The Electric Battery and a Low-Carbon Future
The first true electric battery was invented more than two hundred years ago, but it seems as if the technology’s greatest contributions to society are only just arriving. For two centuries, battery technology advanced in fits and starts, with periods of intense research and innovation and long decades of stagnation. Advancements in battery technology during the periods of focused innovation helped spark the automotive revolution and, over the past few decades, the widespread penetration of personal electronics into so many facets of life. Yet, battery and other electric storage technologies have long held the potential to catalyze a transformation of the energy system as a whole.1 That potential has yet to be realized.
While over the past 50 or so years the technical revolution has sped innovation across a number of fields, from business applications to digital communication to weather prediction to media and nearly everything in between, technological advances in battery storage have not kept pace.2 The microprocessor provides a revealing contrast: in 1971, Intel launched the 4004, widely accepted as the first commercially available microprocessor.3 Since then, as Moore’s law accurately predicted, the numbers of transistors in an integrated circuit in a microprocessor have roughly doubled every two years—a phenomenon that has essentially ushered in the digital revolution.4 Batteries, however, for the most part, are a different story.
The capacity of the common lithium-ion battery (LIB), for instance, only achieved roughly 8 percent gains per year from 1990 through 2010, falling far short of Moore’s law.5 In fact, if Moore’s law applied to batteries, the typical starter battery in a car would today be the size of a coin.6
This isn’t to diminish the benefits that batteries have delivered, but to recognize what more could be. Indeed, throughout the 20th century, batteries served society reliably—powering wristwatches, flashlights, smoke detectors, toys and remote controls, and starting automobiles—but improvements were slow. That is now changing. Since the turn of the century, with the proliferation of laptops and cell phones and other personal electronics, reliable, affordable, and more powerful rechargeable batteries have become integral to the contemporary, digital way of life. Batteries have made possible the mobility of the digital revolution. And in coming years, the electric battery is poised to make possible another, even greater, transformation—of transportation and the very electric power systems that power our lives.7
These advances are coming not a moment too soon. The impacts of greenhouse gas emissions from human activities, mostly derived from energy use, are already being felt, and societies and governments worldwide are feeling warranted pressure to decarbonize energy and transportation systems. This broadening recognition of the need to transition to a lower carbon economy arrived at a symbolic and diplomatic milestone in December of 2015. At the United Nations’ climate summit in Paris, world leaders agreed to a historic global pact on climate change, making an international commitment to hold “the increase in the global average temperature to well below 2°C above pre-industrial levels and pursuing efforts to limit the temperature increase to 1.5°C above pre-industrial levels, recognizing that this would significantly reduce the risks and impacts of climate change.”8 Actually meeting that goal, however, is going to require a historic shift in energy systems, away from greenhouse gas-emitting fossil fuels to low-carbon, renewable resources. In particular, electric and transportation systems have to be rapidly decarbonized in order to keep the concentrations of greenhouse gases in the atmosphere at levels that might hold global warming to under 2°C.9
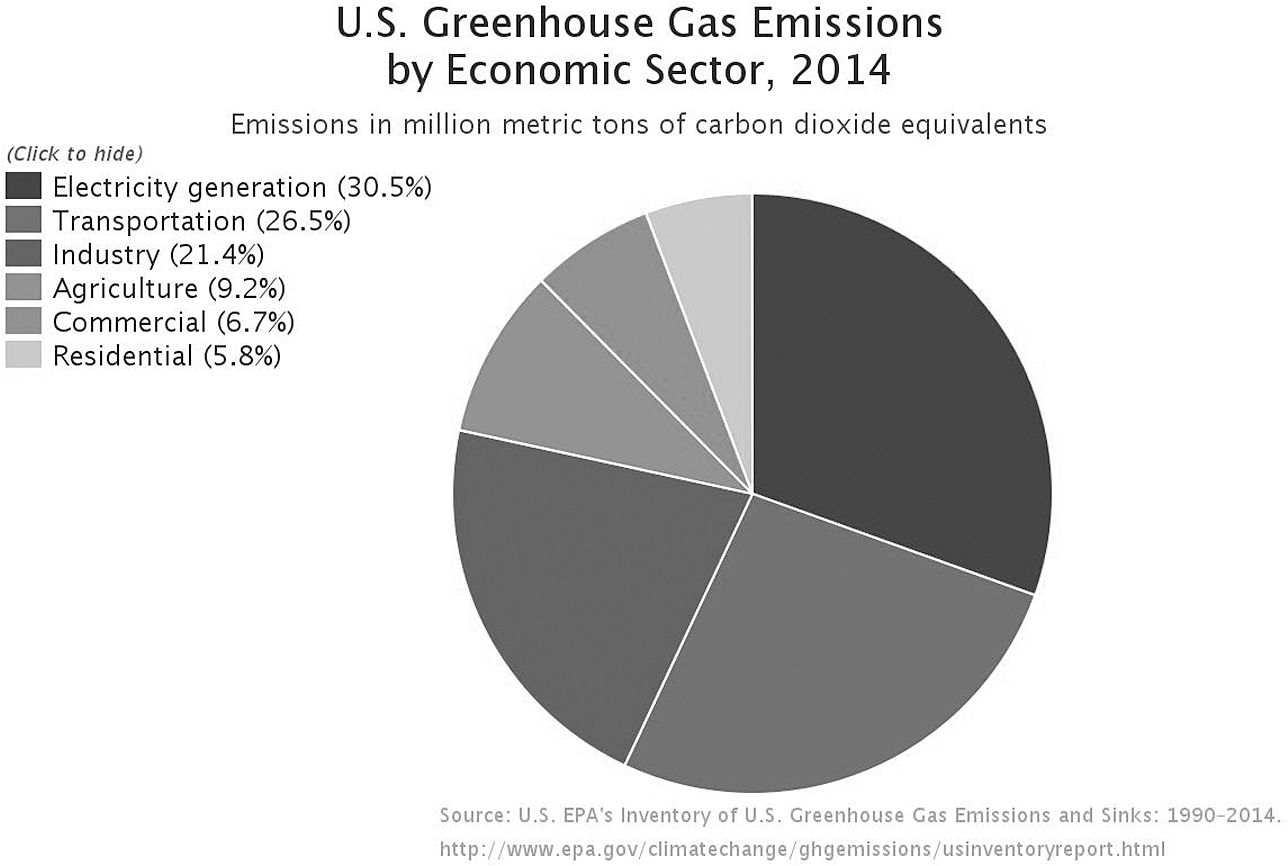
As of 2014 [the most recent data available from the Environmental Protection Agency’s (EPA) greenhouse gas annual inventory at the time of writing], the electricity and transportation sectors represented 56 percent of total greenhouse gas emissions in the United States (see Figure 1.1). Globally, these two sectors represent 39 percent of total greenhouse gas emissions. In order to meet the climate goals agreed upon in the Paris Agreement, countries will have to rapidly convert entire electric sectors to low carbon-emitting resources and to replace gasoline and diesel-combusting vehicles with electric vehicles (EVs) that are plugged into a low-carbon grid. Batteries could also help reduce the climate impacts from other sectors. Consider heating. According to the EPA, greenhouse gases from the “Commercial & Residential” sector that contribute 12 percent of U.S. emissions “arise primarily from fossil fuels burned for heat.” (In fact, 81 percent of all “Commercial & Residential” emissions come from consumption of natural gas, mostly for heating homes.)10 Technologies like air-source heat pumps are increasingly displacing traditional fossil fuel heating systems for homes and businesses. As more heating systems are electrified, a cleaner, lower carbon grid will also effectively reduce emissions.
Figure 1.1 Total U.S. greenhouse gas emissions by economic sector in 2014. (Reprinted from U.S. EPA.)
Similarly, emissions from the industrial sector, which represent 21 percent of greenhouse gases both in the United States and globally, include both direct and indirect emissions. Nearly two-thirds of direct emissions come from the burning of fossil fuels on site for power and heat. Indirect emissions are those produced by a typical power plant to make electricity, which is then used by an industrial facility to power buildings, machinery, and other operations.11 So, in effect, the electricity sector described above doesn’t include the emissions produced to provide the considerable amounts of electricity to industrial customers, nor does it include the electricity produced on site at industrial facilities. A significant amount of this electricity will have to come from low-carbon, renewable resources to meet our climate goals.
Long story short, as Gene Berry of Lawrence Berkeley National Laboratory wrote, “Stabilizing future atmospheric carbon dioxide (CO2) levels at less than a doubling of pre-industrial levels will be a Herculean task, requiring a continuous flow of new carbon free power two to three times greater than today’s energy supply to sustain economic development for a global population approaching 10 billion by the mid-twenty-first century.”12
The battery has emerged as an essential technological component in the push to integrate renewables and decarbonize transportation and the electric grid. To reduce greenhouse gas emissions from many of the highest emitting sectors—electricity, transportation, residential and commercial, and industry—storage of electricity and better batteries are crucial. In fact, electricity storage has been called the “holy grail” for an economy-wide transition to low-carbon, renewable energy sources.13 In order to integrate large amounts of variable, renewable energy generation to the electric grid, better mechanisms to store the energy are necessary.14 Over time, batteries will play a significant role at both the utility grid and household levels. At the grid scale, batteries and other forms of electrical storage will help integrate intermittent renewable sources of energy like wind farms and utility-scaled solar. For homes and businesses, the battery—in both standalone storage packs and in plugged-in EVs—has the potential to make distributed solar (the on-site, often rooftop, deployment of photovoltaics) more reliable, more consumer-friendly, and possibly even more affordable. On the roads, EVs are poised to drastically reduce the amount of oil consumed as gasoline and diesel fuel in personal automobiles and fleets. The electric battery is, of course, the most important single piece of technology in an EV, and the relative success of EVs in the marketplace and on roads worldwide will be determined by the costs and capacities of batteries.
The International Energy Agency states plainly in its most recent Technological Roadmap for Energy Storage that electric batteries and other energy storage technologies “support energy security and climate change goals by providing valuable services in developed and developing energy systems.”15 In short, the electric battery is a core climate solution.
This book will examine the electric battery (and, in Chapter 7, some other forms of electricity storage) in the context of how the technologies will help displace fossil fuel energy consumption with low-carbon renewable resources, in order to achieve national and international emissions reductions goals and avoid the worst fates of climate change.
In the Handbook of Batteries, David Linden and Thomas Reddy define the battery as: “a device that converts the chemical energy contained in its active materials directly into electric energy by means of an electrochemical oxidation-reduction reaction. This type of reaction involves the transfer of electrons from one material to another through an electrical circuit.”16 Batteries can store energy because the chemical reaction inside of them only takes place when there is a flow of electrons, or only when the battery is connected to an electrical circuit. Technically, though the term battery is commonly used, the basic electrochemical unit where the reactions occur is called the voltaic cell, or just cell. Batteries are constructed from one or more voltaic cells, and the overall device is meant to be as compact and sturdy as its application demands.
In Chapter 2, you will take a deeper dive into battery technology and learn about what’s actually going on inside a battery, the various battery technologies, how they’re made, and how to compare and contrast different batteries for different applications. Before getting into the practical details of how batteries work today, however, let’s step back and take a broad look at how the storage of electricity can help integrate renewable energy resources, reduce our dependence on fossil fuels, and help the world charge forward to a low-carbon future.
The Electric Battery as Core Climate Solution
We tend to think of electricity as a source of energy when we flip on a light switch or plug the vacuum into the wall. Technically, however, electricity is a unique form of energy known as an energy carrier; it delivers energy in a useful form to the places that energy is needed to do work.17 Electricity is formally defined as a secondary source of energy, produced by converting the energy from primary sources such as coal, natural gas, nuclear, solar, wind, and hydropower. While the primary sources of energy can be renewable or nonrenewable, electricity itself is neither—it’s just the flow of electrical power or charge.
This flow can be controlled, making electricity the handiest way to harness energy from primary sources and deliver it for convenient use. For electricity to be useful, the charges must flow in orderly ways, known as currents, and currents can be directed through closed paths known as circuits. You may think of the circuits that run from the breaker box through your house, but an electric grid itself is one massive, complex circuit. The development of electrical grids across the industrialized world over the past century has essentially led to the electrification of modern life. In fact, the National Academy of Engineering put the electric grid atop its list of the greatest innovations of the 20th century. (For reference, the automobile ranked second; the Internet, thirteenth.)18
Though we often take it for granted, if not for the convenient delivery of energy through electricity, society would be stuck in a more primitive stage of development—reading by the light from a flame, storing food with ice and salt and jars, and shoveling coal into steam engines.
While it’s hard to dispute how convenient electricity has made energy consumption, there’s a single physical limitation that has stood in the way of even greater electrification of the world’s energy systems: electricity cannot easily be stored.
The physical nature of electricity allows currents to flow essentially at the speed of light, but that current must be tapped into immediately. Electricity is, in effect, an instantaneous transaction; it must be consumed (or converted to do useful work) at the very moment that it is generated (or converted from the primary energy source). While it might be nice to imagine an electrical charge simply looping around a circuit until someone needs to tap into it, that’s simply not how the physics of electricity works. Without a supplemental technology affixed to an electrical circuit, it cannot be stored and saved for later.
This basic scientific truth presents considerable challenges to the electrification of transportation and to the integration of renewable energy resources like solar and wind power. First, let’s consider transportation, and in particular the personal automobile.
How the Battery Begets Cleaner Cars
Fuels like gasoline and diesel are incredibly convenient because they store energy in a liquid form that can be shipped and piped and pumped into a car or truck with no regard for time. The energy content of the gasoline you pump into your car at the local Chevron station is the same as when it left the refinery a few states away, possibly months earlier. If you leave your car at the airport for a week, the gasoline in the tank is still good when you get back. The energy that you need to power that car back home has been readily stored in the liquid fuel. Now if your car needed to run directly off of the electric grid, that electricity would have to be generated at the very instant your foot pushed the accelerator, and you’d have to somehow keep it plugged in while on the highway or road. This is, of course, practically impossible. (That said, many streetcars and subway systems run off direct electric connections, hooked up to overhead wires or a highly charged “third rail.”)
Enter the electric battery. Electric batteries have been in cars for over a century, but with a few exceptions, these lead acid batteries are there to provide the initial burst of current to start the engine and to run a few accessories like radios and windshield wipers. Ever since Henry Ford unveiled his famous Model T, the engines themselves (most of them, at least) have been powered by gasoline or diesel.
Gasoline and diesel, however, are petroleum products, the combustion of which in an engine releases carbon dioxide, which then rises to the atmosphere and contributes to global warming (to say nothing of the other emissions, such as nitrogen oxides, carbon monoxide, and volatile organic compounds, which have a range of negative public health and environmental impacts on the local level). As we noted earlier, transportation is responsible for 26 percent of greenhouse gas emissions in the United States, and 14 percent globally.19 Of all transportation emissions, as of 2006 (the last year the Department of Transportation has made data available), 63 percent came from “light duty” vehicles, or those we commonly think of as personal cars and trucks.20
How to reduce these car and truck emissions? The simplest idea is to get people to drive less. Another is to make internal combustion vehicles more fuel efficient so that they can burn less oil to travel the same amount of miles. Climate experts are in agreement that both of these strategies are important—the “Transport” chapter of the Intergovernmental Panel on Climate Change’s (IPCC) 5th Assessment report includes comprehensive breakdowns of both—and policies and programs are in place or in development around the world to reduce both miles driven and the fuel burned by automotive fleets to drive those miles.
To drastically reduce emissions, however, a broader transition away from internal combustion engines entirely is necessary. After all, for each gallon of gasoline burned, 24 pounds of greenhouse gas emissions are released.21 Emissions from an electric vehicle, by contrast, come from the generation of the electricity that charges the vehicle. The carbon intensity of the grid varies considerably depending on where you plug in, but even in parts of the country where inefficient coal plants dominate electricity generation, the total emissions per mile driven by an electric car are similar to those from a small efficient car powered by an internal combustion engine. While we have invested billions in attempting to make the internal combustion engine more efficient, with only marginal gains, we have a much more technologically clear path for replacing coal plants with low-carbon alternatives.
The electric battery has already made petroleum-free driving possible. In fact, in the early 1900s, lead acid batteries were used to power all-electric cars, and much of the automotive industry was building toward an electric car future before Ford’s Model T changed the world.22 Today, there are currently 23 models of electric vehicles on the U.S. market, and 36 hybrid electric versions.23 Still, to overcome “range anxiety” and to further increase the electric car potential in the country, engineers and entrepreneurs are working on cheaper batteries with more storage capacity. Already, battery costs are coming down dramatically—about 14 percent annually over a recent eight-year span, according to a recent study published in the journal Nature. In 2007, a carmaker would have to dish out $1,000 for a kilowatt-hour’s worth of battery storage in a vehicle. By 2014, that cost had been cut to an average of $410, and as low as $300 per kilowatt-hour for industry leaders.24 In Chapter 4, you’ll read more about the history of electric vehicles and how recent advances in battery technology is making this a truly pivotal moment for personal transportation.
As we wrote earlier, however, an electric car is only as clean and green as the grid it is plugged into. And the climate implications of a lower carbon grid are far greater than “just” the transportation sector alone.
How the Battery Helps Integrate Intermittent Renewables
Just as gasoline and diesel are useful fuels for carrying energy for transportation, coal and natural gas are convenient for powering electricity generation facilities. The trade-off for such convenience, however, is that coal and natural gas are both fossil fuels that emit considerable amounts of greenhouse gas pollution when burned. In 2015, emissions from coal-fired generation represented 71 percent of all carbon dioxide emissions in the electric sector, totaling over 1.3 trillion metric tons.25 Natural gas was responsible for another 530 billion metric tons, or 28 percent of all electric sector emissions. And that’s only the carbon dioxide emissions; these figures don’t include other greenhouse gases, such as methane. The extraction, transport, and combustion of natural gas, until recently considered by many to be a lower emitting “bridge fuel,”26 is known to release vast volumes of methane. Methane is a greenhouse gas that is at least 25 times more efficient at trapping heat in the Earth’s atmosphere than carbon dioxide, but has a shorter life span in the atmosphere.27 But though natural gas emits from 50 to 60 percent less carbon dioxide when combusted in a new, efficient natural gas plant when compared to emissions from a typical new coal plant, the smokestack emissions don’t tell the whole story. The drilling and extraction of natural gas from wells and its transportation in pipelines results in leakage of methane, and recent scientific studies have shown that these can be as high as 9 percent of the total life cycle emissions of natural gas production, far higher than previously estimated.28 These new revelations make natural gas a far less attractive “solution” for climate change as our energy systems necessarily transition away from greenhouse gas pollutants.
In this transition, the single biggest obstacle to powering our homes, businesses, and even the grid with renewable generation is intermittency.29 Though the sun and wind are the two largest sustainable sources of carbon-free power, while harnessing them is getting cheaper by the year, neither can produce electricity that is readily and constantly available whenever it is needed. Solar rays only reach solar panels from sunrise to sunset and only when there aren’t clouds blocking the way. The wind blows inconsistently and often late at night when demand is low and the power generated may not immediately be useful.
For this reason, most homeowners and businesses with distributed solar (like rooftop solar) still feel the need to connect to the grid for reliability and energy around the clock. Similarly, on a larger scale, utilities and grid operators can’t simply displace coal- and natural gas-fired power plants with every megawatt of generating capacity brought online by utility-scaled solar and wind farms.30 The grid still relies on constant generation that is responsive to demand and available at the precise moment of that demand. In fact, the power grid was designed around the concept of large, centralized, easily controlled generators that produce the right amount of electricity at the exact right time to consistently and reliably meet customer demand.31 Intermittent renewables disrupt this convention, as their power fluctuates over multiple time horizons—from rainy seasons to partially cloudy days to sunrises and sunsets and over summer and winter.
For the most part, utilities and grid operators have had to balance new renewable sources with on-demand electric generators that sit ready to produce when they are needed.32 To integrate solar and wind at the scales needed to meet our climate goals, once we get in the ballpark of 40 percent or higher penetration of intermittent renewables on the grid, some degree of storage will be necessary.33 That’s where batteries and other forms of electric storage come into play.
Consider a typical rooftop solar array on an average American house. On a sunny day, it generates the most electricity midday when, unfortunately, it’s most likely that nobody is home, or at least the house isn’t full. These days, the average home’s demand peaks from around 5 p.m. to 9 p.m., when everyone is coming home from work and school, cranking up the air conditioners, the computers, the televisions, and, as the sun goes down, the lights.34 If a residential rooftop solar array could store the electricity generated during the midday hours for the early evening, these households could rely a lot less on their grid ties. That’s not to mention another clear benefit of home storage—keeping the lights on during a blackout. In Chapter 5, we’ll take a deeper dive into how batteries can solve these intermittency issues for homes and other distributed applications.
On a more macro scale, the problem is similar, if a bit more complex. Consider a 200-megawatt solar farm that feeds into the grid. Besides the more basic challenges of matching the supply to the customer demand throughout the local grid, there is the issue of more dynamic intermittency. On a partially cloudy day, this type of larger-scale solar farm can give grid operators the fits, as up to 200 megawatts of electricity are coming on and offline as the clouds pass over the panels.
Wind farms pose the same challenge. Wind doesn’t always blow and spin the turbines when the customers want to use the power, and even when the wind is blowing, it can be irregular and constantly changing the wind farm’s output.
Storing this wind or solar power when it is generated—whether through batteries or alternative means—then lets grid operators tap into the supply when it is needed. In Chapter 6 we’ll take a much closer look at storage at the grid level.
The Electric Battery: A Very Brief History
Before we dive deeper into battery technology and its future uses, let’s take a closer look at its historical development including some of its innovators who have been leading the battery’s charge forward. It’s generally accepted that the electric battery as we currently recognize it was invented by Alessandro Volta in 1800.35 In the mid- to late eighteenth century, the world was fascinated with electricity, and scientists were racing to harness it for practical use. In 1745, static electricity was first stored. Ewald Georg von Kleist of Germany and Dutch scientist Pieter van Musschenbroek both independently (and accidentally) figured out how to store a charge and produce a spark in a jar partially filled with water. Early versions of the Leyden jar (named after Musschenbroek’s city of residence in the Netherlands) were glass jars filled with water and lined with metal foil, with a nail or metal rod protruding down through the lid into the fluid (see Figure 1.2). When an electrostatic generator touched the metal rod, it put a charge into the jar, which could store the charge for hours and deliver a considerable shock to the experimenter who later touched the rod. Von Kleist and Musschenbroek had basically discovered capacitors (which you’ll learn about more in Chapter 7), though they didn’t know it at the time, and neither could ever figure out how exactly the jars stored electricity.36
Figure 1.2 Diagrams of an original Leyden jar (left) with a nail protruding from the fluid-filled jar; a variation (center) with tin foil coating the walls to deliver a greater charge; and a discharger (right) designed so that the experimenters wouldn’t themselves get shocked. (Reprinted from “Lessons in Electricity, IV.” Popular Science Monthly, Volume 9, July 1876.)
Scientists around the world would soon be using Leyden jars to run experiments of their own. Their ranks included Benjamin Franklin, who used the jars while conducting his famous kite experiment in 1752, successfully drawing electricity from lightning.
Still, the Leyden jar only produced an instantaneous jolt of electricity, and any practical use of electricity would require a source of continuous current. In 1800, Volta first delivered that with the electric pile, known today as a Voltaic pile, the forerunner of the modern battery.37
Though Alessandro Volta gets credit for inventing the first true electric battery in 1800, Benjamin Franklin is actually credited for first coining the term battery to describe a device for electricity storage. In a 1749 letter to British friend Peter Collinson, Franklin described a “Machine,” essentially an early capacitor that was clearly inspired by the Leyden jar: “Upon this We made what we call’d an Electrical Battery, consisting of eleven Panes of large Sash Glass, arm’d with thin leaden Plates, pasted on each Side, placed vertically, and supported at two Inches Distance on Silk Cords; with Hooks of thick Leaden Wire one from each Side standing upright, distant from each other; and convenient Communications of Wire and Chain from the giving Side of one Pane to the receiving Side of the other; that so the whole might be charg’d together, and with the same Labour as one single Pane; and another Contrivance to bring the giving Sides, after charging in Contact with one long Wire, and the Receiver with another; which two long Wires would give the Force of all the Plates of Glass at once thro’ the Body of any Animal forming the Circle with them.”38 At the time, the word battery was derived from the French, baterie, which meant “a group of two or more similar objects functioning together,” and was typically used to describe an artillery battery. Franklin co-opted the term to reference the multiple electrochemical cells that were connected together.39
Alessandro Volta and the Voltaic Pile
For decades before inventing what we know as the battery, the Italian scientist Alessandro Volta was experimenting with electricity. In 1775, at the age of 30, Volta invented a machine that, like the Leyden jar, could deliver a charge to other objects. Volta’s invention, however, could transfer multiple charges before needing to be recharged. He called the device the “perpetual electrophorus,” and his fame as a scientist was soon well established.40
The perpetual electrophorus, however, was a mere warm-up for his greatest contribution to science, which would come 25 years later, the result of a long, heated scientific dispute with another Italian, Luigi Galvani. In 1780, Galvani was dissecting frogs and discovered that the specimens, mounted on iron or brass hooks, would twitch when probed with a metal lancet. Galvani believed that he had discovered a new form of energy, generated by the frogs’ muscles themselves, calling it “animal electricity.”41
Volta was immediately enthusiastic and intrigued by the discovery, but soon had doubts about the source of the energy. Mimicking the experiments, Volta found that the muscle twitch could be produced more reliably when two different metals touched the muscle and nerves rather than a single metal. Volta believed that the electricity came from the contact between the two different metals, and not from within the animal itself. The metals themselves were generating the current, Volta argued, and not the frog limbs. Volta would soon prove this theory—that dissimilar metals in contact would produce a charge—to be conclusively correct. (Galvani, it must be noted, wasn’t actually wrong; he had made one of the earliest discoveries of what are now known as “nerve impulses” within animals, a concept he proved when touching the nerves of frog legs and generating muscle twitches without any metal contact whatsoever.)42
If not for his experimental rebuttals of Galvani’s “animal electricity” theory, it’s likely that Volta never would have investigated the electric potentials of different metals. Because there were no instruments sensitive enough to detect weak currents, Volta would test different combinations of metals on his tongue, noting the metallic bitter sensation in his mouth as his saliva, like the frogs’ muscles, conducted modest amounts of electricity.43
Volta, aspiring to show conclusively that the generation of an electric current did not require dead frogs (or any other animal parts), conceived of a now historic experiment. He stacked alternating discs of zinc and copper on top of one another, each separated by a brine-soaked cloth. Then he connected a wire to each end of the pile and a steady current flowed (see Figure 1.3).44
Figure 1.3 Volta’s original illustration from 1800 of the Voltaic pile, below his drawing of the “cups pile” or “crown of cups,” each containing acidic or salt water and connected by metal straps of different metals. (Reprinted from A. Volta. On the Electricity Excited by the Mere Contact of Conducting. London: Philosophical Transactions of the Royal Society, 1800.)
The stack, now called a Voltaic pile, was the first true electric battery. Volta soon found that by stacking different types of metal, he could change the amount of the current produced, and that he could increase the current by adding more disks to the stack. He first published his findings on March 20, 1800, and the impacts of the discovery were immediate.45
A mere six weeks after Volta debuted the battery, two British scientists used a Voltaic pile to generate current to decompose water into hydrogen and oxygen.46 Within a year, Sir Humphry Davy installed the world’s largest electric battery at the Royal Institution of London, producing the first electric light.47 In 1802, a Scottish chemist named William Cruickshank used Volta’s pile to create the first electric battery for mass production, sealing a stack of copper and zinc disks inside a wooden box and filling it with brine.48
Volta was widely celebrated for the discovery, lauded with medals and awards, and, of course, his name immortalized in the unit of electric potential, the volt.
Other inventors would continue to improve upon Volta’s designs and within decades a variety of different battery designs were unveiled to deliver steadier charges for longer periods. The first major leap forward was the Daniell cell in 1836, which didn’t corrode or leak, and was called the “constant battery” for its delivery of a steady current.49 By mid-century, batteries would power then novel technologies like telegraphs, doorbells, and lights.
Planté and the First Rechargeable Battery
For nearly six decades, all batteries were primary, meaning they could be used once until all of the chemical reactants had worn out. In 1859, however, French physician Gaston Planté invented the first rechargeable battery, immersing two rolled up sheets of lead, separated by rubber strips, into an acidic solution. The lead acid battery Planté created is the same basic technology that’s under the hood of your car today. At the time, the Planté cell delivered roughly twice the voltage of the Daniell cell, which was still the best available battery technology. Moreover, the reactions in the Planté cell could be reversed, meaning the battery could be recharged by hooking it up to an external source of electricity.
The Planté cell would soon be improved upon by others—most notably by fellow French scientist Camille Faure, who in 1881 gave the battery much longer life and easily recharging capabilities—and the lead acid battery is still the most popular rechargeable battery in use today.50
Leclanché and the First Dry Cell
In 1866, Georges Leclanché patented a new system that became the technological foundation for much of today’s portable battery industry. Leclanché used zinc as one electrode, a carbon mixed with manganese dioxide as the other, and an ammonium chloride solution acted as the electrolyte. Within two years’ time, the battery was so popular that more than 20,000 of them were employed through the telegraph system, and soon thereafter for telephones, which still required their own power source in the years before centralized electricity generation.51 Though Leclanché’s original battery was wet, meaning it relied on the liquid solution, in the 1880s, a number of inventors nearly simultaneously took the fundamentals of his zinc-carbon cell design and again revolutionized battery technology.
The big leap was the removal of all free liquids, meaning that the battery could be turned and flipped and moved around without fear of it spilling. There is some debate over who was the first to truly invent the dry cell batteries, but German scientist Carl Gassner has the first official patent, dating to 1886.52 Gassner used zinc as the container to house the entire cell and used that sealed zinc container as the anode. Inside, a cathode surrounded a carbon rod, and the electrolyte was safely sealed inside. These dry cell zinc-carbon cells are primary batteries, meaning they cannot be recharged, although they could be produced relatively inexpensively.
Figure 1.4 An advertisement for the Columbia battery. (Reprinted from Hardware and Metal Magazine, March 26, 1921.)
A decade after Gassner filed his patent, an American company, the National Carbon Company, started mass manufacturing disposable, 1.5-volt zinc-carbon dry cells as Columbia batteries, and suddenly the battery transitioned from industry product to consumer good. While you may have never heard of the National Carbon Company, you are probably familiar with its corporate descendant, The Energizer Battery Company.53 For the first half of the 20th century, zinc-carbon Columbia batteries dominated the disposable battery market in the United States (see Figure 1.4).
Invention of the Alkaline Battery: Jungner versus Edison
In 1899, the Swedish scientist Waldemar Jungner invented a rechargeable battery that was the first to use an alkaline electrolyte rather than an acidic solution. Jungner first released nickel-cadmium and silver-cadmium versions, but because of the relatively high costs of cadmium and silver, he also developed a cheaper nickel-iron battery. Jungner obtained a patent for these first-of-their-kind rechargeable alkaline batteries in Sweden in 1899, but focused first on commercializing the nickel-cadmium and silver-cadmium versions because of superior performance. Two years later, in 1901, Thomas Edison took Jungner’s nickel-iron design and patented it in the United States and, with better financial resources, won out a patent dispute with the Swede.54
Edison’s battery ambitions were to develop and mass-produce lightweight, energy-dense batteries that could power automobiles. At the time, gasoline- and diesel-powered cars were considered unreliable and electric cars were being sold in greater numbers. Lead acid batteries dominated the electric vehicle fleet, but Edison thought they were too heavy and the acid was prone to corroding the lead and causing a short life span. Edison released his rechargeable alkaline, nickel-iron battery to great fanfare, but before long the problems were evident. The batteries failed on a number of counts: some leaked, some died quickly. Edison shut down production and spent a few years redesigning the batteries, but by the time he brought the improved versions to market, his friend Henry Ford had released the Model T, and gasoline engines were standardized. Edison’s nickel-iron batteries couldn’t even be used to start Ford’s Model T because they didn’t deliver enough voltage. Edison still managed to market the battery to railroad operators and miners, and the business became quite profitable.55
Jungner may not have made a fortune like Edison, but his nickel-cadmium batteries better withstood the test of time. His battery design was used exclusively by NASA and by other national space programs in the 1960s and is still considered one of the most reliable battery types in the market.56
Alkaline’s True Arrival: Lewis Urry’s Eveready
Though Edison’s alkaline batteries were popular because of their size and cost, their use was limited to industrial applications. For typical household consumers seeking cheap, disposable (primary) batteries, the National Carbon Company’s zinc-carbon Columbia batteries dominated the marketplace despite relatively short life cycles. By mid-century, during the postwar economic boom, the race was on to develop a cheap primary battery with longer life for mass consumption by households in the burgeoning middle class.
In 1955, a Canadian engineer named Lewis Urry was working for National Carbon Company (by then a subsidiary of Union Carbide) and was charged with extending the life span of the commonly used zinc-carbon battery. Urry opted to follow the lead of Jungner and Edison and tested a number of cells that used an alkaline electrolyte. He eventually settled on manganese dioxide and zinc as the different electrode materials, and then came his big breakthrough. “My eureka moment came when I realized that using powdered zinc would give more surface area,” Urry said, describing his epiphany to forgo a solid zinc metal, a discovery that would significantly lengthen the battery’s useful life.57
In 1959, Urry’s disposable alkaline batteries hit the market under the Eveready brand, looking more or less like the throwaway batteries sold today.
Before long, alkaline batteries were in most households and, until the 1980s, Union Carbide controlled a full one-third of global battery sales through the Eveready and Energizer brands.58
Alkalines remain big business today, though companies like Energizer and Duracell have recently started packing different metals and new technology into the familiar AA, AAA, C, and D cells. Today, lithium alternatives to alkaline batteries are on the market, though these disposable batteries are probably the least exciting or important aspect of lithium battery innovation.
Though the earliest experiments with lithium battery technology came way back in the 1910s, it wasn’t until the 1970s that the first lithium batteries were made commercially available. These lithium pioneers were non-rechargeable, however, and the greatest potential of the lithium battery is for rechargeable applications. In the 1980s, some major advances were made on that front. American chemist John Goodenough gets credit for inventing the lithium cobalt oxide battery, the first true lithium-ion battery. In 1980, Goodenough engineered a cobalt oxide cathode, which has become a vital organ of every lithium-ion battery built ever since. A battery loaded with Goodenough’s cathode would produce two to three times as much energy as any other rechargeable battery of its size.59
Japanese chemists working for Asahi Chemical and Sony tweaked Goodenough’s designs and, in 1991, filed for an international patent for a lithium cobalt oxide cell. Soon thereafter, Sony brought the first commercial lithium-ion battery to market. Sales were instantly off the charts, and the batteries enabled Sony to bring smaller, sleeker, handheld video cameras and other portable devices to market.60
In the next chapter, we will further discuss lithium-ion batteries and the current battery landscape.
As you’ve read, batteries have come a long way from the first voltaic piles to the lithium-ion innovations that are currently powering our digital lives. Yet, by all indications, the electric battery’s greatest impacts are still to come as they continue to get cheaper, smaller, and able to hold more energy longer. As battery technology advances and new economies of scale make them ever cheaper, the deployment of the electric battery through the energy system could be unstoppable. Throughout the rest of the chapters of this book, you’ll learn more about how batteries work, the different battery technologies that will power this transition, and how batteries will change transportation and household energy use. You’ll learn about how the grid itself will integrate batteries and other alternative storage technologies to allow for greater reliance on solar and wind, to displace fossil fuel generation, and to deliver cleaner, renewable energy as we charge forward to a low-carbon future.