TABLE 14-1. COMMON SOURCES OF UPPER GASTROINTESTINAL BLEEDING

KNOWLEDGE COMPETENCIES
1. Describe the etiology, pathophysiology, clinical presentation, patient needs, and principles of management for:
• Acute upper gastrointestinal bleeding
• Liver failure
• Acute pancreatitis
• Bowel ischemia
• Bowel obstruction
• Bariatric (gastric bypass surgery)
2. Identify nutritional requirements for enterally fed acutely ill patients.
3. List important interventions to decrease the risk for aspiration pneumonia during enteral feeding.
Life-threatening gastrointestinal (GI) bleeding originates most commonly in the upper GI tract and requires immediate therapy to prevent complications. Although bleeding stops spontaneously in 80% to 90% of cases, patients presenting with sudden blood loss are at risk for decreased tissue perfusion and oxygen-carrying capability. There can be effects on every organ system in the body. Acute upper GI bleeding has a mortality of 6%-13%. Bleeding that originates distal to the ligament of Treitz is considered to be lower GI bleeding which, unlike upper GI bleeding, is not associated with the same morbidity and mortality. Lower GI bleeding is generally a disease of the elderly patient and may be associated with cancer. A poor prognosis with upper GI bleeding is related to age above 65, shock, overall poor health, active bleeding at the time of presentation, elevated creatinine or transaminases, onset of bleeding during hospitalization, and initial low hematocrit. Death is not a direct result of blood loss, but is related to age and comorbidities.
A variety of abnormalities within the GI tract can be the source of upper GI bleeding (Table 14-1). The most common cause of upper GI bleeding is peptic ulcer disease, accounting for 31%-67% of all cases followed by erosive disease, variceal bleeding, esophagitis, cancer, and Mallory-Weis tears. The pathogenesis of peptic ulcer disease is related to hypersecretion of gastric acid, coupled with impaired GI tract mucus secretion. Normally, mucus protects the gastric wall from the erosive effects of acid. Peptic ulcers occur in the stomach and the duodenum, and are characterized by a break in the mucosal layer that penetrates the muscularis mucosa (innermost muscular layer), resulting in bleeding. Infection of the mucosa by Helicobacter pylori, an organism naturally found in the GI tract, also has been implicated in the pathogenesis of peptic ulcer disease.
TABLE 14-1. COMMON SOURCES OF UPPER GASTROINTESTINAL BLEEDING

Gastroesophageal varices develop when there is increased pressure in the portal venous system of the liver. If blood cannot flow easily through the liver because of obstructive disease, it is diverted to collateral channels. These channels are normally low-pressure vessels found in the distal esophagus (esophageal varices), the veins in the proximal stomach (gastric varices), and in the rectal vault (hemorrhoids) (Figure 14-1). Acute upper GI hemorrhage occurs when esophageal and/or gastric varices rupture from increased portal vein pressure (portal hypertension). Esophagogastric varices do not bleed until the portal pressure exceeds 12 mm Hg. Portal hypertension is most commonly caused by primary liver disease (see next section), liver trauma, or thrombosis of the splenic or portal veins. Massive upper GI hemorrhage is associated with these variceal bleeds.
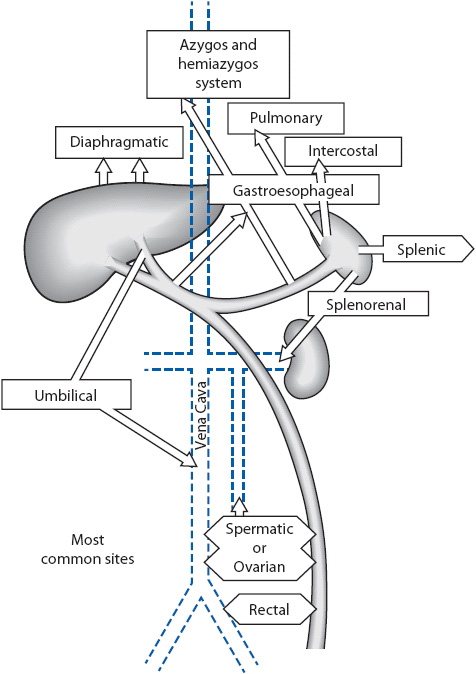
Figure 14-1. The liver with collateral circulation.
Mallory-Weiss syndrome is a linear, nonperforating tear of the gastric mucosa near the gastroesophageal junction. The tear is the result of pressure changes in the stomach that occur with forceful vomiting. Alcohol abuse and inflammatory conditions of the stomach and esophagus are also associated with this disorder. Classically, these tears occur in alcoholic patients who experience intense retching and vomiting associated with binge drinking. However, they may also occur in any patient with a history of repeated emesis.
Hemorrhagic gastritis describes gastric lesions that do not penetrate the muscularis mucosa. These are also referred to as stress ulcers. Onset of bleeding is sudden and is often the first symptom. The causes of gastritis are multifactorial (Table 14-2), but are most commonly associated with nonsteroidal anti-inflammatory drug (NSAID) use, alcohol abuse, and physiologic conditions that cause severe stress (eg, trauma, surgery, burns, severe medical problems). Alcohol and NSAIDs are known to directly disrupt the mucosal defense mechanisms of the stomach (Figure 14-2). Use of NSAIDs is particularly problematic in the elderly and contributes to increased incidence of symptomatic acute upper GI bleeding in this population.
TABLE 14-2. CAUSES OF GASTRITIS
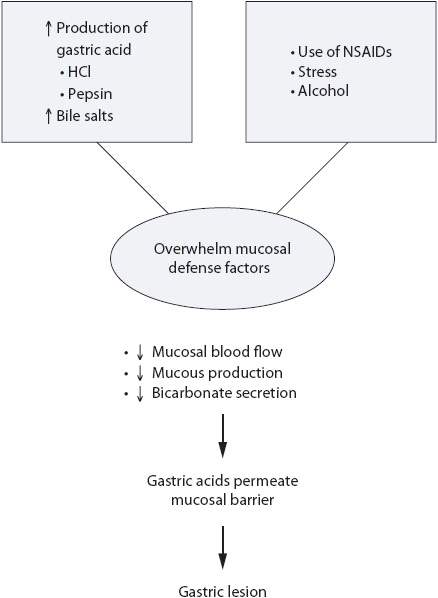
Figure 14-2. Pathogenesis of gastritis.
Regardless of the etiology, upper GI bleeding resulting in a sudden loss of blood volume is associated with decreased venous return to the heart and subsequently cardiac output (CO). The decrease in CO triggers the release of epinephrine and norepinephrine, causing intense vasoconstriction and tissue ischemia (Figure 14-3). In addition, aldosterone and antidiuretic hormones are released, resulting in sodium and water retention. The clinical signs and symptoms of upper GI hemorrhage are directly related to the effects of the decrease in CO and the vasoconstriction response typically seen in hypovolemic shock.
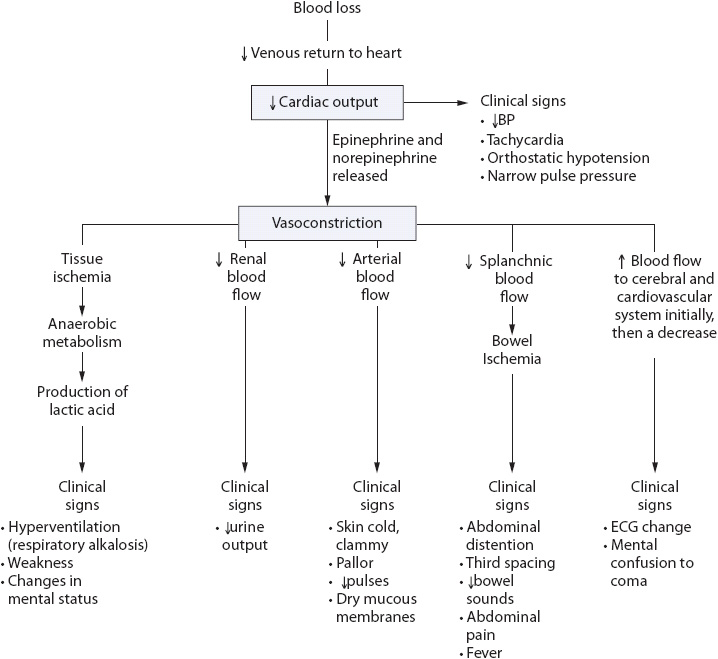
Figure 14-3. Hypovolemic shock.
Individuals may have a history of peptic ulcer disease, tobacco abuse, alcohol abuse, liver disease, severe physiologic stress, NSAID use, anticoagulation or antiplatelet therapy, and/or are older or elderly.
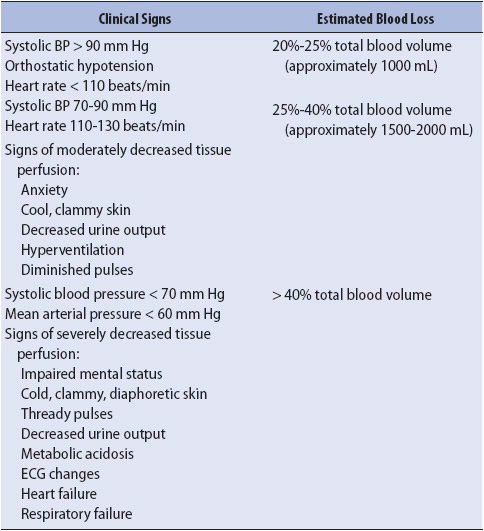
The response of an individual to blood loss will depend on the rate and amount of blood loss, patient’s age, overall health status, and the timing of the initial resuscitation. Specific signs and symptoms include:
• Hematemesis: Bright red blood or coffee ground emesis
• Melena or maroon-colored stools
• Hematochezia
• Nausea
• Epigastric pain
• Abdominal distention
• Bowel sounds increased or decreased
• If blood loss is greater than 25% of blood volume, hypotension (orthostatic), altered hemodynamic values (decreased central venous pressure [CVP], pulmonary capillary wedge pressure [PCWP], mean arterial pressure [MAP], CO)
• Rapid, deep respirations
• Tachycardia
• Fever
• Cold, clammy skin
• Dry mucous membranes
• Decreased pulses
• Weakness
• Decreased urine output
• Anxiety
• Mental status changes
• Restlessness
• Electrocardiographic (ECG) changes consistent with ischemia (eg, ST-segment elevation, arrhythmias)
• Hematocrit may be normal initially, then decreased with fluid resuscitation and blood loss. It is important to note that the hematocrit may not accurately reflect the actual volume of blood loss due to hemodilution and movement of extravascular fluid. The hematocrit decreases as extravascular fluid enters the vascular space in an attempt to restore volume. This process continues for 24 to 72 hours.
• Hemoglobin may also be normal initially, then decreased with fluid resuscitation and blood loss.
• White blood cell count is elevated.
• Platelet count may be decreased depending on amount of blood loss.
• Serum sodium is usually elevated initially due to hemoconcentration.
• Serum potassium is usually decreased with vomiting.
• Serum blood urea nitrogen (BUN) is mildly elevated.
• Serum creatinine is elevated.
• Serum lactate is elevated with severe bleeding.
• Prothrombin time (PT) is usually decreased.
• Activated thromboplastin time (aPTT) is usually decreased.
• Arterial blood gases show respiratory alkalosis (early), then later metabolic acidosis with severe shock and hypoxemia.
• Gastric aspirate shows normal or acidotic pH and is guaiac positive.
The fundamental goal of initial treatment is volume resuscitation. The management of the patient with acute upper GI bleeding focuses on hemodynamic stabilization, identification of the bleeding site, and initiation of definitive medical or surgical therapies to control or stop the bleeding. Measures to decrease anxiety in this patient population are also indicated owing to the severity and sudden onset of GI bleeding.
The initial assessment of the patient with GI bleeding begins with assessment of vital signs, the most reliable reflection of the amount of blood lost. In the presence of hemodynamic instability, resuscitation begins. Risk criteria for patients with acute GI bleed include ongoing bleeding, hemoglobin less than 8 g/dL, transfusion requirement, systolic blood pressure less than 100 mm Hg on presentation, elevated prothrombin time, and alteration in mental status.
1. Monitor and record cardiovascular status (blood pressure, heart rate including orthostatic changes), hemodynamics (CVP, PCWP, CO, MAP), and peripheral pulses.
2. Insert at least two large-bore intravenous (IV) catheters and begin fluid resuscitation with crystalloid solution (eg, normal saline or lactated ringer solution). Administer fluids to maintain MAP at 60 mm Hg or higher.
3. Obtain blood for measurement of hematocrit, hemoglobin, and clotting studies, as well as for a type and cross-match for packed red blood cells (PRBC). Usually 6 units (U) at a minimum are ordered. The initial hematocrit is rarely useful for estimating transfusion requirements. Estimates for the amount of blood loss are most reliably guided by vital sign values (Table 14-3).
4. Administer prescribed IV colloids, crystalloids, or blood products until the patient is stabilized. After the administration of 2 to 3 liters of crystalloid fluids, blood products may be considered during the initial resuscitation if the hemodynamic response is poor. PRBC are used to rapidly increase the hematocrit while providing less volume compared to when whole blood is used. Each unit of PRBC increases the hematocrit by 2% to 3% and improves gas exchange. It may take up to 24 hours after blood is administered for changes to be reflected in the hematocrit values, especially if large amounts of crystalloid solutions were administered during the resuscitation period. In addition to blood, platelets and clotting factors may also be given.
5. Monitor coagulation studies (eg, PT/PTT, platelet count).
6. Monitor fluid balance and renal function (intake and output, daily weight, BUN, creatinine, and hourly urine output).
7. Insert a nasogastric tube if bleeding is massive (> 40% of blood volume) to assess for the rate of bleeding. Placement of a gastric tube in the presence of varices is somewhat controversial and practices vary between institutions. Use of gastric lavage is also controversial. Proponents believe that removing blood clots by gastric lavage is useful in that it allows the stomach to contract and tamponade bleeding vessels. Removal of blood may give some indication of the rate of bleeding and may minimize the chance of pulmonary aspiration. If lavage is ordered, room temperature saline usually is used.
8. Position the patient in the left lateral decubitus position to minimize aspiration associated with hematemesis.
9. Monitor temperature and maintain normothermia. Rapid fluid resuscitation, particularly with blood products, can lead to hypothermia, with interference of normal coagulation. Warming of fluids may be required to prevent hypothermia if traditional measures are insufficient.
10. Prepare for urgent endoscopic therapy if estimated blood loss is greater than 3 U of blood, bright red blood is found in emesis or nasogastric aspirate, or if a variceal bleed is suspected. Usually all other patients will complete endoscopy within 24 hours of admission.
11. Administer supplemental oxygen. Monitor respiratory function. Airway protection with endotracheal intubation to prevent aspiration is indicated in patients with ongoing hematemesis or altered mental status.
TABLE 14-3. ESTIMATING BLOOD LOSS FROM ACUTE GI BLEEDING
Although the history and physical examination are used to differentiate between upper and lower GI bleeding, endoscopic examination is required to determine the exact site of bleeding and will direct future therapy. Endoscopic visualization at the bedside is preferred to allow for early direct visualization of the upper tract during resuscitation measures.
1. The presence of blood in the upper GI tract can make it difficult to identify the bleeding source and deliver treatment. Prokinetic agents facilitate gastric emptying of retained blood and may be administered prior to endoscopy. A recent meta-analysis has shown that when either erythromycin or metoclopramide is given pre-endoscopy, the need for a repeat procedure to identify the bleeding source is reduced.
2. Administer sedation (eg, midazolam [Versed]) as ordered and institute monitoring protocol.
3. Position patient in a left lateral decubitus position to prevent aspiration of GI contents during endoscopy. Have oral-tracheal suction available at the bedside before the procedure begins.
4. Monitor for cardiac ischemia during the examination (eg, ST-segment changes [see Chapter 18, Advanced ECG Concepts], arrhythmias).
Definitive therapies to treat the bleeding differ depending on the cause. A general approach for treatment is summarized in Figure 14-4. In nonvariceal upper GI bleeding, endoscopic treatment is widely accepted as the most effective method to control acute ulcer bleeding and has become the standard for prevention of ulcer rebleeding. Although individual studies have been too small to show significant advantage for endoscopic therapy in reducing mortality, a meta-analysis indicates endoscopic therapy prevents not only rebleeding but also death. Administration of PPIs prior to endoscopy is now routine for patients in whom an ulcer is suspected. The PPIs quickly neutralize acid, which results in stabilization of the blood clot. An acidic environment will inhibit platelet aggregation and lyse an already formed clot. Several therapeutic interventions are available for the endoscopist and include ablative or coagulation therapy (laser, monopolar, bipolar, or multipolar electrocoagulation, and heater probe), pharmacologic therapy also known as sclerotherapy, and mechanical and combination therapies. Pharmacologic treatments are easy to use, inexpensive, and available in most settings. The goal of this treatment is to control bleeding by tamponade, vasoconstriction, and/or an inflammatory reaction after the injection of a variety of agents. Saline alone will compress the vessels. Sclerosants such as alcohol, ethanolamine, and polidocanol cause greater vascular thrombosis, but can result in tissue injury and necrosis and are used less frequently. Epinephrine (1:10,000-1:20,000) provides local tamponade, vasoconstriction, and improved platelet aggregation to promote hemostasis. It is the agent of choice in the United States. Its effects will only last for 20 minutes and therefore requires it be used with an additional more durable treatment.
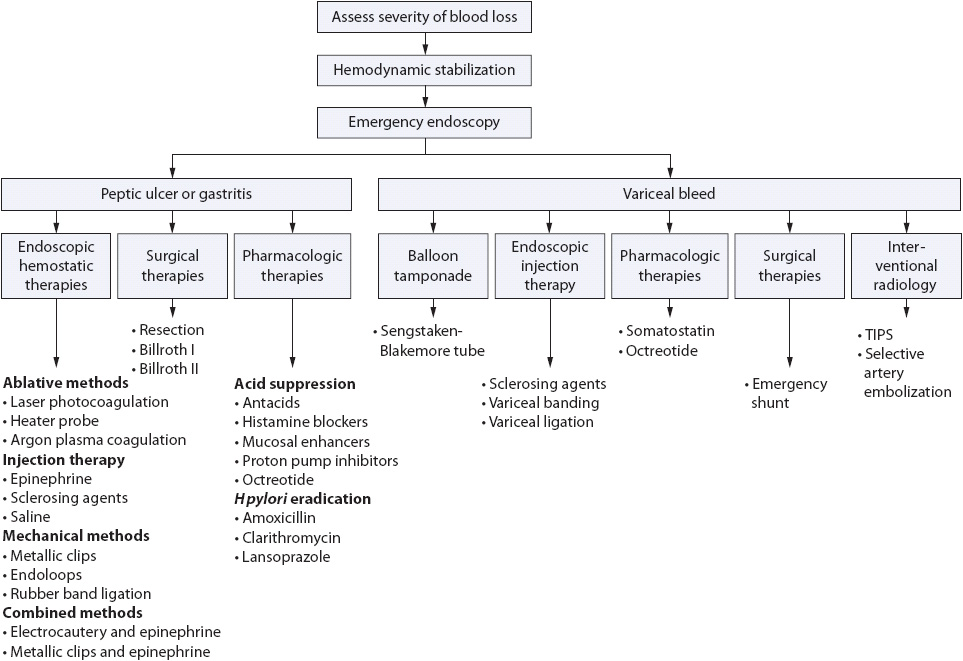
Figure 14-4. Upper GI bleeding treatment guide.
Electrocautery and argon plasma coagulation are examples of ablative treatments and are equally effective. Bleeding vessels can also be mechanically compressed using metallic clips, endoloops, or rubber band ligation. Metallic clips are the mechanical treatment of choice and have been shown to be as effective as other endoscopic techniques. Combination therapy with epinephrine injection has become the standard treatment for actively bleeding ulcers. Adding a second endoscopic treatment, either an ablative therapy or endoclips, significantly reduces the rate of recurrence, need for surgery, and mortality. It is no longer recommended to use epinephrine alone. If the patient rebleeds, a second attempt with endoscopic control is advocated before surgical intervention. Rebleeding is more common in patients with variceal bleeds and is highest initially after admission and for the first 24 hours.
Endoscopy rarely causes serious complications. Risks include GI perforation, precipitation of bleeding, aspiration, respiratory or cardiac compromise, and missed lesions.
Treatment of a Mallory-Weiss tear is supportive therapy. Bleeding episodes are self-limited and the mucosa will heal within 72 hours in 90% of patients.
Today significant bleeding from stress gastritis is rarely encountered. This is due to improvements in the management of shock and sepsis, as well as the prophylactic use of acid-suppressive therapy.
Pharmacologic treatment to reduce portal hypertension may be considered as preparations are underway for emergent upper endoscopy in the patient admitted with variceal upper GI bleeding. In the past, vasopressin was used in combination with nitroglycerin. Somatostatin or its analogue octreotide are now the vasoactive agents of choice. They can induce splanchnic vasoconstriction without the cardiac side effects of vasopressin. Continuous intravenous infusion of these agents results in temporary control of bleeding so that resuscitation, diagnostic, and therapeutic measures can be completed. Pharmacologic treatments are summarized in Table 14-4. At the time of endoscopy, both sclerotherapy and variceal banding or ligation have been shown to control bleeding. Currently, balloon tamponade (Sengstaken-Blakemore tube) is reserved for patients with massive hemorrhage. Once bleeding is controlled, more definitive therapies can be used. Treatment of esophagogastric varices will also include antibiotic prophylaxis for spontaneous bacterial peritonitis. A third-generation cephalosporin is indicated as bacteremia is often present in patients on admission for variceal bleeding.
1. Monitor for complications of endoscopic therapy and/or the sclerosing agents used to treat the ulcer or varix. Complications may include fever and pain because of esophageal spasm, motility disturbances of the esophageal sphincter, and perforation. Systemic complications of endoscopic therapy and/or sclerosing agents also may occur and predominantly affect the cardiovascular and respiratory systems. Cardiovascular effects include heart failure, heart block, mediastinitis and pericarditis. Respiratory effects include aspiration pneumonia, atelectasis, pneumothorax, embolism, and acute respiratory distress syndrome.
2. Institute pharmacologic therapies as prescribed to treat peptic ulcer disease or gastritis (stress ulcers). The most common pharmacologic agents and their actions are reviewed in Table 14-4. PPIs are the drug of choice in patients who have had non-variceal bleeding. They provide a more durable and sustained acid suppression than histamine receptor antagonists. The use of PPIs has been shown in randomized clinical trials to lead to a decrease in recurrent bleeding due to ulcer disease, need for transfusions, surgery, and the length of hospital stay. The use of high dose IV PPIs for 3 days after successful endoscopic treatment has been recommended (80 mg esomeprazole bolus, 8 mg/h continuous infusion). Oral PPIs are recommended for 6-8 weeks after an upper GI bleeding episode to allow for healing of the mucosa. Their use is especially beneficial in patients who use chronic NSAIDs or who have had Helicobacter pylori infection.
3. Administer pharmacologic therapies as prescribed to treat variceal bleeding (Table 14-5). Pharmacologic agents exert their effect by constricting splanchnic blood flow and thereby reducing portal pressure.
4. Intra-aortic balloon pump therapy may be instituted to achieve temporary vascular control in patients in shock. This therapy optimizes blood pressure, increases aortic diastolic pressure, increases coronary flow, and allows time for rapid resuscitation.
5. A tamponade tube, most commonly the Sengstaken-Blakemore tube (Figure 14-5), may be used to emergently decrease blood flow through the varix and to control bleeding so that endoscopy can be performed. Rebleeding is common after deflation or removal. Monitor for complications of this tube, including pulmonary aspiration, rupture of the esophagus, asphyxia, and erosion of the esophageal or gastric wall.
TABLE 14-4. PHARMACOLOGIC THERAPIES FOR ULCER DISEASE/GASTRITIS
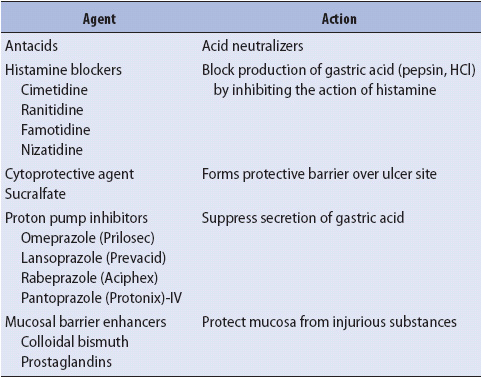
TABLE 14-5. PHARMACOLOGIC THERAPIES FOR VARICEAL UPPER GI BLEEDING
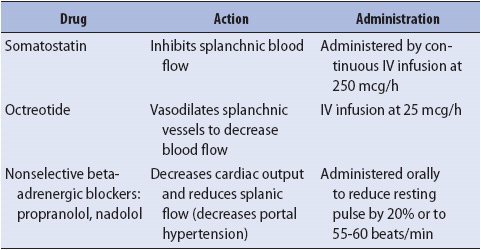
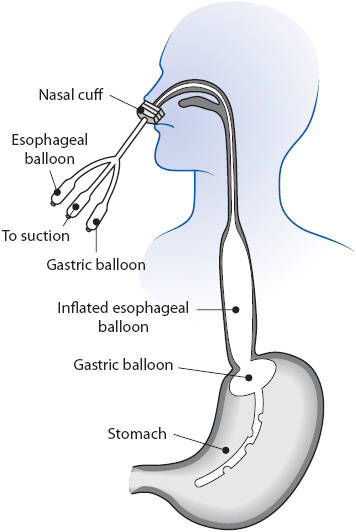
Figure 14-5. Placement of a Sengstaken-Blakemore tube.
Maintain esophageal suction to prevent aspiration. Keep scissors at the bedside to cut and remove the tube if it becomes malpositioned and the tamponade balloon occludes the airway. Endotracheal intubation is usually recommended to prevent pulmonary complications. Release pressure of the esophageal and or gastric balloons at regular intervals to prevent erosions. Administer frequent mouth care and monitor the skin around the tube to prevent necrosis from pressure of the tube.
When variceal bleeding is severe and cannot be controlled endoscopically, emergent portal decompression is achieved with the percutaneous transjugular intrahepatic portosystemic shunt (TIPS).
In the TIPS procedure, a long needle is passed from the right transjugular vein to the hepatic vein into a branch of the portal vein and a stent is placed. This decreases the pressure in the portal vein (decreases portal pressure) and subsequently on the varices to prevent rupture and bleeding.
The advantage of the TIPS procedure is that it can be performed in the interventional radiology department. Complications of the TIPS procedures include puncture of the biliary system, bleeding, infection, and clotting of the stent. Postprocedural systemic failure (septic shock, renal failure) and hepatic encephalopathy (see next section) are also associated complications.
1. Monitor blood pressure, ECG, and pulse oximetry throughout the procedure.
2. Administer preprocedure antibiotic coverage for gram-negative organisms as prophylaxis for sepsis.
3. Provide moderate IV sedation to treat anxiety.
4. Provide pain medication (eg, fentanyl). Certain parts of the procedure, such as balloon dilation of the intrahepatic tract, can be painful.
5. Have lidocaine and atropine available to manage potential complications of the procedure. The vasopressin infusion can cause bradyarrhythmias. Due to the proximity of the hepatic vein to the right ventricle of the heart, ventricular ectopy can be induced during the procedure.
6. Have crystalloids, vasopressors, PRBCs, and fresh frozen plasma readily available to manage hypotension from sepsis, bleeding, or sedation.
7. Have continuous and intermittent suction ready to manage bleeding and airway patency.
Surgery is considered for patients who have massive bleeding that is immediately life threatening and for patients who continue to bleed despite aggressive medical therapies. Surgical therapies for peptic ulcer disease or stress ulcers include gastric resections such as antrectomy, gastrectomy, vagotomy, or combination procedures. An antrectomy or gastrectomy may be performed to decrease the acidity of the duodenum or stomach by removing gastric-acid secreting cells. A vagotomy decreases acid secretion in the stomach by dividing the vagus nerve along the esophagus. Combination procedures are common and one example is the Billroth I, which is a vagotomy and antrectomy with anastomosis of the stomach to the duodenum. A Billroth II consists of a vagotomy, resection of the antrum, and anastomosis of the stomach to the jejunum (Figure 14-6). The latter is preferred over the Billroth I because it does not present the risk for dumping syndrome. Gastric perforations can be treated by simple closure.

Figure 14-6. Billroth I and II procedures.
Surgical decompression of portal hypertension can be accomplished by a procedure called a portacaval shunt. This procedure connects the portal vein to the inferior vena cava, diverting blood from the liver into the vena cava to decrease portal pressure. With the newer interventional radiology techniques this surgery is seldom performed. Liver transplantation can also relieve portal hypertension, but must be considered by weighing the risks vs the benefits in this patient population.
1. Monitor for fluid and electrolyte imbalances postoperatively due to intraoperative fluid loss and the drains inserted to decompress the stomach or to drain the surgical site.
2. Provide for adequate nutrition to promote wound healing.
3. Monitor the appearance of the incision and surrounding tissue.
4. Document and report all wound drainage (color, amount, odor) and complaints of pain or tenderness.
5. Culture any suspicious drainage.
6. Monitor white blood cell count and temperature trends.
When variceal bleeding is severe and cannot be controlled endoscopically, emergent portal decompression is achieved with the percutaneous TIPS.
1. Encourage communication with a calm, interested, and centered approach; for example, “Mr. B, you look nervous (worried) to me. Can you tell me what is bothering you?”
2. Assess the patient’s previous coping skills that were used in similar difficult situations (eg, did family presence, watching TV, listening to music, or using relaxation techniques alleviate anxiety?).
3. Offer appropriate reassurance, facts, and information as requested by the patient. Explain the ICU routine and procedures to the patient. Present information in terms that the patient can understand. Repeat and rephrase the information as necessary. Allow the patient to ask questions.
4. Help the patient to establish a sense of control. Assist the patient to make distinctions among those things he or she can (and should) control (eg, bath time, working on reducing anxiety level) and those things that cannot be controlled (eg, need for vasopressors and monitoring equipment).
5. Guide the patient in discovering that he or she has some control over anxiety and fear. Encourage the patient to participate in breathing and relaxation exercises as a strategy to control the current situation.
The liver has a central role in the body’s metabolism. Metabolic functions include the synthesis of carbohydrates, fats, proteins, and vitamins for nutrition, energy, and key metabolic pathways. Additional processes performed by the liver include the formation of bile, bilirubin metabolism, synthesis of coagulation factors, and detoxification of drugs and toxins. Liver failure may be acute or chronic. Irrespective of the cause of liver injury, inflammation results in damage to hepatocytes, known as “hepatitis.” Injured areas are surrounded by scar tissues leading to fibrosis, and after a period of time progressive fibrosis results in cirrhosis or replacement of the normal hepatic tissue with fibrotic tissue. Chronic liver failure is a slow deterioration that evolves over years leading to cirrhosis. Liver dysfunction potentially can be reversed early as the liver has a regenerative capability; however, fibrotic changes are irreversible resulting in chronic dysfunction and eventual end-stage liver disease.
Acute failure, also known as fulminant hepatic failure, results in a rapid deterioration of liver function in a person without prior liver disease. This cellular insult results in massive cell necrosis leading to a multiorgan dysfunction. Acute liver failure is rare and defined by a coagulation abnormality (usually an INR > 1.5) and encephalopathy without previous liver disease. The time between presentation of either encephalopathy or coagulopathy is usually defined as within 26 weeks.
The leading causes of acute liver failure in the United States and Europe are acetaminophen overdose and idiosyncratic drug reactions, while other causes include viral hepatitis, autoimmune disease, and shock (Table 14-6). Survival in acute liver failure can be categorized into patients in whom intensive care enables recovery of hepatic function and patients who require liver transplantation. Common causes of chronic liver disease include nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, alcoholic liver disease, chronic hepatitis B and C, and hemochromatosis.
TABLE 14-6. COMMON CAUSES OF LIVER FAILURE

Nonalcoholic fatty liver disease is one of the most common causes of chronic liver disease in the Western world. It is associated with obesity, type 2 diabetes, and metabolic syndrome. The spectrum of liver disease associated with this syndrome can range from simple steatosis to advanced fibrosis and cirrhosis. Alcoholic liver injury results from the toxic effects of ethanol on the hepatocytes.
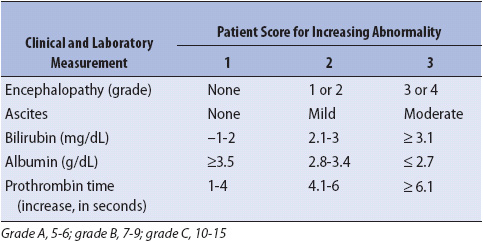
The Child-Pugh classification has served as a long-standing assessment tool to score hepatic function. This classification is based on two clinical variables and three biochemical tests (Table 14-7). Classes A to C are used to define patients from well-compensated disease (Class A) to advanced decompensated disease (Class C).
TABLE 14-7. CHILD-PUGH CRITERIA FOR HEPATIC FUNCTIONAL RESERVE
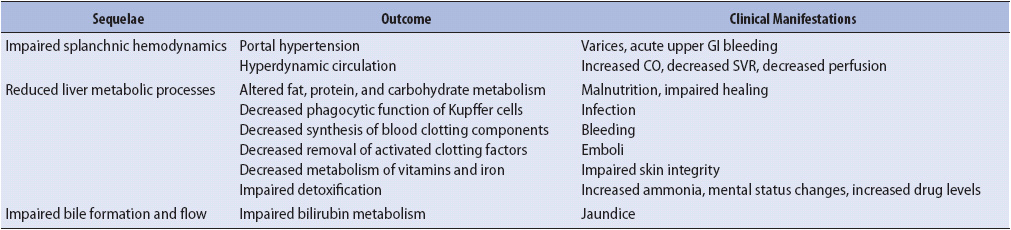
Clinical manifestations are directly related to failure of the liver to perform important metabolic processes (Table 14-8). Complications of liver failure include ascites, hepatic encephalopathy, acute respiratory distress syndrome, electrolyte imbalance, hepatorenal syndrome, and spontaneous bacterial peritonitis.
TABLE 14-8. SEQUELAE OF LIVER FAILURE
Jaundice is secondary to excessive deposition of bilirubin in tissues including skin, mucous membranes, and sclera, resulting in the characteristic yellow discoloration. This deposition of bilirubin represents failure of the liver to adequately uptake, conjugate, and excrete bilirubin. Pruritus is a common associated symptom that can cause much discomfort to patients.
Ascites is the abnormal collection of fluid in the peritoneal cavity. Cirrhosis is the most common cause of ascites and is theorized to be a result of portal hypertension. Increased pressure in the portal system occurs secondary to fibrosis in the liver, causing an obstruction to venous flow. This results in increased nitric oxide, vasodilatation, and renal function compromise, resulting in sodium and water retention. Fluid shifts from the intravascular space into the peritoneal space.
Hepatic encephalopathy defines a spectrum of neuropsychiatric abnormalities that occur with liver failure. Most theories support the pathogenesis that decreased hepatic clearance of certain cerebral toxins results in psychiatric manifestations. Serum ammonia is most often implicated. Ammonia is produced by bacteria in the bowel and in the liver it is converted to urea for excretion. In liver failure, this function of the liver is impaired, allowing ammonia to directly enter the central nervous system. Because ammonia is neurotoxic, as serum ammonia levels rise, the patient often exhibits signs of impaired cerebral functioning or encephalopathy. These signs can range from minor sensory-perceptual changes such as muscle tremors, slurred speech, or slight mental status changes to marked confusion or profound coma.
Classifications of deterioration in brain function have been used, from grade I (mild or episodic drowsiness, impaired concentration/intellect, but arousable and coherent); grade II (increased drowsiness, confusion, and disorientation, but able to arouse); grade III (very drowsy, agitated, disoriented, but able to respond to simple verbal commands); to grade IV (unresponsive except to painful stimuli). Cerebral edema can occur in up to 80% of patients with grade IV encephalopathy. The cause of cerebral edema is poorly understood but recognized as a leading cause of death. Patients with hepatic encephalopathy need to be carefully assessed for other causes of encephalopathy such as sepsis, uremia, acidosis, alcohol withdrawal, hypoxia, and intracerebral bleed.
The major pulmonary complication in liver failure is arterial hypoxemia. The cause has been linked to vascular dilatation in the lung and acute respiratory distress syndrome. Pulmonary edema is also a common finding.
A variety of electrolyte imbalances occur in liver failure. Hypoglycemia develops due to massive hepatic cell necrosis, leading to loss of glycogen stores and diminished glucose release. Hypokalemia may occur from inadequate oral intake, increased potassium losses from vomiting, or from medical interventions (eg, nasogastric suction or diuretic therapy). Hypomagnesemia commonly occurs in conjunction with hypokalemia as there is a close relationship between the movement of these electrolytes. Hypocalcemia is a complication of blood transfusions because the citrate used to anticoagulate stored blood causes calcium depletion. Hypophosphatemia is also commonly associated with acute liver failure. The exact mechanisms remain unknown. Alkalosis and acidosis may both occur.
Hepatorenal syndrome is a unique form of renal failure associated with severe liver disease. This syndrome represents the most frequent fatal complication of liver failure. The pathogenesis is thought to be related to portal hypertension and eventual sustained renal vasoconstriction, resulting in decreased renal perfusion.
Esophageal and gastric varices result from portal hypertension and develop in most patients with advanced cirrhosis. Mortality is significant and prevention with measures including beta-blockers and endoscopic band ligation is employed (see previous section addressing GI bleeding). Similar prominent veins in the abdominal wall and around the umbilicus (caput medusa) may develop.
Spontaneous bacterial peritonitis is defined as an infected ascitic fluid collection without an evident intra-abdominal source. This represents the most common bacterial infection seen and occurs approximately in a quarter of patients admitted with chronic liver failure and ascites. A single microbial organism is usually responsible, such as E coli, and is theorized to be caused by intestinal translocation of organisms that are able to seed the ascitic fluid.
The liver has a multitude of functions including metabolism of carbohydrates, fats, and proteins. The liver also stores essential minerals such as iron, copper, and vitamins A, B12, D, and K.
Malnutrition occurs frequently in patients with hepatic failure due to decreased oral intake and alterations in the metabolism and storage of nutrients. In advanced hepatic failure, the impaired ability to synthesize and store glycogen results in rapid muscle loss even during brief periods of decreased nutrient intake. Patients frequently require vitamin K to normalize PT and PTT, and those with recent ethanol intake should receive intravenous thiamine.
• Exposure to contaminated food, water
• Exposure to blood, body fluids
• Alcohol abuse
Impaired Thought Processes
• Mental status changes (confusion, lethargy)
• Behavioral changes
• Delirium
• Seizures
• Coma
Impaired Gas Exchange
• Hypoxemia
• Pulmonary edema
Fluid Volume Deficit or Excess
• Hypotension
• Skin cool, pale, and dry
• Urine output < 30 mL/h
• Tachycardia
• Dry mucous membranes
Hyperdynamic Circulation
• Arrhythmias
• Fever
• Palmar erythema (flushed palms)
• Jugular vein distension
• Rales
• Murmur
• Increased CO
• Decreased systemic vascular resistance
Altered Nutrition
• Decreased appetite
• Muscle wasting
• Nausea and vomiting
Impaired Liver Metabolism
• Jaundice
• Dry skin
• Ascites
• Total bilirubin > 1 mg/dL
• AST >36 IU/L
• ALT >24 IU/L
• PT >13 seconds
• aPTT > 45 seconds
• Fibrinogen < 200 mg/dL
• Albumin < 3.2 g/dL
• Ammonia > 45 mg/dL
• Ultrasound, endoscopy, endoscopic retrograde cholangiopancreatography (ERCP), liver angiography/biopsy
The management of the patient with liver failure is centered on decreasing the metabolic requirements of the liver, supporting cardiopulmonary status, supporting hematologic and nutritional functions of the liver, and preventing and treating complications.
1. Place the patient on bed-rest to decrease the metabolic needs of the liver. Position the head of the bed at 45° at all times to minimize complications related to ascites. Institute measures to prevent skin breakdown.
2. Monitor drugs that are metabolized or detoxified by the liver, especially narcotics and sedatives.
1. Monitor fluid balance. The patient may have a fluid volume deficit related to portal hypertension, ascites, GI bleeding, or coagulation abnormalities. Fluid overload may be a problem related to sodium excess and hypoalbuminemia.
2. Assist with paracentesis that may be instituted to reduce ascites. Fast removal of fluid via paracentesis requires IV colloid replacement to prevent dehydration. Administer diuretics such as furosemide and spironolactone as prescribed. Weigh patient daily. Monitor abdominal girth when ascites is present.
3. Monitor respiratory status and correlate with arterial blood gas results. Administer oxygen as ordered. Administer sedatives and analgesics cautiously. Assist the patient with maneuvers to improve oxygenation.
1. Monitor for signs of bleeding (eg, gastric contents, stools, urine) and test for occult blood. Observe for petechiae and bruising. Monitor hematologic profile.
2. Administer blood and blood products as ordered.
3. Institute measures for variceal bleeding as needed, including beta blockers.
4. Institute measures to provide for safety and to minimize tissue trauma. Provide for frequent mouth care. Avoid use of rectal tubes.
5. Provide frequent small meals and a bedtime snack containing carbohydrate to prevent muscle wasting. Normal amounts of protein are tolerated in patients who have received appropriate medications for encephalopathy. Consider enteral nutrition (EN) if oral intake is insufficient.
6. Monitor for signs and symptoms of infection. Maintain sterility of invasive lines and tubes. Maintain aseptic technique when performing procedures.
The most common complications of liver failure are hepatic encephalopathy, fluid and electrolyte imbalances, hepatorenal syndrome, and variceal hemorrhage.
1. Observe for changes in mental status. Institute safety measures during periods of mental status changes. Rule out other causes of encephalopathy. Treat precipitating causes.
2. Administer cleansing enemas and cathartics to keep the bowel empty. Lactulose has been a first line treatment to decrease gut ammonia production. Recent research has demonstrated the efficacy of Rifaximin in maintaining remission from hepatic encephalopathy. Monitor patient response to therapy through neurologic assessments and serum ammonia levels. Monitor the use of medications metabolized by the liver.
3. Institute protocols for acute upper GI hemorrhage due to variceal rupture (see previous section).
4. The first and only definitive treatment for hepatorenal syndrome has been liver transplant. Some response has been demonstrated with the use of albumin as an intravascular expander in combination with a vasoconstrictor Terlipressin, another vasopressin analogue.
Efforts to find ways to assist patients with acute liver failure until organ transplantation have led to research in devices that support the liver until an organ is available, or the liver’s regenerative systems recover. All artificial liver support systems involve extracorporeal circulation of the patient’s blood through filters that remove waste products normally filtered by the liver. Currently available liver support systems are not recommended outside of clinical trials.
Liver transplantation has changed the survival of patients with liver failure. The decision to proceed with transplantation requires a detailed assessment and multidisciplinary review. The model for end-stage liver disease, or MELD, is a scoring system that uses serum creatinine, bilirubin, and INR to predict mortality in end-stage liver disease. In 2002, the MELD was adopted as the index to determine transplant priority.
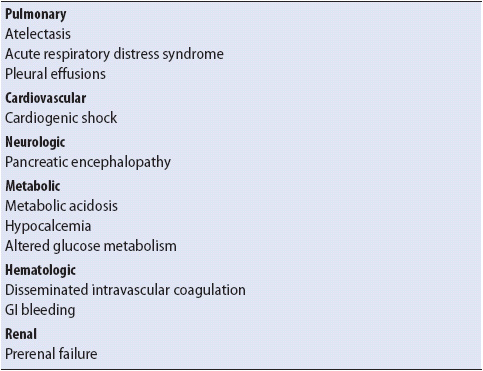
Acute pancreatitis is inflammation of the pancreas resulting from premature activation of pancreatic exocrine enzymes, such as trypsin, phospholipase A, and elastase within the pancreas. The disease ranges in severity from a mild self-limiting form to severe acute pancreatitis. Severe acute pancreatitis is seen in approximately one-fifth of patients with pancreatitis and has a mortality rate of 30%. In the severe acute form, autodigestion and necrosis of the pancreas can occur. This results in the release of inflammatory mediators, which can lead to multisystem failure (see Chapter 11, Multisystem Problems).
The diagnosis of acute pancreatitis is based on at least two of the three following criteria: characteristic abdominal pain or epigastric pain that may radiate to the back; serum amylase or lipase values greater than 3 times the normal range; and characteristic findings on imaging, most often CT imaging. In general, serum lipase is thought to be more sensitive than serum amylase as a marker of pancreatitis. Organ failure and pancreatic necrosis are the two most important markers of severity. To accurately identify the severity of acute pancreatitis, scoring systems have been used such as the acute physiology and chronic health evaluation (APACHE–II).
Recent scoring systems that offer an assessment of disease severity within the first 24 hours have demonstrated clinical usefulness. The bedside index of severity of acute pancreatitis (BISAP) is one example. This tool has demonstrated both accuracy as well as simplicity. The score is calculated on five variables:
1. Blood urea nitrogen greater than 25 mg/dl
2. Impaired mental status with a Glasgow Coma Score greater than 15
3. Presence of systemic inflammatory response syndrome
4. Age greater than 60 and
5. Pleural effusion on imaging
Each variable provides one point and scores of 3, 4, and 5 are associated with hospital mortality of 5.3%, 12.7%, and 22.5% respectively.
The leading causes of acute pancreatitis are alcohol disease and biliary tract disease (stones). Drug-induced causes have been linked to metronidazole, tetracycline, azathioprine, and estrogens. Other less common etiologies are hyperlipidemia, hypercalcemia, infectious, autoimmune, vascular, genetic, pancreatic neoplasms, and idiopathetic.
The pathogenesis of acute pancreatitis is not completely clear. The pancreas normally has a protective mechanism, an enzyme called trypsin inhibitor, to prevent activating enzymes before they reach the duodenum, thereby preventing inflammation of pancreatic cells. Regardless of the etiology, the process of premature activation of pancreatic enzymes is characteristic of pancreatitis, leading to local inflammation and potential necrosis of the pancreas. The activated enzymes can also enter the systemic circulation via the portal vein and lymphatics. This is thought to stimulate platelet-activating factor and humoral systems (kinin, complement, fibrinolysis). This results in multisystem failure with a variety of complications (Table 14-9; see also Chapter 11, Multisystem Problems). Pancreatic abscess, pseudocyst, and necrosis are not uncommon with fulminant forms of the disease.
TABLE 14-9. COMMON MULTISYSTEM COMPLICATIONS OF ACUTE PANCREATITIS
Pancreatic Inflammation
• Acute pain: Severe, relentless, knifelike; midepigastrium or periumbilical
• Abdominal guarding
• Nausea
• Rebound tenderness
• Vomiting
• Abdominal distention
• Hypoactive BSs
Fluid Volume Deficit
• Hypotension
• Tachycardia
• Mental status changes
• Cool, clammy skin
• Decreased urine output
Impaired Gas Exchange
• Decreasing PaO2 (< 60 mm Hg) and SaO2 (< 90%)
• Serum amylase > 100 IU/L
• Serum pancreatic isoamylase > 50%
• Serum lipase > 24 IU/dL
• Serum triglycerides > 150 mg/dL
• Urine amylase > 14 IU/h
• Serum calcium < 8.5 mg/dL
• Serum sodium < 135 mEq/L
• Serum potassium < 3.5 mEq/L
• Serum magnesium < 1.5 mg/dL
• Increased ALT (> 120 U/L), in gallstone pancreatitis
• C-reactive protein (> 120 mg/L)
• Computed tomography (CT)
• ERCP
• MRI
The management of the patient with acute pancreatitis centers on disrupting the cycle of enzyme release of the pancreas and treating complications that can occur with multisystem disease. Interventions within the first 24 hours have been identified as essential to positive outcomes including increased survival. These interventions include scoring severity with BISAP or an alternative scoring system and aggressive fluid resuscitation. Additional principles of management include preventing hypoxemia, resting the pancreas, pain management, and supporting other organ systems that may fail because of mediators released during the inflammatory process.
Patients with acute pancreatitis experience significant hypovolemia as a result of third space losses, vomiting, and vascular permeability related to inflammatory mediators. Hypovolemia can compromise pancreatic circulation and has been linked to pancreatic necrosis. Aggressive fluid and electrolyte replacement is viewed as the key element in the initial management. In severe acute pancreatitis, blood vessels in and around the pancreas may also become disrupted, resulting in hemorrhage.
1. In adults, infusion of intravenous fluids is begun with rates between 250 and 300 mL/h. High-dose fresh frozen plasma is indicated to replace lost circulating proteins. Monitor outcomes of fluid replacement therapy, including blood pressure, heart rate, intake and output, preload indicators (CVP, PCWP), skin turgor, capillary refill, mucous membranes, and urine output (goal of at least 0.5 mL/kg/h).
2. Monitor for signs and symptoms of hemorrhage (low hematocrit and hemoglobin levels). Cullen sign is a bluish discoloration around the umbilical area, and Grey Turner sign is a bluish discoloration around the flanks, indicating blood in the peritoneum. Monitor for increasing abdominal girths.
3. Monitor electrolytes for imbalances related to prolonged vomiting or fluid sequestration. Calcium, sodium, magnesium, and potassium are most commonly affected. Monitor QT intervals on the electrocardiogram and implement seizure precautions with severe hypocalcemia. Hyperglycemia also may be present due to the stress response and impaired secretion of insulin by the islet cells in the inflamed pancreas. Administer an insulin infusion, then sliding scale insulin to obtain a normoglycemic state.
Acute pain is the only universal sign of acute pancreatitis. It is caused by peritoneal irritation from activated pancreatic exocrine enzymes, edema or distention of the pancreas, or interruption of the blood supply to the pancreas. Treatment of pain is a priority because it causes increased exocrine enzyme release by the pancreas, which may worsen the pathologic process.
1. Assess the degree of pain by having the patient use a pain-rating scale.
2. Administer pain analgesics. There is controversy about the use of opiate analgesics (eg, morphine) because they may cause spasm of the sphincter of Oddi, which may worsen the pain. Use a pain rating scale to assess patient outcomes regardless of what is prescribed. Consider scheduled doses or continuous infusion of pain medication for severe pain. Consider epidural analgesia for unrelieved acute pain.
3. Assess patient anxiety and administer sedatives with analgesics.
4. Assist the patient to a position which promotes comfort. The knee-to-chest position often decreases the intensity of the pain.
Preventing stimulation of pancreatic exocrine secretion is a priority to interrupt the cycle of pancreatic inflammation.
1. In the past, avoiding the use of the upper GI tract with oral or gastric feeding was recommended until the patient no longer reported abdominal pain and the serum amylase has returned to normal. That recommendation has been changed.
2. Enteral nutrition with jejunal feedings is often preferred to prevent pancreatic stimulation and enzyme secretion. New research suggests that gastric feedings may be feasible as well.
3. Administer pharmacologic agents as prescribed to block the secretion of pancreatic enzymes. These include anticholinergic agents, cimetidine, and somatostatin.
Local complications in the pancreas include peripancreatic fluid collections, pancreatic pseudocyst, and necrotic collections. In an effort to standardize the recognition and definition of these complications, scoring systems have emerged; one of the most recognized is the Atlanta Classification. Percutaneous or stent therapies to drain the fluids in and around the pancreas and/or surgical resection or debridement may be required, especially if the pancreas becomes infected. Biliary ERCP and laparoscopic cholecystectomy are indicated for gallstone pancreatitis.
Cardiopulmonary complications are the most common multisystem problems. As mentioned, they are thought to be due to pancreatic enzyme–induced mediators. Pancreatic ischemia is also known to promote the release of myocardial depressant factor. This causes decreased myocardial contractility and CO. Surgical therapies such as a pancreatic resection may be performed to prevent systemic complications of acute necrotizing pancreatitis by removing necrotic or infected tissue. In some cases, a pancreatectomy may be performed, but it is associated with considerable mortality.
1. Administer oxygen therapy to maintain arterial oxygen tension and oxygen saturation. Mechanical ventilation with adjunct therapies to promote maximal alveolar gas exchange is often used to manage acute respiratory failure (see Chapter 10, Respiratory System).
2. Administer low-dose dopamine to support myocardial contractility. Dobutamine may also be considered if sepsis is not a complication. Avoid alpha constrictors.
3. Institute measures to prevent infection. Monitor for signs and symptoms of sepsis and initiate appropriate treatment if indicated.
4. Manage coagulopathies (see Chapter 13, Hematologic and Immune Systems).
5. Treat acute tubular necrosis if a complicating factor (see Chapter 15, Renal System).
Major disorders of the intestine include intestinal ischemia. Vascular occlusion of the mesenteric vessels is rare but catastrophic and will result in profound illness. Intestinal ischemia may present as intestinal angina, ischemic colitis, or intestinal infarction. Ischemic colitis is the most common ischemic injury. Ischemia may be acute or chronic. Acute forms are due to sudden and complete arterial occlusion by emboli, thrombosis of atherosclerotic stenosis, small vessel occlusion, or venous thrombosis. Gradual occlusion is better tolerated as there is time for collateral circulation to form. Sudden or acute ischemia is poorly tolerated because the bowel is not protected by collateral circulation. There is an extensive mesenteric collateral circulation which protects against ischemic insults. The colon is particularly susceptible to low-flow states; in particular, the splenic flexure, ileocecal junction, and rectosigmoid.
Intestinal ischemia develops from a compromise in blood flow to the intestine, which is inadequate to meet metabolic demands. It is the result of both hypoperfusion and reperfusion injury. Both the small intestine and the large bowel can be affected. Ischemic colitis will affect segments of the colon with normal colon on either side of the affected area. The right colon is affected 25% of the time, transverse 10%, left 33%, distal colon 25%, and the entire colon 7%. Disease involving the right side of the colon is usually more severe with the patient at risk for having involvement of the small intestine. Three major arterial trunks—the celiac axis, superior mesenteric artery, and inferior mesenteric artery comprise the splanchnic (intestinal) circulation. Ischemic colitis is the most common form of intestinal ischemia. The colon is perfused by the superior mesenteric artery, the inferior mesenteric artery, and branches of the internal iliac arteries.
An acute occlusion is usually the result of a cardiogenic embolus with the superior mesenteric artery most frequently affected. The tissue injury that occurs will result in the release of cellular contents and the by-products of anaerobic metabolism into the general circulation. The ischemic bowel loses protein, electrolytes, and fluid into the lumen and wall of the bowel. The third-space extracellular fluid loss decreases the circulating blood volume. Full-thickness necrosis leads to bowel perforation and peritonitis.
The underlying causes of intestinal ischemia are diverse and include decreased CO, hypovolemia, arrhythmias, hypercoagulable states, mechanical obstruction, vascular disease, and trauma. Predisposing medications include cocaine, cardiac glycosides, and alpha-stimulating sympathomimetic amines (epinephrine, norepinephrine). The elderly patient with systemic atherosclerosis is particularly at risk. In a retrospective study, hyperthyroidism, stroke, and chronic obstructive pulmonary disease were statistically significant independent predictors of mortality.
Signs and symptoms will vary depending on the severity of the ischemia and area and length of intestine affected. The most common signs on admission to the hospital are hematochezia, abdominal pain, and diarrhea.
• Obstruction
• Diabetes mellitus
• Dyslipidemia
• Smoking
• Heart failure
• Aortic or coronary artery bypass surgery
• Shock
• Atrial fibrillation
• Atherosclerosis
• Medications: digitalis, diurectics, NSAIDs, catecholamines, and neurolyptics
• Recurrent indistinct abdominal symptoms
• Anorexia
• Fever
• Tachycardia
• Leukocytosis
• Metabolic acidosis
• Elevated lactate
• Elevated LDH
• Peritoneal signs (abdominal guarding and rebound tenderness)
• Acute onset, colicky, left lower abdominal pain
• Urgent desire to defecate
• Diarrhea
• Cramping
• Abdominal distention
• Decreased BSs
• Hematochezia (bloody stools)
• Abdominal tenderness
• Ileus
• Nausea and vomiting
• Post-prandial pain
• Muscle rigidity
• Fluid volume deficit
Diagnosis is based on clinical findings and supported by radiographic corroboration and colonoscopic evaluation. The use of a barium enema to diagnose acute colonic ischemia is no longer implemented. A CT scan is useful in supporting clinical suspicion and for identifying potential complications. Colonoscopy is the diagnostic modality of choice. It should be performed on an unprepped colon within 48 hours of presentation.
Colonoscopy will identify mucosal abnormalities and biopsies can be obtained. Findings will depend on the stage and severity of the ischemia. The finding of hemorrhagic, dusky mucosa with patches of inflammation is typical. Arteriography is indicated if acute mesenteric ischemia involving the small intestine is suspected. Arteriography can identify the site of occlusion and in addition can facilitate treatment. A cardiac work-up (ECG, holter monitor, transthoracic echocardiogram) are done to exclude a cardiac source for an embolism.
Patient priorities revolve around treating the intravascular fluid volume deficit maximizing and avoiding the use of vasopressors. Most patients will respond to conservative supportive therapy.
Medical treatment for intestinal ischemia will depend on the presentation and severity of the insult. Supportive care is provided with patients placed on bowel rest, antibiotics, and intravenous fluids. Hemodynamic status is optimized and vasoconstrictive drugs are avoided. The patient is monitored for signs of bowel necrosis such as persistent fever, leukocytosis, peritoneal irritation, or protracted pain or bleeding.
In the case of nonocclusive mesenteric insufficiency, a continuous infusion of a vasodilator, such as papaverine, into the superior mesenteric artery can be given intra-arteriorly at the time of arteriography.
Exploratory laparotomy with thromboembolectomy or bypass of the occlusion can be performed if the diagnosis is an acute mesenteric occlusion due to clot or an atherosclerotic plaque. Surgery is also indicated for peritonitis or clinical deterioration (increasing abdominal tenderness, guarding, rebound tenderness, rising temperature, and/or paralytic ileus) with 20% of patients needing surgical intervention for resection of the involved bowel. Current advances in endovascular treatments are increasingly used in patients with chronic mesenteric ischemia. The option for percutaneous transluminal angioplasty with stent placement avoids the risks associated with an open repair.
Bowel obstruction is a common cause for hospitalization and results in 15% of all emergency admissions for abdominal pain and 1.9% of all hospital admissions. Intestinal transit can be affected by either a mechanical or functional obstruction. Mechanical obstructions can be due to lesions which block the internal lumen (luminal or intrinsic) or by lesions which compress the bowel lumen from the outside of the intestine (extrinsic). Mechanical obstructions can be further classified as either a small-bowel obstruction (SBO) or a large-bowel obstruction (LBO); and complete or partial. Complete obstruction always requires surgical management, whereas a partial obstruction can be managed conservatively with serial examinations. Ileus and colonic pseudo-obstruction are categorized as functional obstructions.
Adhesions due to previous surgery are the most common cause of an SBO followed by malignant tumors (peritoneal implants), hernias, and inflammatory bowel disease. Adhesions account for more than 70% of all SBO. Studies have demonstrated that the incidence of SBO is lower in patients who have minimally invasive procedures vs open surgery.
Colorectal cancer is the most common cause of an LBO in the United States with the descending colon and rectosigmoid the most common sites of obstruction. Other causes of a mechanical obstruction owing to intrinsic causes include fecal impaction and foreign bodies. Inflammation (diverticulitis or inflammatory bowel disease), ischemia, intussusception, and anastomotic stricture are also intrinsic etiologies. Extrinsic causes include hernias, abscess, volvulus, or tumors in adjacent organs. Adhesions rarely lead to obstruction of the large bowel.
Early in the course of the obstruction, bowel motility and contractions will increase as the bowel attempts to push contents past the point of obstruction. This can account for diarrhea in the initial presentation. The intestine becomes fatigued, dilates, and contractions are less frequent and intense. Water and electrolytes accumulate in the bowel lumen and lead to dehydration and hypovolemia. Hypochloremia, hypokalemia, and metabolic alkalosis are not uncommon especially if the patient is vomiting or has high nasogastric tube losses. Abdominal distention can compromise respiratory function. In general, with either an SBO or LBO, a segment of the intestine can become trapped and the blood supply can become compromised or strangulated. The blood supply can also be compromised by the increasing tension related to the abdominal distention. Ischemia could result and, if not treated, can lead to bowel necrosis. The cecum is the most common site of colonic ischemia or perforation.
An ileus is intestinal distention and the slowing or absence of the passage of intestinal contents. It is a functional obstruction, so a mechanical cause cannot be identified. Common causes of an ileus are drug induced (anticholinergics, psychotropics, or opiates), metabolic derangements, neurogenic, and infections. Ileus is most common after abdominal operations and persists the longest after colon surgery.
Pseudo-obstruction, also called Ogilvie syndrome, is a condition of distention of the colon with signs and symptoms of obstruction, in the absence of a physical or mechanical cause. Acute colonic pseudo-obstruction (ACPO) is characterized by the absence of intestinal contractility. The exact cause remains unknown. It is commonly seen in hospitalized or institutionalized patients, the elderly, and patients with chronic renal failure, respiratory, cerebral, or cardiovascular disease. It has an unknown prevalence and incidence, and as its name implies, it primarily affects the colon. It is diagnosed only after excluding mechanical LBO. Patients may have a history of unnecessary repeated laparotomy.
Signs and symptoms will vary depending on the cause and location of the obstruction.
• Prior abdominal surgery
• Ischemia
• Hernia
• Abdominal cancer
• Abdominal radiation
• Inflammatory bowel disease
• Failure to pass stool or flatus
• Diarrhea
• Crampy or colicky abdominal pain; sometimes localized to periumbilical and epigastric regions, but usually diffuse
• Abdominal distention
• Generalized tenderness
• Nausea and vomiting
• Bowel sounds may be hyperactive with rushes or may be absent
• Visible peristalsis
• Tympany
• Tachycardia
• Hypotension
• Fever
• Localized tenderness, rebound, guarding (suggest peritonitis)
Plain films of the abdomen will demonstrate whether an obstruction is present. Dilated loops of bowel with air fluid levels are characteristic in the proximal bowel; distal bowel is collapsed. A CT scan of the abdomen and pelvis with oral contrast will show where the obstruction is, identify the transition zone, and demonstrate the etiology. It has become the diagnostic exam of choice. A small-bowel follow-through or water-soluble contrast enema may be necessary. Electrolyte disorders are common due to vomiting and lack of oral intake. The most common electrolyte abnormality is hypokalemia. The patient will exhibit either a metabolic or contraction alkalosis (renal sodium reabsorption in exchange for H+) or metabolic acidosis (GI bicarbonate loss and hypovolemic tissue hypoperfusion).
Treatment options will vary depending on the diagnosis. Initially, a nasogastric tube is placed to decompress the bowel, the intravascular fluid volume deficit is treated with isotonic fluids, electrolyte abnormalities are corrected, bowel rest is initiated, and antiemetics and antibiotics are administered. Long intestinal tubes are no longer indicated and are associated with longer hospital stays and prolonged ileus. A rectal tube can be used to decompress the distal colon in patients with LBO.
Treatment of an ileus is entirely supportive therapy. The most effective treatment is to address the underlying cause. Metabolic or electrolyte abnormalities are corrected and medications which may be producing the ileus are discontinued.
Acute colonic pseudo-obstruction is treated with the administration of neostigmine, a parasympathomimetic agent. It is important that mechanical causes for the obstruction have been excluded before administering the drug. In the treatment of ACPO, 2.5 mg of neostigmine is given intravenously over 3 minutes. The pseudo-obstruction will resolve within less than 10 minutes with the patient passing stool and flatus. If no response occurs, the dose can be repeated 4 hours later. Bradycardia, bronchospasm, and hypotension are side effects of neostigmine and patients must be monitored with telemetry. Atropine should be readily available. Patients with cardiac disease are not the candidates for this treatment. Patients who do not respond to neostigmine should undergo a colonoscopy for decompression. Surgery is only reserved for patients with signs of ischemia, perforation, or whose clinical status deteriorates.
Approximately 90% of all SBOs will resolve spontaneously with supportive therapy. Surgical therapy is required to treat an LBO and may be needed to treat an SBO. Procedures indicated may include lysis of adhesions, reduction of hernias, bypass of obstructions, and resection of affected intestine. Self-expandable metallic colon stents may be placed at the time of colonoscopy to decompress the colon and can be a bridge to elective surgery in patients with a malignancy. A permanent or temporary diverting ileostomy or colostomy may be performed. A flexible sigmoidoscopy can be used initially to decompress a sigmoid volvulus; definitive surgery follows.
1. Administer colloids and crystalloids to treat the fluid volume deficit. Normal saline with potassium supplementation is the replacement fluid of choice. Monitor patient response to fluid resuscitation—hemodynamic parameters (MAP, heart rate), body weight, and intake and output. A Foley catheter is placed to monitor urine output.
2. Administer antimicrobial therapy to treat intra-abdominal infection. Few practitioners will administer antibiotics while the patient is being observed.
3. Position with head of bed elevated to promote lung expansion to relieve pressure from the distended abdomen. Assist with deep breathing exercises to promote lung expansion, mobilization of secretions, and relaxation.
4. Administer analgesics and sedatives for pain management. Avoid excess use of opiates to promote the return of peristalsis. The use of narcotics in the patient in whom a decision has not been made to operate and the diagnosis is uncertain is controversial.
5. Insert a nasogastric tube and apply and maintain suction to drain and decompress the upper GI tract.
6. Monitor and report signs and symptoms of ongoing infection, peritoneal signs, or deterioration in status. Multiple follow-up abdominal radiographs and serial clinical examinations are indicated. Classic symptoms associated with strangulated bowel are leukocytosis, fever, tachycardia, and severe abdominal pain.
7. Provide nutrition as prescribed. Total parenteral nutrition (PN) may be required early in the course of therapy. Enteral therapy should be initiated as early as possible because it promotes the return of peristalsis and may assist in maintaining the gut mucosal barrier function. Enteral nutrition should be used with caution if bowel ischemia is suspected.
Bariatric surgery is increasingly becoming an option for weight reduction for obese individuals who have not been successful with conservative weight loss strategies such as diet, exercise, and pharmacologic therapy. Candidates for bariatric surgery include those patients with a body mass index (BMI) of 40, or a BMI between 35 and 40 in the presence of certain comorbidities, such as diabetes, hypertension, obstructive sleep apnea, and cardiovascular disease.
Because all patients who have bariatric surgery are obese, and many have comorbid diseases, surgical recovery can be particularly challenging. Obesity, diabetes mellitus, coronary artery disease, sleep apnea, and other conditions more common among the obese patients require careful postoperative monitoring.
There are three main types of weight loss surgery: restrictive, malabsorptive, and combined restrictive and malabsorptive. These procedures can be performed via either the laparoscopic or open approach. The majority are done laparoscopically because there is less pain, fewer wound complications, a shorter hospital stay, and quicker recovery. All procedures limit the volume of food eaten and alter gastric emptying. The risk for nutritional deficiencies will vary depending on the surgery performed. The restrictive procedures include the vertical banded gastroplasty (VBG), the laparoscopic adjustable gastric band (LAGB), and more recently the laparoscopic sleeve gastrectomy (LSG). There has been a recent change in the make-up of bariatric surgeries being performed in the United States. The number of laparoscopic sleeve gastrectomies being performed has increased with a reduction seen in the use of the LAGB. The LSG originated as a part of the duodenal switch operation. The laparoscopic Roux-en-Y (LRYGB) is categorized as both restrictive and malabsorptive, and has become the most commonly performed bariatric operation in the United States.
The VBG is done infrequently today, but was popular in the 1980s. The upper stomach near the esophagus is stapled vertically to create a small pouch. A band is placed to restrict the outlet from the pouch. With the LAGB, restriction is accomplished by placing an inflatable silicone band around the antrum of the stomach thereby creating a small pouch. The band is connected to an implanted reservoir under the skin, usually just below the rib cage. The pouch opening can be made smaller or larger by inflating or deflating the band via the reservoir.
The LSG has become an acceptable primary bariatric surgery. The procedure reduces the stomach to about 25% of its original size. A large portion of the stomach is removed following the major curve. The open edges are stapled to form a sleeve or tube with a “banana” shape. The procedure permanently reduces the size of the stomach. Although it is described as a restrictive procedure, recent studies have identified similar metabolic effects as seen with the LRYGB. These effects could potentiate a sense of satiety for patients. Studies demonstrate that weight loss after the LSG to be between that seen with the LAGB and LRYGB. It is a safe surgery which is easy to perform.
The biliopancreatic diversion (BPD), biliopancreatic diversion with duodenal switch (BPD-DS), and duodenal switch (DS) are malabsorptive procedures. These surgeries carry the highest risk for nutritional deficiencies as they result in significant alteration in digestion and malabsorption of protein, vitamins, and minerals. In general, there are three main components of these surgeries: a partial gastrectomy, the common or nutrient limb, and biliopancreatic limb. The common limb is a 50 to 100 cm portion of distal small bowel where limited digestion and absorption occur, while the biliopancreatic limb is created from the remainder of the proximal small bowel and functions to divert digestive juices to the nutrient or common limb.
The laparoscopic Roux-en-Y results in both restriction and malabsorption and is the gold standard surgery for treating obesity. The stomach is separated with a stapler and a 15-mL pouch is created. The small intestine is divided and the distal stomach, duodenum, and first part of the jejunum are bypassed. The distal end of the jejunum is anastomosed to the pouch (gastrojejunostomy) to allow for emptying while the proximal end is connected side to side to the jejunum (jejunojejunostomy) creating a 75- to 150-cm roux limb. The surgery also has a hormonal effect. Removing the gastric fundus, the primary site of ghrelin production, enhances weight loss by reducing appetite.
Standard nursing care of the postoperative patient, including assessment of vital signs and incisions, management of pain, pulmonary exercise, and deep vein thrombosis (DVT) prophylaxis is always implemented. In addition to standard postoperative care, assessment for, and prevention of, complications inherent to bariatric surgery are essential.
Airway obstruction and oxygenation problems are important postoperative concerns following bariatric surgery. A large number of these patients have documented obstructive sleep apnea preoperatively, which places them at higher risk for postoperative respiratory problems. Patients with sleep a pnea will use their CPAP or BiPAP machine while in the hospital to help minimize this risk. The increased risk of postoperative oxygenation problems from anesthesia and postoperative analgesics in this vulnerable group requires careful respiratory monitoring for 24 to 48 hours after surgery.
Leakage of gastric contents at the site of anastomosis is a potentially life-threatening complication and if not recognized early can lead to overwhelming sepsis. Signs and symptoms of an anastomotic leak include fever, left shoulder pain, tachypnea, and tachycardia. Thirst and hypotension are typically appreciated in progressive sepsis. Abdominal pain may occur, but the absence of it does not preclude the possibility of an anastomotic leak. The only sign of a leak may be unexplained tachycardia.
A leak is diagnosed with either a limited upper GI radiograph or a CT scan. A contained leak can be treated with percutaneous drainage. If the leak is not contained, the patient is returned to the operating room for definitive treatment. A leak could result in an intra-abdominal abscess. The key to treating a leak is to identify it early.
Nausea and vomiting should not be considered an expected consequence of bariatric surgery. The cause may be mechanical or behavioral. Vomiting should be very short lived as patients adjust to eating and drinking. Behavioral causes include eating too quickly, overeating, not chewing food well, drinking while eating, or a poor food choice. Dehydration may present as nausea. Anastomotic stricture or another mechanical cause of obstruction must be ruled out. Antiemetics are usually not helpful. If the nausea is due to dehydration, the symptoms will resolve with the administration of intravenous fluids. Counseling the patient will address the behavioral etiologies.
Patients having bariatric procedures are at high risk for pulmonary embolus (PE). Early ambulation postoperatively, which is particularly challenging in this patient population, is important in terms of reducing risk for DVT and PE. Preventing DVT and PE requires a combination of pharmacologic prophylaxis, use of sequential compression devices, and a program of ambulation for the patient. Optimal pain management is important not just for comfort, but to promote mobility. In patients with a prior history of DVT or PE or a history of a clotting disorder, an inferior vena cava filter may be placed preoperatively.
The bariatric surgery patient is at high risk for skin breakdown and poor wound healing. Skin folds harbor moisture, bacteria, and yeast; in addition, the blood supply to adipose tissue is poor. The best skin care is prevention and includes daily inspection of the skin, frequent turning, early ambulation, and special attention to the positioning of catheters and drainage tubes so that they are not hidden with skin folds. Skin care needs to be thorough, paying special attention to the folds under the breasts, back, abdomen, and perineum.
Another important consideration in the care of patients after bariatric surgery is the administration of medications. Because a portion of small bowel has been bypassed, absorption of medications will be impacted. Medications previously given as sustained-released formulations should be given in regular-release form to compensate for the changes in absorption. Tolerance of the gastrointestinal effects of some medications may be altered, and patients should be carefully monitored for a new or changing side effect profile.
Resumption of preoperative diabetic medications, both insulin and oral agents, should also be carefully monitored. Requirements for glucose control change dramatically immediately after surgery and resumption of preoperative doses may lead to significant hypoglycemia. Postoperatively, glucose management should be done with short-acting sliding scale insulin. Many patients will be able to completely discontinue the use of diabetic medications, including insulin, after surgery.
Recovery from bariatric surgery is a lengthy and involved process, extending beyond surgical healing. Patient education is a critical part of acute nursing care. Because anastomotic leak and PE can occur up to 2 weeks after surgery, patients should understand the signs and symptoms to be watchful for at home. Nutritional instruction and dietary progression is an important part of the process, and advancing diet properly is a significant component of reducing nausea, vomiting, and other discomfort for weeks and months after operation. Patients having malabsorptive procedures remain at long-term risk for vitamin and mineral deficiencies, and are best served by a sure understanding of long-term follow-up and dietary supplementation.
The negative consequences of malnutrition have been known for centuries, and there is substantial evidence that malnourished, hospitalized patients have increased morbidity, compromised surgical outcomes, slower ventilator weaning, and increased mortality rates. However, the science of nutrition support for the acutely ill patient is still young. In recent years, several large randomized studies have investigated the timing of nutrition, nutrient needs, and specific nutrients that best affect outcomes, but many important questions related to progressive care nutrition remain unanswered.
There is accumulating evidence that the route of nutrition support can affect morbidity in the acutely ill patient. In addition, protocols and care bundles for the proper initiation and monitoring of patients on nutrition support may reduce complications.
The advent of routine use of PN in the 1970s allowed the provision of large quantities of calories, and protein in an attempt to improve nutrition status. This ill-advised notion of providing supraphysiologic levels of nutrition, or “hyperalimentation,” led to widely published case reports of respiratory failure and hepatic compromise associated with overfeeding. Controlled trials have demonstrated that overfeeding does not provide increased nutritional benefits, and actually has detrimental effects (Table 14-10).
TABLE 14-10. POTENTIAL CONSEQUENCES OF OVERFEEDING OF MACRONUTRIENTSa
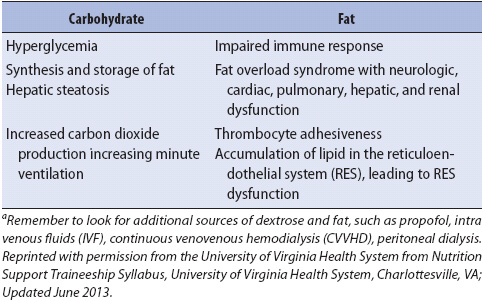
Current recommendations for feeding acutely ill patients suggest approximately 25 total calories/kg/day based on the patient’s ideal body weight, or 27.5 total calories/kg/day in the presence of systemic inflammatory response syndrome. A total of 1.2 to 1.5 g/kg/day of protein is also recommended. In severely malnourished patients, reduced calories (15-20 kcals/kg) are initiated to minimize electrolyte shifts from refeeding. When electrolytes are stable, progression to 30 or more calories/kg may be attempted to improve nutrition status. Close monitoring for tolerance is indicated.
The management of postoperative nutrition begins preoperatively with a comprehensive assessment of nutritional status, identification of psychosocial barriers, and a strong educational component and a plan for consistent follow-up to reinforce principles and measurement of success. Eighty percent of gastric bypass procedures today are malabsorptive or combined restrictive and malabsorptive procedures. As experience and number of surgeries increase, other less well-known deficiencies are starting to emerge. Nutritional supplementation is standard therapy for all bypass operations. Ongoing monitoring and reinforcement of compliance is essential. It is important to note that medication absorption is also altered in these patients. Ethyl alcohol (EtOH) absorption is enhanced, while sustained release and enteric-coated tablets may pass through undissolved, or unutilized. Efficacy of drugs requiring a large volume of food or high-fat meal may be compromised (antifungals, antipsychotics). In addition, reports of increased pregnancies while on birth control have surfaced.
Gastric resection can predispose patients to both nutritional intolerances and deficiencies. Intolerances include dumping syndrome, fat maldigestion, gastric stasis, and lactose intolerance. Combinations of these are most likely responsible for acute postoperative weight loss, the most frequent complication of gastrectomized patients. Nutrient deficiencies can develop months to years after gastric resections and can result in deleterious clinical consequences. Patients are at higher risk of developing osteoporosis and iron- and vitamin B12-deficiency anemia. Decreased acid production and small bowel bacterial overgrowth most likely play a role in the later two. Ongoing nutritional monitoring of these patients will prevent deficiencies and identify those in need of intervention.
Parenteral nutrition is indicated for patients who are malnourished, are at risk for becoming malnourished, and who are unable to receive EN (Table 14-11). PN can be life saving in some cases, but is not without complications and should be used only when necessary (Table 14-12). Prospective trials have demonstrated that the metabolic and infectious complications of PN outweigh the benefits in patients who do not have significant malnutrition. Studies have demonstrated that even short-term PN, used to supplement enteral nutrition in the ICU, has not enhanced benefits and is associated with increased infectious complications and length of stay. The need for PN should be evaluated daily in the inpatient setting, and patients who regain GI function are transitioned to enteral feeding.
TABLE 14-11. INDICATIONS FOR PARENTERAL NUTRITION
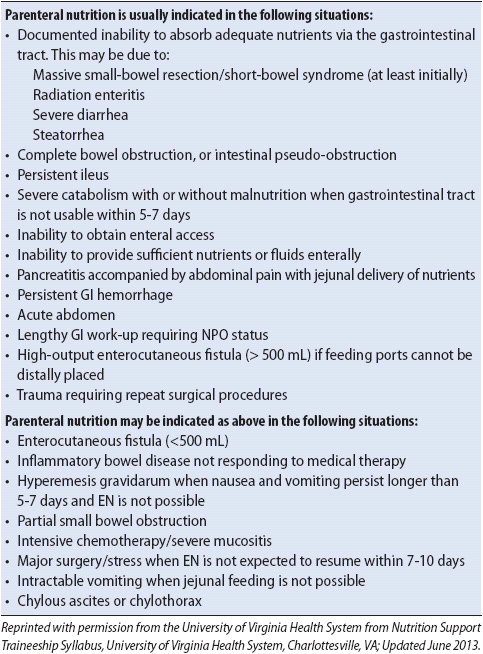
TABLE 14-12. CONTRAINDICATIONS FOR PARENTERAL NUTRITION

Current evidence suggests that EN is the preferred method of feeding the acutely ill patient. It is associated with less infectious complications, expense, and confers some gut protection in terms of immunity, attenuation of systemic response, and atrophy (Table 14-13). Patients who do not receive full amounts of EN have a greater need for rehabilitation services compared to patients receiving full feedings.
TABLE 14-13. BENEFITS OF ENTERAL FEEDING
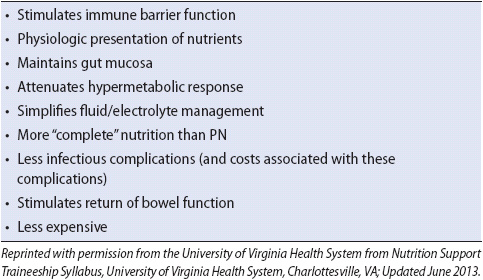
Unfortunately, the delivery of EN is impeded by various situations that occur in progressive care units; for example, EN may be stopped for diagnostic or therapeutic procedures in patients who are unstable, or have clogged or dislodged enteral tubes (Table 14-14). Successful EN is also commonly thwarted by misguided perceptions and beliefs related to the definition of GI “intolerance.” Many clinical responses are based on experiential assumptions and practices, as well as beliefs about how the GI tract functions in acute illness. The following sections review these myths and present facts related to GI tolerance or intolerance of enteral feeding.
TABLE 14-14. COMMON BARRIERS TO OPTIMIZING ENTERAL NUTRITION DELIVERY

Little evidence exists to support the practice of checking gastric residual volume (GRV, the volume of fluid in the stomach), as an indicator of GI tolerance and potential adverse clinical outcomes in enterally fed patients. One of the physiologic functions of the stomach is to act as a reservoir and to control delivery of nutrients into the small bowel. This allows for maximal assimilation with bile salts and pancreatic enzymes. A number of factors contribute to the GRV: endogenous secretions, normal gastric emptying, exogenous fluids, and the cascade effect (Table 14-15).
TABLE 14-15. ABSORPTION AND SECRETION OF FLUID IN THE GI TRACT
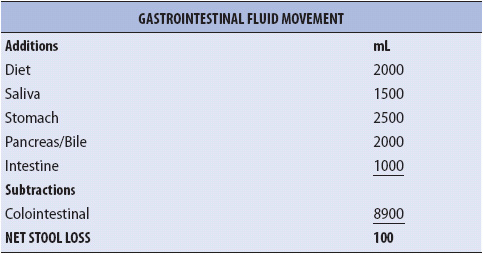
Two to four liters per day of saliva and gastric secretions are produced above the pylorus. Conservatively, this translates into 3 L of fluid that pass through the pylorus every 24 hours (an average of 125 mL/h). Once gastric access is obtained, exogenous medications, water flushes, and EN add to this volume. Commonly in acute care, clinicians expect the stomach to be empty or only contain a very small amount of tube feeding or other liquid upon checking. However, one study demonstrated that 40% of healthy volunteers have an average GRV greater than 100 mL.
The predominant position of progressive care unit patients is supine, preferably with backrest elevation > 30° or higher. In this position, the stomach partially splits over the spine and is mechanically divided into two parts, the fundus (proximal) and the antrum (distal). Because the fundus is the noncontractile portion of the stomach, contents fill the fundus until they “cascade over” the spine into the antrum and finally exit through the pylorus. Thus, if the patient’s feeding port is in the proximal stomach or fundus when the GRV is checked, the aspirated GRV may be erroneous. The GRV in this case may be a function of the patient’s supine positioning rather than decreased GI motility.
The routine practice of checking GRV has not been validated. Some of the factors that make the routine assessment of GRV questionable are listed below:
1. Type of tube (Salem sump vs Dobbhoff-like feeding tube vs a gastrostomy)
2. Location of the gastrostomy on the patient’s abdominal wall (fundus, antrum)
3. Position of the patient when GRV is checked (supine, right or left lateral decubitus, prone)
4. Method of aspiration (20-, 35-, 50-, 60-mL syringe vs gravity drainage vs low constant suction)
5. The volume of the aspirate obtained
6. Disposition of the aspirate (ie, reinfused or discarded)
7. The effects of GI stress prophylaxis medications (proton pump inhibitors) on the production and volume of gastric secretions
8. Lack of data linking elevated GRV to pulmonary aspiration
The practice of measuring GRV is poorly standardized. Furthermore, GRV as a valid measure of EN tolerance or whether the amount of GRV is even linked to the risk of aspiration pneumonia events has yet to be proven. Until more evidence is available, good clinical judgment is needed when evaluating GRV (Table 14-16).
TABLE 14-16. SUGGESTED APPROACHES TO EVALUATE GASTRIC RESIDUAL VOLUME
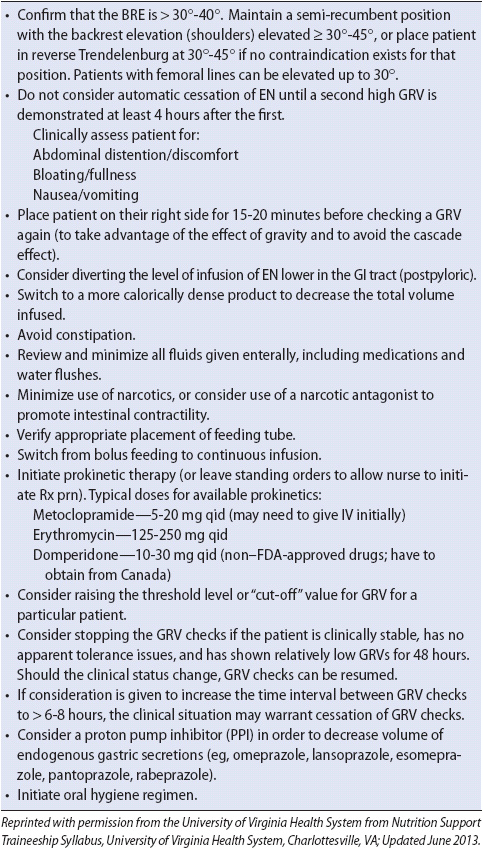
Aspiration is the passage of materials into the airway below the level of the vocal cords. The aspirated material may be saliva, nasopharyngeal secretions, bacteria, food, beverage, gastric contents, or any other liquid or substance. The incidence of aspiration pneumonia from EN is unclear, because it is difficult to identify an aspiration event, and definitions of aspiration vary. Commonly quoted aspiration pneumonia rates in EN patients, however, are between 5% and 36%.
Several methods of evaluating patients for aspiration risk have been popularized through “conventional wisdom.” These include the routine monitoring of GRV (discussed above), evaluation of gag reflex, testing tracheal secretions for the presence of glucose, and the addition of blue food color to feeding formulas.
The gag reflex is the least-reliable protective reflex in ensuring that aspiration does not occur. More important to airway protection are reliable cough and swallow reflexes. The presence of glucose in tracheal secretions is not a specific or sensitive method of detecting aspiration of EN. Tracheal glucose can be positive in patients who are not receiving EN. Finally, some tube-feeding formulas have low glucose concentrations and do not result in a positive test when aspirated.
Several studies have demonstrated that adding blue dye to feeding formulas is not a sensitive method for detecting aspiration and should not be used to indicate aspiration of gastric contents. In addition, some food dyes are mitochondrial toxins leading the Food and Drug Administration to release a Public Health Advisory Report noting the toxicity associated with the use of FD&C Blue No. 1 added to EN solutions.
The position of the patient is one of the primary factors influencing aspiration risk (Table 14-17). Studies have confirmed that aspiration and pneumonia are significantly more likely when patients are supine with the backrest elevation at less than 30°. While the semirecumbent position with backrest elevation of more than 30° cannot guarantee absolute protection against aspiration, it is a method that is inexpensive and relatively easy to accomplish and monitor. Strict use of semirecumbent position is the most consistent and potent means to reduce the likelihood of aspiration.
TABLE 14-17. RISK REDUCTION FOR ASPIRATION PNEUMONIA
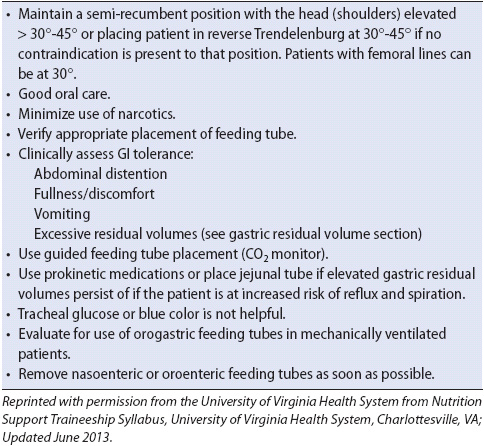
The incidence of aspiration, and subsequently pneumonia, are not affected by the feeding tube size. However, radiographic confirmation of accurate placement is essential. Moreover, there is evidence that pulmonary injury during beside placement of feeding tubes occurs more frequently than is generally appreciated. Using a method to provide feedback that the airway has been inadvertently intubated with the feeding tube can decrease the incidence of injury that may occur before the confirmatory radiograph is obtained. Several studies have reported that use of CO2 detection, electromagnetic guidance, or a preliminary radiograph during placement decreases the incidence of inadvertent pulmonary intubation and injury. It is commonly believed that placing the tip of the feeding tube beyond the pylorus decreases the incidence of aspiration events. However, despite numerous studies and a meta-analysis on the topic, it remains unclear if a properly positioned jejunal tube can reduce aspiration risk. The majority of acutely ill patients in these studies received gastric tube feedings safely and effectively. In studies that used protocols for the prevention of aspiration, very low rates of aspiration pneumonia were demonstrated. From a purely evidence-based standpoint, then the question of jejunal placement of feeding tubes and aspiration risk remains unanswered.
Considering the time and expense associated with jejunal placement of feeding tubes, it is reasonable to use the gastric route unless intolerance is evident. Exceptions to this approach include patients known to be at increased risk for aspiration due to altered anatomy (eg, esophagectomy) or motility (eg, scleroderma, severe gastroparesis). These patients may benefit from jejunally placed tubes.
The delivery rate of the feeding formula may influence aspiration and pneumonia. Bolus administration of 350 mL reduces lower esophageal sphincter pressure, which may precipitate reflux. Continuous EN (transpyloric-duodenal feeds) has been associated with more rapidly attained feeding tolerance, but no significant change in aspiration incidence. In one study, reduced aspiration events were associated with cyclic infusion feedings (16-hour cycle), compared to continuous feedings. The authors postulated that cyclic EN resulted in a reduction of gastric pH and subsequently prevented colonization of gastric contents. However, randomized trials have failed to demonstrate associations between gastric pH, gastric colonization, or pneumonia incidence in patients fed with cyclic vs continuous feedings.
Prokinetic medications have been evaluated to determine whether they improve EN tolerance. Metoclopramide and erythromycin improve gastric emptying, and a combination of these two medications may be superior to either drug alone. However, prokinetic medications do not appear to reduce the incidence of aspiration pneumonia.
Auscultating the abdomen to determine the presence of BS, and thus GI tract function, is a well-entrenched practice yet has never been validated as a marker of GI tract function. However, from a theoretical perspective, if BS were related to peristalsis, the absence of BS may suggest that a functional ileus exists. If nothing is moving through the GI tract (unlikely as 7 L of secretions are produced daily; see Table 14-15), the patient will require gastric decompression. In fact, initiating EN in patients without BS may stimulate the bowel to function normally and BS may emerge in some patients.
Studies that have investigated the effectiveness of auscultation for BS reported that the practice did not improve diet management in postoperative abdominal surgery patients, nor did flatus and bowel movements predict ability to tolerate oral intake. In addition, studies suggest that enteral feeding initiated within the first 24-48 hours of ICU admission with or without the presence of BS is safe.
Auscultation of BS practices in the clinical setting varies. Clinician assessment practices include how the quadrants are auscultated, the frequency of auscultation, time spent listening for sounds, and the interpretation of the sounds. BS are nonspecific and hence are best used in conjunction with the overall clinical assessment of the patient. Suggested approaches to assessment of GI function when BS are absent are found in Table 14-18.
TABLE 14-18. SUGGESTED APPROACHES FOR GI ASSESSMENT OF GI FUNCTION WHEN BOWEL SOUNDS ARE ABSENT
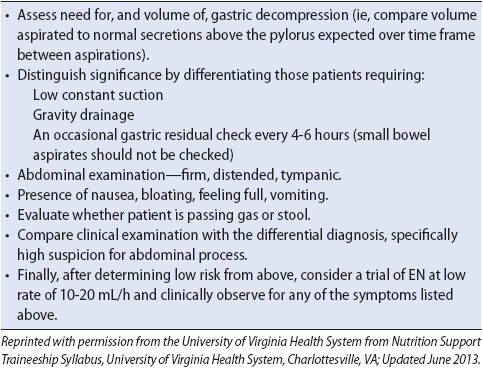
Many factors contribute to nausea and vomiting in the progressive care setting such as medications, the disease process, surgery, procedures, and bedside interventions (eg, during placement of a feeding tube or endotracheal suctioning). After careful assessment and treatment of the underlying cause (Table 14-19), antiemetic coverage may allow the continuation of EN while making the patient more comfortable. It is important that when ordering antiemetics and/or prokinetics, they be scheduled until clinical symptoms abate. Because PRN orders are often not administered for a variety of reasons (eg, patient unable or unaware that the medications may be requested, off floor for procedure, dose is not given), scheduled antiemetics may improve efficacy. However, it is important that the medications be discontinued when no longer necessary to prevent undesirable side effects.
TABLE 14-19. SUGGESTED APPROACHES TO REDUCE NAUSEA AND VOMITING IN ENTERALLY FED PATIENTS

Hypertonic or hyperosmolar formulas are often assumed to be responsible for diarrhea in the EN-fed patient, although no data exists to support this concept. As a result, some believe that diluting formula is an essential step. Diluting formula increases nursing time and the potential for contamination as well as decreasing the nutrient content of the feeding. The GI tract dilutes all foods and fluids ingested (or infused) by secreting saliva, and gastric and pancreatobiliary juices (including bicarbonate) to ensure isotonicity. This occurs whether it is delivered gastrically or jejunally. A clear or full liquid diet is more hypertonic or hyperosomolar than most commercial EN products available in the market as are many medications (Table 14-20). Yet, these are not ordered at one-quarter or half-strength. Because jejunal feedings circumvent the first step in food processing by bypassing the stomach, controversy exists related to how they should be managed. Some believe they should be managed differently than gastric feedings. However, as learned from patients with gastrectomies, where all the food they eat is delivered directly from the esophagus into the jejunum, normal feedings (albeit smaller portions) are consumed without adverse consequences. In those patients requiring frequent or large water boluses, dilution of formula may help, but the flow rate will need to be increased in order to deliver the same amount of nutrition. With this exception, dilution of EN should be avoided.
TABLE 14-20. OSMOLALITY OF SELECTED LIQUIDS AND MEDICATIONS
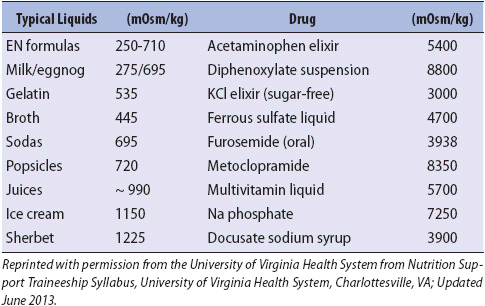
Diarrhea occurs in patients in the hospital setting regardless of oral, EN, or IV nutrition. The assumption that EN is a major cause of diarrhea is a myth. Although definitive evidence is needed to put this notion to rest, numerous studies have suggested other compelling reasons for diarrhea such as concurrent medications, especially those containing sorbitol, and infectious agents (Clostridium difficile in particular). Other commonly cited, but unfounded assumptions for the origin of diarrhea include low serum albumin level, strength, and osmolality of the formula, formula composition, rate of infusion, use of fiber-free EN, and disuse of the GI tract. Recent study on the effects of “fermentable, oligo-, di-, mono-saccharides and polyols” (FODMAPs) suggest that they may adversely affect bowel activity. Found in many enteral formulas, FODMAPs are highly osmotic and fermentable by gut bacteria and have been associated with gas, bloating, cramping, and diarrhea in some patients receiving EN. After potential causes for diarrhea have been ruled out (Table 14-21), medications to slow GI motility may be warranted.
TABLE 14-21. SYSTEMATIC APPROACH WHEN ADDRESSING DIARRHEA IN ENTERALLY FED PATIENTS

Typical infusion rates for the initiation of EN range from 10 to 50 mL/h with increases of 10 to 25 mL every 4 to 24 hours. Unfortunately, like other EN-support practices, little science exists to confirm or refute such regimens. There is limited evidence from work done with healthy volunteers or from studies using faster advancement rates of EN. Only one study has demonstrated that continuous enteral feeding may be started at the final goal rate in acutely ill patients without negative consequences. In the study, feedings started at goal-flow rate did appear to reduce the calorie deficit that frequently accrues in the hospitalized patient. Whether EN runs continuously, nocturnally, during the day, or is given as a bolus, is often institution specific. However, with the increased use of insulin infusions to ensure “tight glucose control,” and thus improve outcomes in acutely ill patients, continuous infusions of EN may protect the patients from hypoglycemic episodes.
It is difficult to achieve goal volumes of EN in acutely ill patients. Frequent interruptions in the delivery of EN are common (see Table 14-14). As a result, it is reasonable to consider “padding” flow rates by basing calculations on < 24 hours to improve delivery of the desired dose. Sample tube-feeding protocols for EN initiation and progression are described in Table 14-22.
TABLE 14-22. EXAMPLE OF EN PROGRESSION REGIMEN

A vast array of EN products are available, including specialty formulas marketed for patients with diabetes, pulmonary, hepatic, and renal failure. Other formulas contain nutrients that may modulate immune function, or have nutrients in their most basic (elemental) form for patients with malabsorptive syndromes. It is important to remember that medical nutrition products are not required to meet the same level of scientific scrutiny as medications before they are marketed. Many believe that adequate outcome data are not available to warrant the use of these expensive products. Prospective, randomized trials have not demonstrated advantages of specialized “pulmonary” or “glucose control” feeding formulas. In fact, in a large randomized multicenter trial of a specialized enteral formula with fish oil and antioxidants for patients with acute lung injury, mortality increased with the specialized feeding compared to standard products. The majority of acutely ill patients may be fed with standard polymeric tube-feeding formulas.
Alhazzani W, Alenezi F, Jaeschke RZ, et al. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2013;41(3):1-13.
Andreyev HJN, Davidson SE, Gillespie C, et al. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut. 2012;61:179-192.
Cappell MS, Friedel D. Acute nonvariceal upper gastrointestinal bleeding: endoscopic diagnosis and therapy. Med Clin N Am. 2008;92:511-550.
Cappell MS, Friedel D. Initial management of acute upper gastrointestinal bleeding: from initial evaluation up to gastrointestinal endoscopy. Med Clin N Am. 2008;92:491-509.
Conrad SA. Acute upper gastrointestinal bleeding in critically ill patients: causes and modalities. Crit Care Med. 2002;30(suppl 6): S365-S368.
D’Amico G, Pietrosi G, Tarantino I, et al. Emergency sclerotherapy versus vasoactive drugs for variceal bleeding in cirrhosis: a Cochrane meta-analysis. Gastroenterology. 2003;124(5):1277-1291.
Dhamija N, Pousman R, Bajwa O, Marik PE. Management of gastrointestinal bleeding. In: Vincent J, Abraham E, Kochanek P, Moore FA, and Fink MP, eds. Textbook of Critical Care, 6th ed, St Louis, MO: Saunders Elsevier; 2011:86-91.
Dworzynski K, Pollit V, Kelsey A, et al. Management of acute upper gastrointestinal bleeding: summary of NICE guidance. BMJ. 2012;344:1-5.
El-Tawil AM. Management of non-variceal upper gastrointestinal tract hemorrhage: controversies and areas of uncertainity. World J Gastroenterol. 2012;18(11):1159-1165.
Green BT, Rockey DC. Acute gastrointestinal bleeding. Semin Gastrointest Dis. 2003;14:44-65.
Holster IL, Kuipers EF. Management of acute nonvariceal upper gastrointestinal bleeding: current policies and future perspectives. World J Gastroenterol. 2012;18(11):1202-1207.
Jairath V, Barkun AN. Improving outcomes from acute upper gastrointestinal bleeding. Gut. 2012;61(9):1246-1249.
Kryzwda E, Andris D, eds. AACN Advanced Critical Care Symposium, Gastroenterology in Critically Ill Patients. April-June 2010;21(2):165-219.
Peter A, Wilcox M. Modern endoscopic therapy of peptic ulcer bleeding. Dig Dis. 2008;26:291-299.
Proctor DD. Critical issues in digestive diseases. Clin Chest Med. 2003;24:623-632.
Rossle M. When endoscopic therapy or pharmacology fails to control variceal bleeding: what should be done? Immediate control of bleeding by TIPS? Langenbecks Arch Surg. 2003;388:155-162.
Schuetz A, Jauch KW. Lower gastrointestinal bleeding: therapeutic strategies, surgical techniques and results. Curr Concepts Clin Surg. 2001;386:17-25.
Sorbi D, Gostout CJ, Peura D, et al. An assessment of the management of acute bleeding varices: a multicenter prospective member-based study. Am J Gastroenterol. 2003;98:2424-2434.
Targownik L, Gralnek IM. A risk score to predict the need for treatment of upper GI hemorrhage. Gastrointest Endoscopy. 2001;54:797-799.
Toubia N, Sanyal A. Portal hypertension and variceal hemorrhage. Med Clin N Am. 2008;92:551-574.
Zhu LL, Xu LC, Chen Y, et al. Poor awareness of preventing aspirin-induced gastrointestinal injury with combined protective medications. World J Gastroenterol. 2012;18(24):3167-3172.
Zuckier L. Acute gastrointestinal bleeding. Semin Nuclear Med. 2003;33:297-311.
Bachir NM, Larson AM. Adult liver transplantation in the United States. Am J Med Sci. June 2012;343(6):462-469.
Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol. Mar 21, 2012;18(11):1166-1175.
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
Dasher K, Trotter JF. Intensive care unit management of liver-related coagulation disorders. Crit Care Clin. July 2012;28(3):389-398.
Foston TP, Carpentar D. Crit Care Nurse N Am. Sept 2010;22(3):395-402.
Koffron A, Stein JA. Liver transplantation: indications, pretransplant evaluation, surgery, and posttransplant complications. Med Clin N Am. 2008;92:861-888.
Lata J. Hepatorenal syndrome. World J Gastroenterol. Sept 28, 2012;18(36):4978-4984.
Lee WM. Recent developments in acute liver failure. Best Practice Res Clin Gastroenterology. Feb 2012;26(1):3-16.
Munoz SJ. Hepatic encephalopathy. Med Clin N Am. 2008;92:795-812.
Munoz SJ. The hepatorenal syndrome. Med Clin N Am. 2008;92:813-837.
Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197.
Rahimi RS, Rockey DC. Complications and outcomes in chronic liver disease. Curr Opin Gastroenterol. 2011;27:204-209.
Rahimi RS, Rockey DC. Complications of cirrhosis. Curr Opin Gastroenterol. 2012;223-229.
Rikkers LF. Surgical complications of cirrhosis and portal hypertension. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. Philadelphia, PA: Saunders Elsevier; 2008:1524-1546.
Rose CF. Ammonia-lowering strategies for the treatment of hepatic encephalopathy. Clin Pharmacol Ther. Sept 2012;92(3):321-331.
Sagi SV, Mittal S, Kasturi KS, Sood GK. Terlipressin therapy for reversal of type 1 hepatorenal syndrome: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2010;25:880-885.
Sibae A, Cappell MS. Accuracy of MELD scores in predicting mortality in decompensated cirrhosis from variceal bleeding, hepatorenal syndrome, alcoholic hepatitis or acute liver failure as well as mortality after non-transplant surgery or TIPS. Dig Dis Sci. April 2011;56(4):977-987.
Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306-317.
Wong F. Recent advances in our understanding of hepatorenal syndrome. Nat Rev Gastroenterology Hepat. May 2012;9(7):381-391.
AGA Institute. AGA institute medical position statement on acute pancreatitis. Gastroenterology. 2007;132:2019-2021.
Anans N, Park JJ, Wu BU. Modern management of acute pancreatitis. Gastroenterol Clin N Am. Mar 2012;41(1):1-8.
Bollen T. Imaging of acute pancreatitis: update of the revised Atlanta classification. Radiol Clin N Am. 2012;50:429-445.
Brisinda G, Vanellla S, Crocco A, et al. Severe acute pancreatitis: advances and insights in assessment of severity and management. Eur J Gastroentel Hepatol. 2011;23:541-551.
Cappell MS. Acute pancreatitis: etiology, clinical presentation, diagnosis, and therapy. Med Clin N Am. 2008;92:889-923.
Cruz-Santamaria DM, Taxonera G, Giner M. Update on pathogenesis and clinical management of acute pancreatitis. World J Gastroint Pathophys. June 2012;15(3):67-70.
Fischer JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterology. Aug 2012;(107):1146-1150.
Mirtallo JM, Forbes A, McClave SA, et al. International consensus guideline for nutrition therapy in pancreatitis. J Parenter Enteral Nutr. May 2012;36(3):284-289.
Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol. 2009;104:966-971.
Talukdar R, Vege SS. Early management of severe acute pancreatitis. Curr Gastroenterol Rep. 2011;13:123-130.
Warndorf MG, Kurtznab JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:705-709.
Wu BU, Bakker OJ, Papachristou GI, et al. Blood urea nitrogen in the early assessment of acute pancreatitis. Arch Intern Med. 2011;171:669-676.
Batke M, Cappell MS. Adynamic ileus and acute colonic pseudo-obstruction. Med Clin N Am. 2008;92:649-670.
Berland T, Oldenburg A. Acute mesenteric ischemia. Curr Gastroenterol Rep. 2008;10:341-346.
Brandt LJ, Feuerstadt P, Blaszka MC. Anatomic patterns, patient characteristics, and clinical outcomes in ischemic colitis: a study of 313 cases supported by histology. Am J Gastroenterol. 2010;105:2245-2252.
Cappell MS, Batke M. Mechanical obstruction of the small bowel and colon. Med Clin N Am. 2008;92:575-597.
Dayton MT, Dempsey DT, Larson GM, Posner AR. New paradigms in the treatment of small bowel obstruction. Curr Prob Surg. 2012;49(11):642-717.
De Giorgio R, Cogliandro RF, Barbara G, et al. Colonic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol Clin N Am. 2011;40:787-807.
Diaz JJ, Bokhari F, Mowery, NT, et al. Guidelines for management of small bowel obstruction. J Trauma. 2008;64:1651-1664.
Edwards MS, Cherr GS, Craven TE, et al. Acute occlusive mesenteric ischemia: surgical management and outcomes. Ann Vasc Surg. 2003;17:72-79.
Feuerstadt P, Brandt LJ. Colon ischemia: recent insights and advances. Curr Gastroenterol Rep. 2010;12:383-390.
Georgescu EF, Carstea D, Dumitrescu D, et al. Ischemic colitis and large bowel infarction: a case report. World J Gastroenterol. 2012;18(39):5640-5644.
Green BT, Tendler DA. Ischemic colitis: a clinical review. Southern Med J. 2005;98:217-222.
McConnell EA. What’s behind intestinal obstruction? Nursing. 2001;31(10):58-63.
McNamara I, Tremelling M, Dunkley I, et al. Management of intestinal obstruction in malignant disease. Clin Med. 2003;3:311-314.
Potluri V, Zhukovsky DS. Recent advances in malignant bowel obstruction: an interface of old and new. Curr Pain Headache Rep. 2003;7:270-278.
Sotiriadis J, Brandt LJ, Behin DS, et al. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol. 2007;102:2247-2252.
ACOG Practice Bulletin No. 105. Bariatric surgery and pregnancy. Obstet Gynecol. 2009;113:1405-1413.
Aills L, Blankenship J, Buffington C, Furtado M, Parrot J. ASMBS allied health nutritional guidelines for the surgical weight loss patient. Surg Obes Relat Dis. 2008;4:S73-S108.
Bankhead R, Boullata J, Brantley S, et al. Enteral nutrition practice recommendations, Enteral Nutrition Practice Recommendations Task Force and the A.S.P.E.N. Board of Directors. JPEN. 2009;33(2):122-167.
Barr J, Hecht M, Flavin KE, et al. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. April 2004;124(4): 1446-1457.
Barrett JS, Gearry RB, Muir JG, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol. 2010;31:874-882.
Booth CM, Heyland DK, Paterson WG. Gastrointestinal promotility drugs in the critical care setting: a systematic review of the evidence. Crit Care Med. 2002;30:1429-1435.
Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta analysis. Am J Clin Nutr. 2001; 74:534-542.
Brozovich M, Read T, Andujar J, et al. Bowel sounds, flatus, and bowel movement do not correlate with tolerance of oral intake following major abdominal surgery: a prospective study (abstract). Dis Colon Rectum. 2005;48(3):625.
Burns SM, Carpenter R, Blevins C, et al. Detection of inadvertent airway intubation during gastric tube insertion: capnography versus a colorimetric carbon dioxide detector. Am J Crit Care. 2006;15(2):188-195.
Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506-517.
Cerra FB, Benitez MR, Blackburn GL, et al. Applied nutrition in ICU patients. A consensus statement of the American College of Chest Physicians. Chest. 1997;111:769-778.
Chan L. Drug therapy-related issues in patients who received bariatric surgery (part I). Prac Gastroenterol. 2010;XXXIV(7):28-32. Available at www.ginutrition.virginia.edu. Accessed March 4, 2013.
Chan L. Drug therapy-related issues in patients who received bariatric surgery (part II). Prac Gastroenterol. 2010;XXXIV(8):24-32. Available at www.ginutrition.virginia.edu. Accessed March 4, 2013.
Dark DS, Pingleton SK, Kerby GR. Hypercapnia during weaning. A complication of nutritional support. Chest. 1985;88:141-143.
Davies AR, Morrison SS, Bailey MJ, et al. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med. 2012;40(8):2342-2348.
de Aguilar-Nascimento JE, Kudsk KA. Clinical costs of feeding tube placement. JPEN J Parenter Enteral Nutr. 2007;31(4): 269-273.
Desachy A, Clavel M, Vuagnat A, et al. Initial efficacy and tolerability of early enteral nutrition with immediate or gradual introduction in intubated patients. Intensive Care Med. 2008;34(6): 1054-1059.
Drover JW, Dhaliwal R, Heyland DK. Small bowel versus gastric feeding in the critically ill patient: results of a meta-analysis. Crit Care Med. 2003;30:A44.
FDA Public Health Advisory FDA/Center for Food Safety & Applied Nutrition. Reports of blue discoloration and death in patients receiving enteral feedings tinted with the dye, FD&C blue NO. September 29, 2003.
Grap MJ, Munro CL, Hummel RS, et al. Effect of backrest elevation on the development of ventilator-associated pneumonia. Am J Crit Care. 2005;14(4):325-332.
Heymsfield SB, Head CA, McManus CB, 3rd, Seitz S, Staton GW, Grossman GD. Respiratory, cardiovascular, and metabolic effects of enteral hyperalimentation: influence of formula dose and composition. Am J Clin Nutr. 1984;40:116-130.
Holzapfel L, Chevret S, Madinier G, et al. Influence of long-term oro- or nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: results of a prospective, randomized, clinical trial. Crit Care Med. 1993;21(8):1132-1138.
Kostadima E, Kaditis AG, Alexopoulos EI, et al. Early gastrostomy reduces the rate of ventilator-associated pneumonia in stroke or head injury patients. Eur Respir J. 2005;26(1):106-111.
Krenitsky J. Gastric versus jejunal feeding: evidence or emotion? Prac Gastroenter. 2006;30(9):46. Available at www.ginutrition.virginia.edu. Accessed March 4, 2013.
Krenitsky J. Immunonutrition—fact, fancy or folly? Prac Gastroenterol. 2006;30(5):47. Available at www.ginutrition.virginia.edu. Accessed March 4, 2013.
Lansford T, Moncure M, Carlton E, et al. Efficacy of a pneumonia prevention protocol in the reduction of ventilator-associated pneumonia in trauma patients. Surg Infect (Larchmt). 2007;8(5):505-510.
Mackenzie SL, Zygun DA, Whitmore BL, et al. Implementation of a nutrition support protocol increases the proportion of mechanically ventilated patients reaching enteral nutrition targets in the adult intensive care unit. J Parenter Enteral Nutr. 2005;29:74-80.
Madsen D, Sebolt T, Cullen L, et al. Listening to bowel sounds: an evidence-based practice project. Am J Nurs. 2005;105(12):40-49.
Marik PE, Zaloga GP. Gastric versus post-pyloric feeding: a systematic review. Crit Care. 2003;7:R46-R51.
Martindale RG, McClave, SA, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009;37(5):1757-1761.
McClave SA, Sexton LK, Spain DA, et al. Enteral tube feeding in the intensive care unit: factors impeding adequate delivery. Crit Care Med. 1999;27:1252-1256.
Metheny NA, Clouse RE. Bedside methods for detecting aspiration in tube fed patients. Chest. 1997;111:724-731.
Miller AD, Smith KM. Medication and nutrient administration considerations after bariatric surgery. Am J Health Syst Pharm. 2006;63(19):1852-1857.
Montejo JC, Minambres E, Bordeje L, et al. Gastric residual volume during enteral nutrition in ICU patients. The REGANE study. Intensive Care Med. 2010;36(8):1386-1393.
Munera-Seeley V, Ochoa JB, Brown N, et al. Use of a colorimetric carbon dioxide sensor for nasoenteric feeding tube placement in critical care patients compared with clinical methods and radiography. Nutr Clin Prac. Jun-Jul 2008;23(3):318-321.
Nguyen NQ, Chapman M, Fraser RJ, et al. Prokinetic therapy for feed intolerance in critical illness: one drug or two? Crit Care Med. 2007;35(11):2561-2567.
O’Donnell K. Small but mighty: selected micronutrient issues in gastric bypass patients. Prac Gastroenterol. 2008;XXXII(5):37. Available at www.ginutrition.virginia.edu. Accessed March 4, 2013.
O’Donnell, K. Severe micronutrient deficiencies in RYGB patients: rare but potentially devastating. Prac Gastroenterol. 2011;XXXV(11):13. Available at www.ginutrition.virginia.edu. Accessed March 4, 2013.
O’Keefe-McCarthy, Santiago C, Lau G. Ventilator-associated pneumonia bundled strategies: an evidence-based practice. WorldViews Evid Based Nurs. 2008;193-204.
Parrish CR. Enteral feeding: the art and the science. Nutr Clin Pract. 2003;18:76.
Parrish CR, Krenitsky J, McCray S. University of Virginia Health System Nutrition Support Traineeship Syllabus. Charlottesville, VA: University of Virginia Medical Center Nutrition Services Department; Updated June 2013.
Parrish CR, McClave S. Checking gastric residual volumes: a practice in search of science? Prac. Gastroenterol. 2008;32(10):33. Available at www.ginutrition.virginia.edu. Accessed March 4, 2013.
Poulard F, Dimet J, Martin-Lefevre L, et al. Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before-after study. J Parenter Enteral Nutr. 2010;34(2):125-130.
Rassias AJ, Ball PA, Corwin HL. A prospective study of tracheopulmonary complications associated with the placement of narrow-bore enteral feeding tubes. Crit Care. 1998;2(1):25-28.
Reignier J, Mercier E, Le Gouge A, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309(3):249-256.
Rice T, Wheeler AP, Thompson BT, et al. Initial trophic vs. full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795-803.
Rice TW, Swope T, Bozeman S, et al. Variation in enteral nutrition delivery in mechanically ventilated patients. Nutrition. 2005; 21(7-8):786-792.
Rice TW, Wheeler AP, Thompson BT, et al; NHLBI ARDS clinical trials network enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574-1581.
Rogers C. Postgastrectomy nutrition. Nutr Clin Pract. 2011;26(2): 126-136.
Saalwachter Schulman A, Sawyer RG. Have you passed gas yet? Time for a new approach to feeding patients postoperatively. Prac Gastroenterol. 2005;32(10):82. Available at www.ginutrition.virginia.edu. Accessed March 4, 2013.
Salord F, Gaussorgues P, Marti-Flich J, et al. Nosocomial maxillary sinusitis during mechanical ventilation: a prospective comparison of orotracheal versus the nasotracheal route for intubation. Intensive Care Med. 1990;16(6):390-393.
Singer P, Anbar R, Cohen J, et al. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011;37(4):601-619.
Sorokin R, Gottlieb JE. Enhancing patient safety during feeding-tube insertion: a review of more than 2,000 insertions. JPEN J Parenter Enteral Nutr. 2006;30(5):440-445.
Talpers SS, Romberger DJ, Bunce SB, Pingleton SK. Nutritionally associated increased carbon dioxide production. Excess total calories vs high proportion of carbohydrate calories. Chest. 1992;102:551-555.
van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367.
van den Broek PWJH, Rasmussen-Conrad EL, Naber AHJ, et al. What you think is not what they get: significant discrepancies between prescribed and administered doses of tube feeding. Brit J Nutr. 2009;101(1):68-71.
van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006;34:396-402.
van Zanten AR, Dixon JM, Nipshagen MD, et al. Hospital-acquired sinusitis is a common cause of fever of unknown origin in orotracheally intubated critically ill patients. Crit Care. 2005;9(5):R583-R590.
Willcutts K. Pre-op NPO and traditional post-op diet advancement: time to move on. Prac Gastroenterol. 2010;XXXIV(12):16. Available at www.ginutrition.virginia.edu. Accessed March 4, 2013.
Yavagal DR, Karnad DR, Oak JL. Metoclopramide for preventing pneumonia in critically ill patients receiving enteral tube feeding: a randomized controlled trial. Crit Care Med. 2000;28(5): 1408-1411.
http://www.ginutrition.virginia.edu. Accessed March 4, 2013.
da Silva L, McCray S. Vitamin B12: no one should be without it. Practic Gastroenterol. 2008;XXXIII(1):34. Accessed March 4, 2013.
DiBaise JK. Small intestinal bacterial overgrowth: nutritional consequences and patients at risk. Prac Gastroenterol. 2008; XXXII(12):15. Accessed March 4, 2013.
McCray S. Lactose intolerance: considerations for the clinician. Prac Gastroenterol. 2003;XXVII(2):21. Accessed March 4, 2013.
Parrish CR, Yoshida C. Nutrition intervention for the patient with gastroparesis: an update. Prac Gastroenterol. 2005;XXIX(8):29. Accessed March 4, 2013.
Radigan A. Post-gastrectomy: managing the nutrition fall-out. Prac Gastroenterol. 2004;XXVIII(6):63. Accessed March 4, 2013.
Ukleja A. Dumping syndrome. Prac Gastroenterol. 2006; XXX(2):32. Accessed March 4, 2013.
Andris DA. Surgical treatment for obesity—ensuring success. J WOCN. 2005;32:393-401.
Boza C, Gamboa C, Salinas J, et al. Laparoscopic roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: a case-control study and 3 years of follow-up. SOARD. 2012;8:243-249.
Buchwalk H. Overview of bariatric surgery. J Am Coll Surg. 2002;194(3):367-375.
Doolen JL, Miller SK. Primary care management of patients following bariatric surgery. J Am Acad Nurse Pract. 2005;17(11):446-450.
Mitka M. Surgery for obesity. JAMA. 2003;289(14):1761-1762.
National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) of the National Institutes of Health. Gastric surgery for severe obesity. Retrieved March 15, 2013 from http://win.niddk.nih.gov/publications/PDFs/gasurg12.04bw.pdf.
Nguyen NT, Nguyen B, Gebhart A, et al. Changes in the makeup of bariatric surgery: anational increase in use of laparoscopic sleeve gastrectomy. J Am Coll Surg. 2013;216(2):252-257.
Parikh M, Issa R, McCrillis A, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy. A systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257(2): 231-237.
Rusch MD, Andris DA. Maladaptive eating and poor dietary compliance after weight loss surgery. NCP. 2007;22(1):41.
Rusch MD, Andris D, Wallace JR. Reasons for failed weight loss surgery. Clin Nutr Insight. 2009;35(1):1.
Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240-245.
Saul D, Stephens D, de Cassia Hofstatter R, et al. Preliminary outcomes of laparoscopic sleeve gastrectomy in a veterans affairs medical center. Am J Surg. 2012;204:e1-e6.
Still CD. Before and after surgery: the team approach to management. J Fam Prac. 2005;54(3). Retrieved March 13, 2013 from http://www.findarticles.com/p/articles/mi_m0689/is_3_54/ai_n13783681
Vidal P, Ramon JM, Goday A, et al. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy as a definitive surgical procedure for morbid obesity. Midterm results. Obes Surg. 2012:e1-e8.
von Drygalski A, Andris DA. Anemia after bariatric surgery: more than just iron deficiency. NCP. 2009;24(2).
von Drygalski A, Andris DA, Nuttleman PR, et al. Anemia after bariatric surgery cannot be explained by iron deficiency alone: results of a large Cohort study. SOARD. 2011;7:151-156.
Watkins BM, Montgomery KF, Ahroni JH, et al. Adjustable gastric banding in an ambulatory surgery center. Obes Surg. 2005;15(7):1045-1049.