brown, paler head and a small white or cream throat patch (often absent). Japanese Sables are often rich yellow or tawny brown with a light grey head, similar to some winter forms of Japanese Marten. Distribution and Habitat Russia (~95% of range), extreme NE Kazakhstan to North Korea and Hokkaido, Japan. Closely tied to temperate debris-rich, dense-canopy forest to 2,200m. Avoids open areas and disturbed habitat. Feeding Ecology Eats mainly small rodents, pikas and Mountain Hare, and also seeds, berries, nuts, invertebrates, birds, fish and freshwater crustaceans. Capable of killing adult Siberian Musk Deer in deep snow. Occasionally kills domestic poultry. Foraging is solitary, cathemeral and mostly terrestrial, although Sables are very agile climbers. Scavenges, mainly from winter-killed ungulates, and caches surplus food. Social and Spatial Behaviour Solitary. Average range size 7.2km2 (♀s) and 13.1km2 (♂s) with little intrasexual overlap (open larch taiga, NE China), and only 1.12km2 (both sexes) with high intrasexual overlap (high-quality forest, Japan). Reproduction and Demography Seasonal. Mating June–August; births April–May (following year). Gestation 236–315 days, with delayed implantation (embryonic development 25–40 days). Litter size 1–5, averaging 2–3. Weaning at around 7–8 weeks. MORTALITY Trapping accounts for most mortality (especially in Russia); Red Fox is a confirmed predator. LIFESPAN 5.5 years in the wild, 15 in captivity. Status and Threats Sable fur is highly sought after; historical overharvest caused widespread declines. Hunting bans and reintroductions have led to recovery; Russian population is now estimated at >2 million. Commercially hunted, mainly in Russia (>700,000 in 2011–12 trapping season), and farmed for fur. Red List LC, population trend Increasing.

| AMERICAN MARTEN Martes americana PACIFIC MARTEN Martes caurina |
HB ♀ 32–40cm, ♂ 36–45cm; T 13.5–23cm; W ♀ 0.3–0.85kg, ♂ 0.47–1.3kg
Formerly classified as 1 species; genetic analyses now separate Pacific Northwest–Rocky Mountains populations as the Pacific Marten. The 2 species are extremely similar in appearance and ecology. Very variable, from tawny beige with dark limbs to uniformly dark chocolate brown. Head is usually paler, buff-brown or greyish. All forms have a cream to yellow throat and chest. Distribution and Habitat American Marten: Canada, Alaska, marginally into Mid-west and NE USA. Pacific Marten: Pacific Northwest coast from S British Columbia to California, and US Rocky Mountains to New Mexico. They are sympatric and hybridise in N Montana and SE Alaska. Both prefer mature temperate forest and woodland with a closed canopy and dense understorey. They avoid open or disturbed habitat, including clear-cuts and recently logged areas. Feeding Ecology Both species eat small rodents, and Snowshoe Hare (mainly during cyclical irruptions). Also eat invertebrates, birds, fish, herptiles, fruits and seeds. They rarely take domestic poultry. Foraging is solitary and mainly nocturno-crepuscular. They hunt terrestrially, arboreally, and under snow. They scavenge from carrion and cache excess food. Social and Spatial Behaviour Solitary and territorial. Average range size 2.3–27.6km2 (♀s) and 4.3–45km2 (♂s). Density estimates 0.4–1.5/km2. Reproduction and Demography Seasonal. Mating July–August; births late March–April (following year). Gestation 220–275 days, with delayed implantation (embryonic development ~40 days). Litter size 1–5, averaging 2–3. Weaning at 6–7 weeks; kits kill small prey at 2.5 months. MORTALITY Adult mortality before trapping 7% (both sexes) increasing to 51% (♀s) to 74% (♂s) during trapping (American Marten, Maine). Mortality in unharvested populations 13–44%, mainly from predation by Bobcat, raptors and other martens. Infanticide by males occurs rarely. LIFESPAN 14.5 years in the wild (typically <5), 15 in captivity. Status and Threats Fur overharvests and forest loss have reduced populations, mainly in New England (American Marten) and coastal W USA (Pacific Marten), where they remain fragmented and rare. Both have benefited from reintroduction projects, especially American Marten. Both: Red List LC, population trend Decreasing.
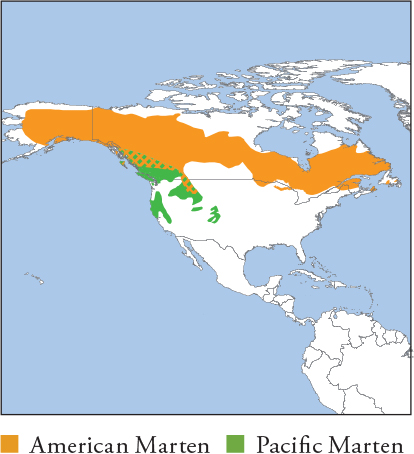
Plate 77

| STONE MARTEN Martes foina |
BEECH MARTEN
HB 40–54cm; T 22–30cm; W 1.1–2.3kg
Typically rich, dark brown with a slightly paler, greyish head, and light tawny underfur, especially on the sides and underparts. Throat has a distinctive white or cream patch that often extends down the front legs. Distribution and Habitat Continental W and C Europe through C Asia, Bhutan, N India, Nepal and N Myanmar to Mongolia and China. Introduced to Wisconsin, USA. Inhabits forest, shrubland, forest edges, hedgerows and rocky hillsides to 4,200m. Occurs near humans, including in densely populated urban areas (W and C Europe). Feeding Ecology Diet varies seasonally and regionally with fluctuating proportions of 2 main food groups: small mammals, especially voles, mice and rabbits; and fruits, including berries. Also eats insects, birds, herptiles, eggs, seeds and other plant items. Urban populations frequently eat commensal birds like pigeons. Sometimes kills domestic poultry. Foraging is solitary and nocturno-crepuscular. Urban Stone Martens readily scavenge from refuse, bird feeders, pet bowls and handouts. Social and Spatial Behaviour Solitary and territorial, with male ranges overlapping multiple female ranges. Ranges tend to be smallest in urban areas, intermediate in rural areas and largest in forested habitat. Range size 0.095–8.8km2, averaging 0.37–0.49km2 (♀s) and 1.11–1.13km2 (♂s) in rural/village areas. Reproduction and Demography Seasonal. Mating July–August; births March–mid-April (following year). Gestation 236–275 days, with delayed implantation (embryonic development ~30 days). Litter size 1–8, averaging 3–4. Weaning at around 6–8 weeks in late May–early June, and dispersal from 6 months. MORTALITY Most known mortality is anthropogenic. LIFESPAN 18.1 years in captivity, much lower in the wild. Status and Threats Widespread and adaptable, reaching high densities in urban habitats. Considered a nuisance in C Europe due to its habit of sheltering in car-engine spaces and chewing leads and hoses, e.g. 160,000 damaged cars in Germany in 2000, for which it is sometimes legally and illegally killed. Hunted for fur in India and Russia. CITES Appendix III – India; Red List LC, population trend Stable.

| PINE MARTEN Martes martes |
EUROPEAN PINE MARTEN, EURASIAN PINE MARTEN
HB 45–58cm; T 16–28cm; W 0.8–1.8kg
Similar to Stone Marten, with which it overlaps in most of W and C Europe, and distinguished by a yellowish throat patch. Distribution and Habitat UK, continental W and C Europe to Fenno-Scandinavia, W Siberia (Russia), Turkey, N Iraq and N Iran. Occurs mainly in mature intact forest, woodland and scrubland with dense understorey. Inhabits coastal shrubland, pasture and grassland with cover, but avoids open areas. Occurs near settlements, but does not readily colonise urban areas as do Stone Martens. Feeding Ecology Preys predominantly on small mammals, especially Field Vole, red-backed voles, field mice, Wood Lemming, squirrels and lagomorphs; a camera-trap set in Poland recorded a marten killing 2 young Red Fox cubs in their den. Other important prey includes invertebrates, birds, herptiles, fruits (including berries), eggs and carrion (mainly wild and domestic ungulate carcasses). Sometimes kills domestic poultry. Foraging is primarily nocturnal and solitary. Scavenges, mainly from carrion and rarely from urban sources. Caches excess food. Social and Spatial Behaviour Solitary and territorial, with male ranges overlapping multiple female ranges. Range size correlates with forest cover and rodent density. Smallest ranges are in mature forest with high rodent abundance, e.g. Poland and Germany; largest known ranges are in open habitat in Finland and Scotland. Average range size 1.4–9.8km2 (♀s) to 2.3–28.6km2 (♂s). Reproduction and Demography Seasonal. Mating July–August; births March–April (following year). Gestation 230–274 days, with delayed implantation (embryonic development ~30 days). Litter size 2–8, averaging 3–5. Weaning at around 6–8 weeks; dispersal from 6 months throughout winter. MORTALITY Annual mortality in protected forest (Poland) is 38.4% (adults and subadults, sexes combined), from canine distemper, winter starvation, poaching, and predation by Eurasian Lynx, Red Fox and raptors. LIFESPAN 5 years in the wild, 17 in captivity. Status and Threats Formerly very heavily hunted for fur, resulting in declines and local extinctions, especially in Russia and Fenno-Scandinavia. Stricter controls have led to recovery, and it is now fairly widespread, but still harvested at questionable levels in some areas. Also illegally persecuted as a pest, the main reason it disappeared from much of the UK, where it is now recovering (especially in Scotland). Red List LC, population trend Stable.

| JAPANESE MARTEN Martes melampus |
YELLOW MARTEN, TSUSHIMA ISLAND MARTEN
HB 47–54.5cm; T 17–22.3cm; W 0.7–1.7kg
Small, slender marten. Rich, dark brown in colour, with a large, rich yellow throat patch; populations on Kyushu and northern Honshu moult in winter to a vivid orange-yellow with a white to pale grey head. Tail sometimes has a white tip. Distribution and Habitat Endemic to Japan (introduced to Hokkaido and Sado for fur, native to other islands). Records from the Korean Peninsula are equivocal. Occurs mainly in broadleaved forest, woodland and subalpine shrubland. Inhabits rural and urban areas with natural forest patches. Feeding Ecology Feeds mainly on small mammals, insects, centipedes, earthworms, spiders, snails, fruits (including berries) and seeds. Considered an important seed disperser in subalpine areas due to the amount of fruit it consumes. Also eats birds, frogs and various plant items. Occasionally takes domestic poultry and is easily trapped with chicks. Foraging is nocturnal and solitary. Social and Spatial Behaviour Adults are solitary and probably territorial; they deposit scats at range borders, with ranges overlapping little within the same sex. Range size 0.5–1km2, similar for females (average 0.63km2) and males (average 0.7km2, Tsushima Island). Reproduction and Demography Seasonal. Mating late July–mid-August; births mid-April–early May (following year). Gestation 230–250 days, with delayed implantation (embryonic development 28–30 days). All known litters number 2 (based on few observations). MORTALITY Rates unknown; main factors on Tsushima Island are roadkills (72%) and feral dogs (9%). LIFESPAN Unknown. Status and Threats Relatively widespread and common within its limited distribution. Main threats are habitat conversion (including to forestry monocultures), roadkills and predation by feral dogs. Legally trapped for fur (not on Tsushima Island) in December–January. Red List LC, population trend Stable.

Plate 78

| NILGIRI MARTEN Martes gwatkinsii |
HB 50–70cm; T 35–50cm; W 1–3kg
Very similar to Yellow-throated Marten and considered by some authors to be the same species; separated as a distinct species in part due to its isolated, discontinuous distribution. Recent genetic analyses, which would help resolve the controversy, are lacking. Appearance of both is similar, but Nilgiri Marten is typically dark brown over the entire upper body and lacks the Yellow-throated Marten’s yellow cape, with golden yellow restricted to throat and chest; the shoulders and torso sometimes tend towards pale rufous-brown. Distribution and Habitat Endemic to the Western Ghats, India, where most records are from 6 mostly disjunct populations, although it may occur in between. Strongly associated with evergreen forest patches and forest–grassland mosaics in undeveloped montane and hilly areas, mostly at medium to high elevations of 800–2,600m, occasionally as low as 120m. Sometimes found in adjoining (within 3km of forest) plantations of tea, coffee, cardamom, acacia and wattle. Feeding Ecology Poorly known. Assumed to be similar to that of Yellow-throated Marten. Has been observed pursuing Indian Giant Squirrel (with which it is sometimes confused due to strikingly similar pelage), Indian Spotted Chevrotain and Bengal Monitor, and eating the nectar of cultivated kapok trees. Raids domestic beehives, thought to be mainly for bee larvae, although honey and honeycomb are doubtless also consumed. Social and Spatial Behaviour Poorly known. Has been sighted singly and in pairs, consistent with the little that is known of Yellow-throated Marten sociality. It is observed and recorded in surveys at markedly lower rates than the Yellow-throated Marten, suggesting it naturally occurs at low densities. Reproduction and Demography Unknown. Status and Threats Distribution is very restricted, calculated to be about 24,500km2, and very fragmented. Thought to be naturally rare based on the frequency of encounters, with an estimated total population of around 1,500 (1,000 of which are mature adults). The Western Ghats are under intense anthropogenic pressure, and further habitat loss and fragmentation are the main threats. Also killed by beekeepers as a perceived pest, and illegally hunted by some communities for meat, although both are considered to have declined in recent years, and the species is thought to be increasing in some areas where persecution was formerly pervasive. CITES Appendix III India; Red List VU, population trend Stable.

| YELLOW-THROATED MARTEN Martes flavigula |
HIMALAYAN YELLOW-THROATED MARTEN, KHARZA
HB 45–65cm; T 37–45cm; W 1.3–3kg
Large marten with a long tail up to 70% the length of its body. Head, nape, hindquarters and tail are normally dark brown with a highly variable tawny-brown cape covering the rest of the upper body, although this is entirely absent in some animals, especially in Peninsular Malaysia, Borneo and Sumatra. Throat and chest are always golden lemon yellow, sometimes extending down the forelimbs. Chin and cheeks are white. Distribution and Habitat From the Russian Far East through the Korean Peninsula and E and S China, including Taiwan, Indochina, Sumatra, Java and Borneo, and extending through the N Indian subcontinent to N Pakistan and N Afghanistan. Inhabits temperate and tropical forests from sea-level to 4,150m. Occurs in secondary forest and plantations, but avoids open anthropogenic habitats. Feeding Ecology Omnivorous, with a wide, opportunistic diet, although detailed studies of feeding ecology are mostly lacking. Eats small mammals, birds (including large ones such as pheasants), reptiles, amphibians, invertebrates, eggs, fruits (including berries), flowers and nectar. Its diet shifts to take advantage of seasonal foods, e.g. focuses on fruits and flowers during spring and summer. Has been observed pursuing large mammals, including Himalayan Tahr, Himalayan Musk Deer and gorals, although the outcome was not observed. A pair was filmed attacking an injured adult Nepal Grey Langur (Jim Corbett NP, India) on the ground, which was unable to flee; the martens eventually lost interest while the langur was still alive. Seven martens were observed feeding on a Chinese Goral, and tahr has been found in scats, but they are extremely unlikely to kill such large prey and are known to take carrion, e.g. one was photographed scavenging the carcass of a Red Muntjac suspected of dying from a snakebite, Huai Kha Khaeg WS, Thailand. A marten was photographed in Chitwan NP, Nepal, carrying a dead adult Small Indian Civet, although it was unclear whether it was killed or scavenged. The nests of social insects, especially wasps and bees, are readily plundered. Beeswax appears in scats throughout the year in Jirisan NP, South Korea, although the remains of honeybees are not, suggesting honey rather than bees is the main food item. Reportedly raids poultry from coops (West Bengal, India). Primarily diurnal, with greater nocturnalism near people, and forages both terrestrially and arboreally; very agile and adept at pursuing prey in trees. Scavenges boiled rice at guardposts in Khao Yai NP, Thailand, and frequents refuse dumps. Social and Spatial Behaviour Poorly known. More often seen in pairs and trios than alone. Pair/group composition is unclear and may comprise females with large kittens, but almost all records involve adult-sized animals, and their frequency of occurrence suggests greater sociality than occurs in other marten species. Also found in larger groups, e.g. at carcasses, which are probably temporary aggregations. Only range estimates (Phu Khieo WS, Thailand) are 8.8km2 (1 ♀) and 1.7–11.8km2 (♂s). Reproduction and Demography Poorly known. Thought to be seasonal. Mating occurs June–August; births March–June (following year). Gestation 220–290 days, with delayed implantation. Litter size 2–5. MORTALITY Unknown. LIFESPAN 14 years in captivity. Status and Threats Widespread and considered secure. Presumably undergoes declines with forest loss and fragmentation. Remains relatively common even in areas with high hunting pressure, e.g. Indochina, perhaps because it has unpleasant-tasting flesh and few communities eat it. The fur is generally not considered valuable; it is hunted for fur in Afghanistan, Pakistan, Russia (Siberia) and North Korea. CITES Appendix III – India; Red List LC, population trend Decreasing.
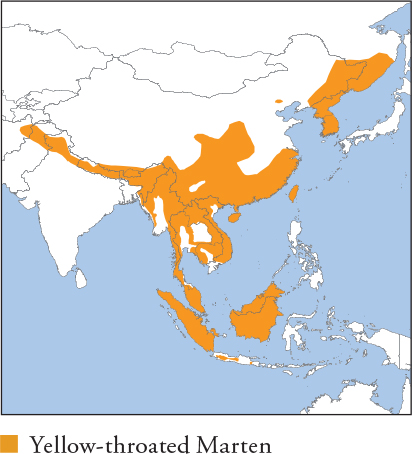
Plate 79

| SMALL-TOOTHED FERRET BADGER Melogale moschata |
CHINESE FERRET BADGER
HB 31.5–42cm; T 13–21.1cm; W 0.8–1.6kg
Ferret badgers show slight external differences between the 4 well-described species, and their taxonomy is in need of review. In 2011, a fifth species, Vietnam or Cuc Phuong Ferret Badger (M. cucphuongensis) was described based on 2 specimens from Cuc Phuong NP, Vietnam. It differs markedly from the other species, having a very distinct elongated skull with a very narrow, slender snout, and largely unmarked brown pelage with creamy-brown underparts. Genetic analysis suggests it is likely a distinct species, although further analysis with more samples is necessary. Its tiny known distribution is also home to Small-toothed Ferret Badger and Large-toothed Ferret Badger. The latter two are sympatric in much of their ranges; they are virtually indistinguishable; the Small-toothed is slightly smaller, generally has a shorter white dorsal stripe and has markedly smaller premolars. Distribution and Habitat S and C China, Taiwan, N Myanmar, Laos, Vietnam and NE India; possibly Bhutan, Cambodia and Thailand. Inhabits forest, woodland, scrub and dense grassland. Tolerates cultivated areas with cover and occurs near human settlements. Feeding Ecology Feeds chiefly on soil-living invertebrates, especially earthworms and insects, as well as fruits and seeds. Less important food includes small mammals, herptiles, carrion and eggs. Not known to prey on poultry. Foraging is solitary and almost exclusively nocturnal. Social and Spatial Behaviour Ranges overlap extensively and mixed-sex groups of up to 4 adults share ranges and setts, suggesting some sociality, including possible maintenance of group ranges as in European Badger, Range size is similar for sexes and averages 1.3km2 (range 0.51–4.7km2). Reproduction and Demography Poorly known. Thought to be seasonal. Mating assumed to be in March (China), but births occur through May–December (Taiwan). Gestation 53–80 days (captivity). Litter size 1–4. MORTALITY Unknown; rabies, canine distemper and SARS coronavirus (rarely) confirmed, but there is no evidence of population impacts. LIFESPAN 10.5 years in captivity. Status and Threats Status poorly known, but the species is widespread and considered common in much of its range. It is used for meat and traditional medicinal practices in Indochina, and heavily hunted in S China; despite this, it apparently remains relatively widespread. Red List LC, population trend Stable.

| LARGE-TOOTHED FERRET BADGER Melogale personata |
BURMESE FERRET BADGER
HB 33–43cm; T 14.5–23cm; W 1.5–3kg
Largest ferret badger, although differences between all species are slight. Dentition is relatively massive. Generally has more white coloration than other species. White dorsal stripe extends to at least the mid-point of the spine, the distal half of the tail is white and body fur has extensive white ‘frosting’. Sometimes considered the same species as Bornean and Javan ferret badgers. Distribution and Habitat SE Nepal, NE India, Myanmar, Thailand, Indochina and S Yunnan, China; presumably Bhutan, although no certain records. Occurs in similar habitats (including anthropogenic habitats) to Small-toothed Ferret Badger. Feeding Ecology Diet less well known than that of Small-toothed Ferret Badger, but assumed to be similar. Considerably more massive dentition suggests it is more predatory on small vertebrates, but this remains unconfirmed. Foraging is almost exclusively nocturnal. Social and Spatial Behaviour Unknown. Most records are of single adults or females with young; it is unknown if it is social or semi-social, as for Small-toothed Ferret Badger. Reproduction and Demography Poorly known. Reputed to be seasonal, with captives giving birth mainly May–June. Litters in captivity 1–3. MORTALITY and LIFESPAN Unknown. Status and Threats Status poorly known, but hunting pressure is very high in most of its range and it rarely appears in camera-trap surveys, e.g. it was not photographed in 8,499 camera-trap days in 2003–06 in Nam Et–Phou Louey PA, Laos. Occurs in a number of protected areas in Thailand. Red List LC, population trend Unknown.

| BORNEAN FERRET BADGER Melogale everetti |
KINABALU FERRET BADGER, EVERETT’S FERRET BADGER
HB 33–45cm; T 14.5–17cm; W 1–2kg
Usually described as mainly brown (rather than greyish), with typical ferret badger markings that are less extensive than in the continental species. The only ferret badger in its range. Distribution and Habitat Endemic to Sabah, Borneo. All certain records are from the Kinabalu massif and Crocker Range region, N Sabah, in montane broadleaved forest habitat at 900–3,700m; a record from lowland forest in E Sabah is erroneous and it is unclear whether it occurs more widely in Borneo. Feeding Ecology Poorly known. Reportedly eats soil-living invertebrates (especially earthworms), lizards, small birds, rodents and fruits. Nocturnal. One record exists of scavenging in roadside refuse dumps. Social and Spatial Behaviour Unknown. Most records are of single adults or females with young. Reproduction and Demography Unknown. Status and Threats Status very poorly known. Extremely small known distribution, estimated at <5,000km2, which includes protected areas, but much of the range is threatened by ongoing conversion to agriculture and hunting. This is possibly Borneo’s most threatened carnivore. Red List EN, population trend Decreasing.

| JAVAN FERRET BADGER Melogale orientalis |
HB 35–40cm; T 14.5–17cm; W 1–2kg
Physically indistinguishable from Bornean Ferret Badger and sometimes considered the same species (and both are sometimes treated as a subspecies of Large-toothed Ferret Badger). The only ferret badger in its range. Distribution and Habitat Endemic to Java and Bali. Formerly thought to be restricted to isolated montane areas, it is now suspected to be more widely distributed across Java and Bali. Preferred habitat is likely mid- to high-altitude primary and secondary forests at 800–2,230m, but it is also recorded in lowlands, including from highly modified habitats such as croplands and rubber plantations near human settlements. Feeding Ecology Diet is assumed to be similar to that of other ferret badgers. Anecdotally reported in association with tourist refuse in Gunung Gede Pangrango NP, W Java, but it is unclear if it scavenges from dumps. Nocturnal. Social and Spatial Behaviour Unknown. Reproduction and Demography Unknown. Status and Threats Status very poorly known. Known distribution is restricted and exposed to a high rate of forest loss and hunting. It has recently started appearing in small numbers in the local novelty pet trade. Red List LC, population trend Unknown.

Plate 80

| PATAGONIAN WEASEL Lyncodon patagonicus |
HURONCITO HB 30–35cm; T 6–9cm; W 0.2–0.25kg
Very small, pale, grizzled grey with chocolate-brown to black underparts. Wide wedge-shaped white crown covers the head, distinguishing it from the considerably larger Lesser Grison, which has a narrow white brow. Distribution and Habitat Endemic to Argentina and a narrow band of C to E Chile. Inhabits cold arid and semi-arid shrubland, steppes and open scrubby woodland from sea-level to 2,000m. Feeding Ecology Poorly known. Thought to hunt mainly small burrowing rodents such as tuco-tucos and mountain cavies; 1 record of predation on Elegant Crested Tinamou. An adult living under the ranger station at Cabo Dos Bahías WR, Argentina, scavenged handouts. The species has a novel series of forepaw muscles never previously described for mammals, possibly enhancing dexterity in handling small prey, although this is speculative. Social and Spatial Behaviour Unknown; assumed to be solitary. Reproduction and Demography Unknown. MORTALITY Black-chested Buzzard-eagle is a known predator. LIFESPAN Unknown. Status and Threats Status essentially unknown. Rarely observed or encountered during wildlife surveys, suggesting it is naturally rare. Red List LC, population trend Unknown.
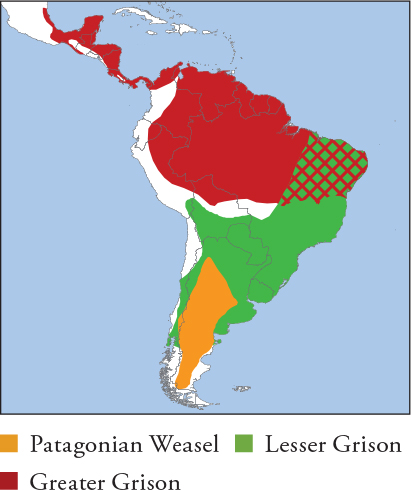
| LESSER GRISON Galictis cuja |
HB 27.3–52cm; T 12–19cm; W 1–2.5kg
Grizzled yellow-grey to brownish-grey upperparts. Black face and underparts bounded by a narrow white or creamy band across the brow to the shoulders. Moves with rapid, low-slung weasel-like movements compared to the Greater Grison’s heavy, bouncing gait (reminiscent of badgers). Distribution and Habitat SE Peru, S Bolivia, and S and E Brazil through Paraguay, Uruguay, Argentina, and C Chile. Occurs in desert, steppes, grassland savannah, shrubland, marshland, woodland and forest from sea-level to 4,200m. Tolerates agricultural and pastoral habitats. Feeding Ecology Diet dominated by small mammals, especially mice, rats and cavies, and introduced European Hare and European Rabbit; often focuses almost entirely on lagomorphs under high availability. Also eats birds, small reptiles, frogs, eggs and invertebrates. Fruits are consumed rarely. Blamed for killing poultry, but depredation is poorly quantified. Cathemeral. Social and Spatial Behaviour Poorly known. Adults are usually observed alone but small groups, including adult pairs and their juveniles, suggest monogamous pair bonds. Up to a dozen animals recorded playing together. Reproduction and Demography Gestation 39 days. Litter size 2–5. Juveniles recorded March–October, suggesting weak seasonality. Mated pairs apparently cooperate to raise kittens. MORTALITY Ocelot and Black-chested Buzzard-eagle are known predators. LIFESPAN Unknown. Status and Threats Widespread. Broad habitat tolerance and considered secure, although status is poorly quantified. Persecuted for killing poultry, often killed on roads (e.g. E Brazil) and by urban feral dogs (e.g. University of São Paulo, Piracicaba, SE Brazil). Red List LC, population trend Unknown.

| GREATER GRISON Galictis vittata |
HB 45–60cm; T 13.5–19.5cm; W 1.4–4kg
Larger than Lesser Grison, with a proportionally shorter tail and paler, grizzled, salt-and-pepper grey fur. The 2 species overlap in E Brazil. Distribution and Habitat E Brazil through N South America to SE Mexico. Occurs in low and mid-elevation forest woodlands, palm savannah, grassland and wetland to 1,500m. Tolerates disturbed forest, plantations, open fields and agricultural land with cover. Feeding Ecology Carnivorous, eating rodents to the size of agoutis, marsupials (including Southern Opossum), reptiles, amphibians, fish, invertebrates and eggs. Captives eat fruits and some plant matter. Sometimes raids domestic poultry. Foraging is cathemeral. Hunting is terrestrial, but readily pursues prey into trees and deep water. Hunts alone, in adult pairs or in small family groups. Social and Spatial Behaviour Assumed to be largely solitary; adult pairs and family groups occur, but sociality is poorly understood. Only range estimate is for 1 female (Venezuela) over 2 months, 4.15km2. Reproduction and Demography Gestation 39–40 days. Litter size 1–4. Juveniles recorded March–October, suggesting weak seasonality. Males often recorded with mothers and kittens, but it is unknown if they assist in raising juveniles. MORTALITY Unknown. LIFESPAN 10.5 years in captivity. Status and Threats Secure over much of its range, but threatened at the extremes, e.g. Mexico and Costa Rica. Tolerant of some disturbance, but hunting pressure and habitat conversion to open agriculture drives local declines. CITES Appendix III – Costa Rica; Red List LC, population trend Stable.

| MARBLED POLECAT Vormela peregusna |
HB 28.8–47.7cm; T 14.5–20.1cm; W 0.3–0.72kg
The sole Eurasian representative of the subfamily Ictonychinae; all members possess aposematic pelage and enlarged anal scent glands used in defensive threat displays. Very dark chocolate brown, with a striking buff-yellow cape dappled with red-brown blotches. Bushy tail grizzled yellow-white, usually with a dark tip. A conspicuous white stripe encircles the face, the tops of the ears are white, and the muzzle and chin are creamy white. Assumes a distinctive arching posture when threatened, followed by ejecting a noxious anal-gland secretion if unheeded. Distribution and Habitat N China, Mongolia, C Asia, the Middle East and SE Europe. Inhabits temperate and arid steppes, grassland, scrubland, rocky upland, salt marshes, semi-desert and open desert habitats. Occurs in cultivated areas, orchards and vegetable gardens near settlements, and in urban parkland. Feeding Ecology Diet dominated by small mammals, especially ground squirrels, jirds, hamsters, voles, rats, mice and rabbits. Also consumes insects (especially during spring–summer flushes), birds, herptiles, snails and fruits. Sometimes kills domestic poultry and rabbits. Foraging is mainly nocturno-crepuscular, solitary and by scent; reputedly has poor eyesight. Eats carrion and scavenges, including raiding larders for smoked meat and cheese. Caches surplus food in burrows. Social and Spatial Behaviour Solitary. Limited data indicate small stable ranges with moderate overlap. Outside the breeding season, adults sometimes fight furiously. Only known range estimates (Israel) 0.5–0.6km2, with little difference between sexes. Reproduction and Demography Seasonal. Mating March–June; births February–May (following year). Gestation 243–327 days, with delayed implantation. Litter size 1–8, averaging 4–5. Mothers reportedly use a unique (for carnivores) distraction display of feigning death and injury, belly-crawling away from hidden pups to divert predators. Weaning at 50–54 days and dispersal at 61–68 days. Females become sexually mature at 3 months; males typically breed after their first year. MORTALITY Poorly known. Most documented mortality is anthropogenic. LIFESPAN Almost 9 years in captivity. Status and Threats Nowhere common, and threatened by conversion of steppe habitats to cultivation, combined with large-scale poisoning of rodents, e.g. China and Mongolia. Killed in small numbers for fur and persecuted for killing poultry. Red List VU, population trend Decreasing.
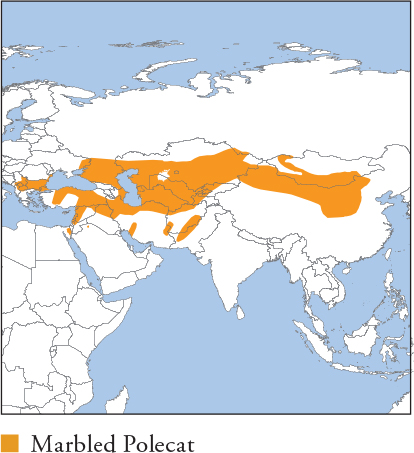
Plate 81

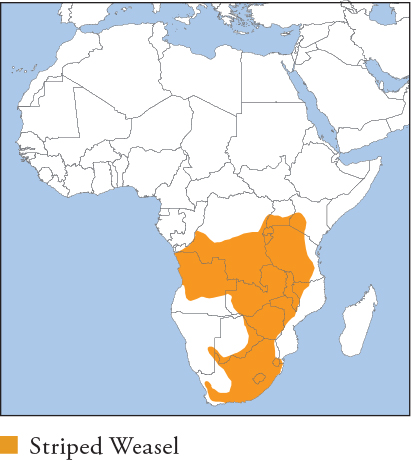
| STRIPED WEASEL Poecilogale albinucha |
AFRICAN STRIPED WEASEL, WHITE-NAPED WEASEL
HB ♀ 24–35cm, ♂ 27–33cm; T 13.8–21.5cm; W ♀ 0.21–0.29kg, ♂ 0.28–0.38kg
Small weasel with a long sinuous body, very short limbs and a long tail. Fur is black with a yellowish-white dorsal stripe starting at the crown; stripe splits into paired stripes that run along each side. Tail is white. Distribution and Habitat Sub-equatorial Africa, from S Kenya and S Uganda to coastal DR Congo, and south to South Africa. Inhabits woodland savannah, grassland, scrubland, forest (its range stops at the limits of the forested Congo Basin) and vegetated semi-arid desert, e.g. the Kalahari. Occurs in plantation, agricultural and pastoral habitats. Feeding Ecology Rodent specialist, hunting mainly small mice, rats and mole rats to its own size; an adult may kill 3–4 rodents a night. Also eats small reptiles, insects and eggs. Foraging is mainly nocturnal, terrestrial and solitary. Forages chiefly by scent, and is well suited to entering small rodent burrows; a powerful burrower, but has not been observed excavating prey. Rodents are killed with a nape bite and vigorous kicking by the hind legs, which may dislocate the neck; large prey is sometimes killed by a throat bite. Caches surplus kills in burrows. Social and Spatial Behaviour Poorly known. Assumed to be solitary; most sightings are of adult individuals or females with pups. Reproduction and Demography Possibly seasonal. Breeding September–April (southern Africa); births from November. Gestation 30–33 days. Litter size 1–3. Weaning at 11 weeks (captivity). Sexual maturity at 8 months. MORTALITY Poorly known. Occasionally killed by Black-backed Jackal, domestic dogs and large owls. Rabies is recorded. LIFESPAN 6 years in captivity. Status and Threats Considered uncommon to rare, but it is inconspicuous and elusive, and there is little accurate information on its status. Killed on roads in rural areas, and highly prized for traditional medicinal use in South Africa. Red List LC, population trend Unknown.
| LIBYAN WEASEL Ictonyx libycus |
SAHARAN STRIPED POLECAT,
NORTH AFRICAN STRIPED WEASEL
HB 20.7–26cm; T 11.4–18cm; W 0.2–0.6kg
Small, compact weasel with a black face, limbs and underparts. White stripes interleaved with variable black interstripes cover the body. Tail is long and white, with interspersed black hairs, and sometimes with a black tip. Fur is longish with a silky appearance. Unbroken white band encircles the face, running from the forehead behind the eyes to the base of the throat; this helps distinguish it from the similar Zorilla. It has well-developed anal glands and secretes a pungent fluid when threatened. Distribution and Habitat N Africa, on the edges of the Sahara in the coastal band of Mediterranean N Africa from Egypt to Mauritania, and through the Sahel from W Mali to the Sudan–Eritrea coast. Scattered records exist across the Sahara itself, but it is unclear if it occurs throughout. Occupies mainly sub-desert habitats such as stony desert, massifs, steppes, oases and sparsely vegetated dunes. Found close to settlements in cultivated areas. Feeding Ecology Poorly known. Thought to feed mainly on small desert rodents, birds, reptiles, eggs and invertebrates. Nocturnal. Social and Spatial Behaviour Unknown. Most records are of single adults; assumed to be solitary. Reproduction and Demography Poorly known. Thought to be seasonal; all records of young occur January–March. Litter size 1–3. MORTALITY Unknown. LIFESPAN 5.5 years in captivity. Status and Threats Status poorly known. Widely distributed and locally abundant in some coastal dune areas. Hunted in Libya and Tunisia in the belief that its body parts increase human male fertility. Red List LC, population trend Unknown.

| ZORILLA Ictonyx striatus |
STRIPED POLECAT
HB 28–38cm; T 16.5–28cm; W ♀ 0.4–1.4kg, ♂ 0.7–1.5kg
Larger than the similar Striped and Libyan weasels. Jet black with 4 white stripes that unite on the crown and run the length of the body to the tail, which is white interspersed with black hairs. Face is distinctively marked with a cluster of 3 white blotches on the forehead and on each temple. Overlaps Libyan Weasel in the Sahel. Ejects a noxious anal secretion when threatened. Distribution and Habitat Throughout sub-Saharan Africa, except the Sahara and Congo Basin. Occurs in a wide variety of habitats from sea-level to 4,000m, including wet and dry woodland savannahs, grassland, forest, dunes, wetland, montane heath, semi-desert and desert. Absent from equatorial forest and desert interiors. Readily inhabits agricultural and cultivated habitats. Feeding Ecology Eats mainly small rodents and insects. Also eats herptiles, birds, chicks, eggs, arachnids and other invertebrates. Largest prey includes Springhare, ground squirrels and large snakes, including venomous species such as cobras. Occasionally kills domestic poultry. Nocturnal and terrestrial. Hunting is solitary, but juveniles sometimes help the mother in subduing large prey such as snakes. Prey is hunted by sight and smell, with rodents and insects often killed in burrows or excavated. Social and Spatial Behaviour Poorly known. Adults are largely solitary. Captive males are intolerant of each other, but females with juveniles tolerate other mother–kitten families in captivity. Reproduction and Demography Poorly known. Reported to give birth mainly November–February in southern Africa, but lactating females are recorded February–October in E Africa. Gestation 36 days. Litter size 1–3, exceptionally to 5 (in captivity, the maximum reared being 3). Weaning at around 8 weeks. Females first breed at 10 months (captivity). MORTALITY Poorly known. Large raptors, especially Martial Eagle and owls, are confirmed predators, and it is frequently killed by domestic dogs in rural areas. LIFESPAN 13.3 years in captivity. Status and Threats Widespread habitat generalist and common to abundant in suitable protected habitat. Roadkills, domestic dogs and persecution for poultry depredation kill significant numbers in rural areas, but probably constitute only a localised threat. Valued in traditional medicinal beliefs in some areas. Red List LC, population trend Stable.

Plate 82

| AMERICAN MINK Neovison vison |
HB ♀ 30–40cm, ♂ 33–43cm; T 12.8–23cm; W ♀ 0.45–1.1kg, ♂ 0.6–2.3kg
Uniformly glossy chestnut-brown to sooty black with slightly paler underparts. The chin is often but not always white; white fur on the chin rarely extends to the upper lip or throat (in contrast to European Mink). American Mink is closely related to American weasels (closest relative is thought to be Long-tailed Weasel) and only distantly related to European Mink. It is classified in its own genus with the now-extinct Sea Mink (N. macrodon), which was formerly distributed on the Atlantic coast of Canada and the US. Distribution and Habitat Most of Canada and the USA, including Alaska; absent from S USA. Introduced for fur in Argentina, Chile and Eurasia, including Japan, where it is invasive and destructive to native wildlife, including the European Mink in Europe. Inhabits densely vegetated waterways, marshes, wetlands, swamp forest and coastal beaches. Feeding Ecology A bold and aggressive predator capable of killing prey much larger than itself, including records of adult swans, geese, gannets and at least one case of a juvenile Harbour Seal, but typical diet comprises small mammals, birds, slow-swimming fish, herptiles, eggs and aquatic invertebrates such as crayfish and crabs. In North America, preys heavily on Muskrat, and population fluctuations of the 2 species are closely linked. An important nest predator of waterfowl and colonially nesting birds such as gulls and terns. Readily preys on domestic poultry. Foraging is solitary, nocturno-crepuscular, and both terrestrial and aquatic. Dives to depths of 6m and swims underwater for up to 35m. Caches surplus food. Social and Spatial Behaviour Solitary. Male ranges overlap 1 or more smaller female ranges. There can be high intrasexual overlap of ranges. Linear range size 1–4.2km (♀s) and 1.5–11.1km (♂s). Density estimates from North American wetlands vary between 1.6–5.4/km2 (Wisconsin) and 25–42/km2 (Louisiana cypress–tupelo swamp). Reproduction and Demography Seasonal. Mating February–April (to early May in Alaska); births April–June. Gestation 39–79 days, with a brief period of delayed implantation (embryonic development 30–32 days). Litter size 2–8, averaging 4–5. Weaning at 7–9 weeks. MORTALITY In North America, deaths are mainly from trapping. LIFESPAN Rarely >3 years in the wild, 8 in captivity. Status and Threats Widespread and common. The most important American fur-bearer and widely trapped, with 400,000–700,000 wild mink harvested each year. Threatened in S Florida by wetland modification and degradation. Red List LC, population trend Stable.

| BLACK-FOOTED FERRET Mustela nigripes |
HB 38–50cm; T 11.4–15cm; W ♀ 0.76–0.85kg, ♂ 0.96–1.1kg
The only ferret native to North America, closely related to Steppe Polecat and Western Polecat, and more distantly to other American Mustela. Yellowish buff on the body, darkening to dark brown on the back. Head is creamy white with a brownish-black mask, white muzzle and chocolate-brown crown. Limbs are chocolate brown to black, and the tail has a black tip. Distribution and Habitat Great Plains of the USA; formerly from S Alberta and Saskatchewan, Canada, to N Mexico. Extinct in the Wild by 1987 and all present populations result from reintroductions. Restricted to short to mid-grass plains and prairies in obligate association with prairie dog colonies. Feeding Ecology Entirely dependent on prairie dogs, which comprise around 90% of the diet. An estimated 0.4–0.6km2 of prairie dog colony is needed to support one Black-footed Ferret, and breeding females need prairie dog densities of at least 1,200 individuals/km2 in core areas to successfully raise pups. The most important prey species is Black-tailed Prairie Dog, followed by White-tailed Prairie Dog and Gunnison’s Prairie Dog (Arizona only). Occasional prey includes small rodents such as deer mice, voles and ground squirrels, as well as cottontail rabbits and White-tailed Jackrabbit. Hunting is mainly nocturno-crepuscular and underground; pursues prairie dogs into their burrows, where most kills occur. Above-ground hunts are less successful; adult prairie dogs often mount an effective defence on the surface. Does not hibernate and hunts hibernating prairie dogs throughout winter. Sometimes caches surplus kills in burrows. Social and Spatial Behaviour Solitary. Adults establish enduring ranges, closely tied to active prairie dog colonies. Same-sex adults avoid each other, but ranges overlap by as much as 42% in areas of high prairie dog density. Male ranges are about twice the size of female ranges. Range sizes 0.23–1.88km2, averaging 0.56–0.65km2 (♀s) and 1.28–1.32km2 (♂s). Reproduction and Demography Seasonal. Mating March–April; births May–June. Gestation 42–45 days. Litter size 1–6, averaging 3–4. Weaning at 6 weeks, and kits venture above ground at 60 days. Dispersal in late autumn at around 5–6 months. MORTALITY Rates of disappearance (including some emigration) are 53–86% annually. Main factors are disease and predation, especially by Coyote and large raptors. LIFESPAN 12 years in captivity, but much lower in the wild. Status and Threats The species was decimated by exotic disease (canine distemper and plague), and the massive anthropogenic-driven decline of prairie dogs. A comprehensive captive breeding and reintroduction effort initiated in 1985 has released ferrets in the 3 native range countries at 29 sites, only 4 of which have established self-sustaining populations, in Arizona, South Dakota and Wyoming. Another 10 populations, all in the US, show limited success or are too recently established to assess. Reintroduction in Canada and Mexico has failed, and the species is again considered extinct in those countries. There are now approximately only 300 adults in the wild, which has declined from a peak of 500 in 2008. Ongoing conversion of prairie grasslands for agriculture limits available habitat to ferrets, but current population declines and reintroduction failures are driven primarily by epidemics of exotic plague. Plague is transmitted by fleas in prairie dog colonies and is now considered endemic across Black-tailed Prairie Dog range. Both Black-footed Ferrets and prairie dogs are vulnerable to infection, so ferrets experience direct mortality and strong indirect effects due to resultant, very dramatic declines (typically >90%) in their prey base. Several hundred ferrets are maintained in captivity in the US. CITES Appendix I; Red List EN, population trend Decreasing.
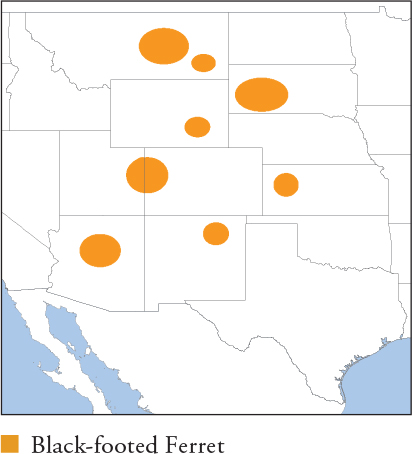
Plate 83

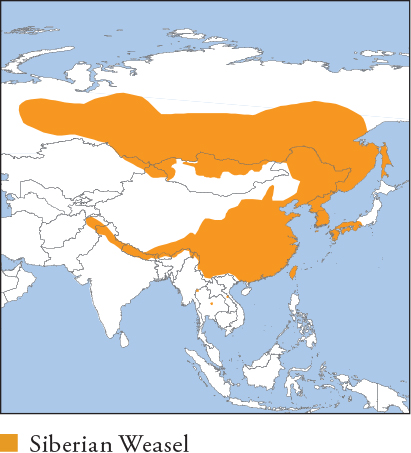
| SIBERIAN WEASEL Mustela sibirica |
SIBERIAN POLECAT, KOLINSKY, HIMALAYAN WEASEL
HB ♀ 25–30.6cm, ♂ 28–40cm; T 13.5–23.5cm; W ♀ 0.36–0.45kg, ♂ 0.43–1.15kg
Formerly classified with the closely related Japanese Weasel; it closely resembles that species but is larger and generally more brightly coloured. Uniformly rich orange-brown with slightly paler underparts. Summer coat tends to be darker brown than winter coat. Face has a dark brown to black mask with a white muzzle and chin. Distribution and Habitat Temperate Asia; C to Far East Russia, N Mongolia, E and S China, Korean Peninsula, and marginally into Nepal, Bhutan and N Indochina. Introduced to, and invasive in, Japan (native on Tsushima Island). Inhabits forest, forest steppe, dense grassland, vegetated scrubland and wetland. Occurs in cultivated areas, plantations and urban areas, but avoids open anthropogenic habitat. Feeding Ecology Small rodents and shrews are the most important prey. Also eats insects, earthworms, crustaceans, herptiles, birds, fledglings, eggs and fruits, including berries. Locally a significant nest predator of colonially nesting birds, e.g. Little Tern (Nakdong Estuary, South Korea), and occasionally raids domestic poultry. In urban habitats, scavenges from refuse, handouts (cakes, bread, etc.) and fish remains from dock areas. Foraging is mainly nocturno-crepuscular and terrestrial, although it swims well. Social and Spatial Behaviour Solitary. Adults live in stable ranges that overlap with those of other adults, but avoid each other, except male–female pairs when breeding. Range size estimates known only from Japan: 0.013–0.017km2 (♀s) and 0.014–0.044km2 (♂s). Reproduction and Demography Seasonal. Mating late February–March; births early April–June. Litter size 2–12, averaging 5–6. Nests in burrows and tree and rock cavities, and under buildings and haystacks in urban and rural areas. MORTALITY Unknown. LIFESPAN 8.8 years in captivity. Status and Threats Widespread and common in many areas. Hunted for fur (legally in Russia) and meat, but hunting is likely a threat only at the range limits, especially in Laos and S China. CITES Appendix III – India; Red List LC, population trend, Stable.
| JAPANESE WEASEL Mustela itatsi |
HB ♀ 22.2–26.5cm, ♂ 26.8-40cm; T 8.3–16.2cm; W ♀ 0.1–0.26kg, ♂ 0.2–0.92kg
Very similar in appearance to the Siberian Weasel and considered the same species until recently. Genetic and morphological evidence indicates they are distinct species that diverged >2 million years ago. Siberian Weasel is introduced in Japan (except Tsushima Island, where it is native), and the 2 species are sympatric and very difficult to tell apart on Kyushu, Shikoku and W Honshu (everywhere west of Nagoya). Japanese Weasel is uniformly orange-brown to tawny brown with a greyish-buff head and throat. Summer coat is typically uniformly darker brown with a greyish-brown to chocolate-brown head and neck. Face has a dark brown mask with a white muzzle and chin. Distribution and Habitat Endemic to Japan, occurring naturally on all large islands, except Hokkaido; introduced to Hokkaido (1880s) and a further 50 small islands in Japan. Introduced to Sakhalin Island, Russia (1932), but thought to be extinct there now. Inhabits most habitats across Japan, especially forest, dense grassland and wetlands from sea-level to 336m. Occurs in agricultural landscapes and peri-urban areas, but apparently intolerant of urbanisation and in large cities is restricted to well-vegetated riparian strips. Feeding Ecology Eats small rodents, insects, earthworms, crustaceans, herptiles, birds, fledglings, eggs and fruits. An urban population living in riverine habitat in outer Tokyo eats mainly fish, insects and fruits. Urban weasels apparently do not utilise anthropogenic foods (in contrast to Siberian Weasel). Foraging is mainly nocturno-crepuscular, terrestrial and semi-aquatic. Social and Spatial Behaviour Solitary, and thought to follow similar patterns to Siberian Weasels. Range size is poorly known, 0.01–0.31km2 (♀s). Reproduction and Demography Seasonal. Mating late winter (February–March); births in spring, April–June. Litter size unknown, assumed to be similar to Siberian Weasel. MORTALITY Unknown. Killed occasionally by domestic dogs. LIFESPAN Unknown. Status and Threats Widespread and common in many areas but it is disappearing from lowlands in western Japan, now occupied by introduced Siberian Weasel (which possibly outcompetes Japanese Weasels or perhaps invades after their urbanisation-driven disappearance). Red List NT, population trend Decreasing.

| STEPPE POLECAT Mustela eversmanii |
STEPPE WEASEL
HB 29–56.2cm; T 7–18.3cm; W ♀ 0.4–0.8kg, ♂ 0.75–1.2kg
Formerly classified with the closely related Western Polecat it strongly resembles that species, but tends to be lighter, with a paler face and fewer dark guard hairs over the body. The 2 species overlap in E Europe and W Russia. The domestic ferret is thought to descend from both Steppe and Western polecats. Distribution and Habitat E Europe through S Russia, NC Asia, Mongolia, and N and C China. Mainly inhabits steppe, grassland and vegetated semi-desert to 2,600m. Generally avoids densely vegetated areas, including most forest types. Occurs in pastures and on farmland, including areas with crops such as corn and cereals. Feeding Ecology Small rodents and lagomorphs are the mainstay, particularly voles, mice, gerbils, hamsters, sousliks, marmots, zokors and pikas. Also eats grassland birds (especially in spring and autumn), including grouse, ptarmigans and pheasants, plus reptiles, fish, eggs and invertebrates. Hunting is mainly nocturno-crepuscular and terrestrial, with most prey captured and killed in burrows. Occasionally scavenges, including from carrion, and caches surplus kills, e.g. up to 50 sousliks in Russian reports. Social and Spatial Behaviour Poorly known. Adults are solitary, with large male ranges overlapping numerous female ranges. Range estimates average 1.27km2 (♀s) and 3.54km2 (♂s; NW Hungary). Reproduction and Demography Seasonal. Mating February–April; births April–June. Gestation 36–41 days. Litter size 4–14, averaging 8–9. Weaning at around 6 weeks; dispersal at 3–4 months. Females first breed at 9–10 months. MORTALITY and LIFESPAN Unknown. Status and Threats Has a wide range, is common in many areas and is globally secure. It has declined significantly in much of its European distribution range since the 1960s, but remains widespread and numerous in its Asian range. Trapping, conversion of grassland habitats, and widespread hunting and poisoning of prey are threats, especially in E Europe, Mongolia and China. Legally hunted for fur in Russia. Red List LC, population trend Decreasing.

Plate 84

| WESTERN POLECAT Mustela putorius |
EUROPEAN POLECAT, COMMON POLECAT, FERRET
HB ♀ 20.5–38.5cm, ♂ 29.5–46cm; T 7–14cm; W ♀ 0.4–0.92kg, ♂ 0.5–1.7kg
Dark chocolate brown to near black, with buff-yellow underfur that is obvious on the sides and neck. Face is buff to silvery white, with a dark mask. The progenitor of the domestic ferret (possibly also with interbreeding from the closely related Steppe Polecat), and they hybridise, producing fertile offspring. Distribution and Habitat W Russia, N Scandinavia throughout W and C Europe, including Britain (absent from Ireland), and extreme N Africa (Morocco and Algeria). Inhabits lowland forest, wooded steppes, vegetated dunes, marshes, meadows and river valleys in open habitat, to 1,500m in Europe (Pyrenees and Alps) and to 2,000m in N Africa (Rif Mountains). Occurs in agricultural areas and close to human settlements. Feeding Ecology Eats mainly small rodents, shrews, lagomorphs, frogs and toads. Other prey includes birds, reptiles, fish, eels, invertebrates and eggs; fruits and other plant items are eaten mainly by young animals. Takes domestic poultry, occasionally becoming a serious pest. Foraging is solitary, mainly nocturno-crepuscular and terrestrial. Scavenges from carrion and occasionally from human refuse, bird feeders and food scraps, especially during winter food shortages. Caches excess food, e.g. 40–120 frogs (W Russia). Social and Spatial Behaviour Solitary. Male ranges overlap 1 or more smaller female ranges. Female ranges tend to be exclusive; male ranges have variable overlap and are most exclusive during the breeding season; ranges are assiduously scent-marked, especially while breeding. Range size 0.65–1.65km (♀s) and 1–3.05km (♂s; linear ranges along riverbanks, Poland), and 0.42–1.21km2 (♀s) and 1.07–2.8km2 (♂s; fragmented forest–agricultural mosaic, Luxembourg). Reproduction and Demography Seasonal. Mating March–June in most of the range, a month earlier in Britain; births April (Britain, May) to early August, peaking mid-July. Gestation 40–43 days, without delayed implantation. Litter size 2–13, averaging 4–6; large litters may be due to hybridisation with ferrets. Weaning at 5–7 weeks. MORTALITY Mostly anthropogenic, especially roadkills, trapping and domestic dogs; starvation is an important factor during severe winters, especially for newly independent subadults. LIFESPAN <5 years in the wild, 14 in captivity. Status and Threats Widespread and relatively common. Persecuted very heavily in the past, which, combined with loss of habitat, led to widespread and severe declines. It is still declining in parts of the range, especially where habitat conversion is advancing in W Europe, but it is recovering in many areas, e.g. Britain and Switzerland. Localised threats include roadkills, prey declines (e.g. dramatic reduction of European Rabbits in Mediterranean range) and persecution as a perceived pest. It is now largely protected from hunting and trapping in most W Europe range states; it is legally trapped in Russia, although intentional trapping pressure is low and most mortality is unintentional ‘by-catch’ during trapping efforts for more valuable fur species such as American Mink and martens. Hybridisation with feral ferrets erodes genetic purity in some areas, e.g. S Britain and N Germany, although not at levels to be considered a major threat. Wild Western Polecats are captured in Algeria (and possibly Morocco) and kept for hunting rabbits, which may constitute a threat if the remaining population is small and isolated, as suspected. Red List LC, population trend Decreasing.

| EUROPEAN MINK Mustela lutreola |
HB ♀ 32–40cm, ♂ 28–43cm; T 12–19cm; W ♀ 0.4–0.6kg, ♂ 0.6–1.1kg
Uniformly glossy chestnut-brown to dark coffee brown with slightly paler underparts. Upper lips, chin and tip of the muzzle are white, sometimes extending down the throat and chest. Very similar in appearance to introduced American Mink, which has replaced European Mink in most of its native distribution. European Minks are slightly smaller, typically with white muzzles and upper lips, whereas white fur in American Minks is usually restricted to the chin, and is often absent entirely. Despite appearances, the 2 species are not closely related; European Mink’s closest relatives are Steppe Polecat and Western Polecat. Distribution and Habitat W Russia, and isolated populations in Belarus, Estonia, Latvia, Ukraine, coastal Romania and the French–Spanish border. Sympatric with introduced American Mink in most of its current range; NW Russia (the Arkhangelsk region and Komi Republic) and the Northern Caucasus region may be exceptions. Rarely more than 100m from fresh water; preferred habitats are slow-flowing streams, lake shores and wetlands that do not completely freeze in winter. Feeding Ecology Semi-aquatic and forages for small, mainly vertebrate prey along waterways, especially small rodents such as European Water Vole, Bank Vole, Wood Mouse and introduced Muskrat. Other prey includes small fish, frogs, crustaceans, aquatic insects, and occasionally birds and reptiles. Foraging is solitary, nocturno-crepuscular, and both terrestrial and aquatic; it is also an excellent swimmer, pursuing prey underwater in dives of 5–20 seconds. Caches surplus kills. Social and Spatial Behaviour Solitary. Large male ranges overlap 1 or more smaller female ranges, with low to moderate overlap within sexes (especially males). Ranges expand in autumn–winter to locate unfrozen water. Linear range size 0.3–5.1km (♀s) and 2.9–11.4km (♂s). Density estimates 2–12/10km of waterway. Reproduction and Demography Seasonal. Mating February–April (early May in captivity); births April–June. Gestation 35–48 days, without delayed implantation (71–76 days reported from fur farms). Litter size 1–8, averaging 4–5. Weaning at 7–9 weeks, at which juveniles can capture small prey. MORTALITY Most recorded mortality is anthropogenic, mainly through trapping. LIFESPAN 10 years in captivity. Status and Threats Formerly distributed throughout Europe (including European Russia) west of the Ural Mountains. Overhunting for fur and habitat destruction (water pollution, damming and draining) have extirpated the species from >95% of its historic range and 18 range countries. The current distribution is now restricted to isolated fragments in Estonia (extinct on the mainland, reintroduced on Hiiumaa Island), France (possibly extinct), Romania, Russia, Spain and Ukraine. The largest numbers are in Russia (<15,000 in discontinuous populations), followed by ~1,000–1,500 in the Danube delta, Romania. All populations are threatened by ongoing habitat degradation, illegal and accidental trapping, persecution and roadkills. Introduction of the larger, competitively aggressive American Mink is thought to be an additional factor. Red List CR, population trend Decreasing.

Plate 85

| LEAST WEASEL Mustela nivalis |
COMMON WEASEL
Includes EGYPTIAN WEASEL M. subpalmata, SICHUAN WEASEL M. russelliana and TONKIN WEASEL M. tonkinensis
HB 11.4–26cm; T 7–9cm; W 0.025–0.3kg
The world’s smallest carnivore, tiny with a short tail without a black tip. Except in southern populations, brown fur moults to pure white in winter; transitional winter forms occur in temperate areas. Sometimes Egyptian Weasel is classified separately in Egypt, but genetic analysis indicates no species-level differences. Classification of Sichuan Weasel (known from 8 specimens from C China) and Tonkin Weasel (1 specimen from N Vietnam) is based on extremely limited data; both are treated here as Least Weasel. Distribution and Habitat Global, from approximately 35–40°N to the Arctic, encompassing Canada, Alaska, NE USA, Eurasia and N Africa. Introduced in New Zealand, Malta, Crete, the Azores and São Tomé. Occurs in virtually all habitats with cover and rodents, from sea-level to 4,000m. Inhabits agricultural and urban areas. Feeding Ecology Small rodent specialist, hunting mainly mice, rats, Meadow Vole, Field Vole, water voles, lemmings and cotton rats. Larger mammals, including lagomorphs, moles and squirrels, are also killed, especially by males. Other food includes birds, fledglings, eggs, herptiles, fish and invertebrates (mainly beetles and earthworms). Takes poultry in rare cases. Foraging is solitary and cathemeral; constantly active to maintain a very fast metabolism, making 5–10 kills/24 hours. Hunting is mostly terrestrial; its tiny tubular body enables hunting in burrows and rodent snow tunnels. Occasionally scavenges carrion during winter. Caches surplus kills in burrows. Social and Spatial Behaviour Solitary with aggressive territorial defence. Breeding males often abandon territories in search of females. Ranges 0.002–0.7km2 (♀s) and 0.006–0.26km2 (♂s). Densities fluctuate widely depending on rodent population cycles, e.g. 2.4–13/km2 (Finland). Reproduction and Demography Aseasonal, peaking in spring to late summer. Gestation 34–37 days, without delayed implantation. Litter size 1–19, averaging 4–10. Weaning at 4–7 weeks; juveniles can kill at 6–7 weeks. Sexual maturity (both sexes) 3–4 months. MORTALITY Populations turn over very rapidly, with annual adult mortality of 75–97%, mainly from food shortages and predation. LIFESPAN Rarely >2 years in the wild, 10 in captivity. Status and Threats Widely distributed, with a broad habitat tolerance. Apparently naturally rare in North America and has declined locally, e.g. UK. Vulnerable to rodenticides, and persecuted intensely in some areas as a predator of game birds. Red List LC, population trend Stable.

| STOAT Mustela erminea |
ERMINE, SHORT-TAILED WEASEL
HB 17–34cm; T 4.2–12cm; W 0.06–0.37kg
Small weasel, rusty-brown to chocolate brown in summer moulting to white in winter, except in southern populations. Tail has a black tip. In North America (called the Short-tailed Weasel), it is smaller with a proportionally shorter tail than sympatric Long-tailed Weasel. Distribution and Habitat Global, from approximately 35°N to the Arctic, throughout Eurasia, North America and Greenland. Introduced in New Zealand. Inhabits a very wide range of habitats, from Arctic tundra to semi-desert, from sea-level to 3,000m. Inhabits farmland. Feeding Ecology Very similar diet to Least Weasel’s, but kills more larger prey, especially rabbits and squirrels. Where lagomorphs are absent, small rodents are the mainstay. Prodigious nest predator of eggs and fledglings, and also eats adult birds, herptiles, invertebrates and fruits. Takes domestic poultry. Foraging is mostly nocturnal and terrestrial, but driven by high energetic requirements that necessitate flexible foraging. Caches surplus prey. Occasionally scavenges from carrion and human refuse. Social and Spatial Behaviour Solitary and territorial. Male ranges overlap multiple female ranges, and adults repel same-sex intruders. Males abandon territories during breeding in search of females. Range size 0.02–1.35km2 (♀s) and 0.08–3.13km2 (♂s). Density estimates 3–10/km2, exceptionally reaching 22/km2 during rodent irruptions. Reproduction and Demography Seasonal. Mating June–August; births April–May (following year). Gestation 223–378 days, with delayed implantation (embryonic development 28–30 days). Litter size 4–18, averaging 6–8. Weaning 4–12 weeks. Females sexually mature at 4–6 weeks, and may be pregnant before they are weaned. MORTALITY Annual mortality 40–54% (3–6-month-old subadults) to 78–83% (2.25–2.5-year-old adults), mainly from prey shortages and predation. LIFESPAN Rarely >3 years in the wild, 10 in captivity. Status and Threats Widely distributed and relatively common to abundant. Legally trapped in much of its range, and persecuted as a predator of game birds and poultry, but resilient to harvest. CITES Appendix III – India; Red List LC, population trend Stable.

| ALTAI WEASEL Mustela altaica |
ALTAI MOUNTAIN WEASEL, ALPINE WEASEL
HB 21.7–28.7cm; T 9–14.5cm; W ♀ 0.12–0.22kg, ♂ 0.22–0.35kg
Pale buff-brown upperparts, darkening slightly in summer, with pale cream to creamy-yellow underparts. Feet conspicuously creamy white, and no black tip to tail. Distribution and Habitat C and N Asia, in the Himalayan, Pamir, Altai and Tien Shan mountains, S Russia, N Mongolia, and C and E China; uncertain but likely in North Korea. Inhabits alpine meadows, grassland, steppes and rocky areas at 1,500–5,200m. Occurs in remote human habitations in sheds, barns and cellars, but avoids degraded agricultural landscapes. Feeding Ecology Poorly known. Small rodents and lagomorphs, especially pikas, zokors, hamsters, sousliks and voles, are probably the main prey. Also eats birds (including ptarmigans), eggs, herptiles, invertebrates and fruits. Forages diurnally, at least where potential predators such as Red Foxes and Stone Martens are mainly nocturnal. Caches surplus kills. Social and Spatial Behaviour Poorly known. Assumed to be solitary and territorial. Reproduction and Demography Poorly known; likely seasonal. Mating February–March (Kazakhstan). Gestation 35–40 days, apparently without delayed implantation. Litter size 2–8, exceptionally to 13. MORTALITY Poorly known. Populations appear to undergo large fluctuations. LIFESPAN Unknown. Status and Threats Declining across much of its range due to habitat degradation from overgrazing by livestock, and agricultural poisoning of prey. Used for folkloric charms in Nepal, where mummified carcasses are believed to prevent infant deaths. CITES Appendix III – India; Red List NT, population trend Decreasing.

Plate 86

| COLOMBIAN WEASEL Mustela felipei |
FELIPE’S WEASEL
HB 21.7–22.5cm; T 11.1–12.2cm; W c.0.12–0.15kg
South America’s smallest weasel. Very small; uniformly very dark brown, including the tail, with pale buff-orange underparts and sometimes a small dark patch on the chest. Easily confused with Long-tailed Weasel, but lacks its black-tipped tail. Natural history and ecology are almost entirely unknown. Distribution and Habitat Restricted to the Andes of Colombia and N Ecuador, where it is known from only 6 physical records and 5 sightings. All records are from high Andean forest at 1,525–2,700m, usually in riparian areas and river valleys (although this is probably a sampling artefact). Feeding Ecology Unknown. Assumed to be a rodent specialist, like other weasels. Social and Spatial Behaviour Unknown. All records are of single animals. Reproduction and Demography Unknown. Status and Threats Occurs in a very restricted distribution and is naturally rare, based on the relative rates it appears in surveys and museum records, e.g. compared to Long-tailed Weasel. Known only from mid- to high-elevation forest habitat, which is under intense pressure in the Andes for forestry and conversion to agriculture or pasture. Local people kill weasels for depredation on poultry and domestic guinea pigs, though it is unclear whether Colombian Weasels are involved. Red List VU, population trend Decreasing.
| LONG-TAILED WEASEL Mustela frenata |
HB ♀ 20.3–22.8cm, ♂ 22.8–26cm; T 7.6–15.2cm; W ♀ 0.8–0.25kg, ♂ 0.16–0.45kg
Long-bodied weasel with a proportionally long tail measuring half to two-thirds the body length. Summer coat is rich, rusty brown to chocolate brown, with creamy-white to rich yellow underparts. Northern populations moult to pure white in winter; tail always has a black tip, regardless of region and season. Southern populations do not moult to white. The species has distinctive white or yellow facial markings in Central America, Mexico and S USA. Distribution and Habitat S Canada, the USA, Mexico, Central America, Venezuela, Colombia, Ecuador, Peru, extreme NW Brazil and N Bolivia. Occurs in virtually all habitats, from arctic–alpine to tropical, and absent only from true desert interiors. Most abundant in open woodland, brushland, riparian grassland, meadows and marshes. Occupies modified habitats, including logged areas, fence rows, fields, cropland and pastures. Feeding Ecology Opportunistic generalist with a wide diet, but rodents, lagomorphs and insectivores are the mainstay. Common prey includes voles, deer mice, cotton rats, wood rats, chipmunks, shrews, moles, pikas, cottontails, Volcano Rabbit (Mexico) and Snowshoe Hare (mainly juveniles). Also takes birds, reptiles (including kingsnakes and Bullsnake), insects and eggs; an important nest predator in some areas. Occasionally preys on small carnivores, mainly other small weasels. Takes domestic poultry, and there is a credible record of predation on 3-day-old piglets. Foraging is mainly nocturno-crepuscular but flexible; diurnalism increases during winter. Does not hibernate. Hunts mainly by sight and smell, ceaselessly investigating burrows, crevices, nests and log hollows for quarry. Prey is subdued by a distinctive hold, in which it tightly entwines the victim with its body and administers a killing bite to the nape (small prey) or a suffocating bite to the throat. Adult weasels make 3–4 kills a day; a Colorado population estimated at 8,000 weasels killed an estimated 11 million prey items (mostly rodents) annually. Caches surplus prey, and scavenges from carrion and human refuse. Social and Spatial Behaviour Solitary, with enduring ranges that overlap, but with exclusive core areas. Adults mostly avoid each other except during the breeding season, when males seek out females and aggressively repel other males. Mixed-sex pairs occasionally associate temporarily outside the breeding season, e.g. sharing burrows. Male ranges encompass 1 or more female ranges, and males attempt to increase their range size during breeding to encounter more females. Average range size 0.52km2 (♀s) and 1.8km2 (♂s; fragmented agricultural habitat, Indiana) to 0.1–0.24km2 (both sexes) in better-quality habitat. Density estimates 0.4–0.8/km2 (agricultural habitat) to 19–38/km2 (high-quality oak forest and marshland). Reproduction and Demography Seasonal. Mating July–August (North America); births April–May (following year). Gestation 205–337 days, with delayed implantation (embryonic development 21–28 days). Litter size 4–9, averaging 6. Kittens weaned at around 35–36 days and independent at 3 months. Females sexually mature at 9–12 weeks; males typically breed in their second year. MORTALITY Rates poorly known, but weasel populations turn over rapidly, with high mortality balanced by high reproduction. Natural mortality is mainly from predation, especially by raptors, foxes and Coyote. LIFESPAN Rarely more than 3 years in the wild. Status and Threats The most widespread mustelid in the western hemisphere, and it has the greatest habitat tolerance of American weasels. Populations fluctuate significantly and rapidly, depending on prey availability; abundance declines under agricultural intensification, prompting local extinctions when combined with low prey numbers. Legally trapped in its North American range, but no longer sought after for commercial trade. Red List LC, population trend Stable.
| AMAZON WEASEL Mustela africana |
TROPICAL WEASEL
HB 24–38cm; T 16–21cm; W c.0.1–0.3kg
Small weasel, uniformly red-brown to dark brown, including the tail. Underparts are pale buff to cream, bisected with a dark brown stripe running from the lower belly to the chest or throat; stripe is discontinuous in 2–3 segments in some individuals. Natural history and ecology are very poorly known. Distribution and Habitat Restricted to the Amazon Basin of N Bolivia, Brazil, E Ecuador and E Peru; possibly more widely distributed, e.g. S Colombia to S French Guiana, but no records. All records come from Amazonian lowland forest. Feeding Ecology Unknown. Assumed to be a rodent specialist, like other weasels. Foraging is possibly diurnal; 1 sighting was at 10:00. Social and Spatial Behaviour Unknown. Most records are of single animals, with 1 sighting of 4 animals, probably a mother–offspring group. Reproduction and Demography Unknown. Status and Threats Status largely unknown. Occurs over a wide range where much of the habitat is well preserved, and is recorded from a number of protected areas. Habitat tolerances are unknown, but deforestation and conversion to agriculture and pasture are considered the primary threats. Red List LC, population trend Unknown.

Plate 87

| YELLOW-BELLIED WEASEL Mustela kathiah |
HB 20–29cm; T 12.5–18cm; W c.0.15–0.3kg
Very small weasel, russet-brown to dark maroon-brown, with rich buff-orange or yellowish underparts and a conspicuously white lower face. Feet sometimes have white flecking or small patches. Natural history and ecology are very poorly known. Distribution and Habitat SE China (about 80% of its range), N and E Indochina, N Myanmar, and the Himalayas from Bhutan to Kashmir. Inhabits mainly temperate and evergreen forest from sea-level to 4,000m. Occurs in highly degraded forest patches and scrub mosaics, e.g. in Hong Kong and N Laos. Feeding Ecology Unknown. Assumed to prey mainly on rodents, other small vertebrates and invertebrates, similar to other weasels. Reported to raid poultry coops on Hainan Island, China. Social and Spatial Behaviour Unknown. All records are of single animals. Reproduction and Demography Unknown. Status and Threats Status poorly known, but the species occurs in highly degraded habitat and in areas that are under intense hunting pressure, e.g. near Phongsaly town, Laos, suggesting that it is tolerant of habitat loss and resilient to anthropogenic pressure. CITES Appendix III – India; Red List LC, population trend Stable.
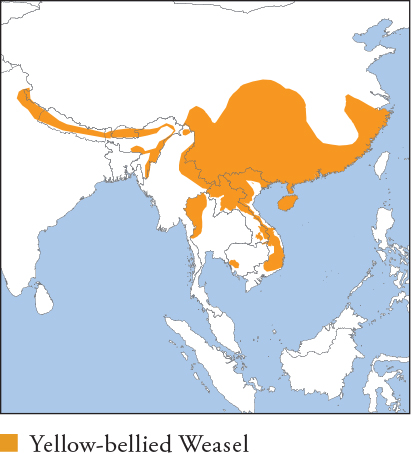
| STRIPE-BACKED WEASEL Mustela strigidorsa |
BACK-STRIPED WEASEL
HB 25–32.5cm; T 13–20.5cm; W 0.7–2kg
Small weasel, dark mahogany-brown to rich russet-brown, with a fine silvery-white or creamy dorsal stripe from the crown to the base of the tail. Cheeks and throat are creamy white to rich yellow-cream, narrowing to a thin yellow line running along the belly to the groin. Natural history and ecology are very poorly known. Distribution and Habitat NE India, N and C Myanmar, S China, N Thailand, N and C Laos, and Vietnam. Most records are from evergreen hill and montane forests to 2,500m, and associated secondary forest, bamboo stands, scrub and grassland. Feeding Ecology Poorly known. One was observed attacking a bandicoot rat estimated at 3 times its size, and another was seen carrying a suspected White-bellied Rat. Local people report that it takes domestic poultry; 2 animals for sale in a Laotian wildlife market were killed apparently while raiding a chicken coop, and the only individual captured for science (NE Thailand) was baited with a live chicken. Social and Spatial Behaviour Unknown. All records are of single animals. Very rarely appears in wildlife surveys, including by camera-trapping, suggesting it is naturally rare, but this remains to be confirmed. Reproduction and Demography Unknown. Status and Threats Status very poorly known. Does not have especially high economic value and appears in wildlife markets moderately frequently, mainly in China, where 3,000–4,000 animals were harvested for fur annually in the 1970s. Sometimes killed as a perceived poultry pest. Red List LC, population trend Stable.

| MALAY WEASEL Mustela nudipes |
HB 30–36cm; T 24–26cm; W c.1kg
Unmistakable weasel, bright orange to reddish brown, usually with a paler orange neck and a creamy-white to pale orange head. Distal half of the tail is pale orange to white. Natural history and ecology are very poorly known. Recorded moving in a very distinctive zigzag pattern with the brightly coloured tail held high like a flag; whether this is typical or associated with fleeing the observer is unknown. Distribution and Habitat Sumatra, Borneo, Peninsular Malaysia and S Thailand; reports from Java are erroneous. Recorded from lowland and hill tropical forests, heath forest, swamp forest, montane forest and montane scrub from sea-level to 1,700m. Sometimes occurs in degraded habitat, including exotic plantations and peri-urban areas. Feeding Ecology Unknown. Diet assumed to be similar to that of other small Mustela weasels, i.e. small rodents, birds, eggs and herptiles. Social and Spatial Behaviour Unknown. Most records are of single animals, with 1 sighting of 2 animals; always on the ground or on ground-level objects such as rocks and logs. Reproduction and Demography Unknown. Status and Threats Status very poorly known. Apparently widespread within its range, but rarely recorded and difficult to see. Has been recorded in degraded habitats, including urban areas, suggesting some tolerance for anthropogenic pressures; its presence in higher-altitude forest buffers it to some extent from the extreme pressures on lowland forest in the Sundaic region. Red List LC, population trend Decreasing.

| INDONESIAN MOUNTAIN WEASEL Mustela lutreolina |
HB 29.7–32.1cm; T 13.6–17cm; W 0.3–0.34kg
Small, dark weasel. Uniformly dark brown with slightly paler underparts, sometimes with inconspicuous creamy-white patches on the chin, throat, upper chest and groin. It is the only dark weasel species on Sumatra and Java. One of the least-known carnivore species; known only from 15 museum records and <5 modern sightings and camera-trap images. Distribution and Habitat Java and S Sumatra (uncertain elsewhere in Sumatra), where it is restricted to highlands at 1,400–3,000m. All records are from hill and montane forests, with a single sighting taking place in alpine scrub above the treeline at 3,000m (Mt Kerinci, Sumatra). Feeding Ecology Unknown. Diet assumed to be similar to that of other small Mustela weasels, i.e. small rodents, birds, eggs and herptiles. Social and Spatial Behaviour Unknown. All records are of single animals, except for the single confirmed wild sighting, which was of 4 animals moving in a ‘train’ fashion, almost certainly a female and 3 large juveniles. Extremely rare in wildlife surveys, including by camera-trapping, suggesting it is naturally scarce, although this remains to be confirmed. Reproduction and Demography Unknown. Status and Threats Status very poorly known. Its montane forest habitat is under significant pressure from illegal forestry and conversion, e.g. to coffee plantations, especially in Java. However, the species’ habitat tolerances and its dependence on undisturbed forest are unclear. Not especially valued for trade, and hunting is less intense in montane habitat than in comparable lowland areas. Red List LC, population trend Stable.

Plate 88

| MARINE OTTER Lontra felina |
CHUNGUNGO
HB 53–79cm; T 30–36.2cm; W 3.2–5.8kg
Smallest South American otter, uniformly glossy, dark brown with a slightly paler muzzle, cheeks and underparts. Broad head has a short muzzle with heavy, long whiskers (for which it is sometimes called the ‘sea cat’), and the tail is relatively short. Distribution and Habitat Restricted to the Pacific coast of South America, patchily distributed from C Peru to Tierra del Fuego and Isla de los Estados, Argentina. Found along rugged rocky coasts rarely more than 100m offshore and 50m inland; occasionally travels up coastal freshwater inlets. Occurs in wharves and groynes near urban areas. Feeding Ecology Feeds primarily on marine crustaceans, fish and molluscs. Also takes freshwater shrimp in coastal rivers. Large prey, e.g. Southern King Crab, is taken ashore to eat, while small prey is consumed at sea; floats on its back to eat, but does not use rock anvils as do Sea Otters. Occasionally hunts on land, primarily chicks of colonially nesting seabirds; actively excavates the burrows of Peruvian Diving Petrel to reach chicks. Foraging is cathemeral and mainly solitary; observed pairs and trios are mother–young groups. Adults congregate amicably at food patches such as fishery dumps. Sometimes scavenges from human fishing waste on shore. Social and Spatial Behaviour Solitary, with facultative territorial behaviour. Often found close together with entirely overlapping ranges, but resident otters do not move, forage or rest together. Adults, especially females, become more territorial and occupy exclusive core areas where food occurs in patches. Range size similar for the sexes, linearly 1.3–4.1km and <110m wide. Reproduction and Demography Poorly known. Pups recorded year-round, peaking September–November (C Chile), although seasonality may be more pronounced further south. Gestation 60–65 days. Litter size 1–4, averaging 2. MORTALITY Poorly known. Most documented deaths are anthropogenic; Killer Whale, sharks and large raptors (on pups) are putative predators. LIFESPAN Unknown. Status and Threats Reduced to an estimated 1,000–2,000 adults in a series of discontinuous populations. Declining due to human overfishing of prey, combined with illegal killing by fishermen as competitors and for fur. Also killed by entanglement in nets and by domestic dogs around docks and urban beaches. Almost extinct in Argentina. CITES Appendix I; Red List EN, population trend Decreasing.
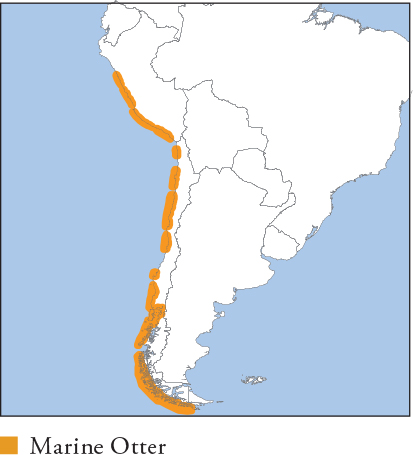
| SOUTHERN RIVER OTTER Lontra provocax |
PATAGONIAN RIVER OTTER, HUILLIN
HB 57–80cm; T 35–43cm; W 8–14.5kg
Medium-sized otter. Mid-brown to dark brown, with a pale greyish muzzle, throat and upper chest, becoming beige on the underparts. Distribution and Habitat Patagonian Argentina and Chile. Occurs in freshwater lakes, rivers and wetland with dense bank vegetation, as well as marine rocky coasts, estuaries and marshes. Avoids disturbed waterways such as canals. Feeding Ecology Crustacean specialist, eating mainly large freshwater crabs and crayfish; small, slow-moving fish are the second-most important food category. Also eats molluscs and, less so, amphibians and birds. Foraging is solitary and cathemeral. Social and Spatial Behaviour Solitary. Ranges are stable, with low overlap among males and greater overlap among females. Range size differs little between sexes, linearly 7.4–22.3km with very small core areas, usually <1.5km, centred around patches of thick vegetation for denning. Density averages 0.73/km of marine coast (S Chile). Reproduction and Demography Thought to be seasonal, depending on location. Pups observed year-round in southern range, but mating occurs December–March, with births thought to occur in July in C Chile. Gestation unknown; assumed to include delayed implantation. Litter size 1–4, averaging 2. MORTALITY and LIFESPAN Unknown. Status and Threats Now known only from 7 discontinuous populations that occupy a fraction of its former range. Serious threats include widespread habitat degradation by water pollution, wetland draining, dredging, canalisation and clearing of bank vegetation, as well as illegal hunting for fur, which is intense in some areas. CITES Appendix I; Red List EN, population trend Decreasing.
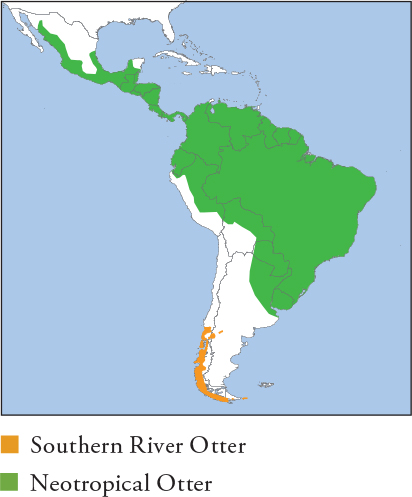
| NEOTROPICAL OTTER Lontra longicaudis |
NEOTROPICAL RIVER OTTER
HB 46–66; T 37–84cm; W 5–12kg (exceptionally to 15kg)
Very similar to Southern River Otter, but generally larger and more heavily built, and with paler fur around the cheeks and muzzle. The 2 species are separated by range. Leucism is recorded (Mexico). Distribution and Habitat N Mexico to Uruguay and N Argentina. Occurs mostly in clear, flowing streams, rivers and lakes in a variety of forest, woodland and scrub habitats, from sea-level usually to 1,500m, exceptionally above 3,000m. In coastal areas, occurs near fresh water such as river outlets and rain-fed lagoons. Avoids silted and sluggish waterbodies. Occurs in suitable habitat near human settlements. Feeding Ecology Eats mainly fish, especially catfish, characins and cichlids, as well as crustaceans, and large aquatic insects and their larvae. Molluscs, amphibians, reptiles, birds and mammals (including rats and Nutria) are opportunistically taken. Fruit is recorded in the diet, but is possibly consumed while eating frugivorous fish. Small prey is eaten in the water, while large prey is carried to shore or tree snags to consume. Foraging is solitary and mainly diurno-crepuscular; becomes nocturnal in areas of human disturbance. Scavenging is suggested by the presence of Capybara fur in scats. Social and Spatial Behaviour Poorly known. Solitary. Adults very actively scent-mark (‘spraint’) prominent features such as logs, root systems, sandbars and rocks, but ranging and territorial patterns are poorly studied. Density estimates 0.8–2.8/km of shore. Reproduction and Demography Semi-seasonal. Mating mainly February–May, but year-round in some areas. Gestation 56–86 days; it is unclear if a short period of delayed implantation accounts for the wide variation. Litter size 1–5, averaging 2–3. Pups start swimming at around 2.5 months. MORTALITY Predation known by anacondas, caimans, piranhas and Jaguar. LIFESPAN Unknown. Status and Threats Species has a wide range, but its status is poorly known. Heavily hunted for fur before 1970, leading to localised extinctions in much of its range. Continued illegal hunting remains a concern, but main threats today are habitat conversion to agriculture and ranching, combined with wetland drainage, damming and water pollution. Sometimes killed intentionally or accidentally by fishermen. CITES Appendix I; Red List NT, population trend Decreasing.

Plate 89

| NORTH AMERICAN OTTER Lontra canadensis |
RIVER OTTER, NEARCTIC OTTER, CANADIAN OTTER
HB 58–73cm; T 31.7–47cm; W 3.4–15.5kg
The only otter in North America except for Sea Otter (coastal W USA and Canada). Uniform pale brown to dark brown, with a pale cream to greyish muzzle, throat and chest. Distribution and Habitat Canada (excluding E Ellesmere and Prince Edward islands) and USA, including most of Alaska. Occurs in all types of non-polluted fresh and coastal waterbodies with well-vegetated or rocky shorelines, including streams, rivers, ponds, lakes, estuaries, marshes, beaches and reservoirs. Feeding Ecology Eats mainly slow-moving, schooling and bottom-dwelling fish, especially carp, minnow, catfish, bowfin and suckers. Crustaceans, especially crayfish, are the second-most important component of the diet. Fast-swimming fish such as salmon are taken infrequently except during spawning runs. Opportunistically eats frogs, reptiles, waterbirds and mammals, mainly Muskrats, Nutria (introduced) and, rarely, North American Beavers (probably juveniles). Occasionally catches small terrestrial mammals in deep snow, including Snowshoe Hares. Foraging is mainly nocturno-crepuscular, with increased diurnalism where it is undisturbed and during winter; active through winter and forages under ice. Hunts alone or in social groups. Can swim to 11km/h and remain underwater for up to 4 minutes. Hunting success poorly known; solitary adult females catch prey in 38% (lake) to 62% (small inlet) of dives. Occasionally scavenges from carrion. Social and Spatial Behaviour Sociality complex and fluid. Adults are generally solitary, with the primary social unit comprising a mother and cubs, but sociality increases with food availability. Mother–cub families may include helpers, either yearlings from previous litters or unrelated young adults. Large clans of up to 18 (mainly males) form in coastal Alaska, probably linked to cooperative hunting of large pelagic fish; females sometimes join clans, but never while raising cubs. Members of social groups are gregarious and play together, e.g. ‘snow-sliding’. Ranges vary from being largely exclusive to overlapping extensively. Average range sizes include 9.6km2 (♀s) to 30.4km2 (♂s; SE Minnesota) and 70km2 (♀s) to 231km2 (♂s; Alberta). Reproduction and Demography Seasonal. Mating December–April; births January–May (following year). Gestation 10–12 months, with delayed implantation (actual gestation 61–63 days). Litter size 1–6. Weaning at 12 weeks. Cubs can swim at 60 days. Sexual maturity at 12–18 months; first breeding rarely before 2–3 years. MORTALITY Most mortality is anthropogenic. The most important natural factor is starvation, mainly in the far north of the range. Adults are occasionally killed by predators ranging from Bald Eagle to Killer Whale. LIFESPAN 13 years in the wild, 25 in captivity. Status and Threats Extirpated from around 75% of its original range by the 1970s due to massive unregulated trapping and degradation of aquatic habitats. Rehabilitation of waterways and reintroduction programmes have restored it to much of its range; it is possibly extinct or very rare in about a third of its historic US distribution, and is extinct in Mexico. Remains susceptible to pollutants and habitat loss. Legally trapped for fur where populations are considered secure, in 29 US states and 11 Canadian provinces. CITES Appendix II; Red List LC, population trend Stable.

| SEA OTTER Enhydra lutris |
HB 100–120cm; T 25–37cm; W ♀ 14.5–32.7kg, ♂ 21.8–45kg
Largest mustelid by weight. Rufous-brown to dark brown, typically with a cream or greyish head, the colour extending variably on the throat and chest. Young pups have light brown woolly fur. It is the only completely marine otter species; can live its entire life at sea. Distribution and Habitat Endemic to the N Pacific, in coastal NE Russia (Kamchatka Peninsula and associated islands), N Japan, British Columbia, Alaska, Washington and California. Inhabits coastal marine environments, typically within 1km of shore. Feeding Ecology Diet overwhelmingly dominated by marine invertebrates such as sea urchins, clams, abalones, mussels and crabs. Squid and octopus are eaten especially during episodic abundances, e.g. California. Fish comprise only occasional prey, but are important to Aleutian Islands populations. Dives up to 100m (usually 25–40m) for an average of 60–120 seconds (up to 260 seconds), and finds prey mostly by sight with its strong underwater vision or by touch. Carries prey to the surface in a fold of skin in its armpit, and breaks open hard-shelled items by smashing them against a rock held on its belly (the rock is also transported in the armpit fold). Between 63% (subadults) and 82% (adults) of foraging dives are successful. Eats 20–33% of its body weight each day to compensate for heat loss to its cold environment. Able to drink sea water. Social and Spatial Behaviour Basically solitary, but with wide regional and temporal variation. Adults mostly occupy enduring ranges, and either space themselves out or overlap extensively depending on food availability. High-quality food patches such as squid flushes promote overlap, and otters sometimes aggregate in large ‘rafts’ of up to 2,000 individuals to feed. During breeding and where females occupy small stable ranges, males establish exclusive territories and exclude other males. Rafts of non-breeding males form in some areas. Average range size 0.8–6.8km2 (♀s) and 0.4–4.8km2 (♂s) in California, and linear 38km (♀s) to 50km (♂s) in Washington. Reproduction and Demography Weakly seasonal; breeding occurs year-round, but births peak May–June (Washington and Alaska), and weakly January–March (California). Gestation 4–12 months (averaging 180–218 days), with delayed implantation. Litter size 1, exceptionally 2. Pups are born at sea, and carried and suckled by the mother at rest; they can swim at 2 months. Weaning and independence coincide at 5–9 months. MORTALITY For pups, mortality varies from 17% (Kodiak Island, Alaska) to 53% (Amchitka Island, Alaska, with limited food). Adults generally have low mortality, e.g. 4–11% (Kodiak Island), mainly from starvation and predation. LIFESPAN 15 (♂s) to 20 (♀s) years in the wild, 30 in captivity. Status and Threats Previously very heavily exploited for its luxuriant fur, which reduced a total estimated population of 300,000 to <2,000 by 1911. Strict protection and reintroductions have restored it to an estimated 125,000, although local declines continue to occur. The main threats are oil spills and other contaminants. Elevated Killer Whale predation in response to seal declines is implicated (though not proven) in declines in the Alaskan Aleutian Islands. Also killed by entanglement in fishing nets and by disease, including exotic disease introduced to waterways by humans. CITES Appendix I California, Appendix II – elsewhere; Red List EN, population trend Decreasing.
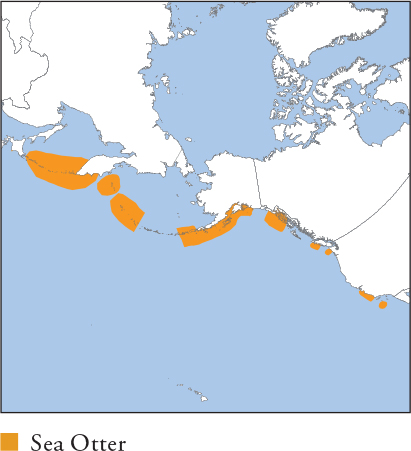
Plate 90

| GIANT OTTER Pteronura brasiliensis |
HB 86.4–130cm; T 45–75cm; W ♀ 22–26kg, ♂ 26–34kg
The largest Latin American mustelid and the world’s second largest (by weight, after Sea Otter). Strongly built, with a very robust, powerful skull and a long, muscular paddle-like tail. Uniformly dark chocolate brown with creamy-white markings on the chin and throat that vary from light stippling to a solid patch that extends onto the chest; markings are unique to individuals. Distribution and Habitat Endemic to South America, from Venezuela to E Paraguay and S Brazil; likely extinct in Argentina and Uruguay. Requires clear, slow-moving waterways or lakes in intact forest, woodland and wetland. Occasionally occupies agricultural canals and artificial reservoirs, but does not tolerate waterways in open and highly disturbed habitats. Feeding Ecology Primarily piscivorous; adults eat around 3kg of fish a day, mainly characins, catfish, perch and cichlids. Most fish captured measure 0.1–0.4m, but Giant Otter is capable of taking catfish measuring more than 1m. Other prey types infrequently recorded include reptiles (including anacondas to 3m, caimans to 1.5m and large turtles), small mammals, birds, crustaceans and molluscs. Hunts in family groups, but with little evidence of cooperation except in subduing large prey; group members provision cubs and large prey is sometimes shared, otherwise individuals mostly eat their own catches. Exclusively diurnal. Small prey is eaten in the water and large prey is taken to the bank or fallen trees to consume. Adults catch up to 3.2 fish/hour. Social and Spatial Behaviour Social and territorial. Lives in highly cohesive family groups comprising a monogamous adult pair and its offspring from 1 or more generations. Groups usually number 4–13; largest recorded groups, numbering to 20, are probably temporary associations of 2 or more families. Groups defend their territories from neighbours, largely by scent-marking and mutual avoidance, but clashes are aggressive and occasionally fatal. Group territories are linearly shaped and spaced along riverbanks or lake edges. Group territory size (linearly) 5.2–32km, averaging 9.3–11.4km. Reproduction and Demography Weakly seasonal. Only the alpha pair breeds in the group. Litters are born in dens in low, sloping banks with dense cover. Births occur mainly in the dry season in areas with marked seasonality, e.g. August–October (Suriname) and April–September (Brazilian Pantanal). Gestation 52–70 days. Litter size 1–5, averaging 2–3. Cubs can swim at 6 weeks and accompany the group as it forages from 3–4 months; weaning at 8–9 months. Dispersal first occurs at 10 months, but usually at 2–3 years; males thought to disperse more often than females. MORTALITY Poorly known. Adults are sometimes killed in intraspecific fights. Black and Spectacled caimans are occasional predators, largely on cubs. LIFESPAN 12.8 years (minimum estimate) in captivity. Status and Threats Formerly heavily hunted for fur, leading to extirpation or declines across much of its range. Hunting is rare today, but its narrow ecological needs and slow reproductive rates make it vulnerable to habitat modification, pollution of waterways by mining contaminants (e.g. mercury), agricultural run-off and overfishing. Persecuted by fishermen as perceived competitors for fish, and caught accidentally in fishing nets. Critically endangered in Ecuador and Paraguay. CITES Appendix I; Red List EN, population trend Decreasing.
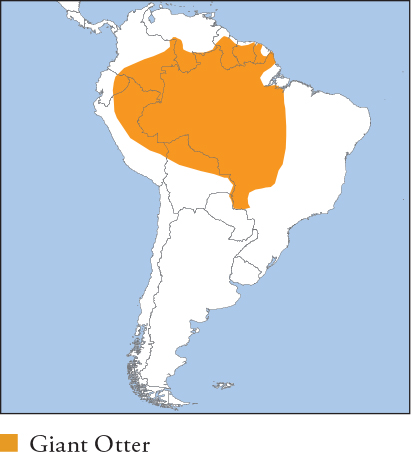
| EURASIAN OTTER Lutra lutra |
EUROPEAN OTTER, COMMON OTTER
Includes JAPANESE OTTER L. nippon
HB ♀ 59–70cm, ♂ 60–90cm; T 35–47cm; W ♀ 6–12kg, ♂ 6–17kg
Medium-sized otter, uniformly dull mid-brown to dark brown with pale cream to white underparts. Population in Japan has been considered a separate species, Japanese Otter (L. nippon), but the evidence is equivocal. Distribution and Habitat Eurasia, from W and N Europe to the Russian Far East and S China; scattered populations across C and Southeast Asia, with isolated populations in S India, Sri Lanka, Sumatra and N Africa. Confirmed in Japan (Tsushima Island) in 2017 after no records since 1979. Inhabits a wide variety of aquatic habitats, always with bank vegetation, rocks or debris, including rivers, streams, lakes, wetland, swamp forest, mangroves and coastal areas, from sea-level to 4,120m. Occupies rural and urban waterbodies only if they have sufficiently intact bank cover. Feeding Ecology Eats principally fish, freshwater amphibians and invertebrates, especially crabs, crayfish and aquatic insects. Occasional prey items consist of birds, including seabirds such as fulmars and guillemots in coastal populations, water voles, rats, rabbits (which are killed in their burrows) and reptiles. Plant matter sometimes appears in the diet, but is probably taken incidentally while consuming prey. Rarely takes carrion. Foraging is largely nocturno-crepsuscular and solitary, except for mothers with attendant cubs. Adults eat approximately 1–1.5kg of fish a day, representing around 12% (♂s) to 28% (♀s with cubs) of their body weight a day. Social and Spatial Behaviour Solitary and territorial. Males maintain large territories encompassing numerous female territories. Females are essentially solitary, but 2–3 adult females (probably related) may share and jointly defend a group territory – although each female maintains an exclusive core area and rarely interacts with the others. Territories are small and mostly exclusive in high-density areas, with size and overlap increasing as density declines. Average territory size (linearly) 7–18.7km (♀s) and 15–38.8km (♂s); individual males are recorded using linear areas extending up to 84km. Density estimates 1/5km (riverbank) to 1/2–3km (lakeshore). Reproduction and Demography Aseasonal or seasonal, depending on seasonality of food availability. Mating in W and N Europe and Russia January–April; births peak April–May. Gestation 60–63 days. Litter size 1–4, exceptionally 5 (captivity). Cubs can swim at 2 months, are weaned at around 4 months and stay with their mother until 10–12 months. Sexual maturity at 18 (♂s) to 24 (♀s) months. MORTALITY Rates poorly known, but the most important factors are roadkills, starvation and intraspecific aggression. LIFESPAN 16 years in the wild (but rarely older than 4), 22 in captivity. Status and Threats Has undergone significant declines in much of its range, due to a combination of overfishing, habitat loss and hunting. Highly vulnerable to canalisation of rivers, clearing of riverine vegetation, dam construction, draining of wetlands and aquaculture, and pollution associated with development. Roadkill is the main mortality factor in most of Europe. Hunted intensively, mainly for traditional medicinal use, in Asia. CITES Appendix I; Red List NT, population trend Decreasing.

Plate 91

| HAIRY-NOSED OTTER Lutra sumatrana |
HB 57.5–82.6cm; T 35–51cm; W 5–8kg
Medium-sized otter, very dark brown with white or yellowish upper lips, chin and throat patch. Very distinctive bulbous muzzle and broad nose covered entirely with fur. The rarest and least known of Asian otters. Distribution and Habitat Endemic to extreme Southeast Asia, with discontinuous populations known from S Vietnam, S and E Cambodia, extreme S Thailand, S Malaysia, Borneo and Sumatra. Recorded mainly from peat swamps, flooded forest, wetland and mangroves, as well as rivers, lakes and mountain streams. Feeding Ecology Thought to eat mainly fish such as gouramis, climbing perch, walking catfish and snakeheads. Water snakes are frequent prey in Thailand. Also eats small numbers of crabs, insects, frogs, lizards, birds and small mammals. Camera-trap records show that it is cathemeral. Reportedly a very fast and agile swimmer that weaves in and out of mangrove roots and bank debris to capture fish. Social and Spatial Behaviour Virtually unknown. Usually reported as solitary, but reliable records exist of small groups numbering to 5, including an observation of 2 adults with a pup. Reproduction and Demography Poorly known. Limited records of pups cluster in November–February, and litters in the wild number 1–2 (based on very few observations). MORTALITY and LIFESPAN Unknown. Status and Threats Very rare, to the point where it was considered extinct until recent ‘rediscoveries’. Now confirmed from a handful of sites, where it is seriously threatened by development and degradation of habitat, combined with widespread poaching for the illegal fur trade in China and, to a lesser degree, for meat and traditional medicine. CITES Appendix II; Red List EN, population trend Decreasing.
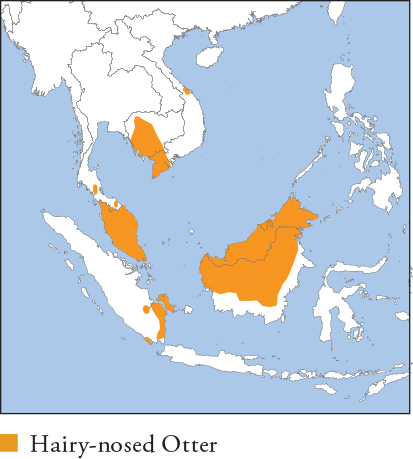
| ASIAN SMALL-CLAWED OTTER Aonyx cinereus |
ORIENTAL SMALL-CLAWED OTTER
HB 36–47cm; T 22.5–27.5cm; W 2.4–3.8kg
Smallest otter, uniformly dark brown, sometimes with an ashy-grey tinge. Greyish-white lower face, throat and upper chest. Claws are vestigial on all the feet. Most closely related to Smooth-coated Otter and African clawless otters (here); hybridisation with the former is known from Singapore. Distribution and Habitat S China through Indochina, Myanmar and Nepal to N India (with isolated populations in E and S India), the Philippines (Palawan), Borneo, Sumatra and Java. Inhabits freshwater bodies from sea-level to 2,000m, including rivers, mountain streams, lakes, peat swamps, mangroves and coastal wetland. Occupies anthropogenic habitats provided there is cover, e.g. rice paddies. Feeding Ecology Specialises in freshwater crabs, followed by small fish such as mudskippers, catfish and gouramis. Also eats snails, aquatic insects, shellfish, amphibians, snakes and small mammals. Captives leave shellfish in the sun until the heat opens them, but it is unknown if wild animals do likewise. Foraging is social and mainly diurno-crepuscular, but nocturnal when disturbed. Hunts by sight and by searching in mud and bank debris with its very dextrous forepaws. Social and Spatial Behaviour Intensely social, living in extended family groups of 2–15, thought to comprise a breeding pair and its offspring of 1 or more generations. Group members inhabit a stable range and are always together, moving, foraging and resting as one. Range sizes and densities are unknown. Reproduction and Demography Monogamous, with breeding apparently restricted to the alpha pair, but reproduction is poorly known. Gestation 60–86 days. Litter size 2–7, averaging 4–5. Captive males help raise pups, and other group members are thought to act as helpers in the wild. MORTALITY Most documented deaths are anthropogenic. LIFESPAN 15 years in captivity. Status and Threats Threatened by habitat degradation, especially from damming, draining of wetlands and peat swamps, water pollution from agricultural run-off, and aquaculture. This is exacerbated by loss of prey from overfishing and pollution, combined with direct persecution; throughout the whole of Asia, all otters are killed for fur, meat and traditional medicine, and as perceived pests by fishermen and shrimp farmers. CITES Appendix II; Red List VU, population trend Decreasing.
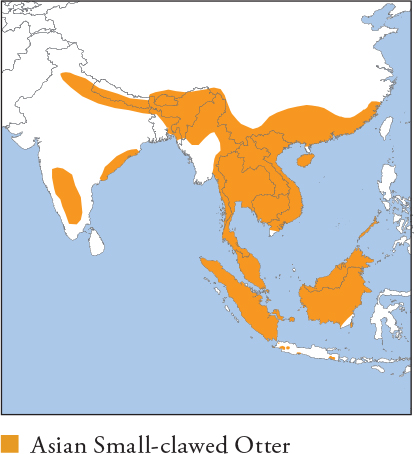
| SMOOTH-COATED OTTER Lutrogale perspicillata |
INDIAN SMOOTH-COATED OTTER, SMOOTH OTTER
HB 59–75cm; T 37–43.2cm; W 7–11kg
Largest Asian otter. Glossy, short, very smooth fur, typically chestnut-brown to dark brown. A creamy-white or yellowish throat patch runs from the upper lips and chin to between the front legs. Distribution and Habitat S Asia, from SE Pakistan through India, Indochina, S China, Borneo, Sumatra and Java; isolated population in SW Iraq. Inhabits lowland and plains waterbodies, including rivers, lakes, wetland, peat swamps and mangroves. Found in rice paddies and artificial ponds. Feeding Ecology Eats mainly fish, often taking introduced pest species such as tilapia and European Carp. Also eats crustaceans (especially in mangroves and rice paddies), snails, insects, frogs, and occasionally birds and rodents such as Ricefield Rat. Foraging is cathemeral, with elevated diurnalism during winter. Forages in family groups, with some evidence of cooperative driving of fish into reeds, where they are caught. In parts of India and Bangladesh, it is tamed and used by fishermen to catch fish and herd them into nets. Social and Spatial Behaviour Highly social, living in groups of 2–11, thought to centre around a female and her offspring from multiple generations. Adult males are sometimes observed with groups, but it is unclear whether they are permanent members. Range size estimated at 7–12km of river to each family group. Density estimates (linearly) 1–1.3/km of shore. Reproduction and Demography Seasonal. Mating August–September; births October–February (India and Nepal); there is some evidence that it breeds year-round under high food availability. Gestation 60–63 days. Litter size 1–5. MORTALITY Poorly known, but groups mount a formidable defence against predators; a credible record exists of a family group fatally injuring an Indian fisherman who had captured a cub in his net. LIFESPAN 20 years in captivity. Status and Threats Fairly widely distributed, but estimated to have declined by >30% over the past 30 years. Vulnerable to the same threats as other Asian otters; being essentially a lowland species, it is particularly vulnerable to the intense development pressure on wetlands throughout S Asia. CITES Appendix II; Red List VU, population trend Decreasing.

Plate 92

| SPOTTED-NECKED OTTER Hydrictis maculicollis |
HB 57.5–76cm; T 38.5–44cm; W ♀ 3.5–4.7kg, ♂ 4.5–6kg (exceptionally to 9kg)
Smallest African otter, chocolate brown, sometimes with a reddish cast. Throat, chest and sometimes belly are marked with white to creamy-white blotches, unique to individuals. Chin and upper lip are white. Formerly classified in Lutra, but recent genetic analyses now place it within the unique genus Hydrictis, most closely related to Sea Otter. Distribution and Habitat Sub-Saharan Africa, from Guinea to Ethiopia, south to South Africa. Inhabits unsilted and unpolluted freshwater habitats with dense bank vegetation, including lakes, streams, rivers and large swamps. Rarely found >10m from water. Feeding Ecology Eats mostly small fish (<20cm), especially cichlids, catfish, barbell, tilapia and introduced trout. Also eats crabs and frogs, especially in the relatively fish-poor waters of southern Africa; diet comprises almost entirely fish in the richer great lakes of C and E Africa. Opportunistically eats aquatic insects, their larvae and occasionally waterbirds. Foraging is solitary or in small social groups, and mostly diurnal with elevated nocturnalism during moonlight. Almost all fishing occurs within 10m of the shore. Around 50% of dives produce a catch, averaging 1 capture every 66 seconds. Scavenging on land not recorded, but takes dead and live fish from fishing nets. Social and Spatial Behaviour Sociality flexible. Adults are often solitary, and small family groups (up to 5) usually comprise a female and kittens, but non-breeding adults (especially males) often associate in larger groups numbering up to 21. Individuals and groups appear to be non-territorial, with high overlap between ranges. Range size averages 5.8km2 (♀s) and 16.2km2 (♂s) in KwaZulu-Natal, South Africa. Reproduction and Demography Apparently seasonal (Lake Victoria); mating is in July and births in September, although litters are recorded at other times elsewhere. Gestation approximately 60 days. Litter size 1–3. MORTALITY Nile Crocodile is considered the main predator, but there are no details. LIFESPAN Unknown. Status and Threats Widespread and numerous in good habitat, especially C and E Africa’s great lakes. However, gradually declining in most of its range due to siltation and pollution. Also drowned in fishing nets, killed by fishermen and valued for traditional medicinal beliefs in many areas. CITES Appendix II; Red List NT, population trend Decreasing.
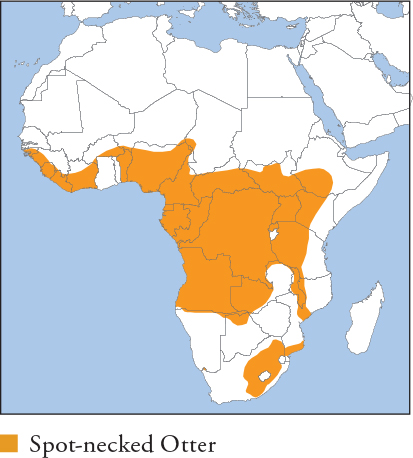
| CAPE CLAWLESS OTTER Aonyx capensis |
AFRICAN CLAWLESS OTTER,
AFRICAN SMALL-CLAWED OTTER
HB ♀ 73–73.6cm, ♂ 76.2–88cm; T 46.5–51.5cm; W ♀ 10.6–16.3kg, ♂ 10–21kg
Large, powerful otter, uniformly mid-brown to dark brown, with a white to pale grey muzzle, throat and upper chest. Feet are partially webbed; the hind paws have small grooming claws, while the forepaws lack claws except for vestigial ‘fingernails’. Distribution and Habitat Most of sub-Saharan Africa; absent from the Congo Basin and from arid SW and NE Africa. Inhabits lakes, rivers, streams, ponds, estuaries, mangroves and marine habitats close to fresh water, which is required for drinking. Relatively tolerant of pollution and siltation, and occupies urban streams, canals and reservoirs. Feeding Ecology Crustacean specialist, eating mainly crabs, crayfish and lobsters. Also eats fish, octopus, frogs and molluscs. Populations in Eastern and Western Cape provinces (South Africa) increase fish consumption during the cold winter, when fish are sluggish and easily caught. Aquatic insects, birds and small mammals (mostly riparian rodents and shrews) are occasionally consumed. Sporadically kills domestic ducks and geese on farmland. Foraging is mainly nocturno-crepuscular, with increased diurnalism where undisturbed, and solitary or in small social groups. Uses its very dextrous forepaws to find and capture prey, mostly in shallow water less than 1.5m deep. Locates and pursues fish mainly by sight. Social and Spatial Behaviour Females are mainly solitary, while males are either solitary or live in small groups numbering up to 5 that occupy a shared range; group members generally travel alone, but regularly meet in a ‘fission–fusion’ pattern, sometimes sharing large prey. Adult female ranges have relatively little overlap, while males vary from little (especially for groups) to extensive overlap. Average territory size (linearly) 17km (♀s) and 42km (♂s) in fresh water, South Africa. Density estimates 1/1.4–5km. Reproduction and Demography Thought to be aseasonal, with birth peaks in the rainy season. Gestation 60–63days. Litter size 1–3. Kittens born with pale smoky-grey woolly fur. They are weaned at around 45–60 days and remain with the mother for up to 12 months. MORTALITY Most known mortality is anthropogenic; Nile Crocodile, Lion and African Fish Eagle occasionally kill otters. LIFESPAN 13 years in captivity. Status and Threats Widespread and tolerant of some habitat modification, but threatened by severe degradation with associated pollution, siltation and eutrophication. Persecuted as a perceived problem on fish farms, and valued for traditional medicine. CITES Appendix I – Cameroon and Nigeria, Appendix II – elsewhere; Red List NT, population trend Decreasing.

| CONGO CLAWLESS OTTER Aonyx congicus |
SWAMP OTTER, CONGO SMALL-CLAWED OTTER
HB 79–97cm; T 41–56cm; W 14–25kg
Very similar to Cape Clawless Otter and sometimes regarded as the same species, but limited genetic and morphological data indicate they are distinct. They overlap at the edges of their respective distributions in Uganda and Rwanda (and possibly elsewhere). Distribution and Habitat Endemic to the Congo Basin; exact range limits unknown, given confusion with Cape Clawless Otter. Inhabits rivers, wetland and swamps in undisturbed rainforest. Not known from marine habitats, but may occur in coastal lagoons and mangroves. Feeding Ecology Diet includes earthworms, frogs, freshwater crabs, fish and aquatic insects. Earthworms are a key component of the diet based on observations from Mbeli Bai, Republic of the Congo, where otters grub in the mud for earthworms with their dextrous forepaws, consuming up to 3 a minute. Diurno-crepuscular where undisturbed. Social and Spatial Behaviour Poorly known. Most sightings are of individuals or mothers with young. Small groups of up to 4 are reported. Forages close to Spotted-necked Otter without conflict (Mbeli Bai). Reproduction and Demography Poorly known. Probably breeds year-round given its equatorial range. Litter size 1–3. Kittens born with pale fur that darkens to adult coloration by 2 months. MORTALITY and LIFESPAN Unknown. Status and Threats Status poorly known. Likely occurs in suitable habitat throughout the Congo Basin, which is well preserved over vast areas. Forest loss and overhunting for bushmeat are primary threats, thought to prompt local declines near settlements. CITES Appendix II; Red List NT, population trend Decreasing.

Plate 93
