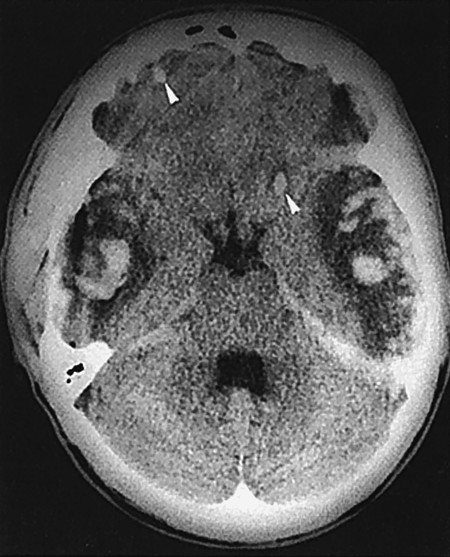
Fig. 6.7 Brain contusion (CT scan). There are extensive hemorrhagic contusions in both temporal lobes and smaller ones in both frontal lobes (arrowheads).
Key Point
Traumatic injuries of the skull and the underlying brain can be of different types and varying severity, depending on the nature and intensity of the causative event. There may be a skull contusion, a skull fracture affecting the cranial vault or the base of the skull, a concussion, brain contusion, an injury to larger-sized blood vessels producing a traumatic hematoma, or any combination of these types of injury.
In more than half of all cases, traumatic brain injury occurs as a component of multiple trauma. The associated injuries commonly affect the face, limbs, spine, chest, abdomen, and pelvis (in descending order of frequency).
The main clinical manifestation of a traumatic brain injury is impaired consciousness, often associated with a memory deficit (retrograde and anterograde amnesia). These problems can be accompanied or followed by neurologic deficits and/or epileptic seizures.
Brain injuries are either closed (i.e., with the dura mater intact) or open (with a wound extending into the subdural compartment or deeper into the brain parenchyma). Their severity is judged clinically and rated numerically on the Glasgow Coma Scale (GCS). Traumatic hematomas can be located within the brain parenchyma (traumatic intracerebral hematoma) or in the epidural or subdural compartments.
Large traumatic hematomas, or extensive damage to the brain parenchyma with accompanying edema, may lead to a rapid rise of intracranial pressure (ICP), causing brain compression and possibly brainstem herniation.
Frequent late complications of severe traumatic brain injury include neuropsychological deficits, personality changes, and symptomatic epilepsy.
Key Point
In the initial phase after trauma, the recognition that a traumatic brain injury is present and its clinical and anatomic assessment (e.g., intracranial hematoma) are vital. The duration and type of the impairment of consciousness are especially important elements of the clinical history. Meticulous history-taking and directed physical examination point the way to proper diagnosis and treatment.
Particular attention must be paid to the following aspects of the history:
The duration of unconsciousness (as reported by eyewitnesses).
The duration of amnesia for events that occurred before the injury (retrograde amnesia) and after it (anterograde amnesia, perhaps accompanied by confusion).
Early epileptic seizures.
Bleeding from the ear or nose (indicating a basilar skull fracture).
Important aspects of the initial physical examination in the emergency room are:
The vital signs, especially pulse and blood pressure (is the patient in shock?).
The level of consciousness: the depth of impairment of consciousness (up to and including deep coma) is assessed numerically with the GCS ( ▶ Table 6.5). This assessment may need to be performed multiple times—in particular, with each change in the patient’s circumstances and surroundings (before and after intubation, transport, arrival in the hospital, etc.).
Cranial injuries: inspection and palpation of the skull, orbital rims, zygomatic arches, jaws.
Cervical spine injuries and other accompanying bodily injuries.
Bleeding and possibly flow of CSF from the nose or ears, or in the pharynx (a CSF leak is conclusive evidence of an open brain injury, while bleeding is not).
Periorbital hematoma, a sign of basilar skull fracture.
Neurologic deficits (impaired pupillary reflexes, visual impairment, nystagmus, deafness, weakness, pyramidal tract signs).
|
Category |
Points |
|
Best verbal response: |
|
|
None |
1 |
|
Unintelligible sounds |
2 |
|
Inappropriate words |
3 |
|
Disoriented |
4 |
|
Oriented |
5 |
|
Eye opening: |
|
|
None |
1 |
|
To painful stimuli |
2 |
|
To auditory stimuli |
3 |
|
Spontaneous |
4 |
|
Best motor response: |
|
|
None |
1 |
|
Abnormal extension |
2 |
|
Abnormal flexion |
3 |
|
Withdraws (pulls away from pain) |
4 |
|
Localizes (fends off painful stimulus) |
5 |
|
Follows commands |
6 |
|
Patient’s overall score |
… |
|
Note: The overall score is the sum of the scores in the three categories. |
|
Key Point
Mild, moderate, and severe traumatic brain injury are distinguished on the basis of the type and duration of the impairment of consciousness.
The severity of traumatic brain injury is assessed on the basis of the clinical manifestations and their classification on the GCS ( ▶ Table 6.6).
|
Mild |
Moderate |
Severe |
|
|
Clinical features |
Loss or impairment of consciousness lasting less than 1 hour, EEG changes lasting less than 24 h |
Loss or impairment of consciousness lasting 1–24 h |
Unconsciousness for more than 24 h, and/or brainstem signs |
|
Score on Glasgow Coma Scale |
13–15 |
9–12 |
3–8 |
|
Possible neurologic findings |
Confusion, amnesia (generally encompassing the traumatic event itself, a short period before it, and the period of posttraumatic confusion) |
Depending on the site and extent of brain injury:
|
|
Mild traumatic brain injury In mild traumatic brain injury, consciousness is only mildly impaired (GCS score: 13–15) and the impairment lasts no longer than 1 hour. The patient may be confused. There may be amnesia for brief periods of time before and after the injury (retro- and anterograde amnesia).
Moderate traumatic brain injury The impairment of consciousness is somewhat more severe (GCS score: 9–12) and longer-lasting. The patient may have neurologic deficits, such as weakness or cranial nerve deficits, and/or epileptic seizures.
Severe traumatic brain injury The GCS score is 8 or lower and the patient is in coma for 1 day or longer. Neurologic deficits are generally seen, as well as signs of intracranial hypotension. Without appropriate treatment, transtentorial herniation of the midbrain and diencephalon may ensue (or, less commonly, herniation of the medulla through the foramen magnum).
Alternative classification An alternative classification of traumatic brain injury, based on pathophysiology rather than clinical severity, consists of the following categories:
Skull contusion.
Commotio cerebri (brain concussion).
Contusio cerebri (brain contusion).
Compressio cerebri (brain compression).
Depending on the clinical situation, the following imaging studies can be performed:
Head CT A head CT (often as a component of whole-body spiral CT) is generally obtained immediately on arrival of the injured patient in the emergency room for the prompt diagnosis of skull fractures, intracranial hemorrhage, brain contusions, cerebral edema, and intracranial air.
Cervical spine CT Cervical spine CT with multiplanar reconstruction is used to diagnose fractures and dislocations of the cervical spine.
Whole-body spiral CT A CT scan of the entire body is obtained in multiply and severely injured patients so that all of their injuries can be detected and classified according to the urgency of treatment.
MRI MRI is more time-consuming than CT and thus plays only a limited role in the acute diagnostic evaluation of head-injured patients. It is a useful means of detecting intracranial hemorrhages and, in particular, diffuse axonal injury in the cerebral white matter, which may be present even in “mild” traumatic brain injury. MRI is preferable to CT for follow-up studies after the initial, acute phase of traumatic brain injury has passed.
Plain X-ray There is no current indication for plain X-rays of the skull in traumatic brain injury. These have been entirely supplanted by CT for the diagnosis of skull fractures. Plain films of the cervical spine and other parts of the skeleton may be useful for various indications and are usually obtained with a whole-body, skeletal and soft-tissue, low-dose X-ray scanner, also called Lodox.
Key Point
The main pathophysiologic types of traumatic brain injury and their manifestations will be presented in this section. Traumatic hematomas will be discussed separately.
The pathophysiologic effects of brain trauma are divided into primary and secondary brain injury, as outlined in ▶ Table 6.7.
|
Primary brain injury (immediate) |
Secondary brain injury (delayed) |
|
Mainly caused by arterial hypotension and hypoxemia as well as intracranial hypertension, leading to ischemia in brain tissue and, in turn, to:
|
A head injury is said to be open only if there is an opening in the dura mater through which the intradural space communicates freely with the outside world, generally with leakage of CSF. Such injuries carry a high risk of early and late infection (meningitis, cerebritis, brain abscess). If the dura mater is intact, the head injury is closed. Thus, a skull fracture directly under a scalp laceration is not necessarily an open injury; it is one only if there is an underlying dural tear.
Patients with simple skull contusions have no evidence of a brain injury, that is, no loss of consciousness or amnesia and normal findings on neurologic examination. Some patients with this syndrome have scalp lacerations, or even skull fractures, and they may suffer from headaches afterward. Adequate therapy consists of a temporary restriction of activity and symptomatic medication, as required (analgesics, antiemetics).
Even mild head trauma can be associated with shearing forces that are strong enough to damage axons in the cerebral white matter and the blood vessels that supply them. Axonal shear injuries in various regions of the brain can lead to the degeneration of neural pathways; their clinical neurologic effects may only be noticed weeks or months after the traumatic event.
“Concussion” is often used as a near-synonym for mild traumatic brain injury. The concussion syndrome consists of a brief period of unconsciousness or impaired consciousness (generally no more than a few minutes), possibly followed by confusion. The periods of retro- and anterograde amnesia, if any, are only brief. Typical accompanying symptoms include headache, dizziness, nausea, and vomiting. There is no neurologic deficit, and it is thus often assumed that there is no underlying structural injury to the brain parenchyma. Nonetheless, T2-weighted MRI and diffusion tensor imaging (see section ▶ 4.2.3, ▶ 4.2.3.2) reveal evidence of axonal damage in some cases. Detailed neuropsychological testing may reveal subtle deficits even in patient who have “only” sustained a concussion; such deficits have been termed “minimal brain injury.” Posttraumatic headache after concussion generally subsides over time.
The clinical distinction between concussion and brain contusion (see later) is not always easy to draw.
The treatment of concussion resembles that of a skull contusion, with transient restriction of activity and symptomatic medication as needed. The patient should not be immobilized any longer than necessary: as long as there is no contraindication (such as hemodynamic instability), the patient should stand up and walk with assistance on the day of injury or in the next few days at latest. Rapidly mobilized patients have less severe postconcussive symptoms with a lesser tendency toward chronification.
Brain contusion is, by definition, a direct injury of the brain parenchyma. It is associated with a long period of unconsciousness and retro- and anterograde amnesia; indeed, the patient may not remember anything for a period of several days surrounding the event. Examination in the acute phase often reveals neurologic deficits, which may persist. Residual anosmia is common (see section ▶ 12.1).
CT or MRI reveals foci of contusion ( ▶ Fig. 6.7, ▶ Fig. 6.8) or intracranial hemorrhage, for example, an acute epidural hematoma ( ▶ Fig. 6.9) or an acute subdural hematoma. Parenchymal injuries can be found both directly underlying the site of the external blow (“coup” injuries) and at the diametrically opposite location in the brain (“contrecoup” injuries). Injuries of the latter type are due to the violent, tissue-distorting force transmitted through the brain tissue at the moment of injury. The pathoanatomic findings in foci of brain contusion include ischemic and hemorrhagic tissue necrosis, small hemorrhages, tears of brain tissue and blood vessels, and secondary brain edema. Lumbar puncture (LP), if performed (generally contraindicated!), yields bloody or xanthochromic CSF.

Fig. 6.7 Brain contusion (CT scan). There are extensive hemorrhagic contusions in both temporal lobes and smaller ones in both frontal lobes (arrowheads).
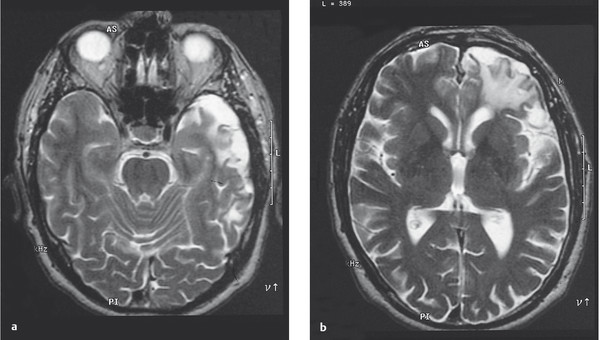
Fig. 6.8 Parenchymal defects 6 years after brain contusion. The T2-weighted MR images reveal cortical defects in the left temporal (a) and frontal lobes (b), accompanied by signal changes in the underlying white matter.
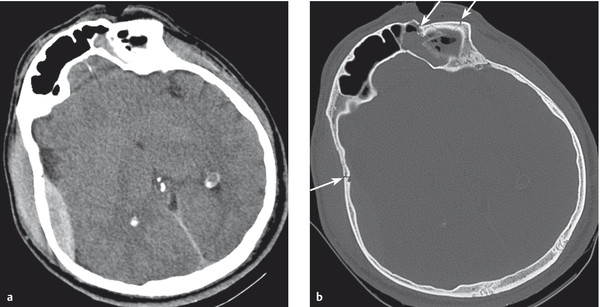
Fig. 6.9 Epidural hematoma (CT scan). There is an epidural hematoma on the right side (a) associated with marked extracranial soft-tissue swelling. (b) The bone window reveals a right-sided skull fracture over the hematoma as well as further fractures in the left frontal bone (arrows). This is the same patient seen in ▶ Fig. 4.1.
MRI scans performed in surviving patients years after a traumatic brain injury with a brain contusion generally reveal a loss of brain substance ( ▶ Fig. 6.8). Neurologic and neuropsychological deficits are usually demonstrable and may persist for life. These and other sequelae are discussed in section ▶ 6.2.7.
Traumatic brain injury causes cerebral edema, whose extent depends on the severity of the injury. Immediately after the traumatic event, water and electrolytes enter the extracellular space (=cytotoxic edema); hours to days later, the blood–brain barrier collapses and vasogenic edema ensues. The result is brain swelling and intracranial hypertension, which, if severe enough, in turn leads to cerebral hypoperfusion and brainstem herniation (see also section ▶ 6.3).
Large brain contusions and extensive traumatic hematomas, combined with the associated secondary brain edema, can cause very rapid and pronounced increases in ICP, leading to brain compression and herniation of the midbrain and diencephalon through the tentorial notch, and/or of the medulla through the foramen magnum. The clinical signs of brainstem herniation are: progressive impairment of consciousness leading to coma; a dilated pupil, initially only on the side of the expansive lesion; flexor and, later, extensor spasms; and, finally, impairment of autonomic regulatory functions (breathing, temperature control, cardiac activity, vascular tone), bilaterally fixed and dilated pupils, and death.
Practical Tip
The clinical severity of traumatic brain injury is correlated with the initially evident extent of structural damage to the skull and brain, but the correlation is not absolute. One patient may have an extensive skull fracture, but no neurologic deficit; another may sustain a relatively minor blow to the head that ruptures a bridging vein and produces a slowly growing subdural hematoma, which then compresses the brain, ultimately leading to coma and death.
Key Point
Traumatic hematomas are due to tears in arteries or veins (see ▶ Fig. 6.11).
Cause and localization Traumatic intracerebral hematomas generally result from the tearing of small blood vessels, most often within contusions of the frontal or temporal lobes.
Clinical features Intracerebral hematomas may exert considerable mass effect; combined with the surrounding edema, they may raise the ICP sufficiently to cause a progressive decline of consciousness and increasingly severe neurologic deficits.
Diagnostic evaluation Intracerebral hematomas are seen as hyperdense areas in a CT scan of the brain.
Treatment The neurosurgical evacuation of large hematomas can be considered, depending on their size and location.
Cause and localization Epidural hematomas ( ▶ Fig. 6.9) are generally produced by traumatic tearing of a dural artery, usually the middle meningeal artery. The tear itself is usually due to a temporoparietal skull fracture, but may occur in the absence of a skull fracture. The blood collection lies between the periosteum and the dura mater.
Clinical features The arterial hemorrhage can compress the brain very rapidly. The following clinical features are typical of epidural hematoma:
A patient who is initially unconscious because of a coexisting brain contusion may fail to awaken because an epidural hematoma has accumulated in the minutes or hours after the injury.
On the other hand, an initially awake or only transiently unconscious patient may rapidly lapse into coma after a so-called “lucid interval” lasting minutes or hours.
The side of the hematoma can often be determined by clinical examination: incipient uncal herniation compresses the ipsilateral oculomotor nerve, causing ipsilateral pupillary dilatation.
Hemiparesis, if present, is generally contralateral to the hematoma.
Diagnostic evaluation If an epidural hematoma is suspected, an immediate head CT is indicated. The hematoma is usually seen as a hyperdense, biconvex zone that is sharply demarcated from the underlying brain tissue.
Treatment Immediate neurosurgical evacuation is necessary to prevent brainstem herniation and death. Patients often make an excellent recovery if they have no underlying brain injury and if the hematoma has been removed promptly.
Subdural hematomas can be acute, subacute, or chronic.
Localization The blood collection lies between the dura mater and the arachnoid and comes about because of a tear in a bridging vein.
Cause An acute subdural hematoma is usually a component of a severe traumatic brain injury with extensive intraparenchymal contusions.
Clinical features Clinical examination alone does not enable a clear-cut distinction between subdural and epidural hematomas: subdural hematoma, too, is characterized by a rapidly progressive decline of consciousness, ipsilateral pupillary dilatation, and contralateral hemiparesis.
Diagnostic evaluation The diagnosis is established by CT: a subdural hematoma is typically seen as a hyperdense or isodense area (depending on the time elapsed since the traumatic event), either crescent-shaped or closely applied to the skull; unlike an epidural hematoma, a subdural hematoma is poorly demarcated from the underlying brain tissue. It may be spread over the entire cerebral hemisphere and may also be found as a layer of blood clot overlying the tentorium.
Practical Tip
Subdural hematomas may be spread over the entire cerebral hemisphere and may also be found as a layer of blood clot overlying the tentorium. Epidural hematomas are generally confined to the space between the cranial sutures.
Treatment Immediate neurosurgical evacuation.
Cause A chronic subdural hematoma may arise in the aftermath of a mild traumatic brain injury or even after a relatively trivial blow to the head, of which the patient may no longer have any recollection. Therapeutic anticoagulation is a risk factor for the development of a chronic subdural hematoma.
Clinical features A few weeks or (rarely) months after the causative event, the patient begins to suffer from increasingly severe headache, fluctuating disturbances of consciousness, confusion, and ultimately progressive somnolence. Hemiparesis, if present, is usually mild, and signs of intracranial hypertension are usually absent.
Diagnostic evaluation The diagnosis is established by CT or MRI ( ▶ Fig. 6.10).
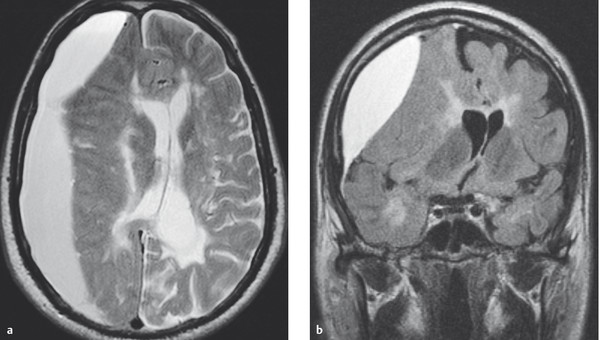
Fig. 6.10 Chronic subdural hematoma in a patient with secondary chronic progressive multiple sclerosis. The hematoma compresses the underlying brain tissue; note also the mainly periventricular signal changes due to MS. On the side of the hematoma, the cerebral sulci cannot be seen; the midline structures of the brain are markedly displaced toward the opposite side. (a) Axial T2-weighted spin-echo MR image. (b) Coronal FLAIR MR image.
Treatment The treatment is by neurosurgical evacuation through one or two burr holes (this is a relatively brief and uncomplicated procedure that can be performed under local anesthesia in cooperative patients).
Key Point
All patients with traumatic brain injury must be carefully clinically observed so that potentially fatal complications can be prevented, in particular, circulatory disturbances (hypotonia, hypoxemia) and intracranial hypertension. In some patients, pressure-measuring devices must be implanted in the brain for continuous, invasive monitoring.
Initial rescue measures The initial rescue measures at the scene of injury include the following, depending on the severity of injury:
Circulatory stabilization with the administration of isotonic fluids (e.g., normal saline; hypotonic fluids are contraindicated, as they can promote cerebral edema) and pressor drugs, if necessary, to sustain a systolic blood pressure of at least 120 mm Hg.
Intubation and artificial ventilation: the airway must be secured, and, depending on the nature of the injury and the patient’s state of consciousness, mask ventilation or intubation may be needed. Supplemental oxygen is given to achieve a saturation level of at least 95% and prevent hypoxia.
Analgesia, sedation, and relaxation: opioids or short-acting general anesthetics such as propofol can be given to sedate the patient and relieve pain (these drugs may cause hypotension, which should be counteracted immediately).
The patient is transported to a trauma center, where imaging studies are performed on arrival to diagnose brain injuries and associated traumatic injuries.
Neurosurgical operations The indications for a neurosurgical procedure include compressive hematomas causing neurologic deficits and/or incipient brain herniation, very extensive injuries to brain tissue, progressive worsening of deficits caused by the traumatic lesion, or otherwise intractable intracranial hypertension.
Mild traumatic brain injury The treatment of mild traumatic brain injury mainly consists of clinical observation.
Note
Any head-injured patient with an impairment of consciousness immediately after the injury (GCS < 15) should be admitted to the hospital.
Patients are generally admitted to the hospital for 24 hours for symptomatic treatment (analgesics and antiemetic drugs, as needed) and intravenous fluid administration until they are able to eat and drink.
Moderate and Severe Traumatic Brain Injury After the initial measures have been taken (possibly including neurosurgical removal of compressive lesions), and depending on the patient’s clinical state, further observation in an intensive care unit or dedicated neurotrauma unit may be needed, with adequate analgesia and sedation, frequent checking of the vital signs and neurologic functions, and, possibly, invasive monitoring of the ICP. Optimal cardiovascular, respiratory, and metabolic conditions should be created, particularly adequate oxygenation and blood pressure, and normoglycemia. Enteral nutrition and antithrombotic prophylaxis increase the probability of survival.
Extensive parenchymal injuries and the associated brain edema usually elevate the ICP. Thus, ICP-reducing measures may need to be taken, including:
Elevation of the head of the bed (generally to 30 degrees).
Hyperventilation.
Osmotherapy (mannitol).
External drainage of CSF.
Neurosurgical removal of compressive hematomas.
Patients with penetrating injuries of the brain or those who have already had an epileptic seizure in the early posttraumatic period should be given anticonvulsant drugs.
Note
All patients with traumatic brain injuries should be carefully clinically monitored. For comatose patients and those with impaired consciousness (particularly if their ICP is not being invasively monitored), regular checks of the size and reactivity of the pupils and of the other brainstem reflexes are mandatory, so that progressive intracranial hypertension can be detected and treated in timely fashion.
Key Point
This section deals with complications that arise later on by pathophysiologic mechanisms coming into effect some time after the initial injury. These include:
Early and late infections.
CSF leaks.
Neurologic deficits (brain and cranial nerves).
Posttraumatic epilepsy.
Malresorptive hydrocephalus.
neuropsychological deficits and personality changes.
A schematic overview of such complications is presented in ▶ Fig. 6.11.
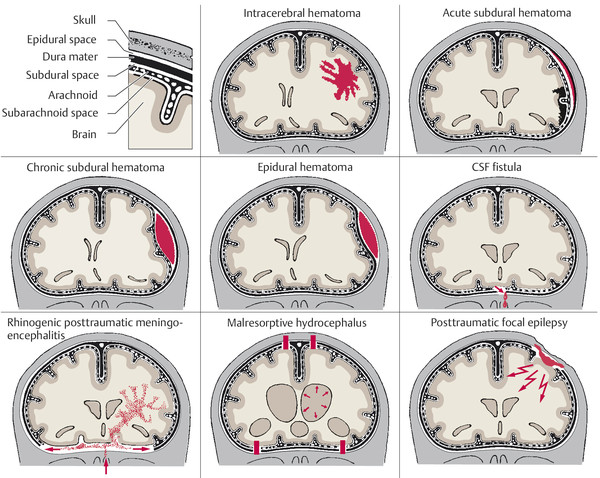
Fig. 6.11 Traumatic hematomas and complications after traumatic brain injury.
Meningitis, empyema, cerebritis, brain abscess Any open or penetrating brain injury (e.g., depressed skull fractures, gunshot wounds) provides a route of access for bacterial contamination of the meningeal spaces and the brain. Early posttraumatic meningitis, subdural empyema, cerebritis, or a brain abscess may appear a few days or weeks after the traumatic event.
Early epileptic seizures These may be present in the early phase and can also persist (late posttraumatic epilepsy).
Hydrocephalus Particularly in patients with severe traumatic brain injury, an impairment of the CSF circulation may lead to hydrocephalus. Patients with hydrocephalus have a higher mortality; some of this excess mortality can probably be reduced by the timely insertion of an external ventricular drain.
Electrolyte disturbances Dysfunction of the hypothalamic–pituitary axis can cause electrolyte disturbances—most commonly, diabetes insipidus because of insufficient secretion of antidiuretic hormone. Hypopituitarism may persist and necessitate hormone substitution.
CSF leak, meningitis, brain abscess A skull base fracture associated with a dural tear may give rise to a persistent CSF leak, manifesting clinically as leakage of clear fluid out of the nose or ear (CSF rhino- or otorrhea) or down the pharynx. The diagnosis can be secured by detection of β2-transferrin in the leaking fluid. CSF leaks are sometimes accompanied by orthostatic headache due to intracranial hypotension (see section ▶ 14.2.4, ▶ 14.2.4.3). If the leak remains undiscovered or untreated, it can serve as a portal for bacterial infection. The patient may present with meningitis (often pneumococcal meningitis) and/or a brain abscess, possibly years after the initial trauma. The presence and exact location of a CSF leak can be demonstrated by MRI and thin-section CT, which may reveal a bony defect or fracture. CSF leaks should be surgically repaired.
Posttraumatic neurologic deficits The commonest cranial nerve deficit after traumatic brain injury is anosmia (see section ▶ 12.1), which is permanent in two-thirds of patients, followed by optic nerve dysfunction (which only rarely improves) and palsies of cranial nerves III, IV, and VI (which usually resolve in 2–3 months). Fractures of the petrous pyramid(s) may cause facial nerve palsy as well as deafness, due to injury either to the vestibulocochlear nerve or to the cochlea itself; when caused by a transverse fracture, deafness is usually permanent. A fracture extending into the jugular foramen may cause a combined palsy of the glossopharyngeal, vagus, and accessory nerves (Siebenmann syndrome).
Focal brain lesions cause deficits according to their localization. Diencephalic lesions often cause diabetes insipidus. Cerebellar lesions cause ataxia, which does not always resolve. Spasticity, due to damage of the motor pathways in the central nervous system (CNS), may be uni- or bilateral.
Posttraumatic epilepsy Posttraumatic epilepsy is seen within 2 years in 80% of the patients who develop it, but it can also arise many years after the initial trauma in rare cases. The seizures may be focal, secondarily generalized, or primarily generalized (cf. section ▶ 9.2).
neuropsychological deficits and personality changes Posttraumatic neuropsychological deficits (variously designated as focal organic brain syndrome, psycho-organic syndrome, or posttraumatic encephalopathy) and personality changes are often the most disabling sequelae of traumatic brain injury for the patient and his or her family. The severity of these problems is positively correlated with the length of the initial loss of consciousness and with the duration of retrograde and anterograde amnesia around the time of the injury. Both short- and long-term memory are impaired and the attention span is shorter than normal. The patient has difficulty coping with complex tasks and situations and is easily fatigued. Impatience, irritability, diminished initiative, poor concentration, and lack of interest ranging to apathy characterize the patient’s behavior.
Note
Traumatic brain injury often has very serious consequences for the patient’s personal and professional life.
Rarer posttraumatic phenomena There may be a persistent (Lhermitte sign paresthesia down the back on flexion of the neck, further described in section ▶ 8.2). Malresorptive hydrocephalus most commonly arises after a traumatic subarachnoid hemorrhage (in either the early or the late posttraumatic phase). It reflects impaired CSF flow and resorption (see section ▶ 6.1.3, ▶ Table 6.3, and section ▶ 6.12.6).
Key Point
The prognosis after traumatic brain injury strongly depends on the severity of the injury on presentation. With an initial Glasgow coma score of 15, the mortality is near 0%; with a score of 3, it is over 80%.
Mild traumatic brain injury has a favorable prognosis; it usually causes no lasting deficit of any kind. After moderate traumatic brain injury, cognitive deficits and focal neurologic deficits (if present) can resolve significantly or even completely, but they can also persist. Survivors of severe traumatic brain injury generally have persistent neurologic deficits, including cognitive deficits. The severity of lasting impairment in such patients is highly variable, however, ranging all the way from the apallic syndrome (persistent vegetative state) to regained independence of function with only mild disability.
Key Point
Intracranial masses rapidly elevate the ICP because the skull is a closed compartment. Intracranial hypertension may arise acutely, that is, in a few minutes or hours (especially in brain hemorrhage), or chronically (for example, when caused by a slowly growing brain tumor). Characteristic manifestations include impairment of consciousness (ranging from somnolence to coma), abnormal breathing, bradycardia, hypertension, opisthotonus, extensor spasms of the limbs, and pupillary dilatation.
Intracranial hypertension impairs cerebral perfusion and CSF circulation and may also result in compression of intracranial structures (e.g., compression of cranial nerves against the base of the skull, occlusive compression of the posterior cerebral artery) and shifting of large portions of the brain within the skull (e.g., herniation of the medial portion of the temporal lobe into the tentorial notch, or of the cerebellar tonsils into the foramen magnum).
Definition Intracranial hypertension is defined as an ICP above 20 mm Hg; in normal individuals, the ICP is generally below 15 mm Hg. Another important quantity is the cerebral perfusion pressure (CPP), which is defined as the mean arterial pressure (MAP) minus the ICP. In normal individuals, the CPP is above 50 mm Hg.
Etiology The common causes of intracranial hypertension are:
Tumor.
Hemorrhage.
Extensive stroke.
Trauma.
Venous sinus thrombosis.
Hydrocephalus.
The cerebral edema that accompanies many of these conditions can exacerbate intracranial hypertension.
The ICP can also be elevated by a variety of other conditions (cf. ▶ Table 6.8).
|
Category |
Causes—particular entities |
Clinical features; remarks |
|
Intracranial mass |
|
Focal neurologic and neuropsychological deficits, headache |
|
Cerebral venous and venous sinus thrombosis |
Infection, preexisting disease (e.g., coagulopathy). Venous sinus thrombosis most commonly arises without any identifiable cause |
Impairment of consciousness, if the internal cerebral veins are involved; headache, epileptic seizures. Venous (sinus) thrombosis may be complicated by secondary intracranial hemorrhage |
|
Infection |
Encephalitis, meningitis, brain abscess |
Fever, meningismus. Causes include herpes simplex encephalitis, neurobrucellosis, and syphilis |
|
Traumatic brain injury |
Contusion, brain edema, intra- or extracerebral hematoma |
Progressively severe manifestations due to brain edema; focal seizures |
|
Impairment of CSF flow or resorption |
|
Headache (possibly ictal), vomiting. Intracranial hypertension is intermittent |
|
Widened lateral ventricles and third ventricle; normal fourth ventricle |
|
|
Spasticity of legs, urinary incontinence, psycho-organic syndrome. Often, prior history of subarachnoid hemorrhage or meningitis |
|
|
Elevation of CSF protein concentration |
For example, in polyradiculitis, spinal tumors (especially schwannoma) |
Lumbar puncture yields CSF with elevated protein |
|
Toxic processes |
Lead poisoning, insecticide poisoning |
Psycho-organic syndrome, anemia, lead line (on gums), sometimes other neurologic and systemic signs and symptoms |
|
Iatrogenic |
Steroids, oral contraceptives, tetracycline |
|
|
Altitude sickness |
Rapid ascent, inadequate oxygen supply, pulmonary and cerebral edema |
Headache, pulmonary edema, retinal hemorrhage, angina pectoris. Descend immediately |
|
Idiopathic intracranial hypertension, pseudotumor cerebri |
ICP elevation of no known cause |
Usually affects obese young women; slit ventricles, sometimes papilledema. A diagnosis of exclusion |
|
Abbreviations: CSF, cerebrospinal fluid; ICP, intracranial pressure. |
||
Pathogenesis The rigid skull and the CSF protect the brain from external mechanical influences. At the same time, however, the skull limits the total volume available for the brain, CSF, cerebral vasculature, and any other intracranially introduced material. If a space-occupying lesion should arise, such as a hematoma or tumor, the ensuing rise in ICP can be counteracted, at least at first by a compensatory exit of venous blood and CSF from the cranial cavity. The amount of added intracranial material that can be tolerated without the development of neurologic symptoms is about 40 mL in younger persons and about 80 mL in the elderly (because of the brain atrophy that often occurs in old age). If volume compensation is exhausted or outpaced by a rapidly expanding lesion, a dangerous rise in ICP occurs. The CPP, which equals the MAP minus the ICP, accordingly falls, and the blood supply to the brain is compromised (or even, in the extreme case, abolished). The brain is damaged not just by the direct mechanical pressure, but also by the ensuing ischemia. Very high ICP leads to herniation of the temporal lobes through the tentorial notch or of the brainstem through the foramen magnum.
▶ Table 6.9 contains an overview of the clinical features and diagnostic evaluation of intracranial hypertension.
|
Type of examination |
Symptoms and signs |
|
|
Physical examination |
Symptoms and signs of intracranial hypertension |
|
|
Signs of impending herniation |
|
|
|
Ocular findings |
|
|
|
Skull X-ray |
Abnormal only in chronic intracranial hypertension: increased digitate markings, enlarged sella turcica with demineralized dorsum sellae, diastasis of one or more cranial sutures in children and adolescents |
|
|
CT/MRI |
Slit ventricles (when elevation of ICP is due to cerebral edema), compressed gyri, periventricular signal change; CT and MRI may reveal the causative lesion for intracranial hypertension (e.g., tumor, hemorrhage) |
|
|
EEG |
Diffusely abnormal, nonspecific |
|
|
LP |
Contraindicated if elevated ICP is suspected. If LP is nonetheless performed, the opening pressure generally exceeds 200-mm CSF but may be normal if CSF flow is blocked at the occipitocervical or spinal level |
|
|
Direct ICP measurement |
Pressure gauge or ventricular drain in the lateral ventricle. Indicated for patients with impaired consciousness and suspected intracranial hypertension |
|
|
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; EEG, electroencephalogram; ICP, intracranial pressure; LP, lumbar pressure; MRI, magnetic resonance imaging. |
||
Practical Tip
An LP should, in general, not be performed if there is clinical suspicion of intracranial hypertension. It may be performed only if the imaging studies and ophthalmoscopic examination have yielded no evidence of acutely elevated ICP with impending brain herniation.
The most important complication of intracranial hypertension is brainstem herniation. With increasing supratentorial mass effect, the mediobasal portion of the temporal lobe is pressed into the tentorial notch, with ensuing herniation and compression of the brainstem (see ▶ Fig. 6.12). The ipsilateral oculomotor nerve is compressed; the loss of its parasympathetic outflow leads to pupillary dilatation (mydriasis). The patient’s consciousness is impaired. Unilateral herniation causes contralateral hemiparesis, and bilateral herniation causes quadriparesis. An infratentorial mass can compress the brainstem directly or cause brainstem herniation downward into the foramen magnum or upward into the tentorial notch.
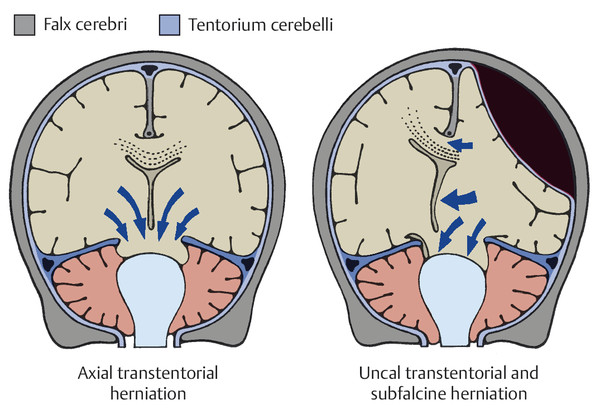
Fig. 6.12 Brainstem herniation. (Reproduced from Mattle H, Mumenthaler M. Neurologie. Stuttgart: Thieme; 2013.)
The treatment of intracranial hypertension consists of general measures to lower the ICP combined with specific treatment of the underlying cause.
General measures
Elevation of the head of the bed to 30 degrees (if the patient is not in shock and the blood pressure is adequate).
Adequate oxygenation with monitoring of the O2 saturation level.
(Restoration of) normoglycemia and normothermia.
Fluid and electrolyte balance (volume administration).
Specific measures
Osmotic agents, such as intravenous mannitol or hypertonic saline, and diuretics, for rapid (but transient) lowering of the ICP.
Sedation if the ICP is severely elevated (GCS > 8), to lessen the brain's metabolic demand for oxygen.
Hyperventilation (if the patient is intubated) to lower the Pco2 and thereby transiently lower the ICP.
Corticosteroids (e.g., dexamethasone, given intravenously) are used to counteract cerebral edema, particularly of the vasogenic type; they are mainly effective against peritumoral and inflammatory brain edema, but not at all against ischemic and traumatic brain edema, which are predominantly of the cytotoxic type.
External ventricular drainage through a catheter inserted into the lateral ventricle; especially useful if intracranial hypertension is due partly or wholly to hydrocephalus.
Hemicraniectomy is sometimes the only way to save the patient’s life, for example, in traumatic brain injury or massive stroke with space-occupying edema.
Hypothermia (cooling the patient) also lowers the ICP; its benefit after traumatic brain injury or stroke remains to be demonstrated.