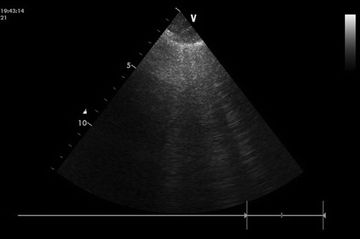
FIGURE 10.1 Three B-lines/comet tails in cardiogenic pulmonary edema. Image courtesy of Dr. Anne-Sophie Beraud, Stanford University Medical Center.
BACKGROUND
No single test can distinguish cardiogenic from non-cardiogenic pulmonary edema. A clear understanding of the physiology of each disease processes, however, enables the clinician to better integrate patient history, exam, and diagnostic tests into a cohesive management strategy. This chapter outlines a systematic approach to the identification of the most common etiologies of acute pulmonary edema. Management details are addressed separately (see Chapters 12 and 14).
ETIOLOGY OF PULMONARY EDEMA
Noncardiogenic pulmonary edema, often referred to as increased permeability pulmonary edema, is caused by an increase in the vascular permeability of the lung—specifically the epithelial barrier—with subsequent movement of protein-rich fluid into the lung intersitium.1 Increased vascular permeability is commonly associated with acute respiratory distress syndrome (ARDS) and can be due to a myriad of pathologies including pneumonia, sepsis, ingestions, and trauma associated with large-volume transfusions. It is also the disease process associated with neurogenic and high-altitude pulmonary edema.
Cardiogenic pulmonary edema results when increased left ventricular end-diastolic and left atrial pressures elevate hydrostatic pressure in the pulmonary capillaries, leading to transmission of protein-poor edema fluid across the lung endothelium and into the alveoli.2 Alveolar edema reduces diffusion capacity, leading to hypoxia and dyspnea. The physiologic stress of the dyspnea results in a catecholamine surge, which produces tachycardia and increased afterload that can, in turn, further augment left-sided pulmonary pressures and exacerbate the edema. Cardiogenic pulmonary edema can result from a variety of pathologies, including acute decompensated heart failure, mitral or aortic valve dysfunction, tachyarrhythmias, and renovascular hypertension.3
CARDIOGENIC PULMONARY EDEMA
Acute decompensated heart failure accounts for more than 650,000 emergency department (ED) visits in the United States per year.4 By itself, acute heart failure is associated with a 5% mortality rate; this number rises to 12% to 15% when the failure produces pulmonary edema.5 Acute decompensated heart failure can result from impaired systolic or diastolic function. Impaired left ventricle (LV) systolic function is associated with coronary artery disease, hypertension, valvular heart disease, viral myocarditis, and dilated cardiomyopathies, as well as with toxins and metabolic disorders such as hypo- and hyperthyroidism. Impaired LV systolic function results in decreased cardiac output, which increases pulmonary capillary pressure and activates the renin–angiotensin–aldosterone system; this, in turn, triggers sodium and fluid retention.6 In diastolic heart failure, the LV becomes less compliant, leading to coronary ischemia (since the coronary arteries fill during diastole), arrhythmias (especially atrial fibrillation), reduced ventricular filling, and increased end-diastolic pressure.
Valvular abnormalities, particularly stenosis of the mitral and aortic valves, are common culprits in acute cardiogenic pulmonary edema. Mitral stenosis, a known complication of rheumatic heart disease, causes an atrial obstruction that leads to pulmonary capillary congestion. Although mitral stenosis generally develops in a chronic manner, stresses such as tachycardia and decreased diastolic filling can lead to acute increases in hydrostatic pressure. Aortic valve stenosis limits LV outflow, resulting in a similar upstream increase in hydrostatic pressure in the pulmonary capillaries.7
Renal artery stenosis is a less common etiology for cardiogenic pulmonary edema. It causes long-standing hypertension, leading to diastolic dysfunction as well as chronic physiologic activation of the renin–angiotensin system with resulting increases in sodium and water retention.8
Of the tachyarrhythmias associated with acute pulmonary edema, atrial fibrillation is the most common. In a study of more than 200 consecutive elderly patients presenting with acute cardiogenic pulmonary edema, approximately 36% had an arrhythmia; 24% were in rapid atrial fibrillation, causing an elevation in end-diastolic pressure and subsequent drop in cardiac output.9 Ventricular arrhythmias can also account for acute cardiogenic pulmonary edema, especially when associated with myocardial ischemia.
NONCARDIOGENIC PULMONARY EDEMA
The functional definition of noncardiogenic edema is the presence of increased vascular permeability, resulting in protein-rich fluid leaking into the pulmonary interstitium and air spaces.1 It is most commonly associated with ARDS, defined as acute bilateral pulmonary edema in the absence of heart failure or other causes of hydrostatic edema.10 With the advent of a therapeutic strategy for ARDS—namely, low tidal volume ventilation—prompt recognition and treatment of this condition are essential.11 A predisposing risk factor is not a diagnostic criterion for ARDS; in fact, 20% of diagnosed cases of ARDS have no identifiable risk factor.12 The most commonly associated conditions are trauma and sepsis;12 others include massive transfusion, aspiration, inhalation injury, and pancreatitis.
Other etiologies of noncardiogenic pulmonary edema likely to be encountered by the emergency physician include neurogenic edema, opiate toxicity, and high-altitude pulmonary edema.13–15 Neurogenic pulmonary edema can be a consequence of seizures, blunt or penetrating head injuries, and cerebral, especially subarachnoid, hemorrhage. Treatment of neurogenic, nonhydrostatic pulmonary edema—thought to be due to catecholamine excess—consists of supportive treatment and management of the underlying brain injury. Opiate toxicity—from narcotics including street drugs (e.g., heroin), hospital-prescribed methadone, intravenous narcotics (e.g., a bolus of fentanyl), and even the narcotic antagonist naloxone—can also precipitate noncardiogenic pulmonary edema; the exact mechanism in this process is, however, unclear. Lastly, high-altitude pulmonary edema can result from rapid ascent to high altitudes. In this case, profound hypoxia leads to pulmonary vasoconstriction, causing capillary leak permeability edema; the primary treatment strategy is descent and supplemental oxygen (see Chapter 54).
DIAGNOSTIC EVALUATION
The diagnosis of pulmonary edema is achieved via patient history and physical exam, chest radiograph, ultrasound, chemistries, and biomarker tests. A cardiogenic etiology is suggested by a history of hypertension, heart failure, aortic or mitral valve disease, or coronary artery disease or its accompanying disease states (e.g., diabetes, hyperlipidemia, obesity). A patient with this history may exhibit findings suggestive of elevated left ventricular end-diastolic pressure, including an S3 gallop, which has a high specificity (90% to 97%) but low sensitivity (9% to 51%) for a reduced ejection fraction.1,16 In addition, the patient may have cool extremities and preferential vasoconstriction resulting from compromised cardiac output. Other physical signs are less reliable indicators of etiology, as they can result from multiple noncardiogenic processes. For example, lower extremity edema can be due to chronic kidney or liver disease, and findings of inspiratory crackles and rhonchi on examination of the lung—while consistent with the finding of pulmonary edema—can also result from aspiration of gastric contents, sepsis, trauma, or recent blood transfusion.
Imaging modalities are useful in the workup of acute pulmonary edema, but often cannot be relied upon to establish etiology. The chest radiograph, almost universally used in the initial workup of dyspnea, can reveal findings highly specific for pulmonary edema—such as the characteristic patterns of cephalization (by which upper lobe vessels are recruited to carry more blood when lower lobe vessels are compressed by increased hydrostatic pressure), interstitial edema, and alveolar edema. However, these findings cannot be used to establish etiology.3 Importantly, in almost 20% of patients with clinically significant heart failure, chest radiograph will show no evidence of pulmonary edema; this is likely because lung fluid must increase 30% before becoming evident radiographically.3,17 Vascular pedicle width (VPW) has also been used to help distinguish between cardiogenic and noncardiogenic causes of pulmonary edema, but its sensitivity and specificity (71% and 66%, respectively) are inadequate for independent use.18 The electrocardiogram (EKG) can also be useful in the initial workup; patients with clinically significant heart failure from various etiologies rarely present with a normal EKG. EKG findings commonly associated with heart failure, as discussed above, include tachycardia (the natural response of the heart to preserve cardiac output in the setting of impaired stroke volume), arrhythmias such as atrial fibrillation, and myocardial infarctions. Finally, a pulmonary artery wedge pressure of ≤18 mmHg—measured using a pulmonary artery catheter (PAC)—has traditionally been part of the definition of noncardiogenic pulmonary edema. Wedge pressure has since been disproved as a useful marker and has fallen out of favor as diagnostic tools less invasive than the PAC have become available.19
In the past decade, bedside, or point-of-care, ultrasound has increasingly been used to evaluate the lungs for various insults, including pulmonary edema, pneumothorax, and pleural effusion. The finding of at least three to six bilateral “B-lines” (vertical lines that extend from the pleural surface and obliterate the A-lines that occur horizontally as reflections of the pleura) has been demonstrated to be up to 95% specific for pulmonary edema and correlates with the radiographic finding of alveolar–interstitial syndrome—a condition most commonly associated with cardiogenic pulmonary edema20–22 (Fig. 10.1). Some caution is advised in the interpretation of these findings, however, as B-lines in clusters can be found in dependent lung zones in up to 28% of normal patients and may be limited or absent in patients with milder forms of pulmonary edema.21–23 Bedside ultrasound to evaluate cardiac function and assess intravascular volume is also increasingly used by the critical care community.24 The combination of LV and right ventricle (RV) assessment, when coupled with an assessment of inferior vena cava size and respiratory variation, can help include or exclude volume overload and heart failure as a likely cause of the pulmonary edema (see Chapters 6 and 7).

FIGURE 10.1 Three B-lines/comet tails in cardiogenic pulmonary edema. Image courtesy of Dr. Anne-Sophie Beraud, Stanford University Medical Center.
Biomarkers, such as B-type natriuretic peptide (BNP), are also useful in determining the etiology of acute pulmonary edema and can help prompt early implementation of targeted interventions, such as diuretics and vasodilators for cardiogenic edema or lung-protective ventilation strategy for nonhydrostatic ARDS.25 A recent study evaluated the utility of BNP in distinguishing cardiogenic from noncardiogenic pulmonary edema and found that a BNP level of 100 pg/mL or less was highly specific for noncardiogenic pulmonary edema in ED patients, with a negative predictive value (NPV) for heart failure of >90%.26 Similarly, a BNP level >500 pg/mL was strongly suggestive of heart failure ([positive predictive value] PPV > 90%).27
Direct analysis of pleural fluid using thoracentesis, although unlikely to be used in a busy ED, is another classic test for distinguishing cardiogenic and noncardiogenic etiology.28 A pleural fluid/serum protein concentration ratio >0.65 has been shown to be over 80% sensitive and specific for noncardiogenic edema in intubated patients being evaluated for ARDS.
CONCLUSION
There is no one test that determines the cause or detects the presence of acute pulmonary edema. A detailed history and physical exam followed by diagnostic testing, including chest radiography, BNP, and ultrasound (cardiac and lung), can help differentiate the cause of acute edema and guide appropriate treatment.
LITERATURE TABLE

CI, confidence interval; NPV, negative predictive value; OR, odds ratio.
1.Ware LB, Matthay MA. Acute pulmonary edema. N Engl J Med. 2005;353:2788–2796.
2.Staub NC. Pulmonary edema. Physiol Rev. 1974;54:678–811.
3.Collins S, Storrow AB, Kirk JD, et al. Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department. Ann Emerg Med. 2008;51(1):45–57.
4.Schappert S, Rechtsteiner E. Ambulatory medical care utilization estimates for 2006. Natl Health Stat Report. 2008;(8):1–29.
5.Mac Sweeney R, McAuley DF, Matthay MA. Acute lung failure. Semin Respir Crit Care Med. 2011;32:607–625.
6.Francis GS, Goldsmith SR, Levine TB, et al. The neurohumoral axis in congestive heart failure. Ann Intern Med. 1984;101(3):370–377.
7.Gandhi SK, Powers JC, Nomeir AM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17–22.
8.Pickering TG. Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis. Lancet. 1988;2(8610):551.
9.Bentancur AG, Rieck J, Koldanov R, et al. Acute pulmonary edema in the emergency department: clinical and echocardiographic survey in an aged population. Am J Med Sci. 2002;323(5):238–243.
10.Ranieri VM, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533.
11.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308.
12.Hudson LD, Milberg JA, Anardi D, et al. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301.
13.Muroi C, Keller M, Pangalu A, et al. Neurogenic pulmonary edema in patients with subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2008;20(3):188.
14.Sporer KA, Dorn E. Heroin-related noncardiogenic pulmonary edema: a case series. Chest. 2001;120(5): 1628.
15.Stream JO, Grissom CK. Update on high-altitude pulmonary edema: pathogenesis, prevention, and treatment. Wilderness Environ Med. 2008;19(4):293–303.
16.Marcus GM, Gerber IL, McKeown BH, et al. Association between phonocardiographic third and fourth heart sounds and objective measures of left ventricular function. JAMA. 2005;293(18):2238–2244.
17.Knudsen CW, Omland T, Clopton P, et al. Diagnostic value of B-type natriuretic peptide and chest radiographic findings in patients with acute dyspnea. Am J Med. 2004;116:363–368.
18.Rice TW, Ware LB, Haponik EF, et al. Vascular pedicle width in acute lung injury: correlation with intravascular pressures and ability to discriminate fluid status. Crit Care. 2011;15(2):R86.
19.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824.
20.Turner JP, Dankoff J. Thoracic ultrasound. Emerg Med Clin North Am. 2012;30(2):451–473.
21.Lichtenstein D, Meziere G, Biderman P, et al. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156(5):1640–1646.
22.Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24(6):689–696.
23.Volpicelli G, Caramello V, Cardinale L, et al. Detection of sonographic B-lines in patients with normal lung or radiographic alveolar consolidation. Med Sci Monit. 2008;14(3):CR122–CR128.
24.Duane PG, Colice GL. Impact of noninvasive studies to distinguish volume overload from ARDS in acutely ill patients with pulmonary edema: analysis of the medical literature from 1966 to 1998. Chest. 2000;118:1709–1717.
25.Noveanu M, Mebazaa A, Mueller C. Cardiovascular biomarkers in the ICU. Curr Opin Crit Care. 2009;15:377–383.
26.Levitt JE, Vinayak AG, Gehlbach BK, et al. Diagnostic utility of B-type natriuretic peptide in critically ill patients with pulmonary edema: a prospective cohort study. Crit Care. 2008;12:R3.
27.Silver MA, Maisel A, Yancy CW, et al. BNP consensus panel 2004: a clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases. Congest Heart Fail. 2004;10(Suppl 3):1–30.
28.Ware LB, Fremont RD, Bastarache JA, et al. Determining the aetiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J. 2010;35(2):331–337.