TABLE 12-1. GLASGOW COMA SCALE
KNOWLEDGE COMPETENCIES
1. Correlate neurologic assessments to patient problems and diagnostic findings.
2. Identify indications, complications, and nursing management for commonly used neurodiagnostic tests.
3. Identify causes of increased intracranial pressure and describe strategies for management.
4. Compare and contrast the pathophysiology, clinical presentation, patient needs, and nursing management for:
• Acute ischemic stroke
• Hemorrhagic stroke
• Seizure disorders
• CNS infections
• Selected neuromuscular diseases
Although there is no single method of performing a neurologic evaluation, a systematic, orderly approach offers the best results. Knowledge of neurologic disease processes and neuroanatomy allows the progressive care nurse to tailor the assessment to individual patients. Obtaining past medical history and history of present illness or injury is essential and includes preexisting neurologic conditions. The time of symptom onset and mechanism of injury have important implications for diagnostic testing and treatment. The administration of any medications that may potentially alter the neurologic examination, especially sedatives and analgesics, is also noted.
Serial assessments, coupled with accurate documentation, allow for detection of subtle changes in neurologic status. Early detection of changes permits rapid intervention and improves patient outcomes. Neurologic assessment in the progressive care unit can be broken down into the following components: level of consciousness, mental status, motor examination, sensory examination, and cranial nerve examination. A baseline examination is established and subsequent assessments are compared. At a minimum, serial neurologic assessment includes level of consciousness, orientation, motor response, and pupil size and reaction to light. Whenever a hand-off of care occurs (for example, at shift change), the care providers perform a neurological examination together to provide an accurate baseline for the nurse assuming care of the patient.
There are two components to level of consciousness: arousal and awareness. Arousal refers to the state of wakefulness; awareness reflects the content and quality of interactions with the environment. Arousal reflects function of the reticular-activating system and brain stem, and awareness indicates functioning of the cerebral cortex. Level of consciousness is assessed on all patients. A change in level of consciousness is the most important indicator of neurologic decline and is immediately acted on by the healthcare team.
Observation of the patient’s behavior, appearance, and ability to communicate is the first step in assessing level of consciousness. If the patient responds meaningfully to the examiner without the need for stimulation, then the patient is described as alert. If stimulation is required, auditory stimuli are used first. If the patient does not rouse to auditory stimuli, tactile stimuli such as a gentle touch or shake are used, followed by painful stimuli if necessary to elicit a response. Accepted methods of central painful stimulus include squeezing the trapezius or other large muscle group. Care is taken to avoid causing tissue trauma. Supraorbital pressure is also an acceptable pain stimulus, but is not used if there is any suspicion of facial fracture. Use of a sternal rub may result in a motor response that is difficult to interpret (see Glasgow Coma Scale [GCS]) and often causes bruising. Nail bed pressure is a commonly used peripheral pain stimulus. Response to central stimulus is more indicative of cerebral function than response to peripheral stimulus. Certain responses to peripheral pain, such as the triple flexion response (stereotypical flexion of the ankle, knee, and hip), can result from a spinal reflex arc and thus may remain present even following death by neurologic criteria (brain death).
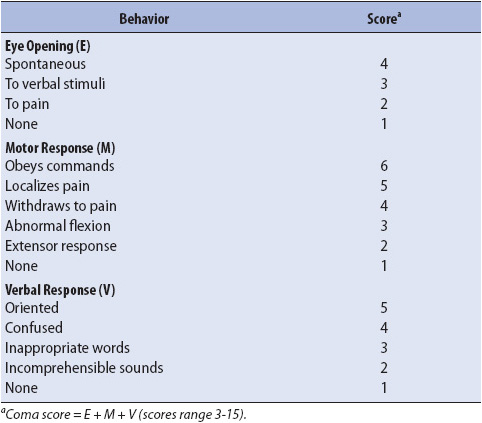
The GCS (Table 12-1) is often used to monitor neurologic status because it provides a standardized approach to assessing and documenting level of consciousness. Response is determined in three categories: eye opening, motor response, and verbal response. The best response in each category is scored, and the results are added to give a total GCS. Scores range from 3 to 15, with 15 indicating a patient that is alert, fully oriented, and following commands.
TABLE 12-1. GLASGOW COMA SCALE

The eye opening score reflects the amount of stimulation that must be applied for the patient to open his eyes. Spontaneous eye opening is the best response, followed by eye opening to verbal stimulation, then eye opening to painful stimulation. Scoring of the eye opening section of the scale can be complicated by orbital trauma and swelling, and this is documented accordingly.
The motor portion of the GCS is the most difficult to assess. Response in each extremity is tested, but only the best motor response is used in calculating a total score. The patient is first asked to follow a command such as “Hold up your thumb” or “Wiggle your toes.” A patient who does not follow commands with his or her extremities is asked to look up and down. In certain neurologic disorders (such as basilar artery stroke or high cervical spinal cord injury), patients may be unable to follow commands with their extremities but still be awake and aware; assessing the ability to look up and down helps identify these individuals. If the patient does not follow commands, then all four extremities are assessed for response to pain stimuli. Upper extremity response to pain is described as localization, withdrawal, decorticate (flexor) posturing, or decerebrate (extensor) posturing. An attempt by the patient to push the stimulus away is clearly localization, but the response is not always easily apparent. Interpretation of patient’s movement is complicated when a sternal rub is used because with both localization and decorticate posturing, the arms move up toward the stimulus. Reaching across the midline of the body to a stimulus (eg, if the right arm comes up to the left shoulder when a left trapezius squeeze is applied) is scored as localization. An easy way to remember decorticate and decerebrate posturing is that decorticate is “into the core,” or flexion, and decerebrate is away from the body, or extension (Figure 12-1). Decorticate posturing signifies damage in the cerebral hemispheres or thalamus. Decerebrate posturing indicates damage to the midbrain or pons. The presence of posturing or a change from decorticate to decerebrate posturing should be brought to the attention of the physician immediately. Motor response to pain in the lower extremities is usually graded as withdrawal or triple flexion. In triple flexion, pain stimulus results in stereotypical flexion of the ankle, knee, and hip. This response can be differentiated from withdrawal by applying the pain stimulus to a different area of the lower extremity (eg, the medial aspect of the calf). If the response is withdrawal, the patient pulls away from the stimulus. If the response is triple flexion, the response is still stereotypical flexion at the ankle, knee, and hip.
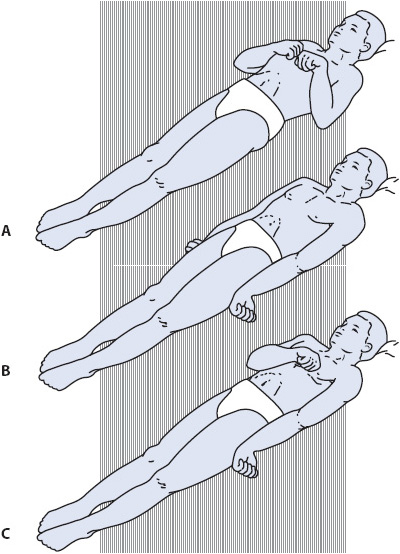
Figure 12-1. Abnormal motor responses. (A) Decorticate posturing. (B) Decerebrate posturing. (C) Decorticate posturing on right side and decerebrate posturing on left side of body. (Reprinted from: Carlson BA. Neurologic clinical assessment. In: Urden LD, Stacy KM, Lough ME, eds. Thelan’s Critical Care Nursing: Diagnosis and Management. St Louis, MO: Mosby; 2002:649.)
The verbal section of the GCS assesses a patient’s ability to speak coherently and with appropriate content. Orientation to person, place, and time is assessed. As mental status declines, orientation to time is lost first, followed by orientation to place. Orientation to person is seldom lost prior to loss of consciousness. Patients with tracheostomies are commonly assigned a verbal score of T and the total GCS is denoted as the sum of the eye opening and motor scores followed by T. Alternatively, the examiner assigns a verbal score based on estimation of the patient’s abilities, often determined by noting the patient’s response when presented with multiple choices.
Although the GCS is frequently used to monitor hospitalized patients, it is important to remember that only a limited amount of information is provided. Additional assessments are necessary to gain an accurate picture of neurologic functioning; these assessments are based on the type of disease process or injury and the part of the central nervous system (CNS) affected.
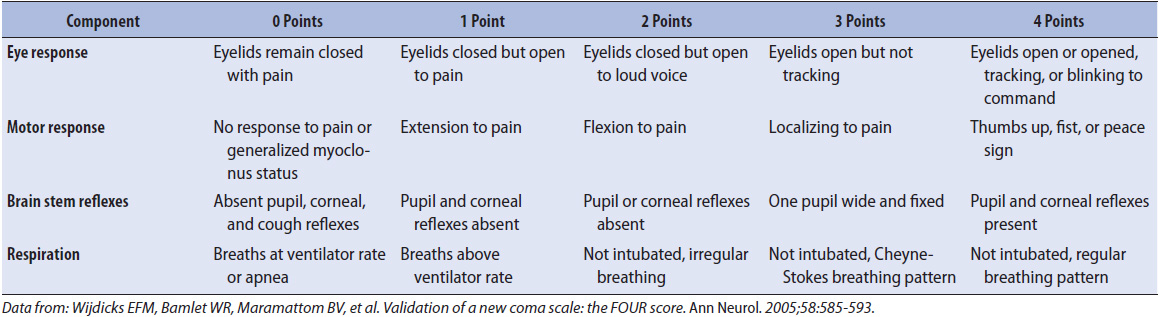
The Full Outline of UnResponsiveness (FOUR) score is another validated tool for the assessment of neurological patients. The FOUR score assigns a value of 0 through 4 in each of four categories: eyes, motor, brain stem reflexes, and respirations. The scores in each category are added together to give a total score of 0 to 16. Table 12-2 provides an overview of the FOUR score, but complete instructions are not included; progressive care nurses who utilize this tool should seek additional information. Because of the inclusion of brain stem reflexes and respiratory pattern, the FOUR score allows the clinician to identify changes in patients with very limited responses.
TABLE 12-2. OVERVIEW OF THE FULL OUTLINE OF UNRESPONSIVENESS (FOUR) SCORE
Although formal measurements of mental status exist, many acutely ill neurologic patients may be unable to complete these assessments because of limited ability to communicate or decreased level of consciousness. Orientation is the component of mental status most often evaluated in the progressive care unit. Other components of mental status assessment include attention/concentration, affect, memory, reasoning, and language function. Attention/concentration, affect, and reasoning are typically assessed informally by simply observing the patient throughout daily care. Short-term memory may be evaluated by giving the patient a list of three items and asking him or her to recall them later. However, deficits are often apparent in informal interactions as well. Difficulty with language can be described as dysarthria (weakness or lack of coordination of the muscles of speech) or aphasia. Aphasia can be either expressive (inability to express thoughts), receptive (inability to comprehend), or global (both expressive and receptive). An individual with expressive aphasia may be able to understand everything that is said but be unable to reply, whereas an individual with receptive aphasia may have nonsensical, fluent speech, but cannot comprehend what is said to him. A patient with dysarthria has slurred speech and is difficult to understand, but the content is appropriate. Dysarthria represents weakness or loss of coordination of the muscles of speech vs a problem with mental status. However, dysarthria often becomes apparent during assessment of mental status and thus is included here.
Delirium is an alteration in mental status that is of particular importance in acutely ill patients, because the development of delirium is associated with worse clinical outcomes and increased hospitalization costs. Delirium is described as hyperactive (restlessness, agitation) or hypoactive (flat affect, apathy, lethargy, decreased responsiveness to the environment). Delirium is characterized by acute changes or fluctuations in mental status, inattention, and cognitive changes or perceptual disturbances. Some patients present with a combination of hyperactive and hypoactive delirium. Delirium is usually rapid in onset and reversible. In contrast, dementia is a progressive, irreversible loss of intellectual or cognitive abilities like reasoning, math, or abstract thinking and develops more slowly. Delirium and dementia are not mutually exclusive; a patient with mild to moderate dementia may exhibit delirium in the unfamiliar environment of the hospital.
Contributing factors to the development of delirium include systemic illness (infection, fever, or metabolic dysfunction), inadequate pain control, electrolyte abnormalities, the administration of medications including benzodiazepines or opioids, sleep deprivation, and withdrawal from alcohol or other substances. Delirium is more common in older patients. The first step in treating delirium is to rule out reversible causes. Nursing strategies to prevent delirium and decrease its effects include reorientation, encouraging progressive mobility, modulating stimulation, providing appropriate cognitive activities, promoting normal sleep-wake cycles, ensuring that assistive devices such as hearing aids and glasses are available, treating pain, and family presence. Family members are educated about delirium and provided with guidance in how to interact with the patient (speak clearly and directly, provide frequent reorientation, and avoid multiple simultaneous conversations). Restraints are not used unless patient or staff safety is compromised, because they only add to the patient’s confusion and apprehension. In addition to environmental controls, medications can be useful in the management of delirium.
Patients with organic brain disease, regardless of specific diagnosis, often exhibit challenging behaviors. Examples include agitation, emotional lability, and disinhibition. This can be very disconcerting to family members, especially when the patient has not exhibited these behaviors previously. Dealing with agitated, confused patients can be frustrating for staff as well. Although medication administration can be necessary to keep the patient safe, many drugs alter neurologic assessment, delay recovery, or even worsen symptoms. Environmental strategies such as decreasing noise and distractions can be very effective and are always used first. If medications are required to maintain safety, they are combined with environmental strategies and used at the lowest dose possible for the shortest time possible.
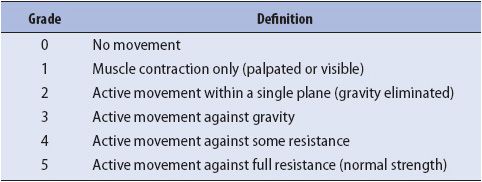
Motor assessment includes muscle size, tone, strength, and involuntary movements such as tics or tremors. Motor function is assessed in each extremity and evaluated for symmetry. In patients who are able to follow commands, pronator drift is an excellent indicator of upper extremity motor function. To assess pronator drift, instruct the patient to close his or her eyes and raise his or her arms with the palms facing the ceiling. A normal response is for the patient to maintain this position until told to stop. Patients with focal motor weakness demonstrate varying degrees of pronator drift. Depending on the severity of weakness, the affected side may drift away from its initial position quickly or slowly, or the palm may simply begin to pronate (Figure 12-2). Further assessment of upper extremity strength involves testing the deltoids, biceps, triceps, and grips. Lower extremity testing includes the hamstrings, quadriceps, dorsiflexion, and plantar flexion. Strength is rated on a 5-point scale (Table 12-3). In patients who do not follow commands, motor assessment consists of first observing the patient for spontaneous movement. If necessary, a pain stimulus is applied and the patient’s response is observed. The response is graded numerically as part of the GCS or FOUR score, but may also be described as purposeful, nonpurposeful, or no response.
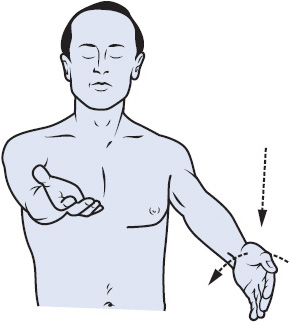
Figure 12-2. Assessment of pronator drift. The patient is asked to hold her or his arms outstretched with the palms supinated and eyes closed. If weakness is present, the weak arm gradually pronates and drifts downward. (Reprinted from: Lindsay KW, Bone I, Callander R. Neurology and Neurosurgery Illustrated. New York, NY: Churchill Livingstone; 1997:19.)
TABLE 12-3. EVALUATION OF MUSCLE STRENGTH
In an awake, alert patient, complete motor assessment includes testing of coordination, an indicator of cerebellar function. Common testing mechanisms include assessment of rapid alternating movements, finger-to-nose testing, and the heel slide test. To test rapid alternating movements, ask the patient to supinate and pronate his or her hands as quickly as possible. In finger-to-nose testing, the patient is instructed to repeatedly touch his or her nose, then the examiner’s finger. To assess the lower extremities, ask the patient to run the heel of his or her foot up and down the shin of the opposite leg as quickly as possible. Patients with cerebellar dysfunction display decreased speed and accuracy on these tests.
There are three basic sensory pathways: pain/temperature, position/vibration, and light touch. Light touch is the pathway most often assessed in the progressive care unit, but may be preserved even if lesions of the spinal cord exist because of overlapping innervation. Because most patients with intracranial lesions report altered sensation in an entire extremity or one side of the body, assessment of light touch is likely to identify these patients. Ask the patient to close his or her eyes, and lightly touch each extremity working distal to proximal. Trunk and facial sensation is also assessed.
When a more comprehensive nursing assessment is indicated, testing for pain and position sense provides useful information. A cotton tip applicator with a wooden stem can be broken and used; the end with the cotton is dull and the broken end is sharp. Touch the patient’s skin lightly in a random pattern and ask the patient to identify the sensation as sharp or dull. Two seconds should elapse between stimuli. To test position sense, or proprioception, move the patient’s index finger or big toe up or down by grasping the digit laterally over the joints. Provide an example of both “up” and “down” positions prior to testing. Repeat these movements in a random order, asking the patient to identify whether the joint is up or down. Always return to the neutral position between movements and carefully grasp the digit to avoid giving the patient clues.
Sensory assessment is performed with the patient’s eyes closed. Documentation of comprehensive sensory assessment is best accomplished using a dermatome chart (Figure 12-3). Areas of abnormal sensation can be marked and tracked over time.
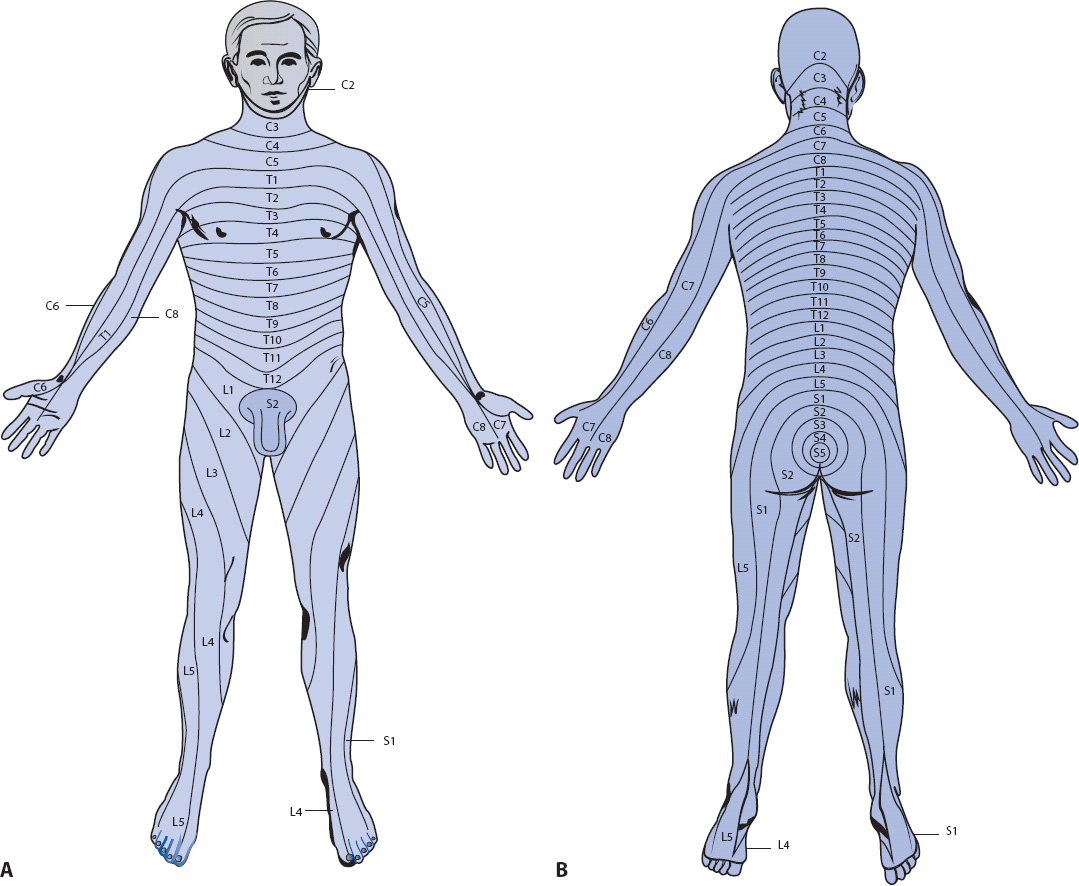
Figure 12-3. Dermatomes. (A) Anterior view. (B) Posterior view. (Reprinted from: Carlson BA. Neurologic anatomy and physiology. In: Urden LD, Stacy KM, Lough ME, eds. Thelan’s Critical Care Nursing: Diagnosis and Management. St Louis, MO: Mosby; 2002:641.)
Assessment of the cranial nerves provides an indication of the integrity of the nerves themselves and of brain stem function. A screening examination based on pupillary response and protective reflexes (corneal, gag, cough) is conducted on all patients. Beyond that, the assessment can be customized to the individual based on pathology and the ability to participate in a more comprehensive exam. Patients with brain stem, cerebellar, or pituitary lesions merit more extensive assessment because of the proximity of the cranial nerves to these structures. The assessments noted below are the most commonly performed tests of cranial nerve function in the progressive care unit. Table 12-4 describes the function of all 12 cranial nerves.
Assessment of pupil size and reaction to light is performed in all patients and provides information about the function of cranial nerves II (optic) and III (oculomotor). Pupils are assessed for size, shape, and reaction to light. These are measured in millimeters, not described by words such as large, small, pinpoint, or blown. Reaction to light is described as brisk, sluggish, or fixed/nonreactive. Both eyes are tested for direct and consensual response. To test direct pupillary response, shine a light directly into one eye and observe the response of the pupil in that eye. A normal response is brisk constriction followed by brisk dilation when the light is withdrawn. To test for consensual pupillary response, shine a light into one eye and observe the pupil of the other eye. It should constrict and dilate similarly. Assessing both direct and consensual response provides information about which cranial nerve (optic or oculomotor, and left or right) is affected. Certain medications can affect pupil size and reactivity; for example, atropine can dilate the pupils and narcotics can cause them to become very constricted. Pupillary changes are often seen late in the course of neurologic decline as increased intracranial pressure (ICP) leads to compression or stretching of cranial nerve III.
The corneal reflex evaluates cranial nerves V (trigeminal) and VII (facial). This test is classically performed with a wisp of cotton lightly drawn across the cornea; a normal response is a blink. A drop of sterile saline can also be used as a stimulus and is less likely to cause corneal abrasions. In alert patients, cranial nerves V and VII can be assessed by testing facial movement and sensation. Movement is assessed by asking the patient to smile, puff out his cheeks with air, and raise his eyebrows. Assessment of facial sensation includes all three branches of cranial nerve V (the trigeminal nerve). The three distributions can be tested by touching the forehead, cheek, and mandible. Patients with cranial nerve VII dysfunction are unable to close the eyelid on the affected side. Strategies to prevent corneal injury include the use of lubricating drops and ointments or taping the lid closed.
The ability to swallow and the gag reflex are controlled by cranial nerves IX (glossopharyngeal) and X (vagus). To assess the gag reflex in a conscious patient, first explain the procedure and be sure the patient does not have a full stomach. Ask the patient to open his mouth and protrude his tongue (this also provides partial assessment of cranial nerve XII, the hypoglossal nerve). Observe the palate for bilateral elevation when the patient says “ahhh.” If the palate does not elevate symmetrically, lightly touch the back of the throat with a tongue blade and observe the response. Both the left and right sides should be tested. To assess the gag reflex in an unconscious patient, use a bite block to keep the patient’s teeth separated, then stimulate the back of the throat with a suction catheter or tongue blade. An intact gag reflex is indicated by forward thrusting of the tongue and sometimes the head. The cough reflex is also controlled by cranial nerves IX and X, and can be assessed by noting spontaneous cough or cough in response to suctioning.
Extraocular eye movements are controlled by muscles innervated by cranial nerves III, IV, and VI. To test extraocular movements, the patient is asked to follow an object (usually the examiner’s finger) through six positions (Figure 12-4). A normal response consists of the eyes moving in the same direction, at the same speed, and in constant alignment (conjugate eye movement). Abnormal eye movements include nystagmus (a jerking, rhythmical movement of one or both of the eyes) or an extraocular palsy (eye movement in one or both eyes is inhibited in a certain direction). Mild nystagmus with extreme lateral gaze may be normal. Dysconjugate gaze, in which the eyes are not aligned, is an abnormal finding.
Figure 12-4. Extraocular eye movements. (A) Extraocular muscles. The eye movement controlled by the muscle is noted in parentheses, along with the associated cranial nerve supply. (B) The six cardinal directions of gaze and associated cranial nerves. (Reprinted from: Carlson BA. Neurologic clinical assessment. In: Urden LD, Stacy KM, Lough ME, eds. Thelan’s Critical Care Nursing: Diagnosis and Management. St Louis, MO: Mosby; 2002:652.)
Vital sign changes due to central nervous system dysfunction occur because of direct brain stem injury, decreased cerebral perfusion, or interruption of nerve pathways. Decreased perfusion causes ischemia and the body’s response is to increase the blood pressure in an attempt to provide more nutrients to the brain. Hypotension is rarely seen except in the terminal stages of brain stem dysfunction or as the result of loss of sympathetic tone in patients with spinal cord injury. Abnormalities in heart rate and rhythm are common, and can be a cause of neurologic decline due to clot formation or inadequate cardiac output, or be a symptom of neurologic dysfunction (such as ST-segment abnormalities following subarachnoid hemorrhage). Respiratory patterns vary widely. Some of the more common patterns are shown in Figure 12-5. It is more important to determine if the patient is ventilating adequately than to determine the specific pattern. Temperature is carefully monitored in patients with acute neurologic dysfunction, because hyperthermia (regardless of infectious or noninfectious etiology) causes increased cerebral metabolic demand. Hypothermia can result from injury to the brain stem or spinal cord.
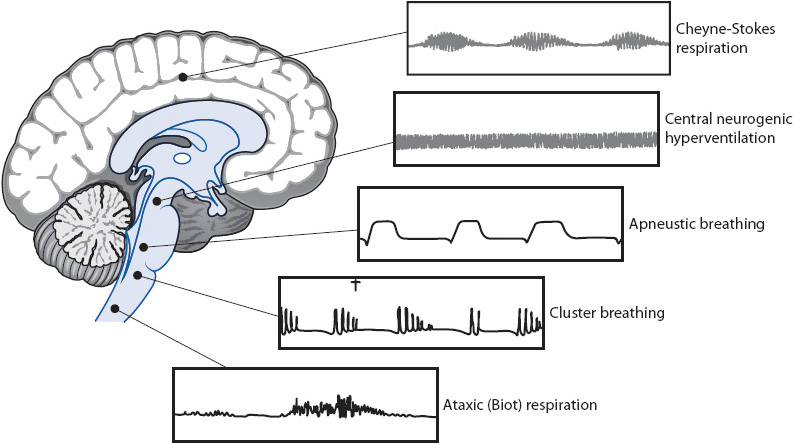
Figure 12-5. Abnormal respiratory patterns associated with increased ICP. Cheyne-Stokes respiration, arising from deep inside the cerebral hemispheres and basal ganglia; central neurogenic hyperventilation, from lower midbrain to middle pons; apneustic breathing, from middle to lower pons; cluster breathing, from upper medulla; and ataxic (Biot) respiration, from medulla. (Reprinted from: Barker E. Intracranial pressure and monitoring. In: Barker E, ed. Neuroscience Nursing: A Spectrum of Care. St Louis, MO: Mosby; 2002:389.)
Cushing response refers to a triad of vital sign changes seen late in the course of neurologic deterioration. The classic triad is marked by widened pulse pressure, bradycardia, and an irregular respiratory pattern. Cushing response is of minimal value in identifying early, significant changes in the patient’s condition, but it is useful to be alert for components of Cushing response (eg, systolic hypertension or change in respiratory pattern).
Lumbar puncture (LP) can be performed for diagnostic or therapeutic purposes. Diagnostic indications for LP include measurement of cerebrospinal fluid (CSF) pressure as an estimation of ICP and sampling of CSF for analysis when central nervous system (CNS) infection, inflammation, or subarachnoid hemorrhage is suspected. Therapeutic indications for LP include drainage of CSF and the placement of tubes for medication administration or ongoing CSF drainage. Examples of disease processes in which LP is used for diagnostic or therapeutic purposes include meningitis, multiple sclerosis, Guillain-Barré syndrome, hydrocephalus, and subarachnoid hemorrhage. Increased ICP is a theoretical contraindication to LP because of the risk for downward herniation of brain tissue due to the pressure gradient created when CSF is removed from the lumbar space. When increased ICP is suspected, a CT scan (described later) may be performed prior to proceeding with the LP. Other contraindications include coagulopathy or infection in the area of skin through which the needle will be introduced. Although recommendations about duration vary based on the specific medication, anticoagulant and antiplatelet medications are often held when LP is planned.
When performing an LP, the clinician locates the L3 to L4 or L4 to L5 intervertebral space and injects a local anesthetic, then inserts a hollow needle with a stylet into the spinal subarachnoid space. The risk of spinal cord injury is minimal because the actual cord ends at L1 and only nerve roots continue below. Proper patient positioning is very important and patients may require sedation if they are unable to remain still. The LP may be performed with the patient sitting up and leaning forward, but a lateral decubitus position is used for most acutely ill patients. The patient lies on his side with his neck flexed forward and his knees pulled up toward the chest. This position widens the intervertebral space, allowing the needle to pass through more easily. The needle is inserted and the stylet is removed. Flow of CSF confirms that the needle is in the spinal subarachnoid space. A manometer is attached to the needle and used to measure an opening pressure. Pressures greater than 20 cm (200 mm) H2O are considered abnormal. If the purpose of the LP is sampling or removal of CSF, the amount of CSF drained varies based on the indication for the procedure, with smaller volumes needed for laboratory analysis than for treatment of hydrocephalus. If the purpose of the procedure is administration of medications or placement of a lumbar drain, the medications will be given or the drain will be placed once needle placement is confirmed by CSF flow.
Normal CSF is clear and colorless. Infection and blood can change the appearance of CSF. In infection, CSF may be cloudy owing to white blood cells and bacteria. Blood causes the CSF to be pink, red, or brown. Although some blood may be present if a small vessel was traumatized during needle insertion, this blood clears as more CSF is drained. Blood due to CNS hemorrhage does not clear. Common tests performed on CSF include analysis of cell counts with differential, glucose, protein, lactate, Gram stain, and culture with sensitivities. Special assays may be requested to look for specific inflammatory or demyelinating disease processes. Once the needle is removed, a small self-adhesive bandage is placed over the insertion site.
Postprocedure care varies with physician preference, hospital protocol, and whether or not the patient complains of headache, but always includes monitoring the insertion site for bleeding, drainage, or hematoma development. Patients may complain of headache (due to loss of CSF), local pain at the insertion site, or pain radiating to the thigh (if a nerve root was hit during the procedure). Flat positioning and increased fluid intake are sometimes recommended after LP but have not been shown to reduce the incidence of post-LP headache. If headache does occur, these strategies are used in combination with analgesic administration. If the headache persists, an autologous blood patch may be used to stop continued CSF leakage.
Computed tomography (CT) is a common diagnostic tool when neurologic dysfunction is suspected. An x-ray beam moves in a 360° arc and a detector measures penetration of the x-ray beam into tissue. Penetration of the x-ray beam varies based on tissue density. The computer translates the collected x-ray beams into images. The result is a series of finely cut pictures showing bony structures, CSF, and brain tissue. Bone is visualized as white because it is most dense. CSF and air are black because of their low density. Brain tissue is seen in varying shades of gray. The appearance of recent intracranial bleeding is white; over time the color darkens as the blood breaks down. CT scans are quick, noninvasive, and easy to perform, and can identify most causes of acute neurologic deterioration, including bleeding, significant edema, and hydrocephalus.
Computed tomography scanning can be performed with a contrast medium to allow for better visualization of lesions such as tumors, abscesses, or vascular abnormalities. CT angiography (CTA) uses scanning during intravenous contrast administration to allow visualization of cerebral blood vessels. CTA is useful in the diagnosis of cerebral vascular anomalies, such as aneurysms or narrowed vessels. A three-dimensional reconstruction of the cerebrovasculature can be created from the images by using a special computer program.
During CT, the patient is placed on a narrow table that is moved up into a donut-shaped gantry. Because the table is narrow, the patient is positioned carefully and secured with padding or straps. Patient movement causes blurry images. Sedation may be required for patients who are unable to cooperate. In patients who receive contrast, assessment of renal function (blood urea nitrogen, creatinine, glomerular filtration rate) is essential because the contrast agent can cause acute kidney injury, especially if the patient is dehydrated or has pre-existing renal compromise, or if given in combination with other nephrotoxic agents. Because the administration of iodinated contrast medium has been associated with lactic acidosis, metformin is discontinued if contrast administration is anticipated.
The primary risks of CT scans result from the use of contrast. Patients with a history of allergic reaction to contrast or iodine require premedication. If contrast dye is administered, intravenous fluids are given before and after the study to decrease the risk of contrast-induced nephropathy (CIN). For more on CIN see Chapter 15, Renal System.
Magnetic resonance imaging (MRI) offers greater anatomic detail than CT scanning without using ionized radiation. The patient is placed in a strong magnetic field and controlled bursts of radio pulse waves are delivered, causing protons within atomic nuclei to resonate. The radiofrequency signals emitted by the resonating nuclei are measured and used to construct images. Cross-sectional images can be obtained in coronal, sagittal, and oblique planes. A contrast agent is sometimes administered, and highlights areas where the blood-brain barrier is disrupted. MRI scans are useful in diagnosing disorders of the brain stem, posterior fossa, and spinal cord, areas that are difficult to fully evaluate with CT. MRI also offers an advantage over CT in the identification of demyelinating disorders such as multiple sclerosis or neurodegenerative diseases. Specific MRI sequences can be used to detect suspected lesions that cannot be seen on CT, such as early cerebral infarction and intramedullary tumors. Magnetic resonance angiography (MRA) uses a specialized computer program to highlight the cerebral vasculature. MRA is useful in the evaluation of suspected arteriovenous malformations (AVMs), aneurysms, and cavernous angiomas. Acute bleeding and bony abnormalities such as fractures can be better visualized using CT. The time requirement for MRI scans is typically longer than that of CT scans, which can be a disadvantage when needing to make treatment decisions based on diagnostic results. In addition, access to the patient is significantly limited during the scan.
All patients must be screened for the presence of implanted or embedded metal prior to MRI. Metallic objects inside the body may become dislodged or slip in the large magnetic tube and can cause patient injury. Most aneurysm clips are now made of nonferrous material and are safe for MRI; it is important to obtain additional information about the device, including when and where it was placed. Orthopedic hardware may also be safe, depending on the part of the body being imaged and the length of time since the hardware was placed. Patients who are either unable to reliably complete the MRI screening or who have a history of impaled metal fragments or shrapnel must have radiographs taken prior to MRI. The MRI magnet can also damage internally magnetized units, such as cardiac pacemakers, causing them to malfunction. An MRI-safe pacemaker is now available but many patients have older devices. Programmable shunts, frequently used for long-term management of hydrocephalus, are affected by the MRI and must be reprogrammed following the procedure. Devices such as medication pumps and nerve/spinal cord stimulators may or may not be MRI safe. At minimum, they need to be turned off before and reprogrammed after the procedure. With all devices and implants, it is important to obtain as much information as possible about the device type and when it was placed, and to report this information to the MRI technologist. Many IV pumps and other types of medical equipment contain metal and cannot be taken into the room where the MRI machine is located. Of note, the same screening precautions apply to the staff member who accompanies the patient to MRI. Any card with a magnetic strip, such as a credit card or even an employee ID, will be damaged by the MRI magnet and is removed. Patient education is important prior to scanning. Patients must be screened closely for any contraindications. In addition, all metal objects, such as jewelry, nonpermanent dentures, prostheses, hairpins, clothing with snaps or zippers, and ECG electrodes with metal snaps must be removed. Transdermal medication patches may also need to be removed. Patients should be advised of the loud “booming” noise of the scanner. Inform patients that the nurse or technician is in full view of them in the scanner and that they can talk to them if they feel uncomfortable on the table. Ensure the safety and comfort of the patient with safety belts and blankets for positioning. Patients who are claustrophobic may need sedation. Open-sided MRI machines are available at some institutions and decrease feelings of claustrophobia. There are no postprocedure interventions associated with MRI. Gadolinium-based contrast agents are sometimes administered in MRI and have been associated with nephrogenic systemic fibrosis (NSF) when given to patients with severe renal insufficiency, so renal function is assessed prior to administration. NSF causes fibrotic changes in the skin and other organs.
Although CTA and MRA are commonly used to assess the cerebrovasculature, catheter angiography remains the gold standard. Cerebral (catheter) angiography is similar to cardiac catheterization. Angiography can be performed for both diagnostic purposes and therapeutic intervention. Blockages or abnormalities of the cerebral circulation can be visualized, aiding in the diagnosis of vascular malformations (such as aneurysms or AVMs) and arterial stenosis. Angioplasty (with or without stent placement) can be performed for narrowed cerebral vessels. Blood vessels can also be therapeutically embolized; this is sometimes done to decrease blood supply to a tumor prior to surgical resection or as treatment for an aneurysm.
During cerebral angiography, a catheter is placed in the femoral or brachial artery and threaded up into the carotid or vertebral arteries, and a radiopaque contrast material is injected. The flow of the contrast material is tracked using radiographic films and fluoroscopy. Patients are kept NPO for 6 hours prior to nonemergent angiography. Coagulation studies are checked on all patients because coagulopathy is a relative contraindication to cerebral angiography. Many patients require sedation during the procedure. General anesthesia may be needed for uncooperative patients because the risk of vessel injury is increased if the patient moves her or his head during the procedure.
Potential complications include neurologic deficit due to injury to an intracranial vessel, allergic reaction to contrast, hematoma formation at the site of catheter insertion, vessel injury (dissection), retroperitoneal hematoma, and vessel spasm following injection of contrast. All patients undergoing cerebral angiography receive hydration because of the large amount of contrast agent used.
Following angiogram, patients are typically kept on bed rest with the head of bed flat for 4 to 6 hours to help prevent hematoma formation at the puncture site. In some cases, a special arterial closure device is used to promote clot formation and allow quicker mobilization, typically after about 2 hours. The amount of time the patient must remain flat is reflected in the postangiography orders. The arterial puncture site is monitored frequently for development of a hematoma, and the neurovascular status of the limb is also checked. Careful monitoring of vital signs and neurologic examination aid in the detection of intra- or extracranial emboli or hemorrhage.
Transcranial Doppler (TCD) ultrasound studies allow visualization of the blood flow through major cerebral blood vessels by directing ultrasonic waves through the thinner parts of the skull bone. A probe that emits ultrasonic waves is placed on the skin. Structures are differentiated based on how much of the wave is reflected back to the probe. A Doppler effect is created when the probe detects moving structures, like red blood cells in a blood vessel. The velocity of blood flow can be calculated. TCDs are noninvasive and can be done at the patient’s bedside. TCDs are used at many institutions to aid in the detection of vasospasm after aneurysmal subarachnoid hemorrhage.
The electroencephalogram (EEG) is a measurement of the brain’s electrical activity. EEG is performed by attaching a number of electrodes to standard locations on the scalp. These electrodes are attached to a recorder, which amplifies and records the activity. EEG is useful in evaluating causes of coma (structural vs metabolic), identifying seizure disorders, and determining the anatomic origin of seizures.
A routine EEG usually lasts 40 to 60 minutes with a portable machine for bedside use. The patient is instructed to lie still with his or her eyes closed. A mild sedative may be prescribed for restless or uncooperative patients, but the interpreter of the EEG must be aware of this because medications may cause changes in the recording. Documentation during the study is done by the technician and may include changes in blood pressure, changes in level of consciousness, medications the patient is currently taking or has taken within 48 hours, patient movement or posturing, and any noxious stimuli introduced. It is best to plan nursing care around the time of the test so that no interventions are done during this examination. When the EEG is complete, the electrodes are removed and any medications that were held prior to the study are resumed. In patients with symptoms concerning for seizure, such as intermittent twitching or fluctuating mental status, a prolonged EEG may be ordered in an attempt to correlate the symptoms with EEG findings. This type of EEG often lasts up to 24 hours, and requires the nurse to note any occurrences of the symptom or behavior thought to represent possible seizure activity.
Continuous EEG monitoring is used to guide treatment in patients with status epilepticus. Patients with status epilepticus are transferred to the intensive care unit for management because invasive airway management and continuous infusions of medications are typically required. Continuous EEG monitoring is also used in the diagnosis and management of intractable or difficult-to-control seizures, usually in conjunction with video monitoring. Continuous monitoring can also be helpful in identifying non-epileptic seizures.
Electromyography (EMG) evaluates the electrical activity of skeletal muscle during movement and rest. Nerve conduction studies (NCS) evaluate peripheral nerve function by measuring the transmission of electrical impulses after stimulation. Conditions in which EMG may aid diagnosis include myopathies and neuropathies, myasthenia gravis (in which the neuromuscular junction is affected), and Guillain-Barré syndrome. The patient may experience some pain related to insertion of the needle electrodes.
The skull in adults is a closed, nondistensible compartment that contains three components: brain parenchyma (80%), blood (10%), and CSF (10%). The Monro-Kellie hypothesis states that to maintain a constant intracranial volume, an increase in any of the three components must be accompanied by a decrease in one or both of the other components. If this reciprocal decrease does not occur, ICP rises. The body is able to compensate for a limited amount of increased intracranial volume by displacement of intracranial venous blood, decreased production of CSF, or displacement of CSF into the spinal subarachnoid space. ICP rises when these compensatory mechanisms have been exceeded (Figure 12-6). Compliance refers to the change in volume needed to result in a given change in pressure and reflects the effectiveness of the compensatory mechanisms. With decreased compliance, a small increase in volume results in a large increase in ICP. Compliance is based on several factors, including the amount of volume increase and the time over which the increase occurs. Smaller increases in volume result in less increase in pressure. Increases in volume that occur over a long period of time are better tolerated than rapid increases because there is time for compensation to occur. Older adults typically have increased compliance because of cerebral atrophy. Increased ICP can result in cerebral hypoperfusion, ischemia, herniation, and eventually death.
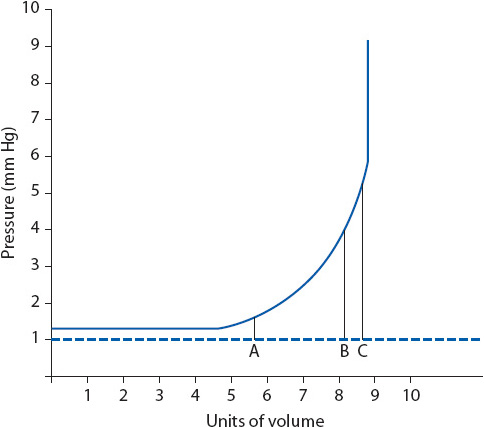
Figure 12-6. Intracranial volume-pressure curve. (A) Pressure is normal, and increases in intracranial volume are tolerated due to compensatory mechanisms. (B) Increases in volume may cause increases in pressure. (C) Small increases in volume may cause large increases in pressure (compensatory mechanisms have been exceeded). (Reprinted from: Mendez KA. Neurologic therapeutic management. In: Urden LD, Stacy KM, Lough ME, eds. Thelan’s Critical Care Nursing: Diagnosis and Management. St Louis, MO: Mosby; 2002:702.)
The brain cannot store oxygen or glucose in significant quantities. Therefore, constant blood flow is required to maintain cerebral metabolism. If cerebral blood flow (CBF) is insufficient, brain cells do not receive sufficient substrate to function and will eventually die. CBF is determined by blood pressure and cerebral vascular resistance.
Autoregulation refers to the ability of cerebral blood vessels to maintain consistent CBF by dilating or constricting in response to changes in blood pressure. Vasodilation occurs in response to decreased blood pressure; increased blood pressure results in vasoconstriction. In persons without neurologic disease, autoregulation allows consistent CBF when mean arterial pressure is 60 to 160 mm Hg. In the injured brain, the autoregulatory response becomes less predictable. When autoregulation is impaired, CBF becomes dependent on systemic arterial pressure.
Cerebral vascular resistance can also be altered through chemoregulatory processes. An increase in the pressure of arterial carbon dioxide (Paco2) produces a lower extracellular pH and causes dilation of cerebral vessels. Conversely, a decrease in Paco2 raises pH and results in cerebral vasoconstriction. Vasodilation also results from Pao2 levels less than 50 or a buildup of metabolic by-products such as lactic acid. Other factors can decrease cerebral vascular resistance and thus alter CBF, including certain anesthetic agents (halothane, nitrous oxide), sodium nitroprusside, and some histamines.
Increased ICP occurs as a result of cerebral edema, mass lesions, increased intracranial blood volume, or increased amounts of CSF. These factors often occur in combination. Pain, suctioning, or an overstimulating environment can also increase ICP.
Cerebral edema is an abnormal accumulation of water or fluid in the intracellular or extracellular space, resulting in increased brain volume. Vasogenic edema results from increased capillary permeability of the vessel walls, which allows plasma and protein to leak into the extracellular space. Cytotoxic edema occurs when fluid collects inside the cells due to failure of cellular metabolism. This causes further breakdown of the cell membrane. Cytotoxic edema can lead to capillary damage, which then results in vasogenic edema.
Mass lesions in the brain parenchyma include brain tumors, hematomas, and abscesses. In addition to raising ICP, mass lesions contribute to ischemia by compression of cerebral vessels.
Venous outflow obstruction can result from compression of the jugular veins (neck flexion, hyperextension, rotation), causing an increase in intracranial blood volume. Increased intrathoracic pressure or increased intra-abdominal pressure (Trendelenburg position, prone position, extreme hip flexion, Valsalva maneuver, coughing, tracheal suctioning) also results in venous outflow obstruction. As discussed previously, cerebral vasodilation occurs as the result of hypoxia, hypercapnia, increased metabolic demands, drug effects, or increased systemic blood pressure combined with autoregulatory failure; these factors cause an overall increase in intracranial blood volume.
Approximately 500 mL of CSF is produced every day. CSF normally flows through the ventricular system into the subarachnoid space where it is absorbed by the arachnoid granulations (Figure 12-7). Obstruction of CSF flow, decreased reabsorption of CSF, or increased production leads to increased intracranial CSF volume (hydrocephalus). Hydrocephalus is referred to as communicating or noncommunicating (also called obstructive). In meningitis or subarachnoid hemorrhage, the arachnoid granulations become clogged with cellular debris and cannot absorb CSF normally, which leads to communicating hydrocephalus. An example of noncommunicating hydrocephalus is obstruction of CSF flow due to a tumor or cyst in the third ventricle of the brain.
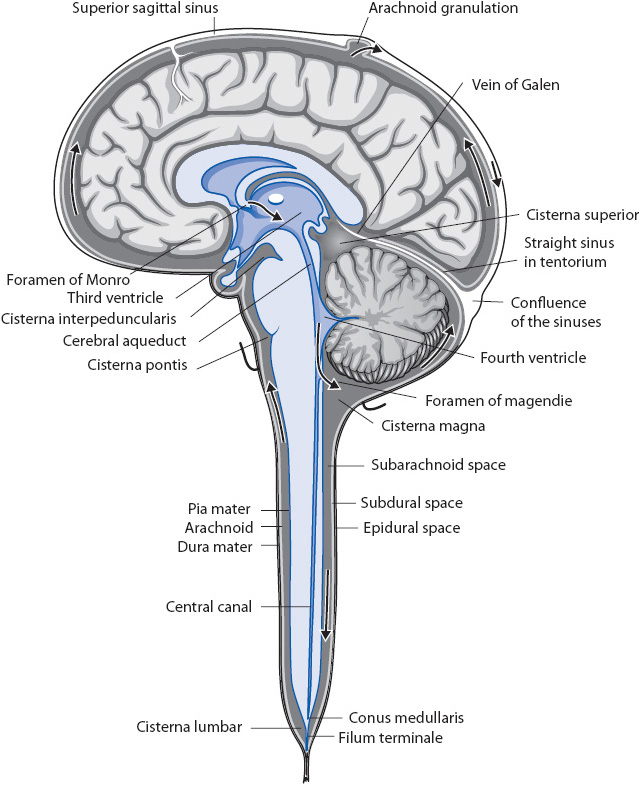
Figure 12-7. Flow of CSF/ventricular system. Drawing illustrates the ventricular system and other structures involved in CSF production, flow, and reabsorption. Arrows indicate the normal direction of flow of CSF. (Reprinted from: Novack CR, Demarest RJ. Meninges, ventricles, and cerebrospinal fluid. In: The Nervous System: Introduction and Review. New York, NY: McGraw-Hill;1986:46.)
Early signs of increased ICP include confusion, restlessness, lethargy, disorientation, headache, nausea or vomiting, and visual abnormalities such as diplopia. Change in level of consciousness is the most important indicator of elevated ICP. The patient may become unable to follow commands and develop motor deficits; abnormal posturing is an ominous sign. Changes in vital signs may occur. Increased systolic blood pressure is the body’s attempt to maintain cerebral perfusion. As ICP worsens, alterations in heart rate or respiratory pattern may also emerge. Pupillary changes are usually late signs of increased ICP. Any of these signs and symptoms requires immediate physician notification. Unless the cause of elevated ICP is known, a CT scan is ordered to evaluate for mass lesions (tumor, blood clot) or hydrocephalus. Invasive ICP monitoring devices are discussed in Chapter 20, Advanced Neurologic Concepts.
Prolonged elevation of ICP may result in cerebral herniation. Folds in the dura mater divide the intracranial cavity into several compartments. Herniation is the distortion and displacement of the brain from one compartment to another, which damages structures and decreases CBF through compression. Classic signs associated with herniation reflect pressure on the brainstem and surrounding structures. Level of consciousness deteriorates, and the patient may demonstrate decorticate or decerebrate posturing. Compression or stretching of the oculomotor nerves (cranial nerve III) causes pupil changes; typically, pupil asymmetry is noted first, followed by a large non-reactive pupil on one side. As compression continues, the other pupil also becomes large and non-reactive and vital sign changes (Cushing response, altered respiratory pattern) occur. When any of these classic signs are noted, emergency action is needed to prevent brain death from occurring.
Management focuses on early recognition of increased ICP, avoiding activities known to elevate ICP, and aggressive treatment if changes in neurologic examination occur. Principles of management of increased ICP follow.
Assess baseline neurologic signs, then reassess frequently and compare to previous findings. Include level of consciousness, coma score, pupillary size and reaction to light, eye movement, and motor and sensory function. Assess vital signs and compare with previous findings to identify trends. Close monitoring of neurologic status facilitates the identification and treatment of complications, such as the development of an epidural or subdural hematoma. In these cases, surgical evacuation of the hematoma may be required. In cases of diffuse cerebral edema, a portion of the skull may be removed to increase compliance and allow the brain to swell outside the contained area of the skull. This procedure is referred to as a craniectomy.
Both hypoxemia and hypercarbia can result in cerebral vasodilation and increased ICP. Hyperventilation is not routinely used to decrease ICP because the resulting decrease in Paco2 may lead to vasoconstriction and worsen cerebral ischemia. Controlled hyperventilation can be used in the setting of impending herniation to “buy time” for other measures to be implemented and take effect.
In the absence of invasive monitoring, blood pressure and fluid management varies based on the underlying disease process. Hypotension is avoided. If the patient is hypotensive, nonglucose-containing fluids are infused to ensure euvolemia; vasopressors may also be required.
Because the venous system of the brain is valveless, increased intrathoracic or intra-abdominal pressure reduces venous return and increases ICP. In general, the head of the bed is elevated to 30°. Hip flexion is minimized. A bowel regimen is used to avoid constipation.
Neck positioning affects venous drainage and can raise ICP. The head and neck are maintained in a neutral position, avoiding flexion, hyperextension, or rotation. Cervical collars are carefully applied to avoid decreasing jugular venous return.
Seizure activity increases cerebral metabolic demand and ICP. The prophylactic use of anticonvulsants is common in neurologically impaired patients at risk for seizures. Additional information on the management of seizures is included later in this chapter.
Fever increases ICP by increasing metabolic demand. For each elevation of 1°C, cerebral metabolic demand increases by approximately 6%. Methods to normalize temperature include antipyretics and air-or water-filled cooling blankets. Shivering increases metabolic demand and is avoided.
Agitation also increases cerebral metabolic demand. Work with other healthcare providers and the patient’s family to maintain a calm, quiet environment. Agitation due to pain is avoided through the use of analgesics. If analgesics or sedatives are used, short-acting agents are preferred because of the importance of on-going neurological assessment. Careful monitoring of respiration is indicated due to the effect of these medications on ventilation; hypoventilation can cause increased ICP.
Cerebrospinal fluid drainage via an intraventricular catheter can be used to lower ICP. Additional information on the use of intraventricular catheters for CSF drainage can be found in Chapter 20, Advanced Neurologic Concepts.
Osmotic diuretics reduce cerebral edema by pulling extracellular fluid from brain tissue into the blood vessels. Mannitol is the most commonly used agent and is given as a bolus dose of 0.25 to 1 g/kg body weight. Mannitol is administered using a filter because it crystallizes easily. Euvolemia is maintained and electrolytes are closely monitored. Some practitioners also use hypertonic saline to increase serum osmolality and pull water into the vascular space.
Corticosteroids (eg, dexamethasone) are useful in decreasing cerebral edema associated with intracranial tumors. Steroids are generally not useful in the management of cerebral edema related to traumatic brain injury or stroke. Potential complications of steroid therapy include gastric irritation or hemorrhage and hyperglycemia.
Other measures to decrease ICP include continuous infusion of analgesics, sedatives, and/or anesthetic agents, the use of neuromuscular blocking agents, and induced barbiturate coma. Patients requiring these interventions are transferred to the intensive care unit for management.
Stroke is the leading cause of death and disability in the United States and worldwide. The brain cannot store oxygen or glucose and therefore requires a constant flow of blood to supply these nutrients. The blood supply to the brain can be altered through several different processes. These include embolism, thrombosis, hemorrhage, and compression or spasm of the vessels. Ischemic stroke due to embolism or thrombus formation accounts for approximately 85% of all strokes. Edema occurs in the area of ischemic or infarcted tissue and contributes to further neuronal cell death. If ischemia is not reversed, neuronal cell death and infarction of brain tissue occurs. The penumbra is an area of tissue that surrounds the core ischemic area. The penumbra receives some blood flow from adjacent vessels but perfusion is marginal. If CBF is improved, the penumbra may recover.
Risk factors for stroke include hypertension, cardiac disease (coronary artery disease, heart failure, atrial fibrillation, endocarditis, patent foramen ovale, myocardial infarction, carotid artery disease), diabetes, increased age, race (African American), male gender, prior stroke, family history, dyslipidemia, hypercoagulability (cancer, pregnancy, high RBCs, sickle cell disease), smoking, obesity, physical inactivity, alcohol or illicit drugs, and some forms of hormone therapy. Transient ischemic attack (TIA) is an important warning sign for stroke. With a TIA, the patient develops stroke symptoms that resolve without tissue infarction. Although most resolve within minutes, an extensive workup to identify treatable causes is warranted with any TIA.
The pathophysiology of stroke varies based on the precipitating event. Thrombosis and embolism formation, described below, result in acute ischemic stroke.
Thrombosis is the most common cause of ischemic stroke and is usually due to atherosclerosis and the formation of plaque within an artery. A thrombus then forms at the site of the plaque and causes brain tissue ischemia along the course of the affected vessel, which results in infarct if not quickly reversed.
Thrombosis because of atherosclerosis of large cerebral vessels results in large areas of infarct. Considerable edema often develops, further increasing ischemia by compressing areas surrounding the infarct. Significant functional deficits are common. If thrombus forms in a smaller branching artery, a lacunar infarct develops. Lacunar infarcts result in smaller areas of neuronal cell death. Deficits are less apparent, unless the infarct is in a crucial area, such as the internal capsule. Patients with a history of atherosclerosis or arteritis are at highest risk for thrombotic strokes. Thrombotic strokes tend to develop during periods of sleep or inactivity, when blood flow is less brisk.
Embolism refers to the occlusion of a cerebral vessel, most often by a blood clot but also by infectious particles, fat, air, or tumor fragments. Embolism is often associated with heart disease that results in bacterial vegetations or blood clots; these vegetations or clots are easily detached from the wall or valves of the heart and then travel to the brain, lodging in a cerebral vessel. Chronic atrial fibrillation, valvular disease, prosthetic valves, cardiomyopathy, and atherosclerotic lesions of the proximal aorta are common causes of embolism. Less common causes include atrial myxomas, patent foramen ovale, and bacterial endocarditis. The fragmented substance easily lodges at the bifurcation of the middle cerebral artery, sometimes breaking apart and traveling further into the cerebral vascular system. The onset of an embolic occlusion is rapid, with symptoms that develop without warning.
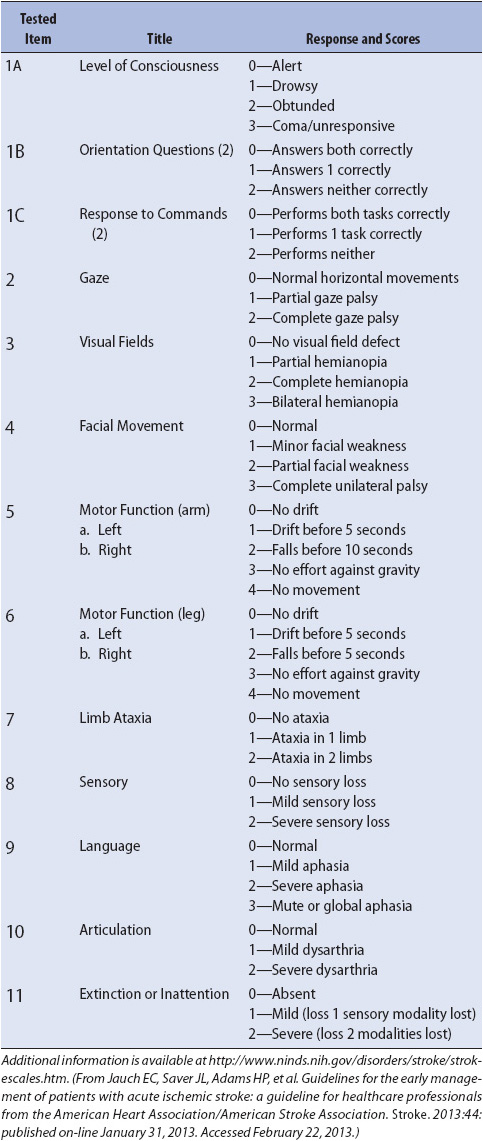
Symptoms of stroke range from very mild to significant loss of functional abilities. Common signs and symptoms include weakness in an extremity or on one side of the body, sensory changes, difficulty speaking or understanding speech, facial droop, headache, and visual changes. Clinical presentation of stroke varies based on the area of ischemia or infarction. The National Institute of Health Stroke Scale (NIHSS) is often used to evaluate and monitor patients after stroke. An overview of the NIHSS scoring system is presented in Table 12-5. Additional training is needed to accurately perform this assessment.
TABLE 12-5. NATIONAL INSTITUTES OF HEALTH STROKE SCALE (NIHSS)
Signs and symptoms occur on the side of the body contralateral to the stroke. Weakness or paralysis occurs in one or both extremities, and sensory loss may be noted. Visual field deficits are also contralateral to the lesion. The patient often displays an ipsilateral gaze preference, in effect “looking to the lesion.” The left hemisphere is dominant in right-handed individuals and many left-handed patients. As the dominant hemisphere, it controls language functions and language-dependent memory. Dominant hemisphere strokes often produce receptive, expressive, or global aphasia. Nondominant hemisphere strokes often cause neglect syndromes in which the patient becomes unaware of the environment and even his or her own body on the contralateral side.
Motor and sensory function may be impaired on one or both sides of the body. Loss of equilibrium, decreased fine motor abilities, and nausea/vomiting are typical. Cranial nerve deficits are common and include dysarthria, nystagmus, dysphagia, and decreased cough reflex. Careful evaluation of airway protection and swallowing ability is essential to determine aspiration risk. Patients with severe deficits often require a feeding tube and potentially a tracheostomy. Because cortical injury is not present, patients maintain a normal mental status and level of alertness unless pressure in the posterior fossa leads to disruption of the reticular activating system.
In patients with cerebellar stroke, obstructive hydrocephalus may occur due to occlusion of the ventricular drainage system by edema. Surgical decompression of the posterior fossa may be necessary and an external ventricular drain may be placed.
Brain stem stroke owing to basilar artery occlusion results in quadriplegia and loss of facial movements (locked-in syndrome). Cognition is intact, and vertical gaze is maintained. These patients will be able to follow commands to look up or down. Early consultation with a speech language pathologist is recommended to help with the development of alternative communication strategies.
The goal of initial diagnostic testing in acute stroke is to rule out intracranial hemorrhage, because treatments for hemorrhagic and ischemic stroke differ significantly. This is typically accomplished by obtaining a noncontrast head CT, which can be quickly and easily obtained, although some centers use MRI for initial testing. Specialized MRI scans (diffusion-weighted imaging, perfusion-weighted imaging) can detect areas of ischemia before they are apparent on CT. MRA detects areas of vascular abnormality, as might be seen with clot due to arterial dissection. Other tests that may be done acutely include cerebral angiography and carotid ultrasound. Transthoracic or transesophageal echocardiography is used to assess cardiac causes of stroke. Hypercoagulable states are detected through laboratory work. All patients who present with stroke receive an ECG, are placed on cardiac monitoring for at least 24 hours, and undergo laboratory evaluation of cardiac biomarkers because of the correlation between cerebrovascular and cardiovascular disease. In addition, conditions that mimic stroke, such as hypoglycemia, must be ruled out.
Stroke is a medical emergency and is treated with the same urgency as acute myocardial infarction. Just as “time is muscle” when the heart is ischemic, “time is brain” when cerebral ischemia occurs. The goals of treatment are to restore circulation to the brain when possible, stop the ongoing ischemic process, and prevent secondary complications. Management principles include the following:
Other conditions may mimic acute ischemic stroke and must be ruled out. Hypoglycemia may cause stroke-like symptoms and is easily detected by using a bedside monitor to check blood glucose. Radiologic tests are performed on all patients with signs and symptoms of stroke to rule out intracranial bleeding. Other conditions that may mimic acute ischemic stroke include toxic or metabolic disorders, migraines, seizures, mass lesions such as brain tumors or abscesses, and psychological disorders.
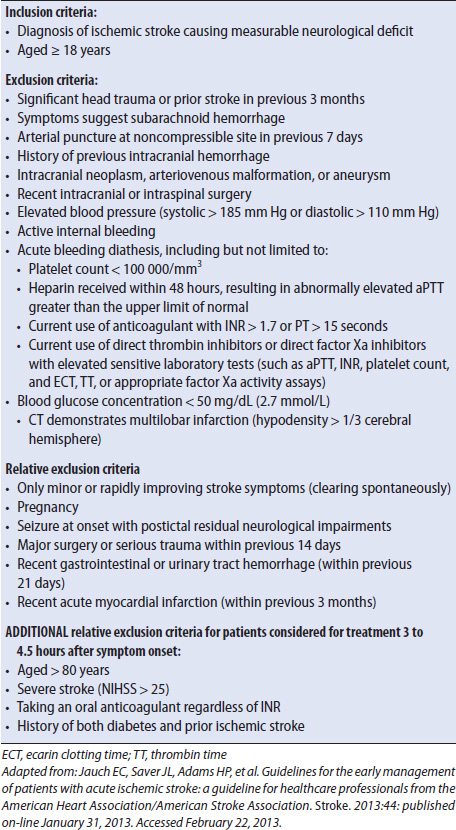
Fibrinolytic therapy is administered in an attempt to restore perfusion to the affected area. IV administration of rtPA is considered in all patients who meet the inclusion/exclusion criteria (Table 12-6) and can be treated within 3 hours of the onset of symptoms. Patients who can be treated between 3 and 4.5 hours after symptom onset can also receive rtPA, although there are several additional exclusion criteria. The recommended dose for rtPA is 0.9 mg/kg, with 10% of the total dose given as a bolus over 1 to 2 minutes followed by the remainder of the dose as an infusion over 1 hour. The maximum dose recommended is 90 mg. In a large-scale study, rtPA administration resulted in improved outcomes at 3 months poststroke. There is an increased risk of intracerebral hemorrhage (ICH) following rtPA administration so frequent neurologic assessments are essential. Vital signs and neurologic checks are done every 15 minutes for the first 2 hours, then every 30 minutes for 6 hours, and then hourly until 24 hours following initial treatment. If neurologic deterioration occurs, rtPA is stopped if still infusing, the physician is notified, and a head CT is performed to assess for bleeding. Following rtPA administration, antiplatelet or anticoagulant medicines are avoided for 24 hours. Placement of nasogastric tubes, bladder catheters, and invasive lines is delayed to decrease the risk of hemorrhage.
TABLE 12-6. INCLUSION AND EXCLUSION CRITERIA FOR TREATMENT WITH RTPA AFTER ACUTE ISCHEMIC STROKE
Endovascular treatment is an option for the management of acute ischemic stroke at some centers. However, the possibility of intra-arterial treatment should not delay the use of intravenous rtPA in patients who are eligible to receive it. Available endovascular therapies include intra-arterial fibrinolysis and mechanical clot extraction or disruption. These treatments, guided by cerebral angiography, must be performed by a physician specially trained in interventional neuroradiology and are not available at all centers. Although rtPA is not FDA-approved for intra-arterial administration, it is sometimes used for patients with middle cerebral artery occlusion who can be treated within 6 hours of the onset of symptoms and are not able to receive intravenous rtPA. Because medication can be infused directly into the thrombus, smaller doses can be used, making this a treatment option for certain patients with exclusion criteria for intravenous rtPA (eg, major surgery in the previous 14 days). Mechanical thrombectomy using a special device may improve recanalization rates when used alone or in combination with fibrinolysis. Care of the patient following endovascular treatment for stroke includes standard post-angiogram monitoring, stroke-specific care, and other interventions as ordered by the physician.
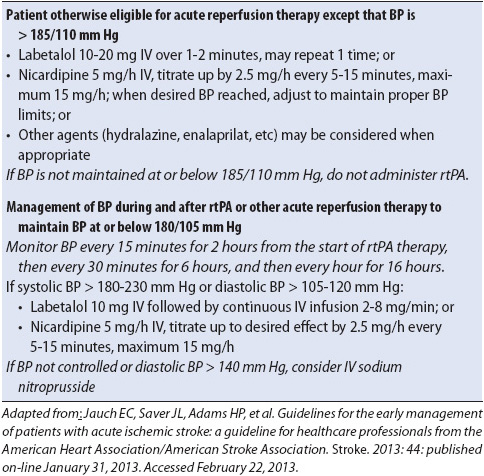
Careful blood pressure management is essential after acute ischemic stroke because a marked or sudden decrease in blood pressure can significantly decrease cerebral perfusion. The physician may elect to hold the patient’s home antihypertensive medications to maximize cerebral blood flow, especially in the first 24 hours after stroke. For patients who are not eligible for fibrinolytic therapy, blood pressure is not treated emergently unless the systolic blood pressure exceeds 220 mm Hg or the diastolic blood pressure exceeds 120 mm Hg. Because of the risk of hemorrhage, blood pressure management is more stringent in patients who are eligible for or who have received fibrinolytic therapy (Table 12-7).
TABLE 12-7. APPROACH TO BLOOD PRESSURE MANAGEMENT AFTER ACUTE ISCHEMIC STROKE IN PATIENTS WHO ARE CANDIDATES FOR REPERFUSION THERAPY
Cerebral edema occurs in the area of infarct and may lead to increased ICP. For further discussion of treatment options, refer to the section on ICP. Hemicraniectomy may be used to alleviate increased ICP in patients with large infarcts, particularly in the distribution of the middle cerebral artery. Aggressive treatment of fever is warranted to avoid increases in cerebral metabolic demand.
Hyperglycemia is associated with worse outcomes after stroke and is treated; lowering blood glucose to 140 to 180 mg/dL is a common goal. Hypoglycemia is deleterious and must be avoided.
Patients are at significant risk for decreased airway maintenance and aspiration following stroke. Decreased level of consciousness, facial weakness, and cranial nerve deficits contribute. Intubation is sometimes necessary during the acute phase, and some patients may need a tracheostomy. Dysphagia is very common after stroke, so careful assessment of swallowing ability is indicated before any oral intake. Many hospitals have dysphagia screening protocols in place, but consultation with the speech language pathologist is often indicated. Placement of a feeding tube may be necessary if the patient is unable to swallow safely.
Deep venous thrombosis is a common complication in stroke patients and may lead to pulmonary embolism. Strategies to decrease risk include elastic compression stockings, intermittent pneumatic compression devices, subcutaneous administration of low-dose anticoagulants, and early progression in activity.
In addition to pneumonia and DVT, patients with stroke are at risk for urinary tract infection (UTI). Indwelling catheters are used only when accurate output is medically necessary and cannot be obtained using alternate methods. When an indwelling catheter is used, it is removed as soon as possible. In patients without indwelling catheters, urinary retention may occur; methods of evaluation include bedside bladder scanning or catheterization for postvoid residual volumes.
The use of antiplatelet and anticoagulant medications varies depending on the size of the infarct, presumed etiology, and whether or not the patient received fibrinolytic therapy. Patients are commonly placed on aspirin within 24 to 48 hours after the initial event and the decision to use other antiplatelet or anticoagulant medications is made on an individual basis. Anticoagulation is typically not used in the acute phase of treatment because it increases the risk of hemorrhagic conversion (development of bleeding within the infarcted tissue), but may be used in certain circumstances.
Carotid endarterectomy is the most common surgical procedure to prevent further ischemic strokes, but is not typically performed in the time period immediately following a stroke due to the risk of reperfusion injury and hemorrhage. Stenosis may also be treated with angioplasty, with or without stent placement.
Other strategies to prevent recurrent stroke include statins for dyslipidemia and behavior modification to address risk factors.
Approximately 15% of all strokes are hemorrhagic. In subarachnoid hemorrhage, bleeding into the subarachnoid space occurs, usually as the result of a ruptured aneurysm. Although subarachnoid hemorrhage is a type of stroke, management issues differ significantly. Subarachnoid hemorrhage is discussed in Chapter 20, Advanced Neurologic Concepts. Here, hemorrhagic stroke refers to intraparenchymal bleeding (also called intracerebral hemorrhage or ICH).
Hypertension is the most common cause of ICH. Other causes include vascular malformations (AVMs or cavernous malformations), coagulopathy, amyloid angiopathy, tumor, vasculitis, venous infarction, and illicit drug abuse. Amyloid angiopathy is most common in patients older than the age of 70. It is a presumed diagnosis in older patients with repeated ICH, but can only be definitively diagnosed by deposits of beta-amyloid protein found in the vessel walls (usually on autopsy). AVM is a common cause of ICH in younger patients (ages 20-40). AVMs are congenital abnormalities in which a tangled mass of blood vessels is present. Within the AVM, the arterial circulation and venous circulation connect without going through a capillary system. Following resolution of the acute ICH, AVMs are treated with endovascular embolization, surgical resection, or stereotactic radiosurgery.
In addition to direct tissue injury, the hematoma formed by ICH displaces nearby brain tissue and causes ischemia through compression. Edema occurs around the site of hemorrhage. If the ICH occurs deep within the cerebral hemispheres, it can rupture into the ventricle (intraventricular hemorrhage). The mortality rate is higher in hemorrhagic stroke than ischemic stroke.
Intracerebral hemorrhage presents with an acute onset of neurologic deficits often associated with a severe headache, nausea/vomiting, decreased consciousness, and sometimes seizures. Neurologic deficits vary based on the area of the brain affected and are similar to the focal deficits experienced by patients with acute ischemic stroke.
Intracerebral hemorrhage is diagnosed using CT scanning or, less commonly, MRI. Tests that may be performed to determine the etiology of the hemorrhage include CTA, MRA, and cerebral angiography.
Initial priorities of care for the patient with ICH include blood pressure control and correction of coagulopathy. Bleeding can continue or recur for several hours after the initial event, so prompt action is essential. Intermittent or continuous intravenous medications are commonly used to keep the systolic blood pressure below 140 to 160 mm Hg. Treatment of coagulopathy is based on the underlying cause of abnormal clotting. Fresh frozen plasma, platelets, vitamin K, or prothrombin complex concentrate may be ordered; regardless of the agent used, the goal is rapid correction of coagulopathy.
Operative management may or may not be indicated based on size and location of hemorrhage. Cerebellar hemorrhage may require a suboccipital craniectomy to evacuate the clot and decrease pressure on vital structures. Intraventricular hemorrhage may cause hydrocephalus, which is treated by placement of an external ventricular drain. Antiepileptic drugs (AEDs) are recommended for patients who experience a seizure or who show electrographic evidence of seizure on EEG. AEDs may also be administered to prevent seizures if the hemorrhage is in a part of the brain associated with seizure risk such as the temporal or frontal lobe.
Similar to patients with acute ischemic stroke, prevention of secondary complications is an essential element of nursing care for patients with ICH. Patients are at risk of aspiration and require careful monitoring of airway clearance, as well as assessment for dysphagia. Meticulous skin care, attention to bowel and bladder management, and prevention of hospital-acquired infections are all important to good outcomes.
Seizures are rapid, repeated bursts of abnormal electrical activity within the brain that result from an imbalance of excitatory and inhibitory impulses. Signs and symptoms depend on the location of the abnormal activity. A seizure is often a symptom or consequence of an underlying neurologic problem, such as a tumor, hemorrhage, trauma, or infection. Systemic disturbances such as hypoxia, hypoglycemia, drug overdose, and drug or alcohol withdrawal may also cause seizures. Many seizures are considered idiopathic, but treatable causes must be ruled out.
During a seizure, the metabolic demands of the brain for oxygen and glucose increase dramatically. The body tries to keep up with these increased requirements by increasing cerebral blood flow (CBF). If CBF does not keep up with demand, neurons revert to anaerobic metabolism, which leads to secondary ischemia and brain injury.
Clinical presentation varies based on the origin and extent of the brain’s abnormal electrical activity. Seizures can be described as focal (starting in one area of the cerebral cortex and limited to one hemisphere) or generalized (rapidly affecting both cerebral hemispheres).
Focal seizures may or may not affect consciousness. Focal seizures that do not impact consciousness (also called simple partial seizures) present with motor activity such as twitching or jerking in an extremity or one side of the face, sensory symptoms such as an unusual taste or smell, or autonomic sensations such as sweating or vomiting.
Focal seizures that alter consciousness (also called complex partial seizures) present with automatisms (smacking the lips, chewing motions, or fidgeting), purposeless activity such as running or arm jerking, or change in affect such as elation or fear. A focal seizure can progress into a bilateral generalized convulsive seizure.
Generalized seizures are characterized by abnormal electrical discharge that rapidly affects both hemispheres. There are several types of generalized seizures:
• Absence: Sudden lapse of consciousness and activity that lasts 3 to 30 seconds. Commonly described as a staring spell.
• Myoclonic: Sudden, brief muscle jerking of one or more muscle groups. Commonly associated with metabolic, degenerative, and hypoxic causes.
• Atonic (also called drop attacks): Sudden loss of muscle tone.
• Clonic: Rhythmic muscle jerking.
• Tonic: Sustained muscle contraction.
• Tonic-clonic: Muscle activity varies between sustained contraction and jerking.
Patients are more likely to be injured during a generalized seizure than during a focal seizure and may complain of generalized muscle aches after the seizure stops if convulsions led to sustained muscle activity.
Status epilepticus indicates prolonged or recurring seizures without a return to baseline mental status. The classic definition of status epilepticus is a seizure or series of seizures lasting longer than 30 minutes, but treatment is typically instituted much sooner and recent guidelines suggest a definition of seizure activity longer than 5 minutes. Status epilepticus is a medical emergency with a significant mortality rate, higher in the elderly or when the seizure is a symptom of an underlying acute process. There are two primary types of status epilepticus: convulsive status epilepticus and nonconvulsive status epilepticus. In convulsive status epilepticus, seizure activity is readily apparent using clinical observation. In nonconvulsive status epilepticus, no outward clinical seizures may be noted but consciousness is impaired and seizure activity is apparent on EEG.
Diagnostic testing for patients with seizures may include:
• Laboratory work to identify electrolyte abnormalities, metabolic disorders, or anti-epileptic drug levels.
• CT to assess for intracranial processes such as an ICH or tumor.
• MRI to look for structural lesions that may indicate a seizure focus.
• LP when an infectious process (eg, meningitis) is the suspected source of seizure activity.
• EEG to evaluate for seizure activity. One normal EEG does not rule out seizure. Prolonged EEG monitoring may be required.
• Continuous video monitoring in conjunction with continuous EEG recordings to correlate clinical phenomena with electrical activity in the brain.
• Intracranial electrodes with continuous EEG monitoring in the evaluation of patients with intractable seizures to identify a focus or foci prior to surgical resection. Intracranial electrodes are inserted via burr holes or a craniotomy.
Management of the patient with seizures focuses on controlling the seizure as quickly as possible, preventing recurrence, maintaining patient safety, and identifying the underlying cause. Observation of seizure type, duration, and any precipitating factors is essential. Following a seizure, patients may experience a period of confusion and altered mental status that slowly resolves. They may complain of a headache or muscle aches. Todd’s paralysis describes continued focal symptoms that can persist for up to 36 hours after a seizure. Because of the risk of missing underlying intracranial pathology, patients with focal neurologic deficits following a seizure are diagnosed with Todd’s paralysis only after other causes have been ruled out.
From a nursing perspective, the first priority is to protect the patient from injury. Ensure a safe environment during the seizure by clearing objects out of the area. Padded side rails are indicated for patients at high risk for seizures. During a seizure, attempting to restrain patient movement may result in injury and is avoided.
Airway management assists with maintaining adequate cerebral oxygenation. Maintaining the airway may depend on stopping the seizure. Positioning the patient on his or her side decreases aspiration. Supplemental oxygen is provided. Nothing should be placed in the patient’s mouth during a seizure. ECG monitoring, continuous pulse oximetry, and blood pressure monitoring are required in patients with prolonged seizures. Hypoglycemia can induce seizure activity, so a glucose level is checked immediately and treated as appropriate.
The average seizure stops within 2 minutes without requiring medication. Patients with prolonged seizures or status epilepticus receive a benzodiazepine such as lorazepam. The second medication given is typically phenytoin or fosphenytoin. Fosphenytoin is converted to phenytoin in the blood and is preferred because it causes less tissue injury should extravasation occur. Both agents can cause cardiovascular side effects, predominately treatment-resistant hypotension. Cardiac and respiratory status should be closely monitored. If seizure activity continues, the patient will require transfer to the intensive care unit for continuous intravenous medications.
The prolonged muscle activity that occurs with prolonged convulsive seizure activity may cause tissue breakdown and lead to rhabdomyolysis. Serum creatine phosphokinase is elevated and myoglobin may be present in the urine. Hydration is essential to avoiding renal dysfunction.
Many patients require ongoing medication for seizure control. Some common medications include levetiracetam, phenytoin, carbamazepine, oxcarbazepine, valproic acid, lamotrigine, and lacosamide. Approximately two-thirds of patients treated with medication are able to attain good seizure control.
Some patients with seizures uncontrolled by medications may be helped by surgery to remove the seizure focus. These patients most often have seizures originating from the temporal lobe. Selection criteria include intractable seizures that significantly impact quality of life and are uncontrolled by medication, an identifiable unilateral focus of seizure activity, and seizure focus in an area where removal will cause no major neurologic deficit. A craniotomy is used to access and excise the seizure focus. The primary complications are hemorrhage and infection. Patients are kept on their previous seizure medications during the postoperative period. About 50% of patients become seizure free after surgery and an additional 30% experience a significant improvement in seizure control.
For patients with intractable seizures who do not have an identifiable focus, placement of a vagus nerve stimulator may be considered. Vagal nerve stimulation reduces seizure duration, frequency, or intensity by providing intermittent electrical stimulation of the vagus nerve. The exact mechanism of action has not been determined.
Meningitis is an acute inflammation of the meninges of the brain and spinal cord. Meningitis can be caused by bacteria, viruses, fungi, or parasites. Risk factors include immunocompromise, trauma, surgery that disrupts the meninges, and crowded living conditions. Signs and symptoms include fever, headache, neck stiffness, irritability, vomiting, photophobia, altered level of consciousness, seizures, weakness, and cranial nerve deficits. Other signs of meningitis include Kernig sign (severe pain in the hamstring with knee extension when the hip is flexed 90°) and Brudzinski sign (involuntary flexion of the knees and hips when the neck is flexed). Many patients with meningococcal meningitis have a characteristic rash (petechial rash that progresses to purple blotches). Diagnostic testing includes LP for opening pressure and CSF analysis, blood cultures, and other laboratory tests to look for infection. CT scanning is performed prior to LP in patients with papilledema or focal neurologic findings. Complications of meningitis include hydrocephalus, cerebral edema, and vasculitis. Nursing priorities include management of elevated ICP, implementation of seizure precautions, and prompt administration of antimicrobial therapy. Delays in antimicrobial therapy are associated with worse outcomes. Isolation may be required until the causative organism is identified and treated; notify the infection control practitioner and follow institutional guidelines.
Encephalitis is inflammation of the brain parenchyma. There are many causes of encephalitis, including arboviruses such as West Nile, but the most common type seen in the United States is encephalitis due to the herpes simplex virus (HSV). HSV encephalitis can result from a new infection, or can represent a reactivation of a preexisting infection. Signs and symptoms include fever, focal or diffuse neurologic changes, headache, and seizures. HSV encephalitis predominately affects the inferior frontal and temporal lobes. Diagnostic testing includes MRI, EEG, and CSF analysis. The diagnosis is often presumed pending specialized testing of the CSF. Empiric therapy is started with an antiviral agent.
An intracranial abscess is a collection of pus in the brain and can be extradural, subdural, or intracerebral. The infective agent enters the brain through the bloodstream, via an opening in the dura (as may occur with a basilar or open skull fracture or following a neurosurgical procedure), or by direct migration from chronic otitis media, poor dentition, frontal sinusitis, or mastoiditis. Signs and symptoms typically develop over a few weeks and may include headache, seizures, fever, neck pain, focal neurologic signs such as hemiparesis, cranial nerve deficits, and change in level of consciousness. Diagnostic testing includes CT with contrast administration, MRI, EEG, and potentially aspiration of the lesion for culture. Treatment includes prolonged antibiotic therapy (usually 6 weeks) and surgical drainage of the abscess.
Although there are a variety of neuromuscular diseases that may result in hospitalization, only a small number of these patients require admission to the progressive care unit. Myasthenia gravis (MG) and Guillain-Barré syndrome (GBS) often cause respiratory muscle weakness and decreased airway clearance. These patients may be admitted to the progressive care unit for close monitoring of respiratory status. They may also be admitted to ventilator weaning units after initial stabilization in the ICU. In addition, patients with chronic progressive neuromuscular diseases such as amyotrophic lateral sclerosis (ALS) may be admitted to the progressive care unit for management of respiratory failure or other complications.
In MG, autoimmune-mediated destruction of acetylcholine receptors results in decreased neuromuscular transmission and muscle weakness. Myasthenia gravis is a chronic disease with periodic exacerbations. Diagnostic testing includes laboratory testing for acetylcholine receptor antibodies, EMG, CT scanning of the chest to evaluate for abnormalities of the thymus, and “Tensilon testing.”
Edrophonium chloride (Tensilon) is a short-acting acetylcholinesterase inhibitor that can be administered intravenously. Improvement in symptoms following edrophonium chloride injection is highly suggestive of MG. Adverse effects of edrophonium chloride include bradycardia, asystole, increased oral and bronchial secretions, and bronchoconstriction.
Treatment for acute MG exacerbation includes plasma exchange or IV immunoglobulin in addition to supportive care. Long-term management may include the administration of anticholinesterase medications, thymectomy, or immunosuppression. Priorities of nursing management during an acute exacerbation include close monitoring of respiratory status and prevention of secondary complications.
Guillain-Barré syndrome causes progressive muscle weakness, sensory loss, and areflexia due to peripheral nerve demyelination. Symptoms generally start in the lower extremities and ascend. Diagnostic studies include LP and nerve conduction studies. Approximately 25% to 40% of patients require mechanical ventilation. Some patients experience autonomic instability characterized by variations in heart rate and blood pressure. Neuropathic pain related to inflammation and demyelination occurs and requires both pharmacologic and nonpharmacologic treatment. In addition to supportive therapy, patients may receive plasma exchange or IV immunoglobulin. Most patients recover with minimal deficits, but may require weeks to months of hospitalization. Nursing priorities include close monitoring of respiratory status and prevention of complications related to prolonged immobility.
Amyotrophic lateral sclerosis is a progressive disease that affects the motor neurons, causing muscle weakness without affecting sensation or cognition. Patients most commonly present with extremity weakness that is asymmetric and more pronounced distally. Bulbar symptoms such as dysarthria and dysphagia may be present initially, or may develop as the disease progresses. The rapidity of progression varies widely among patients. Eventually, ALS causes respiratory failure due to muscle weakness and decreased airway protection.
Patients with ALS may be admitted to the progressive care unit when airway protection becomes problematic and complications such as pneumonia develop, or if they use assisted ventilation (BiPAP or mechanical ventilation) at home and require admission for other complications or procedures. Nursing care focuses on decreasing respiratory complications and other complications of immobility, controlling pain, and providing psychological support.
Alexandrov AW. Transcranial Doppler monitoring. In: Weigand DL, ed. AACN Procedure Manual for Critical Care. 6th ed. St. Louis, Missouri: Saunders, 2011.
Balas MC, Rice M, Chaperon C, et al. Management of delirium in critically ill older adults. Crit Care Nurse. 2012;32:15-26.
Bleck TP. Levels of consciousness and attention. In: Goetz CG, ed. Textbook of Clinical Neurology [electronic version]. 3rd ed. Philadelphia, PA: Saunders; 2007.
Bruno M, Ledoux D, Lambermont B, et al. Comparison of the full outline of UnResponsiveness and Glasgow Liege Scale/Glasgow Coma Scale in an intensive care unit population. Neurocrit Care. 2011;15:447-453.
Brust JCM. Coma. In: Rowland LP, Pedley TA, eds. Merritt’s Neurology [electronic version]. 12th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2010.
Daroff RB, Fenichel GM, Jankovic J, Mazzioatta JC. Bradley’s neurology in clinical practice, Volume I: Principles of Diagnosis and Management [electronic version]. 6th ed. Philadelphia, PA: Saunders; 2012.
Delapaz R. CT and MRI. In: Rowland LP, Pedley TA, eds. Merritt’s Neurology [electronic version]. 12th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2010.
Ely EW, Shintai A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753-1762.
Fletcher JJ, Nathan BR. Cerebrospinal fluid and intracranial pressure. In: Goetz CG, ed. Textbook of Clinical Neurology [electronic version]. 3rd ed. Philadelphia, PA: Saunders; 2007.
Hickey JV, Murphy KP. Neurodiagnostic tests. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices: 2007. AJR. 2007;188:1-27.
Koenigsberg RA, Bianco BA, Faro SH, et al. Neurodiagnostic tools. In: Goetz CG, ed. Textbook of Clinical Neurology [electronic version]. 3rd ed. Philadelphia, PA: Saunders; 2007.
Kramer AA, Wijdicks EFM, Snavely VL, et al. A multicenter prospective study of interobserver agreement using the Full Outline of Unresponsiveness score coma scale in the intensive care unit. Crit Care Med. 2012;40:2671-2676.
Lee K, Fishman RA. Lumbar puncture and cerebrospinal fluid examination. In: Rowland LP, Pedley TA, eds. Merritt’s Neurology [electronic version]. 12th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2010.
Mohr JP, Delapaz R, Rundek T. Neurovascular imaging. In: Rowland LP, Pedley TA, eds. Merritt’s Neurology [electronic version]. 12th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2010.
Neto AS, Nassar AP, Cardoso SO, et al. Delirium screening in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2012;1946-1951.
Stewart-Amidei C, Blissitt PA, Brooks L. Assessment. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
Website for information on delirium and assessment methods: www.icudelirium.org (ICU Delirium and Cognitive Impairment Study Group; Vanderbilt University Medical Center, Veterans Affairs TN Valley Geriatric Research Education and Clinical Center). Accessed February 23, 2013.
Wijdicks EFM, Bamlet WR, Maramottom BV, Manno EM, McClelland RL. Validation of a new coma score: the FOUR score. Ann Neurol. 2005;58:585-593.
Madden LK, March K. Intracranial pressure management. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
March K. Intracranial pressure concepts, cerebral blood flow, and metabolism. In: Bader MK, Littlejohns LR. eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
March K, Olson D, Arbour R. Technology. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
Elijovich L, Patel PV, Hemphill JC. Intracerebral hemorrhage. Sem in Neurol. 2008;28(5):657-667.
Hinkle JL, Guanci MM, Stewart-Amidei C. Cerebrovascular events of the nervous system. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676-685.
Buelow JM, Dean P, Miller W, Plueger M. Epilepsy. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
Engel J, Jr, Weibe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the AANS. Neurology. 2003;60:538-547.
Huff JS, Fountain NB. Pathophysiology and definitions of seizures and status epilepticus. Emerg Med Clin N Am. 2011;29:1-13.
Kennedy PGE. Viral encephalitis. J Neurol. 2005;252:268-272.
Pass M. Central nervous system infections. In: Barker E, ed. Neuroscience Nursing: A Spectrum of Care. 3rd ed. St Louis, MO: Mosby, Inc; 2008.
van de Beek D, Brouwer MC, Thwaites GE, Tunkel AR. Advances in treatment of bacterial meningitis. Lancet. 2012;380:1693-1702.
VanDemark MV, Neatherlin JS, Stewart-Amidei C, Bautista C, Omert T. Infectious and autoimmune processes. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
Burns TM. Guillain-Barré syndrome. Semin Neurol. 2008;28:152-167.
Chiu A, Cocito D, Leone M, et al, and the Piedmonte and Valle d’Aosta Register for Guillain-Barré Syndrome. Guillain-Barré syndrome: a prospective, population-based incidence and outcome survey. Neurology. 2003;60(7):1146-1150.
Polak M, Lorimer M, Koopman W, De Sepulveda LB. Neuromuscular disorders of the nervous system. In: Bader MK, Littlejohns LR, eds. AANN Core Curriculum for Neuroscience Nursing. 5th ed. Glenview, IL: American Association of Neuroscience Nurses; 2010.
Sharshar T, Chevret S, Bourdain F, Raphaël J, for the French Cooperative Group on Plasma Exchange in Guillain-Barré Syndrome: early predictors of mechanical ventilation in Guillain-Barré syndrome. Crit Care Med. 2003;31(1):278-283.
American Association of Critical Care Nurses. Practice alert: delirium assessment and management. Issued November 2011. Available at www.aacn.org. Accessed February 23, 2013.
Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263-306.
Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3-23.
Eaton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack. Stroke. 2009;40:2276-2293.
Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013: 44: published on-line January 31, 2013. Accessed February 22, 2013.
March K, Madden L. Intracranial pressure management. In: Littlejohns LR, Bader MK, eds. AACN-AANN Protocols for Practice: Monitoring Technologies in Critically Ill Neuroscience Patients. Sudbury, MA: Jones and Bartlett Publishers; 2009.
Morgenstern LB, Hemphill JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
Pugh S, Mathiesen C, Meighan M, Summers D, Zrelak P [electronic version]. Guide to the Care of the Hospitalized Patient With Ischemic Stroke: AANN Clinical Practice Guideline. 2nd ed. Glenview, IL: American Association of Neuroscience Nurses; 2011. Available at http://www.aann.org/pubs/content/guidelines.html. Accessed June 1, 2013.
Slazinski T, Anderson TA, Catell E, et al. Care of the patient undergoing intracranial pressure monitoring/external ventricular drainage or lumbar drainage: AANN Clinical Practice Guideline Series. Glenview, IL: American Association of Neuroscience Nurses; 2011.
Summers D, Leonard A, Wentworth D, et al. Comprehensive overview of nursing and interdisciplinary care of the acute ischemic stroke patient. Stroke. 2009;40 2911-2944.
Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303-327.
Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.