TABLE 10-1. BASIC X-RAY DENSITIES

KNOWLEDGE COMPETENCIES
1. Identify various radiologic and pulmonary anatomic features relevant to interpretation of chest x-rays.
2. Describe different systems and principles of management for chest tubes.
3. Describe the etiology, pathophysiology, clinical presentation, patient needs, and principles of management of acute respiratory failure (ARF).
4. Compare and contrast the pathophysiology, clinical presentation, patient needs, and management approaches for common diseases leading to ARF:
• Acute respiratory distress syndrome (ARDS)
• ARF in the chronic obstructive pulmonary disease patient (asthma, emphysema, bronchitis)
• COPD exacerbation
• Acute asthma
• Pulmonary hypertension
• Pneumonia
• Interstitial lung disease
• Pulmonary embolism (PE)
• Venous thromboembolism (VTE)
Chest radiography is an important tool in respiratory assessment, providing visualization of the heart and lungs. Chest x-rays are a complement to bedside assessment. Progressive care nurses need to know basic radiographic concepts and how to optimize portable chest x-ray technique, as well as how to systematically view a chest x-ray image.
Chest x-rays are obtained as part of routine screening procedures, when respiratory disease or an acute change is suspected, to evaluate the status of respiratory abnormalities (eg, pneumothorax, pleural effusion, tumors), to confirm proper invasive tube placement (ie, endotracheal, tracheostomy, or chest tubes, and central line catheters), or following traumatic chest injury.
An x-ray is a form of radiant energy, and a radiographic image is made by x-ray machines. Only a few rays are absorbed by air as beams pass through the atmosphere, whereas all rays are absorbed by metal as the beams attempt to pass through a sheet of metal. When nothing but air lies between the film cassette and the x-ray source, the radiographic image is blackness or radiolucency. If density increases, more beams are absorbed between the film cassette or detector and the x-ray source, and the radiographic image is whiteness or radiopacity. Many institutions are replacing traditional x-ray film with detectors that convert the x-ray energy to a digital radiograph. These can then be stored and distributed in a digital format. As the x-ray beam passes through the patient, the denser tissues absorb more of the beam, and the less dense tissues absorb less of the beam.
The lungs are primarily sacs of air or gas, so normal lungs look black on chest films. Conversely, the skeletal thorax appears white, because bone is very dense and absorbs the most x-rays (Table 10-1). The heart and mediastinum appear gray because those structures are made up of mostly water. Breast tissue is made up of mostly fat and it appears whitish-gray.
The most common method of obtaining a chest x-ray is the posterior-anterior (PA) view. PA chest x-rays are typically done in the radiology department with the machine about 6 ft away from the x-ray film cassette and the patient standing with the anterior chest wall against the x-ray plate and the posterior chest wall toward the x-ray machine. The patient is told to take a deep breath and hold it as the x-ray beam is delivered through the posterior chest wall to the x-ray film cassette. The PA view results in a very accurate, sharp picture of the chest.
Acutely ill patients are rarely able to tolerate the positioning requirements of a PA chest x-ray. Many chest x-rays in progressive care are obtained with an anterior-posterior (AP) view with the patient supine in bed, with or without back rest elevation. With portable AP chest films, the film cassette is placed behind the patient and the x-ray beam is delivered through the anterior chest to the x-ray film. The x-ray machine is only 3 ft away from the patient, which results in greater distortion of chest images, making the AP chest x-ray less accurate than the PA method. Of particular concern is that the heart size is enlarged on an AP film. When viewing chest x-rays, it is important to know whether a PA or an AP view was used to avoid misinterpretation of heart size as cardiomegaly.
Distortions can be minimized by placing the patient in a high Fowler position, or as erect as possible, with the thorax symmetrically placed on the x-ray film cassette. Explain the procedure to the patient and the need to avoid movement. All unnecessary objects lying on the anterior chest (such as ventilator tubing, safety pins, jewelry, ECG wires, nasogastric tubes, etc) are removed or repositioned as possible. If the patient is unconscious, taping the forehead in a neutral position may be necessary, especially in the high Fowler position to avoid mispositioning of the head. All caregivers assisting with the chest x-ray need to protect themselves from radiation exposure by positioning themselves behind the x-ray machine or by using lead aprons covering the neck, chest, and abdomen.
Other chest x-ray views include: (1) lateral views to identify normal and abnormal structures behind the heart, along the spine, and at the base of the lung; (2) oblique views to localize lesions without interference from the bony thorax or to get a better picture of the trachea, carina, heart, and great vessels; (3) lordotic views to better visualize the apical and middle regions of the lungs and to differentiate anterior from posterior lesions; and (4) lateral decubitus (cross-table) views, done with the patient supine or side-lying, to assess for air-fluid levels or free-flowing pleural fluid.
A systematic approach should be used when analyzing a chest x-ray image including reviewing the results. It is important to first make sure that the report and image have been properly labeled (correct name and medical record number), and to identify the right and left sides before viewing the images. If previous images are available, place them next to the new images for comparison. View the chest x-ray from the lateral borders, moving to the medial aspects of the thorax and asking the series of questions found in Table 10-2. Begin the chest x-ray analysis by comparing the right side to the left side using the following sequence (Figures 10-1 and 10-2): (1) soft tissues—neck, shoulders, breasts, and subcutaneous fat; (2) trachea—the column of radiolucency readily visible above the clavicles; (3) bony thorax—note size, shape, and symmetry; (4) intercostal spaces (ICS)—note width and angle; (5) diaphragm—dome-shaped with distinct margins, right dome 1 to 3 cm higher than left dome; (6) pleural surfaces—visceral and parietal pleura appear like a thin, hairlike line along the apices and lateral chest; (7) mediastinum—size varies with age, gender, and size; (8) hila—large pulmonary arteries and veins; (9) lung fields—largest area of the chest and most radiolucent; and (10) catheters, tubes, wires, and line.
TABLE 10-2. STEPS FOR INTERPRETATION OF A CHEST X-RAY FILM

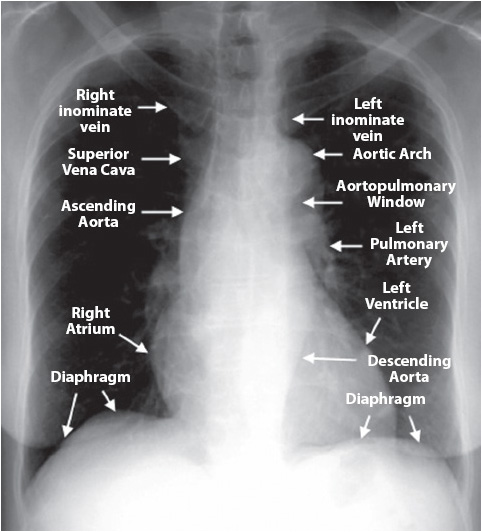
Figure 10-1. Normal chest x-ray with anatomical references. Courtesy of University of Virginia Health Sciences Center, Department of Radiology (From Spencer B. Gay, MD, Juan Olazagasti, MD, Jack W. Higginbotham, MD, et al. Introduction to Chest Radiology. University of Virginia Health Sciences Center–http://www.med-ed.virginia.edu/courses/rad/cxr/anatomy4chest.html)
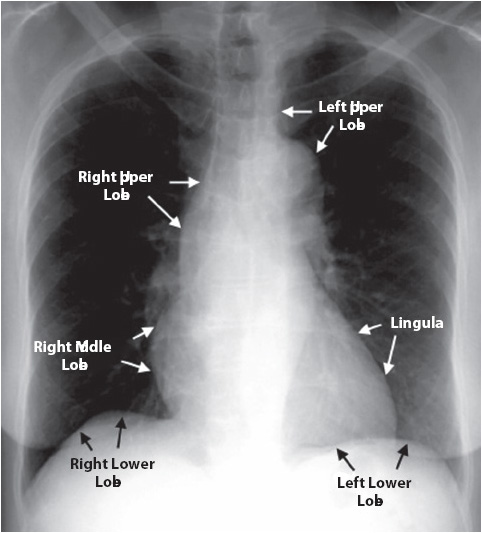
Figure 10-2. Normal chest x-ray with anatomical references. Courtesy of University of Virginia Health Sciences Center, Department of Radiology (From Spencer B. Gay, MD, Juan Olazagasti, MD, Jack W. Higginbotham, MD, et al. Introduction to Chest Radiology. University of Virginia Health Sciences Center–http://www.med-ed.virginia.edu/courses/rad/cxr/anatomy4chest.html)
When the soft tissues are examined, the two sides of the lateral chest should be symmetric. A mastectomy makes one lung look more radiolucent than the other due to the absence of fatty tissue. The trachea should be midline, with the carina visible at the level of the aortic knob or second ICS. The most common cause of tracheal deviation is a pneumothorax, which causes a tracheal and mediastinal shift to the area away from the pneumothorax (Table 10-3, Figures 10-3 and 10-4).
TABLE 10-3. CHEST X-RAY FINDINGS
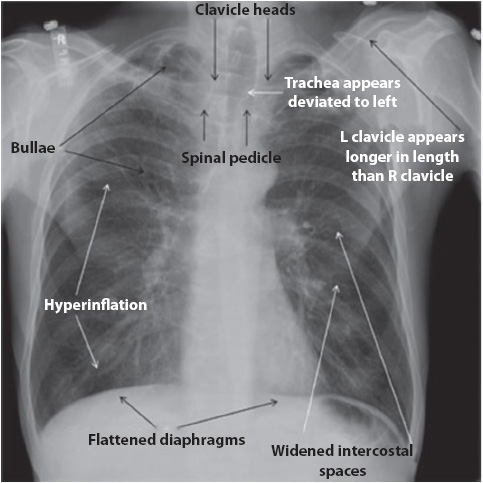
Figure 10-3. COPD, flattened diaphragms, hyperinflation, widened intercostals spaces, apical bullae, and chest rotation. (Reprinted from: Siela D. Chest radiograph evaluation and interpretation. AACN Adv Crit Care. 2008;19:444-473.)
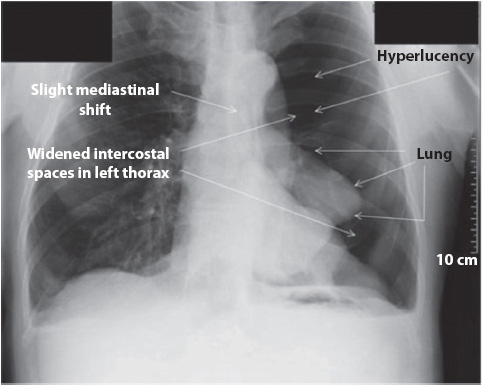
Figure 10-4. Left pneumothorax, hyperlucency, and widened intercostals spaces. (Reprinted from: Siela D. Chest radiograph evaluation and interpretation. AACN Adv Crit Care. 2008;19:444-473.)
Bony thorax inspection reveals general body build. Clavicles should be symmetric and may have an irregular notch or indentation in the inferior medial aspect of the clavicle called a rhomboid fossa, a normal variant. Deformities of the thorax can be detected, such as scoliosis, pectus excavatum (also called funnel chest), or pectus carinatum (also known as pigeon chest). Decreases in the density (less white) of the spine, ribs, and other bones may indicate loss of calcium from the bones due to osteoporosis or long-term steroid dependency. Careful examination of the ICSs and rib angles may indicate pathology. Patients with chronic obstructive pulmonary disease (COPD) have widened ICS and the angle of the ribs to the spine increases to 90° instead of the normal 45° angle because of severe hyperinflation (see Figure 10-3). Conversely, narrowed ICS may be visible in cystic fibrosis patients with severe interstitial fibrosis. Rib fractures, if present, are commonly visible along the lateral borders of the rib cage.
Elevation of the diaphragm can be a result of abdominal distention, phrenic nerve paralysis, or lung collapse. Depression or flattening of the diaphragm can occur when 11 or 12 ribs show on a chest x-ray as a result of COPD or severe hyperinflation due to asthma. Normal costophrenic angles can be seen where the tapered edges of the diaphragm and the chest wall meet. Because breast tissue can obscure the angles in women, these angles are more distinct in men. Obliteration or “blunting” of the costophrenic angle may occur with pleural effusion or atelectasis.
Identification of a pleural space on a chest x-ray is an abnormal finding (see Figure 10-4). The pleural space is not visible unless air (pneumothorax) or fluid (pleural effusion) enters it. These findings commonly are seen in the progressive care unit population.
Two terms often heard regarding the mediastinum are shifting and widening. Mediastinal structures, usually the trachea, bronchi, and heart, can shift with atelectasis, with the shift directed toward the alveolar collapse. Pneumothorax shifts the mediastinum away from the area of involvement. A widening of the mediastinum can indicate several pathologic conditions, such as cardiomegaly, aneurysms, or aortic disruption. Bleeding into the mediastinum, following chest trauma or cardiac surgery, also may cause widening of the mediastinum.
Heart size can be estimated easily by measuring the cardiothoracic ratio on a PA film. It is measured with a PA chest x-ray and is measured by comparing the ratio of the maximal horizontal cardiac diameter to the maximal horizontal thoracic diameter. A normal measurement is less than 50%. Greater percentages are indicative of cardiac enlargement. This method for determining normal heart size is not accurate using AP chest x-rays, the most common type taken of the acutely ill.
The lung fields should be assessed for any areas of increased density (whiteness) or increased radiolucency (blackness), which can indicate an abnormality. Density increases when water, pus, or blood accumulates in the lungs, as in pneumonia (Figure 10-5). Increased radiolucency is caused by increased air in the lungs, as may occur with COPD. A fine line present on the right side of the lung at the sixth rib level (midlung) is a normal finding, representing the horizontal fissure separating the right upper and middle lobes.
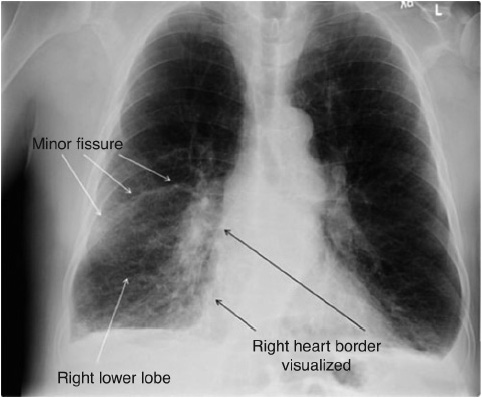
Figure 10-5. Right lower lobe pneumonia with minor fissure visualized. (Reprinted from: Siela D. Chest radiograph evaluation and interpretation. AACN Adv Crit Care. 2008;19:444-473.)
Chest x-rays are frequently obtained in progressive care to confirm proper emergent and nonemergent placement of invasive equipment (endotracheal tubes, central venous catheters including peripherally inserted central catheter [PICC] nasogastric or orogastric tubes, and chest tubes). All invasive tubes have radiopaque lines running the length of the tube that are visible on the x-ray (Figures 10-6 and 10-7).
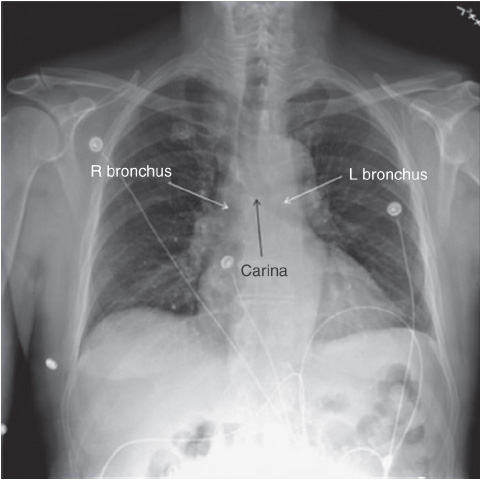
Figure 10-6. Carina and right bronchus. (Reprinted from: Siela D. Chest radiograph evaluation and interpretation. AACN Adv Crit Care. 2008;19:444-473.)
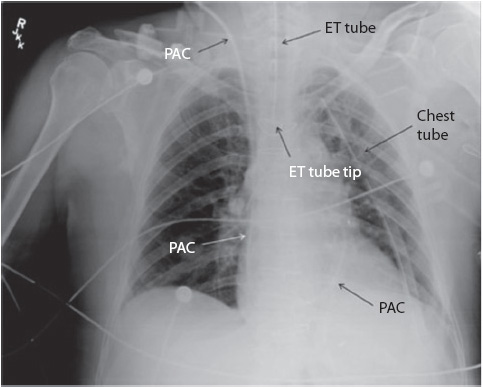
Figure 10-7. Pulmonary artery catheter, endotracheal tube, and left chest tube. (Reprinted from: Siela D. Chest radiograph evaluation and interpretation. AACN Adv Crit Care. 2008;19:444-473.)
All lines should be identified and followed through their paths. The nasogastric or orogastric tube should run the length of the esophagus with the tip of the tube beyond the gastroesophageal junction in the stomach. The stomach can be identified by the radiolucency just under the diaphragm on the left side, which is called the gastric air bubble. Small bore nasoenteric tubes may be positioned with the tip in the stomach or small bowel depending on whether gastric or small bowel feedings are intended. Central line catheters should be viewed with the tip in the superior vena cava.
All items in the chest should be identified, such as temporary or permanent pacing wires, pacing generators, automatic implantable defibrillators, airway stents, tracheostomy tubes, chest tubes, and surgical wires, drains, or clips (see Figure 10-7).
Chest x-rays should be taken after every attempt to insert central venous catheters to detect the presence of an accidental pneumothorax. A common error is to mistake the area above the clavicles as a pneumothorax, especially on AP views.
Two common abnormal x-ray signs frequently discussed are the silhouette sign and the air bronchogram. For any structure to be visible, the density of its edge must contrast with the surrounding density. The loss of contrast is called the silhouette sign. It means that two structures of the same density have come in contact with each other and the borders are lost; for example, the heart is a water density, so if the alveoli near the left heart border fill with fluid, the two densities are the same and there is a loss of contrast and no left heart border. An air bronchogram is air showing through a greater density, such as water (Figure 10-8). The bronchi are not seen on a normal chest x-ray, except for the main-stem bronchi, because they have thin walls, contain air, and are surrounded by air in the alveoli (two structures of the same density). If water surrounds the bronchi, as in pneumonia and pulmonary edema, then the bronchi filled with air are in contrast to the water density and are visible.
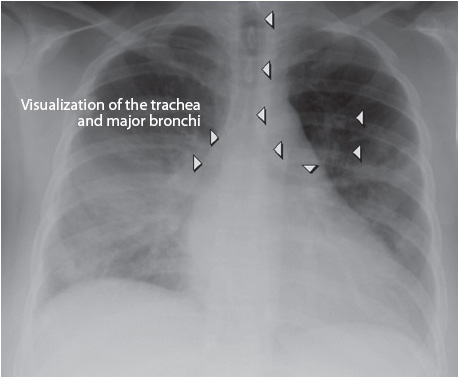
Figure 10-8. Air Bronchogram. (Courtesy of: Yale School of Medicine–http://www.yale.edu/imaging/findings/air_bronchogram/index.html. Accessed September 1, 2013.)
Computed tomography (CT) and magnetic resonance imaging (MRI) allow for the three-dimensional examination of the chest in situations where two-dimensional chest x-rays are insufficient. CT and MRI are particularly advantageous over chest x-rays to evaluate mediastinal and pleural abnormalities, particularly those with fluid collections. Pleural effusions or empyemas, malpositioned or occluded chest tubes, mediastinal hematomas, and mediastinitis are problems for which CT and MRI are more sensitive than chest x-rays.
The need for transportation to the radiology department and positioning restrictions within the scanning devices pose certain risks to acutely ill patients. Of particular concern is the automatic movement of patients during the procedure into and out of the scanning device. Accidental disconnection of invasive devices can easily occur if additional tubing lengths and potential obstructions are not considered. Decreased visualization of patients during the procedures requires vigilant monitoring of cardiovascular and respiratory parameters and devices, as well as establishing a method for conscious patients to alert nearby clinicians in case of difficulties. The strong magnetic field of MRI units may interfere with ventilator performance and a non-magnetic ventilator is required.
Magnetic resonance imaging testing can be a frightening experience for the patient. Anxiety-related reactions, occurring in up to almost one-third of patients, range from mild apprehension to severe anxiety. These reactions can result in cancellation of the test or interference with its results. It is suggested that all patients receive basic information regarding the MRI procedure, including details of the small chamber they will be placed in, the noise and temperature they will experience, and the duration of the procedure. If possible, use of some form of relaxation or music tapes, ear plugs or headsets, and the presence of a family member or friend should be considered. In addition, short-acting anxiolytics should be used for patients who need them.
Pulmonary angiograms are one of the most sensitive tools for diagnosis of pulmonary emboli. A catheter is advanced into the pulmonary artery and contrast material is injected during rapid filming. Emboli appear as filling defects, or dark circumscribed areas, within the white vascular images of the artery.
The invasive nature of this diagnostic test, coupled with potential reactions to the contrast material, restricts its use. As a result the use of computed tomography of the pulmonary arteries (CTPA) is quickly replacing pulmonary angiograms as the gold standard for detecting PE.
Computed tomography of the pulmonary arteries is a less invasive but very specific method of diagnosing a PE. The CTPA only requires a peripheral line through which to inject the contrast material. Similar to the pulmonary angiogram, defects may be readily seen in the pulmonary artery and the study can be done very quickly. This technology is not available in all institutions; however, it is quickly emerging as the diagnostic choice and gold standard for PE detection.
Some still use ventilation-perfusion (V/Q) scans to diagnose a PE although they are also being outdated by the CTPA. A V/Q scan is a nuclear medicine diagnostic tool that requires that medical isotopes are inhaled or injected in order to view the lungs and pulmonary arteries respectively. Generally the perfusion (or blood circulation) part of the test is done first. If there is no defect detected, the scan is read as “low probability.” If the scan detects a defect, then the inhaled (ventilation) portion of the test is done. If no matching defect is seen in the lung, the test is interpreted as “high probability.” But if a “matched defect” is noted (ie, there is a defect in the lung scan that corresponds with that of the perfusion scan), then the interpretation is “indeterminate” or “matched defect.” This may be the result of an atelectasis, pneumonia, or other infiltrate where circulation to that inactive area of the lung is redistributed to other active areas thus resulting in a “matched defect.” In addition to the cumbersome nature of the V/Q scan, the progressive care patient may require both tests (perfusion and ventilation) rather than just one, and the diagnostic yield is often poor.
Chest tubes are commonly used in acutely ill patients to drain air, blood, or fluid from the pleural spaces (pleural chest tubes) or from the mediastinum (mediastinal tubes). Indications for chest tube insertion are varied (Table 10-4), with no contraindications to chest tube insertion because the need to restore lung function supersedes any potential complications associated with insertion. Pleural tube insertion sites vary based on the type of drainage to be removed (air: second ICS, midclavicular line; fluid: fifth or sixth ICS, midaxillary line). Mediastinal tubes are placed during surgery, exiting from the mediastinum below the xiphoid process. Type of chest tube insertions include tube thoracostomy (traditional rigid tubes) or smaller percutaneously inserted catheters (pigtails).
TABLE 10-4. INDICATIONS FOR CHEST TUBE INSERTION

Following insertion, chest tubes are connected to a closed drainage collection system which uses gravity or suction to restore negative pressure in the pleural space and facilitate drainage of fluids or air (Figure 10-9). A Heimlich flutter valve is an alternative to the closed drainage system and consists of a one-way valve that allows air or drainage to collect in a vented drain bag (Figure 10-10). The PleurX® catheter also has a one-way valve and connects as needed to a drainage system (Figure 10-11). Patients may be discharged home with either a Heimlich flutter valve or PleurX catheter for long-term use. Connections to the drainage system must be airtight and secure for proper functioning and to prevent inadvertent entry of air into the pleural space (Figure 10-12). Patency of the system is ensured by avoiding kinking of the drainage tubing, periodic inspection of the tubing for visible clot formation, and gentle squeezing of the tubing between the thumb and index finger.
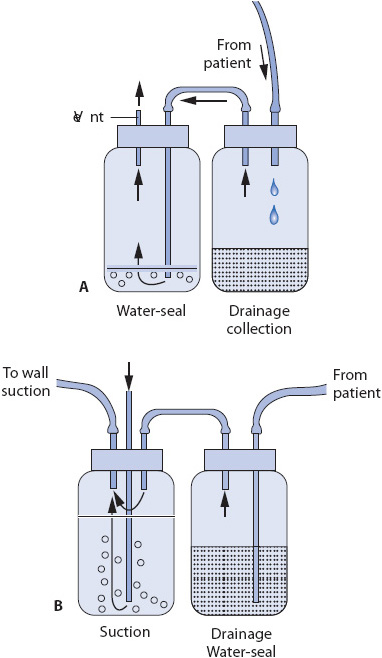
Figure 10-9. Two-bottle chest drainage system. (A) Drainage collection bottle and a water-seal bottle. (B) Water-seal/drainage collection bottle and suction control bottle. (Reprinted from: Luce JM, Tyler ML, Peirson DJ. Intensive Respiratory Care. Philadelphia, PA: WB Saunders; 1984:164, with permission from Elsevier.)
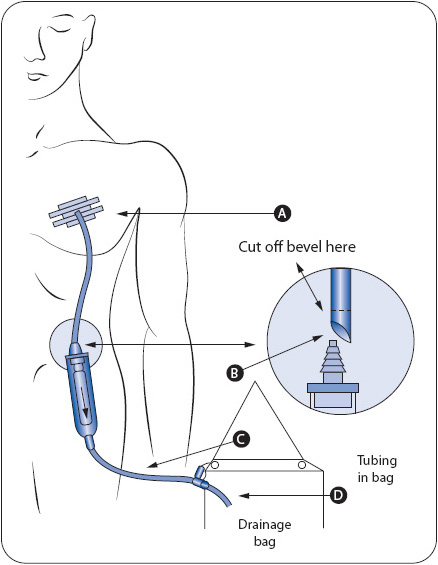
Figure 10-10. Heimlich chest drain valve with connection to drain bag. (From: BD Medical Systems, Franklin Lakes, NJ.)
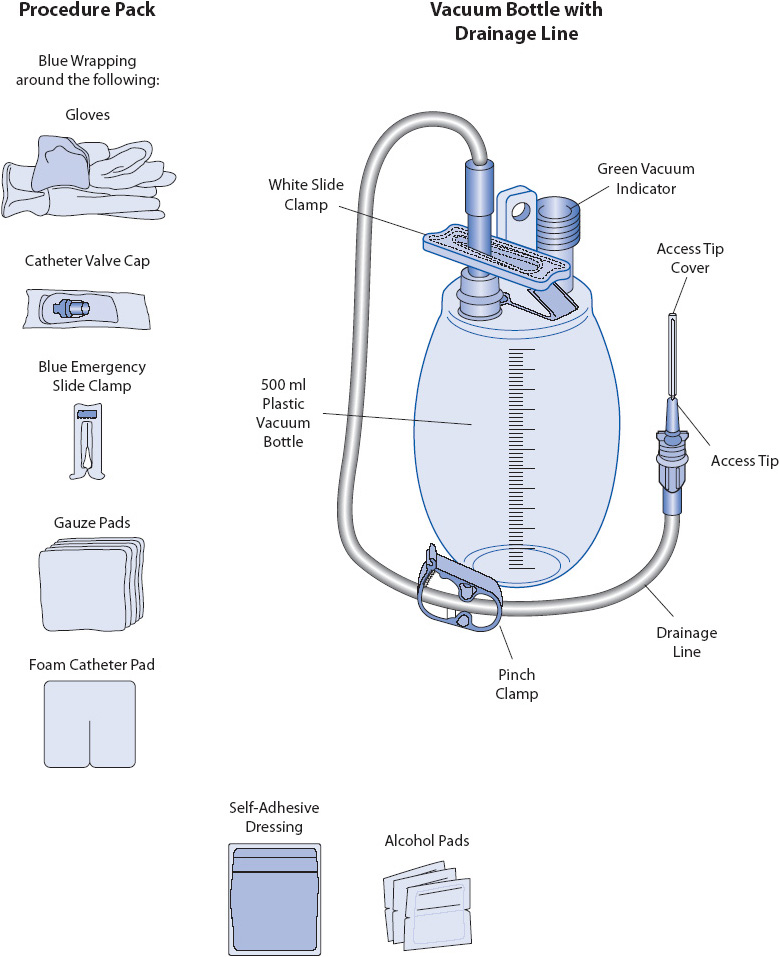
Figure 10-11. Components of a PleurX® drainage kit. (From: Elsevier Baker EM, Melander S. Management of recurrent pleural effusions with a tunneled catheter. Heart Lung. 2010;39:314-318.)
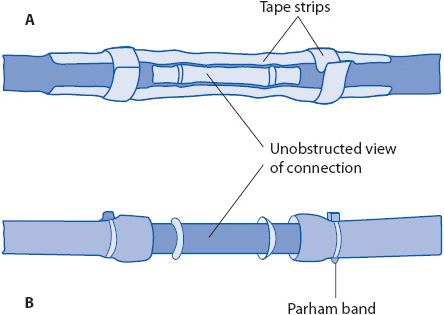
Figure 10-12. Methods for securing connections of chest tube and drainage system. (A) Tape. (B) Parham bands. (Reprinted from: Kersten LD. Comprehensive Respiratory Nursing: A Decision-Making Approach. Philadelphia, PA: WB Saunders; 1989:783, with permission from Elsevier.)
Removal of the chest tube occurs when restoration of lung expansion and fluid or air removal has been accomplished and the underlying lung abnormality has been resolved or corrected. An occlusive dressing at the chest tube removal site is typically used to prevent introduction of air into the pleural space until the skin has formed a protective seal. Analgesic administration is appropriate prior to removal; discomfort associated with removal is often as much or even greater than during insertion.
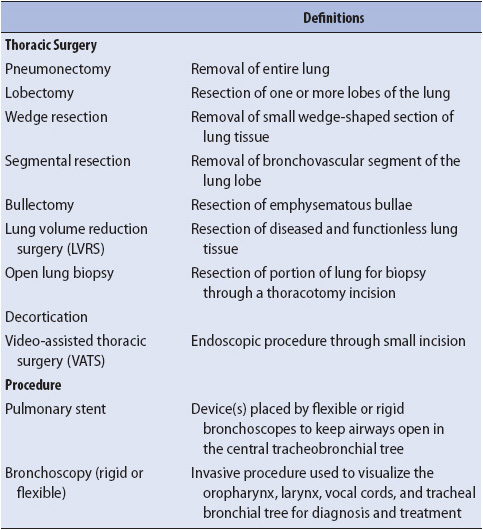
Thoracic surgery and procedures are terms inclusive of a number of procedures involving the thoracic cavity and the lungs. See Table 10-5 for definitions and indications.
TABLE 10-5. THORACIC SURGERY AND PROCEDURES
Management of the patient after lung surgery or post procedure is similar to the patient with trauma to the chest. Refer to the section on thoracic trauma in Chapter 17, Trauma, with the following additions:
The thoracotomy incision is one of the most painful surgical incisions and pain control is an important factor in recovery and prevention of respiratory complications. The routine use of epidural catheters, intercostal blocks, intrapleural local anesthetic administration, or PCA narcotics has improved pain management significantly. Relaxation therapy, deep breathing exercises, and guided imagery may also be effective in helping to reduce pain and anxiety.
• It was once thought that optimal patient ventilation and perfusion matching would be improved and should be prioritized with “good lung” positioned in the dependent position. While blood flow is improved to the dependent lung, patients require frequent repositioning side to side to prevent atelectasis and other complications.
• Early ambulation and sitting at the bedside or in a chair improves diaphragmatic excursion, enhancing ventilation and maximum inflation.
• Deep breathing and use of incentive spirometry is encouraged regularly. These activities both help promote lung reexpansion of collapsed lung tissue and prevent atelectasis.
See section “Chest Tubes” explained earlier.
Each of the case studies below represents a common situation in a progressive care unit—respiratory dysfunction. This rapid onset of respiratory impairment, which is severe enough to cause potential or actual morbidity or mortality if untreated, is termed acute respiratory failure (ARF). Although, the origin of the respiratory failure may be a medical or surgical problem, the management approaches share similar features.
Acute respiratory failure is a change in respiratory gas exchange (CO2 and O2) such that normal cellular function is jeopardized. ARF is defined as a PaO2 less than 60 mm Hg and PaCO2 greater than 50 mm Hg with a pH less than or equal to 7.30. Actual PaO2 and PaCO2 values that define ARF vary, depending on a variety of factors that influence the patient’s normal (or baseline) arterial blood gas values. Factors such as age, altitude, chronic cardiopulmonary disease, or metabolic disturbances may alter the normal blood gas values for an individual, requiring an adjustment to the classic definition of ARF; for example, if PaCO2 levels in a 75-year-old man with COPD are normally 56 mm Hg, ARF would not be diagnosed until pH is less than or equal to 7.30.
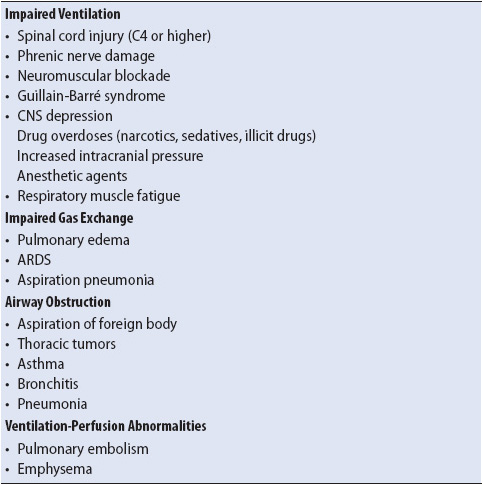
Many abnormalities can lead to ARF (Table 10-6). Regardless of the specific underlying cause, the pathophysiology of ARF can be organized into four main components: impaired ventilation, impaired gas exchange, airway obstruction, and ventilation-perfusion abnormalities.
TABLE 10-6. CAUSES OF ACUTE RESPIRATORY FAILURE IN ADULT
Conditions that disrupt the muscles of respiration or their neurologic control can impair ventilation and lead to ARF (see Table 10-6). Decreased or absent respiratory muscle movement may be due to fatigue from excessive use, atrophy from disuse, inflammation of nerves, nerve damage (eg, surgical damage to the vagus nerve during cardiac surgery), neurologic depression, or progressive disease states such as Guillian Barre or amyotrophic lateral sclerosis (ALS). Impaired respiratory muscle movement decreases movement of gas into the lungs, resulting in alveolar hypoventilation. Inadequate alveolar ventilation causes retention of CO2 and hypoxemia.
Conditions that damage the alveolar-capillary membrane impair gas exchange. Direct damage to the cells lining the alveoli may be caused by inhalation of toxic substances (gases or gastric contents), pneumonia and/or other pulmonary conditions leading to two detrimental alveolar changes. The first is an increase in alveolar permeability, increasing the potential for interstitial fluid to leak into the alveoli and causing noncardiac pulmonary edema (Figure 10-13A). The second alveolar change is a decrease in surfactant production by alveolar type II cells, increasing alveolar surface tension, which leads to alveolar collapse (Figure 10-13B).
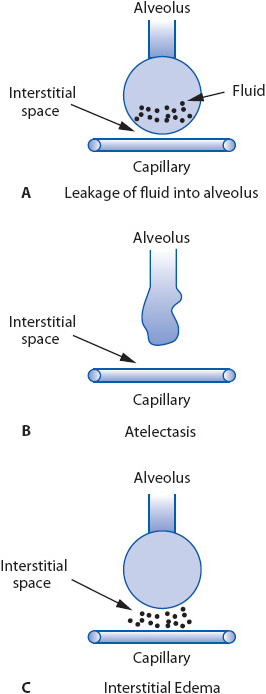
Figure 10-13. Pathophysiologic processes in ARF that impair gas exchange. (A) Increased alveolar membrane permeability. (B) Alveolar collapse from decreased surfactant production. (C) Increased capillary membrane permeability and interstitial edema.
Another cause of impaired gas exchange occurs when fluid leaks from the intravascular space into the pulmonary interstitial space (Figure 10-13C). The excess fluid increases the distance between the alveolus and the capillary, decreasing the efficiency of the gas exchange process. Interstitial edema also compresses the bronchial airways, which are surrounded by interstitial tissue, causing bronchoconstriction. Capillary leakage may occur when pressures within the cardiovascular system are excessively high (eg, in heart failure) or when pathologic conditions elsewhere in the body release biochemical substances (eg, serotonin, endotoxin) that increase capillary permeability.
Conditions that obstruct airways increase resistance to airflow into the lungs, causing alveolar hypoventilation and decreased gas exchange (Figure 10-14). Airway obstructions can result from conditions that: (1) block the inner airway lumen (eg, excessive secretions or fluid in the airways, inhaled foreign bodies), (2) increase airway wall thickness (eg, edema or fibrosis) or decrease airway circumference (eg, bronchoconstriction) as occurs in asthma, or (3) increase peribronchial compression of the airway (eg, enlarged lymph nodes, interstitial edema, tumors).
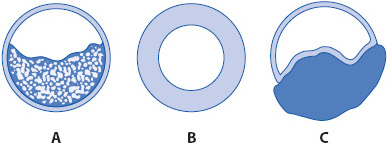
Figure 10-14. Mechanism of airway obstruction. (A) Fluid secretions present within airway. (B) Intraluminal edema narrowing airway diameter. (C) Peribronchial compression of airway.
Conditions disrupting alveolar ventilation or capillary perfusion lead to an imbalance in ventilation and perfusion. This decreases the efficiency of the respiratory gas exchange process (Figure 10-15A). In an effort to keep the ventilation and perfusion ratios balanced, two compensatory changes occur: (1) to avoid wasted alveolar ventilation when capillary perfusion is decreased (eg, with pulmonary embolism [PE]), alveolar collapse occurs to limit ventilation to alveoli with poor or absent capillary perfusion (Figure 10-15B); (2) to avoid capillary perfusion of alveoli that are not adequately ventilated (eg, with atelectasis), arteriole constriction (ie, hypoxic vasoconstriction) occurs and shunts blood away from hypoventilated alveoli to normally ventilated alveoli (Figure 10-15C). As the number of alveolar-capillary units affected by these compensatory changes increases, gas exchange eventually is affected negatively.
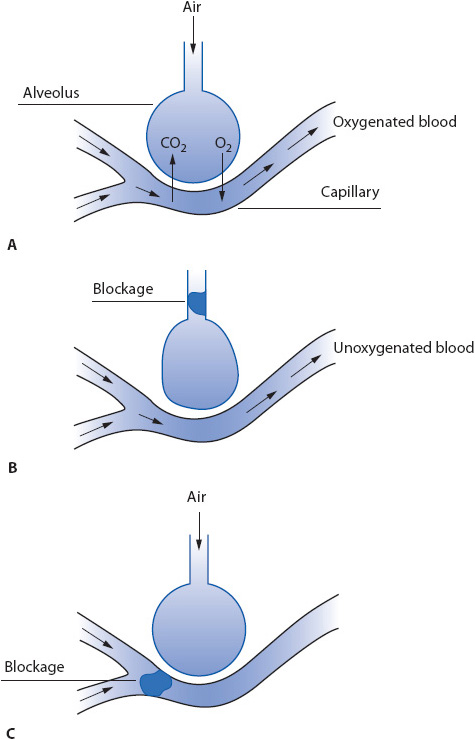
Figure 10-15. Pathophysiologic processes in ARF from ventilation-perfusion abnormalities. (A) Normal ventilation and perfusion relationship. (B) Decreased ventilation and normal perfusion. (C) Normal ventilation and decreased perfusion.
Each of these pathophysiologic changes results in inadequate CO2 removal, O2 absorption, or both. The severity of ARF can be further increased when anxiety and fear of impending death develop, a common consequence of severe dyspnea and hypoxemia. These symptoms increase oxygen demands and the work of breathing, further compromising O2 availability for crucial organ function and depleting respiratory muscle strength.
Signs and Symptoms
• Hypoxemia (PaO2 < 60 mm Hg)
• Restlessness
• Tachypnea
• Dyspnea
• Tachycardia
• Confusion
• Anxiety
• Hypercarbia (PaCO2 > 50 mm Hg)
• Hypertension
• Irritability
• Somnolence (late)
• Cyanosis (late)
• Loss of consciousness (late)
• Pallor or cyanosis of skin
• Use of accessory muscles of respiration
• Abnormal breath sounds (crackles, wheezes)
• Manifestations of primary disease (see description of individual diseases later on)
Diagnostic Tests
• Arterial blood gases—PaO2 less than 60 mm Hg and PaCO2 more than 50 mm Hg; with pH less than or equal to 7.30; or PaO2 and PaCO2 in abnormal range for that individual
• Tests specific to underlying cause (see description of individual diseases later on)
The management of the patient in ARF revolves around four primary areas: improving oxygenation and ventilation, treating the underlying disease state, reducing anxiety, and preventing and managing complications.
Most causes of ARF are treatable, with a return of normal respiratory function following resolution of the pathophysiologic condition. Aggressive support of respiratory function is required, though, until there is resolution of the underlying condition.
1. Provide supplemental O2 to maintain PaO2 greater than 60 mm Hg. The use of noninvasive methods for O2 administration (nasal cannula or face masks) is preferable if acceptable PaO2 levels can be achieved. Continued hypoxemia despite noninvasive O2 delivery methods necessitates intubation and mechanical ventilation and transfer to a critical care unit.
2. Improve ventilation with the administration of bronchodilators, mucolytic agents, and other airway management modalities (suctioning, positioning, mobilization) as indicated. The routine use of chest physiotherapy has not been shown to be supported by the literature and is not recommended.
3. Intubate and initiate mechanical ventilation if noninvasive methods fail to correct hypoxemia and hypercarbia, or if cardiovascular instability develops. The mode of mechanical ventilation, rate, and tidal volume vary, depending on the underlying cause of respiratory failure and a variety of clinical factors.
4. Depending on the cause of ARF, the patient’s response to treatment along with the institution’s resources (specialized respiratory care units), the patient may need to be moved into intensive care for noninvasive ventilation or intubation and mechanical ventilation.
5. If suctioning is required, closely observe for signs and symptoms of complications: oxygenation (SpO2), cardiac arrhythmias, respiratory distress, bronchospasm, increased respiratory rate, increased blood pressure or intracranial pressure, anxiety, pain, or change in mental status. Hyperoxygenate with 100% oxygen using a manual resuscitation bag (MRB) that delivers 100% O2 as well as a PEEP valve when ventilator PEEP levels are more than 5 cm H2O. Suctioning should only be performed when clinically indicated, and never on a routine schedule. The use of in-line suction catheters is encouraged as the catheters do not affect oxygenation as dramatically as complete disconnection, and they decrease the potential contamination of the clinician doing the suctioning.
6. Prior to intrahospital transport, verify adequacy of ventilatory support equipment to maintain cardiopulmonary stability. Verify that PEEP on the transport equipment is maintained.
Correction of the underlying cause of the ARF should be done as soon as possible. See specific management approaches later in the chapter for selected disease states.
Maintain a calm, supportive environment to avoid unnecessary escalation of anxiety. Give brief explanations of activities and approaches being done to relieve ARF. Vigilance and presence of healthcare providers during anxious periods is crucial to avoid panic by patients and visiting family members.
Teach diaphragmatic breathing to slow the rate and increase the depth of respirations. Place one hand on the patient’s abdomen. Instruct the patient to inhale deeply, causing the hand on the abdomen to rise. During exhalation, have the patient feel the hand on the belly sink down toward the spine. Explain that the chest should move minimally. After a minute or two, ask the patient to place his or her hands on the belly to continue the exercise.
If necessary, administer mild doses of anxiolytics (ie, lorazepam or diazepam) that do not depress respiration.
See Chapter 5, Airway and Ventilatory Management, for additional strategies, Chapter 11, Multisystem Problems for preventing selected hospital acquired conditions, and Chapter 14, Gastrointestinal System for GI prophylaxis.
The case study of the patient in a motor vehicle accident is typical of a patient who develops acute respiratory distress syndrome (ARDS). ARDS has a very high morbidity and mortality. It is characterized by non-cardiac pulmonary edema caused by increased alveolar capillary membrane permeability. ARDS affects both lungs and hypoxemia refractory to oxygenation is a hallmark of the condition. ARDS was previously described as the most severe presentation of acute lung injury (ALI) but recently the term ALI has been eliminated in favor of the labels “mild,” “moderate,” and “severe” ARDS. The definition is called the “Berlin Definition of ARDS” and consists of categories that identify the timing of the condition, chest imaging criteria, origin of lung edema, and oxygenation status. The severity stratification of mild, moderate, and severe is based on the PaO2/FiO2 score and PEEP level. The PaO2/FiO2 (also called P/F ratio) ratio is calculated by dividing the PaO2 by the FiO2 (with a decimal; 50% = 0.5).
Risk factors for the development of ARDS can be categorized into conditions that lead to direct damage to the alveolar-capillary membrane (primary causes) and those that are thought to be mediated by cellular or humoral injury to the capillary endothelial wall (secondary causes) (Table 10-7). Whether primary or secondary causes, the pathologic processes involved in ARDS are characterized by excessive alveolar–capillary membrane permeability, interstitial edema, and diffuse alveolar injury (see Figure 10-13). Direct damage to the alveolar membrane can easily occur when toxic substances are inhaled, such as during fires or chemical spills.
TABLE 10-7. PRIMARY AND SECONDARY CAUSES OF ARDS
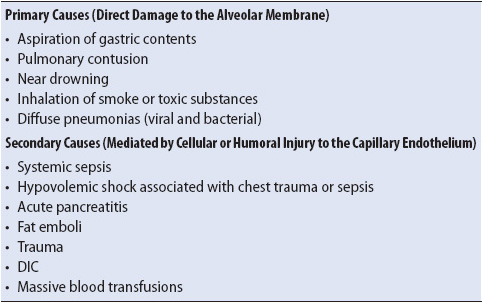
Alveolar and interstitial edema, microatelectasis, and ventilation-perfusion mismatching in ARDS lead to severe hypoxemia and poor lung compliance (“stiff lungs”). In the setting of trauma and sepsis, this abnormality in microvascular permeability occurs in capillary beds throughout the body. Typically, this multisystem organ dysfunction is not clinically apparent, with clinical manifestations isolated to the respiratory system. When multiple organ dysfunction syndrome does occur, it is seen in ARDS patients who develop bacterial infections and sepsis (see Chapter 11, Multisystem Problems).
The ARDS process disrupts normal macrophage function and increases the risk of infection. Mortality and long-term disability from ARDS is high.
Signs and Symptoms
• Dyspnea
• Tachypnea (rates often > 40 breaths/min)
• Intercostal retractions
• Copious secretions
• Panic, fear of impending death
• Crackles and/or wheezes
Diagnostic Tests
• Chest x-ray shows diffuse, bilateral pulmonary infiltrates without increased cardiac size
• PaO2/FiO2 less than or equal to 300 mm Hg
Much of the management of ARDS relies on supportive care and the prevention of complications. To date, interventions to limit the disease progression or reverse the underlying structural defects are not known.
Interventions specific to ARDS to improve oxygenation and ventilation include the following:
1. Administer high FiO2 levels with high-flow system or rebreathing mask. A constant positive airway pressure (CPAP) mask may be tolerated in alert, cooperative patients. Continuous, vigilant monitoring for contraindications of noninvasive CPAP (decreased loss of consciousness, nausea/vomiting, increased dyspnea, or panic) is imperative.
2. Intubation, mechanical ventilation, and transfer to intensive care if cardiovascular instability is present, severe hypoxemia persists, or if fatigue develops. The majority of these patients will need transfer to the ICU for additional management.
Same as previously described for ARF management.
Refer Chapter 5, Airway and Ventilatory Management, for detailed discussion of communication techniques for intubated patients.
1. Minimize cardiovascular instability by careful monitoring; administer fluids to correct hypovolemia.
2. Vasoactive drugs may be required to maintain adequate perfusion.
Individuals with COPD are at high risk for exacerbations and the development of ARF due to progressive airflow limitation with chronic inflammatory airway and lung response. Acute asthma will be discussed in the next section. Altered host defenses, increased secretion volume and viscosity, impaired secretion clearance and airway changes, and common pathophysiologic changes predispose the patient with COPD to acute exacerbations or episodes of ARF requiring hospitalizations.
Any systemic or pulmonary illness can precipitate exacerbations and the development of ARF in patients with COPD. In addition to the etiologies of ARF listed in Table 10-6, diseases or situations that decrease ventilatory drive, muscle strength, chest wall elasticity, or gas exchange capacity, or increase airway resistance or metabolic oxygen requirements can easily lead to ARF in patients with COPD (Table 10-8). The most common precipitating events include:
• Airway infection (pneumonia, bronchitis): Frequent antibiotic administration, hospitalization, and impaired cough and host defenses in COPD increase acute airway infections. Infections are commonly caused by gram-negative enteric bacteria or Legionella, with Haemophilus influenzae and Streptococcus pneumoniae causing acute bronchitis. Moraxella catarrhalis is also a common respiratory organism causing infection in these patients.
• Pulmonary embolus: The high incidence of right ventricular failure in COPD increases the risk of pulmonary embolus from right ventricular mural thrombi.
• Heart failure: In the presence of pulmonary hypertension and right-sided heart failure, treatment of left-sided heart failure is often delayed due to difficulties in early diagnosis.
• Nonadherance to medication regime: The complicated treatment regime for management of COPD, which includes frequent administration of both oral and inhaled agents, frequently leads to underuse of medications.
TABLE 10-8. PRECIPITATING EVENTS OF ACUTE RESPIRATORY FAILURE IN COPD

The development of ARF in COPD patients places a tremendous burden on the pulmonary system. The chronic disease process leads to impairment of ventilation, poor gas exchange, and airway obstruction. The additional burden of an acute disease process, even a relatively minor one, further impairs ventilation and gas exchange and increases airway obstruction. Compensatory mechanisms can easily be overwhelmed, with lethal consequences.
Signs and symptoms are similar to ARF, but usually more pronounced.
Diagnostic Tests
• Chest x-ray: Evidence of COPD (flat diaphragms, hyperinflation of air fields), in addition to x-ray findings specific to the cause of the ARF (see Figure 10-3).
• Arterial blood gases: PaCO2 greater than 50 mm Hg and higher than baseline levels during stable, chronic disease periods.
The presence of chronic respiratory dysfunction and an acute respiratory problem leads to some changes in the typical management of ARF.
Treatment is directed at both the acute precipitating event and the chronic airflow obstruction problems associated with COPD.
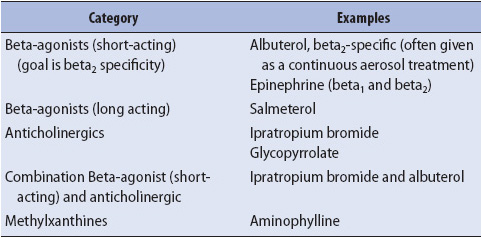
1. Increase airway diameter with bronchodilators and reduce airway edema with corticosteroids. Beta-adrenergic and anticholinergic agents are often used concurrently (Table 10-9). Higher than usual doses may be necessary until the precipitating event is resolved. Systemic corticosteroids are used to decrease airway inflammation and thus bronchospasm, and may enhance secretion clearance as well.
2. Treat pulmonary infections with appropriate antibiotics.
3. Improve secretion removal. Strategies to improve secretion removal include adequate hydration, patient mobilization, coughing, and heated moist aerosolization. The routine use of chest physiotherapy has not been shown to be supported by the literature and is not recommended. Secretions may be thick and tenacious in asthma patients. Monitor response to these therapies and discontinue them if no additional benefits are observed.
TABLE 10-9. BRONCHODILATOR CATEGORIES
Correction of hypoxemia (saturation < 90%) is done by small increases in FiO2 levels, preferably with a controlled O2 delivery device such as a Venturi mask, biphasic intermittent positive airway pressure (BiPAP), or CPAP. Frequent monitoring of arterial blood gases is essential to ensure adequate arterial oxygenation (PaO2 of 55-60 mm Hg or baseline values during nonacute situations) without significantly increasing PaCO2 levels. The administration of oxygen to COPD patients was once felt to eliminate the “hypoxic drive,” putting the patient at risk for hypercarbia, acidosis, and death. This drive is responsible for only approximately 10% of the total drive to breathe. Oxygen should never be withheld and is essential to prevent further deleterious effects of hypoxia and potential organ failure. While it is correct that higher than necessary FiO2 levels may increase PaCO2, this effect occurs by three physiologic mechanisms:
1. The Haldane effect: As hemoglobin becomes desaturated with oxygen, the affinity for carbon dioxide increases. The administration of oxygen then displaces carbon dioxide on hemoglobin and increases carbon dioxide levels in the plasma. Patients with COPD are unable to increase minute ventilation or “blow off” carbon dioxide. This leads to an increase in carbon dioxide, lowering the pH and resulting in a respiratory acidosis.
2. Hypoxic vasoconstriction: This physiologic adaptive mechanism is a response to a decrease in alveolar oxygen and moves capillary blood flow from a closed or atelectatic alveolus to an open alveolus. In patients with COPD, this adaptive mechanism no longer occurs. As a result, dead space ventilation or decreased perfusion (see Figure 10-15C) occurs with resulting increased carbon dioxide levels.
3. Decreased minute ventilation: As a result of increased dead space ventilation with resulting increased carbon dioxide, some COPD patients will decrease their minute ventilation. This decrease will further limit the patient’s inspiratory reserve capacity.
Oxygen administration in COPD patients is necessary to prevent hypoxia and organ failure and should never be withheld. Titration and considerations for mechanical ventilation in the COPD patient with CO2 retention (PaCO2 > 50 mm Hg) should be guided by the pH and PaO2. These include (Figure 10-16):
• pH less than 7.35 with PaO2 greater than 60 mm Hg: consider noninvasive mechanical ventilation or intubation.
• pH greater than 7.35 with PaO2 less than 60 mm Hg: increase oxygen to increase PaO2 to greater than 60 mm Hg. Reassess ABG.
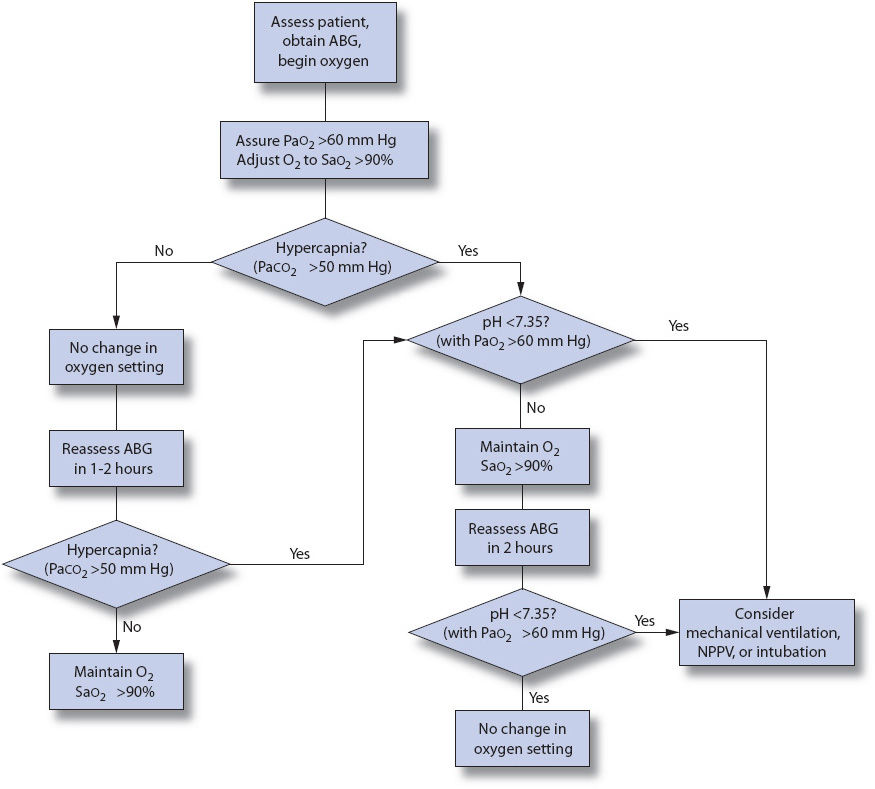
Figure 10-16. Algorithm to correct hypoxemia in an acutely ill COPD patient. ABG: arterial blood gas; NPPV: noninvasive positive pressure ventilation; O2: oxygen; PaCO2: arterial carbon dioxide tension; PaO2: arterial oxygen tension; SaO2: arterial oxygen saturation. (From: American Thoracic Society and European Respiratory Society. Standards for the diagnosis and management of patients with COPD. 2004;183. Available: http://www.thoracic.org/sections/copd/resources. Accessed January 23, 2014.)
Position the patient to maximize ventilatory efforts and relaxation/rest during spontaneous breathing. A high Fowler position and leaning on an overbed table may be a position of comfort.
Relaxation techniques and diaphragmatic, pursed lip breathing may be helpful to decrease anxiety and improve ventilatory patterns. Anxiolytics and other sedatives should be used cautiously to avoid decreasing minute ventilation. COPD patients with ARF may benefit from early use of non-invasive mechanical ventilation.
The decision to intubate and mechanically ventilate the patient is based primarily on the deterioration of mental status, coupled with knowledge of the patient’s baseline pulmonary function and functional status, and the reversibility of the underlying cause. Weaning from mechanical ventilation is frequently more difficult, and in some cases not possible, in the presence of COPD. Informed discussions with the patient and family regarding intubation options should be undertaken. The presence of an advanced directive and power of attorney designee for healthcare decisions can help in guiding clinician’s actions when patients are unable to make treatment decisions themselves (see Chapter 8, Ethical and Legal Considerations).
Typically, patients with COPD have protein-calorie malnutrition, as well as low levels of phosphate, magnesium, and calcium. These chronic nutritional deficits lead to muscle weakness and may interfere with the weaning process if mechanical ventilation becomes necessary. Early enteral feeding (oral or by feeding tube) of these patients is essential to avoid further deterioration in their nutritional status during acute illness and should be initiated as soon as hemodynamically stable. Enteral feeding is preferred over parenteral nutrition due to decreased risk of infectious complications. COPD patients who are malnourished have greater air trapping, lower diffusing capacity, and are less able to mobilize (see Chapter 14, Gastrointestinal System). If used, non-invasive positive pressure ventilation makes oral feeding difficult and the insertion of a small bore nasoenteric tube may be necessary.
In addition to the complications associated with ARF, the following complications are commonly observed in COPD exacerbation patients with ARF:
• Arrhythmia: High incidence of both atrial and ventricular arrhythmia in patients with COPD due to hypoxemia, acidosis, heart disease, medications, and electrolyte abnormalities. Cardiac monitoring and correction of the underlying cause is the goal, with pharmacologic treatment of arrhythmia only for life-threatening situations.
• Pulmonary embolus: High incidence. Observe for signs and symptoms and follow the usual treatment and prevention guidelines.
• GI distention and ileus: Aerophagia is common in dyspneic patients, increasing the incidence of this complication.
• If ventilated (auto-PEEP and barotrauma): High incidence, especially in the elderly and in individuals with high ventilation needs.
• Smoking cessation continues to be the single most-effective way to stop the progression of COPD (Table 10-10).
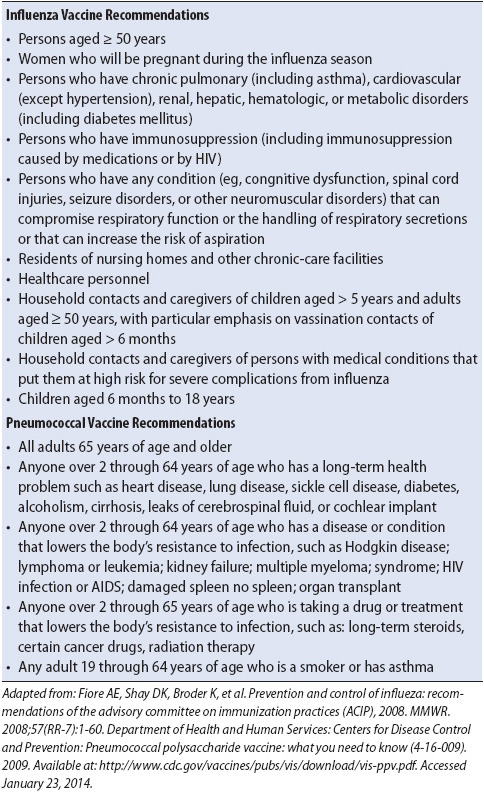
• Immunizations to prevent pneumococcal pneumonia (year round) and influenza (during flu season) remain important preventive measures (Table 10-11).
TABLE 10-10. HELPING SMOKERS QUIT: A GUIDE FOR NURSES

TABLE 10-11. INFLUENZA AND PNEUMOCOCCAL VACCINE RECOMMENDATIONS
Individuals with asthma are at risk for exacerbations that are characterized by a progressive increase in shortness of breath, cough, wheezing, or decrease in expiratory airflow. Acute/severe asthma, status asthmaticus, and asthma attack are also terms that have been used to describe this condition. Asthma differs from COPD in both pathophysiology and therapeutic response and the airway restriction is usually reversible with aggressive treatment (Figure 10-17).
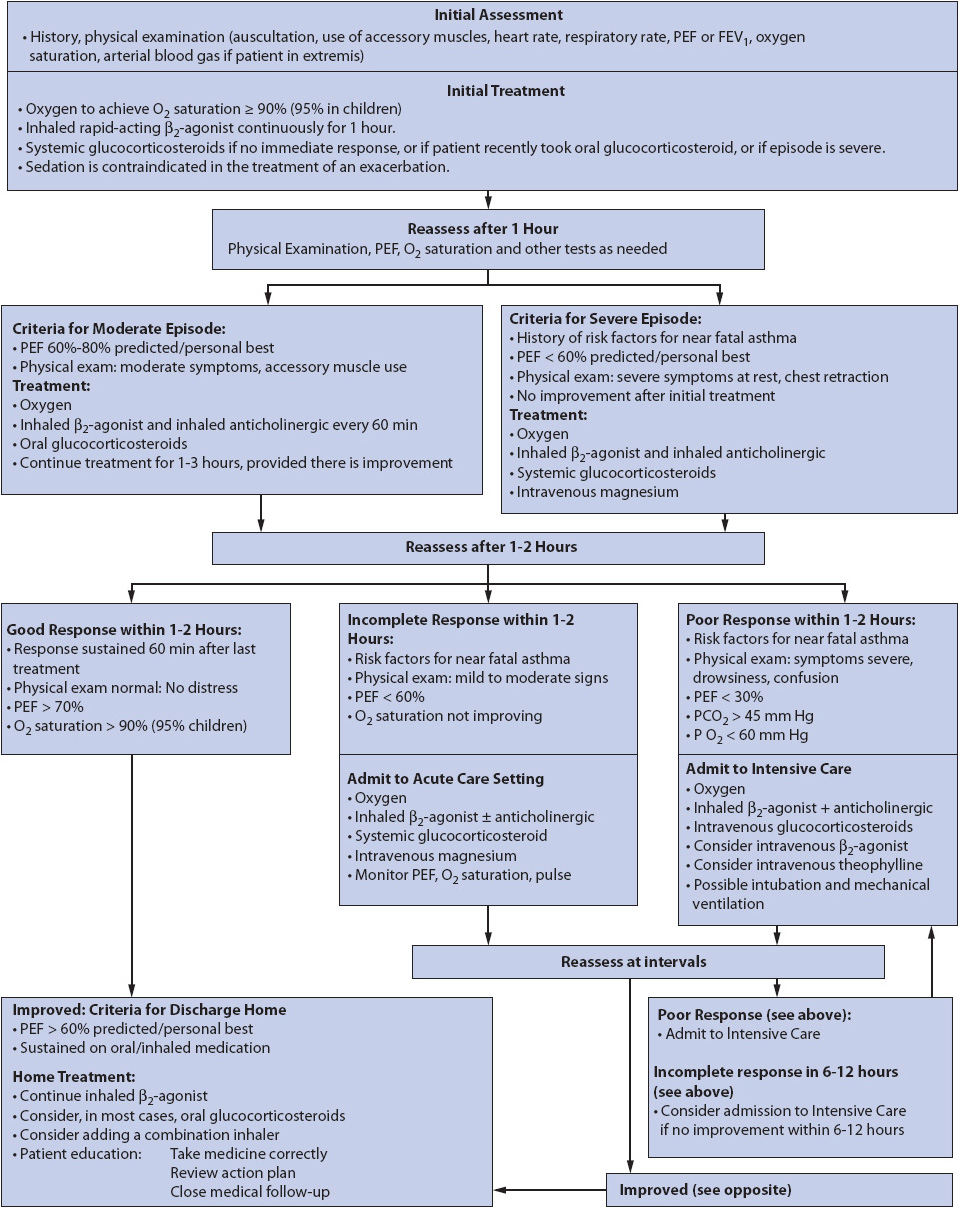
Figure 10-17. Management of asthma exacerbations in acute care setting. From National Heart, Lung, and Blood Institute. Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. 2013. Available at: http://www.ginasthma.org/uploads/users/files/GINA_Report_2012Feb13.pdf. Accessed September 1, 2013.
Asthma exacerbations are first and foremost due to uncontrolled airway inflammation. The pathology results in the severe bronchospasm and increased mucus production present during asthma “attacks,” both of which contribute to the overall airway obstruction. Triggers vary and include infection, inhaled seasonal antigens, foods, exercise, or medications to name just a few. While triggers may stimulate an exacerbation of asthma, they are not causal.
Bronchoconstriction results from mediator release from mast cells and include histamine, prostaglandins, and leukotrienes that contract the smooth muscle. Mucus plugging is thought to be because of eosinophil and shed bronchial epithelial cells as well as impaired mucus transport. Additionally, over time some patients may exhibit airway remodeling (thickening that contributes to airflow narrowing and airflow obstruction) especially if their airway inflammation is not controlled. All of these contribute to the severe and often unrelenting nature of the asthma “attack.”
Some risk factors for the development of an acute severe asthma episode include frequent need for use of their “rescue” inhalers, recent illness, frequent past emergency room visits or hospitalizations, prior intubations and ICU admissions, noncompliance with medical therapy, and inadequate access to healthcare.
Clinical findings are related to severe airflow obstruction and may include the inability to say a whole sentence, shortness of breath, wheezing, pulsus paradoxus, use of accessory muscles of inspiration, diaphoresis, and need to maintain upright position. However, peak flow measurement is one of the best assessment tools for determining the severity of the exacerbation. An absolute peak flow measurement of < 100L/min in an adult generally indicates severe bronchoconstriction, especially in combination with failure to respond to aggressive bronchodilator treatments. These patients are generally admitted to a critical care unit or progressive care unit for close monitoring and aggressive therapy (see Figure 10-17).
• Arterial blood gases: Initial findings may show pH greater than 7.45, PaCO2 less than 35 mm Hg, and mild to moderate hypoxia (respiratory alkalosis). In severe airflow obstruction, findings may progress to pH less than 7.35 and PaCO2 greater than 50 mm Hg (metabolic acidosis).
• Pulsus paradoxus: A decrease of greater than 10 mm Hg in systolic blood pressure during inspiration.
• Pulmonary function tests: FEV1 of less than 20% or peak expiratory flow rate (PEFR) of less than 40% of predicted despite aggressive bronchodilator therapy.
• Spo2: Observe for hypoxia. The SpO2 should be greater than 92%.
Treatment is directed at decreasing airway inflammation, reversal of airflow obstruction, and correction of hypercapnia or hypoxemia if present.
1. Reduce airway inflammation with systemic corticosteroids and provide aggressive bronchodilation. Beta-2 specific bronchodilators (eg, albuterol) are the drug of choice and may be provided continuously by nebulizer through a mouthpiece, mask or if ventilated, through the ventilator circuit. Concomitant use of anticholinergic bronchodilators is generally provided to enhance rapid reversal of bronchospasm. If bronchospasm is refractory to aggressive pharmacologic management (beta-2 specific drugs and anticholinergics), then subcutaneous epinephrine may be used. However, epinephrine should be avoided in adults except in extreme cases as it may precipitate heart attacks, especially in those with pre-existing cardiac disease. The use of magnesium sulfate is not supported in the literature although it is still sometimes used in patients with acute severe asthma.
2. Treat pulmonary infections with appropriate antibiotics.
3. Improve secretion removal. Generally secretions will be easier to mobilize as bronchodilation is enhanced. Until then, strategies are limited. Adequate hydration (generally provided parenterally) is important as the asthma patient is often dehydrated.
4. Once improved, treatment is directed at long-term “control” of the disease.
Education during recovery is crucial to prevent future exacerbations and should focus on self-management techniques such as regular use of their “controller” medications to decrease inflammation, “rescue’ inhalers for bronchodilation, identification and avoidance of “triggers,” and smoking cessation (see Table 10-10). Additionally, provision of pneumococcal and influenza (seasonal) vaccines (see Table 10-11) should be done prior to discharge if they have not already received the vaccines.
Severe hypoxemia should be corrected by providing high FiO2 levels until an adequate oxygen saturation is obtained (90% or greater). Oxygen masks and high flow O2 systems may be used to deliver oxygen. Mechanical ventilation may be necessary if the patient does not respond to more conservative methods. The use of non-invasive ventilation in an asthmatic is discouraged as it may lead to increased hyperinflation and respiratory failure.
Frequent monitoring of arterial blood gases is essential to monitor pH and PaCO2. Helium-oxygen (heliox) mixtures may be used to decrease work of breathing and improve ventilation. Heliox can be administered via mask, or invasive ventilation. Due to the high levels of helium in the heliox mixtures, the use of heliox may be limited in patients with high FiO2 requirements. Position the patient to maximize ventilatory efforts and relaxation/rest during spontaneous breathing. Relaxation techniques may be helpful to decrease anxiety and improve ventilatory patterns. Anxiolytics and other sedatives should not be given unless the patient is intubated. Studies have demonstrated that doing so increases the potential for death. The decision to intubate and mechanically ventilate the patient may be made urgently in patients who are failing to respond to treatment and are fatiguing. If intubation is done the patient should be transferred to a critical care unit for management.
Interstitial lung disease (ILD) is a broad category of over 130 lung disorders that are characterized by fibrosis and/or inflammation of the lungs.
The lung tissue or interstitium is damaged by a known or unknown causes leading to inflammation. The interstitium may include the alveolar space, small airways, blood vessels, and/or the pleura. Fibrosis and scarring then occur with resulting hypoxemia and “stiff lungs.”
Some known causes include:
• Occupational and environmental exposure to irritants, asbestos, silica.
• Infections, tuberculosis.
• Medications, amiodarone, chemotherapy agents.
• Connective tissue or collagen disorders, rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosis.
• Genetic/familial.
Unknown causes are classified as idiopathic pulmonary fibrosis.
Signs and symptoms
• Dyspnea
• Nonproductive cough
• Clubbing
• Lung auscultation fine crackles
• Signs of right-sided heart failure
Diagnostic Tests
• Chest x-ray: May be normal or show lung volume loss
• CT chest: Classic description of honeycombing or “ground glass”
• Lung biopsy: To determine pathogenesis
Treatment is same as previously described for ARF management along with the addition of therapy directed toward the cause of the ILD and may include the following:
• Corticosteroids
• Cytoxic agents
Pulmonary hypertension is a progressive, life-threatening disorder of the pulmonary circulation characterized by high pulmonary artery pressures (> 25 mm Hg). This persistent high pulmonary artery pressure ultimately leads to right ventricular failure. Patients with pulmonary arterial hypertension (PAH) are often on a chronic regimen of therapy to decrease PAH that should not be interrupted during hospitalization. Abrupt cessation of therapy can lead to rebound pulmonary hypertension that can be fatal.
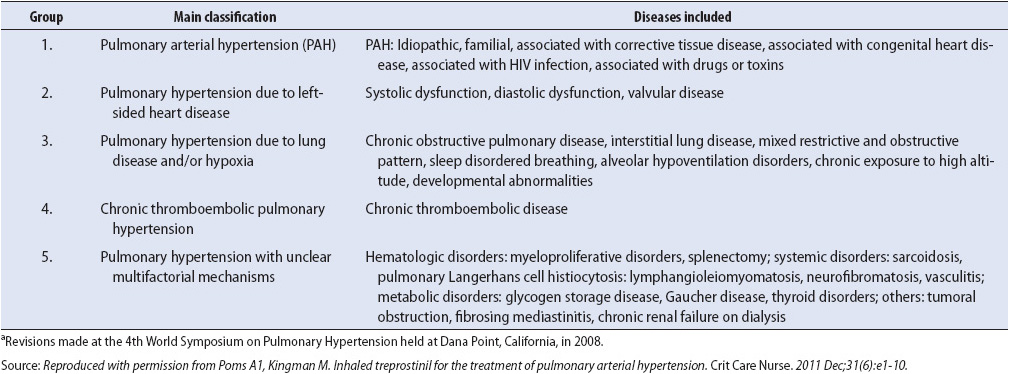
Pulmonary hypertension may result from a number of etiologies (Table 10-12). The pathophysiology is multifactoral with evidence that endothelial dysfunction leads to remodeling of the pulmonary artery vessel wall causing exaggerated vasoconstriction and impaired vasodilatation. This results in decreased blood flow and return of deoxygenated blood to the lungs.
TABLE 10-12. WORLD HEALTH ORGANIZATION CLASSIFICATION OF PULMONARY HYPERTENSIONa
Signs and symptoms include pallor, dyspnea, fatigue, chest pain, and syncope. Cor pulmonale or enlargement of the right ventricle can be a result of pulmonary hypertension and may lead to right ventricular failure (see Table 9-10). The diagnostic strategy is related to both establishing the diagnosis of pulmonary hypertension and if possible the underlying cause.
Diagnostic Tests
• Echocardiogram: Valvular heart disease, left ventricular dysfunction, and intracardiac shunts.
• Chest x-ray: Enlarged hilar and pulmonary arterial shadows and enlargement of the right ventricle.
• 12-lead ECG: Right ventricular strain, right ventricular hypertrophy, and right axis deviation.
• CTPA, Ventilation-perfusion scan, or pulmonary angiogram: These are done to rule out thromboembolism.
• CT chest: Assess for presence or absence of parenchymal lung disease.
• 6-minute-walk test: Measurement of distance used to monitor exercise tolerance, response to therapy, and progression of disease.
• Right-heart cardiac catheterization: Gold standard for diagnosis with vasodilator (adensosine, nitric oxide, epoprostentol) testing for benefit from long-term therapy with calcium channel blockers. Positive response is a decrease in mean PAP of 10 to 40 mm Hg with an increased or unchanged CO from baseline values.
• Serology testing: Antinuclear antibodies.
• Pulmonary function testing: Used to rule out any other diseases contributing to shortness of breath.
• Sleep study: Done as a screen for sleep apnea, which may also contribute to the pulmonary hypertension.
Current treatment options can slow the progression of the disease.
• Long-term anticoagulation therapy to prevent thrombosis.
• Avoidance of beta blockers, decongestants, or other medications that worsen pulmonary hypertension or decrease right heart function.
• Symptom limited physical activity.
• Oxygen to prevent additional pulmonary vasoconstriction due to low oxygen levels. Maintain SaO2 greater than 90% if possible.
• Diuretics to control edema and ascites if right-sided heart failure present.
• Calcium channel blockers only if positive response to vasodilator during cardiac catheterization.
Prostacylin therapy is a potent vasodilator of both the systemic and pulmonary arterial vascular beds and is an inhibitor of platelet aggregation. Patients must be preapproved through their insurance prior to starting these costly medications and be able to self-administer.
• Remodulin (treprostinil sodium) is a continuous subcutaneous or intravenous infusion.
• Veletri (epoprostenol sodium, room temperature stable) is a continuous intravenous infusion.
• Ventavis (iloprost sodium) and Tyvaso (treprostinil) are intermittent inhalation treatments using medication specific nebulizers.
Endothelin receptor antagonists block the neurohormone endothelin from binding in the endothelium and vascular smooth muscle.
• Tracleer (bosentan) and Letairis (ambrisentan) are oral agents.
Phosphodiesterase inhibitors block phosphodiesterase type 5 which is responsible for the degradation of cyclic guanosine monophosphate (cGMP). Increased cGMP concentration results in pulmonary vasculature relaxation; vasodilation in the pulmonary bed and the systemic circulation (to a lesser degree) may occur.
• Revatio (sildenafil) and Adcirca (tadalafil) are oral agents specific for the use in patients with pulmonary hypertension.
Surgical options include the following:
• Atrial septostomy to create a right-to-left shunt to help decompress a failing right ventricle in select patients who are unresponsive to medical therapies. This also leads to significant hypoxemia in an already compromised patient.
• Pulmonary thromboendarterectomy for those with suspected chronic thromboembolic pulmonary hypertension to improve hemodynamics and functional status.
• Lung transplantation is indicated when the pulmonary hypertension has progressed despite optimal medical and surgical therapy.
Respiratory infection is a common cause of ARF. Infections developed before hospitalization (community-acquired), during medical treatment (healthcare acquired), and those acquired during hospitalization (hospital-acquired and ventilator-associated) can lead to significant morbidity and mortality, and require progressive care management. A variety of respiratory infections occur in acutely ill patients, including bronchitis, and pneumonia. This section focuses on pneumonia, the most common respiratory infection and the most common cause of respiratory failure in acutely ill patients.
At high risk for the development of pneumonia are the young, the elderly, those with chronic cardiopulmonary disease, and immunocompromised individuals. In addition, immobility, decreased level of consciousness, and mechanical ventilation place hospitalized patients at high risk for development of hospital-acquired pneumonias. These latter pneumonias are most commonly referred to as ventilator-associated pneumonias or VAPs.
The major routes of entry of causative organisms for pneumonia are aspiration of oropharyngeal or gastric contents into the lungs, inhalation of aerosols or particles containing the organisms, and hematogenous spread of the organism into the lung from another site in the body (Figure 10-18). Most hospital-acquired pneumonias are due to aspiration of bacteria colonizing the oropharynx or upper GI tract. Pneumonia develops when the normal bronchomucociliary clearance mechanism or phagocytic cells are overwhelmed by the number or virulence of organisms aspirated or inhaled into the airways. The proliferation of organisms in the pulmonary parenchyma elicits an inflammatory response, with large influxes of phagocytic cells into the alveoli and airways and production of protein-rich exudates. This inflammatory response impairs the distribution of ventilation and decreases lung compliance, resulting in increased work of breathing and the sensation of dyspnea. Hypoxemia results from the shunting of blood through poorly ventilated areas of pulmonary consolidation. The inflammatory response leads to fever and leukocytosis.
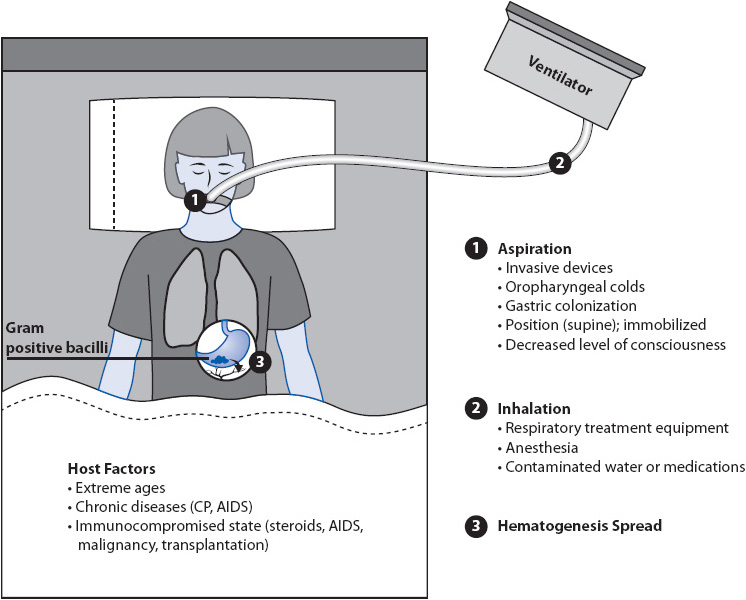
Figure 10-18. Pathogenesis of pneumonia.
Pneumonia also can develop through hematogenous spread, when organisms remote from the lungs gain access to the blood, become lodged in the pulmonary vasculature, and proliferate. Pneumonias with a hematogenous origin usually are distributed diffusely in both lung fields, rather than localized to a single lung or lobe.
Several factors present in acutely ill patients increase the risk for the development of hospital-acquired pneumonias including VAP. Aspiration of oropharyngeal and gastric secretions is increased in the presence of tracheostomy tubes, nasogastric tubes, poor GI motility, gastric distention, and immobility, all of which are common situations in acutely ill patients. Treatments that neutralize the normally acidic gastric contents, such as antacids, H2 blockers, or tube feeding, allow increased growth of gram-negative bacteria in gastric contents. This increases the potential for aspiration of gram-negative bacteria and/or hematogenous spread.
Acutely ill patients at high risk for hospital-acquired pneumonias are those immunocompromised from malignancy, AIDS, and chronic cardiac or respiratory disease; the elderly; or those with depressed alveolar macrophage function (oxygen, corticosteroids). Although a variety of similar organisms cause community-acquired and hospital-acquired pneumonias, their frequency distribution is different (Table 10-13). Of particular concern in hospital-acquired infections is the polymicrobial origin of the pneumonia and the potential for causative organisms to be resistant to antimicrobial therapy.
TABLE 10-13. INFECTIOUS ETIOLOGIC AGENTS IN SEVERE COMMUNITY-ACQUIRED PNEUMONIA REQUIRING INTENSIVE CARE SUPPORT AND HOSPITAL-ACQUIRED PNEUMONIA IN CRITICALLY ILL PATIENTS
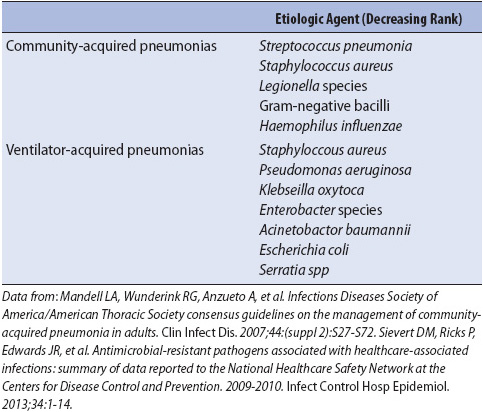
Development of a hospital-acquired pneumonia is a serious complication in acutely ill patients. Increased morbidity and mortality, in addition to increases in progressive care and hospital lengths of stay and costs, make hospital-acquired pneumonias one of the most important sources of negative outcomes for acutely ill patients.
Signs and Symptoms
• Fever
• Cough, typically productive
• Purulent sputum or hemoptysis
• Dyspnea
• Pleuritic chest pain
• Tachypnea
• Abnormal breath sounds (crackles, bronchial breath sounds)
Diagnostic Tests
• Gram stain and culture of sputum for causative organisms. May require fiberoptic bronchoscopy with brush specimen or bronchoalveolar lavage specimen retrieval in situations where pneumonia responds poorly to treatment. This may also be necessary early in admission in those patients who are immunocompromised, such as those with HIV/AIDS. The pneumonias in these immune deficient patients are often due to opportunistic organisms that may require very specific antibiotic coverage.
• New or progressive infiltrates on chest x-ray. Infiltrates may be either localized or diffuse in nature (see Figure 10-5).
• Elevated WBC.
• Abnormal arterial blood gases (hypoxemia, hypocapnia).
Appropriate empirical broad spectrum antimicrobial therapy should be initiated based on likely causative organisms until definitive culture results are obtained. Fluids should be administered to correct hypovolemia and hypotension, if present. Hypotension unresponsive to fluid therapy should alert the clinician to the potential for septic shock.
Similar to ARF management, with the following additions:
• PEEP and CPAP are unlikely to improve oxygenation in the presence of a unilateral pneumonia, and may exacerbate the associated ventilation-perfusion abnormalities. These techniques should be used with caution in pneumonia.
• Voluminous, tenacious respiratory secretions may require endotracheal intubation to assist with clearance. Fiberoptic bronchoscopy may also be required to assist with secretion management.
Although the signs and symptoms of VAP are known, clinical diagnosis is complicated by lack of specific and sensitive criteria. In 2013, the National Healthcare Safety Network lead by the Centers for Disease Control began collecting new surveillance criteria for ventilator-associated events, which include ventilator-associated conditions (VAC), infection-related ventilator-associated conditions (IVAC), possible VAP, and probable VAP (Figure 10-19).
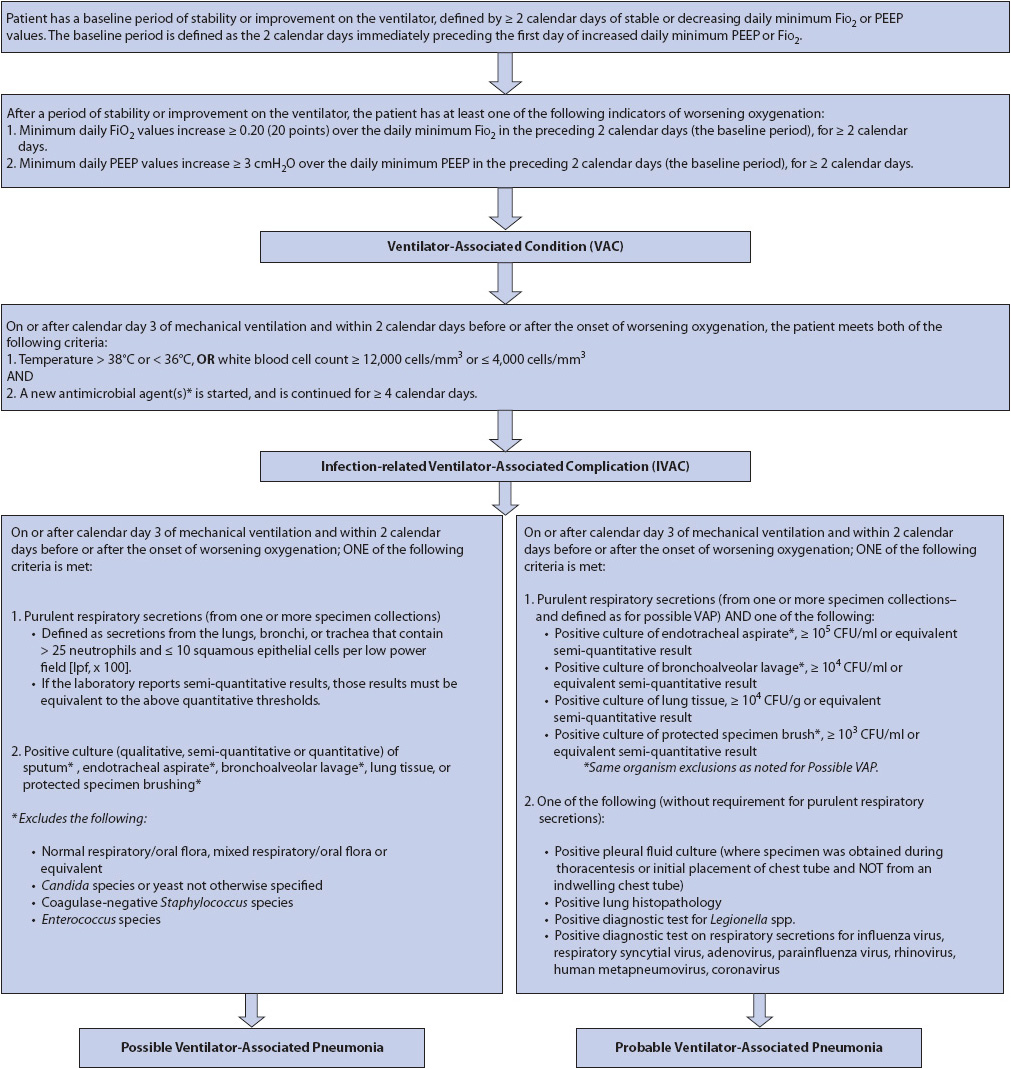
Figure 10-19. Ventilator-Associated Events (VAE) Surveillance Definition Algorithm. (From: the Centers for Disease and Control and Prevention [CDC]). NHSN e-News ventilator-associated event (VAE) surveillance for adults special edition. 2012. Available at: www.cdc.gov/nhsn/psc_da-vae.html.)
In addition to the high morbidity and mortality associated with pneumonia in acutely ill patients, high priority must be given to strategies to prevent the development of hospital-acquired pneumonias. The development of a hospital-acquired pneumonia in an acutely ill patient increases requirements for ventilatory support (mechanical ventilation, oxygen, duration of treatment). It is estimated that a hospital-acquired pneumonia increases hospitalization 4 to 10 days, and increases costs by $20,000 to $40,000 per episode. Prevention strategies (Table 10-14) include the following:
• Decrease the risk of cross-contamination or colonization via the hands of hospitalized personnel. Hand washing is the most effective strategy.
• Decrease the risk of aspiration during enteral nutrition. Avoid supine positioning and keep the head of the bed elevated at all times, unless medically contraindicated. Assess for, and correct, gastric reflux problems. Ambulate as soon as possible.
• Eliminate invasive devices and equipment as soon as possible.
TABLE 10-14. EVIDENCE-BASED PRACTICE GUIDELINES FOR THE PREVENTION OF VENTILATOR-ASSOCIATED PNEUMONIA

• Avoid supine positioning and keep the head of the bed elevated to 30 to 45 degrees at all times.
• Implement a comprehensive oral hygiene program that includes oral suctioning, teeth brushing, and use of oral 0.12% chlorhexidine gluconate for ventilator patients.
• Use sterile technique for tracheal suctioning and suction only when necessary to clear secretions from large airways.
• Maintain a closed system on ventilator/humidifier circuits and avoid pooling of condensation or secretions in the tubing. Do not routinely change the ventilator circuit, except when visibly soiled or malfunctioning. Use sterile water or saline for use with any respiratory equipment.
• Provide nutritional support to improve host defenses.
• Eliminate invasive devices and equipment as soon as possible. Assess weaning readiness daily and limit the use of sedatives (see Chapter 6, Pain and Sedation Management).
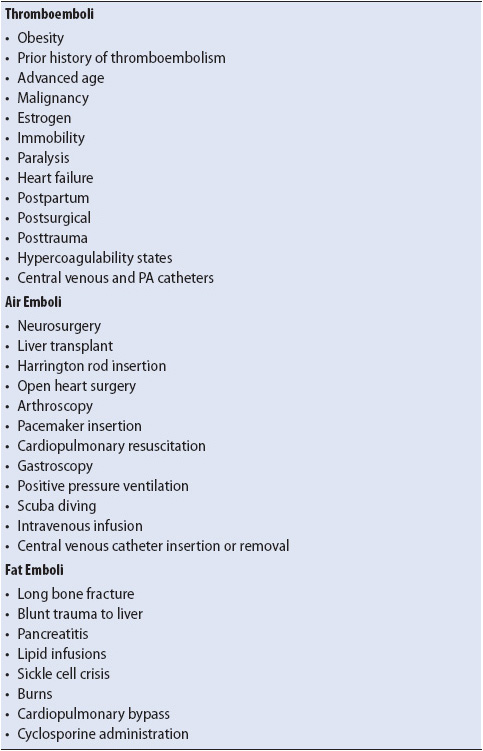
Pulmonary embolism is a complication of deep venous thrombosis (DVT), long bone fracture, or air entering the circulatory system. There are many risk factors for PE (Table 10-15), with acutely ill patients being especially prone due to the presence of central venous catheters, immobility, use of muscle relaxants, and heart failure.
TABLE 10-15. RISK FACTORS FOR THE DEVELOPMENT OF PULMONARY EMBOLISM
Venous thrombi form at the site of vascular injuries or where venous stasis occurs, primarily in the leg or pelvic veins. Thrombi that dislodge travel through the venous circulation until they become wedged in a branch of the pulmonary circulation. Depending on the size of the thrombi, and the location of the occlusion, mild to severe obstruction of blood flow occurs beyond the thrombi.
The primary sequela, and major contributor to mortality, of the pulmonary obstruction is circulatory impairment. The physical obstruction of the pulmonary capillary bed increases right ventricular afterload, dilates the right ventricle, and impedes coronary perfusion. This predisposes the right ventricle to ischemia and right ventricular failure (cor pulmonale).
A secondary consequence of thromboemboli is a mismatching of ventilation to perfusion in gas exchange units beyond the obstruction (see Figure 10-15C), resulting in arterial hypoxemia. This hypoxemia further compromises oxygen delivery to the ischemic right ventricle.
Air or other nonabsorbable gases entering the venous system also travel to the right heart, pulmonary circulation, arterioles, and capillaries. A variety of surgical and non-surgical situations predispose patients to the development of air embolization (see Table 10-15). Damage to the pulmonary endothelium occurs from the abnormal air-blood interface, leading to increased capillary permeability and alveolar flooding. Bronchoconstriction also occurs with air embolization. In addition to hypoxemia, PCO2 removal is also impaired.
Arterial embolization may occur if air passes to the left heart through a patent foramen ovale, present in approximately 30% of the population. Peripheral embolization to the brain, extremities, and coronary perfusion leads to ischemic manifestations in these organs.
Fat enters the pulmonary circulation most commonly when released from the bone marrow following long bone fractures (see Table 10-15). Nontraumatic origins of fat embolization also occur and are thought to be due to the agglutination of low-density lipoproteins or liposomes from nutritional fat emulsions. The presence of fat in the pulmonary circulation injures the endothelial lining of the capillary, increasing permeability and alveolar flooding.
The diagnosis of PE is based primarily on clinical signs and symptoms. Because many of the signs and symptoms are nonspecific, PE frequently is difficult to diagnosis. In acutely ill patients, diagnosis is especially difficult owing to alterations in communication and level of consciousness, and the nonspecific nature of other cardiopulmonary alterations.
Signs and Symptoms
• Dyspnea
• Pleuritic chest pain
• Cough
• Rales
• Apprehension
• Diaphoresis
• Evidence of DVT
• Hemoptysis
• Tachypnea
• Fever
• Tachycardia
• Syncope
• Hypoxia
• Hypotension
Diagnostic Tests
• Chest x-ray: Evaluate for basilar atelectasis, elevation of the diaphragm, and pleural effusion, although most patients have nonspecific findings on chest x-ray; diffuse alveolar filling in air embolism.
• Arterial blood gas analysis: Hypoxemia with or without hypercarbia.
• ECG: Signs of right ventricular strain (right axis deviation, right bundle branch block) or precordial strain; sinus tachycardia.
See earlier discussion of diagnostics for PE.
The key to preventing morbidity and mortality from PE is primarily prevention and secondarily early diagnosis and treatment to prevent reembolization. Objectives include the improvement of oxygenation and ventilation, improvement of cardiovascular function, prevention of reembolization, and prevention of pulmonary embolus.
Oxygen therapy is usually very effective in relieving hypoxemia associated with PE. When cardiopulmonary compromise is severe, the patient may need to be moved into the ICU for intubation and mechanical ventilation to achieve optimal oxygenation.
Controversy exists as to the benefit of vasoactive drug administration (such as norepinephrine and/or inotropic agents) to improve myocardial perfusion of the right ventricle. In severe embolic events, where cardiac failure is profound, additional therapy to hasten clot resolution, such as use of thrombolytic agents and/or intervertional removal of massive emboli may be warranted.
Several strategies are employed to prevent the likelihood of future embolization and cardiopulmonary compromise:
• Limiting activity to prevent dislodgement of additional clots.
• Use of anticoagulation therapy with unfractionated heparin to maintain a PTT 1.5 to 2.5 times the control when no contraindication exists.
• Insertion of vena cava filters to prevent emboli from legs, pelvis, and inferior vena cava from migrating to pulmonary circulation if anticoagulation therapy is contraindicated. Filters are placed percutaneously in the inferior vena cava.
• An important recommendation for the prevention of VTE is awareness and access to a hospital prevention policy including risk assessment (Table 10-16).
• A risk assessment should be done on admission to the unit and discussion daily on rounds should take place. Discussion should also include current VTE prevention intervention, risk for bleeding, and response to treatment.
• If ordered, graduated compression stocking or IPCs (Figure 10-20; Table 10-17) should be in use at all times except when being removed for correct fitting or skin assessment.
• Placement of prophylactic vena cava filters in high-risk patients.
• Early fixation of long bone fractures to prevent fat emboli.
• Early mobilization. As soon as hemodynamic stability is achieved, and there are no other contraindications to mobilization, activity level should begin increasing to include sitting in a chair several times per day and short periods of ambulation.
TABLE 10-16. RISK FACTORS, ASSESSMENT AND THROMBOPROPHYLAXIS FOR VTE
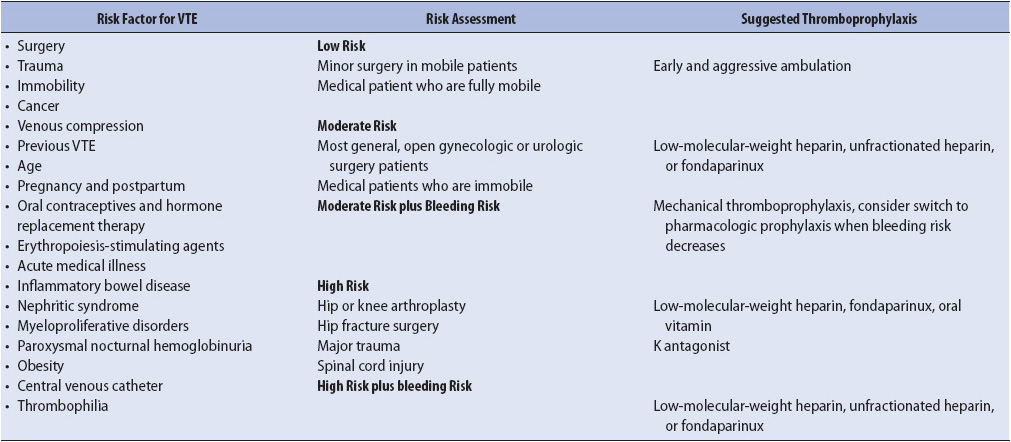
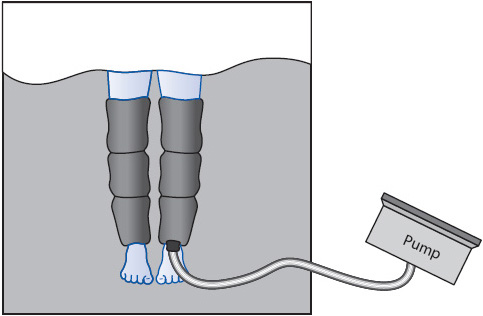
Figure 10-20. Intermittent pneumatic compression (IPC) device for prevention of DVT and PE.
TABLE 10-17. TIPS FOR SAFE AND EFFECTIVE USE OF INTERMITTENT PNEUMATIC COMPRESSION DEVICES

Burns S, ed. Protocols for Practice: Care of the Mechanically Ventilated Patient. Sudbury, MA: Jones and Bartlett Publishers; 2006.
Burns SM. Ventilating patient with acute severe asthma: what do we really know? AACN Adv Crit Care. 2006;17:186-193.
Carlson KK. ed. Advanced Critical Care Nursing. St Louis, MO: Saunders Elsevier; 2008.
Collins PF, Stratton RJ, Elia M. Nutritional support in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1385-1395.
Epstein SK. Noninvasive ventilation to shorten the duration of mechanical ventilation. Resp Care. 2009;54:198-211.
Geiger-Bronsky M, Wilson DJ, eds. Respiratory Nursing: A Core Curriculum. New York, NY: Springer Publishing Company; 2008.
Ginn MB, Cox G, Health J. Evidence-based approach to an inpatient tobacco cessation protocol. AACN Adv Crit Care. 2008;19:268-278.
Halm MA. Relaxation: a self-care healing modality reduces harmful effects of anxiety. Am J Crit Care. 2009;18:169-172.
Louie S, Morrissey BM, Kenyon NJ, Albertson TE, Avdalovic M. The critically ill asthmatic; from ICU to discharge. Clin Rev Allerg Immunol. 2012;43:30-44.
Maki MB, Martin SA, Burns S, Philbrick D, Rauen C. Putting evidence into nursing practice: four traditional practices not supported by evidence. Crit Care Nurse. 2013;33:28-42.
Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731-2740.
McLean B. Acute respiratory failure and intensive measures, Crit Care Nurs Clin N Am. 2012;24:361-375.
Raghavendran K, Napolitano LM. Definition of ALI/ARDS. Crit Care Clin. 2011;27:429-437.
Raoff S, Goulet K, Esan A, Hess DR, Sessler C. Severe hypoxemic respiratory failure, part 2; nonventilatory strategies. Chest. 2010;137:1437-1448.
Rauen CA, Flynn Makic MB, Bridges E. Evidence-based practice habits: transforming research into bedside practice. Crit Care Nurs. 2009;29:46-59.
The ARDS Definition Task Force. Acute respiratory distress syndrome; the Berlin definition. 2012;307:2526-2533.
Urden LD, Stacy KM, Lough ME. Thelan’s Critical Care Nursing: Diagnosis and Management. 6th ed. St Louis, MO:Mosby; 2010.
Connolly MA. Black, white, and shades of gray: common abnormalities in chest radiographs. AACN Clin Issues. 2001;12(2):259-269.
Eisenhuber E, Schaefer-Prokop CM, Prosch H, Schima W. Bedside chest radiography. Resp Care. 2012;57:427-443.
Godoy MC, Leitman BS, deGroot PM, Viahos J, Naidich DP. Chest radiography in the ICU: part 1; evaluation of airway, enteric, and pleural tubes. Am J Roentgenol. 2012;198:563-571.
Sanchez F. Fundamentals of chest x-ray interpretation. Crit Care Nurse. 1986;6:41-52.
Siela D. Chest radiography evaluation and interpretation. Adv Crit Care. 2008;19:444-473.
Lynn-McHale DJ. AACN Procedure Manual for Critical Care, 5th ed. Philadelphia, PA: Elsevier-Saunders; 2011.
AACN Deep Vein Thrombosis Prevention Practice Alert. Aliso Viejo, CA: AACN; 2010. http://www/aacn.org. Accessed February 18, 2013.
AACN VAP Practice Alert. Aliso Viejo, CA: AACN; 2008. http://www.aacn.org. Accessed February 18, 2013.
American College of Chest Physician. Antithrombotic and thrombolytic therapy: American College of Chest Physicians evidence based clinical practice guidelines. 2012 (9th ed). http://journal.publications.chestnet.org/issue.aspx?journalid=99&issueid=23443. Accessed February 21, 2013.
American Thoracic Society and the European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277-304.
American Thoracic Society and the Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
Centers for Disease Control and Prevention. Guidelines for prevention of health-care-associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR. 2004;53(No. RR-3):1-35.
Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-1717.
Filore MC, Jaen CR, Baker TB. Treating tobacco use and dependence: 2008 Update. Quick Reference Guide for Clinicians. Rockville, MD: U.S. Department of Health and Human Services. Public Health Services: April 2009.
Gold Executive Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (Updated 2013). http://www.goldcopd.org/. Accessed February 20, 2013.
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
McClave SA, Martindale RG, Vanek VW. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCCM) and the American Society for Parenteral and Enteral Nutrition (ASPEN). J Parenter Enteral Nutr. 2009;33:277-316.
McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert concensus document on pulmonary hypertension: a report of the American College of Cardiology foundation task force on expert consensus documents and the American Heart Association. Circulation. 2009;119:2250-2294.
National Heart, Lung, and Blood Institute. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma, Full Report 2007. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed February 21, 2013.
National Heart, Lung, and Blood Institute. Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. http://www.ginasthma.org/uploads/users/files/GINA_Report_2012Feb13.pdf. Accessed February 21, 2013.
National Heart, Lung, and Blood Institute. Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed February 21, 2013.
Parshall MB, Schwartzstein RM, Adams L, et al. An official America Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435-452.
Qaseem A, Wilt TJ, Weingberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals Intern Med. 2011;155:179-192.
Raghu G, Collard HR, Egan JJ. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788-824.
Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC), European Respiratory Society (ERS), International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009:34: 1219-1263.
The ARDS Definition Task Force. Acute Respiratory Distress Syndrome: the Berlin Definition. JAMA. 2012;307:2526-2533.