INTRODUCTION
Anxiety is part of a mechanism developed in higher animals to handle adverse situations. The anxiety response can be conceptualized as part of the brain’s alarm system firing during times of perceived danger. The characteristic responses including avoidance, hypervigilance, and increased arousal are implemented to avoid harm.
The evolutionary drive to survive has invariably enhanced a strong anxiety response. Unfortunately, in many individuals, that mechanism is overactive. The alarm fires too frequently. Such people cannot seem to turn down their internal alarm even when the coast is clear.
Diagnostic and Statistical Manual
The Diagnostic and Statistical Manual (DSM) system categorizes anxiety into a multitude of different disorders (generalized anxiety disorder [GAD], obsessive-compulsive disorder [OCD], post-traumatic stress disorder [PTSD], etc.). Although each disorder has unique clinical features, it is not clear whether they actually describe different pathologic states. The disorders have the following similar characteristics:
1. Most patients with an anxiety disorder will have features of the other disorders.
2. Many of the disorders respond to similar treatment interventions: selective serotonin reuptake inhibitors, benzodiazepines, exposure therapy, and so on.
3. There is little evidence to show that different disorders stem from different regions of the brain.
We believe the reaction to perceived threat is mediated in similar pathways in the brain in patients with various anxiety disorders. The one potential exception is OCD, which has unique mechanisms in the brain and will be reviewed separately.
Acute Stress
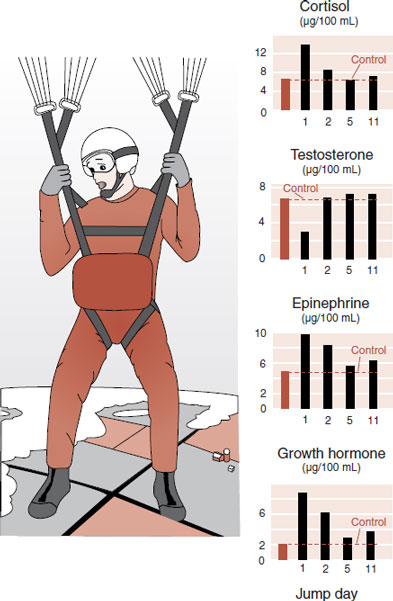
The body’s reaction to an acutely stressful situation is well known to all of us. The characteristic rapid heart rate, dry mouth, and sweaty palms are the body’s response to increased sympathetic activity generated by a stressful situation (see Figure 2.8). Less readily apparent are the endocrine responses to acute stress.
In the late 1970s, Ursin et al. studied the endocrinologic responses of young Norwegian military recruits during parachute training. During the exercise, the recruits repeatedly jumped off a 12 m tower and slid down a long sloping wire to learn the basic skills of parachuting. The training was designed to give the jumper a realistic sensation of the initial free fall. Measures of anxiety and performance skill as well as serum hormone levels were captured at baseline and after each jump.
Initially, anxiety was high and performance was poor. As the days and number of jumps progressed, anxiety subsided and the skills improved. The endocrinologic measures are shown in Figure 22.1. Presumably, the anxiety induces changes in the releasing hormones, which alter the pituitary hormones and ultimately the peripheral hormones (see Figure 7.4). Note how testosterone levels drop with the stress of the jumping, while the other measures increase. Likewise, all return to baseline as the recruits habituate to the task.
While the average individual shows a peak in the endocrine response at the start of a difficult task, which usually subsides as the person gains mastery, this is not true for everyone. Some people have more exaggerated and persistent endocrine reactions. Kirschbaum et al. looked at salivary cortisol levels in 20 men exposed to 5 consecutive days of public speaking. For the total group, the average cortisol levels jumped on the first day and then gradually declined over the following days. However, the men could be divided into two groups: high responders and low responders. Figure 22.2 shows the cortisol levels for these two groups.
FIGURE 22.1  The stress of learning to parachute, on young recruits, results in changes in hormones, which return to baseline as the anxiety decreases. (Adapted from Rosenzweig MR, Breedlove SM, Watson NV. Biological Psychology. 4th ed. Sunderland, MA: Sinauer; 2005. Graphs adapted from Ursin H, Baade E, Levine S. Psychobiology of Stress: A Study of Coping Men. New York, NY: Academic Press; 1978.)
The stress of learning to parachute, on young recruits, results in changes in hormones, which return to baseline as the anxiety decreases. (Adapted from Rosenzweig MR, Breedlove SM, Watson NV. Biological Psychology. 4th ed. Sunderland, MA: Sinauer; 2005. Graphs adapted from Ursin H, Baade E, Levine S. Psychobiology of Stress: A Study of Coping Men. New York, NY: Academic Press; 1978.)
Note how the cortisol in the low responders peaked the first day, but then quickly returned to baseline levels on the following days. These individuals appear to adjust rapidly to the stress of public speaking. The high responders, on the other hand, showed higher, more persistent peaks of cortisol. Such people appear to have a harder time turning off the stress response. Presumably, they have a stronger alarm response originating in the brain, which is driving this endocrine reaction. Alternatively, they have a weak or faulty regulatory system that governs or turns down the response. Or there could be elements of both in some people.
Cortisol has been implicated as a culprit in the development of anxiety after trauma, although not everyone agrees if it is too much or too little. Some researchers believe excess cortisol sensitizes the amygdala and are using mifepristone (RU-486, the “abortion pill”) to block the glucocorticoid receptors after a traumatic event. Other researchers believe the traumatic consolidation of memories is enhanced by insufficient cortisol and they are giving cortisol to patients acutely after trauma to prevent the development of PTSD. Both groups have produced small pilot studies with positive results. The impending larger trials should clear up this paradox.
NEURONAL CIRCUITRY
Multiple lines of research identify the prefrontal cortex (PFC), amygdala, hippocampus, and hypothalamic-pituitary-adrenal (HPA) axis as the regions involved with anxiety (Figure 22.3). However, if there is one organ that represents an alarm system in the brain, it is the amygdala (see Figure 2.7).
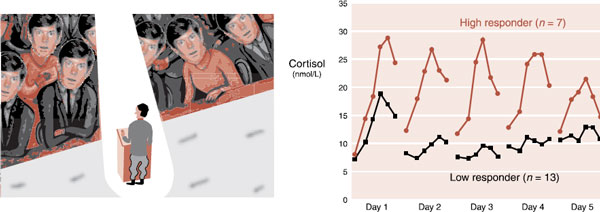
FIGURE 22.2  Men stressed by public speaking over 5 consecutive days showed different patterns of adrenocortical response. (Graph adapted from Kirschbaum C, Prussner JC, Stone AA, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57(5):468-474. Illustration adapted from Images.com.)
Men stressed by public speaking over 5 consecutive days showed different patterns of adrenocortical response. (Graph adapted from Kirschbaum C, Prussner JC, Stone AA, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57(5):468-474. Illustration adapted from Images.com.)
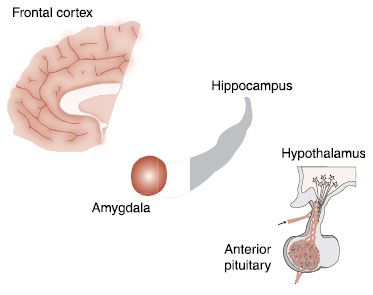
FIGURE 22.3  The important regions of the brain affecting anxiety.
The important regions of the brain affecting anxiety.
In their classic studies with rhesus monkeys in the 1930s, Heinrich Klüver and Paul Bucy identified the amygdala as a structure that is essential for the expression of numerous emotions. They removed large segments of the temporal lobes from wild monkeys and transformed the monkey’s temperament. Aggressive and easily frightened monkeys were changed into docile, calm creatures.
The monkeys virtually became tame. They did not react fearfully to strange humans or even a snake. Of particular interest, the monkeys failed to learn from negative experience. One monkey, bitten by a snake, later approached a snake again as if nothing had happened—a clear example of the survival value of anxiety. The entire constellation of symptoms is called the Klüver-Bucy syndrome and includes a host of other bizarre features, such as hypersexuality and a tendency to transfer objects to the mouth.
In the intervening years, it has been demonstrated that the emotional changes of the Klüver-Bucy syndrome can be elicited with the removal of just the amygdala. Other evidence points to the amygdala as an important region in the recognition and management of fear:
1. Electric stimulation of the amygdala in animals elicits fearful behavior, for example, freezing and tachycardia.
2. Humans with damaged amygdala exhibit impaired fear conditioning.
3. Functional imaging studies of humans show activation of the amygdala during fear learning.
Recognizing Danger
Sensory information enters the brain by way of the thalamus. All neurons carrying auditory and visual information synapse first in the thalamus before being relayed to the appropriate cortical region for analysis. Information about danger is particularly important and needs to be recognized quickly. Work by LeDoux et al. at New York University has shown that the amygdala quickly receives some preliminary information about dangerous events even before it is processed in the cortex.
Figure 22.4 shows an example of a person coming upon a rattlesnake. This life-threatening visual information proceeds from the eyes to the thalamus. However, the thalamus sends fast but rudimentary signals to the amygdala at the same time that the full information is passed back to the visual cortex. The amygdala in turn sends responding signals to the muscles, sympathetic nervous system, and hypothalamus. The person jumps even before being consciously aware of what has been seen. LeDoux has shown with rats that the fear response is preserved even if the neural connections between the thalamus and the cortex are cut. In essence, the animal startles without knowing why.
We all have had the experience of being frightened when seeing something, only to realize that it was just a rope or shadow—not a real threat. It is the fast track from the thalamus directly to the amygdala that causes the false alarm. This is considered to be “unconscious” or preconscious. We jump before we are aware. Patients with anxiety disorders can have exaggerated startle responses. They are burdened with an exaggerated, unconscious reaction to what are actually harmless events.
Anticipatory Anxiety
Some people dread personal interactions in which they will be the focus of attention. Typically, they fear they will embarrass themselves. The anticipatory anxiety can ultimately restrict what they do and limit their social life and career path.
An imaging study looked at the activity in the brain of such patients who were asked to anticipate making a public speech. By subtracting the activity during anticipation from that at rest, they identified the areas of activity. Patients showed greater activity in their amygdala as well as hippocampus and insula. Figure 22.5A shows the functional magnetic resonance imaging (fMRI) slice at the level of the amygdala.
Anticipatory anxiety is not all bad. Figure 22.5B shows the hypothetical upside down U curve that many think represents the benefits and problems with anticipatory anxiety. Some anxiety is beneficial and actually improves performance; however, too much of it is overwhelming and results in a poorer outcome.
FIGURE 22.4  Two representations showing the two tracks that emotionally stimulating sensory information takes to the amygdala after entering the brain through the thalamus. HPA, hypothalamic-pituitary-adrenal. (Adapted from LeDoux JE. Emotion, memory and the brain. Sci Am. 1994;270(6):50-57.)
Two representations showing the two tracks that emotionally stimulating sensory information takes to the amygdala after entering the brain through the thalamus. HPA, hypothalamic-pituitary-adrenal. (Adapted from LeDoux JE. Emotion, memory and the brain. Sci Am. 1994;270(6):50-57.)
Amygdala Memories
There is evidence that primitive emotionally relevant memories are stored in the amygdala. For example, in rodents:
1. Long-term potentiation (LTP) can be induced in the amygdala;
2. Protein synthesis inhibitors injected directly into the amygdala will prevent the formation of fear conditioning; and
3. Chronic stress will induce increased dendritic branching in the amygdala.
These results suggest that the typical structural changes that are observed with memory formation occur in the amygdala in reaction to fearful circumstances. This may be one reason why traumatic events are so persistent. Fearful experiences form quickly, and enduring memories then reside in both the neocortex and the amygdala.
Prefrontal Cortex
One of the central components of anxiety is the feeling that one is not in control. Patients will complain of increased anxiety when they lose control, for example, when the door closes on an airplane or when their social support drives off—anytime they feel trapped. Feeling in control, on the other hand, calms anxiety. The ability to reappraise a difficult situation into more favorable terms is a function of executive control. Rational reappraisal is a central feature of CBT.
TREATMENT
AMYGDALA ACTIVITY
A small study in Sweden looked at the effects of cognitive-behavioral therapy (CBT) and the antidepressant citalopram on brain activity in patients with social phobia. Both treatments were equally effective and both reduced the amygdala activation after 9 weeks of treatment. The degree of amygdala attenuation was associated with clinical improvement 1 year later.
FIGURE 22.5  A. Increased activity at the level of the amygdala when patients with anxiety anticipate making a public speech. B. Hypothetical upside down U curve showing benefits and problems of anticipatory anxiety. (Functional magnetic resonance imaging from Lorberbaum JP, Kose S, Johnson MR, et al. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15(18):2701-2705.)
A. Increased activity at the level of the amygdala when patients with anxiety anticipate making a public speech. B. Hypothetical upside down U curve showing benefits and problems of anticipatory anxiety. (Functional magnetic resonance imaging from Lorberbaum JP, Kose S, Johnson MR, et al. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15(18):2701-2705.)
The ability to cognitively master difficult circumstances and gain control likely resides in the PFC. Clearly, the PFC plays an important role in managing anxiety. However, the exact prefrontal regions involved (medial, lateral, or orbital) are ill-defined, although the medial PFC gets the most attention. The medial prefrontal cortex (mPFC), which includes the anterior cingulate gyrus, is well connected to the amygdala.
In a simple conceptualization, we can imagine that the mPFC applies the brakes to the amygdala. Several lines of evidence highlight the role of the mPFC in anxiety:
1. Lesions of the mPFC in rats reduce the ability to extinguish fears.
2. Stimulation of the mPFC inhibits a learned fear response; that is, the rat does not show anxiety.
3. The mPFC lights up in functional imaging studies when fear is evoked in healthy subjects.
4. Subjects with anxiety disorders have reduced activity in the mPFC.
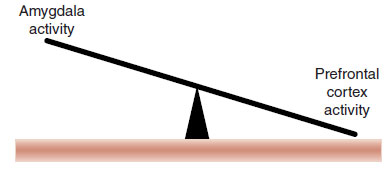
FIGURE 22.6  In simple terms anxiety disorders may be the result of too much activity in the amygdala and not enough activity in the prefrontal cortex.
In simple terms anxiety disorders may be the result of too much activity in the amygdala and not enough activity in the prefrontal cortex.
Taken together, these studies suggest that anxiety in part results from the reciprocal relation between PFC and the amygdala (Figure 22.6). This has been shown clearly in a clever study with traumatized combat veterans and firefighters. The subjects were shown pictures of faces expressing various emotions while in an fMRI (Figure 22.7A). The activity in the brain when the subjects were viewing the happy face was subtracted from the brain activity when viewing the fearful face. The subjects with PTSD were compared with healthy controls.
Figure 22.7B–D shows the results. Note how subjects with PTSD, under the circumstances of the study, show increased activity in their amygdala and decreased activity in their PFC. In essence, their PFC is insufficiently powered and unable to turn down the “alarm” in the amygdala.
Hippocampus
The hippocampus, an area involved with explicit memory acquisition, appears to interact with the amygdala during encoding of emotional memories. Although the exact role of the hippocampus in anxiety disorders remains unclear, it is an area frequently active in imaging studies during fearful situations.
For a number of years, the reduction in volume of the hippocampus in patients with anxiety disorders has been an area of great interest. Several studies have documented smaller hippocampi in anxious patients, presumably due to the toxic effects of an activated HPA axis. However, a unique study with twins provides insight into the cause and effect of hippocampal volume.
Gilbertson et al. recruited 40 pairs of twins, in which one of each pair was exposed to combat in Vietnam and the other had stayed home. (Who knew such a study was possible?) The researchers measured the hippocampal volume of each twin in an MRI. Additionally, the presence and severity of PTSD in the combat-exposed twin was assessed. As with previous reports, the twins who were diagnosed with PTSD had smaller hippocampal volumes (Figure 22.8A). However, the most remarkable finding was that the PTSD score from the combat-exposed twin had a similar correlation with the hippocampal volume of the twin who had stayed at home (Figure 22.8B). In other words, the best prediction of a combat veteran’s level of PTSD and hippocampal size is not his exposure to trauma, but rather the size of his twin’s hippocampus.
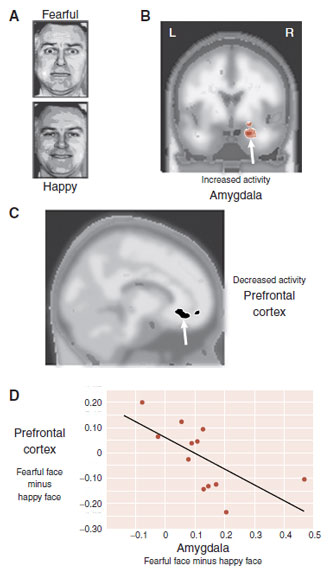
FIGURE 22.7  A. Traumatized subjects and controls are shown pictures of fearful faces and happy faces while in a functional magnetic resonance imaging. The patients show increased activity in the amygdala (B) and decreased activity in the prefrontal cortex (PFC) (C) compared with controls. Activity in the PFC and amygdala (D) has an inverse correlation for the traumatized patients. (A Adapted from Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2(5):352-363. B–D Adapted from Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273-281.)
A. Traumatized subjects and controls are shown pictures of fearful faces and happy faces while in a functional magnetic resonance imaging. The patients show increased activity in the amygdala (B) and decreased activity in the prefrontal cortex (PFC) (C) compared with controls. Activity in the PFC and amygdala (D) has an inverse correlation for the traumatized patients. (A Adapted from Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2(5):352-363. B–D Adapted from Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273-281.)
This finding brings to light one of the great discoveries in neuroscience. It is the interaction between nature and nurture that results in mental illness (Figure 22.9). In other words, those at increased risk for developing PTSD are those individuals with a small hippocampus and exposure to trauma. Neither alone is sufficient.
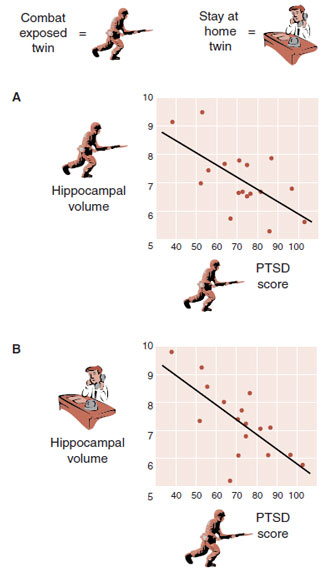
FIGURE 22.8  A. The correlation between hippocampal volume and post-traumatic stress disorder (PTSD) score for the combat-exposed twin. B. The correlation between the stay-at-home twin’s hippocampal volume and the PTSD score of his combat-exposed twin brother. (Adapted from Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242-1247.)
A. The correlation between hippocampal volume and post-traumatic stress disorder (PTSD) score for the combat-exposed twin. B. The correlation between the stay-at-home twin’s hippocampal volume and the PTSD score of his combat-exposed twin brother. (Adapted from Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242-1247.)
FIGURE 22.9  The risk of developing post-traumatic stress disorder (PTSD) is highest among individuals with a small hippocampus and exposure to trauma.
The risk of developing post-traumatic stress disorder (PTSD) is highest among individuals with a small hippocampus and exposure to trauma.
Taken together, these findings suggest an important role for the hippocampus in the development of anxiety. These early preliminary data suggest that there is something neuroprotective about a large hippocampus that mitigates the development of PTSD, even when someone is exposed to unimaginable trauma. Possibly, a large hippocampus is better able to limit the acquisition of haunting memories or more efficient at extinguishing them once they develop.
Two recent twin studies published in 2012 corroborated the Gilbertson findings. In one study with women, a smaller hippocampus correlated with a diagnosis of GAD. In a large study with male twins, hippocampal volume correlated with self-esteem.
NEUROTRANSMITTERS AND CELL BIOLOGY
γ-Aminobutyric Acid
GABA is the major inhibitory neurotransmitter in the brain. Activating GABA neurons calm down the brain. Too much GABA activation causes sluggishness and even coma. The GABA receptor has several sites that bind with other substances, which has the effect of enhancing the inhibitory activity of the GABA neurons. Figure 5.3 shows that ethanol, barbiturates, and benzodiazepines are known to bind with the GABA receptor. It is no wonder that people use and abuse these substances to calm down and reduce anxiety.
ALCOHOLISM AND ANXIETY
There is controversy about the relation between alcoholism and anxiety. Acute alcohol intoxication reduces anxiety. Many clinicians believe that substance abusers are self-medicating their anxiety with alcohol and other calming agents. Longitudinal and genetic studies support this perception for some patients.
However, there is compelling evidence that chronic alcohol exposure induces long-term central nervous system adaptations that cause anxiety. For example, the following have been observed:
1. Withdrawal from alcohol increases anxiety.
2. The anxiety of withdrawal decreases as abstinence persists.
3. Studies on twins have shown that anxiety disorders are common in the alcoholic twin but not the sober twin.
4. Rats exposed to chronic alcohol show alterations in γ-aminobutyric acid (GABA) receptors.
5. Abstaining alcoholics showed a decreased benzodiazepine receptor distribution (see figure).
Abstaining alcoholics show decreased benzodiazepine receptor distribution in frontal cortex and cerebellum compared with controls.
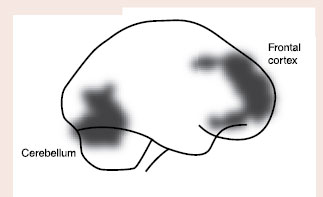
Adapted from Abi-Dargham A, Krystal JH, Anjilvel S, et al. Alterations of benzodiazepine receptors in type II alcoholic subjects measured with SPECT and [123I]iomazenil. Am J Psychiatry. 1998;155(11):1550-1555.
Abnormalities in the benzodiazepine GABA receptor have been implicated as a possible cause of anxiety disorders. Results of the original imaging studies and more recent genetic studies suggest that alterations in the structure or concentration of the benzodiazepine GABA receptor may predispose individuals to anxiety. However, the hypotheses have not been consistently or conclusively established.
Norepinephrine
The norepinephrine (NE) neurons with cell bodies in the locus coeruleus (shown in Figure 4.5) are believed to be part of the stress response system and play an important role in anxiety. The following evidence supports this belief:
1. NE neurons project to the amygdala.
2. Stressed rats show increases in NE release.
3. NE stimulates the release of corticotropin-releasing hormone, which in turn activates the HPA axis.
4. Peripheral NE (the sympathetic branch of the autonomic nervous system) produces somatic symptoms of anxiety: racing heart, sweating, dry mouth, and so on.
With this in mind, blocking NE with the beta-blocker propranolol has been proposed as a treatment to prevent the development of PTSD. That is, giving a beta-blocker in the acute aftermath of trauma (in the ER, on the battle field, etc.) to inhibit the NE stimulation and limit the development of haunting memories. Early small studies reported mild to moderate effects. Subsequent larger, controlled trials have been disappointing.
Brain-Derived Neurotrophic Factor
This brings us back to a topic we first addressed in Chapter 5—LTP. Figures 5.5 to 5.8 show how excessive stimulation alters the signals to the nucleus, which affects gene expression and ultimately changes the structure of the neurons. LTP is an analogy for trauma-induced anxiety (PTSD). With anxiety, we see heightened activity in the amygdala and reduced activity in the PFC and hippocampus. Clearly, something changes in the traumatized anxious brain. The missing ingredient may be growth factor proteins (such as brain-derived neurotrophic factor [BDNF]) that can alter the structure and function of the neural networks.
Animal studies have shown that BDNF is essential for the acquisition and extinction of anxiety:
1. BDNF transcription is increased in the amygdala after fear conditioning.
2. When BDNF is selectively deleted from the hippocampus in mice, they show impaired ability to extinguish conditioned fear.
3. BDNF in the lateral part of the dorsolateral prefrontal cortex (DLPFC) appears to control fear memory formation, while BDNF in the medical DLPFC is necessary for fear extinction.
In summary, this research implies that the development of anxiety and the failure to extinguish it likely occur through the production of growth factor proteins in specific regions of the brain. These conflicting roles for BDNF show how difficult it would be to develop an effective antianxiety treatment, which manipulates the expression of BDNF.
OBSESSIVE-COMPULSIVE DISORDER
OCD is classified as an anxiety disorder in the DSM nomenclature. However, some clinicians conceptualize OCD as a different kind of disorder. They see OCD as one extreme of a spectrum of repetitive behavioral problems: body dysmorphic disorder, certain eating disorders, gambling, and even autism. These conditions are frequently called OC spectrum disorders.
Regardless of the nomenclature controversy, OCD is unique among the anxiety disorders because it is driven by a different neural activity. This was first noticed in the 1930s by the behavioral sequela after von Economo’s encephalitis pandemic from 1917 to 1926. Patients who had recovered from the original infection were often inflicted with OCD or parkinsonism. The basal ganglia were implicated. Symptoms of OCD are also seen with other disorders that affect the basal ganglia: Tourette’s syndrome, Parkinson’s disease, Sydenham’s chorea, and so on.
THE PARADOX OF ANTIDEPRESSANTS AND ANXIETY
If the NE system is part of the anxiety response, why does blocking NE reuptake, and consequently increasing NE at the synapse, relieve anxiety? How can more be less? For example, antidepressants such as the highly noradrenergic imipramine and the NE reuptake inhibitor reboxetine are known to reduce panic attacks. This does not make sense.
This paradox highlights that our understanding of anxiety and how the antidepressants calm the nervous system are in need of further study. Most likely, the antidepressants reduce anxiety by growth or increased strengthening of inhibitory networks.
Early adverse experiences have a significant impact on the later development of anxiety. Harry Harlow established with monkeys the profound negative impact of being raised without a mother or peer relationship. He replaced the mother of infant rhesus monkeys with an inanimate surrogate object during the first months of life. The infant monkeys displayed long-term deficits in social adaptation as well as increased anxiety-related behaviors.
We have discussed examples in which early experience changes neural structures. For example, the following have been observed:
1. Rats raised in standard wire cages have less dendritic branching than those raised in an enriched environment (see Point of Interest, page 90).
2. Licking and grooming by the rat mother effects the pup’s response to stress (see Figure 17.5).
3. Children raised in Romanian orphanages have lower IQs (see Figure 19.8).
A recent study looked at the effect of breast-feeding on anxiety in humans. Children whose parents separated or divorced when the children were between the ages of 5 and 10 years were assessed for anxiety when they turned 10. The children who were breast-fed had less anxiety and more resilience in response to the difficult circumstances. Although there are no details about the effects of breast-feeding on the brain, the results of this study seem strikingly similar to what Michael Meaney found with his high lick and groom mother rats (see Figure 17.8). Extra attention at an early stage of life may be neuroprotective.
Basal Ganglia
The basal ganglia are subcortical structures that are usually associated with movement and motor control. Disruption of the basal ganglia leads to such disorders as Huntington’s disease, Parkinson’s disease, and hemiballismus. However, the exact function of the basal ganglia in OCD remains unclear.
The basal ganglia are made up of interconnected nuclei—the caudate, putamen, and globus pallidus (Figure 22.10). The caudate and putamen together are called the striatum and contains the nucleus accumbens (NAc). Many areas of the cerebral cortex send signals to the basal ganglia, which are relayed to the thalamus, which in turn sends an impulse back to the cortex. This loop is called the cortico-striatal-thalamic-cortical circuit.
Functional imaging studies of obsessing OCD patients have repeatedly shown increased activity in the orbitofrontal cortex, anterior cingulate gyrus, and basal ganglia. It is presumed that these areas light up due to looping signals within the cortico-striatal-thalamic-cortical circuit. However, the particular circuit may depend on the particular ritual: washing, checking, or hoarding seem to activate slightly different regions. Likewise, treatment interventions, whether with psychotherapy or medications, show decreased activity in the basal ganglia for those who respond.
Psychosurgery
There is a long and troubling history of attempts to treat mental illness with neurosurgical interventions (see page 166). Understandably, many are reluctant to even consider neurosurgery as a treatment option for psychiatric diseases. However, there is good evidence that some limited procedures with treatment-resistant patients can diminish OCD symptoms.
Different techniques are preferred in various regions of the world. In the United States, the popular technique is anterior cingulotomy, which is based on the concept of interrupting the circuit between the anterior cingulate gyrus and basal ganglia. Figure 22.11 shows the location of the lesions in postsurgical MRI scans.
Follow-up studies have found that approximately 30% of the anterior cingulotomy patients have a 35% or greater reduction on the Yale-Brown Obsessive Compulsive Scale. Complications include urinary incontinence and seizures, but appear to be infrequent. A different technique used more regularly in Europe is anterior capsulotomy. Although this procedure appears to be more effective, it is also associated with more complications, for example, cognitive and affective dysfunction. More recently, deep brain stimulation has been studied as a way to treat severe and intractable OCD in a manner that is reversible and more flexible. The Food and Drug Administration (FDA) has issued a humanitarian device exemption to allow psychiatrists to treat treatment-resistant OCD patients with deep brain stimulation. The bilateral leads are implanted in a manner to interrupt this pathological cortico-subcortical circuit.
FIGURE 22.10  The structures of the basal ganglia and how they reside within the brain.
The structures of the basal ganglia and how they reside within the brain.
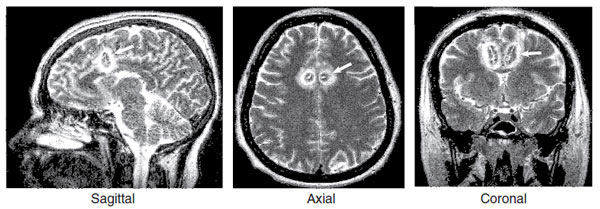
FIGURE 22.11  Three views of anterior cingulotomy for treatment-resistant obsessive-compulsive disorder (OCD). The white arrows show the location of the lesions. The white rings around the lesions are secondary edema from the procedure. (Redrawn from Richter EO, Davis KD, Hamani C, et al. Cingulotomy for psychiatric disease: microelectrode guidance, a callosal reference system for documenting lesion location, and clinical results. Neurosurgery. 2004;54(3):622-628.)
Three views of anterior cingulotomy for treatment-resistant obsessive-compulsive disorder (OCD). The white arrows show the location of the lesions. The white rings around the lesions are secondary edema from the procedure. (Redrawn from Richter EO, Davis KD, Hamani C, et al. Cingulotomy for psychiatric disease: microelectrode guidance, a callosal reference system for documenting lesion location, and clinical results. Neurosurgery. 2004;54(3):622-628.)
DOES MEDICATING ANXIETY PREVENT LEARNING?
Medication and psychotherapy alleviate anxiety through different mechanisms but with equal effectiveness. Combining the two treatment modalities is generally believed to be more efficacious than either of them alone, although this has not been conclusively established. However, some clinicians wonder whether medications limit the learning that is essential for effective psychotherapy.
There are only three large, combined psychotherapy/medication studies for the treatment of anxiety disorders that have long-term follow-up after stopping the medications:
1. Alprazolam and exposure therapy, alone and in combination, for panic disorder
2. CBT, imipramine, or their combination for panic disorder
3. Exposure therapy and sertraline in social phobia
In all three trials, one outcome was the same. When the medication was stopped, the group that received a combination of medication and psychotherapy deteriorated relative to the group that received psychotherapy alone. The figure shows the result for the CBT/imipramine study for panic.
Studies have shown that extinction results from the development of new memories rather than the erasure of the old fearful memories. Does medication limit the development of new memories that are required for effective, enduring psychotherapy? These studies suggest that some therapeutic changes that usually occur in the brain with psychotherapy are impeded by the presence of the psychoactive medication.
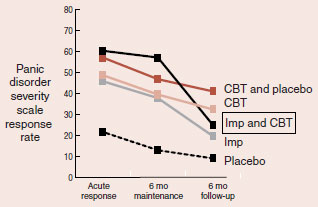
When treatment was stopped after the 6-month maintenance period, the group receiving imipramine (Imp) and cognitive-behavioral therapy (CBT) failed to sustain the benefits of psychotherapy.
1. Marks IM, et al. Brit J Psych. 1993;162:776-787.
2. Barlow DH, et al. JAMA. 2000;283:2529-2536.
3. Haug TT, et al. Brit J Psych. 2003;182:312-318.
QUESTIONS
1. Which hormone initially drops owing to the stress of parachute jumping?
a. Glucocorticoids.
b. Gonadotropins.
c. Growth hormone.
d. Mineralocorticoids.
2. All of the following are true about Klüver-Bucy syndrome, except
a. Made aggressive monkeys tame.
b. Induced hypersexuality.
c. Identified the hippocampus as important for anxiety.
d. Was elicited through removal of most of the temporal lobe.
3. Evidence that the amygdala is important for experiencing fear
a. Ischemic damage increases fear response.
b. Electric stimulation reduces freezing and heart rate.
c. Emotional trauma has no effect on the amygdala.
d. Treatment reduces activity in the amygdala.
4. The recognition of a threatening object includes all of the following, except
a. The brain reacts once all the signals are analyzed.
b. Signals from the amygdala stimulate heart rate.
c. Auditory and visual information proceed to the thalamus.
d. Memories modulate the amygdaloid response.
5. Stimulation of the medial PFC has what effect on a rat’s fear response?
a. Inhibits the expression of anxiety.
c. Increases the activity in the amygdala.
d. Decreases activity in the anterior cingulate gyrus.
6. The best estimate of the volume of a combat veteran’s hippocampus is
a. The total duration of trauma he experienced.
b. The intensity of combat trauma he experienced.
c. The level of glucocorticoids in the morning at rest.
d. The size of his twin’s hippocampus.
7. Medications decrease anxiety in all the following ways, except
a. Increase the GABA inhibitory signal.
b. Increase BDNF production at the NAc.
c. Modulation of the NE neurons.
d. Quieting the amygdala.
8. Imaging studies show increased activity in all the following regions in OCD patients, except
a. Orbitofrontal cortex.
b. Anterior cingulate gyrus.
c. Amygdala.
d. Basal ganglia.
See Answers section at the end of the book.