
MUCH OF THE CANIDS’ FOSSIL RECORD is preserved in the form of bones and teeth. Thus a great deal of anatomical information is lost during fossilization, a process that turns the bones and teeth into something similar in properties to the surrounding rocks and that destroys much of the soft tissues, including muscles, skin, and internal organs. However, fossil bones and teeth fortunately preserve a large amount of anatomical and biochemical information that is extremely valuable in determining an animal’s anatomy, physiology, and, to a certain extent, behavior (figure 4.1). For example, limb bones, an essential component of all mammals’ skeletons, can tell us much about the precise configuration of the limbs, which are important in deducing the animal’s body weight and locomotion repertoire (the ways in which it walked, ran, climbed, or swam). Most skeleton bones also preserve traces of muscle attachments in the form of ridges, crests, and other superficial features that are the basis for the reconstruction of musculature in fossil animals. Starting with such solid information—the fossil evidence—paleontological artists are able to add layers of soft tissue over the skeletal frame in order to re-create an animal’s appearance in life. All the illustrations of extinct mammals in this book were created using a detailed methodology based on skeletal morphology as the starting point for the restoration of unpreserved attributes (figure 4.2). Skull bones also preserve clues about the neural organs (the brain and nervous system) and the sensory organs (for hearing, smelling, and seeing). As part of the food ingestion system, skulls and jaws are also very informative regarding how food was procured and processed (figure 4.3).
When the dietary habits of an extinct carnivoran are studied, however, fossil teeth are the single greatest source of information. To gain an appreciation of the evolutionary history of the canids, one has to begin with some knowledge of the anatomical terms for teeth and bones, which are predominantly the stuff preserved in fossils.

FIGURE 4.1
Gray wolf
Skeleton of the European gray wolf (Canis lupus), with labels showing key anatomical features.
Teeth
DENTAL FORMULA
Among carnivorans, canids have the most conservative (least-modified) teeth, with a bilateral complement of teeth divided into three upper and lower incisors, one upper and lower canine, four upper and lower premolars, two to three upper molars, and three lower molars. With the exception of supernumery teeth in animals such as the bat-eared fox (Otocyon), which grows an extra molar, and extreme cases such as dolphins, which have many more teeth than other mammals, a reduction in the number of teeth has occurred in mammals through evolution in various ways. Paleontologists have devised a system of dental formulas to express the number of teeth in shorthand, such as 3143/3143: the number to the left of the slash represents upper dentition (three incisors, one canine, four premolars, and three molars), and the number to the right denotes lower dentition. The numbers indicate only one side of the jaw, and the number for a full complement of teeth in the mouth must double the dental formula. With the exception of the bear family (Ursidae), the extinct bear dog family (Amphicyonidae), and the Canidae, all other modern families of carnivorans have had fewer teeth (that is, a reduced dental formula) over time, with the cat family (Felidae) in the extreme end of the spectrum: 3121/3121. These reductions in the number of teeth are usually indications of specialized diets moving toward hypercarnivory. Another useful shorthand is the designation of an individual tooth by a combination of a letter—incisor (I or i), canine (C or c), premolar (P or p), and molar (M or m)—and a number (counted from front to back). Thus the entire dentition of a canid can be expressed as I1, I2, I3, C1, P1, P2, P3, P4, M1, and M2 in the upper jaw and i1, i2, i3, c1, p1, p2, p3, p4, m1, m2, and m3 in the lower jaw (see figure 4.3).
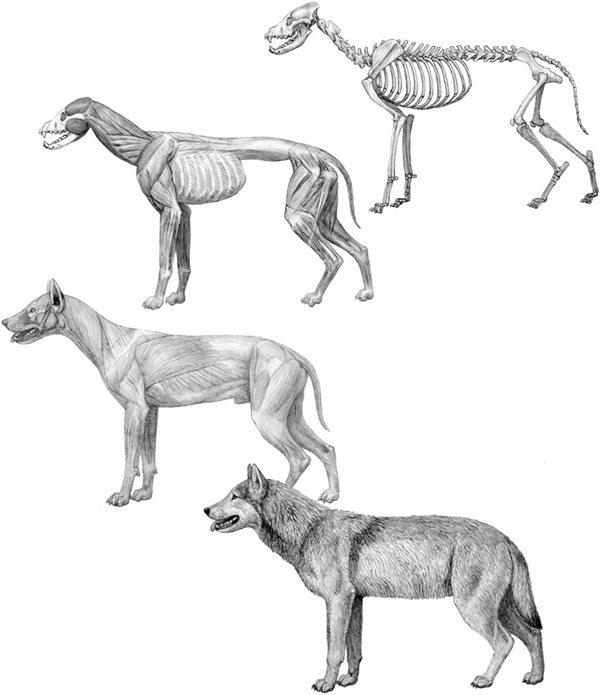
FIGURE 4.2
Dire wolf
Step-by-step reconstruction of the dire wolf (Canis dirus), based on fossils from the late Pleistocene (20,000 years ago) of Rancho la Brea. Top, skeleton; top center, deep muscles; bottom center, superficial muscles; bottom, life appearance. The deep muscle layer is inferred largely from the direct observation of attachment areas on the bones. More superficial layers of tissue require a higher level of inference, and reference to modern related species is important.

FIGURE 4.3
Gray wolf
Top, skull and mandible of the gray wolf (Canis lupus) in lateral view; bottom, skull in palatal view, with the tooth positions labeled.
INCISORS
The incisors are the three small, neatly packed teeth with a flat cutting edge in front of the jaw, those that are clearly visible in front view when an animal opens its mouth. Incisors are rooted in the frontmost part of the jaws—in the premaxillary bone of the upper jaw and the dentary bone (frontmost tip) of the lower jaw. On close examination, there is usually one main cusp on the incisors and two smaller cusps flanking either side (one must make sure to look for these features in a relatively young individual because they are obliterated as the teeth are worn down in older individuals). One of the defining features of the borophagine canids is their possession of one to three small cusps on the side of the upper third incisor.
CANINES
The canines are the fanglike teeth that deliver the killing bites, and carnivorans display their canines for maximum effect in a threatening posture. All canids have prominent canine teeth—which fittingly bear the name of the dog family—in each jaw, and they are found in nearly all mammals (even humans have them, but they are reduced in size and look very much like incisors). The upper canines are technically located at the suture zone of the premaxillary and maxillary bones in the upper jaw and shear with the lower canines, which are slightly in front. In canids, the size of canine teeth tends to be somewhat variable between the sexes. Male canids of fox- to wolf-size species usually have canines that are 3 to 6 percent larger than the females’ canines (Gittleman and Van Valkenburgh 1997).
PREMOLARS
The premolars are the teeth between the canines and the molars. In canids, with the exception of the upper fourth premolar (see “Carnassials”), the premolars are formed by a tall main cusp and one or two smaller accessory cusps in front of and behind the main cusp. As do the incisors and canines, the premolars consist of two sets: the deciduous (or milk) premolars and permanent premolars. The last deciduous premolars often look quite similar to the molars, but the permanent premolars adopt a radically different construction. Canid premolars tend to be stable in most lineages, but among taxa that evolved a bone-crushing adaptation, the premolars (particularly those of the rear end of the series, including the upper carnassials) can be substantially enlarged to become the bone-cracking teeth. Such an adaptation is most obvious in some lineages of the borophagines and, to a lesser extent, the hesperocyonines.
MOLARS
The molars (from Latin mola [mill, millstone]) are the backmost series of teeth. They differ from the premolars in their lack of a deciduous precursor; that is, permanent molars emerge without milk teeth having preceded them. In canids, with the exception of the lower first molar (see “Carnassials”), the molars are formed by a low platform with a series of low cusps closely aligned with each other on the crown surface. These small cusps occlude with each other, so the upper and lower molars function like a millstone. These grindstone-like teeth are commonly well developed in canids (but not as extremely developed as in ursids), and they permit flexibility in a canid diet that mixes meat with vegetable matter and insects. The relative size of the molars is an excellent indicator of the diversity of an animal’s diet.
CARNASSIALS
A specific pair of teeth is very important in defining the order Carnivora: the carnassials, specifically the upper fourth premolar and the lower first molar, which function as the main teeth for cutting the muscles and tendons of killed prey. The carnassials are specially modified in all carnivorans to form a pair of shearing blades that act like scissors. This scissorlike carnassial pair was the most definitive adaptation of the ancestral carnivorans and has been passed down to all descendant lineages. Therefore, the order Carnivora is literally defined by its members’ common possession of a pair of carnassial teeth. In canids, the carnassials are usually well developed enough to handle most of the meat-cutting requirements.
Hypercarnivory Versus Hypocarnivory
The concept of dental adaptation is very important. In 1956, Spanish vertebrate paleontologists Miguel Crusafont-Pairó and Jaime Truyols-Santonja published an insightful study of carnivoran functional morphology in which they categorized carnivorans into hypercarnivores and hypocarnivores based on their dental morphology.
A hypercarnivore is an animal that has elongated the shearing blade of the carnassial teeth at the expense of the grinding part of the dentition (usually molars). The most extreme example of a hypercarnivore is the cat, whose teeth essentially include only the shearing part of the dentition—a pair of long, thin-bladed carnassials—with the grinding part (molars) behind the carnassials being strongly reduced in size (figure 4.4). Such a hypercarnivorous adaptation is thought to be related to a diet that is made up almost exclusively of meat.
In contrast, a hypocarnivore is an animal that has shortened the shearing blade of the carnassial teeth and enlarged the grinding part of the dentition behind the carnassials. The most extreme example of a hypocarnivore is the bear, in which the shearing part of the carnassial teeth is radically reduced and the grinding parts of the molars are extremely broadened (figure 4.5). A hypocarnivore tends to have a far more varied diet that includes meat, insects, fruits, and roots. In the most extreme case of hypocarnivory, the giant panda (Ailuropoda melanoleuca), which is an ursid, has become a dedicated bamboo eater.
In between these two extremes, most of the carnivorans, including the majority of the canids, have teeth that are neither extremely hypercarnivorous nor particularly hypocarnivorous. We call these intermediate forms mesocarnivores (from Latin meso [middle]). In this book, when we discuss hypercarnivorous canids, we refer to canids that have relatively more elongated dental shearing blades and at the same time a relatively reduced grinding part of the dentition, even though these canids are by no means in the same league as the cats in terms of dental specialization (figure 4.6). Likewise, we refer to canids in which the opposite trend has occurred as hypocarnivorous (figure 4.7). But here again, canid hypocarnivory is by no means as extreme as ursid hypocarnivory.
Skulls, Jaws, and Teeth as Indicators of Diet
An important fact of vertebrate paleontology is that dental morphology is highly sensitive to a mammal’s diet. Such a close association between the shape of teeth and the diversity of diet permits paleontologists to learn much about what extinct animals ate. The teeth of canids have changed flexibly toward either hypercarnivory or hypocarnivory during their evolutionary history, which is probably one of the most important reasons for their success as a group in adapting to fast-changing environments. Because of their relatively conservative, unspecialized dentition, ancestral canids (such as Hesperocyon, Archaeocyon, and Leptocyon [chapter 3]) were apparently free to evolve toward either hypercarnivory or hypocarnivory, capitalizing on new opportunities that arose from time to time.

FIGURE 4.4
Leopard
Skull of a typical hypercarnivore, the leopard (Panthera pardus; family Felidae). The cheek teeth in front of and behind the carnassials are either reduced or lost, and the carnassials themselves are narrow, bladelike teeth.
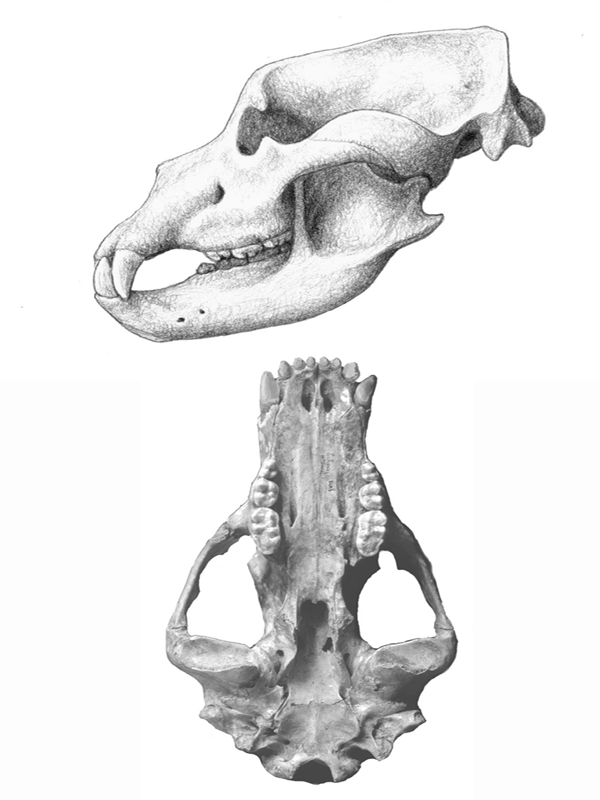
FIGURE 4.5
Cave bear
Skull of a typical hypocarnivore, the cave bear (Ursus spelaeus; family Ursidae). The premolars are reduced or lost, the molars are large and broad, and the carnassials are broadened.
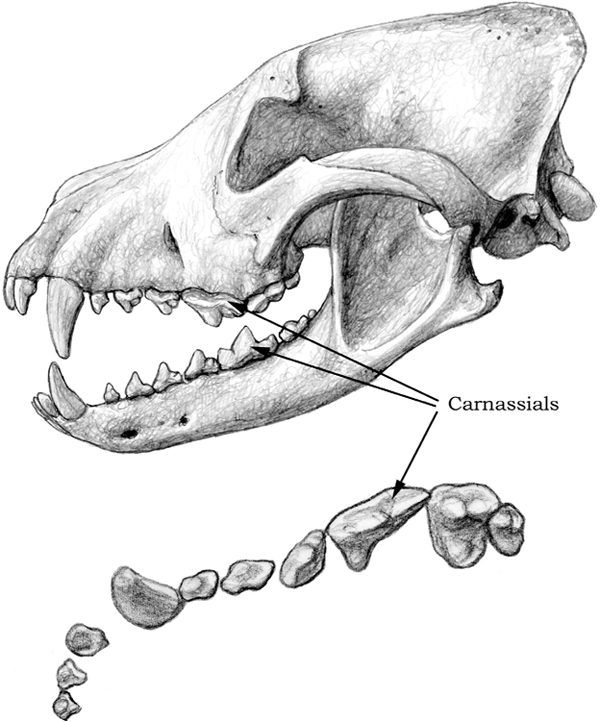
FIGURE 4.6
African hunting dog
Top, skull and mandible of a hypercarnivorous dog, the extant African hunting dog (Lycaon pictus); bottom, detailed occlusal view of the upper-right dentition. Notice the long, bladelike carnassials.
The vertebrate skull serves the all-important function of protecting the brain. However, if protection of the brain were the skull’s only function, then there would be no need for variety in skull shape because a rounded, hard-shelled braincase would be all that any vertebrate needed to shield the brain adequately against common, self-inflicted mechanical damage (as opposed to damage inflicted by predators, which is a different matter). The fact that vertebrate skulls do vary substantially from group to group implies that some other factor is also at play. That factor is often related to food processing.
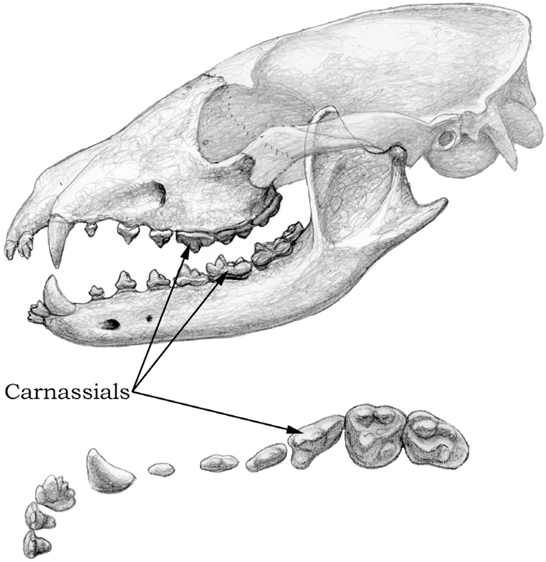
FIGURE 4.7
Cynarctus
Top, skull and mandible of the hypocarnivorous canid Cynarctus (10 Ma); bottom, detailed occlusal view of the upper-right dentition. Notice the relatively small carnassials and the enlarged, broad molars, as well as the extra cusps along the side of the upper third incisor.
As the location of initial food ingestion and mechanical processing, the skull and lower jaw are perhaps the most critical devices in meeting the demands of food intake. For carnivorans, the skull and jaws also aid in the all-important function of killing prey, and it is this function that often defines the overall skull shape in carnivorans. One of the most variable and, by implication, evolutionarily most selected features is the length of the muzzle, or rostrum. It is easy to see differences in this feature among cats and dogs. In general, cats have a much shorter rostrum than do dogs, and this evolutionary divergence can be traced to the very beginning of both families.
LENGTH OF ROSTRUM
Over time from their viverravid beginning, feliform carnivorans have evolved a progressively shorter rostrum. This shortening is associated with the reduction and loss of the anterior premolars (usually first and second premolars) and of the posterior molars (usually second and third molars). The net effect of this shortened rostrum is more posteriorly positioned incisors, canines, and premolars. With the movement of these teeth backward, a more powerful bite was achieved with the same muscles. Such an arrangement is not difficult to understand. When we humans try to crack a hard nut, for example, we tend to place it farther back in our mouths toward the molars, where our temporalis and masseter muscles (the main muscles for closing the mouth) can exert their maximum strength.
The biomechanical principle behind this arrangement is a simple leverage system. Mammalian jaws are anchored on the posterior tip, and this anchoring point (the mandibular condyle) acts as a hinge around which the jaws rotate (as in the common phrase “His jaw dropped”) (figure 4.8). The temporalis and masseter muscles, the main musculature for closing the jaws, are inserted in and around a deeply pocketed area called the masseteric fossa on the ascending ramus (vertical beam rising behind the last molar) of the jaws. The masseteric fossa is always in front of (anterior to) the hinge point, and contractions of the temporalis and masseter muscles acting on the masseteric fossa pull the jaws upward and forward. In such a leverage system, everything else being equal, the closer a food item is to the mandibular condyle (that is, the more posteriorly located the item is), the more powerful a bite on the item would be. Because the killing bite by a carnivoran is almost always delivered by the canine teeth, which are located toward the front tip of the upper and lower jaws, the shortening of jaws can be an effective mechanism in bringing the canines closer to the mandibular condyle, thereby increasing the power of the bite.
As noted, caniform carnivorans have remained conservative in their cranial morphology from the very beginning. Canids inherited this conservative plan and so started out with an unreduced set of teeth. To accommodate the full dentition, the rostrum is relatively long, with the incisors and canines positioned more forward. From this ancestral condition of a relatively long rostrum, canids were flexible enough to be able to evolve toward either a shortened rostrum or a more lengthened rostrum. Hypercarnivorous forms tended to acquire a shorter rostrum, paralleling the condition in cats, and a short rostrum can be seen in several groups of the hesperocyonines and borophagines, although neither ever achieved the extremely shortened jaws seen in the felids (figure 4.9). In the subfamily Caninae, however, a lengthening of the rostrum is evident from the very beginning of the lineage (Leptocyon), and this feature was passed down to all its descendants. A long rostrum increases the area of the nasal cavity (see “Turbinates”) and presumably enhances olfactory functions. Dogs are thus more smell oriented than cats, which are more vision oriented. A lengthening of the rostrum is sometimes also found in mammals that are ant or termite eaters because they use the added length of the rostrum and tongue to reach into narrow spaces. We may speculate about the food habits of early canine progenitors that may have been more insectivorous in their diet, but so far there is little evidence to confirm such speculation.

FIGURE 4.8
Gray wolf
Skull, mandible, and principal jaw-adductor muscles of the gray wolf (Canis lupus). Top, skull and mandible showing the position of the ascending ramus of the mandible, the masseteric fossa, and the mandibular condyle, which is the hinge around which the jaw rotates; center, the temporalis muscle attaches to the top and anterior margin of the ascending ramus of the mandible, and the main direction of its pull (large arrow) is upward and backward; bottom, the insertion of the masseter muscle occupies much of the masseteric fossa, and the main direction of its pull (large arrow) is upward and forward. The force transmitted to the teeth by the contraction of these muscles is greater if the teeth are located closer to the hinge of rotation. Consequently, the shorter a carnivore’s muzzle, the greater the force exerted at the canine tips during the bite.
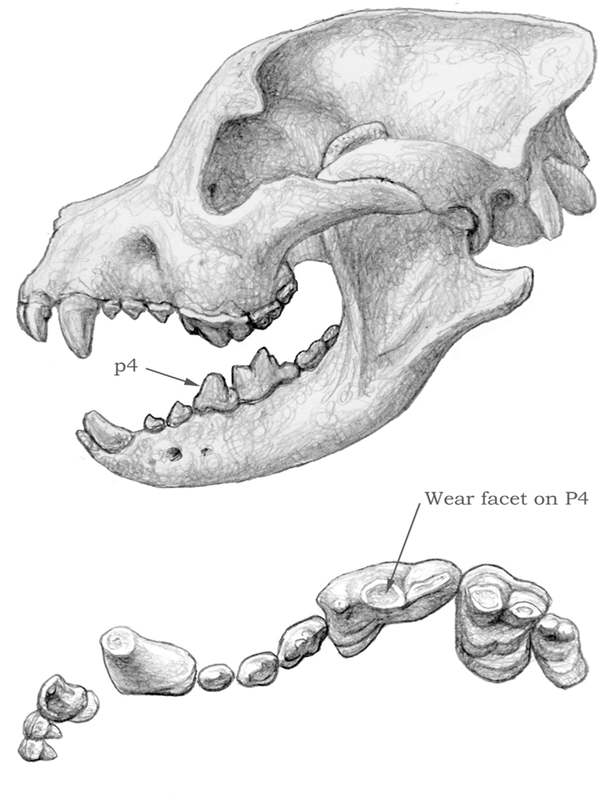
FIGURE 4.9
Borophagus
Skull and dentition of a bone-cracking dog, Borophagus. Notice the shortened rostrum, domed forehead, strengthened mandible, and robust teeth, especially the lower fourth premolar (p4). The upper carnassial (P4) shows horizontal wear associated with bone cracking.
STRENGTH OF JAW
The strength (or the cross-section area) of the jaws is another important factor in their mechanical capabilities. A shorter jaw is generally optimally suited for delivering a powerful bite. Although canid jaws have probably never been as powerful as felid jaws, the less powerful jaws in canids must have been more or less adequate as killing devices, judging from the canids’ success over a very long period of time. However, for those lineages that evolved bone-cracking dentitions, the jaws had to be strengthened in order to cope with the added stress. Thus it is in these lineages—such as Enhydrocyon, Aelurodon, and Borophagus—that the jaws became more massive, with a thickened horizontal ramus (see figure 4.9).
DOME OF HEAD AND FRONTAL SINUS
Hypercarnivorous forms, such as felids, generally have a shortened rostrum and a rather arched skull top as a result of an inflated forehead area. On a lateral (side) view, this arching can be seen as an upward swell of the skullcap above the eyes. Such a domed head may offer some advantages in its reinforcement of the bony structures that anchor the canine teeth. It is seen in all felids and, to a lesser extent, in hyaenids (except for the ant-eating aardwolf [Proteles cristatus]).
Large, hypercarnivorous canid species also tend to have enlarged foreheads. This shape is accomplished by inflation of the frontal sinuses, or the air spaces beneath the frontal bones. Through the expansion of the frontal sinuses, particularly by the upward swelling of the frontal bones, the head often displays a prominent dome in profile view. In extreme cases, the frontal sinus is extended backward toward the back tip (inion) of the skull, as seen in Borophagus diversidens, and, as a result, a large air space exists between the braincase and the parietal bones above it. Swedish vertebrate paleontologist Lars Werdelin (1989) has postulated that such a domed head in bone-cracking canids and hyaenids serves to transmit the great stresses of the premolars to the back of the skull, thereby reducing the bending stress of the rostrum (see figure 4.9).
Ear Bones
Bones in the ear region are the most intricate of the internal skeletal systems in mammals. Modern carnivorans typically have a hard-shelled, rounded bony housing in the head called the tympanic bulla, which, together with the tympanic membrane, holds three tiny ear ossicles (malleus, incus, and stapes) inside. Such a fully ossified enclosure of the middle-ear space is lacking only in the highly conservative African palm civet (Nandinia), which is a basal feliform distantly ancestral to all living catlike carnivorans, such as felids and hyaenids. The tympanic bulla in the palm civet is still partially formed by soft, cartilaginous tissue. A cartilaginous bulla is apparently the ancestral condition for all carnivorans. Indeed, all archaic carnivorans, such as miacids, lacked a bony bulla, and their fossil representatives always have a naked ear region without bony cover (it is possible that some of the early carnivorans may have had partially ossified bullae, but the bullae are not preserved in the fossil record because they were easily separated from the skull or destroyed during fossilization).
Canids were the first among living families of carnivorans to evolve an ossified bulla. From the very beginning, Prohesperocyon sported a hard-shelled tympanic bulla. Furthermore, all canid bullae are partially partitioned by a ridgelike structure called a semiseptum, which seems to work as a strut to reinforce the structural integrity of the bulla rather than to divide the bulla space. Felids, in contrast, have a full septum that divides the bulla into front and back chambers. Whatever the function of the septum, such a structural difference between canids and felids is a convenient way to distinguish these two families when the ear region is preserved in fossil materials (figure 4.10).
A rigid bony housing for the middle-ear space not only protects the delicate ear ossicles in their intricate linkage for sound transmission, but also maintains a fixed volume of air undisturbed by jaw movements (figure 4.11). Some jaw muscles are attached to the bulla, and when in motion, they would deform the bulla if it were flexibly constructed of cartilage; such a disturbance is probably not desirable because of its interference with hearing. The size of the bulla housing and thus the volume of air inside are optimized toward the hearing characteristics of a particular animal. A conspicuously enlarged bulla is present in the fennec fox (Vulpes zerda), of the Sahara and Arabian deserts, which also possesses a greatly enlarged external ear (pinna). A larger volume of air in the bulla appears to be related to the enhancement of low-frequency hearing (just as a larger audio speaker can resonate better for base notes). Enhanced low-frequency hearing in an open, desert environment has apparently been of adaptive value to the fennec fox.
Otarocyon, a basal borophagine canid from the early Oligocene (34 Ma) of western North America, also developed a strikingly large bulla for its small size (chapter 3; see figure 3.13). This extraordinary structure poses a tantalizing question: Why was such a big ear developed in the beginning of canid evolution? Was it in response to a more open, grassy landscape in the early Oligocene, as opposed to a closed, wooded environment in the Eocene (55 to 35 Ma)? Alternatively, could Otarocyon have hunted prey such as rodents and invertebrates in burrows by listening to the low-frequency sounds transmitted through the earth?
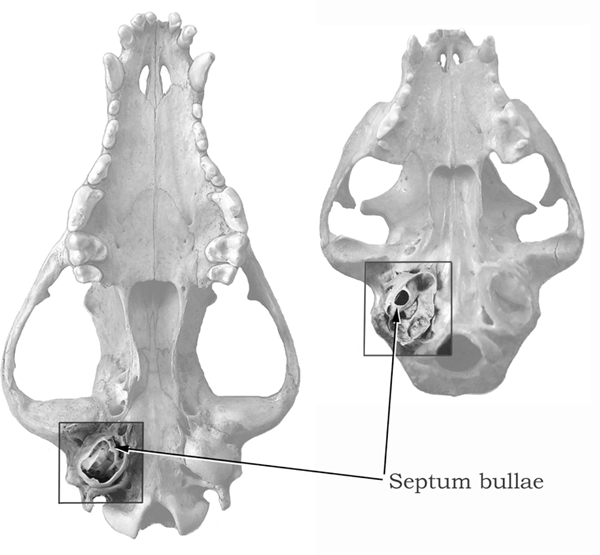
FIGURE 4.10
Comparison of canid and felid skulls
Ventral views of a canid skull (left) and a felid skull (right), with the auditory region highlighted. The bulla has been dissected to show the inner structure, with the dividing wall, or septum.
Turbinates
Modern dogs are famously known for their acute sense of smell; people marvel at rescue dogs and at the ability of sniff dogs to detect trace chemicals that are completely beyond detection by humans. What is not well known by the general public, however, is that the canid nasal cavity features an elaborate set of bones called turbinates, which are associated with odor detection and breathing (figure 4.12). Turbinate bones are delicate, paper-thin scrolls of bony tissue that are outgrowths of the inner walls of the nasal passage (including the maxillary, nasal, and ethmoid bones). The ethmoturbinates are covered mostly by olfactory epithelium, or thin layers of skinlike tissue exposed to the air passageway and packed full of olfactory receptor neurons that transmit the sensing of an odor to the olfactory bulb of the forebrain. Heightened olfactory sensitivity is actually a characteristic of all mammals, and thus the keen sense of smell in canids is inherited from its mammalian ancestors (the relatively obtuse sense of smell in humans is probably a reflection of our increased reliance on our optical senses, such as color and stereo vision).
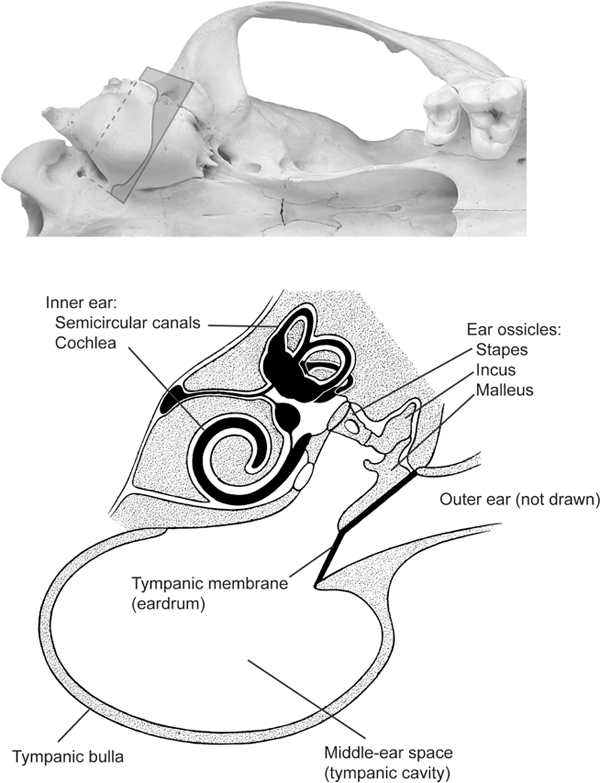
FIGURE 4.11
Anatomy of a canid ear
Ventral view of a coyote skull showing right auditory bulla (top); the rectangle across the bulla indicates the location of the transverse cross sectional view (bottom). The cross section (modified after Evans and Christensen [1979:fig. 19.3]) shows the relationship of the outer-, middle-, and inner-ear regions and three ear ossicles.
Maxilloturbinates and nasoturbinates are covered by respiratory epithelium, a vascular mucous membrane over which the inspired air passes. These turbinates function mainly to warm and moisten the air during inspiration and to conserve heat and water during expiration. On inspiration, the relatively cool and dry incoming air is warmed and saturated with water moisture as it passes over the moist, warm vascular maxilloturbinates. On expiration, the process reverses: warm and saturated air passes back through the just-cooled maxilloturbinates, helping to condense water onto their surface and thereby conserving water for the next breathing cycle. Some studies have suggested that as much as 80 percent of the heat and moisture are retained in the turbinates during expiration through the nose. In canids, the maxilloturbinates, the principal site for heat and moisture exchange, are greatly expanded. The complex and delicate scrolls of maxilloturbinates create an increased surface area within a confined space inside the nasal cavity, thereby increasing the efficiency of heat and water exchange. Such a complex of turbinates may have adaptive values for arid or cold environments, or both, although the size of the turbinates in canids does not strictly reflect the temperature and aridity of their environments. It is nonetheless interesting to speculate on the advantage of an elaborate maxilloturbinate system in arctic canids, such as arctic wolves and foxes, which have the widest distribution among any carnivorans in the vast frozen terrain in northern Eurasia and North America. The arctic faunal realm was an important component in canine evolution in the late Cenozoic (3 Ma) (chapter 6), and canines’ turbinates in this era may have played a key role in their survival in this harsh environment.
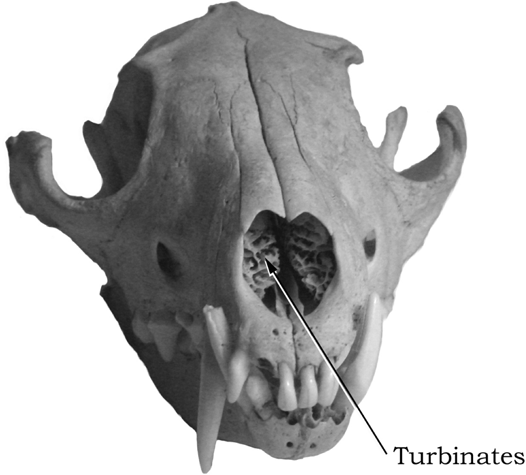
FIGURE 4.12
Red fox
Frontal view of the skull of an extant red fox (Vulpes vulpes), showing the turbinates inside the nasal cavity.
The complexity of canid turbinates is demonstrated in a third purpose for them: the cooling of the blood in the brain. Heat exchange on the maxilloturbinate surfaces cools the blood that supplies the brain via the heart, a process similar to panting in hot weather. In contrast, felids have a countercurrent heat-exchange mechanism that serves to cool arterial blood by immersing arteries within a venous sac called the rete mirabile, which is hidden behind the eyeballs. Maintaining a brain temperature cooler than the core body temperature during high physical exertion is important to all warm-blooded mammals that live in hot climates. Canids are capable of prolonged chases, in contrast to the common ambush predation of felids, and complex turbinates may well have played an important role in enabling this predatory strategy.
Canid maxilloturbinates thus appear to be valuable in both cold and hot climates, which may explain why the sizes of the turbinates in different species of canid do not strictly correlate with the environmental temperature. A large, complex maxilloturbinate system may be adaptive in either situation. It also explains canids’ long noses in contrast to felids’ short noses.
Bone Cracking
Although most of us tend to dismiss the bones on our dinner table as nothing more than a hindrance in our consumption of meat, bones are a source of high-value nutrition in the form of marrow. The center of a hollow long bone (usually various limb bones) is filled with yellow marrow, a fatty substance rich in protein and monounsaturated fats (many cultures use bones for flavoring in cooking because of this internal content). However, the bones themselves also contain an elaborate meshwork of calcium phosphate, and this same sturdy skeleton, built to withstand powerful exertion, is extremely tough to crack. Consequently, not all carnivorans can make use of bone marrow when they consume their prey.
To get at the nutritious marrow, one has to crack open the long bones, which commonly are the hardest bones in the entire skeleton because they bear an animal’s full weight. With the exception of some sea otters that use stones to crack shells, most carnivorans have to crack such bones with their own teeth if they desire what is inside the bones.
Teeth are the hardest biological materials produced in mammals because of the white shiny substance, called enamel, that coats the main body of the tooth (the crown). Made of a crystalline form of calcium phosphate, enamel is harder than bone. However, having a harder substance on the teeth is not enough to handle the bones. Teeth must be sufficiently massive to avoid being shattered during feeding. They also must be situated at the right location to deliver maximum pressure, and there must be a suitable musculature and jaw architecture to withstand the massive strain generated during bone cracking. The best example of such a bone-cracking adaptation is seen in the modern spotted hyena (Crocuta crocuta) of the African savannas. The animal has a massive jaw fitted with extremely robust premolars whose crowns are shaped like a short bullet up to 0.5 inch in diameter (figure 4.13). Because of the reduced number of molars in hyaenids, the premolars are located farther back to allow the best leverage during a bite. Feeding by modern hyenas is perhaps the most efficient of any carnivorans. A pack of hungry hyenas can quickly consume a carcass in a matter of minutes, leaving nothing behind, including the bones. The crushed bones will go through the hyenas’ digestive system and ultimately be excreted as small fragments.
To strengthen the bone-cracking teeth, the enamel’s microstructure is aligned to form a highly elaborate system of Hunter-Schreger Bands (HSB). The enamel in the HSB is woven in a pattern of crisscrossing crystalline fibers that stop cracks when they begin to develop due to high stress, much like a densely woven cloth can resist stress in all directions. Under high magnification (such as using a scanning electronic microscope), the enamel in bone-cracking teeth can be seen to form a zigzag pattern that maximizes their strength (figure 4.14).
During most of the canids’ evolutionary history, they were confined to the North American continent (chapter 7), where hyaenids were not present (only a single genus of hyaenids, Chasmaporthetes, managed to immigrate into North American in the Pliocene [around 5 Ma]). Several lineages of hesperocyonines and borophagines began to develop teeth and jaws capable of cracking bones. Some of them—such as Enhydrocyon, Ectopocynus, and Aelurodon—had multiple heavy premolars on the jaw, like the hyaenids. Others, such as Epicyon and Borophagus, developed a massive lower fourth premolar that was far taller and larger than the preceding premolars. This latter strategy of concentrating stress delivery on a single locus was apparently a highly successful one and gave rise to a long and diverse Epicyon–Borophagus lineage.
Standing Posture
Canids, like hyaenids and some felids, are often pursuit predators, securing food by persistent chasing of prey. That canids are good runners is well known, as seen in greyhound and dog sledge races. Typical of all good runners, canids have long, graceful legs. The advantage of a long leg is obvious: everything else being equal, the longer the legs, the longer the stride. The length of a stride is the distance between steps, and, given the same frequency of stride, a longer stride naturally leads to a faster speed. It is thus almost universally true that the leg length of all fast runners (predator and prey alike) tended to increase in evolution.
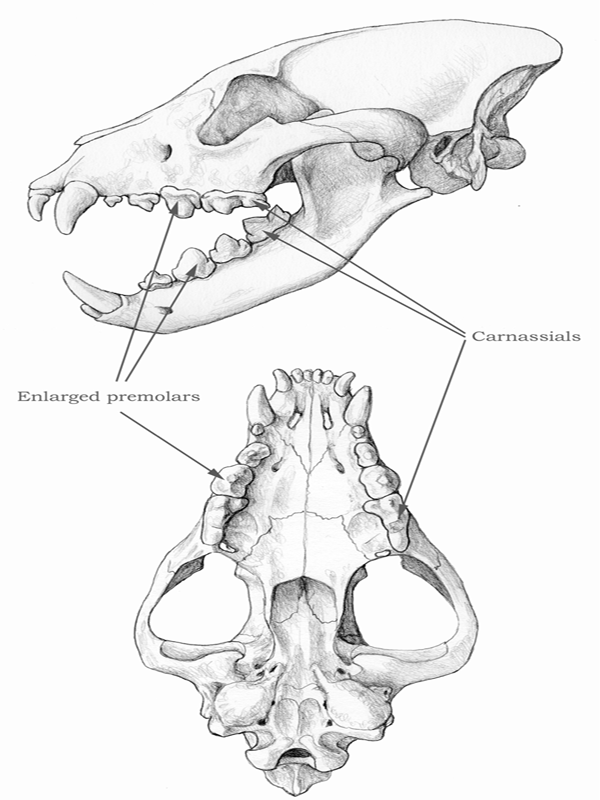
FIGURE 4.13
Spotted hyena
Skull of the extant spotted hyena (Crocuta crocuta). Notice the enlarged, robust premolars, which are placed far back in the jaws thanks to the space gained with the loss of the posterior molars.

FIGURE 4.14
Enamel on the tooth of Borophagus secundus
Enamel microstructure of an upper canine tooth of Borophagus secundus under scanning electronic microscope. Individual “fibers” in the enamel band are enamel crystallites that are woven in intricate ways (HSB) to strengthen the tooth. The image is taken on a cross section shown in the lower diagram. The white scale bar on the lower right corner equals 100 μm. (Image courtesy of John M. Rensberger)
To maximize the benefits of a lengthened leg, the lengthening is almost always concentrated in the distal segments of the limbs (those segments toward the finger and toe tips), rather than the proximal segments (those segments close to the body). For example, the tibia (shin bone) is proportionally more elongated than the femur (thigh bone). The reason for this disproportionate length is that the distal segments have less muscle mass attached to them, and the heavy, lifting muscles are concentrated mostly in the proximal segments. Less muscle means a lighter limb in the distal end, which reduces the angular momentum and thereby eases the muscle exertion required for swinging the limbs.
The adoption of an increasingly more erect posture also increases stride length. Broadly speaking, there are three categories of standing posture: plantigrady, digitigrady, and unguligrady. In a plantigrade posture, the animal walks on the entire palm and sole, and the main joint that marks the beginning of the foot is the ankle joint, as in humans and bears. In a digitigrade posture, however, the animal walks on the fingers and toes, and the palm and heel parts of the hands and feet are lifted into the air. Thus the main joint is the joint between the base of the finger/toe bones and the metapodials just before the former. Among carnivorans, the canids, felids, and hyaenids have adopted a digitigrade posture (figure 4.15). The immediate benefit of such a posture is that a segment of the proximal hand and foot (the metacarpal and metatarsal, respectively) is converted into a segment that contributes to the stride. In an unguligrade posture, the animal walks on the very tip of the fingers and toes (distal phalanges), thus lifting up an additional two segments of the digits (the proximal and medial phalanges). The word unguligrade derives from ungulate, which refers to all hoofed animals that use their terminal phalanges for walking and running, including such well-known examples as horses, sheep, and cows. Thus in the unguligrade posture, the conversion of limb segments is literally and figuratively pushed to the extreme, as in the case of a ballet dancer. The best example of an unguligrade is the horse: its hooves are equivalent to humans’ terminal fingers and toes with nails; the horse stands literally on the very tips of its middle “finger” and “toe.”
In general, digitigrade animals’ hands and feet can be recognized by closely packed or narrower metacarpals and metatarsals, in contrast to the more spread-out appearance of plantigrade animals’ hands and feet. Thus in the former, the metacarpals and metatarsals are nearly parallel to each other, and the distal ends of these bones are nearly touching each other.
The benefit of a more erect posture for runners is fairly obvious: stride length is instantly gained without additional anatomical complexity. But if such a benefit is universal, why didn’t canids (or, for that matter, felids and hyaenids) become unguligrade, as did almost all their prey? Doesn’t a digitigrade or plantigrade posture place a disadvantage on the predators, which have to settle for shorter legs? The answer appears to lie in the fact that carnivorans had to balance their need to increase speed with their need to use their claws as tools for manipulating the prey. A horse’s hooves are nothing but a pad to cushion its steps, and a horse does not have to use its hooves other than for walking and running. Carnivorans, in contrast, use their claws (equivalent to a horse’s hooves) for manipulating, digging, climbing, and even killing.
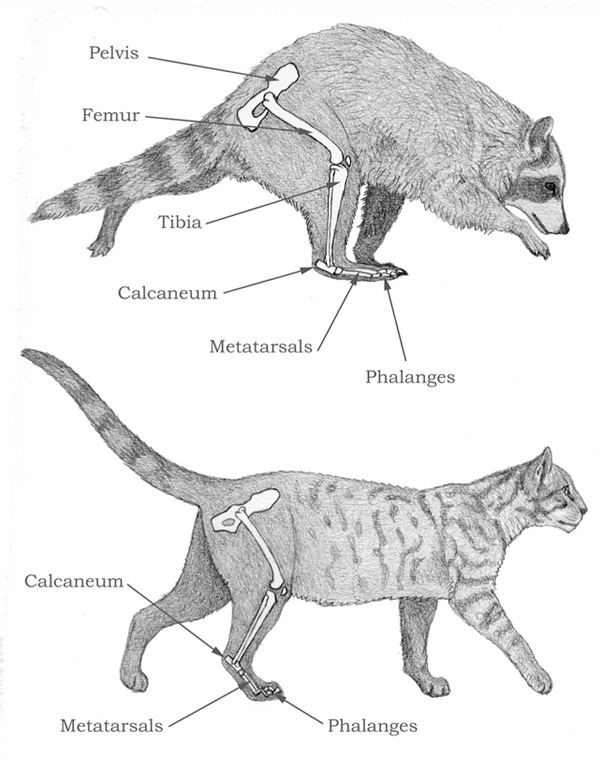
FIGURE 4.15
Comparison of plantigrade and digitigrade posture
Comparison between a plantigrade carnivore, the raccoon (top), and a digitigrade carnivore, the house cat (bottom). Notice that when the foot carries the weight of the body, the calcaneum, or heel bone, is high above the ground for the cat, but close to the ground for the raccoon.
Another important development in fast runners is the loss of peripheral digits. The digits that are not central to bearing weight were reduced and ultimately lost in all ungulates through much of their evolutionary history. Again, a horse serves as a good example. Modern horses have lost all but the single central digit (the middle finger and toe).
From the beginning, canids (such as Hesperocyon) had already acquired a fairly advanced hand and foot, with closely appressed fingers and toes, indicating a semidigitigrade posture. But Hesperocyon retained a relatively well developed first digit (thumb and big toe) and a relatively deep claw. The first digits in later canids were progressively reduced, and the claws became smaller. All modern canids show the loss of a functional first digit, and their hands and feet have four fingers and toes.
In summary, all runners have a combination of lengthened distal limbs, more erect posture, lost lateral digits, and reduced muscle weight in the distal segments. As herbivores, ungulates can often push all these components to the extreme, whereas carnivorans must balance the demand for speed with the need for manipulation of food.
Claws
Claws generally serve three important functions in carnivorans: to help secure prey, to grasp trees when climbing, and, for some, to dig holes in the ground. Recent studies show that ancestral carnivorans were arboreal in the early Cenozoic (around 55 Ma). They had relatively short, strong arms and legs for climbing up and down trees, and flexible wrist and ankle joints for greater degree of rotation of their hands and feet—all necessary for a tree dweller. Often associated with arboreality are deep (in a side view) and sharp claws that can sink into tree bark for a firm grasp. Such sharp claws also come in handy for carnivorans that have to tackle prey of large size.
Claws are formed by a horny sheath (made of the same rigid material as the fingernails of humans) and have bony support beneath the terminal phalanx, or the last segment of the finger bones. The horny material consists of keratin, a colorless protein of the same origin as skin, hair, and horn sheathes in all mammals. Although claws are strong enough to resemble hard tissues such as bones, almost no claw is preserved in fossils because the keratin proteins usually decompose quickly after death. Thus we can only deduce claw shapes from the shape of the underlying bony terminal phalanges (figure 4.16).
One of the most important properties of claws is that they can be retracted into the back of the hands and feet. Retractile claws are an efficient way to preserve the sharpness of the tips while an animal is walking and running on land. Almost all felids have retractile claws that are pulled back when the felids are moving on the ground. They extend their claws when climbing trees or securing prey. The mechanisms that permit a retractile claw are a combination of an elastic lateral dorsal ligament, which passively pulls the claws backward toward the medial phalanges (the segment of finger bone immediately behind the terminal phalanges), and a deep dent on the dorsal surface of the medial phalangeal bone, which accommodates the pulled-back claws. This dent creates an asymmetrical appearance on the medial phalanges when they are looked at from above. Fortunately, this asymmetry is readily apparent in fossil foot bones, allowing paleontologists a ready means to distinguish between retractile and nonretractile claws.

FIGURE 4.16
Comparison of felid and canid phalanges
Phalanges of the central digit of the right forefoot in a cat (left) and in a dog (right), shown disarticulated in dorsal view. The first phalanges are at the bottom of the figure, and the third ones (claw phalanges) are at the top. Notice that the dog’s claw is less compressed laterally and that the cat’s second phalanx has a marked concavity in the lateral margin (marked by an arrow), which is where the third phalanx fits during retraction of the claw. The dog’s second phalanx, in contrast, is almost completely symmetrical.
As noted, a retractile claw originally evolved in ancestral carnivorans as a function of tree dwelling. As later carnivorans quickly discovered, sharp claws are also potent weapons in securing their prey. For example, a modern lion can ride on top of a wildebeest, rodeo style, using its sharp claws to grab hold of its prey’s skin, while trying to deliver a killing bite on the neck. This ability allows a felid to secure its prey by itself. In contrast, canids are unable to cling to their prey with their blunt claws.
In an interesting twist of evolutionary history, canids apparently failed to develop retractile claws early in their history. As they became increasingly more cursorial through the lengthening of their limbs and the adoption of a more erect posture, they lost the ability to retract their claws. According to the fossil record, this change occurred shortly after Hesperocyon, which still possessed a moderately deep claw, but had lost some of the asymmetry of the medial phalanges. From this point on, canids never regained the ability to retract their claws. Their claws protrude during all stages of walking and running, so the claw sheathes become blunt because of excessive wear on the ground. (One can often hear a dog’s footsteps as its claw tips hit the ground, but not a cat’s footsteps, which are silent because the claws are retracted).
Blunt claws are probably not a great hindrance when dealing with prey that are substantially smaller than the predators themselves. These prey can be overpowered by sheer size. When it comes to grappling with larger prey, however, particularly those that are much larger than the predators themselves, the lack of sharp claws can be a shortcoming. In this regard, canids seem handicapped when compared with felids, which as individuals are more capable of securing prey. Such a handicap may be one of the reasons why canids are primarily social hunters, which gives them an advantage over prey (chapter 5).
Neck
In recent years, research by Mauricio Antón (Antón and Galobart 1999) has demonstrated the importance of neck bones and musculature in mammalian predators. There has been considerable variation in the relative length and muscularity of the neck among both extant and extinct dog species, and some of the observed differences are likely to reflect different adaptations. Most species of modern canids have relatively long, straight necks, well muscled but relatively weaker than cats’ necks, which are short and have a strong S curve. Due to the properties of muscle tissue, a shorter muscle contracts more efficiently than a longer muscle, so, other things being equal, a shorter neck can exert more power with less effort. That is why cats’ short necks contribute so well to their positioning the prey’s head and keeping it in place during the killing bite. This function was further emphasized in saber-toothed cats due to the large size of their prey and the need to avoid unexpected stresses during the bite, which could damage the cats’ fragile upper canines.
Not only are dogs’ necks longer than cats’ necks, but the processes for muscle insertion on each cervical (neck) vertebra are smaller in dogs, indicating less powerful muscles. One way in which modern dogs compensate for the mechanical disadvantage of their long necks is the possession of the nuchal ligament, comparable to the one found in ungulates (figure 4.17). The nuchal ligament stretches along the back of the neck from the tips of the anterior thoracic vertebrae, and it helps to support the weight of the head without the need for active muscle contraction, thus saving energy. (There is one important difference, though: in ungulates, the ligament attaches on the back of the skull, but in dogs it attaches on the back of the axis, or second cervical vertebra, so the term nuchal is not so appropriate for this feature in dogs.) No other extant carnivore has a nuchal ligament, and it is intriguing to speculate about the origin of this structure in dogs. Fossils show that some dogs had relatively shorter necks, however, with more developed muscle insertions, and, judging from the morphology of their axis vertebra, lacked the nuchal ligament.
Early members of the subfamily Caninae were the first to show clearly the modern neck morphology associated with the presence of the nuchal ligament, suggesting that the structure is unique to this canid subfamily. Another distinctive trait of such early canines as Eucyon was the lengthening of the leg bones, especially the distal elements of the forelimb, such as the radius and metacarpals. As we have seen, this adaptation occurred for more efficient running, and in the case of Eucyon it most likely had to do with the need to forage across larger territories in search of prey because Eucyon’s habitat was becoming drier and more open, and prey were ever more dispersed. It is tempting to see a connection between the development of longer legs and of a longer neck, but other carnivorans with long legs, such as the cheetah, have short necks. Can we still see a functional connection between these changes?
Indeed, dogs’ foraging method differs from cats’ method in being more scent oriented. Cats depend largely on their eyesight and hearing to detect prey, but, as we know, dogs must “follow their noses” to locate prey. As early canines’ legs became ever longer, a longer neck was necessary to allow them to follow scent trails with their nose close to the ground. The nuchal ligament became an advantageous feature in allowing canines to keep this posture for long periods of time, and the neck muscles in general became reduced as a way to save weight and energy. Reduced muscle power in the neck was not a great problem for these early canines, which hunted relatively small prey. Larger, later members of the family paid a price for the loss of strength in their neck muscles and for the feebleness of their forelimbs, which, although better suited for running, were less suited for handling prey. Nonetheless, these shortcomings were amply compensated for by group-hunting techniques, which all the larger, hypercarnivorous species of modern dogs share.
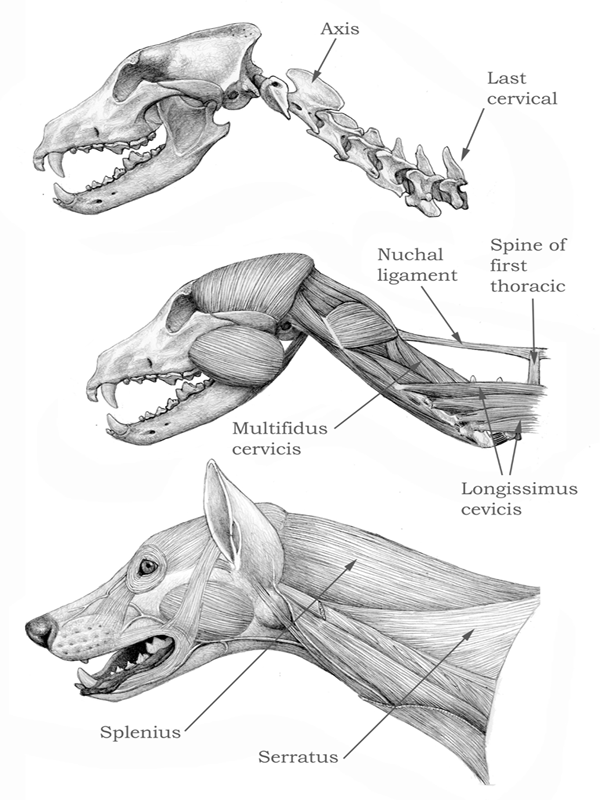
FIGURE 4.17
Gray wolf
Skull and cervical vertebrae (top), deep muscles (center), and superficial muscles of the neck and head (bottom) of the extant gray wolf (Canis lupus). The neck is long and straight, and the nuchal ligament (extending between the spines of the anterior thoracic vertebrae and the back of the axis, or second cervical) helps to keep the neck raised without much muscular effort.
A different condition has been found in the fossils of some species of hypercarnivorous dogs. The neck of Aelurodon is a dramatic example (figure 4.18). Compared with a modern wolf (see figure 4.17), Aelurodon displays a remarkably short, S-shaped neck, with developed processes for muscle insertion, and the shape of its axis suggests the absence of the nuchal ligament. Such features would have been advantageous for such a hunter of large prey and, coupled with relatively shorter, more flexible, and more muscular forelimbs, would have made it more capable of dealing with large prey when hunting either by itself or in small groups. Such a design was easier to develop because the ancestral borophagines had retained a relatively primitive neck design, lacking the nuchal ligament. Modern wolves, dholes, and African hunting dogs would also benefit from such a cervical anatomy, but they are constrained by the morphology of their smaller ancestors. Even so, a detailed study of skeletal proportions (Hildebrand 1952) showed that these three species of big-game hunters have slightly shorter necks than would be expected for their size, when compared with their smaller relatives, such as jackals and coyotes. It would seem, therefore, that some “evolutionary adjustment” is under way.
Brain
Although brains are almost never preserved in the fossil records, they leave a distinct impression on the inner surface of the braincase in the form of ridges and grooves (gyri and sulci, in specialist terminology). Paleontologists can make latex endocasts (replicas) of these impressions, and the results are fairly detailed brain models for comparative purposes. Although paleontologists described natural molds of Hesperocyon 70 years ago (Scott and Jepsen 1936) (when braincase bones are broken in just the right way, the brain morphology is often revealed by such a mold), Leonard Radinsky (1969, 1973) was the first to explore systematically the comparative brain morphology in canids.
The ancestral canid Hesperocyon from the late Eocene to the early Oligocene (35 to 30 Ma) had relatively simple foldings and an underdeveloped frontal region of the brain. This was probably a primitive condition for all carnivorans. By the early Miocene (22 Ma), most canids had an expanded prefrontal cortex of the orbital gyrus immediately behind the olfactory bulb, and this trend continued with further expansions in later canids. Radinsky noticed the presence of a dimple in the coronal gyrus (near the frontal region) in three hypercarnivorous species of social hunters: Canis lupus, Cuon alpinus, and Lycaon pictus. He speculated that a relatively large prorean gyrus in the prefrontal cortex may be related to pack-hunting behavior. Although such studies may suggest interesting patterns, we caution that behavioral patterns are often difficult to correlate with external brain morphology.

FIGURE 4.18
Aelurodon mcgrewi
Skull, cervical vertebrae, and neck muscles of the borophagine dog Aelurodon mcgrewi. Not only were A. mcgrewi ’s neck muscles stronger than the wolf ’s, as shown by the larger processes for attachment in the vertebrae, but the smaller length and the strong S curve of the neck in Aelurodon imply that deep extensor muscles, such as the multifidus cervicis, had a much shorter span from origin to insertion and thus offered a great mechanical advantage. Processes for insertion of other muscles that extend the neck and flex it to the side, such as the longissimus cervicis, extended farther to the sides in Aelurodon, allowing these muscles to twist the neck more efficiently. Even the more superficial extensors, such as the splenius and serratus muscles, were more efficient thanks to their shorter spans.
John L. Gittleman (1986) did an extensive study of relative brain sizes of various carnivorous species and found a significant difference among families of Carnivora. Canids are relatively “brainier” than most other carnivorans except for the ursids. Gittleman attributed such a difference to historical developments in various families of the Carnivora during their early evolution. He also found that the more carnivorous groups (those that ate more meat than other foods) also had larger brains. He speculated that increased brain size in carnivorous species was due to their more complex foraging strategy, involving selection for rapid prey detection, pursuit, capture, and consumption.