César Fernández-de-las-Peñas
Introduction
In clinical practice, trigger point (TrP) release methods cover several manual therapies aimed at eliminating or inactivating TrPs. The latter are mainly described as hypersensitive spots in a taut band of a skeletal muscle painful on stimulation that elicit referred pain (Simons et al. 1999). TrP release methods include direct techniques such as massage, ischemic compression, TrP pressure release, or strain–counterstrain (Dommerholt & McEvoy 2010), and also indirect interventions, e.g. spray and stretch, passive stretching, muscle energy techniques (MET), neuromuscular approaches and/or myofascial induction. Direct techniques are those targeted at connective tissues related to the TrP by applying pressure directly over the TrP, whereas indirect techniques are those targeted at connective tissues related to the taut band and surrounding tissues, including fascia (Dommerholt & McEvoy 2010). An alternative release method is TrP dry needling (TrP-DN; Dommerholt & Fernández-de-las-Peñas 2013). This technique inserts acupuncture filiform and fine needles into the TrP area with the aim of inactivating the TrP. All TrP release methods are complementary because they usually act on different connective tissue levels, including the taut band, TrP area, muscle tissue, and surrounding fascia. In fact, clinicians usually combine different TrP release methods in the same session into a multimodal treatment approach.
Overview
Throughout history TrPs have been referred to by different names (myogelosis, fibrositis, etc.). Although various definitions of TrPs are used among different disciplines, the most commonly accepted definition maintains that ‘TrPs are hypersensitive spots in a taut band of a skeletal muscle that are painful on compression, stretch, overload or contraction of the tissue which respond with a referred pain’
(Simons et al. 1999). Based on their clinical experience, different authors have modified TrP release methods (Simons et al. 1999, Chaitow 2007, Fernández-de-las-Peñas et al. 2011). Different TrP release methods, depending on the amount of pressure applied, presence/absence of pain, duration of the application, position of the tissue (shortened or lengthened), or presence/absence of active contraction of the affected (agonist) or surrounding (antagonist) tissues, are clinically proposed for TrP treatment (Fernández-de-las-Peñas & Pilat 2011).
Several mechanical and neurophysiological mechanisms have been proposed to explain the effects of the different TrP release methods and it is likely that all of them act at the same time.
From a mechanical viewpoint, it has been suggested that mechanical stimulation exerted by TrP release methods can equalize the length of the muscle sarcomeres, can induce longitudinal or transverse mobilization of the taut band, or can induce changes in muscle properties (Dommerholt & McEvoy 2010, Fernández-de-las-Peñas et al. 2011).
•
Potential neurophysiological mechanisms include spinal reflex effects inducing muscle relaxation, muscle hyperemia, stimulation of the gate control theory, or activation of the descending inhibitory pain mechanisms (Dommerholt & McEvoy 2010).
•
These mechanisms are also involved in TrP-DN; however, considering that TrP-DN is a painful intervention and it represents a nociceptive input to the central nervous system (CNS), it is more plausible that the neurophysiological mechanisms are highly relevant for TrP-DN
since painful interventions would be able to activate descending pain inhibition (Dommerholt & Fernández-de-las-Peñas 2013).
•
Finally, recent theories also include mechanisms to the surrounding fascial tissue as it is suggested that TrP release methods, including TrP-DN, can change viscoelastic properties or behavior of the fascial tissue (Langevin 2013). The interaction between TrPs and fascia is based on the premise that muscle perimysium has a high density of myofibroblasts, a common cell found in connective tissue (Schleip et al. 2005), and the role of hyaluronanic acid in myofascial pain (Stecco et al. 2011).
Some systematic reviews have found moderate evidence supporting the use of some TrP release methods for immediate pain relief of TrPs (Vernon & Schneider 2009, Gay et al. 2013) and limited evidence for long-term pain relief; however, it is difficult to draw any clinical conclusion since most studies have investigated single modalities, whereas multimodal approaches are usually used by clinicians. More recent studies have reported that the integration of TrP release methods, including TrP-DN, within multimodal manual therapy programs is effective for some chronic pain conditions such as plantar heel pain, ankle sprain, fibromyalgia syndrome, neck pain, migraine, etc. Future studies are needed to determine the efficacy of TrP release methods included in a biopsychosocial treatment of chronic pain. For instance, it has been observed that a combination of TrP-DN and neuroscience education was more effective than TrP-DN alone for the management of low back pain (Téllez-García et al. 2015).
Objectives
Rather than explaining TrP as a local pathological/anatomical muscle problem, current theories focus on the nociceptive nature of TrPs and their role in perpetuating sensitization mechanisms. The clinical aims of any TrP release method are the inactivation of muscle-associated sensory and motor symptoms and the decrease of nociceptive barrage to the CNS (Fernández-de-las-Peñas et al. 2011). TrP release methods are applied by numerous healthcare professionals, including osteopaths, physicians, chiropractics, physical therapists, dentists, or massage therapists, among others, dependent upon the country and local jurisdictional regulations.
Since there are different TrP release methods, these approaches can be applied in any condition where the muscle or fascial tissues are involved in their etiology. For instance, several studies have included the application of ischemic compression, TrP pressure release, neuromuscular intervention, or myofascial induction in the management of shoulder pain, plantar heel pain, ankle pain, chronic pelvic pain, migraine, lateral epicondylalgia, or tension-type headache.
Assessment
All TrP release methods aim to inactivate active TrPs. The first step in the clinical reasoning process to determine which TrP release method should be used, is the accurate diagnosis of a TrP. In fact, correct TrP diagnosis requires manual ability, training and clinical practice to develop a high degree of reliability in the clinical examination. Typical signs and symptoms include: 1) presence of a hyperirritable spot in a palpable taut band in a skeletal muscle (when accessible to palpation); 2) palpable local twitch response on snapping palpation or DN of the TrP area (when possible); and 3) presence of referred pain elicited by stimulation of the TrP. Additional helpful signs for the diagnosis include muscle weakness, pain on contraction, pain on stretching, a jump sign, autonomic phenomena or motor disturbances (Simons et al. 1999). Although Gerwin et al. (1997) concluded that some muscles are more reliably examined than others, there is no general consensus on the reliability of TrP diagnosis.
TrPs are mainly identified through manual palpation. Clinicians can use either a flat palpation where
the finger or thumb presses the muscle against underlying bone tissue, or a pincer palpation in which a particular muscle is palpated between the clinician’s fingers. Taut band can be identified by palpating perpendicular to the fiber direction, which can elicit a local twitch response (strumming palpation). Once the taut band is located, the clinician moves along the taut band to find a discrete area of intense pain and, sometimes, hardness, which will elicit referred pain.
Once the clinician has localized the TrP, the intervention will depend on the irritability of the tissue, the accessibility of the muscle, and the symptoms. For instance, in patients with higher levels of pain and irritability, the clinician can choose indirect release methods targeting the taut band (e.g. longitudinal strokes or myofascial induction) or pain-free compression methods (e.g. TrP pressure release or strain–counterstrain). In such a patient, the application of TrP-DN may not be the first choice because of the DN-related pain. On the contrary, in patients with lower irritability of the CNS, TrP-DN can be the first therapeutic option, or compression interventions at the pain threshold level applied over the TrP. Nevertheless, clinicians should use a biopsychosocial clinical reasoning to determine which TrP release method is the most suitable for a particular patient.
Mechanisms
Any therapeutic intervention should be evidence-informed and based on scientific evidence, clinicians’ judgments, expertise and clinical decision-making (Dommerholt 2012). It is difficult to determine the exact therapeutic mechanism involved in TrP release methods since mechanical and neurophysiological mechanisms are involved at the same time. In fact, TrP release methods are complementary since they can act at different tissue levels including taut band, TrP region, muscle tissue, and surrounding fascia. The therapeutic aim of TrP release methods is to stop the vicious cycle of the evidence-informed integrated TrP hypothesis (Simons et al. 1999). The updated version of this etiological hypothesis is the most comprehensive framework currently available to explain the TrP formation and to guide the therapeutic management (Gerwin et al. 2004). According to this hypothesis, any TrP release method should focus on decreasing TrP-related symptoms by reversing the observed hypoxia and low pH, and by decreasing the excitability of the muscle nociceptors.
•
From a mechanical viewpoint, it has been proposed that TrP release methods that compress the muscle in a vertical or perpendicular manner equalize the length of the muscle sarcomeres. This effect would be increased if the muscle contracts at the same time (Fernández-de-las-Peñas et al. 2011). Either TrP release intervention can be applied along or across the TrP taut band. By applying these interventions, clinicians will exert transverse or longitudinal mobilization to the taut band and surrounding fascia.
•
It is important to note that the mechanical effects of TrP release methods also involve changes in the viscoelastic properties and/or behavior of surrounding fascia (Langevin 2013). In fact, longitudinal strokes applied along the taut band seem to be very similar neuromuscular technique approaches (Chaitow & DeLany 2008).
•
It has been suggested that the application of a continuous mechanical stimulus, particularly compression or stretching, to soft connective tissue induces a piezoelectric effect, which modifies the ‘gel’ state of the connective tissue to a more solute state. In fact, it has been demonstrated that to produce lasting changes in viscoelastic properties of fascia, mechanical stimulus should be applied for up to 60 seconds (Chaudhry et al. 2007).
•
Hou et al. (2002) investigated the time required for application of TrP pressure release and reported that this intervention is generally applied for 90 seconds. Therefore, it is plausible
that compression interventions aimed at the TrP also induce changes in the surrounding fascia. In such a scenario, some authors have proposed that TrP release methods participate in a similar manner to fascial approaches, involving mechanotransduction processes (Dommerholt 2012).
•
It is also likely that Fascial Manipulation
®
should play a greater role in TrP release as suggested by Stecco (2004). Similarly, connective tissue relaxation appears to require a static stretching to the fascia that is sustained for at least 10 minutes (Langevin 2013).
From a neurophysiological point of view, several theories have been also proposed.
•
Hou et al. (2002) suggested that pain relief may result from reactive hyperemia in the TrP area or a spinal reflex mechanism inducing muscle relaxation. The reactive hyperemia can be related to the reversal of the hypoxia present in the TrP area with the consequent increase in muscle circulation (Gerwin et al. 2004).
•
In addition, muscle relaxation induced by the mechanical stimulus can activate spinal reflex mechanisms including the gate control (Dommerholt & McEvoy 2010).
•
The activation of the gate control is elicited by stimulation of αδ-sensory afferent fibers by the nociceptive mechanical stimulation induced by the TrP release intervention. This activation provokes segmental nociceptive effects over the TrP (Fernández-de-las-Peñas et al. 2011). In fact, current evidence supports that TrP release methods show a localized hypoalgesic effect, by increasing pressure pain thresholds, when compared with no-treatment and sham/inert groups, and their effects are comparable with those of other active treatments (Gay et al. 2013).
•
The segmental anti-nociceptive effect is based on the premise that active TrPs are a source of peripheral nociception since the concentrations of bradykinin, calcitonin gene-related peptide, substance P, tumor necrosis factor-α, interleukins 1β, IL-6 and IL-8, serotonin and norepinephrine were significantly higher near active TrPs than near latent TrP or non-TrP points (Shah et al. 2008). It is not known whether the therapeutic effects of all the TrP release methods, particularly the reduction in referred pain, are related to a reduction of nociceptive afferences to the dorsal horn neurons.
•
A second potential neurophysiological mechanism is the activation of brain areas, particularly descending inhibitory pain mechanisms (Niddam 2009). It seems that nociceptive stimulus applied to the TrP area activates the pain neuromatrix. A recent pilot study reported that TrP compression altered the activity of the autonomic nervous system via the prefrontal cortex to reduce subjective pain (Morikawa et al. 2017).
•
These mechanisms are also mainly involved in TrP-DN; however, considering that TrP-DN is a painful intervention, and therefore represents a nociceptive input to the nervous system, neurophysiological mechanisms are more relevant (Dommerholt & Fernández-de-las-Peñas 2013).
•
It is known that TrP-DN reduces segmental nociceptive input (Srbely et al. 2010). In fact, although no study has investigated the effect of TrP-DN in activation of cortical areas, there is evidence suggesting that the insertion of a needle into acupuncture and non-acupuncture points involves the activation of the limbic system and the descending inhibitory pathways (Dommerholt & Fernández-de-las-Peñas 2013). This hypothesis is partly supported by a study where a single application of TrP-DN decreased
widespread pressure pain sensitivity, a clinical manifestation of central sensitization, in patients with acute neck pain (Mejuto-Váquez et al. 2014).
•
Nevertheless, the mechanical effect of the needling over the fascia should be not ignored, since every time a needle is inserted through the skin towards a TrP, the needle passes through multiple levels of fascia (Langevin 2013).
•
Stretching connective tissue with a (rotating) needle has been shown to stretch and reduce the tissue tension, flatten fibroblasts and remodel the cytoskeleton. Therefore, mechanical stimulation by a needle may activate mechanotransduction (Langevin 2013).
•
Because clinicians induce different movements to the needle during needling, i.e. rotation or in-and-out, it is possible that collagen bundles adhere to the needle, creating a small ‘whorl’ of collagen in the immediate vicinity of the needle (Langevin 2013). Therefore, acupuncture needles can be used to create sustained and localized stretching of subcutaneous and deeper connective tissue layers.
Protocol
Various TrP release methods can be used in clinical practice depending on the patient’s features and clinical experience. They differ in the amount of pressure used, presence/absence of pain, duration of the technique, position of the tissues and employment, or not, of active contraction (Fernández-de-las-Peñas et al. 2011, Fernández-de-las-Peñas & Pilat 2011). Several systematic reviews investigating the effectiveness of TrP release methods (Vernon & Schneider 2009, Gay et al. 2013) and DN (Furlan et al. 2005, Gattie et al. 2017) have been published. The conclusion in relation to manual release methods is that there is evidence supporting their use for short-term relief but not for medium- and long-term follow-ups. The effectiveness of TrP-DN is still controversial although one Cochrane Review found evidence for its use in patients with low back pain (Furlan et al. 2005) and a recent review and meta-analysis concluded that TrP-DN performed by physical therapists is more effective than a no-treatment control or sham dry needling for reducing pain (Gattie et al. 2017). Future studies including long-term follow-ups are clearly needed. Nevertheless, it is difficult to draw clinical conclusions from current evidence since most studies investigated TrP release methods or DN as single modalities, whereas multimodal manual therapy approaches are practiced by clinicians. In fact, current research supports that pain is produced by the brain when there is a perception of bodily danger requiring specific action. Therefore, consideration of the patient’s overall situation is critical for a therapeutic approach. The effects of TrP release methods including TrP-DN cannot be considered independently of the biopsychosocial model and must be approached from a pain science perspective, as it is no longer sufficient to consider any TrP release method strictly as a tool to address local muscle pathology (Dommerholt & Fernández-de-las-Peñas 2013).
In the author’s clinical practice, the pressure level, duration of application and position of the tissue (shortened, stretched) will depend on the degree of sensitization of the CNS presented by the patient and the irritability of the TrP.
•
Hou et al. (2002) suggest alternative compression approaches using low pressure, below pain threshold, for prolonged periods of time (i.e. 90 seconds) or high pressure over the pain threshold (pain tolerance) for shorter periods of time (i.e. 30 seconds).
•
Clinicians should consider that TrP release methods also involve changes in the viscoelastic properties or behavior of surrounding fascia
when they are applied for a duration of at least up to 60 seconds (Chaudhry et al. 2007).
•
Table 21.1
summarizes and
Figure 21.1
illustrates clinical application of different forms of TrP compression: ischemic compression, pressure release, strain–counterstrain or positional release therapy and pulsed or intermittent compression.
•
TrP release methods based on compression interventions can be applied in several chronic and acute pain conditions, including mechanical neck pain, shoulder pain, elbow pain, hip pain, knee pain, temporomandibular pain, headaches, fibromyalgia and whiplash, with the aim of decreasing the pain, improving restricted range of motion and/or improving function.
•
The manual technique depends on the degree of CNS irritability of the patient: in patients with high levels of sensitivity, e.g. fibromyalgia, pain-free TrP release methods, e.g. strain–counterstrain or positional release therapy, can be used, whereas in patients with low levels of sensitivity, e.g. sport players, use of more intense interventions are possible, e.g. ischemic compression (
Fig. 21.1A
).
•
Another TrP release method is massage, which can be performed along (longitudinal strokes) or across (transverse strokes) the taut band. The application of longitudinal strokes along the taut band is sometimes associated with neuromuscular approaches (Chaitow & DeLany 2008; see
Ch. 14
).
•
In this particular approach, longitudinal strokes (glides, slides) are performed over the taut band containing TrPs. The strokes are generally applied with the thumb (
Fig. 21.1B
), elbows or knuckles. In muscles where taut bands can be grasped with pincer palpation, the strokes can be applied with a pincer grip (
Fig. 21.1C
). Again, the degree of pressure and the speed of gliding will depend on the irritability and tone of the tissue. In the author’s clinical experience the best result comes from repetitive, deep and slow strokes (6–10 times) over the affected tissue.
Table 21.1
Different forms of compressive trigger point (TrP) release.
|
Muscle position
|
Degree of compression
|
Time of pressure
|
Duration of the technique
|
|
Ischemic compression
|
Fully lengthened
|
Sufficient to induce moderate pain between 3 and 6–7 (where 10 is maximum)
|
Until pain decreases by around 50–75%
|
Up to 90 seconds
|
|
TrP pressure release
|
Partially lengthened or neutral
|
Usually pain-free (first perception of tissue barrier)
|
Until the therapist perceives TrP or taut band release
|
Up to 90 seconds
|
|
Strain– counterstrain
|
Neurologically silent (usually shortened)
|
Reduction of pain by around 70–80%
|
Constant throughout
|
90 seconds
|
|
Pulsed ischemic compression
|
Neutral or at the ‘first sign of resistance’ barrier, i.e. no lengthening
|
5 seconds compression to induce pain at level 5–6 followed by 3 seconds no pressure – repeated until local or referred pain change or reduction in tissue resistance
|
5 seconds pressure, 3 seconds no pressure, repeated
|
Up to 90 seconds – or until change in pain is reported, or taut band release perceived
|
|
NPRS: Numerical Pain Rate Scale (0–10).
|
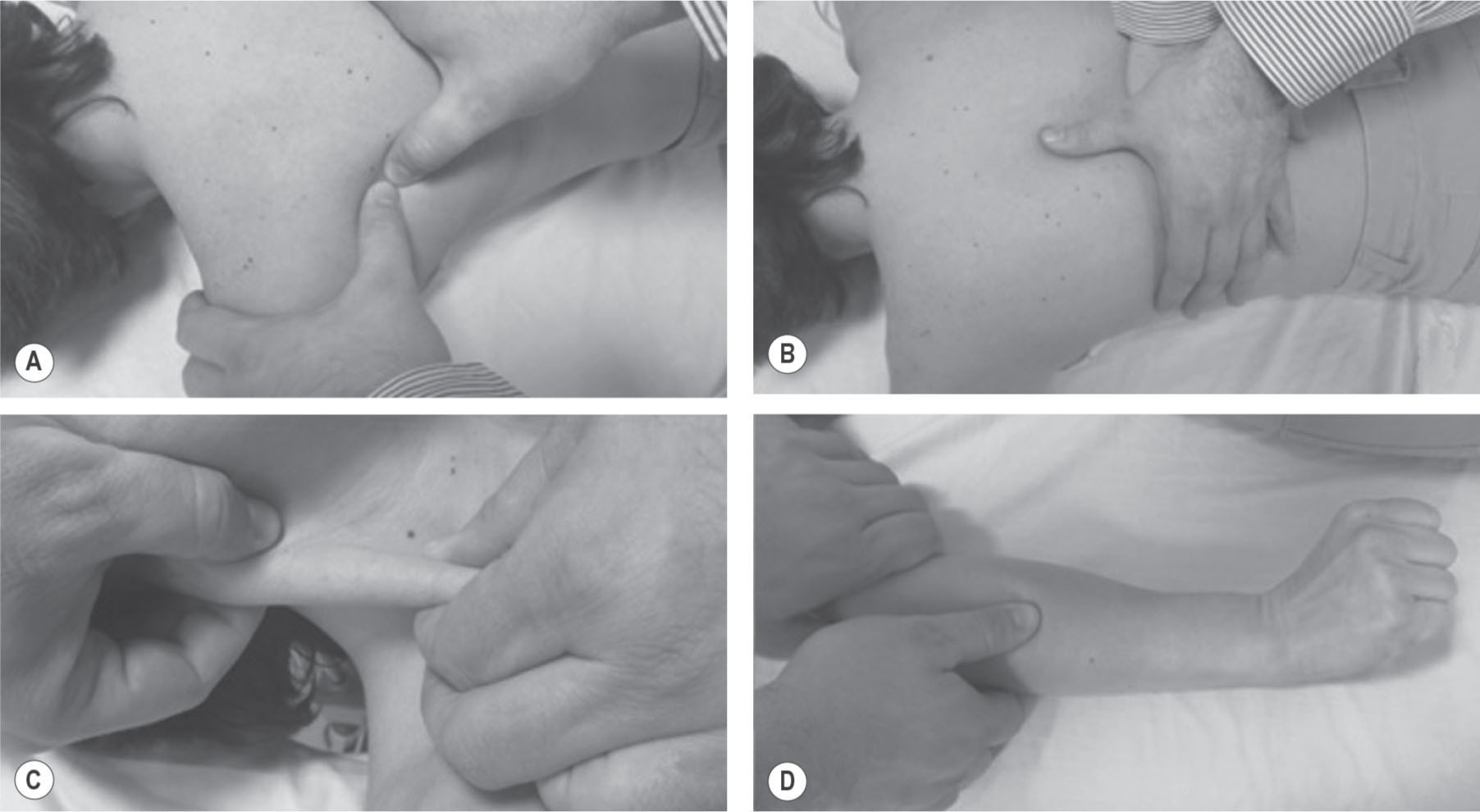
Figure 21.1
Different trigger point (TrP) release methods. (A) Ischemic compression intervention targeted at infraspinatus TrPs. (B) Longitudinal stroke along the taut band over paraspinal muscle TrPs. (C) Centrifugally longitudinal stroke with a pincer palpation along the taut band on the sternocleidomastoid muscle TrPs. (D) Dynamic intervention: longitudinal stroke along the taut band of extensor wrist muscle TrPs as the patient simultaneously and actively extends the wrist
•
Since TrPs are located in active tissues, i.e. muscle or fascia, some patients will benefit from dynamic interventions. In these approaches the clinician will apply any TrP release method, e.g. TrP pressure release or longitudinal strokes, combined with contraction or stretching of the affected or antagonist muscle (Fernández-de-las-Peñas et al. 2011).
•
One possible combination is that during a manual compression, the patient is asked to move the segment through a range of motion. A dynamic technique involves a longitudinal stroke applied by the clinician along the taut band while the patient is asked to move the segment (
Fig 21.1D
).
•
The mechanisms of these techniques are still unknown, but may be related to activation of the intra-fascial Pacinian/Paciniform and the Ruffini mechanoreceptors, which are found in all types of dense proper connective tissues (Schleip et al. 2005).
•
Stretching approaches are also included in TrP release methods: passive stretching (the therapist passively stretches the affected muscle without participation of the patient), active stretching (the patient actively stretches the muscle without participation of the therapist), spray/stretch, or post-isometric relaxation/contract–relax–release (MET).
•
MET involve the accurate localization of forces to the muscle barrier (first sign of resistance of the taut band), a sub-maximal isometric contraction of the muscle against an unyielding counterforce supplied by the therapist, followed by patient relaxation and engagement of a new tissue barrier, or stretching past the previous barrier. The force and duration of the isometric contraction will adapt to the irritability of the tissues ranging from 3 to 9 seconds (see
Ch. 12
).
•
The therapeutic mechanism of the MET may be the combination of a temporary elongation of the connective tissue and plastic changes in the connective tissues.
•
A final non-manual TrP release method is TrP-DN. It is recommended that patients are lying down during any needling procedures, because of the risk of autonomic responses. For every muscle, anatomical landmarks should be first identified, including the margins of the muscle and any relevant bony structures, i.e. the medial and lateral borders of the scapula and the scapular spine when DN infraspinatus muscle TrPs are treated.
•
Although no consensus exists whether disinfection of the skin is necessary, cleaning the surrounding skin with alcohol before any TrP-DN intervention is common practice (Dommerholt & Fernández-de-las-Peñas 2013).
•
For TrP-DN, acupuncture needles in tubes are recommended. The tube is placed on the skin overlying the TrP and the needle is quickly tapped into the skin. The tube is removed, and the needle is moved in and out into the region of the TrP by drawing the needle back to the subcutaneous tissue (never outside of the skin) and redirecting it (
Fig. 21.2
).
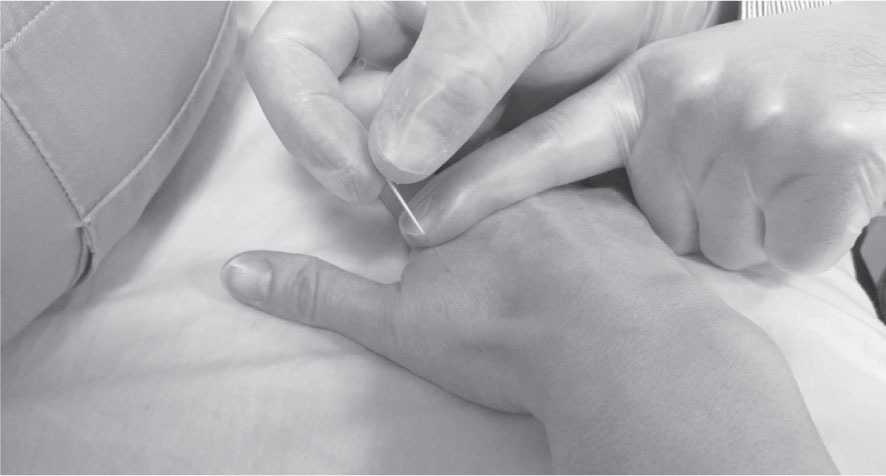
Figure 21.2
Trigger point dry needling (TrP-DN) over adductor pollicis muscle TrP.
Exercise

The clinician, following a clinical reasoning based on the clinical history of the patient, suspects the presence of a TrP in the right sternocleidomastoid muscle. The patient suffers from unilateral headaches located over the temple and the orbit.
The clinician, following a clinical reasoning based on the clinical history of the patient, suspects the presence of a TrP in the right sternocleidomastoid muscle. The patient suffers from unilateral headaches located over the temple and the orbit.

The clinician palpates the right sternocleidomastoid muscle with a pincer palpation all along the muscle searching for the presence of active TrPs. During the examination, the muscle exhibits two painful points in the muscle belly.
The clinician palpates the right sternocleidomastoid muscle with a pincer palpation all along the muscle searching for the presence of active TrPs. During the examination, the muscle exhibits two painful points in the muscle belly.

One of these points elicits referred pain to the head at 6 seconds on compression by the therapist. This referred pain reproduces the headache of the patient (i.e. it is an active TrP). The other point does not refer pain, there is only local pain and tenderness on palpation.
One of these points elicits referred pain to the head at 6 seconds on compression by the therapist. This referred pain reproduces the headache of the patient (i.e. it is an active TrP). The other point does not refer pain, there is only local pain and tenderness on palpation.

The clinician decides to treat the active TrP with manual release methods. The first attempt would be the application of an
ischemic compression technique. The muscle is grasped with a pincer palpation over the TrP and the clinician compresses the muscle until moderate pain appears. The compression is maintained at this level of pressure.
The clinician decides to treat the active TrP with manual release methods. The first attempt would be the application of an
ischemic compression technique. The muscle is grasped with a pincer palpation over the TrP and the clinician compresses the muscle until moderate pain appears. The compression is maintained at this level of pressure.

After 15 seconds of compression the patient comments that the compression elicits referred pain to the head and is highly uncomfortable. The clinician decides to reduce the intensity of the intervention, and to maintain the compression level at the tissue resistance (i.e. TrP pressure release).
After 15 seconds of compression the patient comments that the compression elicits referred pain to the head and is highly uncomfortable. The clinician decides to reduce the intensity of the intervention, and to maintain the compression level at the tissue resistance (i.e. TrP pressure release).

Therefore, the compression is maintained at the tissue resistance level, which is mainly asymptomatic for the patient.
Therefore, the compression is maintained at the tissue resistance level, which is mainly asymptomatic for the patient.

After 20 seconds, the tissue resistance eases and the clinician again increases the level of compression until the next tissue barrier is reached. This procedure is repeated 3–4 times.
After 20 seconds, the tissue resistance eases and the clinician again increases the level of compression until the next tissue barrier is reached. This procedure is repeated 3–4 times.

After the compression intervention, manual palpation of the TrP does not elicit referred pain but the right sternocleidomastoid muscle is still tender and slightly painful because of the TrP taut band. The clinician then decides to apply a longitudinal stroke along the taut band away from the TrP.
After the compression intervention, manual palpation of the TrP does not elicit referred pain but the right sternocleidomastoid muscle is still tender and slightly painful because of the TrP taut band. The clinician then decides to apply a longitudinal stroke along the taut band away from the TrP.

The clinician grasps the muscle with a pincer palpation at the TrP area with both hands. The pressure should be applied at the tissue resistance for 5–10 seconds.
The clinician grasps the muscle with a pincer palpation at the TrP area with both hands. The pressure should be applied at the tissue resistance for 5–10 seconds.

At this moment, a longitudinal stroke along the taut band is applied centrifugally away from the TrP. The speed of the stroke is gentle and smooth at the tissue barrier level. This procedure can be repeated 3–4 times.
At this moment, a longitudinal stroke along the taut band is applied centrifugally away from the TrP. The speed of the stroke is gentle and smooth at the tissue barrier level. This procedure can be repeated 3–4 times.
•
The objective of TrP-DN is to elicit local twitch responses, which are an indication that the TrP is indeed inactivated (Hong 1994). In some muscles or patients, if the local twitch responses are not clearly elicited, the presence of referred pain during TrP-DN can be also considered an indicator of a successful needling (Dommerholt & Fernández-de-las-Peñas 2013).
•
Following TrP-DN, hemostasis must be accomplished to prevent and/or minimize local bleeding. It should be noted that TrP-DN (and all TrP deactivation procedures) should be accompanied by other interventions to restore and maintain range of motion and to facilitate the return to normal function.
References
Chaitow L 2007 Positional release techniques. Churchill Livingstone, Edinburgh
Chaitow L, DeLany J 2008 Clinical application of neuromuscular techniques, 2nd edn. Vol 1: The upper body. Churchill Livingstone, Edinburgh
Chaudhry H et al 2007 Viscoelastic behaviour of human fasciae under extension in manual therapy J Bodywork Mov Ther 11:159–167
Dommerholt J 2012 Trigger point therapy. In: Schleip R, Finley T, Chaitow L, Huijing P (eds) Fascia in manual and movement therapies. Churchill Livingstone Elsevier, Edinburgh
Dommerholt J, McEvoy J 2010 Myofascial trigger point release approach. In: Wise CH (ed) Orthopaedic manual physical therapy: from art to evidence. FA Davis, Philadelphia
Dommerholt J, Fernández-de-las-Peñas C (eds) 2013 Trigger point dry needling: an evidenced and clinical-based approach. Churchill Livingstone Elsevier, London
Fernández-de-las-Peñas C, Pilat A 2011 Soft tissue manipulation approaches to chronic pelvic pain (external). In: Chaitow L, Lovegrove R (eds) Chronic pelvic pain and dysfunction: practical physical medicine. Churchill Livingstone Elsevier, London
Fernández-de-las-Peñas C et al 2011 Manual treatment of myofascial trigger points. In: Fernández-de-las-Peñas C, Cleland J, Huijbregts P (eds) Neck and arm pain syndromes: evidence–informed screening,
diagnosis, and conservative management. Churchill Livingstone Elsevier, London, pp 451–461
Furlan AD et al 2005 Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev 1:CD001351
Gattie et al 2017 The effectiveness of trigger point dry needling for musculoskeletal conditions by physical therapists: A systematic review and meta-analysis. J Orthop Sports Phys Ther 47:133–149
Gay CW et al 2013 Effect of a single session of muscle-biased therapy on pain sensitivity: a systematic review and meta-analysis of randomized controlled trials. J Pain Res 6:7–22
Gerwin RD et al 1997 Inter-rater reliability in myofascial trigger point examination. Pain 69:65–73
Gerwin RD et al 2004 An expansion of Simons’ integrated hypothesis of trigger point formation. Current Pain Head Reports 8:468–475
Hong CZ 1994 Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil 73:256–263
Hou CR et al 2002 Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Arch Phys Med Rehabil 83:1406–1414
Langevin HM 2013 Effects of acupuncture needling on connective tissue. In: Dommerholt J, Fernández-de-las-Peñas C (ed) Trigger point dry needling: an evidenced and clinical-based approach. Churchill Livingston Elsevier, pp 29–32
Mejuto-Vázquez MJ et al 2014 Short-term changes in neck pain, widespread pressure pain sensitivity, and cervical range of motion after the application of trigger point dry needling in patients with acute mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther 44:252–256
Morikawa J et al 2017 Compression at myofascial trigger point on chronic neck pain provides pain relief through the prefrontal cortex and autonomic nervous system: A pilot study. Front Neurosci 11:186
Niddam DM 2009 Brain manifestation and modulation of pain from myofascial trigger points. Curr Pain Headache Rep 13:370–375
Schleip R et al 2005 Active fascial contractility: fascia may be able to contract in a smooth muscle-like manner and thereby influence musculoskeletal dynamics. Med Hyp 65:273–277
Shah J P et al 2008 Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil 89:16–23
Simons DG et al 1999 Travell & Simons’ Myofascial pain and dysfunction: the trigger point manual: the upper half of body. Williams & Wilkins, Baltimore
Srbely JZ et al 2010 Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med 42:463–468
Stecco L 2004 Fascial manipulation for musculoskeletal pain. Piccin, Padova
Stecco C et al 2011 Hyaluronan within fascia in the etiology of myofascial pain. Surgical Radiol Anat 33:891–896
Téllez-García et al 2015 Neuroscience education in addition to trigger point dry needling for the management of patients with mechanical chronic low back pain: A preliminary clinical trial. J Bodyw Mov Ther 19:464–472
Vernon H, Schneider M 2009 Chiropractic management of myofascial trigger points and myofascial pain syndrome: a systematic review of the literature. J Man Physiol Ther 32:14–24



The clinician, following a clinical reasoning based on the clinical history of the patient, suspects the presence of a TrP in the right sternocleidomastoid muscle. The patient suffers from unilateral headaches located over the temple and the orbit.
The clinician, following a clinical reasoning based on the clinical history of the patient, suspects the presence of a TrP in the right sternocleidomastoid muscle. The patient suffers from unilateral headaches located over the temple and the orbit.

The clinician palpates the right sternocleidomastoid muscle with a pincer palpation all along the muscle searching for the presence of active TrPs. During the examination, the muscle exhibits two painful points in the muscle belly.
The clinician palpates the right sternocleidomastoid muscle with a pincer palpation all along the muscle searching for the presence of active TrPs. During the examination, the muscle exhibits two painful points in the muscle belly.

One of these points elicits referred pain to the head at 6 seconds on compression by the therapist. This referred pain reproduces the headache of the patient (i.e. it is an active TrP). The other point does not refer pain, there is only local pain and tenderness on palpation.
One of these points elicits referred pain to the head at 6 seconds on compression by the therapist. This referred pain reproduces the headache of the patient (i.e. it is an active TrP). The other point does not refer pain, there is only local pain and tenderness on palpation.

The clinician decides to treat the active TrP with manual release methods. The first attempt would be the application of an
ischemic compression technique. The muscle is grasped with a pincer palpation over the TrP and the clinician compresses the muscle until moderate pain appears. The compression is maintained at this level of pressure.
The clinician decides to treat the active TrP with manual release methods. The first attempt would be the application of an
ischemic compression technique. The muscle is grasped with a pincer palpation over the TrP and the clinician compresses the muscle until moderate pain appears. The compression is maintained at this level of pressure.

After 15 seconds of compression the patient comments that the compression elicits referred pain to the head and is highly uncomfortable. The clinician decides to reduce the intensity of the intervention, and to maintain the compression level at the tissue resistance (i.e. TrP pressure release).
After 15 seconds of compression the patient comments that the compression elicits referred pain to the head and is highly uncomfortable. The clinician decides to reduce the intensity of the intervention, and to maintain the compression level at the tissue resistance (i.e. TrP pressure release).

Therefore, the compression is maintained at the tissue resistance level, which is mainly asymptomatic for the patient.
Therefore, the compression is maintained at the tissue resistance level, which is mainly asymptomatic for the patient.

After 20 seconds, the tissue resistance eases and the clinician again increases the level of compression until the next tissue barrier is reached. This procedure is repeated 3–4 times.
After 20 seconds, the tissue resistance eases and the clinician again increases the level of compression until the next tissue barrier is reached. This procedure is repeated 3–4 times.

After the compression intervention, manual palpation of the TrP does not elicit referred pain but the right sternocleidomastoid muscle is still tender and slightly painful because of the TrP taut band. The clinician then decides to apply a longitudinal stroke along the taut band away from the TrP.
After the compression intervention, manual palpation of the TrP does not elicit referred pain but the right sternocleidomastoid muscle is still tender and slightly painful because of the TrP taut band. The clinician then decides to apply a longitudinal stroke along the taut band away from the TrP.

The clinician grasps the muscle with a pincer palpation at the TrP area with both hands. The pressure should be applied at the tissue resistance for 5–10 seconds.
The clinician grasps the muscle with a pincer palpation at the TrP area with both hands. The pressure should be applied at the tissue resistance for 5–10 seconds.

At this moment, a longitudinal stroke along the taut band is applied centrifugally away from the TrP. The speed of the stroke is gentle and smooth at the tissue barrier level. This procedure can be repeated 3–4 times.
At this moment, a longitudinal stroke along the taut band is applied centrifugally away from the TrP. The speed of the stroke is gentle and smooth at the tissue barrier level. This procedure can be repeated 3–4 times.