Patrick Charlton and Frank Vriesekoop
CONTENTS
19.3.1 Production of Spent Brewer’s Grain for Animal Feed Purposes
19.3.1.1 Brewer's Grain as an Animal Feedstuff
19.3.1.2 Feeding Quality for Production Animals
19.3.1.3 Opportunities for the Animal Feed Industry
19.3.1.4 Brewer's Grain in Human Food
19.4.1 Brewer’s Yeast Use in Animal Nutrition
19.4.1.3 Practical Uses of Brewer’s Yeast and Its Components
19.5 Brewer’s Yeast in Food Applications
19.5.1 Brewer’s Yeast as a Fat Replacer
19.5.2 Brewer’s Yeast as Glucose Tolerance Factor
19.5.3 Brewer’s Yeast as a Source of Minerals
19.6 Brewer’s Yeast in Health-Related Applications
19.6.1 Brewer’s Yeast as an Antidepressant
19.6.2 Brewer’s Yeast in Wound Healing
19.6.3 Brewer’s Yeast as an Immunomodulator
19.1 INTRODUCTION
Since beer was first produced, the single-minded focus of the brewer has been on the quality, taste, and stability of the beer. The by-products of brewing were of no consequence. However, today with the advent of the “super” breweries, in conjunction with reduced availability of certain feed commodities, the economic recognition of value in hitherto labeled waste streams, and an increase in environmental responsibility with those same waste streams, we have seen an explosive technical development in the treatment and application of brewery by-product and effluent streams for use in animal feed products and, more recently, in human foods. Efficiencies in the brewing process have undoubtedly reduced the volumes of by-product produced, but with today’s larger breweries, the need to process and move by-products out of the brewery quickly and for an optimum price have become significant within brewing logistics and financial performance.
This chapter will review the various sources of by-products throughout the brewing process and their application in both the animal and human food chains, including future technologies to add value to some of these streams. This chapter has been updated based on the chapter in the previous edition of this book written by Nick J. Huige,1 and the authors wish to acknowledge and thank him.
19.2 MALT CULMS
Some breweries today still malt their own barley for beer production. However, much of the malting industry is inherently associated with the brewing industry, and we will include waste streams from the malting industry in this chapter.
Resulting from the core process, in addition to the malted barley, there are several streams of coproducts to the malting process, which are then sold to the animal feed industry for use in complete feeds or sold as straight commodities. This therefore provides the first by-product stream associated with the brewing process.
In the malting process, the grains produce rootlets, which are separated from the malt after kilning. Malt culms contain high levels of protein (up to 25% crude protein) making them a valuable ingredient for animal feed. Culms are low in bulk density as a loose product and so are usually pelletized in a blend with other by-product streams. These streams suitable for mixing with the culms are small malt corns plus barley dust and malt dust. These are combined for production of a pelleted product with medium bulk density (600 kg/m3) and a protein content of approximately 18%. This product is marketed as malt residual pellets.2
It is important to be aware that these by-products may have a higher mycotoxin level than the parent bulks of grain and malt due to the higher concentration of husks and outer parts of the grain, where most mycotoxin contamination occurs. This should be taken into consideration when deciding feeding strategies for these by-products.
The natural process of malting causes metabolism of the barley storage protein hordein into hordenine. In some animals, including horses, hordenine indirectly acts as an adrenergic drug by releasing stored norepinephrine. As such, in hordenine-sensitive animals, it has a positive inotropic effect upon the heart, which increases systolic and diastolic blood pressure, and peripheral blood flow volume.3, 4 Hordenine is therefore classified as a naturally occurring prohibited substance (NOPS) within the equine industry. As such, feeding of any by-products that have been formulated with malt culms should be avoided in equine diets.3
Although relatively small in comparison to other by-product streams, the malting industry is still a significant contributor to the brewing industry by-products. In the United Kingdom, where maltsters process in excess of 2 million metric tons of barley every year, the by-product streams from this account for more than 50,000 metric tons of material entering the animal feed industry.
19.3 SPENT BREWER’S GRAINS
Spent brewer’s grains are a common brewery by-product that is high in protein (more than 20%) and fiber and can be used as supplements for animal feed, replacing other, more expensive feed materials within diet formulations or—in some instances—as foods for human consumption. With regard to animal feed, brewer’s grains may be wet or dry in their final form, with the wet grains typically being sold as a cake for ruminant feeds and the dry grains for monogastrics.
19.3.1 Production of Spent Brewer’s Grain for Animal Feed Purposes
After wort extraction is completed during the mashing process, the remaining grain solids are discharged to a holding vessel and form the brewer’s grain portion used in animal feed. The next process step depends on how the brewer’s grain is sold: wet (BWG), dry (BDG), or partially dewatered (BPG). Wet brewer’s grain comprises between 77% to 81% moisture but typically varies considerably. Other by-products, including grain dust, spent hops, or dead yeast may be blended with BWG, although the moisture content should not exceed 81% to prevent free liquor formation and impairment of the consistency of the final feed material. Due to the high moisture content, the BWG by-product should be immediately transported to the farm for animal feeding purposes. Its high moisture, especially in warmer climates, will encourage the growth of molds, which are typically already present on the grain following harvesting due to field contamination, and the conditions within the by-product will promote their proliferation and the potential for toxin production, which can be harmful to animals and certainly reduce productive performance.
BWG can be partially dewatered and sold as brewer's pressed grain (BPG). If brewer's grain is sold as a dried product (BDG), the wet grain is almost always dewatered prior to drying to reduce costs. Dewatering is accomplished mechanically by means of centrifugation or using screw or roller presses. In general, BWG with a moisture content of 78% to 80% can be dewatered to 63% to 72%. On average, dewatered brewer's grain has 67% moisture.1
Dewatering is generally more difficult when corn grits or excessive amounts of fines are present. The latter (i.e., particles in the range of 0.2 to 0.8 µm) causes dough formation in the by-product and changes in the texture (due to the presence of hordein-type proteins) of the final product that inhibit dewatering.
As seen from the previous discussion, dry matter levels within the brewer’s grain vary depending on processing methods applied. The development of mash filters in the late 1970s allowed for better extraction efficiencies because of smaller particle sizes in the mash and better dewatering, which resulted in a cake of only 50% to 55% moisture.5 The reduced water content of the spent grain from a mash filter has better handling and stability compared to a traditional mash/lauter approach. This allows the manufacturer to dry the brewer’s grain without postmashing dewatering, making it suitable for inclusion into monogastric formulations (i.e., pigs and poultry).
Drying BWG is energy expensive due to the amount of steam generation needed to evaporate the high levels of moisture. Drying is practiced less in European breweries compared to the United States, where energy is often more expensive. Modern dryers use steam to heat rotating disks in a fixed drum dryer, which allow drying at lower costs for producers.6–8
The main problem in operating spent grain dryers is proper moisture control. Where moisture exceeds 14%, the risk of mold growth is always present, leading to hot spots during storage. In addition, overheating can change the chemical bonds within the nutrients, potentially rendering them less nutritionally available or even resistant to digestion in the animal. Linton9 suggested that less than 75% moisture in BWG can make the end product difficult to compact and might lead to oxidative degradation during storage. According to Linton,9 at moisture levels above 80%, free water loss occurs, making storage and handling more difficult. With this in mind, Penrose10 concluded that the ideal moisture for DWG was 73% and proposed to dewater brewer's grain to 69% moisture and then add back slurries, such as surplus yeast, to achieve a final moisture of 73%.
BPG has a moisture level of 65% to 70%, is more palatable, and is easier to handle and store. Due to its lower water levels, freight costs are reduced, making the end product more attractive for on-farm use, although the risk of mycotoxins from mold growth still remain and storage must be carefully considered, especially in warmer weather. Long-term storage of BWG as silage can be feasible,9 but as always, proper harvesting and wrapping is required for this to be successful. The use of modern “gas exchange” silage wraps would be useful in preserving this feedstuff. BWG silage can be used as part of a total mixed ration on farms (TMR) with other materials. A specialty silage product called Maltlage was developed in the United States by Hunt and Spitzer,11 which is a product with about 50% dry matter and is prepared by anaerobic fermentation of a mixture of roughage, vitamins, and minerals to provide essential elements and to buffer the mixture, so that the pH is maintained at 4 to 4.5.
19.3.1.1 Brewer's Grain as an Animal Feedstuff
BWG and BDG are generally considered protein feeds as they consist of greater than 20% crude protein.12 Reviews have reported that brewer’s grains are high in fiber, containing around 17% cellulose, 28% noncellulose polysaccharides, and 28% lignin. This may be useful in ruminant and horse feeds as these animals have a large requirement for fiber in their digestive processes.12 In monogastrics, the high levels of arabinoxylan in the noncellulose components may warrant enzyme addition for optimizing digestion.
Table 19.1 shows the nutritional composition of BWG and BDG products. Energy for ruminants is very similar among the by-products, although it is higher for BDG for monogastrics (poultry).
Table 19.1 Proximate Analysis of Brewery Wet and Dry Grain By-Products (All Values Except Dry Matter Are Shown on a Dry Matter Basis, Energy Expressed as Mcal/kg)
Parameter |
Brewer’s Dry Grains |
Brewer’s Wet Grains |
|---|---|---|
Dry matter (%) |
92 |
23 |
Crude protein (%) |
28 |
27 |
EE (%) |
7.2 |
6.5 |
Ash (%) |
4.0 |
4.8 |
CF (%) |
15 |
15 |
NFE (%) |
45.8 |
46.7 |
DE (cattle) |
3.3 |
3.3 |
NE (lactation) (cattle) |
1.7 |
1.7 |
NE (maintenance) (cattle) |
1.7 |
1.8 |
NE (growth) (cattle) |
1.1 |
1.1 |
ME (poultry) |
2.5 |
2.3 |
Source: Huige, N.J., Brewery by-products and effluents, in Handbook of Brewing, Priest, F.G. and Stewart, G.G., Eds., 2nd ed., CRC Press, Boca Raton, FL, 2006. With permission.
Note: CF, crude fiber; DE, digestible energy; EE, ether extract; ME, metabolizable energy; NE, net energy; NFE, nitrogen free extract.
Crude protein is considered a vague measure of the usefulness of the feed as it is the balance and amounts of amino acids that make up the protein portion that are most important when considering using a feedstuff in a complete formulation. Table 19.2 shows how the amino acid levels in BDG compare to other protein-rich feedstuffs that are available for animal feeds. Soya bean meal is held to be the “gold standard” in animal protein meals. When compared to BDG, absence or lack of various amino acids are easily found, and these would have to be balanced by the addition of synthetic amino acids, which would be part of the economic decision as to whether to use brewer’s grains or not. Some amino acids are present in higher amounts and, as the balance of amino acids is an important consideration in production animals, this would also have to be addressed via the balancer premix utilized within the final complete diet. In particular, brewer's grain is limited in the essential sulfur amino acid methionine and the first limiting amino acid.13, 14
Table 19.2 Levels of Amino Acids in Brewer’s Grains Versus Other Protein Meals Used in Animal Feed
Amino Acid |
Brewer’s Dry Grains |
Brewer’s Yeast |
Distillers Dry Grains + Solubles |
Corn Gluten Meal |
Soya Bean Meal |
|---|---|---|---|---|---|
Alanine |
NA |
NA |
NA |
NA |
5.3 |
Arginine |
5.1 |
4.9 |
3.8 |
3.5 |
7.4 |
Aspartic acid |
NA |
NA |
NA |
NA |
14.0 |
Cysteine |
1.2 |
1.1 |
1.3 |
1.8 |
1.5 |
Glutamic acid |
NA |
NA |
20.5 |
21.3 |
19.9 |
Glycine |
4.4 |
3.8 |
2.8 |
3.8 |
5.1 |
Histidine |
2.3 |
2.4 |
2.4 |
2.5 |
2.6 |
Isoleucine |
5.6 |
4.7 |
5.5 |
5.4 |
5.3 |
Leucine |
9.8 |
7.2 |
8.5 |
18.0 |
8.2 |
Lysine |
3.6 |
7.0 |
2.6 |
2.0 |
6.5 |
Methionine |
1.8 |
1.6 |
1.8 |
2.5 |
1.4 |
Phenylalanine |
5.6 |
4.0 |
6.0 |
7.2 |
5.3 |
Proline |
4.4 |
NA |
10.3 |
8.8 |
6.0 |
Serine |
5.2 |
NA |
5.0 |
4.0 |
5.4 |
Threonine |
3.9 |
4.7 |
3.5 |
3.6 |
4.1 |
Tryptophan |
1.4 |
1.1 |
0.7 |
0.5 |
1.3 |
Tyrosine |
4.5 |
6.3 |
2.4 |
3.0 |
3.2 |
Valine |
6.4 |
5.2 |
5.6 |
5.3 |
5.3 |
Source: Huige, N.J., Brewery by-products and effluents, in Handbook of Brewing, Priest, F.G. and Stewart, G.G., Eds., 2nd ed., CRC Press, Boca Raton, FL, 2006. With Permission.
The relative levels of minerals are shown in Table 19.3, which compares brewer’s grains against other by-products used in animal feeds. Certain minerals are higher in the brewing by-products compared to soya bean meal. This can be useful in formulating diets but would require balancing via the premix used in the feed.
Table 19.3 Comparison of Mineral Composition of Brewery By-Products and Other Protein Feeds
Mineral Levels (as is basis) |
Brewer’s Dry Grains |
Distiller’s Dry Grains |
Soya Bean Meal |
|---|---|---|---|
Calcium (%) |
0.26 |
0.17 |
0.28 |
Chlorine (%) |
0.15 |
0.17 |
0.03 |
Cobalt (ppm) |
0.06 |
0.11 |
0.09 |
Copper (ppm) |
21.0 |
59.0 |
17.0 |
Iron (%) |
0.03 |
0.03 |
0.01 |
Magnesium (%) |
0.15 |
0.21 |
0.27 |
Manganese (ppm) |
37.0 |
29.0 |
27.0 |
Phosphorus (%) |
0.54 |
0.84 |
0.63 |
Potassium (%) |
0.09 |
0.65 |
1.91 |
Selenium (ppm) |
0.70 |
0.38 |
0.10 |
Sodium (%) |
0.24 |
0.04 |
0.18 |
Sulfur (%) |
0.30 |
0.30 |
00.43 |
Source: Huige, N.J., Brewery by-products and effluents, in Handbook of Brewing, Priest, F.G. and Stewart, G.G., Eds., 2nd ed., CRC Press, Boca Raton, FL, 2006. With permission.
Table 19.4 shows the comparison among vitamin levels in brewer’s dry grains and other brewery by-products and soya bean meal. Levels in BDG are considerably lower than the other protein meals, which, as for the aforementioned nutrients, would be rectified during the diet formulation process.
Table 19.4 Vitamin Content of Brewery By-Products and Other Protein Feed (as is Basis)
Vitamin Levels (ppm) |
Brewer’s Dry Grain |
Distiller’s Dry Grains |
Soya Bean Meal |
|---|---|---|---|
Biotin |
0.1 |
0.6 |
0.3 |
Choline |
1,800 |
3,000 |
2,800 |
Folic acid |
0.2 |
0.9 |
0.6 |
Niacin |
44 |
77 |
40 |
Pantothenic acid |
8.5 |
13 |
15 |
Riboflavin |
1.5 |
9.5 |
3.0 |
Thiamine |
0.7 |
3.2 |
4.0 |
Vitamin B6 |
0.7 |
4.6 |
6.0 |
Source: Huige, N.J., Brewery by-products and effluents, in Handbook of Brewing, Priest, F.G. and Stewart, G.G., Eds., 2nd ed., CRC Press, Boca Raton, FL, 2006. With permission.
The nutrient levels listed in the previous tables are averages, although these may vary widely depending upon the source plant materials—especially the level of hops—used during brewing and the processing of the by-product material before it is used in animal feed. Fiber levels can be higher compared to other protein rich feed materials, which is useful in certain animals, such as ruminants and adult pigs, but not for poultry or growing pigs.
19.3.1.2 Feeding Quality for Production Animals
For monogastric animals, fiber may affect the upper digestive tract as it increases transit time, allowing a longer period of the digesta to remain in situ allowing simple sugars, vitamins, and minerals to be adsorbed at higher efficiency. However, under certain circumstances, the presence of increased levels of fiber and an unbalanced amino acid profile can cause issues in digestion and productive performance, respectively.
Research on feeding brewer’s grains to poultry was mainly conducted in the 1970s,15–21 and there is a need for more research using modern feeding strategies and strains of birds as these have changed in the intervening 40 years, with broilers especially becoming larger and more feed efficient over time. However, that being said, the trials conducted on poultry to examine the usefulness of brewer’s grains as a feedstuff within a complete diet formulation show interesting benefits in performance.
In a trial with laying hens, 20% of the diet consisted of BDG in either pelleted or mash feeds and was compared against a commercial control feed that contained no brewing by-products.15 They found that feeding BDG did not change laying performance, although adding lysine numerically increased the rate of production, demonstrating the dilutive effect of this feedstuff and its potential importance regarding balancing amino acids in the feed when BDG is used. No changes in other parameters, including fertility, egg weight, hen body weight, shell quality, or feed consumption were reported. Additional benefits were seen due to the presence of extra selenium from the BDG, in terms of reduced leg abnormality and less occurrence of fatty liver syndrome—both of which pose major economic problems for producers. The feed intake data from this trial were important as it was assumed that the energy of the diet was reduced by adding BDG by 10% to 1,760 kcal/kg, which would be expected to raise consumption to meet requirements. The authors concluded that the metabolic energy values were therefore underestimated for BDG.15 Internal egg quality measurements (Haugh units) were improved with the addition of the by-product. These findings were confirmed by Damron et al.,16 who also reported improved internal egg quality parameters when using up to 10% BDG in feed, with no loss in performance parameters.
Other trials conducted around the same time included brewer’s grains at levels of 10% in the feed.17 This research found that 5% BDG increases egg production significantly but may reduce hen body weight. Their recommendation was a maximum inclusion rate of 10% BDG. Slightly later published work concluded that up to 20% BDG could be used in laying hen diets without compromising laying performance.18 Other trials using 20% BDG in chicken feed decreased low-density lipoprotein cholesterol by 23%,22 which may be important for human consumers in terms of egg nutritional quality. Trials with turkeys showed that BDG could be included in up to 40% of the diet, and increases in egg production and hatchability were noted in turkey breeders receiving this feed.20 However, in work on growing turkeys, this limit was revised to 20%, and the comment was made that added lysine was required to support growth; 15% appeared to be the maximum replacement value without affecting feed conversion.21 For growing meat chickens, Ademosun19 fed broilers until they were eight weeks of age on diets containing differing levels of brewer’s grains and monitored their feed consumption, weight gain, and efficiency of feed conversion. Based on these findings, the author recommended that only 10% of the diet should comprise this feedstuff, due to the limiting effects of the higher fiber. Later trials fed increasing levels of BDG (up to 20% of the diet) to growing broilers and compared their performance to those receiving palm kernel meal or maize offal at the same levels.23 Compared to a control containing no by-products, BDG had the least impact on growth performance, and again their conclusion was that BDG should be limited to a maximum inclusion rate of 10% in feed.
In pig research, trials using increasing levels of BDG have been conducted. Trials reported by Yaakugh et al.,24 used Landrace × Large White pigs during the growing period. They were fed diets containing graded substitutions of BDG for maize in the feed, up to a replacement level of 30%. There was a linear increase in time needed to attain slaughter weight with increasing levels of BDG in the diet and a decrease in feed intake, although the feed conversion ratio was not significantly affected. As every extra day for pig production is a major cost for producers, this is an important consideration when evaluating the cost savings in formulations by using BDG. When carcass characteristics were measured, significant benefits in percentage ham (quadratic improvements with increasing BDG) were observed, alongside a numeric decrease in back fat—both important economic factors in pig production. No other parameters were affected. More recent work with pigs has discussed the potential for using brewer’s grains in their diet and the usefulness of adding specific xylanase enzymes to help digest the fiber portion of the by-product, releasing more energy and nutrients for the animal.25
In ruminants, the presence of higher levels of fiber can be beneficial, especially when included alongside higher protein levels. The fiber acts as an energy source for the animal, while the characteristics of the protein in brewer’s grains allow it to by-pass ruminal degradation, becoming more available for the animal. Due to this, the by-pass protein value for BDG is rated 1.8 times higher compared to soya bean meal. As a result, BDG can offer the same nutritional value when formulated with maize and urea as higher priced feeds containing soya bean meal as the main protein constituent. In addition, the extra fiber contained in the by-product assists in maintaining correct rumen function, which is reliant on adequate fiber intake for optimal fermentation of feed. This has been linked to reduced rumenitis and liver abscesses, which can be detrimental to growth,26, 27 and has also been observed to reduce skin abnormalities in ruminants.28 However, processing brewer’s grains must be conducted in an appropriate manner to maximize their benefit in ruminant animals as the dry version may have a lower feeding value, partly due to palatability and intake issues.29
Table 19.5 shows how BDG can be incorporated into growing beef animal diets without loss of performance.
Table 19.5 Combinations of Equal Feed Value for Growing Beef Cattle
Feedstuff |
Price US$/t |
Soya Bean Meal (lb) |
Brewer’s Dried Grains (lb) |
|---|---|---|---|
Soya bean meal |
160 |
2,000 |
|
Brewer’s dry grains |
70 |
|
1,780 |
Distiller’s dry grains |
116 |
|
|
Corn |
74 |
|
404 |
Urea |
225 |
|
141 |
Cost/ton |
|
$160 |
$93.11 |
Source: Huige, N.J., Brewery by-products and effluents, in Handbook of Brewing, Priest, F.G. and Stewart, G.G., Eds., 2nd ed., CRC Press, Boca Raton, FL, 2006. With permission.
In dairy cows, research has been conducted on the impact of feeding BDG or BWG in rations compared to feeds containing soya bean meal and wheat bran mixtures.30 Milk yield was unaffected by this substitution, although BWG had a better digestibility and was more efficiently used, possibly due to increased solubility and the superior amino acid profile in this product. This again highlights the importance of the form of brewer’s grains for ruminants. Later research showed that spent grains can enhance milk production without influencing fertility,31, 32 which implies a direct nutritional enhancement without affecting the reproductive physiology.
Horses are classed as monogastrics but, in common with ruminants, they have a major requirement for fiber in their diet to maintain hindgut fermentation capacity, from whence they derive the majority of their energy. Trials with BDG by Ott and Richardson33 showed it to be a good feedstuff for inclusion in adult equine rations as energy availability matched or exceeded that of oats when fed at levels of up to 40% in pelleted feeds. Protein digestion was on a par with an oat and soya bean meal mixture. However, in young horses, additional amino acids would be required to maintain correct growth.
19.3.1.3 Opportunities for the Animal Feed Industry
Brewer’s grains certainly offer an attractive alternative as a feedstuff, especially compared to more costly materials such as soya bean meal. Its fiber content and amino acid profile should be considered when substituting it for other ingredients to ensure that nutrient delivery is not compromised. The addition of specific feed enzymes should be part of the strategy of feeding brewer’s grain by-products, especially in monogastrics, to ensure energy levels are not diluted, which would affect growth performance and feed efficiency. In a similar manner, the first limiting amino acid lysine and essential sulfur amino acids must be balanced to ensure no loss in growth rates or interference with liver metabolism. In animals that require fiber intake for the production of energy from fermentation, the data regarding the use of brewer’s grain by-products show that it can have benefits in the feed in terms of digestibility.
Due to the lower costs of brewer’s grain by-products, economic benefits for meat and egg producers can be realized when they are included in complete rations and diets, as long as maximum permitted levels—as deduced from research trials—are followed in order to prevent performance losses.
19.3.1.4 Brewer's Grain in Human Food
The nutritional and functional properties of spent grains have very similar applications in human food as they do in animal feed.34, 35 There have been some relatively small-scale applications of where spent brewer’s grain has been incorporated into high-fiber breads, cookies, and brownies. However, these have not been adopted in large-scale food production for human consumption. The current limitations for the inclusion of spent brewer’s grain in foods appear to be due to undesirable sensory properties of the resultant foods. For instance, when dried, spent brewer’s grain is brownish in color; this limits its application to colored products and the inclusion into traditional grain products (bread, pasta, etc.) due to flavor and textural problems.12 However, some of these potentially negative influences of spent grain have been overcome by an initial lactic acid fermentation of the spent grain before its incorporation into food.36 Despite the relatively poor uptake of spent brewer’s grain into food formulation, it remains a promising additive for future large-scale utilization with the following functional properties of spent brewer's grain flour in foods:
(after Huige1)
Because of these functional properties, spent brewer’s grain flours have tremendous potential to be used in a wide range of products (Table 19.6).
Table 19.6 Potential Use of Spent Brewer’s Grain Products in Food Formulations
High Fiber Fraction |
High Protein Fraction |
||
|---|---|---|---|
Product Usage |
Level of Use |
Product Usage |
Level of Use |
Breakfast cereal flakes |
25–30% |
Cookies and brownies |
Up to 30% |
Granola bars |
20–25% |
Snacks |
Up to 20% |
Muffins |
15–20% |
Breakfast cereals |
Up to 20% |
Specialty breads/rolls |
Up to 15% |
Pasta |
Up to 15% |
Bagels |
Up to 15% |
Pancakes and waffles |
Up to 15% |
Pizza crust |
Up to 15% |
Doughnuts |
Up to 15% |
Pasta |
Up to 10% |
Pastries |
Up to 10% |
Crackers |
Up to 10% |
Breading and batters |
2–5% |
Comminuted meats |
2–5% |
Meat extender |
2–5% |
Nutritious beverages |
2–5% |
Nutritious beverages |
2–5% |
Source: Huige, N.J., Brewery by-products and effluents, in Handbook of Brewing, Priest, F.G. and Stewart, G.G., Eds., 2nd ed., CRC Press, Boca Raton, FL, 2006. With permission.
19.4 SPENT BREWER’S YEAST
Brewer’s yeast is the second largest by-product obtained from the brewing industry. The term “spent brewer’s yeast” is used to describe yeast that is surplus to the brewer’s needs or is no longer needed in the brewing process. The reason why there is an ongoing excess of spent brewer’s yeast coming from the brewing industry is because the beer fermentation process generates approximately four to five times the amount of yeast in excess of the yeast that was added to start the fermentation. This means that for every kg of yeast used to start a fermentation, an additional 4 to 5 kg is generated. In most breweries, a considerable amount of this yeast is reused to start a subsequent fermentation. Nevertheless, a considerable quantity of the yeast generated is surplus to the brewer’s needs, even though it is otherwise suited for repitching in the beer production process. However, the ability to reuse a yeast culture for a series of subsequent beer fermentations is not necessarily unlimited. Depending on the yeast strain and fermentation conditions, the reuse of the same yeast culture can be as extensive as 12 or more cycles, or as limited as 4 to 6 cycles! The limitation on continued reuse stems from either “exhaustion” of the yeast culture or from risks associated with microbial contamination that can become more significant as the yeast reproduces through many generations—both of which affect the quality of the resultant beer and accordingly require the yeast culture to be discarded at some point. Furthermore, some yeast is generated during beer maturation in the postfermentation stages, including yeast obtained from the bottoms of maturation tanks. In Scotland, some excess ale (not lager) yeast is employed in the Scotch whiskey fermentation process. Some distillers consider that this spent brewer’s yeast develops a characteristic flavor in the spirit prior to maturation.
The brewer has no need for this excess or “worn-out” yeast and needs an avenue to dispose of it. Whether the yeast is simply excess or discarded because of starting a new cycle with freshly cultured yeast, it is all generically referred to as “spent brewer’s yeast.” If the brewer produces significant quantities of spent brewer’s yeast, it can be sold and used for other purposes.
When the brewer is looking after the yeast while there is still the potential for reuse of that yeast within the brewing process, the brewer will aim to maintain a high yeast viability level (the concept of yeast viability is addressed in Chapters 8 and 14). This viability is typically 95%+ but can be as low as 85% to 90%. When the brewer wants to reuse this yeast, it is stored under conditions where the yeast has a good chance to retain a high viability level. Once the yeast has been labeled as “spent brewer’s yeast,” typically very little care is taken of it by the brewer to further maintain its viability, unless the purchaser of the spent brewer’s yeast stipulates a set of minimum quality conditions.
This means that the makeup of spent brewer’s yeast as made available to a yeast purchaser can vary substantially! Yeast that is in excess, directly following the completion of fermentation, might well have a viability between 90% and 95%, while yeast that is beyond its cycle limit or becomes contaminated could have a viability well below 85%. Yeast from the bottoms of maturation tanks could potentially have a viability of 50% or less. This yeast, in excess of the needs of the brewer and sold, means that aside of perfectly healthy/viable yeast cells, a significant proportion of what enters the spent yeast container at the brewery could be to some degree in a compromised state.
Spent yeast constitutes a considerable portion of brewery by-product. Although some breweries sell their waste yeast quite successfully for processing into yeast extracts such as Marmite, Vegemite, and Cenovis (details later), many breweries sell their spent yeast as animal feed. With the advent of intelligent waste utilization or relabeling waste as value-added alternative commodities, excess yeast from breweries has great potential to be utilized in the production of health and nutritional value-added alternative commodities.
19.4.1 Brewer’s Yeast Use in Animal Nutrition
Brewer’s yeast is used in various animal feed applications.37–39 Spent brewer’s yeast can be used either in its intact form as live yeast or as dried dead cells. It can be a source of various derivatives with specific activities, such as nucleotides for young animals40 or cell wall material for binding pathogenic bacteria within the gut environment,41, 42 which is important as a replacement for in-feed antibiotics that have been limited or banned in many countries.43, 44
The common brewer’s yeast (Saccharomyces cerevisiae or Saccharomyces pastorianus) has multiple components that can be utilized as an animal feed. Its cell wall contains protein as well as compounds such as mannan-oligosaccharides (MOS), which can be purified into products with specific modes of action, such as pathogen binding via cell surface fimbriae.45 Spent brewer’s yeast, after it has been used for fermentation, contains around 5% MOS.46
Various forms of spent brewer’s yeast exist that can be used in animal feed applications. Liquid brewer’s yeast is 10% to 14% dry matter and contains 40% to 50% protein on a dry matter basis, although in products that have not received further purification and are used directly from breweries, levels can vary widely.47 There are differences among yeast species and strains and the consistency of products in terms of the mode of action and efficacy in animal feeds. Of the many individual species, only a limited number are useful for animal feed applications.
Brewer’s yeast can be in a liquid form, as a wet cake (30% dry matter), or an active dry form (95% dry matter). Active, dry yeast contains around 15 to 25 billion colony forming units (CFU) per gram and may contain different metabolites as a result of fermentation activity during the brewing process where sugar is fermented to alcohol, including peptides, B vitamins, nucleic acids, amino acids, esters, and organic acids (details in Chapter 8). However, the type of alcohol production dictates how much of these metabolites remain in the final yeast culture; for example, for beer making, such metabolites are largely contained in the beer and are not found in the waste yeast. Hence, the nutritional value of basic waste yeast from brewing depends not only on the yeast strain employed but also on what fermentation process has been utilized. Brewer’s yeast may be sold as either “active” yeast or heat-treated (killed) dry yeast. Table 19.7(48− 51) shows the typical makeup of yeast biomass.
Table 19.7 Average Composition of Yeast Biomass
Parameter |
(%DM basis)48 |
(%DM basis)49 |
(%DM basis)50 |
(%DM basis)51 |
|---|---|---|---|---|
Moisture |
2–5 |
5.4 ± 1.2 |
ND |
ND |
Crude protein |
40–52 |
64.9 ± 2.4 |
48.5 |
47.2 |
Nucleic acids |
6–8 |
ND |
7.5 |
7.1 |
Lipids |
4–7 |
0.8 ± 0.1 |
3.5 |
3.5 |
Carbohydrates |
30–37 |
26.9 ± 1.1 |
32.9 |
33.7 |
Sources: Reed, G., and Nagodawithana, T.W., Yeast Technology, 2nd ed., Van Nostrand Reinhold, New York, 1991; Jung, E.Y., et al, J. Food Sci., 76, C272–C278, 2011; Pacheco, M.T.B., et al, J. Nutr. Sci. Vitamin., 43, 601–612, 1997; Caballero‐Córdoba, G.M., and Sgarbieri, V.C., J. Sci. Food Agric., 80, 341–351, 2000. With permission.
Note: ND, not determined.
19.4.1.1 Yeast Components
Although live yeast was initially used in animal feeds, over the last three decades research has focused on its derivatives, such as the yeast cell wall, for meeting specific needs in animals. The cell wall is a rich source of MOS, which can be purified to produce a product with specific pathogen-binding activity in the gut as well as beneficial interactions with the immune system, resulting in appropriate responses to toxic or harmful bacterial colonization. In addition, pregnant mammals have been shown to have increased immunoglobulin levels in colostrum when fed MOS, which enhances early immunity in their young and alleviates problems typically seen at weaning. The cytosol of yeast is rich in nucleotides, which are important in neonatal animals for gut development and have been associated with gut repair in animals with ulceration.
19.4.1.2 Animal Trials
Various published trials have examined the use of brewer’s yeast, fed as a supplement in diets for ruminants, horses, poultry, swine, and fish.38, 52–55 The most numerous trials on purified yeast components are for MOS in poultry studies. These trials have been focused on the comparison of MOS inclusion in feeds on performance and health in poultry with or without antibiotic growth promoters. Meta-analyses have shown that poultry have nearly 2% improvements in weight gain (P < 0.001), 2.25% reduction in feed conversion ratio (P < 0.001), and more than 20% reduction in mortality, to levels less than 5% in the flock when compared with a control diet not supplemented with yeast MOS. No significant differences in performance were seen between MOS and antibiotic supplemented diets, although mortality was still reduced by 17% in the MOS-supplemented chicken flocks.56 Similar benefits have been reported in turkeys.57
In a pig trial, brewer’s yeast was used as a basic source of MOS and was fed at 3% of the diet in two experiments using growing pigs with and without an E. coli K88 challenge.46 Feeding the yeast alone tended to result in higher serum immunoglobulin (Ig) levels in pigs, with or without challenge by pathogens, and total fecal coliforms were consistently lower in pigs fed brewer’s yeast after infection with E. coli K88. Several trials have shown the benefits of MOS on Ig levels in sows’ milk, with knock-on benefits in the resulting piglets.58–60
In horses, the use of live yeast (S. cerevisiae) combined with the yeast cell wall MOS showed increases in digestibility over a 28-day period, with improved fecal scores in horses with a history of sub-clinical colic and diarrheal problems. Fecal score was increased by a factor of 0.8 for those fed the yeast plus MOS by Day 14 on a five-point scale. After 28 days into the trial, the body condition score was improved by 0.5, daily fecal output was significantly less (3.8 kg versus 4.2 kg dry matter basis), and digestibility was increased from 47% to 52% in the yeast and MOS fed group, demonstrating higher digestibility of the diets and reduced diarrheal symptoms.61
In ruminant studies, different strains of live brewer’s yeast were tested for their ability to increase the levels of viable rumen bacteria and hence increase fermentation and energy production.37 Live yeast is useful for rumen microflora moderation as it has the ability to consume excess O2 ingested on forages, which can promote negative aspects of fermentation, resulting in acidosis and reductions in energy release from fiber degradation.37 When added at levels of 1.3 mg/mL to an in vitro ruminant fermentation simulation system (Rusitec), S. cerevisiae NCYC strains 240, NCYC 1026, and the commercial product Yea-Sacc® increased O2 disappearance by 46% to 89% and stimulated beneficial bacterial numbers. Other strains of yeast failed to have any influence on simulated ruminant fermentation characteristics.37
Earlier feeding trials showed that Hereford steers that were fed liquid brewer’s yeast for 98 days, in addition to an existing corn silage ration, had higher dry matter intakes and faster weight gains compared to the control group that did not receive yeast as a supplement.47 Similarly, steers fed S. cerevisiae showed up to 40 times greater numbers in cellulolytic microorganisms compared to animals fed unsupplemented control feeds.62
Carro et al.63 investigated the effect of supplementing S. cerevisiae to three different ruminant diets via a Rusitec in vitro system. Diets were high (70:30), medium (50:50), or low (30:70) forage to concentrate ratio, with or without 15 mg yeast/g dry matter feed. Adding the yeast significantly increased molar proportions of volatile fatty acids (P < 0.05) in the high-forage diet.63 Adding yeast to the high-concentrate/low-forage diet increased dry matter and fiber digestibility (P < 0.01), volatile fatty acid (VFA) production, and protozoal numbers (P < 0.02). Feeding liquid brewer’s yeast to lactating dairy cows altered VFA ratios, increasing propionate, with a positive benefit to milk flavor.64
Research into the use of yeast components in pet food have shown that MOS promotes beneficial bacteria in the hind gut in dogs and increases fiber digestion by more than 10%.65, 66 Strickling67 showed that dogs fed diets supplemented with MOS had reduced levels of the pathogenic Clostridium spp. For immunity, similar benefits were seen in dogs as reported in other species where feeding MOS increased circulating immunoglobulins and lymphocytes.68
The other major components of live yeast are the nucleotides. These are the purine and pyrimidine compounds that form DNA,69 and they are required in all animals for cell production, replication, and repair. Cellular replication is essential for wound healing, repairing tissue damage, and for maintenance of structures, such as the gut lining, which are constantly being sloughed off or are prone to damage from food passage and pathogenic bacterial activity. Immune cells (lymphocytes and erythrocytes) need supplemental nucleotides from feed as they cannot manufacture their own, in contrast to other cells in the body. Trials conducted in animals have shown that feeding nucleotides increased gut wall thickness and improved the maintenance of the gut lining.70, 72 Furthermore, wound healing in rat tissue commenced just four days after supplementing diets with nucleotides.73 The yeast cytosol product NuPro is rich in nucleotides and has been used in young piglet trials, where they received NuPro or plasma protein from seven days of age. Animals fed NuPro had better feed conversion at weaning and higher daily gain compared to those receiving plasma protein. These benefits in weight gain continued in the NuPro group after weaning, leading to a lower feed cost to weight gain ratio (P. Groenewegen, Alltech Swine Technical Manager, personal communication).
19.4.1.3 Practical Uses of Brewer’s Yeast and Its Components
Live yeast and its individual components, from the brewing industry, have great by-product value as specialist animal feed supplements, where their multifactorial activities and proven benefits increase welfare, health, and performance, whether in agricultural species raised for meat, milk, and eggs or for companion and leisure animals. However, the strain of yeast employed and the specific isolation and purification of active components is important in the final efficacy of the product and its suitability in feed systems.
19.5 BREWER’S YEAST IN FOOD APPLICATIONS
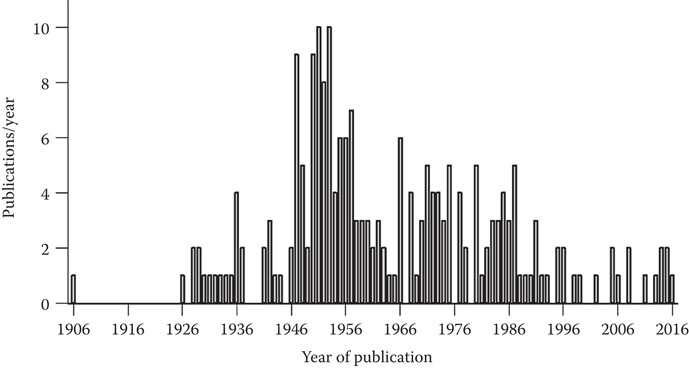
The highly nutritional benefits associated with brewer’s yeast in animal nutrition also apply to human nutrition. It is uncommon to use whole spent brewer’s yeast in human applications; however, extracts and yeast components are used in a wide variety of food applications. Perhaps the better-known applications in some parts of the world are products with brand names such as Marmite and Vegemite. These products are inherently associated with national identities in countries such as Australia, New Zealand, and the United Kingdom. These products have been part of some nations’ histories for well over a century! Although the concept of using spent brewer’s yeast for human consumption was a German-inspired process, the bulk of products and processes to produce them were established in English-speaking countries. In 1902, the first Marmite plant was established in Burton on Trent, which saw a rapid expansion of production across the British Commonwealth into Australia and New Zealand. In 1908, an antipodean Marmite plant came to Australia, which saw production move to New Zealand somewhat later. In 1913, a similar product (Vegex) came on to the market in the United States. A rival brand to Marmite (Vegemite) was launched in Australia in 1923. A separate brand (Bovis) started in Italy in 1925, while Cenovis started in Switzerland in 1931. Although new products have come onto the market in more recent years, all these products are still on the market and are produced from spent brewer’s yeast. The two main brands, Marmite and Vegemite, hold a special place in the hearts of people who grew up with them, they either “love it” or “hate it.” In many instances, liking is due to the fact that people grew up with Marmite and Vegemite, and they would have been unceremoniously presented (particularly at breakfast) as a healthy product full of vitamins—essentially, the ultimate source of goodness! This has been strengthened by the fact that national armies included these products in soldiers’ rations during times of armed conflict in the early- to mid-1900s. Why question the nutritional goodness on what “our proud army” marches? Furthermore, nutritional evidence had shown that people suffering anemia and other vitamin-related deficiencies could be healed by the consumption of a spread made from spent brewer’s yeast.74–79 Furthermore, the nutritional value of yeast spreads intended for human consumption has also been recognized for other purposes. For instance, both Vegemite and Marmite have persistently been used as a nutrient source in microbiological media since 1906 in more than 200 publications over a 110-year period (Figure 19.1), with a significant number of publications during the decade immediately following World War II. The main reason for this was the need for live science research to continue in an age of scarcity.

Figure 19.1 The number of research publications (per year) in which either Marmite or Vegemite was used as the nitrogen source in microbiological growth media for diagnostic purposes.
The production of products such as Vegemite and Marmite commences with the selection of an appropriate quality of spent brewer’s yeast that is substantially free of bacterial contamination and extraneous materials such as filter aids and excessive hop flavors and aromas. The yeast extract processing plants require a reasonably consistent supply of spent brewer’s yeast slurry that fits within fairly tight parameters of dry weight and residual sugar and ethanol. Some producers of yeast extract allow the yeast to grow in order to achieve sufficient supply of raw spent yeast and as a means to reduce residual hop components. When the yeast has been assigned to be received in the autolysis tanks, the spent yeast slurry is heated to an initial 90°C, and salt and enzymes are added after which the hydrolysis process proceeds at 63°C. Both the initial heat and the salt are required to initiate the nascent autolysis process during which yeast enzymes facilitate the hydrolysis of various yeast cell components. The additional enzymes permit particular hydrolytic reactions to be emphasized in order to speed up and steer the hydrolytic process toward specific quality parameters. Following the hydrolysis process, much of the cell wall particulates are removed. The remaining liquid is concentrated to approximately 20% solids before minor particulate matter is removed by means of a rotary drum filter. Further concentrating occurs in multistage vacuum evaporators, after which spices can be added to finish off the flavor profile. This will produce a relatively runny product; the pastier yeast extracts tend to have yeast cell wall materials added back in to achieve the desired spreadability.
Apart from extracts that can be directly consumed, yeast extracts are widely used in food manufacturing as flavor components. The process for the production of yeast extracts for the manufacture of flavor compounds is very similar to that described earlier. However, the hydrolysis process can be steered to yield specific flavor profiles.80–82 The main drivers for the development of flavors are the specific application of enzymes that produce elevated levels of 5' nucleotides to yield pork-specific or chicken-specific flavors.83
Individual components of yeast cells (i.e., yeast cell wall fractions, whole protein fractions, and minerals) are also utilized in the food and allied industries. Furthermore, various nutraceutical and medical applications also exist.
19.5.1 Brewer’s Yeast as a Fat Replacer
The β-glucans and mannoproteins associated with the yeast cell wall have been identified as efficient fat replacers in food formulations.84–90 The proteins associated with the mannoproteins appear to be very effective bioemulsifiers, whereas the phosphomannans enhance the emulsification properties of the mannoproteins.85 The protein fraction of the mannoproteins is the hydrophobic part of the emulsifier, whereas the mannose part of the mannoproteins forms the hydrophilic part of the bioemulsifier. Due to their strong water binding ability, yeast β-glucans are very effective fat replacers, and they have been successfully applied as such in low-fat mayonnaise and cheeses.86, 88, 91 Apart from their role as a fat replacer in low-fat foods, yeast β-glucans have also been found to lower plasma cholesterol levels, while even reducing body fat deposits.92–94
19.5.2 Brewer’s Yeast as Glucose Tolerance Factor
Brewer’s yeast has been recognized as the source of a glucose tolerance factor.95, 96 This glucose tolerance factor improves the rate of glucose removal from the bloodstream in case of a low or insufficient insulin response.97–100 The actual glucose tolerance factor has been identified as trivalent chromium as it is incorporated into yeast proteins. This allows for very efficient absorption of the mineral.101 The use of brewer’s yeast as a source of a glucose tolerance factor has been found to effectively maintain appropriate blood glucose levels in people suffering from noninsulin-dependent diabetes.102–106 Linked to it is the alleviating effect of yeast-chromium on blood glucose for diabetes and the role that yeast-chromium plays in combating acne. It has been shown that acne can be caused by rapid fluctuations in insulin, which can be offset by the administration of brewer’s yeast.107, 108
19.5.3 Brewer’s Yeast as a Source of Minerals
Yeast contains a wide variety of minerals, some of which have shown to be easily accessible when contained within yeast. A clear example is the presence of chromium in yeast (see previous section) and magnesium. One specific trace element that has been shown to accumulate in yeast is selenium.109, 110 Selenium is required by animals to reduce rates of inflammation, to facilitate DNA and hormone synthesis, and to play a role in fertility and reproduction.111 Although deficiencies in selenium in the human diet are rare, the use of yeast as a delivery system for it has been shown to be very efficient, and applications have been found in both human and animal nutrition.109 Similar to the glucose tolerance factor, yeast’s effectiveness as an efficient delivery vehicle is due to the incorporation of the minerals into proteins, which allows for rapid and efficient absorption, and high bio-availability.
19.6 BREWER’S YEAST IN HEALTH-RELATED APPLICATIONS
19.6.1 Brewer’s Yeast as an Antidepressant
Yeast is a rich source of S-adenosyl-L-methionine (SAMe), which has been successfully used112, 113 in the treatment of alcoholism and general liver disorders114–116 as well as in the management of osteoarthritis117 and hot flushes in response to hormonal changes,118 all with no or minimal side effects. As an antidepressant, SAMe acts predominantly through the action of methyl-transferases that shift the methyl group from SAMe to a variety of potential methyl-group acceptors, such as neurotransmitters and other biogenic amines.113 Typically, people suffering from depression have relatively low plasma SAMe levels, and administration of SAMe usually alleviates symptoms of depression. SAMe has been found to be as effective as conventional antidepressant drugs, allowing the use of spent brewer’s yeast-derived SAMe to be a valuable natural alternative to conventional antidepressant drugs.112 Yeast hydrolysate, as a crude preparation, has also been shown to have an antidepressant effect similar to SAMe, which suggests that crude yeast preparations can induce physiological effects similar to highly purified preparations.119–121
19.6.2 Brewer’s Yeast in Wound Healing
Purified yeast glucan has been found to be an effective stimulant of wound healing.122–124 The use of glucan-based skin grafts has shown remarkable improvements in postburn survival through a reduction in postburn hypermetabolism, infection rates, and a more rapid natural wound sealing. Further improvements in wound healing have been made by combining β-glucan with collagen125 or by the use of carboxymethylated β-glucan.126 The latter has been promoted as a cosmeceutical that can act as a skin-protector against potential damage caused by ultraviolet-A (UV-A) exposure.127
19.6.3 Brewer’s Yeast as an Immunomodulator
As mentioned previously, brewer’s yeast has the ability to affect the recovery of wound healing through the action of β-glucans. Most of this activity can be traced back through the apparent priming of innate immune effector cells.128 This will essentially cause the overall immune system to be more effective in combating nonself cell types, such as tumor cells.128–130 This mechanism has been argued to be due to the increased activity of the macrophages.128, 131
19.7 CARBON DIOXIDE
Although ethanol is the principal fermentation product during the production of beer and other alcoholic beverages, carbon dioxide (CO2) is a very close second when it comes to mass conversion of sugar (details in Chapter 8). A portion of this CO2 serves to carbonate the beer while in the fermenter. However, only about 2 to 3 v/v CO2 is needed for saturating the beer in its packaged form. The remainder of the CO2 can be deemed to be in excess for a brewer’s requirement and, as such, could potentially be recovered for other purposes within a brewing environment and/or sold off for nonbrewing applications. Apart from carbonating beer, CO2 is also used to create an oxygen-deficient environment during various postfermentation processes such as the following:
Because most of these processes in which CO2 might be used are at a point where beer is at an almost finished stage, it is important that CO2 purity is monitored continuously to avoid the ingress of impurities.132
Carbon dioxide is predominantly collected from the fermentation vessels. However, when a CO2 collection and purification process is in place, it would make sense to also collect CO2 from storage and transfer tanks, packing lines, and any other process where high levels of nearly pure CO2 are encountered. When collected, CO2 is purified, compressed, liquefied, and stored. Spent CO2 contains various unwanted volatile compounds that either interfere with the production of pure CO2 or are not wanted for inclusion when CO2 is to be used as food grade CO2. Because a sufficiently high CO2 concentration in the off-gas is required for the process to be cost effective, it is imperative that the scheduling of CO2 collection is well-timed during batch fermentations. During early wort fermentation, the oxygen levels are too high and the CO2 levels too low; similar disadvantage periods occur at other times during fermentation. The timing can be judged according to an appropriate decrease in specific gravity (SG), the rise in fermentation temperature, gas-out analysis, or simply based on the time elapsed (i.e., four to six hours into the fermentation).
Purification typically consists of five steps:
Removal of entrained liquids and solids can be facilitated by a seal pot, which consists of a water-containing pot, through which the fermentation outgases bubble. This will strip out any solids or water soluble gasses such as ethanol. The seal pot also prevents flow back of gasses when the fermentation slows down. Alternatively, a scrubber can be employed, where the outgases are sprayed with a mist of water droplets in which entrained solids and water soluble gasses are stripped and drained in a continuous manner, while the gasses continue on to the next purification stage. Entrained oxygen can be separated from the CO2 by rapidly cooling the outgases and allowing the CO2 to be liquefied. CO2 condenses into a liquid at −78°C and O2 remains a gas until it condenses at −183°C. Reducing the temperature below −78°C allows oxygen to be vented from the crude CO2. The next step in the purification stage is the removal of sulfur-containing compounds, which is usually accomplished by passing the crude CO2 over activated carbon.133 Appropriate absorption of sulfur occurs by a fine balance of time and carbon surface area. Only after the sulfur has been removed can any excess nitrogenous compounds be removed. Removing nitrous compounds is facilitated by employing a catalytic converter not unlike the ones used in a car’s exhaust system. The catalytic part is a bed of palladium that facilitates the conversion of NOx to N2. This process requires the input of a small quantity of H2 to achieve the following: NOx + H2 → H2O + N2.
The penultimate step is the removal of water from the gaseous CO2. As mentioned previously, CO2 condenses at −78°C, any residual water will form ice and obstruct the flow of gas. Water-absorbent materials such as alumina-silica, activated alumina, or silica gel can be used to remove water before the CO2 is cooled into a liquid state for convenient storage or transportation. If food grade CO2 is not required, purified or even impure CO2 that is vented from fermenters can be used to neutralize alkaline brewery effluents.1 Vented CO2 can also be collected and pumped into greenhouses, where higher CO2 levels can accelerate plant growth by 20%. Another promising use for surplus CO2 is for fumigating grain silos. Carbon dioxide was found to be an effective nontoxic fumigant that does not leave a residue.1
19.8 CONCLUSION
In a modern brewery, the use and value of by-products presents a significant opportunity to increase the sustainability of the brewing process through capture of potentially environmentally damaging materials and also to increase the overall profitability of the process by creating value from these by-product streams.
Historically, this application has been limited to simple livestock feeding of by-products such as malt culms, brewer’s grains, and waste yeast, limiting the value of these products. However, as raw materials for livestock production become increasingly limited and the availability of technology to further process these products becomes available, the opportunity to increase the value to overall brewery operations will increase.
This chapter has reviewed some of the main areas of opportunity to maximize the use and value of these by-products. The opportunity for further technologies and processing will continue to drive the possibility of increasing by-product value in the brewery. However, the availability and practical use of these opportunities will be limited to those breweries with a large enough by-product volume to warrant the capital investment in such processes. This has led to the development of businesses purchasing by-products from multiple breweries in order to achieve a large enough volume to optimize further processing technologies. It is expected that this area will continue to experience innovative development in the future.
ACKNOWLEDGMENTS
The authors wish to thank and to acknowledge Nick J. Huige, who was the author of this chapter in the previous edition of this book and upon which this updated chapter is based.
REFERENCES
1. Huige, N.J., Brewery by-products and effluents, in Handbook of Brewing, Priest, F.G. and Stewart, G.G., Eds., 2nd ed., CRC Press, Boca Raton, FL, 2006.
2. The Maltsters Association of Great Britain, Malting Coproducts. Valuable Nutritional Ingredients for the Feed Industry. Available from: https://www.ukmalt.com/malting-coproducts (accessed January 7, 2017).
3. Frank, M., Weckman, T.J., Wood, T., Woods, W.E., Tai, C.L., Chang, S.L., Ewing, A., Blake, J.W., and Tobin, T., Hordenine: Pharmacology, pharmacokinetics and behavioural effects in the horse, Equine Vet. J., 22:437–441, 1990.
4. Hapke, H.J. and Strathmann, W., [Pharmacological effects of hordenine], DTW. Deutsche Tierarztliche Wochenschrift, 102:228–232, 1995.
5. Cauwe, Y. and Waesberghe, J.V., Norden HP filter - a new approach to mash filtration, Tech. Q. Master Brew. Assoc. Am., 16:142–147, 1979.
6. Richards, E.A., Energy costs and spent grain drying, Brewers Digest, 51:48, 1976.
7. Tang, Z., Cenkowski, S., and Muir, W.E., Modelling the superheated-steam drying of a fixed bed of brewers’ spent grain, Biosyst. Eng., 87:67–77, 2004.
8. Stroem, L.K., Desai, D.K., and Hoadley, A.F.A., Superheated steam drying of brewer’s spent grain in a rotary drum, Adv. Powder Technol., 20:240–244, 2009.
9. Linton, J.H., Pollution abatement through utilization of wet brewery by-products in livestock feeding, Brewers Digest, November, pp. 42–46, 74, 1973.
10. Penrose, J.D.F., Upgrading grains, Brewer, January, pp. 4–7, 1982.
11. Hunt, L.A. and Spitzer, E.H., Livestock feed composition and method of preparing the same. U.S. Patent No. 3,875,304. 1 Apr. 1975.
12. Mussatto, S.I., Dragone, G., and Roberto, I.C., Brewers' spent graIn: Generation, characteristics and potential applications, J. Cereal Sci., 43:1–14, 2006.
13. Levic, J., Djuragic, O., and Sredanovic, S., Use of new feed from brewery by-products for breeding layers, Romanian Biotechnol. Lett., 15:5559–5565, 2010.
14. Aliyu, S. and Bala, M., Brewer’s spent graIn: A review of its potentials and applications, Afr. J. Biotechnol., 10:324–331, 2011.
15. Jensen, L.S., Chang, C.H., and Maurice, D.V., Improvement in interior egg quality and reduction in liver fat in hens fed brewers dried grains, Poultry Sci., 55:1841–1847, 1976.
16. Damron, B.L., Eldred, A.R., and Harms, R.H., Improvement in interior egg quality by the feeding of brewer’s dried grains, Poultry Sci., 55:1365–1366, 1976.
17. Eldred, A.R., Damron, B.L., and Harms, R.H., Evaluation of dried brewer’s grains and yeast in laying hen diets containing various sulfur amino acid levels, Poultry Sci., 54:856–860, 1975.
18. Harms, R.H., Brewers grains improve interior egg quality, Poultry Int., 15:16, 1976.
19. Ademosun, A.A., Evaluation of brewers’ dried grains in the diets of growing chickens, Brit. Poultry Sci., 14:463–468, 1973.
20. Sullivan, T., Brewers dried grains in rations for starting, growing, finishing and breeder turkeys, USBA Feed Conf. Proc., pp. 71–83, 1977. St. Louis, MO.
21. Sullivan, T.W., Kuhl, H.J., and Holder, D.P., Evaluation of brewers dried grains and yeast in turkey diets, Poultry Sci., 57:1329–1336, 1978.
22. Qureshi, A.A., Chaudhary, V., Weber, F. E., Chicoye, E., and Qureshi, N., Effects of brewer's grain and other cereals on lipid metabolism in chickens, Nutr. Res., 11:159–168, 1991.
23. Onifade, A.A. and Babatunde, G.M., Comparison of the utilisation of plan kernel meal, brewer’s dried grains and maize offal by broiler chicks, Brit. Poultry Sci., 39:245–250, 1998.
24. Yaakugh, I.D.I., Tegbe, T.S.B., Olorunji, S.A.S., and Aduku, A.O., Replacement value of brewer’s dried grains for maize on performance of pigs, J. Sci. Food Agricult., 66:465–471, 1994.
25. Zijlstra, R.T., Owusu-Asiedu, A., and Simmins, P.H., Future of NSP-degrading enzymes to improve utilization of coproducts and gut health in pigs, Livestock Sci., 134:255–257, 2010.
26. Preston, R.L., Vance, R.D., and Cahill, V.R., Energy evaluation of brewers’ grains for growing and finishing cattle, J. Animal Sci., 37:174–178, 1973.
27. Thompson, G.B., Johnson, R., and Hutcheson, D., Evaluation of brewers dried grains in finishing rations in cattle, USBA Feed Conf. Proc., St. Louis, MO pp. 49–65, 1977.
28. Oster, A., A note on the use of brewers’ dried grains as a protein feedstuff for cattle, Animal Prod., 24:279–282, 1977.
29. Linton, J.H. and Sibald, I.R., Acceptability of mixtures of spent yeast and brewers’ grains as components of cattle diets, Brewers Digest, 46:58, 1971.
30. Conrad, H.R. and Rogers, J.A., Comparative nutritive value of brewers wet and dried grains for dairy cattle, USBA Feed Conf. Proc., St. Louis MO, pp. 26–35, 1977.
31. Belibasakis, N.G. and Tsirgogianni, D., Effects of wet brewers’ grains on milk yield, milk composition and blood components of dairy cows in hot weather, Animal Feed Sci. Technol., 57:175–181, 1996.
32. Wenzinger, B., Leistungsminderung und hohe Zellzahlen in einer Milchviehherde nach Verfütterung von hohen Mengen an Biertreber, Schweizer Archiv für Tierheilkunde, 155:515–519, 2013.
33. Ott, E.A. and Richardson, G.L., Effect of protein quality on growing foals, Proc. 5th Equine Nutrition and Physiology Society Symposium, St. Louis, MO, pp. 113–125, 1977.
34. Fărcaş, A., Tofană, M., Socaci, S., Mudura, E., Scrob, S., Salanţă, L., and Mureşan, V., Brewers’ spent grain – A new potential ingredient for functional foods, J. Agroalimentary Proc. Technol., 20:137–141, 2014.
35. Mussatto, S.I., Brewer's spent graIn: A valuable feedstock for industrial applications, J. Sci. Food Agricult., 94:1264–1275, 2014.
36. Waters, D.M., Jacob, F., Titze, J., Arendt, E.K., and Zannini, E., Fibre, protein and mineral fortification of wheat bread through milled and fermented brewers’ spent grain enrichment, Eur. Food Res. Technol., 235:767–778, 2012.
37. Newbold, C.J., Wallace, R.J., and McIntosh, F.M., Mode of action of the yeast Saccharomyces cerevisiae as a feed additive for ruminants, Brit. J. Nutr., 76:249–261, 1996.
38. Ferreira, I.M.P.L.V.O., Pinho, O., Vieira, E., and Tavarela, J.G., Brewer's Saccharomyces yeast biomass: Characteristics and potential applications, Trends Food Sci. Technol., 21:77–84, 2010.
39. Ganguly, S., Dora, K.C., Sarkar, S., and Chowdhury, S., Supplementation of prebiotics in fish feed: A review, Rev. Fish Biol. Fisheries, 23:195–199, 2013.
40. Li, P., Burr, G.S., Goff, J., Whiteman, K.W., Davis, K.B., Vega, R.R., Neill, W.H., and Gatlin, D.M., A preliminary study on the effects of dietary supplementation of brewers’ yeast and nucleotides, singularly or in combination, on juvenile red drum (Sciaenopsocellatus), Aquaculture Res., 36:1120–1127, 2005.
41. Torrecillas, S., Makol, A., Benítez-Santana, T., Caballero, M.J., Montero, D., Sweetman, J., and Izquierdo, M., Reduced gut bacterial translocation in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS), Fish Shellfish Immunol., 30:674–681, 2011.
42. Halas, V. and Nochta, I., Mannan oligosaccharides in nursery pig nutrition and their potential mode of action, Animals, 2:261–274, 2012.
43. White, L.A., Newman, M.C., Cromwell, G.L., and Lindemann, M.D., Brewers’ dried yeast as a source of mannan oligosaccharides for weanling pigs, J. Animal Sci., 80:2619–2628, 2002.
44. Hisano, H., Falcon, R.D., Maria Barrose, M., and Pezzato, E.L., Influence of yeast and yeast derivatives on growth performance and survival of juvenile prawn, Macrobrachium amazonicum, Ciencia Animal Brasileira, 9:657–662, 2008.
45. Spring, P., Wenk, C., Dawson, K.A., and Newman, K.E., The effects of dietary mannan-oligosaccharide on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks, Poultry Sci., 79:205–211, 2000.
46. White, L.A., Newman, M.C., Cromwell, G.L., and Lindemann, M.D., Brewers’ dried yeast as a source of mannan oligosaccharides for weanling pigs, J. Animal Sci., 80:2619–2628, 2000.
47. Grieve, D.G., Feed intake and growth of cattle fed liquid brewer’s yeast, Can. J. Animal Sci., 59:89–94, 1979.
48. Reed, G. and Nagodawithana, T.W., Yeast Technology, 2nd ed., Van Nostrand Reinhold, New York, 1991.
49. Jung, E.Y., Lee, H.S., Choi, J.W., Ra, K.S., Kim, M.R., and Suh, H.J., Glucose tolerance and antioxidant activity of spent brewer's yeast hydrolysate with a high content of cyclo‐his‐pro (CHP), J. Food Sci., 76:C272–C278, 2011.
50. Pacheco, M.T.B., Caballero-Cordoba, G.M., and Sgarbieri, V.C., Composition and nutritive value of yeast biomass and yeast protein concentrates, J. Nutr. Sci. Vitamin., 43:601–612, 1997.
51. Caballero‐Córdoba, G.M. and Sgarbieri, V.C., Nutritional and toxicological evaluation of yeast (Saccharomyces cerevisiae) biomass and a yeast protein concentrate, J. Sci. Food Agricult., 80:341–351, 2000.
52. Castro, C., Pérez-Jiménez, A., Coutinho, F., Pousão-Ferreira, P., Brandão, T.M., Oliva-Teles, A., and Peres, H., Digestive enzymes of meagre (Argyrosomus regius) and white seabream (Diplodus sargus). Effects of dietary brewer's spent yeast supplementation, Aquaculture, 416:322–327, 2013.
53. Zhao, L., Wang, W., Huang, X., Guo, T., Wen, W., Feng, L., and Wei, L., The effect of replacement of fish meal by yeast extract on the digestibility, growth and muscle composition of the shrimp Litopenaeus vannamei, Aquaculture Res., 48(1):311–320, 2015. DOI: 10.1111/are.12883
54. Nocek, J.E., Holt, M.G., and Oppy, J., Effects of supplementation with yeast culture and enzymatically hydrolyzed yeast on performance of early lactation dairy cattle, J. Dairy Sci., 94:4046–4056, 2011.
55. Ralston, S.L., McKinnon, A.O., Squires, E.L., Vaala, W.E., and Varner, D.D., Dietary support for geriatric broodmares and stallions, Equine Reprod., 2:2755–2759, 2011.
56. Hooge, D., Broiler chicken performance may improve with MOS, Feedstuffs, 75:11–13, 2003a.
57. Hooge, D., Dietary mannan-oligosaccharides improve broiler and turkey performance: Meta-analysis of pen trials around the world, in Biotechnology in the Feed Industry Proceedings of Alltech’s 19th Annual Symposium, Lyons, T.P., and Jacques, K.A., Eds., Nottingham University Press, Nottingham, UK, pp. 113–124, 2003.
58. Miguel, J.C., Rodriguez-Zas, S.L., and Pettigrew, J.E., Practical responses to Bio-Mos in nursery pigs: A meta-analyses, in Nutritional Biotechnology in the Feed and Food Industry, Lyons, T.P., and Jacques, K.A., Eds., Nottingham University Press, Nottingham, UK, pp. 425–433, 2002.
59. Lazarevic, M., Spring, P., Shabanovic, M., Tokic, V., and Tucker, T.A., Effect of gut active carbohydrates on plasma IgG concentrations in piglets and calves, Animal, 4:938–943, 2010.
60. Taylor-Pickard, J., McArdle, T., and Icely, S., Effect of feeding Actigen™ to sows during gestation and lactation and on piglet performance, J. Appl. Animal Nutr., 5:1–4, 2016.
61. Ott, E., Influence of Bio-Mos ®, a mannan oligosaccharide supplement, on the immune system of the mare and neonatal foal, in Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech’s 21st Annual Symposium, Lyons, T.P., and Jacques, K.A., Eds., Nottingham University Press, Nottingham, UK, pp. 447–454, 2005.
62. Dawson, K.A., Newman, K.E., and Boling, J.A., Effects of microbial supplements containing yeast and lactobacilli on roughage fed ruminal microbial activities, J. Animal Sci., 68:3392–3398, 1990.
63. Carro, M.D., Lebzien, P., and Rohr, K., Influence of yeast culture on the in vitro fermentation (Rusitec) of diets containing variable portions of concentrates, Animal Feed Sci. Technol., 37:209–220, 1992.
64. Besong, S., Jackson, J.A., Hicks, C.L., and Hemken, R.W., Effects of a supplemental liquid yeast product on feed intake, ruminal profiles, and yield, composition, and organoleptic characteristics of milk from lactating Holstein cows, J. Dairy Sci., 79:1654–1658, 1996.
65. Swanson, K.S. and Fahey, G.C., Prebiotics in companion animal nutrition, in Biotechnology in the feed industry: Proceedings of Alltech’s 18th Annual Symposium, Lyons, T.P., and Jacques, K.A., Eds., Nottingham University Press, Nottingham, UK, pp. 461–474, 2003.
66. Kappel, L., Henk, B., Jowett, P., and Hedhund, C., Effect of Mannan-Oligosaccharide on Diet Component Digestibility and Fermentation Characteristics in Dogs. School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, 1998.
67. Strickling, J.A., Evaluation of oligosaccharide addition to dog diets: Influence on nutrient digestion and microbial populations. Master Thesis. University of Kentucky, Lexington, KY, 1999.
68. Swanson, K.S., Grieshop, C.M., Flickinger, E.A., Merchen, N.R., and Fahey, G.C., Effects of supplemental fructooligosaccharides and mannanoligosaccharides on colonic microbial populations, immune function and fecal odor components in the canine, J. Nutr., 132:1717S–1719S, 2002.
69. Kulkarni, A.D., Rudolph, F.B., and van Buren, C.T., The role of dietary sources of nucleotides in immune function: A review, J. Nutr., 124:1442–1446, 1994.
70. Carver, J.D., Dietary nucleotides: Cellular immune, intestinal and hepatic system effects, J. Nutr., 124:144S–148S, 1994.
71. Carver, J.D., Dietary nucleotides: Effects on the immune and gastrointestinal systems, Acta Paed., 88:83–88, 1999.
72. Grimble, G.K. and Westwood, O.M., Nucleotides as immunomodulators in clinical nutrition, Curr. Opin. Clin. Nutr. Metabol. Care, 4:57–64, 2001.
73. Veerabagu, M.P., Meguid, M.M., Oler, A., and Levine, R.A., Intravenous nucleosides and a nucleotide promote healing of small bowel ulcers in experimental enterocolitis, Digest. Dis. Sci., 41:1452–1457, 1996.
74. Wilcox, W.H., Discussion on the treatment and management of diseases due to dietetic deficiencies, Proc. Royal Soc. Med., London, 13:7–22, 1920.
75. Macpherson, W.G., Herringham, W.P., Elliott, T.R., and Balfour, A., History of the Great War based on official documents, in Medical Services. Diseases of the War, Vol. 1, 1922.
76. Vaughan, J. and Hunter, D., The treatment by Marmite of megalocytic hyperchromic anemia: Occurring in idiopathic stearorrhœea (Coeliac disease), The Lancet, 219:829–834, 1932.
77. Ungley, C., The effect of yeast and wheat embryo in anaemias. 1. Marmite, yestamin, and bemax in megalocytic and nutritional hypochromic anaemias, Quart. J. Med., 2:381–405, 1933.
78. Ungley, C.C., The extrinsic factor in anemia, The Lancet, 228:768, 1936.
79. Wills, L., The nature of the haemopoietic factor in Marmite, The Lancet, 221:1283–1286, 1933.
80. Charpentier, C., Aussenac, J., Charpentier, M., Prome, J.C., Duteurtre, B., and Feuillat, M., Release of nucleotides and nucleosides during yeast autolysis: Kinetics and potential impact on flavour, J. Agricult. Food Chem., 53:3000–3007, 2005.
81. Mahadevan, K. and Farmer, L., Key odor impact compounds in three yeast extract pastes, J. Agricult. Food Chem., 54:7242–7250, 2006.
82. Lin, M., Liu, X., Xu, Q., Song, H., Li, P., and Yao, J., Aroma‐active components of yeast extract pastes with a basic and characteristic meaty flavour, J. Sci. Food Agricult., 94:882–889, 2014.
83. Sombutyanuchit, P., Suphantharika, M., and Verduyn, C., Preparation of 5′-GMP-rich yeast extracts from spent brewer's yeast, World J. Microbiol. Biotechnol., 17:163–168, 2001.
84. Cameron, D.R., Cooper, D.G., and Neufeld, R.J., The mannoprotein of Saccharomyces cerevisiae is an effective bioemulsifier, Appl. Environ. Microbiol., 54:1420–1425, 1998.
85. Barriga, J.A., Cooper, D.G., Idziak, E.S., and Cameron, D.R., Components of the bioemulsifier from S. cerevisiae, Enzyme Microbial Technol., 25:96–102, 1999.
86. Worrasinchai, S., Suphantharika, M., Pinjai, S., and Jamnong, P., β-Glucan prepared from spent brewer's yeast as a fat replacer in mayonnaise, Food Hydrocolloids, 20:68–78, 2006.
87. Santipanichwong, R. and Suphantharika, M., Carotenoids as colorants in reduced-fat mayonnaise containing spent brewer's yeast β-glucan as a fat replacer, Food Hydrocolloids, 21:565–574, 2007.
88. da Silva Araújo, V.B., de Melo, A.N.F., Costa, A.G., Castro-Gomez, R.H., Madruga, M.S., de Souza, E.L., and Magnani, M., Followed extraction of β-glucan and mannoprotein from spent brewer's yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise, Innovat. Food Sci. Emerg. Technol., 23:164–170, 2014.
89. de Melo, A.N.F., de Souza, E.L., da Silva Araujo, V.B., and Magnani, M., Stability, nutritional and sensory characteristics of French salad dressing made with mannoprotein from spent brewer’s yeast, LWT-Food Sci. Technol., 62:771–774, 2015.
90. Borchani, C., Fonteyn, F., Jamin, G., Paquot, M., Thonart, P., and Blecker, C., Physical, functional and structural characterization of the cell wall fractions from baker’s yeast Saccharomyces cerevisiae, Food Chem., 194:1149–1155, 2016.
91. Konuklar, G., Inglett, G.E., Carriere, C.J., and Felker, F.C., Use of a β‐glucan hydrocolloidal suspension in the manufacture of low‐fat Cheddar cheese: Manufacture, composition, yield and microstructure, Int. J. Food Sci. Technol., 39:109–119, 2004.
92. Robbins, E.A. and Seeley, R.D., Cholesterol lowering effect of dietary yeast and yeast fractions, J. Food Sci., 42:694–698, 1977.
93. Nicolosi, R., Bell, S.J., Bistrian, B.R., Greenberg, I., Forse, R.A., and Blackburn, G.L., Plasma lipid changes after supplementation with β-glucan fiber from yeast, Am. J. Clin. Nutr., 70:208–212, 1999.
94. Kim, K.M., Chang, U.J., Kang, D.H., Kim, J.M., Choi, Y.M., and Suh, H.J., Yeast hydrolysate reduces body fat of dietary obese rats, Phytotherapy Res., 18:950–953, 2004.
95. Schwarz, K. and Mertz, W., Chromium (III) and the glucose tolerance factor, Arch. Biochem. Biophys., 85:292–295, 1959.
96. Mertz, W. and Schwarz, K., Relation of glucose tolerance factor to impaired intravenous glucose tolerance of rats on stock diets, Am. J. Physiol., 196:614–618, 1959.
97. Toepfer, E.W., Mertz, W., Polansky, M.M., Roginski, E.E., and Wolf, W.R., Preparation of chromium-containing material of glucose tolerance factor activity from brewer's yeast extracts and by synthesis, J. Agricult. Food Chem., 25:162–166, 1977.
98. Davies, D.M., Holdsworth, E.S., and Sherriff, J.L., The isolation of glucose tolerance factors from brewer’s yeast and their relationship to chromium, Biochem. Med., 33:297–311, 1985.
99. Weksler-Zangen, S., Mizrahi, T., Raz, I., and Mirsky, N., Glucose tolerance factor extracted from yeast: Oral insulin-mimetic and insulin-potentiating agent: In vivo and in vitro studies, Brit. J. Nutr., 108:875–882, 2012.
100. Mirsky, N., Cohen, R., Eliaz, A., and Dovrat, A., Inhibition of diabetic cataract by glucose tolerance factor extracted from yeast, Exp. Biol. Med. (Maywood), 241:817–829, 2016.
101. Vincent, J.B., Comment on purification and characterization of chromium-binding substances from high-chromium yeast, J. Agric. Food Chem., 61:9280–9281, 2013.
102. Grant, A.P. and McMullen, J.K., The effect of brewers’ yeast containing glucose tolerance factor on the response to treatment in Type 2 diabetics. A short controlled study, The Ulster Med. J., 51:110–114, 1982.
103. Offenbacher, E.G. and Pi-Sunyer, F.X., Beneficial effect of chromium-rich yeast on glucose tolerance and blood lipids in elderly subjects, Diabetes, 29:919–925, 1980.
104. Offenbacher, E.G., Rinko, C.J., and Pi-Sunyer, F.X., The effects of inorganic chromium and brewer's yeast on glucose tolerance, plasma lipids, and plasma chromium in elderly subjects, Am. J. Clin. Nutr., 42:454–461, 1985.
105. Cefalu, W.T. and Hu, F.B., Role of chromium in human health and in diabetes, Diabetes Care, 27:2741–2751, 2004.
106. Racek, J., Sindberg, C.D., Moesgaard, S., Mainz, J., Fabry, J., Müller, L., and Rácová, K., Effect of chromium-enriched yeast on fasting plasma glucose, glycated haemoglobin and serum lipid levels in patients with type 2 diabetes mellitus treated with insulin, Biolog. Trace Element Res., 155:1–4, 2013.
107. McCarty, M., High-chromium yeast for acne? Med. Hypotheses, 14:307–310, 1984.
108. Weber, G., Adamczyk, A., and Freytag, S., Treatment of acne with a yeast preparation, Fortschritte der Medizin, 107:563–566, 1989.
109. Lipson, A., Masters, H., O'Halloran, M., Thompson, S., Coveney, J., and Yu, J., The selenium status of children with phenylketonuria: Results of selenium supplementation, J. Paediatr. Child Health, 24:128–131, 1988.
110. Rayman, M.P., The use of high-selenium yeast to raise selenium status: How does it measure up? Brit. J. Nutr., 92:557–573, 2004.
111. Rayman, M.P., The importance of selenium to human health, The Lancet, 356:233–241, 2000.
112. Bressa, G.M., S‐adenosyl‐l‐methionine (SAMe) as antidepressant: Meta‐analysis of clinical studies, Acta Neurologica Scandinavica, 89:7–14, 1994.
113. Mischoulon, D. and Fava, M., Role of S-adenosyl-L-methionine in the treatment of depression: A review of the evidence, Am. J. Clin. Nutr., 76:1158S–1161S, 2002.
114. Lieber, C.S., S-adenosyl-L-methionine: Its role in the treatment of liver disorders, Am. J. Clin. Nutr., 76:1183S–1187S, 2002.
115. Purohit, V. and Russo, D., Role of S-adenosyl-L-methionine in the treatment of alcoholic liver disease: Introduction and summary of the symposium, Alcohol, 27:151–154, 2002.
116. Anstee, Q.M. and Day, C.P., S-adenosylmethionine (SAMe) therapy in liver disease: A review of current evidence and clinical utility, J. Hepatol., 57:1097–1109, 2012.
117. Najm, W.I., Reinsch, S., Hoehler, F., Tobis, J.S., and Harvey, P.W., S-adenosyl methionine (SAMe) versus celecoxib for the treatment of osteoarthritis symptoms: A double-blind cross-over trial, BMC Musculoskel. Disord., 5:6, 2004. DOI: 10.1186/1471-2474-5-6
118. Kadakia, K.C., Loprinzi, C.L., Atherton, P.J., Fee-Schroeder, K.C., Sood, A., and Barton, D.L., Phase II evaluation of S-adenosyl-L-methionine (SAMe) for the treatment of hot flashes, Support. Care Canc., 24:1061–1069, 2016.
119. Kim, J.M., Lee, S.W., Kim, K.M., Chang, U.J., Song, J.C., and Suh, H.J., Anti-stress effect and functionality of yeast hydrolysate SCP-20, Eur. Food Res. Technol., 217:168–172, 2003.
120. Lee, H.S., Jung, E.Y., and Suh, H.J., Chemical composition and anti-stress effects of yeast hydrolysate, J. Med. Food, 12:1281–1285, 2009.
121. Suh, H.J., Kim, S.Y., Chang, U.J., and Kim, J.M., Anti-stress effects of chewing gum prepared with yeast hydrolysate, Eur. Food Res. Technol., 227:331–336, 2008.
122. Kaplan, J.Z., Acceleration of wound healing by a live yeast cell derivative, Arch. Surg., 119:1005–1008, 1984.
123. Wolk, M. and Danon, D., Promotion of wound healing by yeast glucan evaluated on single animals, Med. Biol., 63:73–80, 1984.
124. Crowe, M.J., McNeill, R.B., Schlemm, D.J., Greenhalgh, D.G., and Keller, S.J., Topical application of yeast extract accelerates the wound healing of diabetic mice, J. Burn Care Res., 20:155–162, 1999.
125. Delatte, S.J., Evans, J., Hebra, A., Adamson, W., Othersen, H.B., and Tagge, E.P., Effectiveness of beta-glucan collagen for treatment of partial-thickness burns in children, J. Pediatr. Surg., 36:113–118, 2001.
126. Zülli, F., Suter, F., Biltz, H., Nissen, H.P., and Birman, M., Carboxymethylated β-(1-3)-glucan: A beta glucan from baker's yeast helps protect skin, Cosmet. Toiletries, 111:91–98, 1996.
127. Zülli, F., Suter, F., Blitz, H., and Nissen, H.P., CM-Glucan: A biological response modifier from baker's yeast for skin care, SÖFW. Seifen, Öle, Fette, Wachse, 123:535–541, 1997.
128. Li, B., Allendorf, D.J., Hansen, R., Marroquin, J., Ding, C., Cramer, D.E., and Yan, J., Yeast β-glucan amplifies phagocyte killing of iC3b-opsonized tumor cells via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathway, J. Immunol., 177:1661–1669, 2006.
129. Ghoneum, M. and Gollapudi, S., Apoptosis of breast cancer MCF-7 cells in vitro is induced specifically by yeast and not by fungal mycelia, Anticancer Res., 26:2013–2022, 2006.
130. Cheung, N.K.V. and Modak, S., Oral (1→ 3),(1→ 4)-β-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma, Clin. Canc. Res., 8:1217–1223, 2002.
131. Hansel, R., Polysaccharide, die immune-stimulierend wirking: Eine übersicht über entsprechende fertigarzneimittel, Farmaceutische Tijdschrft voor Belgie., 64:313–326, 1987.
132. Huige, N.J., Charter, W.M., and Wendt, K.W., Measurement and control of oxygen in carbon dioxide, Tech. Q. Master Brew. Assoc. Am., 22:92–98, 1985.
133. Kargari, A. and Ravanchi, M.T., Carbon dioxide: Capturing and utilization, in Greenhouse Gases—Capturing, Utilization and Reduction, Liu, G., Ed., InTech, Rijeka, Croatia, 2012. Available from: http://www.intechopen.com/books/greenhouse-gases-capturing-utilization-and-reduction/carbon-dioxidecapturing-and-utilization
* This Chapter has been updated based on the Chapter 18, “Brewery By-Products and Effluents” by Nick J. Huige in previous version.