Robert Schleip, Divo Gitta Müller
Introduction
In sports science as well as in recent sports education, the prevailing emphasis had been on the classical tirade of muscular training, cardiovascular conditioning and neuromuscular coordination (Jenkins 2005). Comparatively little attention had been given to a specifically targeted training of the involved connective tissues. This common practice is in contrast to the role that the muscular connective tissues play in sports-associated overuse injuries. Whether in running, soccer, baseball, swimming or dancing, the vast majority of associated repetitive strain injuries occur in the collagenous connective tissues – such as tendons, ligaments or joint capsules – which seem to be less adequately prepared and less well adapted to their loading challenge than their muscular or skeletal counterparts (Renström & Johnson 1985, Hyman & Rodeo 2000, Counsel & Breidahl 2010, Prakash et al. 2018).
This chapter offers evidence and insights into the role, and the potential for the training, of the muscular connective (i.e. fascial) tissues, involved in sport and movement activities.
Sports science discovers fascia
A range of sometimes confusing words and terms have been used to describe connective tissues in different contexts (Schleip et al. 2012). This chapter follows the the suggestion for descriptions of ‘the fascial system’ made by the Fascia Nomenclature Committee of the Fascia Research Society (Adstrum et al. 2017, Stecco et al. 2018) since it is most suitable for analyzing multijoint force transmission and the proprioceptive properties of fascial tissues. Here all fibrous collagenous connective tissues, which are part of a body-wide interconnected tensional network, are recognized as parts of the fascial system. Joint capsules, intramuscular connective tissues, ligaments, and tendons are seen merely as local specifications of this tension-resistant fibrous network. Dense planar connective tissues with a multidirectional fiber arrangement, often referred to as ‘proper fascia’, are not regarded as separate functional entities but as gradual continuations of adjacently positioned specialized tissues, such as retinaculae, septi or aponeuroses (Schleip et al. 2012).
While electromyography allowed a precise measurement of muscular activity, until recently the assessment of fascial properties was mostly confined to subjective evaluations. However, advances in ultrasound measurement as well as in histology have resulted in a drastic increase of fascia-related studies within the field of sports medicine. The congress series on ‘Connective tissues in sports medicine’, hosted at Ulm, has defined the dynamic impetus of this rapidly growing field. While earlier studies on Australian kangaroos had already shown that their impressive jumps of up to 13 meters are mostly due to the high storage capacity of their tendons (Kram & Dawson 1998), more recent ultrasound examinations in running and jumping humans revealed that the latter also express a similarly impressive elastic ‘catapult capacity’ in the Achilles tendon and related aponeuroses (Sawicki et al. 2009). At least when assessed in our current Western movement culture, the elastic storage quality in running and hopping tends to be highest between 13 and 16 years of age and then subsequently decreases as we age (Legramandi et al. 2013).
The higher storage capacity of collagenous tissues in young people is associated with a stronger crimp expression within their collagen fibers (Torp et al. 1975). Adequately tailored exercise in older rats has been shown to re-introduce a similar crimp formation as is present in younger ones (Wood et al. 1988). Similarly, it has been shown that the elastic storage capacity in human tendons can be significantly improved by regularly repeated strong mechanical loading of these tissues (Reeves et al. 2006) (
Fig. 8.1
).
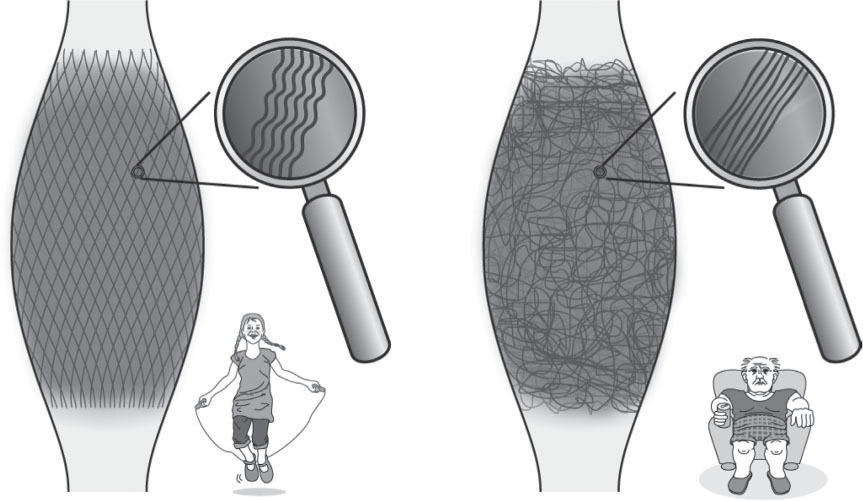
Figure 8.1
Collagen fiber architecture is responsive to mechanical loading. Healthy fasciae (left image) often express a clear two-directional (lattice) orientation of their collagen fiber network and their fibers show a stronger crimp formation. Lack of exercise, on the other hand, tends to induce a multidirectional fiber architecture and a reduction in crimp formation, leading to a loss of springiness and elastic recoil (right image).
Illustration with friendly permission of
FASCIAL-FITNESS.COM
. From Schleip & Baker (eds) 2015 Fascia in sport and movement. Handspring Publishing.
Habitual barefoot running seems to involve a higher storage capacity within the lower leg when compared to shod running (Tam et al. 2014). Interestingly this is less the case when running with minimalistic shoes (Bonacci et al. 2013), possibly due to the role of proprioceptive stimulation involvement by barefoot contact with the ground. However, transition to more natural footwear should be performed more gradually than is even recommended by conservative instructions due to the high likelihood of overuse injuries such as bone marrow edema during the change-over time (Ridge et al. 2013). Renewal of fascial tissues is a relatively slow process, taking several months and more (Babraj et al. 2005, Miller et al. 2005).
Plyometric training, also referred to as ‘jump training’, is hardly new in the field of sports education. The prevailing theory had been that the involved stretch–shortening cycle in these movements leads to increased motor unit activation via the myotatic stretch reflex (Wilk et al. 1993). In contrast to this muscular-oriented explanation focus, a more recent examination demonstrated that a plyometric training for 14 weeks leads to a significant increase in the passive component of the series elastic component (mainly involving the tendon structures) and an associated decrease in the active part of the muscular contraction (Fouré et al. 2011). Congruently, an increase in tendon cross-sectional diameter could be observed in response to plyometric training (Houghton et al. 2013) signifying that the adaptational response included a morphological tissue adaptation within the tendon (
Fig. 8.2
).
Due to methodological difficulties, the high expression of elastic recoil properties of fascial tissues in humans had been proven – until recently – for lower leg structures only. The impressive study by Roach et al. (2013) has now extended that to the human shoulder girdle: his team demonstrated that the ability of humans to throw projectiles at much higher speed – compared with other primates – involves an increased elastic energy storage in the human shoulder girdle structures, and that this capacity is associated with anatomical changes that evolved 2 million years ago during the evolution of the species
Homo erectus,
which probably provided a significant survival benefit in the hunter and gatherer conditions of our ancestors.
Training of elastic recoil properties
The commonly accepted Wolff’s law states that dense connective tissues are able to adapt their morphology to mechanical loading. While this general law had originally been developed with a main focus on skeletal tissues, Davis’ law applies this general principle particularly to soft connective tissues. It proposes that these tissues tend to adapt their architecture to the specific mechanical demands imposed on them, provided that these demands are strong enough, and occur in a regular manner (Nutt 1913).

Figure 8.2
Length changes of fascial elements and muscle fibers in conventional muscle training (A) and in oscillatory movement (B). Note that during a conventional movement (A) the fascial elements do not change their length significantly while the muscle fibers with elastic recoil properties clearly change their length (B). The elastic tendinous (or fascial) elements are shown as springs, the myofibers as straight lines. During movements like hopping or jumping, however, the muscle fibers contract almost isometrically while the fascial elements lengthen and shorten like an elastic yoyo spring.
Illustration adapted from Kawakami et al. (2002). © <
FASCIALNET.COM
>
More recently this concept has been taken further by the mechanostat theory of Harold Frost, emphasizing that tendons, ligament and fascia adapt their cross-sectional diameter and correspondingly also their stiffness in response to the muscular forces imposed onto them (Frost 1972). However, the threshold value of mechanical loading to trigger adaptational effects for tendon is significantly higher for tendons than for muscle. While exercises performed at only 50% of maximum voluntary contraction power are sufficient for triggering an adaptational response in muscle fibers, the involved tendinous connective tissues show very little adaptational response at that impact level; they do require much stronger loads in order to respond. Arampatzis et al. (2007) have shown that for the Achilles tendon and aponeuroses a strain of 4–5% tends to be required for eliciting an adaptational response, while a strain of 2–3% is clearly not sufficient. Note that the high impact loading required for an adaptational response may then be only 35% below the amount of strain at which tendon injuries do occur (Wren et al. 2001). Interestingly, the threshold for an adaptational response of intramuscular fasciae seems to be significantly lower than for tendons (Kjaer et al. 2015).
Once the respective threshold has been reached, the adaptational response of the fibroblasts seems to be largely independent of the quantity of strain application involved in an exercise session. It is possible that only five or 10 elastic bounces may be required for an adaptational response, while adding 100 or more repetitions tends to have very little additional effect (Magnusson et al. 2010) (
Fig. 8.3
).

Figure 8.3
Simplified analogy for the different responsiveness of myofiber and collagen remodeling. Myofibers respond in a dose-dependent manner to the amount of loading. The stronger these fibers are loaded during training, the more effectively the training effect will be in terms of an increased muscle size and strength. In contrast, the training response of collagen renewal works more like a flip switch that turns on a light in an on–off manner. However, the threshold of this flip switch is fairly high and usually demands stronger impacts than are normally applied during daily life situations.
Illustration with friendly permission of
FASCIAL-FITNESS.COM
(modified from
istockphoto.com/0263938
)
.
An inspiring example of a well-conducted fascia training was conducted in Finland with 20 physically active elder men. Here the participants practiced a carefully orchestrated hopping training for 11 weeks which resulted in increased tendon utilization, increased tendon force and increased jumping height; while none of the participants demonstrated any related overloading pathologies. Their fascia-oriented training consisted of several hopping sets of 10 s duration only. This was practiced 3 times per week only and the quantity and intensity of the hopping sets were slowly increased during the 11 weeks (Hoffrén-Mikkola et al. 2015).
Key Point
Playful bouncing and hopping exercises can have beneficial psychological effects and tend to increase elastic recoil properties in related fascial connective tissues. However, it is recommended to accompany these with an enhanced proprioceptive mindfulness, in order to avoid overloading pathologies.
Based on these considerations, suggestions for fascia-oriented training have been described (Schleip & Müller 2013, Schleip & Baker 2015, Mueller & Hertzer 2017). These recommendations consist of four basic application forms, which will be discussed in the remainder of this chapter:
•
elastic recoil
•
fascial stretch
•
fascial release
•
proprioceptive refinement.
Among these, elastic recoil exercises deserve primary attention.
An example of such exercises is given in
Figure 8.4
. Note that for an optimal utilization of elastic recoil properties the main movement should be initiated with a preparatory counter movement in the opposite direction.
•
Figure 8.4
shows a backward stretch and swing of the arms and tool (
Fig. 8.4A
) prior to the forward swinging motion.
•
The subsequent forward movement (
Fig. 8.4B
) is then initiated, involving a proximal body part, such as the pelvis or sternum.
•
Finally, this proximal initiation is followed by the more distal body parts in a sequentially delayed manner, similar to the orchestration in a flexible whip (
Fig. 8.4B–D
).
Figure 8.4
(A–D) Example of an elastic recoil exercise: the Flying Sword. The movement is initiated by a preparatory counter movement (pre-stretch in the opposite direction). Various angular variations are explored during repetitively swinging applications. Illustration with friendly permission of
FASCIAL-FITNESS.COM
.
•
More advanced practitioners can be instructed to experiment with two additional steps before commencing:

Before the preparatory counter movement, a softening and refinement of the somatic perception is initiated. For the duration of one relaxed breath or more the practitioner will go into a state of open attention, checking his body for any unnecessary muscular holding patterns and fostering a sense of curious attention for detecting small details in their perception during the subsequent phases.
Before the preparatory counter movement, a softening and refinement of the somatic perception is initiated. For the duration of one relaxed breath or more the practitioner will go into a state of open attention, checking his body for any unnecessary muscular holding patterns and fostering a sense of curious attention for detecting small details in their perception during the subsequent phases.

This is followed by a subtle multidirectional spatial expansion, which is envisioned to involve an extension of the body-wide ‘diving suit’ being made up of the most superficial layer of dense fasciae covering the whole body. For the thorax this may involve a minute increase in width, depth and length (of only few millimeters each) while it may also be accompanied by an almost invisible extension of the limbs. While this second step is often referred to as ‘iron shirt’ in martial arts practices, it can also be seen as an increase in tensegrity-like omnidirectional pre-tension in the body-wide fascial network (Levin & Martin 2012).
This is followed by a subtle multidirectional spatial expansion, which is envisioned to involve an extension of the body-wide ‘diving suit’ being made up of the most superficial layer of dense fasciae covering the whole body. For the thorax this may involve a minute increase in width, depth and length (of only few millimeters each) while it may also be accompanied by an almost invisible extension of the limbs. While this second step is often referred to as ‘iron shirt’ in martial arts practices, it can also be seen as an increase in tensegrity-like omnidirectional pre-tension in the body-wide fascial network (Levin & Martin 2012).
Interestingly, such expansional movements tend to trigger potentially important perceptual and behavioral alterations. These changes have been shown to result in increased feelings of power as well as in an increased pain tolerance (Peper et al. 2016, Bohns & Wiltermuth 2012).
Listening to the acoustic feedback in many elastic recoil movements can be a helpful guide. The less
sound is created by the practitioner – e.g. in jumping barefoot on the ground – the better. In analogy to the legendary Ninja warriors, who supposedly moved without making any sounds, this feature is referred to as ‘Ninja quality’ in fascial training.
Table 8.1
Steps in an ideal orchestration of fascial elegance
|
Step
|
Emphasis
|
|
Pre 1
|
Open attention
|
|
Pre 2
|
Tensegral expansion
|
|
1
|
Preparatory counter movement
|
|
2
|
Proximal initiation
|
|
3
|
Distal delay
|
Timing and rhythm are crucial in elastic recoil movement. This was impressively shown in the investigation of Heglund et al. (1995) with women from West Africa, who can carry large loads on their heads without any additional energy expenditure due to their pendulum-like storage and release of kinetic energy during gait. When testing these women in a laboratory environment it was revealed that their impressive energy consumption was dependent on walking at their preferred speed. When asked to walk at different speeds than they had intuitively chosen, the women expressed the same load-dependent increase in energy expenditure as was found in untrained Western control subjects.
In elastically swinging recoil motions the ideal speed and rhythm are dependent on the inherent resonant frequency, which itself is a product of the material stiffness as well as the pendulum length. This suggests that a particular musical dance rhythm may allow some dancers to swing, hop and bounce with minimal energy expenditure, while another rhythm may be better suited for other dancers. In addition, a voluntary adjustment of pendulum length and of fascial stiffness (via muscular contraction) may also allow a partial adjustment of the dancer’s resonant frequencies towards the given external rhythm.
This suggests that elastic recoil movements can result in higher loads being safely imposed on fascial tissues. However, this should not be performed without a proper warm-up (as with the other training elements described below) as well as with a heightened somatic mindfulness. Following the findings of Magnusson et al. (2010) such loading is recommended, once or twice per week only, in order to lead towards an optimal collagen renewal.
Stretching recommendations for fascial health
Figure 8.5A
shows an example of a fascia-oriented stretch application. Following the excellent review of pandiculating movements in animals by Bertolucci (2011), the practitioner is asked to explore expansional stretch variations that involve several joints and are felt in large membranous fascial areas. Rather than precisely repeated muscular elongations, angular variations should be explored. While ‘melting’ stretches, in which the elongated muscles are intentionally relaxed, are utilized to reach intramuscular connective tissues as well as extramuscular connections between different muscle bellies, dynamic (more active) stretches can be added in which the elongated muscle fibers are simultaneously activated in order to reach the more serially arranged tendinous structures (Schleip & Müller 2013).
The benefits of such stretching depend on the application context. While static stretches tend to decrease vertical jump height immediately afterwards, dynamic stretching can have a positive effect on this performance (Hough et al. 2009). Dynamic stretching, as examined here, can include mini-bounces
in the long-stretched position, i.e. at the end-range position at which the targeted myofascial tissues are maximally stretched. These mini-bounces should be performed in a soft and proprioceptively precise manner, utilizing sinusoidal speed changes only rather than any abrupt and jerky accelerations or decelerations. Note that during the deceleration phase of such bounces the muscle fibers will be briefly activated in an eccentric manner, while hardly any muscle activation will then be necessary during the subsequent accelerating phase of each mini-bounce. For best results, such gentle mini-bounces should be alternated with phases of melting stretches, performed for at least several breaths and possibly longer.

Figure 8.5
Examples of fascial stretch and fascial release. (A) The so-called ‘cat stretch’ simulates the pandiculations observed in cats and other feline predators. (B) Use of a foam roller on the other side exemplifies the fascial release applications, in which fascial tissues – here the iliotibial tract – can be subjected to sponge-like squeezing in a ‘slow motion’ manner.
Illustrations with friendly permission of
FASCIAL-FITNESS.COM
.
Melting stretches practiced throughout the year can provide health-related range-of-motion benefits (Behm & Chaouachi 2011). A recent study on rats by Corey et al. (2012) demonstrated that static stretching may also have an anti-inflammatory effect when slowly applied to previously inflamed connective tissues.
Fascial release: use of foam rollers
Figure 8.5B
demonstrates a typical fascial self-treatment using a foam roller. Such treatments have shown to improve range of motion and to enhance recovery after intensive exercise (Cheatham et al. 2015, Beardsley & Skarabot 2015, Schroeder & Best 2015). Additionally, in the treated fascial tissues, a decreased arterial stiffness and improved endothelial function have been observed, both of which are most likely enhanced by the documented increased expression of the gaseous transmitter substance nitric oxide (Okamoto et al. 2014).
For a treatment of too flaccid fascial tissues, a more vigorous application using repeated rapid motions can be explored in order to increase local collagen production (Pohl 2010; see
Ch. 7
on connective tissue manipulation). However, when working on chronic scar tissues, hypertoned fascial tissues and pathological adhesions, the implementation of super-slow rolling movements are recommended, which induce a low fluid shear-strain motion on the fibroblasts of the treated tissues (see
Ch. 18
on the treatment of scarring). Such gentle fibroblast stimulation may subsequently trigger release of the enzyme MMP-1, a potent agent for desynthesizing collagen fibers (Zheng et al. 2012).
A crucial and indispensible ingredient: proprioceptive refinement
The body-wide fascial network has been described as a sensory organ (Schleip et al. 2012). Various authors have confirmed the presence of proprioceptive as well as nociceptive nerve endings in fascial tissues (van der Wal 2009, Stecco et al. 2008, Tesarz et al. 2011). Providing mechanical stimulation may stimulate polymodal receptors, belonging to so-called wide-dynamic-range neurons, which tend to provide an analgesic effect on the level of the spinal cord (Wang et al. 2012). Mindful attention to the local tissue stimulation can be a key factor in the therapeutic utilization of this mechanism (Moseley et al. 2008).
Bouncing movements can lead to increased likelihood of injury particularly when performed with an outwardly oriented high performance attitude. Clinical experience has shown that the probability of subsequent strain injuries is highest when showing these movements to middle-aged men with a high achievement orientation, accompanied by a lack of refined somatic perception. Proprioceptive refinement should be fostered in such individuals – and in all fascia-oriented training protocols.
To avoid the dampening function of the reticular formation – a portion of the central nervous system that inhibits the delay of sensory signals, which it considers ‘as expected’ – non-habitual fascial stimulations should be explored, using body positions and joint angulations that are rarely utilized during normal sedentary behavior. In a comparative study by Alexander (2004) an intriguing correlation between joint usage and degenerative joint diseases was observed: the less a joint is utilized in the anatomically available range of motion, the more this joint is prone towards osteoarthritis, possibly due to lack of usage of an available range of joint motion. A chimpanzee-like creative usage of our available joint range of motion could possibly induce a similar healthy tissue physiology as is expressed in our arboreal relatives (
Fig. 8.6
).

Figure 8.6
Simulating ape-like loading of the anatomically available range of joint motion. Towards completion of the long hours of deskwork associated with the writing of this chapter, one of the authors explored a nearby children’s playground for stimulating fascial stretches combined with some monkey-like swinging motions.
©
FASCIALNET.COM
.
Fascial fitness training: an important addition to general health care
Renewal of collagen tissues happens much slower than that of muscle fibers (Babraj et al. 2005). Application of the above described applications will therefore not be visible in a matter of weeks; it usually takes 3–9 months to see the tissue remodeling effects from the outside as well as ‘feel’ them in palpation. However, in contrast to muscular training, the gained effects will not be lost as quickly (e.g. when having to stop training because of health- or work-related reasons) and are therefore of a more long-lasting sustainable quality. Fascial training does not compete with neuromuscular or cardiovascular training, both of which can have very important health effects that are not possible with fascial training only. We suggest our described training principles as an additional component in health-oriented training approaches like prevention and rehabilitation programs as well as athletic and high perfomance training protocols. It promises to lead towards remodeling of the body-wide fascial network in such a way that it works with increased effectiveness and refinement in terms of its kinetic storage capacity as well as a sensory organ for proprioception. Further research is necessary to validate whether it does indeed fulfil its basic promise of an increased protection against repetitive strain injuries in muscular connective tissues.
Key Point
Fascial connective tissues adapt to proper exercise stimulation much more slowly than muscle fibers do. Architectural changes in fascial tissues usually take several months, not weeks, before they can be palpated or be seen in the daily movement orchestration.
References
Adstrum S et al 2017 Defining the fascial system. J Bodyw Mov Ther 21(1):173–177
Alexander CJ 2004 Idiopathic osteoarthritis: time to change paradigms? Skeletal Radiol 33(6):321–324
Arampatzis A, Karamanidis K, Albracht K 2007 Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J Exp Biol 210(Pt 15):2743–2753
Babraj JA et al 2005 Collagen synthesis in human musculoskeletal tissues and skin. J Physiol Endocrinol Metab 289(5):E864–869
Beardsley C, Skarabot J 2015 Effects of self-myofascial release: A systematic review. J Bodyw Movem Ther 19:747–758
Behm DG, Chaouachi A 2011 A review of the acute effects of static and dynamic stretching on performance. Eur J Appl Physiol 111(11): 2633–2351
Bertolucci LF 2011 Pandiculation: nature’s way of maintaining the functional integrity of the myofascial system? J Bodyw Mov Ther 15:268–280
Bohns VK, Wiltermuth SS 2012 It hurts when I do this (or you do that): Posture and pain tolerance. J Exp Soc Psych 48: 341–345
Bonacci J et al 2013 Running in a minimalist and lightweight shoe is not the same as running barefoot: a biomechanical study. Br J Sports Med 47(6):387–392
Cheatham SW, Kober MJ, Cain M, Lee M 2015 The effect of self-myofascial release using a foam roll or roller massager on joint range of motion, muscle recovery, and performance: A systematic review. Int J Sports Phys Ther 10:827–838
Corey SM, Vizzard MA, Bouffard NA et al 2012 Stretching of the back improves gait, mechanical sensitivity and connective tissue inflammation in a rodent model. PLoS One 7:e29831
Counsel P, Breidahl W 2010 Muscle injuries of the lower leg. Semin Musculoskelet Radiol 14:162–175
Fouré A, Nordez A, McNair P, Cornu C 2011 Effects of plyometric training on both active and passive parts of the plantar flexors series elastic component stiffness of muscle-tendon complex. Eur J Appl Physiol 111(3):539–548
Frost MF 1972 The physiology of cartilaginous, fibrous, and bony tissue. C C Thomas, Springfield, IL
Heglund NC, Willems PA, Penta M, Cavagna GA 1995 Energy-saving gait mechanics with head-supported loads. Nature 375(6526): 52–54
Hoffrén-Mikkola M, Ishikawa M, Rantalainen T, Avela J, Komi PV 2015 Neuromuscular mechanics and hopping training in elderly. Eur J Appl Physiol 115:863–877
Hough PA, Ross EZ, Howatson G 2009 Effects of dynamic and static stretching on vertical jump performance and electromyographic activity. J Strength Cond Res 23(2):507–512
Houghton LA, Dawson BT, Rubenson J 2013 Effects of plyometric training on achilles tendon properties and shuttle running during a simulated cricket batting innings. J Strength Cond Res 27(4):1036–1046
Hyman J, Rodeo SA 2000 Injury and repair of tendons and ligaments. Phys Med Rehabil Clin N Am 11:267–288
Jenkins S 2005 Sports science handbook. In: The essential guide to kinesiology. Sport & Exercise Science, vol. 1. Multi-science Publishing, Brentwood
Kawakami Y et al 2002. In vivo muscle fibre behaviour during countermovement exercise in humans reveals a significant role for tendon elasticity. J Physiol 540:635–646
Kjaer M, Jørgensen NR, Heinemeier K, Magnusson SP 2015 Exercise and regulation of bone and collagen tissue biology. Prog Mol Biol Transl Sci 135:259–291
Kram R, Dawson TJ 1998 Energetics and bio mechanics of locomotion by red kangaroos (Macropus rufus). Comp Biochem Physiol B Biochem Mol Biol 120:41–49
Legramandi MA, Schepens B, Cavagna GA 2013 Running humans attain optimal elastic bounce in their teens. Sci Rep 3:1310
Levin P, Martin DC 2012 Biotensegrity: the mechanics of fascia. In: Schleip R, Findley T, Chaitow L, Huijing P (eds) Fascia: the tensional network of the human body. The science and clinical applications in manual and movement therapies. Churchill Livingstone Elsevier, Edinburgh, pp 137–146
Magnusson SP, Langberg H, Kjaer M 2010 The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol 6:262–268
Miller BF, Olesen JL, Hansen M et al 2005 Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567(Pt 3):1021–1033
Moseley GL, Zalucki NM, Wiech K 2008 Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain 137:600–608
Mueller D, Hertzer K 2017 Train your fascia, tone your body: The successful method to form firm connective tissue. Meyer & Meyer Sport, Maidenhead (UK)
Nutt JT 1913 Diseases and deformities of the foot. EB Treat, New York
Okamoto T, Masuhara M, Ikuta K 2014 Acute effects of self-myofascial release using a foam roller on arterial function. J Strength Cond Res 28(1):69–73
Peper E, Booiman A, Lin I-M Harvey R 2016 Increase strength and mood with posture. Biofeedback 44:66–72
Pohl H 2010 Changes in the structure of collagen distribution in the skin caused by a manual technique. J Bodyw Mov Ther 14(1):27–34
Prakash A, Entwisle T, Schneider M et al 2018 Connective tissue injury in calf muscle tears and return to play: MRI correlation. Br J Sports Med 52:929–933
Reeves ND, Narici MV, Maganaris CN 2006 Myotendinous plasticity to aging and resistance exercise in humans. Exp Physiol 91:483–498
Renström P, Johnson RJ 1985 Overuse injuries in sports. A review. Sports Med 2:316–333
Ridge ST et al 2013 Foot bone marrow edema after a 10-wk transition to minimalist running shoes. Med Sci Sports Exerc 45(7):1363–1368
Roach NT, Venkadesan M, Rainbow MJ, Lieberman DE 2013 Elastic energy storage in the shoulder and the evolution of high-speed throwing in Homo. Nature 498(7455):483–486
Sawicki GS, Lewis CL, Ferris DP 2009 It pays to have a spring in your step. Exerc Sport Sci Rev 37:130–138
Schleip R, Jäger H, Klingler W 2012 What is ‘fascia’? A review of different nomenclatures. J Bodyw Mov Ther 16(4):496–502
Schleip R, Müller DG 2013 Training principles for fascial connective tissues: scientific foundation and suggested practical applications. J Bodyw Mov Ther 17(1):103–115
Schleip R, Baker A (eds.) 2015 Fascia in sport and movement. Handspring Publishing, Pencaitland (UK)
Schroeder AN, Best 2015 Is self myofascial release an effective preexercise and recovery strategy? A literature review. Curr Sports Med Reports 14(3):200–208
Stecco C et al 2008 Histological study of the deep fasciae of the limbs. J Bodyw Mov Ther 12(3):225–230
Stecco C et al 2018 Update on fascial nomenclature. J Bodyw Mov Ther 22:354
Tam N, Astephen Wilson JL, Noakes TD, Tucker R 2014 Barefoot running: an evaluation of current hypothesis, future research and clinical applications. Br J Sports Med 48(5):349–355
Tesarz J, Hoheise, U, Wiedenhofer B, Mense S 2011 Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience 194:302–308
Torp S, Arridge RGC, Armeniades CD et al 1975 Structure-property relationships in tendon as a function of age. Colston Papers No. 26. Butterworth, London, pp 197–221
van der Wal J 2009 The architecture of the connective tissue in the musculoskeletal system - an often overlooked functional parameter as to proprioception in the locomotor apparatus. Int J Ther Massage Bodywork 2(4):9–23
Wang W et al 2012 Acute pressure on the sciatic nerve results in rapid inhibition of the wide dynamic range neuronal response. BMC Neurosci 13:147
Wilk KE et al 1993 Stretch-shortening drills for the upper extremities: theory and clinical application. J Orthop Sports Phys Ther 17(5): 225–239
Wood TO, Cooke PH, Goodship AE 1988 The effect of exercise and anabolic steroids on the mechanical properties and crimp morphology of the rat tendon. Am J Sports Med 16:153–158
Wren TA, Yerby SA, Beaupré GS, Carter DR 2001 Mechanical properties of the human achilles tendon. Clin Biomech 16(3): 245–251
Zheng L et al 2012 Fluid shear stress regulates metalloproteinase-1 and 2 in human periodontal ligament cells: involvement of extracellular signal-regulated kinase (ERK) and P38 signaling pathways. J Biomech 45(14):2368–2375






Before the preparatory counter movement, a softening and refinement of the somatic perception is initiated. For the duration of one relaxed breath or more the practitioner will go into a state of open attention, checking his body for any unnecessary muscular holding patterns and fostering a sense of curious attention for detecting small details in their perception during the subsequent phases.
Before the preparatory counter movement, a softening and refinement of the somatic perception is initiated. For the duration of one relaxed breath or more the practitioner will go into a state of open attention, checking his body for any unnecessary muscular holding patterns and fostering a sense of curious attention for detecting small details in their perception during the subsequent phases.

This is followed by a subtle multidirectional spatial expansion, which is envisioned to involve an extension of the body-wide ‘diving suit’ being made up of the most superficial layer of dense fasciae covering the whole body. For the thorax this may involve a minute increase in width, depth and length (of only few millimeters each) while it may also be accompanied by an almost invisible extension of the limbs. While this second step is often referred to as ‘iron shirt’ in martial arts practices, it can also be seen as an increase in tensegrity-like omnidirectional pre-tension in the body-wide fascial network (Levin & Martin 2012).
This is followed by a subtle multidirectional spatial expansion, which is envisioned to involve an extension of the body-wide ‘diving suit’ being made up of the most superficial layer of dense fasciae covering the whole body. For the thorax this may involve a minute increase in width, depth and length (of only few millimeters each) while it may also be accompanied by an almost invisible extension of the limbs. While this second step is often referred to as ‘iron shirt’ in martial arts practices, it can also be seen as an increase in tensegrity-like omnidirectional pre-tension in the body-wide fascial network (Levin & Martin 2012).

