The Technical Challenge: More Powerful and Longer-Lasting Batteries
Prior to our introductory discussion of electric batteries in Chapter One, when you pictured a battery, you probably envisioned the little coppertops that power your flashlights, smoke detectors, and portable electronics. Depending on your experience you might also flash back to the time you had to jump-start your car. Or, you might remember that it is time to plug in your phone or computer to charge it. Each of these images represents a different iteration of battery technology, and all of them are fundamentally integrated into our daily lives.
Our demand for consumer electronics has played a major role in spurring battery technology development. In fact, in the United States we consume around 94 million kilowatt-hours (kWh) just to charge iPhones each year (based on approximately 94 million iPhones in use in the country1 and an estimated 1 kWh per phone per year2). That’s equal to the total amount of electricity used by nearly 9,000 homes in a year.3 While that may have you second-guessing the amount of time you spend on Clash of Clans, you probably owe a debt of gratitude to the iPhone. Advances in smartphone technologies also helped catalyze advances in robotics, medicine, and energy generation, all of which also require improved energy storage.
As discussed in Chapter One, a transition to a low-carbon economy creates the demand for high-capacity, reliable, battery storage devices. And the market is slowly responding. Over the past 10 years battery technologies have become more powerful, more reliable, and less expensive.
This chapter aims to familiarize readers with the basics of battery technology, describe both the staples and the recent innovations in the battery world, identify outstanding technical challenges associated with batteries, and introduce proposed solutions that perhaps will expedite our transition to a low-carbon economy.
Primer: Electricity and Storage
To understand batteries, it is important to first understand the context in which they operate. Batteries do not generate electricity. Although they have recently been associated with renewable energy technologies, such as solar energy, batteries are not a renewable or nonrenewable source of energy. Batteries are simply a common means for storing electricity that has been produced by an external process. Batteries can store renewable electricity but they also store electricity that has been produced by the combustion of fossil fuels or by nuclear power. Batteries are agnostic in regard to the source of electricity they store.
When it comes to our electric grid, batteries as a form of storage are important, since utilities in the United States generate around 4 million gigawatt hours of electricity each year, and, currently, all of it must reach the consumer the instant it is generated.4 The U.S. electric grid, which incorporates all types of electricity generation, is considered one of the engineering marvels of the modern world. But it faces a serious challenge because energy production must instantaneously match energy consumption due to historic challenges that have limited the availability of storage. As a result, the electric grid constantly seeks equilibrium between electricity production and consumption in order to provide us with instantaneous access to things like lights, air conditioning, and Netflix.5 When we cannot be interconnected with the electric grid, storage becomes essential, and the battery has become commonplace as a mobile power source. From today’s digital mobile devices to tomorrow’s electric vehicles, the electric battery has become the essential storage technology when mobility requires us to disconnect from the wired grid. Whether we are considering the future of grid-connected batteries for storing intermittent renewable electricity or as a source of mobile energy to power our personal devices or transportation sources, our understanding of the basics of both electricity and storage is essential.
Energy sustains all life on Earth, and it takes many forms. We use these different types of energy to perform work, such as propelling a car forward or spinning the blades of a fan. Energy is really just the capacity to perform work. At the most basic level, energy falls into two categories: potential energy and kinetic energy.
Potential energy exists when an object could perform work based on the forces acting upon it, but it does not actually do the work. For example, water held by a dam at the top of a cascade has the potential to do work by falling over the cascade due to gravity. Yet, if there is a physical barrier preventing the water from flowing over the falls, then it is not actually doing any work. Similarly, when you pull back the string of a bow, the bow contains elastic potential energy that could be used to shoot an arrow. But you prevent the bow from performing work on the arrow until you are ready to release it.6
Kinetic energy, on the other hand, is the actual energy of motion: the energy that the water has as it tumbles over the falls or the energy of the arrow hurtling toward the target. The total amount of energy contained by an object in motion is directly related to the object’s mass and velocity. In other words, the larger an object is and the faster it is moving, the greater its kinetic energy. Kinetic energy is used to perform work. This work occurs by transferring energy from one object to another. For instance, if the water hits the blades of a turbine as it falls, it transfers some of its energy to the turbine, causing it to spin.7
The transfer of energy is governed by one of the most fundamental laws of the universe: the principle of conservation of energy. The conservation of energy principle states that energy cannot be created or destroyed, only transferred. Therefore, as energy is disseminated throughout the universe, it must always be accounted for. When we think about energy being “lost” due to inefficiencies in systems or engines, that notion is not entirely accurate. Energy is never lost, but it is sometimes transferred away from its intended purpose. While the purpose of placing a turbine in the path of flowing water may be to use the kinetic energy of the water to spin the turbine, some of the kinetic energy will inevitably create translational motion (or vibration) rather than rotational motion. Additional energy will likely go toward generating heat due to the friction of the blades spinning around the axel.8 While the intricate mechanics and mathematics related to conservation of energy are not important for our purposes, it is important to keep in mind the basic principle that humans do not create or destroy energy. We simply harness different types of energy and use them to do work. And just because we are not 100 percent efficient in using energy to do work does not mean that energy is lost; it is merely used for an unintended, and often less useful, purpose.
As energy is transferred, it may also change form. The types of energy that are relevant for this chapter are mechanical energy, chemical energy, and electrical energy. As we have discussed, we use electricity to power our electric grid, but we predominantly use mechanical and chemical energy to create electrical energy.
Mechanical energy is the sum of the potential and kinetic energy contained in an object.9 In the example above, the energy that the flowing water transfers to the turbine is mechanical energy. The mechanical energy in the turbine is then used to do work. Chemical energy is the potential energy stored in molecular bonds. Chemical reactions, such as combustion, release the potential energy and transform the molecules into new configurations. Oftentimes, humans use these types of chemical reactions to create heat and light (whereas heat and light are often considered inefficiencies in the use of mechanical energy). We release stored chemical energy every time we build a fire, drive a car, digest food, or use a battery to power one of our devices.
Finally, electrical energy is the movement of electrons between atoms. Every atom is made up of protons, which are positively charged, neutrons, which are neutral, and electrons, which are negatively charged. The protons and the neutrons are held tightly together by nuclear forces to form the nucleus of the atom. Electrons are much smaller than the protons and neutrons, and they move around the nucleus in well-defined orbits. Each atom has a different number of electrons that orbit at various distances from the nucleus, depending upon the size of the atom. The electrons that are farthest from the nucleus are more weakly held in their orbits by the electromagnetic attraction between the negative and positive charges of the electron and the proton, respectively. Therefore, other forces may influence those electrons. Other forces may cause the electrons to be shared between two atoms or even to move between atoms. The flow of electrons from one atom to another is electricity. The energy released by this flow of electrons is electrical energy.10
Generally speaking, opposite charges attract, meaning that electrons will tend to flow from negatively charged materials to positively charged materials.11 An examination of lightning provides a dramatic example of electron transfer in nature. During an electrical storm, the electrons in the water molecules that make up a cloud are jostled around such that the lower portions of the cloud contain more electrons and are thus negatively charged. Conversely, the water molecules at the top of the cloud have fewer electrons and are thus positively charged. This charge separation creates an electric field within the cloud. As the buildup of negatively charged molecules at the bottom of the cloud increases, it not only increases the intensity of the electric field in the cloud, but it also begins to repel electrons at the surface of the Earth. This process causes a positive charge to accumulate at the surface of the Earth. As the electric fields within the cloud and between the cloud and the Earth intensify, they become so strong that they force the positive and negative charges within surrounding molecules apart. This reduces the attraction between the molecules’ electrons and their nuclei and allows the electrons to be more mobile in the electric field. Aligning the molecular charges in this way and creating more free-flowing electrons make the air more conductive. When the pull of the positive charge from either the top of the cloud or the ground becomes strong enough, the electrons will suddenly flow from the negatively charged portion of the cloud to the positive charge and neutralize the electric field.12 This phenomenon is what we call lightning.
Electrical fields may form naturally, as they do in clouds, to generate lightning. Electrical fields may also be intentionally induced using magnets to influence the movement of the negatively charged electrons. The electrons in most materials spin randomly, so their magnetic forces are cancelled out. Magnets, though, are special materials whose electrons all spin in the same direction. This configuration results in magnetic forces that create two magnetic poles within the material. Opposite poles attract each other while similar poles repel each other, just as with electrical charges. The attraction or repulsion between poles in a magnet creates a magnetic field.13 These magnetic fields, like electrical fields, may be used to move electrons around. In particular, by passing magnets by a highly conductive material (such as copper) at high rates of speed, the resulting magnetic fields force the movement of electrons, thereby creating a current. This process is, in a basic sense, how large-scale generators work.
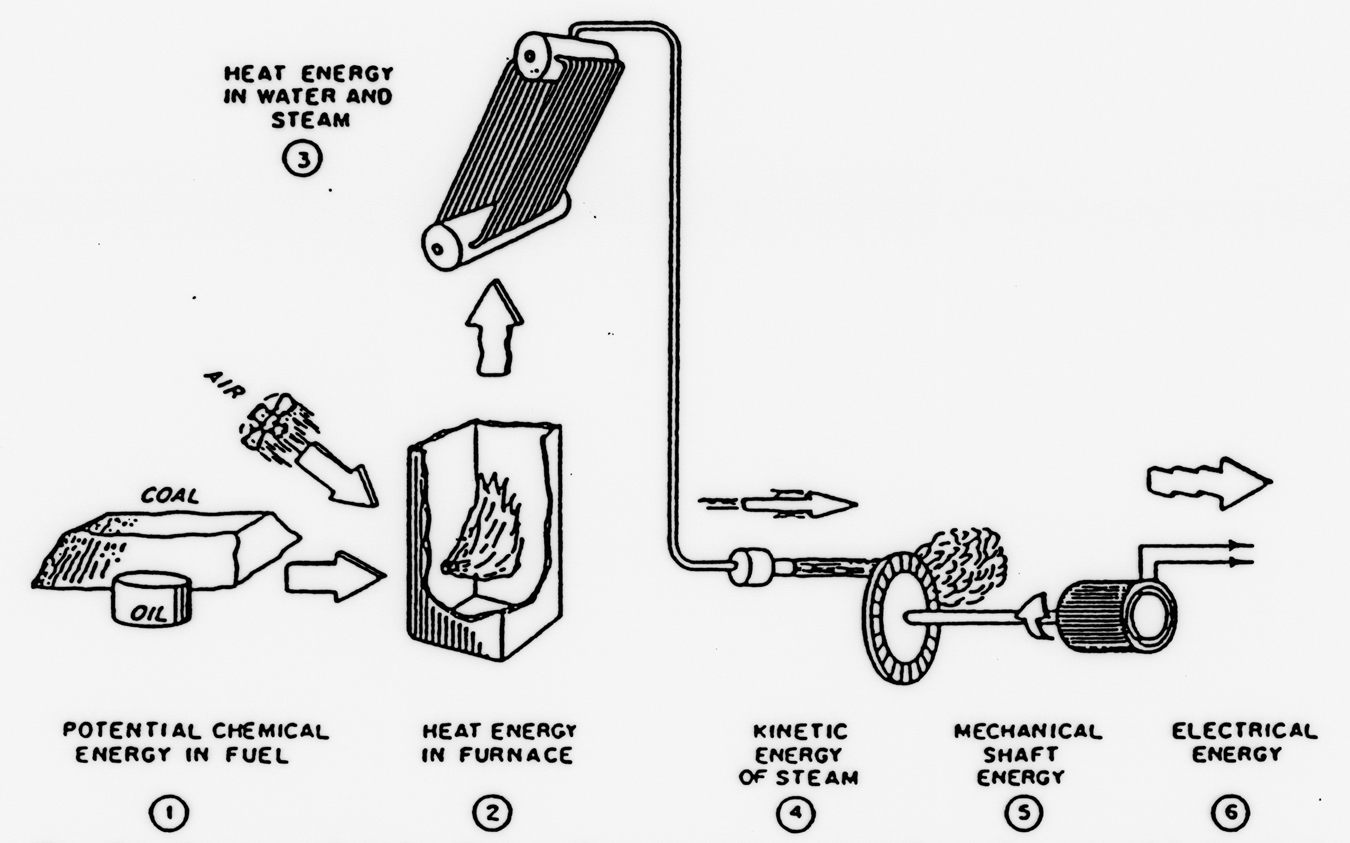
Figure 2.1 In electricity generation facilities, energy undergoes many conversions from potential energy in the fuel source to electrical energy distributed to the grid. (From Library of Congress.)
Generators operate in several phases. First, a generator must capture chemical and mechanical energy. Most utilities in the United States utilize the chemical energy released by combusting fossil fuels to heat water and generate steam. The flow of the steam past the blades of a turbine causes it to spin, resulting in mechanical energy (see Figure 2.1). Alternately, renewable sources such as hydropower and wind bypass the combustion process and directly use the kinetic energy of moving water or air in nature to spin the turbine and generate mechanical energy. Then, the spinning turbine moves magnets past coiled wire, creating a magnetic field. The strong magnetic forces then induce electrons to move through the wire, creating a current.14 Finally, the current is distributed across the country, transferring the chemical or mechanical energy from the generation facility to the end user via electrical energy.
How Does Electricity Transport Energy?
An electrical current generated by any method causes electrons to flow through a conductive path, such as a wire, until it reaches its destination and the electrical energy is used, or until the electrical energy is dissipated to the atmosphere due to resistance. The path that the electricity takes is called a circuit. Electrical grids are effectively massive circuits. They connect electricity generators with electricity consumers. The electricity is distributed across the country and the world via copper or coaxial power lines that connect every electric customer to the grid. When a consumer summons electricity to their outlet by plugging something in, they complete the circuit. The attached device draws the electrical energy to activate a switch, which may be analog or digital. That activated switch then allows the electricity to flow through the rest of the device. Then the electrical energy is used to power the digital components of the device—the circuitry and computers that direct its operation—and some may also be converted back to mechanical energy to power the analog components of the device—such as fans and speakers.15
Battery Basics: What Are the Components of a Battery?
If electricity transfers energy from the generation source to the end use via the flow of electrons, how do batteries fit into the equation? Batteries are essentially modular stores of chemical energy, which can be tapped to convert chemical energy into electrical energy. Thus, batteries are electrochemical power sources.16 When batteries are connected to conductive material in a closed-loop system, they create a circuit through which electrons can flow. In battery-powered devices, inserting the battery serves the same purpose as activating a switch in electricity-powered devices. Inserting the battery (or activating the switch) completes the circuit, allowing electricity to flow through it. The circuit can be very basic or very complex. For example, when you fill your flashlight with D cell Energizers®, you make sure that the batteries are pressed between two conductive materials (usually a spring and a metal plate) so that the circuit is completed. Alternately, when you turn the key in the ignition of your car, you engage a switch that completes a circuit between your car’s battery and the starter engine, which ignites the car’s internal combustion engine.17 The complexity of the circuit usually dictates the size and type of battery that must be used within that circuit.
So how do batteries use chemical energy to induce the flow of electrons? Remember that chemical energy is stored in molecular bonds and may be released when molecules are reconfigured. Every battery is composed of three basic pieces: two electrodes—a cathode and an anode—and an electrolyte. These pieces are housed together in an enclosed space, called a cell. The cathode and anode are separated by the electrolyte, and chemical reactions within the battery cause electrons to accumulate at the anode. This may be compared to the way in which electrons accumulate at the bottom of a cloud during an electrical storm. Since the negatively charged electrons repel each other, they try to get away from one another and move to a more positively charged area. In a battery, the electrons seek to move toward the positively charged cathode, creating electric potential energy. The difference in electric potential energy between two points—for example, between an anode and a cathode—is measured in volts (V).18 Therefore, a 6-volt battery is one in which the difference in electric potential energy between the anode and cathode is 6 volts, or 6 joules per coulomb (meaning that 6 units of energy are transferred per charge).
When a negative charge is built up in a cloud, electrons flow freely and violently as soon as the electric potential energy is released over a conductive path from the negative area to the positive area. Batteries operate in a similar way. The electrons in the anode are separated from the cathode by a semipermeable membrane, allowing only certain molecules to pass through it. But when a battery is introduced into a closed circuit, the electrons can flow along the conductive material from the anode to the cathode outside of the cell. The flow of electrons creates a current (represented in mathematical equations as I) and is measured in amperes (A or amp).19 In this way, the electric potential energy within the battery is converted to electrical energy that can be used to power other devices.
Importantly, though, when electrons flow from the anode to the cathode, the battery cell is fundamentally altered and the electric potential energy is reduced. When there is no longer sufficient electric potential energy within the battery, its ability to create a flow of electrons is depleted. Rechargeable batteries work by reversing the process. Electrical energy from an external power source may be applied to the battery to force the electrons back through a circuit into the anode. This process requires external energy because it induces electrons to move out of equilibrium and congregate together in the anode again. Thus, a rechargeable battery allows for the conversion of electrical energy from a power source into electric potential energy.
Neither discharging nor charging a battery is a spontaneous process when the battery is removed from the circuit. Nevertheless, air itself has some conductive properties (as we saw from the lighting example). Therefore, a small number of electrons may constantly dissipate from the charged battery into the air, depleting the battery’s charge over time. This discharge occurs most frequently with primary batteries but may also occur in secondary batteries. The loss of the electrons from the system reduces the electric potential of the battery, which is why your AA batteries sometimes die even when they have not been fully depleted by use.
How Is Electric Potential Created?
Remember that chemical energy is stored in molecular bonds and can be released and transformed into other types of energy when the molecules are reconfigured. The chemical components of a battery cell are comprised of very precise materials that interact in specific and predictable ways. In particular, battery materials undergo oxidation–reduction reactions (“redox reactions”). In these reactions, one molecule loses electrons and another molecule gains the electrons. These types of reactions occur spontaneously, and they are responsible for combustion, food spoilage, and corrosion.
Redox reactions are comprised of two complementary processes. First, oxidation occurs, resulting in a molecule losing electrons. Then a reduction reaction occurs, resulting in another molecule gaining electrons. These reactions occur in tandem because electrons are most stable when they are part of a molecule, so they will tend to join nearby molecules rather than remain free-floating. In a battery, the chemical reaction between the anode and the electrolyte oxidizes the anode; that is, the conditions cause the anode to lose electrons. The electrons then flow through the circuit to reduce the cathode. In other words, the cathode then gains the newly freed electrons. When both of the electrodes independently interact with the electrolyte, the redox reaction serves to transform the molecules in the electrodes and allow the electrons to flow through the circuit. Creating conditions inside the cells of a battery that enable a redox reaction to take place, creates potential energy, called electric potential. When the circuit is completed and electrons are able to flow from the anode to the cathode, the energy is converted from potential energy to electrochemical energy (see Figure 2.2). The more easily the electrodes are oxidized or reduced, the greater the electric potential is in the battery.20
Figure 2.2 In this simple galvanic cell, the zinc electrode is oxidized as electrons flow away from it toward the copper electrode. The copper electrode is reduced, and to balance the system, charged ions flow through the salt bridge. (Reprinted from Hazmat2.)
There are several mnemonic devices to help understand and remember how redox reactions work. One of the most prevalent is, “Leo the lion says ‘Ger.’ ” In this device, “Leo” is an acronym that stands for “lose electrons, oxidation,” and “Ger” is an acronym that stands for “gain electrons, reduction.” Alternately, just remember, “OIL RIG”: “oxidation is loss, reduction is gain” of electrons. Additionally, to remember which component of the battery undergoes which reaction, just think of the “n” in “anode” as associated with “negative,” whereas the “t” in “cathode” looks like a plus sign, for positive. The negative electrode gets oxidized and sheds electrons, as if it were negatively charged and repelling electrons. And the positive electrode gets reduced by accepting electrons, as if it were positively charged and attracting electrons. (While this is a useful way to remember how redox reactions work, please note that a molecule’s charge and its oxidation state are different.) To add one more level of complexity, materials that are easily oxidized are often called reducing agents because they lead to reduction reactions. Conversely, materials that are easily reduced are called oxidizing agents because they lead to oxidation reactions. The complex electrochemistry at play here is not within the scope of this chapter, but be aware of this terminology, as it may be important when discussing and comparing different batteries.
Electricity Storage: How Are Batteries Quantified and Compared?
So far, we have covered the internal chemistry of primary batteries. Primary batteries are those that are single-use, depletable cells, like your standard AA batteries. Yet, when we discuss battery storage options for transportation and the electric grid, we are referencing secondary batteries. These are rechargeable batteries that can use chemical potential energy to generate electrical energy and can also convert electric energy into chemical potential energy. Nearly all of the battery storage technologies described in this book are secondary batteries.
The composition of a battery and its component materials dictate how a battery may be used and how efficient it will be. There are several metrics that we use to quantify these properties. One of the most basic metrics is the energy density of a battery. Two types of energy density exist: volumetric and gravimetric. Measuring an object’s physical density describes its mass per volume—or volumetric density—so the greater the mass of an object within a fixed volume, the greater its density. Volumetric energy density is similar. It measures the battery’s energy capacity per volume; that is, the amount of energy the battery is capable of generating [in watt-hours (Wh)] per volume [usually liter (L)]. Gravimetric energy density, or specific energy, measures a battery’s energy capacity per mass, or the amount of energy a battery is capable of generating (in Wh) per mass [usually kilograms (kg)]. Generally speaking, battery designers want to achieve the greatest energy density possible. Most devices—whether laptops or electric vehicles—benefit from sleeker, lighter batteries. The more powerful a battery can be while maintaining a manageable size and weight, the more marketable the device will be. Therefore, energy density is one of the most important metrics for designers.
In addition to the energy density, it is important to know the recharge rate and cycle life for a given battery. These metrics describe how a secondary battery is recharged and how many times it may be recharged. The rate at which a battery is charged or discharged is identified by its C rate. This metric normalizes the charge or discharge rate against a battery’s capacity by identifying the current (in amps) at which the particular battery is fully charged or discharged in one hour. Various C rates indicate shorter or longer charge or discharge times: A 2C rate is twice as fast, so the battery will discharge in 30 minutes, whereas a C/2 rate is half as fast, so the battery will discharge in 2 hours. The speed at which a battery charges or discharges is related to the current applied to the battery. For example, if a battery has a capacity of 100 amp-hours, then its 1C rate will be 100 amps. Similarly, a 2C rate for this battery will be 200 amps, and a C/2 rate will be 50 amps.21
A battery’s C rate, or the rate at which it is charged or discharged, will impact the battery’s lifespan. One battery cycle is completed when a fully charged battery is discharged to the allowable level and then recharged. The chemical composition of batteries allows them to provide a relatively high, consistent amount of energy over most of the discharge cycle, but the energy provided drops rapidly toward the end of a discharge cycle (in an exponential decline). Therefore, most batteries are not completely discharged, but instead, some of the energy remains in the battery. This reduces battery stress and increases longevity. Many manufacturers assume a depth of discharge of about 80 percent, meaning only about 80 percent of the energy contained in the battery is discharged in a given cycle. For this reason, a depth of discharge of 80 percent or greater is known as a “deep discharge” or “deep cycling.”22 This assumption accounts for both consumer behavior—because most consumers do not wait to recharge until a battery is completely dead—and design restrictions that prevent operation below a specific voltage.23 Importantly, the higher the C rate, the more stress is imposed on the battery, and the quicker the battery’s voltage is depleted. Thus, for high-load battery uses, such as powering a portable drill, higher depths of discharge are allowed.
The number of cycles a battery can undergo before it fails is known as the battery’s cycle life. Typically, the higher the depth of discharge, the lower the cycle life. In addition to the C rate and the depth of discharge, external factors, such as temperature and humidity, also affect a battery’s actual lifespan. Because of all of these factors, a manufacturer can only estimate how long a battery will last before it must be replaced. The consumer’s use of the battery will ultimately dictate its lifespan.24
Finally, one of the battery metrics most familiar to consumers is the state of charge, which is frequently expressed as the small battery-shaped icon in the upper corner of the device. When the icon is filled in completely, the battery is fully charged. As consumers, we rely quite heavily on our portable devices and, therefore, we often care deeply about that little battery icon. Accurately reflecting the state of charge is crucial to consumers, and failing to do so may lead to many angry online comments and a depletion of brand loyalty. Unfortunately, a battery’s ability to hold a charge or to discharge in a predictable way depends on a variety of factors: the battery’s age, the number of cycles it has completed, its C rate, its depth of discharge, and the temperature at which all this activity occurs. It is extremely difficult to design monitoring systems that track and account for all of these factors. Instead, the state of charge is generally calculated based on measurements of the battery’s voltage or current, which determine the change in capacity over time. Yet, this method is not always very accurate, particularly as the battery ages. Because examining the state of charge is such a critical means by which consumers interact with their devices, but it is often inaccurately predicted, this area of battery technology is ripe for improvement. Many laboratories and facilities around the world are working to improve the prediction of a battery’s state of charge.25
In addition to providing consumers with a more accurate prediction of when they need to plug in their devices, innovators are seeking ways to improve nearly every aspect of battery technology. Utilizing energy storage technologies more efficiently in a wider range of applications may lead to sweeping changes in many industries, from consumer electronics to automobiles to energy generation and distribution, but experts agree that to achieve this impact, battery technologies need to be smaller, cheaper, and more powerful.
For this reason, bright minds all over the world are experimenting with new and different battery chemistries to find the perfect specifications for various uses. Generally speaking, everyone is seeking ways to increase energy density, increase cycle life, and decrease cost. But the relative importance of those different metrics depends on the uses for which the batteries are being developed. For example, batteries designed for large-scale grid integration seeking to achieve load leveling and renewable energy management have a greater need for overall capacity, reliability, and longevity. Conversely, batteries designed for the next all-electric vehicle available to the masses will require improved energy density and affordability. Unfortunately, many existing battery chemistries require trade-offs between these metrics. With that in mind what does the current battery technology landscape look like?
Traditional Battery Chemistries
There are a handful of battery types with which most consumers are likely familiar. Most of the primary batteries that power our older, handheld devices are alkaline batteries. Nearly all AAs, D cells, and other disposable batteries are alkaline batteries, which are dry cell batteries composed of a manganese dioxide cathode and a zinc metal anode (ZnMnO2). Each battery is its own cell, activated when the battery is placed in a device to complete a circuit. The more power the device requires, the more battery cells must be placed in series (positive end to negative end) to increase the available voltage. These battery chemistries create reaction by-products that slowly degrade the cell and reduce the available voltage. The redox reaction between the cathode and the anode in alkaline batteries is not reversible, so the batteries may not be recharged. Thus, when the voltage is no longer high enough to power the device, the battery may be discarded.
Technophiles will note that there have been some advances even in these relatively simple, disposable batteries. Specifically, several different manufacturers have developed water-activated batteries with a variety of similar, magnesium-based chemistries.26 These batteries require the addition of water to complete the redox reaction and generate voltage. Although these batteries are limited in the amount of power they can produce, they have a variety of uses (including powering the “water-activated” rescue beacon on the life vests that come under your seat on an airplane) and tend to be more environmentally friendly, containing fewer heavy metals. Similarly, a number of manufacturers are working to improve metal air batteries.27 These batteries utilize oxygen as the cathode, a pure metal—such as zinc, aluminum, or lithium—as the anode, and an aqueous electrolyte. These batteries are characterized by high-energy densities and low toxicity and have been used in everything from hearing aids (zinc-air) to railway signaling. Yet, they are limited to low power applications and are typically not rechargeable.28
Recently, these small, simple batteries have also been redesigned with rechargeable chemistries. One variation of the secondary AA battery is the nickel cadmium battery (NiCd). This chemistry relies on a nickel hydroxide (Ni(OH)2) cathode and a cadmium (Cd) anode in a potassium hydroxide electrolyte (KOH) to generate a current. NiCd batteries typically charge and discharge at high rates, have relatively high cycle lives, and can be easily stored. Nevertheless, they degrade easily and they require expensive and environmentally hazardous materials. Therefore, they are currently losing market share to chemistries with higher energy densities and improved performance characteristics, such as nickel metal hydride (NiMH) batteries. NiMH batteries have a similar chemistry to NiCd batteries, but simply replace the cadmium with a different metal alloy. This substitution increases the energy density and capacity of the battery and maintains its high cycle life. NiMH batteries are manufactured by the major battery companies and continue to be used in portable electronics and in hybrid electric vehicles, such as the Toyota Prius.29
Speaking of automotive applications, most consumers are also familiar with the car battery. Even if you have never had to replace your battery or jump-start your car, you likely understand that your vehicle requires a lead acid battery that is typically housed near the engine. The lead acid battery is the first rechargeable battery chemistry ever discovered, comprised of lead alloy electrodes in a sulfuric acid electrolyte. Despite the fact that they were discovered more than 150 years ago, these low energy-density secondary batteries are still widely used today. Designers continue to rely on lead acid batteries for a number of reasons: they can handle high-power loads created by actions like cranking a starter engine; they have a low self-discharging rate and therefore maintain their charge for long periods of time; and they work well in cold temperatures. Although these are not the most efficient batteries, and they rely on highly toxic chemicals with negative consequences for environmental and human health, they will likely remain in the picture for the foreseeable future.
Finally, another battery chemistry that has been around for quite a while is the sodium sulfur cell. This battery type was originally developed in the 1960s by Ford and is currently used by the Japanese company NGK for stationary applications. Sodium sulfur batteries utilize a molten sulfur cathode, a molten sodium anode, and a solid sodium alumina electrolyte. This unique chemistry means that the batteries operate at high temperatures, which may cause problems, depending on the use. But these batteries have a very high-energy density, high efficiency, and high cycle life. They are predominantly installed in electric grids as backup power, energy storage for peak shaving, and stabilization for intermittent renewable energy generators.30
Lithium-ion batteries (LIBs) represent the first major leap in battery technology in decades. These batteries have become household items through application in consumer electronics (such as Apple products), in electric vehicles (such as the Tesla Model S), and in many new plug-in hybrids (such as the Chevy Volt). Shifting to lithium-ion battery technologies was a logical step since lithium is the lightest metal on the periodic table, which allows for somewhat higher energy densities. Because lithium ions are so small and light, they can be integrated more easily into other materials through a process called intercalation.31 This process creates a layered structure in which lithium ions can be inserted and removed from a host network of other metals. In most LIBs, solid electrodes composed of a variety of different metals are layered between separators, stacked alternately on top of one another such that the lithium ions flow between the sheets via a liquid electrolyte.32
This type of reaction yields a highly efficient battery with a high cycle life, making it very effective in a wide number of applications. Yet, despite their relatively impressive metrics, LIBs have plateaued in their ability to offer increased capacity, power, and longevity for given weights and costs.33 This especially affects their application in electric vehicles. Therefore, LIBs are an active area of research and innovation for many companies and universities. Some are seeking to tweak the chemical components to unlock better results. For instance, researchers at Stanford and at Oak Ridge National Laboratory are experimenting with aluminum ion configurations, which use similar processes to LIBs.34 Others, including researchers at Samsung, MIT, UC San Diego, and the University of Maryland,35 are opting to introduce a solid electrolyte into the traditional lithium-ion chemistry to increase the battery’s stability, longevity, and safety.36 Still others, such as 24M, a start-up company founded by MIT researchers, are seeking to alter the chemical process itself, hybridizing the solid-state LIB chemistry with that of more advanced flow batteries.37
While innovation continues to propel lithium-ion battery technology forward, other companies are ready to bring the technology to market. Many companies, most notably Tesla Motors, have invested heavily in LIBs. Not all companies believe in the power of the LIB to meet consumers’ increasing demands, but Tesla and many emerging companies38 are betting on LIBs to power vehicles and to provide greater grid stability and demand shifting. In fact, Tesla decided to take production into its own hands by building a $5 billion “Gigafactory” to produce enough LIBs to supply the rising demand for its Model S car and its Powerwall and Powerpack storage systems for residential and commercial deployment. Tesla hopes to drive down costs by at least 30 percent by collapsing the LIB supply chain into a single facility.39
Only time will tell whether LIB development will meet growing consumer demand or whether it will be outpaced by new technologies. Nevertheless, for now, this technology is certainly an integral piece of the energy storage puzzle. The success of particular projects, like the Tesla Gigafactory, will be a bellwether for the future of lithium-ion chemistry.
Leading-Edge Battery Chemistries
One of the most logical next steps in battery technology development is lithium-sulfur chemistry (Li-S). This configuration uses a lithium anode similar to a traditional LIB, but also uses a sulfur carbon cathode and novel electrolytes to create an energy density that may be up to five times greater than LIBs.40 The high theoretical energy density and significantly lower costs of a Li-S battery make the technology extremely promising. Yet, this technology still faces some substantial challenges, particularly with respect to cycle life and stability. Nonetheless, several major companies are working to commercialize Li-S battery technology within the next five years. Sony has made claims that its Li-S battery, to be released in 2020, will improve performance by 40 percent.41 Additionally, a UK company, OXIS Energy, has created a small-scale prototype Li-S battery for use in electric vehicles. The company is working to provide an increased energy density and to improve cycle life before expanding to other markets.42 Finally, NASA is partnering with the University of Maryland (and the LIB company Ampirus) to develop and test Li-S batteries for use in deep space exploration.43
Taking a whole new tack are those companies currently working on flow batteries. This approach represents a fairly significant innovation in battery technology. Flow batteries are secondary batteries that actually bear a passing resemblance to fuel cells. In this type of technology, the redox reaction that defines the battery’s electric capacity is driven by the electrolyte. In traditional batteries, the electrolyte merely facilitates the flow of ions and electrons. But in true redox flow batteries, two different reactive electrolytes (termed the anolyte and the catholyte) are stored in separate tanks and are pumped through the battery stack where they react to generate a current (rather than the anode and the cathode reacting to create a current). This configuration changes the way designers work with batteries, since the capacity depends on the volume of electrolyte stored outside of the cell rather than the size of the cell itself. The configuration also increases the stability and cycle life of the battery because the reactive materials are separated and less able to inadvertently discharge. Finally, the design versatility of redox flow batteries makes them scalable for grid energy storage, with capacities ranging from 500 kWh to 500 MWh.44
True redox flow chemistries include the iron chromium flow battery (ICB) and the vanadium redox flow battery (VRB). The VRB in particular has received some commercial interest,45 especially since 2011 when Pacific Northwest National Laboratory discovered that it could increase the VRB capacity by 70 percent.46 Almost 80 percent of the flow batteries in operation are VRBs, which have shown many of the anticipated benefits associated with flow batteries. But innovation continues; other chemistries are also under development which create true redox flow batteries with less expensive materials,47 and also which create hybrid redox flow batteries.48 These hybrids, like several zinc-based flow batteries, utilize one solid electrode and one reactive electrolyte. In these configurations, the solid electrode is plated within the cell stack and the reactive electrolyte is pumped through the stack, creating the redox reaction and generating the current. These configurations achieve many of the same benefits as true redox flow batteries, particularly with respect to increased design flexibility. Yet, the benefits are typically not realized to the same extent, since one electrode is still solid and embedded in the cell stack.49 While a number of pilot programs exist to test these configurations, we have a while yet to wait for this technology to be widely implemented.
Moving into less well-explored territory, at least one company has commercialized the aqueous hybrid ion (AHI) battery chemistry. The brainchild of Carnegie Mellon’s Jay Whiteacre, the AHI battery utilizes relatively benign and inexpensive materials to create an energy storage solution for large-scale, stationary systems. The manganese-oxide cathode, paired with a carbon composite anode and a saltwater electrolyte, creates a battery with a lower energy density than LIBs, but with a long cycle life and reduced price tag. Whiteacre’s venture capital-funded company, Aquion Energy, has been manufacturing these battery systems since 2012 and currently sells to commercial and residential customers.50
Another cutting-edge battery storage concept is the liquid metal battery, pioneered by MIT’s Donald Sadoway. This chemistry was developed in response to demand for low-cost energy storage for the grid.51 The liquid metal configuration utilizes a closed cell with two molten metal electrodes that naturally separate around a molten salt electrolyte. This approach has the potential to achieve comparable efficiencies to lithium-ion batteries while reducing assembly costs and increasing cycle life (since there are no moving parts and no pressure buildup or other physical alterations associated with the redox reaction).52 In addition to a MIT laboratory dedicated to perfecting the liquid metal battery chemistry, Sadoway is also a founder of Ambri, also a venture capital-funded start-up company dedicated to pursuing commercialization strategies for the technology. While there has been much publicity surrounding the development of the liquid metal battery, the systems have yet to be deployed beyond laboratory scale.
In addition to these private sector advances, the public sector has also been investing in advances in battery technologies through the U.S. Department of Energy (DOE) and through public universities. In particular, a recent collaboration between the DOE and Ohio State University has led to the first demonstrated solar flow battery. This innovation combines redox flow battery configuration with a solar cell, which collects solar energy and stores it as part of the same process. This type of configuration integrates solar electricity generation with electricity storage, reducing the inefficiencies and losses associated with transporting electricity from a solar collector to a battery storage technology. Solar flow batteries and similar technologies may allow future renewable energy installations to leapfrog traditional energy generation and storage designs.53
Beyond the Battery: How Are Batteries Configured for Scalability?
Thus far, this chapter has dealt predominantly with batteries in isolation, discussing the chemistries, interactions, and capacity of a single cell. Yet for batteries to operate functionally in large-scale applications such as grid storage, many of them need to work together. Think about the last time you put batteries in your flashlight or your remote control. When you maneuvered each battery into the tiny space between the spring and the plate, you created a network of batteries so that the total power available to your device would be greater. In this type of application, the batteries are connected in series. This term indicates that the batteries are assembled by connecting the positive terminal—the cathode—of one battery, to the negative terminal—the anode—of the next battery. Allowing the electrons to flow directly from one battery cell to the next in this arrangement increases the available voltage in an additive manner. Therefore, if you have two 1.5-volt AA batteries arranged in series in your remote control, there is a total of 3 volts powering the remote. In this arrangement, however, the amperage rating—or the total capacity—remains the same.54 So even though the available voltage doubles, the electrical capacity of the system will remain the same no matter how many batteries you place in series.55
Alternately, batteries can be arranged in parallel. In this type of configuration, one battery’s cathode is connected to the next battery’s cathode. And the anode is connected to the next anode. The parallel system has the reverse effect: it increases the system’s capacity additively, while the voltage remains the same. Thus, if you were to connect two 6-volt lantern batteries with individual capacities of 12 amp-hours in parallel, the voltage would remain 6 volts, but the capacity of the system would increase to 24 amp-hours. It may be important to increase a battery system’s capacity in order to accommodate higher currents.56
In real-world applications, it is often necessary to increase both the voltage and the capacity of a battery system. In these situations, battery systems may be connected both in series and in parallel. By creating a complicated wiring array of both series and parallel connections between battery cells, you are able to create an additive effect for both the voltage and the capacity. The lithium-ion battery packs deployed in many laptops utilize this type of system where one set of four cells connected in series is connected in parallel to another set of four cells in series, resulting in a total of eight interconnected cells (this configuration is commonly referred to as 4S2P) with a total voltage four times greater than that of an individual cell and a capacity twice as large as that of an individual cell.57 For any battery or battery system, the total electrical power available can be calculated by multiplying the voltage of the system by the current through the system (volts × amps = watts). Or, in the terminology we have been using, volts multiplied by amp-hours (Ah) equals watt-hours. So, that 4S2P LIB powering your laptop, which has a total voltage of about 14 V and a capacity of about 4.8 Ah, can provide just over 67 watt-hours of electricity. In other words, it could power a 60-watt light bulb for just over an hour.
It is not important to get too caught up in the math or the circuitry here, but it is useful to keep in mind that these types of configurations are possible and are often used to build battery storage capacity that meets very specific design criteria. These types of systems are particularly useful for large-scale applications.
Large-scale systems of secondary batteries have been integrated into our electrical grid for years. These are often less sophisticated banks of lead acid batteries that provide a bare minimum level of grid support, stabilizing the flow of electricity for minutes at a time when the demand outpaces the supply. While new iterations of battery technology may have the ability to improve demand management, peak shaving, and renewable energy integration, we must substantially increase the capabilities of large-scale battery storage.58 This can be done first by swapping outdated technologies for more efficient, more energy-dense battery chemistries. Next, it will be important to work toward developing efficient cell configurations that maximize power and efficiency while minimizing the physical footprint and impact of the system. Finally, management systems and other support technologies will help make these types of systems possible, and will help them integrate into a diversified grid in a more streamlined manner.
How Do Batteries Rely on Other Technologies?
Even the most sophisticated battery technologies are likely to fall short of transforming our energy systems if they are implemented without any support. In particular, sensors and other monitoring and management technologies are essential to successfully integrating battery storage into our infrastructure. Many companies59 are now seeking ways to track battery performance at a granular level. Additionally, government agencies are promoting this work. Several years ago, ARPA-E awarded $30 million to 12 different projects aimed at advancing sensing and control technologies to improve safety, performance, and longevity in grid and vehicle batteries.60 Hopefully, the results of those investments will reach consumers soon.
We already discussed one example of how important these ancillary technologies are. Monitoring a battery’s state of charge is imperative, but the techniques for doing so have not yet been perfected. Consumer electronics companies have been working on this issue for decades. More recently, electric vehicle companies have tried to refine the methods for accurately predicting how much battery life remains in a cell. The technology to accurately read or predict the temperature, current, voltage, depth of discharge, and state of charge of an individual cell will catalyze battery deployment to many new applications.
Even when those sensing techniques are robust and available, the industry will still need effective management software to analyze the data. Taking accurate measurements of individual battery cells to determine their state of charge and rate of degradation will generate a lot of raw data. Software developers work alongside battery hardware innovators to build management systems that can direct electricity flows within massive banks of battery cells, optimizing the charging and discharging of each cell. This not only allows for better battery optimization, but also creates systems that can integrate seamlessly into the grid, where electricity demand and supply must be balanced instantaneously.
While these support technologies do not always get the same media attention as the cutting-edge battery chemistries or the sleek devices they power, they are equally essential to the battery revolution.
Where Do Battery Materials Come From?
While some companies61 like Tesla are working to streamline their supply chain, most consumer product companies purchase their batteries, or at least the battery components, from other companies. The battery supply chain, particularly for lithium-ion batteries, is fairly complex and not very well documented. Generally speaking, there are several phases in the supply chain: raw materials, processed materials, battery components, and battery distributors. Because the market is predominantly focused on lithium-ion batteries at the moment, this section will trace lithium-ion battery materials through the supply chain. It is important to note, however, that the wide variety of battery chemistries discussed above will each have independent, widely varying supply chains.
Lithium comes predominantly from three South American countries: Argentina, Chile, and Bolivia.62 After the raw material is extracted and processed, it is transported to a separate, specialized facility to be manufactured into battery components. Currently just three countries—China, Japan, and South Korea—account for 85 percent of the component manufacturing capacity for LIBs.63 The United States is barely on the radar, with only 7 percent of global capacity.64 Yet, Tesla’s Gigawatt factory in Nevada is on track to significantly increase that share. Additionally, the distributions shift slightly when counting exclusively LIB component manufacturing for electric vehicles. Under that scenario, the United States hosts about 17 percent of the global capacity, tied with South Korea.65 The supply chain throughout Asia is by far the most developed, with some vertical integration slowly beginning to take place. Conversely, the supply chain in the United States is immature, and most of the plant operators are relatively new to the industry.66 This may begin to shift as more global companies claim LIB market share and seek to minimize costs through vertical integration. Some companies may be able to reduce costs through this type of strategy by gaining direct access to raw materials and increasing competition. However, the elevated costs of capital and labor, and the more stringent regulatory environment, may continue to impede production growth in the United States for the foreseeable future.67
In contrast to lithium, which is relatively abundant with a supply chain free from global scrutiny, cobalt, another important ingredient in lithium-ion batteries, comes predominantly from the Democratic Republic of the Congo (DRC). In fact, more than half of the world’s supply is mined in that country. What’s more, the Chinese company, Huayou Cobalt, corners the cobalt market in the DRC and sells the raw material to just three LIB component manufacturers. Two of these companies are also based in China; one is based in South Korea. Those three companies then sell manufactured LIB components to at least 16 multinational consumer brands.68 Although this seems like a simplistic supply chain, corporate records for many of these companies, or the lack thereof, make it difficult for many consumer brands to track the raw materials used in their batteries. This becomes problematic in two ways. First, it becomes easier for companies at the beginning of the supply chain to introduce higher prices, or even illegal taxes, on the materials. Second, cobalt mining in the DRC has been linked to severe human rights violations, which are especially difficult to control or correct when the end users are unaware of their connection to such violations.69 Despite the obstacles, Huayou Cobalt, and the industry more generally, have come under heightened scrutiny of late. The consumer-facing companies may soon face greater pressure from their customers and shareholders to implement a better system for tracing the materials used in their products. Companies may also have to prepare for greater government oversight and emphasis on domestic suppliers.
How Are Batteries Manufactured?
Another important consideration for battery innovators is the ease with which next-generation batteries are manufactured. Sony manufactured the original lithium-ion batteries in the 1990s, but the production process came about almost by chance. During this time the music industry was shifting from cassette tapes to compact disks, dramatically impacting Sony’s manufacturing landscape. As much of the company’s equipment and workforce, which had been employed manufacturing cassettes, stood idle, the company realized that it could repurpose those assets. As it turned out, the process for manufacturing the magnetic tape in cassettes is very similar to the original process for manufacturing LIB electrodes. Thus, Sony began manufacturing LIBs and established the manufacturing standard for this technology. Many LIBs are still produced this way.70 But there are some companies breaking the mold. XALT has constructed a LIB facility in Michigan, and Tesla is working toward bringing its Nevada Gigafactory online. Both manufacturing plants offer new innovative approaches to LIB production, but it remains to be seen how dramatically this will increase the batteries’ total price.
As with all disruptive technologies, the industry must continue to improve to reduce costs, increase efficiencies, and thereby increase market share. Lithium-ion battery packs still cost several hundred dollars per kilowatt-hour. This cost is causing the current market to stagnate, since the price remains about four times higher than that of gasoline. Fortunately, only about one-third of the price is driven by material costs. Manufacturing costs actually drive approximately 40 percent of the price of a LIB. This offers the industry some flexibility in trying to become cost competitive with traditional fossil fuels. By designing new methods of manufacturing batteries, innovators may be able to bring prices closer to $100 per kilowatt-hour, which will not only decrease the price of the finished product, but will also decrease the barrier to entry in the battery market. As batteries become easier to manufacture, more innovative start-up companies will be able to enter the industry, challenge entrenched designs, and push the technology to become more efficient and cost competitive more quickly.71
Unfortunately, there are far fewer companies working to improve manufacturing processes than there are companies working to develop a silver bullet battery chemistry. One of the leaders in this part of the field is Yet-Ming Chiang’s 24M. 24M is the second cutting-edge battery company that Chiang, an MIT materials science professor, has founded. In this iteration, he is focused on creating more efficient manufacturing materials, equipment, and processes for a new wave of lithium-based batteries. The company’s pilot scale process produces a cell in less than three minutes, it uses approximately 80 percent less material than conventional LIBs, and it reduces the price to around $100 per kilowatt-hour.72 If this technology is proven at larger scales, then lithium-based battery chemistries may continue to dominate the market in an unpredicted manner.
The need for efficient and inexpensive manufacturing permeates the battery innovation space. It necessitates that innovative chemistries be comprised of relatively affordable materials and that they be relatively simple to assemble. This places companies like Sadoway’s Ambri and Chiang’s 24M at an advantage. Ambri uses liquid metal slurry electrodes that are comprised of low-cost, readily available materials. And the battery components self-assemble in the cell, so all that’s required for manufacturing is to pour the electrodes, electrolyte, and separator into the cell’s framework. Similarly, 24M’s semisolid electrode slurry is easily applied to a film to create the battery cell. Neither company is producing batteries at commercial scale yet, but it is these types of innovations that will drive the battery revolution into its most prosperous days.
Remaining Technological Challenges
The subsequent chapters will highlight some of the barriers that remain to widespread implementation of battery storage technologies. Some of these obstacles are related to the very nature of battery technologies. These include: limitations of material science on achieving greater energy densities and cycling rates, which were discussed here, and problems related to the supply chain and disposal of toxic battery materials, discussed in Chapter 3. Additional barriers exist related to deployment of battery storage technologies to different markets—with transportation technologies addressed in Chapter 4 and grid storage technologies addressed in Chapter 6. Battery storage technologies also face fierce competition, not only within the field for research and development funding, but also externally from other energy storage technologies, discussed further in Chapter 7.
Additionally, technological innovations will never reach their full potential without appropriate enabling environments created by supportive legislation and regulation. Some of the legal and regulatory steps that are underway to support innovation toward a low-carbon economy are discussed in Chapter 8. While there is still room for improvement in the technical application of battery storage technologies, batteries will never be able to achieve their full potential until lawmakers, regulators, and consumers take greater action to demand them.
By transitioning to an electric grid system that integrates a greater amount of potential energy, we can deploy more diverse energy sources, increase reliability, and reduce the strain on our electric grid. There are myriad battery technologies in development that can be integrated into different phases of the electrical grid to begin effectuating these changes. Advanced lithium-ion battery arrays may provide greater longevity for electric vehicles and allow them to better act as distributed grid capacity when not in use. Alternately, sodium sulfur and redox flow configurations may revolutionize utility-scale grid storage to help catalyze the integration of renewable energy generation. Finally, cutting-edge technologies utilizing low-cost materials may dramatically increase affordability and make energy storage accessible to even the most disenfranchised populations. We are in the midst of a battery revolution, with storage technologies poised to dramatically change the way utilities, developers, and regulators approach electricity generation and distribution.