Figure 6-1. Physiologic pathway of pain transmission. (Reprinted from Wild LR, Evans L. Pain. In: Copstead L, ed. Perspectives on Pathophysiology. Philadelphia, PA: WB Saunders;1995:934, with permission from Elsevier.)
KNOWLEDGE COMPETENCIES
1. Describe the elements of pain assessment in progressive care patients.
2. Compare and contrast pain-relieving modalities for the critically ill:
• Nonsteroidal anti-inflammatory drugs
• Opioids, including patient-controlled analgesia
• Epidural analgesia with opioids and/or local anesthetics
• Elastomeric pumps with local anesthetic (LA)
• Nonpharmacologic modalities: distraction, cutaneous stimulation, imagery, and relaxation techniques
3. Identify the important elements of pain control for a patient who is an addict.
4. Describe special considerations for pain management in vulnerable populations such as the elderly.
5. Identify the need for sedation, common sedative drugs, and how to monitor and manage the patient requiring sedation.
Pain management is central to the care of the acutely ill or injured patient. Unfortunately acutely ill patients may not be able to self-report their pain management needs to their healthcare team. Patients identify physical care that promotes pain relief and comfort as an important element of their hospitalization and recovery, especially while in the hospital environment. Providing optimum pain relief for acutely ill patients not only enhances their psychoemotional well-being, but can also help avert additional physiologic injury. This chapter explores a multimodal approach to pain management in acutely ill patients based on the physiologic mechanisms of pain transmission and human responses to pain. Using a multimodal approach, specific pharmacologic and nonpharmacologic pain management techniques are described, including the integral relationships among relaxation, sedation, and pain relief. Strategies also are presented that promote comfort and are easy to incorporate into a plan of care for progressive care patients. Finally, special considerations are delineated for vulnerable populations within the acute care setting.
The pain response is elicited with tissue injuries, whether actual or potential. Undifferentiated free nerve endings, or nociceptors, are the major receptors signaling tissue injury (Figure 6-1). Nociceptors are polymodal and can be stimulated by thermal, mechanical, and chemical stimuli. Nociception refers to the transmission of impulses by sensory nerves, which signal tissue injury.
Figure 6-1. Physiologic pathway of pain transmission. (Reprinted from Wild LR, Evans L. Pain. In: Copstead L, ed. Perspectives on Pathophysiology. Philadelphia, PA: WB Saunders;1995:934, with permission from Elsevier.)
At the site of injury, the release of a variety of neurochemical substances potentiates the activation of peripheral nociceptors. Many of these substances are also mediators of the inflammatory response and they can facilitate or inhibit the pain impulse. These substances include histamine, kinins, prostaglandins, serotonin, and leukotrienes (Figure 6-2).
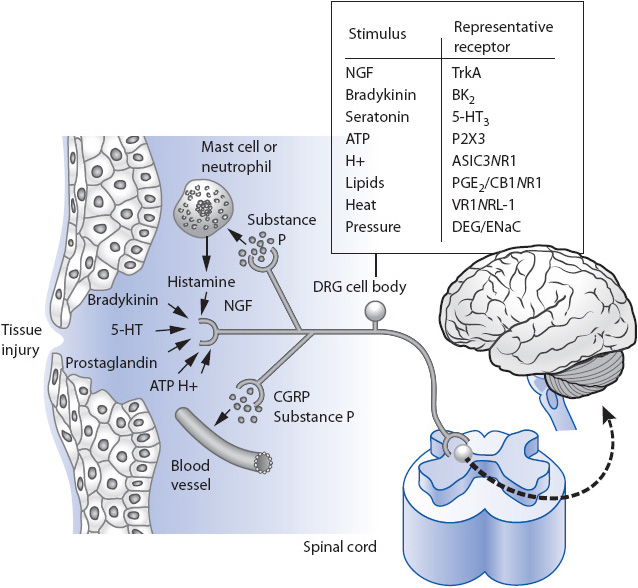
Figure 6-2. Peripheral nociceptors and the inflammatory response at the site of injury. (From Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203-210.)
The nociceptive impulse travels to the spinal cord via specialized, afferent sensory fibers. Small, myelinated A-delta (Aδ) fibers conduct nociceptive signals rapidly to the spinal cord. The A-delta fibers transmit sensations that are generally localized and sharp in quality. In addition to A-delta fibers, smaller, unmyelinated C fibers also transmit nociceptive signals to the spinal cord. Because C fibers are unmyelinated, their conduction speed is much slower than their A-delta counterparts. The sensory quality of signals carried by C fibers tends to be dull and unlocalized (Figure 6-3).
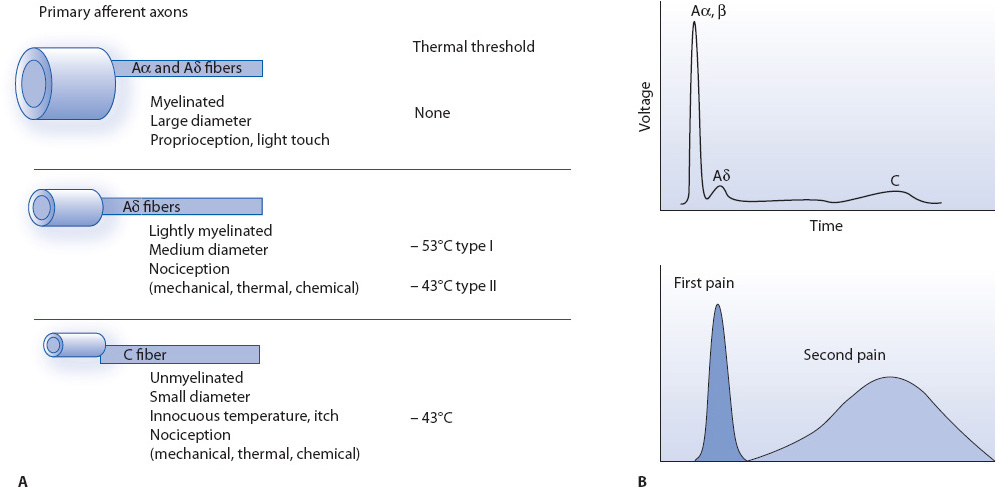
Figure 6-3. Different nociceptors detect different types of pain. (A) Peripheral nerves include small-diameter (Aδ) and medium- to large-diameter (Aα- β) myelinated afferent fibers, as well as small-diameter unmyelinated afferent fibers (C). (B) The fact that conduction velocity is directly related to fiber diameter is highlighted in the compound action potential recording from a peripheral nerve. Most nociceptors are either Aδ or C fibers, and their different conduction velocities (6-25 and ~1.0 m/s, respectively) account for the first (fast) and second (slow) pain responses to injury.
Sensory afferent fibers enter the spinal cord via the dorsal nerve, synapsing with cell bodies of spinal cord interneurons in the dorsal horn (see Figure 6-1). Most of the A-delta and C fibers synapse in laminae I through V, in an area referred to as the substantia gelatinosa. Numerous neuro-transmitters (eg, substance P, glutamate, and calcitonin gene-related peptide) and other receptor systems (eg, opiate, alpha-adrenergic, and serotonergic receptors) modulate the processing of nociceptive inputs in the spinal cord.
Following spinal cord integration, nociceptive impulses travel to the brain via specialized, ascending somatosensory pathways (see Figure 6-1). The spinothalamic tract conducts nociceptive signals directly from the spinal cord to the thalamus. The spinoreticulothalamic tract projects signals to the reticular formation and the mesencephalon in the midbrain, as well as to the thalamus. From the thalamus, axons project to somatosensory areas of the cerebrocortex and limbic fore-brain. The unique physiologic, cognitive, and emotional responses to pain are determined and modulated by the specific areas to which the somatosensory pathways project. The stimulus to the cerebrocortex can also activate the patient’s previous memories of the experience of pain; for example, the thalamus regulates the neurochemical response to pain, and the cortical and limbic projections are responsible for the perception of pain and aversive response to pain, respectively. Similarly, the reticular activating system regulates the heightened state of awareness that accompanies pain. The modulation of pain by activities in these specific areas of the brain is the basis of many of the analgesic therapies available to treat pain.
Human responses to pain can be both physical and emotional. The physiologic responses to pain are the result of hypothalamic activation of the sympathetic nervous system associated with the stress response. Sympathetic activation leads to:
• Blood shifts from superficial vessels to striated muscle, the heart, the lungs, and the nervous system.
• Dilation of the bronchioles to increase oxygenation.
• Increased cardiac contractility.
• Inhibition of gastric secretions and contraction.
• Increase in circulating blood glucose for energy.
Signs and symptoms of sympathetic activation which frequently accompany nociception and pain:
• Increased heart rate
• Increased blood pressure
• Increased respiratory rate
• Pupil dilation
• Pallor and perspiration
• Nausea and vomiting
Although patients experiencing acute pain often exhibit signs and symptoms as noted above, it is critical to note that the absence or presence of any or all of these signs and symptoms does not negate or confirm the presence of pain. In fact some patients, especially those who are seriously ill and with little or no compensatory reserves, may exhibit a shock-like clinical picture in the presence of pain. Patients who are accustomed to underlying chronic pain may have a decreased physiologic response to pain while the actual intensity of the pain remains high (Table 6-1).
TABLE 6-1. TYPES OF PAIN

Acutely ill patients also express pain both verbally and nonverbally. The expressions can take many forms, some of which are subtle cues that could easily be overlooked (Table 6-2). Any signs that may indicate pain warrant further exploration and assessment. Although physiologic and behavioral expressions of acute pain have been described, each person’s response to pain is unique.
Pain assessment is a core element of ongoing surveillance of the acutely ill patient. Self-report of pain intensity and distress should be used whenever possible, especially for patients who can talk or communicate effectively. Regular documentation of pain assessment not only helps monitor the efficacy of analgesic modalities, but also helps ensure communication among caregivers regarding patient’s pain. A variety of tools to assess pain intensity are available. There are three commonly used scales. The numeric rating scale (NRS) uses numbers between 0 and 10 to describe pain intensity; the anchors are “no pain to worst pain imaginable.” Some patients find it easier to use adjectives to describe their pain. The verbal descriptive scale offers patients a standardized list of adjectives to describe their pain intensity. The descriptors are “none,” “mild,” “moderate,” and “severe.” With the visual analogue scale (VAS), a tool developed primarily for research, patients indicate their pain intensity by drawing a vertical line, bisecting a horizontal baseline. The baseline is anchored at either end by the terms “no pain” or “worst pain imaginable.” A numeric conversion is done by measuring the line from the left anchor to the patient’s mark, in millimeters.
Any of these scales can be used with patients who are intubated or unable to speak for other medical reasons; for example, patients can be asked to use their fingers to indicate a number between 0 and 10; similarly, patients can be asked to indicate by nodding their head or pointing to the appropriate adjective or number as they either hear or read the list of choices. With the VAS, the line can be printed on a sheet of paper or marker board and the patients asked to mark the line to indicate their level of pain. While the VAS has been used in acute care patients it may be difficult to use in many as it requires dexterity that may be inhibited by invasive lines, bandages, etc.
Unfortunately, some acutely ill patients are unable to indicate their pain intensity either verbally or nonverbally. In these situations, nurses must often use other clues to assess their patient’s pain. Using a behavioral pain scale provides a guide for identifying and assessing pain in nonverbal patients (Table 6-3). In addition to monitoring physiologic parameters, nurses may also anticipate and recognize clinical situations where pain is likely to occur and use their knowledge of physiology and pathophysiology and experience with other patients with similar problems. By combining their knowledge and experience with well-developed interviewing and observational skills, progressive care nurses can assess patient’s pain effectively and intervene appropriately.
TABLE 6-3. DMC PAIN ASSESSMENT BEHAVIOR SCALE (NONVERBAL) FOR PATIENTS UNABLE TO PROVIDE A SELF-REPORT OF PAIN
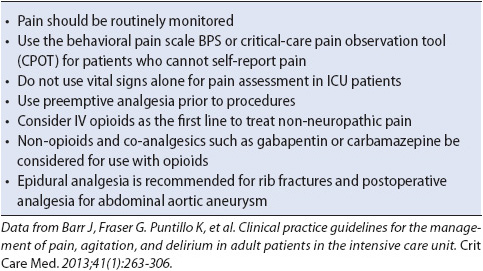
Today there are numerous approaches and modalities available to treat acute pain. Whereas, pharmacologic techniques traditionally have been the mainstay of analgesia, other complementary or nonpharmacologic methods are growing in their acceptance and use in clinical practice. Most modalities used in the treatment of acute pain can be used effectively in patients in progressive care units. Evidence-based practice guidelines to maximize analgesia in acutely ill patients are summarized in Table 6-4.
TABLE 6-4. Evidence-based GUIDELINES: PAIN MANAGEMENT
One of the central goals of pain management is to combine therapies or modalities that target as many of the processes involved in nociception and pain transmission as possible. Analgesic modalities, both pharmacologic and nonpharmacologic, exert their effects by altering nociception at specific structures within the peripheral or central nervous system (CNS), ie, the peripheral nociceptors, the spinal cord, or the brain or by altering the transmission of nociceptive impulses between these structures (Figure 6-4). By understanding where analgesic modalities work, nurses can more effectively select a combination of modalities working at different sites to best treat the source or type of pain patients experience and, subsequently, help patients achieve optimal analgesia.
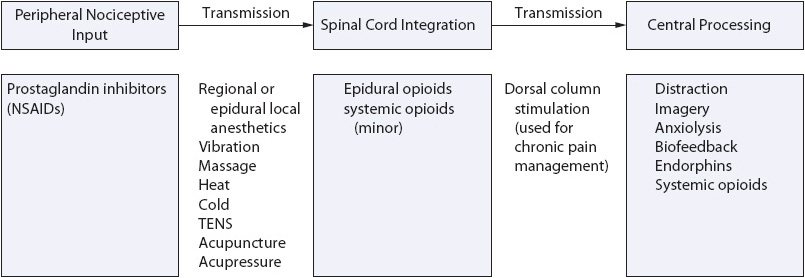
Figure 6-4. A multimodal approach to pain management.
To assist nurses to select and maximize analgesic modalities, for each of the analgesic modalities presented here, there is a brief description of where and how the selected modality works, clinical situations where it can be used most effectively, and strategies for titrating the modality. Finally, because few modalities exert a singular effect, a summary of secondary or side effects commonly associated with them, and strategies to minimize their occurrence, are also addressed.
Nonsteroidal anti-inflammatory drugs (NSAIDs) target the peripheral nociceptors. The NSAIDs exert their effect by modifying or reducing the amount of prostaglandin produced at the site of injury by inhibiting the formation of the enzyme cyclooxygenase, which is also responsible for the breakdown of arachidonic acid and formation of the neurotransmitter prostaglandin. As prostaglandin inhibitors, the NSAIDs have been shown to have opioid-sparing effects and are very effective in managing pain associated with inflammation, trauma to peripheral tissues (eg, soft tissue injuries), bone pain (eg, fractures, metastatic disease), and pain associated with indwelling tubes and drains (eg, chest tubes).
One of the NSAIDs commonly used in the acute care setting is ketorolac tromethamine (Toradol). Ketorolac is currently the only parenteral NSAID preparation available in the United States and can be administered safely via the intravenous (IV) route. Intramuscular administration is not recommended due to the potential for irregular and unpredictable absorption. Recommended dosing for ketorolac is a 30-mg loading dose followed by 15 mg every 6 hours. Like all NSAIDs, ketorolac has a ceiling effect where administration of higher doses offers no additional therapeutic benefit yet significantly increases the risk of toxicity. Another non-opioid alternative to ketorolac is acetaminophen IV for patients who can tolerate the drug and do not have liver disease or other potential contraindications. The Society of Critical Care Medicine (SCCM) recommends the use of adjuvant analgesics such as NSAIDs to reduce opioid analgesic use and reduce opioid-related side effects.
The side effects associated with the use of NSAIDs relate to the function of prostaglandins in physiologic processes in addition to nociception; for example, gastrointestinal (GI) irritation and bleeding may result from NSAID use because prostaglandins are necessary for maintaining the mucous lining of the stomach. Similarly, the enzyme cyclooxygenase is needed for the eventual production of thromboxane, a key substance involved in platelet function. As a result, when NSAIDs are used chronically or in high doses, platelet aggregation may be altered, leading to bleeding problems. NSAID use can also lead to renal toxicity. Cross-sensitivities with other NSAIDs have also been documented (eg, ibuprofen, naproxen, indomethacin, piroxicam, aspirin). For these reasons, ketorolac and other NSAIDs should be avoided for patients who have a history of gastric ulceration, renal insufficiency, and coagulopathies or a documented sensitivity to aspirin or other NSAIDs. In addition, NSAID use is not recommended in patients with heart disease, recent heart bypass surgery, or patients with a history of ischemic attacks or strokes. An alternative to ketorolac for patients who are not good candidates for NSAIDs is intravenous acetaminophen, as noted above. The severity of all NSAID-related side effects increases with high doses or prolonged use. For this reason, ketorolac and other such drugs are designed for short-term modality only and should not be used for more than 5 days.
The principal modality of pain management in the acute care setting continues to be opioids. The SCCM recommends that opioids be considered as first line treatment for non-neuropathic pain. Traditionally referred to as narcotics, opioids produce their analgesic effects primarily by binding with specialized opiate receptors throughout the CNS and thereby altering the perception of pain. Opiate receptors are located in the brain, spinal cord, and GI tract. Although opioids work primarily within the CNS, they also have been shown to have some local or peripheral effects as well. There are at least 45 variations of opiate receptors, which account for the varied response in individual patients.
Opioids are well tolerated by most acutely ill patients and can be administered by many routes including IV, IM, oral, buccal, nasal, rectal, transdermal, and intraspinal. Morphine sulfate is still the most widely used opioid and serves as the gold standard against which others are compared. Other opioids commonly used in the care of the acutely ill include hydromorphone (Dilaudid) and fentanyl (Sublimaze). Opioid polymorphisms may cause opioids to affect patients differently, thus careful use and assessment of the drugs are necessary to determine optimal dosing.
Patient’s responses to opioids, both analgesic responses and side effects, are highly individualized. Just as all the opioid agents have similar pain-relieving potential, all opioids currently available share similar side effect profiles. When side effects do occur, it is important to remember that they are primarily the result of opioid pharmacology, as opposed to the route of administration.
Nausea and vomiting are distressing side effects often related to opioids that, unfortunately, many patients experience. Generally, nausea and vomiting result from stimulation of the chemoreceptor trigger zone (CTZ) in the brain and/or from slowed GI peristalsis. Nausea and vomiting often can be managed effectively with antiemetic modality. Metoclopramide (Reglan), a procainamide derivative, works both centrally at the CTZ and at the GI level to increase gastric motility. However, there are significant risks with metoclopramide use such as the potential for seizures and tardive dyskinesia. These conditions occur more commonly in the elderly and with prolonged use of the drug.
The vestibular system also sends input to the CTZ. For this reason, opioid-related nausea frequently is exacerbated by movement. If patients complain of movement-related nausea, the application of a transdermal scopolamine patch can help prevent and treat opioid-induced nausea. The use of transdermal scopolamine is best avoided in patients older than 60 years because the drug has been reported to increase the incidence and severity of confusion in older patients.
The phenothiazines (ie, prochlorperazine [Compazine], 2.5-10 mg IV) and the butyrophenones (droperidol [Inapsine], 0.625 mg IV) treat nausea through their effects at the CTZ. The serotonin antagonist ondansetron (Zofran) is also effective for treatment of opioid-related nausea. The doses required for postoperative or opioid-related nausea are significantly smaller doses (4 mg IV) than those used with emetogenic chemotherapy.
Pruritus is another opioid-related side effect commonly reported by patients. The actual mechanisms producing opioid-related pruritus are unknown. Although antihistamines can provide symptomatic relief for some patients, the role of histamine in opioid-related pruritus is unclear. One of the drawbacks of using antihistamine agents, such as diphenhydramine (Benadryl), is the sedation associated with their use. In addition, the use of diphenhydramine has been shown to have a 70% increase in cognitive deterioration in geriatric patients. Similar to other opioid side effects, the incidence and severity of pruritus is dose-related and tends to diminish with ongoing use. Another option to treat pruritis is nalbuphine (Nubain), dosed at small doses of 2.5 to 5.0 milligrams IV every 6 hours as needed.
Constipation, another common side effect, results from opioid binding at opiate receptors in the GI tract and decreased peristalsis. The incidence of constipation may be low in acutely ill patients, but it is important to remember that it is likely to be a problem for many patients following the acute initial phase of their illness or injury. The best treatment for constipation is prevention by ensuring adequate hydration, as well as by administering stimulant laxatives and stool softeners, as needed. For patients with opioid induced constipation, the use of methylnaltrexone (Relistor) can be given as a subcutaneous injection for palliative care patients with advanced illness.
Urinary retention can result from increased smooth muscle tone caused by opioids, especially in the detrusor muscle of the bladder. Opioids have no effect on urine production and neither cause nor worsen oliguria.
Opioid modality can result in respiratory depression through its effects on the respiratory centers in the brain stem. Both respiratory rate and the depth of breathing can decrease as a result of opioids, usually in a dose-dependent fashion. Patients at increased risk for respiratory depression include the elderly, those with preexisting cardiopulmonary diseases, patients receiving other respiratory depressive medications such as benzodiazipines, and those who receive large doses. Frequently, the earliest sign of respiratory depression is an increased level of sedation, making this an important component of patient assessment. Other signs and symptoms of respiratory depression include decreased depth of breathing, often combined with slowed respiratory rate, constriction of pupils, hypoxemia, and hypercarbia.
Clinically significant respiratory depression resulting from opiate use is usually treated with IV naloxone (Narcan). Naloxone is an opioid antagonist; it binds with opiate receptors, temporarily displacing the opioid and suspending its pharmacologic effects. As with other medications, naloxone should be administered in very small doses and titrated to the desired level of alertness (Table 6-5). It should be emphasized that the half-life of naloxone is short—approximately 30 to 45 minutes. Careful assessment of the patient should continue and because of its short half-life, additional doses of naloxone may be needed.
TABLE 6-5. ADMINISTRATION OF NALOXONE
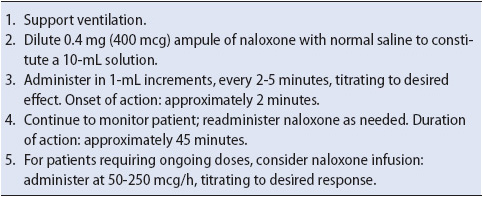
Naloxone should be used with caution in patients with underlying cardiovascular disease. The acute onset of hypertension, pulmonary hypertension, and pulmonary edema with naloxone administration has been reported. Also, naloxone should be avoided in patients who have developed a tolerance to opioids since opioid antagonists can’t precipitate withdrawal or acute abstinence syndrome.
Many acutely ill patients are unable to use the oral route, thus the IV route is used most often. One of the advantages of IV opioids is their rapid onset of action, allowing for easy titration. Loading doses of IV opioids should be administered to achieve an adequate blood level of the drug. Additional doses can then be administered intermittently to maintain analgesic levels.
Many progressive care patients may benefit from the addition of a continuous IV opioid infusion; for example, patients who may not be able to communicate their pain needs effectively, such as those that are mechanically ventilated, are good candidates for continuous opioid infusions. The continuous infusion not only helps achieve the appropriate blood levels, but also can be easily titrated to maintain consistent blood levels. Patients who experience significant fluctuations in analgesia or side effects related to opioid administration may also benefit from the constant blood levels provided by continuous infusions. Whenever possible, the maintenance dose for the infusion should be based on patient’s previous opioid requirements.
Patient-controlled analgesia (PCA) pumps can also be used effectively in the progressive care setting with patients who are alert and able to activate the PCA button. With PCA, patients self-administer small doses of an opioid infusion using a programmable pump. PCA prescriptions typically include a bolus dose of the selected drug, a lockout or delay interval, and either a 1- to 4-hour limit; many of the PCA devices also can be programmed to deliver a basal or background infusion. The bolus dose refers to the amount of the drug the patient receives following pump activation. The initial dose usually ranges between 0.5 and 2.0 mg of morphine or its equivalent. The lockout or delay interval typically ranges between 5 and 10 minutes, which is enough time for the prescribed drug to circulate and take effect, yet allows the patient to easily titrate the medication over time. The 1- to 4-hour limit serves as an additional safety feature by regulating the amount of medication the patient can receive over this period of time.
Assessing whether an acutely ill patient is capable of using a PCA is significant to the success of this analgesic modality. PCA should not be prescribed for the patient who is unable to reliably self-administer pain medication (eg, a patient with a decreased level of consciousness). A patient, however, who is cognitively intact but unable to activate the PCA button due to lack of manual dexterity or strength may utilize a PCA device that has been ergonomically adapted to suit the patient with impaired motor abilities (eg, a pressure switch pad). Lastly, if PCA is prescribed, patients, family members, and visitors should be educated that the patient is the only person to activate the PCA device. Family members and friends may think they are helping by activating the PCA device for the patient and not realize this can produce life-threatening sedation and respiratory depression.
Patients using PCAs usually find a dose and frequency that balances pain relief with other medication-related side effects such as sedation. It is best to start PCA modality after the patient has received loading doses to achieve adequate blood levels of the prescribed opioid. For patients who continue to experience pain while using the PCA pump, the first step in titration is to give an additional loading dose and increase the bolus dose, usually by 25%-50% depending on the pain intensity. If patients continue to have pain in spite of the increased dose, the lockout interval or delay should then be reduced, if possible.
Continuous PCA infusions are no longer recommended for the majority of patients as they increase sedation and do not provide additional pain relief. However, in patients who have preexisting opioid tolerance, a continuous infusion may maintain their baseline opioid requirements while the patient-controlled bolus doses are available to help manage any new pain they experience. The hourly dose of the continuous infusion should be equianalgesic to, and calculated from, patients’ preexisting opioid requirements.
One additional method of reducing pain for acutely ill patients is to combine standard options such as opioids with a regional analgesia. Commonly, this is done with a block during surgery lasting 6 to 8 hours using local anesthetics or a continuous infusion using a small self-continued elastomeric pump. These pumps include the drug reservoir for the local anesthetic (LA) that resembles a filled softball, and there is a preset flow control that allows the LA to infuse at the preset rate. The pump is attached to a catheter that can be placed as a soaker hose configuration along the surgical incision or along a nerve, such as the femoral nerve, for patients undergoing such procedures as a total knee replacement where a continuous flow can be provided for a period of several days. The concentrations of regional analgesics do not cause motor blockade. This technique is especially helpful for thoracotomy patients where pain with respiratory effort may be significantly reduced.
Most often switching from IV to oral opioids is accomplished when acute pain subsides and the patient is able to tolerate oral or enteral nutrition. Patients who receive analgesics by mouth or via the enteral route can experience comparable pain relief to parenteral analgesia with less risk of infection and at lowered cost. Calculating the equianalgesic dose increases the likelihood that the transition to the oral route will be made without loss of pain control. A creative way to wean PCA is to substitute oral or enteral opioid (like morphine or oxycodone) for the amount of drug given by continuous infusion plus one-half of the total dosage of PCA demand doses. Over the next 24 hours, reducing PCA consumption by increasing the lockout period or reducing the bolus size may help transition the patient and narrow the “analgesic gap” between different routes. To prevent opioid overdosage, controlled-release preparations of morphine and oxycodone, designed to be taken less frequently than their immediate-release counterparts, should not be crushed, halved, or administered into enteral feeding tubes to prevent opioid overdosage.
Over the past decade the use of epidural analgesia has grown rapidly. The advantages of epidural analgesia include improved pain control with less sedation, lower overall opioid doses, and generally longer duration of pain management. Epidural analgesia has been associated with a lower morbidity and mortality in acutely ill patients. Both opioids and local anesthetics (LAs), either alone or in combination, commonly are administered via the epidural route. Epidural analgesia may be administered by several methods, including intermittent bolus dosing, continuous infusion, or PCA technology. The mechanisms of action and the resultant clinical effects produced by epidurally administered opioids and LAs are distinct. For this reason, these agents not only are discussed separately, but also should be distinguished when used in clinical practice.
When opioids are administered epidurally, they diffuse into the cerebrospinal fluid and into the spinal cord (Figure 6-5). There, the opioids bind with opiate receptors in the substantia gelatinosa, preventing the release of the neurotransmitter, substance P, and subsequently alter the transmission of nociceptive impulses from the spinal cord to the brain. Because the opioid is concentrated in the areas of high opiate receptor density and where nociceptive impulses are entering the spinal cord, lower doses offer enhanced analgesia, with few, if any, supraspinal effects such as drowsiness.
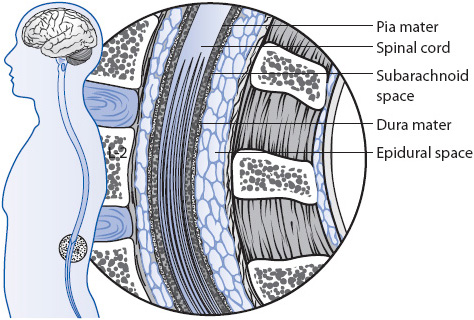
Figure 6-5. Epidural space for catheter placement.
A variety of opioids are commonly used for epidural analgesia including morphine, fentanyl, and hydromorphone. Preservative-free (PF) preparations are usually preferred because some preservative agents can have neurotoxic effects. The opioids can be administered either by intermittent bolus or continuous infusion depending on the pharmacokinetic activity of the selected agent; for example, fentanyl is generally administered via continuous infusion due to its high lipid solubility, resulting in a short duration of action. In contrast, the low lipid solubility of PF morphine results in a delayed onset of action (30-60 minutes) and a prolonged duration of action (6-12 hours). Because of the delayed onset of action taking as long as 60 minutes, PF morphine is recommended for use as a continuous infusion but not as a patient-controlled bolus dose.
The side effects associated with epidural opioids are the same as those described for oral opioids. It is important to remember that side effects are related more closely to the drug administered than by the route of administration; for example, the incidence of nausea and vomiting with epidural morphine is similar to that associated with IV morphine. Although epidural opioids were once feared to be associated with a higher risk of respiratory depression, clinical studies and experience have not confirmed this risk. The incidence of respiratory depression has been reported as being no higher than 0.2%. Risk factors for respiratory depression are similar to those seen with IV opioids: increasing age, high doses, underlying cardiopulmonary dysfunction, obstructive sleep apnea, obesity, and the use of perioperative or supplemental parenteral opioids or other agents causing sedation such as benzodiazipines in addition to epidural opioids.
Epidural opioids can also be combined with dilute concentrations of LAs. When administered in combination, these agents work synergistically, reducing the amount of each agent that is needed to produce analgesia. Whereas epidurally administered opioids work in the dorsal horn of the spinal cord, epidural LAs exert work primarily at the dorsal nerve root by blocking the conduction of afferent sensory fibers. The extent of the blockade is dose related. Higher LA concentrations block more afferent fibers within a given region, resulting in an increased density of the blockade. Higher infusion rates of LA-containing solutions increase the extent or spread of the blockade because more afferent fibers are blocked over a broader region.
Bupivacaine is the LA most commonly used for epidural analgesia and is usually administered in combination with either fentanyl or PF morphine as a continuous infusion. The concentration of bupivacaine used for epidural analgesia usually ranges between 1/16% (0.065 mg/mL) and 1/8% (1.25 mg/mL). These concentrations are significantly lower than those used for surgical anesthesia, which usually range between 1/4% and 1/2% bupivacaine. The type and concentration of opioid used in combination with bupivacaine vary by practitioner and organizational preferences, but usually range between 2 and 5 mcg/mL fentanyl or between 0.02 and 0.04 mg/mL PF morphine. Ropivacaine, a LA alternative to bupivacaine, has a lower profile for causing motor block. For older patients with rib fractures or flail chest, an epidural catheter with LA only may provide positive results with less respiratory compromise and reduced pain.
The side effects accompanying LAs are a direct result of the conduction blockade produced by the agents. Unfortunately, the LA agents are relatively nonspecific in their capacity to block nerve conduction. That is, LAs not only block sensory afferent fibers, but also can block the conduction of motor efferent and autonomic nerve fibers within the same dermatomal regions. Side effects associated with epidural LAs include hypotension—especially postural hypotension from sympathetic blockade—and functional motor deficits from varying degrees of efferent motor fiber blockade. Sensory deficits, including changes in proprioception in the joints of the lower extremities, can accompany epidural LA administration due to the blockade of non-nociceptive sensory afferents.
The extent and type of side effects that can be anticipated with epidural LAs depend on three primary factors: the location of the epidural catheter, the concentration of the LA administered, and the volume or rate of infusion; for example, if a patient has an epidural catheter placed within the midthoracic region, one can anticipate signs of sympathetic nervous blockade, such as postural hypotension, because the sympathetic nerve fibers are concentrated in the thoracic region. In contrast, a patient with a lumbar catheter may experience a mild degree of motor weakness in the lower extremities because the motor efferent and nerves exit the spine in the lumbar region. This usually presents clinically as either heaviness in a lower extremity or an inability to “lock” the knee in place when standing.
Also, as noted, both the concentration and infusion rate of the LA influence the severity and extent of side effects. The density of the blockade and intensity of observed side effects may be increased with high LA concentrations. With higher infusion volumes, greater spread of the LA can be anticipated which can, in turn, lead to a greater number or extent of side effects. If side effects occur, the dose of the LA often is reduced either by decreasing the concentration of the solution or by decreasing the rate.
To maximize epidural analgesia, doses may need to be adjusted. With opioids alone, the dose needed to produce effective analgesia is best predicted by the patient’s age as opposed to body size. Older patients typically require lower doses to achieve pain relief than those who are younger. Small bolus doses of fentanyl (50 mcg) can help safely titrate the epidural dose or infusion to treat pain. Similarly, a small bolus dose of fentanyl can also help treat breakthrough pain that may occur with increased patient activity or procedures. For patients receiving combinations of LAs and opioids, a small bolus dose of the prescribed infusate in conjunction with an increased rate can help titrate pain relief. Recall, however, that increasing the rate of the LA infusion increases the spread of the drug to additional dermatomes, whereas increasing the LA concentration increases the depth or intensity of the blockade and subsequent analgesia.
One of the primary nonpharmacologic techniques for pain management used in the acute care setting is cutaneous stimulation. Cutaneous stimulation produces its analgesic effect by the altering conduction of sensory impulses as they move from the periphery to the spinal cord through the stimulation of the largest sensory afferent fibers, known as the A-alpha (α) and A-beta (β) fibers. The sensory information transmitted by these large fibers is conducted more rapidly than that carried by their smaller counterparts (A-delta (δ) and C fibers) (see Figure 6-3). As a result, nociceptive input from the A-delta and C fibers is believed to be preempted by the sensory input from the non-noxious cutaneous stimuli. Examples of cutaneous stimulation include the application of heat, cold, vibration, or massage. Transcutaneous electrical nerve stimulation units produce similar effects by electrically stimulating large sensory fibers.
Cutaneous stimulation can produce analgesic effects whether used as a complementary modality with other pharmacologic treatments or as an independent treatment modality. Nurses can integrate these modalities easily and safely into analgesic treatment plans for the acutely ill, especially for patients who may be unable to tolerate higher opioid doses. To apply or administer cutaneous stimulation, one simply needs to stimulate sensory fibers anywhere between the site of injury and the spinal cord, but within the sensory dermatome (Figure 6-6). Massage, especially back massage, has additional analgesic benefits; it has been shown to promote relaxation and sleep, both of which can influence patient’s responses to pain.
Distraction techniques such as music, conversation, television viewing, laughter, and deep breathing for relaxation can be valuable adjuncts to pharmacologic modalities. These techniques produce their analgesic effects by sending intense stimuli through the thalamus, midbrain, and brain stem which can increase the production of modulating substances such as endorphins. Also, because the brain can process only a limited amount of incoming signals at any given time, the input provided by distraction techniques competes with nociceptive inputs. This is particularly true for the reticular activating system.
When planning for and using distraction techniques, keep in mind that they are most effective when activities are interesting to the patient (eg, their favorite type of music, television program, or video) and when they involve multiple senses such as hearing, vision, touch, and movement. Activities should be consistent with patient’s energy levels and, most of all, be flexible to meet changing demands.
Imagery is another technique that can be used effectively with acutely ill patients, particularly during planned procedures. Imagery alters the perception of pain stimuli within the brain, promotes relaxation, and increases the production of endorphins in the brain. Patients can use imagery independently or use guided imagery where either a care provider, family member, or friend helps guide the patient in painting an imaginary picture. The more details that can be pictured with the image, the more effective it can be. As with distraction techniques, tapping into multiple sensations is beneficial. Some patients prefer to involve the pain in their picture and imagine it melting or fading away. Other patients may prefer to paint a picture in their mind of a favorite place or activity. Strategies to help guide patients include the use of details to describe the imaginary scene (eg, “smell the fresh scent of the ocean air” or “see the intense red hue of the sun setting beyond the snow-capped mountains”) and the use of relaxing sensory terms such as floating, smooth, dissolving, lighter, or melting. If the patients are able to talk, it can be helpful to have them describe the image they see using appropriate detail, although some patients will prefer not to talk and instead focus on their evolving image. Again, it is important to be flexible in the approach to imagery to maximize its benefits.
Because acutely ill patients experience numerous stressors, most patients benefit from the inclusion of relaxation or anxiolytic therapies. The use of relaxation techniques can help interrupt the vicious cycle involving pain, anxiety, and muscle tension that often develops when pain goes unrelieved. The physiologic response associated with relaxation includes decreased oxygen consumption, respiratory rate, heart rate, and muscle tension; blood pressure may either normalize or decrease.
A wide variety of pharmacologic and nonpharmacologic techniques can be used safely and effectively with progressive care patients to achieve relaxation and/or sedation. Relaxation techniques are simple to use and can be particularly useful in situations involving brief procedures such as turning or minor dressing changes, and following coughing or endotracheal suctioning or other stressful events.
Guided deep breathing and progressive relaxation can be incorporated easily into a plan of care for the progressive care patient. Nurses can coach patients with deep breathing exercises by helping them to focus on and guide their breathing patterns. As patients begin to control their breathing, nurses can work with them to begin progressive relaxation of their muscles. To do this, the nurse can say to the patient as he or she just begins to exhale, “Now begin to relax, from the top of your head to the tips of your toes.” Change the pitch of the voice to be higher for “top of your head,” lower for “tips of your toes,” and be timed such that the final phrase ends as the patient completes exhalation. This procedure capitalizes on the positive aspects of normal body functions, as the body tends to relax naturally during exhalation. This process can and should be practiced during nonstressful periods to augment its efficacy. In fact, teaching and coaching patients to use deep breathing exercises helps equip them with a lifelong skill that can be used any time stressful or painful situations arise.
Probably the single most important aspect of promoting comfort in the ill or injured is the underlying relationship between the patient, the family, and his or her care providers. Family presence at the patient’s bedside has been shown to decrease anxiety and promote healing. Including the people identified by the patient as his or her family support (with a broad definition of family) can provide enormous comfort for the patient resulting in relaxation. Presence not only refers to physically “being there,” but also refers to psychologically “being with” a patient. Although presence has not been well-defined as an intervention protocol, patients regularly describe the importance of the support that their nurses render simply by “being there” and “being with” them.
The pain experience of elderly patients has often been shadowed by myths and misperceptions. Some believe that older patients have less pain because their extensive life experiences have equipped them to cope with discomfort more effectively. This may be true for some individuals—to accept this generalization as truth for all elderly patients is short sighted. In fact, the incidence of and morbidity associated with pain is higher in the elderly than in the general population. Many elderly patients continue to experience chronic pain in addition to any acute pain associated with their illness or injury. Major sources of underlying pain in the elderly include low back pain, arthritis, headache, chest pain, and neuropathies.
Elderly patients often report pain very differently from younger patients due to physiologic, psychological, and cultural changes accompanying age. Some patients may fear loss of control, loss of independence, or being labeled as a “bad patient” if they report pain-related concerns. Also, for some patients the presence of pain may be symbolic of impending death, especially in the acute care setting. In such cases, a patient may be reticent to report his or her pain to a care provider or family member as if to deny pain is to deny death. For reasons such as these, it is important for nurses not only to assure patients about the nature of their pain and the importance of reporting any discomfort. Nurses may also use a variety of pain assessment strategies to incorporate behavioral or physiologic indicators of pain.
Similar strategies are often needed to assess pain in persons who are cognitively impaired. Preliminary reports from ongoing work among nursing home patients suggest that many patients with moderate to severe cognitive impairment are able to report acute pain reliably at the time they are asked. For these patients, pain recall and integration of pain experience over time may be less reliable.
Acutely ill elderly patients can benefit from any of the analgesic therapies discussed. Older patients can tolerate opioids well if the doses are individualized and the patient is monitored for effect. However, it is important to recognize that medication requirements may be reduced in some elderly patients due to age-related renal insufficiency and the potential for decreased renal clearance of the drugs. In addition, they have a reduced muscle to body fat ratio which affects the way that opioids bind and activate in the body. Analgesic requirements are highly individualized and doses should be carefully titrated to achieve pain relief.
The progressive care environment can be uncomfortable and anxiety provoking for patients. Once pain is addressed, anxiolysis may be appropriate to enhance comfort, decrease anxiety, reduce awareness of noxious stimuli, and induce sleep. In some cases, the use of sedatives may be necessary to ensure tolerance of medical modalities, clinical stability, and to protect patients from inadvertent self-harm. While the treatment of anxiety is an important aspect of the care of patients who are acutely ill, frequent dosing methods (infusions or IV boluses) intended to induce a more depressed sensorium (ie, amnesia) in these patients is discouraged. The use of sedation infusions in mechanically-ventilated patients has been associated with negative outcomes such as prolonged mechanical ventilation, increased lengths of stay, and even death. To that end, in critical care and progressive care units where mechanically-ventilated patients are cared for, nurses must focus on how best to minimize infusion use. Daily interruptions of sedation infusions have been associated with improved outcomes and do not appear to incur additional psychological stress. This finding is in direct opposition to the commonly held philosophy that amnesia protects the patient from the psychological stress induced by critical or acute care environments. Further, there is a strong association between sedation infusion use and delirium. Compounding the issue is the fact that those who develop delirium are then at risk for the development of long-term cognitive dysfunction. Because of this finding and other studies demonstrating the positive effect of less sedation use in ventilated patients, recent Society of Critical Care Medicine (SCCM) evidence-based guidelines recommend treating pain first, light sedation if necessary, and the use of nonpharmacologic means of promoting sleep. To ensure that appropriate and adequate anxiolysis is achieved in the acutely ill patient, the nurse must be able to identify the reason for sedation, the drugs most commonly used, the level of sedation required, and how to monitor and manage the sedated patient. Clear identification of the reason for sedation is the first step in the process.
One of the most common reasons for sedative use is to ensure amnesia. Many of the procedures and interventions performed in acute care units may potentially cause pain and anxiety. In anticipation of this, sedatives are proactively administered, often concomitantly with analgesics. Moderate sedation (also called conscious sedation) is commonly employed in these situations; it is discussed later in this chapter.
Ineffective, dyssynchronous, and excessive respiratory effort results in increased work of breathing and oxygen consumption. The reason for the dyssynchronous breathing should be quickly assessed and managed. Efforts should be made to improve tolerance by first treating potential pain and adjusting the ventilator to optimize patient-ventilator interaction. But sedative use in severe cases of patient/ventilator dyssynchrony may also be necessary, and in some cases, lifesaving. These patients are generally transferred to a critical care unit for care management. For more in-depth information on advanced modes of ventilation see Chapter 20 in the AACN Essentials of Critical Care Nursing, 3rd edition.
Anxiety and fear are symptoms that can be experienced by acutely ill patients. However, these symptoms may be difficult to assess in acutely ill patients, especially if they are unable to adequately communicate their feelings secondary to the underlying condition, the presence of an artificial airway, or a reduced sensorium.
When the patient can identify anxiety or fear, the treatment goals are clear. However, in the patient who cannot, the presence of behaviors and signs that are associated with anxiety and/or fear are used as evidence and are the reason sedatives are provided. Manifestations of severe anxiety and/or fear include nonspecific signs of distress such as agitation, thrashing, diaphoresis, facial grimacing, blood pressure elevation, and increased heart rate. These nonspecific signs may also be indicative of pain or may be due to delirium. Pain management requirements must be addressed prior to the administration of sedation in such cases as well as assessing the patient for potential delirium.
Agitation includes any activity that appears unhelpful or potentially harmful to the patient. The patient may be aware of the activity and be able to communicate the reason for the activity; more commonly they are not aware, making it difficult to identify the reason for the agitation. The patient appears distressed and the associated activity includes episodic or continuous nonpurposeful movements in the bed, severe thrashing, attempts to remove tubes, efforts to get out of bed, or other behaviors which may threaten patient or staff safety. Reasons for agitation include pain and anxiety, delirium, preexisting conditions that require pharmacologic interventions (ie, preexisting psychiatric history), withdrawal from certain medications such as benzodiazepines (especially if they have been on them for a long time), and delirium tremens secondary to alcohol withdrawal (see Chapter 11 Multisystem Problems, section on alcohol withdrawal). Patients who experience inadequately controlled agitation face a high risk of morbidity and mortality. Thus potential reasons for the agitation are explored so that appropriate therapy may be initiated.
Sleep deprivation is common among hospitalized patients. Although patients may appear restful, physiologically they may never experience stages of sleep that ensure a “rested” state (ie, rapid eye movement sleep, and stages 2, 3, and 4). These restorative stages of sleep are adversely affected by many factors, including a wide variety of medications. Sleep deprivation is also common among those with pain, discomfort, and anxiety. Additionally, sleep deprivation may be a result of the increased auditory, tactile, and visual stimuli ubiquitous to the hospital environment. The SCCM guidelines recommend the use of nonpharmacologic interventions when possible.
Delirium is more prevalent in acutely ill patients than previously assumed. Patients are especially at risk if they have been on sedatives, especially by infusion for longer than 24 hours, are elderly, have preexisting dementia, a history of hypertension, and high severity of illness at admission. Coma is an independent risk factor for the development of delirium. As noted earlier, the risk of long-term cognitive dysfunction is increased in patients who experience delirium. In the past delirium was commonly associated with agitation. In fact, the agitated presentation of delirium accounts for less than 5% of those who experience the condition. The remainder presents with the hypoactive (calm, quiet) or mixed presentation of the condition. This hypoactive category is underdiagnosed and the associated outcomes are worse than for those with the agitated/active form of delirium. The hallmarks of the condition are disorientation and disorganized thinking. Awareness of the potential for delirium and early recognition are essential for effective management and prevention of undesirable outcomes. Routine assessment of delirium should be done with the use of a valid and reliable delirium-monitoring tool such as the confusion assessment method for the ICU (CAM-ICU) that many acute care units are increasingly adopting for use in selected patients. The drugs of choice for delirium in the acute care setting are described later in this chapter under “Drugs for Delirium” and further discussed in Chapter 7 Pharmacology.
After ensuring that the presence of pain is either ruled out or addressed with the appropriate administration of analgesics, sedatives may be selected based on patient-specific factors such as the level and duration of sedation required. Sedative category summaries follow and comprehensive descriptions of the drugs are discussed in Chapter 7 Pharmacology.
These sedatives have a rapid onset of action and a short duration of effect.
• Midazolam is a popular benzodiazepine that fits in this category. It can be administered intermittently in a bolus IV form or as a continuous infusion. Generally continuous infusions of midazolam are reserved for the critical care unit.
• Ketamine is an IV general anesthetic that produces analgesia, anesthesia, and amnesia without loss of consciousness. It may be given in an IV bolus form, intranasally, or orally. Although contraindicated in those with elevated intracranial pressure, its bronchodilatory properties make it a good choice in those with asthma. A well-known side effect of ketamine is hallucinations; however, these may be prevented with concurrent use of benzodiazepines. It is rarely a first-line sedative of choice, but is commonly used in patients requiring painful, frequent skin debridement procedures (eg, burn patients). The nurse needs to be aware of the hospital policy for use of this medication as some hospitals limit it to physician use only and/or use in ICUs.
These drugs have an intermediate onset of action and duration of effect. However, when given as infusions they may last much longer as they are lipophilic. Generally, continuous infusions of the drug are reserved for the critical care unit.
• Lorazepam is the most common benzodiazepine in acute care and can be administered orally and IV as an intermittent bolus or continuous infusion. When given orally or in a bolus intermittent form, the drug effect is intermediate; however, when used as a continuous infusion (24 hours), its effect is more long term (and it should be considered as such) because awakening may take hours to days to accomplish. Lorazepam, if given frequently or by infusion, may accumulate in those with decreased metabolic function such as the elderly or those with hepatic dysfunction.
• Diazepam, a long acting benzodiazepine, and chlordiazepoxide are infrequently used in acute care; however, they may be selected for treatment of severe alcohol withdrawal. They may be given orally or as an IV bolus.
In the past, the drug of choice for treatment of delirium was haloperidol. However, no evidence supports the use of haloperidol as a pharmacologic agent to reduce the duration of delirium. The drug has been popular in the past because it sedates without significant respiratory depression and is not associated with potential development of tolerance or dependence. It does, however, have potential adverse side effects that must be closely monitored. Extrapyramidal reactions such as dystonia and the potential for neuroleptic malignant syndrome are possible. Another is the effect of haloperidol on QTc intervals; QTc interval monitoring is essential and required when using the drug. Atypical antipsychotics such as risperidone and olanzapine have been used, but little data exist to support widespread use of the drugs for the treatment of delirium. Critical care units are now encouraged to decrease benzodiazepine use due to the association of the drugs with delirium. It is hoped that this will result in less delirium in those transferred to progressive care units as well. In addition, the use of nonpharmacologic interventions may be helpful in decreasing the incidence of delirium in acute care (see Chapter 7 Pharmacology for more on these classes of drugs).
The goal of sedation administration is important to identify (anxiety, sleep, ventilator tolerance, amnesia, etc) and once accomplished an appropriate level of sedation may be determined. When the sedation goal is to produce deep sedation, as in the case of an agitated, ventilated patient with oxygenation problems, intubation and ventilation may be necessary and the patient may need to be transferred to a critical care unit for vigilant monitoring. Monitoring of the patient’s sedation level is often accomplished using sedation scales. Sedation scales have been developed in an effort to assist with the management of sedation in these cases, especially if the sedation requirements are anticipated to last for longer than a few hours. The SCCM evidence-based guidelines recommend the use of two tested and reliable sedation scales that may be used for these patients (see Table 6-6).
A technique referred to as “moderate sedation” (also known as conscious sedation) is common in progressive, acute care, and special procedure units (interventional radiology, endoscopy, etc). It refers to the use of a combination of analgesics and sedatives to minimize discomfort during a procedure while assuring that the patient is able to communicate throughout the procedure. Amnesia is anticipated and often desired. The patient’s ability to maintain a patent airway is central to the decision related to the use of moderate sedation. Patients considered “lowest risk” are generally those who are recommended for the technique, although higher risk individuals may also be recommended based on consultation with the healthcare team. The American Society of Anesthesiology Patient Classification Status is used to determine level of risk (Table 6-7). Institutional guidelines for the use of moderate sedation vary somewhat however, they generally include the use of continuous real-time monitoring such as respiratory rate and pattern, pulse oximetry and heart rhythm in addition to very frequent (ie, every 5 minutes during the procedure) assessment of vitals signs and evaluation of level of consciousness.
TABLE 6-7. ASA Continuum of Depth of Sedation: Definition of General Anesthesia and Levels of Sedation/Analgesia
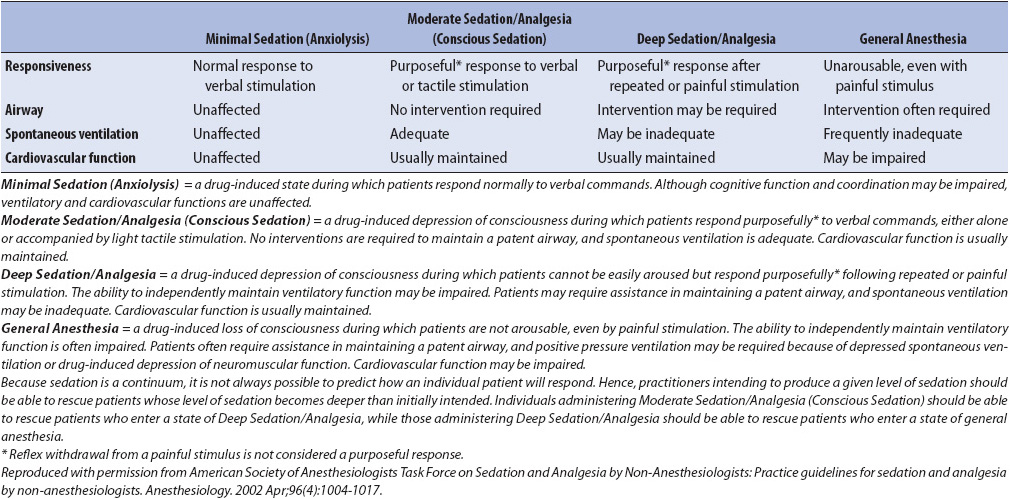
In contrast, there are times when the sedation goal is to produce deeper sedation as in the case of an agitated ventilated patient with oxygenation problems. Sedation scales have been developed in an effort to assist with the management of sedation in these cases, especially if the sedation requirements are anticipated to last for longer than a few hours.
Sedation scales allow the nurse to select a level of sedation for the patient in collaboration with the healthcare team. Descriptors of each level of sedation are provided so that the sedative may be adjusted appropriately. When patients in the progressive care areas require aggressive sedation management (eg, for ventilator intolerance), the scales noted above may be helpful. Sedation monitoring in these cases is done at least hourly and the level of sedation achieved is recorded.
Management of sedation is an essential step in attaining positive outcomes for acutely ill patients. Patients may require sedatives for the treatment of mild to moderate anxiety while in the progressive care unit. Treatment of such anxiety is appropriate and rarely results in adverse effects. Generally the sedatives are provided orally and occasionally as an IV bolus. The doses are adjusted to prevent excessive drowsiness or respiratory depression. Appropriately dosed, use of the sedatives does not interfere with clinical progress such as weaning or rehabilitation. In contrast, it is especially important to consider the effects associated with sedation infusions on outcomes. Generally if a sedation infusion is used in a progressive care unit, its use is short lived and the patient is converted to PO or IV bolus sedation as soon as possible.
In patients who require high levels of sedation to prevent self-harm, sedation infusions and/or frequent IV bolus sedation may be essential. These patients may need to be transferred to a critical care unit if the condition persists.
American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 6th ed. Glenview, IL: American Pain Society; 2008.
American Society of Pain Management Nursing. Core Curriculum for Pain Management Nursing. Dubuque IA: Hunt Publishing; 2009.
Barr J, Fraser G, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306.
Barthelmey O, Limbourg T, Collet J, et al. Impact of non-steroidal anti-inflammatory drugs (NSAIDs) on cardiovascular outcomes in patients with stable atherothrombosis or multiple risk factors. Int J Cardiol. June 28, 2011.
Bavry A, Khaliq A, Gong Y, Handberg E, Cooper-DeHoff R, Pepine C. Harmful effects of NSAIDs among patients with hypertension and coronary artery disease. Am J Med. 2011;124:614-620.
Bennett JS, Daugherty A, Herrington D, Greeneland P, Roberts H, Taubert K. The use of non-steroidal anti-inflammatory drugs (NSAIDs): a science advisory from the American Heart Association. Circulation. 2005;111(13):1713-1716.
Berry P, Covington E, Dahl J, Katz J, Miaskowski C. Pain: current understanding of assessment, management, and treatments. Reston VA: National Pharmaceutical Council, Inc., and the Joint Commission on Accreditation of Healthcare Organizations. 2006.
D’Arcy Y. A Compact Clinical Guide to Acute Pain Management. New York, NY: Springer Publishing, 2011.
Faucett J. Care of the critically ill patient in pain: the importance of nursing. In: Puntillo KA, ed. Pain in the Critically Ill. Gaithersburg, MD: Aspen;1991.
Fine P, Portenoy R. A Clinical Guide to Opioid Analgesia New York, NY: Vendome Group LLC, 2007.
Gardner DL. Presence. In: Bulechek GM, McCloskey JC, eds. Nursing Interventions: Essential Nursing Treatments. Philadelphia, PA: WB Saunders; 1992:316-324.
Gordon DB, Dahl J, Phillips P, et al. The use of “as-needed” range orders for opioid analgesics in the management of acute pain: a consensus statement of the American Society for Pain Management Nursing and the American Pain Society. Pain Manag Nurs. 2004;5:53-58.
Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203-210.
Khatta M. A complementary approach to pain management. Adv Pract Nurs. 2007. Available at www.medscape.com.
Marmo L, D’Arcy Y. A Compact Clinical Guide to Critical Care, ER, and Trauma Pain Management. New York, NY: Springer Publishing, 2013.
Maxam-Moore VA, Wilkie DJ, Woods SL. Analgesics for cardiac surgery patients in critical care: describing current practice. Am J Crit Care. 1994;3:31-39.
Melton S, Liu S. Regional anesthesia techniques. In: S Fishman, J Ballantyne, J Rathmell (eds). Bonica’s Management of Pain, 5th ed, Philadelphia PA: Lippincott Williams and Wilkins, 2010, 92-106.
Morrison RS, Ahronheim JC, Morrison GR, et al. Pain and discomfort associated with common hospital procedures and experiences. J Pain Symptom Manage. 1998, 15:91-101.
Pasternak GW. Molecular biology of opioid analgesia. J Pain Symptom Manag. 2005;29(5S):S2-S9.
Pettigrew J. Intensive nursing care: the ministry of presence. Crit Care Nurs Clin North Am. 1990;2(3):503-508.
Puntillo K. Advances in management of acute pain: great strides or tiny footsteps? Capsules comments. Crit Care Nurs. 1995; 3:97-100.
Puntillo K. Pain experience in intensive care patients. Heart Lung. 1990;19:526-533.
Puntillo K, Weiss SJ. Pain: its mediators and associated morbidity in critically ill cardiovascular surgical patients. Nurs Res. 1994;43:31-36.
Puntillo KA, Morris AB, Thompson CL, et al. Pain behaviors observed during six common procedures: results from Thunder Project II. Crit Care Med. 2004;32(2):421-427.
Puntillo KA, White C, Morris AB, et al. Patients’ perceptions and responses to procedural pain: results from Thunder Project II. Am J Crit Care. 2001;10(4):238-251.
Puntillo KA, Wild LR, Morris AB, et al. Practices and predictors of analgesic interventions for adults undergoing painful procedures. Am J Crit Care. 2002;11(5):415-429.
Puntillo KA, Wilke DJ. Assessment of pain in the critically ill. In: Puntillo KA, ed. Pain in the Critically Ill. Gaithersburg, MD: Aspen; 1991:45-64.
Richman J, Liu S, Courpas C, Wong R, Rowlinson A. McGready J Wu C. Does peripheral nerve block provide superior pain control to opioids? A metanalysis. Anesth and Analg. 2006;102(1):248-257.
Rose L, Smith O, Gelinas C, et al. Critical care nurses’ pain assessment and management practices: a survey in Canada. Am J Crit Care. 2012;21(4):151-259.
Schulz-Stübner S, Boezaart A, Hata JS. Regional analgesia in the critically ill. Crit Care Med. 2005;33:1400-1407.
Stanik-Hutt JA, Soeken KL, Belcher AE, Fontaine DK, Gift AG. Pain experiences of traumatically injured patients in a critical care setting. Am J Crit Care. 2001;10:252-259.
Summer G, Puntillo K. Management of surgical and procedural pain in the critical care setting. Crit Care Clin North Am. 2001;13:233-242.
Sun X, Weissman C. The use of analgesics and sedatives in critically ill patients: physicians’ orders versus medications administered. Heart Lung. 1994;23:169-176.
Thompson C, White C, Wild L, et al. Translating research into practice. Crit Care Nurs Clin North Am. 2001;13:541-546.
Tittle M, McMillan SC. Pain and pain-related side effects in an ICU and on a surgical unit: nurses’ management. Am J Crit Care. 1994;3:25-39.
Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9-17.
Wu CL, Cohen SR, Richman JM, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103:1079-1088.
Brook AD, Ahrens TS, Schaff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27:2609-2615.
Ely W, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;22 (289): 2983-2991.
Girard TD, Pandharipande PP, Ely EW. Review: delirium in the intensive care unit. Crit Care. 2008;12(suppl 3):1-9.
Kress JP, Gehlbach B, Lacy M, et al. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168:1457-1461.
Kress JP, Pohlman A, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-1477.
Kress JP, Pohlman AS, Hall JB. Sedation and analgesia in the intensive care unit. Am J Respir Crit Care Med. 2002;166:1024-1028.
Riker R, Picard J, Fraser G. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999; 27:1325-1329.
Sessler C, Gosnet M, Grap MJ. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338-1344.
American Geriatric Society (AGS). The pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331-1346.
American Society of Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists: an updated report by the American Society of Anesthesiologists Task Force on Sedation and Analgesia by non-Anesthesiologists. Anesthesiology. 2002; 96: 1004-1017.
American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156:1-20.
American Society of Anesthesiologists Taskforce on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting. Anesthesiology, 2004;100(6):1573-1581.
Barr J, Fraser G, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306.
Herr K, Coyne P, Kry T, et al. Pain assessment in the nonverbal patient: position statement with clinical practice recommendations. Pain Manage Nurs. 2006;7(2):44-52.
Woods S. Spiritual and complementary therapies to promote healing and reduce stress. In: Molter NC, ed. AACN’s Protocols for Practice: Creating Healing Environments. 2nd ed. Sudbury, MA: Jones and Bartlett Publishers; 2007.