21
Residual Alkalinity, Malt Acidity, and Mash pH
Or, Everything You Ever Wanted to Know about Mash pH but Were Afraid to Ask
Ignorance is bliss for the beginning masher, because most any water will produce wort when mixed with crushed malt at the right temperature. If you remember the top five brewing priorities from chapter 1, water is not on that list. Let’s be realistic, if you are going to heat up a can of condensed soup, make a box of macaroni and cheese, or make a pot of coffee, you probably won’t give much thought to the water that you are going to make it with. As long as the water tastes good, the food will taste good.
However, let’s say you wanted to brew a truly exceptional cup of coffee; now the taste and composition of the water becomes more important. There is a reason why the foods of master chefs and the beers of master brewers are more highly praised than the rest, and those reasons are attention to detail and control. Their attention to detail and understanding of those details allows them to control the results of the process. Beer is liquid food. We need to understand how the water can affect the brewing process and the taste of the beer in order to be able to exert control. This is where brewing water can get complicated, but I will do my best to lay it all out in a clear, logical stream.
There are six key concepts for understanding how water affects your beer:
- 1. Beer and brewing are food and cooking.
- 2. Know your water source.
- 3. Residual alkalinity is the cornerstone of mash pH.
- 4. Mash pH is the result of the interaction between water chemistry and malt chemistry.
- 5. The mash pH is the cornerstone of beer pH.
- 6. Beer pH is the cornerstone of beer flavor.
Beer and Brewing is Food and Cooking
Beer is food, and the brewing of beer is both a cooking and fermentation process. You, as brewmaster, have the same ability to brew a great beer as a chef does to create a great meal. The key is attention to detail in every aspect of the brewing, or cooking, process: the quality of ingredients, the proportions, and the techniques. There are no shortcuts anymore—everything you do is going to affect the flavor of the beer you make.
The first thing you need to do is consider the flavor of your beer. What aspects or properties control the flavor of food? The answer is pH and seasoning. I like to use spaghetti sauce as an example. A standard commercial spaghetti sauce from the grocery store is not very exciting; it’s probably sweet and bland, and lacks complexity. Children will probably love it, but adults with more refined tastes probably won’t. The other extreme is a spaghetti sauce that was made fresh that morning at a paleo-food Italian restaurant. It will have such bright tomato acidity that you can’t really taste anything else! To make the best spaghetti sauce with a full expression of flavors the pH of the sauce must be balanced, which creates a balance between tomato acid brightness and rich tomato flavor. You cannot have complexity without balance. Once you have balanced the flavors of the sauce with pH, you can fine-tune them further with seasonings to accentuate the specific flavors you prefer.
This is how you need to think about your beer. The pH of the beer controls the way flavors are expressed to your palate, while the water profile—the minerals in the water—are the seasonings for your beer. There are two ways that the minerals season your beer; one is the relative proportions of sulfate and chloride, and the other is the total amount of minerals in the water. We will discuss these aspects more toward the end of the chapter.
Know Your Water Source—a Review
The source of the water dictates the mineral profile of the water. Surface water sources, such as lakes, rivers, and streams, will typically be low in minerals but high in organic matter, such as algae, fish, and lots of microorganisms. Groundwater from aquifers underground tends to be low in organic matter but high in dissolved minerals, such as calcium, sodium, and bicarbonate. Depending on where you live, your source water may be surface water, groundwater, or a mixture of both. The source could change seasonally, such as surface water during the summer and groundwater during the winter. This change in mineral profile can have a big impact on the pH of your mash, wort, and beer.
Surface water sources usually need more microbiological control than groundwater sources, and during the summer a water utility will often increase the chlorine treatments, which can be a problem. However, the low mineral content of surface water makes it easy to add salts to adjust the pH and flavor effects. Groundwater sources often cause calcium carbonate scaling problems for the utility, so they may use lime or other softening treatments to reduce the hardness in the water, which is the opposite of what brewers want. To review, water hardness is determined by the levels of calcium and magnesium ions dissolved in the water, both ions being beneficial for the brewing and fermentation processes. Permanent hardness is calcium and magnesium ions derived from highly soluble salts, such as sulfates and chlorides, because these ions stay in solution. Temporary hardness comes from calcium and magnesium ions derived from carbonate salts, because these are likely to precipitate out as carbonate scale (limescale) when the water is heated or boiled.
Home water softeners remove the calcium and magnesium ions from water but don’t remove the bicarbonate ions, which determine the alkalinity. This is good for the water utility’s pipes, because an alkaline pH (7.0–9.0) is less corrosive than an acidic pH, but it is not as good for our mash, which has a pH target of 5.2–5.6. Alkalinity drives the mash pH up, which can cause the extraction of unpleasant tannin flavors, a harsher bitterness from the hops, and a high beer pH that dulls the overall flavor. This is why you will often see references in brewing text books saying to remove the temporary hardness from the water; it is not because we want to remove the hardness, but because we want to remove the alkalinity that is associated with it.
Alkalinity is the sum of the dissolved carbonate species in the water. Alkalinity is measured by acid titration, that is, by measuring the amount of acid of known concentration required to lower the water’s pH to 4.3, where all of the carbonate species have converted to dissolved carbon dioxide and carbonic acid. This measure of alkalinity by acid titration is called either “total alkalinity,” “general alkalinity,” or “M alkalinity.” By convention, total alkalinity is usually reported in the form “total alkalinity, ppm as CaCO3” (as calcium carbonate), which refers to the potential for calcium carbonate scale (which is important from a water company’s point of view). Both total hardness and total alkalinity are reported as ppm as CaCO3 for this reason. See the sidebar for a discussion of units for mineral concentration.
In order to control your water and its effects on your beer, you need to know what is in it. There are six ions that determine how your brewing water affects your beer and they are: calcium, magnesium, total alkalinity (as CaCO3), sulfate, chloride, and sodium. Let’s look at each in more detail.
Calcium
- • Atomic weight = 40
- • Equivalent weight = 80
- • Recommended range = 50–150 ppm
The calcium ion (Ca2+) is the most important ion in brewing. It is a cofactor in many biochemical reactions in the mash and fermentation. It stabilizes alpha-amylase in the mash at high temperatures and pH. It improves beer clarity via trub coagulation and yeast flocculation. It does not have a flavor but beers taste watery without it. Calcium ions react with malt phosphates to reduce mash pH.
The minimum recommended level for calcium ions in brewing water is 50 ppm for light lagers and ales. Calcium ion levels of 100–150 ppm are generally preferred for good mash and lauter pH stability, although these levels may be too robust for some light beer styles. Calcium ion levels in excess of 200 ppm tend to taste minerally. These concentration values are for the calcium ion itself; for calcium hardness as CaCO3, divide these ppm levels by 20 and multiply by 50.
Magnesium
- • Atomic weight = 24.3
- • Equivalent weight = 12.15
- • Recommended range = 0–40 ppm
The magnesium ion (Mg2+) is the sidekick to the calcium ion, participating in many of the same reactions. It a vital yeast nutrient with a minimum required level of 5 ppm, but usually the malt supplies all the magnesium that the yeast would need. Magnesium can be displaced by calcium in the yeast cell if the calcium ion level in the water is too high, although references do not say what those conditions are. It may be beneficial to add magnesium salts to the water if no magnesium is present. Magnesium ion concentrations greater than 80 ppm are said to contribute a sour bitter flavor to beer, although lower levels of 20–40 ppm are said to enhance the flavor of dark beer styles, such as porter and stout. Magnesium hardness as CaCO3 is equal to the magnesium ion concentration divided by 12.15 and multiplied by 50.
Carbonate Species vs. Water pH @ 20°C

Figure 21.1. The carbonate system consists of carbonate ions (CO32−) at high pH levels, bicarbonate ions (HCO3−) at medium pH levels, and very low levels of carbonic acid (H2CO3) in chemical equilibrium with dissolved carbon dioxide (CO2)aq at low pH levels.
Total Alkalinity as CaCO3
- • Molecular weight = 100
- • Equivalent weight = 50
- • Recommended range = 0–100 ppm
Alkalinity in brewing water is undesirable when brewing most pale beers, but advantageous in some darker brews. Carbonates in water exist in three species (chemical forms), which are carbonate ions (CO32−), bicarbonate ions (HCO3−), and carbonic acid (H2CO3). (Only a very small amount of carbonic acid actually forms; most of it exists as dissolved carbon dioxide.) The relative proportions of these species depend on the water pH. Potable water has a pH between 7.0 and 9.0, often falling between 8.0 and 8.5. The predominant carbonate species in potable water is the bicarbonate ion (HCO3−), which makes up 98% of total carbonate in the pH 8.0–8.5 range. Figure 21.1 shows a graphical representation of the system.
Alkalinity in the form of carbonate activity is the primary buffering system in water, and a large factor in determining the buffering of the mash and wort. Generally, it raises mash pH, which can cause problems for reasons already described. However, this alkalinity can also prevent the mash pH from going too low due to mashing with dark specialty malts, which are naturally acidic. Low levels of carbonate alkalinity (~50 ppm) will also prevent the beer from tasting watery. Higher alkalinity levels (100–150 ppm) can buffer the pH of dark beer styles, such as porter and stout, and prevent them from tasting acrid.
Sulfate
- • Atomic weight = 96
- • Equivalent weight = 48
- • Recommended range = 50–150 ppm for most beer styles
= 150–400 ppm for pale ale and IPA styles
Sulfate ions (SO42−) accentuate the hop character in beer, making it taste drier and crisper. Sulfate ions work better with some hop families and beer styles than others. Pale ales and IPA styles will often use 150 ppm or higher to make the bitterness very assertive and dry. Other styles, such as German helles and Kölsch, must use lower amounts (50–75 ppm) to prevent the bitterness from dominating the soft malt palate of the beer. Noble hops and sulfate ions do not get along well together—sulfate ions seem to bring out very sulfuric flavors in these hops.
Sulfate ions do not affect mash or wort pH.
Chloride
- • Atomic weight = 35.4
- • Equivalent weight = 35.4
- • Recommended range = 50–150 ppm
The chloride ion (Cl−) is not the same as elemental chlorine, and chloride in brewing water will not cause the same sort of flavor problems residual chlorine or chloramine can. Chloride accentuates the maltiness of the beer, making the beer taste fuller and sweeter. Chloride levels greater than 150 ppm can make the beer taste underattenuated or cloying. High concentrations (>300 ppm) of chloride can hurt clarity and flavor stability and cause corrosion of your equipment.
Chloride ions do not affect mash or wort pH.
Sodium
- • Atomic weight = 22.9
- • Equivalent weight = 22.9
- • Recommended range = 0–100 ppm
Sodium ions (Na+) accentuate the malt character of beer, rather like chloride, but you can easily oversalt your beer. Sodium ions can taste metallic in combination with other ions such as calcium, magnesium, bicarbonate, and sulfate. The sodium ion level should be kept to less than 100 ppm in the brewing water. Reverse osmosis is just about the only way to remove sodium from water.
Sodium ions do not affect mash or wort pH.
Water pH
The pH of the water is not important. The pH of the water describes the chemical activity of the water; but that is not what we are interested in—we are interested in the chemical activity and pH of the mash. The only information the water pH can give us is an indication of the balance between the hardness and alkalinity in the water. You can have the same water pH for two completely different mineral profiles that would have very different effects on the mash pH, and subsequently the beer. A high water pH does indicate that the water has more alkalinity than acidity, but it doesn’t tell you what that alkalinity level actually is. It is like the difference between having two children or two gorillas on a seesaw (teeter-totter); children are a lot easier to move (less buffering power) than gorillas. Water pH is useful to know, but you cannot make any real judgments about the suitability of your brewing water, or the effect on mash pH, because of it. It is the actual mineral concentrations in the water that are important; as we will find out in the next section about residual alkalinity.
Residual Alkalinity is the Cornerstone of Mash pH
Water hardness helps lower mash pH, and water alkalinity raises mash pH. The combined effect of this is called residual alkalinity (RA). Residual alkalinity is the most important parameter for understanding how your water is going to affect your beer, because it has a direct effect on the mash pH, and therefore the wort pH and beer pH. Perhaps Dr. David Taylor said it best,
The key point for control of pH throughout the brewing process is during mashing. This is due to the major influence that can be exerted at this stage on the content and format of the buffer systems that will operate subsequently in the wort and beer. (Taylor, 1990, p.135)
Table 21.1—Conversion Factors for Ion Concentrations
| To get | From | Do this |
|---|---|---|
|
Ca2+ mEq/L |
Ca2+ ppm |
Divide by 20. |
|
Ca2+ ppm |
Ca2+ mEq/L |
Multiply by 20. |
|
Ca2+ ppm |
Ca2+ hardness as CaCO3 |
Divide by 50 and multiply by 20. |
|
Ca2+ hardness as CaCO3 |
Ca2+ ppm |
Divide by 20 and multiply by 50. |
|
Ca2+ hardness as CaCO3 |
Total hardness as CaCO3 |
Estimate by assuming that the calcium is often about 4/5 of the total hardness. |
|
CaCO3 mEq/L |
CaCO3 ppm |
Divide by 50. |
|
Alkalinity as CaCO3 |
HCO3− ppm |
Divide by 61 and multiply by 50. |
|
HCO3− mEq/L |
HCO3− ppm |
Divide by 61. |
|
HCO3− ppm |
Alkalinity as CaCO3 |
Divide by 50 and multiply by 61. |
|
Mg2+ mEq/L |
Mg2+ ppm |
Divide by 12.1. |
|
Mg2+ ppm |
Mg2+ mEq/L |
Multiply by 12.1. |
|
Mg2+ ppm |
Mg2+ hardness as CaCO3 |
Divide by 50 and multiply by 12.1. |
|
Mg2+ hardness as CaCO3 |
Mg2+ ppm |
Divide by 12.1 and multiply by 50. |
|
Mg2+ hardness as CaCO3 |
Total hardness as CaCO3 |
Estimate by assuming the magnesium is often about 1/5 of the total hardness. |
|
Total hardness as CaCO3 |
Ca2+ as CaCO3 and Mg2+ as CaCO3 |
Add them. |
Ca2+, calcium ion; CaCO3, calcium carbonate; HCO3−, bicarbonate ion; mEq/L, milliequivalents per liter; Mg2+, magnesium ion; ppm, parts per million.
What Does the Mash pH Do?
As was discussed in chapter 16, the mash pH is the second most important factor, after temperature, for enzyme activity in the mash. The activity rate of enzymes in the mash typically follows a bell-shaped curve about their pH and temperature optima. Enzymes can be denatured by either high temperatures, too high or too low pH, or a combination of both. Denaturing means that the three-dimensional shape of the enzyme irreversibly changes, with the result that the enzyme can no longer interact with the substrate it is supposed to act on, such as starches or proteins. Therefore, mash pH can have a significant impact on starch conversion, soluble and total nitrogen levels, lauterability, fermentability, and yield.
And, as Dr. Taylor notes, it sets up the composition of the wort, including sugars, proteins, and pH buffers, that will affect the performance of all the brewing steps that come after.
Optimum Mash pH
What is the optimum mash pH? Well, it depends. It depends on what parameters you are trying to optimize. There are many different enzyme groups in the mash, each category has several types, and each of which have their own optima. The temperature and pH optima for best protein degradation is not necessarily going to give you the best lauterability or fermentability. The best fermentability may not give you the best yield. Each of these properties will have its own optima. For example, Briggs et al. (1981, 279) state that the best yield is at pH 5.45–5.65, while Bamforth and Simpson (1995) found that it was at pH 5.55–6.05 (note both ranges are measured on room temperature samples). In Technology Brewing and Malting (Kunze 2014, 227), it is stated that the optimum for both alpha- and beta-amylase activity is pH 5.5–5.6, but then later the author says that for the best beer the pH should be 5.2, although I am speculating that the author may have been speaking in the context of pale lagers.
The optimum can vary with temperature in two different ways. First, the pH of every aqueous solution will change with temperature; higher temperatures cause molecules to dissociate more, meaning more ionization occurs and therefore the pH changes. In the case of wort, the generally accepted change is a decrease of 0.3 pH between room temperature and mash temperature (temperature goes up = mash pH goes down). But consider why the mash pH is changing: the constituents of that wort—the ions, peptides, and acids—are changing their activity in response to temperature. It stands to reason that different worts from different styles of beer will experience different amounts of change due to temperature. Experiments have indicated that the pH change between room temperature (68–77°F [20–25°C]) and mash temperature (149–158°F [65–70°C]) can range from 0.27–0.38.1 Therefore, brewing scientists make a point of either declaring what temperature the pH readings were taken at, or measuring the pH at room temperature. All of the professional brewing organizations have standard procedures for measuring mash, wort, and beer pH that specify to measure it at “room temperature,” which is frequently taken to mean 68–77°F (20–25°C).
Second, the optimum pH for a particular process can vary with the temperature the process takes place at. For example, Kolbach and Haase2 determined that the pH optima for starch extraction (from a particular malt) changed non-linearly with temperature; the pH optima changed from 4.9–5.3 at 122°F (50°C), to 5.1–5.5 at 140°F (60°C), and to 5.5–5.9 at 149°F (65°C).
Finally, aside from temperature, the optimum can vary with the agent you are using to adjust the mash pH. Bamforth and Simpson (1995) found that optimum pH for best lauterability changed from 4.4–4.6 when the mash pH was adjusted using sulfuric acid, and to 5.1–5.5 when adjusted using calcium chloride. It is reasonable to assume that a combination of the two agents would have an optimum somewhere in between.
So, what is the answer? There is no single correct answer, it is a range that depends on several factors and the brewer’s priorities. Generally, when we ask what is the optimum mash pH, we are thinking in terms of optimum yield. Notable references agree that the optimum mash pH for yield (conversion and extraction) would seem to fall in the 5.5–5.8 range. However, notable sources also agree that better beer flavor, clarity, and flavor stability are obtained at lower mash pHs. Therefore, the commonly agreed target range for mash pH is 5.2–5.6, as measured at room temperature (68–77°F [20–25°C]). Your specific target within that range will depend on your brewing processes and the recipe or style. Experience3 has demonstrated that pale beers seem to taste better with lower mash pH of 5.2–5.4, while dark beers tend to taste better with a slightly higher mash pH of 5.4–5.6.
Controlling Mash pH
How can we control mash pH? One way is by adjusting the residual alkalinity (RA) of our brewing water using salt and acid additions.
In 1953, German brewing scientist Paul Kolbach determined that 3.5 equivalents (Eq) of calcium ions react with phosphate ions from the malt to release 1 Eq of hydrogen ions that can “neutralize” 1 Eq of alkalinity in water. Magnesium ions, the other ion species contributing to water hardness, also work in the same way as calcium ions, but to a lesser extent, needing 7 Eq to neutralize 1 Eq of alkalinity in water. Neutralization of alkalinity by the chemical reactivity of calcium and magnesium ions does not require enzyme activity or an acid rest. The remaining alkalinity that is not neutralized is the residual alkalinity. As mentioned earlier, residual alkalinity is the result of the competing effects of water hardness and alkalinity on mash pH. When compared to a mash conducted with distilled water (RA = 0), a positive RA value increases mash pH, and a negative RA value (i.e., more hardness than alkalinity) decreases mash pH.
On a per volume basis, this relationship between hardness and alkalinity can be expressed as:
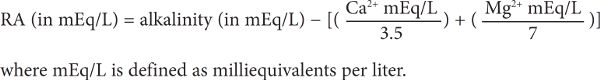
The same relationship can also be expressed in terms of ppm as CaCO3:

And it can be expressed in the units most often seen on municipal water reports, “Total alkalinity as CaCO3,” “Ca ppm,” and “Mg ppm.”
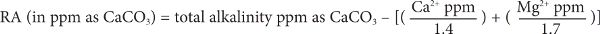
However, Kolbach’s experiments measured the effects of brewing water on wort pH (i.e., after the mash and sparge), using a 1.048 wort with a water-to-grist ratio of about 5 L/kg. More recent experiments4 indicate that the hardness equivalency factor (i.e., the 3.5 Eq value for calcium ions) in the mash not only varies with water-to-grist ratio but also with different types of malt, typically ranging from 2.2 to 3.5. Using a factor of 3.5 represents a more conservative value, that is, the rate at which pH decreases with increasing hardness is less.
The amount of residual alkalinity it takes to change the pH by 0.1 also varies with water-to-grist ratio and malt type. My own experiments with sample mashes using two different base malts and a porter recipe (giving 55 data points including replicates), indicated that the amount of change in RA to effect a change in pH of 0.1 ranged from 25 ppm as CaCO3 at Rv = 8 L/kg (4 qt./lb.) to 100 ppm at Rv = 2 L/kg (1 qt./lb.). The relationship was inversely proportional to Rv, and very nearly equal to 200/Rv. Therefore, a typical number for 0.1 pH change at Rv = 4 L/kg (2 qt./lb.) is about 50 ppm as CaCO3, according to my data.5 Your results may vary.
The Kolbach equation as given above is commonly used to predict the effects of residual alkalinity changes to the mash pH, and has been incorporated in many brewing software applications. Future studies may build on Troester’s and Barth’s work to make a more refined model, but until then the basic Kolbach equation serves to help us understand the basic mechanism we can use to adjust the mash pH. In essence, if you paddle this way, you go forwards; if you paddle that way, you go backwards. The important things to realize are that a) you are in a boat, b) you have a paddle, and c) you can move the boat. Don’t get hung up worrying too much about how fast you are going.
Adjusting Residual Alkalinity
Residual alkalinity is the combined effects of water alkalinity and water hardness on the mash pH. We can adjust either of these quantities with the addition of salts, such as calcium sulfate or sodium bicarbonate, or by adding strong acids or bases.
The following table lists the various brewing salts that can be used to adjust RA. These salts can be added to the brewing water beforehand, or added to the mash. Generally, I prefer to add them to the brewing water beforehand, but some, such as gypsum, can be difficult to dissolve (they need lots of stirring) unless they are added later on to the mash where the pH is lower.
Adjusting Residual Alkalinity with Salt Additions
Salt additions are easy to calculate. Look at your water report to determine the starting concentration of each of the six ions in your source water. You may want to test the water yourself to get more current numbers (see sidebar, “Testing Your Water,” for advice). Table 21.2 gives a list of brewing salts and their ion contributions in both grams per liter (g/L) and grams per gallon (g/gal.). An addition of 1 g/gal. means that you would be adding 1 g of a brewing salt for each gallon of water you are adjusting, so if you have 10 gal. of water then you would be adding 10 g of the brewing salt. The concentrations simply add together. For example, and referring to table 21.2, if your water report lists the calcium ion concentration as 36 ppm, adding 2 g/gal. of calcium chloride (CaCl2) would give you a final concentration of:

Also, keep in mind that by adding calcium chloride you will be increasing the chloride concentration as well. Keeping with the above example of adding 2 g/gal. of calcium chloride and assuming, for example, you had 50 ppm of chloride in the water to start with, your total chloride concentration would be:

Note in the above example that we are adding 2 g of calcium chloride for every gallon of water. Alternatively, you may wish to only add a total of 2 g of calcium chloride to the entire 7 gal. batch of water. The calculation is much the same, except you need to divide the ion concentration by the appropriate volume when adding. For example, when we add 2 g of calcium chloride to source water with existing concentrations of 36 ppm Ca2+ and 50 ppm of Cl−:
For calcium:
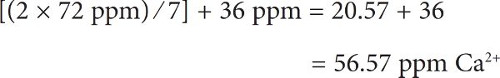
For chloride:

This process is easier when using de-mineralized water (either by distillation or reverse osmosis) as your water source because the mineral ion concentrations are essentially zero. If you dilute your tap water with distilled water, the mineral ion concentrations are effectively divided by whatever proportion of distilled water you use. For example, if you are diluting using one part distilled to one part tap water (i.e., a 50/50 dilution), then your tap water mineral concentrations are cut in half, and any salt additions you make to that diluted brewing source water would be added onto those new values.
Table 21.2—Ion Contributions for Brewing Salt Additions
| Brewing salt | Concn. at 1 g salt per liter (1 g/L) | Concn. at 1 g salt per gallon (1 g/gal.) | Comments |
|---|---|---|---|
|
Calcium carbonate CaCO3 mw = 100 eqw = 50 |
400 ppm Ca2+ 600 ppm CO32− 20 mEq/L alkalinity |
106 ppm Ca2+ 158 ppm CO32− 5.3 mEq/gal. alkalinity |
Will not dissolve in water, but will in mash. However, does not raise mash pH effectively. |
|
Sodium bicarbonate NaHCO3 mw = 84 eqw Na+ = 23 eqw HCO3− = 61 |
273.7 ppm Na+ 710.5 ppm HCO3− 11.8 mEq/L alkalinity |
72.3 ppm Na+ 188 ppm HCO3− 3.04 mEq/gal. alkalinity 150 ppm as CaCO3 |
Dissolves readily, and effective at raising alkalinity and mash pH. Best added to water before mashing. |
|
Ca(OH)2 mw = 74.1 eqw Ca2+ = 20 eqw OH− = 17 |
541 ppm Ca2+ 459 ppm OH− 27 mEq/L alkalinity ΔRA = 19.3 mEq/L |
143 ppm Ca2+ 121 ppm OH− 7.1 mEq/gal. alkalinity ΔRA = 5.1 mEq/gal. 255 ppm as CaCO3 |
Dissolves readily in water. Food-grade pickling lime seems to be good purity. |
|
Sodium hydroxide NaOH mw = 40 eqw Na+ = 23 eqw OH− = 17 |
575 ppm Na+ 425 ppm OH− 25 mEq/L alkalinity |
152 ppm Na+ 112.3 ppm OH− 6.6 mEq/gal. alkalinity 330 ppm as CaCO3 |
Dissolves readily. Raises alkalinity. Caution! Hazardous material! Consult material safety data sheet (MSDS) before use. |
|
Calcium sulfate CaSO4•2H2O mw = 172.2 eqw Ca2+ = 20 eqw SO42− = 48 |
232.8 ppm Ca2+ 557.7 ppm SO42− |
61.5 ppm Ca2+ 147.4 ppm SO42− |
Saturation at room temperature is about 3 g/L. Stir vigorously. Lowers mash pH. |
|
Magnesium sulfate MgSO4•7H2O mw = 246.5 eqw Mg2+ = 12.1 eqw SO42− = 48 |
98.6 ppm Mg2+ 389.6 ppm SO42− |
26.0 ppm Mg2+ 102.9 ppm SO42− |
Saturation at room temperature is about 255 g/L. Lowers mash pH. |
|
Calcium chloride CaCl2•2H2O mw = 147.0 eqw Ca2+ = 20 eqw Cl− = 35.4 |
272.6 ppm Ca 2+ 482.3 ppm Cl− |
72.0 ppm Ca2+ 127.4 ppm Cl− |
Dissolves readily. Lowers mash pH. Food-grade salt may not be high purity. |
|
Magnesium chloride MgCl2•6H2O mw = 203.3 eqw Mg2+ = 12.1 eqw Cl− = 35.4 |
119.5 ppm Mg2+ 348.7 ppm Cl− |
31.6 ppm Mg2+ 92.1 ppm Cl− |
Dissolves readily. Lowers mash pH. Food-grade salt may not be high purity. |
|
Sodium chloride NaCl eqw Na+ = 23 eqw Cl− = 35.4 |
393.4 ppm Na+ 606.6 ppm Cl − |
103.9 ppm Na+ 160.3 ppm Cl− |
Dissolves readily. Avoid iodized salt and anti-caking agents. |
Notes: The contributions are listed equivalently as ppm (mg/L), mEq/L, mEq/gal., or ppm as CaCO3, as applicable.
mw = molar weight in g/mole; eqw = equivalent weight in g/Eq.
Reducing Alkalinity with Acid
You can also use acids to reduce alkalinity and lower mash pH. Acids reduce alkalinity by supplying protons (which are hydrogen ions, H+) to convert all of the carbonate and bicarbonate ions in solution to carbonic acid, and subsequently to carbon dioxide (CO2). Note that the CO2 in solution must be removed from the water for the reaction to be complete. Most of the CO2 will escape as gas when the water is heated and stirred. In commercial breweries, where tuns are usually closed or contained, this CO2 is actively removed by agitation, bubbling with forced air or steam, or spraying. This is in order to prevent CO2 accumulating in enclosed piping or tankage where it can cause severe corrosion problems.
Acid additions to reduce alkalinity are quite simple to calculate if you work in terms of milliequivalents (mEq). The total alkalinity in ppm as CaCO3 is easily converted to milliequivalents per liter by dividing by calcium chloride’s equivalent weight, which is 50. For example, if the total alkalinity of the water is 125 ppm as CaCO3, that would equal 2.5 mEq/L. Adding 1 mEq of acid per liter would therefore reduce the total alkalinity to 1.5 mEq/L, or 75 ppm as CaCO3. However, there are two aspects to consider, specifically:
- 1. How many milliliters of acid is 1 mEq?
- 2. What flavor effect does the acid have?
The answer to the first question is that the amount of acid required depends on the specific acid, and table 18.3 lists dilutions for creating 1 N solutions of several common acids. The “N” stands for normality, which denotes Eq/L. A 1 N solution means that 1 L of the solution supplies 1 Eq, therefore, 1 mL supplies 1 mEq. So if you needed to reduce the alkalinity of 20 L (5.3 gal.) of water by 50 ppm as CaCO3 (i.e., 1 mEq/L), you would add 20 mL of your 1 N acid solution.
When considering the second question, the point is that the acid reaction will replace each equivalent of alkalinity with an equivalent of that acid’s anion (the negatively charged ion, e.g., Cl−). In the case of hydrochloric acid (HCl), this is one way of boosting the chloride level without adding more calcium or magnesium ions. Lactic and citric acids, however, have anions with a characteristic flavor and brewers need to consider whether those flavors will have an impact on the flavor of their beer. Choosing the acid and the final alkalinity is a matter of recipe formulation and may take a bit of trial-and-error. The method presented here reduces the alkalinity without regard to the pH. The purpose is to reduce the level of residual alkalinity, not to arrive at a specific mash pH. For more information on acid additions, see Water: A Comprehensive Guide for Brewers, by Palmer and Kaminski (2013).
Pre-Boiling to Reduce Alkalinity
Boiling has been used for hundreds of years to reduce the alkalinity and hardness of water by decarbonation. Broadly, the way it works is that the rise in water temperature changes the saturation point of all the carbonate species in solution. First the dissolved CO2 fizzes out of the water due to the rise in temperature. This removal of CO2 unbalances the equilibrium between the bicarbonate and carbonic acid in solution, which causes conversion of bicarbonate ions to carbonic acid and aqueous CO2, and in so doing consumes protons. This raises the pH. The increased pH causes some of the remaining bicarbonate ions to convert to carbonate ions. This results in saturation with respect to calcium carbonate, which precipitates. Since the formation of calcium carbonate removes carbonate ions, this causes a further imbalance in the equilibrium, and (in accordance with Le Chatelier’s principle) more bicarbonate ions convert to carbonate ions. Calcium carbonate continues to precipitate until either the calcium ion concentration or bicarbonate ion concentration is about 1 mEq/L, either 20 ppm or 61 ppm, respectively.
Table 21.3—Preparing 1 N Solutions of Common Acids
| Acid | w/w % | Density | Molarity | mL of acid to prepare 1 L of 1 N soln | Anion contribution per mEq/L |
|---|---|---|---|---|---|
|
Hydrochloric |
10 |
1.048 |
2.9 |
348 |
35.4 ppm Cl− |
|
31 |
1.18 |
12.0 |
83.5 |
35.4 ppm Cl− |
|
|
Phosphoric |
10 |
1.05 |
1.1 |
935a |
96 ppm H2PO4− |
|
85 |
1.69 |
14.7 |
68a |
96 ppm H2PO4− |
|
|
Lactic |
88 |
1.209 |
11.8 |
84.7 |
89 ppm lactate (~400 ppm flavor threshold) |
|
Citric |
(powder) |
… |
… |
96 grams in 1 L |
96 ppm citrate (~150 ppm flavor threshold) |
a Phosphoric acid is approximately monoprotic at mash pH.
Note: It is important to understand that the procedure is to dilute the prescribed volume up to a total volume of 1 liter. For example, 348 mL of 10% (w/w) hydrochloric acid would be poured into a volumetric flask, and water added to the flask to make exactly 1 liter.
Take care! Concentrated acids need to be added to a large volume of water that is already in the flask, to avoid exothermic splashing, before being topped up with additional water to the final volume.
This last milliequivalent per liter as CaCO3 does not precipitate and stays in solution. The calcium carbonate that has precipitated exists as microcrystals in suspension, which will eventually grow heavy enough to settle out. According to historical brewing texts,6 the water would typically be boiled for a half hour to allow the CO2 to be fully purged by the steam, and would then be allowed to settle overnight, leaving a white layer of calcium carbonate precipitate on the bottom of the kettle. The reduced-alkalinity water would then be decanted off the sediment for use as brewing liquor. This reaction is limited to water with moderate to high alkalinity, because it requires at least 1 mEq/L of calcium ions (20 ppm) and 1 mEq/L of bicarbonate ions (61 ppm) for the reaction to occur. In fact, unless the water contains significantly more than 1 mEq/L of each, the force driving of the chemical reaction will be low, and the method will be less effective at lowering alkalinity. The higher you can make the pH in this process, that is, the more dissolved CO2 you force out of solution, the more alkalinity you can ultimately remove. This is usually accomplished by bubbling air or steam through the water to agitate it until the pH is 8.5 or more. Water can typically be decarbonated down to a total alkalinity of 50 ppm as CaCO3 without too much trouble, but the concentration of calcium ions in the water is often a limiting factor. The residual calcium ion concentration after softening by boiling can be calculated with the following equation:7

where,
ion concentrations are denoted by square brackets, e.g, [Ca2+] is concentration of Ca2+;
all of the initial (i) and final (f) concentrations are in ppm;
and the factor 3.05 accounts for the conversion between bicarbonate ion and calcium ion equivalents.
The quantity, [HCO3−]f is the estimate of the final bicarbonate concentration, and is assumed to be at least 61 ppm of HCO3−, which is equivalent to total alkalinity of 50 ppm as CaCO3 at roughly pH 8.3. This final bicarbonate concentration of 61 ppm is based on ideal conditions. Using a more conservative value, such as 80 ppm bicarbonate, may be more realistic, allowing for conditions that are not ideal and where the reaction does not proceed to completion. A final bicarbonate concentration between 61 and 80 ppm is more typical when calcium ion concentration is not the limiting factor.
This reaction works best when the total hardness ppm as CaCO3 is greater than the total alkalinity ppm as CaCO3. It also works best if the permanent hardness is greater than the temporary hardness, meaning that there is plenty of calcium in solution to fuel the reaction and nearly all of the bicarbonate ions can be removed, except for that final 1 mEq/L (50 ppm as CaCO3). The best way to increase the permanent-to-temporary hardness ratio is to add calcium sulfate or calcium chloride to the hot water. The salts will also act as nucleation sites and help evolve the CO2 as gas.
This same reaction also happens when the water is merely heated to strike temperature. The difference is that the CO2 may not come out of solution until the water is stirred. This means any calcium carbonate that does precipitate will not have time to settle out, but will remain in suspension and be carried into the mash. The reaction kinetics for calcium carbonate additions to the mash are very slow, typically taking over two hours to change the mash pH. Boiling usually doesn’t affect magnesium ion levels because magnesium carbonate is much more soluble than calcium carbonate.
For example, look at the water profile for the city of Munich:
|
Ionic species |
Ca2+ | Mg2+ | HCO3− | Na+ | Cl− | SO42− | RA |
|---|---|---|---|---|---|---|---|
|
Concn. (ppm) |
77 |
17 |
295 |
4 |
8 |
18 |
177 |
The calcium and bicarbonate concentrations are high, and the residual alkalinity is comparable to Dublin, a city famous for its stouts. How did Munich become renowned for brewing pale Munich helles and amber Oktoberfest beers? One answer may be the decrease in alkalinity from pre-boiling the water. The approximate water composition after boiling would be:
|
Ionic species |
Ca2+ | Mg2+ | HCO3− | Na+ | Cl− | SO42− | RA |
|---|---|---|---|---|---|---|---|
|
Concn. (ppm) |
20 |
17 |
120 |
4 |
8 |
18 |
74 |
Boiling and decanting changes the residual alkalinity of the water from 177 to 74 ppm, and this may be what enables the brewing of lighter-colored styles. Another factor may be the use of acidulated malt, as explained in the sidebar “Using Acidulated Malt.”
Mash pH is Water Chemistry plus Malt Chemistry
The mash pH is the equilibrium between the water chemistry (the residual alkalinity) and the malt chemistry. What is malt chemistry? Well, I’m glad you asked. Every malt contains phosphates, proteins, and acids that affect the chemistry of the mash. Every malt when mashed in distilled water causes a drop in pH from the water’s starting pH of 7.0 to some nominal baseline value. I would like to say that each type of malt has a characteristic baseline pH, but unfortunately this is only generally true. There is a lot of variation in baseline pH (±0.2) between different barley varieties and different maltsters for the same malt type. In base malts, the pH range is typically 5.7–6.0, averaging about 5.8. Specialty malts typically have lower baseline pH values, in the range of 4.0–5.4, with the darkest caramel malts and roast malts having the lowest. In addition, malts with similar baseline pH values can have different buffering power, meaning the capacity to resist changes in pH differs between these malts even if their baseline pH is similar.
The buffering capacity of a malt is measured as milliequivalents per kilogram of alkalinity or acidity to a target pH. Table 21.4 lists the baseline pH and buffering power for several malts to three target mash pH levels. But it must be understood that these are single data points from experiments conducted by Briess Malt & Ingredients Co. in 2015. This data is presented for information only and is not intended to represent the pH values for those malt types in general, nor to represent typical pH values for Briess’ malt types, and certainly not be representative of products from other maltsters. Baseline pH and buffering capacity are not properties that any maltster specifies or controls; modification, yield, and color are much more important to the brewer. The purpose of showing the data in table 21.4 is so that you gain a general understanding of the way different malt types will affect the mash pH. Positive numbers pull mash pH up from the target, negative numbers pull the mash pH down from the target.
How do we use this sort of information? The mash pH is the equilibrium between the residual alkalinity of the water (positive or negative) and the various malts in the grain bill. To understand how this works, assume that we have chosen a target mash pH of 5.4. If we take the values reported in table 21.4, for example, the Pilsen Malt base malt, which has a baseline pH of 5.8 and buffering capacity of 9.2 mEq/kg with respect to the target pH. The base malt represents an alkaline quantity with respect to our mash target, meaning we have to overcome it’s buffering capacity to get down to our target mash pH. The Pilsen Malt’s total buffering capacity is 9.2 mEq/kg multiplied by its weight in the grain bill. Next, let’s assume the recipe has a couple of specialty malts in the grain bill. The first is Caramel Malt 60L with a baseline pH of 4.8 and a buffering capacity of −43.4 mEq/kg with respect to the pH target of 5.4. The second is Chocolate Malt with a baseline pH of 4.7 and a buffering capacity of −39.7 mEq/kg to the target pH of 5.4. These specialty malts represent acidic quantities with respect to our mash pH, meaning we have to overcome their buffering capacities to come up to our target pH of 5.4. The buffering capacity of the brewing water is either alkaline or acidic depending on its RA—alkaline if positive, acidic if negative. Adding up these factors will give you a surplus or deficit of milliequivalents that can be adjusted to zero by adding a strong acid or base, and thus reach your target mash pH. Or to put it another way:

* If the various buffer capacities add up to zero, it means that you will hit your target mash pH. If the number is plus or minus a couple of milliequivalents, you can add the difference with a strong acid or base to hit your mash pH target.
The point of all this is that you understand how the residual alkalinity of the brewing water and the grain bill come together to determine the mash pH. Base malts are alkaline, specialty malts are acidic, and the water can be either. We will explore the water effect more in chapter 22 when we look at famous brewing waters.
Table 21.4—Examples of Baseline pH and Alkalinity/Acidity for Briess Malts
| Briess malt type | Color (°L) | Baseline pH | mEq/kg of alkalinity (+) or acidity (–) to target mash pH of: | ||
|---|---|---|---|---|---|
|
5.2 |
5.4 |
5.6 |
|||
|
Pilsen Malt |
1.5 |
5.8 |
14.8 |
9.2 |
4.5 |
|
Brewers Malt |
1.9 |
5.6 |
11.1 |
5.0 |
0 |
|
Pale Ale Malt |
2.9 |
5.6 |
9.7 |
3.4 |
0 |
|
Red Wheat Malt |
2.8 |
5.8 |
18.9 |
11.1 |
4.8 |
|
Goldpils® Vienna |
3.5 |
5.6 |
13.6 |
7.2 |
1.6 |
|
Ashburne® Mild Malt |
4.4 |
5.5 |
9.4 |
2.6 |
−1.7 |
|
Bonlander® Munich Malt |
12 |
5.5 |
9.0 |
2.6 |
0 |
|
Aromatic Munich Malt |
16 |
5.4 |
5.0 |
0 |
−3.3 |
|
Victory® Malt |
28 |
5.4 |
3.4 |
−0.9 |
−7.8 |
|
Special Roast Malt |
40 |
5.1 |
−2.2 |
−13 |
−20.0 |
|
Caramel Malt 20L |
19 |
5.1 |
−6.0 |
−21.3 |
−26.5 |
|
Caramel Malt 40L |
40 |
4.8 |
−20.7 |
−30.4 |
−41.2 |
|
Caramel Malt 60L |
61 |
4.8 |
−28.9 |
−43.4 |
−74.2 |
|
Caramel Malt 80L |
80 |
4.7 |
−32.8 |
−45.1 |
−58.7 |
|
Caramel Malt 120L |
120 |
4.5 |
−48.8 |
−60.4 |
−72.6 |
|
Extra Special Malt |
126 |
4.6 |
–51.7 |
−67.5 |
−85.5 |
|
Roasted Barley |
292 |
4.7 |
−26.6 |
−38.6 |
−59.2 |
|
Chocolate Malt |
416 |
4.7 |
−29.3 |
−39.7 |
−51.7 |
|
Dark Chocolate Malt |
458 |
4.5 |
−38.8 |
−48.8 |
−57.9 |
|
Black Malt |
471 |
4.6 |
−27.0 |
−36.0 |
−44.1 |
|
Black Barley |
514 |
4.2 |
−38.2 |
−51.8 |
−66.0 |
Source: R. Hansen and J. Geurts, “Specialty malt acidity,” Proceedings of MBAA Annual Conference, Jacksonville, FL, October 8–10, 2015.
Sparge Water Adjustment
Lowering the pH of your sparge water is usually unnecessary, provided your sparge water has sufficient calcium. The phosphates in the mash will react with the calcium and buffer the pH until the wort gravity falls below 1.012. The key is to have enough calcium ions in the water. According to Taylor (1990), a calcium ion concentration of 100–200 ppm is needed to prevent pH rise during sparging, although his data indicate that 50 ppm is sufficient to prevent excessive rise, greater than 5.6, with final total wort pH of 5.4 before the boil. This same trend was observed in a pilot batch of IPA trialed in 2014 at the Ballast Point brewery in Little Italy, San Diego, CA, during the sparging of two mashes using only pale ale malt. The first trial batch used a calcium ion content of 54 ppm and had an RA of −44 ppm as CaCO3. The wort pH rose from 5.39 for the first runnings to 5.54 for the last runnings, and had a wort pH of 5.15 after the boil. The second trial batch had a calcium ion content of 120 ppm and an RA of −99 ppm as CaCO3. The wort pH did not rise during sparging, in fact, it went from 5.35 to 5.33, and the wort pH was 5.07 after the boil.
Generally, if you are building your water from distilled, or adjusting your water to have good calcium ion levels and reduced alkalinity, you will be able to sparge with the same water and not experience excessive pH rise and associated astringency in your beer. If you are not adjusting your water with brewing salts, or you are brewing with highly alkaline water, you may want to acidify it before using it for sparging. A rule of thumb when neutralizing the total alkalinity this way is to divide the total alkalinity, or bicarbonate concentration, by the equivalent weight to determine the number of acid equivalents to add. Be aware that acidifying with phosphoric acid can precipitate the calcium in your water, because the calcium ions react with phosphates in solution to form calcium phosphate. Acidifying to lower pH with phosphoric acid, such as 5.2–5.5, can prevent this. Alternatively, you can use other acids, such as hydrochloric, lactic, and citric acid, that do not have this problem. See appendix B in the Water book by Palmer and Kaminski (2013), for more information on this topic.
If you are mashing a dark beer with several specialty malts using an adjusted water with sufficient calcium and appropriate levels of residual alkalinity, you should be able to sparge with the adjusted water without much rise in pH. Alternatively, you could use a lower RA water (such as distilled water) to sparge with, and make up any water profile differences with brewing salt additions to the boil kettle before starting the boil.
The Mash pH Sets Up the Beer pH
The mash pH continually decreases during the time of the mash as more calcium phosphate precipitate is formed, and as enzyme activity breaks down the malt and releases amino acids and other buffering compounds. The mash pH tends to drop by 0.2 during the course of the mash, so the wort pH in the boil kettle may be between 5.0 and 5.4. See figure 21.2 for a schematic of this process.

Figure 21.2. Change in Wort pH During the Brewing Process. A schematic of the decrease in pH throughout the brewing process.
Wort pH also drops by about 0.3 during the boil. This is due to protein denaturing and coagulation, hop alpha-acid additions, and Maillard reactions. Hop isomerization is increased by higher wort pH, but higher wort pH is not a good way to improve hop utilization, because it results in a harsher, coarser bitterness that is not at all like the hop flavor you want.
The mash pH can affect how much the beer pH drops during fermentation as well. Proteolytic enzyme activity is favored by lower mash pH. There is a trade-off between the amino acids produced during the mash, associated yeast growth, and wort buffering. As yeast ferments the wort, it takes up amino acids and other buffering compounds as nutrients and excretes protons as part of the yeast’s normal biochemical activity. With single-infusion mashes, there is typically a low amount of FAN in the wort, which allows the yeast to grow normally (i.e., not too fast or too slow) but does not give the wort a significant amount of buffering power, so the wort pH drops by about 0.5 pH during fermentation. With the addition of a typical protein rest during the mash more FAN is put into the wort, but not enough to significantly affect yeast growth; however, the extra FAN does give more buffering power and the pH drop is the least seen with this method (about 0.3). With a very long protein rest a lot of FAN is put into the wort, significantly boosting yeast growth and producing a moderate pH drop. Obviously, temperature is a bigger factor for enzymatic activity, but mash pH contributes as well (fig. 21.3).
The mash pH sets the course for the final beer pH. Table 21.5 shows four examples of how the beer pH was changed by varying the residual alkalinity across four different recipes. Fermentation does have a large moderating effect on pH, but the trend is clear.
Beer pH Controls Beer Flavor
Beer pH can be adjusted after fermentation, but the results never seem quite as harmonious as if it had been adjusted in the mash or before fermentation. Generally, beer pH (not including sour beers) ranges between 4.0 and 4.7.
Pale beers seem to taste better with a lower beer pH, between 4.0 and 4.4. A lower beer pH makes the malt character in pale beers taste brighter and the hop character more refined. As the beer pH approaches 4.0, the character becomes sharp and crisp, as it approaches 4.4 it becomes softer.
Figure 21.3. Beer pH vs. Wort FAN. The curve shows the change in beer pH associated with increases in the wort FAN content. (Reproduced from Taylor (1990, fig. 6) with permission.)
Table 21.5—Mash pH and Beer pH Changes following Adjustment of Residual Alkalinity with Calcium Salts and Sodium Bicarbonate
| Reference | Beer style | RA ppm as CaCO3 | Mash pH | Beer pH |
|---|---|---|---|---|
|
Denver NHC 2007a |
Pale ale Pale ale Stout Stout |
−200 −45 −200 −45 |
6.1 5.5 5.4 4.9 |
4.7 4.5 4.6 4.2 |
|
Grand Rapids NHC 2014b |
IPA IPA |
−140 −35 |
5.5 5.25 |
4.6 4.5 |
|
3rd Congresso Technico dos Cervejeiros Artensanais 2016c |
Stout Stout |
−140 −14 |
6.2 5.3 |
4.8 4.1 |
a John Palmer, Rick Bobbit, and Scott Jackson, “Beer Color and Residual Alkalinity: A Practical Example,” Presentation at the AHA National Homebrewers Conference, Denver, June 21–23, 2007.
b John Palmer and Adam Mills, “Brewing Water Effects on Beer Flavor,” Presentation at the AHA National Homebrewers Conference, Grand Rapids, MI, June 12–14, 2014.
c John Palmer, Ronaldo Dutra-Ferreira, and Malcolm Frazer, “Live Brulosophy Experiment,” 3rd Congresso Técnico dos Cervejeiros Artensanais, Florianopolis, Brazil, April 22–23, 2016.
Dark beer styles, particularly those with a high percentage of dark roasted malts, seem to taste better with a higher pH, between 4.3 and 4.7. The higher beer pH softens the malt acidity and opens up the specialty malt flavors, achieving more complexity. Dark beers with a lower pH tend to taste more one dimensional, with the flavors sharpening to a narrower range. This will not taste bad, but the flavor description tends to be summed up in one word, either roasty, coffee, or chocolate. Stouts and porters at the higher end of the pH range tend to taste more layered, and judges’ descriptions will broaden to, for example, roasty with hints of caramel and chocolate. Of course, the flavors that appear depend on the recipe.
Every beer should have a flavor portfolio that includes:
- • malt flavors and aromas;
- • hop flavors and aromas;
- • yeast flavors and aromas.
Every beer recipe has an ideal beer pH where all these flavors are best expressed. If you can’t taste or smell every ingredient, or every ingredient favorably, then you probably haven’t achieved the optimum pH for that beer. You can try adjusting the pH of the final beer with acid and base additions, but it usually works better to adjust the mash pH by 0.1–0.2 and let that change work its way through to the beer, taking the sort of approach best summed up as, “An ounce of prevention is worth a pound of cure.” The brewer’s art is finding that particular pH for a recipe and maintaining it, batch to batch, season to season. Achieving that particular beer pH starts with the water chemistry and the mash pH.
1 A.J. deLange (unpublished data); Hansen and Geurts (2015).
2 P. Kolbach and G.W. Haase, Wochenschrift für Brauerei 56 (1939): 143.
3 John Palmer, Colin Kaminski, Martin Brungard, and AJ DeLange.
4 Troester (2009), Barth and Zaman (2015), and Palmer (2016).
5 Palmer (2016). Recall that Rv is the volume per weight water-to-grist ratio, as detailed in chapter 17.
6 For example, see Sykes and Ling (1907, 410).
7 Martin Brungard, “Water Knowledge,” Bru’n Water, last updated January 8, 2015, http://sites.google.com/site/brunwater/water-knowledge.
