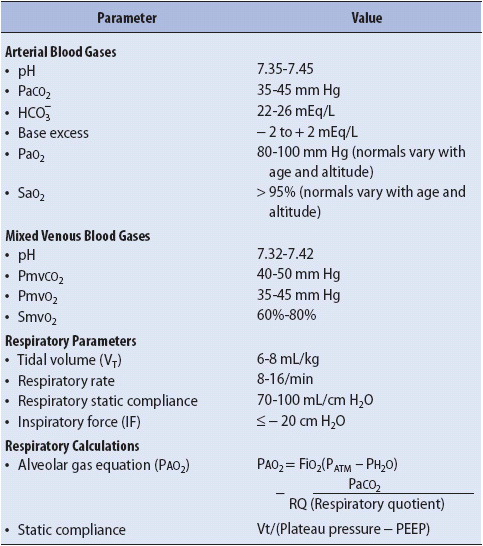
TABLE 5-1. LABORATORY AND CALCULATED RESPIRATORY VALUES
KNOWLEDGE COMPETENCIES
1. Interpret normal and abnormal arterial blood gas results and common management strategies for treatment.
2. Identify indications, complications, and management strategies for artificial airways, oxygen delivery, and monitoring devices.
3. Identify pulmonary and nonpulmonary factors important to the promotion of positive weaning outcomes in long-term mechanically ventilated patients.
4. Describe the concepts of respiratory muscle fatigue, rest, and conditioning as they relate to the mechanically ventilated weaning patient.
5. Identify essential components for the successful design and use of weaning predictors, protocols for weaning trials, and multidisciplinary institutional approaches to the care of long-term mechanically ventilated patients.
Arterial blood gas (ABG) monitoring may be used to assess acid-base balance, ventilation, and oxygenation. An arterial blood sample is analyzed for oxygen tension (PaO2), carbon dioxide tension (PaCO2), and pH using a blood gas analyzer. From these measurements, several other parameters are calculated by the blood gas analyzer, including base excess (BE), bicarbonate (HCO3–), and oxygen saturation (SaO2). Fractional arterial SaO2 can be directly measured if a co-oximeter is available. Normal ABG values analysis are listed in Table 5-1.
Arterial blood gas samples are obtained by direct puncture of an artery, usually the radial artery, or by withdrawing blood through an indwelling arterial catheter system. A heparinized syringe is used to collect the sample to prevent clotting of the blood prior to analysis. Blood gas samples are kept on ice unless there is the ability to immediately analyze to prevent the continued transfer of CO2 and O2 in and out of the red blood cells. ABG analysis equipment is often kept in or near progressive care units to maximize accuracy and decrease the time for reporting of results. Additionally, portable point-of-care devices are available at many hospitals that allow measurement at the bedside. Regardless of the method used to obtain the ABG sample, practitioners should wear gloves and follow universal precautions to prevent exposure to blood during the sampling procedure.
All the pressure monitoring systems used with indwelling arterial catheters have sites where samples of arterial blood can be withdrawn for ABG analysis or other laboratory testing (Figure 5-1). Using the stopcock closest to the catheter insertion site, or the indwelling syringe or reservoir of the needleless systems, a 3- to 5-mL sample of blood is withdrawn to clear the catheter system of any flush system fluid. A 1-mL sample for ABG analysis is then obtained in a heparinized syringe. Any air remaining in the syringe is then removed, an airtight cap is placed on the end of the syringe, and the sample is placed on ice to ensure accuracy of the measurement. The arterial catheter system is then flushed to clear the line of any residual blood.
Figure 5-1. Examples of indwelling arterial catheter systems for blood gas analysis. (A) Closed blood withdrawal system. (B) Open blood withdrawal system. (Courtesy of: Edwards Lifesciences [A].)
Complications associated with this technique for obtaining ABG samples include infection and hemorrhage. Any time an invasive system is used, the potential exists for contamination of the sterile system. The use of needleless systems on indwelling catheter systems decreases patients’ risk for infection, as well as the progressive care practitioners’ risk for accidental needlestick injuries, and should be used whenever feasible. Hemorrhage is a rare complication, occurring when stopcocks are inadvertently left in the wrong position after blood withdrawal or with tubing disconnections. These complications can be avoided by carefully following the proper technique during blood sampling, limiting sample withdrawal to experienced practitioners, assuring connections are tight, and keeping the pressure alarm system of the bedside monitoring system activated at all times.
When indwelling arterial catheters are not in place, ABG samples are obtained by directly puncturing the artery with a needle and syringe. The most common sites for arterial puncture are the radial, brachial, and femoral arteries. Similar to venipuncture, the technique for obtaining an ABG sample is relatively simple, but success in obtaining the sample requires experience.
An Allen test is performed prior to obtaining an ABG by puncture and prior to the insertion of an arterial line into the radial artery. The Allen test requires that the ulnar and radial pulses be occluded for a brief period of time with the forearm held upward to facilitate blood emptying from the hand. Once blanching of the hand is observed, the forearm is placed in a downward position, the ulnar artery is released, and the hand is observed for flushing. If the hand flushes, it is clear that the ulnar artery is capable of supplying blood to the fingers should the radial artery be damaged.
Following location of the pulsating artery and antiseptic preparation of the skin, the needle is inserted into the artery at a 45° angle with the bevel facing upward. The needle is slowly advanced until arterial blood appears in the syringe barrel or the insertion depth is below the artery location. If blood is not obtained, the needle is pulled back to just below the skin and relocation of the pulsating artery is verified prior to advancing the needle again.
As soon as the 1-mL sample of arterial blood is obtained, the syringe is withdrawn and firm pressure quickly applied to the insertion site with a sterile gauze pad. Handheld pressure is maintained for at least 5 minutes and the site inspected for bleeding or oozing. If present, pressure should be reapplied until all evidence of oozing has stopped. Pressure dressings are not applied until hemostasis has been achieved.
As described, all air must be removed from the ABG syringe and an airtight cap applied to the end (remove the needle first). Given the importance of maintaining pressure at the puncture site, it is sometimes helpful to have another practitioner assisting during arterial puncture to ensure appropriate handling of the blood sample.
Complications associated with arterial puncture include arterial vessel tears, air embolism, hemorrhage, arterial obstruction, loss of extremity, and infection. Using proper technique during sampling can dramatically decrease the incidence of these complications. Damage to the artery may be decreased by using a small diameter needle (21-23 gauge in adults) and by avoiding multiple attempts at the same site. After one or two failed attempts at entering the artery, a different site should be selected or another experienced practitioner enlisted to attempt the ABG sampling. All facilities have specific policies and procedures providing guidance on sample acquisition and handling of ABGs and the reader is encouraged to follow their institutional guidelines.
Hemorrhage can occur easily into the surrounding tissues if adequate hemostasis is not achieved with direct pressure. Bleeding into the tissue can range from small blood loss with minimal local damage to large blood loss with loss of distal circulation and even exsanguination. Large blood loss is more commonly seen with femoral punctures and is often the result of inadequate pressure on the artery following needle removal. Bleeding from the femoral artery is difficult to visualize, so significant blood loss can occur before practitioners are alerted to the problem. For this reason, the femoral site is the least preferred site for ABG sampling and is used only when other sites are not accessible.
The need for frequent ABG sampling for ventilation and oxygenation assessment and management may require the insertion of an arterial catheter and monitoring system to decrease the risks associated with repetitive arterial punctures.
The best approach to analyzing the results of ABGs is a systematic one. Analysis is accomplished by evaluating acid-base and oxygenation status. Upon receipt of ABG results, the practitioner first identifies any abnormal values (see Table 5-1). Then a systematic evaluation of acid-base and oxygenation status is done.
Optimal cellular functioning occurs when the pH of the blood is between 7.35 and 7.45. Decreases in pH below 7.35 are termed acidemia, and increases in pH above 7.45 are termed alkalemia. When the amount of acids or bases in the body increases or decreases, the pH changes if the ratio of acids to bases is altered. For example, if acid production increases, and there is no change in the amount of base production, pH decreases. If the base production were to increase as well, as a response to increased acid production, then no change in pH would occur because the ratio of acids to bases would be maintained. Because the body functions best at a pH in the 7.35 to 7.45 range, there are strong systems in place to maintain the balance between acids and bases, even if one of those components is functioning abnormally. Although a variety of regulatory systems are involved in acid-base balance, the bicarbonate (HCO3–) and carbon dioxide (CO2) levels are the primary regulators.
• Metabolic component: HCO3– levels are controlled primarily by the kidneys and have been termed the metabolic component of the acid-base system. By increasing or decreasing the amount of HCO3– excreted in the kidneys, the pH of the blood can be increased or decreased. Changes in HCO3– excretion may take up to 24 hours or longer to accomplish, but can be maintained for prolonged periods.
• Respiratory component: CO2 levels are controlled primarily by the lungs and are termed the respiratory component of the acid-base system. By increasing or decreasing the amount of CO2 excreted by the lungs, the pH of the blood can be increased or decreased. Changes in CO2 excretion can occur rapidly, within a minute, by increasing or decreasing respiration (minute ventilation). Compensation by the respiratory system is difficult to maintain over long periods of time (24 hours).
• Acid-base abnormalities: A variety of conditions may result in acid-base abnormalities (Tables 5-2 and 5-3).
TABLE 5-3. EXAMPLES OF ARTERIAL BLOOD GAS RESULTS
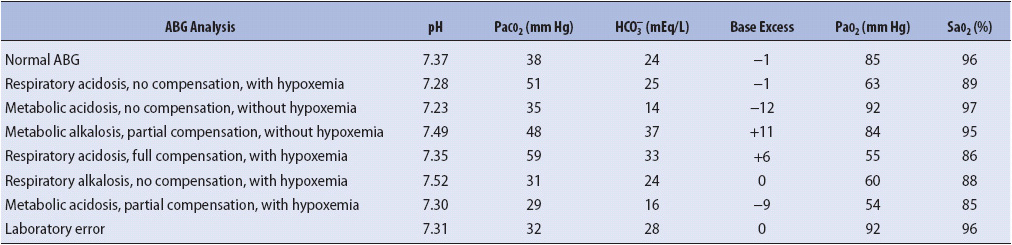
Metabolic alkalemia is present when the pH is above 7.45 and the HCO3– is above 26 mEq/L. In metabolic alkalosis there is either a primary increase in hydrogen ion (H1) loss or HCO3– gain. The respiratory system attempts to compensate for the increased pH by decreasing the amount of CO2 eliminated from the body (alveolar hypoventilation). This compensatory attempt by the respiratory system results in a change in pH, but rarely to a normal value. Clinical situations or conditions that cause metabolic alkalemia include loss of body acids (nasogastric suction of HCl, vomiting, excessive diuretic therapy, steroids, hypokalemia) and ingestion of exogenous bicarbonate or citrate substances. Management of metabolic alkalosis is directed at treating the underlying cause, decreasing or stopping the acid loss (eg, use of antiemetic therapy for vomiting), and replacing electrolytes.
Metabolic acidemia is present when the pH is below 7.35 and the HCO3– is below 22 mEq/L. In metabolic acidosis there is excessive loss of HCO3– from the body by the kidneys or the accumulation of acid. The respiratory system attempts to compensate for the decreased pH by increasing the amount of CO2 eliminated (alveolar hyperventilation). This compensatory attempt by the respiratory system results in a change in pH toward normal. Clinical situations or conditions that cause metabolic acidosis include increased metabolic formation of acids (diabetic ketoacidosis, uremic acidosis, lactic acidosis), loss of bicarbonate (diarrhea, renal tubular acidosis), hyperkalemia, toxins (salicylates overdose, ethylene and propylene glycol, methanol, paraldehyde), and adrenal insufficiency. Management of metabolic acidosis is directed at treating the underlying cause, decreasing acid formation (eg, decreasing lactic acid production by improving cardiac output [CO] in shock), decreasing bicarbonate losses (eg, treatment of diarrhea), removal of toxins through dialysis or cathartics, or administering sodium bicarbonate (NaHCO3) in extreme metabolic acidemia states.
Respiratory alkalemia occurs when the pH is above 7.45 and the PaCO2 is below 35 mm Hg. In respiratory alkalosis, there is an excessive amount of ventilation (alveolar hyperventilation) and removal of CO2 from the body. If these ABG changes persist for 24 hours or more, the kidneys attempt to compensate for the elevated pH by increasing the excretion of HCO3– until normal or near-normal pH levels occur. Clinical situations or conditions that cause respiratory alkalosis include neurogenic hyperventilation, interstitial lung diseases, pulmonary embolism, asthma, acute anxiety/stress/fear, hyperventilation syndromes, excessive mechanical ventilation, and severe hypoxemia. Management of respiratory alkalosis is directed at treating the underlying cause and decreasing excessive ventilation if possible.
Respiratory acidemia occurs when the pH is below 7.35 and the PaCO2 is above 45 mm Hg. In respiratory acidosis there is an inadequate amount of ventilation (alveolar hypoventilation) and removal of CO2 from the body. If these ABG changes persist for 24 hours or more, the kidneys attempt to compensate for the decreased pH by increasing the amount of HCO3– in the body (decreased excretion of HCO3– in the urine) until normal or near-normal pH levels occur. Clinical situations or conditions that cause respiratory acidosis include overall hypoventilation associated with respiratory failure (eg, acute respiratory distress syndrome [ARDS], severe asthma, pneumonia, chronic obstructive pulmonary diseases, and sleep apnea), pulmonary embolism, pulmonary edema, pneumothorax, respiratory center depression, and neuromuscular disturbances in the presence of normal lungs, and inadequate mechanical ventilation. Management of respiratory acidosis is directed at treating the underlying cause and improving ventilation.
Mixed (combined) disturbance is the simultaneous development of a primary respiratory and metabolic acid-base disturbance. For example, metabolic acidosis may occur from diabetic ketoacidosis, with respiratory acidosis occurring from respiratory failure associated with aspiration pneumonia. Mixed acid-base disturbances create a more complex picture when examining ABGs and are beyond the scope of this text.
After determining the acid-base status from the ABG, the adequacy of oxygenation is assessed. Normal values for PaO2 depend on age and altitude. Lower levels of PaO2 are acceptable as normal with increasing age and altitude levels. In general, PaO2 levels between 80 and 100 mm Hg are considered normal on room air.
SaO2 levels are also affected by age and altitude, with values above 95% considered normal. Hemoglobin saturation with oxygen is primarily influenced by the amount of available oxygen in the plasma (Figure 5-2). The S shape to the normal oxyhemoglobin curve emphasizes that as long as PaO2 levels are above 60 mm Hg, 90% or more of the hemoglobin is bound or saturated with O2. Factors that can shift the oxyhemoglobin curve to the right and left include temperature, pH, PaCO2, and abnormal hemoglobin conditions. In general, shifting the curve to the right decreases the affinity of oxygen for hemoglobin, resulting in an increase in the amount of oxygen released to the tissues. Shifting of the curve to the left increases the affinity of oxygen for hemoglobin, resulting in a decreased amount of oxygen released to the tissues.
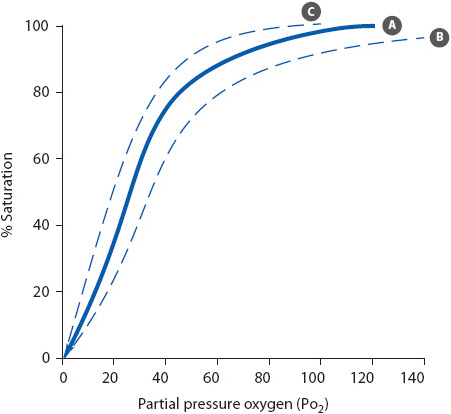
Figure 5-2. Oxyhemoglobin dissociation curve. (A) Normal. (B) Shift to the right. (C) Shift to the left.
A decrease in PaO2 below normal values is hypoxemia. A variety of conditions cause hypoxemia:
• Low inspired oxygen: Usually, the fraction of inspired oxygen concentration (FiO2) is reduced at high altitudes or when toxic gases are inhaled. Inadequate or inappropriately low FiO2 administration may contribute to hypoxic respiratory failure in patients with other cardiopulmonary diseases.
• Overall hypoventilation: Decreases in tidal volume (Vt), respiratory rate, or both reduce minute ventilation and cause hypoventilation. Alveoli are underventilated, leading to a fall in alveolar oxygen tension (PAO2) and increased PaCO2 levels. Causes of hypoventilation include respiratory center depression from drug overdose, anesthesia, excessive analgesic administration, neuromuscular disturbances, and fatigue.
• Ventilation-perfusion mismatch: When the balance between adequately ventilated and perfused alveoli is altered, hypoxemia develops. Perfusion of blood past underventilated alveoli decreases the availability of oxygen for gas exchange, leading to poorly oxygenated blood in the pulmonary vasculature. Examples of this include bronchospasm, atelectasis, secretion retention, pneumonia, pulmonary embolism, and pulmonary edema.
• Diffusion defect: Thickening of the alveolar-capillary membrane decreases oxygen diffusion and leads to hypoxemia. Causes of diffusion defects are chronic disease states such as pulmonary fibrosis and sarcoidosis. Hypoxemia usually responds to supplemental oxygen in conditions of diffusion impairment (eg, interstitial lung disease).
• Shunt: When blood bypasses or shunts past the alveoli, gas exchange cannot occur and blood returns to the left side without being oxygenated. Shunts caused anatomically include pulmonary arteriovenous fistulas or congenital cardiac anomalies of the heart and great vessels, such as tetralogy of Fallot. Physiologic shunts are caused by a variety of conditions that result in closed, nonventilated alveoli such as seen in ARDS.
• Low mixed venous oxygenation: Under normal conditions, the lungs fully oxygenate the pulmonary arterial blood and mixed venous oxygen tension (PmVO2) does not affect PaO2 significantly. However, a reduced PmVO2 can lower the PaO2 significantly when either ventilation-perfusion mismatch or intrapulmonary shunting is present. Conditions that can contribute to low mixed venous oxygenation include low CO, anemia, hypoxemia, and increased oxygen consumption. Improving tissue oxygen delivery by increasing CO or hemoglobin usually improves mixed venous oxygen saturation (SVO2).
Analysis of oxygen and carbon dioxide levels in the venous blood provides additional information about the adequacy of perfusion and oxygen use by the tissues. Venous blood gas analysis, also referred to as a mixed venous blood gas sample, is obtained from the distal tip of a pulmonary artery (PA) catheter or from a central venous pressure (CVP) catheter. Normal values for venous blood gas values are listed in Table 5-1. Central venous oxygen saturation (SCVO2) can be obtained from any central venous catheter with the tip positioned in the superior vena cava. Mixed venous oxygen saturation (SmVO2) can only be obtained from a PA or specialized catheter generally only used in a critical care unit. More information on SmVO2 and SCVO2 monitoring is found in Chapter 4, Hemodynamic Monitoring.
Pulse oximetry is a common method for the continuous, noninvasive monitoring of SaO2. A sensor is applied to skin over areas with strong arterial pulsatile blood flow, typically one of the peripheral fingers or toes (Figure 5-3). Alternative sites include the bridge of the nose, ear, and the forehead (Figure 5-4). The forehead sensor is a reflectance sensor and provides a central monitoring site location. The SaO2 sensor is connected to a pulse oximeter monitor unit via a cable. Light-emitting diodes on one side of the sensor transmit light of two different wavelengths (infrared and red) through arterial blood flowing under the sensor. Depending on the level of oxygen saturation of hemoglobin in the arterial blood, different amounts of light are detected on the other side of the sensor (transmission) or via scattered light on the same side of the light emitters (reflectance). This photo-detection aspect of the sensor transmits information to the microprocessor within the monitor, which then uses various internal software algorithms for calculation and digital display of the oxygen saturation and pulse rate.
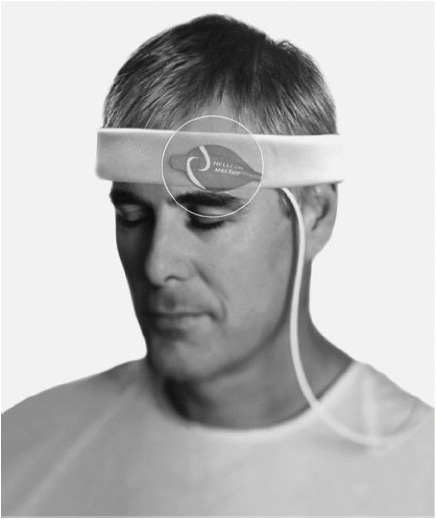
Figure 5-4. Forehead reflectance pulse oximeter sensor. (With permission, Covidien.)
When blood perfusion is adequate and SaO2 levels are greater than 70%, depending on the type of sensor being used and monitoring site, there is generally a close correlation between the saturation reading from the pulse oximeter (SpO2) and SaO2 directly measured from ABGs. In situations where perfusion to the sensor is markedly diminished (eg, peripheral vasoconstriction due to disease, drugs, or hypothermia), the ability of the pulse oximeter to detect a signal may be less than under normal perfusion conditions. Newer generation pulse oximeters have the ability to detect signals during most poor perfusion conditions, as well as certain other sources of signal interference, such as motion or other conditions which create potential for artifact.
Pulse oximetry has several advantages for respiratory monitoring. The ability to have continuous information on the SaO2 level of patients without the need for an invasive arterial puncture decreases infection risks and blood loss from frequent ABG analysis. In addition, these monitors are easy to use, well tolerated by most patients, and portable enough to use during transport.
The major disadvantage of pulse oximeters for assessing oxygen status is that accuracy depends on an adequate arterial pulsatile signal in order for the pulse oximeter to properly function. Clinical situations that decrease the accuracy of the device include:
• Hypotension
• Low CO states
• Vasoconstriction or vasoactive drugs
• Hypothermia
• Movement of the sensor and/or poor skin adherence
Additionally, other sources of potential interference may include direct exposure to ambient light and certain nail polish applications and treatments.
Because these conditions may be found in acutely ill patients, caution is exercised when using pulse oximetry in progressive care units. Proper use (Table 5-4) and periodic validation of the accuracy of the devices with ABG analysis using a co-oximeter instrument is essential to avoid erroneous patient assessment. Routinely used pulse oximeters measure light absorbance at only two wavelengths of light. As such, dyshemoglobinemias such as methemoglobinemia (Met-Hgb) and carboxyhemoglobinemia (CO-Hgb) cannot be measured. Further, the presence of such elevations may cause errors in interpretation of pulse oximetry. Although there are noninvasive devices available for detecting such dyshemoglobinemias, the most widely used and recognized “gold standard” technique for determining the presence of dyshemoglobinemias is co-oximetry via invasive ABG analysis.
A variety of measurements in addition to ABG analysis can be used to further evaluate the acutely ill patient’s respiratory system.
Measurement of selected lung volumes can be easily accomplished at the bedside. Vt, minute ventilation, and negative inspiratory pressure (NIP) are measured with portable, handheld equipment (spirometer and NIP meter, respectively). Lung compliance and alveolar oxygen content can be calculated with standard formulas (see Table 5-1). Frequent trend monitoring of these parameters provides an objective evaluation of the patient’s response to interventions.
Carbon dioxide is a byproduct of cellular metabolism and is transported via the venous blood to the lungs where it is eliminated by the lungs during exhalation. End-tidal CO2 (also referred to as partial pressure of end-tidal CO2:PetCO2) is the concentration of CO2 present at the end of exhalation and is expressed either as a percentage (PetCO2%) or partial pressure (PetCO2 mmHg). The normal range for PetCO2 is typically 1 to 5 mm Hg less than the arterial carbon dioxide tension or PaCO2. For this reason, clinicians have sought to use this noninvasive monitoring method for assessing ventilation status over time. Thus, under conditions of normal ventilation and perfusion (![]() ) matching, the relationship between PetCO2 and PaCO2 is relatively close. However, when
) matching, the relationship between PetCO2 and PaCO2 is relatively close. However, when ![]() relationships are abnormal, this gradient may be as high as 20 mm Hg or more, thus limiting the use of this technology to accurately reflect PaCO2. Assessing the arterial to end-tidal CO2 gradient as a trend may be useful. An increasing gradient reflects a worsening condition and a narrowing gradient may reflect improved ventilation/perfusion matching.
relationships are abnormal, this gradient may be as high as 20 mm Hg or more, thus limiting the use of this technology to accurately reflect PaCO2. Assessing the arterial to end-tidal CO2 gradient as a trend may be useful. An increasing gradient reflects a worsening condition and a narrowing gradient may reflect improved ventilation/perfusion matching.
Currently available end-tidal CO2 monitoring devices fall into one of several categories: colorimetric, capnometric (numeric display only), or capnographic (numeric and graphical display). Colorimetric devices are pH-sensitive, colored paper strips that change color in response to different concentrations of carbon dioxide (Figure 5-5). They are typically used for either initial or intermittent monitoring purposes such as verifying endotracheal tube (ET) placement in the trachea following intubation or in some cases, to rule out inadvertent pulmonary placement of enteral feeding tubes following insertion. A capnometer provides a visual analog or digital display of the concentration of the PetCO2. Capnography includes both capnometry plus the addition of a calibrated graphic recording of the exhaled CO2 on a breath-by-breath basis and is perhaps the most common instrument used for continuous monitoring. Figure 5-6 demonstrates the various phases of a normal carbon dioxide waveform during exhalation.

Figure 5-5. Colorimetric carbon dioxide detector. (With permission, Covidien.)
Figure 5-6. Capnogram waveform phases. Phase A to B: Early exhalation. This represents anatomic dead space and contains little carbon dioxide. Phase B to C: Combination of dead space and alveolar gas. Phase C to D: Exhalation of mostly alveolar gas (alveolar plateau). Phase D: End-tidal point, that is, exhalation of carbon dioxide at maximum point. Phase D to E: Inspiration begins and carbon dioxide concentration rapidly falls to baseline or zero. (With permission, Oridion Systems Ltd., Jerusalem, Israel.)
Capnography devices measure exhaled carbon dioxide using one of several different techniques: infrared spectrography, Raman spectrography, mass spectrometry, or a laser-based technology called molecular correlation spectroscopy as the infrared emission source. The laser creates an infrared emission precisely matching the absorption rate spectrum of CO2 and eliminates the need for moving parts. A capnography device using this technology is shown in Figure 5-7. All capnographs sample and measure expired gases either directly at the patient-ventilator interface (mainstream analysis) or collected and transported via small-bore tubing to the sensor in the monitor (sidestream analysis). Each technique has advantages and disadvantages and the user should strictly follow manufacturer recommendations for optimal performance.
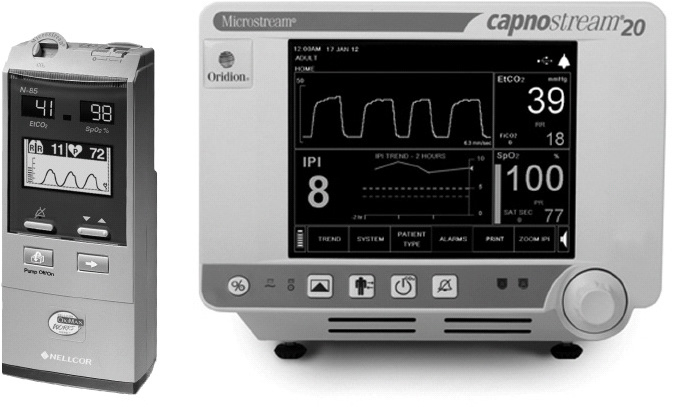
Figure 5-7. Handheld (A) and Bedside (B) combined capnography (sidestream) and pulse oximetry instruments. (With permission, Covidien.)
Clinical application of capnography includes assessment of endotracheal or tracheostomy tube placement, gastric or small bowel tube placement, pulmonary blood flow, and alveolar ventilation, provided ![]() relationships are normal. The 2010-2015 AHA Guidelines for ACLS recommend using quantitative waveform capnography during endotracheal tube placement and in intubated patients during CPR. Waveform capnography allows nurses and other caregivers to monitor CPR quality, optimize chest compressions, and detect return of spontaneous circulation (ROSC) during chest compressions. Assessment of the capnographic waveform alone can yield useful information in detecting ventilator malfunction, response to changes in ventilator settings and weaning attempts, and depth of neuromuscular blockade. It should be noted that although capnography is commonly used in patients with artificial airways, this monitoring technique can also be used in nonintubated patients via a modified nasal/oral sampling cannula. When using capnography in the clinical setting it is important to always follow manufacturer recommendations regarding set-up, maintenance, and troubleshooting of equipment. Institutional policies and protocols regarding clinical management for patient care should also be followed.
relationships are normal. The 2010-2015 AHA Guidelines for ACLS recommend using quantitative waveform capnography during endotracheal tube placement and in intubated patients during CPR. Waveform capnography allows nurses and other caregivers to monitor CPR quality, optimize chest compressions, and detect return of spontaneous circulation (ROSC) during chest compressions. Assessment of the capnographic waveform alone can yield useful information in detecting ventilator malfunction, response to changes in ventilator settings and weaning attempts, and depth of neuromuscular blockade. It should be noted that although capnography is commonly used in patients with artificial airways, this monitoring technique can also be used in nonintubated patients via a modified nasal/oral sampling cannula. When using capnography in the clinical setting it is important to always follow manufacturer recommendations regarding set-up, maintenance, and troubleshooting of equipment. Institutional policies and protocols regarding clinical management for patient care should also be followed.
Maintaining an open and patent airway is an important aspect of progressive care management. Patency can be ensured through conservative techniques such as coughing, head and neck positioning, and alignment. If conservative techniques fail, insertion of an oral or nasal airway or ET tube may be required.
The oropharyngeal airway, or oral bite block, is an airway adjunct used to relieve upper airway obstruction caused by tongue relaxation (eg, postanesthesia or during unconsciousness), secretions, seizures, or biting down on oral ETs (Figure 5-8A). Oral airways are made of rigid plastic or rubber material, semicircular in shape, and available in sizes ranging from infants to adults. The airway is inserted with the concave curve of the airway facing up into the roof of the mouth. The oral airway is then rotated down 180° during insertion to fit the curvature of the tongue and ensure the tongue is not obstructing the airway. The tip of the oropharyngeal airway rests near the posterior pharyngeal wall. For this reason, oral airways are not recommended for use in alert patients because they may trigger the gag reflex and cause vomiting. Oropharyngeal airways are temporary devices for achieving airway patency.
Management of oropharyngeal airways includes frequent assessment of the lips and tongue to identify pressure areas. The airway is removed at least every 24 hours to check for pressure areas and to provide oral hygiene.
The nasopharyngeal airway, or nasal trumpet, is another type of airway adjunct device used to help maintain airway patency, especially in the semiconscious patient (Figure 5-8B). The nasopharyngeal airway is also used to facilitate nasotracheal suctioning. Made of soft malleable rubber or soft plastic, the nasal airway ranges in sizes from 26 to 35 Fr. Prior to insertion, a topical anesthetic (eg, viscous lidocaine), based on hospital policy, may be applied to the nares. The nasopharyngeal airway, lubricated with a water-soluble gel, is gently inserted into one of the nares. The patency of the airway is assessed by listening for, or feeling with your hand, air movement during expiration. The airway should be secured to the nose with a small piece of tape to prevent displacement. Complications of these airways include bleeding, sinusitis, and erosion of the mucous membranes.
Care of the patient with a nasal airway includes frequent assessment for pressure areas and occlusion of the airway with dried secretions. Sinusitis has been documented as a complication. The continued need for the nasal airway is assessed daily and rotation of the airway from nostril to nostril is done on a daily basis. When performing nasotracheal suctioning through the nasal airway, the suction catheter is lubricated with a water-soluble gel to ease passage. Refer to the following discussion on suctioning for additional standards of care.
The laryngeal mask airway (LMA) is an ET tube with a small mask on one end that can be passed orally over the larynx to provide ventilatory assistance and prevent aspiration. Placement of the LMA is easier than intubation using a standard ET tube. Commonly used as the primary airway device in the operating room for certain types of surgical procedures, it should, however, only be considered a temporary airway for patients who require prolonged ventilatory support.
Esophageal tracheal airways are double-lumen airways that allow for rapid airway establishment through either esophageal or tracheal placement. They are used primarily for difficult or emergency intubation and the design permits blind placement without the need for a laryngoscope. The multifunction design permits positive-pressure ventilation, but an ET tube or tracheostomy is eventually needed. The primary advantages to using the airways include less training required to use than standard intubation, no special equipment required, and the cuff provides some protection against aspiration of gastric contents. The tube is contraindicated in responsive patients with intact gag reflexes, patients with known esophageal pathology, and patients who have ingested caustic substances. The tube is sized to the patient’s height.
Artificial airways (oral and nasal ET tubes, tracheostomy tubes) are used when a patent airway cannot be maintained with an adjunct airway device for mechanical ventilation or to manage severe airway obstruction. The artificial airway also protects the lower airway from aspiration of oral or gastric secretions and allows for easier secretion removal.
Endotracheal tubes are made of either polyvinyl chloride or silicone and are available in a variety of sizes and lengths (Figure 5-9A). Standard features include a 15-mm adapter at the end of the tube for connection to various life-support equipments such as mechanical ventilation circuits, closed-suction catheter systems, swivel adapters, or a manual resuscitation bag (MRB). Tubes may be cuffed or uncuffed. For cuffed tubes, air is manually injected into the cuff located near the distal tip of the ET tube through a small one-way pilot valve and inflation lumen. Distance markers are located along the side of the tube for identification of tube position. A radiopaque line is also located on all tubes so as to aid in determining proper position radiographically.
Figure 5-9. Artificial airways. (A) Cuffed endotracheal tube. (B) Cuffed tracheostomy tube. (With permission, Covidien.)
Endotracheal tubes are inserted into the patient’s trachea either through the mouth or nose (Figures 5-10 and 5-11). Orally inserted ET tubes are more common than the nasal route because nasal intubation is associated with sinus infections and are considered an independent risk factor for developing ventilator-associated pneumonia (VAP). With use of the laryngoscope, the upper airway is visualized and the tube is inserted through the vocal cords into the trachea, 2 to 4 cm above the carina. The presence of bilateral breath sounds, along with equal chest excursion during inspiration and the absence of breath sounds over the stomach, preliminarily confirms proper tube placement. An end-tidal CO2 monitor with waveform verification should be used as an immediate assessment for determining tracheal placement. If not available, a colorimetric CO2 detector may be used. A portable chest x-ray verifies proper tube placement. Once proper placement is confirmed, the tube is anchored to prevent movement with either tape or a special ET tube fixation device (Figure 5-12). The centimeter marking of the ET tube at the lip is documented and checked during each shift to monitor proper tube placement.
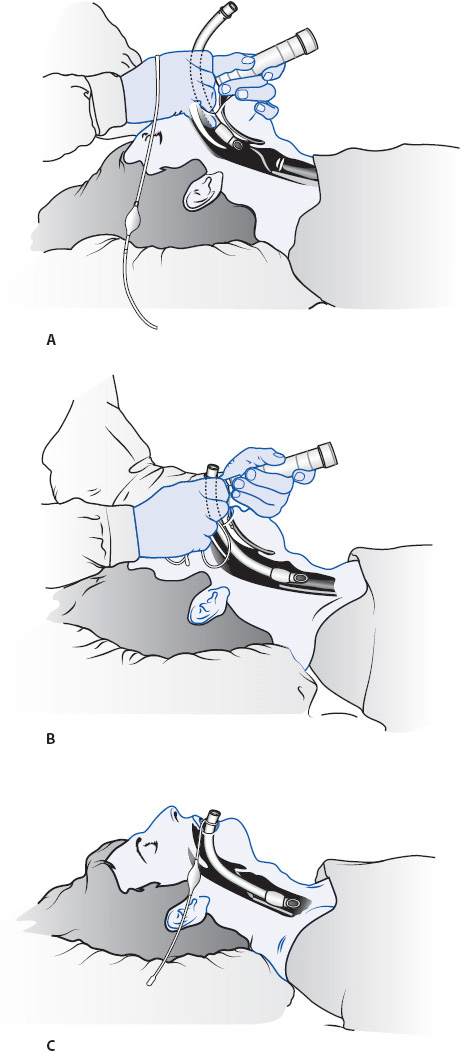
Figure 5-10. Oral intubation with an endotracheal (ET) tube. (A) Insertion of ET tube through the mouth with the aid of a laryngoscope. (B) ET tube advanced through the vocal cords into the trachea. (C) ET tube positioned with the cuff below the vocal cords. (Reprinted from Boggs Wooldridge-King M. AACN Procedure Manual for Progressive Care. 3rd ed. Philadelphia, PA: WB Saunders; 1993: 34-36 with permission from Elsevier.)
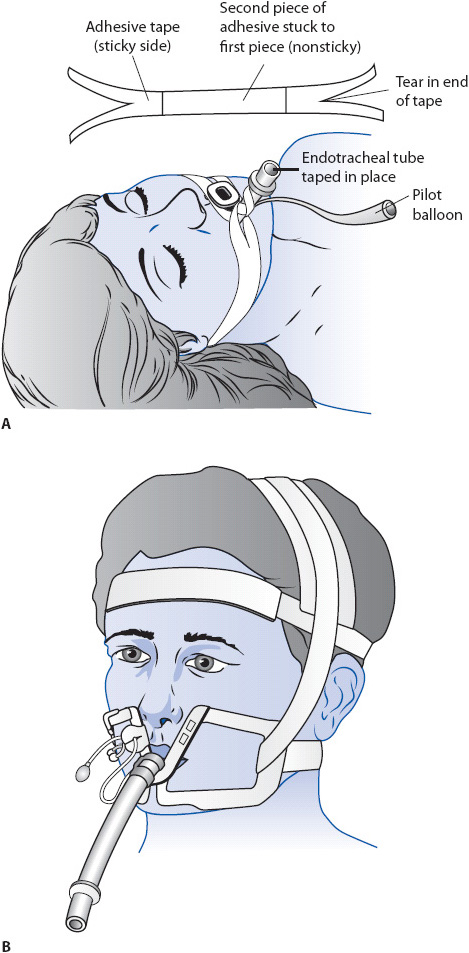
Figure 5-12. Methods for anchoring an endotracheal tube to prevent movement. (A) Taping of an oral ET tube. (Reprinted from Boggs R, Wooldridge-King M. AACN Procedure Manual for Critical Care. 3rd ed. Philadelphia, PA: WB Saunders; 993:108, with permission from Elsevier.) (B) Use of a special fixation device. (Reprinted from Kaplow R, Bookbinder M. A comparison of four endotracheal tube holders. Heart Lung. 1994;23(1):60, with permission from Elsevier.)
Endotracheal tube sizes are typically identified by the tubes’ internal diameter in millimeters (mm ID). The size of the tube is printed on the tube and generally also on the outside packaging. Knowledge of the tube ID is critical; the smaller the mm ID, the higher the resistance to breathing through the tube, thus increasing the work of breathing. The most common ET tube sizes used in adults are 7.0 to 9.0 mm ID.
Endotracheal tubes can in some situations be safely left in place for up to 2-3 weeks, but tracheostomy is often considered following 10 to 14 days of intubation or less. If the need for an artificial airway is anticipated for an extended period of time, a tracheostomy tube may be indicated earlier, but the decision is always individualized. Complications of ET intubation are numerous and include laryngeal and tracheal damage, laryngospasm, aspiration, infection, discomfort, sinusitis, and subglottic injury.
The majority of tracheostomy tubes used in acutely ill patients are made of medical-grade plastic or silicone and come in a variety of sizes (Figure 5-9B). Tracheostomy tubes may be cuffed or uncuffed. As with ET tubes, a standard 15-mm adapter at the proximal end ensures universal connection to MRBs and ventilator circuits. Tracheostomy tubes may be inserted as an elective procedure using a standard open surgical technique in the operating room or at the bedside via a percutaneous insertion. This technique involves a procedure in which a small incision is made in the neck and a series of dilators are manually passed into the trachea over a guide wire, creating a stoma opening through which the tracheostomy tube is inserted into place. Bedside placement obviates the need for patient transport out of the unit and the need for general anesthesia.
Tracheostomies are secured with cotton twill tape or latex-free Velcro latching tube holders attached to openings on the neck flange or plate of the tube. Many tracheostomy tubes have inner cannulae that can be easily removed for periodic cleaning (reusable) or replacement (disposable). Some tracheostomy tubes incorporate an additional opening along the outer tube cannula referred to as a fenestration. A fenestrated tracheostomy tube is sometimes used as an aid for facilitating vocalization by allowing airflow upward and through the vocal cords. A fenestration is not necessary to be able to talk with a tracheostomy tube.
Tracheostomy tubes, in general, are better tolerated by patients than oral or nasal ET tubes in terms of comfort. Further, there are more nutrition and communication options available to patients with tracheostomy tubes than with ET tubes.
Complications of tracheostomies include hemorrhage from erosion of the innominate artery; tracheal stenosis, malacia, or perforation; laryngeal nerve injury; aspiration; infection; air leak; and mechanical problems. Most complications rarely occur with proper management.
Following insertion of an endotracheal or tracheostomy tube, the cuff of the tube is inflated with just enough air to create an effective seal. The cuff is typically inflated with the lowest possible pressure that prevents air leak during mechanical ventilation and decreases the risk of pulmonary aspiration. Cuff pressure is maintained at less than 25 mm Hg (30 cm H2O). Excessive cuff pressure causes tracheal ischemia, necrosis, and erosion, as well as overinflation-related obstruction of the distal airway from cuff herniation. It is important to recognize that even a properly inflated cuffed artificial airway does not completely protect the patient from aspiration of liquids.
There are two common techniques to ensure proper cuff inflation without overinflation: the minimal leak and minimal occlusive volume techniques (MLT and MOV, respectively). The minimal leak technique involves listening over the larynx during positive pressure breaths with a stethoscope while inflating the tube cuff in 1- to 2-mL increments. Inflation continues until only a small air leak, or rush of air, is heard over the larynx during peak inspiration. The minimal leak technique should result in no more than a 50- to 100-mL air loss per breath during mechanical ventilation. The cuff pressure and amount of air instilled into the cuff are recorded following the maneuver.
The minimal occlusive volume cuff inflation technique is similar to the minimal leak technique. Cuff inflation continues, however, until the air leak completely disappears. The amount of air instilled and the cuff pressure are recorded during cuff inflation and periodically to ensure an intracuff pressure of less than 25 mm Hg (30 cm H2O). Manual palpation of the tube pilot balloon does not ensure optimal inflation assessment.
The connection of the ET tube pilot balloon to an intracuff measuring manometer device, such as a manual hand-held cuff inflator, allows for the simultaneous measurement of pressure during inflation or periodic checking (Figure 5-13). The need for excessive pressures to properly seal the trachea may indicate the ET tube diameter is too small for the trachea. In this case, the cuff is inflated to properly seal the trachea until the appropriately sized ET tube can be electively reinserted. At present, evidence of long-term outcomes is lacking to warrant mandatory cuff pressure monitoring. However, until a more definitive statement may be made, the clinician is encouraged to follow tube manufacturer and hospital policy. Current available evidence from clinical and laboratory testing suggests that intra-cuff pressure may be an important contributing factor to the development of complications related to cuffed endotracheal and tracheostomy tubes so attention to proper inflation is encouraged.

Figure 5-13. Portable endotracheal tube cuff inflator and manometer. (Courtesy of: Posey Company, Arcadia, CA.)
Pulmonary secretion removal is normally accomplished by coughing. An effective cough requires a closed epiglottis so that intrathoracic pressure can be increased prior to sudden opening of the epiglottis and secretion expulsion. The presence of an artificial airway such as an ET tube prevents glottic closure and effective coughing, necessitating the use of periodic endotracheal suctioning to remove secretions.
Currently, two methods are commonly used for ET tube suctioning: the closed and open methods. Closed suctioning means the ventilator circuit remains closed while suctioning is performed, whereas open suctioning means the ventilator circuit is opened, or removed, during suctioning. The open method requires disconnection of the ET tube from the mechanical ventilator or oxygen therapy source and insertion of a suction catheter each time the patient requires suctioning. The closed method refers to a closed suction catheter system device that remains attached to the ventilator circuit, allowing periodic insertion of the suction catheter through a diaphragm to suction without removing the patient from the ventilator. Following suctioning, the catheter is withdrawn into a plastic sleeve of the in-line device until the next suctioning procedure.
The need for ET suctioning is determined by a variety of clinical signs and symptoms, such as coughing, increased inspiratory pressures on the ventilator, and the presence of adventitious sounds (rhonchi, gurgling) during chest auscultation. Suctioning may also be performed periodically to ensure airway patency. Suctioning is only done when there is a clinical indication and never on a routine schedule.
Hyperoxygenation with 100% O2 for a minimum of 30 seconds is provided prior to each suctioning episode, whether using an open or closed technique (Table 5-5). Hyperoxygenation helps to prevent decreases in arterial oxygen levels after suctioning. Hyperoxygenation can be achieved by increasing the FiO2 setting on the mechanical ventilator or by using the “suction” button or temporary oxygen-enrichment program available on most microprocessor ventilators. Manual ventilation of the patient using an MRB is not recommended as the best choice and has been shown to be ineffective for providing delivered FiO2 of 1.0. If no other alternative is available to hyperoxygenate, then a MRB can be used. At least 30 seconds of manual breaths with 100% FiO2 are provided before and after each pass of the suction catheter. In spontaneously breathing patients, encourage several deep breaths of 100% O2 before and after each suction pass. The number of suction passes are limited to only those necessary to clear the airway of secretions—usually two or three. The mechanical act of inserting the suction catheter into the trachea can stimulate the vagus nerve and result in bradycardia or asystole. Each pass of the suction catheter should be 10 seconds or less.
TABLE 5-5. STEPS FOR SUCTIONING THROUGH AN ARTIFICIAL AIRWAY

The instillation of 5 to 10 mL of normal saline is no longer advocated during routine ET tube suctioning. This practice was previously thought to decrease secretion viscosity and increase secretion removal during ET tube suctioning. Bolus saline instillation has not been shown to be beneficial and is associated with SaO2 decreases and bronchospasm.
A variety of complications are associated with ET tube suctioning. Decreases in PaO2 have been well documented when no hyperoxygenation therapy is provided with suctioning. Serious cardiac arrhythmias occur occasionally with suctioning, and include bradycardia, asystole, ventricular tachycardia, and heart block. Less severe arrhythmias frequently occur with suctioning and include premature ventricular contractions, atrial contractions, and supraventricular tachycardia. Other complications associated with suctioning include increases in arterial pressure and intracranial pressure, bronchospasm, tracheal wall damage, and nosocomial pneumonia. Many of these complications can be minimized by using sterile technique, vigilant monitoring during and after suctioning, and hyperoxygenation before and after each suction pass.
The reversal or significant improvement of the underlying condition(s) that led to the use of artificial airways usually signals the readiness for removal of the airway. Common indicators of readiness for artificial airway removal include the ability to:
• Maintain spontaneous breathing and adequate ABG values with minimal to moderate amounts of O2 administration (FiO2 < 0.50).
• Protect the airway.
• Clear pulmonary secretions.
Removal of an artificial airway usually occurs following weaning from mechanical ventilatory support (see the discussion on weaning later). Preparations for extubation include an explanation to the patient and family of what to expect, the need to cough, medication for pain, setting up the appropriate method for delivering O2 therapy (eg, face mask, nasal cannula), and positioning the patient with the head of the bed elevated at 30° to 45° to improve diaphragmatic function. Suctioning of the artificial airway is performed prior to extubation if clinically indicated. Obtaining a baseline cardiopulmonary assessment also is important for later evaluation of the response to extubation. Extubation should be performed when full ancillary staff is available to assist if reintubation is required.
Hyperoxygenation with 100% O2 is provided for 30 to 60 seconds prior to extubation in case respiratory distress occurs immediately after extubation and reintubation is necessary. The artificial airway is then removed following complete deflation of the ET or tracheostomy cuff, if present. Immediately apply the oxygen delivery method and encourage the patient to take deep breaths.
Monitor the patient’s response to the extubation. Significant changes in heart rate, respiratory rate, and/or blood pressure of more than 10% of baseline values may indicate respiratory compromise, necessitating more extensive assessment and possible reintubation. Pulmonary auscultation is also performed.
Complications associated with extubation include aspiration, bronchospasm, and tracheal damage. Coughing and deep breathing are encouraged while monitoring vital signs and the upper airway for stridor. Inspiratory stridor occurs from glottic and subglottic edema and may develop immediately or take several hours. If the patient’s clinical status permits, treatment with 2.5% racemic epinephrine (0.5 mL in 3 mL of normal saline) is administered via an aerosol delivery device. If the upper airway obstruction persists or worsens, reintubation is generally required. A reattempt at extubation is usually delayed for 24 to 72 hours following reintubation for upper airway obstruction.
Oxygen is used for any number of clinical problems (Table 5-6). The overall goals for oxygen use include increasing alveolar O2 tension (PaO2) to treat hypoxemia, decreasing the work of breathing, and maximizing myocardial and tissue oxygen supply.
TABLE 5-6. COMMON INDICATIONS FOR OXYGEN THERAPY
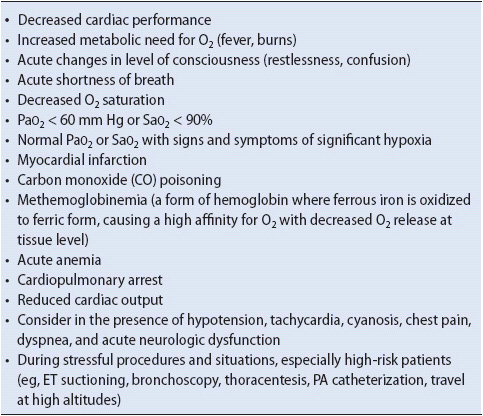
As with any drug, oxygen should be used cautiously. The hazards of oxygen misuse can be as dangerous as the lack of appropriate use. Alveolar hypoventilation, absorption atelectasis, and oxygen toxicity can be life threatening.
Alveolar hypoventilation is underventilation of alveoli, and is a side effect of great concern in patients with chronic obstructive pulmonary disease (COPD) with carbon dioxide retention. As the patient with COPD adjusts to chronically high levels of PaCO2, the chemoreceptors in the medulla of the brain lose responsiveness to high PaCO2 levels. Hypoxemia, then, becomes a primary stimulus for ventilation. However, correction of hypoxemia in the patient with COPD remains important with a target PaO2 of 55 to 60 mm Hg (SaO2 ≥ 90%), despite the presence of hypercapnia.
Absorption atelectasis results when high concentrations of O2 (> 90%) are given for long periods of time and nitrogen is washed out of the lungs. The nitrogen in inspired gas is approximately 79% of the total atmospheric gases. The large partial pressure of nitrogen in the alveoli helps to maintain open alveoli because it is not absorbed. Removal of nitrogen by inspiring 90% to 100% O2 results in alveolar closure because oxygen readily diffuses into the pulmonary capillary.
The toxic effects of oxygen are targeted primarily to the pulmonary and central nervous systems (CNS). CNS toxicity usually occurs with hyperbaric oxygen treatment. Signs and symptoms include nausea, anxiety, numbness, visual disturbances, muscular twitching, and grand mal seizures. The physiologic mechanism is not understood fully but is felt to be related to subtle neural and biochemical changes that alter the electric activity of the CNS.
Pulmonary oxygen toxicity is due to prolonged exposure to high FiO2 levels that may lead to ARDS or bronchopulmonary dysplasia. Two phases of lung injury result. The first phase occurs after 1 to 4 days of exposure to higher O2 levels and is manifested by decreased tracheal mucosal blood flow and tracheobronchitis. Vital capacity decreases because of poor lung expansion and progressive atelectasis persists. The alveolar capillary membrane becomes progressively impaired, decreasing gas exchange. The second phase occurs after 12 days of high exposure. The alveolar septa thickens and an ARDS picture develops, with associated high mortality.
Caring for the patient who requires high levels of oxygen requires astute monitoring by the progressive care nurse. Monitor those patients at risk for absorption atelectasis and oxygen toxicity. Signs and symptoms include nonproductive cough, substernal chest pain, general malaise, fatigue, nausea, and vomiting.
An oxygen concentration of 100% (FiO2 = 1.0) is regarded as safe for short periods of time (< 24 hours). Oxygen concentrations greater than 50% for more than 24 to 48 hours may damage the lungs and worsen respiratory problems. Oxygen delivery levels are decreased as soon as PaO2 levels return to clinically acceptable levels (> 60 mm Hg or higher).
Face masks and nasal cannulas are standard oxygen delivery devices for the spontaneously breathing patient (Figure 5-14). Oxygen can be delivered with a high- or low-flow device, with the concentration of O2 delivered ranging from 21% to approximately 100% (Table 5-7). An example of a high-flow device is the Venturi mask system that can deliver precise concentrations of oxygen (Figure 5-15). The usual FiO2 values delivered with this type of mask are 24%, 28%, 31%, 35%, 40%, and 50%. Often, Venturi masks are useful in patients with COPD and hypercapnia because the clinician can titrate the PaO2 to minimize carbon dioxide retention. An example of a low-flow system is the nasal cannula or prongs. Nasal prongs flow rate ranges are limited to 6 L/min. Flow rates less than 4 L/min need not be humidified. The main advantage of nasal prongs is that the patient can drink, eat, and speak during oxygen administration. The disadvantage is that the exact FiO2 delivered is unknown, because it is influenced by the patient’s peak inspiratory flow demand and breathing pattern. As a general guide, 1 L/min of O2 flow is an approximate equivalent to an FiO2 of 24%, and each additional liter of oxygen flow increases the FiO2 by approximately 4%. Simple oxygen face masks can provide an FiO2 of 34%-50% depending on fit at flow rates from 5-10 L/min. Flow rates should be maintained at 5 L/min or more in order to avoid rebreathing exhaled CO2 that can be retained in the mask. Limitations of using a simple face mask include difficulty in delivering accurate delivery of low concentrations of oxygen and long-term use can lead to skin irritation and potential pressure breakdown. Nonrebreathing masks can achieve higher oxygen concentrations (approximately 60%-80%) than partial rebreathing systems. A one-way valve placed between the mask and reservoir bag with a nonrebreathing system prevents exhaled gases from entering the bag, thus maximizing the delivered FiO2.
Figure 5-14. Noninvasive and invasive methods of O2 delivery. (A) Nasal prongs. (B) Nasal catheter. (C) Face mask. (D) Nonrebreathing mask. (Reprinted from: Kersten L. Comprehensive Respiratory Nursing. Philadelphia, PA: WB Saunders; 1989:608, 609; with permission from Elsevier.)
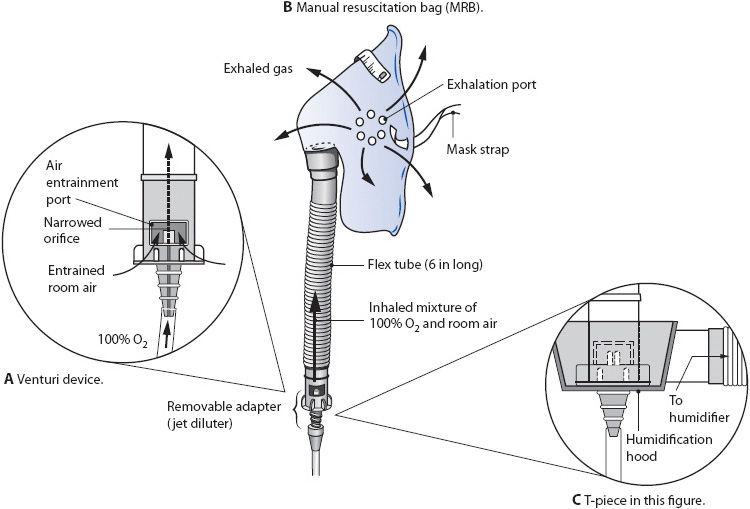
Figure 5-15. (A) Venturi device. (B) Manual resuscitation bag (MRB). (C) T-piece. (Reprinted from: Kersten L. Comprehensive Respiratory Nursing. Philadelphia, PA: WB Saunders; 1989:611, 629, with permission from Elsevier.)
A variation of the nonrebreathing mask without the one-way valves is called a partial rebreathing mask. Oxygen should always be supplied to maintain the reservoir bag at least one-third to one-half full on inspiration. At a flow of 6 to 10 L/min, the system can provide 40% to 70% oxygen. High-flow delivery devices such as aerosol masks or face tents, tracheostomy collars, and t-tube adapters can be used with supplemental oxygen systems. A continuous aerosol generator or large-volume reservoir humidifier can humidify the gas flow. Some aerosol generators cannot provide adequate flows at high oxygen concentrations.
Because conventional low-flow nasal cannulae and oxygen masks are constrained by flow, humidity, and accuracy of delivered inspired oxygen, the recent introduction of high-flow nasal oxygen devices capable of delivering well-humidified blended oxygen (using vapor) across a wide range of oxygen concentrations has been found to be useful in those patients who require a greater degree of support than what is possible by using traditional low flow oxygen devices. These devices provide oxygen at very high flow rates.
Manual resuscitation bags provide 40% to 100% O2 at adult Vt and respiratory rates to an ET tube or tracheostomy tube.
The most common method for delivering oxygen invasively is with a mechanical ventilator. Oxygen can be accurately delivered from 21% to 100% O2. Mechanical ventilation is discussed below in more detail.
Transtracheal oxygen therapy is a method of administering continuous oxygen to patients with chronic hypoxemia. The therapy requires the percutaneous placement of a small plastic catheter into the trachea. The catheter is inserted directly into the trachea above the suprasternal notch under local anesthesia in an outpatient setting. This device allows for low O2 flow rates (< 1-2 L/min) to treat chronic hypoxemia. Advantages of this method for chronic O2 delivery include improved mobility and patient aesthetics because the tubing and catheter, unlike the nasal cannula or face mask, can often be hidden from view, avoidance of nasal and ear irritation from nasal cannulas, decreased O2 requirements, and correction of refractory hypoxemia.
Typically, these patients are managed in the outpatient setting, but occasionally they may be in progressive care units. It is important to maintain the catheter unless specifically ordered to discontinue its use. The stoma formation process takes several weeks and if the catheter is removed, the stoma is likely to close. The catheter is cleaned daily to prevent the formation of mucous plugs. Refer to the manufacturer’s guidelines for further recommendations on care of the catheter while the patient is hospitalized.
Oxygen can also be provided directly to an ET or tracheostomy tube with a T-piece, or blow by, in spontaneously breathing patients who do not require ventilatory support. The T-piece is connected directly to the ET tube or tracheostomy tube 15 mm adapter, providing 21% to 80% O2.
Mechanical ventilation is indicated when noninvasive management modalities fail to adequately support oxygenation and/or ventilation. The decision to initiate mechanical ventilation is based on the ability of the patient to support their oxygenation and/or ventilation needs. The inability of the patient to maintain clinically acceptable CO2 levels and acid-base status is referred to as respiratory failure and is a common indicator for mechanical ventilation. Refractory hypoxemia—which is the inability to establish and maintain acceptable oxygenation levels despite the administration of oxygen-enriched breathing environments—is also a common reason for mechanical ventilation. Table 5-8 presents a variety of physiologic indicators for initiating mechanical ventilation. By monitoring these indicators, it is possible to differentiate stable or improving values from continuing decompensation. The need for mechanical ventilation may then be anticipated to avoid emergent use of ventilatory support.
TABLE 5-8. Indications for Mechanical Ventilation
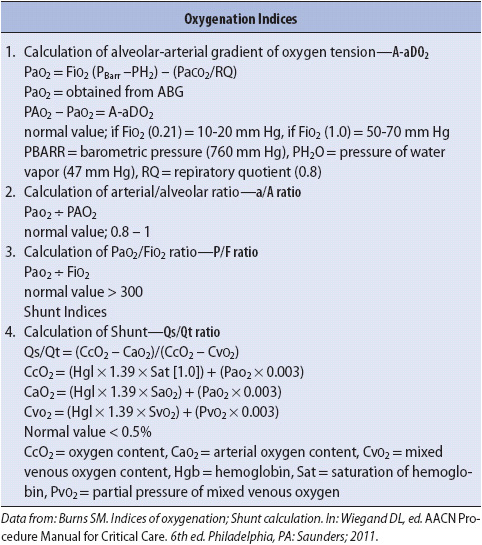
Depending on the underlying cause of the respiratory failure, different indicators may be assessed to determine the need for mechanical ventilation. Many of the causes of respiratory failure, however, are due to inadequate alveolar ventilation and/or hypoxemia, with abnormal ABG values and physical assessment as the primary indicators for ventilatory support.
Mechanical ventilators are designed to partially or completely support ventilation. Two different categories of ventilators are available to provide ventilatory support. Negative-pressure ventilators decrease intrathoracic pressure by applying negative pressure to the chest wall, typically with a shell placed around the chest (Figure 5-16A). The decrease in intrathoracic pressure causes atmospheric gas to be drawn into the lungs. Positive-pressure ventilators deliver pressurized gases into the lung during inspiration (Figure 5-16B). Positive-pressure ventilators can dramatically increase intrathoracic pressures during inspiration, potentially decreasing venous return and CO.
Figure 5-16. Principles of mechanical ventilation as provided by (A) negative-pressure and (B) positive-pressure ventilators.
Negative-pressure ventilators are rarely used to manage acute respiratory problems in progressive care. These devices are typically used for long-term noninvasive ventilatory support when respiratory muscle strength is inadequate to support unassisted, spontaneous breathing. Since the emergence of other, noninvasive modes of positive pressure (eg, BiPAP or bilevel), negative pressure ventilators are infrequently selected (described later in this chapter).
Positive-pressure ventilatory support can be accomplished invasively or noninvasively. Invasive mechanical ventilation is still widely used in most hospitals for supporting ventilation, although noninvasive technologies, which do not require the use of an artificial airway, are becoming more popular. To provide invasive positive-pressure ventilation, intubation of the trachea is required via an ET tube or tracheostomy tube. The ventilator is then connected to the artificial airway with a tubing circuit to maintain a closed delivery system (Figure 5-17). During the inspiratory cycle, gas from the ventilator is usually directed through a heated humidifier or a heat and moisture exchanger (HME) prior to entering the lungs through the ET tube or tracheostomy tube. Contraindications to HME use are listed in Table 5-9. At the completion of inspiration, gas is passively exhaled through the expiratory side of the tubing circuit.
Figure 5-17. Typical setup of a ventilator, closed system tubing circuit, and humidifier connected to an ET tube.
TABLE 5-9. Contraindications to Use of Heated Moisture Exchanger (HME)
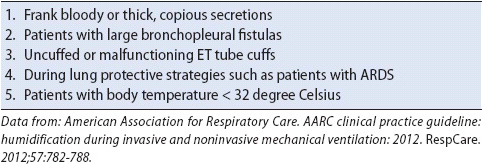
The humidifier located on the inspiratory side of the circuit is necessary to overcome two primary problems. First, the presence of an artificial airway allows gas entering the lungs to bypass the normal upper airway humidification process. Second, the higher flows and larger volumes typically administered during mechanical ventilation require additional humidification to avoid excessive intrapulmonary membrane drying.
Pressure within the ventilator tubing circuit is continuously monitored to alert clinicians to excessively high or low airway pressures. Airway pressure is dynamically displayed on the front of the ventilator control panel.
Traditionally, ventilator circuits have incorporated special water collection cups in the tubing to prevent the condensation from humidified gas from obstructing the tubing. Recently, however, it has become common to use ventilator circuits containing heated wires that run through the inspiratory and expiratory limbs of the circuit. These wires maintain the temperature of the gas at or close to body temperature, significantly reducing the condensation and rainout of humidity in the gas, eliminating the need for in-line water traps. Certain medications, such as bronchodilators or steroids, can also be administered via metered dose inhaler (MDI) or nebulized into the lungs through a low volume aerosol-generating device located in the inspiratory side of the circuit.
The ventilator tubing circuit is maintained as a closed circuit as much as possible to avoid interrupting ventilation and oxygenation to the patient, as well as to decrease the potential for VAP. Avoiding frequent or routine changes of the ventilator circuit also decreases the risk of VAP (see Chapter 10, Respiratory System).
The user interface or control panel of the ventilator usually incorporates three basic sections or areas: (1) control settings for the type and amount of ventilation and oxygen delivery, (2) alarm settings to specify desired high and low limits for key ventilatory measurements, and (3) visual displays of monitored parameters (Figure 5-18). The number and configuration of these controls and displays vary from ventilator model to model, but their function and principles remain essentially the same.

Figure 5-18. Ventilator display control panel. (With permission, Covidien.)
The control settings area of the user interface allows the clinician to set the mode of ventilation, volume, pressure, respiratory rate, FiO2, PEEP level, inspiratory trigger sensitivity or effort, and a variety of other breath delivery options (eg, inspiratory flow rate, inspiratory waveform pattern).
Alarms, which continuously monitor ventilator function, are essential to ensure safe and effective mechanical ventilation. Both high and low alarms are typically set to identify when critical parameters vary from the desired levels. Common alarms include exhaled Vt, exhaled minute volume, FiO2 delivery, respiratory rate, and airway pressures (Table 5-10).
Airway pressures, respiratory rate, exhaled volumes, and the inspiratory to expiratory (I:E) ratio are among the most common visually displayed breath-to-breath values on the ventilator. Airway pressures are monitored during inspiration and exhalation and are often displayed as peak pressure, mean pressure, and end-expiratory pressure. A breath delivered by the ventilator produces higher airway pressures than an unassisted, spontaneous breath by the patient (Figure 5-19). The presence of PEEP is identified by a positive value at the end of expiration rather than 0 cm H2O. Careful observation of the airway pressures provides the clinician with a great deal of information about the patient’s respiratory effort, coordination with the ventilator, and changes in lung compliance.
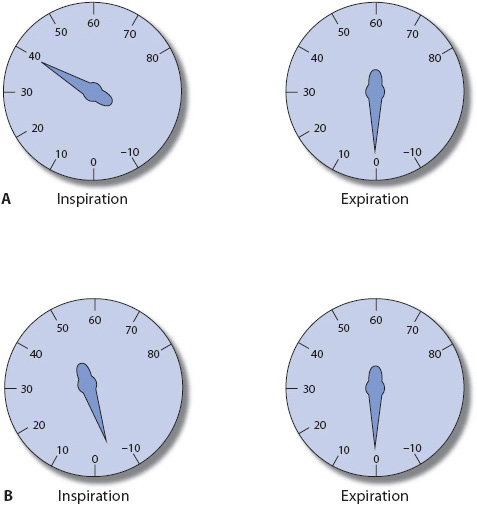
Figure 5-19. Typical airway pressure gauge changes during (A) ventilator-assisted breath and (B) spontaneous breath (cm H2O).
The display of the patient’s exhaled Vt reflects the amount of gas that is returned to the ventilator via the expiratory tubing with each respiratory cycle. Exhaled volumes are measured and displayed with each breath. The patient’s total exhaled minute volume is also often displayed. Exhaled Vts for ventilator-assisted mandatory breaths should be similar (± 10%) to the desired Vt setting selected on the control panel. The Vt of spontaneous breaths, or partially ventilator-supported breaths, however, may be different from the Vt control setting.
The mode of ventilation refers to one of several different methods that a ventilator uses to support ventilation. Modes are often classified as invasive (via an ET tube or tracheostomy tube) or noninvasive (via a face or nasal interface). These modes generate different levels of airway pressures, volumes, and patterns of respiration and, therefore, different levels of support. The greater the level of ventilator support, the less muscle work performed by the patient. This “work of breathing” varies considerably with each of the modes of ventilation and is discussed later in this chapter in the section on respiratory muscle fatigue.
The different modes of ventilation used to support ventilation depend on the underlying respiratory problem and clinical preferences. A brief description of the basic invasive and noninvasive modes of mechanical ventilation follows.
The control mode of ventilation ensures that patients receive a predetermined number and volume of breaths each minute. No deviations from the respiratory rate or Vt settings are delivered with this mode of ventilation. Generally the patient is heavily sedated and/or paralyzed with neuromuscular blocking agents in the ICU to achieve the goal; however, in the progressive care setting the use of these agents is unlikely. Instead the “control” mode might be used in the patient who is paralyzed from a spinal cord injury or has a neuromuscular condition that precludes spontaneous breathing, or in the operating room.
The assist-control mode of ventilation ensures that a predetermined number and volume of breaths is delivered by the ventilator each minute should the patient not initiate respirations at that rate or above. If the patient attempts to initiate breaths at a rate greater than the set minimum value, the ventilator delivers the spontaneously initiated breaths at the prescribed Vt; the patient may determine the total rate. Work of breathing with this mode is variable.
Assist-control ventilation is often used when the patient is initially intubated (because minute ventilation requirements can be determined by the patient), for short-term ventilatory support such as postanesthesia, and as a support mode when high levels of ventilatory support are required. Excessive ventilation can occur with this mode in situations where the patient’s spontaneous respiratory rate increases for nonrespiratory reasons (eg, pain, CNS dysfunction). The increased minute volume may result in potentially dangerous respiratory alkalosis. Changing to a different mode of ventilation or employing sedation may be necessary in these situations.
The synchronized intermittent mandatory ventilation (SIMV) mode of ventilation ensures (or mandates) that a predetermined number of breaths at a selected Vt are delivered each minute. Any additional breaths initiated by the patient are allowed but, in contrast to the assist-control mode, these breaths are not delivered by the ventilator. The patient is allowed to spontaneously breathe at the depth and rate desired until it is time for the next ventilator-assisted, or mandatory, breath. Mandatory breaths are synchronized with the patient’s inspiratory effort, if present, to optimize patient-ventilator synchrony. The spontaneous breaths taken during SIMV are at the same FiO2 as the mandatory breaths.
Originally designated as a ventilator mode for the gradual weaning of patients from mechanical ventilation, the use of a high-rate setting of SIMV can provide total ventilatory support. Reduction of the number of mandatory breaths allows the patient to slowly resume greater and greater responsibility for spontaneous breathing. SIMV can be used for similar indications as the assist-control mode, as well as for weaning the patient from mechanical ventilatory support. It is common to add pressure support to SIMV as a means of decreasing the work of breathing associated with spontaneous breathing.
The work of breathing with this mode of ventilation depends on the Vt and rate of the spontaneous breaths. When the mandatory, intermittent breaths provide the majority of minute volume, the work of breathing by the patient may be less than when spontaneous breathing constitutes a larger proportion of the patient’s total minute volume.
Although strong clinician and institutional biases exist regarding whether to use SIMV or other modes for ventilatory support, little data exist to clarify which mode of ventilation is best. Close observation of the physiologic and psychological response to the ventilatory mode is required, and consideration is given to trials on alternative modes if warranted.
Many ventilators have a mode that allows the patient to breathe spontaneously without ventilator. This is similar to placing the patient on a T-piece or blow-by oxygen setup, except it does have the benefit of providing continuous monitoring of exhaled volumes, airway pressures, and other parameters along with a closed circuit. All the work of breathing is performed by the patient during spontaneous breathing. Use of the ventilator rather than the T-piece during spontaneous breathing actually may slightly increase the work of breathing. This occurs because of the additional inspiratory muscle work that is required to trigger flow delivery for each spontaneous breath. The amount of additional work required varies with different ventilator models.
This mode of ventilation is often identified as continuous positive airway pressure (CPAP), flow-by, or spontaneous (SPONT) on the ventilator. Continuous positive airway pressure (CPAP) is a spontaneous breathing setting with the addition of PEEP during the breathing cycle.
Some ventilators have an additional adjunct that compensates for the resistance secondary to ET tube diameter. It is also called automatic tube compensation (ATC). ATC can be used with ventilatory support or alone with spontaneous breathing.
Pressure support (PS) is a spontaneous breathing mode, available in SIMV and SPONT modes, which maintain a set positive pressure during the spontaneous inspiration. The volume of a gas delivered by the ventilator during each inspiration varies depending on the level of pressure support and the demand of the patient. The higher the pressure support level, the higher the amount of gas delivered with each breath. Higher levels of pressure support can augment the spontaneous Vt and decrease the work of breathing associated with spontaneous breathing. At low levels of support, it is primarily used to overcome the airway resistance caused by breathing through the artificial airway and the breathing circuit. The airway pressure achieved during a pressure support breath is the result of the pressure support setting plus the set PEEP level.
Positive end-expiratory pressure is used in conjunction with any of the ventilator modes to help stabilize alveolar lung volume and improve oxygenation. The application of positive pressure to the airways during expiration may keep alveoli open and prevent early closure during exhalation. Lung compliance and ventilation-perfusion matching are often improved by prevention of early alveolar closure. If alveolar recruitment is not needed and excessive PEEP/CPAP is applied, it may result in adverse hemodynamic (ie, hypotension) or respiratory compromise (ie, auto-PEEP) and lung trauma (ie, barotrauma).
Positive end-expiratory pressure/CPAP is indicated for hypoxemia, which is secondary to diffuse lung injury (eg, ARDS, interstitial pneumonitis). PEEP/CPAP levels of 5 cm Hg or less are often used to provide “physiologic PEEP.” The presence of the artificial airway allows intrathoracic pressure to fall to zero, which is below the usual level of intrathoracic pressure at end expiration (2 or 3 cm H2O).
Use of PEEP may increase the risk of barotrauma due to higher mean and peak airway pressures during ventilation, especially when peak pressures are greater than 40 cm H2O. Venous return and CO may also be affected by these high pressures. If CO decreases with PEEP/CPAP initiation and oxygenation is improved, a fluid bolus may correct hypovolemia. Other complications from PEEP/CPAP increases in intracranial pressure, decreased renal perfusion, hepatic congestion, and worsening of intracardiac shunts.
Bilevel positive airway pressure (ie, BiPAP) is a noninvasive mode of ventilation that combines two levels of positive pressure (PSV and PEEP) by means of a full face mask, nasal mask (most common), or nasal pillows. The ventilator is designed to compensate for leaks in the set-up, and a snug fit is needed, often requiring head or chin straps. This form of therapy can be very labor intensive, requiring frequent assessment of patient tolerance. Full face mask ventilation is cautiously used because the potential for aspiration is high. If full face mask ventilation is chosen, the patient should be able to remove the mask quickly if nausea occurs or vomiting is imminent. Obtunded patients and those with excessive secretions are not good choices for BiPAP ventilation.
A number of options are available with BiPAP and include a spontaneous mode where the patient initiates all the pressure-supported breaths; a spontaneous-timed option, similar to PSV with a backup rate (some vendors call this A/C); and a control mode. The control mode requires the selection of a control rate and inspiratory time.
Bilevel positive airway pressure is used successfully in a wide variety of progressive care patients such as those with sleep apnea, some patients with chronic hypoventilation syndromes, and also to prevent intubation and reintubation following extubation. Use of BiPAP in patients with COPD and heart failure has been associated with decreased mortality and need for intubation. These patients are often difficult to wean from conventional ventilation given their underlying disease processes. Study results also demonstrate that outcomes in immunocompromised patients may also be better with noninvasive ventilation.
Significant complications can arise from the use of any form of mechanical ventilation and can be categorized as those associated with the patient’s response to mechanical ventilation or those arising from ventilator malfunctions. Although the approach to minimizing or treating the complications of mechanical ventilation relate to the underlying cause, it is critical that frequent assessment of the patient, ventilator equipment, and the patient’s response to ventilatory management be accomplished. Many clinicians participate in activities to assess the patient and ventilator, but the ultimate responsibility for ensuring continuous ventilatory support of the patient falls to the progressive care team including the bedside nurse and the respiratory therapist. Critically evaluating clinical indicators such as pH, PaCO2, PaO2, SpO2, heart rate, BP, and so on, in conjunction with patient status and ventilatory parameters, is essential to decrease complications associated with this highly complex technology.
Normal intrathoracic pressure changes during spontaneous breathing are negative throughout the ventilatory cycle. Intrapleural pressure varies from about +5 cm H2O during exhalation to –8 cm H2O during inhalation. This decrease in intrapleural pressure during inhalation facilitates lung inflation and venous return. Thoracic pressure fluctuation during positive-pressure ventilation is opposite to those that occur during spontaneous breathing. The mean intrathoracic pressure is usually positive and increases during inhalation and decreases during exhalation. The use of positive pressure ventilation increases peak airway pressures during inspiration which in turn, increases mean airway pressures. It is this increase in mean airway pressure which may impede venous return to the right atrium, thus decreasing CO. In some patients, this decrease in CO can be clinically significant, leading to increased heart rate and decreased blood pressure and perfusion to vital organs.
Whenever mechanical ventilation is instituted, or when ventilator changes are made, it is important to assess the patient’s cardiovascular response. Approaches to managing hemodynamic compromise include increasing the preload of the heart (eg, fluid administration), decreasing the airway pressures exerted during mechanical ventilation by ensuring appropriate airway management techniques (suctioning, positioning, etc), and by judiciously setting ventilator parameters.
Barotrauma describes damage to the pulmonary system due to alveolar rupture from excessive airway pressures or overdistention of alveoli. Alveolar gas enters the interstitial pulmonary structures causing pneumothorax, pneumomediastinum, pneumoperitoneum, or subcutaneous emphysema. The potential for pneumothorax and cardiovascular collapse requires prompt management of pneumothorax and should be considered whenever airway pressure rises acutely, breath sounds are diminished unilaterally, or blood pressure falls abruptly.
Patients with obstructive airway diseases (eg, asthma, bronchospasm), unevenly distributed lung disease (eg, lobar pneumonia), or hyperinflated lungs (eg, emphysema) are at high risk for barotrauma. Techniques to decrease the incidence of barotrauma include the use of small Vts, cautious use of PEEP, and the avoidance of high airway pressures and development of auto-PEEP in high-risk patients.
Volutrauma describes alveolar damage that results from high pressures resulting from large-volume ventilation in patients with ARDS. A common technique to reduce this risk is the use of smaller Vts (4-6 ml/kg of ideal body weight) and sometimes this is described as the “low stretch” protocol. Different from barotrauma, this damage results in alveolar fractures and flooding of alveoli (non-ARDS, ARDS). It is linked to the use of Vts greater than 6 mL/kg in patients with ARDS.
Auto-PEEP occurs when a delivered breath is incompletely exhaled before the onset of the next inspiration. This gas trapping increases overall lung volumes, inadvertently raising the end-expiratory pressure in the alveoli. The presence of auto-PEEP increases the risk for complications from PEEP. Ventilator patients with COPD (eg, asthma, emphysema) or high respiratory rates are at increased risk for the development of auto-PEEP.
Auto-PEEP, also termed intrinsic PEEP, is difficult to diagnose because it cannot be observed on the airway pressure display at end expiration. The technique for assessment for auto-PEEP varies with different ventilatory models and modes, but typically involves measuring the airway pressure close to the artificial airway during occlusion of the expiratory ventilator circuit during end expiration. This method requires that the patient be completely passive and not trigger a breath; it is not possible to measure auto-PEEP in actively breathing patients. Another technique of monitoring auto-PEEP in actively breathing patients is the use of the flow-time curve displayed by the ventilator. If flow does not return to baseline at the end of exhalation before the next breath starts, the patient has auto-PEEP. Auto-PEEP can be minimized by:
• Maximizing the length of time for expiration (eg, increasing inspiratory flow rates)
• Decreasing obstructions to expiratory flow (eg, using larger diameter ET tubes, eliminating bronchospasm and secretions)
• Avoiding overventilation
Ventilator-associated pneumonia (VAP) is a hospital-acquired complication, and is associated with increased patient morbidity and mortality. Prevention is aimed at avoiding colonization and subsequent aspiration of bacteria into the lower airway. Elevation of the head of the bed (> 30°) and avoiding excessive gastric distention are thought to help minimize the occurrence of aspiration. A specially designed ET tube (Figure 5-20) incorporates a dedicated suction lumen over the ET cuff, which permits continuous or intermittent suctioning of subglottic secretions pooled above the cuff. Removal of the accumulated secretions may be particularly helpful before cuff deflation or manipulation. Studies have demonstrated that the application of continuous aspiration of subglottic secretions with ET tubes only may prevent or delay the onset of VAP. Although subglottic suctioning is now available in tracheostomy tubes, there are currently no recommendations for the use of subglottic suctioning in these tubes. In addition, recent research suggests that oral care protocols including chlorhexidine gluconate 0.12% mouth rinse and tooth brushing to removal plague may be important adjuncts in VAP prevention.
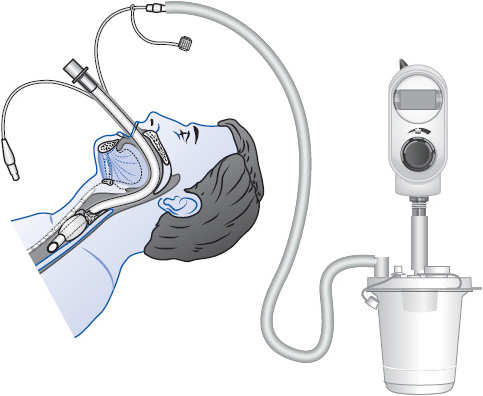
Figure 5-20. Cuffed endotracheal tube with dedicated lumen for continuous aspiration of subglottic secretions accumulated immediately above cuff. The dedicated lumen connector is attached to wall suction. (With permission, Covidien.)
Hyponatremia is a common occurrence following the institution of mechanical ventilation and develops from several factors, including applied PEEP, humidification of inspired gases, hypotonic fluid administration and diuretics, and increased levels of circulating antidiuretic hormone.
Upper gastrointestinal (GI) bleeding may develop secondary to ulceration or gastritis. The prevention of stress ulcer bleeding requires ensuring hemodynamic stability and the administration of proton-pump inhibitors, H2 receptor antagonists, antacids, or cytoprotective agents as appropriate (see Chapter 7, Pharmacology and Chapter 14, Gastrointestinal System, for discussions of GI prophylaxis).
Problems related to the proper functioning of mechanical ventilators, although rare, may have devastating consequences for patients. Many of the alarm systems on ventilators are designed to alert clinicians to improperly functioning ventilatory systems. These alarm systems must be activated at all times if ventilator malfunction problems are to be quickly identified and corrected, and untoward patient events avoided (see Table 5-10).
Many of the “problems” identified with ventilatory equipment are actually related to inappropriate setup or use of the devices. Ventilator circuits that are not properly connected, alarm systems that are set improperly, or inadequate ventilator settings for a particular clinical condition are examples of some of these operator-related occurrences.
There are occasions, however, when ventilator systems do not operate properly. Examples of ventilator malfunctions include valve mechanisms sticking and obstructing gas flow, inadequate or excessive gas delivery, electronic circuit failures in microprocessing-based ventilators, failures with complete shutdown, and power failures or surges in the institution.
The most important approach to ventilator malfunction is to maintain a high level of vigilance to determine if ventilators are performing properly. Ensuring that alarm systems are set appropriately at all times, providing frequent routine assessment of ventilator functioning, and the use of experienced support personnel to maintain the ventilator systems are some of the most crucial activities necessary to avoid patient problems. In addition, whenever ventilator malfunction is suspected, the patient should be immediately removed from the device and temporary ventilation and oxygenation provided with an MRB or another ventilator until the question of proper functioning is resolved. Any sudden change in the patient’s respiratory or cardiovascular status alerts the clinician to consider potential ventilator malfunction as a cause.
The process of transitioning the ventilator-dependent patient to unassisted spontaneous breathing is called weaning from mechanical ventilation. This is a period of time where the requirement for oxygenation and ventilation is decreased, either gradually or abruptly, while monitoring the patient’s response to the resumption of spontaneous breathing. A standardized approach along with weaning readiness criteria has been shown to reduce ventilator days and improve outcomes. Weaning or “liberation” is considered to be complete, or successful, when the patient has been extubated successfully without the need for reintubation within 48 hours. Often, removal of the artificial airway occurs before that time if clinicians are optimistic that the patient’s respiratory status will not deteriorate. The majority of patients intubated and ventilated for short periods of time (< 72 hours) are successfully weaned with the first spontaneous breathing trial (SBT) in the ICU. Additionally, there is a subset of patients with long-term tracheostomy tubes who may require short-term ventilation who are able to wean quickly once the clinical issue requiring mechanical ventilation is resolved. Approximately 30% of patients, however, require extended time periods to successfully complete the weaning process, with some being unable to breathe without partial or complete mechanical ventilation.
Weaning proceeds when the underlying pulmonary disorder that led to mechanical ventilation has sufficiently resolved, and the patient is alert and able to protect the airway. Unnecessary delays in weaning from mechanical ventilation increase the likelihood of complications such as ventilator-induced lung injury, pneumonia, discomfort, and increases in hospitalization costs. Thus, aggressive and timely weaning trials such as SBT are encouraged.
Readiness to wean from short-term mechanical ventilation (STMV) may be assessed with a wide variety of criteria. However, in most institutions, assessment of readiness to wean includes just three or four criteria for most short-term ventilator patients. Along with assessing the patient’s clinical stability, some examples are:
• ABGs within normal limits on minimal to moderate amounts of ventilatory support (FiO2 ≤ 0.50, minute ventilation # 10 L/min, PEEP ≤ 5 cm H2O)
• Negative inspiratory pressure that is more negative than –20 cm H2O
• Spontaneous Vt ≥ mL/kg
• Vital capacity ≥10 mL/kg
• Respiratory rate < 30 breaths/min
• Spontaneous rapid-shallow breathing index < 105 breaths/min/liter
Following selection of the method for weaning (see the discussion below), the actual weaning trial can begin. It is important to prepare both the patient and the progressive care environment properly to maximize the chances for weaning success. Interventions include appropriate explanations of the process to the patient, positioning and medication to improve ventilatory efforts, and the avoidance of unnecessary activities during the weaning trial. Throughout the weaning time, continuous monitoring for signs and symptoms of respiratory distress or fatigue is essential. They include dyspnea, tachypnea, chest abdominal dyssynchrony, anxiety, tachycardia, changes in blood pressure, and changes in oxygenation or ventilation. Many of these indicators are subtle, but careful monitoring of baseline levels before weaning progresses and throughout the trial provides objective indicators of the need to return the patient to previous levels of ventilator support.
The need to temporarily stop the weaning trial is not viewed as, or termed, a failure. Instead it simply suggests that more time needs to be provided to ensure success. A full evaluation of the multiple reasons for inability to wean is necessary, however.
Generally, weaning trials for patients’ ventilated short term in the ICU are accomplished with SBT on T-piece or on the ventilator using CPAP or a low level of pressure support. Readiness for weaning is assessed daily using a “safety screen” which includes such factors as hemodynamic stability, oxygenation status, and improvement in the condition that necessitated the use of mechanical ventilation. Once the patient is assessed as “ready” the spontaneous breathing trial is initiated for a duration of at least 30 minutes but no more than 120 minutes. The trial is stopped should the patient show signs of distress and/or deterioration. A decision to extubate is made with the conclusion of a successful trial. The need for reintubation is associated with increased mortality. Thus, premature attempts at extubation are to be avoided. Some suggest that noninvasive positive-pressure ventilation via face or nasal mask may be useful for patients with respiratory failure following extubation. However, research has demonstrated that this therapy does not prevent the need for reintubation or reduce mortality in these cases.
A variety of methods are available for weaning patients from mechanical ventilation. To date, research on these techniques has not clearly identified any one method as optimal for weaning from short-term mechanical ventilation. Most institutions, however, use one or two approaches routinely. A number of recently published randomized controlled trials demonstrated that the outcomes of patients managed with protocols driven by nonphysician clinicians were better than those managed with standard physician-directed care. Most experts on weaning believe that, with short-term ventilator-dependent patients, the actual method used to wean the patient is less important to weaning success than using a consistently applied protocol strategy.
• T-piece, blow-by, or trach collar: The T-piece method of weaning involves removing the patient from the mechanical ventilator and attaching an oxygen source to the artificial airway with a “T” piece for a SBT. A trach collar also provides oxygen but attaches by elastic strap around the neck instead of directly to the artificial airway. No ventilatory support occurs with this device, with the patient completely breathing spontaneously the entire time this device is connected. The advantage of this method of weaning is that the resistance to breathing is low, because no special valves need to be opened to initiate gas flow. Rapid assessment of the patient’s ability to spontaneously breathe is another purported advantage. Limitations of this SBT are that it may cause ventilatory muscle overload and fatigue. When this occurs, it usually appears early in the SBT, so the patient must be closely monitored during the initial few minutes. A PEEP valve can be added to the T piece; however, similar to trach collar weaning, there are no alarm or backup systems to support the patient should ventilation be inadequate. It is critical to recognize that this technique relies on the clinician to monitor for signs and symptoms of respiratory difficulty and fatigue. Frequently, the FiO2 is increased by at least 10% over the FiO2 setting on the ventilator to prevent hypoxemia resulting from the lower Vt of spontaneous breaths. Patients who fail a SBT should receive a stable, nonfatiguing, comfortable form of ventilatory support for rest following the trial.
• CPAP: The use of the ventilator to allow spontaneous breathing periods without mandated breaths, similar to the T-piece, can be done with the CPAP mode. With this approach, ventilator alarm systems can be used to monitor spontaneous breathing rates and volumes, and a small amount of continuous pressure (5 cm H2O) can be applied if needed. The disadvantage of this approach is that the work of breathing resulting from the need to open the demand valve to receive gas flow for the breath is higher than with the T-piece. For most patients, this slight additional work of breathing is not likely to be a critical factor to their weaning success or failure unless the trial is unduly long. If needed, a low level of pressure support (eg, 5-7 cm H2O) may also be added to offset this workload (CPAP + PS).
• Pressure support: Another method for weaning from ventilation is the use of low level PS ventilation. With this method, patients can spontaneously breathe on the ventilator with a small amount of ventilator support to augment their spontaneous breaths. This technique overcomes some of the resistance to breathing associated with ET tubes and demand valves. The main disadvantage with this approach is that clinicians may underestimate the degree of support to spontaneous breathing provided with this method and prematurely stop the weaning process.
• SIMV: One of the most popular methods of weaning patients in the past, this modality has recently been shown to prolong the duration of mechanical ventilation in comparison to weaning with SBT or pressure support using the SIMV mode. By progressively decreasing the number of mandated breaths delivered by the ventilator, the patient performs more and more of the work of breathing by increasing spontaneous breathing. Advantages to the SIMV mode are the presence of built-in alarms to alert clinicians when ventilation problems occur and, in some modes, the guarantee of a minimum amount of minute ventilation. The disadvantage of SIMV is that each spontaneous breath requires some additional work of breathing to open a valve, which allows gas flow to the patient for the spontaneous breath. SIMV is used either alone or in conjunction with pressure support (SIMV + PS).
In contrast to patients who require short-term (< 3 days) ventilation, those that require long-term mechanical ventilation (defined as > 3 days), may take weeks or even months to liberate from the ventilator. In these long-term mechanically ventilated (LTMV) patients, the weaning process varies and consists of four stages. The first stage is marked by instability and high ventilatory support requirements. During the second stage, called the prewean stage, many physiologic factors continue to require attention, and the patient’s overall status may fluctuate. Ventilatory requirements are less and adjustments are made to maintain oxygenation and acid-base status as well as provide ventilatory muscle conditioning. The third, or weaning stage, is evident when the patient is stable, and rapid progress with weaning trials is possible. Finally, the last stage is called the outcome stage, which consists of extubation, partial or full ventilatory support.
Long-term mechanical ventilation is associated with high morbidity and mortality rates, and institutions lose money on patients ventilated long-term because reimbursement rarely covers the associated costs. As a result, clinicians, scientists, and institutions are interested in testing methods of care delivery that improve the clinical and financial outcomes of the patients. Research in the area of weaning offers guidance to clinicians working with these patients. The following discussions of weaning patients from LTMV address wean assessment, wean planning, and weaning modes and methods, including comprehensive institutional approaches.
Traditionally, the decision about when to begin the weaning process is determined once the condition that necessitates mechanical ventilation is improved or resolved. During this prewean stage, other factors that contribute to wean ability are considered prior to attempting weaning trials. In the past, “traditional” weaning predictors were used in an attempt to determine the optimal timing for extubation. More recently, investigators combined pulmonary elements to improve predictive ability in LTMV patients. An example is the index of rapid shallow breathing, also known as the frequency (fX)/tidal volume (Vt) index, which integrates rate and tidal volume. Unfortunately, predictors have not predicted wean-ability. This is in part because they focus exclusively on pulmonary specific components to the exclusion of other important nonpulmonary factors (Table 5-11). Although the standard weaning criteria are not predictive, the components are helpful for assessing the patient’s overall condition and readiness for weaning.
TABLE 5-11. PULMONARY SPECIFIC WEAN CRITERIA THRESHOLDS
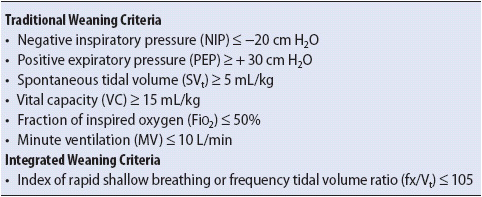
As noted, assessment of weaning potential starts with an evaluation of the underlying reason for mechanical ventilation (sepsis, pneumonia, trauma, and the like). Resolution of the underlying cause is necessary before gains in the weaning process can be expected. However, it is important to remember that resolution alone is frequently not sufficient to ensure successful weaning. Patients who require prolonged mechanical ventilation, sometimes referred to as the “chronically, critically ill,” often suffer from a myriad of conditions that impede weaning. Even with resolution of the disease or condition that necessitated mechanical ventilation, the patient’s overall status is often below baseline (weak, malnourished, etc). Therefore, a systematic, comprehensive approach to weaning assessment is important. One example of a tool that encourages such an approach is the Burns Wean Assessment Program (BWAP) (Table 5-12). The BWAP score is used to track the progress of the patient and keep care planning on target. Factors important to weaning are listed in the BWAP bedside checklist.
Once impediments to weaning are identified, plans that focus on improving the impediments are made in collaboration with a multidisciplinary team. A collaborative approach to assessment and planning greatly enhances positive outcomes in the LTMV patient. However, for care planning to be successful, it must also be systematic. The wean process is dynamic and regular reassessment and adjustment of plans are necessary. Tools like the BWAP can be used to systematically assess and track weaning progress. Other methods that have demonstrated efficacy in assuring consistency in care management and good outcomes for the patients include care delivery models using clinical pathways, protocols for weaning, and institution-wide approaches to managing and monitoring the patients.
A wide variety of weaning modes and methods are available for weaning the patient ventilated short term as described earlier. To date, no data support the superiority of any one mode for weaning those requiring LTMV, however, methods using protocols and other systematic, multidisciplinary approaches do appear to make a difference and are to be encouraged. These methods are described following a discussion of respiratory muscle fatigue, rest, and conditioning because the concepts are integrated into the section on protocols.
Respiratory muscle fatigue is common in ventilated weaning patients and occurs when the respiratory workload is excessive. When the workload exceeds metabolic stores, fatigue and hypercarbic respiratory failure ensue. Examples of those at risk include patients who are hypermetabolic, weak, or malnourished. Signs of encroaching fatigue include dyspnea, tachypnea, chest-abdominal asynchrony, and elevated PaCO2 (a late sign). These signs and symptoms indicate a need for increased ventilatory support. Once fatigued, the muscles require 12 to 24 hours of rest to recover, and careful application of selected modes of ventilation is required.
For the respiratory muscles to recover from fatigue, the inspiratory workload must be decreased. In the case of volume ventilation (eg, assist-control, intermittent mandatory ventilation), this means complete cessation of spontaneous effort, but in the case of pressure ventilation, a high level of PSV may accomplish the necessary “unloading.” Generally, this means increasing the PSV level to attain a spontaneous respiratory rate of 20 breaths per minute or less and the absence of accessory muscle activity. However, in patients with obstructive diseases (eg, asthma and COPD), this higher level of support may result in further hyperinflation and adverse clinical outcomes. If the technique is used in these patients, it should be done cautiously.
Respiratory muscle conditioning employs concepts borrowed from exercise physiology. To condition muscles and attain an optimal training effect from exercise, the concepts of endurance and strength conditioning may be considered. With strength training, a large force is moved a short distance. The muscles are worked to fatigue (short duration intervals) and rested for long periods of time. SBTs on T-piece or CPAP both mimic this type of training because they employ high pressure and low-volume work. Endurance conditioning, which requires that the workload be increased gradually, is easily accomplished with PSV because the level of support can be decreased over time. This kind of endurance training employs low pressure and high-volume work. Central to the application of both conditioning methods is the provision of adequate respiratory muscle rest between trials. Prolonging trials once the patient is fatigued serves no useful purpose and may be extremely detrimental physiologically and psychologically.
Study results suggest that no mode of ventilation is superior for weaning, however, the method of weaning, specifically the use of protocols, decreases variations in care and improves outcomes. Protocols direct caregivers by clearly delineating the protocol components. The protocol components consist of weaning readiness criteria (wean screens), weaning trial method and duration (ie, CPAP, T-piece, or PSV), and definitions of intolerance and respiratory muscle rest.
Spontaneous breathing trials (described earlier), primarily using CPAP or T-piece, are commonly used for the trials. As noted, the duration of such trials is generally between 30 minutes and 2 hours, although in those with tracheotomy tubes the duration may be much longer. While CPAP or T-piece is often used for trials, in most cases the choice between PSV (an endurance mode) and T-piece or CPAP (strengthening modes) is somewhat arbitrary if the protocol is appropriately aggressive and easily understood and applied by the caregivers. There are some conditions that require more selective decision making. One example is that of patients with heart failure. In these patients, the sudden transition from ventilator support to the use of T-piece or CPAP for a SBT may result in an increased venous return during the wean trial that may overwhelm the heart’s ability to compensate. Until appropriate preload and after-load reduction is addressed in these patients, PSV may be a gentler method of weaning. Another example is that of patients with profound myopathies or extremely debilitated states that may benefit from more gradual increases in work such as provided by PSV. Some new pressure modes such as proportional assist ventilation and adaptive support ventilation may potentially decrease the patient’s workload during weaning. More on advanced modes of ventilation, including these, may be found in the AACN Essentials of Critical Care Nursing Book, Chapter 20: Advanced Respiratory Concepts: Modes of Ventilation.
A popular and common sense approach to wean trial progression is to attempt weaning trials during the daytime, allowing the patient to rest at night until the protocol threshold for extubation is reached. In the case of the patient with a tracheostomy, progressively longer episodes of spontaneous breathing, usually on tracheostomy collar or T-piece, are accomplished until tolerated for a specified amount of time. Then, decisions about discontinuation of ventilation and tracheostomy downsizing or decannulation may be made. Wean plans need to be communicated clearly to all members of the healthcare team, and especially the patient, so that the plan is sufficiently aggressive but safe and effective. It is important that the philosophy of weaning is accepted by the healthcare team so that care planning is consistent and effective. Table 5-13 describes some general mechanical ventilator weaning philosophy concepts.
Patients who require LTMV often are affected by a variety of clinical conditions that prolong ventilator duration and other clinical outcomes such as length of stay and death. Research has demonstrated that outcomes of critically ill patients are improved with protocol-directed management.
Randomized controlled trials (RCT) have demonstrated that decreased sedation infusion use and methods to withdraw sedation daily using a “sedation interruption” improve outcomes such as ventilator duration and LOS. In addition, studies linked sedation use (specifically benzodiazepines) to delirium and subsequent cognitive dysfunction, further stimulating a decrease in sedation use in the ventilated patient population. Further emphasizing the importance of decreasing sedation use in these patients, a multicenter RCT combined sedation interruption with a “wake-up and breathe” trial (ie, SBT). In this study, patients assigned to the intervention (sedation interruption and wake-up) had significantly more days of spontaneous breathing, earlier discharge from ICU and hospital, and better 1-year survival than those in the control group.
The “ABCDE” bundle incorporates sedation awakening trials (Figure 5-21) along with other best evidence-based practice for ICU management (Table 5-14). Bundles are a structured method of improving patient care processes and when collectively performed, have resulted in improved patient outcomes.
Figure 5-21. Spontaneous Awakening Trial–SAT. (Data from Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE Bundle” into practice. Crit Care Nurse. 2012;32:35-47.)
The importance of factors such as sedation, delirium, and early mobility to weaning outcomes is essential to understand if the goal of attaining positive outcomes is to be attained. Protocols that assure that these important elements of care are routinely addressed decrease practice variation and improve outcomes.
Critical pathways are used to assure that evidence-based care is provided and that variation in care delivery is reduced. The pathways may be very directive in selected categories of patients, such as in patients with hip replacements, progression can be anticipated by hours or days; however, such specificity is not possible in the ventilated patient. Instead, pathways for the LTMV patient combine elements of care by specific time intervals (ie, begin deep vein thrombosis prophylaxis by day 1) with those that are designated by the stage of illness (ie, patient up to the chair during the prewean stage). In addition to providing an evidence-based blueprint for a wide variety of care elements, the pathways encourage multidisciplinary input and collaboration. In general, they are incorporated into systematic institutional approaches to care of the LTMV patient population.
Given the importance of systematic assessment and care planning, it is not surprising that many institutions have taken a very comprehensive approach to the care for the LTMV patient. Solutions to reduce variation and promote standardization of care are implemented to ensure that best practices are adhered to and good outcomes result.
In one study, an algorithmic approach to weaning was instituted in three adult ICUs and used nurses to manage the process. In another study, advanced practice nurses called “outcomes managers” managed and monitored long-term ventilated patients using a multidisciplinary clinical pathway and protocols for the management of sedation and weaning trials. The two studies demonstrated that statistically significant positive differences in most variables of interest such as ventilator duration, ICU and hospital lengths of stay (LOS), mortality rate, and cost savings were attainable with the approaches.
The healthcare environment is often chaotic. Short LOS and decreased staffing levels affect the continuity of care and contribute to gaps in practice and care planning. Given the complexity of the care of the ventilated patient, it is clear that approaches to care that decrease variation may improve patient outcomes and are to be encouraged.
The complexity of ventilators and the dynamic state of the patient’s clinical condition, as well as the patient’s response to ventilation, create a variety of common problems that may occur during mechanical ventilation. It is crucial that progressive care clinicians be expert in the prevention, identification, and management of ventilator-associated problems in ventilated patients.
During mechanical ventilation, sudden changes in the clinical condition of the patient, particularly respiratory distress, as well as the occurrence of ventilator alarms or abnormal functioning of the ventilator, require immediate assessment and intervention. A systematic approach to each of these situations prevents or minimizes untoward ventilator events (Figure 5-22).
Figure 5-22. Algorithm for management of ventilator alarms and/or development of acute respiratory distress.
The first step is to determine the presence of respiratory distress or hemodynamic instability. If either is present the patient is removed from the mechanical ventilator and manually ventilated with an MRB and 100% O2 for a few minutes. During manual ventilation, a quick assessment of the respiratory and cardiovascular system is made, noting changes from previous status. Clinical improvement rapidly following removal from the ventilator suggests a ventilator problem. Manual ventilation is continued while another clinician corrects the ventilator problem (eg, tubing leaks or disconnections, inaccurate gas delivery) or replaces the ventilator. Continuation of respiratory distress after removal from the ventilator and during manual ventilation suggests a patient-related cause.
Being able to eat “regular food” enhances the ventilated patient’s sense of well being and optimism. However, it is essential that the patient’s ability to swallow is evaluated prior to allowing feeding following extubation (especially in those ventilated long term) and in those who have a tracheostomy tube in place. “Swallow studies” are commonly done in these patients and should be considered for the categories noted above as well as in any patient with a history of aspiration.
Mechanically ventilated patients are unable to speak and communicate verbally due to the presence of a cuffed ET tube or tracheostomy tube. The inability to speak is frustrating for the patient, nurse, and members of the healthcare team. Impaired communication results in patients experiencing anxiety and fear, symptoms that can have a deleterious effect on their physical and emotional conditions. Patients interviewed after extubation reveal how isolated and alone they felt because of their inability to speak.
Patients’ perceptions of communication difficulties related to mechanical ventilation include: (1) inability to communicate, (2) insufficient explanations, (3) inadequate understanding, (4) fears related to potential dangers associated with the inability to speak, and (5) difficulty with communication methods. Except for the problem of inability to vocalize, all of the problems cited by ventilated patients may be resolved easily by progressive care practitioners. For instance, “insufficient explanations” and “inadequate understanding” can be remedied by frequent repetition of all plans and procedures in language that is understandable to a nonmedical person and that takes into account that attention span and cognitive abilities, especially memory, are frequently diminished due to the underlying illness or injury, effects of medications and anesthesia, and the impact of the progressive care environment. Although most messages the ventilated patient needs to communicate lie within a narrow range (“pain,” “hunger,” “water,” and “sleep”), communicating these basic needs is often difficult. Most adults are accustomed to attending to their own basic needs, but in the progressive care unit, not only are they unable to physically perform certain activities, they also may not be able to communicate their needs effectively. Basic needs include such activities as bathing, brushing teeth, combing hair, elimination, eating, drinking, and sleeping. Other examples include simple requests or statements such as “too hot,” “too cold,” “turn me,” “up,” “down,” “straighten my legs,” “my arm hurts,” “I can’t breathe,” and “moisten my lips.”
Patients have described difficulties with communication methods while being mechanically ventilated. This also can be avoided by assessing the patient’s communication abilities. Is the patient alert and oriented? Can the patient answer simple yes and no questions? Does the patient speak English? Can the patient use at least one hand to gesture? Does the patient have sufficient strength and dexterity to hold a pen and write? Are the patient’s hearing and vision adequate? Knowledge of the patient’s communication abilities assists the clinician to identify appropriate communication methods.
Once the most successful communication methods have been identified for a particular patient, they should be written into the plan of care. Continuity among healthcare professionals in their approach to communication with nonvocal patients improves the quality of care and increases patient satisfaction.
A variety of methods for augmenting communication are available and can be classified into two categories: nonvocal treatments (gestures, lip reading, mouthing words, paper and pen, alphabet/numeric boards, flashcards, etc) and vocal treatments (talking tracheostomy tubes and speaking valves). The best way to communicate with the patient who has an artificial airway or who is being mechanically ventilated is still unknown.
Individual patient needs vary and it is recommended that the nurse use a variety of nonvocal treatments (eg, gestures, alphabet board, and paper and pen). Success with communication interventions varies with the diagnosis, age, type of injury or disease, type of respiratory assist devices, and psychosocial factors. For instance, lip reading can be successful in patients who have tracheostomies because the lips and mouth are visible, but in the ET tube patient, where tape and tube holders limit lip movement and visibility, lip reading may be less successful.
Typically the easiest, most common method of communication readily available is the paper and pen. However, the supine position is not especially conducive to writing legibly. The absence of proper eyeglasses, an injured or immobilized dominant writing hand, or lack of strength also can make writing difficult for mechanically ventilated patients. Writing paper should be placed on a firm writing surface (eg, clipboard) with an attached felt-tipped pen that writes in any position. Strength, finger flexibility, and dexterity are required to grasp a pen. Many patients prefer to use a Magic Slate (Western Publishing Co., Racine, WI) or a Magna Doodle (Tyco Industries, Mount Laurel, NJ). These pressure-sensitive, inexpensive toy screens can be purchased at any department store; with them messages can be easily erased, maintaining the privacy of a written message. Although, costly, computer keyboards or touchpads may facilitate writing in patient who are comfortable with “high-tech” solutions.
A phenomenon of decoding exists between patient’s written words (which often look like scribbling) and the nurse’s ability to read what the patient wrote. The majority of the time, the nurse can read the writing of the patient even when it seems indistinguishable to a casual observer. This is due in part to the fact that over 65% of all communication is nonverbal and many contextual cues exist to assist in understanding and communicating effectively.
Another nonvocal method of communication that can be very effective is the deliberate use of gestures. Gestures are best suited for the short-term ventilated patient who is alert and can move at least one hand, even if only minimally. Generally, well-understood gestures are emblematic, have a low level of symbolism, and are easily interpreted by most people.
For example, ventilated patients often indicate that they need suctioning by curving an index finger (to resemble a suction catheter), raising a hand toward the ET tube, and moving their hand back and forth. This is known as an idiosyncratic gesture, a gesture that is used by a particular community, namely, the nurse and the ventilated patient. Other idiosyncratic gestures include “ice chips,” “moisten my mouth,” “spray throat,” “fan,” and “doctor.”
One important aspect of communicating by gesture is to “mirror” the gesture(s) back to the patient, at the same time verbalizing the message or idea conveyed by the patient’s gesture. This mirroring ensures accuracy in interpretation and assists the clinician and patient to form a repertoire to be used successfully in future gestural conversations. When observing a patient’s gestures, stand back from the bed, and watch his or her arms and hands. Most gestures are easily understood, especially those most frequently used by patients (eg, the head nod, indicating “yes” or “no”). Practitioners should ask simple yes-and-no questions, but avoid playing “twenty questions” with ventilated patients because this can be very frustrating. Before trying to guess the needs of ventilated patients, give them the opportunity to use gestures to communicate their needs.
For patients who do not speak English, a picture board is sometimes useful along with well-understood gestures. Picture boards have images of common patient needs (eg, bedpan, glass of water, medications, family, doctor, nurse) that the patient can point to. Picture boards, although commercially available, can be made easily and laminated to more uniquely meet the needs of a specific progressive care population.
Another approach is the use of flash cards that can be purchased or made. Language flash cards contain common words or phrases in English or foreign languages.
If patients with tracheostomy tubes in place have intact organs of speech, they may benefit from vocal treatment strategies like pneumatic and electrical devices, fenestrated tracheostomy tubes, talking tracheostomy tubes, and tracheostomy speaking valves. Several conditions preclude use of vocalization devices, such as neurologic conditions that impair vocalization (eg, Guillain-Barré syndrome), severe upper airway obstruction (eg, head/neck trauma), or vocal cord adduction (eg, presence of an ET tube).
A number of vocal treatments for tracheostomized patients exist. Generally, they require that the cuff be completely deflated to allow for air to be breathed in and out through the mouth and nose as well as around the sides of the tracheostomy. On exhalation the gases pass through the vocal cords allowing for speech. Some use one-way speaking valves (eg, Passy-Muir valve, shown in Figure 5-23) allow for air to be inhaled through the valve but close during exhalation to direct air up past the vocal cords. A fenestrated tracheostomy tube (Figure 5-24) also allows passage of air through the vocal cords. A cap or speaking valve may be used in conjunction with the fenestrated tube to ensure that all exhaled air moves through the vocal cords for vocalization. There have been reported incidences of granuloma tissue development at the site adjacent to the fenestration, which resolves after removal of the tube. In addition, fenestrated ports often become clogged with secretions, again preventing voicing. It is imperative that if the tracheostomy tube is capped, that the cuff of the tracheostomy be completely deflated.
Figure 5-23. Passy-Muir Speaking Valves. (Image courtesy of Passy-Muir, Inc., Irvine, CA.)
Figure 5-24. (A) Fenestrated tracheostomy tube. (B) Opening above the cuff site allowing gas, flow past the vocal cords during inspiration and expiration. (With permission, Covidien.)
Another vocal treatment is the talking tracheostomy tube, which is designed to provide a means of verbal communication for the ventilator-dependent patient. Patients who were otherwise considered to be “unweanable” have been reported to take a renewed interest in the weaning process, and some successfully wean upon hearing their own voice. Currently there are two talking tracheostomy tubes available that maintain a closed system with cuff inflation but differ in how they function.
1. The Portex trachesotomy operates by gas flowing (4-6 L/min) through an airflow line, which has a fenestration just above the tracheostomy tube cuff (Figure 5-25). The air flows through the glottis, thus supporting vocalization if the patient is able to form words with their mouth. However, an outside air source must be provided, which is usually not humidified and the trachea can become dry and irritated. The line for this air source requires diligent cleaning and flushing of the air port to prevent it from becoming clogged. The patients or staff must be able to manually divert air through the tube via a thumb port control.
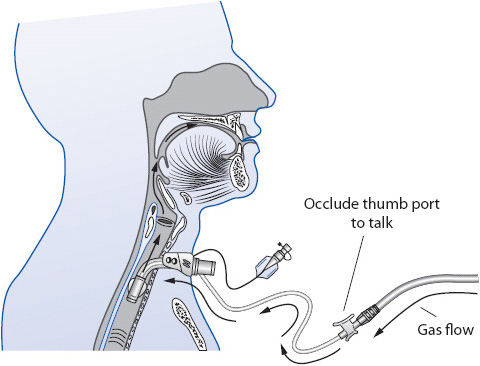
Figure 5-25. Tracheostomy tube with side port to facilitate speech. (With permission Smith Medical, Keen, NH.)
2. The Blom tracheostomy tube system (Figure 5-26) uses a 2-valve system in a specialized speech inner cannula that redirects air and does not require use of an air source. During inhalation the flap valve opens and the bubble valve seals the fenestration preventing air leak to the upper airway. On exhalation, the flap valve closes and the bubble valve collapses to unblock the fenestration to allow air to the vocal cords. An additional component is the exhaled volume reservoir which attaches to the circuit and returns volume to minimize false low expiratory minute volume alarms.
Figure 5-26. Tracheostomy Tube with inner cannula for speaking (Blom Tracheostomy Tube System). (Courtesy of Pulmodyne, Indianapolis, IN [http://www.dolema.com/uploads/6869E_Blom_Brochure.pdf])
The progressive care environment presents many teaching and learning challenges. Patients and families are under a considerable amount of stress, so the nurse must be a very creative teacher and offer communication techniques that are simple, effective, and easy to learn. The desire to communicate with loved ones, however, often makes the family very willing to learn. Frequently, it is the family who makes up large-lettered communication boards, purchases a Magic Slate, or brings in a laptop computer or touchpad for the patient to use. Suggesting that families do this is usually very well received, because loved ones want so desperately to help in some way.
All patients should be informed prior to intubation that they will be unable to speak during the intubation period. A flipchart illustrating what an endotracheal tube or tracheostomy tube is like, with labeling in simple words, may be shown to patients who will be electively intubated (eg, for planned surgery). Practicing with a few nonverbal communication techniques before intubation (eg, gestures, alphabet boards, flash cards) is also beneficial. Another important point to emphasize with patients is that being unable to speak is usually temporary, just while the breathing tube is in place. If preintubation explanations are not feasible or possible, provide these explanations to the intubated patient.
The majority of interventions related to mechanical ventilation focus on maximizing oxygenation and ventilation, and preventing complications associated with artificial airways and the sequelae of assisting the patient’s ventilation and oxygenation with an invasive mechanical device.
• Provide frequent explanations of the purpose of the ventilator.
• Monitor the patient’s response to ventilator therapy and for signs that the patient is dyssynchronous with the ventilator respiratory pattern. The use of graphic displays, common on many ventilator systems, is often a helpful aid to patient assessment.
• Consider ventilator setting changes to maximize synchrony (eg, changes in flow rates, respiratory rates, sensitivities, and/or modes).
• Administer sedative agents judiciously as required to enhance patient/ventilator synchrony.
• Suction only when clinically indicated according to patient assessment.
• Decrease secretion viscosity by maintaining adequate hydration and humidification of all inhaled gases.
• Monitor for signs and symptoms of bronchospasm and administer bronchodilator therapy as appropriate.
• Prevent obstruction of oral ET tubes by using an oral bite block if necessary.
• ABG analysis as appropriate (eg, after some ventilator changes, with respiratory distress or cardiovascular instability, or with significant changes in clinical condition).
• Continuous noninvasive monitoring of SpO2. Validate noninvasive measures with periodic ABG analysis (see Table 5-4).
• Observe for signs and symptoms of decreases in PaO2, increases in PaCO2, and respiratory distress. Development of respiratory distress requires immediate intervention (see Figure 5-23).
• Reposition frequently and develop a mobilization plan to improve ventilation-perfusion relationships and prevent atelectasis.
• Aggressively manage pain, particularly chest and upper abdominal pain, to increase mobility, deep breathing, and coughing (see Chapter 6, Pain and Sedation Management).
• Administer chest physiotherapy for selected clinical conditions (eg, large mucus production, lobar atelectasis).
• Monitor oxygenation status closely during chest physiotherapy for signs and symptoms of arterial desaturation.
• Ensure proper operation of the mechanical ventilator by activation of appropriately set alarms and frequent assessment of device function (commonly checked every 1-2 hours).
• During even brief periods of removal from mechanical ventilation, maintain ventilation and oxygenation with MRB. During intrahospital transport, verify adequacy of ventilatory support equipment, particularly the maintenance of PEEP (when > 10 cm H2O is required) as well as ensuring adequate portable oxygen supply tank pressure. When possible, a portable mechanical ventilator should be used versus MRB.
• Emergency sources of portable oxygen should be readily available in the event of loss of wall oxygen capabilities.
• Systematically assess wean potential and address factors impeding weaning.
• Use a weaning protocol with a “wean screen.” Assure that the patient, family, and key caregivers are aware of weaning trials.
• Stop weaning trial if signs of intolerance emerge.
• Use a systematic evidence-based multidisciplinary approach to weaning.
• Maintain ET or tracheostomy cuff pressures less than 25 mm Hg (30 cm H2O).
• Maintain artificial airway position by securing with a properly fitting holder device or selected tapes. Frequently verify proper ET position by noting ET marking at lip or nares placed after intubation.
• Ensure tape or devices used to secure the artificial airway are properly applied and are not causing pressure areas or skin breakdown. Periodic repositioning of ET tubes may be required to prevent skin integrity problems.
• Use a bite block with oral ET tubes if necessary to prevent accidental biting of the tube.
• Provide frequent mouth care and assess for development of pressure areas from ET tubes. Move the ET from one side of the mouth to the other daily or more frequently if necessary.
• Assess for signs and symptoms of sinusitis with nasal ET tube use (eg, pain in sinus area with pressure, purulent drainage from nares, fever, increased white blood cell count).
• Assess communication abilities and establish at least a method for nonverbal communication (see the discussion of communication previously). Assist family members in using that approach with the patient.
• Anticipate patient needs and concerns in the planning of care.
• Ensure that call lights, bells, or other methods for notifying unit personnel of patient needs are in place at all times.
• Frequently repeat information about communication limitations and how to use different nonverbal communication methods.
• Maintain a calm, supportive environment to avoid unnecessary escalation of anxiety. Provide brief explanations of activities and procedures. The vigilance and presence of healthcare providers during anxiety periods is crucial to avoid panic by patients and visiting family members.
• Teach the patient relaxation techniques to control anxiety.
• Administer mild doses of anxiolytics if needed (eg, lorazepam or diazepam) that do not depress respiration (see Chapter 6, Pain and Sedation Management and Chapter 9, Cardiovascular System).
• Encourage the family to stay with the patient as much as desired and to participate in caregiver activities as appropriate. Presence of a family member provides comfort to the patient and assists the family member to better cope with the illness.
• Promote sleep at night by decreasing light, noise, and unnecessary patient interruptions.
Ahrens T, Sona C. Capnography application in acute and progressive care. AACN Clin Issues. 2003;14:123-132.
Balas MC, Rice M, Chaperon C, et al. Management of delirium in critically ill adults. Crit Care Nurs. 2012;32:15-25.
Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE Bundle” into practice. Crit Care Nurs. 2012;32:35-47.
Berry E, Zecca H. Daily interruptions of sedation: a clinical approach to improve outcomes in clinically ill patients. Crit Care Nurs. 2012;32:43-51.
Branson RD, Mannheimer PD. Forehead oximetry in critically ill patients: the case of a new monitoring site. Respir Care Clin N Am. 2004;10(3):359-367.
Chang L, Wang KK, Chao F. Influence of physical restraint on unplanned extubation of adult intensive care patients: a case-control study. Am J Crit Care. 2008;17:408-415.
Cuccio L, Cerullo E, Paradis H, et al. An evidence-based oral care protocol to decrease ventilator-associated pneumonia. Dimens Crit Care Nurs. 2012;31:301-308.
Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines. Chest. 2006;127:335-371.
Ely EW, Shintani A, Truman B. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753-1762.
Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of crtical illness. Crit Care Med. 2010;38:1513-1520.
Halm M, Amrola R. Effect of oral care on bacterial colonization and ventilator-associated pneumonia. Am J Crit Care. 2009;18:275-278.
Hellstom A, Fagerstom C, Willman A. Promoting sleep by nursing interventions in health care settings: a systematic review. Worldviews Evid Based Nurs. 2011;8:128-142.
Jarachovic M, Mason M, Kerber K, McNett M. The role of standardized protocols in unplanned extubations in a medical intensive care unit. Am J Crit Care. 2011;20:304-311.
Jongerden IP, Rovers MM, Grypdonck MH, Bonten MJ. Open and closed endotracheal suction systems in mechanically ventilated intensive care patients: a meta-analysis. Crit Care Med. 2007;35:260-270.
Kazmarek RM, Stoller JK, Heur AJ, eds. Egan’s Fundamentals of Respiratory Care. 10th ed. St. Louis, MO: Elsevier Mosby; 2013.
Kjonegaard R, Fields W, King ML. Current practice in airway management: a descriptive evaluation. Am J Crit Care. 2009;doi: 10.4037/ajcc2009803.
MacLeod DB, Cortinez LI, Keifer JC, et al. The desaturation response time of finger pulse oximeters during mild hypothermia. Anaesthesia. Jan 2005;60(1):65-71.
Matthews EE. Sleep disturbances and fatigue in critically ill patients. AACN Adv Crit Care. 2011;22:204-224.
Munro CL, Grap MJ, Jones DJ, et al. Chlorhexidine, tooth brushing, and preventing ventilator-associated pneumonia in critically ill adults. Am J Crit Care. 2009;18:428-437.
Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3): S729-767.
Seckel MA. Ask the experts: does the use of a closed suction system help to prevent ventilator-associated pneumonia? Crit Care Nurs. 2008;28(1):65-66.
Seckel MA. Ask the experts: normal saline and mucous plugging. Crit Care Nurs. 2012;32:66-68.
Seckel MA, Schulenburg K. Ask the experts: eating while receiving mechanical ventilation. Crit Care Nurs. 2011;31:95-97.
Stauffer JL. Complications of endotracheal intubation and tracheotomy. Respir Care. 1999;44(7):828-844.
St John RE, Malen JF. Airway management. Crit Care Nurs Clin N Am. 2004;16:413-430.
Stonecypher K. Ventilator-associated pneumonia: the importance of oral care in intubated adults. Crit Care Nurs Q. 2010;33(4):339-347.
Unoki T, Serita A, Grap MJ. Automatic tube compensation during weaning from mechanical ventilation: evidence and clinical implications. Crit Care Nurs. 2008;28:34-42.
Valdez-Lowe C, Ghareeb SA, Artinian NT. Pulse oximetry in adults. AJN. 2009;109(6):52-59.
Aloe K, Ryan M. Creation of an intermediate respiratory care unit to decrease intensive care utilization. JONA. 2009;39:494-498.
Burns SM. Mechanical ventilation and weaning. In: Carlson KK, ed. AACN Advanced Critical Care Nursing. St Louis, MO: Saunders-Elsevier; 2009.
Burns SM. Pressure modes of mechanical ventilation: the good, the bad, and the ugly. AACN Advance Crit Care. 2008;19:399-411.
Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452-2460.
Kane C, York NL. Understanding the alphabet soup of mechanical ventilation. Dimens Crit Care Nurs. 2012;31:217-222.
MacIntyre NR, Branson RD. Mechanical Ventilation. Philadelphia, PA: Saunders; 2009.
Pierce LN. Management of the Mechanically Ventilated Patient. Philadelphia, PA: Saunders-Elsevier; 2007.
Restrepo RD, Walsh BK. Humidification during invasive and noninvasive mechanical ventilation: 2012. Resp Care. 2012;57:782-788.
St. John R. End-tidal CO2 monitoring. In Burns SM. ed. AACN’s Protocols for Practice: Non-Invasive Monitoring Series. Sudbury, MA: Jones and Bartlett; 2006.
Tobin MJ. Principles and Practice of Mechanical Ventilation. New York, NY: McGraw-Hill Medical Publishing Division; 2006.
Unroe M, Kahn JM, Carson SS, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153:167-175.
Walkey AJ, Wiener RS. Use of noninvasive ventilation in patient with acute respiratory failure, 2000-2009. Annals ATS. 2013;10:10-17.
White AC. Long-term mechanical ventilation: management strategies. Resp Care. 2012;57:889-897.
Blackwood B, Alderdice F, Burns K, et al. Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta-analysis. BMJ. 2011;342c7237doi:1-.1136/bmj.b7237.
Boles JM, Blon J, Connors A, et al. Task force: weaning from mechanical ventilation. Eur Respir J. 2007;29:1033-1056.
Bou Aki I, Bou-Khalil P, Kanazi G. Weaning from mechanical ventilation. Curr Opin Anesthesiol. 2012;25:42-47.
Brochard L, Thille AW. What is the proper approach to liberating the weak from mechanical ventilation? Crit Care Med. 2009;37:S410-S415.
Burns SM. Adherence to sedation withdrawal protocols and guidelines in ventilated patients. Clin Nurs Spec. 2012;26:22-8. doi: 10.1097/NUR.0b013e31823bfae8.
Burns SM. Weaning from mechanical ventilation: where were we then, and where are we now? Crit Care Nurs Clin N Am. 2012;24:457-458.
Burns SM, Fisher C, Tribble SS, et al. The relationship of 26 clinical factors to weaning outcome. Am J Crit Care. 2012;21:52-58.
Epstein SK. Weaning from ventilatory support. Curr Opin Crit Care. 2009;15:36-43.
Eskandar N, Apostolakos MJ. Weaning from mechanical ventilation. Crit Care Clin. 2007;23:263-274.
Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126-134.
Haas CF, Loik PS. Ventilator discontinuation protocols. Resp Care. 2012;57:1649-1662.
MacIntyre N. Discontinuing mechanical ventilator support. Chest. 2007;132:1049-1056.
MacIntyre NR. Evidence-based assessments in the ventilator discontinuation process. Resp Care. 2012;57:1611-1618.
McConville JF, Kress JP. Current concepts: weaning patients from the ventilator. NEJM. 2012;367:2233-2239.
Mendes-Tellez PA, Needham DM. Early physical rehabilitation in the ICU and ventilator liberation. Resp Care. 2012;57:1663-1669.
Olff C, Clark-Wadkins C. Tele-ICU partners enhanced evidence-based practice: ventilator weaning intiative. AACN Adv Crit Care. 2012;23:312-322.
Penuelas O, Frutos-Vivar F, Fernandez C, et al. Characteristics and outcomes of ventilated patients according to time to liberation from mechanical ventilation. Am J Respir Crit Care Med. 2011;184:430-437.
Tobin MJ, Guenther SM, Perez W, et al. Konno-Mead analysis of ribcage-abdominal motion during successful and unsuccessful trials of weaning from mechanical ventilation. Am Rev Respir Dis. 1987;135:1320-1328.
White V, Currey J, Botti M. Multidisciplinary team developed and implemented protocols to assist mechanical ventilation weaning: a systematic review of literature. Worldviews Evid Based Nurs. 2011;8:51-59.
Batty S. Communication, swallowing and feeding in the intensive care unit patient. Nurs Crit Care. 2009;14:175-179.
Baumgartner CA, Bewyer E, Bruner D. Management of communication and swallowing in intensive care. AACN Adv Crit Care. 2008;19:433-443.
Happ MB. Communicating with mechanically ventilated patients: state of the science. AACN Clin Issues. 2001;12:247-258.
Windhorst C, Harth R, Wagoner C. Patients requiring tracheostomy and mechanical ventilation: a model for interdisciplinary decision-making. AHSA Leader. 2009;14:10-13.
A Collective Task Force Facilitated by the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Medicine. Evidence-based guidelines for weaning and discontinuing ventilator support. Resp Care. 2002;47:69-90.
American Association of Respiratory Care. AARC clinical practice guideline: capnography/capnometry during mechanical ventilation: 2011. Resp Care. 2011;56:503-509.
American Association for Respiratory Care. AARC clinical practice guideline: care of the ventilator circuit and its relation to ventilator-associated pneumonia. Resp Care. 2003;48:869-879.
American Association of Respiratory Care. AARC clinical practice guideline: endotracheal suctioning of mechanically ventilated patients with artificial airway. Resp Care. 2010;55:758-764.
American Association for Respiratory Care. AARC clinical practice guideline: oxygen therapy for adults in the acute care facility 2002 revision and update. Resp Care. 2002:47;717-720.
American Association for Respiratory Care. AARC clinical practice guideline: removal of the endotracheal tube-2007 revision and update. Resp Care. 2007:52;81-93.
American Association of Critical Care Nurses (AACN). Practice alert: delirium assessment and management. Alisio Viejo, CA: AACN: 2011. www.aacn.org. Accessed March 1, 2013.
American Association of Critical Care Nurses (AACN). Practice alert: oral care in the critically Ill. Alisio Veijo, CA: AACN: 2010. www.aacn.org. Accessed March 1, 2013.
American Association of Critical Care Nurses (AACN). Practice alert: prevention of aspiration. Alisio Viejo, CA: AACN: 2011. www.aacn.org. Assessed March 1, 2013.
American Association of Critical Care Nurses (AACN). Practice alert: ventilator associated pneumonia. Alisio Veijo, CA: AACN: 2008. www.aacn.org. Accessed January 10, 2010.
American Thoracic Society and the Infectious Diseases Society of America. Guidelines for the management of adults with hospital acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
Barden, C, Davis T, Seckel M, et al. C. AACN Tele-ICU Nursing Practice Guidelines. 2013. Available at http://www.aacn.org/wd/practice/docs/tele-icu-guidelines.pdf.
Barr J, Fraser Gl, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263-306.
Burns SM. Practice protocol: weaning from mechanical ventilation. In: Care of the Mechanically Ventilated Patient. 2nd ed. Sudbury, MA: Jones and Bartlett; 2007.
Centers for Disease Control and Prevention. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Health Care Infection Control Practices Advisory Committee. MMWR. 2004;53(No. RR-3):1-35.
Grap MJ. Pulse oximetry. In: Burns SM, ed. AACN’s Protocols for Practice: Noninvasive Monitoring Series. Sudbury, MA: Jones and Bartlett; 2006.
MacIntyre, NR, Cook DJ, Ely EW, Jr., et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(6 suppl):375S-395S.
Muscedere J, Dodek P, Keenan S, et al. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care. 2008;23:126-137.
Pierce LN. Invasive and noninvasive modes and methods of mechanical ventilation. In: Burns SM, ed. AACN’s Protocols for Practice: Care of the Mechanically Ventilated Patient Series. Sudbury, MA: Jones and Bartlett; 2006.
St John RE. End-tidal carbon dioxide monitoring. In: Burns SM, ed. AACN’s Protocols for Practice: Noninvasive Monitoring Series. Sudbury, MA: Jones and Bartlett; 2006.
St John RE, Seckel MA. Airway management. In: Burns SM, ed. AACN’s Protocols for Practice: Care of the Mechanically Ventilated Patient Series. Sudbury, MA: Jones and Bartlett; 2006.
Wiegand DL, ed. AACN Procedure Manual for Critical Care. 6th ed. Philadelphia, PA: Saunders; 2011.