Physiology of the Adrenal Gland
Histology and Embryology
Perrin C. White
The adrenal gland is composed of 2 endocrine tissues: the medulla and the cortex. The chromaffin cells of the adrenal medulla are derived from neuroectoderm, whereas the cells of the adrenal cortex are derived from mesoderm. Mesodermal cells also contribute to the development of the gonads. The adrenal glands and gonads have certain common enzymes involved in steroid synthesis; an inborn error in steroidogenesis in 1 tissue can also be present in the other.
The adrenal cortex of the older child or adult consists of 3 zones: the zona glomerulosa , the outermost zone located immediately beneath the capsule; the zona fasciculata , the middle zone; and the zona reticularis , the innermost zone, lying next to the adrenal medulla. The zona fasciculata is the largest zone, constituting approximately 75% of the cortex; the zona glomerulosa constitutes approximately 15% and the zona reticularis approximately 10%. Glomerulosa cells are small, with a lower cytoplasmic:nuclear ratio, an intermediate number of lipid inclusions, and smaller nuclei containing more condensed chromatin than the cells of the other 2 zones. The cells of the zona fasciculata are large, with a high cytoplasmic:nuclear ratio and many lipid inclusions that give the cytoplasm a foamy, vacuolated appearance. The cells are arranged in radial cords. The cells of the zona reticularis are arranged in irregular anastomosing cords. The cytoplasmic:nuclear ratio is intermediate, and the compact cytoplasm has relatively little lipid content.
The zona glomerulosa synthesizes aldosterone , the most potent natural mineralocorticoid in humans. The zona fasciculata produces cortisol , the most potent natural glucocorticoid in humans, and the zona fasciculata and zona reticularis synthesize the adrenal androgens.
The adrenal medulla consists mainly of neuroendocrine (chromaffin) cells and glial (sustentacular) cells with some connective tissue and vascular cells. Neuroendocrine cells are polyhedral, with abundant cytoplasm and small, pale-staining nuclei. Under the electron microscope, the cytoplasm contains many large secretory granules that contain catecholamines. Glial cells have less cytoplasm and more basophilic nuclei.
The primordium of the fetal adrenal gland can be recognized at 3-4 wk of gestation just cephalad to the developing mesonephros. At 5-6 wk, the gonadal ridge develops into the steroidogenic cells of the gonads and adrenal cortex; the adrenal and gonadal cells separate, the adrenal cells migrate retroperitoneally, and the gonadal cells migrate caudad. At 6-8 wk of gestation, the gland rapidly enlarges, the cells of the inner cortex differentiate to form the fetal zone, and the outer subcapsular rim remains as the definitive zone. The primordium of the adrenal cortex is invaded at this time by sympathetic neural elements that differentiate into the chromaffin cells capable of synthesizing and storing catecholamines. Catechol O -methyltransferase, which converts norepinephrine to epinephrine, is expressed later in gestation. By the end of the 8th wk of gestation, the encapsulated adrenal gland is associated with the upper pole of the kidney. By 8-10 wk of gestation, the cells of the fetal zone are capable of active steroidogenesis.
In the full-term infant, the combined weight of both adrenal glands is 7-9 g. At birth, the inner fetal cortex makes up approximately 80% of the gland and the outer true cortex 20%. Within a few days the fetal cortex begins to involute, undergoing a 50% reduction by 1 mo of age. Conversely, the adrenal medulla is relatively small at birth and undergoes a proportionate increase in size over the first 6 mo after birth. By 1 yr, the adrenal glands each weigh < 1 g. Adrenal growth thereafter results in adult adrenal glands reaching a combined weight of 8 g. The zonae fasciculata and glomerulosa are fully differentiated by about 3 yr of age. The zona reticularis is not fully developed until puberty.
Adrenocorticotropic hormone (ACTH) is essential for fetal adrenal growth and maturation; feedback regulation of ACTH by cortisol is apparently established by 8-10 wk of gestation. Additional factors important in fetal growth and steroidogenesis include placental chorionic gonadotropins and a number of peptide growth factors produced by the placenta and fetus.
Several transcription factors are critical for the development of the adrenal glands. The 3 transcription factors associated with adrenal hypoplasia in humans are steroidogenic factor-1 (SF-1; NR5A1), DAX-1 (dosage-sensitive sex reversal, adrenal hypoplasia congenita, X chromosome; NR0B1), and the GLI3 oncogene. Disruption of SF-1 , encoded on chromosome 9q33, results in gonadal and often adrenal agenesis, absence of pituitary gonadotropes, and an underdeveloped ventral medial hypothalamus. In-frame deletions and frameshift and missense mutations of this gene are associated with 46,XX ovarian insufficiency and 46,XY gonadal dysgenesis. Mutations in the DAX1 gene, encoded on Xp21, result in adrenal hypoplasia congenita and hypogonadotropic hypogonadism (see Chapter 593.1 ). Mutations in GLI3 on chromosome 7p13 cause Pallister-Hall syndrome, other features of which include hypothalamic hamartoblastoma, hypopituitarism, imperforate anus, and postaxial polydactyly.
The postnatal adrenal cortex is not static but, in fact, is continually regenerated from a population of stem or progenitor cells under the adrenal capsule. These cells move radially inward (i.e., centripetally) and can differentiate into zona glomerulosa or fasciculata cells as needed in response to the appropriate trophic stimuli (see Chapter 592.3 ). Several signaling pathways, including sonic hedgehog and Wnt, regulate this process. Sonic hedgehog expression is restricted to the peripheral cortical cells that do not express high levels of steroidogenic genes but give rise to the underlying differentiated cells of the cortex. Wnt/β-catenin signaling maintains the undifferentiated state and adrenal fate of adrenocortical stem/progenitor cells, in part through induction of its target genes DAX1 and inhibin-α, respectively. Adrenal tumors can result from constitutive activation of the Wnt signaling pathway (see Chapter 597 ).
Bibliography
Ferraz-de-Souza B, Achermann JC. Disorders of adrenal development. Endocr Dev . 2008;13:19–32.
Haase M, Willenberg HS, Bornstein SR. Update on the corticomedullary interaction in the adrenal gland. Endocr Dev . 2011;20:28–37.
Kempna P, Fluck CE. Adrenal gland development and defects. Best Pract Res Clin Endocrinol Metab . 2008;22:77–93.
Kim AC, Barlaskar FM, Heaton JH, et al. In search of adrenocortical stem and progenitor cells. Endocr Rev . 2009;30:241–263.
Lourenco D, Brauner R, Lin L, et al. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med . 2009;360:1200–1210.
Adrenal Steroid Biosynthesis
Perrin C. White
Keywords
- Cholesterol
- scavenger receptor class B
- type I (SR-BI)
- steroidogenic acute regulatory (StAR)
- cholesterol side-chain cleavage enzyme
- cytochrome P450
- 3β-hydroxysteroid dehydrogenase
- steroid 21-hydroxylase
- P450 oxidoreductase
- deoxycorticosterone
- aldosterone synthase
- 17α-hydroxylase
- 17-hydroxyprogesterone
- steroid 11β-hydroxylase
- 11β-hydroxysteroid dehydrogenase type 2
Cholesterol is the starting substrate for all steroid biosynthesis (Fig. 592.1 ). Although adrenal cortex cells can synthesize cholesterol de novo from acetate, circulating plasma lipoproteins provide most of the cholesterol for adrenal cortex hormone formation. Receptors for both low-density lipoprotein and high-density lipoprotein cholesterol are expressed on the surface of adrenocortical cells; the receptor for high-density lipoprotein is termed scavenger receptor class B, type I (SR-BI) . Patients with homozygous familial hypercholesterolemia who lack low-density lipoprotein receptors have only mildly impaired adrenal steroidogenesis, suggesting that high-density lipoprotein is the more important source of cholesterol. Cholesterol is stored as cholesteryl esters in vesicles and subsequently hydrolyzed by cholesteryl ester hydrolases to liberate free cholesterol for steroid hormone synthesis.
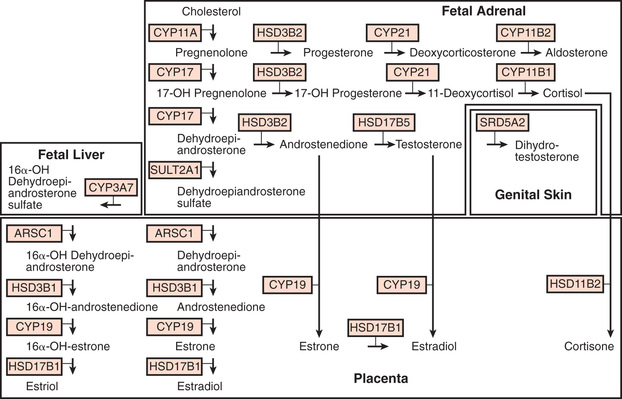
The rate-limiting step of adrenal steroidogenesis is importation of cholesterol across the mitochondrial outer and inner membrane. This requires several proteins, particularly the steroidogenic acute regulatory (StAR) protein. The StAR protein has a very short half-life, and its synthesis is rapidly induced by trophic factors (corticotropin); thus, it is the main short-term (minutes to hours) regulator of steroid hormone biosynthesis.
At the mitochondrial inner membrane, the side chain of cholesterol is cleaved to yield pregnenolone. This is catalyzed by the cholesterol side-chain cleavage enzyme (cholesterol desmolase, side-chain cleavage enzyme, P450scc, CYP11A1; the last term is the current systematic nomenclature), a cytochrome P450 (CYP) enzyme. Like other P450s, this is a membrane-bound hemoprotein with a molecular mass of approximately 50 kDa. It accepts electrons from a reduced nicotinamide adenine dinucleotide phosphate–dependent mitochondrial electron transport system consisting of 2 accessory proteins, adrenodoxin reductase (a flavoprotein) and adrenodoxin (a small protein containing nonheme iron). P450 enzymes use electrons and O2 to hydroxylate the substrate and form H2 O. In the case of cholesterol side-chain cleavage, 3 successive oxidative reactions are performed to cleave the C20,22 carbon bond. Pregnenolone then diffuses out of mitochondria and enters the endoplasmic reticulum. The subsequent reactions that occur depend on the zone of the adrenal cortex.
Zona Glomerulosa
In the zona glomerulosa, pregnenolone is converted to progesterone by 3 β-hydroxysteroid dehydrogenase type 2, an oxidized nicotinamide adenine dinucleotide-dependent enzyme of the short-chain dehydrogenase type. Progesterone is converted to 11-deoxycorticosterone by steroid 21-hydroxylase (P450c21, CYP21), which is another P450. Like other P450s in the endoplasmic reticulum, it uses an electron transport system with only 1 accessory protein, P450 oxidoreductase .
Deoxycorticosterone then reenters mitochondria and is converted to aldosterone by aldosterone synthase (P450aldo, CYP11B2), a P450 enzyme structurally related to cholesterol desmolase. Aldosterone synthase also carries out 3 successive oxidations: 11β-hydroxylation, 18-hydroxylation, and further oxidation of the 18-methyl carbon to an aldehyde.
Zona Fasciculata
In the endoplasmic reticulum of the zona fasciculata, pregnenolone and progesterone are converted by 17α-hydroxylase (P450c17, CYP17) to 17-hydroxypregenolone and 17-hydroxyprogesterone, respectively. This enzyme is not expressed in the zona glomerulosa, which consequently cannot synthesize 17-hydroxylated steroids. 17-hydroxypregnenolone is converted to 17-hydroxyprogesterone and 11-deoxycortisol by the same 3β-hydroxysteroid and 21-hydroxylase enzymes, respectively, as are active in the zona glomerulosa. Thus, inherited disorders in these enzymes affect both aldosterone and cortisol synthesis (see Chapter 594 ). Finally, 11-deoxycortisol reenters mitochondria and is converted to cortisol by steroid 11β-hydroxylase (P450c11, CYP11B1). This enzyme is closely related to aldosterone synthase but has low 18-hydroxylase and nonexistent 18-oxidase activity. Thus, under normal circumstances the zona fasciculata cannot synthesize aldosterone.
Zona Reticularis
In the zona reticularis and to some extent in the zona fasciculata, the 17-hydroxylase (CYP17) enzyme has an additional activity, cleavage of the 17,20 carbon–carbon bond. This converts 17-hydroxypregnenolone to dehydroepiandrosterone (DHEA). DHEA is converted to androstenedione by HSD3B. This may be further converted in other tissues to testosterone and estrogens.
Fetoplacental Unit
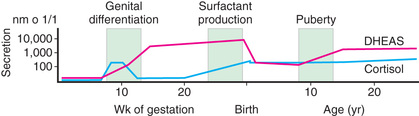
Steroid synthesis in the fetal adrenal varies during gestation (see Figs. 592.1 and 592.2 ). Shortly after the fetal adrenal gland forms (wk 8-10), it efficiently secretes cortisol, which is able to negatively feed back on the fetal pituitary and hypothalamus to suppress ACTH secretion. This is a critical time for differentiation of the external genitalia in both sexes (see Chapter 594.1 ); to prevent virilization, the female fetus must not be exposed to high levels of androgens of adrenal origin, and placental aromatase activity must remain low during this time to minimize conversion of testosterone to estradiol in male fetuses, which would interfere with masculinization. After wk 12, HSD3B activity in the fetal adrenal gland decreases and steroid sulfokinase activity increases. Thus, the major steroid products of the midgestation fetal adrenal gland are DHEA and DHEA sulfate (DHEAS) and, by 16α-hydroxylation in the liver, 16α-hydroxy DHEAS. Aromatase activity increases in the placenta at the same time, and steroid sulfatase activity is high as well. Thus, the placenta uses DHEA and DHEAS as substrates for estrone and estradiol and 16α-OH DHEAS as a substrate for estriol. Cortisol activity is low during the 2nd trimester, which might serve to prevent premature secretion of surfactant by the developing fetal lungs; surfactant levels can affect the timing of parturition. As term approaches, fetal cortisol concentration increases as a result of increased cortisol secretion and decreased conversion of cortisol to cortisone by 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2). Low levels of aldosterone are produced in midgestation, but aldosterone secretory capacity increases near term.
Bibliography
Connelly MA. SR-BI-mediated HDL cholesteryl ester delivery in the adrenal gland. Mol Cell Endocrinol . 2009;300:83–88.
Goto M, Piper Hanley K, Marcos J, et al. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest . 2006;116:953–960.
Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev . 2011;32:81–151.
Monticone S, Auchus RJ, Rainey WE. Adrenal disorders in pregnancy. Nat Rev Endocrinol . 2012;8:668–678.
White PC. Ontogeny of adrenal steroid biosynthesis: why girls will be girls. J Clin Invest . 2006;116:872–874.
Regulation of the Adrenal Cortex
Perrin C. White
Regulation of Cortisol Secretion
Glucocorticoid secretion is regulated mainly by ACTH (corticotropin), a 39-amino-acid peptide that is produced in the anterior pituitary (see Fig. 572.2 ). It is synthesized as part of a larger-molecular-weight precursor peptide known as proopiomelanocortin . This precursor peptide is also the source of β-lipotropin. ACTH and β-lipotropin are cleaved further to yield α- and β-melanocyte–stimulating hormone, corticotropin-like intermediate lobe peptide, γ-lipotropin, β- and γ-endorphin, and enkephalin (see Chapter 572 ).
ACTH is released in secretory bursts of varying amplitude throughout the day and night. The normal diurnal rhythm of cortisol secretion is caused by the varying amplitudes of ACTH pulses. Pulses of ACTH and cortisol occur every 30-120 min, are highest at about the time of waking, are low in late afternoon and evening, and reach their lowest point 1 or 2 hr after sleep begins.
Corticotropin-releasing hormone (CRH), synthesized by neurons of the parvicellular division of the hypothalamic paraventricular nucleus, is the most important stimulator of ACTH secretion. Arginine vasopressin (AVP) augments CRH action. Neural stimuli from the brain cause the release of CRH and AVP (see Chapter 572 ). AVP and CRH are secreted in the hypophyseal-portal circulation in a pulsatile manner. This pulsatile secretion appears to be responsible for the pulsatile (ultradian) release of ACTH. The circadian rhythm of corticotropin release is probably induced by a corresponding circadian rhythm of hypothalamic CRH secretion, regulated by the suprachiasmatic nucleus with input from other areas of the brain. Cortisol exerts a negative feedback effect on the synthesis and secretion of ACTH, CRH, and AVP. ACTH inhibits its own secretion, a feedback effect mediated at the level of the hypothalamus. Thus, the secretion of cortisol is a result of the interaction of the hypothalamus, pituitary, and adrenal glands and other neural stimuli.
ACTH acts through a specific G-protein–coupled receptor (also termed melanocortin receptor-2, encoded by the MCR2 gene) to activate adenylate cyclase and increase levels of cyclic adenosine monophosphate. Cyclic adenosine monophosphate has short-term (minutes to hours) effects on cholesterol transport into mitochondria by increasing expression of StAR protein. The long-term effects (hours to days) of ACTH stimulation are to increase the uptake of cholesterol and the expression of genes encoding the enzymes required to synthesize cortisol. These transcriptional effects occur at least in part through increased activity of protein kinase A, which phosphorylates several transcriptional regulatory factors. MC2R trafficking and signaling are dependent on the MC2R accessory protein (MRAP). Mutations in either MC2R or MRAP can cause familial glucocorticoid deficiency (see Chapter 593 ).
Regulation of Aldosterone Secretion
The rate of aldosterone synthesis, which is normally 100- to 1,000-fold less than that of cortisol synthesis, is regulated mainly by the renin–angiotensin system and by potassium levels, with ACTH having only a short-term effect. In response to decreased intravascular volume, renin is secreted by the juxtaglomerular apparatus of the kidney. Renin is a proteolytic enzyme that cleaves angiotensinogen (renin substrate), an α2 -globulin produced by the liver, to yield the inactive decapeptide angiotensin I. Angiotensin-converting enzyme in the lungs and other tissues rapidly cleaves angiotensin I to the biologically active octapeptide angiotensin II. Cleavage of angiotensin II produces the heptapeptide angiotensin III. Angiotensins II and III are potent stimulators of aldosterone secretion; angiotensin II is a more potent vasopressor agent. Angiotensins II and III occupy a G-protein–coupled receptor activating phospholipase C. This protein hydrolyzes phosphatidylinositol bisphosphate to produce inositol triphosphate and diacylglycerol, which raise intracellular calcium levels and activate protein kinase C and calmodulin-activated kinases. Similarly, increased levels of extracellular potassium depolarize the cell membrane and increase calcium influx through voltage-gated L-type calcium channels. Phosphorylation of transcriptional regulatory factors by calmodulin-activated kinases increases transcription of the aldosterone synthase (CYP11B2) enzyme required for aldosterone synthesis.
Regulation of Adrenal Androgen Secretion
The mechanisms by which the adrenal androgens DHEA and androstenedione are regulated are not completely understood. Adrenarche is a maturational process in the adrenal gland that results in increased adrenal androgen secretion between the ages of 5 and 20 yr. The process begins before the earliest signs of puberty and continues throughout the years when puberty is occurring. Histologically, it is associated with the appearance of the zona reticularis. Whereas ACTH stimulates adrenal androgen production acutely and clearly is the primary stimulus for cortisol release, additional factors have been implicated in the stimulation of the adrenal androgens. These include a relative decrease in expression of HSD3B2 in the zona reticularis and possibly increases in 17,20-lyase activity owing to phosphorylation of CYP17 or increased cytochrome b5 expression.
Bibliography
Guagliardo NA, Yao J, Hu C, et al. Minireview: aldosterone biosynthesis: electrically gated for our protection. Endocrinology . 2012;153:3579–3586.
Hattangady NG, Olala LO, Bollag WB, et al. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol . 2012;350:151–162.
Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev . 2011;32:81–151.
Nakamura Y, Gang HX, Suzuki T, et al. Adrenal changes associated with adrenarche. Rev Endocr Metab Disord . 2009;10:19–26.
Novoselova TV, Jackson D, Campbell DC, et al. Melanocortin receptor accessory proteins in adrenal gland physiology and beyond. J Endocrinol . 2013;217:R1–R11.
Rege J, Rainey WE. The steroid metabolome of adrenarche. J Endocrinol . 2012;214:133–143.
Adrenal Steroid Hormone Actions
Perrin C. White
Keywords
- gluconeogenesis
- epinephrine
- norepinephrine
- hypercalcemia
- osteoporosis
- psychosis
- weight loss
- hypotension
- hyponatremia
- hyperkalemia
- serum and glucocorticoid-regulated kinase
- apparent mineralocorticoid excess syndrome
- dihydrotestosterone
- estrone
- estradiol
- congenital adrenal hyperplasia
- premature adrenarche
- adrenal tumors
- Cushing syndrome
- prednisone, prednisolone
- dexamethasone
Steroid hormones act through several distinct receptors corresponding to the known biologic activities of the steroid hormones: glucocorticoid, mineralocorticoid, progestin, estrogen, and androgen. These receptors belong to a larger superfamily of nuclear transcription factors that include, among others, thyroid hormone and retinoic acid receptors. They have a common structure that includes a carboxyterminal ligand-binding domain and a midregion DNA-binding domain. The latter domain contains 2 zinc fingers, each of which consists of a loop of amino acids stabilized by 4 cysteine residues chelating a zinc ion.
Unliganded glucocorticoid and mineralocorticoid receptors are found mainly in the cytosol in complexes with other proteins. Hormone molecules diffuse through the cell membrane and bind receptors, changing their conformation and causing them to release their cytosolic binding proteins and translocate to the nucleus, where they bind DNA at specific hormone-response elements. Bound receptors can recruit other transcriptional coregulatory factors to DNA.
Whereas different steroids can share bioactivities because of their ability to bind to the same receptor, a given steroid can exert diverse biologic effects in different tissues. The diversity of hormonal responses is determined by the different genes that are regulated by each hormone in different tissues. Additionally, different combinations of coregulators are expressed in different tissues, allowing each steroid hormone to have many different effects. Moreover, enzymes can increase or decrease the affinity of steroids for their receptors and thus modulate their activity. 11β-Hydroxysteroid dehydrogenase type 1 (HSD11B1) converts cortisone, which is not a ligand for the glucocorticoid receptor, to cortisol, which is an active glucocorticoid. This increases local glucocorticoid concentrations in several tissues, especially the liver, where glucocorticoids maintain hepatic glucose output (see Chapter 593.4 ). Overexpression of this enzyme in adipose tissue can predispose to development of obesity. Conversely, HSD11B2 oxidizes cortisol to cortisone, particularly in the kidney, preventing mineralocorticoid receptors from being occupied by high levels of cortisol (see Chapter 593.4 ).
Although corticosteroid receptors mainly act in the nucleus, some responses to both glucocorticoids and mineralocorticoids begin within minutes, an interval too short to be accounted for by increased gene transcription and protein synthesis. Such nongenomic effects can in some cases be mediated by cell membrane–associated isoforms of the classic glucocorticoid and mineralocorticoid receptors, which can couple to a variety of rapid intracellular signaling pathways such as G proteins. Direct interactions with other proteins, such as ion channels, have been documented as well, particularly in the nervous system.
Actions of Glucocorticoids
Glucocorticoids are essential for survival. The term glucocorticoid refers to the glucose-regulating properties of these hormones. However, glucocorticoids have multiple effects on carbohydrate, lipid, and protein metabolism. They also regulate immune, circulatory, and renal function. They influence growth, development, bone metabolism, and central nervous system activity.
In stress situations, glucocorticoid secretion can increase up to 10-fold. This increase is believed to enhance survival through increased cardiac contractility, cardiac output, sensitivity to the pressor effects of catecholamines and other pressor hormones, work capacity of the skeletal muscles, and capacity to mobilize energy stores.
Metabolic Effects
The primary action of the glucocorticoids on carbohydrate metabolism is to increase glucose production by increasing hepatic gluconeogenesis . Glucocorticoids also increase cellular resistance to insulin, thereby decreasing entry of glucose into the cell. This inhibition of glucose uptake occurs in adipocytes, muscle cells, and fibroblasts. In addition to opposing insulin action, glucocorticoids can work in parallel with insulin to protect against long-term starvation by stimulating glycogen deposition and production in liver. Both hormones stimulate glycogen synthetase activity and decrease glycogen breakdown. Glucocorticoid excess can cause hyperglycemia, and glucocorticoid deficiency can cause hypoglycemia.
Glucocorticoids increase free fatty acid levels by enhancing lipolysis, decreasing cellular glucose uptake, and decreasing glycerol production, which is necessary for reesterification of fatty acids. This increase in lipolysis is also stimulated through the permissive enhancement of lipolytic action of other factors such as epinephrine. This action affects adipocytes differently according to their anatomic locations. In the patient with glucocorticoid excess, fat is lost in the extremities but it is increased in the trunk (centripetal obesity), neck, and face (moon facies). This may involve effects on adipocyte differentiation.
Glucocorticoids generally exert a catabolic or antianabolic effect on protein metabolism. Proteolysis in fat, skeletal muscle, bone, lymphoid, and connective tissue increases amino acid substrates that can be used in gluconeogenesis. Cardiac muscle and the diaphragm are almost entirely spared from this catabolic effect.
Circulatory and Renal Effects
Glucocorticoids have a positive inotropic influence on the heart, increasing the left ventricular work index. Moreover, they have a permissive effect on the actions of epinephrine and norepinephrine on both the heart and the blood vessels. In the absence of glucocorticoids, decreased cardiac output and shock can develop; in states of glucocorticoid excess, hypertension is often observed. This may be a result of activation of the mineralocorticoid receptor (see Chapter 593.4 ), which occurs when renal HSD11B2 is saturated by excessive levels of glucocorticoids.
Growth
In excess, glucocorticoids inhibit linear growth and skeletal maturation in children, apparently through direct effects on the epiphyses. However, glucocorticoids are also necessary for normal growth and development. In the fetus and neonate, they accelerate the differentiation and development of various tissues, including the hepatic and gastrointestinal systems, as well as the production of surfactant in the fetal lung. Glucocorticoids are often given to pregnant women at risk for delivery of premature infants in an effort to accelerate these maturational processes.
Immunologic Effects
Glucocorticoids play a major role in immune regulation. They inhibit synthesis of glycolipids and prostaglandin precursors and the actions of bradykinin. They also block secretion and actions of histamine and proinflammatory cytokines (tumor necrosis factor-α, interleukin-1, and interleukin-6), thus diminishing inflammation. High doses of glucocorticoids deplete monocytes, eosinophils, and lymphocytes, especially T cells. They do so at least in part by inducing cell-cycle arrest in the G1 phase and by activating apoptosis through glucocorticoid receptor–mediated effects. The effects on lymphocytes are primarily exerted on T-helper 1 cells and hence on cellular immunity, whereas the T-helper 2 cells are spared, leading to a predominantly humoral immune response. Pharmacologic doses of glucocorticoids can also decrease the size of immunologic tissues (spleen, thymus, and lymph nodes).
Glucocorticoids increase circulating polymorphonuclear cell counts, mostly by preventing their egress from the circulation. Glucocorticoids decrease diapedesis, chemotaxis, and phagocytosis of polymorphonuclear cells. Thus, the mobility of these cells is altered such that they do not arrive at the site of inflammation to mount an appropriate immune response. High levels of glucocorticoids decrease inflammatory and cellular immune responses and increase susceptibility to certain bacterial, viral, fungal, and parasitic infections.
Effects on Skin, Bone, and Calcium
Glucocorticoids inhibit fibroblasts, leading to increased bruising and poor wound healing through cutaneous atrophy. This effect explains the thinning of the skin and striae that are seen in patients with Cushing syndrome.
Glucocorticoids have the overall effect of decreasing serum calcium and have been used in emergency therapy for certain types of hypercalcemia . This hypocalcemic effect probably results from a decrease in the intestinal absorption of calcium and a decrease in the renal reabsorption of calcium and phosphorus. Serum calcium levels, however, generally do not fall below normal because of a secondary increase in parathyroid hormone secretion.
The most significant effect of long-term glucocorticoid excess on calcium and bone metabolism is osteoporosis. Glucocorticoids inhibit osteoblastic activity by decreasing the number and activity of osteoblasts. Glucocorticoids also decrease osteoclastic activity but to a lesser extent, leading to low bone turnover with an overall negative balance. The tendency of glucocorticoids to lower serum calcium and phosphate levels causes secondary hyperparathyroidism. These actions decrease bone accretion and cause a net loss of bone mineral.
Central Nervous System Effects
Glucocorticoids readily penetrate the blood–brain barrier and have direct effects on brain metabolism. They decrease certain types of central nervous system edema and are often used to treat increased intracranial pressure. They stimulate appetite and cause insomnia with a reduction in rapid eye movement sleep. There is an increase in irritability and emotional lability, with an impairment of memory and ability to concentrate. Mild to moderate glucocorticoid excess for a limited period often causes a feeling of euphoria or well-being, but glucocorticoid excess and deficiency can both be associated with clinical depression. Glucocorticoid excess produces psychosis in some patients.
Glucocorticoid effects in the brain are mediated largely through interactions with both the mineralocorticoid and glucocorticoid receptors (sometimes referred to in this context as type I and type II corticosteroid receptors, respectively). Activation of type II receptors increases sensitivity of hippocampal neurons to the neurotransmitter serotonin, which might help explain the euphoria associated with high doses of glucocorticoids. Glucocorticoids suppress release of CRH in the anterior hypothalamus, but they stimulate it in the central nucleus of the amygdala and lateral bed nucleus of the stria terminalis, where it can mediate fear and anxiety states. Glucocorticoids and other steroids might have nongenomic effects by modulating activities of both γ-aminobutyric acid and N -methyl-D -aspartate receptors.
Actions of Mineralocorticoids
The most important mineralocorticoids are aldosterone and, to a lesser degree, 11-deoxycorticosterone; corticosterone and cortisol are normally not important as mineralocorticoids unless secreted in excess. Mineralocorticoids have more limited actions than glucocorticoids. Their major function is to maintain intravascular volume by conserving sodium and eliminating potassium and hydrogen ions. They exert these actions in the kidney, gut, and salivary and sweat glands. Aldosterone can have distinct effects in other tissues. Mineralocorticoid receptors are found in the heart and vascular endothelium, and aldosterone increases myocardial fibrosis in heart failure.
Mineralocorticoids have their most important actions in the distal convoluted tubules and cortical collecting ducts of the kidney, where they induce reabsorption of sodium and secretion of potassium. In the medullary collecting duct, they act in a permissive fashion to allow vasopressin to increase osmotic water flux. Thus, patients with mineralocorticoid deficiency can develop weight loss , hypotension , hyponatremia , and hyperkalemia , whereas patients with mineralocorticoid excess can develop hypertension, hypokalemia, and metabolic alkalosis (see Chapters 593-596 ).
The mechanisms by which aldosterone affects sodium excretion are incompletely understood. Most effects of aldosterone are presumably due to changes in gene expression mediated by the mineralocorticoid receptor, and indeed levels of subunits of both the Na+ , K+ -adenosine triphosphatase and the epithelial sodium channel increase in response to aldosterone. Additionally, aldosterone increases expression of the serum and glucocorticoid-regulated kinase , which indirectly reduces turnover of epithelial sodium channel subunits and thus increases the number of open sodium channels.
The mineralocorticoid receptor has similar affinities in vitro for cortisol and aldosterone, yet cortisol is a weak mineralocorticoid in vivo. This discrepancy results from the action of HSD11B2, which converts cortisol to cortisone. Cortisone is not a ligand for the receptor, whereas aldosterone is not a substrate for the enzyme. Pharmacologic inhibition (as occurs with excessive consumption of licorice) or genetic deficiency of this enzyme allows cortisol to occupy renal mineralocorticoid receptors and produce sodium retention and hypertension; the genetic condition is termed apparent mineralocorticoid excess syndrome .
Actions of the Adrenal Androgens
Many actions of adrenal androgens are exerted through their conversion to active androgens or estrogens such as testosterone , dihydrotestosterone , estrone , and estradiol . In males, <2% of the biologically important androgens are derived from adrenal production, whereas in females approximately 50% of androgens are of adrenal origin. The adrenal contribution to circulating estrogen levels is mainly important in pathologic conditions such as feminizing adrenal tumors. Adrenal androgens contribute to the physiologic development of pubic and axillary hair during normal puberty. They also play an important role in the pathophysiology of congenital adrenal hyperplasia , premature adrenarche , adrenal tumors, and Cushing syndrome (see Chapters 594 , 595 , and 597 ).
In humans, circulating levels of DHEA and DHEAS, the chief adrenal androgens, reach a peak in early adulthood and then decline. This has led to speculation that some age-related physiologic changes might be reversed by DHEA administration, and beneficial effects have been suggested (but not proved) on insulin sensitivity, bone mineral density, muscle mass, cardiovascular risk, obesity, cancer risk, autoimmunity, and the central nervous system.
Synthetic Corticosteroids
Many synthetic analogs of cortisone and hydrocortisone are available. Prednisone and prednisolone are derivatives with an additional double bond in ring A. Similar to cortisone, prednisone is not an active steroid but it is converted to prednisolone by HSD11B1 in the liver. Prednisone and prednisolone are 4-5 times as potent in antiinflammatory and carbohydrate activity but have slightly less effect on retention of water and sodium than cortisol. Halogenated derivatives have different effects. Betamethasone and dexamethasone have 25-40 times the glucocorticoid potency of cortisol but have little mineralocorticoid effect. These analogs are usually used in pharmacologic doses for their antiinflammatory or immunosuppressive properties. The antiinflammatory activity of fludrocortisone is about 15 times that of hydrocortisone, but fludrocortisone is more than 125 times as active a mineralocorticoid; it is used to treat aldosterone deficiency.
Bibliography
Baschant U, Lane NE, Tuckermann J. The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat Rev Rheumatol . 2012;8:645–655.
Fuller PJ, Yao Y, Yang J, et al. Mechanisms of ligand specificity of the mineralocorticoid receptor. J Endocrinol . 2012;213:15–24.
Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol . 2010;10:365–376.
Joels M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev . 2012;64:901–938.
Kim HJ. New understanding of glucocorticoid action in bone cells. BMB Rep . 2010;43:524–529.
Kuo T, Harris CA, Wang JC. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol Cell Endocrinol . 2013;380(1–2):79–88.
Reid DM, Devogelaer J-P, Saag K, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. [and for; the HORIZON investigators] Lancet . 2009;373:1253–1263.
Tahrani AA, Bailey CJ, Del PS, et al. Management of type 2 diabetes: new and future developments in treatment. Lancet . 2011;378:182–197.
van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest . 2009;39:81–93.
Vandevyver S, Dejager L, Tuckermann J, et al. New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology . 2013;154:993–1007.
Wendler A, Albrecht C, Wehling M. Nongenomic actions of aldosterone and progesterone revisited. Steroids . 2012;77:1002–1006.
Adrenal Medulla
Perrin C. White
The principal hormones of the adrenal medulla are the physiologically active catecholamines : dopamine, norepinephrine, and epinephrine (Fig. 592.3 ). Catecholamine synthesis also occurs in the brain, in sympathetic nerve endings, and in chromaffin tissue outside the adrenal medulla. Metabolites of catecholamines are excreted in the urine, principally 3-methoxy-4-hydroxymandelic acid, metanephrine , and normetanephrine . Urinary metanephrines and catecholamines are measured to detect pheochromocytomas of the adrenal medulla and sympathetic nervous system (see Chapter 598 ).
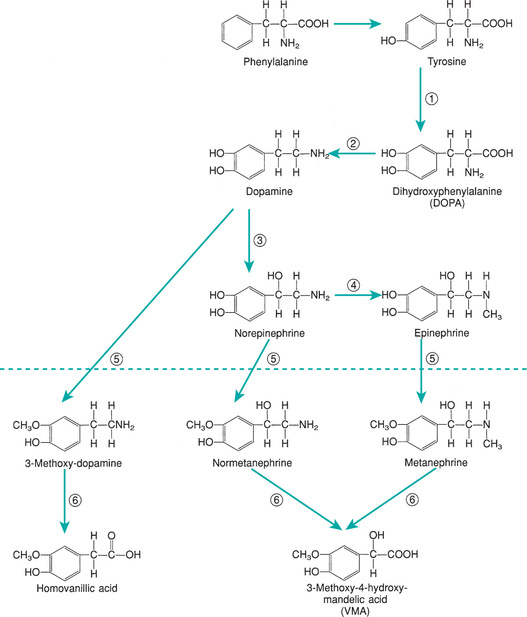
The proportions of epinephrine and norepinephrine in the adrenal gland vary with age. In early fetal stages, there is practically no epinephrine; at birth, norepinephrine remains predominant. However in adults, norepinephrine accounts for only 10–30% of the pressor amines in the medulla.
The effects of catecholamines are mediated through a series of G-protein–coupled adrenergic receptors. Both epinephrine and norepinephrine raise the mean arterial blood pressure, but only epinephrine increases cardiac output. By increasing peripheral vascular resistance, norepinephrine increases systolic and diastolic blood pressures with only a slight reduction in the pulse rate. Epinephrine increases the pulse rate and, by decreasing the peripheral vascular resistance, decreases the diastolic pressure. The hyperglycemic and calorigenic effects of norepinephrine are much less pronounced than are those of epinephrine.
Bibliography
Adams MS, Bronner-Fraser M. Review: the role of neural crest cells in the endocrine system. Endocr Pathol . 2009;20:92–100.
Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev . 2004;56:331–349.
Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul . 2008;42:111–119.