Graham G. Stewart
CONTENTS
14.1 INTRODUCTION
The flavor, aroma, and overall characteristics of any alcoholic beverage (particularly beer) is, in large part, determined by the yeast strain used (further details in Chapter 8), the wort composition (details in Chapters 9, 11, and 15), and the fermentation conditions employed. Yeast properties such as flocculation, fermentation ability (including the uptake of wort sugars, amino acids, small peptides, and ammonium ions), osmotic pressure, ethanol tolerance, and oxygen requirements (details in Chapter 8 and later in this chapter) have a critical impact on fermentation performance. Proprietary strains, belonging to individual breweries, are usually (but not always) jealously guarded and conserved.
The objectives of brewer’s wort fermentation are to consistently metabolize wort constituents into ethanol, carbon dioxide, glycerol, and other fermentation products in order to produce beer with satisfactory quality, stability, and drinkability. Another important objective is to produce yeast crops that can be confidently repitched into subsequent brews in a batch fermentation process.1
Over the years, considerable effort has been made in numerous research and development laboratories (industrial and academic) with an interest in brewing fermentations, to enhance intensity (see Chapter 9) and the efficiency of this stage of the brewing process. One area that has received particular attention is an attempt to improve the efficiency of wort fermentation by evolving it from a batch to a continuous process—details in Section 14.4.
It has already been discussed (Chapter 8) that when yeast is introduced into wort—it is an extremely complex environment,2 compared to other media used in the production of fermentation alcohol (both industrial and potable), and that wort is by far the most intricate.3 When yeast is pitched into wort, it is introduced into a complex environment that consists of simple sugars, dextrins, amino acids, peptides, proteins, vitamins, ions, nucleic acids, and other constituents too numerous to mention. It has already been discussed (Chapter 8) that one of the major advances in brewing science during the past 40 years has been the elucidation of the mechanisms by which yeast cells utilize, in an orderly manner, the plethora of wort nutrients.4
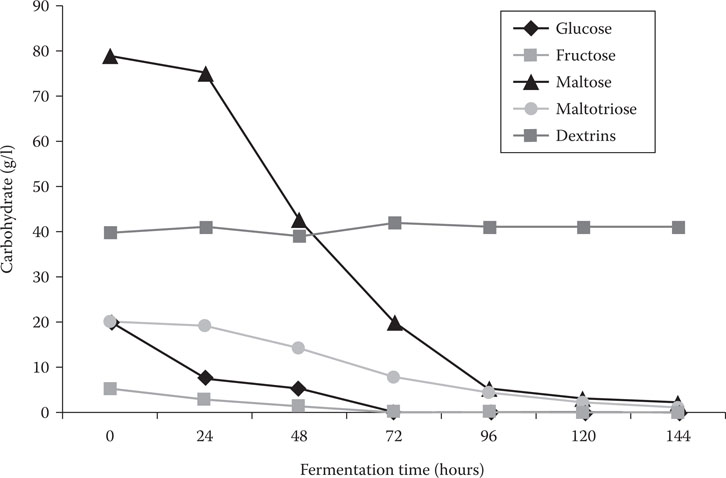
Free amino nitrogen (FAN) is the sum of the individual wort amino acids, ammonium ions, and small peptides (di- and tripeptides). FAN is an important general measure of yeast nutrients that constitute the yeast assimilable nitrogen during brewery fermentations.5 It has already been discussed here (Chapter 8) that wort also contains the fermentable sugars sucrose, fructose, glucose, maltose, and maltotriose, together with dextrin material (Figure 14.1). A typical percentage sugar spectrum of brewer’s wort is shown in Table 14.1.
Table 14.1 Typical Percentage Sugar Spectrum of a Brewer’s Wort
Sugar |
Percentage Composition |
|---|---|
Glucose |
10–15 |
Fructose |
1–2 |
Sucrose |
1–2 |
Maltose |
50–60 |
Maltotriose |
15–20 |
Dextrins |
20–30 |

Figure 14.1 Order of sugar uptake from wort.
14.2 WORT FERMENTATION
The fermentable wort sugars (sucrose, fructose, glucose, maltose, and maltotriose) comprise the largest component of solid material in solution. These five fermentable wort sugars are taken up by brewer’s yeast strains in a distinct order (Figure 14.1). Maltose is by far the largest concentration wort sugar6 followed by maltotriose (Figure 14.2). When glucose concentrations are high (greater than 10% [w/v]), the genes that control maltose metabolism (the MAL genes—discussed in Chapter 8) are repressed. It is only when 40% to 50% of the glucose has been taken up from the wort (this effect is strain dependent) that the metabolism (uptake) of maltose commences.7 This means that a major factor influencing wort fermentation rate is the inhibiting effect that glucose (and to a less extent fructose) has on maltose uptake.
Figure 14.2 Structure of maltose and maltotriose.
In an attempt to overcome this repression, the glucose analogue, 2-deoxy-glucose (2-DOG) has been successfully employed for the selective isolation of spontaneous mutants of various yeasts8 and other fungi9 (Figure 14.3). These 2-DOG mutants are depressed for the production of carbohydrate-hydrolyzing enzymes employing this nonmetabolizable glucose analogue. Derepressed mutants of brewing and other industrial yeast strains have been isolated. These mutants are able to metabolize wort maltose and maltotriose in the presence of glucose. Fermentation and ethanol formation rates in 12°Plato wort were also increased in the 2-DOG mutants when compared to the parental strain (Figure 14.4). In addition, 2-DOG starch mutants of Saccharomyces diastaticus (a yeast variant closely related to ale brewer’s yeast strains that can utilize wort dextrins as a result of the production of an extracellular glucoamylase—further details in Chapter 8) have been isolated that exhibited increased fermentation ability of brewer’s wort, cassava, and corn mash.10
Figure 14.3 Structure of D-glucose and 2-deoxy-D-glucose.
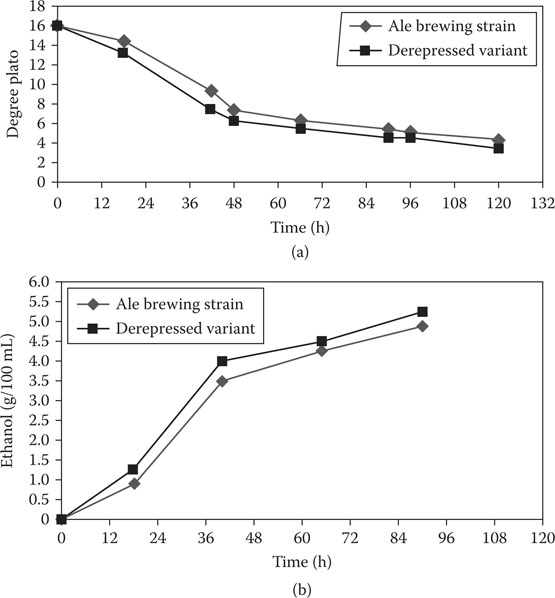
Figure 14.4 Degree Plato reduction (a) and ethanol formation (b) by an ale brewing strain and its 2-deoxy-D-glucose derepressed variant.
All of the Labatt studies in London, Ontario, with 2-DOG mutants, discussed earlier, were conducted with ale and distilling yeast strains. No 2-DOG mutants were isolated from the lager strains that were screened.8 This is the major reason why large-scale trials were not conducted with 2-DOG mutants in a country (Canada) that, by the late 1980s, was predominantly a producer of lager beers. A major Spanish brewing company, with university collaborators, reexamined the use of 2-DOG mutants to ferment 25°Plato wort with lager yeast strains using 2-L European Brewery Convention (EBC) tubes11 and assessed them at a 13°C incubation temperature.12 Improved fermentation capacity, where glucose did not repress maltose uptake, was achieved without changes in the beer flavor profile. However, large-scale fermentation studies (600 hL) with these 2-DOG lager yeast mutants did not result in the required improvements in fermentation efficiency (similar to the results obtained with ale yeast 2-DOG mutants in Canada). As a consequence, further studies with 2-DOG mutants were deferred.
The concluding stages of a brewing wort fermentation overlap with maturation (this involves, mainly, but not exclusively, vicinal diketone (VDK) management and the control of volatile sulfur compounds such as hydrogen sulfide, sulfur dioxide, and a plethora of thiol compounds; details in Chapter 15). It is often difficult to decide where wort fermentation concludes and maturation begins. Indeed, some brewing operations do not mature their beer as a distinct unit process, and the latter stages of fermentation include the following: the uptake of maltotriose and group C amino acids and small peptides, removal of VDK and unwanted sulfur compounds, and—eventually—yeast collection (by flocculation or a centrifuge) for reuse in subsequent fermentations.
14.3 BATCH FERMENTATION
The principal fermentation mode employed globally is batch. As will be described later, there was a brief dalliance in the brewing industry with continuous fermentation. Brewing with batch fermentation is a technique that has predominated for centuries. Although a number of batch fermentation techniques have been employed—for example, horizontal vessels, Yorkshire stone square systems, Burton Union, spherical fermenters, and flat-bottomed sloped rectangular vessels—vertical cylindro-conical vessels (also called Nathan vessels) are the most popular13 and, over the years, increasing capacity vessels have been installed (Figure 14.5) (Table 14.2).
Table 14.2 Fermentation Characteristics of Modern Cylindro-Conical Fermenters
Challenge |
Typical Causes |
Possible Effects |
|---|---|---|
Hot spots |
High outdoor temperatures and sun load. Insufficient heat transfer from tank jackets, inadequate mixing with large tank dimensions |
Yeast stress, variable fermentation conditions, beer instability (haze, poor foam and off-flavors) |
CO2 toxicity |
Premature yeast settling, inadequate mixing, super saturation, tall tanks/high hydrostatic pressure, high fermentation pressures, high-gravity fermentations |
Yeast stress, incomplete and variable fermentations, yeast autolysis, poor foam quality |
Alcohol toxicity |
High gravity brewing |
Yeast stress, premature settling, incomplete fermentations, beer off-flavor |
Premature yeast settling |
Flocculent yeast, inadequate mixing, high-gravity wort |
CO2 toxicity, hot spots, poor access to beer nutrients, yeast stress, incomplete fermentation and poor beer stability |
CO2 super saturation |
High levels of beer CO2, excess foaming |
Yeast stress, off-flavors, product and bitterness loss |

Figure 14.5 Cylindro-conical fermenters at Briggs of Burton.
However, little has changed in basic vessel design. Recent advances mainly relate to automation and improved methods for monitoring and control of the fermentation process. During the past few years, with the advent of larger vessels (5,000 hL plus), the question of nonhomogeneous fermentations within a vessel has come to light. This phenomenon has been termed “stratified fermentation” and has arisen as a result of large multibrew fermenters.
A stratified fermentation is where two or more separate and distinct fermentations occur in a fermenter at the same time.14 These fermentations are layered and marked by boundary zones. Each fermentation has an individual density, temperature, and yeast cell count. A number of conditions during the development of stratification in fermentation are unlikely during maturation (further details later and in Chapter 15):
There are several tools available to detect stratified fermentations:
A number of breweries and research laboratories have assessed the use of a rotary jet mixer (Figure 14.6) in order to avoid stratified fermentation.14 The benefits provided by rotary jet mixing are the result of four primary mechanisms (details in Table 14.2)15:

Figure 14.6 Rotary jet mixer.
Results are positive with more rapid VDK (primarily diacetyl) adjustments (details in Chapter 15), enhanced beer quality, stability, and faster fermentation rates leading to increases in production capacity without excessive capital investment.
14.4 CONTINUOUS FERMENTATION
In the 1950s and 1960s, there was great expectation that significant improvements in process efficiency and overall control would be gained by a move from batch to continuous fermentation in brewing. In addition, another important motive for engaging in continuous processing was economy—particularly the overall cost of the required plant equipment, labor, and real estate.
Although continuous fermentation for the production of beer was first attempted at the turn of the last-but-one century, the processes trialed were unsuccessful. A reawakening of interest was stimulated in the late 1950s as part of the overall postwar reindustrialization. Multivessel systems (Figure 14.7) were in operation on a trial basis in Canada,16 the United States, New Zealand,17 and the United Kingdom.18 This multivessel process was shortly followed in the United Kingdom by a novel tower system (Figure 14.8), which exploited the ability of flocculent yeast strains to sediment efficiently, thus enabling a high concentration of cells to be maintained within the system.18 Both continuous processes opened the specter of more rapid fermentations that had hitherto not been possible with batch systems. In the decade between 1960 and 1970, substantial interest in continuous fermentation occurred in the brewing industry. Enhanced knowledge of brewing/fermentation science (but not enough!), together with advances in engineering and electronic control equipment, offered real hope that continuous fermentation in brewing could be developed into a commercially viable process. It was anticipated that the following advantages would result from the application of continuous fermentation processes in brewing:

Figure 14.7 Multitank stirred continuous wort fermentation.
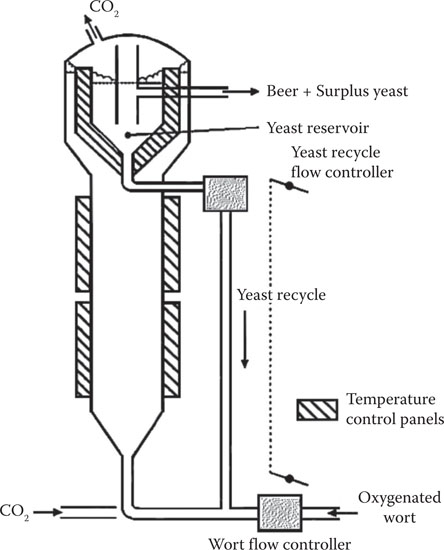
Figure 14.8 Continuous tower fermenter.
The major economic gains were therefore capital investment, labor, and inventory costs. Since the late 1960s and early 1970s, the situation in many countries has radically changed! The exception has been a brewer in New Zealand17 (and experiments with immobilized cell technology procedures in the 1990s—which have been largely unsuccessful). An increase in its use for ale production in the late 1960s proved transitory! Continuous fermentation has never found general acceptance for lager beer production (except for in New Zealand).18
There are a number of reasons why continuous fermentation in brewing was unsuccessful when it was developed over 50 years ago. Basically expressed, batch fermentation was (and still is) simpler in concept! A vessel is cleaned, sterilized, and rinsed, and then filled with wort, pitched with the required quantity of yeast, and oxygenated. The primary fermentation cycle can be preprogrammed with little further attention required until maturation (details in Chapter 15).
Continuous fermentation requires ongoing laboratory monitoring and automatic control of flow rates, temperature gradients, yeast recycle rates, potential bacterial and wild yeast contamination, and oxygen levels. Also, the technical expertise of operators needs to be enhanced. The advent of on-line control and rapid microbiological and analytical methodology has overcome some of these problems (details in Chapters 17 and 21).
Continuous fermentation introduction negatively influenced overall brewing operations. For example, seasonal and weather variations can be a challenge in satisfying customer needs. This flexibility need was exacerbated with the arrival on the market of a plethora of different beer types and brands—low and high alcohol, specialty flavors, dark beers, and so on. However, with today’s ability to produce a spectrum of beer types from a standard neutral beer after “painting” with the following options: malt extract, flavoring materials,19 syrups,20 and/or hop extracts,21 some, but not all, of these problems have been overcome. Today, brewers are able to develop a plethora of liquids from a neutral beer produced by a batch fermentation.22
With one exception (already discussed),17 continuous fermentation processes are not being employed in the brewing process. However, this fermentation technique is being applied to the production of industrial (fuel) ethanol in North America, Brazil, and elsewhere.10
14.5 YEAST COLLECTION
It has already been discussed that yeast collection techniques at the end of batch wort fermentation (also termed cropping) vary depending on whether one is dealing with a traditional ale top cropping fermentation system, a traditional lager bottom fermentation system—cylindro-conical fermenters,21 or a nonflocculent culture in which the yeast is cropped with a centrifuge (details later). With the traditional ale top fermentation system, there are many variations to this procedure—for example, a single, dual, or multistrain yeast system can be employed. The phenomenon of co-flocculation (already discussed in Chapter 8) is an example of two nonflocculent strains that flocculate together.22 The timing of the skimming process can be critical in order to maintain the flocculation characteristics of the ale strains. Traditionally, the first skim or “dirt skim” with trub present is discarded, as is the final skim, usually with the middle skim yeast culture being kept for repitching. With traditional lager bottom fermentation systems, the yeast is deposited on the bottom of the vessel during the closing stages of fermentation. This type of yeast collection is essentially (not completely) nonselective, and the yeast will normally contain entrained trub. The cylindro-conical fermentation system has now been widely adopted for both ale and lager fermentations, and the cycle at the bottom of the fermenter allows for effective removal of the yeast plug.
Yeast cropping can be a time-consuming process stage, particularly if it employs the flocculation properties of a yeast culture, which can be inconsistent.23 One way to overcome some of the cropping problems is to employ a centrifuge to harvest the yeast. Centrifuges have a number of applications during the brewing process,24 but the cropping of yeast with a centrifuge at the end of primary fermentation is currently its principal function. There are a number of advantages to the use of centrifuges. These include a shorter process time, cost reduction (after significant enhanced capital costs of the equipment— Figure 14.9), increased productivity, and reduced wort shrinkage.24 Care must be taken to ensure that elevated temperatures (above 20°C) are not generated during centrifugation and that the centrifuge design ensures minimal dissolved oxygen pickup.

Figure 14.9 Westfalia separator—disc stack centrifuge.
In addition, centrifugation can (under certain circumstances) cause physical damage to yeast cells and, consequently, negatively affect beer physical stability (haze) (Figure 14.10). This is dependent on centrifuge operating parameters. Hydrodynamic forces and yeast cell interactions, within the gap of the centrifuge disc stacks, create collisions among yeast cells producing kinetic energy that causes cellular damage. Release of cell wall mannan during mechanical agitation of yeast slurries, in conjunction with an increase in beer haze, has been well-documented.25
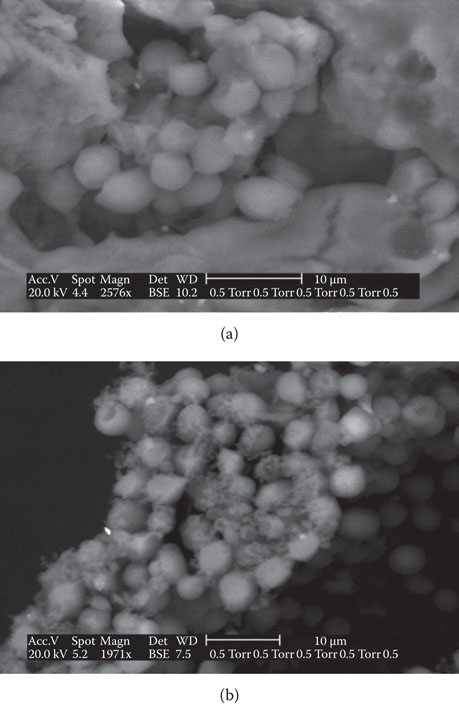
Figure 14.10 Environmental scanning electron microscope of ale yeast strain damage: (a) cells before passing through a disc centrifuge and (b) cells following passage through a disc centrifuge operating at a high g-force.
14.6 YEAST STORAGE
At the end of fermentation, the yeast is cropped for further use employing the flocculating characteristics of the yeast strain or with a centrifuge. However, in this discussion, yeast cropping is considered to be part of fermentation—not yeast management between fermentations. It has already been described that one method of yeast cropping, which is increasing in popularity, is the use of centrifuges, although their use has not been without its problems (details later).26
If a cropped yeast culture is not stored properly, cell consistency suffers, and it will adversely affect fermentation and beer quality. Following cropping, the yeast is stored in a room that is sanitized and that contains a plentiful supply of sterile water and a separate filtered air supply with positive pressure to prevent the entry of contaminants at a temperature of 0ºC. Alternatively, insulated tanks in a dehumidified room can be employed. Also, “off the shelf” yeast storage facilities are available at various working capacities.
Yeast is predominantly stored under 6 inches of beer (sterile water has been employed in the past, but its use is unpopular these days). When high gravity brewing is practiced, it is important to ensure that the ethanol level of the storage beer is decreased to 4% to 6% (v/v) ethanol in order to maintain the viability and vitality of the stored yeast. As more sophisticated systems become available, storage tanks with external cooling (0ºC to 4ºC), equipped with low shear stirring devices, have become popular. The need for low-shear stirring systems has been shown to be important. With high velocity agitation in a yeast storage tank, the yeast cell surface can become disrupted and intracellular proteases (particularly proteinase A [PrA]) are excreted into the suspending medium; this can result in unfilterable mannan hazes in beer27 and poor head retention due to protease hydrolytic activity on foam stability enhancing peptides.28 There are procedures where the yeast is not stored between fermentations. In this case, the yeast is pitched directly from one fermenter to another. This yeast handling procedure occurs with cylindro-conical (vertical) fermenters and is termed “cone to cone yeast pitching.” The procedure was employed by some breweries (particularly in the United Kingdom) in the 1980s and 1990s but currently has limited application because of lack of opportunity and time to conduct quality and contamination studies on the flocculated yeast between fermentations.
One of the factors that will affect fermentation rate is the condition under which the yeast culture is stored between fermentations. Of particular importance in this regard is the influence of temperature during these storage conditions on the cell’s intracellular glycogen level. Glycogen is the major reserve carbohydrate stored within the yeast cell and similar in structure to plant amylopectin (Figure 14.11). It has already been discussed that glycogen serves as a store of biochemical energy during the lag phase of fermentation when the energy demand is intense for the synthesis of lipid compounds such as sterols and unsaturated fatty acids (Figure 14.12). Consequently, it is important that appropriate levels of glycogen (Figure 14.13) and trehalose are maintained during storage so that, during the initial stages of fermentation, the yeast cell is able to synthesize sterols and unsaturated fatty acids and trehalose. Trehalose is a nonreducing disaccharide (Figure 14.14) that plays a protective role in osmoregulation, protection of cells during conditions of nutrient depletion and starvation, and in improving cell resistance to high and low temperatures and to elevated ethanol.
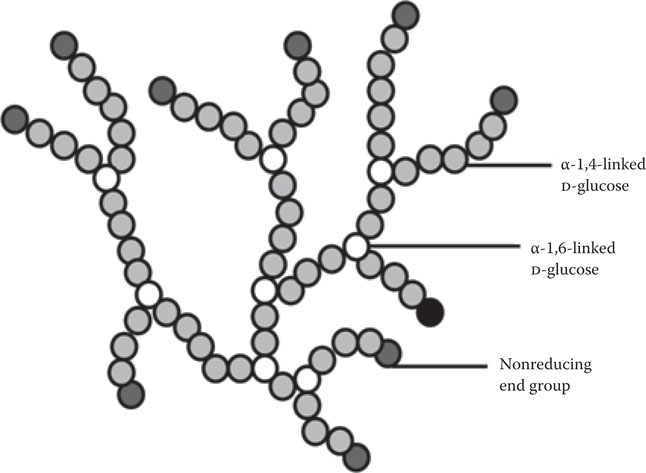
Figure 14.11 Structure of glycogen.
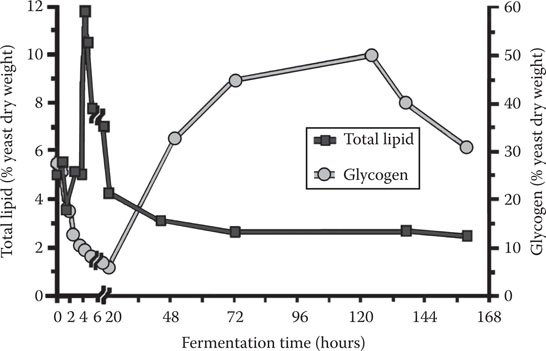
Figure 14.12 Intracellular concentrations of glycogen and lipids in a lager yeast strain during fermentation of a 16° Plato wort.
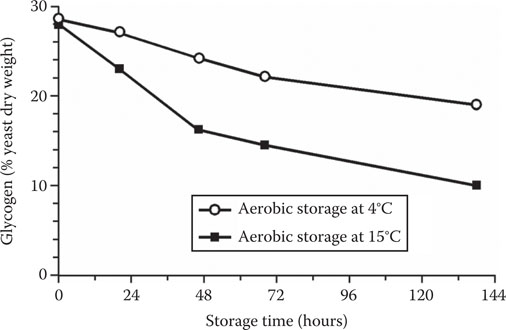
Figure 14.13 The effect of yeast storage temperature on intracellular glycogen concentration.
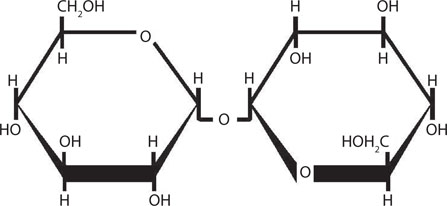
Figure 14.14 Structure of trehalose.
Storage temperature (Figure 14.13) has a direct influence on the rate and extent of glycogen utilization, as might be expected, considering the effect that temperature has upon metabolic rates in general. Although strain-dependent, of particular interest is the fact that, within 48 hours, yeast stored semi-aerobically at 15ºC has only 15% of the original glycogen concentration remaining. Glycogen reductions to this extent will have a profound effect on wort fermentation (Figure 14.15).
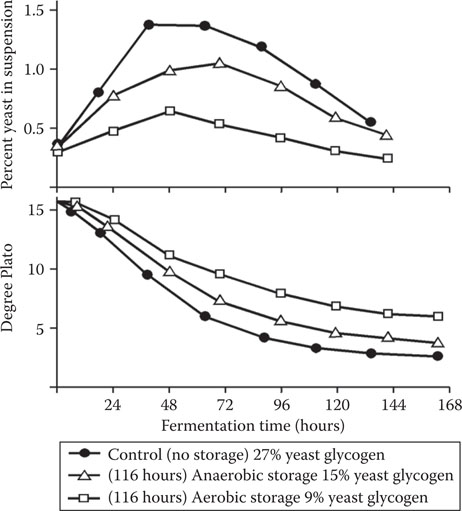
Figure 14.15 The effect of yeast glycogen at pitching on lager wort fermentation characteristics.
The number of times that a yeast crop (generations or cycles) is used for wort fermentations is generally standard practice in a particular brewery. Typically today, a yeast culture is used 6 to 10 times prior to reverting to a fresh culture of the same strain from the pure yeast culture plant. If a particular yeast culture is used beyond the crop specification, fermentation difficulties (e.g., fermentation rate and extent) are sometimes encountered. An example of this effect is when a brewery increases its wort gravity and adopts high gravity brewing procedures. A particular brewing operation, over a 15-year period, increased its wort gravity incrementally. In order to avoid fermentation difficulties, it reduced the number of yeast cycles from a single propagation as follows:
12º Plato wort—more than 20 yeast cycles
14º Plato wort—16 yeast cycles
16º Plato wort—12 yeast cycles
18º Plato wort—8 yeast cycles
Currently, some breweries have adopted a yeast reuse specification of as few as four to six cycles!
The reason why multiple yeast generations can have a negative effect on a culture’s fermentation performance is unclear. However, multiple generations will result in reduced levels of intracellular glycogen and an increase in trehalose, indicating additional stress conditions on yeast cells as the cycles progress (Table 14.3).29
Table 14.3 Concentration of Trehalose and Glycogen in Lager Yeast Following One, Four, and Eight Fermentation Cycles (15° Plato Wort)
Generations (cycles) |
|||
|---|---|---|---|
One |
Four |
Eight |
|
Trehalose a |
8.8 |
9.2 |
11.6 |
Glycogenb |
14.6 |
12.6 |
9.2 |
a μg/g dry weight of yeast.
b mg/g dry weight of yeast.
Yeast storage conditions between brewing fermentations can affect fermentation efficiency and beer quality. Good yeast handling practices should encompass collection and storage procedures, avoid inclusion of oxygen in the slurry, cool the slurry to 2ºC to 4ºC soon after collection, and—perhaps most importantly—ensure that glycogen levels are maintained because of their critical property at the start of wort fermentation.
14.7 YEAST WASHING
Acid washing pitching yeast at pH 2 to 2.2 (with either phosphoric, tartaric, hydrochloric, sulfuric, or nitric acid solutions) usually during the later stages of storage, just prior to being pitched into wort for fermentation, has been employed by many breweries for the past 100 years (and longer) as an effective method for eliminating contaminating bacteria (not wild yeasts) without adversely affecting the physiological quality of the yeast culture. The acid washing regime differs between breweries, with some routinely acid washing their yeast culture after each fermentation cycle, whereas other brewers only acid wash their pitching yeast when there is significant bacterial contamination, and some refrain from acid washing completely. Brewer’s yeast strains are normally resistant to acidic conditions when the washing is conducted properly. However, if other environmental and operating conditions are modified, then the acid resistance of the culture will vary. Simpson and Hammond30 demonstrated that if the temperature of acid washing was greater than 5ºC and/or the ethanol concentration was greater than 8%(v/v), acid washing had a detrimental effect on the culture, causing a decrease in viability and fermentation performance. The physiological condition of the yeast prior to acid washing is an important factor in acid tolerance, with yeast in poor physiological condition prior to washing being more adversely affected by acid washing than healthy yeast.
Acid washing primarily affects the yeast cell envelope containing the physiological systems associated with both the cell wall and the plasma membrane, subsequently decreasing yeast vitality as measured by the acidification power test.31 Studies by Cunningham and Stewart32 have reported that acid washing pitching yeast from high gravity (20° Plato) wort fermentations did not affect the fermentation performance of cropped yeast if it was maintained in good physiological condition. Oxygenation of the yeast at the start of fermentation stimulated yeast growth, leading to a more efficient wort fermentation and, equally important in the context of yeast management between fermentations, producing yeast that was in good physiological condition, permitting it to tolerate exposure to acid washing conditions (phosphoric acid solution at pH 2.2). These data support the findings of Simpson and Hammond,30 who concluded that yeast in poor physiological condition should not be acid washed.
In summary, the dos and do nots for yeast acid washing listed by Simpson and Hammond30 are still appropriate and can be summarized as follows.
The dos of acid washing are:
The do nots of acid washing are:
There are a number of options to acid washing brewer’s yeast:
14.8 YEAST STRESS
During wort fermentation, a yeast culture is exposed to a number of stress conditions; currently, the primary stress factor is the use of high gravity worts33 (details in Chapter 9). During these circumstances, yeast cells are exposed to numerous stresses, including osmotic stress at the beginning of fermentation due to high concentrations of wort sugars and ethanol stress at the end of fermentation.34 Other forms of yeast stress are desiccation (details later), mechanical stress (details later), and thermal stress (hot and cold) (Figure 14.16). The yeast is expected to maintain its metabolic activity during stressful conditions by not only surviving these stresses but by rapidly responding to ensure continued cell viability and vitality.35
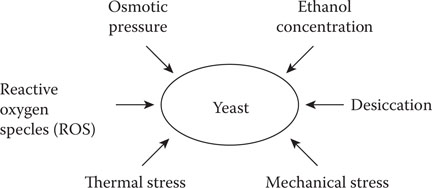
Figure 14.16 Stress factors which promote proteinase A release.
Stress can have a profound and varied effect on yeast cells, including the following:
The balance between PrA occurring in the fermenting wort as a result of cell autolysis and/or enzyme excretion of whole cells is still an unanswered question. Nevertheless, the occurrence of active PrA is important because of its negative effect on beer foam stability.
14.9 DRIED YEAST
Dried yeast has been employed in the baking and distilling industries for more than 50 years.36 However, its use in brewing is relatively recent.37, 38 Among the reasons for this delay are the differences in drying characteristics. Ale yeast strains dry relatively well, whereas lager yeast cultures, when dried, have comparatively lower viabilities. The reasons for the drying differences between these two yeast species are still not fully understood, but the levels of the storage carbohydrates, glycogen, and trehalose have been implicated. Another reason for the delay in adopting dried yeast in brewing is that this yeast is often contaminated with various bacteria and wild yeasts during its production—usually in a baker’s yeast factory. This problem is not as prevalent today as it was 25 years ago.
The use of dried yeast has several advantages and similarities compared to the use of fresh yeast:
It has been reported39 that dried yeast samples exhibited different flocculation and haze formation characteristics when compared to fresh yeast samples. The flocculation rate with fresh and dried ale cultures was rapid, with most of the yeast sedimenting out of suspension within the first minute of the Helm’s sedimentation test,40 with 80% of the culture eventually flocculating out of suspension. The flocculating differences between fresh lager and dried lager samples was more pronounced than with ale strains. Virtually no flocculation took place within a 10-minute test period with the dried yeast samples. This test indicated that the lager dried yeast samples were modified in some way and, as a consequence, exhibited nonflocculent characteristics.
During the studies on flocculation, it was observed that the dried yeast fermentation often left a haze in suspension. Even with ale yeast fermentations, although the yeast flocculated, a haze remained in suspension. This may have been due, in part, to the number of dead cells pitched into the wort. In addition, the fresh yeast fermentation exhibited a foam head, but such a head was absent on the dried yeast samples (both ale and lager cultures). PrA (and other proteinases) is released by dried yeast into the wort in much greater quantities than fresh yeast under similar fermentation conditions.39 PrA release into the wort will have an impact on beer foaming characteristics. The decreased foam stability is due to the hydrolysis of hydrophobic polypeptides by PrA (further details in Chapter 9). Hydrophobic polypeptides are known to be mainly responsible for beer foam stability.41 Leakage of intracellular proteinases from living brewer’s yeast has been demonstrated, particularly when the cells are under stress41 (details in Chapter 2). Indeed, the addition of dead cells (as could be the case with a dried culture) would greatly increase the levels of PrA.
REFERENCES
1. Stewart, G.G. and Russell, I., An Introduction to Brewing Science and Technology. Series III. Brewer’s Yeast, 2nd ed., The Institute of Brewing and Distilling, London, 2009.
2. Stewart, G.G., Hill, A.E., and Russell, I., 125th Anniversary Review—Developments in brewing and distilling yeast strains, J. Inst. Brew., 119:202–220, 2013.
3. Stewart, G.G. and Russell, I., Fermentation – “Black box” of the brewing process, Tech. Q. Master Brew. Assoc. Am., 30:159–168, 1993.
4. Stewart, G.G., Biochemistry of brewing, in Biochemistry of Foods, 3rd ed., Eskin, N.A.M. and Shahidi, F., Eds., Academic Press, London, pp. 291–318, 2013.
5. Lekkas, C., Stewart, G.G., Hill, A., Taidi, B., and Hodgson, J., The importance of free amino nitrogen in wort and beer, Tech. Q. Master Brew. Assoc. Am., 42:113–116, 2005.
6. Stewart, G.G., Studies on the uptake and metabolism of wort sugar during brewing fermentations, Tech. Q. Master Brew. Assoc. Am., 43:265–269, 2006.
7. Stewart, G.G., Glucose, maltose and maltotriose. Do yeast strains care which one? Asia Proc. 31 st Conv. Inst. Brew. Dist. Asia Pacific Section, Paper 03, 2010.
8. Russell, I., Jones, R.M., Berry, D.R., and Stewart, G.G., Isolation and characterization of derepressed mutants of Saccharomyces cerevisiae and Saccharomyces diastaticus, in Biological Research on Industrial Yeasts, Vol II, Stewart, G.G., Russell, I., Klein, R.D., and Hiebsch, R.R., Eds., CRC Press, Boca Raton, FL, pp. 57–65, 1987.
9. Beja da Costa, M. and Van Uden, N., Use of 2-deoxyglucose in the selective isolation of mutants of Trichoderma reesei with enhanced β-glucosidase production, Biotechnol. Bioeng., 22:2429–2432, 1980.
10. Whitney, G.K., Murray, C.R., Russell, I., and Stewart, G.G., Potential cost savings for fuel ethanol production by employing a novel hybrid yeast strain, Biotechnol. Lett., 7:349–354, 1985.
11. Boulton, C. and Quain, D., Brewing Yeast and Fermentation, Blackwell Science, Oxford, 2001.
12. Piña, B., Casso, D., Orive, M., Fité, B., Torrent, J., and Vidal, F., A new source to obtain lager yeast strains adapted to very high gravity brewing: 2-Deoxy-D-glucose resistant clones with fermentation capacity of up to 25° Plato. Proc. 31 st, Eur. Brew. Conv. Congr., Venice, CD Fachverlag Hans Carl, Nürenberg, pp. 186–408, 2007.
13. Nathan, L., Improvements in the fermentation and maturation of beers, J. Inst. Brew., 36:538–550, 1930.
14. Kapral, D., Stratified fermentation—Causes and corrective actions, Tech. Q. Master Brew. Assoc. Am., 45:115–128, 2008.
15. Zdaniewicz, M., Poreda, A., and Tuszynski, T., Rotary jet head—A device for accelerating the fermentation process in brewing, J. Inst. Brew., 122:317–321, 2016.
16. Geiger, K.H. and Compton, J., Continuous fermentation process, U.S. Patent #2,967,107, 1961.
17. Stratton, M.R., Campbell, S.J., and Banks, D.J., Process developments in the continuous fermentation of beer, Asia 23rd Proc. Conv. Inst. Brew Asia Pacific Section, pp. 96–100, 1994.
18. Portno, A.D., Continuous fermentation of brewer’s wort, J. Inst. Brew., 114:45–61, 1968.
19. Coghe, S., D’Hollander, H., Verachtert, H., and Delvaux, F., Impact of dark specialty malts on extract composition and wort fermentation, J. Inst. Brew., 111:51–60, 2005.
20. Helstad, S., Topics in brewing: Brewing adjuncts—Liquid, Tech. Q. Master Brew. Assoc. Am., 50: 99–104, 2013.
21. Roberts, T.R., and Wilson, R.S., Hops, in Handbook of Brewing, 2nd ed., Priest, F.G., and Stewart, G.G., Eds., Taylor and Francis, Boca Raton, FL, pp. 177–279, 2006.
22. Stewart, G.G., Maskell, D.L., and Speers, A., Brewing fundamentals—fermentation, Tech. Q. Master Brew. Assoc. Am., 53:2–22, 2016.
23. Stewart, G.G., Yeast quality assessment, management and culture maintenance, in Brewing Microbiology—Managing Microbes, Ensuring Quality and Valorising Waste, Hill, A.E., Ed., Woodhead Publishing, Cambridge, pp. 11–29, 2015.
24. Chlup, P.H. and Stewart, G.G., Centrifuges in brewing, Tech. Q. Master Brew. Assoc. Am., 48:46–50, 2011.
25. Stoupis, T., Stewart, G.G., and Stafford, R.A., Mechanical agitation and rheological consideration of ale yeast slurry, J. Am. Soc. Brew. Chem., 60:58–62, 2002.
26. Chlup, P.H., Bernard, D., and Stewart, G.G., The disk stack centrifuge and its impact on yeast and beer quality, J. Am. Soc. Brew. Chem., 65:29–37, 2007.
27. Stoupis, T., Stewart, G.G., and Stafford, R.A., Hydrodynamic shear drainage of brewer’s yeast, J. Am. Soc. Brew. Chem., 61:219–228, 2003.
28. Cooper, D.J., Stewart, G.G., and Bryce, J.H., Yeast proteolytic activity during high and low gravity wort fermentations and its effect on heat retention, J. Inst. Brew., 106:197–201, 2000.
29. Odumeru, J.A., D’Amore, T., Russell, I., and Stewart, G.G., Alterations in fatty acid composition and trehalose concentration of Saccharomyces brewing strains in response to heat and ethanol shock, J. Indust. Microbiol., 11:113–119, 1993.
30. Simpson, W.J. and Hammond, J.R., The response of brewing yeast to acid washing, J. Inst. Brew., 95: 347–354, 1989.
31. Kara, B.V., Simpson, W.J., and Hammond, J.R.M., Prediction of the fermentation performance of brewing yeast with the acidification power test, J. Inst. Brew., 94:153–158, 1988.
32. Cunningham, S. and Stewart, G.G., High gravity brewing and acid washing in brewer’s yeast, J. Am. Soc. Brew. Chem., 56:2–7, 1998.
33. Chlup, P.H., Biological and hydrodynamic stress influences on brewing yeast strains’ physiology status during beer production. PhD thesis, Heriot-Watt University, Edinburgh, 2008.
34. Pratt, P.L., Bryce, J.H., and Stewart, G.G., The effects of osmotic pressure and ethanol on yeast viability and morphology, J. Inst. Brew., 109:218–228, 2003.
35. Pratt-Marshall, P.L., Brey, S.E., de Costa, S.D., Bryce, J.H., and Stewart, G.G., High gravity brewing—An inducer of yeast stress, Brew. Guard., 131:22–26, 2002.
36. Pyke, M., The technology of yeast, in The Chemistry and Biology of Yeasts, Cook, E.H., Ed., Academic Press, New York, pp. 535–586, 1958.
37. Fels, S., Reckelbus, B., and Gosselin, Y., Why use dried yeast for brewing your beers? Brewing Distilling Int., 29:17–19, 1998.
38. Debourg, A. and Van Nedervelde, L., The use of dried yeast in the brewing industry, Proc. 27 th Eur. Brew. Conv. Congr., Cannes, IRL Press, Oxford, pp. 751–760, 1999.
39. Finn, D.A. and Stewart, G.G., Fermentation characteristics of dried brewers’ yeast: Effect of drying on flocculation and fermentation, J. Am. Soc. Brew. Chem., 60:135–139, 2002.
40. Helm, E., Nohr, H., and Thorne, R.S.W., Measurement of yeast flocculation and its significance in brewing, Wallerstein Lab. Comm., 16:315–326, 1953.
41. Bamforth, C.W., The foaming properties of beer, J. Inst. Brew., 93:370–383, 1985.