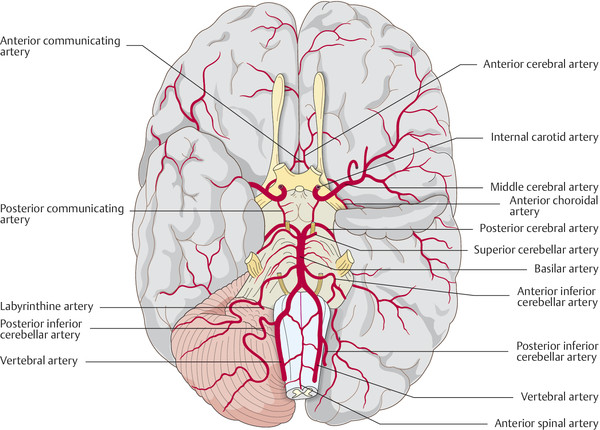
Fig. 6.19 Arteries of the base of the brain. (Reproduced from Bähr M, Frotscher M. Duus' Topical Diagnosis in Neurology. 4th ed. Stuttgart: Thieme; 2005.)
Key Point
The term “stroke” encompasses both ischemic and hemorrhagic disturbances of the cerebral circulation that produce central neurologic deficits of acute or subacute onset. Ischemia accounts for 80 to 85% of stroke, and hemorrhage for 15 to 20%. Nontraumatic intracranial hemorrhage is discussed in section ▶ 6.6).
Roughly 2 per 1,000 persons per year sustain an ischemic stroke; the incidence of stroke rises markedly with age. Women are less commonly affected than men up to age 80, and equally commonly afterward.
Cerebral ischemia is critically impaired perfusion in an area of the brain.
Cerebral ischemia can be classified by
Etiology: ischemia is mostly caused by the blockage of arteries by emboli (arterio-arterial emboli from atherosclerotic stenoses, as well as cardiogenic emboli), macroangiopathy (arteriosclerotic vascular occlusion), or microangiopathy (occlusion of smaller vessels by fibrinoid necrosis, also called “lipohyalinosis”). Impaired venous outflow is a less common cause.
Course: transient ischemic attack (TIA) versus progressive or completed stroke.
Type of infarction: territorial, watershed, border zone, lacunar.
The affected vessel and the resulting vascular syndrome (e.g., middle cerebral artery [MCA] syndrome, posterior cerebral artery syndrome, basilar artery syndrome). Depending on the extent of tissue injury caused by ischemia, the ensuing neurologic deficits may be either transient or permanent.
Every ischemic event calls for thorough diagnostic evaluation to determine the cause, so that recurrences can be prevented. Moreover, appropriate treatment must be given immediately (above all, hemodynamic stabilization and surveillance, thrombolytic treatment where indicated, and recurrence prophylaxis). The diagnosis and treatment of stroke in a specialized institution (a so-called stroke unit or stroke center) is associated with a markedly better outcome.
To understand how the localization and extent of cerebral infarcts depends on the particular artery that is occluded, one must know the anatomy of the territories of the individual vessels, as well as their numerous anastomoses. The anastomotic arterial circle of Willis, at the base of the brain, provides a connection between the carotid and vertebral circulations and between the blood supplies of the right and left cerebral hemispheres ( ▶ Fig. 6.19). The territories of the major cerebral arteries are shown in ▶ Fig. 6.20.

Fig. 6.19 Arteries of the base of the brain. (Reproduced from Bähr M, Frotscher M. Duus' Topical Diagnosis in Neurology. 4th ed. Stuttgart: Thieme; 2005.)
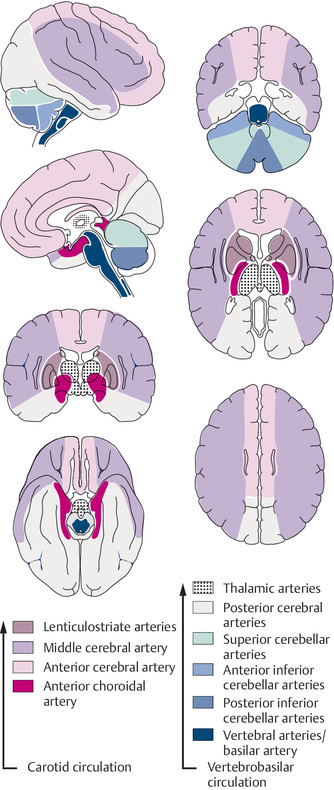
Fig. 6.20 Territories supplied by the individual arteries of the brain.
Glucose is the brain’s nearly exclusive source of energy. The brain accounts for only approximately 2% of body weight but receives approximately 15% of the cardiac output. Regulatory mechanisms ensure that the cerebral perfusion remains constant despite fluctuations in the arterial blood pressure, as long as the latter remains within a certain range. Thus, if the arterial blood pressure should fall, a compensatory dilatation of the cerebral arteries occurs to maintain cerebral perfusion, which is significantly reduced only when the systolic blood pressure falls below 70 mm Hg (or below 70% of the baseline value in hypertensive individuals). Hyperventilation and intracranial hypertension lessen cerebral perfusion, while hypoventilation (i.e., an elevated partial pressure of CO2) increases it.
Relative ischemia and penumbra Normal cerebral perfusion is approximately 58 mL per 100 g of brain tissue per minute. Signs and symptoms of ischemia begin to appear when the perfusion falls below 22 mL per 100 g per minute. In this stage of relative ischemia, the functional metabolism of the affected brain tissue is impaired, but the infarction threshold has not yet been crossed and the tissue can regain its normal function as soon as the perfusion renormalizes. The longer the relative ischemia lasts, however, the less likely it is that normal function will be regained. The zone of tissue in which the local cerebral perfusion lies between the functional threshold and the infarction threshold is called the ischemic penumbra (“partial shadow”). Within the penumbra, brain perfusion is linearly related to the arterial blood pressure.
Note
The penumbra is of major importance in the diagnostic evaluation of stroke, as well as in therapeutic decision-making and prognostication:
Within the penumbra, perfusion is reduced, but diffusion is still normal (perfusion–diffusion mismatch). Thus, imaging studies (above all, MRI) can distinguish it from tissue that has already undergone infarction.
If the occluded vessel is promptly recanalized, the tissue in the penumbra can largely survive and regain its normal function. The penumbra thus represents the tissue at risk for further stroke that may be salvageable by revascularization. Imaging of the penumbra is an important aid to clinical decision-making.
The ischemic penumbra in a patient with an acute MCA occlusion is shown in ▶ Fig. 6.21. A normal cerebral angiogram was presented in an earlier chapter (see ▶ Fig. 4.12). An occlusion of the MCA is seen in ▶ Fig. 6.24.

Fig. 6.21 Visualization of the ischemic penumbra with diffusion-weighted (a) and perfusion-weighted MRI (b). The patient is a 55-year-old man with acute left hemiparesis due to occlusion of the main stem of the right middle cerebral artery. For comparison, diffusion-weighted (c) and perfusion-weighted MRI scans (d) of a 58-year-old man with acute hemianopsia are also shown. (a) The diffusion-weighted image reveals a mottled hyperintense signal in the posterior portion of the right middle cerebral artery territory; most of the territory, however, has a normal diffusion signal. (b) The perfusion-weighted image is based on the time to peak uptake of contrast medium, which is delayed throughout the entire right middle cerebral artery territory. The penumbra is the area of tissue in which perfusion is diminished, but diffusion is normal. If the occluded vessel can be reopened early enough, bringing blood back into the hypoperfused area, the tissue in the penumbra will largely survive and regain its function. (c) Diffusion-weighted MRI for comparison. (d) Perfusion-weighted MRI for comparison. In this case, the area of abnormality is nearly congruent to that seen in (c); thus, there is no penumbra, i.e., the infarction is complete and no brain tissue can now be saved by recanalization.
Total ischemia causes irreversible structural damage of the affected region of the brain. If the blood supply of the entire brain is cut off, unconsciousness ensues in 10 to 12 seconds and cerebral electrical activity, as demonstrated by electroencephalogram (EEG), ceases in 30 to 40 seconds ( ▶ Fig. 6.22). Cellular metabolism collapses, the sodium/potassium pump ceases to function, and interstitial fluid—that is, sodium and water—flows into the cells. The resulting cellular swelling is called cytotoxic cerebral edema. Later, when the blood–CSF barrier collapses, further plasma components, including osmotically active substances, enter the brain tissue; a net flow of fluid from the intravascular space into the intercellular and intracellular spaces then produces vasogenic cerebral edema. In a vicious circle, these two varieties of edema lead to additional compression of brain tissue, thereby impairing the cerebral perfusion still further.
The severity of cerebral ischemia is correlated with its clinical course if untreated. Standardized scales and scores are available for its assessment. The most commonly used scale is the National Institutes of Health Stroke Scale (NIHSS, ▶ Table 6.14).
|
Findings |
0 |
1 |
2 |
3 |
4 |
Points |
|
|
1a |
Level of consciousness |
Awake |
Somnolent |
Stupor |
Coma |
– |
|
|
1b |
Orientation questions: Age? Month? |
2 correct |
1 correct |
0 correct |
– |
– |
|
|
1c |
Commands open and close (1) the eyes and (2) the nonparetic hand |
2 correct |
1 correct |
0 correct |
– |
– |
|
|
2 |
Gaze paresis |
None |
Partial |
Complete |
– |
– |
|
|
3 |
Visual field |
Normal |
Partial hemianopsia |
Complete hemianopsia |
Bilateral hemianopsia/blindness |
– |
|
|
4 |
Central facial palsy |
None |
Mild |
Complete lower half of the face |
Complete upper and lower halves of the face |
– |
|
|
5a |
Left arm motor function |
No sinking when held up for 10 s |
Sinks but does not touch underlying surface |
Sinks onto underlying surface |
No antigravity activity |
No movement at all |
|
|
5b |
Right arm motor function |
||||||
|
6a |
Left leg motor function |
||||||
|
6b |
Right leg motor function |
||||||
|
7 |
Limb ataxia |
None |
One limb affected |
Two limbs affected |
– |
– |
|
|
8 |
Sensation |
Normal |
Partially impaired |
Markedly impaired or lost |
– |
– |
|
|
9 |
Language |
Normal |
Moderate aphasia, communication possible |
Severe aphasia, communication impossible |
Global aphasia, mute |
– |
|
|
10 |
Dysarthria |
None |
Slurred but intelligible speech |
Unintelligible speech (or the patient is mute) |
– |
– |
|
|
11 |
Neglect, inattention |
None |
In one modality |
In more than one modality |
– |
– |
|
|
Total = NIHSS score |
|||||||
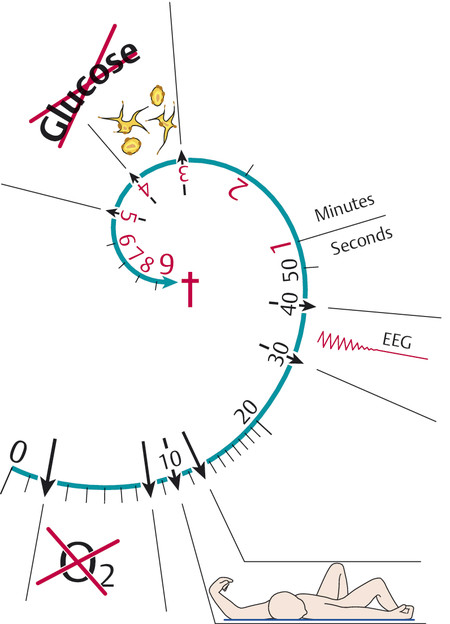
Fig. 6.22 Time course of cerebral ischemia. Diagram of the effect of sudden total deprivation of blood supply to the brain on tissue metabolism, consciousness, the EEG, neuronal morphology, and tissue glucose concentration.
Practical Tip
The term “minor stroke” is commonly used to designate a stroke with only mild motor and/or sensory deficits, with an NIHSS score of 3 points at most (no more than 1 point on any item). Patients with a minor stroke are generally fully awake and alert and neuropsychologically intact. They have a good prognosis.
A stroke with an NIHSS score of more than 15 points has a poor prognosis if untreated and is classified as a “severe stroke.”
Ischemic stroke has multiple causes ( ▶ Fig. 6.23). Embolic events and atherosclerotic stenoses of the major extra- and intracranial arteries play important roles, but there can also be hypertension-induced atherosclerotic changes of the midsized arteries or fibrinoid necrosis (lipohyalinosis) of the small arteries.
A simplified classification by etiology divides ischemic strokes into five classes:
Macroangiopathy: atherosclerosis of large extra- and intracranial vessels, leading to thrombosis in the region of an atherosclerotic plaque, hemodynamic insufficiency in the poststenotic circulation, or arterio-arterial embolism.
Cardiogenic and aortogenic embolism, mainly due to atrial fibrillation, but also as a complication of myocardial infarction, valve replacement, endocarditis, or cardiomyopathy.
Microangiopathy: cerebral small-vessel disease/arteriolosclerosis, usually due to hypertension, most commonly seen in the elderly.
Other etiologies, for example, vasculopathy, dissection, arteritis, coagulopathy, paradoxical embolism, right-to-left shunt.
Undetermined etiology.
▶ Table 6.15 may be a useful aid to the systematic search for the cause of stroke.
|
Cause |
|
|
Atherosclerosis |
|
|
|
|
|
|
Cardiogenic embolism |
Mural thrombus due to myocardial infarction, cardiomyopathy, myocardial aneurysm |
|
Valvular heart disease including rheumatic heart-valve disease, bacterial and nonbacterial endocarditis, prosthetic valves |
|
|
Arrhythmia including atrial fibrillation, sick sinus syndrome, brady- and tachyarrhythmias |
|
|
Atrial myxoma |
|
|
Paradoxical embolism through an open foramen ovale or atrial septal defect |
|
|
Atrial thrombus in aneurysm of the atrial septum |
|
|
Venous and venous sinus thrombosis |
Septic sinus thrombosis |
|
Coagulopathy (e.g., polycythemia, antithrombin deficiency), pregnancy, drugs (oral contraceptives, glucocorticoids) |
|
|
Bland, i.e., without identifiable cause |
|
|
Hematologic diseases |
Thrombophilia due to protein C, protein S, or antithrombin-III deficiency, antiphospholipid antibodies, anticardiolipin antibodies, paroxysmal nocturnal hemoglobinuria |
|
Hemoglobinopathy, e.g., sickle-cell anemia, thalassemia |
|
|
Hyperviscosity syndrome due to polyglobulia, thrombocytosis, leukocytosis, macroglobulinemia, myeloma, polycythemia vera, myeloproliferative syndromes |
|
|
Vasculitis |
Primary CNS vasculitis, granulomatous angiitis of the CNS |
|
Systemic necrotizing vasculitis with CNS involvement, e.g., in periarteritis nodosa, Churg–Strauss syndrome, giant-cell arteritis (polymyalgia rheumatica, temporal arteritis), Takayasu’s arteritis, Wegener granulomatosis, lymphomatoid vasculitis, hypersensitivity vasculitis |
|
|
Connective-tissue diseases and collagenoses with CNS involvement, e.g., systemic lupus erythematosus, scleroderma, rheumatoid arthritis, Behçet disease, mixed connective tissue disease |
|
|
Infectious vasculitis, e.g., due to HIV, tuberculosis, borreliosis, neurosyphilis, fungi, mononucleosis, CMV infection, herpes zoster, hepatitis B, rickettsia, bacterial endocarditis |
|
|
Toxins |
Illicit drugs, e.g., cocaine (also as crack), amphetamines, LSD, heroin |
|
Medications, e.g., sympathomimetic drugs, ergotamines, triptans, intravenous immunoglobulins |
|
|
Nonatherosclerotic vascular diseases |
Dissections of the extra- or intracranial arteries supplying the brain or of the aorta, spontaneous or due (e.g.) to trauma, Marfan syndrome, or fibromuscular dysplasia |
|
Posttraumatic thrombosis or avulsion of arteries supplying the brain |
|
|
Vasospasm after subarachnoid hemorrhage |
|
|
Arteriovenous malformations |
|
|
Hereditary vascular diseases, e.g., Osler–Weber–Rendu disease (hereditary telangiectasia), moyamoya,a CADASIL, and other familial cerebral vasculopathies; fibromuscular dysplasia in neurofibromatosis |
|
|
Pulmonary venous thrombosis |
|
|
Dolichoectasiab |
|
|
Amyloid angiopathy (β-amyloid deposition in the walls of cerebral blood vessels) |
|
|
Various other causes |
Vasospasm, e.g., in migraine, reversible cerebral vasoconstriction syndrome |
|
Metabolic diseases, e.g., homocystinuria, hyperhomocysteinemia, Fabry disease (lysosomal storage disease with ceramide trihexoside deposition in blood vessels), MELAS, and other mitochondrial encephalomyopathies |
|
|
Other sources of emboli, e.g., fat and air emboli, pseudovasculitic syndrome with cholesterol emboli, tumor emboli, distal emboli from giant aneurysms |
|
|
Collagenoses (e.g., in neurofibromatosis) |
|
|
Other pulmonary diseases |
|
|
Iatrogenic stroke |
Angiography and surgery on the carotid arteries, aorta, and heart |
|
Injection of steroid crystals, fat embolism, etc. |
|
|
Liposculpturing (liposuction and reinjection of adipose tissue) |
|
|
Stroke of no identifiable cause |
|
|
Abbreviations: CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukencephalopathy; CNS, central nervous system; HIV, human immunodeficiency virus; LSD, lysergic acid diethylamide; MELAS, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes. aA rare disease, most prevalent in Japan but also seen elsewhere, involving stenosis of the cerebral vessels due to fibrosis of the intima of the distal portion of the carotid artery. Collateral vessels form, resulting in the typical angiographic appearance of a puff of smoke (in Japanese, “moyamoya”). bDilated macroangiopathy with widening and tortuosity of the cerebral blood vessels. |
|
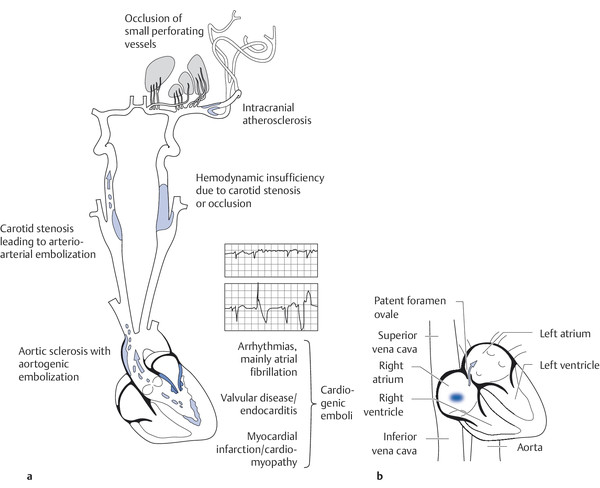
Fig. 6.23 The causes of stroke. (a) The most important causes of stroke. (Reproduced from Mattle H, Mumenthaler M. Neurologie. Stuttgart: Thieme; 2013.) (b) A paradoxical embolus through a patent foramen ovale.
Factors that increase the risk of stroke include the following:
Advanced age.
Positive family history with early onset of atherosclerotic disease (<55 years).
Prior history of cardio- or cerebrovascular disease or peripheral arterial occlusive disease.
Arterial hypertension.
Sedentary habits.
Obesity, truncal obesity, hypercholesterolemia.
Diabetes mellitus.
Sleep apnea syndrome.
Cigarette smoking.
Alcoholism.
Estrogen use (estradiol, mainly in combination with cigarette smoking).
Migraine with aura.
Heart disease, especially atrial fibrillation or flutter.
Stenosis of the cerebral vasculature, especially the internal carotid artery.
Note
The most important risk factors for stroke are aterial fibrillation and arterial hypertension.
Cardiovascular risk factors should be prevented or treated if present. In particular, atrial fibrillation carries a high risk of stroke.
Epidemiology Atrial fibrillation becomes more common with increasing age; it affects approximately 8% of women and 10% of men older than 80 years and causes more than 10% of all cases of ischemic stroke. The risk of having a stroke due to atrial fibrillation increases with the number of other risk factors that are simultaneously present. The CHA2DS2VASc score is an instrument for estimating the annual risk of stroke on the basis of multiple risk factors: age, sex, congestive heart failure, diabetes, arteriopathy, and prior stroke, TIA, or systemic embolism. In most patients, the estimated annual risk of stroke is 2 to 7%.
Pathogenesis of stroke Atrial fibrillation leads to a disturbance of blood flow in the cardiac atria, which are usually enlarged as well. This, in turn, may lead to stasis and thrombus formation, most often in the left atrium. A loose thrombus or part of a thrombus can embolize into the systemic circulation and cause an infarction.
Clinical features Patients with atrial fibrillation who suffer a stroke are older on average than the overall collective of stroke patients. Large territorial infarcts are common in this group and usually fatal. Thus, stroke due to of atrial fibrillation carries a high mortality and survivors are often severely disabled.
Stroke prevention
Note
Anticoagulation can prevent two-thirds of all strokes due to atrial fibrillation.
The new anticoagulants apixaban, edoxaban, and rivaroxaban (factor Xa antagonists) and dabigatran (a thrombin inhibitor) are particularly suitable for the anticoagulation of patients with atrial fibrillation. They are at least as effective as vitamin K antagonists (coumarins).
Epidemiology Arterial dissection, although rare, is the second most common cause of stroke in young adults, after atherosclerosis.
Pathogenesis of stroke An intimal tear leads to splitting of the vascular wall layers. A mural hematoma can occlude the vessel or give rise to an arterio-arterial embolus.
Clinical features Patients with an internal carotid artery dissection can present with the following symptoms and signs:
Pain in the neck, head, or orbit (see also section ▶ 14.2.4).
Horner syndrome (see section ▶ 12.3.5 and ▶ Fig. 12.15).
Lower cranial nerve deficits.
In case of vertebral artery dissection:
Nuchal and occipital pain.
Medullary and cerebellar infarction.
Rarely, basilar artery thrombosis and extensive brainstem infarction.
Treatment Patients with local symptoms, such as pain, can be treated with heparin or acetylsalicylic acid (ASA) and symptomatic pain treatment. In case of stroke, the treatment is the same as for acute stroke of other causes (see section ▶ 6.5.9).
Cerebral hypoperfusion can cause a wide variety of clinical manifestations. In clinical practice, these are often classified by their temporal course and their degree of reversibility or irreversibility ( ▶ Table 6.16). Although classification in this way is useful, it says nothing about the underlying etiology of the ischemic events. Moreover, the boundaries between the listed entities are not sharp.
|
Designation |
Deficits and their duration |
Remarks |
|
TIA |
Transient focal neurologic and/or neuropsychological deficits); transient visual disturbance in one eye (amaurosis fugax). Duration usually 2–15 min, rarely as long as 24 ha |
The current definition of TIA requires the absence of any visual damage to brain tissue in imaging studies. Any such damage classifies the ischemic episode as a stroke whatever its duration |
|
Stroke in evolution, progressive stroke |
Stroke with neurologic deficits that continue to worsen for hours or days after onset |
The cause of progression must be sought: progression may be due to repeated strokes, progression of a thrombosis or embolism, hemorrhagic transformation of an infarct, cerebral edema, hypotension, etc. |
|
Completed stroke |
Established neurologic deficit that is irreversible or only partly reversible |
Rehabilitation enables the improvement or, at least, the retention of residual functional abilities |
|
Abbreviation: TIA, transient ischemic attack. aIn earlier nomenclature, the term “reversible ischemic neurologic deficit” (RIND) designated transient ischemic attacks of longer duration (up to 7 days). This term is now hardly ever used. |
||
Three different basic types of ischemic stroke are distinguished from one another on the basis of the caliber of the vessels involved.
Territorial infarcts are mainly produced by occlusions of the main trunks or major branches of cerebral arteries (cerebral macroangiopathy), which may be due to thrombosis, embolism, or other causes. The infarct includes both cortex and subcortical white matter and sometimes the basal ganglia and thalamus ( ▶ Fig. 6.24, ▶ Fig. 12.2). It is usually possible to infer which vessel has been occluded from the pattern of neurologic deficits that are produced, for example, in strokes involving the territory of the MCA ( ▶ Fig. 6.25) or the posterior cerebral artery.
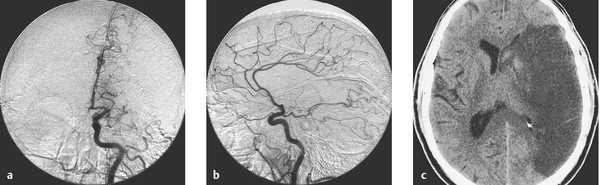
Fig. 6.24 Infarct in the territory of the left middle cerebral artery in a 60-year-old man with acute right hemiplegia. (a) The left carotid angiogram (anteroposterior view) reveals occlusion of the main stem of the middle cerebral artery at its origin. Only the anterior cerebral artery is visualized. (b) The lateral view shows only the pericallosal artery, with its branches, and the posterior cerebral artery, while the middle cerebral artery and its branches are not seen (cf. normal carotid angiogram, ▶ Fig. 4.13). (c) A CT scan obtained 2 days after the onset of symptoms reveals a massive infarct in the territory of the middle cerebral artery, extending from the cortex to the basal ganglia.

Fig. 6.25 Acute territorial infarct in the left basal ganglia due to occlusion of the main trunk of the middle cerebral artery. The affected areas include the striatum and the anterior portion of the internal capsule. (a) FLAIR sequence; (b) diffusion-weighted MR image.
Watershed infarcts (also called border zone infarcts) are infarcts of hemodynamic origin that are likewise due to macroangiopathic processes. Arterial narrowing impairs perfusion in the vulnerable regions at the borders between the territories of two or more arteries ( ▶ Fig. 6.26). If the perfusion pressure is inadequate, infarction ensues.
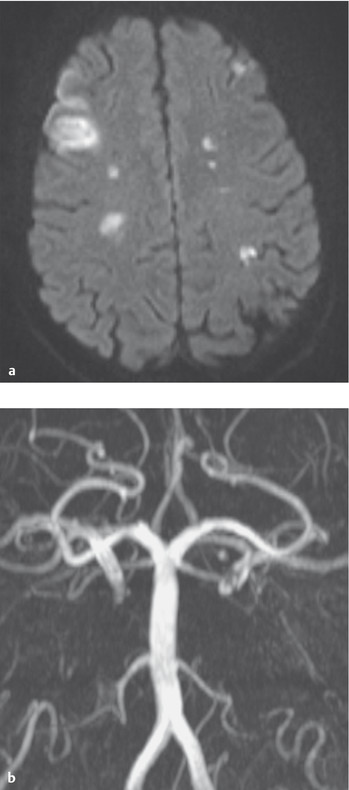
Fig. 6.26 Watershed infarct. (a) Watershed infarcts in the border area between the territories of the anterior and middle cerebral arteries, as seen on diffusion-weighted MRI. (b) A 37-year-old man with bilateral internal carotid artery occlusion due to dissection. In the center of the image, the vertebral arteries and the basilar artery are seen; they provide blood to the anterior circulation via collateral vessels. (Reproduced from Mattle H, Mumenthaler M, Neurologie. Stuttgart: Thieme; 2013.)
Lacunar infarcts are caused by microangiopathy, usually arteriolosclerosis or fibrinoid necrosis (lipohyalinosis) due to hypertension. The infarcts (lacunes) are less than 1.5 cm in diameter and often multiple. They are found mainly in the basal ganglia, thalamus, and brainstem, and sometimes in the cerebral cortex and subcortical white matter ( ▶ Fig. 6.27). Their clinical presentation depends on their number and localization.
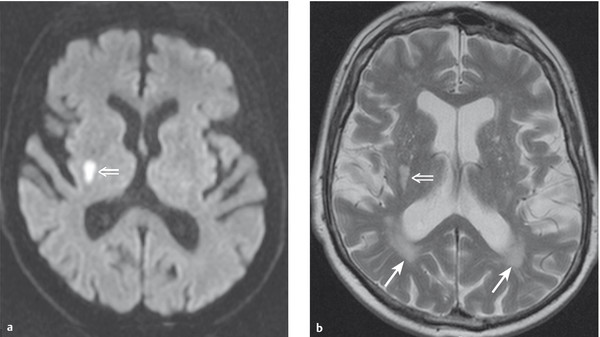
Fig. 6.27 Lacunar infarction in the posterior limb of the right internal capsule. This 72-year-old man with metabolic syndrome presented with an acute left hemisensory syndrome caused by cerebral microangiopathy. (a) The diffusion-weighted MR image shows a hyperintense area corresponding to the infarct (⇒). (b) The T2-weighted image reveals multiple further lesions as well as flatter, rather than rounded, lesions (→) behind the occipital horns that are typical of cerebral microangiopathy (vascular leukencephalopathy).
Multiple subcortical infarcts due to hypertension are the hallmark of subcortical arteriosclerotic encephalopathy, which was once called Binswanger disease and is now generally designated in purely descriptive terms as vascular leukencephalopathy. This entity is associated with vascular dementia (see section ▶ 6.12.5).
Note
Ischemic stroke occurs when persistent ischemia or a complete interruption of the blood supply to a particular area of the brain produces irreversible destruction of brain tissue. The resulting neurologic deficits usually arise quite suddenly (whence the term “stroke”) but in rare cases progress over a longer period of time (“stroke in evolution”). If untreated, they are irreversible, or at most only partly reversible.
The type of neurologic deficit caused by a stroke is predictably related to the site of the ischemic lesion, and thus the site of the lesion can be inferred from the deficit. We will now briefly summarize the clinical manifestations of the major cerebrovascular syndromes and the typical deficits produced by ischemia in circumscribed areas of the brain.
Middle cerebral artery About half of all strokes affect the territory of the MCA. The site of occlusion (main trunk vs. branch of the MCA) determines the clinical manifestations. As a rule, a mainly brachiofacial hemiparesis and hemisensory deficit are found, often accompanied by homonymous hemi- or quadrantanopsia and, in the initial phase, a horizontal gaze palsy toward the side of the hemiparesis. An MCA occlusion on the language-dominant (usually left) side additionally produces aphasia and apraxia, while one on the nondominant side produces impairment of spatial orientation. An occlusion of the main stem of the MCA causes ischemia not only of the cortex, but also of the basal ganglia and internal capsule, producing a more severe contralateral hemiparesis. An infarct involving a large percentage (or all) of the MCA territory can give rise to massive cerebral edema and intracranial hypertension; this is called “malignant MCA infarction.” In the aftermath of the stroke, if the hemiparesis fails to improve over time, or does so only partially, a typical, permanent impairment of gait results: circumduction of the spastically extended lower limb, flexion of the paretic upper limb at the wrist and elbow, and absence of arm swing on the affected side (Wernicke–Mann gait; ▶ Fig. 6.28).
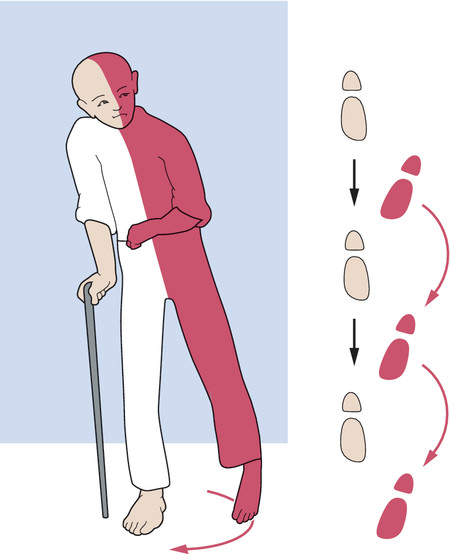
Fig. 6.28 Typical gait disturbance of a hemiplegic patient. Circumduction of the spastically paretic leg because of predominant extensor tone and flexion of the spastically paretic arm at the elbow because of predominant flexor tone.
Practical Tip
Hemiparesis, hemisensory deficit, and homonymous hemianopsia (all of them contralateral to the lesion), along with aphasia in lesions of the dominant hemisphere and hemineglect and impaired spatial perception in lesions of the nondominant hemisphere, constitute the full clinical picture of MCA infarction.
Anterior choroidal artery This vessel is much less commonly the origin of stroke than the MCA. Ischemia in the territory of the anterior choroidal artery causes a homonymous visual field defect, a hemisensory deficit, and, less commonly, hemiparesis. The clinical manifestations resemble those of occlusion of the lenticulostriate arteries (branches of the MCA supplying the basal ganglia and internal capsule). There may also be extrapyramidal motor signs, such as hemiballism.
Anterior cerebral artery Infarction in the territory of this artery, accounting for approximately 5% of all strokes, causes contralateral hemiparesis mainly affecting the lower limb, sometimes accompanied by contralateral ataxia and, if the lesion is left-sided, by apraxia. Occasionally, there may be apathy, abulia (pathologic lack of drive and motivation), and urinary incontinence.
Practical Tip
Watershed infarction (cf. ▶ Fig. 6.26) in the border zone between the territories of the middle and anterior cerebral arteries typically produces contralateral motor and sensory deficits that are most prominent in the lower limb. If the perfusion is critical, these deficits can be more intense when the patient is standing. A tremor that is most prominent in the lower limb can also become apparent when the patient stands.
Ophthalmic artery Transient ischemia in the territory of this vessel produces amaurosis fugax (transient monocular blindness), while longer-lasting ischemia causes retinal infarction. Retinal ischemia is often due to embolism of cholesterol crystals from ulcerating plaques in the internal carotid artery into the ophthalmic artery. Embolized crystals within the arteries of the retina can occasionally be seen by ophthalmoscopy.
Internal carotid artery Stenosis or occlusion of the internal carotid artery can simultaneously cause ischemia of the eye with monocular visual loss (as in ophthalmic artery occlusion) and a large infarct in the territory of the middle cerebral artery. This oculocerebral syndrome is rare, however, as ischemia in the territory of the internal carotid artery usually presents with either monocular visual loss or variably severe hemiparesis and neuropsychological deficits.
Posterior cerebral artery This artery is involved in 10% of all ischemic strokes, often bilaterally (embolism). Proximal occlusion can lead to infarction in the cerebral peduncle, the thalamus, mediobasal portions of the temporal lobe, and the occipital lobe, with a similar constellation of clinical findings to MCA occlusion. The most prominent clinical sign of a distal occlusion (beyond the origin of the posterior communicating artery) is contralateral homonymous hemianopsia, possibly combined with neuropsychological deficits.
Thalamic infarction Thalamic stroke results from occlusion of one of the perforating arteries supplying the thalamus. It usually presents with a contralateral hemisensory deficit, in addition to mild paresis and hemiataxia. The patient’s memory, too, is often impaired.
Basilar artery Occlusion of the main stem or of a branch of the basilar artery causes brainstem, cerebellar, and thalamic signs. Main stem thrombosis generally causes tetraparesis and is often fatal. Ventral pontine infarction can present clinically with locked-in syndrome (described in section ▶ 5.5.5).
The basilar tip syndrome reflects a bilateral midbrain and thalamic infarct, usually due to an embolus that becomes lodged in the distal portion of the basilar artery proximal to its bifurcation into the posterior cerebral arteries. Its typical manifestations are impaired consciousness and upward gaze palsy, sometimes accompanied by a uni- or bilateral hemisensory deficit, ataxia, uni- or bilateral hemianopsia, and neuropsychological deficits such as amnesia and cortical blindness.
Brainstem infarction Brainstem strokes are usually lacunar. They arise in the territory of one or more small perforating arteries that branch off the basilar trunk and can be seen by MRI ( ▶ Fig. 6.29). Their clinical presentation depends on the particular nuclei and fiber tracts that they affect. Brainstem stroke therefore takes many different clinical forms, corresponding to the wide variety of functions served by brainstem structures. As a rule, brainstem stroke causes ipsilateral cranial nerve deficits and a contralateral hemisensory defect and/or hemiparesis.
The large number of brainstem vascular syndromes that have been described and given eponymous names are only rarely seen in “pure” form in clinical practice. The most clinically relevant brainstem syndrome is Wallenberg syndrome ( ▶ Fig. 6.29), which is due to occlusion of the posterior inferior cerebellar artery. Various brainstem syndromes reflecting involvement of different portions of the brainstem are listed in ▶ Table 6.17.
|
Name |
Localization |
Ipsilateral signs |
Contralateral signs |
Special features |
|
Wallenberg syndrome |
Dorsolateral medulla |
Horner syndrome, vocal cord paresis, palatal and posterior pharyngeal paresis, trigeminal nerve deficit, hemiataxia |
Dissociated sensory disturbance (loss of pain and temperature sensation on the trunk and limbs) |
Nystagmus; this syndrome is caused by occlusion of the posterior inferior cerebellar artery |
|
Benedikt syndrome (upper red nucleus syndrome) |
Midbrain, red nucleus |
Oculomotor palsy, sometimes vertical gaze palsy |
Hemiataxia (sometimes), intention tremor, hemiparesis (often without Babinski sign) |
Staggering gait |
|
Weber syndrome |
Midbrain |
Oculomotor nerve palsy |
Hemiparesis |
|
|
Millard–Gubler syndrome |
Caudal pons |
(Peripheral) abducens and facial palsy |
Hemiparesis |
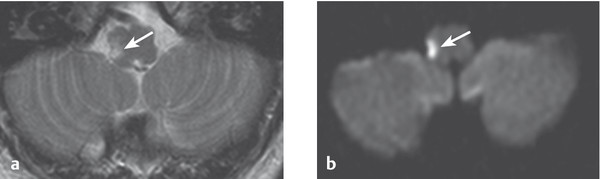
Fig. 6.29 Acute lacunar infarction of the medulla. This 63-year-old man presented with Wallenberg syndrome. (a) T2-weighted spin-echo MR image. (b) Diffusion-weighted MR image that reveals the lesion particularly clearly (→).
Cerebellar infarction Occlusion of the superior, anterior inferior, or posterior cerebellar artery presents with vertigo, nausea, unsteady gait, dysarthria, and often acute headache. The neurologic examination reveals ataxia, dysmetria, and nystagmus. Often, simultaneous infarction of part of the brainstem produces additional brainstem signs. Not uncommonly, edema in and around the infarcted area rapidly leads to a life-threatening elevation of pressure in the posterior fossa, with progressive impairment of consciousness (with or without accompanying occlusive hydrocephalus). A typical MR image of cerebellar stroke is presented in ▶ Fig. 6.30.
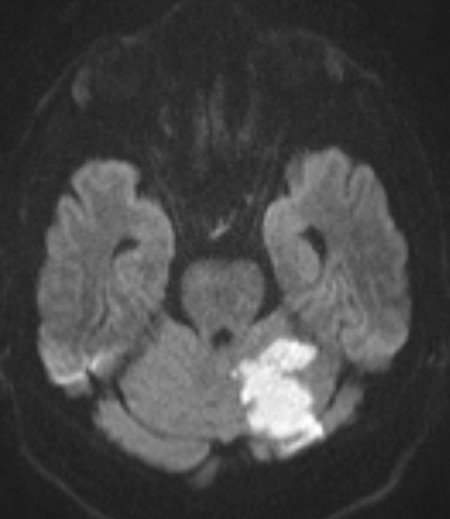
Fig. 6.30 Fresh infarct in the left cerebellar hemisphere, in the distribution of the superior cerebellar artery. Diffusion-weighted MR image.
The acute diagnostic evaluation focuses on the following questions:
Is the stroke ischemic or hemorrhagic in nature?
What are its anatomic site and extent?
What is its cause?
To answer these questions, the following are needed:
A precise history including not only the present illness, but also the past medical history, with special attention to risk factors and systemic illnesses.
A thorough clinical neurologic examination enabling localization of the lesion.
Physical examination of the cardiovascular system (measurement of pulse and blood pressure and auscultation of the heart, the carotid arteries, and perhaps other vessels, depending on the clinical situation; particular attention should be paid to bruits and to any irregularities of the pulse that suggest arrhythmia).
Any patient with a central neurologic deficit of acute onset is likely to have suffered a cerebrovascular event, probably of the ischemic variety. Nonetheless, a CT or MRI is indispensable to differentiate ischemia from hemorrhage and to rule out other, nonvascular etiologies.
Note
The history and physical examination alone cannot reliably differentiate ischemia from hemorrhage as the cause of the deficit; thus, a CT or MRI is needed. All stroke patients should be in a CT or MRI scanner no later than 25 minutes after their arrival in the hospital.
CT Early CT reveals acute brain hemorrhage with high sensitivity (hyperdense lesion). Therefore, all patients with suspected stroke should have a CT scan in the acute phase to determine the further course of their treatment, even though ischemia only becomes evident a few hours later. A perfusion CT with contrast medium can demonstrate the core infarct zone and the penumbra, similarly to MRI. CT is less time-consuming than MRI.
MRI MRI reveals the infarct zone and perifocal edema as soon as the patient begins to experience symptoms, and it displays brainstem and cerebellar infarcts more clearly than CT. Diffusion-weighted MRI clearly reveals the ischemic zone; perfusion MRI, in combination with diffusion-weighted MRI, reveals the penumbra (zone of potentially salvageable tissue; cf. ▶ Fig. 6.21).
Angiographic CT/MRI These studies ( ▶ Fig. 6.31) of the extra- and intracranial vessels can reveal stenosis, occlusion, or collateral circulation. They are generally most useful in the acute phase as an aid to the decision whether to attempt endovascular treatment.
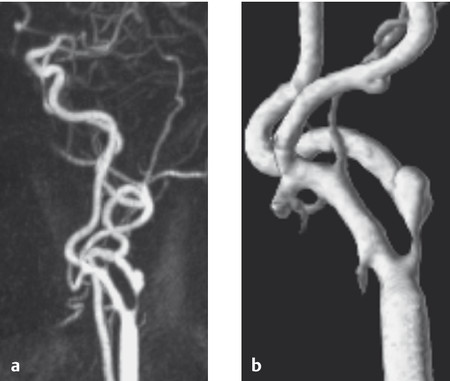
Fig. 6.31 High-grade stenosis of the left internal carotid artery. (a) Contrast-enhanced MR angiography of the cervical vessels shows atherosclerotic stenosis of the internal carotid artery at a typical site distal to its origin from the bifurcation of the common carotid artery. (b) The stenosis is seen even more clearly in the 3D reconstruction.
Neurovascular ultrasound This method, too, can reveal stenosis, occlusion, or collateral circulation; it is more commonly used in routine outpatient evaluation than in the emergency evaluation of acute stroke.
Electrocardiography An ECG may reveal arrhythmia or regional myocardial dysfunction, pointing to a possible cardioembolic event, or else a prior or current (acute) myocardial infarction.
Laboratory tests These are mainly used to identify risk factors, infectious/inflammatory disorders, metabolic disorders, and coagulopathies. The more important tests can generally be performed on an emergency basis.
Electrolytes: sodium, potassium, calcium.
Hemoglobin, complete blood count.
Erythrocyte sedimentation rate, C-reactive protein → inflammation?
Platelet count, prothrombin time, D-dimers if indicated → coagulopathy?
Blood sugar, HbA1c, liver enzymes, lipid profile → risk factors?
Urea, creatinine → renal failure?
Creatine kinase, troponin → cardiac problem?
Syphilis serology.
Depending on the clinical situation, the following tests can also be performed after the acute phase:
Angio-CT, angio-MRI (see ▶ Fig. 4.8., ▶ Fig. 4.9, and ▶ Fig. 6.31), or neurovascular ultrasound (see ▶ Fig. 4.32, ▶ Fig. 4.33) if not already performed in the acute phase.
Echocardiography (transthoracic or transesophageal) to reveal valvular disease and sources of emboli in the heart and aortic arch.
Long-term ECG (24-hour ECG, perhaps event recording with an event or loop recorder, or long-term recording) to demonstrate arrhythmias that can give rise to emboli.
Cerebral angiography (see ▶ Fig. 4.13, ▶ Fig. 4.14, ▶ Fig. 4.15, ▶ Fig. 6.32) to reveal stenosis or occlusion of the cerebral blood vessels or other, rarer types of vascular lesion that cannot be diagnosed by noninvasive means (also performed in the acute phase as a prerequisite to thrombolytic treatment).
Pulse oximetry or apnea test.
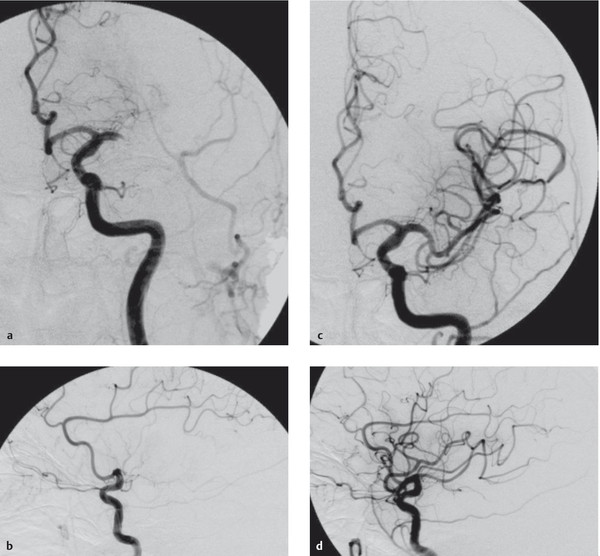
Fig. 6.32 Proximal occlusion of the middle cerebral artery before and after endovascular recanalization. (a,b) Images before recanalization; (c,d) images after recanalization.
Note
Once an ischemic stroke has been diagnosed and an intracerebral hemorrhage has been excluded, the initial goal of treatment is to minimize the amount of brain tissue that will be irreversibly damaged. Brain tissue in the zone of relative ischemia (the penumbra) can be salvaged by prompt restoration of its blood supply. The sooner the lytic treatment can be provided, the better.
Practical Tip
All patients with suspected stroke should be immediately transported to an acute-care hospital with a specialized facility for the treatment of stroke (stroke unit or stroke center). Treatment in a stroke unit or stroke center markedly improves the clinical outcome.
Monitoring The vital signs and neurologic functions should be monitored continuously.
Maintenance of adequate perfusion pressure The blood pressure must be kept relatively high (values up to 200–220 mm Hg systolic and 110 mm Hg diastolic are tolerable; reduction to 180 mm Hg systolic only before intravenous thrombolysis).
Optimization of O2 delivery The airways must be free; in case of hypoxia, supplementary oxygen must be administered and the cause treated. Pneumonia should be prevented or treated if present.
Stabilization of cardiovascular function The patient must be adequately hydrated; heart failure and/or arrhythmia must be treated if present.
Thrombolytic treatment whenever indicated The options include:
Intravenous (systemic) thrombolysis with rtPA (recombinant tissue plasminogen activator) within 4.5 hours of symptom onset (3 is better, 5 the absolute limit), particularly in cases of peripheral rather than main stem vessel occlusion, with clinically mild to moderate stroke.
Intra-arterial (endovascular, interventional) thrombolysis/recanalization up to 6 hours from symptom onset ( ▶ Fig. 6.32), primarily in cases of clinically severe stroke.
Mechanical recanalization within 8 hours of symptom onset.
In cases of severe stroke, or if an interhospital transfer is needed before the patient can be treated, combined intravenous and intra-arterial treatment (so-called bridging treatment) is an option.
If thrombolytic treatment and mechanical recanalization are contraindicated or not possible, ASA is the drug of first choice.
Before rtPA is given, contraindications such as anticoagulation, a fresh trauma, hemorrhage, recent surgery, or endocarditis should be ruled out. Even if rtPA is contraindicated, mechanical recanalization is usually still possible.
Treatment of cerebral edema See the section on cerebral edema.
Treatment of fever and epileptic seizures Pathologic oxygen- and nutrient-consuming metabolic processes such as fever or epileptic seizures must be treated if present.
Optimal blood sugar management Prevention and, if necessary, treatment of hyper- or hypoglycemia.
While the acute measures discussed earlier are being taken, the treatment team should begin to consider further therapeutic strategies to prevent recurrent stroke. Depending on the etiology of the patient’s problem, these can include rehabilitation and prophylactic measures.
Early rehabilitation Mobilization (decubitus prophylaxis), aspiration prophylaxis, physical and occupational therapy, and, if needed, speech therapy.
Prevention of recurrent stroke
General medical treatment:
Minimization of vascular risk profile (optimal treatment of hypertension, diabetes mellitus, hypercholesterolemia, or sleep apnea syndrome, if present, and cessation of smoking).
Treatment of heart failure and/or arrhythmia.
Antithrombotic therapy: the type to be given depends on the etiology of the stroke. The following options are available:
Inhibition of platelet aggregation (mainly aspirin, but also clopidogrel or aspirin with dipyridamole); the treatment begins immediately or 24 hours after intravenous thrombolytic therapy, and is usually continued indefinitely.
Full heparinization: rare, for example, in cases of dissection or venous thrombosis.
Oral anticoagulation (mainly after cardio- or aortoembolic stroke, venous thrombosis, venous sinus thrombosis, or dissections, possibly after ASA has already been given).
Surgical therapy: endarterectomy for high-grade carotid stenosis (in symptomatic patients at any age or in asymptomatic patients younger than 75 years).
Endovascular therapy: stent insertion for high-grade carotid stenosis if endarterectomy is contraindicated or too risky, or in cases of subclavian or intracranial stenosis (likewise in symptomatic patients at any age or in asymptomatic patients younger than 75 years).
In younger patients with paradoxical embolism through a patent foramen ovale, interventional closure of the foramen ovale can be considered.
Definition TIAs are defined as transient focal neurologic manifestations or neuropsychological deficits (e.g., aphasia) without any discernible abnormality in imaging studies. A TIA can also present as a transient visual disturbance in one eye (amaurosis fugax). TIAs generally last 2 to 15 minutes, although, by definition, they may persist for up to 4 hours.
Note
TIAs are not just small and clinically insignificant strokes. They are warning signs and harbingers of what may be a major stroke in the offing. Any patient with a TIA must be neurologically evaluated and treated without delay.
About 20% of all strokes are preceded by one or more TIAs; in about two-thirds of cases, the stroke follows within 3 to 4 days. Patients with vascular risk factors are at particular risk of having a stroke after a TIA, with a greater than 20% risk of stroke in the ensuing 7 days. Patients with TIAs, like stroke patients, should undergo a neurologic diagnostic evaluation and targeted prophylactic treatment as soon as possible, preferably on an emergency basis. These are performed according to the same criteria listed earlier for stroke (see sections ▶ 6.5.8 and ▶ 6.5.9).
Practical Tip
Amaurosis fugax is often due to carotid stenosis. Patients with amaurosis fugax should be evaluated immediately with carotid ultrasound, angio-CT, or angio-MRI.
Note
Besides the much more common arterial disorders just discussed, obstruction to venous flow can also cause cerebral ischemia. Venous obstruction is usually due to thrombosis of the large venous channels draining the brain (venous sinus thrombosis) and of the veins that empty into them (cerebral venous thrombosis).
Etiology and frequency Thromboses of the cerebral veins and venous sinuses are somewhat more common in young women than in men; they account for at most 1% of all cerebral ischemic events.
Localization The superior sagittal sinus is most commonly affected, and the other sinuses and the cortical veins are less commonly affected.
Etiology These thromboses are usually bland, that is, no specific etiology can be identified. They can arise in the postpartum period. A minority of cases are due to infection, either systemic or in the immediate vicinity of the sinus (e.g., chronic otitis); other causes include hypercoagulability states and systemic diseases (e.g., Behçet disease).
Pathogenesis Damming of blood behind a venous obstruction leads to a secondary reduction of arterial inflow and thus to hypoperfusion and infarction. Smaller or larger diapedetic hemorrhages (diapedesis = migration of blood cells through endothelial gaps in the vascular wall because of built-up pressure behind an obstruction) can also occur in the infarcted area (hemorrhagic infarction).
Clinical features The common signs and symptoms are headache, focal or generalized epileptic seizures, papilledema, and sensory and motor deficits, depending on the site of the thrombosis.
Diagnostic evaluation Imaging studies reveal unilateral or bilateral hemorrhagic infarction; the thrombosis itself can usually be seen by MRI, or by contrast-enhanced CT. In rare cases, thrombosis is revealed only by angiography ( ▶ Fig. 6.33). The diagnostic method of choice is MRI.
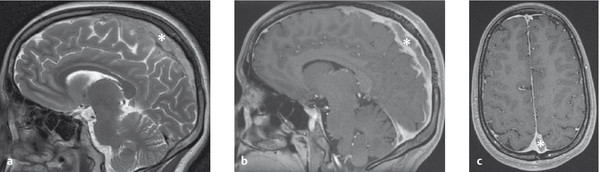
Fig. 6.33 Thrombosis of the superior sagittal sinus (* = thrombus). (a) Sagittal T2-weighted image. (b) The contrast-enhanced sagittal T1-weighted image shows the intraluminal thrombus, as does the axial image (c).
Treatment Anticoagulation (heparin followed by oral anticoagulation), usually for a few months.