Pediatric Heart and Heart-Lung Transplantation
Pediatric Heart Transplantation
Joseph W. Rossano
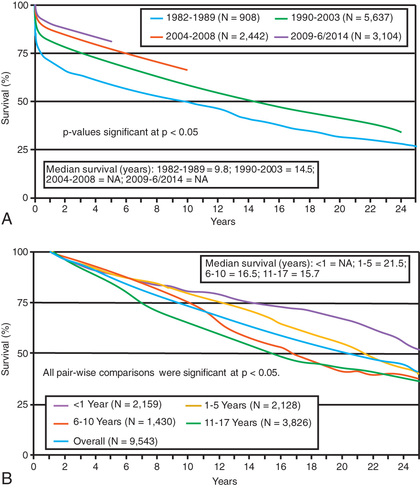
Pediatric heart transplantation is considered the standard therapy that offers long-term survival for end-stage heart disease in children. In adults, ventricular assist devices (VADs) are usually employed as a long-term therapy for patients not eligible for heart transplantation, but in children the vast majority of VADs are used as a bridge to transplantation as opposed to an alternative to transplantation. As of January 2017, almost 9000 heart transplants had been performed on children in the United States since 1988, with about 400 transplants annually; a quarter of these in children <1 yr of age. Survival rates have improved significantly over time, with most of the improvement occurring in the early period after transplant. This period continues to the associated with the greatest risk of death, and many patients who survive the 1st yr after transplant are alive 20 yr later (Fig. 470.1 ). Indeed, a growing number of patients receiving a heart transplant in childhood are approaching their 15, 20, and 30 yr posttransplant anniversaries.
Indications
Heart transplantation is performed (1) in infants and children with end-stage cardiomyopathy who have become refractory to medical therapy, (2) in patients with previously repaired or palliated congenital heart disease (CHD) who have developed ventricular dysfunction or other nonoperable late-term complications, and (3) less frequently in patients with complex CHD—pulmonary atresia with intact septum and coronary arterial stenoses, some forms of hypoplastic left heart syndrome (HLHS)—for whom standard surgical procedures are extremely high risk. Additionally, retransplantation accounts for approximately 5% of transplants annually. Cardiomyopathies account for >50% of heart transplants in pediatric patients older than 1 yr, with the percentage of patients with previously repaired complex CHD at approximately 30%. In infants younger than 1 yr, CHD previously represented >80% of transplants; this has decreased to 60% as standard surgical results for complex CHD (e.g., HLHS) have improved.
Recipient and Donor Selection
Potential heart transplant recipients must be free of serious noncardiac medical problems such as neurologic disease, active systemic infection, severe hepatic or renal disease, and severe malnutrition. Many children with ventricular dysfunction are at risk for the development of pulmonary vascular disease , which if severe enough would also preclude heart transplantation. Therefore, pulmonary vascular resistance (PVR) is measured at cardiac catheterization in heart transplant candidates, both at rest and, if elevated, in response to vasodilators. Patients with fixed elevated PVR are at higher risk for heart transplantation and may be considered candidates for heart-lung transplantation (see Chapter 470.2 ). However, with advances in postoperative management of pulmonary hypertension (e.g., inhaled nitric oxide), many patients with moderate elevation in PVR can undergo heart transplant alone. A comprehensive social services evaluation is an important component of the recipient evaluation. Because of the complex posttransplantation medical regimen, the family must have a history of compliance. Detailed informed consent must be obtained, indicating that the family (and if old enough, the patient) understand the lifelong commitment to immunosuppressive medication and careful monitoring.
Donor shortage is a serious problem for both adults and children. At the national registry of transplant recipients in the United States, the United Network for Organ Sharing (UNOS) , allografts are matched by ABO blood group and body weight. ABO matching may not be required for young infants; the exact age under which ABO tolerance develops has not yet been determined. Patients, especially with a history of CHD, who have undergone prior operations may have antibodies against human leukocyte antigens (HLAs). Patients with elevated anti-HLA antibodies are at risk for a positive crossmatch and early graft dysfunction. These antibodies can also contribute to late graft dysfunction through antibody-mediated rejection and development of cardiac allograft vasculopathy. For patients with these elevated antibodies, there are strategies to avoid a positive crossmatch through a prospective crossmatch or a virtual crossmatch, although this may prolong the waiting list time. Contraindications to organ donation include prolonged cardiac arrest with persistent moderate to severe cardiac dysfunction, ongoing systemic illness or infection, and preexisting severe cardiac disease. Physicians caring for a patient who may be a potential donor should always contact the organ donor coordinator at their institution, who can best judge the appropriateness of organ donation and has experience in interacting with potential donor families. A history of resuscitation alone or reparable CHD is not an automatic exclusion for donation.
The decision of when to place a patient on the transplant waiting list is based on many factors, including poor ventricular function, markedly decreased exercise tolerance as determined by cardiopulmonary exercise testing (see Chapter 450.5 ), poor response to medical heart failure therapy, multiple hospitalizations for heart failure, arrhythmia, progressive deterioration in renal or hepatic function, early stages of pulmonary vascular disease, and poor nutritional status. In patients awaiting transplantation, those with poor left ventricular function are often started on a regimen of anticoagulation to reduce the risk of mural thrombosis and thromboembolism. Patients with progressive heart failure resulting in decreases in end-organ (renal or hepatic) function unresponsive to standard pharmacologic treatment may be candidates for a VAD. The use of these devices has increased dramatically over the last decade, and currently almost half of patients with dilated cardiomyopathy are on VAD support before transplant. VADs can improve hemodynamics and end-organ function, and some patients can even be discharged home on VAD support (see Chapter 469 ).
Perioperative Management
In the classic operation , both donor and recipient hearts were excised so that the posterior portions of the atria containing the venae cavae and pulmonary veins are left intact. The aorta and pulmonary artery are divided above the level of the semilunar valves. The anterior portion of the donor's atria was then connected to the remaining posterior portion of the recipient's atria, thereby avoiding the need for delicate suturing of the venae cavae or pulmonary veins. The donor and recipient great vessels were connected via end-to-end anastomoses. This has been supplanted in many centers by the bicaval anastomosis, with the donor right atrium (and sinus node) left intact and the suture lines at the superior and inferior vena cavae; the left atrial connection is still performed as in the classic procedure.
Heterotopic heart transplantation has been used occasionally for patients with left ventricular cardiomyopathy and elevated PVR. In this operation the donor and recipient hearts are connected in parallel so that the recipient right ventricle (which has hypertrophied over time because of elevated pulmonary pressures) pumps mostly to the lungs, and the donor left ventricle pumps mostly to the body. This operation may be preferable to heart-lung transplant for appropriate candidates (patients with pulmonary hypertension but without parenchymal lung disease, without evidence of right ventricular failure, and without serious CHD). However, this procedure is very uncommon in the current era.
In the immediate postoperative period, immunosuppression is achieved with either a triple-drug or a double-drug regimen, with more centers adopting a minimal corticosteroid or steroid-free regimen. The most common combinations are a calcineurin inhibitor (cyclosporine or tacrolimus) plus an antiproliferative agent (mycophenolate mofetil or azathioprine), plus or minus prednisone . In many centers, induction therapy (usually an antilymphocyte preparation) is added in the 1st week; common agents include antithymocyte globulin (ATG) and the humanized anti–interleukin-2 receptor antibodies (basiliximab). In children who do not experience significant graft rejection, corticosteroids can be gradually eliminated in the early transplant period. Some centers do not use steroids as part of maintenance immunosuppression, but do use them as bolus treatment for acute rejection episodes.
Many pediatric heart transplant recipients can be extubated from endotracheal intubation and mechanical ventilation support within the 1st 48 hr after transplantation and are out of bed in several days. In patients with preexisting high-risk factors, postoperative recovery may be considerably prolonged. For those with preoperative pulmonary hypertension, the use of nitric oxide in the postoperative period can allow the donor right ventricle to hypertrophy in response to elevated pulmonary artery pressures. Occasionally, these patients will require right ventricular assist device support or extracorporeal membrane oxygenation (ECMO).
Diagnosis and Management of Acute Graft Rejection
Posttransplantation management consists of adjusting medications to maintain a balance between the risk of rejection and the side effects of over-immunosuppression. Acute graft rejection is a leading cause of death in pediatric heart transplant recipients. The incidence of acute rejection is greatest in the 1st 3 mo after transplantation and decreases considerably thereafter. Many pediatric patients experience at least 1 episode of acute rejection in the 1st 2 yr after transplantation, although modern immunosuppressive regimens have decreased the frequency of serious rejection episodes. Because the symptoms of rejection can mimic many routine pediatric illnesses (e.g., pneumonia, gastroenteritis), the transplant center should be notified whenever a heart transplant recipient is seen in the pediatrician's office or emergency department for acute illnesses.
Clinical manifestations of acute rejection may include fatigue, fluid retention, fever, diaphoresis, abdominal symptoms, and a gallop rhythm. The electrocardiogram (ECG) may show reduced voltage, atrial or ventricular arrhythmias, or heart block but is usually nondiagnostic. X-ray examination may show an enlarged heart, effusions, or pulmonary edema but typically only in the more advanced stages of rejection. Natriuretic peptide levels are usually increased during episodes of acute rejection. Most rejection episodes occur without any detectable clinical symptoms. On echocardiography, indices of systolic left ventricular function may be decreased; however, these usually do not deteriorate until rejection is at least moderately severe. Techniques to evaluate wall thickening and left ventricular diastolic function have not fulfilled their promise as predictors of early rejection. Most transplant centers do not rely on echocardiography alone for rejection surveillance.
Myocardial biopsy is the most reliable method of monitoring patients for rejection. Biopsy specimens are taken from the right ventricular side of the interventricular septum and can be harvested relatively safely, even in small infants. In infants, surveillance biopsies are usually performed less often and may be as infrequent as once or twice per year. Children may have clinically unsuspected rejection episodes even 5-10 yr after transplantation; most pediatric transplant centers continue routine surveillance biopsies, although at less frequent intervals.
Criteria for grading cardiac rejection are based on a system developed by the International Society for Heart and Lung Transplantation (ISHLT) that takes into account the degree of cellular infiltration and whether myocyte necrosis is present. ISHLT rejection grade 1R is usually mild enough that it is often not treated with bolus steroids, and many of these episodes resolve spontaneously. For patients with ISHLT grade 2R rejection, treatment is instituted with either intravenous (IV) methylprednisolone or a “bump and taper” of oral prednisone. Asymptomatic patients further out from transplant with normal echocardiograms are may treated as outpatients. Patients with grade 3R, or anyone with hemodynamic instability, are admitted to the hospital for IV corticosteroid and potentially more aggressive antirejection therapy. For rejection episodes resistant to steroid therapy, additional therapeutic regimens include antilymphocyte preparation (antithymocyte globulin), methotrexate, or total lymphoid irradiation. Patients with repeated episodes of rejection may also benefit from the switch from cyclosporine to tacrolimus (or vice versa) or the addition of a proliferation single inhibitor (e.g., sirolimus). Refractory rejection is not considered a good indication for retransplantation because of the relatively poor outcomes compared with other indications for retransplantation.
Gene expression profiling of peripheral blood mononuclear cells has been validated in adults as a highly sensitive, moderately selective method of rejection surveillance. These results have not been confirmed in children. Other promising current techniques include the profiling of donor cell–free DNA released in the serum of patients during episodes of graft injury. Progress has also been made in genetic profiling as a means to determine which patients are most at risk for rejection. Children who have single nucleotide polymorphisms (SNPs) leading to greater activity of inflammatory cytokines or decreased activity of regulatory cytokines are at increased risk of rejection.
Some rejection episodes are not associated with a cellular infiltrate on biopsy. These cases of antibody-mediated rejection are mediated by circulating donor-specific antibodies (DSAs) and can be detected by immunostaining of the biopsy specimen for the complement component C4d, for macrophages expressing CD68, and for evidence of histologic damage. Antibody-mediated rejection is less responsive to standard therapies for acute cellular rejection (e.g., bolus corticosteroids) and has been treated with plasmapheresis, intravenous immune globulin (IVIG), the anti-CD20 monoclonal antibody rituximab, and the proteasome inhibitor bortezomib, all with mixed results. The long-term outcome of patients with persistent DSAs is poor, with many having early graft failure.
Complications of Immunosuppression
Infection
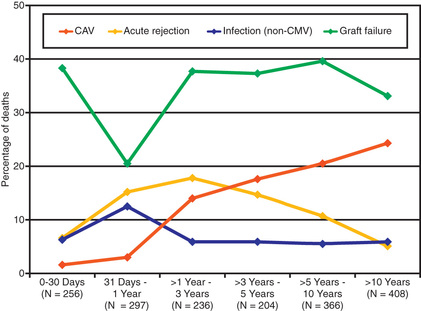
Infection is also a leading cause of death in pediatric transplant patients (Fig. 470.2 ). The incidence of infection is greatest in the 1st 3 mo after transplantation, when immunosuppressive doses are highest. Viral infections are most common and account for as many as 25% of infectious episodes. Cytomegalovirus (CMV) infection was once a leading cause of morbidity and mortality and may occur as a primary infection in patients without previous exposure to the virus or as a reactivation. Severe CMV infection can be disseminated or associated with pneumonitis or gastroenteritis and may provoke an episode of acute graft rejection or graft coronary disease. Most centers use IV ganciclovir or CMV immune globulin (CytoGam), or both, as prophylaxis in any patient receiving a heart from a donor who is positive for CMV or in any recipient who has serologic evidence of previous CMV disease. Oral preparations of ganciclovir with improved absorption profiles are available for chronic therapy and have largely replaced IV preparations for prophylaxis. These regimens have significantly reduced the burden of CMV disease in heart transplant patients. Polymerase chain reaction (PCR) enhances the ability to diagnose CMV infection and monitor the efficacy of therapy serially.
Most normal childhood viral illnesses are well tolerated and do not usually require special treatment. Otitis media and routine upper respiratory tract infections can be treated in the outpatient setting, although fever or symptoms that last beyond the usual course require further investigation. Gastroenteritis , especially with vomiting, can result in greatly reduced absorption of immunosuppressive medications and provoke a rejection episode. In this setting, drug levels should be closely monitored and use of IV medications considered. Gastroenteritis can also be a sign of rejection, so a high index of suspicion must always be maintained. Varicella is another childhood illness of concern for immunosuppressed patients. If a heart transplant recipient acquires clinical varicella infection, treatment with IV acyclovir usually attenuates the illness.
Bacterial infections occur next in frequency after viral, with the lung the most common site of infection, followed by blood, urinary tract, and less often the sternotomy site. Other sources of posttransplantation infection include fungi and protozoa. Many centers use nystatin mouthwash to decrease fungal colonization and trimethoprim-sulfamethoxazole during a patient's corticosteroid prophylaxis to prevent Pneumocystis jiroveci infection.
Growth Retardation
Patients requiring chronic corticosteroid therapy usually have decreased linear growth. Thus, many pediatric transplant programs aim for steroid-free immunosuppression within the 1st yr after transplant. In patients who experience rejection when steroids are withdrawn, alternate-day corticosteroid regimens may result in improved linear growth. Total lymphoid irradiation has also shown promise as a steroid-sparing protocol. Despite these concerns, the majority of long-term survivors of pediatric heart transplantation have normal growth.
Hypertension
Hypertension is common in patients treated with calcineurin inhibitors, caused by a combination of plasma volume expansion and defective renal sodium excretion. Corticosteroids usually potentiate calcineurin-induced hypertension.
Renal Function
Chronic administration of cyclosporine or tacrolimus can lead to a tubulointerstitial nephropathy in adults, but severe renal dysfunction is less common in children. Most pediatric patients gradually have an increase in serum creatinine in the 1st yr after transplantation; if renal dysfunction occurs, it usually responds to a decrease in calcineurin inhibitor (CNI) dosage. The addition of sirolimus, a mammalian target of rapamycin (mTOR) inhibitor, instead of mycophenolate mofetil (MMF) allows a reduction in CNI dose in patients with renal dysfunction, although it is unclear whether this strategy leads to long-term improved renal function. Infection with BK virus, a growing problem in renal transplant patients, has been described as a source of renal dysfunction in heart transplant patients. Fortunately, pediatric heart transplant patients infrequently require renal transplantation.
Neurologic Complications
Neurologic side effects of cyclosporine and tacrolimus include tremor, myalgias, paresthesias, and rarely, seizures. These complications can be treated with reduced doses of medication and occasionally with oral magnesium supplementation. Intracranial infections pose a significant risk, especially because some of the more frequent signs (nuchal rigidity) may be absent in immunosuppressed patients. Potential organisms include Aspergillus, Cryptococcus neoformans, and Listeria monocytogenes. A rare form of encephalopathy known as posterior reversible encephalopathy syndrome (PRES) can occur in patients taking CNIs (cyclosporine or tacrolimus). PRES presents with hypertension, headaches and seizures, requires MRI for diagnosis, and is usually managed by changing CNI or in rare cases eliminating CNIs totally in favor of other immunosuppressive agents (e.g., sirolimus, MMF).
Tumors
One of the serious complications limiting long-term survival in pediatric heart transplant patients is the risk of neoplastic disease. The most common is posttransplant lymphoproliferative disease (PTLD), a condition associated with Epstein-Barr virus (EBV) infection. Patients who are EBV seronegative at transplant (usually infants and young children) are at increased risk of developing PTLD if they subsequently seroconvert, acquiring the virus either from the donor organ or from primary infection. Unlike true cancer, many cases of PTLD respond to a reduction in immunosuppression. A monoclonal antibody directed against the CD20 antigen on activated lymphocytes (rituximab) has been effective against some forms of PTLD. However, PTLD can behave more aggressively, and many patients eventually require chemotherapy. An increased risk of skin cancer requires that children use appropriate precautions when exposed to sunlight.
Cardiac Allograft Vasculopathy
Cardiac allograft vasculopathy (CAV) is a disease of the coronary arteries that occurs in approximately 20% of children 5 yr after transplant. The cause is still unclear, although it is thought to be a form of immunologically mediated vessel injury. Multiple factors, including rejection episodes, infections, hypercholesterolemia, and hyperglycemia, are thought to increase the risk of CAV. Unlike native coronary atherosclerosis, CAV is a diffuse process with a high degree of distal vessel involvement. Because the transplanted heart has been denervated, patients may not experience symptoms such as angina pectoris during ischemic episodes, and the initial manifestation may be cardiovascular collapse or sudden death. Most centers perform coronary angiography annually to screen for coronary abnormalities; some also perform coronary intravascular ultrasound in larger children and adolescents. Standard coronary artery bypass procedures are usually not helpful because of the diffuse nature of the process, although transcatheter stenting can sometimes be effective for isolated lesions. For patients with severe CAV, repeat heart transplantation has been the only effective treatment. Thus, prevention has been the focus of most current research. The cell-cycle inhibitors sirolimus and everolimus have been shown to decrease coronary arterial intimal thickening in adult transplant patients. Other drugs that have been associated with a lower the risk of CAV include the calcium channel blockers (e.g., diltiazem) and the cholesterol-lowering HMG-CoA (3-hydroxy-3-methyl-coenzyme A) reductase inhibitors (e.g., pravastatin, atorvastatin).
Other Complications
Corticosteroids usually result in cushingoid facies, steroid acne, and striae. Cyclosporine can cause a subtle change in facial features, such as hypertrichosis and gingival hyperplasia. These cosmetic features can be particularly disturbing to adolescents and may be the motivation for noncompliance, one of the leading risks for late morbidity and mortality. Most of these cosmetic complications are dose related and improve as immunosuppressive medications are weaned. Tacrolimus does not have the cosmetic side effects of cyclosporine. Osteoporosis and aseptic necrosis are additional reasons for reducing the steroid dosage as soon as possible. Diabetes and pancreatitis are rare but serious complications.
Rehabilitation
Despite the potential risks of immunosuppression, the prospect for rehabilitation in pediatric heart transplant recipients is excellent; most have no functional limitations in their daily lives. They can attend daycare or school and participate in competitive sports and other age-appropriate activities. Standardized measurements of ventricular function are close to normal. Because the transplanted heart is denervated, the increase in heart rate and cardiac output during exercise is slower in transplant recipients, and maximal heart rate and cardiac output responses are mildly attenuated. These subtle abnormalities are rarely noticeable by the patient.
Growth of the transplanted heart is excellent, although a mild degree of ventricular hypertrophy is usually seen, even years after transplantation. The sites of atrial and great vessel anastomoses usually grow without the development of obstruction. In neonates who undergo transplantation for HLHS, however, juxtaductal aortic coarctation may recur.
A serious problem with noncompliance may occur once patients reach adolescence, and life-threatening rejection may result. Early intervention by social workers, counselors, and psychologists may be able to reduce this risk.
Bibliography
Castleberry C, Ryan TD, Chin C. Transplantation in the highly sensitized pediatric patient. Circulation . 2014;129(22):2313–2319.
Colvin MM, Cook JL, Chang P, et al. Antibody-mediated rejection in cardiac transplantation: emerging knowledge in diagnosis and management: a scientific statement from the American Heart Association. Circulation . 2015;131(18):1608–1639.
Fraser CD Jr, Jaquiss RD, Rosenthal DN, et al. Berlin heart study: prospective trial of a pediatric ventricular assist device. N Engl J Med . 2012;367(6):532–541.
Hammond ME, Revelo MP, Miller DV, et al. ISHLT pathology antibody mediated rejection score correlates with increased risk of cardiovascular mortality: a retrospective validation analysis. J Heart Lung Transplant . 2016;35(3):320–325.
Mehra MR, Canter CE, Hannan MM, et al. The 2016 international society for heart lung transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant . 2016;35(1):1–23.
Rossano JW, Dipchand AI, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: nineteenth pediatric heart transplantation report—2016. Focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant . 2016;35(10):1185–1195.
Rossano JW, Jefferies JL, Pahl E, et al. Use of sirolimus in pediatric heart transplant patients: a multi-institutional study from the Pediatric Heart Transplant Study Group. J Heart Lung Transplant . 2017;36(4):429–433.
Stein ML, Bruno JL, Konopacki KL, et al. Cognitive outcomes in pediatric heart transplant recipients bridged to transplantation with ventricular assist devices. J Heart Lung Transplant . 2013;32(2):212–220.
Tran A, Fixler D, Huang R, et al. Donor-specific HLA alloantibodies: impact on cardiac allograft vasculopathy, rejection, and survival after pediatric heart transplantation. J Heart Lung Transplant . 2016;35(1):87–91.
Urschel S, Larsen IM, Kirk R, et al. ABO-incompatible heart transplantation in early childhood: an international multicenter study of clinical experiences and limits. J Heart Lung Transplant . 2013;32(3):285–292.
Heart-Lung and Lung Transplantation
Joseph W. Rossano, Samuel B. Goldfarb
More than 700 heart-lung and 2,200 lung (single or double) pediatric transplants have been performed and reported to ISHLT, with >100 procedures performed annually. The majority of these are lung transplantation, with only 11 heart-lung transplants reported in 2014. Primary indications for lung transplantation include cystic fibrosis, pulmonary hypertension, interstitial lung disease, surfactant deficiencies, and retransplant. Indications for heart-lung transplant include complex CHD along with either pulmonary parenchymal or vascular disease. Patients with normal hearts are candidates for lung transplantation even in the setting of right ventricular dysfunction. This had led to the marked decline in heart-lung transplant procedures in the current era. In some patients with CHD, double-lung transplantation can be performed in combination with repair of intracardiac defects. Patients with cystic fibrosis are not candidates for single-lung grafts because of the risk of infection from the diseased contralateral lung. Patients are selected according to many of the same criteria as for heart transplant recipients (see Chapter 470.1 ).
Posttransplant immunosuppression is usually achieved with a triple-drug regimen, combining a CNI (cyclosporine or tacrolimus) with an antiproliferative agent (MMF or azathioprine) and prednisone. Most patients receive induction therapy with an antithymocyte or anti–T-cell preparation. Unlike patients with isolated heart transplants, patients with lung or heart-lung transplants cannot be weaned totally off steroids. Prophylaxis against P. jiroveci infection is achieved with trimethoprim-sulfamethoxazole or aerosolized pentamidine. Ganciclovir and CMV immune globulin prophylaxis are used as in heart transplant recipients (see Chapter 470.1 ). Antifungal medications are used in the perioperative and posttransplant periods in patients who have pretransplant colonization.
Pulmonary rejection is common in lung or heart-lung transplant recipients, whereas heart rejection is encountered much less often than in patients with isolated heart transplants. Acute rejection occurs in approximately 25% of patients in the 1st yr after transplant. Symptoms of lung rejection may include fever and fatigue, although many episodes are minimally symptomatic. Signs of rejection could include changes in lung function testing and radiographic findings. Surveillance for rejection is performed by monitoring pulmonary function (forced vital capacity; forced expiratory volume in 1 sec [FEV1 ]; forced expiratory flow, midexpiratory phase [FEF25-75% ]), systemic arterial oxygen tension, chest radiographs, chest CT, transbronchial biopsy, and open lung biopsy. In heart-lung transplant recipients, hearts are assessed for rejection similar to the approach described in Chapter 470.1 .
Actuarial survival rates after lung or heart-lung transplantation in children are currently 75–80% at 1 yr and 50% at 5 yr after transplant; improved patient selection and postoperative management are continually improving these survival statistics from prior eras. Some groups, such as patients with CHD in the absence of Eisenmenger syndrome, have particular poor outcomes with transplant. Graft failure and infection are the leading cause of early death, whereas a form of chronic rejection known as bronchiolitis obliterans accounts for almost 50% of late mortality. Other causes of early morbidity and mortality include technical complications, multiorgan failure, primary graft dysfunction, and cardiovascular causes. Additional late complications include the development of late graft failure, malignancies, infection, and other side effects of chronic immunosuppression.
Postoperative indices of cardiopulmonary function and exercise capacity show significant improvement. Problems of donor availability are even more severe with lung transplantation than with isolated heart transplantation. However, significant advances in ex vivo lung perfusion has great potential to expand the number of organs available for transplantation.
Bibliography
Goldfarb SB, Levvey BJ, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: nineteenth pediatric lung and heart-lung transplantation report—2016. Focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant . 2016;35(10):1196–1205.
Hayes D Jr, Sweet SC, Benden C, et al. Transplant center volume and outcomes in lung transplantation for cystic fibrosis. Transpl Int . 2016; 10.1111/tri.12911 .
Keeshan BC, Goldfarb SB, Lin KY, et al. Impact of congenital heart disease on outcomes of pediatric heart-lung transplantation. Pediatr Transplant . 2014;18(2):204–210.
Snell GI, Paraskeva M, Westall GP. Managing bronchiolitis obliterans syndrome (BOS) and chronic lung allograft dysfunction (CLAD) in children: what does the future hold? Paediatr Drugs . 2013;15(4):281–289.
Yeung JC, Krueger T, Yasufuku K, et al. Outcomes after transplantation of lungs preserved for more than 12 h: a retrospective study. Lancet Respir Med . 2017;5(2):119–124.