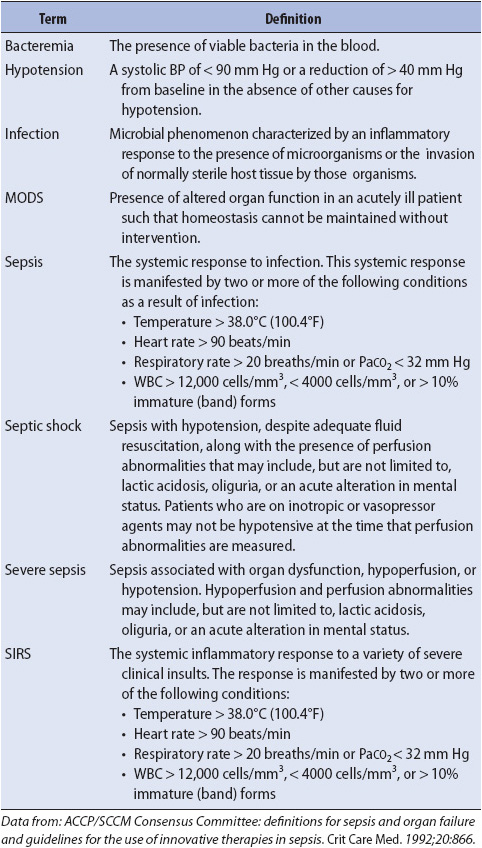
TABLE 11-1. INFLAMMATORY RESPONSES: DEFINITIONS
KNOWLEDGE COMPETENCIES
1. Identify the relationship between the cellular mediators and clinical manifestations of systemic inflammatory response syndrome (SIRS).
2. Describe the etiology, pathophysiology, clinical manifestations, patient needs, and principles of management of SIRS, sepsis, and associated conditions leading to multisystem problems.
3. Compare and contrast the pathophysiology, clinical manifestations, patient needs, and management approaches for multisystem problems resulting from SIRS, sepsis, multiple organ dysfunction, and overdoses.
4. Describe the symptoms and pharmacologic management of the patient suffering from alcohol withdrawal syndrome.
5. Describe treatment considerations for complex wounds and pressure ulcers.
Any acute illness can predispose patients to several complex conditions including sepsis and multiple organ dysfunction syndrome (MODS) (Table 11-1). Sepsis results from an infectious process and represents a systemic response to infection. Sepsis with acute organ dysfunction (severe sepsis) commonly occurs in patients cared for in progressive or critical care units. Sepsis is a serious worldwide healthcare condition that is associated with high mortality rates, despite improvements in the ability to manage infection. Severe sepsis incidence increases annually by 13% with associated mortality rates of 15% to 29%. It is the third most common cause of death in the United States. Patients in any progressive care unit are vulnerable to the condition. Thus, understanding the syndrome and initiating early therapy may prevent deterioration, transfer to a critical care unit, and ultimately death. Systemic inflammatory response syndrome (SIRS) is a systemic response to a clinical insult, such as an infection or burn (Figure 11-1). In some cases, the syndrome may progress to sepsis and MODS. The stimulus for SIRS can be singular or multifactorial. Examples of situations that can precipitate SIRS are burns, trauma, transfusions, pancreatitis, or infection. Following the insult, an inflammatory response is initiated as a normal physiologic response. The inflammatory response consists of vasodilatation, increased microvascular permeability, cellular activation and release of mediators, and coagulation (see Figure 11-1). In SIRS, there is an excessive release of these mediators, which may lead to severe tissue damage, with hypoperfusion of organ systems.
TABLE 11-1. INFLAMMATORY RESPONSES: DEFINITIONS

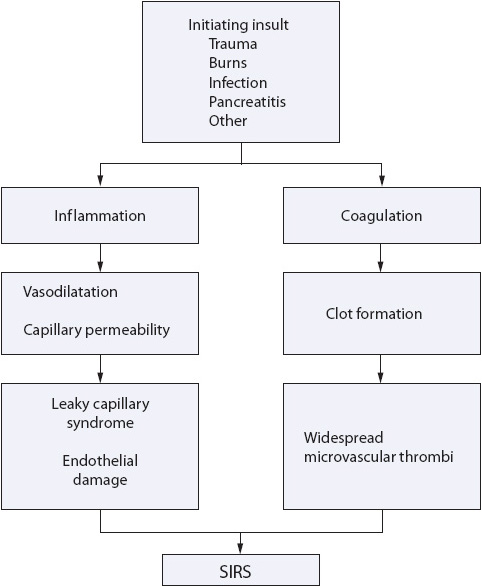
Figure 11-1. SIRS results from activation of interactive cascades of inflammation and coagulation.
Systemic inflammatory response syndrome is manifested in a variety of ways: fever, tachycardia, tachypnea, altered level of consciousness, and decreased urine output. These findings may or may not be the result of an infection. If the response progresses unchecked, the result may be the development of sepsis or dysfunction of one or more organ systems, or MODS. The SIRS, sepsis, and MODS may be thought of as progressively severe conditions along a continuum. The key is early identification of the signs and symptoms of SIRS, and prompt development of a treatment plan to avoid further progression. Early intervention is important to ensure good outcomes in these patients.
Systemic inflammatory response syndrome consists of a series of systemic events that occur in response to an insult to the body. This response is a cellular reaction that initiates a number of mediator-induced responses, and is both inflammatory and immune in nature (Figure 11-2).
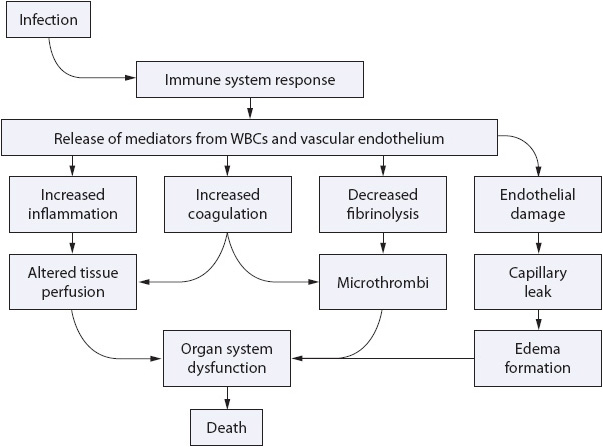
Figure 11-2. Interactive cascade of inflammation and coagulation, leading to endothelium damage, diffuse thrombi, and organ system dysfunction. (Reprinted with permission: Kleinpell R. New initiatives focus on prevention and early recognition of sepsis. Nurs Spectrum. 2004;17[12]:24-26.)
There are essentially four different types of cells that are activated as part of the response to an insult or stimulus: polymorphonuclear cells (neutrophils), macrophages, platelets, and endothelial cells. These cells are activated to become either directly involved in the reaction (ie, platelet aggregation) or are stimulated to produce and release chemical mediators into the circulation, such as cytokines or plasma enzymes. Once activated, “a checks and balances system” is normally in place to control the inflammatory response. In some situations, however, when the response is large or the injury diffuse, local control of the response is lost, leading to excessive mediator release with consequent organ damage. The cellular activation response is highly individualized, and subsequent organ compromise is also variable.
A general understanding of the various mediators responsible for the SIRS is important. Mediators can be divided into five groups: cytokines, plasma enzyme cascades, lipid mediators, toxic oxygen-derived metabolites, and unclassified mediators such as nitric oxide and proteases. These mediators are stimulated after cellular activation in response to a certain stimulus (eg, infection, trauma, pancreatitis). Cytokines are active chemical substances secreted by cells in response to a stimulus. If secreted by lymphocytes, they are called lymphokines, and if secreted by monocytes or macrophages, they are called monokines. Examples of cytokines include tumor necrosis factor, interleukin, interferon, and colony-stimulating factors such as granulocyte colony-stimulating factor.
In addition to cytokines, there is also activation of different enzymatic plasma cascades. Examples of these include the complement cascade and the various coagulation cascades. In addition, there are various lipid mediators that are either stimulated or produced as part of a cellular destructive process. These lipid mediators include arachidonic acid metabolites, leukotrienes, prostaglandins, and platelet-activating factor. Oxygen-derived free radicals are another group of mediators that exert a negative effect as part of the SIRS. Examples of these include hydrogen peroxide and hydroxyl radical. Nitric oxide and proteases are other mediators that are not grouped into any of the previous categories, but are mediators that enhance the SIRS.
In addition to the mediators stimulated as part of the inflammatory and immune responses, mediators related to hormonal stimulation and regulation are also produced. The hormonal response component of the SIRS is characterized by the release of stress hormones (catecholamines, glucagon, cortisol, and growth hormone), suppression of thyroid hormone, and hormonal regulation of fluid and electrolyte balance. Toll-like receptors, or transmembrane proteins that are expressed on various immune cells such as neutrophils and macrophages, have been implicated in ischemia-reperfusion injury that can further alter perfusion and contribute to inflammation.
Sepsis is the manifestation of the SIRS in response to an infectious process (see Table 11-1). The source of infection may be bacterial, viral, fungal, or on rare occasions, rickettsial or protozoal.
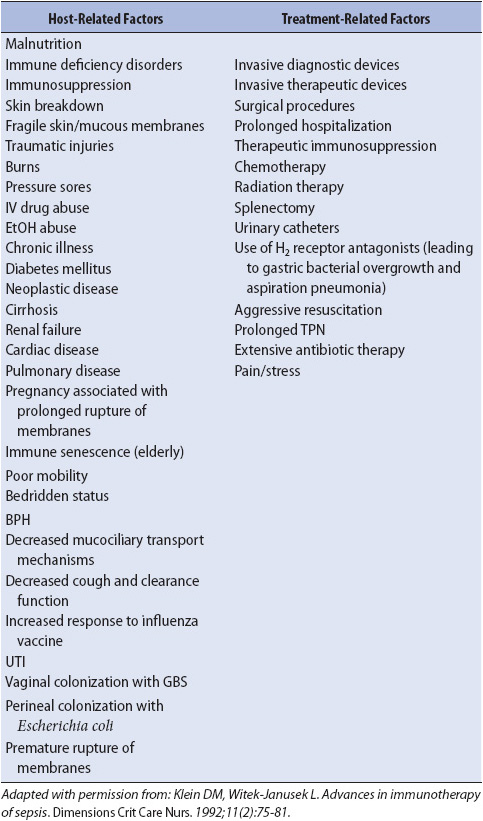
The risk factors for development of sepsis are many and include malnutrition, immunosuppression, prolonged antibiotic use, and the presence of invasive devices (Table 11-2). It is important to remember that a large number of infections in acutely ill patients are hospital acquired and can lead to sepsis. Many of these hospital-acquired infections can be prevented with simple measures. The role of the progressive care nurse is instrumental in preventing hospital acquired infections. Hand washing remains the single most effective method for preventing nosocomial infections. Recent research suggests that relatively simple measures such as ensuring head-of-bed elevation and meticulous mouth care may prevent ventilator-associated pneumonia, a common source of sepsis in acutely ill patients. Therefore, nursing measures to target sepsis prevention as well as early recognition and treatment are important in reducing the high mortality rates associated with severe sepsis (Table 11-3).
TABLE 11-2. RISK FACTORS FOR THE DEVELOPMENT OF SEPSIS
Sepsis can progress to severe sepsis, with organ dysfunction, hypoperfusion, or severe hypotension. Severe sepsis (sepsis that has progressed to cellular dysfunction and organ damage or evidence of hypoperfusion) and septic shock (sepsis with persistent hypotension despite adequate fluid resuscitation) are associated with high mortality rates, despite improvements in the ability to manage infection. Hypoperfusion and perfusion abnormalities that occur in severe sepsis may include oliguria, lactic acidosis, hypoxemia, and alteration in mental status (Table 11-4). Severe sepsis is associated with three integrated responses: activation of inflammation, activation of coagulation, and impairment of fibrinolysis. The result is systemic inflammation, widespread coagulopathy, and microvascular thrombosis, conditions that often lead to multiple organ dysfunction.
TABLE 11-4. SIGNS OF ACUTE ORGAN SYSTEM DYSFUNCTION
Multiple organ dysfunction syndrome (MODS) is the worsening progression of the SIR. If SIRS is allowed to persist unchecked, or becomes overwhelming, the patient develops clinical manifestations of organ dysfunction. The mortality rates for MODS vary depending on the underlying cause, with mortality rates ranging from 50% to 100% as the number of involved organs increases.
Multiple organ dysfunction syndrome can be classified as either primary or secondary. In primary MODS, organ dysfunction is a direct effect of an insult to an organ that has been compromised; for example, aspiration causes lung dysfunction, or acetaminophen overdose causes liver dysfunction. With primary MODS, the onset occurs relatively soon after the insult. In secondary MODS, the organ dysfunction occurs as the result of persistent and prolonged mediator release following an insult such as a thermal burn or pancreatitis. Generally, the time frame for secondary MODS is 7 to 10 days; however, this onset is variable.
Systemic inflammatory response syndrome is the clinical manifestation of two or more of the following conditions:
• Temperature > 38°C (100.4°F) or < 36°C (96.8°F)
• Heart rate > 90 beats/min
• Respiratory rate > 20 breaths/min or PaCO2 < 32 mm Hg
• WBC > 12,000 cells/mm3, < 4000 cells/mm3 or > 10% immature neutrophils (band) forms
Close monitoring and assessment are essential for the detection of early signs of SIRS.
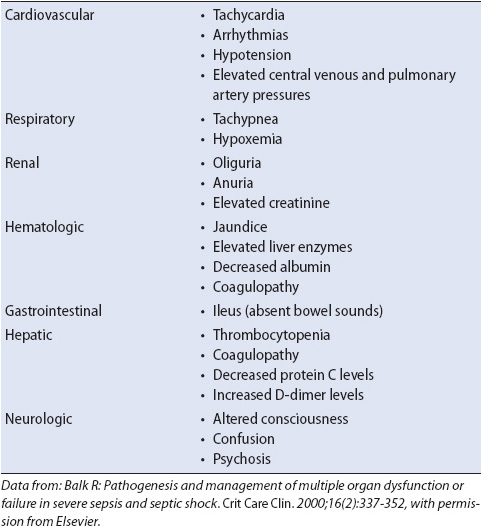
The clinical manifestation of severe sepsis is the result of altered perfusion to vital organ systems. Organ system dysfunction develops due to hypoperfusion and microvascular thrombosis. Table 11-4 summarizes the common manifestations of severe sepsis. Signs of organ system dysfunction include cardiovascular alterations (hypotension, tachycardia, dysrhythmias), respiratory system alterations (tachypnea, hypoxemia), renal system alterations (oliguria, elevated creatinine), hematologic system alterations (thrombocytopenia), gastrointestinal alteration (change in bowel sounds, ileus), hepatic alterations (elevated liver enzymes, jaundice, coagulopathies), and neurologic system alterations (confusion, agitation). Early recognition and treatment are extremely important as the prognosis of patients with severe sepsis is related to the number or organs involved and the severity of dysfunction.
The clinical manifestations of primary and secondary MODS depend on which organs are affected. In patients with severe sepsis, MODS appears to result from a cascade of inflammatory mediators, endothelial injury, altered perfusion, and microcirculatory failure. Mortality in severe sepsis is directly related to the number of failing organ systems and the severity of dysfunction. MODS is regarded as one of the most common causes of death among patients in the ICU.
• Complete blood cell count: White blood cell count > 12,000 cells/mm3, or < 4000 cells/mm3, or > 10% immature bands
• Arterial blood gas: PaCO2 < 32 mm Hg
• Serum lactate: More than 4 mmol/L (36 mg/dL)
• Chest x-ray: May be normal or show signs of infiltrates
• Culture and sensitivity: Generally is positive from a normally sterile source
• Axial computed tomography scan: May be negative or show abscess collection
The treatment of a patient with severe sepsis (SIRS + infection + new organ dysfunction) consists of several objectives: treating or eliminating the underlying cause, maximizing oxygen delivery, and use of evidence-based practice guidelines to include early antibiotic administration and ensure that initial resuscitation, organ system support, and targeted interventions are provided. Additional components of the management plan include providing nutrition and psychological support for the patient and family.
The management plan begins with recognition and treatment of the source or stimulus of the response. Until this is done, no other therapy may be successfully applied. Examples include the drainage of an abscess or the removal of an infected invasive line, vascular graft, or orthopedic device. Once the source (or presumed source) has been identified, empiric antibiotic therapy is initiated and adjusted when definitive culture results are available.
Parallel to the administration of antibiotics are measures to maximize oxygen delivery. The components of oxygen delivery include cardiac output (CO), oxygen saturation (SaO2), hemoglobin (Hgb), and to a lesser extent, partial pressure of oxygen (PaO2). Oxygen demands can be significantly increased in sepsis, especially when patients have fever and/or tachycardia. Administration of supplemental oxygen may help maintain the balance between oxygen supply and oxygen demand.
A significant number of patients with sepsis increase their CO as a compensatory response to meet increased cellular oxygen demands. However, a major pathological problem of sepsis is the increase in the permeability of the capillary bed and vasodilation. As a result, intravascular volume is difficult to maintain and generally transfer to the critical care unit is necessary for aggressive monitoring and management. Protocolized, quantitative resuscitation of patients with severe sepsis with tissue hypoperfusion (defined as hypotension persisting after initial fluid challenge or blood lactate concentration greater than or equal to 4 mmol/L) is recommended. The goals of resuscitation should include all of the following:
1. CVP 8-12 mm Hg
2. MAP > 65 mm Hg
3. Urine output > 0.5 ml/kg/h
4. Superior vena cava oxygenation saturation (SCVO2) or mixed venous oxygen saturation (SVO2) 70% or 65% respectively.
5. Normalization of lactate in patients with elevated lactate levels.
This often necessitates the liberal administration of fluids. A patient may require a combination of both crystalloid and colloid fluid replacement. Pharmacologic support also may be required to maximize CO.
Maintaining SaO2 more than 90% and PaO2 more than 60 mm Hg are acceptable goals.
Sufficient hemoglobin is necessary to ensure adequate oxygen-carrying capacity. Disagreement exists as to the appropriate hemoglobin and hematocrit levels for this type of patient; however, as a general rule, 7 to 9 g of hemoglobin and 21%-27% hematocrit are acceptable depending on the patient’s tolerance.
Decreasing oxygen demand is an important aspect of maximizing oxygen delivery. Methods to reduce oxygen demand include:
1. Reducing tachycardia and tachypnea
2. Reducing hyperthermia
3. Alleviating pain
4. Preventing shivering
5. Providing comfort measures
6. Consolidating activities
7. Placing the patient on mechanical ventilation
By addressing these aspects of supply and demand, unnecessary oxygen consumption may be minimized, thus improving the supply to other tissues in greater need of oxygen.
Notice that there has been no mention of maintaining an optimal blood pressure. The reason for this is that although maintenance of blood pressure is critical, adequate blood pressure does not imply adequate perfusion. For this reason, measurements of oxygen delivery and consumption are used to assess adequacy of perfusion, and not blood pressure alone. There is great variability in perfusion among patients with similar mean arterial pressures (MAPs). A patient with a MAP of 100 mm Hg may not have adequate tissue perfusion. In contrast, a patient with a MAP of 50 may have sufficient tissue perfusion. The point is that an evaluation of perfusion should not be based on pressure assessment alone (this concept is also reviewed in Chapter 4, Hemodynamic Monitoring).
An important objective in the management of sepsis and MODS is to support dysfunctional organ systems. Renal dysfunction, a common sequela of sepsis, is aggressively managed to prevent fluid and electrolyte imbalances, which contribute to the risk of death. Refer the chapter in this book specific to each organ system for approaches used to support failing organs.
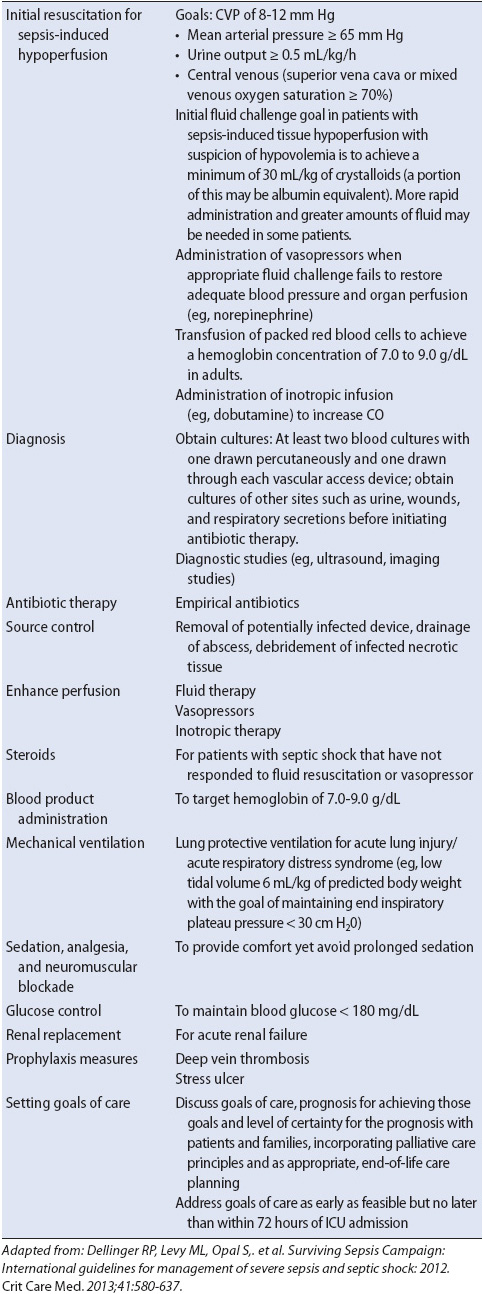
Evidence-based practice guidelines for managing patients with severe sepsis highlight the use of the sepsis bundles (Table 11-5). Key recommendations include initial resuscitation to restore perfusion, organ system support, appropriate diagnostic studies, early administration of broad-spectrum antibiotic therapy, vasopressor and inotropic support, lung protective ventilation strategies, limiting the use of sedation, glucose control, and goal-directed therapy to improve outcomes for patients with severe sepsis (Table 11-6). Several of the evidence-based practice recommendations have direct implications for nursing care because they require monitoring and oversight. Control of glucose (blood glucose, 180 mg/dL) in critically ill patients has been shown to decrease mortality rates and improve outcomes. The use of intravenous insulin to maintain glycemic control requires frequent monitoring of glucose (every 1 hour) and is a nurse-driven intervention.
TABLE 11-5. SURVIVING SEPSIS CAMPAIGN CARE BUNDLES
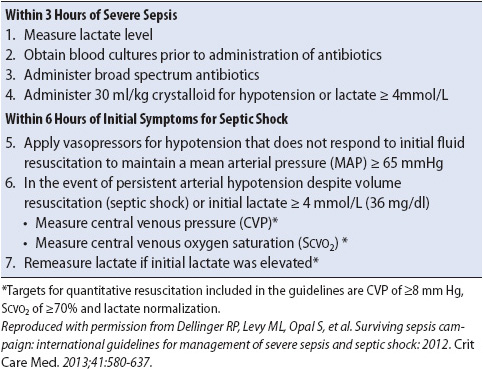
TABLE 11-6. EVIDENCE-BASED PRACTICE GUIDELINES: SURVIVING SEPSIS CAMPAIGN GUIDELINES FOR THE MANAGEMENT OF SEVERE SEPSIS
Enteral nutritional support is the gold standard and preferred route of specialized nutrition support (SNS) delivery unless contraindicated (ASPEN guidelines). If unable to eat, most acutely ill patients can tolerate a standard type of tube feeding or parenteral formula, with rare situations that require feeding modifications (eg, volume overload, organ dysfunction, or gastrointestinal abnormalities). General guidelines for nutritional support include 25 to 35 kcal/kg/day for total caloric intake and 1.5 to 2.0 g protein/kg/day. It is helpful to have a nutrition specialist assist with nutritional planning. Refer Chapter 14, Gastrointestinal System, for more on nutrition.
Chapter 1, Assessment of Progressive Care Patients and Their Families, and Chapter 2, Planning Care for Progressive Care Patients and Their Families, discuss many aspects of psychosocial support of progressive care patients and their families. The updated international sepsis care guidelines identify that setting goals of care, including the discussion of prognosis, should be incorporated into care of the patient with sepsis. Palliative care and end-of-life care planning should also be addressed. The new guidelines identify that a family care conference to discuss goals of care should be addressed as early as feasible, but no later than within 72 hours of ICU admission. Table 11-3 outlines important considerations for nursing care of patients with sepsis or MODS.
Drug or alcohol overdoses, as well as poisonings, can result in multiple organ dysfunction. Overdoses can be deliberate or accidental. Accidental overdose may involve one or multiple substances, and can be acute (eg, inaccurate dosing of pediatric medications) or chronic (eg, inadvertent, unnecessary dosing of asthma medication or over-the-counter medications). The Center for Disease Control and Prevention reports that prescription abuse is the fastest growing drug problem in the United States, with drug overdoses from opioid analgesia misuse among the highest.
The level of intoxication or overdose varies with the element and amount ingested, the time until the patient is treated, and the underlying physical and emotional condition of the patient. The priority of care, as in all emergency situations, is maintenance of the patient’s airway, breathing, and circulation. These patients are often cared for in critical care units, however, some, following the delivery of emergency department care, may be triaged to a progressive care unit for continued therapy and monitoring.
Alcohol overdose is most often seen in alcoholics, in young persons who have not yet reached legal drinking age, or in combination with other drugs as a suicidal gesture. There are four types of alcohol intoxication:
• Ethanol (ethyl or grain alcohol)
• Methanol (wood alcohol)
• Ethylene glycol (antifreeze)
• Isopropyl alcohol (rubbing alcohol)
Alcohol dissolves readily in the lipid components of the plasma membranes of the body, and thus enters the brain quickly, resulting in a rapid effect on the central nervous system. The mechanism of alcohol overdose and withdrawal is complex. Most of the clinical effects can be explained by the interaction of ethanol with various neurotransmitters and neuroreceptors in the brain.
In ethanol intoxication, serum levels range from 200 mg/dL (mild intoxication) to more than 500 mg/dL (coma). A serum alcohol level of 80 mg/dL is the legal upper limit for driving a car in most of the United States.
In the case of methanol intoxication, serum levels range from 50 mg/dL (mild intoxication) to 100 mg/dL (severe intoxication). Metabolic acidosis manifests as a decreased bicarbonate level on arterial blood gas analysis, and indicates that the generation of hydrogen ions by the liver exceeds the ability of the kidney to excrete them. This excess of systemic hydrogen ions results in compensatory hyperventilation, as the body attempts to make the pH more alkaline. Refer Chapter 5, Airway and Ventilatory Management, for further information on acid-base imbalance.
Ethylene glycol intoxication is characterized by neurologic depression, cardiopulmonary complications, pulmonary edema, and renal tubular degeneration. Serum chemistry reveals metabolic acidosis, as described, and renal toxicity. An aggregation of hydrogen ions can result in increased production and accumulation of lactic acid, which tends to impair renal function. Renal toxicity is suspected when the serum pH is less than 7.35, serum creatinine is more than 2.0 mg/dL, and blood urea nitrogen (BUN) is more than 100 mg/dL.
Isopropyl alcohol intoxication is distinguished from other types of alcohol intoxication by the presence of ketoacids in both the urine and serum. Metabolic acidosis is a reflection of excess ketoacids, requiring buffering by the bicarbonate ions.
Excess ingestion of any type of alcohol may cause central nervous system symptoms such as sluggish reflexes, emotional instability, or out-of-character behavior. Amnesia may result for events that occurred during the period of intoxication. Unconsciousness usually occurs before a person can drink enough for fatal consequences to occur, but the rapid consumption of alcohol can cause death by either respiratory depression or aspiration during vomiting. It is estimated that there are 1.2 million hospital admissions for problems related to alcohol abuse with up to 5% of these patients developing delirium tremens requiring medical treatment. There are signs and symptoms that are specific to each type of alcohol ingested. Descriptions follow:
• Acute ethanol intoxication: Muscular incoordination, slurred speech, stupor, hypoglycemia, flushing, seizures, coma, depressed respirations, and hyporeflexia
• Methanol intoxication: Neurologic depression, metabolic acidosis, and visual disturbances
• Ethylene glycol intoxication: Neurologic depression, cardiopulmonary complications, pulmonary edema, and renal tubular degeneration
• Isopropyl intoxication: Neurologic depression, areflexia, respiratory depression, hypothermia, hypotension, and gastrointestinal distress
A differential diagnosis to rule out other medical conditions, such as hypoglycemia or hyperglycemia, which may mimic overdose or intoxication, is an important component of the initial assessment. Because alcohol ingestion interferes with the liver’s ability to produce glucose, alcohol-induced hypoglycemia in the intoxicated patient is fairly common.
Prior to obtaining any diagnostic test, it is extremely important to obtain a history either from the patient, family member, friend, or the person who found the patient to determine the probable substance that was ingested. Once the substance is potentially identified there are diagnostic tests helpful in aiding the treatment of patients following alcohol intoxication. These include:
• Ethanol and methanol serum levels: These are elevated if they were ingested. Most laboratories can run these tests. Isopropyl serum levels are not run as commonly as ethanol and methanol levels.
• Serum creatinine and BUN levels: These may be elevated due to renal dysfunction.
• Liver function studies: The hepatotoxic effects of certain types of alcohol result in abnormal levels.
• Serum glucose and electrolytes: These are often abnormal as described previously.
Patients who suffer from alcohol abuse of dependence may be admitted to a progressive care unit for an unrelated condition or may be admitted for management of withdrawal symptoms. Symptoms of alcohol withdrawal vary and are documented in Table 11-7. The timing related to the emergence of the symptoms also vary and are influenced by concurrent medical illness, daily heavy alcohol use, older age, and abnormal liver function. As a result it is essential to obtain a complete history from the patient and/or family and determine the amount and frequency of alcohol ingested (and last ingested). Use of an alcohol withdrawal symptom assessment form is a helpful way of identifying severity and treatment requirements (Figure 11-3).
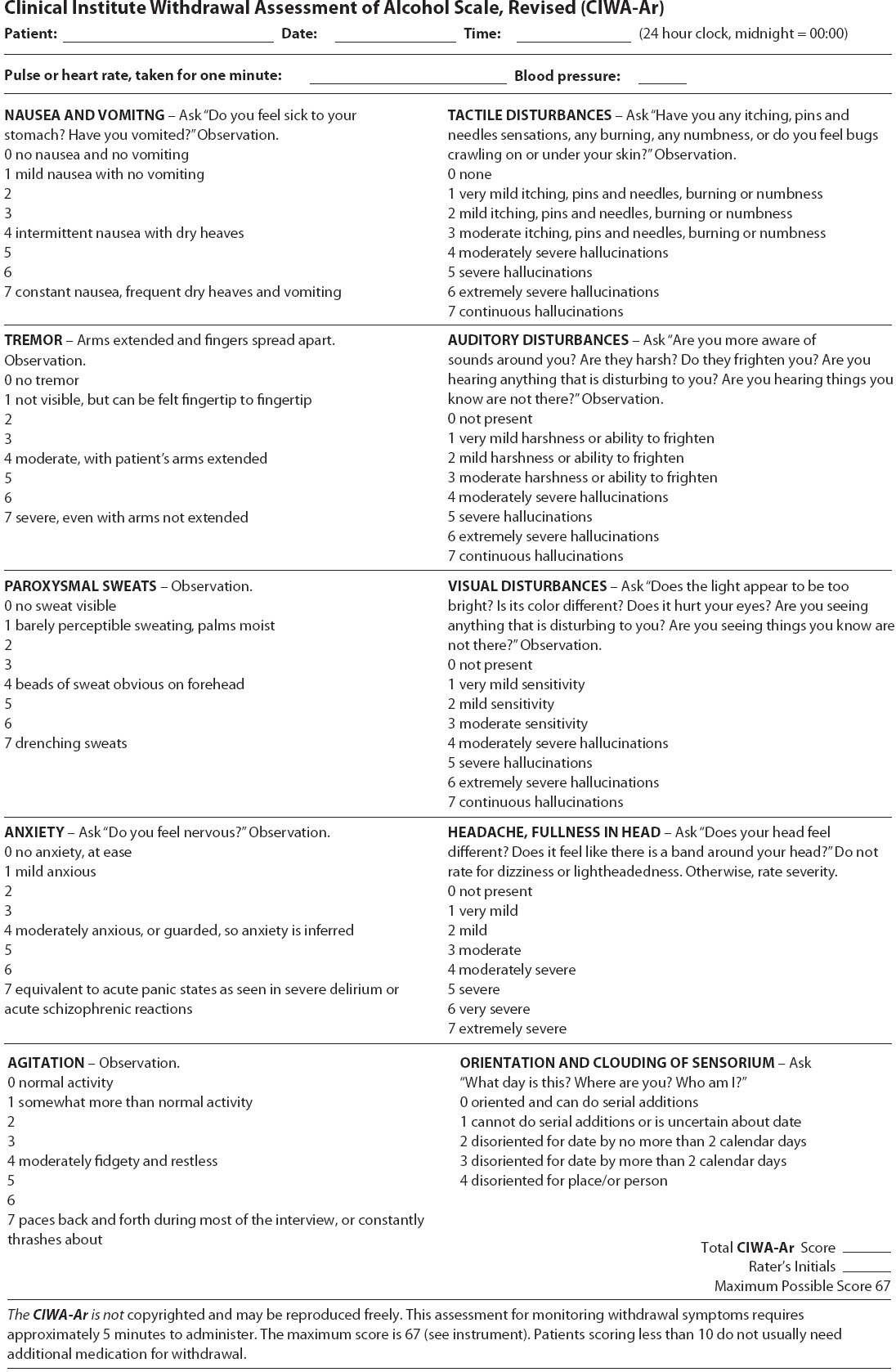
Figure 11-3. Revised Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) scale. (Adapted from: Sullivan JT, Sykora K, SchneidermanJ, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA-AR). Br J Addict. 1989; 84:1353-1357.)
Most treatment management regimens include the use of fluid, electrolyte and nutrition repletion as well as the routine administration of thiamine (to prevent Wernicke encephalopathy), multivitamins, folate, and often magnesium. Benzodiazepines are administered on a set schedule to manage the symptoms of withdrawal and delirium tremens, with additional prn doses used as necessary, to decrease hyperexcitability symptoms which may be life-threatening. Intermediate-acting benzodiazepines are commonly preferred in acute and critical care settings. All benzodiazepines appear similarly effective in the treatment of alcohol withdrawal syndrome. In moderate-to-severe withdrawal, long-acting agents are preferred over short-acting drugs. Adjunctive medications may also be used in some cases to treat agitation (ie, haloperidol-caution, as it lowers seizure threshold) and to decrease autonomic symptoms (ie, beta-blockers, dexmedetomidine, and clonidine).
The use of ethanol or preferably fomepizol for alcohol dehydrogenase (ADH) inhabitation is a mainstay in the management of toxicity due to ingestion of methanol, ethylene glycol, or diethylene glycol. Many patients with chronic alcoholism have clinically significant magnesium deficiency because of malnutrition and chronic diuresis from alcohol ingestion, and electrolyte replacement may be indicated. Alcohol withdrawal management in progressive care requires careful nursing assessment, including alcohol usage history, delirium management, and withdrawal assessment symptoms.
Drug overdose may involve any type of medication. The majority of overdoses involve analgesics, antidepressants, sedatives, opioids, cough and cold drugs, and street drugs (eg, cocaine, crack cocaine, phencyclidine [PCP], D-lysergic acid diethylamide [LSD]). Acetaminophen is the leading cause of overdose. It can lead to hepatocellular damage and is the most common cause of liver transplant. Street drugs are used to induce a relaxed state, elevate mood, or to produce unusual states of consciousness. Psychoactive drugs often are chemically similar to neurotransmitters such as serotonin, dopamine, and norepinephrine, and act by either directly or indirectly altering neurotransmitter-receptor interactions. Medullary inspiratory neurons are highly sensitive to depression by drugs, especially barbiturates and morphine, and death from an overdose of these agents is often secondary to respiratory arrest. Refer Table 11-8 for presenting signs and symptoms of common agents of drug overdose.
TABLE 11-8. SIGNS AND SYMPTOMS OF OVERDOSE
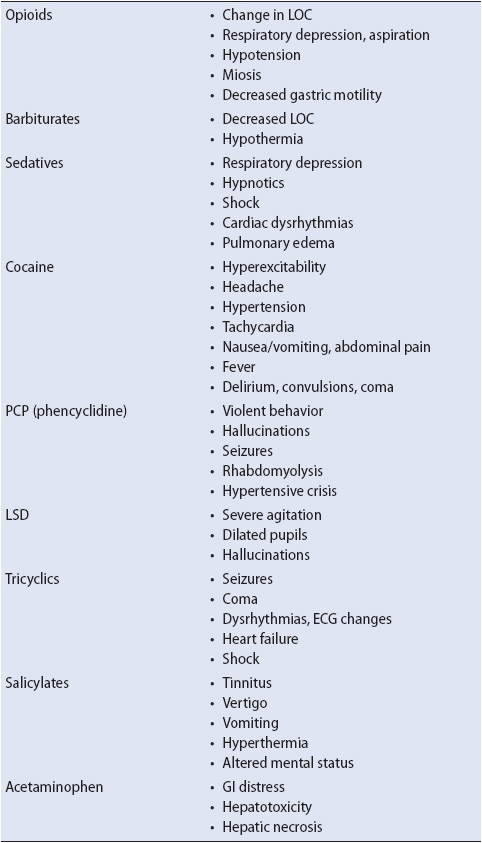
The specific signs and symptoms of drug overdose depend on the substance ingested. However, there are several signs and symptoms that are commonly seen in most patients. These include changes in mental status (typically, decreased level of consciousness), behavioral changes, and respiratory depression. The signs and symptoms of drug overdose for particular drugs are summarized in Table 11-8.
Diagnostic studies for patients following drug overdose include the following:
• Toxicology screen, which can be either broad-spectrum tests, including testing for the presence of such substances as amphetamines, barbiturates, benzodiazepines, and narcotics, or specific screens, if the substance is known. Generally these are urine studies.
• Arterial blood gas and measurement of anion gap, to evaluate oxygenation, ventilation, and the acid-base status respectively.
• Serum glucose and electrolytes, which can be abnormal.
The principles of management of patients following alcohol intoxication or drug overdose are similar. An initial clinical evaluation is conducted with the priority of resuscitating and stabilizing the patient. The principles of management include maintenance of a patent airway, prevention of complications, elimination of ingested substances or toxic metabolites, and maintenance of hemodynamic stability. Specific treatment depends on the agent, route, and amount of exposure, and the severity of overdose.
1. Maintain adequate minute ventilation. Stimulate the patient to breathe. If the patient cannot spontaneously maintain minute ventilation, intubation and mechanical ventilation may be required.
2. Monitor pulse oximetry and blood gas values.
3. Position the patient on their side with the head of the bed elevated > 30° if tolerated.
4. Suction the patient’s airway as needed.
1. Ensure venous access (large-bore peripheral or central access).
2. Administer isotonic fluid to maintain intravascular fluid volume. If hypotension is unresponsive to volume expansion, treatment with vasopressors may be necessary.
3. Obtain a 12-lead ECG and maintain on continuous cardiac monitoring.
4. Treatment of arrhythmias: Supraventricular tachycardia with hypertension due to sympathetic nervous system response can be managed with a combination of beta-blocker and vasodilator therapy (eg, esmolol and nitroprusside), combined alpha- and beta-blocker (labetalol), or a calcium channel blocker (verapamil or diltiazem). Lidocaine or amiodarone may be used for ventricular tachyarrhythmias. This may require transfer to a critical care unit.
1. Measure glucose to rule out hypoglycemia and treat with 50% glucose IV if necessary.
2. Evaluate for carbon monoxide poisoning (carboxyhemoglobin), provide oxygen.
3. Administer thiamine (IV for Wernicke syndrome).
4. Naloxone IV or IM.
5. Flumazenil for benzodiazepine overdose (avoid in those who have potential for seizures).
1. Ipecac is no longer recommended to induce vomiting. There is little evidence that ipecac prevents drug absorption or systemic toxicity. Ipecac is not used as a first-line treatment for most ingested poisons as there is little evidence that it improves the outcome in poisoning cases. Additionally, side effects from ipecac, such as lethargy, may complicate diagnosis and be confused with the effect of other poisons.
2. Gastric lavage: Decreases ingestant absorption and significant amounts of ingested drug can be recovered the closer that lavage is performed to ingestion. Gastric lavage is contraindicated in corrosive ingestions due to risk of gastroesophageal perforation and with hydrocarbons owing to the risk of aspiration-induced hydrocarbon pneumonitis.
3. Activated charcoal: Used with most drugs! Charcoal absorbs ingestants within the gut lumen, allowing the charcoal-toxin complex to be eliminated in the stool. Charcoal is not recommended for patients who have ingested caustic acids and alkalis, alcohols, lithium, or heavy metals.
4. Hemodialysis and hemoperfusion for severe drug intoxication for selected substances: Hemodialysis can be considered for severe poisoning due to methanol, ethylene glycol, salicylates, and lithium. Hemoperfusion, which involves the passage of blood through an absorptive-containing cartridge (usually charcoal), may be indicated for intoxications with carbamazepine, phenobarbital, phenytoin, and theophylline. Therapeutic plasma exchange has also been used to promote rapid lowering of the toxin level.
5. Renal dialysis: Dialysis may be indicated in cases of severe poisoning due to barbiturates, bromide, chloral hydrate, ethanol, ethylene glycol, isopropyl alcohol, lithium, methanol, procainamide, theophylline, salicylates, and possibly heavy metals. Refer Chapter 15, Renal System, for more on renal replacement therapies.
6. Methanol: Practice guidelines have been developed by the American Academy of Clinical Toxicology for the treatment of methanol overdose. Folinic acid (leucovorin) in a dose of 1 mg/kg up to 50 mg every 4 to 6 hours for 24 hours is suggested in methanol poisoning to provide the cofactor for formic acid elimination. Gastric lavage may be considered within 1 hour of ingestion. Activated charcoal does not absorb alcohols, but it may be appropriate to administer if other drugs are suspected. To prevent metabolism of alcohols to toxic metabolites, ethanol can be administered orally or intravenously to maintain a blood concentration of 100 to 150 mg/dL. Hemodialysis is often necessary to remove the alcohol and toxic metabolites and is continued until the acidosis is resolved.
7. Alcohol Withdrawal Syndrome: Assess patients with an alcohol history with a symptom assessment tool to gauge severity of symptoms and to target treatment appropriately. Treat symptoms of alcohol withdrawal and delirium tremens with benzodiazepines on a regular schedule as clinically dictated. Other drugs may be used as adjuncts for treatment of agitation and management of autonomic symptoms such as haloperidol, clonidine, and selected beta-blockers.
Antidotes help counteract the effects of poisons by neutralizing them or by antagonizing their effects. Poisons or conditions with specific antidotes include the following:
• Acetaminophen: N-acetylcysteine
• Opiates: Naloxone
• Benzodiazepines: Flumazenil
• Digoxin: Digiband
• Cyanide: Kelocyanor
• Tricyclic antidepressants: Sodium bicarbonate
• Beta-blockers or calcium channel blockers: Glucagon and calcium
1. Orient the patient to surroundings.
2. Insert a nasogastric tube for stomach decompression and for the delivery of charcoal or other antidotes.
3. Keep the head of the bed elevated to prevent aspiration.
4. Pad bed side rails and restrain the patient as necessary to prevent self-injury.
5. Institute patient safety measures.
6. Provide support to the patient and family.
Complex wounds and pressure ulcers are troubling complications of a hospital stay, especially for acutely ill patients. Complex wounds and pressure ulcers can increase the length of hospitalization, recovery time, and the risk of infection; increase costs of care; and can cause discomfort for patients. Of significance to clinicians is that pressure ulcers are thought to be preventable in most cases and are seen as a reflection of the quality of care provided.
A pressure ulcer is defined by the National Pressure Advisory Panel (NPUAP) as localized injury to the skin and/or underlying tissue usually over a bony prominence, as the result of pressure, or pressure in combination with shear. When a person is immobile the soft tissue is compressed between the skin and the bone. This can lead to ischemia and later tissue death. Typically a pressure ulcer occurs over a bony prominence but can occur anywhere soft tissue is compressed. Pressure ulcer prevalence rates range from 53.2% to 88% and incidence rates vary from 7% to 71.6%, depending on associated risk factors. The most common anatomical sites for pressure ulcers to occur are the sacrum and heels.
There are a number of intrinsic or internal factors related to the risk of pressure ulcer development. Poor nutrition and dehydration contribute to pressure ulcer development because these conditions make tissue more vulnerable to damage. The elderly are at greater risk because of physiologic changes that occur to the skin and tissue with age, such as dermal thinning and the inability of tissue to distribute the mechanical load. Low blood pressure is thought to divert blood away from the skin to more essential organs during critical times. Additionally stress, smoking, surgery, and elevated body temperature are other conditions that can contribute to pressure ulcer development.
According to the NPUAP the six stages of pressures (Table 11-9) are classified as the following:
TABLE 11-9. PRESSURE ULCER CLASSIFICATION BRIEF GUIDE
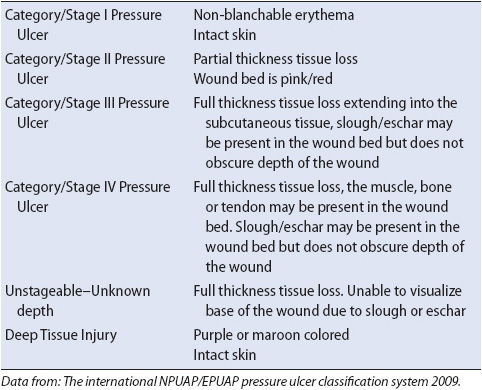
Purple or maroon localized area of discolored intact skin or blood-filled blister due to damage of underlying soft tissue from pressure and/or shear. The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer, or cooler as compared to adjacent tissue. Deep tissue injury may be difficult to detect in individuals with dark skin tones. Evolution may include a thin blister over a dark wound bed. The wound may further evolve and become covered by thin eschar. Evolution may be rapid exposing additional layers of tissue even with optimal treatment.
• Stage I: Intact skin with non-blanchable redness of a localized area usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its color may differ from the surrounding area. The area may be painful, firm, soft, warmer, or cooler as compared to adjacent tissue. Stage I may be difficult to detect in individuals with dark skin tones. May indicate “at risk” persons (a heralding sign of risk).
• Stage II: Partial thickness loss of dermis presenting as a shallow open ulcer with a red pink wound bed, without slough. May also present as an intact or open/ruptured serum-filled blister. Presents as a shiny or dry shallow ulcer without slough or bruising. This stage should not be used to describe skin tears, tape burns, perineal dermatitis, maceration, or excoriation.
Bruising indicates suspected deep tissue injury.
• Stage III: Full thickness tissue loss. Subcutaneous fat may be visible but bone, tendon, or muscle is not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunneling. The depth of a stage III pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput, and malleolus do not have subcutaneous tissue and stage III ulcers can be shallow. In contrast, areas of significant adiposity can develop extremely deep stage III pressure ulcers. Bone/tendon is not visible or directly palpable.
• Stage IV: Full thickness tissue loss with exposed bone, tendon, or muscle. Slough or eschar may be present on some parts of the wound. Often include undermining and tunneling. The depth of a stage IV pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput, and malleolus do not have subcutaneous tissue and these ulcers can be shallow. Stage IV ulcers can extend into muscle and/or supporting structures (eg, fascia, tendon or joint capsule) making osteomyelitis possible. Exposed bone/tendon is visible or directly palpable.
• Unstageable: Full thickness tissue loss in which the base of the ulcer is covered by slough (yellow, tan, gray, green, or brown) and/or eschar (tan, brown, or black) in the wound bed is difficult to evaluate. Until enough slough and/or eschar is removed to expose the base of the wound, the true depth, and therefore stage, cannot be determined. Stable (dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as “the body’s natural (biological) cover” and should not be removed.
Skin assessment, risk assessment, repositioning, nutritional status, and support surfaces are all key pressure ulcer prevention strategies. The two most widely studied and validated risk assessment scales are the Braden and Norton Scales. The Braden scale consists of six subcategories. Each category is scored 1 for the (most at risk) to 4 (least at risk), with the exception of the friction and shear subcategory which is score 1-3. The numbers are added providing a total score that ranges from 4 to 23. If a score falls below 18 the patient is considered at risk for pressure ulcer development. The Norton score includes five parameters: physical condition, mental condition, activity, mobility, and incontinence. The rating for each category is 1-4 with a score potential of 5-20. For both the Braden and Norton scales the lower the score identifies a greater risk of developing a pressure ulcer.
Although the underlying relationship is uncertain, low body weight, poor food intake, and poor nutritional status are all risk factors for the development of pressure ulcers. Patients must have sufficient calories, fluid, and proteins to reduce the risk of development. Patients must also maintain adequate hydration. All patients at nutritional risk should have an evaluation by a registered dietician.
Repositioning should be scheduled for bed and chair bound patients who are at risk of pressure ulcer development. The patient’s overall condition needs to be considered when repositioning. If the patient cannot be turned due to a medical condition then an advanced support surface should be used and attempts made for slight adjustments in position. Heels should be suspended off the surface of the bed to prevent pressure on that area. The use of a pillow or a heel elevation device is also recommended. Support surfaces should be used for those individuals at risk for pressure ulcer development. They are used as adjunct therapy and are not to replace turning and repositioning.
Nosocomial infections, or hospital associated infections (HAI), are estimated to occur in up to 5% of all acute care hospitalizations, or approximately 2 million cases per year. Hospital associated infections have been identified as one of the most serious patient safety issues in healthcare. Two common HAI’s that patients in progressive care units are at risk for include catheter-associated urinary tract infection (CA-UTI) and central line associated blood stream infection (CLA-BSI). Another is hospital acquired pneumonia and ventilator associated events, conditions, and pneumonia (VAE, VAC, VAP, respectively). The pneumonias, their etiology, identification, and management are discussed in Chapter 10 Respiratory System.
Catheter-associated urinary tract infection often results from the presence of an indwelling urinary catheter. Guidelines for the prevention of CA-UTIs issued by the CDC outline several recommendations, including appropriate use of indwelling catheters, education of personnel on proper catheter insertion using aseptic technique and sterile equipment, and maintenance to ensure closed sterile drainage (Table 11.10).
A variety of specialized urethral catheters have been designed to reduce the risk of CA-UTI. These include antiseptic-impregnated catheters and catheters coated with silver alloy or nitrofurazone. Several systematic reviews of the use of antimicrobial urinary catheters in the prevention of CA-UTI have demonstrated a reduction in catheter associated bacteriuria, but consensus on the economic benefit compared to standard catheter use has not been reached. Additional strategies for preventing CA-UTI include removal as early as possible, the use of hand held bladder scanners to evaluate retention, computerized order/entry system prompts to remove the catheters, and education on appropriate need for, and use of, indwelling urinary catheters.
Nursing-related care aspects include thorough assessment to determine need for indwelling catheter use, aseptic insertion technique, indwelling catheter care to minimize infection risk, and astute monitoring of patients with urinary catheters for signs of UTI. All of these are important measures to decrease the risk of CA-UTI.
Central venous catheters (CVCs) are frequently used in hospitalized patients and they carry associated risks, the most common being bloodstream infection (BSI). According to the CDC, up to 250,000 hospital-acquired central line associated blood stream infections (CLA-BSI) occur annually in the US hospitals, with approximately 80,000 of these occurring in ICUs.
A central line associated blood stream infection is defined as the presence of bacteremia in a patient with an intravascular catheter with at least one positive blood culture and clinical signs of infections (ie, fever, chills, and/or hypotension), with no apparent source for the BSI except the catheter. Specific criteria for CLA-BSI include a positive culture with the same organism isolated from the catheter and peripheral blood. A BSI is considered to be associated with a central line if the line was in place during the 48-hour period before development of the BSI. Although CVSs account for only a small percentage of all intravenous lines, they cause most BSIs. The most common mechanism of CLA-BSI is migration of the organism from the insertion site along the surface of the catheter and colonization of its distal part. CLA-BSIs can also occur from contamination of the catheter hub or infusate administered through the device.
Several practices have been evaluated in an attempt to reduce the incidence of CLA-BSI. These include using a standardized catheter insertion technique with a skin preparation such as chlorhexidine and careful maintenance of the catheter. In addition, daily review of catheter necessity, the use of antimicrobial-impregnated dressings, and use of antimicrobial catheters have demonstrated reductions in incidence of CLA-BSI. CLA-BSIs often result from contamination of the catheter during insertion, therefore maximum sterile barrier precautions during insertion are indicated to reduce the incidence of CLA-BSI. Effective barrier precautions include the use of sterile gloves, long-sleeved gowns, full-size drape, masks, and head covers by all personnel involved in the central line insertion procedure.
Additional measures advocated for best practices for CVC care include hand hygiene by washing hands with conventional antiseptic-containing soap and water or with waterless alcohol-based gels or foam before and after palpating insertion sites; and before and after insertion, replacing, accessing, or dressing a CVC. Avoidance of antibiotic ointment at insertion sites, which can promote fungal infections and antibiotic resistance, and restricted use of stopcocks on any tubing other than pressure tubing to minimize contamination are also recommended. Table 11.11 outlines evidence-based strategies for CLA-BSI prevention.
TABLE 11-11. EVIDENCE-BASED STRATEGIES FOR CENTRAL LINE INFECTION PREVENTION

Catheters impregnated or coated with antimicrobials or antiseptics have been shown to decrease the risk of CLA-BSI. Chlorhexidine-impregnated dressings have also been found to reduce the rate of CVC colonization. While evidence for the efficacy of CVC catheters coated with antibacterial or antiseptic agents exists, limited information exists related to their cost-effectiveness. Current CDC recommendations include use of CVC catheters coated with antibacterial or antiseptic agents if unable to decrease the rate to less than 3 infections per 1000 catheter days after implementing standards for insertion and care of the catheter.
Nursing-related care aspects include maximal barrier precautions during CVC insertion; maintenance of central line site to minimize infection risk; prevention of contamination of CVC ports during blood sampling, infusion of intravenous fluids, or medication administration; maintenance of sterile technique for dressing changes; intravenous tubing changes based on protocol guidelines; and astute monitoring of patients with central lines for signs of infection.
Multi-drug resistant organisms (MDRO) are bacteria resistant to current antibiotic therapy. MDROs can cause serious local and systemic infections that can be severely debilitating and even life-threatening. The most common MDROs include methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), tuberculosis (TB), Acinetobacter, and Clostridium difficile infections (C-diff). According to the CDC the prevalence of infections caused by MDROs is on the rise, making early identification, treatment, and prevention of the transmission of MDROs an important area of focus for healthcare providers.
Methicillin-resistant Staphylococcus aureus is a type of staphylococcal organism resistant to traditional antibiotic therapy, including methicillin, oxacillin, amoxicillin, penicillin, and cephalosporins. MRSA can be transmitted by personal contact with contaminated items such as dressings or other infected materials, and can be spread via the hands or equipment of healthcare providers, such as stethoscopes.
Vancomycin-resistant Enterococcus most commonly occurs in hospital and long-term care settings. According to the CDC, persons at-risk for acquiring VRE include those who have been previously treated with the antibiotic vancomycin or other antibiotics for long periods of time, hospitalized persons who have received antibiotics for a long period of time, persons with impaired immune status, those who have had recent surgery or those with invasive catheters. VRE can be passed from person to person by the contaminated hands of caregivers or spread directly to people after they touch surfaces that are contaminated with VRE.
The CDC has identified interventions necessary to control or eradicate MDROs including MRSA and VRE. These categories include administrative support, education, judicious use of antimicrobial agents, MDRO surveillance, infection control precautions, environmental precautions, and decolonization. Additionally, the CDC’s Campaign to Prevent Antimicrobial Resistance recommends judicious use of antibiotics and avoiding excessive duration of antibiotic therapy. General measures to prevent MDROs in healthcare settings include infection prevention measures, early detection of infections, appropriate use of antibiotic therapy, and measures to prevent transmission. Nurses in progressive care units play an important role in the prevention of infections, and in instituting measures to prevent transmission of MDROs. The importance of hand hygiene continues to play a significant role in the prevention of infection and in targeting transmission of MDROs. Some organizations have also implemented the use of contact precautions upon admission of high risk patients for MRSA until proven culture negative. Awareness of specific institutional practices for infection prevention and control including the use of standard and contact precautions, along with education of staff, patients and visitors provide the basis for recommendations for control of MDROs in healthcare settings.
AACN practice alert: Sepsis 2006. www.aacn.org/WD/Practice/Docs/Sepsis-04.2006.pdf. Accessed February 13, 2013.
Aitken LM, Williams G, Harvey M, et al. Nursing considerations to complement the Surviving Sepsis Campaign. Crit Care Med. 2011;39:1800-1818.
Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32:35-47.
Baldwin I, Fealy N. Nursing for Renal Replacement Therapies in the Intensive Care Unit: Historical, Educational, and Protocol Review. Blood Purif. 2009;27:174-181 (DOI:10.1159/000190784).
Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306.
Blackwood B, Wilson-Barnett J. The impact of nurse-directed protocolised-weaning from mechanical ventilation on nursing practice: a quasi-experimental study. J Int Stud. 2007;44:209-226.
Bourgault AM, Ipe L, Weaver J, et al. Development of evidence-based guidelines and critical care nurses: knowledge of enteral feeding. Crit Care Nurse. 2007;27:17-29.
Carmona Monge FJ, Martinez Lareo M, Garcia Gomez S, et al. Effectiveness and safety of goal directed nurse-led blood glucose control in an intensive care unit: a prospective observational study. Enferm Intensiva. 2012;23;11-16.
Chant C, Mustard M, Thorpe KE, Friedrich JO. Nurse vs nomogram-directed glucose control in a cardiovascular intensive care unit. Am J Crit Care. 2012;21:270-279.
Davidson J, Powers K, Hedayat K, et al: Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force. Crit Care Med. 2007;35:605-622.
Dellinger RP, Levy ML, Opal S, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637.
Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013; 41:1167-1174.
Gupta B, Agrawal P, Soni KD, et al. Enteral nutrition practices in the intensive care unit: understanding of nursing practices and perspectives. J Anaesthesiol Clin Pharmacol. 2012;28:41-44.
Institute for Healthcare Improvement. Evaluation for severe sepsis screening tool. http://www.survivingsepsis.org/files/Tools/evaluationforseveresepsisscreeningtool.pdf
Kleinpell R, Aitken L, Schorr CA. Implications of the new international sepsis guidelines for nursing care. Am J Crit Care. 2013;22:212-220.
Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367-374.
Moore LJ, Jones SL, Kreiner LA, et al. Validation of a screening tool for the early identification of sepsis. J Trauma. 2009; 66:1539-1546; discussion 1546-1547.
Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35:1105-1112.
NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108-1118.
O’Connor E, Tragen D, Fahey P et al. Improving blood glucose control during critical illness: a cohort study. J Crit Care. 2010;25:78-83.
Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2011; (3):CD000567. Review.
Pronovost P, Berenholtz S. Improving sepsis care in the intensive care unit: an evidence-based approach. 2004 VHA Research Series. Available: https://www.vha.com/research/public/sepsis_icu.pdf/. Accessed March 16, 2010.
Rivers EP, Ahrens T. Improving outcomes for severe sepsis and septic shock: tools for early identification of at-risk patients and treatment protocol implementation. Crit Care Clin. 2008;23:S1-S47.
Rubinsky M, Clark A. Early enteral nutrition in critically ill patients. Dimens Crit Care Nurs. 2012;31:267-274.
Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207-214.
Amato L, Minozzi S, Vecchi S, Davoli M. Amato, Laura, eds. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev 3(3):2010;CD005063. DOI:10.1002/14651858.CD005063.pub3. PMID 20238336.
Ameres MJ. Acetaminophen (tylenol) poisoning. http://www.emedicinehealth.com/acetaminophen_tylenol_poisoning/article_em.htm. Accessed February 15, 2013.
Anker A. Drug Overdose. http://www.emedicinehealth.com/drug_overdose/page2_em.htm. Accessed February 22, 2013.
Cassidy EM, O’Sullivan I, Bradshaw P, Islam T, Onovo C. Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: a comparison with the standard fixed dose benzodiazepine regimen. Emerg Med J. 2011.
Center for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. JAMA. 2012;307(8):774-776.
Corfee FA. Alcohol withdrawal in the critical care unit. Aust Crit Care. 2011;24(2):110-116.
Karch AM. Nursing 2009 Drug Handbook. Philadelphia, PA: Lippincott, Williams & Wilkins; 2009.
Marraffa JM, Cohen V, Howland MA. Antidotes for toxicological emergencies: a practical review. Am J Health-Syst Pharm. 2012;69(3):199-212.
McKeown NJ, West, PL. Withdrawal syndromes. 2012. http://emedicine.medscape.com/article/819502-overview. Accessed February 25, 2013.
Monte R, Rabunal R, Casariego E, et al. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol. March-April 2010;45(2):151-158.
Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of a2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother. May 2011;45(5):649-657.
Schutt RC, Ronco C, Rosner MH. The role of therapeutic plasma exchange in poisonings and intoxications. Seminars in Dialysis. 2012;25(2):201-206.
Stewart S, Swain S. Assessment and management of alcohol dependence and withdrawal in the acute hospital: concise guidance. Clin Med. 2012;12(3):266-271.
Tetrault JM, O’Connor PG. Substance abuse and withdrawal in the critical care setting. Crit Care Clin. 2008;24:767-788.
Agency for Healthcare Research and Quality. Preventing pressure ulcers in hospitals: a toolkit for improving quality of care. http://www.ahrq.gov/research/ltc/pressureulcertoolkit/putool3a.htm. Accessed May 5, 2012.
Bryant N, Nix Denise. Acute and Chronic Wounds Current Management Concepts. 4th edition, 2010. Elsevier Mosby, St Louis Missouri.
Cox J. Predictors of pressure ulcers in adult critical care patients. Am J Crit Care. 2011;20:364-375.
Guideline for Prevention and Management of Pressure Ulcers, WOCN Clinical Practice Guideline Series, 2010.
Institute for Healthcare (IHI). 5 million lives campaign getting started kit: how to guide. 2008. Cambridge, MA: Author Retrieved February 18, 2013 from http://www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventPressureUlcers.aspx
Jankowski IM. Tips for protecting critically ill patients from pressure ulcers. Crit Care Nurse. 2010;30:S7-S9.
Moore Z, Webster J, Review Group, Cochrane Wounds Group. Dressings and topical agents for preventing ulcers. Cochrane Database Syst Rev. 2011.
Niederhauser A, Lukas CV, Parker V, et al. Comprehensive programs for preventing pressure ulcers: a review of the literature. Adv Skin Wound Care. 2012;15:167-188.
National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel. Pressure ulcer prevention recommendations. In: Prevention and treatment of pressure ulcers: clinical practice guideline. Washington (DC): National Pressure Ulcer Advisory Panel; 2009,21-50.
Shanin ES, Dassen T, Halfens RJ. Incidence, prevention and treatment of pressure ulcers in intensive care patients: a longitudinal study. Int J Nurs Stud. 2009;46:413-421.
Brosnahan J, Jull A, Tracy C. Types of urethral catheters for management of short-term voiding problems in hospitalized adults (Cochrane Review). The Cochrane Database Syst Rev. 2007;1.
Centers for Disease Control Catheter-associated urinary tract infection. http://www.cdc.gov/HAI/ca_uti/uti.html. Accessed February 16, 2013.
Gould VC, Umscheid C, Agarwal RK, et al. Guidelines for the prevention of catheter-associated urinary tract infection 2009. http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009 final.pdf Accessed February 20, 2013.
Gould VC. Catheter association urinary tract infection (CAUTI) prevention toolkit http://www.cdc.gov/HAI/pdfs/toolkits/CAUTItoolkit_3_10.pdf. Accessed February 20, 2013.
Kleinpell RM, Munro CL, Giuliano KK. Targeting health care acquired infections: evidence based strategies. In: Patient Safety and Quality: An Evidence Based Handbook for Nurses. Agency for Healthcare Research and Quality, 2008. http://www.ncbi.nlm.nih.gov/books/NBK2632/. Accessed February 20, 2013.
O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections, 2011. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Accessed February 20, 2013.
Centers for Disease Control and Prevention. Management of multidrug-resistant organisms in healthcare settings, 2006. http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf. Accessed February 20, 2013.
Centers for Disease Control and Prevention. Methicillin resistant Staphylococcus auerus (MRSA) infections http://www.cdc.gov/mrsa/. Accessed February 20, 2013.
Centers for Disease Control and Prevention. Vancomycin-resistant Enterococci in healthcare settings. http://www.cdc.gov/HAI/organisms/vre/vre.html. Accessed February 20. 2013.
Derricott B. Multi-Drug Resistant Organisms (MDROs). 2011. http://www.nursingceu.com/courses/316/index_nceu.html. Accessed February 20, 2013.
Siegel JD, Rhinehart E, Jackson M, et al. The Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug-resistant organisms. In: Healthcare Settings, 2006. http://www.cdc.gov/hicpac/pdf/MDRO/MDROGuideline2006.pdf. Accessed February 20, 2013.