20
ADVANCED NEUROLOGIC CONCEPTS
Dea Mahanes
KNOWLEDGE COMPETENCIES
1. Compare and contrast the pathophysiology, clinical presentation, patient needs, and management approaches for the following conditions:
• Subarachnoid hemorrhage
• Traumatic brain injury
• Acute spinal cord injury
• Brain tumor
2. Describe intracranial monitoring technology and implications for nursing care.
3. Describe the use of lumbar drainage of cerebrospinal fluid and implications for nursing care.
SUBARACHNOID HEMORRHAGE
Etiology, Risk Factors, and Pathophysiology
Subarachnoid hemorrhage (SAH) can result from trauma, aneurysm, or other vascular malformations. This discussion focuses on SAH due to the rupture of an intracranial aneurysm (aSAH). Intracranial aneurysms usually occur in the circle of Willis at arterial bifurcations or trifurcations (Figure 20-1). Aneurysms vary in size and shape; saccular (also called berry) aneurysms are the most common and most amenable to treatment. When an intracranial aneurysm ruptures, blood is forcibly expelled into the subarachnoid space and coats the brain surfaces. A clot may form in the ventricular system or in the brain parenchyma. In some patients, blood in the subarachnoid space causes hydrocephalus by obstructing cerebrospinal fluid (CSF) flow through the ventricles or clogging the arachnoid granulations that absorb CSF. Although the mechanism is not well-understood, arterial narrowing (commonly referred to as “vasospasm”) occurs in a significant number of patients in the days following aneurysm rupture and can cause delayed cerebral ischemia. There are several scales used to grade the severity of aneurysmal subarachnoid hemorrhage (aSAH). The Hunt and Hess scale (Table 20-1) is commonly used in the nursing literature.

Figure 20-1. The circle of Willis as seen from below the brain. (Reprinted from: Perry L, Sands JK. Vascular and degenerative problems of the brain. In: Phipps WJ, Marek JF, Monahan FD, Neighbors M, Sands JK, eds. Medical-Surgical Nursing: Health and Illness Perspectives. St Louis, MO: Mosby; 2003:1365.)
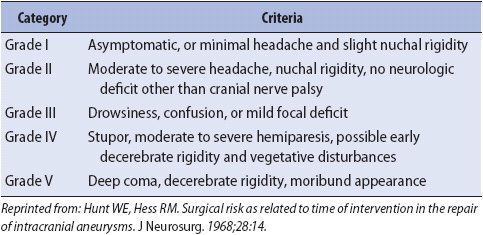
TABLE 20-1. HUNT AND HESS SCALE FOR THE CLASSIFICATION OF PATIENTS WITH INTRACRANIAL ANEURYSMS
Risk factors for intracranial aneurysm formation include smoking, hypertension, family history of intracranial aneurysm, and certain genetic disorders (autosomal dominant polycystic kidney disease, Ehlers-Danlos syndrome). Twenty percent of patients have multiple aneurysms. Risk factors associated with aneurysm rupture include size of the aneurysm, hypertension, smoking, age (risk increases with age, peaking at age 50-60), and the use of stimulants (cocaine, amphetamines). Aneurysmal SAH is more common in men until the age of 50; the incidence is higher in women after age 50 and in the overall population.
Mortality and morbidity associated with aSAH is substantial. Approximately one-third of individuals with aSAH will die either at the time of rupture or during hospitalization. Many survivors are left with significant disability. Predictors of outcome after aSAH include neurologic condition on admission, age, comorbidities, and the amount of blood on the initial CT scan.
Clinical Presentation
Most patients are asymptomatic until the time of aneurysm rupture, but some have prodromal signs such as headache and visual changes. Upon aneurysm rupture, many patients experience a sudden, severe headache, sometimes described as “explosive” or “the worst headache of my life.” Transient or prolonged loss of consciousness can occur. Bystanders may describe seizure-like activity; it is unclear whether this is an actual seizure or abnormal posturing related to a sudden increase in intracranial pressure (ICP). Other common signs and symptoms include nausea and vomiting, stiff neck, blurred vision, mental status changes, and photophobia. Focal deficits, such as hemiparesis, hemiplegia, or aphasia, may also occur.
Diagnostic Tests
Computerized Tomography Scan
A CT scan is used to determine whether subarachnoid hemorrhage has occurred and to assess for hydrocephalus. CT scan will detect subarachnoid blood in almost all patients if performed within the first three days of symptom onset. As the blood in the subarachnoid space starts to break down, the sensitivity of the CT scan decreases. CT angiography (see Chapter 12, Neurologic System) can be performed quickly at the time of the initial scan and may reveal aneurysm location. The amount of blood present on the initial CT scan is predictive of vasospasm risk.
Lumbar Puncture
A lumbar puncture (LP) is performed when CT fails to demonstrate SAH in a patient with a history highly suspicious for SAH. LP is avoided in patients with signs or symptoms of increased ICP due to the risk of herniation. LP is performed at least 6 to 12 hours after the onset of symptoms to allow red blood cells (RBCs) in the CSF to start to break down. This breakdown in RBCs gives a yellow tinge to the CSF after centrifugation. This pigmentation is called xanthochromia and will not be present if blood in the CSF is due to a traumatic LP.
Cerebral Angiography
Although CTA done at the time of the initial CT scan detects many aneurysms, cerebral angiography (catheter angiography) remains the gold standard to identify the location, size, and shape of the aneurysm or other vascular anomalies. The initial angiogram will not reveal an aneurysm in approximately 10% to 20% of patients with SAH. If no aneurysm is seen on the first angiogram, a repeat angiogram after approximately a week will reveal an aneurysm in a small number of these patients. Negative angiogram and a distinct pattern of bleeding on CT scan may also indicate a nonaneurysmal perimesencephalic SAH; patients with this diagnosis have an excellent prognosis. In many patients in whom an aneurysm is detected, angiogram is used to guide endovascular treatment (described later). Angiogram is also used to detect and treat arterial narrowing in patients with neurological decline in the days following aneurysm rupture.
Magnetic Resonance Imaging and Magnetic Resonance Angiography
Magnetic resonance imaging and magnetic resonance angiography (MRA) are used to identify aneurysm location and look for other vascular abnormalities. These studies are especially useful in patients with a negative CT or negative angiogram.
Principles of Management of Aneurysmal Subarachnoid Hemorrhage
Patients who survive the initial rupture of a cerebral aneurysm are at risk for complications that increase their chances for morbidity and death. Primary central nervous system (CNS) complications include rebleeding, hydrocephalus, and delayed cerebral ischemic (DCI) due to arterial narrowing. Arterial narrowing correlates temporally with the breakdown of subarachnoid blood and is due to a combination of arterial spasm and inflammatory changes that thicken the vessel wall. This phenomenon is commonly referred to as “vasospasm.” Although “vasospasm” reflects an incomplete understanding of the pathophysiology leading to DCI, this terminology is commonly used in practice and will be used in this text to reflect arterial narrowing after aSAH.
Rebleeding
Prior to the aneurysm being secured, the biggest risk to the patient is that the aneurysm will bleed again. This risk is highest within the first 24 hours. The probability of death is markedly increased by rebleed. Signs and symptoms of rebleeding include a sudden increase in headache, nausea, and vomiting, decrease in the level of consciousness, and new focal neurologic deficits. The most definitive method to prevent rebleeding is to secure the aneurysm using surgical clipping or endovascular embolization.
In the interim between admission and definitive treatment, strategies such as blood pressure management and prevention of activities that increase blood pressure or ICP are used to decrease the risk of rebleeding. The goal is to treat hypertension without dropping the blood pressure to a level that decreases cerebral perfusion. Systolic BP goals with an upper range of 150 to 160 mm Hg are common. The use of a titratable agent such as nicardipine is recommended.
Bed rest is typically ordered but may be adapted with physician approval to meet patient needs (eg, a patient who becomes anxious and hypertensive when using a bedpan may be allowed to use a bedside commode). Prophylaxis for DVT, including graduated compression stockings and pneumatic compression devices, is implemented. Stool softeners are used to prevent straining due to constipation. Pain is treated with analgesics, usually short-acting narcotics. A calm, quiet environment is maintained. Anxiety is reduced through explanations of care and psychological support. Neurologic assessment is performed hourly (or more frequently if indicated) to promptly identify neurologic changes so that rapid intervention can occur.
Two management options exist to secure the aneurysm and prevent another rupture: surgical clipping of the aneurysm via craniotomy and endovascular embolization of the aneurysm via catheter angiography. Management at a facility that offers both treatment modalities and frequently treats patients with aSAH is recommended to optimize outcomes. The decision to use surgery vs an endovascular procedure is made on the basis of aneurysm location and morphology, comorbidities, and the severity of neurologic deficits on admission. When the aneurysm is amenable to treatment by either modality, endovascular management is generally performed.
The aneurysm is secured as soon as possible, prior to the period of time when patients are most at risk for vasospasm. With the aneurysm secured, standard management strategies for vasospasm can be implemented without the risk of causing additional hemorrhage. Aneurysm surgery is performed via a craniotomy incision. The surgeon carefully dissects tissue away from the aneurysm and places a titanium or titanium alloy clip across the base (Figure 20-2). Different sizes and shapes of clips are available. Following surgery, the patient is initially admitted to the ICU for management. Follow-up radiologic studies may be done, including CT scanning to look for bleeding at the operative site and angiography to evaluate clip position.
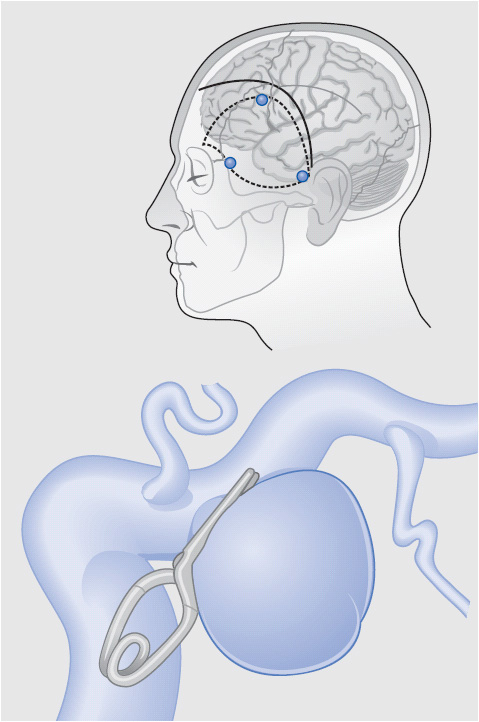
Figure 20-2. Clipping of a posterior communicating artery aneurysm. The clip is placed across the base of the aneurysm so that it can no longer fill with blood, but blood flow can continue through the parent artery. (From: Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928-939.)
Endovascular embolization decreases rebleeding risk by preventing blood flow into the aneurysm. Using cerebral angiography, the interventional radiologist threads a wire with a helical platinum coil at the end into the cerebral vasculature. The coil is manipulated into the body of the aneurysm and detached from the wire using a small electrical current. The neck of the aneurysm must be narrow enough for the coils to be retained in the aneurysm instead of floating back out into the vessel lumen. Figure 20-3 depicts endovascular coil embolization of an aneurysm with a narrow neck (berry or saccular aneurysm). If the neck is wide, special stents may be used to assist with coiling or to span the aneurysm. Multiple coils may be needed to completely fill the aneurysm. The coils cause the aneurysm to clot, preventing blood flow into the aneurysm and decreasing the likelihood of rebleed. The primary risks associated with coil embolization are aneurysmal rupture during the procedure and ischemia related to clot formation in the vessel lumen.
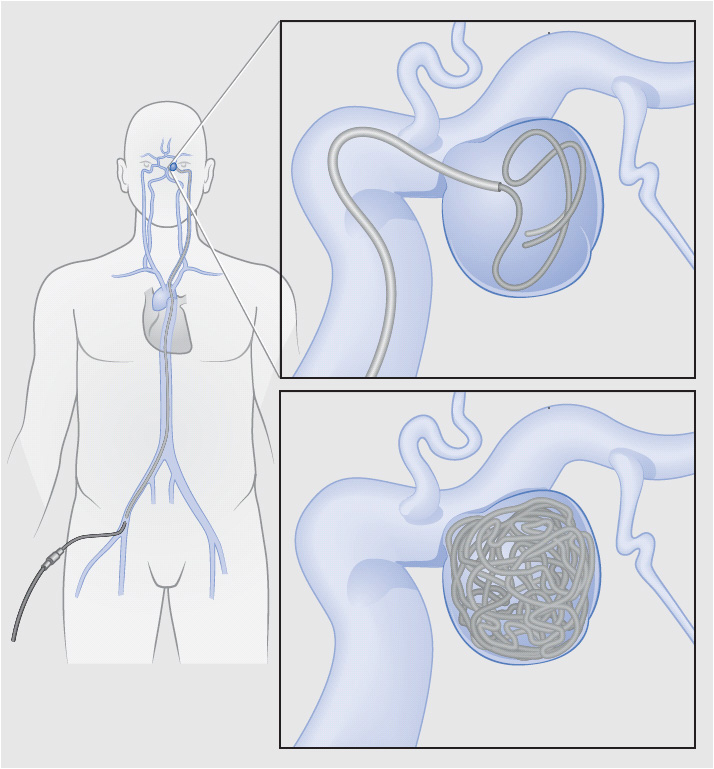
Figure 20-3. Coil embolization of a posterior communicating artery aneurysm. Coils are fed into the aneurysm via a micro-catheter routed from the femoral artery into the cerebral circulation. The coils are then detached into the aneurysm. Multiple coils may be required to occlude the aneurysm. (From: Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928-939.)
Patients with SAH return to the ICU after aneurysm clipping or endovascular treatment. At some institutions, neurologically stable patients without other ICU needs may be transferred to a specialized neuro progressive care unit after 24 to 48 hours to be monitored for vasospasm and other complications.
Hydrocephalus
Subarachnoid hemorrhage disrupts normal CSF flow through two mechanisms. Intraventricular blood may create a blockage in the ventricular drainage system and cause CSF to build up (obstructive or noncommunicating hydrocephalus). In addition, the arachnoid granulations that absorb CSF may become blocked with cellular debris. This results in decreased reabsorption of CSF and communicating hydrocephalus. Signs and symptoms of acute hydrocephalus relate to increased ICP. Acute hydrocephalus after SAH is managed by external ventricular drainage (see section on ICP monitoring and management at the end of this chapter). Some patients later require placement of a ventricular shunt due to continued hydrocephalus.
Late or chronic hydrocephalus can develop weeks after SAH. These patients present with incontinence, gait instability, and cognitive decline. Treatment is placement of a ventricular shunt.
Delayed Cerebral Ischemia (DCI) due to Arterial Narrowing (Vasospasm)
Arterial narrowing occurs in many patients after aSAH and may cause decreased perfusion, potentially leading to DCI and infarction of cerebral tissue. As previously noted, although several mechanisms contribute to arterial narrowing, this phenomenon is commonly referred to as “vasospasm” in clinical practice and this term is used throughout this text. Vasospasm develops 4 to 14 days after initial hemorrhage, with peak incidence around day 7, and is the biggest contributor to morbidity and mortality rates in patients with SAH who survive to hospital admission. Approximately one-third of patients with aneurysmal SAH will develop delayed ischemic neurologic deficits due to vasospasm, and another third will have angiographic evidence of arterial narrowing without neurologic decline. The amount of blood on the initial CT scan is a good predictor of the risk of vasospasm and DCI.
At many institutions, transcranial Doppler studies (TCDs, see Chapter 12, Neurologic System) are used to monitor for the development of vasospasm. TCDs assess blood flow velocity in selected arteries, which will become higher as vessels narrow. TCDs are non-invasive and can be done at the bedside, but accuracy varies based on patient and operator characteristics. CT angiography is also used to look for vasospasm but, as with aneurysm diagnosis, catheter angiography remains the gold standard. Vasospasm is suspected in any patient who develops neurologic decline, especially a decrease in level of consciousness, paresis or paralysis of a limb or side of the body, or aphasia. If any neurologic change is detected, the physician is immediately notified. Early identification of neurologic deficits allows rapid intervention to improve perfusion and prevent infarction.
Maintenance of euvolemia is essential to decrease the risk of DCI. Careful attention to fluid balance is important and must include recognition of insensible fluid loss. Dehydration increases blood viscosity and decreases cerebral perfusion. SAH patients are at risk for dehydration because of cerebral salt wasting, in which excessive sodium is excreted, leading to increased water loss and hypovolemia. If serum sodium falls, volume restriction is contraindicated because of increased risk of cerebral ischemic deficits. Infusion of hypertonic saline is often used for treatment of hyponatremia. Nimodipine, a calcium channel blocker, does not significantly decrease angiographic vasospasm but a large trial showed improved outcomes at 3 months after SAH. If blood pressure drops with the standard dosing regimen of 60 mg orally or via gastric tube every 4 hours, the dose can be divided and 30 mg can be given every 2 hours to decrease the impact on cerebral perfusion.
Historically, DCI due to vasospasm was managed with “Triple-H” therapy, which included hypervolemia, hypertension, and hemodilution. Current evidence-based guidelines support maintenance of euvolemia and the use of induced hypertension if vasospasm occurs. Hypertension is induced using intravenous fluids and vasopressors. Blood pressure goals vary based on the patient response but are typically in the range of 160 to 200 mm Hg. If previously transferred to the progressive care unit, patients requiring treatment for vasospasm will be readmitted to the ICU for management. Vasospasm can also be treated using an endovascular approach with transluminal balloon angioplasty or direct infusion of a calcium channel antagonist such as verapamil into the artery in spasm. There are many ongoing clinical trials related to monitoring and treating arterial narrowing and delayed cerebral ischemia after aSAH.
Additional Management Strategies and Prevention of Complications
At some institutions, prophylactic anticonvulsants are given to patients with SAH for short periods (3 to 7 days) immediately following presentation. Patients who demonstrate clinical or electrographic seizures are treated
according to standard seizure management (see Chapter 12,
Neurologic System) and remain on anticonvulsants throughout hospitalization. Systemic complications of SAH include myocardial dysfunction, cardiac arrhythmias, and neurogenic pulmonary edema; these complications typically occur within hours of the initial hemorrhage. Patients are also at risk for complications of immobility such as infection and DVT.
TRAUMATIC BRAIN INJURY
Etiology, Risk Factors, and Pathophysiology
Major causes of traumatic brain injury (TBI) are falls, motor vehicle accidents (MVAs), and acts of violence. Falls are more common in the elderly, and acts of violence are more prevalent in urban areas. The incidence of TBI is higher in males than females and higher in children ages 0 to 4 years, older adolescents (15 to 19 years old), and adults aged
65 years or older. Rates of hospitalization and death are highest in those older than 75 years. TBI ranges from mild (causing a brief change in consciousness) to very severe (causing prolonged unresponsiveness or even death). TBI severity can be classified using the Glasgow Coma Scale score (GCS, see Chapter 12, Neurologic System). Mild brain injury refers to patients with a GCS score of 13 to 15, moderate indicates a GCS score of 9 to 12, and patients with a score of 8 or less are categorized as having severe brain injury. Although higher GCS is associated with better outcomes, a TBI does not have to be severe to cause long-term impact. Even mild TBI can cause significant functional deficits that become apparent in the weeks and months following injury. Patients with mild TBI and an abnormal CT scan may be admitted to the progressive care unit, or patients admitted to the progressive care unit for other injuries may also be diagnosed with mild TBI. Patients with moderate TBI and a stable or improving neurologic examination are admitted directly to the progressive care unit at some institutions. Patients with severe TBI and most patients with moderate TBI are initially cared for in the ICU, and later transfer to the progressive care unit for continued care.
Mechanism of Injury
Traumatic brain injury occurs as the result of blunt trauma (a direct blow to the head), penetrating trauma (missile or impaled object), or blast injury. Blunt injury occurs as a consequence of:
• Deceleration: The head is moving and strikes a stationary object (eg, pavement).
• Acceleration: A moving object (eg, baseball bat) strikes the head.
• Acceleration-deceleration: The brain moves rapidly within the skull, resulting in a combination of injury-causing forces.
• Rotation: Twisting motion of the brain occurs within the skull, usually due to side impact.
• Deformation/compression: Direct injury to the head changes the shape of the skull, resulting in compression of brain tissue.
In the United States, gunshot wound (GSW) is the most common type of penetrating brain trauma. The degree of injury caused by a GSW varies based on the type of firearm, bullet type, and trajectory of the bullet. Tissue is destroyed by the bullet, and shock waves and cavity formation occur along the bullet’s path. Some bullets will ricochet once inside the skull, creating more tissue destruction. Other causes of penetrating brain injury include nail guns and stab wounds. Surgical management of penetrating trauma to the brain differs from the management of closed injury, but many of the issues relevant to progressive care nurses remain the same.
Awareness of TBI due to blast injury caused by an explosion has increased in recent years. The individual may be hit by flying debris or may be thrown by the force of the blast, causing blunt or penetrating trauma. The brain is also thought to be sensitive to the initial overpressurization wave, with damage occurring as the result of the diffuse impact of intense pressure on brain structures.
Skull Fractures
Skull fractures can result in injury to the underlying brain tissue, or may occur in isolation. Skull fractures are classified as linear, depressed, or basilar.
• Linear skull fractures resemble a line or single crack in the skull. Generally, they are not displaced and require no treatment.
• Depressed skull fractures are characterized by an inward depression of bone fragments. Surgery to elevate the depressed bone may be required. In the case of an open fracture, the wound is also washed out in the operating room to decontaminate the area and decrease the risk of infection.
• Basilar skull fractures involve the base of the skull, including the anterior, middle, or posterior fossa. Clinical manifestations of a basilar skull fracture include periorbital ecchymosis (raccoon’s eyes), mastoid ecchymosis (Battle’s sign), rhinorrhea (CSF or blood leaking from the nose), otorrhea (CSF or blood leaking from the ears), hemotympanum (blood behind the tympanic membrane), conjunctival hemorrhage, and cranial nerve dysfunction. The presence of otorrhea or rhinorrhea indicates a dural tear with increased risk of meningitis. Although most CSF leaks stop spontaneously, those that persist may require surgical repair. Management of CSF leak includes elevating the head of bed, antibiotics, and, occasionally, lumbar drainage of CSF to decrease pressure on the healing dura.
Primary Brain Injury
The damage that results from TBI is due to both the primary insult and secondary injury produced by ongoing intracranial and systemic complications. Primary injury can be described as focal (resulting in local damage at the site of injury) or diffuse (affecting the whole brain). Focal injuries take up space, and can cause tissue compression, increased ICP, brain shift, and herniation. Examples of focal injury include cerebral contusions and hematomas. Diffuse brain injuries involve microscopic damage to cells deep in the white matter. They occur as lateral head motion produces angular movement of the brain within the skull, causing shearing or stretching of axonal nerve fibers. Damage is variable and dependent on the amount of accelerative force transmitted to the brain. Focal and diffuse brain injuries do not typically occur in isolation; for example, a patient with a focal cerebral contusion is also likely to have some component of diffuse brain injury.
Examples of primary injury follow.
• Contusion: Contusions are cortical bruises caused by the brain impacting the inside of the skull. They may be described as coup (occurring at the site of impact) or contrecoup (occurring opposite the site of impact). The frontal and temporal lobes are common sites of contusions. Clinical presentation depends on the site and extent of brain injury. Progressive focal edema and mass effect may result in neurologic deterioration. The severity of injury may not be apparent on the initial CT scan, because bleeding into the contused or lacerated tissue often occurs and results in intracerebral hematoma. Repeat CT scanning may be performed to evaluate for injury progression.
• Epidural hematoma: (EDH, Figure 20-4). EDH is a blood clot located between the dura and the skull. EDH is often associated with skull fractures that lacerate an underlying artery, and is most common in the temporal region due to tearing of the middle meningeal artery. Patients may have a lucid interval especially if the injury is very focal (eg, struck by a baseball or other solid object) and then deteriorate rapidly as the clot expands, displacing brain structures and causing increased ICP. While a lucid interval suggests EDH, many patients do not follow this course. Symptoms of EDH include a decrease in consciousness, headache, seizures, vomiting, hemiparesis, and pupillary dilation. Management includes emergency surgery to evacuate the hematoma.
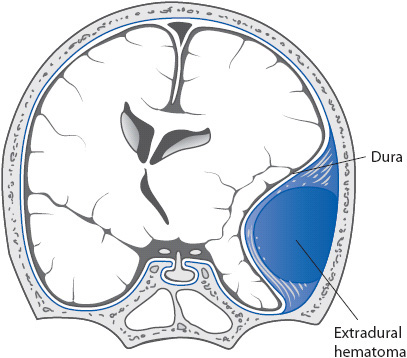
Figure 20-4. Schematic illustration of an epidural hemorrhage. (Reprinted from: Waxman SG. Vascular supply. In: Waxman SG, ed. Clinical Neuroanatomy. New York, NY: Lange Medical Books/McGraw-Hill; 2003:187.)
• Subdural hematoma: (SDH, Figure 20-5.) Bleeding occurs within the subdural space between the dura and arachnoid layer, creating direct pressure on the brain. SDH results from rupture of the bridging veins between the brain and dura, bleeding from contused or lacerated brain tissue, or extension from an intracerebral hematoma. SDH is described as acute if symptoms begin within the first 48 hours after injury. Many patients experience significant symptoms immediately following the injury or much sooner than 48 hours. Patients with acute SDH present with progressive decline in level of consciousness, headache, agitation, and confusion. Motor deficits, pupillary changes, and cranial nerve dysfunction may be seen reflecting the primary brain injury and compressive effects. Treatment of acute SDH consists of evacuation of the hematoma by craniotomy. Blood may also collect in the subdural space more slowly, over days to weeks (subacute SDH) or weeks to months (chronic SDH). The onset of symptoms is insidious because the brain can better compensate for this slow increase in mass. Symptoms include an increasingly severe headache, confusion, drowsiness, and, possibly, seizures, pupillary abnormalities, or motor dysfunction. Predisposing conditions include advanced age, alcoholism, and disorders or treatments that result in prolonged coagulation times. Treatment of subacute or chronic SDH includes evacuation via burr holes or craniotomy.
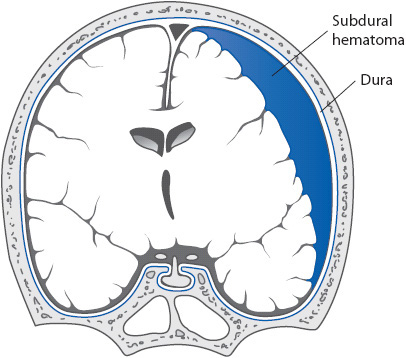
Figure 20-5. Schematic illustration of a subdural hemorrhage. (Reprinted from: Waxman SG. Vascular supply. In: Waxman SG, ed. Clinical Neuroanatomy.
New York, NY: Lange Medical Books/McGraw-Hill; 2003:187.)
• Traumatic subarachnoid hemorrhage: Traumatic SAH can occur alone or in combination with other types of primary brain injury. The risk of symptomatic vasospasm is thought to be less than that associated with aneurysmal SAH, perhaps because the amount of blood seen in the subarachnoid space is typically less with traumatic SAH than when SAH is due to aneurysm rupture. In patients who present with traumatic SAH, the possibility that the patient experienced an aneurysmal SAH (which then caused the traumatic event) should be investigated, especially if the events preceding the trauma are unclear.
• Diffuse injury: Diffuse TBI exists on a continuum from cerebral concussion to severe diffuse axonal injury (DAI). Cerebral concussion is a transient, temporary neurologic dysfunction caused by rapid acceleration-deceleration or by a sudden blow to the head. Symptoms of concussion include headache,
confusion, disorientation, and amnesia; most symptoms resolve without intervention. Patients with severe DAI, also called “shearing injury,” typically experience an immediate and prolonged loss of consciousness and display abnormal posturing. The initial CT scan may appear normal, show signs of diffuse cerebral edema (decreased ventricle size, loss of differentiation between gray and white matter, and loss of sulci), or show very small areas of hemorrhage (punctate hemorrhage). The clinical course and outcome are dependent upon the severity of axonal injury.
Secondary Brain Injury
Patients with moderate to severe TBI are at significant risk for secondary brain injury, defined as ongoing neuronal damage that occurs following TBI as the result of systemic and intracranial complications. Care is directed at minimizing secondary injury by improving the supply of oxygenated blood to the brain and decreasing cerebral metabolic demands. Major contributors to secondary injury include the following:
• Hypoxemia: The brain needs a constant supply of oxygen to function. It is very sensitive to insults that create hypoxemia, such as pneumonia, atelectasis, chest trauma, neurogenic pulmonary edema, airway obstruction, and pulmonary embolus.
• Hypotension: Hypotension (SBP < 90 mm Hg) is associated with increased risk of mortality after TBI. Hypotension decreases cerebral blood flow, resulting in tissue ischemia and buildup of waste products. Mortality risk increases with multiple episodes of hypotension.
• Anemia: Anemia causes secondary injury by decreasing oxygen delivery to the brain. The optimal hematocrit in patients with cerebral insults is not known.
• Hypo- or hyperglycemia: The brain cannot store glucose and is dependent on a constant supply to maintain metabolic function. Hypoglycemia must be avoided because it disrupts this supply and leads to cellular dysfunction. Significant hypoglycemia is uncommon following TBI. Hyperglycemia is more common and is associated with increased mortality; it is unclear whether elevated blood glucose is a marker of injury severity or contributes to pathologic changes that increase mortality.
• Increased metabolic demands: Fever, agitation, and seizures increase metabolic demand. Fever increases ICP and can be due to an infectious process or injury to the hypothalamus.
• Loss of autoregulatory mechanisms: Autoregulatory mechanisms maintain constant cerebral blood flow within a wide range of blood pressures and ICP. The ability to autoregulate blood flow can be lost in the injured brain, increasing susceptibility to ischemia caused by decreased blood flow. The extent of this autoregulatory loss varies among patients.
• Increased intracranial pressure (ICP): Increased ICP negatively affects cerebral perfusion and the viability of neurons. The major sources of increased ICP after brain injury are cerebral edema and expanding lesions, such as hematomas. Edema may be localized to the site of the injury or diffuse, with maximal edema occurring 2 to 5 days after severe TBI.
• Hypo- or hypercapnia: Hypocapnia decreases cerebral blood flow by increasing pH and causing cerebral vasoconstriction. Decreased cerebral blood flow lowers ICP but creates a potentially ischemic state. Hypercapnia results in cerebral vasodilation and increases ICP, which may contribute to secondary injury.
• Biochemical changes: A number of biochemical changes occur following TBI, including the release of excitatory amino acids, free radical production, inflammation, and abnormal calcium shifts. A complete explanation of the processes underlying these changes is beyond the scope of this text. All factors contribute to changes in cellular function and can cause cell death. Much research has been completed in an attempt to stop these biochemical changes and confer neuroprotection; to date, none of these trials has demonstrated significant improvement in outcomes.
Clinical Presentation
Patients with TBI often present with external signs of trauma to the head such as ecchymosis, lacerations, and abrasions. Level of consciousness is the most important indicator of severity of injury and is assessed using the GCS. A decreasing GCS or changes in pupil size, shape, or reactivity indicate neurologic deterioration and warrant immediate physician notification. The type, location, and severity of TBI determine specific neurologic assessment findings. Patients may display hemiparesis, hemiplegia, language deficits, cognitive changes, or behavioral changes. If the injury is severe, the patient may display flexor or extensor posturing, as well as autonomic instability (eg, fever, tachycardia, or hypertension).
Patients with mild TBI do not display focal deficits such as hemiplegia or hemiparesis, but report a variety of physical, cognitive, and emotional symptoms. Signs and symptoms of mild TBI include headache, nausea/vomiting, dizziness, balance disturbance, visual problems, fatigue, and sensitivity to light or sound. Patients often report difficulty concentrating, decreased memory for recent events, slowed thought processes, irritability, anxiety, sadness, and increased emotion. Sleep disturbances are also common following mild TBI, and include both drowsiness/increased need for sleep and difficulty sleeping.
Diagnostic Tests
Computerized Tomography scanning is used to rapidly identify hematomas in need of evacuation. Other bleeding (such as into the subarachnoid space), contusions, skull fractures, and cerebral edema can be detected on CT. MRI is useful in the detection of DAI, brain stem injury, and vascular injury but is not typically included in the initial evaluation. The diagnostic work-up of the TBI patient includes a search for other injuries as appropriate to the mechanism of injury.
Principles of Management of Traumatic Brain Injury
Management priorities for patients with TBI vary based on the severity of injury.
Mild Traumatic Brain Injury (GCS 13-15)
Patients with mild TBI and an abnormal CT scan may be admitted to the progressive care unit for serial neurologic examination, or for monitoring and management of other injuries. These patients require assessment of neurologic status and education regarding possible sequelae of mild TBI, including headaches, difficulty concentrating, dizziness, fatigue, irritability, decreased processing speed, and sleep disturbances. Resources for follow-up are provided to the patient and family. In most cases, the symptoms will resolve, but evaluation by a neuropsychologist or other rehabilitation professional is recommended if symptoms persist.
Moderate Traumatic Brain Injury (GCS 9-12)
Moderate TBI poses a significant challenge to the healthcare team. A number of these patients will decline and require aggressive management similar to patients with severe TBI. Others will improve without intervention. Patients with moderate TBI who are admitted to the progressive care unit should be monitored very closely, with neurological assessment at least hourly. Any change found in neurological examination is immediately acted upon by the healthcare team.
Severe Traumatic Brain Injury (GCS ≤ 8)
The initial management of patients with severe TBI takes place in the ICU and is focused on optimizing functional recovery by minimizing secondary brain injury. In addition, other injuries must be identified and treated. An understanding of the early management of these patients gives the progressive care nurse insight into the patient’s overall hospital course, which is often helpful in supporting families. In addition, these general principles can be applied to any patient with TBI who is experiencing neurologic worsening.
General principles of management for the patient with severe TBI include:
• Airway management: Patients with a GCS of 8 or less require intubation and mechanical ventilation. Patients with TBI are treated with spine precautions until injury to the spinal column can be ruled out, so manual in-line stabilization of the cervical spine is used during intubation. In patients with severe TBI, a tracheostomy is often placed once the patient’s condition has stabilized to allow faster ventilator weaning and facilitate rehabilitation.
• Oxygenation: Hypoxemia worsens secondary brain injury and is avoided. Patients with severe TBI may vomit and aspirate prior to airway placement, or may have thoracic injuries, complicating pulmonary management. The need for aggressive pulmonary care often continues following transfer from the ICU.
• Ventilation: In general, the goal of management is to maintain a normal PaCO2. Hypoventilation causes cerebral vasodilation, which may increase ICP. Prolonged or prophylactic hyperventilation is not recommended because it causes cerebral vasoconstriction, which lowers ICP but may cause cerebral ischemia. Hyperventilation is used for short periods to lower ICP in the setting of acute neurologic worsening while other more definitive measures are implemented.
• Fluid and volume management: The goal of fluid management is to maintain euvolemia. Hypotonic solutions are avoided because they increase cerebral edema. Patients with TBI, especially those with autonomic instability due to DAI, often have large insensible losses due to diaphoresis and fever and are at risk for dehydration. Patients with injury to the hypothalamus or pituitary gland are at risk for diabetes insipidus (DI) or syndrome of inappropriate antidiuretic hormone (SIADH), further complicating fluid management. For more information on DI and SIADH, refer to Chapter 16, Endocrine System.
• Managing increased ICP: Initially, an ICP monitor is placed in patients with severe TBI to help guide management. Treatment is initiated when ICP is sustained above 20 mm Hg (see Special Procedures for Intracranial Pressure at the end of this chapter). These patients are managed in the ICU, but increased ICP can also occur in patients admitted to the progressive care unit with moderate TBI who worsen neurologically, or in response to late complications of severe TBI such as hydrocephalus. Nursing measures to prevent and manage elevations in ICP are discussed in Chapter 12, Neurologic System. Of note, steroids worsen outcome following TBI and should not be given.
• Supporting cerebral perfusion: Hypotension (SBP
< 90 mm Hg) is associated with a poor outcome in TBI patients. Cerebral perfusion pressure (CPP, calculated by subtracting ICP from MAP) is an indirect indicator of cerebral blood flow. Goal CPP may vary slightly based on clinical scenario and other monitors of cerebral perfusion, but a CPP of less than 50 mm Hg is avoided because of cerebral ischemia.
• Preventing increased cerebral oxygen demand: Seizures, fever, and agitation increase cerebral oxygen demand and are avoided. An anticonvulsant is used to prevent posttraumatic seizures during the first 7 days after injury. Continued seizure prophylaxis does not impact the development of posttraumatic seizures and is not recommended. Fever is known to be detrimental to the injured brain. For every 1°C increase in temperature, cerebral metabolism increases by approximately 6%. To prevent additional demands on the injured brain, fever is controlled. It is important to avoid or manage shivering when treating fever because shivering markedly increases cerebral metabolic demand. Agitation also increases cerebral oxygen demand. Strategies to avoid agitation include maintaining a calm, quiet environment and the use of sedating medications. Pain management is very important; short-acting medications are used to allow on-going evaluation of mental status.
Preventing Secondary Complications
Common secondary complications include pneumonia and other infections, deep venous thrombosis (DVT), pulmonary embolism, gastric ulcers, and skin breakdown. Nutrition is started within the first 3 days after injury. DVT prophylaxis is initiated on admission with graduated compression stockings and pneumatic compression devices. Pharmacologic prophylaxis varies by practitioner and type of TBI. Inferior vena caval (IVC) filters are placed in patients who develop DVT and cannot be anticoagulated to decrease the risk of PE.
Complications of immobility are common in patients with TBI. Progression of activity is optimized with early spine clearance. Institutional protocols vary, but typically include a series of spine x-rays, CT scanning, and potentially MRI to rule out injury to the bones and ligaments of the spine.
Promoting Recovery after Traumatic Brain Injury
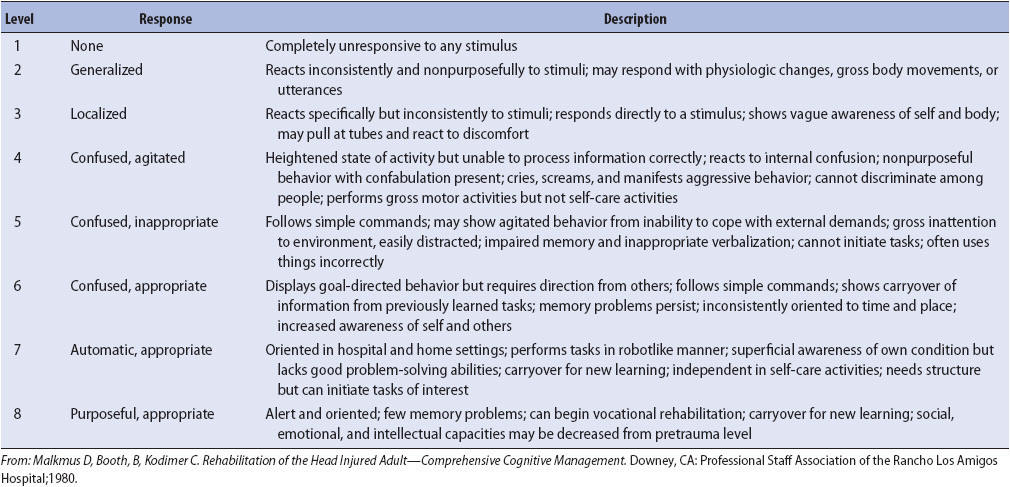
Most patients progress through a series of recovery stages, during which they become more alert, then agitated, then purposeful and more appropriate. The Rancho Los Amigos scale (Table 20-2) is useful in tracking patient recovery, planning interventions, and educating family members. Managing agitation is frequently challenging in patients with TBI. Environmental strategies are very important; stimulation is decreased, the room is kept quiet, and a calm demeanor is maintained by staff and family members. Only one person speaks at a time, and the patient is allowed extra time to respond to questions. Consistent staff members are assigned to care for the patient. All lines and tubes (indwelling bladder catheters, IVs) are removed as soon as possible. Medications are often prescribed as part of managing agitation in patients with TBI but should be used in the smallest doses possible for the shortest time possible because they may slow recovery. Restraints should be avoided unless patient or staff safety is compromised.
TABLE 20-2. RANCHO LOS AMIGOS LEVELS OF COGNITIVE FUNCTIONING SCALE AFTER HEAD TRAUMA
Patients with TBI benefit from a multidisciplinary team approach. Physical therapy, occupational therapy, nutrition, and social work are consulted early in the patient’s hospital course. The speech therapist provides expert assistance with swallowing issues, language, and cognition. A number of other professionals may also be helpful, including the rehabilitation physician and neuropsychologist.
Family Education and Support
Traumatic brain injury alters the life of the injured individual and his or her family forever. The unpredictable nature of recovery from brain injury is difficult to comprehend. Family members may feel that information provided by different caregivers is inconsistent, or that insufficient information is being provided. They express the need to be involved in care—to be “part of the team.”
Nurses can best support families of patients with TBI by providing direct, honest communication (including recognition of the difficulty of predicting prognosis) and by recognizing their need to be present and involved in care. The transition from the ICU to the progressive care unit is often a stressful time for family members. There is less uncertainty about whether or not the patient will live, but the extent of recovery remains unknown. Intensive and progressive care nurses can decrease family members’ anxiety by collaborating to provide continuity of care and education about the stages of recovery.
TRAUMATIC SPINAL CORD INJURY
Etiology, Risk Factors, and Pathophysiology
Common causes of spinal cord injury (SCI) include motor vehicle accidents, falls, acts of violence, and sports-related injuries. The average age at time of injury has increased and is now 41 years. Over 80% of individuals with SCI are male, and approximately 60% of injuries involve the cervical region of the spinal cord. SCI causes varying degrees of paralysis and loss of sensation below the level of injury, and impacts physical, emotional, and social function. Similar to brain injury, deficits are due to both the initial impact (primary injury) and ongoing physiologic changes (secondary injury).
The spinal column consists of stacked vertebrae joined by bony facet joints and intervertebral disks. Ligaments provide structure and support to prevent the vertebrae from moving. The ring-like structure of the stacked vertebrae creates a hollow canal through which the spinal cord runs. SCI occurs when something (eg, bone, disk material, or foreign object) enters the spinal canal and disrupts the spinal cord or its blood supply. Mechanisms of injury include hyperflexion, hyperextension, axial loading/vertical compression, rotation, and penetrating trauma (Figure 20-6). Damage to the spinal cord can be characterized as concussion, contusion, laceration, transection, hemorrhage, or damage to the blood vessels that supply the spinal cord. Concussion causes temporary loss of function. Contusion is bruising of the spinal cord that includes bleeding into the spinal cord, subsequent edema, and possible neuronal death from compression by the edema or damage to the tissue; the extent of neurologic deficits depends on the severity of the contusion. Laceration is an actual tear in the spinal cord that results in permanent injury. Transection is a severing of the spinal cord resulting in complete loss of function below the level of the injury. The most obvious example of cord laceration or transection is penetrating injury that disrupts the cord. Damage to the blood vessels that supply the spinal cord can result in ischemia and infarction, or hemorrhage due to vessel tearing. Regardless of the type of primary injury, secondary insults occur from cellular damage to the spinal cord, vascular damage, structural changes in the gray and white matter, and subsequent biochemical responses. Blood flow to the spinal cord is decreased significantly during the acute phase of injury, resulting in changes in metabolic function, destruction of cell membranes, and the release of free radicals. Patients may develop neurogenic shock following cervical and upper thoracic cord injury. Neurogenic shock results from loss of sympathetic nervous system input from the T1 to L2 area of the spinal cord, which normally increases heart rate and constricts the blood vessel walls. Loss of sympathetic outflow results in bradycardia and decreased vascular resistance. Blood pools in the peripheral vasculature, resulting in hypotension and decreased cardiac output. Neurogenic shock contributes to hypoperfusion and secondary injury.
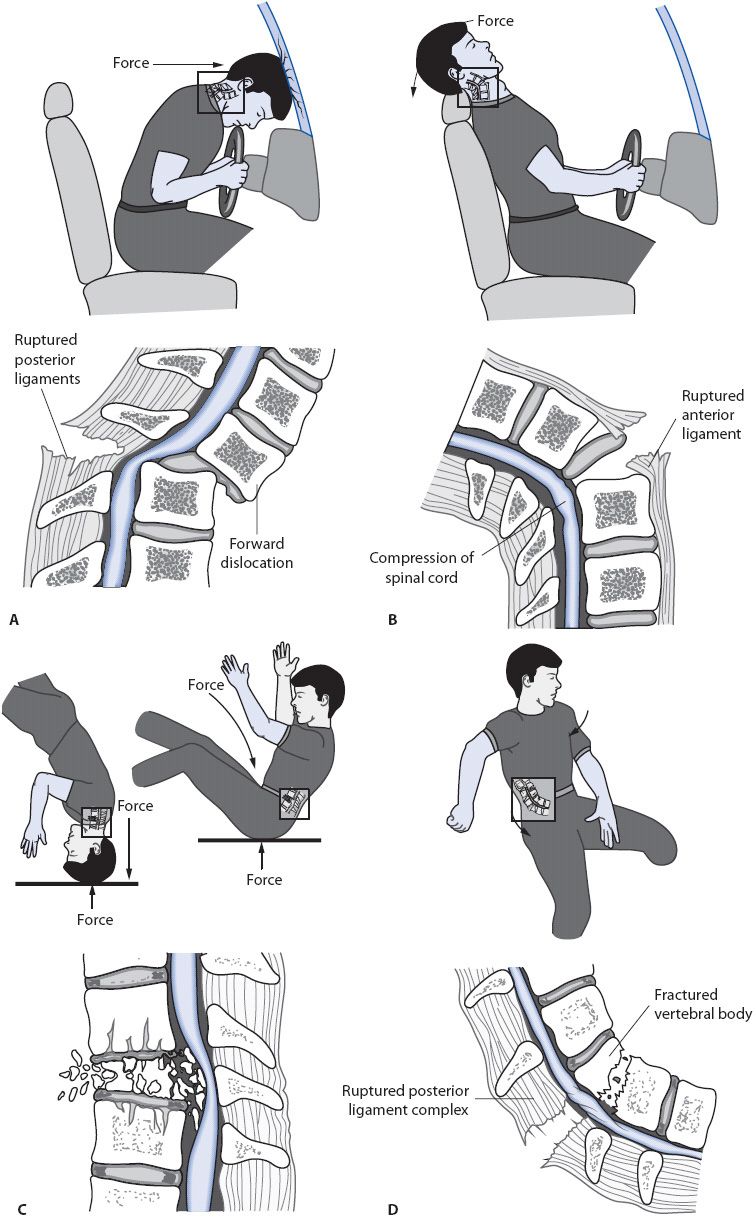
Figure 20-6. Mechanisms of spinal cord injury. (A) Hyperflexion. (B) Hyperextension. (C) Axial loading/vertical compression. (D) Rotation. (Reprinted from: Sands JK. Spinal cord and peripheral nerve problems. In: Phipps WJ, Marek JF, Monahan FD, Neighbors M, Sands JK, eds. Medical-Surgical Nursing: Health and Illness Perspectives. St Louis, MO: Mosby; 2003:1405-1406.)
Clinical Presentation
Assessment of the patient with SCI begins with evaluation of airway, breathing, and circulation, with attention to immobilization of the spine to prevent further injury during the assessment and any subsequent interventions. The focus then shifts to obtaining a baseline assessment of motor and sensory function. Assessment of motor function is performed at least every 4 hours during the acute postinjury period. Decreased motor function may be seen with swelling at the injury site, loss of vertebral alignment, or intrathecal hematoma formation. Changes in function warrant immediate physician notification.
The severity of deficits caused by SCI is determined by whether the injury is complete or incomplete and the level of the spinal cord affected. Acute SCI can result in the temporary suppression of reflexes controlled by segments below the level of injury, a phenomenon referred to as “spinal shock.” Formal determination of complete vs incomplete SCI cannot be made until spinal shock is resolved. Complete SCI results in total loss of sensory and motor function below the level of injury due to complete interruption of motor and sensory pathways. Incomplete SCI results in mixed loss of motor and sensory function because some spinal tracts remain intact. Syndromes associated with incomplete SCI are described in Table 20-3. Deficits caused by SCI relate to the level at which the injury occurs (cervical, thoracic, or lumbar). Cervical and lumbar injuries are more common because these areas have the greatest flexibility and movement. A cervical injury can result in paralysis of all four extremities, or tetraplegia (previously called quadriplegia). Injuries to the thoracic and lumbar areas can result in paraplegia. The American Spinal Injury Association (ASIA) scale (Figure 20-7) is frequently used to assess and document motor and sensory function. Specific functional losses from SCI are summarized in Figure 20-8.
TABLE 20-3. INCOMPLETE SCI SYNDROMES
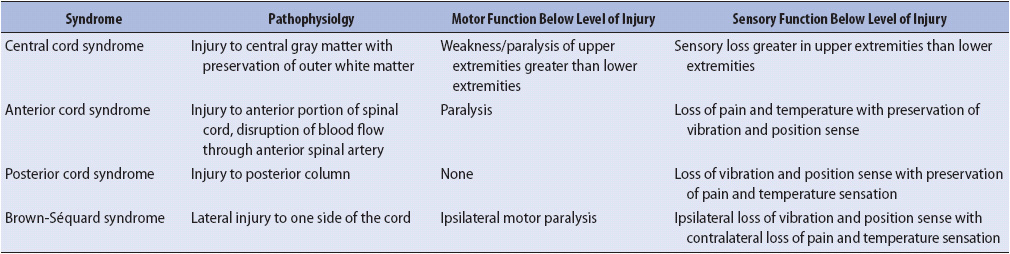
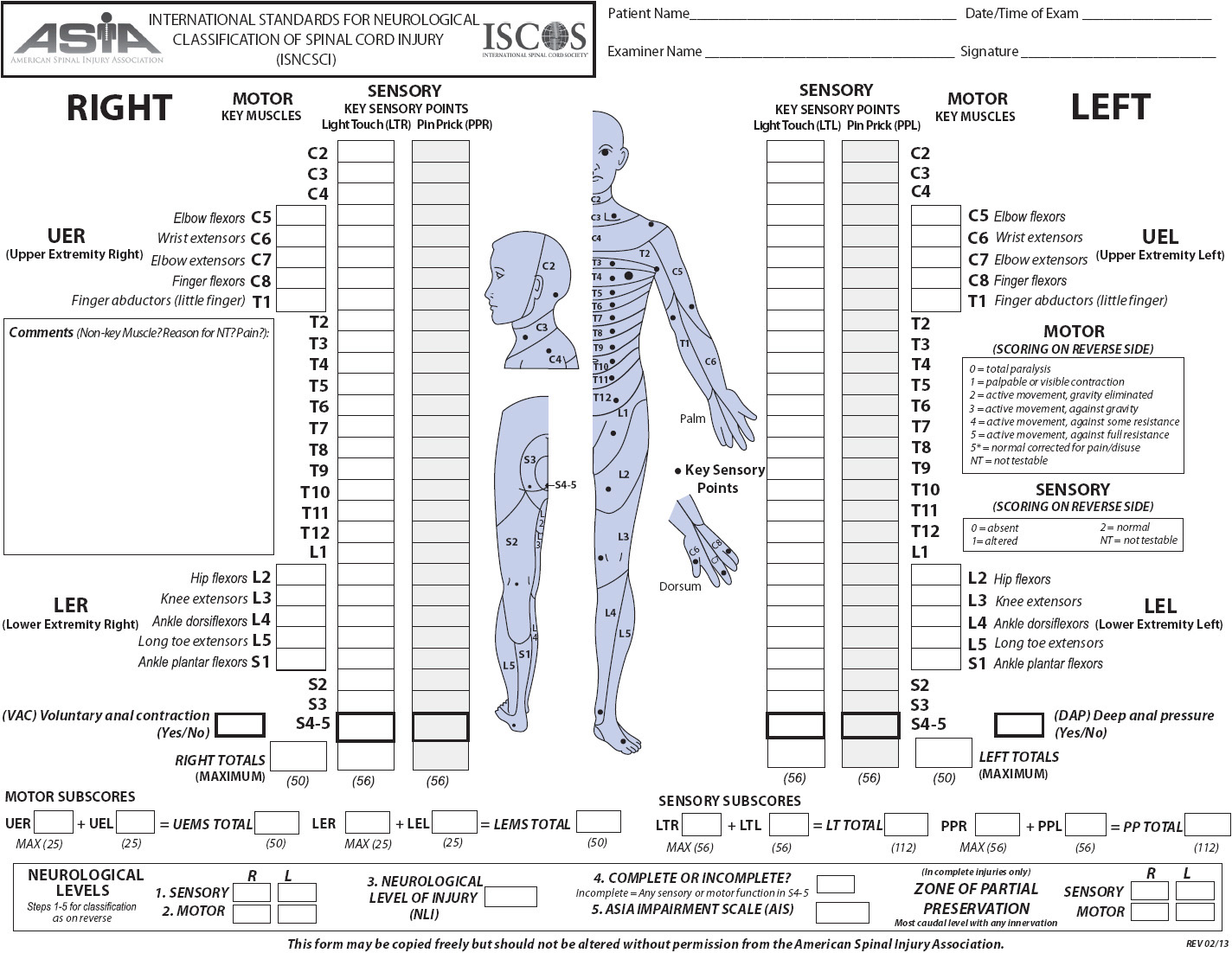
Figure 20-7. ASIA scale for the evaluation of patients with SCI (Copyright American Spinal Injury Association, last update 2/2013) Available at www.asia-spinalinjury.org. Accessed March 5, 2013.
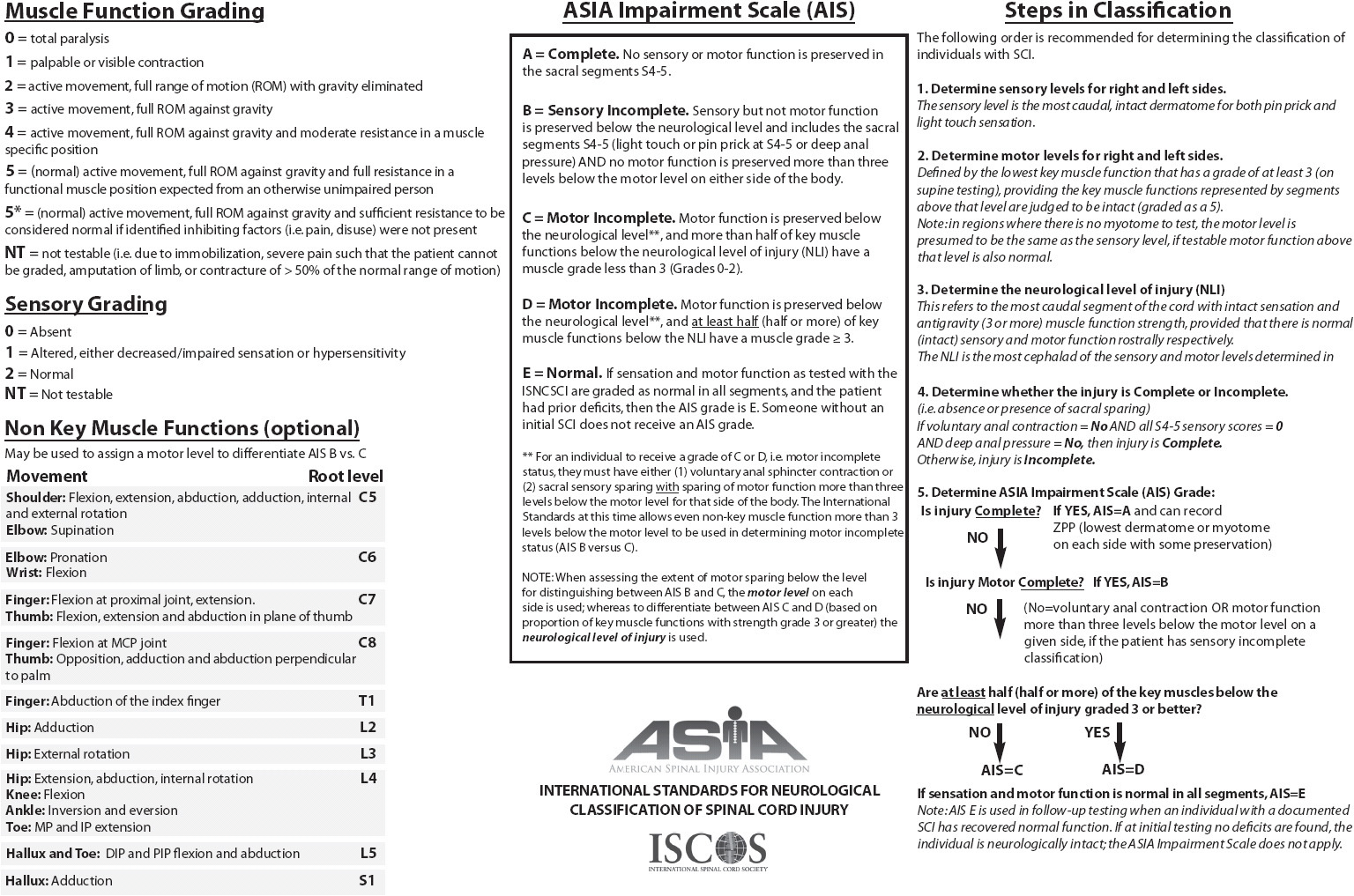
Figure 20-8. Spinal cord injury functional activity chart. (Reproduced with permission from Monahan FD, Phipps WJ, Neighbors M, et al. Phipps’ medical-surgical nursing: Health and illness perspectives, 8th ed. Mosby Elsevier, 2006)
Diagnostic Tests
Immobilization of the spinal column is maintained throughout the trauma evaluation to prevent additional injury. Cervical, thoracic, and lumbar spine x-rays identify the presence of injury to the vertebral column, although these tests are increasingly being replaced with specially constructed CT images. In addition to injury to the vertebral column, CT reveals many injuries to the spinal cord itself, such as bleeding or significant compression. Most patients with suspected SCI undergo an MRI to reveal more subtle signs of injury to the cord and soft tissue, like injury to the supporting ligaments. Injury to the ligaments and spinal cord is possible even without bony abnormalities.
Principles of Management of Acute Spinal Cord Injury
As with brain injury, initial management focuses on decreasing secondary injury and preventing complications. Priorities of management include:
Immobilization and Prevention of Further Injury
Patients with potential SCI are immobilized with a rigid cervical collar and backboard in the prehospital environment and a rigid cervical collar and bed rest in the hospital until injury is ruled out or confirmed clinically and radiographically. Some mattresses (such as air mattresses) do not provide adequate stability to the spinal column; follow manufacturer and institutional guidelines.
Supporting Oxygenation and Ventilation
Altered respiratory function is a major problem for patients with high thoracic or cervical SCI. Impaired oxygenation contributes to secondary injury. The diaphragm is controlled by the phrenic nerve, which exits the spinal cord at the C3 to C5 level. Patients with complete injuries at or above the C2 level require mechanical ventilation due to the loss of diaphragmatic innervation. Patients with cervical or thoracic injuries below the level of diaphragmatic innervation will initiate breaths but still experience respiratory compromise due to paralysis of the intercostal and abdominal muscles. Paralysis of the intercostal muscles causes the chest wall to be flaccid. Contraction of the diaphragm creates a negative pressure in the thoracic cavity and the intercostal muscles retract, decreasing lung capacity. Upright positioning creates further downward displacement of the diaphragm and increases intercostal retraction; flat positioning can improve respiratory function in patients with cervical or thoracic SCI, and abdominal binders can be useful. With time, the intercostal muscles become spastic and the chest wall no longer collapses with inspiration, promoting improved ventilation.
Pulmonary function is closely monitored in patients with cervical and thoracic SCI. Ongoing assessment of maximal inspiratory pressure (MIP) and forced vital capacity allow early identification of impending respiratory failure. In general, a patient who is unable to generate an MIP of at least –20 cm H2O or a vital capacity of greater than 10 to 15 mL/kg requires intubation and mechanical ventilation. Noninvasive ventilation may be considered but does not address problems related to inadequate clearance of secretions.
Hemodynamic Support
Neurogenic shock occurs in patients with cervical or thoracic injury and causes significant hemodynamic alterations, including bradycardia and hypotension. Management focuses on the following:
• Differentiating neurogenic shock from other types of shock. SCI can mask the signs and symptoms of other trauma, including hemorrhage in the abdomen or pelvis.
• Administration of intravenous fluids and vasopressors. Hypotension due solely to neurogenic shock reflects fluid displacement into the vasodilated periphery, not a true lack of fluid volume. As with all trauma patients, adequate fluid resuscitation is important, but continued fluid administration will not correct hypotension and can lead to pulmonary edema or heart failure especially in patients with comorbidities. Norepinephrine is frequently used to counter the loss of sympathetic tone and to provide inotropic and chronotropic support. Limited research shows that maintaining MAP > 85 mm Hg in the week following injury may improve neurologic outcomes. Pending definitive evidence, target blood pressure varies by practitioner. At most institutions, patients requiring vasopressor support due to neurogenic shock or for blood pressure augmentation will be managed in the ICU.
• Monitoring for bradycardia. Patients with SCI above T6 may experience bradycardia. Bradycardia can be profound in patients with cervical injury, even progressing to asystole. Bradycardia occurs more frequently during suctioning; the risk can be lessened but not eliminated by maintaining adequate oxygenation and ventilation. Symptomatic bradycardia is treated with atropine, although some patients require pacemaker placement.
Neuroprotection
There are no currently approved neuroprotective agents that improve outcomes after SCI. Although high-dose methylprednisolone was a part of SCI management for many years, administration is not recommended because there are many associated complications and no convincing benefit. Neuroprotection is an area of ongoing research, and includes both pharmacologic and nonpharmacologic strategies.
Decompression and Stabilization
Early management of SCI includes decompression of the spinal canal and stabilization of the spinal column. In patients with cervical injury, traction may be used to realign the spinal column and relieve pressure on the spinal cord. Traction devices include Gardner-Wells tongs and a halo device (see manufacturer’s literature for more information). Nursing responsibilities during traction placement include patient monitoring, pain management, and administration of sedating agents. Decompression of the spinal cord can also be accomplished surgically. Rapid surgical intervention is indicated for patients with a worsening neurologic examination and ongoing spinal cord compression.
Stabilization of the spinal column does not improve neurologic function but enables the patient to be mobilized without causing additional damage to the spinal cord. In patients who require operative decompression of the spinal canal, the spinal column is stabilized at the time of surgery using rods, screws, or other hardware. For other patients, the timing of surgical stabilization varies. Surgery is commonly performed within 24 hours of injury if the patient’s cardiorespiratory status is stable because early surgery decreases secondary complications and length of stay. Some fractures can be managed without surgery by immobilizing the spinal column and allowing the bones to heal. Immobilization is achieved using a cervical collar, halo vest, or other orthotic device. Skin care is a primary concern for these patients because skin breakdown can occur at contact points with the brace, especially in patients with decreased sensation.
Bladder and Bowel Management
Areflexia caused by spinal shock leads to urinary retention. An indwelling catheter is placed on admission and maintained until the patient is hemodynamically stable and fluid intake is consistent. A program of scheduled intermittent catheterization is then implemented.
A bowel program is initiated soon after admission and typically includes daily stool softeners, glycerin or bisacodyl suppositories, and digital stimulation. For patients with injuries at or above T6, an anesthetic jelly is used to decrease the risk of autonomic dysreflexia (see Complications later in section). The goal of the bowel program is for the patient to have a bowel movement at planned intervals, without incontinence between scheduled evacuations. An effective bowel program decreases constipation, limits incontinence, decreases skin breakdown, and increases the patient’s sense of control.
Managing Pain
Pain following SCI impacts functional recovery and can be challenging to treat. During the immediate postinjury period, many patients complain of musculoskeletal pain and neuropathic pain (described as a burning sensation, paresthesia, or hypersensitivity). Medications prescribed include opiates and muscle relaxants. Antidepressants and anticonvulsants are useful in the treatment of neuropathic pain. Some patients benefit from nonpharmacologic methods such as massage, visual imagery, and diversional activities.
Managing Anxiety
Fear, uncertainty, and anxiety are common emotions following SCI. The psychological and emotional trauma of SCI can be overwhelming. Sudden paralysis does not allow patients or family members to prepare for this major insult. Anxiety results from the hospital environment, feelings of total dependence, sensory deprivation, powerlessness, and an unknown future.
A trusting relationship must be established between the patient and the healthcare team. Use of eye contact, patience, honesty, and consistency are reassuring to the patient. Encouraging self-care within the patient’s abilities decreases feelings of complete dependence. Whenever possible, the patient is allowed choices within the daily care routine. Contracting with the patient may be helpful in setting limits for some patients. The family and significant others are incorporated into the plan of care.
Prevention and Management of Complications
The prevention and effective management of complications maximizes rehabilitation potential. Common complications include:
• Respiratory complications: Respiratory complications are common and contribute to morbidity and mortality. Chest physiotherapy and assisted coughing (“quad” coughing) are used in both ventilated and nonventilated patients. In addition, a mechanical cough assist device (in-exsufflator) can be used to clear secretions. This device imitates a physiologic cough by providing a deep breath via positive pressure followed by negative pressure. Standard measures to prevent hospital-acquired pneumonia, such as head of bed elevation and oral care, are implemented.
• Gastrointestinal problems: Paralytic ileus is common immediately following injury. Initially, an orogastric or nasogastric tube may be placed for decompression, especially in patients with cervical or thoracic injuries. Nutrition (preferably enteral) is started within the first 3 days after injury. Patients with acute SCI are at increased risk for stress ulcers, so it is common to initiate prophylactic medications on admission.
• Skin breakdown: The patient with SCI is at high risk for skin breakdown due to decreased blood flow to the skin and decreased cutaneous response to focal pressure. Meticulous skin care is essential. Skin inspection is performed at least twice daily and pressure reduction strategies are implemented. Early in the hospitalization, the patient who requires assistance with repositioning is encouraged to request that assistance at scheduled intervals. This increases the patient’s sense of control and self-care responsibility, which is associated with improved long-term outcomes.
• Orthostatic hypotension: Blood pools in the lower extremities due to loss of sympathetic vascular tone. Nursing strategies to decrease orthostatic hypotension include application of graduated compression stockings and elastic wraps to the legs, hydration, and gradual progression to an upright position. If these measures are ineffective, medication to raise blood pressure may be ordered.
• Altered thermoregulation: Individuals with SCI at or above the T6 level are unable to conserve heat by vasoconstriction or shivering. Heat loss is compromised by the inability to sweat below the level of injury.
• Deep vein thrombosis: Recommended strategies for prevention during acute hospitalization include mechanical prophylaxis for all patients starting at the time of admission, followed by low-molecular weight heparin or the combination of low-dose unfractionated heparin and intermittent pneumatic compression. To prevent pulmonary embolus secondary to DVT, IVC filters can be placed in patients who develop DVT or who cannot receive pharmacologic prophylaxis.
• Spasticity: During spinal shock, there is a total loss of motor function below the level of injury. Flaccid paralysis progresses to spastic paralysis as spinal shock resolves. Measures to decrease spasticity in the acute postinjury phase include frequent range-of-motion exercises and medications. Occupational and physical therapy are consulted early in the course of hospitalization.
• Autonomic dysreflexia: Autonomic dysreflexia (also called autonomic hyperreflexia) is a life-threatening complication that occurs in individuals with SCI at or above T6 due to unopposed sympathetic response below the level of injury. It can occur any time after spinal shock has resolved. Autonomic dysreflexia results from a variety of stimuli, including overdistended bladder (most common), full rectum, infection, skin stimulation, pressure sores, and pain. The stimulus causes massive vasoconstriction that clinically presents with elevation of blood pressure (relative to the patient’s baseline). Other signs and symptoms include headache, nasal congestion, nausea, blurred vision, flushing and diaphoresis above the level of injury, and feelings of apprehension of anxiety. In some individuals, the only sign of autonomic dysreflexia is elevated blood pressue. Autonomic dysreflexia is a medical emergency and the physician is promptly notified. Treatment includes moving the patient into a sitting position, and promptly identifying and treating the underlying cause (eg, bladder distention, bowel impaction).
Monitor blood pressure and pulse closely and administer short-acting antihypertensive agents as ordered. The timing of pharmacologic intervention varies based on patient characteristics, suspected cause of the event, and institutional guidelines. Careful attention to bowel and bladder management aids in the prevention of autonomic dysreflexia.
Future Spinal Cord Injury Treatment
Currently, much research is focused on SCI. The major areas of investigation include limiting the neuronal damage caused by secondary injury (neuroprotection), enhancing regrowth of neurons (nerve regeneration), and encouraging increased activity of functioning neurons (synaptic plasticity). One resource for patients and families who request information about clinical trials is a web site sponsored by the National Institutes of Health, www.clinicaltrials.gov.
BRAIN TUMORS
Etiology, Risk Factors, and Pathophysiology
The epidemiology of brain tumors varies widely based on tumor type. When all primary CNS tumors are grouped together, the incidence is higher in women than men. This overall gender difference is attributable to increased incidence of meningiomas in women. Prognosis varies based on age (younger patients have a better prognosis), tumor type and degree of differentiation, functional status at diagnosis, and anatomic tumor location. The most common brain tumors are meningiomas, gliomas, and metastatic lesions. Intracranial tumors are classified by distinguishing criteria.
Primary vs Secondary
Primary intracranial tumors originate from the cells and structures in the brain. Secondary or metastatic intracranial tumors originate from structures outside the brain, such as primary tumors of the lung or breast.
Histologic Origins
During the early stage of embryonic development, two types of undifferentiated cells are found—the neuroblasts and the glioblasts. The neuroblasts become neurons. The glioblasts form a variety of cells that support, insulate, and metabolically assist the neurons. The glioblasts are collectively referred to as glial cells and are subdivided into astrocytes, oligodendrocytes, and ependymal cells. This is the basis of a broad category of intracranial tumors called gliomas. Gliomas are subdivided into astrocytomas, oligodendrogliomas, oligoastrocytomas (also called mixed gliomas), and ependymomas. Gliomas are graded based on histologic criteria related to the degree of differentiation from the parent cell. Higher-grade tumors are more malignant. Glioblastoma multiforme (GBM) is a rapidly growing, poorly differentiated tumor. GBM is the most aggressive brain tumor and carries the worst prognosis.
A meningioma is a tumor that arises not from the brain itself, but from the meninges that surround the brain. Meningiomas tend to grow slowly and compress rather than invade the brain. Prognosis is excellent if the tumor is in a surgically accessible location. Neuromas (also called schwannomas) are noninvasive, slow-growing tumors that arise from the Schwann cells, which produce myelin. Pituitary adenomas, located in the pituitary gland, can be secretory or nonsecretory. Secretory tumors increase the production of hormones such as prolactin, growth hormone, adrenocorticotropic hormone, thyrotropin, or gonadotropin. Nonsecretory pituitary tumors cause symptoms through mass effect; patients commonly present with visual changes due to compression of the optic chiasm. Pituitary tumors are treated with pharmacologic agents, surgery, radiation therapy, or a combination of these modalities. The tumors described here are the ones most likely to be encountered in practice; other less common types of brain tumors are beyond the scope of this text.
Anatomic Location
This refers to the actual site of the tumor, such as the frontal lobe, temporal lobe, pons, or cerebellum. Knowing the location of the tumor helps predicting deficits based on the normal functions of that anatomic area. Anatomic location also can refer to the location of the tumor in reference to the tentorium. Supratentorial refers to tumors located above the tentorium (cerebral hemispheres), and infratentorial refers to tumors located below the tentorium (brain stem and cerebellum).
Benign vs Malignant
The distinction between benign and malignant intracranial tumors is based on histologic examination. Tumors made up of well-differentiated cells are “benign” and the prognosis is generally better than if cells are poorly differentiated. However, a histologically benign tumor can be surgically inaccessible. This benign tumor continues to grow and ultimately contributes to a decline in neurologic function and even death. Benign tumors may convert to more histologically malignant types as they develop.
Clinical Presentation
Brain tumors occupy space, causing compression of brain structures, infiltration of tissue that controls functions, and displacement of normal tissue. Brain tumors disrupt the blood-brain barrier and cause cerebral edema. CSF flow may be obstructed by the tumor or edema, leading to hydrocephalus. Tumors are often vascular and may bleed, causing additional neurologic deficits.
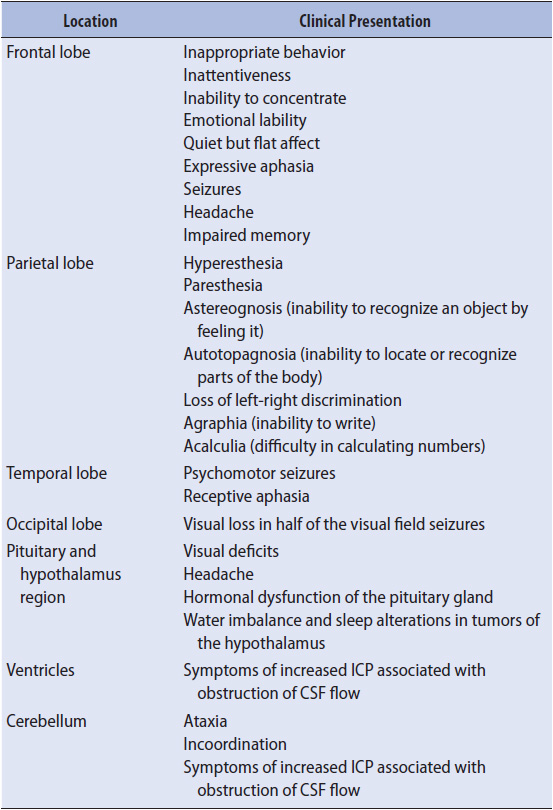
The most common initial signs and symptoms of intracranial tumors are headache, seizures, papilledema, and vomiting. Headache is usually progressive in severity and worse after lying flat, for example, upon awakening from sleep. Clinical presentation may also include decreased level of consciousness, pupillary changes, visual abnormalities, and personality changes. Additional signs and symptoms depend upon the area of the brain that is being compressed or infiltrated (Table 20-4).
TABLE 20-4. CLINICAL PRESENTATION OF BRAIN TUMORS RELATED TO LOCATION
Diagnostic Tests
Computerized Tomography and MRI are used to differentiate tumor from abscess and to identify tumor location and characteristics. Functional MRI detects physiologic changes using MRI scanning during physical and cognitive activity and is helpful in mapping language, sensory, and motor function. Magnetic resonance spectroscopy and positive emission tomography scans evaluate cerebral metabolism and are used to provide information about how aggressive a tumor is (a more aggressive tumor will display higher metabolic activity) and to differentiate necrosis or scarring from tumor. Additional testing includes cerebral angiography, visual field and funduscopic examination, audiometric studies, and endocrine studies. If the lesion is suspected to be metastatic, additional diagnostic tests are done in an attempt to locate the primary tumor site, if not already known. A biopsy of the lesion determines tumor type and degree of differentiation. Biopsy may be performed via a burr hole using stereotactic guidance or may be done as part of a craniotomy for tumor resection.
Principles of Management of Intracranial Tumors
Treatment modalities are used alone or in any combination. Variables considered in selecting appropriate treatment include the type of tumor, its location and size, related symptoms, and the general condition of the patient.
Corticosteroids
A corticosteroid, typically dexamethasone, is administered to decrease vasogenic cerebral edema. Steroids are started in patients with brain tumors when the presence of cerebral edema is noted. Significant improvements in neurologic status can be seen soon after initiation of therapy. Side effects of steroid therapy include gastric irritation, mood swings, fluid retention, hyperglycemia, myopathy, insomnia, and increased risk of infection.
Surgery
The goal of surgery is to resect as much of the tumor as possible with minimal harm to normal tissue. In most cases, a craniotomy is done to provide access for resection. Total resection is curative for some tumor types. Some tumors cannot be completely removed because of location or histologic type. A partial resection of the tumor mass temporarily relieves the symptoms of compression, and increased ICP may be relieved. If CSF flow is obstructed, treatment includes surgical placement of a shunt to reroute CSF from the ventricular system to another part of the body (usually the peritoneal space) where it can be reabsorbed.
Several strategies are available to decrease the morbidity associated with surgery. Intraoperative MRI is available at some centers and is most often used when the lesion is in or near the motor strip, difficult to access, or small and potentially hard to locate. Intraoperative MRI can be used alone or in conjunction with cortical mapping techniques. With cortical mapping, the patient is anesthetized for the initial part of the surgery, then awakened, and asked to perform certain tasks, allowing the surgeon to avoid areas that control speech or motor function. Stereotactic techniques allow targeted biopsy or resection based on previously obtained images.
Most patients undergo elective operations for intracranial tumors and may be admitted to the progressive care unit postoperatively. Postoperative management includes monitoring neurologic status, controlling pain, and preventing and managing complications. Potential complications in the immediate postoperative period include:
• Hematoma formation: Clinical signs include increasing headache, decreasing level of consciousness, and the development of new focal neurologic signs (eg, weakness of an arm or leg). If an intracranial bleed is suspected, a CT scan is obtained. If significant bleeding is found, the patient is returned to the operating room for surgical removal of the hematoma and management of bleeding points.
• Cerebral edema: Postoperative cerebral edema may occur due to the long surgical procedure and/or the retraction of brain tissue to expose the operative area. Cerebral edema is suspected if the patient presents postoperatively with greater neurologic deficits than were present preoperatively. A CT scan is obtained and treatment is initiated to decrease edema. As noted previously, dexamethasone is useful in the management of tumor-related edema.
• Infection: Infection can occur following surgery because of contamination in the operating room or a defect in the dura, which allows communication of the CSF with the atmosphere.
• Deep vein thrombosis: Neurosurgical patients are at increased risk for DVT. Preventive measures to decrease this risk include the use of graduated compression stockings and intermittent pneumatic compression devices, early progression of activity, and low doses of subcutaneous unfractionated heparin.
Radiation Therapy
Radiation therapy preferentially destroys tumor cells because they are rapidly dividing, but affects normal cells also. The treatment dose depends on the histologic type, radioresponsiveness, location of the tumor, and patient tolerance. Increased edema is a common complication of radiation therapy. Patients typically remain on dexamethasone throughout treatment. Special techniques, such as stereotactic radiosurgery or gamma knife radiation, focus concentrated radiation from many directions on the tumor site and reduce radiation to normal tissue.
Chemotherapy
Chemotherapy is used to slow or stop the proliferation of abnormal cells. One commonly used agent in the treatment of high-grade gliomas is temozolomide (Temodar). Temozolimide is administered orally and is generally well-tolerated by patients.
Prevention and Management of Seizures
The incidence of seizures in patients with brain tumors ranges from 20% to 60%. Antiepileptic drugs are often given prophylactically to patients with supratentorial tumors. When seizures do occur, they are managed according to the guidelines described in Chapter 12, Neurologic System. Any seizure in the immediate postoperative period prompts an emergent CT scan to look for hematoma formation.
Special Considerations: Transsphenoidal Resection of Pituitary Tumors
The surgical management of patients with pituitary tumors differs because a transsphenoidal approach may be used (Figure 20-9). Transsphenoidal resection uses a special technique to reach pituitary tumors by going through the sphenoid sinus. An incision may be made under the patient’s upper lip, or an endonasal approach may be used. Because the pituitary gland secretes a number of hormones, endocrine disturbances are common both before and after surgery. Care in the postoperative period is similar to that described for patients undergoing a craniotomy, but certain assessments are emphasized. Because the pituitary gland is located near the optic chiasm, visual acuity and visual field testing is essential. The patient is closely monitored for CSF leak, and is instructed not to blow his or her nose or lean over. Nasal packing, if present, is typically removed by the physician on the first or second postoperative day. Serum cortisol is monitored because the patient will no longer secrete adrenocorticotropic hormone if the anterior pituitary was resected. Close monitoring of fluid balance and electrolytes is required because of the risk of diabetes insipidus (DI). DI is caused by insufficient amounts of antidiuretic hormone (ADH), which is produced by the posterior lobe of the pituitary gland. If ADH is not secreted in sufficient amounts, the patient will produce large volumes of dilute urine. Significant fluid and electrolyte imbalances with dehydration can result. Intake and output are measured frequently (every hour initially). Electrolytes (especially sodium) and urine specific gravity are monitored frequently, typically every 4 hours. Serum and urine osmolality may also be monitored. Management of DI includes allowing the patient to drink fluids as needed to quench thirst. Management may also include IV therapy that correlates with urine output and administration of aqueous vasopressin or desmopressin acetate (DDAVP).
Figure 20-9. Transsphenoidal hypophysectomy. (Reprinted from: Carlson BA: Neurologic disorders. In: Urden LD, Stacy KM, Lough ME, eds. Thelan’s Critical Care Nursing. 4th ed. St Louis, MO: Mosby; 2002:695.)
SPECIAL PROCEDURES: INVASIVE MONITORING OF INTRACRANIAL PRESSURE
Intracranial pressure is most often measured via a catheter inserted into the ventricles or a probe inserted into the brain parenchyma, but can also be measured in the subarachnoid space, epidural space, or subdural space (Figure 20-10). Use of an intraventricular catheter remains the gold standard for ICP measurement. Several systems exist, but the basic setup includes a catheter, transducer (either external or integrated into the catheter), and collection device for CSF. The catheter is placed via a burr hole into the anterior horn of the lateral ventricle. The zero point of the drainage system is leveled at the external landmark of the foramen of Monro and zeroed to atmospheric pressure using manufacturer’s specifications. Slightly different external landmarks for the foramen of Monro are reported in the literature (tragus, halfway between the outer canthus of the eye and the tragus, external auditory meatus); follow institutional protocols to maintain consistency among caregivers. The transducer senses the pressure exerted by the CSF in the ventricles and translates it into a waveform on the monitor. This system is referred to by several names, including external ventricular drain, ventriculostomy, and intraventricular catheter. The advantage of using an intraventricular catheter for monitoring is that CSF can be drained, providing a treatment modality for increased ICP. CSF drainage is controlled by adjusting the height of the system relative to the foramen of Monro. The height of the fluid column in the drainage system creates hydrostatic pressure that opposes ICP. If the drainage system is raised, CSF drainage decreases; when the drainage system is lowered, CSF drainage increases. Rapid drainage of CSF can result in ventricular collapse so CSF is drained in a controlled manner based on a predetermined ICP. This is accomplished by maintaining the drainage system at a specific height, such as 20 cm above the external landmark of the foramen of Monro, or by opening the system to allow CSF drainage only when the ICP exceeds a specified value. CSF drainage is monitored for amount and color. An occlusive dressing is maintained over the catheter site. Risks associated with intraventricular catheter placement include infection and hemorrhage caused by catheter placement. Sterile technique is essential when the catheter is placed and whenever the system is manipulated (for example, to sample CSF).

Figure 20-10. Sites for ICP monitoring. Illustration of possible sites for monitoring intracranial pressure: subarachnoid-subdural, intraventricular, intraparenchymal, and epidural. The “gold standard” remains the intraventricular catheter. (Reprinted from: Lee KR, Hoff JT. Intracranial pressure. In: Youmans JR, ed. Neurological Surgery. Vol 1. Philadelphia, PA: WB Saunders; 1996:505.)
Intracranial pressure is also commonly monitored using a transducer inserted into the brain parenchyma. These monitors are easier to insert and have a lower rate of infection than intraventricular catheters. Leveling to the foramen of Monro is not required. Fiberoptic and strain gauge transducers are connected directly to an independent monitor, which provides an ICP reading. Other technology is available to monitor ICP, including some devices that allow rezeroing after monitor insertion, but these are less commonly used in practice. Normal ICP is 0 to 15 mm Hg in adults.
Intracranial Pressure Waveforms
With continuous ICP monitoring, there are fluctuations in waveforms that correlate with specific physiologic events. Examination of these waveforms can be helpful in evaluating changes in the patient’s condition.
The ICP pulse waveform is a continuous, real-time pressure display that corresponds to each heartbeat. The normal pulse wave has three or more defined peaks representing blood and CSF flow within the cranium: P1 (percussion wave), P2 (tidal wave), and P3 (dicrotic wave).
The pulse waveform at low pressures is a descending saw-toothed pattern with a distinct P1 (Figure 20-11). As mean ICP rises, a progressive elevation of P2 occurs, causing the pulse waveform to appear more rounded. When P2 is equal to or higher than P1, decreased compliance exists (Figure 20-12).
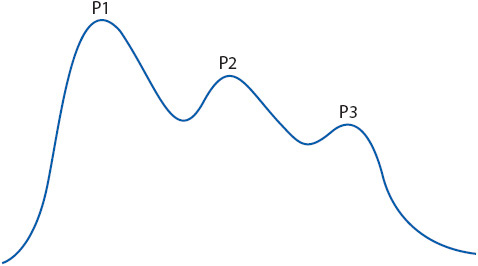
Figure 20-11. Components of a normal ICP waveform.

Figure 20-12. ICP waveform demonstrating decreased compliance.
Trend recordings compress continuous ICP recording data to reflect general trends in ICP over longer time periods (minutes to hours). Three distinct pressure waves have been identified (Figure 20-13). A waves (plateau waves) are sudden increases in pressure lasting 5 to 20 minutes. They begin from a baseline of an already elevated ICP (> 20 mm Hg) and reflect cerebral ischemia. B waves are sharp, rhythmic oscillations of pressure (up to 50 mm Hg) occurring every 0.5 to 2 minutes. They are seen in relationship to fluctuations in the respiratory cycle, such as Cheyne-Stokes respirations. They are not clinically significant, but may progress to A waves. C waves are small rhythmic waves with pressures up to 20 mm Hg occurring 4 to 8 times per minute. They relate to normal changes in systemic arterial pressure, and their clinical significance is unknown.
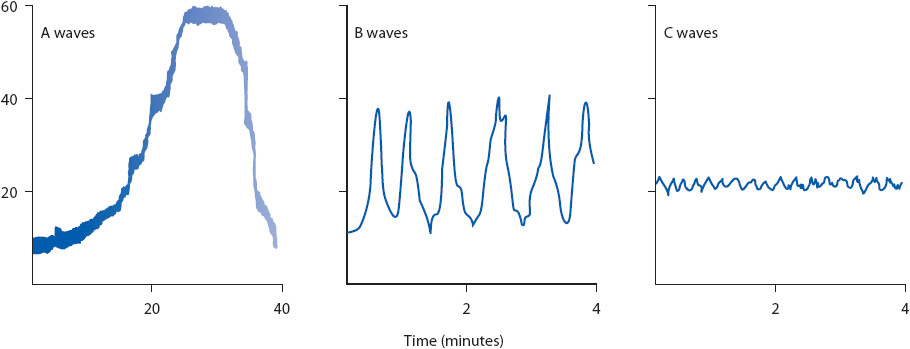
Figure 20-13. ICP trend recordings.
Cerebral perfusion pressure (CPP) is a measurement of the pressure at which blood reaches the brain. CPP is an indirect reflection of CBF. It is calculated by subtracting ICP from mean arterial pressure (CPP = MAP – ICP). Decreased CPP occurs as the result of an increase in ICP, a decrease in mean arterial pressure, or both. A CPP of at least 50 to 60 mm Hg is necessary for adequate cerebral perfusion. CPP below 30 mm Hg results in irreversible neuronal hypoxia.
SPECIAL PROCEDURES: MANAGEMENT OF A PATIENT WITH A LUMBAR DRAIN
Lumbar drains are used in the progressive care unit to manage communicating hydrocephalus, and in patients with CSF leakage following neurosurgery or trauma to decrease pressure against the dura and allow it to heal. Lumbar drains are also used to improve spinal cord perfusion in the immediate post-operative period following thoracoabdominal aneurysm repair. The process for placing a lumbar drain is similar to performing a LP (see Chapter 12, Neurologic System). The physician threads the catheter into the subarachnoid space of the lumbar spine. Nursing responsibilities during placement include assistance with patient positioning and the administration of pain medications and sometimes sedation. Once the lumbar catheter has been placed, it is connected to an external drainage system. The amount of CSF drainage is determined by the height of the system, which corresponds to the amount of pressure within the spinal subarachnoid space. The lumbar drain may be left open so that CSF flows into the collection chamber whenever the pressure in the spinal subarachnoid space exceeds the pressure created by the height of the column of fluid in the drainage system or a certain amount of CSF may be drained every hour. Alternatively, an external transducer can be connected to the system to measure pressure within the spinal subarachnoid space, with orders to allow drainage only when the pressure exceeds a predetermined limit. The decision to keep the system open to drain continuously or to allow CSF to drain only when the pressure exceeds a certain value is made by the physician based on the reason for CSF drainage and provider preference.
The zero reference for leveling the drip chamber and transducer vary based on reason for drainage, provider preference, and institutional protocol. Common sites for leveling include the shoulder, the external auditory meatus, and the level of insertion. Nursing priorities when caring for a patient with a lumbar drain include maintaining the system at the ordered height (eg, 10 cm H2O above the external auditory meatus). If the system is placed below the ordered height, overdrainage of CSF may result and can lead to the development of subdural hemorrhage or even herniation. If the lumbar drainage system is placed too high, CSF drainage may be inadequate for the desired purpose. Other potential complications include infection, bleeding, and sensory deficits related to nerve root injury or irritation. An occlusive sterile dressing is maintained at the insertion site. Practices related to mobilizing the patient with a lumbar drain vary; follow institutional standards and physician order.
SELECTED BIBLIOGRAPHY
Subarachnoid Hemorrhage
Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928-939.
Diringer MN. Management of aneurysmal subarachnoid hemorrhage. Crit Care Med. 2009;37:432-440.
Hinkle JL, Guanci MM, Stewart-Amidei C. Cerebrovascular events of the nervous system. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
Li H, Pan R, Wang H, et al. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke. 2013;44:29-37.
Molyneux AJ, Kerr RSC, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): longterm follow-up. Lancet Neurol. 2009;8:427-433.
Solenski NJ, Haley EC, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Crit Care Med. 1995;23:1007-1017.
Sommargren CE. Electrocardiographic abnormalities in patients with subarachnoid hemorrhage. Am J Crit Care. 2002;11:48-56.
Traumatic Brain Injury
Bond AE, Draeger CRL, Mandleco B, Donnelly M. Needs of family members of patients with severe traumatic brain injury: implications for evidence-based practice. Crit Care Nurs. 2003;23:63-72.
Centers for Disease Control. Blast injuries: traumatic brain injury from explosions. Available at http://emergency.cdc.gov/masscasualties/blastinjury-braininjury.asp. Accessed March 2, 2013.
Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010.
Lombard LA, Zafonte RD. Agitation after traumatic brain injury: considerations and treatment options. Am J Phys Med Rehabil. 2005;84:797-812.
March K, Criddle LM, Wellwood J, et al. Craniocerebral trauma. In: Bader MK, Littlejohns LR, eds. AACN Core Curriculum for Neuroscience Nursing. 54th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
Spinal Cord Injury
Casha S, Christie S. A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma. 2011; 28:1479-1495.
Cotton BA, Pryor JP, Chinwalla I, et al. Respiratory complications and mortality risk associated with thoracic spine injury. J Trauma. 2005;59:1400-1409.
McIlvoy L, Meyer K, Mahanes D, Sachse S, McQuillan KA. Traumatic spine injuries. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. Glenview, IL: American Association of Neuroscience Nurses; 2010.
National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. February 2012. Retrieved March 2, 2013 from https://www.nscisc.uab.edu/.
Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82:803-814.
Brain Tumors
Asthagiri AR, Pouratian N, Sherman J, Ahmed G, Shaffrey ME. Advances in brain tumor surgery. Neurol Clin. 2007;25:975-1003.
Bohan EM, Gallia GL, Bren H. Brain tumors. In: Barker E, ed. Neuroscience Nursing: A Spectrum of Care. 3rd ed. St Louis, MO: Mosby; 2008.
Stewart-Amidei C, Arzbaecher J, Lupica K. Nervous system tumors. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
Intracranial Pressure Monitoring
Madden LK, March K. Intracranial pressure management. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
March K, Olson D, Arbour R. Technology. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
March K, Wellwood J. Intracranial pressure concepts, cerebral blood flow, and metabolism. In: Bader MK, Littlejohns LR. eds. AANN Core Curriculum for Neuroscience Nursing. 45th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010. St Louis: Saunders.
Evidence-Based Guidelines
Alexander S, Gallek M, Presciutti M, Zrelak P. Care of the Patient with Aneurysmal Subarachnoid Hemorrhage: AANN Clinical Practice Guidelines Series [electronic version]. Glenview, IL: American Association of Neuroscience Nurses; 2011. Available at http://www.aann.org/pubs/content/guidelines.html. Accessed April 5, 2013.
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, AANS/CNS Joint Section on Neurotrauma and Critical Care. Guidelines for the Management of Severe Traumatic Brain Injury. New York, NY: Brain Trauma Foundation; 2007.
Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:1711-1737.
Consortium for Spinal Cord Medicine. Early Acute Management in Adults with Spinal Cord Injury: A Clinical Practice Guideline for Health-Care Providers. Washington, DC: Paralyzed Veterans of America; 2008.
Diringer MN, Bleck TP, Hemphill JC, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s multidisciplinary consensus conference. Neurocrit Care. 2011;15:211-240.
Leeper B, Lovasik D. Cerebrospinal drainage systems: external ventricular and lumbar drains. In: Littlejohns LR, Bader MK, eds. AACN-AANN Protocols for Practice: Monitoring Technologies in Critically Ill Neuroscience Patients. Sudbury, MA: Jones and Bartlett Publishers; 2009.
March K, Madden L. Intracranial pressure management. In: Littlejohns LR, Bader MK, eds. AACN-AANN Protocols for Practice: Monitoring Technologies in Critically Ill Neuroscience Patients. Sudbury, Massachusetts: Jones and Bartlett Publishers; 2009.
Mcilvoy L, Meyer K. Nursing Management of Adults With Severe Traumatic Brain Injury: AANN Clinical Practice Guideline Series [electronic version]. Glenview, IL: American Association of Neuroscience Nurses; 2011. Available at http://www.aann.org/pubs/content/guidelines.html. Accessed April 5, 2013.
The American Association of Neurological Surgeons and the Congress of Neurological Surgeons. Guidelines for the management of acute cervical spine and spinal cord injuries from the American Association of Neurological Surgeons and the Congress of Neurological Surgeons. Clin Neurosurg. 2013;72:1-259.
West TA, Bergman K, Biggins MS, et al. Care of the Patient with Mild Traumatic Brain Injury: AANN and ARN Clinical Practice Guideline Series [electronic version]. Glenview, IL: American Association of Neuroscience Nurses and Association of Rehabilitation Nurses. 2011. Available at http://www.aann.org/pubs/content/guidelines.html. Accessed April 5, 2013.

















