Up to this point, we have discussed a number of mechanisms that may explain how the mentally ill brain is different from the normal brain. The immune system is increasingly being viewed as a possible culprit. The body’s reaction to foreign invasion (or mistaken foreign invasion) and the resulting inflammatory response may contribute to the development of some psychiatric disorders.
For a long time, there have been indications of subtle links between mental illness and the immune system. The first antidepressants were developed in the 1950s when it was noted that the antitubercular medication iproniazid had a positive effect on mood. When treated with this medication, even some terminally ill patients became more cheerful, physically active, and hopeful. Ultimately this antibiotic was refined and transformed into the first effective antidepressant: the monoamine oxidase inhibitors.
In 1929 the Swiss psychiatrist Tramer reported an increase in schizophrenic births during the months from December to May. Tramer noted that children born during the winter/spring months were more likely to develop schizophrenia as adults. It is postulated that the seasonal fluctuation is influenced by exposure to infections during the perinatal period, which are more common during the winter months—more on this topic later in the chapter.
Perhaps the oldest link between infection and psychiatric disorders lies with syphilis. This microbe was the cause of about 20% of the hospitalizations for mental illness at the beginning of the last century. In 1910 it was Paul Ehrlich’s “magic bullet,” compound 606, that became the first effective medication for syphilis—the beginning of psychopharmacology. The microbe that causes syphilis, Treponema pallidum, provides a good example of the immune response and the problems this response causes.
THE IMMUNE RESPONSE
The body’s immune system is poised to recognize and attack foreign invaders—anything that is nonself: splinters, bacteria, Fox News, etc. The attack is conducted by a confusing array of cells and proteins—some of which stand guard at all times, while others remain dormant until the alarm is sounded. All the components interact with each other through a litany of signals to rally the defenses when a microscopic terrorist is identified. Likewise the defenses must be turned off when the coast is clear. For the purposes of this book, we can simplify the immune response down to four major components:
1. Cellular—white blood cells (leukocytes)
2. Humoral—antibodies
3. Complement—little torpedoes
4. Cytokines—the molecular signals
All four of these components come into play when the syphilitic bacteria T. pallidum invades the body.
Syphilis
Syphilis is a sexually transmitted disease caused by the corkscrew shaped bacteria T. pallidum (Figure 9.1). When a contagious individual has sex with an uninfected partner, there is about a 30% chance the disease will be transferred. The body’s first line of defense against infection is the skin. T. pallidum spirochete penetrates the dermal barrier by twisting through microabrasion.
The body’s next line of defense is the complement system (Figure 9.2A). The complement system is made up of numerous proteins circulating in the blood poised to coalesce into little torpedoes. When activated by foreign proteins, such as those found on bacteria, the complement molecules lock on to the bacteria. As more and more of these proteins accumulate on the bacteria, the complement can literally drill a hole in the cell wall. This allows fluid to pore in and the bacteria explodes. The trick is to limit the attack to the foreign invaders—to avoid attacking host cells.
Also standing ready to fend off foreign invaders are a variety of white blood cells (leukocytes). Two of these, the macrophage and the neutrophil, are frontline defenders. The macrophage patrols the perimeter of the skin and mucus membranes cleaning up debris and searching for bad guys. (They also are one of the cells that will present foreign antigens to T cells to start the production of antibodies.) Neutrophils circulate in the blood ready to come to the aid of defenseless tissue. They aggressively attack T. pallidum but generally also make a mess of the healthy tissue. The initial physical sign of syphilis—the chancre that develops on the genitalia at the site of the infection—is in large part due to neutrophils. Pus is predominantly dead neutrophils—suicide bombers at the cellular level.

FIGURE 9.1  The bacteria that causes all the problems of syphilis is this annoying spirochete Treponema pallidum seen here in an electron micrograph. (From Centers for Disease Control and Prevention.)
The bacteria that causes all the problems of syphilis is this annoying spirochete Treponema pallidum seen here in an electron micrograph. (From Centers for Disease Control and Prevention.)
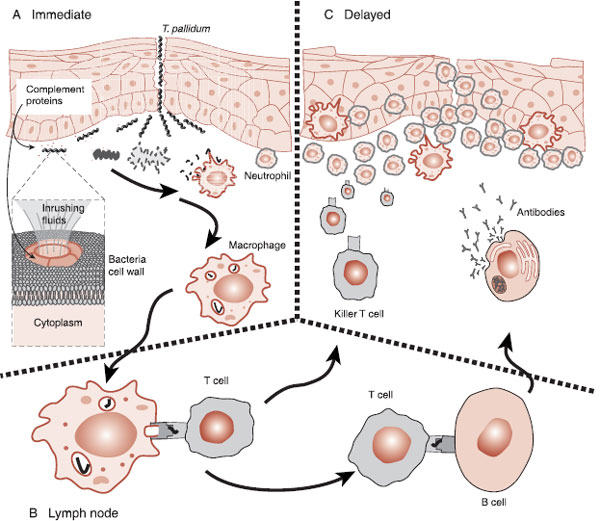
FIGURE 9.2  A. When bacteria invade the body, complement, macrophages, and neutrophils are the first defenders. B. Antigen presenting cells take pieces of the bacteria (antigens) to lymph nodes when the B cells and T cells are activated. C. T cells and B cells, specifically matched to the antigens of the bacteria, follow the cytokines to the site of the injury.
A. When bacteria invade the body, complement, macrophages, and neutrophils are the first defenders. B. Antigen presenting cells take pieces of the bacteria (antigens) to lymph nodes when the B cells and T cells are activated. C. T cells and B cells, specifically matched to the antigens of the bacteria, follow the cytokines to the site of the injury.
After the first phases of syphilis, most people enter a latency phase—usually within 6 months. During this phase, they do not have signs or symptoms of the illness and are not contagious. However, microbes remain hidden within the body and laboratory studies are still positive. Remarkably, the T. pallidum can quietly live undetected in the host for years. How is this possible?
The best explanation for the stealth capabilities of T. pallidum is the relative inertness of the outer membrane. The proteins that protrude through the phospholipid bilayer cell membrane wall (called integral proteins) are few and far between in T. pallidum. It is these proteins that killer T cells and antibodies grab when they attack the bacteria. In comparison, T. pallidum has only 1% of the integral proteins compared with Escherichia coli. So, this bacteria can remain hidden, under the radar so to speak, and not be recognized as different—like little terrorist sleeper cells.
If left untreated, approximately one-third of the people infected with syphilis will cure themselves, another third will remain in the latency phase until they die, and the last third will develop tertiary syphilis—the most serious phase of the infection. Of particular interest for us is neurosyphilis. Neurosyphilis includes inflammation of the meninges, cerebral vasculature, or neural tissue of the brain and/or spinal cord. Although animal studies have shown that T. pallidum can find their way to the central nervous system (CNS) within 18 hours after the initial infection, the serious manifestations of neurosyphilis do not develop for 10 to 50 years.
The important point is this: once the immune system catches up with syphilis in the CNS, it is the inflammatory response—not the bacteria per se—that damages the brain. T. pallidum does not produce a toxin nor directly attacks the cells of the nervous system. It is the overexuberant cellular response to T. pallidum that blocks the vascular support and kills the neurons. The military calls this collateral damage. The incessant neural loss once the immune system is activated leads to personality changes, psychosis, and dementia as well as paralysis, seizures, and death.
TREATMENT
COCAINE VACCINE
Cocaine dependence is a major health problem. There is no Food and Drug Administration (FDA)–approved pharmacotherapy. One research group has developed a vaccine that stimulates the immune system to produce anticocaine antibodies. These antibodies can sequester circulating cocaine and prevent the substance from entering the brain and quelling any euphoria. Early studies have found reduced cocaine intake for addicts whose body produced sufficient antibodies. Future research is focused on eliciting a stronger and more sustained immune response in more patients.
Inflammation
The inflammatory response is the body’s overall reaction to foreign invasion. The signs are swelling, redness, heat, and pain. The mechanisms that cause the inflammation are engorgement of the blood vessels, movement of fluid into the tissue, and, above all, attraction of leukocytes to the infected area. The signals that start the inflammatory process and coordinate the response are the cytokines. (More on the cytokines later in the chapter.)
Adaptive Immunity
A large part of the success of the mammalian defense system results from the capacity of the system to proliferate and attack specific and unique molecular features of the invader. This adaptive immunity commences when pieces of the destroyed bacteria are taken to the lymph nodes and presented to T cells—essentially shopped around to find a good match (Figure 9.2B). When a close match is found, the T cells multiply and head off to attack that specific remnant of the invader, but not before activating the B cells. The B cells with a similar match will start producing antibodies. Antibodies are the “Y”-shaped proteins that can lock on to the specific foreign molecules of the bacteria, virus, or infected cell. Antibodies attached to a foreign cell serve as a signal to other leukocytes, essentially saying that this cell is ready for demolition (Figure 9.2C).
The important points to grasp are the varied mechanisms the body uses to defend against foreign invasion and the need for numerous checks and balances to keep the response under control, that is, to avoid damaging the host cells. As with any battle, “civilian casualties” are unavoidable. War, even at the microscopic level, is a dirty business.
IMMUNITY IN THE BRAIN
The immune response inside the brain is less aggressive than the response normally seen in the rest of the body. Constraining the response is essential because the rejuvenating capacity of the brain is limited and the effects of collateral damage from inflammation are more serious. White blood cells are rarely seen in the CNS. But the brain is not defenseless. Although once thought to be “immune privileged”—that is, able to tolerate the presence of an antigen without eliciting an inflammatory response—the brain is now recognized to have a unique but muted immune response to invaders.
The brain has three layers of protection against foreign invasion.
1. The blood–brain barrier—physical barrier to isolate the brain.
2. Microglia—the macrophage of the brain.
3. Leukocytes infiltration reserved for serious damage.
The distinguishing feature of the CNS immune system is the microglia. The microglia, although considered a nerve cell, actually originate from a different cell line. While neurons, oligodendrocytes, and astrocytes develop from cells of neuroectodermal origin, the microglia come from mesenchymal stem cells (bone, cartilage, and blood cells). Microglia move in and take up residence among the neural cells very early during embryonic development.
The microglia are most closely related to the macrophage and are the central component of the brain’s innate immune system. Like the macrophage, they are dormant until activated, can migrate to the battle zone, and attempt to gobble up whatever should not be there. The microglia respond to chemical signals of danger, emit pro-inflammatory cytokines, and can function as antigen presenting cells—similar to the macrophage. In addition, microglia can also release cytotoxic substances such as hydrogen peroxide to damage the invader. Unfortunately, the hydrogen peroxide also kills neurons.
Neural Prostheses
The protagonist of the 1970s television series The Six-Million Dollar Man was a NASA astronaut whose injured body was rebuilt with mechanical parts. Oh, if only it was this easy. Forty years later, real researchers are still struggling with the basics. A device that could restore simple movements for people with spinal cord injuries would be of great value to those confined to a wheelchair. While still very much in the experimental stages, the goal is to capture the individual’s neural signals generated in the motor cortex and turn them into useful movements (Figure 9.3).
Electrodes implanted directly into the gray matter of the motor cortex are an intrusive element needed to determine the person’s intentions. The electrode captures the electrical activity as the neurons generate action potentials and the signals are sent to a computer. The computer—after substantial training—decodes the signals and moves the external device—in this case a mechanical arm. Problems develop as the brain’s immune system produces a sustained reaction against the foreign body electrode.
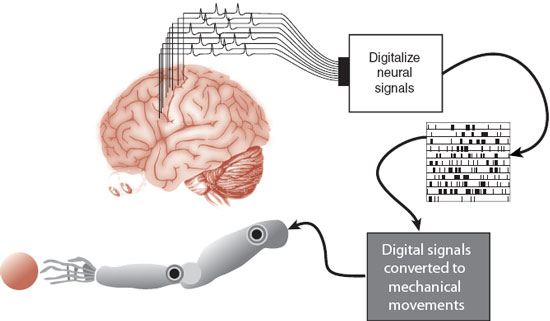
FIGURE 9.3  Electrodes in the motor cortex capture the neural activity of purposeful movement. The signals are digitalized and the computer learns to turn the impulses into movements of the mechanical arm.
Electrodes in the motor cortex capture the neural activity of purposeful movement. The signals are digitalized and the computer learns to turn the impulses into movements of the mechanical arm.
Indwelling Electrodes
The gray matter of the cortex is dense with blood vessels. Invariably as the electrodes are pushed into the brain, the vasculature is ruptured. This breaches the blood–brain barrier and allows the entry of inflammatory proteins as well as macrophages, neutrophils, and T cells. In short, the wound healing process is initiated and neurons—the ones the electrode needs to record—can be damaged or destroyed. While the bloodborne inflammatory response is the most serious, microglia and astrocytes become activated regardless of the vascular damage (Figure 9.4).
Microglia, in their resting state, look like stars with elaborate branching structure. When they sense trouble, they multiply and morph into rounder structures. They also increase their production of cytokines and growth factor proteins. In this activated state, they engulf and digest the cellular debris generated by the injury—acting like their macrophage cousins from the periphery.
Astrocytes usually support the neurons and maintain the integrity of the blood–brain barrier. In response to injury they too become activated. Their main function in response to trouble is to isolate the problem. They produce and release a fibrous substance that plays an essential role in forming a physical barrier around the injury. This barrier is commonly called the glial scar. This is a problem for the whole contraption, since the scar impedes contact between neurons and the electrode.
Glial Scar
Reactive gliosis, the formation of the glial scar, helps to isolate the injury, prevent overwhelming inflammation, and limit cellular degradation. The importance of gliosis was established in mice whose astrocytes were depleted. Small stab wounds in their spinal cords resulted in greater blood–brain barrier disruption, larger leukocyte infiltration, increased neural loss, and more motor deficits than comparable mice with normal astrocytes.
Clearly, the glial scar is essential to limiting CNS damage after trauma. Unfortunately, there is a price to pay. Neurons of warm-blooded animals will not regenerate through the glial scar. This is why patients with severed spinal cords remain paralyzed. The axons on either side of the injury are prevented from reconnecting by the glial scar. Treatments in the future may dissolve the scar once the injury is stabilized, thus allowing axons to regenerate.
CYTOKINES
Cytokines are known as the “hormones of the immune system.” These chemical messengers orchestrate the direction, magnitude, and duration of the inflammatory response. Virtually all the immune cells produce cytokines, although the type and function vary. First discovered in the late 1960s, the number of known cytokines has exploded fills large, small fonted tables. Reasonable readers avoid such material. Suffice it to say that the major cytokines are interferon (IFN), interleukin (IL), and tumor necrosis factor (TNF), although each type has numerous variants designated with numbers or Greek letters.
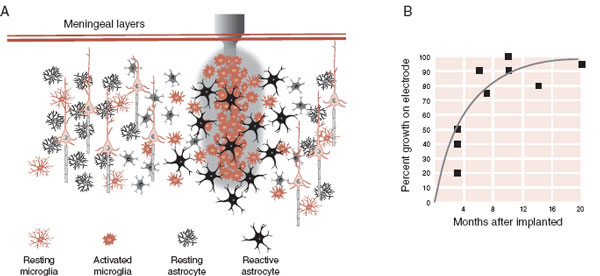
FIGURE 9.4  A. Electrodes implanted in the gray matter elicit an inflammatory response. Activated microglia and astrocytes produce a “scar” that isolates the electrode. B. Within 6 months, 75% of the electrode is surrounded by glial cells. (Adapted from Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J Neurosci Methods. 1998;82:1-15.)
A. Electrodes implanted in the gray matter elicit an inflammatory response. Activated microglia and astrocytes produce a “scar” that isolates the electrode. B. Within 6 months, 75% of the electrode is surrounded by glial cells. (Adapted from Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J Neurosci Methods. 1998;82:1-15.)
Cytokines coordinate the entire response to an infection—from generating a fever to promoting wound healing. However, the primary functions are activating and guiding immune cells. Some of the cytokines activate the white blood cells—induce the cellular feeding frenzy. Other cytokines, in the presence of an antigen, activate T cells and B cells to multiply and go find the pathogen.
Cytokines play an essential role in guiding the immune cells to the battlefield. Activated cells follow the “scent” of the cytokines to their destination (Figure 9.5). Endothelial cells in the blood vessels respond to cytokines by expressing adhesion molecules that “grab” immune cells floating by in the blood. The cells then transform, squeeze through the blood–brain barrier, and follow the scent of the cytokines to find the pathogen.
An essential feature of fighting any infection is the ability to turn off the inflammation when the job is done in order to limit injurious side effects. Cytokines are turned off in several ways. First, cytokines have a short half-life and quickly dissipate anyway. Second, production of cytokines is reduced as the offending antigens disappear. Finally, some cytokines are actually anti-inflammatory and are produced to turn down the immune response.
Neural Plasticity
Cytokines have important functions outside of their role coordinating the immune response. Some of the cytokines of the IL-6 family are known to have signaling functions during development of the normal brain and in response to brain injury. Studies have shown that these cytokines can
- induce differentiation of neural stem cells,
- promote axonal regeneration,
- promote maturation and survival of oligodendrocytes, and
- influence synaptic plasticity.
These unexpected discoveries have generated interest in potentially manipulating cytokines to prevent illness or promote healing. But which cytokines need to be neutralized and which ones enhanced is not easy to determine.
Neurogenesis provides an example of the complexity of manipulating cytokines to promote CNS healing. After a stroke, theoretically it would be beneficial to accelerate neurogenesis. The formation, migration to the site of the injury, and the differentiation into functioning neurons hold great potential as a means to help people recover after cerebral ischemia. Unfortunately, the inflammatory response, also occurring after a stroke, blocks neurogenesis. Consequently, controlling the inflammatory response has been one target postulated for enhancing neurogenesis and recovery. But, here is where it gets complicated; some of the cytokines (such as IL-4 and IFN-γ) also released as part of the inflammatory response actually induce neurogenesis.
The studies with traumatic brain injury (TBI) and stroke highlight the current dilemma with cytokines. They not only contribute to long-term damage but also facilitate healing. Interventions in the future will likely neutralize some cytokines while allowing others to work their magic.
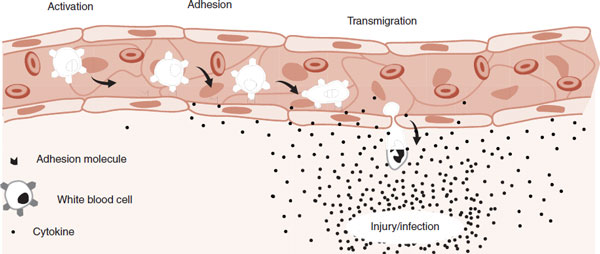
FIGURE 9.5  White blood cells in the capillaries are attracted to the site of an injury. Cytokines released at the injury activate a process that enables leukocytes to adhere to the endothelial cells and then migrate to where they are needed.
White blood cells in the capillaries are attracted to the site of an injury. Cytokines released at the injury activate a process that enables leukocytes to adhere to the endothelial cells and then migrate to where they are needed.
Mental Illness
The pathophysiology of most mental disorders remains a mystery. Dysfunctions of the monoamines, the hypothalamic-pituitary-adrenal (HPA) axis, and the neurotrophins have been discussed as possible mechanisms. Infections and inflammation many also play a role in the development of psychiatric disorders. The capacity of cytokines to affect neural plasticity may contribute to psychiatric illness as collateral damage from a normal immune reaction or some autoimmune activation. If this mechanism is culpable, it opens a whole new avenue for treatment interventions. The most compelling evidence is found with depression and schizophrenia.
Depression
Interferon alpha (IFN-α) for the treatment of hepatitis C makes the most impressive connection between depression and cytokines. Hepatitis C is the major cause of chronic liver disease in the Western world. Only about 15% of people infected with this evasive virus can cure themselves. Of those who develop the chronic infection, 20% to 30% will develop cirrhosis within several decades—2.5% develop hepatic cancer.
The IFN cytokines have anti-viral effects and work in conjunction with T cells to clear viruses. IFN-α was approved for the treatment of hepatitis C in the United States in 1992 and, when given with ribavirin, yields a sustained response rate of about 55%. However, a significant proportion of patients treated with IFN-α develop psychiatric symptoms. About 30% to 50% will develop depression, fatigue, anxiety, irritability, and even hypomania. Prophylactic treatment with antidepressants reduces the psychiatric morbidity in patients receiving IFN-α.
A different example connecting cytokines and depression comes from analyzing the blood of depressed people. Compared with healthy controls, patients with depression typically have higher levels of circulating cytokines. In a meta-analysis of similar studies, the pro-inflammatory IL-1 and IL-6 were elevated in depressed patients. One study found that high levels of C-reactive protein (a nonspecific measure of inflammation) preceded the development of depression in an elderly population. Finally, there is some evidence that high inflammatory markers are predictive of treatment-resistant depression.
What about treating depression with anti-inflammatory medication? One would think that with all the anti-inflammatory medications on the market (prednisone, aspirin, etc.) some bright clinician would have noted positive benefits on mood from one of these medications. Remember, this is how the first antidepressants were serendipitously discovered. So, the lack of a robust effect with the prevalent anti-inflammatory medications is a bit disconcerting. However, there are some studies showing positive effects on mood with anti-inflammatory interventions, which suggest that controlling inflammation may improve mood.
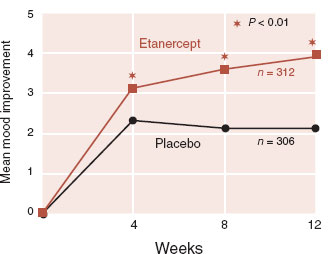
FIGURE 9.6  The TNF inhibitor etanercept significantly improved depressive symptoms in patients with psoriasis as measured by the Beck Depression Inventory. (Adapted from Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.)
The TNF inhibitor etanercept significantly improved depressive symptoms in patients with psoriasis as measured by the Beck Depression Inventory. (Adapted from Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.)
One impressive study was with the TNF inhibitor etanercept in a large placebo-controlled trial for psoriasis. Etanercept is a monoclonal antibody that attaches to and neutralizes the pro-inflammatory cytokine TNF. In this phase III trial, the researchers followed symptoms of depression and fatigue in patients who were ostensibly being treated for psoriasis. Figure 9.6 shows the results for depressive symptoms. Fatigue also improved with active treatment.
TREATMENT
ANTI-INFLAMMATORY ANTIDEPRESSANTS
It turns out that antidepressants as well as mood stabilizers have anti-inflammatory properties. For example, bupropion (Wellbutrin) actually inhibits TNF synthesis! What is the effective component of these medications: increasing monoamines in the synapse, or anti-inflammation, or both?
As we will discuss in Chapter 23, the pathophysiology of schizophrenia appears to involve a disruption of neural growth influenced by genetic predisposition and environmental events. It is possible inflammation is one mechanism that may upset the normal growth of the neurons that results in schizophrenia. While Tamer in 1929 was the first to note a relationship between seasonal births and schizophrenia, it was E. Fuller Torrey who has championed the idea of an infectious etiology. In 1973, Dr. Torrey published a paper in Lancet entitled “Slow and Latent Viruses in Schizophrenia.” His theories were not well received at the time. Now there seem to be several articles on the topic every week.
Initially, the research focused on finding the specific microbe that might cause schizophrenia—cytomegalovirus or Toxoplasma gondii, for example. In the past decade, as with depression, the focus had shifted to inflammation and cytokines. A meta-analysis of 62 studies comparing cytokine levels in 2,200 schizophrenics and 1,800 healthy controls found an increase in three cytokines and a decrease in one in the schizophrenic patients. This analysis suggests that inflammation plays a role in maintaining schizophrenia. However, a more convincing explanation may be the detrimental effect inflammation has on the brain during critical developmental spurts.
An inflammatory reaction when the neurons are sprouting or pruning, such as during the first trimester or adolescence, may discombobulate normal neural growth. One research group has used Finnish medical records from 1947 to 1990 to look at the effect of infection during pregnancy. They located women who were hospitalized in Helsinki with upper urinary tract infections (pyelonephritis) while they were pregnant. Pyelonephritis is a serious infection but one in which the microbe is not transmitted to the fetus—only the mother’s inflammatory response crosses the placenta.
The authors compared the psychiatric outcome in adulthood for the 9,596 children who were exposed to pyelonephritis in utero with 13,808 siblings who were not. As an added variable, they organized the adults according to family history of psychosis. The results are seen in Figure 9.7.
Figure 9.7 shows graphically the additive effect of infection and genes on the development of schizophrenia. The authors wrote, “38% to 46% of the individuals who developed schizophrenia in our sample did so as a result of the synergistic action of prenatal exposure to infection and positive family history.” This study captures what many people believe about schizophrenia and infection—that schizophrenia develops in genetically vulnerable individuals exposed to an infection at a vulnerable time for the brain.
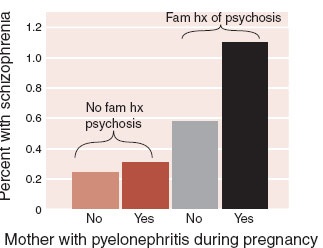
FIGURE 9.7  Mothers who had pyelonephritis while pregnant and have a family history of psychosis are almost five times more likely to have children who develop schizophrenia as adults. (Adapted from Clark MC, Tanskanen A, Huttunen M, et al. Evidence for an interaction between familial liability and exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025-1030.)
Mothers who had pyelonephritis while pregnant and have a family history of psychosis are almost five times more likely to have children who develop schizophrenia as adults. (Adapted from Clark MC, Tanskanen A, Huttunen M, et al. Evidence for an interaction between familial liability and exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025-1030.)
Duality of the Inflammatory Response
One gets the impression that if we could just turn off inflammation we would all live longer, richer, happier lives. If only it were true! TBI provides a good example of the duality of the inflammatory response.
Many people suffer enormous long-term disability after TBI. Increasingly, we are recognizing the profound and enduring effects of minor TBI, particularly in soldiers within close proximity to explosions or professional athletes with multiple concussions. Brain damage following trauma is the result of immediate tissue injury from the impact and secondary injury from the inflammatory response. Considerable damage to the neural structures is caused by edema, cytokine release, and leukocyte infiltration after the initial impact.
Studies with animals have established that limiting the inflammatory response can reduce some long-term deficits from TBI. But not all studies are positive. In one interesting study, brain trauma was inflicted on mice genetically engineered not to express TNF-α (knockout mice). Initially, the knockout mice showed less behavioral impairment than wild-type mice. However, after several weeks, the knockout mice remained significantly impaired while the wild type recovered. It appears, at least with TNF-α, that the inflammatory response is also involved with healing. This may be due to the beneficial effects the cytokines have on neural plasticity.
ANTI-INFLAMMATORY DIET
If one searches “inflammation” in Amazon.com, the screen quickly fills with books extolling the advantages of the anti-inflammatory diet. Is there such a thing? Most people would agree that the Western diet is a catastrophe, causing an epidemic of obesity, heart disease, and diabetes.
There is substantial evidence that a Mediterranean diet (rich in fruits and vegetables, nuts, olive oil, legumes, and fish; moderate in alcohol; and low in red meat, processed meat, refined carbohydrates, and whole-fat dairy products) is healthier for the body. A recent prospective study in Spain found a smaller incidence of depression in subjects with stricter adherence to the Mediterranean diet. OK, but is it anti-inflammatory?
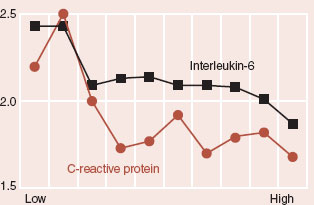
Well, the omega-3 polyunsaturated fatty acids (commonly called fish oils) can reduce the magnitude of the inflammatory response. A prospective analysis of inflammatory markers in post-myocardial infarction survivors compared adherence with the Mediterranean diet to inflammatory markers (C-reactive protein and IL-6; see figure). While the results are not overwhelming, they do suggest that diet may influence the inflammatory system.
AUTOIMMUNE DISEASE
Multiple sclerosis (MS) is the quintessential autoimmune disease. The immune system is designed to tolerate self and attack foreign bodies. Sometimes mistakes are made. MS patients have T cells that react to a protein in the myelin sheath. In theory, this alone should not be a problem. The T cells are in the periphery, while the myelin sheaths are protected behind the blood–brain barrier in the CNS. Additionally, the T cells are no threat as long as they are not activated. But for some people, all these safeguards fail and the T cells go after the myelin in the CNS.
It is likely that a viral infection initiates the process in genetically vulnerable individuals. A closely matching viral infection activates the aberrant T cells and the presence of inflammation allows the leukocytes to slip into the CNS where they attack the myelin. The loss of the myelin and axonal damage can result in a variety of unusual symptoms that wax and wane. Prior to the routine use of brain scans, it was not uncommon for a woman with ambiguous neurological symptoms to be diagnosed with conversion disorder or hysteria before it was recognized months or years later that she actually had MS. (A lesson to remember before we too quickly diagnose patients with personality disorders.)
The development of magnetic resonance imaging (MRI) has changed the way the illness is followed. The focal demyelination in the white matter is easy to identify on MRI. Furthermore, many lesions are clinically silent—meaning the patient is unaware of the problem because the lesion occurs in an area of the brain that does not produce a physical symptom. Figure 9.8 shows how lesions come and go over several months for one patient.
There seem to be different phases of MS. The first phase is one of demyelination with focal symptoms followed by remyelination and recovery. The second phase is one of increasing and unremitting disability due to axonal loss and whole brain volume shrinkage. How quickly one moves from the relapsing/remitting phase to the secondary progressive phase varies from one individual to the next. Of greater relevance to treatment interventions, the first phase appears to have more of an inflammatory component while the secondary progressive phase does not. This becomes apparent in the treatment response.
Prior to 1993, there were no licensed treatments for MS. Now several treatment options exist that focus on reducing symptoms (such as unstable bladder or fatigue) or limiting the inflammatory response. Even more exciting are the beta IFNs—naturally occurring cytokines. They have been reported to reduce relapses by 30%. Unfortunately, long-term follow-up suggests that they have limited effect on the only outcome that really matters: accumulation of disability.
FIGURE 9.8  Serial MRI scans at the same level for the same patient with multiple sclerosis show how lesions appear and recede over the course of 1 year.
Serial MRI scans at the same level for the same patient with multiple sclerosis show how lesions appear and recede over the course of 1 year.
Monoclonal antibodies are a new treatment option being developed for MS. Genetically engineered mice are raised to produce antibodies that target specific molecules on the T cells. When injected, these antibodies latch onto the problematic T-cell molecule, which results in destruction of the cell and reduced immune cell activity. Is that not incredible? In a phase II trial, the monoclonal antibody alemtuzumab reduced relapses by 75% compared with a beta IFN. Early studies suggest that alemtuzumab is most effective in the early phases of MS, but did not change the progression of the illness for those already in the secondary progressive phase—further evidence that the latter phases of MS may have different mechanisms of action.
NEURAL REGULATION OF IMMUNITY
Until now we have been discussing the effects the immune system has on the brain, but actually the influence is bidirectional. The CNS has the capacity to regulate the immune response as well. Generally speaking, the CNS tends to dampen the immune response in order to restore homeostasis and prevent excessive inflammation.
The process starts when cytokines are released by white blood cells and travel to the brain in the plasma (Figure 9.9). The CNS responds through the autonomic nervous and endocrine systems. The sympathetic and parasympathetic branches of the autonomic nervous system modulate the immune response through innervation of the individual immune organs such as the spleen and lymph nodes. The endocrine system exerts its control through the release of sex hormones, thyroid hormone, and, most importantly, cortisol.
In 1950, the Nobel Prize was awarded for the discovery of cortisol. Its extraordinary anti-inflammatory properties were quickly recognized and put to use for rheumatoid arthritis and other inflammatory diseases. Endogenous cortisol provides the most powerful feedback loop through which the CNS modulates the inflammatory response. The importance of cortisol to inhibit an excessive immune response has been demonstrated in animal studies. When animals have their adrenal glands removed and then are inoculated with an infectious agent, they show increased mortality from shock. The unrestrained cytokine and pro-inflammatory responses overwhelm the animal—too much inflammation is detrimental. One of the jobs of the CNS is to keep the immune response from getting out of control. The brain puts the brakes on the immune response by releasing adrenocorticotropic hormone, which in turn stimulates the adrenal release of cortisol (Figure 9.9).
Stress-Induced Immune Dysfunction
Folk wisdom has long recognized that psychological stress takes a toll on one’s physical health. Many years of research support this belief. Studies with new military recruits, anxious medical students, and caregivers of spouses with Alzheimer’s disease have shown the ill effects of stress on the body.
Stress affects the CNS, which in turn alters the signals sent to the body through the endocrine and autonomic systems. Figure 22.1 shows a good example of the endocrine response to the acute stress of learning to parachute. These endocrine and autonomic signals gently suppress the immune response, which in turn puts the subject at greater risk for illness.
FIGURE 9.9  The central nervous system restrains the immune response by way of the autonomic nervous system and endocrine system. ACTH, adrenocorticotropic hormone. (Adapted from Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006:6;318-328.)
The central nervous system restrains the immune response by way of the autonomic nervous system and endocrine system. ACTH, adrenocorticotropic hormone. (Adapted from Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006:6;318-328.)
This connection between stress and poor healing is eloquently shown in animal studies. Mice are stressed by placing them in a restraining tube—overnight, when they are usually most active. In this particular study, the mice were restrained for 12 hours a night for 8 days in a row. On the third day, a small skin wound was opened. Figure 9.10 shows the results of this study for restrained mice compared with controls. The stressed mice had increased corticosterone (cortisol in a mouse), delayed leukocyte movement into the wound, and delayed wound healing.
Studies with humans have shown similar results—particularly patients with depression. Figure 21.3 shows the increased cortisol in depressed patients, suggesting an activated HPA axis. Furthermore, patients with diabetes, cancer, stroke, and myocardial infarction, who also are depressed, are at greater risk for death than similar patients without depression. Is the increased mortality secondary to impaired immunity?
Whitehall Study
The Whitehall study is one of those remarkable large epidemiologic studies that established with hard data what previously had been conjecture. The study followed almost 20,000 male British civil servants for 25 years and correlated the employment grade with health. Simply looking at death, there is a dose–response association between employment grade and mortality (Figure 9.11A). A similar pyramid is seen regardless of whether the death is from for heart disease, neoplasm, stroke, or respiratory disease. Likewise, in one of the many side studies that came out of this project, measures of immune status also correlated with employment status. That is, men at the bottom of the employment pyramid had a more activated immune response as measured by C-reactive protein and circulating white blood cells (Figure 9.11B). Men in the lower employment grades were presumably more “stressed out,” had poorer health, and showed greater immune activity.
Fatigue
Persistent fatigue is a frequent complaint with a long history. In 1869, the neurologist George Beard named the condition neurasthenia—a term that was in DSM-II in the neuroses section but was dropped from psychiatric nomenclature in 1980 with the publication of DSM-III.
FIGURE 9.10  Mice restrained for 12 hours a night over 8 days show an activated HPA axis (A), poorer cellular response to the wound site (B), and delayed wound healing (C). HPA, hypothalamic-pituitary-adrenal. (Adapted from Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64-73.)
Mice restrained for 12 hours a night over 8 days show an activated HPA axis (A), poorer cellular response to the wound site (B), and delayed wound healing (C). HPA, hypothalamic-pituitary-adrenal. (Adapted from Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64-73.)
In the mid-1980s, two practicing internists near Lake Tahoe, Nevada, generated considerable excitement when they reported an epidemic of persistent fatigue associated with elevated titers of the Epstein-Barr virus (EBV). Ultimately, the Centers for Disease Control and Prevention (CDC) disproved EBV as a cause of the illness, but this was the start of what became chronic fatigue syndrome.
Chronic fatigue syndrome captured the attention of patients and clinicians. Many patients, who would never see a psychiatrist, were thrilled to be treated by infectious disease specialists. Researchers, likewise, raced to find the cause. However, all the findings have remained “soft.” While immunologic abnormalities can be found, they are difficult to reproduce and are not diagnostic. Periodically, a new infectious agent is identified by one group only to have other labs fail to duplicate the results. The XMRV retrovirus is the current example.
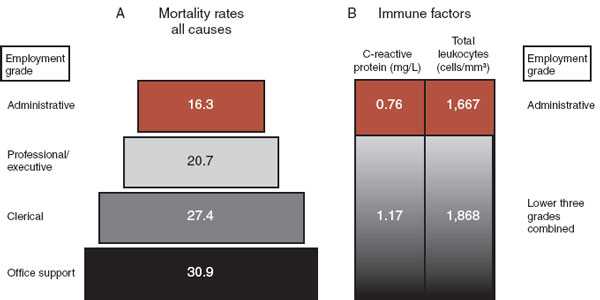
FIGURE 9.11  The Whitehall study showed that employment grade correlated with mortality (A) and immune activation (B) in British civil servants. Men in upper ranks had lower mortality and a more settled immune system.
The Whitehall study showed that employment grade correlated with mortality (A) and immune activation (B) in British civil servants. Men in upper ranks had lower mortality and a more settled immune system.
Many clinicians see chronic fatigue as a psychiatric disorder with similarities to depression. Indeed, antidepressants, cognitive-behavioral therapy, and exercise are effective treatments for depression and chronic fatigue. In general, the medical community has gradually placed chronic fatigue back into a psychosomatic category. However, this chapter should attest to the interplay between the infectious/immunologic and the neuro/psychiatric, which raises the question: Is Chronic fatigue syndrome a stress-induced immunologic deficiency or a psychiatric disorder caused by an infection?
QUESTIONS
1. Treponema pallidum damages the brain
a. Within 18 hours of infection.
b. By targeting pyramidal neurons in the cortex.
c. By releasing a toxin.
d. By inducing an inflammatory response.
2. A cocaine vaccine
a. Reduces immune activation.
b. Stimulates anticocaine antibody production in the CNS.
c. Reduces euphoria.
d. Stimulates anticocaine leukocytes.
3. Cytokines are involved in all of the following except
a. Neural plasticity.
b. Turning off the inflammatory response.
c. Mental illness.
d. Identifying Treponema pallidum.
4. All the following are true of MS except
a. Responds to TNF inhibitors.
b. Can be confused with hysteria.
c. Is a T-cell problem.
d. Induces silent lesions.
5. The CNS reduces the inflammatory response through all of the following except
a. Parasympathetic nervous system.
b. Sympathetic nervous system.
c. Cytokines.
d. Adrenal gland.
See Answers section at the end of the book.