Beer’s Nonbiological Instability
Graham G. Stewart
CONTENTS
20.1 INTRODUCTION
Beer (unlike many alcoholic beverages) is unstable.1 Its properties modify over time. This can occur within a very short time (e.g., foam collapse, the appearance of lightstruck character, and beer gushing) or over longer periods (e.g., biological instability, which can be allied to haze development and flavor deterioration). Beer microbiological control is covered in depth in Chapter 17 and will not be considered further here.
Beer instability (excepting biological instability) can be classified into several categories:
During the past 20 years—and longer, there have been a number of reviews on nonbiological beer instability. There are unlimited reviews on haze,2–4 foam,5 flavor instability,6 flavor instability,7–12 gushing13–15 and lightstruck.16, 17 The elucidation of the mechanisms involved in beer nonbiological instability has been a longstanding research priority of the brewing industry in order to predict and extend beer shelf life.
20.2 PHYSICAL INSTABILITY
This is also known as colloidal instability,1 and it has been subdivided into the following categories:
Precipitates tend to develop when beer is subjected to extremes of temperature. The importance of how temperatures will induce materials to precipitate from solution has been reviewed by Miedl and Bamforth.18 There is no practical opportunity to freeze beer in a brewery to enhance the removal from solution of colloidal materials. The exception is “ice beer” technology.19 The process was very complex and not practical in the long term. However, for a limited time, the image of “ice” appealed to Canadian beer drinkers, especially those who were already used to concentrating their beer out of doors, during winter, in order to freeze and concentrate the alcohol at home! The claim that this “ice” technique resulted in greater smoothness is open to debate. With depleted marketing support, customers concluded that this beer was not notably different from mainstream products, and “ice beer” consumption went into decline! The capital cost of the icing equipment was excessive, as were the operating costs!
Beer exposed to high temperatures may also develop precipitates. Bamforth1 has related his experience in a Middle Eastern market with an alcohol-free beer heated to ambient temperatures exceeding 50°C! This beer developed a gelatinous precipitate as a result of the interaction between isinglass fining material and the foam stabilizer propylene glycol alginate.2 (Further details of these processing aids and additions can be found in Chapter 10.)
Bits are not always associated with the formation of a precipitate and may be difficult to see clearly. This means that they do not always present an obvious problem, as does a distinct turbidity in beer. The preferable way to detect bits is to filter the beer through filter paper and then stain with methylene blue. The method can be quantified by comparing with filter papers containing different quantities of stained bits. In addition, the development of bits can be due to proteins and pentosans that arise as a result of shaking beer inside kegs, bottle, and cans during their shipment globally. Agitation of beer certainly exacerbates clarity problems!20
Haze proper is a uniform lack of beer clarity. It can arise from a number of materials (in addition to the growth of living organisms—details in Chapter 17): starch,21 pentosans,22 oxalic acid,23 and protein-polyphenol complexes.24 Less common causes that have been studied include can lid lubricants25 and dead bacteria arising mainly from malt.26, 27 Haze is divided into “chill haze,” which develops when beer is chilled to 0°C; but this haze material returns to solution when the beer is warmed to 20°C. “Permanent haze” is present in beer at all practical temperatures. The extent to which visible haze is detectable by consumers has been investigated.28, 29 The current importance of beer clarity in bottles and cans is debatable because of consumers’ regrettable propensity to drink beer directly out of the container!
Invisible haze is also called “pseudo haze”30 due to small particles (less than 0.1 µm) that result in high levels of light scatter when haze is measured at 90°C. Causes of it include tiny particles originating in unmodified regions of the starchy endosperm of barley,30 retrograded starch,31 and polysaccharides removed from the surface of yeast cells.32 Also, removal of part of the yeast cell surface during centrifugation can result in haze formation.33
Proteins, polyphenols, and colloidal instability have been highlighted by Outtrup et al.34, 35 with regard to colloidal instability. The most pertinent beer polypeptides with regard to colloidal instability are rich in proline and glutamine and originate from the hordein fraction of barley.
Monomeric polyphenols, such as catechin (Figure 20.1) become haze-potentiating when they are polymerized through oxidation.36 It has been suggested that dimers represent the most potent entities with regards to polyphenol—protein interactions.37

Figure 20.1 A typical beer polyphenol.
All beer proteins have been found to be glycosylated to varying degrees. The molecular size range of the polypeptides that make up beer’s glycoprotein fraction is relatively narrow—10 to 46 kDa. The glycoproteins were found to consist of protein and five (pentoses) and six (hexoses) carbon sugars. Beer glycoproteins have been found to exist in three forms: those responsible for causing haze, those responsible for providing foam stability (details later), and a third group that does not appear to have a role in either physical or foam stability. The glycoproteins responsible for causing haze have a distinct composition.38 The protein component consists mainly of the amino acids proline and glutamic acid, with the carbohydrate component consisting largely of hexose. The silica gel bonding is mainly caused by the amount of proline available. The most important glycoproteins for haze formation are 16.6 and 31.1 kDa in size. This material is definitely glycoprotein as silica does not adsorb free carbohydrates.
The balance between flavonoid polyphenols (tannoids) (Figure 20.1) and sensitive proteins largely dictates physical or colloidal stability. Beers can differ widely in the content of these molecular species, the relative levels of which depend upon raw materials and the process conditions employed. Haze formation is increased by a number of factors,39 but storage temperature has the greatest influence on haze formation because an increase in temperature raises the rate of the reaction. For example, pasteurization accelerates colloidal haze formation. Oxidation (the presence of oxygen) has a large effect on beer haze formation. Extensive oxidation can increase the rate of haze appearance many-fold. Heavy metal ions (particularly iron) can promote the formation of colloidal haze. Movement of beer accelerates haze formation because of the rapid interaction of colloids. As a consequence, transport conditions are important. Light encourages oxidation, including haze formation as well as other reactions (details later).
Beer chill haze consists of a loose bonding of high molecular weight proteins with highly condensed polyphenols (predominantly anthocyanogens). In these loosely bound aggregates, small quantities of carbohydrates and inorganic materials are included. This loose binding is broken on warming. Haze formation occurs as a result of dissolved colloidal particles colliding and hydrogen bonds forming among them. In the course of time, increasingly large aggregates come together until they are visible as haze.
Haze formation correlates with the presence of sensitive proteins (substances that precipitate with tannic acid) and tannoids (polyphenols adsorbed by polyvinylpolypyrrolidone, or PVPP—details in Chapters 10 and 15). The driving force for haze formation is the interaction of hydrophilic groups on these sensitive proteins with polyphenols. There are also hydrophobic proteins in beer. These surface-active species are important in foam formation (details later and in Chapter 9). There are a number of procedures that can be employed to retard or prevent haze formation:
Employing stabilizers can produce beers with a longer shelf life. The main stabilizing agents that can be used singly or together are: silica gel preparations, PVPP, and proteolytic enzymes. Silica gel preparations are important stabilizing agents because they bind hydrophilic polypeptides.38 They are employed in quantities of 50 to 150 g/hL and are usually dosed into the beer prior to filtration. There are two types of silica gel preparations used in brewing: hydrogels, which have a moisture content of more than 30%, and xerogels (dry gels) with a 5% water content. PVPP selectively removes phenol-containing substances. PVPP binds to polyphenols as it has a similar structure to the amino acid proline.40 Both have five-membered saturated, nitrogen-containing rings with amide bonds and no other functional groups. It is not certain whether PVPP binds to the same part of the polyphenol molecule to which polypeptides bind. This selection depends on the pH-sensitive formation of hydrogen bonds that are broken again in alkaline solution with the release of the adsorbed phenol compounds. Regeneration and reuse of PVPP with hot caustic is thought to be effective by some, but not all, brewers—it is an area of considerable polemic! PVPP and silica gel preparations have been used together with good results because both polyphenol and sensitive protein components are removed.41 Proteolytic enzymes are also employed as stabilizing agents, but because of the advent of silica gel preparations, their use today is not as common as it was a number of years ago. The enzyme preparations employed include papain (from papaya), bromelain (from pineapple), and ficin (from figs). These enzyme preparations are not very specific and, as well as hydrolyzing haze-specific proteins, they often hydrolyze the hydrophobic foam-specific polypeptides (details later). Consequently, the use of these enzymes often requires the addition of a foam-enhancing agent such as propylene glycol alginate. The use of propylene glycol alginate has to be treated with care because (as already discussed) hazy beer can result if pH or temperature control is not effective.42 (Further details on beer stabilizing agents are in Chapters 10 and 15.)
20.3 FLAVOR STABILITY
There is little doubt that the most challenging quality problem that brewers currently encounter is the control of beer flavor stability. Any change in fresh beer aroma and/or taste represents flavor instability.10 There are hundreds of molecules in beer that can change to be at concentrations above their flavor threshold. Indeed, the flavor thresholds of many beer staling compounds are very low. For example, 2-nonenal (which imparts a papery aroma to beer) has a flavor threshold of approximately 0.1 μg/L.1
As a consequence of this, it is logical to adopt procedures that minimize changes in the level of all flavor-active molecules in beer.1 The flavor stability of a beer depends primarily on the oxygen content of packaged beer. However, it is now clear that flavor stability is influenced at all stages of the brewing process:43
The role of the aforementioned reaction products in beer flavor staling reactions is ambiguous, and there are reports46 of their positive and negative effects.
In many foods, such as milk, butter, vegetables, vegetable oils, and beverages, staling is caused by the appearance of various unwanted unsaturated carbonyl compounds. It is now becoming increasingly clear that the same is true of beer staling. As already discussed, packaged beer has a limited shelf life. The phenomenon of beer aging or staling has been intensively investigated by the brewing industry with the objective of understanding and controlling it,7 but the mechanism(s) of staling is still not fully understood. The actual compounds responsible for stale flavor vary during prolonged storage as evidenced by changes in the flavor profile of a beer (Figure 20.2).47 The compounds causing the sweetish, leathery character of very old beers have not been fully characterized. However, there is evidence that the papery cardboard character of two- to four-month-old beer is due to unsaturated aldehydes. The most flavor-active aldehyde that has been conclusively proven to rise beyond flavor-threshold levels is trans-2-nonenal.47 Other aldehydes such as nonadienal, decadienal, and undecadienal may also exceed threshold levels.
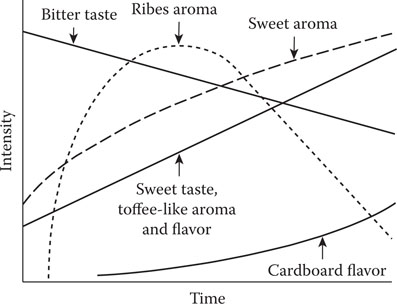
Figure 20.2 Sensory changes in beer flavor during aging.
Although there are many factors that will influence the flavor stability of beer, the oxygen level in the final package is of paramount importance. It is critical that this level in beer, immediately prior to packaging, is as low as possible (less than 100 mg/L) and that oxygen pickup during filling is minimal.
The adverse effects of oxidation on the flavor of finished beer have been known for a long time; some brewers add bisulfites or other antioxidants, such as ascorbic acid, to beer prior to packaging to provide protection against oxygen. This can improve flavor stability. The effectiveness of bisulfite, besides its antioxidant properties and its antimicrobial activity, is also its ability to bind carbonyl compounds (many with unwanted flavors) into flavor-neutral compounds.48 The reaction is reversible, and excess bisulfite will increase yields of the adduct (Figure 20.3)—further details of the effect of sulfur dioxide in beer are in Chapters 15 and 24.
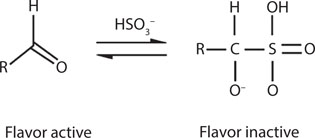
Figure 20.3 Binding of bisulfite to carbonyls.
Bisulfite addition to fresh beer minimizes the increase of free aldehyde concentrations during aging. In addition, when added to stale beer, bisulfite lowers the concentration of free aldehydes and affects the removal of the cardboard flavor. However, over time, the bisulfite will be oxidized to sulfate, thus increasing the concentration of free aldehydes again and restoring the beer’s stale flavor character.48
For many years, attention has been paid to relatively few flavor notes associated with beer staling. For example, Saison and colleagues49 reported that cardboard flavor is linked to 2-nonenal and that methional, 3-methylbutanal, 2-furfuryl ethyl ether, β-damascenone, and acetaldeyde are key contributors to aged beer flavor. However, a holistic method to assay a beer’s freshness or staleness is still not available. The use of taste panels is one obvious answer, but this is not always convenient and relies on the regular availability of trained participants. Consequently, an instrument to conveniently measure “beer freshness” would be welcome!
The detection of reactive oxygen species (ROS) with electron spin resonance (ESR) has been proposed.50–52 The ESR test needs the availability of an expensive instrument, and the results obtained were (and still are) inconsistent.53, 54 As a consequence, the peroxide challenge test (PCT) was developed as an alternative to ESR. The principle of the PCT is to mimic oxidation by titrating beer with hydrogen peroxide. The more hydrogen peroxide a beer can quench, the more resistant it is against oxidation and flavor deterioration. The PCT (contrary to the ESR) allows cost-effective, near real-time, high throughput assessment of beer flavor stability without the use of expensive analytical equipment.12 The PCT is convenient and inexpensive to perform, and the results correlate with ESR measurements and with the organoleptically perceived aged character of a beer sample. It has been demonstrated that aged beer can be distinguished from fresh beer. Even differences among product batches can be identified and benchmarking values for brands and different breweries defined.12 Both PCT and ESR analyses mimic actual perceived beer flavor deterioration adequately. Neither of these analytical methods were able to predict beer flavor deterioration from the analysis of a fresh beer sample. However, it can be concluded that the ESR and the PCT correlate with perceived flavor deterioration, but neither allows a reliable prediction of the speed of flavor deterioration in a given beer based on analysis of the corresponding fresh beer.
20.4 FOAM STABILITY
Many consumers consider the stability of the foam in a glass of beer reflects the quality of the product. The increasing use of adjuncts (unmalted sources of carbohydrate) (details in Chapter 6) and the associated decrease in malt being used today, together with the employment of high-gravity brewing techniques, have had a negative effect on foam values in many beers.55 Also, the regrettable habit of drinkers consuming beer directly out of the bottle or can means that foam stability to them is irrelevant!
There are many foam-promoting compounds in beer, such as iso-α acids from hops, protein/polypeptides, metal ions, and polysaccharides, and all have an important role to play in foam formation and stability. The backbone of foam is protein. Many methods have been tried and extolled for their virtues in the isolation and characterization of foam-positive beer polypeptides. For example, the separation of foaming proteins by hydrophobic interaction chromatography has long been a standard technique.1, 39 Once separated, the proteins are investigated to discover whether they are related to beer foam potential and/or its stability. On the basis of such experiments, it has been proposed that certain sizes of proteins are important in the formation and stabilization of foam. For example, 40, 10, and 8 kDa proteins have been postulated as major foam-stabilizing molecules.56 It is now widely accepted that the polypeptides of greatest hydrophobic character produce the most stable foam, and it is the hydrophobic property that is more important than size.39
The use of high-gravity brewing techniques is essential for the present and future economic viability of the brewing industry internationally (large-scale and craft brewers).55 High-gravity brewing is a procedure that employs wort at higher than normal concentration and therefore requires dilution with deoxygenated water later in the process. By reducing the water employed in the brewhouse, this process increases production capacity without adding to the existing brewing, fermenting, and maturation plants. Therefore, many brewing companies (large and small) worldwide have revised their production processes to accommodate high-gravity brewing procedures as a means to reduce capital expenditure and increase process sustainability. Although this process has many advantages,55, 57 one of the problems that still exists is that beers brewed at higher gravities exhibit poor foam stability.55, 58 (Further details are in Chapter 9.)
The effect of high-gravity brewing on beer head retention with respect to hydrophobic polypeptide levels has been examined using phenyl sepharose liquid chromatography39 throughout the brewing, fermentation, and finishing of high- and low-gravity worts (Figure 20.4).59 It is worthy of note that, recently, Bamforth1 has criticized the accuracy of his own method! In our opinion, this criticism is unwarranted.55
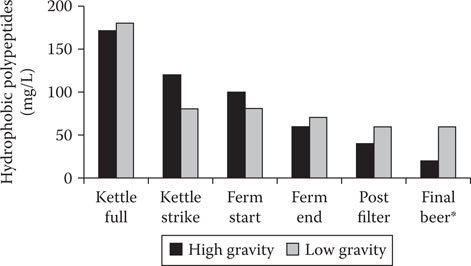
Figure 20.4 Change in the levels of hydrophobic peptides during the brewing process* (final high-gravity beer diluted to 4.5% alcohol by volume).
Three notable features in the hydrophobic polypeptide data are worthy of being highlighted.59, 60 At the kettle full stage (when “runoff” from the lauter tun or mash filter is complete and wort boiling begins), the level of hydrophobic polypeptides was similar in the high-gravity and low-gravity worts despite the use of twice the quantity of malt grist to produce the high-gravity wort. In this case, the high-gravity wort was measured at 20°Plato and the low-gravity wort employed was measured at 10°Plato (1°Plato is equivalent to 1 g of glucose dissolved in 100 mL of distilled water at 20°C). This implies that there was a major failure to extract hydrophobic polypeptides during the high-gravity mash. Also, it is assumed that the hydrophobic polypeptides in wort are obtained from the malt, not from unmalted cereals (adjuncts) such as wheat, corn, and rice (unmalted barley and sorghum could be an exception). However, it has recently been proposed61 that a mannoprotein (Cfg1p) from brewer’s yeast cell walls can stabilize beer foam. The gene that encodes for this protein— CFG1—has been isolated and characterized. This report61 is the first time that a brewer’s yeast beer foaming gene has been cloned and the gene product characterized. Although successful small-scale brewing trials with a yeast strain containing the CFG1 gene have been reported, large-scale fermentations followed by maturation and so on will be necessary to confirm the hypothesis that a yeast cell wall mannoprotein can be responsible for beer foam stability.
There was much greater loss of hydrophobic polypeptides during fermentation of the high-gravity wort so that by the end of fermentation, the hydrophobic polypeptide content of the high-gravity fermented wort was just over 50 mg/L, markedly lower than that of the low-gravity fermented wort (90 mg/L). When the high-gravity brewed beer was diluted to 4.5% alcohol by volume, equivalent to the low-gravity beer, it contained a level of hydrophobic polypeptide less than 50% of the low-gravity brewed beer.60 The head retention of the diluted high-gravity brewed beer was less than that of the low-gravity brewed beer. This contrasts with the low-gravity brewed beer where the hydrophobic polypeptide in this foam accounted for more than 40% of the total polypeptide. Therefore, not only is the polypeptide of the high-gravity brewed beer reduced but so is the hydrophobic content of its foam, which would adversely influence its stability.60, 62 The amino acid profiles of hydrophobic polypeptides recovered from beer foam, unlike polypeptides involved in haze formation (where glutamic acid and proline account for 40% to 50% of the total amino acid composition), have no amino acids present in a distinctive composition.63
It has already been discussed that the fermentation is a key stage where hydrophobic polypeptides are lost during the brewing process (Figure 20.4). Two factors could account for the loss of hydrophobic polypeptides during fermentation. First, fermentation is known to be responsible for the loss of a large quantity of foam-active substances, and this problem is exacerbated during the fermentation of high-gravity worts. This loss is principally due to adsorption of foam onto the side of the fermenter. Second, yeast secretes proteolytic enzymes into the fermenting wort during stress conditions, and these enzymes have a negative effect on the foam stability of the finished beer through the protein degradation (hydrolysis) that occurs during fermentation and storage.64
Analysis of proteinase A activity (using a fluorimetric method described by Kondo et al.65) in wort and beer during the brewing process (Figure 20.5) showed, as would be expected, that freshly boiled wort did not contain enzyme activity. However, during fermentation, proteinase A was secreted into wort by yeast cells. Proteinase A increased throughout fermentation, with the highest enzyme activity occurring at completion of fermentation. Considerably larger quantities of proteinase A were released by the yeast culture during 20°Plato fermentations compared to 10°Plato wort fermentations. During high-gravity brewing, increased stress on the yeast, in the form of both elevated osmotic pressure and ethanol concentrations, stimulated the secretion of proteinase A into the wort during fermentation. It has been shown, using in vitro studies, that both ethanol and increased osmotic pressure (simulated using sorbitol that is not metabolized by brewer's yeast) stimulated the secretion of proteinase by brewer's yeast strains59, 60, 66 (Figure 20.6).
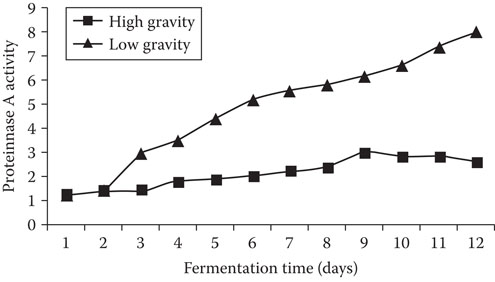
Figure 20.5 The effect of wort-gravity on proteinase A release during fermentation of low- (12°Plato) and high- (20°Plato) gravity worts.
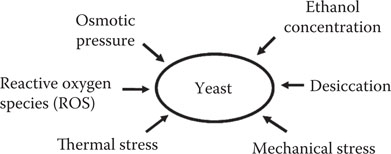
Figure 20.6 Stress factors that promote protein release by brewer’s yeast strains.
20.5 GUSHING
Excess foam in a beer is regarded as deleterious and is known as gushing or “wild beer.” Gushing is the violent, uncontrolled ejection of beer from the package at the time it is opened and involves the loss of a significant portion of the contents (usually at least 50%). There may be two classes of gushing—namely, sporadic and epidemic. Sporadic gushing may occur as a result of minor production deviations that are generally difficult to pinpoint. Epidemic or long-term serious gushing may be caused by several factors. Perhaps the most widely discussed cause is the use of weathered (damp) barley. If barley is harvested when wet, Fusarium or other fungus infections can develop during the malting process, resulting in beer susceptible to serious gushing. The formation of mycotoxins such as deoxynivalenol (DON) has been paralleled with the development of gushing potential. The screening of barley and malt for these metabolites may offer a means of reducing beer gushing problems.67
Other factors, such as increased levels of carbonation or a carbonating system operating without proper controls, can produce beers that have the potential to gush. Calcium oxalate microcrystals in beer are another cause of gushing. These crystals are thought to form nuclei for carbon dioxide gas emissions, but excess treatment and filtration will overcome this cause of gushing. Excessive levels of iron and other nuclei-forming particles such as sediments will also contribute to gushing problems. Beer gushing is not the quality problem that it was 50 years ago.
20.6 LIGHT STABILITY
Beer is sensitive to light, especially in the 350 to 500 nm range. Light at these wavelengths can penetrate clear and green glass containers and cause a nauseous off-flavor in beers bottled in such glass containers and served in these drinking glasses. The beer is said to be “sunstruck” and the aroma and taste referred to as “skunky” (so called because this aroma is similar, but not the same chemical structure, to the anal scent produced by the skunk—a North American rodent). Light instability in beer results from hop components. As already discussed in Chapter 7, hops in brewing have a number of roles: They impart bitterness to beer, provide characteristic hop aromas, suppress growth of certain microorganisms—particularly Gram-positive bacteria, and assist in beer foam stability and contribute polyphenols to the protein-polyphenol complex during wort boiling.
When beer is exposed to light, one of the side chains on the iso-α acids (a component of hops) is cleaved, and the highly reactive radical that is liberated combines with sulfur-containing compounds (Figure 20.7) to produce 3-methyl-2-butene-1-thiol (MBT). MBT has a skunky-like aroma. MBT has a flavor threshold in the order of parts per trillion, making it one of the most flavor-active substances in beer.68
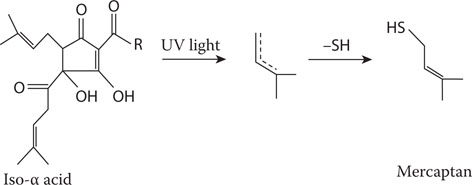
Figure 20.7 Mercaptan formation in light-sensitive iso-α acid.
Specialized hop extracts (produced using liquid carbon dioxide or ethanol as a solvent) have been developed to combat this sensitivity to light.68 In essence, pairs of hydrogen atoms are catalytically added to the isomerized iso-α acids, tetrahydro-iso-α acids. There are three principal types of such extracts (called reduced extracts) currently available on the market: RHO iso-α acids, tetrahydro-iso-α acids, and hexahydro-iso-α acids.
All of these materials are bitter to varying degrees; some improve beer-foam cling and stability and protect beer against lightstruck/sun-induced skunky flavors. Normally, all of these materials are used as a postfermentation addition to achieve maximum benefit and optimum utilization. To achieve complete lightstruck protection, no iso-α acid can be used in any other part of the process. Even repitched yeast56 with iso-α acid absorbed onto its surface will provide sufficient material for photolytic cleavage to occur and the resultant production of MBT and skunky flavors.
20.7 CONCLUSIONS
Beer instability involves a number of complex reactions involving proteins, carbohydrates, polyphenols, metal ions, oxygen, thiols, and carbonyls. There are many diverse types of beer instability involving a number of different microorganisms, chemical species, and reactions. Our understanding of these reactions has progressed over the past 25 years, but we are still far from a complete comprehension of beer instability reaction systems.
REFERENCES
1. Bamforth, C.W., 125th Anniversary Review: The non-biological instability of beer, J. Inst. Brew., 117: 488–497, 2011.
2. Bamforth, C.W., Beer haze, J. Am. Soc. Brew. Chem., 57:81–90, 1999.
3. Leiper, K.A. and Miedl, M., Colloidal stability of beer, in Beer: A Quality Perspective, 1st ed., Bamforth, C.W., Ed., Elsevier Inc., Burlington, pp. 111–162, 2009.
4. Steiner, E., Becker, T., and Gastl, M., Turbidity and haze formation in beer — insights and overview, J. Inst. Brew., 116:360–368, 2010.
5. Evans, D.E. and Bamforth, C.W., Beer foam: Achieving a suitable head, in Beer: A Quality Perspective, 1st ed., Bamforth, C.W., Ed., Elsevier Inc., Burlington, pp. 1–60, 2009.
6. Evans, D.E. and Bamforth, C.W., Beer foam: Achieving a suitable head, in Beer: A Quality Perspective, 1st ed., Bamforth, C.W., Ed., Academic Press, New York, pp. 85–109, 2009.
7. Bamforth, C.W., A critical control point analysis for flavor stability of beer, Tech. Q. Master Brew. Assoc. Am., 41:97–103, 2004.
8. Bamforth, C.W., Beer: Health and Nutrition, Blackwell Publishing, Oxford, UK, 2004.
9. Bamforth, C.W., Brewing Materials and Processes, Academic Press, London, 2016.
10. Bamforth, C.W. and Lentini, A., The flavour stability of beer, in Beer: A Quality Perspective, Bamforth, C.W., Ed., Academic Press, San Diego, CA, pp. 85–109, 2009.
11. Vanderhaegen, B., Neven, H., Verachtert, H., and Derdelinckx, G., The chemistry of beer aging – a critical review, Food Chem., 95:357–381, 2006.
12. Miedl, M., Rogers, P., Clarke, F.M., and Stewart, G.G., The peroxide challenge test: A novel method for holistic time measurement of beer flavour stability, J. Inst. Brew., 117:166–175, 2011.
13. Casey, G.P., Primary versus secondary gushing and assay procedures used to assess malt/beer gushing potential, Tech. Q. Master Brew. Assoc. Am., 33:229–235, 1996.
14. Garbe, L.-A., Schwarz, P., and Ehmer, A., Beer gushing, in Beer: A Quality Perspective, Bamforth, C.W. (Ed.), Academic Press, San Diego, CA, pp. 185–212, 2009.
15. Shokribousjein, Z., Deckers, S.M., Gebruers, K., Lorgouilloux, Y., Baggerman, G., Verachtert, H., Delcour, J. A., Etienne, P., Rock, J.-M., Michiels, C., and Derdelinckx, G., Hydrophobins, beer foaming and gushing, Cerevisia (Belg. J. Brew. Biotechnol.), 35:85–101, 2010.
16. De Keukeleire, D., Heyerick, A., Huvaere, K., Skibsted, L.H., and Andersen, M.L., Beer lightstruck flavour: The full story, Cerevisia, 33:133–144, 2008.
17. Templar, J., Arrigan, K., and Simpson, W.J., Formation, measurement and significance of lightstruck flavor in beer, a review, Brewers’ Digest, 18–25, 1995.
18. Miedl, M. and Bamforth, C.W., The relative importance of temperature and time in the cold conditioning of beer, J. Am. Soc. Brew. Chem., 62:75–78, 2004.
19. Labatt Brewing Company Limited, Improvements in production of fermented malt beverages, U.S. Patent 5,304,384, April, 1984.
20. Walters, M.T., Seefeld, R., Hawthorne, D.B., and Kavanagh, T.E., Composition and kinetics of particle formation in beer post packaging, J. Am. Soc. Brew. Chem., 54:57–61, 1996.
21. Letters, R., Origins of carbohydrates in beer sediments, J. Inst. Brew., 75:54–80, 1969.
22. Coote, N. and Kirsop, B.H., A haze consisting largely of pentosan, J. Inst. Brew., 82:34, 1976.
23. Greif, P. and Schildbach, R., Investigations on the oxalic acid problem in the brewery, Monatsschr, Brauwiss, 31:275–280, 285, 1978.
24. Siebert, K.J., Troukhanova, N.V., and Lynn P.Y., Nature of polyphenols-protein interactions, J. Agric. Food Chem., 44:80–85, 1996.
25. Sharpe, F.R. and Channon, P.J., Beer haze caused by can lid lubricant, Proc. Eur. Brew. Conv. Congr., Madrid, IRL Press, Oxford, pp. 599–606, 1987.
26. Bamforth, C.W., Processing and packaging and their effects on beer stability, Ferment, 1:49–53, 1988.
27. Walker, M.D., Bourne, D.T., and Wenn, R.V., The influence of malt-derived bacteria on the haze and filterability of wort and beer, Proc. Eur. Brew. Conv. Congr., Maastricht, IRL Press, Oxford, pp. 83–89, 1997.
28. Clark, D.T. and Bamforth, C.W., Realistic haze specifications for beer, Tech. Q. Master Brew. Assoc. Am., 44:160–163, 2007.
29. Siebert, K.J., Visual versus instrumental perception of haze – a review, Tech. Q. Master Brew. Assoc. Am., 45:90–98, 2008.
30. Jackson, G. and Bamforth, C.W., Anomalous haze readings due to beta glucans, J. Inst. Brew., 89:155–156, 1983.
31. Wenn, R.V., Wheeler, R.E., and Webb, D.J., The prediction of high haze levels in freshly filtered lager beers responsible for the generation of “invisible” hazes and “bits” in fresh bottled lager, Proc. Eur. Brew. Conv. Congr., Zurich, IRL Press, Oxford, pp. 83, 89, 1989.
32. Lewis, M.J. and Poerwantaro, W.M., Release of haze material from the cell walls of agitated yeast, J. Am. Soc. Brew. Chem., 49:61–65, 1991.
33. Chlup, P.H. and Stewart, G.G., Centrifuges in brewing, Tech. Q. Master Brew. Assoc. Am., 48:46–50, 2011.
34. Outtrup, H., Fogh, R., and Schaumburg, K., The interaction between proanthocyanidins and peptides, Proc. Eur. Brew. Conv. Congr., Madrid, IRL Press, Oxford, pp. 583–590, 1987.
35. Outtrup, H., Haze active peptides in beer. Proc. Eur. Brew. Conv. Congr., Zurich, pp. 609–616, 1989.
36. McMurrough, I., Madigan, D., Kelly, R.J., and Smyth, M.R., The role of flavanoid polyphenols in beer stability, J. Am. Soc. Brew. Chem., 1996, 54:141–148.
37. McMurrough, I., Kelly, R., and Byrne, J., Effect of the removal of sensitive proteins and proanthocyanidins on the colloidal stability of lager beer, J. Am. Soc. Brew. Chem., 50:67–76, 1992.
38. Leiper, K.A., Stewart, G.G., and McKeown, I.P., Beer polypeptides and silica gel. Part I. Polypeptides involved in haze formation, J. Inst. Brew., 109:57–72, 2003.
39. Bamforth, C.W., The foaming properties of beer, J. Inst. Brew., 93:216–219, 1985.
40. Siebert, K.J. and Lynn, P.Y., Mechanisms of beer colloidal stabilization, J. Am. Soc. Brew. Chem., 55: 73–78, 1997.
41. McMurrough, I. and O'Rourke, T., New insight into the mechanism of achieving colloidal stability, Tech. Q. Master Brew. Assoc. Am., 34:271–277, 1997.
42. Jackson, G., Roberts, R.T., and Wainwright, T., Mechanism of beer foam stabilization by propylene glycol alginate, J. Inst. Brew., 86:34–37, 1980.
43. Narziss, L., Miedaner, H., Graf, H., Eichform, P., and Lustig, G., Technological approach to improve flavour stability, Tech. Q. Master Brew. Assoc. Am., 30:48–53, 1993.
44. Irwin, A.J., Barker, R.L., and Pipasts, P., The role of copper, oxygen and polyphenols in beer flavour instability, J. Am. Soc. Brew. Chem., 49:140–149, 1991.
45. Wu, M.J., Rogers, P.J., and Clark, M., 125th Anniversary Review: The role of proteins in beer redox stability, J. Inst. Brew., 118:1–11, 2012.
46. Bright, D., A study of the antioxidant potential of speciality malt. PhD Thesis, Heriot-Watt University, Edinburgh, Scotland, 2001.
47. Dalgleish, C., Flavour stability, Proc. Eur. Brew. Conv. Congr., IRL Press, Oxford, Amsterdam, pp. 623–659, 1997.
48. Barker, R.L., Gracey, D.E.F., Irwin, A.J., Pipasts, P., and Leiska, E., Liberation of staling aldehydes during storage of beer, J. Inst. Brew., 89:411–415, 1983.
49. Saison, D., DeShutter, D.P., Uytenhove, B., Delvaux, F., and Delvaux, F.R., Contribution of staling components to the aged flavour of lager beer by studying their flavour thresholds, Food Chem., 114: 1206–1215, 2009.
50. Uchida, K., Suga, S., and Ono, M., Improvement for oxidative stability of beer – rapid prediction method for beer flavour stability by electron spin resonance spectroscopy, J. Amer. Soc. Brew. Chem., 54:205–211, 1996.
51. Andersen, M.L., Outtrup, H., and Skibsted, L.H., Potential antioxidants in beer assessed by ESR spin trapping, J. Agric. Food. Chem., 48:3106–3111, 2000.
52. Araki, S., Takashio, M., and Shinotsuka, K., A new parameter for determination of the extent of staling in beer, J. Am. Soc. Brew. Chem., 60:26–30, 2002.
53. Uchida, K. and Ono, M., Technological approach to improve beer flavour stability: Analysis of the effect of brewing processes on beer flavour stability by the electron spin resonance method, J. Agric. Soc. Brew. Chem., 58:8–13, 2000.
54. Uchida, K. and Ono, M., Technological approach to improve beer flavour stability: Adjustments of wort aeration in modern fermentation systems using the electron spin resonance method, J. Am. Soc. Brew. Chem., 58:30–36, 2000.
55. Stewart, G.G., Brewing Intensification, American Society of Brewing Chemists, St. Paul, MN, 2014.
56. Lusk, L.T., Goldstein, H., and Ryder, D., Independent role of beer proteins, melanoidins and polysaccharides in foam formation, J. Am. Soc. Brew. Chem., 53:93–103, 1995.
57. Stewart, G.G., High-gravity brewing, Brew. Guard., 128:31–37, 1999.
58. D'Amore, T., Russell, I., and Stewart, G.G., Advances in the fermentation of high-gravity wort, Proc. Eur. Brew. Conv. Congr., Lisbon, IRL Press, Oxford, pp. 337–344, 1991.
59. Cooper, D.J., Stewart, G.G., and Bryce, J.H., Hydrophobic polypeptide extraction during high-gravity mashing—experimental approaches for its improvement, J. Inst. Brew., 104:283–287, 1998.
60. Cooper, D.J., Stewart, G.G., and Bryce, J.H., Some reasons why high-gravity brewing has a negative effect on head retention, J. Inst. Brew., 104:221–228, 1998.
61. Blasco, L., Veiga-Crespo, P., Sanchez-Perez, A., and Villa, T.G., Cloning and characterization of the beer foaming gene CFG1 from Saccharomyces pastorianus, Agric. Food Chem., 60:10796–10807, 2012.
62. Bamforth, C.W., Foam: Method, myth or magic? The Brewer, 81:396–389, 1995.
63. Leiper, K., Beer polypeptides and their selective removal with silica gels, PhD Thesis, Heriot-Watt University, Edinburgh, Scotland, 2002.
64. Stewart, G.G., The chemistry of beer instability, J. Chem. Educ., 81:963–968, 2004.
65. Kondo, H., Yomo, H., Furukubo, S., Fukui, N., Kawasaki, Y., and Nakatani, K., Advanced method for measuring proteinase A in beer, Proc. of the 25th Conv., The Inst. of Brewing, Asia Pacific Section, pp. 119–124, 1998.
66. Lekkas, C., Hill, A.E., Taidi, B., Hodgson, J., and Stewart, G.G., The role of small peptides in brewing fermentations, J. Instit. Brew., 115:134–139, 2009.
67. Heikara, A., Gushing induced by fungi, in European Brewing Convention Monograph VI, Fachverlag Hans Carl, Nürenberg, pp. 251–258, 1980.
68. Wilson, R.J.H., Roberts, I., Smith, R.J., and Bieng, M., Improving hop utilization and flavour control through the use of pre-isomerized products in the brewery, Tech. Q. Master Brew. Assoc. Am., 38:11–21, 2001.