Laboratory Cardiac Evaluation
Radiologic Cardiac Assessment
Daniel Bernstein
Despite the widespread easy access to advanced imaging techniques, such as echocardiography, computed tomography (CT) scan, and magnetic resonance imaging (MRI), the chest x-ray film (radiograph, roentgenogram) remains a highly valuable diagnostic tool and is often the first imaging study performed in a child suspected of having a cardiac defect. It can provide information about cardiac size and shape, pulmonary blood flow (vascularity), pulmonary edema, and associated lung and thoracic anomalies that may be associated with congenital syndromes (e.g., skeletal dysplasias, extra or deficient number of ribs, abnormal vertebrae, previous cardiac surgery). Combined with a careful physical examination, the chest radiograph can help the clinician to establish a diagnosis of congenital heart disease (CHD), as opposed to pulmonary disease, and to narrow the differential diagnosis to specific categories of CHD (e.g., left-to-right shunt lesions vs obstructive lesions).
The most frequently used measurement of cardiac size is the maximal width of the cardiac shadow in a posteroanterior (PA) chest film taken mid-inspiration. A vertical line is drawn down the middle of the sternal shadow, and perpendicular lines are drawn from the sternal line to the extreme right and left borders of the heart; the sum of the lengths of these lines is the maximal cardiac width . The maximal chest width is obtained by drawing a horizontal line between the right and left inner borders of the rib cage at the level of the top of the right diaphragm. When the maximal cardiac width is more than half the maximal chest width (cardiothoracic ratio >50%), the heart is usually enlarged. Cardiac size should be evaluated only when the film is taken during inspiration with the patient in an upright position. A diagnosis of “cardiac enlargement” on expiratory or prone films is a common cause of unnecessary referrals and laboratory studies.
The cardiothoracic ratio is a less useful index of cardiac enlargement in infants than in older children because the horizontal position of the heart may increase the ratio to >50% in the absence of true enlargement. Furthermore, the thymus may overlap not only the base of the heart but also virtually the entire mediastinum, thus obscuring the true cardiac silhouette.
A lateral chest radiograph may be helpful in infants as well as in older children with pectus excavatum or other conditions that result in a narrow anteroposterior (AP) chest dimension. The heart may appear small in the lateral view and suggest that the apparent enlargement in the PA projection was caused by either the thymic image (anterior mediastinum only) or flattening of the cardiac chambers as a result of a structural chest abnormality.
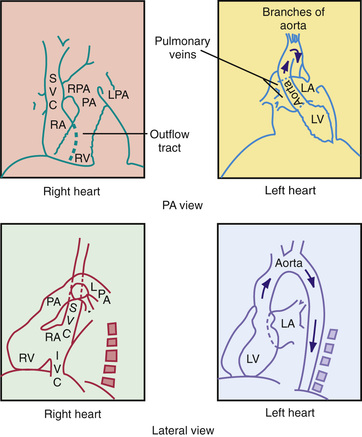
In the PA view the left border of the cardiac shadow consists of 3 convex shadows produced, from above downward, by the aortic knob, the main and left pulmonary arteries, and the left ventricle (Fig. 450.1 ). In cases of moderate to marked left atrial enlargement, the atrium may project between the pulmonary artery and the left ventricle. The right ventricular outflow tract (RVOT) does not contribute to the shadows formed by the left border of the heart. The aortic knob is not as easily seen in infants and children as in adults. The side of the aortic arch (left or right) can often be inferred as being opposite the side of the midline from which the air-filled trachea is visualized. This observation is important because a right-sided aortic arch is often present in cyanotic CHD, particularly in tetralogy of Fallot. Three structures contribute to the right border of the cardiac silhouette. In the view from above, they are the superior vena cava, the ascending aorta, and the right atrium.
Enlargement of cardiac chambers or major arteries and veins results in prominence of the areas in which these structures are normally outlined on the chest radiograph. In contrast, the electrocardiogram is a more sensitive and accurate index of ventricular hypertrophy , which is a thickening of the ventricular wall and may or may not be associated with dilation of the affected cardiac chamber.
The chest radiograph is also an important tool for assessing the degree of pulmonary vascularity. Pulmonary overcirculation is usually associated with left-to-right shunt lesions, whereas pulmonary undercirculation is associated with obstruction of the RVOT. The esophagus is closely related to the great vessels, and a barium esophagogram can help delineate these structures in the initial evaluation of suspected vascular rings, although this has largely been supplanted by CT.
Echocardiographic examination best defines the morphologic features of intracardiac chambers, cardiac valves, and intracardiac shunts. CT is used as an adjunct to echo to evaluate extracardiac vascular morphology. MRI is used most often to provide a more quantitative assessment of ventricular volumes, cardiac function, and shunt and regurgitant fractions than is possible with echo.
Electrocardiography
Daniel Bernstein
Developmental Changes
The marked changes that occur in cardiac physiology and chamber dominance during the perinatal transition (see Chapter 448 ) are reflected in the evolution of the electrocardiogram (ECG) during the neonatal period. Because vascular resistance in the pulmonary and systemic circulations is nearly equal in a term fetus, the intrauterine work of the heart results in an equal mass of both the right and left ventricles. After birth, systemic vascular resistance (SVR) rises when the placental circulation is eliminated, and pulmonary vascular resistance (PVR) falls when the lungs expand. These changes are reflected in the ECG as the right ventricular (RV) wall begins to thin.
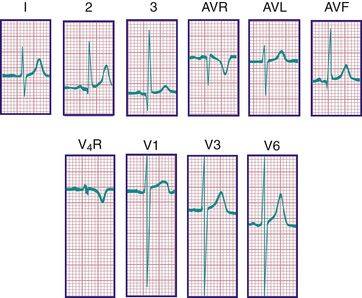
The ECG demonstrates these anatomic and hemodynamic features principally by changes in QRS and T-wave morphologic features. Typically, pediatric ECGs include several additional leads rarely used in adults, such as V3 R and V4 R, which are mirror images of leads V3 and V4 and are important in the evaluation of right ventricular hypertrophy (RVH). On occasion, lead V1 is inappropriately positioned too far leftward to reflect RV forces accurately. This problem is present particularly in premature infants, in whom the electrocardiographic electrode gel may produce contact among all the precordial leads. An additional lead used in children is V7 , located more laterally than V6 and useful for assessing left-sided forces.
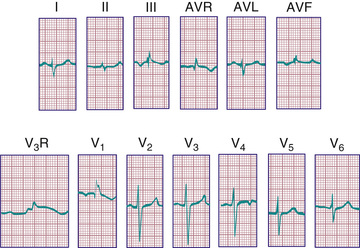
During the 1st postnatal days of life, right axis deviation, large R waves, and upright T waves in the right precordial leads (V3 R or V4 R and V1 ) are the norm (Fig. 450.2 ). As PVR decreases in the 1st few days after birth, the right precordial T waves become negative. In the great majority of cases, this change occurs within the 1st 48 hr of postnatal life. Upright T waves that persist in leads V3 R, V4 R, or V1 beyond 1 wk of life are an abnormal finding indicating RVH or RV strain, even in the absence of QRS voltage criteria. The T wave in V1 should never be positive before 6 yr of age and may remain negative into adolescence or early adulthood. This finding represents one of the most important yet subtle differences between pediatric and adult ECGs and is a common source of error when adult cardiologists interpret pediatric ECGs.
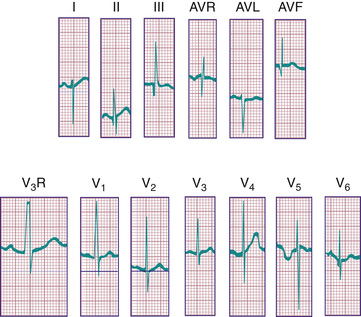
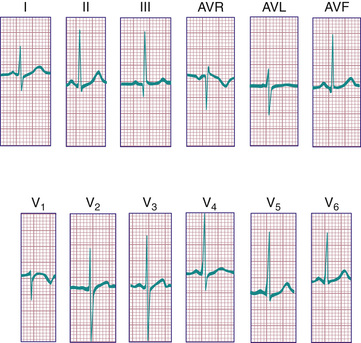
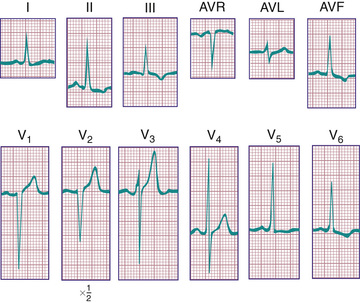
In a newborn the mean QRS frontal-plane axis normally lies in the range of +110 to +180 degrees, reflecting the co-dominance of the fetal right and left ventricles. The right-sided chest leads reveal a larger positive (R ) than negative (S ) wave and may do so for months because the right ventricle remains relatively thick throughout infancy. Left-sided leads (V5 and V6 ) also reflect right-sided dominance in the early neonatal period, when the R:S ratio in these leads may be <1. A dominant R wave in V5 and V6 , reflecting left ventricular (LV) forces, quickly becomes evident within the 1st few days of life (Fig. 450.3 ). As the child matures, the QRS axis gradually shifts leftward, and the RV forces slowly regress. Leads V1 , V3 R, and V4 R display a prominent R wave until 6 mo to 8 yr of age. Most children have an R:S ratio >1 in lead V4 R until age 4 yr. The T waves are inverted in leads V4 R, V1 , V2 , and V3 during infancy and may remain so into the middle of the 2nd decade of life and beyond. The processes of RV thinning and LV growth are best reflected in the QRS-T pattern over the right precordial leads. The diagnosis of RVH or left ventricular hypertrophy (LVH) in a pediatric patient can be made only with an understanding of the normal developmental physiology of these chambers at various ages until adulthood is reached. As the left ventricle becomes dominant, the ECG evolves to the characteristic pattern of older children (Fig. 450.4 ) and adults (Fig. 450.5 ).
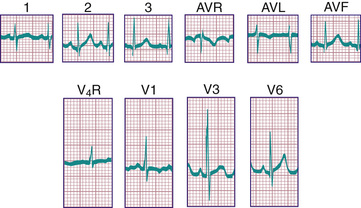
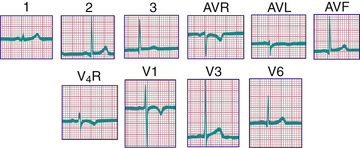
Ventricular hypertrophy may result in increased voltage in the R and S waves in the chest leads. The height of these deflections is governed by the proximity of the specific electrode to the surface of the heart; by the sequence of electrical activation through the ventricles, which can result in variable degrees of cancellation of forces; and by hypertrophy of the myocardium. Because the chest wall in infants and children, as well as in adolescents, may be relatively thin, the diagnosis of ventricular hypertrophy should not be based solely on voltage changes unless those voltages are greatly increased.
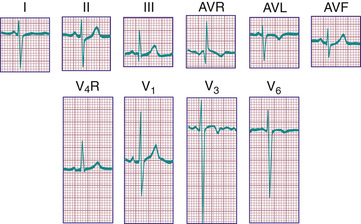
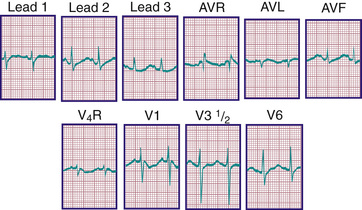
The diagnosis of pathologic RVH is difficult in the 1st wk of postnatal life because physiologic RVH is a normal finding. Serial tracings are often necessary to determine whether marked right axis deviation and potentially abnormal right precordial forces or T waves, or both, will persist beyond the neonatal period (Fig. 450.6 ). In contrast, an adult ECG pattern (see Fig. 450.5 ) seen in a neonate suggests LVH. The exception is a premature infant, who may display a more “mature” ECG than a full-term infant (Fig. 450.7 ), as a result of lower PVR secondary to underdevelopment of the medial muscular layer of the pulmonary arterioles. Some premature infants display a pattern of generalized low voltage across the precordium.
The ECG should always be evaluated systematically to avoid overlooking a minor but important abnormality. One approach is to begin with an assessment of rate and rhythm, followed by a calculation of the mean frontal-plane QRS axis, measurements of segment intervals, assessment of voltages, and lastly assessment of ST and T-wave abnormalities.
Rate and Rhythm
A brief rhythm strip should be examined to assess whether a P wave always precedes each QRS complex. The P-wave axis should then be estimated as an indication of whether the rhythm is originating from the sinus node . If the atria are situated normally in the chest, the P wave should be upright in leads I and aVF and inverted in lead aVR. With atrial inversion (situs inversus ), the P wave may be inverted in lead I. Inverted P waves in leads II and aVF are seen in low atrial, nodal, or junctional rhythms. The absence of P waves indicates a rhythm originating more distally in the conduction system. In this case, the morphologic features of the QRS complexes are important in differentiating a junctional (usually a narrow QRS complex) from a ventricular (usually a wide QRS complex) rhythm.
P Waves
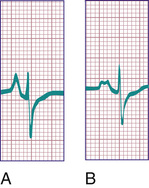
Tall (>2.5 mm), narrow, and spiked P waves are indicative of right atrial enlargement and are seen in congenital pulmonary stenosis, Ebstein anomaly of the tricuspid valve, tricuspid atresia, and sometimes cor pulmonale. These abnormal waves are most obvious in leads II, V3 R, and V1 (Fig. 450.8A ). Similar waves are sometimes seen in thyrotoxicosis. Broad P waves, commonly bifid and sometimes biphasic , are indicative of left atrial enlargement (Fig. 450.8B ). They are seen in some patients with large left-to-right shunts (ventricular septal defect [VSD], patent ductus arteriosus) and with severe mitral stenosis or mitral regurgitation. Left atrial enlargement, however, is one of the most common false-positive readings generated by computerized ECG machines. Flat P waves may be encountered in patients with hyperkalemia.
QRS Complex
Right Ventricular Hypertrophy
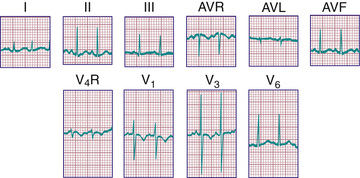
For the most accurate assessment of ventricular hypertrophy, pediatric ECGs should include the right precordial lead V3 R or V4 R, or both. The diagnosis of RVH depends on demonstration of the following changes (see Fig. 450.6 ): (1) a qR pattern in the RV surface leads; (2) a positive T wave in leads V3 R-V4 R and V1 -V3 between ages 6 days and 6 yr; (3) a monophasic R wave in V3 R, V4 R, or V1 ; (4) an rsR′ pattern in the right precordial leads with the 2nd R wave taller than the 1st; (5) age-corrected increased voltage of the R wave in leads V3 R-V4 R or the S wave in leads V6 -V7 , or both; (6) marked right axis deviation (>120 degrees in patients beyond the newborn period); and (7) complete reversal of the normal adult precordial RS pattern. At least 2 of these changes should be present to support a diagnosis of RVH.
Abnormal ventricular loading can be characterized as either systolic (as a result of RVOT obstruction, as in pulmonic stenosis) or diastolic (as a result of increased volume load, as in atrial septal defect [ASD]). These 2 types of abnormal loads result in distinct electrocardiographic patterns. The systolic overload pattern is characterized by tall, pure R waves in the right precordial leads. In older children the T waves in these leads are initially upright and later become inverted. In infants and children <6 yr the T waves in V3 R-V4 R and V1 are abnormally upright. The diastolic overload pattern (typically seen in patients with ASD) is characterized by an rsR′ pattern (Fig. 450.9 ) and a slightly increased QRS duration (which is known as a minor right ventricular conduction delay rather than a true bundle branch block). Patients with mild to moderate pulmonary stenosis may also exhibit an rsR′ pattern in the right precordial leads.
Left Ventricular Hypertrophy
The following features indicate the presence of LVH (Fig. 450.10 ): (1) depression of the ST segments and inversion of the T waves in the left precordial leads (V5 , V6 , and V7 ), known as a left ventricular strain pattern—these findings suggest the presence of a more severe lesion; (2) a deep Q wave in the left precordial leads; and (3) increased voltage of the S wave in V3 R and V1 or the R wave in V6 -V7 , or both. It is important to emphasize that evaluation of LVH should not be based on voltage criteria alone, especially in adolescents and young adults, and within these groups, especially in males. The concepts of systolic and diastolic overload, although not always consistent, are also useful in evaluating LV enlargement. Severe systolic overload of the left ventricle is suggested by straightening of the ST segments and inverted T waves over the left precordial leads; diastolic overload may result in tall R waves, a large Q wave, and normal T waves over the left precordium. Finally, an infant with an ECG that would be considered “normal” for an older child may in fact have LVH.
Bundle Branch Block
A complete right bundle-branch block (prolonged QRS complex which is usually upright with an rSR′ in lead V1; wide S wave in lead V6 ) may be congenital or may be acquired after surgery for CHD, especially when a partial right ventriculotomy has been performed, as in repair of the tetralogy of Fallot. Left bundle branch block (LBBB; prolonged QRS complex, which is usually upright with an rSR′ in lead V6 ; wide S wave in lead V1 ) is less common in children; this pattern is often seen in adults with cardiomyopathy, but much less in children with cardiomyopathy. LBBB may be seen after surgery on the aortic or mitral valve caused by surgical injury to one of the left-sided conduction bundles. Alternatively, a bundle branch block pattern may be indicative of a bypass tract associated with one of the preexcitation syndromes (see Chapter 462 ).
P-R and Q-T Intervals
The duration of the P-R interval shortens with increasing heart rate; thus assessment of this interval should be based on age- and rate-corrected nomograms. A long P-R interval is diagnostic of a first degree heart block , the cause of which may be congenital, postoperative (after open heart surgery), inflammatory (myocarditis, pericarditis, Lyme disease, rheumatic fever), or pharmacologic (digitalis).
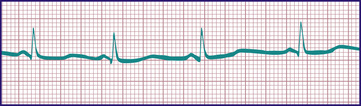
The duration of the Q-T interval varies with the cardiac rate; a corrected Q-T interval (Q-Tc) can be calculated by dividing the measured Q-T interval by the square root of the preceding R-R interval. A normal Q-Tc should be <0.45. It is often lengthened with hypokalemia and hypocalcemia; in the former, a U wave may be noted at the end of the T wave (Fig. 450.11 ). A number of medications can also lengthen the Q-T interval. A congenitally prolonged Q-T interval may also be seen in children with one of the long QT syndromes (Fig. 450.12 ). These patients are at high risk for ventricular arrhythmias, including a form of ventricular tachycardia known as torsades de pointes, and sudden death (see Chapter 462.5 ).
ST Segment and T-Wave Abnormalities
Coronary ischemia, leading to typical ST and T-wave abnormalities seen in adults, is rare in children. A slight elevation of the ST segment (J-point elevation) is often seen in normal teenagers and is attributed to early repolarization of the heart. In pericarditis, irritation of the epicardium may cause elevation of the ST segment, followed by abnormal T-wave inversion as healing progresses. Administration of digitalis is sometimes associated with sagging of the ST segment and abnormal inversion of the T wave.
Depression of the ST segment may also occur in any condition that produces myocardial damage or ischemia, including severe anemia, carbon monoxide poisoning, aberrant origin of the left coronary artery from the pulmonary artery, glycogen storage disease of the heart, myocardial tumors, and mucopolysaccharidoses. An aberrant origin of the left coronary artery from the pulmonary artery may lead to changes indistinguishable from those of acute myocardial infarction in adults. ECG findings of ischemia may be seen in patients with Kawasaki disease who have developed coronary artery aneurysms (see Chapters 191 and 471.1 ). Similar changes may occur in patients with other rare abnormalities of the coronary arteries and in those with cardiomyopathy, even in the presence of normal coronary arteries. These patterns are often misread in young infants because of the unfamiliarity of pediatricians with this “infarct” pattern, and thus a high index of suspicion must be maintained in infants with dilated cardiomyopathy or with symptoms compatible with coronary ischemia (e.g., inconsolable crying).
T-wave inversion may occur in myocarditis and pericarditis, or it may be a sign of either RVH or LVH and ventricular strain. Hypothyroidism may produce flat or inverted T waves in association with generalized low voltage. In hyperkalemia, the T waves are usually of high voltage and are tent shaped (Fig. 450.13 ), although tall T waves can be an early sign in myocardial infarction.
Bibliography
Ahmed H, Czosek RJ, Spar DS, et al. Early repolarization in normal adolescents is common. Pediatr Cardiol . 2017;38(4):864–872.
Garson A. The electrocardiogram in infants and children: a systematic approach . Lea & Febiger: Philadelphia; 1983.
O'Connor M, McDaniel N, Brady WJ. The pediatric electrocardiogram. Part I. Age-related interpretation. Am J Emerg Med . 2008;26:506–512.
O'Connor M, McDaniel N, Brady WJ. The pediatric electrocardiogram. Part III. Congenital heart disease and other cardiac syndromes. Am J Emerg Med . 2008;26:497–503.
Papneja K, Chan AK, Mondal TK, Paes B. Myocardial infarction in neonates: a review of an entity with significant morbidity and mortality. Pediatr Cardiol . 2017;38(3):427–441.
Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J . 2017; 10.1093/eurheartj/ehw631 [Epub ahead of print].
Hematologic Data
Daniel Bernstein
In acyanotic infants with large left-to-right shunts, the onset of heart failure often coincides with the nadir of the normal physiologic anemia of infancy. Increasing the hematocrit in these patients to >40% may decrease shunt volume and result in an improvement in symptoms; however, this form of treatment is generally reserved for infants who are not otherwise surgical candidates (extremely premature infants or those with exceedingly complex CHD for whom only palliative surgery is possible). In these select infants, regular evaluation of the hematocrit and booster transfusions when appropriate may be helpful in improving growth.
Polycythemia is frequently noted in chronically cyanotic patients with right-to-left shunts. Patients with severe polycythemia are in a delicate balance between the risks of intravascular thrombosis and a bleeding diathesis. The most frequent abnormalities include accelerated fibrinolysis, thrombocytopenia, abnormal clot retraction, hypofibrinogenemia, prolonged prothrombin time, and prolonged partial thromboplastin time. The preparation of cyanotic, polycythemic patients for elective noncardiac surgery, such as dental extraction, includes evaluation and treatment of abnormal coagulation.
Because of the high viscosity of polycythemic blood (hematocrit >65%), patients with cyanotic CHD are at risk for the development of vascular thromboses, especially of cerebral veins. Dehydration increases the risk of thrombosis, and thus adequate fluid intake must be maintained during hot weather or intercurrent gastrointestinal illnesses. Diuretics should be used with caution in these patients and may need to be decreased if fluid intake is a concern. Polycythemic infants with concomitant iron deficiency are at even greater risk for cerebrovascular accidents, probably because of the decreased deformability of microcytic red blood cells. Iron therapy may reduce this risk somewhat, but surgical treatment of the cardiac anomaly is the best therapy.
Severely cyanotic patients should have periodic determinations of hemoglobin and hematocrit. Increasing polycythemia, often associated with headache, fatigue, dyspnea, or a combination of these conditions, is one indication for palliative or corrective surgical intervention. In cyanotic patients with inoperable conditions, partial exchange transfusion may be required to treat symptomatic (most often headache or chest pain) individuals whose hematocrit has risen to the 65–70% level. This procedure is not without risk, especially in patients with an extreme elevation in PVR. Because these patients do not tolerate wide fluctuations in circulating blood volume, blood should be replaced with fresh-frozen plasma or albumin.
Echocardiography
Daniel Bernstein
Transthoracic echocardiography (TTE) has replaced invasive studies such as cardiac catheterization for the diagnosis of most forms of CHD. The echocardiographic examination can be used to evaluate cardiac structures in congenital heart lesions using two-dimensional (2D) and three-dimensional (3D) imaging, estimate intracardiac pressures and gradients across stenotic valves and vessels using echo-Doppler and color flow Doppler, quantitate cardiac contractile function (both systolic and diastolic), determine the direction of flow across a defect, examine the integrity of the coronary arteries, and detect the presence of vegetations from endocarditis, as well as the presence of pericardial fluid, cardiac tumors, and chamber thrombi.
Echocardiography may also be used to assist in the performance of interventional procedures, including pericardiocentesis, balloon atrial septostomy (see Chapter 458.2 ), ASD or VSD closure, transcatheter valve implantation, and endocardial biopsy. Transesophageal echocardiography (TEE) is used routinely to monitor ventricular function in patients during surgical procedures and can provide an immediate assessment of the results of surgical repair of congenital heart lesions. A complete TTE examination usually entails a combination of M-mode and 2D and 3D imaging, as well as pulsed, continuous, and color Doppler flow studies. Doppler tissue imaging provides a more quantitative assessment of ventricular systolic and diastolic function.
M-Mode Echocardiography
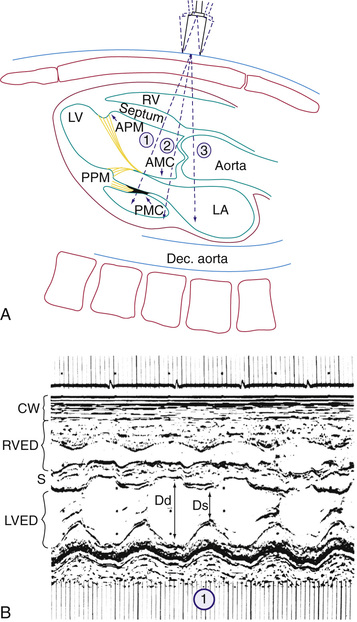
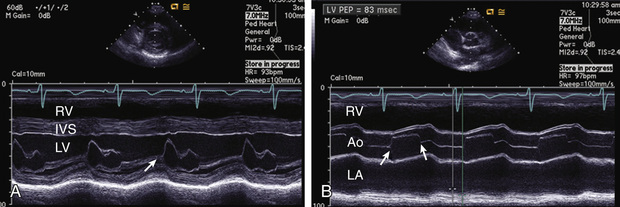
M-mode echocardiography displays a one-dimensional slice of cardiac structure varying over time (Fig. 450.14 ). It is used mostly for the measurement of cardiac dimensions (wall thickness and chamber size) and cardiac function (fractional shortening, wall thickening). M-mode echocardiography is also useful for assessing the motion of intracardiac structures (opening and closing of valves, movement of free walls and septa) and the anatomy of valves (Fig. 450.15 ). The most frequently used index of cardiac function in children is percent fractional shortening (%FS), which contrasts to adults, where ejection fraction is the most common functional measurement. %FS is calculated as (LVED − LVES)/LVED, where LVED is left ventricular dimension at end-diastole and LVES is left ventricular dimension at end-systole. Normal fractional shortening is approximately 28–42%. Other M-mode indices of cardiac function include the mean velocity of fiber shortening (mean VCF ), systolic time intervals (LVPEP = LV preejection period, LVET = LV ejection time), and isovolemic contraction time. M-mode measurements are highly susceptible to errors because of differences in wall motion between different segments of the heart (more frequently seen in adults with ischemic heart disease, but which can be seen in children with congenital and acquired heart disease, especially after surgical repair).
Two-Dimensional Echocardiography
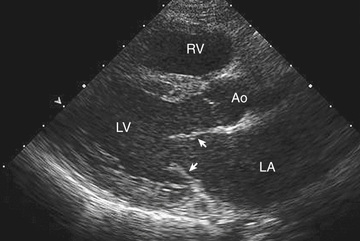
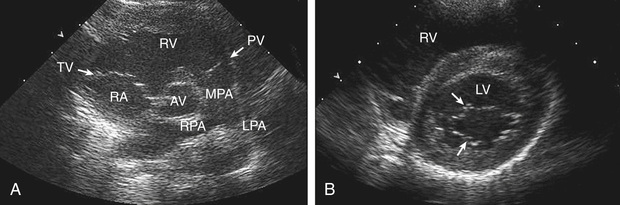
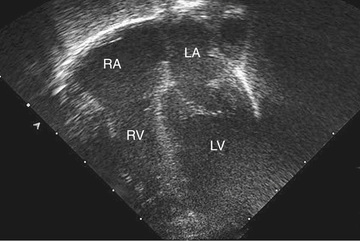
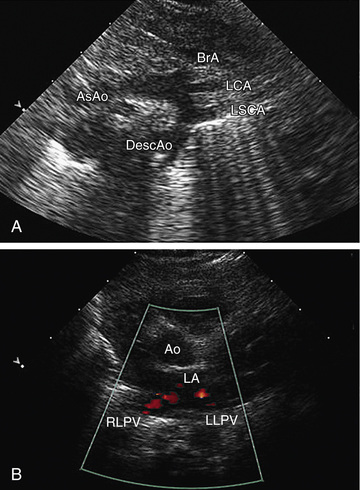
Two-dimensional echocardiography provides a real-time image of cardiac structures. With 2D echocardiography, the contracting heart is imaged in real time using several standard views, including parasternal long axis (Fig. 450.16 ), parasternal short axis (Fig. 450.17 ), apical 4 chamber (Fig. 450.18 ), subcostal (Fig. 450.19 ), and suprasternal (Fig. 450.20 ), each of which emphasizes specific structures. Two-dimensional echocardiography has replaced cardiac angiography for the preoperative diagnosis and follow-up of the vast majority of congenital heart lesions. However, when information from the cardiac examination or other studies is not consistent with the echocardiogram (e.g., size of left-to-right shunt), cardiac catheterization remains an important tool to confirm the anatomic diagnosis and evaluate the degree of physiologic derangement. MRI is also a valuable adjunct to provide a better quantification of ventricular size and function.

Doppler Echocardiography
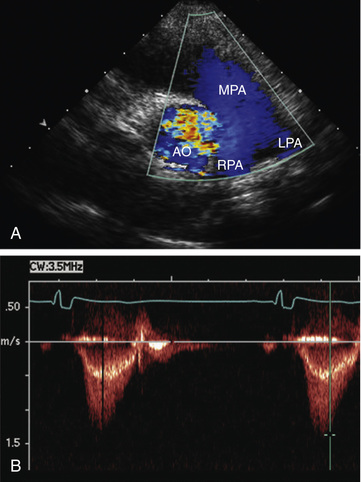
Doppler echocardiography displays blood flow in cardiac chambers and vascular channels based on the change in frequency imparted to a sound wave by the movement of erythrocytes. In pulsed Doppler and continuous wave Doppler, the speed and direction of blood flow in the line of the echo beam change the transducer's reference frequency. This frequency change can be translated into volumetric flow (L/min) data for estimating systemic or pulmonary blood flow and into pressure (mm Hg) data for estimating gradients across the semilunar or atrioventricular valves or across septal defects or vascular communications such as shunts. Color Doppler permits highly accurate assessment of the presence and direction of intracardiac shunts and allows identification of small or multiple left-to-right or right-to-left shunts (Fig. 450.21 ). The severity of valvular insufficiency can be evaluated qualitatively with both pulsed and color Doppler (Fig. 450.22 ). Alterations in venous Doppler flow patterns can be used to detect abnormalities of systemic and pulmonary veins, and alterations of atrioventricular valve Doppler flow patterns can be used to assess ventricular diastolic functional abnormalities, particularly the E/A ratio , the ratio of peak velocity flow in diastole (i.e., the ratio of the early-diastole E wave to the peak velocity flow in late diastole caused by atrial [A] contraction wave).
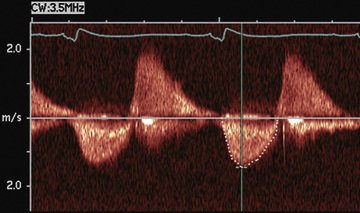
M-mode, 2D, and Doppler echocardiographic methods of assessing LV systolic and diastolic function (e.g., end-systolic wall stress, dobutamine stress echocardiography, Doppler tissue imaging) have proved useful in the serial assessment of patients at risk for the development of both systolic and diastolic ventricular dysfunction and ventricular dyssynchrony (where the coordination of left and right ventricular contraction is abnormal). Such patients include those with cardiomyopathies, those receiving anthracycline drugs for cancer chemotherapy, those at risk for iron overload, and those being monitored for rejection or coronary artery disease after heart transplantation.
Three-Dimensional Echocardiography
Real-time 3D echocardiographic reconstruction is most valuable for the detailed assessment of cardiac morphology (Fig. 450.23 ). Details of valve structure, the size and location of septal defects, abnormalities of the ventricular myocardium, and details of the great vessels, which may not be as readily apparent using 2D imaging, can often be appreciated on 3D echocardiography. Reconstruction of the view that the surgeon will encounter in the operating room makes this technique a valuable adjunct for preoperative imaging.
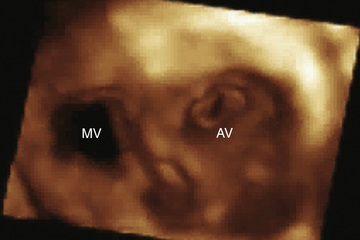
Transesophageal Echocardiography
TEE is an extremely sensitive imaging technique that produces a clearer view of smaller lesions such as vegetations in endocarditis, especially in larger patients. It is useful in visualizing posteriorly located structures such as the atria, aortic root, and atrioventricular valves. TEE is extremely useful as an intraoperative technique for monitoring cardiac function during both cardiac and noncardiac surgery and for screening for residual cardiac defects after the patient is initially weaned from cardiopulmonary bypass but before being disconnected from the bypass circuit. This technique has been especially helpful in evaluating the degree of residual regurgitation or stenosis after valve repairs and in searching for small muscular VSDs that may have been missed during the closure of larger defects. It is always preferable to make the diagnosis of excessive valve regurgitation while the patient is still in the operating room, so that the repair can be revised or the valve replaced, rather than after surgery, when the patient is already in the postoperative care unit. However, hemodynamic measurements made while the chest is open and the patient is still under anesthesia may be different from those made under more normal conditions, as when the patient is ready to be discharged from the hospital.
Fetal Echocardiography
Fetal echocardiography can be used to evaluate cardiac structures or disturbances in cardiac rhythm (Fig. 450.24 ). Obstetricians are attuned to detect gross abnormalities in cardiac structure on routine obstetric ultrasonography (4-chamber view) or may refer the patient because of unexplained hydrops fetalis, a family history of CHD, or a maternal condition associated with fetal cardiac pathology, such as gestational diabetes. Fetal echocardiography can diagnose most significant congenital heart lesions as early as 17-19 wk of gestation; accuracy at this early stage is limited, however, and families should understand that these studies cannot totally eliminate the possibility of CHD. Serial fetal echocardiograms have also demonstrated the importance of flow disturbance in the pathogenesis of CHD; such studies can show the intrauterine progression of a moderate lesion, such as aortic stenosis, into a more severe lesion, such as hypoplastic left heart syndrome (HLHS). M-mode echocardiography can diagnose rhythm disturbances in the fetus and can determine the success of antiarrhythmic therapy administered to the mother. A screening fetal echocardiogram is recommended for women with a previous child or first-degree relative with CHD, for those who are at higher risk of having a child with cardiac disease (e.g., insulin-dependent diabetic patients, women exposed to teratogenic drugs during early pregnancy), and in any fetus in whom a chromosomal abnormality is suspected or confirmed.

Early detection provides the opportunity to counsel and educate the parents about the severity of the cardiac lesion and potential therapeutic or palliative care options. Referral to a high-risk perinatal service is then performed, for further ultrasound screening for associated anomalies of other organs and potential amniocentesis or sequencing of cell-free DNA in maternal blood for karyotyping. For fetuses with ductal dependent lesions, delivery can be planned at a tertiary care center, avoiding the requirement for postnatal transport of an unstable infant. For fetuses with complex CHD at high risk for complications immediately at birth (e.g., HLHS with intact atrial septum), delivery can be arranged with an operating room and surgeon standing by. In utero treatment of CHD is still an experimental procedure, with the most common procedure being aortic balloon valvuloplasty for HLHS. Current results are mixed.
Bibliography
Baker GH, Shirali G, Ringewald JM, et al. Usefulness of live three-dimensional transesophageal echocardiography in a congenital heart disease center. Am J Cardiol . 2009;103:1025–1028.
Benavidez OJ, Gauvreau K, Jenkins KJ, et al. Diagnostic errors in pediatric echocardiography: development of taxonomy and identification of risk factors. Circulation . 2008;117:2995–3001.
Colquitt JL, Pignatelli RH. Strain imaging: the emergence of speckle tracking echocardiography into clinical pediatric cardiology. Congenit Heart Dis . 2016;11(2):199–207.
DeGroff CG. Doppler echocardiography. Pediatr Cardiol . 2002;23:307–333.
Di Carli MF, Geva T, Davidoff R. The future of cardiovascular imaging. Circulation . 2016;133(25):2640–2661.
Friedberg MK, Silverman NH, Moon-Grady AJ, et al. Prenatal detection of congenital heart disease. J Pediatr . 2009;15:26–31.
Frommelt PC. Update on pediatric echocardiography. Curr Opin Pediatr . 2005;17:579–585.
Hijazi ZM, Shivkumar K, Sahn DJ. Intracardiac echocardiography during interventional and electrophysiological cardiac catheterization. Circulation . 2009;119:587–596.
Kluckow M, Seri I, Evans N. Echocardiography and the neonatologist. Pediatr Cardiol . 2008;29:1043–1047.
Parthiban A, Shirali G. Advanced functional echocardiographic imaging of the failing heart in children. Cardiol Young . 2015;25(Suppl 2):94–99.
Exercise Testing
Daniel Bernstein
The normal cardiorespiratory system adapts to the extensive demands of exercise with a several-fold increase in oxygen consumption and cardiac output. Because of the large reserve capacity for exercise, significant abnormalities in cardiovascular performance may be present without symptoms at rest or during ordinary activities. When patients are evaluated in a resting state, significant abnormalities in cardiac function may not be appreciated, or if detected, their implications for quality of life may not be recognized. Permission for children with cardiovascular disease to participate in various forms of physical activity is frequently based on totally subjective criteria. As the importance of aerobic exercise is increasingly recognized, even for children with complex congenital heart lesions, exercise testing can provide a quantitative evaluation of the child's ability to participate safely in both competitive and noncompetitive sports. Exercise testing can also play an important role in evaluating symptoms and quantitating the severity of cardiac abnormalities.
In older children, exercise studies are generally performed on a graded treadmill apparatus with timed intervals of increasing grade and speed. In younger children, exercise studies are often performed on a bicycle ergometer. Many laboratories have the capacity to measure both cardiac and pulmonary function noninvasively during exercise. This allows measurement of both resting and maximal oxygen consumption (VO 2max ) and the point at which anaerobic threshold is reached, which are important indicators of cardiovascular fitness.
As a child grows, the capacity for muscle work is enhanced with increased body size and skeletal muscle mass. All indices of cardiopulmonary function do not increase in a uniform manner. A major response to exercise is an increase in cardiac output, principally achieved through an increase in heart rate, but stroke volume (SV), systemic venous return, and pulse pressure are also increased. Systemic vascular resistance is greatly decreased as the blood vessels in working muscle dilate in response to increasing metabolic demands. As the child becomes older and larger, the response of the heart rate to exercise remains prominent, but cardiac output increases because of growing cardiac volume capacity and thus SV. Responses to dynamic exercise are not dependent solely on age. For any given body surface area, boys have a larger SV than size-matched girls. This increase is also mediated by posture. Augmentation of SV with upright, dynamic exercise is facilitated by the pumping action of working muscles, which overcomes the static effect of gravity and increases systemic venous return.
Dynamic exercise testing defines not only endurance and exercise capacity but also the effect of such exercise on myocardial blood flow and cardiac rhythm. Significant ST segment depression reflects abnormalities in myocardial perfusion, such as the subendocardial ischemia that typically occurs during exercise in children with hypertrophied left ventricles. The exercise ECG is considered abnormal if the ST segment depression is >2 mm and extends for at least 0.06 sec after the J point (onset of the ST segment) in conjunction with a horizontal-, upward-, or downward-sloping ST segment. A decrease in blood pressure before maximal exercise is reached is a risk indicator in patients with hypertrophic cardiomyopathy. Provocation of rhythm disturbances during an exercise study is an important method of evaluating select patients with known or suspected rhythm disorders. The effect of pharmacologic management can also be tested in this manner.
Bibliography
Braden DS, Strong WF. Cardiovascular responses to exercise in childhood. Am J Dis Child . 1990;144:1255–1260.
Cava JR, Danduran MJ, Fedderly RT, et al. Exercise recommendations and risk factors for sudden cardiac death. Pediatr Clin North Am . 2004;51:1401–1420.
James FW, Blomqvist CG, Freed MD, et al. Standards for exercise testing in the pediatric age group: American Heart Association Council on Cardiovascular Disease in the Young, Ad hoc committee on exercise testing. Circulation . 1982;66:1377A–1397A.
Pianosi PT, Liem RI, McMurray RG, et al. Pediatric exercise testing: value and implications of peak oxygen uptake. Children (Basel) . 2017;4(1).
Stephens P. Sudden cardiac death in the young: the value of exercise testing. Cardiol Young . 2017;27(S1):S10–S18.
Stephens P, Paridon SM. Exercise testing in pediatrics. Pediatr Clin North Am . 2004;51:1569–1587.
Washington RL, van Gundy JC, Cohen C, et al. Normal aerobic and anaerobic exercise data for North American school-age children. J Pediatr . 1988;112:223–233.
Cardiac Imaging Studies
Daniel Bernstein
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) are extremely helpful in the diagnosis and management of patients with CHD. These techniques produce tomographic images of the heart in any projection (Fig. 450.25 ), with excellent contrast resolution of fat, myocardium, and lung, as well as moving blood from blood vessel walls. MRI is useful in evaluating areas that are less well visualized by echocardiography, such as distal branch pulmonary artery anatomy and anomalies in systemic and pulmonary venous return.

MRA allows the acquisition of images in several tomographic planes. Within each plane, images are obtained at different phases of the cardiac cycle. Thus, when displayed in a dynamic “cine” format, changes in wall thickening, chamber volume, and valve function can be displayed and analyzed. Blood flow velocity and blood flow volume can be calculated. MRA is an excellent technique for following patients serially after repair of complex CHD, such as tetralogy of Fallot. In these patients, MRA can be used to assess RV volume and mass as well as quantify the amount of regurgitation through either the pulmonary or tricuspid valve. Other MRI techniques, such as myocardial delayed enhancement and tissue T1 weighting, can be used to quantify areas of myocardial scar in patients with cardiomyopathy or in patients after CHD repair, especially tetralogy of Fallot. Magnetic resonance spectroscopy , predominantly a research tool at present, provides a means of demonstrating relative concentrations of high-energy metabolites (adenosine triphosphate, adenosine diphosphate, inorganic phosphate, and phosphocreatine) within regions of the working myocardium.
Computer processing of MRA images allows the noninvasive visualization of the cardiovascular system from inside of the heart or vessels, a technique known as fly-through imaging. These images allow the cardiologist to image the interiors of various cardiovascular structures (Fig. 450.26 ). These techniques are especially helpful in imaging complex peripheral arterial stenoses, especially after balloon angioplasty.
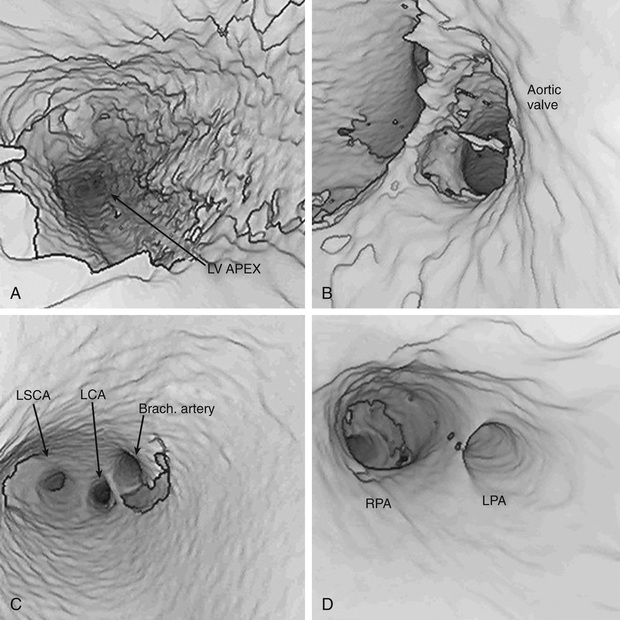
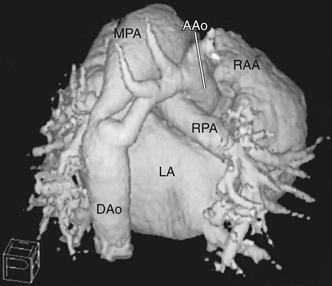
CT scanning can now be used to perform rapid, respiration-gated cardiac imaging in children with resolutions down to 0.5 mm. Three-dimensional reconstruction of CT images is especially useful in evaluating branch pulmonary arteries, anomalies in systemic and pulmonary venous return, and great vessel anomalies such as coarctation of the aorta (Fig. 450.27 ).
Radionuclide angiography may be used to detect and quantify shunts and to analyze the distribution of blood flow to each lung. This technique is particularly useful in quantifying the volume of blood flow distribution between the 2 lungs in patients with abnormalities of the pulmonary vascular tree or after a shunt operation (Blalock-Taussig or Glenn), or to quantify the success of balloon angioplasty and intravascular stenting procedures. Gated blood pool scanning can be used to calculate hemodynamic measurements, quantify valvular regurgitation, and detect regional wall motion abnormalities. Thallium imaging can be performed to evaluate cardiac muscle perfusion. These methods can be used at the bedside of seriously ill children and can be performed serially, with minimal discomfort and low radiation exposure.
Bibliography
Banka P, Geva T. Advances in pediatric cardiac MRI. Curr Opin Pediatr . 2016;28(5):575–583.
Chan FP, Hanneman K. Computed tomography and magnetic resonance imaging in neonates with congenital cardiovascular disease. Semin Ultrasound CT MR . 2015;36(2):146–160.
Feinstein JA, Gatzoulis MA. Use of magnetic resonance imaging and computed tomography. Cardiol Young . 2009;19(Suppl 1):16–22.
Nejatian A, Yu J, Geva T, et al. Aortic measurements in patients with aortopathy are larger and more reproducible by cardiac magnetic resonance compared with echocardiography. Pediatr Cardiol . 2015;36(8):1761–1773.
Prakash A, Powell AJ, Krishnamurthy R, et al. Magnetic resonance imaging evaluation of myocardial perfusion and viability in congenital and acquired pediatric heart disease. Am J Cardiol . 2004;93:657–661.
Wald RM, Haber I, Wald R, et al. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation . 2009;119:1370–1377.
Diagnostic and Interventional Cardiac Catheterization
Daniel Bernstein
The catheterization laboratory, once the site for initial diagnosis of congenital heart disease, has become the center of high-technology interventional procedures, allowing for the nonsurgical repair or palliation of heart defects that once required open heart surgery. Some centers have developed hybrid catheterization laboratories, combining standard fluoroscopic imaging with an operating suite, allowing combined approaches to treat complex congenital heart lesions.
Diagnostic Cardiac Catheterization
Diagnostic catheterization is still performed (1) to assist in the initial diagnosis of some complex congenital heart lesions (e.g., tetralogy of Fallot with pulmonary atresia and major aortopulmonary collateral arteries, pulmonary atresia with intact ventricular septum and coronary sinusoids, HLHS with mitral stenosis); (2) in cases in which other imaging studies are equivocal; (3) in patients for whom hemodynamic assessment is critical (to determine the size of a left-to-right shunt in borderline cases, or to determine the presence or absence of pulmonary vascular disease in an older patient with a left-to-right shunt); (4) between stages of repair of complex CHD (e.g., hypoplastic left or right heart syndromes); (5) for long-term surveillance of patients with complex CHD (e.g., after Fontan palliation for single ventricles); (6) for myocardial biopsy in the diagnosis of cardiomyopathy or in screening for cardiac rejection after cardiac transplantation; and (7) for electrophysiologic study in the evaluation of cardiac arrhythmias (see Chapter 462 ).
Cardiac catheterization should be performed with the patient in as close to a basal state as possible. Conscious sedation or low-level anesthesia is routine. If a deeper level of general anesthesia is required, careful choice of an anesthetic agent is warranted to avoid depression of cardiovascular function and subsequent distortion of the calculations of cardiac output, PVR and SVR, and shunt ratios.
Cardiac catheterization in critically ill infants with CHD should be performed in a center where a pediatric cardiovascular surgical team is available in the event that an operation is required immediately afterward. The complication rate of cardiac catheterization and angiography is greatest in critically ill infants; they must be studied in a thermally neutral environment and treated quickly for hypothermia, hypoglycemia, acidosis, or excessive blood loss.
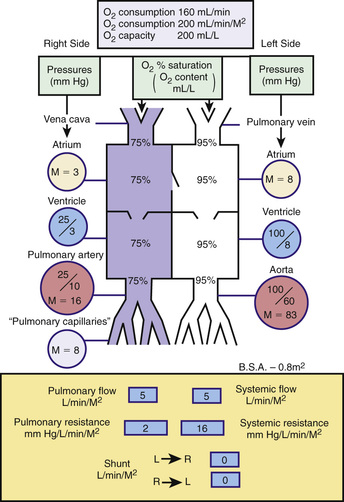
Catheterization may be limited to the right-sided cardiac structures, the left-sided structures, or both the right and left sides of the heart. The catheter is passed into the heart under fluoroscopic guidance through a percutaneous entry point in a femoral or jugular vein. In infants and in a number of older children, the left side of the heart can be accessed by passing the catheter across a patent foramen ovale to the left atrium and left ventricle. If the foramen is closed, the left side of the heart can be catheterized by passing the catheter retrograde via a percutaneous entry site in the femoral artery, or if necessary, via a transatrial septal puncture. The catheter can be manipulated through abnormal intracardiac defects (ASD, VSD). Blood samples are obtained for measuring oxygen saturation in each cardiac chamber or blood vessel, allowing the calculation of shunt volumes. Pressures are measured for calculating gradients, septal defects, or valves and valve areas. Radiopaque contrast is injected to delineate cardiac and vascular structures. A catheter with a thermosensor tip can be used to measure cardiac output by thermodilution. Specialized catheters can be used to measure more sophisticated indices of cardiac function; those with pressure-transducer tips can measure the first derivative of LV pressure (dP/dt). Conductance catheters can be used to generate pressure-volume loops, from which indices of both contractility (end-systolic elastance) and relaxation can be derived, although these are almost exclusively used in research studies. Complete hemodynamics can be calculated, including cardiac output, intracardiac left-to-right and right-to-left shunts, and SVR and PVR. Fig. 450.28 depicts normal circulatory dynamics.
Thermodilution Measurement of Cardiac Output
The thermodilution method for measuring cardiac output is performed with a flow-directed, thermistor-tipped, pulmonary artery (Swan-Ganz) catheter. A known change in the heat content of the blood is induced at one point in the circulation (usually the right atrium or inferior vena cava) by injecting room-temperature saline, and the resultant change in temperature is detected at a point downstream (usually the pulmonary artery). This method is used to measure cardiac output in the catheterization laboratory in patients without shunts. Monitoring cardiac output by the thermodilution method can occasionally be useful in managing critically ill infants and children in an intensive care setting after cardiac surgery or in the presence of shock. In this case, a triple-lumen catheter is used for both cardiac output determination and measurement of pulmonary artery and pulmonary capillary wedge pressure.
Angiocardiography
The major blood vessels and individual cardiac chambers may be visualized by selective angiocardiography, the injection of contrast material into specific chambers or great vessels. This method allows identification of structural abnormalities without interference from the superimposed shadows of normal chambers. Fluoroscopy is used to visualize the catheter as it passes through the various heart chambers. After the cardiac catheter is properly placed in the chamber to be studied, a small amount of contrast medium is injected with a power injector, and cineangiograms are exposed at rates ranging from 15-60 frames/sec. Modern catheterization labs utilize digital imaging technology, allowing for a significant reduction in radiation exposure. Biplane cineangiocardiography allows detailed evaluation of specific cardiac chambers and blood vessels in 2 planes simultaneously with the injection of a single bolus of contrast material. This technique is standard in pediatric cardiac catheterization laboratories and allows one to minimize the volume of contrast material used, which is safer for the patient. Various angled views (e.g., left anterior oblique, cranial angulation) are used to display specific anatomic features optimally in individual lesions.
Rapid injection of contrast medium under pressure into the circulation is not without risk, and each injection should be carefully planned. Contrast agents consist of hypertonic solutions, with some containing organic iodides, which can cause complications, including nausea, a generalized burning sensation, central nervous system symptoms, renal insufficiency, and allergic reactions. For patients with known renal insufficiency who require angiography, there are protocols to protect the kidneys involving prehydration and drugs such as N -acetylcysteine. Intramyocardial injection is generally avoided by careful placement of the catheter before injection. Hypertonicity of the contrast medium may result in transient myocardial depression and a drop in blood pressure, followed soon afterward by tachycardia, an increase in cardiac output, and a shift of interstitial fluid into the circulation. This shift can transiently increase the symptoms of heart failure in critically ill patients.
Interventional Cardiac Catheterization
Catheter treatment is the standard of practice for most cases of isolated pulmonary or aortic valve stenosis as well as for recoarctation of the aorta. A special catheter with a sausage-shaped balloon at the distal end is passed through the obstructed valve. Rapid filling of the balloon with a mixture of contrast material and saline solution results in tearing of the stenotic valve tissue, usually at the site of inappropriately fused raphe. Valvular pulmonary stenosis can be treated successfully by balloon angioplasty ; in most patients, angioplasty has replaced surgical repair as the initial procedure of choice. The clinical results of this procedure are similar to those obtained by open heart surgery, but without the need for sternotomy or prolonged hospitalization. Balloon valvuloplasty for aortic stenosis has also yielded excellent results, although, as with surgery, aortic stenosis often recurs as the child grows, and multiple procedures may thus be required. One complication of both valvuloplasty and surgery is the creation of valvular insufficiency . This complication has more serious implications when it occurs on the aortic vs the pulmonary side of the circulation because regurgitation is less well tolerated at systemic arterial pressures.
Balloon angioplasty is the procedure of choice for patients with restenosis of coarctation of the aorta after earlier surgery. It remains controversial whether angioplasty is the best procedure for native (unoperated) coarctation of the aorta because of reports of late aneurysm formation, and many centers still refer primary coarctation in infants and young children for surgical repair. However, in older patients with previously undiagnosed coarctation, especially those with decreased LV function, primary angioplasty with stent placement may be considered. Other applications of the balloon angioplasty technique include amelioration of mitral stenosis, dilation of surgical conduits (e.g., RV-PA conduits), relief of branch pulmonary artery (PA) narrowing, dilation of systemic or pulmonary venous obstructions, and the long-used balloon atrial septostomy (Rashkind procedure ) for transposition of the great arteries (see Chapter 458.2 ).
Interventional catheterization techniques are being adapted for use in the fetus with lesions such as aortic stenosis to prevent their progression to more complex lesions such as HLHS. In these procedures, after administration of appropriate anesthesia, a needle is passed through the maternal abdominal wall, the uterine wall, and the fetal chest wall and directly into the fetal left ventricle (see Fig. 458.13 ). A coronary angioplasty balloon catheter is passed through the needle and across the stenotic aortic valve, which is then dilated. With the restoration of normal LV blood flow, it is hoped that normal LV growth potential is restored. Midterm results with this technique in a growing number of patients continue to show mixed results, with good ventricular growth leading to a 2-ventricle circulation in approximately 25% of highly preselected patients.
In patients with branch pulmonary artery stenoses, the previously mixed results with balloon angioplasty alone have been enhanced with the use of intravascular stents delivered over a balloon catheter and expanded within the vessel lumen (Fig. 450.29 ). Once placed, the stents can often be dilated to successively greater sizes as the patient grows, although their use in younger infants and children is limited by the extent they can be further expanded. Research into biodissolvable stents may solve this problem in the future. As mentioned, stents are also being used in adolescents and young adults with coarctation of the aorta.

Closure of a small patent ductus arteriosus (PDA) is routinely achieved with catheter-delivered coils (see Fig. 453.11 ), whereas a larger PDA can be closed with a variety of sandwich-type devices. Closure of anomalous vascular connections (coronary fistulas, venovenous collaterals in cyanotic heart lesions) can also be achieved using coils. Secundum ASDs are now routinely closed with a double-disk occluder device (see Fig. 453.3 ). Versions of these devices are currently in clinical trials for closure of surgically difficult-to-reach muscular VSDs and for the more common perimembranous VSD. Catheter-delivered devices may also be used as an adjunct to complex surgical repairs (e.g., dilation or stenting of branch pulmonary artery or pulmonary vein stenosis). High-risk patients undergoing the Fontan operation (see Fig. 457.9 ) often have a small fenestration created between the right and left sides of the circulation to serve as a “pop-off valve” for high right-sided pressure in the early surgical period. Patients with these “fenestrated Fontans” are usually candidates for subsequent closure of the fenestration with a catheter-delivered device.
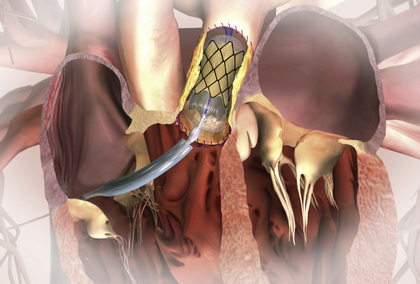
One of the greatest advances in interventional catheterization over the past decade has been transcatheter valve implantation . Typically, a porcine valve is sewn into an expandable stent (commercially available), which is then collapsed around a balloon catheter. The device is positioned across a stenotic or insufficient pulmonary or aortic valve and the balloon inflated, expanding both the stent and the tissue value. The balloon catheter is then removed, leaving the new valve in place, well anchored by the stent to the walls of the main pulmonary artery or aorta. At this time, the most common application in children is replacement of the pulmonary valve (Melody Valve) in patients who have had prior surgery for tetralogy of Fallot (usually because of residual pulmonary insufficiency) (Fig. 450.30 ). In older adults, the most common application is replacement of a stenotic aortic valve, particularly in a patient who is too fragile to undergo open heart surgery. Stent valves have even been placed in the tricuspid position in children with tricuspid insufficiency, although the numbers are currently too small for evaluation as to efficacy and complication rate.
Bibliography
Emmel M, Sreeram N, Bennink G, Sreeram N. Percutaneous tricuspid valve replacement in childhood. Ann Pediatr Cardiol . 2015;8(3):230–232.
Feinstein JA, Kim N, Reddy VM, et al. Percutaneous pulmonary valve placement in a 10-month-old patient using a hand crafted stent-mounted porcine valve. Catheter Cardiovasc Interv . 2006;67:644–649.
Holzer RJ, Hijazi ZM. Transcatheter pulmonary valve replacement: state of the art. Catheter Cardiovasc Interv . 2016;87(1):117–128.
Kutty S, Zahn EM. Interventional therapy for neonates with critical congenital heart disease. Catheter Cardiovasc Interv . 2008;72:663–674.
Lapierre C, Hugues N, Dahdah N, et al. Long-term follow-up of large atrial septal occluder (Amplatzer device) with cardiac MRI in a pediatric population. AJR Am J Roentgenol . 2012;199:1136–1141.
Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol . 2005;45:505–507.
McElhinney DB, Hennesen JT. The Melody® valve and Ensemble® delivery system for transcatheter pulmonary valve replacement. Ann NY Acad Sci . 2013;1291:77–85.
McElhinney DB, Tworetzky W, Lock JE. Current status of fetal cardiac intervention. Circulation . 2010;121(10):1256–1263.
Michelfelder E, Polzin W, Hirsch R. Hypoplastic left heart syndrome with intact atrial septum: utilization of a hybrid catheterization facility for cesarean section delivery and prompt neonatal intervention. Catheter Cardiovasc Interv . 2008;72:983–987.