

Women have been asking me these questions for years. It makes so much sense to measure a baseline level of almost anything before starting a medication designed to change that particular parameter. Clearly, one of the important advantages of modern medicine is our ability to measure objective parameters in the laboratory, with X-rays, MRI, bone-density tests, and other techniques. We then combine this information with the clinical description from the patient about what she is experiencing to determine a diagnosis and a meaningful course of action or treatment plan. For example, if you have clinical symptoms that suggest low thyroid function, we measure the level of thyroid hormones in your blood, both those produced by the gland (T3, T4) and the brain (TSH), and possibly thyroid antibodies. If you need thyroid medication, we then prescribe a low dose and gradually increase it. Then we check a follow-up blood level of TSH to see if the dose is right for you and if not, make further adjustments in the amount you take. This process is done routinely with diabetics on insulin by monitoring the blood levels of fasting glucose and postprandial (after meals) glucose. It is also used in patients on heart medicine, such as digitalis, by monitoring blood levels of the medication at a certain number of hours after a dose. By monitoring blood levels of hormones or medications, doctors are able to use the right amount for your body and minimize the possibility of unwanted side effects. Makes sense, right? So why isn’t this same standard of medical practice used in helping postmenopausal women find the right dose of estrogen therapy?
Why have we had in the United States this “cookbook” approach, with the same Premarin-Provera “recipe” for all postmenopausal women? We do not use the same dose of blood pressure medication for everyone with high blood pressure or the same dose of insulin for all diabetics. Yet, I estimate that over the last fifteen years of my medical practice, nine out of every ten women I’ve seen who were already taking hormone therapy were on 0.625 mg of Premarin for twenty five days a month, and 5 or 10 mg of Provera for Days 16 to 25 every month. Some women said they felt wonderful; others described having “nothing but problems” since starting on these hormones. I was bothered by the fact that almost every woman I saw was on the same dose and type of hormones, and since not everyone was feeling well on them, I began to ask more questions. Why the same dose? “That’s the amount that’s needed.” Why always the same type of estrogen? “That’s what we’ve always used.” Are there any others? “Don’t know of any.” What if women are having side effects? “She can stop, or just take less.” What about rechecking FSH after a woman is on her therapy and see if she has the right amount? “That’s not needed, the FSH never comes back down to normal after menopause.” How do we know; are there any studies on this? “No, there aren’t any studies, we just know that’s the way it is.” Can’t we check blood levels to see if the amount is right? “No, blood levels are useless, hormone levels vary.” That’s my point! They do vary, and we need to know how they vary in relationship to what a woman is experiencing.
I wanted to scream, it was all such nonsense. How could we know if there aren’t any studies? Why doesn’t FSH come back down when a woman is on the right amount of estrogen? That’s how other hormone feedback systems in the body work. This “reasoning” (or lack of it) simply did not make either physiological or medical sense to me. With my patients who were taking hormones and yet were still not feeling well, I started letting them know that checking FSH, estradiol, and testosterone blood levels was an option for them. Even though at that time, we did not have a lot of good information about correlation of blood levels and physical symptoms, I thought it was critical to try and sort this out in order to help my patients feel better. Many women elected to have this done—it made intuitive sense to them. Well, amazing results began to unfold. I found that there was a very good correlation between desirable blood hormonal levels in women who were doing well on their particular regimen. I usually found suboptimal hormonal blood levels in women who were still having a lot of symptoms and/or side effects.
As I continued to research this issue, I found that Dr. Philip Sarrel at Yale had been finding the same thing, as had some European researchers. Dr. Sarrel studied the women who came to the Yale Menopause Center and tabulated the percentage of women who described problems with sexual function as one of the reasons they were seeking a consultation. The numbers speak for themselves as to the magnitude of the impact on women’s lives:
SEXUAL DYSFUNCTION AFTER MENOPAUSE:
YALE MID-LIFE STUDY
Problems Patients Reported: |
Patients Experiencing It |
Decreased sexual desire |
77% |
Reporting of sexual problems |
68% |
Bothered by sexual problems |
64% |
Intercourse (less than/=)1/month |
50% |
Ref: Sarrel: Obstet Gynecol 1990; 75 (suppl. 265–308)
Dr. Sarrel further showed that there seems to be a threshold level of about 50 pg/ml for estradiol with women having sexual problems at menopause: Women with estradiol levels greater than 50 had minimal reports of adverse changes in their sexual function. Women whose estradiol levels were below 50, however, had a dramatic increase in sexual problems of all types, including decreased lubrication, burning and pain with intercourse, difficulty having an orgasm, and diminished quality of orgasm. This was one of the few studies I could find in the literature at that time to clearly demonstrate a relationship between estradiol blood levels and the clinical problems women were describing. Someone else had listened to the patients. I felt validated in my own observations and clinical correlations. In 1998, a study by Drs. Vihtamaki and Tuimala was published in Maturitas, the journal of the International Menopause Society at that time. They found that as many as 45 percent of patients whose self-assessment had been that symptoms were gone, still actually had serum estradiol levels below the currently accepted thresholds for the various protective effects of estradiol on brain, bone, and other target tissues. The authors concluded, as have I in working with thousands of women over the years, that reliable assessment of serum hormone levels is a crucial method to ensure that adequate levels of estradiol are reached to provide the benefits women (and their physicians) are seeking.
The more I listened to my patients and tried to help them feel better, the more I found that blood-level information helped guide me in my recommendations for each individual woman. It also helped her feel that a more rational, logical approach was being used to determine her individual needs. I decided that taking the systematic approach of making treatment decisions based on both clinical symptoms and objective laboratory results made good sense and provided better medical care for my women patients. This approach means that the doses are then designed for the individual woman, and we have objective measures of what is the right amount for her. Women quickly discover that many vague symptoms, for which they may have had to see multiple physicians, often went away when the type and amount of estradiol was right for their individual needs.
If you want to request these blood tests from your physician, what should you be looking for as a desirable target range for estradiol? In my clinical experience, women typically experience their usual energy level, mood, sleep, and memory when serum (blood) levels of estradiol are above 90–100 pg/ml. Levels above this range, up to about 200 or so, are the normal estradiol levels in the first half of the menstrual cycle before women reach menopause. Levels below this are generally too low to maintain a normal feeling of well-being. Recent research has found that estradiol levels below 70–80 pg/ml result in increased bone loss after menopause. There now appears to be a minimum threshold level of about 80–90 pg/ml for estradiol to maintain healthy body function. Below this level, women lose more bone, lose the cardiovascular and brain benefits of estrogen, have more problems sleeping, and also have more loss of bladder and sexual function. I describe what to look for with testosterone levels in chapter 6.
I have been criticized by other physicians for recommending that women have both FSH and estradiol levels checked. They have often said to me that it is “too expensive,” “unreliable,” or “doesn’t tell us anything.” I disagree. This information has made an enormous difference to the women who had been told their symptoms were “all in their head” and who now have a hormone regimen tailored just for them. Many of my patients have also been able to stop the expensive medications for lowering blood pressure and cholesterol, as well as eliminate psychotropic medications when their estradiol levels were again in the optimal ranges. Furthermore, it is difficult to put a price tag on improving someone’s quality of life. I think in the long run it is less expensive and more cost effective to check hormone blood levels than to do all the myriad tests and evaluations that end up being done when hormone problems are not recognized, or for women to undergo a long series of psychotherapy sessions, thinking that the mood changes are just stress or an empty nest or a bad relationship. In my opinion, these blood tests are efficient, informative, and psychologically helpful in identifying a physical cause of disturbing symptoms women frequently experience at midlife and around menopause. I feel strongly that such tests of hormone levels should be available to all women, especially those who have had their ovaries removed.
I am in the process of collecting outcome data on larger numbers of patients to be able to demonstrate to insurance companies that such tests are in fact useful and cost-effective. This research, underway at both HER Place centers, will help clarify these important issues and, I hope, change the way health services are offered to midlife women. Instead of operating on unproved assumptions and old myths, we really need to look at the whole woman and evaluate her endocrine system carefully to rule out hormonal factors contributing to her symptoms as well. If your doctor isn’t listening to your requests, find one who will.
There is so much confusion about estrogens, I would like to describe some of the differences between various types. Bear with me for the chemistry lesson. There are three estrogens found in human females, and the relative amounts of each one present is determined by several factors: genetic makeup, age, amount of body fat, pregnancy, diet, and the presence of any medical condition or lifestyle habits that alter ovarian function. Many women at my seminars think “estrogen” equals “Premarin.” This is not the case. Premarin is only one brand of estrogen among many brands available. I will describe the estrogens commercially available in the U.S. First, some definitions:
17-BETA ESTRADIOL (E2)
This is the predominant natural human estrogen produced by the ovary prior to menopause; it is the primary biologically active estrogen at cell surface receptor sites and also inside the cell at the nucleus receptor sites as I described in chapter 3. It is the major functioning estrogen for our bodies from puberty until menopause, and it is the one responsible for over four hundred functions in the female body. After menopause, we lose the ovary source of 17-beta estradiol, and other body tissues can’t make up for this loss of the most active form of the hormone. We are left with only the estrone made in fat tissue as a poor substitute! Losing the 17-beta estradiol results in the postmenopausal changes in skin, bone, hair, heart/blood vessels, brain, and other organs. Ideally, if you decide to take estrogen therapy after menopause, you would choose a form of 17-beta estradiol to provide exactly the same chemical molecule your ovary had previously made.
There are several brands of 17-beta estradiol commercially available in this country, and all of these brands use a form of estradiol that is derived from soybean or wild yam precursor molecules. These are as follows: Estrace tablets and vaginal cream (FDA approved in 1976), Gynodiol tablets and Alora, Climara, Vivelle, Estraderm transdermal patches (all FDA approved between 1985–1997). Even though some of these have been around a long time, many women and still some physicians seem to have heard about only Premarin. That’s more a testament to effective marketing and prescribing habit than to Premarin being a better estrogen source. There is some 17-beta estradiol in Premarin, but a small amount in comparison to the other ingredients in each tablet. The comparative studies that have been done, although not many, do show that the bioidentical human form of 17-beta estradiol is much better tolerated, with fewer side effects and better improvement in outcome measures (lipids, bone markers, and others).
ESTRONE (E1)
This is the predominant estrogen found in postmenopausal women. Before menopause, estrone is made by body fat, the adrenal glands, the liver, and in the ovary. Estrone is also produced from conversion of estradiol, and vice versa. It serves primarily as a reservoir for the body to make the biologically active 17-beta estradiol, but most of this conversion takes place in functioning ovaries. Estrone (E1) is the form of estrogen that many researchers think may be related to the higher risk of endometrial and breast cancer in older women who are obese. Estrone continues to be produced in the liver and body fat (adipose tissue), and to a smaller extent the adrenal glands, after menopause. The more body fat a woman has (before or after menopause) the more estrone is present. One synthetic brand of estrone is commercially available in the United States for postmenopausal therapy: Ogen (piperazine estrone sulfate). It is not the same chemical structure as estrone made in the human body because it has the piperazine ring structure attached to it. This makes Ogen chemically similar to, but not exactly the same as, human estrone. If you are taking a form of estrone for a postmenopausal estrogen, you may still have some residual symptoms due to the lower amount of estradiol present, and the different chemical ring from the piperazine part of the molecule. I don’t recommend Ogen for women with any kind of muscle or bladder pain syndromes because I have found clinically that this difference in its chemical structure makes it more likely to aggravate the pain problems. The estrogen products that give high levels of estrone and relatively little of the 17-beta estradiol are Premarin, Prem Pro, Prem Phase, Estratab, Estratest, Cenestin, Menest, and Ogen.
ESTRIOL (E3)
This is the weakest of the human estrogens, and is produced by the placenta during pregnancy. Estriol is not normally present in measurable amounts in nonpregnant women. Estriol is biologically the weakest estrogen and has been studied extensively. It really isn’t the “forgotten estrogen” as marketing hype would have you believe; it has been extensively studied in Europe over the last fifty years. Estriol just isn’t used much for menopause therapy because it hasn’t been found to provide the degree of protective effects on bone, heart, brain, and nerves as does our premenopausal 17-beta estradiol. In addition, there are no reputable medical studies showing any protective effect of E3 on breast cancer development, other than the known effect of full-term pregnancy prior to age thirty in reducing breast-cancer risk. Whatever role estriol plays in reducing breast cancer risk is not an independent effect of estriol but rather is thought to occur along with other factors from a full-term pregnancy before age thirty.
For some women, estriol relieves milder symptoms (e.g., vaginal dryness, mild hot flashes), and I describe its use for vaginal symptoms in chapter 12. Estriol has clearly been shown in many studies to have very little benefit on sleep, quite a contrast to what has been shown with 17-beta estradiol. Estriol also does not have the significant beneficial effects found with 17-beta estradiol for improving pain, memory, mood, and the “brain fog” symptoms that are so common in mid-life. I have treated a lot of women who had been put on estriol and suffered from “brain crash” when it didn’t work as well as estradiol on these crucial brain pathways. If you are told that your estriol level is low, that’s a normal finding if you are not pregnant. Another concern that is emerging with more “natural hormone” practitioners recommending estriol is that studies by Whitehead and a number of others over the past twenty years have found that higher doses of estriol (needed to give much symptom relief) will also stimulate the endometrium of the uterus to proliferate just as other estrogens do. Thus, if you take enough estriol to really help your symptoms, you have to still watch for endometrial hyperplasia just as you would if you took any of the estradiol products. Adding estriol to menopause therapy really isn’t “natural” hormone therapy as many compounding pharmacists and alternative practitioners are now promoting; it is simply their desire to sell a product.
EQUINE ESTROGENS
Extracted from the urine of pregnant mares to produce a mixture known as conjugated equine estrogens. The most commonly used brand is Premarin (pregnant mare’s urine). It is interesting to notice that the company making Premarin stopped using the word equine several years ago after PETA (People for the Ethical Treatment of Animals) began their campaign to make consumers aware of the inhumane way pregnant mares are confined during the long periods of urine collection and their foals sold for slaughter. But dropping the equine from the PR materials didn’t change the composition of the product or the way it is collected, so it still contains many estrogens not natural to a woman’s body. I think we should be at least as concerned about what effects occur in human females from these “unnatural” estrogens in our bodies as people are worried about the treatment of the mares.
Actually, the equine estrogens are not just one estrogen as many women think; “conjugated equine estrogen” refers to the entire group of about ten or more different chemical molecules that have different “attachment strengths” for the estradiol receptor sites. Many doctors consider that Premarin is the gold standard for estrogen therapy because it has been on the market longer, and most of the research studies have used only this one type of estrogen to determine estrogen benefits, side effects, and risks. But many people don’t realize that the manufacturer of Premarin is the company that funded much of the research, which is one reason we don’t have more comparative studies using other estrogens. Since the equine estrogens have several components that attach more strongly to the estradiol receptor than does human estradiol, and some of the equine estrogens stay in the body far longer than does estradiol (up to 2–3 months after the last dose), it only makes sense to me that we should study the potential for different effects of horse-derived estrogens on human females. There are many reasons I don’t recommend Premarin, and I will elaborate on these later in this chapter.
All of these estrogens have slightly different chemical structures from the three human forms (estradiol, estrone, estriol), and are therefore more potent, as well as having somewhat different effects and side effects on the body.
The most common form of estrogen found in the birth control pill. Although the ethinyl estradiol in birth control pills is widely used in perimenopausal women who may need better control of irregular cycles and erratic bleeding, it is not widely used in the United States for postmenopausal ERT because it has far greater potency and hepatic effects than does 17-beta estradiol and provides more estrogen effect than is generally needed after a woman reaches menopause. Ethinyl estradiol is also available alone as the brand Estinyl. It is not a birth control pill since it contains only the estrogen and no progestin.
ESTRADIOL VALERATE
About one hundred times more potent than 17-beta estradiol, it is rarely used in the United States, but it is common in Europe for postmenopausal ERT. Estradiol valerate is available for oral or intramuscular delivery. Estradiol valerate was the type of estradiol used in the 1979 Swedish study initially reporting a higher risk of breast cancer in estrogen users. Subsequent analysis and update of the data in 1992 from this group in Sweden did not support the earlier conclusion of higher risk. I elaborate on the flaws in that study in chapter 14.
You need to keep in mind that the estradiol referred to in European research can be either the native human form (17-beta estradiol) or the synthetic and more potent estradiol valerate. It is important not to confuse 17-beta estradiol with estradiol valerate, given their marked differences in potency and estrogenic effects.
PHYTOESTROGENS
These are estrogenic compounds found in several hundred different plants, including soybeans, red clover, grains, and many others. Phytoestrogens are biologically weaker than the native human estrogens and have quite a range of activity at the human estrogen receptors, varying from pure agonist to mixed agonist-antagonist, to full antagonist effects. Some of these different actions are dose and concentration related, and others are related to small chemical changes in the molecular configuration of the molecules. These potency differences may help explain the apparent discrepancy in reports that in China and Japan, where diets are high in phytoestrogens and herbal sources of estrogen are widely used, women do not typically describe hot flashes but do continue to have bone loss after menopause. Japanese menopause researchers have shown clearly that there is an epidemic of osteoporosis in their country; their research indicates that phytoestrogens alone do not provide enough estrogen effect to protect against osteoporosis and decline in cognitive function, even though the plant sources may be helpful for mild symptoms. I describe more aspects of current research on phytoestrogens in chapter 16. Ginseng is often recommended by herbalists as a “natural” source of estrogen. Ginseng, however, can cause high blood pressure, insomnia, anxiety, or agitation if taken in large amounts and, according to current controlled studies, gives little measurable estrogenic effect.
Several recent double-blind, placebo-controlled, prospective studies from the international menopause literature have found that phytoestrogen products were no more effective than placebo even for controlling hot flashes. Although the phytoestrogens are less potent than 17-beta estradiol, it is quite easy with the use of many supplements currently available to produce much higher serum concentrations of the phytoestrogens that competitively inhibit the action of 17-beta estradiol at cellular receptor sites.
Phytoestrogens from soy and wild yam are used in the pharmaceutical industry as the precursor molecules for producing 17-beta estradiol to use in tablets, patches, and creams and also to make the conjugated plant estrogens, Estratab and Cenestin. All of the phytoestrogen precursors require chemical conversion in the laboratory to make the bioidentical human form of 17-beta estradiol, since the human body does not have the enzymes needed for these changes. So just taking phytoestrogen supplement is not going to provide you with the critically necessary 17-beta estradiol.
A group of synthetic organo-solvent, pesticide, and other compounds that mimic some of the actions of estrogen in humans but are not native to the human body and also produce toxic effects. These chemicals, such as DDT, PCB, and a variety of others not normally found in nature, are as a group responsible for a great deal of environmental damage to animals, plants, and water sources. Their potent effect on living organisms is one of the factors responsible for reproductive abnormalities in wildlife and is also postulated as a cause of lower average sperm counts found in human males in recent years. I talk more about these compounds in chapter 14 and what we know about their potential carcinogenic effects. The important thing to keep in mind is that although these chemicals may have some estrogenic effects, their effects are generally negative, and they are not the same estrogen produced by our bodies.
I hear a lot of women talking about wanting to only take natural hormones, and this is the reason often given for wanting to use the wild yam skin cream advertised as a source of progesterone, or dong quai (an herb with estrogenic compounds), or estriol (weakest of the three primary human estrogens) instead of “drugs,” which are “synthetic.” Use of the words natural and synthetic can be very confusing, to patients and doctors alike.
Actually, something synthetic can also be natural, while something natural may be foreign (not native to) the human body. One example is Premarin. It is a “natural” mixture of estrogens because it is made by a biological organism, in this case the horse. But as I said earlier, it contains types of estrogen that are not found in the human body, so it is an “un-natural” estrogen for women. Another example is the “natural” estrogen-type compounds (genistein and others) found in soy and red clover, among others. These are “natural” substances since they come from a biological source, the soy plant. These compounds are “un-natural” for our bodies, however, since we don’t make these same compounds and don’t have the enzymes to change the genistein into 17-beta estradiol.
Making “natural” hormones like what the human body has is a process called “systhesizing,” which must be done in the laboratory. The term synthesis comes from the Greek meaning “a putting together, composition.” In other words, it means “to make something.” Synthetic simply means “produced by synthesis.” In common usage today, synthetic has come to mean “artificial,” but that is not always correct. Synthroid and Estrace are “synthetic” in that they have been made in the laboratory rather than within a biological organism, but they are “natural” in being the exact molecules made by the thyroid and ovary, respectively. Other examples of exact copies of our bodies’ hormones synthesized in the laboratory are Humulin (insulin) and cortisone (cortisol).
The sources for most of these “natural” human hormones are actually plants such as soybeans and yams, with the purified, concentrated extract producing chemical molecules identical to those made in the human body. This resulting 17-beta estradiol, progesterone, or testosterone is then compounded into standardized tablets to regulate the amount of hormone given. Standardization of the dose in each tablet also allows for tailoring the amount given more closely to each individual woman’s needs. I feel this approach is better than trying to get enough active hormone from plant/herbal sources alone, since you really are not able to determine how much you are taking and whether the amount is right for you. There is also the question of whether you are getting additional chemicals native to plants that your human body may not need.
So, when you read ads, newsletters, and books, be an educated consumer, and keep in mind: The important point is not whether a compound is “natural” to plants or horses or whatever, but whether the molecule shape, makeup, and structure is exactly identical to what is made in the human body so that it will fit properly as a “key” in the body’s receptor sites. A compound that meets these requirements is called “bioidentical,” and it becomes like a duplicate key to your car that you have a locksmith make in case you lose the original key. If the locksmith did the job correctly, the duplicate key works exactly the same way as the original. In the case of your hormones, the manufacturer (the ovary) stops making your own hormone at menopause, so a “locksmith” (the laboratory) makes an exact duplicate hormone molecule for you to have to use if you choose to.
I hope this helps clear up some of the confusion. Don’t be misled by clever wording in advertising. Everyone is doing it these days—some pharmaceutical companies are calling their products “natural” because they come from soy plants, since they now know that women want “natural” hormones. Alternative medicine practitioners and compounding pharmacists are calling many things “natural” hormones, including soy compounds that your body has never, ever made. Again, they all are engaging in clever marketing to sell you a particular product. I made up a chart, based on information from various manufacturers, about what is in the various products and what the sources are. If you aren’t sure about what you are taking, or considering taking, check the chart on page 120 to guide you.
We previously used insulins derived from cows (bovine) and pigs (porcine, or pork insulin) to treat diabetics who needed insulin, because we did not have a way to obtain human insulin. These animal insulins were “natural” in that they came from biological sources, but they were not native molecules for the human body, and many times, human diabetics developed allergic reactions or a resistance to the animal insulins. In recent years, scientists have determined the makeup of the human insulin molecule and have been able to synthesize the exact same molecule of human insulin in the laboratory, so that we can now give human diabetics the native human insulin (one brand is Humulin). So, here is one example of a synthetic medication being used because it is the natural one for humans and, therefore, is better tolerated, more effective, and has fewer side effects.
The same is true with estrogen. In the past, we did not have a way to give estrogen orally, because it would be broken down and lost in the digestive process before getting into the bloodstream. About fifty years ago, scientists developed a way to extract estrogens from the urine of pregnant mares, purify the extract containing “conjugated equine estrogens” (CEE), and then coat the estrogens in a matrix of binders (called enteric coating) that allowed the tablet to survive digestion in the stomach, reach the small intestine, be absorbed into the bloodstream, and then produce an estrogenic effect on the body organs. This product, Premarin, has been the primary type of estrogen used in the United States since that time, accounting for about 85 to 90 percent of the prescriptions written for estrogen in the United States. An oral dose of Premarin produces blood levels of the two primary estrogens also found in humans, estradiol and estrone, but it also produces high blood levels of equine estrogens native to horses, also called equilin estrogens. A typical oral dose of 0.625 mg Premarin produces about two thirds of the total circulating estrogens as equilin compounds; only about one third of the total estrogen present is the estrone and estradiol found in humans. I have shown some comparisons on blood levels in the graphs below.
In 1976, the FDA approved a new product (brand name: Estrace) that had been developed as a form of native human estrogen, 17-beta estradiol (derived from soybeans), in a micronized form, which survives digestion and is well absorbed into the bloodstream. Micronization means making the molecule particles small enough that they can be rapidly absorbed into the bloodstream before being broken down by digestive acids and the liver. Many recent studies have shown that the smaller the particle size, the better the absorption and the more reliable the blood levels obtained. Micronization is used to make many therapeutic medications and has been particularly helpful in developing native human forms of estradiol (estrogen), progesterone, and testosterone because these hormones typically were inactivated or destroyed by digestion when taken orally.
The various brands of transdermal estrogen patches listed earlier above are recent innovations to deliver the human 17-beta estradiol in a way that is the most “natural” of all. The patch system allows the estradiol to be absorbed through the skin, directly into the bloodstream, bypassing the “first pass” metabolism in the liver (which breaks down some of the hormone and changes it into other metabolites, making it unavailable for its normal functions). Using the patch means the hormones are delivered to the bloodstream as the ovary did it before menopause, not going through the stomach and liver first. The estradiol patches look like clear circular, oval, or rectangular Band-Aids that stick to the skin and are left in place for several days for the hormones to be slowly absorbed. Then as the hormone delivery is falling, a new patch is put on. Each brand of patch lasts for a slightly different period of time, and women metabolize the hormones at different rates, so it may take a little experimenting to find the change schedule that is right for you.
ESTROGEN BLOOD LEVELS FOLLOWING ORAL DOSE OF 1.25 MG CONJUGATED EQUINE ESTROGENS
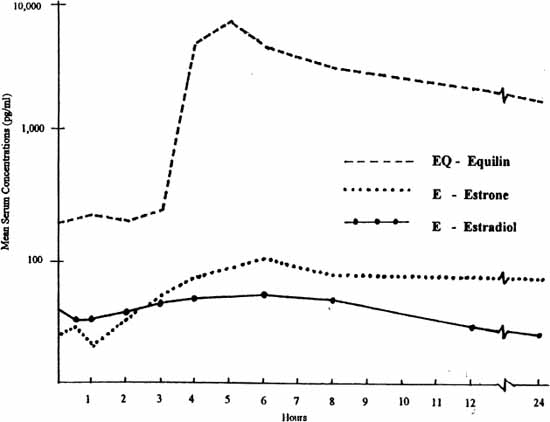
Equilin (EQ) estrogens are derived from horses and are not native to human females. Blood levels of EQ after an oral dose are higher than levels of Human E and E2. Effects of EQ on human females are not well known.
Adapted from Yen, Martin, Burnier, et al, as cited in Whittaker. 1982
SERUM ESTROGEN CONCENTRATIONS FOLLOWING ORAL DOSE OF 2 MG OF MICRONIZED ESTRADIOL
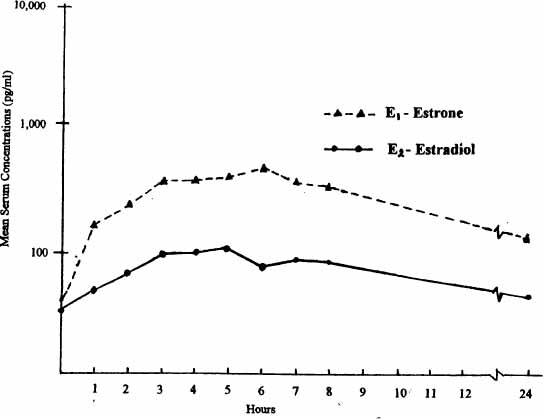
Adapted from Yen, Martin, Burnier, et al, as cited in Whittaker. 1982
The patches have several advantages that I will elaborate on in chapter 15, but generally they keep blood levels of estradiol fairly steady, similar to the hormone production by the ovary. Patches are a very good option for estrogen therapy, with only two primary drawbacks to this form of estradiol: (1) the skin irritation from the adhesive may bother some women, and (2) if you have a very low level of HDL, you may want the extra “plus” of having the oral estrogen stimulate the liver to make more HDL. The patches used in the United States (skin gels and patches in Europe) still give you the beneficial physiological effect of estrogen to maintain the normal level of HDL cholesterol, they just don’t give you the pharmacologic effect of extra liver stimulation to make more HDL that we see with the oral estrogens. The patch may be all that is needed for women who have a normal cholesterol profile. For women who have high total cholesterol and low HDL, however, an oral form of estradiol provides more decrease in total cholesterol and a more significant increase in HDL for cardiovascular protective effects.
COMPONENTS OF ORAL MIXED ESTROGEN PRODUCTS
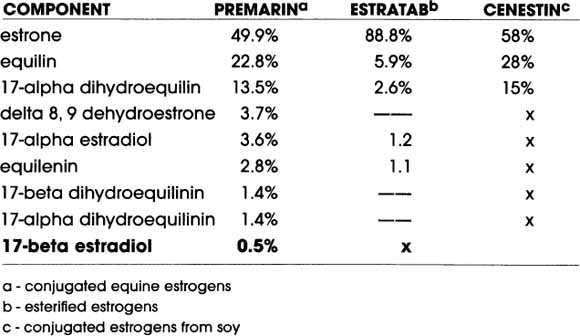
© Elizabeth Lee Vliet, M.D., 2000
Estrace (17-beta estradiol) gives only estrone (made in the liver) and 17-beta estradiol; it does not contain any of the other compounds shown above, thereby reducing the overall estrogen amount delivered per dose when compared to the above types.
SOURCES OF ESTROGENS
Pregnant mares urine |
Premarin |
Soy |
Climara, CES (Canada) |
Yam |
Vivelle, Alora, Estraderm, Estring, |
Yam and soy |
Estrace and Gynodiol (17-beta estradiol), Cenestin (estrone, equilin) |
Plants (incl. soy) |
Estratab (estrone, equilin) |
Plant + Synthetic |
Ogen, generic conjugated estrogens |
Synthetic |
Estinyl, Estrovis, Tace, Dienestrol ethinyl estradiol (in birth control pills) |
© Elizabeth Lee Vliet, M.D., 2000
I have talked about the concept of the body’s chemical messengers (hormones, neurotransmitters, etc.) acting like “keys” in special receptor-site “locks” on the cell membrane. The molecules made by the human body are the specific keys that fit our cell receptor sites. Similar chemical molecules, either from other animals or made in the laboratory, may also work the locks, at least partially the same as the human molecules do. But some of these other chemical molecules may get stuck in the lock and actually block the action of our own human molecular keys. That’s why it is important to understand the different types of hormone preparations and know that the different forms available may have very different effects in your body.
For the steroid hormones, there is an additional receptor site located in the cell nucleus. These receptor sites have been identified for all three primary ovarian hormones. In particular, estrogen receptor sites are found throughout the brain and all organs of the body—skin, blood vessels, bone, heart, intestinal tract, urinary bladder—not just the organs of reproduction. The blood levels of human estrogens include predominately estradiol and, in lower amounts, estrone. Most of an oral dose of estrogen is converted in the blood to estrone or estrone sulfate. But there are important differences between the estrogen circulating in the blood and the estrogen that acts at the cell receptor site. 17-beta estradiol is needed at the receptor site to actually work properly. Estradiol is the estrogen in humans that declines so rapidly after menopause, causing the symptoms of declining estrogen. Estrone is still present in postmenopausal women since it is made in the adrenal glands and in the fat tissue. If estrone were the primary hormone activating the receptor site, then we would NOT expect to see postmenopausal women having the symptoms of estrogen decline. Make sense?
So far, researchers have identified three types of estradiol receptors (ER) in the body: two (ER-alpha and ER-beta) inside the nucleus of cells, and another on the membrane surface of cells. The body’s estrogen receptors need the proper keys of 17-beta estradiol molecules to fit in the locks but the nucleus and membrane receptors each act by a different mechanism to influence the biochemical processes of cells. ER-alpha and ER-beta receptors are found in different types of organs and target tissues throughout the body. They interact in very complicated ways we are just beginning to unravel.
The estradiol receptors in the nucleus works in a more complex manner than the membrane receptors. The estradiol molecule fits into the nuclear receptor and then binds with the cell’s DNA to regulate gene expression. These genes are involved in the synthesis of particular proteins that make up neuropeptides, crucial enzymes, and other chemical messengers. This process is called the genomic hormonal action. An example important in memory regulation is the action of estradiol to trigger the formation of choline acetyltransferase, the enzyme that makes the memory-enhancing chemical messenger, acetylcholine. This effect cannot be investigated in living humans with our current techniques, but it has been shown in studies of rat brains. Estradiol also acts at the nucleus receptor to stimulate messenger RNA inside cells so that neurons in the brain can make proenkephalin, an opiate “messenger” peptide important in pain-reducing pathways. These are just two of many examples of estrogen action at the genomic receptor. Many of the sites of estradiol’s action in the brain, as well as other body organs, work by this process.
Another mechanism of action for estradiol and other hormones is called the nongenomic process. Here, the estradiol binds with a specific “lock” or receptor site on the cell membrane rather than inside the cell. We have not yet discovered the many ways in which these membrane receptors work, but we do know that this mechanism produces much more rapid effects than does the process of gene regulation directed by the nucleus estradiol receptors. Examples in this category seem to be the estradiol stimulation of various neurotransmitters, such as serotonin, dopamine, and GABA. Recent studies in mice indicate that pain pathways in males and females are functionally distinct, and that estradiol is an important regulator of these pathways. Such a significant finding means that we now need to take into account the sex differences in nerve mechanisms regulating pain when we are treating women patients, as well as when doing basic scientific research on pain mechanisms.
SUMMARY: Estradiol Receptors in the Nervous System
 CNS: Concentrated in limbic system
CNS: Concentrated in limbic system
 PNS: Found in spinal cord, peripheral nerves
PNS: Found in spinal cord, peripheral nerves
 Specific for 17-beta estradiol (E2)
Specific for 17-beta estradiol (E2)
 Nerve function is affected by changing E2 levels
Nerve function is affected by changing E2 levels
 17-beta estradiol is the active form at E2 receptor
17-beta estradiol is the active form at E2 receptor
 equilin estrogens: higher affinity at receptors than human E2
equilin estrogens: higher affinity at receptors than human E2
 equilin estrogens may displace 17-beta estradiol at receptors
equilin estrogens may displace 17-beta estradiol at receptors
© Elizabeth Lee Vliet, M.D., 1995
Although many physicians think all the estrogens for ERT are essentially the same, and the manufacturers of the leading products would like you to think they are all the same, they are not. Over two decades ago, two leading menopause researchers in England, Dr. Malcolm Whitehead and Dr. Campbell, did studies of the potencies of the different types of estrogens and the effects of the various estrogens on different target organs in the body. What they found was quite disturbing and has profound implications for women taking various products. They raised some critically important questions back then (1978–1982), but practically speaking, none of their questions and concerns have been addressed with further studies in this country. In fact, as I travel and speak to physician groups, very few of them even know these studies were done and what the outcomes were. Dr. Campbell and Dr. Whitehead’s work has languished in the literature, and their questions are left unanswered.
How does this issue relate to women at risk for heart disease? The work done by Campbell and Whitehead shows that the conjugated equine estrogens are about three times more potent in stimulating the liver production of renin substrate, which is used to make angiotensin in the body, a factor that causes increased blood pressure. Increases in the circulating levels of renin substrate have been proposed as the possible mechanism by which conjugated equine estrogens may elevate blood pressure in some women. Two other estrogens used in this study, micronized estradiol (brand name: Estrace) and piperazine estrone sulfate (Ogen), did not show this elevation of renin substrate. This finding also fits with work published by Geola in 1980, who observed that 1.25 mg of conjugated equine estrogens (brand name: Premarin) daily, caused supraphysiologic (greater than normal) effects on the liver synthesis of renin substrate, physiological (normal) actions on the vagina lining tissue (epithelium), and subphysiological (less than normal) effects on the brain hormones FSH and LH. This correlates with what I have been seeing clinically: The dose of equine estrogens that is adequate for vaginal lubrication may produce a rise in blood pressure, and inadequate effect on brain phenomena like memory. It has to do with different target organs (in this case, liver, vagina, and brain) having very different sensitivities to the various molecular types of estrogens. The findings from studies such as those by Campbell and Whitehead are an additional argument for individualizing the estrogen options for women, rather than using one kind of estrogen and one dose for every woman.
Based on work such as that done by Campbell and Whitehead, it appears that women with existing hypertension would be better served to use one of the native human forms of estrogen, rather than the equine estrogens, in order to avoid the potentially harmful production of high levels of renin substrate. I recently presented this information at a Grand Rounds program for Internal Medicine and Family Medicine physicians at a major medical center. The physician who was head of the hypertension clinic was shocked to hear about these differences in various estrogen potencies on angiotensin. He had not heard about the studies from England and now wants to design a research project in his clinic that will compare blood pressure effects of several types of estrogens. This is the important kind of cross-fertilization of ideas and questions that helps us find better approaches for women.
I have seen many women for evaluation who clearly have had allergic-type reactions to the horse-derived CEE. I also have a significant number of patients who had had problems with vague joint pain syndromes that resolved when I took them off the CEE and prescribed a native human form of 17-beta estradiol. These women had already been evaluated by rheumatologists for possible rheumatoid arthritis, osteoarthritis, lupus, and other diseases that cause joint pain, and had been told “there was nothing wrong.” To me, that statement means simply that there were no laboratory abnormalities that provided a diagnostic label for the joint pain. The joint pain was definitely real for these patients, and it improved with the change in type of estrogen. Whether the pain was due to an adverse allergenic or autoimmune reaction to the horse-derived estrogens, or whether it represented the kind of joint pain that is seen with estrogen deficiency, I cannot say at this point in time. I am suspicious that the joint pain syndrome I have seen so commonly in women on CEE is related to an immunologic reaction to the equilin estrogens, similar in theory to the immunologic reactions seen in diabetics on the animal-derived insulins. Until more physicians and basic science researchers take seriously the descriptions women give about their body experiences on different estrogens, we will not be able to answer these questions.
The data from Campbell and Whitehead, as well as other researchers, has indicated that the equilin (horse) components of conjugated estrogens (equilin and 17-alpha-dihydroequilin) in themselves possess estrogenic activity in the human female, but this has not been studied further to determine whether this activity is beneficial or adverse. I find it incomprehensible that the Campbell–Whitehead research has not prompted more investigation in this country on the potential adverse effects of the equilin estrogens. Even the recently published PEPI studies (Postmenopausal Estrogen and Progestin Intervention trial) used only Premarin as the estrogen, even though this important study compared natural micronized progesterone with a synthetic progestin (Provera) for the first time in the United States. Why aren’t such studies also including a native human form of 17-beta estradiol?
A few years ago, media attention focused on reports of an increased risk of breast cancer in women in the Nurses Health Study who had been on estrogen longer than five years. Another study published in January 2000 showed an increased risk of breast cancer in women taking combination estrogen-progestin therapy compared to estrogen alone. What was never addressed by any of the physicians or health writers commenting on this disturbing information is that the overwhelming majority of women in both of these large-scale studies were using Premarin, alone or with a synthetic progestin (Provera), not 17-beta estradiol. With what we know about (1) how long the equine estrogens stay in the human body (anywhere from eight to fourteen weeks after the last dose), and (2) the stronger “attachment strength” (affinity) of the equine estrogens for the body estradiol receptors (especially the breasts, where estrogens may concentrate in the fat tissue), and (3) the much higher total blood level of equilin estrogens following an oral dose of Premarin, it seems incredible to me that no one is talking about a possible link between accumulations of equine estrogens in breast tissue and the observed higher risk of breast cancer with long-term use. I think this is such an important question that it deserves careful attention and research, but prescribing habits and research protocols are so dominated by the use of one type of estrogen that no one seems to even consider that there may be crucial differences that women need to know.
Then there is just the dimension of women feeling better when they use something more like what their bodies make. Listen to what this young woman had to say after I changed her from PremPhase to natural forms of 17-beta estradiol and progesterone:
Being off the PremPhase has really helped. I feel lots better—have more energy, my sex drive is back, I am not as moody as I was, my memory is definitely better, and I am doing better in school.
She was now taking Estrace 0.5 mg twice a day, and then cycling with natural progesterone orally 200 mg a day for twelve days a month. The additional interesting aspect about her situation is that she was only thirty-four years old, and had been diagnosed with premature menopause by her gynecologist and begun on the PremPhase about two years earlier. No one had done any hormone levels or a check of her bone density, urine bone markers, or lipids to see whether the hormone therapy was doing what it was supposed to do in providing the benefits of estrogen. When I checked all of these measures at her consult, she was shocked to find that on PremPhase (1) her estradiol was far too low at 38 pg/ml (certainly a reason she didn’t feel very good and was having trouble with memory, sleep, and concentration affecting her school performance), (2) her urine bone marker (NTx, 65) was double what it should have been to preserve bone (this should come down to less than 35 if the estrogen therapy is doing its job), and (3) her 8 A.M. cortisol was too high at 17.9, and her fasting lipid profile showed elevated triglycerides. These were ominous findings, especially since she was so young. I wasn’t surprised by this, however, since I commonly find that the progestin in PremPhase and PremPro causes problems with increased triglycerides and higher cortisol. Her low estradiol level helps to explain the “brain fog” and her continuing bone breakdown.
Six months after the change to Estrace and natural progesterone, her 8 A.M. cortisol was completely normal at 9.5, her serum estradiol was 115 pg/ml (drawn at our standard ten to twelve hours following a dose, which is the level we look for), her NTx had come down to 28, and as you saw from her comments, she just felt better!
This is why our work with fine-tuning women’s hormone balance is so rewarding. I can see the “objective” measures change in positive ways, and I enjoy hearing that they feel better and are finding their vitality, energy, and enthusiasm coming back.
Current international menopause research has verified in a variety of settings that it is more important to assess serum estradiol levels than we had previously been taught. Recent studies have shown that as many as 45 percent of patients whose self-assessment had been that symptoms were alleviated, actually still had serum estradiol levels below the currently accepted thresholds for the various protective effects of estradiol on brain, bone, and other target tissues. Reliable assessment of serum hormone levels is one method to ensure that adequate levels of estradiol are reached. Problems as I described with the young woman above could be reduced if we verify adequacy of the hormone replacement our patients are taking and do so with reliable, systematic approaches. Monitoring therapy at appropriate intervals with serum estradiol levels is cost-effective in that it helps to reduce the likelihood of additional medications, or more involved and costly treatments, and helps reduce unpleasant side effects.
Women often comment that they hit their forties and suddenly their contact lenses don’t seem to be tolerable any more, or their hair is falling out, or their skin is so much drier, or they now get “zits” like a teenager. “I’m getting pimples and wrinkles, what’s going on?” said one forty-two-year-old mother. “What’s happening?” Remember, estrogen is Mother Nature’s moisturizer for our body. That means everything from skin to scalp to eyes to mouth to nose to intestinal tract, bladder, and vagina are all affected by increasing dryness as estradiol declines and we are left with more estrone and androgens. How does this show up for you? Dry eyes are the result of loss of estradiol effects on the moisture of the tissue that covers the eyeball, along with other changes as we get older. To fit properly, contact lenses “float” over the eye on a thin film of water. If the surface of the eye isn’t as moist, contact lenses don’t have their usual “floating pad” to ride on, and they burn or feel scratchy. If your eyes are dry, your wearing time decreases. Doctors suggest such dramatic steps as surgically blocking tear ducts without ever thinking about a woman’s hormone balance and its effect on the eye. And it is not just the dryness of your eyes that we need to watch. Current studies have also shown that declines in estradiol are associated with increases in age- related macular degeneration (ARMD), and with the development of glaucoma and cataracts. Research from medical centers in many different countries has shown that estrogen replacement therapy helps to reverse all of these adverse changes on the eyes.
Dry skin is obvious. But less obvious is the loss of collagen as estradiol declines. Collagen gives your skin its elasticity and firmness. When you combine the dryness with loss of collagen, you get the dry, wrinkled, sagging appearance that is associated with aging. It turns out that these changes are not just due to getting older. They are accelerated by loss of the active form of estrogen, estradiol. Since no ones dies of old age of the skin, estrogen effects on skin aging have not been subjected to as many clinical studies as we have seen in more crucial areas, such as estrogen effects on heart, bone, and brain. A carefully designed study from Spain, published in Maturitas in 1992, evaluated the effects of time after menopause on skin collagen content, and the effects of three different estrogen regimens on skin collagen. The researchers showed that skin collagen decreased markedly beyond the forties and after menopause, no matter what age menopause (surgical or natural) occurred, and even if menopause occurred in women younger than forty. This decrease in skin collagen was preventable by the use of all of the estrogen approaches studied, but the transdermal estradiol therapies showed a greater degree of preservation of skin collagen compared to that seen with the conjugated oral estrogens. If you have noticed marked changes in your hair and skin quality as you experience other hormone-related changes, maybe it is time to have your hormone levels and bone density checked. The outer changes you can see may well be a clue to unwanted inner body changes as well.
Thinning, brittle, dry hair is not life threatening, but it certainly is a source of major distress to many women. In fact, losing hair is one of the more common problems women report, right up there with insomnia, fatigue, mood swings, and loss of sex drive. I don’t have space to go into all the causes of alopecia, or hair loss, but some of the common overlooked hormonal causes for women are loss of estradiol, excess testosterone as well as low testosterone, excess DHEA, hypo- and hyperthyroidism. Again, it is surprising to me that dermatologists diagnose “menopausal alopecia” and will prescribe Rogaine for women without even suggesting that hormone levels be checked, or supplemented. Before you go off on tangents of expensive hair loss evaluations, supplements, and medications, at least get a careful and reliable measure of your ovarian and thyroid hormone levels, including thyroid antibodies. In order to be certain of the complete picture with regard to your hormones and hair loss issue, you cannot depend on saliva hormone levels. They simply do not correlate very well with actual hormone delivery to the hair follicle. We have had too many patients whose saliva tests have said they were high in estrogen and low in progesterone, but when I checked the more reliable clinical findings and serum levels, I found the reverse: low estradiol and continuing normal levels of progesterone. The serum hormone tests are what I want to trust in making recommendations for treatment options.
What about those “sinus problems” you have suddenly started having as midlife hit? Remember, the nose and sinus cavities of the face and forehead are lined with a mucous membrane. Similar to the way the loss of estradiol causes dryness of the lining of the vagina, we also see a loss of moisture and healthy mucous lining of the nose and sinuses when estradiol declines. The production of mucous by this lining helps to clear out allergens, bacteria, and viruses from the air you breathe. When estradiol declines, the mucous membrane becomes drier and then doesn’t function as well to clear out all these invaders. Blood flow is decreased as well, due to the constriction of small arteries when estradiol levels fall. If blood flow is diminished, the immune cells and proteins carried by the blood aren’t as available to the tissues to ward off or destroy the invader particles. Taking antihistamines and decongestants every day just makes all this worse, since together they dry the membranes even more and cause more constriction of the blood vessels. A better option is to use natural saline nose sprays to provide needed moisture, and several times a week, do a steam bath (easy—just hold your head, covered with a towel, over a sinkful of hot water and let the steam clear your sinuses and moisturize your face at the same time). Then, get your hormone levels checked and look into whether hormone therapy is more appropriate for you than taking lots of decongestants and antihistamines every day.
I will talk more about estrogen effects on sleep in chapter 11. For now, let me just say that you are not imagining changes in your sleep quality as hormone change hits. Sleep becomes more elusive, it gets more fragmented once you do fall asleep, there are multiple awakenings that you didn’t use to have, you may notice less dreaming and more “restless legs” or muscle twitches, and you wake up feeling exhausted. Sound familiar? Menopausal women commonly develop abnormal breathing during sleep and suddenly start developing sleep apnea syndrome (SAS), a potentially serious sleep disorder, at rates about equal to those seen in men. Prior to menopause, sleep apnea in women is much less common than it is in men. Chronic sleep disruption and loss of sleep leads to daytime fatigue, memory and concentration difficulties, mood problems, muscle pain syndromes and other health problems. If the sleep disturbance is severe, prolonged, and includes significant apneic (stop breathing) spells that cause oxygen loss, the consequences can be even more severe. Sleep apnea is already known to be a significant contributing risk factor for high blood pressure, cardiovascular disease, early morning fatal heart attacks, onset of major depression, and erectile problems in men. The reason such serious consequences of sleep apnea occur are the dangerous drops in oxygen (02 saturation) of the blood when sleep apnea causes you to stop breathing. This drop in oxygen saturation to dangerously low levels happens in both men and women. Menopausal women, however, have an even greater vulnerability than men to sudden death or heart attack during sleep because the estradiol drop in women causes combined effects that make the situation worse. There is the oxygen drop when you stop breathing, added to the effect of nighttime hot flashes causing a rise in catecholamines and instability in heart rate and blood pressure that also affect blood delivery to the heart.
Since our current therapeutic options for sleep apnea are limited to weight loss, surgery, and/or continuous positive airway pressure (CPAP), it would be helpful to know whether hormone changes at menopause, and the use of hormone therapy, play a role in women’s risk of this serious sleep disorder. Drs. Keefe, Watson, and Naftolin conducted a pilot prospective crossover study of the effects of hormone replacement therapy on sleep apnea that was published in 1999 in the Journal of the North American Menopause Society. They performed detailed sleep studies and serum hormone levels both at baseline before hormone therapy and three to four weeks after the women had been on either 17-beta estradiol (E2) 2mg daily alone or together with ten to twelve days of medroxyprogesterone acetate (E2 + P). They did not include a progestin-only group because earlier studies had found no effect on sleep apnea from giving progestin without estrogen. Both E2 alone and with E2 with P regimens were found to decrease sleep apnea to a statistically significant degree in all subjects tested. The investigators concluded that hormone therapy for menopause has a potential role in reducing SAS and its many associated health risks. They also reported another interesting finding: 40 percent of the waking episodes these women had were not associated with vasomotor flushing (“hot flashes”), which suggested that vasomotor flushing and waking episodes are separate and independent manifestations of estrogen decline. It was previously thought that fragmented sleep around menopause was due only to hot flashes at night.
The study confirmed what many other sleep studies have suggested: Ovarian sex steroids, particularly estradiol, have a variety of effects on normal sleep regulation, including direct effects on sleep apnea. It is also significant that patients with SAS, the most severe form of sleep disorder, were used for this study and had the degree of benefit shown. These findings are all the more promising from women with milder forms of sleep disruption, and fit with basic science studies showing that there are many mechanisms by which estradiol with progesterone/progestins interact with neurotransmitters and regulate brain centers involved in sleep pathways. Formal studies like this give further support to “anecdotal” reports from women themselves that their sleep is better when they start a hormone therapy that includes estradiol. With new evidence that hormone therapy containing estrogen can reduce sleep apnea, I think it is even more important for physicians not to dismiss sleep problems in women as “just stress,” and prescribe sleeping pills, such as Ambien, Klonopin, Dalmane, and others. If women actually have sleep apnea, sleeping pills can make the situation even more dangerous by further suppressing breathing during sleep. If your bed partner tells you that you have started snoring at night, or maybe that you seem to stop breathing at times and then “jerk” back in to a loud breathing, I urge you to talk with a physician about having sleep studies to check for sleep apnea, and again, have your estrogen level checked.