Acyanotic Congenital Heart Disease
Left-to-Right Shunt Lesions
Atrial Septal Defect
Daniel Bernstein
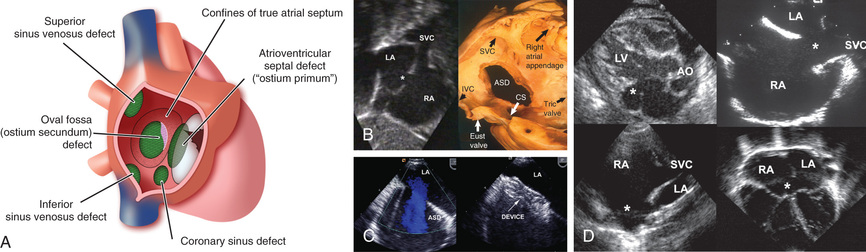
Atrial septal defects (ASDs ) can occur in any portion of the atrial septum—secundum , primum , or sinus venosus —depending on which embryonic septal structure has failed to develop normally (Fig. 453.1 ) (see Chapter 447 ). Less often, the atrial septum may be almost absent, with the creation of a functional single atrium. Isolated secundum ASDs account for approximately 7% of congenital heart defects. The majority of cases of ASD are sporadic; autosomal dominant inheritance does occur as part of the Holt-Oram syndrome (hypoplastic or absent thumbs, radii, triphalangism, phocomelia, first-degree heart block, ASD) or in families with both secundum ASD and heart block (see Table 451.2 ).
An isolated valve-incompetent patent foramen ovale (PFO) is a common echocardiographic finding during infancy. It is usually of no hemodynamic significance and is not considered an ASD; a PFO may play an important role if other structural heart defects are present. If another cardiac anomaly is causing increased right atrial pressure (pulmonary stenosis or atresia, tricuspid valve abnormalities, right ventricular dysfunction), venous blood may shunt across the PFO into the left atrium with resultant cyanosis. Because of the anatomic structure of the PFO, left-to-right shunting is unusual outside the immediate newborn period. In the presence of a large volume load or a hypertensive left atrium (e.g., secondary to mitral stenosis), the foramen ovale may be sufficiently dilated to result in a significant atrial left-to-right shunt. A valve-competent but probe-patent (able to be pushed opened with a catheter) PFO may be present in 15–30% of adults. An isolated PFO does not require surgical treatment, although it may be a risk for paradoxical (right to left) systemic embolization. Device closure of these defects is one treatment option considered in adults with a history of thromboembolic stroke.
Bibliography
Beda RD, Gill EA Jr. Patent foramen ovale: does it play a role in the pathophysiology of migraine headache? Cardiol Clin . 2005;23:91–96.
Benson DW, Silberbach GM, Kavanaugh-McHugh A, et al. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J Clin Invest . 1999;104:1567–1573.
Kharouf R, Luxenberg DM, Khalid O, et al. Atrial septal defect: spectrum of care. Pediatr Cardiol . 2008;29:271–280.
Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol . 2005;45:505–507.
Messé SR, Kent DM. Still no closure on the question of PFO closure. N Engl J Med . 2013;368:1152–1153.
Radzik D, Davignon A, van Doesburg N, et al. Predictive factors for spontaneous closure of atrial septal defects diagnosed in the first 3 months of life. J Am Coll Cardiol . 1993;22:851–853.
Riggs T, Sharp SE, Batton D, et al. Spontaneous closure of atrial septal defects in premature vs full term neonates. Pediatr Cardiol . 2000;21:129–134.
Saito T, Ohta K, Nakayama Y, et al. Natural history of medium-sized atrial septal defect in pediatric cases. J Cardiol . 2012;60:248–251.
Steimle JD, Moskowitz IP. TBX5: a key regulator of heart development. Curr Top Dev Biol . 2017;122:195–221.
Xu YJ, Qiu XB, Yuan F, et al. Prevalence and spectrum of NKX2.5 mutations in patients with congenital atrial septal defect and atrioventricular block. Mol Med Rep . 2017;15(4):2247–2254.
Yew G, Wilson NJ. Transcatheter atrial septal defect closure with the Amplatzer septal occluder: five-year follow-up. Catheter Cardiovasc Interv . 2005;64:193–196.
Ostium Secundum Defect
Daniel Bernstein
An ostium secundum defect in the region of the fossa ovalis is the most common form of ASD and is associated with structurally normal atrioventricular (AV) valves (see Fig. 453.1 ). Mitral valve prolapse has been described in association with this defect but is rarely an important clinical consideration. Secundum ASDs may be single or multiple (fenestrated atrial septum), and openings ≥2 cm in diameter are common in symptomatic older children. Large defects may extend inferiorly toward the inferior vena cava (IVC) and ostium of the coronary sinus, superiorly toward the superior vena cava (SVC), or posteriorly. Females outnumber males 3 : 1 in incidence. Partial anomalous pulmonary venous return (PAPVR), usually of the right upper pulmonary vein, may be an associated lesion.
Pathophysiology
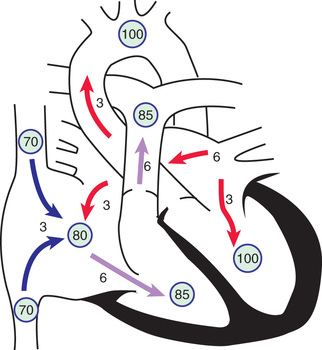
The degree of left-to-right shunting depends on the size of the defect, the relative compliance of the right and left ventricles, and the relative vascular resistance in the pulmonary and systemic circulations. In large defects, a considerable shunt of oxygenated blood flows from the left to the right atrium (Fig. 453.2 ). This blood is added to the usual venous return to the right atrium and is pumped by the right ventricle to the lungs. With large defects, the ratio of pulmonary-to-systemic blood flow (Qp:Qs) is usually between 2 : 1 and 4 : 1. The paucity of symptoms in infants with ASDs is related to the structure of the right ventricle in early life, when its muscular wall is thick and less compliant, thus limiting the left-to-right shunt. As the infant becomes older and pulmonary vascular resistance (PVR) drops, the right ventricular (RV) wall becomes thinner, and the left-to-right shunt across the ASD increases. The increased blood flow through the right side of the heart results in enlargement of the right atrium and ventricle and dilation of the pulmonary artery. The left atrium may also be enlarged as the increased pulmonary blood flow returns to the left atrium, but the left ventricle and aorta are normal in size. Despite the large pulmonary blood flow, pulmonary arterial pressure is usually initially normal because of the absence of a high-pressure communication between the pulmonary and systemic circulations. PVR remains low throughout childhood, although it may begin to increase in adulthood and may eventually result in reversal of the shunt and clinical cyanosis.
Clinical Manifestations
A child with an ostium secundum ASD is most often asymptomatic; the lesion is often discovered inadvertently during physical examination. Even an extremely large secundum ASD rarely produces clinically evident heart failure in childhood. On closer evaluation, however, younger children may show subtle failure to thrive, and older children may have varying degrees of exercise intolerance. Often, the degree of limitation may go unnoticed by the family until after repair, when the child's growth or activity level greatly increases (e.g., “I never knew she could run so fast”).
The physical findings of an ASD are usually characteristic but fairly subtle and require careful examination of the heart, with special attention to the heart sounds. Examination of the chest may reveal a mild left precordial bulge. An RV systolic lift may be palpable at the left sternal border. Sometimes a pulmonic ejection click can be heard. In most patients with an ASD, the characteristic finding is that the second heart sound (S2 ) is widely split and fixed in its splitting during all phases of respiration. Normally, the duration of RV ejection varies with respiration, with inspiration increasing RV volume and delaying closure of the pulmonary valve, widening the S2 split. With an ASD, RV diastolic volume is constantly increased, and ejection time is prolonged throughout all phases of respiration. A systolic ejection murmur is heard; it is usually no greater than a grade 3/6, medium pitched, without harsh qualities, seldom accompanied by a thrill, and best heard at the left middle and upper sternal border. It is produced by the increased flow across the RV outflow tract into the pulmonary artery. Flow across the ASD between the 2 low-pressure atria does not cause an audible murmur. A short, rumbling mid-diastolic murmur produced by the increased volume of blood flow across the tricuspid valve is often audible at the lower left sternal border. This finding, which may be subtle and is heard best with the bell of the stethoscope, usually indicates a Qp:Qs ratio of at least 2 : 1.
Diagnosis
The chest radiograph shows varying degrees of enlargement of the right ventricle and atrium, depending on the size of the shunt. The pulmonary artery is enlarged, and pulmonary vascularity is increased. These signs vary and may not be conspicuous in mild cases. Cardiac enlargement is often best appreciated on the lateral view because the right ventricle protrudes anteriorly as its volume increases. The electrocardiogram (ECG) shows RV volume overload: the QRS axis may be normal or exhibit right axis deviation, and a minor RV conduction delay (rsR′ pattern in the right precordial leads) may be present. Right ventricular hypertrophy would be unusual in the absence of pulmonary hypertension or other lesions (e.g., valvar pulmonic stenosis).
The echocardiogram shows findings characteristic of RV volume overload, including an increased RV end-diastolic dimension and flattening and abnormal motion of the ventricular septum (see Fig. 453.1 ). A normal septum moves posteriorly during systole and anteriorly during diastole (synchronous with the left ventricular contractions). With RV overload and normal PVR, septal motion is either flattened or reversed—that is, anterior movement in systole. The location and size of the ASD are readily appreciated by two-dimensional (2D) scanning, with a characteristic brightening of the echo image seen at the edge of the defect caused by the increased reflectivity of ultrasound at the tissue-blood interface (T-artifact). The shunt is confirmed by pulsed and color flow Doppler. The normal entry of all pulmonary veins into the left atrium should be confirmed.
Patients with the classic features of a hemodynamically significant ASD on physical examination and chest radiography, in whom echocardiographic identification of an isolated secundum ASD is made, need not undergo diagnostic catheterization before repair, with the exception of an older patient, in whom PVR may be a concern. If pulmonary vascular disease is suspected, cardiac catheterization confirms the presence of the defect and allows measurement of the shunt ratio and pulmonary pressure and resistance.
If catheterization is performed, usually at the time of device closure (see Treatment ), the oxygen content of blood from the right atrium will be much higher than that from the SVC. This feature is not specifically diagnostic because it may occur with PAPVR to the right atrium, with a ventricular septal defect (VSD) in the presence of tricuspid insufficiency, with AV septal defects associated with left ventricular–to–right atrial shunts, and with aorta–to–right atrial communications (ruptured sinus of Valsalva aneurysm). Pressure in the right side of the heart is usually normal, but small to moderate pressure gradients (<25 mm Hg) may be measured across the RV outflow tract because of functional stenosis related to excessive blood flow. If the pressure gradient across the pulmonary valve is greater, pathologic pulmonary stenosis is likely present. In children and adolescents, PVR is almost always normal. The shunt is variable and depends on the size of the defect, but it may be of considerable volume (as high as 20 L/min/m2 ). Cineangiography , performed with the catheter through the defect and in the right upper pulmonary vein, demonstrates the defect and confirms the location of the right upper pulmonary venous drainage (normal or aberrant into SVC). Pulmonary angiography demonstrates the defect on the levophase (return of contrast to left side of heart after passing through lungs).
Complications
Secundum ASDs are usually isolated, although they may be associated with PAPVR, pulmonary valvular stenosis, VSD, pulmonary artery branch stenosis, and persistent left SVC, as well as mitral valve prolapse and insufficiency. Secundum ASDs are associated with the autosomal dominant Holt-Oram syndrome. The gene responsible for this syndrome, situated in the region 12q21-q22 of chromosome 12, is TBX5, a member of the T-box transcriptional family. A familial form of secundum ASD associated with AV conduction delay has been linked to mutations in another transcription factor, Nkx2.5. Patients with familial ASD without heart block may carry a mutation in the transcription factor GATA4, located on chromosome 8p22-23 (see Table 451.2 ).
Treatment
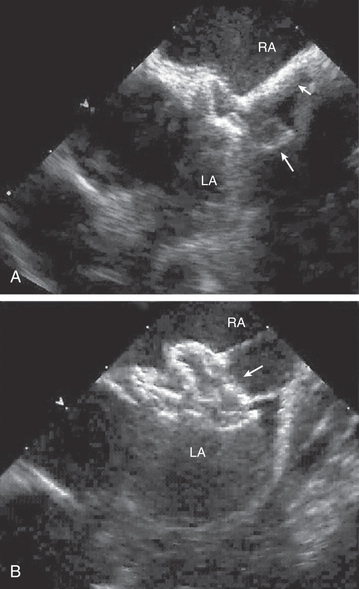
Transcatheter device or surgical closure is advised for all symptomatic patients, as well as for asymptomatic patients with Qp:Qs ratio of at least 2 : 1 and those with RV enlargement. The timing for elective closure is usually after the 1st yr of life and before entry into school. Closure carried out at open heart surgery is associated with a mortality rate of <1%. Repair is preferred during early childhood because surgical mortality and morbidity are significantly greater in adulthood; the long-term risk of arrhythmia caused by chronic atrial dilation is also greater after ASD repair in adults. For most patients, the procedure of choice is percutaneous catheter device closure using an atrial septal occlusion device, implanted transvenously in the cardiac catheterization laboratory (Fig. 453.3 ). The results are excellent, and patients are usually discharged from the hospital the following day. With the latest generation of devices, the incidence of serious complications (e.g., device erosion) is 0.1% and can be decreased by identifying high-risk patients, such as those with a deficient rim of septum in the area where the device would be anchored. Echocardiography can usually determine whether a patient is a good candidate for device closure. In patients with small secundum ASDs and minimal left-to-right shunts without RV enlargement, the consensus is that closure is not required. Infants with small to moderate-sized ASDs can be watched closely, since these defects will often grow smaller in the 1st yr of life. It is unclear at present whether the persistence of a small ASD into adulthood increases the risk for stroke enough to warrant prophylactic closure of all these defects.
Prognosis
Small to moderate-sized ASDs detected in term infants may grow smaller or close spontaneously. Secundum ASDs are well tolerated during childhood, and significant symptoms do not usually appear until the 3rd decade or later. Pulmonary hypertension, atrial dysrhythmias, tricuspid or mitral insufficiency, and heart failure are late manifestations; these symptoms may initially appear during the increased volume load of pregnancy. Infective endocarditis is extremely rare, and antibiotic prophylaxis for isolated secundum ASDs is not recommended.
The results after surgical or device closure in children with moderate-size to large shunts are excellent. Symptoms, if present, disappear rapidly, and growth is frequently enhanced. Heart size decreases to normal, and the ECG shows decreased RV volume load. Late right-sided heart failure and arrhythmias are less common in patients who have had early repair, becoming more common in patients who undergo surgery after 20 yr of age. Although early and midterm results with device closure are excellent, the long-term effects are not yet known. Reports of resolution of migraine headaches in patients after device closure of ASD or PFO are intriguing, suggesting a possible thromboembolic etiology. However, there are also paradoxical reports of patients whose migraines began or worsened after placement of one of these devices.
Sinus Venosus Atrial Septal Defect
Daniel Bernstein
A sinus venosus ASD is situated in the upper part of the atrial septum in close relation to the entry of the superior vena cava (see Fig. 453.1 ). Often, one or more pulmonary veins (usually from the right lung) drain anomalously into the SVC. The SVC sometimes straddles the defect; in this case, some systemic venous blood enters the left atrium, but only rarely does it cause clinically evident cyanosis. The hemodynamic disturbance, clinical picture, ECG, and radiograph are similar to those seen in secundum ASD. The diagnosis can usually be made by echocardiography. If questions remain after echo regarding pulmonary venous drainage, cardiac CT or MRI is usually diagnostic. Cardiac catheterization is rarely required, except in adult patients in whom PVR assessment may be important. Anatomic correction generally requires the insertion of a patch to close the defect while incorporating the entry of any anomalous pulmonary veins into the left atrium. If the anomalous vein drains high in the SVC, the vein can be left intact and the ASD closed to incorporate the SVC mouth into the left atrium. The SVC proximal to the venous entrance is then detached and anastomosed directly to the right atrium. This procedure avoids direct suturing of the pulmonary vein, with less chance of future stenosis. Surgical results are generally excellent. Rarely, sinus venosus defects involve the IVC.
Partial Anomalous Pulmonary Venous Return
Daniel Bernstein
One or several pulmonary veins may return anomalously to the SVC or IVC, right atrium, or coronary sinus and produce a left-to-right shunt of oxygenated blood. Partial anomalous pulmonary venous return (PAPVR) usually involves some or all of the veins from only 1 lung, typically the right. When an associated ASD is present, it is generally of the sinus venosus type but can be secundum (see Chapters 453.2 and 453.3 ). When an ASD is detected by echocardiography, one must always search for associated PAPVR. The history, physical signs, and electrocardiographic and radiologic findings are indistinguishable from those of an isolated ostium secundum ASD. Occasionally, an anomalous vein draining into the IVC is visible on chest radiography as a crescentic shadow of vascular density along the right border of the cardiac silhouette (scimitar syndrome ); in these patients an ASD is not usually present, but pulmonary sequestration or lung hypoplasia and anomalous arterial supply to that lobe are common findings. Total anomalous pulmonary venous return (TAPVR) is a cyanotic lesion and is discussed in Chapter 458.7 . Echocardiography generally confirms the diagnosis. MRI and CT are also useful if there is a question regarding pulmonary venous drainage or in cases of scimitar syndrome. If cardiac catheterization is performed, the presence of anomalous pulmonary veins is demonstrated by selective pulmonary arteriography, and anomalous pulmonary arterial supply to the right lung is demonstrated by descending aortography.
The prognosis for PAPVR is excellent, similar to that for ostium secundum ASDs. When a large left-to-right shunt is present, surgical repair is performed. The associated ASD should be closed in such a way that pulmonary venous return is directed to the left atrium. A single anomalous pulmonary vein without an atrial communication may be difficult to redirect to the left atrium; if the shunt is small and the right ventricle is not enlarged, it may be left unoperated.
Atrioventricular Septal Defects (Ostium Primum and Atrioventricular Canal or Endocardial Cushion Defects)
Daniel Bernstein
The abnormalities encompassed by atrioventricular (AV) septal defects are grouped together because they represent a spectrum of a basic embryologic abnormality, a deficiency of the AV septum . The tricuspid valve sits slightly lower (more toward the cardiac apex) than does the mitral valve, and thus a small portion of septum separates the left ventricle from the right atrium. This is the AV septum that is deficient in all forms of AV septal defect. When the AV septum is absent and there is also an ostium primum defect, the main communication is situated in the lower portion of the atrial septum and overlies the mitral and tricuspid valves. In most cases a cleft in the anterior leaflet of the mitral valve is also noted. The tricuspid valve is usually functionally normal, although some anatomic abnormality of the septal leaflet is present. The ventricular septum is intact.
An AV septal defect, formerly known as an AV canal defect or endocardial cushion defect, consists of a defect of the AV septum and contiguous atrial and ventricular septal defects with a common AV valve. The severity of the AV valve abnormality varies considerably. In the complete form of AV septal defect, a single AV valve is common to both ventricles and consists of an anterior and a posterior bridging leaflet related to the ventricular septum, with a lateral leaflet in each ventricle. The anterior bridging leaflet can be divided into right- and left-sided components or may be single and free floating over the ventricular septum. Complete AV septal defect is common in children with Down syndrome .
Transitional varieties of these defects also occur and include ostium primum defects with clefts in the anterior mitral and septal tricuspid valve leaflets and small VSDs, and less commonly, ostium primum defects with normal AV valves. In some patients the atrial septum is intact, but a VSD is seen in the inlet septum, similar to that found in the complete form of AV septal defect. Sometimes, AV septal defects are associated with varying degrees of hypoplasia of one of the ventricles, known as either left-dominant or right-dominant AV septal defect . If the affected ventricular chamber is too small to establish a 2-ventricle circulation, surgical palliation, aiming for an eventual Fontan procedure, is performed (see Chapters 457.4 and 458.10 ).
Pathophysiology
The basic abnormality in patients with ostium primum defects is the combination of a left-to-right shunt across the atrial defect and mitral (or occasionally tricuspid) insufficiency. The shunt is usually moderate to large, the degree of mitral insufficiency is generally mild to moderate, and pulmonary artery pressure (PAP) is typically normal or only mildly increased. The physiology of this lesion is therefore similar to that of an ostium secundum ASD.
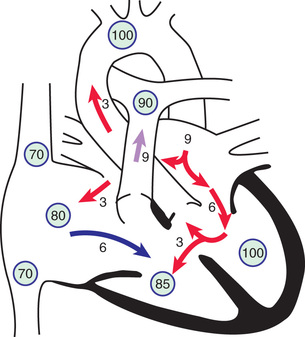
In complete AV septal defects, left-to-right shunting occurs at both the atrial and the ventricular level (Fig. 453.4 ). Additional shunting may occur directly from the left ventricle to the right atrium (known as a Gerbode shunt ) because of absence of the AV septum. Pulmonary hypertension and an early tendency to increase PVR are common. AV valvular insufficiency, which may be moderate to severe, further increases the volume load on one or both ventricles. If the defect is large enough, some right-to-left shunting may also occur at the atrial and ventricular levels and lead to mild arterial desaturation. With time, progressive pulmonary vascular disease increases the right-to-left shunt so that clinical cyanosis develops (Eisenmenger physiology ; see Chapter 460.2 ). The risk for development of pulmonary vascular disease is greater in patients with Down syndrome, and therefore surgical correction is usually considered early in these patients, within the 1st 3-6 mo of life.
Clinical Manifestations
Many children with ostium primum defects are asymptomatic, and the anomaly is discovered during a general physical examination. In patients with moderate shunts and mild mitral insufficiency, the physical signs are similar to those of the secundum ASD, but with an additional apical holosystolic murmur caused by mitral insufficiency.
A history of exercise intolerance, easy fatigability, and recurrent pneumonia may be obtained, especially in infants with large left-to-right shunts and severe mitral insufficiency. In these patients, cardiac enlargement is moderate or marked, and the precordium is hyperdynamic. Auscultatory signs produced by the left-to-right shunt include a normal or accentuated first heart sound (S1 ); wide, fixed splitting of S2 ; a pulmonary systolic ejection murmur sometimes preceded by a click; and a low-pitched, mid-diastolic rumbling murmur at the lower left sternal edge or apex, or both, as a result of increased flow through the AV valves. Mitral insufficiency may be manifested by a harsh (occasionally very high-pitched) apical holosystolic murmur that radiates to the left axilla.
With complete AV septal defects, heart failure and intercurrent pulmonary infection usually appear in infancy. The liver is enlarged, and the infant often develops feeding intolerance and failure to thrive. Cardiac enlargement is moderate to marked, and a systolic thrill is frequently palpable at the lower left sternal border. A precordial bulge and lift may be present as well. S1 is normal or accentuated. S2 is widely split if the pulmonary flow is massive. A low-pitched, mid-diastolic rumbling murmur is audible at the lower left sternal border, indicative of increased blood flow across the right side of the common AV valve, and a pulmonary systolic ejection murmur is produced by the large pulmonary flow. The harsh apical holosystolic murmur of mitral insufficiency may also be present.
Diagnosis
Chest radiographs of children with complete AV septal defects often show moderate to severe cardiac enlargement caused by the prominence of both ventricles and atria. The pulmonary artery is large, and pulmonary vascularity is increased.
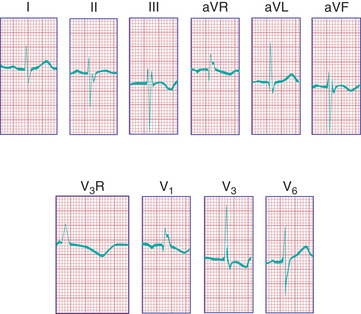
The ECG in patients with a complete AV septal defect is distinctive and usually diagnostic. The principal abnormalities are (1) superior orientation of the mean frontal QRS axis with axis deviation to the right upper quadrant (QRS negative in both lead I and lead aVF), (2) counterclockwise inscription of the superiorly oriented QRS vector loop (manifest by a Q wave in leads I and aVL), (3) signs of biventricular hypertrophy or isolated RV hypertrophy, (4) RV conduction delay (rSR′ pattern in leads V3 R and V1 ), (5) normal or tall P waves, and (6) occasional prolongation of the P-R interval (Fig. 453.5 ).
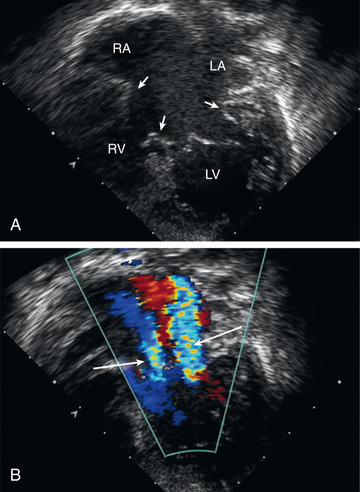
The echocardiogram is diagnostic and shows signs of RV enlargement (Fig. 453.6 ). There is encroachment of the mitral valve into the left ventricular outflow tract; the abnormally low position of the AV valves results in a “gooseneck” deformity of the LVOT. In normal hearts the tricuspid valve inserts slightly more toward the apex than does the mitral valve. In AV septal defects, both valves insert at the same level because of absence of the AV septum. In complete AV septal defects the ventricular septum is also deficient, and the common AV valve is readily appreciated. Pulsed and color flow Doppler echocardiography will demonstrate left-to-right shunting at the atrial, ventricular, or left ventricular–right atrial levels and can be used to semiquantitate the degree of AV valve insufficiency. Echocardiography is useful for determining the insertion points of the chordae of the common AV valve and for evaluating the presence of associated lesions such as patent ductus arteriosus (PDA) or coarctation of the aorta.
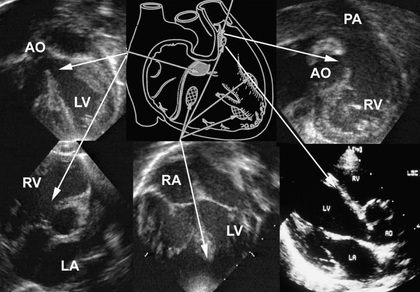
Cardiac catheterization and angiocardiography is rarely required to confirm the diagnosis unless pulmonary vascular disease is suspected, as when diagnosis has been delayed beyond early infancy, especially in patients with Down syndrome in whom the development of pulmonary vascular disease may be more rapid. Catheterization demonstrates the magnitude of the left-to-right shunt, the degree of PVR elevation, and the severity of insufficiency of the common AV valve. By oximetry, the shunt is usually demonstrable at both the atrial and the ventricular level. Arterial oxygen saturation is normal or only mildly reduced unless pulmonary vascular disease is present. Children with ostium primum defects generally have normal or only moderately elevated PAP. Conversely, complete AV septal defects are associated with RV and pulmonary hypertension and, in older patients, increased PVR (see Chapter 460.2 ).
Selective left ventriculography will demonstrate deformity of the common AV valve and distortion of the LVOT caused by the abnormally apical position of this valve (gooseneck deformity). The abnormal anterior leaflet of the mitral valve is serrated, and insufficiency is noted. Direct shunting of blood from the left ventricle to the right atrium may also be demonstrated.
Treatment
Ostium primum defects are approached surgically from an incision in the right atrium. The cleft in the mitral valve is located through the atrial defect and is repaired by direct suture. The defect in the atrial septum is usually closed by insertion of a patch prosthesis. The surgical mortality rate for ostium primum defects is very low.
Surgical treatment of complete AV septal defects is more complex, although highly successful. The postoperative course may be prolonged in infants with severe cardiac failure and in those with pulmonary hypertension. Because of the risk of pulmonary vascular disease developing as early as 6-12 mo of age, surgical intervention must be performed during infancy. Full correction of these defects can be readily accomplished in infancy. Palliation with pulmonary arterial banding , once more common, is reserved for the small subset of patients who have other associated lesions that make early corrective surgery too risky, and may not be as effective in patients with a large amount of AV valve regurgitation. The atrial and ventricular defects are patched, using either 1 or 2 separate patches, and the AV valves are reconstructed. Uncommon complications include surgically induced heart block requiring placement of a permanent pacemaker and excessive LVOT narrowing requiring surgical revision. More often there may be residual tricuspid or mitral regurgitation, which requires long-term surveillance because it may require replacement with a prosthetic valve later in life.
Prognosis
The prognosis for unrepaired complete AV septal defects depends on the magnitude of the left-to-right shunt, degree of PVR elevation, and severity of AV valve insufficiency. Death from cardiac failure during infancy was common before the advent of early corrective surgery. Patients who survived without surgery usually developed pulmonary vascular obstructive disease. Most patients with ostium primum defects and minimal AV valve involvement are asymptomatic or have only minor, nonprogressive symptoms until they reach the 3rd or 4th decade of life, similar to the course of patients with secundum ASDs. Late postoperative complications include atrial arrhythmias and heart block, progressive narrowing of the LVOT requiring surgical revision, and eventual worsening of AV valve regurgitation (usually on the left side) requiring replacement with a prosthetic valve.
Bibliography
Beaton AZ, Pike JI, Stallings C, et al. Predictors of repair and outcome in prenatally diagnosed atrioventricular septal defects. J Am Soc Echocardiogr . 2013;26:208–216.
Becker AE, Anderson RH. Atrioventricular septal defects: what's in a name? J Thorac Cardiovasc Surg . 1982;83:461–469.
Devlin PJ, Backer CL, Eltayeb O, et al. Repair of partial atrioventricular septal defect: age and outcomes. Ann Thorac Surg . 2016;102(1):170–177.
Friedberg MK, Kim N, Silverman NH. Atrioventricular septal defect recently diagnosed by fetal echocardiography: echocardiographic features, associated anomalies, and outcomes. Congenit Heart Dis . 2007;2:110–114.
Malhotra SP, Lacour-Gayet F, Mitchell MB, et al. Reoperation for left atrioventricular valve regurgitation after atrioventricular septal defect repair. Ann Thorac Surg . 2008;86:147–151.
Patel SS, Burns TL, Botto LD, et al. Analysis of selected maternal exposures and non-syndromic atrioventricular septal defects in the National Birth Defects Prevention Study, 1997-2005. Am J Med Genet A . 2012;158A:2447–2455.
Priest JR, Osoegawa K, Mohammed N, et al. De novo and rare variants at multiple loci support the oligogenic origins of atrioventricular septal heart defects. PLoS Genet . 2016;12(4):e1005963.
Spicer DE, Anderson RH, Backer CL. Clarifying the surgical morphology of inlet ventricular septal defects. Ann Thorac Surg . 2013;95:236–241.
Suzuki T, Bove EL, Devaney EJ, et al. Results of definitive repair of complete atrioventricular septal defect in neonates and infants. Ann Thorac Surg . 2008;86:596–602.
Thankavel PP, Ramaciotti C. Isolated mitral cleft in trisomy 21: an initially ‘silent’ lesion. Pediatr Cardiol . 2016;37(2):405–408.
Ventricular Septal Defect
Daniel Bernstein
Ventricular septal defect is the most common cardiac malformation and accounts for 25% of congenital heart disease. Defects may occur in any portion of the ventricular septum, but the most common are of the membranous type (Fig. 453.7 ). These defects are in a posteroinferior position, anterior to the septal leaflet of the tricuspid valve. VSDs between the crista supraventricularis and the papillary muscle of the conus may be associated with pulmonary stenosis and other manifestations of tetralogy of Fallot (see Chapter 457.1 ). VSDs superior to the crista supraventricularis (supracristal ) are less common; these are found just beneath the pulmonary valve and may impinge on an aortic sinus and cause aortic insufficiency (see Chapter 453.7 ). Supracristal VSDs are more common in patients of Asian descent. VSDs in the midportion or apical region of the ventricular septum are muscular in type and may be single or multiple (“Swiss cheese” septum).
Pathophysiology
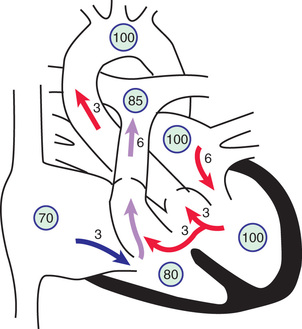
The physical size of the VSD is a major (but not the only) determinant of the size of the left-to-right shunt. When the defect is large, the level of pulmonary vascular resistance (PVR) in relation to systemic vascular resistance (SVR) is the major determinant of the shunt's magnitude. When a small communication is present (usually <5 mm), the VSD is deemed to be pressure restrictive , meaning that right ventricular (RV) pressure is normal or only slightly elevated. The higher pressure in the left ventricle drives the shunt left to right, and the size of the defect limits the magnitude of the shunt. In larger, nonrestrictive VSDs (usually >10 mm), RV and left ventricular (LV) pressures are equalized. In these defects the direction of shunting and the shunt magnitude are determined by the PVR/SVR ratio (Fig. 453.8 ).
After birth in patients with a large VSD, PVR may remain elevated, delaying the normal postnatal decrease, and thus the size of the left-to-right shunt may initially be limited. Because of normal involution of the media of small pulmonary arterioles, PVR begins to fall in the 1st few wk after birth, and the size of the left-to-right shunt then increases. Eventually, a large left-to-right shunt develops, and clinical symptoms become apparent. In most cases during early infancy, PVR is only slightly elevated, and the major contribution to pulmonary hypertension is the large communication allowing exposure of the pulmonary circulation to systemic pressure and the large pulmonary blood flow. With continued exposure of the pulmonary vascular bed to high systolic pressure and high flow, pulmonary vascular obstructive disease eventually develops. When the PVR/SVR ratio approaches 1 : 1, the shunt becomes bidirectional, signs of heart failure abate, and the patient begins to show signs of cyanosis (Eisenmenger physiology ; see Chapter 460.2 ), intermittent at first, but then more constant. In rare infants with a large VSD, more often in those with Down syndrome, PVR never decreases, and symptoms may remain minimal until Eisenmenger physiology becomes evident.
The magnitude of intracardiac shunts is usually described by the Qp:Qs ratio. If the left-to-right shunt is small (Qp:Qs < 1.5 : 1), the cardiac chambers are not appreciably enlarged, and the pulmonary vascular bed is probably normal. If the shunt is large (Qp:Qs > 2 : 1), left atrial and LV volume overload occurs, and RV and pulmonary arterial hypertension may be present if the defect is large. The main pulmonary artery, left atrium, and left ventricle are enlarged.
Clinical Manifestations
The clinical findings of patients with a VSD vary according to the size of the defect and pulmonary blood flow and pressure. Small VSDs with trivial left-to-right shunts and normal pulmonary artery pressure (PAP) are the most common. These patients are asymptomatic, and the cardiac lesion is usually found during routine physical examination. Characteristically, a loud, harsh, or blowing holosystolic murmur is present and heard best over the lower left sternal border, and it is frequently accompanied by a thrill. In a few cases the murmur ends before the second heart sound (S2 ), presumably because of closure of the defect during late systole. A short, loud, harsh systolic murmur localized to the apex in a neonate is often a sign of a tiny VSD in the apical muscular septum. In premature infants the murmur may be heard early because PVR decreases more rapidly.
Large VSDs with excessive pulmonary blood flow and pulmonary hypertension are responsible for signs of congestive heart failure: dyspnea, feeding difficulties, poor growth, profuse perspiration, and recurrent pulmonary infections in early infancy. Cyanosis is usually absent, but duskiness is sometimes noted during infections or crying. Prominence of the left precordium is common, as are a palpable parasternal lift, a laterally displaced apical impulse and apical thrust, and a systolic thrill. The holosystolic murmur of a large VSD is generally less harsh than that of a small VSD and more blowing in nature because of the absence of a significant pressure gradient across the defect. It is even less likely to be prominent in the newborn period. The pulmonic component of S2 may be increased as a result of pulmonary hypertension. The presence of a mid-diastolic, low-pitched rumble at the apex is caused by increased blood flow across the mitral valve and usually indicates a Qp:Qs ratio ≥2 : 1. This murmur is best appreciated with the bell of the stethoscope.
Diagnosis
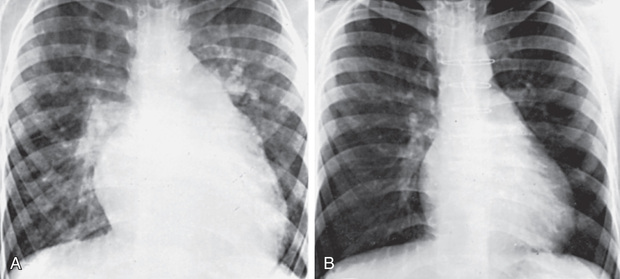
In patients with small VSDs, the chest radiograph is usually normal, although minimal cardiomegaly and a borderline increase in pulmonary vasculature may be observed. The ECG is generally normal but may suggest LV hypertrophy. The presence of RV hypertrophy on ECG is a warning that the defect is not small and that the patient has pulmonary hypertension or an associated lesion such as pulmonic stenosis. In large VSDs the chest radiograph shows gross cardiomegaly with prominence of both ventricles, the left atrium, and the pulmonary artery (Fig. 453.9 ). Pulmonary vascular markings are increased, and frank pulmonary edema, including pleural effusions, may be present. The ECG shows biventricular hypertrophy; the P waves may be notched (indicative of LA enlargement).
The 2D echocardiogram shows the position and size of the VSD (see Fig. 453.7 ). In small defects, especially those of the muscular septum, the defect itself may be difficult to image and is visualized only by color Doppler examination. In defects of the membranous septum , a thin membrane (called a ventricular septal aneurysm but consisting of abnormal tricuspid valve tissue) can partially cover the defect and limit the volume of the left-to-right shunt. Echocardiography is also useful for estimating shunt size by examining the degree of volume overload of the left atrium and left ventricle; in the absence of associated lesions, the extent of their increased dimensions is a good reflection of the size of the left-to-right shunt. Pulsed Doppler examination shows whether the VSD is pressure restrictive by calculating the pressure gradient across the defect. Such calculation allows an estimation of RV pressure and helps determine whether the patient is at risk for the development of early pulmonary vascular disease. The echocardiogram can also be useful to determine the presence of aortic valve insufficiency or aortic leaflet prolapse in the case of supracristal VSDs.
The hemodynamics of a VSD can also be demonstrated by cardiac catheterization, although catheterization currently is performed only when laboratory data do not fit well with the clinical findings or when pulmonary vascular disease is suspected. Oximetry demonstrates increased oxygen content in the right ventricle; because some defects eject blood almost directly into the pulmonary artery (streaming), the full magnitude of the oxygen saturation increase is occasionally apparent only when pulmonary arterial blood is sampled. Small, restrictive VSDs are associated with normal right-sided heart pressures and PVR. Large, nonrestrictive VSDs are associated with equal or near-equal pulmonary and systemic systolic pressure and variable elevations in PVR. Pulmonary blood flow may be 2-4 times systemic blood flow. In patients with such “hyperdynamic pulmonary hypertension,” PAP is at systemic level, but PVR is only minimally elevated because of the high pulmonary blood flow (resistance is equal to pressure divided by flow). However, if left untreated until Eisenmenger syndrome is present, systolic and diastolic PAP will be elevated, but the degree of left-to-right shunting minimal. In these cases, desaturation of blood in the left ventricle is usually encountered. The size, location, and number of ventricular defects can be demonstrated by left ventriculography. Contrast medium passes across the defect(s) to opacify the right ventricle and pulmonary artery. Administration of 100% oxygen with and without nitric oxide can be used to determine whether PVR, if elevated, is still reactive and therefore more likely to decrease after surgical repair.
Treatment
The natural course of a VSD depends to a large degree on the size of the defect. A significant number (30–50%) of small defects close spontaneously, most frequently during the 1st 2 yr of life. Small muscular VSDs are more likely to close (up to 80%) than membranous VSDs (up to 35%). Most defects that close do so before age 4 yr, although spontaneous closure has been reported in adults. VSDs that close often have ventricular septal aneurysm (accessory tricuspid valve) tissue that limits the magnitude of the shunt. Most children with small restrictive defects remain asymptomatic, without evidence of an increase in heart size, PAP, or PVR; a long-term risk is infective endocarditis. Some long-term studies of adults with unoperated small VSDs show an increased incidence of arrhythmia, subaortic stenosis, and exercise intolerance. Guidelines from the Council on Cardiovascular Disease in the Young of the American Heart Association (AHA) state that an isolated, small, hemodynamically insignificant VSD is not an indication for surgery. However, the declining risk of open heart surgery has led some to suggest that all VSDs be closed electively by mid-childhood.
It is less common for moderate or large VSDs to close spontaneously, although even defects large enough to result in heart failure may become smaller, and up to 8% may close completely. More frequently, infants with large defects have repeated episodes of respiratory infection and heart failure despite optimal medical management. Heart failure may be manifested in many of these infants primarily as failure to thrive. Pulmonary hypertension occurs as a result of high pulmonary blood flow. These patients are at risk for pulmonary vascular disease if the defect is not repaired during early infancy.
Patients with VSD are also at risk for the development of aortic valve regurgitation , the greatest risk occurring in patients with a supracristal VSD (see Chapter 453.7 ), where the position of the defect undermines support for the aortic valve right coronary or noncoronary leaflet. A small number of patients with VSD develop acquired infundibular pulmonary stenosis, which then protects the pulmonary circulation from the short-term effects of pulmonary overcirculation and the long-term effects of pulmonary vascular disease. In these patients the clinical picture changes from that of a VSD with a large left-to-right shunt to a VSD with pulmonary stenosis. The shunt may diminish in size, become balanced, or even become a net right-to-left shunt. These patients must be carefully distinguished from those in whom an Eisenmenger physiology develops (see Chapter 460.2 ).
In patients with small VSDs, parents should be reassured of the relatively benign nature of the lesion, and the child should be encouraged to live a normal life, with no restrictions on physical activity. Surgical repair is not recommended. As protection against infective endocarditis, the integrity of primary and permanent teeth should be carefully maintained; with the latest revision of the AHA guidelines, antibiotic prophylaxis is no longer recommended for dental visits or surgical procedures (see Chapter 464 ). These patients can be monitored by a combination of clinical examination and noninvasive laboratory tests until the VSD has closed spontaneously. Echocardiography is used to estimate PAP, screen for the development of LVOT pathology (subaortic membrane or aortic regurgitation), and to confirm spontaneous closure.
In infants with a large VSD, management has 2 goals: control the symptoms of heart failure (see Chapter 469 ) and prevent the development of pulmonary vascular disease. If early treatment is successful, sometimes the shunt may diminish in size with spontaneous improvement, especially during the 1st yr of life. The clinician must be alert not to confuse clinical improvement caused by a decrease in defect size with clinical changes caused by the development of Eisenmenger physiology. Because surgical closure can be carried out at low risk in most infants, medical management should not be pursued in symptomatic infants after an initial unsuccessful trial. Because pulmonary vascular disease can usually be prevented when surgery is performed within the 1st yr of life, even infants with well-controlled heart failure should not have surgery delayed inordinately unless there is evidence that the defect is becoming pressure restrictive.
Indications for surgical closure of a VSD include patients at any age with large defects in whom clinical symptoms and failure to thrive cannot be controlled medically; infants between 6 and 12 mo of age with moderate to large defects associated with pulmonary hypertension, even if the symptoms are controlled by medication; and patients older than 24 mo with a Qp:Qs ratio greater than 2 : 1. Patients with a supracristal VSD of any size are usually referred for surgery because of their higher risk for developing aortic valve regurgitation (see Chapter 453.7 ). Severe pulmonary vascular disease nonresponsive to pulmonary vasodilators is a contraindication to closure of a VSD.
Transcatheter occlusion closure has been used successfully in treating larger muscular VSDs, which may be difficult to access by surgery. Perimembranous VSD catheter closure has a high risk of postprocedural heart block and is currently not routinely performed. Hybrid methods employing a sternotomy with device placement through the anterior wall of the right ventricle under transesophageal echocardiographic and fluoroscopic visualization has been performed in difficult-to-approach muscular defects.
Prognosis
The results of primary surgical repair are excellent, and complications leading to long-term problems (residual ventricular shunts requiring reoperation or heart block requiring a pacemaker) are rare. Palliation with pulmonary artery banding with repair in later childhood, once the standard of care, is now reserved for extremely complicated cases or very premature infants. Surgical risks are somewhat higher for defects in the muscular septum, particularly apical defects and multiple (Swiss cheese–type) VSDs. These patients may require pulmonary arterial banding if symptomatic, with subsequent debanding and repair of multiple VSDs at an older age.
After surgical obliteration of the left-to-right shunt, the hyperdynamic heart becomes quiet, cardiac size decreases toward normal (see Fig. 453.9 ), thrills and murmurs are abolished, and pulmonary artery hypertension regresses. The patient's clinical status greatly improves. Most infants begin to thrive, often quite rapidly after hospital discharge, and cardiac medications are no longer required. Catch-up growth occurs in most patients within the next year. In some patients, after successful surgery, systolic ejection murmurs of low intensity persist for months. The long-term prognosis after surgery is excellent. Patients with a small VSD and those who have undergone surgical closure without residua are considered to be at standard risk for health and life insurance.
Bibliography
Anderson BR, Stevens KN, Nicolson SC, et al. Contemporary outcomes of surgical ventricular septal defect closure. J Thorac Cardiovasc Surg . 2013;145:641–647.
Beekman RH III. Closing the ventricular septal defect because you can: evidence—averse care? J Pediatr . 2007;150:569–570.
Eroglu AG, Atik SU, Sengenc E, et al. Evaluation of ventricular septal defect with special reference to the spontaneous closure rate, subaortic ridge, and aortic valve prolapse II. Pediatr Cardiol . 2017;38(5):915–921.
Glen S, Burns J, Bloomfield P. Prevalence and development of additional cardiac abnormalities in 1448 patients with congenital ventricular septal defects. Heart . 2004;90:1321–1325.
Holzer R, Balzer D, Cao QL, et al. Device closure of muscular ventricular septal defects using the Amplatzer muscular ventricular septal defect occluder: immediate and mid-term results of a U.S. registry. J Am Coll Cardiol . 2004;43:1257–1263.
Hornberger LK, Sahn DJ, Krabill KA, et al. Elucidation of the natural history of ventricular septal defects by serial Doppler color flow mapping studies. J Am Coll Cardiol . 1989;13:1111–1118.
Lim DS, Forbes TJ, Rothman A, et al. Transcatheter closure of high-risk muscular ventricular septal defects with the CardioSEAL occluder: initial report from the CardioSEAL VSD registry. Catheter Cardiovasc Interv . 2007;70:740–744.
Maagaard M, Heiberg J, Asschenfeldt B, et al. Does functional capacity depend on the size of the shunt? A prospective, cohort study of adults with small, unrepaired ventricular septal defects. Eur J Cardiothorac Surg . 2017;51(4):722–727.
Ramaciotti C, Keren A, Silverman NH. Importance of pseudoaneurysms of the ventricular septum in the natural history of isolated perimembranous ventricular septal defects. Am J Cardiol . 1986;57:268–272.
Supracristal Ventricular Septal Defect With Aortic Insufficiency
Daniel Bernstein
A supracristal VSD can be complicated by prolapse of the aortic valve into the defect and aortic insufficiency, which may eventually develop in 50–90% of these patients. Although supracristal VSD accounts for approximately 5% of all patients with VSD, the incidence is higher in Asian children and in males. The VSD, which may be small or moderate in size, is located anterior to and directly below the pulmonary valve in the outlet septum, superior to the muscular ridge known as the crista supraventricularis, which separates the trabecular body of the right ventricle from the smooth outflow portion. The right or, less often, the noncoronary aortic cusp prolapses into the defect and may partially or even completely occlude it. Such occlusion may limit the amount of left-to-right shunting and give the false impression that the defect is not large. Aortic insufficiency is most often not recognized until after 5 yr of age, or even later. Although most common with supracristal VSDs, aortic insufficiency is occasionally associated with VSDs located in the membranous septum.
Early heart failure secondary to a large left-to-right shunt rarely occurs, but without surgery, moderate-to-severe aortic insufficiency and left ventricular failure may eventually ensue. The murmur of a supracristal VSD is usually heard at the mid- to upper left sternal border, as opposed to the lower left sternal border, and it is sometimes confused with that of pulmonic stenosis. A decrescendo diastolic murmur will be appreciated at the upper right or mid-left sternal borders if there is aortic insufficiency. More advanced degrees of aortic insufficiency will be associated with a wide pulse pressure and a hyperdynamic precordium. These clinical findings must be distinguished from PDA or other defects associated with aortic runoff.
The clinical manifestations vary widely, from trivial aortic regurgitation and small left-to-right shunts in asymptomatic children to florid aortic insufficiency and massive cardiomegaly in symptomatic adolescents. Closure of all supracristal ventricular VSDs is usually recommended to prevent the development of aortic regurgitation, even in an asymptomatic child. Patients who already have significant aortic insufficiency require surgical intervention to prevent irreversible left ventricular dysfunction. Surgical options depend on the degree of damage to the valve and for mild insufficiency may include simple closure of the defect to bolster the valve apparatus without touching the valve itself, valvuloplasty for more significant degrees of involvement, and replacement with a prosthesis or homograft or aortopulmonary translocation for severe involvement.
Patent Ductus Arteriosus
Daniel Bernstein
During fetal life, most of the pulmonary arterial blood is shunted right-to-left through the ductus arteriosus into the aorta (see Chapter 448 ). Functional closure of the ductus normally occurs soon after birth, usually within the 1st wk of life, but if the ductus remains patent when PVR falls, aortic blood then is shunted left to right into the pulmonary artery. The aortic end of the ductus is just distal to the origin of the left subclavian artery, and the ductus enters the pulmonary artery at its bifurcation. Female patients with patent ductus arteriosus (PDA) outnumber males 2 : 1. PDA is also associated with maternal rubella infection during early pregnancy, an uncommon occurrence in the vaccination era. PDA is a common problem in premature infants because the smooth muscle in the wall of the preterm ductus is less responsive to high PO 2 and therefore less likely to constrict after birth. In these infants the shunt through a PDA can cause severe hemodynamic derangements and several major sequelae (see Chapter 122.3 ).
When a term infant is found to have a PDA, the wall of the ductus is deficient in both the mucoid endothelial layer and the muscular media, whereas in the premature infant the PDA usually has a normal structure. Thus a PDA persisting beyond the 1st few wk of life in a term infant rarely closes spontaneously or with pharmacologic intervention, whereas if early pharmacologic or surgical intervention is not required in a premature infant, spontaneous closure occurs in most instances. A PDA is seen in 10% of patients with other congenital heart lesions and often plays a critical role in providing a source of pulmonary blood flow when the right ventricular outflow tract is stenotic or atretic (see Chapter 457 ) or in providing systemic blood flow in the presence of aortic coarctation or interruption (see Chapters 454.6 to 454.8 ).
Pathophysiology
Because of the higher aortic pressure postnatally, blood shunts left to right through the ductus, from the aorta to the pulmonary artery. The extent of the shunt depends on the size of the ductus and on the PVR/SVR ratio. If the PDA is small, pressures within the pulmonary artery, the right ventricle, and the right atrium are normal. If the PDA is large, PAP may be elevated to systemic levels during both systole and diastole. Thus, patients with a large PDA are at high risk for the development of pulmonary vascular disease if left unoperated.
Clinical Manifestations
A small PDA is usually asymptomatic and is usually diagnosed by the presence of a heart murmur. A large PDA will result in heart failure similar to that encountered in infants with a large VSD. Retardation of physical growth may be a major manifestation in infants with large shunts. A small PDA is associated with normal peripheral pulses, and a large PDA results in bounding peripheral arterial pulses and a wide pulse pressure , caused by runoff of blood into the pulmonary artery during diastole. Although normal in size when the ductus is small, the heart is moderately or grossly enlarged in cases with a large communication; in these patients the apical impulse is prominent and, with cardiac enlargement, is heaving. A thrill , maximal in the 2nd left interspace, is often present and may radiate toward the left clavicle, down the left sternal border, or toward the apex. It is usually systolic but may also be palpated throughout the cardiac cycle. The classic continuous murmur is described as “machinery-like” in quality. It begins soon after onset of S1 , reaches maximal intensity at the end of systole, and wanes in late diastole. It may be localized to the 2nd left intercostal space or radiate down the left sternal border or to the left clavicle. When PVR is increased, the diastolic component of the murmur may be less prominent or absent. In patients with a large left-to-right shunt, a low-pitched mitral mid-diastolic murmur may be audible at the apex as a result of the increased volume of blood flow across the mitral valve.
Diagnosis
If the left-to-right shunt is small, the ECG is normal; if the ductus is large, LV or biventricular hypertrophy is present. The diagnosis of an isolated, uncomplicated PDA is untenable when RV hypertrophy is present on the ECG.
Radiographic studies in patients with a large PDA show a prominent pulmonary artery with increased pulmonary vascular markings. Cardiac size depends on the degree of left-to-right shunting; it may be normal or moderately to greatly enlarged. The chambers involved are the left atrium and left ventricle. The aortic knob may be normal or prominent.
On echocardiogram the cardiac chambers will be normal in size if the ductus is small. With large shunts, left atrial and LV dimensions are increased. The ductus can easily be visualized directly and its size estimated. Color and pulsed Doppler examinations demonstrate systolic or diastolic (or both) retrograde turbulent flow in the pulmonary artery, and aortic retrograde flow in diastole in the presence of a large shunt (Fig. 453.10 ).
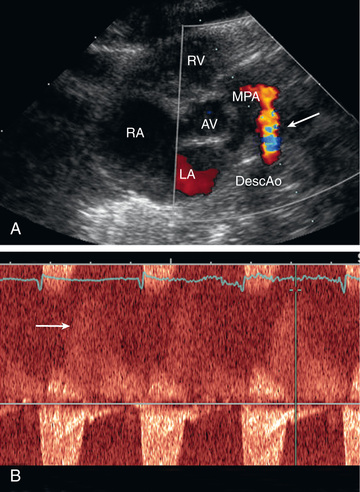
The clinical signs and echocardiographic findings are sufficiently distinctive to allow an accurate diagnosis by noninvasive methods in most patients. In rare patients with atypical findings, cardiac catheterization may be indicated for confirmation of diagnosis. Cardiac catheterization will demonstrate either normal or increased pressure in the right ventricle and pulmonary artery, depending on the size of the ductus. The presence of oxygenated blood shunting into the pulmonary artery confirms the left-to-right shunt. The catheter may pass from the pulmonary artery through the ductus into the descending aorta. Injection of contrast medium into the ascending aorta shows opacification of the pulmonary artery from the aorta and identifies the ductus.
Other conditions can produce systolic and diastolic murmurs in the pulmonic area in an acyanotic patient (see Chapter 449 ). An aorticopulmonary window defect may rarely be clinically indistinguishable from a patent ductus, although in most cases the murmur is only systolic and is loudest at the right rather than the left upper sternal border (see Chapter 453.9 ). A sinus of Valsalva aneurysm that has ruptured into the right side of the heart or pulmonary artery, a coronary arteriovenous fistula, and an aberrant left coronary artery with massive collaterals from the right coronary display dynamics similar to that of a PDA with a continuous murmur and a wide pulse pressure. Truncus arteriosus with torrential pulmonary flow also can present with an aortic runoff physiology. A peripheral arteriovenous fistula also results in a wide pulse pressure, but the distinctive precordial murmur of a PDA is not present. VSD with aortic insufficiency, repaired tetralogy of Fallot, and combined aortic and mitral insufficiency (usually from rheumatic fever) may be confused with a PDA, but the murmurs should be differentiated by their to-and-fro rather than continuous nature. In a to-and-fro murmur there is a quiet segment between the systolic and diastolic components, whereas in a continuous murmur there is flow disturbance throughout the cardiac cycle (even if the murmur is louder during systole than diastole). The combination of a large VSD and a PDA results in findings more like those of an isolated VSD. Echocardiography should be able to eliminate these other diagnostic possibilities.
Prognosis and Complications
Spontaneous closure of the ductus after infancy is extremely rare. Patients with a small PDA may live a normal span with few or no cardiac symptoms, but late manifestations may occur. In patients with a large PDA, cardiac failure most often occurs in early infancy but may occur later in life, even with a moderate-sized communication.
Infective endarteritis may be seen at any age. Pulmonary or systemic emboli may occur. Rare complications include aneurysmal dilation of the pulmonary artery or the ductus, calcification of the ductus, noninfective thrombosis of the ductus with embolization, and paradoxical emboli. Pulmonary hypertension (Eisenmenger syndrome) usually develops in patients with a large PDA who do not undergo ductal closure (see Chapter 460.2 ).
Treatment
Irrespective of age, patients with a PDA require either catheter or surgical closure. In patients with a small PDA, the rationale for closure is prevention of bacterial endarteritis or other late complications. In patients with a moderate to large PDA, closure is accomplished to treat heart failure or prevent the development of pulmonary vascular disease, or both. Once the diagnosis of a moderate to large PDA is made, treatment should not be unduly postponed after adequate medical therapy for cardiac failure has been instituted.
Transcatheter PDA closure is routinely performed in the cardiac catheterization laboratory (Fig. 453.11 ). Small PDAs are generally closed with intravascular coils. Moderate to large PDAs may be closed with an umbrella-like device or with a catheter-introduced sac into which several coils are released. Surgical closure of a PDA can be accomplished by a standard left thoracotomy or using thoracoscopic minimally invasive techniques. The case fatality rate with interventional or surgical treatment is considerably less than 1%; thus closure of the ductus is indicated even in asymptomatic patients. Pulmonary hypertension is not a contraindication to surgery at any age if it can be demonstrated at cardiac catheterization that the shunt flow is still predominantly left to right and that severe pulmonary vascular disease is not present. After closure, symptoms of cardiac failure rapidly disappear. Infants who had failed to thrive usually have immediate improvement in physical development. The pulse and blood pressure return to normal, and the machinery-like murmur disappears. A functional systolic murmur over the pulmonary area may persist; it may represent turbulence in a persistently dilated pulmonary artery. The radiographic signs of cardiac enlargement and pulmonary overcirculation disappear over several months, and the ECG becomes normal.
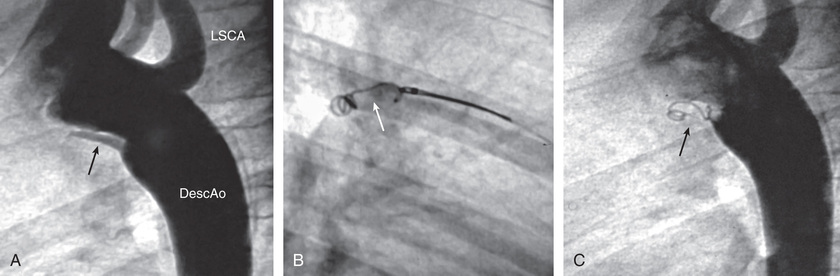
Patent Ductus Arteriosus in Low-Birthweight Infants
See Chapter 122.5 .
Bibliography
Alagarsamy S, Chhabra M, Gudavalli M, et al. Comparison of clinical criteria with echocardiographic findings in diagnosing PDA in preterm infants. J Perinat Med . 2005;33:161–164.
Backes CH, Rivera BK, Bridge JA, et al. Percutaneous patent ductus arteriosus (PDA) closure during infancy: a meta-analysis. Pediatrics . 2017;139(2).
Bergwerff M, DeRuiter MC, Gittenberger-de Groot AC. Comparative anatomy and ontogeny of the ductus arteriosus, a vascular outsider. Anat Embryol (Berl) . 1999;200:559–571.
Rothman A, Lucas VW, Sklansky MS, et al. Percutaneous coil occlusion of patent ductus arteriosus. J Pediatr . 1997;130:447–454.
Aortopulmonary Window Defect
Daniel Bernstein
An aortopulmonary window defect consists of a communication between the ascending aorta and the main pulmonary artery. The presence of pulmonary and aortic valves and an intact ventricular septum distinguishes this anomaly from truncus arteriosus (see Chapter 458.8 ). Symptoms of heart failure appear during early infancy; occasionally, minimal cyanosis is present. The defect is usually large, and the cardiac murmur is usually systolic with an apical mid-diastolic rumble as a result of the increased blood flow across the mitral valve. In the rare instance when the communication is smaller and pulmonary hypertension is absent, the findings on examination can mimic those of a PDA (wide pulse pressure and a continuous murmur at the upper sternal borders). The ECG shows either LV or biventricular hypertrophy. Radiographic studies demonstrate cardiac enlargement and prominence of the pulmonary artery and intrapulmonary vasculature. The echocardiogram shows enlarged left-sided heart chambers; the window defect can best be delineated with color flow Doppler. CT or MR angiography can also be used to visualize the defect (see Chapter 450 , Fig. 450.26 ).
Cardiac catheterization, usually performed in older children to evaluate pulmonary vascular resistance, reveals a left-to-right shunt at the level of the pulmonary artery, as well as hyperkinetic pulmonary hypertension, because the defect is almost always large. Selective aortography with injection of contrast medium into the ascending aorta demonstrates the lesion, and manipulation of the catheter from the main pulmonary artery directly to the ascending aorta is also diagnostic.
An aortopulmonary window defect is surgically corrected during infancy. If surgery is not carried out in infancy, survivors carry the risk of progressive pulmonary vascular obstructive disease, similar to that of other patients who have large intracardiac or great vessel communications.
Coronary Artery Fistula
Daniel Bernstein
A congenital fistula may exist between a coronary artery and an atrium, ventricle (especially the right), or pulmonary artery. Sometimes, multiple fistulas exist. Regardless of the recipient chamber, the clinical signs are similar to those of PDA, although the machinery-like murmur may be more diffuse. If the flow is substantial, the involved coronary artery may be dilated or aneurysmal. The anatomic abnormality is usually demonstrable by color flow Doppler echocardiography and, during catheterization, by contrast injection into the ascending aorta. Small fistulas may be hemodynamically insignificant and may even close spontaneously. If the shunt is large, treatment consists of either transcatheter coil embolization or, for lesions not amenable to catheter intervention, surgical closure of the fistula.
Ruptured Sinus of Valsalva Aneurysm
Daniel Bernstein
When one of the sinuses of Valsalva of the aorta is weakened by congenital or acquired disease, an aneurysm may form and eventually rupture, usually into the right atrium or ventricle. This condition is extremely rare in childhood. The onset is usually sudden. The diagnosis should be suspected in a patient in whom symptoms of acute heart failure develop in association with a new, loud, to-and-fro murmur. Color Doppler echocardiography and cardiac catheterization demonstrate the left-to-right shunt at the atrial or ventricular level. Urgent surgical repair is generally required. This condition is often associated with infective endocarditis of the aortic valve.